-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Prophenoloxidase Activation Is Required for Survival to Microbial Infections in
The melanization reaction is a major immune response in Arthropods and involves the rapid synthesis of a black pigment, melanin, at the site of infection and injury. Melanization requires the activation of proPhenoloxidase, an enzyme that catalyzes the oxidation of phenols to quinones, which polymerize to melanin. The Drosophila genome contains three genes encoding prophenoloxidases (PPO). In this paper, we have generated flies carrying deletions in the PPO1 and PPO2 genes. By analyzing these mutations alone and in combination, we clarify the functions of both prophenoloxidases in humoral melanization. We report that PPO2 composes most of the crystals found in crystal cells, a specific hemocyte cell type. Although PPO1 and PPO2 both contribute to phenoloxidase activity in the insect blood, these PPOs are not fully redundant. Our study shows that PPO1 is involved in the rapid delivery of phenoloxidase activity when required, while PPO2 provides a storage form that can be deployed in a second phase. Some controversy exists in the Drosophila field about the importance of melanization in the Drosophila host defense. Our study demonstrates the important role of PPO1 and PPO2 in the survival to infection with both Gram-positive bacteria and fungi, underlining the importance of melanization in insect immunity.
Published in the journal: . PLoS Pathog 10(5): e32767. doi:10.1371/journal.ppat.1004067
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004067Summary
The melanization reaction is a major immune response in Arthropods and involves the rapid synthesis of a black pigment, melanin, at the site of infection and injury. Melanization requires the activation of proPhenoloxidase, an enzyme that catalyzes the oxidation of phenols to quinones, which polymerize to melanin. The Drosophila genome contains three genes encoding prophenoloxidases (PPO). In this paper, we have generated flies carrying deletions in the PPO1 and PPO2 genes. By analyzing these mutations alone and in combination, we clarify the functions of both prophenoloxidases in humoral melanization. We report that PPO2 composes most of the crystals found in crystal cells, a specific hemocyte cell type. Although PPO1 and PPO2 both contribute to phenoloxidase activity in the insect blood, these PPOs are not fully redundant. Our study shows that PPO1 is involved in the rapid delivery of phenoloxidase activity when required, while PPO2 provides a storage form that can be deployed in a second phase. Some controversy exists in the Drosophila field about the importance of melanization in the Drosophila host defense. Our study demonstrates the important role of PPO1 and PPO2 in the survival to infection with both Gram-positive bacteria and fungi, underlining the importance of melanization in insect immunity.
Introduction
One of the most immediate immune responses in arthropods is the melanization reaction [1], [2]. It involves the rapid synthesis of melanin at the site of infection or injury in order to contain a microbial pathogen as well as to facilitate wound healing. A key enzyme in melanin biosynthesis is phenoloxidase (PO), which catalyzes the oxidation of phenols to quinones, which subsequently polymerize into melanin. PO is usually synthesized as an inactive zymogen called proPO (PPO), which is cleaved to generate active PO as a result of proteolytic cascade activation. Several roles have been ascribed to the melanization reaction in insects [3], [4]. PO activity contributes to wound healing by forming a scab at the epithelial site of injury. By-products of PO activity are reactive oxygen species (ROS), which are thought to contribute to the killing of microbes and pathogens. Finally, melanization participates in the encapsulation reaction against parasites. Deposition of melanin on the parasite forms a physical barrier, allowing the localized and confined production of toxic compounds while ensuring the protection of the host.
Despite extensive genetic studies of the Drosophila immune response, the melanization reaction remains one of its less characterized facets. The Drosophila genome encodes three PPOs. Two of them, PPO1 and PPO2, are found in the crystal cells and possibly in the hemolymph at the larval stage. Crystal cells represent 5% of the hemocyte (blood cells) population of larva [5]–[7]. Upon injury, crystal cells rupture and release PPOs into the hemolymph where they are activated by serine proteases [8]. Although PPO1 and PPO2 are found in the hemolymph compartment in adults, their precise sites of synthesis have not been characterized. Notably, the presence of crystal cells has not yet been established in adult flies [7]. Some reports have suggested that PPO3 is expressed in crystal cells [9] while others suggest an expression in lamellocytes [10], [11]. Lamellocytes are larval hemocytes involved in the encapsulation of parasites such as parasitoid wasps. The production of PPO3 in lamellocytes points to a role of this enzyme in capsule formation. While PPO3 is produced under an active form, both PPO1 and 2 require a proteolytic cleavage to be activated [12]. The cleavage of PPO1 is mediated by a clip-domain serine protease (SP) named Hayan [13]. Hayan also exists as an inactive zymogen that is itself stimulated through a stepwise process involving other serine proteases, whose activities are all controlled by protease inhibitors named serpins. Two clip-domain SPs, MP1 and the crystal cell-specific Sp7 (also referred to as MP2 and PAE), and several serpins have been implicated in the melanization cascade upstream of Hayan [14]–[21]. Inactivation of MP1 or MP2 reduces the level of PO activity after immune challenge, while excessive melanization is usually observed in Serpin-deficient mutants. Studies in other insect species indicate that the SP cascades upstream of PPO are triggered by injury or by pathogen recognition receptors detecting microbial ligands, such as peptidoglycan or β(1,3)-glucan [22]–[24]. Accordingly, full PO activation in Drosophila also requires triggering of Toll pathway-specific pattern-recognition receptors by Gram - positive bacteria (via GNBP1 and PGRP-SA) or fungi (via GNBP3) [25]. These receptors are found in the hemolymph of flies and probably activate PO by SPs distinct from those triggering Toll activation by Spätzle. The melanization cascade is also regulated at the transcriptional level since many transcripts encoding related enzymes, SPs or serpins, are upregulated in the fat body by the Toll and Imd pathways in response to infection [14], [15].
Several studies have analyzed the contribution of melanization to survival of flies with variable results. These studies have used fly stocks carrying either mutations that abolish (almost) all PO hemolymphatic activity (using the uncharacterized mutation Black cells or Hayan1) or reduce it (Sp7 mutation or in vivo RNAi against Sp7 or MP1) [13], [16]–[18], [26]. It was observed that both Black cells and Hayan1 flies exhibit increased susceptibility to large injury [11], [27]. Black cells but not Sp7 flies were shown to be susceptible to the Gram-positive bacterium Enterococcus faecalis [17], [26]. Black cells and Sp7 RNAi flies but not the Sp7 mutants were shown to be susceptible to the entomopathogenic fungus Beauveria bassiana [14], [17], [18]. An extensive study also reports increased susceptibility of the Sp7 mutants to the Gram-negative bacteria Salmonella typhimurium and Gram-positive bacteria Listeria monocytogenes and Staphylococcus aureus [26];although a susceptibility of the Sp7 mutant to S. aureus was not observed in another report [17].
These studies show that our knowledge of the function and regulation of POs in Drosophila is still scarce. One reason is that most studies have used a top-down approach focusing on the most upstream steps of the melanization reaction, which appears ramified. In this study, we have focused on the downstream step of the melanization cascade by generating flies carrying deletions for PPO1 and/or PPO2. By analyzing these mutations alone and in combination, we characterize the functions of both PPOs in the melanization reaction. Our study also confirms and extends previous studies showing an essential role of this reaction against some Gram-positive bacteria and fungi and wasp encapsulation.
Results
A gene-deletion strategy to study Phenoloxidase function
Through homologous recombination, we replaced the entire PPO1 locus (Chromosome II, position 55C) with a copy of the white gene (referred to as PPO1Δ) (Fig. 1A). We also generated a deletion of 5.2 kb removing the entire coding sequence of PPO2 (Chromosome II, position 45A1) by imprecise excision of a Minos transposon (referred to as PPO2Δ). The latter deletion also removes 2 kb of the 3′ non-coding region of the CG13743 gene, which encodes an amino-acid transporter (Fig. 1A). Both PPO deficient lines were backcrossed five times into either w1118 or OregonR to homogenize the genetic background. In this study, we mainly used w1118, PPO deficient flies but obtained similar results with lines backcrossed in OregonR. To explore possible redundancy between the two genes, a double mutant (PPO1Δ, PPO2Δ) was produced by meiotic recombination. The absence of PPO1 and PPO2 transcripts in the respective mutants was confirmed by RT-qPCR (Fig. S1A). We also verified that the PPO2 deletion did not affect the expression of the flanking CG13743 gene (Fig. S1B). Both PPO1Δ and PPO2Δ mutants and the PPO1Δ, PPO2Δ double mutants are viable and do not exhibit any overt developmental or pigmentation defect (Fig. S1C). We noticed that the PPO1Δ, PPO2Δ double mutants have a significantly shorter lifespan (half-life = 24 days, p<0.0001 by Log rank test) than the single mutants and wild-type (Fig. 1B). A similar trend towards reduced lifespan was observed when PPO1Δ, PPO2Δ double mutant flies were raised in germ-free conditions (Fig. S1D), suggesting that early lethality is not due to infection. In this study, we focused on the role of PPOs in the defense against microbial pathogens in larvae and adults.
Fig. 1. Generation and phenotypic characterization of PPO1 and PPO2 deletion mutants. 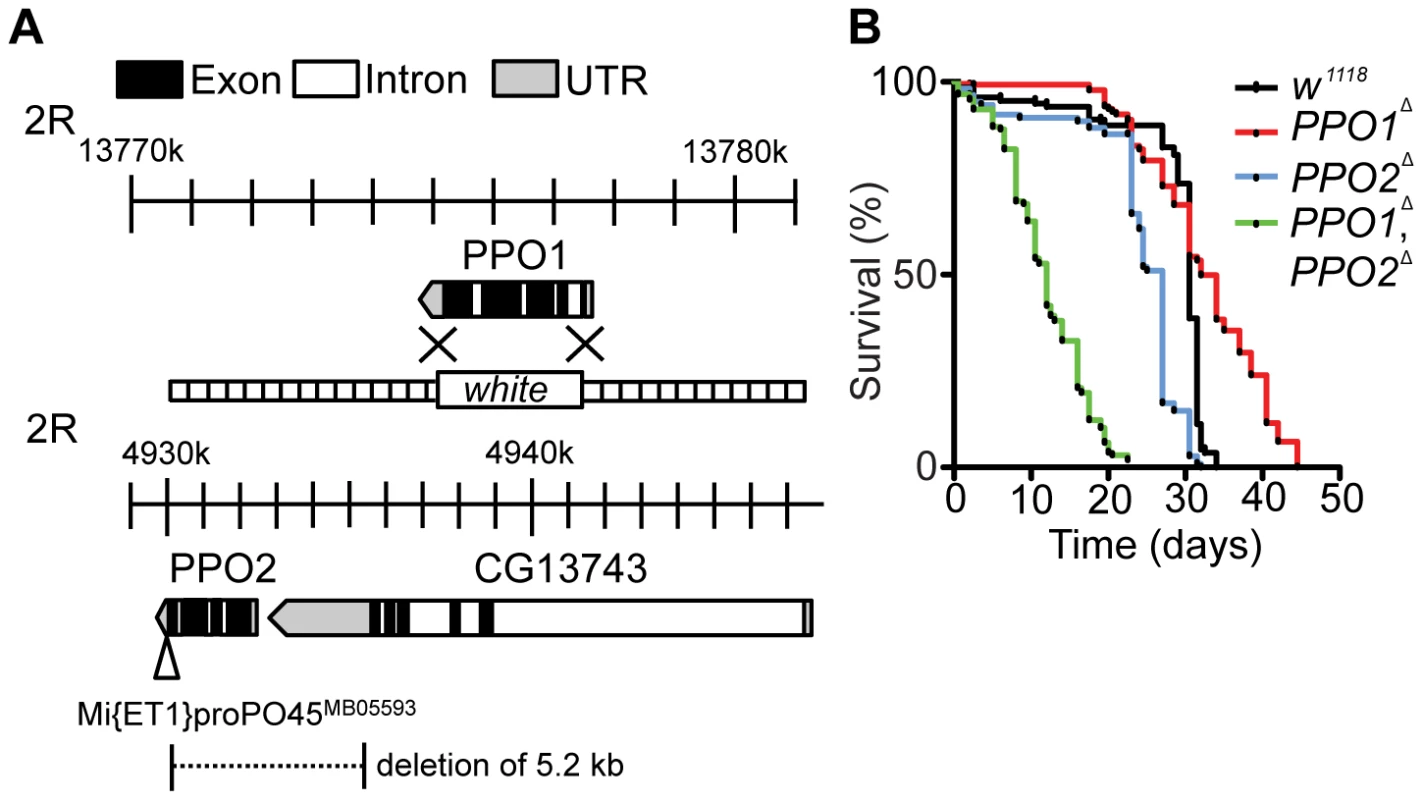
(A) Schematic representation of PPO1Δ and PPO2Δ deletions. The gene map was adapted from FlyBase and includes PPO1 (top) and PPO2 with its neighboring gene CG13743 (bottom). The PPO2 mutant was generated after the mobilization of the transposable element Mi{ET1}PPO2 MB05593 inserted in the 3′ end of PPO2. The imprecise excision deleted a fragment of 5.2 kb including the 3′ non-coding sequence of the neighboring gene CG13743. PPO1Δ mutant flies were generated by homologous recombination of PPO1 with the w gene. The flanking sequences used for recombination (dotted lines) are indicated. (B) Lifespan analysis of unchallenged flies reveals an increased mortality of PPO1Δ, PPO2Δ double mutants (***, p<0.0005). Each survival curve corresponds to one experiment of 3 samples with 20 flies each. p values were calculated using the Log-rank test. PPO2 is stored in the crystals of crystal cells
In Drosophila larvae, PPO1 and 2 are synthetized by specific hemocytes termed crystal cells [5], [28]. Massive release of PPO involves both rupture of crystal cells and dissolution of the crystals [8]. It has long been assumed but never really demonstrated that the crystals within crystal cells are composed of PPO. To clarify this point, we analyzed the morphology of crystal cells in third instar PPO mutant larvae. As crystal cells make up only 5% of all larval hemocytes [6], [7], we used the crystal cell marker lozenge (Lz-Gal4, UAS-GFP) to identify crystal cells in hemolymph preparations of PPO mutants [29]. Fig. 2A–B shows that crystals are present in both wild-type and PPO1 crystal cells (arrows in bright field panels) but in neither PPO2Δ nor PPO1Δ, PPO2Δ double mutants. This indicates that PPO2 is either the major component of the crystals or that it is required for their formation. Heating larvae at 65°C for 10 min induces the spontaneous activation of PPO within the crystal cells [5]. As a consequence of this treatment, crystal cells are easily visualized through the cuticle as black dots. We used this method to analyze how PPO1 and PPO2 contribute to crystal cell melanization. Consistent with our observations, PPO2Δ mutant larvae and PPO1Δ, PPO2Δ double mutants did not show any melanized dots upon heating while PPO1 behaved like wild-type (Fig. 2C and Fig. 2D). PPO2 - specific antibody staining co-localizes with crystals, demonstrating that crystals are indeed composed of PPO2 (Fig. 2B). In contrast, no overt signal could be detected in crystal cells using an antibody that recognizes the native form of PPO1 (Fig. 2A). Nevertheless, we cannot exclude that the anti-PPO1 antibody does not work in immunostaining experiments or that a low amount PPO1 is still present in the crystals but hidden by PPO2. To confirm that crystal cells are the sole source of PPO1 and PPO2 in larvae, we analyzed by Western blot the presence of the PPOs in hemolymph samples of larvae with genetic ablation of crystal cells (genotype: Lz-Gal4, UAS-Bax). Neither PPO1 nor PPO2 were observed in Lz-Gal4, UAS-Bax larvae confirming that the two PPOs are indeed produced by crystal cells (Fig. 2E). Collectively, our study shows that PPO2 is stored in crystal cells while PPO1 is either a minor component of the crystal or most probably released from crystal cells into the hemolymph.
Fig. 2. PPO2 forms the crystals of crystal cells. 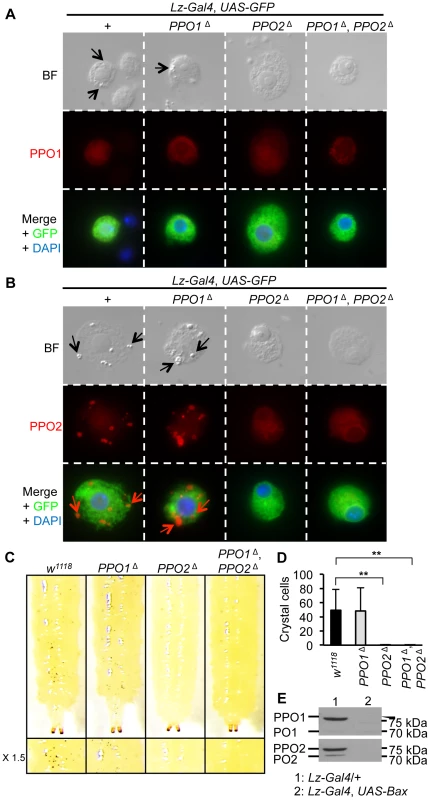
(A) Bright field (BF) and fluorescence micrographs of crystal cells of L3 larvae expressing a Lz-Gal4, UAS-GFP construct combined with the PPO mutations (top panel). Arrows indicate crystals within crystal cells. Immunostaining with antibodies against PPO1 (red), or GFP (green) reveals that PPO1 antibody does not detect PPO1 in the crystals of crystal cells (medium and bottom panels). (B) Immunostaining with antibodies against PPO2 (red), or GFP (green) shows that PPO2 composes the crystals of crystal cells. Specificity of the antibody was confirmed by the absence of signal in crystal cells from PPO2Δ and PPO1Δ, PPO2Δ mutant larvae (medium and bottom panels). Arrows indicate crystals within crystal cells. (C) No melanization of crystal cells was observed in PPO2Δ and PPO1Δ, PPO2Δ double mutant larvae after spontaneous activation of the PPO by incubating larvae at 65°C for 10 minutes. (D) Number of melanized crystal cells found in the posterior region of L3 larvae after heating. No melanized crystal cells were found in PPO2Δ and PPO1Δ, PPO2Δ double mutant larvae. Data were analyzed by t test and values represent the mean ± s.e. of at least 10 different larvae per genotype. (E) PPO1and PPO2 are not detected upon crystal cell depletion. Hemolymph samples were derived from unchallenged F1 progeny of Lz-Gal4, UAS-GFP crossed with UAS-Bax/CyO-GFP larvae. A representative Western Blot analysis using Drosophila anti-PO1 and anti-PO2 antibodies is shown. PO1 and PO2 both contribute to injury-mediated melanization in larvae and adults
Needle injury to wild-type larvae or adults induces a melanization reaction at the wounding site, whose extent is usually proportional to the injury size. This blackening reaction results from de novo synthesis of melanin by PO and is also enhanced by the presence of microbial products [13]. We investigated the role of PPO1 and PPO2 in this process by observing the melanization site 30 min after clean injury in larvae (Fig. 3A). While a reduced melanization spot was observed in PPO1Δ mutants, PPO2Δ mutant larvae displayed a more intense melanization at the wound site compared to wild-type. No melanization spot was observed in injured PPO1Δ, PPO2Δ double mutants. This indicates that both PPO1 and PPO2 contribute to injury-mediated melanization. Our results also suggest that the presence of PPO2 has a slight inhibitory effect on PO activity. A kinetic analysis shows that melanization at the wound site is delayed in PPO1Δ mutants when compared to wild-type (Fig. 3B). This reveals a sequential requirement of the two PPOs: PPO1 provides an immediate source of PO while PPO2 becomes available later.
Fig. 3. Both PPO1 and PPO2 contribute to injury related melanization in larvae. 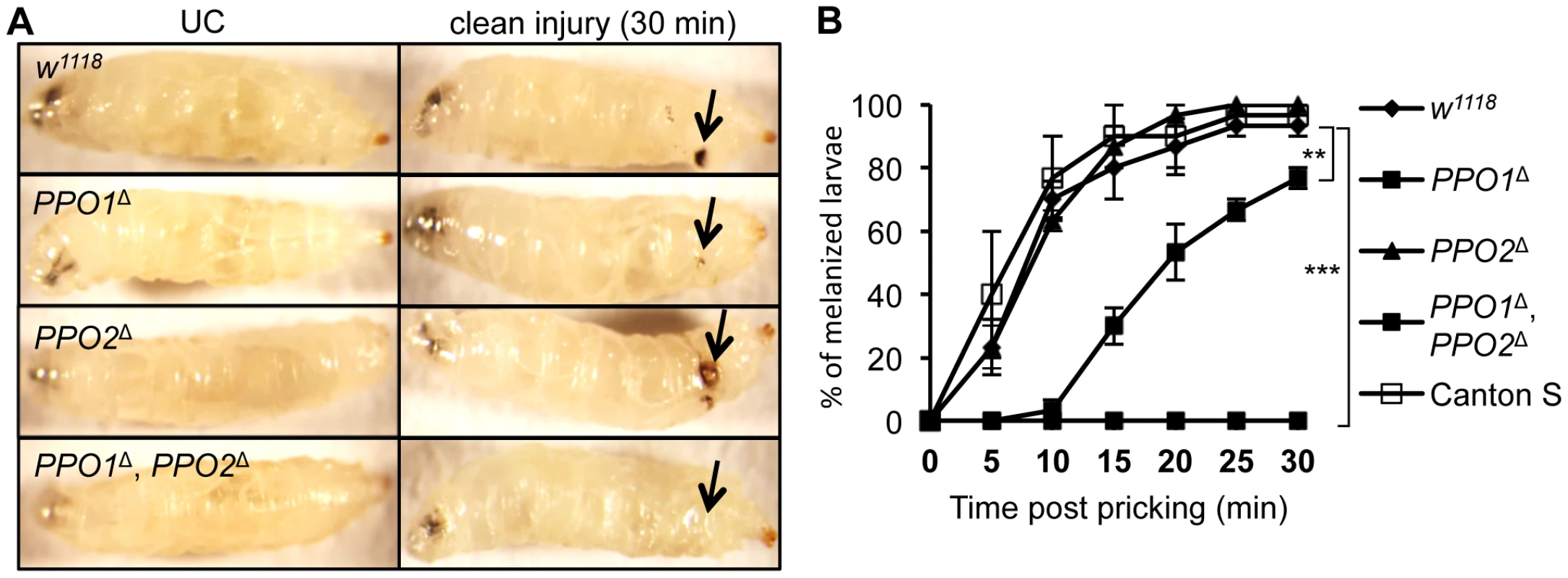
(A) Melanization of larvae after clean injury is abolished in the simultaneous absence of PPO1 and PPO2. A reduced melanization spot was observed in PPO1Δ mutants. PPO2Δ mutant larvae displayed a more intense melanization at the wound site when compared to wild-type. Arrows indicate the pricking site. Larvae were wounded with a tungsten needle and blackening of the wound was recorded 30 minutes later. A representative picture is shown for each genotype. (B) PPO1Δ larvae have a defect in melanization rate upon pricking compared to wild-type and PPO2 mutant larvae. CantonsS larvae were used as an additional wild-type control. Larvae were wounded with a tungsten needle and the presence of a blackening wound was recorded every 5 minutes up to 30 minutes. Data were analyzed using the Log rank test and values represent the mean±s.e. of at least three independent experiments of 10 larvae each. We next investigated the role of PPO in the encapsulation of parasites. Wild-type and PPO mutant second instar larvae were infected with Asobara tabida, a parasitoid wasp that is usually encapsulated in Drosophila melanogaster [30]. Fig. 4 shows that A. tabida is encapsulated in the wild-type and single mutants but not in the PPO1Δ, PPO2Δ double mutants. Staining with phalloidin reveals that the wasp egg is still covered by lamellocytes in the PPO1Δ, PPO2Δ mutant. This confirms that melanization is not required for encapsulation by lamellocytes consistent with a previous report [31].
Fig. 4. Both PPO1 and PPO2 contribute to melanization of wasp capsule. 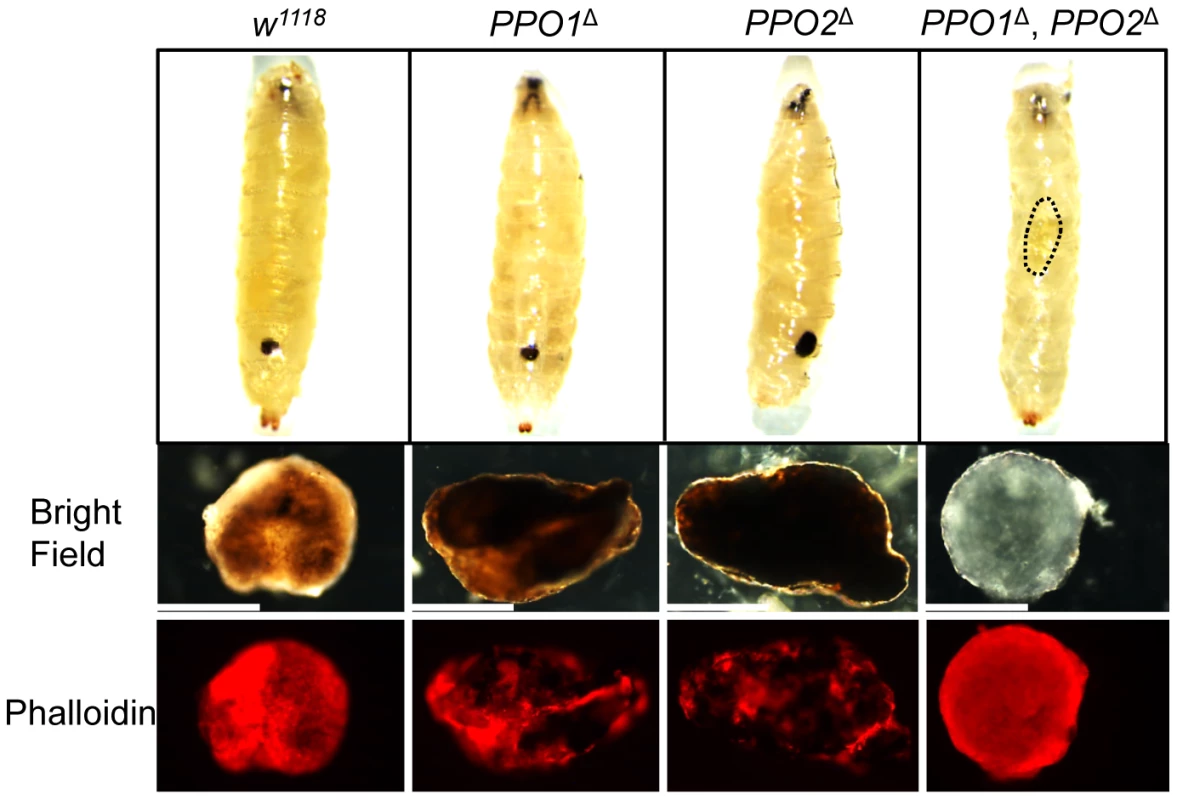
Top panels: representative photos showing infected eggs of A. tabida parasitoid wasp were not melanized in the PPO1Δ, PPO2Δ mutant larvae. Note that A. tabida larvae escape the capsule and develop better in the double-mutant compared to single mutants and wild-type (the position of the larva is indicated with dashed dots). Lower panels: Light micrographs showing melanin deposited on the surface of the eggs of A. tabida removed from the hemocoel of wild-type and PPO mutant larvae at 6 days post-infection. Phalloidin staining reveals the presence of lamellocytes around the egg of wild-type and PPO mutant larvae. Bars = 300 µm. We next focused our attention on the role of PPO1 and PPO2 in adults. As observed in larvae, a reduced melanization spot was observed in PPO1Δ flies while PPO2Δ flies displayed a more intense melanization at the wound site compared to wild - type. No melanization spot was observed in injured PPO1Δ, PPO2Δ flies whatever the microbe used (Fig S2A, S2B, data not shown). We next measured enzymatic PO activity with an L-DOPA assay in adult hemolymph samples from wild-type and PPO mutants. In these experiments, we also included as controls hemolymph samples from Black cells mutant flies which carry an uncharacterized mutation affecting PO activity [5], and from Spn27A1 deficient flies that exhibit constitutive PO activity [14], [15]. Unchallenged wild-type flies showed only a low level of PO activity (Fig. 5A). 4 h after septic injury PO activity increased as previously reported [17]. No significant PO activity was detected in the hemolymph of naive or infected PPO1Δ single and PPO1Δ, PPO2Δ double mutants. PPO2Δ mutants however showed a significantly higher PO activity even in the absence of challenge, which further increased after septic injury. This is consistent with the apparent negative regulatory role of PPO2 observed in injured larvae.
Fig. 5. Increase of PO activity in absence of PPO2. 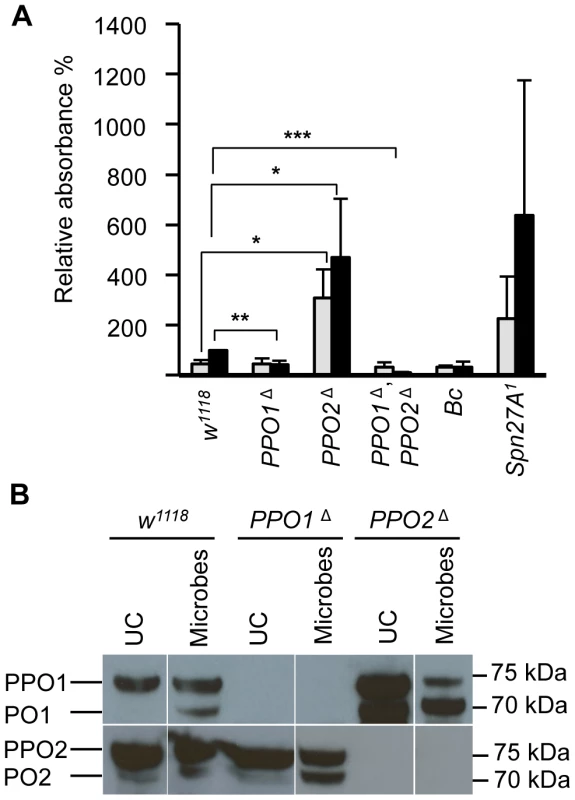
(A) Hemolymph PO activity in unchallenged and wounded adult flies. Spontaneous PO activity is abolished in PPO1Δ, PPO2Δ double mutant flies (***, p<0.0005) and strongly reduced in PPO1Δ mutants (**, p<0.005) compared to wild-type. In PPO2Δ deficient flies, a stronger PO activity is observed even in the absence of wounding (*, p<0.05). Adult flies were punctured and 3 h later their hemolymph was examined for PO activity. Hemolymph of wounded Bc flies and unchallenged Spn27A1 flies was used as control. Data were analyzed by t test and values represent the mean±s.e. of three independent experiments. (B) A representative Western Blot analysis using Drosophila anti-PPO1 (top) and anti-PPO2 (bottom) antibodies. Hemolymph samples were collected from adult flies subjected to injury contaminated with a mixture of Gram-positive Micrococcus luteus and Gram-negative Erwinia carotovora carotovora 15 (E. carotovora) bacteria. Absence of PPO1 and PPO2 in the respective single and double mutant flies is confirmed. Of note, higher levels of the mature form of PPO1 were observed in PPO2Δ mutants consistent with the notion that PPO2 negatively impacts PPO1. Hemolymph was extracted from flies 3 h post wounding. As mentioned above, PPO1 and PPO2 are synthesized as inactive zymogens called prophenoloxidases (PPOs), which are cleaved by SP activity to generate the active form. To determine the relative protein levels of both circulating PPOs and POs in PPO deficient flies, we performed Western blot analyses with hemolymph extracts using anti - PPO1 and anti-PPO2 antisera [13]. The Western blot confirmed the absence of PPOs in the respective mutants. In control wild type flies, PPO1 was detected as a single band of ∼75 kDa, which correlates with its expected molecular weight [13], [17] (Fig. 5B). Septic injury induces the appearance of a shorter band of ∼70 kDa corresponding to the mature form. In the absence of injury, PPO2 was detected as a doublet: a major band of ∼75 kDa corresponding to PPO and a minor band of ∼70 kDa corresponding to the mature form (Fig. 5B). Septic injury also increased the mature form of PPO2. Consistent with the notion that PPO2 exhibits a higher PO activity, PPO2Δ mutants showed higher total amounts of PPO1 and increased levels of the mature form of PPO1 in both naïve and injured flies. Since we did not see a compensatory up-regulation of PPO1 mRNA in PPO2Δ mutant flies (Fig. S1A), PPO1 is likely regulated at post-translational level by PPO2. Collectively, our study shows that both PPO1 and PPO2 contribute to the melanization observed in the hemolymph or at the wound site in flies upon injury.
Epistatic relationships between PPO1, PPO2 and Serpin 27A
Spn27A negatively regulates the hemolymph PPO-activating cascade via inhibition of a yet unidentified SP upstream of Hayan [13]–[15], [32]. Mutations in Spn27A cause lethality and ectopic melanization. We therefore investigated the role of PPO1 and 2 in the Spn27A1 phenotype by recombining the null Spn27A1 mutation with PPO1Δ and/or PPO2Δ. The spontaneous melanization found in Spn27A1 deficient animals is suppressed by the combined absence of PPO1 and PPO2 (Fig. 6). The lethality induced by Spn27A1 deficiency is also largely but not completely rescued in the absence of both PPOs (Fig. S3). We further observed that some 50% of the Spn27A1, PPO1Δ larvae exhibit a characteristic pattern of melanization spots under the epidermis, which could correspond to the attachment sites of sessile hemocytes [6], [33], [34]. Interestingly, the Spn27A1, PPO2Δ double mutants show a stronger melanization phenotype compared to Spn27A1 single mutants with pupae turning black and adults presenting large black spots and wing defects (Fig. 6). Accordingly Spn27A1, PPO2Δ double mutants exhibit higher lethality than Spn27A1 mutants (Fig. S3). These observations are consistent with higher PO activity in PPO2Δ and Spn27A1 mutants compared to the wild-type.
Fig. 6. Epistatic relationships between Serpin 27A and PPOs. 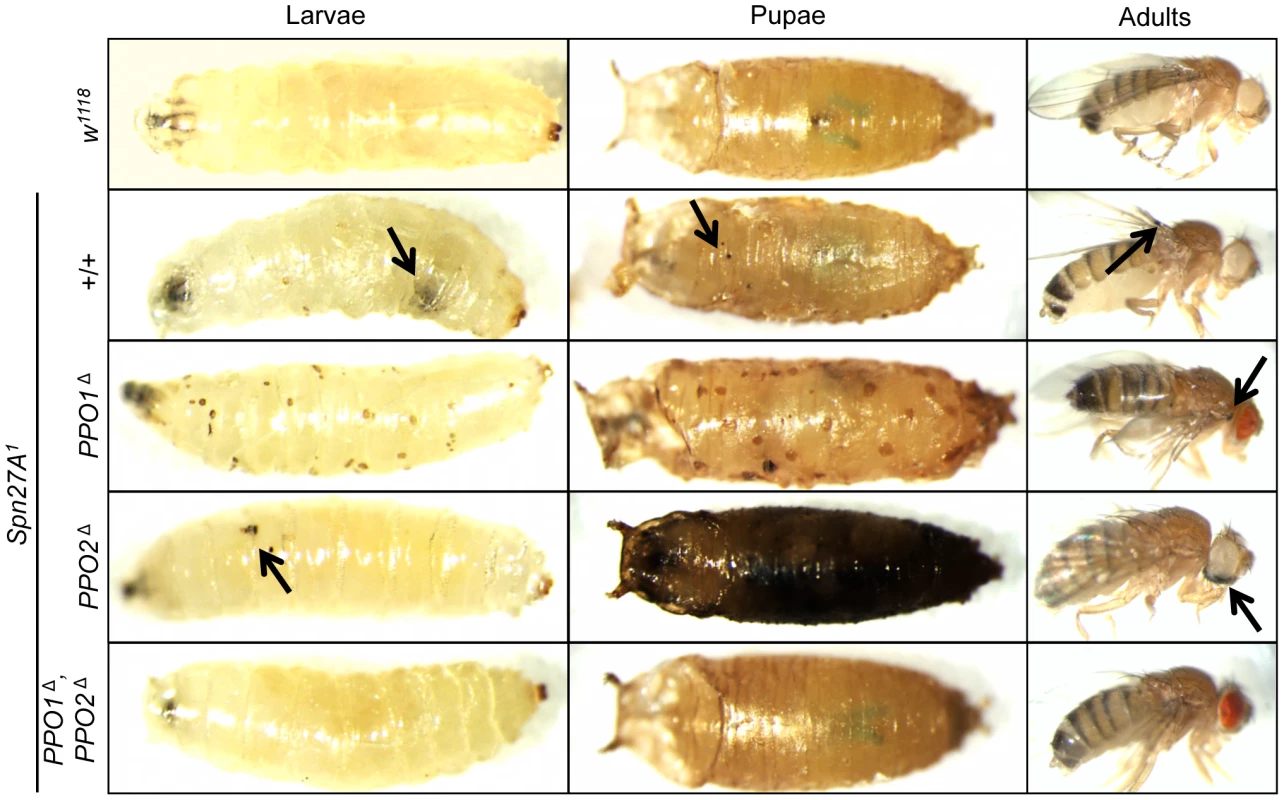
Pictures of larvae (left panel), pupae (center panel) and adults (right panel) were taken in the absence of wounding. Spn27A1 mutants exhibit spontaneous melanization (black arrows) which is suppressed in a PPO1Δ PPO2Δ double mutant background. This uncontrolled melanization is not completely abolished in PPO1Δ, Spn27A1 mutant flies (note the melanized spot indicated by the arrow). A distinctive pattern of melanized spots under the epidermis was observed in Spn27A1, PPO1Δ larvae and pupae. The Spn27A1 melanization was stronger in the absence of PPO2 with pupae turning black and adults presenting black spots and wing defects (not shown). Melanization is dispensable for Toll and Imd pathway activation
Septic injury induces the production of antimicrobial peptides by the fat body [35]. This reaction is regulated at transcriptional level by the Toll and the Imd pathways. Previous studies suggest that antimicrobial peptide gene expression is not affected by melanization [13], [18], [36]. To extend this analysis, we measured the expression of Toll and Imd target genes in single and double PPO mutant adults. We found that PPO mutations have no significant effect on the expression of Diptericin, an antibacterial peptide gene tightly regulated by the Imd pathway, in both unchallenged and Erwinia-infected conditions (Fig. 7A). However, the antifungal peptide gene Drosomycin, a target of the Toll pathway, is induced at higher levels in PPO mutants upon challenge with the Gram-positive M. luteus bacterium (Fig. 7B). To test whether this effect is caused by an increased proliferation of M. luteus in PPO mutants, we repeated this experiment using heat-killed bacteria. Fig. 7C shows that Drosomycin is still induced at significantly higher levels in PPO mutants, with a stronger effect in the double mutant than in single mutants. Since the expression of Diptericin and Drosomycin is controlled by the Imd and Toll pathways, respectively, we conclude that although PPOs are not required for Toll and Imd pathway activation, absence of melanization specifically enhances Toll activation.
Fig. 7. Melanization is not required for Toll and Imd pathway activities. 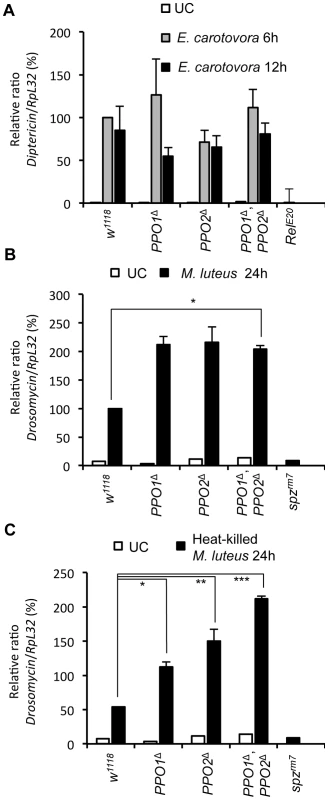
(A) Expression of Diptericin in PPO mutant flies. Total RNA was extracted from animals either uninfected or collected 6 h and 12 h after septic injury with Gram-negative bacteria E. carotovora. Shown are the relative expression levels of Dpt in relation to RpL32. Single and double PPO mutants as well as Bc flies have wild-type Dpt expression levels. The Imd pathway mutant Relish was used as a negative control. (B) Expression of Drosomycin in PPO mutant flies 24 h after septic injury with Gram-positive bacteria M. luteus show that PPO1Δ, PPO2Δ mutant flies (*,p<0.05) have an enhanced Toll pathway activity compared to wild-type. (C) PPO1Δ (*, p<0.05), PPO2Δ (**, p<0.005), and double PPO1Δ, PPO2Δ mutant flies (***, p<0.0005) have an enhanced Toll pathway activity compared to wild- type flies upon infection to heat-inactivated M. luteus. Toll pathway mutant spätzlerm7 was used as a negative control. Shown are the relative expression levels of Drs in relation to RpL32. 100% corresponds to Drs expression level of wild-types flies 24 h after septic injury with the Gram-positive bacteria M. luteus. Data were analyzed using one-way ANOVA and post-test and values represent the mean±s.e. of at least three independent experiments. Melanization contributes to survival to wounding and to infection with Gram - positive bacteria
The in vivo importance of melanization in wound healing and host defense has so far been investigated by perturbing the function of proteases required to activate PPOs or by using the uncharacterized Black cells (Bc) mutation [17], [18], [26], [27], [36], [37]. Our PPO1Δ and PPO2Δ mutants now provide ideal tools to precisely assess the role of PPOs in survival to infection, as well as the individual contribution of PPO1 and PPO2 to host defense. To test the role of PPOs in wound healing, we monitored the survival of flies after mild or severe wounding. This was achieved by perforation of the thorax with clean needles of two different diameters as described in Nam et al. [13]. No significant difference was observed between mutants and wild-type upon mild injury. However, PPO1Δ, PPO2Δ flies but not the single mutants exhibit a higher susceptibility to severe injury compared to wild-type (Fig. 8A–B). This confirms a role of PPOs in wound healing [13], [27] and shows that both PPO1 and PPO2 contribute to the recovery after injury.
Fig. 8. Contribution of PPO1 and PPO2 to wound healing and to host defense. 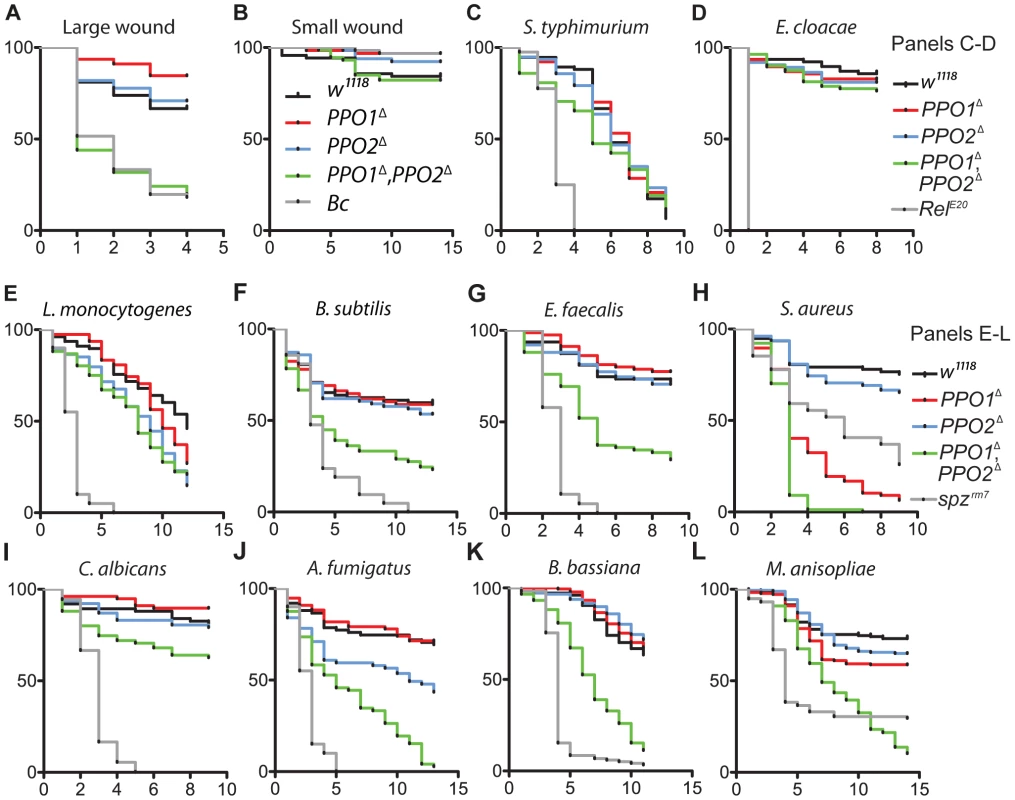
(A, B) Survival rate of flies following injury with clean needle show that concomitant deletion of PPO1 and PPO2 impairs the capacity of flies to survive severe wounding (p<0.0001) compared to wild-type flies. (C, D) PPO1 and PPO2 are not required to survive septic injury with Gram-negative bacteria (S. typhimurium and E. cloacae). (E, F) PPO1Δ, PPO2Δ mutant flies are less resistant to infections with Gram-positive DAP-type bacteria L. monocytogenes (p = 0.0002) and B. subtilis (p<0.0001) compared to wild-type animals. (G, H) PPO1Δ, PPO2Δ mutant flies are severely affected in their capacity to resist infection with Gram-positive Lys-type bacteria E. faecalis and S. aureus (p<0.0001). PPO1Δ and PPO1Δ, PPO2Δ flies are even more susceptible to S. aureus than spzrm7 flies p<0.0001. PPO1Δ mutant flies also exhibit a reduced survival to S. aureus infection (p<0.0001). (I, J) PPO1Δ, PPO2Δ mutant flies exhibit a moderate susceptibility upon septic injury with the yeast C. albicans (p = 0.0101) and a strong susceptibility to injection of A. fumigatus spores (p<0.0001). PPO2Δ mutant flies also die significantly faster from A. fumigatus infection (p = 0.0018 (K, L) Natural infection with entomopathogenic fungi B. bassiana and M. anisopliae reveals that PPO1Δ, PPO2Δ flies have a reduced survival rate compared to wild-type flies (p<0.0001); so did PPO1Δ single mutant flies (p = 0.0084). Black cells (A, B), Relish E20 (C, D) and spätzlerm7 (E, L), respectively, were used as controls. x-axis: Time post-infection in days; y-axis: Percentage of living flies. Data were analyzed by Log-rank test and values are pooled from three independent experiments. Survival to E. faecalis, S. aureus and B. bassiana have been repeated using fly lines carrying the PPO mutations in an OregonR background (Fig. S4C–E). We next used PPO mutant flies to ask to what extent melanization is important for survival to microbial infection, as this question has long been debated [17], [18], [26]. Flies were infected with various Gram-negative and Gram-positive bacteria. The survival rates of PPO flies were similar to wild-type flies after infection with Salmonella typhimurium and Enterobacter cloacae (Fig. 8C, D) while the PPO1Δ, PPO2Δ flies were more susceptible to Erwinia carotovora (Fig. S4A). We next investigated the role of PPOs in the defense against Gram-positive bacteria and compared it to that of the spzrm7 Toll pathway mutant. L. monocytogenes and B. subtilis are two Gram-positive bacteria with diaminopimelic (DAP)-type peptidoglycan that can activate both Toll and Imd pathways [38]. Fig. 8E, F shows that PPO1Δ, PPO2Δ double mutants have an intermediate susceptibility between wild-type and spzrm7 upon infection with these bacteria. Interestingly, PPO flies were also significantly susceptible to infection with Gram-positive bacteria with Lysine-type peptidoglycan (E. faecalis, S. aureus, S. saprophyticus), which are known to activate the Toll pathway exclusively (Fig. 8G, H and S4B–D). Surprisingly, both PPO1Δ and PPO1Δ, PPO2Δ double mutant flies show a higher mortality compared to spzrm7 upon S. aureus infection. Accordingly, the increased susceptibility of PPO mutants to S. aureus was associated with a higher bacterial load indicating that in this specific case, PPOs contribute to pathogen containment (Fig. 9E). In contrast, the persistence of the Gram - negative bacteria E. carotovora and S. typhimurium and the Gram-positive bacteria L. monocytogenesis and E. faecalis was not significantly affected in the PPO1Δ, PPO2Δ double mutants (Fig. 9A–D).
Fig. 9. Microbial persistence in wild-type and PPO1Δ, PPO2Δ double mutant flies. 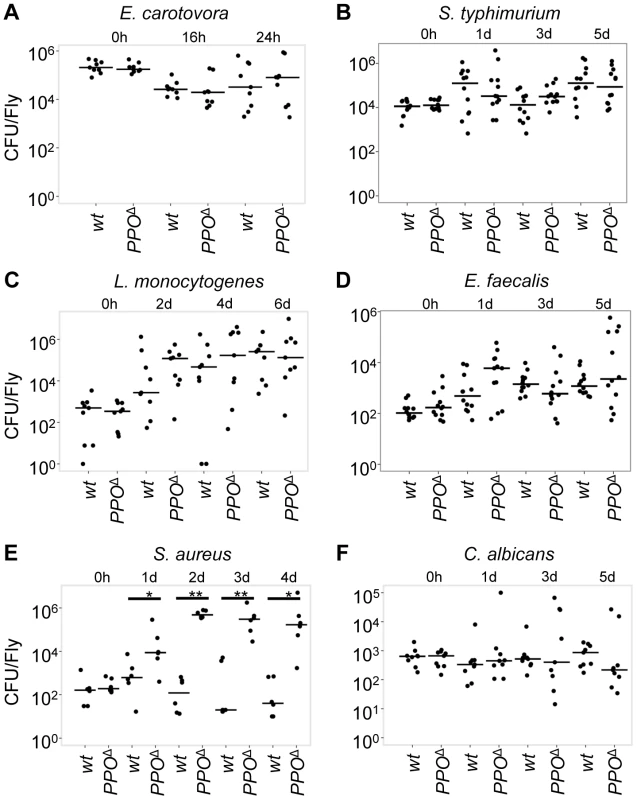
Persistence of E. carotovora (A), S. typhimurium (B), L. monocytogenesis (C), E. faecalis (D), S. aureus (E) and C. albicans (F) were evaluated at different times post- infection in wild-type and PPO1Δ, PPO2Δ mutant flies (referred to as PPOΔ). Statistical analysis reveals no bacterial persistence difference in PPO1Δ, PPO2Δ double mutants compared to wild-type flies upon E. carotovora, S. typhimurium, L. monocytogenesis, E. faecalis and C. albicans infections. However, we noticed an increased counts of S. aureus in PPO1Δ, PPO2Δ double mutants compared to wild-type flies (1 day: p = 0.04113; 2 days: p = 0.002165; 3 days: p = 0.004922; 4 days: p = 0.009524). Bars = median. The number of colony forming units (CFU) is expressed per fly and in a logarithmic scale. Data were analyzed by Wilcoxon test and values are pooled from three independent experiments. Taken together, the survival phenotypes of PPO mutants indicate that PPOs cooperate with the Toll pathway in promoting host defense against Gram-positive bacteria, notably Lysine-type strains.
PPOs contribute to survival to infection with entomopathogenic fungi
We finally investigated the role of melanization in the survival to fungal infections using two infection protocols. In the first, yeast (C. albicans) or spores of a filamentous fungus (A. fumigatus) were injected into the body cavity. In the second, we mimicked natural infection by covering flies with spores of entomopathogenic fungi (B. bassiana, M. anisopliae). Hyphae from germinated spores can penetrate the cuticle and grow within the body cavity, eventually killing flies within several days [39]. PPO mutants exhibit significantly higher susceptibility to injected C. albicans compared to wild-type flies although no effect could be detected on the persistence of this yeast (Fig. 8I and Fig. 9F). This would suggest a role of PPO in the tolerance to C. albicans (i.e. an increased capacity to endure the consequences of infection). More strikingly, injection of spores of A. fumigatus or natural infection with B. bassiana and M. anisopliae lead to a marked vulnerability of PPO1Δ, PPO2Δ double mutants compared to wild-type animals (Fig. 8J–L, S4E).
Collectively, our study demonstrates that melanization significantly contributes to the survival to fungal and Gram-positive bacterial infections. Higher susceptibilities to infection are observed in PPO1Δ, PPO2Δ double mutants and sometimes in PPO1Δ single mutants, consistent with the lower levels of PO activity in these flies.
Discussion
Although melanization is a well-established immune reaction of insects, the precise role of PO genes has never been addressed by loss-of-function mutations. In this study, we have generated deletions of PPO1 and PPO2. We observed that PPO mutants are viable and do not show any pigmentation defect. This is in agreement with the early assumption that another type of enzymes, laccases, but not POs, participate in cuticle tanning [40]. An important finding is that PPO1Δ, PPO2Δ double mutants do not develop any hemolymphatic PO activity upon wounding or following microbial infection. This indicates that PPO1 and PPO2 are the two main sources of PO activity in the hemolymph of Drosophila. Antimicrobial peptide genes such as Diptericin and Drosomycin remain inducible in PPO1Δ, PPO2Δ double mutants. This is consistent with many studies, which showed that PO activity is not required for Toll and Imd pathway activation [13], [18], [36]. Interestingly, Toll pathway activation tends to be stronger in PPO deficient flies, as revealed by a higher level of Drosomycin expression. Higher Toll activity was also observed upon injection of dead bacteria, indicating that this effect is not caused by an increased bacterial proliferation in melanization deficient flies. A possible explanation is that melanin alters the microbes rendering their PAMPs (most probably peptidoglycans) less accessible to pattern-recognition receptors that act upstream of the Toll pathway. Since the PPO and Toll cascades are downstream of the same Pattern-Recognition Receptors [25], another explanation is that loss of one downstream arm frees up effector proteases for the other arm, resulting in over-activation of Toll signaling in PPO mutants.
The contribution of melanization in Drosophila host survival to either injury or microbial infection has been addressed using various mutations: Black cells [17], [27], [36], [37], Sp7 [17], [18], [26] and PPO1Δ, PPO2Δ (this study) (summarized in Table 1). Collectively, Table 1 shows that PPO1Δ, PPO2Δ double mutants, Black cells, and Hayan1 mutants behave the same way in agreement with the fact that they all lack hemolymphatic PO activity. PPO1Δ, PPO2Δ, Black cells, and Hayan1 flies are viable but exhibit increased susceptibility to large injury ([11], [27], this study) and to Gram-positive bacteria ([17], this study) and fungi ([14], [17], this study). Based on these observations, we can conclude that melanization plays a non-redundant role in the defense against Gram-positive bacteria and fungi. This is also supported by studies using the Sp7 mutants [26]. The contribution of PO to survival to Gram-negative infection is less clear as PPO1Δ, PPO2Δ flies survived like wild-type to E. cloacae infection while exhibiting a mild susceptibility to E. carotovora (this study). Nevertheless, Black cells flies have been reported to be more susceptible to A. tumefaciens [17]. Contradictory results have been observed for the survival to S. typhimurium ([26], this study) that could be explained by the strain used. However, previous experiments have shown that flies lacking both a functional Imd pathway and the Black cells mutation die faster to infection with E. coli [18], [36]. Collectively, this suggests that PO contributes to the survival to Gram-negative infection but its effect is either modest or redundant with other mechanisms.
Tab. 1. Contribution of melanization to survival and microbial infection. 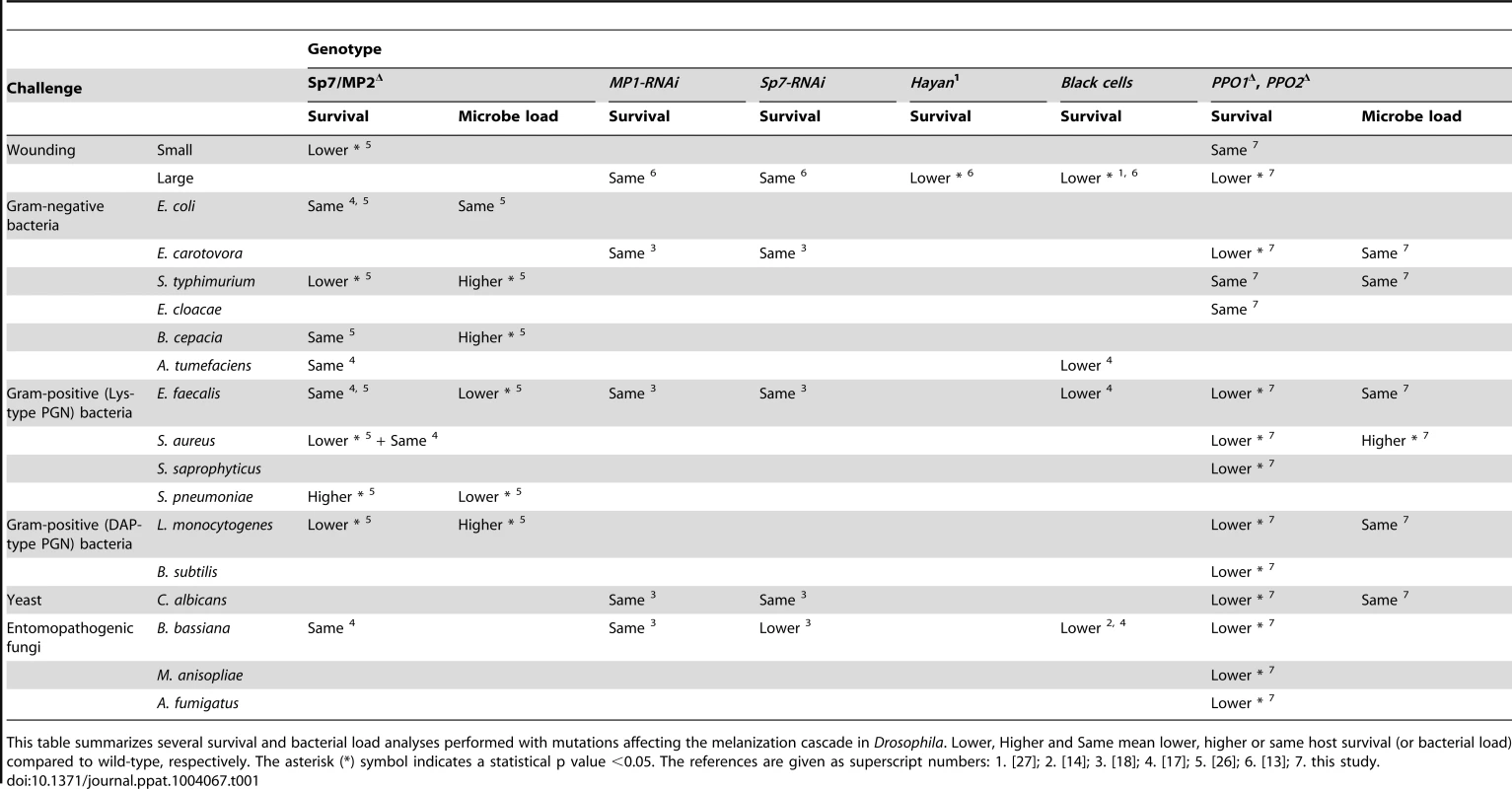
This table summarizes several survival and bacterial load analyses performed with mutations affecting the melanization cascade in Drosophila. Lower, Higher and Same mean lower, higher or same host survival (or bacterial load) compared to wild-type, respectively. The asterisk (*) symbol indicates a statistical p value <0.05. The references are given as superscript numbers: 1. [27]; 2. [14]; 3. [18]; 4. [17]; 5. [26]; 6. [13]; 7. this study. Our persistence analysis did not detect any significant difference in bacterial loads in wild-type and PPO1Δ, PPO2Δ flies upon infection with E. carotovora, L. monocytogenes, E. faecalis and C. albicans, despite an increased susceptibility to these microbes. A first explanation is that PPOs do contribute to tolerance rather than resistance to these micro - organisms [41]. Nevertheless, we cannot exclude that a role in resistance remained undetectable due to the high variability of bacterial load measurements. We point out that work by others [26], which used injection techniques producing less variance demonstrated a role for melanization in the control of L. monocytogenes and S. typhimurium. In contrast, a higher number of bacteria were found in PPOΔ flies infected with S. aureus. Since PPO mutations do not affect the systemic antimicrobial response, this indicates that PO is an immune effector mechanism involved in the resistance to S. aureus. This is in line with several studies that have shown direct microbicidal activity of PO [1], [42], [43]. Except for S. aureus infection, PPO1Δ, PPO2Δ flies tend to cope better with infection than spzrm7 flies, which lack a functional Toll pathway. Since the Toll pathway contributes to PO activation in adults [15], [44], our survival analysis suggests that a significant fraction of Toll-mediated killing is likely caused by PO activity. There are several explanations why PPO mutant flies die faster than Toll pathway mutants upon S. aureus infection. PO could be the only defense mechanism against this bacterium, and the residual PO activity in Toll deficient flies could contribute to survival. A Toll - independent immediate melanization reaction might contain the pathogen and prevent its dissemination at the early steps of infection. Alternatively, the Toll immune response could comprise factors that are deleterious for the host in the absence of melanization. Since phagocytosis also plays an important role in the survival to S. aureus [45]–[47], future studies should investigate the precise link between PPO, phagocytosis and Toll pathway activation during S. aureus infection. Beyond PO's own microbicidal activity, we cannot exclude additional roles for this enzyme, such as for example protection of the host from immunopathology by sequestering microbicidal activities in the immediate proximity of the pathogen. The important role of melanization in the fight against entomopathogenic fungi is consistent with other reports [48] and with the observation that many entomopathogenic fungi have evolved anti-PO activity, as illustrated namely by an active PO suppression by B. bassiana [25].
The number of PPO genes varies among insects from one in the honeybee to ten in Aedes aegypti [49]. This variation in gene number probably reflects differences in the importance of this immune reaction in different insect species. Higher numbers of PPO genes could increase the flexibility in their use allowing complex spatio-temporal deployments of POs at the various insect life stages. Drosophila has three PPOs and our study points to overlapping and distinct functions for all three. Although PPO1 and PPO2 both contribute to hemolymph PO activity, these PPOs are not fully redundant. Our study suggests that PPO1 is involved in the rapid delivery of PO activity when required, while PPO2 provides a storage form that can be deployed in a second phase. This is supported by the observation that melanization at the wound site is delayed in PPO1Δ larvae and adults. Moreover, our data show that PPO2 contributes to the crystals in crystal cells as none were found in PPO2Δ mutants. We hypothesize that PPO1 is also produced in crystal cells but rather than stored is directly secreted into the hemolymph. In our study we observed a precocious activation of PPO1 in the absence of PPO2 pointing to an interaction between both PPOs, most probably within the crystal cells. Crystals would provide an appropriate means of bulk storage and rapid release of concentrated PPO upon crystal cell rupture. Since crystal cells are found in larvae, this might reflect a predominant role of melanization at this stage. Indeed, melanization is thought to play an important role in the encapsulation of parasitoid wasp eggs, which specifically target larval stages. Thus, crystal cells could have evolved as an adaption to release a large quantity of PO in larvae while the rapid delivery of PPO may be less relevant in adults.
Western blot analysis shows an increase of PPO1 and its mature form PO1 in PPO2Δ mutants compared to the wild-type. Since both PPO1 and PPO2 contribute to hemolymphatic PO, we conclude that PPO2 to some extent negatively impacts PPO1 activity. An interaction between PPO1 and PPO2 is suggested by the fact that Black cells, a mutation associated with PPO1, affects both PPO1 and PPO2 as it shows no hemolymph PO activity [5](unpublished data). This higher PO activity in the PPO2Δ mutant could be due to a problem of compartmentalization/secretion of the two enzymes in the crystal cell, a destabilization of PPO1 by PPO2 or to competition between PPO1 and PPO2 for cleavage by the same protease.
It is noteworthy that in both larvae and adults, transcription of PPO genes is not induced by infection or injury, while that of genes encoding other enzymes (dopadecarboxylase, yellow…) or serine proteases involved in the melanization reaction is [50]. This suggests that PO activity is mostly regulated at the post-transcriptional level. PPO3 is a lamellocyte specific PPO [10], [11] that does not require proteolytic cleavage for activation [12]. Our results show that PPO3 does not significantly contribute to hemolymph PO activity since no PO activity was observed in the absence of PPO1 and 2. In addition, we observed that capsules around parasitoid wasp eggs were not melanized in PPO1Δ, PPO2Δ larvae, indicating that PPO3 by itself is not sufficient for this process to occur. Functional characterization of this PPO awaits the generation of a PPO3 mutant. Complex regulatory cascades in the hemolymph converge on the terminal SP Hayan, which is thought to be directly involved in the cleavage of PPO1 and possibly PPO2 [13]. An open question remains whether PPO1 and PPO2 are differentially regulated by these cascades. Our epistatic analysis revealed that most of the Spn27A1 phenotype is suppressed in the combined absence of PPO1 and 2. Previous studies have shown that mutations in the SP gene Hayan also rescue the Spn27A1 phenotype [13]. This is consistent with the observation that Hayan and POs function downstream of Spn27A. We noticed that the removal of PPO1 or PPO2 differentially affects the Spn27A1 phenotype. PPO2, Spn27A1 flies exhibit higher levels of spontaneous PO activity compared to Spn27A1 flies consistent with the notion that PPO2 negatively impacts PPO1. Moreover, PPO2Δ, Spn27A1 and PPO1Δ, Spn27A1 larvae have a distinctive melanization pattern with hemolymphatic melanotic tumors in the former, and conspicuous dots in the latter. These two distinct patterns could arise from the different localization of PPO1 and PPO2 in the hemolymph and the crystal cells respectively, as suggested by our results.
Hemolymphatic melanotic tumors will be observed in PPO2Δ, Spn27A1 as Spn27A could inhibit PPO1 in the hemolymph compartment while melanization in dots could be observed in the PPO1Δ, Spn27A1 background as PPO2 activation could be inhibited by Spn27A inside the crystal cells residing in the hematopoietic sessile niche. The fact that the lethality observed in the absence of Spn27A is largely rescued in the absence of both PPO1 and PPO2 suggests that a significant part of this lethality is caused by the constitutive activation of PO in these mutants. Nevertheless, the residual lethality in Spn27A1 flies devoid of PPO1 and PPO2 indicates the existence of lethality independent of PO activity itself. This could be due to other functions or to damage caused by the presence of activated SPs in the hemolymph.
As a final note, our mechanistic understanding of Drosophila immune effector mechanisms is still very limited. In-depth mechanistic analysis is hampered by the fact that many immune effector genes (such as TEPs, antimicrobial peptide genes, POs) are present as several members and likely function redundantly. This is actually illustrated by the present study since high susceptibility to infection was observed only when two PPOs were knocked-out simultaneously. Fortunately, the development of new knock-out strategies [51], [52], combined with genetics, offers the possibility to dissect the function of each member of a gene family as well as the family's overall contribution. From this point of view, our study definitively confirms the important role of phenoloxidases in insect host defense.
Materials and Methods
Insects stocks and mutant generation
Unless indicated otherwise, w1118 or OregonR flies were used as wild-type controls. The RelishE20 (RelE20), spätzlerm7 (spzrm7), Black cells (Bc) Serpin27A1 (Spn27A1) and UAS-bax/CyO-actin-GFP lines are described in [14], [36], [53], [54]. The Lz-Gal4, UAS-GFP line was obtained from the Bloomington Stock Center. The parasitoid wasp Asobara tabida (Hymenoptera: Braconidae), was reared on PPO1Δ, PPO2Δ double mutant fly stocks at room temperature. After emergence wasps were kept at 16°C and provided with honey until use for experimentation. The PPO1Δ KO line was generated by homologous recombination (Fig.1A) [55]. 4 kb and 4.3 kb of DNA sequence flanking the 5′ and 3′ ends, respectively, of the PPO1 locus (Fig. 1A) were cloned into the p{W25} vector [55]. Flies transformed with p{W25} were used to generate a deletion of PPO1 using a standard crossing protocol. We confirmed by PCR that PPO1 was replaced by the w+ gene in PPO1Δ mutants using forward primer 5′ - CCATCTCCAGAATGCCCCCTTCACT-3′ and reverse primer 5′ - TGCTCAACGGAGTCCTGCGAGTAAT-3′. The PPO2Δ KO line was generated by mobilization of the transposable element Mi{ET1}proPO45 MB05593 inserted in the 3′ end of PPO2 following standard P-element excision protocol. The imprecise excision deleted a fragment of 5.2 kb including the non-coding sequence of the neighboring gene CG13743 without affecting its expression (see Fig.1A and Fig. S1). Primers used to determine the size of deleted fragments can be obtained on request. PPO1Δ, PPO2Δ double mutant flies were generated by standard crosses. Both PPO deficient lines were initially backcrossed five times into a w1118 or OregonR wild-type strain to homogenize the background. Drosophila stocks were maintained at 25°C on standard fly medium. Germ-free animals were generated as described previously [56].
Microorganism culture and infection experiments
The bacterial strains used and their respective optical density of the pellet (O.D.) at 600 nm were: the Gram-negative bacteria Erwinia carotovora 15-GFP (E. carotovora, O.D. 200), Enterobacter cloacae β12 (E. cloacae, O.D. 10) and Salmonella typhimurium pFVP 25.1 (GFP) (S. typhimurium, O.D. 10); the DAP-type PGN containing Gram-positive bacteria Listeria monocytogenes-GFP (BUG2377) (L. monocytogenes, O.D. 0.5) and Bacillus subtilis (B. subtilis, O.D 5); the Lys-type PGN containing Gram-positive bacteria Micrococcus luteus (M. luteus, O.D. 200), Staphylococcus aureus-GFP (S. aureus, O.D.0.5), Staphylococcus saprophyticus (S. saprophyticus, O.D. 5) and Enterococcus faecalis OG1RF+pMV158GFP [57] (E. faecalis, O.D. 0.5); the yeast Candida albicans (C. albicans, O. D. 200). Strains were cultured in Brain-Heart Infusion Broth (BHI - L. monocytogenes), Yeast extract-Peptone-Glucose Broth (C. albicans) or Luria Broth (LB - all others) at 29°C (E. carotovora, M. luteus, C. albicans) or 37°C. Spores of entomopathogenic strains Beauveria bassiana 802 (B. bassiana) and Metarhizium anisopliae KVL 131 (M.anisopliae) were grown on Malt agar plates at 29°C for approximately 3 weeks until sporulation. Systemic infections (septic injury) were performed by pricking adult females in the thorax with a thin needle previously dipped into a concentrated pellet of a bacterial culture or in a suspension of Aspergillus fumigatus (A. fumigatus) spores [58]. Natural infections were initiated by shaking anesthetized flies in a petri dish containing a sporulating culture of entomopathogenic fungi B. bassiana or M. anisopliae. Infected flies were subsequently maintained at 29°C (E. carotovora, M. luteus, B. bassiana, M. anisopliae and A. fumigatus) or at 22°C (all other bacteria) [39]. At least two tubes of 20 flies were used for survival experiments and survival was scored daily. Experiments were repeated at least three times. For lifespan experiments, flies were kept on normal fly medium or on sterilized medium for axenic flies and were flipped every three days. Persistence assay: Flies were infected with the pathogens as described above. Persistence was evaluated at different time points post-infection in triplicates by crushing 5 flies in LB culture medium. Serial dilutions of these extracts were spotted in triplicate on appropriate medium and incubated overnight at 29°C or 37°C. Colonies were counted from spots containing more than 10 colonies. The experiment was repeated three times. For wasp infection, 20 first instar wild-type or PPO larvae were placed in presence of 3 A.tabida females for 2 hours. Parasitized larvae were kept at room temperature until capsule dissection 6 days post-infection. We noticed that the wasp larvae develop better in the PPO1Δ, PPO2Δ mutants compared to the wild-type as A. tabida could be more easily maintained in PPO1Δ, PPO2Δ than in the w1118 or OregonR wild-type stocks.
Wounding experiment
‘Clean’ injury referred to an injury performed with a needle that has been previously sterilized. A low level of bacterial contamination is still possible since the surface of the insect was not sterilized. For adults, two different intensities of physical wounding were used (adapted from [13]). In strong wounding the thorax of the fly was completely penetrated using a sterile needle of ∼50 µm diameter. For survival to clean injury and for imaging of the melanization reaction upon pricking, the thorax of the animal was pricked (as described in infection experiments) using a sterile needle (diameter: ∼5 µm). Pictures were taken three hours post-pricking. Third instar larvae were pricked dorsally using a sterile needle (diameter: ∼5 µm). Pictures of melanized larvae were taken 30 minutes post-injury. For the melanization time-course of larvae (Fig. 3B), the presence of a blackening spot was recorded after pricking, every 5 minutes until 30 minutes. Pictures were captured with a Leica DFC300FX camera and Leica Application Suite.
Hemolymph extraction and PO activity
Larval and adult hemolymph was collected as follows. Fifteen to twenty individuals were placed on a 10 µM filter of an empty mobicol spin column (MOBITEC), covered with glass beads and centrifuged for 20 minutes at 4°C, 10'000 r.pm. Hemolymph was recovered in 50 µl protease inhibitor solution (Roche; one tablet dissolved in 4 ml phosphate-buffered saline, PBS) and protein concentrations adjusted after a Bradford test. Sample volumes were adjusted to 200 µl in 5 mM CaCl2 solution (diluted in protease inhibitor solution, see above) and after addition of 800 µl of L-DOPA solution (20 mM in phosphate buffer pH 6.6) the samples were incubated at 29 °C in the dark. After 30 min, the optical density at 492 nm was measured for each sample against an L-DOPA control containing no hemolymph. Since activation of the proPO system was blocked by the presence of the protease inhibitor, the values reflect the in vivo PO activity at the time of wounding. Each experiment was repeated three times.
Western blots
For Western blots, hemolymph samples were collected from 25 flies in a protease inhibitor solution as described above. Protein concentration of the samples was determined by Bradford assay. 25 µg of protein extract was separated on a 4–20% acrylamide precast Novex gel (Invitrogen) under reducing conditions and transferred to nitrocellulose membranes (Invitrogen iBlot). After blocking in 2% bovine serum albumin in PBT for 1 h, samples were incubated at 4°C overnight with rabbit antibodies against Drosophila PPO1 or PPO2 [13] in a 1∶2000 dilution. Goat anti-rabbit-HRP secondary antibody (Dako) in a 1∶2000 dilution was incubated for 2 h at room temperature. Bound antibody was detected using ECL (GE Healthcare) according to the manufacturer's instructions.
Live imaging and immunofluorescence
For crystal cells imaging, the hemolymph of wild-type or PPO mutant larvae expressing Lz-Gal4, UAS-GFP was immediately fixed on Superfrost Plus adhesive microscope slides (Menzel) with PBS:paraformaldehyde 4% and rinsed with PBS. Lz-Gal4, UAS-GFP expressing cells were stained with rabbit anti-PPO1 (1∶500) or rabbit anti-PPO2 (1∶500) [13] and with mouse anti-GFP (Interchim) diluted in PBT+1% BSA. Anti-PPO1and anti-PPO2 were revealed with an Alexa 594-coupled mouse anti-rabbit (Molecular Probes) diluted to 1∶500 in blocking solution (PBS, 0.1% Tween, 2% BSA). Anti-GFP was revealed with an Alexa 488-coupled mouse anti-mouse (Molecular Probes) diluted to 1∶500 in blocking solution and nuclei were stained with DAPI (Sigma).
For staining of capsules from A. tabida infected larvae, capsules were dissected from 6 day old infected larvae, fixed with PBS:paraformaldehyde 4% and rinsed with PBS. Lamellocytes on capsules were stained with AF594-conjugated Phalloidin (Molecular Probes) diluted 1∶40 in blocking solution. Samples were observed for fluorescence with an Axioplot imager Z1 and Axiocam mRM camera (Zeiss) and a 100/1.30 NA oil immersion objective, with zoom set to 100 (crystal cells) or 20 (capsules). For publication purposes, brightness and contrast were increased identically on control and sample images.
Quantitative RT-PCR
For quantification of mRNA, whole flies were collected at indicated time points. Total fly RNA was isolated from 15 adult flies by TRIzol reagent and dissolved in RNase - free water. Five hundred nanogram total RNA was then reverse-transcribed in 10 µl reaction volume using PrimeScript RT (TAKARA) and random hexamer primers. Quantitative PCR was performed on a LightCycler 480 (Roche) in 96-well plates using the LightCycler 480 SYBR Green I master mix or on a LightCycler 2.0 (Roche) in capillaries using dsDNA dye SYBR Green I (Roche). Primers were as follows: Diptericin forward 5′ - GCTGCGCAATCGCTTCTACT-3′, reverse 5′-TGGTGGAGTGGGCTTCATG-3′; Drosomycin forward 5′-CGTGAGAACCTTTTCCAATATGAT-3′, reverse 5′ - TCCCAGGACCACCAGCAT-3′; RpL32 forward 5′-GACGCTTCAAGGGACAGTATCTG-3′, reverse 5′-AAACGCGGTTCTGCATGAG-3′; PPO1 forward 5′ - TTTTCCCTACTGACAACCTC-3′, reverse 5′ - GGGCTGATAGTCTGCTC-3′; PPO2 forward 5′ - CCCGCCTATACCGAGA-3′, reverse 5′ - CGCACGTAGCCGAAAC-3′; PPO3 forward 5′-GGCGAGCTGTTCTACT-3′, reverse 5′ - GAGGATACGCCCTACTG-3′; CG13743 forward 5′ - GAAGGGCGTAGGGTAT-3′, reverse 5′ - CCATCAGACACATCAGC-3′.
Statistical analysis
Each experiment was repeated independently a minimum of three times (unless otherwise indicated), error bars represent the standard error of the mean of replicate experiments (unless otherwise indicated). Statistical significance of survival and persistence data was calculated with a log-rank or a Wilcoxon test, respectively, and p values are indicated in figure legends. Otherwise statistical significance was calculated with Student's t test or one-way ANOVA followed by post-test and p values of <0.05 = *, <0.005 = **, and <0.0005 = *** were considered significant.
Supporting Information
Zdroje
1. CereniusL, LeeBL, SoderhallK (2008) The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol 29 : 263–271.
2. Tang H (2009) Regulation and function of the melanization reaction in Drosophila. Fly (Austin) 3.
3. LiuH, JiravanichpaisalP, CereniusL, LeeBL, SoderhallI, et al. (2007) Phenoloxidase is an important component of the defense against Aeromonas hydrophila Infection in a crustacean, Pacifastacus leniusculus. J Biol Chem 282 : 33593–33598.
4. NappiA, PoirieM, CartonY (2009) The role of melanization and cytotoxic by - products in the cellular immune responses of Drosophila against parasitic wasps. Adv Parasitol 70 : 99–121.
5. RizkiT, RizkiR, GrellE (1980) A mutant affecting the crystal cells in Drosophila melanogaster. Roux's Arch Dev Biol 188 : 91–99.
6. LanotR, ZacharyD, HolderF, MeisterM (2000) Post-embryonic hematopoiesis in Drosophila. Dev Biol 230 : 243–257.
7. HontiV, CsordasG, KuruczE, MarkusR, AndoI (2014) The cell-mediated immunity of Drosophila melanogaster: Hemocyte lineages, immune compartments, microanatomy and regulation. Dev Comp Immunol 42 : 47–56.
8. BidlaG, DushayMS, TheopoldU (2007) Crystal cell rupture after injury in Drosophila requires the JNK pathway, small GTPases and the TNF homolog Eiger. J Cell Sci 120 : 1209–1215.
9. WaltzerL, FerjouxG, BatailleL, HaenlinM (2003) Cooperation between the GATA and RUNX factors Serpent and Lozenge during Drosophila hematopoiesis. EMBO J 22 : 6516–6525.
10. IrvingP, UbedaJM, DoucetD, TroxlerL, LagueuxM, et al. (2005) New insights into Drosophila larval haemocyte functions through genome-wide analysis. Cell Microbiol 7 : 335–350.
11. NamHJ, JangIH, AsanoT, LeeWJ (2008) Involvement of pro-phenoloxidase 3 in lamellocyte-mediated spontaneous melanization in Drosophila. Mol Cells 26 : 606–610.
12. ChenY, LiuF, YangB, LuA, WangS, et al. (2012) Specific amino acids affecting Drosophila melanogaster prophenoloxidase activity in vitro. Dev Comp Immunol 38 : 88–97.
13. NamHJ, JangIH, YouH, LeeKA, LeeWJ (2012) Genetic evidence of a redox - dependent systemic wound response via Hayan protease-phenoloxidase system in Drosophila. EMBO J 31 : 1253–1265.
14. De GregorioE, HanSJ, LeeWJ, BaekMJ, OsakiT, et al. (2002) An immune - responsive Serpin regulates the melanization cascade in Drosophila. Dev Cell 3 : 581–592.
15. LigoxygakisP, PelteN, JiC, LeclercV, DuvicB, et al. (2002) A serpin mutant links Toll activation to melanization in the host defence of Drosophila. Embo J 21 : 6330–6337.
16. Castillejo-LopezC, HackerU (2005) The serine protease Sp7 is expressed in blood cells and regulates the melanization reaction in Drosophila. Biochem Biophys Res Commun 338 : 1075–1082.
17. Leclerc V, Pelte N, El Chamy L, Martinelli C, Ligoxygakis P, et al. (2005) Prophenoloxidase activation is not required for survival to microbial infections in Drosophila. EMBO Rep.
18. Tang H, Kambris Z, Lemaitre B, Hashimoto C (2006) Two proteases defining a melanization cascade in the immune system of drosophila. J Biol Chem.
19. Scherfer C, Tang H, Kambris Z, Lhocine N, Hashimoto C, et al. (2008) Drosophila Serpin-28D regulates hemolymph phenoloxidase activity and adult pigmentation. Dev Biol.
20. TangH, KambrisZ, LemaitreB, HashimotoC (2008) A serpin that regulates immune melanization in the respiratory system of Drosophila. Dev Cell 15 : 617–626.
21. AhmadST, SweeneyST, LeeJA, SweeneyNT, GaoFB (2009) Genetic screen identifies serpin5 as a regulator of the toll pathway and CHMP2B toxicity associated with frontotemporal dementia. Proc Natl Acad Sci U S A 106 : 12168–12173.
22. YoshidaH, OchiaiM, AshidaM (1986) Beta-1,3-glucan receptor and peptidoglycan receptor are present as separate entities within insect prophenoloxidase activating system. Biochem Biophys Res Commun 141 : 1177–1184.
23. KimCH, KimSJ, KanH, KwonHM, RohKB, et al. (2008) A three-step proteolytic cascade mediates the activation of the peptidoglycan-induced toll pathway in an insect. J Biol Chem 283 : 7599–7607.
24. BidlaG, HaulingT, DushayMS, TheopoldU (2009) Activation of insect phenoloxidase after injury: endogenous versus foreign elicitors. J Innate Immun 1 : 301–308.
25. MatskevichAA, QuintinJ, FerrandonD (2010) The Drosophila PRR GNBP3 assembles effector complexes involved in antifungal defenses independently of its Toll-pathway activation function. Eur J Immunol 40 : 1244–1254.
26. AyresJS, SchneiderDS (2008) A signaling protease required for melanization in Drosophila affects resistance and tolerance of infections. PLoS Biol 6 : 2764–2773.
27. RämetM, LanotR, ZacharyD, ManfruelliP (2001) JNK Signaling Pathway Is Required for Efficient Wound Healing in Drosophila. Dev biol 241 : 145–156.
28. GajewskiKM, SorrentinoRP, LeeJH, ZhangQ, RussellM, et al. (2007) Identification of a crystal cell-specific enhancer of the black cells prophenoloxidase gene in Drosophila. Genesis 45 : 200–207.
29. LebestkyT, ChangT, HartensteinV, BanerjeeU (2000) Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science 288 : 146–149.
30. EslinP, DouryG (2006) The fly Drosophila subobscura: A natural case of innate immunity deficiency. Dev Comp Immunol 30 : 977–983.
31. RizkiRM, RizkiTM (1990) Encapsulation of Parasitoid Eggs in Phenoloxidase - Deficient Mutants of Drosophila-Melanogaster. Journal of Insect Physiology 36 : 523–529.
32. ReichhartJM (2005) Tip of another iceberg: Drosophila serpins. Trends Cell Biol 15 : 659–665.
33. MarkusR, LaurinyeczB, KuruczE, HontiV, BajuszI, et al. (2009) Sessile hemocytes as a hematopoietic compartment in Drosophila melanogaster. Proc Natl Acad Sci U S A 106 : 4805–4809.
34. MakhijaniK, AlexanderB, TanakaT, RulifsonE, BrucknerK (2011) The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development 138 : 5379–5391.
35. LemaitreB, HoffmannJA (2007) The host defense of Drosophila melanogaster. Annu Rev Immunol 25 : 697–743.
36. LemaitreB, Kromer-MetzgerE, MichautL, NicolasE, MeisterM, et al. (1995) A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc Natl Acad Sci USA 92 : 9365–9469.
37. BraunA, HoffmannJA, MeisterM (1998) Analysis of the Drosophila host defense in domino mutant larvae, which are devoid of hemocytes. Proc Natl Acad Sci U S A 95 : 14337–14342.
38. BuchonN, PoidevinM, KwonHM, GuillouA, SottasV, et al. (2009) A single modular serine protease integrates signals from pattern-recognition receptors upstream of the Drosophila Toll pathway. Proc Natl Acad Sci U S A 106 : 12442–12447.
39. LemaitreB, ReichhartJ, HoffmannJ (1997) Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc Natl Acad Sci USA 94 : 14614–14619.
40. ArakaneY, MuthukrishnanS, BeemanRW, KanostMR, KramerKJ (2005) Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning. Proc Natl Acad Sci U S A 102 : 11337–11342.
41. SchneiderDS, AyresJS (2008) Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol 8 : 889–895.
42. ZhaoPC, LiJJ, WangY, JiangHB (2007) Broad-spectrum antimicrobial activity of the reactive compounds generated in vitro by Manduca sexta phenoloxidase. Insect Biochemistry and Molecular Biology 37 : 952–959.
43. KanH, KimCH, KwonHM, ParkJW, RohKB, et al. (2008) Molecular control of phenoloxidase-induced melanin synthesis in an insect. J Biol Chem 283 : 25316–25323.
44. De GregorioE, SpellmanPT, TzouP, RubinGM, LemaitreB (2002) The Toll and Imd pathways are the major regulators of the immune response in Drosophila. Embo J 21 : 2568–2579.
45. KocksC, ChoJH, NehmeN, UlvilaJ, PearsonAM, et al. (2005) Eater, a transmembrane protein mediating phagocytosis of bacterial pathogens in Drosophila. Cell 123 : 335–346.
46. Garg A, Wu LP (2013) Drosophila Rab14 mediates phagocytosis in the immune response to Staphylococcus aureus. Cell Microbiol.
47. ShiratsuchiA, MoriT, SakuraiK, NagaosaK, SekimizuK, et al. (2012) Independent recognition of Staphylococcus aureus by two receptors for phagocytosis in Drosophila. J Biol Chem 287 : 21663–21672.
48. YassineH, KamareddineL, OstaMA (2012) The mosquito melanization response is implicated in defense against the entomopathogenic fungus Beauveria bassiana. PLoS Pathog 8: e1003029.
49. WaterhouseRM, KriventsevaEV, MeisterS, XiZ, AlvarezKS, et al. (2007) Evolutionary dynamics of immune-related genes and pathways in disease - vector mosquitoes. Science 316 : 1738–1743.
50. De GregorioE, SpellmanPT, RubinGM, LemaitreB (2001) Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc Natl Acad Sci U S A 98 : 12590–12595.
51. KondoS, UedaR (2013) Highly improved gene targeting by germline-specific cas9 expression in Drosophila. Genetics 195 : 715–721.
52. Baena-Lopez LA, Alexandre C, Mitchell A, Pasakarnis L, Vincent JP (2013) Accelerated homologous recombination and subsequent genome modification in Drosophila. Development.
53. HedengrenM, AslingB, DushayMS, AndoI, EkengrenS, et al. (1999) Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol Cell 4 : 827–837.
54. GaumerS, GuenalI, BrunS, TheodoreL, MignotteB (2000) Bcl-2 and Bax mammalian regulators of apoptosis are functional in Drosophila. Cell Death Differ 7 : 804–814.
55. GongWJ, GolicKG (2004) Genomic deletions of the Drosophila melanogaster Hsp70 genes. Genetics 168 : 1467–1476.
56. ParedesJC, WelchmanDP, PoidevinM, LemaitreB (2011) Negative Regulation by Amidase PGRPs Shapes the Drosophila Antibacterial Response and Protects the Fly from Innocuous Infection. Immunity 35 : 770–779.
57. GasparF, TeixeiraN, Rigottier-GoisL, MarujoP, Nielsen-LeRouxC, et al. (2009) Virulence of Enterococcus faecalis dairy strains in an insect model: the role of fsrB and gelE. Microbiology 155 : 3564–3571.
58. RomeoY, LemaitreB (2008) Drosophila immunity: methods for monitoring the activity of toll and imd signaling pathways. Methods Mol Biol 415 : 379–394.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge inČlánek Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 5- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Surveillance for Emerging Biodiversity Diseases of Wildlife
- The Emerging Role of Urease as a General Microbial Virulence Factor
- PARV4: An Emerging Tetraparvovirus
- Epigenetic Changes Modulate Schistosome Egg Formation and Are a Novel Target for Reducing Transmission of Schistosomiasis
- The Human Adenovirus E4-ORF1 Protein Subverts Discs Large 1 to Mediate Membrane Recruitment and Dysregulation of Phosphatidylinositol 3-Kinase
- A Multifactorial Role for Malaria in Endemic Burkitt's Lymphoma Pathogenesis
- Structural Basis for the Ubiquitin-Linkage Specificity and deISGylating Activity of SARS-CoV Papain-Like Protease
- Cathepsin-L Can Resist Lysis by Human Serum in
- Epstein-Barr Virus Down-Regulates Tumor Suppressor Expression
- BCA2/Rabring7 Targets HIV-1 Gag for Lysosomal Degradation in a Tetherin-Independent Manner
- The Evolutionarily Conserved Mediator Subunit MDT-15/MED15 Links Protective Innate Immune Responses and Xenobiotic Detoxification
- Suppressor of Cytokine Signaling 4 (SOCS4) Protects against Severe Cytokine Storm and Enhances Viral Clearance during Influenza Infection
- T Cell Inactivation by Poxviral B22 Family Proteins Increases Viral Virulence
- Dynamics of HIV Latency and Reactivation in a Primary CD4+ T Cell Model
- HIV and HCV Activate the Inflammasome in Monocytes and Macrophages via Endosomal Toll-Like Receptors without Induction of Type 1 Interferon
- Virus and Autoantigen-Specific CD4+ T Cells Are Key Effectors in a SCID Mouse Model of EBV-Associated Post-Transplant Lymphoproliferative Disorders
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- Squalene Synthase As a Target for Chagas Disease Therapeutics
- The Contribution of Viral Genotype to Plasma Viral Set-Point in HIV Infection
- Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge in
- Anthrax Lethal Factor as an Immune Target in Humans and Transgenic Mice and the Impact of HLA Polymorphism on CD4 T Cell Immunity
- Ly49C-Dependent Control of MCMV Infection by NK Cells Is -Regulated by MHC Class I Molecules
- Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
- A Large Family of Antivirulence Regulators Modulates the Effects of Transcriptional Activators in Gram-negative Pathogenic Bacteria
- Broad-Spectrum Anti-biofilm Peptide That Targets a Cellular Stress Response
- Malaria Parasite Infection Compromises Control of Concurrent Systemic Non-typhoidal Infection via IL-10-Mediated Alteration of Myeloid Cell Function
- A Role for in Higher Order Structure and Complement Binding of the Capsule
- Hip1 Modulates Macrophage Responses through Proteolysis of GroEL2
- CD8 T Cells from a Novel T Cell Receptor Transgenic Mouse Induce Liver-Stage Immunity That Can Be Boosted by Blood-Stage Infection in Rodent Malaria
- Phosphorylation of KasB Regulates Virulence and Acid-Fastness in
- HIV-Infected Individuals with Low CD4/CD8 Ratio despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened CD8+ T Cell Activation, and Increased Risk of Non-AIDS Morbidity and Mortality
- A Novel Mechanism Inducing Genome Instability in Kaposi's Sarcoma-Associated Herpesvirus Infected Cells
- Structural and Biochemical Characterization Reveals LysGH15 as an Unprecedented “EF-Hand-Like” Calcium-Binding Phage Lysin
- Hepatitis C Virus Cell-Cell Transmission and Resistance to Direct-Acting Antiviral Agents
- Different Modes of Retrovirus Restriction by Human APOBEC3A and APOBEC3G
- TNFα and IFNγ but Not Perforin Are Critical for CD8 T Cell-Mediated Protection against Pulmonary Infection
- Large Scale RNAi Reveals the Requirement of Nuclear Envelope Breakdown for Nuclear Import of Human Papillomaviruses
- The Cytoplasmic Domain of Varicella-Zoster Virus Glycoprotein H Regulates Syncytia Formation and Skin Pathogenesis
- A New Class of Multimerization Selective Inhibitors of HIV-1 Integrase
- Are We There Yet? The Smallpox Research Agenda Using Variola Virus
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
- Dynamic Functional Modulation of CD4 T Cell Recall Responses Is Dependent on the Inflammatory Environment of the Secondary Stimulus
- Bacterial Superantigens Promote Acute Nasopharyngeal Infection by in a Human MHC Class II-Dependent Manner
- Follicular Helper T Cells Promote Liver Pathology in Mice during Infection
- A Nasal Epithelial Receptor for WTA Governs Adhesion to Epithelial Cells and Modulates Nasal Colonization
- Unexpected Role for IL-17 in Protective Immunity against Hypervirulent HN878 Infection
- Human Cytomegalovirus Fcγ Binding Proteins gp34 and gp68 Antagonize Fcγ Receptors I, II and III
- Expansion of Murine Gammaherpesvirus Latently Infected B Cells Requires T Follicular Help
- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Molecular Signatures of Hemagglutinin Stem-Directed Heterosubtypic Human Neutralizing Antibodies against Influenza A Viruses
- The Downregulation of GFI1 by the EZH2-NDY1/KDM2B-JARID2 Axis and by Human Cytomegalovirus (HCMV) Associated Factors Allows the Activation of the HCMV Major IE Promoter and the Transition to Productive Infection
- Inactivation of Fructose-1,6-Bisphosphate Aldolase Prevents Optimal Co-catabolism of Glycolytic and Gluconeogenic Carbon Substrates in
- New Insights into Rotavirus Entry Machinery: Stabilization of Rotavirus Spike Conformation Is Independent of Trypsin Cleavage
- Prophenoloxidase Activation Is Required for Survival to Microbial Infections in
- SslE Elicits Functional Antibodies That Impair Mucinase Activity and Colonization by Both Intestinal and Extraintestinal Strains
- Timed Action of IL-27 Protects from Immunopathology while Preserving Defense in Influenza
- HIV-1 Envelope gp41 Broadly Neutralizing Antibodies: Hurdles for Vaccine Development
- The PhoP-Dependent ncRNA Mcr7 Modulates the TAT Secretion System in
- Cellular Superspreaders: An Epidemiological Perspective on HIV Infection inside the Body
- The Inflammasome Pyrin Contributes to Pertussis Toxin-Induced IL-1β Synthesis, Neutrophil Intravascular Crawling and Autoimmune Encephalomyelitis
- Papillomavirus Genomes Associate with BRD4 to Replicate at Fragile Sites in the Host Genome
- Integrative Functional Genomics of Hepatitis C Virus Infection Identifies Host Dependencies in Complete Viral Replication Cycle
- Co-assembly of Viral Envelope Glycoproteins Regulates Their Polarized Sorting in Neurons
- Targeting Membrane-Bound Viral RNA Synthesis Reveals Potent Inhibition of Diverse Coronaviruses Including the Middle East Respiratory Syndrome Virus
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



