-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaModifier Genes and the Plasticity of Genetic Networks in Mice
Modifier genes are an integral part of the genetic landscape in both humans and experimental organisms, but have been less well explored in mammals than other systems. A growing number of modifier genes in mouse models of disease nonetheless illustrate the potential for novel findings, while new technical advances promise many more to come. Modifier genes in mouse models include induced mutations and spontaneous or wild-derived variations captured in inbred strains. Identification of modifiers among wild-derived variants in particular should detect disease modifiers that have been shaped by selection and might therefore be compatible with high fitness and function. Here we review selected examples and argue that modifier genes derived from natural variation may provide a bias for nodes in genetic networks that have greater intrinsic plasticity and whose therapeutic manipulation may therefore be more resilient to side effects than conventional targets.
Published in the journal: . PLoS Genet 8(4): e32767. doi:10.1371/journal.pgen.1002644
Category: Review
doi: https://doi.org/10.1371/journal.pgen.1002644Summary
Modifier genes are an integral part of the genetic landscape in both humans and experimental organisms, but have been less well explored in mammals than other systems. A growing number of modifier genes in mouse models of disease nonetheless illustrate the potential for novel findings, while new technical advances promise many more to come. Modifier genes in mouse models include induced mutations and spontaneous or wild-derived variations captured in inbred strains. Identification of modifiers among wild-derived variants in particular should detect disease modifiers that have been shaped by selection and might therefore be compatible with high fitness and function. Here we review selected examples and argue that modifier genes derived from natural variation may provide a bias for nodes in genetic networks that have greater intrinsic plasticity and whose therapeutic manipulation may therefore be more resilient to side effects than conventional targets.
A Brief Conceptual History of Modifier Genes
The concept of modifier genes originated with the solution to an early genetic mystery. As early workers tested Mendel's segregation ratios with observations on a wide range of traits and species, several cases of “inconstant inheritance” caused some to question the Mendelian basis of factors underlying these traits [1], [2]. Three cases of particular note are Beaded and truncate wings in fruit flies and pigmentation in hooded rats. In each of these cases, the phenotype varied widely among offspring of parents with established genotypes. Mutant frequencies did not follow Mendelian ratios and—more troubling still—varied among derived lines carrying the same mutation. This led some workers to question whether genes were constant physical units or changed in properties during transmission [3], [4] and was the last serious challenge to the theory of the gene and the generality of Mendelian inheritance. Resolution of this issue came with demonstrations that defined genetic backgrounds could account for the variation in truncate flies [5] and hooded rats [6], [7] and more precisely, by linkage mapping, that discrete genetic loci account for the observed variation in Beaded flies [8]. The term “modifier gene” was coined to indicate a genetic variant identified by its impact on a conditioning mutation, with no obvious phenotype of its own. This is the usage we will follow here (Box 1).
Box 1. What Is a Modifier Gene?
The term has been applied to several classes of genetic activities, some of which overlap. Genetic variations that alter the activity of a protein encoded by a second locus are one class. To the extent that the modifying effect is independent of allelic variation in the gene being modified, this usage is conceptually equivalent to any primary mutation whose phenotypic consequences include loss of interaction with its normal targets (as for a transcription factor and its target genes, or a protein kinase and its substrates). Interactions between mutations that were each previously recognized by their independent phenotypes are another class. While these genetic interactions can be revelatory, one need not invoke the term “modifier” to fully describe both individual and interaction effects, as each locus is identifiable without the other. Quantitative trait loci (QTL) are sometimes referred to as modifiers in the context of a major effect locus, which can be nearer the original meaning, depending on the structure of interactions in the QTL model. For the purposes of this review, we will use “modifier” to mean a genetic variant that is best recognized by its ability to change the phenotypic outcome of an independent “conditioning” variant at another locus. Modifier genes in this usage have no obvious phenotype of their own prior to discovery and are effectively silent—or at least quiet—with respect to the phenotype under study, in the absence of the conditioning mutation.
Early workers quickly appreciated that modifier genes are pervasive across experimental systems and organisms. Modifier genes need not be subtle and can have phenotypic effects as large as the initial conditioning mutation. These early observations contributed to the conceptualization of genetic pathways, prior to knowing the molecular identities of any components. This formal concept remains useful in analyzing genetic networks for sensitive nodes (genes) through which to manipulate mutant phenotypes in genetic disease or experimental biology.
Modifier Genes in Human Disease and Mouse Models: Ripe for Harvest
Modifier genes are also frequent in human disease and often invoked to explain divergent outcomes in genetic disorders with apparently equivalent cause. Among the best examples is cystic fibrosis (CF). CF patients homozygous for the Δ508 allele (approximately half of patients with European ancestry) present with a broad range of organ involvement and clinical severity, much of which is controlled by modifier genes [9]. Despite being among the most common Mendelian disorders and having an unusually common disease allele to minimize heterogeneity, molecularly defined modifiers have only very recently been reported [10], [11]. Just as CF was one of the first major victories in positional cloning of disease genes, its modifier gene network is one of the first to see real progress apart from candidate genes and serendipitous studies. Comparison between modifier studies in human patients and mouse models of CF [12]–[15] will be especially interesting, given replication of at least one syntenic locus between species [16]. Among rarer disorders, some ciliopathy phenotypes (and related developmental abnormalities) may require or be modified by tri-allelic inheritance—requiring three alleles among two or more loci—among functionally related genes [17]–[21]. However, these examples are the exceptions. Most modifier effects in human genetic disorders are much less well understood, and the numbers of available subjects, allelic heterogeneity, and environmental factors that obscure modifier effects in smaller cohorts may limit systematic analyses for a large number of individually rare disorders. This increases the potential value of animal models for both candidate discovery and experimental validation of human modifier genes.
Identification of modifier genes in mouse models offers an opportunity to understand forms of plasticity in mammalian genetic architectures that could be exploited as a preclinical knowledge base in designing therapeutic strategies [22]. A very common finding in mouse models is phenotypic difference between strain backgrounds (e.g., 129 versus C57BL/6 in hybrid lines from gene-targeting experiments), but the sources of variation are not often pursued because of technical and resource constraints. Limited access to these modifier genes is a missed opportunity. Since modifiers are necessarily context dependent, any experimental organism is likely to model only a portion of any particular genetic network in disease. The accuracy of multigenic models likely depends on both the evolutionary plasticity of the network involved and the evolutionary distance between the model and human subjects. From this perspective, with rodents as a sister group to primates, mouse variants that have large effect sizes as modifiers while remaining phenotypically quiet on their own should be useful in identifying points in a genetic network where intervention might have higher therapeutic benefit than collateral cost.
The examples below argue that modifier genes are a field ripe for harvest, particularly with the recent arrival of several new community resources that improve experimental access to genetic variations among common strains. The situation is reminiscent of early days of positional cloning in many respects. Large numbers of loci have been reported and mapped (Figure 1; Table S1), but few have been molecularly identified. Stories of unrewarded efforts have inhibited some investigators from pursuing otherwise tantalizing genetic effects. The two most important parallels, however, are that early successes—highlighting unexpected interactions—show the value of the approach and that new resources and emerging technologies are making the approach both more palatable and more cost effective. With new tools and a critical mass of encouraging results from several laboratories, this is a good time to consider both the value of the waiting crop and the machinery needed for harvest. Instructive examples to date include identification of genetic interactions from candidate genes, from mutagenesis screens, and from natural variants. Understanding modifier gene network architectures in mouse models may provide a powerful functional basis for inferring candidate interactions from human exome and genome sequences, particularly where sample size limits statistical power for independent discovery in clinical samples for Mendelian disorders.
Fig. 1. PubMed references to mouse modifier genes. 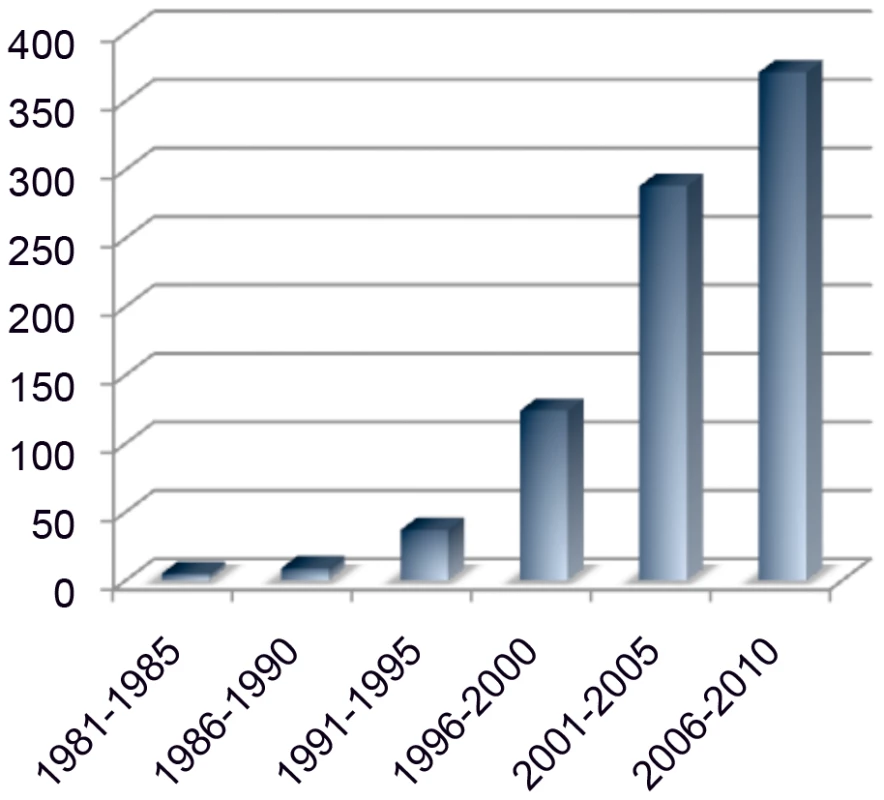
Growing interest in and recognition of modifier genes in mouse models is supported by increased publication rates in 5-year windows for the last 30 years. Mouse modifier papers have increased at a rate faster than the increase of total PubMed citations over the past 15 years. Candidate Genes Are a Useful, but Limited, View of Interactions
Many of the genetic interactions known in mice come from direct tests of candidate interactions. Testing interactions first observed in other species can identify physiological context for gene pairs or networks that are conserved more deeply than the physiology or organ system for which they are most relevant to human disease. For observed binding partners, genetic interactions can test the biological relevance of likely physical contacts. Proposed interactions between mutations with similar phenotypes can also clarify points of convergence between previously separate pathways.
Candidate interactions are often based on homology to interacting genes in other species, including components of developmental signaling pathways, homeotic regulators, and transcription factor cascades in development. Some of these interactions may confirm modifier genes in the strict sense of Box 1, but more often involve interactions between phenotypic null mutations available from other studies. While these are helpful in confirming functional conservation among pathways and defining mammalian contexts that might be relevant to disease, this paradigm is limited in its ability to identify levels of interaction unique to mammalian biology that might be expected from successive genome duplications, rescissions, and neo-functionalization in the lineage leading from ancestral vertebrates to primates and rodents (Euarchontoglires). Indeed, levels of redundancy among paralogous genes in mouse experiments often underscore important differences in trait architectures between mammals and other models. Moreover, focus on just the highly conserved rather than more plastic components of a genetic pathway or network might bias against finding genes that are more readily manipulated without damage to the organism.
Synthetic interactions between environmentally sensitizing alleles are another kind of candidate gene approach. For example, BALB/c and 129S1 mice are sensitive to adriamycin-induced nephropathy and show mitochondrial DNA (mtDNA) depletion in vulnerable organs after adriamycin treatment. Gharavi and colleagues identified a single amino acid substitution in the shared BALB/c and 129S1 allele of Prkdc, which encodes a DNA-activated protein kinase, as the sensitizing variant and asked whether this might be relevant to other mtDNA dysfunctions [23]. The human orthologue of Mpv17, which encodes an inner mitochondrial membrane protein, is mutated in mtDNA depletion syndromes [24], but the corresponding mouse mutation does not produce this phenotype. Papeta et al. showed that Prkdc potentiates mtDNA loss in Mpv17-mutant mice: double mutant mice (but neither single mutant) suffer mtDNA loss and other features of adriamycin-induced nephropathy without adriamycin exposure, providing parallel gene×gene and gene×environment models.
The many successes in modeling the phenotypic consequences of predicted candidate gene interactions in an intact mammal should encourage us to consider what value might be obtained from screens that are less constrained by prior predictions—and therefore capable of identifying novel and unexpected interactions that might catalyze more rapid progress in the often complex genetic architectures relevant to disease.
Spontaneous Modifiers: Volunteers Lead the Way
An early success in using modifier genes to understand a genetic network in mice came from the dilute suppressor (Mregdsu). This modifier arose spontaneously in a non-agouti, dilute stock, suppressing the coat color dilution but with no obvious phenotype of its own [25]. Importantly, dsu similarly suppressed pigmentation phenotypes in mutations at five of 11 classical coat color loci tested [26], [27], indicating a fundamental role in melanosome function. Molecular analysis revealed a spontaneous 11-kb deletion, creating a null allele in a vertebrate-specific gene, subsequently dubbed Melanoregulin [28]. Characterization of Melanoregulin in the context of its genetic suppressor activity revealed a previously unknown molecular function required for pigment granule transfer and other transactions among membrane-bound organelles [29]. This work remains an instructive example as few other strict-sense modifiers have been demonstrated to act on several different mutations and only one mouse modifier, Nxf1Mvb1, has been effective on a larger number.
Spontaneous mutations can also contribute to nominally wild-type inbred backgrounds. The Sodium channel modifier 1 (Scnm1) locus was identified as a strain-dependent modifier of Scn8amed-J, a mutation in a neuronal sodium channel gene responsible for a range of neurological phenotypes [30]. Among several Scn8a mutations, the modifier is specific for the medJ allele, an intronic single nucleotide variant that alters 5′ splice site usage. Positional cloning of the modifier identified a novel gene whose protein plays a direct and previously unsuspected role in pre-mRNA splicing [31], [32]. The sensitizing modifier allele (C57BL/6) encodes a truncated protein (R187X), but is less severe than a subsequently generated null allele [33]. The interaction between Scnm1 and Scn8amed-J appears highly specific, as Scnm1 does not alter general RNA patterns in brain nor modify other tested mutations with similar defects [33]. Because the variant Scnm1 allele is only found in C57 and C58 strains, but not in other strains with otherwise similar Scnm1 haplotypes, it is proposed to have arisen as a spontaneous mutation in a progenitor stock rather than as a wild population variant [32]. The unexpected aspects of mammalian biology identified through the dsu and Scnm1 spontaneous modifiers should encourage further explorations of modifier genes.
Induced Mutations Allow Systematic Screens—with Dramatic Effects
Random mutagenesis to introduce and screen new variants has several advantages for identifying genetic interactions and trait architectures. Ethylnitrosourea (ENU) is especially efficient in mice [34], [35] and in principle can both produce a range of alleles at a given locus and saturate a phenotype for simple loss-of-function alleles at most loci. High-throughput sequencing now rapidly identifies de novo mutations relative to a defined background [36]–[38]. With respect to modifier screens, mutagenesis should in principle identify both strict-sense modifiers and interacting genes with significant independent phenotypes, though the relative proportion may be difficult to predict.
Using epigenetically sensitive Agouti alleles and green fluorescent protein (GFP) transgenes that show variegated expression as phenotype reporters, Whitelaw and coworkers have recovered a substantial collection of ENU-induced Modifier of murine metastable epialleles dominant (MommeD) mutations. This approach creates an “outside-in” interaction network module, with multiple edges (interactions) converging on a single node (conditioning mutation or reporter gene) (Figure 2A). At least five of these mutations have been molecularly identified, encoding core components of chromatin regulatory complexes and a DNA methyltransferase [39]–[42]. The first recessive mutation identified from this screen, MommeR1, is an amino acid replacement allele of transcription factor Foxo3a [43]. Each of these mutations has independent phenotypes in addition to the modifier activity, including recessive lethality for the dominant alleles and female infertility for Foxo3a.
Fig. 2. Modifier gene networks have directional edges between nodes. 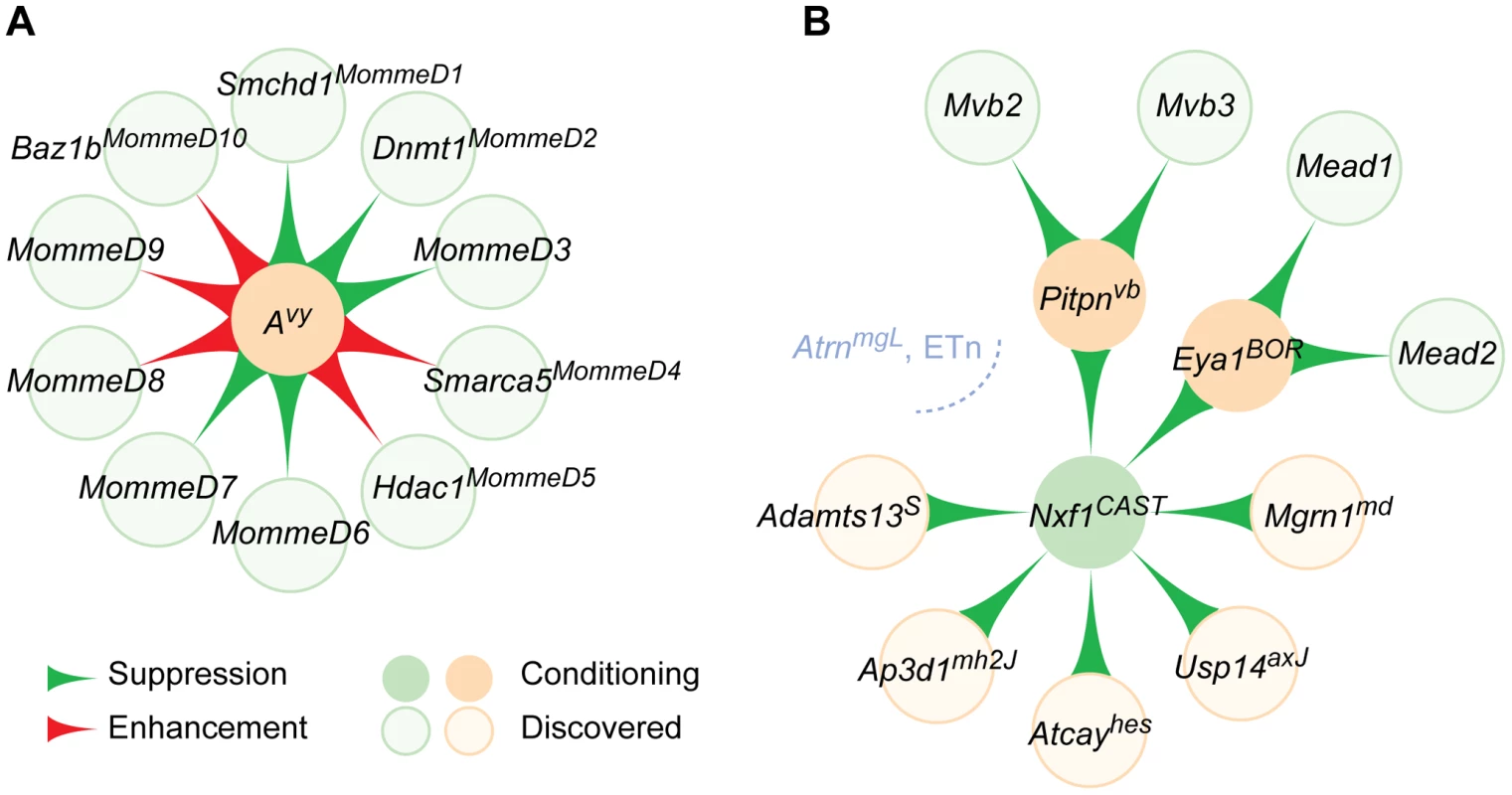
Mouse modifier gene interactions can be diagrammed as nascent modules of an interaction network. (A) Identification of multiple modifier genes for a conditioning mutation through either mutagenesis or linkage analysis of strain variants can be represented as in “outside-in” network module, where one node (the conditioning mutation, beige circle) is a sink hub, acted on by each experimentally discovered modifier (light green circles). MommeD modifiers of the epigenetically sensitive Avy mutation are illustrated as an example. Direction of effect is indicated by the flared edges (connections) between nodes. (B) Validation of modifier mechanisms across independent mutations result in an “inside-out” module, with the shared modifier (green circle) acting as a source hub on several conditioning or test mutations. An incipient network around Nxf1Mvb1, based on shared genetic mechanism, is illustrated. AtrnmgL and several intronic ETn-induced mutations are not affected by Nxf1Mvb1 variation (gray). Pitpnavb and Eya1BOR have additional known modifiers (light green), adding modules to the incipient network. Another instructive example comes from an ENU screen for dominant enhancers of dominant white spotting in a Sox10lacZ/+ model of Waardenburg syndrome [44]. Among 230 pedigrees, Pavan and colleagues recovered three transmissible modifiers of spotting (Mos1,2,3). Remarkably, these three induced mutations mapped to locations distinct from several previously identified modifiers of Sox10 and from each other, suggesting that Sox10 phenotypes are sensitive to perturbations at many positions in an extended genetic network. A collateral phenotype (cephalosyndactyly) and map position strongly suggested Gli3 as the Mos1 gene, which sequence and complementation analysis rapidly confirmed. Mos2 was subsequently identified as a null allele of the RNA regulator Magoh, with severe haploinsufficiency phenotypes in brain development [45].
As illustrated in these examples, chemical mutagenesis allows efficient exploration of genetic networks unconstrained by prior hypothesis. Networks ascertained by this approach, in both examples, comprise modifier alleles that typically have independent deleterious phenotypes. Whether this is more often true of induced mutations or is a property of the specific networks tested, and whether milder alleles at the same genes might retain modifier effects without collateral phenotypes remains to be seen. Alternatively, networks based on variants in natural populations—or nominally wild-type strains—might highlight different genes and network connections, depending on biological trade-offs between modifier effect and collateral phenotypes in any gene's potential allele spectrum and the forces of selection through which a given variant has passed prior to discovery.
Natural Variants: Keys to Genetic Plasticity?
While inbred mouse strains are often referred to as nominally “wild type,” each represents a different sampling of wild (and a few laboratory-derived) alleles across the genome. Inbred strains in aggregate represent an abundant source of captive variation [46], [47] and the vast majority of underlying genetic variants predate laboratory domestication [48]–[50]. This argues that the captive variants—on average—were sufficiently neutral to be sufficiently frequent among wild mice to be incorporated in the common laboratory strains, although some specific instances will be maladaptive by themselves or in combination with other variants. Modifier alleles among the nearly neutral majority of strain variants might be expected to favor, in comparison to mutagenesis experiments, either milder alleles or nodes in the relevant genetic networks with greater intrinsic plasticity—functional variation compatible with normal phenotype. Identification of network nodes with greater genetic plasticity may be especially useful for pointing out experimental and therapeutic approaches with higher likelihood to minimize collateral damage in modifying a specific condition. Three examples of early successes indicate some of the areas to which natural variants might contribute unique findings.
Studies in several laboratories have identified modifiers that alter ApcMin-dependent tumor phenotypes in a genetic cancer model [51]–[58]. Modifier of Min-1 (Mom1) is the most well studied, beginning with its discovery by linkage analysis for intestinal tumor number nearly two decades ago [57]. Based on rough map location and strain distribution, the secreted phospholipase Pla2g2a was proposed as a positional and functionally variant candidate gene, with an apparent null allele caused by a single base insertion in ApcMin sensitive strains [59]. Mom1 is semidominant and analysis of tumor DNA suggested that its effects are not cell autonomous, consistent with its identification as a secreted enzyme. Complementation studies confirmed that Pla2g2a accounted for a large fraction of the variance in tumor number [60] (an additional component, Mom6, was inferred to account for the full effect of the initial linkage [56], adding as a grace note that linkage peaks may be driven both by variants of large effect and by regions containing more than one contributing variant). Elevated expression of human PLA2G2A was subsequently associated with survival in gastric cancer patients [61], providing another example of cross-species validation. Mom1 alleles in mice are widely distributed across inbred strains, suggesting an early origin. Re-sequencing [49], [62] and dense genotyping [48] data provide a partial answer. Review of published data shows that the Mom1 single base insertion is a derived allele that arose on a unique haplotype and that at least 2 Mb of this interval is shared by all characterized Mom1S-allele strains. (Surprisingly, C57BL/6 and wild-derived MOLF/Ei strains share haplotype, 3630/3635 called variants, across 2.4 Mb around Pla2g2a, suggesting contamination of the MOLF/Ei stock with laboratory strains for this interval [63].) While the origin of this Pla2g2a allele is not yet clear, the circumstantial evidence warrants further investigation as a possible wild variant.
The Heart failure modifier 2, one of several mapped Hrtfm loci that modify the course of cardiomyopathy caused by overexpression of calsequestrin [64], was recently identified as an allele of the troponin-interacting kinase gene, Tnni3k, by a combination of fine mapping, strain distribution, and transgenic studies [65]. Molecular studies in a cell culture model implicated a single intronic SNP as the functional variant, creating an alternate 5′ splice donor site that results in a shifted open reading frame in ∼70% of Tnni3k transcripts in calsequestrin-resistant strains. To illustrate potential origin of this strain variant, we tabulated both functionally tested alleles [65] and correlated sequence data from additional strains (Table 1) [62]. This shows a broad distribution of each allele across accepted strain genealogies [66] for a region of modest haplotype diversity, suggesting an origin prior to development of laboratory strains and possibly wild-derived. Interestingly, subsequent work implicates the same Tnni3k variant in susceptibility to coxsackievirus-induced myocarditis [67]. If this variant is confirmed as a wild allele, it will be interesting to determine what factors might account for its accumulation despite the increased risk to cardiac challenges.
Tab. 1. TNNI3 interacting kinase–Heart failure modifier 2 (Tnni3kHrtfm2) variant is widely distributed in laboratory mice. 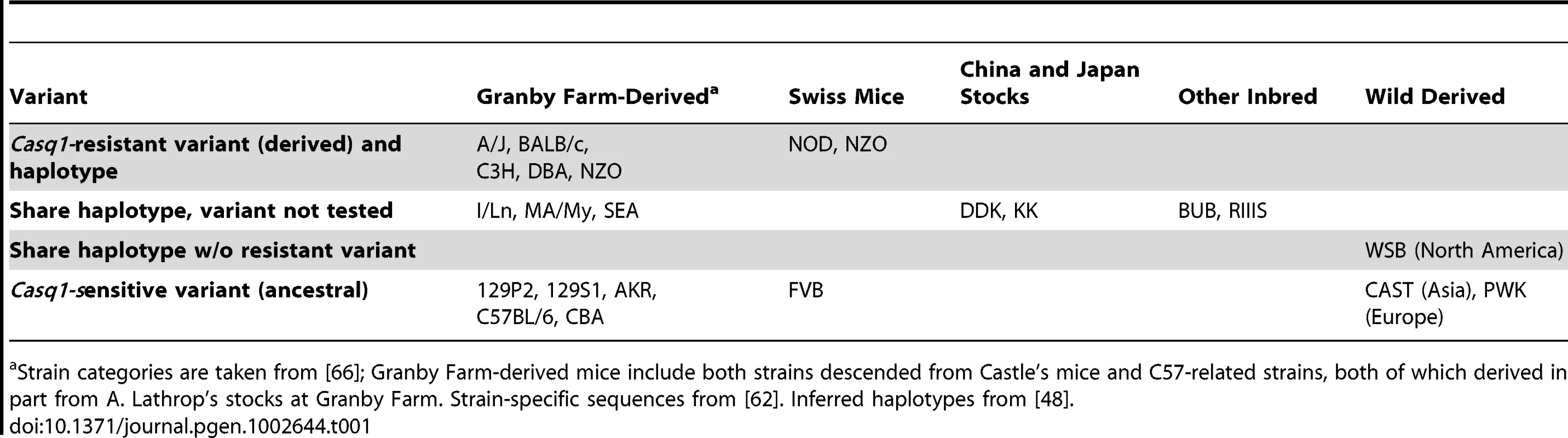
Strain categories are taken from [66]; Granby Farm-derived mice include both strains descended from Castle's mice and C57-related strains, both of which derived in part from A. Lathrop's stocks at Granby Farm. Strain-specific sequences from [62]. Inferred haplotypes from [48]. The Modifier of vibrator 1 was originally detected as a semi-dominant modifier of neurodegeneration and premature death in vibrator mutant animals. The Pitpnavibrator mutation is an IAP-family endogenous retrovirus insertion into an intron of a phosphatidylinositol transfer protein gene, which reduces normal gene expression by competing with splicing of the adjacent exons [68]. Suppression by the CAST/Ei modifier allele occurs by elevating the level and proportion of normally spliced mRNA from the mutant gene. Positional complementation cloning identified the modifier as an amino acid substitution allele of mRNA nuclear export factor Nxf1, suggesting a previously unexpected role for canonical mRNA export machinery in splicing fidelity in at least some contexts [69]. Importantly, this dose-responsive effect on alternative splicing has been demonstrated for six out of seven mutations caused by sense-strand IAP insertions in host gene introns, but none of 15 mutations with other classes of insertional events [70]. This creates an “inside-out” network module, with a modifier node acting on several different mutations, some of which have additional known modifiers (Figure 2B). Of special interest, the suppressing Nxf1 allele was found in a majority of wild castaneus isolates, confirming it as a natural variant, and haplotype analysis suggested directional selection, with a non-conservative amino acid replacement in Nxf1 as the youngest variant on the most common wild haplotype [69].
Among modifiers not yet identified molecularly, the Modifier of dactylaplasia (Mdac) may impact several themes raised above, including selection, specificity, and epigenetic regulation. The functionally tested alleles are both well dispersed across inbred strains [71], [72], suggesting an early—and possibly wild—origin. The modifier acts on two different Dac alleles of Fbxw4, caused by independent insertions of MusD-family retrovirus elements at different sites [73], but has no obvious independent phenotype in reports from several different laboratories. Most interestingly, Mdac alleles alter both DNA methylation and the accumulation of inhibitory histone modifications on the Dac MusD elements [74], raising the intriguing possibility that this locus might act directly in epigenetic mechanisms, without strong collateral phenotypes. This would be interesting both as a phenotypically quiet epigenetic modifier and potentially as a tool for titrating allele strength of other MusD/ETn-induced mutations with similar epigenetic profiles.
Identifying modifiers on the basis of natural variants may require more resources than de novo mutations (once generated), but have the added value of generally highlighting alleles, genes, or networks whose manipulation appears well tolerated by the organism and largely compatible with normal function, having been vetted by selective pressures. In the ultimate goal of finding pressure points through which to modulate disease networks, this may prove advantageous.
Going Forward: Tools for Accessing Strain Variation and Modifier Genes
How readily can we use modifier genes to predict sensitive pathways and nodes for therapeutic interventions? New tools and resources in mouse genetics should help to unclog the pipeline of modifier genes that have been detected but not molecularly defined. Maps of copy number variation [75] and comprehensive genome sequences of the most commonly used strains [62], [76] will be enormously powerful. The combination of predictive sequence variants plus expression data from increasingly facile array and RNA-Seq methods should allow comprehensive consideration of candidate variants across even broadly defined loci. Because modifier genes require the presence of the conditioning mutation, strain resources built for genome-wide shuffling of alleles [77] may have less value for this application (in requiring many crosses to access the variation), while others such as chromosome substitution panels [78], [79] may be useful for isolating individual modifier effects by limiting the need for de novo congenic strain construction. New tools for functional validation at fine scale are also essential to opening the pipeline of modifier gene studies in mice. Recent developments on this front include an increased diversity of strain-specific large-insert BAC libraries (http://bacpac.chori.org/, among others) for transgenic studies, larger-scale tools for engineering ES cells [80]–[82], and most recently germline-potent ES cells from previously refractory strains [83]. These resources, some anticipated a decade ago [84], [85] and some less expected, are only now widely available. This combination of tools and continuing innovations promises deeper insight into mammalian genetic architectures in health and disease, through the lens of modifier genes and mouse genetic diversity.
Supporting Information
Zdroje
1. CarlsonEA 1981 Genes, radiation and society: the life and work of H.J. Muller Ithaca Cornell Universtiy Press 457
2. SturtevantAH 1965 A history of genetics New York Harper & Row
3. CastleWE 1912 The inconstancy of unit-characters. Am Naturalist 46 352 362
4. CastleWE 1916 Further studies on piebald rats and selection. Carnegie Inst Wash Publ No 241 161 192
5. DexterJS 1914 The analysis of a case of continuous variation in Drosophila by a study of its linkage relationships. Am Naturalist 48 712 758
6. CastleWE 1919 Piebald rats and the theory of genes. Proc Natl Acad Sci U S A 5 126 130
7. CastleWE 1951 Variation in the hooded pattern of rats, and a new allele of hooded. Genetics 36 254 266
8. AltenburgEMullerHJ 1920 The genetic basis of truncate wing,-an inconstant and modifiable character in Drosophila. Genetics 5 1 59
9. CuttingGR 2010 Modifier genes in Mendelian disorders: the example of cystic fibrosis. Ann N Y Acad Sci 1214 57 69
10. WrightFAStrugLJDoshiVKCommanderCWBlackmanSM 2011 Genome-wide association and linkage identify modifier loci of lung disease severity in cystic fibrosis at 11p13 and 20q13.2. Nat Genet 43 539 546
11. GuYHarleyITHendersonLBAronowBJVietorI 2009 Identification of IFRD1 as a modifier gene for cystic fibrosis lung disease. Nature 458 1039 1042
12. RozmahelRGyomoreyKPlyteSNguyenVWilschanskiM 1997 Incomplete rescue of cystic fibrosis transmembrane conductance regulator deficient mice by the human CFTR cDNA. Hum Mol Genet 6 1153 1162
13. HastonCKCoreyMTsuiLC 2002 Mapping of genetic factors influencing the weight of cystic fibrosis knockout mice. Mamm Genome 13 614 618
14. HastonCKMcKerlieCNewbiggingSCoreyMRozmahelR 2002 Detection of modifier loci influencing the lung phenotype of cystic fibrosis knockout mice. Mamm Genome 13 605 613
15. HastonCKTsuiLC 2003 Loci of intestinal distress in cystic fibrosis knockout mice. Physiol Genomics 12 79 84
16. ZielenskiJCoreyMRozmahelRMarkiewiczDAznarezI 1999 Detection of a cystic fibrosis modifier locus for meconium ileus on human chromosome 19q13. Nat Genet 22 128 129
17. LouieCMCaridiGLopesVSBrancatiFKispertA 2010 AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis. Nat Genet 42 175 180
18. GirirajanSRosenfeldJACooperGMAntonacciFSiswaraP 2010 A recurrent 16p12.1 microdeletion supports a two-hit model for severe developmental delay. Nat Genet 42 203 209
19. EichersERLewisRAKatsanisNLupskiJR 2004 Triallelic inheritance: a bridge between Mendelian and multifactorial traits. Ann Med 36 262 272
20. BealesPLBadanoJLRossAJAnsleySJHoskinsBE 2003 Genetic interaction of BBS1 mutations with alleles at other BBS loci can result in non-Mendelian Bardet-Biedl syndrome. Am J Hum Genet 72 1187 1199
21. KatsanisNAnsleySJBadanoJLEichersERLewisRA 2001 Triallelic inheritance in Bardet-Biedl syndrome, a Mendelian recessive disorder. Science 293 2256 2259
22. NadeauJHTopolEJ 2006 The genetics of health. Nat Genet 38 1095 1098
23. PapetaNZhengZSchonEABroselSAltintasMM 2010 Prkdc participates in mitochondrial genome maintenance and prevents Adriamycin-induced nephropathy in mice. J Clinical Invest 120 4055 4064
24. SpinazzolaAViscomiCFernandez-VizarraECarraraFD'AdamoP 2006 MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion. Nat Genet 38 570 575
25. SweetHO 1983 Dilute suppressor, a new suppressor gene in the house mouse. J Hered 74 305 306
26. MooreKJSwingDACopelandNGJenkinsNA 1990 Interaction of the murine dilute suppressor gene (dsu) with fourteen coat color mutations. Genetics 125 421 430
27. MooreKJSwingDARinchikEMMucenskiMLBuchbergAM 1988 The murine dilute suppressor gene dsu suppresses the coat-color phenotype of three pigment mutations that alter melanocyte morphology, d, ash and ln. Genetics 119 933 941
28. O'SullivanTNWuXSRachelRAHuangJDSwingDA 2004 dsu functions in a MYO5A-independent pathway to suppress the coat color of dilute mice. Proc Natl Acad Sci U S A 101 16831 16836
29. Damek-PoprawaMDiemerTLopesVSLilloCHarperDC 2009 Melanoregulin (MREG) modulates lysosome function in pigment epithelial cells. J Biol Chem 284 10877 10889
30. KearneyJABuchnerDADe HaanGAdamskaMLevinSI 2002 Molecular and pathological effects of a modifier gene on deficiency of the sodium channel Scn8a (Na(v)1.6). Hum Mol Genet 11 2765 2775
31. HowellVMJonesJMBergrenSKLiLBilliAC 2007 Evidence for a direct role of the disease modifier SCNM1 in splicing. Hum Mol Genet 16 2506 2516
32. BuchnerDATrudeauMMeislerMH 2003 SCNM1, a putative RNA splicing factor that modifies disease severity in mice. Science 301 967 969
33. HowellVMde HaanGBergrenSJonesJMCuliatCT 2008 A targeted deleterious allele of the splicing factor SCNM1 in the mouse. Genetics 180 1419 1427
34. QuwailidMMHugillADearNVizorLWellsS 2004 A gene-driven ENU-based approach to generating an allelic series in any gene. Mamm Genome 15 585 591
35. ConcepcionDSeburnKLWenGFrankelWNHamiltonBA 2004 Mutation rate and predicted phenotypic target sizes in ethylnitrosourea-treated mice. Genetics 168 953 959
36. HiltonJMLewisMAGratiMInghamNPearsonS 2011 Exome sequencing identifies a missense mutation in Isl1 associated with low penetrance otitis media in dearisch mice. Genome Biol 12 R90
37. FairfieldHGilbertGJBarterMCorriganRRCurtainM 2011 Mutation discovery in mice by whole exome sequencing. Genome Biol 12 R86
38. ArnoldCNXiaYLinPRossCSchwanderM 2011 Rapid identification of a disease allele in mouse through whole genome sequencing and bulk segregation analysis. Genetics 187 633 641
39. BlewittMEGendrelAVPangZSparrowDBWhitelawN 2008 SmcHD1, containing a structural-maintenance-of-chromosomes hinge domain, has a critical role in X inactivation. Nat Genet 40 663 669
40. AsheAMorganDKWhitelawNCBruxnerTJVickaryousNK 2008 A genome-wide screen for modifiers of transgene variegation identifies genes with critical roles in development. Genome Biol 9 R182
41. ChongSVickaryousNAsheAZamudioNYoungsonN 2007 Modifiers of epigenetic reprogramming show paternal effects in the mouse. Nat Genet 39 614 622
42. BlewittMEVickaryousNKHemleySJAsheABruxnerTJ 2005 An N-ethyl-N-nitrosourea screen for genes involved in variegation in the mouse. Proc Natl Acad Sci U S A 102 7629 7634
43. YoungsonNAVickaryousNvan der HorstAEppTHartenS 2011 A missense mutation in the transcription factor Foxo3a causes teratomas and oocyte abnormalities in mice. Mamm Genome 22 235 248
44. MateraIWatkins-ChowDELoftusSKHouLIncaoA 2008 A sensitized mutagenesis screen identifies Gli3 as a modifier of Sox10 neurocristopathy. Hum Mol Genet 17 2118 2131
45. SilverDLWatkins-ChowDESchreckKCPierfeliceTJLarsonDM 2010 The exon junction complex component Magoh controls brain size by regulating neural stem cell division. Nature Neurosci 13 551 558
46. StevensJCBanksGTFestingMFFisherEM 2007 Quiet mutations in inbred strains of mice. Trends Mol Med 13 512 519
47. FestingMFW 1996 Origins and characteristics of inbred strains of mice. LyonMFRastanSBrownSDM Genetics variants and strains of the laboratory mouse. 3rd edition Oxford Oxford University Press 1537 1576
48. KirbyAKangHMWadeCMCotsapasCKostemE 2010 Fine mapping in 94 inbred mouse strains using a high-density haplotype resource. Genetics 185 1081 1095
49. FrazerKAEskinEKangHMBogueMAHindsDA 2007 A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature 448 1050 1053
50. WadeCMDalyMJ 2005 Genetic variation in laboratory mice. Nat Genet 37 1175 1180
51. CristRCRothJJLisantiMPSiracusaLDBuchbergAM 2011 Identification of Mom12 and Mom13, two novel modifier loci of Apc (Min) -mediated intestinal tumorigenesis. Cell Cycle 10 1092 1099
52. OikarinenSIClevelandAGCorkKMBynoteKKRafterJJ 2009 Genetic mapping of Mom5, a novel modifier of Apc(Min)-induced intestinal tumorigenesis. Carcinogenesis 30 1591 1596
53. KwongLNShedlovskyABiehlBSClipsonLPaschCA 2007 Identification of Mom7, a novel modifier of Apc(Min/+) on mouse chromosome 18. Genetics 176 1237 1244
54. HainesJJohnsonVPackKSuraweeraNSlijepcevicP 2005 Genetic basis of variation in adenoma multiplicity in ApcMin/+ Mom1S mice. Proc Natl Acad Sci U S A 102 2868 2873
55. SilvermanKAKoratkarRSiracusaLDBuchbergAM 2002 Identification of the modifier of Min 2 (Mom2) locus, a new mutation that influences Apc-induced intestinal neoplasia. Genome Res 12 88 97
56. CormierRTBilgerALillichAJHalbergRBHongKH 2000 The Mom1AKR intestinal tumor resistance region consists of Pla2g2a and a locus distal to D4Mit64. Oncogene 19 3182 3192
57. DietrichWFLanderESSmithJSMoserARGouldKA 1993 Genetic identification of Mom-1, a major modifier locus affecting Min-induced intestinal neoplasia in the mouse. Cell 75 631 639
58. PerreaultNSackettSDKatzJPFurthEEKaestnerKH 2005 Foxl1 is a mesenchymal Modifier of Min in carcinogenesis of stomach and colon. Genes Dev 19 311 315
59. MacPheeMChepenikKPLiddellRANelsonKKSiracusaLD 1995 The secretory phospholipase A2 gene is a candidate for the Mom1 locus, a major modifier of ApcMin-induced intestinal neoplasia. Cell 81 957 966
60. CormierRTHongKHHalbergRBHawkinsTLRichardsonP 1997 Secretory phospholipase Pla2g2a confers resistance to intestinal tumorigenesis. Nat Genet 17 88 91
61. LeungSYChenXChuKMYuenSTMathyJ 2002 Phospholipase A2 group IIA expression in gastric adenocarcinoma is associated with prolonged survival and less frequent metastasis. Proc Natl Acad Sci U S A 99 16203 16208
62. KeaneTMGoodstadtLDanecekPWhiteMAWongK 2011 Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 477 289 294
63. YangHWangJRDidionJPBuusRJBellTA 2011 Subspecific origin and haplotype diversity in the laboratory mouse. Nat Genet 43 648 655
64. WheelerFCFernandezLCarlsonKMWolfMJRockmanHA 2005 QTL mapping in a mouse model of cardiomyopathy reveals an ancestral modifier allele affecting heart function and survival. Mamm Genome 16 414 423
65. WheelerFCTangHMarksOAHadnottTNChuPL 2009 Tnni3k modifies disease progression in murine models of cardiomyopathy. PLoS Genet 5 e1000647 doi:10.1371/journal.pgen.1000647
66. BeckJALloydSHafezparastMLennon-PierceMEppigJT 2000 Genealogies of mouse inbred strains. Nat Genet 24 23 25
67. WiltshireSALeiva-TorresGAVidalSM 2011 Quantitative trait locus analysis, pathway analysis, and consomic mapping show genetic variants of Tnni3k, Fpgt, or H28 control susceptibility to viral myocarditis. J Immunol 186 6398 6405
68. HamiltonBASmithDJMuellerKLKerrebrockAWBronsonRT 1997 The vibrator mutation causes neurodegeneration via reduced expression of PITP alpha: positional complementation cloning and extragenic suppression. Neuron 18 711 722
69. FloydJAGoldDAConcepcionDPoonTHWangX 2003 A natural allele of Nxf1 suppresses retrovirus insertional mutations. Nat Genet 35 221 228
70. ConcepcionDFlores-GarciaLHamiltonBA 2009 Multipotent genetic suppression of retrotransposon-induced mutations by Nxf1 through fine-tuning of alternative splicing. PLoS Genet 5 e1000484 doi:10.1371/journal.pgen.1000484
71. JohnsonKRLanePWWard-BaileyPDavissonMT 1995 Mapping the mouse dactylaplasia mutation, Dac, and a gene that controls its expression, mdac. Genomics 29 457 464
72. ChaiCK 1981 Dactylaplasia in mice a two-locus model for development anomalies. J Hered 72 234 237
73. SidowABulotskyMSKerrebrockAWBirrenBWAltshulerD 1999 A novel member of the F-box/WD40 gene family, encoding dactylin, is disrupted in the mouse dactylaplasia mutant. Nat Genet 23 104 107
74. KanoHKurahashiHTodaT 2007 Genetically regulated epigenetic transcriptional activation of retrotransposon insertion confers mouse dactylaplasia phenotype. Proc Natl Acad Sci U S A 104 19034 19039
75. SheXChengZZollnerSChurchDMEichlerEE 2008 Mouse segmental duplication and copy number variation. Nat Genet 40 909 914
76. YalcinBWongKAgamAGoodsonMKeaneTM 2011 Sequence-based characterization of structural variation in the mouse genome. Nature 477 326 329
77. PhilipVMSokoloffGAckert-BicknellCLStrizMBranstetterL 2011 Genetic analysis in the Collaborative Cross breeding population. Genome Res 21 1223 1238
78. ShaoHBurrageLCSinasacDSHillAEErnestSR 2008 Genetic architecture of complex traits: large phenotypic effects and pervasive epistasis. Proc Natl Acad Sci U S A 105 19910 19914
79. SingerJBHillAEBurrageLCOlszensKRSongJ 2004 Genetic dissection of complex traits with chromosome substitution strains of mice. Science 304 445 448
80. LiMAPettittSJYusaKBradleyA 2010 Genome-wide forward genetic screens in mouse ES cells. Method Enzymol 477 217 242
81. VoehringerDWuDLiangHELocksleyRM 2009 Efficient generation of long-distance conditional alleles using recombineering and a dual selection strategy in replicate plates. BMC Biotechnol 9 69
82. van der WeydenLShaw-SmithCBradleyA 2009 Chromosome engineering in ES cells. Methods in molecular biology 530 49 77
83. ten BergeDKurekDBlauwkampTKooleWMaasA 2011 Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat Cell Biol 13 1070 1075
84. NadeauJH 2001 Modifier genes in mice and humans. Nat Rev Genet 2 165 174
85. HamiltonBAFrankelWN 2001 Of mice and genome sequence. Cell 107 13 16
86. EppigJTBlakeJABultCJKadinJARichardsonJE 2011 The Mouse Genome Database (MGD): comprehensive resource for genetics and genomics of the laboratory mouse. Nucleic Acids Res 40 D881 D886
87. BlakeJABultCJKadinJARichardsonJEEppigJT 2011 The Mouse Genome Database (MGD): premier model organism resource for mammalian genomics and genetics. Nucleic Acids Res 39 D842 D848
Štítky
Genetika Reprodukční medicína
Článek A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target GenesČlánek Population Structure of Hispanics in the United States: The Multi-Ethnic Study of AtherosclerosisČlánek Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms inČlánek Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome AnalysisČlánek Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and MechanismČlánek Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 4- Akutní intermitentní porfyrie
- Srdeční frekvence embrya může být faktorem užitečným v předpovídání výsledku IVF
- Prenatální expozice ftalátům a anogenitální vzdálenost u novorozenců
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
- Kryokonzervace lidských embryí – preferovaná metoda asistované reprodukce
-
Všechny články tohoto čísla
- Runs of Homozygosity Implicate Autozygosity as a Schizophrenia Risk Factor
- Modifier Genes and the Plasticity of Genetic Networks in Mice
- The DSIF Subunits Spt4 and Spt5 Have Distinct Roles at Various Phases of Immunoglobulin Class Switch Recombination
- A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target Genes
- Population Structure of Hispanics in the United States: The Multi-Ethnic Study of Atherosclerosis
- Deep Sequencing of Plant and Animal DNA Contained within Traditional Chinese Medicines Reveals Legality Issues and Health Safety Concerns
- Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms in
- Insulin Signaling Mediates Sexual Attractiveness in
- Progressive Telomere Dysfunction Causes Cytokinesis Failure and Leads to the Accumulation of Polyploid Cells
- Long-Range Chromosome Organization in : A Site-Specific System Isolates the Ter Macrodomain
- Regulation of Budding Yeast Mating-Type Switching Donor Preference by the FHA Domain of Fkh1
- Polyglutamine Toxicity Is Controlled by Prion Composition and Gene Dosage in Yeast
- Patterns of Regulatory Variation in Diverse Human Populations
- Sequence-Specific Targeting of Dosage Compensation in Favors an Active Chromatin Context
- Whole-Exome Sequencing and Homozygosity Analysis Implicate Depolarization-Regulated Neuronal Genes in Autism
- Replication Fork Reversal after Replication–Transcription Collision
- Common Variants at 9p21 and 8q22 Are Associated with Increased Susceptibility to Optic Nerve Degeneration in Glaucoma
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Epigenome-Wide Scans Identify Differentially Methylated Regions for Age and Age-Related Phenotypes in a Healthy Ageing Population
- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Budding Yeast Dma Proteins Control Septin Dynamics and the Spindle Position Checkpoint by Promoting the Recruitment of the Elm1 Kinase to the Bud Neck
- , a Homolog of a Deaf-Blindness Gene, Regulates Circadian Output and Slowpoke Channels
- Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome Analysis
- Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and Mechanism
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
- SPE-44 Implements Sperm Cell Fate
- A Shared Role for RBF1 and dCAP-D3 in the Regulation of Transcription with Consequences for Innate Immunity
- A Companion Cell–Dominant and Developmentally Regulated H3K4 Demethylase Controls Flowering Time in via the Repression of Expression
- The HEN1 Ortholog, HENN-1, Methylates and Stabilizes Select Subclasses of Germline Small RNAs
- Improved Statistics for Genome-Wide Interaction Analysis
- The Probability of a Gene Tree Topology within a Phylogenetic Network with Applications to Hybridization Detection
- Context-Dependent Dual Role of SKI8 Homologs in mRNA Synthesis and Turnover
- Mu Insertions Are Repaired by the Double-Strand Break Repair Pathway of
- Competition between Replicative and Translesion Polymerases during Homologous Recombination Repair in Drosophila
- An Unbiased Assessment of the Role of Imprinted Genes in an Intergenerational Model of Developmental Programming
- Type 2 Diabetes Risk Alleles Demonstrate Extreme Directional Differentiation among Human Populations, Compared to Other Diseases
- Mutations in and Cause “Splashed White” and Other White Spotting Phenotypes in Horses
- Fine-Scale Mapping of Natural Variation in Fly Fecundity Identifies Neuronal Domain of Expression and Function of an Aquaporin
- Dynamics of Brassinosteroid Response Modulated by Negative Regulator LIC in Rice
- Genetic Inhibition of Solute-Linked Carrier 39 Family Transporter 1 Ameliorates Aβ Pathology in a Model of Alzheimer's Disease
- The Functions of Mediator in Support a Role in Shaping Species-Specific Gene Expression
- Patterns of Ancestry, Signatures of Natural Selection, and Genetic Association with Stature in Western African Pygmies
- Dissection of Pol II Trigger Loop Function and Pol II Activity–Dependent Control of Start Site Selection
- PIWI Associated siRNAs and piRNAs Specifically Require the HEN1 Ortholog
- Genome-Wide Patterns of Gene Expression in Nature
- Hypoxia Disruption of Vertebrate CNS Pathfinding through EphrinB2 Is Rescued by Magnesium
- A New Role for Translation Initiation Factor 2 in Maintaining Genome Integrity
- Sex Reversal in C57BL/6J XY Mice Caused by Increased Expression of Ovarian Genes and Insufficient Activation of the Testis Determining Pathway
- The Rac GTP Exchange Factor TIAM-1 Acts with CDC-42 and the Guidance Receptor UNC-40/DCC in Neuronal Protrusion and Axon Guidance
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



