-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
SLX-1 Is Required for Maintaining Genomic Integrity and Promoting Meiotic Noncrossovers in the Germline
Although the SLX4 complex, which includes structure-specific nucleases such as XPF, MUS81, and SLX1, plays important roles in the repair of several kinds of DNA damage, the function of SLX1 in the germline remains unknown. Here we characterized the endonuclease activities of the Caenorhabditis elegans SLX-1-HIM-18/SLX-4 complex co-purified from human 293T cells and determined SLX-1 germline function via analysis of slx-1(tm2644) mutants. SLX-1 shows a HIM-18/SLX-4–dependent endonuclease activity toward replication forks, 5′-flaps, and Holliday junctions. slx-1 mutants exhibit hypersensitivity to UV, nitrogen mustard, and camptothecin, but not gamma irradiation. Consistent with a role in DNA repair, recombination intermediates accumulate in both mitotic and meiotic germ cells in slx-1 mutants. Importantly, meiotic crossover distribution, but not crossover frequency, is altered on chromosomes in slx-1 mutants compared to wild type. This alteration is not due to changes in either the levels or distribution of double-strand breaks (DSBs) along chromosomes. We propose that SLX-1 is required for repair at stalled or collapsed replication forks, interstrand crosslink repair, and nucleotide excision repair during mitosis. Moreover, we hypothesize that SLX-1 regulates the crossover landscape during meiosis by acting as a noncrossover-promoting factor in a subset of DSBs.
Published in the journal: . PLoS Genet 8(8): e32767. doi:10.1371/journal.pgen.1002888
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002888Summary
Although the SLX4 complex, which includes structure-specific nucleases such as XPF, MUS81, and SLX1, plays important roles in the repair of several kinds of DNA damage, the function of SLX1 in the germline remains unknown. Here we characterized the endonuclease activities of the Caenorhabditis elegans SLX-1-HIM-18/SLX-4 complex co-purified from human 293T cells and determined SLX-1 germline function via analysis of slx-1(tm2644) mutants. SLX-1 shows a HIM-18/SLX-4–dependent endonuclease activity toward replication forks, 5′-flaps, and Holliday junctions. slx-1 mutants exhibit hypersensitivity to UV, nitrogen mustard, and camptothecin, but not gamma irradiation. Consistent with a role in DNA repair, recombination intermediates accumulate in both mitotic and meiotic germ cells in slx-1 mutants. Importantly, meiotic crossover distribution, but not crossover frequency, is altered on chromosomes in slx-1 mutants compared to wild type. This alteration is not due to changes in either the levels or distribution of double-strand breaks (DSBs) along chromosomes. We propose that SLX-1 is required for repair at stalled or collapsed replication forks, interstrand crosslink repair, and nucleotide excision repair during mitosis. Moreover, we hypothesize that SLX-1 regulates the crossover landscape during meiosis by acting as a noncrossover-promoting factor in a subset of DSBs.
Introduction
Genomic DNA is subjected to a variety of endogenous and exogenous sources of damage. To repair this DNA damage, the structure-specific endonucleases function to cleave branched DNA structures such as Y forks, 5′-flaps, 3′-flaps, stem loops, bubbles, replication forks (RFs) and Holliday junctions (HJs). SLX-1, a structure-specific nuclease that is highly conserved in eukaryotes, harbors both GIY-YIG-type endonuclease and PHD-type zinc finger domains. Furthermore, SLX1 requires the regulatory subunit SLX4 to perform its nuclease activities in both yeast and humans [1]–[5]. Specifically, the budding yeast Slx1-Slx4 complex, co-purified from Escherichia coli, shows endonuclease activity towards Y forks, 5′-flaps, RFs and HJs [1]. The immunoprecipitation products of fission yeast Slx1-TAP cleave stem loops and HJs [2]. Finally, the human SLX1-SLX4 complex, purified from both human cells and E. coli, cleaves Y forks, 3′-flaps, 5′-flaps, RFs, stem loops and HJs [3]–[5]. However, it is not known whether these endonuclease activities of SLX1 are conserved in other species such as flies and worms.
slx1 was first identified in a synthetic-lethal screen for genes required for the viability of cells lacking Sgs1, the budding yeast RecQ helicase [6]. Sgs1 functions as an anti-recombinase by unwinding and dissolving toxic recombination intermediates, thereby maintaining genome stability [7]. slx1 deletion (slx1Δ) mutants do not exhibit lethality, DNA damage sensitivity or sterility in either budding or fission yeast [2], [6], [8]. However, they exhibit defects in the completion of rDNA replication [2], [9], [10]. Moreover, in humans, the siRNA based-depletion of SLX1 increases both the endogenous and exogenous levels of DNA damage resulting from exposure to ionizing radiation (IR), camptothecin (CPT) and DNA interstrand crosslinking agents [3]–[5], [11]. In humans, SLX4 forms a complex with three structure-specific nucleases, SLX1, XPF-ERCC1 and MUS81-EME1 [3]–[5]. Moreover, individuals carrying mutations in SLX4 exhibit symptoms of Fanconi anemia, a syndrome characterized by chromosomal instability in humans [12]. A mouse knockout of Slx4 also shows chromosomal instability phenotypes similar to those of Fanconi anemia in humans [13]. In budding yeast, Slx4 binds to Slx1 and Rad1XPF in a mutually exclusive manner [6], [14], [15]. We showed that HIM-18, the SLX4 homolog in C. elegans, interacts with SLX-1 and XPF-1 [16]. Furthermore, we found that HIM-18/SLX-4 (referred to herein as HIM-18) and XPF-1 are required for wild type levels of meiotic crossover formation [16]. However, whether SLX-1 is required for meiotic crossover formation remains unknown. Moreover, whether SLX1, XPF and MUS81 function as structure-specific endonucleases either in the same or in different complexes in vivo is also unclear.
Here we show that Caenorhabditis elegans SLX-1 cleaves branched DNA substrates in a HIM-18/SLX-4-dependent manner in vitro. Furthermore, slx-1(tm2644) mutants, which encode for a catalytically inactive (nuclease negative) protein, show accumulation of recombination intermediates in both mitotic and meiotic cells as well as increased sensitivity to DNA damage inducing agents. These results suggest that SLX-1 is required for DNA repair by processing repair intermediates through its nuclease activity. However, while double Holliday junction resolution is required for crossover formation during meiosis, meiotic crossover frequencies were not reduced in slx-1(tm2644) mutants, and instead, crossover distribution was altered compared to wild type. Therefore, although SLX-1 is not an essential nuclease for crossover formation via double Holliday junction resolution between homologous chromosomes during meiotic recombination, our studies reveal that SLX-1 plays a role in regulating crossover distribution. This regulation is not mediated through changes in either the levels or distribution of DNA double-strand breaks (DSBs) along chromosomes. Instead, we propose a model in which SLX-1 regulates meiotic crossover distribution such that they occur at the terminal thirds of chromosomes by promoting DSB repair via a noncrossover pathway along the mid-section of chromosomes.
Results
SLX-1 cleaves branched DNA in a HIM-18–dependent manner
We previously showed that C. elegans SLX-1 interacts with HIM-18 in a yeast two-hybrid assay [16]. To examine whether these two components directly interact with one another, we transiently transfected HEK-293T cells with epitope-tagged HIM-18 and SLX-1 and performed immunoprecipitation experiments. As shown in Figure 1A–1C, full-length HA-SLX-1 associates with full-length Myc-HIM-18, but not with a control Myc tagged protein (Myc-GFP) under the tested conditions. To further characterize the interaction between SLX-1 and HIM-18, we co-expressed the N-terminal domain of SLX-1 that contains its nuclease domain (HA-SLX-1-N; residues 1–272, Figure 1A) with Myc-HIM-18 in 293T cells. In contrast to what was observed with HA-SLX-1 (full-length), Myc-HIM-18 does not associate with HA-SLX-1-N, consistent with yeast two-hybrid results [3]. We also ascertained that the C-terminal domain of SLX-1 that contains its PHD domain (SLX-1-C; residues 273–443) does not interact with HIM-18 (data not shown). These results suggest that the interaction between HIM-18 and SLX-1 is direct and involves both the N-terminal and C-terminal domains of SLX-1.
Fig. 1. C. elegans SLX-1 cleaves branched substrates in a HIM-18–dependent manner. 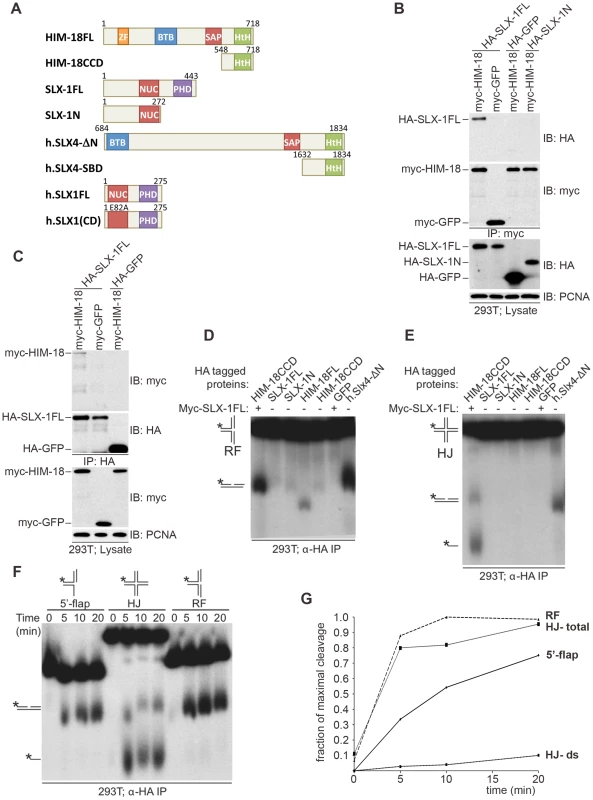
(A) Protein constructs used in this study. Both the length of amino acid residues and domains are shown. FL: full length; CCD: conserved C-terminal domain; SBD: SLX1 binding domain; CD: catalytic dead; ZF: zinc finger; BTB: Broad-Complex, Tramtrack and Bric a brac; SAP: SAF-A/B, Acinus and PIAS; HtH: helix turn helix; NUC: nuclease; PHD: plant homeo domain. (B, C) HIM18 associates with full-length SLX1. (B) The indicated HA- and myc-tagged constructs were transfected into HEK293T cells, the cells lysed and the lysates immuno-precipitated with α-HA resin (Sigma) and immuno-blotted with the indicated antibodies. (C) As in (B), but the lysates were immunoprecipitated with α-myc resin (Sigma). (D–F) Cleavage activity of HIM-18/SLX-1. (D) The indicated proteins were transiently expressed and immuno-purified from 293T cells, incubated with a 32P-end-labeled replication fork (RF) substrate, the products were separated by native gel electrophoresis and visualized by autoradiography. The * indicates the labeled strand. The RF substrate and its major cleavage product are indicated to the left of the gel. (E) Similar to (D), but the Holliday Junction (HJ) was used as the substrate. The HJ substrate and its major cleavage products are indicated to the left of the gel. (F) Time-course to evaluate the cleavage rate of branched DNA substrates by HIM-18/SLX-1. Left panel: The HA-HIM-18CCD/myc-SLX-1 complex was transiently expressed and immuno-purified from 293T cells, incubated with the indicated substrates, the reaction was stopped at the indicated time points and the products separated and visualized as in D and E. (G) Quantification of the autoradiograph on the left using ImageJ software. For HJ, two cleavage rates were calculated: one by taking only the double stranded product into account (HJ-ds), and the other by taking both the double stranded and the single stranded products into account. To examine the roles of HIM-18 and SLX-1 during recombination, we assessed if the HIM-18/SLX-1 complex displayed endonucleolytic activity towards synthetic DNA substrates. HA-HIM-18-CCD (conserved C-terminal domain, residues 548–718) and Myc-SLX-1 were co-expressed in 293T cells and immuno-purified with an α-HA antibody (the CCD domain of HIM-18 was used since it expressed at a higher level than full-length HIM-18). The HA-HIM-18/Myc-SLX-1 complex was incubated with either a radiolabeled replication fork (RF) or a Holliday Junction (HJ) substrate, and the reaction products were separated by native gel electrophoresis and visualized by autoradiography. HA-HIM-18/Myc-SLX-1 exhibited endonucleolytic activity against both RFs and HJs at a level that was comparable to the human HA-SLX4-ΔN complexes purified from 293T cells (Figure 1D and 1E) [5]. Under similar conditions, neither HIM-18 nor SLX-1 alone displayed appreciable catalytic activity against RFs and HJs (Figure 1D and 1E), indicating that SLX-1 is the catalytically active component of the HIM-18/SLX-1 complex. To further characterize its processing activity, we analyzed the substrate preference of HIM-18/SLX-1 and compared its activity against 5′-flap, HJ, and RF substrates. As shown in Figure 1F–1G, time-course experiments revealed that HA-HIM-18/Myc-SLX-1 preferred the RF substrate to the 5′-flap or HJ substrates under the conditions tested. We also observed that HIM-18/SLX-1 had substantially lower activity against the 3′-flap substrate compared to RFs, 5′-flaps, or HJs (Figure S1).
Next, we determined the cleavage sites of each DNA substrate (Figure 2). We determined that HA-HIM-18/myc-SLX-1 cleaved the RF substrate on strand 1 at 2 nucleotides 3′ to the branch point (Figure 2A and 2D). However, in the case of RFs, the cleavage efficiency against the double stranded region (strand 1) was significantly higher than against the same region of the 5′-flap (Figure 2B). In addition, it should be noted that although human HA-SLX4-ΔN purified from 293T cells had a cleavage specificity against RF that was significantly different to that of HA-HIM-18/myc-SLX-1, recombinant human SLX1/SLX4 (SBD) generated RF cleavage products that included the 32 nucleotide product (two nucleotides 3′ to the branch point) that was also produced by the C. elegans protein complex (Figure 2A).
Fig. 2. Cleavage specificity of the C. elegans HIM-18/SLX-1 complex. 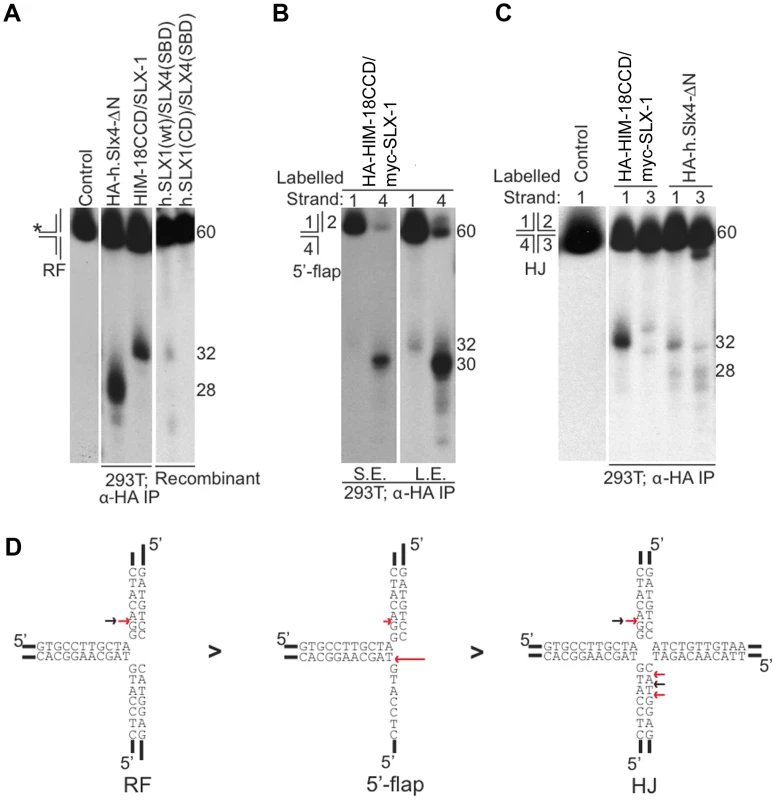
(A) Replication fork (RF) substrate was incubated with the indicated proteins complexes transiently expressed and immuno-purified from 293T cells. The reaction products were separated by denaturing gel electrophoresis to determine the site of cleavage. The length of the labeled oligonucleotide reactants or products determined using oligonucleotide markers (not shown) are indicated to the right of the gel. The * indicates the labeled strand. (B) Similar to A, but the substrate used was the 5′-flap. The image on the right was obtained by exposing the gel for a longer time to indicate cleavage on strand 1. S.E., Short exposure; L.E., Long exposure. (C) Similar to A and B, but the substrate used was the Holliday junction (HJ) 32P-end-labeled at either strand 1 or 3. In (A)–(C) the source of the protein complexes is indicated below the gel image. (D) Structural model of branched substrates indicating cleavage preference and specificity. The red arrow indicates the cleavage site observed with HIM-18/SLX-1 and the black arrow indicates the major cleavage sites observed with the human SLX4-SBD complex [5]. The length of the red arrow is indicative of the cleavage efficiency. HA-HIM-18/myc-SLX-1 purified from 293T cells cleaved the 5′-flap on strand 3, precisely at the junction between the 5′single-stranded arm and the double stranded region of the substrate similar to 5′-flap cleavage by Saccharomyces cerevisiae Slx1-Slx4 [1]. Cleavage also occurred on strand 1 (i.e. the double-stranded region of the 5′-flap), although with much less efficiency than strand 4, at 2 nucleotides 3′ to the branch point (Figure 2B and 2D).
Since HIM-18/SLX-1 displayed significant cleavage activity against HJs (Figure 1D and 1E), we sought to determine if it was in fact a canonical HJ resolvase. A characteristic of bona fide HJ resolvases such as bacterial RuvC, human GEN1 and human SLX1/SLX4, is the ability to cleave opposing strands of a HJ in a symmetric manner to generate products that can be directly ligated [3]–[5], [17], [18]. To test this possibility, we performed cleavage assays against a HJ substrate radiolabeled at either strands 1 or 3 and analyzed the reaction products by denaturing gel electrophoresis. As shown in Figure 2C and 2D, HA-HIM-18/myc-SLX-1 cleaved the HJ substrate at a unique site on strand 1, which was two nucleotides 3′ to the branch point. On strand 3, however, HA-HIM-18/myc-SLX-1 displayed substantially lower cleavage activity compared to strand 1 and cut the HJ at two sites, respectively 1 and 3 nucleotides 3′ to the branch point. This was in contrast to the human HA-SLX4-ΔN complex purified from 293T cells, which cleaves the HJ with perfect symmetry on strands 1 and 3 two nucleotides 3′ to the branch point (Figure 2C and 2D) [5]. These data indicate that the C. elegans HIM-18/SLX-1 complex, though displaying cleavage activity against a HJ substrate, does not appear to function as a bona fide HJ resolvase and is reminiscent of S. cerevisiae and Schizosaccharomyces pombe Slx1-Slx4 endonucleases [1], [2] under the tested conditions.
tm2644 encodes for a catalytically inactive (nuclease-negative) SLX-1
The slx-1(tm2644) mutant, obtained from the Japanese National Bioresource Project, carries a 205 bp deletion encompassing parts of intron 4 and exon 5 which removes the splice acceptor site of the downstream exon 4 (Figure 3A). RT-PCR using a primer set located between the start and stop codons of the slx-1 gene revealed that there are several splice variants containing premature stop codons in slx-1(tm2644) mutants (Figure 3C). Sequence analysis of the RT-PCR products revealed that this alternative splicing does not occur in wild type (Figure 3B). SLX-1 harbors both a GIY-YIG nuclease domain and a PHD finger domain (Figure 3B). 42% of the splice variants lack both the nuclease and PHD finger domains (Figure 3D), whereas 23% contain an intact PHD finger domain (exons 6 and 7) and 35% contain an intact nuclease domain (exons 3 and 4) (Figure 3D). Both exons 3 and 4 carry the conserved catalytic sites of the nuclease (R202 in exon 3 and E243 in exon 4). Moreover, both catalytic sites have been shown to be essential for the nuclease activity of SLX1 both in fission yeast (R34 and E74) [2] and in humans (R41 and E82) [3]. Importantly, the nuclease activity of SLX-1 requires a physical interaction with HIM-18, which is a homolog of human SLX4 (Figure 1D and 1E). However, yeast two-hybrid and immunoprecipitation assays revealed that SLX-1N1–272, which is potentially expressed in slx-1(tm2644) mutants and stems from the only splice variant still carrying the nuclease domain, does not bind to HIM-18 and lacks a nuclease activity (Figure 1A–1E and Figure 3E). Therefore, these results suggest that slx-1(tm2644) mutants are loss-of-function for SLX-1's nuclease activity.
Fig. 3. slx-1(tm2644) mutants express splice variants that lack the C-terminus region required for an interaction with HIM-18, therefore encoding for nuclease-negative variants of SLX-1. 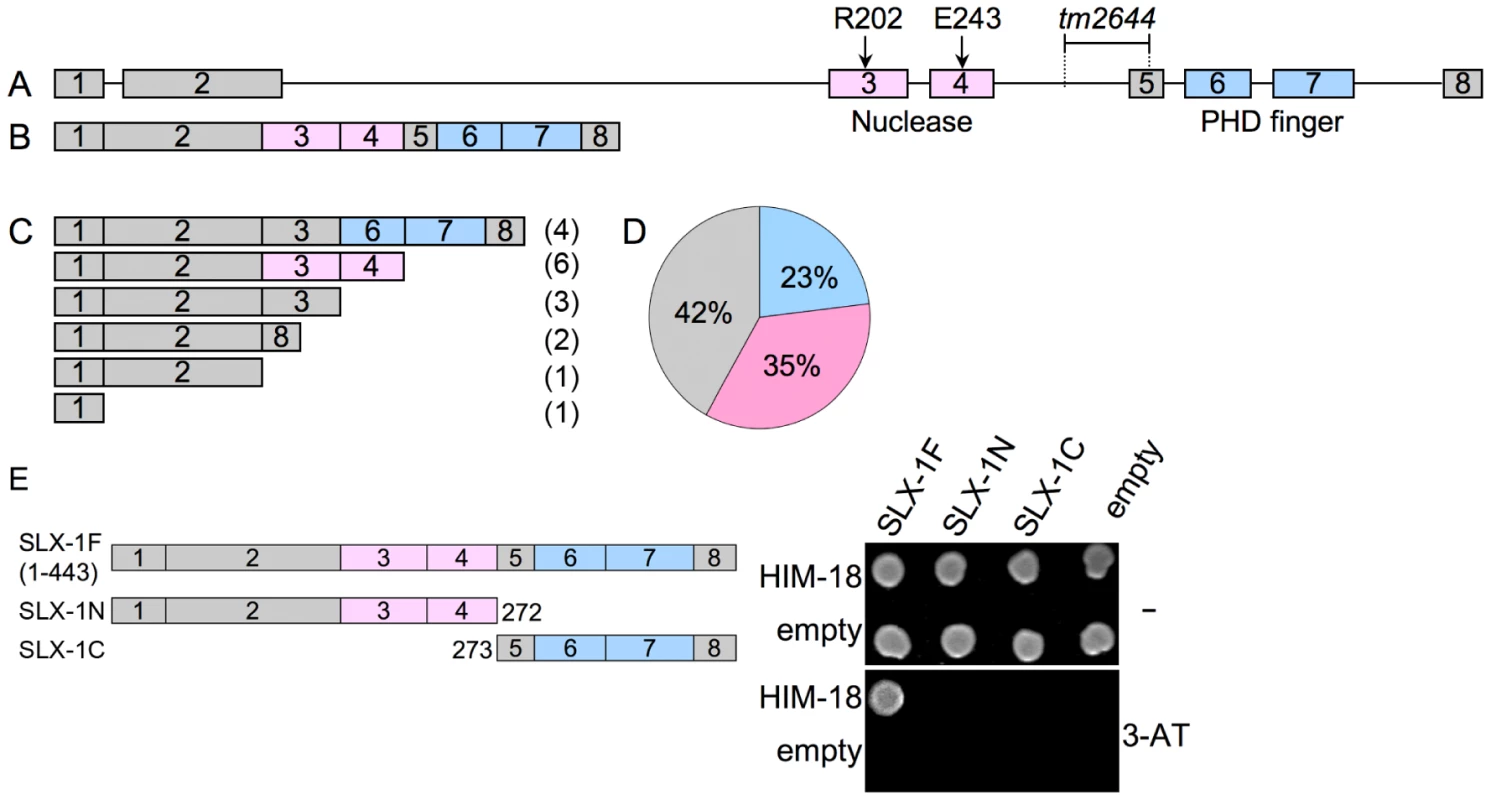
(A) Schematic representation of the slx-1 gene structure. The locations of the nuclease (red) and the PHD finger (blue) domains are shown. R202 and E243 are known catalytic sites of the GIY-YIG nuclease domain. The region deleted in tm2644 is indicated. (B) slx-1 cDNA in wild type. (C) slx-1(tm2644) variant cDNAs. Only start to stop codons are depicted. The number of times each splice variant was detected is indicated in parentheses at the right. (D) The chart indicates the frequency of cDNA variants observed in slx-1(tm2644) mutants. Blue, cDNAs including an intact PHD finger domain (exons 6 and 7). Pink, cDNAs including an intact nuclease domain (exons 3 and 4). Gray, cDNAs carrying neither PHD finger nor nuclease domains. (E) Yeast two-hybrid analysis of interactions between full-length HIM-18 fused to GAL4-AD and full-length or truncated SLX-1 fused to GAL4-DB. Interactions were scored by growth on –HIS+1 mM 3-AT plates. SLX-1 shares overlapping roles with the Bloom syndrome helicase and the XPF-1 and GEN-1 structure-specific endonucleases
To investigate whether slx-1 plays a role in either mitotic development or meiosis, and examine the genetic interactions between slx-1 and other genes implicated in processing recombination intermediates during these cell division programs, we measured the brood size, embryonic lethality, larval arrest and the incidence of males observed among slx-1, him-6/BLM, xpf-1 and gen-1 mutant offspring (Table 1). A decreased brood size is suggestive of increased sterility, whereas either increased embryonic lethality or larval arrest are suggestive of mitotic defects. Finally, a high incidence of males (Him phenotype) is indicative of increased X chromosome nondisjunction and correlates with meiotic defects, whereas a combination of increased embryonic lethality accompanied by a high incidence of males is suggestive of increased aneuploidy resulting from meiotic missegregation of both autosomes and the X chromosome, respectively [19]. slx-1 mutants showed a 32% reduction in brood size (P<0.0001), a 4.6-fold increase in embryonic lethality (P = 0.0006), and a 2-fold increase in larval arrest (P = 0.00197) compared to wild type, supporting a role for slx-1 in mitotic repair. Moreover, a 1.2-fold increase in larval arrest (P = 0.0417) was observed in slx-1;him-18 double mutants compared to him-18 single mutants. This marginally lower significance cutoff most likely indicates that SLX-1 is dependent on HIM-18 during larval development. However, we cannot rule out the possibility that SLX-1's function is not completely HIM-18-dependent during this developmental process. Next, we investigated the genetic interaction of SLX-1 with HIM-6, a C. elegans homolog of yeast Sgs1 and the human Bloom syndrome helicase, which is required for double Holliday junction dissolution [7]. slx-1;him-6 double mutants showed synthetic lethality compared to either single mutant (P<0.0001). These results suggest that SLX-1 and HIM-6 function in parallel or alternate pathways, similar to budding yeast [6] and flies [20]. Finally, we examined the genetic interactions between slx-1 and the structure-specific endonucleases xpf-1 and gen-1, which are a component of the HIM-18 complex and a Holliday junction resolving enzyme, respectively [16], [21]. slx-1;xpf-1 double mutants showed a 2.4-fold increase (P<0.0001) in embryonic lethality and a 3.6-fold increase in larval arrest (P = 0.0029) compared with xpf-1 single mutants. These results suggest that SLX-1 and XPF-1 may have overlapping roles during mitotic development. Interestingly, slx-1;xpf-1;him-18 triple mutants exhibited similar phenotypes to xpf-1;him-18 double mutants, supporting our observation that the nuclease activity of SLX-1 is dependent on HIM-18 and therefore an slx-1 mutation causes no obvious plate phenotypes in an xpf-1;him-18 background. Analysis of slx-1;gen-1 double mutants revealed a 69% reduction in brood size (P<0.0001) and a 5.5-fold increase in larval arrest (P = 0.0079) compared with slx-1 single mutants. These results suggest that SLX-1 and GEN-1 share similar, albeit independent, mitotic roles at least during larval development.
Tab. 1. Plate phenotypes. 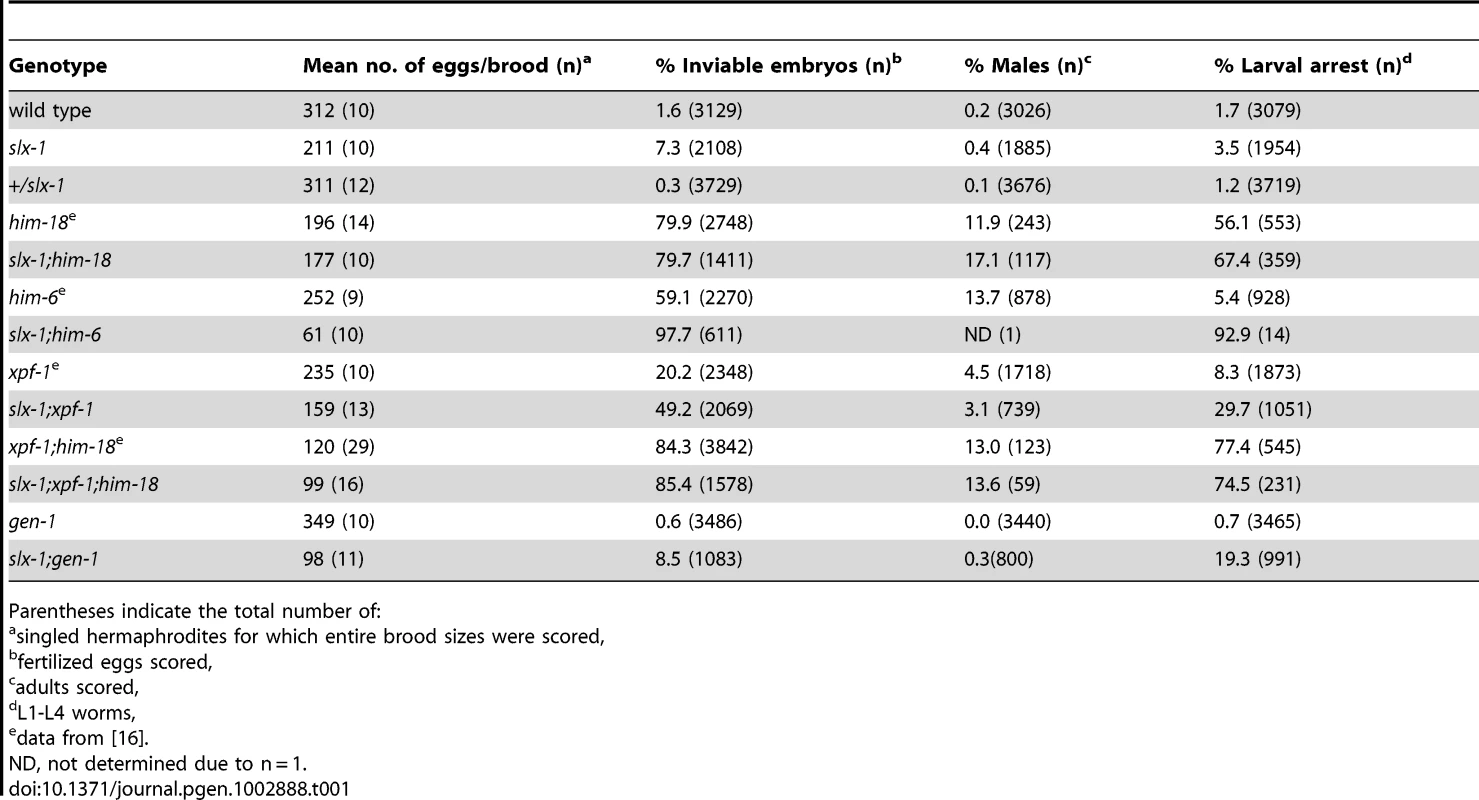
Parentheses indicate the total number of: Recombination intermediates accumulate in slx-1(tm2644) mutants
Homologous recombination provides for the repair of both spontaneous DSBs, stemming from stalled or collapsed replication forks at S phase, and programmed DSBs, produced by SPO-11 during prophase of meiosis I. The organization of nuclei in a temporal and spatial gradient in the C. elegans germline facilitates the identification and analysis of specific stages of both mitotic and meiotic nuclei. Specifically, nuclei at the distal tip end are undergoing mitotic proliferation (zones 1 and 2), nuclei at the transition zone are in the leptotene/zygotene stages of meiosis (zone 3), followed by nuclei in early pachytene (zone 4), mid pachytene (zone 5) and late pachytene (zones 6 and 7) (Figure 4A). To investigate whether SLX-1 is required for the maintenance of genomic integrity in the C. elegans germline, we monitored the levels as well as the kinetics of appearance and disappearance of RAD-51, a protein involved in strand invasion/exchange during DSB repair (Sung, 1994; Colaiacovo et al. 2003). Quantification of RAD-51 foci revealed that these were elevated in both mitotic (zones 1 and 2) and meiotic (zones 3, 6 and 7) nuclei in slx-1 mutants compared to wild type (Figure 4A and Figure S2). Moreover, the mitotic RAD-51 foci persist through late meiotic prophase (late pachytene stage; zone 6) as observed in slx-1;spo-11 double mutants which lack the formation of programmed meiotic DSBs (Figure 4A and Figure S2). However, simple subtraction of the number of RAD-51 foci in slx-1; spo-11 from the number observed in slx-1, as a means of approximating the dynamics of SPO-11-dependent DSBs, also reveals an increase of meiotic recombination intermediates during late pachytene in slx-1 mutants. Further support for a role for SLX-1 in germline DNA repair stems from our analysis of germ cell apoptosis, which was increased 2.3-fold in slx-1 mutants compared to wild type (Figure 4B). Increased germ cell apoptosis was previously shown to occur when an inability to repair DNA damage results in the activation of a DNA damage checkpoint in late pachytene [22]. Taken together, these results suggest that SLX-1 is required for the proper repair of both stalled/collapsed replication forks and meiotic DSBs.
Fig. 4. SLX-1 and HIM-18 are required for both mitotic and meiotic DNA repair. 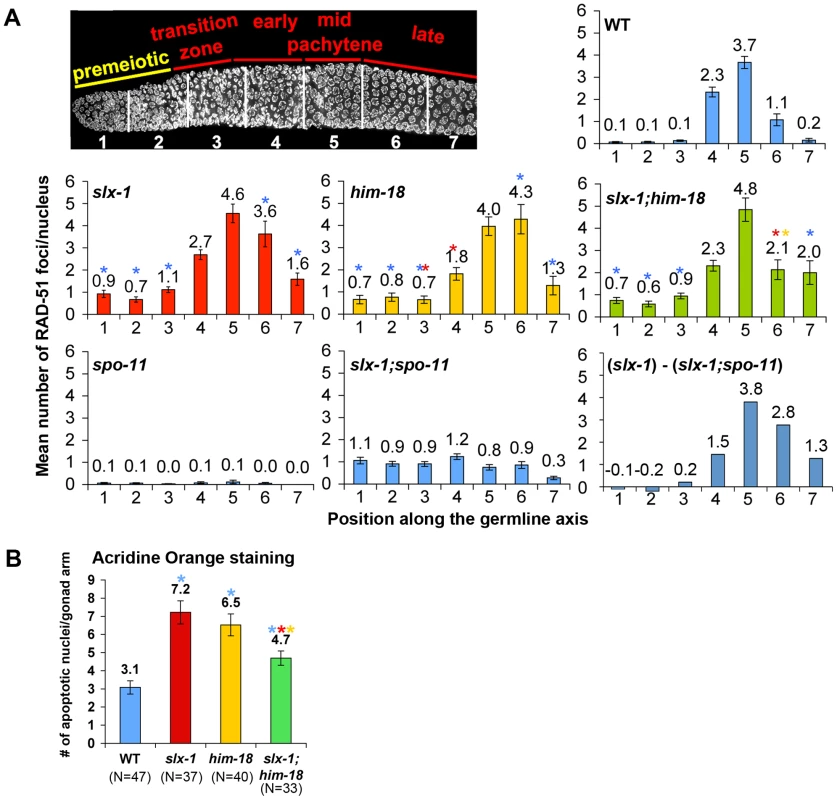
(A) Mean number of RAD-51 foci per nucleus. Histograms depict the quantitation of RAD-51foci in germlines of the indicated genotypes. Quantitative analysis of RAD-51 foci depicted in Figure S3, is represented here as the mean number of RAD-51 foci observed per nucleus (y-axis) on each zone along the germline axis (x-axis) for all indicated genotypes. Error bars represent standard error of the mean. To manifest the levels of meiotic RAD-51 foci, mean number of RAD-51 foci in slx-1;spo-11 double mutants at each zone was subtracted from slx-1 mutants ((slx-1) - (slx-1;spo-11)). Colored asterisks indicate statistical significance between different genotypes (wild type, slx-1, him-18 and slx-1;him-18). (B) Quantitation of germline apoptosis. Apoptotic corpses stained by acridine orange were scored. N = number of gonad arms scored for each genotype. Colored asterisks indicate statistical significance between different genotypes. SLX-1 is required for replication-coupled DNA repair
To further investigate which kinds of DNA damage are repaired by SLX-1-HIM-18 in vivo, we performed a series of DNA damage sensitivity assays by exposing slx-1 mutants to γ-irradiation, which produces DSBs, nitrogen mustard (HN2), which produces DNA inter-strand crosslinks, camptothecin (CPT), which results in single-strand nicks, and UVC, which causes cyclobutane pyrimidine dimers (Figure 5).
Fig. 5. slx-1 mutants exhibit hypersensitivity to nitrogen mustard, camptothecin, and ultraviolet C, but not ionizing radiation. 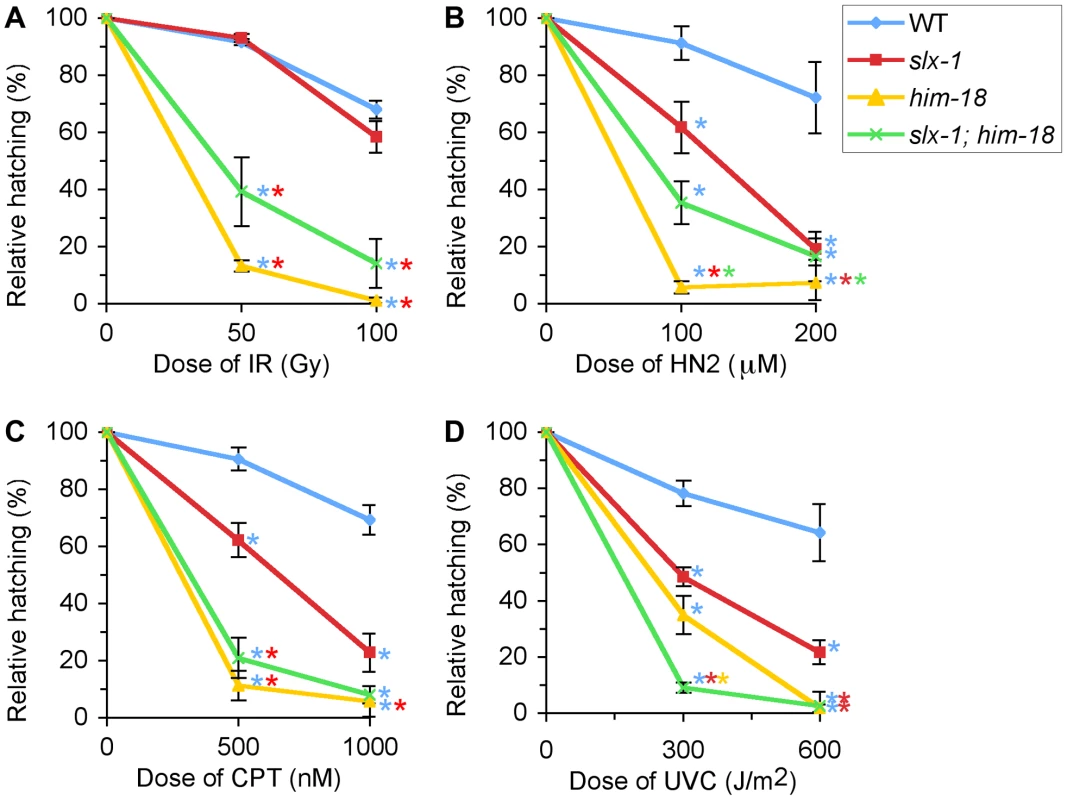
Relative hatching frequencies detected in wild type, slx-1, him-18 and slx-1;him-18 mutants after treatment with the indicated doses of (A) ionizing radiation (IR), (B) nitrogen mustard (HN2), (C) camptothecin (CPT), and (D) ultraviolet C (UVC). Hatching is plotted as a fraction of the hatching observed in untreated animals. Error bars indicate standard error of the mean for ∼20 animals in each of three independent experiments. Colored asterisks indicate statistical significance between different genotypes. After treatment with g-irradiation, no statistically significant, reduction in hatching ratio was observed in slx-1 mutants compared to wild type (P = 0.5512 and P = 0.1455, respectively at 50 and 100 Gy) (Figure 5A). In contrast, hatching ratios were significantly reduced in him-18 (P<0.0001 and P<0.0001) and slx-1;him-18 (P = 0.0004 and P = 0.0002) double mutants after doses of either 50 or 100 Gy of irradiation (Figure 5A). These results suggest that HIM-18 plays a role in the repair of exogenously induced DSBs, which is independent of the nuclease activity of SLX-1.
Exposure to HN2 revealed that slx-1 and slx-1;him-18 mutants share similar hypersensitivity to HN2 compared to wild type at both 100 µM and 200 µM, while him-18 mutants showed more severe hypersensitivity compared to slx-1 and slx-1;him-18 mutants (Figure 5B). These results could be explained by the fact that SLX-1 function is HIM-18-dependent. Therefore, in him-18 mutants ICL repair may be further affected by the presence of inactive SLX-1.
Exposure to CPT revealed hypersensitivity among slx-1 mutants compared to wild type at both 500 nM and 1000 nM doses (Figure 5C). Moreover, him-18 and slx-1;him-18 mutants showed more severe hypersensitivity compared to slx-1 mutants. Since CPT inhibits the removal of topoisomerase I, thereby forming nicked sites after replication [23], these results suggest that SLX-1 is either required for efficient removal of the TOP1-CPT complex or resolution of a HJ intermediate during the re-establishment of a replication fork. Extrapolating from observations made in other species [1], [5], loss of HIM-18 may reduce the nuclease activity of SLX-1, MUS-81 and potentially XPF-1. This would explain why him-18 and slx-1;him-18 mutants show similar hypersensitivity to CPT.
Notably, slx-1, him-18 and slx-1;him-18 mutants showed hypersensitivity to UV (Figure 5D), although the loss of either SLX1 or SLX4 orthologs does not result in hypersensitivity in budding yeast [8], fission yeast [2], flies [24], mouse [13] and human cells [3], [5]. Whereas mutants in several other DNA repair genes such as mus81 and sgs1 in S. cerevisiae [6], [25] have exhibited UV sensitivity, but have no proven direct role in nucleotide excision repair (NER), one can not discard the possibility that in C. elegans, unlike in other species, SLX-1 and HIM-18 may be required for NER.
SLX-1 does not regulate either the levels or the distribution of meiotic DSBs
To investigate whether the higher levels of RAD-51 foci are due to either an increased number of DSBs or a delay of the repair process in slx-1 mutants, we quantified DSB levels by RAD-51 immunostaining in rad-54 mutants, in which DSB repair is blocked and DSB-bound RAD-51 is proposed to be trapped in the germline [26]. In wild type, RAD-51 foci start to increase in nuclei at the entrance into meiosis (zone 3), peak at 3.7 foci/nucleus at mid pachytene (zone 5) and are practically all gone by late pachytene (zone 7) (Figure 4A, Figure 6B and Table S2). In contrast, in rad-54 mutants, higher levels of mitotic RAD-51 foci were observed (0.8 and 1.0 compared to 0.1 at zones 1 and 2, P<0.0001, respectively), and meiotic RAD-51 foci peaked at 75 foci/nucleus at diplotene (−7 oocyte), only being completely absent by late-diakinesis (−1 oocyte) (Figure 6A–6B and Table S2). These three observations: 1) elevated mitotic RAD-51 foci; 2) peak of RAD-51 foci at diplotene; 3) RAD-51 foci only being completely lost by late diakinesis, are different from those described in [26]. They concluded that RAD-51 foci peak at 12/nucleus at early, mid and late pachytene stages in rad-54 mutants. However, both our studies as well as those of others have since revealed a higher number of RAD-51 foci in this mutant background (18–30 and 26–63 foci at mid and late pachytene, respectively [27], [28], this current study). Further support for the number of DSBs we observed in the rad-54 background stems from our analysis of the level of RPA-1-YFP foci in brc-2 mutants, in which DSB repair is blocked and replacement of RPA-1 (replication protein A) by RAD-51 at the resected DSB ends is inhibited in the germline [29] (Figure S4). We observed a similar number of RPA-1-YFP foci (59.1) in brc-2 mutants to that of RAD-51 foci (62.9) in rad-54 mutants in late pachytene nuclei (zone 7). Importantly, we confirmed that the elevated levels of RAD-51 foci we observe in rad-54 mutants at mid and late pachytene, where the events of repair we are focused on take place, are not already at a possible maximum thus obscuring our ability to utilize this mutant background to identify further increases in DSB levels. Specifically, following the formation of additional DSBs by γ-irradiation in rad-54 mutants, we observed 45 foci at 50 Gy compared to 29 foci at 0 Gy in zone 5 (P<0.0001) (Figure S3). slx-1rad-54 double mutants exhibited levels of RAD-51 foci similar to those observed in rad-54 mutants, although RAD-51 foci levels accumulated with slightly faster kinetics than in rad-54 mutants. Furthermore, it is known that elevated levels of meiotic DSBs rescue him-17 mutants, which are deficient in meiotic DSB formation [30], [31]. slx-1;him-17 double mutants did not rescue the him-17 mutant phenotype (Figure 6C–6D). Taken together, these results suggest that the total levels of DSBs are wild type in slx-1 mutants.
Fig. 6. SLX-1 does not regulate either the levels or the distribution of DSBs. 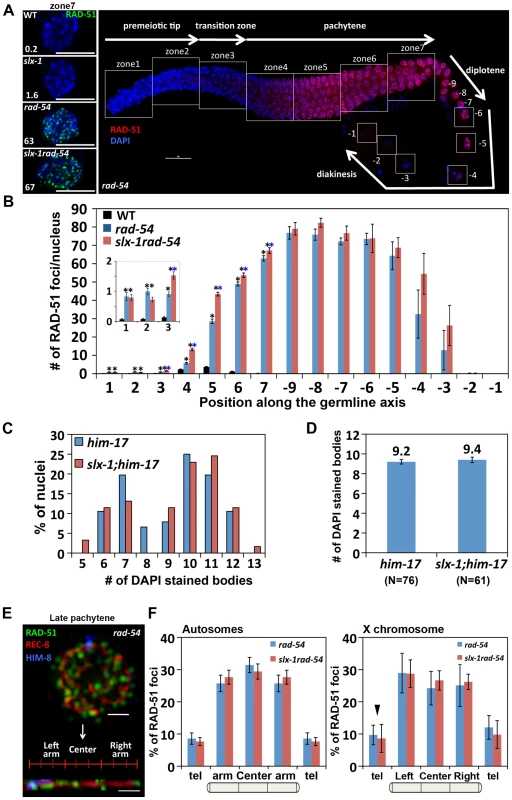
(A) Panels on the left are high magnification images of late pachytene nuclei (zone 7) immunostained with RAD-51 in the indicated genotypes. The average number of RAD-51 foci is shown at the bottom left. Bars, 5 µm. The panel on the right shows a typical immunostaining pattern for RAD-51 in whole mounted gonads of rad-54 mutants. The white squares indicate the seven zones and the diakinesis oocytes (−9 through −1) scored in the analysis. (B) Quantitation of the number of RAD-51 foci in wild type, rad-54 and slx-1rad-54 mutants. Error bars indicate standard error. Colored asterisks indicate statistical significance between different genotypes. (C) Quantitation of DAPI-stained bodies at late diakinesis (−1 and −2 oocytes) in six time outcrossed him-17(ok424) and slx-1;him-17(ok424) mutants. (D) Average number of DAPI-stained bodies in him-17(ok424) and slx-1;him-17(ok424) mutants. Bars indicate standard error. (E) A representative image of a late pachytene nucleus (zone 7) co-immunostained with RAD-51, REC-8 and HIM-8 antibodies and a computationally straightened chromosome axis (bottom) in rad-54 mutants. (F) Distribution of RAD-51 foci along chromosome axes in rad-54 and slx-1rad-54 mutants. RAD-51 foci localization on randomly selected autosomes are quantified in the left panel. The values of RAD-51 distribution on the left and the right arms are averaged as “arm” on the autosomes. The values at the left and the right telomeres are also averaged as “tel” on the autosomes. The distribution of RAD-51 foci on the X chromosome is shown in the panel at the right. Arrowhead indicates pairing center where HIM-8 localizes. Bars indicate standard error. In C. elegans, as in many other species, crossover formation is biased towards the terminal thirds of autosomes [32], [33]. To measure the levels and distribution of DSBs along chromosomes, and determine whether they are altered in slx-1 mutants, we performed three-dimensional traces of chromosomes in late pachytene nuclei by visualizing chromosome axes with an antibody to the meiotic cohesin REC-8 and quantified the levels and distribution of recombination intermediates along these chromosome axes with a RAD-51 antibody, comparing rad-54 and slx-1 rad-54 double mutants (Figure 6E–6F). To distinguish the X chromosome from the autosomes, we identified the X chromosome pairing center end with an anti-HIM-8 antibody [34]. We did not detect a biased distribution of RAD-51 foci along either the arms or the central region of the chromosomes in rad-54 mutants. Therefore, this even distribution of DSBs along the lengths of the chromosomes suggests the existence of mechanisms that inhibit crossover formation after the induction of DSBs at the central region of the chromosomes. Interestingly, both the levels and distribution of RAD-51 foci along chromosome axes were similar between rad-54 and slx-1 rad-54 mutants in both autosomes and the X chromosomes (Figure 6F). These results suggest that SLX-1 does not alter either the overall levels or the distribution of DSBs along either the X chromosomes or the autosomes.
SLX-1 is required for the normal crossover landscape
To investigate whether SLX-1 and HIM-18 are required for meiotic crossover formation, we first observed crossover frequencies in slx-1, him-18 and slx-1;him-18 mutants on both chromosomes IV and X by using the snip-SNP method [16], [35]. Crossover frequencies were not significantly different between wild type and slx-1 mutants on either chromosome (Figure 7A and 7B). However, reduced crossover frequencies were detected in slx-1;him-18 double mutants compared to him-18 single mutants. These data coincide with the observation of a lack of a Him phenotype among slx-1 mutants, whereas slx-1;him-18 mutants show a more severe Him phenotype compared to him-18 single mutants (Table 1). Taken together, these results suggest that SLX-1 is not required to make interhomolog crossovers in normal meiosis, but is partially required on both autosomes and X chromosomes in a him-18 background.
Fig. 7. SLX-1 promotes the regulation of crossover distribution but not crossover frequency. 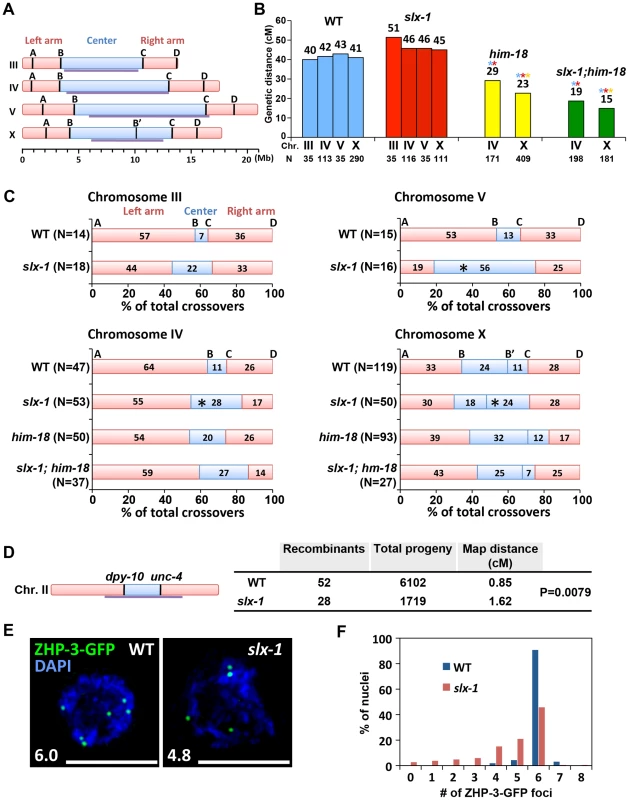
(A) Schematic representation of chromosome domains. Four SNP markers for chromosome III, IV and V and five SNP markers for the X chromosome are shown as A, B, B', C and D. The intervals A–B, B–C and C–D indicate portions of the left arm, center, and right arm, respectively. Purple lines indicate the central domain on which crossover frequency is lower compared to the arms defined by Rockman and Kruglyak [79]. (B) Analysis of crossover frequencies on chromosomes III, IV, V and X in wild type, slx-1, him-18 and slx-1;him-18 mutants. Crossover frequencies are measured between SNP positions A to D. N = number of crossprogeny scored. (C) Analysis of crossover distribution on chromosomes III, IV, V and X on the indicated genotypes. The positions on the chromosomes indicated as left, center and right correspond to intervals A–B, B–C and C–D, respectively as indicated on panel A. Asterisks indicate significant differences compared to wild type. (D) Crossover frequency of wild type and slx-1 mutants between dpy-10 and unc-4 loci on center region of chromosome III. (E) Representative images of ZHP-3-GFP foci in late pachytene nuclei (zone 7) in wild type and slx-1 mutants. The average number of ZHP-3-GFP foci is shown at the bottom left. Bars, 5 µm. (F) Quantitation of ZHP-3-GFP foci in late pachytene nuclei in wild type and slx-1 mutants. Meiotic DSBs, with the exception of the subset designated to be repaired as future interhomolog crossovers, are repaired either by interhomolog noncrossover or intersister pathways [36], [37]. We examined intersister repair by monitoring chromosome morphology in diakinesis oocytes of syp-2 and rec-8 mutants, where either interhomolog interactions or sister chromatid cohesion are impaired, respectively [38]–[40]. We did not observe any additive cytological defects in both slx-1;syp-2 and slx-1;rec-8 double mutants compared with syp-2 or rec-8 single mutants (Figure S5). While these results suggest that SLX-1 may not function during intersister repair, confirmation awaits additional analysis of sister chromatid separation at anaphase where either lagging chromosomes or chromosome bridges might be detected if there are defects in the intersister resolution of dHJs.
We next used the snip-SNP method to assess crossover distribution along chromosomes III, IV, V and X. In slx-1 mutants, the frequency of crossovers detected in the center (intervals B–C) of the autosomes (III, IV and V) is higher (3.1-, 4.2 - and 2.7-fold increases, respectively) than in wild type, whereas it is reduced in the arm regions (intervals A–B and C–D) (Figure 7C and Table S3). Using a pair of morphological markers we further confirmed the occurrence of a higher crossover frequency at the central region of chromosome II in slx-1 mutants compared to wild type (Figure 7D). Crossover distribution along the X chromosome is also different between wild type and slx-1 mutants. At the right portion of the central region of the X chromosome (B'–C), the frequency of crossovers in slx-1 is higher (2.2-fold increase, P = 0.0348) than in wild type. These results suggest that SLX-1 is required for proper crossover distribution along both autosomes and the X chromosome. To further examine the role of SLX-1 in crossover distribution regulation, we measured crossover distribution on chromosomes IV and X in him-18 and slx-1;him-18 double mutants. Crossover levels are increased at the center of chromosome IV in both him-18 and slx-1;him-18 double mutants compared with wild type (1.9 - and 2.5-fold increases, respectively). Moreover, while the observed increases are similar between slx-1 and slx-1;him-18, only a moderate increase is detected in him-18 mutants on chromosome IV (Figure 7C and Table S3). Given that SLX-1 is nearly catalytically dead with regard to its nuclease activity in the absence of HIM-18 in vitro (Figure 1D–1E), the nuclease activity of SLX-1 may not work in him-18 mutants in vivo. Instead, it is possible that other functions of SLX-1, for example the PHD finger-dependent recognition of epigenetic marks, might still work in him-18 mutants. Taken together, these results suggest that the nuclease activity of SLX-1 may be important to maintain proper crossover distribution.
It has been proposed that only one of the various DSB sites along a chromosome is specifically designated as a future interhomolog crossover site [41]. ZHP-3, a homolog of S. cerevisiae Zip3 protein, has been proposed to mark crossover precursor sites during late pachytene stage in C. elegans [16], [42]. To investigate whether crossover designation properly occurs in slx-1 mutants, we compared the numbers of ZHP-3-GFP foci present in pachytene nuclei in wild type and slx-1 mutants. The average number of ZHP-3-GFP foci observed in slx-1 mutants is 80% of those in wild type (Figure 7E). Specifically, while nearly 90% of late pachytene nuclei contain six ZHP-3 foci in wild type, only 45% of nuclei contain six and 55% have less than five ZHP-3-GFP foci in slx-1 mutants (Figure 7F). Given that crossover levels are indistinguishable between wild type and slx-1 mutants, these results suggest that a crossover pathway that is not associated with ZHP-3 foci exists in slx-1 mutants. Alternatively, it could be possible that SLX-1 is required for proper crossover designation.
Discussion
SLX-1 has a structure-specific endonuclease activity
In budding yeast, the substrate preference observed for recombinant Slx1-Slx4 is 5′-flaps>Y forks>RFs>mobile HJs>3′-flaps>fixed HJs (the latter involves an asymmetric cut given the non-ligatable processed substrate that is then detected) (Flott and Brill 2003). In fission yeast, the Slx1 immunoprecipitation product cuts stem loops and HJs (symmetric cut) [2]. Finally, in humans, SLX1/SLX4 exhibits preference for 5′flaps and HJs>RFs>3′-flaps [3], [5].
In this current study, we determined that in C. elegans, the preference of SLX-1-HIM-18 is for RFs>5′-flaps>HJs>3′-flaps (Figure 1F–1G and Figure 2D). The slight difference observed in the order of substrate preference in C. elegans compared to those in yeast and humans is thought to originate from either the difference of the growth temperature or the difference of the length of the N-terminal domain of SLX-1 (168 amino acids compared to 9 amino acids in both the yeast and human orthologs) (Figure 1A). Future analysis may require performing the in vitro nuclease assay at 20°C, which is the optimum temperature for growth of C. elegans, and using an N-terminal truncated SLX-1. Similar to other organisms, the nuclease activity of SLX-1 depends on HIM-18 (Figure 1D–1E). This endonuclease activity potentially affects the homologous recombination machinery during ICL-repair, break-induced replication and NER. During prophase of meiosis I, homologous recombination occurs between homologous chromosomes. Double Holliday junction resolution is important for crossover formation, however the HJ resolvase activity of the SLX-1-HIM-18 complex is not required for meiotic crossover formation (Figure 7B). Potentially, other structure-specific endonucleases such as XPF-1, which interacts with HIM-18 [16], MUS-81 and GEN-1 [21], may act coordinately to resolve dHJs during meiotic recombination.
The mitotic roles of SLX-1 in various DNA damage repair pathways
We showed that slx-1 mutants were hypersensitive to several kinds of DNA damaging agents (Figure 4C and Figure 8A). Notably, both slx-1 and him-18 mutants showed hypersensitivity to UV. This phenotype is different from that observed in budding yeast, fission yeast, flies, mice and humans [2], [3], [5], [8], [13], [24]. SLX-1 has a GIY-YIG nuclease domain also present in UvrC in E. coli where it is important for making an incision 3′ to the damage site (cyclobutane pyrimidine dimer, CPD) during the NER process [43], [44]. Additionally, the N-terminal domain of C. elegans SLX-1 is longer than that of its homologs in other organisms, so it is possible that the N-terminal region confers the NER function of SLX-1. XPF is largely known as a repair factor for the NER pathway, including in C. elegans [45], [46]. XPF exists in two types of complexes in human cells, one is a 2M Dalton complex containing SLX1, SLX4, MUS81, EME1 and ERCC1, the other is the XPF-ERCC1 heterodimer [4]. Therefore, it remains to be determined whether XPF-1, SLX-1 and HIM-18 make single or heterologous complexes during different DNA damage responses or at different stages of the cell cycle.
Fig. 8. The levels of H2AK5ac are similar between wild type and slx-1 mutants. 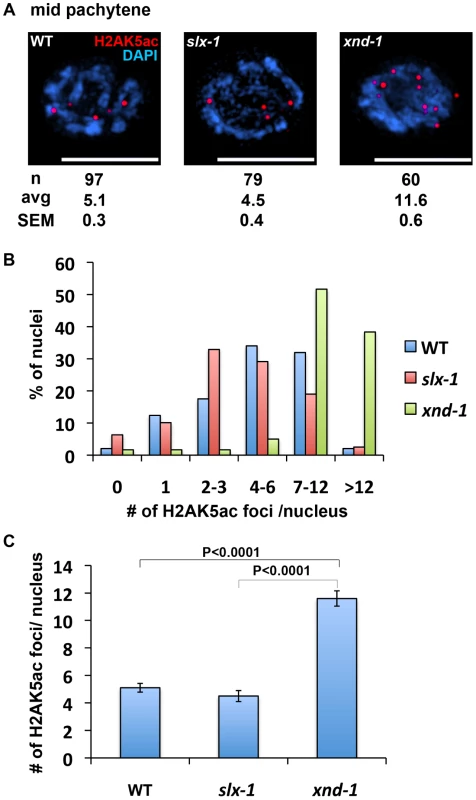
(A) Representative images of mid-pachytene nuclei stained with anti H2AK5ac in wild type, slx-1 and xnd-1 mutants. Bars, 5 µm. (B) Quantitation of H2AK5ac foci at mid pachytene in wild type, slx-1 and xnd-1 mutants. (C) Average number of H2AK5ac foci/nucleus in wild type, slx-1 and xnd-1 mutants. Bars indicate standard error. It is thought that the dual incisions surrounding an interstrand crosslink are performed by MUS81, XPF or FAN1 [47]–[52]. In this study, we raise the possibility that SLX-1 might have the activity required for the incision based on the HN2 hypersensitivity observed in slx-1 mutants (Figure 5B and Figure 8A). Surprisingly, the mutation in slx-1 partially suppressed the HN2 induced DNA damage sensitivity observed in him-18 mutants (Figure 5B). It has been reported that SLX1 represses the nuclease activity towards RFs and 3′-flaps of MUS81 and XPF in human cells [4]. Therefore, in the him-18 mutant, SLX-1 might repress the incision activity of MUS81 and XPF or other nucleases such as FAN1. Given that HIM-18 carries sites potentially recognized by kinases and ubiquitin/SUMO conjugating enzymes [16], that both human and yeast Slx4 are phosphorylated by ATM/ATR [53], and that human SLX4 is phosphorylated by PLK1 [5], it is possible that post-translational modifications of HIM-18 might modulate its ability to regulate the nuclease activity of the components of the HIM-18 complex either directly or indirectly. In addition to HN2 hypersensitivity, both accumulation of RAD-51 foci in late pachytene (zone 6) and germ cell apoptosis in him-18 mutants are partially rescued by the slx-1 mutation (Figure 4A and 4B). One possible explanation is that SLX-1 might be deregulated in the absence of HIM-18 in these cases. In him-18 mutants, inactive SLX-1 might inhibit these repair pathways. In slx-1;him-18 double mutants, since there is no inhibition by SLX-1, the him-18 phenotype is partially suppressed. Further studies will reveal the regulation of the HIM-18 complex in each DNA repair pathway.
Catalytic subunits of a HJ resolvase for meiotic crossover formation
We showed that SLX-1 is not required for wild type levels of crossover formation during meiotic recombination (Figure 7B). It is believed that there are two possible pathways that can lead to crossover formation, one is double Holliday junction resolution [54] and the other is a “nick/counternick” mechanism [55]. A possible explanation for why crossover frequency is not changed in slx-1 mutants is that SLX-1 and other structure specific nucleases, such as GEN-1, MUS-81 and XPF-1, are partially redundant and can compensate for each other with regards to the activity of crossover formation. This is supported in part by the observations that gen-1 mutants are fertile [21] (Table 1), mus-81 mutants do not enhance X chromosome non-disjunction [16] and xpf-1 mutants exhibit only a mild reduction in crossover levels [16]. Moreover, slx-1 enhances the developmental defects observed in xpf-1 and gen-1 mutants, supporting the idea that functions of SLX-1 are partially redundant with those of GEN-1 and XPF-1 (Table 1). Furthermore, a recent study suggests that MUS81, SLX4 and GEN1 can compensate for lack of the BLM helicase in human cells by resolving HJs in somatic Bloom's syndrome cells [56]. Therefore, further investigation of the genetic interactions between these structure-specific endonucleases may reveal whether there are redundancies for the activities of Holliday junction resolution during meiotic recombination. Another aspect to consider is that excess crossovers generated in wild type following X-ray exposure are dependent on MUS-81 in C. elegans, although MUS-81 is not required for physiological crossover formation during meiosis [57]. mus-81 and slx-1 mutants share a couple of phenotypes that are not observed in either xpf-1 or gen-1 mutants, such as the elevated levels of RAD-51 foci observed during mitotic proliferation (Figure 4A and Figure S2) [16] and the synthetic lethality with him-6 (Table 1) (Saito et al., unpublished results). Therefore, it remains to be determined whether SLX-1 is also required for crossover formation under an excess of DSBs resulting from IR treatment in a manner similar to mus-81 mutants.
SLX-1 might function as either a noncrossover-specific resolvase of double Holliday junctions or an epigenetic reader during meiosis
How is crossover distribution regulated in C. elegans meiosis? Recently, it was reported that crossover distribution is shifted from the arms to the center of chromosomes in xnd-1 mutants, a phenotype reminiscent to that we observed in our current analysis of slx-1 mutants [58]. Hyperacetylation of histone H2A lysine 5 is one of the features of the xnd-1 mutants. However, the acetylation levels of H2AK5 are similar to those of wild type in slx-1 mutants (Figure 7). Therefore, the hyperacetylation of H2AK5 is not the cause of the change of crossover distribution in slx-1 mutants.
Based on the analysis of both DSB and crossover distribution in slx-1 mutants, we propose a model in which SLX-1 inhibits crossover formation at the center of the chromosomes during meiotic recombination (Figure 9B). While crossover formation is biased to the arms in wild type, surprisingly we found that DSBs are more evenly distributed along chromosome axes in wild type. Only one of the DSBs, presumably the one marked by ZHP-3, is converted into an interhomolog crossover at one of the arm regions. All other DSBs are repaired either by intersister repair or interhomolog noncrossover formation. Interestingly, the number of ZHP-3 foci is reduced to 80% of wild type levels in slx-1 mutants, and nevertheless the total number of crossovers is similar between wild type and slx-1 mutants (Figure 7B, 7E, 7F). These data suggest that there is a pathway not associated with ZHP-3 foci to make a crossover in slx-1 mutants. Whether this pathway leads to crossover formation at the center region of the chromosomes and whether these ZHP-3 foci-independent crossovers depend on MUS-81, which is known to make ZHP-3 foci-independent crossovers when there is an excess of DSBs [57], are issues that remain to be solved.
Fig. 9. A model for how SLX-1 functions as a structure-specific endonuclease in various genome maintenance pathways. 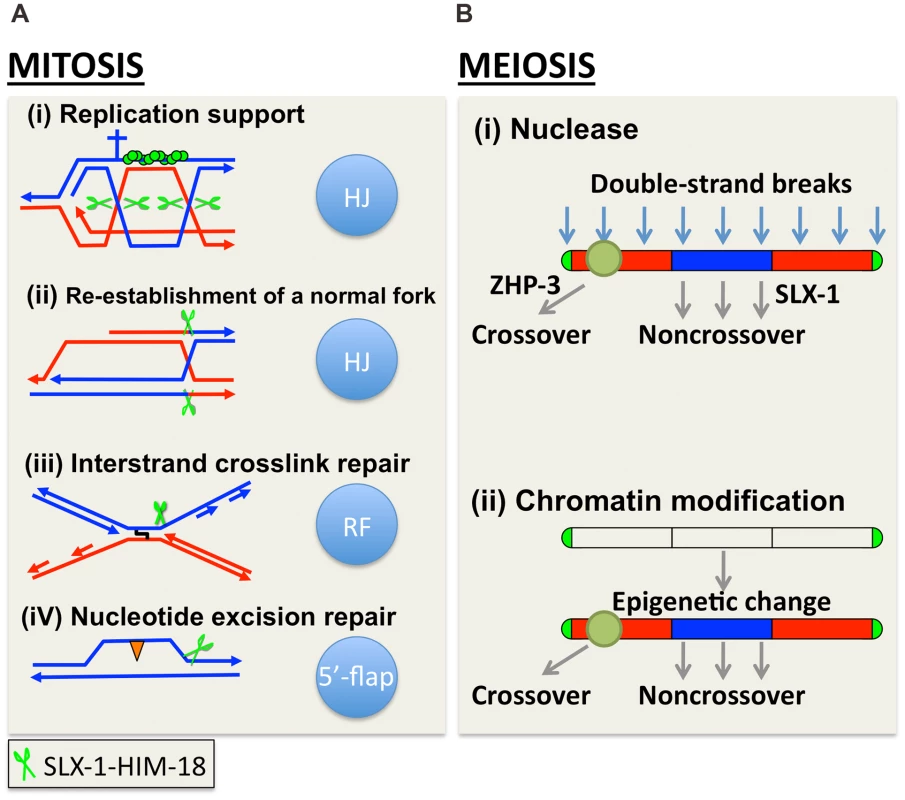
(A) Mitotic role of SLX-1. DNA repair pathways and the nuclease activity of SLX-1 are indicated. The HIM-18-dependent nuclease activity of SLX-1 is required for replication-coupled DNA repair such as during the re-establishment of a normal fork, DNA interstrand crosslink (ICL) repair, and nucleotide excision repair (NER). (A-i) The SLX-1-HIM-18 complex supports the RAD-51-mediated (green circles) repair pathway employed at replication forks. Specifically, SLX-1-HIM-18 resolves Holliday junctions when replication forks stall or collapse during S phase. Green scissors represent the SLX-1-HIM-18 nuclease complex. The cross represents a replication block. (A-ii) HIM-18-SLX-1 resolves HJs during re-establishment of a normal replication fork when CPT-induced single-ended DSBs are generated. (A-iii) The SLX-1-HIM-18 complex induces an incision at DNA ICLs when replication forks collide at an ICL. (A-iv) SLX-1-HIM-18 repairs UV-induced CPDs (orange triangles) via the replication coupled repair and NER pathways. Although it is thought that XPF and XPG make the 5′ and 3′ incisions, respectively, flanking the CPD lesion during the NER pathway in other organisms, there may be a possibility that SLX-1 also makes the 3′ incision, which is reminiscent of its 5′-flap cleavage activity. (B) SLX-1 is required for proper crossover distribution during meiotic recombination. Two alternative models are presented. (B-i) SLX-1 is a noncrossover specific Holliday junction resolvase. One of the evenly distributed DSBs are selected as a crossover precursor at one of the arm regions and marked by ZHP-3. DSBs located at the center of the chromosomes are repaired as noncrossovers via double Holliday junction resolution, synthesis-dependent strand annealing (SDSA) or intersister repair. SLX-1 might convert a dHJ into a noncrossover product by same sense dHJ resolution at the mid-section of chromosomes. (B-ii) Although the function of the PHD finger of SLX-1 is unknown, it is possible that a SLX-1-dependent modification or recognition of chromosome domains contributes to the regulation of crossover distribution. In yeast, the crossover/noncrossover decision is made very early, prior to or during the formation of stable single-end invasion (SEI) intermediates, and therefore earlier than dHJ formation [59]. Once a dHJ is formed, it is usually converted into a crossover in yeast meiosis. However, it is not known whether this tendency is conserved in other organisms. We hypothesize that DSBs introduced at the mid-section of chromosomes are converted into a noncrossover product either via a synthesis-dependent strand annealing (SDSA) pathway or via symmetric resolution of a dHJ in wild type C. elegans. Based on the HJ cleavage activities we observed for SLX-1, SLX-l might have a role in converting a dHJ into a noncrossover product via same sense resolution of a dHJ arising at the mid section of chromosomes during meiotic recombination (Figure 9B).
In addition to its nuclease domain, SLX-1 has a PHD finger. This type of domain is largely known to be involved in chromatin remodeling, transcriptional control and ubiquitin/SUMO E3 ligase activity. Chromosome arms, where crossovers happen at a higher frequency, are marked by methylated histone H3 lysine 9 (H3K9me) during early embryogenesis and the L3 larval stage in C. elegans [60], [61]. However, it is not yet known whether this kind of epigenetic mark is also observed during meiotic recombination or whether other kinds of epigenetic marks delimitate the chromosome arms and the central region in C. elegans. One possibility is that the PHD finger of SLX-1 is involved in epigenetic change/read and somehow divides the arms and central regions along chromosomes (Figure 9B). It will be important to investigate what kinds of epigenetic marks may be read by the PHD finger of SLX-1, and how this may regulate crossovers.
Taken together, our analysis indicates that SLX-1 is required for several kinds of mitotic DNA repair pathways and reveals a role for this protein in the regulation of meiotic crossover distribution thereby promoting the maintenance of genomic integrity. Importantly, our study leads us to propose a model in which SLX-1 functions as a noncrossover promoting factor at the crossover cold regions during meiotic recombination.
Materials and Methods
C. elegans genetics
C. elegans strains were cultured at 20°C under standard conditions [32]. The N2 Bristol strain was used as the wild-type background. The following mutations and chromosome rearrangements were used in this study: LGI: slx-1(tm2644), rad-54(ok615), hT2[bli-4(e937) let-?(q782) qIs48](I; III); LGII: xpf-1(e1487), dpy-10(e128), unc-4(e120), mIn1[dpy-10(e128) mIs14](II); LGIII: him-18(tm2181), brc-2(tm1086), xnd-1(ok709), qC1[dpy-19(e1259) glp-1(q339) qIs26] (III); LGIV: Ppie-1::zhp-3::gfp, spo-11(ok79), him-6(ok412), rec-8(ok978), nT1[unc-?(n754) let-? qIs50] (IV; V), nT1[qIs51] (IV; V); LGV: him-17(ok424), syp-2(ok307) [16], [19], [32], [42], [62]–[67]. Transgenes: opIs263 [Prpa-1::RPA-1-YFP::3′-UTR] [68].
The slx-1 allele, tm2644, is predicted to encode for a catalytically inactive (nuclease-negative) protein. We also tried to knockdown slx-1 both by RNAi and by generating slx-1(tm2644)/Df trans-heterozygotes. RNAi utilizing feeding clones generated from either SLX-1 cDNA (Vidal ORFeome library; [69], [70]) or genomic DNA (Ahringer RNAi library; [71], [72]), did not result in depletion in either wild type or slx-1(tm2644) mutant backgrounds. Attempts to generate a slx-1/Df trans-heterozygote for classical genetic characterization of the slx-1(tm2644) allele were hindered because the only available deficiency encompassing that gene, sDf4, involves a free chromosome duplication that interferes with this analysis.
Plasmids, cell culture, and antibodies
HIM-18 and SLX-1 open reading frames in either pENTR or pDONR derivatives were transferred to the indicated expression vectors (pDEST-myc or pDEST-HA) using the Gateway cloning system (Invitrogen) and sequence validated. 293T cells were grown in Dulbecco Modified Eagle medium (DMEM) supplemented with 10% (v/v) FBS (Invitrogen), 100 units of penicillin per ml, and 0.1 mg streptomycin per ml. Antibodies against HA (16B12; Covance) and Myc (9E10; Santa Cruz) epitopes were utilized.
Protein interaction analysis and in vitro cleavage assays
For protein interaction studies, the indicated proteins were expressed in HEK293T cells using Lipofectamine (Invitrogen) and after 24–48 h, cells were lysed in 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.5% NP-40, 10 mM NaF, 1 mM EDTA+protease inhibitors (ROCHE), and cleared lysates used for immunoprecipitation with the indicated antibodies. Immune complexes were washed 4–5X with lysis buffer, re-suspended in SDS laemli buffer and were subjected to polyacrylamide gel separation and immunoblotting with the indicated antibodies. Recombinant GST-h.SLX1/His-h.SLX4(SBD) was purified as previously described [5]. 5′32P-labeled DNA substrates (5′-flap, 3′-flap, Holliday Junction, and Replication Fork) were prepared as previously described [5]. The sequences used for the preparation of labeled substrates are presented in Table S1. Radiolabeled substrates were incubated with the indicated immune complexes expressed and purified as described above. Immune complexes were washed 3X in cleavage buffer (50 mM Tris pH 8.0, 5 mM MgCl2, 40 mM NaCl, 1 mM DTT, 100 µg/ml BSA) prior to initiating the reaction. After 30 min at 37°C, reaction mixtures were treated with 25 mM EDTA and 1% Proteinase K in 10% SDS prior to electrophoresis on either 8% polyacrylamide gels (native) or 12% polyacrylamide-urea gels (denaturing). Reaction products were visualized by autoradiography and quantified with ImageJ software.
Time course analysis for RAD-51 foci
Quantitative analysis of RAD-51 foci was performed as in [38] except that all seven zones composing the germline were scored. 2–3 germlines were scored for each genotype. The average number of nuclei scored per zone for a given genotype was as follows, ± standard deviation: zone 1, n = 79±24; zone 2, n = 93±38; zone 3, n = 103±34; zone 4, n = 93±28; zone 5, n = 82±18; zone 6, n = 64±14; and zone 7, n = 58±13. Statistical comparisons between genotypes were performed using the two-tailed Mann-Whitney test, 95% confidence interval (C.I.).
Measurements of RAD-51 distribution
C. elegans gonads were fixed and stained with rabbit α-RAD-51 (SDIX) (1∶20,000), mouse α-REC-8 (Abcam) (1∶100) and guinea pig α-HIM-8 (1∶100). Chromosome axes were traced in 3D along the REC-8 signal and straightened by using either Priism [73] or softWoRx (Applied Precision). For each chromosome axis, positions of the RAD-51 foci were measured with softWoRx. Statistical comparisons between genotypes were performed using the two-tailed Mann-Whitney test, 95% confidence interval (C.I.).
DNA damage sensitivity experiments
To assess ionizing radiation (IR) sensitivity, animals (∼19 hours post L4 stage) were treated with 0, 50 or 100 Gy of IR from a Cs137 source at a dose rate of 1.86 Gy/min. For UVC sensitivity, animals were placed on the UV stratalinker 2400 (Stratagene) and exposed to 0, 300 or 600 J/m2. For nitrogen mustard (HN2) sensitivity, young adult animals were treated with 0, 100 or 200 µM of HN2 (mechlorethamine hydrochloride; Sigma) in M9 buffer containing E. coli OP50 with slow shaking in the dark for 19 hours. Treatment with camptothecin (CPT; Sigma) was similar, but with doses of 0, 500 or 1000 nM. Following treatment with IR, UVC, HN2 or CPT, animals were plated to allow recovery for 3 hours. For all damage sensitivity experiments, 21 animals were plated 7 per plate and hatching was assessed for 4 hours after the recovery. After 1.5 days, hatched worms and dead eggs were counted. Each damage condition was replicated at least three times in independent experiments.
Quantitative analysis of germ cell apoptosis
22–24 hour post-L4 hermaphrodites were stained with acridine orange (AO) for 2 hours and mounted under coverslips in 5 µl of a 15 mM sodium azide solution on 1.5% agarose pads. Apoptotic nuclei stained with AO were observed in the late pachytene region of the germline with a Leica DM5000 B fluorescence microscope. Between 33 and 47 gonads were scored for each genotype. Statistical comparisons between genotypes were performed using the two-tailed Mann-Whitney test, 95% C.I.
Determining crossover frequencies and distribution
Meiotic crossover frequencies were assayed utilizing single-nucleotide polymorphisms (SNP) markers as in [74], except that +/+ worms were used as a control. PCR and DraI restriction digests of single worm lysates were performed as described in [75]. The following DraI SNP primers were utilized: A (uCE3-637), B (CE3-127), C (snp_Y39A1), D (uCE3-1426) for chromosome III, A (uCE4-515), B (pkP4055), C (snp_F49E11), D (pkP4099) for chromosome IV, A (pkP5076), B (snp_Y61A9L), C (pkP5129), D (snp_Y17D7B) for chromosome V, and A (pkP6143), B (pkP6105), B' (snp_F11A1), C (pkP6132), D (uCE6-1554) for the X chromosome. Statistical analysis was performed using the two-tailed Fisher's Exact test, 95% C.I., as in [26] (Table S4).
Recombination analysis using visible markers was performed as in [76] and recombination frequencies were calculated as in [77].
Yeast two-hybrid analysis
The yeast two-hybrid assay was performed according to [78]. cDNA of HIM-18 full length, SLX-1 full length, SLX-1N1–272 and SLX-1C273–443 were cloned into the Gateway donor vector (pDONR223). Each construct was then subcloned into 2 µ Gateway destination vectors pVV213 (activation domain (AD), LEU2+) and pVV212 (Gal4 DNA binding domain (DB), TRP1+). AD-Y and DB-X fusions were transformed into MATa Y8800 and MATa Y8930 yeast strains, respectively. These yeast strains have three reporter genes: GAL2-ADE2, met2::GAL7-lacZ and LYS2::GAL1-HIS3. MATa Y8800 and MATα Y8930 were mated on YPD plates and diploids carrying both plasmids were selected on SC-Leu-Trp plates. The interactions were assessed by growth on -His+1 mM 3-AT plates at 30°C.
Supporting Information
Zdroje
1. FrickeWM, BrillSJ (2003) Slx1-Slx4 is a second structure-specific endonuclease functionally redundant with Sgs1-Top3. Genes Dev 17 : 1768–1778.
2. CoulonS, GaillardPH, ChahwanC, McDonaldWH, YatesJR3rd, et al. (2004) Slx1-Slx4 are subunits of a structure-specific endonuclease that maintains ribosomal DNA in fission yeast. Mol Biol Cell 15 : 71–80.
3. FekairiS, ScaglioneS, ChahwanC, TaylorER, TissierA, et al. (2009) Human SLX4 is a Holliday junction resolvase subunit that binds multiple DNA repair/recombination endonucleases. Cell 138 : 78–89.
4. MunozIM, HainK, DeclaisAC, GardinerM, TohGW, et al. (2009) Coordination of structure-specific nucleases by human SLX4/BTBD12 is required for DNA repair. Mol Cell 35 : 116–127.
5. SvendsenJM, SmogorzewskaA, SowaME, O'ConnellBC, GygiSP, et al. (2009) Mammalian BTBD12/SLX4 assembles a Holliday junction resolvase and is required for DNA repair. Cell 138 : 63–77.
6. MullenJR, KaliramanV, IbrahimSS, BrillSJ (2001) Requirement for three novel protein complexes in the absence of the Sgs1 DNA helicase in Saccharomyces cerevisiae. Genetics 157 : 103–118.
7. WuL, HicksonID (2003) The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature 426 : 870–874.
8. LiF, DongJ, PanX, OumJH, BoekeJD, et al. (2008) Microarray-based genetic screen defines SAW1, a gene required for Rad1/Rad10-dependent processing of recombination intermediates. Mol Cell 30 : 325–335.
9. KaliramanV, BrillSJ (2002) Role of SGS1 and SLX4 in maintaining rDNA structure in Saccharomyces cerevisiae. Curr Genet 41 : 389–400.
10. CoulonS, NoguchiE, NoguchiC, DuLL, NakamuraTM, et al. (2006) Rad22Rad52-dependent repair of ribosomal DNA repeats cleaved by Slx1-Slx4 endonuclease. Mol Biol Cell 17 : 2081–2090.
11. AndersenSL, BergstralhDT, KohlKP, LaRocqueJR, MooreCB, et al. (2009) Drosophila MUS312 and the vertebrate ortholog BTBD12 interact with DNA structure-specific endonucleases in DNA repair and recombination. Mol Cell 35 : 128–135.
12. StoepkerC, HainK, SchusterB, Hilhorst-HofsteeY, RooimansMA, et al. (2011) SLX4, a coordinator of structure-specific endonucleases, is mutated in a new Fanconi anemia subtype. Nat Genet 43 : 138–141.
13. CrossanGP, van der WeydenL, RosadoIV, LangevinF, GaillardPH, et al. (2011) Disruption of mouse Slx4, a regulator of structure-specific nucleases, phenocopies Fanconi anemia. Nat Genet 43 : 147–152.
14. ItoT, ChibaT, OzawaR, YoshidaM, HattoriM, et al. (2001) A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc Natl Acad Sci U S A 98 : 4569–4574.
15. FlottS, AlabertC, TohGW, TothR, SugawaraN, et al. (2007) Phosphorylation of Slx4 by Mec1 and Tel1 regulates the single-strand annealing mode of DNA repair in budding yeast. Mol Cell Biol 27 : 6433–6445.
16. SaitoTT, YoudsJL, BoultonSJ, ColaiacovoMP (2009) Caenorhabditis elegans HIM-18/SLX-4 interacts with SLX-1 and XPF-1 and maintains genomic integrity in the germline by processing recombination intermediates. PLoS Genet 5: e1000735 doi:10.1371/journal.pgen.1000735.
17. DunderdaleHJ, BensonFE, ParsonsCA, SharplesGJ, LloydRG, et al. (1991) Formation and resolution of recombination intermediates by E. coli RecA and RuvC proteins. Nature 354 : 506–510.
18. IpSC, RassU, BlancoMG, FlynnHR, SkehelJM, et al. (2008) Identification of Holliday junction resolvases from humans and yeast. Nature 456 : 357–361.
19. HodgkinJ, HorvitzHR, BrennerS (1979) Nondisjunction Mutants of the Nematode CAENORHABDITIS ELEGANS. Genetics 91 : 67–94.
20. AndersenSL, KuoHK, SavukoskiD, BrodskyMH, SekelskyJ (2011) Three structure-selective endonucleases are essential in the absence of BLM helicase in Drosophila. PLoS Genet 7: e1002315 doi:10.1371/journal.pgen.1002315.
21. BaillyAP, FreemanA, HallJ, DeclaisAC, AlpiA, et al. (2010) The Caenorhabditis elegans homolog of Gen1/Yen1 resolvases links DNA damage signaling to DNA double-strand break repair. PLoS Genet 6: e1001025 doi:10.1371/journal.pgen.1001025.
22. GartnerA, MilsteinS, AhmedS, HodgkinJ, HengartnerMO (2000) A conserved checkpoint pathway mediates DNA damage–induced apoptosis and cell cycle arrest in C. elegans. Mol Cell 5 : 435–443.
23. PommierY, RedonC, RaoVA, SeilerJA, SordetO, et al. (2003) Repair of and checkpoint response to topoisomerase I-mediated DNA damage. Mutat Res 532 : 173–203.
24. YildizO, MajumderS, KramerB, SekelskyJJ (2002) Drosophila MUS312 interacts with the nucleotide excision repair endonuclease MEI-9 to generate meiotic crossovers. Mol Cell 10 : 1503–1509.
25. InterthalH, HeyerWD (2000) MUS81 encodes a novel helix-hairpin-helix protein involved in the response to UV - and methylation-induced DNA damage in Saccharomyces cerevisiae. Mol Gen Genet 263 : 812–827.
26. MetsDG, MeyerBJ (2009) Condensins regulate meiotic DNA break distribution, thus crossover frequency, by controlling chromosome structure. Cell 139 : 73–86.
27. NottkeAC, Beese-SimsSE, PantalenaLF, ReinkeV, ShiY, et al. (2011) SPR-5 is a histone H3K4 demethylase with a role in meiotic double-strand break repair. Proc Natl Acad Sci U S A 108 : 12805–12810.
28. RosuS, LibudaDE, VilleneuveAM (2011) Robust crossover assurance and regulated interhomolog access maintain meiotic crossover number. Science 334 : 1286–1289.
29. MartinJS, WinkelmannN, PetalcorinMI, McIlwraithMJ, BoultonSJ (2005) RAD-51-dependent and -independent roles of a Caenorhabditis elegans BRCA2-related protein during DNA double-strand break repair. Mol Cell Biol 25 : 3127–3139.
30. ReddyKC, VilleneuveAM (2004) C. elegans HIM-17 links chromatin modification and competence for initiation of meiotic recombination. Cell 118 : 439–452.
31. TsaiCJ, MetsDG, AlbrechtMR, NixP, ChanA, et al. (2008) Meiotic crossover number and distribution are regulated by a dosage compensation protein that resembles a condensin subunit. Genes Dev 22 : 194–211.
32. BrennerS (1974) The genetics of Caenorhabditis elegans. Genetics 77 : 71–94.
33. BarnesTM, KoharaY, CoulsonA, HekimiS (1995) Meiotic recombination, noncoding DNA and genomic organization in Caenorhabditis elegans. Genetics 141 : 159–179.
34. PhillipsCM, WongC, BhallaN, CarltonPM, WeiserP, et al. (2005) HIM-8 binds to the X chromosome pairing center and mediates chromosome-specific meiotic synapsis. Cell 123 : 1051–1063.
35. WicksSR, YehRT, GishWR, WaterstonRH, PlasterkRH (2001) Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat Genet 28 : 160–164.
36. BzymekM, ThayerNH, OhSD, KlecknerN, HunterN (2010) Double Holliday junctions are intermediates of DNA break repair. Nature 464 : 937–941.
37. GoldfarbT, LichtenM (2010) Frequent and efficient use of the sister chromatid for DNA double-strand break repair during budding yeast meiosis. PLoS Biol 8: e1000520 doi:10.1371/journal.pbio.1000520.
38. ColaiacovoMP, MacQueenAJ, Martinez-PerezE, McDonaldK, AdamoA, et al. (2003) Synaptonemal complex assembly in C. elegans is dispensable for loading strand-exchange proteins but critical for proper completion of recombination. Dev Cell 5 : 463–474.
39. PasierbekP, JantschM, MelcherM, SchleifferA, SchweizerD, et al. (2001) A Caenorhabditis elegans cohesion protein with functions in meiotic chromosome pairing and disjunction. Genes Dev 15 : 1349–1360.
40. SeversonAF, LingL, van ZuylenV, MeyerBJ (2009) The axial element protein HTP-3 promotes cohesin loading and meiotic axis assembly in C. elegans to implement the meiotic program of chromosome segregation. Genes Dev 23 : 1763–1778.
41. Martinez-PerezE, ColaiacovoMP (2009) Distribution of meiotic recombination events: talking to your neighbors. Curr Opin Genet Dev 19 : 105–112.
42. BhallaN, WynneDJ, JantschV, DernburgAF (2008) ZHP-3 acts at crossovers to couple meiotic recombination with synaptonemal complex disassembly and bivalent formation in C. elegans. PLoS Genet 4: e1000235 doi:10.1371/journal.pgen.1000235.
43. TruglioJJ, RhauB, CroteauDL, WangL, SkorvagaM, et al. (2005) Structural insights into the first incision reaction during nucleotide excision repair. EMBO J 24 : 885–894.
44. Van HoutenB, CroteauDL, DellaVecchiaMJ, WangH, KiskerC (2005) ‘Close-fitting sleeves’: DNA damage recognition by the UvrABC nuclease system. Mutat Res 577 : 92–117.
45. McDanielLD, SchultzRA (2008) XPF/ERCC4 and ERCC1: their products and biological roles. Adv Exp Med Biol 637 : 65–82.
46. ParkHK, SuhD, HyunM, KooHS, AhnB (2004) A DNA repair gene of Caenorhabditis elegans: a homolog of human XPF. DNA Repair (Amst) 3 : 1375–1383.
47. HanadaK, BudzowskaM, ModestiM, MaasA, WymanC, et al. (2006) The structure-specific endonuclease Mus81-Eme1 promotes conversion of interstrand DNA crosslinks into double-strands breaks. EMBO J 25 : 4921–4932.
48. NiedernhoferLJ, OdijkH, BudzowskaM, van DrunenE, MaasA, et al. (2004) The structure-specific endonuclease Ercc1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol Cell Biol 24 : 5776–5787.
49. KratzK, SchopfB, KadenS, SendoelA, EberhardR, et al. (2010) Deficiency of FANCD2-associated nuclease KIAA1018/FAN1 sensitizes cells to interstrand crosslinking agents. Cell 142 : 77–88.
50. LiuT, GhosalG, YuanJ, ChenJ, HuangJ (2010) FAN1 acts with FANCI-FANCD2 to promote DNA interstrand cross-link repair. Science 329 : 693–696.
51. MacKayC, DeclaisAC, LundinC, AgostinhoA, DeansAJ, et al. (2010) Identification of KIAA1018/FAN1, a DNA repair nuclease recruited to DNA damage by monoubiquitinated FANCD2. Cell 142 : 65–76.
52. SmogorzewskaA, DesettyR, SaitoTT, SchlabachM, LachFP, et al. (2010) A genetic screen identifies FAN1, a Fanconi anemia-associated nuclease necessary for DNA interstrand crosslink repair. Mol Cell 39 : 36–47.
53. MatsuokaS, BallifBA, SmogorzewskaA, McDonaldER3rd, HurovKE, et al. (2007) ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316 : 1160–1166.
54. SzostakJW, Orr-WeaverTL, RothsteinRJ, StahlFW (1983) The double-strand-break repair model for recombination. Cell 33 : 25–35.
55. GaillardPH, NoguchiE, ShanahanP, RussellP (2003) The endogenous Mus81-Eme1 complex resolves Holliday junctions by a nick and counternick mechanism. Mol Cell 12 : 747–759.
56. WechslerT, NewmanS, WestSC (2011) Aberrant chromosome morphology in human cells defective for Holliday junction resolution. Nature 471 : 642–646.
57. YoudsJL, MetsDG, McIlwraithMJ, MartinJS, WardJD, et al. (2010) RTEL-1 enforces meiotic crossover interference and homeostasis. Science 327 : 1254–1258.
58. WagnerCR, KuerversL, BaillieDL, YanowitzJL (2010) xnd-1 regulates the global recombination landscape in Caenorhabditis elegans. Nature 467 : 839–843.
59. BishopDK, ZicklerD (2004) Early decision; meiotic crossover interference prior to stable strand exchange and synapsis. Cell 117 : 9–15.
60. GersteinMB, LuZJ, Van NostrandEL, ChengC, ArshinoffBI, et al. (2010) Integrative analysis of the Caenorhabditis elegans genome by the modENCODE project. Science 330 : 1775–1787.
61. LiuT, RechtsteinerA, EgelhoferTA, VielleA, LatorreI, et al. (2011) Broad chromosomal domains of histone modification patterns in C. elegans. Genome Res 21 : 227–236.
62. AustinJ, KimbleJ (1989) Transcript analysis of glp-1 and lin-12, homologous genes required for cell interactions during development of C. elegans. Cell 58 : 565–571.
63. DernburgAF, McDonaldK, MoulderG, BarsteadR, DresserM, et al. (1998) Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 94 : 387–398.
64. EdgleyML, RiddleDL (2001) LG II balancer chromosomes in Caenorhabditis elegans: mT1(II;III) and the mIn1 set of dominantly and recessively marked inversions. Mol Genet Genomics 266 : 385–395.
65. FergusonEL, HorvitzHR (1985) Identification and characterization of 22 genes that affect the vulval cell lineages of the nematode Caenorhabditis elegans. Genetics 110 : 17–72.
66. McKimKS, PetersK, RoseAM (1993) Two types of sites required for meiotic chromosome pairing in Caenorhabditis elegans. Genetics 134 : 749–768.
67. WickyC, AlpiA, PassannanteM, RoseA, GartnerA, et al. (2004) Multiple genetic pathways involving the Caenorhabditis elegans Bloom's syndrome genes him-6, rad-51, and top-3 are needed to maintain genome stability in the germ line. Mol Cell Biol 24 : 5016–5027.
68. StergiouL, EberhardR, DoukoumetzidisK, HengartnerMO (2011) NER and HR pathways act sequentially to promote UV-C-induced germ cell apoptosis in Caenorhabditis elegans. Cell Death Differ 18 : 897–906.
69. ReboulJ, VaglioP, RualJF, LameschP, MartinezM, et al. (2003) C. elegans ORFeome version 1.1: experimental verification of the genome annotation and resource for proteome-scale protein expression. Nat Genet 34 : 35–41.
70. LameschP, MilsteinS, HaoT, RosenbergJ, LiN, et al. (2004) C. elegans ORFeome version 3.1: increasing the coverage of ORFeome resources with improved gene predictions. Genome Res 14 : 2064–2069.
71. FraserAG, KamathRS, ZipperlenP, Martinez-CamposM, SohrmannM, et al. (2000) Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408 : 325–330.
72. KamathRS, FraserAG, DongY, PoulinG, DurbinR, et al. (2003) Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421 : 231–237.
73. ChenH, CybornW, SedatJW, AgardDA (1992) PRIISM: an integrated system for display and analysis of three-dimensional microscope images. Proceedings of SPIE 1660 : 784–790.
74. NabeshimaK, VilleneuveAM, HillersKJ (2004) Chromosome-wide regulation of meiotic crossover formation in Caenorhabditis elegans requires properly assembled chromosome axes. Genetics 168 : 1275–1292.
75. DavisMW, HammarlundM, HarrachT, HullettP, OlsenS, et al. (2005) Rapid single nucleotide polymorphism mapping in C. elegans. BMC Genomics 6 : 118.
76. KellyKO, DernburgAF, StanfieldGM, VilleneuveAM (2000) Caenorhabditis elegans msh-5 is required for both normal and radiation-induced meiotic crossing over but not for completion of meiosis. Genetics 156 : 617–630.
77. MacQueenAJ, ColaiacovoMP, McDonaldK, VilleneuveAM (2002) Synapsis-dependent and -independent mechanisms stabilize homolog pairing during meiotic prophase in C. elegans. Genes Dev 16 : 2428–2442.
78. WalhoutAJ, VidalM (2001) High-throughput yeast two-hybrid assays for large-scale protein interaction mapping. Methods 24 : 297–306.
79. RockmanMV, KruglyakL (2009) Recombinational landscape and population genomics of Caenorhabditis elegans. PLoS Genet 5: e1000419 doi:10.1371/journal.pgen.1000419.
Štítky
Genetika Reprodukční medicína
Článek Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma GenomesČlánek Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail ExpansionČlánek Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid CellsČlánek Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug ActivationČlánek The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin DynamicsČlánek Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 8- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
-
Všechny články tohoto čísla
- Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma Genomes
- Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail Expansion
- Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid Cells
- Chromosome Territories Meet a Condensin
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Fine-Mapping and Initial Characterization of QT Interval Loci in African Americans
- Genome Patterns of Selection and Introgression of Haplotypes in Natural Populations of the House Mouse ()
- A Combinatorial Amino Acid Code for RNA Recognition by Pentatricopeptide Repeat Proteins
- Advances in Quantitative Trait Analysis in Yeast
- Experimental Evolution of a Novel Sexually Antagonistic Allele
- Variation of Contributes to Dog Breed Skull Diversity
- , a Gene Involved in Axonal Pathfinding, Is Mutated in Patients with Kallmann Syndrome
- A Single Origin for Nymphalid Butterfly Eyespots Followed by Widespread Loss of Associated Gene Expression
- Cryptocephal, the ATF4, Is a Specific Coactivator for Ecdysone Receptor Isoform B2
- Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug Activation
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
- A Sexual Ornament in Chickens Is Affected by Pleiotropic Alleles at and , Selected during Domestication
- Use of Allele-Specific FAIRE to Determine Functional Regulatory Polymorphism Using Large-Scale Genotyping Arrays
- Novel Loci for Metabolic Networks and Multi-Tissue Expression Studies Reveal Genes for Atherosclerosis
- The Genetic Basis of Pollinator Adaptation in a Sexually Deceptive Orchid
- Uncovering the Genome-Wide Transcriptional Responses of the Filamentous Fungus to Lignocellulose Using RNA Sequencing
- Inheritance Beyond Plain Heritability: Variance-Controlling Genes in
- The Metabochip, a Custom Genotyping Array for Genetic Studies of Metabolic, Cardiovascular, and Anthropometric Traits
- Reprogramming to Pluripotency Can Conceal Somatic Cell Chromosomal Instability
- Condensin II Promotes the Formation of Chromosome Territories by Inducing Axial Compaction of Polyploid Interphase Chromosomes
- PTEN Negatively Regulates MAPK Signaling during Vulval Development
- A Dynamic Response Regulator Protein Modulates G-Protein–Dependent Polarity in the Bacterium
- Population Genomics of the Facultatively Mutualistic Bacteria and
- Components of a Fanconi-Like Pathway Control Pso2-Independent DNA Interstrand Crosslink Repair in Yeast
- Polysome Profiling in Liver Identifies Dynamic Regulation of Endoplasmic Reticulum Translatome by Obesity and Fasting
- Stromal Liver Kinase B1 [STK11] Signaling Loss Induces Oviductal Adenomas and Endometrial Cancer by Activating Mammalian Target of Rapamycin Complex 1
- Reprogramming of H3K27me3 Is Critical for Acquisition of Pluripotency from Cultured Tissues
- Transgene Induced Co-Suppression during Vegetative Growth in
- Hox and Sex-Determination Genes Control Segment Elimination through EGFR and Activity
- A Quantitative Comparison of the Similarity between Genes and Geography in Worldwide Human Populations
- Minibrain/Dyrk1a Regulates Food Intake through the Sir2-FOXO-sNPF/NPY Pathway in and Mammals
- Comparative Analysis of Regulatory Elements between and by Genome-Wide Transcription Start Site Profiling
- Simple Methods for Generating and Detecting Locus-Specific Mutations Induced with TALENs in the Zebrafish Genome
- S Phase–Coupled E2f1 Destruction Ensures Homeostasis in Proliferating Tissues
- Cell-Nonautonomous Signaling of FOXO/DAF-16 to the Stem Cells of
- The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin Dynamics
- Comparative Analysis of the Genomes of Two Field Isolates of the Rice Blast Fungus
- Role of Mex67-Mtr2 in the Nuclear Export of 40S Pre-Ribosomes
- Genetic Modulation of Lipid Profiles following Lifestyle Modification or Metformin Treatment: The Diabetes Prevention Program
- HAL-2 Promotes Homologous Pairing during Meiosis by Antagonizing Inhibitory Effects of Synaptonemal Complex Precursors
- SLX-1 Is Required for Maintaining Genomic Integrity and Promoting Meiotic Noncrossovers in the Germline
- Phylogenetic and Transcriptomic Analysis of Chemosensory Receptors in a Pair of Divergent Ant Species Reveals Sex-Specific Signatures of Odor Coding
- Reduced Prostasin (CAP1/PRSS8) Activity Eliminates HAI-1 and HAI-2 Deficiency–Associated Developmental Defects by Preventing Matriptase Activation
- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
- and Link Transcription of Phospholipid Biosynthetic Genes to ER Stress and the UPR
- CDK9 and H2B Monoubiquitination: A Well-Choreographed Dance
- Rare Copy Number Variations in Adults with Tetralogy of Fallot Implicate Novel Risk Gene Pathways
- Ccdc94 Protects Cells from Ionizing Radiation by Inhibiting the Expression of
- NOL11, Implicated in the Pathogenesis of North American Indian Childhood Cirrhosis, Is Required for Pre-rRNA Transcription and Processing
- Human Developmental Enhancers Conserved between Deuterostomes and Protostomes
- A Luminal Glycoprotein Drives Dose-Dependent Diameter Expansion of the Hindgut Tube
- Melanophore Migration and Survival during Zebrafish Adult Pigment Stripe Development Require the Immunoglobulin Superfamily Adhesion Molecule Igsf11
- Dynamic Distribution of Linker Histone H1.5 in Cellular Differentiation
- Combining Comparative Proteomics and Molecular Genetics Uncovers Regulators of Synaptic and Axonal Stability and Degeneration
- Chemical Genetics Reveals a Specific Requirement for Cdk2 Activity in the DNA Damage Response and Identifies Nbs1 as a Cdk2 Substrate in Human Cells
- Experimental Relocation of the Mitochondrial Gene to the Nucleus Reveals Forces Underlying Mitochondrial Genome Evolution
- Rates of Gyrase Supercoiling and Transcription Elongation Control Supercoil Density in a Bacterial Chromosome
- Mutations in a P-Type ATPase Gene Cause Axonal Degeneration
- A General G1/S-Phase Cell-Cycle Control Module in the Flowering Plant
- Multiple Roles and Interactions of and in Development of the Respiratory System
- UNC-40/DCC, SAX-3/Robo, and VAB-1/Eph Polarize F-Actin during Embryonic Morphogenesis by Regulating the WAVE/SCAR Actin Nucleation Complex
- Epigenetic Remodeling of Meiotic Crossover Frequency in DNA Methyltransferase Mutants
- Modulating the Strength and Threshold of NOTCH Oncogenic Signals by
- Loss of Axonal Mitochondria Promotes Tau-Mediated Neurodegeneration and Alzheimer's Disease–Related Tau Phosphorylation Via PAR-1
- Acetyl-CoA-Carboxylase Sustains a Fatty Acid–Dependent Remote Signal to Waterproof the Respiratory System
- ATXN2-CAG42 Sequesters PABPC1 into Insolubility and Induces FBXW8 in Cerebellum of Old Ataxic Knock-In Mice
- Cohesin Rings Devoid of Scc3 and Pds5 Maintain Their Stable Association with the DNA
- The MicroRNA Inhibits Calcium Signaling by Targeting the TIR-1/Sarm1 Adaptor Protein to Control Stochastic L/R Neuronal Asymmetry in
- Rapid-Throughput Skeletal Phenotyping of 100 Knockout Mice Identifies 9 New Genes That Determine Bone Strength
- The Genes Define Unique Classes of Two-Partner Secretion and Contact Dependent Growth Inhibition Systems
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Variation of Contributes to Dog Breed Skull Diversity
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



