-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
MAP Kinase Phosphatase-2 Plays a Critical Role in Response to Infection by
In this study we generated a novel dual specific phosphatase 4 (DUSP4) deletion mouse using a targeted deletion strategy in order to examine the role of MAP kinase phosphatase-2 (MKP-2) in immune responses. Lipopolysaccharide (LPS) induced a rapid, time and concentration-dependent increase in MKP-2 protein expression in bone marrow-derived macrophages from MKP-2+/+ but not from MKP-2−/− mice. LPS-induced JNK and p38 MAP kinase phosphorylation was significantly increased and prolonged in MKP-2−/− macrophages whilst ERK phosphorylation was unaffected. MKP-2 deletion also potentiated LPS-stimulated induction of the inflammatory cytokines, IL-6, IL-12p40, TNF-α, and also COX-2 derived PGE2 production. However surprisingly, in MKP-2−/− macrophages, there was a marked reduction in LPS or IFNγ-induced iNOS and nitric oxide release and enhanced basal expression of arginase-1, suggesting that MKP-2 may have an additional regulatory function significant in pathogen-mediated immunity. Indeed, following infection with the intracellular parasite Leishmania mexicana, MKP-2−/− mice displayed increased lesion size and parasite burden, and a significantly modified Th1/Th2 bias compared with wild-type counterparts. However, there was no intrinsic defect in MKP-2−/− T cell function as measured by anti-CD3 induced IFN-γ production. Rather, MKP-2−/− bone marrow-derived macrophages were found to be inherently more susceptible to infection with Leishmania mexicana, an effect reversed following treatment with the arginase inhibitor nor-NOHA. These findings show for the first time a role for MKP-2 in vivo and demonstrate that MKP-2 may be essential in orchestrating protection against intracellular infection at the level of the macrophage.
Published in the journal: . PLoS Pathog 6(11): e32767. doi:10.1371/journal.ppat.1001192
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001192Summary
In this study we generated a novel dual specific phosphatase 4 (DUSP4) deletion mouse using a targeted deletion strategy in order to examine the role of MAP kinase phosphatase-2 (MKP-2) in immune responses. Lipopolysaccharide (LPS) induced a rapid, time and concentration-dependent increase in MKP-2 protein expression in bone marrow-derived macrophages from MKP-2+/+ but not from MKP-2−/− mice. LPS-induced JNK and p38 MAP kinase phosphorylation was significantly increased and prolonged in MKP-2−/− macrophages whilst ERK phosphorylation was unaffected. MKP-2 deletion also potentiated LPS-stimulated induction of the inflammatory cytokines, IL-6, IL-12p40, TNF-α, and also COX-2 derived PGE2 production. However surprisingly, in MKP-2−/− macrophages, there was a marked reduction in LPS or IFNγ-induced iNOS and nitric oxide release and enhanced basal expression of arginase-1, suggesting that MKP-2 may have an additional regulatory function significant in pathogen-mediated immunity. Indeed, following infection with the intracellular parasite Leishmania mexicana, MKP-2−/− mice displayed increased lesion size and parasite burden, and a significantly modified Th1/Th2 bias compared with wild-type counterparts. However, there was no intrinsic defect in MKP-2−/− T cell function as measured by anti-CD3 induced IFN-γ production. Rather, MKP-2−/− bone marrow-derived macrophages were found to be inherently more susceptible to infection with Leishmania mexicana, an effect reversed following treatment with the arginase inhibitor nor-NOHA. These findings show for the first time a role for MKP-2 in vivo and demonstrate that MKP-2 may be essential in orchestrating protection against intracellular infection at the level of the macrophage.
Introduction
The mitogen-activated protein (MAP) kinase phosphatases (MKPs) are a family of dual specific phosphatases which regulate the functional activity of the major MAP kinase subfamilies through tyrosine and threonine dephosphorylation [1]. At least eleven isoforms exist each with different structures, subcellular distributions, substrate specificity and mechanisms of regulation. For example, the prototypic MKP-1 is induced by a wide variety of extracellular signals, is strictly nuclear located, and is able to dephosphorylate all MAP kinases, whereas MKP-3 is constitutively expressed, cytosolic and selective for extracellular regulated kinase (ERK) above the other major MAP kinases, c-Jun N-terminal kinase (JNK) and p38 MAP kinase. Thus, the action of one or more MKP is essential for the tight regulation of MAP kinase activity and subsequent functional responses mediated by a vast array of extracellular stimuli [1].
A number of MKPs have been implicated in the regulation of disease. Dysfunction or changes in expression of MKPs is a feature of a number of cancers [1], whilst roles in gluconeogenesis, insulin resistance and diabetes have also been established [2], [3]. Recent evidence also implicates a role in the regulation of immune responses [4]. Deletion of MKP-1 [5] and PAC-1 [6] have been shown to both enhance and reduce LPS mediated cellular responses respectively, whilst MKP-5 is thought to regulate adaptive immunity via effects upon T-cells [7]. Furthermore, one of the main anti-inflammatory effects of dexamethasone is attributed to the induction of MKP-1 and the subsequent inhibition of p38 MAP kinase [8].
MAP kinase phosphatase-2 (MKP-2) is a class I DUSP [9], induced by growth factors, hormones and stress agents such as hydrogen peroxide. It is nuclear located due to two nuclear location sequences [10] and dephosphorylates ERK and JNK in vitro [11] whilst being ineffective for p38 MAP kinase despite binding strongly to this kinase [12]. Cellular studies suggest the potential of cell type specificity as stable or conditional over-expression of MKP-2 selectively inhibits JNK in epithelial cells types [13], [14]. Although often described as a surrogate to MKP-1, MKP-2 has been demonstrated to have roles in cellular apoptosis and senescence [13], [15]. A number of recent indirect studies have also indirectly implicated MKP-2 in cancer [16]. However, the function of MKP-2 in the immune system remains uncharacterised due to the lack of suitable MKP-2 (DUSP-4) knockout mouse models.
In this study we examined the function of the MKP-2 (DUSP4) gene using a novel MKP-2 knockout mouse. In macrophages derived from MKP-2−/− mice, we find that its deletion results in enhanced JNK and p38 MAP kinase activation but not, as expected, increased ERK phosphorylation. Increased IL-6, IL-12, TNFα and PGE2 production suggested that MKP-2 may modify the innate immune response in a manner similar to that observed in the MKP-1 deletion model. However, in contrast to these changes we observed marked reduction in inducible nitric oxide (iNOS) expression and enhanced arginase-1 activation indicative of an additional regulatory function. We found that that bone-marrow derived macrophages from MKP-2−/− mice were more susceptible to infection with the intracellular parasite Leishmania (L.) mexicana than macrophages from their wild-type counterparts. The enhanced susceptibility of MKP-2−/− macrophages was reversed using the arginase inhibitor nor-NOHA. Consequently following infection with L. mexicana MKP-2−/− mice had limited ability to control lesion development and parasite growth. This was related to a significant down-regulation of specific Th1 activity in MKP-2 deficient mice. Taken together, our results demonstrate for the first time that MKP-2 plays a functional role in limiting immune responses associated with the macrophage lineage and indicates for the first time that MKP-2 is not a functionally redundant DUSP in vivo.
Results
MKP-2 deletion in macrophages
The MKP-2 deletion strategy is outlined in Figure 1A. Using targeted homologous recombination exons 2–4 were removed. Therefore, most of the open reading frame including the phosphatase catalytic domain and the 3′UTR region of the DUSP4 gene was deleted. Although exon1, which comprises the 5′UTR, the start codon and the KIM domain, remained unchanged, the occurrence of a truncated protein was unlikely since the poly A tail necessary for any kind of protein synthesis was removed. Deletion was confirmed by Southern Blotting (insert), which identified both the wild type 9.9 kB fragment and the mutated 8.3 kB fragment and by PCR (not shown). In Panels B & C, bone marrow derived macrophages were assessed for the presence of MKP-2 protein by Western blotting. In wild type cells, LPS stimulated MKP-2 expression in a concentration-dependent manner, giving a maximum response at approximately 1 µg/ml LPS (Panel B). Induction was also time dependent reaching a peak at 2 h following LPS treatment (Panel C). No induction was observed in macrophages derived from MKP-2−/− mice.
Fig. 1. Generation of mice lacking DUSP4/MKP-2 gene by targeted homologous recombination. 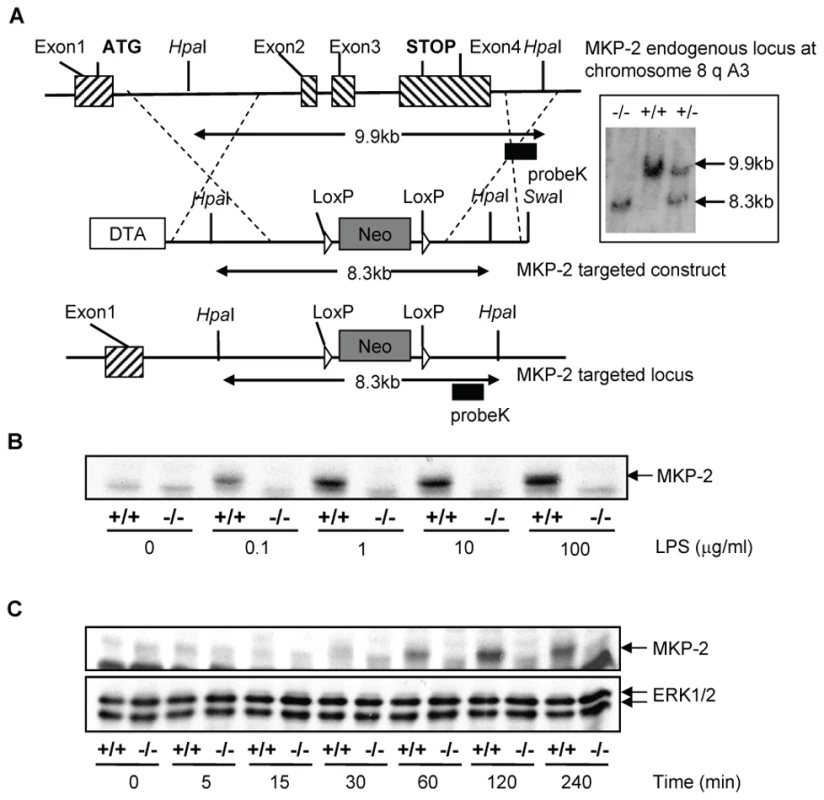
(Panel A): Schematic showing the DUSP4/MKP-2 gene locus, the targeted construct and the resulting targeted allele. Recombination events are indicated by dashed lines and show the replacement of an 8.3 kb SwaI DUSP4/MKP-2 genomic fragment containing exons 2–4 by the PGK-Neo cassette. SwaI and HpaI described the restriction sites for the respective enzymes. The pGK-Neo cassette is flanked by LoxP sites. DTA represents the negative selection cassette. (Inset) An example of the 3′ southern blot analysis of mouse tail tip genomic DNA following digestion with HpaI using an external Probe K as indicated in panel A. The autoradiography revealed the 9.9 kb (wild type) and 8.3 kb (targeted) fragments representing the two different alleles discriminating wild type, heterozygote or homozygote mutant animals. B & C: Concentration (LPS µg/ml for 60 min) and time dependent (LPS 100 ng/ml) expression of MKP-2 in bone marrow derived macrophages. The blot represents at least 4 individual experiments. MKP-2 deletion enhances MAP kinase signalling
We then examined the effect of MKP-2 deletion on LPS-induced kinase phosphorylation (Figure 2) as MKP-2 has previously been shown to dephosphorylate ERK and JNK in vitro [11]. In MKP-2−/− macrophages, LPS-stimulated JNK phosphorylation was potentiated and prolonged (Panel A *p<0.05). Enhanced JNK activity was confirmed by JNK in vitro kinase assay and Western blotting for serine-63 phosphorylation of c-Jun (Supplementary Figure S1). Surprisingly, LPS-stimulated p38 MAP kinase phosphorylation (Panel B) was also found to be enhanced in MKP-2 −/− mice, despite p38 MAP kinase not being a recognised substrate for MKP-2 in vitro [11]. In contrast, ERK activation was not altered in MKP-2−/− macrophages at any time point studied or over different concentration ranges (Panel C). Preliminary results, however, showed a lack of ERK translocation to the nucleus (results not shown) suggesting a lack of access of ERK to the phosphatase rather than a lack of MKP-2 activity on ERK as the explanation for this finding.
Fig. 2. MKP-2 deletion enhances LPS -stimulated MAP kinase phosphorylation in macrophages. 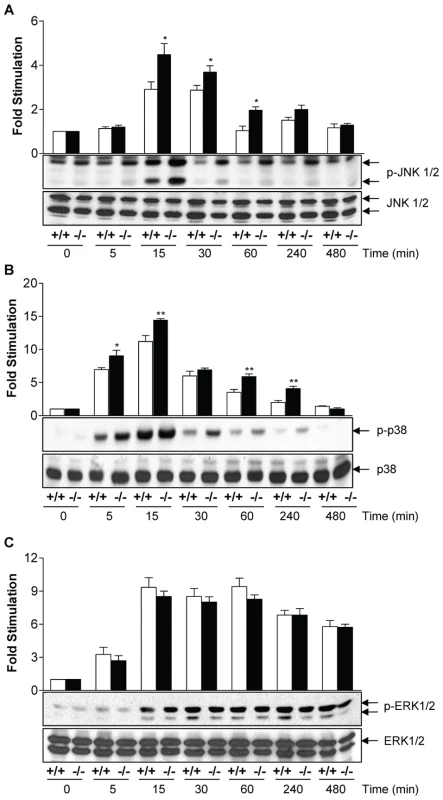
Cells were incubated with LPS (100 ng/ml) for the indicated time and whole cell lysates were prepared, and assessed for JNK (Panel A), p38 MAP kinase (Panel B) or ERK (Panel C) phosphorylation or total levels by Western blotting as outlined in the Methods section. Each blot is representative of 4 individual experiments. * P<0.05 relative to MKP-2+/+ macrophages. Cytokine induction modified in MKP-2 knockout macrophages
Having established that enhanced kinase activation occurred in macrophages from MKP-2−/− mice, we also assessed the consequences of MKP-2 deletion on the expression and release of key cytokines, known to be regulated by MAP kinase activation. Figure 3 shows cytokine production in macrophages derived from both wild type and MKP-2−/− mice. Panel A shows that LPS induced a substantial increase in IL-6 production manifest at 6–8 h and reaching a peak after 24 h. MKP-2 deletion had little effect upon basal levels, however, but markedly enhanced the rate and magnitude of production stimulated by LPS (24 hr: MKP-2+/+ = 1.83±0.01 ng/ml, MKP-2−/− = 4.44±0.83 n = 4, P<.005). Similar results were observed for both IL-12, which was assayed as IL-12p40/70 (Panel B), and TNFα (Panel C) although production reached a peak at 48 h and the degree of potentiation, whilst significant, was less than that for IL-6. In contrast, LPS stimulated IL-10 production was markedly reduced in MKP-2 deficient macrophages (Panel D).
Fig. 3. Cytokine production in macrophages derived from MKP-2−/− mice. 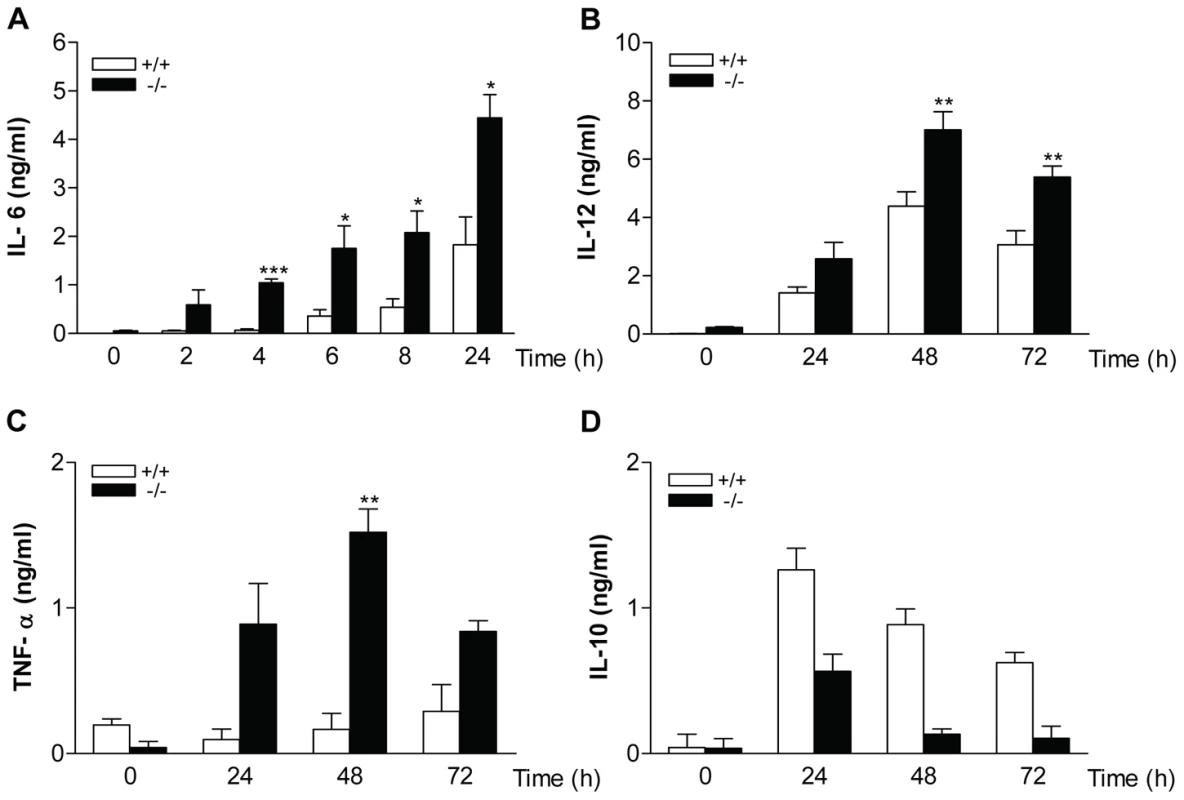
Cells were stimulated with LPS (100 ng/ml) for the indicated times and supernatants assessed for either IL-6 (panel A), IL-12 (Panel B), TNFα (Panel C) or IL-10 (Panel D) as outlined in the Methods section. Each value represents the mean ± SEM from at least 4 individual experiments. * P<0.05 compared to MKP-2 +/+ macrophages. Reciprocal effects upon iNOS and arginase-1 following MKP-2 deletion
We then assessed the expression of a number of inflammatory proteins following MKP-2 deletion (Figure 4). Panel A shows that in MKP-2−/− macrophages the rate of onset of cyclo-oxygenase-2 (COX-2) expression was increased in response to LPS, relative to wild type controls, an outcome predicted by previous studies assessing MAP kinase regulation of COX-2 expression and consistent with enhanced JNK and p38 MAP kinase activation. A difference in induction was observed as early as 2 and 4 h and was consistent with increased PGE2 production (Panel A and histogram D). We also assayed inducible nitric oxide synthase (iNOS) expression following incubation with LPS for up to 24 h. However, rather than being enhanced, iNOS expression was strongly inhibited relative to wild type controls and this was reflected in reduced formation of nitric oxide - derived nitrate and nitrite (Figure 4, Panel B and histogram E). This strong inhibitory effect was reproduced when IFN-γ was used to induce iNOS instead of LPS (Supplementary Figure S2). IFN-γ is known to regulate iNOS expression via the JAK/STAT pathway but no differences where found in the activation of these intermediates in MKP-2+/+ and MKP-2−/− macrophages (Supplementary Figure S2). We also determined if other macrophage proteins were down regulated to a similar extent, specifically arginase-1, a protein stimulated through the alternative macrophage activation pathway and utilising the same substrate, L-arginine, as iNOS (Panel C). Basal arginase-1 expression and activity was considerably higher in MKP-2−/− macrophages, and equivalent to 24 h of IL-4 stimulation in MKP-2+/+ samples (Panel C and histogram F). These results contrasted greatly with the expression of iNOS, which is indicative of classical macrophage activation and shows that the MKP-2−/− macrophages intrinsically have a unique profile of inflammatory protein expression.
Fig. 4. Differential effects of MKP-2 deletion upon COX-2, iNOS and Arginase-1 expression in LPS-stimulated macrophages. 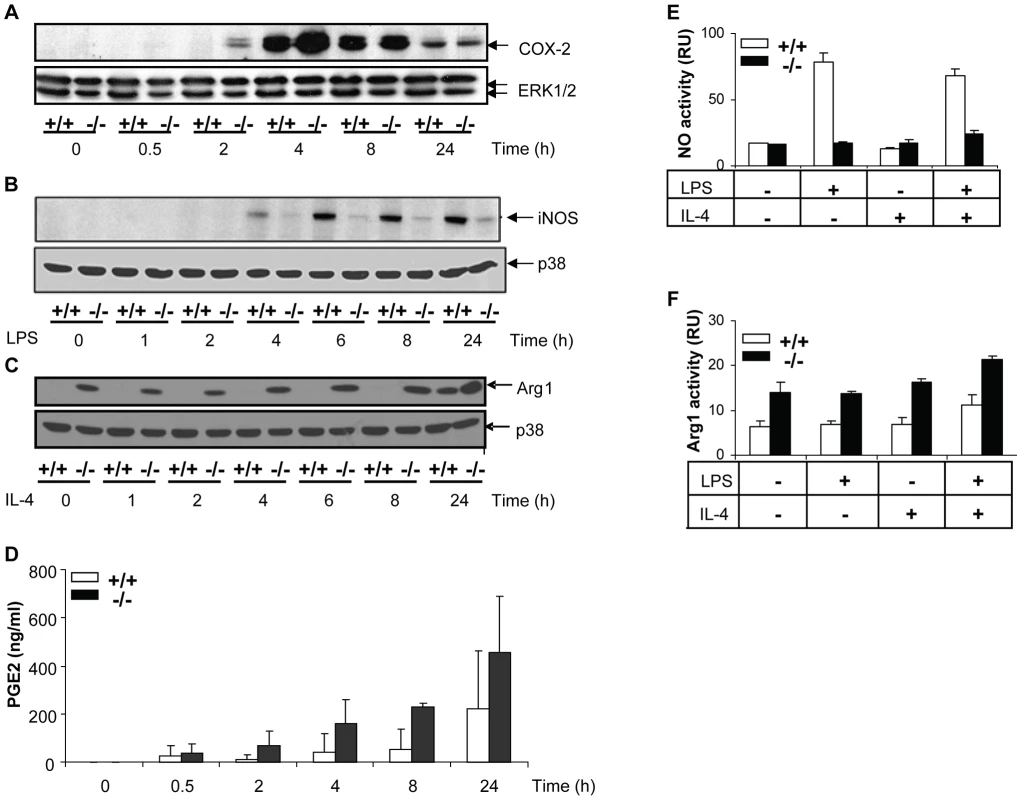
Cells were stimulated with LPS (100 ng/ml) for the indicated times and whole cell lysates were prepared, and assessed for COX-2 (Panel A), iNOS (Panel B) and Arginase-1 (Panel C) by Western blotting as outlined in Materials and Methods. Blot is representative of three individual experiments. Samples were assessed for associated PGE2 histogram (D), Nitrite (E) and arginase activity (F). MKP-2 deficiency results in increased susceptibility to Leishmania mexicana infection
Changes in MAP kinase signalling and iNOS activity are implicated in the ability of mice to resist infection with Leishmania [17], [18], [19], [20], [21], [22]. However, while the signalling and associated cytokine changes in MKP2−/− mice would favour increased protection, the unexpected changes in iNOS and arginase expression would favour disease progression. As L. mexicana induces an intermediate disease phenotype in C57BL/6 and B6/129 mice whereby lesion growth is controlled, but fails to heal [23], we used this pathogen to test the ultimate influence of MKP-2 on disease outcome.
Initially, we tested the effect of Leishmania mexicana promastigotes on cellular MAP kinase signalling responses and iNOS induction (Figure 5) to confirm that both LPS, via TLR-4, and promastigotes mediate common kinase signalling cassettes and related functional end points. We initially found that in wild type macrophages, promastigotes activated ERK, JNK and p38 MAP kinase (Panel A). However, in TLR-4 deficient macrophages, both ERK and JNK phosphorylation were abolished in response to promastigotes, with a substantial reduction in p38 MAP kinase. In TLR-4−/− macrophages the LPS-induced responses were also reduced, but this not by as much as the reduction seen in promastigote-induced responses. This is probably because our commercial source of LPS may have been contaminated with bacterial lipoproteins resulting in some activation of TLR-2. Nevertheless, these data suggest that both agents act principally through TLR-4. MKP-2 deletion had little effect upon the ERK response (Panel B) or p38 MAP kinase signalling (not shown) in response to promastigotes and JNK phosphorylation was only marginally increased although not significantly (Panel C). Despite activation of these kinases, promastigotes alone failed to significantly induce MKP-2 protein (data not shown) and had no effect upon iNOS synthesis alone or in response to LPS (Panel D). Furthermore, promastigotes did not significantly increase the already high levels of arginase-1 activity in MKP-2−/− macrophages (arginase activity, µg/ml: MKP-2+/+ control = 125.4±14.3, L. mexicana = 101.1±13.0; MKP-2−/− control = 345.8±17.10, L. mexicana = 392±23.5 n = 3).
Fig. 5. Macrophage activation by Leishmania mexicana. 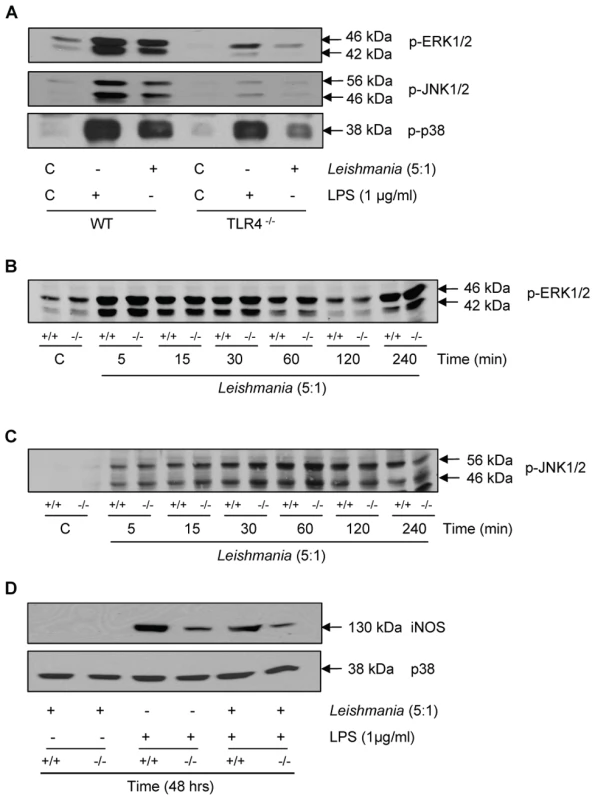
In Panel A macrophages from wild type (WT) and TLR-4 deletion mice (TLR-4−/−) were incubated with LPS (100 ng/ml) or promastigotes (5∶1) for 30 min. In panels B and C, MKP-2+/+ macrophages (+/+) and MKP-2−/−(−/−) were stimulated with promastigotes for the times indicated. In Panel D cells were incubated promatigotes alone or in combination with LPS for 48 h. Samples were assessed for ERK, JNK and p38 MAP kinase phosphorylation or iNOS expression as indicated. Each blot is representative of three individual experiments. Nevertheless, despite promastigotes being unable to modify MKP-2 expression per se, we found that MKP-2 deletion had a significant effect in vivo following Leishmania mexicana infection. Upon injection into the footpad with L. mexicana, MKP-2−/− mice developed progressively growing lesions and could not limit lesion growth unlike their wild-type counterparts (Figure 6A). Lesions grew more rapidly in MKP-2+/+ mice compared with MKP-2−/− mice in the first 4 weeks of infection but the parasite burdens remained higher at this site in MKP-2−/− mice than their wild-type counterparts throughout infection (Figure 6B). At week 15, the Th1 response, as measured by antigen specific IgG2a and IFN-γ production (Figures 7A and B), was significantly reduced in MKP-2−/− compared with MKP-2+/+ mice confirming that the MKP-2−/− mice were defective in their ability to control parasite growth. No differences in the Th2 response were noted with specific IgG1 (Figure 7A) and whole IgE as well as IL-4, IL-13, and IL-10 production (data not shown), all being similar in MKP-2−/− and MKP-2+/+ mice. There was no evidence of an intrinsic T cell defect in MKP-2−/− mice as splenocytes from infected MKP-2−/− and MKP-2+/+ mice produced similar levels of IFN-γ upon stimulation with anti-CD3 (Figure 7C).
Fig. 6. MKP-2 deficiency increases susceptibility to L. mexicana infection in the footpad. 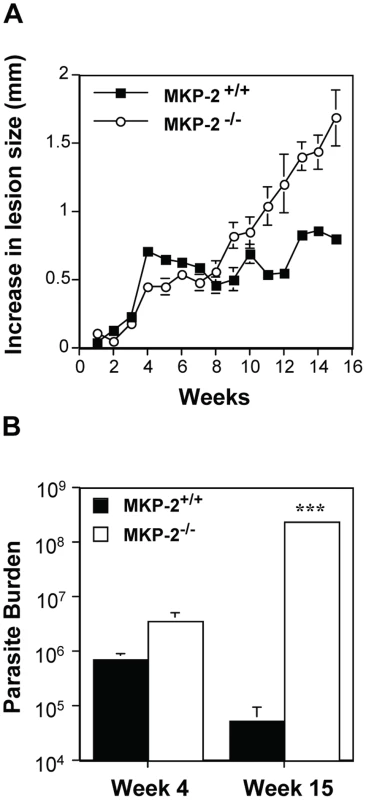
Infection was with 5×106 L. mexicana stationary phase promastigotes into the footpad and susceptibility was measured by mean ± SEM lesion growth (Panel A), and parasite burdens (***p<0.001) (Panel B). MKP-2−/− (n = 8), unlike MKP-2+/+ mice (n = 7) were unable to control lesion growth (Panel A) and this was reflected in the comparative parasite burdens (Panel B) in the footpad. Representative results from 3 similar experiments. Fig. 7. MKP-2 deficiency results in a limited Th1 response following footpad infection with L. mexicana. 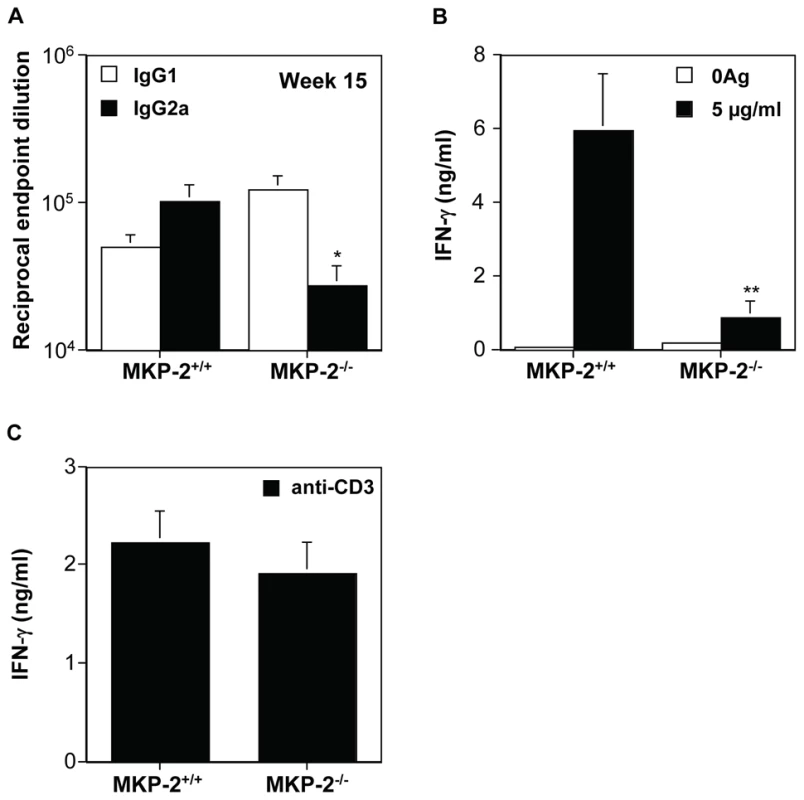
Th1 responses were measured by specific serum IgG2a antibody (*p<0.05) (Panel A), and antigen induced splenocyte IFN-γ production (**p<0.01) (Panel B).Results are expressed as mean ± SEM of MKP-2−/− (n = 8) and MKP-2+/+ (n = 7) mice. MKP-2 deficiency did not directly impair the ability of T cells to mount a Th1 response as anti-CD3 stimulation of splenocytes from both infected and non-infected MKP-2+/+ and MKP-2−/− mice induced similar IFN-γ production (Panel C). Representative results from 3 similar experiments. The growth of L. mexicana is subject to different immunological controls at different sites. The non-healing response to L. mexicana in infected footpads is associated with deficient IFN-γ production, independent of a Th2 response, whereas in the back rump the host response to infection is entirely Th2 dependent [24]. Consequently, we monitored the growth of L. mexicana in the shaven back rump of MKP-2−/− and MKP-2+/+ mice (Figure 8). Lesions appeared earlier and lesion growth was more rapid in MKP-2−/− mice (Figure 8A). The increased susceptibility of MKP-2−/− mice over wild-type animals was associated with expanded Th2 responses in these animals as measured by significantly increased specific IgG1 production (Figure 8B) and increased ConA induced splenocyte IL-4 (Figure 8D) and IL-13 (Figure 8E) production. At the same time there were no significant differences between infected MKP-2−/− and MKP-2+/+ in IFN-γ production (Figure 8C) and specific IgG2a levels (Figure 8B). As with footpad infections there was no indication of an intrinsic T cell defect as measured by anti-CD3 splenocyte cytokine production (results not shown).
Fig. 8. MKP-2 deficiency results in increased susceptibility and an enhanced Th2 response following infection in the rump with L. mexicana. 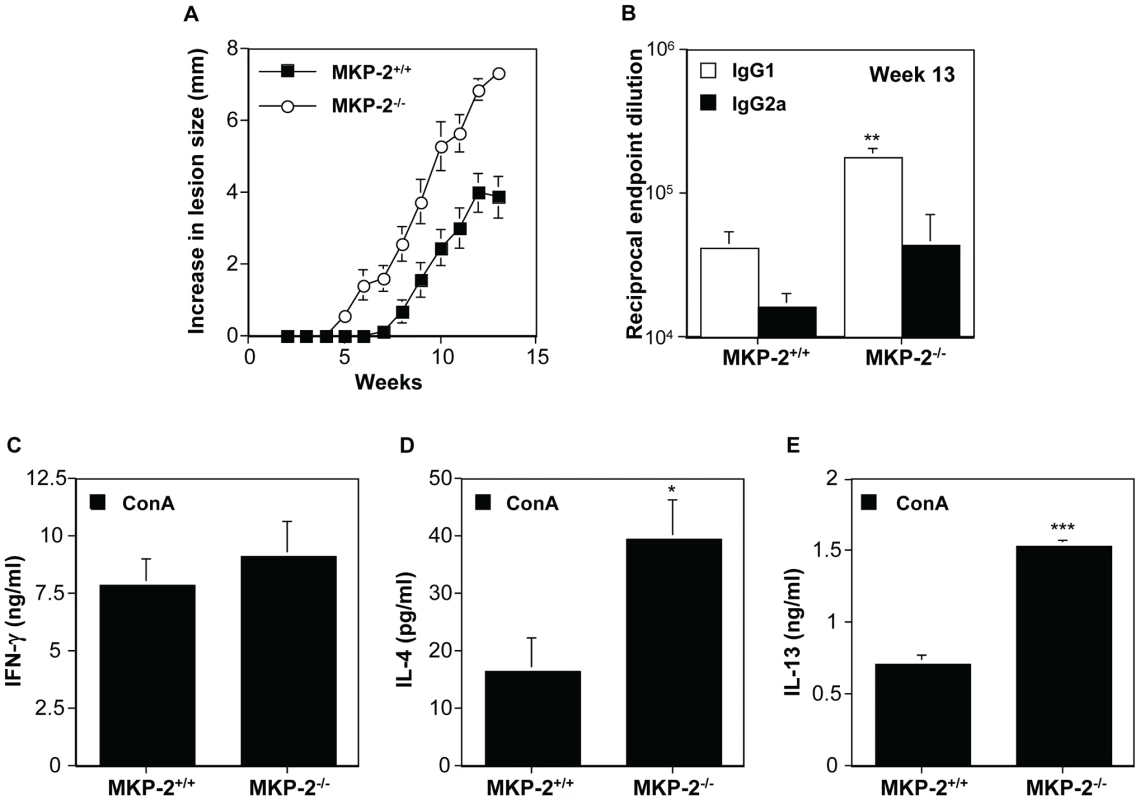
Lesions developed quicker and were of greater size in MKP-2−/− (n = 6) mice compared with MKP-2+/+ (n = 6) mice (Panel A). Enhanced Th2 responses in MKP-2−/− mice were measured by increased specific serum IgG1 antibody production compared with wild-type counterparts (**p<0.01) (Panel B). In addition while ConA induced splenocyte IFN-γ production was similar in MKP2−/− and MKP-2+/+ mice (Panel C), IL-4 (*p<0.05) (Panel D) and IL-13 (***p<0.001) (Panel E) levels were significantly elevated in MKP-2−/− animals. Results are expressed as mean ± SEM of MKP-2−/− and MKP-2+/+ mice. MKP-2 deficient bone marrow-derived macrophages have impaired ability to control the growth of L. mexicana
To confirm that the intrinsic defect in infectivity was at the level of the host macrophage we therefore compared the growth of L. mexicana parasites in MKP-2−/− and MKP-2+/+ bone marrow derived macrophages. Macrophages were infected with promastigotes at a multiplicity of infection (M.O.I) of 5 parasites/macrophage and growth monitored at 4, 24, 48 and 72 h post-infection in resting as well as LPS+IFN - γ stimulated macrophages (Figure 9). Macrophages from MKP-2−/− mice were significantly more permissive to infection than MKP-2+/+ macrophages as measured by the percentage of cells infected by 4 h (p<0.01) (Figure 9A). Similarly, parasite growth was significantly enhanced in MKP2−/− macrophages compared with MKP-2+/+ macrophages under non-stimulated conditions up to 72 h post-infection (Figure 9B). MKP-2−/− macrophages were, however, able to control parasite growth following IFN - γ+LPS stimulation although only to a level comparable with non-stimulated macrophages derived from MKP-2+/+ bone marrow. This would be consistent with data in figure 5 showing that after 48 h there is measurable iNOS expressed in MKP-2−/− KO macrophages in response to LPS although much less than in MKP-2+/+ macrophages.
Fig. 9. MKP-2 deficiency results in impaired ability of macrophages to control L. mexicana infection. 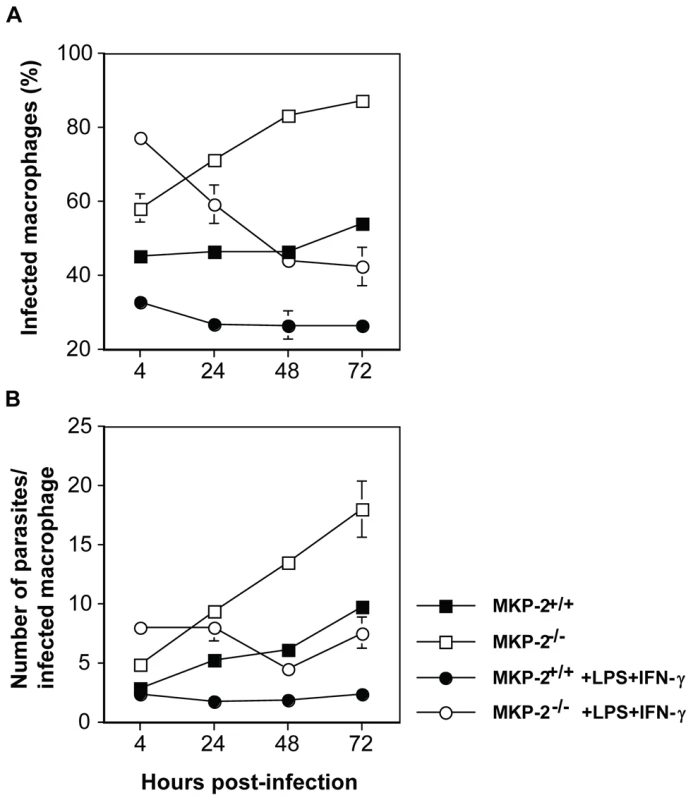
Both the percentage of infected macrophages (p<0.005) (Panel A) and the numbers of parasites/infected macrophage (p<0.01) (Panel B) were significantly greater in non-stimulated bone marrow derived-macrophages from MKP2−/− donors compared with MKP-2+/+ mice by 72 h post-infection. While stimulation with 100 ng LPS + 100U IFN-γ significantly inhibited both the percentage (Panel A) of infected macrophages and the mean number of parasites/infected macrophage (Panel B) in the cultures derived from MKP-2−/− mice compared with non-stimulated MKP-2−/− macrophages this was only to levels comparable with those found in non-stimulated macrophages from MKP-2+/+ mice at 72 h post-infection. Each figure represents 3 experiments (mean ± SEM of triplicate determinations). L-arginine inhibition reverses the effect of MKP-2 deficiency on parasite growth
Figure 10 shows the effect upon NO production and parasite infection following the treatment with the arginase inhibitor Nω-hydroxy-nor-Arginine (nor-NOHA). Initially, we assessed NO release to confirm that arginase inhibition had been effective in altering, as predicted, the levels of NO in macrophages (Panel A). In LPS+IFN-γ -stimulated MKP-2−/− macrophages NO levels were, as predicted, low, however following nor-NOHA treatment, levels increased significantly equivalent to the level observed for wild type macrophages. In addition, treatment of MKP-2+/+ macrophages with nor-NOHA failed to significantly increase the levels of NO further. When assessing parasite growth in MKP-2−/− and MKP-2+/+ macrophages following nor-NOHA pre-treatment we found that changes in arginase activity altered infectivity (Panel B). In MKP-2−/− macrophages, infectivity was high relative to wild type as expected. However, infectivity was significantly reduced in response to nor-NOHA, to a level which was not significantly different from MKP-2+/+ macrophages. Inhibition of NO using L-NAME also increased the infectivity of the parasite in MKP-2+/+ macrophages suggesting NO is a determinant of macrophage resistance to infectivity with L. Mexicana promastigotes. Furthermore, L-NAME treatment of infected MKP-2−/− macrophages resulted in rapid macrophage destruction and release of parasites (>90%) making it impossible to quantify changes in infectivity. This finding nevertheless re-enforced the idea of increased sensitivity of MKP-2−/− macrophages to infection per se.
Fig. 10. Arginase inhibition reverses the susceptibility of MKP-2−/− macrophages to control L. mexicana infection. 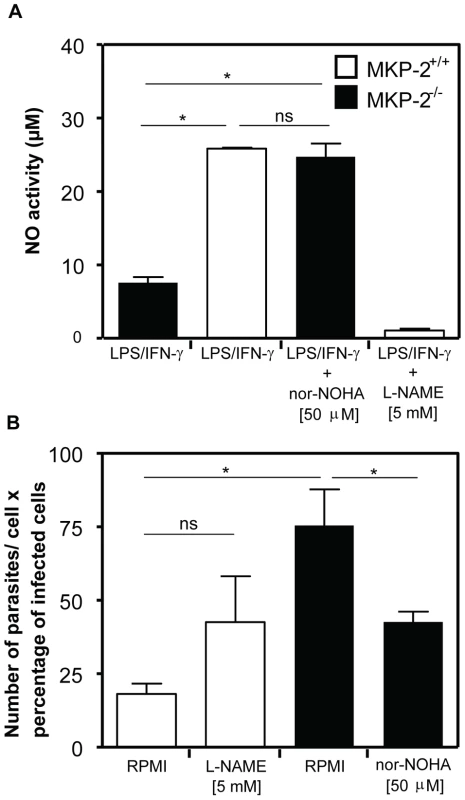
Macrophages were pre-treated with or without inhibitors for 60 min as indicated prior to activation with LPS (100 ng/ml) plus IFN-γ (100 U/ml) and samples assayed for NO after 48 hr (Panel A). In panel B following treatment with inhibitors macrophages were incubated with L. mexicana promastigotes (5∶1) and survival measured after 48 h as outlined in the Material and Methods section. Data is displayed as a composite of the number of parasites per cell and the percentage of infected cells.Results are expressed as mean ± SEM. *P<0.05 compared to vehicle treated cells (RPMI). Discussion
This study demonstrates for the first time a novel immune function for MKP-2 in vivo primarily caused by changes in the ability of macrophages to induce innate immune responses. In particular, MKP-2 deletion gives rise to a novel phenotype associated with decreased iNOS and increased arginase-1 activity which makes MKP-2 deficient macrophages more intrinsically susceptible to infection with the intracellular parasite L. mexicana. Infection of MKP-2 deficient mice not only results in increased disease susceptibility and parasite growth in vivo compared to their wild-type counterparts but it is also associated with either inhibition of a Th1 response or promotion of a Th2 response appropriate to enhance infection at the site utilised.
In characterising the novel MKP-2−/− model, we clearly demonstrate that following gene deletion JNK phosphorylation and activity was enhanced. This is consistent with the original studies in vitro [11] and more recent studies using either stable or conditional expression of MKP-2 [13], [14]. ERK activation was, however, not enhanced in MKP-2−/− macrophages despite being a substrate for MKP-2, due to compartmentalisation of ERK to the cytosol, generating a substrate selective function for MKP-2 in this cell type. Unexpectedly, we found that phosphorylation of p38 MAP kinase was also enhanced despite this kinase not being susceptible to dephosphorylation by MKP-2 in vitro. A recent cellular study, however, has indirectly implicated involvement of MKP-2 in regulating AMP kinase and p38 MAP kinase-dependent gluconeogenesis [25] and our findings support this data. Our data show that predictions based on in vitro data can be misleading and indeed study of other MKP deletion mice also shows discordance between substrate specificity in vitro and kinase regulation in whole cells. Whilst MKP-1 is effective against all three major MAP kinases, enhanced p38 MAP kinase is observed in MKP-1−/− macrophages [26], whilst in cells derived from PAC-1 knockout mice, ERK phosphorylation is inhibited despite ERK being a specific substrate for PAC-1 in vitro [6].
Our studies demonstrated that enhanced JNK and p38 MAP kinase activation where reflected at the level of cytokine synthesis. Evidence strongly supports a role for p38 MAP kinase in the expression of IL-6, TNFα and IL-12 [27], [28], [29]. In contrast, a role for JNK is less well defined and cell type specific involvement is implicated [27], [29]. Our studies also correlate well with those obtained with the MKP-1 deletion which implicates p38 MAP kinase as the main in vivo substrate for MKP-1 and shows this kinase to be linked to cytokine release [26], [30]. This would suggest that MKP-2 deletion, in a manner similar to MKP-1 [26], [30] could enhance innate immune responses in vitro and also possibly in vivo.
This possibility was contradicted by other findings. Whilst we found up regulation of COX-2 and associated PGE2 in MKP-2 deletion mice, results also observed in MKP-1 deletion mice, surprisingly expression of iNOS was ablated. Studies show that iNOS expression is enhanced following MKP-1 deletion in vitro [31] and associated with increased mortality in vivo [32]. This suggests the potential for MKP-2 deletion, unlike ablation of MKP-1, to protect against NO induced mortality. Furthermore, we find that basal arginase 1 expression and associated arginase activity is markedly increased in MKP-2 deletion mice, a response which would also mediate a reduction in NO formation, this is again different to the findings in MKP-1−/− macrophages [31]. The molecular mechanisms underpinning the reciprocal regulation of these two inflammatory proteins are at present unclear, as none of the cognate signalling pathways regulating iNOS or arginase induction were found to be negatively affected. Thus, there is a potential for a direct action of MKP-2 within the nucleus which may directly regulate transcription. Recently, MKP-1 has been implicated in the regulation of histone phosphorylation in the nucleus [33] suggesting nuclear functions for the MKPs other than direct MAP kinase regulation.
Overall our studies in vitro therefore reveal that in the absence of MKP-2 macrophages take on a functional profile which can be directed towards either a type-1 or type-2 phenotype. Our in vivo studies enabled us to demonstrate which of these profiles predominate in vivo. Leishmania infection has a well recognized ability to subvert the development of Th1 responses partly via effects upon MAP kinase signalling [17], [20], [34]. Most of these studies have implicated MAP kinase involvement at the level of the host macrophage or dendritic cell, but JNK activation via T cells has also been shown to negatively regulate Th2 cells [34] in a healing response. Therefore, we hypothesised, given the enhanced p38 and JNK activation of MPK-2−/− mice on the C57BL/6 background, that such animals would develop a healing phenotype against this organism. MKP-2 negatively regulated IL-12, IL-6 and TNF-α expression and positively regulates IL-10 confirming this hypothesis. Currently iNOS induction and the subsequent release of NO is considered protective against intracellular infection with many organisms including Leishmania species [19]. In addition, arginase-1, which competes for the same substrate as iNOS, has been shown to promote disease progression not only against Leishmania (in both healing and non-healing strains of mice [18]), but also Mycobacterium and Toxoplasma gondii [35]. As MKP-2 deficiency downregulates iNOS, but upregulates arginase-1 expression and activity this suggests that, counter intuitively, MKP-2−/− animals would be more susceptible to Leishmania infection.
Our studies indeed show that MKP2−/− mice are, in fact, more susceptible to infection with L. mexicana and it is the consequence of changes in iNOS and arginase rather than changes in kinase mediated inflammatory cytokine signalling which dictates the subsequent in vivo response to MKP-2 deletion. The differential effects of Leishmania infection in MKP-2−/− macrophages relative to MKP-2+/+ is not due to Leishmania interacting with the cell in a different way to LPS, both agents utilised TLR-4 during the early stages of stimulation. Furthermore, the fact that Leishmania mexicana alone does not induce MKP-2 points to another potentially indirect effect of MKP-2 deletion not linked to changes in kinase activity. Nevertheless, following footpad infection with L. mexicana, lesion growth was increased and parasite burden enhanced, outcomes associated with reduced IFN-γ production. Of significance it has recently been demonstrated that during L. major infections high local arginase levels at the site of infection mediate L-arginine depletion, which results in impaired local CD4+ T cell function particularly IFN-γ production [21], [22]. The importance of arginase in modulating the virulence of L. mexicana is also highlighted by the fact that arginase null-mutant L. mexicana has attenuated virulence in vitro and in vivo suggesting that the parasite arginase depletes host L-arginine available for iNOS activity [36], [37]. Significantly, mice infected in the footpad with arginase null-mutant L. mexicana have increased antigen induced IFN - γ production. Thus changes in arginase-1 expression in MKP-2−/− macrophage can be easily linked to observed changes in infectivity and immune responses.
Our study is one of the first to reveal an in vivo function for MKP-2 and indicates that MKP-2 does not act as a surrogate to the more extensively studied MKP-1. Our results reveal the potential of MKP-2 to participate in opposing regulatory mechanisms. The first mechanism is based on upregulation of JNK and p38 MAP kinase signalling and is associated with enhanced cytokine expression. The second regulatory mechanism however, involves changes in iNOS and arginase-1 expression, associated with the alternative activated macrophage pathway, is not readily associated with modulation in kinase signalling but possibly involves a different molecular target. The in vivo consequences of this second action can be clearly seen during Leishmania infection where there is down-regulation of Th1 and/or up-regulation of Th2 responses, distinguishing MKP-2 from any of the other MKPs believed to play a role in immune function.
Materials and Methods
Ethics statement
All animal procedures conformed to guidelines from The Home Office of the UK government. All work was covered by two Home Office licences: PPL60/3929, “mechanism of control of parasite infection” and PPL60/3439, “genetic models of cancer and inflammation”.
Materials
Dulbecco's modified Eagle's medium and foetal calf serum (FCS) were purchased from Invitrogen. RPMI 164 and LPS were from Sigma Aldrich. Antibodies against p-ERK, and MKP-2 were obtained from Santa Cruz. All other phospho-antibodies were purchased from Biosource International Inc. (USA) and secondary antibodies from Jackson Immuno Research Laboratories Inc (PA, USA). The TLR-4 deficient mice were obtained from Professor Akira S. Osaka University, Japan.
Methods
Generation of MKP-2 deficient mice
The deletion of the MKP-2 gene was performed in collaboration with Genoway, Lyon, France using standard procedures. The short arm and the long arm flanking both side of the cluster of exon 2–4 of the mouse MKP-2 gene was obtained by PCR using the following primers: for the small homology arm: 5′-GTGCCTGGTTCTGTGTGTGTCTGTTCTCC-3′ for the forward primer and 5′-TCTTACAGCCCTCTTTCCTCACGGTCG-3′ for the reverse primer producing a PCR fragment of 3009 bp. For the long homology arm: 5′-CTTTAGGAGCGACGGCCAGGAACACAGG-3′for the forward primer and 5′-ACCCTGCCACACAGGTTGGAGCAAGG-3′ for the reverse primer producing a PCR fragment of 6336 bp. Both arms were introduced into a PBS vector in either side of the neomycin cassette. The final vector was transfected into 129Sv mouse embryonic stem cell. Selection was made to select only the clones which had homologous recombination events first by PCR and then using Southern blotting for the short and the long arm on the construct. Male chimeras were obtained and crossed with C57Bl/6 female to obtain the F1 generation. The F1 generation was screened for germ line transmission of the mutation. Heterozygous mice were backcrossed against C57Bl/6 and genotyped using Southern blotting and PCR.
Generation of mouse macrophages
Bone marrow derived macrophages were isolated from femurs of 3 months old MKP-2−/−or +/+ mice and grown in DMEM, containing 20% (v/v) heat-inactivated FCS supplemented and 30% L cell-conditioned medium supplemented with 5 mM L-Glutamine, 100 U/ml penicillin, 1 µg/ml streptomycin. Adherent cells were harvested and then seeded in either 12 well (1×106 cells/ml) or 96 well (2×106 or 2×105 cells/ml) plates with RPMI 1641 supplemented with 10% FCS.
Western blotting
Proteins (15 µg per lane) were separated by 10% SDS-PAGE and transferred onto nitrocellulose. The membranes were blocked for non-specific binding for 2 h in 2% BSA (w/v) diluted in NATT buffer 50 mM Tris-HCl, 150 mM NaCl, 0.2% (v/v) Tween-20. The blots were then incubated overnight with 50 ng/ml primary antibody diluted in 0.2% BSA (w/v) in NATT buffer. The blots were washed with NATT buffer for 90 min and incubated with HRP-conjugated secondary antibody (20 ng/ml in 0.2% BSA (w/v) diluted in NaTT buffer) for 2 h. After a further 90 min wash, the blots were subjected to ECL reagent and exposed to Kodak X-ray film.
Leishmania mexicana parasites and infection model
L. mexicana (MYNC/BZ/62/M379) was maintained by serial passage of amastigotes inoculated into the shaven rumps of BALB/c mice. Infections were initiated using stationary phase promastigotes grown in TC100 insect medium (Sigma, St. Louis, USA) supplemented with v/v 10% FCS (Harlan Sera-Lab Ltd., Crawley). Promastigotes (5×106 in 25 µl) were inoculated subcutaneously into the hind footpad or subcutaneously into the shaven back rump. Female 6–8 week old MKP-2−/− and MKP-2+/+ mice were used in each experiment. Increase in footpad size was measured using a dial gauge micrometer and rumps with a slide gauge micrometer at weekly intervals. Parasites were enumerated from lesions as previously described [23].
Macrophage infection with L. mexicana promastigotes
One hundred microlitres of bone marrow-derived MKP-2−/− or MKP-2+/+ macrophages (2×106/ml) in RPMI 1641 supplemented with 10% FCS were added to each well of a 24-well tissue culture plate (Techno Plastic Products, Trasadingen, Switzerland) containing round 13-mm cover slips. Cells were infected by adding 100 µl L. mexicana stationary phase promastigotes at a parasite: host cell ratio of 5 : 1 and incubating the cells for 4 h at 33°C The medium was then changed to remove unattached parasites and replenished with 100 µl fresh medium or medium containing IFN-γ or LPS alone or in combination. Cells were fixed in methanol (Banford Laboratories, Norden Rochdale, UK) and stained with 10% (v/v) aqueous Giemsa stain (BDH Laboratory Supplies) at 4, 24, 48 and 72 h post infection so that the percentage of cells infected and the number of parasites/100 infected macrophages could be determined by microscopy. To inhibit arginase or iNOS activity, cells were pre-treated for 1 h with 50 µM Nω-hydroxy-nor-Arginine (nor-NOHA, Calbiochem) or 5 mM N (G)-nitro-L - arginine methyl ester (L-NAME, Sigma), respectively. L. mexicana promastigotes were added at an M.O.I. of 5 and plates were incubated at 34°C. After 48 h, medium was removed, cells fixed with methanol and subsequently stained with Giemsa. Coverslips were mounted onto microscopic glass slides and parasite number and infection rate for a total of 200 macrophages per coverslip was assessed using a bright field microscope.
Immunological analysis
ELISA was routinely used for detection of IL-6, IL-12, TNFα and PGE2 in macrophages and L. mexicana specific-IgG1 and -IgG2a and total IgE in the plasma of infected mice as previously described [38]. Splenocytes were cultured in 96-well plates as previously described [39] and IFN-γ, IL-10, IL-13 and IL-4 production measured by capture ELISA.
Measurement of arginase activity
Arginase activity was measured using an assay based on a reaction with α-isonitrosopropiophenon (ISPF). Briefly, macrophages were grown on 24 - well plates, exposed to agonist as appropriate and harvested in 50 µl lysis buffer (50 mM Tris-HCl, 10 mM MnCl2, 0·1% Triton X-100, 5 µg/ml pepstatin A, 5 µg/ml aprotinin, and 5 µg/ml antipain hydrochloride, pH 7.4). Arginine hydrolysis was carried out by incubating cell lysates with 25 µl of 0·5 M L-arginine (pH 9.7) at 37°C for 60 min. The reaction was terminated by adding 400 µL of an acid solution (H2SO4, H3PO4 and H2O in a ratio of 1∶3∶7 and 25 µL of a 9% solution of ISPF). Samples along with known urea standards were incubated at 95°C for 45 minutes, and then allowed to cool for 10 min in the dark. Aliquots were added to wells of a 96 well plate and absorbance read at 540 nm on a Spectromax 190 plate reader.
NO measurement in cell culture supernatants
Cell culture medium supernatant was collected and nitrite was analysed as a measure for NO production using the Griess reagent. Equal volumes of Griess reagent (1% (w/v) sulphanilamide/ 0.1% (w/v) N-(1-naphtyl)ethylenediamine dihydrochloride/ 2.5% (v/v) H3PO4) and cell culture supernatant were mixed and incubated at room temperature in the dark for 10 min. Absorbance was read at 540 nm on a Spectromax 190 plate reader. Nitrite production was determined using NaNO2 as standard.
Statistical analysis
Statistical significance was determined between groups using the Mann-Whitney U test for endpoint antibody titrations, as well as Student's t-test and one-way ANOVA with Dunnett's post test where appropriate. All experiments were performed at least three times with similar findings.
Supporting Information
Zdroje
1. KeyseSM
2008 Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev 27 253 261
2. WuJJ
RothRJ
AndersonEJ
HongEG
LeeMK
2006 Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metab 4 61 73
3. XuH
YangQ
ShenM
HuangX
DembskiM
2005 Dual specificity MAPK phosphatase 3 activates PEPCK gene transcription and increases gluconeogenesis in rat hepatoma cells. J Biol Chem 280 36013 36018
4. HammerM
MagesJ
DietrichH
ServatiusA
HowellsN
2006 Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J Exp Med 203 15 20
5. ZhaoQ
WangX
NelinLD
YaoY
MattaR
2006 MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med 203 131 140
6. JeffreyKL
BrummerT
RolphMS
LiuSM
CallejasNA
2006 Positive regulation of immune cell function and inflammatory responses by phosphatase PAC-1. Nat Immunol 7 274 283
7. ZhangY
BlattmanJN
KennedyNJ
DuongJ
NguyenT
2004 Regulation of innate and adaptive immune responses by MAP kinase phosphatase 5. Nature 430 793 797
8. FurstR
SchroederT
EilkenHM
BubikMF
KiemerAK
2007 MAPK phosphatase-1 represents a novel anti-inflammatory target of glucocorticoids in the human endothelium. Faseb J 21 74 80
9. Misra-PressA
RimCS
YaoH
RobersonMS
StorkPJ
1995 A novel mitogen-activated protein kinase phosphatase. Structure, expression, and regulation. J Biol Chem 270 14587 14596
10. SlossCM
CadalbertL
FinnSG
FullerSJ
PlevinR
2005 Disruption of two putative nuclear localization sequences is required for cytosolic localization of mitogen-activated protein kinase phosphatase-2. Cell Signal 17 709 716
11. ChuY
SolskiPA
Khosravi-FarR
DerCJ
KellyK
1996 The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J Biol Chem 271 6497 6501
12. ChenP
HutterD
YangX
GorospeM
DavisRJ
2001 Discordance between the binding affinity of mitogen-activated protein kinase subfamily members for MAP kinase phosphatase-2 and their ability to activate the phosphatase catalytically. J Biol Chem 276 29440 29449
13. CadalbertL
SlossCM
CameronP
PlevinR
2005 Conditional expression of MAP kinase phosphatase-2 protects against genotoxic stress-induced apoptosis by binding and selective dephosphorylation of nuclear activated c-jun N-terminal kinase. Cell Signal 17 1254 1264
14. RobinsonCJ
SlossCM
PlevinR
2001 Inactivation of JNK activity by mitogen-activated protein kinase phosphatase-2 in EAhy926 endothelial cells is dependent upon agonist-specific JNK translocation to the nucleus. Cell Signal 13 29 41
15. TresiniM
LorenziniA
TorresC
CristofaloVJ
2007 Modulation of replicative senescence of diploid human cells by nuclear ERK signaling. J Biol Chem 282 4136 4151
16. SiebenNL
OostingJ
FlanaganAM
PratJ
RoemenGM
2005 Differential gene expression in ovarian tumors reveals Dusp 4 and Serpina 5 as key regulators for benign behavior of serous borderline tumors. J Clin Oncol 23 7257 7264
17. ForgetG
GregoryDJ
WhitcombeLA
OlivierM
2006 Role of host protein tyrosine phosphatase SHP-1 in Leishmania donovani-induced inhibition of nitric oxide production. Infect Immun 74 6272 6279
18. NadererT
McConvilleMJ
2008 The Leishmania-macrophage interaction: a metabolic perspective. Cell Microbiol 10 301 308
19. WeiXQ
CharlesIG
SmithA
UreJ
FengGJ
1995 Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375 408 411
20. YangZ
MosserDM
ZhangX
2007 Activation of the MAPK, ERK, following Leishmania amazonensis infection of macrophages. J Immunol 178 1077 1085
21. ModolellM
ChoiBS
RyanRO
HancockM
TitusRG
2009 Local suppression of T cell responses by arginase-induced L-arginine depletion in nonhealing leishmaniasis. PLoS Negl Trop Dis 3 e480
22. MunderM
MollinedoF
CalafatJ
CanchadoJ
Gil-LamaignereC
2005 Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood 105 2549 2556
23. BuxbaumLU
DeniseH
CoombsGH
AlexanderJ
MottramJC
2003 Cysteine protease B of Leishmania mexicana inhibits host Th1 responses and protective immunity. J Immunol 171 3711 3717
24. McMahon-PrattD
AlexanderJ
2004 Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol Rev 201 206 224
25. BerasiSP
HuardC
LiD
ShihHH
SunY
2006 Inhibition of Gluconeogenesis through Transcriptional Activation of EGR1 and DUSP4 by AMP-activated Kinase. J Biol Chem 281 27167 27177
26. SalojinKV
OwusuIB
MillerchipKA
PotterM
PlattKA
2006 Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J Immunol 176 1899 1907
27. FiebichBL
AkundiRS
BiberK
HamkeM
SchmidtC
2005 IL-6 expression induced by adenosine A2b receptor stimulation in U373 MG cells depends on p38 mitogen activated kinase and protein kinase C. Neurochem Int 46 501 512
28. GuoX
GerlRE
SchraderJW
2003 Defining the involvement of p38alpha MAPK in the production of anti - and proinflammatory cytokines using an SB 203580-resistant form of the kinase. J Biol Chem 278 22237 22242
29. KrauseA
HoltmannH
EickemeierS
WinzenR
SzamelM
1998 Stress-activated protein kinase/Jun N-terminal kinase is required for interleukin (IL)-1-induced IL-6 and IL-8 gene expression in the human epidermal carcinoma cell line KB. J Biol Chem 273 23681 23689
30. ChiH
BarrySP
RothRJ
WuJJ
JonesEA
2006 Dynamic regulation of pro - and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A 103 2274 2279
31. NelinLD
WangX
ZhaoQ
ChicoineLG
YoungTL
2007 MKP-1 switches arginine metabolism from nitric oxide synthase to arginase following endotoxin challenge. Am J Physiol Cell Physiol 293 C632 640
32. WangX
MengX
KuhlmanJR
NelinLD
NicolKK
2007 Knockout of Mkp-1 enhances the host inflammatory responses to gram-positive bacteria. J Immunol 178 5312 5320
33. KinneyCM
ChandrasekharanUM
YangL
ShenJ
KinterM
2009 Histone H3 as a novel substrate for MAP kinase phosphatase-1. Am J Physiol Cell Physiol 296 C242 249
34. ConstantSL
DongC
YangDD
WyskM
DavisRJ
2000 JNK1 is required for T cell-mediated immunity against Leishmania major infection. J Immunol 165 2671 2676
35. El KasmiKC
QuallsJE
PesceJT
SmithAM
ThompsonRW
2008 Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol 9 1399 1406
36. GaurU
RobertsSC
DalviRP
CorralizaI
UllmanB
2007 An effect of parasite-encoded arginase on the outcome of murine cutaneous leishmaniasis. J Immunol 179 8446 8453
37. RobertsSC
TancerMJ
PolinskyMR
GibsonKM
HebyO
2004 Arginase plays a pivotal role in polyamine precursor metabolism in Leishmania. Characterization of gene deletion mutants. J Biol Chem 279 23668 23678
38. SatoskarA
BrombacherF
DaiWJ
McInnesI
LiewFY
1997 SCID mice reconstituted with IL-4-deficient lymphocytes, but not immunocompetent lymphocytes, are resistant to cutaneous leishmaniasis. J Immunol 159 5005 5013
39. PollockKG
ConacherM
WeiXQ
AlexanderJ
BrewerJM
2003 Interleukin-18 plays a role in both the alum-induced T helper 2 response and the T helper 1 response induced by alum-adsorbed interleukin-12. Immunology 108 137 143
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease AgentČlánek TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease SusceptibilityČlánek The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive ParasiteČlánek Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Patients with Discordant Responses to Antiretroviral Therapy Have Impaired Killing of HIV-Infected T Cells
- A Molecular Mechanism for Eflornithine Resistance in African Trypanosomes
- Tyrosine Sulfation of the Amino Terminus of PSGL-1 Is Critical for Enterovirus 71 Infection
- Autoimmunity as a Predisposition for Infectious Diseases
- The Subtilisin-Like Protease AprV2 Is Required for Virulence and Uses a Novel Disulphide-Tethered Exosite to Bind Substrates
- Structural Analysis of HIV-1 Maturation Using Cryo-Electron Tomography
- Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease Agent
- Interferon-Inducible CXC Chemokines Directly Contribute to Host Defense against Inhalational Anthrax in a Murine Model of Infection
- TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease Susceptibility
- The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive Parasite
- CO Acts as a Signalling Molecule in Populations of the Fungal Pathogen
- SV2 Mediates Entry of Tetanus Neurotoxin into Central Neurons
- MAP Kinase Phosphatase-2 Plays a Critical Role in Response to Infection by
- Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
- Potentiation of Epithelial Innate Host Responses by Intercellular Communication
- Fcγ Receptor I Alpha Chain (CD64) Expression in Macrophages Is Critical for the Onset of Meningitis by K1
- ANK, a Host Cytoplasmic Receptor for the Cell-to-Cell Movement Protein, Facilitates Intercellular Transport through Plasmodesmata
- Analysis of the Initiating Events in HIV-1 Particle Assembly and Genome Packaging
- Evolution of Linked Avirulence Effectors in Is Affected by Genomic Environment and Exposure to Resistance Genes in Host Plants
- Structural Basis of HIV-1 Neutralization by Affinity Matured Fabs Directed against the Internal Trimeric Coiled-Coil of gp41
- Hepatitis C Virus (HCV) Evades NKG2D-Dependent NK Cell Responses through NS5A-Mediated Imbalance of Inflammatory Cytokines
- Host Cell Invasion and Virulence Mediated by Ssa1
- Global Gene Expression in Urine from Women with Urinary Tract Infection
- Should the Human Microbiome Be Considered When Developing Vaccines?
- HapX Positively and Negatively Regulates the Transcriptional Response to Iron Deprivation in
- Enhancing Oral Vaccine Potency by Targeting Intestinal M Cells
- Herpes Simplex Virus Reorganizes the Cellular DNA Repair and Protein Quality Control Machinery
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- Cryo Electron Tomography of Native HIV-1 Budding Sites
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- Modelling the Evolution and Spread of HIV Immune Escape Mutants
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
- Platelet-Activating Factor Receptor Plays a Role in Lung Injury and Death Caused by Influenza A in Mice
- Genetic and Structural Basis for Selection of a Ubiquitous T Cell Receptor Deployed in Epstein-Barr Virus Infection
- Ubiquitin-Regulated Nuclear-Cytoplasmic Trafficking of the Nipah Virus Matrix Protein Is Important for Viral Budding
- Pneumolysin Activates the NLRP3 Inflammasome and Promotes Proinflammatory Cytokines Independently of TLR4
- Immune Evasion by : Differential Targeting of Dendritic Cell Subpopulations
- Survival in Selective Sand Fly Vector Requires a Specific -Encoded Lipophosphoglycan Galactosylation Pattern
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



