-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Germline Polymorphism of DNA Polymerase Beta Induces Genomic Instability and Cellular Transformation
Several germline single nucleotide polymorphisms (SNPs) have been identified in the POLB gene, but little is known about their cellular and biochemical impact. DNA Polymerase β (Pol β), encoded by the POLB gene, is the main gap-filling polymerase involved in base excision repair (BER), a pathway that protects the genome from the consequences of oxidative DNA damage. In this study we tested the hypothesis that expression of the POLB germline coding SNP (rs3136797) in mammalian cells could induce a cancerous phenotype. Expression of this SNP in both human and mouse cells induced double-strand breaks, chromosomal aberrations, and cellular transformation. Following treatment with an alkylating agent, cells expressing this coding SNP accumulated BER intermediate substrates, including single-strand and double-strand breaks. The rs3136797 SNP encodes the P242R variant Pol β protein and biochemical analysis showed that P242R protein had a slower catalytic rate than WT, although P242R binds DNA similarly to WT. Our results suggest that people who carry the rs3136797 germline SNP may be at an increased risk for cancer susceptibility.
Published in the journal: . PLoS Genet 8(11): e32767. doi:10.1371/journal.pgen.1003052
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003052Summary
Several germline single nucleotide polymorphisms (SNPs) have been identified in the POLB gene, but little is known about their cellular and biochemical impact. DNA Polymerase β (Pol β), encoded by the POLB gene, is the main gap-filling polymerase involved in base excision repair (BER), a pathway that protects the genome from the consequences of oxidative DNA damage. In this study we tested the hypothesis that expression of the POLB germline coding SNP (rs3136797) in mammalian cells could induce a cancerous phenotype. Expression of this SNP in both human and mouse cells induced double-strand breaks, chromosomal aberrations, and cellular transformation. Following treatment with an alkylating agent, cells expressing this coding SNP accumulated BER intermediate substrates, including single-strand and double-strand breaks. The rs3136797 SNP encodes the P242R variant Pol β protein and biochemical analysis showed that P242R protein had a slower catalytic rate than WT, although P242R binds DNA similarly to WT. Our results suggest that people who carry the rs3136797 germline SNP may be at an increased risk for cancer susceptibility.
Introduction
DNA Polymerase β (Pol β) is the main polymerase involved in the base excision repair pathway (BER), the pathway responsible for repairing up to 20,000 endogenous lesions per cell per day [1], [2]. Pol β is a bifunctional polymerase, containing both deoxyribose phosphate (dRP) lyase and nucleotidyl transferase activities (reviewed in [3]). One or both of these activities are essential, as Pol β knockout mice die shortly after birth [4].
Two germline SNPs of the POLB gene (rs12678588and rs3136797) have been previously identified, and the variant alleles have been shown to be present in specific populations [5], [6]. The rs12678588 SNP results in a nonsynonymous amino acid substitution of glutamine for arginine at residue 137 (R137Q). In the wild-type (WT) protein, Arg137 is methylated by the protein arginine N-methyltransferase 1 (PRMT1), leading to a reduction in proliferating cell nuclear antigen (PCNA) binding [7]. R137Q is a slow polymerase with decreased BER activity in cell extracts, and cells expressing this variant have increased formation of AP sites following methyl methanesulfonate (MMS) exposure [8]. Little is known about the biochemical and cellular characteristics of the rs3136707 SNP, in which the proline at residue 242 is altered to arginine (P242R) or its role in human health. Carriers of this allele include populations from Eastern Europe [6]. Interestingly, patients heterozygous for this allele exhibited decreased survival when treated for either lung cancer or lymphoma [9], [10]. Additionally, this residue is located at the base of Loop II, a region that has been shown by us to be critical for polymerase activity and fidelity [11]–[13].
In this study, we tested the hypothesis that the P242R germline POLB variant has a functional phenotype that could drive carcinogenesis. We found that expression of a cDNA encoding the P242R protein in both human and mouse cells induce chromosomal aberrations and cellular transformation. We also show that purified P242R protein is a slow polymerase that binds DNA tightly. In combination, our results suggest that cells expressing P242R accumulate BER intermediates that result in the induction of DSBs and chromosomal aberrations that lead to cellular transformation. Our results also indicate that the P242R germline variant of Pol β could result in aberrant BER in carriers of the allele, potentially leading to increased cancer predisposition.
Results
Expression of P242R Induces Chromosomal Aberrations
Previous work on Pol β has shown that expression of certain tumor-specific single amino acid variants can induce genomic instability in the form of chromosomal aberrations [14], [15]. Therefore, we characterized chromosomal aberrations in MCF10A normal human epithelial cells expressing the germline variant P242R. We generated stable MCF10A cell lines expressing C-terminally HA-tagged Pol β-WT or P242R at equal levels to the endogenous WT protein (Figure S1A) and scored metaphase spreads from each of these lines. Figure 1A–1B shows examples of metaphase spreads from MCF10A cells expressing WT or P242R Pol β, respectively. MCF10A cells expressing P242R had increased amounts of chromosomal fusions and fragments compared to cells expressing the WT (Figure 1C). To determine if P242R was a mutator in cells, we performed the λcII forward mutation assay using a C127λb clonal cell line as we describe [16]. Briefly, these cells carry the λ genome and express either P242R or WT Pol β. The phage λ genome is packaged from isolated genomic DNA and the mutant frequency is obtained by infection of E. coli using differential plating conditions as we describe [16]. The spectra of mutations induced by P242R and WT Pol β are generated by sequencing purified plaques. We found that expression of P242R does not induce an increased frequency of point mutations nor a mutation spectrum different from that of WT Pol β, suggesting that it is not a mutator polymerase (Table S1 and Figure S2). Therefore, our results suggest that expression of P242R induces genomic instability in the form of chromosomal aberrations.
Fig. 1. Chromosomal aberrations in P242R-expressing cells. 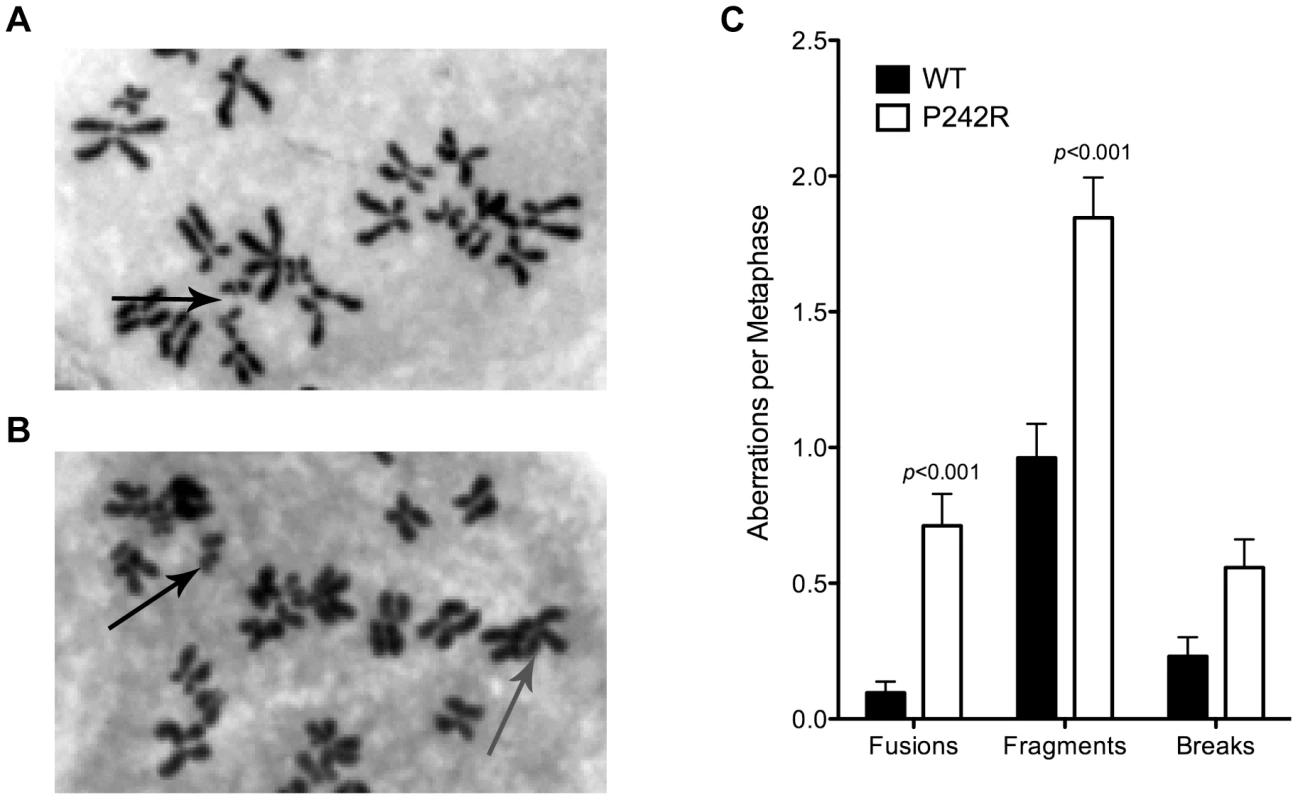
Representative metaphase spread of MCF10A expressing (A.) WT or (B.) P242R Pol β. Chromosomal fusions are shown with the gray arrow and fragments are shown with black arrows. C. Number of aberrations per metaphase. A total of at least 50 metaphases were scored for each cell line. Single-Strand and Double-Strand Break Accumulation in P242R-Expressing Cells
A key role of Pol β is to fill gaps that arise from excision of DNA damage during BER. Chromosomal aberrations are known to arise from breaks in the DNA and aberrant BER can lead to the accumulation of DNA breaks [17]. Therefore, we wished to determine whether treatment with the alkylating agent methylmethane sulfonate (MMS) induces the formation of SSB and DSB BER intermediates in MCF10A cells expressing P242R. MMS induces DNA base damage that is repaired by the BER pathway and therefore treatment of cells with MMS leads to an increase in substrates recognized by Pol β. We used the alkaline comet assay to quantify SSBs induced by MMS. Following treatment with MMS for 30 min, cells expressing P242R have a significantly higher level of SSBs than cells expressing WT Pol β (Figure 2A) (p<0.001). Even after cells were allowed to recover from the MMS treatment for 30 or 60 min, greater levels of SSBs were still observed in P242R cells compared to WT cells (p<0.001). In fact, the percentage of tail DNA was increased in P242R cells allowed to recover compared to P242R cells treated with MMS for 30 min without any recovery (p<0.001) suggesting that cells expressing P242R were unable to efficiently repair the damage or were continuing to accumulate damage. Although the alkaline comet assay measures SSBs, DSBs could also be present. To determine if this was the case, we treated cells with MMS for 30 min and allowed them to recover as in the comet assay, except we monitored γH2AX staining as an indicator of DSB formation. Two to about five percent of cells expressing either WT or P242R exhibited DSBs when treated with MMS for 30 followed by a recovery period for 0, 30 or 60 min. (Figure 2B). Because the levels of DSBs are similar in cells expressing WT or P242R using this protocol, our results suggest that the comet assay is not predominantly detecting DSBs. Importantly, DSBs are not observed to accumulate in P242R-expressing cells unless they are treated for at least 2 hrs with MMS. We then treated the MCF10A pools expressing WT or P242R with MMS for two hours and analyzed γH2AX staining as an indicator of DSB formation. We also stained with propidium iodide for cell cycle analysis. We found that exposure to MMS for 2 hours increased the levels of DSBs in both WT and P242R-expressing cells although MMS induced significantly more DSBs in P242R cells compared to WT Pol β (Figure 2C) (p<0.001). Moreover, cells expressing WT Pol β began to repair the damage after 2 hours following treatment (p<0.05) whereas cells expressing P242R continued to exhibit γH2AX staining, suggesting that these cells continued to accumulate DSBs longer and/or had delayed repair compared to cells expressing WT Pol β. In fact, WT Pol β expressing cells are able to repair the damage after 2–4 hours and return to background levels of γH2AX staining whereas even after a 4 hour recovery, γH2AX positive foci were observed in cells expressing P242R Pol β (Figure S3). Strikingly, we also observe a significant increase in γH2AX staining in untreated cells expressing P242R Pol β compared to WT (p<0.05) (Figure 2C). The differences observed were not due to varying levels of expression as western analysis shows that WT and P242R were expressed at similar levels in these cells (Figure S1A). Furthermore, although γH2AX positive cells were observed in all three phases of the cell cycle, cells expressing P242R had a significantly higher percentage of γH2AX cells in both S and G2/M phases (Figure 2D) (p<0.001) suggesting that the DSBs may be formed, in part, during DNA replication.
Fig. 2. Accumulation of BER intermediates in MCF10A cells expressing P242R Pol β. 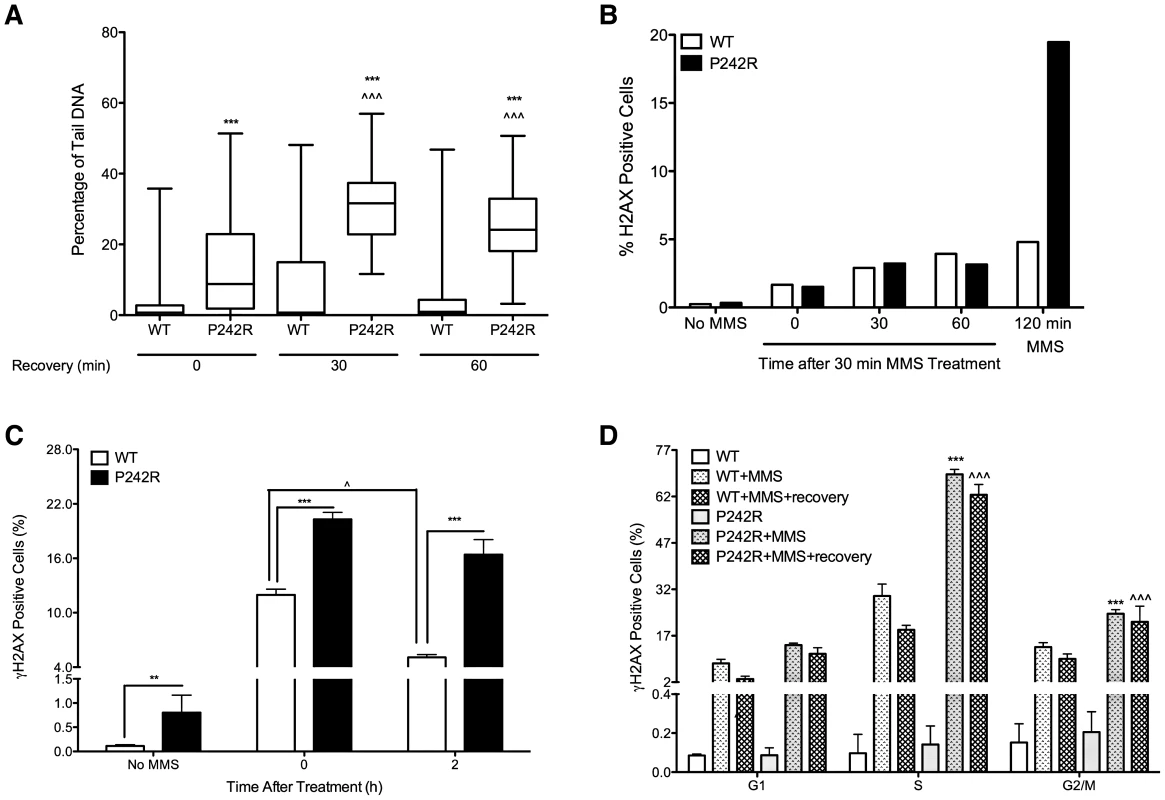
A. MCF10A pools expressing WT or P242R Pol β were treated with 2 mM MMS for 30 minutes and allowed to recover for 0, 30, or 60 min and single-strand breaks (SSBs) were analyzed by comet assay. The percentage of tail DNA is plotted on the Y-axis. B. MCF10A pools expressing WT or P242R Pol β were treated with 2 mM MMS for 30 min and allowed to recover for 0, 30, or 60 min, stained with γH2AX antibody, and analyzed by flow cytometry. Cells were treated for 120 min as a positive control. C–D. MCF10A pools expressing WT or P242R Pol β were treated with 2 mM MMS for 2 h and allowed to recover for 0 or 2 hours. Cells were stained with γH2AX antibody and propidium iodide to assess the levels of double-strand breaks (DSBs) and the cell cycle phase, respectively, and analyzed by flow cytometry. Data are plotted as the mean ± SEM. Data are plotted as the mean ± SEM (n = 3). A and C. ** and *** denote p<0.01 and 0.001, respectively. ∧ denotes p<0.05 comparing 0 vs 2 h recovery within each cell line. ∧∧∧ denotes p<0.001 comparing 30 or 60 min recovery to 0 recovery. D. *** denotes p<0.001 comparing WT+MMS to P242R+MMS in each phase of the cell cycle. ∧ and ∧∧∧ denote p<0.05 and 0.001, respectively, comparing WT+MMS+recovery to P242R+MMS+recovery in each phase of the cell cycle. Expression of P242R Pol β in Mouse and Human Cells Induces Cellular Transformation
We tested the hypothesis that the genomic instability resulting from expression of the germline Pol β P242R protein induces cellular transformation. We generated clonal C127λb cell lines expressing exogenous HA-tagged human Pol β (WT and P242R) at approximately equal levels to endogenous Pol β in a tetracycline-repressible manner (Figure S1B). In the focus formation assay, untransformed cells will grow to confluence forming a monolayer (Figure 3A), while transformed cells will continue to grow after reaching confluence, forming foci (Figure 3B). Expression of P242R Pol β induced cellular transformation whereas expression of WT Pol β did not (Figure 3C–3D). To confirm transformation in these lines, we used a soft agar growth assay in which transformed cells that are capable of anchorage-independent growth will grow when plated on soft agar, while non-transformed cells will not. This assay confirms the results of the focus formation assay, showing that expression of P242R induces anchorage independent growth (Figure 3E) (p<0.01 and 0.05 for P242R clones 5 and 15, respectively). Next, we assessed anchorage independent growth in the human MCF10A cells. Similar to the C127λb clonal lines, MCF10A pools expressing P242R also displayed increased numbers of cells able to grow in soft agar compared to WT (Figure 3F) (p<0.05). Additionally, MCF10A cells expressing P242R had an increased rate of proliferation, another hallmark of cancer cells (Figure 3G) (p<0.05). Together, these data suggest that expression of Pol β P242R induces cellular transformation in both mouse and human cells.
Fig. 3. Expression of P242R induces cellular transformation. 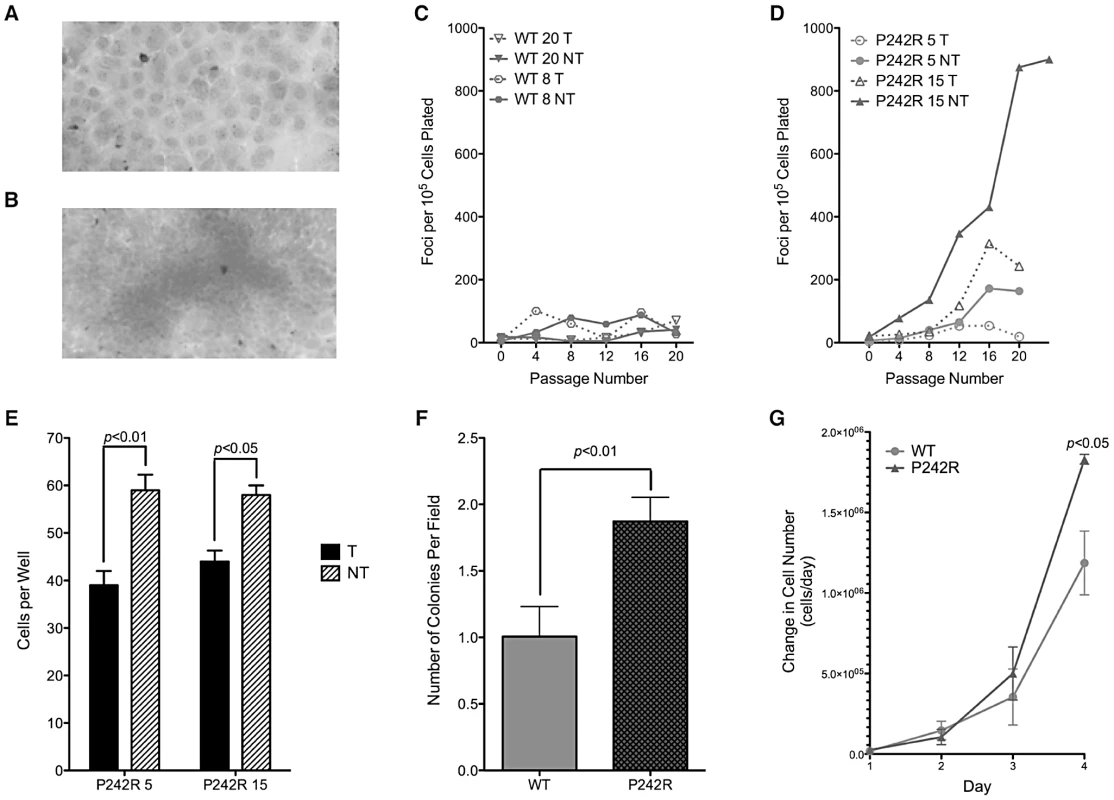
A. Representative image of crystal violet stained untransformed cells. Note the clear monolayer of cell growth. B. Representative image of a focus. Note the accumulation of cells as marked by dark staining. C–D. Focus formation assay with two clonal cell lines either inducing (NT, solid line) or not inducing (T, dashed line) exogenous (C.) WT or (D.) P242R Pol β. Mean foci per 105 cells plated (± standard deviation of plating replicates) plotted against passage number. E. Anchorage independent growth assay with P242R clonal cell lines from the focus formation assay. Cells per field (± standard error) are plotted on the Y-axis. F. Anchorage independent growth assay with MCF10A pools expressing WT or P242R. The number of colonies per field are plotted on the Y-axis. G. WT or P242R MCF10A pools were plated in 5 dishes at a density of 25,000 or 50,000 cells per dish. Cells were trypsinized and counted each day. Data are plotted as the mean ± SEM of the change in cell number (n = 3). Expression of P242R in WT MEFs Does Not Confer Sensitivity to MMS
The accumulation of BER intermediates suggests that P242R may not function in the gap-filling step as well as WT Pol β. Expression of P242R in the Pol β−/− mouse embryonic fibroblasts (MEFs) partially rescued cellular survival in response to treatment with MMS, albeit not as well as expressing WT Pol β (Figure 4A). The reduced ability of P242R to rescue cells from the effects of MMS compared to WT implies that P242R has a partially impaired BER function. However, the rs3136797 SNP, encoding P242R, is present as a heterozygous allele and rarely as a homozygous allele [6]. In addition, the cell lines we employed for our studies, namely, C127λb and MCF10A, both express WT Pol β. Therefore, we investigated whether expression of the P242R variant in the presence of WT Pol β sensitizes cells to MMS, as has been shown for the polymerase-dead E295K variant [15]. We conducted clonogenic survival assays using pools of Pol β+/+ MEFs and MCF10A cells expressing either WT or P242R Pol β or empty vector. Expression of P242R only slightly sensitized cells to high concentrations of MMS in a Pol β proficient background (Figure 4B–4C). In combination with our chromosomal aberration studies, this suggests that some of the cells harboring genomic instability are likely to survive and could become transformed.
Fig. 4. P242R Pol β confers slight sensitivity to MMS compared to WT. 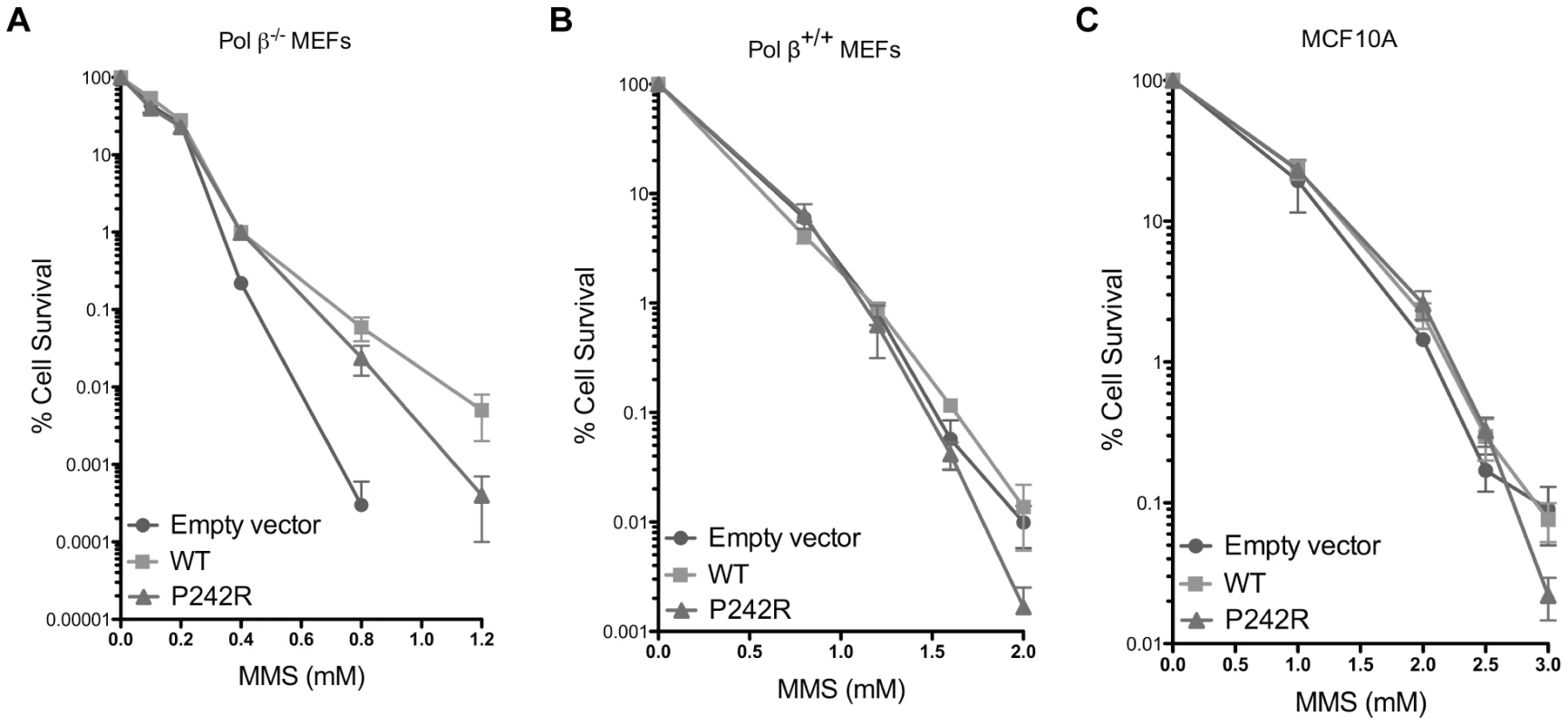
Clonogenic survival assays were conducted with (A.) Pol β−/− MEFs, (B.) Pol β+/+ MEFs, or (C.) MCF10A pools expressing WT or P242R Pol β. Filled circles represent results from pools expressing empty vector, filled squares represent pools expressing WT Pol β, and filled triangles represent pools expressing P242R Pol β. Data are plotted as the mean ± SEM (n = 3). The P242R Germline Variant Is a Slow Polymerase
The recombinant WT and P242R proteins were purified from E. coli and studied in a presteady-state burst assay. This assay used a radiolabeled 1 bp gapped DNA substrate, the preferred substrate for Pol β. Both proteins fit a biphasic burst of product formation, typical of Pol β activity (Figure 5A). However, P242R had decreased initial burst (kobs = 14±2 and 7.5±0.2 sec−1, WT and P242R, respectively) and steady-state rate compared to WT (kss = 3.3±0.5 and 1.7±0.2 sec−1, WT and P242R, respectively). This decreased activity was not due to decreased DNA binding, as the gel electrophoretic mobility shift assay showed that the P242R variant binds 1-bp gapped DNA with similar affinity to WT (Kd = 5±1 and 5±1 nM, WT and P242R, respectively) (Figure 5B). Together, our data suggest that the slow rate of DNA synthesis catalyzed by P242R could result in unfilled gaps that lead to genomic instability and cellular transformation.
Fig. 5. The P242R germline variant of Pol β is slow and binds DNA tightly. 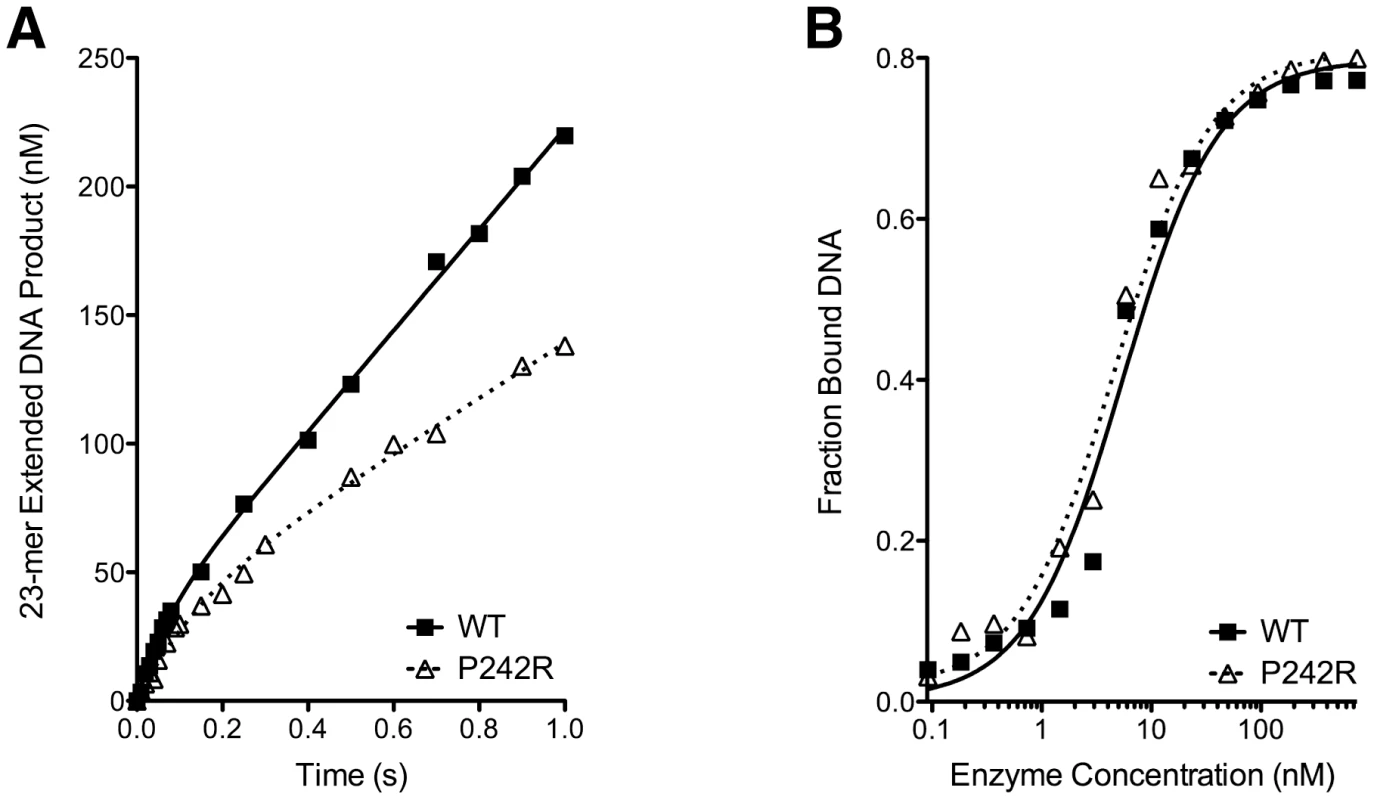
A. Representative results from a presteady-state burst assay. Results for the WT are shown as filled squares fit with a solid curve. Results for the P242R are shown as open triangles fit with a dashed curve. The assay was repeated four times for each protein. B. Representative results from a gel electrophoretic mobility shift assay. Results for the different proteins are shown as in A. Discussion
Previous work from our laboratory showed that the rs3136797 SNP is present at maximum frequencies of 2.4% and is predominantly found in Eastern European populations and Keralites [6]. This polymorphism is one of two in the POLB gene that results in a missense mutation and the allele with the rs3136797 SNP encodes the P242R Pol β protein. Using haplotype analysis, this allele was shown to be evolutionarily distinct from other POLB SNPs studied and its low frequency suggests that few homozygotes exist. Given that Pol β plays a critical role in the repair of endogenous DNA damage and functions to maintain genome stability, we tested the hypothesis that the P242R germline variant has a functional phenotype related to carcinogenesis. Our work shows that expression of P242R Pol β in the presence of WT Pol β induces genomic instability and cellular transformation in both mouse and human cells. Our results are consistent with the interpretation that the people who carry the P242R Pol β SNP are at increased risk for cancer.
P242R Is Located in a Region of Pol β That Is Critical for Activity
Pro242 of Pol β is located at the base of Loop II, a structure that we previously showed to be important for Pol β activity and fidelity [11], [12]. Loop II is highly flexible, solvent exposed, and quite far away from the active site of Pol β. Mutations in amino acids within this loop result in decreased nucleotide discrimination and fidelity and induce mutations [11], [12], [18], [19]. In addition, alterations in the amino acid sequence of the loop results in a reduced catalytic rate [12]. Pro242 is conserved between all members of the Pol X polymerase family [20], [21]. Prolines can cause rigidity in the protein structure and this characteristic may help to anchor this flexible loop. We previously proposed that the specific geometry of this loop, and particularly of residue 242, is important for maintaining the β-sheet structure of the Pol β active site. A change from Pro to Arg could disrupt the overall structure of the loop. Thus, it is not surprising to find that alteration of residue 242 from Pro to Arg results in an enzyme with low DNA polymerase activity.
Accumulation of BER Intermediates Play a Role in Cellular Transformation Induced by P242R
Cancer is a disease of aging. We suggest that slow accumulation of genomic instability over 50–60 years may occur in people carrying the P242R germline variant and that genomic instability could lead to cancer. P242R catalyzes single nucleotide gap filling at a rate half that of WT Pol β, although the protein binds to DNA with similar affinity as WT Pol β (Figure 5). Because P242R is a slow polymerase, gaps and SSBs undergoing repair with P242R may accumulate at levels somewhat higher than those repaired by WT Pol β. Indeed, treatment of cells with MMS for 30 min leads to significantly increased SSB levels in cells expressing P242R versus WT (Figure 2A), even after the cells have had a chance to recover. At pH>12.6, the alkaline comet assay detects SSBs, BER intermediates from incomplete repair, and alkali-labile sites [20], [21], but others report that it also detect DSBs [22]. Monitoring of γH2AX under our conditions suggests the presence of few DSBs (Figure 2B). Our results suggest that SSBs and single nucleotide gaps accumulate in P242R-expressing cells, likely as a result of the slow gap filling activity of P242R.
Treatment of cells for 2 hours with MMS results in the appearance of greater levels of DSBs in cells expressing P242R versus WT Pol β, and we show that they form predominantly during S-phase. These results suggest after two hours of MMS treatment, the BER system becomes overwhelmed with DNA damage, resulting in fewer gaps being filled by Pol β and leading to DSB formation upon encounter of the replication fork by a gap. However, cells expressing WT Pol β recover from this treatment more quickly than cells expressing P242R. This is likely due to deficient gap filling by P242R. Our results are also consistent with the possibility that treatment with MMS for 2 hours induces frank DSBs that are more rapidly repaired by WT versus P242R Pol β. We do not favor this explanation because to date there is no evidence for Pol β having a direct role in the repair of DSBs. In addition, our cell cycle data suggests that the DSBs accumulate during S-phase, which is consistent with the idea that they originate as a result of replication of a break or gap in the DNA. This mechanism has been suggested to explain similar findings that have been reported in Pol β−/− cells, where MMS treatment in G1 leads to unrepaired gaps that can form DSBs when cells enter G2/M [23]. Additionally, treatment of cells with a low dose of MMS and a PARP inhibitor, which effectively inhibits BER, resulted in DSB formation primarily during S-phase [24].
We have also shown that expression of P242R induces an elevated frequency of chromosomal aberrations compared to cells expressing WT Pol β (Figure 1). We suggest that these aberrations arise as the result of the persistent accumulation of BER intermediates in cultured cells (Figure 2), and that the presence of this type of genomic instability leads to cellular transformation (Figure 3). We show that cells expressing P242R undergo cellular transformation as demonstrated by formation of foci, anchorage-independent growth, and an increased rate of proliferation. The increase in proliferation suggests that critical cell cycle check points may be disrupted and cells are growing without effectively repairing the DNA. This uncontrolled cellular growth and replication of damaged DNA can induce the genomic instability we observe in these cells.
Cells Expressing P242R Accumulate Endogenous DSBs
For many of the experiments we performed we increased the levels of DNA damage over endogenous levels by treating with MMS, which would be expected to increase the amounts of Pol β substrates, namely, single nucleotide gaps in cells, in order to be able to detect breaks and aberrations. However, we find that the levels of endogenous DSBs are increased in cells expressing P242R compared to cells expressing only WT Pol β (Figure 2B). Since Pol β is responsible for repairing at least 20,000 lesions/cell/day of endogenous damage, it is probable that humans expressing the P242R SNP may have more unresolved lesions compared to those with two WT alleles. Our finding of endogenous DSBs in cells expressing P242R suggests that over time, cells harboring this variant would incur more DSBs than cells without P242R even in the absence of exogenous DNA damage. We envision that the majority of DSBs are repaired accurately but that some of them are not, leading to deletions and insertions if repaired by non-homologous end joining, gene fusions, or other types of genomic instability, which could lead to cancer. Environmental exposures and/or diagnostic procedures over a P242R-carrying individual's lifetime could serve to enhance the rate or levels of genomic instability and perhaps decrease the latency of cancer.
P242R and Cancer Therapy
Given that the P242R variant is rare (maximum allele frequency 2.4%) [6], epidemiologic studies have had limited success determining the role of this variant in human health [25]. It has been suggested that the 242Arg allele is associated with poor prognosis in lung cancer and lymphoma patients [9], [10], but the mechanism is unknown. One possibility is that the slow rate of DNA synthesis of P242R might be expected to enhance cancer cell death in the presence of DNA damage induced by radio - and chemotherapies, because many DNA gaps would remain unfilled. Indeed, we have shown that expression of P242R in Pol β-deficient MEFs does not rescue cells as completely as WT Pol β. This suggests that in the presence of P242R alone, for example in a homozygote who carries two alleles of P242R, would lead to cell death as a result of treatment with alkylating agents. However, expression of P242R in the presence of WT Pol β, as would be the case with a heterozygotic individual, only slightly sensitizes cells to high concentrations of MMS (Figure 4). This suggests that the majority of cells expressing both P242R and WT survive treatment with alkylating agents. We suggest that these survivors could have increased levels of genomic instability, based upon our results showing that when treated with MMS, cells expressing both P242R and WT Pol β accumulate BER intermediates and have an increased frequency of chromosomal aberrations. Thus, treatment of cells expressing both of these proteins could lead to cellular transformation or more aggressive disease.
In conclusion, our results show that the presence of the P242R germline variant contributes to the increase in chromosomal aberrations and cellular transformation. Therefore, individuals carrying this germline variant may have increased cancer susceptibility, suggesting that aberrant BER at the level of the germline could be a driver of carcinogenesis.
Materials and Methods
Chemicals and Reagents
All ultrapure deoxynucleoside triphosphates (dNTPs) were purchased from New England Biolabs. [γ-32P] ATP (5 mCi) and ATP were purchased from Amersham Biosciences and Sigma-Aldrich, respectively. All oligonucleotides used for the in vitro biochemical assays were purchased from Keck Biotechnology Research Center at Yale University and purified as described [26]. All oligonucleotides used for cloning and PCR were purchased from Invitrogen and are shown in Table S2.
Plasmids and Cloning
Human Pol β cDNA (Genbank accession NM_002690) was cloned into the pET28a expression plasmid (Novagen) for expression with an N-terminal 6×His tag. WT Pol β cDNA sequence was verified by sequencing at the Keck DNA Sequencing Facility at Yale University. For cell culture experiments, human Pol β cDNA with a C-terminal hemagluttinin (HA) tag was cloned into the pRVYTet-Sis retroviral vector as described [14][15]. The 242Arg variant was introduced into the human WT (242Pro) Pol β cDNA sequence using site-directed mutagenesis (Stratagene) following the manufacturer's protocols.
Bacterial Strains, Mammalian Cell Lines, and Cell Culture
For cloning of Pol β, E. coli DH5αMCR with the genotype mcrA (mrr-hsdRMS-mcrBC)φ80ΔlacZ(M15) (lacZYA-argF)U169 deoR recA1 endA1 phoA supE44 thi-1 gyrA96 relA1 was used. Human Pol β was expressed in Rosetta(DE3) cells (Novagen). For the λcII forward mutagenesis assay, lysogen strain NM759 [27] was used for the preparation of sonication extracts and BHB2688 [28], [29] was used for the freeze-thaw extracts. E. coli strain G1250 hflA::Tn5 hflB29 was used for selection and harvesting of packaged phage harboring mutations in the cII gene.
Mouse embryonic fibroblast (MEF) cell lines 92TAg (Pol β+/+) and 88TAg (Pol β−/−) were gifts from Leona Samson (Massachusetts Institute of Technology) [30], [31]. These cells were maintained in high-glucose Dulbecco modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen), 1% penicillin-streptomycin (Invitrogen) and 1% L-glutamine and grown at 37°C in a 5% CO2 humidified incubator.
MCF10A cells are immortalized, non-transformed epithelial cells derived from human mammary tissue (ATCC). These cells were maintained in DMEM/F12 medium (Invitrogen) supplemented with 5% horse serum (Invitrogen), 1% penicillin-streptomycin, epidermal growth factor (20 ng/ml), hydrocortisone (0.5 µg/ml), cholera toxin (100 ng/ml), insulin (10 µg/ml) (Sigma-Aldrich) and grown at 37°C in a 5% CO2 humidified incubator.
C127 cells have been described [32]. The C127λ cells were made by a procedure similar to the one described in [28] with the following exceptions. The λsup-Fneo vector (kind gift from Dr. Peter Glazer, Yale University School of Medicine) was transfected into C127 cells using FuGene 6 (Roche). Single clones were selected in 900 mg/mL of G418 and expanded. A dot blot was used as described [28] to identify clones carrying the λsup-Fneo DNA. The C127λb clone was used in the experiments described here. C127λb cells were grown in Dulbecco modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen), 1% penicillin-streptomycin (Invitrogen), and 600 µg/ml G418 at 37°C in a 5% CO2 humidified incubator.
The GP2-293 virus packaging cell line (Clontech) was used for retrovirus preparation. These cells were maintained in Dulbecco modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen), 1% L-glutamine (Invitrogen), 1% penicillin-streptomycin (Invitrogen) and 1 mM HEPES (Invitrogen).
Transfection, Infection, and Expression Analysis
Human Pol β WT and P242R constructs were packaged into retrovirus using the GP2-293 packaging line. pRVYTet and pVSV-G plasmids were co-transfected into GP2-293 cells using standard calcium phosphate transfection, cells were grown for 72 hours, and retrovirus was harvested.
To infect C127λb, 92TAg (Pol β+/+), 88TAg cells (Pol β−/−), and MCF10A, cells were grown to approximately 30% confluence and infected with retrovirus in the presence of 4 µg/ml polybrene. Cells were incubated overnight in fresh media with 4 µg/ml polybrene. For selection of pools, cells were split 1∶3 the day after infection and cells with the integrated construct were selected with 220 µg/ml hygromycin B for the C127λb and MEFs and 15 µg/ml hygromycin B for the MCF10A cells. For generation of stable clones, C127λb cells were split at several dilutions following infection and selected with 250 µg/ml hygromycin B. Single cell clones were grown and selected using cloning rings. Clonal cell lines were propagated in the presence of 160 µg/ml hygromycin B.
Expression of exogenous HA-tagged Pol β was verified by Western blot. Cells were passed in parallel in the presence or absence of tetracycline. Approximately 80–90% confluent cells were harvested by scraping with hot SDS Loading Buffer (50 mM Tris pH 6.8, 100 mM DTT, 2% SDS 10% glycerol). Lysates were boiled for 10 minutes and run on a 10% acrylamide SDS-PAGE gel. Proteins were transferred to nitrocellulose membrane using a semi-dry transfer apparatus and probed using monoclonal mouse anti-Pol β antibody (Abcam #1831).
Genomic Instability Analysis
Four 10 cm dishes were seeded with 106 cells per dish each per cell line and grown at 37°C 5% CO2 overnight. The cells were fed fresh medium and colcemid (Invitrogen) was added to a final concentration of 100 ng/ml. MCF10A pools were incubated in colcemid for three hours before harvesting by mitotic shakeoff. Cells were harvested via centrifugation, washed twice with 1× PBS, and resuspended dropwise in 0.8% sodium citrate. Following lysis, cells were incubated at 37°C for 30 minutes before fixing in Carnoy's Fixative (75% methanol, 25% acetic acid). Finally, cells were dropped onto microscope slides, dried and stained with 5% KaryoMax Giemsa stain (Invitrogen). Well-spread metaphases were identified under 100× objective (Zeiss). Images were taken using Spot Camera software (Diagnostic Instruments). Metaphase spreads were de-identified and scored by eye for chromosomal fusions, breaks, acentromeric chromosomes and fragments.
Single-Cell Gel Electrophoresis Assay (Comet Assay)
Equal numbers of cells (4×105) were plated in 60 mm dishes. The following day, the cells were treated with 2 mM MMS for 30 min. After treatment, the cells were prepared and analyzed immediately according to published procedures [33] using Cometslides (Trevigen Cat # 4250-200-03). Image analysis of 100–125 cells was performed using CometScore software (TriTek, Sumerduck, VA). Data are represented as mean ± SEM (n = ).
Flow Cytometry
MCF10A cells expressing WT or P242R Pol β were untreated or treated with 2 mM MMS for 2 h. Cells were rinsed then with PBS and replaced with fresh media. Cells were allowed to recover for 0 h and 2 h post treatment. Cells were harvested by trypsinization, washed once with PBS, and pelleted. The pellet was resuspended by adding 70% ice cold ethanol dropwise while vortexing. Cells were fixed overnight at −20°C. The cells were incubated with primary phospho-γH2AX antibody (Millipore 05-636) 1∶500 overnight at 4°C. Following the incubation, cells were washed twice with PBS and incubated with anti-mouse secondary antibody conjugated to FITC 1∶500 for 1 h at room temperature. Cells were washed twiced with PBS and resuspended in 500 µl PI/RNase staining buffer (BD Pharmingen). Fluorescence was analyzed by flow cytometry using the BD FACSCalibur and analyzed using FlowJo 8.8.6 software.
Cellular Transformation
The focus formation assay was conducted as in [15]. Briefly, cells were passaged every 3–4 days in the presence of 160 µg/ml hygromycin B and the presence (non-induced) or absence (induced) of 3 µg/ml tetracycline. Every four passages, 1×10 5 cells were seeded into each of four T25 flasks. These cells were fed every 3–4 days with media containing or lacking 3 µg/ml tetracycline. After 21 days, the cells were stained with 0.25% crystal violet. The presence of foci was also monitored by microscopic examination as described previously [14], [15]. Anchorage independent growth was assessed as previously described [14]. Approximately 1×104 MCF10A cells were mixed with media containing 0.7% noble agar (USB). This mixture was poured onto a layer of media containing 1.0% noble agar in a well of a 6-well dish. Cells were fed twice weekly for 4 weeks. The number of colonies present in each of five microscope fields per well from a total of 6 wells per experiment were counted after 4 weeks of growth.
Clonogenic Survival
Cells (2.2×105) from MEF pools were seeded into 60 mm dishes and incubated for 48 hours. Cells were treated with varying concentrations of MMS for two hours followed by trypsinization and plating at dilutions. Treated cells were grown for 9–11 days before staining with 0.25% crystal violet. Colonies were scored by eye at 4× magnification. Only colonies with more than 50 cells were counted and all experiments were repeated at least twice. For MCF10A cells, various concentrations of cells were plated in 6 well dishes and were allowed to attach overnight. Cells were treated with varying concentrations of MMS for two hours. Following treatment, cells were rinsed with PBS and replaced with fresh media. Cells were allowed to grow for 12–14 days before staining with crystal violet as detailed above.
Cellular Proliferation
MCF10A pools were plated at a density of 25,000 or 50,000 cells per 10 cm dish. Cells were counted every day for 5 consecutive days. Data were plotted as change in cell number per day.
Protein Expression and Purification
pET28a plasmids with human Pol β with 242Pro (WT) and 242Arg (242Arg) cDNA were transformed into Rosetta(DE3) cells. Protein expression was induced by addition of 1.0 mM isopropyl β-D-thiogalactopyranoside (IPTG) and grown at 37°C for 2 hours before harvesting via centrifugation. Cell pellets were dried and stored at −80°C. Protein induction was verified by using 10% SDS-PAGE stained with Coommassie Blue.
Protein was purified using fast protein liquid chromatography. Crude proteins were run through a HiTrap Chelating HP column (GE Healthcare) charged with Ni2+ using a linear imidazole gradient (from 5 to 500 mM) in 40 mM Tris-HCl pH 8.0, 500 mM NaCl. The His-fusion proteins eluted at approximately 277 mM imidazole over five or six fractions of two ml each. The fractions were combined, concentrated to less than one milliliter and diluted into nine milliliters low salt buffer (50 mM Tris-HCl pH 8 .0, 1 mM EDTA, 10% glycerol, 0.1 M NaCl). The diluted protein was then run on a SP HP column (GE Healthcare) using a linear NaCl gradient from 100 mM to 2000 mM. Purified protein fractions eluted at approximately 1000–1200 mM NaCl in two fractions, which were combined and concentrated to less than one milliliter. Glycerol was added to a final concentration of 10–15% and aliquots were flash frozen in liquid N2 and stored at −80°C. All proteins were purified to >90% homogeneity based on Coomassie Blue staining of 10% SDS-PAGE gels.
Preparation of DNA Substrates
Oligonucleotides were synthesized by the W.M. Keck facility and purified by polyacrylamide gel electrophoresis as described previously [34]. Briefly, primer oligos were radiolabelled with γ-32P ATP using T4 polynucleotide kinase (New England Biolabs) and downstream oligos were kinased using non-radioactive ATP. Kinased oligonucleotides were purified using Microspin columns (Biorad). Primer, template, and downstream oligos were annealed by denaturing at 95°C for 5 minutes, slow cooling to 50°C for 30 minutes, holding at 50°C for 20 minutes, and then resting on ice. Complete substrate annealing was confirmed using 12% native polyacrylamide gel electrophoresis and visualized using autoradiography.
Pre-Steady State Burst
Radiolabeled 1 bp gapped DNA (300 nM 45AG [35]) and Pol β (100 nM) were combined with the correct dNTP and 10 mM MgCl2 in a KinTek Chemical Quench-Flow apparatus at 37°C. The reactions were quenched by the addition of 0.5 M EDTA. The reaction products were separated on a 20% denaturing polyacrylamide gels, visualized, and quantified using a Storm 860 Phosphorimager with ImageQuant software. Data were fitted to the burst equation:
where A is the amplitude, kobs is the observed rate constant of the exponential phase, and kss is the rate constant of the linear phase [36].Gel Electrophoretic Mobility Shift Assay
The DNA binding constant was determined by gel electrophoretic mobility shift assay as described previously [34]. The dissociation constant for DNA (KD) was determined by fitting the fraction bound protein (Y) versus protein concentration with the equation:
wherer Y is the amount of bound protein, m is a scaling factor, and b is the apparent minimum Y value [37].λcII Forward Mutagenesis Assay
High molecular weight DNA was harvested from C127λb cells using standard phenol chloroform extraction follwed by dialysis against TE buffer as previously described [16]. Sonication and freeze-thaw packaging extracts were prepared as in [28], [29] using NM759 and BHB2688 E. coli strains. To rescue the λ vector from the DNA, approximately 5 µg of high molecular weight genomic DNA was added to 60 µl of sonication extracts mixed with 40 µl of freeze-thaw extracts. Reactions were incubated at 32°C for 90 minutes. An additional 100 µl of packaging extracts was added and the reactions were incubated for an additional 90 minutes at 32°C. For cII gene mutation detection, the in vitro packaged phage were diluted, adsorbed in G1250 bacteria, and plated in 0.4% top agar on TB plates. Plates were incubated at 37°C overnight to determine the infection titre, because due to a termperature-sensitive mutation in the cII gene in the phage vector, all phage are lytic and form plaques at this termperature. Plates were also incubated at 24°C. The WT phage do not form plaques at this temperature whereas phage with mutations in the cII gene will. cII mutants were obtained from at least three independent packages. Mutant plaques were replated in fresh cultures. Phage DNA was harvested and used as a template for PCR. The cII genes were amplified by PCR and sequenced at the W.M. Keck Sequencing facility.
Statistics
Two-tailed t-tests and two-way analysis of variance (ANOVA) were used as appropriate to determine whether the mean of each cell line was different from the empty vector cells. Bonferroni's post hoc test was used to determine significant differences between the means of each group. All statistics were performed using GraphPad Prism version 5 (GraphPad Software, San Diego, CA). Data are represented as mean ± SEM.
Supporting Information
Zdroje
1. LindahlT (1993) Instability and decay of the primary structure of DNA. Nature 362 : 709–715.
2. BarnesDE, LindahlT (2004) Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu Rev Genet 38 : 445–476.
3. BeardWA, WilsonSH (2006) Structure and mechanism of DNA polymerase Beta. Chem Rev 106 : 361–382.
4. SugoN, ArataniY, NagashimaY, KubotaY, KoyamaH (2000) Neonatal lethality with abnormal neurogenesis in mice deficient in DNA polymerase beta. Embo J 19 : 1397–1404.
5. MohrenweiserHW, XiT, Vazquez-MatiasJ, JonesIM (2002) Identification of 127 amino acid substitution variants in screening 37 DNA repair genes in humans. Cancer Epidemiol Biomarkers Prev 11 : 1054–1064.
6. YamtichJ, SpeedWC, StrakaE, KiddJR, SweasyJB, et al. (2009) Population-specific variation in haplotype composition and heterozygosity at the POLB locus. DNA Repair (Amst) 8 : 579–584.
7. El-AndaloussiN, ValovkaT, ToueilleM, HassaPO, GehrigP, et al. (2007) Methylation of DNA polymerase beta by protein arginine methyltransferase 1 regulates its binding to proliferating cell nuclear antigen. Faseb J 21 : 26–34.
8. GuoZ, ZhengL, DaiH, ZhouM, XuH, et al. (2009) Human DNA polymerase beta polymorphism, Arg137Gln, impairs its polymerase activity and interaction with PCNA and the cellular base excision repair capacity. Nucleic Acids Res 37 : 3431–3441.
9. MatakidouA, el GaltaR, WebbEL, RuddMF, BridleH (2007) Genetic variation in the DNA repair genes is predictive of outcome in lung cancer. Hum Mol Genet 16 : 2333–2340.
10. SellickGS, WadeR, RichardsS, OscierDG, CatovskyD, et al. (2008) Scan of 977 nonsynonymous SNPs in CLL4 trial patients for the identification of genetic variants influencing prognosis. Blood 111 : 1625–1633.
11. LinGC, JaegerJ, EckertKA, SweasyJB (2009) Loop II of DNA polymerase beta is important for discrimination during substrate binding. DNA Repair (Amst) 8 : 182–189.
12. LinGC, JaegerJ, SweasyJB (2007) Loop II of DNA polymerase beta is important for polymerization activity and fidelity. Nucleic Acids Res 35 : 2924–2935.
13. SawayaMR, PrasadR, WilsonSH, KrautJ, PelletierH (1997) Crystal structures of human DNA polymerase beta complexed with gapped and nicked DNA: evidence for an induced fit mechanism. Biochemistry 36 : 11205–11215.
14. SweasyJB, LangT, StarcevicD, SunKW, LaiCC, et al. (2005) Expression of DNA polymerase {beta} cancer-associated variants in mouse cells results in cellular transformation. Proc Natl Acad Sci U S A 102 : 14350–14355.
15. LangT, DalalS, ChikovaA, DiMaioD, SweasyJB (2007) The E295K DNA polymerase beta gastric cancer-associated variant interferes with base excision repair and induces cellular transformation. Mol Cell Biol 27 : 5587–5596.
16. LangT, MaitraM, StarcevicD, LiSX, SweasyJB (2004) A DNA polymerase beta mutant from colon cancer cells induces mutations. Proc Natl Acad Sci U S A 101 : 6074–6079.
17. MaW, WestmorelandJW, GordeninDA, ResnickMA (2011) Alkylation base damage is converted into repairable double-strand breaks and complex intermediates in G2 cells lacking AP endonuclease. PLoS Genet 7: e1002059 doi:10.1371/journal.pgen.1002059
18. KosaJL, SweasyJB (1999) The E249K mutator mutant of DNA polymerase beta extends mispaired termini. J Biol Chem 274 : 35866–35872.
19. DalalS, KosaJL, SweasyJB (2004) The D246V mutant of DNA polymerase beta misincorporates nucleotides: evidence for a role for the flexible loop in DNA positioning within the active site. J Biol Chem 279 : 577–584.
20. Garcia-DiazM, BebenekK, KrahnJM, BlancoL, KunkelTA, et al. (2004) A structural solution for the DNA polymerase lambda-dependent repair of DNA gaps with minimal homology. Mol Cell 13 : 561–572.
21. Garcia-DiazM, DominguezO, Lopez-FernandezLA, de LeraLT, SanigerML, et al. (2000) DNA polymerase lambda (Pol lambda), a novel eukaryotic DNA polymerase with a potential role in meiosis. J Mol Biol 301 : 851–867.
22. OlivePL, BanathJP (2006) The comet assay: a method to measure DNA damage in individual cells. Nat Protoc 1 : 23–29.
23. PascucciB, RussoMT, CrescenziM, BignamiM, DogliottiE (2005) The accumulation of MMS-induced single strand breaks in G1 phase is recombinogenic in DNA polymerase beta defective mammalian cells. Nucleic Acids Res 33 : 280–288.
24. HeacockML, StefanickDF, HortonJK, WilsonSH (2010) Alkylation DNA damage in combination with PARP inhibition results in formation of S-phase-dependent double-strand breaks. DNA Repair (Amst) 9 : 929–936.
25. MorenoV, GemignaniF, LandiS, Gioia-PatricolaL, ChabrierA, et al. (2006) Polymorphisms in genes of nucleotide and base excision repair: risk and prognosis of colorectal cancer. Clin Cancer Res 12 : 2101–2108.
26. StarcevicD, DalalS, SweasyJ (2005) Hinge residue Ile260 of DNA polymerase beta is important for enzyme activity and fidelity. Biochemistry 44 : 3775–3784.
27. JakubczakJL, MerlinoG, FrenchJE, MullerWJ, PaulB, et al. (1996) Analysis of genetic instability during mammary tumor progression using a novel selection-based assay for in vivo mutations in a bacteriophage lambda transgene target. Proc Natl Acad Sci U S A 93 : 9073–9078.
28. GlazerPM, SarkarSN, SummersWC (1986) Detection and analysis of UV-induced mutations in mammalian cell DNA using a lambda phage shuttle vector. Proc Natl Acad Sci U S A 83 : 1041–1044.
29. GuntherEJ, MurrayNE, GlazerPM (1993) High efficiency, restriction-deficient in vitro packaging extracts for bacteriophage lambda DNA using a new E.coli lysogen. Nucleic Acids Res 21 : 3903–3904.
30. SobolRW, HortonJK, KuhnR, GuH, SinghalRK, et al. (1996) Requirement of mammalian DNA polymerase-beta in base-excision repair. Nature 379 : 183–186.
31. SobolRW (2007) DNA polymerase beta null mouse embryonic fibroblasts harbor a homozygous null mutation in DNA polymerase iota. DNA Repair (Amst) 6 : 3–7.
32. LowyDR, RandsE, ScolnickEM (1978) Helper-independent transformation by unintegrated Harvey sarcoma virus DNA. J Virol 26 : 291–298.
33. OlivePL, BanathJP (2006) The comet assay: a method to measure DNA damage in individual cells. Nature protocols 1 : 23–29.
34. MurphyDL, KosaJ, JaegerJ, SweasyJB (2008) The Asp285 variant of DNA polymerase beta extends mispaired primer termini via increased nucleotide binding. Biochemistry 47 : 8048–8057.
35. MurphyDL, JaegerJ, SweasyJB (2011) A triad interaction in the fingers subdomain of DNA polymerase beta controls polymerase activity. J Am Chem Soc 133 : 6279–6287.
36. YamtichJ, StarcevicD, LauperJ, SmithE, ShiI, et al. (2010) Hinge residue I174 is critical for proper dNTP selection by DNA polymerase beta. Biochemistry 49 : 2326–2334.
37. DalalS, ChikovaA, JaegerJ, SweasyJB (2008) The Leu22Pro tumor-associated variant of DNA polymerase beta is dRP lyase deficient. Nucleic Acids Res 36 : 411–422.
Štítky
Genetika Reprodukční medicína
Článek The Covariate's DilemmaČlánek Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene SignallingČlánek Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 11- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- The Covariate's Dilemma
- Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene Signalling
- Plan B for Stimulating Stem Cell Division
- Discovering Thiamine Transporters as Targets of Chloroquine Using a Novel Functional Genomics Strategy
- Is a Modifier of Mutations in Retinitis Pigmentosa with Incomplete Penetrance
- Evolutionarily Ancient Association of the FoxJ1 Transcription Factor with the Motile Ciliogenic Program
- Genome Instability Caused by a Germline Mutation in the Human DNA Repair Gene
- Transcription Factor Oct1 Is a Somatic and Cancer Stem Cell Determinant
- Controls of Nucleosome Positioning in the Human Genome
- Disruption of Causes Defective Meiotic Recombination in Male Mice
- A Novel Human-Infection-Derived Bacterium Provides Insights into the Evolutionary Origins of Mutualistic Insect–Bacterial Symbioses
- Trps1 and Its Target Gene Regulate Epithelial Proliferation in the Developing Hair Follicle and Are Associated with Hypertrichosis
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Population-Based Resequencing of in 10,330 Individuals: Spectrum of Genetic Variation, Phenotype, and Comparison with Extreme Phenotype Approach
- HP1a Recruitment to Promoters Is Independent of H3K9 Methylation in
- Transcription Elongation and Tissue-Specific Somatic CAG Instability
- A Germline Polymorphism of DNA Polymerase Beta Induces Genomic Instability and Cellular Transformation
- Interallelic and Intergenic Incompatibilities of the () Gene in Mouse Hybrid Sterility
- Comparison of Mitochondrial Mutation Spectra in Ageing Human Colonic Epithelium and Disease: Absence of Evidence for Purifying Selection in Somatic Mitochondrial DNA Point Mutations
- Mutations in the Transcription Elongation Factor SPT5 Disrupt a Reporter for Dosage Compensation in Drosophila
- Evolution of Minimal Specificity and Promiscuity in Steroid Hormone Receptors
- Blockade of Pachytene piRNA Biogenesis Reveals a Novel Requirement for Maintaining Post-Meiotic Germline Genome Integrity
- RHOA Is a Modulator of the Cholesterol-Lowering Effects of Statin
- MIG-10 Functions with ABI-1 to Mediate the UNC-6 and SLT-1 Axon Guidance Signaling Pathways
- Loss of the DNA Methyltransferase MET1 Induces H3K9 Hypermethylation at PcG Target Genes and Redistribution of H3K27 Trimethylation to Transposons in
- Genome-Wide Association Studies Reveal a Simple Genetic Basis of Resistance to Naturally Coevolving Viruses in
- The Principal Genetic Determinants for Nasopharyngeal Carcinoma in China Involve the Class I Antigen Recognition Groove
- Molecular, Physiological, and Motor Performance Defects in DMSXL Mice Carrying >1,000 CTG Repeats from the Human DM1 Locus
- Genomic Study of RNA Polymerase II and III SNAP-Bound Promoters Reveals a Gene Transcribed by Both Enzymes and a Broad Use of Common Activators
- Long Telomeres Produced by Telomerase-Resistant Recombination Are Established from a Single Source and Are Subject to Extreme Sequence Scrambling
- The Yeast SR-Like Protein Npl3 Links Chromatin Modification to mRNA Processing
- Deubiquitylation Machinery Is Required for Embryonic Polarity in
- dJun and Vri/dNFIL3 Are Major Regulators of Cardiac Aging in Drosophila
- CtIP Is Required to Initiate Replication-Dependent Interstrand Crosslink Repair
- Notch-Mediated Suppression of TSC2 Expression Regulates Cell Differentiation in the Intestinal Stem Cell Lineage
- A Combination of H2A.Z and H4 Acetylation Recruits Brd2 to Chromatin during Transcriptional Activation
- Network Analysis of a -Mouse Model of Autosomal Dominant Polycystic Kidney Disease Identifies HNF4α as a Disease Modifier
- Mitosis in Neurons: Roughex and APC/C Maintain Cell Cycle Exit to Prevent Cytokinetic and Axonal Defects in Photoreceptor Neurons
- CELF4 Regulates Translation and Local Abundance of a Vast Set of mRNAs, Including Genes Associated with Regulation of Synaptic Function
- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- The Genomes of the Fungal Plant Pathogens and Reveal Adaptation to Different Hosts and Lifestyles But Also Signatures of Common Ancestry
- A Genome-Scale RNA–Interference Screen Identifies RRAS Signaling as a Pathologic Feature of Huntington's Disease
- Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
- Population Genomic Scan for Candidate Signatures of Balancing Selection to Guide Antigen Characterization in Malaria Parasites
- Tissue-Specific Regulation of Chromatin Insulator Function
- Disruption of Mouse Cenpj, a Regulator of Centriole Biogenesis, Phenocopies Seckel Syndrome
- Genome, Functional Gene Annotation, and Nuclear Transformation of the Heterokont Oleaginous Alga CCMP1779
- Antagonistic Gene Activities Determine the Formation of Pattern Elements along the Mediolateral Axis of the Fruit
- Lung eQTLs to Help Reveal the Molecular Underpinnings of Asthma
- Identification of the First ATRIP–Deficient Patient and Novel Mutations in ATR Define a Clinical Spectrum for ATR–ATRIP Seckel Syndrome
- Cooperativity of , , and in Malignant Breast Cancer Evolution
- Loss of Prohibitin Membrane Scaffolds Impairs Mitochondrial Architecture and Leads to Tau Hyperphosphorylation and Neurodegeneration
- Microhomology Directs Diverse DNA Break Repair Pathways and Chromosomal Translocations
- MicroRNA–Mediated Repression of the Seed Maturation Program during Vegetative Development in
- Selective Pressure Causes an RNA Virus to Trade Reproductive Fitness for Increased Structural and Thermal Stability of a Viral Enzyme
- The Tumor Suppressor Gene Retinoblastoma-1 Is Required for Retinotectal Development and Visual Function in Zebrafish
- Regions of Homozygosity in the Porcine Genome: Consequence of Demography and the Recombination Landscape
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
- Polyadenylation-Dependent Control of Long Noncoding RNA Expression by the Poly(A)-Binding Protein Nuclear 1
- A Unified Method for Detecting Secondary Trait Associations with Rare Variants: Application to Sequence Data
- Genetic and Biochemical Dissection of a HisKA Domain Identifies Residues Required Exclusively for Kinase and Phosphatase Activities
- Informed Conditioning on Clinical Covariates Increases Power in Case-Control Association Studies
- Biochemical Diversification through Foreign Gene Expression in Bdelloid Rotifers
- Genomic Variation and Its Impact on Gene Expression in
- Spastic Paraplegia Mutation N256S in the Neuronal Microtubule Motor KIF5A Disrupts Axonal Transport in a HSP Model
- Lamin B1 Polymorphism Influences Morphology of the Nuclear Envelope, Cell Cycle Progression, and Risk of Neural Tube Defects in Mice
- A Targeted Glycan-Related Gene Screen Reveals Heparan Sulfate Proteoglycan Sulfation Regulates WNT and BMP Trans-Synaptic Signaling
- Dopaminergic D2-Like Receptors Delimit Recurrent Cholinergic-Mediated Motor Programs during a Goal-Oriented Behavior
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání





