-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Bacterial Superantigens Promote Acute Nasopharyngeal Infection by in a Human MHC Class II-Dependent Manner
Streptococcus pyogenes is the most common cause of bacterial pharyngitis, also known as ‘strep throat’. However, this organism is also responsible for a range of other important human illnesses including necrotizing fasciitis and rheumatic heart disease (RHD). Indeed, complications from RHD and invasive infections by S. pyogenes are responsible for over one half million deaths per year in the world. S. pyogenes produces potent toxins called superantigens (SAgs), also known as the scarlet fever or erythrogenic toxins. SAgs have been studied for many years, yet we don't understand what purpose SAgs play in the life cycle of S. pyogenes. Rather than studying SAgs in the context of serious streptococcal disease, we studied the role of SAgs in a nasopharyngeal infection model. Our work demonstrates that for S. pyogenes to efficiently infect mice, the mice must express a human protein that is a receptor for the SAgs, and that S. pyogenes must produce SAgs. We further show that immunizing against SAgs prevents nasopharyngeal infection. This work demonstrates that SAgs are important factors for establishing infection by S. pyogenes and that SAgs may be potential candidates for inclusion within a S. pyogenes vaccine.
Published in the journal: . PLoS Pathog 10(5): e32767. doi:10.1371/journal.ppat.1004155
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004155Summary
Streptococcus pyogenes is the most common cause of bacterial pharyngitis, also known as ‘strep throat’. However, this organism is also responsible for a range of other important human illnesses including necrotizing fasciitis and rheumatic heart disease (RHD). Indeed, complications from RHD and invasive infections by S. pyogenes are responsible for over one half million deaths per year in the world. S. pyogenes produces potent toxins called superantigens (SAgs), also known as the scarlet fever or erythrogenic toxins. SAgs have been studied for many years, yet we don't understand what purpose SAgs play in the life cycle of S. pyogenes. Rather than studying SAgs in the context of serious streptococcal disease, we studied the role of SAgs in a nasopharyngeal infection model. Our work demonstrates that for S. pyogenes to efficiently infect mice, the mice must express a human protein that is a receptor for the SAgs, and that S. pyogenes must produce SAgs. We further show that immunizing against SAgs prevents nasopharyngeal infection. This work demonstrates that SAgs are important factors for establishing infection by S. pyogenes and that SAgs may be potential candidates for inclusion within a S. pyogenes vaccine.
Introduction
S. pyogenes (commonly known as the β-hemolytic Group A Streptococcus) is a prominent bacterial pathogen that causes a diverse range of clinical manifestations. Globally, S. pyogenes is responsible for over 600 million cases of pharyngitis, and more than one half million deaths primarily from complications of autoimmune rheumatic heart disease and invasive infections [1]. In addition, approximately 12% of school-aged children are asymptomatic carriers of this organism [2], and this ‘carriage’ state can last for years without the development of disease [3]. Although humans remain the only known natural reservoir for S. pyogenes, closely related streptococci such as Streptococcus canis and Streptococcus dysgalactiae lack host-specific adaptation. This indicates that the ancestor to S. pyogenes was unlikely to be human specific, and this further suggests that a primary feature in the evolution of S. pyogenes was the stringent adaptation to the human host [4]. Although morbidity associated with S. pyogenes is dependent on the ability to colonize and transmit within human populations, the molecular basis of the human specific tropism by S. pyogenes remains poorly understood.
One group of ‘trademark’ virulence factors produced by S. pyogenes are the bacterial superantigens (SAgs), also commonly referred to as the erythrogenic toxins or the streptococcal pyrogenic exotoxins [5]. There are at least 14 genetically distinct streptococcal SAgs [6] often encoded within mobile, or putatively mobile, genetic elements [7]–[9]. Thus, different S. pyogenes strains typically encode for distinct repertoires of multiple SAgs. These toxins function by engaging lateral surfaces of both MHC class II (MHC-II) molecules and T cell receptor (TCR) β-chains [10]; these unconventional interactions can force the activation of enormous numbers of T cells. Indeed, human MHC-II molecules are known host factors fundamental to the development of severe streptococcal disease [11]–[14], and the ability of MHC-II to modulate severity of invasive streptococcal disease has been linked directly to SAgs [12], [15]. Although SAgs are well recognized in the pathogenesis of scarlet fever [16], [17] and the streptococcal toxic shock syndrome (TSS) [18], [19], in what context these toxins contribute to the fitness and life cycle of S. pyogenes is unknown. Thus, since a major biological niche for S. pyogenes is the upper respiratory tract, we hypothesized that SAgs have likely evolved to function in the context of asymptomatic nasopharyngeal colonization and/or pharyngitis, rather than in the context of severe invasive disease.
Here we show that mice expressing human MHC-II molecules are highly susceptible to acute nasopharyngeal infection by S. pyogenes. Furthermore, we demonstrate that S. pyogenes MGAS8232 requires the streptococcal pyrogenic exotoxin A (SpeA) SAg to cause acute nasopharyngeal infection. In addition, immunization with toxoid SpeA is protective for nasopharyngeal infection by wild-type S. pyogenes MGAS8232. This work indicates that the streptococcal SAgs play an important role in the life cycle of S. pyogenes by promoting the initial stages of infection, and that these toxins should be further considered as potential vaccine targets to prevent S. pyogenes nasopharyngeal carriage.
Results
Human Leukocyte Antigen (HLA) molecules are critical host factors for nasal infection by S. pyogenes
S. pyogenes infection of the nasopharynx in mice is a model for pharyngeal infection in humans [20], [21]. We first evaluated the influence of human MHC-II on mouse nasopharyngeal infection by S. pyogenes. Nasal inoculation of C57BL/6 (B6) mice with ∼1×108 CFU of S. pyogenes MGAS8232 resulted in very low bacterial recovery from the nasal mucosa [herein referred to as the complete nasal turbinates (cNT)] at 48 h (Figure 1A). However, ‘humanized’ B6 mice that express HLA-DR4 (DR4-B6 mice) contained ∼100-fold more CFUs of S. pyogenes compared to wild-type B6 mice, and mice that expressed HLA-DQ8 (DQ8-B6 mice), or both HLA-DR4 and HLA-DQ8 (herein referred to as HLA-B6 mice), contained ∼10,000-fold more CFUs than wild-type B6 mice (Figure 1A). In a time course analysis of this acute infection model, ∼5×103 bacterial CFUs of S. pyogenes MGAS8232 were recovered at 24 h, which increased by ∼100-fold at 48 h, and were subsequently cleared after one week (Figure 1B). Importantly, MGAS8232 did not become invasive as viable S. pyogenes cells were not recovered from the spleen (Figure 1B) or blood (data not shown) in HLA-B6 mice at any time point. These data reveal that human MHC-II molecules are important host factors for acute nasopharyngeal infection by S. pyogenes.
Fig. 1. MHC-II is an important determinant of S. pyogenes nasopharyngeal infection in mice. 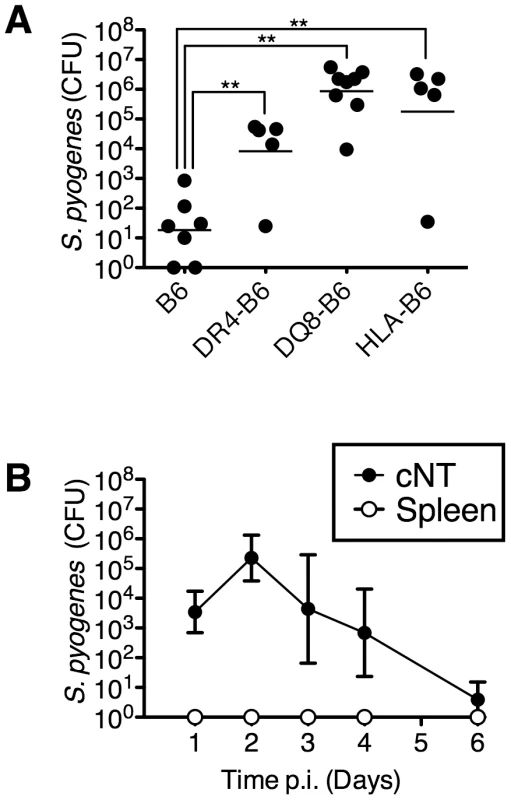
(A) Individual mice were nasally inoculated with ∼1×108 bacterial colony forming units (CFU) with MGAS8232 and nasopharyngeal CFUs were assessed at 48 h. Each symbol represents CFUs from an individual mouse. (B) Time-course analysis of MGAS8232 inoculated HLA-B6 mice. CFUs were determined from the cNTs and spleens (n≥3 per group) at the indicated time points. Statistical significance is displayed as **p<0.01 by Student's t-test. HLA-B6 mice are responsive to SpeA and SmeZ
To investigate the potential role of SAgs for nasopharyngeal infection in HLA-mice, we mined the genome of S. pyogenes MGAS8232 and confirmed that this strain encodes for six genetically distinct SAgs (SpeA, SpeC, SpeG, SpeL, SpeM and SmeZ) [22]. The mature coding region for each SAg gene was cloned, recombinant SAgs (rSAg) were expressed and purified, and each was shown to activate human T cells (data not shown). To assess activity of the rSAgs in HLA-mice, ex vivo splenocyte activation was evaluated. B6 splenocytes demonstrated little capacity for activation by all six rSAgs as measured by mouse IL-2 production and proliferative responses (Figure 2A). However, splenocytes from HLA-B6 mice showed enhanced responses to both the SpeA and SmeZ rSAgs in a dose-dependent manner (Figure 2B). These data indicate that both SpeA and SmeZ function in HLA-B6 mice and suggest that SAgs encoded within S. pyogenes MGAS8232 may contribute to the ability of S. pyogenes to infect the nasopharynx of HLA-mice.
Fig. 2. HLA-B6 mice are responsive to SpeA and SmeZ. 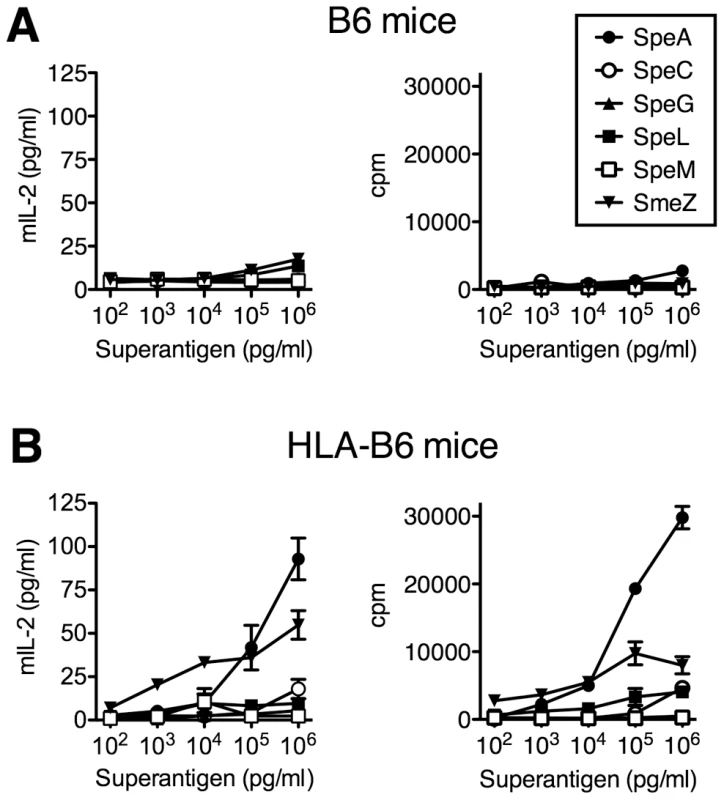
Splenocytes (2×105/well) from (A) B6 or (B) HLA-B6 mice were treated with serial 10-fold titrations of indicated SAgs. Mouse IL-2 (mIL-2) production was assessed at 18 h, and proliferation, expressed as count per minute (cpm), was assessed after 72 hours. Data shown is from a representative experiment completed in triplicate. Construction of superantigen deficient S. pyogenes
In order to determine empirically if SAgs were direct contributors to the enhanced nasopharyngeal infection phenotype in HLA-mice, a series of precise, in-frame deletions were generated within the coding regions for each SAg gene in S. pyogenes MGAS8232 (Figure 3A). As speL and speM are encoded in tandem, these SAgs were deleted together. In addition, we generated a complete SAg deletion strain lacking the coding regions for all six SAg genes (MGAS8232 ΔSAg) (Figure 3A). Each of the S. pyogenes deletion strains grew comparably to wild-type MGAS8232 in vitro (Figure 3B). Furthermore, the various mutants did not show any alterations in protease (SpeB) activity (Figure 3C). To evaluate SAg production in these strains in vitro, we generated rabbit polyclonal antibodies to each rSAg. The specific rabbit antisera recognized each of the expected SAgs without cross-reacting with others (Figure 3D). Next, we assessed in vitro production of the SAg proteins for the wild-type and isogenic SAg deletion strains. The production of both SpeC and SpeL from wild-type S. pyogenes MGAS8232 was clearly detectable by Western blot analysis, while SpeA was weakly detected (Figure 3E). Importantly, for each of these three SAgs, the toxin was not made by the appropriate deletion mutant. To evaluate SAg activity from the different S. pyogenes mutants, we tested the supernatants from the isogenic S. pyogenes strains for the ability to activate HLA-B6 mice splenocytes. Consistent with the Western blot analysis (Figure 3E), and the ability of select SAgs to activate these cells (Figure 2B), the largest reduction in activity for the individual deletion strains was for MGAS8232 ΔSpeA, whereas the MGAS8232 ΔSAg mutant did not activate splenocytes above background levels (Figure 3F).
Fig. 3. Generation of isogenic S. pyogenes genetic mutants lacking superantigen genes. 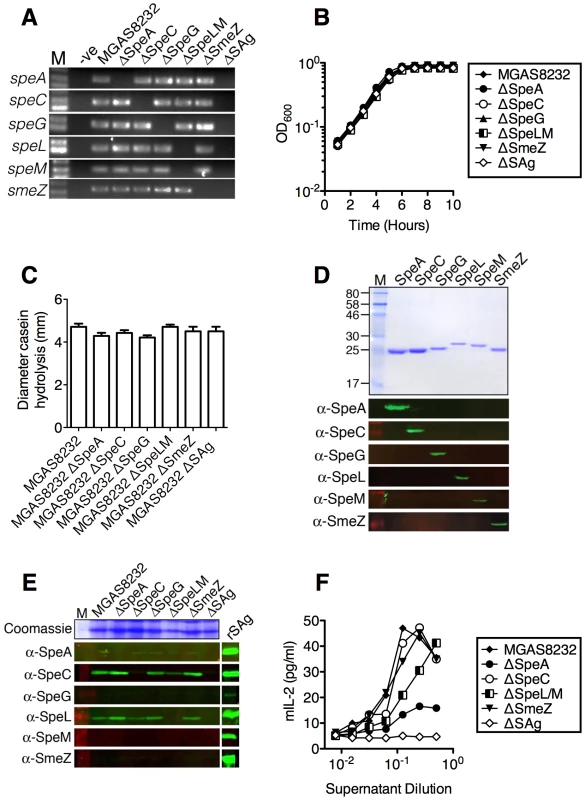
Individual SAg genes were systemically deleted in S. pyogenes MGAS8232 as detailed in Materials and Methods. (A) Genetic SAg deletions were confirmed by PCR with primers internal to each gene. Growth curves (B) and protease activity (C) of the indicated mutants. (D) Recombinant SAgs (SDS-PAGE; top panel) and Western blot experiments (bottom six panels) to determine specificity of rabbit polyclonal antisera to distinct recombinant SAg proteins. (E) Streptococcal SAg deletion strains were assessed for production of each SAg (100-fold concentrated supernatants) by Western blotting. (F) Activation potential using mouse IL-2 (mIL-2) assays using supernatant titrations to stimulate splenocytes (2×105/well) from HLA-B6 mice. The SpeA superantigen is required for S. pyogenes MGAS8232 acute nasopharyngeal infection in HLA-mice
We next evaluated all of the isogenic S. pyogenes MGAS8232 strains for the ability to infect HLA-B6 mice. MGAS8232 ΔSpeA demonstrated a striking reduction in bacterial CFUs recovered at 48 h compared to wild-type MGAS8232 (Figure 4A). Although some variability was seen for the other mutants in vivo (ΔSpeC, ΔSpeG, ΔSpeLM and ΔSmeZ), multiple animal experiments demonstrated that each was capable of infecting the HLA-mice to wild-type levels. MGAS8232 ΔSAg however, resembled wild-type MGAS8232 recovery in B6 mice (Figures 1A and 4A). In order to genetically complement the MGAS8232 ΔSAg strain, and to determine if a single SAg could promote infection, we introduced the wild-type speA gene (and the corresponding speA promoter) into the chromosome of MGAS8232 ΔSAg between the pepO and tsf genes. The wild-type SpeA complemented strain enhanced infection significantly above MGAS8232 ΔSAg that was not statistically different from wild-type MGAS8232 (Figure 4A). To assess the requirement for SAg activity for the infection phenotype, we constructed a SpeA MHC-II binding mutant based on a structural model of SpeA in complex with HLA-DQ8 (Figure 4B). This model predicted SpeA Tyr100 would interact with the conserved MHC-II α-chain Lys39 and both of these equivalent residues in the staphylococcal enterotoxins, and HLA-DR1, respectively, have been shown to be important for SAg function [23], [24]. Recombinant SpeAY100A (Figure 4C) demonstrated ∼100-fold reduction in potency relative to wild-type SpeA (Figure 4D) and thus we introduced speAY100A into MGAS8232 ΔSAg in a similar fashion to the wild-type speA complementation experiment. The speAY100A-complemented strain was significantly reduced compared to the wild-type SpeA complemented stain, and although CFUs were not statistically increased over MGAS8232 ΔSAg, there was a trend for enhanced infection by ∼1 log. We believe this potential increase may reflect the residual activity present in the SpeAY100A mutant (Figure 4A). Furthermore, using qRT-PCR we confirmed that the in vitro expression of speAY100A was similar to speAWT between the two complemented strains (data not shown). These collective data indicate that the enhanced nasopharyngeal infection by S. pyogenes MGAS8232 in HLA-B6 mice is due to the production of the SpeA exotoxin, and that SAg function is required.
Fig. 4. Superantigens are critical for S. pyogenes nasopharyngeal infection in mice. 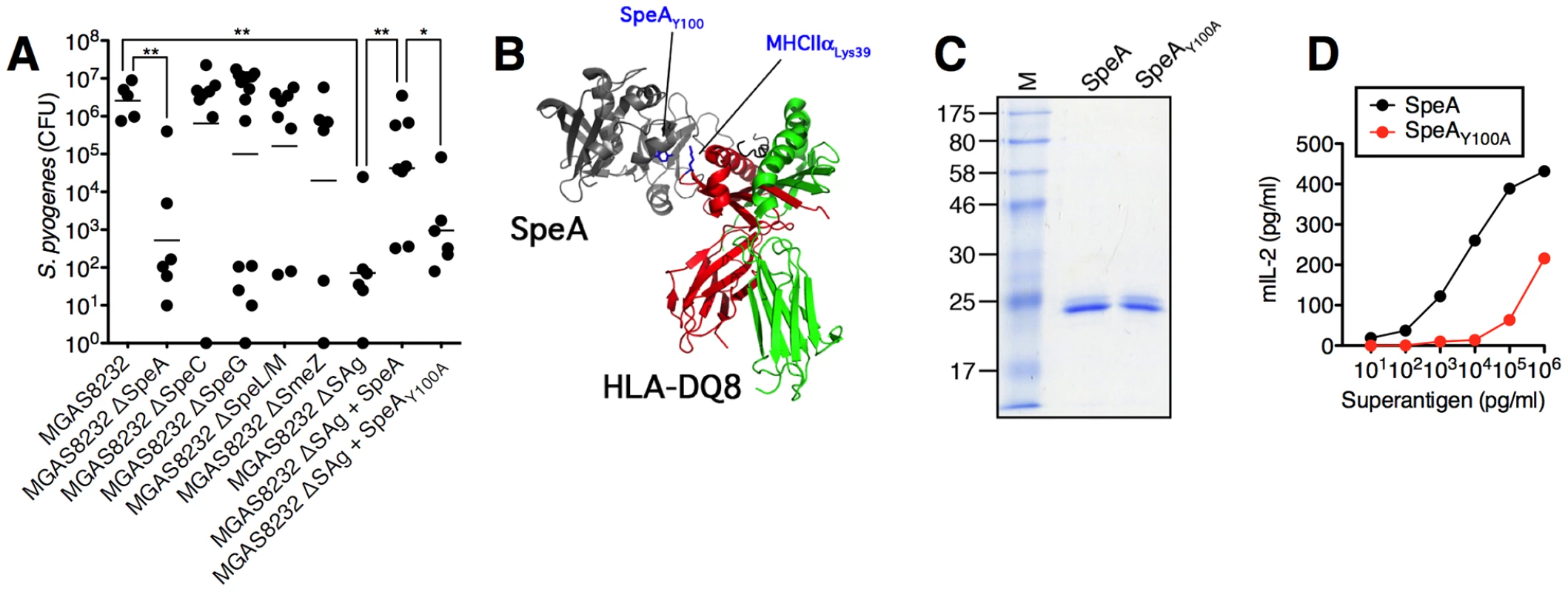
(A) Individual HLA-B6 mice were nasally inoculated with the indicated MGAS8232 strains [∼1×108 (range 0.6–1.4×108) CFUs, and nasopharyngeal CFUs were assessed at 48 h. Each symbol represents an individual mouse. (B) Ribbon diagram model of SpeA in complex with human MHC II with relevant amino acids highlighted. (C) Recombinant SpeA proteins (2 µg). (D) Dose response of SpeA and SpeAY100A for activation of HLA-B6 splenocytes (1×106/well). Mouse IL-2 (mIL-2) production was assessed at 18 h. Statistical significance is displayed as *p<0.05 or **p<0.01 by Student's t test. S. pyogenes infects the nasal turbinates and induces superantigen-dependent inflammation
To gain insight into the S. pyogenes infection process in HLA-B6 mice, we generated coronal sections of the nasal passage for sham, wild-type MGAS8232, or MGAS8232 ΔSAg treated mice, at both 24 and 48 h. Sections were stained with H&E, as well as DAPI (blue) and an anti-S. pyogenes fluorescent antibody (red). As predicted, there was no evidence of S. pyogenes infection seen in the sham treated mice at 24 or 48 h (Figure 5A). Both wild-type MGAS8232 and MGAS8232 ΔSAg demonstrated sparse, but detectable S. pyogenes cells, within the upper nasal turbinates at 24 h (Figure 5A). This was consistent with bacterial counts from MGAS8232 ΔSAg infected mice at 24 h (975±475 CFU/cNT; n = 3 mice) that did not differ significantly from wild-type MGAS8232 infected mice (Figure 1B). Consistent with the CFU data (Figure 4A), very few MGAS8232 ΔSAg cells were detected microscopically at 48 h, whereas wild-type MGAS8232 produced a robust infection that was localized to the upper nasal turbinates (Figure 5A; 48 h MGAS8232 boxed insets). H&E-stained sections from 48 h post-infection (n = 5 mice per group, 2 sections per mouse) were scored in a blinded fashion for the presence and severity of mucus, red blood cells, and nucleated cellular debris on the surface of the respiratory epithelium and neuroepithelium. This analysis revealed no significant findings for the sham treated mice, and mice infected with MGAS8232 ΔSAg displayed only mild neutrophilic infiltration in the sub-epithelial spaces, and mild epithelial disruption (Figure 5B). However, mice infected with wild-type MGAS8232 demonstrated significant signs of inflammation including sloughed cellular debris into the nasal cavity lumen, and marked neutrophilic infiltration and epithelial cell disruption with evidence of cocci on the epithelial surface, and evidence of hypersecretory activity from the neuroepithelium (Figure 5B). Flow cytometric analysis of total cNT preparations from mice treated with the 3 groups (n = 4 mice per group) however, revealed few overall changes in immune cell percentages, although a significant decrease in the dendritic cell (DC) (CD11c+) population was revealed in the wild-type MGAS8232 infected animals compared with MGAS8232 ΔSAg infect mice, and the analysis also showed a trend for increased neutrophils (GR1+) in wild-type MGAS8232 infected animals (Figure S1) that was consistent with the histological analyses. We did not observe any changes in immune cell populations in the spleen or lymph nodes (Figure S1). However, cytokine analysis of homogenized cNTs demonstrated an early inflammatory type or TH1-skewed response of the wild-type MGAS8232 infected mice compared with the MGAS8232 ΔSAg strain, including enhanced production of IL1α, IL-2, IL-6 and IL-17, as well as the chemokines KC, IP-10 and MCP-1 (Figures 5C and S2). By 48 h, the wild-type MGAS8232 infected mice displayed a robust chemokine response consistent with the high numbers of bacterial cells by this time point. Taken together, these data are consistent with an early SAg-dependent inflammatory environment within the nasal turbinates where S. pyogenes could both survive and rapidly expand to high numbers over the initial 48 h of infection.
Fig. 5. Nasopharyngeal infection of HLA-B6 mice with wild-type S. pyogenes MGAS8232 localizes to the nasal turbinates and induces a SAg-dependent inflammatory response. 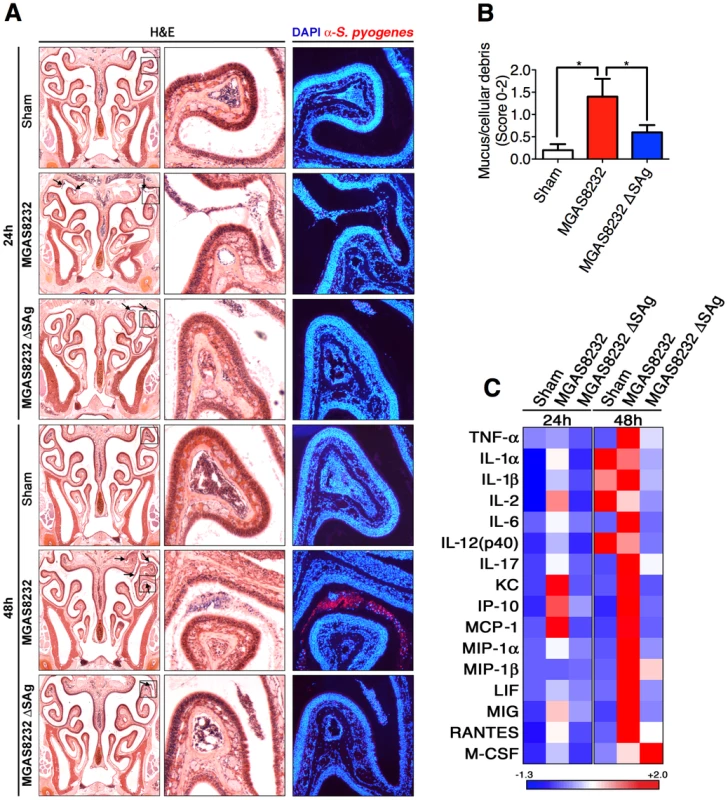
(A) Coronal 5 µm sections of mouse heads stained with H&E stain or with DAPI (blue) and α-S. pyogenes (red) antibody. Shown are representative sections at 24 h and 48 h time points for sham treated, wild-type S. pyogenes MGAS8232 and MGAS8232 ΔSAg strains. Right panels are close up views from the boxed sections. Arrows indicate inflammatory cells containing S. pyogenes. (B) Histological assessment of nasal tissue sections from S. pyogenes MGAS8232 and MGAS8232 ΔSAg infected HLA-mice. Differences in pathological severity were assessed by examination of H&E stained sections at 48 h. Sections (n = 5 per group) were scored (0–2) for the presence and severity of mucus, red blood cells, and nucleated cellular debris on the surface of the respiratory epithelium. Statistical significance is displayed as *p<0.05 by Student's t test. (C) Heat map of multiplex cytokine arrays from treatment groups as in Panel A (n≥3 mice per group). Data shown represent the mean cytokine responses that displayed significant differences within each time group. Values for each row were normalized to have a mean of zero and a variance of one. Corresponding quantitative data and statistical analyses are shown in Figure S2. Superantigen-neutralizing antibodies inhibit nasopharyngeal infection by S. pyogenes in HLA-B6 mice
Given the prominent nasopharyngeal infection phenotype for S. pyogenes MGAS8232 that was SpeA-dependent (Figures 4A and 5A), we tested the ability of humoral immunity to SpeA to influence nasopharyngeal infection by S. pyogenes MGAS8232 (Figure 6A). We vaccinated HLA-mice with the SpeAY100A toxoid and these mice developed high anti-SpeA IgG titres compared with sham-vaccinated mice (Figure 6B). Following nasal inoculation with ∼1×108 CFU of wild-type MGAS8232, SpeAY100A-vaccinated mice showed a dramatic reduction in infection (Figure 6C) that was similar to the MGAS8232 ΔSpeA mutant (Figure 4A), while sham-vaccinated mice were infected efficiently (Figure 6C). These data indicate that humoral immunity to appropriate SAgs can inhibit nasopharyngeal infection in SAg-sensitive mice.
Fig. 6. Vaccination with toxoid SAg inhibits S. pyogenes nasopharyngeal infection in mice. 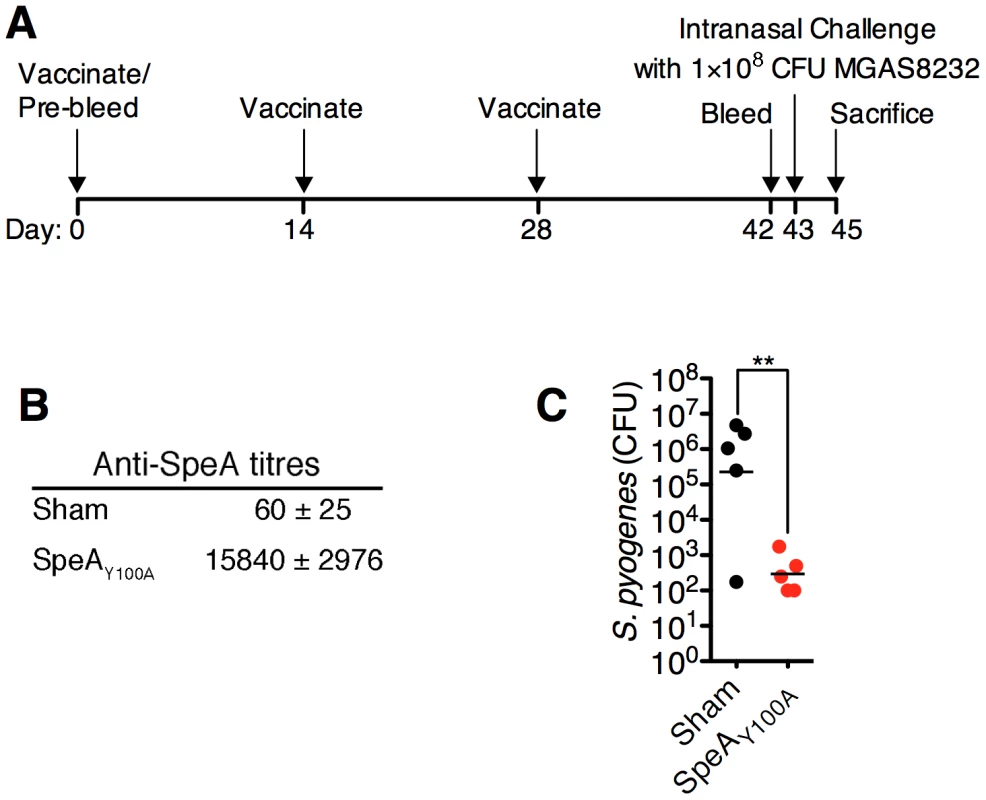
(A) Time-line of vaccination protocol. (B) Anti-SpeA IgG antibody titres (mean ± SEM) from HLA-B6 mice (n = 5 mice per group), either sham vaccinated, or vaccinated with SpeAY100A as described in Materials and Methods. (C) Sham or SpeAY100A vaccinated HLA-B6 mice were nasally inoculated with ∼1×108 bacterial CFUs of S. pyogenes MGAS8232. Nasopharyngeal CFUs were assessed at 48 h. Each symbol represents an individual mouse. Statistical significance is displayed as **p<0.01 by Student's t-test. Discussion
Bacterial SAgs from S. pyogenes are well recognized as important virulence factors for the development of serious toxin-mediated diseases such as the streptococcal TSS [5]. However, given that streptococcal TSS is rare, and since the disease has a very high mortality rate (∼45%) [25], it is unlikely that the induction of streptococcal TSS provides an evolutionary advantage for S. pyogenes. Thus, the actual biological function of these toxins has remained an enigma. We reasoned that since a major niche for S. pyogenes is the epithelial surfaces of the nasopharynx, we sought to evaluate the influence of SAgs in this context. Herein, we provide important evidence that acute infection of the upper respiratory tract by S. pyogenes MGAS8232 is enhanced dramatically in mice expressing human MHC-II, and that this phenotype is directly attributed to SpeA. A logical interpretation of these findings is that the true biological function of SAgs, in terms of the life cycle of S. pyogenes, is to promote the initial establishment of infection in humans.
This work also provides further evidence that host MHC-II molecules are a central component of the human specific tropism of S. pyogenes. Human MHC-II [11]–[14], human plasminogen [26], [27], and human CD46 [28] are each known to contribute to the development of invasive streptococcal disease, although the influence of these latter host factors have not yet been tested in a nasopharyngeal infection model. S. pyogenes produces a hyaluronic acid capsular polysaccharide [29] that is important for nasopharyngeal colonization in mice [30], and the hyaluronic acid binding receptor CD44 is an important receptor for S. pyogenes [31]. However, the conserved amino acid sequences, structures, and binding affinities of human and mouse CD44 for hyaluronan [32] would argue against a specific role of CD44 in the human-specific tropism of S. pyogenes.
Although there were no significant differences in CFUs at 24 h comparing wild-type S. pyogenes and the SAg-deficient strain, wild-type MGAS8232 was able to expand rapidly between 24 h to 48 h by ∼2 logs (Figure 1B), which was entirely consistent with the immunofluorescence staining of the nasal passages (Figure 5A). Although there were few differences in immune cell percentages from the cNT by 48 h, other than a clear decrease in CD11c+ leukocytes for wild-type MGAS8232 infected mice (Figure S1), S. pyogenes did induce a SAg-dependent Th1/inflammatory cytokine response, which was accompanied by the influx of innate immune cells by 48 h (Figure 5A). Selective depletion of CD11c+ DCs in mice resulted in enhanced dissemination of S. pyogenes from a subcutaneous infection into lymph nodes and the liver, demonstrating that DCs are contributors to host defence against S. pyogenes [33]. However, some strains of S. pyogenes can also induce DC apoptosis in a streptolysin O-dependent manner [34], which may have contributed to the decrease in CD11c+ populations. Nevertheless, we favour the hypothesis that S. pyogenes has provoked a localized and SAg-mediated cytokine response that resulted in a state of transient immunosuppression allowing S. pyogenes to escape myeloid cell mediated killing. In support of this hypothesis, staphylococcal enterotoxin B has been demonstrated in vivo to induce a transient (∼48 h) Vβ-unrestricted immunosuppressive response in T cells with the inability to produce IL-2, that also caused decreased numbers of splenic CD11c+ dendritic cells [35]. These responses were very consistent with mouse cytokine responses (Figure 5C) and immune cell analyses (Figure S1) of MGAS8232 infected HLA-B6 mice. By 48 h, however, a robust IL-6 and IL-17 response was produced (Figures 5C and S2), concurrent with the high numbers of S. pyogenes, and each of these cytokines are known to be important for S. pyogenes control [21], [36]. Alternatively, inflammation induced epithelial cell damage within the nasal turbinates (Figure 5B) may have promoted access to host cell adhesive factors to allow for the initial establishment of infection. The very weak cytokine response to the MGAS8232 ΔSAg strain was somewhat surprising, yet this finding may support this second hypothesis where in the absence of functional SAg, S. pyogenes are rapidly cleared through primarily mucociliary clearance mechanisms. Although S. pyogenes is capable of internalization into epithelial cells, the evidence indicates that S. pyogenes does not replicate efficiently within epithelial cells [37], [38], and thus we do not predict a role for enhanced intracellular survival based on SAg expression.
SAgs have also been studied directly in the context of live invasive streptococcal disease using defined genetic knockout strains. Earlier work utilizing a S. pyogenes myositis model in HLA-DQ8 transgenic mice demonstrated clear Vβ-specific alterations during infection, as well as SpeA-dependent conjunctivitis, hyperplasia of the lymph nodes and spleen, and T cell infiltration into the liver, yet the SpeA knockout strain demonstrated no difference in overall mortality compared with the wild-type counterpart [14]. Additionally, although SmeZ is dominant in the speA - and speC-negative M89 isolate S. pyogenes H293, genetic disruption of smeZ did not alter bacterial clearance or mortality in a peritoneal infection model [39]. Thus, a picture has emerged where individual SAgs may not contribute to S. pyogenes survival during invasive disease. Nevertheless, streptococcal SAgs can cause TSS directly in experimental animal models [40]–[42], human MHC-II molecules contribute to mouse mortality during invasive infections [12], [14], and in severe invasive human infections, streptococcal TSS is a major contributor to overall mortality [25].
In the nasal infection model presented here, despite the high number of bacterial cells recovered from wild-type S. pyogenes infected HLA-B6 mice at 48 h, S. pyogenes did not become invasive (Figure 1B), and the infection was reduced to very low levels after about 7 days. In humans, symptomatic pharyngitis infections typically last for about one week without antibiotic treatment [43], and thus the presented model appears to be a reasonable approximation of acute upper respiratory tract infection in humans. However, whether the model could replicate a longer term asymptomatic colonization state is unlikely, as many patients can harbour S. pyogenes for extended time periods, potentially lasting over 2 years [3].
Although our data demonstrate that SpeA is critical for the infection phenotype in HLA-B6 mice, the genome of S. pyogenes MGAS8232 also encodes for 5 other SAgs, each which is known to activate human T cells [44]–[47]. Although SpeC was the predominant SAg secreted from MGAS8232 in vitro (Figure 3E), the lack of a phenotype for the MGAS8232 ΔSpeC mutant was somewhat predicted, as this SAg does not activate murine T cells [48] (Figure 2). Although recombinant SmeZ did potently activate splenocytes from HLA-B6 mice, and SmeZ is the primary immunoactive SAg for some S. pyogenes strains [39], we did not detect expression of SmeZ in vitro from wild-type MGAS8232 (Figure 3E), which likely contributed to the inability of SmeZ to compensate functionally in the MGAS8232 ΔSpeA strain. However, the MGAS8232 ΔSAg strain complemented with wild-type SpeA did not appear to fully restore the infection phenotype, and the lack of SmeZ production could potentially be responsible for this result. The remaining SAgs showed very weak activity for the activation of splenocytes from HLA-mice and thus they do not play an important role for infection in this model.
The vaccination experiments provide further evidence, independent of the genetic deletion strains, of the critical role played by SpeA during the infection. Neutralization of SAg activity by antibodies is consistent with clinical evidence that has established a link between the lack of streptococcal SAg-neutralizing antibodies and the development of streptococcal TSS [49]-[51]. Also, neutralizing antibodies are known to protect against experimental STSS in rabbits [40], [41]. Thus, pre-existing anti-SAg antibodies may potentially inhibit infection by specific strains of S. pyogenes, yet S. pyogenes could theoretically circumvent this through up-regulation of additional SAgs for which neutralizing antibodies are absent. Since it is clear that different strains of S. pyogenes encode different repertoires of SAgs [7], S. pyogenes may also potentially alter SAg expression patterns to engage different host MHC-II molecules.
The SAgs remain well-recognized virulence factors for S. pyogenes. However, this work demonstrates that their genuine contribution to the life cycle of this pathogen is likely to promote the establishment of pharyngitis, or potentially asymptomatic colonization, in genetically susceptible individuals expressing SAg-responsive MHC-II molecules. The redundancy of SAgs within S. pyogenes has also remained unexplained, and this work further illustrates that this may exist, in part, to overcome the highly polymorphic nature of human MHC-II molecules, and also to avoid natural host immunity to SAgs. Thus, SAgs are important contributors to the complex genotype-phenotype relationship that exists between S. pyogenes and humans, and these toxins should be considered further as valid targets for vaccination studies to impede the enormous burden of disease by this versatile pathogen.
Materials and Methods
Ethics statement
All animal experiments were in accordance with the Canadian Council on Animal Care Guide to the Care and Use of Experimental Animals. The animal protocol (#2009-038) was approved by the Animal Use Subcommittee at Western University.
Bacteria
S. pyogenes MGAS8232 is an M18 serotype that was isolated from a patient with acute rheumatic fever in Utah in 1987 and the genome has been sequenced and fully annotated [22]. S. pyogenes strains were grown in Todd Hewitt media supplemented with 1% (w/v) yeast extract. Deletions were made for all of the SAg genes using the pG+host5 system [52], [53]. All recombinant plasmids were built with standard molecular procedures using Escherichia coli XL1-blue as the cloning host [54]. Briefly, deletion constructs were generated by amplification of ∼500 bp of DNA on either side of the relevant SAg gene (Primers are listed in Table S1) and cloned into pG+host5. Flanking DNA included the first and last 8 codons for each SAg gene to generate precise, markerless and in frame deletions of each SAg gene. Plasmids were electroporated (Bio-Rad Gene Pulser XCell) into S. pyogenes MGAS8232 and single crossover integrations were selected at 40°C under erythromycin (1 µg ml−1) selection. PCR confirmed single crossover integrations were subcultured without antibiotics at 30°C and single clones were screened for a loss of erythromycin resistance, and double crossover gene disruptions were confirmed by PCR. In each case, appropriate PCR products were sequenced to confirm the expected deletion. All mutants were confirmed to lack growth alterations using Bioscreen C (Piscataway, NJ, USA) assays. To assess for protease activity, S. pyogenes strains were grown on dialyzed brain heart infusion agar containing 1.5% skim milk. Five microliters of OD600 0.1 S. pyogenes mutants were inoculated into 2 mm holes punched in the plates and incubated at 37°C for 24 hours. The ability of each strain to hydrolyze casein was assessed by the diameter of the zones of clearing. For speA and speAY100A complementation experiments, wild-type and mutant speA from MGAS8232 (including the native promoter) were individually ‘knocked in’ using the pG+host5 system to the non-coding region between genes encoding endopeptidase O (pepO) and elongation factor-Ts (tsf) and confirmed by PCR and DNA sequencing.
Superantigens and antibodies
Genes encoding for SpeA, SpeG, SpeL, SpeM, and SmeZ, lacking nucleotides encoding the predicted signal peptides, were PCR amplified and cloned into a modified pET-28a vector to introduce an engineered tobacco etch virus (TEV) protease cleavage site downstream from a His6 tag. Cloning of SpeC into the pET-41a vector has been described [55], and SmeZ was cloned in a similar manner to SpeC. All recombinant SAgs were produced by 200 µM isopropyl β-D-1-thiogalactopyranoside (IPTG)-induced expression in E. coli BL21(DE3), purified via nickel affinity chelation chromatography, and His6 tags were removed using autoinactivation resistant His6::TEV protease [56], as described [57], [58]. Proteins were run on 12% separating SDS-PAGE gels and Western blots were visualized on a LI-COR Odyssey (LI-COR Biosciences) using IRDye800 conjugated donkey anti-rabbit IgG as the secondary antibody (Rockland Inc.). All recombinant SAgs ran as discrete homogenous bands by SDS-PAGE (Figure 3D). Purified and lyophilized SpeA, SpeC, SpeG, SpeL, SpeM, and SmeZ were used to generate polyclonal rabbit antibodies from a commercial source (ProSci Incorporated, USA).
Nasopharyngeal infection model
HLA-expressing humanized mice (B6-DR4, B6-DQ8, B6-DR4/DQ8) have been previously described [12], [59], [60]. These mice were bred in a barrier facility at Western University and were routinely genotyped for the appropriate transgene(s). The mouse nasopharyngeal infection model has been described [61], with modifications. Briefly, mice were used at 9–13 weeks. Freshly grown exponential phase S. pyogenes cells (OD600 0.2–0.3) were washed and suspended in Hanks balanced saline solution [HBSS; a total of ∼1×108 (range 0.6–1.4×108) CFU per 15 µl] and 7.5 µl was used to inoculate each nostril under Forane (isoflurane, USP) inhalation anesthetic (Baxter Corporation). Sham treated mice only received HBSS. Mice were sacrificed at the noted time points, and the cNTs, including the nasal associated lymphoid tissue, nasal turbinates, and maxillary sinuses were removed. Tissue was homogenized in HBSS, serially diluted, and plated on Trypticase Soy Agar with 5% Sheep Blood plates (Becton, Dickinson and Company, MD, USA), or used for cytokine analysis, or flow cytometry.
Ex vivo experiments
For splenocyte activation experiments, cells were harvested from B6 or HLA-B6 mouse spleens, treated with ammonium-chloride-potassium (ACK) lysis buffer (15 mM NH4CL, 10 mM KHCO3, 0.1 mM EDTA) and suspended (2×105 cells per well) in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Sigma-Aldrich), 0.1 mm minimal essential medium (MEM) non-essential amino acids, 2 mm L-glutamine, 1 mm sodium pyruvate, 100 U ml−1 penicillin, 100 µg ml−1 streptomycin (all from Gibco Life Technologies) and 50 µM β-mercaptoethanol (Sigma). SAgs were added at the indicated concentrations and mouse IL-2 was determined after 18 h by enzyme-linked immunosorbent assay (eBioscience). Proliferation was measure by the addition of 1 µCi/well [3H]thymidine after 72 h and after another 18 h cells were harvested on fiberglass filters and [3H]thymidine incorporation was assessed on a 1450 Microbeta liquid scintillation counter (Wallac).
Molecular modelling
The SpeA Tyr100→Ala mutation was predicted to disrupt the low-affinity MHC-II binding domain of SpeA based on a model of the crystal structure of SpeA in complex with HLA-DQ8. To generate this model, the structures of SpeA (PDB: 1FNU) [62] and HLA-DQ8 (PDB: 1JK8) [63] were superpositioned onto the known SEC3:HLA-DR1 complex (PDB: 1JWM) [64], and visualized using Pymol (pymol.sourceforge.net). Mutagenesis of speA was conducted using megaprimer-PCR (Table S1) to introduce the SpeA Tyr100→Ala mutation.
Histology and immunofluorescence
Mice were fully anesthetized with Forane (isoflurane, USP) inhalation anaesthetic (Baxter Corporation) and perfused through the heart with sterile PBS containing heparin using a Gilson Minipuls 3 peristaltic pump (Middletown, WI, U.S.A) at a constant flow rate. Mice were then perfused with 10% neutral buffered formalin (BDH, VWR, West Chester, PA, USA) through the peristaltic pump. The head was soaked in 10 volumes of formalin for 24 hours and re-suspended in Shandon TBD-2 Decalcifier (TBD; Thermo Scientific, Kalamazoo, MI, USA) for 96 hours. TBD-decalcified heads were placed in formalin for 48 hours and washed with 1× PBS, and resuspended in 10-volumes of 1× PBS twice daily for 4 days, washed in 70% ethanol twice, and stored in 10-volumes of 70% ethanol. Cassettes were processed in Leica ASP300 fully enclosed paraffin wax tissue processor overnight using the ‘bone’ program and embedded in paraffin wax. Heads were serially sectioned between the first and second molar on a HM335E Microtome (Leica) into 5 micron sections, mounted on Fisherbrand Superfrost Plus microscope slides (Fisher Scientific, Fair Lawn, NJ, USA) and dried at 45°C for 48 h prior to storage/staining. Tissues were stained with H&E in a Leica Autostainer XL.
H&E stained slides were evaluated by an experienced mouse pathologist in a blinded fashion. The relative amount of mucus present covering the epithelia, the presence of red blood cells, and the presence of nucleated cellular debris on the surface of the epithelia was assessed and the presence and severity of these findings were used to assign a score of zero to two points to each of two sections per mouse (n = 5 mice per group). The scores were averaged to determine differences in histological pathology in the mice. Fluorescence staining was done with adjacent serial sections using a Goat α-S. pyogenes polyclonal (NB200-643; Novus Biologicals) at 1∶100 dilution, and donkey α-Goat Alexflour 595 (A-11058; Invitrogen) at 1∶1000 dilution. Images were captured using an upright BX61 fluorescent microscope (Olympus).
Flow cytometry
For flow cytometry analysis, isolated cells (from cNTs, lymph nodes, or spleens) were aliquoted at 500,000 cells per 5 ml tube, and pre-treated with Fc block (hybridoma clone 2.4G2) prior to cell staining. Staining was done in panels using the following antibodies: α-CD3-APC (clone 145-2C11), α-CD3-PE Cy7 (clone 53–7.3), α-CD8 PE Cy7 (clone 53–6.7), α-CD45 PE (clone 30-F11), α-CD19 (clone MB19-1), α-NK1.1 (clone PK136), α-CD11c (clone N418), α-F4/80 (clone BM8), and α-GR1 (clone RB6-8C5) (all from eBioScience); α-CD4 APC Cy7 (clone GK1.5; Biolegend); and α-CD45 Alexafluor 700 (clone 30-F11; BD Biosciences). Dead cells were excluded using 7-AAD (BD Biosciences). Antibodies to stain cells for each panel were added, mixed, and incubated on ice in the dark for 30 minutes. Cells were washed twice with 1× PBS +5% FBS and resuspended in 500 µl of 1× PBS +5% FBS. Stained cells were run on a BD FACS Canto II flow cytometer (BD Biosciences). Standard compensations were used for each tissue using FACSdiva software.
Multiplex cytokine analysis
Cytokine concentrations were determined from cNT homogenates isolated from mice treated with HBSS (sham), wild-type S. pyogenes MGAS8232, or isogenic S. pyogenes MGAS8232 ΔSAg strains at either 24 or 48 hours post-infection in HLA-B6 mice. Multiplex bead arrays were performed using the Mouse Cytokine 32-plex Discovery Array (Eve Technologies). Heat maps were generated using the Matrix2png algorithm [65] and data is shown as the average cytokine responses from 3–4 mice per group. Quantitative data from the cytokine analyses are shown in Figure S2.
Vaccination experiments
For vaccination experiments, 6–8 week old HLA-B6 mice were injected subcutaneously with 1 µg of recombinant SpeAY100A (or sham) emulsified in Imject Alum Adjuvant (Thermo Fisher Scientific Inc.) every 2 weeks for a total of three injections. Two weeks following the last injection, mice were bled for antibody titers as determined by direct ELISA against 1 µg wild-type SpeA per well, and calculated as the reciprocal of the lowest serum dilution with readings 4-fold above background. Mice were challenged with ∼1×108 CFU wild-type MGAS8232 as described above 24 hours after the final bleed and CFUs were determined 48 h post infection.
Statistical analyses
When appropriate, individual data points, or the mean ± SEM, are shown, and p values were calculated using the Student's t-test with Prism software (GraphPad). A p value of less than 0.05 was determined to be statistically significant.
Supporting Information
Zdroje
1. CarapetisJR, SteerAC, MulhollandEK, WeberM (2005) The global burden of group A streptococcal diseases. Lancet Infect Dis 5 : 685–694.
2. ShaikhN, LeonardE, MartinJM (2010) Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics 126: e557–564.
3. JohnsonDR, KurlanR, LeckmanJ, KaplanEL (2010) The human immune response to streptococcal extracellular antigens: clinical, diagnostic, and potential pathogenetic implications. Clin Infect Dis 50 : 481–490.
4. LefebureT, RichardsVP, LangP, Pavinski-BitarP, StanhopeMJ (2012) Gene repertoire evolution of Streptococcus pyogenes inferred from phylogenomic analysis with Streptococcus canis and Streptococcus dysgalactiae. PloS One 7: e37607.
5. McCormickJK, YarwoodJM, SchlievertPM (2001) Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol 55 : 77–104.
6. CommonsRJ, SmeestersPR, ProftT, FraserJD, Robins-BrowneR, et al. (2013) Streptococcal superantigens: Categorization and clinical associations. Trends Mol Med 20 : 48–62.
7. BeresSB, MusserJM (2007) Contribution of exogenous genetic elements to the group A Streptococcus metagenome. PloS One 2: e800.
8. OkumuraK, ShimomuraY, MurayamaSY, YagiJ, UbukataK, et al. (2012) Evolutionary paths of streptococcal and staphylococcal superantigens. BMC Genomics 13 : 404.
9. PanchaudA, GuyL, CollynF, HaenniM, NakataM, et al. (2009) M-protein and other intrinsic virulence factors of Streptococcus pyogenes are encoded on an ancient pathogenicity island. BMC Genomics 10 : 198.
10. SundbergEJ, DengL, MariuzzaRA (2007) TCR recognition of peptide/MHC class II complexes and superantigens. Semin Immunol 19 : 262–271.
11. KotbM, Norrby-TeglundA, McGeerA, El-SherbiniH, DorakMT, et al. (2002) An immunogenetic and molecular basis for differences in outcomes of invasive group A streptococcal infections. Nat Med 8 : 1398–1404.
12. NoohMM, El-GengehiN, KansalR, DavidCS, KotbM (2007) HLA transgenic mice provide evidence for a direct and dominant role of HLA class II variation in modulating the severity of streptococcal sepsis. J Immunol 178 : 3076–3083.
13. Norrby-TeglundA, ChatellierS, LowDE, McGeerA, GreenK, et al. (2000) Host variation in cytokine responses to superantigens determine the severity of invasive group A streptococcal infection. Eur J Immunol 30 : 3247–3255.
14. SriskandanS, UnnikrishnanM, KrauszT, DewchandH, Van NoordenS, et al. (2001) Enhanced susceptibility to superantigen-associated streptococcal sepsis in human leukocyte antigen-DQ transgenic mice. J Infect Dis 184 : 166–173.
15. LlewelynM, SriskandanS, PeakmanM, AmbrozakDR, DouekDC, et al. (2004) HLA class II polymorphisms determine responses to bacterial superantigens. J Immunol 172 : 1719–1726.
16. DickGF, DickGH (1924) A skin test for susceptibility to scarlet fever. JAMA 82 : 256–266.
17. SchlievertPM, BettinKM, WatsonDW (1979) Reinterpretation of the Dick test: role of group A streptococcal pyrogenic exotoxin. Infect Immun 26 : 467–472.
18. ConeLA, WoodardDR, SchlievertPM, TomoryGS (1987) Clinical and bacteriologic observations of a toxic shock-like syndrome due to Streptococcus pyogenes. N Engl J Med 317 : 146–149.
19. StevensDL, TannerMH, WinshipJ, SwartsR, RiesKM, et al. (1989) Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med 321 : 1–7.
20. ParkHS, CostalongaM, ReinhardtRL, DombekPE, JenkinsMK, et al. (2004) Primary induction of CD4 T cell responses in nasal associated lymphoid tissue during group A streptococcal infection. Eur J Immunol 34 : 2843–2853.
21. WangB, DileepanT, BriscoeS, HylandKA, KangJ, et al. (2010) Induction of TGF-beta1 and TGF-beta1-dependent predominant Th17 differentiation by group A streptococcal infection. Proc Natl Acad Sci U S A 107 : 5937–5942.
22. SmootJC, BarbianKD, Van GompelJJ, SmootLM, ChausseeMS, et al. (2002) Genome sequence and comparative microarray analysis of serotype M18 group A Streptococcus strains associated with acute rheumatic fever outbreaks. Proc Natl Acad Sci U S A 99 : 4668–4673.
23. ThibodeauJ, LabrecqueN, DenisF, HuberBT, SekalyRP (1994) Binding sites for bacterial and endogenous retroviral superantigens can be dissociated on major histocompatibility complex class II molecules. J Exp Med 179 : 1029–1034.
24. UlrichRG, BavariS, OlsonMA (1995) Staphylococcal enterotoxins A and B share a common structural motif for binding class II major histocompatibility complex molecules. Nat Struct Biol 2 : 554–560.
25. O'BrienKL, BeallB, BarrettNL, CieslakPR, ReingoldA, et al. (2002) Epidemiology of invasive group A Streptococcus disease in the United States, 1995–1999. Clin Infect Dis 35 : 268–276.
26. Sanderson-SmithML, DinklaK, ColeJN, CorkAJ, MaamaryPG, et al. (2008) M protein-mediated plasminogen binding is essential for the virulence of an invasive Streptococcus pyogenes isolate. FASEB J 22 : 2715–2722.
27. SunH, XuY, SitkiewiczI, MaY, WangX, et al. (2012) Inhibitor of streptokinase gene expression improves survival after group A Streptococcus infection in mice. Proc Natl Acad Sci U S A 109 : 3469–3474.
28. MatsuiH, SekiyaY, NakamuraM, MurayamaSY, YoshidaH, et al. (2009) CD46 transgenic mouse model of necrotizing fasciitis caused by Streptococcus pyogenes infection. Infect Immun 77 : 4806–4814.
29. SugaharaK, SchwartzNB, DorfmanA (1979) Biosynthesis of hyaluronic acid by Streptococcus. J Biol Chem 254 : 6252–6261.
30. WesselsMR, BronzeMS (1994) Critical role of the group A streptococcal capsule in pharyngeal colonization and infection in mice. Proc Natl Acad Sci U S A 91 : 12238–12242.
31. CywesC, StamenkovicI, WesselsMR (2000) CD44 as a receptor for colonization of the pharynx by group A Streptococcus. J Clin Invest 106 : 995–1002.
32. BanerjiS, WrightAJ, NobleM, MahoneyDJ, CampbellID, et al. (2007) Structures of the Cd44-hyaluronan complex provide insight into a fundamental carbohydrate-protein interaction. Nat Struct Mol Biol 14 : 234–239.
33. LoofTG, RohdeM, ChhatwalGS, JungS, MedinaE (2007) The contribution of dendritic cells to host defenses against Streptococcus pyogenes. J Infect Dis 196 : 1794–1803.
34. CortesG, WesselsMR (2009) Inhibition of dendritic cell maturation by group A Streptococcus. J Infect Dis 200 : 1152–1161.
35. MurailleE, De SmedtT, AndrisF, PajakB, ArmantM, et al. (1997) Staphylococcal enterotoxin B induces an early and transient state of immunosuppression characterized by V beta-unrestricted T cell unresponsiveness and defective antigen-presenting cell functions. J Immunol 158 : 2638–2647.
36. DileepanT, LinehanJL, MoonJJ, PepperM, JenkinsMK, et al. (2011) Robust antigen specific Th17 T cell response to group A Streptococcus is dependent on IL-6 and intranasal route of infection. PLoS pathogens 7: e1002252.
37. LaPentaD, RubensC, ChiE, ClearyPP (1994) Group A streptococci efficiently invade human respiratory epithelial cells. Proc Natl Acad Sci U S A 91 : 12115–12119.
38. SchragerHM, RheinwaldJG, WesselsMR (1996) Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J Clin Invest 98 : 1954–1958.
39. UnnikrishnanM, AltmannDM, ProftT, WahidF, CohenJ, et al. (2002) The bacterial superantigen streptococcal mitogenic exotoxin Z is the major immunoactive agent of Streptococcus pyogenes. J Immunol 169 : 2561–2569.
40. McCormickJK, TrippTJ, OlmstedSB, MatsukaYV, GahrPJ, et al. (2000) Development of streptococcal pyrogenic exotoxin C vaccine toxoids that are protective in the rabbit model of toxic shock syndrome. J Immunol 165 : 2306–2312.
41. RoggianiM, StoehrJA, OlmstedSB, MatsukaYV, PillaiS, et al. (2000) Toxoids of streptococcal pyrogenic exotoxin A are protective in rabbit models of streptococcal toxic shock syndrome. Infect Immun 68 : 5011–5017.
42. WelcherBC, CarraJH, DaSilvaL, HansonJ, DavidCS, et al. (2002) Lethal shock induced by streptococcal pyrogenic exotoxin A in mice transgenic for human leukocyte antigen-DQ8 and human CD4 receptors: implications for development of vaccines and therapeutics. J Infect Dis 186 : 501–510.
43. DagnelieCF, van der GraafY, De MelkerRA (1996) Do patients with sore throat benefit from penicillin? A randomized double-blind placebo-controlled clinical trial with penicillin V in general practice. Br J Gen Pract 46 : 589–593.
44. BarsumianEL, SchlievertPM, WatsonDW (1978) Nonspecific and specific immunological mitogenicity by group A streptococcal pyrogenic exotoxins. Infect Immun 22 : 681–688.
45. KamezawaY, NakaharaT, NakanoS, AbeY, Nozaki-RenardJ, et al. (1997) Streptococcal mitogenic exotoxin Z, a novel acidic superantigenic toxin produced by a T1 strain of Streptococcus pyogenes. Infect Immun 65 : 3828–3833.
46. ProftT, MoffattSL, BerkahnCJ, FraserJD (1999) Identification and characterization of novel superantigens from Streptococcus pyogenes. J Exp Med 189 : 89–102.
47. SmootLM, McCormickJK, SmootJC, HoeNP, StricklandI, et al. (2002) Characterization of two novel pyrogenic toxin superantigens made by an acute rheumatic fever clone of Streptococcus pyogenes associated with multiple disease outbreaks. Infect Immun 70 : 7095–7104.
48. LiPL, TiedemannRE, MoffatSL, FraserJD (1997) The superantigen streptococcal pyrogenic exotoxin C (SPE-C) exhibits a novel mode of action. J Exp Med 186 : 375–383.
49. BasmaH, Norrby-TeglundA, GuedezY, McGeerA, LowDE, et al. (1999) Risk factors in the pathogenesis of invasive group A streptococcal infections: role of protective humoral immunity. Infect Immun 67 : 1871–1877.
50. ErikssonBK, AnderssonJ, HolmSE, NorgrenM (1999) Invasive group A streptococcal infections: T1M1 isolates expressing pyrogenic exotoxins A and B in combination with selective lack of toxin-neutralizing antibodies are associated with increased risk of streptococcal toxic shock syndrome. J Infect Dis 180 : 410–418.
51. Norrby-TeglundA, PauksensK, HolmSE, NorgrenM (1994) Relation between low capacity of human sera to inhibit streptococcal mitogens and serious manifestation of disease. J Infect Dis 170 : 585–591.
52. BiswasI, GrussA, EhrlichSD, MaguinE (1993) High-efficiency gene inactivation and replacement system for gram-positive bacteria. J Bacteriol 175 : 3628–3635.
53. JiY, McLandsboroughL, KondaguntaA, ClearyPP (1996) C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect Immun 64 : 503–510.
54. Sambrook J, Russell DW (2001) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press.
55. Nur-ur RahmanAK, BonsorDA, HerfstCA, PollardF, PeirceM, et al. (2011) The T cell receptor beta-chain second complementarity determining region loop (CDR2beta) governs T cell activation and Vbeta specificity by bacterial superantigens. J Biol Chem 286 : 4871–4881.
56. KapustRB, TozserJ, FoxJD, AndersonDE, CherryS, et al. (2001) Tobacco etch virus protease: mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency. Protein Eng 14 : 993–1000.
57. BrouillardJN, GuntherS, VarmaAK, GryskiI, HerfstCA, et al. (2007) Crystal structure of the streptococcal superantigen SpeI and functional role of a novel loop domain in T cell activation by group V superantigens. J Mol Biol 367 : 925–934.
58. GuntherS, VarmaAK, MozaB, KasperKJ, WyattAW, et al. (2007) A novel loop domain in superantigens extends their T cell receptor recognition site. J Mol Biol 371 : 210–221.
59. ItoK, BianHJ, MolinaM, HanJ, MagramJ, et al. (1996) HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J Exp Med 183 : 2635–2644.
60. HillJA, BellDA, BrintnellW, YueD, WehrliB, et al. (2008) Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice. J Exp Med 205 : 967–979.
61. ParkHS, FrancisKP, YuJ, ClearyPP (2003) Membranous cells in nasal-associated lymphoid tissue: a portal of entry for the respiratory mucosal pathogen group A Streptococcus. J Immunol 171 : 2532–2537.
62. EarhartCA, VathGM, RoggianiM, SchlievertPM, OhlendorfDH (2000) Structure of streptococcal pyrogenic exotoxin A reveals a novel metal cluster. Protein Sci 9 : 1847–1851.
63. LeeKH, WucherpfennigKW, WileyDC (2001) Structure of a human insulin peptide-HLA-DQ8 complex and susceptibility to type 1 diabetes. Nat Immun 2 : 501–507.
64. SundbergEJ, AndersenPS, SchlievertPM, KarjalainenK, MariuzzaRA (2003) Structural, energetic, and functional analysis of a protein-protein interface at distinct stages of affinity maturation. Structure 11 : 1151–1161.
65. PavlidisP, NobleWS (2003) Matrix2png: a utility for visualizing matrix data. Bioinformatics 19 : 295–296.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge inČlánek Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 5- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Surveillance for Emerging Biodiversity Diseases of Wildlife
- The Emerging Role of Urease as a General Microbial Virulence Factor
- PARV4: An Emerging Tetraparvovirus
- Epigenetic Changes Modulate Schistosome Egg Formation and Are a Novel Target for Reducing Transmission of Schistosomiasis
- The Human Adenovirus E4-ORF1 Protein Subverts Discs Large 1 to Mediate Membrane Recruitment and Dysregulation of Phosphatidylinositol 3-Kinase
- A Multifactorial Role for Malaria in Endemic Burkitt's Lymphoma Pathogenesis
- Structural Basis for the Ubiquitin-Linkage Specificity and deISGylating Activity of SARS-CoV Papain-Like Protease
- Cathepsin-L Can Resist Lysis by Human Serum in
- Epstein-Barr Virus Down-Regulates Tumor Suppressor Expression
- BCA2/Rabring7 Targets HIV-1 Gag for Lysosomal Degradation in a Tetherin-Independent Manner
- The Evolutionarily Conserved Mediator Subunit MDT-15/MED15 Links Protective Innate Immune Responses and Xenobiotic Detoxification
- Suppressor of Cytokine Signaling 4 (SOCS4) Protects against Severe Cytokine Storm and Enhances Viral Clearance during Influenza Infection
- T Cell Inactivation by Poxviral B22 Family Proteins Increases Viral Virulence
- Dynamics of HIV Latency and Reactivation in a Primary CD4+ T Cell Model
- HIV and HCV Activate the Inflammasome in Monocytes and Macrophages via Endosomal Toll-Like Receptors without Induction of Type 1 Interferon
- Virus and Autoantigen-Specific CD4+ T Cells Are Key Effectors in a SCID Mouse Model of EBV-Associated Post-Transplant Lymphoproliferative Disorders
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- Squalene Synthase As a Target for Chagas Disease Therapeutics
- The Contribution of Viral Genotype to Plasma Viral Set-Point in HIV Infection
- Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge in
- Anthrax Lethal Factor as an Immune Target in Humans and Transgenic Mice and the Impact of HLA Polymorphism on CD4 T Cell Immunity
- Ly49C-Dependent Control of MCMV Infection by NK Cells Is -Regulated by MHC Class I Molecules
- Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
- A Large Family of Antivirulence Regulators Modulates the Effects of Transcriptional Activators in Gram-negative Pathogenic Bacteria
- Broad-Spectrum Anti-biofilm Peptide That Targets a Cellular Stress Response
- Malaria Parasite Infection Compromises Control of Concurrent Systemic Non-typhoidal Infection via IL-10-Mediated Alteration of Myeloid Cell Function
- A Role for in Higher Order Structure and Complement Binding of the Capsule
- Hip1 Modulates Macrophage Responses through Proteolysis of GroEL2
- CD8 T Cells from a Novel T Cell Receptor Transgenic Mouse Induce Liver-Stage Immunity That Can Be Boosted by Blood-Stage Infection in Rodent Malaria
- Phosphorylation of KasB Regulates Virulence and Acid-Fastness in
- HIV-Infected Individuals with Low CD4/CD8 Ratio despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened CD8+ T Cell Activation, and Increased Risk of Non-AIDS Morbidity and Mortality
- A Novel Mechanism Inducing Genome Instability in Kaposi's Sarcoma-Associated Herpesvirus Infected Cells
- Structural and Biochemical Characterization Reveals LysGH15 as an Unprecedented “EF-Hand-Like” Calcium-Binding Phage Lysin
- Hepatitis C Virus Cell-Cell Transmission and Resistance to Direct-Acting Antiviral Agents
- Different Modes of Retrovirus Restriction by Human APOBEC3A and APOBEC3G
- TNFα and IFNγ but Not Perforin Are Critical for CD8 T Cell-Mediated Protection against Pulmonary Infection
- Large Scale RNAi Reveals the Requirement of Nuclear Envelope Breakdown for Nuclear Import of Human Papillomaviruses
- The Cytoplasmic Domain of Varicella-Zoster Virus Glycoprotein H Regulates Syncytia Formation and Skin Pathogenesis
- A New Class of Multimerization Selective Inhibitors of HIV-1 Integrase
- Are We There Yet? The Smallpox Research Agenda Using Variola Virus
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
- Dynamic Functional Modulation of CD4 T Cell Recall Responses Is Dependent on the Inflammatory Environment of the Secondary Stimulus
- Bacterial Superantigens Promote Acute Nasopharyngeal Infection by in a Human MHC Class II-Dependent Manner
- Follicular Helper T Cells Promote Liver Pathology in Mice during Infection
- A Nasal Epithelial Receptor for WTA Governs Adhesion to Epithelial Cells and Modulates Nasal Colonization
- Unexpected Role for IL-17 in Protective Immunity against Hypervirulent HN878 Infection
- Human Cytomegalovirus Fcγ Binding Proteins gp34 and gp68 Antagonize Fcγ Receptors I, II and III
- Expansion of Murine Gammaherpesvirus Latently Infected B Cells Requires T Follicular Help
- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Molecular Signatures of Hemagglutinin Stem-Directed Heterosubtypic Human Neutralizing Antibodies against Influenza A Viruses
- The Downregulation of GFI1 by the EZH2-NDY1/KDM2B-JARID2 Axis and by Human Cytomegalovirus (HCMV) Associated Factors Allows the Activation of the HCMV Major IE Promoter and the Transition to Productive Infection
- Inactivation of Fructose-1,6-Bisphosphate Aldolase Prevents Optimal Co-catabolism of Glycolytic and Gluconeogenic Carbon Substrates in
- New Insights into Rotavirus Entry Machinery: Stabilization of Rotavirus Spike Conformation Is Independent of Trypsin Cleavage
- Prophenoloxidase Activation Is Required for Survival to Microbial Infections in
- SslE Elicits Functional Antibodies That Impair Mucinase Activity and Colonization by Both Intestinal and Extraintestinal Strains
- Timed Action of IL-27 Protects from Immunopathology while Preserving Defense in Influenza
- HIV-1 Envelope gp41 Broadly Neutralizing Antibodies: Hurdles for Vaccine Development
- The PhoP-Dependent ncRNA Mcr7 Modulates the TAT Secretion System in
- Cellular Superspreaders: An Epidemiological Perspective on HIV Infection inside the Body
- The Inflammasome Pyrin Contributes to Pertussis Toxin-Induced IL-1β Synthesis, Neutrophil Intravascular Crawling and Autoimmune Encephalomyelitis
- Papillomavirus Genomes Associate with BRD4 to Replicate at Fragile Sites in the Host Genome
- Integrative Functional Genomics of Hepatitis C Virus Infection Identifies Host Dependencies in Complete Viral Replication Cycle
- Co-assembly of Viral Envelope Glycoproteins Regulates Their Polarized Sorting in Neurons
- Targeting Membrane-Bound Viral RNA Synthesis Reveals Potent Inhibition of Diverse Coronaviruses Including the Middle East Respiratory Syndrome Virus
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



