-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAutoimmunity as a Predisposition for Infectious Diseases
article has not abstract
Published in the journal: . PLoS Pathog 6(11): e32767. doi:10.1371/journal.ppat.1001077
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1001077Summary
article has not abstract
Autoimmunity refers to an inappropriate immune response against self-components of the host that results in pathological conditions. Autoimmune diseases are characterized by an activation of autoreactive T and B cells, are associated in some cases with the production of pathogenic autoantibodies against self-molecules, culminating in inflammation and tissue damage. The reasons for the breakdown of tolerance mechanisms leading to autoimmunity are not clearly known. However, a combination of genetic, immunological, and environmental factors plays a critical role in the pathogenesis of autoimmunity [1]–[5].
Autoimmunity Can Predispose to Infectious Diseases
During the course of autoimmunity, autoantibodies that can neutralize key components of the immune system that are essential in mounting anti-microbial responses may be produced (Figure 1). These autoantibodies might either exacerbate ongoing infectious diseases or predispose the individual to an increased risk of bacterial, viral, and opportunistic fungal infections. For example, cytokines play a critical role in the process of mounting anti-microbial responses due to their ability to regulate the innate and adaptive immune systems, in polarizing T cell responses, and by acting as effector molecules. Thus, IL-12 mediates Th1 cell differentiation and IL-4 influences Th2 differentiation. IL-6, IL-21, TGF-β, IL-1β, and IL-23 are critical for the differentiation and expansion of Th17 cells. Th1 cells produce cytokines IFN-γ and IL-2, and confer protection against intracellular pathogens (viruses and intracellular bacteria such as Mycobacterium and Salmonella). Th2 cells produce IL-4, IL-5, and IL-13, and are important to clear extracellular pathogens and parasites. Th-17 cells secrete IL-17, IL-21, and IL-22, and provide protection against several extracellular pathogens, including fungi such as Candida (Figure 1) [6]–[9]. In addition, type I IFNs have a critical role in anti-viral immunity and in modulating T and B cell responses. Therefore, it can be conceived that the development of neutralizing antibodies against any of these cytokines as a consequence of autoimmunity affects the cellular functions and clearance of pathogens and predisposes the host to infectious diseases. This is further supported by reports of a high prevalence of infections in autoimmune patients treated with neutralizing monoclonal antibodies to inflammatory cytokines. Patients with rheumatoid arthritis, Crohn's disease, or psoriasis treated on a chronic basis with monoclonal antibodies to TNF-α are predisposed to mycobacterial, listerial, and viral infections [10]–[12].
Fig. 1. Host immune response to pathogens and predisposition to infections due to autoimmunity. 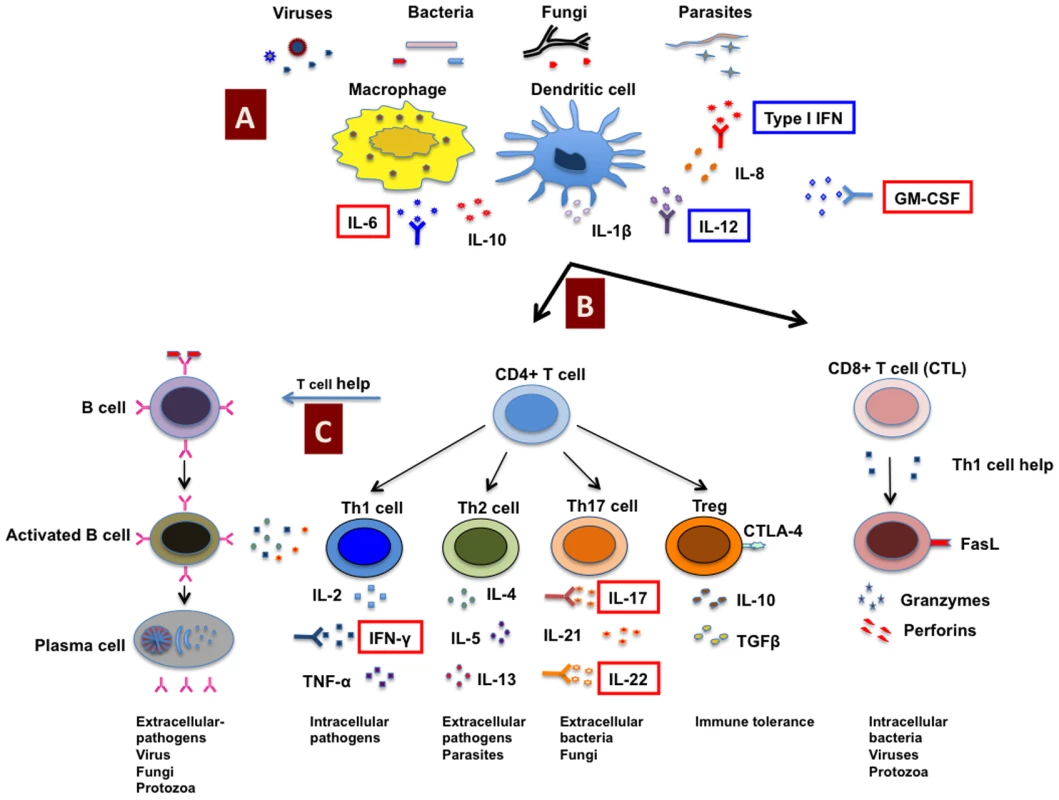
Antigens from invading pathogens are recognized and presented by innate immune cells (A) such as macrophages and dendritic cells to CD4+ and CD8+ T cells (CTL) (B). CD8+ T cells recognize endogenous antigens presented by MHC class I molecules and exert cytotoxic functions upon activation. CD4+ T cells recognize antigens presented in the context of MHC class II molecules, and under the influence of innate cells and cytokine milieu, CD4+ T cells can be polarized into different subsets such as Th1, Th2, Th17, and regulatory T cells (Tregs) that secrete distinct cytokines. CD4+ T cells provide help to B cells to produce antigen-specific antibodies (C). However, due to autoimmunity, neutralizing autoantibodies can be produced against any of these key components of the immune system critical for mounting anti-microbial responses and might either predispose to an increased risk of bacterial, viral, and opportunistic fungal infections or exacerbate the ongoing infectious diseases. Indeed, in patients with infections, the occurrence of neutralizing autoantibodies against several key cytokines such as IFN-γ, IL-6, GM-CSF, IL-17, and IL-22 (highlighted in red boxes) that interfere with the host immune response to pathogens have been demonstrated. In addition, autoantibodies are also reported against type I IFNs and IL-12 that might play role in predisposition to infections (highlighted in blue boxes). CTLA-4, cytotoxic T lymphocyte antigen-4; CTL, cytotoxic T lymphocyte; FasL, Fas ligand; GM-CSF, granulocyte/macrophage–colony stimulating factor. Specific Examples of Autoimmunity Favoring Infectious Diseases
Several reports have now demonstrated the occurrence of neutralizing autoantibodies against cytokines in patients with infections. These reports thus provide a pointer towards a previously unknown link between autoimmune responses and predisposition to infectious diseases.
A correlation between neutralizing autoantibodies to IFN-γ and mycobacterial infections has been reported [13]–[20]. Moreover, the clinical features of patients with anti-IFN-γ immunoglobulin G (IgG) are analogous to those with genetic defects in the IFN-γ/IL-12 pathway, which is characterized by progressive or disseminated infection with mycobacteria of low virulence, indicating that anti-IFN-γ IgG induces an acquired immunodeficiency state and predisposes to mycobacterial infections [13]–[20]. These anti-IFN-γ IgG neutralized IFN-γ in whole blood culture, inhibited IFN-γ-dependent phosphorylation of STAT-1 and production of TNF-α and IL-12 by normal peripheral blood mononuclear cells (PBMCs) and macrophages, and inhibited HLA-DR expression in normal monocytes [14]–[17]. In another study, one patient's serum was shown to inhibit IFN-γ-mediated upregulation of MHC class I on Jurkat cells [20]. Given the critical role of the type I cytokine pathway in the immune response to mycobacterial infections [21], these reports provide direct evidence for how anti-IFN-γ autoantibodies can affect protective anti-mycobacterial immunity.
Recurrent staphylococcal cellulitis and subcutaneous abscesses were reported in a child with autoantibodies against IL-6 [22]. These anti-IL-6 autoantibodies inhibited IL-6-mediated STAT3 phosphorylation and C-reactive protein (CRP) production in Hep3B cells. Since IL-6 is pivotal for CRP induction, these results indicated that anti-IL-6 autoantibodies contributed to the lack of CRP response in this patient during staphylococcal infections. In addition, IL-6 - deficient mice have been shown to be susceptible to various pyogenic infections, including Streptococcus pneumoniae, Pseudomonas aeruginosa, and Klebsiella pneumoniae [23]–[26]. Interestingly, autoantibodies to IL-6 were not identified in other patients with severe staphylococcal diseases and hence suggest that anti-IL-6 autoantibodies were not generated due to molecular mimicry with Staphylococcus aureus. In addition, the course of clinical events in the patient was suggestive of an occurrence of anti-IL-6 autoantibodies that preceded staphylococcal infection.
Patients suffering from pulmonary alveolar proteinosis (PAP) present with neutralizing antibodies against granulocyte/macrophage colony–stimulating factor (GM-CSF) and show high mortality due to infection [27]. GM-CSF has a key role in enhancing the antimicrobial activities of neutrophils and macrophages by augmenting the expression of CD11b, an adhesion molecule that mediates neutrophil adhesion to endothelial cells, and hence promoting the recruitment of neutrophils to the site of infection; promoting the differentiation of macrophages and dendritic cells (DCs); and by priming the phagocytosis and bactericidal activities of these cells. Low levels of GM-CSF autoantibodies are present in healthy individuals. These autoantibodies are implicated in scavenging and neutralizing free GM-CSF and to regulate myeloid cell functions and GM-CSF-mediated inflammation and autoimmunity [28]. However, active PAP patients have high amounts of GM-CSF autoantibodies that impair the antimicrobial functions of neutrophils, macrophages, and the expression of CD11b [29]. In addition, these autoantibodies exist abundantly in the lungs, and by effectively blocking GM-CSF binding to its receptor, they specifically inhibit alveolar macrophage differentiation, phagocytosis, and surfactant catabolism [30], [31]. Patient-derived GM-CSF autoantibodies reproduced PAP in experimental non-human primate and murine models [29], [32], while individuals with mutations in GM-CSF receptor are also affected with PAP [33]. These results thus confirm the causal relationship between defective GM-CSF function, autoantibodies, and PAP.
Th17 cytokines are implicated in protection against fungal infections, including Candida at mucosal surfaces, and hence neutralizing antibodies to Th17 cytokines can predispose to fungal infections [8], [34], [35]. Interestingly, neutralizing autoantibodies against Th17 cell cytokines IL-17A, IL-17F, and IL-22 have been reported in chronic mucocutaneous candidiasis (CMC) patients with autoimmune polyendocrinopathy syndrome-1 (APS-1) or thymoma [36], [37]. Of particular importance, the autoantibody titers were high before the onset of CMC. Further, individuals with mutations in STAT3 and IL-12RB1 showed impaired development of Th17 cells and higher susceptibility to candidiasis [38].
In addition to the above examples, autoantibodies to type I IFNs (such as IFN-α2, IFN-ω), IL-12, and TNF-α were also identified in patients with autoimmune and rheumatic diseases and in those with chronic infections [39]. Intractable (even fatal) infections in myasthenia gravis patients with thymoma might be related to high titers of anti-IL-12 and anti-IFNα autoantibodies that can reduce an IFN-γ response with a bias towards an IL-4 response [40].
Taken together, anti-cytokine autoantibodies induce an acquired immune-compromised state that predisposes the host to infections. Although autoantibodies to several cytokines are relatively widespread, they rarely neutralize to a significant extent [39]. Further, anti-cytokine autoantibodies do not seem to have co-distribution, and cytokines do have redundant functions; hence, severe infections are not common unless as described above, and neutralizing autoantibodies are developed against specific cytokines that are key in an anti-microbial response.
In view of these findings, we suggest that patients with uncontrolled or repeated infections despite antimicrobial therapy should be considered for screening and evaluating autoimmunity. Although reported examples are of autoantibodies to cytokines, the occurrence of autoantibodies that target either molecules implicated in the recognition of pathogens (such as Toll-like receptors and lectin receptors) or antigen presenting and co-stimulatory molecules cannot be ruled out. Indeed, genetic defects or polymorphisms in pattern recognition receptors and their signaling pathways and susceptibility to infections have been reported [41]–[44].
Enigma of Induction of Anti-Cytokine Autoantibodies
Despite the reports of anti-cytokine antibodies in several malignant or infectious diseases and their low titers in healthy individuals, the high titers are predominant in autoimmune diseases [39]. Consensually, anti-cytokine antibodies against type I IFNs, IL-12, IL-17, and IL-22 are found in APS-1 or myasthenia gravis patients associated with or without thymoma [36], [37], [45]. Here, AIRE (autoimmune regulator), a novel gene that regulates peripheral self-antigen expression in medullary thymic epithelia and DCs, is mutated, leading to disturbed self-tolerance mechanisms. Thus, APS-1 patients may display autoantibodies against type I IFNs and IL-17 cytokines as a result of impaired AIRE-dependent tolerance induction. Further, extensive work by the Meager and Willcox group provided clues toward autoimmunizing mechanisms and innate cells (plasmacytoid and myeloid DCs) in the induction of anti-cytokine antibodies to type I IFNs and IL-12 [39], [40], [45].
Therefore, it is probable that autoantibodies are produced as a consequence of infections and these autoantibodies subsequently exacerbate the infectious diseases or, alternatively, a cryptic autoimmunity develops due to unknown reasons that predispose the individual to infections. Infectious agents and vaccines are often thought to be one of the environmental factors that induce autoimmunity either by molecular mimicry, epitope spreading, bystander activation of immune system, or polyclonal activation of immune cells [2], [3]. It is thus likely that in case of chronic persistent diseases such as tuberculosis, a pathogen might trigger the autoimmune process by one of these mechanisms. Indeed, the majority of patients with autoantibodies and mycobacterial infections originated from disease-endemic areas [13]–[20]. Therefore, dissection of underlying causes of autoimmunity such as genetic polymorphisms, gene deficiency, or environmental factors might shed light on these unanswered questions.
Therapeutic Options for Autoimmunity-Associated Infectious Diseases
Therapeutic strategies for autoimmunity-associated infectious diseases should be aimed at controlling the infection as well as inhibiting the autoimmune response: blocking autoantibody-producing B cells and neutralizing autoantibodies. In this context, a combination of anti-microbial agents and immunosuppressive treatments represents a classical line of therapy for autoimmunity-associated infectious diseases. Plasmapheresis that removes autoantibodies or supplementing exogenous cytokines (against which autoantibodies have developed) are other potential therapeutic strategies. However, such therapeutic strategies do not eliminate the source of autoantibodies, i.e., autoantibody-producing B cells and plasma cells.
Autoantibody-producing B cells can be eliminated by B cell–targeted therapies (such as monoclonal antibodies to CD20, CD19, and CD22 or to B cell-activating factor (BAFF) [46]–[48]). However, repeated cycles of B cell–targeted therapies can lead to a reduction in total immunoglobulin level and predisposition to serious infections [49], [50]. Also, these therapeutic agents do not target antibody-producing plasma cells.
In view of proven safety and efficacy in diverse autoimmune diseases, polyclonal intravenous immunoglobulin (IVIg) in combination with anti-microbial agents represents an attractive therapy for autoimmunity-associated infections [51]. IVIg targets both cellular and soluble mediators of autoimmunity and inhibits the disease by multi-pronged mutually nonexclusive mechanisms such as neutralization of anti-cytokine autoantibodies by broad-spectrum anti-idiotypic antibodies, induction of B cell tolerance, inhibition of cellular proliferation, regulation of immunoglobulin repertoire, suppression of innate antigen presenting cells and inhibition of T cell help to B cells, and expansion of CD4+CD25+ regulatory T cells, the cells that are critical for maintaining immune tolerance and to suppress autoimmunity [51], [52]. Since IVIg is obtained from pooled plasma of several thousand healthy blood donors, based on the exposure of donors to infectious diseases and vaccinations, IVIg contains antibodies to a wide range of infectious agents, and hence these anti-microbial antibodies within IVIg preparations can directly neutralize pathogens [53]. However, determining an effective dose regimen and duration of IVIg therapy needs further investigation.
In our opinion, considering all therapeutic options, a “triple” combination of anti-microbial agents, B cell–targeted therapies, and IVIg represents the most appropriate and ideal method for treating autoimmunity-associated infectious diseases. Indeed, the combination of B cell–targeted therapies and IVIg has been successfully used in several autoimmune and inflammatory diseases [54], [55].
Accession Numbers/ID Numbers for Genes and Proteins: UniProtKB
The UniProt (http://www.uniprot.org/) accession numbers for genes and proteins discussed in this paper are IL-17A, Q16552; IL-17F, Q96PD4; IL-21, Q9HBE4; IL-22, Q9GZX6; IL-23, Q9NPF7; IL-12RB2, Q99665; IFN-γ, P01579; GM-CSF, P01587; IL-6, P05231; IL-4, P05112; IL-5, P05113; IL-13, P35225; BAFF (BLyS), Q9Y275; IFN-α2, P01563; IFN-ω, P05000; TNF-α, P01375; IL-1β, P01584; IL-12 p40 (IL-12B), P29460; IL-12 p35 (IL-12A), P29459; AIRE, O43918; STAT3, P40763; HLA-DR, O19685; CD20, P11836; CD11b, P11215; CD19, P15391; CD22, P20273.
Zdroje
1. ShoenfeldY
1994 Idiotypic induction of autoimmunity: a new aspect of the idiotypic network. FASEB J 8 1296 1301
2. KivityS
Agmon-LevinN
BlankM
ShoenfeldY
2009 Infections and autoimmunity—friends or foes? Trends Immunol 30 409 414
3. Agmon-LevinN
PazZ
IsraeliE
ShoenfeldY
2009 Vaccines and autoimmunity. Nat Rev Rheumatol 5 648 652
4. InvernizziP
GershwinME
2009 The genetics of human autoimmune disease. J Autoimmun 33 290 299
5. HewagamaA
RichardsonB
2009 The genetics and epigenetics of autoimmune diseases. J Autoimmun 33 3 11
6. ZhuJ
PaulWE
2008 CD4 T cells: fates, functions, and faults. Blood 112 1557 1569
7. BettelliE
KornT
OukkaM
KuchrooVK
2008 Induction and effector functions of T(H)17 cells. Nature 453 1051 1057
8. DubinPJ
KollsJK
2008 Th17 cytokines and mucosal immunity. Immunol Rev 226 160 171
9. AimaniandaV
HaenslerJ
Lacroix-DesmazesS
KaveriSV
BayryJ
2009 Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol Sci 30 287 295
10. DinarelloCA
2003 Anti-cytokine therapeutics and infections. Vaccine 21 Suppl 2 S24 34
11. WinthropKL
ChillerT
2009 Preventing and treating biologic-associated opportunistic infections. Nat Rev Rheumatol 5 405 410
12. Marodi L, Casanova JL Can primary immunodeficiencies help to provide insights into infectious risks of therapeutic antibodies? Nat Rev Immunol 10 299 300
13. MadariagaL
AmurrioC
MartinG
Garcia-CebrianF
BicandiJ
1998 Detection of anti-interferon-γ autoantibodies in subjects infected by Mycobacterium tuberculosis. Int J Tuberc Lung Dis 2 62 68
14. DoffingerR
HelbertMR
Barcenas-MoralesG
YangK
DupuisS
2004 Autoantibodies to interferon-γ in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin Infect Dis 38 e10 14
15. HoflichC
SabatR
RosseauS
TemmesfeldB
SlevogtH
2004 Naturally occurring anti-IFN-γ autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenenans. Blood 103 673 675
16. KampmannB
HemingwayC
StephensA
DavidsonR
GoodsallA
2005 Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-γ. J Clin Invest 115 2480 2488
17. PatelSY
DingL
BrownMR
LantzL
GayT
2005 Anti-IFN-γ autoantibodies in disseminated nontuberculous mycobacterial infections. J Immunol 175 4769 4776
18. TanakaY
HoriT
ItoK
FujitaT
IshikawaT
2007 Disseminated Mycobacterium avium complex infection in a patient with autoantibody to interferon-γ. Intern Med 46 1005 1009
19. KoyaT
TsubataC
KagamuH
KoyamaK
HayashiM
2009 Anti-interferon-γ autoantibody in a patient with disseminated Mycobacterium avium complex. J Infect Chemother 15 118 122
20. BaerleckenN
JacobsR
StollM
SchmidtRE
WitteT
2009 Recurrent, multifocal Mycobacterium avium-intercellulare infection in a patient with interferon-γ autoantibody. Clin Infect Dis 49 e76 78
21. CasanovaJL
AbelL
2002 Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol 20 581 620
22. PuelA
PicardC
LorrotM
PonsC
ChrabiehM
2008 Recurrent staphylococcal cellulitis and subcutaneous abscesses in a child with autoantibodies against IL-6. J Immunol 180 647 654
23. van der PollT
KeoghCV
GuiraoX
BuurmanWA
KopfM
1997 Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis 176 439 444
24. van EnckevortFH
SweepCG
SpanPN
NeteaMG
HermusAR
2001 Reduced adrenal response and increased mortality after systemic Klebsiella pneumoniae infection in interleukin-6-deficient mice. Eur Cytokine Netw 12 581 586
25. ColeN
BaoS
StapletonF
ThakurA
HusbandAJ
2003 Pseudomonas aeruginosa keratitis in IL-6-deficient mice. Int Arch Allergy Immunol 130 165 172
26. DiaoH
KohanawaM
2005 Endogenous interleukin-6 plays a crucial protective role in streptococcal toxic shock syndrome via suppression of tumor necrosis factor alpha production. Infect Immun 73 3745 3748
27. KitamuraT
TanakaN
WatanabeJ
Uchida, KanegasakiS
1999 Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med 190 875 880
28. UchidaK
NakataK
SuzukiT
LuisettiM
WatanabeM
2009 Granulocyte/macrophage-colony-stimulating factor autoantibodies and myeloid cell immune functions in healthy subjects. Blood 113 2547 2556
29. UchidaK
BeckDC
YamamotoT
BerclazPY
AbeS
2007 GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med 356 567 579
30. UchidaK
NakataK
TrapnellBC
TerakawaT
HamanoE
2004 High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood 103 1089 1098
31. NakataK
KanazawaH
WatanabeM
2006 Why does the autoantibody against granulocyte-macrophage colony-stimulating factor cause lesions only in the lung? Respirology 11 Suppl S65 69
32. SakagamiT
UchidaK
SuzukiT
CareyBC
WoodRE
2009 Human GM-CSF autoantibodies and reproduction of pulmonary alveolar proteinosis. N Engl J Med 361 2679 2681
33. SuzukiT
SakagamiT
RubinBK
NogeeLM
WoodRE
2008 Familial pulmonary alveolar proteinosis caused by mutations in CSF2RA. J Exp Med 205 2703 2710
34. ContiHR
ShenF
NayyarN
StocumE
SunJN
2009 Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 206 299 311
35. LinL
IbrahimAS
XuX
FarberJM
AvanesianV
2009 Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog 5 e1000703 doi:10.1371/journal.ppat.1000703
36. KisandK
Boe WolffAS
PodkrajsekKT
TserelL
LinkM
2010 Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med 207 299 308
37. PuelA
DoffingerR
NatividadA
ChrabiehM
Barcenas-MoralesG
2010 Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med 207 291 297
38. de BeaucoudreyL
PuelA
Filipe-SantosO
CobatA
GhandilP
2008 Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med 205 1543 1550
39. MeagerA
WadhwaM
DilgerP
BirdC
ThorpeR
2003 Anti-cytokine autoantibodies in autoimmunity: preponderance of neutralizing autoantibodies against interferon-α, interferon-ω and interleukin-12 in patients with thymoma and/or myasthenia gravis. Clin Exp Immunol 132 128 136
40. ZhangW
LiuJL
MeagerA
Newsom-DavisJ
WillcoxN
2003 Autoantibodies to IL-12 in myasthenia gravis patients with thymoma; effects on the IFN-γ responses of healthy CD4+ T cells. J Neuroimmunol 139 102 108
41. van de VosseE
van DisselJT
OttenhoffTH
2009 Genetic deficiencies of innate immune signalling in human infectious disease. Lancet Infect Dis 9 688 698
42. FerwerdaB
FerwerdaG
PlantingaTS
WillmentJA
van SprielAB
2009 Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med 361 1760 1767
43. GlockerEO
HennigsA
NabaviM
SchafferAA
WoellnerC
2009 A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med 361 1727 1735
44. CawsM
ThwaitesG
DunstanS
HawnTR
LanNT
2008 The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog 4 e1000034 doi:10.1371/journal.ppat.1000034
45. MeagerA
VisvalingamK
PetersonP
MollK
MurumagiA
2006 Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med 3 e289 doi:10.1371/journal.pmed.0030289
46. EdwardsJC
CambridgeG
2006 B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol 6 394 403
47. TedderTF
2009 CD19: a promising B cell target for rheumatoid arthritis. Nat Rev Rheumatol 5 572 577
48. DornerT
RadbruchA
BurmesterGR
2009 B-cell-directed therapies for autoimmune disease. Nat Rev Rheumatol 5 433 441
49. KorhonenR
MoilanenE
2009 Anti-CD20 antibody rituximab in the treatment of rheumatoid arthritis. Basic Clin Pharmacol Toxicol 106 13 21
50. McDonaldV
LeandroM
2009 Rituximab in non-haematological disorders of adults and its mode of action. Br J Haematol 146 233 246
51. KazatchkineMD
KaveriSV
2001 Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med 345 747 755
52. Tha-InT
BayryJ
MetselaarHJ
KaveriSV
KwekkeboomJ
2008 Modulation of the cellular immune system by intravenous immunoglobulin. Trends Immunol 29 608 615
53. BayryJ
Lacroix-DesmazesS
KazatchkineMD
KaveriSV
2004 Intravenous immunoglobulin for infectious diseases: back to the pre-antibiotic and passive prophylaxis era? Trends Pharmacol Sci 25 306 310
54. AhmedAR
SpigelmanZ
CavaciniLA
PosnerMR
2006 Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin. N Engl J Med 355 1772 1779
55. VoAA
LukovskyM
ToyodaM
WangJ
ReinsmoenNL
2008 Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med 359 242 251
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease AgentČlánek TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease SusceptibilityČlánek The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive ParasiteČlánek Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
-
Všechny články tohoto čísla
- Patients with Discordant Responses to Antiretroviral Therapy Have Impaired Killing of HIV-Infected T Cells
- A Molecular Mechanism for Eflornithine Resistance in African Trypanosomes
- Tyrosine Sulfation of the Amino Terminus of PSGL-1 Is Critical for Enterovirus 71 Infection
- Autoimmunity as a Predisposition for Infectious Diseases
- The Subtilisin-Like Protease AprV2 Is Required for Virulence and Uses a Novel Disulphide-Tethered Exosite to Bind Substrates
- Structural Analysis of HIV-1 Maturation Using Cryo-Electron Tomography
- Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease Agent
- Interferon-Inducible CXC Chemokines Directly Contribute to Host Defense against Inhalational Anthrax in a Murine Model of Infection
- TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease Susceptibility
- The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive Parasite
- CO Acts as a Signalling Molecule in Populations of the Fungal Pathogen
- SV2 Mediates Entry of Tetanus Neurotoxin into Central Neurons
- MAP Kinase Phosphatase-2 Plays a Critical Role in Response to Infection by
- Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
- Potentiation of Epithelial Innate Host Responses by Intercellular Communication
- Fcγ Receptor I Alpha Chain (CD64) Expression in Macrophages Is Critical for the Onset of Meningitis by K1
- ANK, a Host Cytoplasmic Receptor for the Cell-to-Cell Movement Protein, Facilitates Intercellular Transport through Plasmodesmata
- Analysis of the Initiating Events in HIV-1 Particle Assembly and Genome Packaging
- Evolution of Linked Avirulence Effectors in Is Affected by Genomic Environment and Exposure to Resistance Genes in Host Plants
- Structural Basis of HIV-1 Neutralization by Affinity Matured Fabs Directed against the Internal Trimeric Coiled-Coil of gp41
- Hepatitis C Virus (HCV) Evades NKG2D-Dependent NK Cell Responses through NS5A-Mediated Imbalance of Inflammatory Cytokines
- Host Cell Invasion and Virulence Mediated by Ssa1
- Global Gene Expression in Urine from Women with Urinary Tract Infection
- Should the Human Microbiome Be Considered When Developing Vaccines?
- HapX Positively and Negatively Regulates the Transcriptional Response to Iron Deprivation in
- Enhancing Oral Vaccine Potency by Targeting Intestinal M Cells
- Herpes Simplex Virus Reorganizes the Cellular DNA Repair and Protein Quality Control Machinery
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- Cryo Electron Tomography of Native HIV-1 Budding Sites
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- Modelling the Evolution and Spread of HIV Immune Escape Mutants
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
- Platelet-Activating Factor Receptor Plays a Role in Lung Injury and Death Caused by Influenza A in Mice
- Genetic and Structural Basis for Selection of a Ubiquitous T Cell Receptor Deployed in Epstein-Barr Virus Infection
- Ubiquitin-Regulated Nuclear-Cytoplasmic Trafficking of the Nipah Virus Matrix Protein Is Important for Viral Budding
- Pneumolysin Activates the NLRP3 Inflammasome and Promotes Proinflammatory Cytokines Independently of TLR4
- Immune Evasion by : Differential Targeting of Dendritic Cell Subpopulations
- Survival in Selective Sand Fly Vector Requires a Specific -Encoded Lipophosphoglycan Galactosylation Pattern
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



