-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of
Animals need to couple growth with nutritional availability for proper development and physiology, which leads to better survival. Nutritional information is mostly perceived by peripheral organs, particularly metabolic organs such as adipose tissue and gut, before being relayed to the brain, which modulates physiological responses. Hormonal signaling ensures this organ-to-organ communication, and defects in this endocrine regulation in humans often cause diseases including obesity and diabetes. In the fruit fly Drosophila melanogaster, adipose tissue (the “fat body”) has been suggested to play an important role in coordinating growth with metabolism. Here, we show that the Drosophila CCHamide-2 (CCHa2) gene, expressed in the fat body and gut, encodes a nutrient-sensitive peptide hormone. The CCHa2 peptide signals to neuroendocrine cells in the brain that produce Drosophila insulin-like peptides (Dilps) through its receptor (CCHa2-R) and promotes the production of Dilps. Mutants of both CCHa2 and CCHa2-R display severe growth retardation during larval stages. These results suggest that CCHa2 and CCHa2-R functionally connect peripheral tissues with the brain, and that CCHa2/CCHa2-R signaling coordinates the animal’s growth with its nutritional conditions by regulating its production of insulin-like peptides.
Published in the journal: . PLoS Genet 11(5): e32767. doi:10.1371/journal.pgen.1005209
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005209Summary
Animals need to couple growth with nutritional availability for proper development and physiology, which leads to better survival. Nutritional information is mostly perceived by peripheral organs, particularly metabolic organs such as adipose tissue and gut, before being relayed to the brain, which modulates physiological responses. Hormonal signaling ensures this organ-to-organ communication, and defects in this endocrine regulation in humans often cause diseases including obesity and diabetes. In the fruit fly Drosophila melanogaster, adipose tissue (the “fat body”) has been suggested to play an important role in coordinating growth with metabolism. Here, we show that the Drosophila CCHamide-2 (CCHa2) gene, expressed in the fat body and gut, encodes a nutrient-sensitive peptide hormone. The CCHa2 peptide signals to neuroendocrine cells in the brain that produce Drosophila insulin-like peptides (Dilps) through its receptor (CCHa2-R) and promotes the production of Dilps. Mutants of both CCHa2 and CCHa2-R display severe growth retardation during larval stages. These results suggest that CCHa2 and CCHa2-R functionally connect peripheral tissues with the brain, and that CCHa2/CCHa2-R signaling coordinates the animal’s growth with its nutritional conditions by regulating its production of insulin-like peptides.
Introduction
Organisms need to coordinate growth and metabolism with their nutritional status to ensure proper development and the maintenance of homeostasis. In multicellular animals, nutritional information is mostly perceived by peripheral organs. It is subsequently relayed to other peripheral organs or to the central nervous system (CNS), which generates appropriate physiological and behavioral responses. Endocrine systems ensure this type of organ-to-organ communication via hormonal signals secreted from specialized glandular cells. For example, mammalian insulin is secreted from pancreatic β-cells in response to high blood glucose levels; insulin is then received by its receptor in the liver as well as in many other tissues to promote glucose uptake and anabolism, thereby reducing blood sugar levels [1]. In a similar manner, leptin secreted from adipose tissues is received by the hypothalamus, where it acts to alter energy expenditure and food intake [2] [3] [4] [5]. Caloric restriction reduces the secretion of leptin, leading to both an increase in appetite and a decrease in energy expenditure, which is known to be an adaptive response to starvation [6]. These findings demonstrate the significance of peripheral tissues in the maintenance of homoeostasis [7]. However, only a few peripheral hormones have been identified, and the mechanisms by which they regulate an organism's development or physiology in response to external stimuli remain elusive.
It has been reported that the endocrine system of Drosophila melanogaster allows adipose tissue, known as the fat body, to communicate with the CNS in a manner similar to that observed in mammals. This signaling depends on nutritional conditions and ultimately couples growth and metabolism with nutritional status. To date, two pathways have been described. In one pathway described from larvae, the fat body-specific down-regulation of either the Slimfast (Slif) amino acid transporter or the Target of Rapamycin (TOR) nutrient-sensing pathway affects systemic growth, suggesting that a hitherto unidentified amino acid-dependent signal(s) is secreted by the fat body for proper growth control [8]. In a second pathway that was identified in adults, Unpaired-2 (Upd2), which is a functional analogue of leptin, was identified as another fat body-derived growth regulator [9]. The expression of upd2 is both sugar - and lipid-sensitive and is apparently independent of the amino acid-activated TOR pathway [9]. Although no signaling molecules that act downstream of the Slif/TOR pathway have been identified yet, these fat body-derived signals ultimately regulate the production of insulin-like peptides (Drosophila insulin-like peptides; Dilps) secreted from the brain [10] [9].
Dilps are evolutionarily conserved peptide hormones with functions similar to those of mammalian insulin/insulin-like growth factor (IGF), including the control of tissue growth and blood sugar levels in response to nutritional conditions [11,12] [13] [14] [15] [16]. Eight dilp genes exist in the Drosophila melanogaster genome [11] [15] [16]. Unlike mammalian insulin, which is secreted from the pancreas, the major Dilps (Dilp2, -3, and -5) are specifically expressed in bilateral clusters of neurosecretory cells [insulin-producing cells (IPCs)] located in the anteromedial region of the brain hemispheres [11] [12]. With regard to the regulation of insulin-like peptides, the knockdown of the Slif/TOR pathway or upd2 in the larval fat body results in the down-regulation of Dilp2 secretion [9,10]. Upd2, a type-I cytokine, activates the JAK/STAT pathway through its receptor Domeless (Dome) [17] [18]. Dome is expressed in the GABAergic neurons juxtaposed to the IPCs in the adult brain. Activation of Dome by Upd2 blocks GABAergic inhibition of the IPCs and thereby facilitates Dilp secretion [9]. Therefore, signaling from peripheral tissues to the brain appears to be essential for the regulation of organismal growth and metabolism in response to nutrition availability in Drosophila melanogaster.
In this study, we investigated the roles of CCHa2 and its receptor in growth control in Drosophila melanogaster. CCHa2 was identified as a bioactive peptide that activates a G protein-coupled receptor (GPCR) encoded by CG14593 (now named CCHa2-R) [19]. Strong expression of CCHa2 in the larval fat body and gut motivated us to examine the roles of CCHa2 and its receptor in nutrient sensing and growth control. By generating mutants of CCHa2 and CCHa2-R, we show that CCHa2/CCHa2-R signaling from the periphery to the CNS can control the synthesis and secretion of Dilps. Our results demonstrate that CCHa2 is a novel hormone derived from peripheral tissues and that CCHa2/CCHa2-R form an additional afferent hormonal signaling pathway that coordinates systemic growth with nutrition availability.
Results
CCHa2 is a selective nutrition-sensitive hormone derived from peripheral tissues
We first measured the expression of CCHa2 mRNA in larval tissues using quantitative RT-PCR (RT-qPCR). As shown in Fig 1A, CCHa2 was predominantly detected in the fat body and the gut, with only very low expression detected in the CNS. We also examined CCHa2 expression using CCHa2 antisera, which detected punctate staining in the cytoplasm of the fat cells (Fig 1B and 1C). CCHa2 immunoreactivity was also detected in endocrine cells in the gut as previously reported [20] (Fig 1F). These signals were specific for CCHa2, because they were absent in CCHa2 mutants (Fig 1D and 1E and 1G).
Fig. 1. CCHa2 is a selective nutrient-sensitive hormone derived from peripheral tissues. 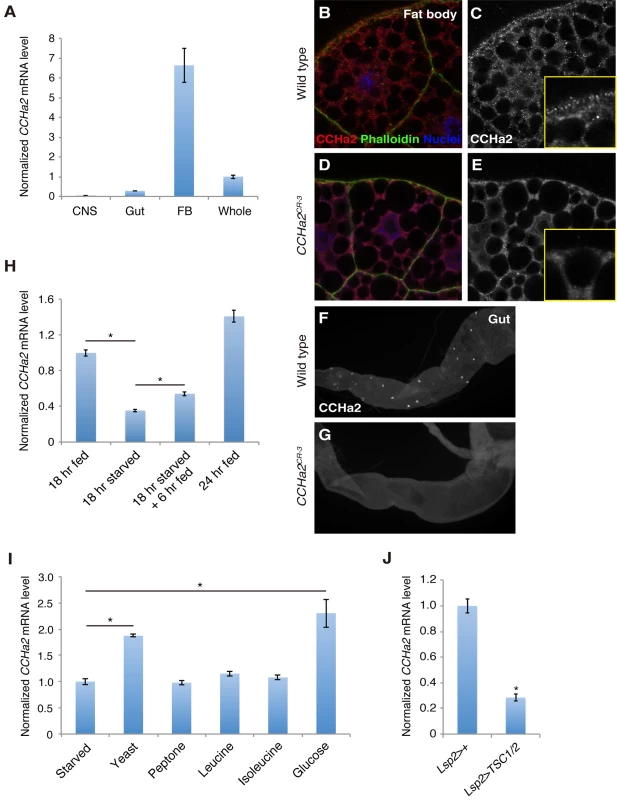
(A) RT-qPCR was performed on RNA extracted from larval tissues. CCHa2 is predominantly expressed in the larval fat body. (B-G) Anti-CCHa2 immunostaining of the fat body (B-E) and gut (F, G) from wild-type and CCHa2 mutants. Insets in C and E are high-magnification images of the rim of the fat cell. The punctate staining in B and C that is absent from D and E is the CCHa2 signal; the dimmer uniform signal is non-specific background staining, since it also appears in the tissue from CCHa2-null animals (E). (H) Effects of nutritional status on CCHa2 expression. Third-instar larvae were raised under the indicated nutritional conditions, and CCHa2 mRNA levels were tested by RT-qPCR using whole-animal RNA extracts. CCHa2 expression was decreased by starvation and recovered by re-feeding the starved larvae with yeast paste. (I) Nutrient requirements for CCHa2 expression. Third-instar larvae starved for 18 hours were re-fed with the indicated nutrients for 6 hours, and CCHa2 mRNA levels in whole animals were measured by RT-qPCR. Yeast and glucose significantly promoted CCHa2 expression. (J) Effects of fat-body-specific TOR pathway manipulation on CCHa2 expression. The TOR pathway was inhibited by expressing TSC1/2 in the fat body using Lsp2-GAL4. CCHa2 mRNA levels in whole animals were measured by RT-qPCR. We next examined the nutritional dependence of CCHa2 expression. In this assay, third-instar larvae [72 hours after egg laying (AEL)] were starved for 18 hours on water agar plates, and the relative amount of CCHa2 mRNA in the whole animal was quantified. After starvation, the expression of CCHa2 was significantly decreased, but levels recovered when the larvae were re-fed with yeast paste (Fig 1H).
To determine which nutrient signal controls CCHa2 expression, larvae were re-fed with different substances after starvation. Interestingly, both yeast and glucose induced CCHa2 expression (Fig 1I). It has been reported that the TOR nutrition-sensing pathway is activated by amino acids but not by glucose [10]. Nonetheless, we tested the involvement of this pathway in CCHa2 regulation in the fat body. When the TOR pathway was blocked in the fat body by the overexpression of signaling components TSC1/2, CCHa2 expression was significantly reduced (Fig 1J). (It should be noted that the knockdown of the TOR pathway in the fat body severely affects larval growth [8]; therefore, Lsp2-GAL4, which is expressed in the fat body at the wandering stage – after the growth period [13] – was used to overexpress TSC1/2 in order to avoid secondary effects from a systemic growth defect.) These observations suggest that CCHa2 expression is responsive to glucose and the TOR pathway. Given that glucose alone is sufficient to promote CCHa2 expression, CCHa2 appears to be distinct from the currently unidentified fat body-derived factor previously proposed to act downstream of the Slif/TOR pathway [10].
The receptor for CCHa2 is expressed in the brain
We performed RT-qPCR assays to examine the larval expression pattern of CCHa2-R (CG14593), which encodes the receptor for CCHa2. As shown in Fig 2A, CCHa2-R mRNA was detected specifically in the larval CNS. Fluorescent in situ hybridization (FISH) of larval brains with a CCHa2-R antisense RNA probe yielded signal in a subset of cells located at the anteromedial region of the brain (Fig 2B and 2C), which contains several types of neuroendocrine cells, including the IPCs [21]. To increase sensitivity for the mapping of CCHa2-R expression, its pattern was indirectly visualized using a GAL4 construct that recapitulates endogenous CCHa2-R expression. For this purpose, we established a transgenic fly line that carries an ~80-kb genomic region containing the entire CCHa2-R coding region, as well as substantial flanking sequence. The coding portion of the first CCHa2-R coding exon was replaced with sequence encoding the strong GAL4::p65 transcriptional activator (Fig 2E; CCHa2-R-GAL4::p65). Consistent with the endogenous expression pattern (Fig 2B and 2C), strong GFP signals were detected in the anteromedial region of the brain in transgenic flies in which CCHa2-R-GAL4::p65 drove the expression of UAS-nls::GFP (CCHa2-R>nlsGFP) (Fig 2F and 2G). Low levels of GFP expression were also observed in a number of cells in the brain and the ventral nerve cord (VNC) (Fig 2F and 2G), in which no CCHa2-R expression was detected by FISH, probably due to its lower sensitivity. When the transgenic larval brains were co-stained with anti-Dilp2 antibody, CCHa2-R>nlsGFP colocalized with the Dilp2 immunostaining (Fig 2H). We also found that neighboring peptidergic neurons that contain neuropeptide F (NPF) and SIFamide (SIFa), both also showed CCHa2-R>nlsGFP-expression (S1 Fig). Thus, CCHa2-R is expressed in a few types of neuroendocrine cells including the IPCs.
Fig. 2. The CCHa2 receptor is expressed in the brain including the IPCs. 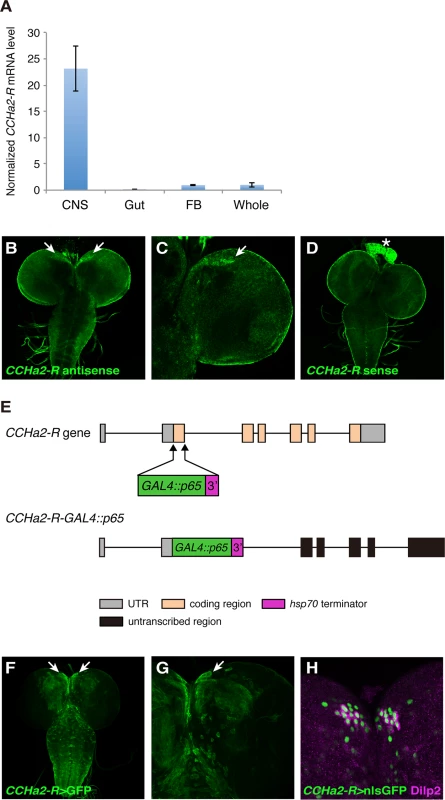
(A) RT-qPCR was performed on RNA extracted from larval tissues. CCHa2-R is specifically expressed in the CNS. (B-D) CCHa2-R mRNA was detected by in situ hybridization; signals were amplified by the TSA system. CCHa2-R mRNA is expressed in cells located in the anteromedial region of the brain (arrows in B, C). (D) Sense probe control for B and C. Signals in the ring gland (asterisk) and the CNS surface are non-specific background staining. (E) Schematic drawing of the CCHa2-R-GAL4::p65 construct. (F-G) Expression of membrane-targeted GFP driven by CCHa2-R-GAL4::p65. GFP was detected in the anteromedial cells (arrows). (H) Nuclear-localized GFP driven by CCHa2-R-GAL4::p65 is present in all the IPCs, which are marked with anti-Dilp2. Neighboring GFP-labeled cells express NPF or SIFamide (S1 Fig). CCHa2-R mutations affect the production of insulin-like peptides in the brain
Since CCHa2-R is expressed in the IPCs, we examined whether CCHa2/CCHa2-R signaling is involved in insulin regulation. We generated two mutant CCHa2-R alleles, both expected to be nulls. Using gene targeting by homologous recombination [22] [23], most of the CCHa2-R coding region – from the translation-initiating methionine through the middle of the 7th transmembrane domain of the encoded GPCR – was deleted, generating the CCHa2-RKO51-2 allele (Figs 3A and S2A). In a second scheme, transcription activator-like effector nucleases (TALENs) were targeted to sequences near the translation-initiation site of CCHa2-R to create a frame-shift mutation in the CCHa2-R coding region [24]. By injecting pairs of TALEN-encoding mRNAs into wild-type embryos, we generated the CCHa2-RTAL-34 frameshift allele (Figs 3B and S2B and S2C). Although these two mutant alleles are both homozygous-viable, we used them in transheterozygous combination (i.e., CCHa2-RKO51-2/CCHa2-RTAL-34) to avoid the effects of any unexpected secondary mutations.
Fig. 3. Generation of CCHa2-R mutants. 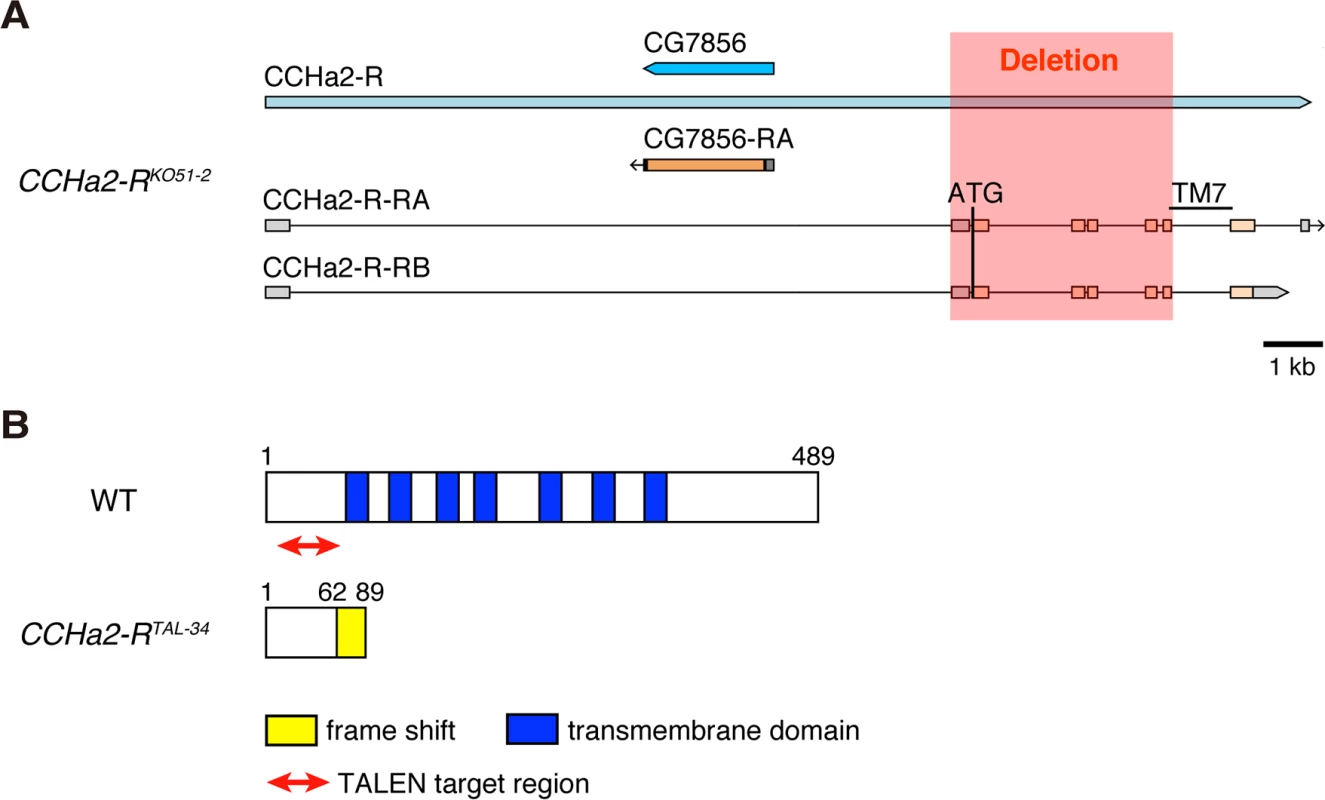
(A) The CCHa2-RKO51-2 allele was generated by gene targeting using homologous recombination. In CCHa2-RKO51-2, a 4-kb deletion spanning exons 2 through 6 removes a region from the initiating methionine through the middle of 7th transmembrane domain. (B) The CCHa2-RTAL-34 allele was created using TALENs. A 74-bp deletion was created in the first coding exon of the CCHa2-R gene, resulting in a frame-shift at amino acid position 62 and a truncation of the protein. These mutant alleles were first used to examine whether CCHa2-R is required for the transcription of two Drosophila insulin-like peptide (dilp) genes. It has been reported that dilp2, dilp3, and dilp5 are expressed in IPCs [11] [12]. As previously reported, the expression level of dilp3 is very low during the growth period (S3A Fig) [13] [14], and neither growth nor developmental timing is altered in dilp3-null mutants [25]. Nonetheless, we tested whether dilp3 is under the control of CCHa2-R signaling. As shown in S3B Fig, dilp3 mRNA levels were not altered by CCHa2-R mutations. In contrast, dilp2 and dilp5 are highly expressed in feeding larvae and have substantial influence over larval growth [13] [14] [25]. Therefore, we focused on these two Dilps. For this analysis, total RNA extracted from whole larvae was used, as dilp2 and dilp5 are predominantly expressed in the CNS during the larval stages examined [26,27]. In mid-L3 (96 hours AEL) larvae, dilp2 expression was not significantly altered by the loss of CCHa2-R (Fig 4A). In contrast, the expression of dilp5 mRNA was remarkably reduced in CCHa2-R mutant larvae, compared to control larvae heterozygous for either the CCHa2-RKO51-2 or CCHa2-RTAL-34 allele, regardless of gender (Fig 4B). It was reported that starvation or Slif/TOR inhibition down-regulates the secretion of Dilp2, but not its transcription [28] [10]. We therefore examined the protein levels of Dilp2 in IPCs by staining larval brains with anti-Dilp2. It has been shown previously that, when dilp2 transcription is constant, increased Dilp2 within the cytoplasm of IPCs reflects decreased Dilp2 release into the hemolymph, and intracellular Dilp2 has been used as a sign of Dilp2 retention in the IPCs [10]. Consistent with this, stronger Dilp2 signals were observed in the IPCs of starved wild-type larvae than in those under fed conditions (Fig 4C). When CCHa2-R mutant larvae were fed, their IPCs showed increased Dilp2 immunoreactivity, which was much stronger than that observed in starved or fed control larvae (Fig 4C). To accurately compare the signals, the Dilp2 signal intensity in the IPCs for each condition was normalized against signals of membrane-bound GFP expressed under the control of the dilp2-GAL4 driver as an internal control (because, as noted above, dilp2 transcription is unaltered in the mutants). The quantification results clearly show that levels of Dilp2 protein in the mutant IPCs were significantly higher than those in the wild type (Fig 4D). Dilp5 protein levels were also quantified by the same methods in wild-type and CCHa2-R mutant IPCs. Despite a ~40% reduction in dilp5 mRNA in the mutant IPCs (Fig 4B), Dilp5 protein levels only dropped by about 20%, suggesting decreased release (Fig 4E and 4F). These results suggest that the secretion of both Dilp2 and Dilp5 is severely affected by the loss of CCHa2-R. We also noticed that Dilp2 levels in mutant IPCs under starved conditions were slightly higher than those under fed conditions (Fig 4C and 4D), suggesting that CCHa2-R is not the sole nutrient-sensitive Dilp regulator. Analysis of feeding activity using dyed yeast demonstrated no significant differences in dye ingestion between wild-type and CCHa2-R mutants (S4 Fig), suggesting that down-regulation of CCHa2/CCHa2-R signaling does not affect larval feeding behaviors. Taken together, these results show that CCHa2-R plays a crucial role in regulating the synthesis and secretion of insulin-like peptides in IPCs.
Fig. 4. CCHa2-R mutations affect the production of Dilp2 and Dilp5 in IPCs. 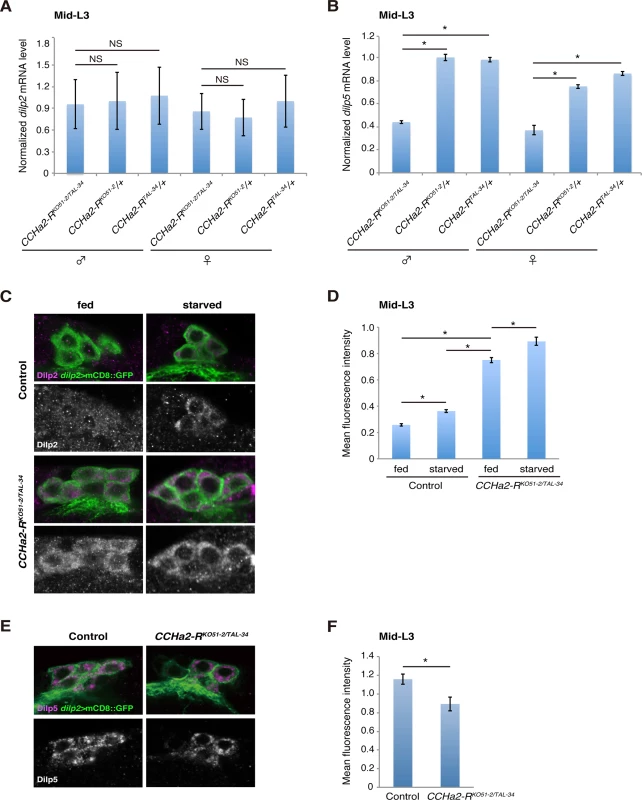
Transcript and protein levels of brain Dilps were examined in mid-third-instar larvae (96 hours AEL). (A) Relative expression level of dilp2 mRNA in whole larval RNA extracts was quantified by RT-qPCR. Transcription of dilp2 was unaffected by the loss of CCHa2-R. (B) Relative expression level of dilp5 mRNA in whole larval RNA extracts was quantified by RT-qPCR. Expression of dilp5 was significantly reduced in CCHa2-R mutants. (C) Dilp2 in the IPCs was detected by Dilp2 antibody in wild-type and CCHa2-R mutants in which the IPCs were labeled by dilp2-GAL4-driven UAS-mCD8::GFP. Wild-type and CCHa2-R mutant larvae were either fed or starved for 24 hours before staining. (D) Dilp2 levels in the IPCs were quantified. Fluorescence intensities for Dilp2 were normalized against those for GFP on the same confocal section (n = 52, 48, 51, 45 cells for fed control, starved control, fed mutants, and starved mutants, respectively). (E) Dilp5 in the IPCs was detected by anti-Dilp5 antibody in wild-type and CCHa2-R mutants, in which the IPCs were labeled by dilp2-GAL4 driving UAS-mCD8::GFP. (F) Dilp5 levels in the IPCs were quantified (n = 37 and 36 for fed control and fed mutants, respectively). Peripheral tissue-derived CCHa2 induces dilp5 expression in the brain
To examine whether CCHa2 acts together with CCHa2-R in the control of Dilps in vivo, we generated animals carrying a defective CCHa2 gene using the CRISPR/Cas system [29]. Short guide RNA was targeted to the sequence corresponding to the CCHa2 peptide-coding region, resulting in the isolation of putative null alleles for CCHa2 (CCHa2CR-1, CCHa2CR-2, and CCHa2CR-3) (Figs 5A and S5). All of these alleles displayed virtually the same phenotypes, so the results for CCHa2CR-1 are shown as representative. First, RT-qPCR analyses showed that the expression of dilp5 mRNA was significantly reduced in CCHa2CR-1/Df hemizygotes compared to heterozygous control larvae regardless of sex (Fig 5B). These results are similar to those seen in CCHa2-R mutants (compare Figs 4B and 5B). Consistent with these reduced dilp5 levels, the body weight of mid-third-instar larvae (96 hours AEL) was markedly lower in the CCHa2 hemizygotes than in the heterozygous control regardless of sex (Fig 5C). These results suggest that CCHa2 and CCHa2-R act together to regulate dilp5 expression in the brain.
Fig. 5. Peripheral tissue-derived CCHa2 induces dilp5 expression in the brain. 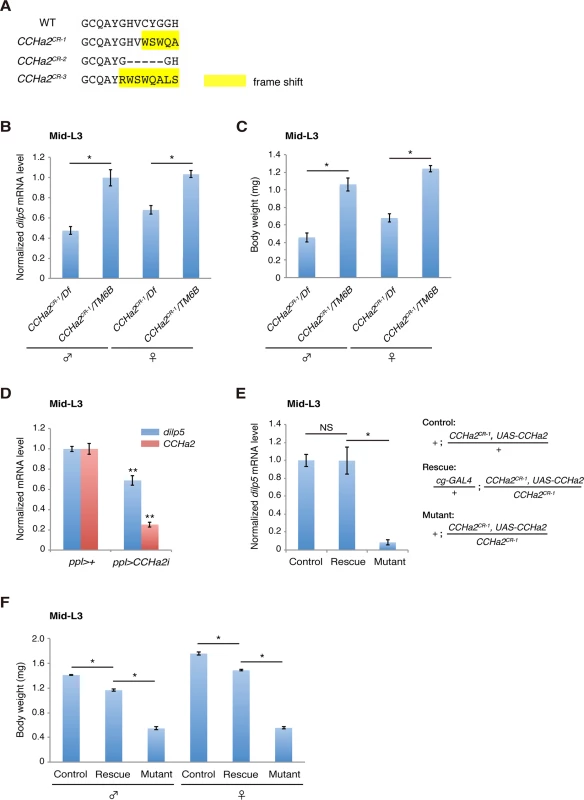
(A) Amino acid sequences of the CCHa2 peptide formed in the CCHa2 mutant alleles. (B) dilp5 mRNA levels in mid-third-instar larvae (96 hours AEL) were quantified by RT-qPCR using whole larval RNA extracts. dilp5 transcription was down-regulated in CCHa2 mutants. (C) Larval body weight was measured at the mid-third-instar larval stage (96 hours AEL; 5 to 10 larvae per batch, n = 3 batches). CCHa2 mutant larvae weighed significantly less than wild-types. (D) CCHa2 was knocked down in the fat body and gut using the ppl-GAL4 driver, and dilp5 mRNA levels were quantified by RT-qPCR. dilp5 expression was significantly reduced in the CCHa2-knockdown larvae. (E) CCHa2 was over-expressed in fat bodies of CCHa2 mutant animals using the cg-GAL4 driver, and dilp5 mRNA levels in the CNS were quantified by RT-qPCR. dilp5 transcription was completely restored by CCHa2 expression in the fat body. (F) The body weight of rescued animals (96 hours AEL; 5 to 10 larvae per batch, n = 3 batches). Body weight was mostly rescued by CCHa2 expression in the fat body. To clarify whether peripheral tissues are responsible for the CCHa2-dependent regulation of dilps in the brain, CCHa2 was specifically knocked down in the fat body and gut using targeted RNAi driven by the ppl-GAL4 driver [8] [30]. As shown in Fig 5D, dilp5 mRNA levels were significantly reduced in CCHa2-knockdown larvae, indicating that peripheral tissue-derived CCHa2 activates dilp5 expression in the brain. To further confirm the importance of CCHa2 signaling from the periphery to the CNS, we expressed CCHa2 in the fat body in the CCHa2 mutant background using the cg-GAL4 driver [30]. To ensure the detection of brain-specific changes in dilp5 expression, RNA extracted from the brain was used for RT-qPCR. As shown in Fig 5E, CCHa2 expression in the fat body completely restored dilp5 expression in the brain of CCHa2 mutants. Consistent with the rescued dilp5 expression, the body weight of these larvae was mostly recovered (Fig 5F). These results demonstrate that CCHa2 released from peripheral tissues controls dilp5 expression in the brain.
CCHa2/CCHa2-R signaling forms a direct link between peripheral tissues and the brain
To clarify whether CCHa2 directly signals to the brain, calcium imaging was performed using ex vivo culture of larval brains expressing the fluorescent calcium sensor GCaMP6s [31] in the IPCs. Dissected wild-type or CCHa2-R mutant brains were immersed in phosphate buffered saline (PBS). Peptides were added to the culture medium, and fluorescence changes from GCaMP6s were measured by live imaging. As shown in Fig 6A and 6B, signal intensities in wild-type IPCs were dramatically increased upon CCHa2 administration (S1 Movie), indicating that no other tissues are required for relaying the CCHa2 signal to the CNS. In contrast, no such increase in signal was observed in CCHa2-R mutant brains upon CCHa2 administration (S2 Movie). The difference in fluorescence intensities between wild-type and CCHa2-R mutant IPCs became significant as early as 2 minutes after CCHa2 administration and lasted at least for 7 minutes (S6 Fig). No increase in signal intensity was observed in wild-type brains treated with ghrelin or nociceptin (S3 and S4 Movies, respectively), mammalian peptide hormones which have no homologues in Drosophila. These results indicate that the observed activation of the IPCs is a specific response to CCHa2 mediated by CCHa2-R.
Fig. 6. CCHa2 directly activates IPCs in the brain. 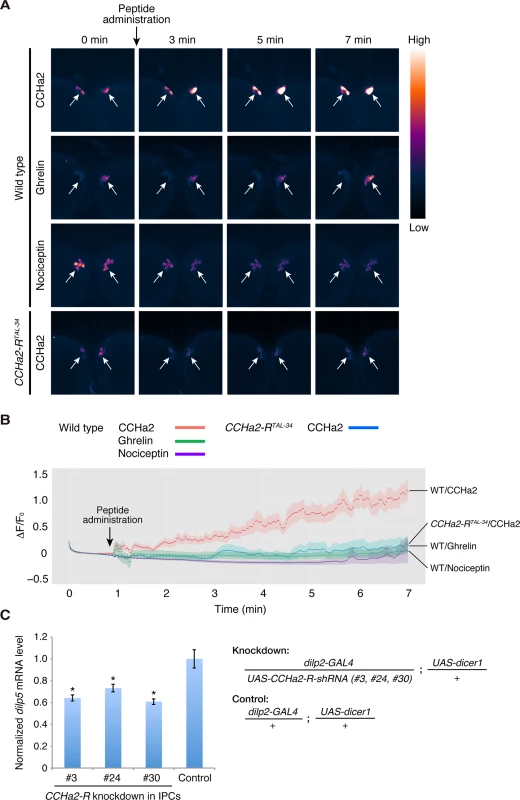
(A, B) Calcium imaging of larval brain explants. Brains dissected from late-third-instar larvae were exposed to peptide hormones: wild-type (dilp2-GAL4/UAS-GCaMP6s) and CCHa2-R mutant (dilp2-GAL4, CCHa2-RTAL-34/UAS-GCaMP6s, CCHa2-RTAL-34) brains were challenged with CCHa2; wild-type brains were additionally tested with mammalian ghrelin and nociceptin. GCaMP6s signals in IPCs were measured by confocal microscopy at 4 Hz. Pseudocolored images of selected time points are displayed in (A). ΔF/F0 at each time point is plotted in (B). (C) CCHa2-R was knocked down specifically in the IPCs using UAS-CCHa2-R-shRNA driven by dilp2-GAL4, and dilp5 mRNA levels were quantified by RT-qPCR. Expression of dilp5 was significantly reduced in the CCHa2-R-knockdown larvae. To examine whether the CCHa2 signal directly activates the IPCs, rather than being relayed by other neurons, CCHa2-R was knocked down specifically in the IPCs. We generated three UAS-CCHa2-R-shRNA lines (#3, 24, 30; S7 Fig). When any one of these shRNAs was specifically expressed in the IPCs, dilp5 mRNA levels were significantly decreased (Fig 6C). These results demonstrate that CCHa2-R is specifically required in IPCs for those cells’ response to the CCHa2 ligand, and suggest that CCHa2 secreted from the peripheral tissues directly signals to IPCs without being relayed by other tissues.
CCHa2-R mutants show defects in larval growth and developmental delay
Since insulin-like peptides are major growth hormones in flies, we anticipated that abnormal expression and secretion of Dilps in the CCHa2-R mutants would lead to growth defects. To test this hypothesis, wild-type and mutant animals were weighed at time points from larval through adult stages. As predicted, CCHa2-R transheterozygous mutants weighed markedly less than heterozygous CCHa2-RTAL-34/+ control larvae from 72 to 108 hours AEL (Fig 7A). However, after 108 hours AEL, the mutant larvae showed rapid weight gain, surpassing wild-type weight at 120 hours AEL (Fig 7A). The body weight of the mutant larvae decreased from 144 hours AEL onward, resulting in pupae and adults of normal weight. In concert with these growth defects, CCHa2-R mutants displayed a developmental delay during the larval stages, with the feeding period extended for about 24 hours before entering the wandering stages. This prolonged feeding period resulted in an increase in body weight and delay in pupation and eclosion (Fig 7B). Since broad dilp2 overexpression caused lethality [11], we were unable to examine whether these developmental delays are a consequence of growth defects or whether developmental timing is regulated independently of growth. These results nevertheless suggest that CCHa2-R is a major growth regulator until around 108 hours AEL, after which time other mechanisms may operate to accelerate the growth of the mutant larvae.
Fig. 7. CCHa2-R mutants show growth defects and developmental delay. 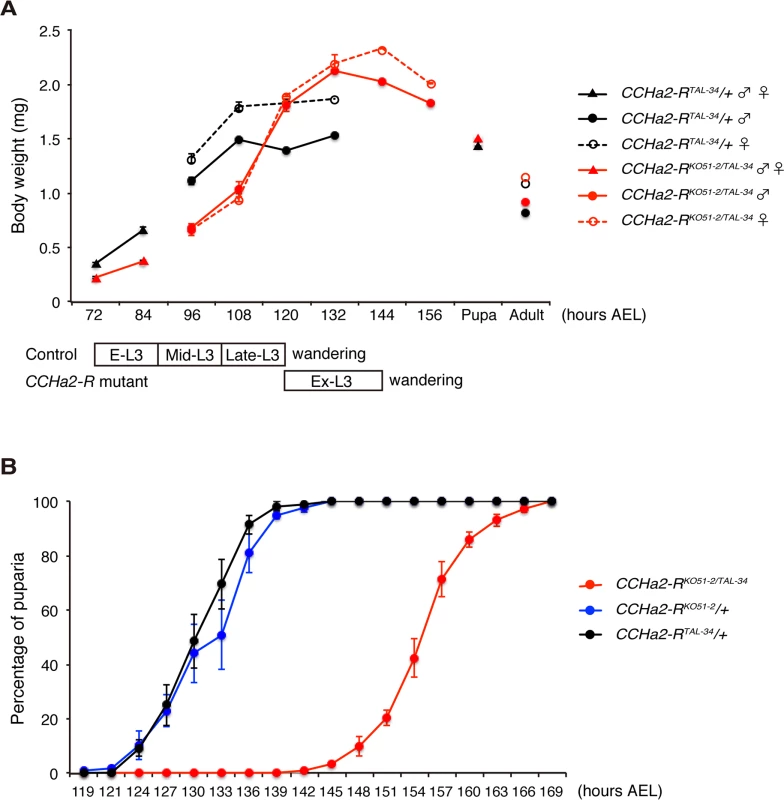
(A) Body weight was measured in batches of 10 to 35 animals, and the average weight per animal was plotted (n = 3 batches). (B) Timing of pupation of 40 animals was examined every 3 hours, and the averages of three experiments were plotted. Both the down-regulation of dilp5 expression (Fig 8A) and the retention of Dilp2 within the IPCs (Fig 8B and 8C) persisted in the CCHa2-R mutants through the last day of the extended larval stage (up to 144 hours AEL). These results support our conclusion that the reduction in Dilp2 and Dilp5 is not the consequence of feeding defects, because these larvae show abnormal Dilp regulation regardless of active feeding and growth. It has been reported that brain-derived Dilps (Dilp2, -3, and -5) are received by an insulin receptor (InR) in the fat body, leading to transcriptional repression of dilp6 in that tissue [25]. Hence, the reduction in Dilp expression in the brain in CCHa2-R mutants could lead to up-regulation of dilp6 transcription in the fat body. Consistent with this idea, we found that dilp6 mRNA levels were elevated in CCHa2-R mutant larvae (Fig 8D). Furthermore, we found that the removal of dilp6 from the CCHa2-R mutants abolished growth recovery between 96 to 120 hours AEL (Fig 8E). Taken together, these findings indicate that CCHa2/CCHa2-R signaling is a major regulator of Dilps until the mid - to late third-instar larval stages and that the growth recovery observed in later-stage mutants is due to the up-regulation of dilp6 resulting from the impairment of Dilp expression in the brain.
Fig. 8. Growth recovery in CCHa2-R mutants is due to the up-regulation of dilp6. 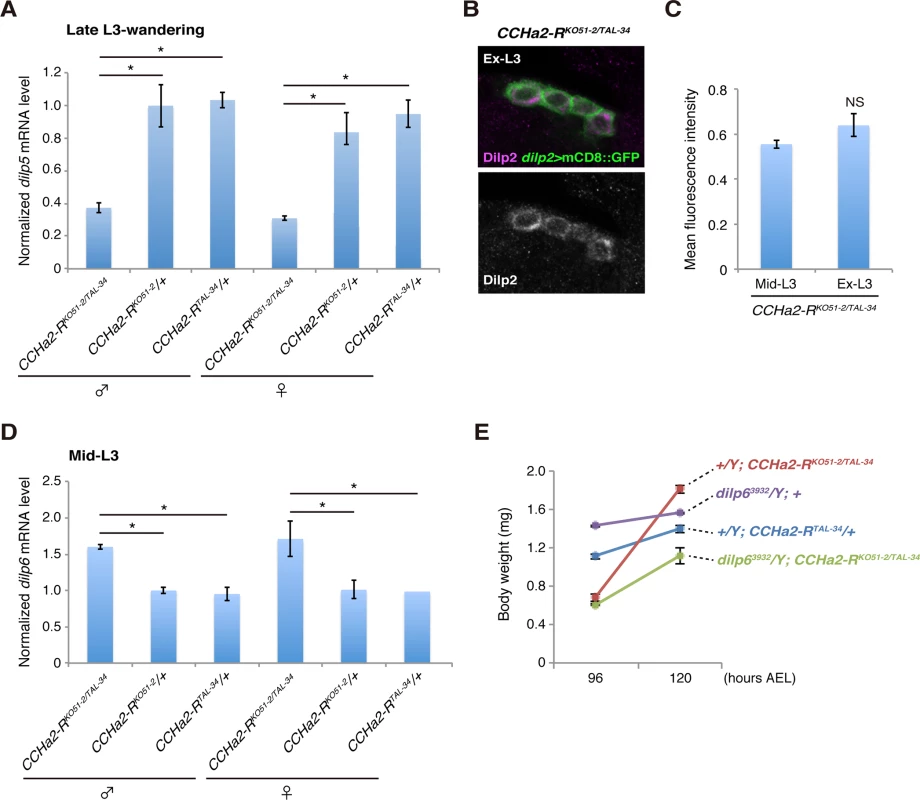
(A) Relative amounts of dilp5 mRNA were quantified by RT-qPCR using whole animal RNA extracts from larvae at the late third-instar to wandering stages. The dilp5 mRNA level was significantly reduced in CCHa2-R mutants. (B, C) Dilp2 immunoreactivity in the IPCs was compared in CCHa2-R mutants at the mid-third instar (Mid-L3) and extended larval stages (Ex-L3). IPCs expressing dilp2>mCD8::GFP were doubly stained with antibodies against Dilp2 and GFP. Fluorescence intensities of the Dilp2 signals were unchanged in Mid-L3 and Ex-L3 larvae (n = 50 and 28 cells for Mid-L3 and Ex-L3 larvae, respectively). (D) Relative amounts of dilp6 mRNA were quantified by RT-qPCR using whole-animal RNA extracts from third-instar larvae (Mid-L3, 96 hours AEL). Transcription of dilp6 was up-regulated in CCHa2-R mutants. (E) Body weight of dilp6, CCHa2-R double mutants and control animals was measured at 96 and 120 hours AEL, and the average weight per animal was plotted (5 to 10 larvae per batch, n = 3 batches). Discussion
Some peripheral tissues act as monitors of the nutritional environment and metabolic status. Thus, communication between peripheral organs, particularly from metabolic organs such as adipose tissues or the gut, and the brain is imperative for proper development and the maintenance of homeostasis. Here, we have demonstrated that signaling from peripheral tissues to the CNS mediated by the CCHa2 hormone and its receptor is required for the proper regulation of growth in response to nutritional conditions.
The CCHa2 peptide is a nutrient-specific Dilp regulator
A previous study suggested the existence of an amino acid-sensitive Dilp regulator(s) in larvae [10]. This as-yet-unidentified Dilp regulator(s) is regulated by the Slif/TOR pathway, and leucine and isoleucine, positive regulators of TOR signaling, are sufficient to promote the secretion of Dilp2 in both in vivo and ex vivo co-cultures of brain and fat bodies [10]. Our results demonstrated that the TOR pathway is required for CCHa2 expression during the larval stages (Fig 1J). However, feeding with amino acids, including leucine and isoleucine, was insufficient to promote CCHa2 expression (Fig 1I). CCHa2 expression was, however, induced by feeding with glucose (Fig 1I). Therefore, unlike the amino acid-dependent Dilp regulator(s) predicted by Géminard et al. [10], CCHa2 was found to be primarily sensitive to glucose. Some biological substances are produced by the metabolism of specific nutrients. For example, pyrimidine or purine bases are synthesized from amino acids. Therefore, it is possible that CCHa2 is down-regulated when glucose is abundant but other nutrients are not available, to limit growth in inhospitable environments. The reduction of CCHa2 mRNA in TOR-pathway knockdown larvae may recapitulate this scenario (Fig 1J).
In addition to CCHa2, Upd2 was reported to be a glucose-sensitive Dilp regulator expressed in the fat body [9]. The expression of upd2 in adult flies is up-regulated by feeding with a high-glucose or high-lipid diet. CCHa2 and Upd2, however, responded differently when the TOR pathway was disturbed: whereas CCHa2 expression was down-regulated in TOR-pathway-knockdown larvae, upd2 was up-regulated by the inhibition of the TOR pathway in adults [9]. Furthermore, the time course of CCHa2/CCHa2-R signaling is distinct from that of Upd2/Dome signaling. Disruption of upd2 down-regulated animals’ growth from larval to adult stages [9], whereas CCHa2-R mutations reduced growth until late-L3 stages, after which growth was recovered, leading to adults of normal size (Fig 7A). This growth recovery resulted from up-regulation of dilp6 expression (Fig 8D and 8E), which appears to be a consequence of dysregulated brain Dilps. The lack of growth recovery in upd2-knockdown animals in spite of abnormal Dilp production remains unexplained. Nevertheless, these results indicate that Drosophila melanogaster possesses multiple insulin regulators that have different nutrient sensitivities. Multi-input Dilp regulation might be advantageous under the imbalanced nutritional conditions that arise in the wild, and this could represent a general strategy for animal growth regulation.
In mammals, different hormones are secreted in response to long-term or short-term metabolic changes. For instance, gut-derived cholecystokinin, glucagon-like peptide-1, and PYY3-36, as well as stomach-derived ghrelin, all of which control feeding behavior, are secreted in response to food ingestion [32]. These hormones respond to acute metabolic changes and immediately signal to the feeding center in the brain. On the other hand, the synthesis or secretion of leptin and adiponectin is affected by the amount of lipid stored in adipocytes [33] [34], suggesting that leptin and adiponectin respond to long-term changes in metabolic status. The expression of CCHa2 responds to yeast and glucose within 6 hours (Fig 1H), indicating that CCHa2 mediates relatively rapid changes in metabolic status. Thus, it appears that CCHa2 functions as a short-acting metabolic regulator analogous to the mammalian gut - or stomach-derived hormones described above, and that Drosophila melanogaster CCHa2 might have an important role in the maintenance of energy homeostasis under volatile nutritional conditions.
Mechanisms for the regulation of brain Dilps by CCHa2/CCHa2-R signaling
The results from the calcium imaging experiments using brain explants and IPC-specific CCHa2-R knockdown strongly suggest that CCHa2 crosses the blood-brain barrier (BBB) to regulate the IPCs, although the underlying mechanism remains elusive. The Drosophila BBB consists of two different glial cell layers composed of either the perineurial glia (PG) or the subperineurial glia (SPG) [35] [36]. The SPG cell layer, which is adjacent to the neurons of the brain, forms septate junctions, which function as a barrier to separate the humoral space and the brain, analogously to the mammalian tight junctions formed between endothelial cells. Although several studies have identified important molecules involved in the formation of these septate junctions[36,37] [38] [39], little is known about functional aspects of the BBB [40]. CCHa2 could provide an ideal model for the study of BBB function as well as drug delivery across the BBB.
These experiments also show that peripheral tissue-derived CCHa2 directly activates IPCs in the brain. In mammals, direct sensing of blood glucose levels by pancreatic β-cells is a major trigger for insulin secretion. In these cells, glucose metabolism inhibits the ATP-dependent potassium channel (KATP channel) and opens voltage-dependent calcium channels (VDCCs), resulting in the exocytosis of insulin-containing granules [41]. The KATP channel also seems to be involved in insulin secretion in Drosophila IPCs [42]. Interestingly, a group of Gαs - and Gαq/11-coupled GPCRs can also activate the insulin secretion pathway in mammals [43]. The closest mammalian homologues of CCHa2-R—the Bombesin-related receptor subtypes 3, 1, and 2 (also known as gastrin-releasing-peptide receptor)—signal through Gαq/11 [44] [45] [46] [47] [48]. The slow rise in [Ca2+] in the IPCs in response to CCHa2 application is consistent with CCHa2-R’s mediation of Dilp release through the same pathway.
In contrast to Dilp2, dilp5 is also regulated by CCHa2/CCHa2-R signaling at the transcriptional level. Although the expression of dilp5 in the IPCs is activated by the conserved transcription factors Dachshund and Eyeless [27], whether CCHa2-R regulates these factors in IPCs remains unknown.
Overexpression of CCHa2-R in IPCs using the GAL4/UAS system displayed inhibitory effects on dilp5 expression, which prevented us from investigating whether direct CCHa2-R activation in IPCs is sufficient for Dilp regulation. CCHa2-R expression in the brain is not specific to IPCs but occurs in other central neurons (Fig 2B, 2C, 2F and 2G). Therefore, although we have shown that CCHa2-R expression in the IPCs is required for full dilp5 expression, it is possible that there may also be additional indirect pathways by which CCHa2 may up-regulate the Dilps. Although BBB glial cells are proposed to receive as-yet-unidentified signal(s) from the fat body and re-activate neural stem cells in the brain by secreting Dilp6 [49] [50], CCHa2-R>nlsGFP was undetectable in the BBB glial cells (S1G–S1I Fig). Thus BBB cells are unlikely to receive CCHa2 signals or to relay the signals to the IPCs.
Roles of bombesin family receptor signaling
The closest mammalian homologue of CCHa2-R is Brs3, an orphan GPCR, which is a member of the bombesin-like peptide receptor family [51]. Brs3-deficient mice develop obesity in association with a reduced metabolic rate and elevated feeding activity [52]. Interestingly, Brs3 is expressed in pancreatic β-cells both in mice and humans [53]. However, its involvement in insulin regulation has been controversial. Only if Brs3 knockout adult mice become obese (especially after 23 weeks old) do their plasma insulin levels increase [52]. Since hyper-insulinemia is generally observed in genetically obese mice, the elevation of insulin is most likely the consequence of the obesity rather than the loss of Brs3 function [52]. On the other hand, a Brs3 agonist promoted insulin secretion in both rodent insulinoma cell lines and in islets isolated from wild-type but not Brs3 mutants [53]. Our vigorous genetic approach combined with direct observations of Dilp production in IPCs provides the first evidence, to our knowledge, that Bombesin-related receptor signaling activated by its endogenous ligand promotes insulin production.
Materials and Methods
Fly strains and diets
The following fly stocks were used: Oregon-R, y w, dilp2-GAL4 [12], Lsp2-GAL4 [13] [54], ppl-GAL4 [8] [30], cg-GAL4 [30] [55], UAS-TSC1/2 [56], UAS-CCHa2, UAS-dilp2 [11], UAS-CCHa2 RNAi (VDRC-102257), UAS-GCaMP6s [31], UAS-CCHa2-R-shRNA #3, #24, #30 (see below), UAS-dicer1 (see below), and dilp63932 [13]. CCHa2-RKO51-2 and CCHa2-RTAL-34 are putative null alleles, which were generated by gene targeting using homologous recombination and TALEN, respectively (see below). CCHa2CR-1, CCHa2CR-2, and CCHa2CR-3 were generated using the CRISPR/Cas9 system (see below). Df(3R)Exel7320 (Exelixis) was used as a deficiency that uncovers CCHa2.
Flies were raised at 25°C on regular fly food containing (per liter) 46 g yeast extract, 70 g cornmeal, 100 g glucose, and 6 g agar. For starvation experiments, third-instar larvae (72 hours AEL) were cultured on water agar plates for 18 hours. The starved larvae were later re-fed with different nutrients (yeast paste, 46 g/L peptone, 10% glucose, 0.3% L-isoleucine, or 0.2% L-leucine).
Mutagenesis
CCHa2-RKO51-2 was generated by gene targeting using homologous recombination [22] [23]. A 2.4-kb fragment upstream of the second exon and a 2.5-kb fragment downstream of the 6th exon were amplified by PCR and then cloned into the NheI and SpeI sites of the pGX-attP-WN vector [23]. The targeting vector was integrated into the fly genome to generate a donor line. We performed gene targeting as described in Zhou et al. (2012) [23] and obtained the CCHa2-RKO51-2 mutation in which the region from the first methionine through the middle of the 7th transmembrane domain was removed (Figs 3 and S2). CCHa2-RTAL-34 was generated by inducing double-strand breaks at the CCHa2 locus using TALEN [24]. A Golden Gate TALEN kit (Addgene) was used to generate two sets of RVD plasmids corresponding to the sequences found in the first coding exon of the CCHa2-R gene (1L, 1R, 2L, and 2R; S2 Fig).
1L: TAGTACCGTATGTGCCC
1R: GGAGACGTACATTGTCA
2L: TGCTGTACACGCTCATCTTC
2R: GGCAACGGCACGCTGGTCATCA
For in vitro transcription, the RVD fragments were cloned into the pCS2TAL3DDD or pCS2TAL3RRR vector (a gift from K. Hoshijima, The University of Utah). Capped and polyadenylated RNAs were synthesized by in vitro transcription from linearized 1L, 1R, 2L, and 2R plasmids, and they were mixed for injection into y w embryos. To detect mutated DNA, the target region was amplified by PCR from genomic DNA of mutant candidates, and the amplified fragments were re-annealed. The resulting fragments were digested by T7 endonuclease I (NEB). Sequencing of the mutated fragment revealed that the CCHa2-RTAL-34 mutation is a 74-bp deletion causing a frame-shift mutation at amino acid position 62 (Figs 3 and S2).
CCHa2 mutants were generated using the germline-specific CRISPR/Cas9 system as described in Kondo and Ueda (2013) [29] (S5 Fig). The following sgRNA target was used for the mutagenesis of the CCHa2 gene. Break points of the mutants were determined as described above.
CCHa2 (88): GCCTACGGTCATGTGTGCTACGG
Vector construction
The CCHa2-R-GAL4::p65 construct (Fig 2E) was generated with bacterial artificial chromosome (BAC) recombineering techniques [57] in P[acman] BAC clone CH321-87C13 [58] (Children’s Hospital Oakland Research Institute, Oakland, CA). A landing-site cassette was created by flanking the selectable marker in pSK+-rpsL-kana [59] with 5' GAL4 and 3' HSP70 UTR arms from pBPGUw [60], a gift of G. Rubin. Fifty-base CCHa2-R-specific genomic homology arms were added by PCR using the primers below (genomic homology in lower case, cassette homology in upper case):
CCHa2-R-GAL4-F: 5'-tagaaacaccattgagacatcttgcccaggagcagctccctcctccccacATGAAGCTACTGTCTTCTATCGAACAAGC
CCHa2-R-HSP70-R: 5'-acttccccaccttctgcgggacccccacagtgcgtgatatatccacttacGATCTAAACGAGTTTTTAAGCAAACTCACTCCC
The cassette was recombined into the BAC and then replaced with full-length GAL4::p65-HSP70 amplified from pBPGAL4.2::p65Uw [61] (a gift of G. Rubin) in a second recombination. The final BAC was verified by sequencing the recombined regions and was integrated into the attP40 site [62] (Genetic Services, Inc., Cambridge, MA).
UAS-CCHa2 was generated by cloning the coding region of the CCHa2 gene into the EcoRI site of the pUAST vector. Flies were transformed with the UAS-CCHa2 vector by P-element-mediated transformation to generate the UAS-CCHa2 stock.
UAS-CCHa2-R-shRNA lines were generated as described previously [63]. The following oligonucleotide pairs were annealed and cloned into the NheI and EcoRI sites of the pWALIUM20 vector. The UAS-shRNA constructs were integrated into the attP40 site [62].
#3 Top strand: 5'-CTAGCAGTGGCTGATCTGTTGGTTATATTTAGTTATATTCAAGCATAAATATAACCAACAGATCAGCCGCG
#3 Bottom strand: 5'-AATTCGCGGCTGATCTGTTGGTTATATTTATGCTTGAATATAACTAAATATAACCAACAGATCAGCCACTG
#24 Top strand: 5'-CTAGCAGTCGATTGTCTACACGCAGGAAATAGTTATATTCAAGCATATTTCCTGCGTGTAGACAATCGGCG
#24 Bottom strand: 5'-AATTCGCCGATTGTCTACACGCAGGAAATATGCTTGAATATAACTATTTCCTGCGTGTAGACAATCGACTG
#30 Top strand: 5'-CTAGCAGTCGAACTGACTTGGAGTTATGTTAGTTATATTCAAGCATAACATAACTCCAAGTCAGTTCGGCG
#30 Bottom strand: 5'-AATTCGCCGAACTGACTTGGAGTTATGTTATGCTTGAATATAACTAACATAACTCCAAGTCAGTTCGACTG
In order to amplify the efficiency of shRNA-mediated gene knockdown, UAS-dicer1 was constructed. The dicer1 fragment was amplified by PCR using the dicer1 cDNA as a template (a gift from Q. Liu, University of Texas Southwestern Medical Center, and Y. Tomari, University of Tokyo). The following primers were used with Q5 DNA polymerase (NEB):
Dcr-1 5': 5'-GGGGTACCAAAATGGCGTTCCACTGGTGCG-3'
Dcr-1 3': 5'-GGAGATCTTAGTCTTTTTTGGCTATCAAGC-3'
The dicer1 fragment was cloned into the KpnI and BglII sites of the UASp-K10attB vector (a gift from B. Suter, University of Bern), and the UAS-dicer1 construct was integrated into the attP2 site.
Quantitative RT-PCR
Total RNA from whole larvae or larval tissues was extracted using a PureLink RNA Mini Kit (Life Technologies). cDNAs were prepared by reverse-transcribing 1 μg of total RNA using ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo), and quantitative RT-PCR was performed using Thunderbird qPCR Mix (Toyobo). Expression levels were normalized against those of rp49. The following primers were used:
CCHa2 forward: 5′-AGTGCAGTTGGACTTTGGTAGTGT
CCHa2 reverse: 5′-AGGGATGCTGTTTAGCATCTATGAC
CCHa2R forward: 5′-CTCACTGTCTTTACTGCGGTGAT
CCHa2R reverse: 5′-CCACCATGAACTTTGCATACTC
dilp2 forward: 5′-GTATGGTGTGCGAGGAGTAT
dilp2 reverse: 5′-TGAGTACACCCCCAAGATAG
dilp3 forward: 5′-GTCCAGGCCACCATGAAGTTGTGC
dilp3 reverse: 5′-CTTTCCAGCAGGGAACGGTCTTCG
dilp5 forward: 5′-TGTTCGCCAAACGAGGCACCTTGG
dilp5 reverse: 5′-CACGATTTGCGGCAACAGGAGTCG
dilp6 forward: 5′-TGCTAGTCCTGGCCACCTTGTTCG
dilp6 reverse: 5′-GGAAATACATCGCCAAGGGCCACC
rp49 forward: 5′-AGTATCTGATGCCCAACATCG
rp49 reverse: 5′-CAATCTCCTTGCGCTTCTTG
Immunostaining and FISH
Antibody staining was conducted as described previously [64], except that the gut samples were fixed in 4% formaldehyde in PBS for 4 hours. Rabbit anti-CCHa2 (1 : 1,000) [19], rabbit anti-Dilp2 (1 : 2,500; T. Ida), rabbit anti-Dilp2 (1 : 2,000, a gift from T. Nishimura, RIKEN Center for Developmental Biology) [27], rabbit anti-Dilp5 (1 : 2000, a gift from T. Nishimura) [27], mouse anti-Repo c8D12 (1 : 100; DSHB), mouse anti-GFP (1 : 500; Invitrogen), and rabbit anti-NPF (1 : 500; Ray Biotech, Inc) were used as primary antibodies, with anti-mouse-Alexa 488 (1 : 500; Invitrogen), anti-mouse-Cy3 (1 : 500; Jackson ImmunoResearch), anti-rabbit-Alexa 488 (1 : 500; Invitrogen), and anti-rabbit-Cy3 (1 : 500; Jackson ImmunoResearch) used as secondary antibodies. Alexa 488-conjugated phalloidin (Invitrogen) and TO-PRO-3 (Invitrogen) were used to label the cell membrane and nuclei, respectively. FISH was performed as described in Lehmann and Tautz [65] with a modification designed to amplify signals. Digoxigenin-labeled RNA probes were detected using the TSA Plus Fluorescein Kit (PerkinElmer). For the detection of CCHa2-R mRNA, the signals were further amplified using mouse anti-FITC (1 : 1,000; Jackson ImmunoResearch) and anti-mouse-Alexa 488 antibodies. Images were analyzed by LSM700 confocal microscopy (Zeiss) or DM5000B fluorescence microscopy with a DFC500 CCD camera (Leica).
Fluorescence quantification
To quantify Dilp2 and Dilp5 levels in IPCs, CNS samples were doubly stained with anti-Dilp2 or anti-Dilp5 and anti-GFP antibodies. Fluorescence images were acquired using a LSM700 confocal microscope (Zeiss). Constant laser power and scan settings were used to image the control and mutant samples. To quantify Dilp levels, Dilp and GFP signal intensities were measured on the same section using the ImageJ software (NIH). Dilp signals were normalized against the GFP signal intensities.
Calcium imaging
dilp2-GAL4/UAS-GCaMP6s and dilp2-GAL4, CCHa2-RTAL-34/UAS-GCaMP6s, CCHa2-RTAL-34 animals were used for imaging. Late-third-instar larvae were dissected in PBS. The ring gland and imaginal disks were removed from the brain. Dissected brains were immersed in 200 μl of PBS and tethered with tungsten wire (0.125 mm diameter, A-M Systems, Inc.) to the bottom of a culture dish (35x10 mm, Falcon). Custom-synthesized CCHa2 (SCRUM Inc.) and synthetic ghrelin and nociception (Peptide institute Inc.) were used. The ligand was pipetted directly into the bath in a volume of 100 μl to yield a final concentration of 10–9 M. Imaging was performed with a microscope (Axio Imager.A2, Carl Zeiss) equipped with a spinning disc confocal head (CSU-W1, Yokogawa). We used a 20x water-immersion objective lens (NA = 0.5; Carl Zeiss) mounted with a piezoelectric-activated lens mover (P-725K085 PIFOC, Physik Instrumente GmbH & Co. KG). GCaMP6s signals were excited with a 488-nm laser at 512 x 512 pixel resolution and collected at 250 ms/frame using an EM-CCD camera (ImagEM512, Hamamatsu Photonics) in water-cooled mode. For each IPC within an optical section, regions of interest (ROIs) were selected over multiple IPC somata using the ImageJ software (National Institutes of Health). Raw intensity values for GCaMP6s emission were recorded as mean pixel intensities (value range: 0–65,535) for each ROI at each time point and exported from ImageJ. GCaMP6s signal averaged over 30 frames before stimulation was taken as F0, and ΔF/F0 was calculated for each time point.
Analysis of body weight and developmental timing
Larvae were synchronized at hatching (24 hours AEL) and cultured with regular fly food. Ten to thirty-five larvae were collected and weighed at each time point. For the analysis of developmental timing, pupation of synchronized larvae was examined every three hours. Results are shown as the averages of triplicated experiments.
Feeding assay
The food-ingestion assay was modified from Edgecomb et al. [66]. In brief, 10 larvae were fed with yeast paste containing 1% Brilliant Blue FCF (Wako) for 1 hour or 2 hours. After rinsing with water, larvae were quickly frozen and homogenized in 200 μL of PBS (pH 7.0). The homogenates were centrifuged for 16,000 x g for 10 min, and the supernatants were analyzed spectrophotometrically for absorbance at 625 nm. Results are shown as the averages of triplicated experiments.
Statistics
The data in each graph were presented as means ± SEM. Two-tailed t-test was used to evaluate the significance of the results between two samples. For multiple comparisons, one-way ANOVA was applied, then pair-wise comparison was performed by Tukey-Kramer (Figs 1H, 4D and 5E and 5F) or Dunnett test (Fig 1I). For the calcium imaging data, Kruskal-Wallis rank sum test was performed for comparison followed by Holm-Bonferroni post-hoc test between four groups of samples (S6 Fig). A significance level of p<0.05 was used for all tests, which was marked by an asterisk in the figure.
Supporting Information
Zdroje
1. Nandi A, Kitamura Y, Kahn CR, Accili D (2004) Mouse models of insulin resistance. Physiol Rev 84 : 623–647. 15044684
2. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, et al. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372 : 425–432. 7984236
3. Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, et al. (1995) Identification and expression cloning of a leptin receptor, OB-R. Cell 83 : 1263–1271. 8548812
4. Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, et al. (1996) Abnormal splicing of the leptin receptor in diabetic mice. Nature 379 : 632–635. 8628397
5. Li C, Ioffe E, Fidahusein N, Connolly E, Friedman JM (1998) Absence of soluble leptin receptor in plasma from dbPas/dbPas and other db/db mice. J Biol Chem 273 : 10078–10082. 9545355
6. Frederich RC, Lollmann B, Hamann A, Napolitano-Rosen A, Kahn BB, et al. (1995) Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest 96 : 1658–1663. 7657836
7. Friedman JM, Halaas JL (1998) Leptin and the regulation of body weight in mammals. Nature 395 : 763–770. 9796811
8. Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, et al. (2003) A nutrient sensor mechanism controls Drosophila growth. Cell 114 : 739–749. 14505573
9. Rajan A, Perrimon N (2012) Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell 151 : 123–137. doi: 10.1016/j.cell.2012.08.019 23021220
10. Geminard C, Rulifson EJ, Leopold P (2009) Remote control of insulin secretion by fat cells in Drosophila. Cell Metab 10 : 199–207. doi: 10.1016/j.cmet.2009.08.002 19723496
11. Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, et al. (2001) An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol 11 : 213–221. 11250149
12. Rulifson EJ, Kim SK, Nusse R (2002) Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296 : 1118–1120. 12004130
13. Okamoto N, Yamanaka N, Yagi Y, Nishida Y, Kataoka H, et al. (2009) A fat body-derived IGF-like peptide regulates postfeeding growth in Drosophila. Dev Cell 17 : 885–891. doi: 10.1016/j.devcel.2009.10.008 20059957
14. Slaidina M, Delanoue R, Gronke S, Partridge L, Leopold P (2009) A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev Cell 17 : 874–884. doi: 10.1016/j.devcel.2009.10.009 20059956
15. Colombani J, Andersen DS, Leopold P (2012) Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science 336 : 582–585. doi: 10.1126/science.1216689 22556251
16. Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M (2012) Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science 336 : 579–582. doi: 10.1126/science.1216735 22556250
17. Boulay JL, O'Shea JJ, Paul WE (2003) Molecular phylogeny within type I cytokines and their cognate receptors. Immunity 19 : 159–163. 12932349
18. Wright VM, Vogt KL, Smythe E, Zeidler MP (2011) Differential activities of the Drosophila JAK/STAT pathway ligands Upd, Upd2 and Upd3. Cell Signal 23 : 920–927. doi: 10.1016/j.cellsig.2011.01.020 21262354
19. Ida T, Takahashi T, Tominaga H, Sato T, Sano H, et al. (2012) Isolation of the bioactive peptides CCHamide-1 and CCHamide-2 from Drosophila and their putative role in appetite regulation as ligands for G protein-coupled receptors. Front Endocrinol (Lausanne) 3 : 177. doi: 10.3389/fendo.2012.00177 23293632
20. Veenstra JA, Ida T (2014) More Drosophila enteroendocrine peptides: Orcokinin B and the CCHamides 1 and 2. Cell Tissue Res.
21. Park D, Veenstra JA, Park JH, Taghert PH (2008) Mapping peptidergic cells in Drosophila: where DIMM fits in. PLoS One 3: e1896. doi: 10.1371/journal.pone.0001896 18365028
22. Rong YS, Golic KG (2000) Gene targeting by homologous recombination in Drosophila. Science 288 : 2013–2018. 10856208
23. Zhou W, Huang J, Watson AM, Hong Y (2012) W::Neo: a novel dual-selection marker for high efficiency gene targeting in Drosophila. PLoS One 7: e31997. doi: 10.1371/journal.pone.0031997 22348139
24. Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, et al. (2011) Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res 39: e82. doi: 10.1093/nar/gkr218 21493687
25. Gronke S, Clarke DF, Broughton S, Andrews TD, Partridge L (2010) Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet 6: e1000857. doi: 10.1371/journal.pgen.1000857 20195512
26. Chintapalli VR, Wang J, Dow JA (2007) Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet 39 : 715–720. 17534367
27. Okamoto N, Nishimori Y, Nishimura T (2012) Conserved role for the Dachshund protein with Drosophila Pax6 homolog Eyeless in insulin expression. Proc Natl Acad Sci U S A 109 : 2406–2411. doi: 10.1073/pnas.1116050109 22308399
28. Ikeya T, Galic M, Belawat P, Nairz K, Hafen E (2002) Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol 12 : 1293–1300. 12176357
29. Kondo S, Ueda R (2013) Highly improved gene targeting by germline-specific Cas9 expression in Drosophila. Genetics 195 : 715–721. doi: 10.1534/genetics.113.156737 24002648
30. Rewitz KF, Yamanaka N, O'Connor MB (2010) Steroid hormone inactivation is required during the juvenile-adult transition in Drosophila. Dev Cell 19 : 895–902. doi: 10.1016/j.devcel.2010.10.021 21145504
31. Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, et al. (2013) Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499 : 295–300. doi: 10.1038/nature12354 23868258
32. Cummings DE, Overduin J (2007) Gastrointestinal regulation of food intake. J Clin Invest 117 : 13–23. 17200702
33. Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, et al. (1995) Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med 1 : 1311–1314. 7489415
34. Yamauchi T, Kamon J, Waki H, Murakami K, Motojima K, et al. (2001) The mechanisms by which both heterozygous peroxisome proliferator-activated receptor gamma (PPARgamma) deficiency and PPARgamma agonist improve insulin resistance. J Biol Chem 276 : 41245–41254. 11533050
35. DeSalvo MK, Mayer N, Mayer F, Bainton RJ (2011) Physiologic and anatomic characterization of the brain surface glia barrier of Drosophila. Glia 59 : 1322–1340. doi: 10.1002/glia.21147 21351158
36. Stork T, Engelen D, Krudewig A, Silies M, Bainton RJ, et al. (2008) Organization and function of the blood-brain barrier in Drosophila. J Neurosci 28 : 587–597. doi: 10.1523/JNEUROSCI.4367-07.2008 18199760
37. Faivre-Sarrailh C, Banerjee S, Li J, Hortsch M, Laval M, et al. (2004) Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development 131 : 4931–4942. 15459097
38. Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, et al. (1996) A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell 87 : 1059–1068. 8978610
39. Banerjee S, Pillai AM, Paik R, Li J, Bhat MA (2006) Axonal ensheathment and septate junction formation in the peripheral nervous system of Drosophila. J Neurosci 26 : 3319–3329. 16554482
40. Bainton RJ, Tsai LT, Schwabe T, DeSalvo M, Gaul U, et al. (2005) moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila. Cell 123 : 145–156. 16213219
41. Henquin JC (2009) Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia 52 : 739–751. doi: 10.1007/s00125-009-1314-y 19288076
42. Fridell YW, Hoh M, Kreneisz O, Hosier S, Chang C, et al. (2009) Increased uncoupling protein (UCP) activity in Drosophila insulin-producing neurons attenuates insulin signaling and extends lifespan. Aging (Albany NY) 1 : 699–713. 20195385
43. Blad CC, Tang C, Offermanns S (2012) G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat Rev Drug Discov 11 : 603–619. doi: 10.1038/nrd3777 22790105
44. Ryan RR, Weber HC, Mantey SA, Hou W, Hilburger ME, et al. (1998) Pharmacology and intracellular signaling mechanisms of the native human orphan receptor BRS-3 in lung cancer cells. J Pharmacol Exp Ther 287 : 366–380. 9765358
45. Ryan RR, Weber HC, Hou W, Sainz E, Mantey SA, et al. (1998) Ability of various bombesin receptor agonists and antagonists to alter intracellular signaling of the human orphan receptor BRS-3. J Biol Chem 273 : 13613–13624. 9593699
46. Qin X, Qu X, Coy D, Weber HC (2012) A selective human bombesin receptor subtype-3 peptide agonist mediates CREB phosphorylation and transactivation. J Mol Neurosci 46 : 88–99. doi: 10.1007/s12031-011-9675-3 22127929
47. Benya RV, Wada E, Battey JF, Fathi Z, Wang LH, et al. (1992) Neuromedin B receptors retain functional expression when transfected into BALB 3T3 fibroblasts: analysis of binding, kinetics, stoichiometry, modulation by guanine nucleotide-binding proteins, and signal transduction and comparison with natively expressed receptors. Mol Pharmacol 42 : 1058–1068. 1336112
48. Nishino H, Tsunoda Y, Owyang C (1998) Mammalian bombesin receptors are coupled to multiple signal transduction pathways in pancreatic acini. Am J Physiol 274: G525–534. 9530154
49. Chell JM, Brand AH (2010) Nutrition-responsive glia control exit of neural stem cells from quiescence. Cell 143 : 1161–1173. doi: 10.1016/j.cell.2010.12.007 21183078
50. Sousa-Nunes R, Yee LL, Gould AP (2011) Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila. Nature 471 : 508–512. doi: 10.1038/nature09867 21346761
51. Hewes RS, Taghert PH (2001) Neuropeptides and neuropeptide receptors in the Drosophila melanogaster genome. Genome Res 11 : 1126–1142. 11381038
52. Ohki-Hamazaki H, Watase K, Yamamoto K, Ogura H, Yamano M, et al. (1997) Mice lacking bombesin receptor subtype-3 develop metabolic defects and obesity. Nature 390 : 165–169. 9367152
53. Feng Y, Guan XM, Li J, Metzger JM, Zhu Y, et al. (2011) Bombesin receptor subtype-3 (BRS-3) regulates glucose-stimulated insulin secretion in pancreatic islets across multiple species. Endocrinology 152 : 4106–4115. doi: 10.1210/en.2011-1440 21878513
54. Roignant JY, Carre C, Mugat B, Szymczak D, Lepesant JA, et al. (2003) Absence of transitive and systemic pathways allows cell-specific and isoform-specific RNAi in Drosophila. RNA 9 : 299–308. 12592004
55. Asha H, Nagy I, Kovacs G, Stetson D, Ando I, et al. (2003) Analysis of Ras-induced overproliferation in Drosophila hemocytes. Genetics 163 : 203–215. 12586708
56. Tapon N, Ito N, Dickson BJ, Treisman JE, Hariharan IK (2001) The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell 105 : 345–355. 11348591
57. Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG (2005) Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res 33: e36. 15731329
58. Venken KJ, Carlson JW, Schulze KL, Pan H, He Y, et al. (2009) Versatile P[acman] BAC libraries for transgenesis studies in Drosophila melanogaster. Nat Methods 6 : 431–434. doi: 10.1038/nmeth.1331 19465919
59. Wang S, Zhao Y, Leiby M, Zhu J (2009) A new positive/negative selection scheme for precise BAC recombineering. Mol Biotechnol 42 : 110–116. doi: 10.1007/s12033-009-9142-3 19160076
60. Pfeiffer BD, Jenett A, Hammonds AS, Ngo TT, Misra S, et al. (2008) Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci U S A 105 : 9715–9720. doi: 10.1073/pnas.0803697105 18621688
61. Pfeiffer BD, Ngo TT, Hibbard KL, Murphy C, Jenett A, et al. (2010) Refinement of tools for targeted gene expression in Drosophila. Genetics 186 : 735–755. doi: 10.1534/genetics.110.119917 20697123
62. Bischof J, Maeda RK, Hediger M, Karch F, Basler K (2007) An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A 104 : 3312–3317. 17360644
63. Ni JQ, Zhou R, Czech B, Liu LP, Holderbaum L, et al. (2011) A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat Methods 8 : 405–407. doi: 10.1038/nmeth.1592 21460824
64. Moore LA, Broihier HT, Van Doren M, Lehmann R (1998) Gonadal mesoderm and fat body initially follow a common developmental path in Drosophila. Development 125 : 837–844. 9449666
65. Lehmann R, Tautz D (1994) In situ hybridization to RNA. Methods Cell Biol 44 : 575–598. 7535885
66. Edgecomb RS, Harth CE, Schneiderman AM (1994) Regulation of feeding behavior in adult Drosophila melanogaster varies with feeding regime and nutritional state. J Exp Biol 197 : 215–235. 7852903
Štítky
Genetika Reprodukční medicína
Článek Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1Článek A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria ParasiteČlánek The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of CentrosomesČlánek Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In VivoČlánek MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle CellsČlánek PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune ResponsesČlánek Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA SynthesisČlánek The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .Článek Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 5- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Genomic Heritability: What Is It?
- Triglyceride-Increasing Alleles Associated with Protection against Type-2 Diabetes
- Epistasis Is a Major Determinant of the Additive Genetic Variance in
- Genetic Regulation of Bone Metabolism in the Chicken: Similarities and Differences to Mammalian Systems
- Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1
- The Philosophical Approach: An Interview with Ford Doolittle
- Downregulation of the Host Gene by miR-92 Is Essential for Neuroblast Self-Renewal in
- A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria Parasite
- Y Fuse? Sex Chromosome Fusions in Fishes and Reptiles
- Regulation of Active DNA Demethylation by a Methyl-CpG-Binding Domain Protein in
- Overlapping Patterns of Rapid Evolution in the Nucleic Acid Sensors cGAS and OAS1 Suggest a Common Mechanism of Pathogen Antagonism and Escape
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Genetic Architecture of Abdominal Pigmentation in
- Whole Genome DNA Binding Analysis of the Bacterial Replication Initiator and Transcription Factor DnaA
- The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of Centrosomes
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- Burden Analysis of Rare Microdeletions Suggests a Strong Impact of Neurodevelopmental Genes in Genetic Generalised Epilepsies
- Cell Cycle Control by the Master Regulator CtrA in
- Myopathic Lamin Mutations Cause Reductive Stress and Activate the Nrf2/Keap-1 Pathway
- Monoallelic Loss of the Imprinted Gene Promotes Tumor Formation in Irradiated Mice
- Phylum-Level Conservation of Regulatory Information in Nematodes despite Extensive Non-coding Sequence Divergence
- Clustering and Negative Feedback by Endocytosis in Planar Cell Polarity Signaling Is Modulated by Ubiquitinylation of Prickle
- Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In Vivo
- MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle Cells
- β-Catenin Signaling Biases Multipotent Lingual Epithelial Progenitors to Differentiate and Acquire Specific Taste Cell Fates
- A Simple Auxin Transcriptional Response System Regulates Multiple Morphogenetic Processes in the Liverwort
- Parallel Gene Expression Differences between Low and High Latitude Populations of and .
- The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of
- Characterization of TCF21 Downstream Target Regions Identifies a Transcriptional Network Linking Multiple Independent Coronary Artery Disease Loci
- PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune Responses
- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Coronary Artery Disease Associated Transcription Factor TCF21 Regulates Smooth Muscle Precursor Cells That Contribute to the Fibrous Cap
- Rescue of DNA-PK Signaling and T-Cell Differentiation by Targeted Genome Editing in a Deficient iPSC Disease Model
- Disruption of Transcriptional Coactivator Sub1 Leads to Genome-Wide Re-distribution of Clustered Mutations Induced by APOBEC in Active Yeast Genes
- Yeast Killer Elements Hold Their Hosts Hostage
- Keeping in Shape the Dogma of Mitochondrial DNA Maternal Inheritance
- Extreme-Depth Re-sequencing of Mitochondrial DNA Finds No Evidence of Paternal Transmission in Humans
- Trading Places—Switching Frataxin Function by a Single Amino Acid Substitution within the [Fe-S] Cluster Assembly Scaffold
- Natural Variation Identifies , a Universal Gene Required for Cell Proliferation and Growth at High Temperatures in
- Mutations in Gene Are Associated with Predisposition to Breast Cancer
- The Whole of a Scientific Career: An Interview with Oliver Smithies
- Cell Specific eQTL Analysis without Sorting Cells
- Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA Synthesis
- Systemic Regulation of RAS/MAPK Signaling by the Serotonin Metabolite 5-HIAA
- Reprogramming LCLs to iPSCs Results in Recovery of Donor-Specific Gene Expression Signature
- The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .
- Genetic Mechanism of Human Neutrophil Antigen 2 Deficiency and Expression Variations
- Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
- Early Lineage Priming by Trisomy of Leads to Myeloproliferation in a Down Syndrome Model
- Turning into a Frataxin-Dependent Organism
- Accounting for Experimental Noise Reveals That mRNA Levels, Amplified by Post-Transcriptional Processes, Largely Determine Steady-State Protein Levels in Yeast
- Biological Significance of Photoreceptor Photocycle Length: VIVID Photocycle Governs the Dynamic VIVID-White Collar Complex Pool Mediating Photo-adaptation and Response to Changes in Light Intensity
- CTXφ Replication Depends on the Histone-Like HU Protein and the UvrD Helicase
- Disruption of miR-29 Leads to Aberrant Differentiation of Smooth Muscle Cells Selectively Associated with Distal Lung Vasculature
- Notch Is Required in Adult Sensory Neurons for Morphological and Functional Plasticity of the Olfactory Circuit
- Post-transcriptional Regulation of Keratinocyte Progenitor Cell Expansion, Differentiation and Hair Follicle Regression by
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
- Casein Kinase 1 and Phosphorylation of Cohesin Subunit Rec11 (SA3) Promote Meiotic Recombination through Linear Element Formation
- Fibroblast Growth Factor 9 Regulation by MicroRNAs Controls Lung Development and Links Loss to the Pathogenesis of Pleuropulmonary Blastoma
- Auxin-Mediated Transcriptional System with a Minimal Set of Components Is Critical for Morphogenesis through the Life Cycle in
- The Broad-Spectrum Antiviral Protein ZAP Restricts Human Retrotransposition
- The 4E-BP Caf20p Mediates Both eIF4E-Dependent and Independent Repression of Translation
- Turning into a Frataxin-Independent Organism
- Promotion of Bone Morphogenetic Protein Signaling by Tetraspanins and Glycosphingolipids
- Essential Role of the ESX-5 Secretion System in Outer Membrane Permeability of Pathogenic Mycobacteria
- The Zinc-Finger Antiviral Protein ZAP Inhibits LINE and Alu Retrotransposition
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



