-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
The presence of the FoF1-ATP synthase in every aerobic organism suggests that evolution has settled on a basic blueprint for the complex rotary motor capable of synthesizing life’s universal energy currency—ATP. However, compared to yeast and mammalian models of the FoF1-ATP synthase, several recent studies have reported unique structural and functional features of this complex from organisms representing the clades of Chromalveolata, Archaeplastida and Excavata. One of the most striking cases is observed in trypanosomes, important parasites of humans and animals. Notably, the FoF1-ATP synthase/ATPase switches from synthesizing ATP in the insect vector life stage to hydrolyzing ATP in their mammalian hosts to generate the essential mitochondrial membrane potential (Δψm). Moreover, this indispensable FoF1-ATPase contains up to 14 trypanosome-specific subunits. Here we characterize one such novel subunit, ATPaseTb2. We demonstrate that this subunit is crucial for the survival of the infectious stage of trypanosomes, part of the fully assembled FoF1-complex and it is essential for maintaining the Δψm. Given the enzyme’s irreplaceable function and extraordinary composition, we believe that the FoF1-ATPase is an attractive drug target.
Published in the journal: . PLoS Pathog 11(2): e32767. doi:10.1371/journal.ppat.1004660
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004660Summary
The presence of the FoF1-ATP synthase in every aerobic organism suggests that evolution has settled on a basic blueprint for the complex rotary motor capable of synthesizing life’s universal energy currency—ATP. However, compared to yeast and mammalian models of the FoF1-ATP synthase, several recent studies have reported unique structural and functional features of this complex from organisms representing the clades of Chromalveolata, Archaeplastida and Excavata. One of the most striking cases is observed in trypanosomes, important parasites of humans and animals. Notably, the FoF1-ATP synthase/ATPase switches from synthesizing ATP in the insect vector life stage to hydrolyzing ATP in their mammalian hosts to generate the essential mitochondrial membrane potential (Δψm). Moreover, this indispensable FoF1-ATPase contains up to 14 trypanosome-specific subunits. Here we characterize one such novel subunit, ATPaseTb2. We demonstrate that this subunit is crucial for the survival of the infectious stage of trypanosomes, part of the fully assembled FoF1-complex and it is essential for maintaining the Δψm. Given the enzyme’s irreplaceable function and extraordinary composition, we believe that the FoF1-ATPase is an attractive drug target.
Introduction
Trypanosomes are unicellular flagellates from the order Kinetoplastida, which is comprised of some of the most devastating human pathogens in the world. For example, in sub-Saharan Africa, infection from Trypanosoma brucei gambiense and T. b. rhodesiense causes Human African Trypanosomiasis, which is almost always fatal if left untreated [1]. The latest WHO reports estimate that there are 10,000 new cases annually in endemic regions. Meanwhile, a third subspecies, T. b. brucei, infects livestock and therefore negatively affects the human population through malnutrition and economic hardships [2,3].
T. brucei parasites have a complex life cycle, alternating between the mammalian host and the insect vector, a tse-tse fly. During this environmental switch, the protist undergoes rapid and dramatic changes in cell morphology and metabolism [4–6]. In particular, the single mitochondrion undergoes extensive remodelling, which reflects the adaptability of the parasite to consume different carbon sources based on their availability [4]. The procyclic (insect) form (PF) of trypanosomes catabolizes amino acids and maintains a well-developed mitochondrion with abundant cristae, Krebs cycle enzymes and a complete oxidative phosphorylation pathway. This pathway includes enzymatic complexes that generate a mitochondrial (mt) membrane potential (Δψm) that is coupled to ATP synthesis by the FoF1-ATP synthase [5]. In contrast, the bloodstream form (BF) of this parasite populates the glucose-rich fluids (e.g. blood and spinal fluid) of its vertebrate host, allowing them to utilize just glycolysis for ATP production. This results in a drastically reduced mitochondrion that lacks significant cristae, key enzymes of the Krebs cycle and the cytochrome-containing respiratory complexes that pump protons into the inner mt membrane space [6,7]. Despite this reduction, the BF mitochondrion is still an active organelle, holding vital processes e.g. lipid metabolism [8], ion homeostasis [9], calcium signalling [10,11], FeS cluster assembly [12] and acetate production for de novo lipid biosynthesis [13]. Importantly, in the absence of proton-pumping respiratory complexes III and IV, the indispensable Δψm is sustained mainly by the hydrolytic activity of the FoF1-ATPase. Thus, this complex possesses an essential, unique and irreplaceable function in BF mitochondria [14].
In other eukaryotes, this reverse activity of the FoF1-ATP synthase is observed only rarely, for very brief moments of time and under very specific conditions (i.e. during oxygen deprivation or in response to damaged or mutated mt respiratory proteins). When the function of the respiratory complexes is compromised, the Δψm falls below a physiological threshold and is restored by the reverse proton pumping activity of the FoF1-ATPase, which is powered by ATP hydrolysis. The hydrolytic activity of the catalytic F1-ATPase is also essential for exceptional cells that lack mtDNA (ρ° cells). These cells do not express several core subunits of the membrane embedded Fo-moiety (subunits 6, 8 and 9 in yeast, subunits a and A6L in bovine) of the FoF1-ATPase, notably those that are components of the proton pore. Thus, the matrix protruding F1-ATPase energizes the inner mt membrane by coupling ATP hydrolysis with the exchange of ADP3- for ATP4- by the ATP/ADP carrier (AAC) [15]. The same mechanism for producing the Δψm is utilized by trypanosomes that lack a mt genome, which is called a kinetoplast [16]. These naturally occuring dyskinetoplastic forms (Dk) of T. brucei (e.g. T. b. evansi or T. b.equiperdum) cause economically significant diseases in horses, camels, and water buffaloes. Remarkably, these parasites are not able to switch to the insect stage and are transmitted mechanically by bloodsucking insects or by coitus [17]. The mtDNA-lacking trypanosomes can also be chemically induced in the laboratory (e.g. Dk T. brucei EATRO164) [18]. Interestingly, each of the Dk cell lines characterized so far, bear one of several different compensatory mutations in the nuclear encoded subunit γ that enable the Δψm to be generated independently of the Fo-moiety [14,16,19].
In general, the FoF1-ATP synthase complex consists of two functionally distinct enzymatic segments: the hydrophilic F1 catalytic moiety and the membrane-bound Fo pore. Both of these subcomplexes are linked together by the central and peripheral stalks. The central stalk rotates with the c-ring when protons are allowed to pass through the Fo pore, located between the c-ring and subunit a. In contrast to the rotation of the central stalk, the stationary peripheral stalk plays a crucial role in keeping the catalytic F1 headpiece static, thus resisting the rotational torque. The eubacterial F1-moiety consists of the catalytic domain and the central stalk, which are comprised of five subunits in a stoichiometry of α3, β3, γ1, δ1, ε1. The Fo-moiety is composed of the oligomeric c10–15 ring and a single subunit a joined together with two copies of subunit b, which extend from the membrane and form the base of the peripheral stalk. The composition of the eukaryotic enzyme has been determined mainly from detailed studies of FoF1-ATP synthase purified from the mitochondria of Bos taurus and Saccharomyces cerevisiae, members of the clade Opisthokonta. These enzymes contain homologous prokaryotic-like core components, but also incorporate additional eukaryotic specific subunits involved in the structure, oligomerization and regulation of the complex [20]. These novel subunits have been assigned to various regions of the FoF1-ATP synthase: i) subunit ε and inhibitory factor 1 (IF1) bind to the F1-moiety; ii) the small hydrophobic subunit 8 (subunit A6L in bovine) is located in the membrane interacting with subunit a; iii) the soluble subunit h (subunit F6 in bovine), oligomycin sensitivity-conferring protein (OSCP) and hydrophilic subunit d are assigned to the peripheral stalk; iv) subunits e, f, g, all containing one transmembrane helix, are regarded as accessory peripheral stalk proteins that predominantly reside in the inner membrane where they presumably stabilize the utmost hydrophobic subunit 6 (or subunit a in bovine), thereby assisting the peripheral stalk with its stator function [21–23].
Recently, however, several studies have reported unique structural and functional features of the FoF1-ATP synthase from medically relevant parasites and other organisms that represent the clades of Chromalveolata [24], Archaeplastida [25] and Excavata [26]. In Plasmodium falciparum, a member of the phylum Apicomplexa, the genomic data indicates the likely presence of all eukaryotic F1 subunits and some of the Fo and stator subunits [27]. Conspicuously absent are the subunits a and b, which are crucial to the function of all known FoF1-ATP synthases. These subunits are also missing in the ATP synthase of P. yoelii and Tetrahymena thermophila, the latter a member of the sister phylum Ciliophora, both of which have been shown experimentally to possess ATP synthase activity [28–30]. This suggest that ciliates and apicomplexan species employ highly divergent or novel subunits to fulfil the functions of the classical subunits a and b. Another example of an unusual FoF1-ATP synthase complex comes from studies of the chlorophycean algea Chlamydomonas reinhardtii and Polytomella sp. that determined the complex contains up to 9 unique subunits (Asa1-Asa9) that either form an innovative peripheral stator or are responsible for complex dimerization [31].
Trypanosoma FoF1-ATP synthase consists of the well conserved F1-moiety comprised of subunits α, β, γ, δ, ε and the trypanosome-specific subunit p18 [26,32], and the less characterized Fo pore and peripheral stalk where only subunits c, a and OSCP were identified at the gene or protein level [26,33]. Additionally, the complex contains up to 14 Kinetoplastida-specific subunits that lack homology to any of the previously described subunits. Therefore, their position within the complex and their function is unknown. Interestingly, these recently identified FoF1-ATP synthase subunits specific for T. brucei (ATPaseTb) may represent early evolutionary attempts to create the functional and structural components of the eukaryotic accessory subunits for this early divergent species. Previously, two of these novel subunits, ATPaseTb1 (Tb10.70.7760) and ATPaseTb2 (Tb927.5.2930), were shown to be essential for the proper function and structural integrity of FoF1-ATP synthase in the procyclic form of this parasite [26].
Here, we have extended this study and functionally characterized ATPaseTb2 in the disease-relevant stage of T. brucei and Dk T. b evansi. Our results demonstrate that the membrane-bound ATPaseTb2 protein is indispensable for the survival of BF and Dk trypanosomes because it is crucial for maintaining the Δψm in these cells. Furthermore, the unexpected observation of assembled FoF1-ATPase complexes in two different strains of trypanosomes lacking mt DNA suggests that the composition of the Dk FoF1-ATPase complex is more similar to the BF FoF1-ATPase complex than previously thought.
Results
Bioinformatics reveals that ATPaseTb2 contains a putative transmembrane domain and a region homologous to the bovine subunit d
ATPaseTb2 is annotated as a hypothetical protein in the TriTrypDB database (www.tritrypdb.org) and its mt localization was predicted by the presence of an N-terminal mt targeting sequence assigned by Mitoprot II v1.101 [34] (probability 0.823). Strikingly, this subunit has no detectable homologs outside Kinetoplastida based on a similarity sequence search (e.g. BLAST). To uncover any homologous relationships for protein sequences that do not share high enough sequence identity, but might have similar secondary structure, we employed the HHpred toolkit (http://toolkit.tuebingen.mpg.de), which is based on an HMM-HMM comparison that reveals homologous relationships even if the sequences share less than 20% sequence identity [35]. All of the available Kinetoplastida ATPaseTb2 homologs obtained from the TriTrypDB (S1A Fig.) were analyzed using HHpred software. Among the first five structural homologs detected was the bovine FoF1-ATP synthase subunit d, with a probability of 44.7 and a P-value of 0.0021. Then a consensus sequence generated from an alignment of this 92 amino acids (aa) region from all identified kinetoplastida homologs was resubmitted for HHpred analysis. This search returned a significant hit to the bovine subunit d with a probability of 78.1 and a P-value of 0.0002 (S1B Fig.). This bovine FoF1-ATP synthase subunit is comprised of 160 aa and resides within the peripheral stalk where it interacts with all the other three components of the peripheral stalk (b, F6 and OSCP). This subunit is predominantly hydrophilic, contains several long alpha-helices and is essential for the enzymatic function [23,36,37]. The region of similarity to subunit d falls in the middle of the ATPaseTb2 sequence and it is followed by a putative trans-membrane (TM) domain (aa 239–254) that was predicted by MEMSAT-SVM software (http://bioinf.cs.ucl.ac.uk/psipred/) [38] with a -0.738 prediction score (Fig. 1). This prediction is intriguing because a typical eukaryotic subunit d is not directly attached to the membrane, but rather it interacts with the soluble region of the membrane-bound subunit b via three coiled-coil interactions [23]. Thus, ATPaseTb2 may represent a novel subunit of the membrane-bound peripheral stalk that possibly depicts an early divergent functional alternative of the stator by creating a chimera of subunits b and d.
Fig. 1. Bioinformatics reveal that ATPaseTb2 possesses a region of low homology to ATPase subunit d. 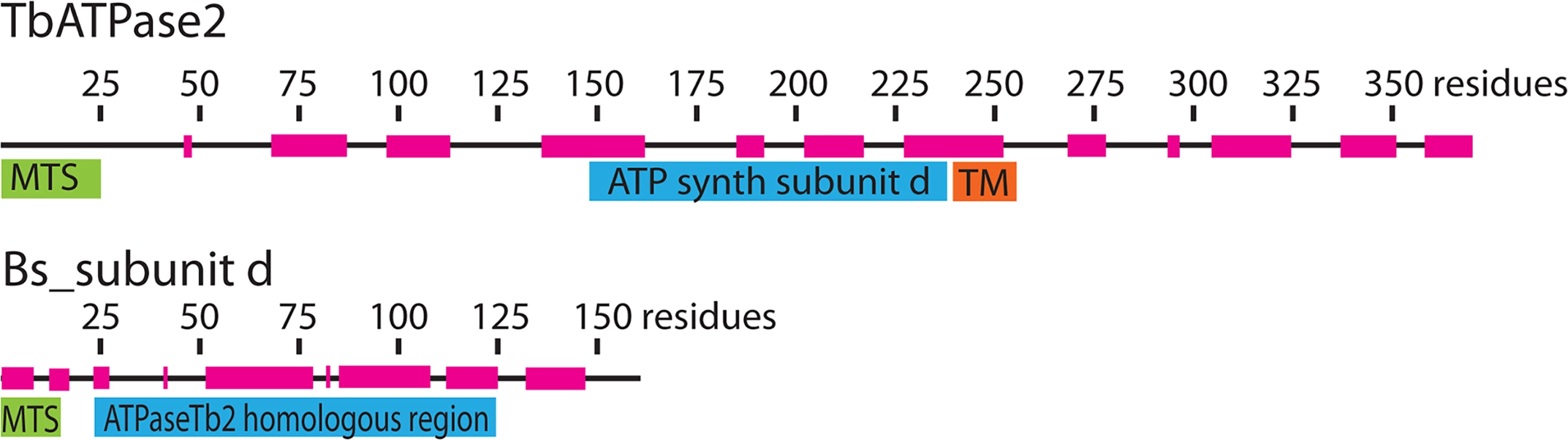
A schematic representation depicting the ATPaseTb2 protein (370 aminoacids (AA)) and the Bos taurus subunit d (Bs_sub d, 161 AA) was created using Adobe Illustrator CS5.1. The mitochondrial targeting signals (MTS, green) were predicted by Mitoprot II v1.101. The transmembrane domain (TM, orange) within the ATPaseTb2 sequence was predicted by MEMSAT-SVM software. The homologous region (blue) to Bs_sub d was determined using HHpred toolkit. The regions of alpha-helices (magenta) depicted on the amino acid sequence line were predicted by software from EMBOSS garnier. ATPaseTb2 is a mt membrane-bound protein expressed in various forms of T. brucei
The mitochondrial localization, as well as the structural and functional association of ATPaseTb2 with the FoF1-ATP synthase in PF T. brucei cells was previously described [26]. Because the activity of this complex significantly differs between the insect and mammalian stage of the parasite, we were interested if the ATPaseTb2 protein is also expressed in trypanosomes residing in the bloodstream of their host. Therefore, we cultured PF427 (insect stage), BF427 (bloodstream forms) and the laboratory-induced dyskinetoplastic Dk164 T. brucei cells, in addition to the naturally occurring Dk T.b.evansi. Whole cell lysates from PF427, BF427, Dk164 and T.b.evansi cells were fractionated by SDS-PAGE and analyzed by western blot using a specific polyclonal antibody against ATPaseTb2 (Fig. 2A). Interestingly, ATPaseTb2 is expressed in all four cell types; however, its expression is significantly decreased in BF427 and even more reduced in Dk164 and T.b.evansi cells. A similar expression pattern for F1-ATPase subunit β and mt chaperone Hsp70 was also observed between PF, BF and Dk cells (Fig. 2A). These results are in agreement with the proposed reduction of the mitochondria size, function and activity in the various bloodstream forms of T. brucei [39].
Fig. 2. ATPaseTb2 is expressed in PF427, BF427, Dk164 T. brucei and T. b. evansi cells and it is a membrane-bound protein. 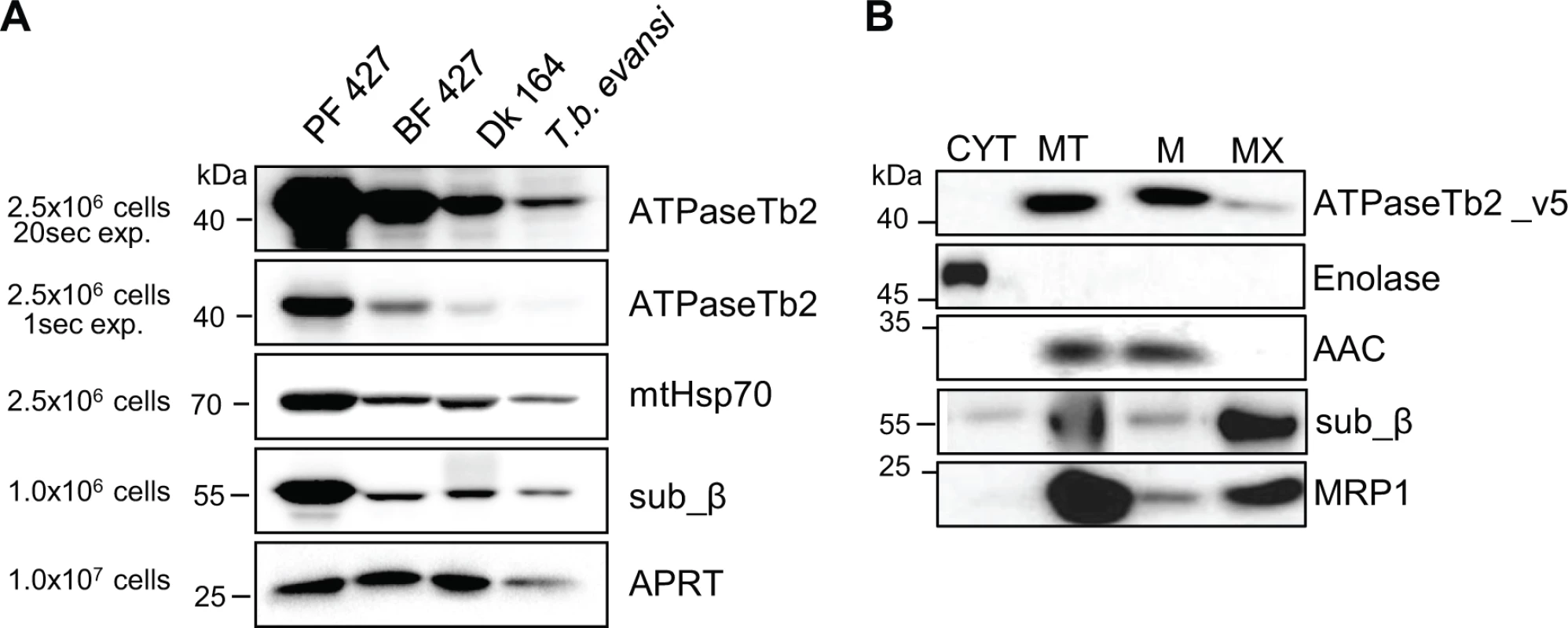
A) The steady state abundance of ATPaseTb2 (two different exposure times), subunit β, mtHsp70 and cytosolic adenosine phosphoribosyl transferase (APRT) was determined in PF427, BF427, Dk164 and T. b. evansi cells by western blot analysis of whole cell lysates from 2.5x106 cells or 1x106 cells (for subunit β and APRT) resolved on SDS-PAGE gels. The pertinent sizes of the protein marker are indicated on the left. B) Subcellular localization of ATPaseTb2 was determined using BF cells expressing v5-tagged ATPaseTb2. Cytosolic (CYT) and mitochondrial (MT) fractions were obtained by hypotonic lysis. Mt pellets were further treated with Na2CO3 and spun at a high-speed to obtain mt membrane (M) and matrix (MX) fractions. Purified fractions were analyzed by western blot with the following antibodies: anti-v5 (ATPaseTb2_v5), anti-enolase (cytosol), anti-AAC (mt inner membrane), anti-β (F1-ATPase subunit) and anti-MRP1 (mt matrix). The relevant sizes of the protein marker are indicated on the left. To experimentally investigate the subcellular localization of ATPaseTb2 within the bloodstream T. brucei cell, the ATPaseTb2 gene product was C-terminally tagged with a v5-tag and inducibly expressed using tetracycline (tet). Cytosolic and mitochondrial fractions were isolated from a hypotonic lysate of cells over-expressing the ATPaseTb2_v5 protein. The mitochondrial fractions were then treated with digitonin to create mitoplasts that were further incubated with sodium carbonate (Na2CO3) to obtain mt membrane and mt matrix fractions. The subsequent western analyses established the purity of the extracted fractions as the cytosolic enolase, the mt inner membrane carrier protein (AAC) and the matrix-localized guide RNA binding protein (MRP1) were confined within their respective subcellular fractions. Notably, ATPaseTb2 was predominantly localized in the mt membrane fraction, while subunit β of the matrix-soluble F1-ATPase was released into the matrix fraction (Fig. 2B), demonstrating that ATPaseTb2 is firmly embedded in the inner mt membrane.
ATPaseTb2 is a bona fide subunit of FoF1-ATPase monomers and higher oligomers
To confirm that ATPaseTb2 is a genuine subunit of both the BF and Dk FoF1-ATPase, we employed the same strategy applied previously for PF cells [26]. Therefore, we first created BF cell lines in which either the ATPaseTb2 or the F1 subunit p18 were inducibly expressed with a C-terminal tag. Complexes assembled with a tagged protein were then purified using a one-step IgG affinity purification, followed by treatment with TEV protease to release bound complexes from the IgG beads. The TEV eluates were further analyzed by western blot for the presence of the tagged proteins, known FoF1-ATPase subunits, and AAC (Fig. 3A). Importantly, the presence of the tagged subunits ATPase Tb2 and p18 in the respective cell lines were confirmed using a specific antibody against the c-myc epitope that comprises part of the tag. In addition, the ATPaseTb2_TAP eluate contained FoF1-ATPase subunits β, p18 and ATPaseTb1. Vice versa, the p18_TAP eluate contained subunits ATPaseTb2, ATPaseTb1 and subunit β. While AAC has been described to associate with mammalian FoF1-ATPase under certain conditions [40], it is not a core component of the enzyme and the lack of its signal indicates the stringency of this technique. The TAP tag purification from non-induced cells was used as a negative control to verify that the detected proteins do not bind non-specifically to the charged beads. To analyze the composition of the Dk FoF1-ATPase, p18 tagged complexes were purified and subjected to the same set of antibodies described for the BF complexes (Fig. 3B). These results also depicted the same interactions seen in Fig. 3A. Therefore, the co-purification of ATPaseTb2 with known ATPase subunits p18 and β validates that it is an authentic constituent of the FoF1-ATPase complex in both BF and Dk cells.
Fig. 3. ATPaseTb2 is a bona fide subunit of the monomeric and multimeric FoF1-ATPases. 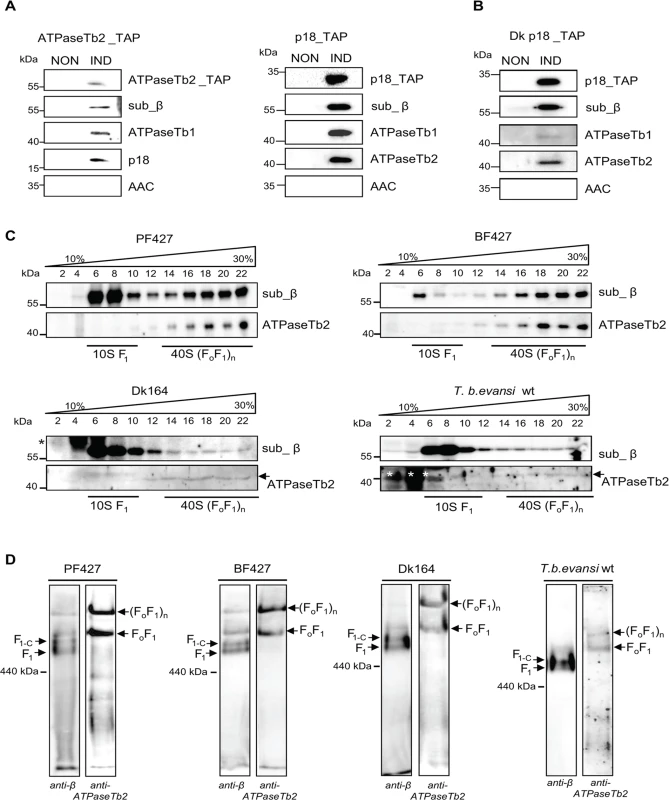
A) ATPaseTb2_TAP and p18_TAP tagged complexes were purified from BF cells using one-step IgG affinity chromatography from non-induced (NON) and 2 days induced (IND) cells containing the regulatable ectopic TAP tagged protein. The tagged protein complexes were eluated by TEV protease, fractionated on SDS-PAGE and examined by western blot analyzes. The presence of the tagged ATPaseTb2 and p18 subunits was verified using a c-myc antibody (top panels: ATPaseTb2_TAP and p18_TAP). The known FoF1-ATPase subunits (sub β, p18 and ATPase_Tb1) were detected using specific antibodies. The lack of signal for AAC serves as a control for specificity of the used method. The applicable sizes of the protein marker are indicated on the left. B) p18_TAP tagged complexes were purified from dyskinetoplastic T. b. evansi as described above for BF cells and subjected to the same set of antibodies. C) The sedimentation profile of F1- and FoF1-ATPase complexes was determined using glycerol gradient sedimentation. Hypotonically purified mitochondria from PF427, BF427, Dk164 and T. b. evansi cells were lysed with 1% Triton X-100 and fractionated on a 10–30% glycerol gradient. The glycerol gradient fractions were collected, fractionated by SDS-PAGE and analyzed by western blots. Western analyzes with an anti-β antibody depicted the sedimentation profile of the F1-ATPase and monomeric/multimeric FoF1-ATP synthase complexes, whereas the anti- ATPaseTb2 antibody only immunodecorates this protein within the monomeric/multimeric FoF1-ATPase complexes. The subdivision of the various structural forms of the FoF1-ATPase complexes are underlined as determined in [26]. The glycerol gradient fractions and the sizes of the protein marker are indicated. Nonspecific bands visible in Dk164 and T. b. evansi gradients are indicated by asterisks. D) The native F1- and FoF1-ATPase complexes were visualized using hrCNE. Purified mitochondria from PF427, BF427, Dk164 and T.b.evansi cells were lysed with digitonin (4 mg/mg), fractionated on a 3%-12% hrCNE and blotted onto a nitrocellulose membrane. The F1-ATPase (F1), the F1-ATPase bound with the c-ring (F1+C) and the monomeric FoF1/multimeric (FoF1)n complexes were all visualized using specific polyclonal antibodies against either subunit β or ATPaseTb2. The size of ferritin from the equine spleen (440 kDa) is indicated on the left. The sedimentation profile of FoF1-ATP synthase in 10–30% glycerol gradients (GG) was previously published and revealed two distinct regions representing the F1-moiety and the FoF1-monomer and multimeric complexes [26]. In order to specify if ATPaseTb2 is a component of the F1-moiety or the FoF1-complex, the glycerol gradient sedimentation profile was determined using mt lysates from all four cell types: PF427, BF427, Dk164 and T.b.evansi. In correlation with the literature, a new specific antibody raised against the F1-subunit β depicted both regions, which are defined as ∼10S (fractions 6–10), and ∼40S (fractions 14–22) (Fig. 3C). A different sedimentation pattern was revealed when a specific antibody recognizing ATPaseTb2 detected only the 40S region (fractions 14–22), representing the FoF1-monomer and -multimers. These findings suggest that the novel subunit is not a component of the F1-moiety, but rather a member of the fully assembled complex. Furthermore, compelling results hinting at the function of this hypothetical protein were also obtained when the mt lysates of Dk164 and T. b. evansi cells were fractionated on similar gradients. While the majority of the signal for subunit β was predictably detected in fractions 6–10, representing the F1-ATPase complex, there was also a weak signal detected in higher S values. These same ∼40S complexes were identified using the ATPaseTb2 antibody (Fig. 3C). This indicates that in the absence of subunit a, the F1-moiety is still attached to the mt membrane via a central stalk, a ring of subunit c and the peripheral stalk. Since the Fo subunit a is missing in these cells, the attachment of this complex to the membrane is presumably not as strong as in BF cells. Therefore, upon treatment with detergent to lyse the mitochondria, a majority of F1-ATPase is released and detected in lower S values while a small portion of the FoF1-complexes is preserved and sediments at higher S value fractions. Consequently, our data suggests that previously undetected membrane-bound FoF1-complexes are present in the mitochondria of the Dk trypanosomes.
To verify our intriguing results, the FoF1-ATP synthase/ATPase complex was examined by an alternative method involving high resolution clear native electrophoresis (hrCNE) followed by western blot analysis. Results obtained using the antibody against subunit β revealed four predominant bands in both the PF427 and BF427 samples, representing various forms of the F1-moiety and FoF1-complexes (Fig. 3D). The lowest band likely corresponds to the F1-ATPase (subunits α, β, γ,δ,ε), while the one above it seemingly represents F1-ATPase with a ring of subunit c, as it has been described for the mammalian complex [41]. Noticeably, in each sample the antibody against ATPaseTb2 immunodetected only two major bands corresponding to the FoF1-monomer and -multimers. When the mt lysates of Dk164 and T. b. evansi cells were fractionated on a hrCNE, subunit β was mainly detected in the lower bands representing the F1-moiety and the FoF1-monomer, while only a weaker band was observed at the size depicting FoF1-multimers. But western blot analysis with the ATPaseTb2 antibody clearly demonstrated the existence of higher assemblies of the FoF1-ATPase complexes (Fig. 3D). Thus, ATPaseTb2 is a membrane-bound subunit of the monomeric and multimeric FoF1-ATP synthase/ATPase complex in all four T. brucei cell types examined. Furthermore, no significant differences in size were observed between the FoF1-monomeric and -multimeric complexes detected in PF427, BF427 and Dk cells, indicating that subunit a might be the only missing component of the assembled FoF1-ATPase complex in dyskinetoplastic cells.
Silencing of ATPaseTb2 inhibits cell growth by decreasing the Δψm in BF T. brucei
To assess the importance of ATPaseTb2 in the infective stage of T. brucei and to evaluate its functional association with FoF1-ATPase, an ATPaseTb2 RNA interference (RNAi) cell line was created. The expression of dsRNA was triggered by the addition of tet to the culture medium and the in vitro growth of the ATPaseTb2 knock-down (KD) cells was inspected for seven days (Fig. 4A). Strikingly, a significant growth phenotype was already detected in RNAi induced cells on just the second day of tet addition. Presumably, the powerful selection forces acting on these cells missing a critical protein resulted in the emergence of a subpopulation that was no longer responsive to RNAi induction after only 120 hours, leading to the growth recovery of the culture (Fig. 4A). This phenomenon is often reported for RNAi experiments in BF T. brucei [42].
Fig. 4. RNAi silencing of ATPaseTb2 inhibits the growth and mt membrane potential of BF T. brucei. 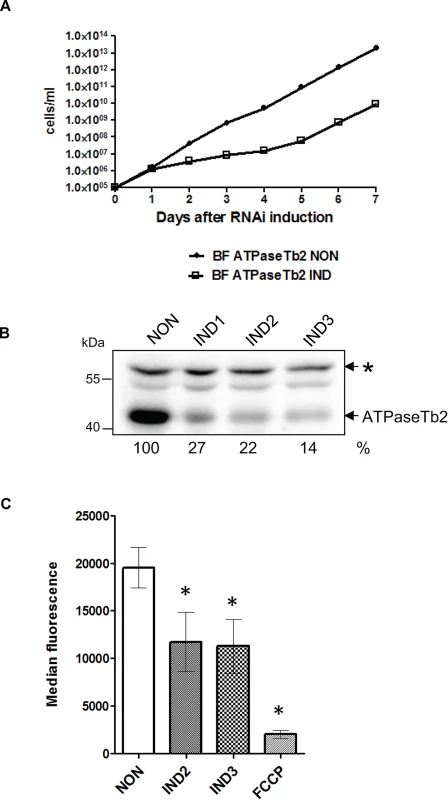
A) Growth curves of the noninduced (NON) and RNAi induced (IND) ATPaseTb2 RNAi bloodstream T. brucei cell lines were measured for 7 days. Cells were maintained in the exponential growth phase (between 105 and 106 cells/ml) and the cumulative cell number was calculated from cell densities adjusted by the dilution factor needed to seed the cultures at 105 cells/ml each day. The figure is representative of three independent RNAi-inductions. B) The steady-state abundance of ATPaseTb2 in non-induced (NON) RNAi cells and in cells induced with tet for 1, 2 and 3 days (IND1, IND2, IND3) was determined by western blot analysis using a specific ATPaseTb2 antibody. The non-specific band (marked with an asterisk) detected on the same membrane served as a loading control. The numbers beneath the blots represent the abundance of immunodetected ATPaseTb2 expressed as a percentage of the non-induced samples after normalizing to the loading control. The relevant sizes of the protein marker are indicated on the left. The figure is a representative western blot from three independent RNAi-inductions. C) Using Mitotracker Red CMX-Ros, the mt membrane potential was measured by flow cytometry in non-induced (NON) ATPaseTb2 RNAi cells and cells induced for 2 or 3 days (IND2 and IND3). The median fluorescence for each sample is depicted on the y-axis of the column graph. The results are means ± s.d. (n = 3). *P< 0.05, Student’s t-test. The targeted KD of ATPaseTb2 was confirmed by western blot analysis of whole cell lysates harvested from an equivalent number of cells for ATPaseTb2 RNAi non-induced and induced cells throughout the RNAi time course (Fig. 4B). Western blot analysis using the antibody against ATPaseTb2 exhibited a reduction of the targeted protein by 73% at day 2 of RNAi induction. A non-specific band detected on the same membrane was used to determine equal loading of the samples.
With the efficient knockdown of ATPaseTb2 verified, an in vivo assay was employed to measure the Δψm in the ATPaseTb2 KD cell population (Fig. 4C), since the Δψm is generated by the FoF1-ATPase in BF T. brucei [9]. Flow cytometry analysis was used to determine the changes observed in these cells stained with Mitotracker Red CMX-Ros, whose fluorescent intensity is proportionally dependent on the strength of the Δψm. A substantial decrease of the Δψm in the KD cell population was observed two days (IND2) after RNAi induction, coinciding with the first time point to display a significant growth inhibition. Importantly, this result confirms the vital function of ATPaseTb2 within the FoF1-ATPase.
Depleting ATPaseTb2 does not significantly alter ATP hydrolysis capabilities
To assess if the decrease in the Δψm is the result of an impaired ATP hydrolytic activity of the enzyme, the total ATPase activity was measured in mt lysates with or without azide, an inhibitor of the catalytic F1-ATPase [43]. Typically, the specific F1-ATPase activity represents ∼ 35–45% of total mt ATPase activity. Our results indicate that neither the total ATPase nor azide-sensitive activities were significantly altered between the non-induced and ATPaseTb2 RNAi-induced cells (Fig. 5A). ATPase activity can also be visualized by in-gel activity staining when mitochondria from these cells are solubilized with dodecyl maltoside and separated on a 2–12% blue native PAGE (BNE) [41]. The specific ATPase staining revealed two major bands in non-induced cells, the predominant lower one representing the activity produced from the F1-moiety and the less significant higher band representing the hydrolytic activity of the monomeric FoF1-ATPase (Fig. 5B). It is important to note that solubilizing mitochondria with any detergent can result in unintended structural consequences not found under normal physiological conditions, especially for a large complex comprised of a matrix moiety and a membrane embedded region. Although detergents tend to dissociate the F1-moiety from FoF1-ATPase complexes, it is intriguing that there is still a portion of ATP hydrolysis being produced from the complete FoF1-ATPase in the non-induced BF cells. Conspicuously, this activity is absent when ATPaseTb2 RNAi is induced (Fig. 5B).
Fig. 5. ATPaseTb2 depletion does not appreciably affect F1-ATPase activity in BF T. 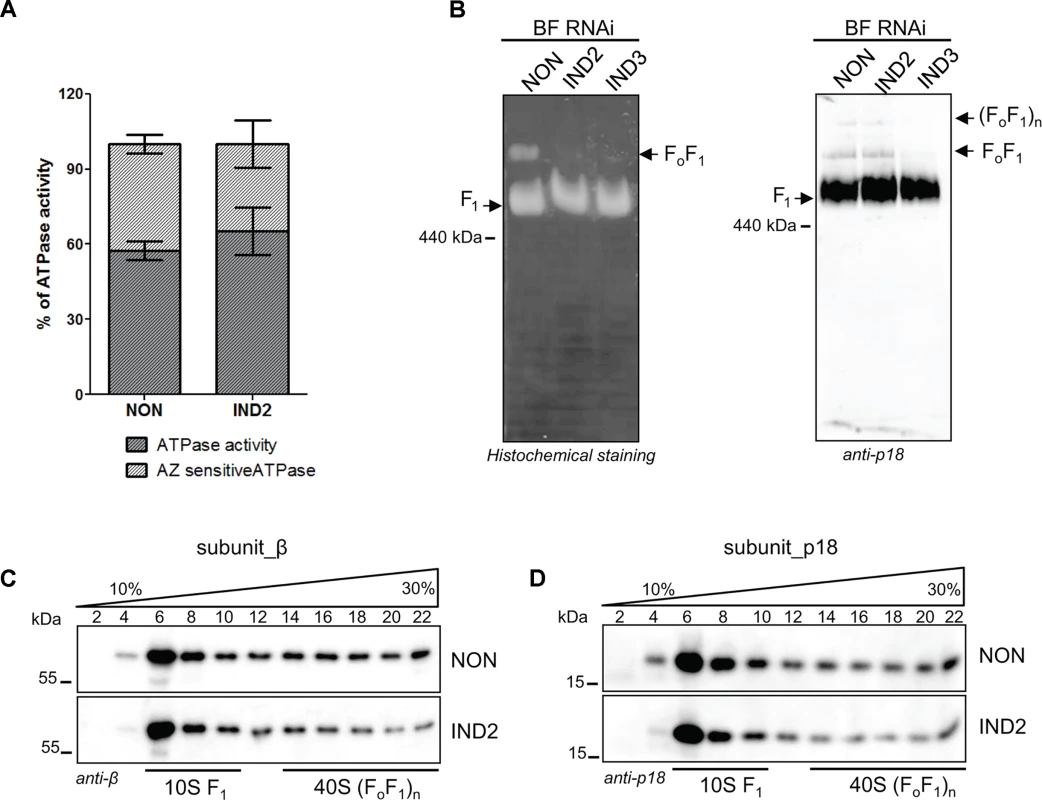
brucei cells, but it does significantly diminish the stability of FoF1-ATPase complexes. A) Employing the Sumner ATPase assay, the F1-ATPase hydrolytic activity was measured in ATPaseTb2 RNAi cells either not induced (NON) or induced for 2 days (IND2). Crude mt vesicles were obtained by digitonin extraction and the ATPase activity was assayed by measuring the release of free phosphates. The specific F1-ATPase inhibitor, azide (AZ, 2 mM), was added as indicated. The total amount of free-phosphate created from all ATPase enzymes present in the sample was set at 100% (hatched column). The azide-sensitive activity representing the F1-ATPase is depicted in dark grey. The results are means ± s.d. (n = 4). B) In-gel ATP hydrolysis activity of FoF1-ATPase was visualized after ATPaseTb2 reduction. Mitochondria from RNAi non-induced cells (NON) and cells induced for 2 (IND2) and 3 (IND3) days were lysed with 2% dodecyl maltoside. Equal amounts of lysed mitochondrial proteins (100 μg) were fractionated on a 2%-12% BNE and the FoF1-ATPase activity was visualized by in-gel histochemical staining resulting in a white lead phosphate precipitate. Positions of F1-ATPase and monomeric FoF1-ATPase are depicted. The size of equine spleen ferritin (440 kDa) is indicated. C) The stability of FoF1-ATPase complexes upon ATPaseTb2 silencing was examined using hrCNE. Mitochondria from RNAi non-induced cells (NON) and cells induced for 2 (IND2) and 3 (IND3) days were lysed by digitonin (4 mg/mg). Equal amounts of lysed mitochondrial proteins (20 μg) were fractionated on a 3%-12% hrCNE, blotted onto a nitrocellulose membrane and probed with the anti-p18 antibody. Positions of F1-ATPase and monomeric and dimeric FoF1-ATPases are depicted by arrows. The size of ferritin from equine spleen (440 kDa) is indicated. D) The sedimentation profile of FoF1-ATPase complexes was examined using western blot analysis of glycerol gradient fractions. Mitochondria from RNAi non-induced cells (NON) and cells induced for 2 days (IND2) were lysed with 1% Triton X-100. An equal amount of the cleared lysates (3,3 mg) were loaded on a manually poured 10–30% glycerol gradient. Western analyzes with anti-β and anti-p18 antibodies depicted the sedimentation profile of the FoF1-ATPase complexes. The manually fractionated glycerol gradient fractions are labelled and sizes of the protein marker are indicated. Loss of ATPaseTb2 disrupts the FoF1-ATPase complexes in BF T. brucei
The absence of activity for the FoF1-monomeric complex might be explained by complex instability. Thus, the structural integrity of the coupled FoF1-complex was further analyzed using hrCNE, which were loaded with digitonin-lysed mitochondria from non-induced and ATPaseTb2 RNAi induced cells. After transferring the separated native protein complexes to a nitrocellulose membrane, a specific antibody against the p18 subunit detected the F1-moiety as well as both monomeric and oligomeric FoF1-complexes (Fig. 5C). Interestingly, we repeatedly observed that the abundance of the monomeric and oligomeric complexes is decreased after RNAi induction and by day 3 these complexes are not detected at all. These results were complemented by the sedimentation profile of FoF1-ATPase complexes on glycerol gradients. Mitochondria were isolated from non-induced RNAi cells and cells induced for two days (IND2). These were then lysed by 1% Triton X-100 and an equal amount of mt protein from each sample was fractionated on a 10–30% GG. The resolved fractions were then analyzed by western blot and the anti-β and anti-p18 antibodies demonstrated the effect of the ATPaseTb2 KD on the FoF1-ATPase sedimentation profile. As indicated in Fig. 5D and the corresponding scanning densitometry results listed in S2 Fig., two days of RNAi induction resulted in a diminished amount of β and p18 signals from the higher S-values (IND2 panels), while the GG sedimentation pattern of RNAi non-induced cells was more similar to wild type BF427 (see Fig. 3C). These results are in agreement with the PF RNAi ATPaseTb2 cell lines, in which the stability of the monomeric and multimeric FoF1-ATP synthases was significantly affected upon RNAi [26].
Using a variety of methods employing various detergent types, we have established that the ATPaseTb2 subunit is an essential component of the FoF1-ATPase monomer and higher oligomers in the bloodstream stage of T. brucei. Furthermore, the depletion of ATPaseTb2 diminishes the abundance of membrane-bound FoF1-ATPase complexes, which directly initiates a substantial decrease in the Δψm that manifests as a strong growth phenotype.
ATPaseTb2 depletion affects cell growth, the Δψm and FoF1-ATPase integrity in Dk T. b. evansi
To further dissect the primary role of the membrane-bound ATPaseTb2 subunit, we investigated the effect of targeted gene silencing in transgenic Dk T. b. evansi, which facilitate the tet inducible expression of dsRNA. These Dk cells were transfected with an ATPaseTb2 RNAi vector and several positive clones were screened for a growth phenotype. As shown in Fig. 6A, the surprising inhibition of cell growth in RNAi induced cells appeared two days after tet induction and the doubling time remained reduced throughout the whole ten day experiment. Furthermore, western analysis of whole cell lysates harvested over the RNAi time course exhibited a significant reduction of ATPaseTb2 in the RNAi induced cells beginning on day 1 (Fig. 6B), while the analysis of a non-specific band served as the loading control. Interestingly, if the only role of the subunit was to stabilize the proton pore, it presumably would not be essential in cells that lack mtDNA and have adapted to the loss of a functioning proton pore. However, these results suggest that ATPaseTb2 is important for FoF1-ATPase complexes in these unique cells and thus this cell line was further exploited to characterize the function of ATPaseTb2.
Fig. 6. Cell growth, Δψm maintenance and the stability of FoF1-ATPase complexes are all affected by the loss of ATPaseTb2 in dyskinetoplastic T.b. evansi. 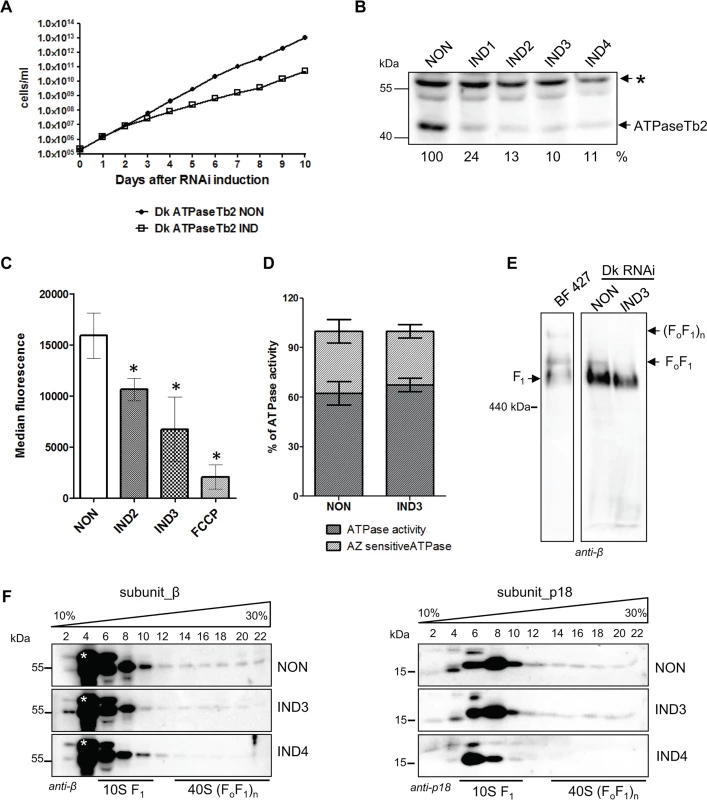
A) Growth curves of the non-induced (NON) and induced (IND) ATPaseTb2 RNAi Dk T. b. evansi cell lines were measured for 10 days. Cells were maintained in the exponential growth phase (between 105 and 106 cells/ml) and the cumulative cell number represents the cell density adjusted by the daily dilution factor. The figure is representative of three independent RNAi-inductions. B) The steady-state abundance of ATPaseTb2 in non-induced (NON) cells and cells harvested 1, 2, 3 and 4 days post RNAi induction (IND1, IND2, IND3, IND4) was determined by western blot analysis using a specific ATPaseTb2 antibody. Mt Hsp70 served as a loading control. The numbers underneath the ATPaseTb2 panel represent the abundance of immunodetected protein expressed as a percentage of the non-induced samples after normalizing to the loading control. The pertinent sizes of the protein marker are indicated on the left. The figure is a representative western blot from three independent RNAi-inductions. C) The Δψm was measured in non-induced (NON) ATPaseTb2 RNAi cells and cells induced for 2 and 3 days (IND2 and IND3) by flow cytometry using Mitotracker Red CMX-Ros. The results are means ± s.d. (n = 3). *P< 0.05, Student’s t-test. D) The F1-ATPase hydrolytic activity was measured for induced (IND3) or non-induced Dk ATPaseTb2 RNAi cells as described in Fig. 5A. The results are means ± s.d. (n = 4). E) The stability of FoF1-ATPase complexes upon ATPaseTb2 depletion was examined using hrCNE. Mitochondria from wild type BF427, ATPaseTb2 RNAi T. b. evansi non-induced cells (NON) and cells induced for 3 days (IND3) were lysed by digitonin (4 mg/mg). Equal amounts of lysed mitochondrial proteins (20 μg) were fractionated on a 3%-12% hrCNE, blotted onto a nitrocellulose membrane and probed with an anti-β antibody. Positions of F1-ATPase and monomeric and multimeric FoF1-ATPases are depicted by arrows. The size of the equine spleen ferritin (440 kDa) is indicated on the left. F) The sedimentation profile of FoF1-ATPase complexes was examined using western blot analysis of glycerol gradient fractions. Mitochondria from RNAi non-induced cells (NON) and cells induced for 3 and 4 days (IND3 and IND4) were lysed by 1% Triton X-100 and an equal amount of the cleared samples (1.3 mg) were loaded on a 10–30% glycerol gradient. Western analyzes with anti-β and anti-p18 antibodies depicted the sedimentation profile of the FoF1-ATPase complexes. The glycerol gradient fractions are labelled and the relevant sizes of the protein marker are indicated. A nonspecific band only visible in Dk164 gradients is indicated by an asterisk. Since the Δψm in Dk cells is generated using the hydrolytic activity of the F1-moiety coupled with ATP/ADP translocation, the Δψm was examined in ATPaseTb2 non-induced and RNAi induced cell populations. As shown in Fig. 6C, a reduction of the Δψm was detected in the RNAi induced cell population after 2 (IND2) and 3 days (IND3). Since the reduction of the Δψm already appears at day 2 of tet induction and the growth phenotype does not truly manifest until day 3, we postulate that the observed decrease of the Δψm is a direct effect of ATPaseTb2 silencing and not a consequence of decreased cell viability. Furthermore, to assess if this decreased Δψm is caused by an impairment of the F1-ATPase hydrolytic activity, the total ATPase activity was measured in mt lysates with or without azide. Similar to BF RNAi ATPaseTb2 cells (Fig. 5A), no changes in total or azide-sensitive activities were observed between the non-induced and ATPaseTb2 RNAi-induced cells (Fig. 6D).
The structural integrity of Dk FoF1-ATPase complexes was then investigated using mild non-denaturing hrCNE to fractionate mitochondrial proteins purified from BF427 cells and Dk cells either induced for ATPaseTb2 RNAi or not. A western blot containing BF427 mitochondria as a reference sample was probed with an anti-β antibody and used as marker to visualize each state of the F1-moiety, either isolated by itself or being partnered with the monomeric and multimeric FoF1-complexes. Strikingly, while a majority of the β subunit signal in Dk RNAi non-induced cells was observed in the region of isolated F1-ATPase, we also consistently detected an irrefutable signal corresponding to the FoF1-monomeric complex, presumably assembled intact with the exception of subunit a (Fig. 6E). While there is evidence of mammalian rho cells containing a similar complex [44–46], this is the first time it has been demonstrated in the comparable Dk trypanosomatids. Furthermore, the induction of ATPaseTb2 RNAi in the Dk cells led to the disappearance of the monomeric complex, leaving only the F1-ATPase to be detected on the western blot (Fig. 6E). Once again, the evidence indicates that ATPaseTb2 is important for the structural integrity of the FoF1-ATPase and its depletion in Dk T.b. evansi cells leads to the disruption of the unique membrane-bound FoF1-ATPase complexes.
To corroborate this observation, we purified mitochondria from Dk T. b. evansi non-induced and ATPaseTb2 RNAi-induced cells and lysed them with Triton X-100. These gently disrupted lysates were then fractionated on glycerol gradients, which revealed that a majority of the signal from subunits β and p18 was located within fractions 6–10, representing the F1-moiety (Fig. 6F). Nevertheless, weaker bands representing the FoF1-ATPase complexes (fractions 14–22) were again detected in the non-induced samples (Fig. 6F NON panels). Noticeably, these same protein markers in the mt fractionation of ATPaseTb2 RNAi induced cells displayed a significant reduction in fractions 14–22 (Fig. 6F IND4 panels), where the FoF1 - monomer/oligomer sediments. This observation is quite surprising since the main role of this enzyme is to create the Δψm, presumably using only the hydrolytic activity of the matrix facing F1-moiety. In an attempt to determine if a portion of the F1-ATPase is membrane associated and that the disruption of this attachment is the reason for the observed decrease in the Δψm and the detected growth phenotype, we decided to visualize these complexes in situ using immunogold labelling followed by electron microscopy.
Loss of ATPaseTb2 leads to the dissociation of F1-ATPase from the mt membrane
Immunogold labeling with a primary anti-β antibody was performed on ultrasections of Dk T. b. evansi non-induced (NON) and ATPaseTb2 RNAi induced cells for 3 (IND3) and 5 (IND5) days. Electron micrographs illustrate that Dk trypanosomes have a simple and reduced mitochondrion (S3A Fig.), lacking both cristae and a typical kinetoplast structure, as previously reported for the dyskinetoplastic T. b. evansi [17]. Whenever these reduced double-membrane organelles could be unequivocally identified, we tallied the number of immunogold beads either in the close proximity of the mt membrane or in the matrix (S3A Fig.). Strikingly, 70% of the gold particles identified were found associated with the mt membrane in the NON images (S3B Fig.). From the 113 images captured from either NON, IND3 or IND5 samples, we performed a Chi-squared analysis on the immunogold beads associated with the mt membrane to determine if their distribution is random (S3C Fig.). With two degrees of freedom, the calculated χ2 = 43.5 (p < 0.0001), which signifies that the difference between the observed and expected particles was statistically significant. These values were then plotted to determine the relative labelling index (RLI = Nobs/ Nexp) for the mt membrane associated gold particles (S3D Fig.). This analysis reveals that the observed number of gold particles found in close proximity to the mt membrane for the NON electron micrographs is significantly greater than if the particles were randomly distributed (RLI = 1), while the opposite is true for the IND5 samples. Since we understand the general subjectivity of this method, we performed this experiment in a blinded study and only considered a gold bead to be associated with the mt membrane if it was in the immediate proximity of the membrane. For the very few scored beads that could potentially fall in a membrane-bound grey zone, it should be noted that the physical distance from the subunit β antigen to the gold particle can be up to 15–20 nm, not to mention that subunit β itself is projected from the membrane by subunit γ by as much as 13 nm [47,48]. Furthermore, very few immunogold beads were detected outside of defined mt structures and each mt image only contained 1–8 immunogold beads (average of 2.4 beads/mt image), suggesting that the labelling was very specific for our abundant mt antigen. It is important to note, that utilizing an assay that does not require the use of any detergents, we demonstrated that a majority of the F1-ATPase is located within close proximity of the mt membrane and this association is disrupted when ATPaseTb2 is depleted.
Discussion
The T. brucei FoF1-ATPase has obtained unique properties in comparison to its eukaryotic counterparts. The occurrence of novel subunits combined with the lack of typical eukaryotic subunits that compose the Fo membrane-bound moiety and the peripheral stalk is intriguing from an evolutionary viewpoint. Because these atypical subunits lack significant homology to known proteins, questions arise concerning their authenticity as genuine subunits of the complex and what their function and localization within the complex might be. Here, we focused on the functional characterization of one of the trypanosomatid specific subunits, ATPaseTb2 in the bloodstream stages of T. brucei. Notably, we determined that its function is essential for maintaining the normal growth rate of the infectious stage of T. brucei and also for the important veterinary dyskinetoplastic parasite, T. b. evansi. Additional analyses demonstrated that the ATPaseTb2 is membrane embedded and a component of the FoF1-ATPase that is present in both BF and Dk cells. Furthermore, the depletion of this Fo-moiety subunit results in a decreased Δψm and a loss of FoF1-ATPase complexes. Combined with bioinformatic tools that predict a transmembrane domain and identify a low homology to subunit d, we suggest that ATPaseTb2 might be a component of the peripheral stalk of the FoF1-ATPase.
The peripheral stalk is indispensable for ATP synthesis as it serves to impede the movement of the catalytic α3β3 headpiece while the proton motive force rotates the c-ring and the connected asymmetrical central stalk in a way that imposes conformational changes in the catalytic nucleotide binding sites of the β subunits. The peripheral stator is also essential when the complex harnesses the energy provided from ATP hydrolysis to drive the rotation of the enzyme in reverse, allowing protons to be pumped across the mt inner membrane to produce the Δψm when physiological conditions dictate.
The bovine and yeast peripheral stalk is composed of four subunits, OSCP, b, d, and F6/h (bovine/yeast nomenclature). Subunit b (∼20kDa protein) contains two trans-membrane segments at the N-terminus, while the rest of the protein is hydrophilic—protruding into the matrix and interacting with OSCP and subunit d [23,49–51]. The interaction between subunit b and OSCP is stabilized by subunit F6 [22]. The predominantly hydrophilic subunit d interacts with all three mentioned subunits and it has been shown to be essential for the function of the FoF1-complex. The yeast knock-out of subunit d was characterized by the de-attachment of the catalytic F1-ATPase from the protonophoric sector, the loss of detectable oligomycin sensitive ATPase activity and the absence of subunit a in the Fo-moiety [36]. Notably, of these four subunits, the only homolog identified in the T. brucei genome is OSCP and its protein product was identified as a bona fide subunit of the purified FoF1-ATP synthase [26]. Homologs for subunits b, d, and F6 are missing and most likely have been replaced by the novel proteins that associate with the T. brucei FoF1-ATP synthase. Here we demonstrated that the ATPaseTb2 subunit is a membrane-bound protein, containing one predicted transmembrane domain, with a large hydrophilic region extending into the matrix that possesses a low homology to the bovine subunit d. In accordance with other yeast or bovine subunits of the peripheral stalk, the down-regulation of ATPaseTb2 affects the stability of the FoF1-complex and its oligomycin sensitivity (this study, [26]).
Considering the relatively large molecular mass of ATPaseTb2 (43 kDa), it is plausible to speculate that ATPaseTb2 functionally represents the membrane-bound subunit of the peripheral stalk fused with subunit d, offering an early attempt by eukaryotes to add layers of complexity that will allow for greater adaptability. It is noteworthy to mention that a species-specific architectural variant of the peripheral stalk was also proposed for colorless and green algae, where the novel subunits Asa2, Asa4 and Asa7 fulfil a structural role in forming the peripheral stalk [52,53]. Other discrepancies from the typical eukaryotic enzyme model can be found in the ciliate Tetrahymena thermophila, representing the superphylum of Alveolates, where the homolog of the conserved subunit b has so far not been identified in the genome. Instead, three novel proteins detected in this ATP synthase complex have been proposed to substitute for subunit b [28]. Thus, it seems that the composition and overall structural appearance of the FoF1-ATP synthase from organisms representing different lineages other than Opisthokonts, might be more diverse than previously thought and deserve more attention from basic researchers to elucidate alternate evolutionary solutions to a common thread of life.
T. brucei is a member of the Excavata clade and represents a desirable model to study the function and regulation of the FoF1-ATP synthase/ase complex since it utilizes the proton motive force to produce ATP in the PF life stage, while in the BF stage it hydrolyzes ATP to pump the protons necessary to create the Δψm. Moreover, dyskinetoplastic T. b. evansi employs the hydrolytic activity of the F1-ATPase and the electrogenic exchange of ADP3- for ATP4- by the AAC as yet another strategy to generate the Δψm (Fig. 7). Therefore, it was surprising that the ATPaseTb2 protein was detected in all four examined cell types (PF, BF, Dk 164 and T. b. evansi), albeit at a much lower abundance in BF and Dk cells, but that is in full agreement with the reduced mitochondria previously defined for these two forms [39]. Furthermore, when PF and BF mt lysates were resolved by GG and hrCNE, ATPaseTb2 was only detected when co-localized with the monomeric and oligomeric FoF1-ATP synthase/ase complexes. Interestingly, similar results were obtained from the mt lysates of cells lacking mtDNA. Considering that only the F1-ATPase was previously assumed to be important for maintaining the Δψm in the Dk trypanosomes, the presence of these complexes is intriguing. Nevertheless, a proteomics study performed with osteosarcoma 143B ρ° cells revealed that in addition to the F1-ATPase subunits, subunit d of the peripheral stalk was also identified [54]. In a similar project involving fibroblast MRC5 ρ° cells, the oligomycin insensitive complex was purified and determined to contain several Fo (b and c) subunits along with a couple of peripheral stalk subunits (OSCP, d). Notably, this complex was loosely associated with the mt membrane [45]. Furthermore, several additional studies have shown that the F1-ATPase in yeast or mammalian cells lacking a mt genome (i.e. mammalian subunits a/ATP6, A6L; yeast subunits 6, 8 and 9) is membrane associated, with the attachment most likely occurring via the central and/or peripheral stalk [44,46,55,56]. Our data also suggest that since ATPaseTb2 membrane-bound complexes (monomeric and multimeric) do not display significant differences in their native size or sedimentation values, then the mt encoded subunit a might be the only missing subunit from these complexes. Such a uniquely structured complex can be explained by the current model for the assembly of the FoF1-ATPase, in which the Fo subunit a is the last protein incorporated into the enzyme to ensure that it is able to function properly before introducing a complete proton pore. Furthermore, this integration is dependent on the presence of subunit b of the peripheral stalk [57].
Fig. 7. Schematic representation of the FoF1-ATP synthase/ATPase complex in trypanosome mitochondria. 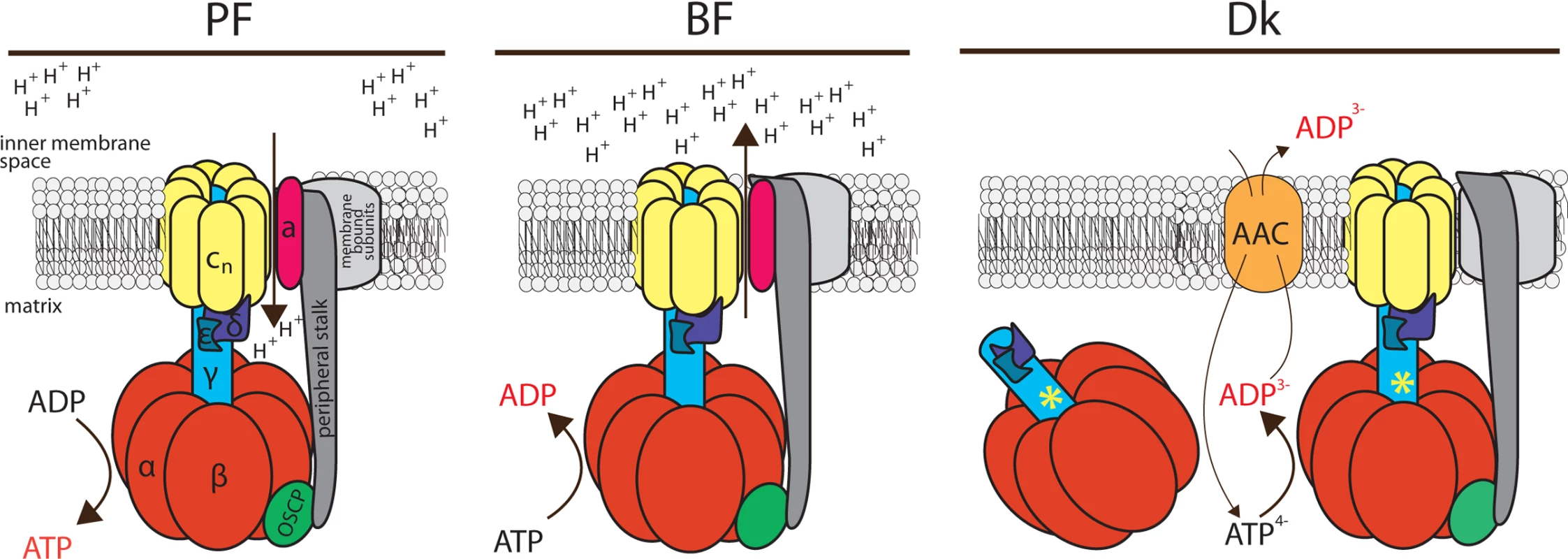
The FoF1-ATP synthase possesses the conventional function in the procyclic form (PF) of the parasite, coupling transmembrane proton transfer with ATP synthesis. In contrast, this enzymatic complex works in reverse in the bloodstream form (BF) of the parasite, hydrolyzing ATP to generate the Δψm in the absence of canonical cytochrome-containing respiratory complexes. This hydrolytic activity is also utilized in the dyskinetoplastid (Dk) form, which lacks mitochondrial DNA and thus a proton pore. Therefore, in Dk cells, enhanced ATP hydrolysis by a mutated F1-moiety (a necessary mutation in the γ subunit is marked with the asterisk) provides substrate for the ATP/ADP carrier (AAC) and the Δψm is produced by the electrogenic exchange of ADP3- for ATP4-. Furthermore, we present that a minor fraction of F1-ATPase still appears to be associated with Fo and that association may be functionally important for maintaining the Δψm. Orthologues of the colored subunits (α, β, γ, δ, ε, OSCP, a and c) have been annotated in trypanosomes. In addition to OSCP, the peripheral stalk (dark grey) is usually composed of subunits b, F6 and d, but these homologs have not been identified in the T. brucei genome. Also, conspicuously absent are subunits A6L, e, f and g, which are all membrane-bound (light grey). RNAi silencing of ATPaseTb2 in BF cells caused a strong growth phenotype concurring with a decreased Δψm. Importantly, ATPaseTb2 also proved to be essential for maintaining the normal growth rate and the Δψm of Dk cells. Combined with the hrCNE and glycerol gradient sedimentation assays that revealed a decrease of the high molecular weight complexes, but not the F1-ATPase, our studies suggest that these membrane-bound enzymes might significantly contribute to the membrane potential in Dk cells. The biological significance of this localization of the F1-ATPase might be to efficiently coordinate the activity of this enzyme with its substrate transporter, both of which are responsible for producing the Δψm in Dk. By restricting the F1-ATPase to the mt membrane, it keeps it within the close vicinity of its biochemically functional partner, the AAC. In this way, it helps ensure that the hydrolysis of ATP results in a relatively concentrated region of substrate for the mt membrane embedded AAC, especially in a mitochondrion lacking defined cristae and the microenvironments they create. Indeed, there is precedence for such a close spatial collaboration to increase efficiency, as an actual physical interaction between these two enzymes has been reported as the ATP synthasome in mammals [40,58]. We are currently building a set of tools to perform additional functional assays and imaging techniques that we hope will further resolve the function of the FoF1-ATPase complexes in these pathogenic trypanosomes.
Materials and Methods
Plasmid construction
The ATPaseTb2 (Tb927.5.2930) RNAi constructs targeted an 825 bp fragment of the gene that was PCR amplified from T. brucei strain 427 genomic DNA with the following oligonucleotides: FW—CACGGATCCATGCGCCGTGTATC, REV-CACCTCGAGTTCGGCCCGATC. Utilizing the BamHI and XhoI restriction sites inherent in the primers (underlined), this fragment was cloned into the p2T7-TA blue plasmid [59] (used to create a BF RNAi cell line) and the p2T7–177 plasmid [60] (used to generate a Dk RNAi cell line). For the inducible expression of ATPaseTb2 fused with a C-terminal 3xv5 tag, the ATPaseTb2 coding sequence was PCR amplified (Fw: ACAAAGCTTATGC GCCGTGTATC, Rev: ATAGGATCCGTGATGGGCCTTTTTC) and cloned into the pT7_v5 vector using HindIII and BamHI restriction enzymes [61]. The pLEW79MHTAP vectors for the tet inducible expression of ATPaseTb2 and Tb927.5.1710 (FoF1-ATPase subunit p18) TAP-tagged proteins were previously described in [26].
Trypanosoma culture conditions and generation of cell lines
Bloodstream form T. brucei brucei Lister 427 strain, stable acriflavin-induced dyskinetoplastic T. b. brucei EATRO 164 [18] and dyskinetoplastic T. b. evansi Antat 3/3 [62] were all grown in vitro at 37°C and 5% CO2 in HMI-9 media containing 10% FBS. The BF427 single marker cell line [63] and a T. b. evansi cell line [14], both constitutively expressing the ectopic T7 RNA polymerase and tet repressor (TetR were used for the tet inducible expression of dsRNA and the TAP - and v5-tagged proteins. Each plasmid was linearized with NotI enzyme and transfected into the appropriate cell line as described previously [63]. Linearized p2T7–177 was targeted to the minichromosome 177 basepair repeat region, while linear pTv5 and pLEW79MHTAP vectors were integrated into the rDNA intergenic spacer region [60,61,63]. The induction of RNAi and ectopically expressed tagged proteins was triggered by the addition of 1 μg/ml of tet into the media. Cell densities were measured using the Z2 Cell Counter (Beckman Coulter Inc.). Throughout the analyses, cells were maintained in the exponential mid-log growth phase (between 1x105 to 1x106 cells/ml).
Tandem affinity purification (TAP) of tagged complexes
The TAP protocol was generously provided by L. Jeacock and A. Schnaufer (personal communication) and optimized for BF cells. Briefly, 2x108 cells were harvested and lysed for 20 min on ice with 1% Triton X-100 in an IPP150 buffer (150 mM NaCl, 0,1% NP40, 10mM Tris-HCl pH 8.0) containing Complete protease inhibitors (Roche). The lysates were then cleared by centrifugation (16,000 g, 15 min, 4°C). Meanwhile, IgG antibodies were covalently bound to Dynabeads M-270 Epoxy (Invitrogen) using a protocol previously described [64]. Charged beads were blocked with 1% BSA and then incubated with the cleared cell lysates (4°C for 2 hours, constantly rotating). The beads were then washed three times with IPP150 and once with a TEVCB buffer (150 mM NaCl, 0.1% NP40, 0.5 mM EDTA, 1 mM DTT, 10 mM Tris-HCl, pH 8.0). Finally, the bound protein complexes were released by AcTEV protease (Invitrogen) cleavage and the eluate was analyzed by SDS-PAGE.
Isolation of mt vesicles
Crude mt vesicles were obtained by hypotonic lysis as described in detail earlier [65]. Briefly, cell pellets from ∼2x109 cells were washed with SBG (150 mM NaCl, 20 mM glucose, 20 mM NaHPO4, pH 7.9), resuspended in DTE (1 mM Tris, 1 mM EDTA, pH 8.0) and homogenized in a Dounce homogenizer. Alternatively, for a smaller scale hypotonic isolation, cell pellets from ∼1x109 cells were washed in NET buffer (150 mM NaCl, 100 mM EDTA, 10 mM Tris, pH 8.0), resuspended in DTE and homogenized by passing through a 25G needle. To re-create the physiological isotonic environment, 60% sucrose was immediately added to the lysed cells to reach a final concentration of 250 mM. Samples were spun down at 15,000 g, 10 min, 4°C to clear the soluble cytoplasmic material from the lysates. The resulting pellets were resuspended in STM (250 mM sucrose, 20 mM Tris pH 8.0, 2 mM MgCl2), supplemented with a final concentration of 3 mM MgCl2 and 0.3 mM CaCl2 before incubating with 5 μg/ml DNase I for 1hr on ice. Then an equal volume of STE buffer (250 mM sucrose, 20 mM Tris pH 8.0, 2 mM EDTA pH 8.0) was added and the material was centrifuged at 15,000 g, 10 min, 4°C. Pellets enriched with the mt membrane vesicles were washed in STE and kept at -70°C.
SDS-PAGE and Western blot
Protein samples were separated on SDS-PAGE, blotted onto a PVDF membrane (PALL) and probed with the appropriate monoclonal (mAb) or polyclonal (pAb) antibody. This was followed by incubation with a secondary HRP-conjugated anti-rabbit or anti-mouse antibody (1 : 2000, BioRad). Proteins were visualized using the Pierce ECL system (Genetica/Biorad) on a ChemiDoc instrument (BioRad). When needed, membranes were stripped at 50°C for 30 min in a stripping buffer (62.5 mM Tris pH 6.8, 100 mM mercapthoethanol, 2% SDS) and re-probed. The PageRuler prestained protein standard (Fermentas) was used to determine the size of detected bands. Primary antibodies used in this study were: mAb anti-v5 epitope tag (1 : 2000, Invitrogen), mAb anti-mtHsp70 (1 : 2000) [66], pAb anti-AAC (1 : 2000) [67], pAb anti-MRP1 (1 : 1000) [68], pAb anti-APRT (1 : 1000) and pAb anti-enolase (1 : 1000) [69]. Antibodies against ATPaseTb2 (1 : 1000), ATPaseTb1 (1 : 1000), subunit β (1 : 2000) and subunit p18 (1 : 1000) were prepared for the purpose of this study. The open reading frames of the respective genes without their predicted mt localization signal were cloned into the E. coli expression plasmid, pSKB3. The proteins were overexpressed in BL21 E. coli cells and purified under native conditions (subunit β) or denatured conditions (ATPaseTb2, ATPaseTb1 and p18) using a 6 M guanidinium lysis buffer and 8 M urea binding buffer. The denatured proteins were then refolded with a step-wise dialysis procedure that included 0.5 M arginine in the refolding buffer. Native and refolded antigens were sent to Pineda (Antikörper-Service, Germany) or Davids Biotechnologie (Regensburg, Germany) for polyclonal antibody production.
Mt membrane potential (Δψm) measurement
The Δψm was determined utilizing the red-fluorescent stain Mitotracker Red CMX-Ros (Invitrogen). Cells in the exponential growth phase were stained with 100 nM of the dye for 30 min at 37°C. Cells were pelleted (1,300 g, 10 min, RT), resuspended in 2 ml of PBS (pH 7.4) and immediately analyzed by flow cytometry (BD FACS Canto II Instrument). For each sample, 10,000 events were collected. Treatment with the protonophore FCCP (20 μM) was used as a control for mt membrane depolarization. Data were evaluated using BD FACSDiva (BD Company) software.
F1 - ATPase assay
ATPase activity was measured with the Sumner assay, which is based on the release of free phosphate when ATP is hydrolyzed by the enzyme as described earlier [14,70]. Briefly, crude mt lysates were obtained from 2x108cells by SoTe/digitonin extraction (0.015% digitonin, 0.6 M Sorbitol, 2 mM EDTA, 20 mM Tris-HCl pH 7.5). Mt pellets were resuspended in an assay buffer (200 mM KCl, 2 mM MgCl2, Tris-HCl pH 8.0) and the 20 min reaction was initiated by the addition of ATP to a final concentration of 5 mM. Where indicated, samples were pre-treated with the F1-ATPase specific inhibitor, sodium azide (2 mM) for 10 min at 37°C. The 100 μl enzymatic reactions were deproteinated by the addition of 1.9 μl of 70% perchloric acid. After a 30 min incubation on ice, the samples were spun down (16,000g, 10 min, 4°C) and 90 μl of the supernatant was incubated for 10 min with 0,5 ml of Sumner reagent (8.8% FeSO4.7H2O, 375 mM H2SO4, 6.6% (NH4)Mo7O24.4H2O)[62]. 200 μl was then transferred to a 96 well plate and the absorbance was measured at 610 nm using a Tecan Infinite plate reader (Infinite M200Pro, Tecan). To calibrate the assay, a standard curve was calculated from the absorbance values of linear inorganic phosphate samples (0–2 mM).
In-gel histochemical staining of F1-ATPase activity
Blue native PAGE (BNE) of mt lysates, followed by in-gel activity staining was adapted from published protocols [26,32]. Briefly, mt vesicles from ∼2x109 cells were resuspended in a mt lysis buffer (0,75 M amino-n-caproic acid—ACA, 50 mM Bis-Tris, 0,5 mM EDTA, pH 7.0), lysed with 2% dodecylmaltoside (DDM) for 1hr on ice and then cleared by centrifugation (16,000g for 30 min, 4°C). The protein concentration of each mt lysate was determined by a Bradford assay (BioRad), so that 100 μg of total mt protein could be mixed with 1 M ACA and 5% Coomassie brilliant blue G-250 before being loaded on a 2–12% Bis-Tris BNE gel. Immediately after the run (3 hr, 100 V, 4°C), the gel was incubated overnight in an ATPase reaction buffer (35 mM Tris pH 8.0, 270 mM glycine, 19mM MgSO4, 0.3% Pb(NO3)2, 11 mM ATP).
High resolution clear native PAGE (hrCNE)
The protocol for hrCNE was adapted from published studies [71,72]. In summary, crude mt vesicles from ∼5x108 cells were resuspended in a mt lysis buffer (2 mM ACA, 50 mM Imidazole-HCl, 1 mM EDTA, 50 mM NaCl, pH 7) and lysed for one hour on ice with 4 mg digitonin/1 mg protein. The samples were spun down at 16,000 g for 30 min and the cleared lysate protein concentrations were determined by a Bradford assay. Samples were mixed with a 5x loading dye (0.1% Ponceau-S, 50% glycerol) and loaded onto a 3%-12% native gradient gel. After electrophoresis (3 hr, 100 V, 4°C), the resolved mt lysates were transferred onto a nitrocellulose membrane (overnight, 20 V, 4°C) and probed with selected antibodies.
Na2CO3 submitochondrial fractionation
Na2CO3 extraction of mt membranes was adapted from a previously published protocol [73]. Mt vesicles from 1x109 cells were isolated by hypotonic lysis as described above. The resulting supernatant from the 25G needle homogenization step was kept as a cytosolic fraction (CYTO). The mt pellet was further treated with digitonin (80 μg/ml) for 15 min on ice to disrupt the mt outer membrane. The material was then cleared by centrifugation (12,000 g, 20 min, 4°C) and the pelleted mitoplasts were resuspended in 0.1 M Na2CO3 buffer (pH 11.5) and incubated for 30 min on ice. A final ultracentrifugation step (100,000 g, 4°C for 1 hr) performed in an SW50Ti rotor of a Beckman Instrument resulted in a supernatant comprised of proteins from the mt matrix (MX), including stripped peripheral membrane proteins, and a pellet containing integral proteins isolated from the mt membrane fraction (M).
Glycerol gradient (GG) sedimentation
Hypotonically purified mt vesicles from ∼2.5x109 cells were resuspended in a GG lysis buffer (10 mM Tris, pH 7.2, 10 mM MgCl2, 200 mM KCl, 1mM DTT) and lysed with 1% Triton X-100 (30 min, on ice). The lysates were cleared by a centrifugation step (2x 16,000 g, 30 min, 4°C) and the protein concentration was determined by a Bradford assay. Mt cleared lysates were resolved by ultracentrifugation (Beckman Instrument, SW40 rotor) at 38,000 g for 5 hr on 11ml 10–30% GG, which was poured manually or using the Gradient Station (Biocomp) according to the manufacturer’s protocol. The glycerol gradients were then fractionated either manually or with the Gradient station and 500μl fractions were stored at -70°C.
Immunogold staining of ultrathin sections and transmission electron microscopy
Cells (∼5x107) were pelleted (1300 g, 10 min, RT), washed in PBS (pH 7.4) and immediately fixed in a 4% formaldehyde/ 0.1 M phosphate buffer. Samples were then dehydrated at -10°C through a series of seven steps that increased the concentration of ethanol from 30% to100%, pausing at each step for 1 hour. Next, the samples were embedded in LR White Resin (Electron Microscopy Sciences) and polymerized (UV light, 48 hours at -10°C). Ultrathin sections were prepared by the Ultracut UCT ultramicrotome (Leica) and mounted on nickel grids. Prepared sections were blocked in 5% BSA, labelled with primary anti-β antibody (1 : 10 dilution), washed three times with PBS and incubated with protein A conjugated to 10 nm gold particles (1 : 100 dilution, Aurion). The immunogold labelled grids were contrasted, carbon coated and examined by the TEM (JEM-1010, Jeol). Grids, which were immunolabeled with only the protein A conjugated to 10nM gold particles, were used as a negative control.
Quantification and statistical analysis of immunogold labelling
The number of gold particles was statistically evaluated as described earlier [12,74]. Using ImageJ software (NIH, USA), a grid consisting of squares (test points, P) with constant size (16,105 μm2) was superimposed randomly on the electron micrographs of identified mitochondria. All test squares and immunogold particles within the immediate proximity to the mt membrane were counted separately for each micrograph. In order to statistically evaluate the labelling, all observed gold particles (Nobs) and all test points (P) from 113 images captured from NON, IND3 and IND5 samples were tallied. Expected numbers of gold particles (Nexp) for each sample were calculated as (total sum Nobs x P)/total sum P. To determine if the difference between the observed and expected particles was significant and not due to random fluctuations, a Chi-squared analysis χ2 = (Nobs—Nexp)2/ Nexp was performed using GraphPad QuickCalcs calculator (www.graphpad.com/quickcalcs). In addition, the relative labeling index (RLI), where the predicted RLI = 1 for random labelling, was calculated as RLI = Nobs/ Nexp.
Gene IDs for the genes and proteins mentioned in this study
ATPaseTb2 (Tb927.5.2930), ATPaseTb1 (Tb927.10.520), TbAAC (Tb927.10.14820/30/40), p18 (Tb927.5.1710), mtHsp70 (Tb927.6.3740), MRP1 (Tb927.11.1710), APRT (Tb927.7.1780), enolase (Tb427.10.2890), subunit β (Tb927.3.1380).
Supporting Information
Zdroje
1. Jamonneau V, Ilboudo H, Kabore J, Kaba D, Koffi M, et al. (2012) Untreated human infections by Trypanosoma brucei gambiense are not 100% fatal. PLoS Negl Trop Dis 6: e1691. doi: 10.1371/journal.pntd.0001691 22720107
2. Stuart K, Brun R, Croft S, Fairlamb A, Gurtler RE, et al. (2008) Kinetoplastids: related protozoan pathogens, different diseases. J Clin Invest 118 : 1301–1310. doi: 10.1172/JCI33945 18382742
3. Steverding D (2008) The history of African trypanosomiasis. Parasit Vectors 1 : 3. doi: 10.1186/1756-3305-1-3 18275594
4. Bringaud F, Riviere L, Coustou V (2006) Energy metabolism of trypanosomatids: adaptation to available carbon sources. Mol Biochem Parasitol 149 : 1–9. 16682088
5. Besteiro S, Barrett MP, Riviere L, Bringaud F (2005) Energy generation in insect stages of Trypanosoma brucei: metabolism in flux. Trends Parasitol 21 : 185–191. 15780841
6. Hannaert V, Bringaud F, Opperdoes FR, Michels PA (2003) Evolution of energy metabolism and its compartmentation in Kinetoplastida. Kinetoplastid Biol Dis 2 : 11. 14613499
7. Brown SV, Hosking P, Li J, Williams N (2006) ATP synthase is responsible for maintaining mitochondrial membrane potential in bloodstream form Trypanosoma brucei. Eukaryot Cell 5 : 45–53. 16400167
8. Guler JL, Kriegova E, Smith TK, Lukes J, Englund PT (2008) Mitochondrial fatty acid synthesis is required for normal mitochondrial morphology and function in Trypanosoma brucei. Mol Microbiol 67 : 1125–1142. doi: 10.1111/j.1365-2958.2008.06112.x 18221265
9. Nolan DP, Voorheis HP (1992) The mitochondrion in bloodstream forms of Trypanosoma brucei is energized by the electrogenic pumping of protons catalysed by the F1F0-ATPase. Eur J Biochem 209 : 207–216. 1327770
10. Huang G, Vercesi AE, Docampo R (2013) Essential regulation of cell bioenergetics in Trypanosoma brucei by the mitochondrial calcium uniporter. Nat Commun 4 : 2865. doi: 10.1038/ncomms3865 24305511
11. Vercesi AE, Docampo R, Moreno SN (1992) Energization-dependent Ca2+ accumulation in Trypanosoma brucei bloodstream and procyclic trypomastigotes mitochondria. Mol Biochem Parasitol 56 : 251–257. 1484549
12. Kovarova J, Horakova E, Changmai P, Vancova M, Lukes J (2014) Mitochondrial and nucleolar localization of cysteine desulfurase Nfs and the scaffold protein Isu in Trypanosoma brucei. Eukaryot Cell 13 : 353–362. doi: 10.1128/EC.00235-13 24243795
13. Mazet M, Morand P, Biran M, Bouyssou G, Courtois P, et al. (2013) Revisiting the central metabolism of the bloodstream forms of Trypanosoma brucei: production of acetate in the mitochondrion is essential for parasite viability. PLoS Negl Trop Dis 7: e2587. doi: 10.1371/journal.pntd.0002587 24367711
14. Schnaufer A, Clark-Walker GD, Steinberg AG, Stuart K (2005) The F1-ATP synthase complex in bloodstream stage trypanosomes has an unusual and essential function. EMBO J 24 : 4029–4040. 16270030
15. Buchet K, Godinot C (1998) Functional F1-ATPase essential in maintaining growth and membrane potential of human mitochondrial DNA-depleted rho degrees cells. J Biol Chem 273 : 22983–22989. 9722521
16. Dean S, Gould MK, Dewar CE, Schnaufer AC (2013) Single point mutations in ATP synthase compensate for mitochondrial genome loss in trypanosomes. Proc Natl Acad Sci U S A 110 : 14741–14746. doi: 10.1073/pnas.1305404110 23959897
17. Brun R, Hecker H, Lun ZR (1998) Trypanosoma evansi and T. equiperdum: distribution, biology, treatment and phylogenetic relationship (a review). Vet Parasitol 79 : 95–107. 9806490
18. Stuart KD (1971) Evidence for the retention of kinetoplast DNA in an acriflavine-induced dyskinetoplastic strain of Trypanosoma brucei which replicates the altered central element of the kinetoplast. J Cell Biol 49 : 189–195. 4102002
19. Lai DH, Hashimi H, Lun ZR, Ayala FJ, Lukes J (2008) Adaptations of Trypanosoma brucei to gradual loss of kinetoplast DNA: Trypanosoma equiperdum and Trypanosoma evansi are petite mutants of T. brucei. Proc Natl Acad Sci U S A 105 : 1999–2004. doi: 10.1073/pnas.0711799105 18245376
20. Walker JE (2013) The ATP synthase: the understood, the uncertain and the unknown. Biochem Soc Trans 41 : 1–16. doi: 10.1042/BST20110773 23356252
21. Devenish RJ, Prescott M, Rodgers AJ (2008) The structure and function of mitochondrial F1F0-ATP synthases. Int Rev Cell Mol Biol 267 : 1–58. doi: 10.1016/S1937-6448(08)00601-1 18544496
22. Walker JE, Dickson VK (2006) The peripheral stalk of the mitochondrial ATP synthase. Biochim Biophys Acta 1757 : 286–296. 16697972
23. Dickson VK, Silvester JA, Fearnley IM, Leslie AG, Walker JE (2006) On the structure of the stator of the mitochondrial ATP synthase. EMBO J 25 : 2911–2918. 16791136
24. Vaidya AB, Mather MW (2009) Mitochondrial evolution and functions in malaria parasites. Annu Rev Microbiol 63 : 249–267. doi: 10.1146/annurev.micro.091208.073424 19575561
25. Lapaille M, Escobar-Ramirez A, Degand H, Baurain D, Rodriguez-Salinas E, et al. (2010) Atypical subunit composition of the chlorophycean mitochondrial F1FO-ATP synthase and role of Asa7 protein in stability and oligomycin resistance of the enzyme. Mol Biol Evol 27 : 1630–1644. doi: 10.1093/molbev/msq049 20156838
26. Zikova A, Schnaufer A, Dalley RA, Panigrahi AK, Stuart KD (2009) The F(0)F(1)-ATP synthase complex contains novel subunits and is essential for procyclic Trypanosoma brucei. PLoS Pathog 5: e1000436. doi: 10.1371/journal.ppat.1000436 19436713
27. Mather MW, Henry KW, Vaidya AB (2007) Mitochondrial drug targets in apicomplexan parasites. Curr Drug Targets 8 : 49–60. 17266530
28. Balabaskaran Nina P, Dudkina NV, Kane LA, van Eyk JE, Boekema EJ, et al. (2010) Highly divergent mitochondrial ATP synthase complexes in Tetrahymena thermophila. PLoS Biol 8: e1000418. doi: 10.1371/journal.pbio.1000418 20644710
29. Nishi A, Scherbaum OH (1962) Oxidative phosphorylation in synchronized cultures of Tetrahymena pyriformis. Biochim Biophys Acta 65 : 419–424. 13938753
30. Uyemura SA, Luo S, Vieira M, Moreno SN, Docampo R (2004) Oxidative phosphorylation and rotenone-insensitive malate - and NADH-quinone oxidoreductases in Plasmodium yoelii yoelii mitochondria in situ. J Biol Chem 279 : 385–393. 14561763
31. Vazquez-Acevedo M, Cardol P, Cano-Estrada A, Lapaille M, Remacle C, et al. (2006) The mitochondrial ATP synthase of chlorophycean algae contains eight subunits of unknown origin involved in the formation of an atypical stator-stalk and in the dimerization of the complex. J Bioenerg Biomembr 38 : 271–282. 17160464
32. Hashimi H, Benkovicova V, Cermakova P, Lai DH, Horvath A, et al. (2010) The assembly of F(1)F(O)-ATP synthase is disrupted upon interference of RNA editing in Trypanosoma brucei. Int J Parasitol 40 : 45–54. doi: 10.1016/j.ijpara.2009.07.005 19654010
33. Chi TB, Brown BS, Williams N (1998) Subunit 9 of the mitochondrial ATP synthase of Trypanosoma brucei is nuclearly encoded and developmentally regulated. Mol Biochem Parasitol 92 : 29–38. 9574907
34. Claros MG, Vincens P (1996) Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem 241 : 779–786. 8944766
35. Biegert A, Mayer C, Remmert M, Soding J, Lupas AN (2006) The MPI Bioinformatics Toolkit for protein sequence analysis. Nucleic Acids Res 34: W335–339. 16845021
36. Norais N, Prome D, Velours J (1991) ATP synthase of yeast mitochondria. Characterization of subunit d and sequence analysis of the structural gene ATP7. J Biol Chem 266 : 16541–16549. 1832157
37. Walker JE, Runswick MJ, Poulter L (1987) ATP synthase from bovine mitochondria. The characterization and sequence analysis of two membrane-associated sub-units and of the corresponding cDNAs. J Mol Biol 197 : 89–100. 2890767
38. Nugent T, Jones DT (2009) Transmembrane protein topology prediction using support vector machines. BMC Bioinformatics 10 : 159. doi: 10.1186/1471-2105-10-159 19470175
39. Vertommen D, Van Roy J, Szikora JP, Rider MH, Michels PA, et al. (2008) Differential expression of glycosomal and mitochondrial proteins in the two major life-cycle stages of Trypanosoma brucei. Mol Biochem Parasitol 158 : 189–201. doi: 10.1016/j.molbiopara.2007.12.008 18242729
40. Ko YH, Delannoy M, Hullihen J, Chiu W, Pedersen PL (2003) Mitochondrial ATP synthasome. Cristae-enriched membranes and a multiwell detergent screening assay yield dispersed single complexes containing the ATP synthase and carriers for Pi and ADP/ATP. J Biol Chem 278 : 12305–12309. 12560333
41. Meyer B, Wittig I, Trifilieff E, Karas M, Schagger H (2007) Identification of two proteins associated with mammalian ATP synthase. Mol Cell Proteomics 6 : 1690–1699. 17575325
42. Chen Y, Hung CH, Burderer T, Lee GS (2003) Development of RNA interference revertants in Trypanosoma brucei cell lines generated with a double stranded RNA expression construct driven by two opposing promoters. Mol Biochem Parasitol 126 : 275–279. 12615326
43. Bowler MW, Montgomery MG, Leslie AG, Walker JE (2006) How azide inhibits ATP hydrolysis by the F-ATPases. Proc Natl Acad Sci U S A 103 : 8646–8649. 16728506
44. Appleby RD, Porteous WK, Hughes G, James AM, Shannon D, et al. (1999) Quantitation and origin of the mitochondrial membrane potential in human cells lacking mitochondrial DNA. Eur J Biochem 262 : 108–116. 10231371
45. Garcia JJ, Ogilvie I, Robinson BH, Capaldi RA (2000) Structure, functioning, and assembly of the ATP synthase in cells from patients with the T8993G mitochondrial DNA mutation—Comparison with the enzyme in Rho(0) cells completely lacking mtDNA. Journal of Biological Chemistry 275 : 11075–11081. 10753912
46. Wittig I, Meyer B, Heide H, Steger M, Bleier L, et al. (2010) Assembly and oligomerization of human ATP synthase lacking mitochondrial subunits a and A6L. Biochim Biophys Acta 1797 : 1004–1011. doi: 10.1016/j.bbabio.2010.02.021 20188060
47. Stock D, Leslie AG, Walker JE (1999) Molecular architecture of the rotary motor in ATP synthase. Science 286 : 1700–1705. 10576729
48. Strauss M, Hofhaus G, Schroder RR, Kuhlbrandt W (2008) Dimer ribbons of ATP synthase shape the inner mitochondrial membrane. EMBO J 27 : 1154–1160. doi: 10.1038/emboj.2008.35 18323778
49. Arnold I, Pfeiffer K, Neupert W, Stuart RA, Schagger H (1998) Yeast mitochondrial F1F0-ATP synthase exists as a dimer: identification of three dimer-specific subunits. EMBO J 17 : 7170–7178. 9857174
50. Burger G, Lang BF, Braun HP, Marx S (2003) The enigmatic mitochondrial ORF ymf39 codes for ATP synthase chain b. Nucleic Acids Res 31 : 2353–2360. 12711680
51. Heazlewood JL, Whelan J, Millar AH (2003) The products of the mitochondrial orf25 and orfB genes are FO components in the plant F1FO ATP synthase. FEBS Lett 540 : 201–205. 12681508
52. Miranda-Astudillo H, Cano-Estrada A, Vazquez-Acevedo M, Colina-Tenorio L, Downie-Velasco A, et al. (2014) Interactions of subunits Asa2, Asa4 and Asa7 in the peripheral stalk of the mitochondrial ATP synthase of the chlorophycean alga Polytomella sp. Biochim Biophys Acta 1837 : 1–13. doi: 10.1016/j.bbabio.2013.08.001 23933283
53. van Lis R, Mendoza-Hernandez G, Groth G, Atteia A (2007) New insights into the unique structure of the F0F1-ATP synthase from the chlamydomonad algae Polytomella sp. and Chlamydomonas reinhardtii. Plant Physiol 144 : 1190–1199. 17468226
54. Chevallet M, Lescuyer P, Diemer H, van Dorsselaer A, Leize-Wagner E, et al. (2006) Alterations of the mitochondrial proteome caused by the absence of mitochondrial DNA: A proteomic view. Electrophoresis 27 : 1574–1583. 16548050
55. Orian JM, Hadikusumo RG, Marzuki S, Linnane AW (1984) Biogenesis of mitochondria: defective yeast H+-ATPase assembled in the absence of mitochondrial protein synthesis is membrane associated. J Bioenerg Biomembr 16 : 561–581. 6242247
56. Paul MF, Velours J, Arselin de Chateaubodeau G, Aigle M, Guerin B (1989) The role of subunit 4, a nuclear-encoded protein of the F0 sector of yeast mitochondrial ATP synthase, in the assembly of the whole complex. Eur J Biochem 185 : 163–171. 2553400
57. Rak M, Gokova S, Tzagoloff A (2011) Modular assembly of yeast mitochondrial ATP synthase. EMBO J 30 : 920–930. doi: 10.1038/emboj.2010.364 21266956
58. Chen C, Ko Y, Delannoy M, Ludtke SJ, Chiu W, et al. (2004) Mitochondrial ATP synthasome: three-dimensional structure by electron microscopy of the ATP synthase in complex formation with carriers for Pi and ADP/ATP. J Biol Chem 279 : 31761–31768. 15166242
59. Alibu VP, Storm L, Haile S, Clayton C, Horn D (2005) A doubly inducible system for RNA interference and rapid RNAi plasmid construction in Trypanosoma brucei. Mol Biochem Parasitol 139 : 75–82. 15610821
60. Wickstead B, Ersfeld K, Gull K (2002) Targeting of a tetracycline-inducible expression system to the transcriptionally silent minichromosomes of Trypanosoma brucei. Mol Biochem Parasitol 125 : 211–216. 12467990
61. Surve S, Heestand M, Panicucci B, Schnaufer A, Parsons M (2012) Enigmatic presence of mitochondrial complex I in Trypanosoma brucei bloodstream forms. Eukaryot Cell 11 : 183–193. doi: 10.1128/EC.05282-11 22158713
62. Borst P, Fase-Fowler F, Gibson WC (1987) Kinetoplast DNA of Trypanosoma evansi. Mol Biochem Parasitol 23 : 31–38. 3033499
63. Wirtz E, Leal S, Ochatt C, Cross GA (1999) A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol Biochem Parasitol 99 : 89–101. 10215027
64. Oeffinger M, Wei KE, Rogers R, DeGrasse JA, Chait BT, et al. (2007) Comprehensive analysis of diverse ribonucleoprotein complexes. Nat Methods 4 : 951–956. 17922018
65. Panigrahi AK, Ogata Y, Zikova A, Anupama A, Dalley RA, et al. (2009) A comprehensive analysis of Trypanosoma brucei mitochondrial proteome. Proteomics 9 : 434–450. doi: 10.1002/pmic.200800477 19105172
66. Panigrahi AK, Zikova A, Dalley RA, Acestor N, Ogata Y, et al. (2008) Mitochondrial complexes in Trypanosoma brucei: a novel complex and a unique oxidoreductase complex. Mol Cell Proteomics 7 : 534–545. 18073385
67. Singha UK, Peprah E, Williams S, Walker R, Saha L, et al. (2008) Characterization of the mitochondrial inner membrane protein translocator Tim17 from Trypanosoma brucei. Mol Biochem Parasitol 159 : 30–43. doi: 10.1016/j.molbiopara.2008.01.003 18325611
68. Vondruskova E, van den Burg J, Zikova A, Ernst NL, Stuart K, et al. (2005) RNA interference analyses suggest a transcript-specific regulatory role for mitochondrial RNA-binding proteins MRP1 and MRP2 in RNA editing and other RNA processing in Trypanosoma brucei. J Biol Chem 280 : 2429–2438. 15504736
69. Hannaert V, Albert MA, Rigden DJ, da Silva Giotto MT, Thiemann O, et al. (2003) Kinetic characterization, structure modelling studies and crystallization of Trypanosoma brucei enolase. Eur J Biochem 270 : 3205–3213. 12869196
70. Law RH, Manon S, Devenish RJ, Nagley P (1995) ATP synthase from Saccharomyces cerevisiae. Methods Enzymol 260 : 133–163. 8592441
71. Wittig I, Karas M, Schagger H (2007) High resolution clear native electrophoresis for in-gel functional assays and fluorescence studies of membrane protein complexes. Mol Cell Proteomics 6 : 1215–1225. 17426019
72. Acestor N, Zikova A, Dalley RA, Anupama A, Panigrahi AP, et al. (2011) Trypanosoma brucei Mitochondrial Respiratome: Composition and organization in procyclic form. Molecular Cell Proteomics resubmitted.
73. Acestor N, Panigrahi AK, Ogata Y, Anupama A, Stuart KD (2009) Protein composition of Trypanosoma brucei mitochondrial membranes. Proteomics 9 : 5497–5508. doi: 10.1002/pmic.200900354 19834910
74. Mayhew TM, Lucocq JM (2008) Quantifying immunogold labelling patterns of cellular compartments when they comprise mixtures of membranes (surface-occupying) and organelles (volume-occupying). Histochem Cell Biol 129 : 367–378. doi: 10.1007/s00418-007-0375-6 18180944
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek 2014 Reviewer Thank YouČlánek Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water LabelingČlánek High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 2- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- 2014 Reviewer Thank You
- A Case for Two-Component Signaling Systems As Antifungal Drug Targets
- Prions—Not Your Immunologist’s Pathogen
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
- Livestock-Associated : The United States Experience
- The Neurotrophic Receptor Ntrk2 Directs Lymphoid Tissue Neovascularization during Infection
- The Intracellular Bacterium Uses Parasitoid Wasps as Phoretic Vectors for Efficient Horizontal Transmission
- CD200 Receptor Restriction of Myeloid Cell Responses Antagonizes Antiviral Immunity and Facilitates Cytomegalovirus Persistence within Mucosal Tissue
- Phage-mediated Dispersal of Biofilm and Distribution of Bacterial Virulence Genes Is Induced by Quorum Sensing
- CXCL9 Contributes to Antimicrobial Protection of the Gut during Infection Independent of Chemokine-Receptor Signaling
- Mitigation of Prion Infectivity and Conversion Capacity by a Simulated Natural Process—Repeated Cycles of Drying and Wetting
- Approaches Reveal a Key Role for DCs in CD4+ T Cell Activation and Parasite Clearance during the Acute Phase of Experimental Blood-Stage Malaria
- Revealing the Sequence and Resulting Cellular Morphology of Receptor-Ligand Interactions during Invasion of Erythrocytes
- Crystal Structures of the Carboxyl cGMP Binding Domain of the cGMP-dependent Protein Kinase Reveal a Novel Capping Triad Crucial for Merozoite Egress
- Non-redundant and Redundant Roles of Cytomegalovirus gH/gL Complexes in Host Organ Entry and Intra-tissue Spread
- Characterization of Metabolically Quiescent Parasites in Murine Lesions Using Heavy Water Labeling
- A Working Model of How Noroviruses Infect the Intestine
- CD44 Plays a Functional Role in -induced Epithelial Cell Proliferation
- Novel Inhibitors of Cholesterol Degradation in Reveal How the Bacterium’s Metabolism Is Constrained by the Intracellular Environment
- G-Quadruplexes in Pathogens: A Common Route to Virulence Control?
- A Rho GDP Dissociation Inhibitor Produced by Apoptotic T-Cells Inhibits Growth of
- Manipulating Adenovirus Hexon Hypervariable Loops Dictates Immune Neutralisation and Coagulation Factor X-dependent Cell Interaction and
- The RhoGAP SPIN6 Associates with SPL11 and OsRac1 and Negatively Regulates Programmed Cell Death and Innate Immunity in Rice
- Lymph-Node Resident CD8α Dendritic Cells Capture Antigens from Migratory Malaria Sporozoites and Induce CD8 T Cell Responses
- Coordinated Function of Cellular DEAD-Box Helicases in Suppression of Viral RNA Recombination and Maintenance of Viral Genome Integrity
- IL-33-Mediated Protection against Experimental Cerebral Malaria Is Linked to Induction of Type 2 Innate Lymphoid Cells, M2 Macrophages and Regulatory T Cells
- Evasion of Autophagy and Intracellular Killing by Human Myeloid Dendritic Cells Involves DC-SIGN-TLR2 Crosstalk
- CD8 T Cell Response Maturation Defined by Anentropic Specificity and Repertoire Depth Correlates with SIVΔnef-induced Protection
- Diverse Heterologous Primary Infections Radically Alter Immunodominance Hierarchies and Clinical Outcomes Following H7N9 Influenza Challenge in Mice
- Human Adenovirus 52 Uses Sialic Acid-containing Glycoproteins and the Coxsackie and Adenovirus Receptor for Binding to Target Cells
- Super-Resolution Imaging of ESCRT-Proteins at HIV-1 Assembly Sites
- Disruption of an Membrane Protein Causes a Magnesium-dependent Cell Division Defect and Failure to Persist in Mice
- Recognition of Hyphae by Human Plasmacytoid Dendritic Cells Is Mediated by Dectin-2 and Results in Formation of Extracellular Traps
- Essential Domains of Invasins Utilized to Infect Mammalian Host Cells
- High Heritability Is Compatible with the Broad Distribution of Set Point Viral Load in HIV Carriers
- Yeast Prions: Proteins Templating Conformation and an Anti-prion System
- A Novel Mechanism of Bacterial Toxin Transfer within Host Blood Cell-Derived Microvesicles
- A Wild Strain Has Enhanced Epithelial Immunity to a Natural Microsporidian Parasite
- Control of Murine Cytomegalovirus Infection by γδ T Cells
- Dimorphism in Fungal Pathogens of Mammals, Plants, and Insects
- Recognition and Activation Domains Contribute to Allele-Specific Responses of an Arabidopsis NLR Receptor to an Oomycete Effector Protein
- Direct Binding of Retromer to Human Papillomavirus Type 16 Minor Capsid Protein L2 Mediates Endosome Exit during Viral Infection
- Characterization of the Mycobacterial Acyl-CoA Carboxylase Holo Complexes Reveals Their Functional Expansion into Amino Acid Catabolism
- Prion Infections and Anti-PrP Antibodies Trigger Converging Neurotoxic Pathways
- Evolution of Genome Size and Complexity in the
- Antibiotic Modulation of Capsular Exopolysaccharide and Virulence in
- IFNγ Signaling Endows DCs with the Capacity to Control Type I Inflammation during Parasitic Infection through Promoting T-bet+ Regulatory T Cells
- Identification of Effective Subdominant Anti-HIV-1 CD8+ T Cells Within Entire Post-infection and Post-vaccination Immune Responses
- Viral and Cellular Proteins Containing FGDF Motifs Bind G3BP to Block Stress Granule Formation
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Cytoplasmic Actin Is an Extracellular Insect Immune Factor which Is Secreted upon Immune Challenge and Mediates Phagocytosis and Direct Killing of Bacteria, and Is a Antagonist
- A Specific A/T Polymorphism in Western Tyrosine Phosphorylation B-Motifs Regulates CagA Epithelial Cell Interactions
- Within-host Competition Does Not Select for Virulence in Malaria Parasites; Studies with
- A Membrane-bound eIF2 Alpha Kinase Located in Endosomes Is Regulated by Heme and Controls Differentiation and ROS Levels in
- Cytosolic Access of : Critical Impact of Phagosomal Acidification Control and Demonstration of Occurrence
- Role of Pentraxin 3 in Shaping Arthritogenic Alphaviral Disease: From Enhanced Viral Replication to Immunomodulation
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- HITS-CLIP Analysis Uncovers a Link between the Kaposi’s Sarcoma-Associated Herpesvirus ORF57 Protein and Host Pre-mRNA Metabolism
- Molecular and Functional Analyses of a Maize Autoactive NB-LRR Protein Identify Precise Structural Requirements for Activity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Control of Murine Cytomegalovirus Infection by γδ T Cells
- ATPaseTb2, a Unique Membrane-bound FoF1-ATPase Component, Is Essential in Bloodstream and Dyskinetoplastic Trypanosomes
- Rational Development of an Attenuated Recombinant Cyprinid Herpesvirus 3 Vaccine Using Prokaryotic Mutagenesis and In Vivo Bioluminescent Imaging
- Telomeric ORFS in : Does Mediator Tail Wag the Yeast?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



