-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Enhancing Oral Vaccine Potency by Targeting Intestinal M Cells
The immune system in the gastrointestinal tract plays a crucial role in the control of infection, as it constitutes the first line of defense against mucosal pathogens. The attractive features of oral immunization have led to the exploration of a variety of oral delivery systems. However, none of these oral delivery systems have been applied to existing commercial vaccines. To overcome this, a new generation of oral vaccine delivery systems that target antigens to gut-associated lymphoid tissue is required. One promising approach is to exploit the potential of microfold (M) cells by mimicking the entry of pathogens into these cells. Targeting specific receptors on the apical surface of M cells might enhance the entry of antigens, initiating the immune response and consequently leading to protection against mucosal pathogens. In this article, we briefly review the challenges associated with current oral vaccine delivery systems and discuss strategies that might potentially target mouse and human intestinal M cells.
Published in the journal: . PLoS Pathog 6(11): e32767. doi:10.1371/journal.ppat.1001147
Category: Review
doi: https://doi.org/10.1371/journal.ppat.1001147Summary
The immune system in the gastrointestinal tract plays a crucial role in the control of infection, as it constitutes the first line of defense against mucosal pathogens. The attractive features of oral immunization have led to the exploration of a variety of oral delivery systems. However, none of these oral delivery systems have been applied to existing commercial vaccines. To overcome this, a new generation of oral vaccine delivery systems that target antigens to gut-associated lymphoid tissue is required. One promising approach is to exploit the potential of microfold (M) cells by mimicking the entry of pathogens into these cells. Targeting specific receptors on the apical surface of M cells might enhance the entry of antigens, initiating the immune response and consequently leading to protection against mucosal pathogens. In this article, we briefly review the challenges associated with current oral vaccine delivery systems and discuss strategies that might potentially target mouse and human intestinal M cells.
Advantages and Challenges Surrounding Mucosal Vaccines
The mucosal immune system is a critical line of defense against infectious diseases, as the majority of infections are initiated at mucosal sites [1]–[3]. Therefore, the induction of specific immune responses at mucosal sites may be able to control infections at their point of entry into the body. Over the past few decades, several candidate vaccines have been designed and tested by various mucosal routes in pre-clinical or clinical trials. Although the mucosal immune system comprises several anatomically remote and functionally distinct compartments, it is firmly established that the oral ingestion or intranasal administration of antigens induces humoral and cellular responses not only at the site of antigen exposure but also in other mucosal compartments [4], [5]. This is due to the dissemination of antigen-sensitized precursor B and T lymphocytes from the inductive (e.g., intestinal Peyer's patches) to the effector sites such as the above mentioned glands. However, not all inductive sites display comparable ability to induce equal responses at all effector sites. Despite several advantages, as compared to systemic injections, the delivery of vaccines by mucosal routes, particularly through the genitals or rectum, has not been shown to be very practical in human trials [6]–[8]. In addition, it is hard to administer a mucosal vaccine through the genital tract, as the immunological features of the female reproductive tract, in particular, alter dramatically in response to hormonal fluctuations during the menstrual cycle [9]–[11]. In addition, both male and female genital tracts lack inductive mucosal sites analogous to intestinal Peyer's patches [12]. Furthermore, rectal vaccinations have been shown to induce only modest and localized immune responses, and are not very effective in larger animals and humans [13], [14]. The pitfalls in quantifying effector cells in rectal tissues, combined with the intricacies of the inoculation route, are some other major challenges associated with rectal immunization. Therefore, in order to advance a mucosal vaccine for human use, the routes of administration appear to be limited to oral and nasal administration.
Nasally delivered vaccines are easy to administer and have been shown to be more promising for inducing both mucosal as well as systemic immune responses [15]–[17]. It should be stressed that the immune system of the upper respiratory tract (nasal cavity, oropharynx, trachea, and large bronchi) and lower respiratory tract (bronchioli and alveoli) display marked differences with respect to the dominance of Ig isotypes and induction of humoral immune responses. While the induction of dominant IgA responses in the upper respiratory tract is of importance in the protection at this locale, the lower respiratory tract is the domain of antibodies, of the IgG isotype of circulatory origin. Consequently, systemic immunizations with, for example, pneumococcal polysaccharide vaccines, induce protective immune responses. A nasal spray influenza vaccine (FluMist) containing live attenuated influenza has been approved for human use since 2003 [18], [19]. In an HIV study, macaques that were intranasally vaccinated with SHIV-capturing nanospheres demonstrated elevated levels of IgA and IgG antibodies [20]. Additionally, these vaccinated macaques showed a higher frequency of CD4 +T cells and lower viral loads compared to control macaques after a SHIV challenge. However, two human clinical trials involving nasal administration of HIV-1-derived antigens were recently terminated due to safety concerns. The potential for side effects such as Bell's palsy and damage to the olfactory nerves and the nasal epithelium have been cause for concern; however, these side effects could have occurred due to the use of highly reactogenic adjuvants and not because of the route of administration [21]–[23]. The possibility of such side effects, and the reason that the gastrointestinal (GI) tract is the first line of defense against mucosal pathogens, has led many scientists to pursue oral vaccination. The advantages and disadvantages of each route of mucosal immunization are summarized in Table 1. In this article, the advantages, challenges, and pitfalls with this route of vaccination are addressed. We also briefly review current options for oral delivery systems and approaches that have been explored to improve the uptake of potential vaccines. Oral vaccines have the ability to induce both mucosal and systemic immune responses and are safer, easier to administer, and do not require sterile needles and syringes [24]–[27]. Therefore, oral vaccines could more easily meet the needs of affected people in developing countries, where access to trained medical professionals is frequently limited. Although oral vaccines have several attractive features, the limited numbers of approved oral vaccines attest to the challenges associated with mucosal vaccine design. Studies involving oral vaccine use have been limited due to several challenges, such as difficulties in the collection and processing of external secretions, a lack of standardized assays, the induction of tolerance, the stability of antigens in the harsh conditions of the GI tract, and the antigen–microbial interactions that are continuously occurring in the large intestine [28], [29]. It is for these reasons that only a limited number of oral vaccines are currently licensed, compared to many parenteral vaccines.
Tab. 1. Advantages and Disadvantages of Each Route of Mucosal Immunization Is Summarized. 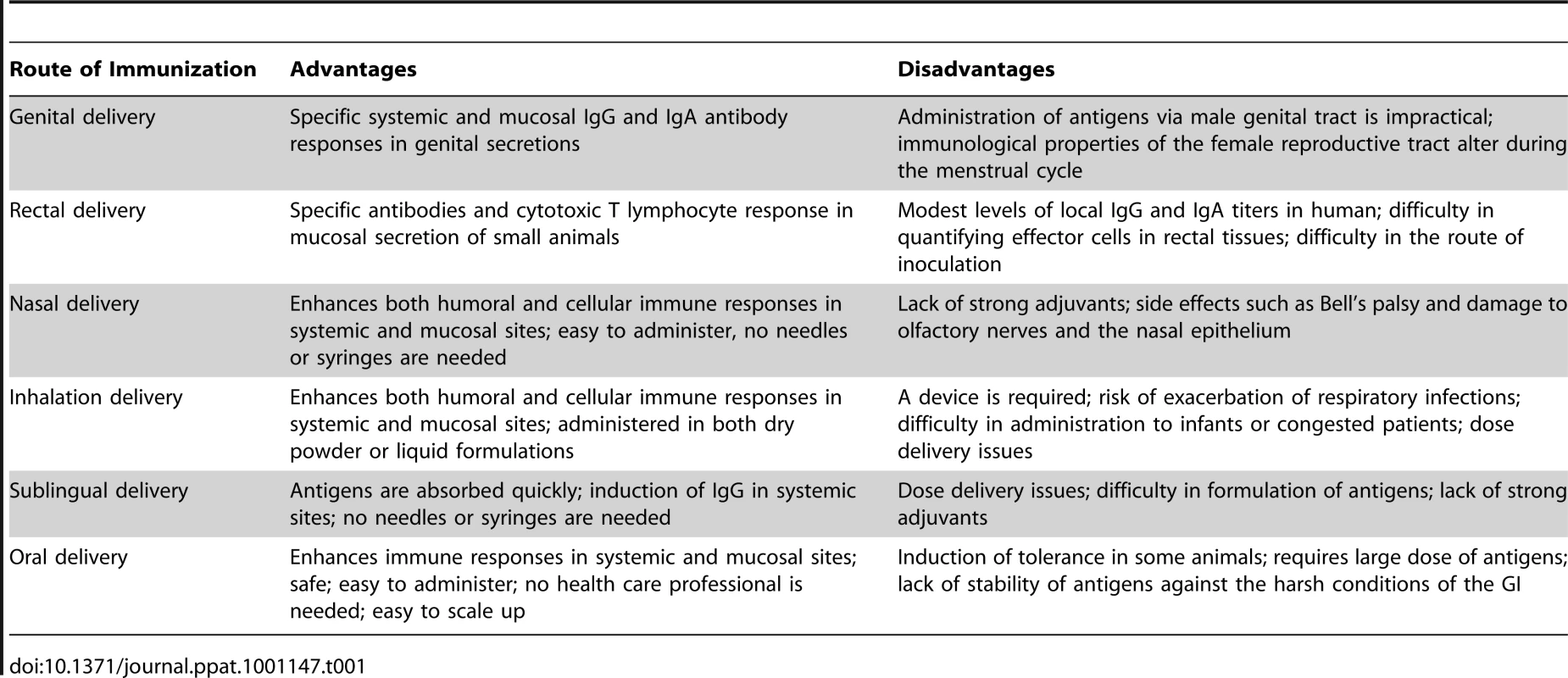
Oral Vaccine Delivery Systems
Recombinant or attenuated strains of various bacteria such as Salmonella, Escherichia coli, Listeria, Shigella, and Lactobacilli have been used as a vectors to deliver antigens into the gut-associated lymphoid tissue (GALT) [30]–[35]. While some interesting results have been reported for these oral delivery systems, immune responses against the vectors eventually predominated over time [36], [37]. In addition, glycosylated antigens cannot be produced in bacteria [38]. Furthermore, over 1014 microorganisms of >20,000 species reside in the large intestine [43]. Such a large competing population would greatly diminish the chances of colonization and subsequent induction of a vigorous immune response through such vector microorganisms. Oral delivery of live attenuated recombinant viruses such as adenoviruses (Ad), poxviruses, influenza, herpes viruses, and polioviruses encoding specific antigens has been also tested in several oral vaccine studies. While these viral vectors showed promising results, pre-existing immunity to these viruses may prevent their ability to deliver desired antigens.
Oral delivery of DNA vaccines encoding various antigens has also been evaluated in various animal studies [1], [2], [39]–[41]. DNA vaccines contain unmethylated CpG motifs with binding activity to TLR9 receptors. This characteristic assists in activating a variety of cells including dendritic cells (DCs), macrophages, monocytes, and splenocytes [42]. The TLR9 signaling pathway leads to IL-1β and INF-γ secretion, polarizing the immune response to a Th1 type. One of the pitfalls associated with DNA vaccines is the low uptake of DNA from the intestinal tract, which consequently limits B and T cell immune responses [43].
Over the past few years, specific T and B cell epitopes have been characterized in tumor and viral antigens. Synthesis of peptide epitopes for use as a vaccine requires an understanding of T and B cell immunodominant epitopes in the protein structure, and their interaction with major histocompatibility complexes (MHCs) or human leukocyte antigen (HLA) complexes [44]–[48]. The design and development of immunodominant multivalent epitopes representing diverse HLA types is an attractive strategy against hypervariable viruses such as HIV-1 and hepatitis C virus (HCV). One of the pitfalls with this approach is that peptide vaccines are not immunogenic alone, and thus require carriers and potent adjuvants to enhance their immunogenicity. The use of lipidated peptide immunogens is one of several strategies currently being pursued for the improvement of peptide immunogenicity [49]–[51]. Previous studies have demonstrated that the presence of lipid moieties on peptides prolongs the duration of antigen presentation, enhances cytosolic uptake of peptide immunogens, activates innate immunity due to a TLR2 agonist effect, and differentiates non-activated B cells into immunoglobulin-secreting plasma cells [52]–[55]. Although no commercialized peptide vaccine is yet available, this approach has shown promising results in animal studies [56]. Oral delivery of peptide vaccines has been evaluated in pre-clinical and clinical trials. In a phase I study, 33 HIV-seronegative volunteers were primed orally three times with a V3 peptide derived from HIV-1 isolate MN, followed by a systemic boosting [57]. While no broad humoral or cellular immune responses were detected, the results could prove helpful in the further development of orally administered peptide vaccines.
Plant-based oral vaccines are another delivery system that has been tested in recent years [58]–[61]. Seed crops such as rice, maize, and soybean appear to be suitable expression and delivery systems that offer several advantages, such as resistance to intestinal enzymes, rapid scale-up of exogenous antigens, low-cost production, and a decreased risk of contamination by human pathogens [62], [63]. In a mouse study, MucoRice-expressed cholera toxin subunit B (CTB) was administered orally to animals, and specific immune responses and neutralizing activity in both systemic and mucosal compartments were detected [64]. Interestingly, immunized animals with MucoRice-CTB demonstrated protection from an oral challenge with cholera toxin compared to control animals. In a similar study conducted in a non-human primate model, cynomolgus macaques received orally administered MucoRice-CTB. Animals were found to have CT-specific, neutralizing antibodies, and high levels of systemic IgG and intestinal IgA antibodies [65].
Over the past few years, several oral vaccine delivery vehicles such as liposomes, dendrimers, multiple emulsions, immune stimulating complexes (ISCOMs), biodegradable polymers such as poly (lactide-co-glycolide acid), and dendrimers have also been identified [66]–[70]. Antigens, adjuvants, and targeting molecules could be incorporated individually or in combination into these microparticles. These vehicles may thus act as immunostimulants while preventing the degradation of immunogens by enzymes in the GI tract. These particulate formulations might also interact with microfold (M) cells and release immunogens slowly, consequently promoting phagocytosis. Some microparticle studies have shown that the addition of polymers such as chitosan might increase the interaction of antigens with the intestinal mucosal surface [66], [67]. The efficacy of these microparticles has been tested in several animal studies and in a limited number of clinical trials. In a human trial, five volunteers were orally immunized with a surface Enterotoxigenic Escherichia coli (ETEC) polymeric protein (CS6) associated with a biodegradable polymer, poly-lactide-co-glycolide (PLG) [68]. Oral administration of these microparticles was safe, and four out of five volunteers showed IgA responses and a 3.5-fold increase in the levels of serum IgG antibody responses.
In a study by Frey et al. [69], oral administration of CTB was tested as a model for enhancing antigen uptake by intestinal epithelial cells. CTB was chosen as it promotes immune responses when co-administered orally, and its receptor (ganglioside GM1) is present on all intestinal epithelial cell surfaces. In vivo results in rabbits showed that soluble CTB-FITC (diameter of 6.4 nm) was able to bind to apical membranes of both enterocytes and M cells. Whereas CTB coupled to colloidal gold (diameter of 28.8 nm) bound only to M cells and not enterocytes, CTB-coated microparticles (diameter of 1.13 µm) failed to bind to either rabbit enterocytes or rabbit M cells. In a study by Mann et al. [70], two different sizes of a liposome-entrapped influenza antigen were delivered orally in a mouse model. The group of mice that was orally immunized with larger liposomes (60–350 nm and 400–2,500 nm) showed a greater Th1 bias, serum IgG2a production, and antigen-induced splenocyte IFN-γ production, compared to mice having received liposomes 10–100 nm in size. While this study also showed that microparticle size is an important factor associated with particle uptake, the size of the microparticles was quite different from a previous study.
However, sizing is not the only issue with these microparticles. A variety of additional parameters, including the ratio and quantity of chemical components, the amount of encapsulated antigen, hydrophobicity, the ionic surface charge, the type of associated adjuvants, and the dose of administration are also crucial, and should be optimized. In this context, the association of M cell–targeting ligands on the surface of the delivery vehicles might also enhance the binding specificity to intestinal Peyer's patches. In the next section, we briefly describe the M cell surface markers that could be considered in a strategy to enhance capture and uptake of orally administered vaccines.
Targeting the Apical Surface of M Cells
M cells are specialized epithelial cells that predominantly reside in the follicle-associated epithelium (FAE) overlying Peyer's patches. M cells also reside in other sections of the intestinal tract such as the colon and rectum [71]–[74]. M cells are identifiable by their flattened apical surfaces, fewer numbers of cytoplasmic lysosomes, greater numbers of mitochondria, and the absence of glycocalyx covering their surfaces. It also appears that mouse M cells express particular surface markers, compared to enterocytes, such as β1 integrin or α-L-fucose-specific (L-fucose) lectin [75]. In contrast to enterocytes, M cells take up antigens or microorganisms from the intestinal lumen (Figure 1) by phago-, endo-, or pinocytosis and transcytosis, and deliver them to the underlying immune system of the mucosae. This phenomenon also occurs by other mechanisms, for instance in intestinal DCs; however, this will not be discussed here. M cells are not limited to the GALTs, and are also present in other mucosal tissues such as nasopharyngeal-associated lymphoid tissue (NALT) and bronchus-associated lymphoid tissues (BALT) and tonsils [76], [77]. It has been shown that M cells in NALT are a major site of virus entry as well as vaccine delivery; however, limited studies have been reported with regards to the roles of NALT and BALT in the uptake and transport of vaccine-delivered antigens.
Fig. 1. Schematic diagram of intestinal epithelium showing M cells, Peyer's patches, intestinal epithelial cells, and pathway of Ag transport. 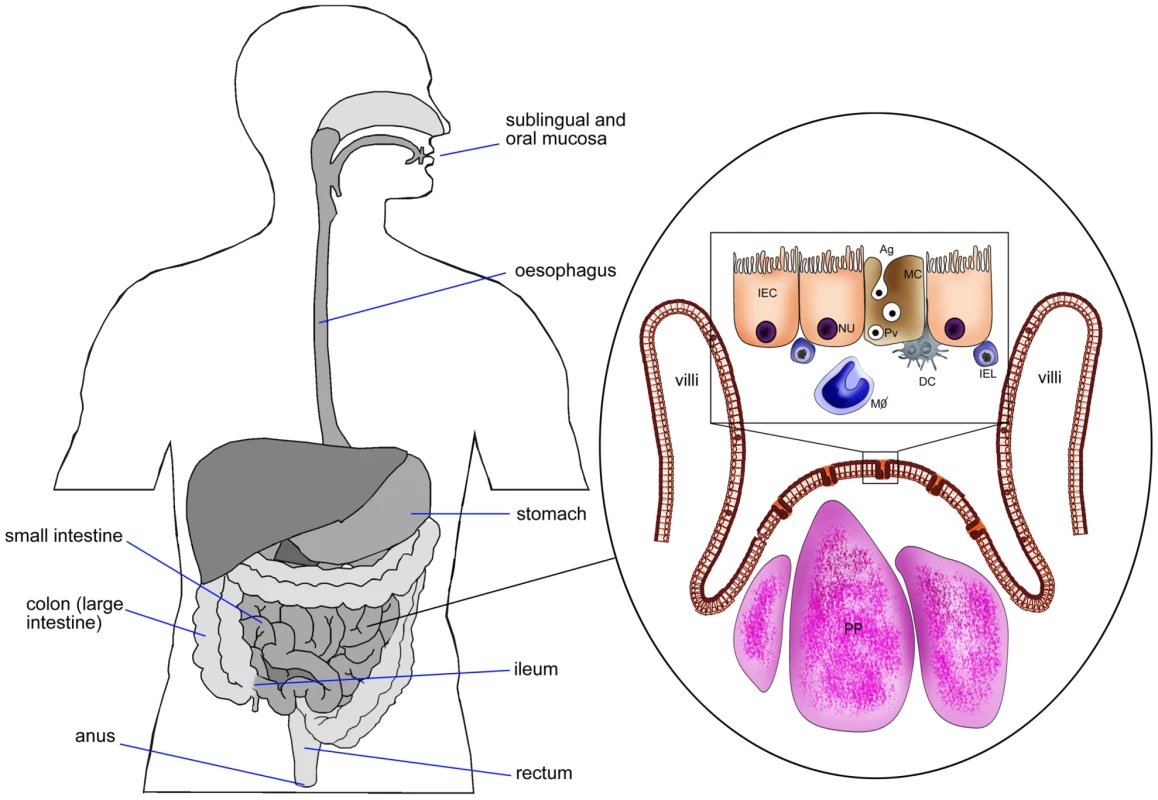
DC, dendritic cells; IEC, intestinal epithelial cell (NU, nucleus); MC, M cell; IEL, intra epithelial lymphocytes; PP, Peyer's patches; MΦ, macrophages; Pv, particulate Ag in pinocytic vesicle of M cell. The ability of M cells in Peyer's patches to take up and transcytose diverse numbers of microorganisms to antigen-presenting cells (APCs) have made M cells an ideal target for vaccine delivery to the mucosal immune system [78]–[80]. It is estimated that only 1 out of 10 million epithelial cells in the intestinal tract is an M cell (approximately 5% in humans and 10% in mice) [81]. Due to these low numbers of M cells, several approaches have been attempted to enhance M cell targeting. It has been indicated that M cell numbers in Peyer's patches are increased after exposure to Streptococcus pneumonia R36a [82]. However, these increased numbers of M cells may uptake all antigens in the intestinal epithelium and not just the antigens of interest, consequently increasing the probability of inducing food allergies and inflammatory diseases. Therefore, it might be more reasonable to target the existing M cells in Peyer's patches than to try to amplify their numbers.
Targeting specific receptors on the apical surface of M cells may have the ability to specifically increase the uptake and presentation of antigens, consequently initiating the immune response and inducing protection against infectious challenge. To date, only limited numbers of M cell receptors and their ligands have been identified, and most of these receptors are not only expressed in M cells but in neighboring enterocytes as well. Some important pathogen recognition receptors (PRRs), such as toll-like receptor-4 (TLR-4), platelet-activating factor receptor (PAFR), and α5β1 integrin, are expressed on the surface of human and mouse M cells [83]–[85]. These innate immune system molecules interact with pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide, lipotechoic acid, peptidoglycan, and bacterial flagellin. This interaction is crucial for the translocation of bacteria across the lumen. Consequently, targeting PRRs might be a suitable strategy for enhancing the uptake of orally administered vaccines by M cells. This interaction activates several signaling pathways that may play important roles in M cell functions. For instance, M cells take up many enteropathogenic microorganisms, such as Yersinia spp., via the α5β1 integrin, and inhibition of this adhesion molecule significantly inhibits transcytosis of M cells [86]–[88]. While PRRs are also expressed on neighboring enterocytes (a challenge in targeting only M cells), the expression patterns of these receptors are varied. For instance, α5β1 integrin is dispersed on the lateral and basolateral surfaces of enterocytes, while in M cells, α5β1 is distributed only on the apical surface.
Lectin-binding studies in experimental animals have shown that M cells express on their surface a particular glycosylation pattern [89], [90]. Several studies showed that Ulex europaeus agglutinin-1 (UEA-1), a lectin specific for α-l-fucose residues, selectively binds to M cells in murine Peyer's patches [91]–[94]. In a study by Manocha et al. [94], the UEA-1 coated on the surface of microparticles encoding HIV genes had the capability to bind to the apical surface of M cells. In another study, by Chionh et al. [95], oral vaccination in a mouse model with killed whole Helicobacter pylori and UEA-1 or Campylobacter jejuni and UEA-1 induced protective responses against live challenge. However, M cell glycosylation patterns are not common to all species, and it remains to be seen whether it can be used to effectively target human M cells [96]. Human M cells have proven to be largely anonymous, as it has been difficult to isolate enough of such cells for further characterization and functional evaluation. Therefore, the specific receptor requirements for human M cells and how to specifically target these receptors remains a challenge. In recent years, a few in vitro human M cell models have been established [97], [98]. One of the most common M cell–like models is comprised of co-cultures of human colon carcinoma cells (Caco-2) along with human lymphoblastoid B cell lines (Raji B cells) [99], [100]. This in vitro model has been used to study the morphology and expression of M cell surface markers and antigen absorption, and to screen oral drug/vaccine delivery systems, as it closely imitates human M cells. While these M cell–like models have been used to attempt to further understand human M cells, one of the concerns of this model pertains to its over-simplification of in vivo events, as well as the lack of signaling factors from other immune cells such as T cells that are required for the formation and optimal function of M cells.
Microarray and three-dimensional imaging of specific molecules associated with M cells has revealed that a surface marker called glycoprotein 2 (GP2) is expressed on both human and mouse M cells [101], [102]. It appears that GP2 plays an important role in molecular mechanisms responsible for antigen uptake by M cells. GP2 serves as a transcytotic apical receptor on the surface of M cells that specifically binds to type I pili on bacterial outer membranes (FimH) [101]. Elimination of GP2 reduced the entry and uptake of bacteria into Peyer's patches and decreased T cell proliferative and antibody responses. Altogether, these results suggest that the GP2 protein might be a promising vaccine target for immunizing against infectious diseases. Several studies have shown that FimH adhesion–based vaccines are able to prevent infection by impeding colonization, enhancing humoral immune responses, and blocking bacterial attachment [103]–[105]. It would be exciting to determine if FimH could direct other antigens to M cells as well.
In an interesting study by Giannasca et al. [106], Peyer's patches were biopsied from volunteers with blood groups type O (two individuals) and type A (one individual). The binding and cellular localizations of 31 lectins and ten anticarbohydrate monoclonal antibodies from biopsy samples were performed by histochemistry and compared to the nearby enterocytes on Peyer's patches. Lectin and antibody results revealed a higher expression of carbohydrates on enterocytes than M cells. Some lectins and antibodies such as OPA and anti-Lewis A also reacted with both M cells and enterocytes. Interestingly, only one (anti-sialyl Lewis A) out of the 41 tested lectins or antibodies largely reacted with human M cells (∼80%) and bound only weakly to the FAE enterocytes (∼20%). While a larger number of human tissue specimens are required to confirm this oligosaccharide repertoire, an anti-sialyl Lewis A–mediated vaccine delivery system might be appropriate approach to enable M cell–targeted mucosal vaccines in humans.
In a study by Misumi et al. [107], the capability of tetragalloyl-D-lysine dendrimer (TGDK) to target M cells was examined in an in vitro human M–like cell culture and a rhesus macaque animal model. The results indicated that TGDK specifically bound to a human intestinal M–cell like model under in vitro conditions and was delivered from the M cell surface to the basolateral area. To examine the in vivo effect of TGDK on M cell targeting, rhesus macaques were orally administered with enteric coated capsules containing TGDK-conjugated multiantigens at weeks 0, 2, and 6. ELISA from feces samples of immunized macaques indicated a high level of IgA antibody responses. Conversely, the control macaques did not induce specific IgA in fecal samples. Furthermore, the immunized macaques with TGDK-conjugated multiantigens also showed neutralizing activity against SIV infection. These results concluded that TGDK transports from the lumen into intestinal M cells, and can consequently be considered for use in mucosal vaccine delivery in humans and non-human primates.
Mucosal Immune Responses and Mucosal Tolerance
Repeated oral administration of large doses of antigen in animal models result in decreased or abrogated T cell–mediated responses to a subsequent systemic immunization with the same antigen [108]. This phenomenon prompts a question concerning the possible induction of mucosal tolerance by mucosally delivered vaccines. Importantly, for vaccine efficacy the dominant target of oral tolerance is the T and not the B cell compartment. As a matter of fact, initial mucosal administration of antigens by the oral or nasal routes primes for B cell responses in parallel with diminished T cell responses in humans as well as in animals [109]. Thus, vaccines whose protective effect is dependent on the induction of antibodies (which is the target of all currently used vaccines in humans) are not likely to diminish their efficacy by mucosal administration of antigens. Furthermore, pre-existing immune responses induced by systemic immunization cannot be attenuated or suppressed by subsequent mucosal administration of the same antigen [109]. However, initial mucosal immunization of immunologically naïve subjects (e.g., with HIV-1 vaccines) might have the undesirable effect of diminishing cell-mediated responses, including cytotoxic T cell–dependent immunity. Thus, the temporal sequence of immunization with initial systemic priming and mucosal boosting as well as the use of certain adjuvants is likely to prevent the induction of mucosal tolerance.
Concluding Remarks
Over the past few decades, oral immunization has been extensively studied due to its many attractive features. The immunological potential, absorption, or limitation in the uptake of antigens, as well as the characteristic distribution of functional cell types in the GI tract, have made it a vital target in the development of oral vaccines. The phenomenon of tolerance is a crucial challenge to overcome in the development of effective oral vaccines. Experimental animal studies have indicated that oral administration of antigens targets the systemic T cell compartment, diminishes cell-mediated immune responses, and induces tolerance. This phenomenon might lead to the induction of cytokines such as TGF-β and IL-10, and consequently enhance antigen-specific antibody responses such as IgA and IgG. While the humoral immune response is critical in the control of some mucosal pathogens, its effect might be questionable on other mucosal pathogens such as HIV and HCV where cell-mediated immune responses may play a larger role. Opponents to this tolerance hypothesis, including the authors of this article, believe that tolerance is not an issue in humans, as it occurs through a completely different mechanism. Furthermore, some clinical studies have showed that a combination of oral priming and systemic boosting might activate both humoral and cellular arms of the immune system. On the other hand, we think that the absence of a potent oral vaccine might be due to other challenges, including antigen degradation by proteolytic enzymes, the low dose of antigen absorbed, a lack of potent mucosal adjuvants, and not actively directing antigens to M cells. To overcome these issues, further work regarding oral vehicle delivery systems that protect antigens and specifically target M cells is required. Targeting M cells by mimicking the entry of mucosal pathogens such as E. coli, Salmonella, and Yersinia may reflect the in vivo binding specificity required by orally administered antigens. Regarding this aspect, a number of studies showed that these pathogens bind to specific lectins expressed on the apical surface of M cells. The binding of orally administered vaccines to M cell lectins was further studied in murine models and indicated that α-L-fucose-lectin (UEA-1) is able to bind specifically to M cells and, to a lesser degree, enterocytes. However, the characterization of murine M cells by this lectin-binding pattern did not reflect the glycosylation patterns present on human M cells. Unfortunately, human M cell features, function, and differentiation from neighboring enterocytes are not well understood.
Based on previous studies, by using tetragalloyl-D-lysine dendrimers, a monoclonal antibody targeting GP2, or using a monoclonal antibody targeting sialyl Lewis A, it might be possible to more specifically direct oral delivery systems to human M cells. However, as these molecules are also expressed on neighboring enterocytes (albeit at lower levels), it will likely be difficult to devise an ideal oral delivery system for targeting human M cells. The understanding of human M cell function, identification of more specific apical surface molecules, and the improvement of intestinal M cell–like models are crucial for the design and further development of M cell–targeted vaccines.
Zdroje
1. McGheeJR
MesteckyJ
DertzbaughMT
EldridgeJH
HirasawaM
1992
The mucosal immune system: from fundamental concepts to vaccine development.
Vaccine
10
75
88
2. McGheeJR
KiyonoH
1992
Mucosal immunity to vaccines: current concepts for vaccine development and immune response analysis.
Adv Exp Med Biol
327
3
12
3. Xu-AmanoJ
BeagleyKW
MegaJ
FujihashiK
KiyonoH
1992
Induction of T helper cells and cytokines for mucosal IgA responses.
Adv Exp Med Biol
327
107
117
4. MesteckyJ
1987
The common mucosal immune system and current strategies for induction of immune responses in external secretions.
J Clin Immunol
7
265
276
5. AziziA
GhunaimH
Diaz-MitomaF
MesteckyJ
2010
Mucosal HIV vaccines: a holy grail or a dud?
Vaccine
28
4015
4026
6. ParrEL
ParrMB
1990
A comparison of antibody titres in mouse uterine fluid after immunization by several routes, and the effect of the uterus on antibody titres in vaginal fluid.
J Reprod Fertil
89
619
625
7. HanebergB
KendallD
AmerongenHM
ApterFM
KraehenbuhlJP
1994
Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces.
Infect Immun
62
15
23
8. KozlowskiPA
Cu-UvinS
NeutraMR
FlaniganTP
1997
Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women.
Infect Immun
65
1387
1394
9. SentmanCL
MeadowsSK
WiraCR
ErikssonM
2004
Recruitment of uterine NK cells: induction of CXC chemokine ligands 10 and 11 in human endometrium by estradiol and progesterone.
J Immunol
173
6760
6766
10. WiraCR
RossollRM
2003
Oestradiol regulation of antigen presentation by uterine stromal cells: role of transforming growth factor-beta production by epithelial cells in mediating antigen-presenting cell function.
Immunology
109
398
406
11. LuFX
MaZ
RourkeT
SrinivasanS
McChesneyM
1999
Immunoglobulin concentrations and antigen-specific antibody levels in cervicovaginal lavages of rhesus macaques are influenced by the stage of the menstrual cycle.
Infect Immun
67
6321
6328
12. MesteckyJ
MoldoveanuZ
RussellMW
2005
Immunologic uniqueness of the genital tract: challenge for vaccine development.
Am J Reprod Immunol
53
208
214
13. HolmgrenJ
CzerkinskyC
2005
Mucosal immunity and vaccines.
Nat Med
11
S45
S53
14. LagranderieM
WinterN
BalazucAM
GicquelB
GheorghiuM
1998
A cocktail of Mycobacterium bovis BCG recombinants expressing the SIV Nef, Env, and Gag antigens induces antibody and cytotoxic responses in mice vaccinated by different mucosal routes.
AIDS Res Hum Retroviruses
14
1625
1633
15. BergquistC
JohanssonEL
LagergardT
HolmgrenJ
RudinA
1997
Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina.
Infect Immun
65
2676
2684
16. DurraniZ
McInerneyTL
McLainL
JonesT
BellabyT
1998
Intranasal immunization with a plant virus expressing a peptide from HIV-1 gp41 stimulates better mucosal and systemic HIV-1-specific IgA and IgG than oral immunization.
J Immunol Methods
220
93
103
17. HirabayashiY
KurataH
FunatoH
NagamineT
AizawaC
1990
Comparison of intranasal inoculation of influenza HA vaccine combined with cholera toxin B subunit with oral or parenteral vaccination.
Vaccine
8
243
248
18. NarayanKM
DelRC
2010
Comparative efficacy of influenza vaccines.
N Engl J Med
362
179
180
19. FioreAE
BridgesCB
CoxNJ
2009
Seasonal influenza vaccines.
Curr Top Microbiol Immunol
333
43
82
20. MiyakeA
AkagiT
EnoseY
UenoM
KawamuraM
2004
Induction of HIV-specific antibody response and protection against vaginal SHIV transmission by intranasal immunization with inactivated SHIV-capturing nanospheres in macaques.
J Med Virol
73
368
377
21. StoweJ
AndrewsN
WiseL
MillerE
2006
Bell's palsy and parenteral inactivated influenza vaccine.
Hum Vaccin
2
110
112
22. LewisDJ
HuoZ
BarnettS
KromannI
GiemzaR
2009
Transient facial nerve paralysis (Bell's palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin.
PLoS ONE
4
e6999
doi:10.1371/journal.pone.0006999
23. MutschM
ZhouW
RhodesP
BoppM
ChenRT
2004
Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland.
N Engl J Med
350
896
903
24. BaumannU
2008
Mucosal vaccination against bacterial respiratory infections.
Expert Rev Vaccines
7
1257
1276
25. BrangerCG
Torres-EscobarA
SunW
PerryR
FetherstonJ
2009
Oral vaccination with LcrV from Yersinia pestis KIM delivered by live attenuated Salmonellaenterica serovar Typhimurium elicits a protective immune response against challenge with Yersinia pseudotuberculosis and Yersinia enterocolitica.
Vaccine
27
5363
5370
26. FooksAR
2000
Development of oral vaccines for human use.
Curr Opin Mol Ther
2
80
86
27. KostrzakA
CervantesGM
GuetardD
NagarajuDB
Wain-HobsonS
2009
Oral administration of low doses of plant-based HBsAg induced antigen-specific IgAs and IgGs in mice, without increasing levels of regulatory T cells.
Vaccine
27
4798
4807
28. GrdicD
SmithR
DonachieA
KjerrulfM
HornquistE
1999
The mucosal adjuvant effects of cholera toxin and immune-stimulating complexes differ in their requirement for IL-12, indicating different pathways of action.
Eur J Immunol
29
1774
1784
29. CzerkinskyC
HolmgrenJ
2009
Enteric vaccines for the developing world: a challenge for mucosal immunology.
Mucosal Immunol
2
284
287
30. HallLJ
ClareS
PickardD
ClarkSO
KellyDL
2009
Characterisation of a live Salmonella vaccine stably expressing the Mycobacterium tuberculosis Ag85B-ESAT6 fusion protein.
Vaccine
27
6894
6904
31. CasiniE
1972
[Bacterial vaccines for oral administration and local mechanisms of immunity].
Ann Sclavo
14
547
553
32. LiangS
HosurKB
NawarHF
RussellMW
ConnellTD
2009
In vivo and in vitro adjuvant activities of the B subunit of Type IIb heat-labile enterotoxin (LT-IIb-B5) from Escherichia coli.
Vaccine
27
4302
4308
33. BoyerJD
RobinsonTM
MaciagPC
PengX
JohnsonRS
2005
DNA prime Listeria boost induces a cellular immune response to SIV antigens in the rhesus macaque model that is capable of limited suppression of SIV239 viral replication.
Virology
333
88
101
34. PerdigonG
AlvarezS
Nader de MaciasME
Pesce de Ruiz HolgadoAA
1988
[Adjuvant activity of lactic bacteria: perspectives for its use in oral vaccines].
Rev Argent Microbiol
20
141
146
35. PouwelsPH
LeerRJ
ShawM
Heijne den Bak-GlashouwerMJ
TielenFD
1998
Lactic acid bacteria as antigen delivery vehicles for oral immunization purposes.
Int J Food Microbiol
41
155
167
36. WellsJM
MercenierA
2008
Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria.
Nat Rev Microbiol
6
349
362
37. TuckerSN
TingleyDW
ScallanCD
2008
Oral adenoviral-based vaccines: historical perspective and future opportunity.
Expert Rev Vaccines
7
25
31
38. MesteckyJ
NguyenH
CzerkinskyC
KiyonoH
2008
Oral immunization: an update.
Curr Opin Gastroenterol
24
713
719
39. FuJ
BianG
ZhaoB
DongZ
SunX
2009
Enhancing efficacy and mucosa-tropic distribution of an oral HIV-PsV DNA vaccine in animal models.
J Drug Target
17
803
812
40. NingJF
ZhuW
XuJP
ZhengCY
MengXL
2009
Oral delivery of DNA vaccine encoding VP28 against white spot syndrome virus in crayfish by attenuated Salmonella typhimurium.
Vaccine
27
1127
1135
41. CazorlaSI
BeckerPD
FrankFM
EbensenT
SartoriMJ
2008
Oral vaccination with Salmonella enterica as a cruzipain-DNA delivery system confers protective immunity against Trypanosoma cruzi.
Infect Immun
76
324
333
42. KindrachukJ
PotterJ
WilsonHL
GriebelP
BabiukLA
2008
Activation and regulation of toll-like receptor 9: CpGs and beyond.
Mini Rev Med Chem
8
590
600
43. ForsmanA
UshameckisD
BindraA
YunZ
BlombergJ
2003
Uptake of amplifiable fragments of retrotransposon DNA from the human alimentary tract.
Mol Genet Genomics
270
362
368
44. Van KaerL
Ashton-RickardtPG
EichelbergerM
GaczynskaM
NagashimaK
1994
Altered peptidase and viral-specific T cell response in LMP2 mutant mice.
Immunity
1
533
541
45. ToesRE
NussbaumAK
DegermannS
SchirleM
EmmerichNP
2001
Discrete cleavage motifs of constitutive and immunoproteasomes revealed by quantitative analysis of cleavage products.
J Exp Med
194
1
12
46. YorkIA
GoldbergAL
MoXY
RockKL
1999
Proteolysis and class I major histocompatibility complex antigen presentation.
Immunol Rev
172
49
66
47. MoXY
CascioP
LemeriseK
GoldbergAL
RockK
1999
Distinct proteolytic processes generate the C and N termini of MHC class I-binding peptides.
J Immunol
163
5851
5859
48. AziziA
Diaz-MitomaF
2007
Viral peptide immunogens: current challenges and opportunities.
J Pept Sci
13
776
786
49. DuesbergU
von demBA
KirschningC
MiyakeK
SauerbruchT
2002
Cell activation by synthetic lipopeptides of the hepatitis C virus (HCV) —core protein is mediated by toll like receptors (TLRs) 2 and 4.
Immunol Lett
84
89
95
50. YamashitaY
MaedaY
TakeshitaF
BrennanPJ
MakinoM
2004
Role of the polypeptide region of a 33 kDa mycobacterial lipoprotein for efficient IL-12 production.
Cell Immunol
229
13
20
51. SchroderNW
HeineH
AlexanderC
ManukyanM
EckertJ
2004
Lipopolysaccharide binding protein binds to triacylated and diacylated lipopeptides and mediates innate immune responses.
J Immunol
173
2683
2691
52. JacksonDC
LauYF
LeT
SuhrbierA
DeliyannisG
2004
A totally synthetic vaccine of generic structure that targets Toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses.
Proc Natl Acad Sci U S A
101
15440
15445
53. VossS
UlmerAJ
JungG
WiesmullerKH
BrockR
2007
The activity of lipopeptide TLR2 agonists critically depends on the presence of solubilizers.
Eur J Immunol
37
3489
3498
54. SumikawaY
AsadaH
HoshinoK
AzukizawaH
KatayamaI
2006
Induction of beta-defensin 3 in keratinocytes stimulated by bacterial lipopeptides through toll-like receptor 2.
Microbes Infect
8
1513
1521
55. SirskyjD
Diaz-MitomaF
GolshaniA
KumarA
AziziA
2010
Innovative bioinformatic approaches for developing peptide-based vaccines against hypervariable viruses.
Immunol Cell Biol
E-pub ahead of print. 11 May 2010
56. AziziA
AndersonDE
TorresJV
OgrelA
GhorbaniM
2008
Induction of broad cross-subtype-specific HIV-1 immune responses by a novel multivalent HIV-1 peptide vaccine in cynomolgus macaques.
J Immunol
180
2174
2186
57. LambertJS
KeeferM
MulliganMJ
SchwartzD
MesteckyJ
2001
A Phase I safety and immunogenicity trial of UBI microparticulate monovalent HIV-1 MN oral peptide immunogen with parenteral boost in HIV-1 seronegative human subjects.
Vaccine
19
3033
3042
58. TacketCO
2009
Plant-based oral vaccines: results of human trials.
Curr Top Microbiol Immunol
332
103
117
59. StreatfieldSJ
2005
Delivery of plant-derived vaccines.
Expert Opin Drug Deliv
2
719
728
60. StreatfieldSJ
2005
Plant-based vaccines for animal health.
Rev Sci Tech
24
189
199
61. HammondRW
NemchinovLG
2009
Plant production of veterinary vaccines and therapeutics.
Curr Top Microbiol Immunol
332
79
102
62. WalmsleyAM
ArntzenCJ
2000
Plants for delivery of edible vaccines.
Curr Opin Biotechnol
11
126
129
63. HaqTA
MasonHS
ClementsJD
ArntzenCJ
1995
Oral immunization with a recombinant bacterial antigen produced in transgenic plants.
Science
268
714
716
64. NochiT
TakagiH
YukiY
YangL
MasumuraT
2007
Rice-based mucosal vaccine as a global strategy for cold-chain - and needle-free vaccination.
Proc Natl Acad Sci U S A
104
10986
10991
65. NochiT
YukiY
KatakaiY
ShibataH
TokuharaD
2009
A rice-based oral cholera vaccine induces macaque-specific systemic neutralizing antibodies but does not influence pre-existing intestinal immunity.
J Immunol
183
6538
6544
66. AminM
JaafariMR
TafaghodiM
2009
Impact of chitosan coating of anionic liposomes on clearance rate, mucosal and systemic immune responses following nasal administration in rabbits.
Colloids Surf B Biointerfaces
74
225
229
67. BorgesO
TavaresJ
deSA
BorchardG
JungingerHE
Cordeiro-da-SilvaA
2007
Evaluation of the immune response following a short oral vaccination schedule with hepatitis B antigen encapsulated into alginate-coated chitosan nanoparticles.
Eur J Pharm Sci
32
278
290
68. KatzDE
DeLorimierAJ
WolfMK
HallER
CasselsFJ
2003
Oral immunization of adult volunteers with microencapsulated enterotoxigenic Escherichia coli (ETEC) CS6 antigen.
Vaccine
21
341
346
69. FreyA
GiannascaKT
WeltzinR
GiannascaPJ
ReggioH
1996
Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting.
J Exp Med
184
1045
1059
70. MannJF
ShakirE
CarterKC
MullenAB
AlexanderJ
2009
Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection.
Vaccine
27
3643
3649
71. OwenRL
JonesAL
1974
Epithelial cell specialization within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles.
Gastroenterology
66
189
203
72. HathawayLJ
KraehenbuhlJP
2000
The role of M cells in mucosal immunity.
Cell Mol Life Sci
57
323
332
73. GebertA
GokeM
RothkotterHJ
DietrichCF
2000
[The mechanisms of antigen uptake in the small and large intestines: the roll of the M cells for the initiation of immune responses].
Z Gastroenterol
38
855
872
74. KuhnEM
KaupFJ
1996
Morphological characteristics of the ileal Peyer's patches in the rhesus macaque: a histological and ultrastructural study.
Anat Histol Embryol
25
65
69
75. ClarkMA
HirstBH
JepsonMA
1998
M-cell surface beta1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer's patch M cells.
Infect Immun
66
1237
1243
76. GebertA
PabstR
1999
M cells at locations outside the gut.
Semin Immunol
11
165
170
77. GebertA
FassbenderS
WernerK
WeissferdtA
1999
The development of M cells in Peyer's patches is restricted to specialized dome-associated crypts.
Am J Pathol
154
1573
1582
78. HolmgrenJ
CzerkinskyC
2005
Mucosal immunity and vaccines.
Nat Med
11
S45
S53
79. YukiY
KiyonoH
2009
Mucosal vaccines: novel advances in technology and delivery.
Expert Rev Vaccines
8
1083
1097
80. BraydenDJ
JepsonMA
BairdAW
2005
Keynote review: intestinal Peyer's patch M cells and oral vaccine targeting.
Drug Discov Today
10
1145
1157
81. KuoleeR
ChenW
2008
M cell-targeted delivery of vaccines and therapeutics.
Expert Opin Drug Deliv
5
693
702
82. MeynellHM
ThomasNW
JamesPS
HollandJ
TaussigMJ
1999
Up-regulation of microsphere transport across the follicle-associated epithelium of Peyer's patch by exposure to Streptococcus pneumoniae R36a.
FASEB J
13
611
619
83. TyrerP
RuthFA
KydJ
HarveyM
SizerP
2002
Validation and quantitation of an in vitro M-cell model.
Biochem Biophys Res Commun
299
377
383
84. MannJF
FerroVA
MullenAB
TetleyL
MullenM
2004
Optimisation of a lipid based oral delivery system containing A/Panama influenza haemagglutinin.
Vaccine
22
2425
2429
85. ChouMY
HartvigsenK
HansenLF
FogelstrandL
ShawPX
2008
Oxidation-specific epitopes are important targets of innate immunity.
J Intern Med
263
479
488
86. ScibelliA
MatteoliG
RopertoS
AlimentiE
DipinetoL
2005
Flavoridin inhibits Yersinia enterocolitica uptake into fibronectin-adherent HeLa cells.
FEMS Microbiol Lett
247
51
57
87. SaltmanLH
LuY
ZahariasEM
IsbergRR
1996
A region of the Yersinia pseudotuberculosis invasin protein that contributes to high affinity binding to integrin receptors.
J Biol Chem
271
23438
23444
88. SinhaB
FrancoisPP
NusseO
FotiM
HartfordOM
1999
Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin alpha5beta1.
Cell Microbiol
1
101
117
89. JangMH
KweonMN
IwataniK
YamamotoM
TeraharaK
2004
Intestinal villous M cells: an antigen entry site in the mucosal epithelium.
Proc Natl Acad Sci U S A
101
6110
6115
90. KozlowskiPA
WilliamsSB
LynchRM
FlaniganTP
PattersonRR
2002
Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle.
J Immunol
169
566
574
91. GuptaPN
KhatriK
GoyalAK
MishraN
VyasSP
2007
M-cell targeted biodegradable PLGA nanoparticles for oral immunization against hepatitis B.
J Drug Target
15
701
713
92. LavelleEC
GrantG
PusztaiA
PfullerU
O'HaganDT
2000
Mucosal immunogenicity of plant lectins in mice.
Immunology
99
30
37
93. WangX
KochetkovaI
HaddadA
HoytT
HoneDM
2005
Transgene vaccination using Ulex europaeus agglutinin I (UEA-1) for targeted mucosal immunization against HIV-1 envelope.
Vaccine
23
3836
3842
94. ManochaM
PalPC
ChitralekhaKT
ThomasBE
TripathiV
2005
Enhanced mucosal and systemic immune response with intranasal immunization of mice with HIV peptides entrapped in PLG microparticles in combination with Ulex Europaeus-I lectin as M cell target.
Vaccine
23
5599
5617
95. ChionhYT
WeeJL
EveryAL
NgGZ
SuttonP
2009
M-cell targeting of whole killed bacteria induces protective immunity against gastrointestinal pathogens.
Infect Immun
77
2962
2970
96. GiannascaPJ
GiannascaKT
LeichtnerAM
NeutraMR
1999
Human intestinal M cells display the sialyl Lewis A antigen.
Infect Immun
67
946
953
97. KerneisS
BogdanovaA
KraehenbuhlJP
PringaultE
1997
Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria.
Science
277
949
952
98. GramLK
RistGM
LennernasH
SteffansenB
2009
Impact of carriers in oral absorption: Permeation across Caco-2 cells for the organic anions estrone-3-sulfate and glipizide.
Eur J Pharm Sci
37
378
386
99. LimJS
NaHS
LeeHC
ChoyHE
ParkSC
2009
Caveolae-mediated entry of Salmonella typhimurium in a human M-cell model.
Biochem Biophys Res Commun
390
1322
1327
100. GullbergE
LeonardM
KarlssonJ
HopkinsAM
BraydenD
2000
Expression of specific markers and particle transport in a new human intestinal M-cell model.
Biochem Biophys Res Commun
279
808
813
101. HaseK
KawanoK
NochiT
PontesGS
FukudaS
2009
Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response.
Nature
462
226
230
102. TeraharaK
YoshidaM
IgarashiO
NochiT
PontesGS
2008
Comprehensive gene expression profiling of Peyer's patch M cells, villous M-like cells, and intestinal epithelial cells.
J Immunol
180
7840
7846
103. LangermannS
MollbyR
BurleinJE
PalaszynskiSR
AugusteCG
2000
Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli.
J Infect Dis
181
774
778
104. PoggioTV
La TorreJL
ScodellerEA
2006
Intranasal immunization with a recombinant truncated FimH adhesin adjuvanted with CpG oligodeoxynucleotides protects mice against uropathogenic Escherichia coli challenge.
Can J Microbiol
52
1093
1102
105. BouckaertJ
BerglundJ
SchembriM
DeGE
CoolsL
2005
Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin.
Mol Microbiol
55
441
455
106. GiannascaPJ
GiannascaKT
LeichtnerAM
NeutraMR
1999
Human intestinal M cells display the sialyl Lewis A antigen.
Infect Immun
67
946
953
107. MisumiS
MasuyamaM
TakamuneN
NakayamaD
MitsumataR
2009
: Targeted delivery of immunogen to primate m cells with tetragalloyl lysine dendrimer.
J Immunol
182
6061
6070
108. FariaAM
WeinerHL
2005
Oral tolerance.
Immunol Rev
206
232
259
109. MesteckyJ
RussellMW
ElsonCO
2007
Perspectives on mucosal vaccines: is mucosal tolerance a barrier?
J Immunol
179
5633
5638
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease AgentČlánek TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease SusceptibilityČlánek The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive ParasiteČlánek Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Patients with Discordant Responses to Antiretroviral Therapy Have Impaired Killing of HIV-Infected T Cells
- A Molecular Mechanism for Eflornithine Resistance in African Trypanosomes
- Tyrosine Sulfation of the Amino Terminus of PSGL-1 Is Critical for Enterovirus 71 Infection
- Autoimmunity as a Predisposition for Infectious Diseases
- The Subtilisin-Like Protease AprV2 Is Required for Virulence and Uses a Novel Disulphide-Tethered Exosite to Bind Substrates
- Structural Analysis of HIV-1 Maturation Using Cryo-Electron Tomography
- Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease Agent
- Interferon-Inducible CXC Chemokines Directly Contribute to Host Defense against Inhalational Anthrax in a Murine Model of Infection
- TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease Susceptibility
- The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive Parasite
- CO Acts as a Signalling Molecule in Populations of the Fungal Pathogen
- SV2 Mediates Entry of Tetanus Neurotoxin into Central Neurons
- MAP Kinase Phosphatase-2 Plays a Critical Role in Response to Infection by
- Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
- Potentiation of Epithelial Innate Host Responses by Intercellular Communication
- Fcγ Receptor I Alpha Chain (CD64) Expression in Macrophages Is Critical for the Onset of Meningitis by K1
- ANK, a Host Cytoplasmic Receptor for the Cell-to-Cell Movement Protein, Facilitates Intercellular Transport through Plasmodesmata
- Analysis of the Initiating Events in HIV-1 Particle Assembly and Genome Packaging
- Evolution of Linked Avirulence Effectors in Is Affected by Genomic Environment and Exposure to Resistance Genes in Host Plants
- Structural Basis of HIV-1 Neutralization by Affinity Matured Fabs Directed against the Internal Trimeric Coiled-Coil of gp41
- Hepatitis C Virus (HCV) Evades NKG2D-Dependent NK Cell Responses through NS5A-Mediated Imbalance of Inflammatory Cytokines
- Host Cell Invasion and Virulence Mediated by Ssa1
- Global Gene Expression in Urine from Women with Urinary Tract Infection
- Should the Human Microbiome Be Considered When Developing Vaccines?
- HapX Positively and Negatively Regulates the Transcriptional Response to Iron Deprivation in
- Enhancing Oral Vaccine Potency by Targeting Intestinal M Cells
- Herpes Simplex Virus Reorganizes the Cellular DNA Repair and Protein Quality Control Machinery
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- Cryo Electron Tomography of Native HIV-1 Budding Sites
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- Modelling the Evolution and Spread of HIV Immune Escape Mutants
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
- Platelet-Activating Factor Receptor Plays a Role in Lung Injury and Death Caused by Influenza A in Mice
- Genetic and Structural Basis for Selection of a Ubiquitous T Cell Receptor Deployed in Epstein-Barr Virus Infection
- Ubiquitin-Regulated Nuclear-Cytoplasmic Trafficking of the Nipah Virus Matrix Protein Is Important for Viral Budding
- Pneumolysin Activates the NLRP3 Inflammasome and Promotes Proinflammatory Cytokines Independently of TLR4
- Immune Evasion by : Differential Targeting of Dendritic Cell Subpopulations
- Survival in Selective Sand Fly Vector Requires a Specific -Encoded Lipophosphoglycan Galactosylation Pattern
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



