-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPreferential Binding to Elk-1 by SLE-Associated Risk Allele Upregulates Expression
Immunoregulatory cytokine interleukin-10 (IL-10) is elevated in sera from patients with systemic lupus erythematosus (SLE) correlating with disease activity. The established association of IL10 with SLE and other autoimmune diseases led us to fine map causal variant(s) and to explore underlying mechanisms. We assessed 19 tag SNPs, covering the IL10 gene cluster including IL19, IL20 and IL24, for association with SLE in 15,533 case and control subjects from four ancestries. The previously reported IL10 variant, rs3024505 located at 1 kb downstream of IL10, exhibited the strongest association signal and was confirmed for association with SLE in European American (EA) (P = 2.7×10−8, OR = 1.30), but not in non-EA ancestries. SNP imputation conducted in EA dataset identified three additional SLE-associated SNPs tagged by rs3024505 (rs3122605, rs3024493 and rs3024495 located at 9.2 kb upstream, intron 3 and 4 of IL10, respectively), and SLE-risk alleles of these SNPs were dose-dependently associated with elevated levels of IL10 mRNA in PBMCs and circulating IL-10 protein in SLE patients and controls. Using nuclear extracts of peripheral blood cells from SLE patients for electrophoretic mobility shift assays, we identified specific binding of transcription factor Elk-1 to oligodeoxynucleotides containing the risk (G) allele of rs3122605, suggesting rs3122605 as the most likely causal variant regulating IL10 expression. Elk-1 is known to be activated by phosphorylation and nuclear localization to induce transcription. Of interest, phosphorylated Elk-1 (p-Elk-1) detected only in nuclear extracts of SLE PBMCs appeared to increase with disease activity. Co-expression levels of p-Elk-1 and IL-10 were elevated in SLE T, B cells and monocytes, associated with increased disease activity in SLE B cells, and were best downregulated by ERK inhibitor. Taken together, our data suggest that preferential binding of activated Elk-1 to the IL10 rs3122605-G allele upregulates IL10 expression and confers increased risk for SLE in European Americans.
Published in the journal: . PLoS Genet 9(10): e32767. doi:10.1371/journal.pgen.1003870
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003870Summary
Immunoregulatory cytokine interleukin-10 (IL-10) is elevated in sera from patients with systemic lupus erythematosus (SLE) correlating with disease activity. The established association of IL10 with SLE and other autoimmune diseases led us to fine map causal variant(s) and to explore underlying mechanisms. We assessed 19 tag SNPs, covering the IL10 gene cluster including IL19, IL20 and IL24, for association with SLE in 15,533 case and control subjects from four ancestries. The previously reported IL10 variant, rs3024505 located at 1 kb downstream of IL10, exhibited the strongest association signal and was confirmed for association with SLE in European American (EA) (P = 2.7×10−8, OR = 1.30), but not in non-EA ancestries. SNP imputation conducted in EA dataset identified three additional SLE-associated SNPs tagged by rs3024505 (rs3122605, rs3024493 and rs3024495 located at 9.2 kb upstream, intron 3 and 4 of IL10, respectively), and SLE-risk alleles of these SNPs were dose-dependently associated with elevated levels of IL10 mRNA in PBMCs and circulating IL-10 protein in SLE patients and controls. Using nuclear extracts of peripheral blood cells from SLE patients for electrophoretic mobility shift assays, we identified specific binding of transcription factor Elk-1 to oligodeoxynucleotides containing the risk (G) allele of rs3122605, suggesting rs3122605 as the most likely causal variant regulating IL10 expression. Elk-1 is known to be activated by phosphorylation and nuclear localization to induce transcription. Of interest, phosphorylated Elk-1 (p-Elk-1) detected only in nuclear extracts of SLE PBMCs appeared to increase with disease activity. Co-expression levels of p-Elk-1 and IL-10 were elevated in SLE T, B cells and monocytes, associated with increased disease activity in SLE B cells, and were best downregulated by ERK inhibitor. Taken together, our data suggest that preferential binding of activated Elk-1 to the IL10 rs3122605-G allele upregulates IL10 expression and confers increased risk for SLE in European Americans.
Introduction
The gene cluster that includes interleukin 10 (IL10), IL19, IL20 and IL24 is located on chromosome 1q31-32, a genomic region that is linked with susceptibility to systemic lupus erythematosus (SLE, OMIM 152700) [1], [2]. Recent genome-wide association (GWA) and follow-up replication studies in European ancestry have identified an association between the minor allele of rs3024505, a SNP located at 1 kb downstream of IL10, and increased risk for SLE [3], inflammatory bowel disease (IBD) including both Crohn's disease (CD) [4] and ulcerative colitis (UC) [5], [6], and decreased risk for type 1 diabetes [7], [8]. In addition, SNPs in the second intron (rs1518111) and the promoter region (rs1800871) of IL10 have been reported to be associated with Behçet's disease (BD) in GWAS of Turks [9] and Japanese [10]. These findings indicate IL10 as a common susceptibility locus shared by SLE and several other autoimmune diseases.
Dysregulation of IL-10 family cytokines contributes to autoimmune disease and tissue damage (reviewed in [11]). IL-10 is an important immunoregulatory cytokine with a wide variety of functions in T cells, B cells, natural killer cells, dendritic cells and macrophages [12]. The observations of elevated serum IL-10 levels in SLE patients correlating with increased disease activity [13], [14], and promising findings of anti-IL-10 monoclonal antibody treatment in patients with SLE [15] support a pivotal role for IL-10 in the pathogenesis of SLE. Of interest, elevated IL-10 levels were also reported in first-degree relatives of SLE patients [16], [17], suggesting that levels of IL10 expression may be determined genetically.
In this study, we fine mapped the IL10 gene cluster for genetic association with SLE in 15,533 case and control subjects from four diverse ancestries, identified a causal variant rs3122605 at IL10 5′ upstream using both genetic and functional assays, and explored the underlying molecular mechanism in explaining the elevated IL-10 levels in patients with SLE associated with increased disease activity.
Results
Association of four IL10 SNPs with SLE susceptibility in European Americans
To fine map the IL10 gene cluster, we genotyped 19 tag SNPs in 15,533 case and control subjects from four ancestries, including European American (EA, 3,820 cases vs. 3,412 controls), African American (AA, 1,670 vs. 1,904), Asian (AS, 1,252 vs. 1,249) and Amerindian/Hispanic (HS, 1,445 vs. 781). Each SNP was assessed for the association with SLE susceptibility under a logistic regression model adjusted for gender and global ancestry (Figure 1A). In the largest EA dataset, rs3024505 located at 1 kb downstream of IL10 exhibited the strongest association with SLE (minor allele frequency of 18.2% in cases vs. 14.8% in controls, P = 2.7×10−8, OR [95%CI] = 1.30 [1.19–1.43]), which exceeded the GWAS significance level of P<5×10−8 (Table S1). To identify additional SLE-associated SNPs, we performed SNP imputation using 1000 Genomes Project data as a reference. In EA, a total of 109 well-imputed SNPs spanning 154 kb from IL10 downstream to FAIM3 were assessed for association with SLE. Of them, three imputed SNPs (rs3122605, rs3024493 and rs3024495 located at 9.2 kb upstream, intron 3 and 4 of IL10, respectively), which were in tight linkage disequilibrium (LD, r2>0.9) with the genotyped SNP rs3024505, were strongly associated with SLE and remained significant after Bonferroni correction (rs3122605: P = 1.3×10−8, OR = 1.34 [1.21–1.48]; rs3024493: P = 5.0×10−8, OR = 1.29 [1.18–1.42]); rs3024495: P = 1.0×10−7, OR = 1.29 [1.17–1.41]) (Figure 1B and Table S1). None of the genotyped or imputed SNPs was significantly associated with SLE in three non-European datasets including AS, AA and HS after Bonferroni correction (Table S2). These data confirmed that IL10 is a risk locus for SLE in EA, and thus we subsequently focused on EA only to identify the causal variant(s).
Fig. 1. SNPs of the IL10 gene cluster associated with SLE in European Americans. 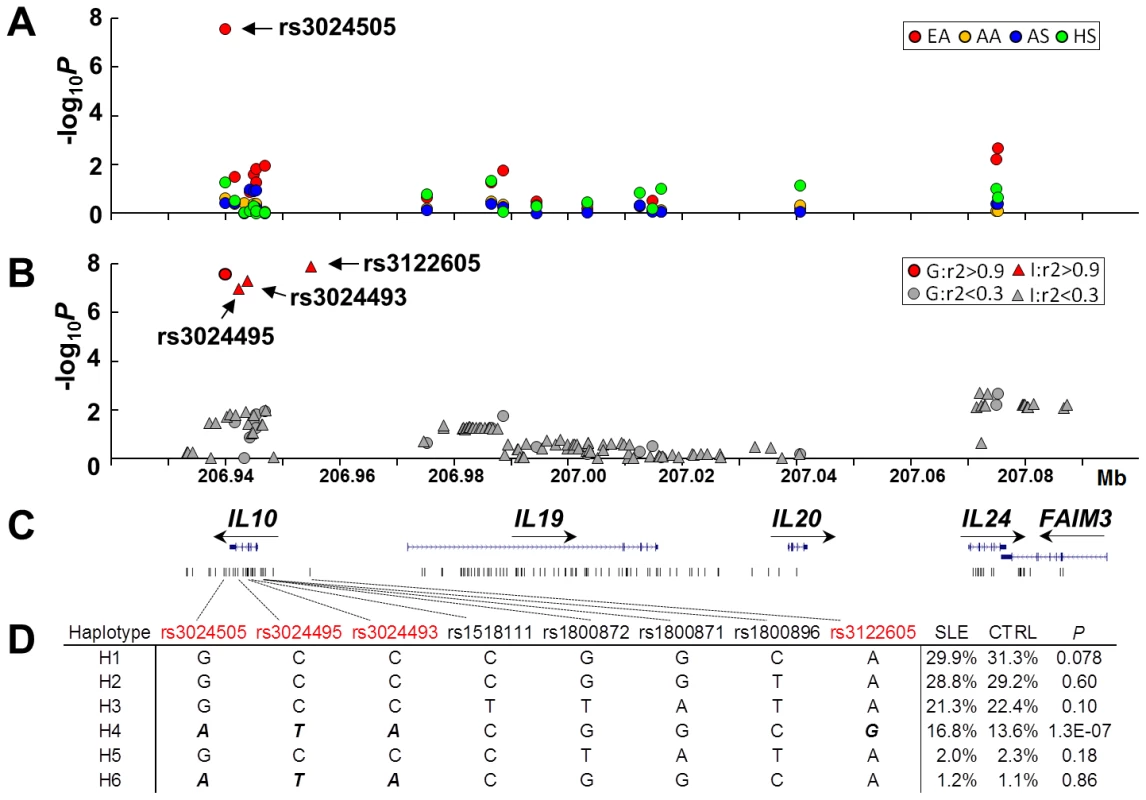
(A) Association of 19 genotyped SNPs with SLE in EA (red), AA (yellow), AS (blue) and HS (green). Allelic P value (−log10P) of each SNP was plotted against its genomic position. (B) Association of 19 genotyped and an additional 109 imputed SNPs with SLE in EA. Genotyped and imputed SNPs were indicated as circles and triangles, respectively. Based on its pairwised LD strength with rs3024505, each SNP was highlighted as red (r2>0.9) or grey (r2<0.9). (C) Genomic structure of the IL10 gene cluster and the location of each SNP. (D) Haplotypic analysis in EA. Haplotypes were constructed using four SLE-associated SNPs shown in Figure 1B (rs3024505, rs3024495, rs3024493 and rs3122605), three previously reported SLE-associated SNPs (rs1800872, rs1800871 and rs1800896 in the promoter of IL10) and rs1518111 (the T allele associated with Bechet's disease). Risk alleles of four SLE-associated SNPs shown in Figure 1B were bolded and italicized. IL10 promoter SNPs rs1800872 (also named as −592T/G), rs1800871 (−819G/A) and rs1800896 (−1082T/C), which were identified to be associated with elevated IL-10 production and SLE susceptibility in some, but not all of previous studies (reviewed in [18], [19]), showed nominal association with SLE in our EA dataset (Table S1). The BD-associated SNP rs1518111 showing effect on decreased IL-10 levels [9] was not associated with SLE in EA (Table S1). We performed haplotypic analysis to investigate relationships between these four previously reported SNPs and rs3024505, rs3024495, rs3024493 and rs3122605. Only the haplotype H4 carrying risk alleles of rs3024505, rs3024495, rs3024493 and rs3122605 was strongly associated with SLE (frequency of 16.8% in cases vs. 13.6% in controls, P = 1.3×10−7) (Figure 1D).
Due to strong LD among rs3122605, rs3024493, rs3024495 and rs3024505 in EA ancestry, their associations with SLE were highly correlated and could not be distinguished from each other using the conditional haplotype-based association test (Table S1). Conditioning on rs3122605, rs3024493, rs3024495 and rs3024505, respectively, association signals (P<0.05) of all other SNPs within the IL10 gene cluster were completely eliminated (Table S1), suggesting that these four SNPs within tight LD could capture all associations of the IL10 cluster region with SLE in EA. Of note, searching +/−200 kb of IL10 based on the 1000 Genomes Project data, we found that rs61815643 located at 10.3 kb upstream of IL10 was also in strong LD with rs3122605 (r2 = 0.9) in European subjects, which suggested that this SNP might account for association signals detected within the IL10 gene cluster. However, because the imputation quality of rs61815643 did not reach the threshold of information score >0.9, it was not included for association test in this study.
Taken together, our data provide evidence supporting IL10 as a risk locus for SLE in EA and the underlying causal variant(s) might be or tagged by rs3122605, rs3024505, rs3024495 and rs3024493.
Dose-dependent association between SLE-risk allele and elevated IL10 expression levels
To explore potential functional consequences of the SLE-associated SNPs (rs3122605, rs3024493, rs3024495 and rs3024505), we assessed their genetic effects on influencing IL10 expression. IL10 mRNA levels in peripheral blood mononuclear cells (PBMC) and IL-10 protein levels in plasma from EA subjects were measured by quantitative real-time PCR and ELISA, respectively. Using rs3122605 as a surrogate of the other three SNPs, we compared IL10 expression levels among subjects carrying different genotypes of rs3122605. In control subjects, the SLE-risk (G) allele of rs3122605 was dose-dependently associated with elevated IL10 expression at both mRNA (n = 55; P = 0.001, R2 = 0.18 in linear regression) and protein (n = 116; P = 3.3×10−7, R2 = 0.21) levels (Figure 2). Consistently, dose-dependent association of the risk allele with elevated IL10 expression was also observed in patients with SLE at both mRNA (n = 58; P = 6.4×10−6, R2 = 0.31) and protein (n = 132; P = 1.4×10−6, R2 = 0.16) levels (Figure 2).
Fig. 2. Dose-dependent association of rs3122605 risk G-allele with elevated levels of IL10 mRNA and protein. 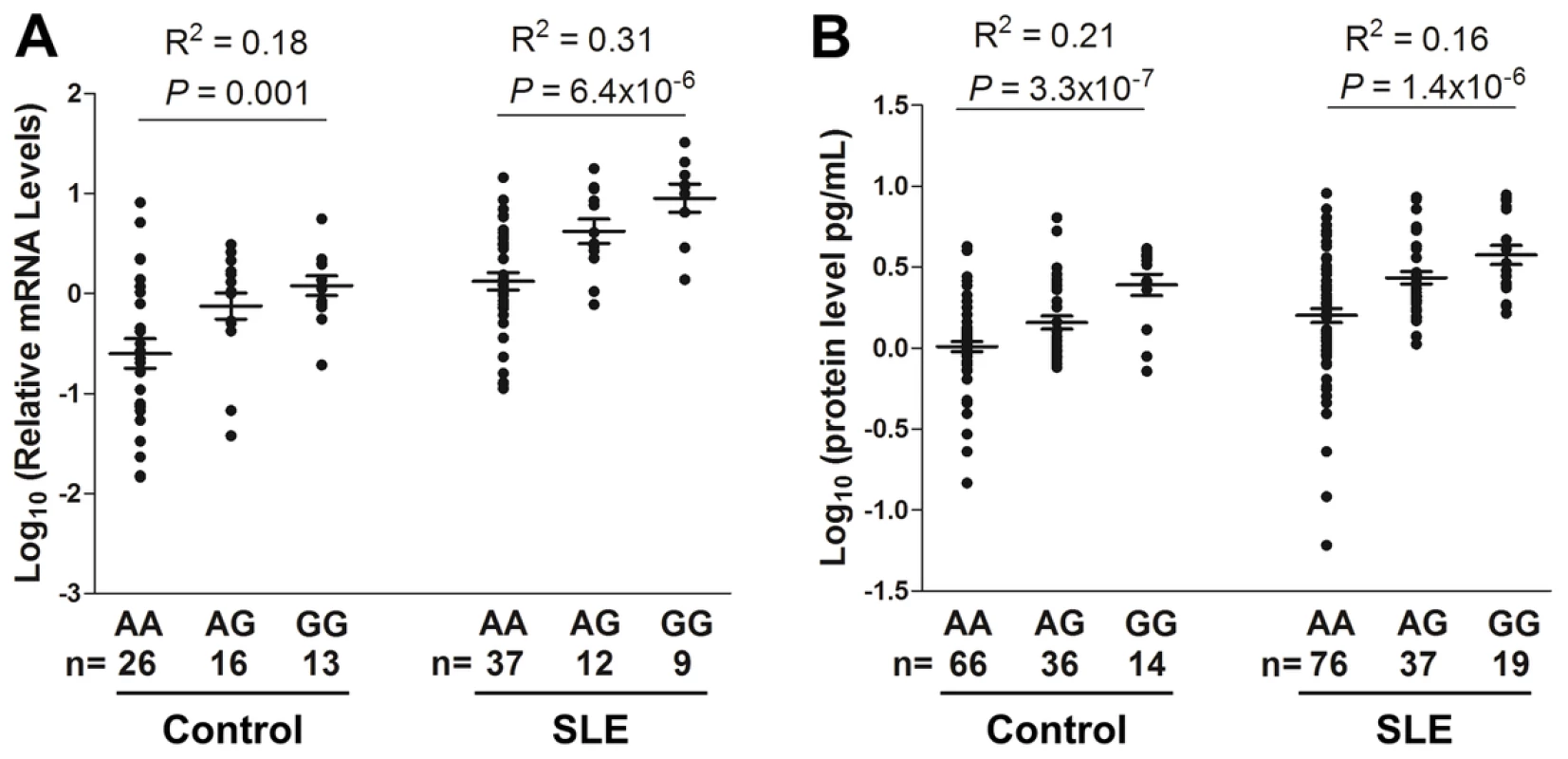
IL10 mRNA (A) and protein levels (B) were measured in PBMCs and plasma from EA SLE patients and healthy controls, respectively. Each symbol represents an individual and horizontal lines indicate mean ± SEM values. Compared to healthy controls, higher IL10 expression was observed in patients with SLE carrying the same genotype at both mRNA (genotype AA: P = 1.3×10−4, AG: P = 4.3×10−4, GG: P = 3.9×10−5, cases vs. controls in t test) and protein (AA: P = 4.6×10−4, AG: P = 4.9×10−6, GG: P = 0.047) levels (Figure 2), probably due to the activated immune status of SLE patients.
The risk allele of rs3122605 creates a novel binding site to transcription factor Elk-1 at 5′upstream of IL10
We hypothesized the presence of transcription factors activated in SLE patients upregulating IL10 expression and prepared nuclear extracts of peripheral blood lymphocytes from active SLE patients (defined as SLEDAI score≥4) [20], [21] to perform electrophoretic mobility shift assays (EMSA) for testing allelic differences in transcription factor binding conferred by the SLE-associated SNPs rs3122605, rs3024505, rs3024495 and rs3024493. Because we could not exclude the possibility that rs61815643 is a SLE-risk SNP affecting IL10 expression, it was also tested by EMSA.
Upon incubation with nuclear extracts, specific mobility-shift bands were only detected using the oligodeoxynucleotide probe containing the risk, but not the non-risk allele of rs3122605 (Figure 3A). Of interest, no specific binding of nuclear proteins was observed with the oligodeoxynucleotide probes containing either the risk or the non-risk allele of rs3024505, rs3024493, rs3024495 and rs61815643 (Figure S1). In silico analysis using the program TFSEARCH indicated that the risk allele of rs3122605 might create a novel binding site of the transcription factor Elk-1 (ETS-like transcription factor 1). We validated this prediction by showing that the addition of polyclonal rabbit IgG anti-ElK-1 antibody produced a super shift band only to the probe containing the risk but not the non-risk allele of rs3122605 in EMSA (Figure 3A).
Fig. 3. Preferential binding of rs3122605-risk allele to Elk-1, which is a transcription factor activated in peripheral lymphocytes of SLE patients. 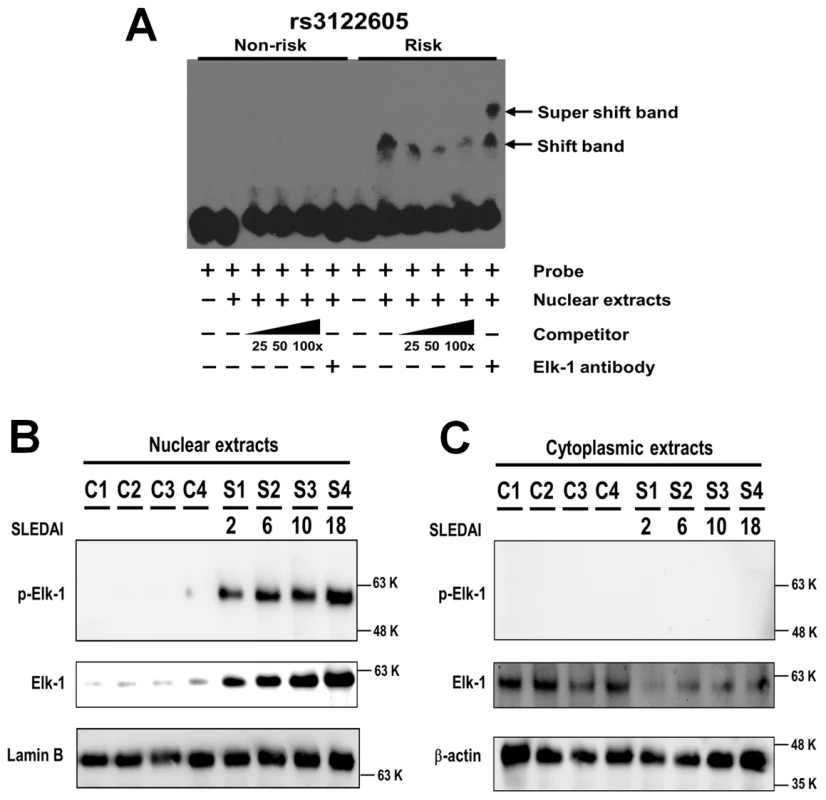
(A) Specific binding of transcription factor Elk-1 to the risk allele of rs3122605 in EMSA. Mobility-shift bands were produced by the oligodeoxynucleotide probes containing the risk allele of rs3122605 incubated with nuclear extracts of peripheral blood lymphocytes from active SLE patients, and the addition of anti-Elk-1 antibody generated a super shift band. The data are representative of two independent experiments. (B, C) Nuclear retention of activated p-Elk-1 in PBMCs from SLE patients. The presence of Elk-1 and p-Elk-1 in nuclear (B) and cytoplasmic (C) extracts of PBMCs from SLE patients and healthy controls was measured using Western blot. Lamin B (B) and β-actin (C) were used as loading controls. The data are representative of two independent experiments. Taken together, these data showed that rs3122605, the risk allele of which binds to Elk-1 in peripheral blood lymphocytes from SLE patients with active disease, is more likely to be the causal variant upregulating IL10 expression than the other four candidate SNPs.
Aberrant activation of Elk-1 in the nuclei of PBMCs from patients with SLE
Elk-1 is activated through phosphorylation and the phosphorylated Elk-1 (p-Elk-1) translocates into the nucleus to induce gene transcription [22]. To investigate the role of Elk-1 in SLE, we compared the distribution and activation of Elk-1 in PBMCs between SLE cases (n = 4) and healthy controls (n = 4) using Western blot. The amount of total Elk-1 was higher in nuclear (Figure 3B) but lower in cytoplasmic extracts (Figure 3C) of cases than controls. Of interest, p-Elk-1 was detected only in nuclear extracts of cases but not in controls (Figure 3B) and not in cytoplasmic extracts of either cases or controls (Figure 3C). These data suggest that Elk-1 is aberrantly activated and accumulates in nuclei of SLE PBMCs. Furthermore, the amount of total Elk-1 and p-Elk-1 appeared to be increased in SLE patients with higher SLEDAI scores (Figure 3B).
Co-expression of IL-10 and p-Elk-1 increases with SLE disease activity in B cells and could be best down-regulated by ERK inhibitor
Using flow cytometry, we quantified the co-expression of IL-10 and p-Elk-1 in specific cell subsets of PBMCs including CD3+ T cells, CD19+ B cells and CD14+ monocytes from healthy controls and SLE patients and showed representative data plotted in two-dimensional dot plots in Figure 4A. Compared to healthy controls (n = 3), percentages of IL-10+p-Elk-1+ cells were significantly increased in B (n = 11), T cells (n = 12) and monocytes (n = 12) from SLE patients (Student's t-test: P = 0.013, 0.012 and 0.012, respectively, Figure 4B). Active SLE patients had significantly elevated IL-10+p-Elk-1+ double positive B cells compared to non-active SLE patients (n = 7 vs. 4, P = 0.038). Similar trends for association with SLE disease activity were detected in IL-10+p-Elk-1+ T cells and monocytes, but the difference was not statistically significant (P = 0.74 and 0.57, respectively).
Fig. 4. Co-expression of p-Elk-1 and IL-10 in PBMCs. 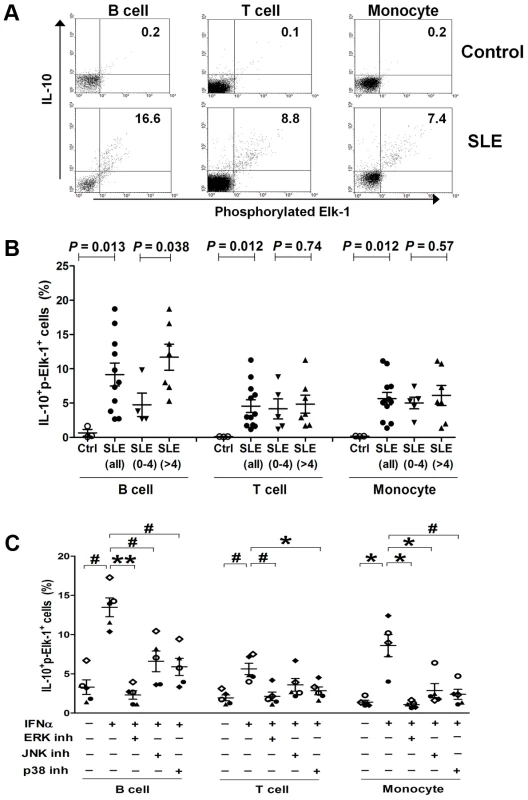
(A) Quantification of co-expression of p-ELK-1 and IL-10 in B cells (CD19+), T cells (CD3+) and monocytes (CD14+), respectively, by flow cytometry. Numbers in upper quadrants indicate the percentages of double positive (IL-10+p-Elk-1+) cells. (B) Increased proportions of IL-10+p-Elk-1+ cells in B, T cells and monocytes from SLE patients compared to controls, and increased IL-10+p-Elk-1+ cells in B cells from active compared to inactive SLE patients. Each symbol represents an individual and horizontal lines indicate mean ± SEM values. (C) Decreased proportions of IL-10+p-Elk-1+ cells with inhibition of Elk-1 activation. Normal PBMCs were incubated with IFNα in the presence or absence of MAPK inhibitor ERK (PD 98059), JNK (SP 600125) or p38 (SB 203580), and IL-10+p-Elk-1+ cells were quantified in B, T cells and monocytes, respectively. Each symbol represents an individual and horizontal lines indicate mean ± SEM values. P*≤0.01, P#<0.005, P**<0.0001 (Student's t test) for the comparison of indicated groups. Elk-1 is known to be activated by mitogen-activated protein kinases (MAPK), including ERK (extracellular-signal-regulated kinases), JNK (c-Jun N-terminal kinases) and p38. We wondered which MAPK inhibitor could best down-regulate the elevated co-expression of IL-10 and p-Elk-1 in PBMCs. Because of the low expression of IL-10 in freshly isolated control PBMCs (Figure S2), we stimulated control PBMCs with IFN-α, a pivotal cytokine upregulated in most SLE patients [23], to mimic SLE PBMCs and incubated them with the MAPK inhibitors specific to ERK (PD 98059), JNK (SP 600125) or p38 (SB 203580), respectively. IFN-α-stimulation could significantly increase the percentage of IL-10+p-Elk-1+ cells in B, T cells and monocytes (n = 5, P = 0.0002, 0.0018 and 0.0079, respectively, Figure 4C), and such increase could be best down-regulated by the addition of the ERK inhibitor (P = 2.8×10−5, 0.0046 and 0.0079 in B, T cells and monocytes, respectively, Figure 4C). To a lesser extent, the p38 inhibitor also significantly suppressed IFN-α-induced double positive cells in all three cell subsets (P = 0.0016, 0.01 and 0.0039 in B, T cells and monocytes, respectively). Under our experimental conditions, the JNK inhibitor significantly inhibited the percentage of IL-10+p-Elk-1+ cells in B cells and monocytes (P = 0.0048 and 0.009, respectively), but not in T cells (P = 0.10).
Discussion
Our data provide strong evidence for a dose-dependent association between SLE-predisposing IL10 genotypes and corresponding mRNA and protein levels of IL-10, and identify one underlying molecular mechanism to explain previous findings of elevated IL-10 serum levels in SLE patients that positively correlated with increased disease activity. In addition to confirming the previously reported association with SLE at the IL10 3′ downstream SNP (rs3024505) in European Americans, we identified a SLE-associated risk haplotype, defined by the minor alleles of four SNPs in tight LD, rs3024505, rs3024495, rs3024493 and rs3122605, which could best explain the association with SLE and capture underlying causal variant(s) within the IL10 gene cluster in EA ancestry. The minor allele of rs3122605, which tags the IL10 SLE-risk haplotype, exhibited a dose-dependent association with elevated IL10 expression at both mRNA levels in PBMCs and protein levels in plasma samples from SLE patients and healthy controls, suggesting that these four SLE-associated SNPs may act by influencing IL10 regulation. Further functional studies showed that only rs3122605 was experimentally validated to confer preferential allele-binding to the transcription factor Elk-1 present in SLE PBMCs, hence the most likely functional variant present on the IL10 risk haplotype. Compared to normal PBMCs, nuclear localization of activated p-Elk-1 was observed only in SLE PBMCs. Co-expression of p-Elk-1 and IL-10 was significantly increased in all SLE PBMC subsets compared to normal PBMC subsets. Of interest, SLE patients with active disease had higher double positive (p-Elk-1+IL-10+) B cells than those with inactive disease. These data suggested that nuclear accumulation of activated Elk-1 in SLE peripheral lymphocytes contributes to overproduction of IL-10 in SLE patients associated with disease activity.
An abnormally high production of IL-10 in patients with SLE has been consistently demonstrated in many studies (reviewed in [18]), but the underlying molecular mechanism remains less well-characterized. The observation that healthy relatives of SLE patients also exhibit increased levels of IL-10 [16], [24], [25] suggests a possibility of genetically regulated IL-10 production. A number of genetic polymorphisms in the IL10 promoter region have been reported [26]–[30], in particular, three SNPs shown in Figure 1D located at −1082 (rs1800896, C/T), −819 (rs1800871, G/A) and −592 (rs1800872, G/T) have been inconsistently associated with IL-10 production levels and risk of SLE (reviewed in [18], [19]). Identification of SNPs in the −1.3 to −4 kb region of the IL10 promoter associated with both IL-10 production phenotypes and SLE susceptibility [31] suggested that further evaluating the contribution of SNPs in the more distal promoter of IL10 might be warranted. Consistently, the SNP (rs3122605) we identified that tags the SLE-risk haplotype in EA ancestry and confers genetic effect on IL10 expression is located at 9.2 kb upstream of IL10. According to the ENCODE Project, rs3122605, rs3024505, rs3024493 and rs61815643 were located within DNasel hypersensitive and transcription factor binding sites in at least one cell type (as shown in UCSC genome browser), suggesting that each of them may affect gene expression through interaction with regulatory elements. Given that gene regulation by genetic variants often occurs within the specific cell types most relevant to the disease phenotype [32], we used nuclear extracts from PBMCs of active SLE patients to perform EMSA assays and found only the SLE-risk allele of rs3122605 preferentially binds to the transcription factor Elk-1. Therefore, our data support rs3122605 as the most likely causal variant on the SLE-associated haplotype and implicate a potential importance for Elk-1-mediated upregulation of IL10 expression in SLE patients, particularly in those patients carrying the risk allele of rs3122605.
Elk-1 is a member of the Ets oncogene family of transcription factors characterized by a conserved DNA-binding domain and a C-terminal activation domain containing multiple phosphorylation sites targeted by three major MAP kinase pathways [33]. Different phosphorylation patterns of Elk-1 mediated through activation of MAPK signaling cascades by distinct external stimuli are important for Elk-1 to execute its physiologic functions [34]–[37]. We used an antibody to measure phosphorylation of Elk-1 at S383, and detected the presence of Elk-1 pS383 in the nuclei of SLE but not normal PBMCs. Quantification of IL-10 and p-Elk-1 co-expression confirmed a higher proportion of IL-10+p-Elk-1+ cells in SLE than normal PBMC subsets. These findings imply that Elk-1 in nuclei of SLE PBMCs has been biologically activated likely due to a higher baseline immune activation status, which may enhance ability of Elk-1 to regulate IL10 transcription. In the absence of microenvironmental activation in control PBMCs, Elk-1 remains within the cytoplasm and less translocated into nuclei, limiting its regulation effects. This hypothesis may in part explain the observation of higher IL-10 expression in SLE patients than healthy controls even if they carry the same risk genotype of rs3122605. In support of this possibility, previous studies revealed an increased expression of activation markers on peripheral lymphocytes of SLE patients, including phosphorylated ERK, JNK and p38 which are prerequisite for subsequent activation of Elk-1 [38], [39]. In addition, our data showed a significantly increased proportion of IL-10+p-Elk-1+ cells in normal PBMC subsets (Figure 4C) when exposed to IFN-α which may induce a partial activation phenotype in lymphocytes mimicking that of SLE.
We used healthy control, rather than SLE, PBMCs for testing effects of MAPK inhibitors on co-expression of IL-10 and p-Elk-1, because disease activity and medications of SLE patients might confound the results and leucopenia of SLE patients could limit the amount of PBMCs available for our experiments. In all three cell subsets of PBMCs, inhibition of ERK could best suppress IFN-α induced increase in IL-10 and p-Elk-1 co-expression, highlighting the importance for phosphorylated Elk-1 via ERK signaling in regulation of IL10 transcription. The ERK-dependent activation of Elk-1 has been clearly demonstrated in neuronal cells (reviewed in [40]) in which phosphorylation of Elk-1 at S383/389 by ERK is tightly linked to its activation and nuclear translocation and inhibition of phosphorylation results in cytoplasmic Elk-1 retention, limiting its transcriptional properties [22].
Elk-1, like all members of Ets-domain containing transcription factors, can bind genomic regions similarly as well as uniquely to regulate distinct classes of target genes [41]. Another member of the Ets family, Ets-1, has a dual function in regulating IL10 gene expression acting as both a transcriptional activator with the binding partner Sp-1 in HIV-1Tat-induced IL10 transcription in THP-1 cells [42], and as a repressor interacting with histone deacetylase 1 (HDAC1) in Th1 cells [43]. Increasing evidence indicates the involvement of Ets-1 in the pathogenesis of SLE: (1) ETS1 has been identified as a risk locus for SLE in GWAS [44], [45] and the risk allele is associated with decreased levels of ETS1 transcripts in healthy control PBMCs [45]; (2) Ets-1 is critical in maintaining B cell identity and its absence drives terminal differentiation of B cells into immunoglobulin-secreting plasma cells [46], [47]; (3) Ets-1 functions as a cofactor for T-bet essential for Th1 effector function and differentiation [48]; (4) Ets-1 negatively regulates Th17 cell differentiation and Ets-1 deficiency results in elevated production of IL-17 and IL-17-related cytokines by Th17 cells [49]. These emerging findings support an important role of Ets family transcription factors in the development of SLE manifestations.
Previous findings indicated that increased production of IL-10 by SLE PBMCs was mainly derived from B cells [16], [50]–[53]. One explanation might be due to elevated expression of toll-like receptor 9 (TLR9) on B cells of SLE patients with active disease, as the study showed that TLR9-CpG interaction could enhance the production of anti-dsDNA antibody and IL-10 [54]. B cell receptor (BCR) stimulation or BCR-TLR9 costimulation have been shown to activate the Erk pathway in B cells of NZB×NZW F1 mice [55]. A plausible explanation of elevated co-expression of IL-10 and p-Elk-1 in B cells from SLE patients, especially during active disease, might be attributable to activated ERK and Elk-1 involved in the BCR-dependent IL-10 production in SLE B cells.
Overexpression of B-cell-derived IL-10 contributes to the pathogenesis of SLE likely dependent on its ability to promote B cell proliferation, differentiation and autoantibody production [56], [57]. Recently identified IL-10 producing regulatory B cells (Bregs) may exert immunosuppressive effects to modulate murine lupus [58]–[63]. The phenotypic markers of human Breg cells have not reached consensus; they may be enriched in CD19+CD24hiCD38hi and CD24hiCD27+ peripheral blood cells [64], [65]. Interestingly, CD19+CD24hiCD38hi B cells from SLE patients produce less IL-10 upon stimulation and are functionally impaired in suppressive capacity [64]. Thus, it seems unlikely that Bregs are the major producer of elevated IL-10 we observed in SLE patients.
The minor allele of rs3024505 showed consistently higher frequency in SLE patients than controls in all four ancestries, but only reached statistical significance for association with SLE in EA. Upon considering different genetic models, the additive model yielded the best genotypic association in EA (P = 2.7×10−8, OR [95%CI] = 1.30 [1.19–1.43]). Given the lack of evidence for genetic heterogeneity across EA, AS, AA and HS (P = 0.66 for Q statistic), the lack of significant association between rs3024505 and risk of SLE in AS, AA and HS might be due to low minor allele frequency and small sample size. Under the assumption that the minor allele of rs3024505 confers genetic risk with an odds ratio of 1.3 (determined in EA), the power to detect a significant association (P<0.05) for EA samples reaches 100%, whereas it is only 31% in AS, 55% in AA and 58% in HS datasets. According to the 1000 Genome Project data, the LD strength between rs3024505 and our proposed causal SNP rs3122605 is similarly strong in Asians (r2 = 1.0) and Americans/Hispanics (r2 = 0.8) as in Europeans (r2 = 0.85), but not in Africans (r2 = 0.1), suggesting that rs3122605 can be tagged by rs3024505 in non-Europeans except for the African-derived population.
It is possible that other SLE-associated variant(s) specific for AS, AA or HS failed to be captured by SNPs used in this study due to different LD pattern in each ancestry. Among non-European populations, SLE GWAS conducted in AA and HS are not in the currently available literature. To our knowledge, there have been four published SLE GWA studies conducted in AS, including two Chinese [44], [45], one Japanese [66] and one Korean GWAS [67], and a meta-analysis study based on Chinese GWAS [68]. In these studies, the Illumina Human 610 BeadChip is the commonly used genotyping platform, which contains nine IL10 SNPs (including rs3024505) and can capture 23 of the 38 common SNPs (MAF>1%) within +/−5 kb of IL10 with r2>0.9 in Asians (according to the 1000 Genome Project data). Because none of these studies have reported association signals of IL10 SNPs with SLE, current data are consistent with our findings that the IL10 locus is not a strong genetic risk factor for SLE in AS. Taken together, the association of IL10 with SLE in non-EA ancestries awaits further investigation.
We have searched four publically available cis-eQTL datasets conducted in immune cells from healthy Europeans [69]–[72] but found no convincing evidence to support the presence of another SNP that can better capture the association signal of IL10 expression trait in these datasets than our data of SLE-associated rs3024505/rs3122605. In addition, there was no convincing evidence to support the association of rs3024505 with differential expression of other genes within +/−1 Mb flanking region of IL10.
Given that the SNP rs3024505 confers increased risk for both SLE and IBD and could be tagged by rs3122605, it is possible that patients with IBD carrying the risk allele of rs3024505 may exhibit high serum levels of IL-10. There is evidence supporting elevated circulating IL-10 levels in both CD and UC patients that positively associated with disease activity [73]–[75], similarly to previous reports in SLE patients. However, genetically engineered mice exhibiting low to no IL-10 signaling in the intestinal tract develop severe IBD manifestations [76]–[78], supporting a pivotal role of IL-10 in down-regulation of inflammation. Increased levels of circulating IL-10 may be elicited by chronic inflammation in IBD, but may not be sufficiently strong to dampen intestinal inflammation [75], raising the possibility of defective IL-10 signaling at sites of organ damage in patients with SLE.
In conclusion, by characterizing genetic variations within the IL10 gene cluster region, we have identified the IL10 upstream SNP rs3122605 as the best likely causal variant responsible for association with SLE in European Americans. The SLE-associated rs3122605 G-allele preferentially binds to the activated Elk-1 conferring elevated IL10 expression. The observation that SLE patients, particular those with increased disease activity, showed enhanced activation of Elk-1 in nuclei and elevated co-expression of IL-10 and phospho-Elk-1 in peripheral lymphocytes highlights the involvement of aberrant Elk-1 signaling in development of SLE and suggests potential targeting therapy for disease amelioration.
Materials and Methods
Ethics statement
This study was approved by the Institutional Review Boards (IRBs) or the ethnic committees at the institutions where subjects were recruited. All subjects were enrolled after informed consent had been obtained. The overall study was approved by the IRB of the Oklahoma Medical Research Foundation (OMRF).
Subjects
To test the association of IL10 family genes with SLE, we used a large collection of case-control subjects from the collaborative Large Lupus Association Study 2 (LLAS2) [79], including European American (EA: 4,248 cases vs. 3,818 controls), African American (AA: 1,724 vs. 2,024), Asian (AS: 1,328 vs. 1,348) and Hispanic enriched for the Amerindian-European admixture (HS: 1,622 cases vs. 887 controls). African Americans included 286 Gullahs (155 cases vs. 131 controls), who are subjects with African ancestry. Asians were composed primarily of Koreans (906 cases and 1,012 controls) but also included Chinese, Japanese, Taiwanese and Singaporeans. Cases were defined by meeting at least four of the 1997 American College of Rheumatology (ACR) revised criteria for the classification of SLE [80].
To test functional consequences of SLE-associated variants, SLE patients and healthy controls of European ancestry were recruited at the University of California, Los Angeles and through the Lupus Family Registry and Repository (LFRR, lupus.omrf.org) for blood donations.
Genotyping and quality control
DNA samples were processed at the Lupus Genetics Studies Unit of OMRF. SNP genotyping was performed using an Illumina custom bead array on the iSCAN instrument for 19 tag SNPs covering over 135 kb of IL10-IL24 region and 347 admixture informative markers (AIMs). SNPs meeting the following criteria were included for subsequent genetic association tests: well-defined cluster scatter plots, SNP call rate >90%, minor allele frequency >1%, total proportion missing <5%, P>0.05 for differential missing rate between cases and controls, and Hardy-Weinberg proportion (HWP) test with a P>0.01 in controls and P>0.0001 in cases.
Subjects with individual genotyping missing rate >10% (due to low quality), shared identity by descent >0.4 or showing mismatch between the reported and estimated gender were removed. The global ancestry of each subject was estimated based on genotype of AIMs, using principal components analysis (PCA) [81] and ADMIXMAP [82]–[84], as described in another LLAS2 study [85], and then genetic outliers were removed.
Finally, a total of unrelated 15,533 subjects including EA (3,820 cases vs. 3,412 controls), AA (1,670 vs. 1,904; composed of 92.5% African Americans and 7.5% Gullahs), AS (1,252 vs. 1,249; composed of 74.6% of Koreans, 16.1% of Chinese and subjects from Japan and Singapore) and HS (1,445 vs. 781) were analyzed for 19 SNPs.
SNP imputation
Imputation was performed using IMPUTE 2.1.2 [86], with SNP genotypes of 379 Europeans (CEU, TSI, GBR, FIN and IBS), 246 Africans (YRI, ASW and LWK), 286 Asians (CHB, JPT and CHS) and 181 Americans (MXL, PUR and CLM) from the 1000 Genomes Project (version 3 of the phase 1 integrated data, March 2012 release) as references in imputation for our EA, AA, AS and HS subjects, respectively. Imputed genotypes had to meet the threshold of information score >0.9, as well as the quality control criteria as described above. After imputation, we obtained an additional 109 SNPs for EA, 45 for AA, 80 for AS and 64 for HS (the number varied due to different LD structure) for further analysis.
Real-time quantitative PCR
Total RNA was purified with TRIzol reagent (Life Technologies) from PBMCs of EA individuals (58 SLE cases and 55 healthy controls) and reverse-transcribed into cDNA with SuperScript II Reverse Transcriptase kit (Life Technologies). Messenger RNA levels of IL10 and a housekeeping gene RPLP0 were measured by quantitative real-time PCR using TaqMan assays (IL10 probe: Hs00961622_m1; RPLP0 probe: Hs99999902_m1, Applied Biosystems). All samples were run in triplicate. Relative IL10 mRNA levels were normalized to that of RPLP0, calculated by the 2−ΔΔCt method and Log10 transformed.
Enzyme-linked immunosorbent assay (ELISA)
Plasma IL-10 levels from 132 SLE patients and 116 healthy controls of EA ancestry were measured by ELISA (R&D systems).
Cell cultures
To examine whether inhibition of MAPK pathway may affect co-expression of IL-10 and p-Elk-1, control PBMCs (1×106) were cultured in growth medium with or without interferon alpha (IFNα) (1000 U/ml; PBL Biomedical Laboratories) in the presence or absence of one MAPK inhibitor (EMD Millipore), PD 98059 (20 µM; ERK/MEK inhibitor), SP 600125 (20 µM; JNK inhibitor) or SB 203580 (10 µM; p38-MAPK pathway inhibitor), respectively. Addition of Brefeldin A (eBiosciense) to cells in culture blocks intracellular protein (IL-10) transport processes.
Flow cytometry
The patients with SLE recruited in this part of study were evaluated for disease activity by the SLE Disease Activity Index (SLEDAI) 2000 [87] at the time of blood draw, and SLEDAI≥4 was considered as active disease [20], [21]. Freshly isolated or cultured PBMCs were incubated with mouse reference serum to block nonspecific binding to Fcγ receptors and then incubated with peridinin chlorophyll protein (PerCP)-conjugated anti-human CD3, allophycocyanin (APC)-conjugated anti-human CD19 and phycoerythrin (PE)-conjugated anti-human CD14 (eBiosciense) to identify T cell, B cell and monocyte subpopulations, respectively. For intracellular staining of IL-10 and p-Elk-1, cells were fixed with IC Fixation Buffer (eBiosciense), washed with Permeabilization Buffer (eBiosciense), and stained with fluorescein isothiocyanate (FITC)-conjugated anti-human IL-10 (eBiosciense) and PE - or Alexa Fluor647-conjugated anti-phospho-Elk-1 antibody (BD Biosciences). Background fluorescence was assessed using appropriate isotype - and fluorochrome-matched control antibodies. Cells were collected and analyzed by FACSCalibur flow cytometer equipped with the manufacturer's software (CellQuest; BD Biosciences).
Bioinformatic prediction of transcription factors, electrophoretic mobility shift assay (EMSA) and supershift assay
Bioinformatic analysis using the program TFSEARCH (conducted on 01/18/2011) showed predicted binding to Elk-1 and STRE in DNA sequence containing the minor but not the major allele of rs3122605 (Figure S3 and S4). In addition, HSF, ADR1 and MZF1 were predicted to bind with sequence containing either allele of rs3122605. Given that we were interested in identifying transcription factors that preferentially bind to the minor allele of rs3122605, and that the STRE (stress response element) binding factor includes two yeast transcription factors, Msn2p and Msn4p, we prioritized to test Elk-1 using EMSA.
EMSA and supershift assays were performed as previously described [88]. Nuclear extracts were prepared from peripheral blood lymphocytes of SLE patients with active disease using NE-PER Nuclear Extraction Reagent (Thermo scientific) and incubated with biotin-labeled oligodeoxynucleotides (synthesized by Integrated DNA Technologies, depicted in Table S3). EMSAs were performed with the LightShift Chemiluminescent EMSA kit (Thermo scientific). The antibody used in the supershift reactions was polyclonal rabbit anti-human Elk-1 (Santa Cruz Biotechnology).
Immunoblot analyses
Cytoplasmic or nuclear proteins from PBMCs were prepared using NE-PER Nuclear Extraction Reagent (Thermo scientific). Following SDS/PAGE, proteins were transferred onto Immobilon-P membrane (Millipore). After blocking with membrane blocking solution (Invitrogen, Life Technologies), the membrane was successively incubated with the anti-Elk-1 (Santa Cruz Biotechnology) or anti-phospho-Elk-1 (Cell Signaling Technology) primary antibody and the horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology). Blots were developed using the ECLPlus Western Blotting Detection System (GE Healthcare), visualized with ChemiDoc XRS imager and analyzed by Quantity One software (BIO-RAD). β-actin or Lamin B was used as internal control.
Statistical analysis
Allelic association tests in each ancestral group and conditional haplotype-based association tests in EA ancestry were performed by PLINK v1.07 software [89] under a logistic regression model adjusted for gender and the first three principal components estimated using AIMs. The Bonferroni corrected P-value threshold was adjusted to P<3.9×10−4 ( = 0.05/128 SNPs in EA). Pairwised LD values between SNPs and haplotypic association with SLE were evaluated using Haploview 4.2 [90]. The linear regression test was used to evaluate the association of SNP genotypes with IL-10 mRNA or protein levels. The Student's t-test was used to compare the mean values between two groups. A P value<0.05 was considered to be statistically significant.
Supporting Information
Zdroje
1. JohannesonB, LimaG, von SalomeJ, Alarcon-SegoviaD, Alarcon-RiquelmeME (2002) A major susceptibility locus for systemic lupus erythemathosus maps to chromosome 1q31. Am J Hum Genet 71 : 1060–1071.
2. WuH, BoackleSA, HanvivadhanakulP, UlgiatiD, GrossmanJM, et al. (2007) Association of a common complement receptor 2 haplotype with increased risk of systemic lupus erythematosus. Proc Natl Acad Sci U S A 104 : 3961–3966.
3. GatevaV, SandlingJK, HomG, TaylorKE, ChungSA, et al. (2009) A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat Genet 41 : 1228–1233.
4. FrankeA, McGovernDP, BarrettJC, WangK, Radford-SmithGL, et al. (2010) Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet 42 : 1118–1125.
5. FrankeA, BalschunT, KarlsenTH, SventoraityteJ, NikolausS, et al. (2008) Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet 40 : 1319–1323.
6. AndersonCA, BoucherG, LeesCW, FrankeA, D'AmatoM, et al. (2011) Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet 43 : 246–252.
7. BarrettJC, ClaytonDG, ConcannonP, AkolkarB, CooperJD, et al. (2009) Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 41 : 703–707.
8. PlagnolV, HowsonJM, SmythDJ, WalkerN, HaflerJP, et al. (2011) Genome-wide association analysis of autoantibody positivity in type 1 diabetes cases. PLoS Genet 7: e1002216.
9. RemmersEF, CosanF, KirinoY, OmbrelloMJ, AbaciN, et al. (2010) Genome-wide association study identifies variants in the MHC class I, IL10, and IL23R-IL12RB2 regions associated with Behcet's disease. Nat Genet 42 : 698–702.
10. MizukiN, MeguroA, OtaM, OhnoS, ShiotaT, et al. (2010) Genome-wide association studies identify IL23R-IL12RB2 and IL10 as Behcet's disease susceptibility loci. Nat Genet 42 : 703–706.
11. HofmannSR, Rosen-WolffA, TsokosGC, HedrichCM (2012) Biological properties and regulation of IL-10 related cytokines and their contribution to autoimmune disease and tissue injury. Clin Immunol 143 : 116–127.
12. PestkaS, KrauseCD, SarkarD, WalterMR, ShiY, et al. (2004) Interleukin-10 and related cytokines and receptors. Annu Rev Immunol 22 : 929–979.
13. ParkYB, LeeSK, KimDS, LeeJ, LeeCH, et al. (1998) Elevated interleukin-10 levels correlated with disease activity in systemic lupus erythematosus. Clin Exp Rheumatol 16 : 283–288.
14. HoussiauFA, LefebvreC, Vanden BergheM, LambertM, DevogelaerJP, et al. (1995) Serum interleukin 10 titers in systemic lupus erythematosus reflect disease activity. Lupus 4 : 393–395.
15. LlorenteL, Richaud-PatinY, Garcia-PadillaC, ClaretE, Jakez-OcampoJ, et al. (2000) Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum 43 : 1790–1800.
16. LlorenteL, Richaud-PatinY, CoudercJ, Alarcon-SegoviaD, Ruiz-SotoR, et al. (1997) Dysregulation of interleukin-10 production in relatives of patients with systemic lupus erythematosus. Arthritis Rheum 40 : 1429–1435.
17. GrondalG, KristjansdottirH, GunnlaugsdottirB, ArnasonA, LundbergI, et al. (1999) Increased number of interleukin-10-producing cells in systemic lupus erythematosus patients and their first-degree relatives and spouses in Icelandic multicase families. Arthritis Rheum 42 : 1649–1654.
18. BeebeAM, CuaDJ, de Waal MalefytR (2002) The role of interleukin-10 in autoimmune disease: systemic lupus erythematosus (SLE) and multiple sclerosis (MS). Cytokine Growth Factor Rev 13 : 403–412.
19. OkamotoA, FujioK, OkamuraT, YamamotoK (2011) Regulatory T-cell-associated cytokines in systemic lupus erythematosus. J Biomed Biotechnol 2011 : 463412.
20. GladmanDD, UrowitzMB, KagalA, HallettD (2000) Accurately describing changes in disease activity in Systemic Lupus Erythematosus. J Rheumatol 27 : 377–379.
21. YeeCS, FarewellVT, IsenbergDA, GriffithsB, TehLS, et al. (2011) The use of Systemic Lupus Erythematosus Disease Activity Index-2000 to define active disease and minimal clinically meaningful change based on data from a large cohort of systemic lupus erythematosus patients. Rheumatology (Oxford) 50 : 982–988.
22. LavaurJ, BernardF, TrifilieffP, PascoliV, KappesV, et al. (2007) A TAT-DEF-Elk-1 peptide regulates the cytonuclear trafficking of Elk-1 and controls cytoskeleton dynamics. J Neurosci 27 : 14448–14458.
23. BanchereauJ, PascualV (2006) Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 25 : 383–392.
24. van der LindenMW, WestendorpRG, SturkA, BergmanW, HuizingaTW (2000) High interleukin-10 production in first-degree relatives of patients with generalized but not cutaneous lupus erythematosus. J Investig Med 48 : 327–334.
25. GrondalG, KristjansdottirH, GunnlaugsdottirB, ArnasonA, LundbergI, et al. (1999) Increased number of interleukin-10-producing cells in systemic lupus erythematosus patients and their first-degree relatives and spouses in Icelandic multicase families. Arthritis Rheum 42 : 1649–1654.
26. EskdaleJ, WordsworthP, BowmanS, FieldM, GallagherG (1997) Association between polymorphisms at the human IL-10 locus and systemic lupus erythematosus. Tissue Antigens 49 : 635–639.
27. MehrianR, QuismorioFPJr, StrassmannG, StimmlerMM, HorwitzDA, et al. (1998) Synergistic effect between IL-10 and bcl-2 genotypes in determining susceptibility to systemic lupus erythematosus. Arthritis Rheum 41 : 596–602.
28. D'AlfonsoS, RampiM, BocchioD, ColomboG, Scorza-SmeraldiR, et al. (2000) Systemic lupus erythematosus candidate genes in the Italian population: evidence for a significant association with interleukin-10. Arthritis Rheum 43 : 120–128.
29. MokCC, LanchburyJS, ChanDW, LauCS (1998) Interleukin-10 promoter polymorphisms in Southern Chinese patients with systemic lupus erythematosus. Arthritis Rheum 41 : 1090–1095.
30. RoodMJ, KeijsersV, van der LindenMW, TongTQ, BorggreveSE, et al. (1999) Neuropsychiatric systemic lupus erythematosus is associated with imbalance in interleukin 10 promoter haplotypes. Ann Rheum Dis 58 : 85–89.
31. GibsonAW, EdbergJC, WuJ, WestendorpRG, HuizingaTW, et al. (2001) Novel single nucleotide polymorphisms in the distal IL-10 promoter affect IL-10 production and enhance the risk of systemic lupus erythematosus. J Immunol 166 : 3915–3922.
32. TrynkaG, SandorC, HanB, XuH, StrangerBE, et al. (2013) Chromatin marks identify critical cell types for fine mapping complex trait variants. Nat Genet 45 : 124–130.
33. SharrocksAD (2001) The ETS-domain transcription factor family. Nat Rev Mol Cell Biol 2 : 827–837.
34. RaoVN, ReddyES (1994) elk-1 proteins interact with MAP kinases. Oncogene 9 : 1855–1860.
35. LiW, MarshallC, MeiL, DzubowL, SchmultsC, et al. (2005) Srcasm modulates EGF and Src-kinase signaling in keratinocytes. J Biol Chem 280 : 6036–6046.
36. SharmaA, CallahanLM, SulJY, KimTK, BarrettL, et al. (2010) A neurotoxic phosphoform of Elk-1 associates with inclusions from multiple neurodegenerative diseases. PLoS One 5: e9002.
37. MorrisJF, SulJY, KimMS, Klein-SzantoAJ, SchochetT, et al. (2013) Elk-1 phosphorylated at threonine-417 is present in diverse cancers and correlates with differentiation grade of colonic adenocarcinoma. Hum Pathol 44 : 766–776.
38. GrammerAC, FischerR, LeeO, ZhangX, LipskyPE (2004) Flow cytometric assessment of the signaling status of human B lymphocytes from normal and autoimmune individuals. Arthritis Res Ther 6 : 28–38.
39. WongCK, WongPT, TamLS, LiEK, ChenDP, et al. (2009) Activation profile of intracellular mitogen-activated protein kinases in peripheral lymphocytes of patients with systemic lupus erythematosus. J Clin Immunol 29 : 738–746.
40. BesnardA, Galan-RodriguezB, VanhoutteP, CabocheJ (2011) Elk-1 a transcription factor with multiple facets in the brain. Front Neurosci 5 : 35.
41. OdrowazZ, SharrocksAD (2012) ELK1 uses different DNA binding modes to regulate functionally distinct classes of target genes. PLoS Genet 8: e1002694.
42. LiJC, LauAS (2007) A role for mitogen-activated protein kinase and Ets-1 in the induction of interleukin-10 transcription by human immunodeficiency virus-1 Tat. Immunology 121 : 337–348.
43. LeeCG, KwonHK, SahooA, HwangW, SoJS, et al. (2012) Interaction of Ets-1 with HDAC1 represses IL-10 expression in Th1 cells. J Immunol 188 : 2244–2253.
44. HanJW, ZhengHF, CuiY, SunLD, YeDQ, et al. (2009) Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet 41 : 1234–1237.
45. YangW, ShenN, YeDQ, LiuQ, ZhangY, et al. (2010) Genome-Wide Association Study in Asian Populations Identifies Variants in ETS1 and WDFY4 Associated with Systemic Lupus Erythematosus. PLoS Genet 6: e1000841.
46. EyquemS, CheminK, FasseuM, ChopinM, SigauxF, et al. (2004) The development of early and mature B cells is impaired in mice deficient for the Ets-1 transcription factor. Eur J Immunol 34 : 3187–3196.
47. WangD, JohnSA, ClementsJL, PercyDH, BartonKP, et al. (2005) Ets-1 deficiency leads to altered B cell differentiation, hyperresponsiveness to TLR9 and autoimmune disease. Int Immunol 17 : 1179–1191.
48. GrenninglohR, KangBY, HoIC (2005) Ets-1, a functional cofactor of T-bet, is essential for Th1 inflammatory responses. J Exp Med 201 : 615–626.
49. MoisanJ, GrenninglohR, BettelliE, OukkaM, HoIC (2007) Ets-1 is a negative regulator of Th17 differentiation. J Exp Med 204 : 2825–2835.
50. LlorenteL, Richaud-PatinY, WijdenesJ, Alcocer-VarelaJ, MaillotMC, et al. (1993) Spontaneous production of interleukin-10 by B lymphocytes and monocytes in systemic lupus erythematosus. Eur Cytokine Netw 4 : 421–427.
51. LlorenteL, Richaud-PatinY, FiorR, Alcocer-VarelaJ, WijdenesJ, et al. (1994) In vivo production of interleukin-10 by non-T cells in rheumatoid arthritis, Sjogren's syndrome, and systemic lupus erythematosus. A potential mechanism of B lymphocyte hyperactivity and autoimmunity. Arthritis Rheum 37 : 1647–1655.
52. al-JanadiM, al-DalaanA, al-BallaS, al-HumaidiM, RaziuddinS (1996) Interleukin-10 (IL-10) secretion in systemic lupus erythematosus and rheumatoid arthritis: IL-10-dependent CD4+CD45RO+ T cell-B cell antibody synthesis. J Clin Immunol 16 : 198–207.
53. CsiszarA, NagyG, GergelyP, PozsonyiT, PocsikE (2000) Increased interferon-gamma (IFN-gamma), IL-10 and decreased IL-4 mRNA expression in peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE). Clin Exp Immunol 122 : 464–470.
54. NakanoS, MorimotoS, SuzukiJ, NozawaK, AmanoH, et al. (2008) Role of pathogenic auto-antibody production by Toll-like receptor 9 of B cells in active systemic lupus erythematosus. Rheumatology (Oxford) 47 : 145–149.
55. SchickelJN, PasqualiJL, SoleyA, KnappAM, DecossasM, et al. (2012) Carabin deficiency in B cells increases BCR-TLR9 costimulation-induced autoimmunity. EMBO Mol Med 4 : 1261–1275.
56. RoussetF, GarciaE, DefranceT, PeronneC, VezzioN, et al. (1992) Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A 89 : 1890–1893.
57. LlorenteL, Richaud-PatinY (2003) The role of interleukin-10 in systemic lupus erythematosus. J Autoimmun 20 : 287–289.
58. YangM, RuiK, WangS, LuL (2013) Regulatory B cells in autoimmune diseases. Cell Mol Immunol 10 : 122–132.
59. BlairPA, Chavez-RuedaKA, EvansJG, ShlomchikMJ, EddaoudiA, et al. (2009) Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol 182 : 3492–3502.
60. HaasKM, WatanabeR, MatsushitaT, NakashimaH, IshiuraN, et al. (2010) Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J Immunol 184 : 4789–4800.
61. WatanabeR, IshiuraN, NakashimaH, KuwanoY, OkochiH, et al. (2010) Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol 184 : 4801–4809.
62. ScapiniP, LamagnaC, HuY, LeeK, TangQ, et al. (2011) B cell-derived IL-10 suppresses inflammatory disease in Lyn-deficient mice. Proc Natl Acad Sci U S A 108: E823–832.
63. TeichmannLL, KashgarianM, WeaverCT, RoersA, MullerW, et al. (2012) B cell-derived IL-10 does not regulate spontaneous systemic autoimmunity in MRL.Fas(lpr) mice. J Immunol 188 : 678–685.
64. BlairPA, NorenaLY, Flores-BorjaF, RawlingsDJ, IsenbergDA, et al. (2010) CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 32 : 129–140.
65. IwataY, MatsushitaT, HorikawaM, DililloDJ, YanabaK, et al. (2011) Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 117 : 530–541.
66. OkadaY, ShimaneK, KochiY, TahiraT, SuzukiA, et al. (2012) A genome-wide association study identified AFF1 as a susceptibility locus for systemic lupus eyrthematosus in Japanese. PLoS Genet 8: e1002455.
67. LeeHS, KimT, BangSY, NaYJ, KimI, et al. (2013) Ethnic specificity of lupus-associated loci identified in a genome-wide association study in Korean women. Ann Rheum Dis [Epub ahead of print].
68. YangW, TangH, ZhangY, TangX, ZhangJ, et al. (2013) Meta-analysis followed by replication identifies loci in or near CDKN1B, TET3, CD80, DRAM1, and ARID5B as associated with systemic lupus erythematosus in Asians. Am J Hum Genet 92 : 41–51.
69. FairfaxBP, MakinoS, RadhakrishnanJ, PlantK, LeslieS, et al. (2012) Genetics of gene expression in primary immune cells identifies cell type-specific master regulators and roles of HLA alleles. Nat Genet 44 : 502–510.
70. GrundbergE, SmallKS, HedmanAK, NicaAC, BuilA, et al. (2012) Mapping cis - and trans-regulatory effects across multiple tissues in twins. Nat Genet 44 : 1084–1089.
71. DimasAS, DeutschS, StrangerBE, MontgomerySB, BorelC, et al. (2009) Common regulatory variation impacts gene expression in a cell type-dependent manner. Science 325 : 1246–1250.
72. StrangerBE, MontgomerySB, DimasAS, PartsL, StegleO, et al. (2012) Patterns of cis regulatory variation in diverse human populations. PLoS Genet 8: e1002639.
73. SzkaradkiewiczA, MarciniakR, Chudzicka-StrugalaI, WasilewskaA, DrewsM, et al. (2009) Proinflammatory cytokines and IL-10 in inflammatory bowel disease and colorectal cancer patients. Arch Immunol Ther Exp (Warsz) 57 : 291–294.
74. WangAH, LamWJ, HanDY, DingY, HuR, et al. (2011) The effect of IL-10 genetic variation and interleukin 10 serum levels on Crohn's disease susceptibility in a New Zealand population. Hum Immunol 72 : 431–435.
75. KucharzikT, StollR, LugeringN, DomschkeW (1995) Circulating antiinflammatory cytokine IL-10 in patients with inflammatory bowel disease (IBD). Clin Exp Immunol 100 : 452–456.
76. KuhnR, LohlerJ, RennickD, RajewskyK, MullerW (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75 : 263–274.
77. SpencerSD, Di MarcoF, HooleyJ, Pitts-MeekS, BauerM, et al. (1998) The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. J Exp Med 187 : 571–578.
78. GlockerEO, KotlarzD, BoztugK, GertzEM, SchafferAA, et al. (2009) Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med 361 : 2033–2045.
79. ScofieldRH, KaufmanKM (2012) Mapping susceptibility gene in systemic lupus erythematosus. Methods Mol Biol 900 : 11–24.
80. HochbergMC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40 : 1725.
81. PriceAL, PattersonNJ, PlengeRM, WeinblattME, ShadickNA, et al. (2006) Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38 : 904–909.
82. HoggartCJ, ParraEJ, ShriverMD, BonillaC, KittlesRA, et al. (2003) Control of confounding of genetic associations in stratified populations. Am J Hum Genet 72 : 1492–1504.
83. HoggartCJ, ShriverMD, KittlesRA, ClaytonDG, McKeiguePM (2004) Design and analysis of admixture mapping studies. Am J Hum Genet 74 : 965–978.
84. McKeiguePM (2005) Prospects for admixture mapping of complex traits. Am J Hum Genet 76 : 1–7.
85. LessardCJ, AdriantoI, KellyJA, KaufmanKM, GrundahlKM, et al. (2011) Identification of a systemic lupus erythematosus susceptibility locus at 11p13 between PDHX and CD44 in a multiethnic study. Am J Hum Genet 88 : 83–91.
86. HowieBN, DonnellyP, MarchiniJ (2009) A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 5: e1000529.
87. GladmanDD, IbanezD, UrowitzMB (2002) Systemic lupus erythematosus disease activity index 2000. J Rheumatol 29 : 288–291.
88. TanW, WuH, ZhaoJ, DerberLA, LeeDM, et al. (2010) A functional RANKL polymorphism associated with younger age at onset of rheumatoid arthritis. Arthritis Rheum 62 : 2864–2875.
89. PurcellS, NealeB, Todd-BrownK, ThomasL, FerreiraMA, et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 : 559–575.
90. BarrettJC, FryB, MallerJ, DalyMJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21 : 263–265.
Štítky
Genetika Reprodukční medicína
Článek Defending Sperm FunctionČlánek How to Choose the Right MateČlánek Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental TransitionČlánek Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in SpermatogenesisČlánek The Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate EmbryogenesisČlánek Multiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time inČlánek Playing the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral MyelinationČlánek A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase inČlánek Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 10- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- Defending Sperm Function
- How to Choose the Right Mate
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
- Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental Transition
- A Novel Actin mRNA Splice Variant Regulates ACTG1 Expression
- Tracking Proliferative History in Lymphocyte Development with Cre-Mediated Sister Chromatid Recombination
- Correlated Occurrence and Bypass of Frame-Shifting Insertion-Deletions (InDels) to Give Functional Proteins
- Chimeric Protein Complexes in Hybrid Species Generate Novel Phenotypes
- Loss of miR-10a Activates and Collaborates with Activated Wnt Signaling in Inducing Intestinal Neoplasia in Female Mice
- Both Rare and Copy Number Variants Are Prevalent in Agenesis of the Corpus Callosum but Not in Cerebellar Hypoplasia or Polymicrogyria
- Reverse PCA, a Systematic Approach for Identifying Genes Important for the Physical Interaction between Protein Pairs
- Partial Deletion of Chromosome 8 β-defensin Cluster Confers Sperm Dysfunction and Infertility in Male Mice
- Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in Spermatogenesis
- Coordinate Regulation of Mature Dopaminergic Axon Morphology by Macroautophagy and the PTEN Signaling Pathway
- Cooperation between RUNX1-ETO9a and Novel Transcriptional Partner KLF6 in Upregulation of in Acute Myeloid Leukemia
- Mobility of the Native Conjugative Plasmid pLS20 Is Regulated by Intercellular Signaling
- FliZ Is a Global Regulatory Protein Affecting the Expression of Flagellar and Virulence Genes in Individual Bacterial Cells
- Specific Tandem Repeats Are Sufficient for Paramutation-Induced Trans-Generational Silencing
- Condensin II Subunit dCAP-D3 Restricts Retrotransposon Mobilization in Somatic Cells
- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- The Insulator Homie Promotes Expression and Protects the Adjacent Gene from Repression by Polycomb Spreading
- Human Intellectual Disability Genes Form Conserved Functional Modules in
- Coordination of Cell Proliferation and Cell Fate Determination by CES-1 Snail
- ORFs in Drosophila Are Important to Organismal Fitness and Evolved Rapidly from Previously Non-coding Sequences
- Different Roles of Eukaryotic MutS and MutL Complexes in Repair of Small Insertion and Deletion Loops in Yeast
- The Spore Differentiation Pathway in the Enteric Pathogen
- Acceleration of the Glycolytic Flux by Steroid Receptor Coactivator-2 Is Essential for Endometrial Decidualization
- The Human Nuclear Poly(A)-Binding Protein Promotes RNA Hyperadenylation and Decay
- Genome Wide Analysis Reveals Zic3 Interaction with Distal Regulatory Elements of Stage Specific Developmental Genes in Zebrafish
- Xbp1 Directs Global Repression of Budding Yeast Transcription during the Transition to Quiescence and Is Important for the Longevity and Reversibility of the Quiescent State
- The Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate Embryogenesis
- Incorporating Motif Analysis into Gene Co-expression Networks Reveals Novel Modular Expression Pattern and New Signaling Pathways
- The Bacterial Response Regulator ArcA Uses a Diverse Binding Site Architecture to Regulate Carbon Oxidation Globally
- Direct Monitoring of the Strand Passage Reaction of DNA Topoisomerase II Triggers Checkpoint Activation
- Multiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time in
- A Reversible Histone H3 Acetylation Cooperates with Mismatch Repair and Replicative Polymerases in Maintaining Genome Stability
- ALS-Associated Mutations Result in Compromised Alternative Splicing and Autoregulation
- Robust Demographic Inference from Genomic and SNP Data
- Preferential Binding to Elk-1 by SLE-Associated Risk Allele Upregulates Expression
- Rad52 Sumoylation Prevents the Toxicity of Unproductive Rad51 Filaments Independently of the Anti-Recombinase Srs2
- The Serum Resistome of a Globally Disseminated Multidrug Resistant Uropathogenic Clone
- Identification of 526 Conserved Metazoan Genetic Innovations Exposes a New Role for Cofactor E-like in Neuronal Microtubule Homeostasis
- SUMO Localizes to the Central Element of Synaptonemal Complex and Is Required for the Full Synapsis of Meiotic Chromosomes in Budding Yeast
- Integrated Enrichment Analysis of Variants and Pathways in Genome-Wide Association Studies Indicates Central Role for IL-2 Signaling Genes in Type 1 Diabetes, and Cytokine Signaling Genes in Crohn's Disease
- Genome-Wide High-Resolution Mapping of UV-Induced Mitotic Recombination Events in
- Genome-Wide Analysis of Cell Type-Specific Gene Transcription during Spore Formation in
- Playing the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral Myelination
- Two Portable Recombination Enhancers Direct Donor Choice in Fission Yeast Heterochromatin
- Mining the Human Phenome Using Allelic Scores That Index Biological Intermediates
- Yeast Tdh3 (Glyceraldehyde 3-Phosphate Dehydrogenase) Is a Sir2-Interacting Factor That Regulates Transcriptional Silencing and rDNA Recombination
- A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase in
- A Review of Bacteria-Animal Lateral Gene Transfer May Inform Our Understanding of Diseases like Cancer
- High Throughput Sequencing Reveals Alterations in the Recombination Signatures with Diminishing Spo11 Activity
- Partitioning the Heritability of Tourette Syndrome and Obsessive Compulsive Disorder Reveals Differences in Genetic Architecture
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- A GDF5 Point Mutation Strikes Twice - Causing BDA1 and SYNS2
- Systematic Unraveling of the Unsolved Pathway of Nicotine Degradation in
- Natural Genetic Variation of Integrin Alpha L () Modulates Ischemic Brain Injury in Stroke
- Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
- Evolutionary Change within a Bipotential Switch Shaped the Sperm/Oocyte Decision in Hermaphroditic Nematodes
- Limiting of the Innate Immune Response by SF3A-Dependent Control of MyD88 Alternative mRNA Splicing
- Multiple Signaling Pathways Coordinate to Induce a Threshold Response in a Chordate Embryo
- Distinct Regulatory Mechanisms Act to Establish and Maintain Pax3 Expression in the Developing Neural Tube
- Genome Wide Analysis of Narcolepsy in China Implicates Novel Immune Loci and Reveals Changes in Association Prior to Versus After the 2009 H1N1 Influenza Pandemic
- Mismatch Repair Genes and Modify CAG Instability in Huntington's Disease Mice: Genome-Wide and Candidate Approaches
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- Hsp70-Hsp40 Chaperone Complex Functions in Controlling Polarized Growth by Repressing Hsf1-Driven Heat Stress-Associated Transcription
- Function and Evolution of DNA Methylation in
- Stimulation of mTORC1 with L-leucine Rescues Defects Associated with Roberts Syndrome
- Transcription Termination and Chimeric RNA Formation Controlled by FPA
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



