-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Different Roles of Eukaryotic MutS and MutL Complexes in Repair of Small Insertion and Deletion Loops in Yeast
DNA mismatch repair greatly increases genome fidelity by recognizing and removing replication errors. In order to understand how this fidelity is maintained, it is important to uncover the relative specificities of the different components of mismatch repair. There are two major mispair recognition complexes in eukaryotes that are homologues of bacterial MutS proteins, MutSα and MutSβ, with MutSα recognizing base-base mismatches and small loop mispairs and MutSβ recognizing larger loop mispairs. Upon recognition of a mispair, the MutS complexes then interact with homologues of the bacterial MutL protein. Loops formed on the primer strand during replication lead to insertion mutations, whereas loops on the template strand lead to deletions. We show here in yeast, using oligonucleotide transformation, that MutSα has a strong bias toward repair of insertion loops, while MutSβ has an even stronger bias toward repair of deletion loops. Our results suggest that this bias in repair is due to the different interactions of the MutS complexes with the MutL complexes. Two mutants of MutLα, pms1-G882E and pms1-H888R, repair deletion mispairs but not insertion mispairs. Moreover, we find that a different MutL complex, MutLγ, is extremely important, but not sufficient, for deletion repair in the presence of either MutLα mutation. MutSβ is present in many eukaryotic organisms, but not in prokaryotes. We suggest that the biased repair of deletion mispairs may reflect a critical eukaryotic function of MutSβ in mismatch repair.
Published in the journal: . PLoS Genet 9(10): e32767. doi:10.1371/journal.pgen.1003920
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003920Summary
DNA mismatch repair greatly increases genome fidelity by recognizing and removing replication errors. In order to understand how this fidelity is maintained, it is important to uncover the relative specificities of the different components of mismatch repair. There are two major mispair recognition complexes in eukaryotes that are homologues of bacterial MutS proteins, MutSα and MutSβ, with MutSα recognizing base-base mismatches and small loop mispairs and MutSβ recognizing larger loop mispairs. Upon recognition of a mispair, the MutS complexes then interact with homologues of the bacterial MutL protein. Loops formed on the primer strand during replication lead to insertion mutations, whereas loops on the template strand lead to deletions. We show here in yeast, using oligonucleotide transformation, that MutSα has a strong bias toward repair of insertion loops, while MutSβ has an even stronger bias toward repair of deletion loops. Our results suggest that this bias in repair is due to the different interactions of the MutS complexes with the MutL complexes. Two mutants of MutLα, pms1-G882E and pms1-H888R, repair deletion mispairs but not insertion mispairs. Moreover, we find that a different MutL complex, MutLγ, is extremely important, but not sufficient, for deletion repair in the presence of either MutLα mutation. MutSβ is present in many eukaryotic organisms, but not in prokaryotes. We suggest that the biased repair of deletion mispairs may reflect a critical eukaryotic function of MutSβ in mismatch repair.
Introduction
DNA mismatch repair (MMR) is a major repair system in organisms ranging from bacteria to humans. The discovery that MMR defects cause the most common form of inherited colon cancer underscored the importance of this repair pathway to human health [1]–[6]. In eukaryotes, MMR involves recognition of mismatches created during replication by protein complexes that are homologues of bacterial MutS, followed by downstream processing events involving homologues of bacterial MutL [7]–[9]. There are two main recognition complexes, MutSα, a heterodimer consisting of Msh2 and Msh6 that recognizes base-base mismatches and small loops, and MutSβ, a heterodimer consisting of Msh2 and Msh3 that recognizes mainly loops [7]–[10].
The exact role that MutSβ plays in MMR is not clear. Loss of MutSβ causes only a weak mutator effect unless the assay is specific for insertion or deletion (in/del) mutations [11], [12]. In general, there seems to be much less MutSβ protein than MutSα protein in yeast and human cells [13]–[15]; however, a recent report suggests that the relative amounts of MutSα and MutSβ vary in mouse tissues, with some tissues containing more MutSβ than MutSα [16]. A number of organisms such as Drosophila melanogaster and Caenorhabditis elegans apparently have no MutSβ although they have MutSα [17]. Most analysis of MutSβ MMR function has tended to center on its repair of loops compared to the repair of base-base mismatches by MutSα. However, two early studies of MutSβ and microsatellite instability in yeast found a surprising difference in loop repair and loss of MutSβ compared to loss of MutSα [18], [19]. For example, using an assay for dinucleotide repeat slippage, Sia et al. found more insertions than deletions in wild-type cells, whereas complete loss of MMR resulted in approximately equal numbers of insertions and deletions; strikingly, cells containing only MutSα had many more deletions than insertions whereas cells containing only MutSβ had many more insertions than deletions [18]. The authors concluded that loops on the primer strand were repaired differently from loops on the template strand.
The role of MutL proteins in MMR is less well understood, although they act downstream of initial mismatch detection [7]–[9]. In both yeast and mammalian cells, there are three MutL complexes: MutLα, MutLβ, and MutLγ [7]–[9]. Downstream processing usually involves MutLα, in yeast a heterodimer of Mlh1 and Pms1 [7]–[9]. In yeast, it appears that both MutLβ (consisting of Mlh1 and Mlh2) and MutLγ (Mlh1 and Mlh3) play a role in correction of deletion mutations, although the effect is minor and depends on a sensitive assay [20], [21]. Although MutL proteins are not thought to have any specific recognition of mismatches, two mutations in PMS1, pms1-G882E and pms1-H888R, were shown to result in substantial increases in +1 insertions but had essentially no effect on repair of base-base mismatches or deletions [22].
Biochemical analysis has given no information about how MMR could differentiate between mismatches that would lead to insertion versus deletions. The experiments above that have indicated that MMR might repair insertion and deletion loops differently have been rather limited, and we wished to examine in/del mutagenesis in an environment in which sequence context, transcriptional strand, and replication strand could be controlled. We had previously found that we could generate insertion mutations of various sizes and compositions in vivo via single-strand oligonucleotide (oligo) transformation that was subject to MMR [23]. In that case, the oligos produced loops on the primer strand of replication that in the absence of repair led to insertion mutations; we had not tested whether oligos could induce loops on the template strand of replication that would lead to deletion mutations. Here we show that, in the absence of MMR, oligo transformation can be used to induce template-strand loops that lead to deletion mutations (deletion loops) with essentially the same efficiency as primer-strand loops that lead to insertion mutations (insertion loops). Using this assay we find that, when only MutSα is present, insertion loops are repaired with a greater efficiency than deletion loops, whereas in the presence of only MutSβ, insertion loops are poorly repaired, but deletion loops are efficiently repaired. Deletion loops are repaired almost as efficiently in strains containing pms1-G882E or pms1-H888R as in wild type strains, whereas insertion loops are not repaired. Surprisingly, repair of deletion loops in pms1-G882E or pms1-H888R mutant strains has a major dependence on MutLγ. Our data indicate that the biased repair of insertion versus deletion loops is dependent on interactions with the MutL proteins. We suggest that these properties of MMR can best be understood in an evolutionary sense in which MutSα represents the functions of bacterial MutS to repair base-base mismatches and in/del mismatches with a bias toward insertions, whereas MutSβ represents a new function present in some eukaryotes that complements MutSα function with respect to repair of deletion mismatches, chiefly through a different interaction with MutL proteins.
Results
An assay for in/del mutations
We had previously used oligo transformation to study insertion mutations using the lys2ΔA746 frameshift reversion assay that requires restoration of a −1 frameshift in a region of the LYS2 gene indifferent to amino acid sequence [23], [24]. We wanted to study the effects of MMR on deletion mutations as well as insertion mutations, but, because of the different affinities in binding of loop sizes by MutSα and MutSβ, it was necessary to compare the effects of insertion and deletion mismatches of the same size, requiring the use of two complementary reversion assays. We therefore used both the −1 lys2ΔA746 frameshift allele and the +1 frameshift allele lys2ΔBgl in the same LYS2 region [23]–[25]. In order to have reversion windows with known orientations relative to a dependable origin of replication and to have the different frameshift alleles as similar as possible, we used the LYS2 genes inserted in both orientations (“same” and “opposite”) at the HIS4 locus previously described [26] and inserted the frameshift alleles as described in MATERIALS AND METHODS. The −1 lys2ΔA746 frameshift allele was used to study +1 and −2 loops, and the +1 frameshift allele lys2ΔBgl was used to study −1 and +2 loops. The overall scheme for the assay is illustrated in Figure 1.
Fig. 1. An assay for loop repair. 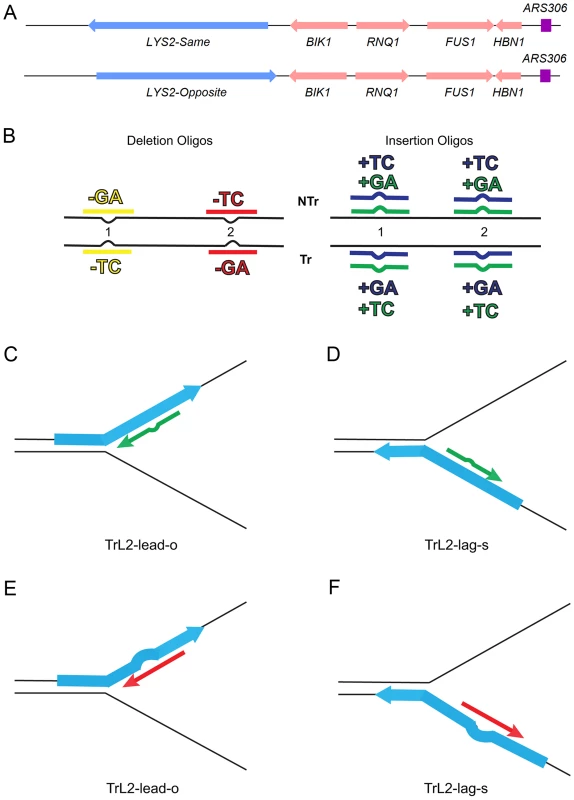
(A) The initial strains used to construct the assay for in/del loop repair were a set of isogenic strains containing the LYS2 gene replacing the HIS4 gene near the ARS306 origin of replication as shown above [26]. “Same” and “Opposite” refer to the orientation of the LYS2 gene relative to the orientation of the original HIS4 gene [26]. The wild-type LYS2 sequences were subsequently replaced with sequences to create either the −1 frameshift allele lys2ΔA746 or the +1 frameshift allele lys2ΔBgl [23]–[25]. (B) The −1 lys2ΔA746 and the +1 lys2ΔBgl frameshift alleles can be reverted to wild-type by a compensating addition or deletion of nucleotides anywhere within an approximately 200-bp reversion window [23]–[25]. Oligos with sequences corresponding to two different locations within the reversion window of the mutant alleles and ranging in size from 31–36 nt were used to produce Lys+ revertants (Table S4). The colors indicated are those used in subsequent figures and also in Table S4. The red and yellow oligos induce a 2-nt loss in lys2ΔA746 strains and the blue and green oligos insert 2 nt into lys2ΔBgl strains. Single-stranded oligos are used for transformation, and can therefore have the sequence of either the transcribed (Tr) or non-transcribed strand (NTr). Oligos inducing 1-nt in/del mutations follow a similar color and naming scheme (see text for details). (C–F) Oligos transform by serving as primers for subsequent replication, on either the leading or lagging strands of replication. If the mismatch created by the oligo is not removed during replication, a reverting frameshift will result in the next round of replication. Additional nucleotides in the oligo will create a primer-strand loop and thus an insertion mutation; missing nucleotides in the oligo will create a loop on the template strand and thus lead to a deletion mutation. (C) and (D) indicate that the same oligo (an oligo with the sequence of the transcribed strand (Tr) in location 2 (L2) adding a sequence of TC) will anneal to the leading strand of replication in lys2ΔBgl strains of the Opposite orientation (TrL2-lead-o) or to the lagging strand in strains of the Same orientation (TrL2-lag-s). (E) and (F) show the same process for an oligo inducing a deletion of GA in lys2ΔA746 strains by annealing on the leading strand in strains of the Opposite orientation (TrL2-lead-o) or on the lagging strand of strains in the Same orientation (TrL2-lag-s). The efficiency of recognition by MMR is known to be dependent not only on the mispaired bases, but also on the sequence context surrounding the mispaired bases [10], [27]. Therefore we used a collection of oligos that created different mispairs, in two sequence contexts (Figure 1B). Because we transform with single-stranded oligos, the oligos can have the sequence of the transcribed strand and create a TC or GA insertion loop or have the sequence of the nontranscribed strand and create a TC or GA insertion loop, all in otherwise the same sequence context. This was done in two different locations within the reversion windows of the lys2 mutant alleles. Deletion loops are created by transforming with oligos lacking certain bases contained in the template strand; therefore different deletion loop sequences cannot be created in the same sequence context using the same strains.
Oligos induce 2-nt insertion or deletion mutations with approximately the same efficiency in the absence of MMR
As detailed in Materials and Methods, an oligo was transformed into a given strain in three independent experiments, and the average number of transformants over background reversion events was determined. All oligos were transformed into two strains with opposite orientations of the LYS2 gene relative to the nearby origin of replication so that the effect of loops on the leading versus lagging strand could be assessed. The results of transformation with a selected set of oligos in strains containing or lacking certain components of MMR are given in Figure 2 and the full set of results is given in Figure S1.
Fig. 2. Effect of MMR on 2-nt in/del mismatches. 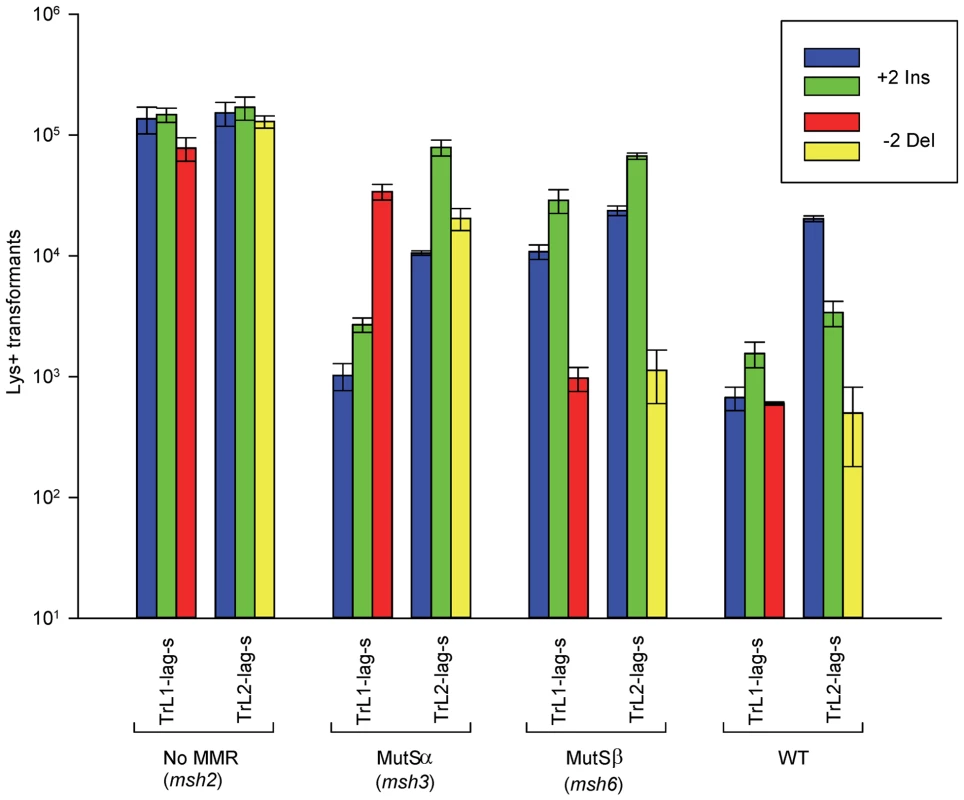
The mean number of Lys+ revertants, with standard deviation, is shown for the indicated oligo and strain combination. The coloring is explained in Figure 1 and oligo sequences are given in Table S4. TrL1 and TrL2 refer to oligos with the sequence of the transcribed strand in Location 1 and 2, respectively. For the Tr oligos, annealing to the lagging strand occurs in strains with the Same orientation (Lag-s). The fewer transformants obtained for a given oligo and strain combination, the better the repair for the mismatch created by the oligo. Oligos creating insertion loops are transformed into lys2ΔBgl strains and oligos creating deletion loops are transformed into lys2ΔA746 strains. As an example, all TrL1 oligos are essentially identical in sequence, with the exception that the “blue” oligo inserts a +GA loop, the “green” oligo inserts a +TC loop, and the “red” oligo causes a 2-nt −GA deletion loop in the template strand opposite the location of the + loops in the other two oligos. There is no active MMR in msh2 strains, whereas msh3 strains have MutSα present and msh6 strains contain MutSβ. Several patterns can be observed in the results in strains lacking MMR (msh2). Each pair of oligos differing only in the insertion bases gave results that were generally not statistically different from one another. Comparing any set of oligos (e.g. TrL1-Lag-s), the difference between insertions and deletions was generally less than 2-fold, with a mixture of insertions, deletions, or neither predominating. Finally, as we have observed previously [23], [28], in all cases the number of insertions or deletions was greater when targeted to the lagging strand than to the leading strand, by an average of approximately 6-fold in these experiments. Therefore we can conclude that, in the absence of MMR, insertion and deletion mutations can be created at approximately equal efficiencies by oligos.
MutSα and MutSβ have opposite effects on 2-nt insertion versus deletion mispairs
In contrast to oligo transformation in the absence of MMR, one can see quite different patterns of transformation in strains containing only MutSα (msh3 strains) or MutSβ (msh6 strains) in Figure 2. To compare the effect of MMR on transformation, we divided the average number of revertants obtained in the absence of MMR by the average number of revertants obtained in a given MMR background to give a Repair Ratio (Table 1). The larger the Repair Ratio, the more effectively the loop created by the oligo was removed. The results in strains containing only MutSβ (msh6 strains) are very consistent, as can be seen in Figures 2 and S1 and Table 1. In every case, deletion mispairs were corrected much more efficiently than insertion mispairs; in Table 1, the Repair Ratios for insertions range from 1 to 13 and for deletions from 59 to 310. The results in strains containing only MutSα (msh3 strains) were more varied. Uniformly, deletion mispairs are poorly repaired, with a range of Repair Ratios of 2 to 9 (Table 1). Insertion mispairs are repaired with a wide range of efficiencies of 2 to 130 (Table 1). The one consistent difference is that within the same sequence context, a GA sequence in the loop is always repaired more efficiently than a TC. However, when only MutSα is present, insertion loops are repaired overall with much greater efficiency than deletion loops. Additionally, in the presence of only MutSα, insertion loops are repaired with somewhat greater efficiency when the loop is on the lagging strand compared to the leading strand, with an average ratio of 1.6, whereas, when only MutSβ is present, deletion loops on the leading strand are repaired 1.6-fold more efficiently than on the lagging strand, a difference we previously found under other circumstances [23]. The difference between these two ratios is statistically significant as determined by a Mann-Whitney rank sum test (P = 0.038). A median measure of the insertion and deletion loop Repair Ratios is given in Table 2, which illustrates the differing biases of MutSα and MutSβ.
Tab. 1. Repair Ratios for 2-nt in/del mispairs. 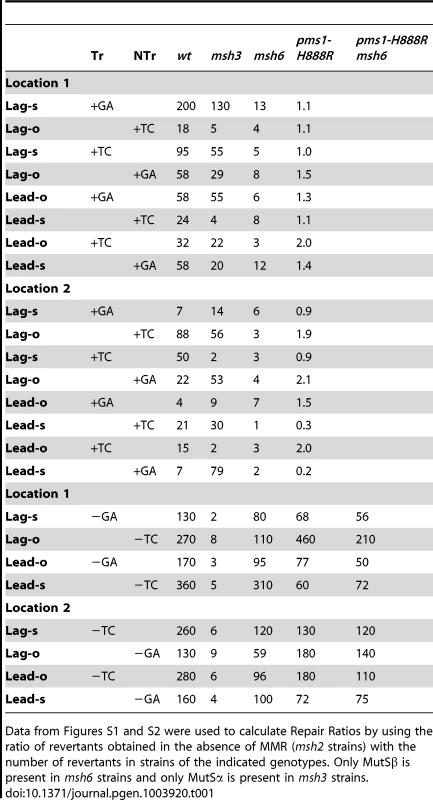
Data from Figures S1 and S2 were used to calculate Repair Ratios by using the ratio of revertants obtained in the absence of MMR (msh2 strains) with the number of revertants in strains of the indicated genotypes. Only MutSβ is present in msh6 strains and only MutSα is present in msh3 strains. Tab. 2. Median Repair Ratios for 2-nt in/del mismatches. 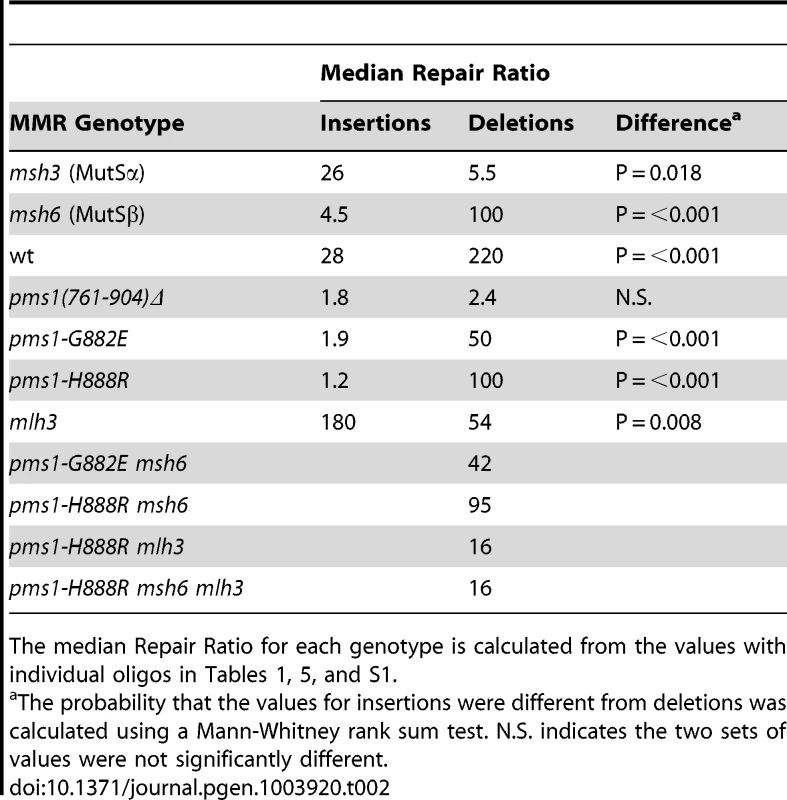
The median Repair Ratio for each genotype is calculated from the values with individual oligos in Tables 1, 5, and S1. Deletion mispairs of 2 nt are more efficiently corrected than insertion mispairs in wild-type strains
There is inherently more error associated with measurement of revertants in cells that are wild type for MMR, as the number of revertants can be decreased by over two orders of magnitude to quite low numbers. However, a consistent pattern emerges as observed both in Figures 2 and S1 and in Tables 1 and 2: 2-nt deletion mispairs are corrected more efficiently than insertion mispairs in strains wild type for MMR. Deletion mispairs are corrected with an efficiency somewhat greater than that of cells containing MutSβ alone (usually less than two-fold), presumably reflecting the ability of MutSα to recognize deletion mispairs, albeit at a much lower efficiency than does MutSβ. Insertion mispairs are generally corrected with an efficiency greater than that observed in cells with MutSα alone, although in 5 cases, insertion mispairs were corrected less efficiently than in MutSα cells, and in one other case about the same (Table 1). One explanation for those situations could be a dilution in MutSα molecules due to Msh3 pairing with some of the Msh2 [13], [14]. A dinucleotide repeat stability assay previously showed that 2-nt deletions were repaired with a greater efficiency than insertions in strains wild-type for MMR [18], [19].
Two mutations in PMS1, pms1-G882E and pms1-H888R, result in repair deficiency of 2-nt insertions
A screen for mutations in PMS1 found two mutants that resulted in large increases in +1 insertions but had no effect on deletions [22]. We tested those mutations in our assay system to see if they would have a similar effect on 2-nt in/del mispairs. The results are shown in Figures 3 and S2.
Fig. 3. The effect of mutations in PMS1 on 2-nt in/del mispairs. 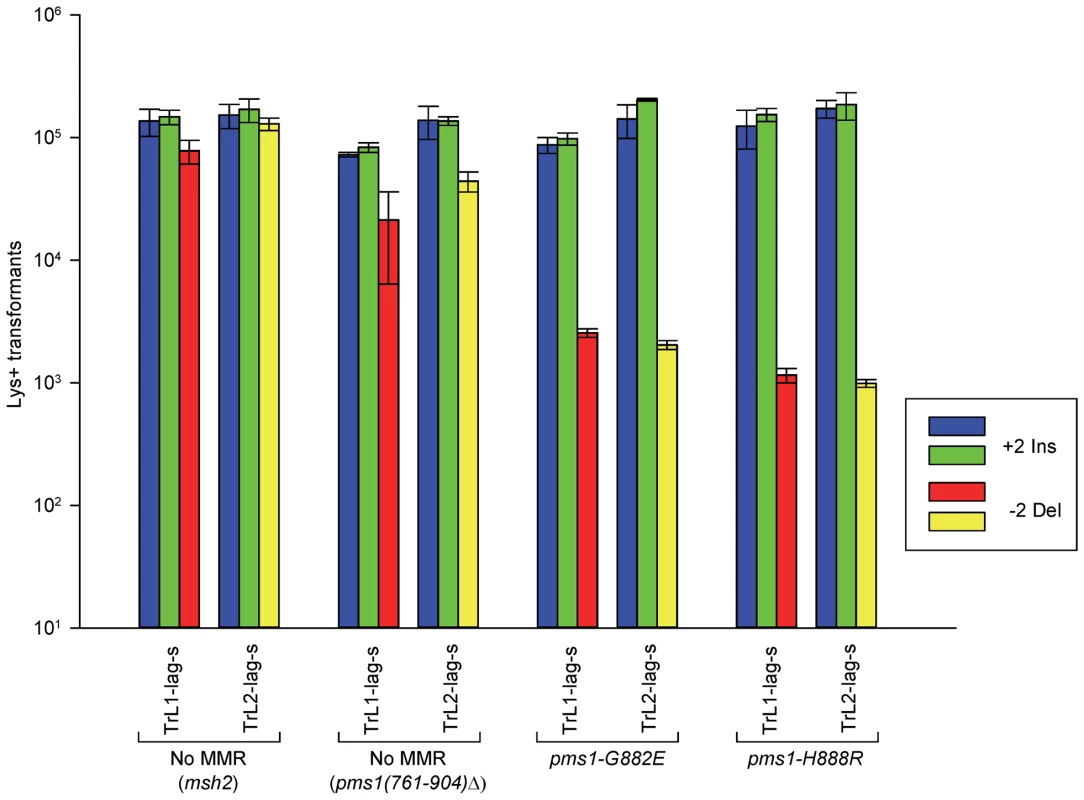
Oligos were transformed into strains of the indicated genotypes and analyzed as in Figure 2; the msh2 results are those given in Figure 2. As described in Materials and Methods, the pms1(761-904)Δ mutant was a precursor in construction of the two PMS1 point mutations; terminal deletions of that length have previously been shown to be nonfunctional [29]. Pms1 is needed for most repair, as the pms1(761-904)Δ strains behave similarly to the msh2 strains. However, the msh2 strains generally had more transformants, averaging 1.7-fold more insertions and 2.5-fold more deletions (Table S1), suggesting that some in/del repair might be mediated by complexes lacking Pms1. Strains containing either of the two PMS1 point mutations show an extreme difference in repair of insertion versus deletion mispairs that is evident in Figures 3 and S2 and given quantitatively in Tables 1 and S1. Both mutant strains repair deletion mispairs but have little effect on insertion mispairs. The median effect of each mutation is presented in Table 2. The effect of the two mutations, pms1-G882E and pms1-H888R are similar, but the pms1-H888R mutants appear to have a more distinctive effect, with almost no repair of insertion mispairs but more repair of deletion mispairs than the pms1-G882E mutants. Because there is very little repair of deletion mispairs in the absence of Pms1 (Table S1), the pms1 point mutants must be functional in deletion repair.
Similar MMR effects are observed in 1-nt in/del mispairs
Previously, the evidence for the differential effect of MutSα and MutSβ on in/del mutations came from a dinucleotide repeat assay, although an assay using one particular mononucleotide repeat indicated that the loss of either MutSα or MutSβ led to an increase mainly of deletions [18]. The pms1-G882E and pms1-H888R mutations had only been examined with mononucleotide repeats [22]. Therefore we wanted to examine whether the effects we observed on 2-nt in/del mispairs would be observed in similar 1-nt in/del mismatches. For that survey, we used only oligos in one location, and the results are presented in Figures 4 and S3; quantitative comparisons are given in Table 3.
Fig. 4. Effect of MMR on 1-nt in/del mismatches. 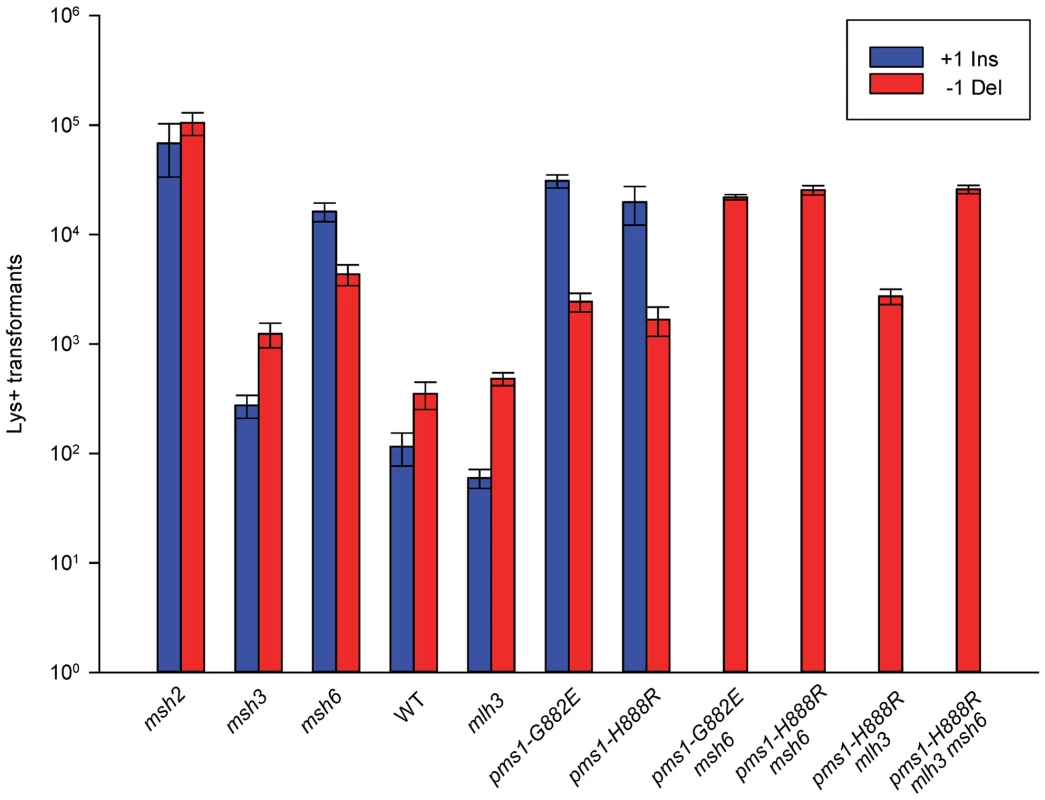
TrL1 Oligos were transformed into Same-orientation strains of the indicated genotypes and analyzed as in Figure 2 (TrL1-Lag-s). For 1-nt in/del mismatches, oligos creating insertion loops are transformed into lys2ΔA746 strains and oligos creating deletion loops are transformed into lys2ΔBgl strains. Only MutSβ is present in msh6 strains and only MutSα is present in msh3 strains. Tab. 3. In/del Repair Ratios for 1 nt mispairs. 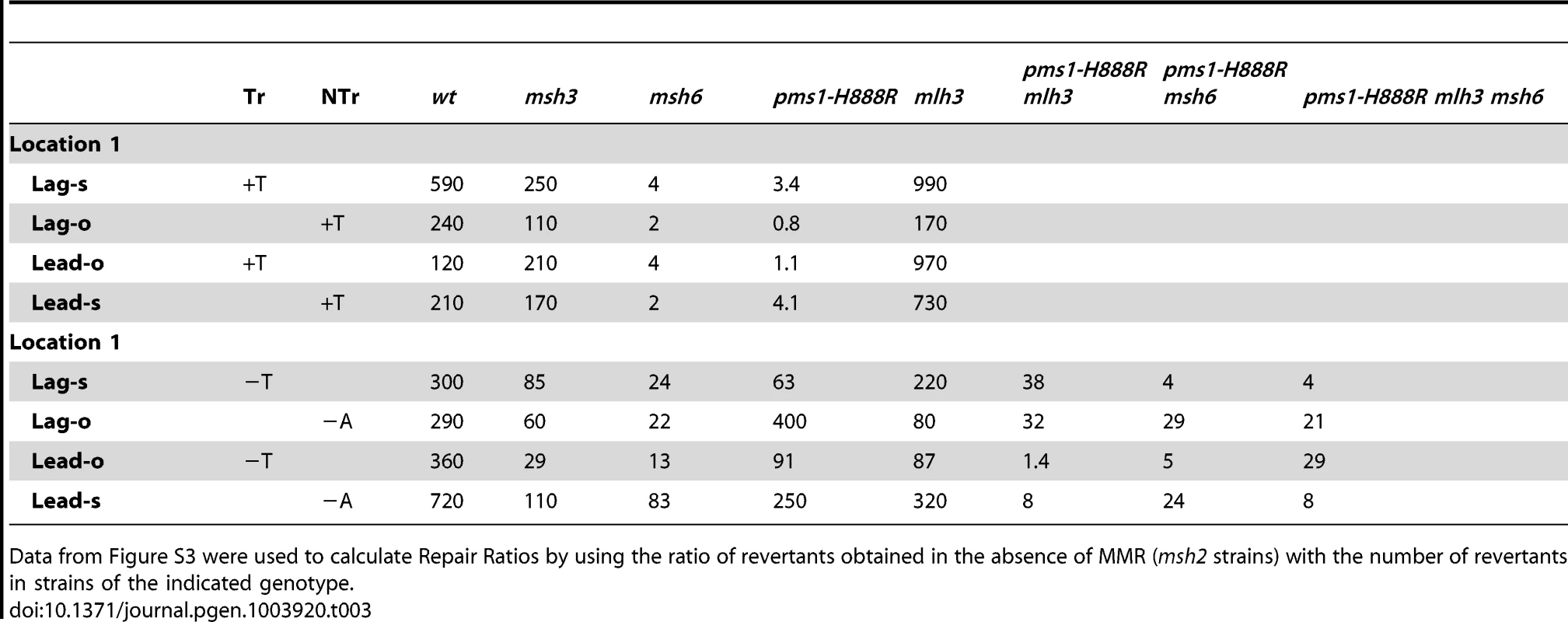
Data from Figure S3 were used to calculate Repair Ratios by using the ratio of revertants obtained in the absence of MMR (msh2 strains) with the number of revertants in strains of the indicated genotype. There are similarities to the results with 2-nt in/del mismatches in terms of the opposing biases for insertions versus deletions, but the quantitative results differ, presumably due to the relatively greater affinity of MutSα recognition for 1-nt loops over 2-nt loops, and the correspondingly lower recognition of MutSβ for 1-nt loops compared to 2-nt loops. MutSα has an overall much greater effect on suppression of 1-nt in/del mismatches than does MutSβ, and MutSα has substantial activity on 1-nt deletion loops in contrast to its activity on 2-nt deletion loops (Figures 4 and S3). Even so, MutSα has a consistently greater activity toward 1-nt insertion mismatches, whereas the MutSβ activity is the reverse. In contrast, the pms1-G882E and pms1-H888R mutants have about the same lack of insertion repair as exhibited on 2-nt in/del mispairs (Tables 3, S2). However, deletion repair in the pms1-G882E and pms1-H888R mutants is much more efficient than that in strains containing only MutSβ, indicating the involvement of MutSα in 1-nt deletion loop repair. The median Repair Ratios are given in Table 4 and illustrate that in contrast to the situation with 2-nt loops, there is relatively more repair of deletions with MutSα only and relatively less repair of deletions with MutSβ only, and in wild-type cells insertions and deletion mismatches are corrected with indistinguishable efficiency.
Tab. 4. Median Repair Ratios for 1-nt in/del mismatches. 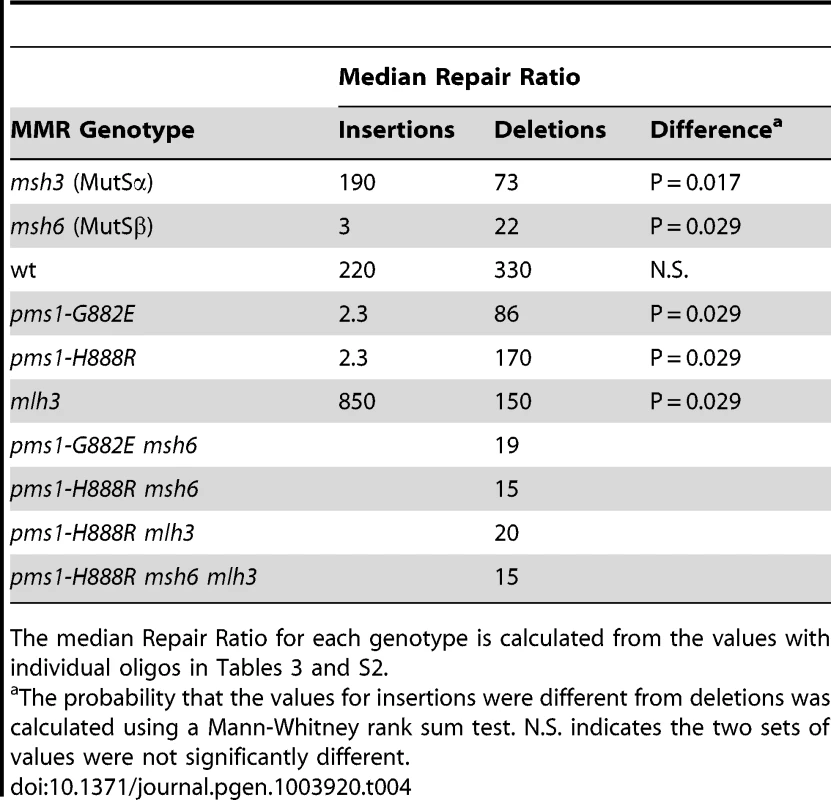
The median Repair Ratio for each genotype is calculated from the values with individual oligos in Tables 3 and S2. The interaction of MutSα and PMS1 mutations in in/del repair
How can the specificity of the pms1-G882E and pms1-H888R mutations best be understood? The pms1-G882E and pms1-H888R mutant strains appeared to be similar to msh6 strains lacking MutSα for 2-nt deletion repair; we therefore examined strains containing both msh6 deletions and pms1 mutations to determine if they appeared to be in the same pathway. Because the pms1 mutations fail to repair insertion loops, we could only examine the effect on deletion loop mispairs. The results are given in Figures 5 and S4 and Tables 1, 2, and S1 for 2-nt deletions. Results in the double mutants, pms1-H888R msh6 and pms1-G882E msh6, are not distinguishable from the single pms1 mutant results (Table 2).
Fig. 5. Effect of Mlh3 on 2-nt deletion mispairs. 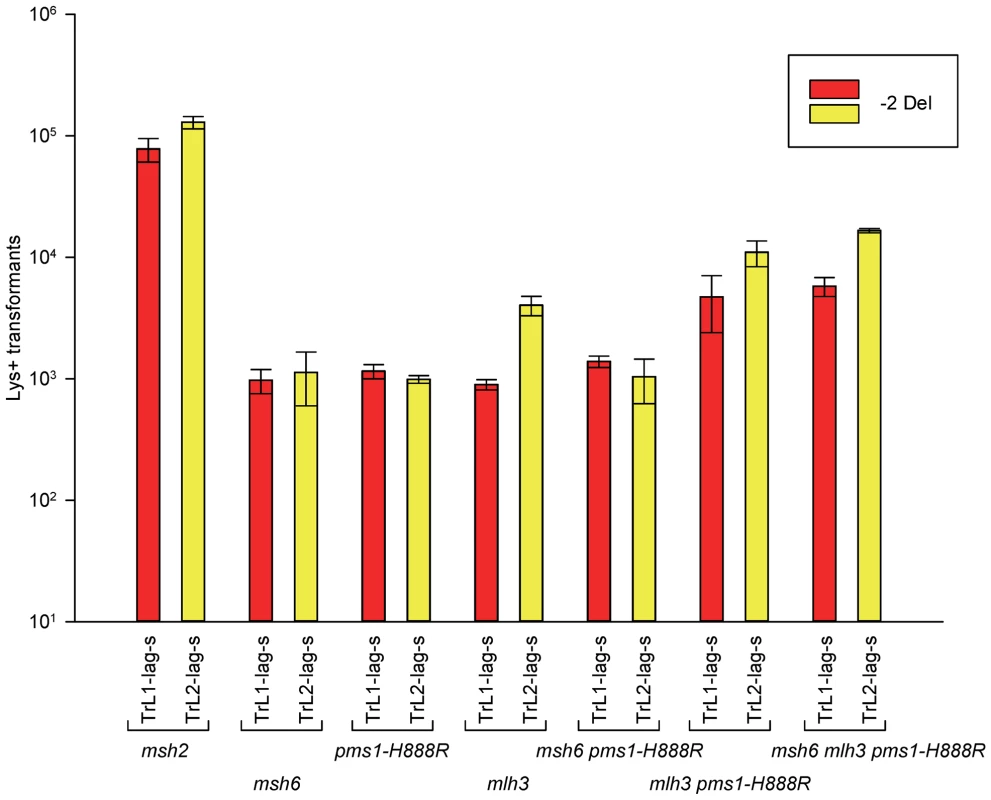
Oligos were transformed into strains of the indicated genotypes and analyzed as in Figure 2. (Data for msh2, msh6, and pms1-H888R are from Figures 2 and 3.) For 1-nt deletion loop repair, MutSα is much more important than in 2-nt loop repair and as noted above, the repair in the pms1 mutants is more efficient than in the presence of only MutSβ. Repair of 1-nt deletion loops in the double pms1-G882E msh6 and pms1-H888R msh6 mutants is much lower than in the single pms1 mutants (Figure 4; Tables 3, S2), indicating that much of the deletion loop repair in the pms1 mutants must be due to the action of MutSα (Table 4).
The role of MutLγ in in/del repair
In order to determine if MutLγ (composed of Mlh1 and Mlh3 subunits [21]) might be involved in some of the observed repair, we examined strains with an MLH3 deletion. The results for 2-nt in/del mispairs are given in Figures 5 and S4 and Tables 2 and 5. It is evident that MutLγ is not involved in repair of insertion mispairs, as Repair Ratios actually increased in the absence of MutLγ (P = 0.019) (perhaps due to a somewhat increased amount of MutLα). An mlh3 deletion resulted in an approximately 4-fold decrease in repair of deletion mispairs (Table 2) (P = <0.001). Those results were expected given the limited effect previously found for mlh3 deletions [10], [20], [21]. The pms1-H888R mutation has less than a 2-fold effect on deletion repair, so one would have anticipated that the double mutant would be similar to the mlh3 mutant. Such was not the case as seen in Tables 2 and 5. The double mutant had an almost 13-fold reduction in deletion repair compared to wild type. The difference between repair in mlh3 and pms1-H888R mlh3 strains is significant, with P = <0.001.
Tab. 5. The effect of Mlh3 on Repair Ratios for 2-nt in/del mispairs. 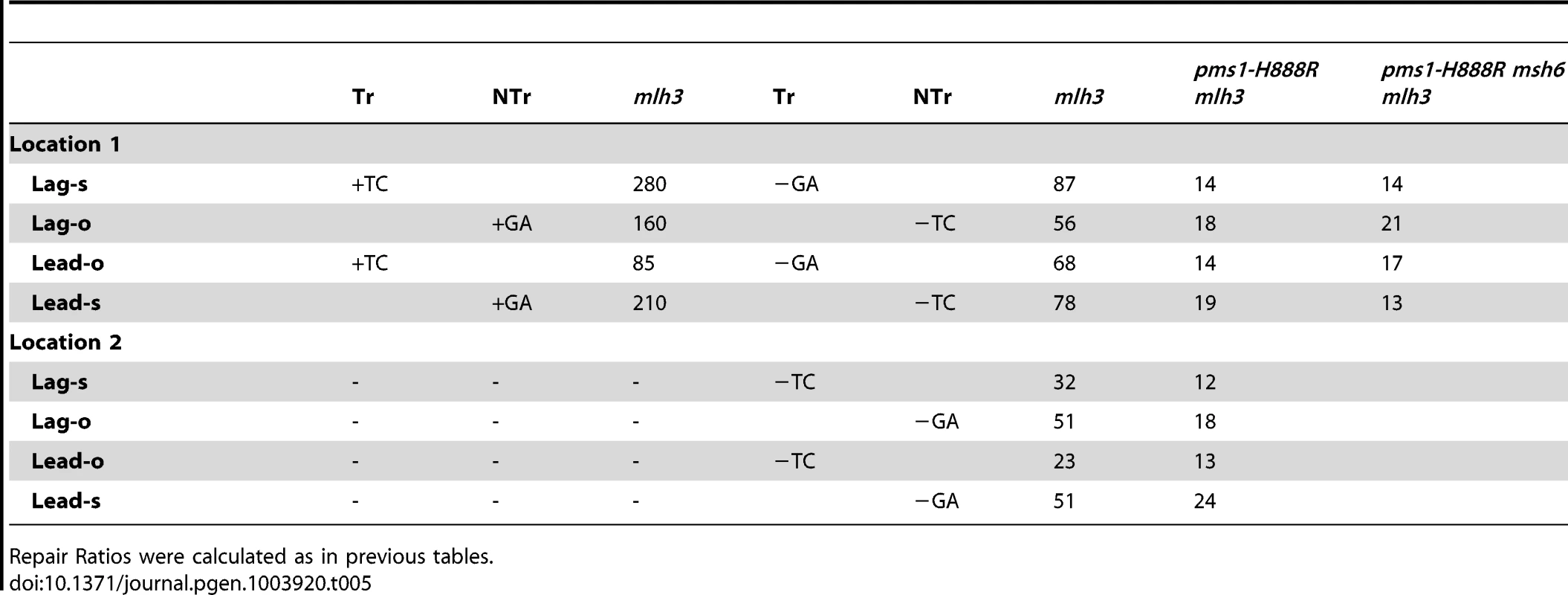
Repair Ratios were calculated as in previous tables. The same pattern was found in 1-nt in/del mismatch repair. A single mlh3 deletion has a relatively small effect on in/del repair, slightly raising the efficiency of insertion repair compared to wild type and slightly decreasing the efficiency of deletion repair, although the difference in both cases is marginally significant (P = 0.05) (Tables 3 and 4). The pms1-H888R mutant has robust deletion repair, but the double mutant pms1-H888R mlh3 was reduced by 20 fold in deletion repair (the difference is significant, with P = 0.029); deleting msh6 had no further effect (Tables 3 and 4). This result was particularly surprising, as MutSα is responsible for much of the 1-nt deletion repair and yet MutLγ has been thought to work only with MutSβ [21].
Discussion
The biases we find here for repair of in/del mispairs had been previously observed in two different systems: a dinucleotide repeat assay for MutSα and MutSβ [18], [19] and frameshift reversion assays for the pms1 mutants [22]. Given the limited scope of each of those experiments, it was not clear whether the results reflected a general property of the proteins involved, or were influenced by the DNA sequences involved in the particular assays used. Our results with a completely different assay system and with a variety of different sequences and gene strands and orientations lend confidence that our observations reflect an inherent difference in repair of insertion versus deletion loops by MMR.
For 2-nt in/del mismatches, strains containing only MutSβ provide the clearest picture of a bias. As shown in Table 1, the repair of all insertion loops tested is poor, ranging from 1 to 13-fold, and the repair of all tested deletion loops is robust, ranging from 60 to 300-fold. Although MutSβ has a measurable effect on repair of most insertions, it is only deletions for which it has a substantial effect. The effect in strains containing only MutSα is a bit more complex. The repair of deletion loops is uniformly low, ranging from 2 to 9-fold (Table 1). The repair of insertion loops is much more variable, with most (13/16) being repaired by a factor of 15 to 200-fold. The greater variability of repair initiated by MutSα compared to MutSβ is presumably a function of a stronger effect of sequence specificity [27]. The median Repair Ratios calculated in Table 2 indicate the remarkable difference between in/del repair mediated by MutSα and MutSβ although the individual data in Table 1 serve as a useful reminder that the particular repair of a given sequence can be quite variable.
Our analysis of repair of 1-nt in/del mispairs was not as extensive as that for 2-nt in/del mispairs, but the results we obtained reveal a similar pattern (Tables 3 and 4). Presumably due to the greater affinity for MutSα for 1-nt in/del mismatches compared to 2-nt in/del mismatches (and the converse for MutSβ) the overall effect of MutSα on repair of both insertion and deletion mismatches is much greater than for 2-nt in/del mismatches, and the effect of MutSβ for 1-nt deletion mismatches is much less than for 2-nt mismatches (Table 4 compared to Table 2). However, one can see that for all tested combinations, insertion loops are repaired more efficiently than deletion loops when only MutSα is present, and the reverse when only MutSβ is present. In this context, it is interesting to observe the overall effect on in/del mismatches as observed in strains wild type for MMR. For 2-nt in/del mismatches, one sees that on average deletion mispairs are repaired significantly better than insertion mispairs (Table 2), whereas for 1-nt mispairs, there is no consistent bias in repair (Table 4), reflecting the relatively greater effect of MutSα on repair.
How can the difference in repair of insertion versus deletion loops be explained? For that question, the existing evidence from biochemistry is not very helpful, as no biochemical experiments have been done in which the strands of duplex DNA that have been used could be identified as primer or template strands in a replication complex. Recent structural studies reveal that MutSβ binding to in/del mismatches takes place in a very different manner from MutSα or MutS binding to mismatches [30], [31]. Biochemical experiments have measured binding affinities, but what we measure here are overall repair efficiencies, for which the binding of MutSα or MutSβ is just the first step.
After MutSα or MutSβ binding, the next step in MMR is an association with a MutL protein complex, which is usually MutLα. If the repair efficiencies we measure were purely the result of MutSα or MutSβ binding efficiencies, then we would expect that any mutant of MutLα would have equivalent effects on in/del mismatch repair. However, the PMS1 mutations we characterize, pms1-G882E and pms1-H888R, show extreme bias in in/del mismatch repair. As observed in Table 1, the effects of both mutations are approximately the same, with the pms1-H888R mutation showing a bit stronger effect. Strains with the pms1-H888R mutation show almost no repair of any insertion, but very robust repair of deletion mispairs, ranging from 60 to 460-fold. A similar effect is seen for 1-nt in/del mispairs (Table 3). The initial characterization of those two mutations showed that they had only a modest effect on overall mutation rate and that their mutator effect was primarily on frameshift deletions [22]. Our results with 2-nt in/del mispairs suggested that the pms1-H888R mutant behaved very much like strains containing only MutSβ, and the double-mutant strains did not seem to be significantly different from either of the single mutants (Table 2). However, as seen in Table 4, the situation is quite different with 1-nt in/del mispairs, as the double mutant strains are much worse at repair of deletions than either pms1 mutant strain, indicating that much of the deletion repair in the pms1-H888R or pms1-G882E mutant is due to MutSα activity.
It is not clear how the two pms1 mutants affect MMR. The two pms1 mutations map into a region described as an Mlh1-interaction region [29], but the interaction of the mutant proteins with Mlh1 was not found to be defective as judged by a two-hybrid assay [22]. A recent structure of the S. cerevisiae MutLα C-terminal domain permits a much better understanding of the location of the mutations within the MutLα protein [32]. At the time of the Erdeniz et al. paper, the initiating ATG codon was thought to be in a location such that the length of the Pms1 protein would be 904 aa. However, a genomic analysis found a different ATG codon to be the correct initiation site for translation, leading to a predicted protein length of 873 aa [33]. With that numbering, the two Pms1 mutations would be G851E and H857R. The crystal structure shows that the H857 residue is centered in the β8 β-sheet that is part of one of the most important regions of the heterodimerization interface, Patch 1 [32]. The G851 residue sits just outside the β8 β-sheet and so it is reasonable to suppose that a mutation in that residue could affect Pms1-Mlh1 interaction. One of the zinc atoms in the endonuclease site is stabilized by C848 and H850 [32]. That would put the G851 residue close to the endonuclease site, making it possible that the G851E substitution might interfere with the binding of the zinc atom and thus affect endonuclease activity. However, there is no indication in the structure that the H857 residue would influence endonuclease activity, and as we found above, the H857R mutation has a more distinctive mutator effect than the G851E mutation. Both of the mutations were found to have essentially wild-type base-base MMR activity [22], and as Pms1 endonuclease activity is crucial for MMR function [34], we consider it highly unlikely that the effects of the two mutations is on the endonuclease activity of Pms1.
In accordance with previous results, we find that the absence of Mlh3 leads to somewhat less effective repair of deletion mispairs (Tables 2 and 4) [20], [21]. The repair of insertion mispairs in an mlh3 background is more robust than in a wild-type background, suggesting that the loss of Mlh3 might lead to a somewhat greater amount of MutLα in the cell, with correspondingly greater repair of insertions. That view is consistent with the previous observation that overexpression of Mlh3 appears to result in lower levels of MutLα [35]. The surprise was the deletion repair observed in pms-H888R mlh3 mutants. Given the small effect of each individual mutation on deletion repair, one would have expected deletion repair to be robust in that mutant background. Instead, repair of both 1-nt and 2-nt deletion mispairs was synergistically compromised (Tables 2 and 4). Based on the prior results with the pms1-H888R mutants, it appeared that only insertion repair was compromised [22]. Our results suggest a different possibility: although the pms1-H888R mutant is functional for base mismatch repair, it functions relatively poorly in in/del mismatch repair. One possible explanation for this hypothesis involves the finding that MutS complexes recognizing mismatches are responsible for loading multiple copies of MutLα onto DNA [36]. MutSα recognizing a base-base mispair can interact with the pms1-H888R mutant to create a functional complex. However because of the orientation of the proteins mediated by their binding to PCNA, neither MutSα nor MutSβ when recognizing an insertion mispair can interact properly with the pms1-H888R mutant complex and there is very little insertion repair. When MutSβ recognizes a deletion mispair, the complex is positioned so that it is able to interact with the pms1-H888R mutant MutLα, although relatively poorly, giving Repair Ratios of 16–20 (Tables 2 and 4). This interaction is facilitated by MutLγ interacting with MutSβ, which then helps recruit multiple molecules of the pms1-H888R mutant complex.
Repair of 2-nt deletion loops by MutSα is poor (Repair Ratio of 5.5, Table 2); however repair of 1-nt deletion loops by MutSα is much more robust (Repair Ratio of 73, Table 4), although still less than insertion loop repair. Repair of 1-nt deletion loops in the pms1-H888R mutant is much greater than repair with only MutSβ present (Repair Ratio of 170 compared to 22, Table 4), suggesting that much of the repair in the pms1-H888R mutant must be by MutSα. The fact that repair of 1-nt deletion loops in the pms1-H888R mlh3 background drops to the level of repair when only MutSβ is present suggests that MutSα-directed repair in the presence of the pms1-H888R mutation involves MutLγ. The very modest effect of the mlh3 mutation by itself shows that normal MutSα-directed repair of 1-nt deletion loops does not use MutLγ; confirmation of this suggestion would require additional experiments. One issue that has not been clear from previous experiments because of the modest effect of MutLγ on repair is whether there were certain mismatches that required MutLγ function, perhaps instead of MutLα, or whether the action of MutLγ always required MutLα and any mismatch was potentially susceptible to MutLγ function. Because each of our assays examines only one particular mismatch and because we see a strong effect in the mlh3 pms1-H888R background, we can draw several conclusions. 1) MutLγ functions only in repair of deletion loops and not insertion loops. 2) Any deletion loop is susceptible to being aided in repair by MutLγ. 3) MutLγ-mediated repair also requires MutLα. These conclusions do not mean that the effect of MutLγ deletion would be the same for all deletion loops: for both 1-nt and 2-nt deletion loops there is a range of about 4-fold in Repair Ratios, suggesting that certain mismatches could be more dependent for MutLγ on their repair.
The above model, while compatible with our results, makes several predictions that may however prove difficult to study. The first is that the bias in repair of insertions compared to deletions is ultimately a function of the MutL complexes and not the recognition by MutS complexes. A role for MutLγ in the repair of some deletion mispairs had previously been detected [20], [21], so the idea that MutL complexes could be biased in in/del repair is not without precedent. Secondly, the bias observed in in/del repair mediated by MutSα and MutSβ indicate that they contact MutLα differently such that a deletion mispair recognized by MutSβ is more likely to be repaired than if the same mispair were recognized by MutSα, and vice versa for insertion mispairs. A major question then is how the MutS and particularly the MutL components could be oriented such that an insertion mispair was recognized differently from a deletion mispair.
An important part of the explanation likely involves interactions of MMR proteins with the proliferating cell nuclear antigen, PCNA. PCNA is one of a family of DNA sliding clamps that encircles DNA, is essential for replication, and has binding sites for many proteins, including the replicative polymerases [37] and there is evidence that it can act as a scaffold to coordinate MMR through consecutive protein-protein interactions [38]. PCNA is required for MMR at a step preceding DNA resynthesis [39], [40], and MMR interactions with PCNA could be responsible for strand discrimination [41], [42]. A variety of experiments demonstrated direct interactions of PCNA with Mlh1, Msh3, and Msh6, and those interactions were important for proper MMR [40], [43]–[46]. It is clear that interaction with PCNA is not sufficient to drive MMR, as there are other processes occurring. For example, engineering a mutation that blocked MutSα conformational change upon mismatch binding demonstrated that such change was necessary for MutLα binding [47]. PCNA is asymmetrical with respect to the replication fork, and this asymmetry can result in specific MutLα loading and subsequent endonucleolytic activation and thus proper strand discrimination as has been observed in human MMR [42]. Importantly for this work, experiments with various PCNA mutants suggested that the interactions of PCNA are different for Msh3 compared to Msh6 [48]. In addition, it has been recently shown in humans that in contrast with MutSα, PCNA and MutLα have the same binding site on MutSβ, suggesting that the interaction of MutSβ with PCNA and MutLα would be sequential [49]. These considerations suggest a mechanism by which the recognition of, for example, an insertion loop could be different for MutSα compared to MutSβ because of their different orientation to the duplex bulge due to their different PCNA interaction. It is not clear how subsequent interactions with MutL complexes are handled. In vitro studies suggest that MutSα is bound to PCNA on homoduplex DNA, and, when a mispair is encountered, the interaction with PCNA is either lost or changed [50]. The next step of interaction with MutL complexes could be sequential for both MutS complexes, with a loss of the MutS interactions [38], but given the different nature of the MutS complex interactions with PCNA [49], the nature of the interactions of MutSα and MutSβ with MutLα is likely to be very different.
It is surprising to find that insertion and deletion mispairs are repaired with differing biases and that MutSα and MutSβ exhibit opposite biases for such repair. What might account for the development of an MMR system that would function in such a manner? A recent analysis was done of multiple strains of over 40 bacterial and archaeal species. It was found that in species with no MMR system, expansions and contractions of simple sequence repeats were equally likely, whereas in species containing MMR systems, there was a bias toward contraction of simple sequence repeats [51]. Thus, it appears that bacterial and archaeal MMR systems, like yeast strains containing only MutSα, repair insertions better than deletions. It is possible that such a bias could have an evolutionary advantage, tending to reduce the length of simple sequence repeats. Although most eukaryotic species seem to have an MMR system, not all have a MutSβ; in fact two favorite model organisms, D. melanogaster and C. elegans, lack MutSβ, although they both have MutSα [17]. Structural evidence also shows that MutSα binds mismatches in a manner similar to MutS, whereas MutSβ binds mismatches quite differently [30]. This analysis would suggest that MutSα represents the bacterial MutS activity, whereas MutSβ represents a new activity in which the bias toward repair of deletion mispairs may have been equally or more important than the recognition of larger loops. Many eukaryotic organisms have abundant simple sequence repeats, including those in exons, and the addition of a more robust activity repairing potential deletion mispairs would help preserve those repeats in the genome. This new MutSβ activity, due to the MSH3 gene, not only had a recognition specificity different from that of MutSα, but interacted in a somewhat different manner with PCNA and MutLα and the new MutLγ complex that apparently does not usually interact with MutSα [21]. Domain swap experiments have shown that the mismatch recognition domain of Msh3 is not necessary for interaction with MutLγ, but rather another part of the Msh3 protein present in MutSβ [52].
Given the high degree of conservation, in both sequence and function, between MMR systems in yeast and mammalian cells, our results likely apply also to mammalian cells, although the experiments to test that are much more difficult to carry out. Repeat stability is a concern for mammalian cells, both in terms of various trinucleotide repeat diseases and in cancer [53], [54]. In various trinucleotide repeat diseases, there is a strong involvement with MMR, but the effects are complicated [53]. In a mouse model of Friedreich ataxia which has GAA repeats, repeat instability was increased in the absence of MMR and there were enhanced deletions in the absence of MutSβ and an enhancement of both deletions and insertions in the absence of MutSα, with a relatively greater increase in insertions [55]. Those results are consistent with the activities we report here. However, repeat instability of other types of trinucleotide repeats shows a different effect, with MMR appearing to be required for expansion, for example [53]. Although there is not a complete understanding of such effects, many of them involve MutSβ and interactions with larger loops. For example, there are certain types of loops that are repairable by MutSβ and others such as CAG loops in which the loop appears to maintain MutSβ binding, thus preventing repair [56]. However, in an in vitro assay, 1 or 2 repeats of CTG/CAG were repaired in a process requiring MutSβ, but not larger loops, or substrates that contained multiple loops on both strands [57].
Some of the first analyses of MMR genes in humans demonstrated that defects in MMR led to Lynch syndrome or hereditary nonpolyposis colorectal cancer and that such cells manifested a greatly enhanced microsatellite instability [1], [2]. Although the overall mutator effect of deficiencies in MMR is likely important in tumor formation and progression, genes containing exonic microsatellite sequences are a particularly susceptible target as any alteration in such sequences will likely lead to a strong phenotype [54], [58], [59]. Additionally there is some evidence that microsatellite repeats within introns and in 5′ and 3′ untranslated regions could also contribute to carcinogenesis [54]. Not only is the distribution of different tumor types generally different in MMR-defective mice compared to humans, but there is a marked difference depending on the particular defect in MMR [54], [60]. Our results provide additional information on possible reasons for those differences. Part of the difference between the distribution of tumor types in mouse and human is likely due to the difference in the existence and sequence of regions in cancer target genes susceptible to in/del formation. Although we are able to induce approximately equal frequencies of insertion or deletion mispairs in the absence of MMR, spontaneous formation of primer or template loops could be at least partially a function of sequence, sequence context, and replication on the leading versus lagging strand, thus also implicating the relation of the gene to replication origin. Because there is plasticity in use of replication origins, the same gene could be replicated differently depending on tissue type [61]. Not only could the formation of a loop be influenced by its sequence and location near an origin, but as we have demonstrated previously [23] and also find here, there is a bias in repair by MutSα and MutSβ depending on the replication strand. There is some variability with MutSβ with different oligos, but there is even more pronounced variability with MutSα, with almost a 100-fold difference in repair between the best - and worst-repaired oligo (Table 1). In both yeast and human cells, there seems to be generally more MutSα than MutSβ in cells, so the likelihood of repair of a given in/del will depend on how well it is recognized by MutSα or MutSβ, which could depend on a variety of factors including sequence and perhaps location, whether it is an insertion or deletion loop, and on which replication strand it appears on. If there turns out to be significant variability in the relative amounts of MutSα and MutSβ in various tissues, as has been found in mouse [16], the likelihood of repair could depend on tissue type. We demonstrate here the surprising finding that although the recognition of in/del mispairs is due to the MutS complex, it is the interaction with the MutL complex that biases the efficiency of repair of an insertion versus deletion mispair. Thus mutations in the genes encoding MutLα could influence not only the efficiency of repair but its bias in repair of in/del mispairs.
Materials and Methods
S. cerevisiae strains and oligos
The genotypes of strains used in these experiments can be found in Table S3. All strains were derivatives of SJR2259 and SJR22609 [26] with LYS2 moved into HIS4 location. Mutant lys2 alleles either with [+1] (lys2SΔBgl and lys2OΔBgl) or [−1] (lys2SΔA746 and lys2OΔA746) frameshifts were then introduced by two-step allele replacement [62] using plasmids pSR125 [63] or pSR786 [64] respectively. ‘S’ and ‘O’ refer to the orientation of the LYS2 gene - the same or opposite orientation relatively to original HIS4 orientation (Figure 1A). Gene deletions were made using a PCR fragment generated from the collection of yeast gene deletions [65]. The pms1 point mutations were made using the delitto perfetto method [66]. The pCORE cassette was inserted into the PMS1 gene using primers GCP735 and GCP736 (Table S4) creating the pms1(761-904)Δ mutant. The pCORE cassette was then replaced by transformation with a PCR product from strain NEY398 or NEY402 [22] using primers GCP737 and GCP738 (Table S4). Oligos for transformation were gel purified (Eurofins MWG Operon) and are listed in Table S4.
Transformation with oligos
Transformation by electroporation was performed essentially as described previously [28], [67]. An overnight culture of yeast cells (0.5 ml) was inoculated into 25 ml of YPAD [68], incubated with shaking at 30° to an A600 of 1.3–1.5, washed twice with cold H2O, and once with cold 1 M sorbitol. After the final centrifugation, all solution was removed from the cells and 150 µL of cold 1 M sorbitol added to resuspend the cells. After addition of 200 pmol oligo and 50 ng of pRS314 [69] plasmid DNA, the solution was mixed and transferred into a 2-mm gap electroporation cuvette and electroporated at 1.55 kV, 200 Ω, and 25 uF (BTX Harvard Apparatus ECM 630). Immediately after electroporation, the cell suspension was added into 5 ml YPAD to recover for 2 h with shaking at 30°. Then cells were centrifuged, washed with H2O, and plated on synthetic dextrose (SD) medium lacking lysine [68]. The number of Trp+ transformants resulting from the pRS314 plasmid served as a useful marker of successful transformations, but was not consistent enough to be used as an internal standard for transformation efficiency. In order to determine background reversion, the same strains were electroporated as described but without adding oligos. For each oligo and strain combination, three independent experiments were performed, and the mean and standard deviation of the number of total transformants calculated.
Supporting Information
Zdroje
1. FishelR, LescoeMK, RaoMRS, CopelandNG, JenkinsNA, et al. (1993) The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell 75 : 1027–1038.
2. LeachFS, NicolaidesNC, PapadopoulosN, LiuB, JenJ, et al. (1993) Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell 75 : 1215–1225.
3. HemminkiA, PeltomäkiP, MecklinJ-P, JärvinenH, SalovaaraR, et al. (1994) Loss of the wild type MLH1 gene is a feature of hereditary nonpolyposis colorectal cancer. Nature Genet 8 : 405–409.
4. NicolaidesNC, PapadopoulosN, LiuB, WeiY-F, CarterKC, et al. (1994) Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature 371 : 75–80.
5. PapadopoulosN, NicolaidesNC, WeiY-F, RubenSM, CarterKC, et al. (1994) Mutation of a mutL homolog in hereditary colon cancer. Science 263 : 1625–1629.
6. BronnerCE, BakerSM, MorrisonPT, WarrenG, SmithLG, et al. (1994) Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature 368 : 258–261.
7. LiGM (2008) Mechanisms and functions of DNA mismatch repair. Cell Res 18 : 85–98.
8. IyerRR, PluciennikA, BurdettV, ModrichPL (2006) DNA mismatch repair: functions and mechanisms. Chem Rev 106 : 302–323.
9. JiricnyJ (2006) The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol 7 : 335–346.
10. HarringtonJM, KolodnerRD (2007) Saccharomyces cerevisiae Msh2-Msh3 acts in repair of base-base mispairs. Mol Cell Biol 27 : 6546–6554.
11. StrandM, EarleyMC, CrouseGF, PetesTD (1995) Mutations in the MSH3 gene preferentially lead to deletions within tracts of simple repetitive DNA in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 92 : 10418–10421.
12. NewL, LiuK, CrouseGF (1993) The yeast gene MSH3 defines a new class of eukaryotic MutS homologues. Mol Gen Genet 239 : 97–108.
13. MarraG, IaccarinoI, LettieriT, RoscilliG, DelmastroP, et al. (1998) Mismatch repair deficiency associated with overexpression of the MSH3 gene. Proc Natl Acad Sci USA 95 : 8568–8573.
14. DrummondJT, GenschelJ, WolfE, ModrichP (1997) DHFR/MSH3 amplification in methotrexate-resistant cells alters the hMutSα/hMutSβ ratio and reduces the efficiency of base-base mismatch repair. Proc Natl Acad Sci USA 94 : 10144–10149.
15. GhaemmaghamiS, HuhW, BowerK, HowsonRW, BelleA, et al. (2003) Global analysis of protein expression in yeast. Nature 425 : 737–741.
16. TomeS, SimardJP, SleanMM, HoltI, MorrisGE, et al. (2013) Tissue-specific mismatch repair protein expression: MSH3 is higher than MSH6 in multiple mouse tissues. DNA Repair (Amst) 12 : 46–52.
17. LinZ, NeiM, MaH (2007) The origins and early evolution of DNA mismatch repair genes–multiple horizontal gene transfers and co-evolution. Nucleic Acids Res 35 : 7591–7603.
18. SiaEA, KokoskaRJ, DominskaM, GreenwellP, PetesTD (1997) Microsatellite instability in yeast: Dependence on repeat unit size and DNA mismatch repair genes. Mol Cell Biol 17 : 2851–2858.
19. JohnsonRE, KovvaliGK, PrakashL, PrakashS (1996) Requirement of the yeast MSH3 and MSH6 genes for MSH2-dependent genomic stability. J Biol Chem 271 : 7285–7288.
20. HarfeBD, MinesingerBK, Jinks-RobertsonS (2000) Discrete in vivo roles for the MutL homologs Mlh2p and Mlh3p in the removal of frameshift intermediates in budding yeast. Curr Biol 10 : 145–148.
21. Flores-RozasH, KolodnerRD (1998) The Saccharomyces cerevisiae MLH3 gene functions in MSH3-dependent suppression of frameshift mutations. Proc Natl Acad Sci USA 95 : 12404–12409.
22. ErdenizN, DudleyS, GealyR, Jinks-RobertsonS, LiskayRM (2005) Novel PMS1 alleles preferentially affect the repair of primer strand loops during DNA replication. Mol Cell Biol 25 : 9221–9231.
23. KowYW, BaoG, ReevesJW, Jinks-RobertsonS, CrouseGF (2007) Oligonucleotide transformation of yeast reveals mismatch repair complexes to be differentially active on DNA replication strands. Proc Natl Acad Sci USA 104 : 11352–11357.
24. HarfeBD, Jinks-RobertsonS (1999) Removal of frameshift intermediates by mismatch repair proteins in Saccharomyces cerevisiae. Mol Cell Biol 19 : 4766–4773.
25. GreeneCN, Jinks-RobertsonS (1997) Frameshift intermediates in homopolymer runs are removed efficiently by yeast mismatch repair proteins. Mol Cell Biol 17 : 2844–2850.
26. KimN, AbdulovicAL, GealyR, LippertMJ, Jinks-RobertsonS (2007) Transcription-associated mutagenesis in yeast is directly proportional to the level of gene expression and influenced by the direction of DNA replication. DNA Repair (Amst) 6 : 1285–1296.
27. MarsischkyGT, KolodnerRD (1999) Biochemical characterization of the interaction between the Saccharomyces cerevisiae MSH2-MSH6 complex and mispaired bases in DNA. J Biol Chem 274 : 26668–26682.
28. RodriguezGP, RomanovaNV, BaoG, RoufNC, KowYW, et al. (2012) Mismatch repair dependent mutagenesis in nondividing cells. Proc Natl Acad Sci USA 109 : 6153–6158.
29. PangQS, ProllaTA, LiskayRM (1997) Functional domains of the Saccharomyces cerevisiae Mlh1p and Pms1p DNA mismatch repair proteins and their relevance to human hereditary nonpolyposis colorectal cancer-associated mutations. Mol Cell Biol 17 : 4465–4473.
30. GuptaS, GellertM, YangW (2012) Mechanism of mismatch recognition revealed by human MutSβ bound to unpaired DNA loops. Nat Struct Mol Biol 19 : 72–78.
31. DowenJM, PutnamCD, KolodnerRD (2010) Functional studies and homology modeling of Msh2-Msh3 predict that mispair recognition involves DNA bending and strand separation. Mol Cell Biol 30 : 3321–3328.
32. GueneauE, DherinC, LegrandP, Tellier-LebegueC, GilquinB, et al. (2013) Structure of the MutLalpha C-terminal domain reveals how Mlh1 contributes to Pms1 endonuclease site. Nat Struct Mol Biol 20 : 461–468.
33. KellisM, PattersonN, EndrizziM, BirrenB, LanderES (2003) Sequencing and comparison of yeast species to identify genes and regulatory elements. Nature 423 : 241–254.
34. KadyrovFA, HolmesSF, AranaME, LukianovaOA, O'DonnellM, et al. (2007) Saccharomyces cerevisiae MutLα Is a mismatch repair endonuclease. J Biol Chem 282 : 37181–37190.
35. NishantKT, PlysAJ, AlaniE (2008) A mutation in the putative MLH3 endonuclease domain confers a defect in both mismatch repair and meiosis in Saccharomyces cerevisiae. Genetics 179 : 747–755.
36. HombauerH, CampbellCS, SmithCE, DesaiA, KolodnerRD (2011) Visualization of eukaryotic DNA mismatch repair reveals distinct recognition and repair intermediates. Cell 147 : 1040–1053.
37. MoldovanGL, PfanderB, JentschS (2007) PCNA, the maestro of the replication fork. Cell 129 : 665–679.
38. LeeSD, AlaniE (2006) Analysis of interactions between mismatch repair initiation factors and the replication processivity factor PCNA. J Mol Biol 355 : 175–184.
39. JohnsonRE, KovvaliGK, GuzderSN, AminNS, HolmC, et al. (1996) Evidence for involvement of yeast proliferating cell nuclear antigen in DNA mismatch repair. J Biol Chem 271 : 27987–27990.
40. UmarA, BuermeyerAB, SimonJA, ThomasDC, ClarkAB, et al. (1996) Requirement for PCNA in DNA mismatch repair at a step preceding DNA resynthesis. Cell 87 : 65–73.
41. ChenC, MerrillBJ, LauPJ, HolmC, KolodnerRD (1999) Saccharomyces carevisiae pol30 (proliferating cell nuclear antigen) mutations impair replication fidelity and mismatch repair. Mol Cell Biol 19 : 7801–7815.
42. PluciennikA, DzantievL, IyerRR, ConstantinN, KadyrovFA, et al. (2010) PCNA function in the activation and strand direction of MutLα endonuclease in mismatch repair. Proc Natl Acad Sci USA 107 : 16066–16071.
43. AminNS, NguyenMN, OhS, KolodnerRD (2001) exo1-Dependent mutator mutations: Model system for studying functional interactions in mismatch repair. Mol Cell Biol 21 : 5142–5155.
44. BowersJ, TranPT, JoshiA, LiskayRM, AlaniE (2001) MSH-MLH complexes formed at a DNA mismatch are disrupted by the PCNA sliding clamp. J Mol Biol 306 : 957–968.
45. ClarkAB, ValleF, DrotschmannK, GaryRK, KunkelTA (2000) Functional interaction of proliferating cell nuclear antigen with MSH2-MSH6 and MSH2-MSH3 complexes. J Biol Chem 275 : 36498–36501.
46. Flores-RozasH, ClarkD, KolodnerRD (2000) Proliferating cell nuclear antigen and Msh2p-Msh6p interact to form an active mispair recognition complex. Nature Genet 26 : 375–378.
47. HargreavesVV, PutnamCD, KolodnerRD (2012) Engineered disulfide-forming amino acid substitutions interfere with a conformational change in the mismatch recognition complex Msh2-Msh6 required for mismatch repair. J Biol Chem 287 : 41232–41244.
48. LauPJ, Flores-RozasH, KolodnerRD (2002) Isolation and characterization of new proliferating cell nuclear antigen (POL30) mutator mutants that are defective in DNA mismatch repair. Mol Cell Biol 22 : 6669–6680.
49. IyerRR, PluciennikA, GenschelJ, TsaiMS, BeeseLS, et al. (2010) MutLα and proliferating cell nuclear antigen share binding sites on MutSβ. J Biol Chem 285 : 11730–11739.
50. LauPJ, KolodnerRD (2003) Transfer of the MSH2.MSH6 complex from proliferating cell nuclear antigen to mispaired bases in DNA. J Biol Chem 278 : 14–17.
51. KumarP, NagarajaramHA (2012) A study on mutational dynamics of simple sequence repeats in relation to mismatch repair system in prokaryotic genomes. J Mol Evol 74 : 127–139.
52. ShellSS, PutnamCD, KolodnerRD (2007) Chimeric Saccharomyces cerevisiae Msh6 protein with an Msh3 mispair-binding domain combines properties of both proteins. Proc Natl Acad Sci USA 104 : 10956–10961.
53. LopezCA, ClearyJD, PearsonCE (2010) Repeat instability as the basis for human diseases and as a potential target for therapy. Nat Rev Mol Cell Biol 11 : 165–170.
54. ShahSN, HileSE, EckertKA (2010) Defective mismatch repair, microsatellite mutation bias, and variability in clinical cancer phenotypes. Cancer Res 70 : 431–435.
55. EzzatizadehV, PintoRM, SandiC, SandiM, Al-MahdawiS, et al. (2012) The mismatch repair system protects against intergenerational GAA repeat instability in a Friedreich ataxia mouse model. Neurobiol Dis 46 : 165–171.
56. LangWH, CoatsJE, MajkaJ, HuraGL, LinY, et al. (2011) Conformational trapping of mismatch recognition complex MSH2/MSH3 on repair-resistant DNA loops. Proc Natl Acad Sci U S A 108: E837–E844.
57. PanigrahiGB, SleanMM, SimardJP, GileadiO, PearsonCE (2010) Isolated short CTG/CAG DNA slip-outs are repaired efficiently by hMutSβ, but clustered slip-outs are poorly repaired. Proc Natl Acad Sci USA 107 : 12593–12598.
58. GuoJ, ZhengL, LiuW, WangX, WangZ, et al. (2011) Frequent truncating mutation of TFAM induces mitochondrial DNA depletion and apoptotic resistance in microsatellite-unstable colorectal cancer. Cancer Res 71 : 2978–2987.
59. DuvalA, HamelinR (2002) Mutations at coding repeat sequences in mismatch repair-deficient human cancers: Toward a new concept of target genes for instability. Cancer Res 62 : 2447–2454.
60. HeganDC, NarayananL, JirikFR, EdelmannW, LiskayRM, et al. (2006) Differing patterns of genetic instability in mice deficient in the mismatch repair genes Pms2, Mlh1, Msh2, Msh3 and Msh6. Carcinogen 27 : 2402–2408.
61. HansenRS, ThomasS, SandstromR, CanfieldTK, ThurmanRE, et al. (2010) Sequencing newly replicated DNA reveals widespread plasticity in human replication timing. Proc Natl Acad Sci USA 107 : 139–144.
62. RothsteinR (1990) Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. Methods Enzymol 194 : 281–301.
63. DattaA, Jinks-RobertsonS (1995) Association of increased spontaneous mutation rates with high levels of transcription in yeast. Science 268 : 1616–1619.
64. AbdulovicAL, MinesingerBK, Jinks-RobertsonS (2008) The effect of sequence context on spontaneous Pol ζ -dependent mutagenesis in Saccharomyces cerevisiae. Nucleic Acids Res 36 : 2082–2093.
65. WinzelerEA, ShoemakerDD, AstromoffA, LiangH, AndersonK, et al. (1999) Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285 : 901–906.
66. StoriciF, LewisLK, ResnickMA (2001) In vivo site-directed mutagenesis using oligonucleotides. Nat Biotechnol 19 : 773–776.
67. RodriguezGP, SongJB, CrouseGF (2012) Transformation with oligonucleotides creating clustered changes in the yeast genome. PLoS ONE 7: e42905.
68. ShermanF (1991) Getting started with yeast. Methods Enzymol 194 : 3–21.
69. SikorskiRS, HieterP (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122 : 19–27.
Štítky
Genetika Reprodukční medicína
Článek Defending Sperm FunctionČlánek How to Choose the Right MateČlánek Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental TransitionČlánek Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in SpermatogenesisČlánek The Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate EmbryogenesisČlánek Multiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time inČlánek Playing the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral MyelinationČlánek A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase inČlánek Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 10- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Defending Sperm Function
- How to Choose the Right Mate
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
- Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental Transition
- A Novel Actin mRNA Splice Variant Regulates ACTG1 Expression
- Tracking Proliferative History in Lymphocyte Development with Cre-Mediated Sister Chromatid Recombination
- Correlated Occurrence and Bypass of Frame-Shifting Insertion-Deletions (InDels) to Give Functional Proteins
- Chimeric Protein Complexes in Hybrid Species Generate Novel Phenotypes
- Loss of miR-10a Activates and Collaborates with Activated Wnt Signaling in Inducing Intestinal Neoplasia in Female Mice
- Both Rare and Copy Number Variants Are Prevalent in Agenesis of the Corpus Callosum but Not in Cerebellar Hypoplasia or Polymicrogyria
- Reverse PCA, a Systematic Approach for Identifying Genes Important for the Physical Interaction between Protein Pairs
- Partial Deletion of Chromosome 8 β-defensin Cluster Confers Sperm Dysfunction and Infertility in Male Mice
- Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in Spermatogenesis
- Coordinate Regulation of Mature Dopaminergic Axon Morphology by Macroautophagy and the PTEN Signaling Pathway
- Cooperation between RUNX1-ETO9a and Novel Transcriptional Partner KLF6 in Upregulation of in Acute Myeloid Leukemia
- Mobility of the Native Conjugative Plasmid pLS20 Is Regulated by Intercellular Signaling
- FliZ Is a Global Regulatory Protein Affecting the Expression of Flagellar and Virulence Genes in Individual Bacterial Cells
- Specific Tandem Repeats Are Sufficient for Paramutation-Induced Trans-Generational Silencing
- Condensin II Subunit dCAP-D3 Restricts Retrotransposon Mobilization in Somatic Cells
- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- The Insulator Homie Promotes Expression and Protects the Adjacent Gene from Repression by Polycomb Spreading
- Human Intellectual Disability Genes Form Conserved Functional Modules in
- Coordination of Cell Proliferation and Cell Fate Determination by CES-1 Snail
- ORFs in Drosophila Are Important to Organismal Fitness and Evolved Rapidly from Previously Non-coding Sequences
- Different Roles of Eukaryotic MutS and MutL Complexes in Repair of Small Insertion and Deletion Loops in Yeast
- The Spore Differentiation Pathway in the Enteric Pathogen
- Acceleration of the Glycolytic Flux by Steroid Receptor Coactivator-2 Is Essential for Endometrial Decidualization
- The Human Nuclear Poly(A)-Binding Protein Promotes RNA Hyperadenylation and Decay
- Genome Wide Analysis Reveals Zic3 Interaction with Distal Regulatory Elements of Stage Specific Developmental Genes in Zebrafish
- Xbp1 Directs Global Repression of Budding Yeast Transcription during the Transition to Quiescence and Is Important for the Longevity and Reversibility of the Quiescent State
- The Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate Embryogenesis
- Incorporating Motif Analysis into Gene Co-expression Networks Reveals Novel Modular Expression Pattern and New Signaling Pathways
- The Bacterial Response Regulator ArcA Uses a Diverse Binding Site Architecture to Regulate Carbon Oxidation Globally
- Direct Monitoring of the Strand Passage Reaction of DNA Topoisomerase II Triggers Checkpoint Activation
- Multiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time in
- A Reversible Histone H3 Acetylation Cooperates with Mismatch Repair and Replicative Polymerases in Maintaining Genome Stability
- ALS-Associated Mutations Result in Compromised Alternative Splicing and Autoregulation
- Robust Demographic Inference from Genomic and SNP Data
- Preferential Binding to Elk-1 by SLE-Associated Risk Allele Upregulates Expression
- Rad52 Sumoylation Prevents the Toxicity of Unproductive Rad51 Filaments Independently of the Anti-Recombinase Srs2
- The Serum Resistome of a Globally Disseminated Multidrug Resistant Uropathogenic Clone
- Identification of 526 Conserved Metazoan Genetic Innovations Exposes a New Role for Cofactor E-like in Neuronal Microtubule Homeostasis
- SUMO Localizes to the Central Element of Synaptonemal Complex and Is Required for the Full Synapsis of Meiotic Chromosomes in Budding Yeast
- Integrated Enrichment Analysis of Variants and Pathways in Genome-Wide Association Studies Indicates Central Role for IL-2 Signaling Genes in Type 1 Diabetes, and Cytokine Signaling Genes in Crohn's Disease
- Genome-Wide High-Resolution Mapping of UV-Induced Mitotic Recombination Events in
- Genome-Wide Analysis of Cell Type-Specific Gene Transcription during Spore Formation in
- Playing the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral Myelination
- Two Portable Recombination Enhancers Direct Donor Choice in Fission Yeast Heterochromatin
- Mining the Human Phenome Using Allelic Scores That Index Biological Intermediates
- Yeast Tdh3 (Glyceraldehyde 3-Phosphate Dehydrogenase) Is a Sir2-Interacting Factor That Regulates Transcriptional Silencing and rDNA Recombination
- A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase in
- A Review of Bacteria-Animal Lateral Gene Transfer May Inform Our Understanding of Diseases like Cancer
- High Throughput Sequencing Reveals Alterations in the Recombination Signatures with Diminishing Spo11 Activity
- Partitioning the Heritability of Tourette Syndrome and Obsessive Compulsive Disorder Reveals Differences in Genetic Architecture
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- A GDF5 Point Mutation Strikes Twice - Causing BDA1 and SYNS2
- Systematic Unraveling of the Unsolved Pathway of Nicotine Degradation in
- Natural Genetic Variation of Integrin Alpha L () Modulates Ischemic Brain Injury in Stroke
- Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
- Evolutionary Change within a Bipotential Switch Shaped the Sperm/Oocyte Decision in Hermaphroditic Nematodes
- Limiting of the Innate Immune Response by SF3A-Dependent Control of MyD88 Alternative mRNA Splicing
- Multiple Signaling Pathways Coordinate to Induce a Threshold Response in a Chordate Embryo
- Distinct Regulatory Mechanisms Act to Establish and Maintain Pax3 Expression in the Developing Neural Tube
- Genome Wide Analysis of Narcolepsy in China Implicates Novel Immune Loci and Reveals Changes in Association Prior to Versus After the 2009 H1N1 Influenza Pandemic
- Mismatch Repair Genes and Modify CAG Instability in Huntington's Disease Mice: Genome-Wide and Candidate Approaches
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- Hsp70-Hsp40 Chaperone Complex Functions in Controlling Polarized Growth by Repressing Hsf1-Driven Heat Stress-Associated Transcription
- Function and Evolution of DNA Methylation in
- Stimulation of mTORC1 with L-leucine Rescues Defects Associated with Roberts Syndrome
- Transcription Termination and Chimeric RNA Formation Controlled by FPA
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



