-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Principal Genetic Determinants for Nasopharyngeal Carcinoma in China Involve the Class I Antigen Recognition Groove
Nasopharyngeal carcinoma (NPC) is an epithelial malignancy facilitated by Epstein-Barr Virus infection. Here we resolve the major genetic influences for NPC incidence using a genome-wide association study (GWAS), independent cohort replication, and high-resolution molecular HLA class I gene typing including 4,055 study participants from the Guangxi Zhuang Autonomous Region and Guangdong province of southern China. We detect and replicate strong association signals involving SNPs, HLA alleles, and amino acid (aa) variants across the major histocompatibility complex-HLA-A, HLA –B, and HLA -C class I genes (PHLA-A-aa-site-62 = 7.4×10−29; P HLA-B-aa-site-116 = 6.5×10−19; P HLA-C-aa-site-156 = 6.8×10−8 respectively). Over 250 NPC-HLA associated variants within HLA were analyzed in concert to resolve separate and largely independent HLA-A, -B, and -C gene influences. Multivariate logistical regression analysis collapsed significant associations in adjacent genes spanning 500 kb (OR2H1, GABBR1, HLA-F, and HCG9) as proxies for peptide binding motifs carried by HLA - A*11 : 01. A similar analysis resolved an independent association signal driven by HLA-B*13 : 01, B*38 : 02, and B*55 : 02 alleles together. NPC resistance alleles carrying the strongly associated amino acid variants implicate specific class I peptide recognition motifs in HLA-A and -B peptide binding groove as conferring strong genetic influence on the development of NPC in China.
Published in the journal: . PLoS Genet 8(11): e32767. doi:10.1371/journal.pgen.1003103
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003103Summary
Nasopharyngeal carcinoma (NPC) is an epithelial malignancy facilitated by Epstein-Barr Virus infection. Here we resolve the major genetic influences for NPC incidence using a genome-wide association study (GWAS), independent cohort replication, and high-resolution molecular HLA class I gene typing including 4,055 study participants from the Guangxi Zhuang Autonomous Region and Guangdong province of southern China. We detect and replicate strong association signals involving SNPs, HLA alleles, and amino acid (aa) variants across the major histocompatibility complex-HLA-A, HLA –B, and HLA -C class I genes (PHLA-A-aa-site-62 = 7.4×10−29; P HLA-B-aa-site-116 = 6.5×10−19; P HLA-C-aa-site-156 = 6.8×10−8 respectively). Over 250 NPC-HLA associated variants within HLA were analyzed in concert to resolve separate and largely independent HLA-A, -B, and -C gene influences. Multivariate logistical regression analysis collapsed significant associations in adjacent genes spanning 500 kb (OR2H1, GABBR1, HLA-F, and HCG9) as proxies for peptide binding motifs carried by HLA - A*11 : 01. A similar analysis resolved an independent association signal driven by HLA-B*13 : 01, B*38 : 02, and B*55 : 02 alleles together. NPC resistance alleles carrying the strongly associated amino acid variants implicate specific class I peptide recognition motifs in HLA-A and -B peptide binding groove as conferring strong genetic influence on the development of NPC in China.
Introduction
Nasopharyngeal carcinoma (NPC) is an epithelial malignancy with highly variable incidence rates around the world. An estimated 84,400 incident cases of NPC and 51,600 deaths occurred in 2008 with the highest incidence in South-Eastern Asia, relative to the Americas, Europe, Africa, and Central and Eastern Asia [1]. An early indicator of NPC development is the occurrence of immunoglobulin (Ig)A antibodies to Epstein-Barr virus (EBV) capsid antigens (EBV-IgA/VCA). [2], [3] NPC incidence for individuals expressing IgA/VCA antibodies were 31.7 times higher than the incidence in the age matched general population. [4] Linkage and family studies indicated that genetic predisposition also plays an important role in NPC onset and susceptibility. [5], [6] Among host genetic markers implicated as associated with NPC, the highly variable class I human leukocyte antigen (HLA) genes on chromosome 6 (6p21.3) have shown a strong and consistent association with NPC risk. [7], [8], [9], [10], [11], [12], [13]_ENREF_7 HLA class I association studies across mainland China [11], [13], Taiwan [10], [12], and Singapore [8] have consistently demonstrated that HLA-A*11 and B*13 are associated with NPC resistance, while A*02 (A*02 : 07), A*33, B*46 and B*58 are associated with increased NPC susceptibility (Table S1). Genome-wide association studies (GWAS) have been applied to numerous complex diseases to implicate common risk variants through well powered genetic studies [14], [15], [16]. Recent GWAS also affirmed a strong HLA influence on NPC incidence and implicated four additional non-HLA genes, however extensive linkage disequilibrium across the gene dense HLA region have confounded identification of the causal association gene(s). [17], [18], [19]
To refine and extend these reports, we explore here the operative factors of genetic association for this disease in a comprehensive four step study utilizing: 1) A GWAS utilizing 591,458 SNPs resolved by Affymetrix 6.0 genotyping platform to identify gene regions associated with NPC; 2) SNP genotyping to replicate the top signals in a second independent NPC cohort; 3) High resolution HLA molecular genotyping to identify specific alleles and haplotypes associated with NPC; and 4) Amino acid variant analysis to fine map the major genetic determinants associated with this disease. The analyses demonstrates that two independent association signals, specifying peptide binding grove motifs in HLA-A and in HLA –B drive the signals tracked by scores of SNP and amino acid variants that are association proxies for the HLA class I NPC association.
Results
We performed a GWAS with 1104 southern Chinese individuals from NPC-phase II study cohorts [20], [21] (See Materials and Methods) using the Affymetrix Genome-Wide SNP 6.0 genotyping platform. After SNP - and sample-base quality control (Table S2), 591,458 SNPs genotyped in 1043 study participants (567 cases and 476 controls; Table S3, line I). Principal components analysis confirmed that all samples came from individuals of Southern Chinese ancestry (Figures S1, S2, S3, S4, S5). A quantile-quantile plot of the observed p-values showed a clear deviation from the null distribution which suggested that the most significant lower p-values are smaller than those expected by chance and likely reflect genetic association (Figure S6). The GWAS allele associations suggested a strong influence in the HLA-A region of chromosome 6 and weaker signals on chromosome 16 and 17 (Figure 1A).
Fig. 1. NPC associations of GWAS and Taqman validation. 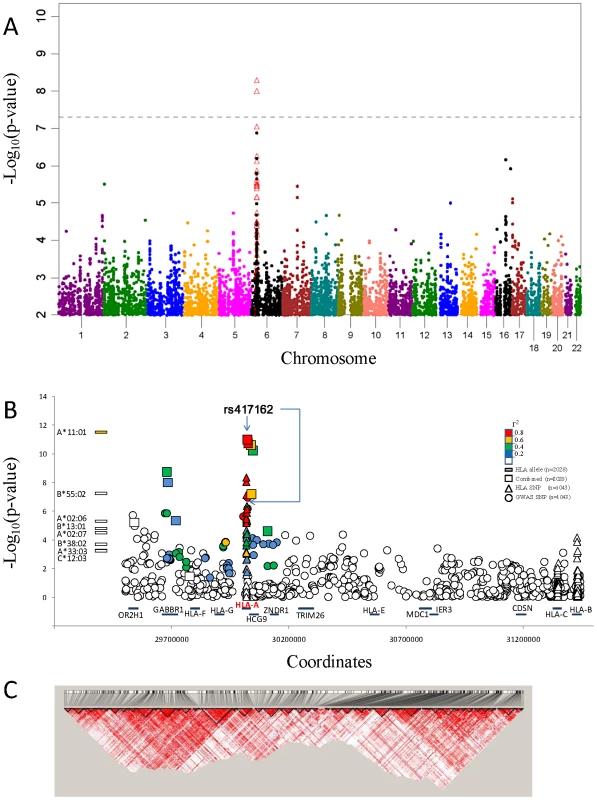
A.) Manhattan plot of GWAS P value association results of 591,458 SNP allele genotypes versus chromosome coordinate position (N = 1043 study participants; Row I in Table S3). Association p-values (-log10 transformed) are calculated by logistic regression in additive logistic model and plotted by genomic position. Association p-values for HLA SNP that were assessed by HLA sequence base typing for the same 1043 individuals are indicated by open red triangles (see text). B.) NPC association signal for significant HLA alleles (left) and included SNP variants on Chromosome 6.The –log10 p values, calculated with the logistic regression model, in GWAS and combined association tests are shown, SNPs are ordered according to the location on chromosome 6 HLA-A region. Color coded indicate the LD value (r2) of each variant with the most significant SNP rs417162. C.) Disequilibrium coefficient values for SNPs genotyped in the HLA region for NPC GWAS (N = 1043), generated with the use of Haploview software. Twenty-four SNPs (Table S4) with p-values less than 5×10−5 in 16 association tests (Table S5) and sixteen previous GWAS reported NPC associated SNPs (Table S6) and were selected for replication. Replication SNP genotyping was conducted in an independent Chinese cohort that included 356 NPC cases and 629 controls (Table S3, line II). Six of 40 SNPs that showed genome-wide significant NPC association and replication were within 500 kb of each other in the MHC region of chromosome 6 (Table 1, Table S7). The most significant SNP rs417162 (Pcombined = 1.1×10−11, OR = 0.61) is located within the HLA-A locus, while four additional replicated SNPs were within adjacent genes, GABBR1 and HCG9.
Tab. 1. GWAS and validation of SNPs association data in two independent NPC cohorts. 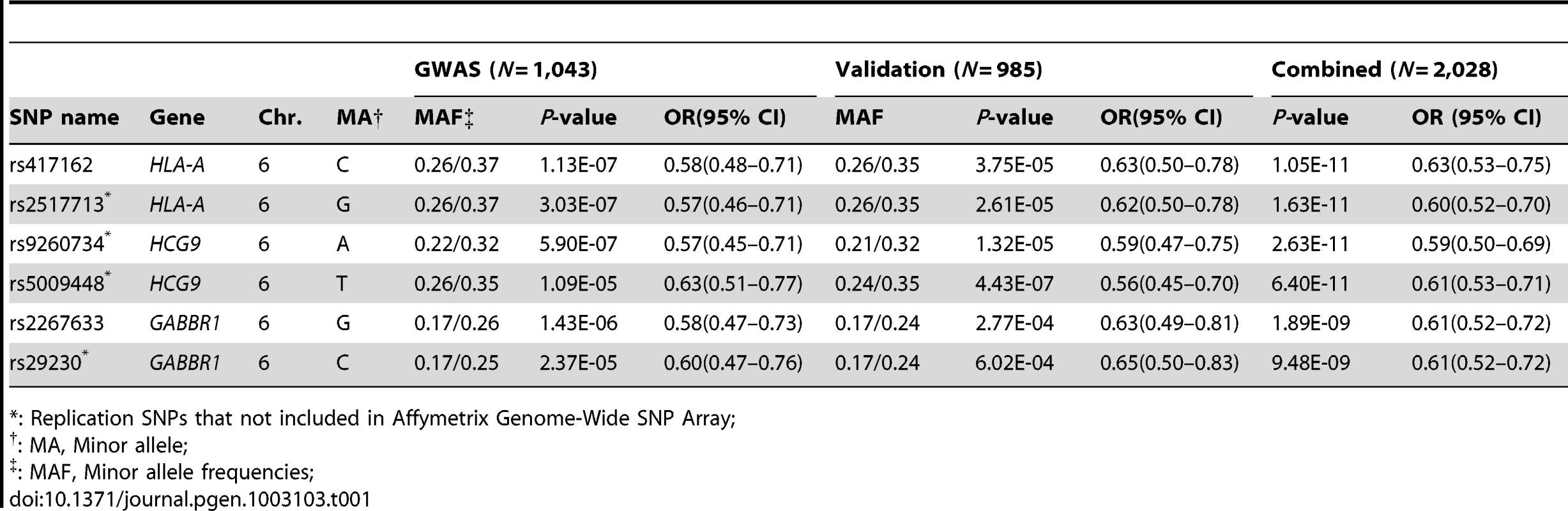
: Replication SNPs that not included in Affymetrix Genome-Wide SNP Array; A fine-grain view of the pattern of GWAS SNPs around the HLA-A locus is illustrated in Figure 1B. An extensive cluster of associated HLA-A region SNPs that approach or exceed genome-wide association threshold p-values (p<10−8) is apparent within 500 kb including associations in the adjacent HCG9 and GABBR1 genes (Figure 1C). The strong linkage disequilibrium (LD) across the HLA region is well known, which raises the question whether these HLA region associations represent single, multiple independent, or LD-proxy driven associations.
To characterize the HLA association with NPC in finer detail, high-resolution molecular HLA genotyping was performed on 4055 study participants; 1043 subjects in the discovery cohort, 985 subjects in the replication cohort, and 2027 subjects that comprise the remainder of cohort (Table S3). [20], [21] NPC cases, controls with EBV-IgA/VCA positive, and controls with EBV-IgA/VCA negative were examined for association of HLA alleles and HLA haplotypes with NPC risk and EBV IgA/VCA antibody status. Three HLA alleles, A*11 : 01, B*38 : 02 and B*55 : 02 showed the most significant association with NPC (P = 1.7×10−19, P = 7.0×10−11, and P = 1.6×10−10 respectively; Table 2). In addition to the strong HLA-A and -B associations, there was a moderate association of HLA-C*12 : 02 allele as well (P = 4.3×10−5; Table 2). NPC associated HLA-A-B-C haplotypes which included HLA allele combinations from Table 2 were also apparent (Table S8).
Tab. 2. Gene frequencies (%) of the HLA-A, -B, and -C alleles detected and association analysis (N = 4,055).# 
: Amino acid motifs from Lund et al., 2004. Functional variation of different MHC molecules to bind peptides and activate effector cells in the immune system underlies their association with disease. [22], [23]_ENREF_18 To identify the specific site driving the NPC HLA associations, HLA gene sequence was translated to identify amino acid variants using a web-based software of the Immunology Database and Analysis Portal (ImmPort) system [24]. Genetic association of 284 detected HLA amino acid variant within the three HLA class I genes implicated the most significant NPC association of glutamine (Gln, Q) at amino acid position 62 of HLA-A gene (P = 1.2×10−24, OR = 0.59; Figure 2A; Tables S9 and S10) which marks HLA-A*11, however there are 25 additional amino acid sites I in HLA-A that also show exceed genome wide significance (P<_ENREF_1810−8; Figure 2A). The HLA-B signal centered on amino acid Leucine (Leu, L) at amino acid position −16 and 116 (P = 1.7×10−13 and 2.4×10−13, OR = 0.65 and 0.63; Table S10), which marks B*13 : 01 and B*55 : 02. A far less significant association was observed for the amino acid residue Tryptophan (Trp, W) at amino acid position 156 for HLA-C (P = 1.4×10−9, OR = 0.47;). Amino acid residues that correspond to the antigenic peptide binding groove residues showed the strongest association (See color code in Figure 2A–2C) suggesting that the peptide binding groove and function are major genetic factors for NPC risk.
Fig. 2. NPC associations of HLA alleles and amino acid variants. 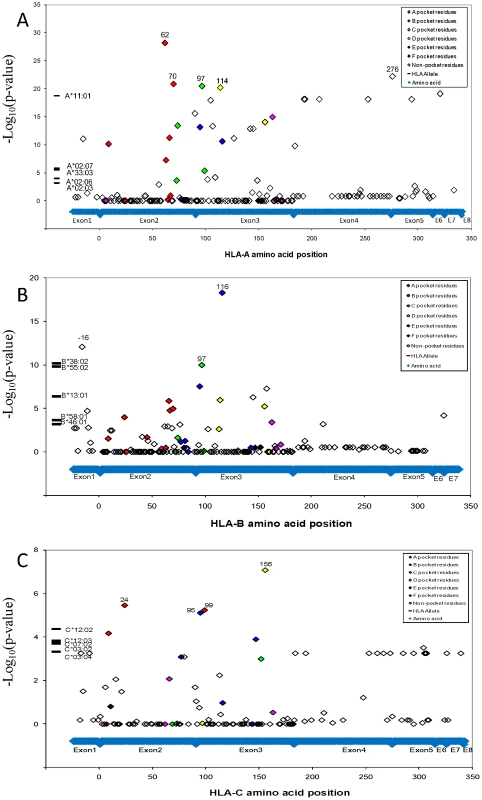
A.) NPC associations of alleles and amino acid variants at HLA-A locus; B.) NPC associations of alleles and amino acid variants at HLA-B locus; C.) NPC associations of alleles and amino acid variants at HLA-C locus. Genetic association of HLA alleles and amino acid sites were calculated (N = 4055 study participants; Line V in Table S3). For amino acid positions with more than two alleles, p-value for the omnibus test that tests all amino acid alleles simultaneously (with >1 degrees of freedom) for association to control. Given the plethora and complexity of HLA genetic associations plus the extensive LD within HLA, we attempted to resolve which HLA region SNPs and aa-variants represent proxy variants for one or more functional sites (i.e. they were tracking by LD) and which represent independent (non-LD) association signals using a multivariate logistic regression analyses [25]. Strongly associated aa-variants (Figure 2 and in Tables S9 and S10; e.g HLA-A-62Gln) were analyzed in a multivariate logistical regression analysis adjusting statistically for non-random influence of each of the adjacent aa-variants (Figure 3; also in Table S11). A dramatic reduction of association p-value significance for the strongest HLA-A aa variant, HLA-A-62Gln, is observed when this model is adjusted for adjacent aa-variants within and about the HLA-A gene but are not diminished by adjusting for variants in HLA-B or HLA-C. Thus, we conclude that there is a single association signal in HLA-A tracked by several dozen proxy aa/SNP variants within the HLA-A region. When HLA-A*11 : 01 is the index allele, the extreme NPC association signal is diminished to 0.1–0.01 by HLA-A aa variants as well as each SNP in the genes adjacent to HLA-A locus (See Figure 3B and HLA-A*11 : 01 column in Table S11). This multivariate dependence plus the knowledge that HLA-A*11 : 01 carries the five strongly significant associated aa variants (62Gln, 276Leu, 114Arg, 70Gln, and 97Ile) in the peptide binding groove and reaches the highest significance in allele level would support the conclusion that the causal association is driven by the HLA-A*11 : 01 allele (Table 2, Table S10, Figure S7).
Fig. 3. Proxy variant analysis for the strongest aa-variants or HLA allele in HLA-A, in HLA-B, and in HLA-C. 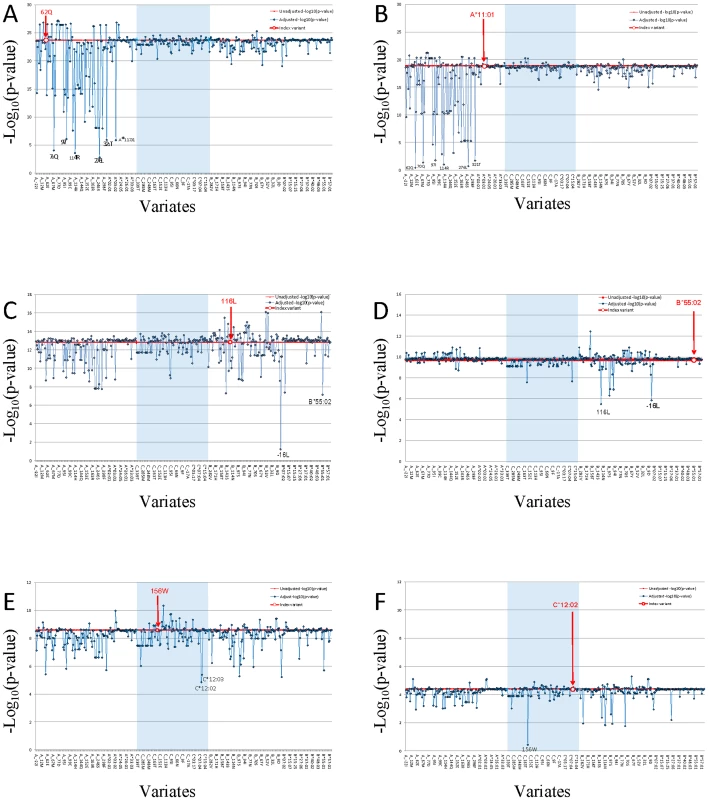
Genetic association of each HLA amino acid variant was calculated. Study participants in both phase I and phase II (N = 4055 study participants; Line V in Table S3). Multivariate conditional logistic regression analysis was performed to compute amino acid variants (A, C, and E) or HLA alleles (B, D, and F) association p-value. The HLA typing data set (N = 4055 study participants; Line V in Table S3) were used PLINK to examine the residual effect of index amino acid variant or HLA allele while using other amino acid variant or HLA allele as a covariate, and we adjusted the results for age and gender. The index amino acid variant or HLA allele was marked with bold red font. The red line indicated unadjusted –log10 p of index. Three HLA class I genes regions were separated by light blue block of HLA-C gene between HLA-A and HLA-B. The X-axis is amino acid or HLA allele covariate, ranking by their coordinate. HLA alleles were group together ranking by allele names in each HLA class I gene locus. The Y-axis is the –log10p of index variant adjust by covariate. Independent variants should not change the adjusted p-values from the strong unadjusted values of the index variant, while LD-proxies would reduce their p-values appreciably depending upon the strength of LD. A multivariate logistical regression analysis for HLA-B variants indicates that HLA-B associations are independent from the HLA-A signals and driven by two amino acid sites in strong LD with each other (HLA-B: -16Leu and 116Leu; P = 1.7×10−13 and 2.4×10−13) (Figure 3C and Table S9). The most significant HLA-B signal is located at amino acid position 116 (Figure 2B). The amino acid variant HLA-B-116Leu is present in the two strongly associated protective HLA-B alleles B*13 : 01 and B*55 : 02, but the encoded amino acid in the associated suscetible allele HLA-B*38 : 02 is Phenylalanine (Table S9). It is also relevant that the same location of HLA-B amino acid position 116 has also been definitively implicated as the single aa site that drives high susceptibility of the HLA-B*35 association with very rapid AIDS progression in HIV-1 infected European Americans. [26], [27] It seems that this variant influences HLA peptide repertoire recognition and/or presentation for both HIV and EBV infections. The amino acid substitution in the heavy chain at position 116 could abolish the ability of P9 picket of HLA-B*35 : 01 to bind tyrosine but preferentially accommodate smaller hydrophobic residues such as methionine, valine, or leucine at the carboxy-terminal anchor had been shown by peptide-binding assays. [28]
The HLA-C signal is ten logs weaker than HLA-A or HLA-B and is diminished slightly by adjusting for HLA-A or HLA-B variants (Figure 3E, Table S11). Further, the most significant HLA-C alleles (HLA-C*03 : 02 and -C*12 : 02) track HLA-A and -B alleles in the haplotype analyses (Table S8), suggesting the HLA-C association are likely proxies of the stronger HLA-B and -A associations. We interpret these cumulative data as suggestive that there are two robust independent HLA association signals with NPC development: HLA-A including at least five amino acid position in 62Gln, 70Gln, 97Ile, 114Arg and 276Leu carried by HLA-A*11 : 01 and HLA-B including the -16Leu and 116Leu-bearing alleles.
Our GWAS analysis also provided an opportunity to inspect regions of the genome outside HLA that had been implicated in previous NPC studies. The results (Table S6 and Table 1) offer strong supportive confirmation of SNPs in the HLA-A gene region (including the adjacent HCG9, and GABBR1 genes) as suggested by previous GWAS. [18], [19] However, our SNP replication (Table S6) and multivariate logistical regression analysis (Figure 3; Table S11) indicate that all these associations are by and large proxies for the primary functional aa variants association in our cohort. We also replicated the TNFRSF19, MDS1-EVI1, CDNK2A/2B gene associations [19] in our cohort (p = 1.5×10−5; 5.0×10−5 and 5.6×10−3 respectively) although these genes did not achieved genome wide significance (Table S6). The ITGA9 association reported by Ng et al14 was not replicated in our cohort (Table S6).
Discussion
We present and interpret a 1 M SNP GWAS, in subjects from Guangxi Zhuang Autonomous Region and Guangdong province of southern China, where perhaps the highest recorded NPC incidence has been found. [3], [4], [20], [21] Multiple genome wide significant association signals were evident with the HLA gene region and in a few other chromosomal regions (Figure 1A). Because the HLA region is complex and displays extensive LD, we sought to resolve the causal association signals with several different approaches. These included replication in an independent cohort from the same area, sequence based gene typing of the HLA-A, -B and -C genes, and analysis of sequence based nucleotide alleles as well as 284 amino acid site variants across the HLA genes (Figure 2). We compared association signals of SNPs, aa variants, HLA - alleles defined by molecular typing and associated HLA –A, -B and C haplotypes. To resolve the operative variants from proxies that track signals by LD, we enlisted a multivariate logistical regression of alleles and site variants with the strongest signals (Figure 3, Table S11). Finally we revisited and attempted replication in our cohort reports from other NPC gene associations including GWAS recently published, [17], [18], [19] (TNFRSF19 -CHR 13, MDS1-EVI1-CHR 3, and CDNK2A/2B - CHR 9; Table S6) affirming gene influence that are important in this disease.
In the present study, two independent powerful association signals within the HLA region were resolved for NPC, amidst a background of scores of adjacent associated LD-proxy variants. The first influence involved the HLA-A*11 : 01 allele sequence and function, specifically in the peptide binding groove, which recognizes invading antigens. This conclusion derives from several lines of evidence: 1.) HLA-A*11 : 01 is a common allele in the populations (F = 0.25) and is the only “protective” allele with genome wide significant HLA-A signal (OR = 0.59; P = 1.7×10−19; Table 1) the strongest of all HLA alleles. 2.) HLA-A*11 : 01 is included in the significantly associated protective HLA haplotypes (Table S8); 3.) 100% of associated SNPs and aa variants about HLA-A, including those in adjacent genes, namely the HCG9, and GABBR1 loci, are proxies HLA-A*11 : 01(Figure 3; Table S11); 4.) HLA-A*11 : 01 carries five strongly significant associated aa variants (62Gln, 276Leu, 114Arg, 70Gln, and 97Ile) in its peptide binding groove (Table S10). Taken together, the HLA-A association is centered on HLA-A*11 : 01 allele function and tracked by internal and closely linked proxy aa and SNP variants.
It may also be relevant that the sequence of HLA-A*11 : 01 allele (F = 0.25 in this population) differs by only one amino acid residue (Lys19Glu) from that of the HLA-A*11 : 02 allele (F = 0.04), yet HLA-A *11 : 02 shows no apparent association with NPC onset. Both HLA-A*11 : 01 and HLA-A*11 : 02 alleles share a unique peptide binding motif signature of “.[YT]……[K-]” (Table 2) and an identical sequence within the defined residues of the antigen recognition site. [23] Since the only Lys19Glu residue difference between the two HLA-A*11 alleles is outside the peptide binding region, the possibility of an alternative mechanisms for NPC pathogenesis, e.g. HLA-A/KIR innate immunity involvement [29] or dendritic cell interaction, [30] should be considered and explored in future studies.
We further demonstrate an independent HLA-B signal derived from three representive alleles, two protective alleles (B*13 : 01 and B*55 : 02) and a suscetible allele B*38 : 02. Both HLA-A and -B associations involve functional variants in the antigenic recognition site. The strongest HLA-B aa site implicated is identical to the single aa site that mediates HLA-B*35 rapid AIDS progression reported previously. [26], [27], [30] All the NPC associations were genome wide significant in one or more analyses, replicated internally in independent Guangxi cohorts and externally in other genetic association studies in Asia. Our study demonstrates a powerful genetic influence on NPC onset in Chinese people, implicates explicit HLA alleles, peptide recognition motifs, and aa variants that confer strong genetic influence on the development of NPC in China. HLA disease associations are likely to involve multiple mechanisms. A recent study in HIV disease showed that allelic diversity of HLA-C can cause variation in the level of surface expression of the HLA-C molecule, which in turn affects viral load control and disease progression [31], perhaps through both HLA-restricted CTL responses and HLA/KIR-mediated NK cell activities. The functional basis for HLA associations with NPC should be explored fully, now that the genetic basis of this disease is well-characterized, in hopes of explaining the complex HLA association with NPC in the Chinese population.
Materials and Methods
Ethics statement
This study were approved by institutional ethics review committees at the relevant organizations, and conducted with the IRB approval (NIH IRB -02-C-N056). Informed consent was obtained from all study participants.
Study cohorts
A total of 4055 study subjects (1405 NPC cases and 2650 controls, Table S3) were recruited in two independent collection phases: phase I -April 2000 to June 2001 and phase II-November 2004 to October 2005, from the Guangxi autonomous region and Guangdong province of southern China. [20] All study subjects were of Han ethnic origin and reside in the catchment area of the Xijiang River. IgA antibodies to EBV capsid antigen (EBV-IgA/VCA) were confirmed by serologic testing for all the subjects at the time of study enrollment. In phase I, the case group included 356 unrelated patients with biopsy-confirmed NPC. The mean age was 50.1 years (range 19–80), 95.5% of them were EBV-IgA/VCA antibody positive. Controls included case spouses or geographically matched residents who were NPC free at the time of study enrollment. An additional 422 adult children of the study subjects were enrolled for haplotype inference and for quality control assessment, but they were excluded in association analyses. In phase II, the case group included 1049 unrelated patients with biopsy-confirmed NPC. The mean age was 46.3 years (range 10–77), 96.3% of them were EBV-IgA/VCA antibody positive. Two distinct NPC-free control groups were included; one group was positive (N = 1001) and the other negative (N = 1020) for the EBV-IgA/VCA antibody. The mean ages were 46.1 and 46.6, for the antibody positive and negative controls groups. All study subjects were self-reported Guangxi or Guangdong provincial ancestry for either maternal or paternal ancestry for at least three generations.
Genome-wide SNPs genotyping
A total of 598 NPC cases and 506 controls were randomly selected from phase II enrollment cohort for GWAS analysis. DNA was extracted from whole blood by traditional phenol/chloroform method with phase Lock Gel tube (Qiagen, MaXtract High Density, catalog # 129065). The genome-wide genotyping experiments were conducted by using the Affymetrix Genome-Wide SNP Array 6.0 genotyping platform. 325 nanograms of DNA per sample were prepared for both Sty1 and Nsp1 restriction enzyme digestion for this assay, genotyping in according to the manufacturer's instructions.
Quality control
Patient DNA
Genotyping analysis of GWAS samples was performed in Genotyping Console 3.0.2 for first-pass quality control. The contrast quality control (CQC) metrics were computed by the Affymetrix software. We attempted to ascertain genotypes for 1,104 NPC study subjects. 11 samples failed genotyping or were removed because of failing CQC (<0.4) or call rate (<90%). An additional 17 samples failed to meet further QC filters which included heterozygosity < = 25% and at least one enzyme specific CQC value (Nsp1 or Sty1) >1. 24 samples were removed because the genotypes determined in the GWAS were discordant with genotypes previously determined by the Laboratory of Genomic Diversity for candidate gene studies. The gender of the samples was determined from the heterozygosity of X chromosome SNPs (Affymetrix and PLINK software) and by the ratio of the mean intensity of the copy number probes on the Y chromosome to the mean intensity of a subset of copy number probes on the X chromosome (Affymetrix software). Four samples were removed because the gender determined from the genotypes was discordant with the gender provided by the cohort. Identity by descent (IBD) statistics computed using PLINK software were used to detect cryptic familial relationships. Four first degree relationships were observed (3 full sib pairs and 1 parent-offspring pair). For each of these pairs, the sample with the lowest call rate was removed. A fifth sample was removed because the IBD statistics were consistent with that individual having a first cousin relationship with five other subjects. After sample filtering, a number of 1043 subjects were remained for further analyses (Table S2).
SNPs
Genotypes were ascertained for the 934,968 SNPs on the Genome-wide SNP 6.0 platform using the command line option of Affymetrix software. NetAffx version 30 was used for SNP annotation. This data set uses map positions based on the NCBI Build 36.1/UCSC hg18 human genome assembly. Unsupported SNPs, QC SNPs, non-autosomal SNPs, and remaining redundant SNPs were identified from the annotation data set and removed. Genotypes from 8 CEPH and 10 NPC mother/father/offspring trios were checked for errors in Mendelian inheritance using PLINK software. SNPs having 2 or more errors in either group of samples were rejected. Per-SNP call rate, Hardy-Weinberg test statistics, and minor allele frequencies were computed for 1,043 NPC study samples (see Table S2A) using PLINK software. SNPs not meeting the criteria shown in the table were removed (Table S2B).
Replication genotyping
Validation and replication genotyping of significant SNPs from our GWAS and from other studies was performed using the ABI Taqman genotyping assays by design in accordance with the manufacturer's instructions. The sequence detection software (SDS2.2, Applied Biosystems, Foster City, CA, USA) was used for allelic discrimination and confirmed the good quality of genotyping.
HLA typing
High resolution HLA molecular typing was performed for all 1,405 unrelated NPC cases and 2,650 unrelated controls from both enrollment cohorts. HLA class I alleles were characterized using a PCR-SSOP (sequence-specific oligonucleotide probe) typing protocol developed by the 13th International Histocompatibility Workshop [32] for the first enrollment study cohort (N = 985), and using a DNA sequence-based typing (SBT) protocol in the second enrollment study cohort (N = 3070). The sequencing analysis was performed using the ABI Big Dye Terminator Cycle Sequencing Kit and the ABI3730xl DNA analyzer (Applied Biosystems, Foster City, CA). HLA alleles were assigned on the basis of the sequence database of known alleles with the help of the ASSIGN software developed by Conexio Genomics (Conexio Genomics, Western Australia, Australia). Ambiguous heterozygous genotypes were resolved by additional PCR and sequencing procedures using allele-specific PCR primers to selectively amplify only one of the two alleles.
Haplotype of HLA-A, HLA-B and HLA-C allelic combinations were assessed using 422 children of the phase I study subjects in 179 patients and 379 controls. Based on expectation maximization algorithm to generate maximum likelihood estimation haplotype, we observed 90% accuracy on HLA-A-B-C haplotypes, 91% on HLA-A-B and HLA-A-C haplotypes, and 99% on HLA-B-C haplotypes. For the remaining NPC cases and controls, HLA haplotypes were assigned by population-based estimation methods of PROC HAPLOTYPE in SAS/Genetics package.
HLA amino acid variant definition
Because the most significant NPC associated SNPs is located on the HLA class I region (see Results), an amino acid analysis was carried out to evaluate the role of functional relative amino acid residues in HLA associations. From our high resolution HLA genotyping results, we were able to define corresponding amino acid sequences for all study subjects. The amino acid variants in HLA class I genes were defined by using web-based software of the Immunology Database and Analysis Portal (ImmPort) system [24].
Proxy SNP–variant analysis
We have used the method of testing each variant for reduced-p-values in multi-variants models resulting from co-linearity of variants to recognize LD and independence of signals in the association of NPC with HLA-A, -B and -C. Multicollinearity in logistic regression models is a result of strong correlations between variables. The existence of multicollinearity (high r2) between variants inflates the variances of the parameter estimates. That will likely result in lowered p-values for a given SNP that was determined to be in significant association with NPC when tested signally. We used a reduction in significance as an indicator that two variants were in strong LD, and therefore not independent signals as has been done by recent authors [18]. This gives us a general idea about independence of the signals within HLA and adjacent genes within the context of the disease association. However, we recognize that although multicollinearity may lower magnitudes of regression coefficient estimates and resulting p-value significance in cases of LD, this method may be subject to error such as when a rare SNP on a haplotype does not have a large effect on the model. These methods are provided as an indicator of independence, but not as a definitive measure in our understanding of the disease.
Statistical analysis
We performed logistic regression model analysis for all SNPs passing the quality control filters, using a Cochran-Armitage trend, co-dominant, dominant, recessive, and allelic model taking the number of copies of the rare allele 0, 1 or 2, as the explanatory variable. The comparisons were conducted between NPC cases and NPC free controls, NPC cases and NPC-free but EBV-IgA/VCA antibody positive controls (EP controls), NPC cases and NPC-free but EBV-IgA/VCA-antibody negative controls (EN controls), EP controls and EN controls respectively. Population structure/stratification was assessed using the Principal Components Analysis (PCA) module of Eigensoft software [33]. Study samples were first run together with HapMap individuals of European, African, and Asian descents to identify any potential admixed individuals. Later, PCA analyses of only the study samples were conducted. Initially all autosomal SNPs that passed the quality control filters were used to estimate the contribution of each SNP to the top ten eigenvectors. Previously reported correlated genome regions [19] (such as on chromosomes 6, 8, and 11) were observed and excluded from the following PCA analyses. Moreover, to avoid any confounders due to LD among the SNPs, the genotype data was pruned to 90 K independent SNPs distributed throughout the genome by PLINK prior to the PCA analyses. The logistic regression analysis performed using PLINK [25], controlling for gender, age and the first three eigenvectors; the significance was evaluated using the log likelihood test. SNPs were sorted according to the lowest P-value in a combined set of samples in one of these models. The chi-square tests were used for testing case-control association for allele effects. HLA allele frequencies were calculated based on observed genotypes; HLA-A, -B and -C haplotype were assigned based on maximum likelihood estimation using the SAS/Genetics HAPLOTYPE procedure. The effect of HLA alleles on the development of NPC and EBV-IgA/VCA antibody was evaluated by computing odds ratios (OR) and 95% confidence intervals (CI) using logistic regression. For HLA allele and haplotype test, P values were calculated by logistic regression and then corrected by the Bonferroni, which was multiplied by the number of all detected alleles or haplotypes. Significance was considered at P<0.05 after correction.
Supporting Information
Zdroje
1. JemalA, BrayF, CenterMM, FerlayJ, WardE, et al. (2011) Global cancer statistics. CA Cancer J Clin 61 : 69–90.
2. HenleG, HenleW (1976) Epstein-Barr virus-specific IgA serum antibodies as an outstanding feature of nasopharyngeal carcinoma. Int J Cancer 17 : 1–7.
3. ZengY, ZhangLG, LiHY, JanMG, ZhangQ, et al. (1982) Serological mass survey for early detection of nasopharyngeal carcinoma in Wuzhou City, China. Int J Cancer 29 : 139–141.
4. ZengY, ZhangLG, WuYC, HuangYS, HuangNQ, et al. (1985) Prospective studies on nasopharyngeal carcinoma in Epstein-Barr virus IgA/VCA antibody-positive persons in Wuzhou City, China. Int J Cancer 36 : 545–547.
5. NgWT, YauTK, YungRW, SzeWM, TsangAH, et al. (2005) Screening for family members of patients with nasopharyngeal carcinoma. Int J Cancer 113 : 998–1001.
6. CoffinCM, RichSS, DehnerLP (1991) Familial aggregation of nasopharyngeal carcinoma and other malignancies. A clinicopathologic description. Cancer 68 : 1323–1328.
7. SimonsMJ, WeeGB, ChanSH, ShanmugaratnamK, DayNE, et al. (1975) Immunogenetic aspects of nasopharyngeal carcinoma (NPC) III. HL-a type as a genetic marker of NPC predisposition to test the hypothesis that Epstein-Barr virus is an etiological factor in NPC. IARC Sci Publ 249–258.
8. ChanSH, DayNE, KunaratnamN, ChiaKB, SimonsMJ (1983) HLA and nasopharyngeal carcinoma in Chinese–a further study. Int J Cancer 32 : 171–176.
9. LuSJ, DayNE, DegosL, LepageV, WangPC, et al. (1990) Linkage of a nasopharyngeal carcinoma susceptibility locus to the HLA region. Nature 346 : 470–471.
10. HildesheimA, AppleRJ, ChenCJ, WangSS, ChengYJ, et al. (2002) Association of HLA class I and II alleles and extended haplotypes with nasopharyngeal carcinoma in Taiwan. J Natl Cancer Inst 94 : 1780–1789.
11. HuSP, DayNE, LiDR, LubenRN, CaiKL, et al. (2005) Further evidence for an HLA-related recessive mutation in nasopharyngeal carcinoma among the Chinese. Br J Cancer 92 : 967–970.
12. YuKJ, GaoX, ChenCJ, YangXR, DiehlSR, et al. (2009) Association of human leukocyte antigens with nasopharyngeal carcinoma in high-risk multiplex families in Taiwan. Hum Immunol 70 : 910–914.
13. TangM, ZengY, PoissonA, MartiD, GuanL, et al. (2010) Haplotype-dependent HLA susceptibility to nasopharyngeal carcinoma in a Southern Chinese population. Genes Immun 11 : 334–342.
14. ReillyMP, LiM, HeJ, FergusonJF, StylianouIM, et al. (2011) Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet 377 : 383–392.
15. SeshadriS, FitzpatrickAL, IkramMA, DeStefanoAL, GudnasonV, et al. (2010) Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA 303 : 1832–1840.
16. YangJJ, PlengeRM (2011) Genomic technology applied to pharmacological traits. JAMA 306 : 652–653.
17. NgCC, YewPY, PuahSM, KrishnanG, YapLF, et al. (2009) A genome-wide association study identifies ITGA9 conferring risk of nasopharyngeal carcinoma. J Hum Genet 54 : 392–397.
18. TseKP, SuWH, ChangKP, TsangNM, YuCJ, et al. (2009) Genome-wide association study reveals multiple nasopharyngeal carcinoma-associated loci within the HLA region at chromosome 6p21.3. Am J Hum Genet 85 : 194–203.
19. BeiJX, LiY, JiaWH, FengBJ, ZhouG, et al. (2010) A genome-wide association study of nasopharyngeal carcinoma identifies three new susceptibility loci. Nat Genet 42 : 599–603.
20. GuoXC, ScottK, LiuY, DeanM, DavidV, et al. (2006) Genetic factors leading to chronic Epstein-Barr virus infection and nasopharyngeal carcinoma in South East China: study design, methods and feasibility. Hum Genomics 2 : 365–375.
21. GuoX, JohnsonRC, DengH, LiaoJ, GuanL, et al. (2009) Evaluation of non-viral risk factors for nasopharyngeal carcinoma in a high-risk population of Southern China. International Journal of Cancer 124 : 2942–2947.
22. BjorkmanPJ, SaperMA, SamraouiB, BennettWS, StromingerJL, et al. (1987) The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature 329 : 512–518.
23. LundO, NielsenM, KesmirC, PetersenAG, LundegaardC, et al. (2004) Definition of supertypes for HLA molecules using clustering of specificity matrices. Immunogenetics 55 : 797–810.
24. KarpDR, MarthandanN, MarshSG, AhnC, ArnettFC, et al. (2010) Novel sequence feature variant type analysis of the HLA genetic association in systemic sclerosis. Hum Mol Genet 19 : 707–719.
25. PurcellS, NealeB, Todd-BrownK, ThomasL, FerreiraMA, et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 : 559–575.
26. GaoX, NelsonGW, KarackiP, MartinMP, PhairJ, et al. (2001) Effect of a single amino acid change in MHC class I molecules on the rate of progression to AIDS. N Engl J Med 344 : 1668–1675.
27. ThammavongsaV, SchaeferM, FilzenT, CollinsKL, CarringtonM, et al. (2009) Assembly and intracellular trafficking of HLA-B*3501 and HLA-B*3503. Immunogenetics 61 : 703–716.
28. SteinleA, FalkK, RotzschkeO, GnauV, StevanovicS, et al. (1996) Motif of HLA-B*3503 peptide ligands. Immunogenetics 43 : 105–107.
29. BashirovaAA, ThomasR, CarringtonM (2011) HLA/KIR restraint of HIV: surviving the fittest. Annu Rev Immunol 29 : 295–317.
30. HuangJ, GoedertJJ, SundbergEJ, CungTD, BurkePS, et al. (2009) HLA-B*35-Px-mediated acceleration of HIV-1 infection by increased inhibitory immunoregulatory impulses. J Exp Med 206 : 2959–2966.
31. KulkarniS, SavanR, QiY, GaoX, YukiY, et al. (2011) Differential microRNA regulation of HLA-C expression and its association with HIV control. Nature 472 : 495–498.
32. Hurley C (2005) IHWG Technology Core Joint Report; Hansen JA, Dupont B, editors: Immunobiology of the Human MHC: Proceedings of the 13th International Hisotcompatibility Workshop and Conference, Fred Hutchinson Cancer Research.
33. PriceAL, PattersonNJ, PlengeRM, WeinblattME, ShadickNA, et al. (2006) Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38 : 904–909.
Štítky
Genetika Reprodukční medicína
Článek The Covariate's DilemmaČlánek Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene SignallingČlánek Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 11- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
-
Všechny články tohoto čísla
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- The Covariate's Dilemma
- Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene Signalling
- Plan B for Stimulating Stem Cell Division
- Discovering Thiamine Transporters as Targets of Chloroquine Using a Novel Functional Genomics Strategy
- Is a Modifier of Mutations in Retinitis Pigmentosa with Incomplete Penetrance
- Evolutionarily Ancient Association of the FoxJ1 Transcription Factor with the Motile Ciliogenic Program
- Genome Instability Caused by a Germline Mutation in the Human DNA Repair Gene
- Transcription Factor Oct1 Is a Somatic and Cancer Stem Cell Determinant
- Controls of Nucleosome Positioning in the Human Genome
- Disruption of Causes Defective Meiotic Recombination in Male Mice
- A Novel Human-Infection-Derived Bacterium Provides Insights into the Evolutionary Origins of Mutualistic Insect–Bacterial Symbioses
- Trps1 and Its Target Gene Regulate Epithelial Proliferation in the Developing Hair Follicle and Are Associated with Hypertrichosis
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Population-Based Resequencing of in 10,330 Individuals: Spectrum of Genetic Variation, Phenotype, and Comparison with Extreme Phenotype Approach
- HP1a Recruitment to Promoters Is Independent of H3K9 Methylation in
- Transcription Elongation and Tissue-Specific Somatic CAG Instability
- A Germline Polymorphism of DNA Polymerase Beta Induces Genomic Instability and Cellular Transformation
- Interallelic and Intergenic Incompatibilities of the () Gene in Mouse Hybrid Sterility
- Comparison of Mitochondrial Mutation Spectra in Ageing Human Colonic Epithelium and Disease: Absence of Evidence for Purifying Selection in Somatic Mitochondrial DNA Point Mutations
- Mutations in the Transcription Elongation Factor SPT5 Disrupt a Reporter for Dosage Compensation in Drosophila
- Evolution of Minimal Specificity and Promiscuity in Steroid Hormone Receptors
- Blockade of Pachytene piRNA Biogenesis Reveals a Novel Requirement for Maintaining Post-Meiotic Germline Genome Integrity
- RHOA Is a Modulator of the Cholesterol-Lowering Effects of Statin
- MIG-10 Functions with ABI-1 to Mediate the UNC-6 and SLT-1 Axon Guidance Signaling Pathways
- Loss of the DNA Methyltransferase MET1 Induces H3K9 Hypermethylation at PcG Target Genes and Redistribution of H3K27 Trimethylation to Transposons in
- Genome-Wide Association Studies Reveal a Simple Genetic Basis of Resistance to Naturally Coevolving Viruses in
- The Principal Genetic Determinants for Nasopharyngeal Carcinoma in China Involve the Class I Antigen Recognition Groove
- Molecular, Physiological, and Motor Performance Defects in DMSXL Mice Carrying >1,000 CTG Repeats from the Human DM1 Locus
- Genomic Study of RNA Polymerase II and III SNAP-Bound Promoters Reveals a Gene Transcribed by Both Enzymes and a Broad Use of Common Activators
- Long Telomeres Produced by Telomerase-Resistant Recombination Are Established from a Single Source and Are Subject to Extreme Sequence Scrambling
- The Yeast SR-Like Protein Npl3 Links Chromatin Modification to mRNA Processing
- Deubiquitylation Machinery Is Required for Embryonic Polarity in
- dJun and Vri/dNFIL3 Are Major Regulators of Cardiac Aging in Drosophila
- CtIP Is Required to Initiate Replication-Dependent Interstrand Crosslink Repair
- Notch-Mediated Suppression of TSC2 Expression Regulates Cell Differentiation in the Intestinal Stem Cell Lineage
- A Combination of H2A.Z and H4 Acetylation Recruits Brd2 to Chromatin during Transcriptional Activation
- Network Analysis of a -Mouse Model of Autosomal Dominant Polycystic Kidney Disease Identifies HNF4α as a Disease Modifier
- Mitosis in Neurons: Roughex and APC/C Maintain Cell Cycle Exit to Prevent Cytokinetic and Axonal Defects in Photoreceptor Neurons
- CELF4 Regulates Translation and Local Abundance of a Vast Set of mRNAs, Including Genes Associated with Regulation of Synaptic Function
- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- The Genomes of the Fungal Plant Pathogens and Reveal Adaptation to Different Hosts and Lifestyles But Also Signatures of Common Ancestry
- A Genome-Scale RNA–Interference Screen Identifies RRAS Signaling as a Pathologic Feature of Huntington's Disease
- Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
- Population Genomic Scan for Candidate Signatures of Balancing Selection to Guide Antigen Characterization in Malaria Parasites
- Tissue-Specific Regulation of Chromatin Insulator Function
- Disruption of Mouse Cenpj, a Regulator of Centriole Biogenesis, Phenocopies Seckel Syndrome
- Genome, Functional Gene Annotation, and Nuclear Transformation of the Heterokont Oleaginous Alga CCMP1779
- Antagonistic Gene Activities Determine the Formation of Pattern Elements along the Mediolateral Axis of the Fruit
- Lung eQTLs to Help Reveal the Molecular Underpinnings of Asthma
- Identification of the First ATRIP–Deficient Patient and Novel Mutations in ATR Define a Clinical Spectrum for ATR–ATRIP Seckel Syndrome
- Cooperativity of , , and in Malignant Breast Cancer Evolution
- Loss of Prohibitin Membrane Scaffolds Impairs Mitochondrial Architecture and Leads to Tau Hyperphosphorylation and Neurodegeneration
- Microhomology Directs Diverse DNA Break Repair Pathways and Chromosomal Translocations
- MicroRNA–Mediated Repression of the Seed Maturation Program during Vegetative Development in
- Selective Pressure Causes an RNA Virus to Trade Reproductive Fitness for Increased Structural and Thermal Stability of a Viral Enzyme
- The Tumor Suppressor Gene Retinoblastoma-1 Is Required for Retinotectal Development and Visual Function in Zebrafish
- Regions of Homozygosity in the Porcine Genome: Consequence of Demography and the Recombination Landscape
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
- Polyadenylation-Dependent Control of Long Noncoding RNA Expression by the Poly(A)-Binding Protein Nuclear 1
- A Unified Method for Detecting Secondary Trait Associations with Rare Variants: Application to Sequence Data
- Genetic and Biochemical Dissection of a HisKA Domain Identifies Residues Required Exclusively for Kinase and Phosphatase Activities
- Informed Conditioning on Clinical Covariates Increases Power in Case-Control Association Studies
- Biochemical Diversification through Foreign Gene Expression in Bdelloid Rotifers
- Genomic Variation and Its Impact on Gene Expression in
- Spastic Paraplegia Mutation N256S in the Neuronal Microtubule Motor KIF5A Disrupts Axonal Transport in a HSP Model
- Lamin B1 Polymorphism Influences Morphology of the Nuclear Envelope, Cell Cycle Progression, and Risk of Neural Tube Defects in Mice
- A Targeted Glycan-Related Gene Screen Reveals Heparan Sulfate Proteoglycan Sulfation Regulates WNT and BMP Trans-Synaptic Signaling
- Dopaminergic D2-Like Receptors Delimit Recurrent Cholinergic-Mediated Motor Programs during a Goal-Oriented Behavior
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



