-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAsynchronous Replication, Mono-Allelic Expression, and Long Range -Effects of
Mammalian chromosomes initiate DNA replication at multiple sites along their length during each S phase following a temporal replication program. The majority of genes on homologous chromosomes replicate synchronously. However, mono-allelically expressed genes such as imprinted genes, allelically excluded genes, and genes on female X chromosomes replicate asynchronously. We have identified a cis-acting locus on human chromosome 6 that controls this replication-timing program. This locus encodes a large intergenic non-coding RNA gene named Asynchronous replication and Autosomal RNA on chromosome 6, or ASAR6. Disruption of ASAR6 results in delayed replication, delayed mitotic chromosome condensation, and activation of the previously silent alleles of mono-allelic genes on chromosome 6. The ASAR6 gene resides within an ∼1.2 megabase domain of asynchronously replicating DNA that is coordinated with other random asynchronously replicating loci along chromosome 6. In contrast to other nearby mono-allelic genes, ASAR6 RNA is expressed from the later-replicating allele. ASAR6 RNA is synthesized by RNA Polymerase II, is not polyadenlyated, is restricted to the nucleus, and is subject to random mono-allelic expression. Disruption of ASAR6 leads to the formation of bridged chromosomes, micronuclei, and structural instability of chromosome 6. Finally, ectopic integration of cloned genomic DNA containing ASAR6 causes delayed replication of entire mouse chromosomes.
Published in the journal: . PLoS Genet 9(4): e32767. doi:10.1371/journal.pgen.1003423
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003423Summary
Mammalian chromosomes initiate DNA replication at multiple sites along their length during each S phase following a temporal replication program. The majority of genes on homologous chromosomes replicate synchronously. However, mono-allelically expressed genes such as imprinted genes, allelically excluded genes, and genes on female X chromosomes replicate asynchronously. We have identified a cis-acting locus on human chromosome 6 that controls this replication-timing program. This locus encodes a large intergenic non-coding RNA gene named Asynchronous replication and Autosomal RNA on chromosome 6, or ASAR6. Disruption of ASAR6 results in delayed replication, delayed mitotic chromosome condensation, and activation of the previously silent alleles of mono-allelic genes on chromosome 6. The ASAR6 gene resides within an ∼1.2 megabase domain of asynchronously replicating DNA that is coordinated with other random asynchronously replicating loci along chromosome 6. In contrast to other nearby mono-allelic genes, ASAR6 RNA is expressed from the later-replicating allele. ASAR6 RNA is synthesized by RNA Polymerase II, is not polyadenlyated, is restricted to the nucleus, and is subject to random mono-allelic expression. Disruption of ASAR6 leads to the formation of bridged chromosomes, micronuclei, and structural instability of chromosome 6. Finally, ectopic integration of cloned genomic DNA containing ASAR6 causes delayed replication of entire mouse chromosomes.
Introduction
Morphological differences between mitotic chromosomes residing within the same cell were first described in mammalian cells over forty years ago (reviewed in [1]). In addition, numerous reports have described an abnormal chromosomal phenotype affecting single or a few chromosomes in mitotic preparations from tumor-derived and other established cell lines. For example, Dr. Harald zur Hausen described an under-condensed appearance of individual chromosomes during mitosis in cell lines derived from 7 different leukemia patients [2]. The individual under-condensed chromosomes synthesized DNA after the normally condensed chromosomes had finished replication, indicating that the under-condensed chromosomes were extremely late replicating, with DNA synthesis extending into the G2 phase. These observations have been extended to include chromosomes present in numerous tumor-derived and permanent cell lines from several different mammalian species [3], [4], [5]. Furthermore, the under-condensed and late replicating phenotype present in tumor-derived cells occurred only on certain rearranged chromosomes and not on others, suggesting that the under-condensed and late replicating phenotype was associated with certain chromosomal rearrangements [5]. A detailed analysis of the replication-timing defect on under-condensed chromosomes indicated that they exhibit a delay in replication timing (DRT), which is characterized by a >2 hour delay in both the initiation and the completion of DNA synthesis along the entire length of the chromosome [5]. Chromosomes with DRT also display a delay in mitotic chromosome condensation (DMC), which is characterized by an under-condensed appearance during mitosis, delayed recruitment of Aurora B kinase, and a delay in the mitotic phosphorylation of serine 10 of histone H3 [5], [6]. The DRT/DMC phenotype was also detected on ∼5% of inter-chromosomal translocations induced by exposure to ionizing radiation (IR) [7]. Taken together, these observations indicate that DRT/DMC occurs on certain rearranged chromosomes and is a common phenotype in cancer cells and in cells exposed to IR.
We have developed a chromosome engineering system that allows for the systematic analysis of human chromosomes with DRT/DMC [6], [7], [8], [9]. This system relies on site-specific recombinases to generate precise chromosomal rearrangements. Using this system we previously identified four balanced translocations, each displaying DRT/DMC on one of the two derivative chromosomes [8]. Subsequently, we found that translocations or deletions at a discrete locus on human chromosome 6 result in DRT/DMC. The deletions that cause DRT/DMC on chromosome 6 disrupt a large intergenic non-coding RNA gene named ASynchronous replication and Autosomal RNA on chromosome 6, or ASAR6 [9]. ASAR6 displays random mono-allelic expression in immortalized cell lines, and as the name implies, displays asynchronous replication between alleles. In addition, the asynchronous replication of ASAR6 is coordinated with other asynchronous loci on the long arm of chromosome 6. Thus, the early replicating chromosome for ASAR6 is also the early replicating chromosome for several nearby mono-allelic genes, indicating that these genes display cis-coordinated asynchronous replication. However, the early replicating chromosome for ASAR6 was on the same homolog as the later replicating alleles for other random asynchronously replicating loci located at a distance on the long arm of chromosome 6, indicating that the coordination of asynchronous replication with these other genes was in trans [9].
ASAR6 shares many physical and functional similarities with the large non-coding RNA gene Xist, which is located within the X inactivation center [10], [11]. For example, ASAR6 and Xist represent large non-coding RNA genes that display random mono-allelic expression, asynchronous replication, and they both control the expression of other mono-allelic genes in cis [9], [10]. In addition, deletion of the Xist gene from somatic cells isolated from adult mice results in a late replication phenotype that is similar to the DRT phenotype caused by disruption of ASAR6 [9], [12], [13]. Thus, the chromosomal phenotypes associated with ASAR6 disruption are remarkably similar to the phenotypes associated with disruption of Xist in adult somatic cells (see Table 1) [8], [9], [13].
Tab. 1. Phenotypes associated with gene disruption. 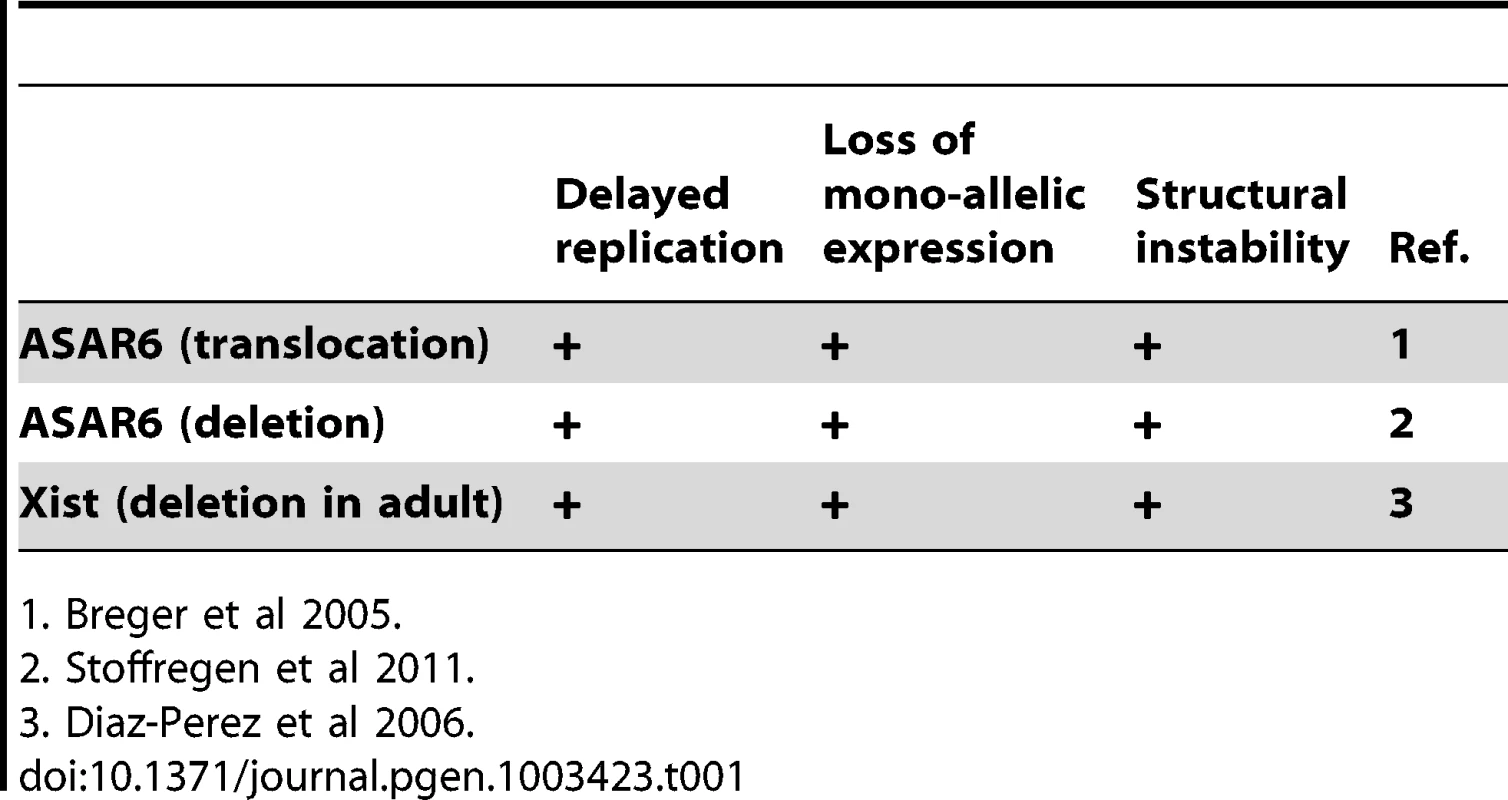
1. Breger et al 2005. In this report we mapped the domain of asynchronous replication surrounding ASAR6, and found that it resides within an ∼1.2 mb domain containing five other protein coding and non-coding genes at 6q16.1. In contrast to other nearby mono-allelic genes, ASAR6 is expressed from the later replicating allele, which is another characteristic shared with Xist [14]. We also found that the asynchronous replication of ASAR6 is also coordinated with other asynchronously replicating loci on the short arm of chromosome 6, indicating that the coordination in replication timing extends across the centromere. In addition, we found that ASAR6 RNA is transcribed by RNA Polymerase II, even though it is not spliced nor polyadenlyated. Furthermore, we found that chromosomes with a disruption of ASAR6 appear as bridged chromosomes between daughter nuclei, are often found in micronuclei, and experience frequent secondary rearrangements, indicating that ASAR6 functions to maintain the structural integrity of chromosome 6 via a cis acting mechanism. Finally, we found that ectopic integration of cloned genomic DNA containing ASAR6 can cause delayed replication of entire mouse chromosomes.
Results
ASAR6 resides within an ∼1.2 mb domain of asynchronous replication
Mono-allelic expression with random choice between maternal and paternal alleles defines genes subject to X-inactivation and ∼5–10% of autosomal genes [10], [15], [16]. One defining characteristic of mono-allelically expressed genes is asynchronous replication timing between alleles [17], [18], [19], [20]. We previously found that ASAR6 displays asynchronous replication in primary human fibroblasts, and that three genes located near ASAR6 also display asynchronous replication [9]. In addition, the asynchronous replication of ASAR6 and these nearby genes is coordinated in cis. However, the boundaries of this asynchronously replicating domain were not defined in this earlier study. Therefore, to characterize this cis-coordinated domain of asynchronous replication timing further, we examined the extent of asynchronous replication of loci extending centromeric and telomeric from ASAR6. For this analysis we used a “single dot-double dot” FISH assay [21] on primary human skin fibroblasts. This assay utilizes a methanol/acetic acid fixation, which destroys the nuclear structure and allows for a relatively accurate analysis of replication timing [19], [22]. Using a probe to a particular chromosomal site, some cells display two single hybridization dots, indicating that neither allele has replicated (a ss pattern), other cells display two double dots, indicating that both alleles have replicated (a dd pattern), and a third class of cells contains one single dot and one double dot (a sd pattern), indicating that only one of the two alleles has replicated (see Figure 1A–1C). For the analysis of the ASAR6 domain we used two-color FISH to examine two loci simultaneously and scored cells that presented the sd pattern for both loci. If the two loci show asynchronous replication that is coordinated in cis the double dots for both loci will be on the same chromosome [[17]; see Figure 1D–1G]. In contrast, if the two loci are coordinated in trans the double dots will be on opposite chromosomes [9]. Furthermore, if the two loci are not coordinated, the double dots for both loci will be on the same chromosome 50% of the time. Table 2 shows the results of our analysis and indicates that ASAR6 resides within an ∼1.2 megabase domain of cis-coordinated asynchronous replication surrounded by synchronously replicating DNA. Figure 1H illustrates the positions of the probes used during this analysis and the extent of asynchronous and synchronous replication surrounding ASAR6.
Fig. 1. Asynchronous replication of the ASAR6 domain. 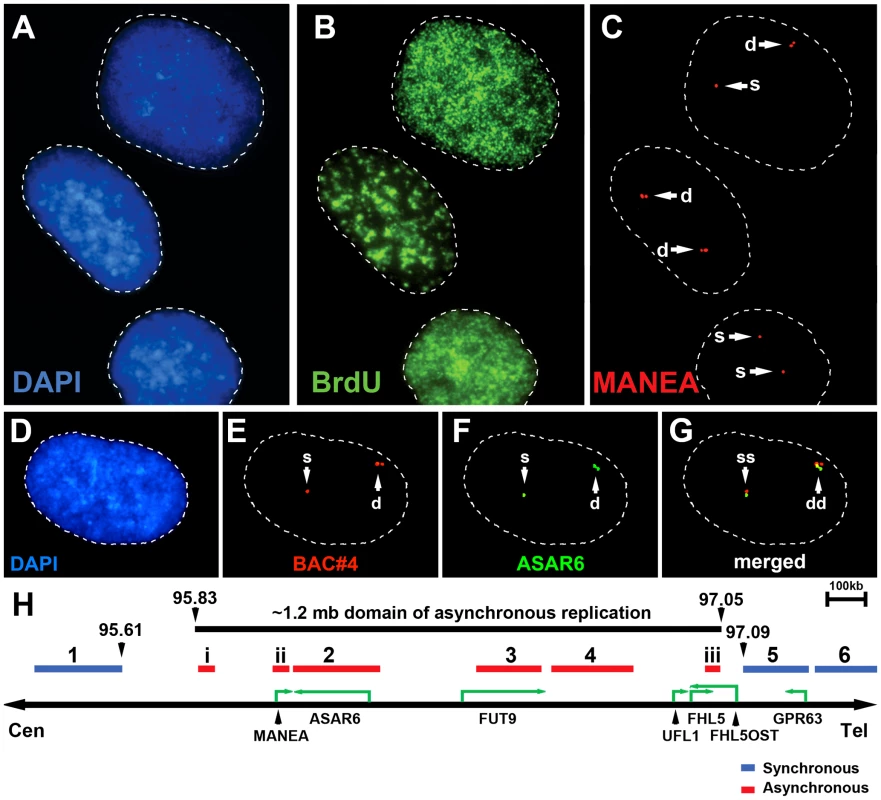
Low passage human skin fibroblasts were analyzed using the “single dot-double dot” assay [21]. A–C) Cells were processed for FISH using a Fosmid containing the MANEA gene (G248P8476B7) and for DNA synthesis using BrdU incorporation. A) DNA was stained with DAPI (blue). B) DNA synthesis was detected using an antibody against BrdU (green). C) DNA FISH was used to detect MANEA (red). Single dots (s) represent un-replicated loci and double dots (d) represent replicated loci. Approximately 35% of cells were BrdU positive. Arrows mark the location of the FISH signals. D–G) Two-color DNA FISH assay for coordination in asynchronous replication. Low passage primary human skin fibroblasts were processed for DNA FISH using BAC RP11-374I15 as probe (ASAR6, green in F and G) plus BAC RP11-959I6 (red in E and G, and BAC#4 in H). The percentage of cells that simultaneously displayed the sd pattern for both probes (ss/dd) was determined in 100 cells (see Table 2). A–G) Dashed lines show the outline of the nuclei. DNA was stained with DAPI (blue). Arrows mark the location of the DNA FISH signals. H) Schematic diagram of the ∼1.2 mb region of chromosome 6 that displays cis coordination in asynchronous replication. The positions of the boundaries between synchronous and asynchronously replicating probes are shown in megabases. The probes that showed asynchronous replication are shown in red and the probes that showed synchronous replication are shown in blue. BAC RP11-922A18 (1 in panel H), BAC RP11-374I15 (2 in panel H) BAC RP11-75N19 (3 in panel H), BAC RP11-959I6 (4 in panel H), BAC CTD 2183011 (5 in panel H), BAC CTC-385F17 (6 in panel H), Fosmid G248P85637C5 (i in panel H), G248P8476B7 (ii in panel H), or G248P86054G4 (iii in panel H). Green arrows mark the location and direction of transcription of the indicated genes. Tab. 2. Coordinated asynchronous replication timing by single-double assay. 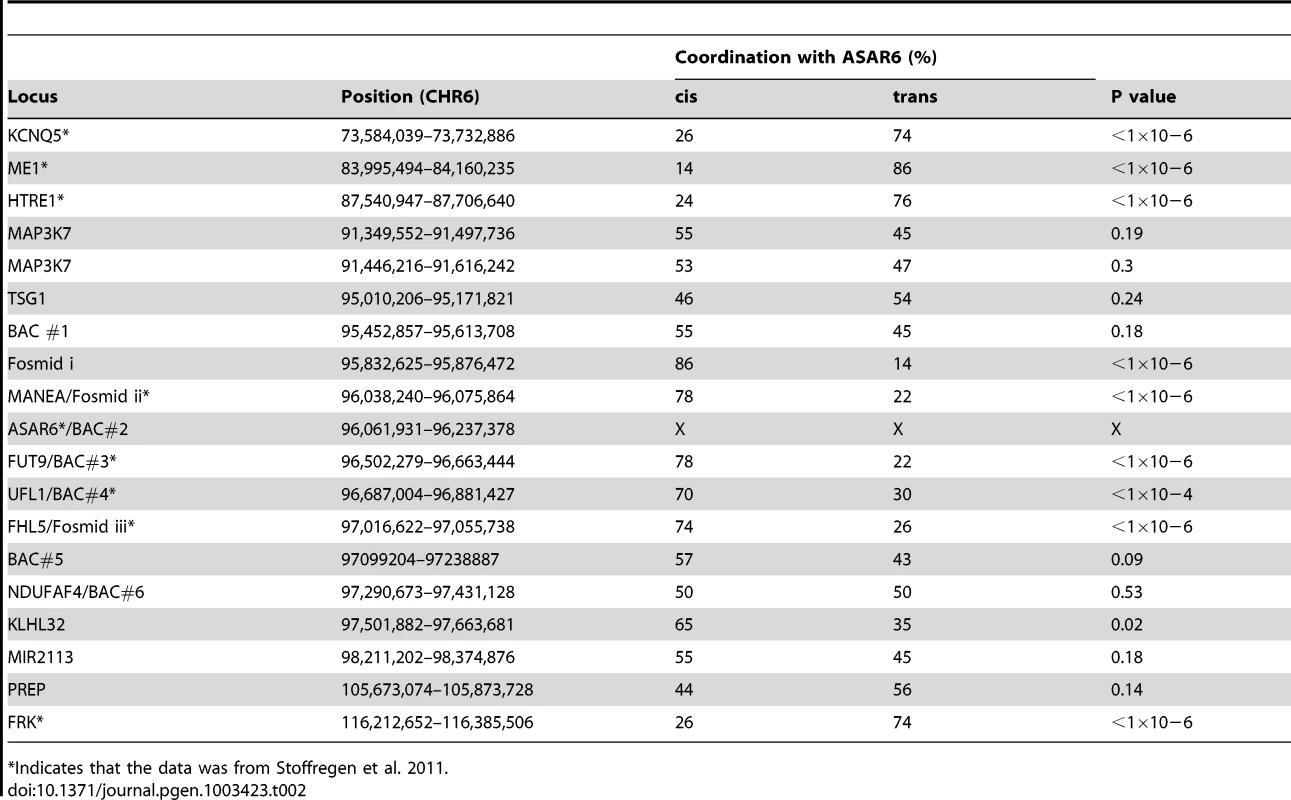
Indicates that the data was from Stoffregen et al. 2011. Asynchronous replication on chromosome 6 is coordinated in cis and in trans
One limitation of the “single dot-double dot” assay is that the asynchronous replication of loci greater than 50 mb apart are difficult to score, as a signal coming from the paternal allele of one locus may be closer to the maternal allele of the other locus. Therefore, to assay the random asynchronous replication of chromosome 6 loci at the whole chromosome level we utilized a second replication-timing assay known as Replication Timing-Specific Hybridization, or ReTiSH [23]. In the ReTiSH assay, cells are labeled with BrdU for different times and then harvested during metaphase (see Figure 2A). Regions of chromosomes that incorporate BrdU are visualized by a modification of Chromosome Orientation-Fluorescence In Situ Hybridization (CO-FISH), where the replicated regions (BrdU-labeled) are converted to single stranded DNA and then hybridized directly with specific probes [24]. Since metaphase chromosomes are analyzed for hybridization signals located on the same chromosome in metaphase spreads, the physical distance between the two loci is not a limitation of the ReTiSH assay [23]. To verify this methodology in our hands, we analyzed the hybridization patterns of ribosomal RNA genes. The rDNA clusters are located on human chromosomes 13, 14, 15, 21 and 22, and are known to display asynchronous replication between alleles [23]. In addition, to assay asynchronous replication in a second human cell type we used primary blood lymphocytes (PBLs) for this analysis. PBLs were exposed to BrdU for either 14 or 6 hours. As expected, we detected hybridization of an 18S rDNA probe to all 10 chromosomes at the 14 hour time point, but hybridization of this rDNA probe to only 5 chromosomes, representing single copies each of chromosomes 13, 14, 15, 21, and 22, at the 6 hour time point (Figure S1). Therefore, this assay allows us to identify early and late replicating chromosomes with respect to asynchronously replicating loci. Next, we used a two-color hybridization scheme to simultaneously detect ASAR6 and other asynchronous loci on chromosome 6. This assay also included a chromosome 6 centromeric probe to unambiguously identify both chromosome 6 s. Because centromeric heterochromatin is late replicating, centromeric probes hybridize to both copies of each chromosome at the 14 and 6 hour time points [23]. As observed with the “single dot-double dot” assay ([9]; and see Table 2 and Figure 1), we found that the asynchronous replication of ASAR6 was coordinated in cis with the closely linked genes FUT9 and UFL1 (Figure 2B and 2C; and Table 3). We also found that the asynchronous replication of ASAR6 was coordinated with the ME1 locus; however the coordination was in trans (Table 3). Thus, the earlier replicating ASAR6 allele is on the same chromosome as the later replicating ME1 allele, which was also seen previously using the “single dot-double dot” assay in primary fibroblasts [9]. In addition, we found that the asynchronous replication of ASAR6 was also coordinated in cis with two loci on the short arm of chromosome 6, the HLA locus and an olfactory receptor (OR) gene cluster (Figure 2D and 2E; and Table 3). To confirm the trans coordination along chromosome 6 using additional probes, we assayed the ME1 and HLA loci simultaneously using ReTiSH. As expected we found that the later replicating ME1 allele was on the same chromosome as the earlier replicating chromosome for the HLA locus, and vise versa (Figure 2F and 2G; and Table 3). These data indicate that human chromosome 6 contains loci that display asynchronous replication that is coordinated both in cis and in trans, and that some of these loci are separated by >87 megabases of genomic DNA and located on either side of the centromere (Figure S2).
Fig. 2. Coordinated asynchronous replication timing on chromosome 6. 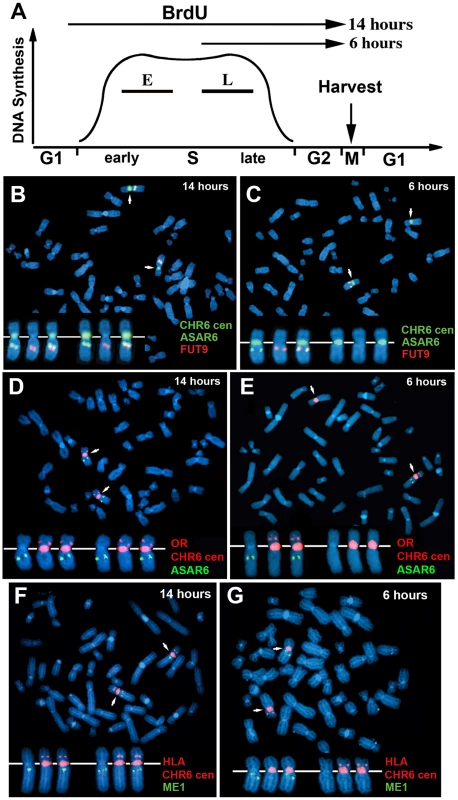
A) Schematic representation of the ReTiSH assay. PBLs were exposed to BrdU during the entire length of S phase (14 hours) or only during late S phase (6 hours). The ReTiSH assay can distinguish between alleles that replicate early (E) and late (L) in S phase. B–G) Mitotic spreads were hybridized with three different FISH probes. First, each ReTiSH assay included a centromeric probe to chromosome 6. Arrows mark the centromeric signals in each panel. Each assay also included BAC probes representing asynchronously replicating loci on chromosome 6. The insets in each panel show the two chromosome 6 s aligned at their centromeres. B and C) ReTiSH assay using an ASAR6 BAC (RP11-374I15; green) plus a FUT9 BAC (RP11-75N19; red). The ASAR6 and FUT9 BACs show hybridization signals to the same chromosome 6 at the 6 hour time point. D and E) ReTiSH assay using an ASAR6 BAC (RP11-374I15; green) plus a BAC (RP11-150A6; red) containing the olfactory receptor genes OR14J1, OR5V1, OR12D3 and OR12D2. The ASAR6 BAC and the OR BAC show hybridization signals to the same chromosome 6 s at the 6 hour time point. F and G) ReTiSH assay using an HLA BAC (RP11-166F24; red) plus a ME1 BAC (RP11-703G14; green). HLA and ME1 show hybridization signals on the opposite chromosome 6 s at the 6 hour time point, indicating that HLA and ME1 display trans coordination of asynchronous replication. Tab. 3. Coordinated asynchronous replication timing by ReTiSH. 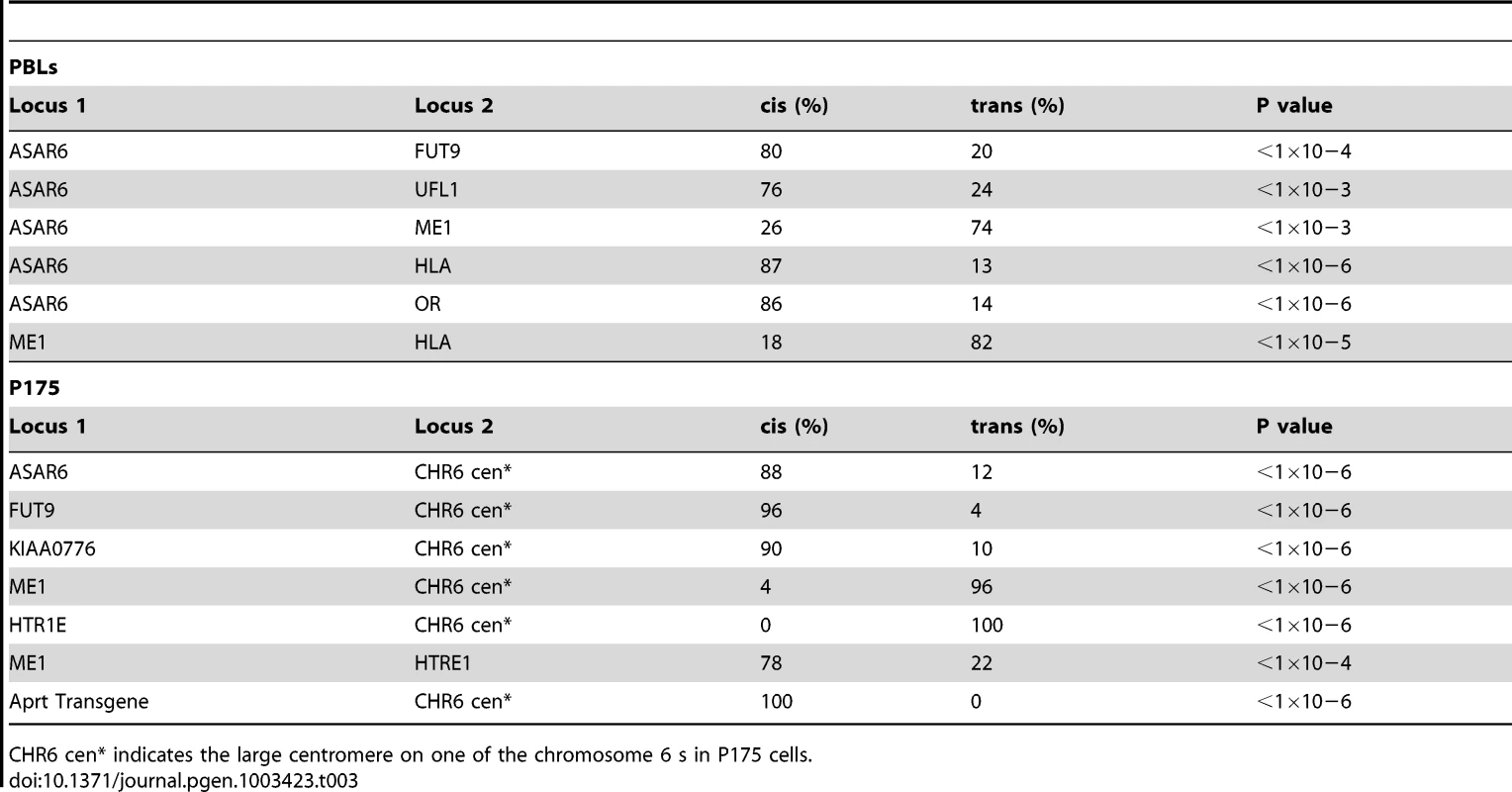
CHR6 cen* indicates the large centromere on one of the chromosome 6 s in P175 cells. The expressed allele of ASAR6 replicates after the silent allele
All mono-allelically expressed genes share the property of asynchronous replication. Typically, the earlier-replicating alleles of these mono-allelically expressed genes are the expressed alleles. However, one unusual characteristic of the XIST gene is that the silent allele on the active X chromosome replicates before the expressed allele on the inactive X chromosome [14]. Therefore, to determine if ASAR6 shares this property with XIST we analyzed the asynchronous replication of ASAR6 in cells where the active and inactive alleles could be identified. For this analysis we used the ReTiSH assay in the clonal cell line P175, where ASAR6 expression is mono-allelic and the actively transcribed chromosome for ASAR6 contains a transgene insertion [9]. To confirm that the ReTiSH assay can detect asynchronous replication in P175 cells we first analyzed the hybridization patterns of the rDNA loci. As expected, we detected hybridization of an rDNA probe to all of the rDNA containing chromosomes at the 14 hour time point, but hybridization of the rDNA probe to only 5 chromosomes, representing single copies of chromosomes 13, 14, 15, 21, and 22, at the 5 hour time point (Figure S3A and S3B). In addition, P175 cells contain a centromeric polymorphism on chromosome 15, which allowed for an unambiguous distinction between the two homologs. Thus, using a centromeric probe to chromosome 15 we found that the rDNA probe hybridized to the smaller centromere containing chromosome 15 at the 5 hour time point, indicating that the chromosome with the smaller centromere contains the later replicating rDNA allele (Figure S3C and S3D). Therefore, the ReTiSH assay allows us to identify the earlier and later replicating alleles of loci on homologous chromosomes in P175 cells. Next, to identify the earlier and later replicating alleles of ASAR6 we used a similar two-color FISH assay to detect replication of ASAR6 plus other loci along chromosome 6. For this analysis we also took advantage of a chromosome 6 centromeric polymorphism, which allowed for an unambiguous distinction between the two chromosome 6 homologs in P175 cells. Table 3 shows the results of this analysis and indicates that the later replicating allele of ASAR6 (FISH positive at the 5 hour time point) is linked to the chromosome 6 with the larger centromere (Figure 3A and 3B; and Table 3). In addition, the larger chromosome 6 centromere is linked to the Aprt transgene (Figure 3C and 3D; and Table 3). Because the Aprt transgene is inserted in the chromosome 6 that expresses ASAR6 [9], we conclude that ASAR6 is expressed from the later replicating allele. A similar analysis of the asynchronous replication timing of the protein-coding gene FUT9 indicated that the later replicating allele is also linked to the larger centromere of chromosome 6 (Figure 3E and 3F; and Table 3). These data indicate that the asynchronous replication of the closely linked genes ASAR6 and FUT9 is coordinated in cis in P175 cells. However, because FUT9 is expressed from the opposite chromosome as ASAR6 [9], FUT9 is expressed from the earlier replicating allele. Furthermore, ReTiSH assays on additional asynchronous loci indicated that asynchronous replication along chromosome 6 is coordinated both in cis and in trans in P175 cells (Figure 3E–3H; Figure S3E and S3F; and Table 3), and these data are consistent with our observations in primary skin fibroblasts (Figure 1H, Table 2; and [9]) and PBLs (Figure S2; and Table 3).
Fig. 3. ASAR6 is expressed from the later replicating allele. 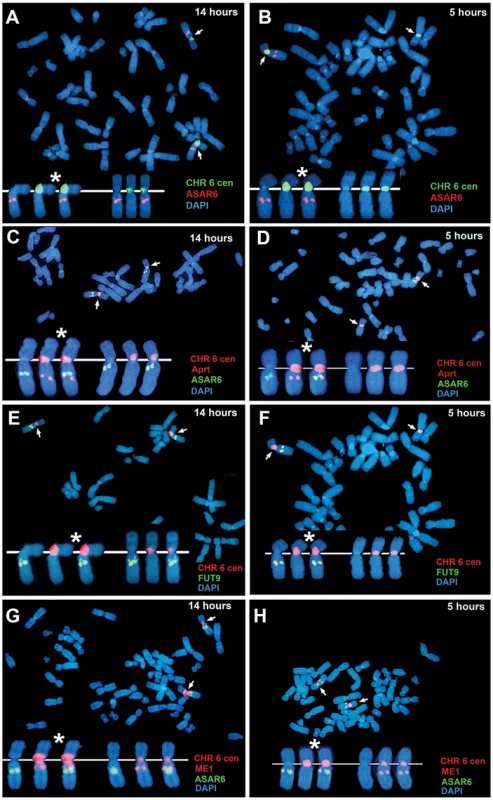
P175 cells were exposed to BrdU for 14 or 5 hours. Cultures were harvested for mitotic cells and processed for ReTiSH. A–H) Mitotic spreads were hybridized simultaneously with either two or three different FISH probes. First, each ReTiSH assay included a chromosome 6 centromeric probe, which hybridizes to both chromosome 6 s at both time points. Arrows mark the centromeric signals in each panel. P175 cells contain a chromosome 6 centromeric polymorphism so that one of the chromosomes displays a much larger FISH signal (*). Each assay included BAC probes representing asynchronously replicating loci on chromosome 6. The insets in each panel show the two chromosome 6 s aligned at their centromeres. A and B) ReTiSH assay using an ASAR6 BAC (RP11-374I15; red) plus a chromosome 6 centromeric probe (green). The ASAR6 BAC hybridized to the chromosome 6 with the larger centromere at the 5 hour time point. C and D) ReTiSH assay using an ASAR6 BAC (RP11-374I15; green) plus a probe to the Aprt transgene (red), which is integrated into only one of the chromosome 6 s [9]. The Aprt transgene is present on the chromosome 6 with the larger centromeric signal (and see Table 3). E and F) ReTiSH assay using a FUT9 BAC (RP11-75N19; green) plus a chromosome 6 centromeric probe (red). The FUT9 BAC hybridized to the chromosome 6 with the larger centromere at the 5 hour time point. G and H) ReTiSH assay using an ASAR6 BAC (RP11-374I15; green) plus a ME1 BAC (RP11-703G14; red). ASAR6 and ME1 show hybridization signals on the opposite chromosome 6 s at the 5 hour time point. ASAR6 is mono-allelically expressed in primary blood lymphocytes
We previously detected mono-allelic expression of ASAR6 in immortalized human lymphoblastoid cell lines, which involved the isolation of proliferating clones derived from single cells [9]. These clonal cell lines expressed either the maternal or the paternal allele of ASAR6, indicating that ASAR6 is subject to random mono-allelic expression. In order to determine if ASAR6 is mono-allelically expressed in primary human tissues, we used RNA-DNA FISH to assay expression of ASAR6 in primary cultures of human PBLs. We detected single sites of RNA hybridization in >95% of cells expressing ASAR6 (Figure 4A–4I; and see Figure S4 for each probe in separate images). In addition, the hybridization signal detected from ASAR6 is limited to a relatively small region of the nucleus, indicating that ASAR6 RNA does not coat chromosome 6 in PBLs.
Fig. 4. Mono-allelic expression of ASAR6 in human PBLs. 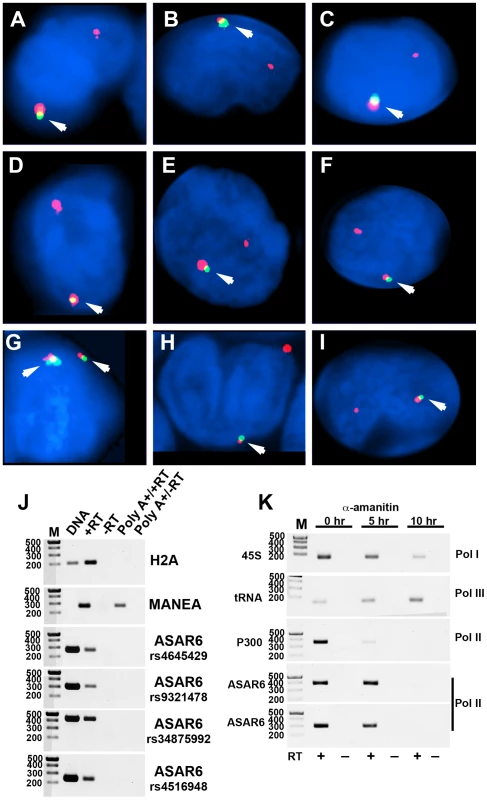
A–I). RNA-DNA FISH for expression of ASAR6. PBLs were subjected to RNA FISH (green) using a Fosmid (G248P86031A6) probe for ASAR6. Slides were subsequently re-fixed and processed for DNA FISH (red) using BAC RP11-959I6, located distal to FUT9 (BAC#4 in Figure 1H). In regions of the slide where the FISH worked well, the RNA FISH probe detected a positive signal in >80% of the cells. DNA was stained with DAPI. Arrows mark the location of the RNA signals. Two sites of RNA hybridization were detected in <5% of cells (see Figure S4 for each probe in separate images). Panel G shows an example of bi-allelic expression of ASAR6. J) ASAR6 RNA is not polyadenlylated. Total RNA extracted from P175 cells was subjected to two rounds of Poly A selection followed by reverse transcriptase reactions (RT) in the presence (+) or absence (−) of reverse transcriptase followed by semi-quantitative PCR using primers to histone H2A, MANEA, or ASAR6. Genomic DNA was used as positive control. The primers used to detect MANEA cDNA span an intron and therefore do not result in a product in the genomic DNA lane. Four independent PCRs, from four different regions of ASAR6 (each spanning a different SNP; rs4645429, rs9321478, rs34875992, rs4516948) were used to detect ASAR6 RNA. The sizes of the DNA ladder (M) are indicated in base pairs. K) ASAR6 is transcribed by RNA Polymerase II. P175 cells were exposed to 20 ug/mL of α-amanitin for 0, 5, and 10 hours. Total RNA was subjected to reverse transcriptase reactions (RT) in the presence (+) or absence (−) of reverse transcriptase followed by semi-quantitative PCR using primers to 45S RNA, a tRNA, P300 and ASAR6. The sizes of the DNA ladder (M) are indicated in base pairs. Two independent PCRs, from two different regions of ASAR6 (associated with SNPs rs34875992 [top] and rs9321478 [bottom]) were used to detect ASAR6 RNA. During our original characterization of ASAR6 RNA, using RT-PCR assays with primers that spanned many different regions of ASAR6 covering ∼200 kb of genomic DNA, we failed to detect any evidence for the presence of introns ([9]; and data not shown). In addition, to determine if ASAR6 RNA is polyadenylated we subjected total RNA to two rounds of Poly A selection and subsequently assayed ASAR6 RNA by RT-PCR. Figure 4J shows that ASAR6 RNA was not detected in the Poly A+ fraction. Similarly, RNA expressed from the non-polyadenylated RNA for histone H2A was also not detected in the Poly A+ fraction. In contrast, RNA from the protein-coding gene MANEA was detected in the Poly A+ fraction. Therefore, ASAR6 RNA is not polyadenylated. Furthermore, cell fractionation studies indicated that ASAR6 RNA is restricted to the nuclear compartment (E.P.S. and M.J.T. data not shown), which is consistent with our RNA-DNA FISH analysis (Figure 4A–4I; and [9]). Consistent with these observations, RNA-seq data from the Encode project found that expression of ASAR6 RNA is enriched in the nuclear Poly A - fraction [[25]; and see Figure S5B and S5C].
The observations described above suggest that ASAR6 RNA does not contain introns nor does it contain a Poly A tail, which raises the question of which RNA polymerase is responsible for transcribing ASAR6. Therefore, to determine if ASAR6 RNA is the product of RNA Polymerase II we treated cells with α-amanitin, which is a selective inhibitor of RNA Polymerase II [26], and assayed expression of ASAR6 RNA using a semi-quantitative RT-PCR assay. The results of this analysis are shown in Figure 4K, and indicate that ASAR6 RNA is indeed sensitive to α-amanitin. Similarly, RNA expressed from the protein-coding gene P300 is also sensitive to α-amanitin treatment. In contrast, expression of 45S RNA (an RNA Polymerase I product) and a tRNA (an RNA Polymerase III product) was not inhibited by α-amanitin. We conclude that ASAR6 is transcribed by RNA Polymerase II. In addition, this analysis indicated that the half-life of ASAR6 RNA is approximately 5 hours, and is much longer than the half-life of P300 RNA (∼2 hours), which is a spliced and polyadenylated RNA Polymerase II product. Thus, ASAR6 RNA represents an unusual RNA Polymerase II product that it is not spliced, not polyadenlyated, yet is not rapidly degraded [27].
Delineation of the critical sequences of ASAR6 required for replication delay of chromosome 6
We previously found that nested deletions in chromosome 6, originating from an integrated loxP cassette in the P175 cell line, resulted in delayed replication timing of chromosome 6 [9]. This analysis indicated that deletions ranging in size from ∼30 mb to as small as ∼76 kb resulted in delayed replication. Interestingly, all of the deletions that cause delayed replication result in the removal of the 5′ region of the ASAR6 gene (see Figure S5A). Therefore, we next determined if a smaller deletion, which would leave the 5′ end of ASAR6 intact, would result in DRT. For this analysis we used homologous recombination, using a rAAV gene targeting strategy [28], to introduce a second loxP site into chromosome 6 of P175 cells. Subsequent Cre expression resulted in the deletion of ∼47 kb upstream of ASAR6 (see Figure S6).
We next assayed the replication timing of the chromosome 6 s present in two independent isolates of cells containing this ∼47 kb deletion in chromosome 6. Cultures were incubated with BrdU for 4.5 hours and mitotic cells were harvested, processed for BrdU incorporation and subjected to FISH using a chromosome 6 paint as probe. Comparing the BrdU incorporation patterns between chromosome 6 s in multiple cells indicated that the two copies of chromosome 6 incorporated similar amounts of BrdU, indicating that the chromosome 6 s containing this relatively small deletion retain normal replication timing (Figure 5A–5D). In contrast, a similar analysis of cells containing a larger ∼76 kb deletion showed a large difference in BrdU incorporation, consistent with a delay in replication timing of >2 hours of one of the chromosome 6 s (Figure 5E–5H). Therefore, these two deletions define the critical region required for the DRT phenotype of chromosome 6, and represents ∼29 kb of genomic DNA containing the 5′ region of ASAR6 (see Figure S5A).
Fig. 5. Delineation of the critical region required for DRT on chromosome 6. 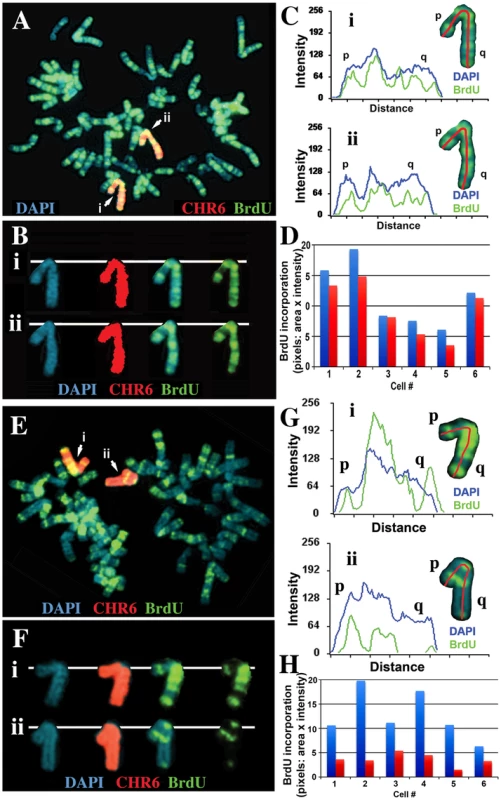
Cells containing either an ∼47 kb deletion (ΔAAV-1d) or an ∼76 kb deletion (Δ175-23a) at the ASAR6 locus (see Figure 7A and S5A) were incubated with BrdU for 4.5 hours, harvested, and processed for FISH using a chromosome 6 paint (red) as probe and for BrdU incorporation using an antibody against BrdU (green). The DNA was stained with DAPI (blue). A) An example of this analysis from the ΔAAV-1d (∼47 kb deletion). The two chromosome 6 s are indicated with arrows, and arbitrarily assigned i or ii. B) The two chromosome 6 s from panel A, were cut out and aligned with each color displayed separately or in combination. C) Pixel intensity profiles of the BrdU incorporation (green), and DAPI (blue) staining along the two chromosome 6 s from panel A. D) Quantification of the BrdU incorporation in chromosome 6 s. The red and blue bars represent the two chromosomes identified by the chromosome 6 paint in six different cells. E) An example of this analysis from the Δ175-23a cells (∼76 kb deletion). The two chromosome 6 s are indicated with arrows, and arbitrarily assigned i or ii. F) The two chromosome 6 s from panel E, were cut out and aligned with each color displayed separately. G) Pixel intensity profiles of the BrdU incorporation (green), and DAPI (blue) staining along the two chromosome 6 s from panel E. H) Quantification of the BrdU incorporation in chromosome 6 s. The red and blue bars represent the two chromosomes identified by the chromosome 6 paint in six different cells, the late replicating chromosome contains a deletion of ∼76 kb [9]. Disruption of ASAR6 results in instability of chromosome 6
We previously found that chromosomes with DRT/DMC cause a 30–80 fold increase in the rate at which secondary rearrangements occur on the affected chromosome, indicating that DRT/DMC causes genomic instability [8]. During the characterization of chromosomes containing deletions of ASAR6 we noticed numerous secondary rearrangements affecting chromosome 6. Figure 6A and 6B show examples of these secondary rearrangements in cells with an engineered deletion of ASAR6. In addition, chromosome 6 was occasionally observed as a bridged chromosome between two daughter nuclei (Figure 6C). Secondary rearrangements and bridged chromosomes involving chromosome 6 were not detected in cultures of P175 cells or in subclones of P175 cells in the absence of ASAR6 disruption (not shown; and see [8]). In addition, we found that cells containing a deletion of the ASAR6 gene show a 10 fold increase in the frequency of micronuclei that hybridize to a chromosome 6 paint probe [0.5% (5/1000) of cells prior to deletion and 5.0% (50/1000) after deletion] (Figure 6D–6G). Furthermore, we also detected micronuclei that did not hybridize with the chromosome 6 paint in cells that also contained chromosome 6 positive micronuclei (Figure 6F and 6G). Because chromosome 6 DNA is often translocated to other chromosomes in these ASAR6 deleted clones (Figure 6A and 6B), and these secondary rearrangements can also display DRT/DMC [8], we could not determine if the micronuclei that do not hybridize with the chromosome 6 paint (Figure 6G) did not originate from one of these secondary rearrangements with DRT/DMC. Regardless, these data indicate that DNA from other chromosomes within the same cells as a DRT/DMC chromosome can also be segregated into micronuclei. These observations indicate that ASAR6 functions to maintain structural integrity and proper mitotic segregation of chromosome 6 via a cis acting mechanism, and that the instability induced by DRT/DMC on one chromosome can affect the stability of other chromosomes within the same cell.
Fig. 6. Disruption of ASAR6 leads to instability of chromosome 6. 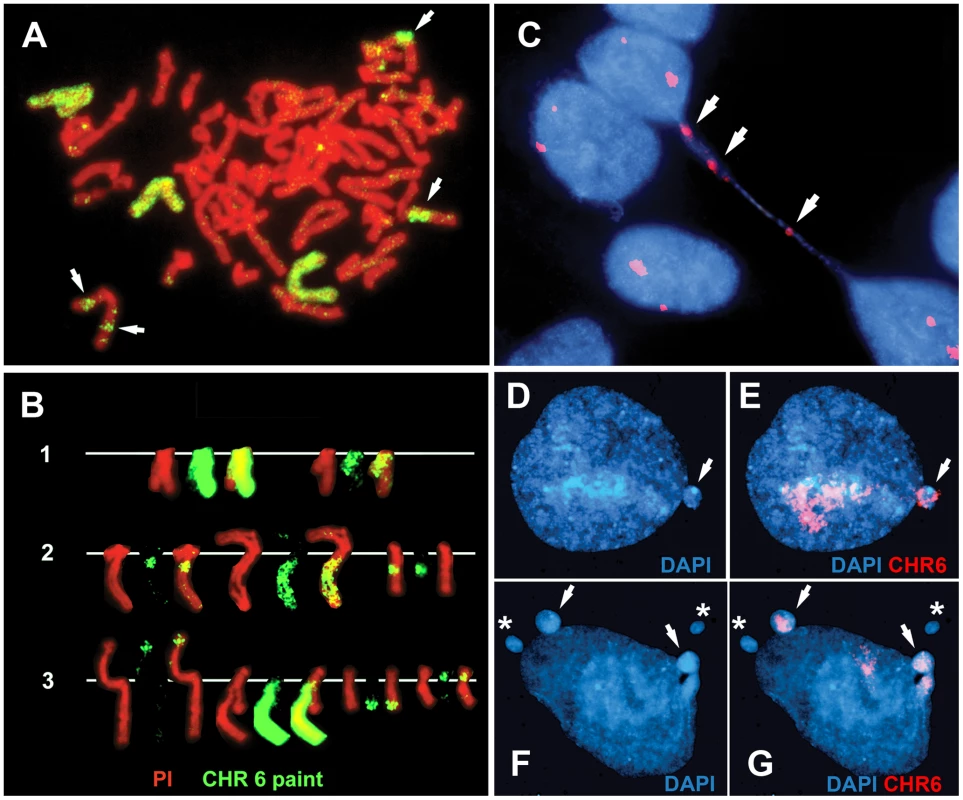
(A) An example of a mitotic spread containing three different translocations involving chromosome 6. Cells containing an engineered deletion of ASAR6 were processed for FISH using a chromosome 6 paint as probe (green). The DNA was stained with propidium iodide. The arrows mark the locations of three different rearrangements involving chromosome 6. B) Partial karyotypes from three different mitotic spreads (1–3). The DNA was stained with propidium iodide. Notice that each cell contained different rearrangements involving chromosome 6. C) An example of a bridged chromosome between two daughter nuclei. Cells containing a disruption of ASAR6 were processed for FISH using a chromosome 6 centromeric probe (red). The DNA was stained with DAPI. Arrows mark the sites of hybridization to the chromosome 6 centromeric probe on the bridged chromosome. Chromosome bridges involving chromosome 6 were detected in approximately 1/500 metaphase spreads. D–G) Examples of micronuclei derived from chromosome 6. Cells containing a deletion of ASAR6 were processed for FISH using a chromosome 6 paint as probe (red). The DNA was stained with DAPI. Arrows mark micronuclei that hybridized to the chromosome 6 probe. Asterisks mark micronuclei that did not hybridize to the chromosome 6 paint. Ectopic integration of an ASAR6 transgene delays replication timing of mouse chromosomes
One well-characterized activity of the Xist gene is its ability to delay DNA replication timing in cis when ectopically integrated into chromosomes [reviewed in [29]. This activity is not restricted to ES cells, as ectopic integration of either human or mouse XIST/Xist into the chromosomes of differentiated mammalian cell lines can delay replication of entire chromosomes [30], [31], [32], [33]. Therefore, to determine if ASAR6 also displays this activity we tested whether ectopic integration of cloned genomic DNA from the ASAR6 locus can cause delayed replication timing of mouse chromosomes. For this analysis we used a BAC that contains ∼180 kb of genomic DNA spanning the critical region of the ASAR6 locus required for DRT (see Figure 7A and Figure S5A). Prior to transfection, the BAC was modified by recombineering [34] to contain a Hygromycin B resistance gene to enable positive selection in mammalian cells. Mouse cells were transfected with the modified BAC and subjected to selection in media containing Hygromycin B. Individual clones were isolated and analyzed for BAC integration sites, BAC copy number, and replication timing of the affected chromosome.
Fig. 7. Ectopic integration of ASAR6 results in delayed replication of mouse chromosomes. 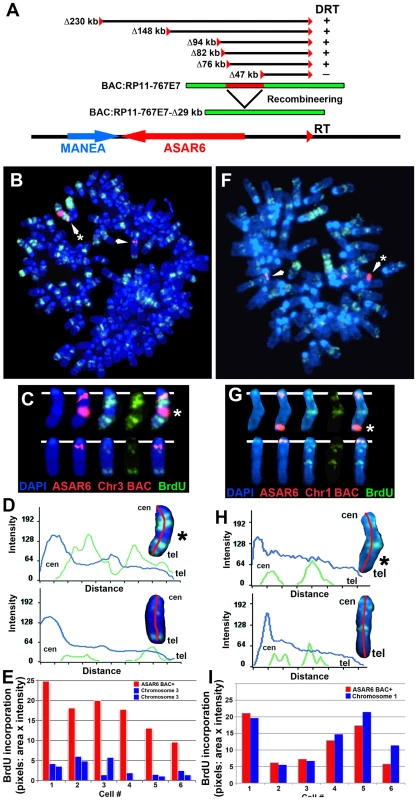
A) Schematic representation of the ASAR6 BAC (RP11-767E7) used for integration into mouse chromosomes. The approximate locations of the loxP integration sites (red triangles) in P175 cells, deletions [in kilobases (Δkb)], and the location of the ASAR6 BAC (green) are indicated. The ASAR6 BAC was modified using recombineering to contain a deletion of the ∼29 kb (red) critical region identified by our deletion analysis. B–E) Cells containing a multicopy array of the ASAR6 BAC integrated into mouse chromosome 3 were incubated with BrdU for 2.5 hours, harvested for mitotic cells, and processed for FISH using the ASAR6 BAC (RP11-767E7) plus a mouse chromosome 3 BAC (RP23-430A13) as probes (both in red), and for BrdU incorporation using an antibody against BrdU (green). The DNA was stained with DAPI (blue). The chromosome 3 s are indicated by arrows. C) The chromosome 3 s from panel B, were cut out and aligned with each color displayed separately or in combination. D) Pixel intensity profiles of the BrdU incorporation (green), and DAPI (blue) staining along the chromosome 3 s from panel B are shown. Note that the chromosome 3 with the ASAR6 BAC integration (*) contains much more BrdU incorporation, indicating that it displays delayed replication. E) Quantification of the BrdU incorporation in six different cells. The red bars represent the chromosome containing the ASAR6 BAC and the blue bars represent the normal chromosome 3 s in six different cells. Most of the cells in this clone contained three chromosome 3 s. The values represent the total number of pixels (area×intensity)×1000. F–I) Cells containing a multicopy array of the ASAR6 BAC containing an ∼29 kb deletion (see panel A, RP11-767E7-Δ29 kb) integrated into mouse chromosome 1 were incubated with BrdU for 3.0 hours, harvested for mitotic cells, and processed for FISH using the ASAR6 BAC (RP11-767E7) plus a mouse chromosome 1 BAC (RP23-34K7) as probes (both in red), and for BrdU incorporation using an antibody against BrdU (green). The DNA was stained with DAPI (blue). The chromosome 1 s are indicated with arrows. The chromosome with the ASAR6 BAC is marked with an asterisk (*). G) The chromosome 1 s from panel F, were cut out and aligned with each color displayed separately or in combination. H) Pixel intensity profiles of the BrdU incorporation (green), and DAPI (blue) staining along the chromosome 1 s from panel F are shown. Note that the chromosome 1 containing the ASAR6 BAC (Δ29 kb) shows similar BrdU incorporation as the normal chromosome 1. I) Quantification of the BrdU incorporation in six different cells. The red bars represent the chromosome containing the ASAR6 BAC (Δ29 kb) and the blue bars represent the normal chromosome 1 s in six different cells. The values represent the total number of pixels (area×intensity)×1000. Figure 7B shows the replication timing analysis of a clone containing a multicopy array (∼20 copies) of the ASAR6 BAC integrated into mouse chromosome 3. Cultures were incubated with BrdU for 2.5 hours and mitotic cells were harvested, processed for BrdU incorporation and subjected to FISH using a mouse chromosome 3 BAC (located near the centromere) plus the ASAR6 BAC as probes. The FISH signal from the chromosome 3 centromeric region allowed us to identify all of the chromosome 3 s, and the presence or absence of the ASAR6 BAC allowed us to distinguish between the integrated and non-integrated chromosomes, respectively. Comparing the BrdU incorporation pattern between chromosome 3 s in multiple cells indicated that the chromosome containing the ASAR6 BAC was delayed in replication timing (Figure 7B–7E). Delayed replication was also detected in a second clone containing ectopic integration of the ASAR6 BAC into a different mouse chromosome (data not shown), indicating that integration of the ASAR6 BAC into different mouse chromosomes results in delayed replication.
Our chromosome engineering studies created a set of nested deletions at the ASAR6 locus and defined an ∼29 kb region of ASAR6 DNA, that when deleted, results in DRT/DMC on human chromosome 6 (see Figure 7A and Figure S5A). To determine if this critical region is also required for delayed replication following ectopic integration, we deleted this ∼29 kb region from the ASAR6 BAC (see Figure 7A) using recombineering strategies and reintroduced the deleted BAC into mouse chromosomes. Figure 7F–7I shows the results of the replication timing analysis on a clone containing ∼20 copies of the deleted BAC integrated into mouse chromosome 1. This analysis indicated that the integrated chromosome 1 does not display delayed replication. An analysis of similar integrations of the deleted BAC into three other mouse chromosomes similarly did not display delayed replication (data not shown). In total, we detected delayed replication in 2 out of 3 ectopic integrations of the intact ASAR6 BAC and in 0 out of 4 ectopic integrations of the deleted BAC. While it is not possible to conclude that the BAC with the ∼29 kb deletion cannot induce delayed replication upon ectopic integration, especially with the limited number of integrations assayed, our observations suggest that the ∼29 kb critical region of ASAR6 is necessary to prevent DRT in its native location on chromosome 6 and is sufficient to cause DRT when integrated at ectopic locations.
Discussion
Mammalian cells replicate their genomes every cell cycle during a defined replication-timing program. It is clear that the determinants of replication timing are not encoded within the sequence of the origins of replication, but rather the timing of origin firing is dictated by chromosomal location [35], [36]. Recent studies indicate that at least half of the genome is subject to changes in the temporal sequence of DNA replication during development [37], [38]. The current thinking is that replication timing is directly linked to complex higher-order features of chromosome architecture [39], [40]. However, both the mechanisms and the significance of this temporal replication program remain poorly defined.
Asynchronous replication represents an epigenetic state that is established early in development, and is not dependent on gene expression [17], [19], [20]. An additional feature of many autosomal mono-allelic genes is that they do not appear separately in the genome, but rather they appear in groups that occupy relatively large chromosomal domains [18]. We found that ASAR6 resides within an ∼1.2 mb domain of cis-coordinated asynchronous replication timing, which includes at least five other genes located at 6q16.1. In addition, we found that ASAR6 is expressed from the later replicating allele, which is in contrast to other mono-allelically expressed genes located within this same domain.
Previous studies have shown that most if not all of the genes residing on the inactive X chromosome display late replication [14], [41], [42], [43], indicating that the asynchronous replication of X linked genes in female cells is coordinated in cis. The observations described here are consistent with previous reports showing that, like X chromosomes, autosome pairs also display coordination in their asynchronous replication of random mono-allelically expressed genes [17], [20], [23]. In this report we found that human chromosome 6 displays coordinated asynchronous replication between loci separated by >87 mb of genomic DNA, and between loci on either side of the centromere. However, we found that chromosome 6 contains loci that display both cis and trans coordination of asynchronous replication. Another important feature of asynchronous replication is that it can be observed in all cell types. Thus, we found that chromosome 6 displays both cis and trans coordination of asynchronously replicating loci in two different human cell types, primary skin fibroblasts and PBLs [also see [9]]. These observations represent the first example of a mammalian chromosome displaying both cis and trans coordination of asynchronous replication.
In general, early replicating regions of the genome are correlated with transcriptional activity. While parallels between X inactivation and the cis coordinated replication asynchrony of autosomal mono-allelic genes have been made [17], [18], [20], [23], [44], [45], previous reports found that not all random mono-allelically expressed genes are expressed from the same homolog [15], [16], [46]. One obvious presumption from these studies is that the later replicating alleles of some of these mono-allelic genes represent the actively transcribed alleles. However, the asynchronous replication of these ‘non-coordinated’ mono-allelically expressed genes was not assayed in these earlier studies. Therefore, our results suggest a possible explanation for the apparent ‘non-coordinated’ expression pattern of autosomal mono-allelic genes. Thus, it is possible that all autosomes display a pattern of cis and trans coordination in asynchronous replication similar to human chromosome 6. Therefore, the apparent non-coordinated expression pattern of some autosomal mono-allelic genes may still be correlated with earlier and later replicating alleles. Therefore, a detailed analysis of the coordinated asynchronous replication in combination with allele-specific expression assays within the same cells is required to determine if mono-allelic expression is indeed originating from the later replicating alleles.
The observation of trans coordination of asynchronous replication on chromosome 6 indicates that there is a reciprocal relationship between the asynchronous replication of certain chromosome 6 loci so that their earlier replicating alleles are always on the same homolog as the later replicating alleles for other loci. This reciprocal relationship appears to affect the entire chromosome, as trans coordination is observed with loci that are on either side of the chromosome 6 centromere. The reason for this reciprocal relationship between these asynchronously replicating loci is currently not known. One possibility is that asynchronous replication of certain genes may be the consequence of cis acting chromosomal elements that reside within the same chromosome domain. These cis acting elements may have functions, e.g. regulating chromosome-wide replication timing, that don't involve transcriptional regulation directly but do require asynchronous replication for their activity. Thus, a gene located within the same domain as one of these cis acting elements may simply be responding to the late replication of the domain, and consequently transcriptional silencing on one allele may be a secondary affect of the epigenetic state associated with the later replicating domain.
Previous work indicates that the ASAR6 and Xist genes share many characteristics, including: 1) they both express large non-coding RNAs, 2) they both display random mono-allelic expression, 3) they both display asynchronous replication that is coordinated with other linked mono-allelic genes, 4) disruption of either gene results in delayed replication timing and instability of entire chromosomes in cis, and 5) disruption of either gene results in the transcriptional activation of the previously silent alleles of linked mono-allelic genes [9], [10], [12], [13]. In addition, in this report we found that ASAR6 is expressed from the later replicating allele, which is also a feature of Xist [14]. Furthermore, another well-characterized activity contained within the Xist gene is the ability to delay replication timing of entire chromosomes upon ectopic integration of cloned genomic DNA [reviewed in [29]]. In this report we found that ectopic integration of cloned genomic DNA containing ASAR6 also has the ability to delay replication of mouse chromosomes following ectopic integration. Furthermore, we found that the ability of ASAR6 transgenes to delay replication at ectopic locations occurred only when multiple copies of the transgene were integrated, which was also observed with Xist genomic transgenes [30], [33], [47], [48], [49]. However, there are also some notable differences between ASAR6 and Xist. For example, the Xist non-coding RNA physically “coats” the inactive X chromosome, while the ASAR6 RNA does not appear to coat chromosome 6 in adult somatic tissues [9]. In addition, Xist RNA is expressed in all female adult tissues (reviewed in [10], [11]), while expression of ASAR6 is limited to only a subset of adult tissues [9]. Furthermore, we found that even though ASAR6 RNA is transcribed by RNA Polymerase II it is not polyadenlyated and does not appear to contain introns, which is in contrast to the polyadenlyated and spliced Xist RNA [50]. Additional studies suggest that Xist RNA mediates silencing and late replication of the inactive X chromosome via direct interactions with chromatin associated RNA binding proteins [51], [52], [53]. Additional studies designed to interrogate whether or not ASAR6 RNA interacts with these RNA interacting proteins should reveal whether or not ASAR6 also shares these activities.
We previously found that chromosomes with DRT/DMC cause a 30–80 fold increase in the rate at which secondary rearrangements occur on the affected chromosome, indicating that DRT/DMC causes genomic instability [5], [8]. In addition, we found that chromosomes with DRT/DMC are delayed in their recruitment of Aurora B kinase, are frequently unattached to the mitotic spindle, activate the spindle assembly checkpoint, and are often seen as lagging chromosomes during mitosis [6]. In this report we found that disruption of ASAR6 leads to several abnormalities involving chromosome 6, including: chromosomal bridges between daughter nuclei, segregation into micronuclei, and numerous secondary rearrangements. Thus, we propose a model for the instability of individual chromosomes that includes: 1) delayed replication timing of individual chromosomes caused by genetic disruption of cis-acting loci (e.g. ASAR6 or Xist); 2) delayed recruitment of Aurora B kinase resulting in delayed mitotic chromosome condensation; 3) delayed mitotic spindle attachment leading to chromosome missegregation, bridged chromosomes, and the formation of micronuclei; 4) the onset of mitotic chromosome condensation prior to the completion of DNA synthesis leading to stalled replication forks; and 5) multiple rearrangements generated at the stalled replication forks via replicative mechanisms (Figure S7; also see [1]).
Recently, the phenomenon of “chromothripsis” was described as a new mechanism for generating complex chromosome rearrangements in cancer cells [54]. Chromothripsis appears to be a cataclysmic event in which one or a few chromosomes are fragmented and then reassembled in a haphazard manner. The authors of the original chromothripsis paper proposed that either exposure to IR or telomere dysfunction was responsible for the multiple rearrangements affecting individual chromosomes [54]. Alternatively, recent work from Dr. David Pellman's lab suggests that missegregation of chromosomes, caused by transient mitotic spindle disruption, can result in lagging chromosomes, micronucleus formation, late replication, and ‘pulverization’ of individual chromosomes [55]. In addition, the complex chromosome rearrangements associated with “genomic disorders” in humans were recently found to resemble chromothripsis [56], [57]. Sequencing the breakpoints at the complex rearrangements identified characteristic features, including small templated insertions of nearby sequences and microhomologies, suggestive of replicative processes [56]. Thus, sequencing the genomes of cells containing disruption of ASAR6 should reveal whether or not the mechanisms that are functioning to generate rearrangements on DRT/DMC chromosomes are similar or distinct from those responsible for chromothripsis-type rearrangements.
The DRT/DMC phenotype has been detected on chromosome rearrangements involving many different human and mouse chromosomes [5], [7], [8], [9], [13]. Therefore, it seems likely that all mammalian chromosomes contain loci that function to regulate chromosome-wide replication timing of individual chromosomes. Given the similarities in structure and function of the two loci characterized to date, Xist and ASAR6, we propose that all mammalian chromosomes contain ‘inactivation/stability centers’ that function to maintain proper replication timing, mitotic chromosome condensation, mono-allelic gene expression and stability of individual chromosomes. Under this scenario every mammalian chromosome contains four distinct types of cis-acting elements, origins of replication, centromeres, telomeres, and ‘inactivation/stability centers”, all functioning to ensure proper replication, segregation and stability of individual chromosomes.
Materials and Methods
Cell culture
Low passage primary human skin fibroblasts were obtained from ATCC and cultured in DMEM plus 10% fetal bovine serum (Hyclone). Primary blood lymphocytes were isolated after venipuncture into a Vacutainer CPT (Becton Dickinson, Franklin Lakes, NJ) per the manufacturer's recommendations and grown in 5 mL RPMI 1640 (Life Technologies) supplemented with 10% fetal bovine serum (Hyclone) and 1% phytohemagglutinin (Life Technologies). P175 cells are a human APRT deficient cell line derived from the HT1080 fibrosarcoma [58], and were grown in DMEM (Gibco) supplemented with 10% fetal bovine serum (Hyclone). P175 derivatives were grown as above with the addition of 500 mg/ml Geneticin (Gibco), 200 mg/ml Hygromycin B (Calbiochem), and/or 10 ug/ml Blasticidin S HCl (Invitrogen). The deletion-line derivatives were grown in DMEM supplemented with 10% dialyzed fetal bovine serum (Hyclone), 10 mg/ml azaserine (Sigma) and 10 mg/ml adenine (Sigma) to facilitate selection for Aprt-expressing cells. Mouse C2C12 cells were used for the BAC integration assays, and were grown in DMEM supplemented with 10% fetal bovine serum. Clones containing BAC integrations were isolated following selection in media containing 200 mg/ml Hygromycin B. All cells were grown in a humidified incubator at 37°C in a 5% carbon dioxide atmosphere.
DNA FISH
Trypsinized cells were centrifuged at 1,000 rpm for 10 minutes in a swinging bucket rotor. The cell pellet was re-suspended in 75 mM potassium chloride for 15–30 minutes at 37°C, re-centrifuged at 1,000 rpm for 10 minutes and fixed in 3∶1 methanol∶acetic acid. Fixed cells were added drop-wise to microscope slides to generate mitotic chromosome spreads using standard methods [59]. Slides with mitotic spreads were baked at 85°C for 20 minutes and then treated with 0.1 mg/ml RNAase for 1 hour at 37°C. After RNAase treatment, the slides were washed in 2×SSC (1×SSC is 150 mM NaCl and 15 mM sodium citrate) with 3 changes for 3 minutes each and dehydrated in 70%, 90%, and 100% ethanol for 3 minutes each. The slides were denatured in 70% formamide in 2×SSC at 70°C for 3 min and whole chromosome paints were used according to the manufacturer's recommendations and hybridization solutions (American Laboratory Technologies and Vysis). Detection of digoxigenin-dUTP probes utilized a three-step incubation of slides with sheep FITC-conjugated anti-digoxigenin antibodies (Roche) followed by rabbit FITC-conjugated anti-sheep antibodies (Roche) followed by goat FITC-conjugated anti-rabbit antibodies (Jackson Laboratories). Slides were stained with DAPI (12.5 mg/ml) or propidium iodide (0.3 mg/ml), cover slipped, and viewed under UV fluorescence with appropriate filters (Olympus).
Centromeric, BAC, and Fosmid probes: Mitotic chromosome spreads were prepared as described above. Slides were treated with RNase at 100 ug/ml for 1 h at 37°C and washed in 2×SSC and dehydrated in 70%, 90% and 100% ethanol. Chromosomal DNA was denatured at 75°C for 3 minutes in 70% formamaide/2×SSC, followed by dehydration in ice cold 70%, 90% and 100% ethanol. BAC and Fosmid DNAs were nick-translated using standard protocols to incorporate biotin-11-dUTP or digoxigenin-dUTP (Invitrogen). BAC and Fosmid DNAs were directly labeled with Cy3-dUTP, FITC-dUTP, Spectrum Orange-dUTP or Spectrum Green_dUTP (Vysis, Abbott Laboratories) using nick-translation or random priming using standard protocols. Final probe concentrations varied from 40–60 ng/ul. Centromeric probe cocktails (Vysis) plus BAC or Fosmid DNAs were denatured at 75°C for 10 minutes and prehybridized at 37°C for 30 minutes. Probes were applied to slides and incubated overnight at 37°C. Post-hybridization washes consisted of three 3-minute rinses in 50% formamide/2×SSC, three 3-minute rinses in 2×SSC, and finally three 3-minute rinses in PN buffer (0.1 M Na2HPO4+0.0 M NaH2PO4, ph 8.0, +2.5% Nonidet NP-40), all at 45°C. Signal detection was carried out as described [60]. Amplification of biotinylated probe signal utilized alternating incubations of slides with anti-avidin (Vector) and FITC-Extravidin (Sigma). Slides were then counterstained with either propidium iodide (2.5 ug/ml) or DAPI (15 ug/ml) and viewed under UV fluorescence (Olympus).
RNA–DNA FISH
Cells were plated on microscope slides treated with concanavalin A (Sigma) at ∼50% confluence and incubated for 4 hours in complete media in a 37°C humidified CO2 incubator. Slides were rinsed 1 time with sterile RNase free PBS. Slides were incubated for 30 seconds in CSK buffer (100 mM NaCl, 300 mM Sucrose, 3 mM MgCl2,10 mM Pipes, ph 6.8), 5 minutes in CSK buffer plus 0.1% Triton X-100, and then for an addition 30 seconds in CSK buffer at room temperature. Cells were fixed in 4% paraformaldehyde in PBS for 10 minutes at room temperature. Slides were rinsed in 70% ETOH and stored in 70% ETOH at 4°C until use. Just prior to use, slides were dehydrated through an ETOH series (70%, 90% and 100%) and allowed to air dry. Denatured probes were prehybridized with Cot-1 DNA at 37°C for 30 min. Slides were hybridized at 37°C for 14–16 hours. Slides were washed as follows: 3 times in 50% formamide/2×SSC at 42°C for 5 minutes, 3 times in 2×SSC at 42°C for 5 minutes, 3 times in 4×SSC/0.1% Tween 20 at room temperature for 3 minutes. Slides were then fixed in 4% paraformaldehyde in PBS for 5 minutes at room temperature, and briefly rinsed in 2×SSC at room temperature. The slides were then dehydrated in 70%, 90% and 100% ETOH, and then processed for DNA FISH, including the RNAase treatment step, as described above. Slides were then counterstained with either propidium iodide (2.5 ug/ml) or DAPI (15 ug/ml) and viewed under UV fluorescence (Olympus). Z-stack images were generated using a Cytovision workstation.
Replication timing assay
The BrdU replication timing assay was performed on exponentially dividing cultures essentially as described [61]. Briefly, asynchronously growing cells were exposed to 20 ug/ml of BrdU (Sigma) for 2.0, 2.5, 3.0 4.5, and 5 hours. Mitotic cells were harvested in the absence of colcemid, treated with 75 mM KCl for 15–30 minutes at 37°C, fixed in 3∶1 methanol∶acetic acid and dropped on wet ice cold slides. The chromosomes were denatured in 70% formamide in 2×SSC at 70°C for 3 minutes and processed for DNA FISH, as described above. The incorporated BrdU was then detected using a FITC-labeled anti-BrdU antibody (Becton Dickinson). Slides were stained with propidium iodide (0.3 mg/ml), cover slipped, and viewed under UV fluorescence. Due to the variability in the length of G2 between cells in different clones, the time point at which 50% of the mitotic cells contained BrdU incorporation was used for quantitative analysis. All images were captured with an Olympus BX Fluorescent Microscope using a 100× objective, automatic filter-wheel and Cytovision workstation. Individual chromosomes were identified with either chromosome-specific paints or centromeric probes in combination with BACs from the deleted regions. Utilizing the Cytovision workstation, each chromosome was isolated from the metaphase spread and a line drawn along the middle of the entire length of the chromosome. The Cytovision software was used to calculate the pixel area and intensity along each chromosome for each fluorochrome occupied by the DAPI and BrdU (FITC) signals. The total amount of fluorescent signal was calculated by multiplying the average pixel intensity by the area occupied by those pixels.
ReTiSH
We used the ReTiSH assay essentially as described [23]. Briefly, unsynchronized, exponentially growing cells were treated with 30 µM BrdU (Sigma) for 6 or 5 and 14 hours. Colcemid (Sigma) was added to a final concentration of 0.1 µg/mL for 1 h at 37°C. Cells were trypsinized, centrifuged at 1,000 rpm, and resuspended in prewarmed hypotonic KCl solution (0.075 M) for 40 min at 37°C. Cells were pelleted by centrifugation and fixed with methanol-glacial acetic acid (3∶1). Fixed cells were drop gently onto wet, cold slides and allowed to air-dry. Slides were treated with 100 µg/ml RNAse A at 37°C for 10 min. Slides were rinsed briefly in d2H20 followed by fixation in 4% formaldehyde at room temperature for 10 minutes. Slides were incubated with pepsin (1 mg/mL in 2 N HCl) for 10 min at 37°C, and then rinsed again with d2H20 and stained with 0.5 µg/µL Hoechst 33258 (Sigma) for 15 minutes. Slides were flooded with 200 µl 2×SSC, coversliped and exposed to 365-nm UV light for 30 min using a UV Stratalinker 2400 transilluminator (Stratagene). Slides were rinsed with d2H20 and drained. Slides were incubated with 100 µl of 3 U/µl of ExoIII (Fermentas) in ExoIII buffer for 15 min at 37°C. The slides were then processed directly for DNA FISH as described above, except with the absence of a denaturation step.
Semi-quantitative RT–PCR
Total RNA was extracted using Trizol (Invitrogen) reagent. For Poly A selection, two rounds of selection were carried out using a PolyATract kit (Promega). Total RNA or Poly A selected RNA was subjected to reverse transcriptase reactions using Superscript III (Invitrogen) according to the manufacturers instructions. PCRs were carried out with a first cycle of 2 minutes at 95°C, 45 seconds at 58°C and 1 minute at 72°C followed by 28–30 cycles of 30 seconds at 95°C, 45 seconds at 58°C and 1 minute at 72°C. The conditions were chosen so that none of the PCRs reached a plateau at the end of the amplification protocol, i.e. they were in the exponential phase of amplification. Each set of reactions always included a genomic DNA positive control, and a no sample and a no reverse transcriptase negative controls. The PCR products were resolved on 1% agarose gels and stained with ethidium bromide. The gels were photographed under UV illumination, and the resulting image was inverted using Photoshop (Adobe).
Recombineering
SW102 cells [62] were electroporated with 4 µg of purified BAC DNA at 1.35 kV and 600 ohms with a capacitance of 10 µF using a 0.1 cm gap cuvette. Bacterial cells were selected in 12.5 µg/mL Chloramphenicol (Cam) and 12.5 µg/mL Tetracycline (Tet) for 48 hours at 30 degrees C. To insert a Hygromycin resistance gene (HygR) into BACs we used a counter-selection modification strategy. SW102 cells are normally resistant to Streptomycin (Str). We first introduced DNA into the BAC that conferred Str sensitivity and Kanamycin (Kan) resistance to the cells and then replaced it with DNA containing HygR and an Ampicillan (Amp) resistance gene. Upon Amp resistance the cells then revert back to Str resistance and Kan sensitivity. The PCR product used in the first recombineering step was generated by amplifying rpsL-neo template DNA (GeneBridges, Dresden, Germany) with the rpsL-neo Forward (5′-CTTATCGATGATAAGCTGTCAAACATGAGAATTGATCCGGAACCCTTAATGGCCTGGTGATGATGGCGGGATCG-3′) and rpsL-neo Reverse (5′-CCGATGCAAGTGTGTCGCTGTCGACGGTGACCCTATAGTCGAGGGACCTATCAGAAGAACTCGTCAAGAAGGCG-3′) primers. This PCR product contains 50 bp of homology to the pBACe3.6 vector on each end. Prior to electroporation, the PCR product was digested with DpnI, phenol/chloroform extracted, ethanol precipitated. Cells containing the BAC were combined with 2 µL PCR product and electroporated as mentioned above. Cells were plated on LB/agar plates containing 15 µg/mL Cam and 15 µg/mL Kan. Cam+Kan resistant cells were selected for Str sensitivity and correct targeting was confirmed by restriction enzyme digestion and PCR. To replace the rpsL-neo DNA with the HygR gene, we used the protocol outlined above with some modifications. The PCR product used for recombination was generated by amplifying loxP-hygro-amp in pCR2.1 with Hyg Forward (5′-CCGATGCAAGTGTGTCGCTGTCGACGGTGACCCTATAGTCGAGGGACCTACAGGAAACAGCTATGACCATG-3′) and Hyg Reverse (5′-CTTATCGATGATAAGCTGTCAAACATGAGAATTGATCCGGAACCCTTAATTGTAAAACGACGGCCAGT-3′) primers. Before induction, cells were grown in 15 µg/mL Cam and 15 µg/mL Kan and following electroporation cells were plated on dishes containing 15 µg/mL Cam, 50 µg/mL Str and 15 µg/mL Amp. Correct targeting was confirmed by restriction enzyme digestion and PCR.
To make 29 kb deletion, we used a galK selection method that has been described previously (REF: PMID 15731329). Briefly, the galK cassettee was amplified with the primers galK For (5′-AAGTGTGCACATATGTGTTAGATGAAATATTGAGAAGGAACTTGAGTAAACCCTGTTGACAATTAATCATCGGCA-3′) and galK Rev (5′-TCATAATATGCATGGTAGGAAGTCTCCAGGAACTGACCCGTATAACAGGATTCAGCACTGTCCTGCTCCTT-3′). Electroporated PCR product into SW102+RP11-767E7+Hyg cells using the protocol described above. Following electroporation and recovery, cells were spun down and pellet was washed twice with M9 media and plated on M63 plates. Incubated at 32 degrees C for 6 days. Clones were grown up and analyzed for the presence of the deletion. This deletes chr6 : 96203250–96232818 (GRCh37/hg19).
Supporting Information
Zdroje
1. ThayerMJ (2012) Mammalian chromosomes contain cis-acting elements that control replication timing, mitotic condensation, and stability of entire chromosomes. Bioessays 2012.
2. zur HausenH (1967) Chromosomal changes of similar nature in seven established cell lines derived from the peripheral blood of patients with leukemia. J Natl Cancer Inst 38 : 683–696.
3. KatoH, SandbergAA (1968) Chromosome pulverization in human cells with micronuclei. J Natl Cancer Inst 40 : 165–179.
4. MilesCP, O'NeillF (1969) 3H labeling patterns of permanent cell line chromosomes showing pulverization or accentuated secondary constrictions. J Cell Biol 40 : 553–561.
5. SmithL, PlugA, ThayerM (2001) Delayed Replication Timing Leads to Delayed Mitotic Chromosome Condensation and Chromosomal Instability of Chromosome Translocations. Proc Natl Acad Sci U S A 98 : 13300–13305.
6. ChangBH, SmithL, HuangJ, ThayerM (2007) Chromosomes with delayed replication timing lead to checkpoint activation, delayed recruitment of Aurora B and chromosome instability. Oncogene 26 : 1852–1861.
7. BregerKS, SmithL, TurkerMS, ThayerMJ (2004) Ionizing radiation induces frequent translocations with delayed replication and condensation. Cancer Research 64 : 8231–8238.
8. BregerKS, SmithL, ThayerMJ (2005) Engineering translocations with delayed replication: evidence for cis control of chromosome replication timing. Hum Mol Genet 14 : 2813–2827.
9. StoffregenEP, DonleyN, StaufferD, SmithL, ThayerMJ (2011) An autosomal locus that controls chromosome-wide replication timing and mono-allelic expression. Hum Mol Genet 20 : 2366–2378.
10. PayerB, LeeJT (2008) X chromosome dosage compensation: how mammals keep the balance. Annu Rev Genet 42 : 733–772.
11. AuguiS, NoraEP, HeardE (2011) Regulation of X-chromosome inactivation by the X-inactivation centre. Nat Rev Genet 12 : 429–442.
12. Diaz-PerezS, OuyangY, PerezV, CisnerosR, RegelsonM, et al. (2005) The element(s) at the nontranscribed Xist locus of the active X chromosome controls chromosomal replication timing in the mouse. Genetics 171 : 663–672.
13. Diaz-PerezSV, FergusonDO, WangC, CsankovszkiG, TsaiSC, et al. (2006) A deletion at the mouse Xist gene exposes trans-effects that alter the heterochromatin of the inactive X chromosome and the replication time and DNA stability of both X chromosomes. Genetics 174 : 1115–1133.
14. GartlerSM, GoldsteinL, Tyler-FreerSE, HansenRS (1999) The timing of XIST replication: dominance of the domain. Hum Mol Genet 8 : 1085–1089.
15. ZwemerLM, ZakA, ThompsonBR, KirbyA, DalyMJ, et al. (2012) Autosomal monoallelic expression in the mouse. Genome Biol 13: R10.
16. GimelbrantA, HutchinsonJN, ThompsonBR, ChessA (2007) Widespread monoallelic expression on human autosomes. Science 318 : 1136–1140.
17. EnsmingerAW, ChessA (2004) Coordinated replication timing of monoallelically expressed genes along human autosomes. Hum Mol Genet 13 : 651–658.
18. GoldmitM, BergmanY (2004) Monoallelic gene expression: a repertoire of recurrent themes. Immunol Rev 200 : 197–214.
19. MostoslavskyR, SinghN, TenzenT, GoldmitM, GabayC, et al. (2001) Asynchronous replication and allelic exclusion in the immune system. Nature 414 : 221–225.
20. SinghN, EbrahimiFA, GimelbrantAA, EnsmingerAW, TackettMR, et al. (2003) Coordination of the random asynchronous replication of autosomal loci. Nat Genet 33 : 339–341.
21. SeligS, OkumuraK, WardDC, CedarH (1992) Delineation of DNA replication time zones by fluorescence in situ hybridization. EMBO J 11 : 1217–1225.
22. AzuaraV, BrownKE, WilliamsRR, WebbN, DillonN, et al. (2003) Heritable gene silencing in lymphocytes delays chromatid resolution without affecting the timing of DNA replication. Nat Cell Biol 5 : 668–674.
23. SchlesingerS, SeligS, BergmanY, CedarH (2009) Allelic inactivation of rDNA loci. Genes Dev 23 : 2437–2447.
24. GoodwinE, MeyneJ (1993) Strand-specific FISH reveals orientation of chromosome 18 alphoid DNA. Cytogenet Cell Genet 63 : 126–127.
25. ConsortiumEP (2011) A user's guide to the encyclopedia of DNA elements (ENCODE). PLoS Biol 9: e1001046 doi:10.1371/journal.pbio.1001046.
26. MeihlacM, KedingerC, ChambonP, FaulstichH, GovindanMV, et al. (1970) Amanitin binding to calf thymus RNA polymerase B. FEBS Lett 9 : 258–260.
27. WyersF, RougemailleM, BadisG, RousselleJC, DufourME, et al. (2005) Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 121 : 725–737.
28. HirataR, ChamberlainJ, DongR, RussellDW (2002) Targeted transgene insertion into human chromosomes by adeno-associated virus vectors. Nat Biotechnol 20 : 735–738.
29. MinksJ, BrownCJ (2009) Getting to the center of X-chromosome inactivation: the role of transgenes. Biochem Cell Biol 87 : 759–766.
30. HallLL, ByronM, SakaiK, CarrelL, WillardHF, et al. (2002) An ectopic human XIST gene can induce chromosome inactivation in postdifferentiation human HT-1080 cells. Proc Natl Acad Sci U S A 99 : 8677–8682.
31. ChowJC, HallLL, LawrenceJB, BrownCJ (2002) Ectopic XIST transcripts in human somatic cells show variable expression and localization. Cytogenet Genome Res 99 : 92–98.
32. RasmussenTP, WutzAP, PehrsonJR, JaenischRR (2001) Expression of Xist RNA is sufficient to initiate macrochromatin body formation. Chromosoma 110 : 411–420.
33. LeeJT, JaenischR (1997) Long-range cis effects of ectopic X-inactivation centres on a mouse autosome. Nature 386 : 275–279.
34. MarinelliLJ, HatfullGF, PiuriM (2012) Recombineering: A powerful tool for modification of bacteriophage genomes. Bacteriophage 2 : 5–14.
35. HirataniI, TakebayashiS, LuJ, GilbertDM (2009) Replication timing and transcriptional control: beyond cause and effect–part II. Curr Opin Genet Dev 19 : 142–149.
36. SchwaigerM, SchubelerD (2006) A question of timing: emerging links between transcription and replication. Curr Opin Genet Dev 16 : 177–183.
37. RybaT, HirataniI, LuJ, ItohM, KulikM, et al. (2010) Evolutionarily conserved replication timing profiles predict long-range chromatin interactions and distinguish closely related cell types. Genome Res 20 : 761–770.
38. YaffeE, Farkash-AmarS, PoltenA, YakhiniZ, TanayA, et al. (2010) Comparative analysis of DNA replication timing reveals conserved large-scale chromosomal architecture. PLoS Genet 6: e1001011 doi:10.1371/journal.pgen.1001011.
39. DeS, MichorF (2011) DNA replication timing and long-range DNA interactions predict mutational landscapes of cancer genomes. Nat Biotechnol 29 : 1103–1108.
40. GilbertDM, TakebayashiSI, RybaT, LuJ, PopeBD, et al. (2011) Space and time in the nucleus: developmental control of replication timing and chromosome architecture. Cold Spring Harb Symp Quant Biol 75 : 143–153.
41. TorchiaBS, CallLM, MigeonBR (1994) DNA replication analysis of FMR1, XIST, and factor 8C loci by FISH shows nontranscribed X-linked genes replicate late. Am J Hum Genet 55 : 96–104.
42. GomezM, BrockdorffN (2004) Heterochromatin on the inactive X chromosome delays replication timing without affecting origin usage. Proc Natl Acad Sci U S A 101 : 6923–6928.
43. HirataniI, RybaT, ItohM, RathjenJ, KulikM, et al. (2009) Genome-wide dynamics of replication timing revealed by in vitro models of mouse embryogenesis. Genome Res 20 : 155–169.
44. KruegerC, MorisonIM (2008) Random monoallelic expression: making a choice. Trends Genet 24 : 257–259.
45. ZakharovaIS, ShevchenkoAI, ZakianSM (2009) Monoallelic gene expression in mammals. Chromosoma 118 : 279–290.
46. LiSM, ValoZ, WangJ, GaoH, BowersCW, et al. (2012) Transcriptome-wide survey of mouse CNS-derived cells reveals monoallelic expression within novel gene families. PLoS ONE 7: e31751 doi:10.1371/journal.pone.0031751.
47. LeeJT, LuN, HanY (1999) Genetic analysis of the mouse X inactivation center defines an 80-kb multifunction domain. Proc Natl Acad Sci U S A 96 : 3836–3841.
48. HerzingLB, RomerJT, HornJM, AshworthA (1997) Xist has properties of the X-chromosome inactivation centre. Nature 386 : 272–275.
49. HeardE, MongelardF, ArnaudD, AvnerP (1999) Xist yeast artificial chromosome transgenes function as X-inactivation centers only in multicopy arrays and not as single copies. Mol Cell Biol 19 : 3156–3166.
50. BrockdorffN, AshworthA, KayGF, McCabeVM, NorrisDP, et al. (1992) The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell 71 : 515–526.
51. HasegawaY, BrockdorffN, KawanoS, TsutuiK, NakagawaS (2010) The matrix protein hnRNP U is required for chromosomal localization of Xist RNA. Dev Cell 19 : 469–476.
52. ZhaoJ, SunBK, ErwinJA, SongJJ, LeeJT (2008) Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 322 : 750–756.
53. GanesanS, SilverDP, DrapkinR, GreenbergR, FeunteunJ, et al. (2004) Association of BRCA1 with the inactive X chromosome and XIST RNA. Philos Trans R Soc Lond B Biol Sci 359 : 123–128.
54. StephensPJ, GreenmanCD, FuB, YangF, BignellGR, et al. (2011) Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 144 : 27–40.
55. CrastaK, GanemNJ, DagherR, LantermannAB, IvanovaEV, et al. (2012) DNA breaks and chromosome pulverization from errors in mitosis. Nature 2012.
56. LiuP, ErezA, NagamaniSC, DharSU, KolodziejskaKE, et al. (2011) Chromosome catastrophes involve replication mechanisms generating complex genomic rearrangements. Cell 146 : 889–903.
57. KloostermanWP, GuryevV, van RoosmalenM, DuranKJ, de BruijnE, et al. (2011) Chromothripsis as a mechanism driving complex de novo structural rearrangements in the germline. Hum Mol Genet 20 : 1916–1924.
58. ZhuY, ByeS, StambrookPJ, TischfieldJA (1994) Single-base deletion induced by benzo[a]pyrene diol epoxide at the adenine phosphoribosyltransferase locus in human fibrosarcoma cell lines. Mutat Res 321 : 73–79.
59. Helm S (1995) Cancer Cytogenetics. New York: Wiley-Liss.
60. Trask B, Pinkel D (1990) Flow cytometry; Crissman HA, Darzynkiewica Z, editors. New York: Academic Press.
61. SmithL, ThayerM (2012) Chromosome replication timing combined with fluorescent in situ hybridization. JOVE in press.
62. WarmingS, CostantinoN, CourtDL, JenkinsNA, CopelandNG (2005) Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res 33: e36.
63. ValenE, PascarellaG, ChalkA, MaedaN, KojimaM, et al. (2009) Genome-wide detection and analysis of hippocampus core promoters using DeepCAGE. Genome Res 19 : 255–265.
Štítky
Genetika Reprodukční medicína
Článek The G4 GenomeČlánek Mondo/ChREBP-Mlx-Regulated Transcriptional Network Is Essential for Dietary Sugar Tolerance inČlánek RpoS Plays a Central Role in the SOS Induction by Sub-Lethal Aminoglycoside Concentrations inČlánek Tissue Homeostasis in the Wing Disc of : Immediate Response to Massive Damage during DevelopmentČlánek Disruption of TTDA Results in Complete Nucleotide Excision Repair Deficiency and Embryonic LethalityČlánek DJ-1 Decreases Neural Sensitivity to Stress by Negatively Regulating Daxx-Like Protein through dFOXO
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 4- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
-
Všechny články tohoto čísla
- Epigenetic Upregulation of lncRNAs at 13q14.3 in Leukemia Is Linked to the Downregulation of a Gene Cluster That Targets NF-kB
- A Big Catch for Germ Cell Tumour Research
- The Quest for the Identification of Genetic Variants in Unexplained Cardiac Arrest and Idiopathic Ventricular Fibrillation
- A Nonsynonymous Polymorphism in as a Risk Factor for Human Unexplained Cardiac Arrest with Documented Ventricular Fibrillation
- The Hourglass and the Early Conservation Models—Co-Existing Patterns of Developmental Constraints in Vertebrates
- Smaug/SAMD4A Restores Translational Activity of CUGBP1 and Suppresses CUG-Induced Myopathy
- Balancing Selection on a Regulatory Region Exhibiting Ancient Variation That Predates Human–Neandertal Divergence
- The G4 Genome
- Extensive Natural Epigenetic Variation at a Originated Gene
- Mouse Oocyte Methylomes at Base Resolution Reveal Genome-Wide Accumulation of Non-CpG Methylation and Role of DNA Methyltransferases
- The Environment Affects Epistatic Interactions to Alter the Topology of an Empirical Fitness Landscape
- TIP48/Reptin and H2A.Z Requirement for Initiating Chromatin Remodeling in Estrogen-Activated Transcription
- Aconitase Causes Iron Toxicity in Mutants
- Tbx2 Terminates Shh/Fgf Signaling in the Developing Mouse Limb Bud by Direct Repression of
- Mondo/ChREBP-Mlx-Regulated Transcriptional Network Is Essential for Dietary Sugar Tolerance in
- Sex-Differential Selection and the Evolution of X Inactivation Strategies
- Identification of a Tissue-Selective Heat Shock Response Regulatory Network
- Phosphorylation-Coupled Proteolysis of the Transcription Factor MYC2 Is Important for Jasmonate-Signaled Plant Immunity
- RpoS Plays a Central Role in the SOS Induction by Sub-Lethal Aminoglycoside Concentrations in
- Six Homeoproteins Directly Activate Expression in the Gene Regulatory Networks That Control Early Myogenesis
- Rtt109 Prevents Hyper-Amplification of Ribosomal RNA Genes through Histone Modification in Budding Yeast
- ATP-Dependent Chromatin Remodeling by Cockayne Syndrome Protein B and NAP1-Like Histone Chaperones Is Required for Efficient Transcription-Coupled DNA Repair
- Iron-Responsive miR-485-3p Regulates Cellular Iron Homeostasis by Targeting Ferroportin
- Mutations in Predispose Zebrafish and Humans to Seminomas
- Cytotoxic Chromosomal Targeting by CRISPR/Cas Systems Can Reshape Bacterial Genomes and Expel or Remodel Pathogenicity Islands
- Tissue Homeostasis in the Wing Disc of : Immediate Response to Massive Damage during Development
- All SNPs Are Not Created Equal: Genome-Wide Association Studies Reveal a Consistent Pattern of Enrichment among Functionally Annotated SNPs
- Functional 358Ala Allele Impairs Classical IL-6 Receptor Signaling and Influences Risk of Diverse Inflammatory Diseases
- The Tissue-Specific RNA Binding Protein T-STAR Controls Regional Splicing Patterns of Pre-mRNAs in the Brain
- Neutral Genomic Microevolution of a Recently Emerged Pathogen, Serovar Agona
- Genetic Requirements for Signaling from an Autoactive Plant NB-LRR Intracellular Innate Immune Receptor
- SNF5 Is an Essential Executor of Epigenetic Regulation during Differentiation
- Dialects of the DNA Uptake Sequence in
- Reference-Free Population Genomics from Next-Generation Transcriptome Data and the Vertebrate–Invertebrate Gap
- Senataxin Plays an Essential Role with DNA Damage Response Proteins in Meiotic Recombination and Gene Silencing
- High-Resolution Mapping of Spontaneous Mitotic Recombination Hotspots on the 1.1 Mb Arm of Yeast Chromosome IV
- Rod Monochromacy and the Coevolution of Cetacean Retinal Opsins
- Evolution after Introduction of a Novel Metabolic Pathway Consistently Leads to Restoration of Wild-Type Physiology
- Disruption of TTDA Results in Complete Nucleotide Excision Repair Deficiency and Embryonic Lethality
- Insulators Target Active Genes to Transcription Factories and Polycomb-Repressed Genes to Polycomb Bodies
- Signatures of Diversifying Selection in European Pig Breeds
- The Chromosomal Passenger Protein Birc5b Organizes Microfilaments and Germ Plasm in the Zebrafish Embryo
- The Histone Demethylase Jarid1b Ensures Faithful Mouse Development by Protecting Developmental Genes from Aberrant H3K4me3
- Regulates Synaptic Development and Endocytosis by Suppressing Filamentous Actin Assembly
- Sensory Neuron-Derived Eph Regulates Glomerular Arbors and Modulatory Function of a Central Serotonergic Neuron
- Analysis of Rare, Exonic Variation amongst Subjects with Autism Spectrum Disorders and Population Controls
- Scavenger Receptors Mediate the Role of SUMO and Ftz-f1 in Steroidogenesis
- DNA Double-Strand Breaks Coupled with PARP1 and HNRNPA2B1 Binding Sites Flank Coordinately Expressed Domains in Human Chromosomes
- High-Resolution Mapping of H1 Linker Histone Variants in Embryonic Stem Cells
- Comparative Genomics of and the Bacterial Species Concept
- Genetic and Biochemical Assays Reveal a Key Role for Replication Restart Proteins in Group II Intron Retrohoming
- Genome-Wide Association Studies Identify Two Novel Mutations Responsible for an Atypical Hyperprolificacy Phenotype in Sheep
- The Genetic Correlation between Height and IQ: Shared Genes or Assortative Mating?
- Comprehensive Assignment of Roles for Typhimurium Genes in Intestinal Colonization of Food-Producing Animals
- An Essential Role for Zygotic Expression in the Pre-Cellular Drosophila Embryo
- The Genome Organization of Reflects Its Lifestyle
- Coordinated Cell Type–Specific Epigenetic Remodeling in Prefrontal Cortex Begins before Birth and Continues into Early Adulthood
- Improved Detection of Common Variants Associated with Schizophrenia and Bipolar Disorder Using Pleiotropy-Informed Conditional False Discovery Rate
- Site-Specific Phosphorylation of the DNA Damage Response Mediator Rad9 by Cyclin-Dependent Kinases Regulates Activation of Checkpoint Kinase 1
- Npc1 Acting in Neurons and Glia Is Essential for the Formation and Maintenance of CNS Myelin
- Identification of , a Retrotransposon-Derived Imprinted Gene, as a Novel Driver of Hepatocarcinogenesis
- Aag DNA Glycosylase Promotes Alkylation-Induced Tissue Damage Mediated by Parp1
- DJ-1 Decreases Neural Sensitivity to Stress by Negatively Regulating Daxx-Like Protein through dFOXO
- Asynchronous Replication, Mono-Allelic Expression, and Long Range -Effects of
- Differential Association of the Conserved SUMO Ligase Zip3 with Meiotic Double-Strand Break Sites Reveals Regional Variations in the Outcome of Meiotic Recombination
- Focusing In on the Complex Genetics of Myopia
- Continent-Wide Decoupling of Y-Chromosomal Genetic Variation from Language and Geography in Native South Americans
- Breakpoint Analysis of Transcriptional and Genomic Profiles Uncovers Novel Gene Fusions Spanning Multiple Human Cancer Types
- Intrinsic Epigenetic Regulation of the D4Z4 Macrosatellite Repeat in a Transgenic Mouse Model for FSHD
- Bisphenol A Exposure Disrupts Genomic Imprinting in the Mouse
- Genetic and Genomic Architecture of the Evolution of Resistance to Antifungal Drug Combinations
- Transposable Elements Are Major Contributors to the Origin, Diversification, and Regulation of Vertebrate Long Noncoding RNAs
- Functional Dissection of the Condensin Subunit Cap-G Reveals Its Exclusive Association with Condensin I
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The G4 Genome
- Neutral Genomic Microevolution of a Recently Emerged Pathogen, Serovar Agona
- The Histone Demethylase Jarid1b Ensures Faithful Mouse Development by Protecting Developmental Genes from Aberrant H3K4me3
- The Tissue-Specific RNA Binding Protein T-STAR Controls Regional Splicing Patterns of Pre-mRNAs in the Brain
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



