-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Burden Analysis of Rare Microdeletions Suggests a Strong Impact of Neurodevelopmental Genes in Genetic Generalised Epilepsies
Epilepsy affects about 4% of the general population during lifetime. The genetic generalised epilepsies (GGEs) represent the most common group of epilepsies with predominant genetic aetiology, accounting for 20% of all epilepsies. Despite their strong heritability, the genetic basis of the majority of patients with GGE remains elusive. Genomic microdeletions constitute a significant source of genetic risk factors for epilepsies. The present genome-wide burden analysis in 1,366 European patients with GGE and 5,234 ancestry-matched controls explored the role of large and rare microdeletions (size ≥ 400 kb, frequency < 1%) in the complex genetic architecture of common GGE syndromes. Our results revealed a 2-fold excess of microdeletions in GGE patients relative to the population controls, 2) a 7-fold increased burden for known hotspot microdeletions (15q11.2, 15q13.3, 16p13.11, 22q11.2) previously associated with a wide range of neurodevelopmental disorders, and 3) a more than 4-fold enrichment of microdeletions carrying a gene implicated in neurodevelopmental disorders. Our findings reinforce emerging evidence that genes affected by microdeletions in GGE patients have a strong impact in fundamental neurodevelopmental processes and dissect novel candidate genes involved in epileptogenesis.
Published in the journal: . PLoS Genet 11(5): e32767. doi:10.1371/journal.pgen.1005226
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005226Summary
Epilepsy affects about 4% of the general population during lifetime. The genetic generalised epilepsies (GGEs) represent the most common group of epilepsies with predominant genetic aetiology, accounting for 20% of all epilepsies. Despite their strong heritability, the genetic basis of the majority of patients with GGE remains elusive. Genomic microdeletions constitute a significant source of genetic risk factors for epilepsies. The present genome-wide burden analysis in 1,366 European patients with GGE and 5,234 ancestry-matched controls explored the role of large and rare microdeletions (size ≥ 400 kb, frequency < 1%) in the complex genetic architecture of common GGE syndromes. Our results revealed a 2-fold excess of microdeletions in GGE patients relative to the population controls, 2) a 7-fold increased burden for known hotspot microdeletions (15q11.2, 15q13.3, 16p13.11, 22q11.2) previously associated with a wide range of neurodevelopmental disorders, and 3) a more than 4-fold enrichment of microdeletions carrying a gene implicated in neurodevelopmental disorders. Our findings reinforce emerging evidence that genes affected by microdeletions in GGE patients have a strong impact in fundamental neurodevelopmental processes and dissect novel candidate genes involved in epileptogenesis.
Introduction
The epilepsies comprise a clinically heterogeneous group of neurological disorders defined by recurrent spontaneous seizures due to paroxysmal excessive and synchronous neuronal activity in the brain [1]. Epilepsy affects about 4% of the general population during their lifetime [2] and about 40% of all epilepsies are thought to have a strong genetic contribution. The genetic generalised epilepsies (GGEs) represent the most common group of epilepsies with predominant genetic aetiology, accounting for 20% of all epilepsies [3]. Their clinical features are characterised by unprovoked generalised seizures with age-related onset, generalised spike and wave discharges on the electroencephalogram and no evidence for an acquired cause [4,5]. Despite their strong familial aggregation and heritability [6–9], the genetic architecture of common GGE syndromes is likely to display a biological spectrum, in which a small fraction (1–2%) follows monogenic inheritance, whereas the majority of GGE patients presumably display an oligo-/polygenic predisposition with extensive genetic heterogeneity [10]. Although causative mutations for rare GGE with monogenic inheritance have been identified in genes primarily affecting neuronal excitability, synaptic transmission, and neurodevelopmental processes [11,12], the genetic basis of the majority of patients with GGE remains largely unsolved.
Genomic copy number variations (CNVs) constitute a significant source of genetic risk factors for common focal and generalised epilepsies [13–20]. By targeted screening of rearrangements at genomic hotspots associated with neurodevelopmental disorders [21], we previously identified recurrent microdeletions at 15q11.2, 15q13.3 and 16p13.11 as important genetic risk factors of common GGE syndromes [14,16,17,22–24]. The microdeletions at 15q13.3 and 16p13.11 represent the most prevalent genetic determinants of GGE identified so far [14,16]. In addition, we were able to show that non-hotspot exonic microdeletions in three brain-expressed genes encoding gephyrin (GPHN) [25], neurexin 1 (NRXN1) [26] and the RNA-binding protein FOX1 (RBFOX1) [27] confer susceptibility of GGE. Although the GGE-associated microdeletions identified to date are individually rare (<1%), they cumulatively account for a significant fraction of the genetic burden in more than 3% of patients with common GGE syndromes [14–16,22].
In the present genome-wide burden analysis, we used the Affymetrix SNP 6.0 array to screen large (≥ 400 kb) and rare (< 1%) autosomal microdeletions with high calling confidence (≥ 200 markers) in European case-control cohorts of 1,366 GGE patients and 5,234 population controls. We aimed to: 1) assess the genetic burden of large and rare microdeletions in common GGE syndromes, 2) evaluate the contribution of recurrent hotspot and unique microdeletions to the genetic burden of GGE, and 3) identify novel candidate genes for GGE. Specifically, we tested the hypothesis whether microdeletions affecting genes involved in neurodevelopmental processes account for a significant fraction of the genetic risk of GGE syndromes.
Results
Burden analysis of autosomal microdeletions
We identified 103 microdeletions in 100 out of 1,366 GGE patients compared to 214 microdeletions in 208 out of 5,234 controls (S1 Table). Overall, 7.3% of patients with GGE carried at least one microdeletion compared to 4.0% in controls (P = 1.77 x 10–7; OR = 1.91, 95%-CI: 1.48–2.46) (Table 1). We observed a marginal increase in microdeletion frequency in the GGE patients when we considered only microdeletions affecting either at least one protein-coding RefSeq gene (n = 18,299; P = 5.86 x 10–7; OR = 1.95, 95%-CI: 1.48–2.57) or at least one brain-expressed gene (n = 8,878; P = 1.38 x 10–7; OR = 2.19, 95%-CI: 1.61–2.98) (Table 1). Likewise, the median size of microdeletions was larger in the GGE patients (713 kb; interquartile range (IQR) = 523 kb—1,537 kb) compared to controls (589 kb; IQR = 488–930 kb; P = 3.99 x 10–3; Wilcoxon-Mann-Whitney-Test). The number of individuals carrying at least two microdeletions did not differ significantly between the GGE patients (n = 3) and controls (n = 6; P = 0.40, Fisher´s exact test). The microdeletion burden was similar for males (7.2%) and females (7.4%) affected by GGE (P = 0.91; OR = 0.97, 95%-CI: 0.63–1.52). The distribution of GGE subsyndromes did not differ between 100 GGE patients carrying a microdeletion (33 JME, 50 CAE/JAE, 17 EGTCS/EGMA) and the group of 1,266 GGE patients without a large and rare microdeletion (507 JME, 548 CAE/JAE, 211 EGTCS/EGMA; P > 0.15).
Tab. 1. Microdeletion burden analysis. 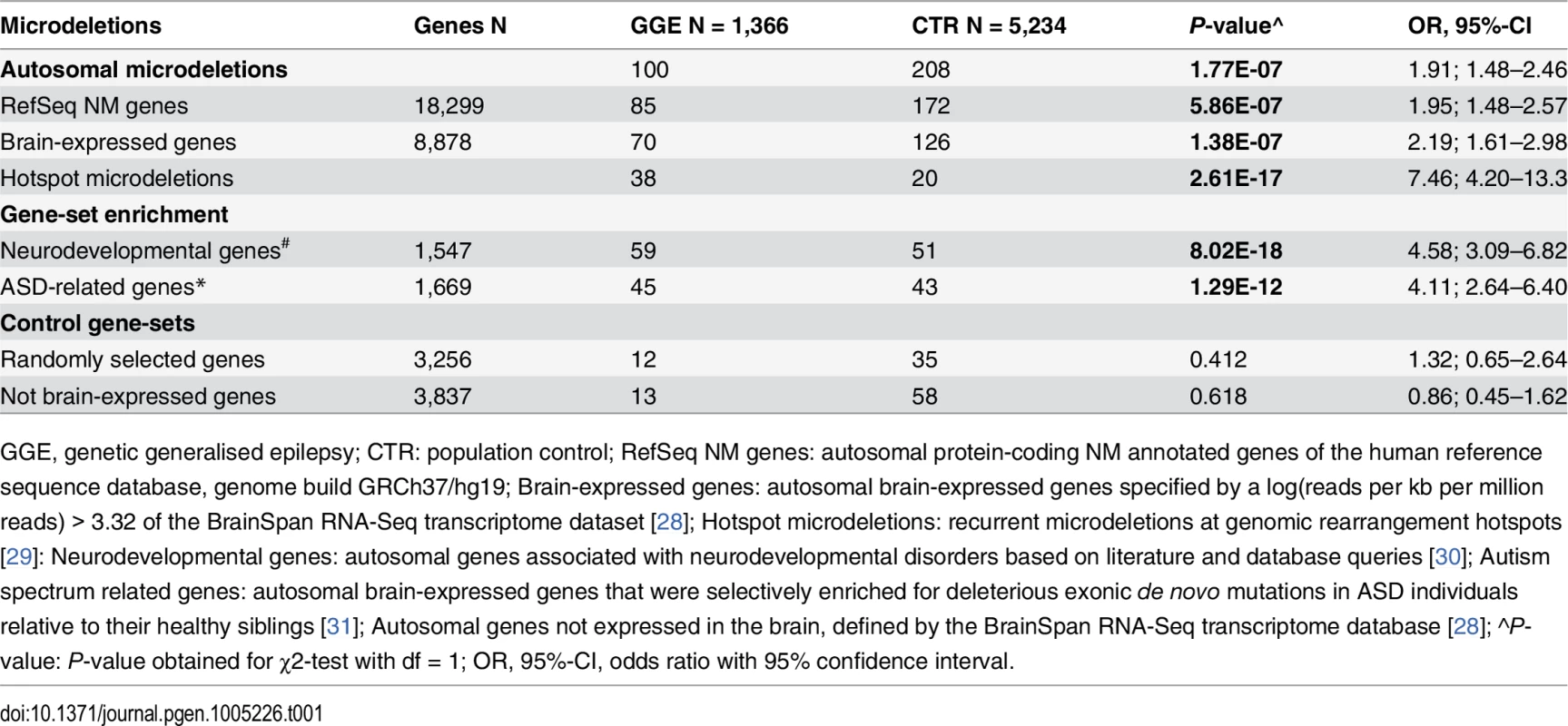
GGE, genetic generalised epilepsy; CTR: population control; RefSeq NM genes: autosomal protein-coding NM annotated genes of the human reference sequence database, genome build GRCh37/hg19; Brain-expressed genes: autosomal brain-expressed genes specified by a log(reads per kb per million reads) > 3.32 of the BrainSpan RNA-Seq transcriptome dataset [28]; Hotspot microdeletions: recurrent microdeletions at genomic rearrangement hotspots [29]: Neurodevelopmental genes: autosomal genes associated with neurodevelopmental disorders based on literature and database queries [30]; Autism spectrum related genes: autosomal brain-expressed genes that were selectively enriched for deleterious exonic de novo mutations in ASD individuals relative to their healthy siblings [31]; Autosomal genes not expressed in the brain, defined by the BrainSpan RNA-Seq transcriptome database [28]; ^P-value: P-value obtained for χ2-test with df = 1; OR, 95%-CI, odds ratio with 95% confidence interval. GGE-related spectrum of microdeletions
The spectrum of 103 microdeletions identified in 100 GGE patients comprised: 1) 38 (36.9%) recurrent microdeletions at seven known genomic rearrangement hotspots previously associated with a wide range of neurodevelopmental disorders [29], 2) 27 (26.2%) genic microdeletions that were detected only in the GGE patients, 3) 16 (15.5%) microdeletions without a protein-coding RefSeq gene and that were not present in the controls, and 4) 22 (21.4%) non-hotspot microdeletions which overlap with the microdeletions identified in the controls (S1 Table). Most prominent was the 7.5-fold excess of recurrent hotspot microdeletions in the GGE patients compared to the controls (P = 2.61 x 10–17; OR = 7.46, 95%-CI: 4.20–13.33; χ2-test, df = 1) (Table 2). Overall, 2.8% (n = 38) of 1,366 GGE patients carried one of the known recurrent microdeletions at 1q21.1 (n = 1), 15q11.2 (n = 13), 15q13.3 (n = 11), 16p11.2 (n = 1), 16p12 (n = 3), 16p13.11 (n = 6) and 22q11.2 (n = 3), whereas these hotspot microdeletions were observed only in 0.4% (n = 20) of 5,234 population controls (S1 and S2 Figs). Significant associations with GGE patients were found for single hotspot microdeletions at 15q11.2 (P = 4.18 x 10–4; OR = 3.58; 95%-CI: 1.58–8.09, χ2-test, df = 1), 15q13.3 (P = 2.89 x 10–8, Fisher´s exact test), 16p13.11 (P = 1.48 x 10–3; OR = 11.48, 95%-CI: 2.05–116.5, Fisher´s exact test), and 22q11.2 (P = 8.85 x 10–3, Fisher´s exact test). All hotspot microdeletions in GGE patients identified by SNP arrays were validated by TaqMan qPCR. Altogether, the present findings highlight the cumulative impact of the recurrent microdeletions at 15q11.2, 15q13.3, 16p13.11 and 22q11.2 on the genetic risk of common GGE syndromes.
Tab. 2. Recurrent microdeletions at rearrangement hotspots. 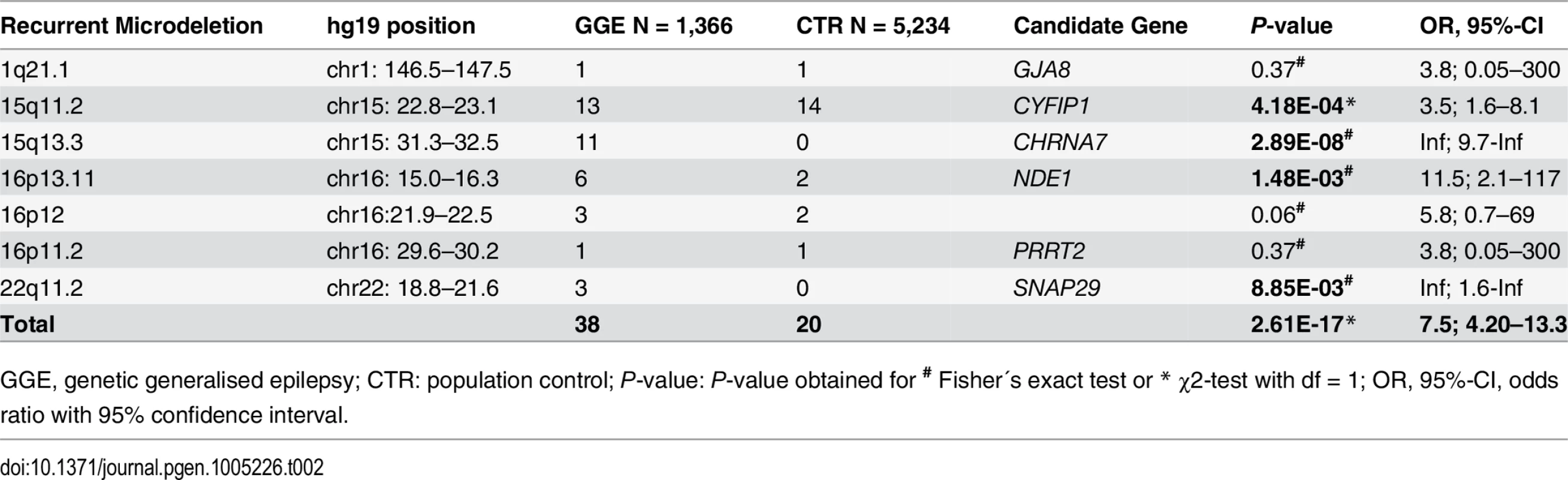
GGE, genetic generalised epilepsy; CTR: population control; P-value: P-value obtained for # Fisher´s exact test or * χ2-test with df = 1; OR, 95%-CI, odds ratio with 95% confidence interval. Besides the recurrent hotspot microdeletions, we identified 27 GGE patients carrying a genic microdeletion that was not observed in the controls (Table 3 and S1 Table and S3 Fig). These microdeletions affected 158 protein-coding RefSeq genes and exhibited an enrichment of genes previously associated with epilepsy (NRXN1, RBFOX1, PCDH7, KCNA2, EPM2A, RORB, PLCB1) and neuropsychiatric disorders (DPYD, CADM2, PARK2, GRM8, TSNARE1, TPH2, MACROD2) (Table 3). Microdeletions involving NRXN1 exons 1–2 were observed in two GGE patients with genetic absence epilepsies [26]. In addition, two partially overlapping microdeletions were identified in the chromosomal region 8q24.3 encompassing the genes encoding the t-SNARE domain containing 1 protein (TSNARE1; chr8 : 143,293,441–143,484,601) and the brain-specific angiogenesis inhibitor 1 (BAI1; chr8 : 143,545,376–143,626,368). All other unique microdeletions occurred only once. The microdeletions affecting the neuronal genes, NRXN1 and RBFOX1, have been reported in two previous publications [26,27].
Tab. 3. Gene-disrupting microdeletions found only in patients with genetic generalised epilepsy. 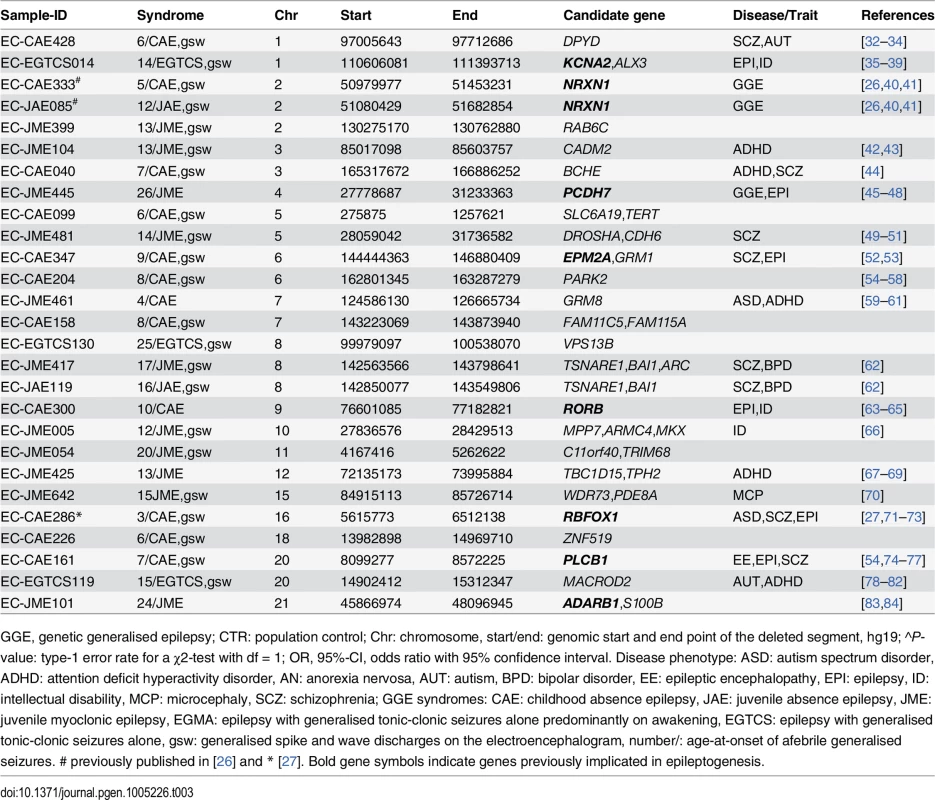
GGE, genetic generalised epilepsy; CTR: population control; Chr: chromosome, start/end: genomic start and end point of the deleted segment, hg19; ^P-value: type-1 error rate for a χ2-test with df = 1; OR, 95%-CI, odds ratio with 95% confidence interval. Disease phenotype: ASD: autism spectrum disorder, ADHD: attention deficit hyperactivity disorder, AN: anorexia nervosa, AUT: autism, BPD: bipolar disorder, EE: epileptic encephalopathy, EPI: epilepsy, ID: intellectual disability, MCP: microcephaly, SCZ: schizophrenia; GGE syndromes: CAE: childhood absence epilepsy, JAE: juvenile absence epilepsy, JME: juvenile myoclonic epilepsy, EGMA: epilepsy with generalised tonic-clonic seizures alone predominantly on awakening, EGTCS: epilepsy with generalised tonic-clonic seizures alone, gsw: generalised spike and wave discharges on the electroencephalogram, number/: age-at-onset of afebrile generalised seizures. # previously published in [26] and * [27]. Bold gene symbols indicate genes previously implicated in epileptogenesis. Gene-set enrichment analyses of neurodevelopmental genes
To explore the hypothesis whether neurodevelopmental genes affected by the microdeletions have an impact on the genetic risk of common GGE syndromes, we performed enrichment analyses of the deleted genes, using two previously published sets of genes implicated in neurodevelopmental disorders (ND): 1) ND-related genes (n = 1,547) compiled by literature and database queries [30], and 2) genes implicated in autism spectrum disorder (ASD-related genes) comprising 1,669 brain-expressed genes with an enrichment of deleterious exonic de novo mutations in ASD [31]. Microdeletions carrying at least one ND-related gene were 4.6-fold enriched in the GGE patients as compared to the controls (P = 8.02 x 10–18; OR = 4.58, 95%-CI: 3.09–6.82) (Table 1). Likewise, microdeletions encompassing at least one ASD-related gene showed a 4.1-fold excess in the GGE patients relative to the controls (P = 1.29 x 10–12; OR = 4.11, 95%-CI: 2.64–6.40) (Table 1). To explore the impact of neurodevelopmental genes that are not covered by the recurrent hotspot microdeletions, we combined the ND - and ASD-related gene lists [30,31] and removed all genes affected by observed recurrent hotspot microdeletions. Non-recurrent microdeletions carrying at least one of the 2,495 selected ND/ASD-related genes showed a 2.3-fold excess in GGE patients (n = 1,328) compared to control subjects (n = 5,214), when individuals with recurrent hotspot microdeletions were excluded (P = 4.56 x 10–4; OR = 2.48, 95%-CI: 1.42–4.30). To rule out an artificial enrichment of microdeletions in the GGE patients, we compiled two control gene assemblies comprising: 1) 3,256 randomly selected autosomal protein-coding RefSeq genes, and 2) 3,837 autosomal protein-coding RefSeq genes not expressed in the brain [28]. Both control gene assemblies did not show evidence for an increase of the microdeletion burden in GGE patients compared to controls (P > 0.40) (Table 1).
Functional enrichment and network analyses
The Disease Association Protein-Protein Link Evaluator (DAPPLE v2.0) tool [85] was applied to identify significant physical connectivity among proteins encoded by genes affected by microdeletions. Therefore, we separately tested the gene assemblies for the GGE patients and the control subjects. Based on an initial regional query we extracted 191 seed genes from 103 microdeletions found in the GGE patients and 221 seed genes from 214 microdeletions observed in controls. There was an overlap of 61 genes between the two assemblies. DAPPLE network analyses revealed a significant enrichment for direct connections between the seed genes (P = 0.01) in the GGE microdeletion carriers, while the control gene network did not show evidence for an enrichment (P = 0.40). Finally, in GGE we found eleven genes with significant connectivity: PLCB1 (P = 0.002), GRM1 (P = 0.002), ARC (P = 0.002), CNTN6 (P = 0.015), CHL1 (P = 0.033), BAI1 (P = 0.033), CYFIP1 (P = 0.040), TRIP13 (P = 0.042), MAPK3 (P = 0.044), GJ8 (P = 0.048), and KCNA2 (P = 0.050) (S4 Fig).
Utilising the Enrichr tool [86], functional enrichment analysis of the gene assembly affected by the microdeletions in the GGE patients revealed a significant enrichment of the MGI Mammalian Phenotype term "abnormal emotion/affect behaviour" (MP:0002572; Padj = 1.30 x 10–3) and the GO biological process term “cognition” (GO:0050890; Padj = 0.012) (Table 4). Enrichr network analysis identified one significant PPI Hub in the GGE patients based on an enrichment of nine deleted genes (ARC, TJP1, MAPK3, MYH11, EXOC3, NRXN1, PARK2, PLCB1, GRM1) among 219 network genes (Padj = 0.018), for which GRIN2B encodes the shared interacting protein.
Tab. 4. Functional gene enrichment and network analysis. 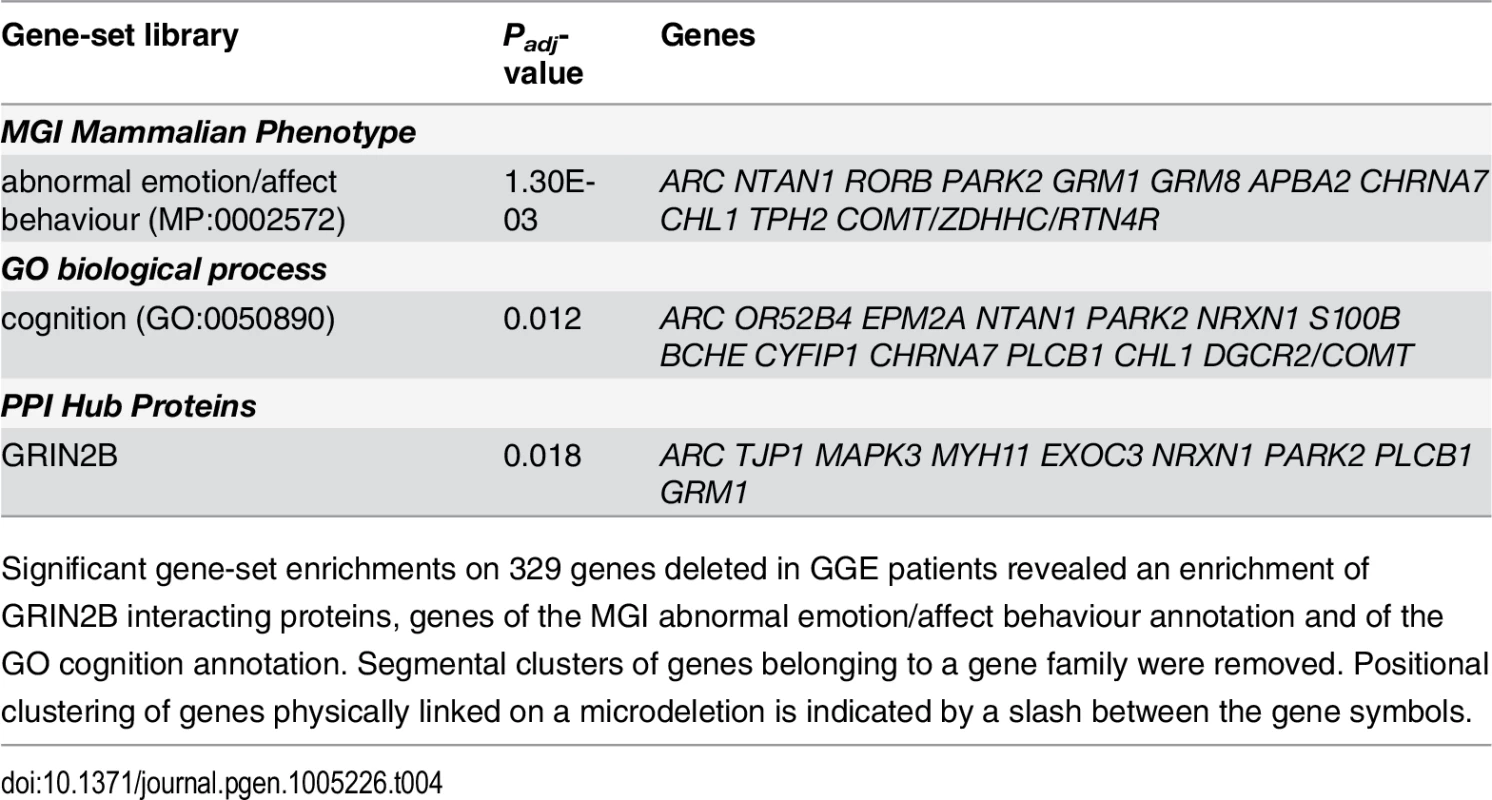
Significant gene-set enrichments on 329 genes deleted in GGE patients revealed an enrichment of GRIN2B interacting proteins, genes of the MGI abnormal emotion/affect behaviour annotation and of the GO cognition annotation. Segmental clusters of genes belonging to a gene family were removed. Positional clustering of genes physically linked on a microdeletion is indicated by a slash between the gene symbols. Discussion
High burden driven by recurrent hotspot microdeletions
The present burden analysis applied a screening strategy that focused on both large (≥ 400 kb, ≥ 200 markers) and rare (< 1%) autosomal microdeletions to ensure a high calling accuracy [87] and to enrich pathogenic microdeletions among confounding benign copy number polymorphisms [88–90]. We found a significant 1.9-fold excess of microdeletions in the patients with GGE compared to the controls (Table 1). Overall, 7.3% of the 1,366 GGE patients carried at least one microdeletion compared to 4.0% in 5,234 controls. These findings highlight the important impact of microdeletions on the genetic susceptibility of common GGE syndromes with an attributable risk of about 3.3%.
The spectrum of 103 microdeletions identified in the GGE patients contained a high proportion (36.9%) of recurrent microdeletions at genomic rearrangement hotspots, known to play a pathogenic role in a wide range of neuropsychiatric disorders including epilepsy [13,91,92]. In total, 2.8% of the GGE patients carried one of the known pathogenic hotspot microdeletions at 1q21.1, 15q11.2, 15q13.3, 16p11.2, 16p12, 16p13.11 and 22q11.2 (Table 2), whereas these hotspot microdeletions were found only in 0.4% of the population controls (S1 and S2 Figs). Although these hotspot microdeletions are individually rare (< 1%), they collectively result in a 7.5-fold increased burden in the GGE patients and a population-attributable risk of about 2.4%. A previous genome-wide CNV search in epilepsies observed a similar cumulative prevalence of recurrent hotspot microdeletions in 3.5% out of 399 GGE patients [16]. Likewise, a targeted screening of the microdeletions at 15q11.2, 15q13.3 and 16p13.11 showed a cumulative frequency of 3.1% in 359 GGE patients and an even higher frequency of 10% in 60 GGE patients with intellectual disability [17]. Several other CNV studies targeting these genomic rearrangement hotspots also emphasised a substantial impact of recurrent microdeletions at 15q11.2, 15q13.3 and 16p13.11 in the pathogenesis of GGE and other epilepsies [14–20,22–24,93,94]. To our knowledge, this is the first study demonstrating a significant association of the recurrent microdeletion at 22q11.2 with GGE. Re-evaluation of the clinical records of three GGE patients carrying a 22q11.2 microdeletion revealed additional congenital and developmental features fitting to known conditions of the 22q11.2 deletion syndrome (OMIN 188400/192430). GGE patient (EC-EGMA094) had a moderate psychomotoric retardation, patient (EC-EGTCS145) was affected by a cleft palate and an atrial septal defect, and patient (EC-EGTCS044) had a mild impairment of his motoric coordination during childhood, moderate learning disabilities and hypocalcaemia, highlighting the 22q11.2 deletion syndrome as a multisystem disorder with high penetrance and variable phenotypic spectrum [95]. According to our ascertainment scheme [96], the present GGE patients with recurrent microdeletions did not exhibit severe intellectual disability or severe psychiatric comorbidities at the age of exploration but may evolve psychiatric disorders at later age. Considering the published CNV studies of epilepsies [14–20,24], meta-analyses may demonstrate an association of the less frequent recurrent hotspot microdeletions at 16p11.2 and 16p12 with GGE. Haploinsufficiency of CYFIP1 at 15q11.2 [97], CHRNA7 at 15q13.3 [98], NDE1 at 16p13.11 [99] and PRRT2 at 16p11.2 [100] has been implicated as risk-conferring mechanism for epilepsy and other neurodevelopmental phenotypes [88,89,91].
Functional-enrichment, pathway and network analyses showed significant connectivity of genes affected by microdeletions in GGE patients (S4 Fig) and a significant enrichment for the MGI Molecular Function category "abnormal emotion/affect behaviour" (MP:0002572) as well as the GO biological process term “cognition” (GO:0050890). The protein-protein interaction analyses highlight several genes that have been implicated in epileptogenesis (CYFIP1, GRIN2B, KCNA2, NRXN1, PLCB1) [14,16,26,39,74,75,97] and neurodevelopmental processes (ARC, GRM1, PARK2) [51,52,55,57–59].
Enrichment of microdeletions involving neurodevelopmental genes
In line with our neurodevelopmental hypothesis, we found a significant 4.6-fold excess of microdeletions carrying at least one ND-related gene [30] and a 4.1-fold enrichment of microdeletions affecting at least one ASD-related gene [31] in the GGE patients compared to the control subjects. In contrast, the two control gene assemblies did not show an increase of the microdeletion burden in GGE patients compared to controls (P > 0.40). Accordingly, the intriguing enrichment of ND - and ASD-related genes demonstrates that genes involved in neurodevelopmental processes play an important role in the epileptogenesis of common GGE syndromes. Notably, the moderate overlap of the previously published assemblies of ND - and ASD-related genes implicates a large number of neurodevelopmental genes contributing to the risk of common GGE syndromes and extensive genetic heterogeneity. The emerging overlap of gene-disrupting microdeletions and the rapidly evolving landscape of loss-of-function gene mutations in rare and common epilepsy syndromes will facilitate the prioritisation of causal epilepsy genes and the elucidation of the leading molecular pathways of epileptogenesis [101,102].
Non-hotspot microdeletions implicating potential GGE genes
We identified 27 gene-covering microdeletions in non-hotspot genomic regions that were present only in GGE patients (Table 3 and S3 Fig). These autosomal microdeletions involved several genes previously implicated in epilepsy and neurodevelopmental disorders. Although it remains challenging to distinguish benign and pathogenic microdeletions, several of these contain plausible candidate genes for epilepsy. Of particular interest were seven genes at seven microdeletion loci that have been associated with epilepsy.
Three of the epilepsy-associated microdeletions have been reported in two previous publications demonstrating an association of microdeletions affecting the 5´-terminal exons of the neuronal genes encoding the adhesion molecule neurexin 1 (NRXN1; 2p16.3, chr2 : 50,145,642–51,259,673, hg19) and the splicing regulator RNA-binding protein fox-1 homolog (RBFOX1; 16p13.3, chr16 : 5,289,468–7,763,341, hg19) [26,27]. The microdeletions involving NRXN1 exons 1–2 were observed in two female GGE patients with genetic absence epilepsies [26]. The 5´-terminal untranslated RBFOX1 exons 1–2 were deleted in a female patient with childhood absence epilepsy [27]. Deleterious mutations and microdeletions of the genes, NRXN1 and RBFOX1, have been reported in a large number of patients with a broad range of neuropsychiatric disorders, who were frequently also affected by epilepsy [40,41,54,72,81]. A recent study demonstrated that the splicing regulator Rbfox1 controls neuronal excitation in the mammalian brain and the Rbfox1 knockout in mice results in an increased susceptibility to spontaneous and kainic acid-induced seizures [71]. Furthermore, molecular, cellular, and clinical evidence supports a pivotal role of RBFOX1 in human neurodevelopmental disorders [73,103].
A 3.45 Mb microdeletion harbouring the protocadherin PCDH7 gene (chromosomal location: 4p15.1, chr4 : 30,721,950–31,148,422, hg19) was found in a female GGE subject with juvenile myoclonic epilepsy. An international GWAS meta-analysis including 8,696 epilepsy patients and 26,157 controls highlights PCDH7 as susceptibility gene for epilepsy in general and GGE syndromes in particular [45]. The PCHD7 gene encodes a calcium-dependent adhesion protein that is expressed in neurons of thalamocortical circuits and the hippocampus [46]. PCDH7 has been implicated as neuronal target gene of MECP2 [47], the gene for Rett syndrome (OMIM #312750), which manifests as a progressive neurodevelopmental disorder with recurrent seizures. Moreover, mutations in the X-chromosomal protocadherin gene PCDH19 cause epilepsy and intellectual disability in females [48]. These lines of evidence suggest an involvement of PCDH7 in epileptogenesis.
A 788 kb microdeletion involving the Shaker-like voltage-gated potassium channel gene KCNA2 (1p13, chr1 : 111,136,002–111,174,096, hg19) was identified in a male GGE patient with generalised tonic-clonic seizures starting at the age of 14. The Kv1 subfamily plays an essential role in the initiation and shaping of action potentials, influencing action potential firing patterns and controlling neuronal excitability as well as seizure susceptibility [36,38,39]. De novo loss - or gain-of-function mutations in KCNA2 have been identified to cause human epileptic encephalopathy [39]. Furthermore, the Kcna2 knockout mice exhibit spontaneous seizures and have a reduced life span [35,37].
One female GGE patient with childhood absence epilepsy carried a 2.4 Mb microdeletion in the chromosomal region 6q24.6 encompassing two neuronally expressed genes encoding the metabotropic glutamate receptor type 1 (GRM1; chr6 : 146,348,917–146,758,734, NM_001278065, hg19) and laforin (EPM2A; chr6 : 145,946,439–146,056,991, NM_005670, hg19). Deleterious mutations in the GRM1 gene have been found in patients with schizophrenia [52]. Also, familial segregation analysis of deleterious non-synonymous sequence variants revealed a co-segregation with multiple neuropsychiatric conditions including epilepsy in some families. Recessive mutations/microdeletions of EPM2A cause progressive myoclonic epilepsy type 2A (Lafora disease, OMIM #254780) [53].
A 582 kb microdeletion encompassing exon 1 of the gene encoding the RAR-related orphan receptor B (RORB; 9q21.13, chr9 : 77,112,251–77,303,533, NM_006914, hg19) was found in a male patient with childhood absence epilepsy, overlapping with the critical region of a novel microdeletion syndrome at 9q21.13 characterised by intellectual disability, speech delay, facial dysmorphisms and epilepsy [63]. The RORB gene is a strong candidate for the neurological phenotype because RORB was deleted in all affected individuals [63], it is expressed in the cerebral cortex and thalamus, and genetic associations of RORB with bipolar disorder [64] and verbal intelligence [65] have been reported.
The gene encoding the enzyme phospholipase C-beta 1 (PLCB1; 20p12.3, chr20 : 8,112,911–8,865,546, hg19) was partially deleted (exons 1–3, NM_015192, hg19) in a male GGE patient with childhood absence epilepsy. PLCB1 catalyses the generation of inositol 1,4,5-trisphoshate and diacylglycerol from phosphatidylinositol 4,5-bisphosphate, a key step in the intracellular transduction of many extracellular signals. Homozygous microdeletions of chromosome 20p12.3, disrupting the promoter region and first three coding exons of PLCB1, have previously been reported in two consanguineous families with early infantile epileptic encephalopathy [74]. Mutation analysis of a family with severe intractable epilepsy and neurodevelopmental delay revealed compound heterozygous mutations in PLCB1 composed of a 476 kb microdeletion encompassing PLCB1 and a deleterious PLCB1 splice site mutation [75]. Girirajan et al. [54] found an enrichment of microdeletions and duplications involving the PLCB1 gene in individuals with autism. Together, these findings implicate that the PLCB1 gene contributes to the genetic risk of neurodevelopmental disorders including epilepsy.
In addition to the epilepsy-associated microdeletions, nine deleted genes have been previously implicated as genetic risk factors in a broad range of neuropsychiatric disorders. Unique hemizygous microdeletions in GGE patients involved DPYD/1p13.3 [32–34], CADM2/3p12.1 [43], BCHE/3q26.1 [44], PARK2/6q24 [54,55,57,58], GRM8/7q31.33 [59–61]. TSNARE1/8q24.3 [62], MPP7-ARMC4-MKX/10p12.1 [66], TPH2/12q21.1 [67–69], MACROD2/20p12.1 [78–81], and ADARB1/21q22.3 [83,84]. Notably, overlapping microdeletions encompassing TSNARE1 at chromosome 8q24.3 in two GGE patients indicate its potential role in epileptogenesis. A recent GWAS meta-analysis of psychiatric disorders identified TSNARE1 as susceptibility gene for schizophrenia, schizoaffective and bipolar disorders [62]. While the function of TSNARE1 remains elusive, bioinformatic predictions suggest a vertebrate-specific function in synaptic vesicle exocytosis [104]. Further studies will be necessary to disentangle the pathogenic genes and to elucidate their molecular pathways in neurodevelopmental disorders and epileptogenesis.
Summary
Our burden analysis of large and rare autosomal microdeletions (size ≥ 400 kb, frequency < 1%) revealed: 1) a nearly 2-fold excess of microdeletions in GGE patients relative to the population controls, 2) a 7-fold increased burden for known hotspot microdeletions previously associated with neurodevelopmental disorders, and 3) a more than 4-fold enrichment of microdeletions carrying a gene implicated in neurodevelopmental disorders. Recurrent microdeletions at seven genomic rearrangement hotspots accounted for 37% of all microdeletions identified in the GGE patients and predominantly contributed to the excess of microdeletions in GGE patients. Comorbidity of GGE with other neurodevelopmental disorders, such as intellectual disability, ASD and schizophrenia, may result in even higher prevalence of recurrent hotspot microdeletions [17] and emphasises a valuable diagnostic contribution to the clinical management of these severely affected comorbid patients with GGE. The remarkable phenotypic variability observed for the recurrent hotspot microdeletions suggests a shared susceptibility of a wide range of neuropsychiatric disorders and GGE [105]. Several genes affected by microdeletions that were found only in GGE patients highlight novel candidate genes for GGE. Altogether, the present findings reinforce converging lines of evidence that genes affected by microdeletions in GGE patients reside in fundamental neurodevelopmental processes.
Materials and Methods
Case-control cohorts
The study protocol was approved by the local institutional review boards of the contributing clinical centres. All study participants provided written informed consent. Genomic DNA samples of all study participants were processed by the Affymetrix SNP 6.0 array. For the genome-wide CNV burden analysis, we did not include individuals with excessive CNV counts (> 50 autosomal deletions per individual for deletions spanning > 40 kb in size and covering > 20 markers). In addition, we excluded all Affymetrix SNP 6.0 array data derived from lymphoblastoid cell lines because of the clonal source of the DNA which is prone to CNV artefacts compared to genomic DNA samples derived from blood cells [21]. All study participants were of self-reported North-Western European origin.
Unrelated GGE patients of European descent were ascertained through the primary diagnosis of a common GGE syndrome according to the classification of the International League Against Epilepsy [1,4]. The standardised protocols for phenotyping of GGE syndromes as well as inclusion and exclusion criteria are available online at: http://portal.ccg.uni-koeln.de/ccg/research/epilepsy-genetics/sampling-procedure/ [96]. GGE patients with a history of severe major psychiatric disorders (autism spectrum disorder, schizophrenia, affective disorder: recurrent episodes requiring pharmacotherapy or treatment in a hospital), or severe intellectual disability (no basic education, permanently requiring professional support in their daily life) were excluded. The GGE cohort comprised 1,366 patients (853 females, 513 males) with the following age-related GGE syndromes: childhood absence epilepsy (CAE, n = 398), juvenile absence epilepsy (JAE, n = 191), unspecified genetic absence epilepsy (GAE, n = 9), juvenile myoclonic epilepsy (JME, n = 540), epilepsies with generalised tonic-clonic seizures (GTCS) alone predominantly on awakening (EGMA, n = 94), and epilepsies with recurrent unprovoked GTCS alone starting before the age of 26 (EGTCS, n = 134). These 1,366 GGE patients were collected from Austria (n = 142), Belgium (n = 39), Denmark (n = 97), Germany (n = 801) and the Netherlands (n = 287). Notably, 1,052 of the GGE patients and 3,022 population controls investigated in the present study were part of a previous study that investigated six target microdeletions at genomic rearrangement hotspots [14].
Affymetrix SNP 6.0 data from 5,234 German population controls (2,559 females, 2,675 males) were obtained from three epidemiologically based cohorts: 1) KORA cohort from South Germany (n = 1,507) [106], 2) PopGen cohort from North Germany (n = 1,143) [107], and 3) SHIP cohort from East Germany (n = 2,584) [108]. The population controls were unscreened for epilepsy or major neuropsychiatric disorders. EIGENSTRAT principal component analysis [109] was applied to remove ancestry outliers and to match for European ancestry of the case-control cohorts [96].
CNV analysis and screening of autosomal microdeletions
Genomic DNA samples were investigated by the Affymetrix Genome-Wide Human SNP Array 6.0 (Affymetrix, Santa Clara, CA, USA). CNV analysis was performed as previously described [14,22], using the Birdsuit algorithm implemented in the Affymetrix Genotyping Console version 4.1.1. All annotations refer to the genome build GRCh37/hg19. The present genome-wide burden analysis focused on rare and large autosomal microdeletions to ensure a high reliability of the microdeletion calls [87] and to enrich pathogenic microdeletions [88–90]. Therefore, we filtered out autosomal microdeletions with high calling confidence according to the following criteria: a) size ≥ 400 kb, b) coverage of ≥ 200 probe sets, and c) microdeletion frequency < 1% in the entire study sample. The microdeletion size of at least 400 kb was selected because all known pathogenic hotspot microdeletions identified in neurodevelopmental disorders exceed this size in CNV scans with the Affymetrix SNP Array 6.0 [29,88–90]. We did not include microduplications in the present burden analysis because the accuracy of CNV detection is lower for microduplications compared to microdeletions [110]. In particular, genomic DNA samples with substantial degradation are prone to spurious microduplication calls. Moreover, microduplications seem to exert pathogenic effects less frequently compared to microdeletions [88]. We excluded microdeletions with an overlap of > 10% with 12 chromosomal regions prone to artificial CNV calls according to a recently published "artefact list" [111]. For all QC-filtered microdeletions identified by SNP array screening, the segmental log2 ratios of the signal intensities and the SNP heterozygosity state were visually inspected by the Chromosome Analysis Suite v1.2.2 (Affymetrix, Santa Clara, CA, USA) to exclude spurious microdeletion calls. Validation of all 38 recurrent hotspot microdeletions and four GGE-associated microdeletions identified by SNP arrays in the GGE patients was carried out by real-time quantitative PCR (qPCR) according to the manufacturer´s instructions (Life Technologies, Carlsbad, CA, USA).
Burden analyses
Overall burden analyses were carried out for three assemblies of autosomal microdeletions: 1) any microdeletion, 2) genic microdeletions encompassing at least one protein-coding RefSeq gene, defined by the largest NM gene transcript (n = 18,299, hg19), and 3) microdeletions affecting a brain-expressed gene (n = 8,878), specified by a log(RPKM) > 3.32 of the BrainSpan RNA-Seq transcriptome dataset (http://www.brainspan.org/) [28].
Specifically, we tested the hypothesis whether microdeletions affecting genes involved in neurodevelopmental processes account for a significant fraction of genetic risk of GGE syndromes. Therefore, we investigated two recently published assemblies of genes associated with neurodevelopmental disorders (ND): 1) ND-related genes compiling 1,547 genes that were associated with neuropsychiatric disorders, autism candidate genes and genes of known genomic disorders based on literature and database queries [30], and 2) ASD-related genes comprising 1,669 brain-expressed genes that were selectively enriched for deleterious exonic de novo mutations in ASD individuals relative to their healthy siblings [31]. To evaluate a spurious enrichment of microdeletions in the GGE patients relative to the population controls, we tested two control gene assemblies comprising: 1) 3,256 randomly selected autosomal genes, and 2) 3,837 autosomal genes not expressed in the brain [28], defined by the BrainSpan RNA-Seq transcriptome dataset. ND - and ASD-related genes, genes located in genomic rearrangement hotspots, or the artefact list were removed from the compiled control gene assemblies.
Functional enrichment and network analyses
Functional-enrichment tests, pathway and network analyses were performed with the Disease Association Protein–Protein Link Evaluator version 2.0 program (DAPPLE v2.0; http://www.broadinstitute.org/mpg/dapple/dappleTMP.php; [85]) and the gene-set enrichment tool Enrichr (http://amp.pharm.mssm.edu/Enrichr/index.html; [86]). Therefore, we compiled two lists of genes affected by microdeletions in either the GGE patients (number of genes; n = 329; n = 191 regional seed genes) or the controls (n = 428 genes; n = 221 regional seed genes). There was an overlap of 103 genes (n = 61 seed genes) in both gene lists. To explore potential physical interactions among proteins encoded by deleted genes, DAPPLE uses experimentally validated, protein-protein interaction (PPI) databases to identify network and protein connectivity. Empirically, 1,000 random networks were generated by permutation to determine whether the connectivity of each seed protein with the PPI reference network was greater than that expected by chance.
The gene-set enrichment tool Enrichr was applied separately to explore patient and control lists of genes affected by microdeletions for an overlap with pathway gene-set libraries, specifically the database PPI Hub Proteins [112], and gene-set libraries created from Gene Ontology [113] as well as MGI Mammalian Phenotype terms [114]. A pathway or ontology term was considered as significantly enriched if the false discovery rate (FDR, Benjamini-Hochberg) was lower than 5% for an assembly of more than two genes and occurred only in the GGE patients but not in the controls.
Statistical analyses
Burden analysis was performed by comparisons of the frequency of autosomal microdeletions in GGE patients and controls. The P-values and corresponding odds ratios (ORs) with the 95%-confidence intervals were calculated with a two-sided χ2-test or Fisher´s exact test if appropriate. The Wilcoxon-Mann-Whitney-Test was applied to compare differences in the genomic size of microdeletions. In addition, the individual burden of microdeletions was assessed for comparisons of microdeletion size. Nominal two-sided P-values < 0.05 were considered significant.
Supporting Information
Zdroje
1. Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. (2010) Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 51 : 676–685. doi: 10.1111/j.1528-1167.2010.02522.x 20196795
2. Hauser WA, Annegers JF, Kurland LT (1993) Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia 34 : 453–468. 8504780
3. Jallon P, Loiseau P, Loiseau J (2001) Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CAROLE study. Coordination Active du Reseau Observatoire Longitudinal de l' Epilepsie. Epilepsia 42 : 464–475. 11440341
4. Commission on Classification and Terminology of the International League Against Epilepsy (1989) Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 30 : 389–399. 2502382
5. Nordli DR Jr. (2005) Idiopathic generalized epilepsies recognized by the International League Against Epilepsy. Epilepsia 46 Suppl 9 : 48–56. 16302875
6. Berkovic SF, Howell RA, Hay DA, Hopper JL (1998) Epilepsies in twins: genetics of the major epilepsy syndromes. Ann Neurol 43 : 435–445. 9546323
7. Kjeldsen MJ, Kyvik KO, Christensen K, Friis ML (2001) Genetic and environmental factors in epilepsy: a population-based study of 11900 Danish twin pairs. Epilepsy Res 44 : 167–178. 11325572
8. Vadlamudi L, Milne RL, Lawrence K, Heron SE, Eckhaus J, Keay D, et al. (2014) Genetics of epilepsy: The testimony of twins in the molecular era. Neurology 83 : 1042–1048. doi: 10.1212/WNL.0000000000000790 25107880
9. Peljto AL, Barker-Cummings C, Vasoli VM, Leibson CL, Hauser WA, Buchhalter JR, et al. (2014) Familial risk of epilepsy: a population-based study. Brain 137 : 795–805. doi: 10.1093/brain/awt368 24468822
10. Dibbens LM, Heron SE, Mulley JC (2007) A polygenic heterogeneity model for common epilepsies with complex genetics. Genes Brain Behav 6 : 593–597. 17559416
11. Poduri A, Lowenstein D (2011) Epilepsy genetics—past, present, and future. Curr Opin Genet Dev 21 : 325–332. doi: 10.1016/j.gde.2011.01.005 21277190
12. Pandolfo M (2013) Pediatric epilepsy genetics. Curr Opin Neurol 26 : 137–145. doi: 10.1097/WCO.0b013e32835f19da 23449174
13. Scheffer IE, Mefford HC (2014) Epilepsy: Beyond the single nucleotide variant in epilepsy genetics. Nat Rev Neurol 10 : 490–491. doi: 10.1038/nrneurol.2014.146 25112510
14. de Kovel CG, Trucks H, Helbig I, Mefford HC, Baker C, Leu C, et al. (2010) Recurrent microdeletions at 15q11.2 and 16p13.11 predispose to idiopathic generalized epilepsies. Brain 133 : 23–32. doi: 10.1093/brain/awp262 19843651
15. Heinzen EL, Radtke RA, Urban TJ, Cavalleri GL, Depondt C, Need AC, et al. (2010) Rare deletions at 16p13.11 predispose to a diverse spectrum of sporadic epilepsy syndromes. Am J Hum Genet 86 : 707–718. doi: 10.1016/j.ajhg.2010.03.018 20398883
16. Mefford HC, Muhle H, Ostertag P, von Spiczak S, Buysse K, Baker C, et al. (2010) Genome-wide copy number variation in epilepsy: novel susceptibility loci in idiopathic generalized and focal epilepsies. PLoS Genet 6: e1000962. doi: 10.1371/journal.pgen.1000962 20502679
17. Mullen SA, Carvill GL, Bellows S, Bayly MA, Trucks H, Lal D, et al. (2013) Copy number variants are frequent in genetic generalized epilepsy with intellectual disability. Neurology 81 : 1507–1514. doi: 10.1212/WNL.0b013e3182a95829 24068782
18. Striano P, Coppola A, Paravidino R, Malacarne M, Gimelli S, Robbiano A, et al. (2012) Clinical significance of rare copy number variations in epilepsy: a case-control survey using microarray-based comparative genomic hybridization. Arch Neurol 69 : 322–330. doi: 10.1001/archneurol.2011.1999 22083797
19. Dimassi S, Labalme A, Lesca G, Rudolf G, Bruneau N, Hirsch E, et al. (2014) A subset of genomic alterations detected in rolandic epilepsies contains candidate or known epilepsy genes including GRIN2A and PRRT2. Epilepsia 55 : 370–378. doi: 10.1111/epi.12502 24372385
20. Reinthaler EM, Lal D, Lebon S, Hildebrand MS, Dahl HH, Regan BM, et al. (2014) 16p11.2 600 kb Duplications confer risk for typical and atypical Rolandic epilepsy. Hum Mol Genet 23 : 6069–6080. doi: 10.1093/hmg/ddu306 24939913
21. Itsara A, Cooper GM, Baker C, Girirajan S, Li J, Absher D, et al. (2009) Population analysis of large copy number variants and hotspots of human genetic disease. Am J Hum Genet 84 : 148–161. doi: 10.1016/j.ajhg.2008.12.014 19166990
22. Helbig I, Mefford HC, Sharp AJ, Guipponi M, Fichera M, Franke A, et al. (2009) 15q13.3 microdeletions increase risk of idiopathic generalized epilepsy. Nat Genet 41 : 160–162. doi: 10.1038/ng.292 19136953
23. Dibbens LM, Mullen S, Helbig I, Mefford HC, Bayly MA, Bellows S, et al. (2009) Familial and sporadic 15q13.3 microdeletions in idiopathic generalized epilepsy: precedent for disorders with complex inheritance. Hum Mol Genet 18 : 3626–3631. doi: 10.1093/hmg/ddp311 19592580
24. Olson H, Shen Y, Avallone J, Sheidley BR, Pinsky R, Bergin AM, et al. (2014) Copy number variation plays an important role in clinical epilepsy. Ann Neurol 75 : 943–958. doi: 10.1002/ana.24178 24811917
25. Dejanovic B, Lal D, Catarino CB, Arjune S, Belaidi AA, Trucks H, et al. (2014) Exonic microdeletions of the gephyrin gene impair GABAergic synaptic inhibition in patients with idiopathic generalized epilepsy. Neurobiol Dis 67 : 88–96. doi: 10.1016/j.nbd.2014.02.001 24561070
26. Moller RS, Weber YG, Klitten LL, Trucks H, Muhle H, Kunz WS, et al. (2013) Exon-disrupting deletions of NRXN1 in idiopathic generalized epilepsy. Epilepsia 54 : 256–264. doi: 10.1111/epi.12078 23294455
27. Lal D, Trucks H, Moller RS, Hjalgrim H, Koeleman BP, de Kovel CG, et al. (2013) Rare exonic deletions of the RBFOX1 gene increase risk of idiopathic generalized epilepsy. Epilepsia 54 : 265–271. doi: 10.1111/epi.12084 23350840
28. Pinto D, Delaby E, Merico D, Barbosa M, Merikangas A, Klei L, et al. (2014) Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet 94 : 677–694. doi: 10.1016/j.ajhg.2014.03.018 24768552
29. Carvill GL, Mefford HC (2013) Microdeletion syndromes. Curr Opin Genet Dev 23 : 232–239. doi: 10.1016/j.gde.2013.03.004 23664828
30. Krumm N, O'Roak BJ, Karakoc E, Mohajeri K, Nelson B, Vives L, et al. (2013) Transmission disequilibrium of small CNVs in simplex autism. Am J Hum Genet 93 : 595–606. doi: 10.1016/j.ajhg.2013.07.024 24035194
31. Uddin M, Tammimies K, Pellecchia G, Alipanahi B, Hu P, Wang Z, et al. (2014) Brain-expressed exons under purifying selection are enriched for de novo mutations in autism spectrum disorder. Nat Genet 46 : 742–747. doi: 10.1038/ng.2980 24859339
32. Xu B, Ionita-Laza I, Roos JL, Boone B, Woodrick S, Sun Y, et al. (2012) De novo gene mutations highlight patterns of genetic and neural complexity in schizophrenia. Nat Genet 44 : 1365–1369. doi: 10.1038/ng.2446 23042115
33. Carter MT, Nikkel SM, Fernandez BA, Marshall CR, Noor A, Lionel AC, et al. (2011) Hemizygous deletions on chromosome 1p21.3 involving the DPYD gene in individuals with autism spectrum disorder. Clin Genet 80 : 435–443. doi: 10.1111/j.1399-0004.2010.01578.x 21114665
34. Willemsen MH, Valles A, Kirkels LA, Mastebroek M, Olde Loohuis N, Kos A, et al. (2011) Chromosome 1p21.3 microdeletions comprising DPYD and MIR137 are associated with intellectual disability. J Med Genet 48 : 810–818. doi: 10.1136/jmedgenet-2011-100294 22003227
35. Brew HM, Gittelman JX, Silverstein RS, Hanks TD, Demas VP, Robinson LC, et al. (2007) Seizures and reduced life span in mice lacking the potassium channel subunit Kv1.2, but hypoexcitability and enlarged Kv1 currents in auditory neurons. J Neurophysiol 98 : 1501–1525. 17634333
36. Wimmer VC, Reid CA, So EY, Berkovic SF, Petrou S (2010) Axon initial segment dysfunction in epilepsy. J Physiol 588 : 1829–1840. doi: 10.1113/jphysiol.2010.188417 20375142
37. Robbins CA, Tempel BL (2012) Kv1.1 and Kv1.2: similar channels, different seizure models. Epilepsia 53 Suppl 1 : 134–141. doi: 10.1111/j.1528-1167.2012.03484.x 22612818
38. Pena SD, Coimbra RL (2015) Ataxia and myoclonic epilepsy due to a heterozygous new mutation in KCNA2: proposal for a new channelopathy. Clin Genet 87: e1–3. doi: 10.1111/cge.12542 25477152
39. Syrbe S, Hedrich UB, Riesch E, Djemie T, Muller S, Moller RS, et al. (2015) De novo loss - or gain-of-function mutations in KCNA2 cause epileptic encephalopathy. Nat Genet, doi: 10.1038/ng.3239 [Epub ahead of print].
40. Bena F, Bruno DL, Eriksson M, van Ravenswaaij-Arts C, Stark Z, Dijkhuizen T, et al. (2013) Molecular and clinical characterization of 25 individuals with exonic deletions of NRXN1 and comprehensive review of the literature. Am J Med Genet B Neuropsychiatr Genet 162B: 388–403. doi: 10.1002/ajmg.b.32148 23533028
41. Dabell MP, Rosenfeld JA, Bader P, Escobar LF, El-Khechen D, Vallee SE, et al. (2013) Investigation of NRXN1 deletions: clinical and molecular characterization. Am J Med Genet A 161A: 717–731. doi: 10.1002/ajmg.a.35780 23495017
42. Albayrak O, Putter C, Volckmar AL, Cichon S, Hoffmann P, Nothen MM, et al. (2013) Common obesity risk alleles in childhood attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 162B: 295–305. doi: 10.1002/ajmg.b.32144 23533005
43. Casey JP, Magalhaes T, Conroy JM, Regan R, Shah N, Anney R, et al. (2012) A novel approach of homozygous haplotype sharing identifies candidate genes in autism spectrum disorder. Hum Genet 131 : 565–579. doi: 10.1007/s00439-011-1094-6 21996756
44. Lesch KP, Selch S, Renner TJ, Jacob C, Nguyen TT, Hahn T, et al. (2011) Genome-wide copy number variation analysis in attention-deficit/hyperactivity disorder: association with neuropeptide Y gene dosage in an extended pedigree. Mol Psychiatry 16 : 491–503. doi: 10.1038/mp.2010.29 20308990
45. International League Against Epilepsy Consortium on Complex Epilepsies (2014) Genetic determinants of common epilepsies: a meta-analysis of genome-wide association studies. Lancet Neurol 13 : 893–903. doi: 10.1016/S1474-4422(14)70171-1 25087078
46. Kim SY, Mo JW, Han S, Choi SY, Han SB, Moon BH, et al. (2010) The expression of non-clustered protocadherins in adult rat hippocampal formation and the connecting brain regions. Neuroscience 170 : 189–199. doi: 10.1016/j.neuroscience.2010.05.027 20541594
47. Miyake K, Hirasawa T, Soutome M, Itoh M, Goto Y, Endoh K, et al. (2011) The protocadherins, PCDHB1 and PCDH7, are regulated by MeCP2 in neuronal cells and brain tissues: implication for pathogenesis of Rett syndrome. BMC Neurosci 12 : 81. doi: 10.1186/1471-2202-12-81 21824415
48. Dibbens LM, Tarpey PS, Hynes K, Bayly MA, Scheffer IE, Smith R, et al. (2008) X-linked protocadherin 19 mutations cause female-limited epilepsy and cognitive impairment. Nat Genet 40 : 776–781. doi: 10.1038/ng.149 18469813
49. Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, et al. (2003) The nuclear RNase III Drosha initiates microRNA processing. Nature 425 : 415–419. 14508493
50. Inoue YU, Asami J, Inoue T (2008) Cadherin-6 gene regulatory patterns in the postnatal mouse brain. Mol Cell Neurosci 39 : 95–104. doi: 10.1016/j.mcn.2008.05.020 18617008
51. Jimenez-Mateos EM, Henshall DC (2013) Epilepsy and microRNA. Neuroscience 238 : 218–229. doi: 10.1016/j.neuroscience.2013.02.027 23485811
52. Ayoub MA, Angelicheva D, Vile D, Chandler D, Morar B, Cavanaugh JA, et al. (2012) Deleterious GRM1 mutations in schizophrenia. PLoS One 7: e32849. doi: 10.1371/journal.pone.0032849 22448230
53. Minassian BA, Lee JR, Herbrick JA, Huizenga J, Soder S, Mungall AJ, et al. (1998) Mutations in a gene encoding a novel protein tyrosine phosphatase cause progressive myoclonus epilepsy. Nat Genet 20 : 171–174. 9771710
54. Girirajan S, Dennis MY, Baker C, Malig M, Coe BP, Campbell CD, et al. (2013) Refinement and discovery of new hotspots of copy-number variation associated with autism spectrum disorder. Am J Hum Genet 92 : 221–237. doi: 10.1016/j.ajhg.2012.12.016 23375656
55. Glessner JT, Wang K, Cai G, Korvatska O, Kim CE, Wood S, et al. (2009) Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature 459 : 569–573. doi: 10.1038/nature07953 19404257
56. Gaier ED, Eipper BA, Mains RE (2014) Pam heterozygous mice reveal essential role for Cu in amygdalar behavioral and synaptic function. Ann N Y Acad Sci 1314 : 15–23. doi: 10.1111/nyas.12378 24593825
57. Scheuerle A, Wilson K (2011) PARK2 copy number aberrations in two children presenting with autism spectrum disorder: further support of an association and possible evidence for a new microdeletion/microduplication syndrome. Am J Med Genet B Neuropsychiatr Genet 156B: 413–420. doi: 10.1002/ajmg.b.31176 21360662
58. La Cognata V, Iemmolo R, D'Agata V, Scuderi S, Drago F, Zappia M, et al. (2014) Increasing the Coding Potential of Genomes Through Alternative Splicing: The Case of PARK2 Gene. Curr Genomics 15 : 203–216. doi: 10.2174/1389202915666140426003342 24955028
59. Elia J, Glessner JT, Wang K, Takahashi N, Shtir CJ, Hadley D, et al. (2012) Genome-wide copy number variation study associates metabotropic glutamate receptor gene networks with attention deficit hyperactivity disorder. Nat Genet 44 : 78–84. doi: 10.1093/jncimonographs/lgs016 22623599
60. Prasad A, Merico D, Thiruvahindrapuram B, Wei J, Lionel AC, Sato D, et al. (2012) A discovery resource of rare copy number variations in individuals with autism spectrum disorder. G3 (Bethesda) 2 : 1665–1685. doi: 10.1534/g3.112.004689 23275889
61. Asadollahi R, Oneda B, Joset P, Azzarello-Burri S, Bartholdi D, Steindl K, et al. (2014) The clinical significance of small copy number variants in neurodevelopmental disorders. J Med Genet 2 : 1665–1685.
62. Sleiman P, Wang D, Glessner J, Hadley D, Gur RE, Cohen N, et al. (2013) GWAS meta analysis identifies TSNARE1 as a novel Schizophrenia / Bipolar susceptibility locus. Sci Rep 3 : 3075. doi: 10.1038/srep03075 24166486
63. Boudry-Labis E, Demeer B, Le Caignec C, Isidor B, Mathieu-Dramard M, Plessis G, et al. (2013) A novel microdeletion syndrome at 9q21.13 characterised by mental retardation, speech delay, epilepsy and characteristic facial features. Eur J Med Genet 56 : 163–170. doi: 10.1016/j.ejmg.2012.12.006 23279911
64. McGrath CL, Glatt SJ, Sklar P, Le-Niculescu H, Kuczenski R, Doyle AE, et al. (2009) Evidence for genetic association of RORB with bipolar disorder. BMC Psychiatry 9 : 70. doi: 10.1186/1471-244X-9-70 19909500
65. Ersland KM, Christoforou A, Stansberg C, Espeseth T, Mattheisen M, Mattingsdal M, et al. (2012) Gene-based analysis of regionally enriched cortical genes in GWAS data sets of cognitive traits and psychiatric disorders. PLoS One 7: e31687. doi: 10.1371/journal.pone.0031687 22384057
66. Mroczkowski HJ, Arnold G, Schneck FX, Rajkovic A, Yatsenko SA (2014) Interstitial 10p11.23-p12.1 microdeletions associated with developmental delay, craniofacial abnormalities, and cryptorchidism. Am J Med Genet A 164A: 2623–2626. doi: 10.1002/ajmg.a.36627 25073539
67. Cichon S, Winge I, Mattheisen M, Georgi A, Karpushova A, Freudenberg J, et al. (2008) Brain-specific tryptophan hydroxylase 2 (TPH2): a functional Pro206Ser substitution and variation in the 5'-region are associated with bipolar affective disorder. Hum Mol Genet 17 : 87–97. 17905754
68. Mosienko V, Beis D, Pasqualetti M, Waider J, Matthes S, Qadri F, et al. (2014) Life without brain serotonin: Reevaluation of serotonin function with mice deficient in brain serotonin synthesis. Behav Brain Res 227C: 78–88.
69. Mosienko V, Matthes S, Hirth N, Beis D, Flinders M, Bader M, et al. (2014) Adaptive changes in serotonin metabolism preserve normal behavior in mice with reduced TPH2 activity. Neuropharmacology 85 : 73–80. doi: 10.1016/j.neuropharm.2014.05.015 24863038
70. Colin E, Huynh Cong E, Mollet G, Guichet A, Gribouval O, Arrondel C, et al. (2014) Loss-of-Function Mutations in WDR73 Are Responsible for Microcephaly and Steroid-Resistant Nephrotic Syndrome: Galloway-Mowat Syndrome. Am J Hum Genet 95 : 637–648. doi: 10.1016/j.ajhg.2014.10.011 25466283
71. Gehman LT, Stoilov P, Maguire J, Damianov A, Lin CH, Shiue L, et al. (2011) The splicing regulator Rbfox1 (A2BP1) controls neuronal excitation in the mammalian brain. Nat Genet 43 : 706–711. doi: 10.1038/ng.841 21623373
72. Zhao WW (2013) Intragenic deletion of RBFOX1 associated with neurodevelopmental/neuropsychiatric disorders and possibly other clinical presentations. Mol Cytogenet 6 : 26. doi: 10.1186/1755-8166-6-26 23822903
73. Fogel BL, Wexler E, Wahnich A, Friedrich T, Vijayendran C, Gao F, et al. (2012) RBFOX1 regulates both splicing and transcriptional networks in human neuronal development. Hum Mol Genet 21 : 4171–4186. doi: 10.1093/hmg/dds240 22730494
74. Kurian MA, Meyer E, Vassallo G, Morgan NV, Prakash N, Pasha S, et al. (2010) Phospholipase C beta 1 deficiency is associated with early-onset epileptic encephalopathy. Brain 133 : 2964–2970. doi: 10.1093/brain/awq238 20833646
75. Ngoh A, McTague A, Wentzensen IM, Meyer E, Applegate C, Kossoff EH, et al. (2014) Severe infantile epileptic encephalopathy due to mutations in PLCB1: expansion of the genotypic and phenotypic disease spectrum. Dev Med Child Neurol 56 : 1124–1128. doi: 10.1111/dmcn.12450 24684524
76. McOmish CE, Burrows EL, Howard M, Hannan AJ (2008) PLC-beta1 knockout mice as a model of disrupted cortical development and plasticity: behavioral endophenotypes and dysregulation of RGS4 gene expression. Hippocampus 18 : 824–834. doi: 10.1002/hipo.20443 18493969
77. Lo Vasco VR, Cardinale G, Polonia P (2012) Deletion of PLCB1 gene in schizophrenia-affected patients. J Cell Mol Med 16 : 844–851. doi: 10.1111/j.1582-4934.2011.01363.x 22507702
78. Anney R, Klei L, Pinto D, Regan R, Conroy J, Magalhaes TR, et al. (2010) A genome-wide scan for common alleles affecting risk for autism. Hum Mol Genet 19 : 4072–4082. doi: 10.1093/hmg/ddq307 20663923
79. Lionel AC, Crosbie J, Barbosa N, Goodale T, Thiruvahindrapuram B, Rickaby J, et al. (2011) Rare copy number variation discovery and cross-disorder comparisons identify risk genes for ADHD. Sci Transl Med 3 : 95ra75.
80. Kohannim O, Hibar DP, Stein JL, Jahanshad N, Hua X, Rajagopalan P, et al. (2012) Discovery and Replication of Gene Influences on Brain Structure Using LASSO Regression. Front Neurosci 6 : 115. doi: 10.3389/fnins.2012.00115 22888310
81. Bradley WE, Raelson JV, Dubois DY, Godin E, Fournier H, Prive C, et al. (2010) Hotspots of large rare deletions in the human genome. PLoS One 5: e9401. doi: 10.1371/journal.pone.0009401 20195527
82. Jones RM, Cadby G, Blangero J, Abraham LJ, Whitehouse AJ, Moses EK (2014) MACROD2 gene associated with autistic-like traits in a general population sample. Psychiatr Genet 24 : 241–248. doi: 10.1097/YPG.0000000000000052 25360606
83. Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N, et al. (2000) Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature 406 : 78–81. 10894545
84. Vesely C, Tauber S, Sedlazeck FJ, Tajaddod M, Haeseler A, Jantsch MF (2014) ADAR2 induces reproducible changes in sequence and abundance of mature microRNAs in the mouse brain. Nucleic Acids Res 42 : 12155–12168. doi: 10.1093/nar/gku844 25260591
85. Rossin EJ, Lage K, Raychaudhuri S, Xavier RJ, Tatar D, Benita Y, et al. (2011) Proteins encoded in genomic regions associated with immune-mediated disease physically interact and suggest underlying biology. PLoS Genet 7: e1001273. doi: 10.1371/journal.pgen.1001273 21249183
86. Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, et al. (2013) Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14 : 128. doi: 10.1186/1471-2105-14-128 23586463
87. Pinto D, Darvishi K, Shi X, Rajan D, Rigler D, Fitzgerald T, et al. (2011) Comprehensive assessment of array-based platforms and calling algorithms for detection of copy number variants. Nat Biotechnol 29 : 512–520. doi: 10.1038/nbt.1852 21552272
88. Watson CT, Marques-Bonet T, Sharp AJ, Mefford HC (2014) The genetics of microdeletion and microduplication syndromes: an update. Annu Rev Genomics Hum Genet 15 : 215–244. doi: 10.1146/annurev-genom-091212-153408 24773319
89. Cooper GM, Coe BP, Girirajan S, Rosenfeld JA, Vu TH, Baker C, et al. (2011) A copy number variation morbidity map of developmental delay. Nat Genet 43 : 838–846. doi: 10.1038/ng.909 21841781
90. Cooper NJ, Shtir CJ, Smyth DJ, Guo H, Swafford AD, Zanda M, et al. (2015) Detection and correction of artefacts in estimation of rare copy number variants and analysis of rare deletions in type 1 diabetes. Hum Mol Genet 24 : 1774–1790. doi: 10.1093/hmg/ddu581 25424174
91. Grayton HM, Fernandes C, Rujescu D, Collier DA (2012) Copy number variations in neurodevelopmental disorders. Prog Neurobiol 99 : 81–91. doi: 10.1016/j.pneurobio.2012.07.005 22813947
92. McLysaght A, Makino T, Grayton HM, Tropeano M, Mitchell KJ, Vassos E, et al. (2014) Ohnologs are overrepresented in pathogenic copy number mutations. Proc Natl Acad Sci U S A 111 : 361–366. doi: 10.1073/pnas.1309324111 24368850
93. Mefford HC, Mulley JC (2010) Genetically complex epilepsies, copy number variants and syndrome constellations. Genome Med 2 : 71. doi: 10.1186/gm192 20923578
94. Helbig I, Swinkels ME, Aten E, Caliebe A, van 't Slot R, Boor R, et al. (2014) Structural genomic variation in childhood epilepsies with complex phenotypes. Eur J Hum Genet 22 : 896–901. doi: 10.1038/ejhg.2013.262 24281369
95. Fung WL, Butcher NJ, Costain G, Andrade DM, Boot E, Chow EW, et al. (2015) Practical guidelines for managing adults with 22q11.2 deletion syndrome. Genet Med, doi: 10.1038/gim.2014.175 [Epub ahead of print].
96. EPICURE Consortium, EMINet Consortium, Steffens M, Leu C, Ruppert AK, Zara F, et al. (2012) Genome-wide association analysis of genetic generalized epilepsies implicates susceptibility loci at 1q43, 2p16.1, 2q22.3 and 17q21.32. Hum Mol Genet 21 : 5359–5372. doi: 10.1093/hmg/dds373 22949513
97. Pathania M, Davenport EC, Muir J, Sheehan DF, Lopez-Domenech G, Kittler JT (2014) The autism and schizophrenia associated gene CYFIP1 is critical for the maintenance of dendritic complexity and the stabilization of mature spines. Transl Psychiatry 4: e423.
98. Schaaf CP (2014) Nicotinic acetylcholine receptors in human genetic disease. Genet Med 16 : 649–656. doi: 10.1038/gim.2014.9 24556925
99. Paciorkowski AR, Keppler-Noreuil K, Robinson L, Sullivan C, Sajan S, Christian SL, et al. (2013) Deletion 16p13.11 uncovers NDE1 mutations on the non-deleted homolog and extends the spectrum of severe microcephaly to include fetal brain disruption. Am J Med Genet A 161A: 1523–1530. doi: 10.1002/ajmg.a.35969 23704059
100. Djemie T, Weckhuysen S, Holmgren P, Hardies K, Van Dyck T, Hendrickx R, et al. (2014) PRRT2 mutations: exploring the phenotypical boundaries. J Neurol Neurosurg Psychiatry 85 : 462–465. doi: 10.1136/jnnp-2013-305122 24101679
101. Hoischen A, Krumm N, Eichler EE (2014) Prioritization of neurodevelopmental disease genes by discovery of new mutations. Nat Neurosci 17 : 764–772. doi: 10.1038/nn.3703 24866042
102. Coe BP, Witherspoon K, Rosenfeld JA, van Bon BW, Vulto-van Silfhout AT, Bosco P, et al. (2014) Refining analyses of copy number variation identifies specific genes associated with developmental delay. Nat Genet 46 : 1063–1071. doi: 10.1038/ng.3092 25217958
103. Bill BR, Lowe JK, Dybuncio CT, Fogel BL (2013) Orchestration of neurodevelopmental programs by RBFOX1: implications for autism spectrum disorder. Int Rev Neurobiol 113 : 251–267. doi: 10.1016/B978-0-12-418700-9.00008-3 24290388
104. Smith JJ, Sumiyama K, Amemiya CT (2012) A living fossil in the genome of a living fossil: Harbinger transposons in the coelacanth genome. Mol Biol Evol 29 : 985–993. doi: 10.1093/molbev/msr267 22045999
105. Coe BP, Girirajan S, Eichler EE (2012) A genetic model for neurodevelopmental disease. Curr Opin Neurobiol 22 : 829–836. doi: 10.1016/j.conb.2012.04.007 22560351
106. Wichmann HE, Gieger C, Illig T, Group MKS (2005) KORA-gen—resource for population genetics, controls and a broad spectrum of disease phenotypes. Gesundheitswesen 67 Suppl 1: S26–30. 16032514
107. Krawczak M, Nikolaus S, von Eberstein H, Croucher PJ, El Mokhtari NE, Schreiber S (2006) PopGen: population-based recruitment of patients and controls for the analysis of complex genotype-phenotype relationships. Community Genet 9 : 55–61. 16490960
108. Volzke H, Alte D, Schmidt CO, Radke D, Lorbeer R, Friedrich N, et al. (2011) Cohort profile: the study of health in Pomerania. Int J Epidemiol 40 : 294–307. doi: 10.1093/ije/dyp394 20167617
109. Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D (2006) Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38 : 904–909. 16862161
110. Zhang D, Qian Y, Akula N, Alliey-Rodriguez N, Tang J, Bipolar Genome S, et al. (2011) Accuracy of CNV Detection from GWAS Data. PLoS One 6: e14511. doi: 10.1371/journal.pone.0014511 21249187
111. Jacquemont S, Coe BP, Hersch M, Duyzend MH, Krumm N, Bergmann S, et al. (2014) A higher mutational burden in females supports a "female protective model" in neurodevelopmental disorders. Am J Hum Genet 94 : 415–425. doi: 10.1016/j.ajhg.2014.02.001 24581740
112. Berger SI, Posner JM, Ma'ayan A (2007) Genes2Networks: connecting lists of gene symbols using mammalian protein interactions databases. BMC Bioinformatics 8 : 372. 17916244
113. Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. (2000) Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25 : 25–29. 10802651
114. Smith CM, Finger JH, Hayamizu TF, McCright IJ, Eppig JT, Kadin JA, et al. (2007) The mouse Gene Expression Database (GXD): 2007 update. Nucleic Acids Res 35: D618–623. 17130151
Štítky
Genetika Reprodukční medicína
Článek Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1Článek A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria ParasiteČlánek The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of CentrosomesČlánek Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In VivoČlánek MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle CellsČlánek PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune ResponsesČlánek Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA SynthesisČlánek The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .Článek Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 5- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Intrauterinní inseminace a její úspěšnost
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
-
Všechny články tohoto čísla
- Genomic Heritability: What Is It?
- Triglyceride-Increasing Alleles Associated with Protection against Type-2 Diabetes
- Epistasis Is a Major Determinant of the Additive Genetic Variance in
- Genetic Regulation of Bone Metabolism in the Chicken: Similarities and Differences to Mammalian Systems
- Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1
- The Philosophical Approach: An Interview with Ford Doolittle
- Downregulation of the Host Gene by miR-92 Is Essential for Neuroblast Self-Renewal in
- A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria Parasite
- Y Fuse? Sex Chromosome Fusions in Fishes and Reptiles
- Regulation of Active DNA Demethylation by a Methyl-CpG-Binding Domain Protein in
- Overlapping Patterns of Rapid Evolution in the Nucleic Acid Sensors cGAS and OAS1 Suggest a Common Mechanism of Pathogen Antagonism and Escape
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Genetic Architecture of Abdominal Pigmentation in
- Whole Genome DNA Binding Analysis of the Bacterial Replication Initiator and Transcription Factor DnaA
- The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of Centrosomes
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- Burden Analysis of Rare Microdeletions Suggests a Strong Impact of Neurodevelopmental Genes in Genetic Generalised Epilepsies
- Cell Cycle Control by the Master Regulator CtrA in
- Myopathic Lamin Mutations Cause Reductive Stress and Activate the Nrf2/Keap-1 Pathway
- Monoallelic Loss of the Imprinted Gene Promotes Tumor Formation in Irradiated Mice
- Phylum-Level Conservation of Regulatory Information in Nematodes despite Extensive Non-coding Sequence Divergence
- Clustering and Negative Feedback by Endocytosis in Planar Cell Polarity Signaling Is Modulated by Ubiquitinylation of Prickle
- Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In Vivo
- MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle Cells
- β-Catenin Signaling Biases Multipotent Lingual Epithelial Progenitors to Differentiate and Acquire Specific Taste Cell Fates
- A Simple Auxin Transcriptional Response System Regulates Multiple Morphogenetic Processes in the Liverwort
- Parallel Gene Expression Differences between Low and High Latitude Populations of and .
- The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of
- Characterization of TCF21 Downstream Target Regions Identifies a Transcriptional Network Linking Multiple Independent Coronary Artery Disease Loci
- PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune Responses
- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Coronary Artery Disease Associated Transcription Factor TCF21 Regulates Smooth Muscle Precursor Cells That Contribute to the Fibrous Cap
- Rescue of DNA-PK Signaling and T-Cell Differentiation by Targeted Genome Editing in a Deficient iPSC Disease Model
- Disruption of Transcriptional Coactivator Sub1 Leads to Genome-Wide Re-distribution of Clustered Mutations Induced by APOBEC in Active Yeast Genes
- Yeast Killer Elements Hold Their Hosts Hostage
- Keeping in Shape the Dogma of Mitochondrial DNA Maternal Inheritance
- Extreme-Depth Re-sequencing of Mitochondrial DNA Finds No Evidence of Paternal Transmission in Humans
- Trading Places—Switching Frataxin Function by a Single Amino Acid Substitution within the [Fe-S] Cluster Assembly Scaffold
- Natural Variation Identifies , a Universal Gene Required for Cell Proliferation and Growth at High Temperatures in
- Mutations in Gene Are Associated with Predisposition to Breast Cancer
- The Whole of a Scientific Career: An Interview with Oliver Smithies
- Cell Specific eQTL Analysis without Sorting Cells
- Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA Synthesis
- Systemic Regulation of RAS/MAPK Signaling by the Serotonin Metabolite 5-HIAA
- Reprogramming LCLs to iPSCs Results in Recovery of Donor-Specific Gene Expression Signature
- The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .
- Genetic Mechanism of Human Neutrophil Antigen 2 Deficiency and Expression Variations
- Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
- Early Lineage Priming by Trisomy of Leads to Myeloproliferation in a Down Syndrome Model
- Turning into a Frataxin-Dependent Organism
- Accounting for Experimental Noise Reveals That mRNA Levels, Amplified by Post-Transcriptional Processes, Largely Determine Steady-State Protein Levels in Yeast
- Biological Significance of Photoreceptor Photocycle Length: VIVID Photocycle Governs the Dynamic VIVID-White Collar Complex Pool Mediating Photo-adaptation and Response to Changes in Light Intensity
- CTXφ Replication Depends on the Histone-Like HU Protein and the UvrD Helicase
- Disruption of miR-29 Leads to Aberrant Differentiation of Smooth Muscle Cells Selectively Associated with Distal Lung Vasculature
- Notch Is Required in Adult Sensory Neurons for Morphological and Functional Plasticity of the Olfactory Circuit
- Post-transcriptional Regulation of Keratinocyte Progenitor Cell Expansion, Differentiation and Hair Follicle Regression by
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
- Casein Kinase 1 and Phosphorylation of Cohesin Subunit Rec11 (SA3) Promote Meiotic Recombination through Linear Element Formation
- Fibroblast Growth Factor 9 Regulation by MicroRNAs Controls Lung Development and Links Loss to the Pathogenesis of Pleuropulmonary Blastoma
- Auxin-Mediated Transcriptional System with a Minimal Set of Components Is Critical for Morphogenesis through the Life Cycle in
- The Broad-Spectrum Antiviral Protein ZAP Restricts Human Retrotransposition
- The 4E-BP Caf20p Mediates Both eIF4E-Dependent and Independent Repression of Translation
- Turning into a Frataxin-Independent Organism
- Promotion of Bone Morphogenetic Protein Signaling by Tetraspanins and Glycosphingolipids
- Essential Role of the ESX-5 Secretion System in Outer Membrane Permeability of Pathogenic Mycobacteria
- The Zinc-Finger Antiviral Protein ZAP Inhibits LINE and Alu Retrotransposition
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



