-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Inactivation of Fructose-1,6-Bisphosphate Aldolase Prevents Optimal Co-catabolism of Glycolytic and Gluconeogenic Carbon Substrates in
The development of new chemotherapies targeting Mycobacterium tuberculosis (Mtb) will benefit from genetic evaluation of potential drug targets and a better understanding of the pathways required by Mtb to establish and maintain chronic infections. We employed a genetic approach to investigate the essentiality of fructose-1,6-bisphosphate aldolase (FBA) for growth and survival of Mtb in vitro and in mice. A conditional fba mutant revealed that Mtb requires FBA for growth in the acute phase and for survival in the chronic phase of mouse infections. In vitro essentiality of fba was strictly condition-dependent. An FBA deletion mutant (Δfba) required a balanced combination of carbon substrates entering metabolism above and below the FBA-catalyzed reaction for growth and died in media with single carbon sources and in mouse lungs. Death of Δfba in vitro was associated with the perturbation of intracellular metabolites. These studies highlight how a conditional fba mutant helped identify conditions in which FBA is dispensable for growth of Mtb, evaluate FBA as a potential target for eliminating persistent bacilli and offer insight into metabolic regulation of carbon co-catabolism in Mtb.
Published in the journal: . PLoS Pathog 10(5): e32767. doi:10.1371/journal.ppat.1004144
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004144Summary
The development of new chemotherapies targeting Mycobacterium tuberculosis (Mtb) will benefit from genetic evaluation of potential drug targets and a better understanding of the pathways required by Mtb to establish and maintain chronic infections. We employed a genetic approach to investigate the essentiality of fructose-1,6-bisphosphate aldolase (FBA) for growth and survival of Mtb in vitro and in mice. A conditional fba mutant revealed that Mtb requires FBA for growth in the acute phase and for survival in the chronic phase of mouse infections. In vitro essentiality of fba was strictly condition-dependent. An FBA deletion mutant (Δfba) required a balanced combination of carbon substrates entering metabolism above and below the FBA-catalyzed reaction for growth and died in media with single carbon sources and in mouse lungs. Death of Δfba in vitro was associated with the perturbation of intracellular metabolites. These studies highlight how a conditional fba mutant helped identify conditions in which FBA is dispensable for growth of Mtb, evaluate FBA as a potential target for eliminating persistent bacilli and offer insight into metabolic regulation of carbon co-catabolism in Mtb.
Introduction
Metabolism is an important aspect of all host–pathogen interactions [1]. Mycobacterium tuberculosis (Mtb) has evolved to replicate and survive for decades within diverse host granulomas, including caseating, fibrotic and cavitating lesions, each providing a different environment [2], [3]. Mtb's ability to adapt to multiple environments may in part be attributed to its metabolic flexibility and its capacity to efficiently catabolize multiple carbon sources simultaneously [4]–[6]. Fatty acids and lipids are major carbon sources available to Mtb in vivo [7]–[10], but Mtb also has access to glucose and glycolytic 3-carbon compounds, requires trehalose import for virulence and can utilize CO2 as source of carbon [6], [11]–[13]. Mtb lacks classical carbon catabolite repression and it remains to be identified how co-catabolism of multiple carbon sources is regulated to achieve optimal growth [4].
Knowledge about Mtb's metabolism benefits the understanding of tuberculosis pathogenesis and can identify potential new targets for chemotherapeutic interventions, as Mtb mutants lacking metabolic enzymes are among the most attenuated in the mouse model of tuberculosis [8], [10], [14]–[17]. Fructose bisphosphate aldolase (FBA) is central to glycolysis and gluconeogenesis (Figure S1) and has been the focus of structural, enzymatic, and drug developmental studies [18]–[22]. Evidence supporting FBA's requirement for optimal growth came from the failure to isolate mutants with transposon insertions in fba, the gene encoding FBA, in standard in vitro culture conditions [23]. Also, previous attempts to delete fba from the Mtb chromosome by homologous recombination have failed [18], [19]. A conditional fba mutant generated in the attenuated H37Ra strain revealed that growth in either glucose - or succinate-containing media was strictly dependent upon induction of FBA expression, demonstrating that FBA is an essential enzyme for both glycolysis and gluconeogenesis [19]. Moreover, FBA is expressed in Mtb during mouse and guinea pig infections, and increased amounts were identified in culture filtrates during hypoxia, a condition which persistent Mtb is thought to encounter in the host [19], [24]. FBA has recently been shown to be secreted by Mtb and to bind host plasminogen, potentially enabling FBA to play a metabolism-independent role in host-pathogen interactions [19]. Importantly, Mtb FBA differs from human FBA by its reaction mechanism. FBAs catalyze the reversible cleavage of fructose-1,6-bisphosphate (FBP) to yield dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (G3P). However, class I FBAs such as the human enzyme produce a Schiff-base reaction intermediate whereas class II FBAs, to which the Mtb enzyme belongs, require a divalent metal cation such as Zn2+ to stabilize the enolate intermediate [25]. Although some bacteria express both class I and class II enzymes, Mtb lacks an annotated class I FBA [19], [26]. Class I FBA activity in Mtb has been reported in earlier studies, but could not be detected by others [19], [27], [28]. The difference in catalytic mechanism of FBA from humans compared to that of Mtb enabled the design of bacteria-targeting class II-specific FBA inhibitors and work is ongoing to improve their efficacy [20], [22], [29], [30].
Our goal was to investigate the importance of Mtb's FBA in acute and chronic mouse infections and to further characterize the basis for its in vitro essentiality. To achieve these goals we generated Mtb strains in which FBA expression was tightly regulated by a tunable dual-control (DUC) genetic switch that combines transcriptional silencing and controlled protein depletion [31]. We found that essentiality of FBA was condition-dependent, and could be overcome by availability of two carbon sources entering metabolism above and below the FBA-catalyzed step. However this was dependent on the specific ratio of the glycolytic and gluconeogenic carbon sources, because metabolism of butyrate by a strain lacking fba was dependent on Mtb's ability to efficiently co-catabolize glucose. Mtb relied on FBA for both growth during acute and persistence during chronic mouse infections.
Results
FBA is required for growth in glycolytic and gluconeogenic carbon sources and for growth and persistence in mice
To investigate the role of FBA in vitro and in vivo we generated an Mtb strain in which expression of FBA is regulated by the recently described DUC switch [31], so that anhydrotetracycline (atc) or doxycycline (doxy) trigger transcriptional repression of the fba gene and simultaneous degradation of the FBA protein. We first introduced a second copy of fba with a strong promoter (Psmyc-fba) [32] via an integrative plasmid into the Mtb chromosome and then deleted the native copy. After confirming fba deletion by Southern blot (Figure S2), we generated FBA-DUC by replacing Psmyc-fba with a DAS+4-tagged fba gene, whose transcription was controlled by a TetR-regulated promoter. The DAS+4-tag allowed for proteolytic inactivation of FBA. Immunoblot analysis confirmed FBA depletion upon addition of atc (Figure S3). Similar to previous findings with an H37Ra FBA-TetON mutant [19], growth of H37Rv FBA-DUC with single carbon sources was inhibited when FBA was depleted by the addition of atc (Figure 1A–C). Unexpectedly, however, growth of FBA-DUC was unaffected by atc in media containing glucose and a second carbon source, such as butyrate, or glycerol (Figure 1D,E).
Fig. 1. FBA depletion inhibits growth of Mtb in single carbon sources, but not in the presence of both a glycolytic and a gluconeogenic carbon source. 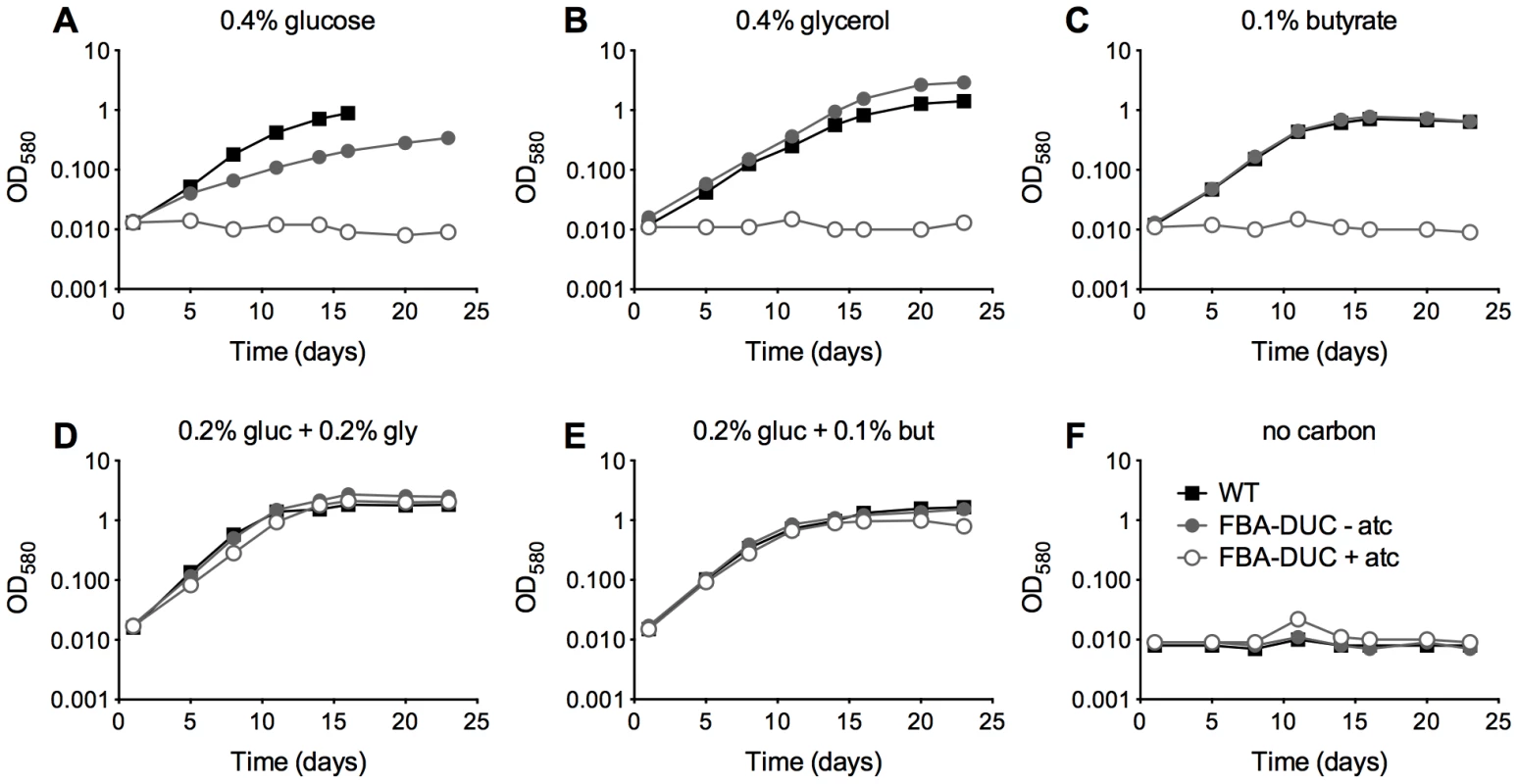
Growth of WT (black squares) and FBA-DUC in the absence (grey circles) and presence (open circles) of 500 ng/ml atc in carbon defined media containing (A) 0.4% glucose (B) 0.4% glycerol (C) 0.1% butyrate (D) 0.2% glucose and 0.2% glycerol (E) 0.2% glucose and 0.1% butyrate and (F) no carbon. Bacteria were cultured in 25 cm2 flasks. Data are representative of at least two independent experiments. To assess if FBA is required in vivo, we infected mice with WT and FBA-DUC and monitored growth and survival in lungs and spleens (Figure 2). Mice infected with FBA-DUC were fed doxy-containing chow starting at the day of infection, on day 10, and on day 35 post-infection to determine whether FBA is required for growth and persistence of Mtb. FBA-DUC failed to replicate in lungs of mice fed with doxy starting on the day of infection and was undetectable by CFU determinations at day 10 post-infection (Figure 2A). No pathological signs of infection were observed in lungs of these mice (not shown) and no CFU were detectable in their spleens. When doxy administration was started later, CFU declined rapidly in lungs and spleens of both acutely and chronically infected mice. Decreases in CFU were accompanied by decreases in lung pathology (Figure 2B). In mice that did not receive doxy, FBA-DUC replicated and persisted similar to WT. Together these data establish that Mtb requires FBA activity for growth during acute and persistence during chronic mouse infections.
Fig. 2. FBA is required for replication and persistence of Mtb in mice. 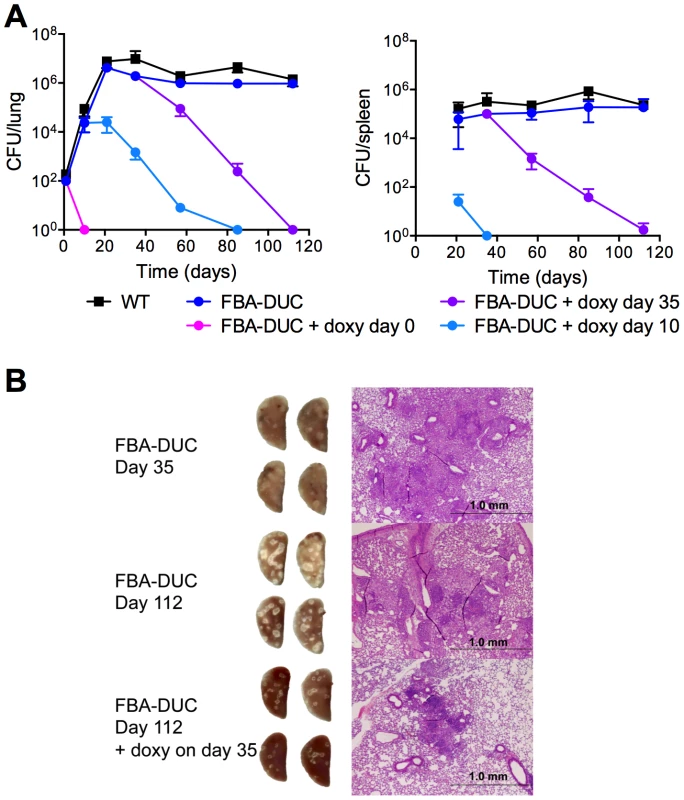
(A) Growth and survival of WT (squares) and FBA-DUC (circles) in mouse lungs (left panel) and spleens (right panel). Mice infected with FBA-DUC received doxy-containing food from the day of infection (day 0), day 10, day 35 or not at all as indicated. CFU were not detected in spleens from mice infected with FBA-DUC and treated with doxy starting day 0. The limit of detection was 4 CFU in lungs and spleens. Data are means ± SD of four mice, except for three data points which derive from 3 or 2 mice due to the appearance of atc/doxy resistant revertants in the lungs (day 57 FBA-DUC+doxy day 10, day 85 FBA-DUC+doxy day 10 and day 112 FBA-DUC+doxy day 35). (B) Gross pathology and H&E staining of lung sections from mice infected with FBA-DUC. Lungs were isolated at day 35 (upper panel) and day 112 (middle and lower panel) from mice not treated and administered doxy starting day 35 post infection. A second short course infection experiment reproduced the phenotype of FBA-DUC in mice not treated or treated with doxy starting on the day of infection. FBA essentiality is carbon source-dependent
Growth of FBA-DUC with atc in dual carbon source media (Figure 1) suggested that FBA may not be essential in all conditions and that its essentiality may be carbon source-dependent. Therefore, we next attempted to delete fba by replacing the integrative, plasmid containing Psmyc-fba with a plasmid that did not contain fba. Δfba candidates grew readily on agar plates containing a carbon source combination - glucose and glycerol - that permitted growth of FBA-DUC with atc (Figure 3A). No Δfba candidates were obtained on standard Mtb agar plates with glycerol and Middlebrook OADC, a supplement containing oleic acid and glucose as carbon sources. However, doubling the glucose concentration resulted in growth of colonies albeit with slower growth rate than in the absence of oleic acid (Figure 3A). Southern blot and immunoblot confirmed that the Δfba candidates were true knockout strains (Figure S2, S3). As observed on agar plates, growth of Δfba in liquid culture was carbon source-dependent, although Δfba replicated in oleic acid-containing medium following a twenty-day lag phase (Figure 3B). This delayed growth was not from complete fba suppressor mutants because these bacteria were not capable of growth with a single carbon source (not shown). These experiments demonstrated that essentiality of FBA is conditional and suggested that Mtb lacking FBA (Δfba) has specific carbon source requirements.
Fig. 3. FBA essentiality is carbon source dependent. 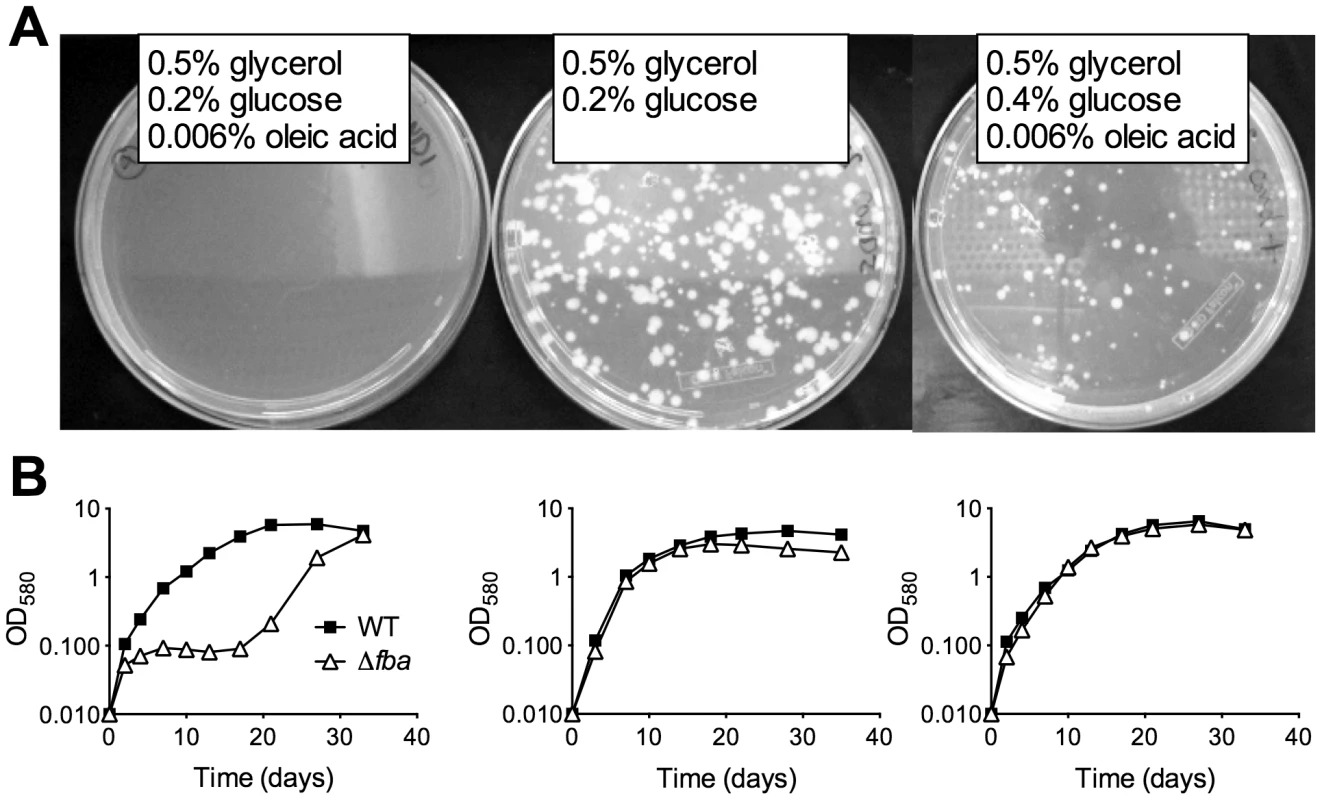
(A) Growth of replacement transformants of Mtb Psmyc-fba-fba::hyg with a plasmid not containing fba and thus resulting in Δfba on agar plates containing the indicated carbon sources. (B) Growth of Δfba in 7H9 base liquid media with identical carbon sources as in the above plate conditions. Indeed, similar to the effects of conditional FBA-depletion, complete inactivation of FBA by deletion of fba caused a failure to replicate with a single carbon source but did not drastically affect growth with two carbon sources that enter central carbon metabolism above and below the FBA catalyzed step (Figure 4A). Growth of the complemented strain (Δfba-comp) was similar to that of WT in all conditions tested, demonstrating that the observed growth phenotypes were caused by the lack of FBA. CFU analysis of Δfba in single carbon sources revealed that Δfba died rapidly, with a 5 log decrease in CFU in 7 days and no detectable CFU by day 14 in media containing glycerol, butyrate, or acetate as sole carbon sources (Figure 4B, data not shown for acetate). Death in glucose-containing medium was slower, but also substantial. In contrast, Δfba survived with only a 10-fold decrease in CFU in base media without added carbon. Thus, the presence of a single carbon source resulted in killing of Δfba and the kinetics of death were dependent on the specific carbon source suggesting that the mechanism leading to death of Δfba in glucose may be different from that induced by glycerol or butyrate feeding below the FBA catalyzed step.
Fig. 4. FBA is required for growth and survival in single carbon sources. 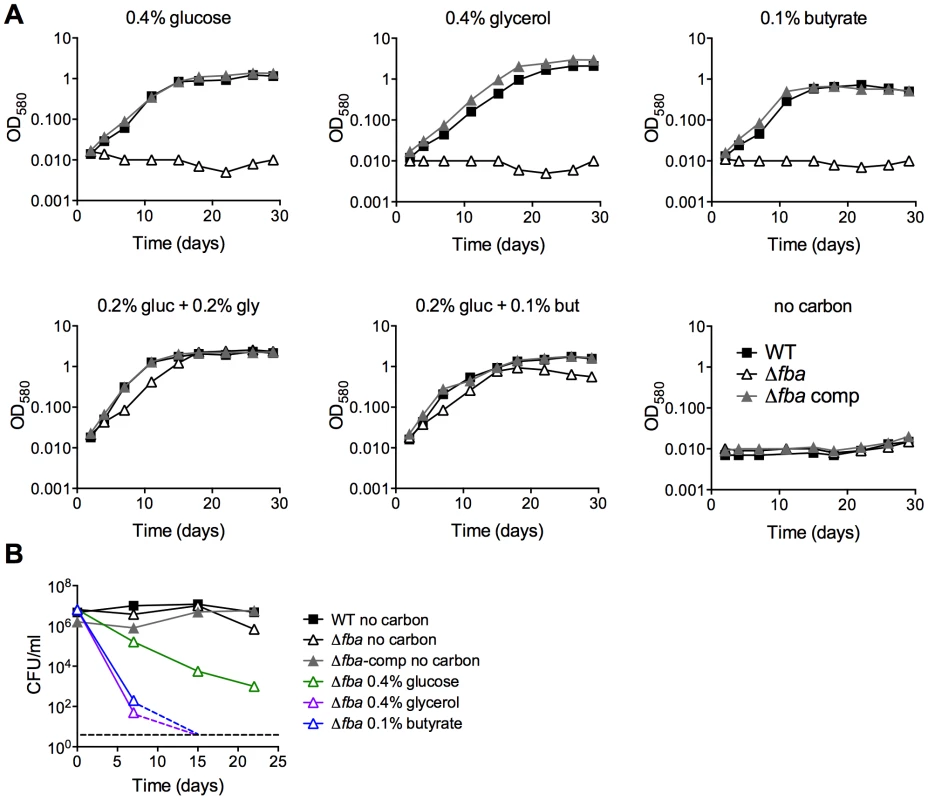
(A) Growth measured by absorbance of WT (black squares), Δfba (open triangles) and Δfba-comp (grey triangles) in carbon-defined media containing glucose, glycerol and butyrate at the indicated concentrations. Bacteria were cultured in 25 cm2 flasks. (B) Survival measured by culturing for CFU on growth-permissive agar plates of the indicated Mtb strains in media with single carbon sources and no carbon addition at different time points post-inoculation. Stippled lines indicate that next data point was below the limit of detection. Δfba failed to replicate in resting bone marrow derived mouse macrophages (BMDM) in contrast to WT and Δfba-comp (Figure 5A). Activation of macrophages with IFNγ reduced replication of WT and Δfba-comp and did not affect survival of Δfba. In mice, Δfba did not establish an infection and was cleared from mouse lungs by day 10 post infection (Figure 5B). Thus, the metabolic environment in phagosomes of macrophages ex vivo and in mouse lungs did not support growth of Mtb in the absence of FBA and resulted in killing of Δfba in mice. The lack of death of Δfba in isolated macrophages suggests that the intracellular environment in macrophages ex vivo does not exactly mimic that faced by Mtb in macrophages inside the lung.
Fig. 5. FBA is required for replication in macrophages and growth and survival in mouse lungs. 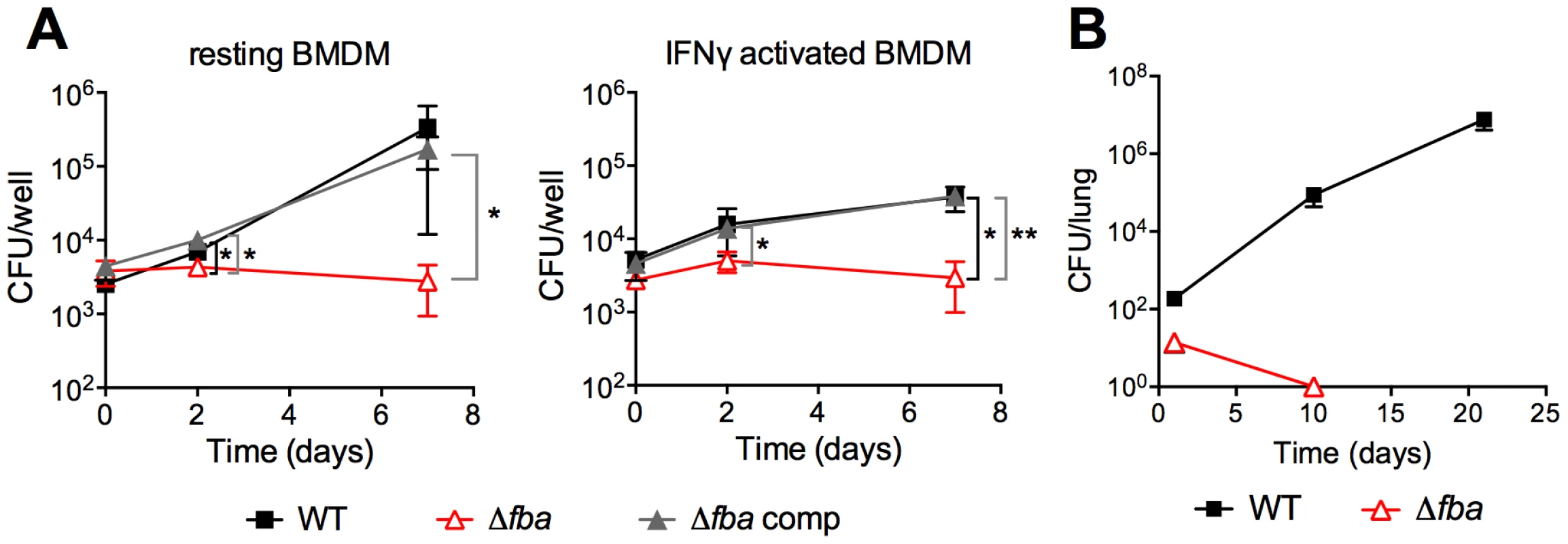
(A) Bacterial loads in resting and IFNγ activated mouse bone marrow derived macrophages (BMDM) infected with carbon-starved WT, Δfba and Δfba-comp. Data are means +/− SD of triplicate wells. *P≤0.05; **P≤0.005 by Student's t-test (B) Growth and survival of WT and Δfba in mouse lungs. Data are means +/− SD from four mice. Limit of detection was 4 CFU and Δfba was not detectable on days 10 and 21. Metabolic consequences of fba deletion
To better understand the impact of loss of FBA on Mtb metabolism we used liquid chromatography/mass spectrometry to measure metabolite levels in WT, Δfba, and Δfba-comp cultured on filters on top of agar plates containing 0.2% glucose, 0.2% glycerol, or a combination of 0.2% glucose and 0.2% glycerol (Figure 6). Following exposure to glucose alone, Δfba accumulated high levels of hexose-phosphate (P) and depleted its triose-P and phosphoenolpyruvate (PEP) pools. These metabolic changes are consistent with a defect in glucose metabolism due to the absence of FBA activity. Pentose-P and sedoheptulose-P pools were also significantly increased in Δfba suggesting either increased flux through the pentose phosphate (PP) pathway or decreased metabolism of PP pathway intermediates. In contrast, exposure to glycerol as sole carbon source resulted in depletion of hexose-P and sedoheptulose-P pools while triose-P levels were increased. The presence of both glucose and glycerol reversed most metabolic changes in Δfba except for the triose-P pool, which remained elevated, but not to the same extent as with glycerol as sole carbon source. These experiments demonstrate that metabolism of single carbon sources by Δfba significantly altered intracellular metabolite concentrations, which was accompanied by cell death (Figure 4). The buildup of PP pathway metabolites was consistent with increased flux into the PP pathway during culture on glucose; however, the PP pathway could not act as an efficient bypass to overcome loss of FBA.
Fig. 6. Metabolic consequences of fba deletion. 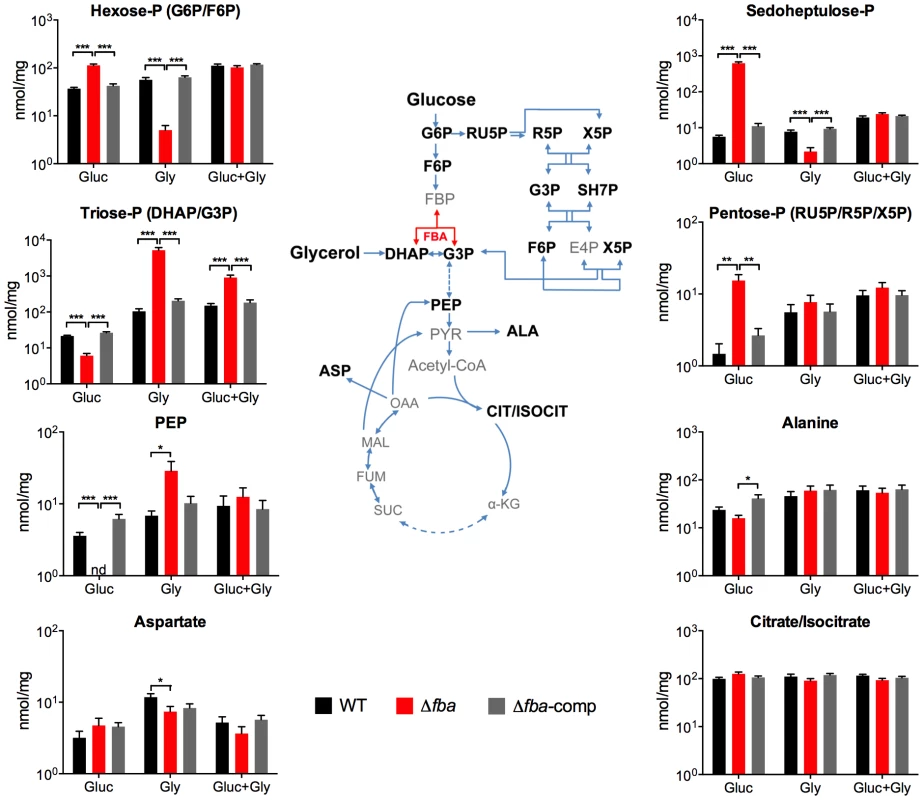
Intrabacterial pool sizes of selected metabolites (nmol/mg protein) in the indicated Mtb strains after 24 hours culture on media containing either glucose (Gluc), glycerol (Gly) or a combination of both. nd = not detected. All values are means of measurements from two independent experiments, each performed with triplicate cultures ± SEM. *P≤0.05; **P≤0.005; ***P≤0.0005 by Student's t-test. ALA, alanine; α-KG, α-ketoglutarate; ASP, aspartate; CIT/ISOCIT, citrate/isocitrate; DHAP, dihydroxyacetone phosphate; E4P, erythrose 4-phosphate; FBA, fructose-1,6-bisphosphate aldolase; F6P, fructose 6-phosphate; FBP, fructose 1,6-bisphosphate; FUM, fumarate; G3P, glyceraldehyde 3-phosphate; G6P, glucose 6-phosphate; MAL, malate; OAA, oxaloacetate; PEP, phosphoenolpyruvate; PYR, pyruvate; R5P, ribose 5-phosphate; RU5P, ribulose 5-phosphate, SH7P, sedoheptulose 7-phosphate; SUC, succinate; X5P, xylulose 5-phosphate. Only a specific balance of carbon sources can compensate for the lack of FBA
Growth of Δfba required the presence of two carbon sources entering metabolism above and below the FBA catalyzed reaction and on agar plates was dependent on the amount of glucose in media containing glycerol and oleic acid (Figure 3). We therefore sought to investigate whether FBA might regulate a specific balance of glycolytic and gluconeogenic metabolism. WT Mtb replicated with glucose as sole carbon source and even low concentrations of butyrate enhanced its growth as previously reported [4] up to 0.4% when butyrate became toxic (Figure 7A). In contrast, when provided with 0.2% glucose, Δfba required at least 0.05% butyrate for growth, which was maximal with 0.1% but did not reach that of WT suggesting that in these conditions Mtb's capacity to metabolize butyrate is limited by FBA deficiency.
Fig. 7. Mtb lacking FBA requires a balanced carbon diet for growth. 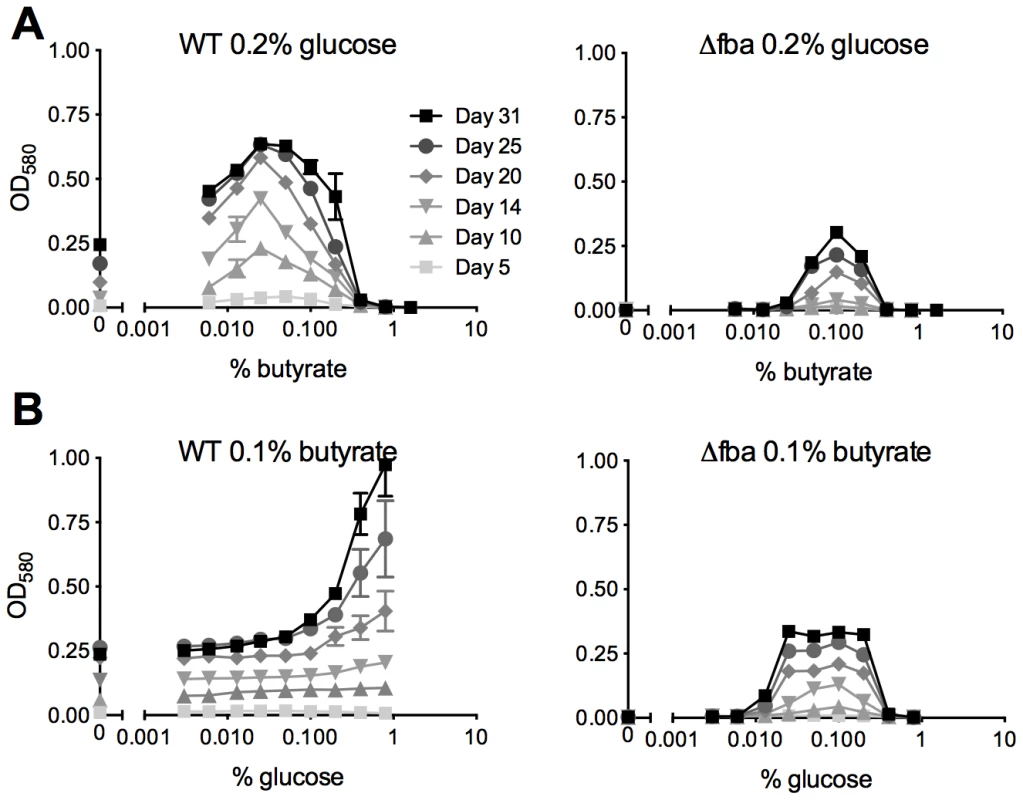
Growth of WT and Δfba was in media containing (A) 0.2% glucose with varying concentrations of butyrate and (B) 0.1% butyrate with varying concentrations of glucose. Bacteria were cultured in 96-well plates and absorbance was measured at the indicated time points. Data are means of triplicate cultures +/− SEM and representative of two independent experiments. With a fixed concentration of 0.1% butyrate, WT growth increased with increasing amounts of glucose (Figure 7B). Growth of Δfba, however, plateaued with 0.025% glucose and was inhibited at concentrations above 0.2%. Inefficient glucose metabolism thus limited growth of Δfba in the presence of butyrate suggesting that FBA facilitates the efficient catabolism of butyrate by driving glycolysis. Together these data imply that the efficiency of glucose metabolism determines Mtb's capacity to co-catabolize butyrate.
Vulnerability of Mtb to partial FBA depletion depends on the carbon source
Given the in vivo essentiality in acute and chronic mouse infections, our final goal was to evaluate to what degree FBA has to be depleted to result in growth inhibition and whether this is dependent on the available carbon source. FBA-DUC grew slower than WT in media with 0.4% glucose even in the absence of atc (Figure 1) and in glucose FBA-DUC was more sensitive to atc-induced growth inhibition than in butyrate-containing media (Figure S4). The reduced growth in glucose-containing, atc-free media (Figure 1,8A) was likely due to the low FBA amounts expressed in FBA-DUC, which were reduced by approximately 87% compared to WT even in the absence of atc (Figure 8B). Addition of 6.3 ng/ml atc depleted FBA by approximately 97% and abolished growth of FBA-DUC in glucose containing medium. In contrast, depletion by 87% or 97% was insufficient to reduce growth of FBA-DUC in medium with 0.1% butyrate as the sole carbon source. Addition of 25 ng/ml atc, which reduced FBA expression below the level of detection, was required to inhibit growth with butyrate. Thus, Mtb was more sensitive to FBA depletion when grown with glucose as sole carbon source than when metabolizing butyrate emphasizing that vulnerability to depletion of an essential protein can be dependent on the growth condition.
Fig. 8. Vulnerability of Mtb to FBA depletion depends on the carbon source. 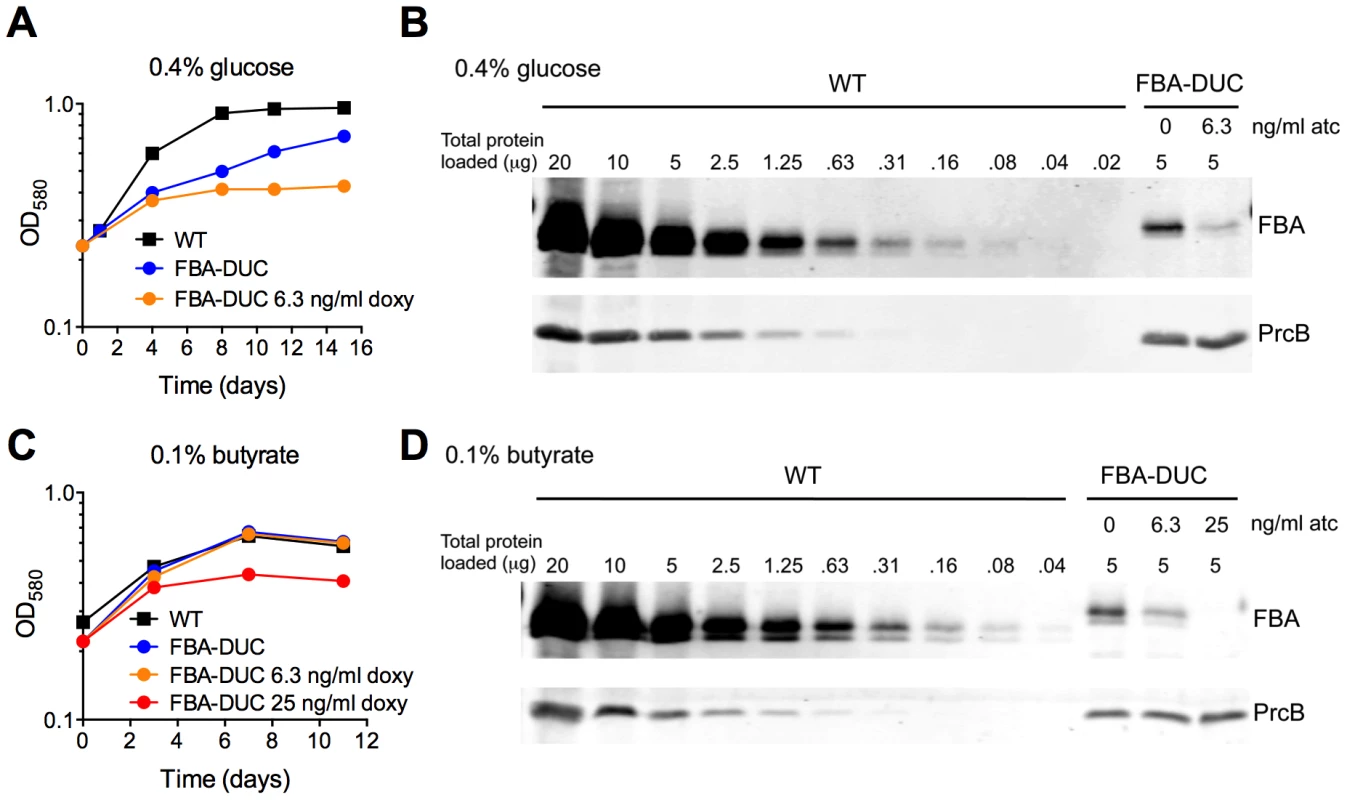
Growth of WT and FBA-DUC without and with indicated amounts of atc in (A) 0.4% glucose or (C) 0.1% butyrate. (B) FBA immunoblot in protein extracts from 0.4% glucose cultures on day 15 after atc addition. (D) FBA immunoblot in protein extracts from 0.1% butyrate cultures on day 11 after atc addition. PrcB served as loading control. Discussion
The experiments reported here enhance our understanding of Mtb carbon metabolism and are relevant to tuberculosis drug development. Mtb fba has previously been shown to be required for growth on standard media [19], [33] and FBA inhibitors are under development [22], [29], [30], but to our knowledge no FBA-specific inhibitor has been effective at inhibiting growth of live Mtb. Furthermore, it was unknown whether FBA is essential for growth or survival of Mtb in vivo. Using a genetic approach we evaluated FBA as a potential drug target by assessing its essentiality for growth and persistence during mouse infections. Depletion of FBA after establishment of chronic infection led to complete clearance of viable Mtb in mouse lungs and spleens, which promotes FBA as a potential therapeutic target for killing of persistent bacilli.
Unexpectedly, FBA was not essential in all conditions in vitro, and a conditional fba mutant was critical to identify conditions in which Δfba could be isolated. This deletion mutant was able to replicate when provided with combinations of carbon substrates entering metabolism above and below the FBA-catalyzed reaction. Thus, it will be crucial to determine the efficacy of FBA inhibitors against live Mtb in carbon conditions where Mtb is vulnerable to FBA inactivation in contrast to standard mycobacterial liquid culture medium, which contains a combination of glucose and glycerol making FBA dispensable.
Earlier studies have highlighted Mtb's ability to co-catabolize multiple carbon sources in vitro and in macrophages [4]–[6]. This is in contrast to other bacteria such as E. coli, which exhibits diauxic growth in the presence of multiple carbon sources resulting from their sequential metabolism [34], [35]. Here, we provide further evidence for Mtb's co-catabolism and offer insight into its metabolic regulation. Growth of WT Mtb on glucose and on butyrate, which requires β-oxidation for conversion into acetyl-CoA mimicking the fate of long chain fatty acids, was enhanced in a dose responsive manner by addition of the respective other carbon source. Growth of Δfba only occurred with two carbon substrates, feeding into either side of the FBA reaction. Additionally, deletion of FBA rendered Mtb extraordinarily sensitive to the relative concentrations of carbon sources in the medium. In media containing glucose and butyrate, Δfba was unable to efficiently utilize glucose to enhance its growth while growth of WT scaled with glucose. Metabolism of increasing amounts of butyrate was also restricted shown by Δfba's limited growth despite the presence of 0.2% glucose. FBA thus facilitates efficient butyrate catabolism through its ability to metabolize glucose and FBA inactivation resulted in suboptimal co-catabolism of glucose and butyrate.
The mechanism of death of Δfba in carbon-unbalanced media remains to be determined but may be due to buildup of FBA substrates at higher concentrations of glucose or butyrate, as well as regulatory mechanisms allowing Mtb to sense carbon levels and respond accordingly. Indeed, the inability of Δfba to grow on standard Mtb plate medium containing glucose, glycerol and oleic acid was overcome by the addition of 0.2% additional glucose, which was not required for growth in nearly identical medium lacking oleic acid.
Inactivation of FBA in E. coli prevented growth with glucose but not with glycerol and succinate as carbon substrates [36], [37]. Accumulation of sugar phosphates including the FBA substrate fructose-1,6-bisphosphate (FBP) is thought to be the cause of this growth inhibition, which is supported by suppressor mutations that prevent FBP buildup and allow growth on glucose [38]. With our metabolite extraction protocol we did not detect FBP, however other sugar phosphates including hexose-P, sedoheptulose-P and pentose-P increased dramatically in Δfba cultured on glucose, while triose-P and PEP accumulated on glycerol. The accumulation of these metabolites was not observed when both glucose and glycerol were available to Δfba, except for the triose-P pools, which remained elevated but much less than in the glycerol condition. It is thus possible that the accumulation of phosphorylated metabolites is cause for the death of Δfba in conditions where glycolytic and gluconeogenic carbon flow is unbalanced, such as with single carbon substrates or in mixes with an abundance of a glycolytic or gluconeogenic substrate.
How potent would an FBA inhibitor need to be to affect Mtb growth? We measured the vulnerability of Mtb to FBA depletion in single glycolytic and gluconeogenic carbon sources where FBA is essential. Although depleting FBA by 97% did not affect growth with butyrate as sole carbon source, it prevented growth with glucose and 87% inhibition was sufficient to reduce growth with glucose. These data reveal a differential susceptibility of Mtb to FBA depletion, depending on the available carbon source. Antibiotic targets vary widely in how much inhibition is required to stop replication or induce death and not all effective drug targets are highly vulnerable to inhibition [39]. Specific target-drug interactions can contribute to the efficacy of a compound. We cannot predict how effectively FBA must be depleted to abolish replication in vivo, but the WT-like phenotype of FBA-DUC without doxy suggests that 13% of WT FBA levels are sufficient for normal growth and persistence in mice. The fast killing of Mtb following further FBA depletion in mouse lungs and spleens suggests that it is an effective target during acute and chronic mouse infections. During infections FBA may also have an additional, metabolism-independent function in Mtb's interaction with the host as it can be secreted and bind to human plasminogen [19].
The requirement of FBA for growth and persistence in mice suggests that in vivo Mtb either faces single carbon sources or lacks access to the growth permissive ratio of carbon sources that can compensate for the lack of FBA. This is likely due to an abundance of fatty acids and lipids which are predominant carbon sources available to Mtb in vivo [7]–[10]. It is unknown if FBA is essential for growth and persistence of Mtb in humans; experimental animal models that more closely mimic human TB pathology [40] would help address this question. Given that human granulomas contain lipid-rich foamy macrophages and build up lipids in the caseum [41], [42], it is plausible that Mtb requires FBA also during human infection.
Materials and Methods
Ethics statement
Mouse studies were performed following National Institutes of Health guidelines for housing and care of laboratory animals and performed in accordance with institutional regulations after protocol review and approval by the Institutional Animal Care and Use Committee of Weill Cornell Medical College (protocol # 0601-441A, Conditional Expression of Mycobacterial Genes).
Strains, media and culture conditions
M. tuberculosis H37Rv strains were grown in Middlebrook 7H9 liquid media containing 0.5% bovine serum albumin fraction V, 0.2% glucose, 0.2% glycerol, 0.085% NaCl, and 0.05% tyloxapol without shaking in 5% CO2 at 37°C. For carbon-defined growth curves, Mtb was cultured in Sauton's base media modified to be carbon-limited, containing 0.05% potassium phosphate monobasic, 0.05% magnesium sulfate heptahydrate, 0.2% citric acid, 0.005% ferric ammonium citrate, 0.05% ammonium sulfate, 0.0001% zinc sulfate, and 0.05% tyloxapol at pH 7.4. For solid media, Middlebrook 7H10 media with 0.5% glycerol and 10% Middlebrook OADC supplement (final concentration of 0.5% bovine serum albumin fraction V, 0.2% glucose, 0.085% NaCl, 0.006% oleic acid, 0.0003% catalase) or self-made ADNaCl (final concentration of 0.5% bovine serum albumin fraction V, 0.2% glucose, 0.085% NaCl) was used. Carbon sources glucose, glycerol, sodium acetate and butyric acid, were added at indicated concentration (%wt/vol or %vol/vol, depending on stock). When appropriate, hygromycin B (50 µg/ml), streptomycin (10 µg/ml), kanamycin (25 µg/ml), and/or zeocin (25 µg/ml) were added. Anhydrotetracycline (atc) was added at the indicated concentrations and replenished at half the initial concentration in liquid cultures every 4–5 days for growth curves but not vulnerability assays. For survival assays, bacterial culture samples were taken from growth curve cultures at the time-points indicated and plated for CFU. For metabolomic profiling, Mtb was seeded at OD580∼1 on 0.22 µM nitrocellulose filters (1 ml per filter) and cultured on Middlebrook 7H10 agar medium containing 0.5% bovine serum albumin fraction V, 0.085% NaCl, 0.2% glucose, and 0.2% glycerol for 5 days. Filters were then transferred to similar plates with defined carbon sources: 0.2% glucose, 0.2% glycerol, or both together, each at 0.2%. Mtb was harvested 24 hours later by metabolic quenching in cold acetonitrile∶methanol∶H2O (40∶40∶20) and mechanically lysed using a bead beater as described [4], [10].
Generation of mutant strains
M. tuberculosis H37Rv was transformed with a plasmid expressing fba under the control of a strong promoter P1 (Psmyc [32]) that integrates into the phage attL5 site in the Mtb genome. In this fba merodiploid strain deletion of native fba was achieved by allelic exchange using specialized transducing phage phAE87 as previously described [10]. After confirming removal of native fba by Southern blot, replacement transformations of the attL5 insets were performed to generate FBA-DUC and Δfba. In FBA-DUC, fba contained a C-terminal DAS+4 tag and a plasmid in the phage tweety site that allowed inducible expression of SspB as described [31]. In Δfba the attL5 site carries a kanamycin-resistant plasmid not containing fba. All plasmids were constructed using Gateway Cloning Technology (Invitrogen) using BP and LR recombinase reactions following the manufacturers instructions. The complemented mutant is Δfba transformed with a plasmid that integrates in the attL5 site expressing fba from the P1 promoter.
Metabolomics using Liquid Chromatography-Mass Spectrometry
M. tuberculosis metabolites were separated and detected in a Agilent Accurate Mass 6220 TOF coupled to an Agilent 1200 Liquid Chromatography system using a Cogent Diamond Hydride Type C column (Microsolve Technologies) using solvents and configuration as described [43]. Metabolites were quantified by standard curves generated with authentic chemicals spiked into homologous mycobacterial lysates. Quantified metabolite concentrations were normalized to bacterial biomass of individual samples determined by measuring residual protein content (BCA Protein Assay kit; Pierce).
Immunoblot analysis for vulnerability assay
Protein extracts were prepared from bacterial pellets from 30 ml cultures at indicated time points in specified media. Briefly, cultures were washed with phosphate buffered saline (PBS), 0.05% Tween 80 and resuspended in 1 ml PBS, 1× protease inhibitor cocktail (Roche). Cells were lysed by bead beating three times at 4500 rpm for 30 seconds with 0.1 mm Zirconia/Silica beads. Beads and cell walls were removed through centrifugation (11000× g/10 min/4°C) and the supernatant was filtered through a 0.2 µm SpinX column (Corning). Lysate protein concentrations were determined using a DC Protein Assay Kit (Bio-Rad). For immunoblots 20-0.02 µg protein extracts were separated by SDS-PAGE, transferred to nitrocellulose membranes and probed overnight with rabbit antisera FBA [19] (1∶2000 dilution in 1∶1 PBS/Odyssey Blocking Buffer (LI-COR Biosciences), 0.1% Tween20) or PrcB (1∶18,000 dilution in 1∶1 PBS/Odyssey Blocking Buffer (LI-COR Biosciences), 0.1% Tween20). As secondary antibody IRDye 680 Donkey anti-Rabbit IgG(H+L) (LI-COR Biosciences) was used. Proteins were detected using the Odyssey Infrared Imaging System (LI-COR Biosciences).
Mouse and macrophage infections
Female C57BL/6 mice (Jackson Laboratory) were infected by aerosol using an inhalation exposure system (Glas-Col) and early-log-phase M. tuberculosis cultures as single-cell suspensions in PBS to deliver 100 to 200 bacilli per mouse. Doxycycline containing food (2000 ppm, Research Diets) was given to mice starting at the indicated time-points. Serial dilutions of lungs and spleens homogenates were cultured on 7H10 plates containing ADNaCl to determine CFU at the indicated time points. The left lobe of each lung was fixed in 10% buffered formalin, further processed for histopathology and stained with hematoxilyn and eosin. We isolated and infected bone marrow-derived mouse macrophages as previously described [44].
Supporting Information
Zdroje
1. EhrtS, RheeK (2013) Mycobacterium tuberculosis metabolism and host interaction: mysteries and paradoxes. Curr Top Microbiol Immunol 374 : 163–188 doi:__10.1007/82_2012_299
2. LinPL, RodgersM, SmithL, BigbeeM, MyersA, et al. (2009) Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun 77 : 4631–4642 doi:10.1128/IAI.00592-09
3. BarryCE, BoshoffHI, DartoisV, DickT, EhrtS, et al. (2009) The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Micro 7 : 845–855 doi:10.1038/nrmicro2236
4. de CarvalhoLPS, FischerSM, MarreroJ, NathanC, EhrtS, et al. (2010) Metabolomics of Mycobacterium tuberculosis reveals compartmentalized co-catabolism of carbon substrates. Chemistry & Biology 17 : 1122–1131 doi:10.1016/j.chembiol.2010.08.009
5. BesteDJV, BondeB, HawkinsN, WardJL, BealeMH, et al. (2011) C Metabolic Flux Analysis Identifies an Unusual Route for Pyruvate Dissimilation in Mycobacteria which Requires Isocitrate Lyase and Carbon Dioxide Fixation. PLoS Pathog 7: e1002091 doi:10.1371/journal.ppat.1002091
6. BesteDJV, NöhK, NiedenführS, MendumTA, HawkinsND, et al. (2013) 13C-Flux Spectral Analysis of Host-Pathogen Metabolism Reveals a Mixed Diet for Intracellular Mycobacterium tuberculosis. Chemistry & Biology 20 : 1012–1021 doi:10.1016/j.chembiol.2013.06.012
7. BlochH, SegalW (1956) Biochemical differentiation of Mycobacterium tuberculosis grown in vivo and in vitro. J Bacteriol 72 : 132–141.
8. Muñoz-ElíasE, McKinneyJ (2005) Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat Med 11 : 638–644.
9. PandeyAK, SassettiCM (2008) Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci USA 105 : 4376–4380 doi:10.1073/pnas.0711159105
10. MarreroJ, RheeKY, SchnappingerD, PetheK, EhrtS (2010) Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection. Proc Natl Acad Sci USA 107 : 9819–9824 doi:10.1073/pnas.1000715107
11. MarreroJ, TrujilloC, RheeKY, EhrtS (2013) Glucose Phosphorylation Is Required for Mycobacterium tuberculosis Persistence in Mice. PLoS Pathog 9: e1003116 doi:10.1371/journal.ppat.1003116.s006
12. KalscheuerR, WeinrickB, VeeraraghavanU, BesraGS, JacobsWR (2010) Trehalose-recycling ABC transporter LpqY-SugA-SugB-SugC is essential for virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci USA 107 : 21761–21766 doi:10.1073/pnas.1014642108
13. WatanabeS, ZimmermannM, GoodwinMB, SauerU, BarryCE, et al. (2011) Fumarate reductase activity maintains an energized membrane in anaerobic Mycobacterium tuberculosis. PLoS Pathog 7: e1002287 doi:10.1371/journal.ppat.1002287
14. McKinneyJ, zu BentrupK, Muñoz-ElíasE, MiczakA, ChenB, et al. (2000) Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406 : 735–738.
15. BlumenthalA, TrujilloC, EhrtS, SchnappingerD (2010) Simultaneous analysis of multiple Mycobacterium tuberculosis knockdown mutants in vitro and in vivo. PLoS ONE 5: e15667 doi:10.1371/journal.pone.0015667
16. KalscheuerR, SysonK, VeeraraghavanU, WeinrickB, BiermannKE, et al. (2010) Self-poisoning of Mycobacterium tuberculosis by targeting GlgE in an alpha-glucan pathway. Nat Chem Biol 6 : 376–384 doi:10.1038/nchembio.340
17. VenugopalA, BrykR, ShiS, RheeK, RathP, et al. (2011) Virulence of Mycobacterium tuberculosis depends on lipoamide dehydrogenase, a member of three multienzyme complexes. Cell Host Microbe 9 : 21–31 doi:10.1016/j.chom.2010.12.004
18. PeganS, RuksereeK, FranzblauS, MesecarA (2009) Structural basis for catalysis of a tetrameric class IIa fructose 1, 6-bisphosphate aldolase from Mycobacterium tuberculosis. J Mol Biol 386 : 1038–1053.
19. la Paz Santangelo deM, GestPM, GuerinME, CoinçonM, PhamH, et al. (2011) Glycolytic and non-glycolytic functions of Mycobacterium tuberculosis fructose-1,6-bisphosphate aldolase, an essential enzyme produced by replicating and non-replicating bacilli. J Biol Chem 286 : 40219–40231 doi:10.1074/jbc.M111.259440
20. LabbéG, KrismanichAP, de GrootS, RasmussonT, ShangM, et al. (2012) Development of metal-chelating inhibitors for the Class II fructose 1,6-bisphosphate (FBP) aldolase. J Inorg Biochem 112 : 49–58 doi:10.1016/j.jinorgbio.2012.02.032
21. PeganSD, RuksereeK, CapodagliGC, BakerEA, KrasnykhO, et al. (2013) Active Site Loop Dynamics of a Class IIa Fructose 1,6-Bisphosphate Aldolase from Mycobacterium tuberculosis. Biochemistry 52 : 912–925 doi:10.1021/bi300928u
22. CapodagliGC, SedhomWG, JacksonM, AhrendtKA, PeganSD (2014) A Noncompetitive Inhibitor for Mycobacterium tuberculosis's Class IIa Fructose 1,6-Bisphosphate Aldolase. Biochemistry 53 : 202–213 doi:10.1021/bi401022b
23. GriffinJE, GawronskiJD, DejesusMA, IoergerTR, AkerleyBJ, et al. (2011) High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog 7: e1002251 doi:10.1371/journal.ppat.1002251
24. RosenkrandsI, SlaydenR, CrawfordJ, AagaardC, BarryCIII, et al. (2002) Hypoxic response of Mycobacterium tuberculosis studied by metabolic labeling and proteome analysis of cellular and extracellular proteins. J Bacteriol 184 : 3485.
25. RamsaywakPC, LabbéG, SiemannS, DmitrienkoGI, GuillemetteJG (2004) Molecular cloning, expression, purification, and characterization of fructose 1,6-bisphosphate aldolase from Mycobacterium tuberculosis–a novel Class II A tetramer. Protein Expr Purif 37 : 220–228 doi:10.1016/j.pep.2004.05.011
26. ColeST, BroschR, ParkhillJ, GarnierT, ChurcherC, et al. (1998) Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393 : 537–544.
27. BaiNJ, PaiMR, MurthyPS, VenkitasubramanianTA (1974) Effect of oxygen tension on the aldolases of Mycobacterium tuberculosis H37Rv. FEBS Lett 45 : 68–70.
28. BaiN, PaiM, MurthyP, VenkitasubramanianT (1982) Fructose-bisphosphate aldolases from mycobacteria. Methods Enzymol 90 : 241–250.
29. DaherR, TherisodM (2010) Highly Selective Inhibitors of Class II Microbial Fructose Bis-phosphate Aldolases. ACS Medicinal Chemistry Letters 1 : 101–104 doi:10.1021/ml100017c
30. DaherR, CoinçonM, FonvielleM, GestPM, GuerinME, et al. (2010) Rational design, synthesis, and evaluation of new selective inhibitors of microbial class II (zinc dependent) fructose bis-phosphate aldolases. J Med Chem 53 : 7836–7842 doi:10.1021/jm1009814
31. KimJ-H, O'BrienKM, SharmaR, BoshoffHIM, RehrenG, et al. (2013) A genetic strategy to identify targets for the development of drugs that prevent bacterial persistence. Proc Natl Acad Sci USA 110 : 19095–19100 doi:10.1073/pnas.1315860110
32. EhrtS, GuoXV, HickeyCM, RyouM, MonteleoneM, et al. (2005) Controlling gene expression in mycobacteria with anhydrotetracycline and Tet repressor. Nucleic Acids Res 33: e21 doi:10.1093/nar/gni013
33. GriffinJE, PandeyAK, GilmoreSA, MizrahiV, MckinneyJD, et al. (2012) Cholesterol Catabolism by Mycobacterium tuberculosis Requires Transcriptional and Metabolic Adaptations. Chemistry & Biology 19 : 218–227 doi:10.1016/j.chembiol.2011.12.016
34. Kovárová-KovarK, EgliT (1998) Growth kinetics of suspended microbial cells: from single-substrate-controlled growth to mixed-substrate kinetics. Microbiol Mol Biol Rev 62 : 646–666.
35. GörkeB, StülkeJ (2008) Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Micro 6 : 613–624 doi:10.1038/nrmicro1932
36. BöckA, NeidhardtFC (1966) Isolation of a Mutant of Escherichia coli with a Temperature-sensitive Fructose-1,6-Diphosphate Aldolase Activity. J Bacteriol 92 : 464–469.
37. BöckA, NeidhardtFC (1966) Properties of a Mutant of Escherichia coli with a Temperature-sensitive Fructose-1,6-Diphosphate Aldolase. J Bacteriol 92 : 470–476.
38. SchreyerR, BöckA (1973) Phenotypic suppression of a fructose-1,6-diphosphate aldolase mutation in Escherichia coli. J Bacteriol 115 : 268–276.
39. WeiJ-R, KrishnamoorthyV, MurphyK, KimJ-H, SchnappingerD, et al. (2011) Depletion of antibiotic targets has widely varying effects on growth. Proc Natl Acad Sci USA 108 : 4176–4181 doi:10.1073/pnas.1018301108
40. Dartois V (2010) Immunopathology of tuberculosis disease across species. In: Leong FJ, Dartois V, Dick T, editors. A Color Atlas of Comparative Pathology of Pulmonary Tuberculosis. CRC Press.
41. PeyronP, VaubourgeixJ, PoquetY, LevillainF, BotanchC, et al. (2008) Foamy Macrophages from Tuberculous Patients' Granulomas Constitute a Nutrient-Rich Reservoir for M. tuberculosis Persistence. PLoS Pathog 4: e1000204 doi:10.1371/journal.ppat.1000204.t003
42. KimM-J, WainwrightHC, LocketzM, BekkerL-G, WaltherGB, et al. (2010) Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol Med 2 : 258–274 doi:10.1002/emmm.201000079
43. EohH, RheeKY (2013) Multifunctional essentiality of succinate metabolism in adaptation to hypoxia in Mycobacterium tuberculosis. Proc Natl Acad Sci USA 110 : 6554–6559 doi:10.1073/pnas.1219375110
44. VandalOH, PieriniLM, SchnappingerD, NathanCF, EhrtS (2008) A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat Med 14 : 849–854 doi:10.1038/nm.1795
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge inČlánek Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 5- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Surveillance for Emerging Biodiversity Diseases of Wildlife
- The Emerging Role of Urease as a General Microbial Virulence Factor
- PARV4: An Emerging Tetraparvovirus
- Epigenetic Changes Modulate Schistosome Egg Formation and Are a Novel Target for Reducing Transmission of Schistosomiasis
- The Human Adenovirus E4-ORF1 Protein Subverts Discs Large 1 to Mediate Membrane Recruitment and Dysregulation of Phosphatidylinositol 3-Kinase
- A Multifactorial Role for Malaria in Endemic Burkitt's Lymphoma Pathogenesis
- Structural Basis for the Ubiquitin-Linkage Specificity and deISGylating Activity of SARS-CoV Papain-Like Protease
- Cathepsin-L Can Resist Lysis by Human Serum in
- Epstein-Barr Virus Down-Regulates Tumor Suppressor Expression
- BCA2/Rabring7 Targets HIV-1 Gag for Lysosomal Degradation in a Tetherin-Independent Manner
- The Evolutionarily Conserved Mediator Subunit MDT-15/MED15 Links Protective Innate Immune Responses and Xenobiotic Detoxification
- Suppressor of Cytokine Signaling 4 (SOCS4) Protects against Severe Cytokine Storm and Enhances Viral Clearance during Influenza Infection
- T Cell Inactivation by Poxviral B22 Family Proteins Increases Viral Virulence
- Dynamics of HIV Latency and Reactivation in a Primary CD4+ T Cell Model
- HIV and HCV Activate the Inflammasome in Monocytes and Macrophages via Endosomal Toll-Like Receptors without Induction of Type 1 Interferon
- Virus and Autoantigen-Specific CD4+ T Cells Are Key Effectors in a SCID Mouse Model of EBV-Associated Post-Transplant Lymphoproliferative Disorders
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- Squalene Synthase As a Target for Chagas Disease Therapeutics
- The Contribution of Viral Genotype to Plasma Viral Set-Point in HIV Infection
- Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge in
- Anthrax Lethal Factor as an Immune Target in Humans and Transgenic Mice and the Impact of HLA Polymorphism on CD4 T Cell Immunity
- Ly49C-Dependent Control of MCMV Infection by NK Cells Is -Regulated by MHC Class I Molecules
- Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
- A Large Family of Antivirulence Regulators Modulates the Effects of Transcriptional Activators in Gram-negative Pathogenic Bacteria
- Broad-Spectrum Anti-biofilm Peptide That Targets a Cellular Stress Response
- Malaria Parasite Infection Compromises Control of Concurrent Systemic Non-typhoidal Infection via IL-10-Mediated Alteration of Myeloid Cell Function
- A Role for in Higher Order Structure and Complement Binding of the Capsule
- Hip1 Modulates Macrophage Responses through Proteolysis of GroEL2
- CD8 T Cells from a Novel T Cell Receptor Transgenic Mouse Induce Liver-Stage Immunity That Can Be Boosted by Blood-Stage Infection in Rodent Malaria
- Phosphorylation of KasB Regulates Virulence and Acid-Fastness in
- HIV-Infected Individuals with Low CD4/CD8 Ratio despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened CD8+ T Cell Activation, and Increased Risk of Non-AIDS Morbidity and Mortality
- A Novel Mechanism Inducing Genome Instability in Kaposi's Sarcoma-Associated Herpesvirus Infected Cells
- Structural and Biochemical Characterization Reveals LysGH15 as an Unprecedented “EF-Hand-Like” Calcium-Binding Phage Lysin
- Hepatitis C Virus Cell-Cell Transmission and Resistance to Direct-Acting Antiviral Agents
- Different Modes of Retrovirus Restriction by Human APOBEC3A and APOBEC3G
- TNFα and IFNγ but Not Perforin Are Critical for CD8 T Cell-Mediated Protection against Pulmonary Infection
- Large Scale RNAi Reveals the Requirement of Nuclear Envelope Breakdown for Nuclear Import of Human Papillomaviruses
- The Cytoplasmic Domain of Varicella-Zoster Virus Glycoprotein H Regulates Syncytia Formation and Skin Pathogenesis
- A New Class of Multimerization Selective Inhibitors of HIV-1 Integrase
- Are We There Yet? The Smallpox Research Agenda Using Variola Virus
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
- Dynamic Functional Modulation of CD4 T Cell Recall Responses Is Dependent on the Inflammatory Environment of the Secondary Stimulus
- Bacterial Superantigens Promote Acute Nasopharyngeal Infection by in a Human MHC Class II-Dependent Manner
- Follicular Helper T Cells Promote Liver Pathology in Mice during Infection
- A Nasal Epithelial Receptor for WTA Governs Adhesion to Epithelial Cells and Modulates Nasal Colonization
- Unexpected Role for IL-17 in Protective Immunity against Hypervirulent HN878 Infection
- Human Cytomegalovirus Fcγ Binding Proteins gp34 and gp68 Antagonize Fcγ Receptors I, II and III
- Expansion of Murine Gammaherpesvirus Latently Infected B Cells Requires T Follicular Help
- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Molecular Signatures of Hemagglutinin Stem-Directed Heterosubtypic Human Neutralizing Antibodies against Influenza A Viruses
- The Downregulation of GFI1 by the EZH2-NDY1/KDM2B-JARID2 Axis and by Human Cytomegalovirus (HCMV) Associated Factors Allows the Activation of the HCMV Major IE Promoter and the Transition to Productive Infection
- Inactivation of Fructose-1,6-Bisphosphate Aldolase Prevents Optimal Co-catabolism of Glycolytic and Gluconeogenic Carbon Substrates in
- New Insights into Rotavirus Entry Machinery: Stabilization of Rotavirus Spike Conformation Is Independent of Trypsin Cleavage
- Prophenoloxidase Activation Is Required for Survival to Microbial Infections in
- SslE Elicits Functional Antibodies That Impair Mucinase Activity and Colonization by Both Intestinal and Extraintestinal Strains
- Timed Action of IL-27 Protects from Immunopathology while Preserving Defense in Influenza
- HIV-1 Envelope gp41 Broadly Neutralizing Antibodies: Hurdles for Vaccine Development
- The PhoP-Dependent ncRNA Mcr7 Modulates the TAT Secretion System in
- Cellular Superspreaders: An Epidemiological Perspective on HIV Infection inside the Body
- The Inflammasome Pyrin Contributes to Pertussis Toxin-Induced IL-1β Synthesis, Neutrophil Intravascular Crawling and Autoimmune Encephalomyelitis
- Papillomavirus Genomes Associate with BRD4 to Replicate at Fragile Sites in the Host Genome
- Integrative Functional Genomics of Hepatitis C Virus Infection Identifies Host Dependencies in Complete Viral Replication Cycle
- Co-assembly of Viral Envelope Glycoproteins Regulates Their Polarized Sorting in Neurons
- Targeting Membrane-Bound Viral RNA Synthesis Reveals Potent Inhibition of Diverse Coronaviruses Including the Middle East Respiratory Syndrome Virus
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



