-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
, a Homolog of a Deaf-Blindness Gene, Regulates Circadian Output and Slowpoke Channels
Many aspects of behavior and physiology are under circadian control. In Drosophila, the molecular clock that regulates rhythmic patterns of behavior has been extensively characterized. In contrast, genetic loci involved in linking the clock to alterations in motor activity have remained elusive. In a forward-genetic screen, we uncovered a new component of the circadian output pathway, which we have termed dyschronic (dysc). dysc mutants exhibit arrhythmic locomotor behavior, yet their eclosion rhythms are normal and clock protein cycling remains intact. Intriguingly, dysc is the closest Drosophila homolog of whirlin, a gene linked to type II Usher syndrome, the leading cause of deaf-blindness in humans. Whirlin and other Usher proteins are expressed in the mammalian central nervous system, yet their function in the CNS has not been investigated. We show that DYSC is expressed in major neuronal tracts and regulates expression of the calcium-activated potassium channel SLOWPOKE (SLO), an ion channel also required in the circadian output pathway. SLO and DYSC are co-localized in the brain and control each other's expression post-transcriptionally. Co-immunoprecipitation experiments demonstrate they form a complex, suggesting they regulate each other through protein–protein interaction. Furthermore, electrophysiological recordings of neurons in the adult brain show that SLO-dependent currents are greatly reduced in dysc mutants. Our work identifies a Drosophila homolog of a deaf-blindness gene as a new component of the circadian output pathway and an important regulator of ion channel expression, and suggests novel roles for Usher proteins in the mammalian nervous system.
Published in the journal: . PLoS Genet 8(4): e32767. doi:10.1371/journal.pgen.1002671
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002671Summary
Many aspects of behavior and physiology are under circadian control. In Drosophila, the molecular clock that regulates rhythmic patterns of behavior has been extensively characterized. In contrast, genetic loci involved in linking the clock to alterations in motor activity have remained elusive. In a forward-genetic screen, we uncovered a new component of the circadian output pathway, which we have termed dyschronic (dysc). dysc mutants exhibit arrhythmic locomotor behavior, yet their eclosion rhythms are normal and clock protein cycling remains intact. Intriguingly, dysc is the closest Drosophila homolog of whirlin, a gene linked to type II Usher syndrome, the leading cause of deaf-blindness in humans. Whirlin and other Usher proteins are expressed in the mammalian central nervous system, yet their function in the CNS has not been investigated. We show that DYSC is expressed in major neuronal tracts and regulates expression of the calcium-activated potassium channel SLOWPOKE (SLO), an ion channel also required in the circadian output pathway. SLO and DYSC are co-localized in the brain and control each other's expression post-transcriptionally. Co-immunoprecipitation experiments demonstrate they form a complex, suggesting they regulate each other through protein–protein interaction. Furthermore, electrophysiological recordings of neurons in the adult brain show that SLO-dependent currents are greatly reduced in dysc mutants. Our work identifies a Drosophila homolog of a deaf-blindness gene as a new component of the circadian output pathway and an important regulator of ion channel expression, and suggests novel roles for Usher proteins in the mammalian nervous system.
Introduction
In diverse phyla, circadian systems act to synchronize changes in arousal and internal physiology to optimal time periods for feeding, courtship, and other ethologically relevant behaviors. In Drosophila, the molecular basis of the internal clock that drives such rhythmic alterations in behavior has been extensively characterized [1]. Molecular and genetic approaches have demonstrated that a transcriptional negative-feedback loop lies at the heart of the clock, in which the transcription factors CLOCK and CYCLE activate expression of their own repressors, PERIOD (PER) and TIMELESS [1]. In combination with additional modulatory feedback loops and post-translational regulatory mechanisms, oscillatory activation of CLOCK/CYCLE leads to temporally controlled expression of a wide range of clock-controlled genes, thus altering the functional properties of clock neurons in a time-dependent manner [2]–[6].
In contrast to the core clock mechanism, only a small number of genes that act downstream of the clock have been identified, including pigment dispersing factor (pdf), pdf receptor (pdfr), neurofibromatosis-1 (nf1), slowpoke (slo), narrow abdomen (na), and ebony [7]–[14]. Two of these output genes encode voltage-gated ion channels, SLO and NA, suggesting that modulation of neuronal excitability is an essential component of circadian output. Of the two channels, the electrophysiological properties and cellular consequences of SLO channels have been defined in much greater detail. SLO is a member of the BK (big K+) family of voltage-gated Ca2+-activated potassium channels, and generates non-inactivating K+ currents with high single-channel conductance [15], [16]. SLO channels act to repolarize the membrane potential during action potentials, and Drosophila slo mutants thus exhibit broader action potentials in flight muscles and cultured neurons [17]–[19]. Intriguingly, BK channel function is critical for circadian behavior in both Drosophila and mammals. Drosophila slo mutants are arrhythmic, yet restoring SLO expression in clock neurons does not robustly rescue rhythmic behavior, suggesting that SLO acts downstream of clock cells [2], [7]. Mammalian BK channels are also required for clock output from the suprachiasmatic nucleus (SCN), and contribute to the silencing of SCN neurons during the night [20].
Consistent with the key role of ion channels in the control of neuronal physiology and behavior, regulators of ion channel function have also been found to modulate behavioral outputs. For example, SLEEPLESS, a positive regulator of Shaker potassium channels, strongly affects sleep in Drosophila [21]. Here we identify a novel SLO-binding protein, which we have termed DYSCHRONIC (DYSC). dysc mutants exhibit arrhythmic locomotor activity but normal eclosion rhythms and wild-type molecular oscillations in clock neurons, suggesting dysc is specifically required for circadian locomotor output. Intriguingly, DYSC is the closest Drosophila homolog of Whirlin, a PDZ (PSD-95/DLG/ZO-1) domain-containing protein mutated in Type II Usher syndrome, a human deaf-blindness disease [22], [23]. Through targeted rescue experiments, we demonstrate that DYSC acts downstream of clock cells to control locomotor output. We show that DYSC co-localizes with SLO in major neuronal tracts in the brain, and that the two proteins form a complex to regulate each other's expression post-transcriptionally. Furthermore, SLO-dependent potassium currents are significantly reduced in dysc neurons in the adult brain. Our results define a novel channel regulator required for rhythmic alterations in behavior and suggest new roles for Whirlin in the mammalian brain.
Results
dyschronic Is Required for Circadian Locomotor Output in Drosophila
In an ongoing forward-genetic screen for sleep and circadian mutants, we identified an arrhythmic mutant line resulting from a P-element insertion, which we named dyschronics168 (dyscs168). Most dyscs168 homozygotes were arrhythmic in constant-dark (DD) conditions, with a minority showing weak rhythmicity (Figure 1A and Table 1). To assess whether dysc regulates circadian behavior in general or locomotor behavior specifically, we examined circadian patterns of eclosion from the pupal case, a behavior that is dependent on correct output from small ventral lateral neurons (s-LNvs), a subset of clock neurons required for rhythmic locomotion in DD [12], [24]. Interestingly, despite clear arrhythmic locomotor patterns in dysc mutants, we observed an eclosion rhythm that closely mirrored wild-type controls (Figure 1B), suggesting that s-LNv output is unimpaired in dysc mutants.
Fig. 1. The <i>dysc</i> mutation defines a novel circadian output gene. 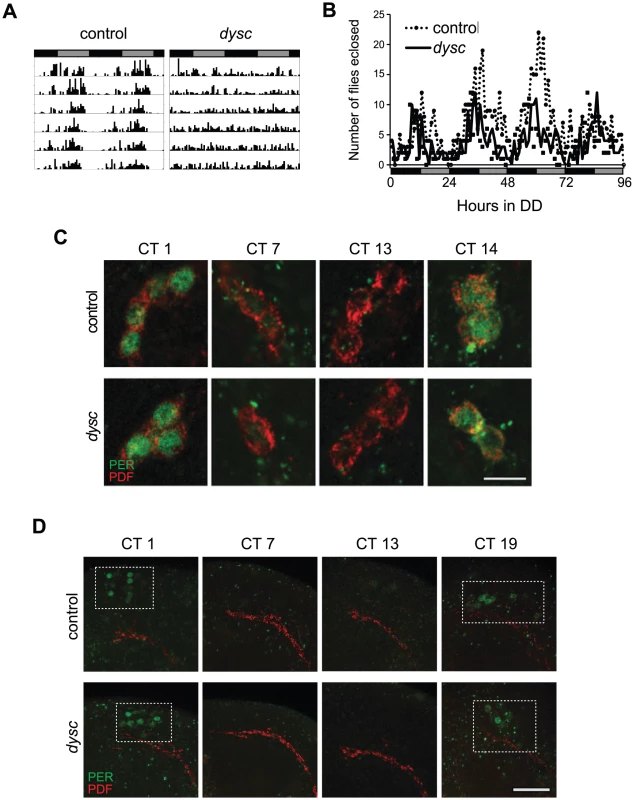
Tab. 1. Free-running circadian locomotor rhythm phenotypes in DD. 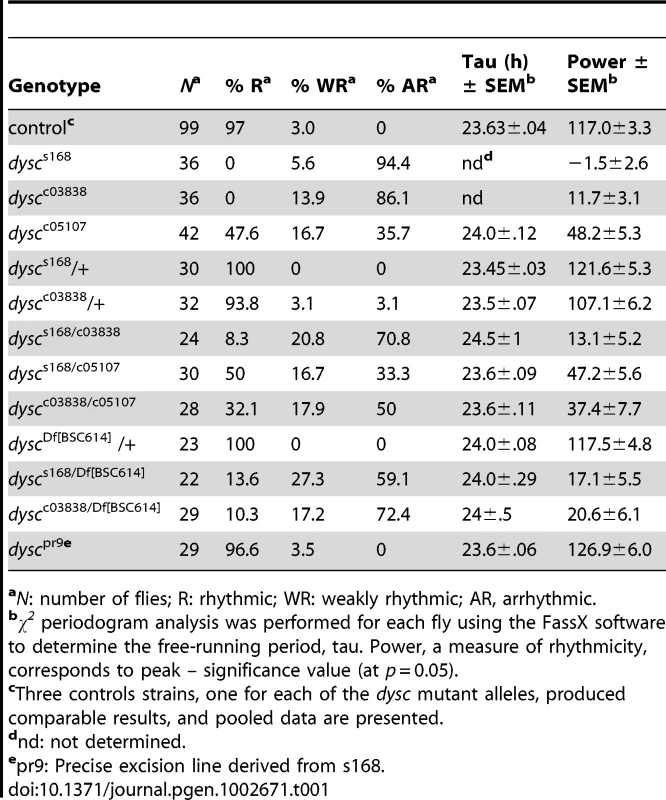
aN: number of flies; R: rhythmic; WR: weakly rhythmic; AR, arrhythmic. To determine whether DYSC functions as a component of the clock, we next assessed the integrity of molecular oscillations in clock neurons in dysc mutants. We examined PER cycling in two sets of clock neurons: s-LNvs and a cluster of dorsal neurons (DN1s), which has been proposed to be a direct target of output from the s-LNvs [25]. In dysc mutants, we found that daily cycling of PER expression was indistinguishable from wild-type controls in both sets of clock neurons (Figure 1C, 1D). Similarly, PER levels exhibited wild-type oscillatory patterns in head extracts of dysc mutants (Figure S1). Since the bulk of PER protein in head extracts derives from eye tissue, this suggests that the molecular clock is unimpaired in the periphery as well. These findings establish that core clock function is normal in dysc mutants, and thus identify DYSC as a constituent of the circadian locomotor output pathway.
dysc Is a Drosophila Homolog of whirlin, a Human Deaf-Blindness Gene
We mapped the s168 P-element insertion to an intron in a previously uncharacterized locus, CG34400 (Figure 2A). The dysc transcription unit generates two predominant classes of mRNAs via alternative splicing: multiple long isoforms and a short isoform (Figure 2A). Full-length dysc transcripts encode proteins containing three PDZ domains (Figure 2B), a motif commonly associated with scaffolding proteins [26], while the short DYSC isoform lacks the C-terminal PDZ domain. Intriguingly, comparative genomics identified dysc as the closest Drosophila homolog of whirlin (USH2D, DFNB31), a gene implicated in type II Usher syndrome (USH2) in humans [22]. Like dysc, the mammalian whirlin locus encodes several distinct splice-forms, including those corresponding to long dysc isoforms as well as one containing the C-terminal PDZ domain alone [22], [27] (www.ensembl.org; Figure 2B).
Fig. 2. dysc is the Drosophila ortholog of whirlin, a member of the mammalian Usher complex. 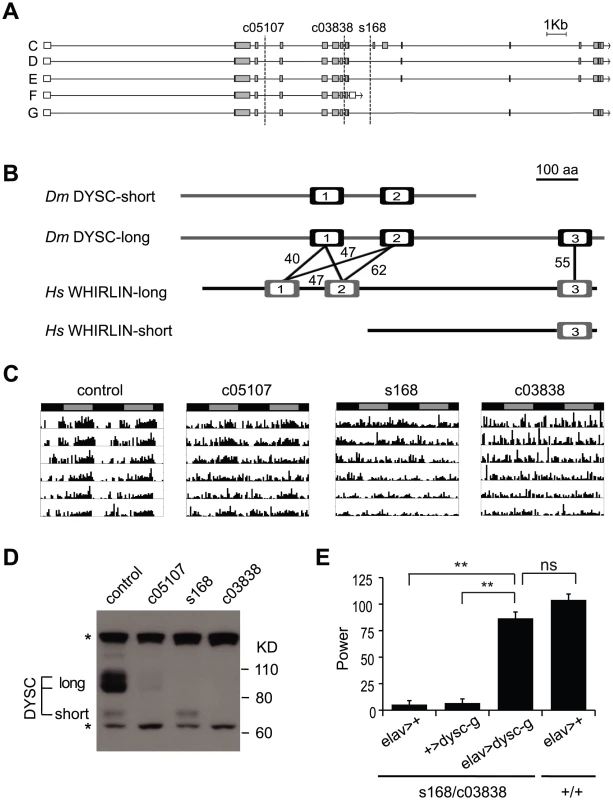
(A) Schematic illustrating differentially spliced isoforms arising from the dysc transcription unit, and the insertion sites (dotted lines) of the three alleles used in this study. (B) Isoform F yields a shorter isoform containing two PDZ domains (open boxes), while the remaining isoforms contain three PDZ domains. Percentage identity between the PDZ domains of DYSC and human Whirlin are shown. The whirlin transcription unit, like dysc, generates long and short isoforms, as well as one containing the C-terminal PDZ domain alone. (C) Representative actograms of individual control and dyscc05107, dyscs168 and dyscc03838 homozygous flies in DD. (D) Western blot showing DYSC long and short isoform expression in the above genotypes. MAPK bands are shown for loading control. * non-specific labeling. (E) Pan-neuronal expression by elav-Gal4 of a UAS-dysc transgene encoding a long DYSC isoform (Isoform G) in a dyscs168/c03838 trans-heterozygotic background increased the power, or strength, of circadian rhythmicity relative to driver-alone and transgene-alone controls to levels statistically indistinguishable from a wild-type control containing the elav-Gal4 driver. See also Table 2. Error bars represent SEM. ** p<0.0001; ns: not significant; two-tailed t-test with Bonferroni correction. USH2 is characterized by early-age hearing loss due to alterations in ear cell stereocilia formation followed by progressive blindness resulting from photoreceptor degeneration [23]. Genetic ablation of whirlin in mice also leads to hearing loss coupled with abnormal photoreceptor development [27], [28]. In addition to sensory tissues, Whirlin is also expressed in the mammalian central nervous system [29], [30], yet the function of Whirlin in the brain is unclear. The identification of dysc thus provides a platform in a genetically amenable model organism to investigate novel functions of a Whirlin homolog.
Loss of DYSC Results in Arrhythmic Locomotor Behavior
We performed several independent genetic experiments to establish that specific disruption of the dysc locus is indeed causative of the arrhythmic phenotype in dysc mutants. We obtained two additional P-element insertions in dysc (c03838 and c05107) (Figure 2A). Like dyscs168, most dyscc03838 mutants were arrhythmic in DD (Figure 2C and Table 1). On the other hand, dyscc05107 mutants exhibited a milder circadian phenotype, with some showing robust rhythmicity (Figure 2C and Table 1). To analyze the effects of the three separate P-element insertions on DYSC expression, we generated a polyclonal antibody to DYSC. Part of the DYSC antigen is common to all isoforms, and thus the antibody is expected to recognize all isoforms. As expected, in wild-type adult head extracts, we observed multiple bands corresponding to the predicted long isoforms, and a single band at the expected size of the short isoform (Figure 2D). Western blotting further revealed differential effects of the three P-element insertions on the expression of DYSC isoforms (Figure 2D). The dyscc05107 insertion acts as a hypomorphic allele, leaving expression of all DYSC isoforms reduced but still detectable. In contrast, dyscc03838 renders expression of all DYSC isoforms undetectable, and is therefore a null or a strong hypomorphic allele. The remaining DYSC expression in dyscc05107 homozygotes is thus likely to be sufficient to partially rescue rhythmic behavior. While dyscs168 also renders the long isoforms undetectable, it leaves expression of the short isoform intact. Our finding that the s168 mutation causes as strong a circadian phenotype as c03838 suggests that the short isoform is not sufficient for rhythmic behavior and that the C-terminal PDZ domain plays an important role in circadian rhythms.
In addition to assessing rhythmicity in DD conditions, we also examined locomotor patterns of dysc mutants in 12 h light∶dark (LD) conditions (Figure S2A). In contrast to wild-type flies, dyscs168 and dyscc03838 homozygotes did not exhibit anticipation of lights-on, further suggesting that output of the morning oscillator (which drives rhythmic behavior in DD) is impaired by loss of DYSC. Hypomorphic dyscc05107 flies showed normal morning anticipation, while anticipation of lights-off was maintained in all dysc allelic backgrounds (Figure S2A). Overall daytime and nighttime activity in LD conditions was greater in dysc mutants relative to wild type controls (Figure S2B). This is in contrast to climbing defects observed in dysc mutants (Figure S2C), and suggests that although dysc flies have some motor problems, overall inactivity is not a contributing factor for arrhythmicity.
All dysc alleles were recessive; trans-heterozygotic combinations of the three alleles were largely arrhythmic; and heterozygosity for both dyscs168 and dyscc03838 in combination with a deficiency removing the dysc locus also resulted in arrhythmia (Table 1). In addition, precise excision of the dyscs168 P-element restored wild-type patterns of locomotion (Table 1), indicating that the P-element insertion is responsible for arrhythmicity. Finally, to test whether transgenic expression of dysc could restore rhythmic behavior, we generated flies carrying a UAS-dysc transgene encoding a long isoform of DYSC. Pan-neuronal expression of the UAS-dysc transgene in dysc mutants was sufficient to rescue rhythmic behavior (Figure 2E and Table 2). Consistent with the fact that dyscs168 homozygotes, which express normal levels of the short isoform, are arrhythmic, transgenic expression of a short DYSC isoform did not restore rhythmicity in dyscc03838 mutants (Table 2; see Figure S3 for expression levels of long and short dysc transgenes). Over-expression of either the long or short isoforms of DYSC in a wild-type background did not affect circadian rhythmicity (Table 2). These results comprehensively demonstrate that DYSC is required for circadian alterations in locomotor activity, and furthermore indicate that correct circadian output requires DYSC expression in the nervous system.
Tab. 2. Phenotypic consequences of targeted restoration and over-expression of DYSC. 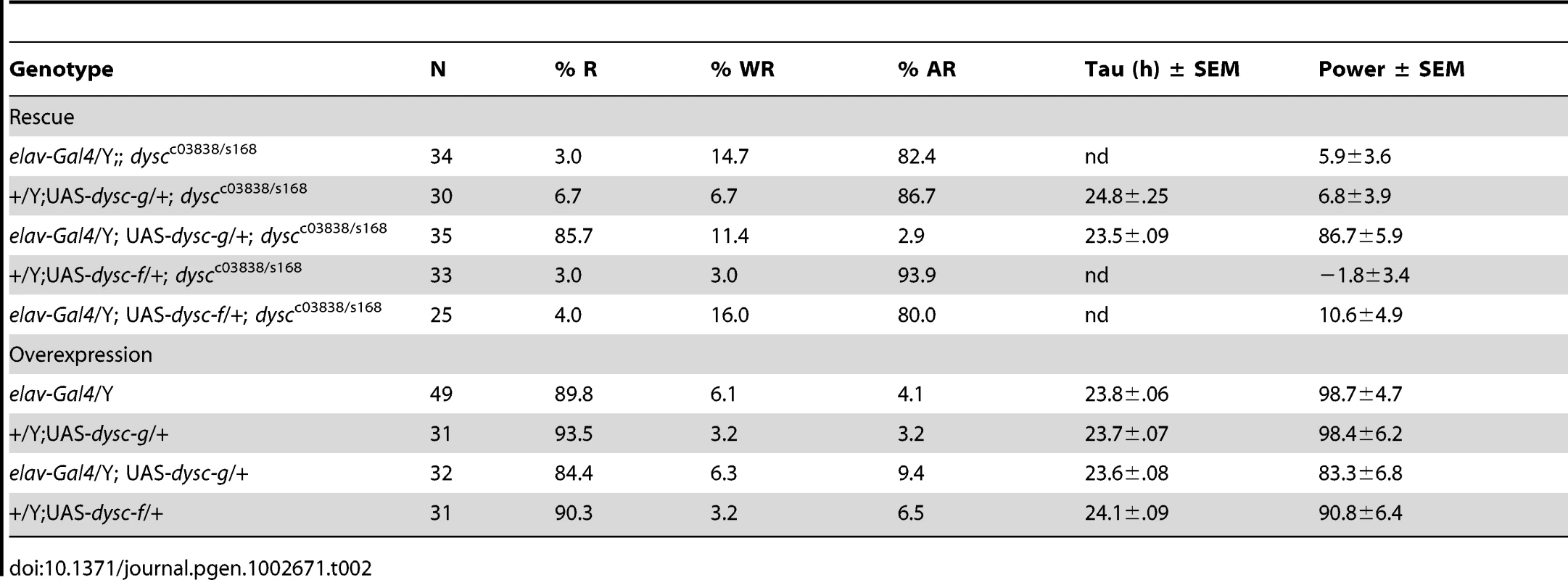
DYSC Is Enriched in Major Neuronal Tracts in the Drosophila Nervous System
We next examined DYSC expression in the adult Drosophila nervous system by performing whole-mount immuno-staining of the adult brain. DYSC-specific immuno-reactivity was enriched in major neuronal tracts, i.e., dense bundles of neuronal processes, throughout the central brain (Figure 3A). Interestingly, in the mushroom bodies, ellipsoid body and antennal lobes, DYSC expression was broader than in other regions. For example, DYSC expression was observed throughout the mushroom body including the lobes, peduncle, and calyx, although not in the cell bodies (Figure 3A). In fact, no cell-body expression of DYSC was detected in any brain region. To define the cell-body locations of DYSC-expressing neurons, we generated transgenic flies carrying Gal4 under the control of the dysc promoter. Consistent with the widespread expression of DYSC, GFP expression driven by dysc-Gal4 was detected in many cell bodies in the brain (Figure S4A), including subsets of clock neurons (Figure S4B), and expression of DYSC using the dysc-Gal4 driver restored rhythmic behavior in dysc mutants (Figure 3B).
Fig. 3. DYSC is enriched in neuronal tracts and is required downstream of the clock cells. 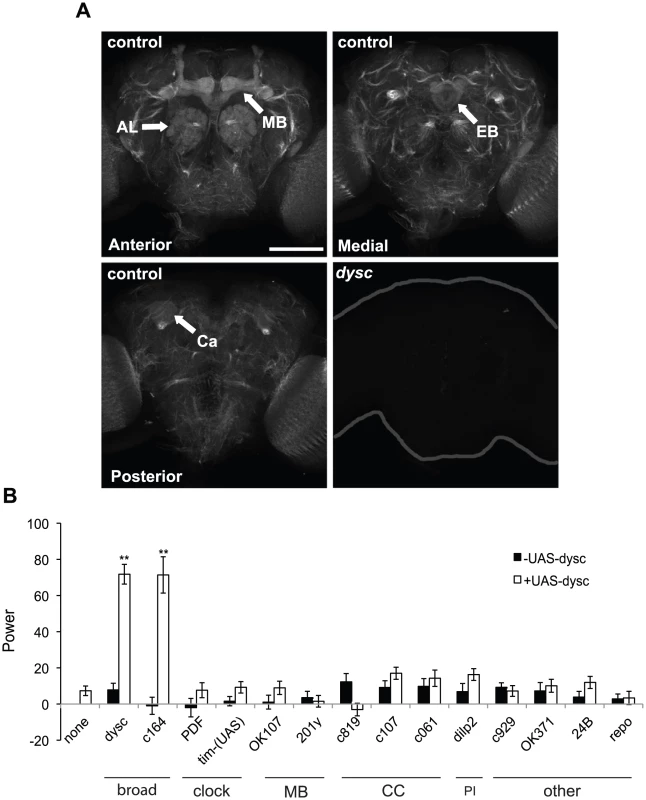
(A) Strong DYSC staining was observed in major neuronal tracts throughout the central brain in adult control flies, as well as in the antennal lobes (AL), mushroom bodies (MB) and ellipsoid body (EB). Upper panels show confocal projections spanning the anterior and medial compartments of the adult brain, and lower left panel shows a single 2 µm slice to illustrate DYSC expression in the posterior calyx (Ca) of the mushroom bodies. Similar DYSC immuno-reactivity was not observed in dyscc03838 homozygous brains (lower right panel), confirming the specificity of the antibody. Scale bar represents 100 µm. (B) Targeted rescue of the dysc circadian phenotype using the UAS-dysc transgene (Isoform G). Data are from homozygous dyscc03838 or trans-heterozygous dyscc03838/s168 flies. In addition to dysc mutants carrying a UAS-dysc transgene insertion alone, driver-alone controls were used for each driver line. The power of rhythmicity in DD is shown for each genotype (N≥21, except for pdf-Gal4 driver control, for which N = 17). MB: mushroom bodies; CC: central complex; PI: pars intercerebralis. c929- and OK371-Gal4 lines drive expression in peptidergic and glutamatergic neurons, respectively. 24B- and repo-Gal4 lines are tissue-specific drivers for muscle and glia, respectively. Error bars represent SEM. ** p<0.0001; two-tailed t-test with Bonferroni correction. To examine whether DYSC expression was under circadian control, as has previously been documented for certain output genes [2], [13], we examined DYSC expression and localization at various circadian time-points. These experiments revealed that DYSC protein levels in head extracts were not subject to circadian cycling, nor was any obvious temporal alteration in DYSC expression and localization in the adult brain observed (Figure S5). However, we cannot rule out the possibility that DYSC undergoes circadian regulation in a subset of cells.
DYSC Is Required Downstream of the Central Clock for Rhythmic Behavior
We attempted to narrow down the key DYSC-expressing cells required for circadian locomotor behavior using a targeted rescue strategy (Figure 3B). Complementing our pan-neuronal rescue data, transgenic expression of DYSC in muscle or glial cells did not restore rhythmic behavior (Figure 3B). Expression of DYSC in PDF - or TIM-expressing clock neurons also failed to rescue the circadian phenotype (Figure 3B). We next attempted to rescue dysc mutant arrhythmicity via targeted expression of UAS-dysc to major centers in the Drosophila nervous system. Expression of DYSC in the central complex, pars intercerebralis or the mushroom bodies, regions of the Drosophila brain implicated in motor control and complex behaviors [31]–[33], was insufficient to restore rhythmicity. Whereas c164-Gal4, a driver widely used for expression in motor neurons, robustly rescued circadian behavior, OK371-Gal4, which drives expression in glutamatergic neurons, including motor neurons, did not (Figure 3B). c164-Gal4 drives expression in several brain regions in addition to motor neurons [34] (Figure S6A), but not in the ellipsoid body, a region important for locomotor behavior [32]. Co-staining with PER shows that it also drives expression in a few clock cells (Figure S6B). Given that dysc-Gal4 drives expression in many clock cells (Figure S4B) and that a recent study identified dysc as a potential direct target of CLK [35], DYSC may function in both clock and non-clock cells. However, our results clearly show that DYSC expression in clock cells alone is not sufficient to restore rhythmicity. Combined with our data indicating that DYSC does not affect clock protein oscillations (Figure 1), this suggests that DYSC is required downstream of clock neurons.
Collectively, these results indicate a role for DYSC in an intermediary circuit between the central clock neurons and motor neurons, and further suggest that the cellular requirements for DYSC in the circadian output circuit are likely to be structurally complex and not easily recapitulated using restricted driver lines.
DYSC Is Required for Expression of SLO Channels
In mammalian photoreceptors and cochlear stereocilia, Whirlin, the mammalian DYSC homolog, forms a scaffolding complex to properly localize the transmembrane proteins Usherin and Very large G-protein-coupled receptor 1 (VLGR1) [27], [29], [36], [37]. We hypothesized that DYSC might also be required for appropriate transmembrane protein localization in the Drosophila nervous system. Since Usher proteins have previously been shown to interact with ion channels [38], we focused on SLO, a Ca2+-activated potassium channel, which is required for clock output in flies and mammals [2], [7], [20].
Using a new anti-SLO antibody, we observed clear enrichment of SLO in major neuronal tracts throughout the central brain (Figure 4A). SLO staining in neuronal tracts was not detected in slo4 mutants (Figure 4A), confirming the specificity of the antibody. Intriguingly, DYSC and SLO exhibited a high degree of co-localization in neuronal tracts (Figure 4B). Unlike DYSC, however, we did not observe strong SLO staining in the mushroom body lobes, calyx or the ellipsoid body (Figure S7). Given the degree of overlapping expression, we examined whether SLO expression was altered in dysc mutants. Remarkably, SLO staining in neuronal tracts was undetectable in dysc mutants (Figure 4A), and the only remaining SLO signal within the brain was localized to the mushroom body peduncle. To test whether voltage-gated potassium channels in general were affected in dysc mutants, we examined the expression and localization of the A-type potassium channel, Shaker. In contrast to SLO, Shaker protein levels and localization within the brain were unaffected in dysc flies (Figure 4C–4D). Thus, DYSC specifically regulates the expression of a potassium channel subtype.
Fig. 4. DYSC regulates expression of SLO. 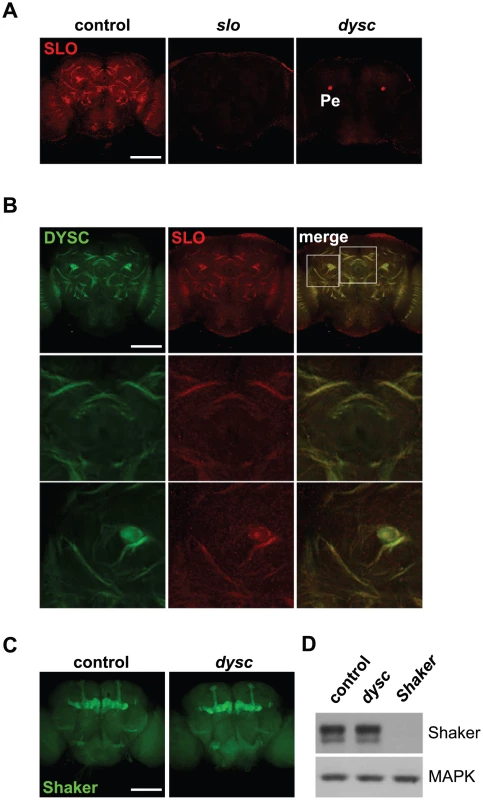
(A) Maximum-intensity projections of confocal sections illustrating enrichment of SLO in neuronal tracts in the medial compartment of the central brain of wild-type control (left panel), slo4 (middle) and dyscc03838 (right) males. SLO staining in neuronal tracts was undetectable in both slo4 and dyscc03838 mutants. However, we still observed robust SLO immuno-reactivity in the mushroom body peduncle (Pe) of dyscc03838 homozygotes. Scale bar represents 100 µm. (B) DYSC and SLO show strong co-localization in the adult brain. Upper panels, 2 µm confocal slice of a medial section of the adult brain. Middle and lower panels show magnified images of regions indicated in the upper right panel. (C–D) Shaker localization and expression is not altered in dysc mutants. (C) Confocal projection of Shaker expression in adult control and dyscc03838 male brains. (D) Shaker protein expression in head extracts of wild-type control, dyscc03838, and ShakerDf flies. MAPK was used as a loading control. The experiment was performed three times with similar results. SLO-Dependent Currents Are Markedly Reduced in dysc Mutants
To assess the cellular consequences of the regulation of SLO by DYSC, we performed in vivo whole-cell patch-clamp electrophysiology on adult dilp2-positive neurons in wild-type and dyscs168 adult brains. These neurons are located in the pars intercerebralis (PI) and have previously been shown to express a SLO-dependent Ca2+-activated non-inactivating potassium current [39]. We chose these neurons for their easy accessibility, and because neurons in the PI were positively labeled by the dysc-Gal4 driver (Figure S4A). Voltage-dependent outward potassium currents were evoked by depolarizing voltage steps in the whole-cell recording mode (Figure 5A). To examine the proportion of non-inactivating potassium component (which includes SLO channels) in the total outward current, we initially applied voltage pulses to pulse potentials ranging from −60 mV to +50 mV from a holding potential of −70 mV. Subsequently, outward currents from the same neuron were evoked via a similar protocol but from a holding potential of −30 mV. Inactivating channels are predominantly inactivated when the membrane potential is held at −30 mV, and non-inactivating channels (including SLO) can thus be isolated from the total outward current. We observed that the outward current at +50 mV in wild-type neurons showed a moderate reduction when the membrane potential was held at −30 mV relative to −70 mV (Figure 5A–5B); in dysc mutants, the outward current showed a much greater reduction when the membrane potential was held at −30 mV (Figure 5A–5B), indicating a loss of non-inactivating currents in dysc mutants.
Fig. 5. Reduced SLO channel currents in dysc mutants. 
(A) Voltage-dependent outward currents were recorded from dilp2 neurons in wild-type (upper panels) and dyscs168 adult brains (middle panels) in the whole cell patch recording mode. Whole-cell outward currents were evoked by 150 ms depolarizing voltage steps to −60 mV to +50 mV in 10 mV increments (bottom panels), from a holding potential of −70 mV (left) and −30 mV (right) in the same neuron. (B) Quantification of the effect of holding potential in wild-type flies and dysc mutants. Control dilp2 neurons: N = 7; dyscs168 dilp2 neurons: N = 5. * p<0.05, one-tailed Mann-Whitney U-test. (C) Effects of bath application of 2 mM CaCl2 and 1 mM TEA on outward currents in wild-type dilp2 neurons (upper panels) and dyscs168 dilp2 neurons (lower panels). Outward currents were generated by repetitive pulses to a single voltage, +50 mV, from the holding potential of −30 mV (left). Peak current amplitudes plotted against time are also shown (right). Black and gray bars indicate application of CaCl2 and TEA, respectively. (D–E) Quantification of the changes in current elicited by addition of 2 mM CaCl2 (D) or 1 mM TEA (E) in control and dyscs168 dilp2 neurons. Application of 2 mM CaCl2 induces a smaller increase in outward currents in dyscs168 dilp2 neurons relative to wild-type (D). The extent of inhibition by TEA is also far lower in dyscs168 dilp2 neurons (E). Control dilp2 neurons: N = 4; dyscs168 dilp2 neurons: N = 3. Error bars represent SEM. * p<0.05, one-tailed Mann-Whitney U-test. To determine what proportion of the non-inactivating outward current is carried by SLO potassium currents, we examined the effect of extracellular Ca2+ on outward currents in dilp2-neurons, since the SLO channel is highly activated by intracellular Ca2+ that enters through Ca2+ channels. In wild-type neurons, adding 2 mM CaCl2 significantly potentiated the non-inactivating component of the current (Figure 5C–5D). In contrast, the non-inactivating currents in dysc dilp2-neurons exhibited only a slight increase upon addition of CaCl2. Furthermore, while the application of 1 mM tetraethylammonium (TEA), a blocker of SLO channels [40], reduced the total outward current by 63% in wild-type neurons, it produced only a 17% reduction in dysc neurons (Figure 5C and 5E). Thus, the non-inactivating outward current in dilp2-neurons is predominantly carried by SLO channels, and is markedly reduced in dysc mutants. These results are in accord with our data showing greatly reduced SLO channel expression in dysc mutants (Figure 4).
DYSC and SLO Form a Mutually Dependent Complex
Finally, we asked if DYSC and SLO exhibit a mutually dependent relationship, since such co-dependence has been demonstrated between Whirlin and its binding partners VLGR1 and Usherin [27], [36], [37]. Interestingly, DYSC in neuronal tracts was largely undetectable in slo4 mutants, yet DYSC expression in the mushroom body, ellipsoid body and antennal lobes, areas that do not robustly express SLO, remained intact (Figure 6A–6B). In fact, we noted a significant increase in DYSC levels in the mushroom body lobes in slo4 mutants relative to controls (α/β-lobes: increase = 33.1±8%, p<0.05, Mann-Whitney U-test; γ-lobes: increase = 41.9±8%, p<0.001; controls: n = 13 brains, slo4: n = 10). The mechanistic basis for this increase in DYSC levels is unclear. One possibility is that in the mushroom bodies, DYSC has a mutually dependent relationship with an unidentified protein that is upregulated in the absence of SLO, which leads to an increase in DYSC.
Fig. 6. SLO and DYSC form a mutually dependent complex. 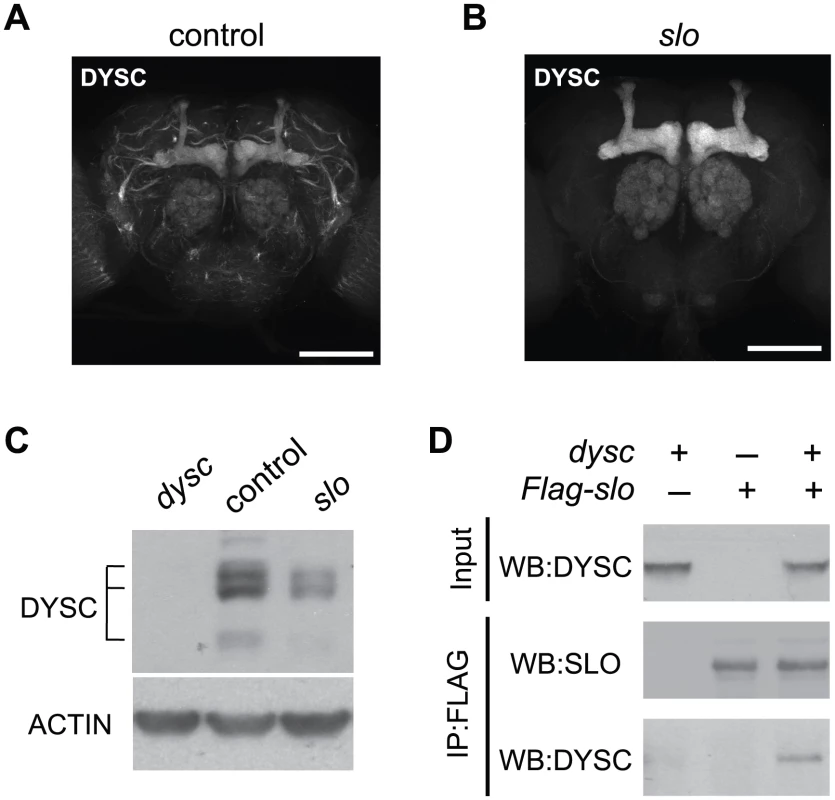
(A–B) Maximal intensity confocal projections showing DYSC expression in wild-type control (A) and slo4 (B) adult brains. Relative to control flies, DYSC expression in neuronal tracts is severely reduced in slo4 mutants. In contrast, DYSC expression in the mushroom body lobes, antennal lobes and ellipsoid body remains intact in slo4 mutants. Scale bar represents 100 µm. (C) Loss of SLO reduces DYSC expression in head extracts. Both long and short isoforms of DYSC show a clear reduction in slo4 mutants. Actin was used as a loading control. (D) DYSC physically interacts with SLO in HEK-tsA cells. Cultured cells were transfected with constructs encoding DYSC and FLAG-tagged SLO, or either construct alone. Cell extracts were immuno-precipitated (IP) using an anti-FLAG antibody, and subjected to western blotting (WB) using an anti-DYSC or anti-SLO antibody. Loss of SLO also resulted in a substantial reduction in total head DYSC protein levels (Figure 6C). SLO and DYSC thus exhibit a reciprocal requirement for expression in neuronal tracts. We did not observe any reduction of dysc mRNA levels in slo4 mutants, nor any change in slo mRNA levels in dysc mutants (Figure S8), indicating that the mutual regulation of DYSC and SLO is a post-transcriptional effect.
The reciprocal requirement of SLO and DYSC suggests formation of a stable complex. To examine whether the two proteins can physically interact, we performed co-immunoprecipitation experiments in human embryonic kidney (HEK-tsA) cells. When co-expressed with SLO, DYSC was co-immunoprecipitated with SLO, but not when expressed without SLO (Figure 6D). These data suggest that the two proteins regulate each other's expression through direct protein-protein interaction. In summary, our data identify DYSC as a novel binding partner and regulator of SLO, and suggests that the arrhythmic phenotype exhibited by dysc mutants is in part due to a loss of SLO channels.
Discussion
Here we describe a novel mutant, dysc, which exhibits arrhythmic locomotor patterns in DD conditions. Analysis of clock protein oscillations and eclosion rhythms in dysc mutants, as well as targeted rescue of the arrhythmic dysc phenotype, all indicate that dysc is a crucial constituent of the circadian output pathway and acts downstream of the core clock.
One intriguing aspect of dysc function is its ontology. Comparative genomics identifies dysc as the closest Drosophila homolog of whirlin, a loci associated with nonsyndromic deafness and Type II Usher syndrome (USH2) in humans [22], [41]. USH is a genetically heterogeneous disorder associated with alterations in cochlear stereocilia structure, vestibular dysfunction and retinitis pigmentosa, resulting in deaf-blindness with varying ages of onset [23]. Our study demonstrates that Usher proteins can also play a crucial role in complex behaviors. This is intriguing given the broad expression of many Usher proteins in the mammalian nervous system, and the lack of functional roles ascribed to the Usher interactome in the brain [29], [30]. Our data also point to a plausible mechanism by which an Usher protein homolog regulates a behavioral output: the control of ion channel expression. This parallels the role of several other PDZ domain-containing proteins in both the mammalian and Drosophila nervous systems, such as members of the PSD-95 family, which serve to cluster potassium channels at axons and synapses [42]–[44].
Recent data indicate that the Usher interactome also includes ion channels. Harmonin, a PDZ-containing protein linked to USH1, co-localizes with and negatively regulates the Cav1.3 voltage-gated calcium channel in inner hair cells [38]. We identify a novel interaction between an Usher protein homolog and the SLO Ca2+-activated potassium channel. DYSC physically interacts with SLO, and in the absence of DYSC, SLO expression in neuronal tracts as well as SLO currents in dilp2-neurons in vivo are markedly reduced. DYSC's influence on other potassium channels appears to be limited, since Shaker expression was unaffected in dysc mutants, and in dysc dilp2-neurons robust outward potassium currents were still detected, albeit with a reduced non-inactivating component caused by the loss of SLO expression. Thus, in contrast to the relationship between Harmonin and Cav1.3 [38], DYSC positively regulates SLO expression in the Drosophila brain. Usher proteins and their homologs can therefore both promote and inhibit ion channel function in a subtype-specific manner. It is also noteworthy that both Harmonin's and DYSC's effect on cellular physiology via control of Cav1.3 and SLO respectively is to reduce the excitability of the cell and synaptic output. Thus, one question arising from these studies is whether Usher proteins generally act to negatively tune neuronal excitability. Further studies investigating potential interactions between Usher proteins and other ion channels will help to shed light on this intriguing issue.
We also demonstrate a mutually dependent relationship between DYSC and SLO. In mammals, this finding is paralleled by similar relationships between several Usher proteins and their binding partners [27], [37], [38], and between potassium channels and their associated proteins [45], [46]. Interestingly, whereas loss of SLO greatly reduces DYSC levels in major neuronal tracts in most brain regions, it has an opposite effect in the mushroom body, ellipsoid body, and antennal lobes. In addition, SLO expression is detectable only in the peduncle of the mushroom bodies in dysc mutants. These findings raise the possibility that DYSC has a mutually dependent relationship with different proteins depending on the cell type. Given the broad expression in the brain and its interaction with SLO, DYSC is likely to have pleiotropic effects on behavior. SLO is involved in multiple complex behaviors, including the production of courtship songs and ethanol sensitivity [47], [48]. It will be interesting to investigate whether DYSC is also involved in these behaviors.
SLO channels have previously been implicated in the circadian output circuit [7], suggesting a mechanism by which DYSC affects rhythmic behavior. Previous work has demonstrated that loss of SLO de-synchronizes clock protein oscillations in DN clusters [7]. In contrast, in dysc mutants the molecular clock is unaffected in these neurons. It is possible that a sufficient level of SLO remains in dysc mutants to maintain clock protein cycling. Given our results and the previous finding that restoring SLO in clock cells is not sufficient for a full rescue of the arrhythmic phenotype of slo mutants [7], it is likely that SLO performs an important role in the intermediate circuit between the clock and motor neurons where DYSC is required, as well as in clock cells. We propose that DYSC links the central clock output to locomotor activity by regulating membrane excitability, in part through its control of SLO expression. Thus, precise control of neuronal excitability is required not only for correct clock neuron function [49], [50], but also in downstream circuits that connect clock cells to motor neuron targets.
In conclusion, we have identified a novel Drosophila ion channel regulator and human disease gene homolog that impacts complex behavior. In addition to shedding new light on genetic components of the circadian output pathway, our results suggest new roles for Whirlin in the mammalian nervous system. It will be interesting to determine if whirlin mutants are also arrhythmic, and whether Whirlin similarly regulates SLO expression in the mammalian brain.
Materials and Methods
Drosophila Strains
Flies were reared on standard food containing cornmeal, yeast, and molasses. The s168 mutant strain was isolated from an ongoing screen for sleep and circadian mutants. Novel strains carrying random insertions of the P[XP] transposable element in a white (iso31) background were generated using the Δ2–3 transposase. Sleep and circadian behavior was assayed as previously described [51]. Inverse PCR revealed that the s168 line carries a P-element insertion in the dysc locus. Two additional P-element insertion alleles of dysc, c05107 and c03838, were obtained from the Exelixis collection at the Harvard Medical School. All three alleles were backcrossed to the iso31 strain at least 5 times, and balanced mutant and sibling control lines were established. The BSC614 deficiency line that removes the dysc locus and OK371-, c819-, c107-, and c061-Gal4 lines were obtained from the Bloomington Stock Center. c164-Gal4 was obtained from L. Griffith (Brandeis University). Other drivers and the Shaker deficiency line were obtained as previously described [46], [52].
Precise excision lines were derived from the s168 line by a transposase-mediated mobilization of the P element. We identified three precise excision lines by PCR amplification and sequencing. Preliminary results indicated that they had similar circadian behavior, and data from one of them are presented. We screened ∼150 excision lines, but were unable to obtain imprecise excision lines that remove coding regions.
Behavioral Assays
To monitor circadian locomotor behavior, 2 - to 5-day old male flies, entrained to a 12 h∶12 h LD cycle for at least 3 days, were put into glass tubes containing 5% sucrose and 2% agar, and their activity was monitored using the Drosophila Activity Monitoring System (Trikinetics) at 25°C. For quantification of circadian behavior, activity counts were collected in 30-min bins over a 6-day period in DD. Actograms were generated using ClockLab (Actimetrics), and circadian period and power of rhythmicity were determined using Fly Activity Analysis Suite for Mac OSX (FaasX, M. Boudinot). The power of rhythmicity is defined as the difference between the χ2 value and the significance value at p = 0.05. Flies with power of less than 25 were considered arrhythmic, between 25 and 50, weakly rhythmic, and over 50, rhythmic. Circadian period was determined for rhythmic flies only, whereas power of rhythmicity was determined for all flies, including arrhythmic and weakly rhythmic ones. Locomotor patterns in 12 h∶12 h LD conditions were calculated as follows: single-fly activity was monitored over a three day period and averaged to generate a mean 24 h activity plot. This activity plot was then further averaged across the experimental population. For analysis of eclosion behavior, pupae entrained to a 12 h∶12 h LD cycle throughout development were taped to eclosion monitors (Trikinetics) using double-sided tape. Data were collected in 1-h bins over a 4-day period in DD at 25°C. Climbing assays were performed as described previously [46].
Transgenic Fly Lines
Fly head mRNA was extracted using the Ultraspec RNA Isolation System (Biotecx) and reverse transcribed using High Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems). To generate the UAS-dysc construct, dysc cDNA was PCR-amplified in two pieces using two sets of primers: for N-terminus: 5′-CTG AAT TCC CAG CAG TGT AAT GC-3′ and 5′-CGA GAA AGG ATT GCC CAT T-3′; for C-terminus: 5′-CGA TCC GGA CTG ATG ATT G-3′ and 5′-CTG GTA CCG GCA GGG CAA GC-3′. The N - and C-terminus fragments were subcloned into the TOPO TA-cloning vector (Invitrogen), sequenced, and subsequently inserted into the pUAST vector. The cloned cDNA represents a novel isoform, differing slightly from other long isoforms in FlyBase (www.flybase.org) through alternative splicing, and we have designated it Isoform G. The C-terminus of the short isoform (F) was amplified using the following primers: 5′-CGA TCC GGA CTG ATG ATT G-3′ and 5′-CTG GTA CCT AAG TGT ATA TAG TGT CTG-3′. To generate the dysc-Gal4 construct, approximately 4.5 Kb upstream of the transcriptional start site was PCR-amplified from genomic DNA using the following primers: 5′-TCC TGC CTC TGG ATC CCG CCA CGT TG-3′ and 5′-ACG CGG CCG CGG CTT CAA ACC AAA TCA GC-3′. The PCR fragment was inserted into the pPT-Gal vector. Transgenic fly lines carrying the UAS-dysc or dysc-Gal4 construct were generated by standard germline transformation in the iso31 background (Rainbow Transgenics).
Antibody Production and Western Blot Analysis
The rat polyclonal antibody to DYSC (TJR43) was raised against a portion of the DYSC protein fused to N - and C-terminal 6× HIS tags. A PCR fragment amplified using the primers 5′-CCG AAT TCT GCA CCT CCA TCG A-3′ and 5′-CCT TCG ATA GCA ATA CCT CGA GTT-3′ was inserted into the pET-28a vector. Protein expression and purification was performed at the Protein Expression Facility of Wistar Institute. The antibody recognizes both long and short isoforms of DYSC. The rabbit polyclonal antibody to SLO (763) was a generous gift from I. Levitan, and will be described elsewhere. While the antibody detected SLO-specific signal in immuno-staining assays, it could not detect SLO on Western blots due to masking by a non-specific band present in slo4 mutants.
Western blot experiments were carried out essentially as described [46] except that fly heads were homogenized and lysed in 2× SDS sample buffer containing 5% β-mercaptoethanol. Antibodies to PER (PA1139), DYSC (TJR43), SLO (763), and Shaker (UPR55) [46] were used at 1∶1000. Antibodies to MAPK (Sigma) and β-ACTIN (Abcam) were used at 1∶10,000. Western blot experiments were repeated at least three times except as noted, and representative blots are shown.
Immunohistochemistry
To examine cycling of PER and PDF in the central clock cells, young female flies (1–4 days old) were entrained to a 12 h∶12 h LD cycle for at least 3 days, and were collected at indicated times during the second day in DD. Dissected brains were fixed in 4% paraformaldehyde, and incubated overnight in antibodies to PER (UPR34) and PDF (HH74) [24] diluted 1∶1000. PER and PDF levels were judged through visual inspection. For DYSC and SLO staining, male flies were used. Rat anti-DYSC and rabbit anti-SLO antibodies were used at 1∶400 and 1∶1000, respectively. Fluorophore-conjugated secondary antibodies were obtained from Invitrogen. Brains stained with PER and PDF antibodies were imaged with a Leica TCS-SP5 confocal microscope, and those stained with DYSC and SLO antibodies were imaged with an Olympus Fluoview confocal microscope. Samples for comparison were processed at the same time and imaged with the same settings at sub-saturation intensities. At least five brains were examined per condition. To quantify DYSC levels in the mushroom body lobes, average pixel intensities in the α/β - and γ-lobes of the mushroom bodies were determined in each brain hemisphere using Image J. For each pair of lobes, a mean value was calculated, yielding a single value for α/β - and γ-lobes for each brain. Data from paired batches of control and slo4 brains were normalized to the mean of the controls for each batch.
Co-Immunoprecipitation
For co-immunoprecipitation (co-IP) experiments, the coding region of the G isoform of dysc was inserted into the pcDNA3 expression vector using standard molecular biology techniques. The O isoform of SLO (www.flybase.org) was tagged with the 3×FLAG epitope via PCR-driven overlap extension, and was cloned into the pcDNA3 vector. HEK-tsA cells were transfected with various combinations of dysc and Flag-slo constructs (330 ng each) in 60 mm Petri dishes using Effectene (Qiagen). pCDNA3 vector DNA was included in some conditions to make the total amount of DNA equal in all conditions, and pIRES-GFP (330 ng) was included in all conditions to monitor transfection efficiency. Co-IP was performed essentially as described [46] except that cells were lysed in extraction buffer containing 50 mM KCl, 10 mM HEPES, 2 mM EDTA, 5 mM Tris at pH 7.5, 1% Triton X-100, 10% glycerol, 10 µg/mL leupeptin, 10 µg/mL aprotinin, 2 µg/mL pepstatin A, 0.5 mM PMSF, 1 mM Na3VO4, 10 mM r-nitrophenyl phosphate, pH 7.5, and an antibody to FLAG (Sigma) was used.
Real-Time Reverse-Transcriptase (RT) PCR
cDNAs from fly heads were generated as described above. Real-time RT-PCR was performed using SYBR green (Applied Biosystems) with the following primers: 5′-CGG CAT TTG CGT TAA AGG AG-3′ and 5′-GAG ATG TAG ACG CCT AAG CCT GAG-3′ for dysc, and 5′-GTC GTA CGG AAT GCT GTG CA-3′ and 5′-GAG CTG GTG TCC CTG AAT CG-3′ for slo. Both sets of primers recognize regions common to all isoforms.
In Vivo Electrophysiology
Dilp2-positive neurons were labeled by driving a membrane-tagged GFP (CD8::GFP) using the dilp2-Gal4 driver, expressed in either a wild-type or dyscs168 background. For in vivo patch recording from PI neurons [33], [39] flies were anesthetized with CO2 and glued ventral side down to a glass coverslip. The coverslip was placed in a chamber containing extracellular solution (101 mM NaCl, 3 mM KCl, 4 mM MgCl2, 1.25 mM NaH2PO4, 20.7 mM NaHCO3, 5 mM glucose [pH 7.2]) and then the cuticle was peeled off using fine forceps to expose the surface of the brain. The chamber was placed on the stage of an Olympus BX51 fluorescent microscope, and PI neurons were identified by their location and fluorescence. Patch-recording electrodes (WPI) were fire polished, and had resistances from 3 to 4 MΩ when filled with intracellular solution (102 mM K-gluconate, 17 mM NaCl, 2 mM CaCl2, 0.5 mM MgCl2, 5 mM EGTA, 10 mM HEPES, pH 7.2). Standard techniques were used to record macroscopic currents in the whole-cell voltage-clamp mode with an Axopatch 200A amplifier (Molecular Devices). Data were digitized with a Digidata 1322A interface (Molecular Devices) and stored on a PC hard drive for further analysis with pClamp9 software (Molecular Devices).
Statistical Analysis
For comparison of rhythm strength between pairs of conditions, Student's t-tests (unpaired, two-tailed) were performed with Bonferroni correction for multiple comparisons. When comparing multiple experimental genotypes to controls, one-way ANOVA with Dunnett post-hoc tests were used. For electrophysiology data, Mann-Whitney U-tests were performed. Significance values were calculated using Kaleidograph (Synergy Software) or Excel (Microsoft).
Supporting Information
Zdroje
1. AlladaRChungBY 2010 Circadian organization of behavior and physiology in Drosophila. Annu Rev Physiol 72 605 624
2. CerianiMFHogeneschJBYanovskyMPandaSStraumeM 2002 Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J Neurosci 22 9305 9319
3. McDonaldMJRosbashM 2001 Microarray analysis and organization of circadian gene expression in Drosophila. Cell 107 567 578
4. NagoshiESuginoKKulaEOkazakiETachibanaT 2010 Dissecting differential gene expression within the circadian neuronal circuit of Drosophila. Nat Neurosci 13 60 68
5. SheebaVGuHSharmaVKO'DowdDKHolmesTC 2008 Circadian - and light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons. J Neurophysiol 99 976 988
6. CaoGNitabachMN 2008 Circadian control of membrane excitability in Drosophila melanogaster lateral ventral clock neurons. J Neurosci 28 6493 6501
7. FernandezMPChuJVillellaAAtkinsonNKaySA 2007 Impaired clock output by altered connectivity in the circadian network. Proc Natl Acad Sci U S A 104 5650 5655
8. HyunSLeeYHongSTBangSPaikD 2005 Drosophila GPCR Han is a receptor for the circadian clock neuropeptide PDF. Neuron 48 267 278
9. LearBCLinJMKeathJRMcGillJJRamanIM 2005 The ion channel narrow abdomen is critical for neural output of the Drosophila circadian pacemaker. Neuron 48 965 976
10. LearBCMerrillCELinJMSchroederAZhangL 2005 A G protein-coupled receptor, groom-of-PDF, is required for PDF neuron action in circadian behavior. Neuron 48 221 227
11. MertensIVandingenenAJohnsonECShaferOTLiW 2005 PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron 48 213 219
12. RennSCParkJHRosbashMHallJCTaghertPH 1999 A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99 791 802
13. SuhJJacksonFR 2007 Drosophila ebony activity is required in glia for the circadian regulation of locomotor activity. Neuron 55 435 447
14. WilliamsJASuHSBernardsAFieldJSehgalA 2001 A circadian output in Drosophila mediated by neurofibromatosis-1 and Ras/MAPK. Science 293 2251 2256
15. PerezGLagruttaAAdelmanJPToroL 1994 Reconstitution of expressed KCa channels from Xenopus oocytes to lipid bilayers. Biophys J 66 1022 1027
16. AdelmanJPShenKZKavanaughMPWarrenRAWuYN 1992 Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron 9 209 216
17. ElkinsTGanetzkyB 1988 The roles of potassium currents in Drosophila flight muscles. J Neurosci 8 428 434
18. ElkinsTGanetzkyBWuCF 1986 A Drosophila mutation that eliminates a calcium-dependent potassium current. Proc Natl Acad Sci U S A 83 8415 8419
19. SaitoMWuCF 1991 Expression of ion channels and mutational effects in giant Drosophila neurons differentiated from cell division-arrested embryonic neuroblasts. J Neurosci 11 2135 2150
20. MeredithALWilerSWMillerBHTakahashiJSFodorAA 2006 BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci 9 1041 1049
21. KohKJoinerWJWuMNYueZSmithCJ 2008 Identification of SLEEPLESS, a sleep-promoting factor. Science 321 372 376
22. MburuPMustaphaMVarelaAWeilDEl-AmraouiA 2003 Defects in whirlin, a PDZ domain molecule involved in stereocilia elongation, cause deafness in the whirler mouse and families with DFNB31. Nat Genet 34 421 428
23. ReinersJNagel-WolfrumKJurgensKMarkerTWolfrumU 2006 Molecular basis of human Usher syndrome: deciphering the meshes of the Usher protein network provides insights into the pathomechanisms of the Usher disease. Exp Eye Res 83 97 119
24. MyersEMYuJSehgalA 2003 Circadian control of eclosion: interaction between a central and peripheral clock in Drosophila melanogaster. Curr Biol 13 526 533
25. ZhangLChungBYLearBCKilmanVLLiuY 2010 DN1(p) circadian neurons coordinate acute light and PDF inputs to produce robust daily behavior in Drosophila. Curr Biol 20 591 599
26. NourryCGrantSGBorgJP 2003 PDZ domain proteins: plug and play! Sci STKE 2003 RE7
27. YangJLiuXZhaoYAdamianMPawlykB 2010 Ablation of whirlin long isoform disrupts the USH2 protein complex and causes vision and hearing loss. PLoS Genet 6 e1000955 doi:10.1371/journal.pgen.1000955
28. MburuPKikkawaYTownsendSRomeroRYonekawaH 2006 Whirlin complexes with p55 at the stereocilia tip during hair cell development. Proc Natl Acad Sci U S A 103 10973 10978
29. van WijkEvan der ZwaagBPetersTZimmermannUTe BrinkeH 2006 The DFNB31 gene product whirlin connects to the Usher protein network in the cochlea and retina by direct association with USH2A and VLGR1. Hum Mol Genet 15 751 765
30. LeinESHawrylyczMJAoNAyresMBensingerA 2007 Genome-wide atlas of gene expression in the adult mouse brain. Nature 445 168 176
31. KeeneACWaddellS 2007 Drosophila olfactory memory: single genes to complex neural circuits. Nat Rev Neurosci 8 341 354
32. StraussR 2002 The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol 12 633 638
33. CrockerAShahidullahMLevitanIBSehgalA 2010 Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron 65 670 681
34. SlawsonJBKuklinEAEjimaAMukherjeeKOstrovskyL 2011 Central regulation of locomotor behavior of Drosophila melanogaster depends on a CASK isoform containing CaMK-like and L27 domains. Genetics 187 171 184
35. AbruzziKCRodriguezJMenetJSDesrochersJZadinaA 2011 Drosophila CLOCK target gene characterization: implications for circadian tissue-specific gene expression. Genes Dev 25 2374 2386
36. AdatoALefevreGDelpratBMichelVMichalskiN 2005 Usherin, the defective protein in Usher syndrome type IIA, is likely to be a component of interstereocilia ankle links in the inner ear sensory cells. Hum Mol Genet 14 3921 3932
37. MichalskiNMichelVBahloulALefevreGBarralJ 2007 Molecular characterization of the ankle-link complex in cochlear hair cells and its role in the hair bundle functioning. J Neurosci 27 6478 6488
38. GregoryFDBryanKEPangrsicTCalin-JagemanIEMoserT 2011 Harmonin inhibits presynaptic Cav1.3 Ca(2) channels in mouse inner hair cells. Nat Neurosci 14 1109 1111
39. ShahidullahMReddySFeiHLevitanIB 2009 In vivo role of a potassium channel-binding protein in regulating neuronal excitability and behavior. J Neurosci 29 13328 13337
40. ShenKZLagruttaADaviesNWStandenNBAdelmanJP 1994 Tetraethylammonium block of Slowpoke calcium-activated potassium channels expressed in Xenopus oocytes: evidence for tetrameric channel formation. Pflugers Arch 426 440 445
41. EbermannISchollHPCharbel IssaPBecirovicELamprechtJ 2007 A novel gene for Usher syndrome type 2: mutations in the long isoform of whirlin are associated with retinitis pigmentosa and sensorineural hearing loss. Hum Genet 121 203 211
42. ArnoldDBClaphamDE 1999 Molecular determinants for subcellular localization of PSD-95 with an interacting K+ channel. Neuron 23 149 157
43. OgawaYHorreshITrimmerJSBredtDSPelesE 2008 Postsynaptic density-93 clusters Kv1 channels at axon initial segments independently of Caspr2. J Neurosci 28 5731 5739
44. ZitoKFetterRDGoodmanCSIsacoffEY 1997 Synaptic clustering of Fascilin II and Shaker: essential targeting sequences and role of Dlg. Neuron 19 1007 1016
45. FoegerNCMarionneauCNerbonneJM 2010 Co-assembly of Kv4 {alpha} subunits with K+ channel-interacting protein 2 stabilizes protein expression and promotes surface retention of channel complexes. J Biol Chem 285 33413 33422
46. WuMNJoinerWJDeanTYueZSmithCJ 2010 SLEEPLESS, a Ly-6/neurotoxin family member, regulates the levels, localization and activity of Shaker. Nat Neurosci 13 69 75
47. CowmeadowRBKrishnanHRAtkinsonNS 2005 The slowpoke gene is necessary for rapid ethanol tolerance in Drosophila. Alcohol Clin Exp Res 29 1777 1786
48. PeixotoAAHallJC 1998 Analysis of temperature-sensitive mutants reveals new genes involved in the courtship song of Drosophila. Genetics 148 827 838
49. NitabachMNBlauJHolmesTC 2002 Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell 109 485 495
50. NitabachMNWuYSheebaVLemonWCStrumbosJ 2006 Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. J Neurosci 26 479 489
51. WuMNKohKYueZJoinerWJSehgalA 2008 A genetic screen for sleep and circadian mutants reveals mechanisms underlying regulation of sleep in Drosophila. Sleep 31 465 472
52. JoinerWJCrockerAWhiteBHSehgalA 2006 Sleep in Drosophila is regulated by adult mushroom bodies. Nature 441 757 760
Štítky
Genetika Reprodukční medicína
Článek A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target GenesČlánek Population Structure of Hispanics in the United States: The Multi-Ethnic Study of AtherosclerosisČlánek Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms inČlánek Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome AnalysisČlánek Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and MechanismČlánek Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 4- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
-
Všechny články tohoto čísla
- Runs of Homozygosity Implicate Autozygosity as a Schizophrenia Risk Factor
- Modifier Genes and the Plasticity of Genetic Networks in Mice
- The DSIF Subunits Spt4 and Spt5 Have Distinct Roles at Various Phases of Immunoglobulin Class Switch Recombination
- A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target Genes
- Population Structure of Hispanics in the United States: The Multi-Ethnic Study of Atherosclerosis
- Deep Sequencing of Plant and Animal DNA Contained within Traditional Chinese Medicines Reveals Legality Issues and Health Safety Concerns
- Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms in
- Insulin Signaling Mediates Sexual Attractiveness in
- Progressive Telomere Dysfunction Causes Cytokinesis Failure and Leads to the Accumulation of Polyploid Cells
- Long-Range Chromosome Organization in : A Site-Specific System Isolates the Ter Macrodomain
- Regulation of Budding Yeast Mating-Type Switching Donor Preference by the FHA Domain of Fkh1
- Polyglutamine Toxicity Is Controlled by Prion Composition and Gene Dosage in Yeast
- Patterns of Regulatory Variation in Diverse Human Populations
- Sequence-Specific Targeting of Dosage Compensation in Favors an Active Chromatin Context
- Whole-Exome Sequencing and Homozygosity Analysis Implicate Depolarization-Regulated Neuronal Genes in Autism
- Replication Fork Reversal after Replication–Transcription Collision
- Common Variants at 9p21 and 8q22 Are Associated with Increased Susceptibility to Optic Nerve Degeneration in Glaucoma
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Epigenome-Wide Scans Identify Differentially Methylated Regions for Age and Age-Related Phenotypes in a Healthy Ageing Population
- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Budding Yeast Dma Proteins Control Septin Dynamics and the Spindle Position Checkpoint by Promoting the Recruitment of the Elm1 Kinase to the Bud Neck
- , a Homolog of a Deaf-Blindness Gene, Regulates Circadian Output and Slowpoke Channels
- Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome Analysis
- Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and Mechanism
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
- SPE-44 Implements Sperm Cell Fate
- A Shared Role for RBF1 and dCAP-D3 in the Regulation of Transcription with Consequences for Innate Immunity
- A Companion Cell–Dominant and Developmentally Regulated H3K4 Demethylase Controls Flowering Time in via the Repression of Expression
- The HEN1 Ortholog, HENN-1, Methylates and Stabilizes Select Subclasses of Germline Small RNAs
- Improved Statistics for Genome-Wide Interaction Analysis
- The Probability of a Gene Tree Topology within a Phylogenetic Network with Applications to Hybridization Detection
- Context-Dependent Dual Role of SKI8 Homologs in mRNA Synthesis and Turnover
- Mu Insertions Are Repaired by the Double-Strand Break Repair Pathway of
- Competition between Replicative and Translesion Polymerases during Homologous Recombination Repair in Drosophila
- An Unbiased Assessment of the Role of Imprinted Genes in an Intergenerational Model of Developmental Programming
- Type 2 Diabetes Risk Alleles Demonstrate Extreme Directional Differentiation among Human Populations, Compared to Other Diseases
- Mutations in and Cause “Splashed White” and Other White Spotting Phenotypes in Horses
- Fine-Scale Mapping of Natural Variation in Fly Fecundity Identifies Neuronal Domain of Expression and Function of an Aquaporin
- Dynamics of Brassinosteroid Response Modulated by Negative Regulator LIC in Rice
- Genetic Inhibition of Solute-Linked Carrier 39 Family Transporter 1 Ameliorates Aβ Pathology in a Model of Alzheimer's Disease
- The Functions of Mediator in Support a Role in Shaping Species-Specific Gene Expression
- Patterns of Ancestry, Signatures of Natural Selection, and Genetic Association with Stature in Western African Pygmies
- Dissection of Pol II Trigger Loop Function and Pol II Activity–Dependent Control of Start Site Selection
- PIWI Associated siRNAs and piRNAs Specifically Require the HEN1 Ortholog
- Genome-Wide Patterns of Gene Expression in Nature
- Hypoxia Disruption of Vertebrate CNS Pathfinding through EphrinB2 Is Rescued by Magnesium
- A New Role for Translation Initiation Factor 2 in Maintaining Genome Integrity
- Sex Reversal in C57BL/6J XY Mice Caused by Increased Expression of Ovarian Genes and Insufficient Activation of the Testis Determining Pathway
- The Rac GTP Exchange Factor TIAM-1 Acts with CDC-42 and the Guidance Receptor UNC-40/DCC in Neuronal Protrusion and Axon Guidance
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



