-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGenome-Wide Patterns of Gene Expression in Nature
Organisms in the wild are subject to multiple, fluctuating environmental factors, and it is in complex natural environments that genetic regulatory networks actually function and evolve. We assessed genome-wide gene expression patterns in the wild in two natural accessions of the model plant Arabidopsis thaliana and examined the nature of transcriptional variation throughout its life cycle and gene expression correlations with natural environmental fluctuations. We grew plants in a natural field environment and measured genome-wide time-series gene expression from the plant shoot every three days, spanning the seedling to reproductive stages. We find that 15,352 genes were expressed in the A. thaliana shoot in the field, and accession and flowering status (vegetative versus flowering) were strong components of transcriptional variation in this plant. We identified between ∼110 and 190 time-varying gene expression clusters in the field, many of which were significantly overrepresented by genes regulated by abiotic and biotic environmental stresses. The two main principal components of vegetative shoot gene expression (PCveg) correlate to temperature and precipitation occurrence in the field. The largest PCveg axes included thermoregulatory genes while the second major PCveg was associated with precipitation and contained drought-responsive genes. By exposing A. thaliana to natural environments in an open field, we provide a framework for further understanding the genetic networks that are deployed in natural environments, and we connect plant molecular genetics in the laboratory to plant organismal ecology in the wild.
Published in the journal: . PLoS Genet 8(4): e32767. doi:10.1371/journal.pgen.1002662
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002662Summary
Organisms in the wild are subject to multiple, fluctuating environmental factors, and it is in complex natural environments that genetic regulatory networks actually function and evolve. We assessed genome-wide gene expression patterns in the wild in two natural accessions of the model plant Arabidopsis thaliana and examined the nature of transcriptional variation throughout its life cycle and gene expression correlations with natural environmental fluctuations. We grew plants in a natural field environment and measured genome-wide time-series gene expression from the plant shoot every three days, spanning the seedling to reproductive stages. We find that 15,352 genes were expressed in the A. thaliana shoot in the field, and accession and flowering status (vegetative versus flowering) were strong components of transcriptional variation in this plant. We identified between ∼110 and 190 time-varying gene expression clusters in the field, many of which were significantly overrepresented by genes regulated by abiotic and biotic environmental stresses. The two main principal components of vegetative shoot gene expression (PCveg) correlate to temperature and precipitation occurrence in the field. The largest PCveg axes included thermoregulatory genes while the second major PCveg was associated with precipitation and contained drought-responsive genes. By exposing A. thaliana to natural environments in an open field, we provide a framework for further understanding the genetic networks that are deployed in natural environments, and we connect plant molecular genetics in the laboratory to plant organismal ecology in the wild.
Introduction
Organisms in the real world are continuously exposed to multiple environmental signals and must respond appropriately to dynamic, fluctuating conditions found in nature [1]. Dynamic environmental signals can differ spatially and temporally during an individual's life cycle with different degrees of predictability, and it is in the context of complex natural environments that genetic regulatory networks actually function and evolve. The response to dynamic fluctuating environments is particularly critical for sessile organisms such as plants that cannot react behaviorally to adverse conditions but must respond by modulating development and physiology to cope with changing conditions [2], [3].
Temperature, water levels, biotic interactions and resource availability are just some key environmental conditions that cue organismal responses, and there have been significant advances in dissecting how these and other ecological signals are transduced by the organism to appropriate gene expression levels that may ultimately determine phenotypes [2]–[9]. With very few exceptions, however, studies on molecular genetic responses to the environment are undertaken in homogenous controlled laboratory conditions. The natural world, in contrast, is anything but controlled, and understanding how genes are regulated in natural ecological settings in the midst of fluctuating environmental signals remains a key objective of the new fields of ecological genomics and systems biology [6].
Arabidopsis thaliana has become one of the key plant model species, not only for studies of genetics and development but for ecology and evolution as well [10]–[12]. This species is a weedy annual plant, occupying disturbed habitats such as the margins of agricultural fields as well as natural ruderal environments. It is native to Europe and Central Asia [13], but has extended its range to include eastern and northwestern portions of the United States [13], [14]. A large proportion of natural populations adopt the spring annual strategy, with germination and flowering in spring [15]. Arabidopsis thaliana displays a wide range of ecological relationships, including within - and between-species interactions and adaptations to abiotic environments. It responds physiologically and developmentally to a variety of environmental cues, including light, daylength, vernalization, nutrient and water levels [10], [11], [15], and can be affected by bacterial and fungal pathogens [16], and by insect herbivory [17].
Despite the role of A. thaliana as a model plant system, remarkably little is known about the phenotypic range and performance of this species in the wild. Focusing on studies in natural field conditions may thus provide opportunities for a more comprehensive view, not only of ecological processes in this species, but also of development and physiology not possible in controlled laboratory experimentation. Indeed, a few field studies of A. thaliana have begun to shed light on the ecological genetics and natural selection in this species in field conditions [18]. Other field studies have looked at natural selection for and costs of herbivory defense traits [19]–[21], seasonal germination timing [22], fitness costs of R gene polymorphisms [23], the role of epistasis in fitness-related traits [24], and the genetic architecture of flowering time [25], [26].
Although it is clear that organismal phenotypes and the genetic architecture of various traits differ between controlled laboratory and field conditions, the extent to which patterns of gene expression is modulated in the wild is not at all understood. There are a large number of global gene expression studies in A. thaliana [27]–[31], and some notable investigations include a comprehensive developmental expression map [29], a cell-specific expression atlas of the root [28] and studies of circadian clock gene regulation [27].
All these studies, however, were undertaken in controlled laboratory conditions. Global gene expression studies of plant species in field conditions [32]–[42] demonstrate that there are significant transcription level differences between controlled and field growth conditions. A study in A. thaliana used responses to increased CO2 and ozone levels in Free Air CO2 Enrichment (FACE) environment [32]. From this study, >1,000 transcripts were either up - or down-regulated between controlled versus field ambient conditions compared with high vs. low CO2 or ozone levels, and there was a preponderance of genes associated with general defense reactions, secondary metabolism, redox control, energy provision, protein turnover, signaling and transcription [32]. More detailed experimental studies have also managed to connect specific genes with phenotypes; for example, seasonal flowering time response in A. halleri in the wild has been shown to be associated with expression of the FLC gene [43].
To contribute to our understanding of the ecological genomics and systems biology of plants in the wild, we determined genome-wide gene expression profiles in the shoot throughout the life cycle of the model plant A. thaliana under natural field conditions. We chose two distinct A. thaliana accessions Bayreuth-0 or Bay-0 (originally from a fallow field in Bayreuth, Germany) and Shakdara or Sha (from a mountainous site at Pamiro-Alay, Tajikistan) because there are genetic [44] and genomic resources [45], [46] in these accessions that can be further used to dissect molecular mechanisms of environmental response. Our study allowed us to identify genes that significantly vary across the spring life cycle of these two accessions and determine patterns of transcriptional co-regulation in field conditions. We found that in addition to accession and flowering stage, temperature and precipitation appear to be correlated with large-scale gene expression patterns in the field, and a large number of co-expressed gene clusters are enriched in loci responsive to several abiotic and biotic stresses. Our results suggest that stress-responsive loci are not only adaptive for extreme environments, but are deployed during the life cycle of A. thaliana to deal with normally fluctuating environments.
Results
A large fraction of protein-coding genes are expressed in the Arabidopsis shoot in the wild
We assayed RNA from replicate pools of shoot samples for genome-wide gene expression of A. thaliana across its life cycle in the complex and natural conditions of the field using the Affymetrix ATH1 gene chip. The field experiment spanned the seedling (∼4-leaf) stage to flowering in the late spring of 2008 at the Cold Spring Harbor Laboratory field site (see Figure 1). During this experimental period, daily temperatures ranged from a mean low of 8.7°C to a mean high of 23.7°C. Of the 30 days that the plants were outside, it rained only 8 days, with precipitation levels during these days ranging from 2.5 to 31.8 mm.
Fig. 1. Field environmental conditions and total gene expression in A. thaliana. 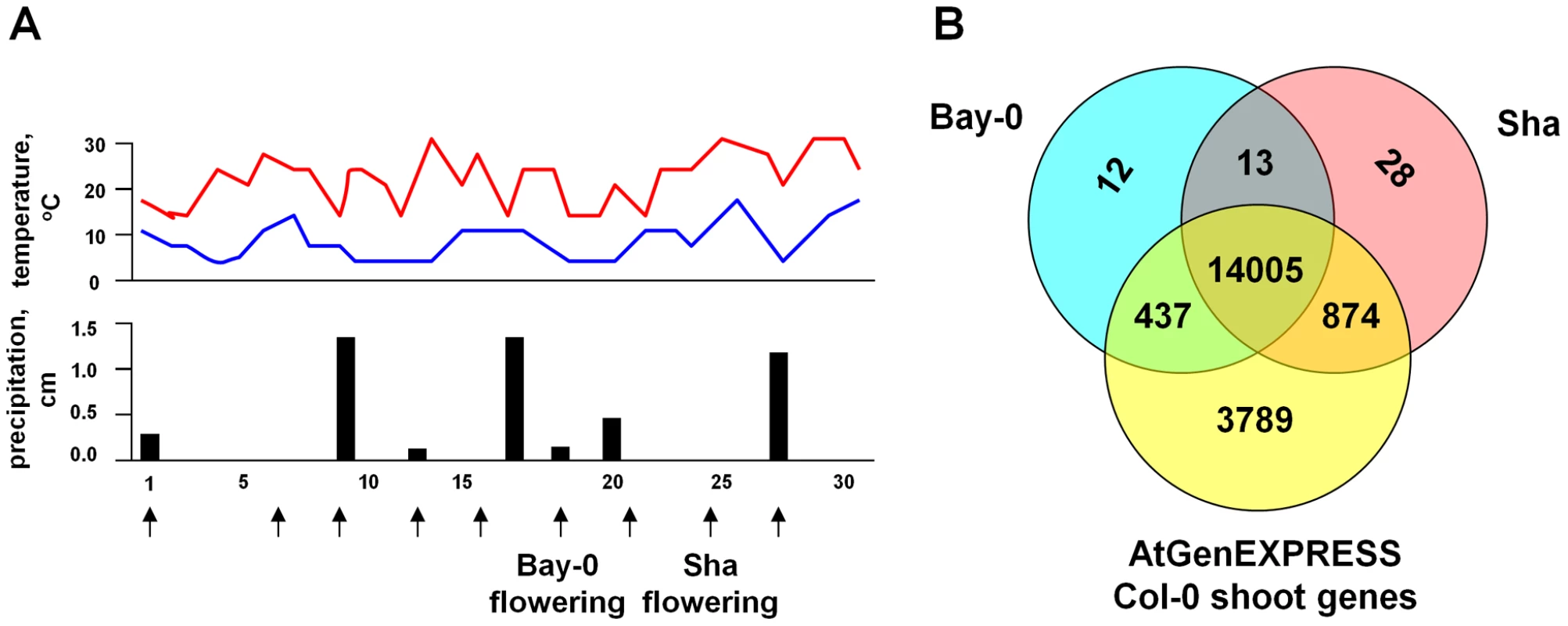
(A) The top graph shows the daily maximum (red) and minimum temperatures (blue), and the lower graph shows the daily levels of precipitation in the field during the experiment. The sampling days (given as days after germination) are shown in the bottom. The arrows indicate the sampling points, and the onset of flowering for Bay-0 and Sha in the experiment are shown. (B) The total number of genes expressed in Bay-0 and Sha in the field, compared to those observed for Col-0 shoot tissue in the ATGenExpress data set [29]. Despite the possibility of environmental heterogeneity in this outdoor field site, the replicates for the eight Bay-0 and ten Sha samples produced very similar results (mean pairwise correlation = 0.98, see Figure S1), which is comparable to replication quality observed in controlled laboratory experiments [29]. Genes were designated as expressed if they were observed in all three replicates at a timepoint by the Affymetrix Microarray Suite 5 (MAS 5) algorithm [47], and we found that 47% to 58% of genes in Bay-0 and 45% to 61% in Sha were expressed at each timepoint. In total, we found that ∼67% of genes were expressed in at least one accession for at least one time point (see Figure S2).
We compared our results to those observed in the ATGenExpress [29] data set. In total, we detected 15,369 genes in at least one accession (∼67% of genes), which is less than the 19,105 genes detected in the 48 comparable vegetative and flower tissue samples from wild type Col-0 in the ATGenExpress data set. Only 53 genes that were not found in the Col-0 shoot expression atlas showed expression in the Bay-0/Sha field dataset. The reduced number of detected genes in our experiment could reflect the fact that the ATGenExpress is a compendium of experiments done under multiple experimental conditions, some of which may not be relevant to the field conditions under which we conducted our study. Moreover, the ATGenExpress analysis has greater power to detect expression level differences given the larger sample sizes in that study [29].
The majority of the genes that were expressed in Col-0 overlapped with both Bay-0 and Sha samples (14,005 genes) (see Figure 1B). Across the Bay-0 and Sha field samples, expression for 7,459 genes (∼33%) were undetected, of which more than half were unannotated loci (4,626 genes in Bay-0 and 4,415 in Sha, FDR<0.05; see Figure S3). Twelve other GO terms were significantly over-represented among these unexpressed genes across the two accessions, including defense response and transcriptional regulation genes.
The flowering of A. thaliana in the field provides an internal validation of the observed gene expression patterns, since several genes have been identified that are associated with flowering and flower development. We created a heat map of groups of unique gene expression patterns (defined by cluster analysis, description of analysis below) for which more than 50% of the variance was explained by flowering status in both Bay-0 and Sha (see Figure 2). As expected, these clusters contain several floral developmental genes, including AP1 [48], AP3 [49], PI [50], AG [51], STK [52], and SEP2 [53]. The observation that the expression of these floral genes increase upon flowering provides confidence that our results in the field are consistent with expectations based on previous developmental genetic studies [29].
Fig. 2. Heat map for Bay-0 genes that show differential expression during flowering. 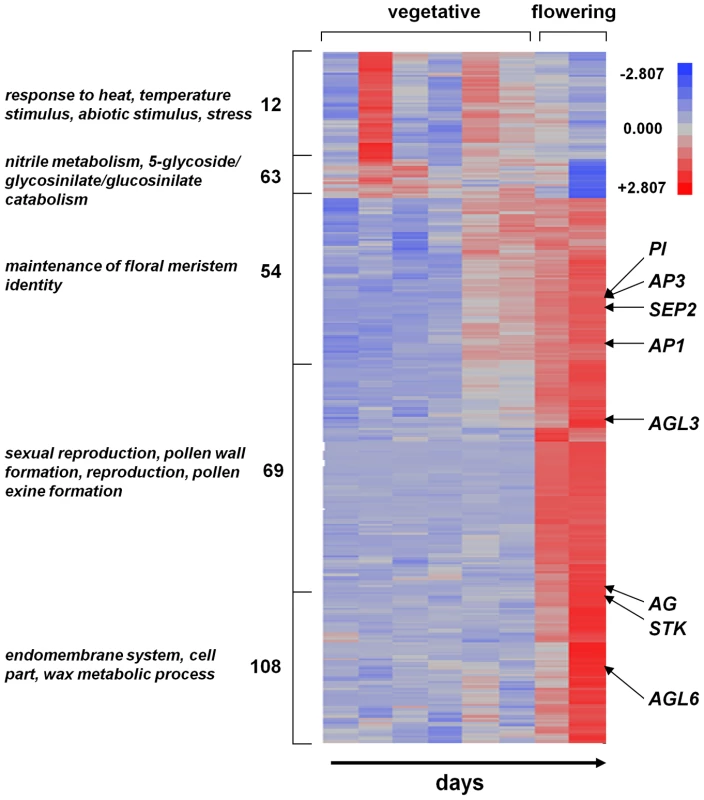
The numbers listed to the left indicate the cluster number identified in the silhouette analyses. The four most significant (i.e., lowest p values) GO term categories enriched for each cluster are shown on the left. The vegetative and flowering samples are indicated at the top, and the rows corresponding to various flower development genes are shown. Several developmental genes associated with bolting and flower development are highlighted. Scale: from brightest blue equals most down-regulated to brightest red equals most up-regulated. Strong differentiation in genome-wide gene expression patterns between A. thaliana accessions
While we expected to find that the flowering states of A. thaliana (vegetative vs. flowering) are transcriptionally distinct, we also found that natural genotypic differences between accessions are an equally important component of genome-wide differences in gene expression patterns under field conditions. We ran a principal variance components analysis (PVCA) [54] of the ∼22,800 genes expressed across the combined Bay-0 and Sha data set to examine whether gene expression is explained by accession or flowering status (vegetative or flowering). This approach first reduces the dimensionality of the data set with principal components analysis (PCA), and then computes variance components by fitting a mixed linear model to each principal component (PCi = accession+flowering status+accession-by-flowering status+error). For each factor in the model, the variance components are averaged across all of the principal components, but weighted by the eigenvalues for the corresponding principal component.
Principal component 1 (PC1all) explained 18% of the variation in genome-wide gene expression and clearly distinguished the two accessions, while vegetative vs. flowering states were demarcated along PC2all (16%) and PC3all (10%). The mixed linear model of the principal components attributed approximately equal amount of the transcriptional variance to accession (39%) and flowering status (38%; see Figure 3). We modeled the effects of accession and flowering status on gene expressions with a mixed model analysis of variance (ANOVA): gene expression = accession+flowering status+accession-by-flowering status+error. The analysis indicated that the two accessions differed significantly in 3,344 genes (∼14% of the transcripts; see Figure 3), which is within the range previously found for several accessions [55]. This apparent discrepancy between the number of genes that significantly vary between accessions and the fraction of transcriptional variance explained by accession indicates that a small fraction of genes can explain a large amount of expression variation, which has been shown in other comparisons between accessions [56].
Fig. 3. Components of transcriptional variance in A. thaliana in the field. 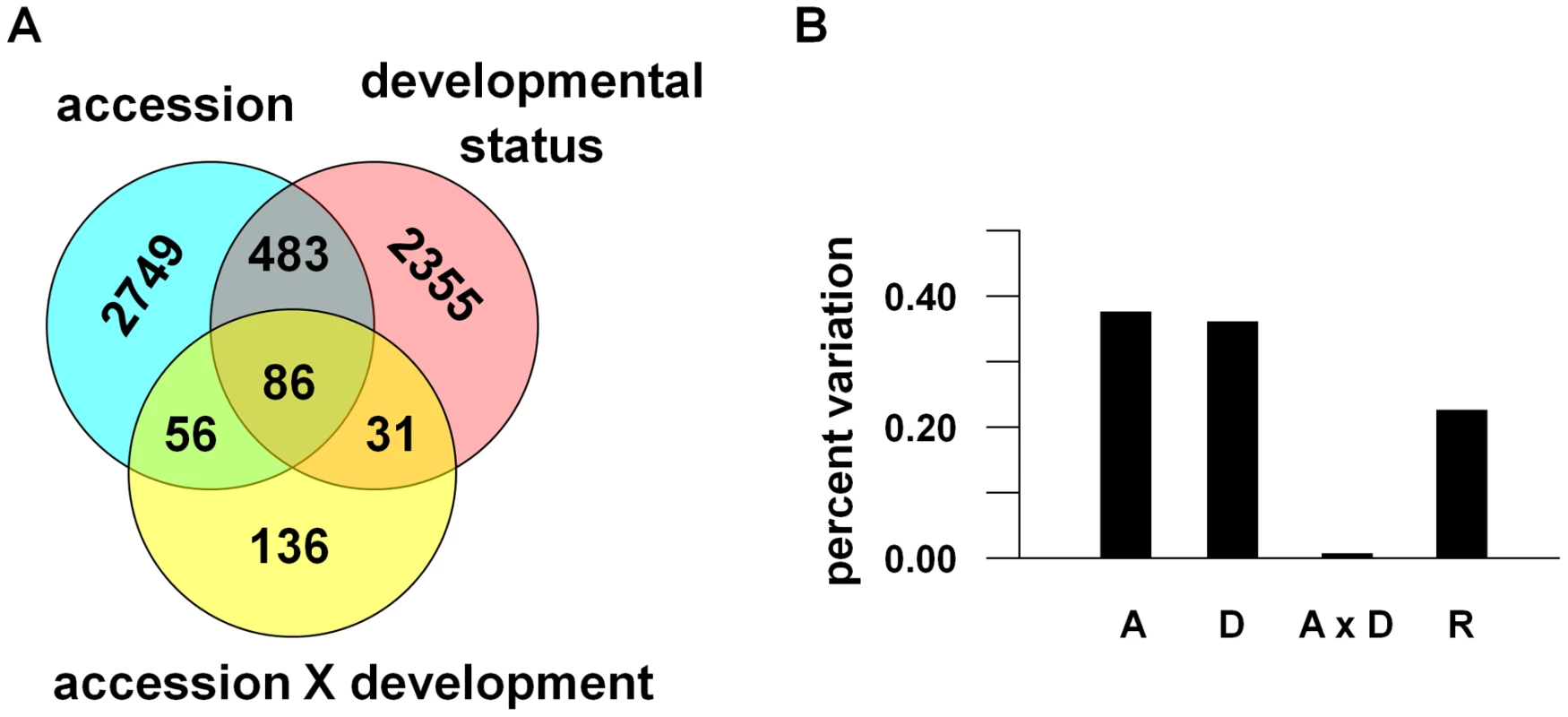
(A) The number of genes that significantly differ between Bay and Sha accessions, flowering status, and that show an accession-by-flowering status (development) interaction. (B) The percent of the transcriptional variance explained by these factors. In addition, 2,955 genes (13% of transcripts) significantly differed between vegetative and bolting shoots, 569 of these were also different between accessions, and 117 of these also showed a significant interaction effect between accession and flowering status (see Figure 3). The overall amount of variance explained by the interaction term was very small (<0.01%; only 309 genes total). These results suggest that while the flowering states of A. thaliana (vegetative vs. flowering) are transcriptionally distinct, natural genotypic differences between accessions show equally significant genome-wide differences in gene expression patterns under field conditions.
A gene ontology (GO) enrichment analysis on the combined data for the main effects of accession, flowering status and the interaction of these two terms showed accession differences are enriched for genes in the sulfate assimilation, glucosinolate and glutathione biosynthesis pathways, while unannotated and translation genes were underrepresented (FDR q<0.05, see Table S1). There are 21 GO categories overrepresented between vegetative and flowering states, including genes that are associated with development, pollen exine formation, and sexual reproduction.
Using the same PVCA and mixed model ANOVA approach, we also looked at global trends in gene expression observed within each of the two accessions by examining how variation in genome-wide transcription levels is explained by various environmental factors. We examined the effects of age, flowering status (i.e., vegetative vs. flowering), minimum and maximum daily temperatures, and daily precipitation, using the mixed linear model: gene expression = age+flowering status+minimum temperature+maximum temperature+precipitation+error (see Figure 4).
Fig. 4. Environmental components of transcriptional variance in A. thaliana in the field. 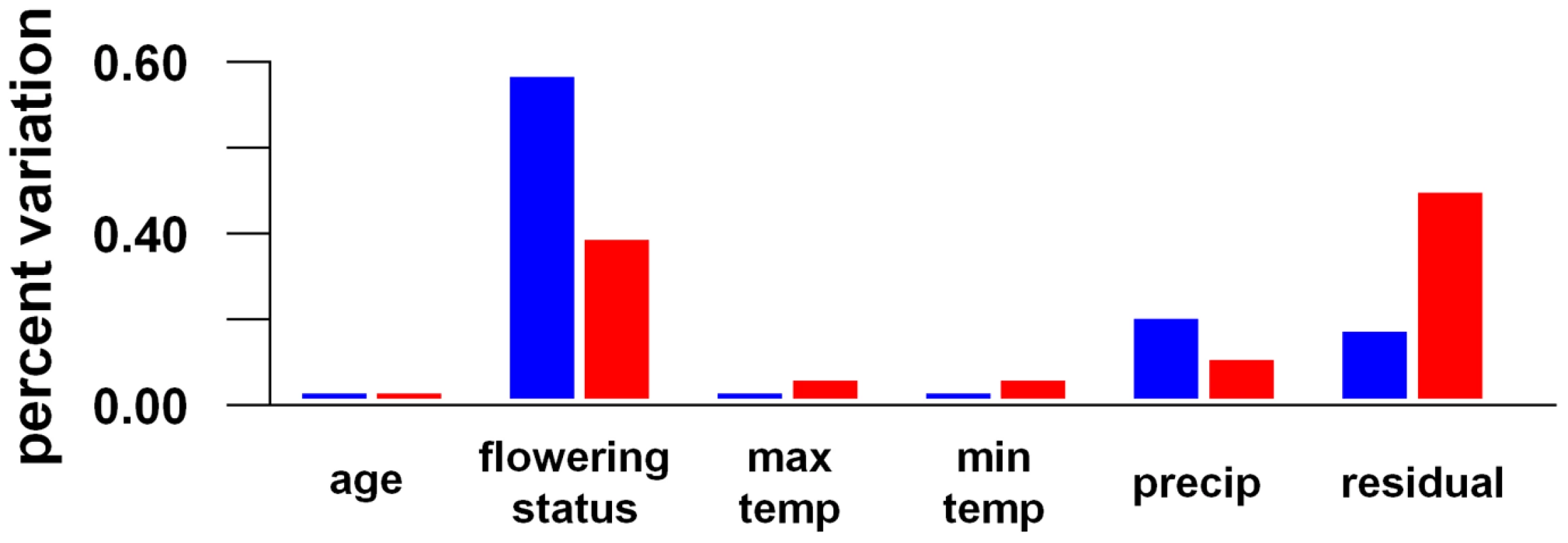
The blue indicates Bay-0 and the red Sha. Temperature and precipitation were the only environmental measurements we were able to obtain from the field site. Another environmental variable was daylength, but this was correlated with age (i.e., increasing daylength during the field experiment). We found weak correlations between the other environmental features during the field study: maximum and minimum daily temperatures (r2 = 0.17; p<0.007), maximum temperature and precipitation (r2 = 0.26; p<0.001), and minimum temperature and precipitation (r2 = 0; p<0.77).
Looking at each accession separately, flowering status explained more than 50% of the variance in expression across the genome for Bay-0 and over 30% of the variance for Sha. The only environmental variable that explained a substantial portion of transcriptional variance was precipitation (23% in Bay-0, 13% in Sha); plant age, minimum and maximum temperature explained negligible levels of variance (see Figure 4).
Significant variation in gene expression across the A. thaliana life cycle in the field
Genes whose expression levels are variable in time across the life cycle in the field are of great interest, since they may provide insights on the transcriptomic response to development and environment (i.e., transcriptomic plasticity). An alternative approach to ANOVA that may be more appropriate for significance testing of time course microarray data fits a cubic spline to gene expression levels across time and tests for significant deviation from an invariant gene expression pattern [57], [58]. Using this approach, 12,599 genes in Bay-0 and 15,824 genes in Sha (FDR of q<0.05) display significant variation in time. GO analyses of these genes found enrichment for genes associated with metabolism, microtubule-based processes, heat and stress response, and transport processes. While the larger number of time-variable genes in Sha could reflect higher developmental and environmental transcriptomic plasticity (i.e. Bay-0 might be more robust), it more likely reflects the larger number of time-points sampled in Sha and its consequent exposure to a wider range of environments due to later flowering.
Several co-expressed gene clusters in the wild are enriched for abiotic and biotic stress-inducible genes
A major goal of this study is to identify genes and gene clusters that may be associated with fluctuations in natural environmental conditions in the field. One approach we took was to identify gene clusters in the expression profiles, and correlate these with environmental factors and previously published microarray studies of abiotic/biotic stress responses.
We identified distinct co-expressed gene clusters in the life cycle of these accessions in the wild. We used the mean expression values of significantly time-variable genes found at an FDR of q<0.01 to isolate strong signatures of response to environmental factors. Under this criterion, we analyzed 3,827 genes in Bay-0 and 8,215 genes in Sha using the silhouette method [59], to identify 109 co-expressed gene clusters in Bay-0 and 188 clusters in Sha (see Figure 5). This number of clusters was similar to that identified by K-means clustering with a correlation of between 0.75 and 0.8 in Bay-0 (105 and 163 distinct clusters) and between 0.7 and 0.75 in Sha (169 and 277 distinct clusters).
Fig. 5. Gene expression clusters in A. thaliana field transcriptomes. 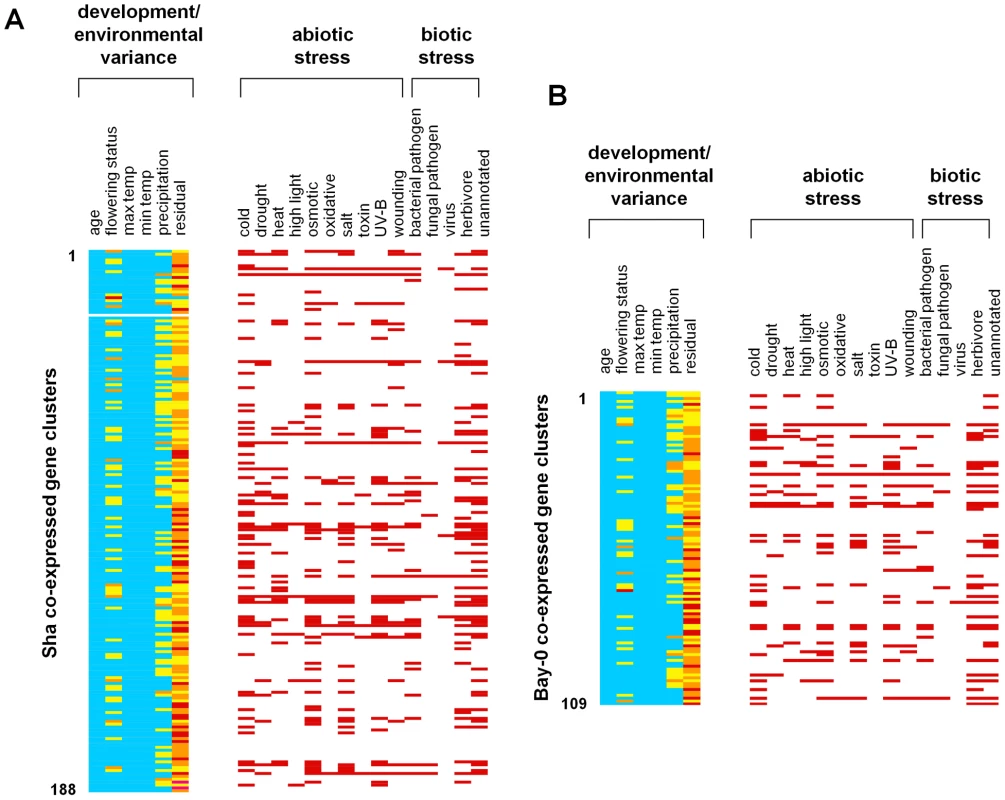
Silhouette analysis defined (A) 188 clusters in Sha and (B) 109 clusters in Bay-0. Each row indicates a specific cluster. The components of the transcriptional variance explained by environmental (precipitation, maximum and minimum daily temperatures) and developmental factors (age and flowering status) are shown. The percent of the transcriptional variance explained by a factor is shown in color (blue, <25%; yellow, between 25–50%; orange, between 50–75%; red, >75%). We also examined each cluster for enrichment of genes shown to be differentially expressed in previously-studied transcriptome analyses. A red bar indicates that the cluster is enriched in genes differentially expressed under that stress condition. For each cluster, we used the PVCA approach to fit the mixed linear model: gene expression = age+flowering status+minimum temperature+maximum temperature+precipitation+error. There were several clusters that showed >50% of the variance explained by flowering status, although no cluster showed transcriptional effects due to plant age. Among the environmental responses, there were several clusters for which more than 50% of the variation was explained by precipitation – indicating up - or down-regulation of genes under high precipitation (>25 mm but not at <10 mm). While several clusters showed a small percentage of the variance explained by either minimum or maximum daily temperature, no cluster showed more than 50% of the variance explained by these factors suggesting that the genes responding specifically to temperature may be more dispersed across the structure of clusters we identified. We also compared the genes in these clusters to published microarray studies on gene expression under known environmental stresses. These studies encompass various abiotic and biotic stresses that affect gene expression, including high light [60], cold, drought, heat, osmotic stress, oxidative stress, salt, genotoxins, UV-B exposure, wounding [61], and infection by RNA virus [62], [63], bacterial pathogens, fungi and herbivores [64]. Combining these data sets, more than half of the genes on the ATH1 array were associated with at least one stress (13,153 of 22,800 genes). Enrichment in specific clusters for genes associated with differential expression in each of these stress responses could provide clues about the ecological factors that drive the underlying field expression patterns.
An intriguing result of the stress annotation analysis was that many gene clusters appeared to contain genes that responded to multiple laboratory stress treatments. For example, six co-expressed gene clusters in Bay-0 and ten clusters in Sha appeared to contain genes that were responsive to nine or more stresses (see Figure 5), suggesting that these loci were associated with generalized stress responses. These include several genes encoding heat-shock proteins (e.g, Hsp70, Hsp101, Hsp17.6), the cold - and ABA inducible gene kin1, cold-regulated genes cor15a and b, and the stress-responsive LT16 and sti1-like protein-coding loci. These general stress response clusters included both biotic and abiotic stresses in all but one of the Bay and one of the Sha clusters, which were enriched for only abiotic stresses. In general, we found strong overlap between response to abiotic and biotic stresses. For example in Bay, 28 clusters were enriched for both types of stress, while 15 were enriched for only abiotic stresses and five only for biotic stresses. In Sha, 42 clusters were enriched for both types of stress, with 36 clusters enriched only for abiotic stresses and 11 clusters enriched only for biotic stresses.
On the other hand some clusters were enriched for response to only one specific stress: for example nine Sha clusters were enriched only for response to cold, four clusters were enriched only for response to osmotic stress, two clusters were enriched only for response to UV-B radiation, and five clusters were enriched only for response to herbivory (see Figure 5).
Principal component analysis of vegetative stage gene expression in the wild
Our analysis looked at expression across both vegetative and reproductive stages of the life cycle. Given the strong effect of flowering status on gene expression patterns, we also undertook a principal component analysis of gene expression on the vegetative stages in the field, and examined correlations with environmental conditions of the PC scores. A subset of genes significantly expressed in both accessions across vegetative stages, were analyzed to minimize the differences due to flowering stage (i.e. PC1all = 17.9% of the variance), and accession (PC2all = 15.6% of the variance). Thus a total of 14 samples with three replicates each (6 samples from Bay-0 and 8 samples from Sha) and 8,954 genes were analyzed by principal component analysis.
Ten vegetative stage principal components (PCveg) captured ∼70% of the variation between accessions and across time points. To visualize the trends of the variation, the mean PCveg values for each accession were plotted against their corresponding time points (see Figure 6). Only the first five vegetative stage principal components are shown as they captured more than 50% of the transcriptional variation. PC1veg, PC2veg and PC5veg showed no significant differences between Bay-0 and Sha. In contrast PC3veg and PC4veg showed differences across most time points between accessions, suggesting that these principal components still capture some of the expression differences due to genetic background. This indicates that the subset of genes significantly expressed in vegetative stages was not sufficient to account for accession effects (i.e. PC1all), but it uncovers other trends of gene expression variation that have an environmental basis.
Fig. 6. Vegetative stage principal component analyses of A. thaliana field transcriptome. 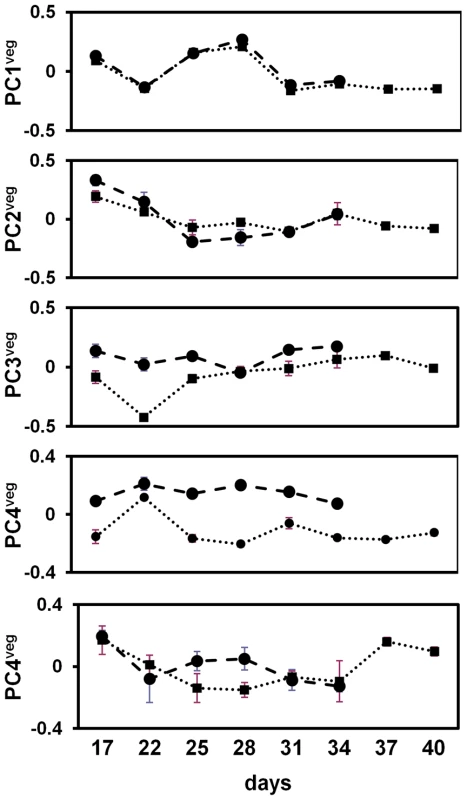
The first five principal components are indicated. Principal component scores are on the y-axis and sampling days on the x-axis. The dashed line gives the PC score for Bay-0 and the dotted line for Sha. At each sampling day, the mean PC score across the three replicates are given for Bay-0 (circles) and Sha (squares), with standard error bars (+/− 1 SE). To assess possible environmental or developmental associations with these principal components of gene expression, we used multivariate regression to regress PCveg values on measured variables (see Table 1). Minimum daily temperature was not considered in the model because it was co-linear with maximum daily temperature. PC1veg was significantly negatively correlated with maximum daily temperatures (β = −0.025, p<0.001) and marginally significant with RLN (β = 0.025, p<0.049), explaining a significantly large proportion of the variance (adjusted r2 = 0.87, p<0.0001). PC2veg was significantly correlated to daily precipitation levels (β = −0.17, p<0.001) and age (β = −0.014, p<0.001), which explained significant proportions of the variance (adjusted r2 = 0.53, p<0.0001). The third principal component of global vegetative gene expression did not show a significant linear correlation with any of the environmental factors or plant development. These results predict that genes correlated to PC1veg might be related to temperature responses, whereas those that correlate to PC2veg might be related to water availability/drought responses.
Tab. 1. Characteristics of the major vegetative stage principal components of A. thaliana genome-wide gene expression in the field. 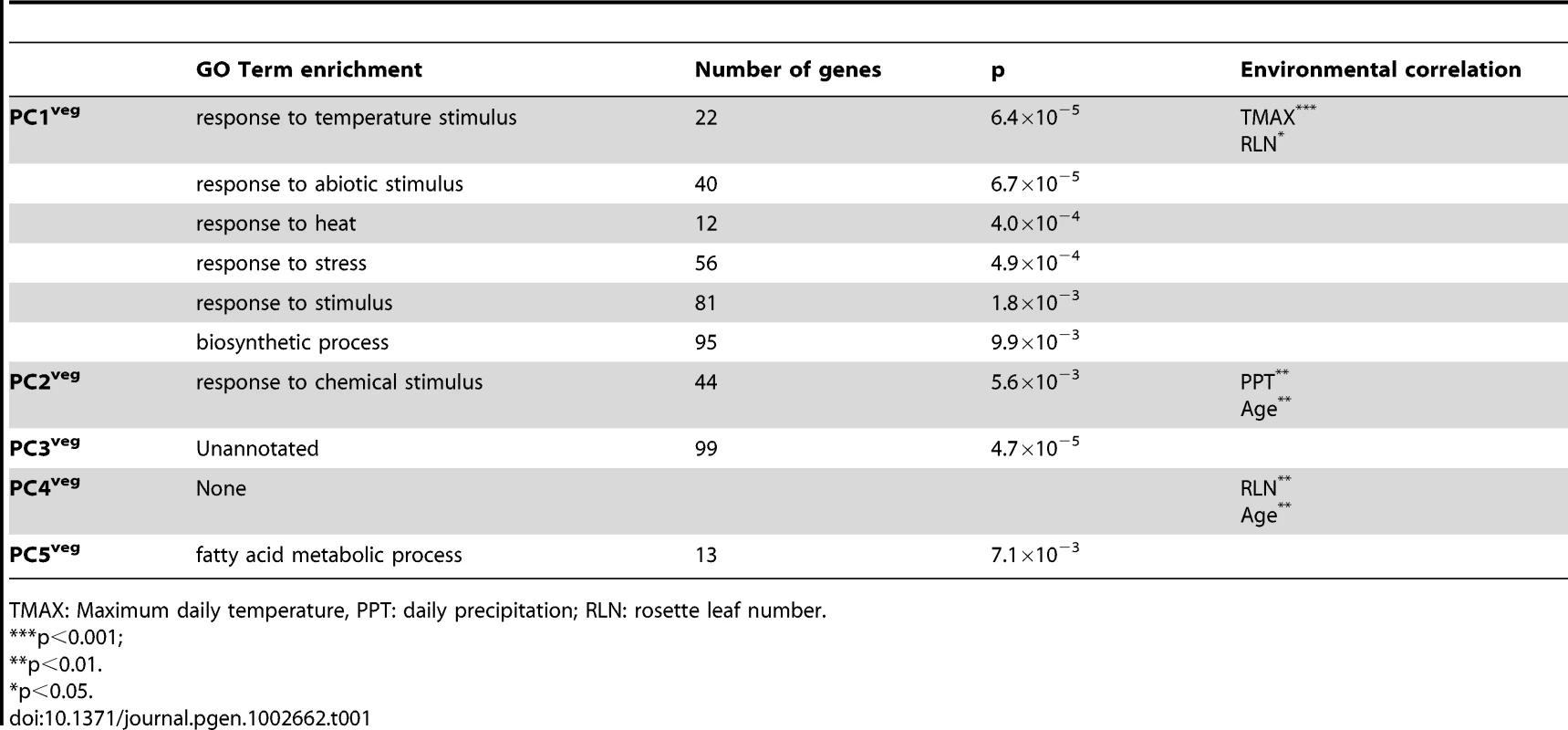
TMAX: Maximum daily temperature, PPT: daily precipitation; RLN: rosette leaf number. We examined the gene sets for gene ontology (GO) term enrichments (p≤0.01; hypergeometric distribution) to identify significantly over-represented functional gene classes in Virtual Plant 1.0 [65] (see Table 1). First, the entire set of 8,954 genes that showed significant time series expression differences in the vegetative stages, was analysed for GO term enrichment; these showed cell part, membrane, plasma membrane, response to chemical stimulus, response to stimulus, response to abiotic stimulus, intracellular part, membrane bounded organelle, and intracellular membrane-bounded organelle as over-represented gene ontology categories.
In order to understand what gene functions are associated with each principal component, we conducted GO term enrichment analyses for gene sets associated with each PCveg. We selected genes that showed extreme PC loadings for each of the PCveg axes (upper and lower 2.5 percent of the quantile distributions); thus we selected the 5% of the genes that showed the best correlation to each PCveg. The results of the analyses on each vegetative stage principal component showed that genes strongly associated with PC1veg are mainly from the GO categories response to temperature stimulus, response to abiotic stimulus, response to heat, response to stress, response to stimulus, which is consistent with the observation of maximum daily temperature and temperature fluctuation explaining a significant proportion of the variance in this principal component of expression. Genes with strong loadings on PC2veg are orthogonal (uncorrelated) to genes in PC1veg, and were over-represented in the GO categories response to chemical stimulus genes, which might reflect growth and stress responses regulated by common hormone signaling cascades [66] rather than the enrichment in the 8954 genes dataset. Genes strongly associated with PC3veg are typically unannotated genes, but 32 are transposable elements. PC5veg was associated with genes related to fatty acid metabolism [67], while genes with high loadings in PC4veg did not show overrepresentation from any GO category. Gene lists for PC1veg and PC2veg (2.5 and 5% of quantiles) are shown in the Tables S2, S3, S4, S5.
Expression of the temperature response regulatory network in the vegetative stage in the wild
It has recently been shown that approximately half of the transcript responses to ambient temperature in A. thaliana are regulated by the chromatin remodeling gene ARP6 [68]. This gene, formerly known as ESD1, encodes a subunit of the SWR1 complex required for insertion of the alternative histone H2A.Z into nucleosomes [69], [70]. ARP6 regulates global response to ambient temperature in part by modulating nucleosome occupancy of H2A.Z [68].
The ARP6 gene was associated with PC1veg, and its expression was significantly correlated with this principal component (r2 = 0.49, p<0.001; see Figure 7), consistent with PC1veg being correlated with temperatures in the field. One other temperature-regulated gene is the heat shock protein HSP70 [71], which is also regulated by ARP6 [68]. Like ARP6, the expression of the HSP70 gene was significantly correlated with PC1veg (r2 = 0.73, p<0.001; see Figure 7).
Fig. 7. Gene expression in A. thaliana under field conditions correlated with PC1veg. 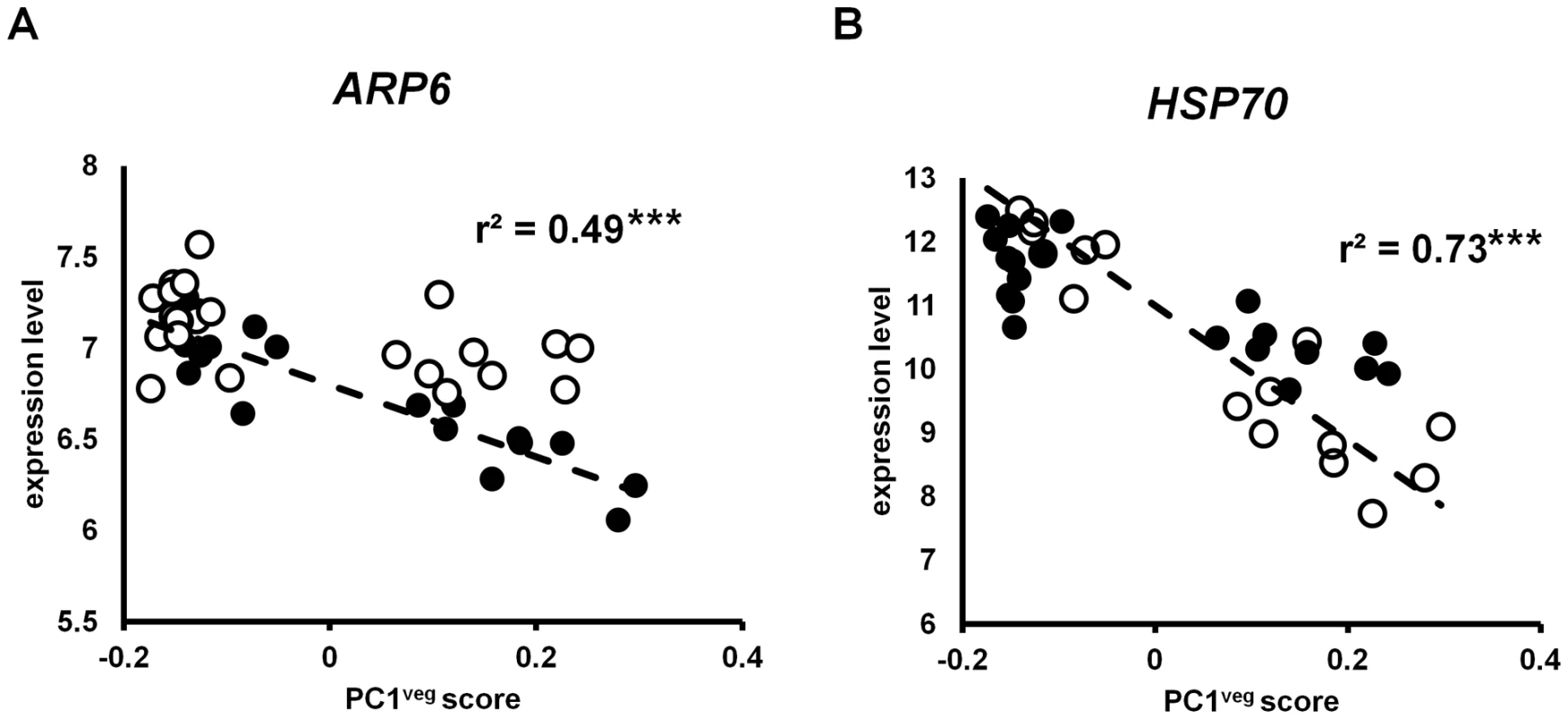
Each point is the LS mean expression level of (A) ARP6 and (B) HSP70 in Bay-0 (open circles) and Sha (closed circles) for each time point. The dashed line shows the linear regression through the data. *** = p<0.001. Our results and those of Kumar and Wigge [68] both suggest that ARP6 may regulate global temperature responses in the field by controlling nucleosome dynamics. We identified other genes that may be co-regulated with ARP6 by finding loci whose expression in the field matched the absolute expression pattern of ARP6 in our data, using Pavlidis template matching [72]. Previous studies have shown that temperature explains 47% of the variation in ARP6 expression [r2 = 0.47; 68]. We used this threshold in our template matching analysis, and found that out of the 8,954 genes in our analysis, ∼40% (3,583 genes) in Bay-0 and ∼24% (2,118 genes) in Sha displayed expression profiles that were correlated to the ARP6 field expression template at this threshold cut-off. These are consistent with previous studies that suggest that ARP6 can control responses to temperature for a large number (∼5,000 genes) in A. thaliana [68].
To gain further understanding about the functions of these genes that are co-regulated with ARP6, we looked for over-represented GO term categories. One GO term that was enriched was response to heat with 47 genes in Bay-0 (p<10−6), and 27 genes in Sha (p<0.0003). The other enriched category was unannotated genes, with 573 genes in Bay-0 (p<10−8) and 339 genes in Sha (p<0.00015), out of which 67 and 47 genes respectively are transposable elements. The latter suggests that ARP6 activity in the wild might be regulating other functions still to be described, and that transposable element activity may be triggered in part by environmental temperature fluctuations.
ABA hormone signaling gene expression is associated with daily precipitation levels in pre-bolting plants
The second global vegetative principal component (PC2veg) was correlated to precipitation and fluctuating temperatures, and was significantly associated with genes involved in chemical stimulus response (p<0.007). Although among the genes associated with PC2veg were those that are linked to auxin, cytokinin, gibberellic acid and brassinosteroid hormones, genes involved in the hormone abscisic acid (ABA) made up the largest fraction of hormone-associated loci in this principal component. This is noteworthy since ABA synthesis and signaling are known to mediate stress responses to water availability and osmotic stress, including drought and salinity stress responses, and PC2veg is significantly correlated with daily precipitation levels (see Table 1).
Among the ABA-associated genes significantly associated with PC2veg was ROP10, which encodes a plasma membrane-bound rho-related GTPase protein that negatively regulates ABA responses [73], [74]. ROP10 expression was significantly correlated with PC2veg (r2 = 0.42, p<0.001; see Figure 8). To identify other genes that are co-regulated with ROP10, we obtained the genes that matched its absolute expression pattern using Pavlidis template matching. Unlike the temperature response analysis, we did not use previous data to guide our choice of correlation coefficient; we arbitrarily used a correlation coefficient of 0.7 in this analysis. Using this criterion, we identified many more genes co-regulated with ROP10 in Bay-0 (273 genes) than in Sha (18 genes). Consistent with our finding of ABA hormone associated genes in PC2veg, and the significant correlation of this vegetative state principal component with daily precipitation levels, we found two genes in Bay-0 (At1g52080 and At5g61820) and one gene in Sha (At1g01470) that appear to be regulated by ABA levels [74]. Other ABA-associated genes correlated with PC2veg include AAO3 (r2 = 0.71, p<0.001; see Figure 8), which encodes an enzyme that catalyzes the last step of ABA biosynthesis in leaves [75], and P5CS2 (r2 = 0.55, p<0.001) which encodes the rate-limiting enzymes for ABA-associated accumulation of proline under water stress [76], [77].
Fig. 8. Gene expression in A. thaliana under field conditions correlated with PC2veg. 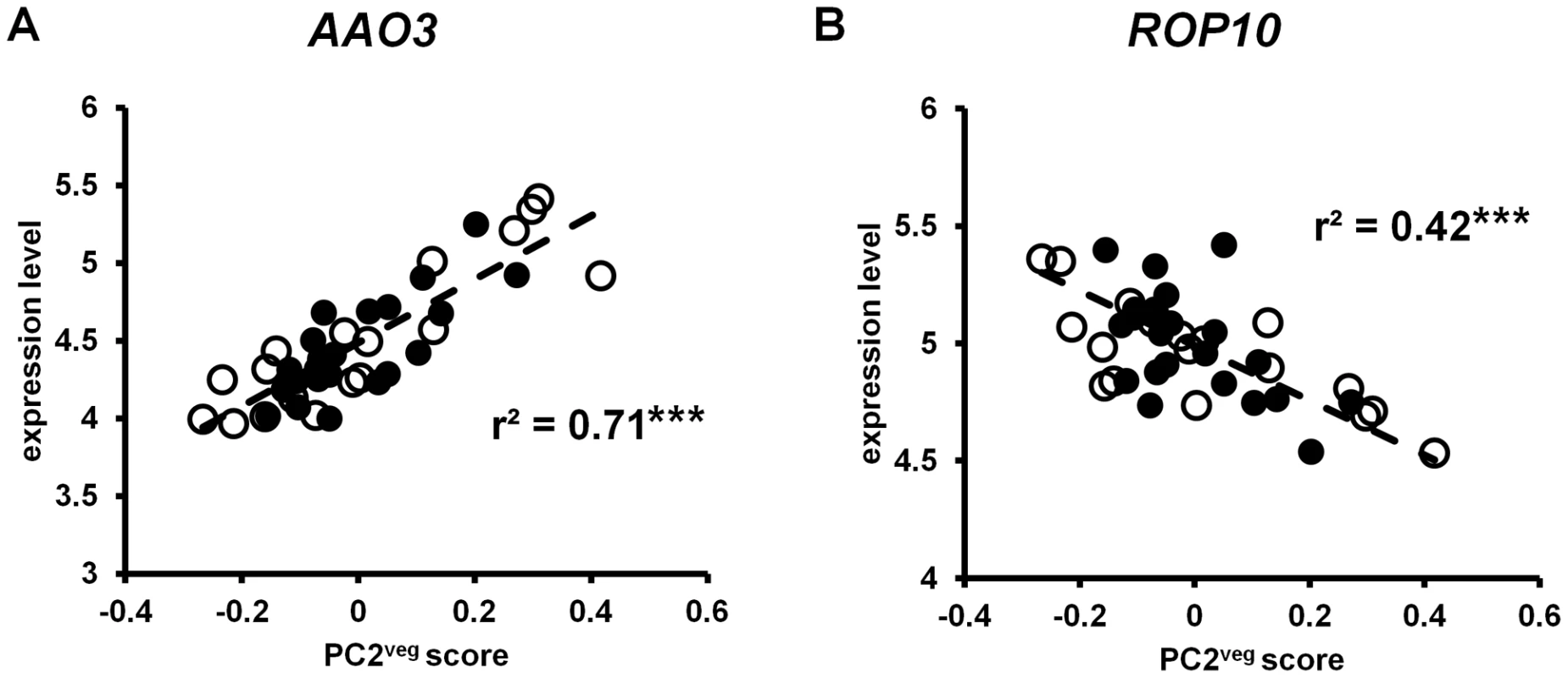
Each point is the LS mean expression level of (A) ROP10 and (B) AAO3 in Bay-0 (open circles) and Sha (closed circles) for each timepoint. The dashed line shows the linear regression through the data. *** = p<0.001. Discussion
We have determined genome-wide expression profiles throughout the life cycle of the model plant A. thaliana under ecological field conditions to examine the nature of the transcriptome under the complex environment of a natural climatic season. Our analysis indicates that a majority of the genes in the A. thaliana genome show significant changes in gene expression throughout its life cycle in the field, and that accession is an important component of transcriptional variation among individuals. There are also clear effects of flowering status, as the onset of flowering not surprisingly leads to large-scale changes in transcriptional patterns in the A. thaliana shoot, with several genes associated with shoot bolting and floral development increasing in expression (see Figure 2). Despite the complexity in natural environments, transcriptional patterns are clearly organized into ∼100–200 co-expressed gene clusters in our A. thaliana accessions. Genetic studies have identified genetic networks that underlie plant responses to abiotic stress, including networks associated with drought responses [78], heat stress [79], [80] and cold responses [81]. Many of these networks contain genes that responded to multiple, laboratory-induced environmental stresses that have been identified by previous microarray studies in plants, including transcriptional responses to temperature [81]–[83] drought [61], [82], salt stress [83], metals [84], [85], nutrients [86], [87] and biotic challenges [88], [89]. Indeed, many of our inferred field gene expression clusters contain genes responsive to multiple environmental factors (see Figure 5), suggesting that these clusters may be responding to the complex conditions in field settings. It should be noted that the spring field environment in which our plants grew were not extreme in either temperature or precipitation levels, and our findings indicate that previously described stress genes may be associated simply with responses to normal environmental fluctuations that plants generally experience during their life cycle.
We were able to obtain measurements for daily temperature and precipitation from our field site, and it appears that both of these factors are significantly correlated with gene expression patterns. Precipitation was correlated with global gene expression patterns across the full development of the plant, while temperature was correlated with expression only in pre-bolting plants. This is unlikely to provide the full picture of responses to environmental conditions in the field, since for some genes, expression is probably associated with (i) complex, nonlinear responses to these environments, (ii) interactions between environmental signals, and (iii) responses to unmeasured environmental conditions. Future studies should provide greater breadth and resolution in environmental measurements. As more data becomes available, more complex relationships between gene expression and environmental features, including complex interactions between different variables, can be explored.
Despite the limitations of our environmental analyses, they confirm the role of several genes and gene sets to field environmental fluctuations, including ARP6 [68], [90] and HSP70 [71] to temperature, and ROP10 [73] and AAO3 [75] to precipitation. We did examine whether gene function for some of these loci showed fitness effects under variable or stressed environments (see Text S1, Figure S4 and Table S6). Using T-DNA insertion mutants for 14 genes that are associated with PC1veg and PC2veg, we compared fruit number in mutant vs. wild-type lines under fluctuating temperature or decreased water environments. We only saw a significant environmentally-dependent effect of accession under changes in water availability, associated with the genes AA03 and ALDH7B4 [93]. Contrary to expectations, however, the decrease in fruit numbers in mutant vs. wild-type lines was observed in benign (and not stressful) environments (see Figure S4 and Table S6). More intensive studies with a larger sample of genes may yet reveal fitness consequences of other ecologically-relevant genes identified in our analysis.
While there have been tremendous strides in understanding the molecular genetic networks underlying plant phenotypes, we still know very little about what happens in natural wild environments. Indeed, there is growing interest in the study of the genetics of adaptation in ecological field environments, especially as related to climatic variables [94], [95]. As we begin to study the genetic networks associated with plant environmental responses [91], [92], we can correlate molecular networks with gene expression profiles in the wild, identify natural variation in genes and genetic networks and associated microevolutionary adaptations, and establish relationships between gene functions and organismal phenotypes [6]. This will allow us to link gene functions to ecologically relevant responses of plants to their lives in the wild, providing the foundation for the study of ecological genomics and ecological systems biology that can illuminate the molecular basis of species adaptations to real-world environments.
Materials and Methods
Study material and sites
We chose Bay-0 and Sha because they are rapid-cycling spring annuals that germinate and flower under Northeastern US field conditions [93]. These accessions have been genotyped at >1,000 gene fragments and thus have a large amount of SNP markers available for further genetic characterization [45], and are the progenitors of a recombinant inbred mapping population with over 165 core lines that can be used for future QTL mapping studies [44]. The field site at the Cold Spring Harbor Laboratory greenhouses has similar, but milder climatic conditions to Bristol, Rhode Island, which is the site of previous A. thaliana field projects [25], [93]–[95], and we had previously grown Bay-0 and Sha accessions in this field site in the fall/winter/spring of 2006–2007.
Sampling and microarray experiments
We stratified seeds of Bay-0 and Sha for four days at 4°C and planted them in flats in a mixture of equal parts topsoil, sand and peat moss. We left the seeds under domes to germinate in an ambient temperature greenhouse for 5 days, and moved them outside on day 10 to acclimate the plants before transplanting to the field. On day 13 (27 April 2008 at the ∼4-leaf stage), we planted seedlings in a 2-m2 field grid marked off every 2 cm2, and distributed the two accessions across the grid in a completely randomized design. For each accession, we collected three replicates of the entire shoot of two individuals every three days, between 4.5–5.0 hours after sunrise, starting five days after transplant until bolting was observed for each accession (see Figure 1A). We collected replicates for each sample within a 15–20 minute window in a given day. We recorded bolting day as the day when at least 50% of the plants of an accession had initiated bolting. On this day for each accession (sample 6 for Bay-0 and sample 8 for Sha), we collected three sets of replicates from both bolted and non-bolted individuals. Three days after this bolting date for each accession, we collected the final sample of bolted shoots. Bolting occurred at 34 days for Bay-0, and we collected six vegetative and two bolting timepoints. The Sha accession bolted at 40 days after planting, and we collected eight vegetative and two bolting timepoints.
We collected a total of 18 samples in triplicate except for in Sha, where one replicate was lost in the sixth sample timepoint. For each replicate of each sample, we extracted total RNA using the RNAEasy plant mini kit (QIAGEN), using all of the above ground tissue for both plants. In the later stages of development, the total material for reach replicate exceeded the limits recommended for the Qiagen spin columns. In those cases, we used twice as much RLT buffer to suspend the frozen finely ground tissue and transferred half of the lysate to each of two spin columns. The two halves of the sample were kept separate through the collection, but were combined before quantification and hybridization to the microarray chips. The New York University Medical Center Genome Core Facility performed the hybridization of RNA and scanning to Affymetrix ATH1 chips according to manufacturer's protocols (Affymetrix).
Data analysis
In order to compare our results to those observed in the ATGenExpress [29] data set, we used the Affymetrix Microarray Suite 5 (MAS 5) algorithm [47], [96] to determine if genes were expressed at any time point. Genes were considered expressed if they were observed in all three replicates of a sample. We used JMP/Genomics with the SAS statistical package (Version 9.1.3 for Windows; SAS Institute, Cary, NC, USA) and Virtual Plant 1.0 [65] for all initial PVCA, PCA, correlations, ANOVA and Gene Ontology (GO) analyses. Because the ATH1 microarray was designed based on the Col-0 accession, different single feature polymorphisms (SFPs) for probes within each probe set may exist for Bay-0 and for Sha, and probe mis-hybridization may occur when examining the transcriptome of Bay-0 and Sha [97]. To correct for this problem, we ran analyses on different imports of the raw (.cel file) data filtering the appropriate probes that contained SFPs for Bay-0, Sha, or for both depending on the analysis. After importing the raw data into JMP/Genomics with the appropriate filter, we background transformed the data with RMA across the collection of microarrays. Raw expression was summarized by probeset with a median polish and log2 transformation. We used the TAIR 9 annotation file to obtain the probe to gene matches.
We used principal variance components analyses [PVCA; 54] to examine global expression trends associated with accession and flowering status. The PVCA approach first reduces the dimensionality of the data set with PCA, and then computes variance components by fitting a mixed linear model to each principal component, treating each factor of interest in the model as a random effect (including continuous variables). We used the model PCi = accession+flowering status+accession-by-flowering status+error, where i indicates each principal component, starting with 1 and continuing through all principal components calculated in the PCA. The variance component for each factor is obtained by a weighted averaging across the values calculated for each principal component, weighted by the eigenvalues for the corresponding principal component. We used the same factors in a mixed model ANOVA to directly fit the model to gene expression (ANOVA model: gene expression = accession+flowering status+accession-by-flowering status+error). We also used the same PVCA and mixed model ANOVA approach to examine a larger model that incorporated age and the environmental factors maximum daily temperature, minimum daily temperature and daily precipitation (ANOVA model: PCi or gene expression = age+flowering status+minimum temperature+maximum temperature+precipitation+error) within each accession.
Finally, we used the EDGE program designed for significance testing of time course microarray data that fits a cubic spline to gene expression levels across time and tests for significant deviation from an invariant gene expression pattern [57], [58]. We ran a GO analysis in JMP Genomics to identify association with gene ontology categories for each cluster.
Cluster analysis
We used K-means clustering on the mean expression values of the significant genes from a stringent pairwise ANOVA analysis (FDR q<0.01) for Bay-0 (3,827 genes) and Sha (8,215 genes). Although an r = 0.7 has been arbitrarily used in other microarray analyses to define the number of clusters within a data set, we used the silhouette function in MATLAB (MathWorks 2009) to find an appropriate number of distinct clusters of genes that behave similarly across the data sets [59]. The silhouette statistic is a pairwise method of evaluating the amount of similarity of individuals within a cluster compared to each of the individuals within each of the other clusters. By running this statistic on an increasing number of clusters, the silhouette approach allowed us to identify an appropriate number of clusters given the structure of the data [98]. We identified the appropriate number of distinct clusters within each accession when the average silhouette value of the worst cluster became 0 and each successive increase in the number of clusters continued to show that the average silhouette value of the worst cluster was 0 or less than zero. We also ran GO enrichment analysis to identify association with gene ontologies for each cluster using JMP Genomics. To identify which clusters are associated with age, flowering status or environmental factors, we ran PVCA on all of the genes in each cluster separately for all Bay-0 and Sha clusters.
Stress annotation analysis
We created a functional annotation file based on previously published microarray data that had reported differential regulation of specific genes in response to several abiotic and biotic stresses. These include 118 high light response genes [60]; 4,972 cold, 1,562 drought, 3,990 heat, 5,842 osmotic, 511 oxidative, 5,148 salt, 1,219 toxins, 3,792 UVB and 1,771 wounding response genes [61]. Biotic stresses include 97 [62] and 3,687 [63] RNA virus response genes; 2,034 bacterial pathogen response genes, 151 fungal pathogen response genes, 2,397 herbivore response genes [64]. Combining these data sets, more than half of the genes on the ATH1 array were associated with at least one stress (13,153 of 22,800 genes).
Principal component analyses of vegetative stages
We performed a more detailed principal component analysis (PCA) in vegetative stages using JMP Genomics (SAS). Probes were normalized and centered to zero to determine the main trends of the variation across the samples. PCA was done using only genes that are significantly expressed (q<0.05) in both Bay-0 and Sha in vegetative stages (8,954 genes, representing 40% of the original dataset). Thus, trends in gene expression in each sample can be represented as PC loadings (i.e., PC1veg, PC2veg, PC3veg) in an allometric gene expression scale. To assess whether trends in gene expression were influenced by environmental factors or development, we ran a multivariate regression analysis using the PCveg scores as a response variable to maximum daily temperature (TMAX), daily precipitation (PPT), rosette leaf number (RLN), rosette diameter (RD), and plant age in the following model [PCveg = TMAX+PPT+RLN+RD+age+error]. Variance inflation factors were below 10, indicating low co-linearity between variables. We ran the regression analysis on the first five PC axis scores using an FDR of 0.05.
To identify genes contributing to each PC axis, we selected genes that showed extreme PC loadings for each of the PCveg from the upper and lower 2.5% of the quantile distributions in the PC loadings, which identified a subset of 448 genes in each PCveg. Because the PCs are orthogonal axes, the genes driving the variation in PC1veg do not intersect with the genes in PC2veg and PC3veg. The gene lists were analyzed for GO enrichment (p≤0.01) to identify significantly over-represented functional gene classes in Virtual Plant 1.0 [65].
Several genes in these gene lists caught our attention, including ARP6 and ROP10. To obtain lists of genes that are co-expressed with ARP6 and ROP10, Pavlidis Template Matching was done using the Multiple Array Viewer software, with absolute correlation coefficients as thresholds [72].
Supporting Information
Zdroje
1. GibsonG 2008 The environmental contribution to gene expression profiles. Nat Rev Genet 9 575 581
2. PigliucciMSchlichtingCDJonesCSSchwenkK 1996 Developmental reaction norms: the interactions among allometry, ontogeny and plasticity. Plant Species Biol 11 69 85
3. LeakeyADBAinsworthEABernardSMMarkelzCRJOrtD 2009 Gene expression profiling: opening the black box of plant ecosystem responses to global change. Global Change Biol 15 1201 1213
4. KammengaJEHermanMAOuborgNJJohnsonLBreitlingR 2007 Microarray challenges in ecology. Trends Ecol Evol 22 273 279
5. ChapmanMALeebens-MackJHBurkeJM 2008 Positive Selection and expression divergence following gene duplication in the sunflower CYCLOIDEA gene family. Mol Biol Evol 25 1260 1273
6. RichardsCLHanzawaYEhrenreichIPuruggananMD 2009 Perspectives on ecological and evolutionary systems biology. GutierrezRACoruzziGM Annual Plant Reviews: Plant Systems Biology Oxford, UK Blackwell Publishing
7. AndersonJTMitchell-OldsT 2011 Ecological genetics and genomics of plant defenses: Evidence and approaches. Funct Ecol 25 312 324
8. De BoerTEBirlutiuABochdanovitsZTimmermansMJTNDijkstraTMH 2011 Transcriptional plasticity of a soil arthropod across different ecological conditions. Mol Ecol 20 1144 1154
9. VandersteenW 2011 Detecting gene expression profiles associated with environmental stressors within an ecological context. Mol Ecol 20 1322 1323
10. KoornneefMAlonso-BlancoCVreugdenhilD 2004 Naturally ocurring genetic variation in Arabidopsis thaliana. Ann Rev Plant Biol 55 141 172
11. ShimizuKKPuruggananMD 2005 Evolutionary and ecological genomics of Arabidopsis. Plant Physiol 138 578 584
12. ShindoCBernasconiGHardtkeCS 2007 Natural genetic variation in Arabidopsis: Tools, traits and prospects for evolutionary ecology. Ann Bot 99 1043 1054
13. HoffmannMHGlaßASTomiukJSchmuthsHFritschRM 2003 Analysis of molecular data of Arabidopsis thaliana (L.) Heynh. (Brassicaceae) with Geographical Information Systems (GIS). Mol Ecol 12 1007 1019
14. SamisKEHeathKDStinchcombeJR 2008 Discordant longitudinal clines in flowering time and phytochrome C in Arabidopsis thaliana. Evolution 62 2971 2983
15. PigliucciM 1998 Developmental phenotypic plasticity: Where internal programming meets the external environment. Curr Op Plant Biol 1 87 91
16. GossEBergelsonJ 2007 Fitness consequences of infection of Arabidopsis thaliana with its natural bacterial pathogen Pseudomonas viridiflava. Oecologia 152 71 81
17. KliebensteinDPedersenDBarkerBMitchell-OldsT 2002 Comparative analysis of quantitative trait loci controlling glucosinolates, myrosinase and insect resistance in Arabidopsis thaliana. Genetics 161 325 332
18. BenningtonCStrattonD 1998 Field tests of density-and frequency-dependent selection in Erigeron annuus (Compositae). Am J Bot 85 540
19. MauricioRRausherMD 1997 Experimental manipulation of putative selective agents provides evidence for the role of natural enemies in the evolution of plant defense. Evolution 78 1301 1310
20. MauricioRRausherMDBurdickDS 1997 Variation in the defense strategies of plants: Are resistance and tolerance mutually exclusive? Ecology 78 1301 1311
21. MauricioR 1998 Cost of resistance to natural enemies in field populations of the annual Arabidopsis thaliana. Am Nat 151 20 28
22. DonohueKDornLGriffithCKimEAguileraA 2005 The evolutionary ecology of seed germination of Arabidopsis thaliana: Variable natural selection on germination timing. Evolution 59 758 770
23. TianDTrawMBChenJQKreitmanMBergelsonJ 2003 Fitness costs of R-gene-mediated resistance in Arabidopsis thaliana. Nature 423 74 77
24. MalmbergRLHeldSWaitsAMauricioR 2005 Epistasis for fitness-related quantitative traits in Arabidopsis thaliana grown in the field and in the greenhouse. Genetics 171 2013 2027
25. WeinigCUngererMCDornLAKaneNCToyonagaY 2002 Novel loci control variation in reproductive timing in Arabidopsis thaliana in natural environments. Genetics 162 1875 1884
26. CaicedoALStinchcombeJROlsenKMSchmittJPuruggananMD 2004 Epistatic interaction between Arabidopsis FRI and FLC flowering time genes generates a latitudinal cline in a life history trait. Proc Nat Acad Sci USA 101 15670 15675
27. HarmerSLHogeneschJBStraumeMChangH-SHanB 2000 Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290 2110 2113
28. BirnbaumKShashaDEWangJYJungJWLambertGM 2003 A gene expression map of the Arabidopsis root. Science 302 1956 1960
29. SchmidMDavisonTSHenzSRPapeUJDemarM 2005 A gene expression map of Arabidopsis thaliana development. Nat Genet 37 501 506
30. BradySMLongTABenfeyPN 2006 Unraveling the dynamic transcriptome. Plant Cell 18 2101 2111
31. GalbraithDWBirnbaumK 2006 Global studies of cell type-specific gene expression in plants. Ann Rev Plant Biol 57 451 475
32. MiyazakySFredricksenMHollisKCPoroykoVShepleyD 2004 Transcript expression profiles of Arabidopsis thaliana grown under controlled conditions and open-air elevated concentrations of CO2 and of O3. Field Crops Res 90 47 59
33. GuptaPDuplessisSWhiteHKarnoskyDFMartinF 2005 Gene expression patterns of trembling aspen trees following long-term exposure to interacting elevated CO2 and tropospheric O3. New Phytol 167 129 142
34. TaylorGStreetNRTrickerPJSjodinAGrahamL 2005 The transcriptome of Populus in elevated CO2. New Phytol 167 143 154
35. YangSHLoopstraCA 2005 Seasonal variation in gene expression for loblolly pines (Pinus taeda) from different geographical regions. Tree Physiol 25 1063 1073
36. AinsworthEARogersAVodkinLOWalterASchurrU 2006 The effects of elevated CO2 concentration on soybean gene expression. An analysis of growing and mature leaves. Plant Physiol 142 135 147
37. DruartNRodriuez-BueyMBarron-GraffordGSjodinABhaleraoR 2006 Molecular targets of elevated [CO2] in leaves and stems of Pupolus deltoides: implications for future tree growth and carbon sequestration. Func Plant Biol 33 121 131
38. SchmidtDDBaldwinIT 2006 Transcriptional responses of Solanum nigrum to methyl jasmonate and competition: A glasshouse and field study. Func Ecol 20 500 508
39. LiPAinsworthEALeakeyADBUlanovALozovayaV 2008 Arabidopsis transcript and metabolite profiles: Ecotype-specific responses to open-air elevated CO2. Plant Cell Env 31 1673 1687
40. LeakeyADBXuFGillespieKMMcGrathJMAinsworthEA 2009 Genomic basis for stimulated respiration by plants growing under elevated carbon dioxide. Proc Nat Acad Sci USA 106 3597 3602
41. TallisMJLinYRogersAZhangJStreetNR 2010 The transcriptome of Populus in elevated CO2 reveals increades anthocyanin biosynthesis during delayed autumnal senescence. New Phytol 186 415 428
42. TraversSETangZCarageaDGarrettKAHulbertSH 2010 Variation in gene expression of Andropogon gerardii in response to altered environmental conditions associated with climate change. J Ecol 98 374 383
43. AikawaSKobayashiMJSatakeAShimizuKKKudohH 2010 Robust control of the seasonal expression of the Arabidopsis FLC gene in a fluctuating environment. Proc Nat Acad Sci USA 107 11632 11637
44. LoudetOChaillouSCamilleriCBouchezDDaniel-VedeleF 2002 Bay-0×Shahdara recombinant inbred line population: a powerful tool for the genetic dissection of complex traits in Arabidopsis. Theor Appl Genet 104 1173 1184
45. NordborgMHuTTIshinoYJhaveriJToomajianC 2005 The pattern of polymorphism in Arabidopsis thaliana. PLoS Biol 3 e196 doi:10.1371/journal.pbio.0030196
46. CaoJSchneebergerKOssowskiSGuntherTBenderS 2011 Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nat Genet 43 956 963
47. GentlemanRCareyVBatesDBolstadBDettlingM 2004 Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol 5 R80
48. MandelMAGustafson-BrownCSavidgeBYanofskyMF 1992 Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature 360 273 277
49. JackTBrockmanLLMeyerowitzEM 1992 The homeotic gene APETALA3 of Arabidopsis thaliana encodes a MADS box and is expressed in petals and stamens. Cell 68 683 697
50. GotoKMeyerowitzEM 1994 Function and regulation of the Arabidopsis floral homeotic gene PISTILLATA. Genes Dev 8 1548 1560
51. YanofskyMFMaHBowmanJLDrewsGNFeldmannKA 1990 The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 346 35 39
52. RounsleySDDittaGSYanofskyMF 1995 Diverse roles for MADS Box genes in Arabidopsis development. Plant Cell 7 1259 1269
53. MaHYanofskyMFMeyerowitzEM 1991 AGL1-AGL6, an Arabidopsis gene family with similarity to floral homeotic and transcription factor genes. Genes Dev 5 484 495
54. LiJBushelPRChuT-MWolfingerRD 2009 Principal variance components analysis: Estimating batch effects in microarray gene expression data. SchererA Batch Effects and Noise in Microarray Experiments Chichester, UK John Wiley & Sons, Ltd 141 154
55. KliebensteinDJWestMALvan LeeuwenHKimKDoergeRW 2006 Genomic survey of gene expression diversity in Arabidopsis thaliana. Genetics 172 1179 1189
56. GanXStegleOBehrJSteffenJGDreweP 2011 Multiple reference genomes and transcriptomes for Arabidopsis thaliana. Nature 477 419 423
57. StoreyJDXiaoWLeekJTTompkinsRGDavisRW 2005 Significance analysis of time course microarray experiments. Proc Natl Acad Sci USA 102 12837 12842
58. LeekJTMonsenEDabneyARStoreyJD 2006 EDGE: Extraction and analysis of differential gene expression. Bioinformatics 22 507 508
59. KaufmanLRousseeuwPJ 1990 Finding groups in data: An introduction to cluster analysis Hoboken John Wiley & Sons
60. RosselJBWilsonIWPogsonBJ 2002 Global changes in gene expression in response to high light in Arabidopsis. Plant Physiol 130 1109 1120
61. KilianJWhiteheadDHorakJWankeDWeinlS 2007 The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. The Plant Journal 50 347 363
62. WhithamSAQuanSChangH-SCooperBEstesB 2003 Diverse RNA viruses elicit the expression of common sets of genes in susceptible Arabidopsis thaliana plants. Plant J 33 271 283
63. BabuMGriffithsJHuangT-SWangA 2008 Altered gene expression changes in Arabidopsis leaf tissues and protoplasts in response to Plum pox virus infection. BMC Genomics 9 325
64. De VosMVan OostenVRVan PoeckeRMPVan PeltJAPozoMJ 2005 Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant-Microbe Interact 18 923 937
65. KatariMSNowickiSDAceitunoFFNeroDKelferJ 2010 VirtualPlant: A software platform to support systems biology research. Plant Physiol 152 500 515
66. PauwelsLBarberoGFGeerinckJTillemanSGrunewaldW 2010 NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464 788 791
67. BerberichTHaradaMSugawaraKKodamaHIbaK 1998 Two maize genes encoding ω-3 fatty acid desaturase and their differential expression to temperature. Plant Mol Biol 36 297 306
68. KumarSVWiggePA 2010 H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell 140 136 147
69. KoborMSVenkatasubrahmanyamSMeneghiniMDGinJWJenningsJL 2004 A protein complex containing the conserved Swi2/Snf2-related ATPase Swr1p deposits histone variant H2A.Z into euchromatin. PLoS Biol 2 e131 doi:10.1371/journal.pbio.0020131
70. MizuguchiGShenXLandryJWuW-HSenS 2004 ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 303 343 348
71. SungDYVierlingEGuyCL 2001 Comprehensive expression profile analysis of the Arabidopsis Hsp70 gene family. Plant Physiol 126 789 800
72. SaeedAIBhagabatiNKBraistedJCLiangWSharovVHoweEA 2006 TM4 microarray software suite. Methods Enzymol 411 134 193
73. ZhengZ-LNafisiMTamALiHCrowellDN 2002 Plasma membrane-associated ROP10 small GTPase is a specific negative regulator of abscisic acid responses in Arabidopsis. Plant Cell 14 2787 2797
74. XinZZhaoYZhengZ-L 2005 Transcriptome analysis reveals specific modulation of abscisic acid signaling by ROP10 small GTPase in Arabidopsis. Plant Physiol 139 1350 1365
75. SeoMPeetersAJMKoiwaiHOritaniTMarion-PollA 2000 The Arabidopsis aldehyde oxidase 3 (AAO3) gene product catalyzes the final step in abscisic acid biosynthesis in leaves. Proc Natl Acad Sci USA 97 12908 12913
76. AbrahamERigoGSzekelyGNagyRKonczC 2003 Light-dependent induction of proline biosynthesis by abscisic acid and salt stress is inhibited by brassinosteroid in Arabidopsis. Plant Mol Biol 51 363 372
77. SzékelyGÁbrahámECséplőÁRigóGZsigmondL 2008 Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J 53 11 28
78. ShinozakiKYamaguchi-ShinozakiK 2007 Gene networks involved in drought stress response and tolerance. J Exp Bot 58 221 227
79. SwindellWHuebnerMWeberA 2007 Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genomics 8 125
80. SwindellWRHuebnerMWeberAP 2007 Plastic and adaptive gene expression patterns associated with temperature stress in Arabidopsis thaliana. Heredity 99 143 150
81. Yamaguchi-ShinozakiKShinozakiK 2006 Trascriptional regulatory networks in cellular responses and tolerance to dehydration and cold stress. Ann Rev Plant Biol 57 781 803
82. DenbyKGehringC 2005 Engineering drought and salinity tolerance in plants: lessons from genome-wide expression profiling in Arabidopsis. Trends Biotech 23 547 552
83. RabelloAGuimaraesCRangelPda SilvaFSeixasD 2008 Identification of drought-responsive genes in roots of upland rice (Oryza sativa L). BMC Genomics 9 485
84. van de MortelJEAlmar VillanuevaLSchatHKwekkeboomJCoughlanS 2006 Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiol 142 1127 1147
85. OgawaINakanishiHMoriSNishizawaN 2009 Time course analysis of gene regulation under cadmium stress in rice. Plant and Soil 325 97 108
86. PalencharPKouranovALejayLCoruzziG 2004 Genome-wide patterns of carbon and nitrogen regulation of gene expression validate the combined carbon and nitrogen (CN)-signaling hypothesis in plants. Genome Biol 5 R91
87. LianXWangSZhangJFengQZhangL 2006 Expression profiles of 10,422 genes at early stage of low nitrogen stress in rice assayed using a cDNA microarray. Plant Mol Biol 60 617 631
88. SchenkPMKazanKWilsonIAndersonJPRichmondT 2000 Coordinated plant defense response in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA 97 11655 11660
89. EhltingJChowriraSMattheusNAeschlimanDArimuraG-I 2008 Comparative transcriptome analysis of Arabidopsis thaliana infested by diamond back moth (Plutella xylostella) larvae reveals signatures of stress response, secondary metabolism, and signalling. BMC Genomics 9 154
90. Martin-TrilloMLázaroAPoethigRSGómez-MenaCPiñeiroMA 2006 EARLY IN SHORT DAYS 1 (ESD1) encodes ACTIN-RELATED PROTEIN 6 (AtARP6), a putative component of chromatin remodelling complexes that positively regulates FLC accumulation in Arabidopsis. Development 133 1241 1252
91. Fournier-LevelAKorteACooperMDNordborgMSchmittJ 2011 A map of local adaptation in Arabidopsis thaliana. Science 334 86 89
92. HancockAMBrachiBFaureNHortonMWJarymowyczLB 2011 Adaptation to climate across the Arabidopsis thaliana genome. Science 334 83 86
93. Korves ToniaMSchmid KarlJCaicedo AnaLMaysCStinchcombe JohnR 2007 Fitness effects associated with the major flowering time gene FRIGIDA in Arabidopsis thaliana in the field. Am Nat 169 E141 E157
94. WeinigCDornLAKaneNCGermanZMHalldorsdottirSS 2003 Heterogeneous selection at specific loci in natural environments in Arabidopsis thaliana. Genetics 165 321 329
95. StinchcombeJRWeinigCUngererMOlsenKMMaysC 2004 A latitudinal cline in flowering time in Arabidopsis thaliana modulated by the flowering time gene FRIGIDA. Proc Natl Acad Sci USA 101 4712 4717
96. AFFYMETRIXAffymetrix, editor 2002 Affymetrix Microarray Suite User Guide; Santa Clara, CA
97. BorevitzJOHazenSPMichaelTPMorrisGPBaxterIR 2007 Genome-wide patterns of single-feature polymorphism in Arabidopsis thaliana. Proc Nat Acad Sci USA 104 12057 12062
98. RousseeuwPJ 1987 Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J Compt Appl Math 20 53 65
Štítky
Genetika Reprodukční medicína
Článek A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target GenesČlánek Population Structure of Hispanics in the United States: The Multi-Ethnic Study of AtherosclerosisČlánek Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms inČlánek Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome AnalysisČlánek Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and MechanismČlánek Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 4- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
- Délka menstruačního cyklu jako marker ženské plodnosti
-
Všechny články tohoto čísla
- Runs of Homozygosity Implicate Autozygosity as a Schizophrenia Risk Factor
- Modifier Genes and the Plasticity of Genetic Networks in Mice
- The DSIF Subunits Spt4 and Spt5 Have Distinct Roles at Various Phases of Immunoglobulin Class Switch Recombination
- A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target Genes
- Population Structure of Hispanics in the United States: The Multi-Ethnic Study of Atherosclerosis
- Deep Sequencing of Plant and Animal DNA Contained within Traditional Chinese Medicines Reveals Legality Issues and Health Safety Concerns
- Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms in
- Insulin Signaling Mediates Sexual Attractiveness in
- Progressive Telomere Dysfunction Causes Cytokinesis Failure and Leads to the Accumulation of Polyploid Cells
- Long-Range Chromosome Organization in : A Site-Specific System Isolates the Ter Macrodomain
- Regulation of Budding Yeast Mating-Type Switching Donor Preference by the FHA Domain of Fkh1
- Polyglutamine Toxicity Is Controlled by Prion Composition and Gene Dosage in Yeast
- Patterns of Regulatory Variation in Diverse Human Populations
- Sequence-Specific Targeting of Dosage Compensation in Favors an Active Chromatin Context
- Whole-Exome Sequencing and Homozygosity Analysis Implicate Depolarization-Regulated Neuronal Genes in Autism
- Replication Fork Reversal after Replication–Transcription Collision
- Common Variants at 9p21 and 8q22 Are Associated with Increased Susceptibility to Optic Nerve Degeneration in Glaucoma
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Epigenome-Wide Scans Identify Differentially Methylated Regions for Age and Age-Related Phenotypes in a Healthy Ageing Population
- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Budding Yeast Dma Proteins Control Septin Dynamics and the Spindle Position Checkpoint by Promoting the Recruitment of the Elm1 Kinase to the Bud Neck
- , a Homolog of a Deaf-Blindness Gene, Regulates Circadian Output and Slowpoke Channels
- Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome Analysis
- Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and Mechanism
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
- SPE-44 Implements Sperm Cell Fate
- A Shared Role for RBF1 and dCAP-D3 in the Regulation of Transcription with Consequences for Innate Immunity
- A Companion Cell–Dominant and Developmentally Regulated H3K4 Demethylase Controls Flowering Time in via the Repression of Expression
- The HEN1 Ortholog, HENN-1, Methylates and Stabilizes Select Subclasses of Germline Small RNAs
- Improved Statistics for Genome-Wide Interaction Analysis
- The Probability of a Gene Tree Topology within a Phylogenetic Network with Applications to Hybridization Detection
- Context-Dependent Dual Role of SKI8 Homologs in mRNA Synthesis and Turnover
- Mu Insertions Are Repaired by the Double-Strand Break Repair Pathway of
- Competition between Replicative and Translesion Polymerases during Homologous Recombination Repair in Drosophila
- An Unbiased Assessment of the Role of Imprinted Genes in an Intergenerational Model of Developmental Programming
- Type 2 Diabetes Risk Alleles Demonstrate Extreme Directional Differentiation among Human Populations, Compared to Other Diseases
- Mutations in and Cause “Splashed White” and Other White Spotting Phenotypes in Horses
- Fine-Scale Mapping of Natural Variation in Fly Fecundity Identifies Neuronal Domain of Expression and Function of an Aquaporin
- Dynamics of Brassinosteroid Response Modulated by Negative Regulator LIC in Rice
- Genetic Inhibition of Solute-Linked Carrier 39 Family Transporter 1 Ameliorates Aβ Pathology in a Model of Alzheimer's Disease
- The Functions of Mediator in Support a Role in Shaping Species-Specific Gene Expression
- Patterns of Ancestry, Signatures of Natural Selection, and Genetic Association with Stature in Western African Pygmies
- Dissection of Pol II Trigger Loop Function and Pol II Activity–Dependent Control of Start Site Selection
- PIWI Associated siRNAs and piRNAs Specifically Require the HEN1 Ortholog
- Genome-Wide Patterns of Gene Expression in Nature
- Hypoxia Disruption of Vertebrate CNS Pathfinding through EphrinB2 Is Rescued by Magnesium
- A New Role for Translation Initiation Factor 2 in Maintaining Genome Integrity
- Sex Reversal in C57BL/6J XY Mice Caused by Increased Expression of Ovarian Genes and Insufficient Activation of the Testis Determining Pathway
- The Rac GTP Exchange Factor TIAM-1 Acts with CDC-42 and the Guidance Receptor UNC-40/DCC in Neuronal Protrusion and Axon Guidance
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



