-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSusceptibility to Anthrax Lethal Toxin-Induced Rat Death Is Controlled by a Single Chromosome 10 Locus That Includes
Anthrax lethal toxin (LT) is a bipartite protease-containing toxin and a key virulence determinant of Bacillus anthracis. In mice, LT causes the rapid lysis of macrophages isolated from certain inbred strains, but the correlation between murine macrophage sensitivity and mouse strain susceptibility to toxin challenge is poor. In rats, LT induces a rapid death in as little as 37 minutes through unknown mechanisms. We used a recombinant inbred (RI) rat panel of 19 strains generated from LT-sensitive and LT-resistant progenitors to map LT sensitivity in rats to a locus on chromosome 10 that includes the inflammasome NOD-like receptor (NLR) sensor, Nlrp1. This gene is the closest rat homolog of mouse Nlrp1b, which was previously shown to control murine macrophage sensitivity to LT. An absolute correlation between in vitro macrophage sensitivity to LT-induced lysis and animal susceptibility to the toxin was found for the 19 RI strains and 12 additional rat strains. Sequencing Nlrp1 from these strains identified five polymorphic alleles. Polymorphisms within the N-terminal 100 amino acids of the Nlrp1 protein were perfectly correlated with LT sensitivity. These data suggest that toxin-mediated lethality in rats as well as macrophage sensitivity in this animal model are controlled by a single locus on chromosome 10 that is likely to be the inflammasome NLR sensor, Nlrp1.
Published in the journal: . PLoS Pathog 6(5): e32767. doi:10.1371/journal.ppat.1000906
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000906Summary
Anthrax lethal toxin (LT) is a bipartite protease-containing toxin and a key virulence determinant of Bacillus anthracis. In mice, LT causes the rapid lysis of macrophages isolated from certain inbred strains, but the correlation between murine macrophage sensitivity and mouse strain susceptibility to toxin challenge is poor. In rats, LT induces a rapid death in as little as 37 minutes through unknown mechanisms. We used a recombinant inbred (RI) rat panel of 19 strains generated from LT-sensitive and LT-resistant progenitors to map LT sensitivity in rats to a locus on chromosome 10 that includes the inflammasome NOD-like receptor (NLR) sensor, Nlrp1. This gene is the closest rat homolog of mouse Nlrp1b, which was previously shown to control murine macrophage sensitivity to LT. An absolute correlation between in vitro macrophage sensitivity to LT-induced lysis and animal susceptibility to the toxin was found for the 19 RI strains and 12 additional rat strains. Sequencing Nlrp1 from these strains identified five polymorphic alleles. Polymorphisms within the N-terminal 100 amino acids of the Nlrp1 protein were perfectly correlated with LT sensitivity. These data suggest that toxin-mediated lethality in rats as well as macrophage sensitivity in this animal model are controlled by a single locus on chromosome 10 that is likely to be the inflammasome NLR sensor, Nlrp1.
Introduction
Anthrax lethal toxin (LT), a major virulence factor of Bacillus anthracis, is composed of two proteins, lethal factor (LF) and protective antigen (PA). PA binds to cellular receptors and facilitates LF entry into the cytosol (for review see [1]). LF is a protease which cleaves and inactivates members of the mitogen-activated protein kinase kinase (MAPKK or MEK) family, resulting in proliferation arrest in most cell types and a unique, rapid (<90 min), caspase-1 dependent lysis of mouse macrophages from certain inbred strains through poorly characterized mechanisms (for review see [2]).
In mouse macrophages, sensitivity to LT-mediated lysis is a dominant trait that maps to the highly polymorphic Nlrp1b (Nalp1b) gene on chromosome 11 [3]. Mouse Nlrp1b (mNlrp1b) has five alleles that correlate with LT sensitivity or resistance in macrophages, and it is one of three tandem Nlrp1 paralogs on chromosome 11 [3]. mNlrp1b, the paralog controlling LT sensitivity, is a NOD-like receptor (NLR) which, when activated, leads to assembly of the inflammasome, a multiprotein complex responsible for the activation of caspase-1 [4]. The mNlrp1b inflammasome-mediated activation of caspase-1 is necessary for murine macrophage cell death in response to LT [3], [5]–[7]. Furthermore, expression of mNlrp1b from LT-sensitive macrophages together with caspase-1 is sufficient to render other cell types sensitive to the effects of LT [8].
Unlike human Nlrp1 (hNlrp1), mNlrp1b (despite the acronym representing NLR family, pyrin domain containing 1b) lacks an N-terminal pyrin domain. The pyrin domain is required for hNlrp1 binding to the inflammasome adaptor protein ASC, and mNlrp1b is not believed to interact with this adaptor [9]. However, mNlrp1b does have the NACHT (nucleotide oligomerization), LRR (leucine-rich repeat), and CARD (caspase recruitment) domains commonly found in NLR proteins (for recent reviews see: [10], [11]). It is unclear how polymorphisms in the mNlrp1b protein result in such striking variation in the ability of LT to activate caspase-1 (and subsequently induce cell death) in murine macrophages.
While the mNlrp1b inflammasome requirement for murine macrophage death in response to LT is well established, it is unclear if this inflammasome is involved in LT-mediated death of animals. LT injection into rodents induces an atypical vascular collapse, replicating the shock state associated with anthrax disease [12]–[14]. Susceptibility in mice, however, is controlled by multiple loci [15], and macrophage sensitivity does not control animal susceptibility to LT [16]. Furthermore, factors such as adrenal function can also modulate LT toxicity in mice [17]. Thus, the molecular basis for the death induced by LT in mice is currently unknown.
The rapid LT-mediated death of the Fischer rat [18] can occur in as little as 37 minutes through a unique vascular shock [19], [20]. In rats, left ventricular failure accompanied by a rapid accumulation of pleural fluid (a hallmark of anthrax disease) is typically associated with LT-mediated death [12], [21], [22]. In contrast, LT-induced murine death occurs by vascular collapse over a longer period of days [13], [15]. Early targeting of cardiac function by LT has also recently been demonstrated in mice [23]. The role of MEK cleavage and/or inflammasome components in vascular collapse induced by LT in rodents is also currently unknown. Thus, studies of determinant molecular pathways would be greatly assisted if genomic targets controlling susceptibility were identified.
Strain-specific variations in macrophage and animal sensitivity to LT were previously noted for four rat strains [24]. Toxicity testing of first filial (F1) progeny from crosses of LT-sensitive (Brown Norway and F344) and resistant (Lewis and Wistar Kyoto) strains led to the conclusion that toxin sensitivity exhibited a dominant mode of inheritance [24]. Further, the authors concluded that the inter-cross results were consistent with LT sensitivity in rats being determined by a single, dominant gene [24].
In the current report, we used the HXB/BXH recombinant inbred (RI) rat collection, developed by two gender-reciprocal matings of the Wistar Kyoto-related strain, the SHR/Ola rat (an LT-resistant rat) with a Brown Norway congenic (BN-Lx, an LT-sensitive rat) [25], [26] as an ideal genetically-derived animal model to map LT sensitivity of rats. We report that susceptibility of rats to anthrax LT maps to a single locus on chromosome 10 that contains the Nlrp1 (rNlrp1) gene. Furthermore, LT sensitivity of a large number of rat strains was found to perfectly correlate with their macrophage sensitivity to toxin. Sequence analysis of rNlrp1 from twelve rat strains identified specific variations within a limited 100-aa N-terminal region of rNlrp1 that correlate perfectly with LT sensitivity. Taken together, these data suggest that a single locus on chromosome 10, likely rNlrp1, controls both rat macrophage sensitivity to anthrax LT as well as rat death in response to this toxin.
Results
Susceptibility to LT in the rat correlates with macrophage sensitivity to toxin
Twelve inbred rat strains and their bone marrow-derived macrophages (BMDMs) were tested for sensitivity to anthrax LT (Figure 1A). Both the rats and their corresponding BMDMs exhibited a qualitative dichotomous phenotype. Thus, macrophages were either sensitive or resistant to toxin, and rats showed an identical pattern, either succumbing within 60 minutes or remaining completely resistant to systemic toxin treatment (Figure 1A). For all the inbred rat strains, sensitivity of BMDMs was predictive of animal susceptibility to toxin. This result is notably different from what was previously observed in a comparison of mouse strains, where correlation between mouse strain susceptibilities to LT and their macrophage sensitivities was not found [15]. We and others have proposed that multiple genetic loci control LT susceptibility in mice [15], [27].
Fig. 1. LT sensitivity in rat strains correlates perfectly with macrophage sensitivity. 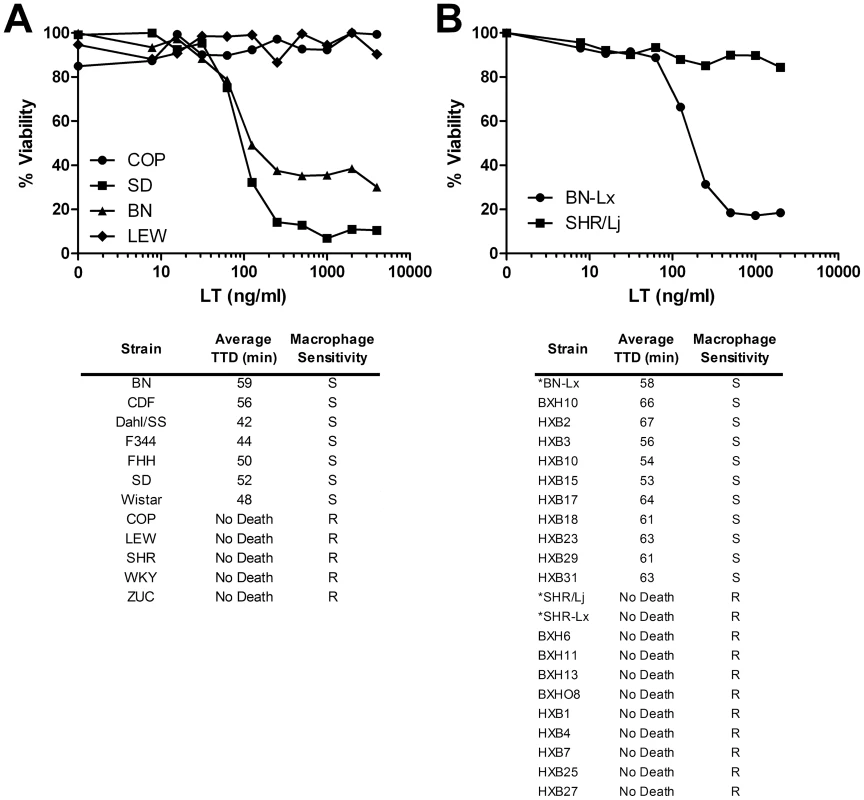
(A, B) Top panels show representative cytotoxicity assays for rat macrophages treated with toxin. BMDMs were treated with various concentrations of LT and cell viability was assessed after 3 h by MTT assay as described in Materials and Methods. Lower panels show average rat times to death (TTD) after LT treatment (100 µg, IV, n = 3 or n = 4/strain), and the macrophage LT sensitivity phenotypes (S = sensitive, R = resistant). (*)s indicate progenitor strains and a related congenic strain. Recombinant inbred rat screen identifies the LT susceptibility locus in rats
The absence of intermediate sensitivities in the rats supported the possibility that LT sensitivity in rats is controlled by a single gene [24]. This fact suggested that a recombinant inbred (RI) rat strain panel derived from LT-sensitive and LT-resistant progenitors could be used to map the LT susceptibility locus. RI strains allow linking of allelic variation at specific chromosomal loci to particular phenotypes. The widely used HXB/BXH RI rat collection was developed by two gender-reciprocal matings of the hypertensive SHR/Ola rat (an LT-resistant rat; “H” alleles) with a Brown Norway (BN) congenic expressing polydactylyl luxate syndrome (BN-Lx, an LT-sensitive rat; “B” alleles) [25], [26]. This RI panel has been successfully used for identification of quantitative trait loci that control a range of phenotypes, including cardiovascular function, insulin resistance and multiple behavioral traits (for review see [26]). We tested nineteen HXB/BXH RI rat strains and their macrophages for sensitivity to toxin. Ten of nineteen strains were sensitive, and once again the LT sensitivity of their isolated macrophages correlated perfectly with animal susceptibility (Figure 1B). Differences in PA receptor function were ruled out as progenitor strains had similar sensitivity to an LF-Pseudomonas exotoxin A fusion protein (FP59), which requires PA for cell entry but induces lethality by inhibition of protein synthesis (data not shown).
Analyses of LT sensitivity phenotypes in the context of published marker data for all chromosomes of the RI rat panel [28] pointed to the existence of a single sensitivity locus on chromosome 10, with the strongest linkage being to marker D10Rat102 (52.5 M), where marker genotypes matched sensitivity and resistance to LT in all but two rat strains (P = 0.001) (Figure 2A and Figure S1). An abridged set of markers (O. Seda and L. Sedova, unpublished) that were mapped to the initial marker set for agreement (P. Flodman et al., unpublished) indicated that the genotype at another marker (D10Rat77, 56.9 M) was fully consistent with the LT phenotype for all genotyped RI strains (P = 0.00005; Figure 2A). We noted that rNlrp1 (58.0 M), the ortholog for the murine LT macrophage sensitivity locus, lies within 1.1 M of D10Rat77 (Figure 2B). Analysis of SNP data in the region between marker D10Rat77 and marker D10Rat80 (also from the unpublished abridged data set) showed a perfect correlation between genotype and LT phenotype in all rat strains for the region surrounding rNlrp1 (P = 0.00001; Figure 2C). The boundaries of the LT susceptibility locus were determined by SNP analyses of all RI strains to lie between SNP Cpn_10055303964 at 55.3 M and WKYc98d01_s1_778 at 58.2 M (http://gscan.well.ox.ac.uk/gsBleadingEdge/rat.snp.selector.cgi). Analyses of SNPs within this locus comparing RI progenitor strains to several of the previously characterized inbred strains (COP, LEW, WKY, Dahl/SS, and F344) found only 7 individual SNPs that perfectly correlated with sensitivity among these LT sensitive and LT resistant strains. Three of these SNPs lie very close to rNlrp1 (rat101_030_o22.q1ca_511 at 57.8 M, rat102_003_p19.q1ca_444 at 57.9 M, and J577324 at 58.1 M), further supporting rNlrp1 as the leading candidate sensitivity locus among a small number of candidate genes. In view of the prior demonstration that mNlrp1b controls mouse macrophage sensitivity to LT, it was not surprising that the mapping data identified a locus containing rNlrp1 as determining LT sensitivity in rat macrophages. However, a single locus control of animal death was not anticipated.
Fig. 2. Mapping of the LT sensitivity locus to chromosome 10. 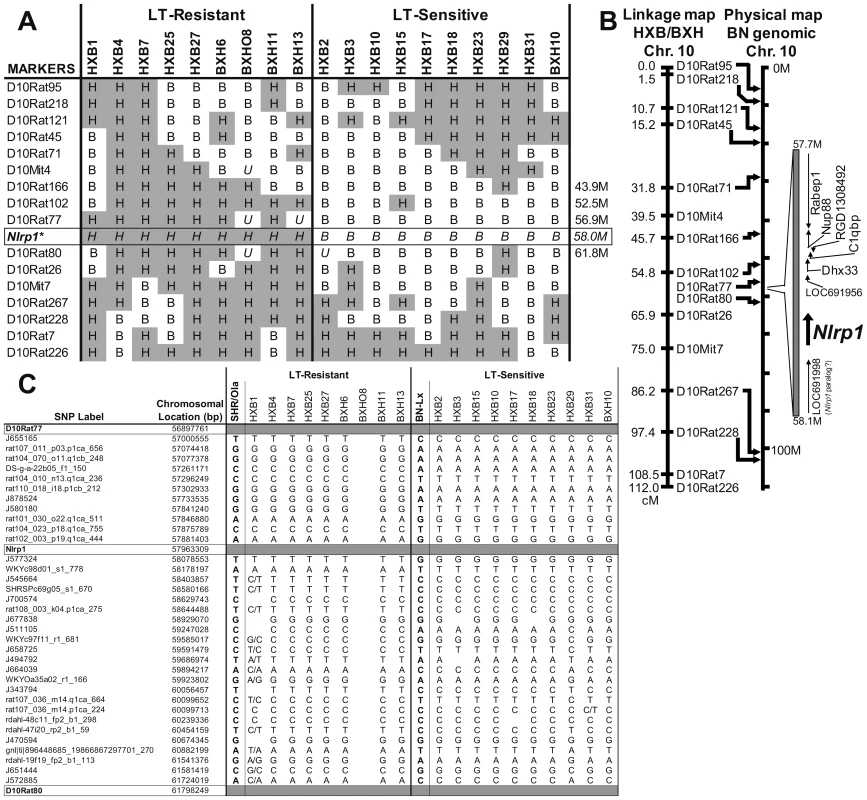
(A) Strain distribution patterns at several markers on chromosome 10 (a complete collection based on published data [28] for all chromosomes is found in Figure S1). Genotypes are SHR-like (H; gray) or BN-Lx-like (B; white). U indicates unknown genotypes. (*) indicates predicted genotypes. (B) Linkage map for markers on chromosome 10. Markers with known locations in the genomic data for chromosome 10 of the Brown Norway rat (BN/SsNMcw; RGSC 3.4) are mapped to their approximate physical locations on the chromosome (indicated with arrows). Expansion indicates the region from 57.7 M to 58.1 M of chromosome 10 and selected open reading frames. (C) SNP data between markers D10Rat77 and D10Rat80 (flanking rNlrp1) for all RI strains (except BXHO8) and their progenitors. SNP heterozygosity is indicated by dual base designation. BLAST searches (http://blast.ncbi.nlm.nih.gov/Blast.cgi) using the predicted BN rNlrp1 (allele 1, see later sections) sequence also identified a potential rNlrp1 paralog (GenBank accession: XM_001080760 “similar to NACHT, leucine rich repeat and PYD containing 1” at LOC691998 in the Rat Genome Sequencing Consortium (RGSC) v3.4, or alternatively, XM_001080056 at LOC687768 in the Celera assembly) located immediately adjacent to rNlrp1 with a predicted protein sequence that has 76% aa identity with the BN rNlrp1 sequence. RT-PCR analyses utilizing intraexonic and intron-spanning primers specific to this paralog showed that it exists in all strains except Copenhagen (COP) but is not expressed in macrophages and thus it is unlikely that this paralog is involved in macrophage toxicity (Figure S2).
rNlrp1 N-terminal sequence in rats correlates with LT sensitivity
The perfect correlation of macrophage sensitivity and animal susceptibility to toxin, along with the established role of mNlrp1b in controlling murine macrophage sensitivity suggested that rNlrp1 was the best candidate for control of sensitivity. We next sequenced rNlrp1 cDNA from twelve rat strains in order to identify sensitivity-correlated variations. BMDMs isolated from ten strains analyzed previously (Figure 1) as well as the progenitor strains for the RI panel (Figure 2) were used as sources of mRNA for sequencing. The sequencing identified a 3657-bp coding region corresponding to a 1219-aa rNlrp1 protein. By aligning the cDNA sequences to the BN genomic sequence data we determined that the rNlrp1 mRNA is formed through splicing of 14 exons, arranged like those of mNlrp1b [3]. Conserved Domain Database (CDD) searches indicated that rNlrp1 contains the same functional NACHT (nucleotide oligomerization; pfam05729), LRR (leucine-rich repeat; cd00116), and CARD (caspase recruitment; pfam00619) domains as mNlrp1b and hNlrp1 (Figure 3). rNlrp1 is similar to mNlrp1b in lacking a pyrin domain. Sequence alignments showed there to be two sensitivity-associated (“sensitive”) alleles (1 and 2) and three resistance-associated (“resistant”) alleles (3,4,5) among the studied strains (Figure 3 and Figure S3). The differences within these two groups turn out to be minor. The protein encoded by the second sensitive allele, allele 2, differs from allele 1 at a single amino acid (Asn61 to Lys61), but this substitution is also found in resistant alleles 3–5. More interestingly, alleles 3 and 4 both contain sequences corresponding to the N-terminal region of resistant allele 5 and several polymorphisms associated with the C-terminus of sensitive allele 1. Thus, the predicted proteins in these two rats combine a few elements of both a resistant and a sensitive rNlrp1, making it unlikely that the NACHT, LRR and CARD domains determine LT susceptibility. A single difference between resistant alleles 3 and 4 was found, where a substitution results in a Gln to Arg change (Arg561). Allele 5, however, is similar to allele 4 and contains this Arg561 residue, indicating that this residue is also unlikely to be associated with resistance to LT. Thus, we conclude that all the polymorphisms that correlate perfectly with LT sensitivity lie in the 100-aa N-terminus of rNlrp1. Unfortunately, no information is available about the function of this region in rodent Nlrp1 proteins. There is no homologous region in hNlrp1, which instead harbors an N-terminal pyrin domain (absent from rodent Nlrp1 proteins) [10].
Fig. 3. Allelic variations in rNlrp1 correlate with LT sensitivity. 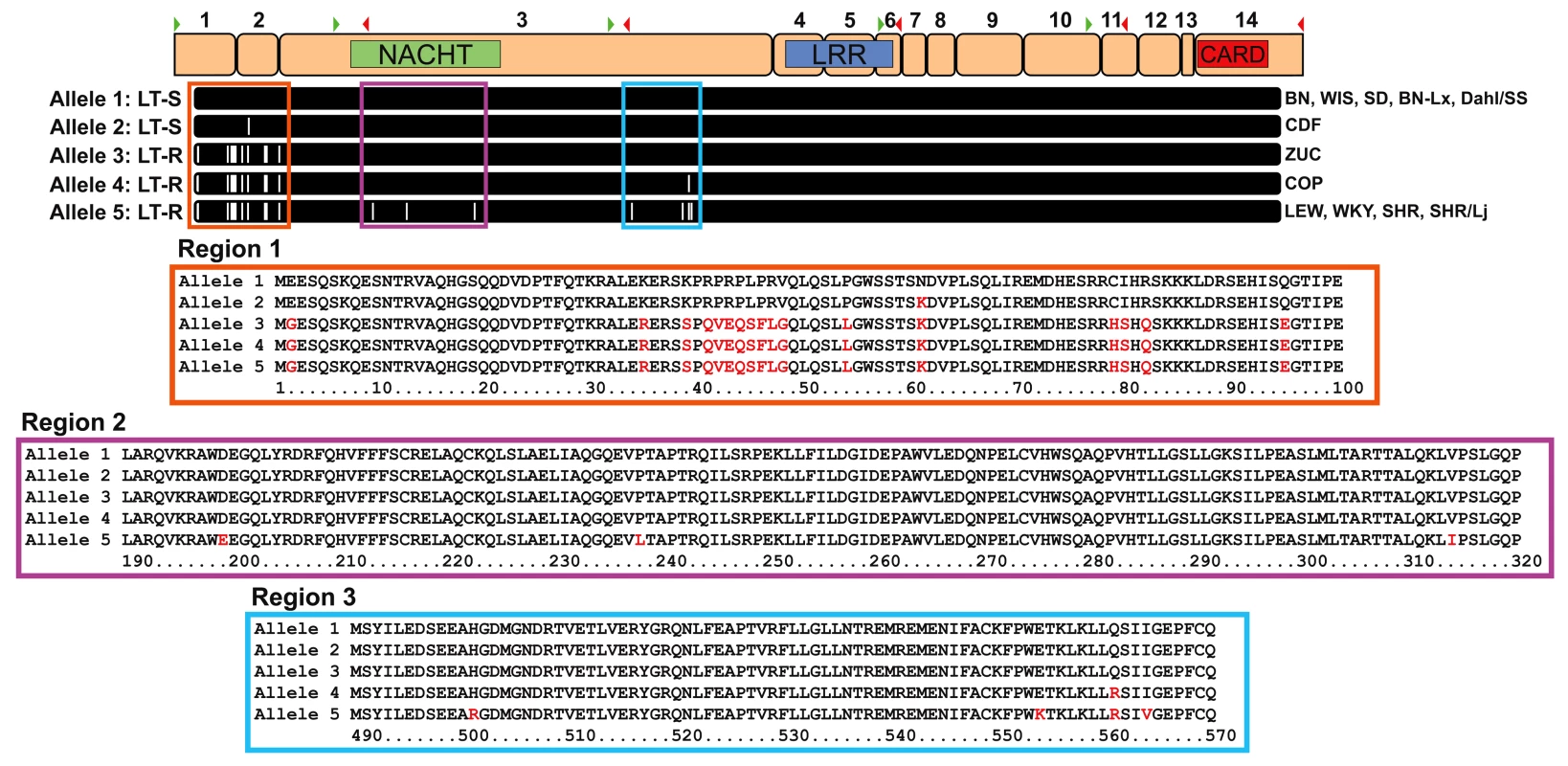
Proposed exon structure of the mature mRNA for rNlrp1 is shown at the top. Exons are numbered above and locations of forward (green arrowhead) and reverse (red arrowhead) primers for the primary sequencing reactions are indicated. Approximate domain locations are shown for NACHT (green), LRR (blue), and CARD (red). Amino acid alignments for the five alleles of rNlrp1 are aligned to the exon structure. White hashes are indicative of amino acid changes relative to protein encoded by allele 1. Expansions of the three regions of interest are also shown with alignments of the five alleles. Red letters identify residues that differ from those in the protein encoded by allele 1. Discussion
In the work reported here, a RI rat panel was used to identify the LT susceptibility locus for both rats and their macrophages. Analyses of LT sensitivity in several rat strains as well as the RI panel identified a complete correlation between macrophage and rat sensitivity to the toxin. This locus, on chromosome 10, contains rNlrp1, which is the homolog for the mouse mNlrp1b gene, previously proven to be critical for determining murine macrophage susceptibility to LT. Sequence analyses of rNlrp1 in primary macrophages from twelve rat strains identified five polymorphic alleles. Surprisingly, the few polymorphisms that correlated with LT macrophage and animal sensitivity in rats were located within the first 100 aa of rNlrp1, in an area of undefined function, and not within the previously described Nlrp1 functional domains (NACHT, LRR and CARD).
The mapping data strongly suggests (with a P = 0.000001) that this rNlrp1-containing region of chromosome 10 is the LT sensitivity locus for both rats and their macrophages. Among the approximately 250 microsatellite markers previously characterized for this RI set [28] and new previously unpublished markers sets, we found that marker D10Rat77 on chromosome 10 had an absolute genotypic correlation with the LT sensitivity phenotype. SNP analysis in this region also confirms the marker data and shows perfect correlation for LT susceptibility within the locus containing rNlrp1 (genome-wide empiric p-value = 0.001). Our mapping data does not rule out the possibility that another gene very closely linked to rNlrp1 could be mediating LT's effects in the rat. However, two additional lines of evidence argue against this possibility. First, rNlrp1 aa sequence variations between several inbred rat strains unrelated to the RI panel progenitors correspond perfectly with the sensitivity phenotypes. Second, an absolute correlation was found between macrophage sensitivity and animal susceptibility for 34 rat strains. Considering the established role of mNlrp1b in control of murine macrophage sensitivity, it is unlikely that a different gene controls macrophage sensitivity in rats. However we cannot exclude the possibility that whole animal susceptibility is controlled by multiple closely-linked genes within the single chromosome 10 locus identified in this study, and that these genes are inherited in a fashion such that polymorphisms associated with sensitivity in rNlrp1 are also always found in another candidate gene. Historically, a similar issue plagued the identification of mNlrp1b as the mouse macrophage sensitivity locus for LT. The sensitivity locus in mice was first identified as the closely linked Kif1c gene, which presented almost perfect polymorphism correlations with sensitivity [29]. In the absence of a transgenic rat model providing definitive proof linking the rNlrp1 gene to rat death, we believe the mapping and sequence data presented here strongly support rNlrp1 as the most likely determinant of LT sensitivity.
Gene predictions and BLAST searches identified a potential paralog immediately adjacent to rNlrp1, but this paralog is not expressed at the mRNA level, at least in macrophages. Similarly, of the three tandem mNlrp1 paralogs found in mice, only mNlrp1b was shown to be expressed in the LT-sensitive 129S1/SvImJ macrophage, and expression of this paralog was sufficient to confer LT sensitivity to resistant mouse macrophages and fibroblasts [3], [8]. However, the other two mouse paralogs are expressed in a number of inbred strains, further complicating analyses of mouse susceptibility. Curiously, phylogenetic analyses indicate that the predicted rNlrp1 paralog sequence is distant from the other rat Nlrp1 sequences and is more similar to the mouse and human Nlrp1 sequences (Figure S4).
The highly polymorphic nature of the mNlrp1b alleles has made it difficult to associate specific polymorphisms with the macrophage sensitivity phenotype in mice. Fortunately, there are far fewer differences in rNlrp1 between sensitive and resistant rat strains. Sequence differences that correlated with phenotypic differences were found only within the extreme N-terminal region of rNlrp1, and not in the domains (NACHT, LRR, and CARD) which have recognized roles in Nlrp1 function. This surprising finding draws attention to the N-terminal domains of rodent Nlrp1 proteins, absent in hNlrp1, which instead contain a pyrin domain at the N-terminus [11]. The pyrin domain in hNlrp1 is required for association with the inflammasome adaptor protein ASC, which is not part of the LT-induced mNlrp1b inflammasome complex [9]. Interestingly, all human macrophages tested to date have been LT-resistant (unpublished observations), a behavior that might relate to the absence of the N-terminal pyrin domain in rodent Nlrp1. However, hNlrp1 polymorphisms are now being identified and associated with a number of human diseases [30]–[32], so it may be necessary to test a larger number of donors to identify any LT-sensitive hNlrp1 alleles. As the N-terminus in hNlrp1 plays an important role in protein-protein interactions, it is tempting to postulate that the N-terminal 100 aa of rodent Nlrp1 proteins may also interact with other cellular components to modulate function.
The perfect correlation of rat macrophage LT sensitivity to that of the animals might at first suggest that the lysis of macrophages in vivo causes the rapid death of LT-injected rats. However, this is unlikely to be the case, for several reasons. Rat macrophages begin to die only 2 h after treatment in vitro with saturating toxin doses, whereas the rats may die in as little as 37 min [20]. Initial studies in LT-treated mice were interpreted as showing that death (which occurs only after 2–3 days) resulted from cytokines released following macrophage lysis [33]. However, more extensive later studies showed that mice harboring resistant macrophages also succumb to LT through a vascular collapse that is similar to that in mice with sensitive macrophages [13], and the correlation within mouse strains between the LT sensitivities of isolated macrophages and the animals is poor [13], [15]. Studies with mNlrp1b transgenic mice confirm that macrophage and animal susceptibility to LT are not correlated [16]. Preliminary studies in our laboratory suggest that cell types other than macrophages control the lethal response to LT (data not shown). Consistent with this view, Nlrp1 has recently been demonstrated to play a functional role in a number of cell types, including neuronal cells [34]–[37]. Furthermore, it should be noted that LT-induced death in both rats and mice has recently been associated with early changes in cardiac function [21], [23]. Thus, it is possible that LT targeting of rNlrp1 function in the heart plays a role in the rapid lethality phenotype. A better understanding of the distribution and function of different Nlrp1 isoforms in various cell types is needed to fully understand the mechanisms by which LT may influence Nlrp1 activity, and whether this gene alone is sufficient for control of animal susceptibility to toxin.
In summary, we present data mapping the control of rapid LT-induced rat death to a single chromosome 10 locus. This locus contains the polymorphic rNlrp1 gene, which is the best candidate for conferring sensitivity to macrophages, and possibly to animals. As such, this is the first suggestion that an inflammasome NLR protein may directly control animal lethality. While both the mechanistic basis for the rapid LT-induced lethality in the rat and direct proof of rNlrp1-mediated rat death require further experimentation, identification of the limited polymorphisms within rNlrp1 that correlate perfectly with LT sensitivity suggest a starting point for analysis of the possible role this protein may play in controlling rapid rat death in response to LT.
Materials and Methods
Ethics statement
All animal experiments were performed in strict accordance with guidelines from the NIH and the Animal Welfare Act, under protocols approved by the Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Animal studies
PA, LF, and FP59 were purified from B. anthracis [38]–[40]. The LF used here is a recombinant protein having an N-terminal sequence beginning HMAGG. Doses and concentrations of LT given for each experiment correspond to that of each toxin component (i.e., 1 µg/ml LT is 1 µg/ml PA +1 µg/ml LF and 100 µg LT is 100 µg PA +100 µg LF). Rats purchased from Charles River Laboratories (Wilmington, MA) were maintained there as either inbred or long-term outbred colonies. Rats strains used included (with strain designations, abbreviations and inbred/outbred status): Brown Norway (BN/Crl; BN; inbred), Fischer CDF (F344/DuCrl; CDF; inbred), SASCO Fischer (F344/NCrl; F344; inbred), Dahl Salt Sensitive (SS/JrHsdMcwiCrl; Dahl/SS; inbred), Lewis (LEW/Crl; LEW; inbred), Wistar (Crl:WI; WIS; outbred), Wistar Kyoto (WKY/NCrl; WKY; inbred), Sprague Dawley (CRL:SD; SD; outbred), Spontaneously Hypertensive Rat (SHR/NCrl; SHR; inbred), Copenhagen (COP/CrCrl; COP; inbred), Zucker-Lean (Crl:ZUC-Leprfa; ZUC; outbred) and Fawn Hooded Hypersensitive (FHH; inbred). The recombinant inbred (RI) rat strain panel used in this study was derived from the progenitor strains BN-Lx and SHR/Ola (indicated to be genetically equivalent to SHR/Lj used in this study) [25], [26]. The microsatellite marker genotypes and linkage maps for the RI panel were most recently characterized by one of our laboratories [28]. Additional microsatellite markers were identified in progenitors and mapped across the RI strains (P. Flodman et al., unpublished; O. Seda and L. Sedova, unpublished) by PCR. Marker data were correlated with SNP genotypes available through the Wellcome Trust Centre for Human Genetics STAR Rat SNP Selector (http://gscan.well.ox.ac.uk/gsBleadingEdge/rat.snp.selector.cgi).
Adult female RI rats (9–12 weeks old) of the 19 strains of the HXB/BXH set were rederived, bred, and maintained at the University of California, San Diego, and shipped to Bethesda, MD, for toxin testing and bone marrow collection. The progenitor strains and a congenic strain, SHR-Lx, were included in the analysis. Rats were acclimated for four-five days prior to experiments. For all rat LT challenge studies, female rats (130–160 g) were injected with LT (100 µg, IV) and monitored continuously for 5 h followed by a 24-h check of surviving animals. This dose of toxin represents 10× LD100 for the sensitive F344 and CDF rats when using the well-characterized toxin prepared in our laboratory.
Cell culture
L929 mouse fibroblast cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 10 mM HEPES, and 50 µg/ml gentamicin (all obtained from Invitrogen, Carlsbad, CA) at 37°C in 5% CO2. Bone marrow-derived macrophages (BMDMs) were cultured in complete DMEM (as described above) with 30% L929 cell culture supernatant. BMDMs were grown for 7–9 days to allow time for differentiation before use in assays.
Cytotoxicity assays
BMDMs were plated in 96-well plates 24 h prior to assays at 90% confluence. For basic macrophage LT sensitivity testing, cells were exposed to LT at the indicated concentrations and times. Viability was assessed by addition of MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide] (USB Corporation, Cleveland, OH) to a final concentration of 0.6 mg/ml in DMEM. Following 30–45 min of incubation with MTT dye, cell culture medium was removed and cells were dissolved with 0.5% SDS, 25 mM HCl in 90% isopropanol and A570 was measured. Percent viabilities were calculated relative to medium-treated controls.
Statistical analyses
Statistical analyses were performed using SAS (ver. 9.1.3, SAS Institute Inc., Cary, NC). Association between genotype and phenotype across the RI strains was assessed for each marker using Fisher's exact test. Nominal p-values for the tests of association are reported without correction for multiple comparisons. The genome-wide significance of the linkage findings was assessed using MapManager QTXb20 to calculate an empiric p-value based on 10,000 permutations [41].
rNlrp1 sequencing
RNA was isolated from BMDMs by a TRIZOL extraction method according to manufacturer's protocol (Invitrogen). RNA was reverse transcribed using the SuperScript III First Strand Synthesis System (Invitrogen). Sequencing primers were designed to cover the full coding sequence of rNlrp1 using the predicted rNlrp1 mRNA sequence (GenBank accession: XM_340835) from the Rat Genome Sequencing Consortium BN rat genomic sequence data (RGSC v3.4, GenBank accession: NW_047334). The primary sequencing reactions consisted of amplifying five overlapping cDNA regions (locations indicated in Figure 3 and primer sequences are provided in Table S1). All PCR was performed with the TaKaRa Ex Taq (TAKARA Bio Inc., Otsu, Japan). PCR products were purified using PureLink PCR purification kits (Invitrogen) and sequenced on Applied Biosystems 3730xl DNA analyzers at MACROGEN USA (Rockville, MD). Additional primers and reactions were used for confirmation of specific regions and clarification of the overlaps for the primary reactions (Table S1). Sequences were assembled and analyzed using the Lasergene program suite (DNASTAR, Inc., Madison, WI). Alignments were created using ClustalX (http://www.clustal.org/) and phylogenetic trees were visualized with TreeViewX (v0.5; http://darwin.zoology.gla.ac.uk/~rpage/treeviewx/index.html). Exon structure was determined by aligning the cDNA sequences to the BN genomic data by a BLAT search (http://genome.ucsc.edu/cgi-bin/hgBlat).
Accession codes
Amino acid sequences used in alignments and phylograms included a potential rNlrp1 paralog predicted sequence (XP_001080760.1), hNlrp1 isoform 1 (NP_127497), C57BL/6J mNlrp1a (AAZ40527), C57BL/6J mNlrp1c (AAZ40528), BALB/cJ mNlrp1b Allele 1 (AAZ40509), C57BL/6J mNlrp1b Allele 2 (AAZ40517), NOD/LtJ mNlrp1b Allele 3 (AAZ40521), DBA/2J mNlrp1b Allele 4 (AAZ40523), and CAST/EiJ mNlrp1b Allele 5 (AAZ40526). cDNA GenBank sequences for new rNlrp1 sequences determined in this work are as follows: HM060628 (BN), HM060629 (BN-Lx), HM060630 (COP), HM060631 (Dahl/SS), HM060632 (CDF), HM060633 (LEW), HM060634 (SD), HM060635 (SHR), HM060636 (SHR/Lj), HM060637 (WIS), HM060638 (WKY), HM060639 (ZUC).
Supporting Information
Zdroje
1. YoungJA
CollierRJ
2007 Anthrax toxin: receptor-binding, internalization, pore formation, and translocation. Annu Rev Biochem 76 243 265
2. MoayeriM
LepplaSH
2009 Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol Aspects Med 30 439 455
3. BoydenED
DietrichWF
2006 Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet 38 240 244
4. MartinonF
BurnsK
TschoppJ
2002 The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10 417 426
5. MuehlbauerSM
EveringTH
BonuccelliG
SquiresRC
AshtonAW
2007 Anthrax lethal toxin kills macrophages in a strain-specific manner by apoptosis or caspase-1-mediated necrosis. Cell Cycle 6 758 766
6. FinkSL
BergsbakenT
CooksonBT
2008 Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc Natl Acad Sci U S A 105 4312 4317
7. WickliffeKE
LepplaSH
MoayeriM
2008 Anthrax lethal toxin-induced inflammasome formation and caspase-1 activation are late events dependent on ion fluxes and the proteasome. Cell Microbiol 10 332 343
8. LiaoKC
MogridgeJ
2009 Expression of Nlrp1b inflammasome components in human fibroblasts confers susceptibility to anthrax lethal toxin. Infect Immun 77 4455 4462
9. NourAM
YeungYG
SantambrogioL
BoydenED
StanleyER
2009 Anthrax lethal toxin triggers the formation of a membrane-associated inflammasome complex in murine macrophages. Infect Immun 77 1262 1271
10. FranchiL
EigenbrodT
Munoz-PlanilloR
NunezG
2009 The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 10 241 247
11. MartinonF
MayorA
TschoppJ
2009 The inflammasomes: guardians of the body. Annu Rev Immunol 27 229 265
12. BeallFA
DalldorfFG
1966 The pathogenesis of the lethal effect of anthrax toxin in the rat. J Infect Dis 116 377 389
13. MoayeriM
HainesD
YoungHA
LepplaSH
2003 Bacillus anthracis lethal toxin induces TNF-á-independent hypoxia-mediated toxicity in mice. J Clin Invest 112 670 682
14. CuiX
MoayeriM
LiY
LiX
HaleyM
2004 Lethality during continuous anthrax lethal toxin infusion is associated with circulatory shock but not inflammatory cytokine or nitric oxide release in rats. Am J Physiol Regul Integr Comp Physiol 286 R699 R709
15. MoayeriM
MartinezNW
WigginsJ
YoungHA
LepplaSH
2004 Mouse susceptibility to anthrax lethal toxin is influenced by genetic factors in addition to those controlling macrophage sensitivity. Infect Immun 72 4439 4447
16. TerraJK
CoteCK
FranceB
JenkinsAL
BozueJA
2010 Cutting edge: Resistance to Bacillus anthracis infection mediated by a lethal toxin sensitive allele of Nalp1b/Nlrp1b. J Immunol 184 17 20
17. MoayeriM
WebsterJI
WigginsJF
LepplaSH
SternbergEM
2005 Endocrine perturbation increases susceptibility of mice to anthrax lethal toxin. Infect Immun 73 4238 4244
18. BeallFA
TaylorMJ
ThorneCB
1962 Rapid lethal effect in rats of a third component found upon fractionating the toxin of Bacillus anthracis. J Bacteriol 83 1274 1280
19. EzzellJW
IvinsBE
LepplaSH
1984 Immunoelectrophoretic analysis, toxicity, and kinetics of in vitro production of the protective antigen and lethal factor components of Bacillus anthracis toxin. Infect Immun 45 761 767
20. GuptaPK
MoayeriM
CrownD
FattahRJ
LepplaSH
2008 Role of N-terminal amino acids in the potency of anthrax lethal factor. PLoS ONE 3 e3130
21. WatsonLE
KuoSR
KatkiK
DangT
ParkSK
2007 Anthrax toxins induce shock in rats by depressed cardiac ventricular function. PLoS ONE 2 e466
22. KuoSR
WillinghamMC
BourSH
AndreasEA
ParkSK
2008 Anthrax toxin-induced shock in rats is associated with pulmonary edema and hemorrhage. Microb Pathog 44 467 472
23. MoayeriM
CrownD
DorwardDW
GardnerD
WardJM
2009 The heart is an early target of anthrax lethal toxin in mice: a protective role for neuronal nitric oxide synthase (nNOS). PLoS Pathog 4 e1000456
24. NyeSH
WittenburgAL
EvansDL
O'connorJA
RomanRJ
2007 Rat survival to anthrax lethal toxin is likely controlled by a single gene. Pharmacogenomics J 8 16 22
25. PravenecM
KlirP
KrenV
ZichaJ
KunesJ
1989 An analysis of spontaneous hypertension in spontaneously hypertensive rats by means of new recombinant inbred strains. J Hypertens 7 217 221
26. PrintzMP
JiroutM
JaworskiR
AlemayehuA
KrenV
2003 Genetic models in applied physiology. HXB/BXH rat recombinant inbred strain platform: a newly enhanced tool for cardiovascular, behavioral, and developmental genetics and genomics. J Appl Physiol 94 2510 2522
27. McAllisterRD
SinghY
Du BoisWD
PotterM
BoehmT
2003 Susceptibility to anthrax lethal toxin is controlled by three linked quantitative trait loci. Am J Pathol 163 1735 1741
28. JiroutM
KrenovaD
KrenV
BreenL
PravenecM
2003 A new framework marker-based linkage map and SDPs for the rat HXB/BXH strain set. Mamm Genome 14 537 546
29. WattersJW
DewarK
LehoczkyJ
BoyartchukV
DietrichWF
2001 Kif1C, a kinesin-like motor protein, mediates mouse macrophage resistance to anthrax lethal factor. Curr Biol 11 1503 1511
30. JinY
MaillouxCM
GowanK
RiccardiSL
LabergeG
2007 NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med 356 1216 1225
31. TaiebA
2007 NALP1 and the inflammasomes: challenging our perception of vitiligo and vitiligo-related autoimmune disorders. Pigment Cell Res 20 260 262
32. MagittaNF
Boe WolffAS
JohanssonS
SkinningsrudB
LieBA
2009 A coding polymorphism in NALP1 confers risk for autoimmune Addison's disease and type 1 diabetes. Genes Immun 10 120 124
33. HannaPC
AcostaD
CollierRJ
1993 On the role of macrophages in anthrax. Proc Natl Acad Sci U S A 90 10198 10201
34. FeldmeyerL
KellerM
NiklausG
HohlD
WernerS
2007 The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol 17 1140 1145
35. de Rivero VaccariJP
LotockiG
MarcilloAE
DietrichWD
KeaneRW
2008 A molecular platform in neurons regulates inflammation after spinal cord injury. J Neurosci 28 3404 3414
36. AbulafiaDP
de Rivero VaccariJP
LozanoJD
LotockiG
KeaneRW
2009 Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cereb Blood Flow Metab 29 534 544
37. de Rivero VaccariJP
LotockiG
AlonsoOF
BramlettHM
DietrichWD
2009 Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab 29 1251 1261
38. ParkS
LepplaSH
2000 Optimized production and purification of Bacillus anthracis lethal factor. Protein Expr Purif 18 293 302
39. VarugheseM
ChiA
TeixeiraAV
NichollsPJ
KeithJM
1998 Internalization of a Bacillus anthracis protective antigen-c-Myc fusion protein mediated by cell surface anti-c-Myc antibodies. Mol Med 4 87 95
40. AroraN
KlimpelKR
SinghY
LepplaSH
1992 Fusions of anthrax toxin lethal factor to the ADP-ribosylation domain of Pseudomonas exotoxin A are potent cytotoxins which are translocated to the cytosol of mammalian cells. J Biol Chem 267 15542 15548
41. ManlyKF
CudmoreRHJr
MeerJM
2001 Map Manager QTX, cross-platform software for genetic mapping. Mamm Genome 12 930 932
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Mouse Senile Amyloid Fibrils Deposited in Skeletal Muscle Exhibit Amyloidosis-Enhancing ActivityČlánek The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced MucositisČlánek Crystal Structure of HIV-1 gp41 Including Both Fusion Peptide and Membrane Proximal External RegionsČlánek Demonstration of Cross-Protective Vaccine Immunity against an Emerging Pathogenic Ebolavirus Species
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 5- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Quorum Sensing Inhibition Selects for Virulence and Cooperation in
- The HMW1C Protein Is a Glycosyltransferase That Transfers Hexose Residues to Asparagine Sites in the HMW1 Adhesin
- Analysis of Virion Structural Components Reveals Vestiges of the Ancestral Ichnovirus Genome
- Mouse Senile Amyloid Fibrils Deposited in Skeletal Muscle Exhibit Amyloidosis-Enhancing Activity
- Global Migration Dynamics Underlie Evolution and Persistence of Human Influenza A (H3N2)
- The Type III Effectors NleE and NleB from Enteropathogenic and OspZ from Block Nuclear Translocation of NF-κB p65
- VEGF Promotes Malaria-Associated Acute Lung Injury in Mice
- Identification of a Mutant PfCRT-Mediated Chloroquine Tolerance Phenotype in
- The Early Stage of Bacterial Genome-Reductive Evolution in the Host
- Host-Detrimental Role of Esx-1-Mediated Inflammasome Activation in Mycobacterial Infection
- Elevation of Intact and Proteolytic Fragments of Acute Phase Proteins Constitutes the Earliest Systemic Antiviral Response in HIV-1 Infection
- The Pleiotropic CymR Regulator of Plays an Important Role in Virulence and Stress Response
- Alternative Sigma Factor σ Modulates Prophage Integration and Excision in
- Effect of Neuraminidase Inhibitor–Resistant Mutations on Pathogenicity of Clade 2.2 A/Turkey/15/06 (H5N1) Influenza Virus in Ferrets
- Massive APOBEC3 Editing of Hepatitis B Viral DNA in Cirrhosis
- NK Cells and γδ T Cells Mediate Resistance to Polyomavirus–Induced Tumors
- Is Genetically Diverse in Animals and Appears to Have Crossed the Host Barrier to Humans on (At Least) Two Occasions
- Adenylate Cyclase Toxin Mobilizes Its β Integrin Receptor into Lipid Rafts to Accomplish Translocation across Target Cell Membrane in Two Steps
- The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced Mucositis
- HIV-1 Transmitting Couples Have Similar Viral Load Set-Points in Rakai, Uganda
- Few and Far Between: How HIV May Be Evading Antibody Avidity
- Galectin-9/TIM-3 Interaction Regulates Virus-Specific Primary and Memory CD8 T Cell Response
- Perforin Expression Directly by HIV-Specific CD8 T-Cells Is a Correlate of HIV Elite Control
- The Set3/Hos2 Histone Deacetylase Complex Attenuates cAMP/PKA Signaling to Regulate Morphogenesis and Virulence of
- Infidelity of SARS-CoV Nsp14-Exonuclease Mutant Virus Replication Is Revealed by Complete Genome Sequencing
- Combining ChIP-chip and Expression Profiling to Model the MoCRZ1 Mediated Circuit for Ca/Calcineurin Signaling in the Rice Blast Fungus
- Internalin B Activates Junctional Endocytosis to Accelerate Intestinal Invasion
- A Complex Small RNA Repertoire Is Generated by a Plant/Fungal-Like Machinery and Effected by a Metazoan-Like Argonaute in the Single-Cell Human Parasite
- Opc Invasin Binds to the Sulphated Tyrosines of Activated Vitronectin to Attach to and Invade Human Brain Endothelial Cells
- Muc2 Protects against Lethal Infectious Colitis by Disassociating Pathogenic and Commensal Bacteria from the Colonic Mucosa
- PdeH, a High-Affinity cAMP Phosphodiesterase, Is a Key Regulator of Asexual and Pathogenic Differentiation in
- Isolates with Antimony-Resistant but Not -Sensitive Phenotype Inhibit Sodium Antimony Gluconate-Induced Dendritic Cell Activation
- The Microbiota and Allergies/Asthma
- Environmental Factors Determining the Epidemiology and Population Genetic Structure of the Group in the Field
- Prolonged Antigen Presentation Is Required for Optimal CD8+ T Cell Responses against Malaria Liver Stage Parasites
- Crystal Structure of HIV-1 gp41 Including Both Fusion Peptide and Membrane Proximal External Regions
- Susceptibility to Anthrax Lethal Toxin-Induced Rat Death Is Controlled by a Single Chromosome 10 Locus That Includes
- Demonstration of Cross-Protective Vaccine Immunity against an Emerging Pathogenic Ebolavirus Species
- Effective, Broad Spectrum Control of Virulent Bacterial Infections Using Cationic DNA Liposome Complexes Combined with Bacterial Antigens
- High Multiplicity Infection by HIV-1 in Men Who Have Sex with Men
- The -Specific Human Memory B Cell Compartment Expands Gradually with Repeated Malaria Infections
- EBV Promotes Human CD8 NKT Cell Development
- Persistent Growth of a Human Plasma-Derived Hepatitis C Virus Genotype 1b Isolate in Cell Culture
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Quorum Sensing Inhibition Selects for Virulence and Cooperation in
- The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced Mucositis
- Crystal Structure of HIV-1 gp41 Including Both Fusion Peptide and Membrane Proximal External Regions
- Susceptibility to Anthrax Lethal Toxin-Induced Rat Death Is Controlled by a Single Chromosome 10 Locus That Includes
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



