-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced Mucositis
Mucositis, also referred to as mucosal barrier injury, is one of the most debilitating side effects of radiotherapy and chemotherapy treatment. Clinically, mucositis is associated with pain, bacteremia, and malnutrition. Furthermore, mucositis is a frequent reason to postpone chemotherapy treatment, ultimately leading towards a higher mortality in cancer patients. According to the model introduced by Sonis, both inflammation and apoptosis of the mucosal barrier result in its discontinuity, thereby promoting bacterial translocation. According to this five-phase model, the intestinal microbiota plays no role in the pathophysiology of mucositis. However, research has implicated a prominent role for the commensal intestinal microbiota in the development of several inflammatory diseases like inflammatory bowel disease, pouchitis, and radiotherapy-induced diarrhea. Furthermore, chemotherapeutics have a detrimental effect on the intestinal microbial composition (strongly decreasing the numbers of anaerobic bacteria), coinciding in time with the development of chemotherapy-induced mucositis. We hypothesize that the commensal intestinal microbiota might play a pivotal role in chemotherapy-induced mucositis. In this review, we propose and discuss five pathways in the development of mucositis that are potentially influenced by the commensal intestinal microbiota: 1) the inflammatory process and oxidative stress, 2) intestinal permeability, 3) the composition of the mucus layer, 4) the resistance to harmful stimuli and epithelial repair mechanisms, and 5) the activation and release of immune effector molecules. Via these pathways, the commensal intestinal microbiota might influence all phases in the Sonis model of the pathogenesis of mucositis. Further research is needed to show the clinical relevance of restoring dysbiosis, thereby possibly decreasing the degree of intestinal mucositis.
Published in the journal: . PLoS Pathog 6(5): e32767. doi:10.1371/journal.ppat.1000879
Category: Review
doi: https://doi.org/10.1371/journal.ppat.1000879Summary
Mucositis, also referred to as mucosal barrier injury, is one of the most debilitating side effects of radiotherapy and chemotherapy treatment. Clinically, mucositis is associated with pain, bacteremia, and malnutrition. Furthermore, mucositis is a frequent reason to postpone chemotherapy treatment, ultimately leading towards a higher mortality in cancer patients. According to the model introduced by Sonis, both inflammation and apoptosis of the mucosal barrier result in its discontinuity, thereby promoting bacterial translocation. According to this five-phase model, the intestinal microbiota plays no role in the pathophysiology of mucositis. However, research has implicated a prominent role for the commensal intestinal microbiota in the development of several inflammatory diseases like inflammatory bowel disease, pouchitis, and radiotherapy-induced diarrhea. Furthermore, chemotherapeutics have a detrimental effect on the intestinal microbial composition (strongly decreasing the numbers of anaerobic bacteria), coinciding in time with the development of chemotherapy-induced mucositis. We hypothesize that the commensal intestinal microbiota might play a pivotal role in chemotherapy-induced mucositis. In this review, we propose and discuss five pathways in the development of mucositis that are potentially influenced by the commensal intestinal microbiota: 1) the inflammatory process and oxidative stress, 2) intestinal permeability, 3) the composition of the mucus layer, 4) the resistance to harmful stimuli and epithelial repair mechanisms, and 5) the activation and release of immune effector molecules. Via these pathways, the commensal intestinal microbiota might influence all phases in the Sonis model of the pathogenesis of mucositis. Further research is needed to show the clinical relevance of restoring dysbiosis, thereby possibly decreasing the degree of intestinal mucositis.
Introduction
Mucositis, also referred to as mucosal barrier injury, is one of the most debilitating side effects of radiotherapy and chemotherapy treatment [1]. It is characterized by both inflammation and cell loss in the epithelial barrier lining the gastrointestinal tract [2], [3]. Clinically, mucositis is associated with bacteremia, malnutrition, the use of total parenteral nutrition, and an increment in the use of intravenous analgesics. These complications all lead to longer hospitalizations and increasing health care costs. Moreover, mucositis is a frequent reason for reducing the dosages of chemotherapeutics or to postpone chemotherapy treatment, ultimately leading towards a higher mortality in cancer patients [2], [4].
Historically, research has focused on oral mucositis. More recently, attention has been drawn towards the pathophysiology and clinical symptoms of intestinal mucositis, which is characterized by symptoms like nausea, bloating, vomiting, abdominal pain, and severe diarrhea [5], [6].
According to the model introduced by Sonis, five phases are important in the pathophysiology of mucositis: (1) the formation of reactive oxygen species leading to the activation of nuclear factor kappa B (NFκB) during the initiation phase, (2) the induction of messenger molecules such as tumor necrosis factor alpha (TNFα), resulting in treatment-related tissue inflammation and apoptosis during the upregulation/message generation phase, (3) the amplification of messenger molecules in the amplification/signaling phase, leading to more inflammation and apoptosis, (4) discontinuity of the epithelial barrier resulting from apoptosis during the ulcerative phase, thereby promoting bacterial translocation, and (5) a spontaneous healing phase, characterized by cell proliferation [3]. According to this five-phase model, the intestinal microbiota plays no role in the pathophysiology of mucositis. However, research has implicated a role for the commensal intestinal microbiota in several local and systemic inflammatory diseases like inflammatory bowel disease, pouchitis, radiotherapy-induced diarrhea, atopic disease, obesity, and diabetes [7]–[11]. Recent studies have also shown that both chemotherapeutics and (prophylactically used) antibiotics do have an effect on intestinal microbial composition [12]–[14]. Moreover, the effects of the changing commensal intestinal microbiota on the development and severity of mucositis are being unravelled. Research has shown that bacteria play a role in the metabolism of certain chemotherapeutics. The outgrowth of these bacteria might lead to the formation of active toxic metabolites of the chemotherapeutic drug, which directly affects the progression of intestinal mucositis [13]. However, the commensal intestinal microbiota might also have beneficial effects on the development of intestinal mucositis, as the mere presence of resident intestinal bacteria might offer protection against its development. In this review, we propose and discuss five pathways in the development of mucositis that are potentially influenced by the commensal intestinal microbiota: 1) the inflammatory process and oxidative stress, 2) intestinal permeability, 3) the composition of the mucus layer, 4) the resistance towards harmful stimuli and epithelial repair mechanisms, and 5) the activation and release of immune effector molecules (Figures 1 and 2).
Fig. 1. The epithelial barrier is comprised of a single layer of epithelial cells intertwined by tight junctions. 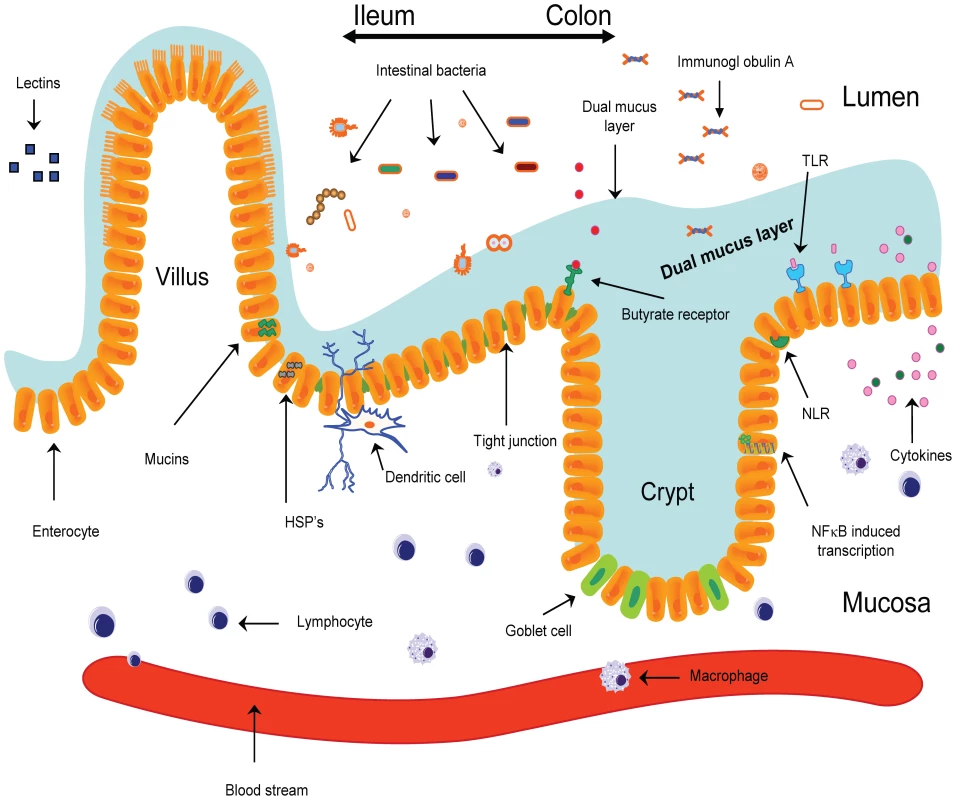
The mechanical barrier is increased further by a mucus layer. Binding of bacteria to TLRs present on epithelial cells results in the activation of NFκB, ultimately resulting in the release of pro-inflammatory and anti-inflammatory cytokines. After phagocytosis, bacterial products are internalized and then are recognized by receptors of the NOD family (NLRs), resulting in the modulation of the inflammatory response. Dendritic cells are capable of internalizing bacteria sampled from the lumen, after which bacteria are presented to immune effector cells. HSPs, heat shock proteins; NLR, NOD-like receptor; sIgA, secretory immunoglobulin A; TLR, Toll-like receptor. Fig. 2. The resident microbiota interferes in the process of mucositis. 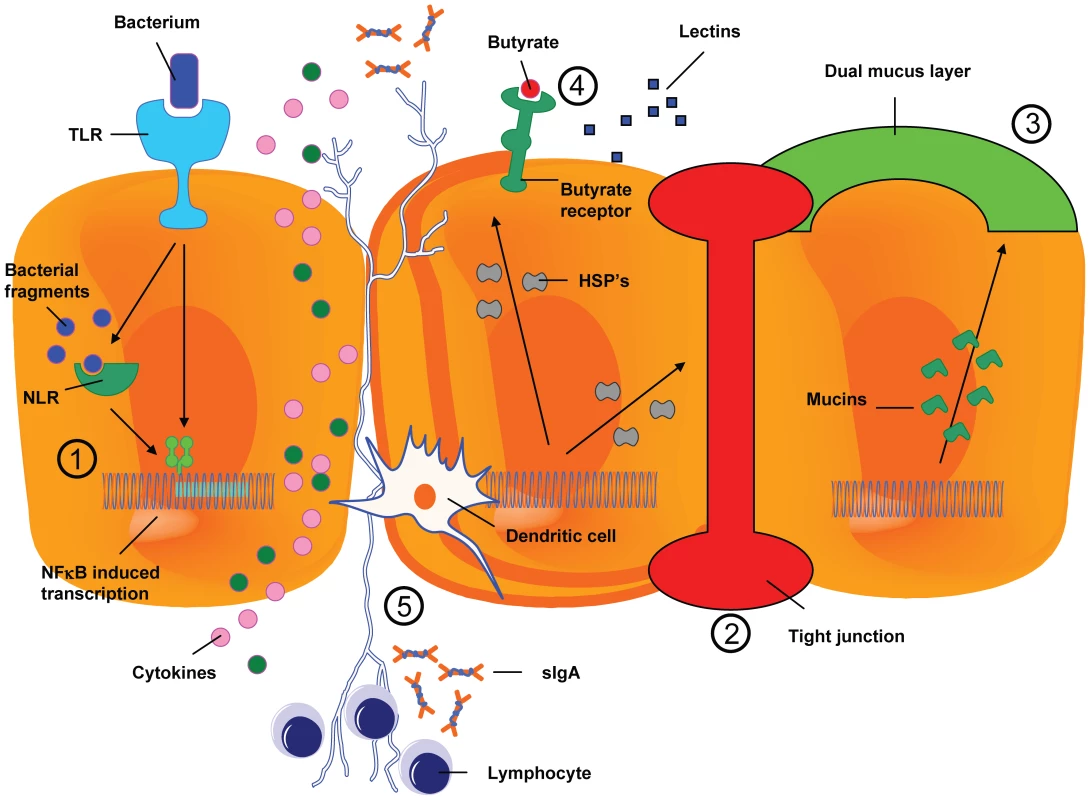
Depicted are five possible ways in which intestinal bacteria can attenuate or aggrevate mucositis: 1) influencing the inflammatory process, 2) influencing intestinal permeability, 3) influencing the composition of the mucus layer, 4) influencing resistance to harmful stimuli and enhancing epithelial repair, and finally, 5) the activation and release of immune effector molecules. Host–Microbe Interaction
A detailed review of the communication pathways between the intestinal microbiota and the human host is beyond the scope of this article and this communication is therefore only shortly reviewed.
The epithelial barrier lining the gastrointestinal tract is composed of a single layer of epithelial cells intertwined by tight junctions [15]. These epithelial cells have two important functions. Firstly, they form a mechanical barrier separating the inside of the human body from the outside world. Secondly, they are essential in the communication between the human body and the intestinal microbiota [16]–[18].
An important aspect of these two functions of the epithelial cells is the dual mucus layer at the apical side of the epithelial cells [19], [20]. The inner layer strengthens the epithelial barrier, whereas the loose outer layer is proposed to be important in the communication between epithelial cells and microbiota [20], [21].
With respect to the communication between microbes and the gut, two groups of receptors are thought to be important in the communication between the human body and the resident microbiota: the Toll-like receptor (TLR) family and the nucleotide oligomerisation domain (NOD) receptor family [22]–[25]. Both groups of receptors play an important role in the genesis and modulation of the inflammatory response. The TLRs are present at the outer membrane of the epithelial cells. Bacteria are recognized by the extracellularly located part of TLRs, leading to activation of NFκB [23], [25]. In turn, activation of NFκB results in the development of an inflammatory response. So far, multiple members of the TLR family have been described in mammals. The most extensively researched receptors are TLR-2, TLR-3, TLR-4, TLR-5, and TLR-9 [16], [23], [26]–[32]. TLR-2 is activated by peptidoglycan, a part of the cell wall of gram-positive bacteria, whereas TLR-4 is activated by lipopolysaccharide (LPS), a substance of gram-negative microorganisms. TLR-3 is activated by viral DNA, TLR-9 is activated by bacterial DNA, and TLR-5 is activated by the protein flagellin, present in flagellated bacteria. After binding to TLRs, bacteria are processed and bacterial parts are transported intracellularly. Here they bind to receptors of the NOD family. It is believed that activation of NOD receptors modulates the inflammatory response activated by TLR binding [22]. This theory is supported by the fact that NOD−/ − mice are profoundly susceptible to intestinal inflammation [33], [34]. Moreover, mutations in NOD2 are associated with the development of Crohn's disease in humans [35]–[37].
Not only epithelial cells, but also local dendritic cells are thought to play a role in the initiation and/or modulation of intestinal inflammation and, in addition, in the induction of tolerance [38]–[40]. Dendritic cells sample bacteria from the intestinal lumen, after which these bacteria are transported to the local lymph nodes. Here, the bacteria are presented to immune cells, whose activation can result in the activation of the innate and adaptive immune system. Why certain microbial stimuli result in tolerance where others induce an inflammatory response is still largely unknown.
Pathways Describing the Role of Commensal Intestinal Microbiota in Mucositis
1) Influencing the Inflammatory Process and Modulating Oxidative Stress
The healthy human intestine is characterized by a state of low-grade inflammation. The resident microbiota guarantees a constant exposure to TLR ligands such as peptidoglycan, LPS, and bacterial DNA. This ensures a continuous basal activation of downstream signaling pathways, resulting in low-grade physiological inflammation [25], [27]. Paradoxically, commensal bacteria are also capable of suppressing more severe inflammatory responses, and their disappearance may even result in incremental inflammation [41]–[46]. For example, Bacteroides thetaiotaomicron and Bifidobacterium infantis both decrease NFκB activation [43], [47], leading to a decrease in endotoxin levels and plasma interleukin (IL)-6 levels [45]. The Clostridium XIVa group has been proposed to attenuate intestinal inflammation by exerting an effect on polyamine secretion, which in turn regulates the expression of TLR-2 [28], [48].
Bacteria or bacterial parts, as well as their secreted products, relieve inflammatory symptoms. For example, Faecalibacterium prausnitzii secretes a substance capable of decreasing NFκB activation. This so far unidentified substance induces the production of the anti-inflammatory IL-10, thereby attenuating inflammation. B. infantis also secretes an unidentified product that attenuates colitis in mice [46], [49]. Several intestinal bacteria produce short chain fatty acids (SCFAs), with butyrate being the most thoroughly investigated. Butyrate is produced by F. prausnitzii and Clostridium XIVa and has been shown to have profound anti-inflammatory effects [50]–[54]. Substitution of butyrate attenuates inflammatory symptoms in (diversion) colitis and chemotherapy-induced mucositis in vivo in mice [46], [55]–[58]. Moreover, butyrate not only attenuates inflammation, but also reduces intestinal permeability and stimulates the activation of immune effector molecules.
In short, multiple intestinal bacteria are capable of decreasing NFκB activation, resulting in a diminished production of inflammatory cytokines. The exact nature and relevance of the relationship between chemotherapy-induced mucositis, inflammation, and intestinal microbiota is subject to ongoing research.
2) Influencing Intestinal Permeability
Intestinal permeability increases after chemotherapy treatment, and has been shown to be one of the hallmarks of the third and fourth phases of mucositis as reported by Sonis [2], [3], [6]. One of the mechanisms resulting in a chemotherapy-induced increase in permeability is probably villous atrophy. Atrophy leads to an increase of intestinal permeability, as has been shown both in vivo and in vitro [59]. However, the resident intestinal microbiota has also been proposed to influence intestinal permeability [26], [60]. Indeed, several commensal bacteria have been shown to improve the epithelial barrier function both in vitro and in vivo, although not all in vivo studies were able to confirm these improvements [59], [61]–[65]. For example, TLR-2 ligands stimulate the phosphorylation of protein kinase C, leading to a decrease in intestinal permeability [26]. This decrease in permeability is proposed to be the result of changes in tight junctions. Administration of bifidobacteria is associated with an enhanced expression of proteins forming tight junctions [49], and has been shown to decrease intestinal permeability [64]. Both bifidobacteria and lactobacilli have been shown to increase tight junction protein expression and restore intestinal permeability [66]–[68].
Another factor contributing to attenuating intestinal permeability is the bacterial induction of heat shock proteins (HSPs). These HSPs are thought to preserve the viability of epithelial cells in stress conditions [69]–[71], thereby reducing intestinal permeability.
Finally, the bacterial production of SCFAs is associated with a reduction in intestinal permeability. This effect of SCFAs is also proposed to be mediated by an increase in epithelial cell viability [52], [58], [72].
Epithelial cell loss is a hallmark of the third phase of the five-phase mucositis model, eventually resulting in an increased permeability. The commensal intestinal microbiota attenuates cellular atrophy and increases tight junction strength. Therefore, we propose that changes in the commensal intestinal microbiota influence the third phase of mucositis. This way, the commensal intestinal microbiota might influence the eventual severity of mucositis encountered in the ulcerative phase.
3) Influencing the Composition of the Mucus Layer
As mentioned before, the mucus layer covering the intestinal epithelium strengthens the mechanical epithelial barrier. The protective mucus layer is comprised of glycoproteins, trefoil factors, and mucins. These mucins are produced by goblet cells, which are specialized epithelial cells [73]. The composition of the mucus layer is important in the protection against bacterial infections and inflammation. For example, it has been shown that mucin type 2 knockout mice develop severe colitis after harmful stimuli, in contrast to mice capable of producing mucin 2. Furthermore, in animals lacking mucin 2, bacteria are detected deep down in the normally sterile crypts of the intestine [20], [74].
The commensal intestinal microbiota is proposed to play a role in the maintenance of the mucus layer. Indeed, the absence of these intestinal microbiota is associated with a decrease in goblet cells, which are also smaller in size [75]. Furthermore, the thickness of the mucus layer is decreased in animals devoid of intestinal microbiota.
The genes encoding mucins are directly regulated by bacteria and their products [76]–[78], and in response to intestinal microbes and/or their secreted products the secretion of mucus increases [76], [79]. For example, both Lactobacillus rhamnosus Gorbach and Goldin (GG) and Lactobacillus plantarum increase the expression of MUC-2 and MUC-3 genes, and Lactobacillus acidophilus upregulates MUC-2 gene expression [77], [80]. Furthermore, bacteria producing butyrate are thought to play a role in the composition of the mucus layer, as butyrate is capable of increasing mucin synthesis as well [52].
The commensal resident microbiota not only interferes with the expression of MUC genes, but also interferes with the expression and/or activity of cell glycosyltransferases. These enzymes induce changes in the carbohydrate repertoire of mucins, which might change their efficacy in bacterial defense [81], [82].
Thus, the intestinal microbiota influences the composition of the mucus layer covering the epithelium, thereby increasing the strength of the epithelial barrier. A strengthened barrier decreases the risk of bacterial translocation, thereby possibly attenuating inflammation present in the ulcerative phase of the Sonis mucositis model.
4) Influencing Resistance to Harmful Stimuli and Influencing Epithelial Repair
The commensal intestinal microbiota contributes to epithelial repair. In germ-free animals, the mitotic index and cell turnover of epithelial cells are lower as compared to normally colonized animals [83], [84]. Moreover, the transit time of epithelial cells migrating towards the top of the intestinal villi is prolonged [85]. These changes result in a retarded renewal, i.e., a retarded repair, of the intestinal epithelium.
Bacterial induction of NFκB not only controls the physiological state of low-grade inflammation in the intestine, it also stimulates the repair of, for example, mechanical-induced epithelial damage [86]. The importance of bacterial ligands in this process is shown in TLR-4−/ − epithelial cells. These cells, which are not capable of recognizing the resident microbiota, exhibit severe repair defects in response to harmful chemical stimuli. This is probably due to a reduced capacity of NFκB-induced cytoprotective factors such as HSPs and IL-6 [25], [29]. When TLR ligands were administered to germ-free mice, this was sufficient to protect them against artificially induced colitis [25].
Bacteria acting as TLR ligands are not the only ones that play an important role in increasing the resistance towards harmful stimuli and enhancing epithelial repair. Again, butyrate plays an important role. Butyrate stimulates the migration of epithelial cells, thereby enhancing mucosal healing [52], [72]. Other bacterial products, such as the peptides secreted by L. rhamnosus GG, have been shown to inhibit cytokine-induced apoptosis and promote cell growth, thereby also enhancing mucosal repair [87].
Therefore, we again propose that the commensal intestinal microbiota might attenuate the epithelial damage in the third phase of mucositis. As the commensal intestinal microbiota stimulates epithelial repair mechanisms, it can be hypothesized that the microbiota also attenuates mucositis by influencing the healing phase of mucositis.
5) Influencing the Production and Release of Immune Effector Molecules
The commensal intestinal microbiota regulates the expression and release of immune effector molecules. These molecules are pivotal for maintaining intestinal homeostasis [27], [88]–[90]. For example, if the contact between microbiota and intestinal epithelium suddenly increases, the expression of RegIIIγ increases. This C-type lectin has antimicrobial activity and limits bacterial translocation. Furthermore, it maintains intestinal integrity and homeostasis [89], [90].
Another immune effector molecule influenced by the resident microbiota is immunoglobulin A (IgA). IgA is produced by mucosa-associated immune effector cells [81], [90], [91]. Intestinal microbiota is capable of regulating the expression of IgA, which in turn regulates the composition of the intestinal microbiota. For example, suppletion of bifidobacteria is associated with an increase in the expression of secretory IgA [92].
Both live bacteria and their products are capable of upregulating immune effector molecules. For example, SCFAs such as butyrate regulate the production of cathelicidins, which exhibit broad-spectrum anti-bacterial activity against potential pathogens [93].
By influencing the expression and release of immune effector molecules, the commensal intestinal microbiota regulates itself and maintains homeostasis in the intestinal tract. In the end, this will positively influence all five phases described in Sonis's mucositis model.
Conclusion; an Extended Five-Phase Model for Mucositis
Although the protective role of commensal intestinal bacteria in human disease is increasingly being appreciated, research concerning the relationship between intestinal bacteria and chemotherapy-induced mucositis is still scarce. Most studies that investigate the role of bacteria in human disease have focused on inflammatory bowel disease, which is caused by a chronic inflammatory process instead of the acute damage induced by chemotherapeutics.
In the model introduced by Sonis to explain the pathogenesis of radiotherapy-induced and chemotherapy-induced mucositis, the resident intestinal microbiota played no role [3]. However, recently it has been shown that chemotherapy treatment is associated with a decrease in the number of anaerobic bacteria and a decrease in microbial diversity [14], [94]. Furthermore, the resident intestinal bacteria have been shown to play a role in radiotherapy-induced diarrhea [10]. Moreover, research has shown that a decreasing microbial diversity coincides in time with the development of severe chemotherapy-induced mucositis (M. van Vliet et al., unpublished data). We hypothesize that the commensal intestinal microbiota might play a pivotal role in both radiotherapy-induced and chemotherapy-induced mucositis when the intestine is irradiated or when chemotherapeutics are used that deregulate intestinal microbial homeostasis, as the disappearance of the intestinal microbiota will minimize their protection of enterocytes against harmful stimuli. Further research is needed to show whether the commensal intestinal bacteria should be incorporated as a meaningful factor in Sonis's five-phase model for mucositis. Theoretically, the commensal intestinal microbiota could influence all phases of the pathogenesis of mucositis: the initiation phase, the phase of upregulation and message generation, the phase of amplification and signalling, the ulcerative phase, and the healing phase.
Further research will also have to show the clinical relevance of restoring dysbiosis, thereby possibly decreasing the degree of intestinal mucositis. This would not only increase the quality of life of patients, but could also positively influence treatment intensity, probably decreasing the morbidity and mortality of cancer patients. Completely restoring dysbiosis might be a clinical problem, since whole live bacteria used as probiotics have already been described as causing invasive infections in immunocompromised patients and were associated with increased mortality in patients with severe pancreatitis [95]–[98]. However, it has been shown that substitution of bacterial parts instead of whole live bacteria might be sufficient to attenuate local and systemic inflammation without the risk of invasive infections [30], [99], [100].
Zdroje
1. BellmLA
EpsteinJB
Rose-PedA
MartinP
FuchsHJ
2000 Patient reports of complications of bone marrow transplantation. Support Care Cancer 8 33 39
2. BlijlevensNM
DonnellyJP
De PauwBE
2000 Mucosal barrier injury: biology, pathology, clinical counterparts and consequences of intensive treatment for haematological malignancy: an overview. Bone Marrow Transplant 25 1269 1278
3. SonisST
2004 The pathobiology of mucositis. Nat Rev Cancer 4 277 284
4. SonisST
OsterG
FuchsH
BellmL
BradfordWZ
2001 Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol 19 2201 2205
5. BlijlevensNM
DonnellyJP
DePauwBE
2005 Inflammatory response to mucosal barrier injury after myeloablative therapy in allogeneic stem cell transplant recipients. Bone Marrow Transplant 36 703 707
6. LutgensLC
BlijlevensNM
DeutzNE
DonnellyJP
LambinP
2005 Monitoring myeloablative therapy-induced small bowel toxicity by serum citrulline concentration: a comparison with sugar permeability tests. Cancer 103 191 199
7. CaniPD
BibiloniR
KnaufC
WagetA
NeyrinckAM
2008 Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57 1470 1481
8. FrankDN
St AmandAL
FeldmanRA
BoedekerEC
HarpazN
2007 Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 104 13780 13785
9. GosselinkMP
SchoutenWR
van LieshoutLM
HopWC
LamanJD
2004 Eradication of pathogenic bacteria and restoration of normal pouch flora: comparison of metronidazole and ciprofloxacin in the treatment of pouchitis. Dis Colon Rectum 47 1519 1525
10. ManichanhC
VarelaE
MartinezC
AntolinM
LlopisM
2008 The gut microbiota predispose to the pathophysiology of acute postradiotherapy diarrhea. Am J Gastroenterol 103 1754 1761
11. TurnbaughPJ
LeyRE
MahowaldMA
MagriniV
MardisER
2006 An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444 1027 1031
12. EdlundC
NordCE
2000 Effect on the human normal microflora of oral antibiotics for treatment of urinary tract infections. J Antimicrob Chemother 46 Suppl 1 41 48
13. StringerAM
GibsonRJ
BowenJM
LoganRM
AshtonK
2009 Irinotecan-induced mucositis manifesting as diarrhoea corresponds with an amended intestinal flora and mucin profile. Int J Exp Pathol 90 489 499
14. van VlietMJ
TissingWJ
DunCA
MeessenNE
KampsWA
2009 Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin Infect Dis 49 262 270
15. PowellDW
1981 Barrier function of epithelia. Am J Physiol 241 G275 G288
16. CarioE
2005 Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2. Gut 54 1182 1193
17. MedzhitovR
2007 Recognition of microorganisms and activation of the immune response. Nature 449 819 826
18. SartorRB
2008 Microbial influences in inflammatory bowel diseases. Gastroenterology 134 577 594
19. AtumaC
StrugalaV
AllenA
HolmL
2001 The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol 280 G922 G929
20. JohanssonME
PhillipsonM
PeterssonJ
VelcichA
HolmL
2008 The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 105 15064 15069
21. SwidsinskiA
Loening-BauckeV
TheissigF
EngelhardtH
BengmarkS
2007 Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut 56 343 350
22. ConstansA
2005 Giving a nod2 the right target. The scientist 19 24 25
23. DoyleSL
O'NeillLA
2006 Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol 72 1102 1113
24. FranchiL
WarnerN
VianiK
NunezG
2009 Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev 227 106 128
25. Rakoff-NahoumS
PaglinoJ
Eslami-VarzanehF
EdbergS
MedzhitovR
2004 Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118 229 241
26. CarioE
GerkenG
PodolskyDK
2004 Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 127 224 238
27. CarioE
2008 Therapeutic impact of toll-like receptors on inflammatory bowel diseases: a multiple-edged sword. Inflamm Bowel Dis 14 411 421
28. ChenJ
RaoJN
ZouT
LiuL
MarasaBS
2007 Polyamines are required for expression of Toll-like receptor 2 modulating intestinal epithelial barrier integrity. Am J Physiol Gastrointest Liver Physiol 293 G568 G576
29. FukataM
MichelsenKS
EriR
ThomasLS
HuB
2005 Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol 288 G1055 G1065
30. RachmilewitzD
KatakuraK
KarmeliF
HayashiT
ReinusC
2004 Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 126 520 528
31. Vicente-SuarezI
TakahashiY
ChengF
HornaP
WangHW
2007 Identification of a novel negative role of flagellin in regulating IL-10 production. Eur J Immunol 37 3164 3175
32. Vijay-KumarM
WuH
AitkenJ
KolachalaVL
NeishAS
2007 Activation of toll-like receptor 3 protects against DSS-induced acute colitis. Inflamm Bowel Dis 13 856 864
33. KobayashiKS
ChamaillardM
OguraY
HenegariuO
InoharaN
2005 Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307 731 734
34. WatanabeT
KitaniA
MurrayPJ
WakatsukiY
FussIJ
2006 Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity 25 473 485
35. HampeJ
CuthbertA
CroucherPJ
MirzaMM
MascherettiS
2001 Association between insertion mutation in NOD2 gene and Crohn's disease in German and British populations. Lancet 357 1925 1928
36. HugotJP
ChamaillardM
ZoualiH
LesageS
CezardJP
2001 Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411 599 603
37. OguraY
BonenDK
InoharaN
NicolaeDL
ChenFF
2001 A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411 603 606
38. BjorckP
BeilhackA
HermanEI
NegrinRS
EnglemanEG
2008 Plasmacytoid dendritic cells take up opsonized antigen leading to CD4+ and CD8+ T cell activation in vivo. J Immunol 181 3811 3817
39. HapfelmeierS
MullerAJ
StecherB
KaiserP
BarthelM
2008 Microbe sampling by mucosal dendritic cells is a discrete, MyD88-independent step in DeltainvG S. Typhimurium colitis. J Exp Med 205 437 450
40. NiessJH
ReineckerHC
2006 Dendritic cells in the recognition of intestinal microbiota. Cell Microbiol 8 558 564
41. BorodyTJ
WarrenEF
LeisS
SuraceR
AshmanO
2003 Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol 37 42 47
42. FrickJS
SchenkK
QuitadamoM
KahlF
KoberleM
2007 Lactobacillus fermentum attenuates the proinflammatory effect of Yersinia enterocolitica on human epithelial cells. Inflamm Bowel Dis 13 83 90
43. KellyD
CampbellJI
KingTP
GrantG
JanssonEA
2004 Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol 5 104 112
44. KhanMA
MaC
KnodlerLA
ValdezY
RosenbergerCM
2006 Toll-like receptor 4 contributes to colitis development but not to host defense during Citrobacter rodentium infection in mice. Infect Immun 74 2522 2536
45. O'HaraAM
O'ReganP
FanningA
O'MahonyC
MacsharryJ
2006 Functional modulation of human intestinal epithelial cell responses by Bifidobacterium infantis and Lactobacillus salivarius. Immunology 118 202 215
46. SokolH
PigneurB
WatterlotL
LakhdariO
Bermudez-HumaranLG
2008 Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 105 16731 16736
47. BegAA
2004 ComPPARtmentalizing NF-kappaB in the gut. Nat Immunol 5 14 16
48. MatsumotoM
BennoY
2007 The relationship between microbiota and polyamine concentration in the human intestine: a pilot study. Microbiol Immunol 51 25 35
49. EwaschukJB
DiazH
MeddingsL
DiederichsB
DmytrashA
2008 Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol 295 G1025 G1034
50. BarcenillaA
PrydeSE
MartinJC
DuncanSH
StewartCS
2000 Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol 66 1654 1661
51. DuncanSH
HoldGL
HarmsenHJ
StewartCS
FlintHJ
2002 Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int J Syst Evol Microbiol 52 2141 2146
52. HamerHM
JonkersD
VenemaK
VanhoutvinS
TroostFJ
2008 Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 27 104 119
53. HamerHM
JonkersDM
BastA
VanhoutvinSA
FischerMA
2008 Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr
54. NanceyS
BienvenuJ
CoffinB
AndreF
DescosL
2002 Butyrate strongly inhibits in vitro stimulated release of cytokines in blood. Dig Dis Sci 47 921 928
55. Di SabatinoA
MoreraR
CiccocioppoR
CazzolaP
GottiS
2005 Oral butyrate for mildly to moderately active Crohn's disease. Aliment Pharmacol Ther 22 789 794
56. HarigJM
SoergelKH
KomorowskiRA
WoodCM
1989 Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med 320 23 28
57. RamosMG
BambirraEA
CaraDC
VieiraEC
Alvarez-LeiteJI
1997 Oral administration of short-chain fatty acids reduces the intestinal mucositis caused by treatment with Ara-C in mice fed commercial or elemental diets. Nutr Cancer 28 212 217
58. VenkatramanA
RamakrishnaBS
ShajiRV
KumarNS
PulimoodA
2003 Amelioration of dextran sulfate colitis by butyrate: role of heat shock protein 70 and NF-kappaB. Am J Physiol Gastrointest Liver Physiol 285 G177 G184
59. WangQ
WangXD
JeppssonB
AnderssonR
KarlssonB
1996 Influence of colostomy on in vivo and in vitro permeability of the rat colon. Dis Colon Rectum 39 663 670
60. SamonteVA
GotoM
RavindranathTM
FazalN
HollowayVM
2004 Exacerbation of intestinal permeability in rats after a two-hit injury: burn and Enterococcus faecalis infection. Crit Care Med 32 2267 2273
61. EutameneH
LamineF
ChaboC
TheodorouV
RochatF
2007 Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J Nutr 137 1901 1907
62. HeymanM
TerpendK
MenardS
2005 Effects of specific lactic acid bacteria on the intestinal permeability to macromolecules and the inflammatory condition. Acta Paediatr Suppl 94 34 36
63. QinHL
ZhengJJ
TongDN
ChenWX
FanXB
2008 Effect of Lactobacillus plantarum enteral feeding on the gut permeability and septic complications in the patients with acute pancreatitis. Eur J Clin Nutr 62 923 930
64. StratikiZ
CostalosC
SevastiadouS
KastanidouO
SkouroliakouM
2007 The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum Dev 83 575 579
65. ZengJ
LiYQ
ZuoXL
ZhenYB
YangJ
2008 Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 28 994 1002
66. LiuQ
NobaekS
AdawiD
MaoY
WangM
2001 Administration of Lactobacillus plantarum 299v reduces side-effects of external radiation on colon anastomotic healing in an experimental model. Colorectal Dis 3 245 252
67. QinHL
ShenTY
GaoZG
FanXB
HangXM
2005 Effect of lactobacillus on the gut microflora and barrier function of the rats with abdominal infection. World J Gastroenterol 11 2591 2596
68. MoorthyG
MuraliMR
DevarajSN
2008 Lactobacilli facilitate maintenance of intestinal membrane integrity during Shigella dysenteriae 1 infection in rats. Nutrition
69. AijazS
Sanchez-HerasE
BaldaMS
MatterK
2007 Regulation of tight junction assembly and epithelial morphogenesis by the heat shock protein Apg-2. BMC Cell Biol 8 49
70. ArvansDL
VavrickaSR
RenH
MuschMW
KangL
2005 Luminal bacterial flora determines physiological expression of intestinal epithelial cytoprotective heat shock proteins 25 and 72. Am J Physiol Gastrointest Liver Physiol 288 G696 G704
71. MatsuoK
ZhangX
OnoY
NagatomiR
2009 Acute stress-induced colonic tissue HSP70 expression requires commensal bacterial components and intrinsic glucocorticoid. Brain Behav Immun 23 108 115
72. VenkatramanA
RamakrishnaBS
PulimoodAB
1999 Butyrate hastens restoration of barrier function after thermal and detergent injury to rat distal colon in vitro. Scand J Gastroenterol 34 1087 1092
73. MoncadaDM
KammanadimintiSJ
ChadeeK
2003 Mucin and Toll-like receptors in host defense against intestinal parasites. Trends Parasitol 19 305 311
74. Van der SluisM
De KoningBA
De BruijnAC
VelcichA
MeijerinkJP
2006 Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131 117 129
75. KandoriH
HirayamaK
TakedaM
DoiK
1996 Histochemical, lectin-histochemical and morphometrical characteristics of intestinal goblet cells of germfree and conventional mice. Exp Anim 45 155 160
76. Caballero-FrancoC
KellerK
De SimoneC
ChadeeK
2007 The VSL#3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol 292 G315 G322
77. KimY
KimSH
WhangKY
KimYJ
OhS
2008 Inhibition of Escherichia coli O157:H7 attachment by interactions between lactic acid bacteria and intestinal epithelial cells. J Microbiol Biotechnol 18 1278 1285
78. MattarAF
TeitelbaumDH
DrongowskiRA
YongyiF
HarmonCM
2002 Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model. Pediatr Surg Int 18 586 590
79. BarceloA
ClaustreJ
MoroF
ChayvialleJA
CuberJC
2000 Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut 46 218 224
80. MackDR
AhrneS
HydeL
WeiS
HollingsworthMA
2003 Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 52 827 833
81. BourliouxP
KoletzkoB
GuarnerF
BraescoV
2003 The intestine and its microflora are partners for the protection of the host: report on the Danone Symposium “The Intelligent Intestine,” held in Paris, June 14, 2002. Am J Clin Nutr 78 675 683
82. HooperLV
GordonJI
2001 Commensal host-bacterial relationships in the gut. Science 292 1115 1118
83. RollsBA
TurveyA
CoatesME
1978 The influence of the gut microflora and of dietary fibre on epithelial cell migration in the chick intestine. Br J Nutr 39 91 98
84. WebbP
ChananaAD
CronkiteEP
LaissueJA
JoelDD
1980 Comparison of DNA renewal in germ-free and conventional mice using [125I]iododeoxyuridine and [3H]thymidine. Cell Tissue Kinet 13 227 237
85. SavageDC
SiegelJE
SnellenJE
WhittDD
1981 Transit time of epithelial cells in the small intestines of germfree mice and ex-germfree mice associated with indigenous microorganisms. Appl Environ Microbiol 42 996 1001
86. KarraschT
SteinbrecherKA
AllardB
BaldwinAS
JobinC
2006 Wound-induced p38MAPK-dependent histone H3 phosphorylation correlates with increased COX-2 expression in enterocytes. J Cell Physiol 207 809 815
87. YanF
CaoH
CoverTL
WhiteheadR
WashingtonMK
2007 Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132 562 575
88. AyabeT
SatchellDP
WilsonCL
ParksWC
SelstedME
2000 Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 1 113 118
89. CashHL
WhithamCV
BehrendtCL
HooperLV
2006 Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313 1126 1130
90. StroberW
2006 Immunology. Unraveling gut inflammation. Science 313 1052 1054
91. Di GiacintoC
MarinaroM
SanchezM
StroberW
BoirivantM
2005 Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol 174 3237 3246
92. WangZ
XiaoG
YaoY
GuoS
LuK
2006 The role of bifidobacteria in gut barrier function after thermal injury in rats. J Trauma 61 650 657
93. MullerCA
AutenriethIB
PeschelA
2005 Innate defenses of the intestinal epithelial barrier. Cell Mol Life Sci 62 1297 1307
94. StringerAM
GibsonRJ
LoganRM
BowenJM
YeohAS
2009 Gastrointestinal microflora and mucins may play a critical role in the development of 5-Fluorouracil-induced gastrointestinal mucositis. Exp Biol Med (Maywood) 234 430 441
95. BesselinkMG
van SantvoortHC
BuskensE
BoermeesterMA
van GoorH
2008 Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 371 651 659
96. CannonJP
LeeTA
BolanosJT
DanzigerLH
2005 Pathogenic relevance of Lactobacillus: a retrospective review of over 200 cases. Eur J Clin Microbiol Infect Dis 24 31 40
97. LedouxD
LabombardiVJ
KarterD
2006 Lactobacillus acidophilus bacteraemia after use of a probiotic in a patient with AIDS and Hodgkin's disease. Int J STD AIDS 17 280 282
98. LiongMT
2008 Safety of probiotics: translocation and infection. Nutr Rev 66 192 202
99. KatakuraK
LeeJ
RachmilewitzD
LiG
EckmannL
2005 Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest 115 695 702
100. RachmilewitzD
KarmeliF
TakabayashiK
HayashiT
Leider-TrejoL
2002 Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology 122 1428 1441
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Mouse Senile Amyloid Fibrils Deposited in Skeletal Muscle Exhibit Amyloidosis-Enhancing ActivityČlánek Crystal Structure of HIV-1 gp41 Including Both Fusion Peptide and Membrane Proximal External RegionsČlánek Demonstration of Cross-Protective Vaccine Immunity against an Emerging Pathogenic Ebolavirus Species
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 5- Jak souvisí postcovidový syndrom s poškozením mozku?
- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Quorum Sensing Inhibition Selects for Virulence and Cooperation in
- The HMW1C Protein Is a Glycosyltransferase That Transfers Hexose Residues to Asparagine Sites in the HMW1 Adhesin
- Analysis of Virion Structural Components Reveals Vestiges of the Ancestral Ichnovirus Genome
- Mouse Senile Amyloid Fibrils Deposited in Skeletal Muscle Exhibit Amyloidosis-Enhancing Activity
- Global Migration Dynamics Underlie Evolution and Persistence of Human Influenza A (H3N2)
- The Type III Effectors NleE and NleB from Enteropathogenic and OspZ from Block Nuclear Translocation of NF-κB p65
- VEGF Promotes Malaria-Associated Acute Lung Injury in Mice
- Identification of a Mutant PfCRT-Mediated Chloroquine Tolerance Phenotype in
- The Early Stage of Bacterial Genome-Reductive Evolution in the Host
- Host-Detrimental Role of Esx-1-Mediated Inflammasome Activation in Mycobacterial Infection
- Elevation of Intact and Proteolytic Fragments of Acute Phase Proteins Constitutes the Earliest Systemic Antiviral Response in HIV-1 Infection
- The Pleiotropic CymR Regulator of Plays an Important Role in Virulence and Stress Response
- Alternative Sigma Factor σ Modulates Prophage Integration and Excision in
- Effect of Neuraminidase Inhibitor–Resistant Mutations on Pathogenicity of Clade 2.2 A/Turkey/15/06 (H5N1) Influenza Virus in Ferrets
- Massive APOBEC3 Editing of Hepatitis B Viral DNA in Cirrhosis
- NK Cells and γδ T Cells Mediate Resistance to Polyomavirus–Induced Tumors
- Is Genetically Diverse in Animals and Appears to Have Crossed the Host Barrier to Humans on (At Least) Two Occasions
- Adenylate Cyclase Toxin Mobilizes Its β Integrin Receptor into Lipid Rafts to Accomplish Translocation across Target Cell Membrane in Two Steps
- The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced Mucositis
- HIV-1 Transmitting Couples Have Similar Viral Load Set-Points in Rakai, Uganda
- Few and Far Between: How HIV May Be Evading Antibody Avidity
- Galectin-9/TIM-3 Interaction Regulates Virus-Specific Primary and Memory CD8 T Cell Response
- Perforin Expression Directly by HIV-Specific CD8 T-Cells Is a Correlate of HIV Elite Control
- The Set3/Hos2 Histone Deacetylase Complex Attenuates cAMP/PKA Signaling to Regulate Morphogenesis and Virulence of
- Infidelity of SARS-CoV Nsp14-Exonuclease Mutant Virus Replication Is Revealed by Complete Genome Sequencing
- Combining ChIP-chip and Expression Profiling to Model the MoCRZ1 Mediated Circuit for Ca/Calcineurin Signaling in the Rice Blast Fungus
- Internalin B Activates Junctional Endocytosis to Accelerate Intestinal Invasion
- A Complex Small RNA Repertoire Is Generated by a Plant/Fungal-Like Machinery and Effected by a Metazoan-Like Argonaute in the Single-Cell Human Parasite
- Opc Invasin Binds to the Sulphated Tyrosines of Activated Vitronectin to Attach to and Invade Human Brain Endothelial Cells
- Muc2 Protects against Lethal Infectious Colitis by Disassociating Pathogenic and Commensal Bacteria from the Colonic Mucosa
- PdeH, a High-Affinity cAMP Phosphodiesterase, Is a Key Regulator of Asexual and Pathogenic Differentiation in
- Isolates with Antimony-Resistant but Not -Sensitive Phenotype Inhibit Sodium Antimony Gluconate-Induced Dendritic Cell Activation
- The Microbiota and Allergies/Asthma
- Environmental Factors Determining the Epidemiology and Population Genetic Structure of the Group in the Field
- Prolonged Antigen Presentation Is Required for Optimal CD8+ T Cell Responses against Malaria Liver Stage Parasites
- Crystal Structure of HIV-1 gp41 Including Both Fusion Peptide and Membrane Proximal External Regions
- Susceptibility to Anthrax Lethal Toxin-Induced Rat Death Is Controlled by a Single Chromosome 10 Locus That Includes
- Demonstration of Cross-Protective Vaccine Immunity against an Emerging Pathogenic Ebolavirus Species
- Effective, Broad Spectrum Control of Virulent Bacterial Infections Using Cationic DNA Liposome Complexes Combined with Bacterial Antigens
- High Multiplicity Infection by HIV-1 in Men Who Have Sex with Men
- The -Specific Human Memory B Cell Compartment Expands Gradually with Repeated Malaria Infections
- EBV Promotes Human CD8 NKT Cell Development
- Persistent Growth of a Human Plasma-Derived Hepatitis C Virus Genotype 1b Isolate in Cell Culture
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Quorum Sensing Inhibition Selects for Virulence and Cooperation in
- The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced Mucositis
- Crystal Structure of HIV-1 gp41 Including Both Fusion Peptide and Membrane Proximal External Regions
- Susceptibility to Anthrax Lethal Toxin-Induced Rat Death Is Controlled by a Single Chromosome 10 Locus That Includes
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



