-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDefending Sperm Function
article has not abstract
Published in the journal: . PLoS Genet 9(10): e32767. doi:10.1371/journal.pgen.1003889
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1003889Summary
article has not abstract
A New Role for ß-defensins
ß-defensins are a large family of cationic peptides with antimicrobial activities in vitro against Gram-positive and Gram-negative bacteria, fungi, and enveloped viruses [1]. As such, they are thought to be an important component of the innate immune system and are expressed by cells such as phagocytes and epithelia that are involved in host defence against microbial infections. The defensins form pores within the target membranes allowing the movement of small molecules across the plasma membrane and causing decreased bacterial viability. Several ß-defensins are expressed by epithelial cells of the caput epididymis where they may have a role in innate immunity of the urogenital system [2] or protecting sperm from immunorecognition [3]. To date, however, the exact role of ß-defensins in contributing to innate immunity in vivo has not been unequivocally established. It is possible that ß-defensins may have other important physiological functions beyond their antimicrobial activities. Indeed, a mutation in the Defb103 defensin gene causes a dominant black coat colour in dogs [4]. The paper in this issue of PLOS Genetics by Yu S. Zhou and colleagues contributes to our understanding of the function of ß-defensins in vivo by showing that they also have a role in sperm function [5]. To my knowledge, this is the first unequivocal demonstration of a ß-defensin phenotype in transgenic knock-out mice.
Events at Fertilization
Spermatozoa show exquisite structural adaptations for their destiny of egg fertilization. The genetic material is tightly compacted into a streamlined head, the mid-piece is packed with mitochondrial powerhouses, and a long tail provides efficient propulsion. The head is covered by the acrosome, a vesicular cap derived from the Golgi that contains enzymes required for fertilization. Freshly ejaculated sperm are motile but not capable of fertilization, as they need to undergo a process called capacitation, which involves biochemical and metabolic changes. These changes include removal of cholesterol from the sperm membranes. Capacitation allows the sperm to bind to the ZP3 protein, a component of the outer zona pellucida layer of the egg. Binding to ZP3 activates T-type and TRPC2 membrane channels that allow Ca2+ entry into the sperm to enable acrosome exocytosis. In addition, a cation channel (CatSper) found in the tail region of the sperm that has a promiscuous activation profile can also allow Ca2+ entry and may also play a role in facilitating the acrosome reaction. The acrosome reaction is an essential step to allow the sperm to fuse with the plasma membrane of the egg to complete fertilization. Fertilization causes release of Ca2+ from intracellular stores in the egg, which induces exit from meiotic arrest and exocytosis of cortical granules to prevent multiple sperm entry.
ß-defensins Act as a Brake to Sperm Activation
In the article by Zhou et al. [5], the authors have identified that some ß-defensins are essential for normal sperm function and male fertility in the mouse. There are at least 50 ß-defensins genes in the mouse genome with a cluster of 31 located on chromosome 8. Analysis of the function of individual genes in vivo would be a Herculean task and might be compromised by functional redundancy such as in mice deficient for Defb1, which show only mild defects [6]. To overcome this problem, the authors took the pragmatic approach of deleting several genes at once. Deletion of a cluster of nine ß-defensin genes (Defb1, Defb50, Defb2, Defb10, Defb9, Defb11, Defb15, Defb35, and Defb13) produced mutant offspring with no obvious gross phenotype or increased inflammatory profiles under normal animal housing conditions. Mutant female mice were fertile, but males were infertile even though histological analysis showed normal spermatogenesis and sperm numbers in the epididymides.
The cause of the infertility was related to multiple defects in the sperm that would compromise their fertilization ability. The sperm showed increased fragility and decreased motility, probably caused by a disrupted microtubule structure in the tail. Around 20% of sperm isolated from the cauda (tail) part of the epididymis showed precocious sperm capacitation, as judged by the appearance of zonadhesin protein on the surface of the sperm. Twenty to twenty-eight percent of the sperm also showed a premature acrosome reaction which normally only occurs when sperm bind to the ZP3 protein. Consistent with the premature acrosome reaction, the mutant sperm showed increased levels of intracellular Ca2+ compared to normal sperm.
Unanswered Questions
The data clearly identify a novel function for ß-defensins in sperm activation and male fertility. It is not yet known, however, whether these ß-defensins play a similar role in humans. Five of the nine ß-defensin genes that were deleted in the mouse have no human orthologues, so it is possible that these effects may be restricted to the mouse. Alternatively, the four genes that are conserved in both humans and mice (DEFB1/Defb1, DEFB106/Defb15, DEFB105Defb35, and DEFB107/Defb13) are all expressed in the human testes based on RNAseq expression profiling, so if any of these genes are responsible for the phenotype then they may show functional conservation between humans and mice.
It is also not known what the individual contribution of each of the deleted ß-defensin genes might be towards the phenotype. It is possible that the phenotype might be caused by the loss of only one of the nine ß-defensins genes. Analysis of the expression profile of each of these genes in the epididymis may provide a clue as to which is the most important. For example, the Defb11 gene is specific to the mouse and shows expression in epididymal epithelia. It might be possible to evaluate the effect of each ß-defensin protein by adding it to mutant sperm and testing whether it can prevent premature capacitation.
Another important question is to understand the mechanism by which these ß-defensins prevent premature activation of the sperm. The usual sequence of events in the sperm prior to fertilization is shown in Figure 1. Normally Ca2+ entry occurs after binding to the egg ZP3 protein although some Ca2+ can also enter by promiscuous activation of CatSper channels. The authors speculate that the ß-defensins might inhibit Ca2+ channel activity in a way similar to the SPINK3 protein found in seminal fluid. The cationic nature of the ß-defensins will facilitate binding to the sperm plasma membrane where they would be well-placed to interfere with Ca2+ channel opening. In addition, the ß-defensins may prevent premature sperm capacitation by inhibiting cholesterol removal from the sperm membranes or preventing hyperpolarization. Biochemical and electrophysiological studies should be able to test these hypotheses and provide a better insight into how ß-defensins modulate sperm activation.
Fig. 1. Events at egg fertilization and the possible roles of ß-defensin proteins in regulating this process. 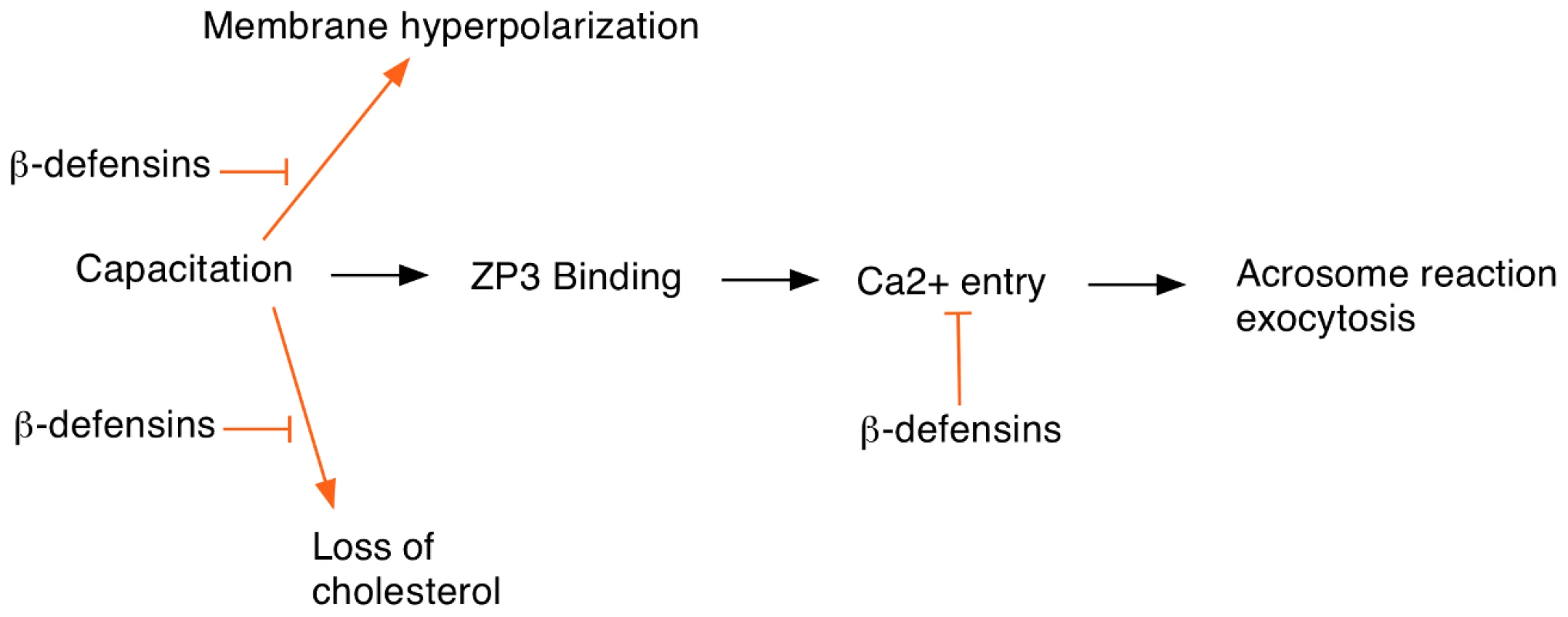
Sperm capacitation is required for egg fertilization and involves several cellular changes including loss of cholesterol from the sperm head and increased membrane hyperpolarization. ß-defensin proteins may inhibit these events to help prevent premature sperm capacitation. In addition, ß-defensins might also block Ca2+ entry, which is required for the acrosome reaction.
Zdroje
1. GanzT (2003) Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 3 : 710–720.
2. YamaguchiY, NagaseT, MakitaR, FukuharaS, TomitaT, et al. (2002) Identification of multiple novel epididymis-specific beta-defensin isoforms in humans and mice. J Immunol 169 : 2516–2523.
3. YudinAI, GeneraoSE, TollnerTL, TreeceCA, OverstreetJW, et al. (2005) Beta-defensin 126 on the cell surface protects sperm from immunorecognition and binding of anti-sperm antibodies. Biol Reprod 73 : 1243–1252.
4. CandilleSI, KaelinCB, CattanachBM, YuB, ThompsonDA, et al. (2007) A ß-defensin mutation causes black coat color in domestic dogs. Science 318 : 1418–1423.
5. ZhouYS, WebbS, LetticeL, TardifS, KilanowskiF, et al. (2013) Partial deletion of chromosome 8 ß-defensin cluster confers sperm dysfunction and infertility in male mice. PLoS Genet 9 e1003826. doi: 10.1371/journal.pgen.1003826
6. MorrisonG, KilanowskiF, DavidsonD, DorinJ (2002) Characterization of the mouse beta defensin 1, Defb1, mutant mouse model. Infect Immun 70 : 3053–3060.
Štítky
Genetika Reprodukční medicína
Článek How to Choose the Right MateČlánek Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental TransitionČlánek Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in SpermatogenesisČlánek The Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate EmbryogenesisČlánek Multiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time inČlánek Playing the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral MyelinationČlánek A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase inČlánek Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 10- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
- Délka menstruačního cyklu jako marker ženské plodnosti
-
Všechny články tohoto čísla
- Defending Sperm Function
- How to Choose the Right Mate
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
- Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental Transition
- A Novel Actin mRNA Splice Variant Regulates ACTG1 Expression
- Tracking Proliferative History in Lymphocyte Development with Cre-Mediated Sister Chromatid Recombination
- Correlated Occurrence and Bypass of Frame-Shifting Insertion-Deletions (InDels) to Give Functional Proteins
- Chimeric Protein Complexes in Hybrid Species Generate Novel Phenotypes
- Loss of miR-10a Activates and Collaborates with Activated Wnt Signaling in Inducing Intestinal Neoplasia in Female Mice
- Both Rare and Copy Number Variants Are Prevalent in Agenesis of the Corpus Callosum but Not in Cerebellar Hypoplasia or Polymicrogyria
- Reverse PCA, a Systematic Approach for Identifying Genes Important for the Physical Interaction between Protein Pairs
- Partial Deletion of Chromosome 8 β-defensin Cluster Confers Sperm Dysfunction and Infertility in Male Mice
- Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in Spermatogenesis
- Coordinate Regulation of Mature Dopaminergic Axon Morphology by Macroautophagy and the PTEN Signaling Pathway
- Cooperation between RUNX1-ETO9a and Novel Transcriptional Partner KLF6 in Upregulation of in Acute Myeloid Leukemia
- Mobility of the Native Conjugative Plasmid pLS20 Is Regulated by Intercellular Signaling
- FliZ Is a Global Regulatory Protein Affecting the Expression of Flagellar and Virulence Genes in Individual Bacterial Cells
- Specific Tandem Repeats Are Sufficient for Paramutation-Induced Trans-Generational Silencing
- Condensin II Subunit dCAP-D3 Restricts Retrotransposon Mobilization in Somatic Cells
- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- The Insulator Homie Promotes Expression and Protects the Adjacent Gene from Repression by Polycomb Spreading
- Human Intellectual Disability Genes Form Conserved Functional Modules in
- Coordination of Cell Proliferation and Cell Fate Determination by CES-1 Snail
- ORFs in Drosophila Are Important to Organismal Fitness and Evolved Rapidly from Previously Non-coding Sequences
- Different Roles of Eukaryotic MutS and MutL Complexes in Repair of Small Insertion and Deletion Loops in Yeast
- The Spore Differentiation Pathway in the Enteric Pathogen
- Acceleration of the Glycolytic Flux by Steroid Receptor Coactivator-2 Is Essential for Endometrial Decidualization
- The Human Nuclear Poly(A)-Binding Protein Promotes RNA Hyperadenylation and Decay
- Genome Wide Analysis Reveals Zic3 Interaction with Distal Regulatory Elements of Stage Specific Developmental Genes in Zebrafish
- Xbp1 Directs Global Repression of Budding Yeast Transcription during the Transition to Quiescence and Is Important for the Longevity and Reversibility of the Quiescent State
- The Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate Embryogenesis
- Incorporating Motif Analysis into Gene Co-expression Networks Reveals Novel Modular Expression Pattern and New Signaling Pathways
- The Bacterial Response Regulator ArcA Uses a Diverse Binding Site Architecture to Regulate Carbon Oxidation Globally
- Direct Monitoring of the Strand Passage Reaction of DNA Topoisomerase II Triggers Checkpoint Activation
- Multiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time in
- A Reversible Histone H3 Acetylation Cooperates with Mismatch Repair and Replicative Polymerases in Maintaining Genome Stability
- ALS-Associated Mutations Result in Compromised Alternative Splicing and Autoregulation
- Robust Demographic Inference from Genomic and SNP Data
- Preferential Binding to Elk-1 by SLE-Associated Risk Allele Upregulates Expression
- Rad52 Sumoylation Prevents the Toxicity of Unproductive Rad51 Filaments Independently of the Anti-Recombinase Srs2
- The Serum Resistome of a Globally Disseminated Multidrug Resistant Uropathogenic Clone
- Identification of 526 Conserved Metazoan Genetic Innovations Exposes a New Role for Cofactor E-like in Neuronal Microtubule Homeostasis
- SUMO Localizes to the Central Element of Synaptonemal Complex and Is Required for the Full Synapsis of Meiotic Chromosomes in Budding Yeast
- Integrated Enrichment Analysis of Variants and Pathways in Genome-Wide Association Studies Indicates Central Role for IL-2 Signaling Genes in Type 1 Diabetes, and Cytokine Signaling Genes in Crohn's Disease
- Genome-Wide High-Resolution Mapping of UV-Induced Mitotic Recombination Events in
- Genome-Wide Analysis of Cell Type-Specific Gene Transcription during Spore Formation in
- Playing the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral Myelination
- Two Portable Recombination Enhancers Direct Donor Choice in Fission Yeast Heterochromatin
- Mining the Human Phenome Using Allelic Scores That Index Biological Intermediates
- Yeast Tdh3 (Glyceraldehyde 3-Phosphate Dehydrogenase) Is a Sir2-Interacting Factor That Regulates Transcriptional Silencing and rDNA Recombination
- A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase in
- A Review of Bacteria-Animal Lateral Gene Transfer May Inform Our Understanding of Diseases like Cancer
- High Throughput Sequencing Reveals Alterations in the Recombination Signatures with Diminishing Spo11 Activity
- Partitioning the Heritability of Tourette Syndrome and Obsessive Compulsive Disorder Reveals Differences in Genetic Architecture
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- A GDF5 Point Mutation Strikes Twice - Causing BDA1 and SYNS2
- Systematic Unraveling of the Unsolved Pathway of Nicotine Degradation in
- Natural Genetic Variation of Integrin Alpha L () Modulates Ischemic Brain Injury in Stroke
- Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
- Evolutionary Change within a Bipotential Switch Shaped the Sperm/Oocyte Decision in Hermaphroditic Nematodes
- Limiting of the Innate Immune Response by SF3A-Dependent Control of MyD88 Alternative mRNA Splicing
- Multiple Signaling Pathways Coordinate to Induce a Threshold Response in a Chordate Embryo
- Distinct Regulatory Mechanisms Act to Establish and Maintain Pax3 Expression in the Developing Neural Tube
- Genome Wide Analysis of Narcolepsy in China Implicates Novel Immune Loci and Reveals Changes in Association Prior to Versus After the 2009 H1N1 Influenza Pandemic
- Mismatch Repair Genes and Modify CAG Instability in Huntington's Disease Mice: Genome-Wide and Candidate Approaches
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- Hsp70-Hsp40 Chaperone Complex Functions in Controlling Polarized Growth by Repressing Hsf1-Driven Heat Stress-Associated Transcription
- Function and Evolution of DNA Methylation in
- Stimulation of mTORC1 with L-leucine Rescues Defects Associated with Roberts Syndrome
- Transcription Termination and Chimeric RNA Formation Controlled by FPA
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



