-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
TNFα and IFNγ but Not Perforin Are Critical for CD8 T Cell-Mediated Protection against Pulmonary Infection
Bacterial pneumonia is one of the most common causes of death worldwide. Pulmonary infection of bacterium Yersinia pestis, the causative agent of plague, results in pneumonic plague and is extremely lethal. Mouse models of pulmonary Y. pestis infection are considered translational tools for the development of pneumonic plague countermeasures and studies of the basic mechanisms of immune defense against acutely lethal pulmonary bacterial infections. Here, we used several methods to investigate the functions that CD8 T cells exert to confer protection against pulmonary Y. pestis infection and evaluated their relative contributions. We found that although CD8 T cells have the ability to kill Y. pestis-infected cells, a function called cytotoxicity, this function is not required for CD8 T cells to protect against Y. pestis infection. In contrast, protection depends upon the ability of CD8 T cells to produce the cytokines TNFα and IFNγ, and mice whose T cells cannot produce these two cytokines are not protected. Therefore, we conclude that cytokine production, not cytotoxicity, is essential for CD8 T cell-mediated control of pulmonary Y. pestis infection and we suggest that assays detecting cytokine production may be useful correlates of vaccine efficacy against plague and other acutely lethal septic bacterial pneumonias.
Published in the journal: . PLoS Pathog 10(5): e32767. doi:10.1371/journal.ppat.1004142
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004142Summary
Bacterial pneumonia is one of the most common causes of death worldwide. Pulmonary infection of bacterium Yersinia pestis, the causative agent of plague, results in pneumonic plague and is extremely lethal. Mouse models of pulmonary Y. pestis infection are considered translational tools for the development of pneumonic plague countermeasures and studies of the basic mechanisms of immune defense against acutely lethal pulmonary bacterial infections. Here, we used several methods to investigate the functions that CD8 T cells exert to confer protection against pulmonary Y. pestis infection and evaluated their relative contributions. We found that although CD8 T cells have the ability to kill Y. pestis-infected cells, a function called cytotoxicity, this function is not required for CD8 T cells to protect against Y. pestis infection. In contrast, protection depends upon the ability of CD8 T cells to produce the cytokines TNFα and IFNγ, and mice whose T cells cannot produce these two cytokines are not protected. Therefore, we conclude that cytokine production, not cytotoxicity, is essential for CD8 T cell-mediated control of pulmonary Y. pestis infection and we suggest that assays detecting cytokine production may be useful correlates of vaccine efficacy against plague and other acutely lethal septic bacterial pneumonias.
Introduction
Plague, one of the world's most deadly infectious diseases, has killed hundreds of millions of humans during three major pandemics [1]. It is caused by the Gram-negative facultative intracellular bacterium Yersinia pestis, which is naturally maintained in rodent reservoirs. Fleas transmit Y. pestis between rodents and to other mammals. Human infections typically result from fleabites as well, but a pneumonic form of plague can spread from human to human via infectious respiratory droplets. Pneumonic plague is fulminant and nearly always fatal unless treated with antibiotics within 24 h of symptom onset. Although natural outbreaks of pneumonic plague are uncommon, the high mortality rate, small window for treatment, existence of antibiotics-resistant strains, and potential for use as an airborne biological weapon fosters research aimed at the development of effective countermeasures.
Mouse models of pulmonary Y. pestis infection are considered translational tools for the development of pneumonic plague countermeasures because the pathology of plague in rodents is highly similar to that observed in humans. Analogous septic pneumonias caused by more common bacteria, including members of the Klebsiella, Streptococcus, and Staphylococcus species, are leading causes of death worldwide [2], [3]. Thus, murine models of plague also provide tools for studying basic mechanisms of immune defense against acutely lethal bacterial infections that seed the human lung and then disseminate to cause septic morbidity.
Ab-based subunit vaccines composed of the Y. pestis F1 and LcrV proteins provide rodents and some nonhuman primates with substantial protection against pulmonary Y. pestis infection [4]. Despite inducing high titer Ab responses, these vaccines fail to induce adequate protection in all nonhuman primates, most notably in African green monkeys [4], [5], [6]. This observation raises the possibility that Abs may not suffice to protect humans against pneumonic plague. Recent studies indicate T cells also contribute to protection against pulmonary Y. pestis infection in mice and the cytokines TNFα, IFNγ and IL-17 are required for optimal T cell-mediated protection [7], [8]. For example, B cell-deficient mice vaccinated with live attenuated Y. pestis are protected against lethal challenge, and depleting T cells or neutralizing TNFα and IFNγ at the time of challenge fully abolishes the protection [7]. TNFα and IFNγ also contribute to Ab-mediated protection in wild-type mice: the passive protection conferred by therapeutic administration of F1 and LcrV-specific mAb and the active protection conferred by immunization with a recombinant F1/LcrV vaccine are both abolished by neutralization of TNFα and IFNγ [9], [10]. Together, these findings suggest that pneumonic plague vaccines should also aim to induce cellular immunity that produces cytokines, in addition to inducing Ab-mediated humoral immunity.
CD8 T cells are critical for defense against a variety of pathogens, including viruses, protozoa and bacteria [11], [12]. The effector functions used by CD8 T cells to resist pathogens include secretion of cytokines like TNFα and IFNγ and Ag-specific cytolysis of infected cells [11], [12]. Recently we identified a dominant and protective T cell epitope recognized by Y. pestis-specific CD8 T cells [13]. Immunizing wild-type C57BL/6 mice with this single peptide, YopE69–77, primes Ag-specific CD8 T cells that suffice to confer protection against lethal pulmonary Y. pestis challenge [13]. Moreover, immunizing mice with YopE69–77 also elicits a CD8 T cell response that protects against lethal intragastric challenge with Yersinia pseudotuberculosis, the enteropathogen from which Y. pestis evolved [14]. A prior study showed that CD8 T cells and perforin, a key molecule for cytolysis, are required to protect naïve mice against attenuated Y. pseudotuberculosis infection [15]. However, the relative contributions of cytokines and cytolysis to CD8 T cell-mediated anti-Yersinia immunity in vivo have never been reported. Furthermore, it is not yet clear whether T cells or other cell types produce the TNFα and IFNγ required for effective T cell-mediated defense against plague.
In this study, we examined the effector functions of YopE69–77-specific CD8 T cells during pulmonary Y. pestis infection, and investigated their relative contributions to protection mediated by YopE69–77 immunization. Using a combination of gene-deficient mice, Ab-mediated depletion, cell transfers, and bone marrow chimeric mice, we demonstrate that YopE69–77-specific CD8 T cells exhibit perforin-dependent cytotoxicity in vivo, but perforin is dispensable for YopE69–77-specific CD8 T cell-mediated protection. In contrast, TNFα and IFNγ are critical for protection and YopE69–77-mediated protection is severely impaired when production of TNFα and IFNγ by CD8 T cells is simultaneously suppressed.
Results
Immunization with YopE69–77 confers protection against Y. pestis
Prior studies established that intranasal immunization with YopE69–77, a dominant CD8 T cell epitope of the Y. pestis YopE protein, confers C57BL/6 mice with CD8 T cell-mediated protection against pulmonary Y. pestis infection [13]; however, the mechanism of protection has yet to be defined. To investigate the relative contributions of cytokines and cytolysis to YopE69–77-specific CD8 T cell-mediated protection, we first examined the protective efficacy of YopE69–77 immunization. Wild-type C57BL/6 mice were immunized intranasally with adjuvant cholera toxin (CT) alone or adjuvant with 1 µg of YopE69–77 and then challenged intranasally with Y. pestis. As previously described [13], immunization with YopE69–77 conferred protection against 20 median lethal dose (MLD) of Y. pestis strain D27, with 80% YopE69–77-immunized mice surviving the lethal challenge (Figure 1A). Immunization with YopE69–77 also conferred significant protection against a higher dose (200 MLD) challenge of Y. pestis strain D27 (Figure 1B), with 67% mice surviving. Moreover, immunization with YopE69–77 even sufficed to confer modest yet significant protection against intranasal challenge with fully virulent pigmentation locus-positive Y. pestis strain CO92 (Figure 1C). Although 75% of YopE69–77-immunized mice ultimately succumbed to the fully virulent challenge, protection was evidenced by both a 1.5-day prolongation in median survival time and an increase in the overall survival rate (Figure 1C). These data indicate that YopE69–77 immunization confers significant CD8 T cell-mediated protection against lethal pulmonary challenge with multiple Y. pestis strains. To investigate the mechanism of CD8 T cell-mediated defense in vivo, we focused on the model employing 20 MLD of Y. pestis strain D27 challenge, as this model showed the greatest degree of protection.
Fig. 1. Immunization with YopE69–77 peptide protects mice against Y. pestis. 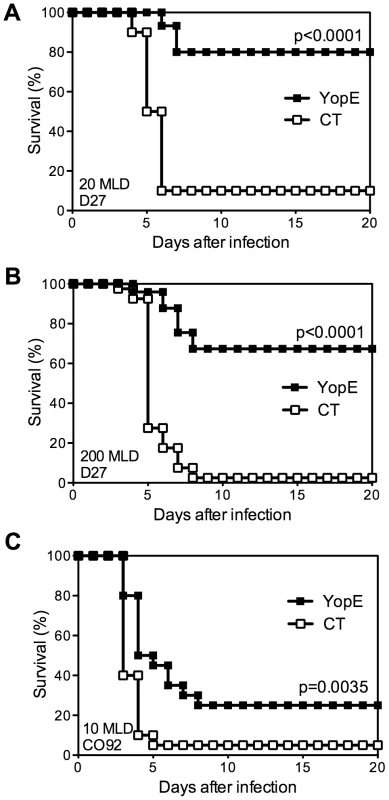
Wild-type C57BL/6 mice were immunized intranasally with CT adjuvant alone (CT) or CT mixed with YopE69–77 peptide (YopE) and then challenged intranasally with (A) 20 MLD (2×105 CFU) or (B) 200 MLD (2×106 CFU) Y. pestis strain D27 or (C) 10 MLD (1×104 CFU) Y. pestis strain CO92. In comparison with CT–immunized mice (n = 10–40), YopE69–77–immunized mice (n = 15–39) exhibited significantly increased survival. Data were pooled from 2–5 independent experiments. Mice immunized with YopE69–77 exhibit perforin-dependent cytotoxic activity
To test whether immunization with YopE elicits a CTL response, we measured the ability of YopE67–77-specific CD8 T cells to lyse peptide-pulsed autologous primary lymphocytes using a standard in vivo CTL assay [16]. Naïve splenocyte target cells from CD45.1 congenic C57BL/6 mice were pulsed with YopE69–77 peptide and labeled with a high concentration of CFSE. Control target cells were pulsed with OVA257–264 peptide and labeled with a low concentration of CFSE. The pulsed cells were then mixed at a one-to-one ratio and injected intravenously into wild-type C57BL/6 mice that had been previously immunized. Approximately 20 h later, splenocytes were harvested and the proportion of transferred target cells labeled with high or low levels of CFSE was determined by flow cytometry. The proportion of cells recovered from naïve C57BL/6 mice was used as the comparator (Figure 2A, a).
Fig. 2. Mice immunized with YopE69–77 exhibit perforin-dependent cytotoxic activity. 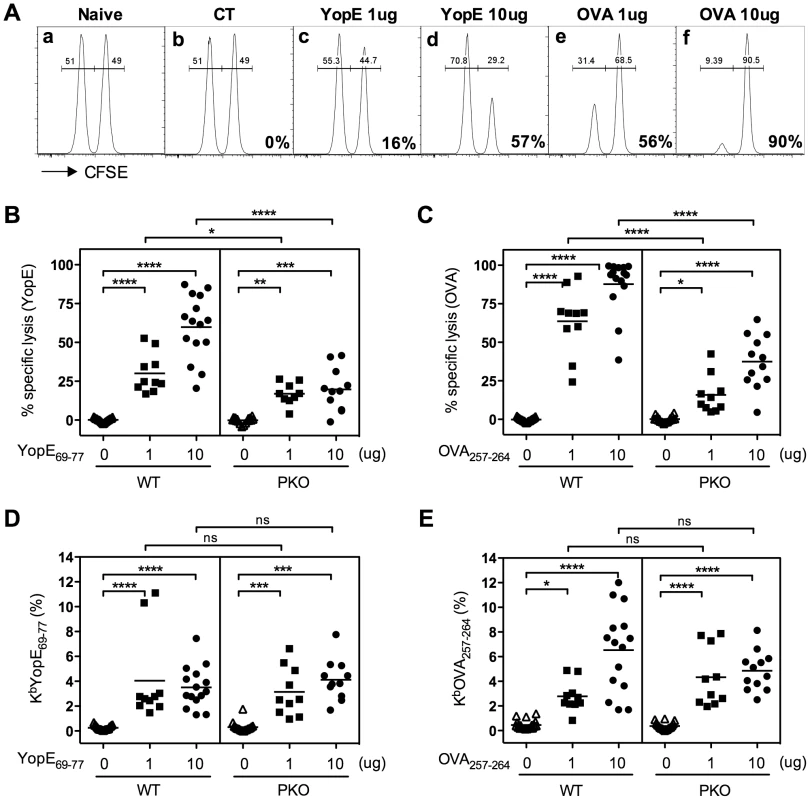
Wild-type (WT) and perforin-deficient (PKO) C57BL/6 mice were immunized with CT adjuvant alone or CT mixed with 1 or 10 µg of YopE69–77 or OVA257–264 peptides. Splenocytes from naïve congenic WT mice (CD45.1+) that were either pulsed with YopE69–77 peptide and labeled with 10 µM of CFSE or pulsed with OVA257–264 peptide and labeled with 1 µM of CFSE were mixed together at a 1∶1 ratio and injected into the immunized recipient mice. Splenocytes of recipient mice were then collected 20–22 h later and stained for congenic marker. The target cells (CD45.1+CD45.2−) were gated and the proportion of each CFSE-labeled population was analyzed by flow cytometry. The percent specific lysis was then calculated as described in the Materials and Methods. (A) Representative plots of the flow cytometry analysis of naïve or immunized WT recipient mice. The numbers on the lower right corner of the plots depict the percentage of specific lysis of YopE69–77-pulsed target cells or OVA257–264-pulsed target cells. (B and C) The percentages of specific lysis of YopE69–77-pulsed target cells by YopE69–77-immunized mice (B) or OVA257–264-pulsed target cells by OVA257–264-immunized mice (C). In comparison with WT mice, PKO mice displayed significantly decreased cytotoxicity (one-way ANOVA). Data shown are pooled from 5 independent experiments. (D and E) The percentages of PBL staining positive for CD8 and KbYopE69–77 (D) or KbOVA257–264 (E) tetramers two days before the cytotoxicity assay. In comparison with WT mice, PKO mice immunized with the same amount of peptide have comparable Ag-specific CD8 T cell frequencies (one-way ANOVA). Data shown are pooled from 5 independent experiments. As expected, near equal proportions of both target cell populations were recovered from mice immunized with CT adjuvant alone, indicating that no specific lysis was taking place (Figure 2A, b). Cells harvested from YopE69–77-immunized mice had lower proportions of CFSEhigh target cells, indicating that the YopE69–77-pulsed target cells had been lysed in vivo (Figure 2A, c and d). In contrast, cells harvested from OVA257–264-immunized mice had lower proportions of CFSElow target cells, indicating that the OVA257–264-pulsed target cells were killed (Figure 2A, e and f). Mice immunized with 10 µg of peptide (Figure 2A, d and f) exhibited stronger in vivo cytotoxic activity than mice immunized with 1 µg of peptide (Figure 2A, c and e). Together, these data suggest that immunization with YopE69–77 primes T cells that have the ability to specifically kill YopE69–77-pulsed target cells in vivo.
Perforin can play a critical role in CD8 T cell-mediated cytotoxicity, and perforin-deficient mice display increased susceptibility to many viral, bacterial and protozoan infections [11], [12], [17]. To investigate whether perforin contributes to YopE69–77-specific CD8 T cell-mediated cytotoxicity, Ag-pulsed target cells were transferred into immunized perforin-deficient mice and the CTL assay was performed. The YopE69–77 - and OVA257–264-immunized perforin-deficient mice showed significantly reduced cytotoxic activity against YopE69–77 - and OVA257–264-pulsed target cells, respectively, in comparison with immunized wild-type mice (Figures 2B and 2C). The reduced cytotoxicity could not be explained by reduced T cell priming, as the peripheral blood of the immunized wild-type and perforin-deficient mice harbored comparable percentages of YopE69–77-specific (Figure 2D) or OVA257–264-specific (Figure 2E) CD8 T cells. These data demonstrate that YopE69–77-specific CD8 T cells have the ability to kill YopE69–77-pulsed target cells in vivo in a perforin-dependent manner.
Perforin is dispensable for YopE69–77-specific CD8 T cell-mediated protection against Y. pestis and Y. pseudotuberculosis
We next investigated whether perforin is required for protection against lethal Y. pestis challenge. Wild-type or perforin-deficient mice were immunized with YopE69–77 and then challenged intranasally with 20 MLD of Y. pestis strain D27. As shown in Figure 3A, most control mice immunized with adjuvant alone or with control peptide OVA257–264 succumbed to Y. pestis challenge, whereas most wild-type and perforin-deficient mice immunized with YopE69–77 survived. Similar results were observed when mice were immunized with either 1 µg or 10 µg YopE69–77 (Figure 3A zand data not shown). Measurements of bacterial burden in lung and liver tissues at day 4 after Y. pestis challenge showed significantly decreased numbers of CFU in both the YopE69–77-immunized wild-type and perforin-deficient mice, as compared with control mice immunized with adjuvant alone or with OVA257–264 (Figure 3B). Neither the survival data nor the bacterial burden data revealed a significant role for perforin in the YopE69–77-specific CD8 T cell-mediated protection against Y. pestis. Therefore, although the YopE69–77-specific CD8 T cells exhibit perforin-dependent cytotoxic activity in vivo (Figure 2), perforin is dispensable for protection against lethal Y. pestis challenge.
Fig. 3. Perforin is dispensable for YopE69–77–specific CD8 T cell-mediated protection against Y. pestis and Y. pseudotuberculosis. 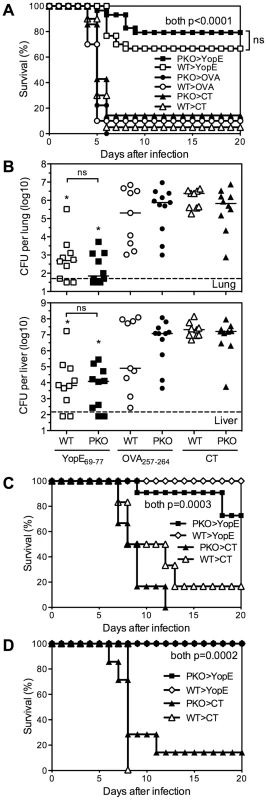
Wild-type (WT) and perforin-deficient (PKO) C57BL/6 mice were immunized intranasally with CT adjuvant alone, or CT mixed with YopE69–77 or OVA257–264 peptides and then challenged with (A and B) 20 MLD (2×105 CFU) Y. pestis strain D27 intranasally, (C) 10 MLD (5×109 CFU) Y. pseudotuberculosis strain 32777 intragastrically or (D) 10 MLD (1.2×102 CFU) Y. pseudotuberculosis strain 32777 intravenously. (A) Y. pestis survival data pooled from 3 independent experiments (n = 9–30 mice/group). (B) Day 4 bacterial burden in lung and liver tissues after Y. pestis challenge (Kruskal-Wallis test, compared with CT- or OVA257–264–immunized PKO or WT mice). Data are pooled from 2 independent experiments (n = 9–11 mice/group). Solid bar depicts median; broken line depicts the limit of detection. (C and D) Y. pseudotuberculosis survival data (n = 6–7 mice/group for CT, n = 10–11 mice/group for YopE). Data were pooled from 2 independent experiments. We previously demonstrated that immunization with YopE69–77 also protects against lethal intragastric challenge of mice with Y. pseudotuberculosis [14]. Other investigators reported that naïve perforin-deficient mice display increased susceptibility to intravenous challenge with an attenuated Y. pseudotuberculosis strain [15]. To investigate whether perforin is required for YopE69–77-specific CD8 T cell-mediated protection against Y. pseudotuberculosis, we immunized wild-type and perforin-deficient mice and challenged intragastrically or intravenously with 10 MLD of virulent Y. pseudotuberculosis strain 32777. We observed that both wild-type and perforin-deficient mice immunized with YopE69–77 were specifically protected against intragastric and intravenous Y. pseudotuberculosis challenge (Figures 3C and 3D, respectively). These data indicate that perforin is also dispensable for YopE69–77-specific CD8 T cell-mediated protection against Y. pseudotuberculosis.
TNFα and IFNγ are critical for YopE69–77-specific CD8 T cell-mediated protection against Y. pestis
To investigate roles for cytokines in CD8 T cell-mediated protection against Y. pestis, we immunized TNFα - or IFNγ-deficient mice with YopE69–77 and then challenged with 20 MLD of Y. pestis strain D27. While most YopE69–77-immunized wild-type mice survived the challenge, almost all YopE69–77-immunized TNFα-deficient mice and IFNγ-deficient mice succumbed (Figure 4A and 4B). Notably, the wild-type and IFNγ-deficient mice generated comparable frequencies of YopE69–77-specific CD8 T cells after immunization, and despite their impaired survival, the TNFα-deficient mice consistently generated higher frequencies of specific cells (data not shown), implying that cytokine deficiency does not quantitatively impair the expansion and/or persistence of YopE69–77-specific CD8 T cells. These results suggest that TNFα and IFNγ play critical roles during YopE69–77-specific CD8 T cell-mediated protection against pulmonary Y. pestis infection.
Fig. 4. TNFα and IFNγ are critical for T cell-mediated protection against Y. pestis. 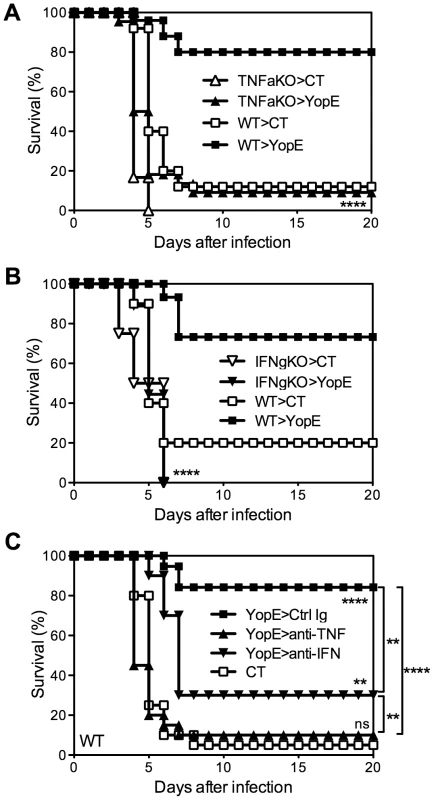
(A and B) Wild-type (WT), TNFα-deficient (TNFaKO), IFNγ-deficient (IFNgKO) C57BL/6 mice were immunized intranasally with CT adjuvant alone or CT mixed with YopE69–77 peptide and then challenged intranasally with 20 MLD Y. pestis strain D27. In comparison with YopE69–77–immunized WT mice (n = 15–25), YopE69–77–immunized TNFα-deficient (n = 22) and IFNγ-deficient (n = 18) mice exhibited significantly reduced survival. Data were pooled from 3–5 independent experiments. (C) Wild-type C57BL/6 mice were immunized intranasally with CT alone or CT mixed with YopE69–77 peptide and then challenged intranasally with 20 MLD Y. pestis strain D27. One day before the challenge, the YopE69–77-immunized mice received neutralizing mAb specific for TNFα (anti-TNF), IFNγ (anti-IFN), or an isotype-matched mAb (Ctrl Ig). In comparison with CT-immunized mice (n = 20), YopE69–77-immunized mice treated with isotype-matched mAb (n = 19) or IFNγ-neutralizing mAbs (n = 10) but not TNFα-neutralizing mAb (n = 20) were protected against Y. pestis challenge. Data were pooled from 4 independent experiments. To assess whether protection mediated by YopE69–77-specific CD8 T cells requires cytokines at the time of challenge, YopE69–77-immunized wild-type mice were treated with neutralizing mAb specific for TNFα or IFNγ. Control mice were treated with an isotype-matched mAb of irrelevant specificity. As shown in Figure 4C, while mice treated with mAb to neutralize IFNγ at the time of challenge displayed decreased but still significant protection (p<0.01), nearly all mice treated with mAb to neutralize TNFα succumbed. These results suggest that production of TNFα is particularly critical at the time of pulmonary Y. pestis challenge.
TNFα produced by either macrophages/neutrophils or T cells is dispensable for YopE69–77-specific CD8 T cell-mediated protection against Y. pestis
A variety of cell types can produce TNFα during the immune response to infection. To assess whether a specific cell type is responsible for producing the critical TNFα during CD8 T cell-mediated defense against Y. pestis, we performed studies with previously described mice possessing cell-type-specific deficiencies in TNFα expression [18]. Control mice and mice deficient for TNFα expression in either the macrophage/neutrophil lineage (MN-TNF KO) or the T cell lineage (T-TNF KO) were immunized with YopE69–77. Immunized TNF-floxed littermate control mice largely survived challenge with 20 MLD of Y. pestis strain D27 (Figure 5). Unexpectedly, both the MN-TNF KO mice and the T-TNF KO mice were also protected by YopE69–77 immunization, with 90% and 80% surviving the lethal challenge, respectively (Figures 5A and 5B, respectively). These data suggest that TNFα derived from either macrophages/neutrophils or T cells can suffice to confer YopE69–77-mediated protection against pulmonary Y. pestis challenge.
Fig. 5. Selective depletion of TNFα from either macrophages/neutrophils or T cells does not impact the protection conferred by YopE69–77 immunization. 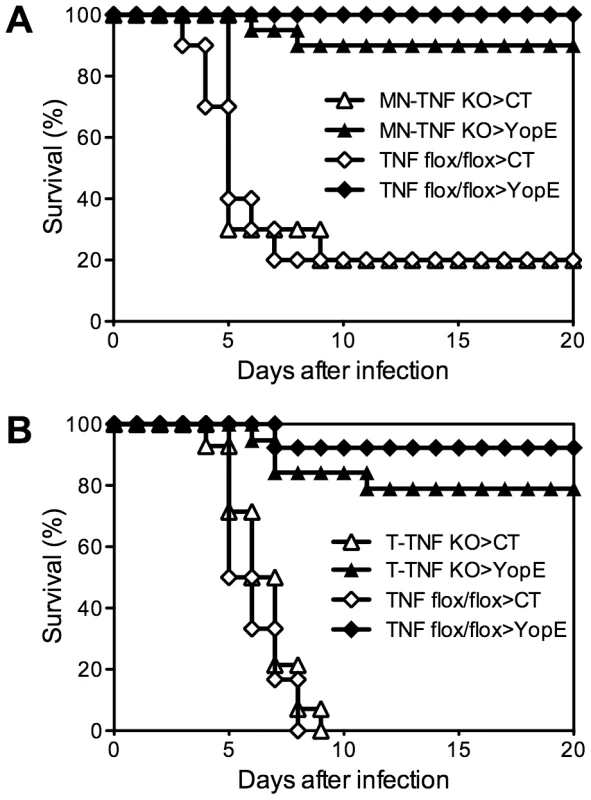
Mice with (A) monocyte/neutrophil-specific (MN-TNF KO) or (B) T cell-specific (T-TNF KO) deletion of TNFα were immunized with CT adjuvant alone or CT mixed with YopE69–77 peptide and challenged intranasally with 20 MLD Y. pestis strain D27. Littermate TNF-floxed (TNF flox/flox) mice were used as controls. In comparison with CT-immunized mice (n = 10–14), YopE69–77-immunized MN-TNF KO mice (n = 20) and T-TNF KO mice (n = 19) were protected against Y. pestis challenge (p<0.0001) with no significant difference from YopE69–77-immunized TNF flox/flox mice (n = 12–13 mice/group). Data were pooled from 3 (A) and 5 (B) independent experiments. Neither TNFα nor IFNγ production by T cells is critical for YopE69–77-specific CD8 T cell-mediated protection against Y. pestis
To definitively assess whether CD8 T cells are required to produce TNFα or IFNγ, we next assayed protection conferred by primed CD8 T cells isolated from YopE69–77-immunized TNFα - or IFNγ-deficient mice. Specifically, wild-type, TNFα-deficient, or IFNγ-deficient mice were immunized with YopE69–77 and their CD8+ splenic T cells were purified by magnetic beads and injected intravenously into naïve wild-type recipient mice, which were then challenged with 20 MLD of Y. pestis strain D27. The mice that received CD8+ T cells from YopE69–77-immunized wild-type mice showed significantly improved survival as compared with mice that received CD8+ T cells from control adjuvant-immunized mice (p<0.0001), with 43% of the mice surviving (Figure 6A). Mice that received CD8+ T cells from YopE69–77-immunized TNFα - or IFNγ-deficient mice displayed more modest but significant protection (both p<0.05), with 29% and 27% surviving the challenge, respectively. Thus, YopE69–77-specific CD8 T cells lacking the capacity to produce either TNFα or IFNγ can confer significant protection against Y. pestis.
Fig. 6. TNFα and IFNγ produced by YopE69–77-specific CD8 T cells have complementary roles during protection against Y. pestis. 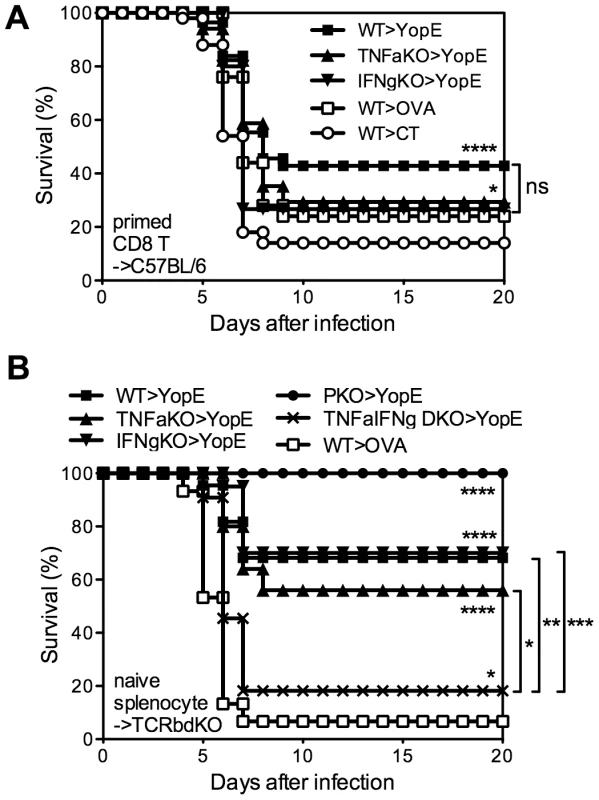
(A) Wild-type (WT), TNFα-deficient (TNFaKO) or IFNγ-deficient (IFNgKO) C57BL/6 mice were immunized with CT mixed with YopE69–77 peptide. WT mice immunized with CT adjuvant only or CT mixed with OVA257–264 peptide were used as controls. CD8+ splenocytes were then purified and transferred intravenously to naïve WT C57BL/6 mice, which were challenged intranasally with 20 MLD Y. pestis strain D27 the next day. In comparison with mice that received CD8+ T cells from CT-immunized WT mice (n = 50), mice that received CD8+ T cells from YopE69–77-immunized WT (n = 56), TNFα-deficient (n = 17) and IFNγ-deficient (n = 15) mice were protected against Y. pestis challenge. Notably, mice that received CD8+ T cells from OVA257–264-immunized WT mice (n = 25) were also protected (p<0.05). Data were pooled from 7 independent experiments. (B) Splenocytes isolated from naïve WT, TNFαKO, IFNγKO, perforin-deficient (PKO) or TNFα/IFNγ-deficient (TNFαIFNγ DKO) mice were transferred to TCRβδ-deficient mice. The mice were then immunized with CT mixed with YopE69–77. Control mice received naïve WT splenocytes and were immunized with CT mixed with OVA257–264 peptide. Mice were then challenged intranasally with 20 MLD Y. pestis strain D27. In comparison with control mice (n = 15), YopE69–77 immunized mice that received WT (n = 22), TNFαKO (n = 25), IFNγKO (n = 20), or PKO (n = 11) splenocytes were protected against Y. pestis challenge. YopE69–77-immunized mice that received TNFαIFNγ DKO splenocytes were also protected (n = 11) but the survival was significantly lower in comparison with the mice received WT, TNFαKO or IFNγKO splenocytes. Data were pooled from 3 independent experiments. In our experience, the transfer of primed T cells followed shortly thereafter by an infectious challenge can sometimes provide non-specific protection (data not shown), perhaps owing to the potential of effector and memory CD8 T cells to rapidly secrete IFNγ in response to IL-12 and IL-18 in the absence of cognate antigen [19]. In fact, the mice that received CD8+ T cells from OVA257–264-immunized wild-type mice displayed some protection, with 24% surviving the challenge (Figure 6A). Moreover, the protection in this primed T cell transfer model was relatively weak, as compared with our prior studies of intact mice, thus making it difficult to study the mechanism. Additionally, these YopE69–77-specific CD8 T cells were primed in an environment where cytokine was deficient. In an attempt to overcome these possible caveats to the experiments shown in Figure 6A, we next developed a model to study cytokine-deficient T cells that were primed in situ. First, we isolated naïve splenocytes from wild-type or gene-deficient mice and transferred them to TCRβδ-deficient mice, which lack T cells, thus creating chimeric mice in which primed T cells could only arise from cytokine-deficient donor splenocytes. Then, we immunized and challenged these splenocyte chimeras. The chimeras that received wild-type splenocytes showed significant YopE-specific protection (p<0.0001); 70% of the YopE69–77-immunized mice survived and only 7% of the control OVA257–264-immunized mice survived (Figure 6B). All the YopE69–77-immunized mice that received splenocytes from perforin-deficient mice also survived, further confirming that perforin is dispensable for protection against Y. pestis mediated by primed CD8 T cells (Figure 6B). YopE69–77-immunized mice that received splenocytes from TNFα-deficient or IFNγ-deficient mice also displayed significant protection (both p<0.0001), exhibiting 56% and 70% survival, respectively. Thus, mouse models employing either the transfer of primed CD8+ T cells (Figure 6A) or the priming of CD8+ T cells in situ (Figure 6B) demonstrate that YopE69–77-specific CD8 T cells lacking the capacity to produce either TNFα or IFNγ can confer significant protection against Y. pestis.
TNFα and IFNγ produced by YopE69–77-specific CD8 T cells show complementary roles in protecting against Y. pestis
The observation that YopE69–77-specific CD8 T cells lacking either TNFα or IFNγ exhibit slightly impaired protection suggested that the TNFα and IFNγ produced by CD8 T cells may have complementary roles. To explore this possibility, we generated TNFα and IFNγ double-deficient (TNFα/IFNγ-deficient) mice. We observed that T cell-deficient mice that received splenocytes from TNFα/IFNγ-deficient mice were poorly protected by YopE69–77 immunization (Figure 6B), with only 18% surviving lethal Y. pestis challenge. The observation that further removing the remaining cytokine from either the TNFα - or IFNγ-deficient mice leads to a significant and substantial decrease in survival suggests complementary roles for TNFα and IFNγ produced by CD8 T cells.
In the splenocyte chimera model shown in Figure 6B, the cytokine-deficient splenic T cells had developed in the thymuses of cytokine-deficient mice, perhaps imprinting them with altered functions. To overcome this potential concern, a final set of studies was performed using mixed bone marrow chimeras. Lethally irradiated TCRβδ-deficient mice were reconstituted with a mixture of bone marrow cells isolated from gene-deficient or wild-type mice. In each case, 75% of the bone marrow cells came from TCRβδ-deficient mice, thereby enabling reconstitution of wild-type elements of all leukocyte populations except for T cells. The remaining 25% of the bone marrow cells came from wild-type mice or gene-deficient mice, thereby enabling the development of wild-type or gene-deficient T cells, respectively. Six weeks after reconstitution, the mice were immunized and then challenged with 20 MLD of Y. pestis strain D27. As shown in Figure 7A, 88% of the YopE69–77-immunized mice reconstituted with wild-type T cells were protected, while all of the OVA257–264-immunized mice reconstituted with wild-type T cells succumbed. Likewise, all YopE69–77-immunized mice reconstituted without T cells (i.e. mice that received only TCRβδ-deficient bone marrow) succumbed to challenge infection. Consistent with Figures 6, the YopE69–77-immunized chimeric mice reconstituted with TNFα-, IFNγ - or perforin-deficient T cells all showed significantly improved protection when compared with mice lacking T cells (all p<0.0001), indicating that production of TNFα-, IFNγ - or perforin by T cells is not absolutely essential. Indeed, the degree of protection observed in YopE69–77-immunized chimeric mice reconstituted with TNFα - or perforin-deficient T cells did not differ significantly from that observed in mice reconstituted with wild-type T cells (Figure 7A). Although the YopE69–77-immunized chimeric mice reconstituted with IFNγ-deficient T cells displayed significantly increased protection in comparison with mice lacking T cells, the degree of protection was significantly reduced in comparison with mice reconstituted with wild-type T cells (p<0.001, Figure 7A). Notably, survival was further reduced in mice reconstituted with TNFα/IFNγ-deficient T cells (p<0.05 in compared with mice reconstituted with IFNγ-deficient T cells), with only 18% of mice surviving the lethal challenge (Figure 7A).
Fig. 7. YopE69–77-specific CD8 T cells lacking the capacity to produce TNFα and IFNγ fail to protect mice and control bacterial burden. 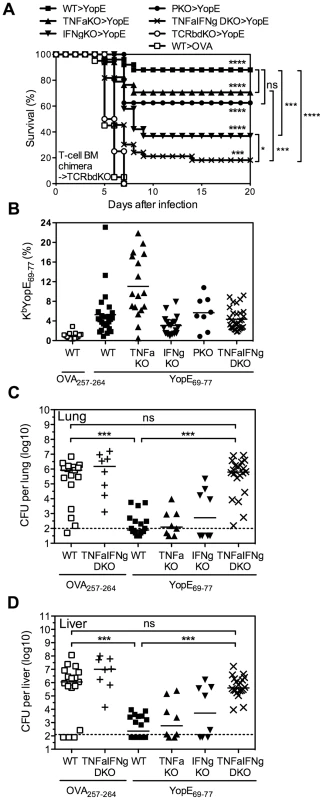
TCRβδ-deficient (TCRbdKO) mice were lethally irradiated and reconstituted with 75% TCRβδKO bone marrow cells and 25% of either WT, TNFαKO, IFNγKO, PKO, or TNFαIFNγ DKO bone marrow cells. Six weeks later they were immunized with CT mixed with YopE69–77 or OVA257–264 and then challenged intranasally with 20 MLD Y. pestis strain D27. (A) Survival. In comparison with OVA257–264-immunized mice reconstituted with WT T cells (n = 20), the YopE69–77-immunized chimeric mice reconstituted with WT (n = 25), TNFαKO (n = 17), IFNγKO (n = 19), PKO (n = 8) or TNFαIFNγ DKO (n = 33) T cells all showed significant protection. (B) The percentage of CD8+ T cells that stained positive for MHC class I tetramer KbYopE69–77 in PBL on the day before challenge. Solid bar depicts the mean. All groups of chimeric mice that were immunized with YopE69–77 had significantly increased frequency of KbYopE69–77+CD8+ T cells in compared with the chimeric mice immunized with OVA257–264 (p<0.001). YopE69–77-immunized chimeric mice reconstituted with TNFαKO T cells had significantly higher frequency of KbYopE69–77+CD8+ T cells in compared with YopE69–77-immunized chimeric mice reconstituted with WT T cells (p<0.01). Data for (A) and (B) are pooled from 6 independent experiments. (C and D) Bacterial burden in lung (C) and liver (D) tissues was measured at day 4 after challenge (Kruskal-Wallis test). Data are pooled from 3 independent experiments. Solid bar depicts median; broken line depicts the limit of detection. It is worth noting that we confirmed the efficiency of bone marrow reconstitution and YopE immunization in mice prior to Y. pestis infection by measuring the frequency of CD4 T cells, CD8 T cells, and YopE69–77-specific CD8 T cells in the peripheral blood. In spite of slightly lower frequency of CD8 T cells in the peripheral blood of mice reconstituted with TNFα-, IFNγ - or TNFα/IFNγ-deficient T cells than that of mice reconstituted with wild-type T cells (data not shown), the frequency of YopE69–77-specific CD8 T cells, as measured by KbYopE69–77 tetramer, was not lower than that of mice reconstituted with wild-type T cells (Figure 7B). In fact, mice reconstituted with TNFα-deficient T cells generated even more YopE69–77-specific CD8 T cells than mice reconstituted with wild-type T cells, even though they exhibited reduced survival (Figure 7A).
Subsequently, we generated bone marrow chimeras, immunized and challenged them as described above, and then euthanized mice at day 4 after challenge to measure bacterial burden. This analysis revealed significantly decreased bacterial CFU in lung (Figure 7C) and liver (Figure 7D) tissues of YopE69–77-immunized chimeric mice reconstituted with wild-type, TNFα - or IFNγ-deficient T cells, as compared with OVA257–264-immunized chimeric mice reconstituted with wild-type T cells. Thus, deficient production of either TNFα or IFNγ by T cells does not impair their ability to control bacteria. In contrast, we observed significantly increased numbers of bacterial CFU in lung and liver tissues of YopE69–77-immunized chimeric mice reconstituted with TNFα/IFNγ-deficient T cells, as compared with YopE69–77-immunized chimeric mice reconstituted with wild-type T cells (Figure 7C and 7D). In fact, the burden of YopE69–77-immunized chimeric mice reconstituted with TNFα/IFNγ-deficient T cells achieved levels approaching those of OVA257–264-immunized chimeric mice reconstituted with either wild-type or TNFα/IFNγ-deficient T cells, which were not protected (Figure 7A).
Discussion
In this study, we conducted experiments to better understand the mechanisms of immunization-induced protective CD8 T cell responses and their relative contributions to defense against pulmonary Y. pestis infection. We extended our prior work [13] and demonstrated that immunization with YopE69–77 suffices to confer remarkable protection against pigmentation locus-deficient Y. pestis strain D27 (Figures 1A and 1B). YopE69–77-immunized mice can withstand as high as 200 MLD challenge (Figure 1B). Intranasal administration of Y. pestis strain D27 causes mortality in mice but does not cause the fulminant pneumonia characteristic of fully virulent Y. pestis [20]. However, we also found that YopE69–77-immunized mice were significantly protected against intranasal infection with the fully virulent Y. pestis strain CO92 (Figure 1C). To our knowledge, this is the first report that T cell-mediated immunity can protect against fully virulent Y. pestis infection. Notably, the protection was incomplete, suggesting that YopE69–77-specific CD8 T cells alone may be insufficient to combat fully virulent Y. pestis strains.
Taking advantage of the robust protection observed in mice infected with 20 MLD Y. pestis strain D27, we manipulated immune responses to identify effector functions that are critical for CD8 T cell-mediated anti-Yersinia immunity in vivo. Researchers typically use gene-deficient mice, adoptive cell transfer models and Ab-mediated depletion methods to demonstrate roles for different effector functions of cellular immunity during host defense against microbial infections. There are caveats to each approach and determining the relative contribution of each specific effector function by any particular type of immune cell can be technically challenging. First, effector functions usually are not unique to a particular cell type. Both CD8 T cells and NK cells utilize perforin-mediated cell killing, whereas TNFα and/or IFNγ can be produced by CD4 T cells, CD8 T cells, NK cells, and some innate cells such as macrophages and neutrophils. Second, the removal of any particular effector function can inadvertently alter cell differentiation, homeostasis, and/or the immunodominance hierarchies of Ag-specific cells. For example, IFNγ-deficient and perforin-deficient mice exhibit altered expansion, contraction and immunodominance of Ag-specific CD8 T cells during Listeria infection [21]. Third, effector and memory Ag-specific CD8 T cells have the potential to rapidly secrete IFNγ in response to IL-12 and IL-18 in the absence of cognate Ag [19], hence transferring primed effector/memory Ag-specific cells to mice followed by infection could confer innate protection in an Ag nonspecific manner. In an effort to address these complexities and overcome the limitations of any single methodology, we used multiple approaches to examine the contributions of potential effector mechanisms used by CD8 T cells to combat septic pneumonic plague.
YopE69–77-immunized perforin-deficient mice, which display severely impaired Ag-specific cytotoxic activity in vivo (Figure 2), are resistant to lethal Y. pestis and Y. pseudotuberculosis challenge (Figure 3). Likewise, the levels of protection provided by wild-type and perforin-deficient YopE69–77-specific CD8 T cells are indistinguishable in T cell chimeric mice (Figure 6B and 7A). These data strongly suggest that perforin does not contribute substantially to CD8 T cell-dependent adaptive host defense against Yersinia. In contrast, a recent publication by Bergman et al. suggested that perforin can be critical for protection against Y. pseudotuberculosis infection [15]. They studied naïve mice infected with an attenuated strain of Y. pseudotuberculosis and reported that perforin deficiency impaired protection, as evidenced by greater bacterial burden in the spleens and livers. When the surviving perforin-deficient mice were challenged with a virulent strain of Y. pseudotuberculosis, the “immunized” perforin-deficient mice only exhibited significantly impaired protection if animals without detectable bacteria were excluded from the analysis. Since we found no evidence that perforin deficiency impairs either bacterial clearance or survival in YopE69–77-immunized mice, the combined data suggest that perforin may be important for primary defense against Yersinia but can be dispensable for adaptive defense.
While perforin deficiency has little impact on protection mediated by YopE69–77-specific CD8 T cells, we note that perforin deficiency did not completely abolish all cytotoxicity in our model (Figure 2B). Thus, we cannot exclude the possibility that the residual cytotoxicity, which is independent of perforin, may contribute to adaptive defense against Yersinia. Future studies will need to assess whether such other cytotoxic pathways, for example, Fas/FasL and TNF/TNF receptors, contribute to YopE69–77-specific CD8 T cell-mediated protection.
In contrast to perforin deficiency, cytokine deficiency dramatically impairs anti-Yersinia immunity mediated by YopE69–77-specific CD8 T cells. Data presented in this study highlights the complexity of cytokine-mediated protection. YopE69–77-immunized mice that completely lack the ability to produce either TNFα or IFNγ succumb to infection, suggesting that each of these cytokines are absolutely required for CD8 T cell-mediated anti-Yersinia immunity (Figures 4A and 4B). However, the data in Figure 4C reveal that IFNγ is not absolutely required for protection at the time of challenge. It implies that IFNγ may have a role in the development of YopE69–77-specific CD8 T cells that have the capacity to combat Y. pestis. Moreover, the data in Figure 6 reveal that IFNγ production by T cells is not required for resistance to Y. pestis, implying that cell types other than T cells must be capable of producing enough IFNγ to confer modest yet significant protection. In contrast, chimeric mice in which the T cells cannot produce IFNγ exhibit significant impairments in survival after YopE immunization and Y. pestis challenge (Figure 7A). While we cannot completely exclude the possibility that cell reconstitution in mice deficient for T cell-derived IFNγ is suboptimal, the frequency of YopE69–77-specific CD8 T cells in the peripheral blood prior to challenge is similar in wild-type mice and chimeric mice whose T cells cannot produce IFNγ (Figure 7B). Since other cells in the environment are capable of producing IFNγ in that model, the data suggest that a cell-intrinsic defect resulting from the loss of IFNγ influences some quality of differentiating CD8 T cells. One possibility is that mice whose T cells cannot produce IFNγ may have reduced TNFα production or responsiveness. Indeed, IFNγ has been shown to enhance TNF receptor expression [22].
In contrast to IFNγ, Ab-mediated depletion of TNFα at the time of Y. pestis challenge abrogates nearly all protection in YopE69–77-immunized mice, suggesting that TNFα is absolutely required for protection at the time of challenge (Figure 4C). Cells of the macrophage, neutrophil and T cell lineages can each produce TNFα. However, TNFα production by T cells is not absolutely critical for resistance to Y. pestis, implying that cell types other than CD8 T cells must be capable of producing enough TNFα to confer modest yet significant protection (Figures 5B, 6 and 7). We found that TNFα from macrophages/neutrophils is not absolutely required for protection either, at least not when the mice have intact IFNγ production (Figure 5A). These observations imply that TNFα from one cell type can substitute for the loss of TNFα in another cell type in pulmonary Y. pestis infection. Alternatively, residual production of TNFα from cells other than T cells and macrophages/neutrophils could also contribute to protection.
In contrast to the dramatic impact of Ab-mediated depletion of TNFα at the time of challenge, the impact of depleting CD8 T cell-derived TNFα seems to be modest (Figures 6 and 7). One caveat is that we repeatedly observed a higher frequency of YopE69–77-specific CD8 T cells in the peripheral blood of mice whose T cells could not produce TNFα (Figure 7B and data not shown). Thus, it is conceivable that impairments in protection caused by the absence of T cell-derived TNFα can be restored, at least partially, by an increase in number of YopE69–77-specific CD8 T cells.
While depleting either TNFα or IFNγ from CD8 T cells impairs protection to a certain extent, simultaneously depleting both cytokines additionally compromises protection. Specifically, chimeric mice whose T cells can produce neither cytokine display significantly reduced survival when compared with mice whose T cells cannot produce either TNFα or IFNγ (Figures 6B and 7A). Some TNFα production from either T cells or non-T cells, in concert with intact IFNγ production, appears to be sufficient for near optimal protection (Figure 5). However, the data suggest that TNFα and IFNγ production by CD8 T cells work complementarily to confer optimal protection. One likely mechanism by which these cytokines contribute to host survival is through the control of bacterial burden (Figures 7C and 7D). TNFα and IFNγ are known to activate macrophages and neutrophils to produce reactive nitrogen and oxygen intermediates that assist in the control of intracellular bacteria. Adding purified TNFα and IFNγ to the macrophage cultures can overcome Y. pestis-induced suppression of macrophage anti-bacterial activity in vitro [23]. These cytokines can also stimulate neutrophils to secret antimicrobial molecules to control extracellular bacteria.
Although depleting both TNFα and IFNγ produced by T cells dramatically compromises the protection mediated by YopE69–77-specific CD8 T cells, it does not completely abolish the protection. We did observe a minimal of 20% host survival, which is significant in comparison with OVA257–264-immunized wild-type mice and reproducible. This finding implies that while CD8 T cell-derived TNFα and IFNγ are critical for host defense against Y. pesitis, they may not be the sole players. Despite the observation that perforin is dispensable for CD8 T cell-mediated protection against Y. pestis (Figure 3), its function may contribute to host survival when the two key effector functions of CD8 T cells are absent. Moreover, other mediators that CD8 T cells can produce during infection may also contribute to protection. For example, CD8 T cell production of macrophage inflammatory protein-1α (MIP-1α/CCL3) is required for anti-Listeria immunity [24], [25].
One could argue that in our splenocyte transfer and bone marrow chimera models (Figures 6B and 7A, respectively), CD4 T cells share the same defect with CD8 T cells. However, we found that CD4 T cells are not required for YopE69–77-mediated protection, since YopE69–77-immunized CD4-deficient mice were protected as well as YopE69–77-immunized wild-type mice (unpublished data). Thus, we think it unlikely that a deficiency of CD4 T cell-derived cytokines accounts for the lost of protection we observe in these models.
CD8 T cells can utilize a variety of mechanisms to protect against infectious agents. Understanding the effective and critical functions of the CD8 T cell response against pathogens that cause septic bacterial pneumonias and defining the combinations of effector functions that optimally combat such pathogens should improve our ability to design effective vaccines. Our study shows that YopE69–77-specific CD8 T cells exhibit perforin-dependent cytotoxic activity in vivo, but this effector function does not appear to be required for anti-Yersinia immunity. In contrast, production of TNFα and IFNγ is essential for CD8 T cell-mediated protection. Interestingly, the time at which these cytokines must be produced appears to differ, as does their essential cellular sources. Overall, the data suggest that CD8 T cell-derived TNFα and IFNγ exert complementary functions and CD8 T cells require both cytokines to provide optimal defense against pulmonary Y. pestis infection. Our study further suggests cytokine-producing CD8 T cells may be a valuable addition to subunit plague vaccines and assays detecting Ag-specific TNFα production may be a particularly useful correlate of plague vaccine efficacy. Given that septic bacterial pneumonias are a leading cause of death, it will be interesting to determine whether cytokine production, rather than cytolysis, is generally more important for T cell-mediated defense against septic bacterial pneumonias.
Materials and Methods
Ethics statement
All animal studies were conducted in accordance with the Guide for Care and Use of Laboratory Animals of the National Institutes of Heath and approved by Trudeau Institute Animal Care and Use Committee (IACUC protocols # 02-022 and 02-161).
Mice
Wild-type C57BL/6, B6.129S7-Ifngtm1Ts (IFNγ-deficient), B6.129S-Tnftm1Gkl (TNFα-deficient), C57BL/6-Prf1tm1Sdz (perforin-deficient), B6.129P2-Tcrbtm1Mom Tcrdtm1Mom (TCRβδ-deficient), B6.SJL-Ptprca Pepcb/BoyJ (CD45.1) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and then bred in the specific-pathogen-free Trudeau Institute Animal Breeding Facility. TNFα/IFNγ double-deficient mice were generated at Trudeau Institute. Mice deficient for TNFα production in macrophage and neutrophils (MN-TNF KO mice) or T cells (T-TNF KO mice) were generated by crossing mice with TNF “floxed” genes to mice carrying the LysM-Cre or CD4-Cre transgenes, respectively [18]. All strains are on the C57BL/6 background. Experimental mice were matched for age and sex, and first immunized between 6 and 10 weeks of age.
Bacteria and infections
Pigmentation locus-deficient Y. pestis strain KIM D27 was originally provided by Dr. Robert Brubaker (Michigan State University, East Lansing, MI). Strain D27 bacilli from frozen glycerol stocks were grown overnight at 26°C in Bacto heart infusion broth (Difco Laboratories, Detroit, MI) supplemented with 2.5 mM CaCl2. Cultures were then diluted to an OD of 0.1 at 620 nm, regrown for 3–4 h at 26°C, quantified by OD measurement, and then washed and resuspended in saline at the desired concentration. The numbers of bacterial CFU in the inocula were confirmed by plating. Infections were performed by applying 30 µl to the nares of mice that were lightly anesthetized with isoflurane. The intranasal median lethal dose (MLD) for strain D27 is approximately 1×104 CFU when the bacteria are grown and administered as described above [26].
The fully virulent pigmentation locus-positive Y. pestis strain CO92 NR-641 was obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH. Bacteria from frozen glycerol stocks were grown overnight at 26°C in Bacto heart infusion broth. Cultures were then diluted to an OD of approximately 0.1 at 620 nm, regrown for 2.5–3 h at 26°C, quantified by OD measurement, and prepared at the desired concentration. The numbers of bacterial CFU in the inocula were confirmed by plating. Infections were performed by applying 25 µl to the nares of mice that were lightly anesthetized with isoflurane. Preliminary studies established that the intranasal MLD for strain CO92 is approximately 1×103 CFU when the bacteria are grown and administered as described above.
Y. pseudotuberculosis serotype O:1 strain 32777 bacilli from frozen glycerol stocks were grown overnight at 26°C in Luria-Bertani medium to reach an OD of approximately 1.2 at 600 nm. The cultures were then washed and resuspended in saline to achieve the desired concentration. For intragastric infections, mice were fasted for 20–21 h and then inoculated with 200 µl of Y. pseudotuberculosis through a 20-gauge feeding needle. For intravenous infections, mice were injected with 200 µl of Y. pseudotuberculosis via the tail vein. The intragastric and intravenous MLD for strain 32777 is approximately 5×108 CFU and 12 CFU, respectively, when the bacteria are grown and administered as described above.
Immunization with YopE and ovalbumin peptides
Unless otherwise indicated, mice were lightly anesthetized with isoflurane and immunized intranasally on days 0, 7 and 21 with 15 µl saline solution containing 1 µg cholera toxin (CT; List Biological Laboratory, Campbell, CA) as adjuvant and 1 µg YopE69–77 peptide (H2N-SVIGFIQRM-OH; New England Peptide, Gardner, MA) or control OVA257–264 peptide (H2N-SIINFEKL-OH). Mice were then challenged with 20 or 200 MLD of Y. pestis strain D27, 10 MLD of Y. pestis strain CO92 or 10 MLD of Y. pseudotuberculosis strain 32777 at day 37. In some experiments, PBL were collected by mandibular bleeding at day 35 to assay Ag-specific CD8 T cells by flow cytometry.
In vivo cytotoxicity assay
Splenocytes harvested from naïve CD45.1+ congenic mice were used as target cells. After lysing red blood cells using ammonium chloride buffer, the splenocytes were pulsed with either YopE69–77 or OVA257–264 peptide (10 µM) for 1 h at 37°C in complete medium (DMEM supplemented with 10% heat-inactivated FCS, 2 mM L-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 1% penicillin-streptomycin and 55 µM 2-mercaptoethanol). After washing and resuspending in Hank's balanced salt solution, YopE69–77-pulsed cells were incubated with 10 µM CFSE and OVA257–264-pulsed cells were incubated with 1 µM CFSE for 10z min in a 37°C water bath. CFSE labeling was then stopped by adding FBS to a final concentration of 20%. Cells were washed twice with complete medium and mixed at a 1∶1 ratio. A total of 2×107 cells were then injected intravenously into immunized recipient mice. Spleen cells were collected 20–22 h later and stained with PE-conjugated anti-CD45.1 mAb, biotin-conjugated anti-CD45.2 mAb and allophycocyanin-conjugated streptavidin. The frequency of each CFSE-labeled population in the target cell gate (CD45.1+CD45.2−) was determined by flow cytometry. Cells injected into naïve C57BL/6 mice were used as controls. The percent specific lysis was determined by the following formula: [1-(ratio of cells recovered from naïve mice/ratio of cells recovered from infected mice)]×100. For YopE-specific lysis, the ratio of recovery of OVA257–264-pulsed cells to YopE69–77-pulsed cells = (percentage of CFSElow cells)/(percentage of CFSEhigh cells). For OVA-specific lysis, the ratio of recovery of YopE69–77-pulsed cells to OVA257–264-pulsed cells = (percentage of CFSEhigh cells)/(percentage of CFSElow cells).
MHC class I tetramer reagent and staining
The allophycocyanin-conjugated MHC class I peptide tetramers KbYopE69–77 and Kb OVA257–264 were supplied by the NIH Tetramer Facility and the Trudeau Institute Molecular Biology Core Facility, respectively. PBL were obtained from mandibular blood collected in heparin-containing eppendorf tubes. Red blood cells were lysed by treatment with ammonium chloride. Enumeration of YopE69–77-specific CD8 T cells by KbYopE69–77 tetramer staining was described previously [13]. In brief, cells were treated with 1 µg Fc block (clone 2.4G2) for 10 min at 4°C, washed, and incubated with tetramers for 1 h at room temperature. After washing again, cells were stained with anti-CD4-FITC (clone RM4–5) and anti-CD8-PE (clone 53-6.7) for 30 min at 4°C. Data were collected on a BD Bioscience FACSCanto II and analyzed using FlowJo software.
Measurement of survival and bacteria burden
For survival studies, mice were monitored at least once daily after infection. Unresponsive or recumbent mice were considered moribund and euthanized. For measurement of bacterial burden, mice were euthanized by carbon dioxide asphyxiation at the indicated day after infection. Liver and lung tissues were harvested and homogenized in saline. Serial dilutions of the homogenates were plated on blood agar and incubated at 26°C for 48 h.
Cytokine neutralization
Mice were treated with 1 mg mAb specific for TNFα (clone XT3.11, rat IgG1) or IFNγ (clone XMG1.2, rat IgG1) diluted in PBS. The mAb were administered intraperitoneally on the day before challenge infection. Control mice received 1–1.6 mg of isotype-matched rat IgG1 mAb (clone HRPN).
Cell purifications and transfers
For primed CD8+ T cell transfers, donor mice were immunized intranasally as described above on days 0, 7 and 21. At day 36, splenocytes were harvested, red blood cells were lysed and cell suspensions were applied to nylon wool columns to enrich T cells. After incubation at 37°C for 90 min, the nonadherent cells were eluted and CD8+ T cells were purified by magnetic activated cell sorting (MACS; Miltenyi Biotec Inc., Auburn, CA) according to the manufacturer's instructions. The purified cells were incubated at 37°C for 90 min followed by centrifugation to release beads from cells. The purity of CD8+ donor T cells was ∼95%. A total of 1×107 CD8+ T cells were injected intravenously into naïve wild-type C57BL/6 recipient mice. The following day, the recipient mice were challenged with Y. pestis strain D27.
For naïve splenocyte transfers, donor splenocytes were isolated and red blood cells were lysed. After washing, 5×107 cells were injected intravenously into naïve TCRβδ-deficient recipient mice. The recipient mice were then immunized and challenged with Y. pestis strain D27 as described above.
Mixed bone marrow chimeras
TCRβδ-deficient mice were lethally irradiated (950 rad provided in two doses) and reconstituted with a total of 1×107 donor bone marrow cells. The donor cells comprised bone marrow cells from TCRβδ-deficient mice mixed with bone marrow cells from wild-type C57BL/6 mice or the indicated gene-deficient mice at a 3 to 1 ratio. Chimeric mice were allowed to reconstitute for 6 weeks before they were immunized and challenged with Y. pestis strain D27.
Statistics
Statistical analyses were performed using the computer program Prism 5 (GraphPad Software). Survival data were analyzed by log-rank tests. Bacterial burden data were analyzed by nonparametric Kruskal-Wallis test followed by Dunn's post test; CFU that fell below the detection limit of our assay were assigned values 0.2 log below the detection limit. All other data were analyzed by Student t test or one-way ANOVA as indicated. *p<0.5, **p<0.01, ***p<0.001, ****p<0.0001; ns, not significant.
Zdroje
1. PerryRD, FetherstonJD (1997) Yersinia pestis–etiologic agent of plague. Clin Microbiol Rev 10 : 35–66.
2. AnevlavisS, BourosD (2010) Community acquired bacterial pneumonia. Expert Opin Pharmacother 11 : 361–374.
3. PelegAY, HooperDC (2010) Hospital-acquired infections due to gram-negative bacteria. N Engl J Med 362 : 1804–1813.
4. SmileyST (2008) Current challenges in the development of vaccines for pneumonic plague. Expert Rev Vaccines 7 : 209–221.
5. BashawJ, NorrisS, WeeksS, TrevinoS, AdamoviczJJ, et al. (2007) Development of in vitro correlate assays of immunity to infection with Yersinia pestis. Clin Vaccine Immunol 14 : 605–616.
6. QueneeLE, CilettiNA, ElliD, HermanasTM, SchneewindO (2013) Prevention of pneumonic plague in mice, rats, guinea pigs and non-human primates with clinical grade rV10, rV10-2 or F1-V vaccines. Vaccine 29 : 6572–6583.
7. SzabaFM, KummerLW, WilhelmLB, LinJS, ParentMA, et al. (2009) D27-pLpxL, an avirulent strain of Yersinia pestis, primes T cells that protect against pneumonic plague. Infect Immun 77 : 4295–4304.
8. LinJS, KummerLW, SzabaFM, SmileyST (2011) IL-17 contributes to cell-mediated defense against pulmonary Yersinia pestis infection. J Immunol 186 : 1675–1684.
9. KummerLW, SzabaFM, ParentMA, AdamoviczJJ, HillJ, et al. (2008) Antibodies and cytokines independently protect against pneumonic plague. Vaccine 26 : 6901–6907.
10. LinJS, ParkS, AdamoviczJJ, HillJ, BliskaJB, et al. (2010) TNFalpha and IFNgamma contribute to F1/LcrV-targeted immune defense in mouse models of fully virulent pneumonic plague. Vaccine 29 : 357–362.
11. HartyJT, TvinnereimAR, WhiteDW (2000) CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol 18 : 275–308.
12. WongP, PamerEG (2003) CD8 T cell responses to infectious pathogens. Annu Rev Immunol 21 : 29–70.
13. LinJS, SzabaFM, KummerLW, ChromyBA, SmileyST (2011) Yersinia pestis YopE contains a dominant CD8 T cell epitope that confers protection in a mouse model of pneumonic plague. J Immunol 187 : 897–904.
14. ZhangY, MenaP, RomanovG, LinJS, SmileyST, et al. (2012) A protective epitope in type III effector YopE is a major CD8 T cell antigen during primary infection with Yersinia pseudotuberculosis. Infect Immun 80 : 206–214.
15. BergmanMA, LoomisWP, MecsasJ, StarnbachMN, IsbergRR (2009) CD8(+) T cells restrict Yersinia pseudotuberculosis infection: bypass of anti-phagocytosis by targeting antigen-presenting cells. PLoS Pathog 5: e1000573.
16. WonderlichJ, ShearerG, LivingstoneA, BrooksA (2006) Induction and measurement of cytotoxic T lymphocyte activity. Curr Protoc Immunol Chapter 3: Unit 3 11.
17. KagiD, LedermannB, BurkiK, SeilerP, OdermattB, et al. (1994) Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369 : 31–37.
18. GrivennikovSI, TumanovAV, LiepinshDJ, KruglovAA, MarakushaBI, et al. (2005) Distinct and nonredundant in vivo functions of TNF produced by T cells and macrophages/neutrophils: protective and deleterious effects. Immunity 22 : 93–104.
19. BergRE, CrossleyE, MurrayS, FormanJ (2003) Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med 198 : 1583–1593.
20. Lee-LewisH, AndersonDM (2010) Absence of inflammation and pneumonia during infection with nonpigmented Yersinia pestis reveals a new role for the pgm locus in pathogenesis. Infect Immun 78 : 220–230.
21. BadovinacVP, TvinnereimAR, HartyJT (2000) Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science 290 : 1354–1358.
22. TsujimotoM, YipYK, VilcekJ (1986) Interferon-gamma enhances expression of cellular receptors for tumor necrosis factor. J Immunol 136 : 2441–2444.
23. LukaszewskiRA, KennyDJ, TaylorR, ReesDG, HartleyMG, et al. (2005) Pathogenesis of Yersinia pestis infection in BALB/c mice: effects on host macrophages and neutrophils. Infect Immun 73 : 7142–7150.
24. Narni-MancinelliE, CampisiL, BassandD, CazarethJ, GounonP, et al. (2007) Memory CD8+ T cells mediate antibacterial immunity via CCL3 activation of TNF/ROI+ phagocytes. J Exp Med 204 : 2075–2087.
25. CookDN, SmithiesO, StrieterRM, FrelingerJA, SerodyJS (1999) CD8+ T cells are a biologically relevant source of macrophage inflammatory protein-1 alpha in vivo. J Immunol 162 : 5423–5428.
26. ParentMA, BerggrenKN, KummerLW, WilhelmLB, SzabaFM, et al. (2005) Cell-mediated protection against pulmonary Yersinia pestis infection. Infect Immun 73 : 7304–7310.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge inČlánek Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 5- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Surveillance for Emerging Biodiversity Diseases of Wildlife
- The Emerging Role of Urease as a General Microbial Virulence Factor
- PARV4: An Emerging Tetraparvovirus
- Epigenetic Changes Modulate Schistosome Egg Formation and Are a Novel Target for Reducing Transmission of Schistosomiasis
- The Human Adenovirus E4-ORF1 Protein Subverts Discs Large 1 to Mediate Membrane Recruitment and Dysregulation of Phosphatidylinositol 3-Kinase
- A Multifactorial Role for Malaria in Endemic Burkitt's Lymphoma Pathogenesis
- Structural Basis for the Ubiquitin-Linkage Specificity and deISGylating Activity of SARS-CoV Papain-Like Protease
- Cathepsin-L Can Resist Lysis by Human Serum in
- Epstein-Barr Virus Down-Regulates Tumor Suppressor Expression
- BCA2/Rabring7 Targets HIV-1 Gag for Lysosomal Degradation in a Tetherin-Independent Manner
- The Evolutionarily Conserved Mediator Subunit MDT-15/MED15 Links Protective Innate Immune Responses and Xenobiotic Detoxification
- Suppressor of Cytokine Signaling 4 (SOCS4) Protects against Severe Cytokine Storm and Enhances Viral Clearance during Influenza Infection
- T Cell Inactivation by Poxviral B22 Family Proteins Increases Viral Virulence
- Dynamics of HIV Latency and Reactivation in a Primary CD4+ T Cell Model
- HIV and HCV Activate the Inflammasome in Monocytes and Macrophages via Endosomal Toll-Like Receptors without Induction of Type 1 Interferon
- Virus and Autoantigen-Specific CD4+ T Cells Are Key Effectors in a SCID Mouse Model of EBV-Associated Post-Transplant Lymphoproliferative Disorders
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- Squalene Synthase As a Target for Chagas Disease Therapeutics
- The Contribution of Viral Genotype to Plasma Viral Set-Point in HIV Infection
- Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge in
- Anthrax Lethal Factor as an Immune Target in Humans and Transgenic Mice and the Impact of HLA Polymorphism on CD4 T Cell Immunity
- Ly49C-Dependent Control of MCMV Infection by NK Cells Is -Regulated by MHC Class I Molecules
- Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
- A Large Family of Antivirulence Regulators Modulates the Effects of Transcriptional Activators in Gram-negative Pathogenic Bacteria
- Broad-Spectrum Anti-biofilm Peptide That Targets a Cellular Stress Response
- Malaria Parasite Infection Compromises Control of Concurrent Systemic Non-typhoidal Infection via IL-10-Mediated Alteration of Myeloid Cell Function
- A Role for in Higher Order Structure and Complement Binding of the Capsule
- Hip1 Modulates Macrophage Responses through Proteolysis of GroEL2
- CD8 T Cells from a Novel T Cell Receptor Transgenic Mouse Induce Liver-Stage Immunity That Can Be Boosted by Blood-Stage Infection in Rodent Malaria
- Phosphorylation of KasB Regulates Virulence and Acid-Fastness in
- HIV-Infected Individuals with Low CD4/CD8 Ratio despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened CD8+ T Cell Activation, and Increased Risk of Non-AIDS Morbidity and Mortality
- A Novel Mechanism Inducing Genome Instability in Kaposi's Sarcoma-Associated Herpesvirus Infected Cells
- Structural and Biochemical Characterization Reveals LysGH15 as an Unprecedented “EF-Hand-Like” Calcium-Binding Phage Lysin
- Hepatitis C Virus Cell-Cell Transmission and Resistance to Direct-Acting Antiviral Agents
- Different Modes of Retrovirus Restriction by Human APOBEC3A and APOBEC3G
- TNFα and IFNγ but Not Perforin Are Critical for CD8 T Cell-Mediated Protection against Pulmonary Infection
- Large Scale RNAi Reveals the Requirement of Nuclear Envelope Breakdown for Nuclear Import of Human Papillomaviruses
- The Cytoplasmic Domain of Varicella-Zoster Virus Glycoprotein H Regulates Syncytia Formation and Skin Pathogenesis
- A New Class of Multimerization Selective Inhibitors of HIV-1 Integrase
- Are We There Yet? The Smallpox Research Agenda Using Variola Virus
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
- Dynamic Functional Modulation of CD4 T Cell Recall Responses Is Dependent on the Inflammatory Environment of the Secondary Stimulus
- Bacterial Superantigens Promote Acute Nasopharyngeal Infection by in a Human MHC Class II-Dependent Manner
- Follicular Helper T Cells Promote Liver Pathology in Mice during Infection
- A Nasal Epithelial Receptor for WTA Governs Adhesion to Epithelial Cells and Modulates Nasal Colonization
- Unexpected Role for IL-17 in Protective Immunity against Hypervirulent HN878 Infection
- Human Cytomegalovirus Fcγ Binding Proteins gp34 and gp68 Antagonize Fcγ Receptors I, II and III
- Expansion of Murine Gammaherpesvirus Latently Infected B Cells Requires T Follicular Help
- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Molecular Signatures of Hemagglutinin Stem-Directed Heterosubtypic Human Neutralizing Antibodies against Influenza A Viruses
- The Downregulation of GFI1 by the EZH2-NDY1/KDM2B-JARID2 Axis and by Human Cytomegalovirus (HCMV) Associated Factors Allows the Activation of the HCMV Major IE Promoter and the Transition to Productive Infection
- Inactivation of Fructose-1,6-Bisphosphate Aldolase Prevents Optimal Co-catabolism of Glycolytic and Gluconeogenic Carbon Substrates in
- New Insights into Rotavirus Entry Machinery: Stabilization of Rotavirus Spike Conformation Is Independent of Trypsin Cleavage
- Prophenoloxidase Activation Is Required for Survival to Microbial Infections in
- SslE Elicits Functional Antibodies That Impair Mucinase Activity and Colonization by Both Intestinal and Extraintestinal Strains
- Timed Action of IL-27 Protects from Immunopathology while Preserving Defense in Influenza
- HIV-1 Envelope gp41 Broadly Neutralizing Antibodies: Hurdles for Vaccine Development
- The PhoP-Dependent ncRNA Mcr7 Modulates the TAT Secretion System in
- Cellular Superspreaders: An Epidemiological Perspective on HIV Infection inside the Body
- The Inflammasome Pyrin Contributes to Pertussis Toxin-Induced IL-1β Synthesis, Neutrophil Intravascular Crawling and Autoimmune Encephalomyelitis
- Papillomavirus Genomes Associate with BRD4 to Replicate at Fragile Sites in the Host Genome
- Integrative Functional Genomics of Hepatitis C Virus Infection Identifies Host Dependencies in Complete Viral Replication Cycle
- Co-assembly of Viral Envelope Glycoproteins Regulates Their Polarized Sorting in Neurons
- Targeting Membrane-Bound Viral RNA Synthesis Reveals Potent Inhibition of Diverse Coronaviruses Including the Middle East Respiratory Syndrome Virus
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



