-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Lung eQTLs to Help Reveal the Molecular Underpinnings of Asthma
Genome-wide association studies (GWAS) have identified loci reproducibly associated with pulmonary diseases; however, the molecular mechanism underlying these associations are largely unknown. The objectives of this study were to discover genetic variants affecting gene expression in human lung tissue, to refine susceptibility loci for asthma identified in GWAS studies, and to use the genetics of gene expression and network analyses to find key molecular drivers of asthma. We performed a genome-wide search for expression quantitative trait loci (eQTL) in 1,111 human lung samples. The lung eQTL dataset was then used to inform asthma genetic studies reported in the literature. The top ranked lung eQTLs were integrated with the GWAS on asthma reported by the GABRIEL consortium to generate a Bayesian gene expression network for discovery of novel molecular pathways underpinning asthma. We detected 17,178 cis- and 593 trans- lung eQTLs, which can be used to explore the functional consequences of loci associated with lung diseases and traits. Some strong eQTLs are also asthma susceptibility loci. For example, rs3859192 on chr17q21 is robustly associated with the mRNA levels of GSDMA (P = 3.55×10−151). The genetic-gene expression network identified the SOCS3 pathway as one of the key drivers of asthma. The eQTLs and gene networks identified in this study are powerful tools for elucidating the causal mechanisms underlying pulmonary disease. This data resource offers much-needed support to pinpoint the causal genes and characterize the molecular function of gene variants associated with lung diseases.
Published in the journal: . PLoS Genet 8(11): e32767. doi:10.1371/journal.pgen.1003029
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003029Summary
Genome-wide association studies (GWAS) have identified loci reproducibly associated with pulmonary diseases; however, the molecular mechanism underlying these associations are largely unknown. The objectives of this study were to discover genetic variants affecting gene expression in human lung tissue, to refine susceptibility loci for asthma identified in GWAS studies, and to use the genetics of gene expression and network analyses to find key molecular drivers of asthma. We performed a genome-wide search for expression quantitative trait loci (eQTL) in 1,111 human lung samples. The lung eQTL dataset was then used to inform asthma genetic studies reported in the literature. The top ranked lung eQTLs were integrated with the GWAS on asthma reported by the GABRIEL consortium to generate a Bayesian gene expression network for discovery of novel molecular pathways underpinning asthma. We detected 17,178 cis- and 593 trans- lung eQTLs, which can be used to explore the functional consequences of loci associated with lung diseases and traits. Some strong eQTLs are also asthma susceptibility loci. For example, rs3859192 on chr17q21 is robustly associated with the mRNA levels of GSDMA (P = 3.55×10−151). The genetic-gene expression network identified the SOCS3 pathway as one of the key drivers of asthma. The eQTLs and gene networks identified in this study are powerful tools for elucidating the causal mechanisms underlying pulmonary disease. This data resource offers much-needed support to pinpoint the causal genes and characterize the molecular function of gene variants associated with lung diseases.
Introduction
Recent genome-wide association studies (GWAS) have identified loci that harbor susceptibility genes for asthma and other pulmonary conditions [1]–[16]. Many of the genes at these loci have unknown function and have not previously been considered biologically plausible candidates for disease pathogenesis. Extensive linkage disequilibrium (LD) within these loci makes it difficult to identify the actual susceptibility genes, let alone which genetic variants are responsible for altered expression or function of their protein products. Moreover, the associated polymorphisms can only explain a relatively small proportion of the variability of the phenotype in the population [9] and of its heritability [17].
Integrative genomics is a promising new approach to identify true causal genes and variants. By using gene expression as a phenotype and examining how DNA polymorphisms contribute to both gene expression (expression quantitative trait loci – eQTLs) and disease phenotype, true causal relationships can be discovered [18]–[20]. In the present study we performed genome-wide genotyping and lung-specific gene expression on a large dataset of lung tissue (1,111 human subjects) to explore effects of genetic variation on gene expression and their joint relationship to asthma. Although the lung tissue came from a heterogeneous group of subjects, the discovery of lung tissue eSNPs could elucidate the causal molecular pathways in a variety of pulmonary disorders.
Results
Demographic characteristics of study participants
Demographic and clinical data for the 1,111 patients who passed clinical, genotyping and gene expression quality control assessments are summarized in Table 1 according to the three sites of recruitment, Laval University, University of British Columbia, and University of Groningen (henceforth referred to as Laval, UBC, and Groningen, respectively). The majority of the subjects were smokers or former-smokers. There were some differences in the clinical characteristics across the sites including age, lung function and smoking status. To account for this heterogeneity between sites, we performed a meta-analysis rather than a pooled analysis for eQTL discovery. The overall analysis workflow is illustrated in Figure 1.
Fig. 1. Overall study design and analysis workflow. 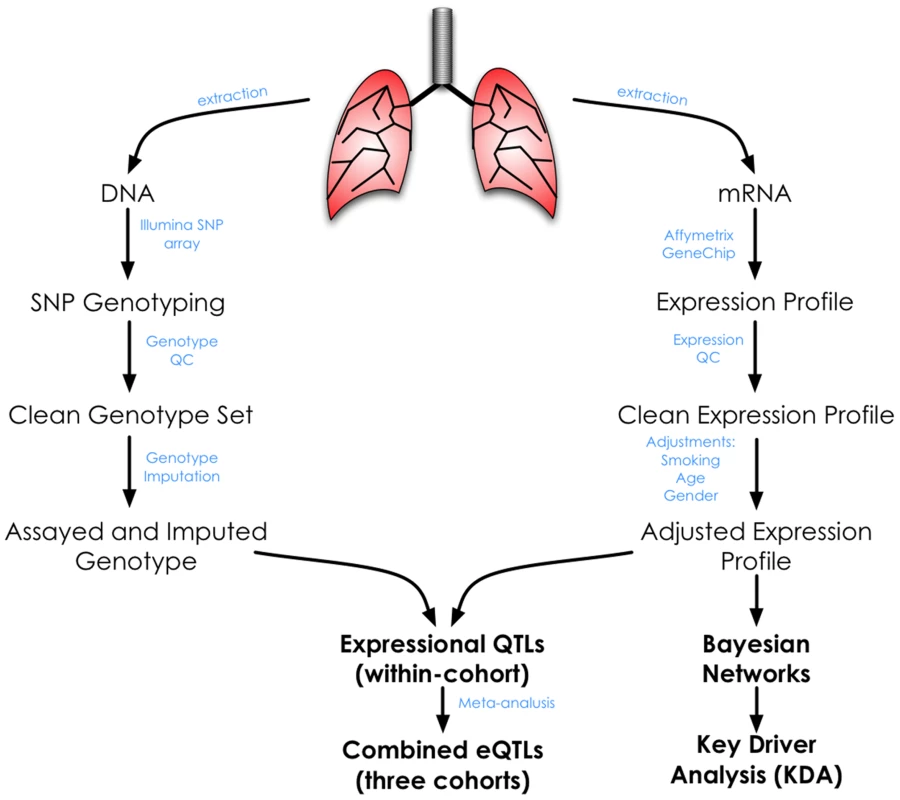
Tab. 1. Demographic and clinical characteristics of study cohort. 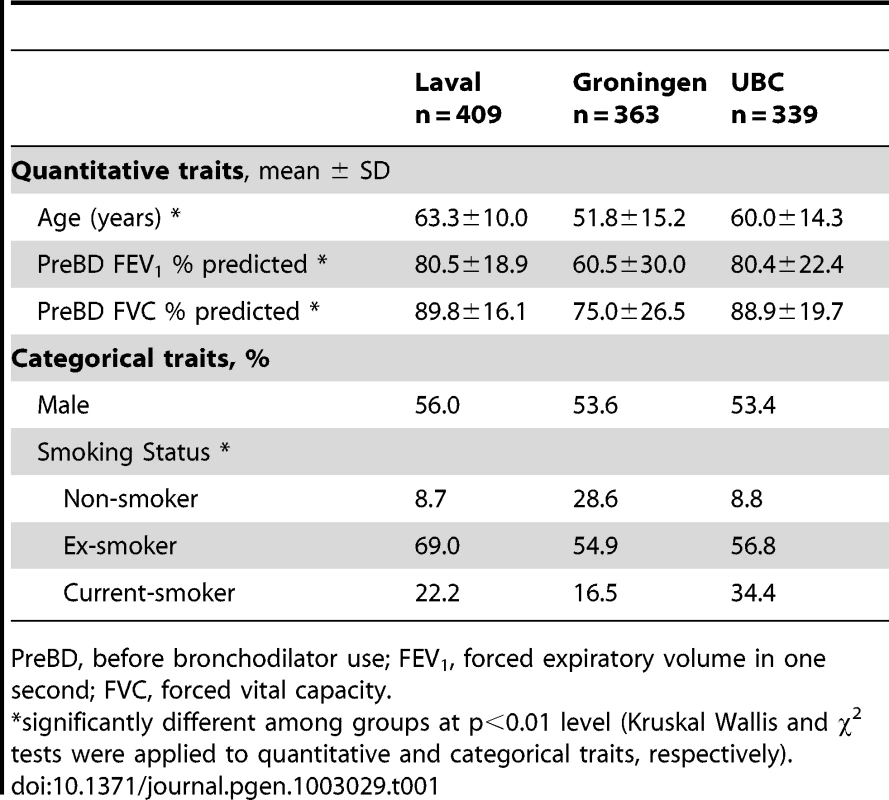
PreBD, before bronchodilator use; FEV1, forced expiratory volume in one second; FVC, forced vital capacity. Genome-wide association analysis for expression traits (eQTL)
We identified cis - and trans-acting eQTLs using established methods [19]. For simplicity we assumed that each transcript could have at most one cis-eQTL. We considered all association signals from SNPs within 1 Mb up and downstream, of the transcription probeset as a single cis-eQTL. Trans-eQTLs were defined as association signals from SNPs located greater than 1 Mb from the probeset. The eSNP was identified as the SNP that was most significantly associated (lowest P value) with the expression trait. A summary of the eQTLs identified at 10% false discovery rate (FDR) in the three cohorts as well as the meta-analysis are reported in Table 2. There were variations in the numbers of eQTLs across the cohorts. For example, we identified 10,630, 5,655 and 7,953 cis-eQTLs in the Laval, Groningen and UBC cohorts, respectively. There were also variations in the number of detectable trans-eQTLs (Table 2). The P values reported for the trans-eQTLs are lower than those for cis-eQTLs, owing to the higher statistical threshold required to achieve statistical significance. In general, a single SNP (eQTL peak) explained between 10 and 20% of the transcript's expression variance for cis - and trans-eQTLs, respectively (Table S1). However 6.8% of the cis acting SNPs explained more than 30% of the variance in the transcript and 15.3% of the trans acting SNPs explained more than 30% of a transcript's variance (Figure S1).
Tab. 2. Summary of expression QTLs detected at 10% false discovery rate. 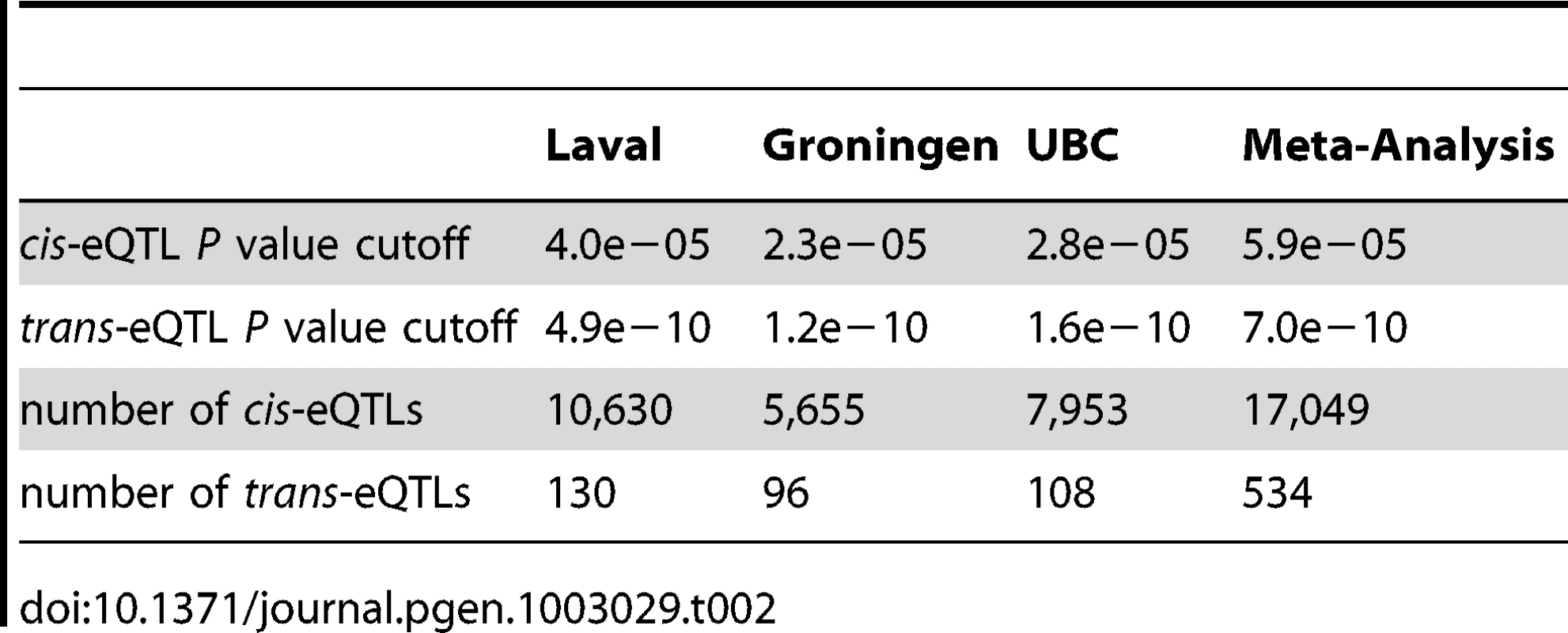
Further, we investigated the consistency of the eQTLs derived from the cohorts. Rather than simply comparing the existence of eQTLs, we adopted replication criteria similar to GWAS. A successful replication was defined as an eQTL in which the relationship between the same SNP and gene expression (with the same sign) was observed in more than one cohort at a P of ≤1×10−4. Using this criterion, 54.5% of the Laval cis-eQTLs were replicated in at least one other cohort and 79.5% of the Laval trans-eQTLs were replicated in another cohort. With respect to the Groningen eQTLs, the replication rates of the cis - and trans-eQTLs were 72.3% and 86.0%, respectively. The replication rates of the UBC cis - and trans-eQTLs were 65.4% and 90.5%, respectively. The eQTLs with higher R2values (variance of gene expression level explained) were more likely to be replicated than those with lower R2 values. There was a higher replication rate with trans-eQTLs than with cis-eQTLs.
The meta-analysis revealed many more eQTLs owing to the increased sample size (Table 2). We identified 17,049 cis-eQTLs and 534 trans-eQTL and the details of all eQTLs are listed in Table S2. 68.7% of these cis-eQTLs and 31.7% of trans-eQTLs were identified in at least one cohort, suggesting that the meta-analysis detected many additional eQTLs. The ‘non-combinability’ among cohorts is quantified in terms of Cochran's Q and the τ statistics (Table S2). Cochran's Q, was calculated as the weighted sum of squared differences among individual study effects and the pooled effect across studies, with the weights proportional to the inverse of variance of the eQTL effect. Q follows a chi-square statistic with k-1 degrees of freedom (k is the number of studies), and by these means, we calibrated the p value (termed Q.pvalue). We detected modest degrees of heterogeneity among the three cohorts. 16% of the QTLs had Q.pvalue<0.05 and 2% of the eQTLs showed Q.pvalue<0.001. We also present the τ statistic, which is the moment-based estimate of the between-study variance and is not dependent on the number of studies. Both the fixed effect and random effect meta-analysis results are presented in Table S2, and we used the fix effect results in the downstream analysis. We previously showed that the number of eQTLs detectable at a certain FDR was related directly to the sample size (i.e. statistical power) [21]. The meta-analysis yielded a greater relative increase in the number of trans activating eQTLs than of cis-eQTLs, suggesting that sample size is particularly important for detecting trans-eQTLs. Among the 51,627 non-control probesets on our array, 33.0% showed a cis-eQTL. 74.0%, 64.4%, 56.4% of the 51,627 probesets were “present” in at least 20%, 50%, and 80% of the tissue samples. Using these three cutoffs, 40.2%, 42.5%, and 43.4% of the “present” transcripts were found to be controlled by cis-eSNPs, respectively. Accordingly we believe that we have identified the majority of the strong lung cis-eQTLs.
As reported previously [22], [23], eQTLs were often found with the expression of more than one gene underlying a GWAS signal (Table 3). For example, the rs7216389-T allele on chromosome 17q was reported to increase asthma risk with an odds ratio (OR) of 1.45 [10]. This SNP was significantly associated with the expression levels of four genes, ORMDL3, GSDMA, GSDMB, and CRKRS (Figure 2a). Although ORMDL3 was originally suggested [10] to be the gene mediating rs7216389-T's association with asthma, this gene demonstrated the weakest eQTL signal of the four genes in our dataset. Rs7216389-T was positively associated with expression levels of ORMDL3 (P = 1×10−7), CRKRS (P = 1.76×10−9) and GSDMB (P = 4×10−15), consistent with the results of Moffat et al. [10] However, this same allele was inversely related to the level of expression of GSDMA (P = 8.78×10−25) (Figure 2b). The strongest eSNP in the 17q asthma susceptibility locus was rs3859192 located in intron 6 of the GSDMA gene governing the expression levels of this gene (P = 3.55×10−151) (Figure 2b).
Fig. 2. Lung eQTLs found on chromosome 17q21. 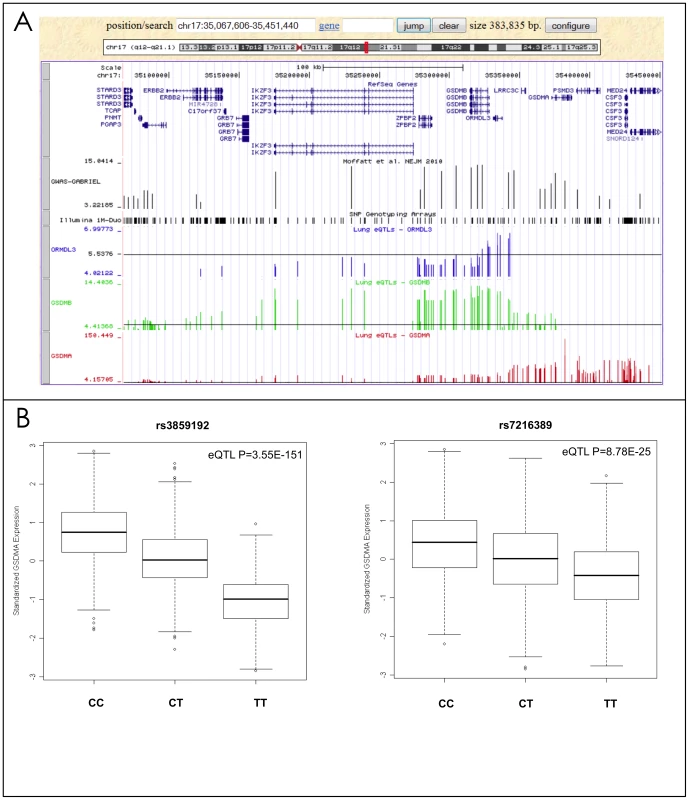
(a) The upper panel is a region of chromosome 17q21 from the UCSC browser showing the genes located in this region. The next panel shows the results from the GABRIEL consortium [9] (each bar represents a SNP and the y-axis shows −log10 P values for association with asthma). The following three panels show the lung eQTL results for expression levels of ORMDL3 (blue bars), GSDMB (green bars), and GSDMA (red bars), respectively. The y-axis represents −log10 P values derived from the meta-analysis of gene expression. The black horizontal lines are drawn at P = 0.05. (b) Boxplots of lung gene expression levels for GSDMA according to genotype groups for SNPs rs3859192 and rs7216389 in 1,111 subjects. Tab. 3. Expression eQTL underlying Asthma GWAS hits. 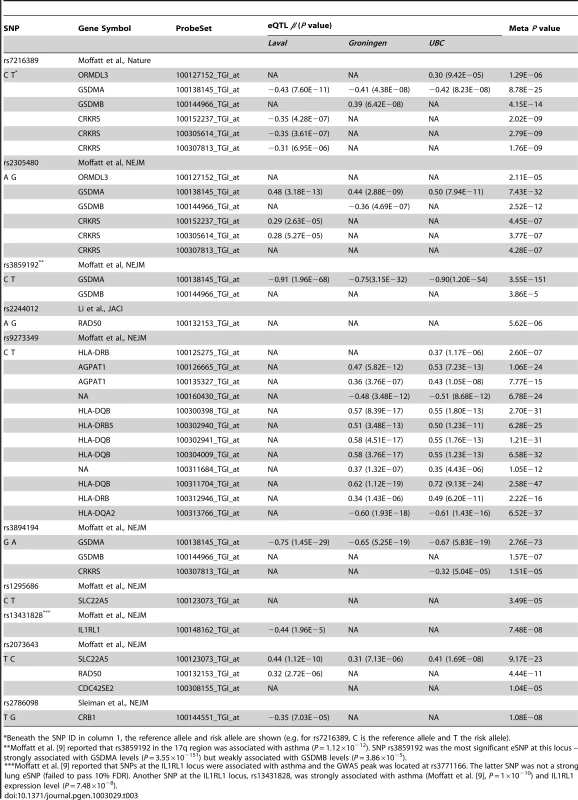
Beneath the SNP ID in column 1, the reference allele and risk allele are shown (e.g. for rs7216389, C is the reference allele and T the risk allele). To determine the cellular source and relative expression of GSDMB and GSDMA in human lung we performed real-time PCR, and Western blots on primary normal airway epithelial cells and immunohistochemistry on formalin-fixed normal lung tissue (see Text S1 for details). Figure 3 shows abundant mRNA and protein expression of GSDMA but little GSDMB from primary human lung epithelial cells (Figure 3a and 3b respectively). Figure 3c shows that GSDMA is expressed in both apical and basal airway epithelial cells in the conducting airways.
Fig. 3. Relative expression of GSDMA and GSDMB. 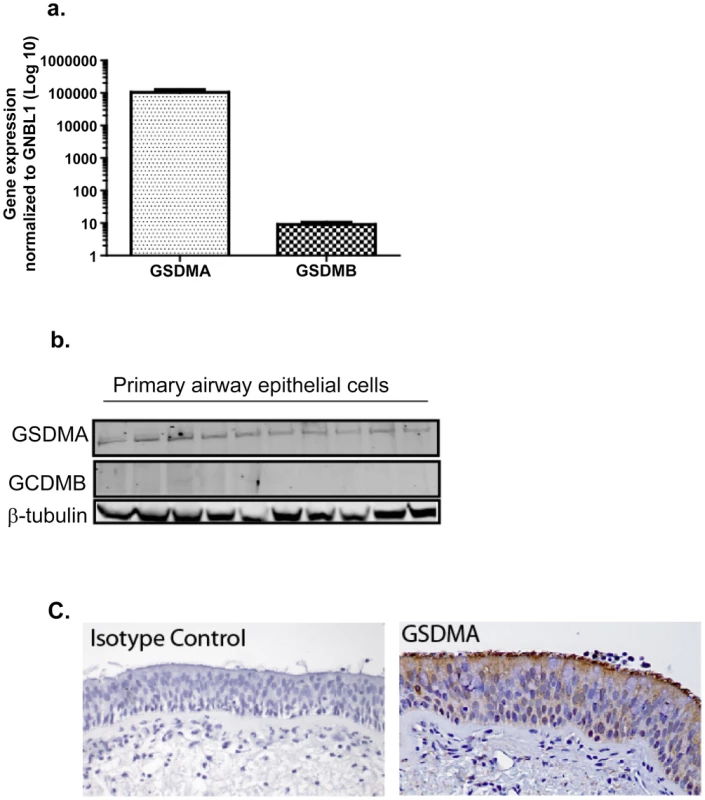
Primary human airway epithelial cells in monolayer culture were analyzed for; (a) the relative abundance of mRNA for GSDMA and GSDMB (n = 7) and (b) Protein expression of GSDMA and GSDMB by Western blot normalized to expression of β-tubulin (n = 10). (c) Representative image of GSDMA expression in human conducting airway by immunohistochemistry, which shows expression of GSDMA in both basal and apical cells within the airway epithelium. While most GWAS publications only report the top signals, the GABRIEL study [9] released the asthma association results on all SNPs investigated, allowing an in-depth analysis. For this analysis, we did not limit the list of eSNPs to the most significant SNP for each probeset; all cis eSNPs which passed the 10% FDR for any probe-set were considered. Using this strategy 60,530 of the 567,589 GABRIEL SNPs were eSNPs in lung tissue. These 60,530 eSNPs were enriched for significant association with asthma in the GABRIEL study (Figure 4). This is consistent with previous studies showing that SNPs associated with complex traits are more likely to be eQTLs [24].
Fig. 4. Q–Q plot for the GABRIEL meta-analysis among eSNPs found in the lung eQTL study. 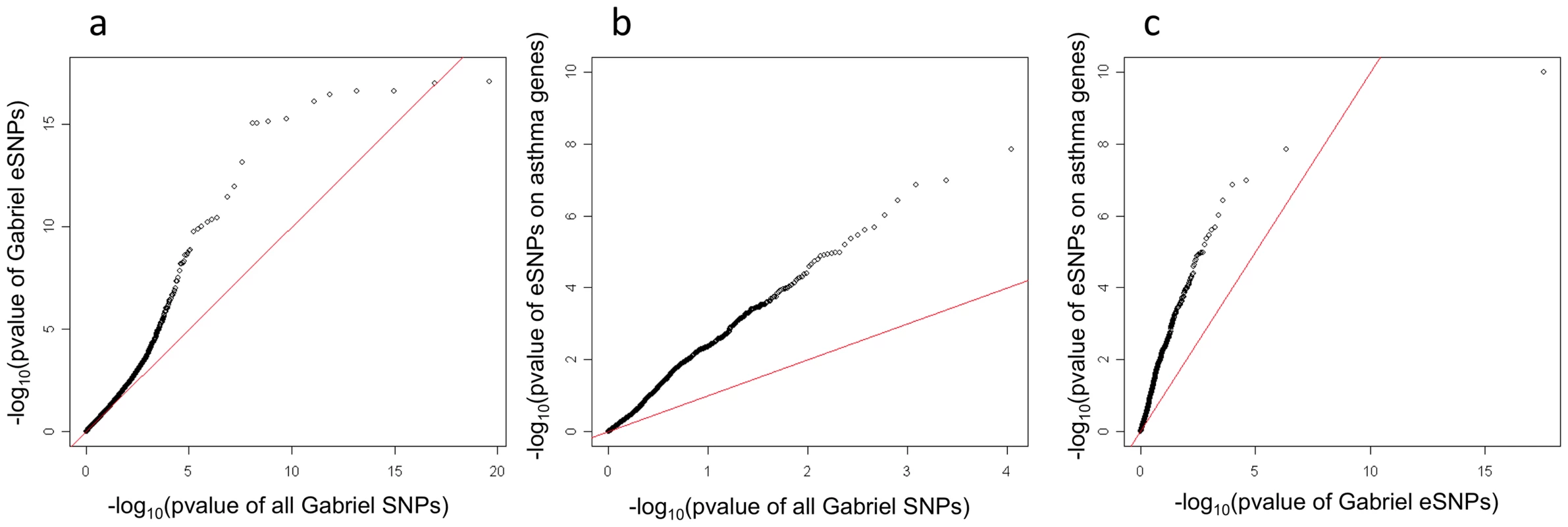
Among all SNPs surveyed by the GABRIEL meta-analysis, 60,530 were eSNPs in the Lung eQTL study and considered in this Q-Q plot. As is apparent eQTLs were enriched for lower P values (enrichment P value<2.2E−16). Panel (a) is GABRIEL eSNPs plotted against GABRIEL SNPs, panel (b) is eSNPs of canonical asthma genes plotted against GABRIEL SNPs and panel (c) is eSNPs of canonical asthma genes plotted against GABRIEL eSNPs. Key Driver Analyses (KDA) of networks
One of the drawbacks of GWAS is the reliance on a large number of statistical tests, which puts the threshold for significance at an extremely low level thereby increasing the chance of missing real associations. Given that asthma is a pulmonary disorder it is reasonable to assume that important molecular drivers are expressed in lung tissue. Therefore, instead of filtering primarily by P values to identify loci/genes that explain asthma, we filtered the loci in the GABRIEL [9] dataset by their status as a cis acting eSNP. Specifically, all SNPs from the GABRIEL study associated with asthma with P<0.01 were translated into genes via our lung eSNP list. A total of 7,613 SNPs were linked to the expression of 739 unique genes.
Using Bayesian networks constructed on gene expression data we attempted to find the molecular underpinnings of asthma. For this discussion we define a Bayesian network as a probabilistic graphical model that represents a set of random variables (gene expression in this case) and their conditional dependencies (edges) via a directed acyclic graph (see Text S1). The workflow is illustrated in Figure S2. We first identified each gene from the GABRIEL GWAS dataset and collected all genes within 3 edges of that gene on the Bayesian Networks and found the largest coherent sub-network (i.e. the largest network with an observable path between every gene in the network) that contained the highest proportion of GABRIEL genes (Figure S3). We choose to use 3 edges so that we had enough genes to lead to a reasonable biological annotation of the genes in the network, i.e. limiting to 2 edges is too restrictive and results in a small number of relevant genes; on the other hand, using 4 edges leads to broad gene sets and makes it hard to pinpoint pathways. From this sub-network, we performed Key Driver Analyses (KDA) to capture key regulators of asthma [25], [26]. KDA is a method that captures the “hub nodes” conditioning on the direction that one gene influences over other genes and isolating those genes with the strongest influence over the entire network. KDA determines the regulatory components in a directed network for a particular set of genes (i.e. the genes whose expression levels were control by GABRIEL GWAS SNPs). With this analysis, we identified well documented asthma candidate genes [27] that drive genes discovered in GABRIEL. These genes are denoted as canonical asthma genes. Figure 5 shows the top 6 key driver genes (yellow nodes) that control the asthma canonical genes (blue nodes) as well as other genes that are one edge away from the key driver genes (grey nodes). The network when annotated with GeneGo intuitively is described as “Immune Response” (the number of overlap genes is 25, from a 1,346 genes set, with the background being 16,606, P = 3.53×10−20). The KDA data in Table S3 shows the six genes that remain significant after adjustments for multiple testing.
Fig. 5. The meta-analysis Bayesian network with the top 6 KDA genes and their nearest neighbors. 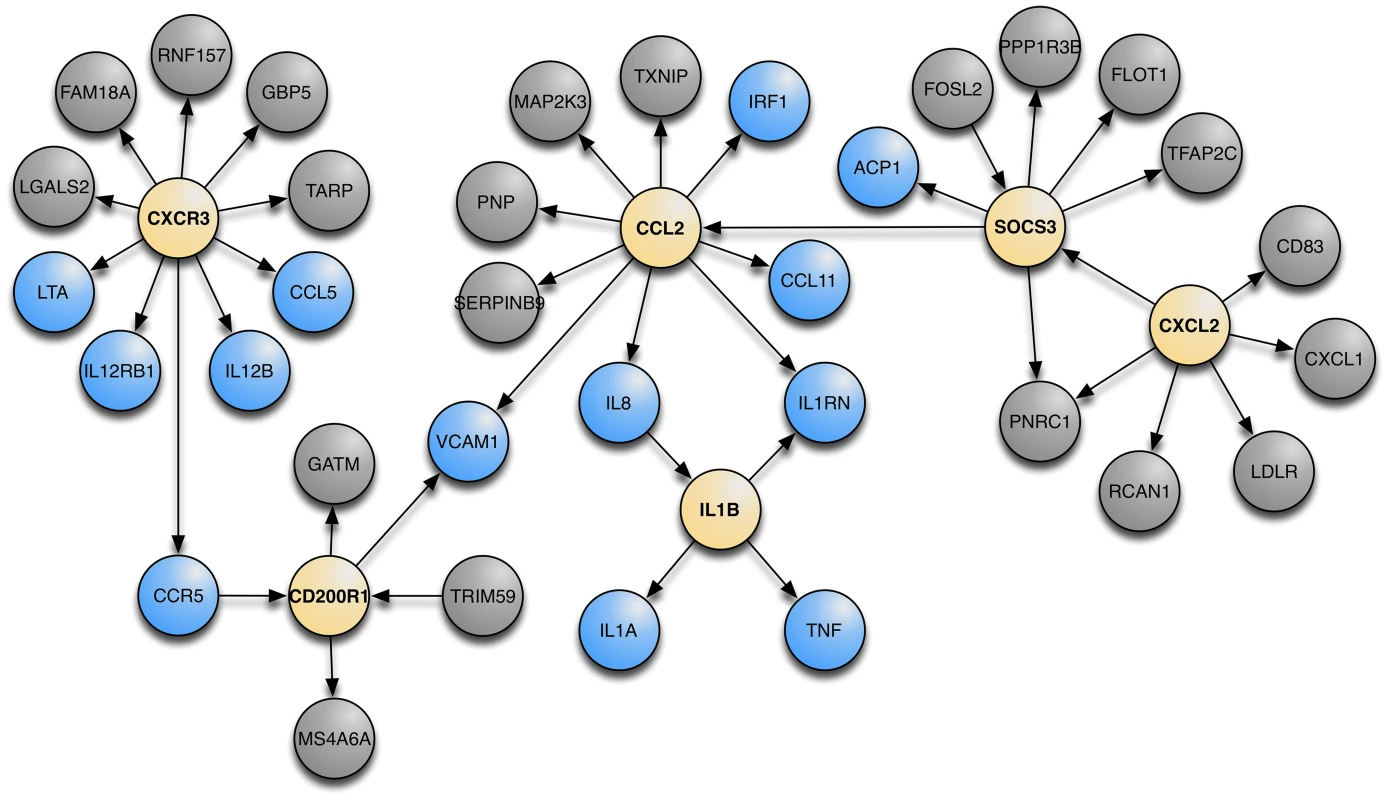
KDA genes are in yellow, canonical genes are in blue, and all other genes are in grey. P values for each of the key driver genes are in Table S3. Network biology
Networks provide a means for data integration. Above we used the networks to define a central node of the network that drives asthma by integrating lung Bayesian networks, asthma GWAS and literature (canonical genes) to arrive at set of genes that drive the asthma genes in the lung Bayesian network. Similarly we can use the Bayesian networks to rank competing genetic hypotheses around the molecular basis of asthma. It has been shown that the neighborhood milieu of proteins in a network can predict the probable function of a protein for which no function is known [28]. Following the same logic we assessed the sub-network surrounding each candidate causal gene for asthma. The gene with the highest connection to other asthma genes is most likely a causal asthma gene. Chromosomes 2q12 and 17q21 both have strong GWAS hits for asthma. However, these loci harbor a number of genes that could potentially explain these hits10,11. Following the workflow shown in Figure S4 [27], we drew inference on causal genes underlying asthma GWAS signals on these two chromosomes (Table 4). Clearly IL1RL1's subnetwork is enriched for canonical asthma genes (P = 1.8×10−07) making it the most likely gene driving the asthma association on 2q12. The result for 17q21 is less clear, but still points away from ORMDL3, the gene first suggested as causal by Moffatt et al. [10]. The sub-network around GSDMA is significantly enriched for canonical genes (P = 0.005) suggesting that GSDMA is the most likely driver behind the asthma association on 17q21.
Tab. 4. Driver genes behind the asthma association on 17q21 and 2q12. 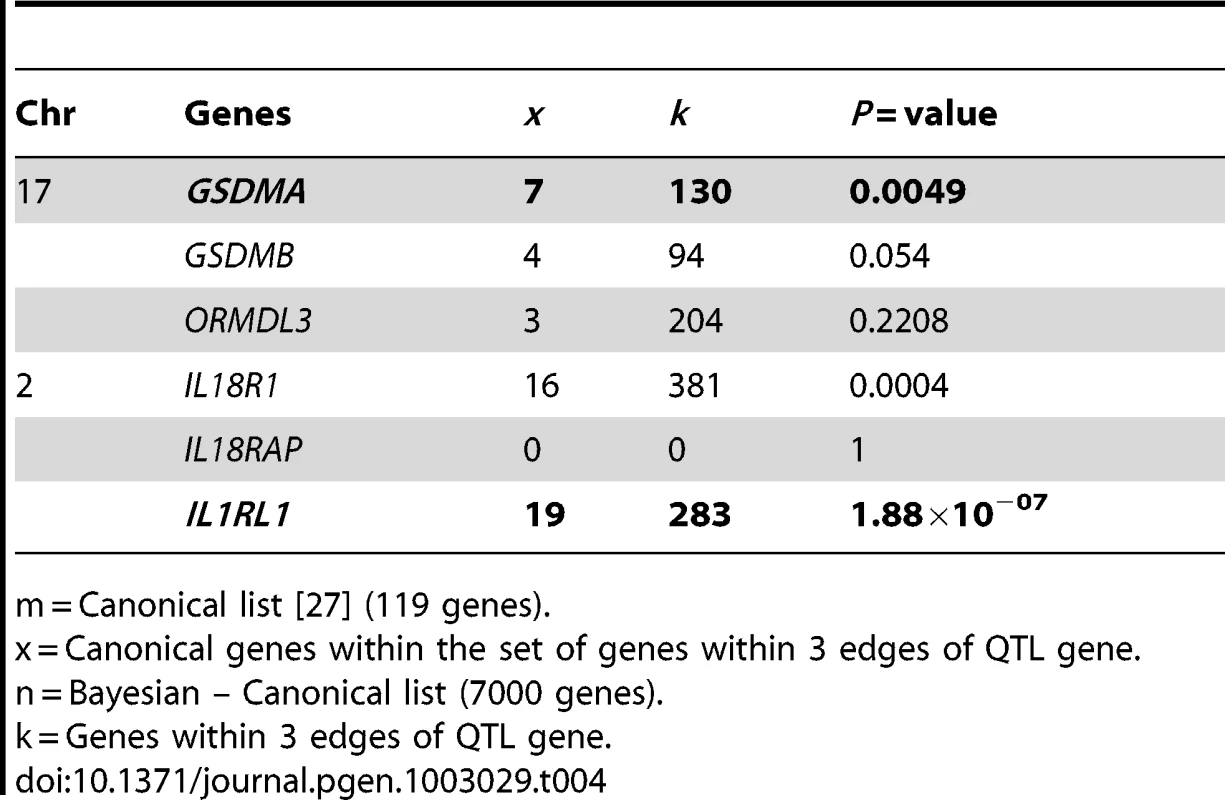
m = Canonical list [27] (119 genes). Discussion
One of the primary goals of genetic studies is to identify genes and pathways which contribute to susceptibility to human diseases and towards this goal many GWA studies have discovered SNPs in novel genes [1]–[16]. However, the mechanism by which these SNPs lead to disease susceptibility cannot be directly inferred from GWAS. The discovery of eQTLs has been shown to be a powerful tool in addressing this gap [18]–[20]. Although eQTL analysis of readily available tissue types such as peripheral blood leukocytes and lymphoblastoid cell lines has contributed to the understanding of how genes modulate risk of respiratory diseases, the discovery of lung-specific eQTL is probably more revealing in understanding the pathogenesis of respiratory disease. In this paper, we determined genetic variation and interrogated gene expression in a large collection (n = 1,111) of human lung samples to systematically characterize the genetic architecture of gene expression in this tissue. Owing to the large sample size, we discovered over 17,000 eQTLs. These eQTLs are gene variants that directly or indirectly govern gene expression in the lung and the data set represents a unique resource which we have made available for the use of lung researchers.
To illustrate the power of the resource we merged these data with SNPs known to be strongly associated with asthma and were able to identify the most likely causal gene variants. Chromosome 17q21 is the most consistent locus associated with asthma [9], [10], [29]–[36]. In the original GWAS study [10], the SNPs associated with asthma in the 17q21 susceptibility locus were also associated with transcript levels of ORMDL3 in lymphoblastoid cell lines, suggesting that ORDML3 was the causal gene. However, more refined analysis of the same samples, exploiting data generated by the 1000 genomes project, suggested that GSDMB, in close proximity to ORMDL3, could be the causal gene [9]. Another eQTL study performed in white blood cell RNA samples suggested that many SNPs in the 17q21 regions are associated with transcript levels of both ORMDL3 and GSDMB [32]. Verlaan et al. [37] showed that SNPs in the region demonstrate domain-wide cis-regulatory effects suggesting long-range chromatin interactions and they found allele-specific differences in nucleosome distribution and binding of the insulator protein CTCF. A recent study also showed that asthma risk alleles on 17q21 were associated with increased ORMDL3 and GSDMA gene expression and elevated IL-17 secretion in cord blood mononuclear cells [38]. To refine the causal gene/variant, we have interrogated our eQTL results within this locus. Interestingly, the SNPs most strongly associated with the expression of ORMDL3 are located in the promoter (rs4794820) and intron 1 (rs12603332) of the ORMDL3 gene, but the P values are not convincing (most significant SNP rs4794820 P = 1.0×10−7). In addition the SNP rs4794820 is more strongly associated with mRNA expression levels of GSDMB (P = 9.3×10−12) and GSDMA (P = 2.2×10−46). Rs4794820 is located between ORMDL3 and GSDMA on 17q21 (see Figure 2a for the location of genes). Considering the transcription orientation of ORMDL3, GSDMA, and GSDMB, rs4794820 lies in the promoter of the three genes and is possibly a pleiotropic regulatory variant or, more likely, in LD with such a regulatory element. We observed a concordance between SNPs associated with transcript levels of ORMDL3, GSDMB and GSDMA (Figure 2a). These genes are likely co-regulated, as proposed previously [32], [37]. However, the most compelling eQTL on 17q21 was observed in the association between the asthma susceptibility SNP (rs3859192 – p = 1.11−12 for association with asthma) and the level of expression of GSDMA (P = 3.55×10−151, Figure 2b). In fact, many SNPs on 17q21 were strongly associated with the expression of GSDMA (Figure 2a). Thus our data strongly suggest that the SNPs across this whole locus are associated with asthma because they modulate GSDMA expression in the lung, clearly showing the value of our lung eQTL dataset to refine previous GWAS hits for asthma. It should be noted that correlations between GWAS and eQTL results must be interpreted with caution. Here we related eQTLs in the lung to GWAS studies for asthma which is an airway disease but which is known to involve other tissue types including immune cells. The genetic control of gene expression is tissue specific. Regulatory variants for GSDMA identified in the lung may regulate a different gene(s) in another relevant tissue or cell-type for asthma. More functional work in multiple tissues and cell-types measuring gene expression with or without various stimuli will be required to confirm the causal asthma gene on 17q21.
The case for GSDMA as the susceptibility gene underlying the 17q GWAS signal is supported by biologic plausibility. GSDMA is a member of the gasdermin family of proteins first identified in the mouse [39]. They have been reported as being expressed in the upper gastrointestinal tract and skin where they are involved in the regulation of apoptosis and act as tumor suppressors [40]. Further support for GSDMA as an asthma susceptibility gene is our observation that GSDMA is robustly expressed in human airway epithelium (Figure 3). In addition its network neighborhood is enriched in genes involved in immune responses. We examined the genes around GSDMA using Bayesian and co-expression networks, and performed functional annotation of these genes (Table S4a–S4b). The GSDMA network neighborhood is enriched for a number of immune response pathways, which are highly relevant to asthma.
By integrating asthma GWAS results with our eSNP and Bayesian network, we were able to identify a network of 34 genes that highlights the molecular underpinnings of asthma (Figure 5 and Table S3). This network of genes is annotated as inflammatory response by the online tool DAVID [41] with an enrichment score of 3.27 (P = 7×10−6). Of particular interest is one Key Driver node, SOCS3 (suppressor of cytokine signaling 3). SOCS3 belongs to the SOCS family of genes that are cytokine-inducible negative regulators of cytokine signaling and play an important role in TH2-mediated allergic responses through control of the balance between TH1 and TH2 cells. It is implicated in both asthma and atopic dermatitis, as well as in regulating serum IgE levels [42]. Moriwaki and colleagues found that down-regulation of Socs3 in ovalbumin sensitized mice caused attenuation of eosinophilia and airway hyperresponsiveness generated by ovalbumin challenge [43].
Important discoveries in the field of asthma were made using eQTL mapping in lymphoblastoid cell lines [10]. In this study, we used lung tissue to map lung eQTLs, which is likely the most relevant tissue to study the genetics of lung diseases like asthma. Ideally one would want to relate SNPs to gene expression and to disease phenotype using tissue or cells from individuals affected by the disease of interest [44]. In this study, only a small percentage of the subjects had self-reported asthma. It should be noted that eQTL mapping in liver tissues was successful to identify new susceptibility genes for type 1 diabetes, coronary artery disease and blood lipid levels [19], [20], [45]. These discoveries were made regardless of the medical condition of patients from whom the liver tissues were explanted. Similarly, lung tissue from any source (normal or diseased) is a valid approach to identify the genetic variants that influence gene expression irrespective of disease status. The heterogeneous nature of the tissue we profiled with varying environmental exposures (e.g. smoking) and disease status (e.g. lung cancer) would not be expected to generate associations between SNPs and gene expression but would rather tend to introduce noise into these relationships.
In summary, the present study reports on a comprehensive set of lung eQTLs that complement previous large scale studies of eQTLs in other tissue types [18], [19] and which can be used to shed light on GWAS findings in lung diseases. Using the results of the largest asthma GWA study as an example we show how the lung tissue eQTL dataset can be used to identify the most likely causal genes and pathways. This dataset constitutes an invaluable tool to provide new insights into the pathogenesis of other lung diseases such as chronic obstructive pulmonary disease, lung cancer and cystic fibrosis.
Materials and Methods
Ethics statement
All lung tissue samples were obtained in accordance with Institutional Review Board guidelines at the three sites: Laval University (Quebec, Canada), University of British-Columbia (Vancouver, Canada) and Groningen University (Groningen, The Netherlands). All patients provided written informed consent and the study was approved by the ethics committees of the Institut universitaire de cardiologie et de pneumologie de Québec and the UBC-Providence Health Care Research Institute Ethics Board for Laval and UBC, respectively. The study protocol was consistent with the Research Code of the University Medical Center Groningen and Dutch national ethical and professional guidelines (“Code of conduct; Dutch federation of biomedical scientific societies”; http://www.federa.org).
Lung tissues collection
Lung tissue was collected from patients who underwent lung resectional surgery at the three participating sites; Laval University, University of British Columbia, and University of Groningen. Lung specimens from the Laval site were taken from the Respiratory Health Network Tissue Bank of the FRQS (www.rsr.chus.qc.ca). Subjects' enrollment and lung tissues processing at the three sites are described in Text S1.
Genome-wide gene expression and genotyping
Gene expression profiles were obtained using a custom Affymetrix array (see GEO platform GPL10379) testing 51,627 non-control probesets. DNA samples were genotyped on the Illumina Human1M-Duo BeadChip array. 409, 363, and 339 patients had both genotyping and gene expression data that passed standard quality controls (see Text S1) in Laval, Groningen, and UBC, respectively. These final datasets (total n = 1,111) were used to discover eQTLs and to identify eSNPs.
Statistical analysis
The normalized expression data were adjusted for age, gender and smoking status in a robust linear model to accommodate potential outliers in expression level (Figure 1). In parallel, we performed genotype QC to exclude SNPs of low call rate (<0.9) and deviating from Hardy-Weinberg equilibrium (P<1×10−6). Genotype imputations were based on the cleaned sets. In the end, assayed and imputed genotypes were used to identify cis and trans acting expression quantitative trait loci (eQTLs) following a method similar to that previously described in Schadt et al. [19]. The strategy for assembling of the asthma candidate gene list (Table S5) and for constructing Bayesian networks, and co-expression modules are given in Text S1.
Supporting Information
Zdroje
1. ChoudhryS, TaubM, MeiR, Rodriguez-SantanaJ, Rodriguez-CintronW, et al. (2008) Genome-wide screen for asthma in Puerto Ricans: evidence for association with 5q23 region. Hum Genet 123 : 455–468.
2. DeWanAT, TricheEW, XuX, HsuLI, ZhaoC, et al. (2010) PDE11A associations with asthma: results of a genome-wide association scan. J Allergy Clin Immunol 126 : 871–e879, 871-873, e879.
3. GudbjartssonDF, BjornsdottirUS, HalapiE, HelgadottirA, SulemP, et al. (2009) Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet 41 : 342–347.
4. HancockDB, RomieuI, ShiM, Sienra-MongeJJ, WuH, et al. (2009) Genome-wide association study implicates chromosome 9q21.31 as a susceptibility locus for asthma in mexican children. PLoS Genet 5: e1000623.
5. HimesBE, HunninghakeGM, BaurleyJW, RafaelsNM, SleimanP, et al. (2009) Genome-wide association analysis identifies PDE4D as an asthma-susceptibility gene. Am J Hum Genet 84 : 581–593.
6. HuiJ, OkaA, JamesA, PalmerLJ, MuskAW, et al. (2008) A genome-wide association scan for asthma in a general Australian population. Hum Genet 123 : 297–306.
7. KimSH, ChoBY, ParkCS, ShinES, ChoEY, et al. (2009) Alpha-T-catenin (CTNNA3) gene was identified as a risk variant for toluene diisocyanate-induced asthma by genome-wide association analysis. Clin Exp Allergy 39 : 203–212.
8. MathiasRA, GrantAV, RafaelsN, HandT, GaoL, et al. (2010) A genome-wide association study on African-ancestry populations for asthma. J Allergy Clin Immunol 125 : 336–e334, 336-346, e334.
9. MoffattMF, GutIG, DemenaisF, StrachanDP, BouzigonE, et al. (2010) A large-scale, consortium-based genomewide association study of asthma. N Engl J Med 363 : 1211–1221.
10. MoffattMF, KabeschM, LiangL, DixonAL, StrachanD, et al. (2007) Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature 448 : 470–473.
11. OberC, TanZ, SunY, PossickJD, PanL, et al. (2008) Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med 358 : 1682–1691.
12. SleimanPM, FloryJ, ImielinskiM, BradfieldJP, AnnaiahK, et al. (2010) Variants of DENND1B associated with asthma in children. N Engl J Med 362 : 36–44.
13. WeidingerS, GiegerC, RodriguezE, BaurechtH, MempelM, et al. (2008) Genome-wide scan on total serum IgE levels identifies FCER1A as novel susceptibility locus. PLoS Genet 4: e1000166.
14. HirotaT, TakahashiA, KuboM, TsunodaT, TomitaK, et al. (2011) Genome-wide association study identifies three new susceptibility loci for adult asthma in the Japanese population. Nat Genet
15. TorgersonDG, AmplefordEJ, ChiuGY, GaudermanWJ, GignouxCR, et al. (2011) Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet
16. NoguchiE, SakamotoH, HirotaT, OchiaiK, ImotoY, et al. (2011) Genome-Wide Association Study Identifies HLA-DP as a Susceptibility Gene for Pediatric Asthma in Asian Populations. PLoS Genet 7: e1002170.
17. ManolioTA, CollinsFS, CoxNJ, GoldsteinDB, HindorffLA, et al. (2009) Finding the missing heritability of complex diseases. Nature 461 : 747–753.
18. EmilssonV, ThorleifssonG, ZhangB, LeonardsonAS, ZinkF, et al. (2008) Genetics of gene expression and its effect on disease. Nature 452 : 423–428.
19. SchadtEE, MolonyC, ChudinE, HaoK, YangX, et al. (2008) Mapping the genetic architecture of gene expression in human liver. PLoS Biol 6: e107.
20. TeslovichTM, MusunuruK, SmithAV, EdmondsonAC, StylianouIM, et al. (2010) Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466 : 707–713.
21. HaoK, SchadtEE, StoreyJD (2008) Calibrating the performance of SNP arrays for whole-genome association studies. PLoS Genet 4: e1000109.
22. MontgomerySB, DermitzakisET (2011) From expression QTLs to personalized transcriptomics. Nat Rev Genet 12 : 277–282.
23. MurphyA, ChuJH, XuM, CareyVJ, LazarusR, et al. (2010) Mapping of numerous disease-associated expression polymorphisms in primary peripheral blood CD4+ lymphocytes. Hum Mol Genet 19 : 4745–4757.
24. NicolaeDL, GamazonE, ZhangW, DuanS, DolanME, et al. (2010) Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genet 6: e1000888.
25. TranL, ZhangB, ZhangZ, ZhangC, XieT, et al. (2011) Inferring Causal Genomic Alterations in Breast Cancer Using Gene Expression Data. BMC Systems Biology 5 : 121.
26. ZhuJ, ZhangB, SmithEN, DreesB, BremRB, et al. (2008) Integrating large-scale functional genomic data to dissect the complexity of yeast regulatory networks. Nat Genet 40 : 854–861.
27. BosséY, HudsonTJ (2007) Toward a comprehensive set of asthma susceptibility genes. Annu Rev Med 58 : 171–184.
28. SharanR, UlitskyI, ShamirR (2007) Network-based prediction of protein function. Mol Syst Biol 3 : 88.
29. BisgaardH, BonnelykkeK, SleimanPM, BrasholtM, ChawesB, et al. (2009) Chromosome 17q21 gene variants are associated with asthma and exacerbations but not atopy in early childhood. Am J Respir Crit Care Med 179 : 179–185.
30. FerreiraMA, McRaeAF, MedlandSE, NyholtDR, GordonSD, et al. (2010) Association between ORMDL3, IL1RL1 and a deletion on chromosome 17q21 with asthma risk in Australia. Eur J Hum Genet
31. GalanterJ, ChoudhryS, EngC, NazarioS, Rodriguez-SantanaJR, et al. (2008) ORMDL3 gene is associated with asthma in three ethnically diverse populations. Am J Respir Crit Care Med 177 : 1194–1200.
32. HalapiE, GudbjartssonDF, JonsdottirGM, BjornsdottirUS, ThorleifssonG, et al. (2010) A sequence variant on 17q21 is associated with age at onset and severity of asthma. Eur J Hum Genet 18 : 902–908.
33. MadoreAM, TremblayK, HudsonTJ, LapriseC (2008) Replication of an association between 17q21 SNPs and asthma in a French-Canadian familial collection. Hum Genet 123 : 93–95.
34. SleimanPM, AnnaiahK, ImielinskiM, BradfieldJP, KimCE, et al. (2008) ORMDL3 variants associated with asthma susceptibility in North Americans of European ancestry. J Allergy Clin Immunol 122 : 1225–1227.
35. TavendaleR, MacgregorDF, MukhopadhyayS, PalmerCN (2008) A polymorphism controlling ORMDL3 expression is associated with asthma that is poorly controlled by current medications. J Allergy Clin Immunol 121 : 860–863.
36. WuH, RomieuI, Sienra-MongeJJ, LiH, del Rio-NavarroBE, et al. (2009) Genetic variation in ORM1-like 3 (ORMDL3) and gasdermin-like (GSDML) and childhood asthma. Allergy 64 : 629–635.
37. VerlaanDJ, BerlivetS, HunninghakeGM, MadoreAM, LariviereM, et al. (2009) Allele-specific chromatin remodeling in the ZPBP2/GSDMB/ORMDL3 locus associated with the risk of asthma and autoimmune disease. Am J Hum Genet 85 : 377–393.
38. LluisA, SchedelM, LiuJ, IlliS, DepnerM, et al. (2011) Asthma-associated polymorphisms in 17q21 influence cord blood ORMDL3 and GSDMA gene expression and IL-17 secretion. J Allergy Clin Immunol 127 : 1587–e1586, 1587-1594, e1586.
39. SaekiN, KuwaharaY, SasakiH, SatohH, ShiroishiT (2000) Gasdermin (Gsdm) localizing to mouse Chromosome 11 is predominantly expressed in upper gastrointestinal tract but significantly suppressed in human gastric cancer cells. Mamm Genome 11 : 718–724.
40. SaekiN, UsuiT, AoyagiK, KimDH, SatoM, et al. (2009) Distinctive expression and function of four GSDM family genes (GSDMA-D) in normal and malignant upper gastrointestinal epithelium. Genes Chromosomes Cancer 48 : 261–271.
41. Huang daW, ShermanBT, LempickiRA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4 : 44–57.
42. SekiY, InoueH, NagataN, HayashiK, FukuyamaS, et al. (2003) SOCS-3 regulates onset and maintenance of T(H)2-mediated allergic responses. Nat Med 9 : 1047–1054.
43. MoriwakiA, InoueH, NakanoT, MatsunagaY, MatsunoY, et al. (2011) T cell treatment with small interfering RNA for suppressor of cytokine signaling 3 modulates allergic airway responses in a murine model of asthma. Am J Respir Cell Mol Biol 44 : 448–455.
44. HernandezDG, NallsMA, MooreM, ChongS, DillmanA, et al. (2012) Integration of GWAS SNPs and tissue specific expression profiling reveal discrete eQTLs for human traits in blood and brain. Neurobiol Dis 47 : 20–28.
45. MusunuruK, StrongA, Frank-KamenetskyM, LeeNE, AhfeldtT, et al. (2010) From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature 466 : 714–719.
Štítky
Genetika Reprodukční medicína
Článek The Covariate's DilemmaČlánek Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene SignallingČlánek Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 11- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- The Covariate's Dilemma
- Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene Signalling
- Plan B for Stimulating Stem Cell Division
- Discovering Thiamine Transporters as Targets of Chloroquine Using a Novel Functional Genomics Strategy
- Is a Modifier of Mutations in Retinitis Pigmentosa with Incomplete Penetrance
- Evolutionarily Ancient Association of the FoxJ1 Transcription Factor with the Motile Ciliogenic Program
- Genome Instability Caused by a Germline Mutation in the Human DNA Repair Gene
- Transcription Factor Oct1 Is a Somatic and Cancer Stem Cell Determinant
- Controls of Nucleosome Positioning in the Human Genome
- Disruption of Causes Defective Meiotic Recombination in Male Mice
- A Novel Human-Infection-Derived Bacterium Provides Insights into the Evolutionary Origins of Mutualistic Insect–Bacterial Symbioses
- Trps1 and Its Target Gene Regulate Epithelial Proliferation in the Developing Hair Follicle and Are Associated with Hypertrichosis
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Population-Based Resequencing of in 10,330 Individuals: Spectrum of Genetic Variation, Phenotype, and Comparison with Extreme Phenotype Approach
- HP1a Recruitment to Promoters Is Independent of H3K9 Methylation in
- Transcription Elongation and Tissue-Specific Somatic CAG Instability
- A Germline Polymorphism of DNA Polymerase Beta Induces Genomic Instability and Cellular Transformation
- Interallelic and Intergenic Incompatibilities of the () Gene in Mouse Hybrid Sterility
- Comparison of Mitochondrial Mutation Spectra in Ageing Human Colonic Epithelium and Disease: Absence of Evidence for Purifying Selection in Somatic Mitochondrial DNA Point Mutations
- Mutations in the Transcription Elongation Factor SPT5 Disrupt a Reporter for Dosage Compensation in Drosophila
- Evolution of Minimal Specificity and Promiscuity in Steroid Hormone Receptors
- Blockade of Pachytene piRNA Biogenesis Reveals a Novel Requirement for Maintaining Post-Meiotic Germline Genome Integrity
- RHOA Is a Modulator of the Cholesterol-Lowering Effects of Statin
- MIG-10 Functions with ABI-1 to Mediate the UNC-6 and SLT-1 Axon Guidance Signaling Pathways
- Loss of the DNA Methyltransferase MET1 Induces H3K9 Hypermethylation at PcG Target Genes and Redistribution of H3K27 Trimethylation to Transposons in
- Genome-Wide Association Studies Reveal a Simple Genetic Basis of Resistance to Naturally Coevolving Viruses in
- The Principal Genetic Determinants for Nasopharyngeal Carcinoma in China Involve the Class I Antigen Recognition Groove
- Molecular, Physiological, and Motor Performance Defects in DMSXL Mice Carrying >1,000 CTG Repeats from the Human DM1 Locus
- Genomic Study of RNA Polymerase II and III SNAP-Bound Promoters Reveals a Gene Transcribed by Both Enzymes and a Broad Use of Common Activators
- Long Telomeres Produced by Telomerase-Resistant Recombination Are Established from a Single Source and Are Subject to Extreme Sequence Scrambling
- The Yeast SR-Like Protein Npl3 Links Chromatin Modification to mRNA Processing
- Deubiquitylation Machinery Is Required for Embryonic Polarity in
- dJun and Vri/dNFIL3 Are Major Regulators of Cardiac Aging in Drosophila
- CtIP Is Required to Initiate Replication-Dependent Interstrand Crosslink Repair
- Notch-Mediated Suppression of TSC2 Expression Regulates Cell Differentiation in the Intestinal Stem Cell Lineage
- A Combination of H2A.Z and H4 Acetylation Recruits Brd2 to Chromatin during Transcriptional Activation
- Network Analysis of a -Mouse Model of Autosomal Dominant Polycystic Kidney Disease Identifies HNF4α as a Disease Modifier
- Mitosis in Neurons: Roughex and APC/C Maintain Cell Cycle Exit to Prevent Cytokinetic and Axonal Defects in Photoreceptor Neurons
- CELF4 Regulates Translation and Local Abundance of a Vast Set of mRNAs, Including Genes Associated with Regulation of Synaptic Function
- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- The Genomes of the Fungal Plant Pathogens and Reveal Adaptation to Different Hosts and Lifestyles But Also Signatures of Common Ancestry
- A Genome-Scale RNA–Interference Screen Identifies RRAS Signaling as a Pathologic Feature of Huntington's Disease
- Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
- Population Genomic Scan for Candidate Signatures of Balancing Selection to Guide Antigen Characterization in Malaria Parasites
- Tissue-Specific Regulation of Chromatin Insulator Function
- Disruption of Mouse Cenpj, a Regulator of Centriole Biogenesis, Phenocopies Seckel Syndrome
- Genome, Functional Gene Annotation, and Nuclear Transformation of the Heterokont Oleaginous Alga CCMP1779
- Antagonistic Gene Activities Determine the Formation of Pattern Elements along the Mediolateral Axis of the Fruit
- Lung eQTLs to Help Reveal the Molecular Underpinnings of Asthma
- Identification of the First ATRIP–Deficient Patient and Novel Mutations in ATR Define a Clinical Spectrum for ATR–ATRIP Seckel Syndrome
- Cooperativity of , , and in Malignant Breast Cancer Evolution
- Loss of Prohibitin Membrane Scaffolds Impairs Mitochondrial Architecture and Leads to Tau Hyperphosphorylation and Neurodegeneration
- Microhomology Directs Diverse DNA Break Repair Pathways and Chromosomal Translocations
- MicroRNA–Mediated Repression of the Seed Maturation Program during Vegetative Development in
- Selective Pressure Causes an RNA Virus to Trade Reproductive Fitness for Increased Structural and Thermal Stability of a Viral Enzyme
- The Tumor Suppressor Gene Retinoblastoma-1 Is Required for Retinotectal Development and Visual Function in Zebrafish
- Regions of Homozygosity in the Porcine Genome: Consequence of Demography and the Recombination Landscape
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
- Polyadenylation-Dependent Control of Long Noncoding RNA Expression by the Poly(A)-Binding Protein Nuclear 1
- A Unified Method for Detecting Secondary Trait Associations with Rare Variants: Application to Sequence Data
- Genetic and Biochemical Dissection of a HisKA Domain Identifies Residues Required Exclusively for Kinase and Phosphatase Activities
- Informed Conditioning on Clinical Covariates Increases Power in Case-Control Association Studies
- Biochemical Diversification through Foreign Gene Expression in Bdelloid Rotifers
- Genomic Variation and Its Impact on Gene Expression in
- Spastic Paraplegia Mutation N256S in the Neuronal Microtubule Motor KIF5A Disrupts Axonal Transport in a HSP Model
- Lamin B1 Polymorphism Influences Morphology of the Nuclear Envelope, Cell Cycle Progression, and Risk of Neural Tube Defects in Mice
- A Targeted Glycan-Related Gene Screen Reveals Heparan Sulfate Proteoglycan Sulfation Regulates WNT and BMP Trans-Synaptic Signaling
- Dopaminergic D2-Like Receptors Delimit Recurrent Cholinergic-Mediated Motor Programs during a Goal-Oriented Behavior
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



