-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Mitigation of Prion Infectivity and Conversion Capacity by a Simulated Natural Process—Repeated Cycles of Drying and Wetting
Prion diseases such as chronic wasting disease and scrapie are emerging in North America at an increasing rate. Infectious prions are introduced into the environment from both living and dead animals where they can bind to soil. Little information is available on the effect of prion inactivation under conditions that would be found in the natural environment. In this study, we exposed both unbound and soil-bound prions to repeated cycles of drying and wetting to simulate ambient environmental conditions. We found evidence of prion inactivation in both unbound and soil bound prions. The influence of repeated cycles of drying and wetting are dependent on the prion strain and soil type used and, interestingly, prions bound to soil were more susceptible to inactivation. This is the first report of natural environmental processes mitigating prion infectivity. This data suggests that the total environmental prion load is a balance between input and natural clearance.
Published in the journal: . PLoS Pathog 11(2): e32767. doi:10.1371/journal.ppat.1004638
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004638Summary
Prion diseases such as chronic wasting disease and scrapie are emerging in North America at an increasing rate. Infectious prions are introduced into the environment from both living and dead animals where they can bind to soil. Little information is available on the effect of prion inactivation under conditions that would be found in the natural environment. In this study, we exposed both unbound and soil-bound prions to repeated cycles of drying and wetting to simulate ambient environmental conditions. We found evidence of prion inactivation in both unbound and soil bound prions. The influence of repeated cycles of drying and wetting are dependent on the prion strain and soil type used and, interestingly, prions bound to soil were more susceptible to inactivation. This is the first report of natural environmental processes mitigating prion infectivity. This data suggests that the total environmental prion load is a balance between input and natural clearance.
Introduction
Prion diseases, also known as transmissible spongiform encephalopathies (TSEs), are a group of fatal neurodegenerative diseases which impact a number of species including cattle (bovine spongiform encephalopathy, BSE), sheep and goats (scrapie), deer, elk and moose (chronic wasting disease, CWD), and humans (Creutzfeldt-Jakob disease, CJD, and others) [1]. The infectious agent of prion diseases, PrPSc, is a misfolded isoform of a non-infectious cellular prion protein, PrPC. CWD and scrapie prions can remain infectious over long time periods [2–5] in the environment. The increasing incidence and geographic range of CWD in cervids and its unknown host range makes this disease of particular concern in North America.
Prions enter the environment from infected hosts. Prions are shed into the environment via antler velvet [6], blood, saliva [7], urine [8–10], feces [11, 12], and birthing matter [13]. Prions also enter the environment through decomposition of infected animal carcasses [5]. Prions can be present in these excreta during the presymptomatic phase of disease, therefore, infected animals can shed prions into the environment over wide areas of the host’s home range. The amount of infectivity that is introduced into the environment is difficult to assess since prion titer is operationally defined with the route of infection, the age of the animal, the number of doses, and the PrP genotype of the host all making significant contributions in establishment of infection [14–17].
After release from an infected host, PrPSc binds to a wide range of soil and soil minerals [18–20]. Clay and clay soils have higher affinity for prions and adsorb PrPSc at a faster rate compared to sand or sandy soils [18, 19]. Binding of PrPSc to soil in a competitive matrix such as brain homogenate is slow and reduced compared to non-competitive environments (i.e. purified PrPSc) [18, 19]. Once bound to soil, prions remain highly infectious although soil-induced changes in in vitro PrPSc conversion efficiency and infectivity in animals have been measured [21, 22]. Further work is needed to fully elucidate the effect of PrPSc binding to surfaces on prion infectivity and transmission.
The contribution of soil bound prions to the natural transmission of prion disease is incompletely understood. Modeling studies conducted in CWD-endemic areas indicate that locations with a preponderance of organic soils correspond with an increased incidence of CWD [23–25]. Since the soil type that is bound to PrPSc has a relatively small influence on prion infectivity, other factors may play a role in prion transmission. Prions are primarily retained in surface soils [26] and the close contact of ruminant animals with soils renders soil-bound prions a likely source for prion disease transmission through ingestion or inhalation [27–30]. Therefore, PrPSc binding to soil may increase the bioavailability of prions for transmission. Inactivation of soil-bound prions will be required to control and prevent the spread of prion diseases in the environment.
Prion degradation under environmentally-relevant conditions is poorly understood. To date, the majority of studies have investigated degradation and inactivation of prions that are not bound to soil. Microorganisms and isolated enzymes, sometimes associated with harsh digestion conditions (high temperature and extreme pH), effectively reduce PrPSc abundance [31–34]. Exposure of prions to intact lichens at room temperature and neutral pH can reduce the abundance of PrPSc [35]. The loss of PrPSc immunoreactivity does not always correspond with a measurable reduction of prion infectivity [33, 36] therefore, studies that rely solely on changes in PrPSc abundance must be interpreted with caution. Prionzyme, a commercially-available enzyme, degraded soil-adsorbed prions under environmentally-relevant conditions and is the first evidence to suggest that mitigation of soil-bound prions is possible [37, 38].
Prions retained in surface soils are exposed to ambient environmental processes that have the potential to inactivate prions. Naturally-occurring cycles of drying and wetting alter soil aggregate stability and can influence interactions between soil particulate organic matter and dissolved organic compounds [39–41]. They can also change microbial activity and population dynamics [39, 40, 42]. Additionally, dehydration can unfold the native protein structure [43]. It is not known if these processes alter the biologic properties of soil-bound prions. To address this important question, we investigated the effects of repeated cycles of drying and wetting on the fitness of prions bound to various soil types.
Results
Repeated cycles of drying and wetting reduced the abundance of total protein in hamster brain homogenate
The abundance of total protein in the brain homogenate (BH) of hamster infected with HY TME before or after binding to silty clay loam (SCL) was shown in Fig. 1. The amount of total protein of unadsorbed BH was significantly reduced (p<0.05) by 13% (Fig. 1). After binding to SCL, the amount of total protein remained unchanged (Fig. 1) suggesting protection of proteins from degradation of repeated drying and wetting by adsorption to soil surface. Repeated cycles of wetting and drying did not result in changes in pH or conductivity.
Fig. 1. Reduced total protein abundance in HY TME brain homogenate. 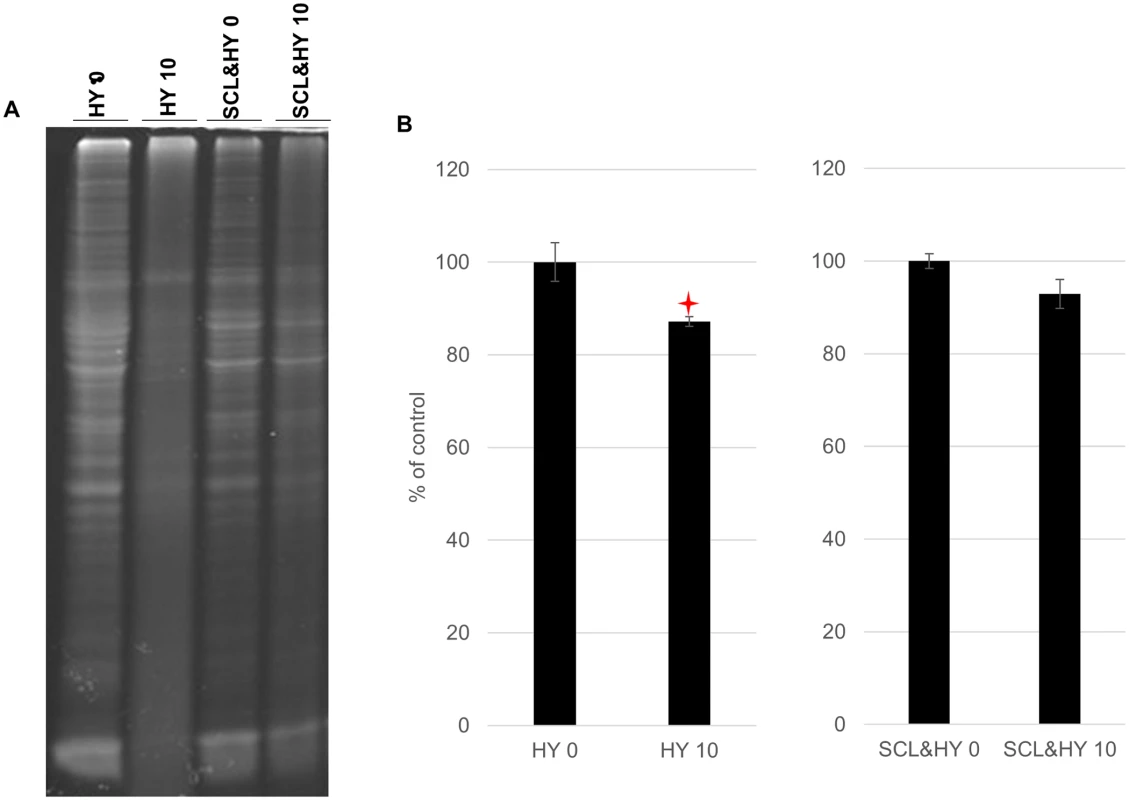
UV scanned polyacrylamide gel stained with SYPRO Ruby (A) and quantification of total proteins (B). Samples were not digested with proteinase K. Star indicates significant difference (p<0.05; n = 3) between treated and untreated sample. Repeated cycles of drying and wetting alter the resistance of HY PrPSc to digestion with proteinase K
Soil - or soil mineral-adsorbed HY PrPSc (HY) was prepared as described in Table 1 and subjected to 0 (control), 1, and 10 repeated cycles of drying and wetting. Significant differences (p>0.05) were not observed in normalized PrPSc immunoreactivity between samples after 1 drying and wetting cycle (Dry 1) compared to the negative control (no drying and wetting treatment, Dry 0) (Fig. 2). PrPSc abundance was significantly decreased (p<0.05) between the negative control and 10 repeated cycles of drying and wetting of unbound HY, silty clay loam (SCL)-, bentonite-, and silicon dioxide (SiO2)-adsorbed HY (Fig. 2). The average reductions are 51%, 53%, 72%, and 73%, respectively (Fig. 2B). The PrPSc abundance of sandy loam soil (SLS)-adsorbed and sand-adsorbed HY PrPSc were not significantly (p>0.05) changed following 10 repeated cycles of drying and wetting (Fig. 2).
Tab. 1. Preparation of soil bound HY TME, DY TMEa, and elk CWDa PrPSc. 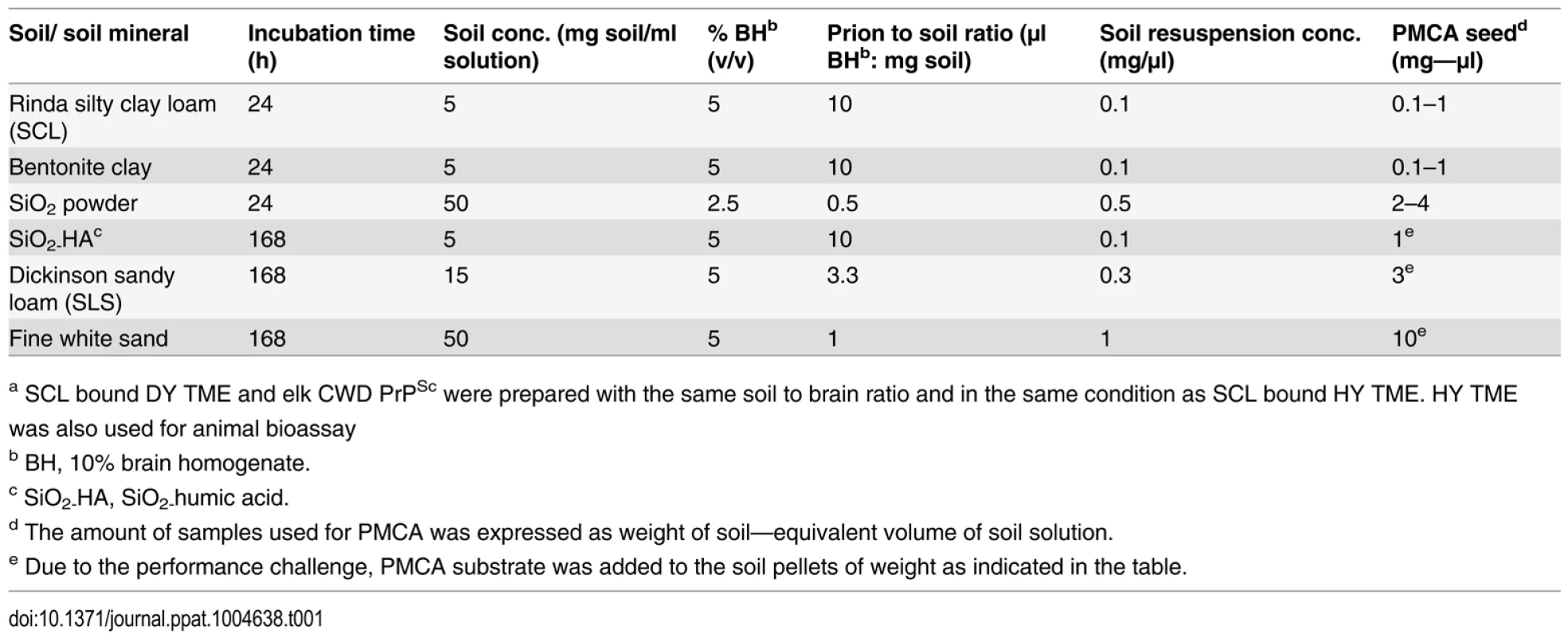
a SCL bound DY TME and elk CWD PrPSc were prepared with the same soil to brain ratio and in the same condition as SCL bound HY TME. HY TME was also used for animal bioassay Fig. 2. Repeated cycles of drying and wetting alter the resistance of HY PrPSc to digestion with proteinase K. 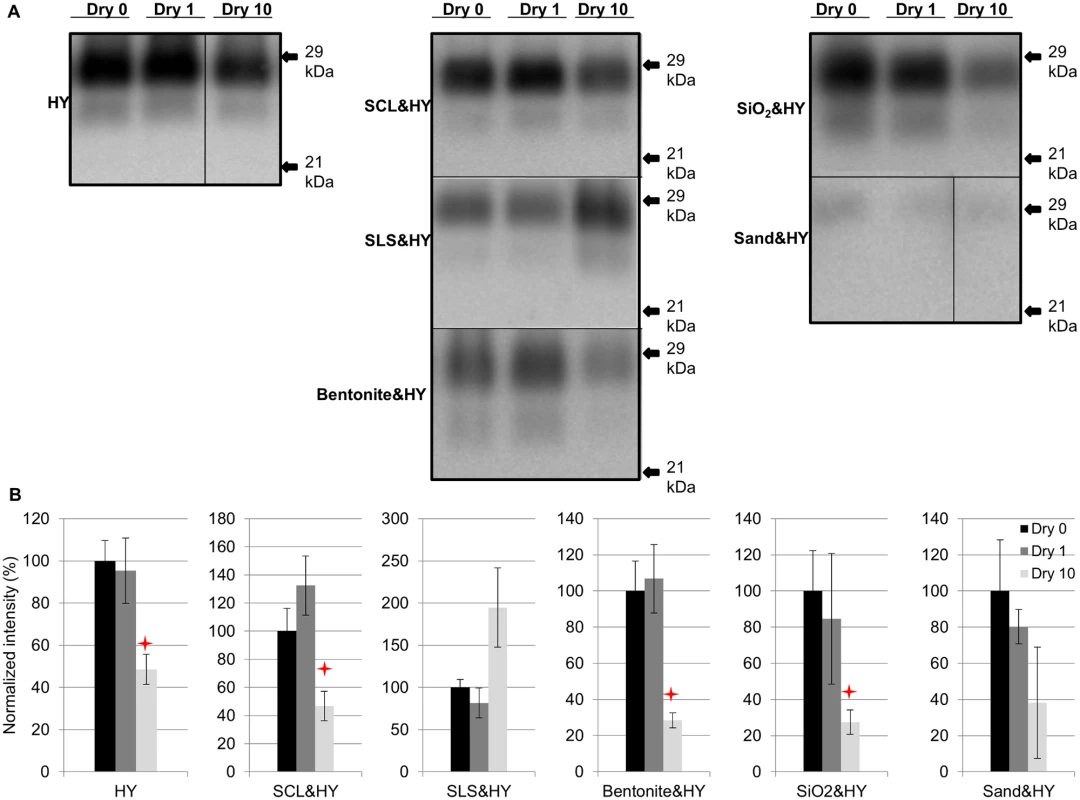
Western blot (A) and quantification (B) of PK digested HY PrPSc alone or adsorbed to soil before (Dry 0) and after 1 (Dry 1) and 10 (Dry 10) serial rounds of drying and wetting. Migration of 29 and 21 kDa molecular weight marker is indicated on the right of the Western blot. Star indicates significant difference (p<0.05; n = 3) between treated and untreated sample. Repeated cycles of drying and wetting reduced the PMCA conversion efficiency of soil bound HY TME
After 1 round of PMCA, PrPSc amplification in all samples subjected to 1 cycle of drying and wetting was not significantly (p>0.05) different compared to unbound HY (Fig. 3). The amplification of unbound HY, SCL-, SiO2-, bentonite-, and sand-adsorbed HY subjected to 10 drying/wetting cycles was significantly (p<0.05) reduced by 48%, 95%, 100% (negative value for bentonite-HY was corrected to 0%), 74%, and 95%, respectively (Fig. 3). However, the PMCA conversion efficiency of SiO2 HA-adsorbed HY was not changed (p>0.05) after 10 cycles of drying and wetting (Fig. 3). Sandy loam soil inhibits HY PrPSc PMCA conversion independent of HY adsorption resulting in low PrPSc abundance (Fig. 3).
Fig. 3. Repeated cycles of drying and wetting reduced the PMCA conversion efficiency of soil bound HY TME. 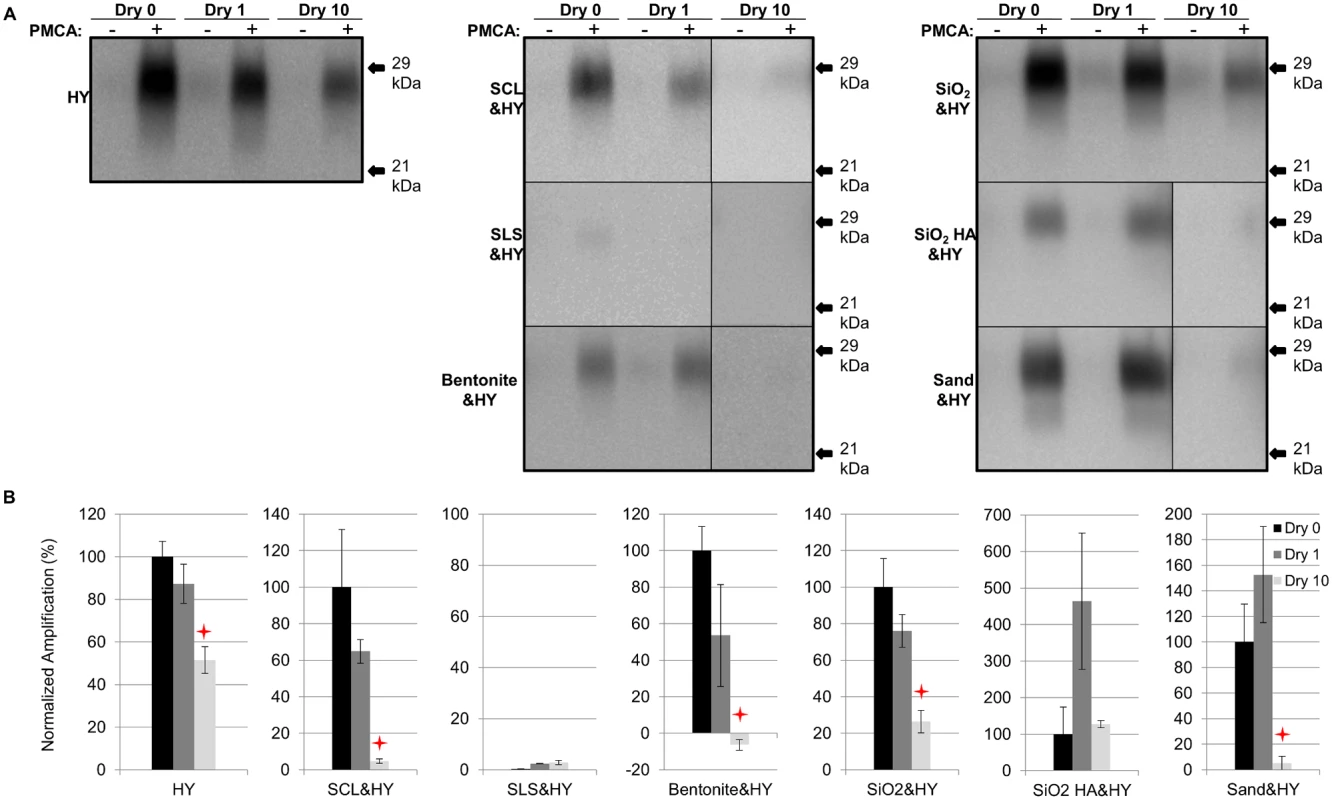
Western blot (A) and quantification (B) of PMCA amplification (1 round) of HY PrPSc alone or adsorbed to soil before (Dry 0) and after 1 (Dry 1) and 10 (Dry 10) serial rounds of drying and wetting. Migration of 29 and 21 kDa molecular weight marker is indicated on the right of the Western blot. Star indicates significant difference (p<0.05; n = 3) between treated and untreated sample. Influence of repeated cycles of drying and wetting on proteinase K resistance and amplification efficiency of DY TME
Samples were prepared as described in Table 1. A significant (p>0.05) difference in PrPSc immunoreactivity was not observed for unbound DY or SCL-adsorbed DY after 1 drying and wetting cycle compared to the control (Fig. 4A and 4C). A significant (p<0.05) reduction in PrPSc abundance of 48% was observed for unbound DY treated with 10 cycles of drying and wetting while the PrPSc abundance of SCL-bound DY was not significantly (p>0.05) changed (Fig. 4A and 4C). After 3 rounds of PMCA, DY and SCL-bound DY amplified to similar (p>0.05) levels after 1 cycle of drying and wetting compared to controls (Fig. 4B and 4D). When exposed to 10 drying/wetting cycles, a significant reduction (p<0.05) in amplification was only observed for SCL-bound DY by 68% and not for unbound DY (Fig. 4B and 4D).
Fig. 4. Influence of repeated cycles of drying and wetting on proteinase K resistance and amplification efficiency of DY TME. 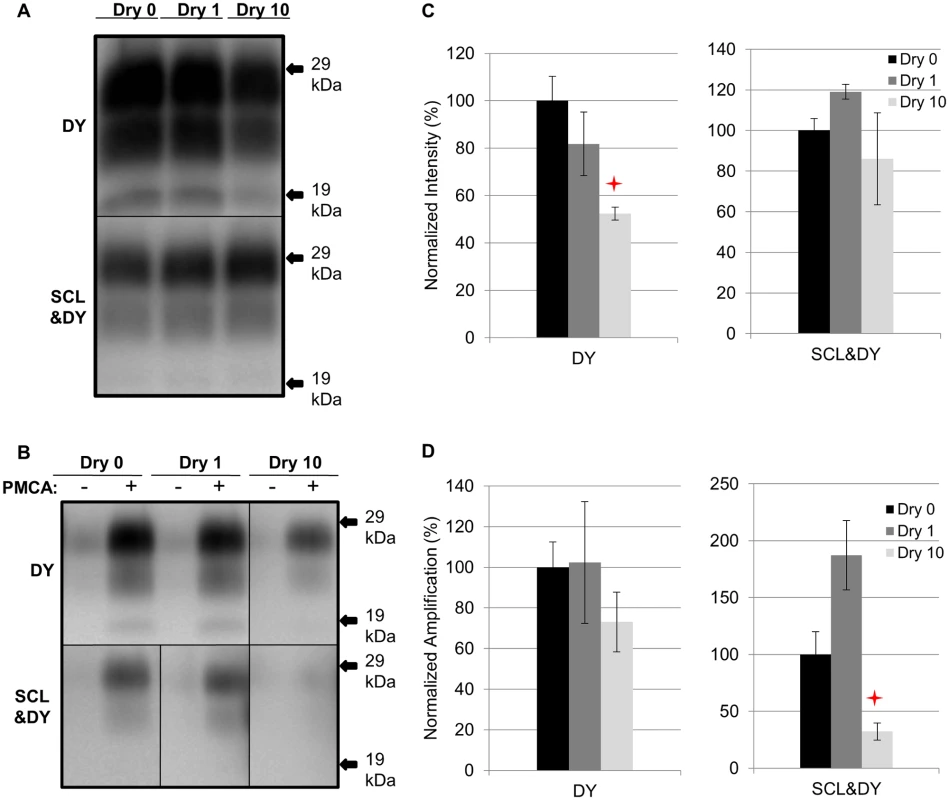
Western blot (A and B) and quantification (C and D) of PK digested and PMCA amplification (3 rounds) of DY PrPSc alone or adsorbed to SCL before (Dry 0) and after 1 (Dry 1) and 10 (Dry 10) serial rounds of drying and wetting. Negative PMCA samples were diluted from corresponding PMCA seeding with a dilution factor of 80. Migration of 29 and 19 kDa molecular weight marker is indicated on the right of the Western blot. Star indicates significant difference (p<0.05; n = 3) between treated and untreated sample. Influence of repeated cycles of drying and wetting on proteinase K resistance and amplification efficiency of CWD
SCL-adsorbed CWD was prepared as described in Table 1. Compared to the untreated samples (Dry 0), the PrPSc abundance did not significantly (p>0.05) differ between SCL-bound and unbound CWD with up to 10 cycles of drying and wetting (Fig. 5A and 5C). The PMCA conversion efficiency of unbound CWD after 3 rounds of PMCA was not significantly (p>0.05) different through 10 repeated cycles of drying and wetting compared to controls (Fig. 5B and 5D). In contrast, a significant (p<0.05) reduction of 83% in PMCA conversion efficiency of SCL-bound CWD after 10 cycles of drying and wetting treatment was observed (Fig. 5B and 5D).
Fig. 5. Influence of repeated cycles of drying and wetting on proteinase K resistance and amplification efficiency of elk CWD. 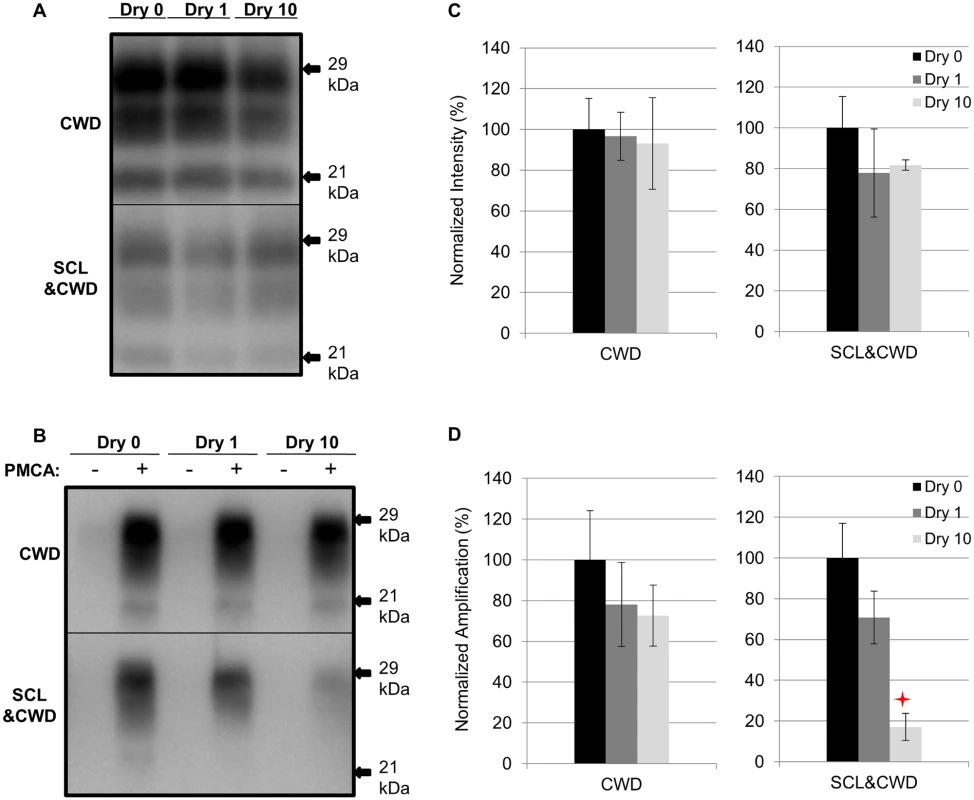
Western blot (A and B) and quantification (C and D) of PK digested and PMCA amplification (3 rounds) of elk CWD PrPSc alone or adsorbed to SCL before (Dry 0) and after 1 (Dry 1) and 10 (Dry 10) serial rounds of drying and wetting. Migration of 29 and 21 kDa molecular weight marker is indicated on the right of the Western blot. Star indicates significant difference (p<0.05; n = 3) between treated and untreated sample. Sorption of HY to soil reduces the number of wet dry cycles that result in a decrease in PMCA conversion activity
Unbound HY or SCL-adsorbed HY were subjected to 3, 5 or 7 repeated rounds of drying and wetting. Samples were then subjected to 1 round of PMCA and the abundance of amplified PrPSc was quantified. Unbound HY had similar (p>0.05) conversion efficiency at 1, 3, 5 and 7 repeated cycles of drying and wetting. However, 10 cycles of repeated drying and wetting resulted in a significant (p<0.05) reduction in HY PrPSc amplification compared to the sample treated with 1 cycle of drying and wetting (Fig. 6A and 6C). In contrast, after 3 repeated rounds of drying and wetting of SCL-bound HY amplification was significantly (p<0.05) inhibited (Fig. 6B and 6D). These results demonstrate that, under the conditions tested, binding to SCL enhances the reduction in conversion efficiency induced by repeated cycles of drying and wetting.
Fig. 6. Sorption of HY to soil reduces the number of wet dry cycles that result in a decrease in PMCA conversion activity. 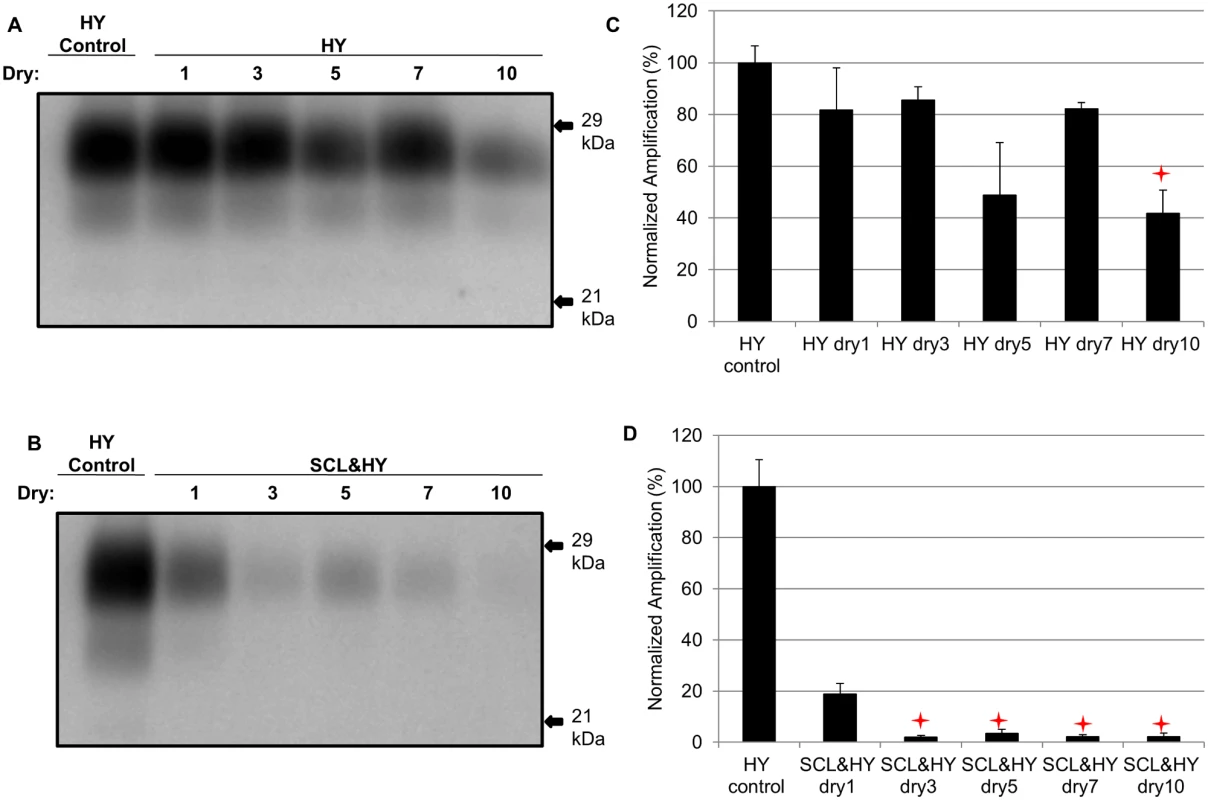
Western blot (A and B) and quantification (C and D) of PMCA amplification (1 round) of HY PrPSc alone or adsorbed to SCL after 1, 3, 5, 7, and 10 serial rounds of drying and wetting. Migration of 29 and 21 kDa molecular weight marker is indicated on the right of the Western blot. Star indicates significant difference (p<0.05; n = 3) between multiple drying/wetting treated and single drying/wetting treated sample. PMCA conversion efficiency is decreased by sorption to soil
Unabsorbed and SCL-adsorbed HY was subject to 0 or 10 serial rounds of wetting and drying. The samples were adjusted to equalize the abundance of PrPSc between the samples. Ten-fold serial dilutions, ranging from 10-2 to 10-8, of these samples were subject to one round of PMCA. PMCA reactions seeded with untreated HY-SCL resulted in detectable PrPSc though the 10-4 dilution, while PMCA reaction seeded with an equal amount of HY-SLC PrPSc that was treated with 10 serial rounds of wetting and drying resulted in detectable PrPSc though the 10-2 dilution (Fig. 7). These results indicate that 10 serial rounds of wetting and drying reduce the specific activity of SCL absorbed HY PrPSc by two logs.
Fig. 7. Reduced PMCA conversion coefficient of SCL-HY after repeated cycles of drying and wetting. 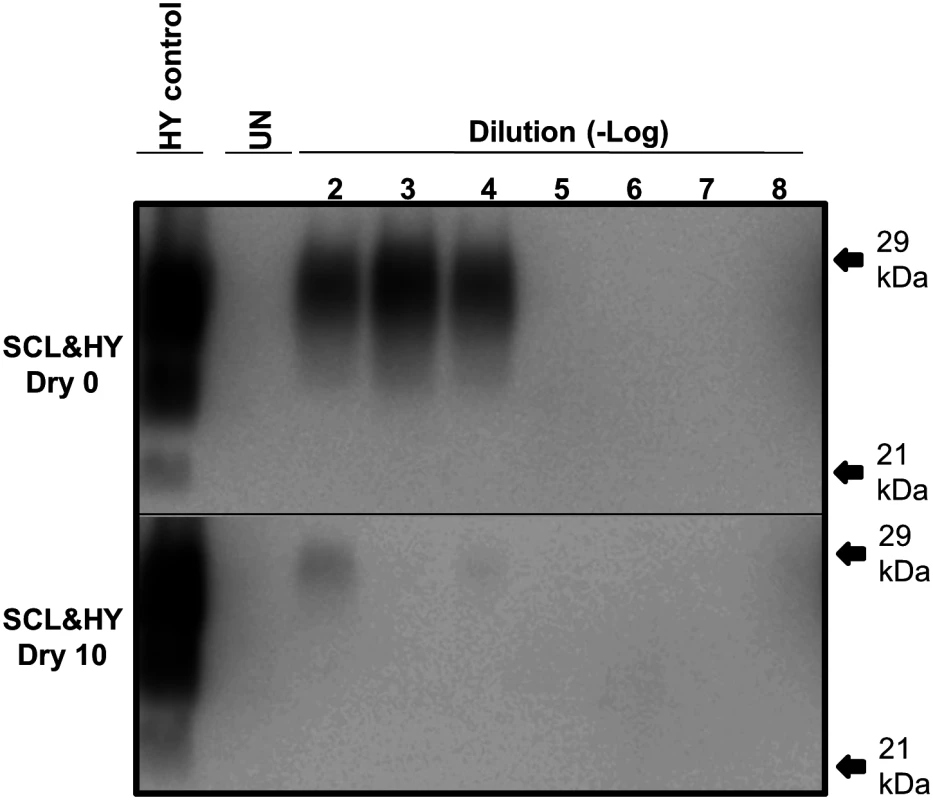
Western blot of PMCA amplification of 10-fold serial dilutions of standardized SCL-HY PrPSc before (Dry 0) or after 10 (Dry 10) rounds of drying and wetting. Samples in replicates (n = 3) were tested. Migration of 29 and 21 kDa molecular weight marker is indicated on the right of the Western blot. Repeated cycles of drying and wetting extend the incubation period of prion infection
Selected drying and wetting treated samples and untreated controls were intracerebrally inoculated into Syrian hamsters. Incubation periods for hamsters inoculated with HY, SCL-, SiO2-, and SLS-bound HY subjected to 0, 1, or 10 drying/wetting cycles are summarized in Table 2 and the survival results are presented in Fig. 8. Consistent with PK-resistance and PMCA results (Fig. 2 and 3), the incubation period of hamsters inoculated with SCL-HY subjected to 1 cycle of drying and wetting did not significantly (p>0.05) differ compared to hamsters inoculated with untreated SCL-HY (Fig. 8 and Table 2). The incubation period of HY - and SCL-HY-inoculated hamsters subjected to 10 cycles of drying/wetting was significantly (p<0.05) extended 13 days compared to that of hamsters inoculated with the untreated control (Fig. 8 and Table 2). This extension of the incubation period is consistent with a 2 log reduction in prion titer [21]. The incubation period of hamsters inoculated with SLS-HY and SiO2-HY treated with 10 cycles did not significantly (p>0.05) differ compared to hamsters inoculated with untreated control (Fig. 8 and Table 2).
Tab. 2. Incubation periods of hamsters intracerebrally inoculated with unbound and soil-bound PrP<sup>Sc</sup>. 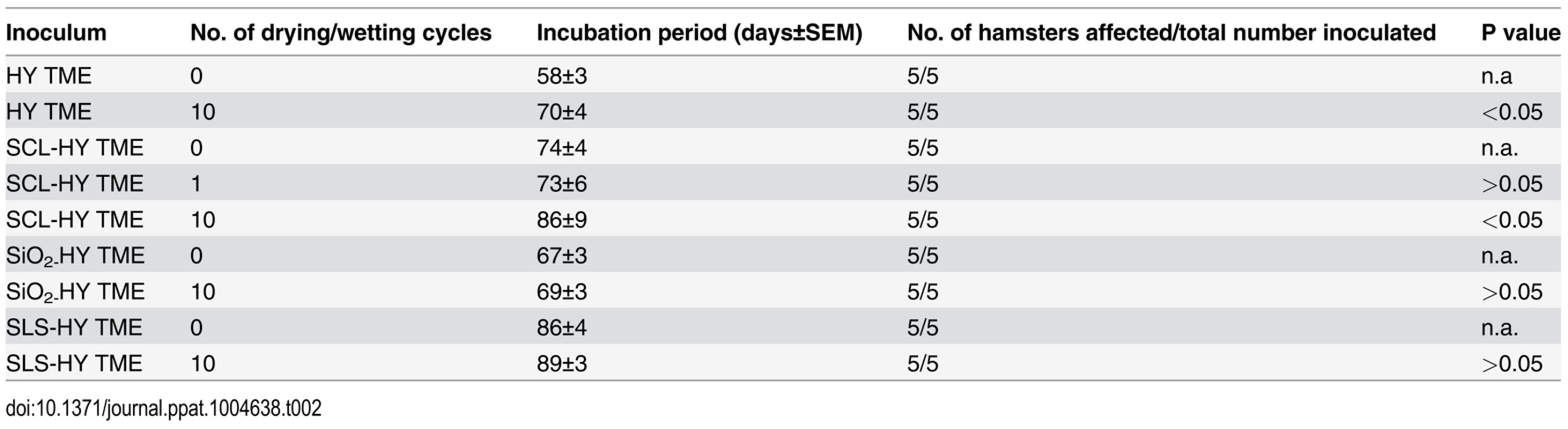
Fig. 8. Repeated cycles of drying and wetting extend the incubation period of prion infection. 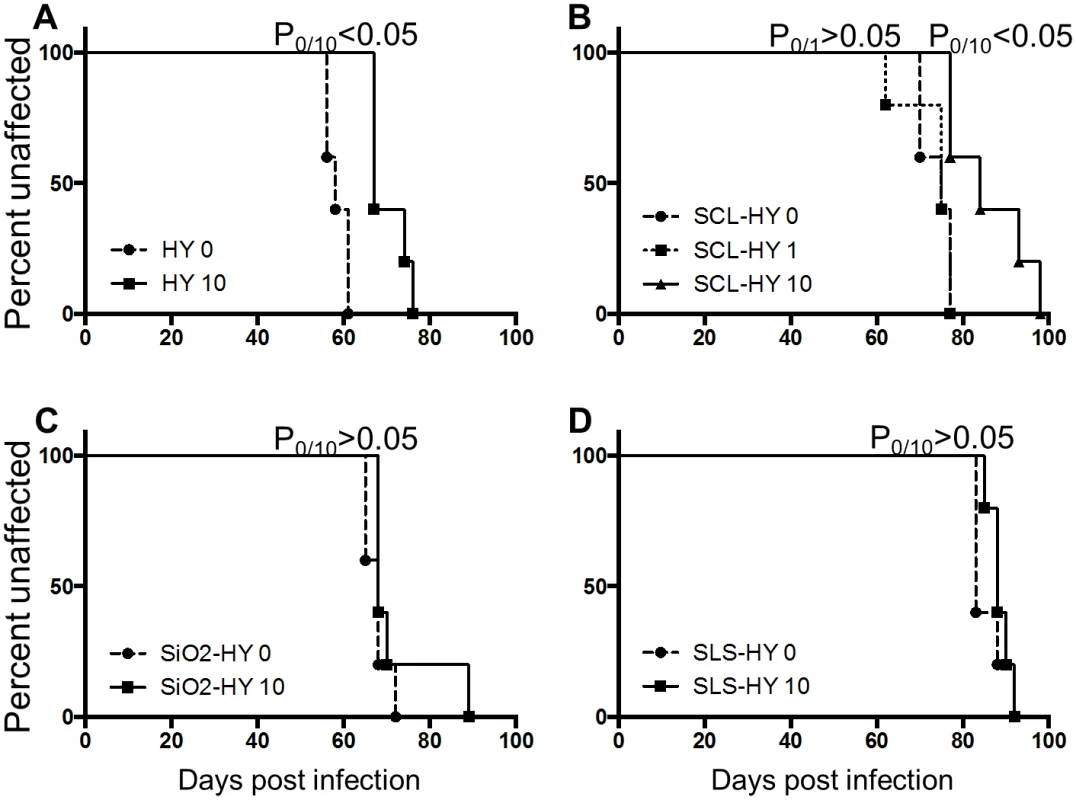
Survival curve of hamsters inoculated with untreated (0) or drying/wetting treated (1 or 10) HY (A), SCL-HY (B), SiO2-HY (C), and SLS-HY (D). 5 hamsters (n = 5) were used for each sample. P0/1 or P0/10 designated p value derived from the comparison of incubation periods of hamsters inoculated with untreated (0) and 1 or 10 cycle(s) of drying and wetting treated samples. Discussion
Prions have been detected in the environment [2–5, 44, 45] and they can survive for years in soils [2–5]. Prion shedding and persistence in the environment is well documented, however, little is known about environmental degradation of prions. In this study we investigated if prion infectivity is mitigated by natural environmental conditions. Under natural conditions of repeated wetting and drying we found evidence of PrPSc degradation, decreased PrPSc conversion activity, and an increase in prion incubation period suggesting reduced prion infectivity. This effect was dependent on the soil type that was bound to PrPSc and the source of prions, underscoring the complexity of the interaction of prions with soil.
Reduced PrPSc abundance after repeated cycles of drying and wetting
Repeated cycles of drying and wetting degraded not only PrPSc but also other proteins in the brain homogenate whereas binding to soil prior to repeated cycles of wetting and drying eliminated this effect. (Fig. 1). While the exact mechanism of prion protein degradation due to repeated cycles of drying and wetting are not known, several possibilities exist. We hypothesize that exposure to repeated cycles of drying and wetting results in protein conformational changes that render PrPSc more susceptible to degradation. Loss of water and changes in ion concentrations and pH in solution may occur during dehydration which can affect the secondary structure of proteins [43, 46–48], however, changes in ionic strength or pH were not observed between cycles in this study. Although poorly understood, changes in soil properties such as surface charge or cation exchange capacity occur after repeated drying and wetting cycles [49, 50] that can result in desorption and/or reorganization of adsorbed compounds including proteins.
Alternatively, consolidation of the bonding between soil and the adsorbed PrPSc after drying may make PrPSc desorption more difficult resulting in reduced PrPSc detection and prion infectivity. The implication of this possibility is that PrPSc desorption is required for prion conversion and western blot detection. This explanation is consistent with previous findings that PrPSc attached to stainless steel surfaces (dried onto surface or as incubation solution) becomes more resistant to decontamination when exposed to extended drying compared with no drying or wet storage condition [51, 52]. We speculate more compact stacking of PrPSc aggregates on the surface may occur during drying as water molecules evaporate, minimizing PrPSc exposure to the surroundings.
Repeated drying and wetting renders soil-bound prion less infectious
The reduced PMCA conversion coefficient in combination with the extended incubation period in hamster bioassay compared to untreated prions (Fig. 3 and 8, Table 2) are consistent with a 2 log reduction in prion infectivity of soil-bound prions [21] after 10 cycles of drying/wetting. While both PMCA and bioassay data are in agreement with a 2 log reduction in titer following 10 cycles of drying/wetting, since titer was not directly calculated, this value should be interpreted with caution. It is unknown if additional cycles of wetting will further reduce infectivity or if complete prion inactivation is possible. The observed reduction in infectivity is not entirely due to loss of PrPSc. When standardized for the amount of starting PrPSc, the PMCA conversion coefficient of HY bound to SCL that has not been treated to repeated cycles of wetting and drying is two orders of magnitude greater compared to HY-SCL that has been repeatedly wetted and dried for 10 cycles (Fig. 6). Since the reduction in the specific activity of PrPSc is enhanced when HY is bound to SCL, we hypothesize at the PrPSc-SCL interface, the wetting and drying process is altering a property of PrPSc that renders it less infectious.
Effect of repeated drying and wetting on the properties of soil-bound prions is soil type and strain dependent
Changes in PrPSc abundance and PMCA conversion efficiency after repeated cycles of drying and wetting are observed for a subset of unbound and soil-bound PrPSc. HY and DY PrPSc are more susceptible to PK digestion following repeated cycles of drying and wetting compared to CWD (Figs. 2, 3, 4, and 5). Binding of DY PrPSc to SCL protects DY PrPSc by enhancing PK resistance but results in a reduction in DY and CWD PrPSc conversion activity (Figs. 4 and 5). Adsorption of the same PrPSc to different soils resulted in a greater or lesser effect of repeated drying/wetting on PrPSc properties (Figs. 2 and 3). Factors that may contribute to the variation include soil surface properties and soil-prion bonding. Since the percentage of sand and clay in a soil resulted in different soil stability changes in response to drying/wetting cycles we hypothesize that clay-clay and clay-sand interactions can indirectly affect the soil-prion interface [41]. Overall, this dynamic can affect which prion strains persist and the relative titer in any given environment. Since the relative ratios of prion strains can affect strain emergence, the environment would be expected to have a strong influence on the overall dynamics of transmission and strain emergence.
Environmental factors affecting the efficiency of repeated drying and wetting
Since prions are likely to be immobile in surface soils [26], soil-bound prions are readily accessible to ambient weather conditions which can include changes in surface soil moisture. In some CWD-endemic areas such as Phantom Valley (Latitude: 40.4°N; Longitude: 105.9°W) close to Rocky Mountain National Park, the number of moisture change in surface soil (soil depth is 0.5 m) can be up to 28 per month [53]. Based on our findings, drying/wetting cycles which repeatedly change soil moisture may be a natural degradation pathway for soil-bound prions. Additionally, wetting and drying can change microbial activity in soils [39, 40] which may affect prion-soil interactions. Ambient temperature likely does not contribute to natural prion degradation since most heat induced decontamination (incomplete inactivation) occurs at temperatures well over 100°C [54–57]. Enzymes secreted from soil microorganisms, such as serine proteases, may be auxiliary for soil-bound prion degradation and inactivation, however, they were only found to be effective at high temperature, high pH or both [58, 59]. Overall, this study provides the first evidence that natural processes can reduce prion infectivity. Since the total environmental prion load is a balance between addition of prions to the environment and clearance of prions from the environment, efforts to limit prion input into the environment may positively affect this balance and have meaningful results in reducing environmental transmission of prion diseases. Additionally, the soil composition and hydrology of an area may shape the overall transmission dynamics and alter strain prevalence of prions.
Materials and Methods
Prion sources and tissue preparation
Prion-infected brain tissues were collected from hamsters infected with either the hyper (HY) or drowsy (DY) strain of transmissible mink encephalopathy (TME) or from a naturally infected elk with CWD agent as described previously [20]. Infected brains were homogenized to 10% (w/v) in Dulbecco’s phosphate-buffered saline (DPBS) without Ca2+ or Mg2+ (Mediatech, Herndon, VA) using strain-dedicated Tenbroeck tissue grinders (Kontes, Vineland, NJ). Uninfected Syrian hamster and uninfected Tg(CerPrP)1536 mouse brain was homogenized to 10% (w/v) in ice-cold PMCA conversion buffer (DPBS [pH 7.5] containing 5 mM EDTA, 1% (v/v) Triton X-100, and a complete protease inhibitor tablet [Roche Diagnostics, Mannheim, Germany]). The homogenate was centrifuged at 460×g for 30 seconds and the supernatant was collected and stored at -80°C.
Prion adsorption to soils and soil minerals
Soils and soil minerals used in this study included sterile Rindasilty clay loam (SCL) soil (a VerticEpiaqualf); sterile Dickinson sandy loam soil (SLS, a TypicHapludoll); sodium bentonite clay (CETCO, Arlington Heights, IL); silicon dioxide powder (Sigma Aldrich, St. Louis, MO); humic acid (HA)-coated silica gel particles (SiO2 HA); and gamma-irradiated fine white sand (Fisher Scientific, Pittsburgh, PA). Physicochemical properties of these soils and soil minerals have been described previously [19, 38]. To obtain soil bound prions, 10% brain homogenate (BH) was added to soil and for each soil or soil mineral, the incubation time and prion to soil ratios, were selected based on previous studies [19, 20] (Table 1). Each BH-soil combination was prepared in triplicate. The BH-soil mixture was rotated at 24 rpm (Mini Labroller, Edison, NJ) at room temperature. Samples were removed after incubation and centrifuged at 100× g for 5min. The supernatant was removed and the pellets were washed a minimum of three times with 1× DPBS. The soil pellets were resuspended in 1× DPBS at concentrations described in Table 1 and were stored at -80°C until use. HY TME, DY TME, and elk CWD BH were used as unbound controls.
Drying and wetting treatment
Each sample was placed in an uncapped 200 µL PCR tube (Thermo Scientific) and incubated at 40°C. Samples were dried and weighed periodically until there was less than a 0.5% change in weight resulting in a minimum of 7 hr drying time. To perform consistently, around 12 hours’ drying is selected for one cycle. Dried samples were rehydrated with 10 µl of ultrafiltered deionized water and mixed thoroughly. The drying followed by rewetting constituted one drying/wetting cycle. Conductivity and pH were measured with an Oakton 700 bench top meter using a 3-point calibration curve. After the desired number of treatment cycles, samples were stored at -80°C until use.
Animal bioassay
Intracerebral (i.c.) inoculations of Syrian hamsters (Harlan Sprague-Dawley, Indianapolis, IN) were conducted as described previously [30, 60]. Silty clay loam soil HY TME (untreated, and 1 - and 10-drying/wetting-cycle treated) and silicon dioxide powder adsorbed HY TME (untreated, and 10-drying/wetting-cycle treated) were selected as inocula. The incubation period was determined as the length of time in days between inoculation and the onset of clinical signs of HY TME.
PMCA
Protein misfolding cyclic amplification (PMCA) was performed as described previously [61]. Sonication was performed with a Misonix (Farmingdale, NY) 4000 sonicator with amplitude set to level 75, generating an average output of 160 W during sonication treatment. Samples were diluted with 10% (w/v) uninfected hamster or elk brain homogenate at 1 : 100 for HY TME and CWD, and 1 : 20 for DY TME for the first round. After one round, homogenate from round 1 was diluted at 1 : 20 for HY TME, 1 : 10 for elk CWD, and 1 : 1 for DY TME for the subsequent rounds. Each round was performed at 37°C for either 24 hr for HY TME and DY TME consisted of 144 cycles of 5 s sonication followed by 9min 55s of incubation or 48 hr for elk CWD consisted of 288 cycles with the same sonication/incubation time as HY TME and DY TME. Before each PMCA round, an aliquot was placed at -80°C as an unsonicated control. Samples containing only 10% (w/v) uninfected brain homogenate were included with each PMCA round as negative controls.
Western blot analysis
Western blot analysis was performed as described previously [62]. Briefly, samples were incubated and digested with 22.5 µg/ml (HY TME/DY TME) or 45 µg/ml (elk CWD) proteinase K (PK) (Roche Diagnostics Corporation, Indianapolis, IN) at 37°C for 30 min (HY TME/DY TME) or 1 hr (CWD) with constant agitation. The PK digestion was terminated by boiling in 1x SDS-PAGE sample buffer (final concentration). The samples were size fractionated with 12.5% SDS-PAGE and transferred to a polyvinylidenedifluoride membrane (NuPage; Invitrogen, Carlsbad, CA). The membrane was blocked with 5% w/v nonfat dry milk in 1× TTBS (Bio-Rad Laboratories, Hercules, CA) for 30 min. Hamster samples were immunoblotted with MAb 3F4 (Chemicon, Temecula, CA; 1 : 10,000). Elk/Tg(CerPrP)1536 samples were immunoblotted with 8H4 (1 : 10,000). The blots were developed with Supersignal West Femto maximum sensitivity substrate, according to the manufacturer’s instructions (Pierce, Rockford, IL), imaged on a 4000R imaging station (Kodak, Rochester, NY), and analyzed using Kodak (New Haven, CT) molecular imaging software, V.5.0.1.27. Densities of sample replicate (n≥3) intensities were standardized to brain homogenate controls on the same gel to control for inter-gel variance. PMCA amplification is determined as the absolute difference of intensity density between unamplified and sonicator-amplified samples. For each type of soil, unbound prions and untreated samples (Dry 0) were used as controls to determine the effect of drying/wetting (Dry 1 and Dry 10) on PrPSc seeding efficiency. Intensities of the same control were averaged out through the entire study. Statistical analysis (Student’s t test with Welch’s correction, two-tailed P value) was performed using Prism 6.0 (GraphPad Software, Inc., San Diego, CA) by separately comparing samples with each treatment to the untreated control or samples with the least treatment.
SYPRO Ruby gel staining
Separated proteins in the polyacrylamide gel were stained with SUPRO Ruby following the protocol provided by the manufacturer. Briefly, the gel was placed in a clean plastic dish and incubated in a fixative solution containing 10% methanol and 7% acetic acid at room temperature with gentle agitation for two 15 minutes. Then the gel was incubated in undiluted SYPRO Ruby staining solution overnight without being exposed to light. Stained gel was then transferred to a clean plastic dish and washed with 10% methanol and 7% acetic solution for two times followed by one time rinse with MQ H2O. The gel was imaged in an electrophoresis gel imaging imager cabinet (Bio-rad Universal Hood ii) using UV epi-illumination and analyzed by a 1-D analysis software Quantity One version 4.6.7. The lightness density of samples of interest were compared and significance was analyzed with build-in t test (two-tailed p value with unequal variance) in Microsoft Excel 2013.
Ethics statement
All procedures involving animals were approved by Creighton University Institutional Animal Care and Use Committee (protocol number 0 872 and 0881) and comply with the Guide for the Care and Use of Laboratory Animals.
Zdroje
1. Prusiner SB (2004) Prion Biology and Diseases.; Prusiner SB, editor. NY: Cold Spring Harbor. 8865151
2. Brown P, Gajdusek DC (1991) Survival of scrapie virus after 3 year’s interment. Lancet 337 : 269–270. doi: 10.1016/0140-6736(91)90873-N 1671114
3. Georgsson G, Sigurdarson S, Brown P (2006) Infectious agent of sheep may persist in the environment for at least 16 years. J Gen Virol 87 : 3737–3740. doi: 10.1099/vir.0.82011-0 17098992
4. Miller MW, Williams ES (2003) Horizontal prion transmission in mule deer. Nature 425 : 35–36. doi: 10.1038/425035a 12955129
5. Miller MW, Williams ES, Hobbs NT, Wolfe LL (2004) Environmental source of prion transmission in mule deer. Emerg Infect Dis 10 : 1003–1006. doi: 10.3201/eid1006.040010 15207049
6. Angers RC, Seward TS, Napier D, Green M, Hoover E, et al. (2009) Chronic Wasting Disease Prions in Elk Antler Velvet. Emerging Infectious Diseases 15 : 696–703. doi: 10.3201/eid1505.081458 19402954
7. Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, et al. (2006) Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314 : 133–135. doi: 10.1126/science.1132661 17023660
8. Kariv-Inbal Z, Ben-Hur T, Grigoriadis NC, Engelstein R, Gabizon R (2006) Urine from scrapie-infected hamsters comprises low levels of prion infectivity. Neurodegener Dis 3 : 123–128. doi: 10.1159/000094770 16954698
9. Murayama Y, Yoshioka M, Okada H, Takata M, Yokoyama T, et al. (2007) Urinary excretion and blood level of prions in scrapie-infected hamsters. J Gen Virol 88 : 2890–2898. doi: 10.1099/vir.0.82786-0 17872544
10. Seeger H, Heikenwalder M, Zeller N, Kranich J, Schwarz P, et al. (2005) Coincident scrapie infection and nephritis lead to urinary prion excretion. Science 310 : 324–326. doi: 10.1126/science.1118829 16224026
11. Maluquer de Motes C, Grassi J, Simon S, Herva ME, Torres JM, et al. (2008) Excretion of BSE and scrapie prions in stools from murine models. Vet Microbiol 131 : 205–211. doi: 10.1016/j.vetmic.2008.02.014 18395370
12. Safar JG, Lessard P, Tamguney G, Freyman Y, Deering C, et al. (2008) Transmission and detection of prions in feces. J Infect Dis 198 : 80–89. doi: 10.1086/588193 18505383
13. Race R, Jenny A, Sutton D (1998) Scrapie infectivity and proteinase K-resistant prion protein in sheep placenta, brain, spleen, and lymph node: implications for transmission and antemortem diagnosis. J Infect Dis 178 : 949–953. doi: 10.1086/515669 9806020
14. Kincaid AE, Bartz JC (2007) The nasal cavity is a route for prion infection in hamsters. Journal of Virology 81 : 4482–4491. doi: 10.1128/JVI.02649-06 17301140
15. Gough KC, Baker CA, Rees HC, Terry LA, Spiropoulos J, et al. (2012) The Oral Secretion of Infectious Scrapie Prions Occurs in Preclinical Sheep with a Range of PRNP Genotypes. Journal of Virology 86 : 566–571. doi: 10.1128/JVI.05579-11 22013047
16. Jacquemot C, Cuche C, Dormont D, Lazarini F (2005) High incidence of scrapie induced by repeated injections of subinfectious prion doses. Journal of Virology 79 : 8904–8908. doi: 10.1128/JVI.79.14.8904-8908.2005 15994784
17. Defaweux V, Dorban G, Antoine N, Piret J, Gabriel A, et al. (2007) Neuroimmune connections in jejunal and ileal Peyer’s patches at various bovine ages: potential sites for prion neuroinvasion. Cell and Tissue Research 329 : 35–44. doi: 10.1007/s00441-007-0396-4 17406903
18. Johnson CJ, Phillips KE, Schramm PT, McKenzie D, Aiken JM, et al. (2006) Prions adhere to soil minerals and remain infectious. Plos Pathog 2: e32. doi: 10.1371/journal.ppat.0020032 16617377
19. Saunders SE, Bartz JC, Bartelt-Hunt SL (2009) Prion protein adsorption to soil in a competitive matrix is slow and reduced. Environ Sci Technol 43 : 7728–7733. doi: 10.1021/es900502f 19921886
20. Saunders SE, Bartz JC, Bartelt-Hunt SL (2009) Influence of prion strain on prion protein adsorption to soil in a competitive matrix. Environ Sci Technol 43 : 5242–5248. doi: 10.1021/es900502f 19708348
21. Saunders SE, Shikiya RA, Langenfeld K, Bartelt-Hunt SL, Bartz JC (2011) Replication efficiency of soil-bound prions varies with soil type. J Virol 85 : 5476–5482. doi: 10.1128/JVI.00282-11 21430062
22. Johnson CJ, Pedersen JA, Chappell RJ, McKenzie D, Aiken JM (2007) Oral transmissibility of prion disease is enhanced by binding of soil particles. Plos Pathog 3: e93. doi: 10.1371/journal.ppat.0030093 17616973
23. Saunders SE, Bartz JC, Bartelt-Hunt SL (2012) Soil-mediated prion transmission: Is local soil-type a key determinant of prion disease incidence? Chemosphere 87 : 661–667. doi: 10.1016/j.chemosphere.2011.12.076 22265680
24. Walter WD, Walsh DP, Farnsworth ML, Winkelman DL, Miller MW (2011) Soil clay content underlies prion infection odds. Nat Comm 2 : 200. doi: 10.1038/ncomms1203
25. O’Hara Ruiz M, Kelly AC, Brown WM, Novakofski JE, Mateus-Pinilla NE (2013) Influence of landscape factors and management decisions on spatial and temporal patterns of the transmission of chronic wasting disease transmission in white-tailed deer. Geospatial health 8. 24258897
26. Jacobson KH, Lee S, Somerville RA, McKenzie D, Benson CH, et al. (2010) Transport of the Pathogenic Prion Protein through Soils. Journal of Environmental Quality 39 : 1145–1152. doi: 10.2134/jeq2009.0137 20830901
27. Saunders SE, Bartelt-Hunt SL, Bartz JC (2012) Occurrence, Transmission, and Zoonotic Potential of Chronic Wasting Disease. Emerging Infectious Diseases 18 : 369–376. doi: 10.3201/eid1803.110685 22377159
28. Smith CB, Booth CJ, Pedersen JA (2011) Fate of Prions in Soil: A Review. Journal of Environmental Quality 40 : 449–461. doi: 10.2134/jeq2010.0412 21520752
29. Beyer WN, Connor EE, Gerould S (1994) Estimates of soil ingestion by wildlife. Journal of Wildlife Management 58 : 375–382. doi: 10.2307/3809405
30. Kincaid AE, Bartz JC (2007) The nasal cavity is a route for prion infection in hamsters. J Virol 81 : 4482–4491. doi: 10.1128/JVI.02649-06 17301140
31. Huang H, Spencer JL, Soutryine A, Guan J, Rendulich J, et al. (2007) Evidence of degradation of abnormal prion protein in tissues from sheep with scrapie during composting. Can J Vet Res 71 : 34–40. 17193880
32. Langeveld JP, Wang JJ, Van de Wiel DF, Shih GC, Garssen GJ, et al. (2003) Enzymatic degradation of prion protein in brain stem from infected cattle and sheep. J Infect Dis 188 : 1782–1789. doi: 10.1086/379664 14639552
33. Scherbel C, Pichner R, Groschup MH, Mueller-Hellwig S, Scherer S, et al. (2006) Degradation of scrapie associated prion protein (PrPSc) by the gastrointestinal microbiota of cattle. Vet Res 37 : 695–703. doi: 10.1051/vetres:2006024 16820134
34. McLeod AH, Murdoch H, Dickinson J, Dennis MJ, Hall GA, et al. (2004) Proteolytic inactivation of the bovine spongiform encephalopathy agent. Biochem Bioph Res Co 317 : 1165–1170. doi: 10.1016/j.bbrc.2004.03.168 15094392
35. Johnson CJ, Bennett JP, Biro SM, Duque-Velasquez JC, Rodriguez CM, et al. (2011) Degradation of the disease-associated prion protein by a serine protease from lichens. Plos One 6: e19836. doi: 10.1371/journal.pone.0019836 21589935
36. Scherbel C, Pichner R, Groschup MH, Mueller-Hellwig S, Scherer S, et al. (2007) Infectivity of scrapie prion protein PrPSc following in vitro digestion with bovine gastrointestinal microbiota. Zoonoses Public Hlth 54 : 185–190. doi: 10.1111/j.1863-2378.2007.01040.x 17542960
37. Saunders SE, Bartz JC, Vercauteren KC, Bartelt-Hunt SL (2011) An enzymatic treatment of soil-bound prions effectively inhibits replication. Appl Environ Microbiol 77 : 4313–4317. doi: 10.1128/AEM.00421-11 21571886
38. Saunders SE, Bartz JC, Vercauteren KC, Bartelt-Hunt SL (2010) Enzymatic digestion of chronic wasting disease prions bound to soil. Environ Sci Technol 44 : 4129–4135. doi: 10.1021/es903520d 20450190
39. Cosentino D, Chenu C, Le Bissonnais Y (2006) Aggregate stability and microbial community dynamics under drying-wetting cycles in a silt loam soil. Soil Biology & Biochemistry 38 : 2053–2062. doi: 10.1016/j.soilbio.2005.12.022
40. Denef K, Six J, Bossuyt H, Frey SD, Elliott ET, et al. (2001) Influence of dry-wet cycles on the interrelationship between aggregate, particulate organic matter, and microbial community dynamics. Soil Biology & Biochemistry 33 : 1599–1611. doi: 10.1016/S0038-0717(01)00076-1
41. Singer MJ, Southard RJ, Warrington DN, Janitzky P (1992) Stability of synthetic sand clay aggregates after wetting and drying cycles Soil Science Society of America Journal 56 : 1843–1848.
42. Ma K, Conrad R, Lu YH (2013) Dry/Wet Cycles Change the Activity and Population Dynamics of Methanotrophs in Rice Field Soil. Applied and Environmental Microbiology 79 : 4932–4939. doi: 10.1128/AEM.00850-13 23770899
43. Kuntz IDJ, Kauzmann W (1974) Hydration of proteins and polypeptides. Adv Protein Chem 28. doi: 10.1016/S0065-3233(08)60232-6 4598824
44. Nichols TA, Pulford B, Wyckoff AC, Meyerett C, Michel B, et al. (2009) Detection of protease-resistant cervid prion protein in water from a CWD-endemic area. Prion 3 : 171–183. doi: 10.4161/pri.3.3.9819 19823039
45. Maddison BC, Baker CA, Terry LA, Bellworthy SJ, Thorne L, et al. (2010) Environmental Sources of Scrapie Prions. Journal of Virology 84 : 11560–11562. doi: 10.1128/JVI.01133-10 20739536
46. Wu ZY, Bertram HC, Kohler A, Bocker U, Ofstad R, et al. (2006) Influence of aging and salting on protein secondary structures and water distribution in uncooked and cooked pork. A combined FT-IR microspectroscopy and H-1 NMR relaxometry study. Journal of Agricultural and Food Chemistry 54 : 8589–8597.
47. Mauri AN, Anon MC (2006) Effect of solution pH on solubility and some structural properties of soybean protein isolate films. Journal of the Science of Food and Agriculture 86 : 1064–1072. doi: 10.1002/jsfa.2457
48. Pretzer D, Schulteis BS, Smith CD, Vandervelde DG, Mitchell JW, et al. (1991) Stability of the thrombolytic protein fibrolase—effect of temperature and pH on activity and conformation. Pharmaceutical Research 8 : 1103–1112. doi: 10.1023/A:1015842032164 1788155
49. Lundquist EJ, Jackson LE, Scow KM (1999) Wet-dry cycles affect dissolved organic carbon in two California agricultural soils. Soil Biology & Biochemistry 31 : 1031–1038. doi: 10.1016/S0038-0717(99)00017-6
50. Jablonowski ND, Linden A, Koeppchen S, Thiele B, Hofmann D, et al. (2012) Dry-wet cycles increase pesticide residue release from soil. Environmental Toxicology and Chemistry 31 : 1941–1947. doi: 10.1002/etc.1851 22782855
51. Lemmer K, Mielke M, Pauli G, Beekes M (2004) Decontamination of surgical instruments from prion proteins: in vitro studies on the detachment, destabilization and degradation of PrPSc bound to steel surfaces. Journal of General Virology 85 : 3805–3816. doi: 10.1099/vir.0.80346-0 15557254
52. Secker TJ, Herve R, Keevil CW (2011) Adsorption of prion and tissue proteins to surgical stainless steel surfaces and the efficacy of decontamination following dry and wet storage conditions. Journal of Hospital Infection 78 : 251–255. doi: 10.1016/j.jhin.2011.03.021 21658801
53. International soil moisture network. https://ismn.geo.tuwien.ac.at/data-access/. Accessed on June 11, 2014.
54. Hunter GD, Millson GC (1964) Studies on the heat stability and chromatographic behaviour of the scrapie agent. J Gen Microbiol 37 : 251–258. doi: 10.1099/00221287-37-2-251 14247749
55. Mould DL, Dawson AM (1970) The response in mice to heat treated scrapie agent. J Comp Pathol 80 : 595–600. doi: 10.1016/0021-9975(70)90057-5 4992639
56. Brown P, Rohwer RG, Green EM, Gajdusek DC (1982) Effect of chemicals, heat, and histopathological processing on high infectivity hamster-adapted scrapie virus. J Infect Dis 145 : 683–687. doi: 10.1093/infdis/145.2.683 6804575
57. Rohwer RG (1984) Virus-like sensitivity of the scrapie agent to heat inactivation. Science 223 : 600–602. doi: 10.1126/science.6420887 6420887
58. Pilon JL, Nash PB, Arver T, Hoglund D, VerCauteren KC (2009) Feasibility of infectious prion digestion using mild conditions and commercial subtilisin. Journal of Virological Methods 161 : 168–172. doi: 10.1016/j.jviromet.2009.04.040 19467265
59. Mitsuiki S, Hui Z, Matsumoto D, Sakai M, Moriyama Y, et al. (2006) Degradation of PrPSc by keratinolytic protease from Nocardiopsis sp TOA-1. Bioscience Biotechnology and Biochemistry 70 : 1246–1248. doi: 10.1271/bbb.70.1246
60. Bessen RA, Marsh RF (1992) Identification of 2 biologically distince strains of transmissible mink encephalopathy in hamsters Journal of General Virology 73 : 329–334.
61. Shikiya RA, Ayers JI, Schutt CR, Kincaid AE, Bartz JC (2010) Coinfecting prion strains compete for a limiting cellular resource. J Virol 84 : 5706–5714. doi: 10.1128/JVI.00243-10 20237082
62. Bartz JC, Kramer ML, Sheehan MH, Hutter JA, Ayers JI, et al. (2007) Prion interference is due to a reduction in strain-specific PrPSc levels. J Virol 81 : 689–697. doi: 10.1128/JVI.01751-06 17079313
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek 2014 Reviewer Thank YouČlánek Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water LabelingČlánek High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 2- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- 2014 Reviewer Thank You
- A Case for Two-Component Signaling Systems As Antifungal Drug Targets
- Prions—Not Your Immunologist’s Pathogen
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
- Livestock-Associated : The United States Experience
- The Neurotrophic Receptor Ntrk2 Directs Lymphoid Tissue Neovascularization during Infection
- The Intracellular Bacterium Uses Parasitoid Wasps as Phoretic Vectors for Efficient Horizontal Transmission
- CD200 Receptor Restriction of Myeloid Cell Responses Antagonizes Antiviral Immunity and Facilitates Cytomegalovirus Persistence within Mucosal Tissue
- Phage-mediated Dispersal of Biofilm and Distribution of Bacterial Virulence Genes Is Induced by Quorum Sensing
- CXCL9 Contributes to Antimicrobial Protection of the Gut during Infection Independent of Chemokine-Receptor Signaling
- Mitigation of Prion Infectivity and Conversion Capacity by a Simulated Natural Process—Repeated Cycles of Drying and Wetting
- Approaches Reveal a Key Role for DCs in CD4+ T Cell Activation and Parasite Clearance during the Acute Phase of Experimental Blood-Stage Malaria
- Revealing the Sequence and Resulting Cellular Morphology of Receptor-Ligand Interactions during Invasion of Erythrocytes
- Crystal Structures of the Carboxyl cGMP Binding Domain of the cGMP-dependent Protein Kinase Reveal a Novel Capping Triad Crucial for Merozoite Egress
- Non-redundant and Redundant Roles of Cytomegalovirus gH/gL Complexes in Host Organ Entry and Intra-tissue Spread
- Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water Labeling
- A Working Model of How Noroviruses Infect the Intestine
- CD44 Plays a Functional Role in -induced Epithelial Cell Proliferation
- Novel Inhibitors of Cholesterol Degradation in Reveal How the Bacterium’s Metabolism Is Constrained by the Intracellular Environment
- G-Quadruplexes in Pathogens: A Common Route to Virulence Control?
- A Rho GDP Dissociation Inhibitor Produced by Apoptotic T-Cells Inhibits Growth of
- Manipulating Adenovirus Hexon Hypervariable Loops Dictates Immune Neutralisation and Coagulation Factor X-dependent Cell Interaction and
- The RhoGAP SPIN6 Associates with SPL11 and OsRac1 and Negatively Regulates Programmed Cell Death and Innate Immunity in Rice
- Lymph-Node Resident CD8α Dendritic Cells Capture Antigens from Migratory Malaria Sporozoites and Induce CD8 T Cell Responses
- Coordinated Function of Cellular DEAD-Box Helicases in Suppression of Viral RNA Recombination and Maintenance of Viral Genome Integrity
- IL-33-Mediated Protection against Experimental Cerebral Malaria Is Linked to Induction of Type 2 Innate Lymphoid Cells, M2 Macrophages and Regulatory T Cells
- Evasion of Autophagy and Intracellular Killing by Human Myeloid Dendritic Cells Involves DC-SIGN-TLR2 Crosstalk
- CD8 T Cell Response Maturation Defined by Anentropic Specificity and Repertoire Depth Correlates with SIVΔnef-induced Protection
- Diverse Heterologous Primary Infections Radically Alter Immunodominance Hierarchies and Clinical Outcomes Following H7N9 Influenza Challenge in Mice
- Human Adenovirus 52 Uses Sialic Acid-containing Glycoproteins and the Coxsackie and Adenovirus Receptor for Binding to Target Cells
- Super-Resolution Imaging of ESCRT-Proteins at HIV-1 Assembly Sites
- Disruption of an Membrane Protein Causes a Magnesium-dependent Cell Division Defect and Failure to Persist in Mice
- Recognition of Hyphae by Human Plasmacytoid Dendritic Cells Is Mediated by Dectin-2 and Results in Formation of Extracellular Traps
- Essential Domains of Invasins Utilized to Infect Mammalian Host Cells
- High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
- Yeast Prions: Proteins Templating Conformation and an Anti-prion System
- A Novel Mechanism of Bacterial Toxin Transfer within Host Blood Cell-Derived Microvesicles
- A Wild Strain Has Enhanced Epithelial Immunity to a Natural Microsporidian Parasite
- Control of Murine Cytomegalovirus Infection by γδ T Cells
- Dimorphism in Fungal Pathogens of Mammals, Plants, and Insects
- Recognition and Activation Domains Contribute to Allele-Specific Responses of an Arabidopsis NLR Receptor to an Oomycete Effector Protein
- Direct Binding of Retromer to Human Papillomavirus Type 16 Minor Capsid Protein L2 Mediates Endosome Exit during Viral Infection
- Characterization of the Mycobacterial Acyl-CoA Carboxylase Holo Complexes Reveals Their Functional Expansion into Amino Acid Catabolism
- Prion Infections and Anti-PrP Antibodies Trigger Converging Neurotoxic Pathways
- Evolution of Genome Size and Complexity in the
- Antibiotic Modulation of Capsular Exopolysaccharide and Virulence in
- IFNγ Signaling Endows DCs with the Capacity to Control Type I Inflammation during Parasitic Infection through Promoting T-bet+ Regulatory T Cells
- Identification of Effective Subdominant Anti-HIV-1 CD8+ T Cells Within Entire Post-infection and Post-vaccination Immune Responses
- Viral and Cellular Proteins Containing FGDF Motifs Bind G3BP to Block Stress Granule Formation
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Cytoplasmic Actin Is an Extracellular Insect Immune Factor which Is Secreted upon Immune Challenge and Mediates Phagocytosis and Direct Killing of Bacteria, and Is a Antagonist
- A Specific A/T Polymorphism in Western Tyrosine Phosphorylation B-Motifs Regulates CagA Epithelial Cell Interactions
- Within-host Competition Does Not Select for Virulence in Malaria Parasites; Studies with
- A Membrane-bound eIF2 Alpha Kinase Located in Endosomes Is Regulated by Heme and Controls Differentiation and ROS Levels in
- Cytosolic Access of : Critical Impact of Phagosomal Acidification Control and Demonstration of Occurrence
- Role of Pentraxin 3 in Shaping Arthritogenic Alphaviral Disease: From Enhanced Viral Replication to Immunomodulation
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- HITS-CLIP Analysis Uncovers a Link between the Kaposi’s Sarcoma-Associated Herpesvirus ORF57 Protein and Host Pre-mRNA Metabolism
- Molecular and Functional Analyses of a Maize Autoactive NB-LRR Protein Identify Precise Structural Requirements for Activity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Control of Murine Cytomegalovirus Infection by γδ T Cells
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



