-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Subtilisin-Like Protease AprV2 Is Required for Virulence and Uses a Novel Disulphide-Tethered Exosite to Bind Substrates
Many bacterial pathogens produce extracellular proteases that degrade the extracellular matrix of the host and therefore are involved in disease pathogenesis. Dichelobacter nodosus is the causative agent of ovine footrot, a highly contagious disease that is characterized by the separation of the hoof from the underlying tissue. D. nodosus secretes three subtilisin-like proteases whose analysis forms the basis of diagnostic tests that differentiate between virulent and benign strains and have been postulated to play a role in virulence. We have constructed protease mutants of D. nodosus; their analysis in a sheep virulence model revealed that one of these enzymes, AprV2, was required for virulence. These studies challenge the previous hypothesis that the elastase activity of AprV2 is important for disease progression, since aprV2 mutants were virulent when complemented with aprB2, which encodes a variant that has impaired elastase activity. We have determined the crystal structures of both AprV2 and AprB2 and characterized the biological activity of these enzymes. These data reveal that an unusual extended disulphide-tethered loop functions as an exosite, mediating effective enzyme-substrate interactions. The disulphide bond and Tyr92, which was located at the exposed end of the loop, were functionally important. Bioinformatic analyses suggested that other pathogenic bacteria may have proteases that utilize a similar mechanism. In conclusion, we have used an integrated multidisciplinary combination of bacterial genetics, whole animal virulence trials in the original host, biochemical studies, and comprehensive analysis of crystal structures to provide the first definitive evidence that the extracellular secreted proteases produced by D. nodosus are required for virulence and to elucidate the molecular mechanism by which these proteases bind to their natural substrates. We postulate that this exosite mechanism may be used by proteases produced by other bacterial pathogens of both humans and animals.
Published in the journal: . PLoS Pathog 6(11): e32767. doi:10.1371/journal.ppat.1001210
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001210Summary
Many bacterial pathogens produce extracellular proteases that degrade the extracellular matrix of the host and therefore are involved in disease pathogenesis. Dichelobacter nodosus is the causative agent of ovine footrot, a highly contagious disease that is characterized by the separation of the hoof from the underlying tissue. D. nodosus secretes three subtilisin-like proteases whose analysis forms the basis of diagnostic tests that differentiate between virulent and benign strains and have been postulated to play a role in virulence. We have constructed protease mutants of D. nodosus; their analysis in a sheep virulence model revealed that one of these enzymes, AprV2, was required for virulence. These studies challenge the previous hypothesis that the elastase activity of AprV2 is important for disease progression, since aprV2 mutants were virulent when complemented with aprB2, which encodes a variant that has impaired elastase activity. We have determined the crystal structures of both AprV2 and AprB2 and characterized the biological activity of these enzymes. These data reveal that an unusual extended disulphide-tethered loop functions as an exosite, mediating effective enzyme-substrate interactions. The disulphide bond and Tyr92, which was located at the exposed end of the loop, were functionally important. Bioinformatic analyses suggested that other pathogenic bacteria may have proteases that utilize a similar mechanism. In conclusion, we have used an integrated multidisciplinary combination of bacterial genetics, whole animal virulence trials in the original host, biochemical studies, and comprehensive analysis of crystal structures to provide the first definitive evidence that the extracellular secreted proteases produced by D. nodosus are required for virulence and to elucidate the molecular mechanism by which these proteases bind to their natural substrates. We postulate that this exosite mechanism may be used by proteases produced by other bacterial pathogens of both humans and animals.
Introduction
Dichelobacter nodosus is a Gram negative, anaerobic rod that is the principal causative agent of ovine footrot, a debilitating disease of the hoof of ruminants. The disease results in significant costs to the worldwide sheep industry due to a reduction in meat and wool production and the expenditure associated with prevention and treatment programs [1], [2], [3]. Footrot is characterized by the separation of the keratinous hoof from the underlying tissue, resulting in severe lameness and loss of body condition [4], [5]. The severity of the disease can vary from benign footrot, which presents as an interdigital dermatitis that does not progress, to virulent footrot, which results in severe under-running of the horn of the hoof and the separation of the hoof from the underlying tissue [1]. Type IV fimbriae are an essential virulence factor [6], [7]; it also has been suggested that three closely-related secreted subtilisin-like proteases produced by D. nodosus may be required for virulence [8], [9]. In strains that cause virulent footrot these proteases are called acidic protease isoenzymes 2 and 5 from virulent strains (AprV2 and AprV5) and basic protease from virulent strains (BprV). In benign strains the comparable proteases are termed AprB2, AprB5 and BprB. All of these proteases are synthesised as inactive precursors with an N-terminal pre-pro-region, a serine protease domain and a C-terminal domain of unknown function. The active protease is produced by cleavage of the N-terminal pre-pro region and the C-terminal domain [10], [11], [12]. The protease domains have significant sequence identity to members of the subtilase family of serine proteases (54% identity with closest homologue from Dehalococcoides sp. VS), but sequence alignments indicate several insertions in the D. nodosus proteases [13].
Previous studies suggested that these proteases may represent the key difference between virulent and benign strains of D. nodosus; proteases secreted by virulent isolates have a greater thermostability and elastase activity (as monitored on elastin agar plates) than those of benign strains and it is postulated that this difference may relate to their in vivo activity against host tissue. These features are utilized in diagnostic tests to distinguish between virulent and benign footrot [14], [15], [16]. Comparison of the protease sequences from the virulent strain A198 with the benign strain C305 revealed that within the mature protease domain there is a single amino acid difference between AprV2 and AprB2 [12], and between AprV5 and AprB5 [11], and 96% sequence identity between BprV and BprB [17].
In this multidisciplinary study we set out to determine the role of these proteases in virulence and to determine the molecular basis for their function. We constructed isogenic protease mutants and characterized their protease activity and virulence. We showed that AprV2 was required for a virulent D. nodosus isolate to cause disease. Determination of the crystal structure of AprV2 revealed the presence of a novel exosite loop. This combined genetic and structural approach has permitted a comprehensive investigation of the secreted protease component of a pathogenic organism, and furthermore provided novel insight into how subtilisin-like proteases may have been hijacked by pathogenic microorganisms to degrade extracellular matrix components.
Results
Protease activity of D. nodosus
To assess the contribution of each of the three extracellular proteases to the overall protease activity of the virulent D. nodosus isolate VCS1703A, separate chromosomal mutants of each protease gene were constructed by allelic exchange events that involved double crossovers. To confirm that the observed phenotypes resulted from these mutations, the mutants were complemented by insertion of the wild-type protease genes into the chromosome. Quantitative protease assays of culture supernatants, using azocasein as the substrate, showed that mutation of the aprV5 and aprV2 genes reduced total protease activity by 71% and 39%, respectively (Figure 1A). Complementation with the respective wild-type genes returned total protease activity to wild-type levels; however, it was also observed that the complemented aprV5 strain tended to lose protease activity upon repeated subculture. Since only a 12% reduction (P<0.05) was observed in the bprV mutant, BprV does not appear to make a major contribution to total protease activity. These results indicate that AprV5, either directly or indirectly, makes the major contribution to total extracellular protease activity.
Fig. 1. Protease activity of D. nodosus wild-type and protease mutants. 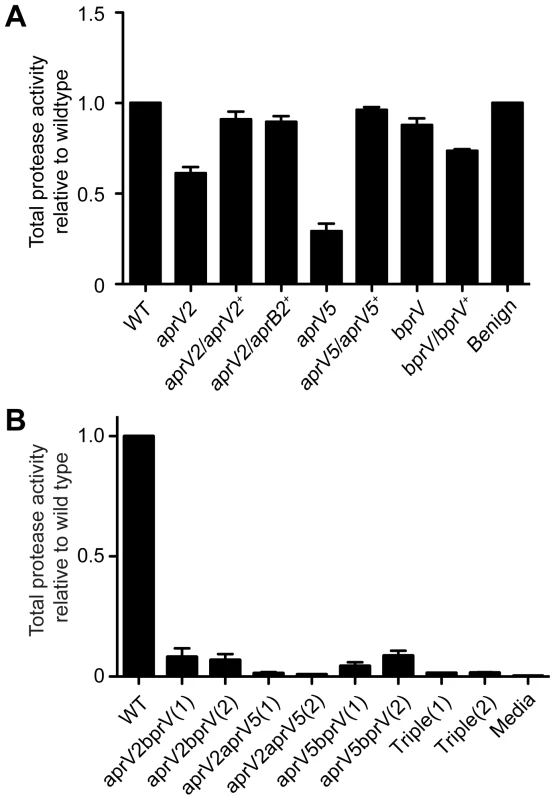
Total protease activity of 40 h culture supernatants was measured using azocasein as the substrate. The protease activity is expressed relative to the wild-type activity. Means and standard error of the mean (s.e.m.) are shown. (A) Total protease activity of the protease mutants and their complemented derivatives. Each of the mutants had significantly reduced (P<0.05, n = 3, t-test) protease activity compared to the wild-type strain VCS1703A, while the complemented strains, except for the bprV complemented strain, were not significantly different to wild-type. WT: Wild-type VCS1703A, Benign: the benign isolate CS101. (B) Total protease activity of double and triple protease mutants. WT: Wild-type VCS1703A, Triple: the aprV2 aprV5 bprV triple mutant. The designation (1) and (2) represents independently derived mutants. Other genotypes are as indicated. The protease activity of each of these mutants was significantly different to wild-type (P<0.05, n = 3, t-test). To confirm the individual contribution of each protease gene to the overall protease activity of VCS1703A, double and triple mutants were also constructed. Only very low levels of protease activity were observed in the aprV2bprV and aprV5bprV double mutants (Figure 1B). Negligible protease activity was detected in the triple mutant and the aprV2aprV5 double mutant. The reduction in total protease activity for the double mutants was greater than the combined reduction in the total protease activity for the single mutants suggesting that the secreted proteases either act synergistically to degrade target substrates or that one or more of the proteases may be involved in the activation of the other proteases, or both.
The ability of D. nodosus to digest insoluble elastin in an agar medium has been used as a diagnostic test to distinguish virulent and benign strains. Virulent isolates digest elastin within seven to ten days, while benign strains show no digestion after >21 days incubation [14]. Analysis of the wild type, the protease mutants and the complemented strains on elastin agar showed that the aprV2 mutant was unable to digest elastin, even after 30 days incubation, whereas both the aprV5 and bprV mutants were able to digest elastin at wild-type levels, showing clearing after 10 days (Figure S1A). Complementation of the aprV2 mutation restored the ability to digest elastin. In addition, the aprV5bprV mutants still had elastase activity. These results provided evidence that the AprV2 protease was responsible for the elastase activity. Note that the levels of elastase activity in culture supernatants were not high enough for detection in quantitative elastase assays.
Sequence analysis of the aprV2 and aprB2 genes has shown there is only one amino acid difference (Y92R) between the two mature proteases [12]. To see what effect complementing the aprV2 mutant with the aprB2 gene would have on the protease phenotype of the resultant strain we inserted the aprB2 gene from the benign strain CS101 into the site of the disrupted aprV2 gene. Analysis of this strain in the azocasein assay showed that its total protease activity was not significantly different to the wild type (Figure 1A). However, this complemented strain had the in vitro phenotype of a benign strain since it had no elastase activity (Figure S1A), and its protease thermostability profile (Figure S1B) was that expected of a benign isolate [16].
The apparent difference in elastase activity between AprV2 and AprB2 was assessed in vitro using an Elastin-Congo Red substrate and purified recombinant proteins. Under these conditions, the ability of AprB2 to degrade the substrate was significantly less than AprV2 (p<0.05, Figure 2). Both proteases displayed similar activity against the soluble chromogenic elastase substrate, N-Methoxysuccinyl-Ala-Ala-Pro-Val p-nitroanilide (AAPVn), with kinetic parameters that were of the same order of magnitude (Table 1). These results suggest that the Y92R substitution does not contribute directly to catalysis at the active site of the enzyme.
Fig. 2. Elastase activity of AprV2, AprB2 and mutants. 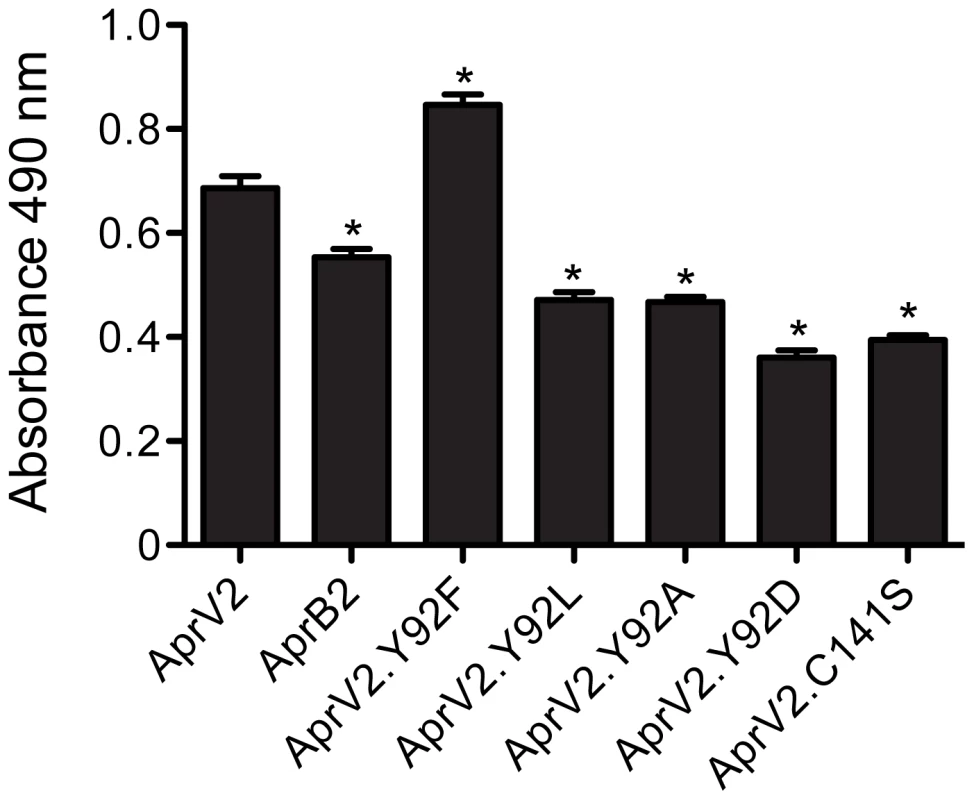
For quantitative measurement of elastase activity of recombinant AprV2, AprB2 (AprV2.Y92R) and protease mutants, purified protease was incubated with Elastin-Congo Red at 25°C for 19 h. Elastin degradation was detected spectroscopically at 490 nm. The mean and s.e.m are shown (* p<0.001, n = 3, one-way ANOVA compared to AprV2). Tab. 1. Activity of AprV2, AprB2 and mutants against the elastin like peptide AAPV(n). 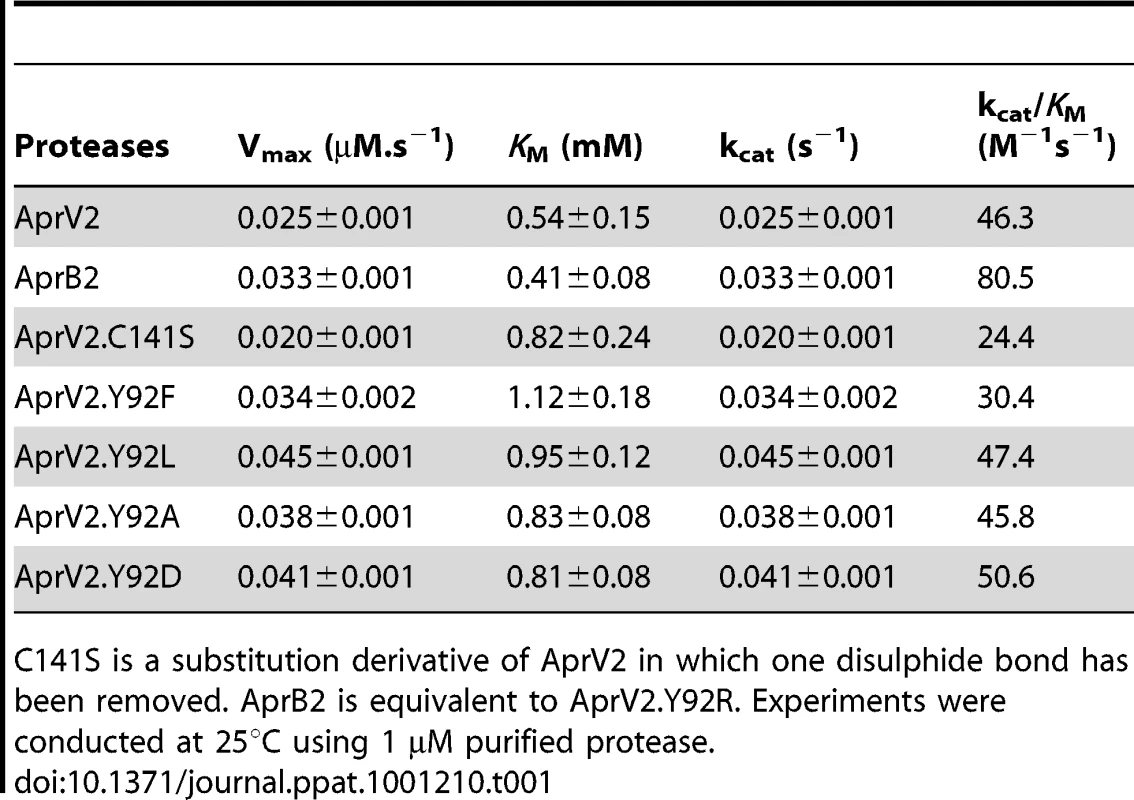
C141S is a substitution derivative of AprV2 in which one disulphide bond has been removed. AprB2 is equivalent to AprV2.Y92R. Experiments were conducted at 25°C using 1 µM purified protease. AprV2 is essential for virulence in sheep
To determine the role in disease of each of the proteases, virulence testing in sheep was carried out on the wild type, the aprV2, aprV5 and bprV mutants and their corresponding complemented strains, using our standard procedure [6], [7]. These experiments represented a rigorous test of the ability of these bacteria to cause disease since such pen-based trials often magnify the ability of less virulent isolates to cause disease. Comparative analysis of the footrot scores of sheep infected with the wild-type strain and the aprV2 mutant revealed a significant difference (P<0.0001); the aprV2 mutant was effectively avirulent (Figure 3). Complementation of the aprV2 mutant with the wild-type aprV2 gene restored the wild-type virulence profile, fulfilling molecular Koch's postulates. To our surprise, complementation of the aprV2 mutant with aprB2 also restored a virulent phenotype, indicating that AprV2-mediated elastase activity was not required for virulence. Analysis of isolates obtained from infected lesions confirmed that they had the expected phenotypic and genotypic properties.
Fig. 3. Virulence testing of isogenic isolates in sheep. 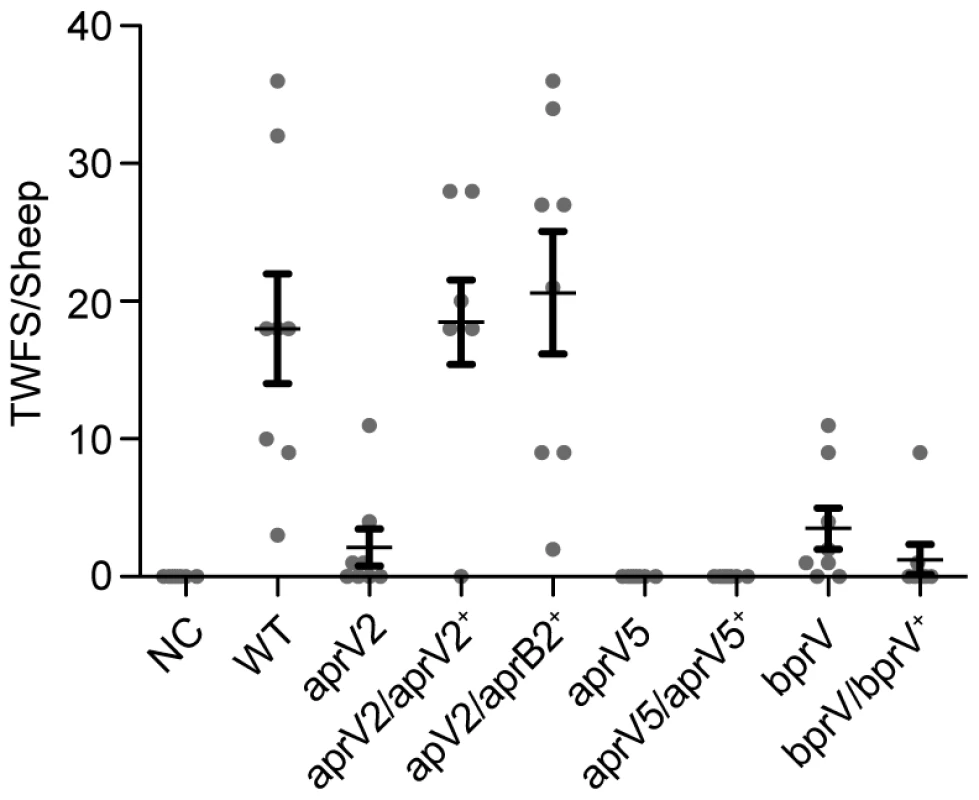
Sheep were challenged with the wild type, the three protease mutants and their complemented derivatives in a blind pen trial. The total weighted foot score (TWFS) for individual sheep at week three is shown. The mean and the s.e.m. for each group are shown. Each mutant is significantly different to the wild-type, while the aprV2/aprV2+ and aprV2/aprB2+ complemented strains are not significantly different to wild-type (P<0.05, n = 8, one-way ANOVA). NC: negative control, not infected with bacteria. The aprV5 and bprV mutants were also avirulent (Figure 3), but complementation of these strains with the respective wild-type genes did not restore virulence, which was unexpected. Extensive sequencing of each of the protease genes in these strains showed them to be intact. However, upon subculture, protease secretion and/or elastin digestion by the aprV5 and bprV complemented strains were variable, as was their ability to undergo twitching motility, a property that is essential for virulence [7]. Therefore, we suggest that the complemented derivatives were genetically unstable and that secondary mutations were being selected in these strains. Consequently, no meaningful conclusions can be drawn from the aprV5 and bprV sheep virulence experiments.
Identification of the native substrates of AprV2
Since the elastase activity of AprV2 was not required for virulence it was of interest to determine which hoof proteins were degraded by Aprv2 and AprB2. Fragments of hoof from a disease-free sheep were exposed to recombinant AprV2 and AprB2 and solubilised proteins were identified. AprV2 degraded type I keratin, serum albumin and the beta subunit of haemoglobin (Figure 4). Importantly, the hoof digestion pattern produced by AprB2 was similar to that produced by AprV2 (Figure 4), which is consistent with results of the virulence trials.
Fig. 4. Degradation of sheep hoof by recombinant protease. 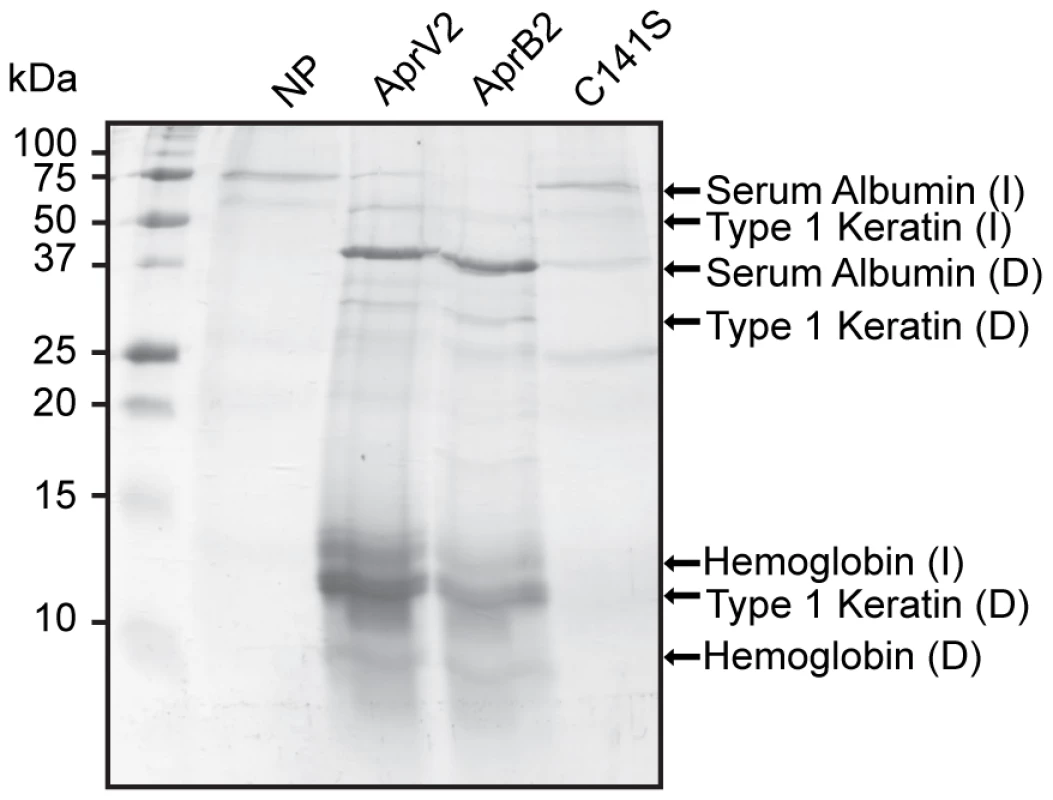
The activity of AprV2, AprB2 and AprV2.C141S on fragments of hoof isolated from a disease free sheep. C141S is a substitution derivative of AprV2 in which one disulphide bond has been removed. The degradation products were visualised by SDS-PAGE. Degraded proteins were identified by in-gel tryptic digest followed by LCMS. I: intact proteins; D: degraded proteins; NP: no protease added. AprV2 contains a novel disulfide tethered extended loop
To investigate how the single amino acid difference (Y92R) between the active forms of AprV2 and AprB2 alters the substrate specificity of AprB2 we determined the crystal structures of AprV2 and AprB2 to 2.0 Å and 1.7 Å, respectively (Table 2; Figure 5A,B). The two structures were very similar (RMSD of 0.28 Å for 339 Cα) (Figure 5C) and therefore we will describe the structure with reference to AprV2.
Fig. 5. Crystal structure of AprV2. 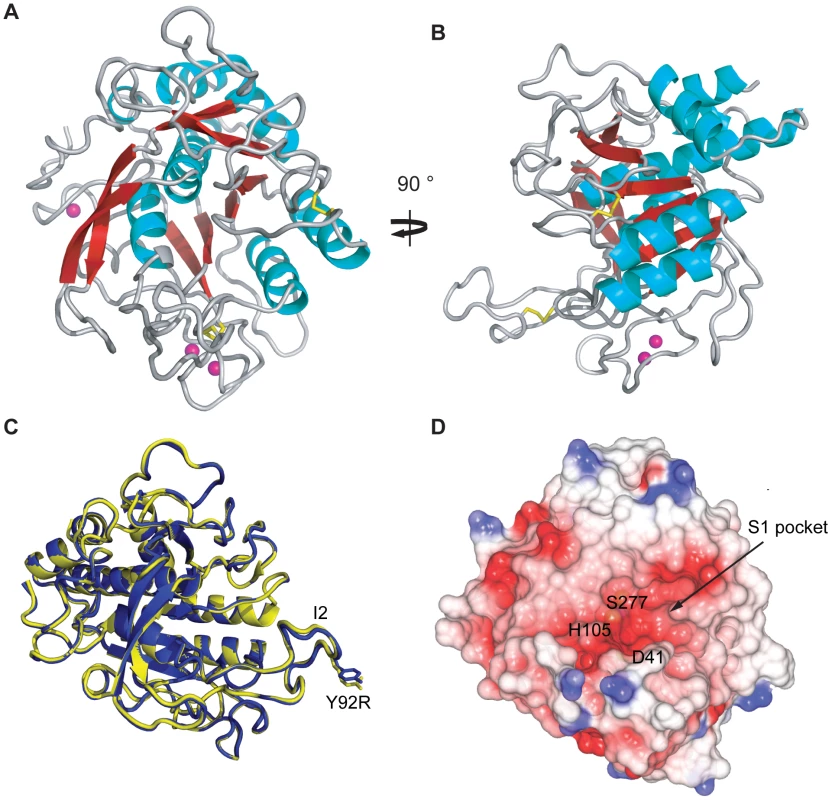
(A) and (B) Cartoon representation of AprV2. Two views differing by 90° are shown. Disulphide bonds are shown in yellow stick representation. Calcium ions found in the crystal structure are shown as pink spheres. (C) An overlay of the crystal structures of AprV2 (blue) and AprB2 (yellow). The structures are shown as a Cα trace. The I2 loop is labelled. Tyr 92 in AprV2 and Arg 92 in AprB2 are shown in stick representation and labelled. (D) Electrostatic potential surface of AprV2 generated using CCP4mg [50]. Positively charged electrostatic potential is coloured blue and negatively charged electrostatic potential is coloured red. The location of the active site residues is indicated. The location of the S1 binding pocket is indicated. (A), (B) and (C) were prepared using PyMol [51]. The structural alignment in (C) was prepared using MUSTANG [52]. Secondary structure elements were calculated using stride [53]. Tab. 2. Structure refinement statistics for AprV2, AprB2 and AprV2.C141S. 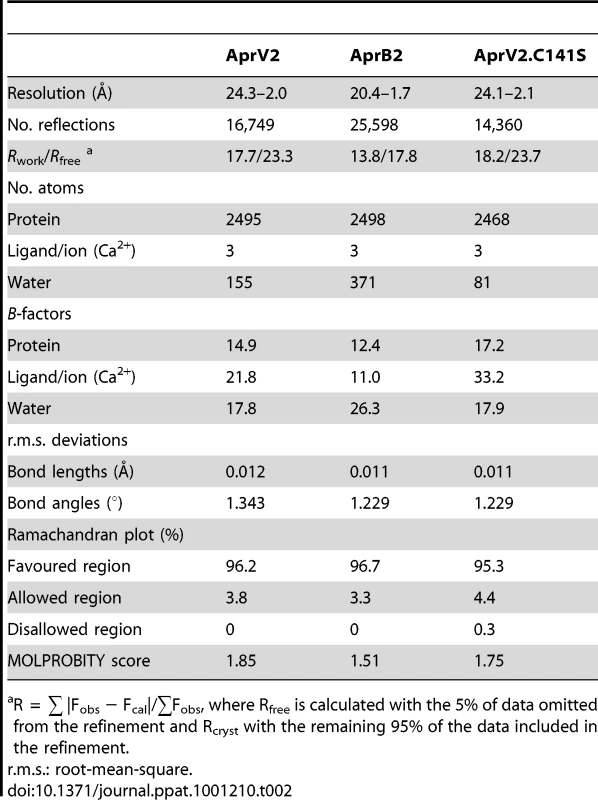
R = ∑ |Fobs − Fcal|/∑Fobs, where Rfree is calculated with the 5% of data omitted from the refinement and Rcryst with the remaining 95% of the data included in the refinement. AprV2 adopts a subtilisin-like fold consisting of a curved six-stranded parallel beta sheet sandwiched between two and five alpha helices (Figure 5A, B). A two stranded anti-parallel beta hairpin runs perpendicular to the plane of the central beta sheet. The proteases have two disulphide bonds, Cys89-Cys141 and Cys183-Cys220 and three calcium binding sites (Figure S2). The proposed catalytic triad (Asp41, His105 and Ser277) of AprV2 is located at the C-terminal edge of the beta sheet. The most striking feature of the substrate binding site is a large, elongated S1 binding pocket, which is lined by residues 177–180, 204–208, 215 and 218, and appears capable of accommodating bulky side-chains such as phenylalanine (Figure 5D). This finding is consistent with previous studies, which have shown that AprV2 preferentially cleaves after phenylalanine or leucine residues [18].
Comparison with other subtilisin-like proteases reveals several major insertions (termed I1-I4) in the loops that surround the active site cleft (Figure 6A, B; Figure S3). Most notable is the large well ordered I2 loop (residues 82–102) that is tethered to the subtilisin-like fold by a disulphide bond between Cys141 and Cys89 (Figure 6). The loop is well defined in the electron density, with low B-factors, suggesting that it has limited mobility in the crystal structure (Figure 6C). However, the apparent stability of the I2 loop is likely to arise from crystal packing and it is uncertain if this conformation would be favoured in solution. Surprisingly, the single amino acid difference between AprV2 and AprB2 (Y92R) is located at the tip of this extended loop, ∼27 Å from the active site serine (S277). A PSI-BLAST search revealed that the additional loops present in AprV2 may be conserved in other extracellular proteases (Figure S3B).
Fig. 6. AprV2 contains a novel disulfide tethered loop. 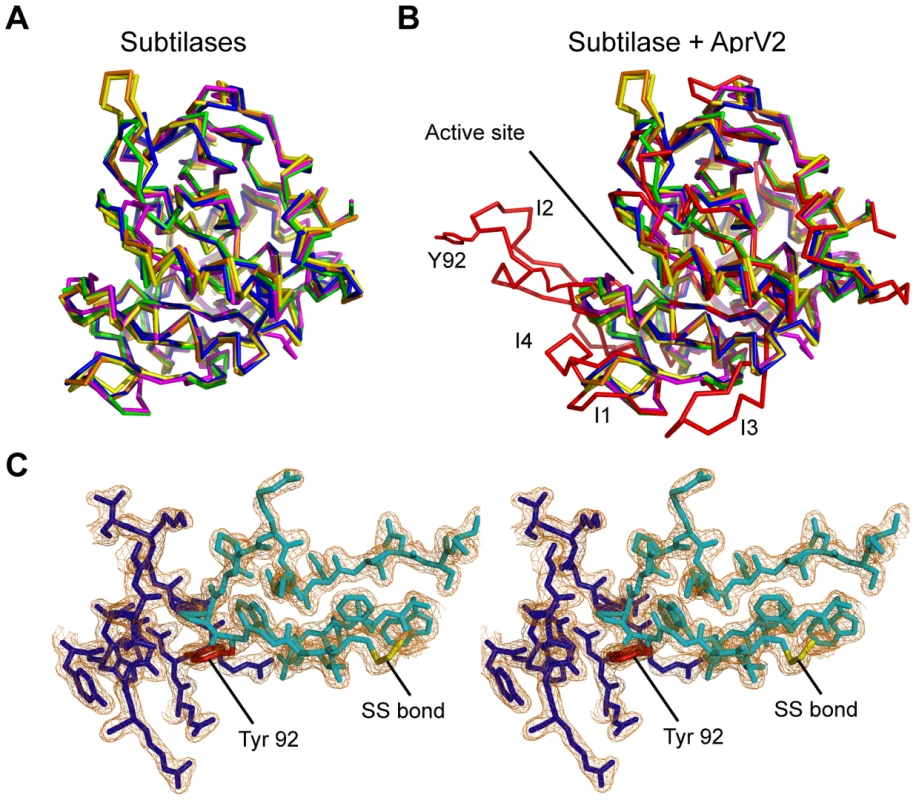
(A) Overlay of the crystal structures of AkP (magenta; 1DBI), thermitase (green; 1THM), Carlsburg subtilisn (yellow; 1AF4), BPN' (orange; 1SUP) and savinase (blue; 1SVN). (B) Overlay of the crystal structures of AprV2 (red) with AkP, thermitase, Carlsburg subtilisn, BPN' and savinase (coloured as in (a)). The structures were superimposed using the A chains only and are shown as a Cα trace. The I1, I2, I3 and I4 loops are labelled. Tyr 92 in AprV2 and Arg 92 are shown in stick representation and labelled. (C) Stereo view of a 2|Fo|−|Fc| electron density map depicting the disulfide tethered I2 loop of the AprV2 protease. The map is contoured at 1.2 σ. Water molecules have been removed for clarity. The conformation of this loop (cyan) is stabilised by inter and intramolecular contacts with molecules generated by symmetry coloured blue. The alignments in (A) and (B) were generated using MUSTANG [52]. Secondary structure elements were calculated using stride [53]. The figure was prepared using PyMol [51]. We constructed an I2 loop truncation mutant, AprV2Δ83–99, but the resultant protein was not functional, therefore we investigated the role of the I2 loop using site-directed mutagenesis. We targeted residue 92 as the Y92R substitution reduced the ability of AprB2 to degrade Elastin-Congo Red (Figure 2), while maintaining its ability to degrade the elastin-like peptide AAPVn (Table 1). We used site-directed mutagenesis to convert Tyr92 to Asp, Ala, Leu or Phe and examined the ability of the resultant proteins to degrade insoluble Elastin-Congo Red. While the presence of a negative charge (Asp), positive charge (Arg) or smaller hydrophobic (Ala and Leu) side-chain decreased elastin degradation, the Phe substitution increased the elastase activity of the enzyme (Figure 2). We also examined the ability of these mutants to degrade AAPVn (Table 1), fibronectin (Figure S4) or hoof material (Figure S5). No major differences were discernable. Based on these data we conclude that an aromatic ring is required at position 92 for maximal activity against insoluble elastin, but that this activity is not related to the hoof digestion observed in a footrot lesion.
The Cys89-Cys141 disulphide bond is required for optimal activity
To test the importance of the Cys89-Cys141 disulfide bond for proteolytic activity, we also used site-directed mutagenesis to convert Cys141 to Ser141. Although this AprV2.C141S derivative was still active against small peptide substrates (Table 1), we noted that the ability of this enzyme to degrade fibronectin, insoluble elastin and proteins from sheep hoof was reduced. Notably, wild-type AprV2 was able to break down fibronectin in 48 h, whereas at the corresponding time point AprV2.C141S-treated fibronectin was still intact (Figure 7A). The elastinolytic activity of the C141S protease was also approximately two-fold lower than wild type (Figure 2). Finally, the ability of AprV2.C141S to degrade sheep hoof material was significantly reduced compared to AprV2 and AprB2 (Figure 4). Together these data suggest that the integrity of the I2 loop and the Cys89-Cys141 disulfide bond is important for maximal AprV2 protease activity.
Fig. 7. Characterisation of AprV2.C141S derivative. 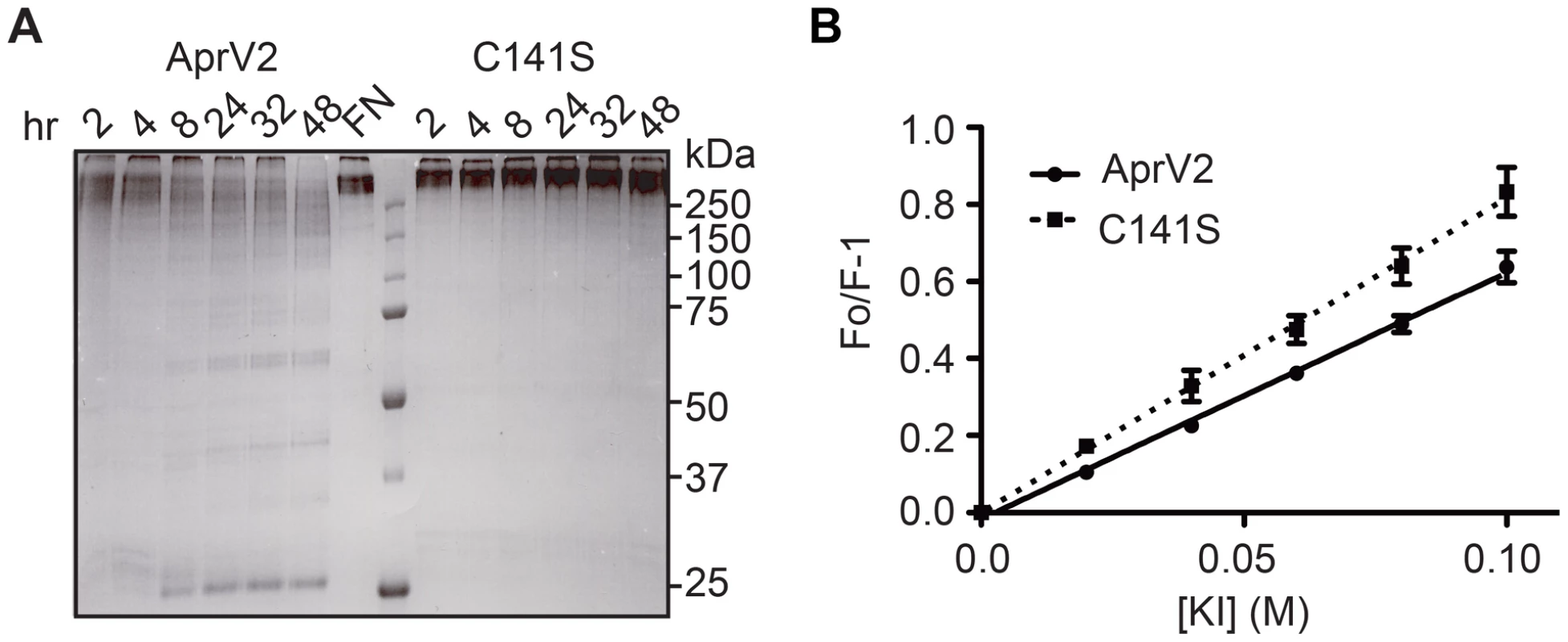
(A) Degradation of fibronectin by purified AprV2 and AprV2.C141S at 25°C. The degradation of fibronectin over time was assessed by SDS-PAGE analysis. (B) Stern-Volmer plot for iodide quenching of tryptophan in AprV2 and AprV2.C141S. Purified protease was incubated with increasing amounts of KI, and the fluorescence emission intensity was recorded. The lines represent a least squares fit of the experimental data as described previously [49]. The mean and s.e.m from three independent experiments are shown. Note that in these figures AprV2.C141S is shown as C141S. To determine whether the C141S substitution affects the conformation/mobility of the I2 loop we determined the crystal structure of AprV2.C141S to 2.1 Å (structure refinement statistics in Table 2). The structure of AprV2.C141S was very similar to that of AprV2, overlaying with an RMSD of 0.21 Å for 339 Cα. The structure confirmed the absence of the Cys89-Cys141 disulfide bond and revealed that while the I2 loop had a slightly different structure to that of AprV2 there were no significant differences in the structure of the active site or primary substrate binding site (Figure 8A and S6). The average B factors for the I2 loops in AprV2 and AprV2.C141S were 14.6 Å2 and 28.4 Å2, respectively, indicating that the I2 loop is more mobile in the substituted protease (Figure 8B,C). Given that the conformation of the I2 loop is stabilized by crystal packing in all three structures we investigated whether the I2 loop was more mobile in solution in AprV2.C141S using intrinsic tryptophan fluorescence spectroscopy; the I2 loop contains two tryptophans. Steady state fluorescence quenching data showed that the wild-type protease was more protected from quenching by potassium iodide than the C141S enzyme (Figure 7B), confirming that the conformation of the I2 loop in AprV2.C141S is different to that in the wild type.
Fig. 8. Crystal structure of AprV2.C141S. 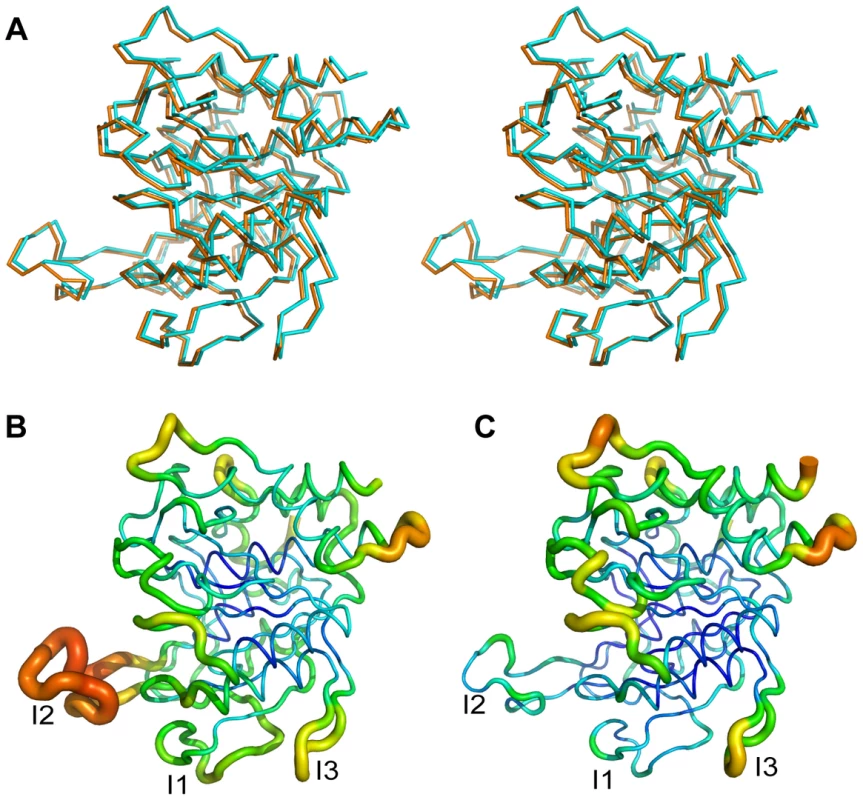
(A) Overlay of AprV2 (orange) with AprV2.C141S (cyan) shown in stereo. The structures are shown as a Cα trace. (B) and (C) Ribbon representation of AprV2.C141S (B) and AprV2 (C) coloured and sized according to B-factor. High B-factors are coloured red and shown as thicker ribbon. Low B-factors are coloured blue and shown as thinner ribbon. The alignment was generated using the program MUSTANG [52]. The figure was prepared using Pymol [51]. Discussion
Although the extracellular proteases of D. nodosus have been considered for many years to be potential virulence factors [9], their importance in the pathogenesis of disease had not been established. The analysis of their role in disease has always been complicated by the fact each isolate produces three very closely related proteases. The genetic approach utilized here has now provided clear evidence that the AprV2 protease is essential for virulence. This conclusion is based on data that showed that an aprV2 mutant was unable to cause footrot in sheep, unlike the isogenic wild-type strain, and that the ability to cause disease was restored to wild-type levels in the complemented derivative. No definitive conclusions could be drawn from the virulence testing of the aprV5 and bprV mutants. However, it is likely that the AprV5 and BprV proteases also play a role in disease, especially since the three secreted proteases appear to act synergistically, with the double protease mutants, aprV2aprV5, aprV2bprV, and aprV5bprV, showing lower secreted protease activity than that expected based on the secreted protease activity of the individual mutants (Figure 1B). It remains to be elucidated how this synergism arises, although it could occur at the processing, secretion or substrate level.
Benign and virulent strains of D. nodosus can be differentiated by phenotypic analysis of their extracellular proteases, including analysis of their elastase activity and thermostability [14], [16], [19], [20]. We now have established that AprV2 is responsible for the elastase activity of the virulent isolate VCS1703A. Purified AprB2, which differs in sequence from AprV2 only at residue 92, is less efficient at degrading elastin. Therefore, it was important to examine the effect on virulence of complementing the aprV2 mutant with aprB2. This AprV2−AprB2+ strain was benign by the standard laboratory tests used to differentiate virulent and benign strains. Unexpectedly, it was virulent in the sheep footrot trial, producing disease that was indistinguishable in footrot severity from that caused by the isogenic wild-type strain. Since this strain still produces both AprV5 and BprV we conclude that the presence of one benign protease (AprB2) in combination with two virulent proteases (AprV5 and BprV) is not sufficient to make a strain benign even though in a laboratory diagnostic context the strain would be designated as benign. Therefore, although it appears that the properties of AprV2 and AprB2 are responsible for the differentiation of benign and virulent strains in laboratory tests, there clearly are other virulence factors, such as the other virulent proteases, that contribute to virulent disease.
We have shown that AprV2 mediates the degradation of keratin (Figure 4), a component of the ovine hoof that confers physical protection and tissue integrity. This finding suggests that AprV2 has a direct role in destroying the keratin layer of the ovine hoof, a characteristic feature of virulent footrot. The initial site of D. nodosus attachment during infection is at the epidermal layer of the interdigital skin and degradation of keratin in this area by AprV2 is likely to be required to break through the skin horn junction, allowing the subsequent under-running of the horn by D. nodosus.
An intriguing feature identified in the structures of AprV2 and AprB2 is the disulphide-tethered I2 loop. This loop is located next to, and partially occludes, the substrate binding site of the enzyme (Figure 6). The single amino acid difference between AprV2 and AprB2 is located at the tip of the loop. We have shown that residue 92 is a key determinant of elastase activity. Although, substitution of Tyr92 with Arg reduced the degradation of insoluble elastin, no significant differences in either KM or Vmax were observed for hydrolysis of the elastin-like peptide, AAPVn. This result suggests that residue 92 does not contribute to catalysis at the active site of the enzyme. Instead, the reduced elastinolytic activity of AprB2 is likely to arise from impaired enzyme-substrate interactions at a site distal to the active site. We therefore propose that the I2 loop functions as an exosite, mediating the formation of a stable enzyme-substrate complex. The disulphide bond tethering the I2 loop appears to be important for this function since its disruption alters the mobility of the loop, which significantly reduces the ability of the protease to degrade fibronectin, insoluble elastin and other proteins from the sheep hoof.
We have identified several serine proteases that like AprV2 also appear to contain large insertions between the β1 strand and α2 helix (the location of the I1 and I2 loops) (Figure S3). These enzymes include MprA, from Burkholderia pseudomallei (the causative agent of melioidosis), TgSUB1 and TgSUB2, from Toxoplasma gondii (the causative agent of toxoplasmosis) and PfSUB1 from the malaria parasite Plasmodium falciparum. MprA degrades physiologically relevant proteins and may play a role in causing the lung damage associated with melioidosis [21], [22], however, it has only a minor role in virulence [23]. PfSUB1 and TgSUB2 appear to be critical for parasite survival [24], [25]. The presence of the I2-like insertions in these proteins suggests that the mechanism of exosite loop-mediated proteolysis used by the D. nodosus secreted proteases may represent a mechanism of substrate recognition that is utilized by other bacterial proteases. Studies investigating the structure of these proteins along with the function of their I2-like insertions will shed some light on this hypothesis and may lead to the development of improved diagnostic reagents and the identification of novel vaccine and drug targets.
Materials and Methods
Construction and characterization of D. nodosus mutants and complemented strains
Strains and plasmids are detailed in Table 3. D. nodosus strains were routinely grown in an anaerobic chamber (Coy Laboratory Products Inc.) as described previously [6].
Tab. 3. Bacterial strains and plasmids. 
*Independently derived mutants. To construct the single mutants, suicide plasmids were inserted into D. nodosus strain VCS1703A by natural transformation [6]. The aprV2 and bprV mutants were complemented by transforming the mutants with the relevant plasmids, which reconstituted the disrupted gene and inserted a different resistance marker. The aprV5 mutant was complemented by inserting an intact copy of aprV5 and a kanamycin resistance marker into one of the three rrnA operons. The aprV2bprV double mutant was constructed by inserting the bprV suicide plasmid into an aprV2 mutant, and the aprV2aprV5 double mutant constructed by inserting the aprV2 suicide plasmid into an aprV5 mutant. An aprV5bprV double mutant was constructed by inserting a suicide plasmid into the wild-type strain, which disrupted both genes when a double crossover event occurred. Finally, a triple aprV2aprV5bprV mutant was constructed by inserting the aprV5bprV suicide plasmid into the aprV2 mutant.
All mutants and complemented strains were confirmed by PCR and Southern hybridizations. PCR-RFLP analysis of the omp gene family was used to confirm that mutants were derived from the wild-type strain [26]. Elastase activity and protease thermostability assays for the differentiation of benign and virulent strains of D. nodosus were as described previously [14], [16], [27].
Virulence testing in sheep
Virulence testing in sheep was performed as before [6], [7]. The sheep were randomly allocated into nine groups of eight sheep and challenged blind with the various strains. A plain agar challenge was used as the negative control. The feet of all animals were examined and scored for footrot lesions at the start of the trial and then at weekly intervals using a standard lesion scoring method [28], [29]. The total weighted foot score (TWFS) was used to provide an unambiguous overall footrot score for each animal [29]. The trial was carried out in a PC2 containment facility at Elizabeth Macarthur Agricultural Institute in accordance with the guidelines of the Australian Government Office of the Gene Technology Regulator and the Elizabeth Macarthur Agricultural Institute Animal Ethics Committee.
Measurement of protease activity
All assays were performed in 20 mM Tris-HCl pH 8 and 5 mM CaCl2 (buffer A) with the exception of the Elastin-Congo Red elastase assay, which was performed in 25 mM Bis-Tris pH6.5, 150 mM NaCl, 5 mM CaCl2 and 5% glycerol. Quantitative determination of total protease activity or elastase activity was carried out using azocasein [26] or Elastin-Congo Red [30] assays, respectively. Degradation of AAPVn by recombinant protease (1 µM) was measured at 25°C as described [31]. KM and Vmax were determined by plotting initial velocities against AAPVn concentration and fitted by non-linear regression (Prism). Fibronectin degradation was measured by incubating human fibronectin (1 µM, BD Biosciences) with recombinant protease (0.1 µM) at 25°C. Cleavage products were visualised by SDS-PAGE. Proteolytic degradation of hoof was determined by incubating dissected hoof material (2.2% (w/v) in buffer A) from a disease-free sheep with recombinant protease (110 µg/ml) at 25°C. Samples were taken over a 16 hour period and degradation products were visualised by SDS-PAGE.
Proteolytic digestion of hoof and substrate identification by in-gel tryptic digestion and LCMS
Hoof material (14% (w/v) in buffer A) from a disease-free sheep was incubated with 100 µg of recombinant protease at 25°C for 18 h. Degradation products were separated by SDS-PAGE. The bands were excised and subjected to in-gel tryptic digestion and the digests analysed by LC-MS/MS using a HCT ULTRA ion trap mass spectrometer (BrukerDaltonics) coupled online with a 1200 series capillary HPLC (Agilent technologies). Proteins were identified by searching the LC-MS/MS data against the National Center for Biotechnology Information (NCBI) non-redundant and Swiss-Prot databases using the MASCOT search engine (version 2.1, Matrix Science Inc.) with all taxonomy selected.
Protein production, crystallisation and data collection
AprV2 and AprB2 were purified and crystallised as before [32]. Data collection statistics have been reported [32]. The expression construct for AprV2.C141S was generated using the Quikchange site-directed mutagenesis kit (Stratagene) and pET22b.AprV2 as the template. Expression, purification and crystallisation of AprV2.C141S were as for AprV2. Data collection statistics for AprV2.C141S are in Table S1.
Structure determination and refinement
Unless stated otherwise, all programs used for structural and crystallographic analysis were located within the CCP4 interface [33] to the CCP4 suite [34]. Manual building and maximum likelihood refinement were carried out using COOT [35] and REFMAC5 [36], respectively. The protease structures were solved by molecular replacement using PHASER [37]. A search model for AprB2 was derived from the coordinates of Bacillus Ak.1 protease (PDB code 1DBI [38]), identified using the FFAS server [39]. The search model was generated using the SCRWL server and consisted of all conserved side-chains with the remaining non-alanine/glycine residues truncated at the Cγ atom [40]. The initial model of AprB2 was subject to several iterations of manual building and refinement. The model was then subjected to automatic building using ARP/wARP [41] before the structure was completed by more cycles of manual building and refinement. The refined AprB2 structure with the I2 loop deleted was used as the MR search model for AprV2 and AprV2.C141S. The initial models were subjected to simulated annealing using PHENIX [42], [43]. Successive rounds of manual building and refinement incorporating TLS [44] generated the final models. Water molecules were added to all models using ARP/warp v 5.0 [45], [46]. Structure validation was carried out using MolProbity [47] and COOT [35]. Refinement statistics for the structures determined are presented in Table 2. The coordinates and structure factors are available from the Protein Data Bank (2LPA; 2LPC; 2LPC). Raw data and images are available from TARDIS (www.tardis.edu.au) [48].
Fluorescence quenching experiments
Recombinant protease (0.5 µM in buffer A) was incubated with increasing amounts of quenching solution (2 M KI and 1 mM Na2S2O3) and the change in the fluorescence emission intensity of the tryptophan residues (λex290 nm/λem340 nm) was measured using a Perkin-Elmer LS50B spectrofluorometer. The data were analysed as before [49].
Ethics statement
The sheep virulence experiments were carried out in a PC2 containment facility at the Elizabeth Macarthur Agricultural Institute in accordance with the guidelines of the Australian Government Office of the Gene Technology Regulator and the Elizabeth Macarthur Agricultural Institute Animal Ethics Committee. These experiments were approved by the Elizabeth Macarthur Agricultural Institute Animal Ethics Committee.
Supporting Information
Zdroje
1. StewartDJ
1989 Footrot of sheep.
EgertonJR
YongWK
RiffkinGG
Footrot and foot abscess of ruminants Boca Raton CRC Press 5 45
2. GreenLE
GeorgeTR
2008 Assessment of current knowledge of footrot in sheep with particular reference to Dichelobacter nodosus and implications for elimination or control strategies for sheep in Great Britain. Vet J 175 173 180
3. WaniSA
SamantaI
2006 Current understanding of the aetiology and laboratory diagnosis of footrot. Vet J 171 421 428
4. EgertonJR
RobertsDS
ParsonsonIM
1969 The aetiology and pathogenesis of ovine footrot. I. A histological study of the bacterial invasion. J Comp Pathol 81 179 185
5. StewartDJ
ClarkBL
JarretRG
1984 Differences between strains of Bacteroides nodosus in their effects on the severity of footrot, bodyweight and wool growth on Merino sheep. Aust Vet J 61 349 352
6. KennanRM
DhungyelOP
WhittingtonRJ
EgertonJR
RoodJI
2001 The type IV fimbrial subunit gene (fimA) of Dichelobacter nodosus is essential for virulence, protease secretion, and natural competence. J Bacteriol 183 4451 4458
7. HanX
KennanRM
DaviesJK
ReddacliffLA
DhungyelOP
2008 Twitching motility is essential for virulence in Dichelobacter nodosus. J Bacteriol 190 3323 3335
8. KorttAA
RiffkinMC
FocaretaA
StewartDJ
1993 Amino acid sequence of extracellular acidic protease V5 of Dichelobacter nodosus, the causative organism of ovine footrot. Biochem Mol Biol Int 29 989 998
9. BillingtonSJ
JohnstonJL
RoodJI
1996 Virulence regions and virulence factors of the ovine footrot pathogen, Dichelobacter nodosus. FEMS Microbiol Lett 145 147 156
10. LilleyGG
StewartDJ
KorttAA
1992 Amino acid and DNA sequences of an extracellular basic protease of Dichelobacter nodosus show that it is a member of the subtilisin family of proteases. Eur J Biochem 210 13 21
11. RiffkinMC
FocaretaA
EdwardsRD
StewartDJ
KorttAA
1993 Cloning, sequence and expression of the gene (aprV5) encoding extracellular serine acidic protease V5 from Dichelobacter nodosus. Gene 137 259 264
12. RiffkinMC
WangLF
KorttAA
StewartDJ
1995 A single amino-acid change between the antigenically different extracellular serine proteases V2 and B2 from Dichelobacter nodosus. Gene 167 279 283
13. SiezenRJ
LeunissenJA
1997 Subtilases: the superfamily of subtilisin-like serine proteases. Protein Sci 6 501 523
14. StewartDJ
1979 The role of elastase in the differentiation of Bacteroides nodosus infections in sheep and cattle. Res Vet Sci 27 99 105
15. DepiazziLJ
HendersonJ
PenhaleWJ
1990 Measurement of protease thermostability, twitching motility and colony size of Bacteroides nodosus. Vet Microbiol 22 353 363
16. PalmerMA
1993 A gelatin test to detect activity and stability of proteases produced by Dichelobacter nodosus. Vet Microbiol 36 113 122
17. LilleyGG
RiffkinMC
StewartDJ
KorttAA
1995 Nucleotide and deduced protein sequence of the extracellular, serine basic protease gene (bprB) from Dichelobacter nodosus strain 305: comparison with the basic protease gene (bprV) from virulent strain 198. Biochem Mol Biol Int 36 101 111
18. KorttAA
StewartDJ
1994 Properties of the extracellular acidic proteases of Dichelobacter nodosus. Stability and specificity of peptide bond cleavage. Biochem Mol Biol Int 34 1167 1176
19. DepiazziLJ
RichardsRB
1979 A degrading proteinase test to distinguish benign and virulent ovine isolates of Bacteroides nodosus. Aust Vet J 55 25 28
20. LiuD
YongWK
1993 Use of elastase test, gelatin gel test and electrophoretic zymogram to determine virulence of Dichelobacter nodosus isolated from ovine foot rot. Res Vet Sci 55 124 129
21. LeeMA
LiuY
2000 Sequencing and characterization of a novel serine metalloprotease from Burkholderia pseudomallei. FEMS Microbiol Lett 192 67 72
22. ChinC-Y
OthmanR
NathanS
2007 The Burkholderia pseudomallei serine protease MprA is autoproteolytically activated to produce a highly stable enzyme. Enzyme Microb Technol 40 370 377
23. ValadeE
ThibaultFM
GauthierYP
PalenciaM
PopoffMY
2004 The PmlI-PmlR quorum-sensing system in Burkholderia pseudomallei plays a key role in virulence and modulates production of the MprA protease. J Bacteriol 186 2288 2294
24. YeohS
O'DonnellRA
KoussisK
DluzewskiAR
AnsellKH
2007 Subcellular discharge of a serine protease mediates release of invasive malaria parasites from host erythrocytes. Cell 131 1072 1083
25. MillerSA
ThathyV
AjiokaJW
BlackmanMJ
KimK
2003 TgSUB2 is a Toxoplasma gondii rhoptry organelle processing proteinase. Mol Microbiol 49 883 894
26. KennanRM
DhungyelOP
WhittingtonRJ
EgertonJR
RoodJI
2003 Transformation-mediated serogroup conversion of Dichelobacter nodosus. Vet Microbiol 92 169 178
27. KorttAA
BurnsJE
StewartDJ
1983 Detection of the extracellular proteases of Bacteroides nodosus in polyacrylamide gels: a rapid method of distinguishing virulent and benign ovine isolates. Res Vet Sci 35 171 174
28. EgertonJR
RobertsDS
1971 Vaccination against ovine foot-rot. J Comp Pathol 81 179 185
29. WhittingtonRJ
NichollsPJ
1995 Grading the lesions of ovine footrot. Res Vet Sci 58 26 34
30. OhmanDE
CryzSJ
IglewskiBH
1980 Isolation and characterization of Pseudomonas aeruginosa PAO mutant that produces altered elastase. J Bacteriol 142 836 842
31. BetsuyakuT
NishimuraM
YoshiokaA
TakeyabuK
MiyamotoK
1996 Elastin-derived peptides and neutrophil elastase in bronchoalveolar lavage fluid. Am J Respir Crit Care Med 154 720 724
32. WongW
KennanR
RosaldoCJ
RoodJI
WhisstockJ
2010 Crystallization of the virulent and benign subtilisin-like proteases from the ovine foot-rot pathogen, Dichelobacter nodosus. Acta Crystallogr F 66 289 293
33. PottertonE
BriggsP
TurkenburgM
DodsonE
2003 A graphical user interface to the CCP4 program suite. Acta Crystallogr D Biol Crystallogr 59 1131 1137
34. Collaborative Computational Project N 1994 The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr 50 760 763
35. EmsleyP
CowtanK
2004 Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60 2126 2132
36. MurshudovGN
VaginAA
DodsonEJ
1997 Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr 53 240 255
37. McCoyAJ
Grosse-KunstleveRW
StoroniLC
ReadRJ
2005 Likelihood-enhanced fast translation functions. Acta Crystallogr D Biol Crystallogr 61 458 464
38. SmithCA
ToogoodHS
BakerHM
DanielRM
BakerEN
1999 Calcium-mediated thermostability in the subtilisin superfamily: the crystal structure of Bacillus Ak.1 protease at 1.8 A resolution. J Mol Biol 294 1027 1040
39. JaroszewskiL
RychlewskiL
LiZ
LiW
GodzikA
2005 FFAS03: a server for profile--profile sequence alignments. Nucleic Acids Res 33 W284 288
40. CanutescuAA
ShelenkovAA
DunbrackRLJr
2003 A graph-theory algorithm for rapid protein side-chain prediction. Protein Sci 12 2001 2014
41. LangerG
CohenSX
LamzinVS
PerrakisA
2008 Automated macromolecular model building for X-ray crystallography using ARP/wARP version 7. Nat Protoc 3 1171 1179
42. AfoninePV
Grosse-Kunstleve
AdamsRW
2005 The Phenix refinement framework. CCP4 Newsletter 42 Contribution 8
43. BrungerAT
KuriyanJ
KarplusM
1987 Crystallographic R Factor Refinement by Molecular Dynamics. Science 235 458 460
44. PainterJ
MerrittEA
2006 Optimal description of a protein structure in terms of multiple groups undergoing TLS motion. Acta Crystallogr D Biol Crystallogr 62 439 450
45. PerrakisA
SixmaTK
WilsonKS
LamzinVS
1997 wARP: improvement and extension of crystallographic phases by weighted averaging of multiple-refined dummy atomic models. Acta Crystallogr D Biol Crystallogr 53 448 455
46. LamzinVS
WilsonKS
1993 Automated refinement of protein models. Acta Crystallogr D Biol Crystallogr 49 129 147
47. DavisIW
Leaver-FayA
ChenVB
BlockJN
KapralGJ
2007 MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res 35 W375 383
48. AndroulakisS
SchmidbergerJ
BateMA
DeGoriR
BeitzA
2008 Federated repositories of X-ray diffraction images. Acta Crystallogr D Biol Crystallogr D64 810 814
49. LehrerSS
1971 Solute perturbation of protein fluorescence. The quenching of the tryptophyl fluorescence of model compounds and of lysozyme by iodide ion. Biochemistry 10 3254 3263
50. PottertonL
McNicholasS
KrissinelE
GruberJ
CowtanK
2004 Developments in the CCP4 molecular-graphics project. Acta Crystallogr D Biol Crystallogr 60 2288 2294
51. DeLanoWL
2002 The PyMOL User's Manual Scientific D, editor. San Carlos, CA, USA
52. KonagurthuAS
WhisstockJC
StuckeyPJ
LeskAMPS
2006 MUSTANG: A multiple structural alignment algorithim. Proteins: Structure, Function and Bioinformatics 64 559 574
53. FrishmanD
ArgosP
1995 Knowledge-based protein secondary structure assignment. Proteins 23 566 579
54. VieiraJ
MessingJ
1982 The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19 259 268
55. WangRF
KushnerSR
1991 Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100 195 199
56. GallagherT
OliverJ
BottR
BetzelC
GillilandGL
1996 Subtilisin BPN' at 1.6 A resolution: analysis for discrete disorder and comparison of crystal forms. Acta Crystallogr 52 1125 1135
57. GouetP
CourcelleE
StuartDI
MetozF
1999 ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics 15 305 308
58. ThompsonJD
HigginsDG
GibsonTJ
1994 Clustal W: improving the sensitivity of progressive multiple sequence alignments through sequence weighing, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res 22 4673 4680
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease AgentČlánek TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease SusceptibilityČlánek The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive ParasiteČlánek Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Patients with Discordant Responses to Antiretroviral Therapy Have Impaired Killing of HIV-Infected T Cells
- A Molecular Mechanism for Eflornithine Resistance in African Trypanosomes
- Tyrosine Sulfation of the Amino Terminus of PSGL-1 Is Critical for Enterovirus 71 Infection
- Autoimmunity as a Predisposition for Infectious Diseases
- The Subtilisin-Like Protease AprV2 Is Required for Virulence and Uses a Novel Disulphide-Tethered Exosite to Bind Substrates
- Structural Analysis of HIV-1 Maturation Using Cryo-Electron Tomography
- Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease Agent
- Interferon-Inducible CXC Chemokines Directly Contribute to Host Defense against Inhalational Anthrax in a Murine Model of Infection
- TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease Susceptibility
- The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive Parasite
- CO Acts as a Signalling Molecule in Populations of the Fungal Pathogen
- SV2 Mediates Entry of Tetanus Neurotoxin into Central Neurons
- MAP Kinase Phosphatase-2 Plays a Critical Role in Response to Infection by
- Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
- Potentiation of Epithelial Innate Host Responses by Intercellular Communication
- Fcγ Receptor I Alpha Chain (CD64) Expression in Macrophages Is Critical for the Onset of Meningitis by K1
- ANK, a Host Cytoplasmic Receptor for the Cell-to-Cell Movement Protein, Facilitates Intercellular Transport through Plasmodesmata
- Analysis of the Initiating Events in HIV-1 Particle Assembly and Genome Packaging
- Evolution of Linked Avirulence Effectors in Is Affected by Genomic Environment and Exposure to Resistance Genes in Host Plants
- Structural Basis of HIV-1 Neutralization by Affinity Matured Fabs Directed against the Internal Trimeric Coiled-Coil of gp41
- Hepatitis C Virus (HCV) Evades NKG2D-Dependent NK Cell Responses through NS5A-Mediated Imbalance of Inflammatory Cytokines
- Host Cell Invasion and Virulence Mediated by Ssa1
- Global Gene Expression in Urine from Women with Urinary Tract Infection
- Should the Human Microbiome Be Considered When Developing Vaccines?
- HapX Positively and Negatively Regulates the Transcriptional Response to Iron Deprivation in
- Enhancing Oral Vaccine Potency by Targeting Intestinal M Cells
- Herpes Simplex Virus Reorganizes the Cellular DNA Repair and Protein Quality Control Machinery
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- Cryo Electron Tomography of Native HIV-1 Budding Sites
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- Modelling the Evolution and Spread of HIV Immune Escape Mutants
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
- Platelet-Activating Factor Receptor Plays a Role in Lung Injury and Death Caused by Influenza A in Mice
- Genetic and Structural Basis for Selection of a Ubiquitous T Cell Receptor Deployed in Epstein-Barr Virus Infection
- Ubiquitin-Regulated Nuclear-Cytoplasmic Trafficking of the Nipah Virus Matrix Protein Is Important for Viral Budding
- Pneumolysin Activates the NLRP3 Inflammasome and Promotes Proinflammatory Cytokines Independently of TLR4
- Immune Evasion by : Differential Targeting of Dendritic Cell Subpopulations
- Survival in Selective Sand Fly Vector Requires a Specific -Encoded Lipophosphoglycan Galactosylation Pattern
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



