-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Transcription Termination and Chimeric RNA Formation Controlled by FPA
Alternative cleavage and polyadenylation influence the coding and regulatory potential of mRNAs and where transcription termination occurs. Although widespread, few regulators of this process are known. The Arabidopsis thaliana protein FPA is a rare example of a trans-acting regulator of poly(A) site choice. Analysing fpa mutants therefore provides an opportunity to reveal generic consequences of disrupting this process. We used direct RNA sequencing to quantify shifts in RNA 3′ formation in fpa mutants. Here we show that specific chimeric RNAs formed between the exons of otherwise separate genes are a striking consequence of loss of FPA function. We define intergenic read-through transcripts resulting from defective RNA 3′ end formation in fpa mutants and detail cryptic splicing and antisense transcription associated with these read-through RNAs. We identify alternative polyadenylation within introns that is sensitive to FPA and show FPA-dependent shifts in IBM1 poly(A) site selection that differ from those recently defined in mutants defective in intragenic heterochromatin and DNA methylation. Finally, we show that defective termination at specific loci in fpa mutants is shared with dicer-like 1 (dcl1) or dcl4 mutants, leading us to develop alternative explanations for some silencing roles of these proteins. We relate our findings to the impact that altered patterns of 3′ end formation can have on gene and genome organisation.
Published in the journal: . PLoS Genet 9(10): e32767. doi:10.1371/journal.pgen.1003867
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003867Summary
Alternative cleavage and polyadenylation influence the coding and regulatory potential of mRNAs and where transcription termination occurs. Although widespread, few regulators of this process are known. The Arabidopsis thaliana protein FPA is a rare example of a trans-acting regulator of poly(A) site choice. Analysing fpa mutants therefore provides an opportunity to reveal generic consequences of disrupting this process. We used direct RNA sequencing to quantify shifts in RNA 3′ formation in fpa mutants. Here we show that specific chimeric RNAs formed between the exons of otherwise separate genes are a striking consequence of loss of FPA function. We define intergenic read-through transcripts resulting from defective RNA 3′ end formation in fpa mutants and detail cryptic splicing and antisense transcription associated with these read-through RNAs. We identify alternative polyadenylation within introns that is sensitive to FPA and show FPA-dependent shifts in IBM1 poly(A) site selection that differ from those recently defined in mutants defective in intragenic heterochromatin and DNA methylation. Finally, we show that defective termination at specific loci in fpa mutants is shared with dicer-like 1 (dcl1) or dcl4 mutants, leading us to develop alternative explanations for some silencing roles of these proteins. We relate our findings to the impact that altered patterns of 3′ end formation can have on gene and genome organisation.
Introduction
Eukaryotic mRNA 3′ ends are defined by a protein complex that cleaves pre-mRNA in close association with RNA polymerase II (Pol II) and adds a poly(A) tail to the free 3′ end [1], [2]. This event is closely associated with transcription termination, since cleavage exposes the 5′ end of the nascent RNA to a 5′–3′ exonuclease that degrades the RNA up to the exit channel of Pol II, hence contributing to termination [3]. However, termination is the least understood aspect of the transcription cycle [3] and at a sub-set of mammalian genes, cleavage and polyadenylation occur post-transcriptionally because rapid cleavage of nascent RNA at co-transcriptional cleavage (CoTC) sites downstream of the poly(A) signal promotes termination and the release of pre-mRNA from the chromatin template [4].
The selection of alternative cleavage and polyadenylation sites defines different 3′ ends within pre-mRNAs transcribed from a single gene and can therefore affect function by determining coding potential and the inclusion of regulatory sequence elements. Termination efficiency can also affect transcript levels, possibly because termination facilitates recycling of transcription complexes [5]–[7]. Therefore, the processes of cleavage, polyadenylation and termination are important stages at which gene expression can be regulated. However, the widespread nature of this control has only become apparent relatively recently [1], [2].
A less well-studied phenomenon, which suggests an additional role for regulated 3′ end formation and transcription termination, is the existence of chimeric transcripts formed between exons of neighbouring genes encoded on the same chromosomal strand [8]–[10]. Specific chimeric RNAs are conserved in vertebrates [10], regulated under certain conditions [11] and occur recurrently in cancerous tissues [12], [13]. However, regulatory processes controlling chimeric RNA formation are poorly understood.
The spen family protein FPA is a trans-acting regulator of RNA 3′ end formation, but is not a conserved component of either the splicing or the cleavage and polyadenylation apparatus [14]. First identified as a regulator of flower development, FPA enables flowering by ultimately limiting the expression of the floral repressor FLC [15]. Intriguingly, several viable A. thaliana mutants disrupted in factors that mediate RNA 3′ end formation are late flowering as a result of the specific misregulation of FLC [16]–[18]. The mechanism by which FPA controls FLC is unknown, but increased FLC transcription in fpa mutants is accompanied by increased levels of alternatively processed non-coding antisense RNAs (asRNAs) at the FLC locus [14]. The biological role of FPA is not restricted to flowering, since FPA also influences other aspects of development [19], [20]. In addition, fpa mutants were isolated in a screen designed to identify factors required for RNA silencing [21]. However, the involvement of FPA in endogenous RNA silencing pathways is currently unclear because the apparent misregulation of an endogenous RNA silencing target (the SINE retroelement AtSN1) in fpa mutants can be explained by read-through resulting from defective 3′ end formation at an upstream gene [14].
There is intense interest in determining how the widespread alternative patterns of RNA 3′ end formation can be regulated and what the consequences of disrupting specific regulators may be. We recently defined genome-wide patterns of cleavage and polyadenylation in A. thaliana using direct RNA sequencing (DRS), thereby refining our understanding of 3′ end formation in this model organism [22]. DRS can define the site of RNA cleavage and polyadenylation with an accuracy of ±2 nt in the absence of errors induced by reverse transcriptase, internal priming, ligation or amplification [22], [23]. In this study, we set out to answer two questions by quantifying shifts in RNA 3′ end formation between wild-type (WT) and fpa mutants with DRS: (1) could we clarify the roles of FPA in plant biology (particularly in relation to flowering and RNA silencing)?; and (2) could we define generic consequences of defective RNA 3′ end regulation that would be of broad relevance? Here we identify the abundance and sites of 3′ end formation of RNAs transcribed antisense to the floral regulator FLC, but do not detect evidence of a widespread role for FPA in RNA-mediated chromatin silencing. We identify the generic consequences of disrupting regulated RNA 3′ end formation, prominent among which is the formation of specific chimeric RNAs between exons of otherwise separate and well-characterised genes. In addition, we make the unexpected but related discovery that transcription termination defects in fpa are shared at some of the same loci in both dcl1 and dcl4 mutants. Consequently, we suggest an alternative explanation involving defective upstream termination for the previously reported DCL1-mediated silencing of overlapping gene pairs [24].
Results
Loss of FPA function alters patterns of gene expression
We subjected total RNA purified from three biological replicates of WT A. thaliana [Columbia-0 (Col-0) accession] and fpa-7 loss-of-function mutants to DRS. RNA was prepared from 14-day-old whole seedlings. A total of 22,560,508 WT and 24,383,585 fpa-7 reads that map polyadenylated RNA 3′ ends were aligned uniquely to the most recent A. thaliana genome release (currently TAIR10). A summary of the read statistics is given in Table S1. In each genotype, the vast majority of reads mapped to 3′ untranslated regions (UTRs) of protein-coding genes (Figure 1A–B). The DRS data can be visualised using aligned reads available at www.compbio.dundee.ac.uk/polyADB/.
Fig. 1. Distribution of DRS reads. 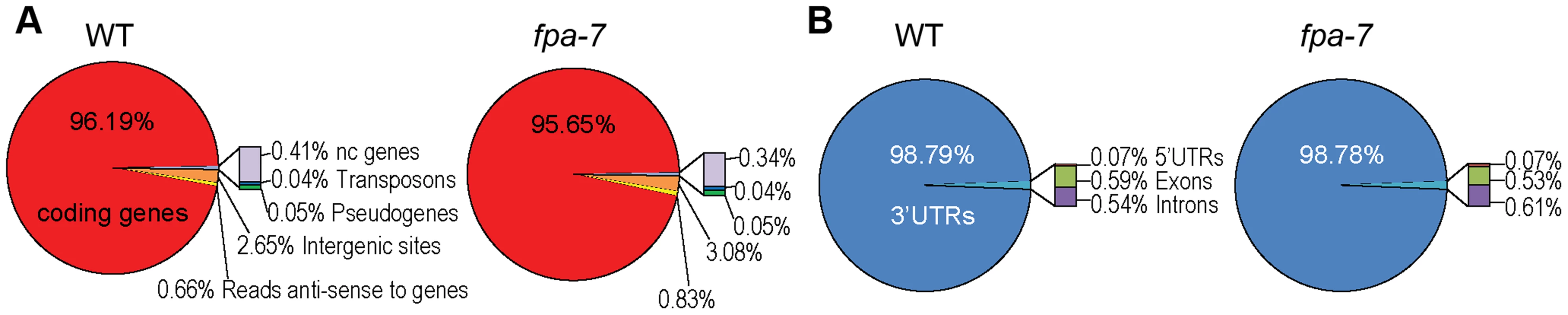
(A) Genome-wide distribution after re-annotation in wild-type (WT) and fpa-7. (B) Distribution of DRS reads mapping to protein-coding genes after re-annotation. We first asked whether DRS could reveal changed patterns of gene expression between genotypes by measuring the difference in read counts mapped to annotated protein-coding genes. We used DESeq [25] to detect differential gene expression between the WT and fpa mutant DRS datasets. The expression of 18,406 protein-coding genes was detected in WT A. thaliana; DESeq analysis suggested that 1,114 genes were differentially expressed in the fpa-7 mutant (Table S2), with the vast majority being up - or down-regulated by less than two-fold (Figure 2A). Since fpa mutants flower late, a number of gene expression changes in fpa are either predictable or already established. For example (and consistent with our expectations), down-regulation of the floral pathway integrator SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1; fold change = 0.13, P = 10e−96) and up-regulation of the floral repressor FLC (fold change = 27, P = 3.10e−139) were readily detected (Figures 2B–C and S1A).
Fig. 2. Differentially expressed genes between wild-type and fpa-7. 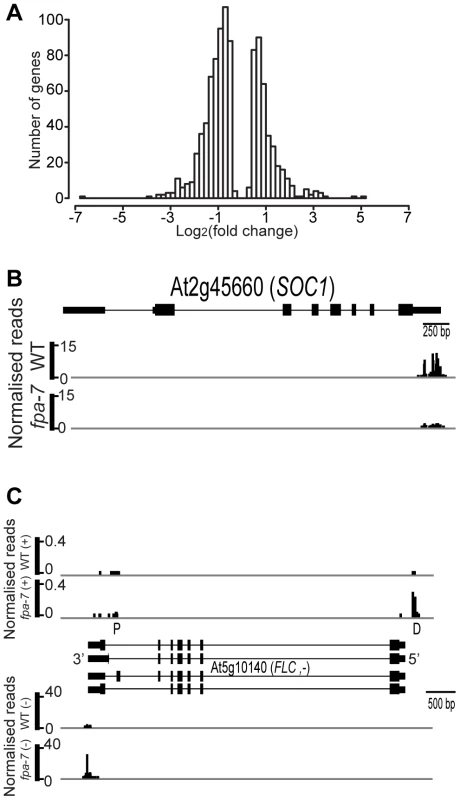
(A) Histogram of log2 fold change profiles for protein-coding genes differentially expressed (DE) between wild-type (WT) and fpa-7. (B) Reads mapping to the locus encoding SOC1. The SOC1 gene is orientated 5′–3′. (C) Reads mapping to the locus encoding FLC. Normalised reads are presented for WT and fpa. The top panel displays the reads corresponding to the FLC asRNAs and corresponds to the (+) strand while the bottom panel displays the reads corresponding to FLC mRNA and corresponds to the (−) strand. The FLC gene is orientated 3′–5′, while the reads corresponding to FLC asRNAs are orientated 5′–3′. Proximally polyadenylated ‘P’ FLC asRNAs were detected in fpa-7 and WT by the low number of reads at surrounding sites, while the distal ‘D’ cleavage sites were clearly defined. Exons are denoted by rectangles, UTRs by adjoining narrower rectangles and introns by lines. Images of normalised read alignments were made using the Integrated Genome Browser [55] and correspond to combined reads from the three sequenced biological replicates for each genotype. fpa mutants contain increased numbers of asRNA transcripts cleaved antisense to the FLC promoter
We previously reported that increased read-through of asRNA transcripts through the FLC locus in fpa mutants correlates with increased sense strand transcription [14]; this seemingly counterintuitive finding was confirmed here by DRS (Figures 2C and S1A). DRS identified the preferred sites of asRNA cleavage and polyadenylation (Figure 2C), indicated that asRNA expression is approximately 100-fold lower than FLC sense strand expression (Figure 2C) and showed that, in fpa mutants, increased levels of sense strand FLC expression are associated with increased levels of asRNAs cleaved and polyadenylated antisense to the FLC promoter (Figures 2C and S1A). In contrast to another report [18], DRS data did not indicate reduced levels of cleavage and polyadenylation at proximal sites in the asRNAs in fpa mutants, although this interpretation would benefit from a greater sequencing depth (Figure 2C). A single nucleotide polymorphism (SNP) associated with variation in flowering time and asRNA expression level [26] maps to the distal poly(A) signal of preferred cleavage sites antisense to the FLC promoter (Figure S1B). These DRS data clarify multiple 3′ end processing events at the FLC locus for the first time and are therefore valuable for understanding how the recurrent identification of 3′ end processing factors might affect FLC expression [14], [16]–[18].
FPA does not play a widespread role in RNA-mediated chromatin silencing
Although FPA has been reported to play a widespread role in RNA-mediated chromatin silencing [21], we found statistically significant increases in fpa-7 DRS counts at only 28 of the 31,189 transposons and 3,903 transposable element genes annotated in TAIR10 (Table S3). Since we had previously found that the apparent involvement of FPA in silencing the SINE retroelement AtSN1 could be explained by read-through resulting from defective termination at an upstream Pol II gene [14], [21], we asked whether reads mapping to annotated transposons reflect a genuine loss of silencing or whether they could also be explained by read-through. We found, for example, that DRS reads mapping to an apparently up-regulated transposable element gene (At5g10670) did indeed result from read-through from the upstream protein-coding gene At5g10690 (Figure 3A–C). Therefore, at least some of the relatively small number of reads mapping to transposons in this study may also be explained by read-through events. Clearly, not all misregulated transposons will be polyadenylated and so they will not be detected here, but the results of this genome-wide analysis are inconsistent with the suggestion that FPA plays a widespread role in RNA-mediated chromatin silencing. This conclusion is supported by a recent DNA methylation analysis of A. thaliana silencing mutants that included fpa-7 and found no evidence of FPA affecting RNA-dependent DNA methylation (RdDM) target sites [27].
Fig. 3. Differentially expressed transposons between wild-type and fpa-7. 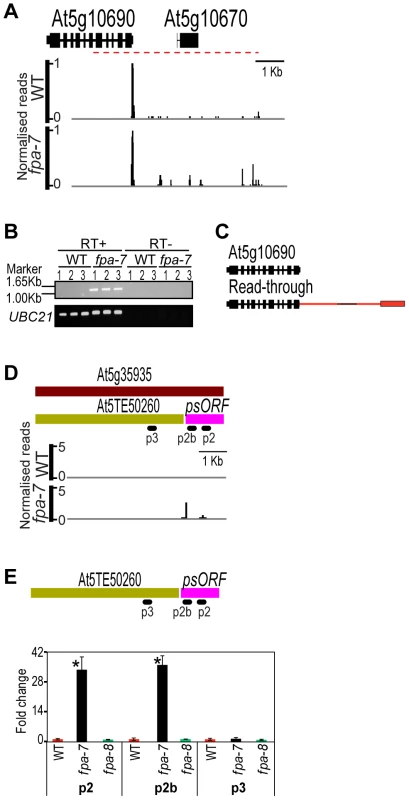
(A) Differential expression of the transposable element gene (At5g10670) in fpa-7. (B) Read-through contiguous RNAs were validated by RT-PCR (red dashed line). Three biological replicates (1, 2 and 3) were used for each genotype: wild-type (WT) and fpa-7. UBIQUITIN LIGASE 21 (UBC21) was used as a control. RT-PCR products were separated on agarose gels and stained with ethidium bromide. (C) Transcripts are either cleaved and polyadenylated in the annotated 3′UTR or at the intergenic sites, as determined by sequencing the cloned RT-PCR products. Red rectangles represent the 3′UTR specific to the read-through transcript and red lines represent 3′UTR introns. (D) Differential expression of the transposable element gene (At5g35935) in fpa-7. Recent re-annotation of At5g35935 [12], [13], [28], [29] defines two transcription units within it: the recently arisen pseudogene psORF and the transposon At5TE50260. DRS data reveal that silencing of psORF is lost in fpa-7. (E) RT-qPCR analysis of psORF in fpa-7 and fpa-8 mutant alleles. Silencing of psORF (p2 and p2b) is lost in fpa-7 but not in fpa-8. Data are the means ± SEM obtained for three independent PCR amplifications of three biological replicates. The y-axis shows the fold change relative to WT (WT set to 1) after normalisation to UBC21 gene expression. Location of the RT-qPCR amplicon is displayed on the left panel. *, P<0.05; Student's t-test. Normalised reads mapping to the different loci are presented for WT and fpa. Genes are orientated 5′–3′; exons are denoted by rectangles, UTRs by adjoining narrower rectangles and introns by lines. Images of normalised read alignments were made using the Integrated Genome Browser [55] and correspond to combined reads from the three sequenced biological replicates for each genotype. The number of DRS reads mapping to the transposable element At5g35935 was significantly different between WT and fpa-7 (Figure 3D; fold change = 33, P = 2.63e−32). Recent re-annotation of At5g35935 defined a newly arisen pseudogene psORF (pseudogene small open reading frame) within this sequence [28], [29], and it is the increased expression in fpa-7 of psORF, rather than the transposon, that is detected by DRS (Figure 3D–E). According to recently published DNA methylation data [27], psORF is demethylated in fpa-7 mutants (Figure S2A), and we found no evidence that read-through from an upstream gene could account for the increased number of DRS reads detected here in fpa-7. These findings therefore raised the possibility that FPA functions to silence this newly arisen pseudogene. However, we did not detect misregulation of psORF in a second allele, fpa-8 (Figure 3E). It has recently been shown that de novo originated A. thaliana genes might be prone to epigenetic variation in the early stages of their formation [30]. Accordingly, apparent changes in the DNA methylation or expression of such sequences may be a coincidence of the different genetic backgrounds analysed [28], [29]. Such epigenetic variation might also explain why misregulation of antisense RNAs at the newly acquired helitron transposable element At1TE93275 [28], previously reported in fpa mutants [31], was not detected in this fpa-7 dataset (Figure S2B). Consequently, particularly careful analysis is required for the identification of authentic factors mediating the silencing of such newly originated sequences.
FPA affects intronic cleavage site selection and intergenic read-through
Besides refining our understanding of previously proposed roles for FPA in flowering and RNA silencing, we sought to identify the generic consequences of disrupting a regulator of RNA 3′ end formation. For example, one might predict that 3′ end formation within intronic sites and conventional 3′UTRs would be altered in fpa mutants. Cleavage and polyadenylation within intronic sequences outside the 3′UTR can have profound consequences on gene function by truncating mRNA coding potential. FPA effects autoregulation in this way by mediating selection of the promoter proximal intronic cleavage site within its own pre-mRNA [14]. We therefore asked whether FPA controls alternative polyadenylation at other intronic sites. We applied our data-smoothing and peak-finding algorithms to define cleavage sites [22] and estimated differential usage of these sites using DESeq. The validity of this approach was supported by the finding that previously identified intronic alternative polyadenylation events within FPA (Figure 4A; P = 7.10e−11), but not FCA were dependent on FPA function [14], [22]. Unexpectedly, additional intronic cleavage sites were also detected in FPA RNA itself, but only in the transfer (T)-DNA-induced fpa-7 allele (Figure 4A) and not in ethyl methanesulfonate (EMS)-induced alleles such as fpa-8 [21], suggesting that T-DNA insertions can trigger cryptic cleavage and polyadenylation (Figure S3A–F). These allele-dependent distinctions in patterns of FPA polyadenylation were indicated by previous RNA gel blot hybridisations [14]. Reduced selection of intronic cleavage sites in 13 genes and increased selection of intronic cleavage sites in another 25 genes were found in fpa-7 (Table S4), indicating that FPA ultimately promotes cleavage at some sites, but represses it at others.
Fig. 4. FPA affects intronic cleavage site selection and intergenic read-through. 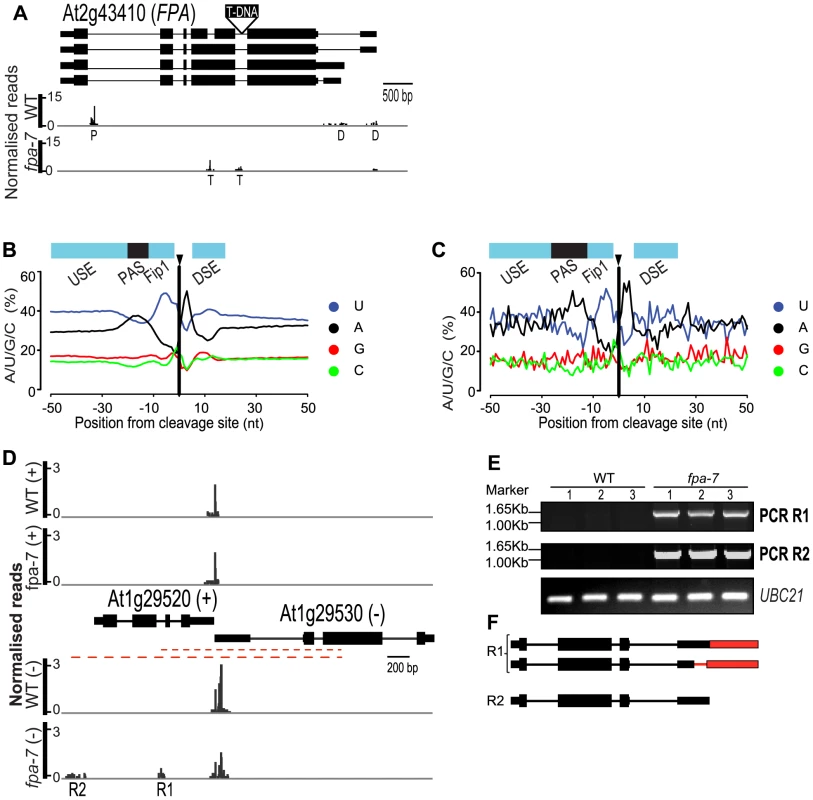
(A) Reads mapping to the locus encoding FPA. Promoter proximal ‘P’ and distal ‘D’ alternative poly(A) sites are indicated, as are the poly(A) sites ‘T’ resulting from the T-DNA insertion in fpa-7. (B, C) Nucleotide composition profiles around cleavage sites within annotated genes (B) and at intergenic sites (C) display alternating A- and U-rich sequences. USE, upstream sequence element; PAS, poly(A) signal; Fip1, the U-rich sequence upstream of the cleavage site is the proposed binding site of FIP1 [58]; DSE, downstream sequence element. (D–F) Example of intergenic DRS reads mapping antisense to a coding gene. Normalised reads mapping to the At1g29520–At1g29530 loci are displayed in (D). The upper panel shows reads mapping to the (+) strand 3′ end of At1g29520, while the lower panel shows reads mapping to the (−) strand 3′ end of At1g29530. (E) R1 and R2 contiguous RNAs were validated by RT-PCR (red dashed lines) with poly(A)+ RNA. RT-PCR products were separated on agarose gels and stained with ethidium bromide. Three biological replicates (1, 2 and 3) were used for each genotype: wild-type (WT) and fpa-7. (F) Transcripts are either cleaved and polyadenylated in the annotated 3′UTR or at the intergenic sites, as determined by sequencing the cloned RT-PCR products. Red narrower rectangles represent regions specific to the read-through transcript and red lines the 3′UTR introns. Images of normalised read alignments were made using the Integrated Genome Browser [55] and correspond to combined reads from the three sequenced biological replicates for each genotype. In order to validate this data analysis, we investigated some of the deduced intronic alternative polyadenylation changes in more detail (Figure S4). For example, we detected shifts in alternative polyadenylation at IBM1 (Increase in BONSAI Methylation 1), a gene encoding a histone demethylase specific for H3K9 dimethylation and monomethylation [32]. Expression of the active enzyme was recently shown to be controlled by alternative polyadenylation, which in turn is dependent on DNA methylation at this locus (Figure S4A) [33] since 3′ end formation occurs exclusively at proximal poly(A) sites in mutants that disrupt CG and CHG DNA methylation [33]. In contrast, our DRS data suggest that cleavage occurs almost exclusively at the IBM1 distal site in fpa mutants (Figure S4B; P = 0.03); this was confirmed by RNA gel blot hybridisation (Figure S4C). FPA and DNA methylation mediated by METHYLTRANSFERASE 1 (MET1) and the plant-specific chromomethylase CMT3 therefore appear to have opposing effects on poly(A) site choice in IBM1 pre-mRNA transcribed through intragenic heterochromatin.
We previously showed that FPA affects 3′ end formation not only within introns but also at conventional 3′UTRs, thus causing intergenic read-through of Pol II transcripts [14]. This discovery was recently extended by a tiling array analysis of fpa fca double mutants [31]. Consistent with these previous studies, our DRS analysis mapped reads to intergenic sequences (defined here as regions between protein-coding genes) in fpa mutants. DESeq analysis identified 61 up-regulated intergenic regions downstream of down-regulated genes and 109 up-regulated intergenic regions downstream of genes with unchanged expression in fpa-7 (Table S5). The amount of polyadenylated read-through RNAs relative to upstream annotated genes varied (Figure S5A). We validated potential intergenic read-through events in detail (Figures S5B–Q and S6) and identified three different types of events: first, extended 3′UTRs (Figure S5F–N); second, cryptic splicing event(s) generating altered 3′UTR sequences (Figure S6); and third, read-through accompanied by cryptic splicing that alter the protein-coding sequence of the upstream gene, revealing that intergenic read-through is not necessarily benign (Figure S5O–Q).
DRS extends previous studies by identifying the cleavage and polyadenylation sites of read-through RNAs. We analysed the sequence features of intergenic cleavage sites and, although the relatively small size of the fpa read-through dataset makes the nucleotide profile surrounding intergenic cleavage sites appear somewhat noisy, an alternating pattern of A - and U-rich regions flanking the cleavage site and a prominent A-rich region around −20 was detected (Figure 4B–C). We previously showed that the distinguishing feature of preferred and non-preferred cleavage sites within the same 3′UTR is the relative prominence of the A-rich peak located approximately 20 nt upstream of the cleavage site [22] (Figure 4B). Because the corresponding A-rich peak is prominent here, we suggest that 3′ end formation of read-through RNAs in fpa mutants takes place at strong poly(A) signals located within intergenic regions.
We identified 14 read-through RNAs in fpa mutants that result in transcription of novel RNAs antisense to expressed protein-coding genes (Table S6), but in no case was this associated with down-regulation of sense strand mRNA expression. For example, although we could validate read-through from At1g29530 that generated new antisense RNA against the sense strand-encoded At1g29520, there was no change in the sense strand expression of At1g29520 (Figure 4D–F). Since increased expression of RNA cleaved and polyadenylated antisense to the FLC promoter correlates with increased sense strand FLC mRNA expression, we next asked whether other genes differentially expressed in fpa-7 are associated with increased detectable cleavage and polyadenylation of RNA antisense to their promoters. This analysis (Table S7) identified four cases (including FLC) in which sense strand gene expression was increased and 13 cases in which it was decreased when increased DRS reads mapping antisense to promoters in fpa-7 were detected. Overall, we conclude that novel asRNAs generated in fpa mutants are not necessarily associated with gene silencing. However, we also identify loci where detailed experimental analysis is required to uncover potentially distinct regulatory consequences of novel asRNAs.
FPA affects chimeric RNA formation
Among those RNAs affected by FPA, we identified cases in which tandem genes showed reciprocal changes in read abundance. For example, in the fpa-7 mutant we found a reduction in the number of reads aligning to the 3′ end of At3g59060 (PHYTOCHROME INTERACTING FACTOR 5, PIF5) compared to WT, but the number of reads mapping to the 3′ end of the downstream gene At3g59050 (POLYAMINE OXIDASE 3, PA03) was increased (Figure 5A). We asked whether these read differences could be explained by defective 3′ end formation at PIF5 in fpa mutants, leading to chimeric RNAs being cleaved and polyadenylated within the 3′UTR of PA03. Consistent with this idea, RT-PCR analysis using primers anchored in PIF5 and PA03 (and thus spanning the intergenic region) detected the formation of chimeric RNAs specifically in fpa mutants (Figure 5B–C). RNA gel blot analysis confirmed this, with a probe to the 5′ end of PIF5 revealing 60% read-through into RNAs of increased size relative to PIF5 (Figure 5D). Three major hybridising signals specific to fpa mutants were detected using probes spanning the intergenic region, the PA03 3′UTR and the different exons and UTRs of PIF5 (Figure 5D–E). A combination of RT-PCR, 5′ rapid amplification of cDNA ends (RACE), RNA gel blot and cloning approaches identified two of these (α and β) as chimeric RNAs that differ as a result of a cryptic splicing event (Figure 5E–G), while the third comprised two similar sized RNAs with 5′ ends mapping to either the 3′UTR of PIF5 (γ) or the intergenic sequence (γ′) (Figure 5E–G). The differential sensitivities of these RNAs to tobacco acid pyrophosphatase (TAP) suggest that γ is capped, but γ′ is not (Figure 5F). None of the chimeric RNAs altered the deduced PIF5 open reading frame, but they did effectively extend the 3′UTR from 211 nt to 2,720 nt in α and to 1,680 nt in β chimeric transcripts. In contrast, native PA03 expression is undetectable in fpa mutants (Figure 5D). The 5′ end of γ RNAs aligned to the PIF5 3′UTR (Figure 5F,G) but, as judged by 5′RACE, was distinct from all of the 3′ ends that mapped to the PIF5 3′UTR (Figure 5G). This suggests that γ RNAs did not result from cleavage of chimeric RNAs followed by capping. Instead, we found differences between WT and fpa mutants in H3K4me3, a chromatin modification associated with transcription start sites [34], across PIF5 and PA03, with a decrease in H3K4me3 at the 5′ end of PA03 and an additional H3K4me3 peak detected at the 3′ end of PIF5 in fpa-8 (Figure 6A). These data are consistent with a shift in the PA03 transcription start site accompanying the chimeric RNAs detected here in fpa mutants.
Fig. 5. PIF5–PA03, an example of chimeric RNA formation controlled by FPA. 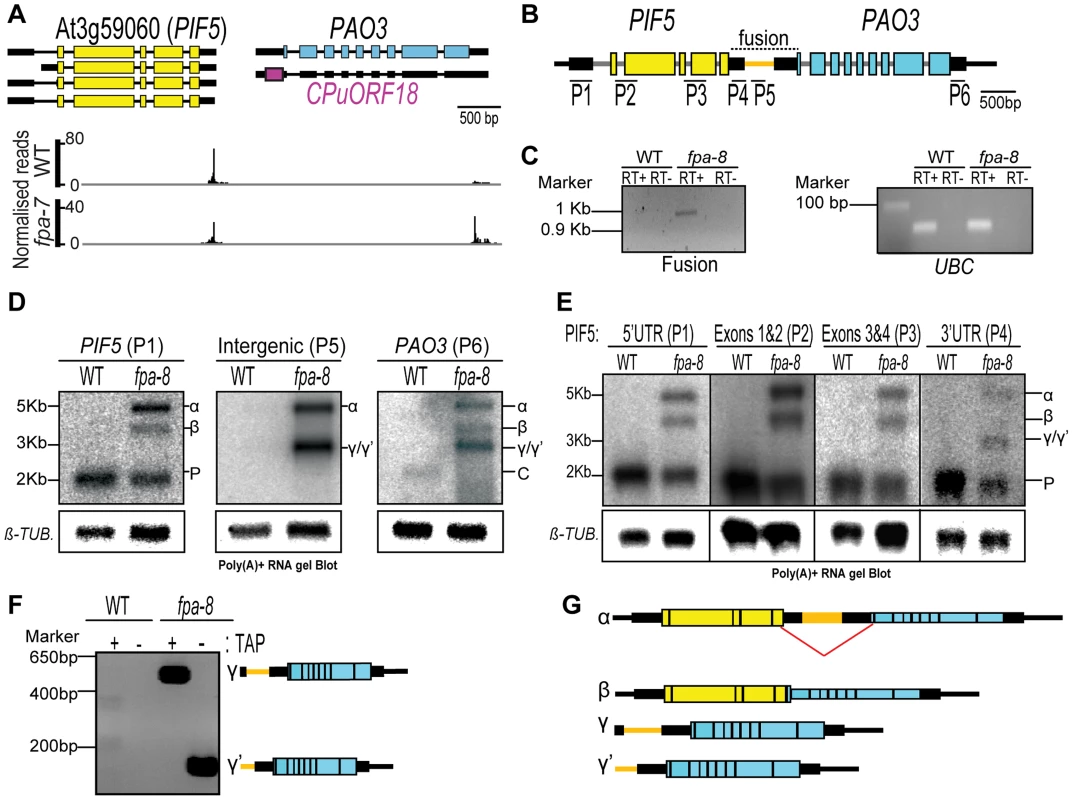
(A) Normalised reads mapping to the locus encoding PIF5–PA03. Exons are denoted by coloured rectangles, UTRs by adjoining narrower rectangles and introns by lines. The image of normalised read alignments was made using the Integrated Genome Browser [55] and corresponds to combined reads from the three sequenced biological replicates for each genotype. (B) Location of RNA gel blot probes are indicated by numbers (P1–P6) and the tested fusion region by a dotted line. (C) RT-PCR analysis of a contiguous RNA between PIF5 and PA03 detected in fpa-8. UBIQUITIN LIGASE 21 (UBC) was used as a control. (D,E) RNA gel blot analysis of PIF5–PA03 chimeric RNAs in wild-type (WT) and fpa-8. P, PIF5; C, PA03 transcripts. β-TUBULIN (β-TUB.) was used as an internal control. Probes used are shown in (B). (F) 5′RACE analysis of the γ and γ′ RNAs with or without tobacco acid pyrophosphatase (TAP) treatment. PCR products were separated on an agarose gel and stained with ethidium bromide. (G) Schematic representation of the different RNAs expressed at the PIF5 locus in fpa mutants. The splicing event occurring in the β chimeric RNA is shown by a red line. Fig. 6. PIF5–PA03, an example of chimeric RNA formation controlled by FPA. 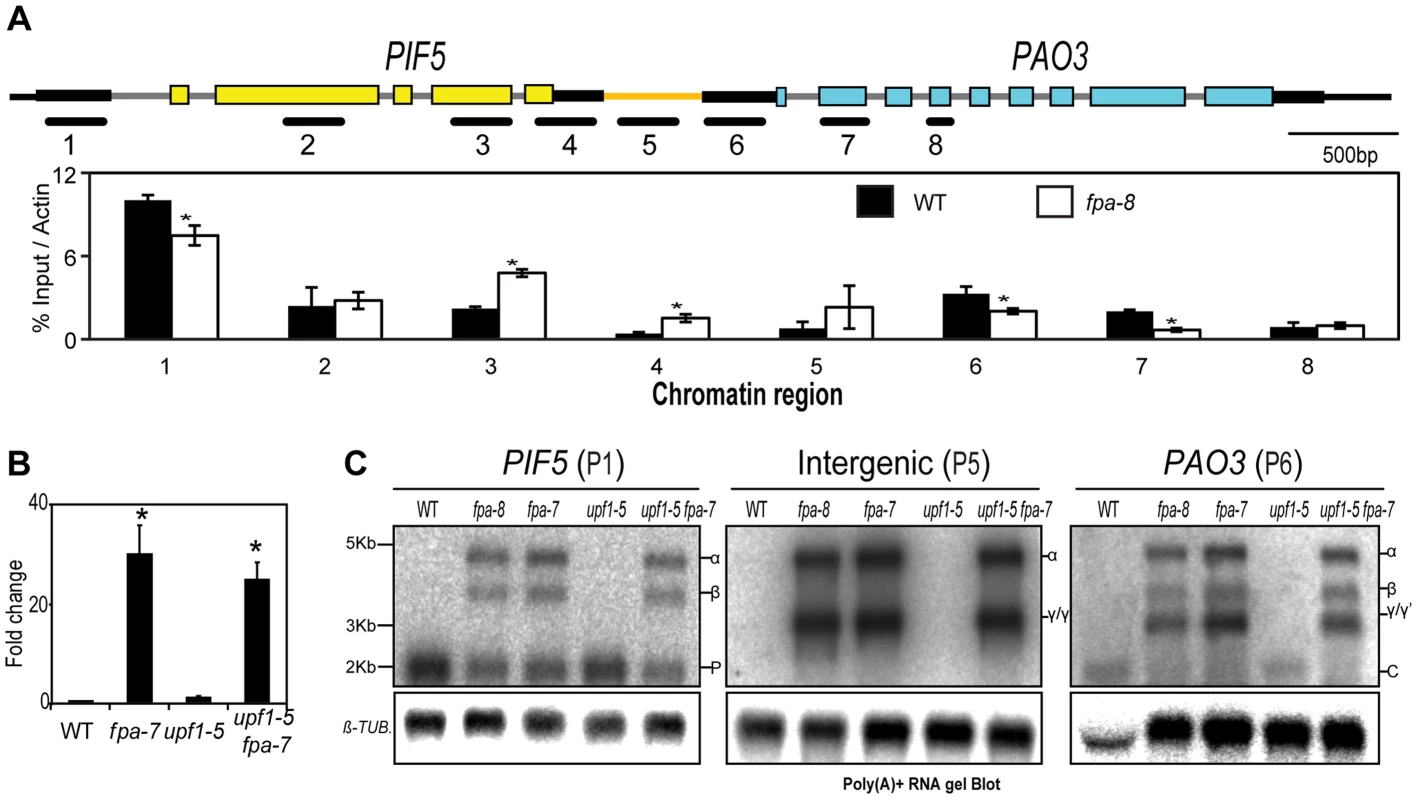
(A) H3K4me3 Chromatin ImmunoPrecipitation (ChIP) analysis of genomic regions at the PIF5 locus. γ RNAs result from a shift in the transcription start site. Black lines depict the chromatin regions analysed by qPCR. Histograms show means ± SEM obtained for enrichment calculated by percentage input normalised against actin for three PCR amplifications. *, P<0.05; Student's t-test. (B) RT-qPCR analysis of PIF5–PA03 chimeric RNAs in fpa and upf1 mutants. Data are the means ± SEM obtained for three independent PCR amplifications of three biological replicates. The y-axis shows the fold change relative to wild-type (WT; (WT set to 1) after normalisation to UBC21 gene expression. Location of the RT-qPCR amplicon is displayed in Figure S7D. *, P<0.05; Student's t-test. (C) RNA gel blot analysis of PIF5–PA03 chimeric RNAs in fpa and upf1 mutants. In addition to being detected in single fpa-7 and fpa-8 mutant alleles, chimeric PIF5–PA03 RNAs were detected in an early-flowering flc-3 fpa-7 double mutant but were generally not found in other late-flowering mutants (Figure S7A–C), revealing that they result from a specific lack of FPA and not indirectly from late flowering. We did detect low levels of chimeric RNAs in late-flowering pcfs4 mutants (Figure S7C) in which a protein related to the core cleavage, polyadenylation and termination factor Pcf11 is disrupted [17]. We investigated whether FPA may mediate this effect indirectly by determining whether splicing of PIF5 pre-mRNA is perturbed, since splicing is intimately connected to RNA 3′ end formation [1]. However, we found no evidence for changes in either the fidelity or efficiency of PIF5 pre-mRNA splicing in fpa mutants (Figure S7D–F). We also investigated whether FPA affects the turnover of chimeric RNAs, since they comprise long 3′UTRs with multiple introns downstream of in-frame stop codons that might normally be degraded by nonsense-mediated RNA decay (NMD) [35]. However, we found no evidence of stabilised read-through RNAs in mutant backgrounds defective in the NMD factor UPF1 [36] (Figure 6B–C), and indeed found that chimeric RNAs appear to escape NMD (Figure 6C). PIF5 is one of a family of critical growth regulators in A. thaliana and is closely related to PIF4, with which it shares some functions [37]. PIF4 and PIF5 may have arisen from a duplication event since both have related downstream polyamine oxidase loci (PIF4-PA02 and PIF5-PA03) that have conserved peptide open reading frames in their 5′UTRs (CPuORF17 and CPuORF18, respectively). Despite these similarities, chimeric RNAs were found at PIF5, but not PIF4 (Figure S7G) in fpa mutants, and not dcl1 mutants (Figure S7H) underscoring the specificity of FPA-mediated effects on chimeric RNA formation.
Chimeric RNA formation was detected at four other tandem gene pairs (Figures S8–S11) following the development of an algorithm based on reciprocal DRS read abundance at tandem protein-coding genes (Tables S8,S9). In each case, the resulting chimeric RNA encodes the open reading frame for the same upstream gene combined with a different effective 3′UTR (Figure S8C). Remarkably, one chimeric RNA isoform appears to result from a splicing event that excised almost the entire coding sequence of the downstream At1g02470 gene from the chimeric transcript. In all cases, while there was evidence of chimeric RNA in WT, the level of chimeric RNAs was increased in fpa mutants. Although our algorithm probably underestimates the number of chimeric RNAs formed in fpa mutants because it only considers neighbouring protein-coding genes, it is clear that FPA affects specific chimeric RNA formation at a limited number of sites in the genome.
Defective termination in fpa mutants can occur at the same loci in dcl mutants
Read-through was detected at the 3′ end of the flowering regulator FCA (At4g16280) in fpa mutants. Failsafe termination at this site was recently shown to depend upon DCL4 [38]. FCA read-through RNAs similar to those previously found in dc14 mutants were detected here in fpa mutants (Figure 7A–C), revealing that, even in the presence of DCL4, failsafe termination fails in the absence of FPA. The expression of DCL4 itself is unaffected by FPA (Table S2).
Fig. 7. Characterisation of intergenic read-through RNAs in fpa and dc11 mutants. 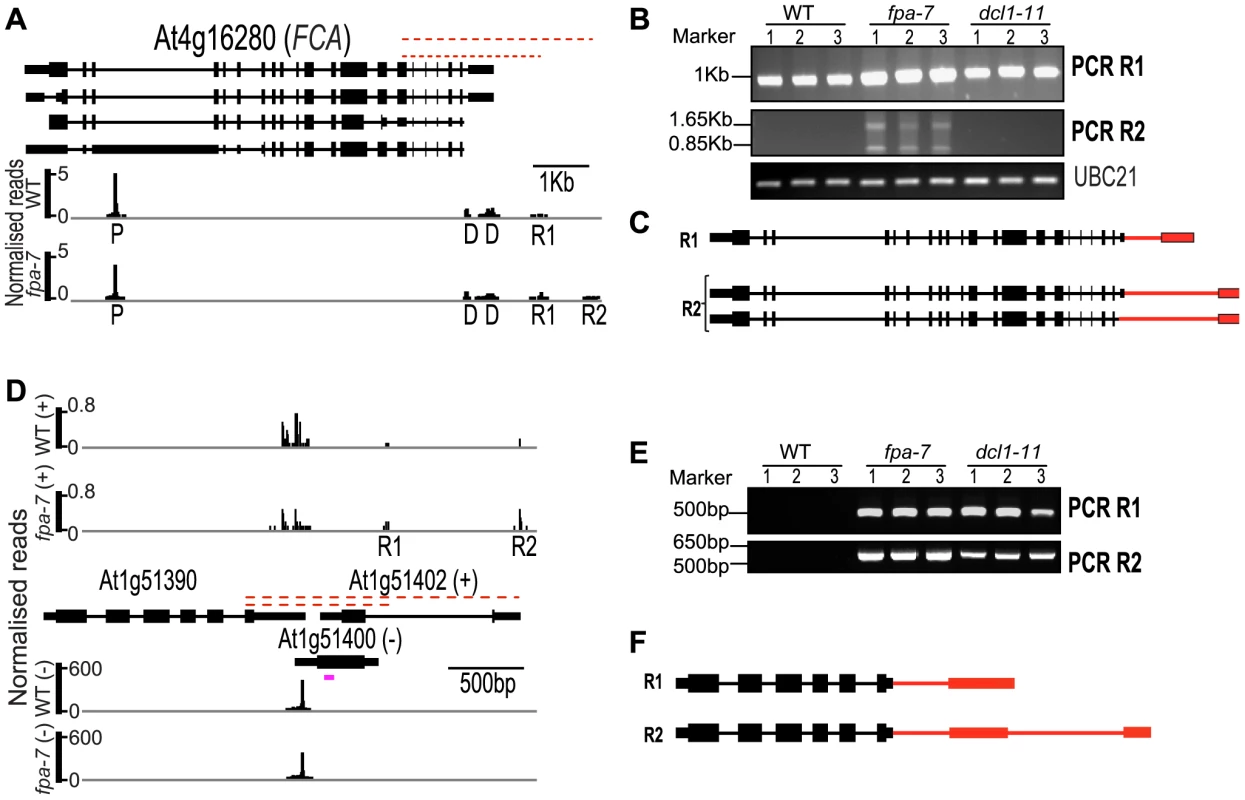
(A–C) Characterisation of FCA intergenic read-through RNAs. (A) Normalised reads mapping to the locus encoding FCA. (B) Identification of R1 and R2 contiguous RNAs. Three biological replicates (1, 2 and 3) were used for each genotype: wild-type (WT), fpa-7 and dc11–11. (C) Description of the R1 and R2 read-through transcripts. (D–F) Characterisation of At1g51390 read-through RNAs. (D) Normalised reads mapping to At1g51390–At1g51402–At1g51400. (E) Identification of R1 and R2 contiguous RNAs. Three biological replicates (1, 2 and 3) were used for each genotype: WT, fpa-7 and dc11–11. (F) Description of the R1 and R2 read-through transcripts. The red dashed lines indicate the regions amplified by RT-PCR on reverse-transcribed poly(A)+ RNAs. Red narrower rectangles represent 3′UTR parts specific to the read-through transcript and red lines the 3′UTR introns. RT-PCR products were separated on agarose gels and stained with ethidium bromide. The purple line indicates the location and strand detected by the probe, which was used previously [24]. The image of normalised read alignments was made using the Integrated Genome Browser [55] and corresponds to combined reads from the three sequenced biological replicates for each genotype. Exons are denoted by coloured rectangles, UTRs by adjoining narrower rectangles and introns by lines. We next asked whether this connection between FPA and DCL proteins extends to other loci. DRS indicated that fpa read-through RNAs are found at genomic regions where DCL-dependent small RNAs, described as natural antisense transcript siRNAs (nat-siRNAs), are also found [24]. We first validated the existence of these read-through RNAs in fpa mutants, using RT-PCR to reveal read-through at the 3′ end of At1g51390, for example (Figure 7D). A DCL1-dependent nat-siRNA1g51400 has recently been mapped to this genomic region [24]. Using RT-PCR analysis of poly(A)+ RNA, we identified similar read-through RNAs in dc11–11 downstream of At1g51390 (Figure 7D–F). This finding is consistent with DCL1 ultimately controlling transcription termination. Expression of the annotated downstream gene At1g51402 has previously been reported to increase in dc11 mutants, which has been taken as evidence of nat-siRNA-mediated gene silencing [24]. However, our findings suggest that this detectable increase in At1g51402 expression can be explained instead by read-through from the upstream gene. We also detected differences in 3′ end formation at other loci in fpa and dc11 mutants, although read-through at FCA was unaffected by DCL1 (Figure 7B). We therefore conclude that FPA and DCL proteins control termination at overlapping sets of target genes.
Discussion
The impact of alternative polyadenylation and transcription termination is underexplored, but widespread changes in poly(A) site choice reveal it to be an important level at which gene expression can be regulated. Here we used DRS to assess the consequences of disrupting regulated 3′ end formation dependent on the spen family protein FPA.
The function of FPA in flowering ultimately depends on its control of the floral repressor FLC [15]. The recurrent identification of late-flowering mutants with elevated FLC expression, in which other factors that mediate RNA cleavage and polyadenylation are also disrupted, suggests a critical role for RNA 3′ end formation in FLC regulation [16]–[18]. However, 3′ end formation of FLC mRNA itself appears to be largely unaffected by these mutations. Instead, attention has focused on a potential role for asRNA processing in FLC regulation because polyadenylated asRNA expression at the FLC locus is also changed in these mutants [14], [18], [39]. Importantly, it is unclear whether these asRNAs are the cause of, the consequence of or simply coincide with FLC regulation. For example, the positive correlation of sense and asRNA expression detected here may be a consequence of gene-looping events that juxtapose promoters and terminators that are each characterised by nucleosome-free regions [40]. The embedded nature of these asRNAs within the FLC locus makes experimental separation of these events particularly challenging. DRS sheds new light on these complex issues because it can unequivocally score the strand of origin and simultaneously define and quantify multiple sites of RNA 3′ end formation [22], [23]. DRS analysis provided little evidence of altered asRNA proximal site cleavage between WT and fpa mutants, while increased levels of asRNAs that read-through to cleavage sites antisense to the FLC promoter were clearly detected in fpa mutants. These findings are not easily reconciled with a model that suggests proximal processing of asRNA triggers FLC chromatin silencing [18]. Clearly, much remains to be explained about FLC regulation and, while the DRS data might restrict some models of interpretation, greater sequencing depth and characterisation of further RNA species are also required. For example, it is unclear whether RNAs engaged in R-loops at this locus (within a region that overlaps the proximally polyadenylated FLC asRNAs) [41] would be detected by DRS.
A second proposed function for FPA is in RNA-mediated chromatin silencing [21]. Our DRS data, together with recently reported DNA methylation data [27], show that FPA does not play a widespread role in RNA-mediated chromatin silencing at RdDM sites. This clarification is important because a perceived role in RNA silencing has influenced interpretations of how FPA might function. Our data do not explain why FPA was identified in a mutant screen for factors required for RNA silencing [21]. We could show that a novel asRNA, which may be generated by defective termination in fpa mutants, was not obligatorily associated with reduced sense strand expression, but we also identified cases in which increased cleavage and polyadenylation antisense to promoter regions in fpa-7 mutants was associated with either increased or reduced sense strand expression. Therefore, one explanation for the identification of FPA as a factor required for RNA silencing may be that defective 3′ end formation, arising either within the transgenes used for the screen or at endogenous genes close to the site of transgene insertion, affects the efficacy of the silencing trigger from the transgene itself [21]. We did identify misregulation of psORF, a recently acquired pseudogene [28], [29], in one fpa mutant allele. The silencing of such recently duplicated sequences in different backgrounds is poorly studied, but recent work suggests that epialleles may exist in either different accessions or different generations. In other words, de novo originated genes might be prone to epigenetic variation in the early stages of their formation [30]. Since we only detected misregulation of psORF in fpa-7 and not in fpa-8, and also did not detect misregulation of asRNAs at the newly acquired At1TE93275 transposable element in this fpa-7 dataset, it is unclear whether FPA is genuinely involved in the control of such newly arisen sequences.
Besides addressing specific biological roles of FPA in A. thaliana, we also asked whether this analysis could reveal generic consequences of defective RNA 3′ end formation. We detected changes in intronic cleavage sites, intergenic read-through and chimeric RNA formation, thus defining a range of events that inform future analyses of the impact of such regulators in other species. These findings also have broader implications because they suggest how 3′ end formation might affect the evolution of gene structure and gene order through regulated 3′UTR sequences, new exon combinations or de novo evolution of new genes. We (and others [31]) discovered that intergenic read-through to new poly(A) sites in fpa mutants is often associated with cryptic splicing events, consequently generating mRNAs with a diversity of potential 3′UTRs. Furthermore, in some cases read-through was associated with cryptic splicing events that disrupted upstream protein-coding exons, resulting in an impact on protein function that may be more immediately obvious. The strong intergenic poly(A) signals (activated here in fpa mutants) may derive from genuine alternative polyadenylation, unannotated genes or transposons or may instead reflect the evolution of high quality intergenic poly(A) sites to trap runaway read-through RNAs. Such read-through to intergenic poly(A) signals may contribute to the de novo evolution of new or orphan genes from non-coding genomic regions [42].
One strikingly clear shift in poly(A) site choice was detected at IBM1. Alternative polyadenylation of IBM1 pre-mRNA controls the activity of the histone demethylase encoded by this gene [33]. IBM1 poly(A) site choice is also dependent on DNA methylation: the IBM1 intronic DNA sequence downstream of the proximal poly(A) site is heavily methylated and alternative polyadenylation is profoundly altered in met1 and cmt3 mutants [33]. Relatively little is known about the impact of DNA methylation and intragenic heterochromatin on co-transcriptional pre-mRNA processing, but alternative splicing was recently shown to be affected by DNA methylation [43]. In addition, alternative polyadenylation of the imprinted H13 locus in mice involves interplay between two poly(A) sites and DNA methylation at an internal promoter that separates them [44]. How directly involved DNA methylation and FPA are in poly(A) site choice at IBM1 still needs to be established. However, the opposing alternative polyadenylation phenotypes of met1 and cmt3, on the one hand, and fpa mutants, on the other, suggest that A. thaliana IBM1 could be a genetically tractable model system to dissect the interplay between DNA methylation, intragenic heterochromatin and poly(A) site selection. It will be interesting to determine whether other FPA-dependent alternative polyadenylation events are also related to DNA methylation. It may be relevant that methyl-CpG binding domain protein 9 (MDB9) binds to regions of FLC chromatin coincident with the sites of alternative polyadenylation of FLC asRNAs and influences FLC expression and DNA methylation [45].
A striking consequence of read-through we discovered in fpa mutants was the formation of chimeric RNAs. Conceptually related chimeric RNAs have previously been discovered [8]–[10], but no specific trans-acting factor mediating their formation has been found. Therefore, one of the advances we make here is to reveal that a consequence of losing regulated 3′ end formation can be the transcription of specific chimeric RNAs. We do not yet know whether FPA-dependent chimeric RNAs are regulated in vivo or are simply the consequence of loss-of-function in fpa mutants. Regardless, chimeric RNAs have been overlooked in genome-wide studies of 3′ end formation until now [46]–[48]. However, the formation of chimeric RNAs may account for phenotypes resulting from defects in mediators of this process or be a consequence of global changes in poly(A) site choice that distinguish quiescent from proliferating cells [49], [50]. Since chimeric RNAs bring together different exon combinations, they have the potential to generate novel biological functions. Consistent with this idea, specific chimeric RNAs are conserved in different vertebrates [10]. An additional function or consequence of chimeric RNA formation that we detected here may be transient silencing of downstream genes by interference with transcription [51] or pre-mRNA processing. By discovering a trans-acting factor that affects specific chimeric RNAs, it should now also become possible to design switches that reversibly convert two genes into one in a controllable way and that may have biotechnological applications.
Although it is clear that chimeric RNAs were formed at only a limited number of sites in fpa mutants, it is likely that our algorithm identifying reciprocal changes in expression at tandem protein-coding genes underestimated the number of potential chimeric RNAs. For example, Pol II read-through could result in complex transcripts comprised of more than two genes (including non-protein-coding RNAs), which terminate at a site other than the 3′ end of an annotated protein-coding gene. Indeed, a transcript previously detected in fpa fca double mutants [31], which can also be described as a chimeric RNA, results from read-through at the 3′ end of At1g55805 into At1g55800 and is cleaved within a downstream intergenic space.
A clear molecular phenotype associated with loss-of-function fpa mutants is the stable accumulation of RNAs that are cleaved and polyadenylated in intergenic regions, revealing that FPA ultimately promotes transcription termination. Our analysis of these read-through events led us to make the unexpected discovery that FPA and DCL proteins share termination targets. DCL4, an RNase III-like protein previously established to function in processing small RNAs, was recently also shown to function in transcription termination at the FCA locus [38]. One interpretation of these data is that DCL4 mediates ‘failsafe’ termination by cleaving RNA downstream of the poly(A) site(s) at potential CoTC sites and thus facilitates access of the 5′–3′ exonuclease that disrupts the transcribing Pol II. We discovered the same read-through events at the FCA locus in fpa mutants, revealing that termination at this locus requires not only DCL4 but also FPA. Since FPA does not affect DCL4 expression, this suggests that termination at certain loci requires multiple, specific trans-acting factors in addition to the torpedo exonuclease and the cleavage and polyadenylation machinery [3], [52], [53]. We discovered that the connection between FPA, DCL proteins and termination was not limited to DCL4, since DCL1 may ultimately affect termination at some loci that are also regulated by FPA. Notably, we detected read-through downstream of genes with validated DCL1-dependent antisense nat-siRNAs [24]. DCL proteins may directly cleave nascent RNA to mediate termination, as recently suggested [38] (with small RNAs such as nat-siRNAs simply being the by-products of this cleavage), or may cleave antisense RNAs and thus generate siRNAs that guide subsequent nascent sense strand cleavage and Pol II termination by Argonaute proteins. Notably, read-through at the FCA locus in dc14 mutants also occurs antisense to an overlapping gene, At4g16270 [38]. Here we clarify the suggestion that nat-siRNAs function in trans to silence At1g51402 gene expression [24] by revealing that defective termination at the upstream gene in dc11 mutants can explain these findings. This set of discoveries raises the general question of whether some aspects of dcl mutant phenotypes are a direct consequence of defects in Pol II transcription termination.
By focusing our analysis on polyadenylated RNA 3′ ends, we set out to investigate the generic consequences of disrupting a regulator of alternative polyadenylation. We have documented examples of such alterations here, but it will now be interesting to study the mechanism by which FPA mediates this control. Crucial to this is the identification of the immediate, not simply ultimate, targets of FPA function. Furthermore, sequencing RNA from individual cells of different tissues and across circadian rhythms of expression is likely to be required for the comprehensive identification of transcripts affected by FPA.
We recently proposed that the 3′ ends of thousands of A. thaliana genes require re-annotation [22]. The more in-depth DRS data we present here reinforces this finding. Although we developed an algorithm designed to assign DRS reads to incompletely annotated gene models, this automated approach used in isolation is not meant to be definitive [22]. Ultimately, the analysis of gene expression would benefit from the development of a technology that directly sequences entire RNA molecules at depth. In the current absence of such a technique, it is likely that accurately revising A. thaliana genome annotation will require diverse datasets that include DRS and, for example, RNA-seq and chromatin modification data. The meta-analysis of data from these different technologies nevertheless requires appropriate alignment software specific for each sequencing technology. Single molecule sequencing is more susceptible to mismatches and deletions and so requires indel-aware alignment software. A recent analysis of DRS data is fundamentally flawed because it used a version of the Bowtie alignment software with parameters that are wholly inappropriate for DRS data analysis [54]. Although global analyses of pre-mRNA processing require an improved understanding and annotation of the A. thaliana transcriptome, it is likely that de novo annotation of control transcriptomes prepared in parallel, rather than improved genome annotation alone, will be required to comprehensively analyse changes in the sequences and levels of RNA molecules resulting from altered RNA processing.
Materials and Methods
Plant material and growth conditions
The T-DNA insertion line fpa-7 (SALK_021959) and the flc-3 mutant were provided by R. Amasino (Madison). The fpa-8 mutant (induced by EMS and containing a point mutation leading to a premature stop codon) and fy-2 were provided by C. Dean (John Innes Centre), and fve-3 was provided by J. Martínez-Zapater (Madrid). The WT strain Col-0 and the T-DNA insertion lines upf1–5 (SALK_112922), sr45–1 (SALK_004132) and flk-1 (SALK_007750), as well as ld-1, were obtained from NASC (UK). fld-3 and ref6–3 were provided by S. Michaels (Indiana University) and Y.S. Noh (Seoul National University), respectively. A. thaliana WT Col-0, fpa-7 and fpa-8 mutant seeds were sown in MS10 plates, stratified for 2 days at 4°C and germinated in a controlled environment at a constant temperature of 24°C under 16 h light/8 h dark conditions. Seedlings were harvested 14 days after transfer to 24°C.
RNA procedures
Samples were prepared for DRS as described previously [22] and RNA gel blot analyses were carried out as described [14]. For RT-PCR and RT-qPCR, RNA was isolated using TRI Reagent (Sigma-Aldrich) followed by DNase I treatment (Invitrogen), and reverse transcription was primed with oligo(dT)15 using M-MLV reverse transcriptase (Promega). RT-qPCR was carried out as previously described [14]. For validation of read-through transcripts, fragments were cloned into the pGEM-T Easy (Promega) vector and then sequenced. 5′RACE was performed on 250 ng of poly(A)+ RNA from WT and fpa-8 backgrounds using the FirstChoice RLM-RACE Kit (Ambion), with or without TAP treatment, according to the manufacturer's instructions. Fragments obtained by 5′RACE were cloned into the pGEM-T Easy (Promega) vector and then sequenced.
Aligning and filtering procedures for DRS data
Sequencing datasets described in this study have been deposited at the European Nucleotide Archive (ENA): Study, PRJEB3993; accession no, ERP003245. Raw DRS sequences were aligned using open source HeliSphere software (version 1.1.498.63, available free from http://sourceforge.net/projects/openhelisphere/files/helisphere/).
The indexDPgenomic aligner was run with the following parameters: seed_size = 18; num_errors = 1; weight = 16; best_only = 1; max_hit_duplication = 25; percent_error = 0.2; read_step = 4; min_norm_score = 4.2; and strands = both. We discarded globally non-unique alignments and selected one alignment randomly if there were several non-unique local alignments mapped to a genetic region. Reads with more than four indels were deleted, and read alignments were refined using an iterative multiple alignment procedure. DRS reads containing low complexity genomic regions, identified by DustMasker from the Blast+ 2.2.24 package, were discarded, as previously described [22].
Data normalisation
In order to compare WT and fpa mutant data, we used read-per-million (RPM) normalisation, i.e. each DRS read was assigned a weight in such a way that the sum of all weights in a sample condition was equal to 1×106. We called the weights ‘normalised reads’. All images of normalised read alignments were made using the Integrated Genome Browser [55] and correspond to combined reads from the three sequenced biological replicates for each genotype.
Algorithm for predicted re-annotation of coding genes
An algorithm that accommodates intergenic DRS reads in automated gene re-annotation was carried out as previously described [22], but based on the WT data described in this study.
Differential expression analyses
The DESeq (version 1.8.2) package [25] was used to search for differentially expressed (DE) genes and poly(A) peaks. DESeq estimates the variance of expression levels for a set of genomic features (genes, intergenic regions or poly(A) peaks) based on read count data within the features in several biological replicates from two different genotypes. This package then calculates P values for the features to be non-DE based on the hypothesis that replicated expression levels of the features are distributed according to negative binomial distribution. These P values were adjusted using Benjamini-Hochberg multiple testing corrections [56]. We prepared raw un-normalised DRS read counts for all replicates of the two genotypes. In order to curb uncertainty due to low read counts, we set a minimal read count per replicate of 15 raw reads for protein-coding genes and transposons, 11 raw reads for intergenic regions, and 8 raw reads for poly(A) peaks. Reads with counts lower than the limit in different replicates were excluded from the DE analyses. These cutoffs were established by maximising the number of DE features identified between WT and fpa-7.
Differentially expressed coding gene analysis
Counts of DRS reads mapping to re-annotated protein-coding genes were prepared for three replicates of each genotype (WT and fpa). A total of 18,406 protein-coding genes were identified. Once the requirement of a minimum raw read count of 15 was applied to all replicates, 15,081 protein-coding genes remained for the DE analysis. Table S2 summarises normalised mean expression values for both genotypes, as well as fold change and P values calculated by DESeq for each coding gene with a P value of <0.01 to be considered as differentially expressed. Table S3 shows the same data for transposons and transposable gene elements.
Analysis of antisense RNAs at differentially expressed coding gene promoters
We defined promoter regions as the 2 Kb regions upstream of the transcription start site of protein-coding genes. The analysis was based on prepared poly(A) peaks for intergenic read-through analysis and our list of DE coding genes (Table S2). P values between WT and fpa were calculated for the regions antisense to DE gene promoter, with at least 11 raw reads in a replicate. So-called promoter regions with P values<0.05 in the fpa mutants were classified as DE genes with antisense transcripts that overlap with their promoters.
Putative chimeric RNA analysis
Uniquely aligned and filtered reads and re-annotated coding genes were used for this analysis. We prepared a list of neighbouring genes with normalised read counts for every gene for both genotypes (WT and fpa). We selected gene pairs with expression levels higher than 0.5 RPM (this corresponds to roughly 11–12 raw reads within a gene) for both genes in the pair and for both genotypes. Pairs with a DESeq P value of <0.01 for which the upstream gene was down-regulated and the downstream gene was up-regulated in the fpa mutant were considered as candidates for chimeric RNA formation.
Gaussian smoothing of DRS data and peak-finding algorithm
We prepared a dataset of poly(A) sites, corresponding to the 3′ ends of aligned and filtered DRS reads, by applying smoothing and peak-finding algorithms as previously described [22].
Analysis of differentially expressed intergenic read-throughs
We defined intergenic regions as regions between protein-coding genes and excluded genomic regions of transposable gene elements, pseudogenes and non-coding genes. The analysis was based on uniquely aligned, filtered and smoothed poly(A) sites and our re-annotation of coding genes. We separately prepared poly(A) peaks for each replicate, clustered poly(A) peaks within a 4-bp window and selected poly(A) peaks with at least 0.5 RPM expression. We removed poly(A) peaks within protein-coding genes, transposable gene elements, pseudogenes and non-coding genes. Since we did not re-annotate the transposable gene elements, pseudogenes and non-coding genes, we also excluded poly(A) peaks within 50 bp downstream of the 3′ ends of these genomic features. We assigned two parameters to every intergenic region: the total sum of poly(A) peaks within the region (and for each replicate); and the position of the centroid of the poly(A) peaks in a region as Pcentroid = ΣEiPi/ΣEi, where Ei is the expression level of ith poly(A) peak within each region and Pi is the genomic coordinate of the peak. P values between WT and fpa were calculated for intergenic regions with at least 11 raw reads in a replicate. At the final step, intergenic regions with P values<0.05 and centroid positions not within 30 bp downstream of the coding gene 3′ end in the fpa mutants were classified as DE. The 30-bp offset corresponds to the median length of DRS reads; by applying this additional filter we therefore excluded situations where intergenic reads were mainly grouped near the 3′ end of the upstream gene and hence may belong to the gene.
Analysis of differentially expressed poly(A) peaks
In this analysis, we compared expression levels of individual poly(A) peaks in the two genotypes rather than the total reads aligned to a gene. The comparison of peaks is complicated by over-calling peaks (i.e. peaks being identified where there should be no peak) and the fact that the centre of biologically equivalent peaks may be called in slightly different positions in different genotypes as a consequence of the varied read depth and the peak-calling algorithm. Figure S12 summarises the process we developed to overcome these issues and systematically identify equivalent peaks between WT and fpa-7.
Having identified peak pairs, we applied DESeq to all pairs with at least 8 raw reads in a replicate. This gave 86,699 poly(A) peak pairs. Peak pairs with a P value of <0.01 were considered to be DE in the fpa-7 mutant. These comprised 6% of the selected poly(A) peaks: 2,538 down-regulated and 2,671 up-regulated. Due to the data partition between two neighbouring peaks in one genotype and/or incorrect peak matching, our algorithms can produce ‘false positives’. For example, in Figure S13, if we examine the most highly expressed poly(A) peak in the gene, we see there is another poly(A) peak downstream of the peak in WT and nothing in fpa-7. The peak-finding algorithm incorrectly combined poly(A) sites belonging to the bump on the right-hand slope of the peak to the most highly expressed poly(A) peak in fpa-7 and created a new peak in WT. Since this WT peak was matched to 0 in fpa-7, it was wrongly classified as DE by DESeq. We therefore designed an algorithm to identify and exclude these ‘false positive’ peaks: if a poly(A) peak was up-regulated in one genotype, we looked for poly(A) peaks within an 8-bp window in the other genotype; if we found poly(A) peaks in the genotype that were not associated with the DE poly(A) peak and the summed expression of the peaks was significantly different (20% higher than the associated poly(A) peak only), then we defined this DE poly(A) peak as a ‘false positive’. This led to 37% of down-regulated and 32% of up-regulated DE peaks being excluded from further analysis. We also applied this method to select intronic cleavage sites that were differentially used in fpa-7 compared to WT. We then only considered peaks that comprised >10% of expression at a particular gene and manually excluded sites that mapped to 3′UTR introns.
3′RACE
3′RACE was performed using the FirstChoice RLM-RACE Kit Protocol (Ambion) according to the manufacturer's instructions. The reaction was started with 250 ng poly(A)+ RNA. Multiple PCR products were purified, cloned into the pGEM-T Easy vector (Promega) and sequenced.
Chromatin ImmunoPrecipitation (ChIP) analysis
ChIP was performed as previously described [57]. Anti-H3K4me3 monoclonal antibodies were obtained from Diagenode (MAb-152–050).
Primers used in this study
All primers used in this study are listed in Table S10.
Supporting Information
Zdroje
1. Di GiammartinoDC, NishidaK, ManleyJL (2011) Mechanisms and consequences of alternative polyadenylation. Mol Cell 43 : 853–866 doi:10.1016/j.molce1.2011.08.017
2. ProudfootNJ (2011) Ending the message: poly(A) signals then and now. Genes Dev 25 : 1770–1782 doi:10.1101/gad.17268411
3. KuehnerJN, PearsonEL, MooreC (2011) Unravelling the means to an end: RNA polymerase II transcription termination. Nat Rev Mol Cell Biol 12 : 283–294 doi:10.1038/nrm3098
4. NojimaT, DienstbierM, MurphyS, ProudfootNJ, DyeMJ (2013) Definition of RNA Polymerase II CoTC Terminator Elements in the Human Genome. Cell Rep 3 : 1080–1092 doi:10.1016/j.celrep.2013.03.012
5. WestS, ProudfootNJ (2009) Transcriptional termination enhances protein expression in human cells. Mol Cell 33 : 354–364 doi:10.1016/j.molce1.2009.01.008
6. MapendanoCK, Lykke-AndersenS, KjemsJ, BertrandE, JensenTH (2010) Crosstalk between mRNA 3′ end processing and transcription initiation. Mol Cell 40 : 410–422 doi:10.1016/j.molce1.2010.10.012
7. PadmanabhanK, RoblesMS, WesterlingT, WeitzCJ (2012) Feedback regulation of transcriptional termination by the mammalian circadian clock PERIOD complex. Science 337 : 599–602 doi:10.1126/science.1221592
8. AkivaP, ToporikA, EdelheitS, PeretzY, DiberA, et al. (2006) Transcription-mediated gene fusion in the human genome. Genome Res 16 : 30–36 doi:10.1101/gr.4137606
9. ParraG, ReymondA, DabbousehN, DermitzakisET, CasteloR, et al. (2006) Tandem chimerism as a means to increase protein complexity in the human genome. Genome Res 16 : 37–44 doi:10.1101/gr.4145906
10. PrakashT, SharmaVK, AdatiN, OzawaR, KumarN, et al. (2010) Expression of conjoined genes: another mechanism for gene regulation in eukaryotes. PLoS One 5: e13284 doi:10.1371/journal.pone.0013284.t002
11. DalzielM, KolesnichenkoM, Neves dasRP, IborraF, GodingC, et al. (2011) Alpha-MSH regulates intergenic splicing of MC1R and TUBB3 in human melanocytes. Nucleic Acids Res 39 : 2378–2392 doi:10.1093/nar/gkq1125
12. MaherCA, Kumar-SinhaC, CaoX, Kalyana-SundaramS, HanB, et al. (2009) Transcriptome sequencing to detect gene fusions in cancer. Nature 458 : 97–101 doi:10.1038/nature07638
13. KannanK, WangL, WangJ, IttmannMM, LiW, et al. (2011) Recurrent chimeric RNAs enriched in human prostate cancer identified by deep sequencing. Proc Natl Acad Sci USA 108 : 9172–9177 doi:10.1073/pnas.1100489108
14. HornyikC, TerziLC, SimpsonGG (2010) The spen family protein FPA controls alternative cleavage and polyadenylation of RNA. Dev Cell 18 : 203–213 doi:10.1016/j.devce1.2009.12.009
15. MichaelsSD, AmasinoRM (2001) Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization. Plant Cell 13 : 935–941.
16. SimpsonGG, DijkwelPP, QuesadaV, HendersonI, DeanC (2003) FY is an RNA 3′ end-processing factor that interacts with FCA to control the Arabidopsis floral transition. Cell 113 : 777–787.
17. XingD, ZhaoH, XuR, LiQQ (2008) Arabidopsis PCFS4, a homologue of yeast polyadenylation factor Pcf11p, regulates FCA alternative processing and promotes flowering time. Plant J 54 : 899–910 doi:–10.1111/j.1365–313X.2008.03455.x
18. LiuF, MarquardtS, ListerC, SwiezewskiS, DeanC (2010) Targeted 3′ processing of antisense transcripts triggers Arabidopsis FLC chromatin silencing. Science 327 : 94–97 doi:10.1126/science.1180278
19. VeleyKM, MichaelsSD (2008) Functional redundancy and new roles for genes of the autonomous floral-promotion pathway. Plant Physiol 147 : 682–695 doi:10.1104/pp.108.118927
20. BäurleI, DeanC (2008) Differential interactions of the autonomous pathway RRM proteins and chromatin regulators in the silencing of Arabidopsis targets. PLoS One 3: e2733 doi:10.1371/journal.pone.0002733
21. BäurleI, SmithL, BaulcombeDC, DeanC (2007) Widespread role for the flowering-time regulators FCA and FPA in RNA-mediated chromatin silencing. Science 318 : 109–112 doi:10.1126/science.1146565
22. SherstnevA, DucC, ColeC, ZacharakiV, HornyikC, et al. (2012) Direct sequencing of Arabidopsis thaliana RNA reveals patterns of cleavage and polyadenylation. Nat Struct Mol Biol 19 : 845–852 doi:10.1038/nsmb.2345
23. OzsolakF, KapranovP, FoissacS, KimSW, FishilevichE, et al. (2010) Comprehensive polyadenylation site maps in yeast and human reveal pervasive alternative polyadenylation. Cell 143 : 1018–1029 doi:10.1016/j.ce11.2010.11.020
24. ZhangX, XiaJ, LiiY, Barrera-FigueroaB, ZhouX, et al. (2012) Genome-wide analysis of plant nat-siRNAs reveals insights into their distribution, biogenesis and function. Genome Biol 13: R20 doi:––10.1186/gb-2012–13–3-r20
25. AndersS, HuberW (2010) Differential expression analysis for sequence count data. Genome Biol 11: R106 doi:10.1186/gb-2010-11-10-r106
26. CousthamV, LiP, StrangeA, ListerC, SongJ, et al. (2012) Quantitative modulation of polycomb silencing underlies natural variation in vernalization. Science 337 : 584–587 doi:10.1126/science.1221881
27. StroudH, GreenbergMVC, FengS, BernatavichuteYV, JacobsenSE (2013) Comprehensive analysis of silencing mutants reveals complex regulation of the Arabidopsis methylome. Cell 152 : 352–364 doi:10.1016/j.ce11.2012.10.054
28. GarciaD, GarciaS, PontierD, MarchaisA, RenouJ-P, et al. (2012) Ago hook and RNA helicase motifs underpin dual roles for SDE3 in antiviral defense and silencing of nonconserved intergenic regions. Mol Cell 48 : 109–120 doi:10.1016/j.molce1.2012.07.028
29. PontierD, PicartC, RoudierF, GarciaD, LahmyS, et al. (2012) NERD, a plant-specific GW protein, defines an additional RNAi-dependent chromatin-based pathway in Arabidopsis. Mol Cell 48 : 121–132 doi:10.1016/j.molce1.2012.07.027
30. SilveiraAB, TrontinC, CortijoS, BarauJ, Del BemLEV, et al. (2013) Extensive natural epigenetic variation at a de novo originated gene. PLoS Genet 9: e1003437 doi:10.1371/journal.pgen.1003437.s005
31. SonmezC, BäurleI, MagusinA, DreosR, LaubingerS, et al. (2011) RNA 3′ processing functions of Arabidopsis FCA and FPA limit intergenic transcription. Proc Natl Acad Sci U S A 108 : 8508–8513 doi:10.1073/pnas.1105334108
32. SazeH, ShiraishiA, MiuraA, KakutaniT (2008) Control of genic DNA methylation by a jmjC domain-containing protein in Arabidopsis thaliana. Science 319 : 462–465 doi:10.1126/science.1150987
33. RigalM, KeveiZ, PélissierT, MathieuO (2012) DNA methylation in an intron of the IBM1 histone demethylase gene stabilizes chromatin modification patterns. EMBO J 31 : 2981–2993 doi:10.1038/emboj.2012.141
34. ZhangX, BernatavichuteYV, CokusS, PellegriniM, JacobsenSE (2009) Genome-wide analysis of mono-, di - and trimethylation of histone H3 lysine 4 in Arabidopsis thaliana. Genome Biol 10: R62 doi:––10.1186/gb-2009–10–6-r62
35. MühlemannO, EberleAB, StalderL, Zamudio OrozcoR (2008) Recognition and elimination of nonsense mRNA. Biochim Biophys Acta 1779 : 538–549 doi:10.1016/j.bbagrm.2008.06.012
36. Arciga-ReyesL, WoottonL, KiefferM, DaviesB (2006) UPF1 is required for nonsense-mediated mRNA decay (NMD) and RNAi in Arabidopsis. Plant J 47 : 480–489 doi:–10.1111/j.1365–313X.2006.02802.x
37. LeivarP, QuailPH (2011) PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci 16 : 19–28 doi:10.1016/j.tplants.2010.08.003
38. LiuF, BakhtS, DeanC (2012) Cotranscriptional role for Arabidopsis DICER-LIKE 4 in transcription termination. Science 335 : 1621–1623 doi:10.1126/science.1214402
39. LiuF, QuesadaV, CrevillénP, BäurleI, SwiezewskiS, et al. (2007) The Arabidopsis RNA-binding protein FCA requires a lysine-specific demethylase 1 homolog to downregulate FLC. Mol Cell 28 : 398–407 doi:10.1016/j.molce1.2007.10.018
40. JacquierA (2009) The complex eukaryotic transcriptome: unexpected pervasive transcription and novel small RNAs. Nat Rev Genet 10 : 833–844 doi:10.1038/nrg2683
41. SunQ, CsorbaT, Skourti-StathakiK, ProudfootNJ, DeanC (2013) R-Loop stabilization represses antisense transcription at the Arabidopsis FLC locus. Science 340 : 619–621 doi:10.1126/science.1234848
42. TautzD, Domazet-LošoT (2011) The evolutionary origin of orphan genes. Nat Rev Genet 12 : 692–702 doi:10.1038/nrg3053
43. ShuklaS, KavakE, GregoryM, ImashimizuM, ShutinoskiB, et al. (2011) CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature 479 : 74–79 doi:10.1038/nature10442
44. WoodAJ, SchulzR, WoodfineK, KoltowskaK, BeecheyCV, et al. (2008) Regulation of alternative polyadenylation by genomic imprinting. Genes Dev 22 : 1141–1146 doi:10.1101/gad.473408
45. YaishMWF, PengM, RothsteinSJ (2009) AtMBD9 modulates Arabidopsis development through the dual epigenetic pathways of DNA methylation and histone acetylation. Plant J 59 : 123–135 doi:10.1111/j.1365 313X.2009.03860.x
46. JenalM, ElkonR, Loayza-PuchF, van HaaftenG, KühnU, et al. (2012) The poly(A)-binding protein nuclear 1 suppresses alternative cleavage and polyadenylation sites. Cell 149 : 538–553 doi:10.1016/j.ce11.2012.03.022
47. MartinG, GruberAR, KellerW, ZavolanM (2012) Genome-wide analysis of pre-mRNA 3′ end processing reveals a decisive role of human cleavage factor I in the regulation of 3′ UTR length. Cell Rep 1 : 753–763 doi:10.1016/j.celrep.2012.05.003
48. JohnsonSA, KimH, EricksonB, BentleyDL (2011) The export factor Yra1 modulates mRNA 3′ end processing. Nat Struct Mol Biol 18 : 1164–1171 doi:10.1038/nsmb.2126
49. SandbergR, NeilsonJR, SarmaA, SharpPA, BurgeCB (2008) Proliferating cells express mRNAs with shortened 3′ untranslated regions and fewer microRNA target sites. Science 320 : 1643–1647 doi:10.1126/science.1155390
50. MayrC, BartelDP (2009) Widespread shortening of 3′UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell 138 : 673–684 doi:10.1016/j.ce11.2009.06.016
51. MagrangeasF, PitiotG, DuboisS, Bragado-NilssonE, ChérelM, et al. (1998) Cotranscription and intergenic splicing of human galactose-1-phosphate uridylyltransferase and interleukin-11 receptor alpha-chain genes generate a fusion mRNA in normal cells. Implication for the production of multidomain proteins during evolution. J Biol Chem 273 : 16005–16010.
52. CuiM, AllenMA, LarsenA, MacmorrisM, HanM, et al. (2008) Genes involved in pre-mRNA 3′-end formation and transcription termination revealed by a lin-15 operon Muv suppressor screen. Proc Natl Acad Sci USA 105 : 16665–16670 doi:10.1073/pnas.0807104105
53. AlénC, KentNA, JonesHS, O'SullivanJ, ArandaA, et al. (2002) A role for chromatin remodeling in transcriptional termination by RNA polymerase II. Mol Cell 10 : 1441–1452.
54. ZhangX, LiiY, WuZ, PolishkoA, ZhangH, et al. (2013) Mechanisms of small RNA generation from cis-NATs in response to environmental and developmental cues. Mol Plant 6 : 704–715 doi:10.1093/mp/sst051
55. NicolJW, HeltGA, BlanchardSG, RajaA, LoraineAE (2009) The Integrated Genome Browser: free software for distribution and exploration of genome-scale datasets. Bioinformatics 25 : 2730–2731 doi:10.1093/bioinformatics/btp472
56. BenjaminiY, HochbergY (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57 : 289–300.
57. WierzbickiAT, HaagJR, PikaardCS (2008) Noncoding transcription by RNA polymerase Pol IVb/Pol V mediates transcriptional silencing of overlapping and adjacent genes. Cell 135 : 635–648 doi:10.1016/j.ce11.2008.09.035
58. JanCH, FriedmanRC, RubyJG, BartelDP (2011) Formation, regulation and evolution of Caenorhabditis elegans 3′UTRs. Nature 469 : 97–101 doi:10.1038/nature09616
Štítky
Genetika Reprodukční medicína
Článek Defending Sperm FunctionČlánek How to Choose the Right MateČlánek Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental TransitionČlánek Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in SpermatogenesisČlánek The Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate EmbryogenesisČlánek Multiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time inČlánek Playing the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral MyelinationČlánek A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase inČlánek Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 10- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- Defending Sperm Function
- How to Choose the Right Mate
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
- Conserved Translatome Remodeling in Nematode Species Executing a Shared Developmental Transition
- A Novel Actin mRNA Splice Variant Regulates ACTG1 Expression
- Tracking Proliferative History in Lymphocyte Development with Cre-Mediated Sister Chromatid Recombination
- Correlated Occurrence and Bypass of Frame-Shifting Insertion-Deletions (InDels) to Give Functional Proteins
- Chimeric Protein Complexes in Hybrid Species Generate Novel Phenotypes
- Loss of miR-10a Activates and Collaborates with Activated Wnt Signaling in Inducing Intestinal Neoplasia in Female Mice
- Both Rare and Copy Number Variants Are Prevalent in Agenesis of the Corpus Callosum but Not in Cerebellar Hypoplasia or Polymicrogyria
- Reverse PCA, a Systematic Approach for Identifying Genes Important for the Physical Interaction between Protein Pairs
- Partial Deletion of Chromosome 8 β-defensin Cluster Confers Sperm Dysfunction and Infertility in Male Mice
- Genome-Wide and Cell-Specific Epigenetic Analysis Challenges the Role of Polycomb in Spermatogenesis
- Coordinate Regulation of Mature Dopaminergic Axon Morphology by Macroautophagy and the PTEN Signaling Pathway
- Cooperation between RUNX1-ETO9a and Novel Transcriptional Partner KLF6 in Upregulation of in Acute Myeloid Leukemia
- Mobility of the Native Conjugative Plasmid pLS20 Is Regulated by Intercellular Signaling
- FliZ Is a Global Regulatory Protein Affecting the Expression of Flagellar and Virulence Genes in Individual Bacterial Cells
- Specific Tandem Repeats Are Sufficient for Paramutation-Induced Trans-Generational Silencing
- Condensin II Subunit dCAP-D3 Restricts Retrotransposon Mobilization in Somatic Cells
- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- The Insulator Homie Promotes Expression and Protects the Adjacent Gene from Repression by Polycomb Spreading
- Human Intellectual Disability Genes Form Conserved Functional Modules in
- Coordination of Cell Proliferation and Cell Fate Determination by CES-1 Snail
- ORFs in Drosophila Are Important to Organismal Fitness and Evolved Rapidly from Previously Non-coding Sequences
- Different Roles of Eukaryotic MutS and MutL Complexes in Repair of Small Insertion and Deletion Loops in Yeast
- The Spore Differentiation Pathway in the Enteric Pathogen
- Acceleration of the Glycolytic Flux by Steroid Receptor Coactivator-2 Is Essential for Endometrial Decidualization
- The Human Nuclear Poly(A)-Binding Protein Promotes RNA Hyperadenylation and Decay
- Genome Wide Analysis Reveals Zic3 Interaction with Distal Regulatory Elements of Stage Specific Developmental Genes in Zebrafish
- Xbp1 Directs Global Repression of Budding Yeast Transcription during the Transition to Quiescence and Is Important for the Longevity and Reversibility of the Quiescent State
- The Integrator Complex Subunit 6 (Ints6) Confines the Dorsal Organizer in Vertebrate Embryogenesis
- Incorporating Motif Analysis into Gene Co-expression Networks Reveals Novel Modular Expression Pattern and New Signaling Pathways
- The Bacterial Response Regulator ArcA Uses a Diverse Binding Site Architecture to Regulate Carbon Oxidation Globally
- Direct Monitoring of the Strand Passage Reaction of DNA Topoisomerase II Triggers Checkpoint Activation
- Multiple bHLH Proteins form Heterodimers to Mediate CRY2-Dependent Regulation of Flowering-Time in
- A Reversible Histone H3 Acetylation Cooperates with Mismatch Repair and Replicative Polymerases in Maintaining Genome Stability
- ALS-Associated Mutations Result in Compromised Alternative Splicing and Autoregulation
- Robust Demographic Inference from Genomic and SNP Data
- Preferential Binding to Elk-1 by SLE-Associated Risk Allele Upregulates Expression
- Rad52 Sumoylation Prevents the Toxicity of Unproductive Rad51 Filaments Independently of the Anti-Recombinase Srs2
- The Serum Resistome of a Globally Disseminated Multidrug Resistant Uropathogenic Clone
- Identification of 526 Conserved Metazoan Genetic Innovations Exposes a New Role for Cofactor E-like in Neuronal Microtubule Homeostasis
- SUMO Localizes to the Central Element of Synaptonemal Complex and Is Required for the Full Synapsis of Meiotic Chromosomes in Budding Yeast
- Integrated Enrichment Analysis of Variants and Pathways in Genome-Wide Association Studies Indicates Central Role for IL-2 Signaling Genes in Type 1 Diabetes, and Cytokine Signaling Genes in Crohn's Disease
- Genome-Wide High-Resolution Mapping of UV-Induced Mitotic Recombination Events in
- Genome-Wide Analysis of Cell Type-Specific Gene Transcription during Spore Formation in
- Playing the Field: Sox10 Recruits Different Partners to Drive Central and Peripheral Myelination
- Two Portable Recombination Enhancers Direct Donor Choice in Fission Yeast Heterochromatin
- Mining the Human Phenome Using Allelic Scores That Index Biological Intermediates
- Yeast Tdh3 (Glyceraldehyde 3-Phosphate Dehydrogenase) Is a Sir2-Interacting Factor That Regulates Transcriptional Silencing and rDNA Recombination
- A Minimal Nitrogen Fixation Gene Cluster from sp. WLY78 Enables Expression of Active Nitrogenase in
- A Review of Bacteria-Animal Lateral Gene Transfer May Inform Our Understanding of Diseases like Cancer
- High Throughput Sequencing Reveals Alterations in the Recombination Signatures with Diminishing Spo11 Activity
- Partitioning the Heritability of Tourette Syndrome and Obsessive Compulsive Disorder Reveals Differences in Genetic Architecture
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- A GDF5 Point Mutation Strikes Twice - Causing BDA1 and SYNS2
- Systematic Unraveling of the Unsolved Pathway of Nicotine Degradation in
- Natural Genetic Variation of Integrin Alpha L () Modulates Ischemic Brain Injury in Stroke
- Evolutionary Tuning of Protein Expression Levels of a Positively Autoregulated Two-Component System
- Evolutionary Change within a Bipotential Switch Shaped the Sperm/Oocyte Decision in Hermaphroditic Nematodes
- Limiting of the Innate Immune Response by SF3A-Dependent Control of MyD88 Alternative mRNA Splicing
- Multiple Signaling Pathways Coordinate to Induce a Threshold Response in a Chordate Embryo
- Distinct Regulatory Mechanisms Act to Establish and Maintain Pax3 Expression in the Developing Neural Tube
- Genome Wide Analysis of Narcolepsy in China Implicates Novel Immune Loci and Reveals Changes in Association Prior to Versus After the 2009 H1N1 Influenza Pandemic
- Mismatch Repair Genes and Modify CAG Instability in Huntington's Disease Mice: Genome-Wide and Candidate Approaches
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- Hsp70-Hsp40 Chaperone Complex Functions in Controlling Polarized Growth by Repressing Hsf1-Driven Heat Stress-Associated Transcription
- Function and Evolution of DNA Methylation in
- Stimulation of mTORC1 with L-leucine Rescues Defects Associated with Roberts Syndrome
- Transcription Termination and Chimeric RNA Formation Controlled by FPA
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dominant Mutations in Identify the Mlh1-Pms1 Endonuclease Active Site and an Exonuclease 1-Independent Mismatch Repair Pathway
- Eleven Candidate Susceptibility Genes for Common Familial Colorectal Cancer
- The Histone H3 K27 Methyltransferase KMT6 Regulates Development and Expression of Secondary Metabolite Gene Clusters
- A Mutation in the Gene in Labrador Retrievers with Hereditary Nasal Parakeratosis (HNPK) Provides Insights into the Epigenetics of Keratinocyte Differentiation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



