-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHIV-1 Envelope gp41 Broadly Neutralizing Antibodies: Hurdles for Vaccine Development
article has not abstract
Published in the journal: . PLoS Pathog 10(5): e32767. doi:10.1371/journal.ppat.1004073
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004073Summary
article has not abstract
A primary correlate of protection for most effective viral vaccines is induction of antibodies with potent virus neutralization [1]. HIV-1 differs from other viruses for which successful vaccines have been made, because as a highly mutable, integrating retrovirus, it is resistant to both immune responses and antiretroviral therapy upon establishment of a latently infected CD4+ T-cell pool [2]. Thus, to prevent infection, an HIV-1 vaccine must induce protective immunity that is active during transmission [3]. Broadly neutralizing antibodies (bnAbs) are targeted to conserved regions of the HIV-1 envelope (Env) and neutralize a broad spectrum of HIV-1 quasi-species [4]. When passively infused in rhesus macaques [5]–[8] or transduced in humanized mice [9] preceding challenge, bnAbs robustly prevent infection, suggesting they can protect if present during transmission. However, bnAbs are made in a minority of HIV-1–infected individuals years after infection and cannot be elicited by current immunization regimens [3]. Thus, identifying impediments to bnAb induction to devise better immunization strategies is a central goal for HIV-1 vaccine development.
Potential Structural and Immunological Roadblocks to BnAb Induction
The advent of high-throughput recombinant antibody technology has facilitated isolation of bnAbs with remarkable breadth and potency from HIV-1–infected individuals [10] and has re-invigorated structure-based vaccine design efforts [4]. We now have atomic-level descriptions of multiple bnAb epitopes and extensive knowledge of four general, vulnerable Env regions in which they cluster: the gp41 membrane proximal external region (MPER), the gp120 CD4-binding site (CD4bs), and two sites enriched for quaternary proteoglycan epitopes, one associated with the first/second hypervariable loops (V1/V2-glycan), the other around the third hypervariable loop (V3-glycan) [3], [4], [10]. Despite this progress, efforts to engineer immunogens capable of presenting neutralizing epitopes still fail to induce bnAbs [4], making it increasingly apparent that more traditional approaches, i.e., those aimed at overcoming limited bnAb epitope accessibility resulting from steric and conformational hindrances [3] or eliminating immunogenically dominant non-neutralizing epitopes [11], while important, cannot alone solve the HIV-1 bnAb induction problem.
Therefore, attention has instead shifted to the host for insight into why bnAbs are so difficult to induce. This re-focus is derived from observations that two gp41 bnAbs exhibited features suggesting immune tolerance mechanisms limited their induction [12], [13]. One of these features, in vitro poly/autoreactivity, has reasonable concordance with immune tolerance, as suggested by the observation that in vitro poly/autoreactivity of the normal human repertoire progressively wanes at developmental stages, coinciding with previously defined tolerance checkpoints [14] (reviewed in [15]). A second feature, extended antibody combining regions (HCDR3s), can also invoke counterselection of B-cells, either during peripheral development [16] or prior to B-cell receptor (BCR) expression [17], especially when as accentuated as in the V1/V2-glycan class of bnAbs [4], [15]. We now know from knock-in (KI) models of MPER-targeting bnAbs that such traits can invoke profound immune tolerance [18]–[22], and other recent studies have revealed this bnAb class may be modulated by additional contributory immunoregulatory factors: either genetic determinants like VH allelic variation [23] and overlapping MHC class II–restricted CD4+ TH/bnAb epitopes [24] or environmental influences, such as shaping of the B-cell repertoire by incidental antigen exposure [25]. The isolation of over 100 bnAbs has not only confirmed the presence of long HCDR3s and/or poly/autoreactivity in most, but has revealed a third feature in all: unusually high somatic mutation levels that may also be associated with self-reactivity [26] and, if required by vaccination, cannot be achieved by existing immunization protocols [10], [26].
Evidence for Tolerance Control of Self-Reactive BnAbs
4E10 and 2F5, HIV-1 Env gp41 bnAbs with adjacent, linear epitopes in the MPER and two of only three bnAbs to have been directly isolated from HIV-1–infected patients between 1993 and 2009, were identified to have traits associated with negative selection, i.e., long HCDR3s and poly/autoreactivity [12]. Based on this, we proposed that bnAb responses were impaired by immune tolerance [12], [13]. Recently, we and others have examined this hypothesis by following B-cell development in KI mice that express 4E10 or 2F5 V(D)J rearrangements [18]–[22]. These mice exhibited striking blockades in generating immature B-cells, a phenotype characteristic of central clonal deletion and similar to KI mice expressing BCRs with high affinities to known self-antigens [27]–[29]. Furthermore, residual 2F5 and 4E10 KI B-cells poorly express and signal through their BCRs [19]–[22], resembling unresponsive (anergic) B-cells [30]. Overall, these results indicate that the self-reactivity of the 2F5 and 4E10 bnAbs is sufficient to induce profound immune tolerance-mediated negative selection in vivo.
Recently, conserved vertebrate host antigens recognized by 2F5 and 4E10 have been identified: kynureninase and splicing factor 3b subunit-3 (SF3B3), respectively [31]. Interestingly, 2F5's bnAb epitope ELDKWA is identical to an alpha-helical motif in kynureninase [31], and B-cell escape clones from 2F5 KI mice selectively and stringently purge ELDKWA binding [20], [32]. In contrast, 4E10's bnAb epitope NWF(N/D)IT is not present in SF3B3, and relative to 2F5, 4E10 exhibits considerably more polyreactivity [31] with high avidity for lipids [13]. Thus, for certain bnAbs, like 2F5, viral mimicry may involve substantial sequence overlap between host and bnAb epitopes.
These studies [19]–[21], [31], [32] challenge the widely held immunologic notion that self - and viral epitope specificities are distinct, and they raise an intriguing question: to what extent does self-mimicry generally impact antiviral responses? Regarding bnAb responses specifically, a key question for HIV-1 vaccine development will be whether immune tolerance limits induction of all MPER+ bnAbs and to what extent it limits bnAbs targeting other Env regions. The recent identification of 10E8, a potent MPER+ bnAb lacking in vitro polyreactivity [33], argues against the former question, but only if standard autoimmune assays for poly/autoreactivity reflect bona fide physiological self-reactivity. Indeed, since work using protein arrays has identified 10E8 specificity for select host-antigens (Kelsoe G, Haynes BF, unpublished results, manuscript in preparation), generation of 10E8 KI mice will be needed to resolve this issue. Also noteworthy is that bnAb b12, originally reported to be poly/autoreactive [13], lacks tolerizing self-reactivity in KI mice [34]. Although b12's origin from a phage library limits the physiological relevance of this example, it nevertheless reinforces the importance of using bnAb KI models to confirm in vivo effects of poly/autoreactivity measurements.
A key, related issue regarding how tolerizing self-reactivity impairs bnAb induction is when it is acquired or lost in various bnAb lineages. Since KI mice carrying reverted (unmutated ancestor [UA]) 2F5 BCRs undergo profound central deletion (Verkoczy L, Haynes BF, unpublished results, manuscript in preparation), bnAbs like 2F5 likely have self-reactivity encoded in their primary VDJ rearrangement (or, at least, early during affinity maturation, given the caveat in predicting its inferred HCDR3 using best-probability estimates), while others like CH103 and 4E10, whose UA BCRs lack bnAb and self-epitope specificity in vitro (Haynes BF, unpublished results, and [35], [36]) may be tolerized in the periphery. Even bnAbs, like VRC01 and 10E8, that may lack tolerizing self-reactivity and bear considerably more mutations than polyreactive bnAbs to similar epitopes could themselves be products of tolerized bnAb lineages (see below). Thus, definitively evaluating the extent to which self-reactivity limits bnAb lineages will likely require assessing in vivo tolerizing effects of gene-targeted V(D)J specificities of not only bnAbs but also their lineage ancestors. In this regard, higher-throughput KI methodologies, based on RAG blastocyst complementation [37], could significantly accelerate such analysis.
BnAbs Arise after Extensive Somatic Mutation and Virus Evolution during HIV-1 Infection
A hallmark of B-cells is their ability to interact with exogenous antigen and to produce specific Abs with high affinity. This process, affinity maturation (AM), occurs in germinal centers by two linked mechanisms, IgV region somatic hypermutation and affinity-dependent selection. In secondary responses to conventional antigen, somatic mutation levels are normally restricted, because excess mutation decreases affinity and cell survival [38]. In contrast, bnAbs accumulate high (15%–48%) mutation frequencies [4], which may be required for neutralizing activity by promoting structural flexibility in bnAb V(D)J framework regions [39]. That all bnAbs identified thus far originate from subjects infected with HIV-1 for 2–4 years suggests that these remarkable mutation frequencies are a product of highly convoluted, yet-to-be understood AM pathways [26], but how and why this occurs has been elusive.
Studies examining the in vivo evolution of clonal bnAb lineages during chronic HIV-1 infection [35], [40] now provide insight into how selection pressures by Env sequence diversification impacts this process. In a study by Liao et al., the entire evolutionary footprint of CH103 (a CD4bs+ bnAb derived from a donor that seroconverted after ∼2.5 years) and its virus targets were molecularly elucidated [35]. Interaction of this bnAb lineage UA BCR with the transmitted founder virus Env resulted in intense co-evolution by successive viral escape mutants and mutant BCR intermediates, culminating in viral diversification focused to the restricted CH103 epitope, immediately preceding final acquisition of neutralizing breadth.
These studies, and recent findings that most experimentally reverted bnAb UAs lack neutralizing epitope affinity [26], [34], [41], provide one plausible explanation for why bnAbs accumulate so much mutation: naïve BCRs need to be extensively modified to meet unusually demanding structural requirements for acquiring breath and/or potency, i.e., significant affinity to bnAb epitopes. Could alternative explanations account for this degree of mutation in some bnAbs? That bnAbs with in vitro poly/autoreactivity tend to be less mutated relative to non-poly/autoreactive bnAbs for similar epitopes [4], [15] raises the intriguing possibility that tolerance and extensive AM, in bnAbs whose epitopes have structural overlap with self-antigens, are causally linked. Two mechanisms by which tolerizing self-reactivity of bnAbs could drive unusually high mutation rates have been proposed [26], [31], [32]. First, negative selection during early development may forbid unmutated BCRs that bind self-mimicking bnAb epitopes to participate in conventional AM, thus creating “holes in the repertoire” that necessitate recruitment of weakly crossreactive, previously mutated B-cell clones to achieve bnAb specificity via AM. The second non-exclusive possibility is that there is close, but not complete, structural overlap between self - and bnAb epitopes. Thus, already-mutated B-cells with neutralizing specificity attempt to escape anergy via selection for mutations at critical V(D)J residues that remove self-reactivity but maintain bnAb epitope affinity, a requirement that may take multiple rounds of mutation and selection to accomplish.
Developing Novel BnAb-Based HIV-1 Vaccine Strategies
Current HIV-1 vaccination strategies induce antibody mutation profiles (∼4%–5%) similar to autologous neutralizing antibodies in acute HIV infection and most human antibodies produced in 2° responses to infection [10]. Thus, the apparent requirement for polyreactivity and/or extensive mutation for generating bnAb function [39], [42], [43] comes at an expensive cost: creation of disfavored and/or complex AM pathways that existing vaccine approaches cannot recapitulate. Recently, a B-cell lineage immunogen design approach has been proposed to overcome this (Figure 1) [26]. This strategy builds on two assumptions: (1) the best immunogens bind naïve BCRs with the highest affinity, and (2) serial immunization with distinct immunogens can recreate Env-guided AM pathways that generate bnAbs during infection. The general approach involves priming with an immunogen that binds a given bnAb lineage's inferred UA BCR (to initiate B-cell responses), followed by serial boosting with immunogens optimized to bind inferred intermediate ancestor (IA) BCRs. Perhaps the most straightforward and physiological use of this strategy for development of HIV-1 vaccine candidates would involve sequential use of Env proteins identified from serial isolates of known bnAb lineages to drive evolutionary intermediates [35]. Variations of this approach have also been recently proposed, involving in vitro selection and/or mutational methodologies to synthetically engineer immunogens that bind both UA and mature BCRs from bnAb lineages to common epitopes [44], [45], and more generally, analogous vaccine strategies against other pathogens have also recently been described [46].
Fig. 1. B-cell lineage-based approach to vaccine design. 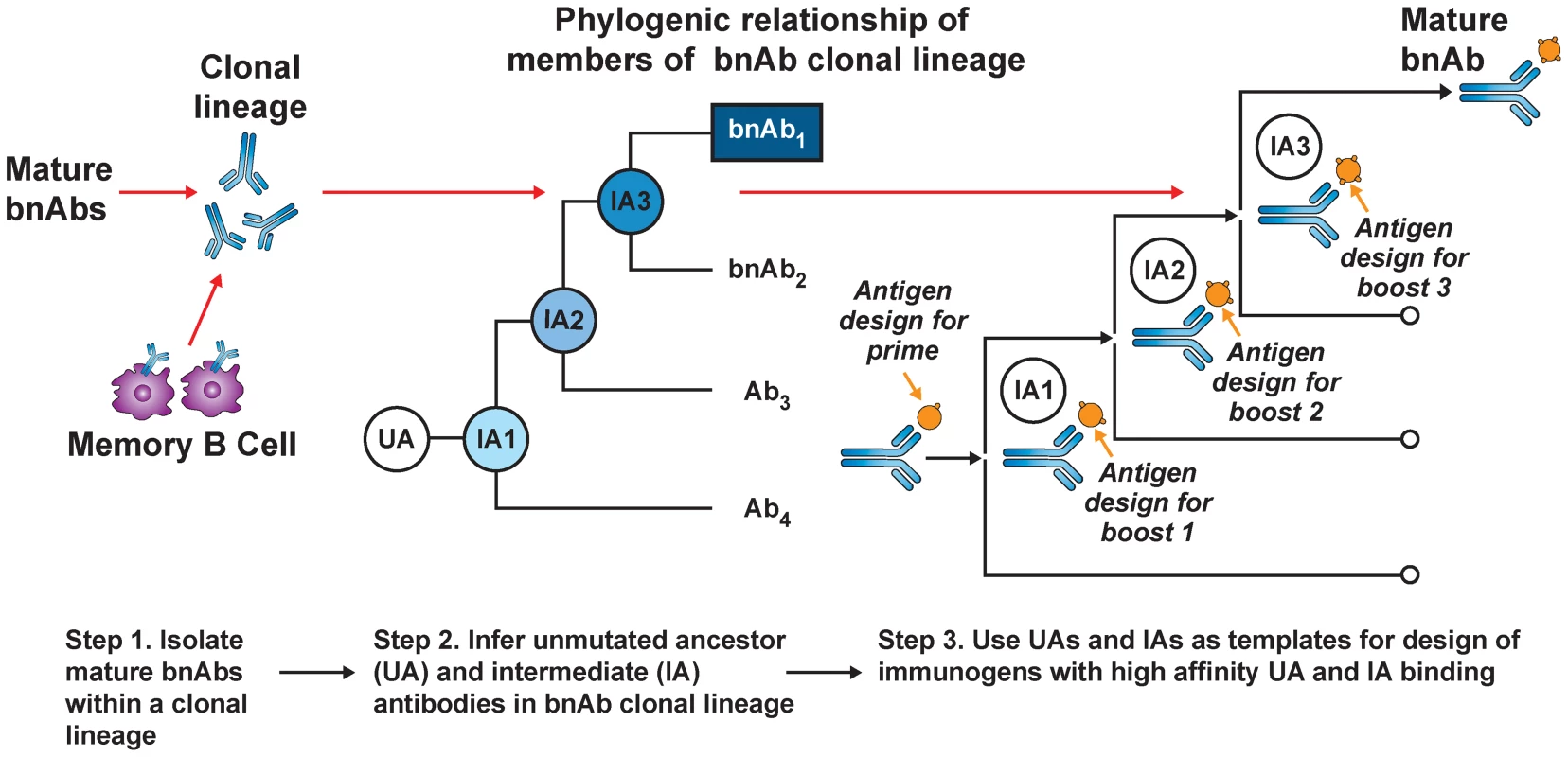
Mature bnAbs can be isolated from HIV-1–infected donors using modern methods such as memory B-cell culture or sorting of antigen-specific B-cells. Based on the known bnAb sequence, next generation sequencing can be used to find numerous clonal relatives of the mature bnAb. If appropriate longitudinal samples are available, it is possible to infer the full antibody lineage, including the UA and IAs. The expressed UA and IA sequences can then be used as templates for the design of HIV-1 immunogens with high-affinity binding. As the antibody lineage is known to evolve in response to viral evolution, it may be possible to design sequential immunogens with high-affinity binding for the UA and IA, thus guiding the antibody response toward the mature antibody with broad neutralizing activity (with permission from [4]). Conclusions
Although our studies suggest host mimicry by HIV-1 Env gp41 neutralization epitopes limits bnAb induction [19]–[21], [31], [32], they also indicate such epitopes can be used in vaccine regimens designed to successfully target anergic bnAb-specific B-cells [32]. Furthermore, poly/autoreactivity is part of normal antibody responses [43], and evidence from passive protection or in vitro pathogenicity assays thus far does not suggest adverse effects would result from inducing bnAbs with tolerizing self-reactivity [4]. However, the requirement for high mutation levels [39] may be the most daunting problem facing HIV-1 vaccine development and may necessitate identifying and exploiting lineages requiring relatively fewer mutations to acquire bnAb specificity. Finally, since single bnAbs readily select HIV-1 escape mutants [4], [35], but certain combinations of individual bnAbs targeting distinct Env regions (for example, V1/V2-glycan and CD4bs-specific bnAbs) confer near-complete breadth [10], a successful vaccine will also need to incorporate approaches capable of inducing multiple types of bnAbs.
Zdroje
1. PlotkinS (2010) Correlates of protection induced by vaccination. Clin Vaccine Immunol 17 : 1055–1065.
2. WeiX, DeckerJM, WangS, HuiH, KappesJC, et al. (2003) Antibody neutralization and escape by HIV-1. Nature 422 : 307–312.
3. McElrathMJ, HaynesBF (2010) Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity 33 : 542–554.
4. MascolaJR, HaynesBF (2013) HIV-1 neutralizing antibodies: understanding nature's pathways. Immunol Rev 254 : 225–44.
5. HessellAJ, PoignardP, HunterM, HangartnerL, TehraniDM, et al. (2009) Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med 15 : 951–954.
6. HessellAJ, RakaszEG, PoignardP, HangartnerL, LanducciG, et al. (2009) Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog 5: e1000433.
7. MoldtB, RakaszEG, SchultzN, Chan-HuiPY, SwiderekK, et al. (2012) Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci USA 109 : 18921–18925.
8. BarouchDH, WhitneyJB, MoldtB, KleinF, OliveiraTY, et al. (2013) Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503 : 224–228.
9. BalazsAB, ChenJ, HongCM, RaoDS, YangL, et al. (2012) Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 481 : 81–84.
10. BonsignoriM, AlamSM, LiaoHX, VerkoczyL, TomarasGD, et al. (2012) HIV-1 antibodies from infection and vaccination: insights for guiding vaccine design. Trends Microbiol 20 : 532–539.
11. StamatatosL, MorrisL, BurtonDR, MascolaJR (2009) Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nature Med 15 : 866–870.
12. HaynesBF, FlemingJ, St ClairEW, KatingerH, StieglerG, et al. (2005) Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 308 : 1906–1908.
13. HaynesBF, MoodyMA, VerkoczyL, KelsoeG, AlamSM (2005) Antibody polyspecificity and neutralization of HIV-1: a hypothesis. Hum Antibodies 14 : 59–67.
14. WardemannH, YurasovS, SchaeferA, YoungJW, MeffreE, et al. (2003) Predominant autoantibody production by early human B cell precursors. Science 301 : 1374–1377.
15. VerkoczyL, KelsoeG, MoodyMA, HaynesBF (2011) Role of immune mechanisms in induction of HIV-1 broadly neutralizing antibodies. Curr Opin Immunol 23 : 383–390.
16. MeffreE, MililiM, Blanco-BetancourtC, AntunesH, NussenzweigMC, et al. (2001) Immunoglobulin heavy chain expression shapes the B cell receptor repertoire in human B cell development. J Clin Invest 108 : 879–886.
17. Von BoehmerH, MelchersF (2010) Checkpoints in lymphocyte development and autoimmune disease. Nat Immunol 11 : 176–183.
18. VerkoczyL, DiazM, HollTM, OuyangY-B, Bouton-VervilleH, et al. (2010) Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance. Proc Natl Acad Sci USA 107 : 181–186.
19. VerkoczyL, ChenY, Bouton-VervilleH, ZhangJ, DiazM, et al. (2011) Rescue of HIV-1 broad neutralizing antibody-expressing B cells in 2F5 VH×VL knockin mice reveals multiple tolerance controls. J Immunol 187 : 3785–3797.
20. ChenY, ZhangJ, HwangK-K, Bouton-VervilleH, XiaS-M, et al. (2013) Common tolerance mechanisms, but distinct cross-reactivities associated with gp41 and lipids, limit production of HIV-1 broad neutralizing antibodies 2F5 and 4E10. J Immunol 191 : 1260–1275.
21. Doyle-CooperC, HudsonKE, CooperAB, OtaT, SkogP, et al. (2013) Immune Tolerance Negatively Regulates B Cells in Knock-In Mice Expressing Broadly Neutralizing HIV Antibody 4E10. J Immunol 191 : 3186–3191.
22. FintonKA, LarimoreK, LarmanHB, FriendD, CorrentiC, et al. (2013) Autoreactivity and exceptional CDR plasticity (but not unusual polyspecificity) hinder elicitation of the anti-HIV antibody 4E10. PLoS Pathog 9: e1003639.
23. AlamSM, LiaoHX, DennisonSM, JaegerF, ParksR, et al. (2011) Differential reactivity germ line allelic variants of a broadly neutralizing HIV-1 antibody to a gp41 fusion intermediate conformation. J Virol 85 : 11725–11731.
24. ZhangJ, AlamSM, Bouton-VervilleH, ChenY, NewmanA, et al. (2014) Modulation of non-neutralizing gp41 responses by an MHC-restricted TH epitope overlapping those of MPER broad neutralizing antibodies. J Immunol 192 : 1693–1706.
25. LiaoHX, ChenX, MunshawS, ZhangR, MarshallDJ, et al. (2011) Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. J Exp Med 208 : 2237–2249.
26. HaynesBF, KelsoeG, HarrisonSC, KeplerTB (2012) B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat Biotech 30 : 423–433.
27. NemazeeD, BurkiK (1989) Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature 337 : 562–566.
28. EriksonJ, RadicMZ, CamperSA, HardyRR, CarmackC, et al. (1991) Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature 349 : 331–334.
29. HartleySB, CrosbieJ, BrinkR, KantorAB, BastenA, et al. (1991) Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature 353 : 765–769.
30. GoodnowCC (1992) Transgenic mice and analysis of B-cell tolerance. Ann Rev Immunol 10 : 489–518.
31. YangG, HollTM, LiuY, LiX, NicelyN, et al. (2013) Identification of autoantigens recognized by the 2F5 and 4E10 broadly neutralizing HIV-1 antibodies. J Exp Med 210 : 241–256.
32. VerkoczyL, ChenY, ZhangJ, Bouton-VervilleH, NemanA, et al. (2013) Induction of HIV-1 broad neutralizing antibodies in 2F5 knock-in mice: selection against MPER-associated autoreactivity limits T-dependent responses. J Immunol 191 : 2538–2550.
33. HuangJ, OfekG, LaubL, LouderMK, Doria-RoseNA, et al. (2012) Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491 : 406–412.
34. OtaT, Doyle-CooperC, CooperAB, DooresKJ, Aoki-OtaM, et al. (2013) B cells from Knock-in Mice Expressing Broadly Neutralizing HIV Antibody b12 Carry an Innocuous B Cell Receptor Responsive to HIV Vaccine Candidates. J Immunol 191 : 3179–85.
35. LiaoH-X, LynchR, ZhouZ, GaoG, AlamSM, et al. (2013) Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 496 : 469–76.
36. OtaT, Doyle-CooperC, CooperAB, HuberM, FalkowskaE, et al. (2012) Anti-HIV B Cell Lines as Candidate Vaccine Biosensors. J Immunol 189 : 4816–4824.
37. ChenJ, LansfordR, StewartV, YoungF, AltFW (1993) RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc Natl Acad Sci USA 90 : 4528–4532.
38. BatistaFD, NeubergerMS (1998) Affinity Dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity 8 : 751–759.
39. KleinF, DiskinR, ScheidJF, GaeblerC, MouquetH, et al. (2013) Somatic mutations of the immunoglobulin framework are generally required for broad and potent neutralization. Cell 153 : 126–138.
40. MoorePL, GrayES, WibmerCK, BhimanJN, NonyaneM, et al. (2012) Evolution of an HIV glycan-dependent broadly neutralizing antibody epitope through immune escape. Nat Med 18 : 1688–1692.
41. HootS, McGuireAT, CohenKW, StrongRK, HangartnerL, et al. (2013) Recombinant HIV envelope proteins fail to engage germline versions of anti-CD4bs bnAbs. PLoS Pathog 9: e1003106.
42. AlamSM, MorelliM, DennisonSM, LiaoHX, ZhangR, et al. (2009) Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc Natl Acad Sci USA 106 : 20234–20239.
43. MouquetH, ScheidJF, ZollerMJ, KrogsgaardM, OttRG, et al. (2010) Polyreactivity increases the apparaent affinity of anti-HIV antibodies by hereoligation. Science 467 : 591–595.
44. JardineJ, JulienJ-P, MenisS, OtaT, KalyuzhniyO, et al. (2013) Rational HIV immunogen design to target specific germline B cell receptors. Science 340 : 711–716.
45. McGuireAT, HootS, DreyerAM, LippyA, StuartA, et al. (2013) Engineering HIV envelope protein to activate germline B cell receptors of broadly neutralizing anti-CD4 binding site antibodies. J Exp Med 210 : 655–663.
46. DormitzerPR, GrandiG, RappuoliR (2012) Structural vaccinology starts to deliver. Nat Rev Microbiol 10 : 807–813.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge inČlánek Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 5- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Surveillance for Emerging Biodiversity Diseases of Wildlife
- The Emerging Role of Urease as a General Microbial Virulence Factor
- PARV4: An Emerging Tetraparvovirus
- Epigenetic Changes Modulate Schistosome Egg Formation and Are a Novel Target for Reducing Transmission of Schistosomiasis
- The Human Adenovirus E4-ORF1 Protein Subverts Discs Large 1 to Mediate Membrane Recruitment and Dysregulation of Phosphatidylinositol 3-Kinase
- A Multifactorial Role for Malaria in Endemic Burkitt's Lymphoma Pathogenesis
- Structural Basis for the Ubiquitin-Linkage Specificity and deISGylating Activity of SARS-CoV Papain-Like Protease
- Cathepsin-L Can Resist Lysis by Human Serum in
- Epstein-Barr Virus Down-Regulates Tumor Suppressor Expression
- BCA2/Rabring7 Targets HIV-1 Gag for Lysosomal Degradation in a Tetherin-Independent Manner
- The Evolutionarily Conserved Mediator Subunit MDT-15/MED15 Links Protective Innate Immune Responses and Xenobiotic Detoxification
- Suppressor of Cytokine Signaling 4 (SOCS4) Protects against Severe Cytokine Storm and Enhances Viral Clearance during Influenza Infection
- T Cell Inactivation by Poxviral B22 Family Proteins Increases Viral Virulence
- Dynamics of HIV Latency and Reactivation in a Primary CD4+ T Cell Model
- HIV and HCV Activate the Inflammasome in Monocytes and Macrophages via Endosomal Toll-Like Receptors without Induction of Type 1 Interferon
- Virus and Autoantigen-Specific CD4+ T Cells Are Key Effectors in a SCID Mouse Model of EBV-Associated Post-Transplant Lymphoproliferative Disorders
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- Squalene Synthase As a Target for Chagas Disease Therapeutics
- The Contribution of Viral Genotype to Plasma Viral Set-Point in HIV Infection
- Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge in
- Anthrax Lethal Factor as an Immune Target in Humans and Transgenic Mice and the Impact of HLA Polymorphism on CD4 T Cell Immunity
- Ly49C-Dependent Control of MCMV Infection by NK Cells Is -Regulated by MHC Class I Molecules
- Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
- A Large Family of Antivirulence Regulators Modulates the Effects of Transcriptional Activators in Gram-negative Pathogenic Bacteria
- Broad-Spectrum Anti-biofilm Peptide That Targets a Cellular Stress Response
- Malaria Parasite Infection Compromises Control of Concurrent Systemic Non-typhoidal Infection via IL-10-Mediated Alteration of Myeloid Cell Function
- A Role for in Higher Order Structure and Complement Binding of the Capsule
- Hip1 Modulates Macrophage Responses through Proteolysis of GroEL2
- CD8 T Cells from a Novel T Cell Receptor Transgenic Mouse Induce Liver-Stage Immunity That Can Be Boosted by Blood-Stage Infection in Rodent Malaria
- Phosphorylation of KasB Regulates Virulence and Acid-Fastness in
- HIV-Infected Individuals with Low CD4/CD8 Ratio despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened CD8+ T Cell Activation, and Increased Risk of Non-AIDS Morbidity and Mortality
- A Novel Mechanism Inducing Genome Instability in Kaposi's Sarcoma-Associated Herpesvirus Infected Cells
- Structural and Biochemical Characterization Reveals LysGH15 as an Unprecedented “EF-Hand-Like” Calcium-Binding Phage Lysin
- Hepatitis C Virus Cell-Cell Transmission and Resistance to Direct-Acting Antiviral Agents
- Different Modes of Retrovirus Restriction by Human APOBEC3A and APOBEC3G
- TNFα and IFNγ but Not Perforin Are Critical for CD8 T Cell-Mediated Protection against Pulmonary Infection
- Large Scale RNAi Reveals the Requirement of Nuclear Envelope Breakdown for Nuclear Import of Human Papillomaviruses
- The Cytoplasmic Domain of Varicella-Zoster Virus Glycoprotein H Regulates Syncytia Formation and Skin Pathogenesis
- A New Class of Multimerization Selective Inhibitors of HIV-1 Integrase
- Are We There Yet? The Smallpox Research Agenda Using Variola Virus
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
- Dynamic Functional Modulation of CD4 T Cell Recall Responses Is Dependent on the Inflammatory Environment of the Secondary Stimulus
- Bacterial Superantigens Promote Acute Nasopharyngeal Infection by in a Human MHC Class II-Dependent Manner
- Follicular Helper T Cells Promote Liver Pathology in Mice during Infection
- A Nasal Epithelial Receptor for WTA Governs Adhesion to Epithelial Cells and Modulates Nasal Colonization
- Unexpected Role for IL-17 in Protective Immunity against Hypervirulent HN878 Infection
- Human Cytomegalovirus Fcγ Binding Proteins gp34 and gp68 Antagonize Fcγ Receptors I, II and III
- Expansion of Murine Gammaherpesvirus Latently Infected B Cells Requires T Follicular Help
- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Molecular Signatures of Hemagglutinin Stem-Directed Heterosubtypic Human Neutralizing Antibodies against Influenza A Viruses
- The Downregulation of GFI1 by the EZH2-NDY1/KDM2B-JARID2 Axis and by Human Cytomegalovirus (HCMV) Associated Factors Allows the Activation of the HCMV Major IE Promoter and the Transition to Productive Infection
- Inactivation of Fructose-1,6-Bisphosphate Aldolase Prevents Optimal Co-catabolism of Glycolytic and Gluconeogenic Carbon Substrates in
- New Insights into Rotavirus Entry Machinery: Stabilization of Rotavirus Spike Conformation Is Independent of Trypsin Cleavage
- Prophenoloxidase Activation Is Required for Survival to Microbial Infections in
- SslE Elicits Functional Antibodies That Impair Mucinase Activity and Colonization by Both Intestinal and Extraintestinal Strains
- Timed Action of IL-27 Protects from Immunopathology while Preserving Defense in Influenza
- HIV-1 Envelope gp41 Broadly Neutralizing Antibodies: Hurdles for Vaccine Development
- The PhoP-Dependent ncRNA Mcr7 Modulates the TAT Secretion System in
- Cellular Superspreaders: An Epidemiological Perspective on HIV Infection inside the Body
- The Inflammasome Pyrin Contributes to Pertussis Toxin-Induced IL-1β Synthesis, Neutrophil Intravascular Crawling and Autoimmune Encephalomyelitis
- Papillomavirus Genomes Associate with BRD4 to Replicate at Fragile Sites in the Host Genome
- Integrative Functional Genomics of Hepatitis C Virus Infection Identifies Host Dependencies in Complete Viral Replication Cycle
- Co-assembly of Viral Envelope Glycoproteins Regulates Their Polarized Sorting in Neurons
- Targeting Membrane-Bound Viral RNA Synthesis Reveals Potent Inhibition of Diverse Coronaviruses Including the Middle East Respiratory Syndrome Virus
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



