-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaFew and Far Between: How HIV May Be Evading Antibody Avidity
article has not abstract
Published in the journal: . PLoS Pathog 6(5): e32767. doi:10.1371/journal.ppat.1000908
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1000908Summary
article has not abstract
HIV-1 Consistently Evades the Humoral Immune Response
More than 25 years have passed since the discovery of HIV type 1, the causative agent of AIDS, and the first vaccine candidate to exhibit evidence for protection against infection was reported only recently [1]. However, the extent and mode of protection are still under debate [2]. Thus, a vaccine that effectively stimulates complete protective immunity by the cellular branch (cytotoxic T lymphocytes) and/or the humoral branch (antibodies) of the immune system has yet to emerge. Among the millions of people who have received treatment for the disease and the many more who have tested HIV positive, there exists no definitive case in which a potent neutralizing antibody response enabled an infected individual to successfully clear or control the infection. In a small percentage of cases, individuals will exhibit a natural ability to suppress viral replication and progression of the disease. However, the explanation for the existence of this rare phenotype has primarily converged on a robust cellular immune response, with evidence generally lacking for a significant contribution to viral control by antibodies [3]–[5].
Structural features of the HIV envelope spike are critical to its unusual ability to escape neutralizing antibodies. However, many of the identified features are not unique to this virus. Here, we propose another strategy HIV employs to evade antibodies: the low density of envelope spikes, a distinguishing feature when compared with viruses to which protective neutralizing antibody responses are consistently raised, directly impedes bivalent binding by immunoglobulin G (IgG) antibodies. The result is a minimization of avidity, normally used by antibodies to achieve high affinity binding and potent neutralization, thereby expanding the range of mutations that allow HIV to evade antibodies. Understanding limitations to avidity may be essential to the design of anti-HIV vaccines and therapies.
The HIV Spike Structure and Its Rapid Mutation Facilitate Antibody Evasion
Tremendous effort has been devoted to understanding why HIV so effectively evades antibodies. Accepted explanations include rapid mutation of the two glycoproteins that comprise the envelope spike, gp120 and gp41, and structural features that enable the spike to hide conserved epitopes from antibodies. These structural features include a shield of host-derived carbohydrates [6], conformational masking [7], steric occlusion [8], the protection of conserved regions at interfaces by oligomerization or in narrow pockets [9]–[11], and the presence of highly variable flexible loops that shield conserved epitopes on the envelope spike [9], [12]. In addition, it was recently hypothesized that a lack of germline genes capable of maturing into potent anti-HIV antibodies may represent holes in the potential antibody repertoire [13].
While the importance of the envelope spike's structural attributes to limiting antibody potency are well established, they are not unique to HIV. For example, the receptor binding sites of both rhinovirus and influenza are narrow pockets predicted to be inaccessible to antibodies [14], and mutation, loop decoys, and glycan shielding have all been implicated in antibody evasion by influenza [15], [16]. Nevertheless, these viruses and many others and/or the vaccines that have been developed against them elicit potent neutralizing antibody responses that significantly contribute to their clearance or provide sterilizing immunity [17].
What distinguishes HIV from other viruses in relation to antibody-mediated neutralization? Is it simply that HIV is more adept at employing the evasion strategies outlined above? While it is clear that HIV is superbly adapted for evading antibodies based on these strategies (as described in recent reviews [15], [18]), we propose an additional contributing factor in its ability to escape neutralization by antibodies [19], which is based on recent data that describe the spatial arrangement of spikes on its surface. The reasoning is rooted in an inherent limitation to the architecture of an antibody as it relates to avidity, which in this context refers to the ability of a bivalent antibody to simultaneously bind two epitopes tethered to the same surface [20]. We begin with comparisons of available neutralization data and the spatial arrangements of envelope spikes for HIV and other viruses, then present a discussion of avidity and the factors that influence it, and end with speculations on how a greater understanding of the factors that aid or inhibit avidity might be used to further inform vaccine design.
Comparison of Monovalent and Bivalent Binding of Antibodies to Viruses
Most of the neutralizing activity in the sera of HIV-positive individuals can be attributed to antibodies of the IgG subclass [21], [22], which represent the predominant class of immunoglobulin in blood. IgG antibodies are composed of an Fc region fused to two identical Fabs (Figure 1). Antigens bind to the tip of each Fab, which present the unique surfaces that define the epitope specificity of the antibody. While the immune system can draw from an almost unlimited sequence library to change the specificity of the Fabs, the antibody architecture is relatively constant, including the range of permissible end-to-end distances between the Fabs. The Fabs are linked to the Fc region by a flexible hinge, which typically allows a 10–15-nm center-to-center separation between the antigen-binding sites of the two Fabs for IgGs.
Fig. 1. Scale model of an IgG antibody. 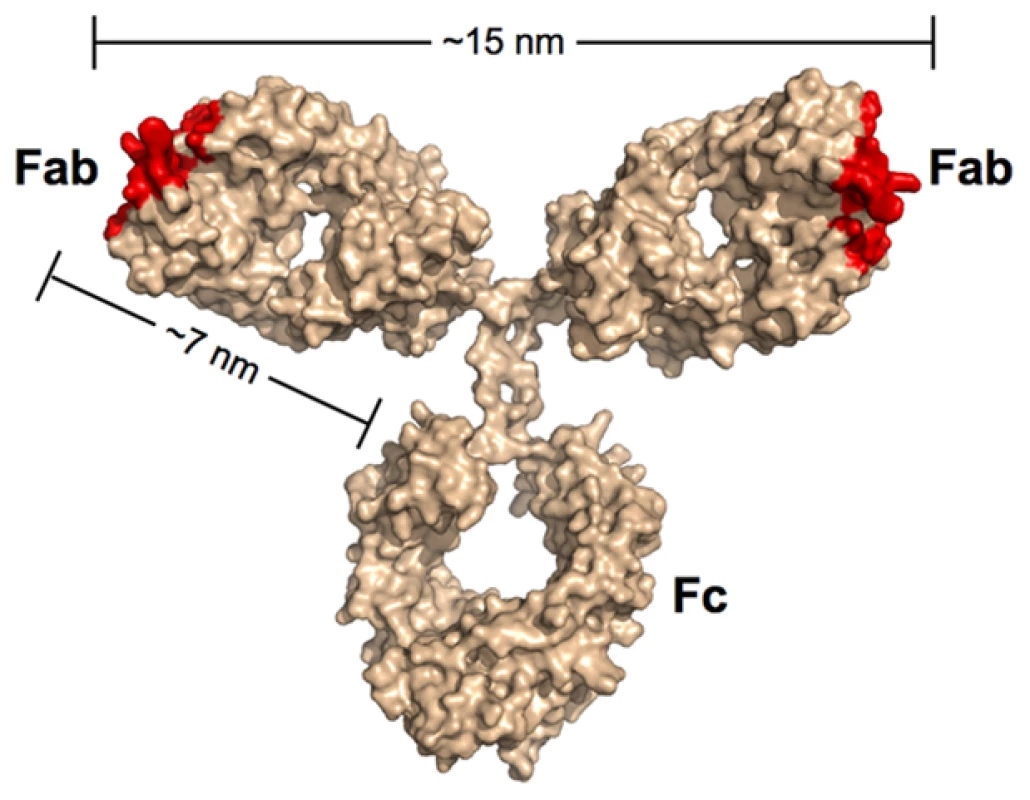
Red denotes the locations of antigen recognition sites. A longer than typical separation distance (17 nm) was reported for the structure of intact b12 IgG [69]. The longer distance resulted in part from an unusually long CDR3 loop protruding from the antigen-combining site of each Fab. As this loop wraps around the CD4-binding loop on gp120 [64], the effective separation distance on this IgG and other antibodies with protruding CDR3 loops would be ∼15 nm. In the context of antibodies, the term avidity refers to their ability to bind two physically linked antigens simultaneously (e.g., to the surface of the same virus). The result of avidity can be a dramatic increase in the strength of the binding as compared to a monovalent 1∶1 interaction such that once bound, the antibody interaction with antigen becomes essentially irreversible over biologically relevant time scales [23]. Antibodies have been shown to bind bivalently to non enveloped viruses such as rhinovirus and poliovirus, which contain a rigid icosahedrally symmetric outer protein shell with closely spaced epitopes. Thus, rhinovirus and poliovirus saturate with 30 IgGs bound via both Fabs to 60 repeating epitopes created by 30 2-fold symmetry axes [24], [25]. An early demonstration of the importance of avidity in IgG binding to poliovirus revealed that using papain to digest the antibody and create monovalent Fabs led to a substantial increase in the molar concentration required to inhibit infection in vitro [25]. By contrast, a limited role for avidity in neutralization of HIV by some antibodies is suggested by the relatively modest increases in neutralization potencies of IgGs as compared to their corresponding Fabs [26]–[28]. In addition, conversion of the broadly neutralizing anti-HIV antibodies 2F5 and 4E10 from IgGs with two combining sites to dimeric IgA (four Fabs) and/or pentameric IgM (ten Fabs) either did not improve their neutralization efficiencies or abrogated activity altogether [29], [30]. Similarly, we have observed equivalent neutralization potencies for the anti-HIV antibody b12 when tested as an IgA, IgM, or IgG (P. Gnanapragasm, R. Galimidi, J. Klein, A. West, Jr., and P. Bjorkman, unpublished data).
One way to quantitatively assess the effects of antibody avidity is to compare the neutralization potency values of a Fab and its parental IgG. We define the molar neutralization ratio (MNR) as the concentration in an in vitro neutralization assay at which a Fab achieves 50% inhibition of viral infectivity (IC50) divided by the IC50 for the parental IgG. If an antibody binds only monovalently to the viral surface (i.e., it is incapable of cross-linking epitopes on the virus), it would inhibit at an approximately 2-fold lower concentration than the Fab (MNR = 2) because the IgG has twice the number of antigen-binding sites [31]. MNRs greater than 2 suggest avidity effects resulting from the IgG cross-linking epitopes on the virus. Results from published studies show high MNRs for respiratory syncytial virus (RSV) [32] and influenza [31], [33] (Figure 2), suggesting that antibodies can take advantage of avidity effects to bind to enveloped viruses. However, a compilation of the highest reported MNRs we could find for antibodies against HIV [26]–[28] shows that neutralizing antibodies, including those that serve as models for the types of antibodies that researchers would most like to elicit with an HIV vaccine, yield relatively low MNRs (Figure 2). This suggests a general limitation to bivalent binding of IgGs to HIV. We propose that the spatial distribution of envelope spikes on HIV, combined with the distribution of protein epitopes on the spike trimer, explains the predominantly monovalent binding of anti-HIV antibodies, which in turn limits the ability of the humoral immune response to prevent viral escape by mutation.
Fig. 2. Bar graph of the highest reported molar neutralization ratios (MNRs). 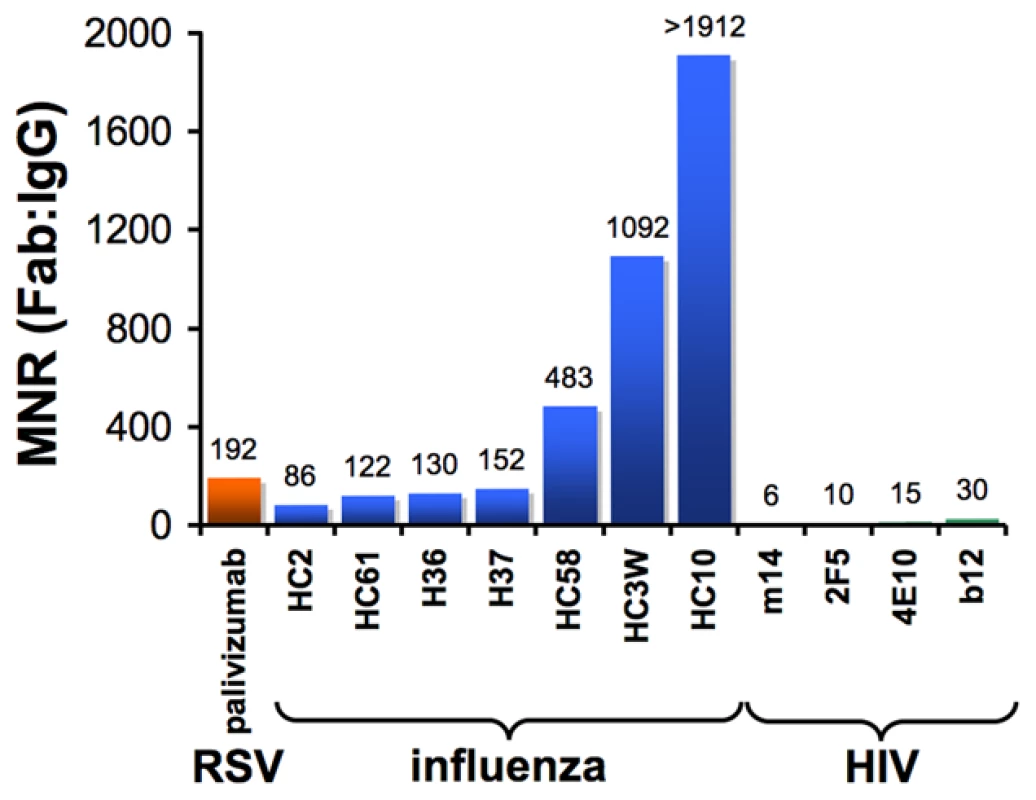
MNRs were reported for monoclonal antibodies against HIV [26], [27], [28], RSV [32], and influenza [31], [33]. The MNR for each antibody was calculated as the IC50 of the Fab divided by the IC50 of the IgG derived from in vitro neutralization assays (IC90s were reported for some influenza IgG/Fab comparisons [31], but IC50 ratios would be nearly the same because the slopes of the inhibition curves were similar). MNRs for a particular IgG/Fab combination can vary with the strain of virus being tested because the degree to which cross-linking can benefit an IgG depends on the affinity of the Fab for its antigen. Differences in size between a Fab and IgG may also influence the MNR if steric factors play a role in the neutralization mechanism of a particular antibody. However, this effect is probably minor, as (Fab)′2 fragments generally exhibit similar neutralization potencies to their parental IgGs [31], [33]. Not shown are high MNR values (∼70) derived for IgG/Fab comparisons involving HIV virions with a gp41 cytoplasmic tail truncation [70]. The tail deletion, which is rarely observed in vivo, has been suggested to increase the mobility of envelope trimers [70] and/or increase the number of spikes per virion [71], so its effects on intra-spike cross-linking are not well understood. HIV Envelope Spikes Are Present at Low Density
Enveloped viruses such as HIV contain an outer shell composed of a cell-derived lipid membrane displaying embedded antigens that were acquired during budding from the host cell. Consequently, enveloped viruses generally lack the structural elements of non-enveloped viruses that enforce a symmetric arrangement of antigens in non-enveloped viruses. Electron micrographs of enveloped viruses for which antibody-mediated neutralization is known to be critical to the control and/or elimination of infection [17], [34] generally reveal a high density of envelope spikes. For example, influenza type A virus incorporates ∼450 spikes per virus particle spaced at intervals ≤10 nm [35] (Figure 3A). Similarly, measles, RSV, and hepatitis B virions include large numbers of closely spaced spikes (Figure 3B–3D). Indeed, the high densities of repetitive, identical epitopes on the surfaces of non-enveloped icosahedral viruses and enveloped viruses such as vesticular stomatitis, rabies, influenza, and Sindbis allow induction of T cell–independent B cell activation during the elicitation of the humoral immune response [36]. In striking contrast, biochemical studies and cryo-electron tomography (cryo-ET) reconstructions showed that HIV, although similar in size to influenza type A, has an average of ∼14 spikes per virus particle (the full range from published studies is four to 35 spikes) [37]–[41] (Figure 3E). Despite the dearth of envelope spikes, HIV remains infectious, as it has been shown that as few as four spikes are sufficient for viral attachment [42], and possibly fewer may be needed to achieve fusion with the target cell membrane [43], [44].
Fig. 3. Comparison of enveloped viruses and nearest neighbor distances for HIV envelope spikes. 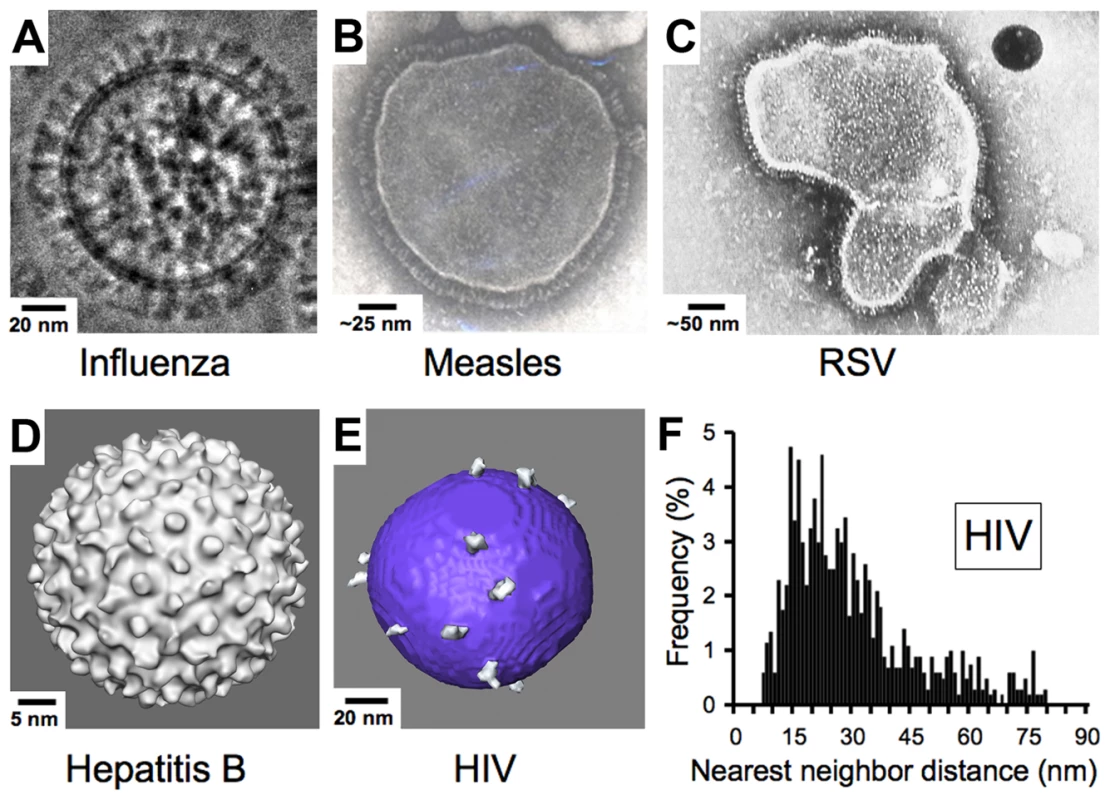
(A) Influenza type A virus. Image provided by Drs. Masashi Yamaguchi and Kuniaki Nagayama. (B) Measles virus. Image reproduced with permission from Dr. Shmuel Rozenblatt from http://www.tau.ac.il/lifesci/departments/biotech/members/rozenblatt/figures.html. (C) RSV (image credit: US Centers for Disease Control and Prevention). (D) Hepatitis B virus. Image provided by Drs. Kelly Dryden and Mark Yeager. (E) HIV type 1. Image provided by Drs. Ping Zhu and Kenneth Roux. See also [38]. Many schematic pictures of HIV in textbooks and on Web sites show more spikes per virion. Some of these figures were based on early electron micrographs of a mutant simian immunodeficiency virus containing a higher number of spikes per viral particle [72]. Others were based on the incorrect assumption that HIV exhibits icosahedral symmetry. (F) Distribution of nearest neighbor distances between HIV spikes derived from cryo-ET analyses of 40 HIV virions. Data were taken from [38]. Although some spike clustering was reported [38], the virions exhibited a large distribution of nearest neighbor distances between spikes (7–80 nm center to center). Low Spike Density and Spike Structure Impede Bivalent Binding by IgGs to HIV
What are the consequences of the low number of envelope spikes on HIV virions to antibody binding? Cryo-ET studies of HIV particles allowed an analysis of nearest neighbor distances between individual spikes, revealing that the low number of envelope spikes also translates to a low spike surface density. Thus, the majority of nearest neighbor distances fall outside of the range of the two Fabs of an IgG [38] (Figure 3F) as previously predicted [45], leaving a minority of HIV envelope spikes available for cross-linking by a bivalent antibody. Inter-spike cross-linking might still be possible if spikes were able to freely diffuse within the viral membrane, but analyses of cryo-ET data [38] and evidence for interactions between the cytoplasmic tail of gp41 and the matrix protein of HIV [46], [47] suggest that the arrangement of spikes on a virus particle is likely to be static over time periods relevant to neutralization.
Cross-linking within a spike trimer (intra-spike cross-linking) represents another way to achieve bivalent binding of an IgG. However, cryo-ET structures of HIV spike trimers bound to Fabs [39] and molecular modeling based on crystal structures [27], [48] suggest that bivalent binding within a single trimeric spike is also unlikely, at least for antibodies directed against gp41 or the CD4-binding site of gp120. Therefore, most anti-HIV antibodies probably bind only one epitope per spike. Anti-carbohydrate antibodies may be an interesting exception: since a single spike subunit contains many carbohydrate attachment sites, an anti-carbohydrate antibody can bind using both Fabs to adjacent carbohydrate sites within a spike monomer. Although antibodies that recognize viral carbohydrates are rare because viral carbohydrates are usually non-immunogenic, one broadly neutralizing antibody against HIV, IgG 2G12, presents its two Fabs as a single domain-swapped structure that recognizes a constellation of viral carbohydrates within gp120 [49] and appears to be unusually effective in conferring protection against infection in vivo [50]. A naturally occurring dimeric form of IgG 2G12 composed of four Fabs and two Fcs was recently found to exhibit a 100 - to 160-fold average increased molar neutralization potency over its monomeric form, with an increase of ≥500-fold against seven of the 21 strains tested [51], suggesting an enhanced ability to cross-link carbohydrate epitopes on a single envelope spike. Another exception might be represented by a new class of highly potent and broadly neutralizing anti-HIV antibodies, which include PG9 and PG16 [52]. The location of the proposed epitope for these antibodies, at the top of the envelope spike, might allow both Fabs of a single IgG to bind the same spike trimer.
How Avidity Can Enhance Antibody Potency: A Theoretical Examination
The affinity of a monomeric Fab, given by the equilibrium dissociation constant (KD), is equal to the dissociation rate constant (koff), divided by the association rate constant (kon). Overall, avidity manifests as an increase in the observed affinity of an IgG (a decrease in the KD) when binding two tethered antigens such that saturation of a surface can be achieved at lower concentrations as compared to a monovalent Fab. The affinity increase is mostly due to a reduction in the observed dissociation rate for the IgG such that binding to antigen becomes virtually irreversible over time scales relevant to the lifetime of a pathogen [23]. The effects of avidity on the affinity of an antibody can be modeled as a two-step reaction involving one antibody molecule and two epitopes tethered to the same surface (Figure 4A). After becoming tethered to its target through the first Fab, the small reaction volume of the second forward reaction serves to increase the second reaction rate [53], but this second binding step can only occur if the second binding site lies within the volume that is accessible to the free Fab arm. A corollary of the model for avidity is that the potency of a neutralizing IgG that can bind bivalently to two epitopes simultaneously on the same surface of a pathogen will primarily depend on the magnitude of kon. In contrast, the potency of a Fab or an IgG that binds monovalently will depend on the magnitudes of both the kon and the koff.
Fig. 4. Bivalent binding model and effect of dissociation rate on neutralization in bivalent and monovalent binding. 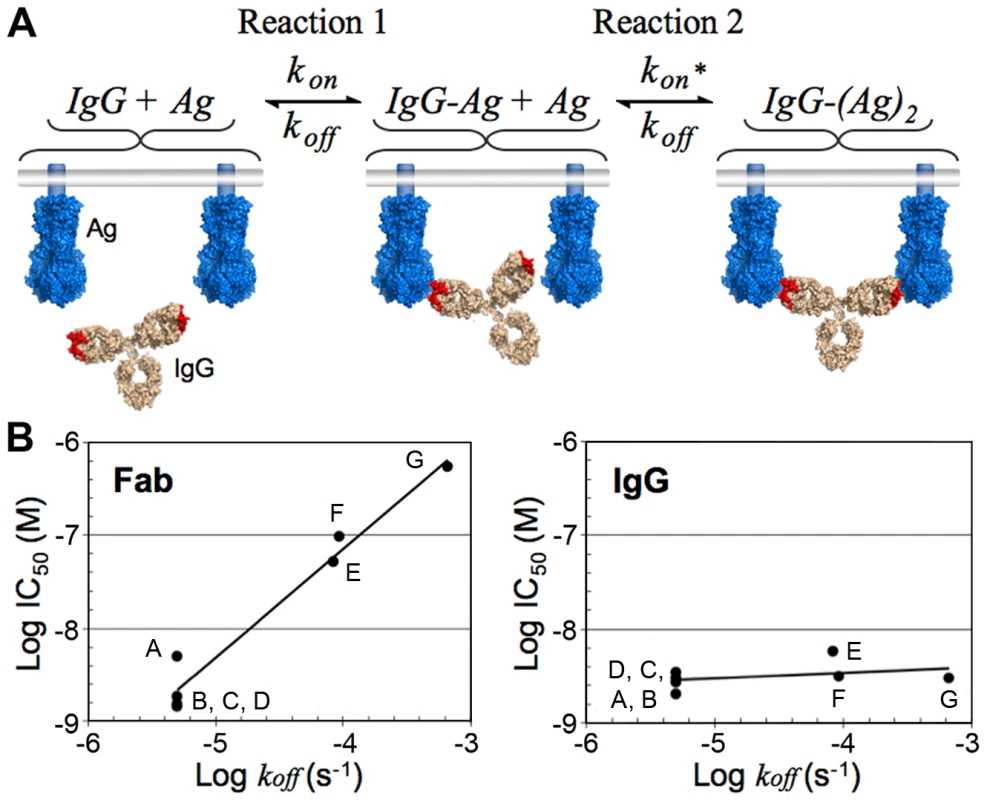
(A) Schematic of the step-wise bivalent binding model of an IgG to two envelope spikes (Ag, antigen) tethered to the same surface (kon, association rate constant; koff, dissociation rate constant; kon*, enhanced association rate constant resulting from the small reaction volume of Reaction 2). (B) Comparison of the effect of the Fab dissociation rate constant (koff) on the neutralization potency of Fab (left) and IgG (right) variants of palivizumab, a monoclonal antibody against RSV. Adapted from Table 1 in [32]. The names of the Fab/IgG pairs were changed to A–G for clarity. A, AFFFd; B, AFFYd; C, AFSFd; D, AFFGd; E, W100F; F, S32A; G, wild type (see [32] for an explanation of mutant nomenclature). Note that these results suggest that high affinity Fabs with slow dissociation rates (e.g., Fabs selected by techniques such as phage display) may not exhibit increased neutralization potencies, particularly against a densely packed virus, when converted to bivalent IgGs. The predicted insensitivity of an IgG to changes in koff under conditions permissible to bivalent binding was demonstrated for palivizumab, a monoclonal antibody directed against RSV [32], an enveloped virus with a high spike density (Figure 3C). A comparison of neutralization potencies of Fabs and their parental IgGs for a library of antibody variants derived from palivizumab demonstrated that mutations that decreased koff did not change the potency of the corresponding IgG but did increase the neutralization potency of the Fab [32] (Figure 4B). Furthermore, as predicted by avidity effects, mutations that increased kon served to increase the neutralization potencies of both the Fab and the IgG [32]. Thus, for cases in which efficiently cross-linking the surface of a virus is likely (e.g., RSV or influenza), an antibody can maintain a relatively unchanged neutralization potency even as the virus accumulates mutations that increase its koff. However, in cases in which efficient cross-linking is unlikely (e.g., HIV), the virus can escape antibody-mediated neutralization with mutations that weaken either rate constant, resulting in a virus that can more easily escape the humoral immune system during the course of an infection.
How Understanding Limitations to Avidity Can Inform the Design of Anti-HIV Vaccines and Therapies
The vertebrate immune system is remarkable in its ability to respond to and clear infections. Unfortunately, the relatively fixed distance between the two antigen-binding sites of an IgG and a reliance on avidity as a mechanism to achieve higher affinities makes it susceptible to evasion by pathogens that employ high mutation rates coupled with low antigen densities. When compared to the antigen densities present on the surfaces of viruses to which neutralizing antibody responses can be consistently raised (Figure 3A–3D), it seems an unlikely coincidence that HIV—a virus that is among the most adept at evading antibody-mediated neutralization—also stands out as having an unusually high mutation rate and an unusually low density of surface envelope spikes with apparently restricted mobility. Thus, it is tempting to speculate that whereas antibodies evolved to form a bivalent structure that enhances binding to pathogen surfaces through avidity effects, HIV evolved a low spike density designed to specifically thwart bivalent binding by antibodies.
In the initial immune response to a particular variant of HIV, it is likely that IgGs will exhibit sufficiently slow dissociation rates and high enough affinities to exert selective pressure even when binding monovalently, whether by neutralization of virus particles or by recruiting effector functions against infected cells. However, faced with a target to which bivalent binding is predominantly impossible, antibody potency will be susceptible to escape by a wider range of mutations: ones that serve to decrease the rate of association as well as ones that serve to increase the rate of dissociation. The immune system may respond with revisions to the antibody repertoire, but the rate at which new antibodies are made will be easily outpaced by the virus's rate of mutation.
Without the buffering effect against escape by mutation that avidity provides, it is likely that immunogens derived from HIV will need to be specifically tailored to focus the antibody response against only the most conserved epitopes—a key objective that has already been identified by many research groups [54], [55]. Viewed through the lens of avidity considerations, a general deficiency in bivalent binding will impose the additional requirement that broadly neutralizing antibodies still exhibit high affinities for their epitopes when binding monovalently. An alternative approach, as others have proposed [56], [57], may lie in eliciting anti-carbohydrate antibodies, as the high density of glycans on each gp120 monomer should enable efficient bivalent binding to individual envelope spikes. Thus, new immunogens designed to elicit antibodies capable of intra-spike cross-linking—either carbohydrate epitopes within or between spike monomers, or protein epitopes between spike monomers—may prove critical to the induction of a broadly cross-reactive neutralizing antibody response.
Using available crystallographic [9], [58]–[66] and electron microscopy data [35], [37]–[39], [67], [68], it might also be possible to engineer novel bivalent and multivalent antibody architectures that are capable of intra-spike cross-linking by increasing the reach between Fabs using insertions in the hinge region of an IgG that adopt extended conformations [19], although they would need to be administered via passive immunization or gene therapy. Carbohydrate-binding reagents specific for HIV (perhaps based on the anti-carbohydrate antibody 2G12) might be a logical starting point, as multimerization of 2G12 has been shown to significantly enhance its neutralization potency [30], [51]. These engineering approaches, as well as the design of immunogens able to elicit intra-spike cross-linking antibodies, could hold a significant advantage in that either approach would make the low spike density on HIV irrelevant to neutralization potency.
Zdroje
1. Rerks-NgarmS
PitisuttithumP
NitayaphanS
KaewkungwalJ
ChiuJ
2009 Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361 2209 2220
2. DolinR
2009 HIV vaccine trial results–an opening for further research. N Engl J Med 361 2279 2280
3. KoupRA
SafritJT
CaoY
AndrewsCA
McLeodG
1994 Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol 68 4650 4655
4. MiguelesSA
SabbaghianMS
ShupertWL
BettinottiMP
MarincolaFM
2000 HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci U S A 97 2709 2714
5. McMichaelAJ
Rowland-JonesSL
2001 Cellular immune responses to HIV. Nature 410 980 987
6. WeiX
DeckerJM
WangS
HuiH
KappesJC
2003 Antibody neutralization and escape by HIV-1. Nature 422 307 312
7. KwongPD
DoyleML
CasperDJ
CicalaC
LeavittSA
2002 HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420 678 682
8. LabrijnAF
PoignardP
RajaA
ZwickMB
DelgadoK
2003 Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J Virol 77 10557 10565
9. KwongPD
WyattR
RobinsonJ
SweetRW
SodroskiJ
1998 Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393 648 659
10. WyattR
DesjardinE
OlshevskyU
NixonC
BinleyJ
1997 Analysis of the interaction of the human immunodeficiency virus type 1 gp120 envelope glycoprotein with the gp41 transmembrane glycoprotein. J Virol 71 9722 9731
11. MooreJP
SodroskiJ
1996 Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol 70 1863 1872
12. StarcichBR
HahnBH
ShawGM
McNeelyPD
ModrowS
1986 Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell 45 637 648
13. XiaoX
ChenW
FengY
ZhuZ
PrabakaranP
2009 Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: implications for evasion of immune responses and design of vaccine immunogens. Biochem Biophys Res Commun 390 404 409
14. RossmannMG
1989 The canyon hypothesis. Hiding the host cell receptor attachment site on a viral surface from immune surveillance. J Biol Chem 264 14587 14590
15. KwongPD
WilsonIA
2009 HIV-1 and influenza antibodies: seeing antigens in new ways. Nat Immunol 10 573 578
16. SkehelJJ
StevensDJ
DanielsRS
DouglasAR
KnossowM
1984 A carbohydrate side chain on hemagglutinins of Hong Kong influenza viruses inhibits recognition by a monoclonal antibody. Proc Natl Acad Sci U S A 81 1779 1783
17. PlotkinSA
2008 Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis 47 401 409
18. Karlsson HedestamGB
FouchierRA
PhogatS
BurtonDR
SodroskiJ
2008 The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat Rev Microbiol 6 143 155
19. KleinJS
2009 Investigations in the design and characterization of HIV-1 neutralizing molecules. Pasadena California Institute of Technology. 166
20. JanewayCA
TraversP
WalportM
SchlomchikMJ
2005 Immunobiology. New York, , NY Garland Science Publishing
21. ScheidJF
MouquetH
FeldhahnN
SeamanMS
VelinzonK
2009 Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458 636 640
22. TomarasGD
YatesNL
LiuP
QinL
FoudaGG
2008 Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol 82 12449 12463
23. MattesMJ
2005 Binding parameters of antibodies: pseudo-affinity and other misconceptions. Cancer Immunol Immunother 54 513 516
24. SmithTJ
OlsonNH
ChengRH
ChaseES
BakerTS
1993 Structure of a human rhinovirus-bivalently bound antibody complex: implications for viral neutralization and antibody flexibility. Proc Natl Acad Sci U S A 90 7015 7018
25. IcenogleJ
ShiwenH
DukeG
GilbertS
RueckertR
1983 Neutralization of poliovirus by a monoclonal antibody: kinetics and stoichiometry. Virology 127 412 425
26. ZhangMY
XiaoX
SidorovIA
ChoudhryV
ChamF
2004 Identification and characterization of a new cross-reactive human immunodeficiency virus type 1-neutralizing human monoclonal antibody. J Virol 78 9233 9242
27. KleinJS
GnanapragasamPN
GalimidiRP
FoglesongCP
WestAPJr
2009 Examination of the contributions of size and avidity to the neutralization mechanisms of the anti-HIV antibodies b12 and 4E10. Proc Natl Acad Sci U S A 106 7385 7390
28. OfekG
TangM
SamborA
KatingerH
MascolaJR
2004 Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J Virol 78 10724 10737
29. KunertR
WolbankS
StieglerG
WeikR
KatingerH
2004 Characterization of molecular features, antigen-binding, and in vitro properties of IgG and IgM variants of 4E10, an anti-HIV type 1 neutralizing monoclonal antibody. AIDS Res Hum Retroviruses 20 755 762
30. WolbankS
KunertR
StieglerG
KatingerH
2003 Characterization of human class-switched polymeric (immunoglobulin M [IgM] and IgA) anti-human immunodeficiency virus type 1 antibodies 2F5 and 2G12. J Virol 77 4095 4103
31. SchofieldDJ
StephensonJR
DimmockNJ
1997 Variations in the neutralizing and haemagglutination-inhibiting activities of five influenza A virus-specific IgGs and their antibody fragments. J Gen Virol 78 (Pt 10) 2431 2439
32. WuH
PfarrDS
TangY
AnLL
PatelNK
2005 Ultra-potent antibodies against respiratory syncytial virus: effects of binding kinetics and binding valence on viral neutralization. J Mol Biol 350 126 144
33. EdwardsMJ
DimmockNJ
2001 Hemagglutinin 1-specific immunoglobulin G and Fab molecules mediate postattachment neutralization of influenza A virus by inhibition of an early fusion event. J Virol 75 10208 10218
34. PantaleoG
KoupRA
2004 Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat Med 10 806 810
35. YamaguchiM
DanevR
NishiyamaK
SugawaraK
NagayamaK
2008 Zernike phase contrast electron microscopy of ice-embedded influenza A virus. J Struct Biol 162 271 276
36. BachmannMF
ZinkernagelRM
1996 The influence of virus structure on antibody responses and virus serotype formation. Immunol Today 17 553 558
37. ZhuP
ChertovaE
BessJJr
LifsonJD
ArthurLO
2003 Electron tomography analysis of envelope glycoprotein trimers on HIV and simian immunodeficiency virus virions. Proc Natl Acad Sci U S A 100 15812 15817
38. ZhuP
LiuJ
BessJJr
ChertovaE
LifsonJD
2006 Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature 441 847 852
39. LiuJ
BartesaghiA
BorgniaMJ
SapiroG
SubramaniamS
2008 Molecular architecture of native HIV-1 gp120 trimers. Nature 455 109 113
40. ChertovaE
BessJWJr
CriseBJ
SowderIR
SchadenTM
2002 Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), Is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol 76 5315 5325
41. LayneSP
MergesMJ
DemboM
SpougeJL
ConleySR
1992 Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology 189 695 714
42. SougratR
BartesaghiA
LifsonJD
BennettAE
BessJW
2007 Electron tomography of the contact between T cells and SIV/HIV-1: implications for viral entry. PLoS Pathog 3 e63 doi:10.1371/journal.ppat.0030063
43. YangX
KurtevaS
RenX
LeeS
SodroskiJ
2005 Stoichiometry of envelope glycoprotein trimers in the entry of human immunodeficiency virus type 1. J Virol 79 12132 12147
44. MagnusC
RusertP
BonhoefferS
TrkolaA
RegoesRR
2009 Estimating the stoichiometry of human immunodeficiency virus entry. J Virol 83 1523 1531
45. McInerneyTL
McLainL
ArmstrongSJ
DimmockNJ
1997 A human IgG1 (b12) specific for the CD4 binding site of HIV-1 neutralizes by inhibiting the virus fusion entry process, but b12 Fab neutralizes by inhibiting a postfusion event. Virology 233 313 326
46. YuX
YuanX
MatsudaZ
LeeTH
EssexM
1992 The matrix protein of human immunodeficiency virus type 1 is required for incorporation of viral envelope protein into mature virions. J Virol 66 4966 4971
47. BhatiaAK
KaushikR
CampbellNA
PontowSE
RatnerL
2009 Mutation of critical serine residues in HIV-1 matrix result in an envelope incorporation defect which can be rescued by truncation of the gp41 cytoplasmic tail. Virology 384 233 241
48. LuftigMA
MattuM
Di GiovineP
GeleziunasR
HrinR
2006 Structural basis for HIV-1 neutralization by a gp41 fusion intermediate-directed antibody. Nat Struct Mol Biol 13 740 747
49. ScanlanCN
PantophletR
WormaldMR
Ollmann SaphireE
StanfieldR
2002 The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1–>2 mannose residues on the outer face of gp120. J Virol 76 7306 7321
50. HessellAJ
RakaszEG
PoignardP
HangartnerL
LanducciG
2009 Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog 5 e1000433 doi:10.1371/journal.ppat.1000433
51. WestAPJr
GalimidiRP
FoglesongCP
GnanapragasamPN
Huey-TubmanKE
2009 Design and expression of a dimeric form of human immunodeficiency virus type 1 antibody 2G12 with increased neutralization potency. J Virol 83 98 104
52. WalkerLM
PhogatSK
Chan-HuiPY
WagnerD
PhungP
2009 Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326 285 289
53. BonginiL
FanelliD
PiazzaF
De Los RiosP
SannerM
2007 A dynamical study of antibody-antigen encounter reactions. Phys Biol 4 172 180
54. StamatatosL
MorrisL
BurtonDR
MascolaJR
2009 Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med 15 866 870
55. PantophletR
WilsonIA
BurtonDR
2004 Improved design of an antigen with enhanced specificity for the broadly HIV-neutralizing antibody b12. Protein Eng Des Sel 17 749 758
56. BurtonDR
StanfieldRL
WilsonIA
2005 Antibody vs. HIV in a clash of evolutionary titans. Proc Natl Acad Sci U S A 102 14943 14948
57. PantophletR
BurtonDR
2006 GP120: target for neutralizing HIV-1 antibodies. Annu Rev Immunol 24 739 769
58. ChanDC
FassD
BergerJM
KimPS
1997 Core structure of gp41 from the HIV envelope glycoprotein. Cell 89 263 273
59. WeissenhornW
DessenA
HarrisonSC
SkehelJJ
WileyDC
1997 Atomic structure of the ectodomain from HIV-1 gp41. Nature 387 426 430
60. KwongPD
WyattR
MajeedS
RobinsonJ
SweetRW
2000 Structures of HIV-1 gp120 envelope glycoproteins from laboratory-adapted and primary isolates. Structure 8 1329 1339
61. ChenB
VoganEM
GongH
SkehelJJ
WileyDC
2005 Structure of an unliganded simian immunodeficiency virus gp120 core. Nature 433 834 841
62. HuangCC
TangM
ZhangMY
MajeedS
MontabanaE
2005 Structure of a V3-containing HIV-1 gp120 core. Science 310 1025 1028
63. HuangCC
LamSN
AcharyaP
TangM
XiangSH
2007 Structures of the CCR5 N terminus and of a tyrosine-sulfated antibody with HIV-1 gp120 and CD4. Science 317 1930 1934
64. ZhouT
XuL
DeyB
HessellAJ
Van RykD
2007 Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature 445 732 737
65. ChenL
KwonYD
ZhouT
WuX
O'DellS
2009 Structural basis of immune evasion at the site of CD4 attachment on HIV-1 gp120. Science 326 1123 1127
66. DiskinR
MarcovecchioPM
BjorkmanPJ
2010 Structure of a clade C HIV-1 gp120 bound to CD4 and CD4-induced antibody reveals anti-CD4 polyreactivity. Nat Struct Mol Biol. doi:10.1038/nsmb.1796
67. ZanettiG
BriggsJA
GrunewaldK
SattentauQJ
FullerSD
2006 Cryo-electron tomographic structure of an immunodeficiency virus envelope complex in situ. PLoS Pathog 2 e83 doi:10.1371/journal.ppat.0020083
68. BennettA
LiuJ
Van RykD
BlissD
ArthosJ
2007 Cryoelectron tomographic analysis of an HIV-neutralizing protein and its complex with native viral gp120. J Biol Chem 282 27754 27759
69. SaphireEO
ParrenPW
PantophletR
ZwickMB
MorrisGM
2001 Crystal structure of a neutralizing human IGG against HIV-1: a template for vaccine design. Science 293 1155 1159
70. CrooksET
JiangP
FrantiM
WongS
ZwickMB
2008 Relationship of HIV-1 and SIV envelope glycoprotein trimer occupation and neutralization. Virology 377 364 378
71. ZinglerK
LittmanDR
1993 Truncation of the cytoplasmic domain of the simian immunodeficiency virus envelope glycoprotein increases env incorporation into particles and fusogenicity and infectivity. J Virol 67 2824 2831
72. JohnstonPB
DubayJW
HunterE
1993 Truncations of the simian immunodeficiency virus transmembrane protein confer expanded virus host range by removing a block to virus entry into cells. J Virol 67 3077 3086
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Mouse Senile Amyloid Fibrils Deposited in Skeletal Muscle Exhibit Amyloidosis-Enhancing ActivityČlánek The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced MucositisČlánek Crystal Structure of HIV-1 gp41 Including Both Fusion Peptide and Membrane Proximal External RegionsČlánek Demonstration of Cross-Protective Vaccine Immunity against an Emerging Pathogenic Ebolavirus Species
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 5- Stillova choroba: vzácné a závažné systémové onemocnění
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Prof. Martin Matějovič: Sepse je umělý konstrukt, hledání univerzálního biomarkeru je zřejmě slepá cesta
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
-
Všechny články tohoto čísla
- Quorum Sensing Inhibition Selects for Virulence and Cooperation in
- The HMW1C Protein Is a Glycosyltransferase That Transfers Hexose Residues to Asparagine Sites in the HMW1 Adhesin
- Analysis of Virion Structural Components Reveals Vestiges of the Ancestral Ichnovirus Genome
- Mouse Senile Amyloid Fibrils Deposited in Skeletal Muscle Exhibit Amyloidosis-Enhancing Activity
- Global Migration Dynamics Underlie Evolution and Persistence of Human Influenza A (H3N2)
- The Type III Effectors NleE and NleB from Enteropathogenic and OspZ from Block Nuclear Translocation of NF-κB p65
- VEGF Promotes Malaria-Associated Acute Lung Injury in Mice
- Identification of a Mutant PfCRT-Mediated Chloroquine Tolerance Phenotype in
- The Early Stage of Bacterial Genome-Reductive Evolution in the Host
- Host-Detrimental Role of Esx-1-Mediated Inflammasome Activation in Mycobacterial Infection
- Elevation of Intact and Proteolytic Fragments of Acute Phase Proteins Constitutes the Earliest Systemic Antiviral Response in HIV-1 Infection
- The Pleiotropic CymR Regulator of Plays an Important Role in Virulence and Stress Response
- Alternative Sigma Factor σ Modulates Prophage Integration and Excision in
- Effect of Neuraminidase Inhibitor–Resistant Mutations on Pathogenicity of Clade 2.2 A/Turkey/15/06 (H5N1) Influenza Virus in Ferrets
- Massive APOBEC3 Editing of Hepatitis B Viral DNA in Cirrhosis
- NK Cells and γδ T Cells Mediate Resistance to Polyomavirus–Induced Tumors
- Is Genetically Diverse in Animals and Appears to Have Crossed the Host Barrier to Humans on (At Least) Two Occasions
- Adenylate Cyclase Toxin Mobilizes Its β Integrin Receptor into Lipid Rafts to Accomplish Translocation across Target Cell Membrane in Two Steps
- The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced Mucositis
- HIV-1 Transmitting Couples Have Similar Viral Load Set-Points in Rakai, Uganda
- Few and Far Between: How HIV May Be Evading Antibody Avidity
- Galectin-9/TIM-3 Interaction Regulates Virus-Specific Primary and Memory CD8 T Cell Response
- Perforin Expression Directly by HIV-Specific CD8 T-Cells Is a Correlate of HIV Elite Control
- The Set3/Hos2 Histone Deacetylase Complex Attenuates cAMP/PKA Signaling to Regulate Morphogenesis and Virulence of
- Infidelity of SARS-CoV Nsp14-Exonuclease Mutant Virus Replication Is Revealed by Complete Genome Sequencing
- Combining ChIP-chip and Expression Profiling to Model the MoCRZ1 Mediated Circuit for Ca/Calcineurin Signaling in the Rice Blast Fungus
- Internalin B Activates Junctional Endocytosis to Accelerate Intestinal Invasion
- A Complex Small RNA Repertoire Is Generated by a Plant/Fungal-Like Machinery and Effected by a Metazoan-Like Argonaute in the Single-Cell Human Parasite
- Opc Invasin Binds to the Sulphated Tyrosines of Activated Vitronectin to Attach to and Invade Human Brain Endothelial Cells
- Muc2 Protects against Lethal Infectious Colitis by Disassociating Pathogenic and Commensal Bacteria from the Colonic Mucosa
- PdeH, a High-Affinity cAMP Phosphodiesterase, Is a Key Regulator of Asexual and Pathogenic Differentiation in
- Isolates with Antimony-Resistant but Not -Sensitive Phenotype Inhibit Sodium Antimony Gluconate-Induced Dendritic Cell Activation
- The Microbiota and Allergies/Asthma
- Environmental Factors Determining the Epidemiology and Population Genetic Structure of the Group in the Field
- Prolonged Antigen Presentation Is Required for Optimal CD8+ T Cell Responses against Malaria Liver Stage Parasites
- Crystal Structure of HIV-1 gp41 Including Both Fusion Peptide and Membrane Proximal External Regions
- Susceptibility to Anthrax Lethal Toxin-Induced Rat Death Is Controlled by a Single Chromosome 10 Locus That Includes
- Demonstration of Cross-Protective Vaccine Immunity against an Emerging Pathogenic Ebolavirus Species
- Effective, Broad Spectrum Control of Virulent Bacterial Infections Using Cationic DNA Liposome Complexes Combined with Bacterial Antigens
- High Multiplicity Infection by HIV-1 in Men Who Have Sex with Men
- The -Specific Human Memory B Cell Compartment Expands Gradually with Repeated Malaria Infections
- EBV Promotes Human CD8 NKT Cell Development
- Persistent Growth of a Human Plasma-Derived Hepatitis C Virus Genotype 1b Isolate in Cell Culture
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Quorum Sensing Inhibition Selects for Virulence and Cooperation in
- The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced Mucositis
- Crystal Structure of HIV-1 gp41 Including Both Fusion Peptide and Membrane Proximal External Regions
- Susceptibility to Anthrax Lethal Toxin-Induced Rat Death Is Controlled by a Single Chromosome 10 Locus That Includes
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



