-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Malaria Parasite Infection Compromises Control of Concurrent Systemic Non-typhoidal Infection via IL-10-Mediated Alteration of Myeloid Cell Function
Non-typhoidal Salmonella serotypes (NTS) most frequently cause diarrheal disease, which is self-limiting. However, in sub-Saharan Africa, NTS is one of the most common causes of life-threatening bloodstream infections. Individuals with these bloodstream infections frequently have an underlying condition such as HIV in adults or malaria in children. We used a mouse model to investigate why malaria predisposes children to invasive NTS infections. Our results implicate an anti-inflammatory response induced by malaria parasites via the cytokine IL-10 in promoting increased growth of bacteria that have disseminated from the intestine to other organs of the body. This response is beneficial in that it prevents death from malaria, but has an adverse effect on phagocytic cells, blocking their ability to control growth of bacteria that have disseminated from the intestine to other organs of the body.
Published in the journal: . PLoS Pathog 10(5): e32767. doi:10.1371/journal.ppat.1004049
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004049Summary
Non-typhoidal Salmonella serotypes (NTS) most frequently cause diarrheal disease, which is self-limiting. However, in sub-Saharan Africa, NTS is one of the most common causes of life-threatening bloodstream infections. Individuals with these bloodstream infections frequently have an underlying condition such as HIV in adults or malaria in children. We used a mouse model to investigate why malaria predisposes children to invasive NTS infections. Our results implicate an anti-inflammatory response induced by malaria parasites via the cytokine IL-10 in promoting increased growth of bacteria that have disseminated from the intestine to other organs of the body. This response is beneficial in that it prevents death from malaria, but has an adverse effect on phagocytic cells, blocking their ability to control growth of bacteria that have disseminated from the intestine to other organs of the body.
Introduction
In immunocompetent individuals, NTS serotypes are associated with gastroenteritis, a localized infection with low mortality that manifests as diarrhea, vomiting and intestinal cramping. However, immunocompromised individuals can develop life-threatening NTS bacteremia [1], [2]. Epidemiological associations suggest that the most common immunocompromising conditions predisposing to pediatric NTS bacteremia in sub-Saharan Africa are malnutrition and acute or recent malaria [1], [3]–[5]. The magnitude of the public health problem posed by NTS bacteremia is little publicized but it contributes considerably to morbidity and mortality in Sub-Saharan Africa [6]. For example, NTS are currently the most common blood isolates from children [3], [5], [7] and the second most common cause of pediatric meningitis in Malawi [8], resulting in mortality rates of approximately 50%, despite antibiotic therapy [9]. A factor complicating treatment of invasive NTS is the high prevalence of multidrug resistance [1], [10]–[13]. While the occurrence of NTS bacteremia in pediatric malaria patients is well documented, little is known about immunologic mechanisms underlying increased susceptibility to NTS bacteremia. This study was undertaken to identify mechanisms affecting the outcome of NTS infection in the setting of underlying malaria.
Results
Underlying P. yoelii infection leads to increased levels of systemic infection with S. Typhimurium, but reduced pyogenic inflammation
Since early studies on malaria patients demonstrated reduced responses to lipopolysaccharide (LPS) [14], and sensing of S. Typhimurium LPS via Toll-like receptor (TLR) 4 is crucial to control of NTS infection [15], we reasoned that malaria parasite infection might blunt innate immune responses required to control invasive bacteria. To test the idea that defective inflammatory responses in malaria could increase susceptibility to systemic infection, we used a mouse model to study the effects of malaria on inflammatory responses to NTS in a mouse strain (CBA) that was genetically resistant to lethal infection with both pathogens. To induce malaria in this model, mice were inoculated intraperitoneally (IP) with blood-stage Plasmodium yoelii subsp. nigeriensis (P. yoelii), a model for acute malaria. Maximal parasitemia and anemia were allowed to develop before intragastric (IG) inoculation with the NTS strain Salmonella enterica serotype Typhimurium 14028 (S. Typhimurium, Fig. 1A). Co-infection did not affect the levels of malaria parasite infection (Fig. 1B). However, co-infected mice exhibited increased bacterial loads of S. Typhimurium in the liver by 2 days post infection, as well as a more rapid increase in bacterial colonization between days 2 and 4, as compared to mice infected with S. Typhimurium alone (Fig. 1C). By 4 days post inoculation of S. Typhimurium, high numbers of bacteria were found in the blood of co-infected mice, while very few bacteria were detected in the circulation of mice infected only with S. Typhimurium (Fig. 1C). Co-infection with P. yoelii also resulted in elevated systemic colonization with a Malawian S. Typhimurium isolate, which belongs to a distinct sequence type circulating in sub-Saharan Africa [11] (Fig. S1 in Text S1). The rapid increase in systemic colonization of co-infected mice inoculated with S. Typhimurium by the IG route could result from increased intestinal permeability [16], as well as from increased replication of bacteria once they reach systemic sites. To distinguish between these possibilities, we performed an experiment in which mice were inoculated via the IP route with S. Typhimurium, to bypass the intestine (Fig. 1E and 1F). Co-infected mice inoculated IP with S. Typhimurium exhibited a similar rapid increase in bacteria within the liver (Fig. 1E) and blood (Fig. 1F) to mice inoculated via the IG route, suggesting that co-infection with malaria parasites promoted increased replication of S. Typhimurium at systemic sites.
Fig. 1. Increased systemic replication of S. Typhimurium (STm) during concurrent P. yoelii (Py) infection. 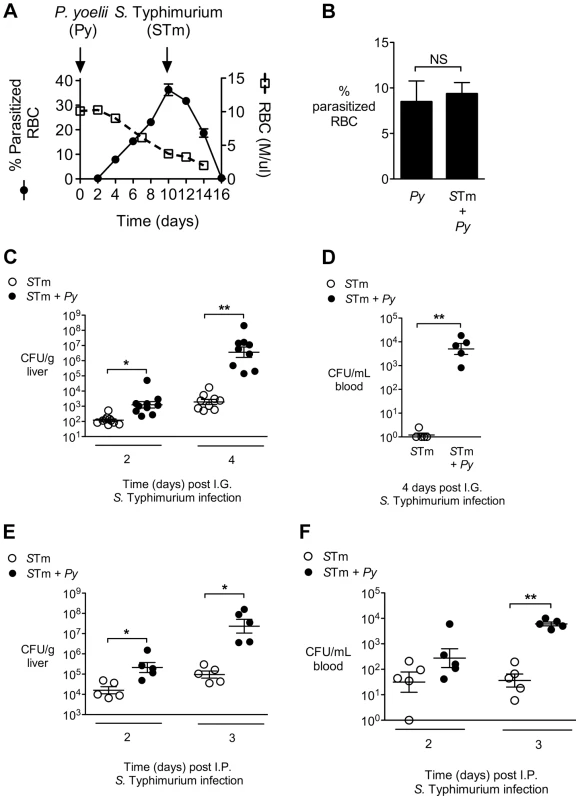
A, Parasitemia and anemia in P. yoelii-infected mice (n = 4). Arrows indicate time points at which P. yoelii and S. Typhimurium were inoculated. B, Comparison of parasitemia at 14 d after P. yoelii infection in P. yoelii-infected mice and mice co-infected for 4 d with S. Typhimurium (n = 5). Data are shown as mean ± SEM. C, Colonization (CFU) of the liver at 2 and 4 days after IG infection of P. yoelii-infected or uninfected CBA mice with S. Typhimurium (n = 5–10). Results are from 2 independent experiments. D, CFU of S. Typhimurium in the blood 4 days after IG infection of CBA mice (n = 5). Panels, B, C and D are compiled from the two independent experiments. E–F, CFU in liver (E) and blood (F) at 2 and 3 days after IP infection of P. yoelii-infected or uninfected CBA mice (n = 5). Dots represent individual mice and bars represent the mean ± SEM. Statistical significance was determined using an unpaired Student's t test on log-transformed values and is indicated as *, P<0.05; **, P<0.01. To determine whether underlying malaria affected inflammatory responses to disseminated NTS, histopathological comparison of inflammatory responses in the livers of S. Typhimurium-infected mice and mice co-infected with P. yoelii was performed. Despite nearly 10,000-fold higher bacterial numbers in the livers of the co-infected mice, this group had both significantly fewer microabscesses, and smaller-sized lesions, than mice infected only with S. Typhimurium. In a previous study, in which we inoculated mice simultaneously with S. Typhimurium and P. yoelii and evaluated inflammatory changes at d5 post infection, we found no effect of malaria parasite infection on S. Typhimurium-induced microabscess formation [17]. This difference between the previous study and our current results suggested that the blunting effect of malaria parasite infection on microabscess formation occurs later in the course of parasite infection. The blunting of microabscess formation observed in our current study suggested that a reduced inflammatory response to invasive S. Typhimurium in the liver could contribute to the increased bacterial load in this tissue during co-infection (Fig. 2A).
Fig. 2. Decreased neutrophil-mediated pathology in the liver of co-infected mice. 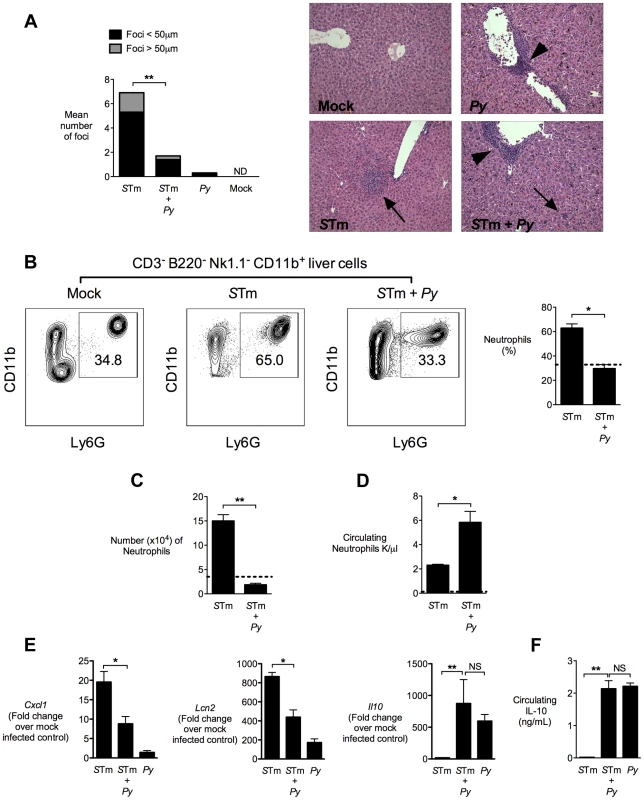
A, Quantification of inflammatory lesions in the liver and representative micrographs of H&E stained liver tissue from mock-infected mice, mice infected with P. yoelii, or co-infected mice 4 days after IG inoculation of S. Typhimurium in CBA mice (n = 5–10). Arrows represent pyogranulomatous lesions and arrowheads represent perivascular monocytic infiltrates. Results are from the experiment shown in figure 1B. B, Representative flow cytometry plots for neutrophil frequency in singlet live CD3− B220− NK1.1− CD11b+ liver cells. Right panel shows quantification of neutrophils from S. Typhimurium and co-infected mice (n = 4–5). Dotted line represents mock-infected mice (n = 2). The gating strategy used for generation of these results is shown in Fig. S2 in Text S1. C, Number of neutrophils (singlet live CD3− B220− NK1.1− CD11b+ Ly6G+) per 4×106 liver cells determined by AccuCount beads. Results are from data shown in figure 2D. D, Number of circulating neutrophils (K/µl) determined by complete blood counts. Results are from data shown in figure 2C. E, Expression of Cxcl1, Lcn2 and Il10 in liver tissue of CBA mice 4 days after IG inoculation with S. Typhimurium (n = 4–9). Data expressed as fold change over mock-infected control. Results are from the experiment shown in figure 1B. F, Circulating IL-10 measured 2 days after IG infection with S. Typhimurium. Results are from the experiment shown in figure 1B. Data bars represent the mean +SEM. Statistical significance was determined using an unpaired Student's t test (A–D, F) or ANOVA with Tukey's post test (E) on log-transformed values and is indicated as *, P<0.05; **, P<0.01. To determine whether the reduced size of inflammatory lesions was the result of decreased influx of neutrophils into the liver, we analyzed neutrophil infiltration by flow cytometry of cell suspensions from livers that were perfused with saline to remove circulating neutrophils (Fig. 2B and 2C). As expected, in livers of mice infected only with S. Typhimurium, a marked neutrophil influx was measured, compared to mock-infected controls. In contrast, in P. yoelii-infected mice, significantly fewer neutrophils were found in the liver after S. Typhimurium infection (Fig. 2B and 2C), which was consistent with the reduced inflammation observed by histopathology (Fig. 2A). The reduced infiltration of neutrophils into the liver of co-infected mice coincided with an increase in circulating neutrophils, compared to the mice infected with S. Typhimurium only (Fig. 2D). These results suggested that malaria parasite infection impaired recruitment of neutrophils that are needed to control S. Typhimurium infection. To test this idea, we measured expression of KC (Cxcl1), a CXC chemokine that recruits neutrophils to sites of infection (Fig. 2E). Cxcl1 expression was strongly reduced in the liver of P. yoelii co-infected mice, compared to mice infected with S. Typhimurium alone (Fig. 2E), suggesting that reduced chemokine expression may be a factor contributing to impaired neutrophil recruitment. Expression of a second inflammation-associated gene, Lcn2 encoding the siderophore-scavenging peptide Lipocalin-2, was also reduced in co-infected mice. This reduction of Lcn2 expression could reflect reduction in two different cellular sources of Lipocalin-2, since Lipocalin-2 is produced both by S. Typhimurium-infected macrophages [18] and by neutrophils during malaria parasite infection [19], [20]. While Salmonella-induced pathology was blunted by co-infection, other pathologic changes induced by P. yoelii, including perivascular inflammation, hepatomegaly and induction of Tumor necrosis factor alpha (Tnfa) remained intact, or were increased by co-infection, compared to infection with S. Typhimurium alone (Fig. 2 and Fig. S3 in Text S1).
IL-10 elicited by malaria parasite infection contributes to increased systemic colonization
In malaria, induction of the immunoregulatory cytokine interleukin-10 (IL-10) has been shown to prevent excessive and potentially fatal pathology in murine models of cerebral malaria [21]–[24], and this important role of IL-10 has been corroborated by observational data from malaria patients [25]–[28]. In mice infected with P. yoelii or mice co-infected with P. yoelii and S. Typhimurium, but not in mice infected with S. Typhimurium alone, high levels of circulating IL-10 were measured (Fig. 2F). Similarly, in the liver, induction of Il10 expression was observed in both P. yoelii-infected and co-infected mice, but not in mice infected only with S. Typhimurium (Fig. 2E). The similar levels of IL-10 detected in P. yoelii infection and co-infection indicated that the malaria parasites, rather than S. Typhimurium, were eliciting IL-10 production in this model (Fig. 2E and 2F).
To determine whether parasite-induced IL-10 contributed to the increased systemic loads of S. Typhimurium in co-infected mice, we blocked the effects of IL-10 using a neutralizing antibody (Fig. 3A). In co-infected mice, neutralization of IL-10 reduced bacterial loads in both the liver and in the blood, suggesting a contribution of this cytokine to the increased systemic levels of S. Typhimurium during co-infection. In contrast, in mice infected with S. Typhimurium only, IL-10 depletion had no effect on bacterial colonization, suggesting that the effect of IL-10 blocking in this experiment was specific to co-infection (Fig. 3A).
Fig. 3. IL-10 contributes to increased systemic loads of S. Typhimurium in malaria parasite-infected CBA mice. 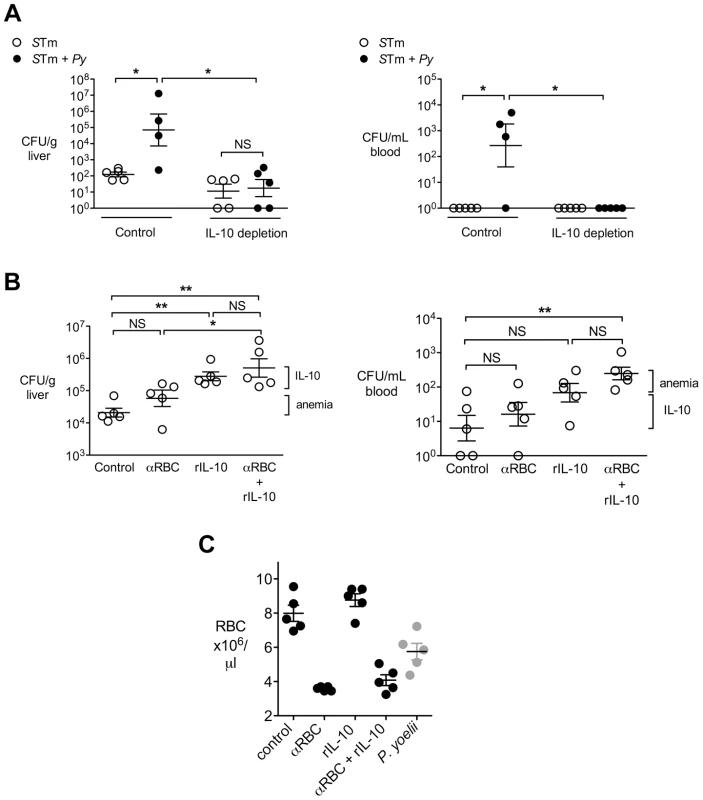
A, S. Typhimurium and co-infected CBA mice as described in Fig. 1A, were depleted of IL-10 using a blocking antibody, or treated with a control antibody. At 2 d post-S. Typhimurium infection, CFU of S. Typhimurium were enumerated in liver and blood (n = 4–5). Statistical significance was determined using an unpaired Student's t test on log-transformed values, or a Mann-Whitney U test for groups that include all zero values, and is indicated as *, P<0.05; **, P<0.01. B, Mice were treated with either an anti-RBC antibody to induce anemia, recombinant IL-10 (rIL-10) or both. At 4 days post-S. Typhimurium infection, CFU were enumerated in liver and blood (n = 5). Dots represent individual mice and bars represent the mean ± SEM. Brackets indicate the contributions of anemia and IL-10 to increased bacterial colonization. C, Levels of anemia in experimental groups from (B) at 4 d post IG infection. The number of circulating RBC in mice infected with P. yoelii at 14 d after inoculation is given as a reference. Statistical significance was determined using one-way ANOVA with a Tukey's post test *, P<0.05; **, P<0.01. IL-10 and anemia act together to increase susceptibility to systemic S. Typhimurium infection
In the experiments shown above, mice infected with P. yoelii developed severe anemia (Fig. 1A) as well as high IL-10 levels (Fig. 2F). Studies in Africa that established a link between NTS bacteremia and malaria reported an association between pediatric NTS bacteremia and severe malarial anemia or recent malaria [3], [29], [30]. To determine whether parasite-induced anemia could have an additive effect with parasite-induced IL-10, we determined the effects of anemia and IL-10, both individually and in combination, on susceptibility to systemic S. Typhimurium infection (Fig. 3B). Mice were administered recombinant IL-10 (rIL-10), or anti-red blood cell (α-RBC) IgG to induce anemia, or received both treatments. All mice treated with α-RBC antibodies developed severe anemia comparable to that induced by P. yoelii infection (Fig. 3C). However, when compared to mock-treated controls, a significant increase in bacteremia was observed only in mice treated with both rIL-10 and α-RBC antibodies (Fig. 3B, right panel). Similarly, compared to treatment with α-RBC antibodies, an additive effect of IL-10 treatment was observed on colonization of the liver (Fig. 3B, left panel). This interpretation of an additive effect of both factors would be consistent with our previous results suggesting that induction of anemia resulted in lower levels of NTS bacteremia than parasite infection [17].
IL-10 elicited by malaria parasite infection blunts hepatic inflammatory responses induced by Salmonella
To determine the effect of IL-10 on inflammation induced by S. Typhimurium, we used mice deficient for Il10, which were on a C57BL/6 strain background. An important difference between the C57BL/6 strain and the CBA strain used for our previous experiments is that C57BL/6 mice have a defective Slc11a1 (also known as Nramp1) allele, which reduces the ability of resident<1?tlb 10.5pt?> macrophages to control systemic S. Typhimurium infection [31]–[35]. Experiments with P. chabaudi showed that CBA and C57BL/6 mice are both able to control malaria parasite infection, therefore use of the C57BL/6 strain was not expected to alter susceptibility to NTS bacteremia via an effect on malaria parasite infection [36].
Co-infection of C57BL/6 mice, with the same dose of bacteria and parasites used in CBA mice, resulted in 10–100-fold higher levels of S. Typhimurium in the liver at 2d after inoculation with S. Typhimurium, compared to CBA mice (Fig. 4A and Fig. 1B). In this set of experiments, mice progressed rapidly to lethal morbidity between days 2–3. Further, at 2d post infection, in contrast with the CBA mice, no difference in colonization with S. Typhimurium was observed in the liver of C57BL/6 mice between groups infected only with S. Typhimurium and co-infected with P. yoelii and S. Typhimurium (Fig. 4A). Compared to control mice co-infected with P. yoelii, the Il10−/− mice had reduced loads of S. Typhimurium in the liver (Fig. 4A). Since the levels of bacterial colonization between S. Typhimurium-infected mice and malaria parasite co-infected mice were similar, we examined the effect of malaria parasite co-infection on inflammatory responses to S. Typhimurium in the liver. In a similar manner to what was observed in CBA mice (Fig. 2), the co-infected C57BL/6 mice exhibited blunting of Cxcl1 and Lcn2 expression (Fig. 4B). However in co-infected Il10−/− mice this blunting of proinflammatory cytokine expression was absent (Fig. 4C). As reported previously for P. chabaudi infection [37], the co-infected Il10−/− mice had lower P. yoelii parasitemia (Fig. 4D), suggesting that part of the effect of IL-10 deficiency on inflammatory responses may be an indirect effect of reduced parasite infection. These results suggested that induction of Il10 expression by malaria parasite infection led directly or indirectly to blunting of inflammatory responses elicited by S. Typhimurium in the liver.
Fig. 4. IL-10 contributes to blunting of neutrophil chemokines in livers of malaria parasite-infected C57BL/6 mice. 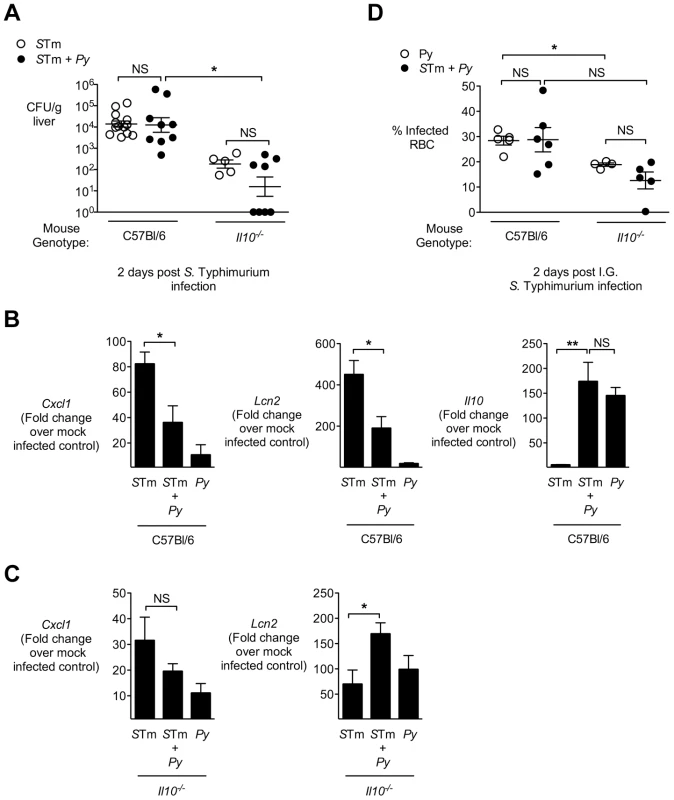
A, Mice deficient for IL-10 (Il10−/−) or controls (C56BL/6) were infected with either S. Typhimurium or co-infected as described in Fig. 1A. At 2 days post-S. Typhimurium infection, CFU of S. Typhimurium was enumerated in liver (n = 5–13). Dots represent individual mice. B, Expression of Cxcl1, Lcn2 and Il10 in liver tissue of control mice, 2 days after S. Typhimurium infection (n = 5–13). Results are from data shown in figure 4A. C, Expression of Cxcl1 and Lcn2 in liver tissue of Il10−/− mice, 2 days after S. Typhimurium infection (n = 5–8). Results are from data shown in figure 4A. D, Effect of co-infection with S. Typhimurium in C57BL/6 and Il10−/− mice on malaria parasitemia. C57BL/6 and Il10−/− and mice were inoculated IP with blood stage P. yoelii, then at day 10, were inoculated IG with S. Typhimurium. Parasitemia was determined at day 12 (2 days after S. Typhimurium infection). Bars represent the mean +SEM (n = 4–6). Data from control mice are compiled from 3 independent experiments and Il10−/− from 1 experiment. Statistical significance was determined using an unpaired Student's t test (A, D) or one-way ANOVA with Tukey's post test (B, C) on log-transformed values and is indicated as *, P<0.05; **, P<0.01. Malaria parasite-induced IL-10 acts on myeloid cells to increase systemic NTS infection
S. Typhimurium is found within liver macrophages (also known as Kupffer cells) during infection [38], and these cells play an important role in control of systemic S. Typhimurium burden [15], [39]. We therefore hypothesized that the IL-10-dependent increase in systemic colonization of S. Typhimurium during co-infection could result from an effect of IL-10 on resident macrophages [40]. To determine whether macrophage responsiveness to IL-10 promotes disseminated NTS infection, we bred mice that were deficient for IL-10 receptor (IL-10R) expression on monocytes/macrophages and neutrophils (LysM-cre+/−×IL10R1flox/flox) on a C57BL/6 strain background. Groups of LysM-cre−/− littermate controls and LysM-cre+/− (conditionally IL-10R-deficient) mice were then inoculated with S. Typhimurium by the IP route or co-infected with P. yoelii and S. Typhimurium. Since our earlier results showed that the co-infected C57BL/6 mice experienced rapid lethal morbidity and high systemic loads of S. Typhimurium (Fig. 4), we reduced the inoculum of both S. Typhimurium and P. yoelii in these experiments, as described in the Materials and Methods. At 2 days post S. Typhimurium infection, the LysM-cre−/− controls had significantly more bacteria in both the liver (Fig. 5A) and in the blood (Fig. 5B). In contrast, co-infection with P. yoelii had no effect on the ability of the mice deficient for IL-10R expression in myeloid cells to control systemic S. Typhimurium infection (Fig. 5A and 5B). The improved control of systemic S. Typhimurium infection in the conditionally IL-10R-deficient mice did not appear to be an indirect effect of reduced parasitemia, since at day 14 post P. yoelii infection, no significant difference in parasitemia was observed between the LysM-cre+/− and the LysM-cre−/− control groups (Fig. 5C).
Fig. 5. Malaria parasite-induced IL-10 acts on myeloid cells. 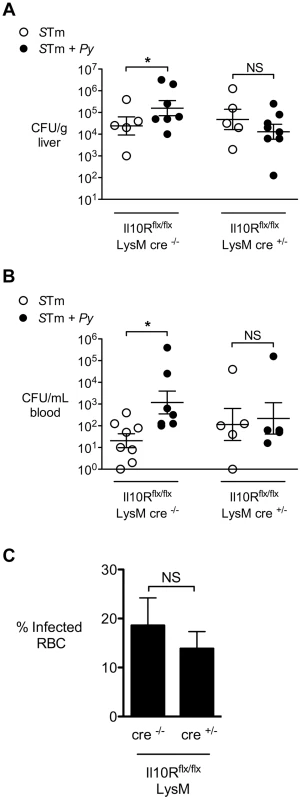
Mice conditionally deficient for IL-10 receptor (Il10R) on myeloid cells (Il10Rf/f LysM-cre) or Cre-negative littermate controls were infected with S. Typhimurium or co-infected with S. Typhimurium and P. yoelii. Bacterial colonization was assessed 2 days after IP inoculation with S. Typhimurium and 12 days after P. yoelii inoculation (n = 5–8). A, Colonization of liver tissue by S. Typhimurium. B, S. Typhimurium bacteremia. C, Parasitemia in Cre+/− and Cre−/− mice co-infected with P. yoelii and S. Typhimurium. Dots represent individual mice and bars represent the mean ± SEM. Data are compiled from 2 independent experiments. Statistical significance was determined using an unpaired Student's t test on log-transformed values and is indicated as *, P<0.05; **, P<0.01. Concurrent malaria parasite infection alters gene expression in tissue macrophages
During chronic infection S. Typhimurium has been found to persist preferentially within M2, or alternatively activated macrophages [41], as well as in hemophagocytic macrophages expressing M2 activation markers [42], therefore we tested the idea that malaria might polarize liver macrophages to an M2 phenotype. To this end, we analyzed expression profiles of alternative activation markers in the CD11b+ cell-enriched liver fraction of CBA mice infected IG with S. Typhimurium only, or co-infected with S. Typhimurium at 14 d after P. yoelii infection. At 4 d after IG S. Typhimurium infection, macrophages were positively selected from liver homogenates using CD11b-immmunomagnetic beads (Fig. 6). The CD11b+ fraction from co-infected mice contained 50-fold more bacteria than in mice infected with S. Typhimurium only, suggesting that at least part of the increased bacterial load in the liver is associated with macrophages (Fig. 6A).
Fig. 6. Liver CD11b+ macrophages exhibit increased association with S. Typhimurium and an altered phenotype during malaria parasite co-infection. 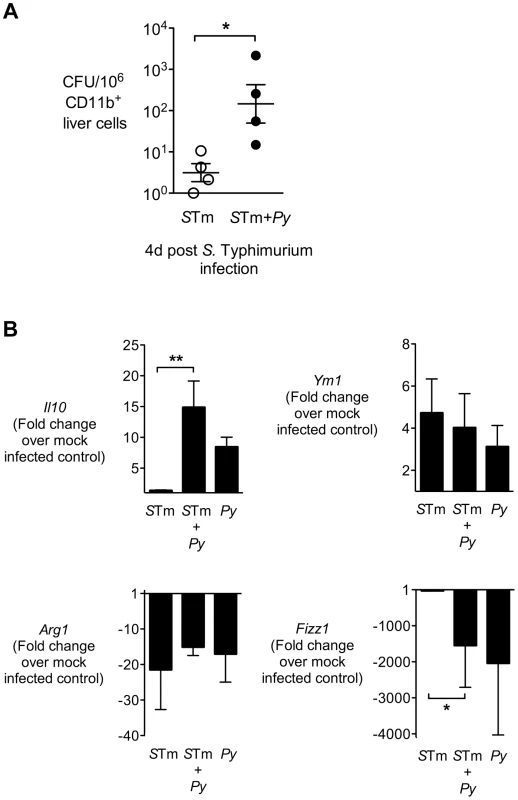
A, CD11b+ liver cells were enriched by positive selection from S. Typhimurium-infected and co-infected CBA mice at 4 days post-S. Typhimurium infection (n = 4). CFU of S. Typhimurium was enumerated from 106 CD11b+ liver cells. CD11b+ liver cells, B, Expression of Il10, Ym1, Fizz1, and Arg1 in CD11b+ liver cells was determined by qRT-PCR. Data are expressed as fold change over mock-infected control. All mice were co-infected as described in Fig. 1A. Dots represent individual mice and bars represent the mean ± SEM. Statistical significance was determined using an unpaired Student's t test (A) or one-way ANOVA with Tukey's post test (B) on log-transformed values and is indicated as *, P<0.05; **, P<0.01. Analysis of gene expression in the CD11b+ cell fraction revealed these cells did not have elevated expression of typical alternatively activated macrophage markers such as Fizz1, Arg1, or Ym1 (Fig. 6B) as well as mannose receptor (not shown). In contrast, Il10, a marker of regulatory macrophages, was expressed more highly in the CD11b+ liver cell fraction of mice infected with P. yoelii or co-infected with P. yoelii and S. Typhimurium (Fig. 6B), suggesting that malaria may polarize liver macrophages to a M2-like regulatory phenotype [43].
Macrophages are a source of IL-10 during co-infection
To determine the importance of macrophage IL-10 production for malaria-mediated susceptibility to disseminated infection, we bred mice that were conditionally deficient for IL-10 production by cells of the myeloid lineage, which includes neutrophils and monocytes/macrophages. To this end, C57BL/6 mice expressing a LysM-cre allele (specific for myeloid cells) were bred to mice homozygous for a floxed Il10 allele (IL10flox/flox) [44]. Conditionally IL-10 deficient mice, or their respective Cre-negative littermate controls were co-infected with S. Typhimurium and P. yoelii as described above (Fig. 7). At 2 days post IP infection with S. Typhimurium, the co-infected, Cre-negative littermate controls had on average 50-fold higher (P = 0.08) loads of S. Typhimurium in the liver compared to mice infected with S. Typhimurium only, and a 1000-fold higher burden of S. Typhimurium in the circulation (P<0.05; Fig. 7A). In contrast, in the cre-positive mice that were conditionally deficient for IL-10 production by macrophages and neutrophils, no difference in S. Typhimurium colonization was observed between co-infected and S. Typhimurium-infected groups of mice. Unlike what we observed with completely IL-10 deficient mice (Fig. 4D), the improved ability of the conditionally IL-10 deficient mice to control systemic S. Typhimurium infection could not be attributed to an indirect effect of a reduction in P. yoelii infection, since the conditionally deficient mice actually had a nonsignificant increase in circulating parasites (Fig. 7B). Elimination of macrophage/neutrophil IL-10 production led to a striking reduction of Il10 expression in the liver of co-infected mice, indicating that in this tissue, LysM-expressing cells are the major producers of IL-10 (Fig. 7C). Together, these results suggested that malaria parasite-induced production of IL-10 by macrophages and/or neutrophils is a mechanism that compromises control of systemic bacterial infection in the co-infected mice.
Fig. 7. Macrophage/neutrophil derived IL-10 contributes to increased systemic S. Typhimurium burden during concurrent malaria parasite infection. 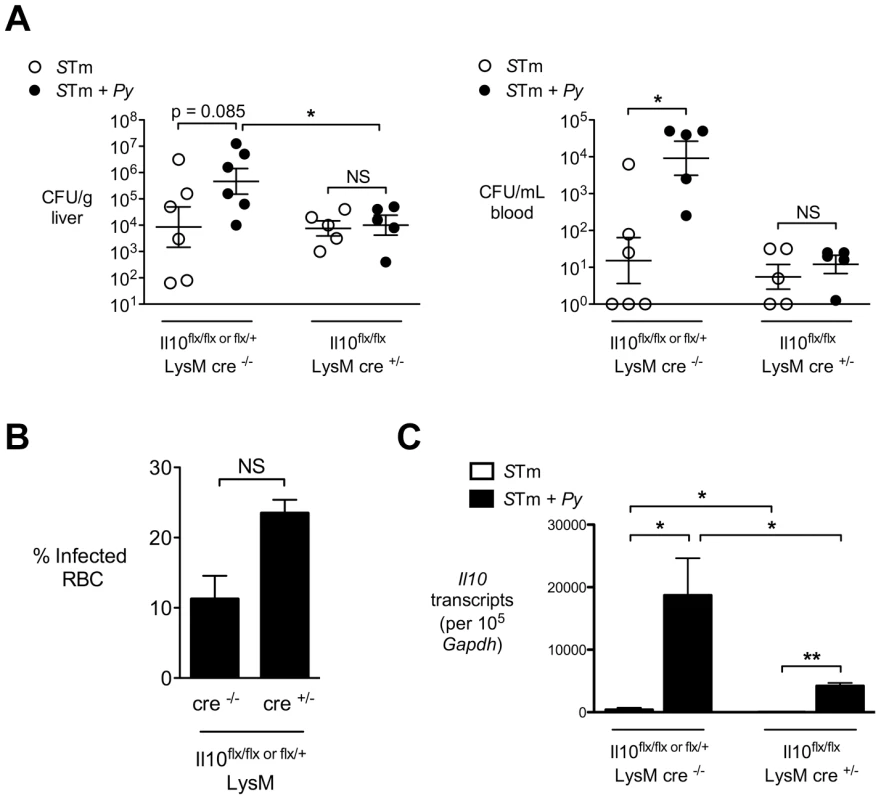
Mice conditionally deficient for IL-10 expression on myeloid cells (Il10f/f LysM-cre) or Cre-negative littermate controls were infected with S. Typhimurium or co-infected with S. Typhimurium and P. yoelii. Bacterial colonization was assessed 2 days after IP inoculation with S. Typhimurium and 12 days after P. yoelii inoculation (n = 5–6). A, Colonization of liver tissue (left panel) and blood (right panel) by S. Typhimurium. B, Parasitemia in Cre+/− and Cre−/− mice co-infected with P. yoelii and S. Typhimurium C,. Expression of Il10 in livers of Il10f/f LysM-cre mice or littermate controls at 12 after P. yoelii infection and 2 days after S. Typhimurium infection. Bars represent the mean +SEM (n = 5). Dots represent individual mice and bars represent the mean ± SEM. Data are compiled from 2 independent experiments. Statistical significance was determined using an unpaired Student's t test on log-transformed values and is indicated as *, P<0.05; **, P<0.01. Since the LysM-cre allele used to delete Il10 in the conditional knockout mice is expressed in both monocytes/macrophages and neutrophils our results suggested that production of IL-10 by both cell types could compromise immunity to S. Typhimurium in the mouse. However, the lack of neutrophil influx into tissues of S. Typhimurium and P. yoelii-coinfected CBA mice (Fig. 2B) as well as a lack of Il10 induction in bone marrow neutrophils during P. yoelii infection (Fig. S4 in Text S1) suggest that macrophage-derived IL-10 is more important than neutrophil-derived IL-10 in suppressing control of systemic S. Typhimurium infection in the malaria parasite-infected mice.
In the context of murine malaria models, CD4 T cells were shown to be an important cellular source of immunomodulatory IL-10 [37], [45]. We therefore asked whether IL-10 produced by CD4 T cells contributed to increased infection with S. Typhimurium. To this end, IL10flox/flox×CD4-cre mice (conditionally deficient for IL-10 production by T cells; [46]), and their respective littermate controls were co-infected with S. Typhimurium and P. yoelii as described above. Similar to a previous report [37], mice conditionally for IL-10 production by CD4 T cells had a trend for lower parasitemia during co-infection (Fig. S5A in Text S1). However in the liver, the CD4 T cell-specific defect in IL-10 had no effect on expression of Il10 in tissue, suggesting that at this site, T cells are not a major source of IL-10 during co-infection. (Fig. S5B in Text S1). Consistent with this result, IL-10 production by CD4 T cells had no effect on control S. Typhimurium infection during malaria co-infection (Fig. S5C in Text S1).
In summary, our results show that IL-10 induced by malaria parasite infection acts on phagocytic cells to shift their activation state toward a regulatory, IL-10 producing phenotype. As a result, these cells lose their ability to control S. Typhimurium infection. This compromise of tissue macrophage function may contribute to the increased susceptibility of individuals with malaria to disseminated NTS infection.
Discussion
The results of our study show that in the context of malaria parasite infection, the host's ability to control systemic S. Typhimurium infection is compromised. One factor contributing to increased infection levels of S. Typhimurium during co-infection with malaria is induction of IL-10. In murine malaria models, the protective role of IL-10 in dampening excessive inflammation is well-established [21]–[23]. The sources of immunomodulatory IL-10 in the P. yoelii and P. chabaudi malaria models are populations of CD4 T cells [17], [37], [45].
Thus, in murine malaria models, production of IL-10 serves to limit damage caused by the inflammatory response to malaria parasites. Induction of IL-10 expression by intestinal helminths has also been shown to have beneficial effects by inhibiting colitis in a murine model of inflammatory bowel disease [47]. While in the context of co-infection with malaria parasites and NTS, immunoregulation by IL-10 could prevent inflammatory pathology and resulting organ damage, a clear downside of this regulation is a reduced ability to limit bacterial burden at systemic sites (Fig. 2). An important target of parasite-induced IL-10 was cells of the myeloid lineage, most likely macrophages. A shift was observed in the phenotype of macrophages within the liver toward a population that produced IL-10, which would be consistent with a subset of M2 or alternatively activated macrophages [43], [48]. IL-10 production by macrophages was also an essential determinant of susceptibility to systemic infection (Fig. 7 and Fig. S6 in Text S1), suggesting that in the context of co-infection, macrophages both produce and respond to IL-10. These results are consistent with a previous study that identified IL-10 producing macrophages in the spleen during P. chabaudi infection [37]. Previous studies have implicated heme, released via lysis of infected erythrocytes, or hemozoin, the product of hemoglobin degradation by malaria parasites, in induction of IL-10 expression by macrophages, but the mechanism by which this occurs is unknown [49], [50].
Since S. Typhimurium is an intracellular pathogen that resides within macrophages during infection, IL-10 produced by macrophages might act locally on tissue macrophages to generate a more permissive niche for intracellular S. Typhimurium infection. Both alternatively activated macrophages and hemophagocytic macrophages express an M2 phenotype that renders them more permissive for intracellular replication of S. Typhimurium [41], [42], [51], suggesting that in the setting of malaria, IL-10 induced by parasite infection could contribute to a shift in macrophage activation state, thereby providing a more favorable environment for S. Typhimurium infection. At the cellular level, multiple effects of the tissue cytokine environment could promote increased intracellular S. Typhimurium infection. For example, compared to M1 or classically activated macrophages, M2 macrophages undergo a metabolic shift that increases intracellular glucose availability, and this has been shown to promote intracellular growth of both S. Typhimurium and Brucella abortus [41], [52]. Further, in vitro studies have shown that blocking of IL-10 in cultured macrophages leads to an increased localization of two other intracellular pathogens, Mycobacterium tuberculosis and Brucella abortus, to LAMP1-positive compartments, and this corresponded with reduced intracellular replication of these bacteria [53], [54]. These studies, together with a study demonstrating increased phagolysosomal fusion of S. Typhimurium-containing vacuoles after IL-10 neutralization in trophoblast-like cell cultures [55], suggest that in vivo, IL-10 induced by malaria parasite infection might alter membrane traffic in tissue macrophages as well. It will be interesting to perform a more detailed characterization of functional changes to the macrophage population in vivo that are induced by malaria parasite infection.
This IL-10 dependent defect in macrophage function is likely to act together with additional immune defects caused by malaria. Recently it was shown by Cunnington et al., that hemolysis caused by malaria parasite infection suppresses neutrophil function [56], [57]. Neutrophils play an important role in controlling systemic Salmonella infection, especially in the liver [58]. S. Typhimurium replication within macrophages induces pyroptosis, a form of inflammatory cell death, resulting in its release from macrophages [59]–[61]. Uptake and killing of these extracellular bacteria by neutrophils is critical in preventing their spread to other cells [62]. Thus IL-10-induced suppression of neutrophil recruitment and macrophage function likely synergize with this neutrophil defect to increase susceptibility to disseminated NTS infection in the setting of malaria (Fig. S6 in Text S1). Additional parasite-induced changes to host physiology that have been documented in malaria, including depletion of complement and reduction in intestinal barrier function, may also play a role in promoting systemic NTS disease [16], [63], [64].
In conclusion, our results provide evidence that malaria parasite-induced IL-10, a response that limits pathology in the context of malaria, compromises innate immune responses to S. Typhimurium, a prevalent invasive bacterial infection, by compromising the function of phagocytic cells. Further studies will be needed to determine whether this mechanism occurs in human malaria patients and whether it compromises responses to other invasive pathogens.
Materials and Methods
Additional methods are provided in Text S1.
Ethics statement
Experiments with mice were carried out in strict accordance with the recommendations in the Guide for Care and Use of Laboratory Animals of the National Institute of Health and were approved by the Institutional Animal Care and Use Committees at the University of California at Davis under protocols 15702 and 16597.
Mouse strains
Specific pathogen free 6–8 week-old female CBA/J, C57BL/6J and C57BL/6J IL10−/− (B6.129P2-Il10tm1Cgn/J; [65]) mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). Mice with a floxed Il10 allele (B6-Il10tm3Cgn; [44]), Il10R allele (B6-Il10ratm1.1Jack, [66]) and LysM-cre mice or CD4-cre mice, [67] used to generate mice conditionally deficient for synthesis of IL-10 or IL-10R1, were described previously. For all conditionally deficient mice, Cre-negative littermates carrying the floxed allele of interest were used as controls. Mice were maintained under specific pathogen-free conditions by the UC Davis Center for Laboratory Animal Science, receiving irradiated rodent chow and sterile drinking water ad libitum. IL10-deficient mice were evaluated before use for signs of inflammatory bowel disease, and mice with rectal prolapse, abnormal fecal pellets or failure to gain weight at the normal rate for C57BL/6 mice were excluded from the study. Whole blood was collected with heparinized syringes and complete blood counts were analyzed by the UC Davis Comparative Pathology Laboratory.
Plasmodium yoelii nigeriensis (P. yoelii)
Parasite stocks were obtained from the Malaria Research and Reference Reagent Resource and the species was confirmed by DNA sequencing of merozoite surface protein-1 (MSP-1) [17]. Parasite stocks were made by passage in CD-1 mice. For co-infection experiments in CBA and C57BL/6, mice were inoculated intraperitoneally (IP) on day 0 with approximately 4×107 infected red blood cells (iRBCs) in 0.1 ml of saline. For co-infection experiments in conditionally deficient strains, mice were inoculated with approximately 4×106 iRBCs. Mock-infected controls were injected IP with an equivalent amount of blood from uninfected CD-1 mice.
Salmonella enterica serotype Typhimurium
S. Typhimurium strain IR715 (pHP45Ω), resistant to nalidixic acid, ampicillin and streptomycin, was used for this study [68], [69]. To ensure consistent infection of resistant CBA mice with S. Typhimurium, intragastrically (IG) inoculated mice received 20 mg of streptomycin (Sigma) by gavage 24 h prior to infection. Mice received either 0.1 ml of sterile Luria-Bertani (LB) broth or 1×108 colony forming units (CFU) of Salmonella in 0.1 ml of LB broth by gastric gavage. Inocula were cultured for 16 h aerobically with selective pressure (50 mg/L carbenicillin) at 37°C. For some experiments, S. Typhimurium strain D23580, a multidrug-resistant bloodstream isolate from a Malawian child with malaria and NTS bacteremia [11], was used. For IP inoculation, mice received 100–500 CFU of S. Typhimurium in phosphate-buffered saline (PBS) or an equivalent volume of PBS.
Histopathology
Histological samples were collected at the time of necropsy. 5 µm sections were cut from formalin fixed paraffin embedded tissues and stained with hematoxylin and eosin. A trained pathologist (MNX) performed histopathology scoring in a blinded fashion to quantify inflammatory lesions in liver tissue.
In vivo antibody-mediated anemia and administration of recombinant IL-10
Uninfected mice were administered IP either 600 µg of rabbit anti-mouse RBC IgG (Rockland) in 0.2 ml PBS at Days 4, 6, 10 and 11 or 3 µg of mouse IL-10 recombinant protein (eBioscience) in 0.1 ml PBS every 12 hours starting at Day 8.5 to Day 13.5, or both treatments combined. Control mice received 600 µg of non-specific rabbit IgG (Rockland) in 0.2 ml PBS, or 0.1 ml PBS concurrently.
Liver CD11b+ cell isolation
Cells were isolated using CD11b MicroBeads (Miltenyi Biotec) according to the manufacturer's protocol. Briefly, gallbladders were removed aseptically, and livers were digested with collagenase type II to obtain a single cell suspension. Hepatocytes were removed by centrifugation and CD11b+ cells were positively selected by a magnetic column.
Flow cytometry
Flow cytometry analysis was performed for the detection of neutrophils in livers from S. Typhimurium and co-infected CBA mice 4 days post IG bacterial infection. Single cell suspensions of liver tissue were obtained according to protocol described by Miltenyi Biotec for the preparation of single-cell suspensions from mouse liver. Briefly, livers were perfused with 10 mL of PBS (GIBCO) by cardiac puncture, gallbladders were removed aseptically, and livers were digested with collagenase type IV for 30 minutes at 37°C. Tissue was dissociated using a gentleMACS Dissociator (Miltenyi Biotec) and passed through a 70 µm filter. Hepatocytes were removed by centrifugation and red blood cells were lysed using ACK buffer (Lonza). Liver cells were resuspended in PBS and counted. 4×106 cells/mouse were resuspended in 2 mL of PBS and stained with Aqua Live/Dead cell discriminator (Invitrogen) according to the manufacturer's protocol. After Live/Dead staining, cells were washed with PBS and resuspended in 50 µL of PBS containing 1% bovine serum albumin and 2 mM EDTA (fluorescence-activated cell sorter [FACS] buffer). Cells were stained with a FC receptor blocking antibody, anti-CD16/CD32 (eBioscience), for 5 minutes at 4°C and then stained with a cocktail of anti-B220 PE (BD Biosciences), anti-CD3 PE (BD Biosciences), anti-NK1.1 PE (Biolegend), anti-CD11b PECy7 (eBioscience), and Ly6G PerCp/Cy5.5 (Biolegend) for 30 minutes at 4°C. Cells were washed in FACS buffer, fixed with 4% formaldehyde for 30 minutes at 4°C, and then resuspended in FACS buffer. 50 µL of SPHERO AccuCount Fluorescent Particles 10.1 µm (Spherotech) were added to each sample prior to analysis in order to determine absolute counts. Calculation of absolute counts was performed according to manufacturer's protocol. Flow cytometry analysis was performed using an LSRII apparatus (Becton Dickinson), and 7.5×105 events were collected per mouse. Resulting data were analyzed using FlowJo software (Treestar, inc. Ashland, OR). Gates were based on Fluorescence-Minus-One (FMO) controls.
Statistical analysis
The statistical significance of differences between groups was determined by a Student's t test or one-way ANOVA on logarithmically transformed data. For data sets in which all values were the same (zero), statistical significance was determined by a Mann-Whitney U test. A P value of 0.05 or less was considered to be significant. All data were analyzed using two-tailed tests.
Supporting Information
Zdroje
1. FeaseyNA, DouganG, KingsleyRA, HeydermanRS, GordonMA (2012) Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379 : 2489–2499.
2. GordonMA (2008) Salmonella infections in immunocompromised adults. J Infect 56 : 413–422.
3. BronzanRN, TaylorTE, MwenechanyaJ, TemboM, KayiraK, et al. (2007) Bacteremia in Malawian children with severe malaria: prevalence, etiology, HIV coinfection, and outcome. J Infect Dis 195 : 895–904.
4. NielsenMV, SarpongN, KrumkampR, DekkerD, LoagW, et al. (2012) Incidence and characteristics of bacteremia among children in Rural Ghana. PLoS One 7: e44063.
5. WalshAL, PhiriAJ, GrahamSM, MolyneuxEM, MolyneuxME (2000) Bacteremia in febrile Malawian children: clinical and microbiologic features. Pediatr Infect Dis J 19 : 312–318.
6. ReddyEA, ShawAV, CrumpJA (2010) Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis 10 : 417–432.
7. SigauqueB, RocaA, MandomandoI, MoraisL, QuintoL, et al. (2009) Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J 28 : 108–113.
8. MolyneuxEM, WalshAL, MalengaG, RogersonS, MolyneuxME (2000) Salmonella meningitis in children in Blantyre, Malawi, 1996–1999. Ann Trop Paediatr 20 : 41–44.
9. MolyneuxEM, MankhamboLA, PhiriA, GrahamSM, ForsythH, et al. (2009) The outcome of non-typhoidal salmonella meningitis in Malawian children, 1997–2006. Ann Trop Paediatr 29 : 13–22.
10. KariukiS, RevathiG, KariukiN, KiiruJ, MwituriaJ, et al. (2006) Characterisation of community acquired non-typhoidal Salmonella from bacteraemia and diarrhoeal infections in children admitted to hospital in Nairobi, Kenya. BMC Microbiol 6 : 101.
11. KingsleyRA, MsefulaCL, ThomsonNR, KariukiS, HoltKE, et al. (2009) Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res 19 : 2279–2287.
12. OkoroCK, KingsleyRA, ConnorTR, HarrisSR, ParryCM, et al. (2012) Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat Genet 44 : 1215–1221.
13. OundoJO, MuliF, KariukiS, WaiyakiPG, IijimaY, et al. (2002) Non-typhi salmonella in children with severe malaria. East Afr Med J 79 : 633–639.
14. PerryJA, OlverCS, BurnettRC, AveryAC (2005) Cutting edge: the acquisition of TLR tolerance during malaria infection impacts T cell activation. J Immunol 174 : 5921–5925.
15. Vazquez-TorresA, VallanceBA, BergmanMA, FinlayBB, CooksonBT, et al. (2004) Toll-like receptor 4 dependence of innate and adaptive immunity to Salmonella: importance of the Kupffer cell network. J Immunol 172 : 6202–6208.
16. ChauJY, TiffanyCM, NimishakaviS, LawrenceJA, PakpourN, et al. (2013) Malaria-associated L-arginine deficiency induces mast cell-associated disruption to intestinal barrier defenses against nontyphoidal Salmonella bacteremia. Infect Immun 81 : 3515–3526.
17. RouxCM, ButlerBP, ChauJY, PaixaoTA, CheungKW, et al. (2010) Both hemolytic anemia and malaria parasite-specific factors increase susceptibility to Nontyphoidal Salmonella enterica serovar typhimurium infection in mice. Infect Immun 78 : 1520–1527.
18. NairzM, FritscheG, BrunnerP, TalaszH, HantkeK, et al. (2008) Interferon-gamma limits the availability of iron for intramacrophage Salmonella typhimurium. Eur J Immunol 38 : 1923–1936.
19. MohammedAO, ElghazaliG, MohammedHB, ElbashirMI, XuS, et al. (2003) Human neutrophil lipocalin: a specific marker for neutrophil activation in severe Plasmodium falciparum malaria. Acta Trop 87 : 279–285.
20. ZhaoH, KonishiA, FujitaY, YagiM, OhataK, et al. (2012) Lipocalin 2 bolsters innate and adaptive immune responses to blood-stage malaria infection by reinforcing host iron metabolism. Cell Host Microbe 12 : 705–716.
21. KossodoS, MonsoC, JuillardP, VeluT, GoldmanM, et al. (1997) Interleukin-10 modulates susceptibility in experimental cerebral malaria. Immunology 91 : 536–540.
22. LiC, CorralizaI, LanghorneJ (1999) A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect Immun 67 : 4435–4442.
23. LinkeA, KuhnR, MullerW, HonarvarN, LiC, et al. (1996) Plasmodium chabaudi chabaudi: differential susceptibility of gene-targeted mice deficient in IL-10 to an erythrocytic-stage infection. Exp Parasitol 84 : 253–263.
24. KobayashiF, MoriiT, MatsuiT, FujinoT, WatanabeY, et al. (1996) Production of interleukin 10 during malaria caused by lethal and nonlethal variants of Plasmodium yoelii yoelii. Parasitol Res 82 : 385–391.
25. AyimbaE, HegewaldJ, SegbenaAY, GantinRG, LechnerCJ, et al. (2011) Proinflammatory and regulatory cytokines and chemokines in infants with uncomplicated and severe Plasmodium falciparum malaria. Clin Exp Immunol 166 : 218–226.
26. MirghaniHA, EltahirHG, TMAE, MirghaniYA, ElbashirMI, et al. (2011) Cytokine profiles in children with severe Plasmodium falciparum malaria in an area of unstable malaria transmission in central Sudan. J Trop Pediatr 57 : 392–395.
27. LutyAJ, PerkinsDJ, LellB, Schmidt-OttR, LehmanLG, et al. (2000) Low interleukin-12 activity in severe Plasmodium falciparum malaria. Infect Immun 68 : 3909–3915.
28. WaltherM, WoodruffJ, EdeleF, JeffriesD, TongrenJE, et al. (2006) Innate immune responses to human malaria: heterogeneous cytokine responses to blood-stage Plasmodium falciparum correlate with parasitological and clinical outcomes. J Immunol 177 : 5736–5745.
29. GrahamSM, WalshAL, MolyneuxEM, PhiriAJ, MolyneuxME (2000) Clinical presentation of non-typhoidal Salmonella bacteraemia in Malawian children. Trans R Soc Trop Med Hyg 94 : 310–314.
30. MabeyDC, BrownA, GreenwoodBM (1987) Plasmodium falciparum malaria and Salmonella infections in Gambian children. J Infect Dis 155 : 1319–1321.
31. LissnerCR, SwansonRN, O'BrienAD (1983) Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol 131 : 3006–3013.
32. O'BrienAD, RosenstreichDL, TaylorBA (1980) Control of natural resistance to Salmonella typhimurium and Leishmania donovani in mice by closely linked but distinct genetic loci. Nature 287 : 440–442.
33. PlantJ, GlynnAA (1976) Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis 133 : 72–78.
34. HarringtonKA, HormaecheCE (1986) Expression of the innate resistance gene Ity in mouse Kupffer cells infected with Salmonella typhimurium in vitro. Microb Pathog 1 : 269–274.
35. VidalS, TremblayML, GovoniG, GauthierS, SebastianiG, et al. (1995) The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J Exp Med 182 : 655–666.
36. StevensonMM, LyangaJJ, SkameneE (1982) Murine malaria: genetic control of resistance to Plasmodium chabaudi. Infect Immun 38 : 80–88.
37. Freitas do RosarioAP, LambT, SpenceP, StephensR, LangA, et al. (2012) IL-27 promotes IL-10 production by effector Th1 CD4+ T cells: a critical mechanism for protection from severe immunopathology during malaria infection. J Immunol 188 : 1178–1190.
38. Richter-DahlforsA, BuchanAM, FinlayBB (1997) Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med 186 : 569–580.
39. FriedmanRL, MoonRJ (1977) Hepatic clearance of Salmonella typhimurium in silica-treated mice. Infect Immun 16 : 1005–1012.
40. AraiT, HiromatsuK, NishimuraH, KimuraY, KobayashiN, et al. (1995) Effects of in vivo administration of anti-IL-10 monoclonal antibody on the host defence mechanism against murine Salmonella infection. Immunology 85 : 381–388.
41. EiseleNA, RubyT, JacobsonA, ManzanilloPS, CoxJS, et al. (2013) Persistent Salmonella infection is controlled by PPARd, a host regulator of fatty acid metabolism. Cell Host Microbe 14 : 171–82 doi:10.1016/j.chom.2013.07.010
42. McCoyMW, MorelandSM, DetweilerCS (2012) Hemophagocytic macrophages in murine typhoid fever have an anti-inflammatory phenotype. Infect Immun 80 : 3642–3649.
43. MosserDM, EdwardsJP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8 : 958–969.
44. SieweL, Bollati-FogolinM, WickenhauserC, KriegT, MullerW, et al. (2006) Interleukin-10 derived from macrophages and/or neutrophils regulates the inflammatory response to LPS but not the response to CpG DNA. Eur J Immunol 36 : 3248–3255.
45. CouperKN, BlountDG, WilsonMS, HafallaJC, BelkaidY, et al. (2008) IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog 4: e1000004.
46. RoersA, SieweL, StrittmatterE, DeckertM, SchluterD, et al. (2004) T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med 200 : 1289–1297.
47. SetiawanT, MetwaliA, BlumAM, InceMN, UrbanJFJr, et al. (2007) Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine. Infect Immun 75 : 4655–4663.
48. MantovaniA, SicaA, SozzaniS, AllavenaP, VecchiA, et al. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25 : 677–686.
49. CambosM, BazinetS, AbedE, Sanchez-DardonJ, BernardC, et al. (2010) The IL-12p70/IL-10 interplay is differentially regulated by free heme and hemozoin in murine bone-marrow-derived macrophages. Int J Parasitol 40 : 1003–1012.
50. DeshpandeP, ShastryP (2004) Modulation of cytokine profiles by malaria pigment–hemozoin: role of IL-10 in suppression of proliferative responses of mitogen stimulated human PBMC. Cytokine 28 : 205–213.
51. HandWL, King-ThompsonNL (1983) Effect of erythrocyte ingestion on macrophage antibacterial function. Infect Immun 40 : 917–923.
52. XavierMN, WinterMG, SpeesAM, den HartighAB, NguyenK, et al. (2013) PPARgamma-Mediated Increase in Glucose Availability Sustains Chronic Brucella abortus Infection in Alternatively Activated Macrophages. Cell Host Microbe 14 : 159–170.
53. XavierMN, WinterMG, SpeesAM, NguyenK, AtluriVL, et al. (2013) CD4+ T cell-derived IL-10 promotes Brucella abortus persistence via modulation of macrophage function. PLoS Pathog 9: e1003454.
54. O'LearyS, O'SullivanMP, KeaneJ (2011) IL-10 Blocks Phagosome Maturation in Mycobacterium tuberculosis-infected Human Macrophages. American Journal of Respiratory Cell and Molecular Biology 45 : 172–180.
55. NguyenT, RobinsonN, AllisonSE, CoombesBK, SadS, et al. (2013) IL-10 produced by trophoblast cells inhibits phagosome maturation leading to profound intracellular proliferation of Salmonella enterica Typhimurium. Placenta 34 : 765–774.
56. CunningtonAJ, NjieM, CorreaS, TakemEN, RileyEM, et al. (2012) Prolonged Neutrophil Dysfunction after Plasmodium falciparum Malaria Is Related to Hemolysis and Heme Oxygenase-1 Induction. J Immunol 189 : 5336–46 doi:10.4049/jimmunol.1201028
57. CunningtonAJ, de SouzaJB, WaltherM, RileyEM (2012) Malaria impairs resistance to Salmonella through heme - and heme oxygenase-dependent dysfunctional granulocyte mobilization. Nat Med 18 : 120–127.
58. ConlanJW (1996) Neutrophils prevent extracellular colonization of the liver microvasculature by Salmonella typhimurium. Infect Immun 64 : 1043–1047.
59. BrozP, MonackDM (2011) Molecular mechanisms of inflammasome activation during microbial infections. Immunol Rev 243 : 174–190.
60. FinkSL, CooksonBT (2007) Pyroptosis and host cell death responses during Salmonella infection. Cell Microbiol 9 : 2562–2570.
61. MiaoEA, RajanJV, AderemA (2011) Caspase-1-induced pyroptotic cell death. Immunol Rev 243 : 206–214.
62. MiaoEA, LeafIA, TreutingPM, MaoDP, DorsM, et al. (2010) Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 11 : 1136–1142.
63. GreenwoodBM, BruetonMJ (1974) Complement activation in children with acute malaria. Clin Exp Immunol 18 : 267–272.
64. KrettliAU, NussenzweigV, NussenzweigRS (1976) Complement alterations in rodent malaria. Am J Trop Med Hyg 25 : 34–41.
65. KuhnR, LohlerJ, RennickD, RajewskyK, MullerW (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75 : 263–274.
66. PilsMC, PisanoF, FasnachtN, HeinrichJM, GroebeL, et al. (2010) Monocytes/macrophages and/or neutrophils are the target of IL-10 in the LPS endotoxemia model. Eur J Immunol 40 : 443–448.
67. ClausenBE, BurkhardtC, ReithW, RenkawitzR, ForsterI (1999) Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 8 : 265–277.
68. StojiljkovicI, BaumlerAJ, HeffronF (1995) Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster. J Bacteriol 177 : 1357–1366.
69. PrentkiP, KrischHM (1984) In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29 : 303–313.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge inČlánek Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 5- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Surveillance for Emerging Biodiversity Diseases of Wildlife
- The Emerging Role of Urease as a General Microbial Virulence Factor
- PARV4: An Emerging Tetraparvovirus
- Epigenetic Changes Modulate Schistosome Egg Formation and Are a Novel Target for Reducing Transmission of Schistosomiasis
- The Human Adenovirus E4-ORF1 Protein Subverts Discs Large 1 to Mediate Membrane Recruitment and Dysregulation of Phosphatidylinositol 3-Kinase
- A Multifactorial Role for Malaria in Endemic Burkitt's Lymphoma Pathogenesis
- Structural Basis for the Ubiquitin-Linkage Specificity and deISGylating Activity of SARS-CoV Papain-Like Protease
- Cathepsin-L Can Resist Lysis by Human Serum in
- Epstein-Barr Virus Down-Regulates Tumor Suppressor Expression
- BCA2/Rabring7 Targets HIV-1 Gag for Lysosomal Degradation in a Tetherin-Independent Manner
- The Evolutionarily Conserved Mediator Subunit MDT-15/MED15 Links Protective Innate Immune Responses and Xenobiotic Detoxification
- Suppressor of Cytokine Signaling 4 (SOCS4) Protects against Severe Cytokine Storm and Enhances Viral Clearance during Influenza Infection
- T Cell Inactivation by Poxviral B22 Family Proteins Increases Viral Virulence
- Dynamics of HIV Latency and Reactivation in a Primary CD4+ T Cell Model
- HIV and HCV Activate the Inflammasome in Monocytes and Macrophages via Endosomal Toll-Like Receptors without Induction of Type 1 Interferon
- Virus and Autoantigen-Specific CD4+ T Cells Are Key Effectors in a SCID Mouse Model of EBV-Associated Post-Transplant Lymphoproliferative Disorders
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- Squalene Synthase As a Target for Chagas Disease Therapeutics
- The Contribution of Viral Genotype to Plasma Viral Set-Point in HIV Infection
- Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge in
- Anthrax Lethal Factor as an Immune Target in Humans and Transgenic Mice and the Impact of HLA Polymorphism on CD4 T Cell Immunity
- Ly49C-Dependent Control of MCMV Infection by NK Cells Is -Regulated by MHC Class I Molecules
- Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
- A Large Family of Antivirulence Regulators Modulates the Effects of Transcriptional Activators in Gram-negative Pathogenic Bacteria
- Broad-Spectrum Anti-biofilm Peptide That Targets a Cellular Stress Response
- Malaria Parasite Infection Compromises Control of Concurrent Systemic Non-typhoidal Infection via IL-10-Mediated Alteration of Myeloid Cell Function
- A Role for in Higher Order Structure and Complement Binding of the Capsule
- Hip1 Modulates Macrophage Responses through Proteolysis of GroEL2
- CD8 T Cells from a Novel T Cell Receptor Transgenic Mouse Induce Liver-Stage Immunity That Can Be Boosted by Blood-Stage Infection in Rodent Malaria
- Phosphorylation of KasB Regulates Virulence and Acid-Fastness in
- HIV-Infected Individuals with Low CD4/CD8 Ratio despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened CD8+ T Cell Activation, and Increased Risk of Non-AIDS Morbidity and Mortality
- A Novel Mechanism Inducing Genome Instability in Kaposi's Sarcoma-Associated Herpesvirus Infected Cells
- Structural and Biochemical Characterization Reveals LysGH15 as an Unprecedented “EF-Hand-Like” Calcium-Binding Phage Lysin
- Hepatitis C Virus Cell-Cell Transmission and Resistance to Direct-Acting Antiviral Agents
- Different Modes of Retrovirus Restriction by Human APOBEC3A and APOBEC3G
- TNFα and IFNγ but Not Perforin Are Critical for CD8 T Cell-Mediated Protection against Pulmonary Infection
- Large Scale RNAi Reveals the Requirement of Nuclear Envelope Breakdown for Nuclear Import of Human Papillomaviruses
- The Cytoplasmic Domain of Varicella-Zoster Virus Glycoprotein H Regulates Syncytia Formation and Skin Pathogenesis
- A New Class of Multimerization Selective Inhibitors of HIV-1 Integrase
- Are We There Yet? The Smallpox Research Agenda Using Variola Virus
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
- Dynamic Functional Modulation of CD4 T Cell Recall Responses Is Dependent on the Inflammatory Environment of the Secondary Stimulus
- Bacterial Superantigens Promote Acute Nasopharyngeal Infection by in a Human MHC Class II-Dependent Manner
- Follicular Helper T Cells Promote Liver Pathology in Mice during Infection
- A Nasal Epithelial Receptor for WTA Governs Adhesion to Epithelial Cells and Modulates Nasal Colonization
- Unexpected Role for IL-17 in Protective Immunity against Hypervirulent HN878 Infection
- Human Cytomegalovirus Fcγ Binding Proteins gp34 and gp68 Antagonize Fcγ Receptors I, II and III
- Expansion of Murine Gammaherpesvirus Latently Infected B Cells Requires T Follicular Help
- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Molecular Signatures of Hemagglutinin Stem-Directed Heterosubtypic Human Neutralizing Antibodies against Influenza A Viruses
- The Downregulation of GFI1 by the EZH2-NDY1/KDM2B-JARID2 Axis and by Human Cytomegalovirus (HCMV) Associated Factors Allows the Activation of the HCMV Major IE Promoter and the Transition to Productive Infection
- Inactivation of Fructose-1,6-Bisphosphate Aldolase Prevents Optimal Co-catabolism of Glycolytic and Gluconeogenic Carbon Substrates in
- New Insights into Rotavirus Entry Machinery: Stabilization of Rotavirus Spike Conformation Is Independent of Trypsin Cleavage
- Prophenoloxidase Activation Is Required for Survival to Microbial Infections in
- SslE Elicits Functional Antibodies That Impair Mucinase Activity and Colonization by Both Intestinal and Extraintestinal Strains
- Timed Action of IL-27 Protects from Immunopathology while Preserving Defense in Influenza
- HIV-1 Envelope gp41 Broadly Neutralizing Antibodies: Hurdles for Vaccine Development
- The PhoP-Dependent ncRNA Mcr7 Modulates the TAT Secretion System in
- Cellular Superspreaders: An Epidemiological Perspective on HIV Infection inside the Body
- The Inflammasome Pyrin Contributes to Pertussis Toxin-Induced IL-1β Synthesis, Neutrophil Intravascular Crawling and Autoimmune Encephalomyelitis
- Papillomavirus Genomes Associate with BRD4 to Replicate at Fragile Sites in the Host Genome
- Integrative Functional Genomics of Hepatitis C Virus Infection Identifies Host Dependencies in Complete Viral Replication Cycle
- Co-assembly of Viral Envelope Glycoproteins Regulates Their Polarized Sorting in Neurons
- Targeting Membrane-Bound Viral RNA Synthesis Reveals Potent Inhibition of Diverse Coronaviruses Including the Middle East Respiratory Syndrome Virus
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



