Two Portable Recombination Enhancers Direct Donor Choice in Fission Yeast Heterochromatin
Mating-type switching in fission yeast results from gene conversions of the active mat1 locus by heterochromatic donors. mat1 is preferentially converted by mat2-P in M cells and by mat3-M in P cells. Here, we report that donor choice is governed by two portable recombination enhancers capable of promoting use of their adjacent cassette even when they are transposed to an ectopic location within the mat2-mat3 heterochromatic domain. Cells whose silent cassettes are swapped to mat2-M mat3-P switch mating-type poorly due to a defect in directionality but cells whose recombination enhancers were transposed together with the cassette contents switched like wild type. Trans-acting mutations that impair directionality affected the wild-type and swapped cassettes in identical ways when the recombination enhancers were transposed together with their cognate cassette, showing essential regulatory steps occur through the recombination enhancers. Our observations lead to a model where heterochromatin biases competitions between the two recombination enhancers to achieve directionality.
Published in the journal:
. PLoS Genet 9(10): e32767. doi:10.1371/journal.pgen.1003762
Category:
Research Article
doi:
https://doi.org/10.1371/journal.pgen.1003762
Summary
Mating-type switching in fission yeast results from gene conversions of the active mat1 locus by heterochromatic donors. mat1 is preferentially converted by mat2-P in M cells and by mat3-M in P cells. Here, we report that donor choice is governed by two portable recombination enhancers capable of promoting use of their adjacent cassette even when they are transposed to an ectopic location within the mat2-mat3 heterochromatic domain. Cells whose silent cassettes are swapped to mat2-M mat3-P switch mating-type poorly due to a defect in directionality but cells whose recombination enhancers were transposed together with the cassette contents switched like wild type. Trans-acting mutations that impair directionality affected the wild-type and swapped cassettes in identical ways when the recombination enhancers were transposed together with their cognate cassette, showing essential regulatory steps occur through the recombination enhancers. Our observations lead to a model where heterochromatin biases competitions between the two recombination enhancers to achieve directionality.
Introduction
Fission yeast cells switch mating type by directed recombination events where the information in the expressed mat1 locus is replaced with information copied from one of two silent loci, mat2 or mat3 (reviewed in [1]). The system allows investigating multiple facets of recombination, including effects of chromatin structure on recombination and mechanisms of donor choice: how is a particular DNA template selected for recombination when several are available in a cell?
The mat1, mat2 and mat3 loci are linked in the mating-type region (Figure 1). mat1 determines the mating type of the cell by expressing two divergent regulatory genes, Pi and Pc in P cells (mat1-P allele), Mi and Mc in M cells (mat1-M allele; [2]). Silent information for the P and M mating types is stored at respectively mat2 ∼17 kb centromere-distal to mat1, and mat3 ∼29 kb centromere-distal to mat1 [3]–[5]. The mating-type specific information at mat1, mat2 and mat3 is flanked by short homology boxes, the centromere-distal H1 box and the centromere-proximal H2 box [2]. Other elements are specific for mat2 and mat3 [2],[6],[7] (Figure 1). mat2 and mat3 are furthermore embedded in a ∼20 kb heterochromatic domain that spans the mat2-mat3 interval and extends on both sides to inverted repeat boundaries [8],[9]. This domain has been studied extensively. It provides one of the best characterized model systems for how heterochromatic regions can be established and maintained. In this domain, histones are hypoacetylated, histone H3 is methylated at lysine 9 (H3K9me) in an RNA interference-dependent manner, and chromodomain proteins of the HP1 family are associated with the modified histones [8],[10]–[15]. The HP1-like chromodomain protein Swi6 interacts with numerous protein complexes believed to modulate heterochromatin formation, gene silencing and recombination, in ways that remain to a large extent undefined in particular regarding roles in recombination [14],[16]–[19].
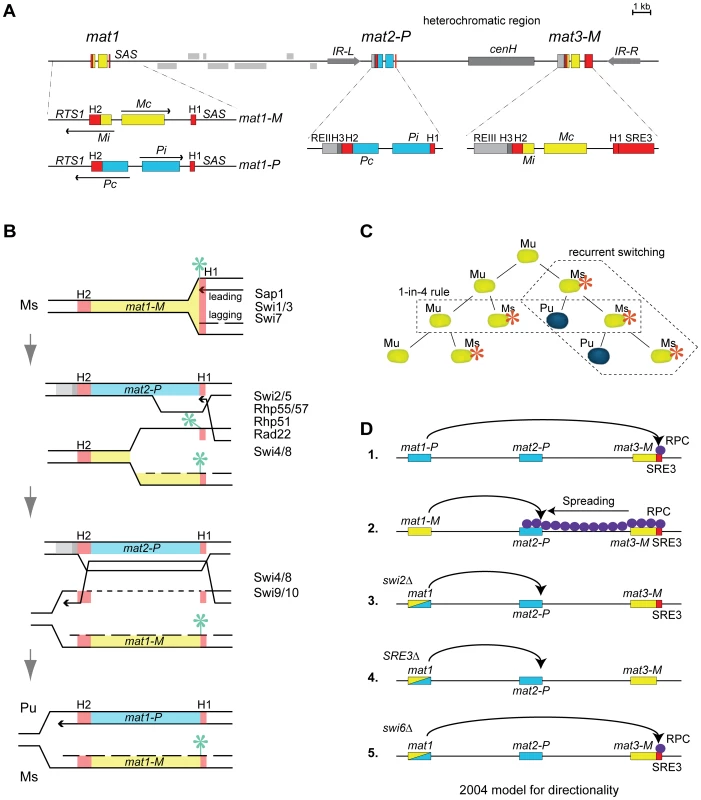
Interconversions of the mat1 locus between mat1-P and mat1-M lead to mating-type switching (reviewed in [1]). The conversions are coupled to DNA replication which reaches mat1 from a centromere-distal origin [20],[21]. Switching is initiated by the introduction of a strand-specific imprint in the lagging strand, resulting from the incorporation of two ribonucleotides or a nick between the mat1 H1 homology box and the mating-type specific information [20],–[28]. In the following rounds of DNA replication, the imprint is placed again on the chromatid made by lagging-strand synthesis, generating a lineage of imprinted, switchable cells [24],[29]. While lagging-strand synthesis propagates the imprinted mat1 locus in this lineage, leading-strand synthesis produces switched progeny (Figure 1B). At each division, leading-strand synthesis proceeds through the mat1 H1 homology box and stops at the imprint creating a single-ended double-strand break (DSB) or other recombinogenic molecule with a free 3′end [25],[30]. The free 3′end invades the H1 box of one of the silent loci which is then used instead of mat1 as template for leading-strand synthesis [29],[31]. This heals the break. Resolution of the recombination intermediate occurs within the H2 homology box with the help of the Swi4/8 and Swi9/10 gene products, producing a switched mat1 locus [5],[32]–[36]. The newly-switched mat1 locus does not carry an imprint hence it does not switch at the following S phase, however the chromatid made by lagging-strand synthesis acquires an imprint and starts a new lineage of switchable cells.
A choice of information is made in all switchable cells such that either mat2-P or mat3-M is used as donor to replicate and convert mat1. At this step, mat2-P and mat3-M are not picked at random. Switchable mat1-M cells preferentially use mat2-P whereas switchable mat1-P cells use mat3-M [23],[37],[38]. Coupled with the mechanism of switching outlined above, these directed choices produce a reproducible pattern of mating-type switching where (1) one out of four grand-daughters of a newly-switched cell has a high probability of having a switched mating-type (80–90%; one-in-four rule) and (2) once a cell becomes switchable the probability of recurrent switches in its lineage is very high (80–90%; recurrent-switching rule; Figure 1C).
Previous studies have revealed the importance of donor location and chromatin structure in donor choice [39]–[42]. Strains in which the mat2 and mat3 contents were swapped from mat2-P mat3-M (h90 configuration) to mat2-M mat3-P (h09 configuration) switch inefficiently to the opposite mating-type [39]. Mutations in the chromodomain protein Swi6, the H3K9 methyltransferase Clr4, or the Clr4-complex subunits Clr7 and Clr8 have opposite effects on switching in the h09 and h90 mating-type regions indicating chromatin structure favors use of mat2 in M cells and use of mat3 in P cells [39],[42]. The phenotypes of these mutants suggest that unproductive homologous switching occurs in h09 cells where mat1-M is converted by mat2-M and mat1-P by mat3-P instead of the productive heterologous switching occurring in h90 cells.
The strand exchange taking place at H1 is likely to be a determining step in donor choice. The Swi2 and Swi5 proteins are believed to facilitate this step together with Rad22, the fission yeast RAD52 homolog [43] and Rhp51, the S. pombe RecA homolog [44],[45]. The imprint, detected as a chromosomal fragile site, is formed at mat1 in swi2 and swi5 mutants but a subsequent step in the conversion process fails [5]. Consistent with this step being strand-invasion at the donor loci Swi2 interacts physically with both Swi5 and Rhp51 [17] and Swi5 facilitates Rhp51-mediated strand exchange in vitro [46]–[49]. Combined with the observation that Swi2 interacts with Swi6 [17], the properties of Swi2 and Swi5 place these factors close to the point where donor selection takes place.
A model for the directionality of mating-type switching combining effects of chromatin structure and targeted recruitment of recombination proteins was proposed in [41] (Figure 1; referred to as 2004 model below). In this model, the search for a donor starts at mat2. If the Swi2/Swi5 recombination-promoting complex (RPC) is encountered at mat2, mat2 is used to convert mat1. If RPC is not at mat2, the search proceeds to mat3 and mat3, which is constitutively associated with RPC, is used to convert mat1. The constitutive association of mat3 and RPC observed in both P and M cells is proposed to occur through a DNA element named SRE [41] that we will call SRE3 below to reflect its proximity to mat3. RPC is localized at SRE3 in P cells – ensuring that mat3 is used in P cells - but spreads from SRE3 all the way to mat2 in M cells – ensuring that mat2 is used in M cells. Spreading of RPC from SRE3 to mat2 requires Swi6. A recent addition to the model proposes that the spreading is driven by a greater abundance of the Swi2 and Swi5 proteins in M cells resulting from the positive regulation of the swi2 and swi5 genes by the M-specific transcription factor Mc [50]. Alternatively, Mc might stimulate the production of a shorter form of Swi2 expressed in P cells through alternative promoter usage [51].
The directionality model summarized above [41] provides a framework for investigations of mating-type switching, although several critical steps in it have no documented mechanism. One unexplained feature is that the search for a donor should start at mat2. This step is important because it accounts for mat2 being used in M cells when Swi2/Swi5 is present at both mat2-P and mat3-M. The model proposes that a higher-order chromatin structure helps choosing mat2 by placing it near mat1 but how this occurs remains unknown. Another aspect of the model that has not been documented experimentally is the physical interaction between SRE3 and Swi2. This is also a crucial element because the model is centered on SRE3 being the sole entry point for Swi2 in the mating-type region, accounting for the observation that Swi2 was not detected in the mating-type region of SRE3Δ strains by ChIP [41]. Here, we report further investigations on the directionality of mating-type switching bearing on these and other points. Our results challenge the notions that SRE3 is the sole entry point for Swi2, that Swi2 reaches mat2 by spreading from SRE3, and that the search for a donor starts at mat2. Instead, our results show that directionality requires two recombination enhancers, SRE2 and SRE3, whose ability to stimulate recombination in a cell-type specific manner is not tied to a specific location in the mating-type region. We present evidence that directionality results from competitions between SRE2 and SRE3, governed by cell-type specific chromatin structures.
Results
SRE3-independent effects of Swi2 and Swi5 on the directionality of mating-type switching indicate the presence of a second recombination enhancer
The SRE3 element was described as the entry point at which the Swi2/Swi5 complex associates with the mating-type region [41]. Following this initial association, proposed to take place in both cell types, Swi2 could remain at SRE3 in P cells or spread to mat2 in M cells. Support for this mechanism is provided by ChIP experiments that failed to detect Swi2 anywhere in the mating-type region in strains lacking SRE3 [41]. We examined the model further through a simple genetic test. If the directed association of Swi2 with the mating-type region is abolished in SRE3Δ cells the mating-type bias in SRE3Δ cells should not be altered by deletion of swi2.
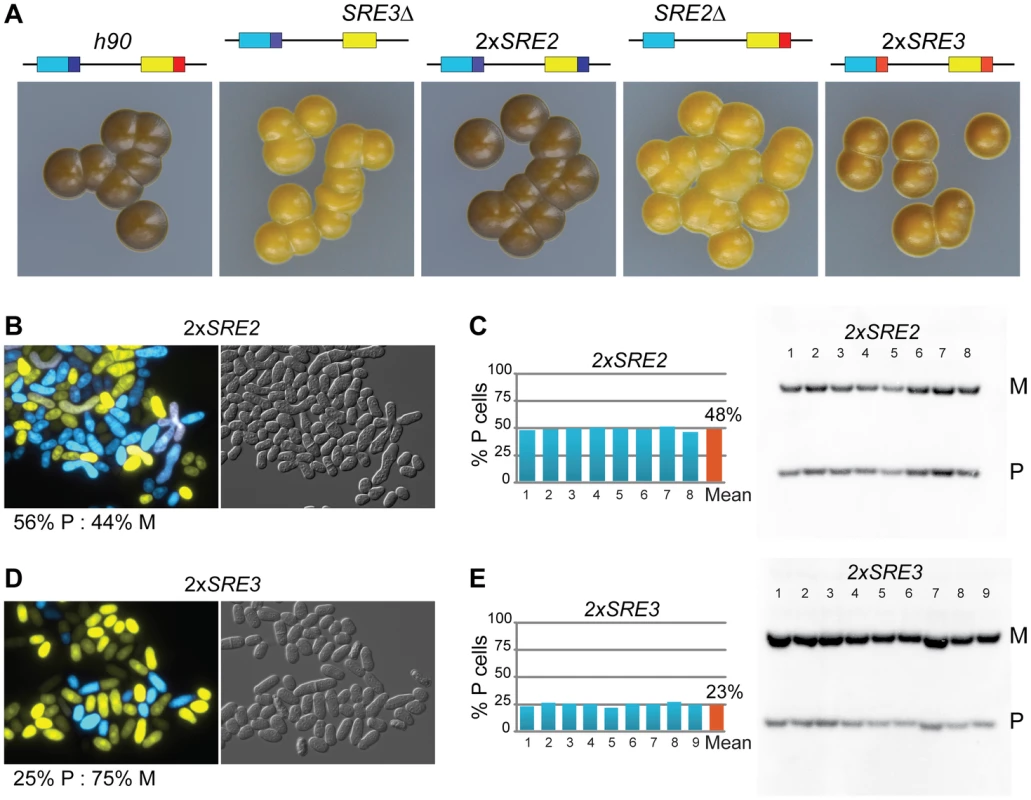
In S. pombe, the efficiency of mating-type switching can be estimated by staining sporulated colonies with iodine vapors. Efficiently-switching strains produce colonies that are stained darkly by iodine vapors because they contain many spores while poorly-switching strains produce lightly-stained colonies [52]. The predominant mating-type in cell populations can be further determined by quantifying the content of mat1 by Southern blot or PCR. In addition, we developed here a reporter system in which M cell express YFP and P cells express CFP allowing typing individual cells with a fluorescence microscope (Figure 1D). Sporulated SRE3Δ colonies were stained lightly by iodine and colonies did not stain at their junctions indicating preferential use of one donor (Figure 2B). Southern blotting, competitive PCR, and fluorescent typing all showed that SRE3Δ cells contain predominantly the mat1-P allele (P∶M = 82∶18 by Southern blot; P∶M = 88∶12 by cell count; Figure 2; competitive PCR not shown). The SRE3Δ strain used for these analyses was made in our laboratory [7] hence these results confirm the observations in [41] with an independent strain and support the conclusion of these authors that mat2-P is the preferred donor in SRE3Δ cells.
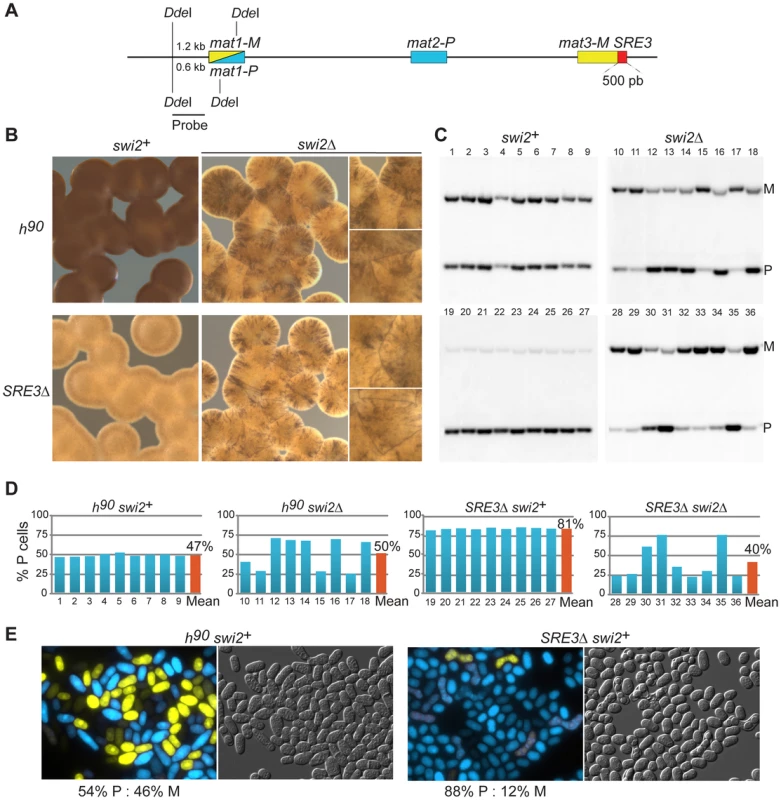
We assessed the effect of deleting swi2 in both wild-type h90 cells and SRE3Δ cells (Figure 2). Iodine staining indicated that deletion of swi2 severely affected switching efficiency in both backgrounds: h90 swi2Δ and SRE3Δ swi2Δ cells formed streaky colonies staining at their junctions showing that cells are predominantly of the M mating-type in some colonies and predominantly of the P mating type in other colonies (Figure 2B). We measured P∶M ratios in nine independent cultures of respectively h90 swi2+; h90 swi2Δ; SRE3Δ swi2+; and SRE3Δ swi2Δ cells by Southern blot (Figure 2C). While all h90 swi2+ cultures had balanced P∶M ratios and all SRE3Δ swi2+ cultures were predominantly of the P mating-type, large fluctuations in P∶M ratios were observed in h90 swi2Δ and SRE3Δ swi2Δ cultures. The strong phenotypic variability observed in h90 swi2Δ cultures disagrees with the report [41] that h90 swi2Δ cells contain predominantly mat1-P and that the switching pattern of h90 swi2Δ cells is indistinguishable from switching in h90 SRE3Δ cells. Further, the clear phenotypic differences we observed between SRE3Δ swi2+ (P≫M in all colonies; 81% P cells averaged over 9 cultures) and SRE3Δ swi2Δ strains (variegated phenotype; 40% P cells averaged over 9 cultures) is not predicted in [41]. Similarly, we observed culture-to-culture variations with, if any, a bias towards M cells in h90 swi5Δ cultures (72% M cells averaged over 9 cultures; Figure S1) in contrast to [50] who found that h90 swi5Δ cells are predominantly P. As for the deletion of swi2+, deletion of swi5+ abrogated the preferential use of mat2-P in SRE3Δ cells (Figure S1). We conclude that the RPC is necessary for the efficient and preferential choice of mat2-P in SRE3Δ cells. Since this represents a situation where RPC cannot reach mat2-P by spreading from SRE3, this result does not support the spreading model and suggests instead that other DNA element(s) or factors attract Swi2 and Swi5 independently of SRE3.
Chromosomal deletions adjacent to mat2-P impair switching, defining the SRE2 recombination enhancer
While systematically introducing deletions in the mating-type region we found that a set of nested deletions on the centromere-distal side of mat2-P affected switching, defining a ∼500 bp element adjacent to the H1 box, SRE2. Deletion of SRE2 caused a pronounced switching defect (Figure 3). Sporulated SRE2Δ colonies were stained very lightly by iodine vapors and they did not stain at their junctions; a Southern blot testing mat1 content in nine independent cultures indicated a large predominance of M cells in all cultures; and the existence of a strong bias towards M cells was also supported by fluorescence microscopy (Figure 3). Identical phenotypes were obtained when SRE2Δ colonies were seeded from P or M spores confirming efficient asymmetric switching favoring mat3-M (data not shown). The location of SRE2 relative to mat2 is similar to the location of SRE3 relative to mat3 but no extensive sequence similarities were noted between SRE2 and SRE3. Both elements are A-T rich (75% for SRE2 and 72% for SRE3 over 492 bp). The authors of a recent study [51] noticed like us that a deletion adjacent to mat2-P prevented efficient use of mat2-P however the study did not characterize the element further. Several observations reported below argue against deletion of SRE2 simply preventing use of mat2 as a donor. They suggest instead that SRE2 regulates donor choice.
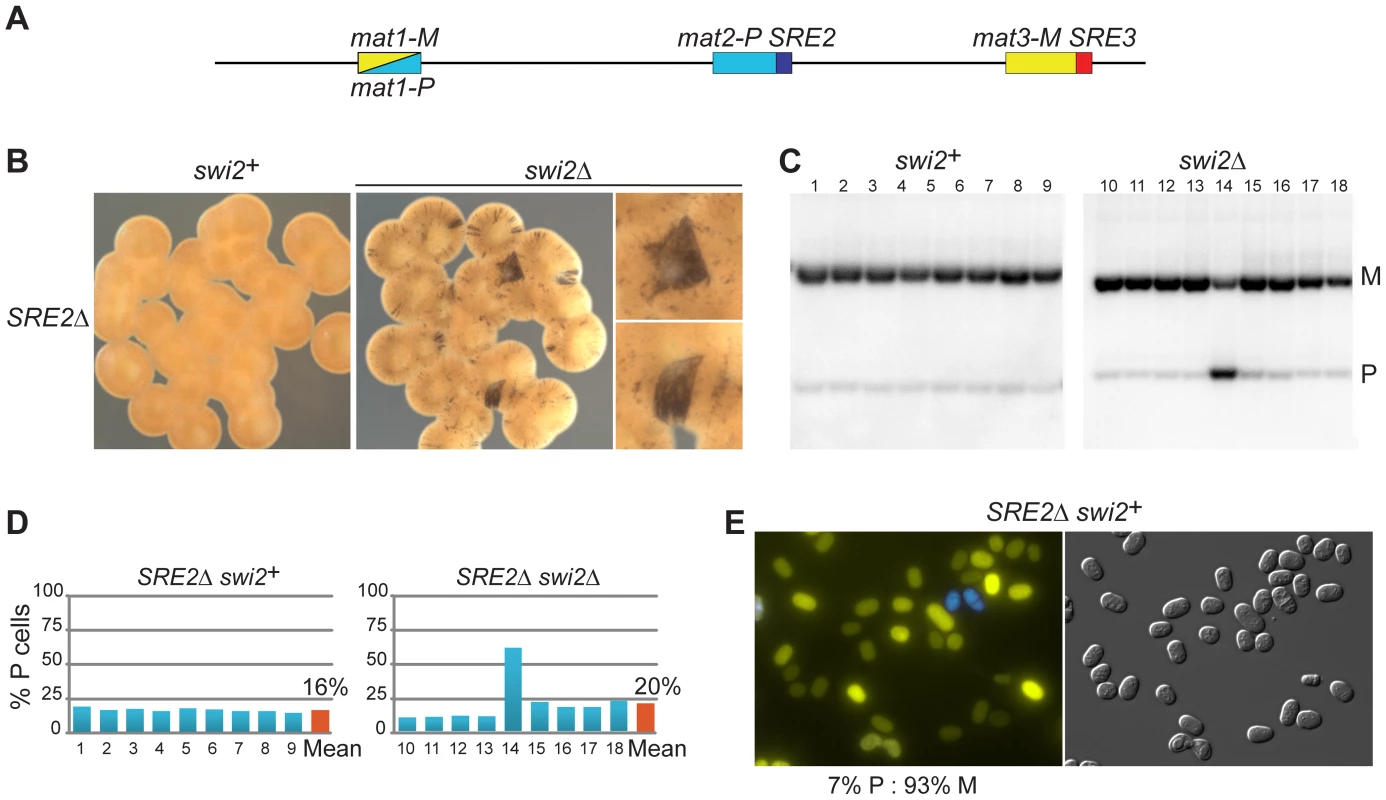
As for the strains examined above, deleting swi2+ affected switching in SRE2Δ cells. Two types of sporulated colonies were observed following iodine staining, light colonies with a few dark streaks containing mostly M cells, and more rare darker colonies containing a greater proportion of P cells (Figure 3; 80% M cell averaged over nine colonies). Deletion of swi5 produced a similar phenotype (77% M cell averaged over nine colonies; Figure S1; Southern blot quantifications are summarized in Figure S2.). These phenotypes are consistent with mat3-M remaining a preferred donor in SRE2Δ cells even when RPC is not present in the cells. This again fails to support the 2004 model, where mat2-P is the preferred donor when RPC is not present due to higher-order chromatin structure. Alternatively, SRE2 might be responsible for the higher-order structure postulated by the model.
We investigated the association of Swi2 with parts of the mating-type region by ChIP (Figure S3). In unswitchable mat1-M cells, where mat2-P is perhaps poised for switching, Swi2 was detected at mat2-P and at SRE2 as previously reported [41]. In our experiments, Swi2 was also detected at these locations in SRE3Δ cells consistent with an SRE3-independent mode of recruitment to the mating-type region. This recruitment appeared facilitated by SRE2 since the association of Swi2 with mat2 was reduced in SRE2Δ cells (primer pairs 44, 46 and ‘SRE2Δ’ in M cells, Figure S3).
SRE2 and SRE3 direct donor choice independently of their location
A deletion reducing the use of a donor cassette is not on its own evidence that the deletion removed a directionality element. We explored the possibility that SRE2 and SRE3 are genuine directionality elements by engineering h09 cells. The donor loci are mat2-M mat3-P in the h09 mating-type region [39]. The cassette contents are precisely exchanged between the H2 and H1 homology boxes placing mat2-M near SRE2 and mat3-P near SRE3. This arrangement results in inefficient switching to the opposite mating-type ([39]; Figure 4). The h09 strain provides a useful tool to study directionality since it allows designing experiments in which the tested outcome is improved switching rather than loss of switching. We tested whether swapping SRE2 and SRE3 in h09 cells improved heterologous switching.
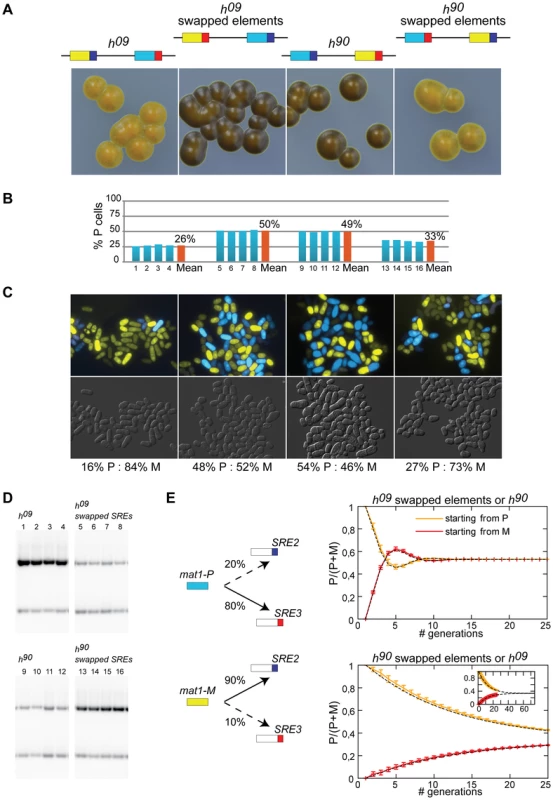
Figure 4 shows that h09 cells with swapped elements switched mating-type very efficiently and produced populations with equal proportions of P and M cells. Their sporulated colonies were undistinguishable from h90 colonies. Their mat1 content examined by Southern blot was evenly balanced and fluorescence microscopy confirmed equal proportions of P and M cells in colonies (Figure 4). Conversely h90 cells with swapped SRE elements switched mating-type poorly, produced mainly mat1-M cells as h09 cells with unswapped elements do, and formed colonies very similar to h09 colonies (Figure 4). Together these experiments show that the PSRE2 MSRE3 combination (whether mat2-PSRE2 mat3-MSRE3 in wild-type h90 cells with native elements or mat2-MSRE3 mat3-PSRE2 in h09 cells with swapped elements) leads to balanced use of the two cassettes while the PSRE3 MSRE2 combination (whether mat2-MSRE2 mat3-PSRE3 in h09 cells with native elements or mat2-PSRE3 mat3-MSRE2 in h90 cells with swapped elements) leads to inefficient heterologous switching. We conclude from these observations that SRE2 and SRE3 behave as directionality elements responsible for the balanced heterologous switching observed in h90 cells. P cells select the cassette adjacent to SRE3 while M cells select the cassette adjacent to SRE2 and SRE2 and SRE3 can both be recognized ectopically when their location relative to mat1 has been swapped.
Asymmetries
Should SRE2 and SRE3 be the sole determinants of directionality and should their action be fully symmetrical, h09 cells with native SRE elements and h90 cells with swapped SRE elements would engage in futile cycles where mat1-P selects mat3-PSRE3 (in h09) or mat2-PSRE3 (in h90 cells with swapped elements) and mat1-M selects mat2-MSRE2 (in h09) or mat3-MSRE2 (in h90 cells with swapped elements; Figure 4). Two types of colonies would be formed, one type containing predominantly P cells, the other predominantly M cells. This is not what is observed. Both h09 cells with native elements and h90 cells with swapped elements form populations where M cells predominate (Figure 4) indicating preferential choice of the cassette adjacent to SRE2. The fact that the bias is towards the MSRE2 cassette in both cases even though the MSRE2 cassette occupies different locations in the two strains shows that a preponderant cause for the bias is location independent.
The mechanisms responsible for directionality are likely to fail occasionally, allowing the ‘wrong’ donor to be selected. We reasoned that a small error rate would not have a strong impact on the overall composition of h90 cell populations, but the same error rate could have more profound consequences in h09 populations because the mistakes would lead to changes in mating-type that would subsequently be stably propagated through homologous switching. We modeled a situation where P cells use predominantly SRE3 to select a donor for switching, while M cells select predominantly SRE2 (Figure 4). We allowed a low occurrence of mistakes in both cell types, where P cells occasionally use SRE2 (20% of attempted switches) while M cells use SRE3 more rarely (10% of attempted switches). As expected such a bias leads to an accumulation of M cells in both h09 cells with native elements and h90 cells with swapped elements supporting the hypothesis that aberrant choices contribute to the preponderance of M cells in these strains. We note that in addition to SRE2 being more promiscuous than SRE3, the cassette content in the MSRE2 combination might facilitate use of MSRE2 over PSRE3 in P cells.
SRE2 and SRE3 enhance recombination in both cell types
As a way of testing the extent to which P cells can use SRE2 we replaced SRE3 with SRE2 (mat2-PSRE2 mat3-MSRE2 strain referred to as 2×SRE2). The 2×SRE2 strain switched mating-type efficiently as judged from its dark iodine staining and balanced ratio of P and M cells (Figure 5). 2×SRE2 populations contained 48% P cells according to Southern blot, 56% P cells according to microscopy. The phenotype of the 2×SRE2 strain shows that P cells are proficient in the use of the SRE2 element in mat3-MSRE2 otherwise P cells would accumulate in the population of 2×SRE2 cells. To illustrate this further SRE3Δ colonies are shown near the 2×SRE2 strain for comparison in Figure 5. SRE2 at mat3-M considerably improves heterologous switching showing that P cells use SRE2. Even though 2×SRE2 cells switch mating-type efficiently, mating-type selectivity in 2×SRE2 is not as in wild-type leading us to propose that donor choice is randomized in 2×SRE2 rather than directional. A differential behavior of 2×SRE2 and wild-type mating-type region is shown for example in the next section where h90 swi6Δ and 2×SRE2 swi6Δ strains clearly differ from each other. Similarly we replaced SRE2 with SRE3 (mat2-PSRE3 mat3-MSRE3 strain referred to as 2×SRE3). The 2×SRE3 strain produced a mixture of P and M cells which shows that M cells can use SRE3, however with a bias towards M cells (Figure 5). 2×SRE3 populations contained 23% P cells as estimated from Southern blot, 25% estimated from microscopy. Together with the results presented above for the 2×SRE2 strain these ratios indicate that M cells are not as proficient at using mat2-PSRE3 as P cells are at using mat3-MSRE2. In summary SRE2 can stimulate recombination of donor loci with mat1 efficiently not only in M cells but also in P cells whereas SRE3 is more active in P cells than in M cells. The ability of each element to function in both cell types shows that these elements are not strictly dependent on cell-type-specific factors to stimulate recombination.
Effects of chromatin — mechanisms of recombination enhancement by SRE2 and SRE3
A remarkable aspect of mating-type switching is that the donor loci are in heterochromatin. We asked whether and how the ability of the SRE elements to stimulate recombination was affected by heterochromatin through epistasis analyses using cells lacking the chromodomain protein Swi6.
Deletion of swi6+ in h09 or h90 cells with native or swapped elements radically altered donor choice (compare Figure 4 and 6). Populations of h90 cells or h09 cells with swapped elements went from balanced P∶M ratios (49% and 50% P resp.) to containing predominantly M cells (87% and 84% resp.). Conversely the M bias in populations of h09 cells or h90 cells with swapped elements was abrogated by swi6Δ. In all cases, the changes reflected that use of the cassette adjacent to SRE2 was greatly decreased in favor of the cassette adjacent to SRE3 following deletion of swi6+, as indicated in Figure 6. These phenotypes show that Swi6 biases donor choice towards the cassette controlled by SRE2, or away from the cassette controlled by SRE3, whether the cassette contains the P or M information, and whether it is located at mat2 or mat3.
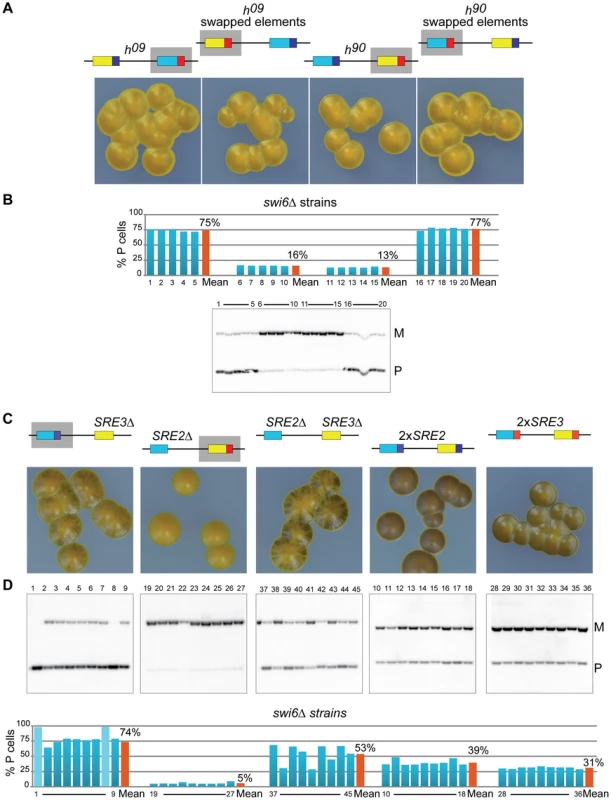
Reduced selection of SRE2 in h90 swi6Δ cells depended on the presence of SRE3 in the same cells. No change in preferred donor was observed in h90 SRE3Δ cells following deletion of swi6+, SRE2 kept being used (compare SRE3Δ in Figure 2 to SRE3Δ swi6Δ in Figure 6; mat1-P predominates in both). This indicated that SRE2 could stimulate recombination at mat2-PSRE2 in the absence of Swi6 when SRE3 was not present. Very inefficient switching in SRE2Δ SRE3Δ swi6Δ cells confirmed that the selection of mat2-PSRE2 in SRE3Δ swi6Δ cells depended on SRE2 (Figure 6; inefficient switching in the SRE2Δ SRE3Δ swi6Δ strain produces colonies staining at their junctions and large fluctuations in P/M ratios). Similarly, use of mat3-MSRE3 in SRE2Δ swi6Δ cells required SRE3 (compare SRE2Δ swi6Δ with SRE2Δ SRE3Δ swi6Δ in Figure 6). In summary these phenotypes show that both SRE2 and SRE3 can stimulate recombination in the absence of Swi6. Competitions between the two enhancers drive donor selection both in the absence and presence of Swi6. In the absence of Swi6 SRE3 is preferred over SRE2. When present, Swi6 biases donor selection towards SRE2.
Discussion
Some forms of recombination occur with an extraordinary efficiency in heterochromatin as illustrated by fission yeast mating-type switching. In mating-type switching, a euchromatic locus, mat1, undergoes productive recombinogenic interactions with a heterochromatic partner in every other dividing cell. Not only are these recombination events frequent, they are also exquisitely fine-tuned such that a specific donor is selected for each conversion of mat1. Our work identifies small, portable, DNA elements responsible for donor choice and provides new insights into the mechanisms responsible for the directionality of switching. Some of our observations differ from previous reports [41],[50]. We discuss here these discrepancies and use our findings to build a new model for the directionality of mating-type switching.
The SRE elements direct donor choice ectopically
Cells in which the silent-cassette contents are swapped (h09) switch mating-type inefficiently, indicating cells fail to choose heterologous donors when the donors are not at their endogenous location [39]. Here, we find that a crucial aspect of donor location is proximity of the donors to their respective recombination enhancers, SRE2 and SRE3. The determining role of SRE2 and SRE3 in donor selection was revealed by the high efficiency of switching in h09 cells when the SRE elements were swapped concomitantly with the contents of mat2 and mat3 (Figure 4). Heterologous donors could be found efficiently even when they were not at their endogenous location, provided the coupling with their cognate recombination enhancers was maintained.
The fact that h09 cells with swapped SRE elements switch well has strong implications for the 2004 model. The 2004 model is a two-component model integrating effects of donor positioning relative to mat1 (in the model the recombinogenic DSB at mat1 encounters mat2 before it encounters mat3) and presence of RPC (the first RPC-associated donor encountered is used; Figure 1). In h09 cells with swapped elements a search starting at mat2 would encounter SRE3 first. SRE3 being the proposed nucleation site for RPC, constitutively associated with RPC in both cell types, mat2-MSRE3 should be selected preferentially in both cell types which is clearly not the case. Our observations show instead that M cells choose SRE2 and P cells choose SRE3 when these elements are present, independently of their location.
One way of reconciling the portability of the SRE elements with the 2004 model is to propose that SRE2 is responsible both for the higher-order chromatin structure that brings mat2 close to mat1 in this model and also for directing the spreading of Swi2 away from SRE3 in M cells. While such roles for SRE2 should be envisioned and tested, other observations we made suggest that Swi2 does not reach mat2 by spreading from SRE3.
RPC catalyzes asymmetric switching in situations where RPC was not previously detected by ChIP and in the absence of SRE3
ChIP experiments reported in previous publications have detected large, cell-type specific variations in the association of RPC with the mating-type region [41],[50]. RPC was detected over the entire mat2-mat3 interval in M cells but the association was restricted to SRE3 in P cells [41]. In cells lacking SRE3, RPC was not detected at all [41]. While these strikingly differential associations hint to some relevance for directionality, how the associations lead to the selection of a specific donor is not straightforward. RPC associations do not on their own determine which cassette will be used since the association of RPC with SRE3 is cell-type independent. Here, we found that RPC catalyzes switching even in situations where RPC was not previously detected by ChIP [41] and in the absence of SRE3. In our experiments, the pronounced bias towards the P mating-type displayed by SRE3Δ cells was abolished in SRE3Δ swi2Δ cells and in SRE3Δ swi5Δ cells, showing RPC is necessary for the preferential use of mat2-P in SRE3Δ cells (Figure 2 and Figure S1). Not only is this epistatic relationship not predicted by the 2004 model – the model predicts that the SRE3Δ swi2Δ double mutant should switch like SRE3Δ – but the 2004 model specifically relies on swi2Δ and SRE3Δ cells having identical phenotypes, which is also contradicted by our results (Figure 2).
Based on our genetic evidence we suggest that ChIP has failed to detect interactions between Swi2 and the mating-type region that are relevant to directionality. Difficulties in detecting the association of Swi2 with the mating-type region might be due to the fact that Swi2 is not an abundant protein, that relevant interactions occur in a short window of the cell cycle, or to the fact that ChIP experiments have been conducted in switching-defective cells lacking elements at mat1 that might participate in directionality as indicated in [24]. Unlike [41], we observed that in M cells Swi2 remained associated with mat2-P and SRE2 in the absence of SRE3 (Figure S3). A core feature in the 2004 model is that Swi2 spreads from SRE3 to mat2 in P cells. Spreading of a protein along the chromatin fiber can be difficult to distinguish from other mechanisms that might lead to the same final associations. Binding at multiple sites might give the appearance of spreading from one of the sites. Here, we suggest that Swi2 does not have to spread from SRE3 to facilitate switching at SRE2.
Effects of chromatin on the ability of SRE2 and SRE3 to stimulate recombination
We observed that both SRE2 and SRE3 can stimulate recombination in the absence of Swi6. While populations of SRE2Δ swi6Δ cells were predominantly M and populations of SRE3Δ swi6Δ cells were predominantly P these biases were lost in populations of SRE2Δ SRE3Δ swi6Δ cells (Figure 6C–D) showing SRE3 stimulates recombination with mat3-M in SRE2Δ swi6Δ cells and SRE2 stimulates recombination with mat2-P in SRE3Δ swi6Δ cells. We furthermore observed that competitions between SRE2 and SRE3 take place in swi6Δ cells when both elements are present. While SRE3Δ swi6Δ populations were predominantly P (Figure 6C–D), reflecting choice of SRE2, h90 swi6Δ populations were predominantly M (Figure 6A–B), reflecting choice of SRE3, from which we conclude that SRE3 outcompetes SRE2 in h90 swi6Δ cells. The switching phenotypes of h09 swi6Δ; h09 with swapped elements swi6Δ; and h90 with swapped elements swi6Δ cells all show that SRE3 is preferred over SRE2 in swi6Δ cells when both elements are present (Figure 6A–B).
Swi6 exerts major effects on mating-type switching through SRE2 and SRE3. Comparing Figure 4 and Figure 6A–B shows that Swi6 tilts the relative efficiency of the two elements, allowing SRE2 to be preferred over SRE3 in M cells. We suggest that this effect is key to directionality. Several lines of evidence have established that heterochromatin differs in the mating-type region of P and M cells making heterochromatin a good candidate for providing cell-type specificity in mating-type switching. Ectopic reporters are more strongly repressed in M cells than in P cells, whether the reporters are near mat2 or mat3 [7],[53], (G. Thon unpublished data) and consistently more Swi6 is detected over the entire mat2-mat3 region in M cells than in P cells [8]. These differences between P and M cells are likely to reflect differential associations of various protein complexes over the entire mating-type region, including but not limited to Swi6, Swi2 and Swi5 [14],[16]–[19],[54]. Global changes over the entire region would account for our observation that the effects of Swi6 on SRE2 and SRE3 were independent of donor location (Figure 6). The model for the directionality of switching outlined below proposes that differences in the chromatin structure of P and M cells determine which recombination enhancer is used in each cell type.
Model for the directionality of mating-type switching
We propose a simple model for the directionality of mating-type switching that takes into account our observations (Figure 7). This model is an alternative to models where the recombination enhancers favor cell-type specific interactions between the donor loci and mat1 through DNA looping but it is not incompatible with looping models.
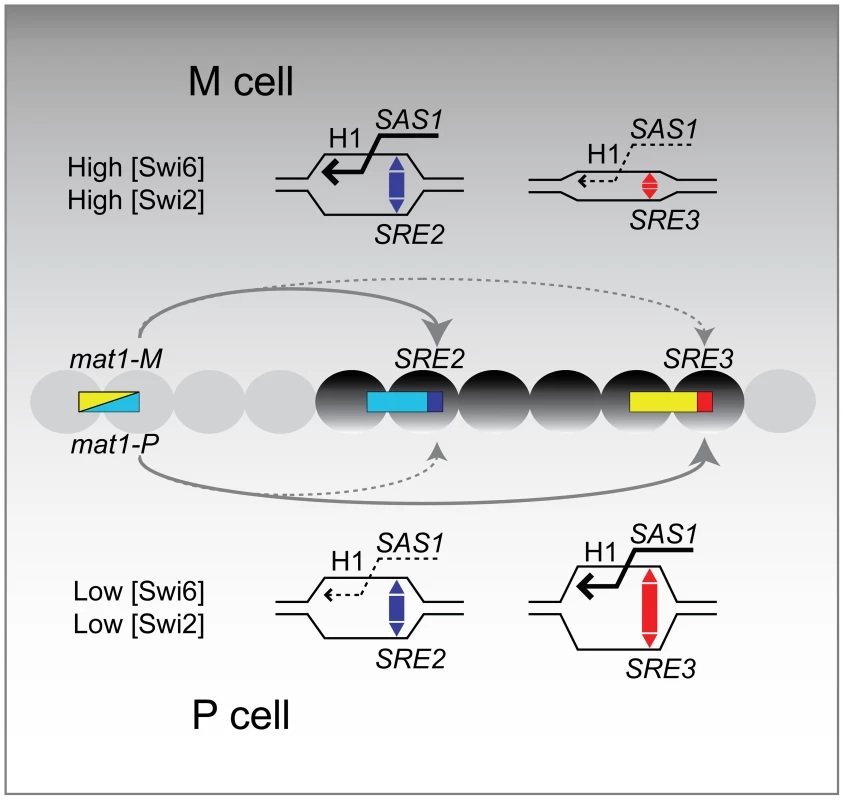
In the proposed model SRE2 and SRE3 compete to capture the free DNA end generated at mat1. When Swi6 and associated factors are in comparatively low abundance in the mating-type region as is the case in P cells, SRE3 stimulates recombination at its adjacent H1 homology box more efficiently than SRE2. When Swi6 and associated factors are in greater abundance in the mating-type region, as is the case in M cells, SRE2 is more efficient than SRE3.
Several mechanisms can be envisioned for how SRE2 and SRE3 might facilitate strand invasion at their adjacent H1 box in a chromatin-dependent manner. SRE2 and SRE3 might have an intrinsic ability to facilitate D-loop formation as suggested by their low melting temperature (predicted from 72–75% AT content and data not shown). Indeed, evidence has been presented that SRE2 can form a heteroduplex with DNA adjacent to the mat1 H1 box [33]. Swi6 could modulate the ability of SRE2 and SRE3 to stimulate strand-invasion at H1 through changes in chromatin structure. Swi6 binds to nucleosomes methylated at H3K9 and it oligomerizes. The association of Swi6 with chromatin per se might constrain the topology of DNA around H1 and the SRE elements in a way that would alter D-loop induction by the SRE elements and depend on the concentration of Swi6. Other, non mutually-exclusive effects of SRE2 and SRE3 could be through the positioning of nucleosomes. Swi6 might induce the local sliding or displacement of nucleosomes through one of its associated ATP-dependent chromatin remodeling complexes (RSC, Ino80, FACT; [18],[19]) thereby altering the ability of a recombination enhancer to increase H1 accessibility. Finally, direct interactions might take place between the recombination enhancers and recombination factors such as Swi2 as suggested in the case of Swi2 and SRE3 [41]. Directionality would occur if SRE3 had a higher affinity for Swi2 than SRE2 but a lower peak efficiency than SRE2 when stimulating recombination in the context of heterochromatin. At low concentration of Swi2, SRE3 but not SRE2 would be associated with Swi2, promoting invasion of its adjacent cassette. At high concentrations of Swi2, SRE2 would not only be associated with Swi2 but it would use its associated Swi2 more efficiently than SRE3, leading to preferred choice of SRE2 over SRE3. Low association of Swi6 and Swi2 in the mating-type region of P cells would promote invasion of the SRE3-adjacent cassette. High association of Swi6 and Swi2 in the mating-type region of M cells would promote invasion of the SRE2-adjacent cassette.
How pre-existing chromatin structures affect recombination and DSB repair is poorly understood in spite of a great relevance for the maintenance of genome integrity in all eukaryotes. Competitions between donors for gene conversions [55],[56] and regional, cell-type specific, control of recombination [57]–[59] were observed in S. cerevisiae similar to what we observed here. Indeed, much of our knowledge on the effects of chromatin on recombination was acquired using S. cerevisiae [59]–[61]. Our characterization of the fission yeast SRE elements opens the field for further in vivo and in vitro studies of recombination regulation in other chromatin contexts.
Materials and Methods
Standard procedures were used to construct and examine S. pombe strains. The details of the strain constructions, Southern blots and microscopy are presented in Text S1 (Extended experimental procedures). Strain genotypes are listed in Table S1 and oligonucleotide sequences in Table S2.
Supporting Information
Zdroje
1. KlarAJ (2007) Lessons learned from studies of fission yeast mating-type switching and silencing. Annu Rev Genet 41: 213–236.
2. KellyM, BurkeJ, SmithM, KlarA, BeachD (1988) Four mating-type genes control sexual differentiation in the fission yeast. EMBO J 7: 1537–1547.
3. BeachDH (1983) Cell type switching by DNA transposition in fission yeast. Nature 305: 682–687.
4. BeachDH, KlarAJ (1984) Rearrangements of the transposable mating-type cassettes of fission yeast. EMBO J 3: 603–610.
5. EgelR, BeachDH, KlarAJ (1984) Genes required for initiation and resolution steps of mating-type switching in fission yeast. Proc Natl Acad Sci USA 81: 3481–3485.
6. ThonG, CohenA, KlarAJ (1994) Three additional linkage groups that repress transcription and meiotic recombination in the mating-type region of Schizosaccharomyces pombe. Genetics 138: 29–38.
7. ThonG, BjerlingKP, NielsenIS (1999) Localization and properties of a silencing element near the mat3-M mating-type cassette of Schizosaccharomyces pombe. Genetics 151: 945–963.
8. NomaK, AllisCD, GrewalSI (2001) Transitions in distinct histone H3 methylation patterns at the heterochromatin domain boundaries. Science 293: 1150–1155.
9. ThonG, BjerlingP, BunnerCM, Verhein-HansenJ (2002) Expression-state boundaries in the mating-type region of fission yeast. Genetics 161: 611–622.
10. EkwallK, JaverzatJP, LorentzA, SchmidtH, CranstonG, et al. (1995) The chromodomain protein Swi6: a key component at fission yeast centromeres. Science 269: 1429–14231.
11. HallIM, ShankaranarayanaGD, NomaK, AyoubN, CohenA, et al. (2002) Establishment and maintenance of a heterochromatin domain. Science 297: 2232–2237.
12. ShankaranarayanaGD, MotamediMR, MoazedD, GrewalSI (2003) Sir2 regulates histone H3 lysine 9 methylation and heterochromatin assembly in fission yeast. Curr Biol 13: 1240–1246.
13. SadaieM, IidaT, UranoT, NakayamaJ (2004) A chromodomain protein, Chp1, is required for the establishment of heterochromatin in fission yeast. EMBO J 23: 3825–3835.
14. YamadaT, FischleW, SugiyamaT, AllisCD, GrewalSI (2005) The nucleation and maintenance of heterochromatin by a histone deacetylase in fission yeast. Mol Cell 20: 173–185.
15. HansenKR, HazanI, ShankerS, WattS, Verhein-HansenJ, et al. (2011) H3K9me-independent gene silencing in fission yeast heterochromatin by Clr5 and histone deacetylases. PLoS Genet 7: e1001268.
16. NonakaN, KitajimaT, YokobayashiS, XiaoG, YamamotoM, et al. (2002) Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nat Cell Biol 4: 89–93.
17. AkamatsuY, DziadkowiecD, IkeguchiM, ShinagawaH, IwasakiH (2003) Two different Swi5-containing protein complexes are involved in mating-type switching and recombination repair in fission yeast. Proc Natl Acad Sci USA 100: 15770–15775.
18. MotamediMR, HongEJ, LiX, GerberS, DenisonC, et al. (2008) HP1 proteins form distinct complexes and mediate heterochromatic gene silencing by nonoverlapping mechanisms. Mol Cell 32: 778–790.
19. FischerT, CuiB, DhakshnamoorthyJ, ZhouM, RubinC, et al. (2009) Diverse roles of HP1 proteins in heterochromatin assembly and functions in fission yeast. Proc Natl Acad Sci USA 106: 8998–9003.
20. DalgaardJZ, KlarAJ (1999) Orientation of DNA replication establishes mating-type switching patterns in S. pombe. Nature 400: 181–184.
21. DalgaardJZ, KlarAJ (2001) A DNA replication-arrest site in RTS1 regulates imprinting by determining the direction of replication at mat1 in S. pombe. Genes Dev 15: 2060–2068.
22. KlarAJ (1987) Differentiated parental DNA strands confer developmental asymmetry on daughter cells in fission yeast. Nature 326: 466–470.
23. KlarAJ (1990) The developmental fate of fission yeast cells is determined by the pattern of inheritance of parental and grandparental DNA strands. EMBO J 9: 1407–1415.
24. HolmesAM, KaykovA, ArcangioliB (2005) Molecular and cellular dissection of mating-type switching steps in Schizosaccharomyces pombe. Mol Cell Biol 25: 303–311.
25. VengrovaS, DalgaardJZ (2004) RNase-sensitive DNA modification(s) initiates S. pombe mating-type switching. Genes Dev 18: 794–804.
26. VengrovaS, DalgaardJZ (2006) The wild-type Schizosaccharomyces pombe mat1 imprint consists of two ribonucleotides. EMBO Rep 7: 59–65.
27. ArcangioliB (1998) A site- and strand-specific DNA break confers asymmetric switching potential in fission yeast. EMBO J 17: 4503–4510.
28. KaykovA, ArcangioliB (2004) A programmed strand-specific and modified nick in S. pombe constitutes a novel type of chromosomal imprint. Curr Biol 14: 1924–1928.
29. ArcangioliB (2000) Fate of mat1 DNA strands during mating-type switching in fission yeast. EMBO Rep 1: 145–150.
30. KaykovA, HolmesAM, ArcangioliB (2004) Formation, maintenance and consequences of the imprint at the mating-type locus in fission yeast. EMBO J 23: 930–938.
31. Yamada-InagawaT, KlarAJ, DalgaardJZ (2007) Schizosaccharomyces pombe switches mating type by the synthesis-dependent strand-annealing mechanism. Genetics 177: 255–265.
32. FleckO, HeimL, GutzH (1990) A mutated swi4 gene causes duplications in the mating-type region of Schizosaccharomyces pombe. Curr Genet 18: 501–509.
33. FleckO, MichaelH, HeimL (1992) The swi4+ gene of Schizosaccharomyces pombe encodes a homologue of mismatch repair enzymes. Nucleic Acids Res 20: 2271–2278.
34. FleckO, RudolphC, AlbrechtA, LorentzA, SchärP, et al. (1994) The mutator gene swi8 effects specific mutations in the mating-type region of Schizosaccharomyces pombe. Genetics 138: 621–632.
35. RödelC, JupitzT, SchmidtH (1997) Complementation of the DNA repair-deficient swi10 mutant of fission yeast by the human ERCC1 gene. Nucleic Acids Res 25: 2823–2827.
36. RudolphC, KunzC, ParisiS, LehmannE, HartsuikerE, et al. (1999) The msh2 gene of Schizosaccharomyces pombe is involved in mismatch repair, mating-type switching, and meiotic chromosome organization. Mol Cell Biol 19: 241–250.
37. MiyataH, MiyataM (1981) Mode of conjugation in homothallic cells of Schizosaccharomyces pombe. J Gen Appl Microbiol 27: 365–371.
38. EgelR, EieB (1987) Cell lineage asymmetry in Schizosaccharomyces pombe: Unilateral transmission of a high-frequency state of mating-type switching in diploid pedigrees. Curr Genet 12: 429–433.
39. ThonG, KlarAJ (1993) Directionality of fission yeast mating-type interconversion is controlled by the location of the donor loci. Genetics 134: 1045–1054.
40. GrewalSI, KlarAJ (1997) A recombinationally repressed region between mat2 and mat3 loci shares homology to centromeric repeats and regulates directionality of mating-type switching in fission yeast. Genetics 146: 1221–1238.
41. JiaS, YamadaT, GrewalSI (2004) Heterochromatin regulates cell type-specific long-range chromatin interactions essential for directed recombination. Cell 119: 469–480.
42. ThonG, HansenKR, AltesSP, SidhuD, SinghG, et al. (2005) The Clr7 and Clr8 directionality factors and the Pcu4 cullin mediate heterochromatin formation in the fission yeast Schizosaccharomyces pombe. Genetics 171: 1583–1595.
43. OstermannK, LorentzA, SchmidtH (1993) The fission yeast rad22 gene, having a function in mating-type switching and repair of DNA damages, encodes a protein homolog to Rad52 of Saccharomyces cerevisiae. Nucleic Acids Res 21: 5940–5944.
44. GrishchukAL, KraehenbuehlR, MolnarM, FleckO, KohliJ (2004) Genetic and cytological characterization of the RecA-homologous proteins Rad51 and Dmc1 of Schizosaccharomyces pombe. Curr Genet 44: 317–328.
45. MurayamaY, KurokawaY, MayanagiK, IwasakiH (2008) Formation and branch migration of Holliday junctions mediated by eukaryotic recombinases. Nature 451: 1018–1021.
46. HarutaN, KurokawaY, MurayamaY, AkamatsuY, UnzaiS, et al. (2006) The Swi5-Sfr1 complex stimulates Rhp51/Rad51- and Dmc1-mediated DNA strand exchange in vitro. Nat Struct Mol Biol 13: 823–830.
47. AkamatsuY, TsutsuiY, MorishitaT, SiddiqueMS, KurokawaY, et al. (2007) Fission yeast Swi5/Sfr1 and Rhp55/Rhp57 differentially regulate Rhp51-dependent recombination outcomes. EMBO J 26: 1352–1362.
48. KurokawaY, MurayamaY, Haruta-TakahashiN, UrabeI, IwasakiH (2008) Reconstitution of DNA strand exchange mediated by Rhp51 recombinase and two mediators. PLoS Biol 6: e88.
49. KuwabaraN, MurayamaY, HashimotoH, KokabuY, IkeguchiM, et al. (2012) Mechanistic insights into the activation of Rad51-mediated strand exchange from the structure of a recombination activator, the Swi5-Sfr1 complex. Structure 20: 440–449.
50. MatsudaE, Sugioka-SugiyamaR, MizuguchiT, MehtaS, CuiB, et al. (2011) A homolog of male sex-determining factor SRY cooperates with a transposon-derived CENP-B protein to control sex-specific directed recombination. Proc Natl Acad Sci USA 108: 18754–18759.
51. YuC, BonaduceMJ, KlarAJ (2012) Going in the right direction: mating-type switching of Schizosaccharomyces pombe is controlled by judicious expression of two different swi2 transcripts. Genetics 190: 977–987.
52. BreschC, MullerG, EgelR (1968) Genes involved in meiosis and sporulation of a yeast. Mol Gen Genet 102: 301–306.
53. AyoubN, GoldshmidtI, CohenA (1999) Position effect variegation at the mating-type locus of fission yeast: a cis-acting element inhibits covariegated expression of genes in the silent and expressed domains. Genetics 152: 495–508.
54. Aguilar-ArnalL, MarsellachFX, AzorínF (2008) The fission yeast homologue of CENP-B, Abp1, regulates directionality of mating-type switching. EMBO J 27: 1029–1038.
55. KlarAJ, HicksJB, StrathernJN (1982) Directionality of yeast mating-type interconversion. Cell 28: 551–561.
56. CoïcE, MartinJ, RyuT, TaySY, KondevJ, et al. (2011) Dynamics of homology searching during gene conversion in Saccharomyces cerevisiae revealed by donor competition. Genetics 189: 1225–1233.
57. WuX, HaberJE (1996) A 700 bp cis-acting region controls mating-type dependent recombination along the entire left arm of yeast chromosome III. Cell 87: 277–285.
58. SzetoL, FafaliosMK, ZhongH, VershonAK, BroachJR (1997) Alpha2p controls donor preference during mating type interconversion in yeast by inactivating a recombinational enhancer of chromosome III. Genes Dev 11: 1899–1911.
59. HaberJE (2012) Mating-type genes and MAT switching in Saccharomyces cerevisiae. Genetics 191: 33–64.
60. LiJ, CoïcE, LeeK, LeeCS, KimJA, et al. (2012) Regulation of budding yeast mating-type switching donor preference by the FHA domain of Fkh1. PLoS Genet 8: e1002630.
61. SinhaM, PetersonCL (2009) Chromatin dynamics during repair of chromosomal DNA double-strand breaks. Epigenomics 1: 371–385.
Štítky
Genetika Reprodukční medicínaČlánek vyšel v časopise
PLOS Genetics
2013 Číslo 10
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Srdeční frekvence embrya může být faktorem užitečným v předpovídání výsledku IVF
- Primární hyperoxalurie – aktuální možnosti diagnostiky a léčby
- Mateřský haplotyp KIR ovlivňuje porodnost živých dětí po transferu dvou embryí v rámci fertilizace in vitro u pacientek s opakujícími se samovolnými potraty nebo poruchami implantace
- Příjem alkoholu a menstruační cyklus
Nejčtenější v tomto čísle
- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
