-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
MIG-10 Functions with ABI-1 to Mediate the UNC-6 and SLT-1 Axon Guidance Signaling Pathways
Extracellular guidance cues steer axons towards their targets by eliciting morphological changes in the growth cone. A key part of this process is the asymmetric recruitment of the cytoplasmic scaffolding protein MIG-10 (lamellipodin). MIG-10 is thought to asymmetrically promote outgrowth by inducing actin polymerization. However, the mechanism that links MIG-10 to actin polymerization is not known. We have identified the actin regulatory protein ABI-1 as a partner for MIG-10 that can mediate its outgrowth-promoting activity. The SH3 domain of ABI-1 binds to MIG-10, and loss of function of either of these proteins causes similar axon guidance defects. Like MIG-10, ABI-1 functions in both the attractive UNC-6 (netrin) pathway and the repulsive SLT-1 (slit) pathway. Dosage sensitive genetic interactions indicate that MIG-10 functions with ABI-1 and WVE-1 to mediate axon guidance. Epistasis analysis reveals that ABI-1 and WVE-1 function downstream of MIG-10 to mediate its outgrowth-promoting activity. Moreover, experiments with cultured mammalian cells suggest that the interaction between MIG-10 and ABI-1 mediates a conserved mechanism that promotes formation of lamellipodia. Together, these observations suggest that MIG-10 interacts with ABI-1 and WVE-1 to mediate the UNC-6 and SLT-1 guidance pathways.
Published in the journal: . PLoS Genet 8(11): e32767. doi:10.1371/journal.pgen.1003054
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003054Summary
Extracellular guidance cues steer axons towards their targets by eliciting morphological changes in the growth cone. A key part of this process is the asymmetric recruitment of the cytoplasmic scaffolding protein MIG-10 (lamellipodin). MIG-10 is thought to asymmetrically promote outgrowth by inducing actin polymerization. However, the mechanism that links MIG-10 to actin polymerization is not known. We have identified the actin regulatory protein ABI-1 as a partner for MIG-10 that can mediate its outgrowth-promoting activity. The SH3 domain of ABI-1 binds to MIG-10, and loss of function of either of these proteins causes similar axon guidance defects. Like MIG-10, ABI-1 functions in both the attractive UNC-6 (netrin) pathway and the repulsive SLT-1 (slit) pathway. Dosage sensitive genetic interactions indicate that MIG-10 functions with ABI-1 and WVE-1 to mediate axon guidance. Epistasis analysis reveals that ABI-1 and WVE-1 function downstream of MIG-10 to mediate its outgrowth-promoting activity. Moreover, experiments with cultured mammalian cells suggest that the interaction between MIG-10 and ABI-1 mediates a conserved mechanism that promotes formation of lamellipodia. Together, these observations suggest that MIG-10 interacts with ABI-1 and WVE-1 to mediate the UNC-6 and SLT-1 guidance pathways.
Introduction
Axons navigate to their targets in the developing nervous system by making a series of responses to extracellular guidance cues [1]–[6]. Several conserved families of guidance cues have been identified, including the netrins and slits. These guidance cues activate receptors on the growth cone at the tip of the growing axon, causing a directional response that steers the axon either towards or away from the source of guidance cue. A key component of the mechanism that drives the directional response to guidance cues is the asymmetric accumulation of f-actin within the growth cone. For instance, in vitro growth cone turning assays have shown that actin is asymmetrically polymerized to the side of the growth cone closest to a source of netrin, which is thought to cause the growth cone to turn towards the source of netrin [7]. Likewise, asymmetric actin distribution has also been observed in growth cones migrating in vivo [8], [9].
Although many actin regulatory proteins have been implicated in the control of growth cone morphology, we do not understand how these proteins are coordinated to cause a directional response to guidance cues [10]. For example, the WVE-1 (Wave) complex activates the ARP2/3 actin-nucleating complex to control the formation of growth cone filopodia [11], [12]. Furthermore, loss of function of WVE-1 or ARP2/3 components causes defects in axon guidance [11], [13], [14]. However, we do not know how the activity of this complex is controlled to give rise to the asymmetry that underlies growth cone guidance. In particular, it is difficult to understand how shallow gradients of guidance cues could be transformed into the sharply polarized outgrowth-promoting activity that is required for a directional response.
MIG-10 may provide a key to understanding how guidance signals are transformed into sharply localized actin-based outgrowth activity. MIG-10 is a cytoplasmic outgrowth-promoting protein that becomes sharply localized in response to the UNC-6 guidance cue [15]–[19]. The role of MIG-10 in guidance has been studied in the HSN neuron of C. elegans, which extends an axon ventrally, towards a source of UNC-6 guidance cue. In response to UNC-6, the UNC-40 (DCC) receptor becomes asymmetrically localized to the side of the cell closest to the source of UNC-6. This in turn, leads to the asymmetric localization of MIG-10 to the side of the cell closest to the source of UNC-6. MIG-10 has an outgrowth-promoting activity, thereby causing axon growth towards the source of UNC-6. However, the mechanisms that mediate the outgrowth-promoting activity of MIG-10 are not understood.
Although MIG-10 and its homolog lamellipodin are thought to play a major role in inducing actin polymerization, the mechanisms that mediate this effect are not known [20]. Knockdown of lamellipodin in fibroblasts results in a severe reduction in polymerized f-actin, with large areas devoid of the normal meshwork of f-actin. The Ena/VASP actin regulatory proteins can physically interact with lamellipodin. However, loss of all Ena/VASP function in fibroblasts does not produce the severe defects in actin polymerization that occur with knockdown of lamellipodin. Likewise, in C. elegans axon guidance, complete loss of Ena/VASP (UNC-34) function results in axon guidance defects that are far weaker than those observed after complete loss of MIG-10 function [18]. Furthermore, complete loss of UNC-34 function does not reduce the outgrowth-promoting activity of MIG-10 [16]. Together, these observations indicate that the actin-polymerizing activity of lamellipodin and MIG-10 must be explained primarily through interaction with an effector other than UNC-34 (Ena/VASP).
Here, we present evidence that the outgrowth-promoting activity of MIG-10 is mediated through interactions with ABI-1 and WVE-1. Furthermore, our genetic data indicate that MIG-10 functions with both ABI-1 and WVE-1 to mediate the UNC-6 and SLT-1 guidance signaling pathways. ABI-1 and WVE-1 are part of a well-characterized complex that promotes actin polymerization by activating the actin-nucleating activity of the ARP2/3 complex [21]–[26]. Thus, our observations suggest a model where MIG-10 interacts with ABI-1 and WVE-1 to direct actin polymerization in response to guidance cues.
Results
Identification of ABI-1 as a potential component of the MIG-10 scaffold
MIG-10 is hypothesized to serve as a scaffold that can asymmetrically localize outgrowth-promoting proteins. However, the identities of these outgrowth-promoting proteins are unknown. We hypothesized that these outgrowth-promoting proteins are likely to bind to the polyproline motifs in MIG-10. Therefore, we searched for proteins that include polyproline-binding domains (SH3, WW, and EVH1 domains) for potential function in the MIG-10 pathway. To do this, we constructed an RNAi sublibrary for genes that encode polyproline-binding domains and screened for RNAi clones that phenocopy loss of mig-10 function in the HSN neuron (Figure 1). The HSN axon normally migrates ventrally to the ventral nerve cord (Figure 1B). In mig-10 null mutants, 44±4.4% of the HSN axons migrate anteriorly before turning ventrally [18]. We observed this same phenotype in mig-10(RNAi) animals, but with a penetrance of 21.6±4.6% (see Figure 1C). From our screen, we identified abi-1(RNAi), which had a penetrance of 15.1±3.6% HSN guidance defects (see Figure 1D). The migration of the HSN cell body was also affected by mig-10(RNAi) and abi-1(RNAi). However, previous analysis has indicated that HSN axon guidance defects are not secondary consequences of defects in cell body migration [18], [27].
Fig. 1. MIG-10 interacts physically with ABI-1. 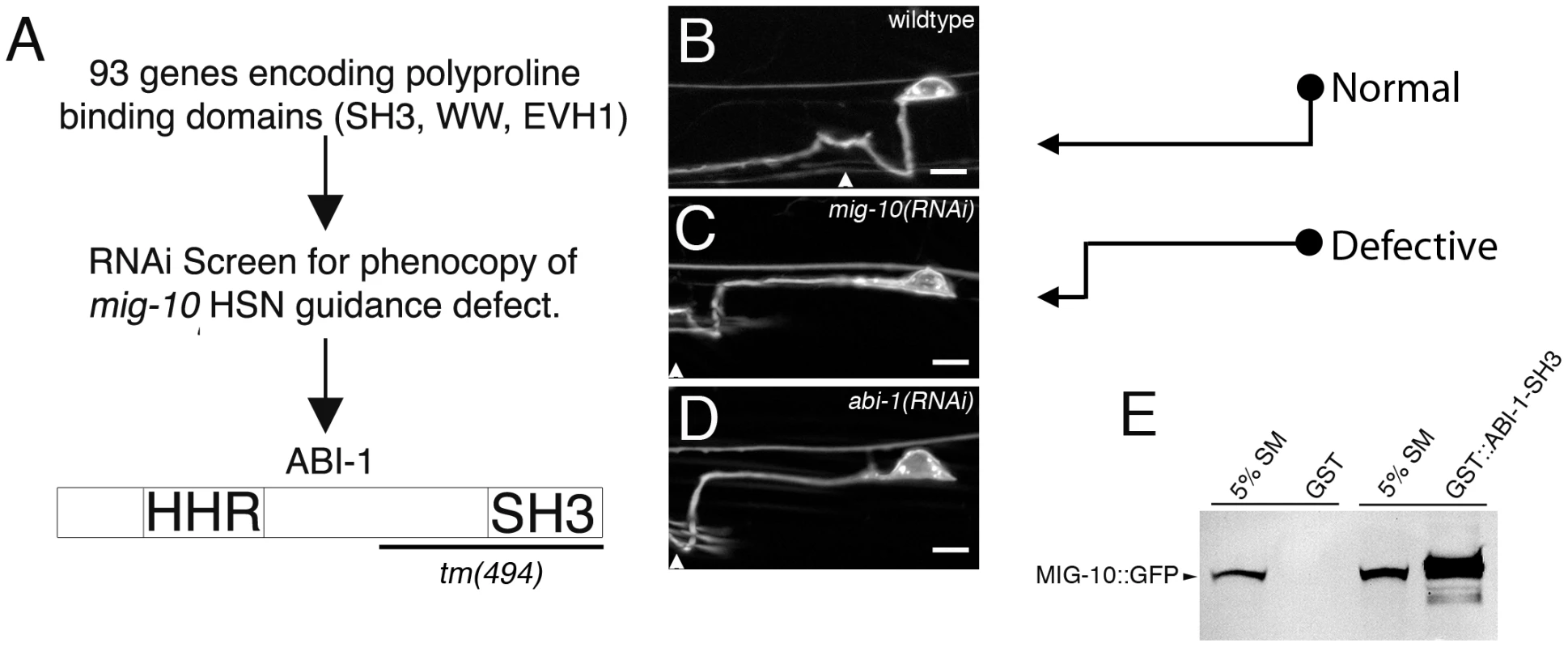
(A) Diagram of the RNAi screening strategy used to identify ABI-1. A sublibrary of RNAi clones encoding proline-binding domains (SH3, WW, EVH1) was created. Each clone was screened for the ability to phenocopy the HSN ventral guidance defect observed in mig-10 loss of function mutants. This strategy led to the identification of ABI-1 as a potential interaction partner for MIG-10. In this study we have utilized the abi-1(tm494) allele, which is predicted to truncate the ABI-1 protein as indicated by the bracket. (B) Example of HSN axon in wild-type animals. The axon makes a direct ventral migration. (C) Example of HSN ventral guidance defect observed in mig-10(RNAi) animals. The axon migrates laterally prior to turning ventrally. (D) Example of HSN ventral guidance defect observed in abi-1(RNAi) animals. The HSN axon was observed with an unc-86::myrGFP transgene. Arrowheads mark approximate position of the vulva. Note that the migration of the HSN cell body was also affected by mig-10(RNAi) and abi-1(RNAi). However, previous analysis has indicated that HSN axon guidance defects are not secondary consequences of defects in cell body migration [18], [27]. Scale bars represent 5 µm. (E) MIG-10 binds to the SH3 domain of ABI-1. MIG-10::GFP was incubated with the SH3 domain of ABI-1 fused to GST (GST::ABI-1-SH3) or GST as a control. Bound material was detected by western blotting with an antibody to GFP. For reference, an amount equivalent to 5% of the MIG-10::GFP starting material was run on a gel (5% SM). Since ABI-1 has an SH3 domain, and MIG-10 has consensus-binding sites for SH3 domains, we tested for a physical interaction between the SH3 domain of ABI-1 and MIG-10 (Figure 1E). We found that MIG-10 binds to the SH3 domain of ABI-1 fused to GST (GST::ABI-1-SH3). By contrast, MIG-10 did not bind to GST alone. Two concurrent studies have also found that MIG-10 can bind to full length ABI-1, using yeast 2 hybrid and also co-immunoprecipitation [28], [29]. Together, these observations identify ABI-1 as a potential member of the MIG-10 outgrowth-promoting complex.
ABI-1 functions in UNC-6 and SLT-1 signaling
The AVM and PVM neurons are ideal for studying ventral guidance because their axons are guided ventrally by both attraction towards a source of UNC-6 guidance cue and by repulsion from a source of the SLT-1 guidance cue. Previous work with these neurons has indicated that MIG-10 functions in both the UNC-6 and SLT-1 signaling pathways [16], [17]. In these experiments, null alleles were used to remove function of either the UNC-6 or SLT-1 guidance cues. Since unc-6; slt-1 double null mutants exhibit guidance defects that are nearly fully penetrant, UNC-6 and SLT-1 are thought to be the predominant guidance cues responsible for AVM and PVM axon guidance [16], [17], [30], [31]. Therefore, the function of either guidance cue can be assayed by removing the function of the other cue. For example, a null mutation in mig-10 can enhance guidance defects in the unc-6 null mutant background, indicating that mig-10 functions in SLT-1 signaling. Likewise, a null mutation in mig-10 can also enhance defects in the slt-1 null mutant background, indicating that mig-10 functions in UNC-6 signaling. To determine if ABI-1 also functions in UNC-6 and SLT-1 signaling, we repeated these experiments with an abi-1 loss of function mutation.
To determine if ABI-1 functions in the UNC-6 signaling pathway, we examined abi-1(tm494); slt-1(eh15) double mutants. The abi-1(tm494) mutation is a hypomorphic loss of function allele [32], whereas the slt-1(eh15) is a null allele. In this slt-1 null mutant background, the AVM and PVM axons are guided by attraction towards UNC-6. Thus, any enhancement of guidance defects in the abi-1; slt-1 double mutant would indicate that ABI-1 functions in the UNC-6 pathway. Indeed, we found that in abi-1; slt-1 double mutants, both AVM and PVM ventral guidance errors were enhanced relative to slt-1 single mutants, indicating that ABI-1 is involved in the UNC-6 attractive signaling pathway (Figure 2A–2D).
Fig. 2. ABI-1 is involved in both UNC-6 and SLT-1 signaling pathways. 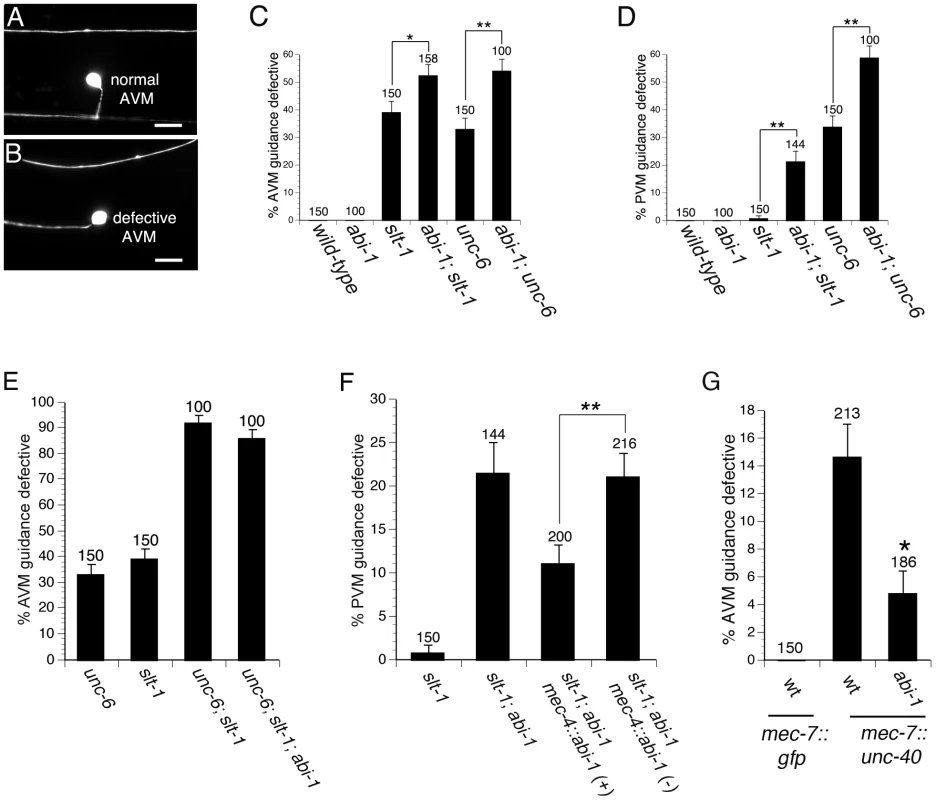
(A) Example of AVM neuron with normal ventral guidance. (B) Example of AVM neuron with defective ventral guidance. (C–D) Genetic interactions between unc-6 and abi-1 as well as between slt-1 and abi-1 in the AVM (C) and PVM (D), indicate that ABI-1 functions in both the UNC-6 and SLT-1 signaling pathways. (E) Loss of abi-1 function does not enhance defects in the unc-6; slt-1 mutant background. (F) ABI-1 functions cell autonomously to mediate axon guidance. A mec-4::abi-1 transgene suppresses PVM ventral axon guidance defects in slt-1; abi-1 double mutants. mec-4::abi-1(−) represents animals that have lost the mec-4::abi-1 transgene during mitosis. These animals serve as controls and were scored simultaneously on the same slides as the mec-4::abi-1(+) animals, which carry the transgene. Data was combined from 2 independently derived transgenic lines, cueEx1 and cueEx2, which showed similar results. (G) The abi-1(tm494) loss of function mutation suppresses guidance defects in AVM neurons overexpressing UNC-40. Scale bars are 5 µM. Error bars represent standard error of the proportion. Brackets indicate statistically significant difference, Z test for proportions (*p<0.05, **p<0.005). The AVM axon was visualized with the zdIs5 transgene (mec-4::gfp). Scale bars are 10 µM. Alleles used were unc-6(ev400) null, slt-1(eh15) null, and abi-1(tm494) loss of function. To determine if ABI-1 functions in the SLT-1 signaling pathway, we examined abi-1(tm494); unc-6(ev400) double mutants. In this unc-6 null mutant background, these axons are guided by repulsion from SLT-1. Both AVM and PVM axon guidance errors were significantly enhanced by loss of abi-1 function in abi-1; unc-6 double mutants, indicating that ABI-1 is involved in the SLT-1 repulsive signaling pathway (Figure 2A–2D). Despite being involved in both UNC-6 and SLT-1 signaling, we did not observe any AVM or PVM guidance defects in abi-1 single mutants, suggesting that ABI-1, like MIG-10, functions redundantly with other proteins to mediate UNC-6 and SLT-1 signaling.
Although double mutant analysis suggests that the AVM and PVM axons are guided predominately by UNC-6 and SLT-1, we can not exclude the possibility of a third guidance pathway. To determine if ABI-1 might function in a third pathway, we constructed an unc-6; slt-1; abi-1 triple mutant. Neither AVM nor PVM axon guidance defects were enhanced in the unc-6; slt-1; abi-1 triple mutant relative to the unc-6; slt-1 double mutant (Figure 2E and Figure S1). These observations do not support a role for ABI-1 in a third guidance pathway.
To determine if ABI-1 functions cell autonomously to mediate the axon guidance, we conducted a transgenic rescue experiment in abi-1; slt-1 double mutants (Figure 2F). The mec-4 promoter was used to drive expression of ABI-1 in the six touch neurons, including the PVM neuron. Transgenic expression of ABI-1 in the PVM neuron partially rescued the ventral guidance defects in the abi-1; slt-1 double mutants. By contrast, siblings that had lost the transgenic array did not show rescue of the ventral guidance defects. These observations indicate that ABI-1 functions cell autonomously to mediate the UNC-6 signaling pathway.
Since we found that ABI-1 functions in the UNC-6 signaling pathway, we also wanted to determine if ABI-1 functions downstream of UNC-40, the receptor for UNC-6. To determine if ABI-1 functions downstream of UNC-40, we used a mec-7::unc-40 transgene to create an UNC-40 gain of function phenotype. The mec-7::unc-40 transgene caused ventral axon guidance defects in the AVM and PVM neurons (Figure 2G and Figure S2). These guidance defects are suppressed by loss of abi-1 function in both the AVM and PVM neurons. These observations suggest that ABI-1 functions downstream of UNC-40.
MIG-10 functions with ABI-1 and WVE-1 to mediate ventral guidance of the HSN axon
The physical association between MIG-10 and ABI-1 suggests that they could function together to regulate axon guidance. To study genetic interactions between mig-10 and abi-1, we used the HSN ventral axon migration, since single mutants in abi-1 or mig-10 produce guidance defects in this neuron. Homozygous mig-10(ct41) null mutants have guidance defects with a penetrance of 44±4.4% [18]. Homozygous abi-1(tm494) hypomorphic loss of function mutants have HSN guidance defects with a penetrance of 14±3.4% (n = 100). To ask if ABI-1 and MIG-10 function together, we used dosage sensitive genetic analysis (Figure 3A). Both the abi-1 and mig-10 mutations were recessive, as neither mig-10 heterozygotes (mig-10/+) nor abi-1 heterozygotes (abi-1/+) had guidance errors in excess of wild-type animals. To test for a genetic interaction between mig-10 and abi-1, we examined HSN axon guidance in animals transheterozygous for mutations in mig-10 and abi-1, that is containing one mutant and one wild-type copy of each of these genes (mig-10/+; abi-1/+). These transheterozygous mutants had HSN guidance defects with a penetrance of 10.7±1.8% (Figure 3A). Consistent with the physical association between MIG-10 and ABI-1, these observations indicate that ABI-1 functions with MIG-10 to regulate axon guidance.
Fig. 3. Dosage-sensitive genetic interactions indicate that MIG-10, ABI-1, and WVE-1 function together to mediate axon guidance. 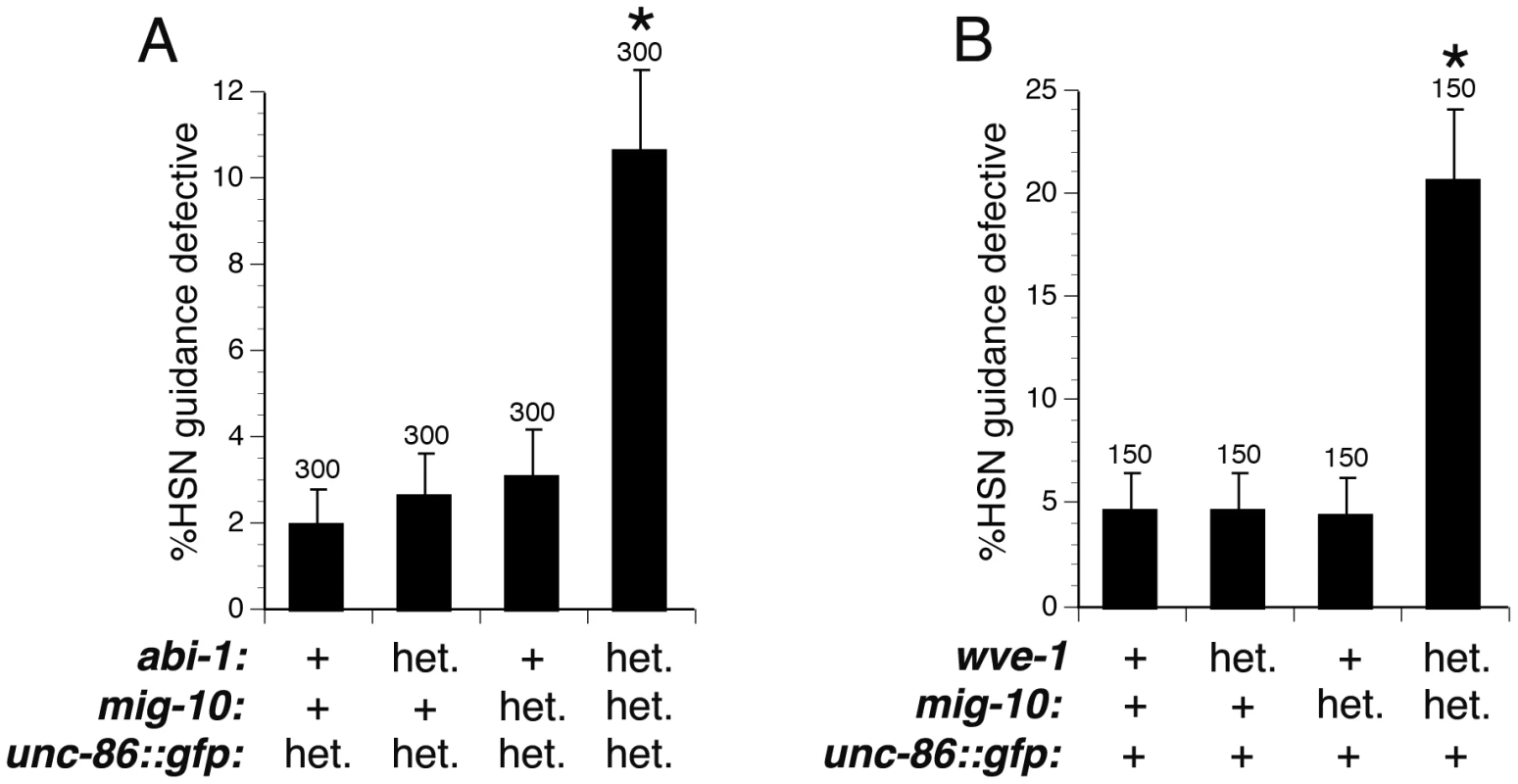
(A) Transheterozygous genetic interaction between abi-1(tm494) and mig-10(ct41). HSN ventral guidance in animals heterozygous for either abi-1(tm494) or mig-10(ct41) was comparable to those in wild-type animals. Animals transheterozygous for abi-1(tm494) and mig-10(ct41) had significantly greater ventral guidance errors compared to wild-type animals. Heterozygous animals were constructed by crossing unc-86::myrgfp males with abi-1(tm494) or mig-10(ct41) hermaphrodites and scoring F1 cross progeny. Transheterozygous animals were constructed by crossing abi-1(tm494); unc-86::myrgfp males with mig-10(ct41) hermaphrodites and scoring F1 cross progeny. (B) Transheterozygous genetic interaction between wve-1(ok3308) and mig-10(ct41). HSN ventral guidance was comparable to wild-type in animals heterozygous for wve-1(ok3308) or mig-10(ct41). HSN ventral guidance in animals heterozygous for either wve-1(ok3308) or mig-10(ct41) was comparable to those in wild-type animals. Animals transheterozygous for wve-1(ok3308) and mig-10(ct41) had significantly greater ventral guidance errors compared to wild-type animals. Similar results were obtained using the wve-1(ne350) allele instead of the wve-1(ok3308) allele. The hT2 balancer covers both wve-1 and mig-10 and was used to construct wve-1 heterozygotes, mig-10 heterozygotes, and the wve-1; mig-10 transheterozygotes. The kyIs262 transgene (unc-86::myrgfp) was used for observing the HSN axon. For the labels in both (A) and (B), “het.” means heterozygous for the mutant or for the unc-86::myrgfp transgene. Whereas, “+” means homozygous for the wild-type gene or homozygous for the unc-86::myrgfp transgene. Brackets indicate statistically significant difference between transheterozygotes and single heterozygotes, Z test for proportions (*p<0.0005). Since mig-10 interacts genetically with abi-1, we also wanted to test for interaction between mig-10 and wve-1. Studies of the mammalian homologs of ABI-1 and WVE-1, known as Abi1 and Wave, have indicated that Abi1 binds to Wave to enhance its ability to promote lamellipodial protrusion by activating the ARP2/3 complex to promote nucleation and branching of f-actin [21], [22], [24], [25]. Likewise, genetic studies in C. elegans have indicated that ABI-1 and WVE-1 are required for subcellular enrichment of f-actin and for the formation of cellular protrusions during cell migration [23]. To determine if WVE-1 is involved in HSN ventral guidance, we examined homozygous wve-1(ok3308) mutants that had been maternally rescued. We found that these wve-1 mutants had HSN guidance defects with a penetrance of 13±3.3% (n = 150), suggesting that WVE-1 is involved in HSN ventral guidance. To determine if MIG-10 functions with WVE-1, we tested for dosage-sensitive genetic interaction between mig-10 and wve-1 (Figure 3B). Both wve-1(ok3308) and mig-10(ct41) are recessive, as neither mig-10 heterozygotes (mig-10/+) nor wve-1 heterozygotes (wve-1/+) had guidance errors in excess of wild-type animals. Animals transheterozygous for mig-10(ct41) and wve-1(ok3308), (mig-10)/+; wve-1/+), had HSN guidance errors with a penetrance of 20.7±3.3% (Figure 3B). We repeated this experiment with the wve-1(ne350) allele [23] and found that animals transheterozygous for mig-10(ct41) and wve-1(ne350) had HSN guidance errors with a penetrance of 18±2.7% (n = 200). Together, these results indicate that MIG-10 functions with ABI-1 to regulate guidance.
ABI-1 and WVE-1 mediate the outgrowth-promoting activity downstream of MIG-10
Previous work has indicated that MIG-10 has an outgrowth-promoting activity and that the actin regulatory protein UNC-34 can interact with MIG-10 [16], [17]. However, complete loss of UNC-34 function does not reduce the outgrowth-promoting activity of MIG-10, indicating that UNC-34 does not account for MIG-10's outgrowth promoting activity.
Since ABI-1 and WVE-1 are part of a complex that can promote lamellipodial protrusion, we asked if ABI-1 and WVE-1 can mediate the outgrowth-promoting activity of MIG-10. To test this hypothesis, we determined if loss of WVE-1 function or loss of ABI-1 function could suppress the outgrowth-promoting activity of MIG-10. The ALM neuron normally has a single axon that grows towards the anterior (Figure 4A). Transgenic expression of MIG-10 in the ALM neuron causes the growth of a second posterior process (Figure 4B). The growth of this second process is suppressed by loss of function mutations in abi-1 or wve-1 (Figure 4C). By contrast, max-2(nv162), a likely null mutation, had no effect on the growth of the second process. The lack of an effect of the max-2 mutation is expected because MAX-2 is thought to regulate axon guidance by functioning in a pathway that is parallel to MIG-10 [18]. Together, these observations indicate that ABI-1 and WVE-1 function downstream of MIG-10 to mediate its outgrowth-promoting activity.
Fig. 4. ABI-1 and WVE-1 mediate outgrowth-promoting activity downstream of MIG-10. 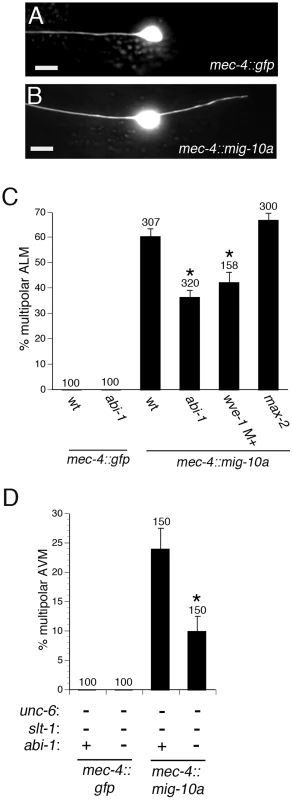
(A) Example of normal ALM neuron with a single anterior axon. (B) Example of ALM multipolar defect caused by transgenic expression of MIG-10A by the mec-4::mig-10a transgene. (C) Loss of function mutations abi-1(tm494) and wve-1(ok3308) suppress MIG-10 transgenic expression phenotype. The max-2(nv162) mutation, a likely null, does not suppress the MIG-10 transgenic expression phenotype. The wve-1(ok3308) mutants were maternally rescued. The AVM axon was visualized with a zdIs5 transgene (mec-4::gfp). (D) The abi-1(tm494) loss of function mutation suppresses the AVM multipolar phenotype that results from transgenic expression of MIG-10 in the unc-6; slt-1 double null mutant background. *Statistically significant difference compared to wild-type or unc-6; slt-1 double mutant, z-test for proportions (p<0.005). We also examined a role for ABI-1 in mediating the MIG-10 outgrowth-promoting activity in the AVM and PVM neurons. In these neurons, the outgrowth-promoting activity of MIG-10 can be oriented by either the UNC-6 or SLT-1 guidance cues [17]. Thus, transgenic expression of MIG-10 does not cause multipolar outgrowth in the wild-type, unc-6 null, or slt-1 null backgrounds. However, transgenic expression of MIG-10 does produce multipolar outgrowth in the unc-6; slt-1 double null background. We found that the abi-1(tm494) loss of function mutation significantly suppresses the MIG-10 outgrowth activity in the unc-6; slt-1 double null mutant background in the AVM and PVM neurons (Figure 4D and Figure S3). These results further support our conclusion that ABI-1 mediates the outgrowth-promoting activity of MIG-10.
ABI-1 (Abi1) mediates a conserved pathway that promotes formation of lamellipodia downstream of MIG-10
Overexpression of C. elegans MIG-10 in cultured mammalian cells induces the formation of lamellipodia, suggesting that MIG-10 can interact with a conserved pathway to promote the formation of lamellipodia [17]. To determine if ABI-1 might be a part of that conserved pathway, we asked if the mammalian homolog of ABI-1 (Abi1) is required for the lamellipodia-forming activity of MIG-10 in cultured mammalian cells. To address this question, we knocked down expression of mammalian ABI-1 (Abi1) in HEK293 cells expressing MIG-10::GFP (Figure 5). Control cells expressing GFP have a round morphology with only minimal lamellipodia (Figure 5A, 5D). Expression of MIG-10::GFP induces the formation of lamellipodia (Figure 5B, 5D), which is significantly reduced by co-expression of an Abi1 shRNA (Figure 5C, 5D). By contrast, co-expression of a scrambled control shRNA had no effect on lamellipodia formation (Figure 5D). These results indicate that MIG-10 promotes the formation of lamellipodia in mammalian cells by functioning with a conserved pathway that includes mammalian Abi1.
Fig. 5. ABI-1 mediates the lamellipodia-forming activity of MIG-10 in cultured HEK293 cells. 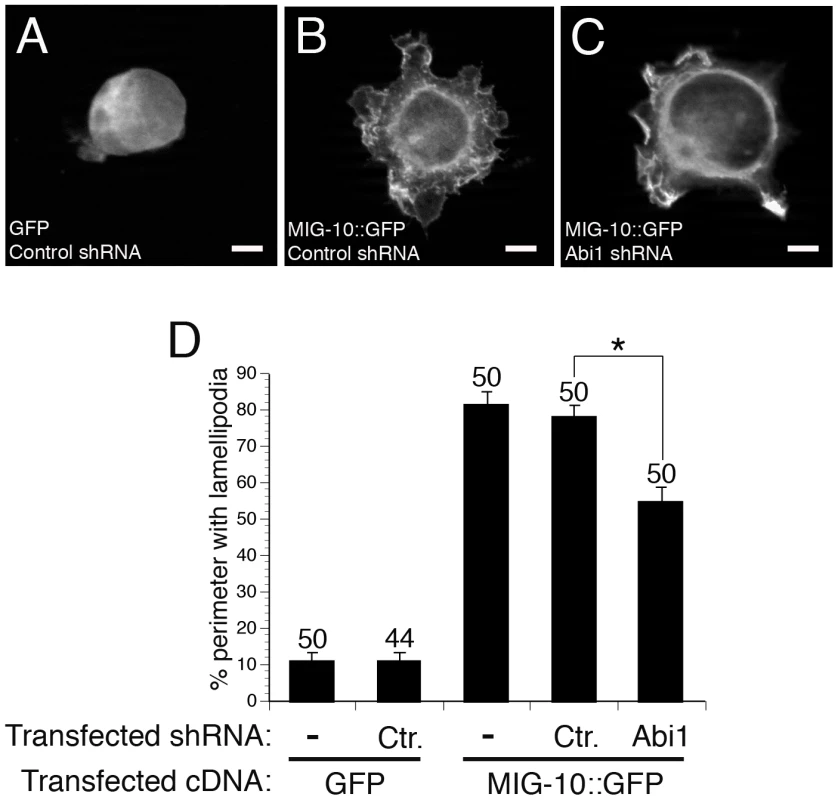
(A) Example of cell transfected with GFP and control shRNA. (B) Example of cell transfected with MIG-10::GFP and control shRNA. (C) Example of cell transfected with MIG-10::GFP and Abi1 shRNA. (D) Knockdown of Abi1 suppresses the lamellipodia-forming activity of MIG-10. Graph shows the average cell perimeter with lamellipodia. “Ctr.” means cells transfected with scrambled control shRNA. “−” means cells were not transfected with any shRNA. “Abi1” means cells were transfected with the PAV197 shRNA against Abi1. Error bars represent the standard error of the mean. *Bracket indicates statistically significant difference, t-test (p<0.0001). Scale bars are 5 µm. Discussion
Several actin regulatory proteins have been implicated in the control of growth cone morphology [10]. A few of these have been implicated in the directional response to specific guidance cues. However, little is known about how actin regulatory proteins interact with one another to establish a directional response to guidance cues. Here, we uncover an interaction between the MIG-10 cytoplasmic scaffold and the ABI-1 actin regulatory protein. This interaction could help to explain how actin polymerization is spatially regulated during guidance, since MIG-10 is asymmetrically localized in response to guidance cues [15], [18]. Concurrent work has indicated that the interaction between MIG-10 and ABI-1 can also regulate excretory canal morphogenesis and synapse formation [28], [29], Moreover, recent work has also shown that axon guidance cues and receptors are involved in regulating the WVE-1 complex during embryonic morphogenesis [33]. Together, these observations suggest that the interactions between axon guidance signaling components and the WVE-1 actin regulatory complex may be important in multiple aspects of actin-dependant developmental processes.
Genetic studies of UNC-6 (netrin) signaling in C. elegans have revealed a direction-sensing module that includes the UNC-40 receptor, PI 3-Kinase, Rac and MIG-10 [15]–[19]. UNC-6 is secreted from ventrally localized cells and is thought to form a gradient that causes the HSN axon to migrate ventrally. In response to the UNC-6 gradient, UNC-40 becomes asymmetrically localized to the ventral side of the HSN cell. UNC-40 is thought to initiate signaling events that involve activation of Rac and production of PI(3,4)P2 by PI 3-Kinase. MIG-10 binds to both Rac and PI(3,4)P2 and thus becomes asymmetrically localized to the ventral side of the cell. This direction-sensing module is reminiscent of chemotaxis in neutrophils, where Rac and PI 3-Kinase are thought to form a positive feedback loop that transforms directional information from shallow gradients of chemotactic cues into sharply localized directional signal that promotes actin-based motility [34], [35]. Likewise, we propose that UNC-40, Rac, PI 3-Kinase and MIG-10 form a direction-sensing module that can transform a shallow gradient of UNC-6 into a sharply localized outgrowth-promoting activity.
Our current results provide a link that connects MIG-10 to an actin polymerization-promoting complex, thereby explaining how the directional information encoded by asymmetric localization of MIG-10 can be transformed into directed axon outgrowth. We have found that MIG-10 interacts with ABI-1 and WVE-1 to mediate axon guidance. Previous work has indicated that ABI-1 binds to WVE-1 to promote its ability to activate the ARP2/3 complex [21]. The ARP2/3 complex can nucleate actin branches, thereby producing the meshwork of actin that is thought to provide the force that drives motility [36]. Therefore, the interaction between MIG-10 and ABI-1 can explain how MIG-10 is able to spatially direct outgrowth activity. We have been unable to visualize ABI-1::GFP in the HSN neuron. However, studies of Abi1 in mammalian cells indicate that it is located at the leading edge of migrating cells [37]. Since we have found that ABI-1 functions with MIG-10, it is likely that ABI-1 localizes to the leading edge of the HSN neuron. Alternatively, ABI-1 might be localized throughout the cell. However, since MIG-10 is localized to the leading edge, the functional interaction between MIG-10 and ABI-1 would still be confined to the leading edge.
Previous work has implicated ABI-1 and WVE-1 in axon guidance, but the guidance cues were not known [11], [13], [14]. Our current work indicates that ABI-1 and WVE-1 are involved in both the attractive UNC-6 signaling pathway and the repulsive SLT-1 signaling pathway. Despite being involved in both UNC-6 and SLT-1 signaling, the single abi-1 loss of function mutant does not have any defects in AVM or PVM guidance, suggesting that ABI-1 functions redundantly with other proteins to mediate guidance in these neurons. The lack of AVM and PVM guidance defects in abi-1(tm494) mutants might also be due to the fact that this is a hypomorphic allele [32]. In fact, recent work has indicated that RNAi depletion of GEX-3, another member of the WVE-1 complex, can cause mild guidance defects in the AVM [33]. In our study, the role of ABI-1 in axon guidance is revealed in abi-1; unc-6 or abi-1; slt-1 double mutants, in which guidance information has been reduced to create a sensitized genetic background. Mutations in several other axon guidance genes (including null alleles) also give only weak or non-existent phenotypes as single mutants, but are enhanced by unc-6 or slt-1 null mutations. These mutations include mig-10(null), age-1(maternally rescued), unc-34(null), ced-10(hypomorphic), unc-115(null) and madd-2(null) 16–18,30,31. Together, these observations suggest that guidance signaling functions with a great deal of redundancy in these neurons.
Our results, when considered with previous findings, suggest that CED-10, MIG-10, ABI-1 and WVE-1 may be organized into a complex or complexes that features redundant physical interactions (see Figure S4). Our previous and current results suggest that CED-10 can interact with MIG-10 and that MIG-10 interacts with ABI-1 [18]. Previous studies have indicated that CED-10 can interact with a subcomplex that contains Sra1 (GEX-2) and Nap1 (GEX-3) [21]. This GEX-2/GEX-3 subcomplex interacts with ABI-1. Thus, ABI-1 could be redundantly bound by both MIG-10 and the GEX-2/GEX-3 subcomplex. This redundant binding could occur within a single complex or could occur within separate complexes (see Figure S4). Discrimination between these two possible configurations will require biochemical and structural studies. We propose that guidance signaling molecules may be organized into networks that include redundant physical interactions. This redundancy could provide a more robust mechanism for the control of the actin polymerization. This model is consistent with the observation that mutations in genes that encode guidance signaling proteins (such as mig-10, age-1, unc-34, ced-10, unc-115 and madd-2), generally lead to only weak or nonexistent guidance defects [16]–[18], [30], [31].
In our study, ABI-1 is shown to act in both the UNC-6 and SLT-1 pathways. Several other intracellular outgrowth-promoting proteins have also been implicated in both attractive and repulsive signaling pathways including MIG-10, Rac, Pak, UNC-34 and Abl [16], [17], [38]–[41]. In addition, overexpression of the repulsive UNC-5 receptor can promote neurite outgrowth in cultured cells [42]. Together, these observations suggest that a common set of outgrowth-promoting proteins are involved in both attractive and repulsive responses. A likely explanation for the dual roles of outgrowth-promoting proteins is that they could be oriented by both attractive and repulsive signals, thereby promoting growth towards or away from the source of guidance cue, respectively. Thus, the difference between attraction and repulsion could be in where and how a common set of outgrowth-promoting proteins are localized.
Finally, ABI-1 may link to multiple upstream signaling modules to mediate distinct aspects of axon growth. For instance, recent work has found that the UNC-53 scaffold protein interacts with ABI-1 to promote longitudinal axon growth in C. elegans [32]. In unc-53 loss of function mutants, longitudinal, but not circumferential axon growth is disrupted. Conversely, MIG-10 is thought to mediate circumferential, but not longitudinal axon growth [16], [17]. These observations suggest that the mechanisms that control actin polymerization to drive longitudinal outgrowth are distinct from those that control actin polymerization to drive circumferential guidance. We propose that for the process of circumferential axon guidance, ABI-1 links to the MIG-10 scaffold. By contrast, for the process of longitudinal axon extension, ABI-1 links to the UNC-53 scaffold. Therefore, each of these distinct upstream regulatory processes can both utilize the same actin regulatory complex.
Materials and Methods
Strains
AGC1: slt-1(eh15); abi-1(tm494); cueEx1, AGC2: slt-1(eh15); abi-1(tm494) cueEx2, AGC3: abi-1(tm494); cueIs3, AGC4: cueIs3, AGC5: cueIs3; wve-1(ok3308)/hT2 [bli4(e937); let-?(q782); qIs48], AGC6: slt-1(eh15); abi-1(tm494) cueEx4, AGC8: wve-1(ok3308)/hT2 [bli4(e937); let-?(q782); qIs48]; kyIs262, AGC9: mig-10(ct41)/hT2 [bli4(e937); let-?(q782); qIs48]; kyIs262, AGC10: mig-10(ct41)/hT2 [bli4(e937); let-?(q782); qIs48]; wve-1(ok3308)/hT2; kyIs262, AGC12: abi-1(tm494); slt-1(eh15); zdIs5, AGC13: abi-1(tm494); unc-6(ev400); zdis5, AGC14: abi-1(tm494); zdIs5, AGC15: cueIs3; max-2(nv162), AGC16: wve-1(ne350)/hT2; mig-10(ct41)/hT2, AGC18: unc-6(ev400); slt-1(eh15); abi-1(tm494); zdIs5, AGC19: unc-6(ev400); slt-1(eh15); abi-1(tm494); urEx305. AGC20: cueIs7; abi-1(tm494); zdIs5, AGC21: cueIs7; zdIs5. The wve-1(ne350) allele was a gift from Martha Soto [23], [43]. The wve-1(ok3308) allele was obtained from the CGC and out-crossed 3 times. We found that the ok3308 mutation behaved as a zygotic sterile. The abi-1(tm494) hypomorphic allele was provided by Shohei Mitani.
Transgenes
cueEx1 and cueEx2 [mec-4::abi-1; odr-1::dsred] were created by injecting pAGC2 at 25 ng/ul and odr-1::dsred at 50 ng/ul. urEx305 [mec-4::mig-10a; flp-20::gfp] was created as described previously [17]. cueIs3 [mec-4::mig-10a; flp-20::gfp] was created by integrating urEx305 with a gamma radiation source. cueEx4 [mec-7::abi-1; odr-1::dsred] was created by injecting pAGC3 at 25 ng/ul and odr-1::dsred at 50 ng/ul. kyIs262 [unc-86::myrgfp] was kindly provided by Cori Bargmann. zdIs5 [mec-4::gfp] was obtained from the CGC. evEx344 [mec-7::unc-40; unc-129::gfp] was a gift from Joseph Culotti. cueIs7 was created by integrating evEx344.
DNA constructs
pAGC2 contains the mec-4::abi-1 and was created by amplifying the abi-1 cDNA using the following primers fwd: gcagcagctagccaccatgagtgttaatgatcttcaag and rev: gcagcaggtacctcatactggaactacgtagtttc. The PCR product was cut with NheI and KpnI and ligated into the Nhe1 and Kpn1 sites of pIM207 [17], [44].
pAGC3 contains mec-7::abi-1 and was created by using Nhe1 and Kpn1 to subclone the abi-1 cDNA from pAGC2 into pIM211 [17].
pAGC4 contains GST::abi-1-SH3 and was created by using the following primers to amplify DNA encoding the SH3 domain of ABI-1: fwd:gccacaagcgatccagtctctttgatacgagtgct and rev: gccacaaggaattctcatactggaactacgtagtt. The PCR product was cut with BamHI and EcoRI and cloned into the same sites of pGEX-2T.
PAV197 contains an shRNA construct for Abi1 and PAV16 contains a scrambled control shRNA. Both of these constructs were kindly provided by Giorgio Scita [21].
GST binding assay
GST binding assays were performed as described previously [45]. Briefly, a GST fusion with the SH3 domain of ABI-1 (GST::ABI-1-SH3) was prepared by transforming BL21(DE3) cells with pAGC4. Cell lysates containing MIG-10::GFP were prepared by transfecting HEK293 cells with a plasmid encoding MIG-10::GFP and lysing cells 24 hours later. The GST::ABI-1-SH3 fusion protein was purified and coupled to glutathione-sepharose. Cell lysates were added to the glutathione-GST::ABI-1-SH3 complex and incubated for 2 hours. The glutathione-sepharose-protein complexes were washed three times with 0.1% Triton X-100, boiled in loading buffer and run on and SDS PAGE gel. Bound proteins were detected with an antibody against GFP.
Cell culture
HEK293 cells were grown in DMEM with 10% FBS. Fugene 6 was used to transfect or co-transfect cells with the appropriate plasmids. For co-transfection experiments 2 µg of DNA encoding the appropriate shRNA was mixed 1 µg of DNA encoding GFP or MIG-10::GFP. Cells were transfected in plastic dishes and allowed to grow for 72 hours after transfection. Next, cells were removed from the plastic dishes and were replated onto glass coverslips coated with polylysine. After 24 hours, the cells were fixed with paraformaldehyde and mounted for observation and analysis.
Analysis of phenotypes
For analysis of axon guidance phenotypes, animals were mounted on a 5% agarose pad and observed with a 40× objective. For AVM and PVM ventral guidance, an axon was scored as defective if it failed to reach the ventral nerve cord. For HSN ventral guidance, an axon was scored as defective if it traveled laterally for a distance equivalent to 2 cell bodies or more prior to migrating ventrally. For analysis of multipolarity in the ALM, AVM and PVM neurons, a cell was scored as defective if it had a posterior axon that was longer than 2 cell bodies.
Supporting Information
Zdroje
1. Lai Wing SunK, CorreiaJP, KennedyTE (2011) Netrins: versatile extracellular cues with diverse functions. Development 138 : 2153–2169.
2. KolodkinAL, Tessier-LavigneM (2010) Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol 3.
3. RaperJ, MasonC (2010) Cellular strategies of axonal pathfinding. Cold Spring Harb Perspect Biol 2: a001933.
4. ChedotalA, RichardsLJ (2010) Wiring the brain: the biology of neuronal guidance. Cold Spring Harb Perspect Biol 2: a001917.
5. O'DonnellM, ChanceRK, BashawGJ (2009) Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci 32 : 383–412.
6. BashawGJ, KleinR (2010) Signaling from axon guidance receptors. Cold Spring Harb Perspect Biol 2: a001941.
7. MarsickBM, FlynnKC, Santiago-MedinaM, BamburgJR, LetourneauPC (2010) Activation of ADF/cofilin mediates attractive growth cone turning toward nerve growth factor and netrin-1. Dev Neurobiol 70 : 565–588.
8. NorrisAD, LundquistEA (2011) UNC-6/netrin and its receptors UNC-5 and UNC-40/DCC modulate growth cone protrusion in vivo in C. elegans. Development 138 : 4433–4442.
9. O'ConnorTP, BentleyD (1993) Accumulation of actin in subsets of pioneer growth cone filopodia in response to neural and epithelial guidance cues in situ. J Cell Biol 123 : 935–948.
10. DentEW, GuptonSL, GertlerFB (2011) The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol 3.
11. NorrisAD, DyerJO, LundquistEA (2009) The Arp2/3 complex, UNC-115/abLIM, and UNC-34/Enabled regulate axon guidance and growth cone filopodia formation in Caenorhabditis elegans. Neural Dev 4 : 38.
12. KorobovaF, SvitkinaT (2008) Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells. Mol Biol Cell 19 : 1561–1574.
13. ShakirMA, JiangK, StruckhoffEC, DemarcoRS, PatelFB, et al. (2008) The Arp2/3 activators WAVE and WASP have distinct genetic interactions with Rac GTPases in Caenorhabditis elegans axon guidance. Genetics 179 : 1957–1971.
14. ZallenJA, CohenY, HudsonAM, CooleyL, WieschausE, et al. (2002) SCAR is a primary regulator of Arp2/3-dependent morphological events in Drosophila. J Cell Biol 156 : 689–701.
15. AdlerCE, FetterRD, BargmannCI (2006) UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat Neurosci 9 : 511–518.
16. ChangC, AdlerCE, KrauseM, ClarkSG, GertlerFB, et al. (2006) MIG-10/lamellipodin and AGE-1/PI3K promote axon guidance and outgrowth in response to slit and netrin. Curr Biol 16 : 854–862.
17. QuinnCC, PfeilDS, ChenE, StovallEL, HardenMV, et al. (2006) UNC-6/netrin and SLT-1/slit guidance cues orient axon outgrowth mediated by MIG-10/RIAM/lamellipodin. Curr Biol 16 : 845–853.
18. QuinnCC, PfeilDS, WadsworthWG (2008) CED-10/Rac1 mediates axon guidance by regulating the asymmetric distribution of MIG-10/lamellipodin. Curr Biol 18 : 808–813.
19. QuinnCC, WadsworthWG (2008) Axon guidance: asymmetric signaling orients polarized outgrowth. Trends Cell Biol 18 : 597–603.
20. KrauseM, LeslieJD, StewartM, LafuenteEM, ValderramaF, et al. (2004) Lamellipodin, an Ena/VASP ligand, is implicated in the regulation of lamellipodial dynamics. Dev Cell 7 : 571–583.
21. InnocentiM, ZucconiA, DisanzaA, FrittoliE, ArecesLB, et al. (2004) Abi1 is essential for the formation and activation of a WAVE2 signalling complex. Nat Cell Biol 6 : 319–327.
22. KundaP, CraigG, DominguezV, BaumB (2003) Abi, Sra1, and Kette control the stability and localization of SCAR/WAVE to regulate the formation of actin-based protrusions. Curr Biol 13 : 1867–1875.
23. PatelFB, BernadskayaYY, ChenE, JobanputraA, PooladiZ, et al. (2008) The WAVE/SCAR complex promotes polarized cell movements and actin enrichment in epithelia during C. elegans embryogenesis. Dev Biol 324 : 297–309.
24. RogersSL, WiedemannU, StuurmanN, ValeRD (2003) Molecular requirements for actin-based lamella formation in Drosophila S2 cells. J Cell Biol 162 : 1079–1088.
25. SteffenA, RottnerK, EhingerJ, InnocentiM, ScitaG, et al. (2004) Sra-1 and Nap1 link Rac to actin assembly driving lamellipodia formation. EMBO J 23 : 749–759.
26. SotoMC, QadotaH, KasuyaK, InoueM, TsuboiD, et al. (2002) The GEX-2 and GEX-3 proteins are required for tissue morphogenesis and cell migrations in C. elegans. Genes & development 16 : 620–632.
27. AlexanderM, ChanKK, ByrneAB, SelmanG, LeeT, et al. (2009) An UNC-40 pathway directs postsynaptic membrane extension in Caenorhabditis elegans. Development 136 : 911–922.
28. McSheaMA, SchmidtKL, DubukeML, BaldigaCE, SullenderME, et al. (2012) Abelson interactor-1 (ABI-1) interacts with MRL adaptor protein MIG-10 and is required in guided cell migrations and process outgrowth in C.elegans. Dev Biol in press. doi: 10.1016/j.ydbio.2012.09.017.
29. StavoeAKH, NelsonJC, Martínez-VelázquezLA, KleinM, SamuelADT, et al. (2012) Synaptic Vesicle Clustering Requires a Distinct MIG-10/Lamellipodin Isoform and ABI-1 downstream from Netrin. Genes and Development 26 : 2206–2221.
30. GitaiZ, YuTW, LundquistEA, Tessier-LavigneM, BargmannCI (2003) The netrin receptor UNC-40/DCC stimulates axon attraction and outgrowth through enabled and, in parallel, Rac and UNC-115/AbLIM. Neuron 37 : 53–65.
31. YuTW, HaoJC, LimW, Tessier-LavigneM, BargmannCI (2002) Shared receptors in axon guidance: SAX-3/Robo signals via UNC-34/Enabled and a Netrin-independent UNC-40/DCC function. Nat Neurosci 5 : 1147–1154.
32. SchmidtKL, Marcus-GueretN, AdeleyeA, WebberJ, BaillieD, et al. (2009) The cell migration molecule UNC-53/NAV2 is linked to the ARP2/3 complex by ABI-1. Development 136 : 563–574.
33. BernadskayaYY, WallaceA, NguyenJ, MohlerWA, SotoMC (2012) UNC-40/DCC, SAX-3/Robo, and VAB-1/Eph Polarize F-Actin during Embryonic Morphogenesis by Regulating the WAVE/SCAR Actin Nucleation Complex. PLoS Genet 8: e1002863 doi:10.1371/journal.pgen.1002863.
34. BourneHR, WeinerO (2002) A chemical compass. Nature 419 : 21.
35. WeinerOD (2002) Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass. Curr Opin Cell Biol 14 : 196–202.
36. PollardTD (2007) Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct 36 : 451–477.
37. StradalT, CourtneyKD, RottnerK, HahneP, SmallJV, et al. (2001) The Abl interactor proteins localize to sites of actin polymerization at the tips of lamellipodia and filopodia. Curr Biol 11 : 891–895.
38. BashawGJ, KiddT, MurrayD, PawsonT, GoodmanCS (2000) Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell 101 : 703–715.
39. FanX, LabradorJP, HingH, BashawGJ (2003) Slit stimulation recruits Dock and Pak to the roundabout receptor and increases Rac activity to regulate axon repulsion at the CNS midline. Neuron 40 : 113–127.
40. ForsthoefelDJ, LieblEC, KolodziejPA, SeegerMA (2005) The Abelson tyrosine kinase, the Trio GEF and Enabled interact with the Netrin receptor Frazzled in Drosophila. Development 132 : 1983–1994.
41. LebrandC, DentEW, StrasserGA, LanierLM, KrauseM, et al. (2004) Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron 42 : 37–49.
42. PicardM, PetrieRJ, Antoine-BertrandJ, Saint-Cyr-ProulxE, VillemureJF, et al. (2009) Spatial and temporal activation of the small GTPases RhoA and Rac1 by the netrin-1 receptor UNC5a during neurite outgrowth. Cell Signal 21 : 1961–1973.
43. XiongH, MohlerWA, SotoMC (2011) The branched actin nucleator Arp2/3 promotes nuclear migrations and cell polarity in the C. elegans zygote. Dev Biol 357 : 356–369.
44. XuY, RenXC, QuinnCC, WadsworthWG (2011) Axon response to guidance cues is stimulated by acetylcholine in Caenorhabditis elegans. Genetics 189 : 899–906.
45. QuinnCC, ChenE, KinjoTG, KellyG, BellAW, et al. (2003) TUC-4b, a novel TUC family variant, regulates neurite outgrowth and associates with vesicles in the growth cone. J Neurosci 23 : 2815–2823.
46. ChenZ, BorekD, PadrickSB, GomezTS, MetlagelZ, et al. Structure and control of the actin regulatory WAVE complex. Nature 468 : 533–538.
Štítky
Genetika Reprodukční medicína
Článek The Covariate's DilemmaČlánek Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene SignallingČlánek Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 11- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- The Covariate's Dilemma
- Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene Signalling
- Plan B for Stimulating Stem Cell Division
- Discovering Thiamine Transporters as Targets of Chloroquine Using a Novel Functional Genomics Strategy
- Is a Modifier of Mutations in Retinitis Pigmentosa with Incomplete Penetrance
- Evolutionarily Ancient Association of the FoxJ1 Transcription Factor with the Motile Ciliogenic Program
- Genome Instability Caused by a Germline Mutation in the Human DNA Repair Gene
- Transcription Factor Oct1 Is a Somatic and Cancer Stem Cell Determinant
- Controls of Nucleosome Positioning in the Human Genome
- Disruption of Causes Defective Meiotic Recombination in Male Mice
- A Novel Human-Infection-Derived Bacterium Provides Insights into the Evolutionary Origins of Mutualistic Insect–Bacterial Symbioses
- Trps1 and Its Target Gene Regulate Epithelial Proliferation in the Developing Hair Follicle and Are Associated with Hypertrichosis
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Population-Based Resequencing of in 10,330 Individuals: Spectrum of Genetic Variation, Phenotype, and Comparison with Extreme Phenotype Approach
- HP1a Recruitment to Promoters Is Independent of H3K9 Methylation in
- Transcription Elongation and Tissue-Specific Somatic CAG Instability
- A Germline Polymorphism of DNA Polymerase Beta Induces Genomic Instability and Cellular Transformation
- Interallelic and Intergenic Incompatibilities of the () Gene in Mouse Hybrid Sterility
- Comparison of Mitochondrial Mutation Spectra in Ageing Human Colonic Epithelium and Disease: Absence of Evidence for Purifying Selection in Somatic Mitochondrial DNA Point Mutations
- Mutations in the Transcription Elongation Factor SPT5 Disrupt a Reporter for Dosage Compensation in Drosophila
- Evolution of Minimal Specificity and Promiscuity in Steroid Hormone Receptors
- Blockade of Pachytene piRNA Biogenesis Reveals a Novel Requirement for Maintaining Post-Meiotic Germline Genome Integrity
- RHOA Is a Modulator of the Cholesterol-Lowering Effects of Statin
- MIG-10 Functions with ABI-1 to Mediate the UNC-6 and SLT-1 Axon Guidance Signaling Pathways
- Loss of the DNA Methyltransferase MET1 Induces H3K9 Hypermethylation at PcG Target Genes and Redistribution of H3K27 Trimethylation to Transposons in
- Genome-Wide Association Studies Reveal a Simple Genetic Basis of Resistance to Naturally Coevolving Viruses in
- The Principal Genetic Determinants for Nasopharyngeal Carcinoma in China Involve the Class I Antigen Recognition Groove
- Molecular, Physiological, and Motor Performance Defects in DMSXL Mice Carrying >1,000 CTG Repeats from the Human DM1 Locus
- Genomic Study of RNA Polymerase II and III SNAP-Bound Promoters Reveals a Gene Transcribed by Both Enzymes and a Broad Use of Common Activators
- Long Telomeres Produced by Telomerase-Resistant Recombination Are Established from a Single Source and Are Subject to Extreme Sequence Scrambling
- The Yeast SR-Like Protein Npl3 Links Chromatin Modification to mRNA Processing
- Deubiquitylation Machinery Is Required for Embryonic Polarity in
- dJun and Vri/dNFIL3 Are Major Regulators of Cardiac Aging in Drosophila
- CtIP Is Required to Initiate Replication-Dependent Interstrand Crosslink Repair
- Notch-Mediated Suppression of TSC2 Expression Regulates Cell Differentiation in the Intestinal Stem Cell Lineage
- A Combination of H2A.Z and H4 Acetylation Recruits Brd2 to Chromatin during Transcriptional Activation
- Network Analysis of a -Mouse Model of Autosomal Dominant Polycystic Kidney Disease Identifies HNF4α as a Disease Modifier
- Mitosis in Neurons: Roughex and APC/C Maintain Cell Cycle Exit to Prevent Cytokinetic and Axonal Defects in Photoreceptor Neurons
- CELF4 Regulates Translation and Local Abundance of a Vast Set of mRNAs, Including Genes Associated with Regulation of Synaptic Function
- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- The Genomes of the Fungal Plant Pathogens and Reveal Adaptation to Different Hosts and Lifestyles But Also Signatures of Common Ancestry
- A Genome-Scale RNA–Interference Screen Identifies RRAS Signaling as a Pathologic Feature of Huntington's Disease
- Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
- Population Genomic Scan for Candidate Signatures of Balancing Selection to Guide Antigen Characterization in Malaria Parasites
- Tissue-Specific Regulation of Chromatin Insulator Function
- Disruption of Mouse Cenpj, a Regulator of Centriole Biogenesis, Phenocopies Seckel Syndrome
- Genome, Functional Gene Annotation, and Nuclear Transformation of the Heterokont Oleaginous Alga CCMP1779
- Antagonistic Gene Activities Determine the Formation of Pattern Elements along the Mediolateral Axis of the Fruit
- Lung eQTLs to Help Reveal the Molecular Underpinnings of Asthma
- Identification of the First ATRIP–Deficient Patient and Novel Mutations in ATR Define a Clinical Spectrum for ATR–ATRIP Seckel Syndrome
- Cooperativity of , , and in Malignant Breast Cancer Evolution
- Loss of Prohibitin Membrane Scaffolds Impairs Mitochondrial Architecture and Leads to Tau Hyperphosphorylation and Neurodegeneration
- Microhomology Directs Diverse DNA Break Repair Pathways and Chromosomal Translocations
- MicroRNA–Mediated Repression of the Seed Maturation Program during Vegetative Development in
- Selective Pressure Causes an RNA Virus to Trade Reproductive Fitness for Increased Structural and Thermal Stability of a Viral Enzyme
- The Tumor Suppressor Gene Retinoblastoma-1 Is Required for Retinotectal Development and Visual Function in Zebrafish
- Regions of Homozygosity in the Porcine Genome: Consequence of Demography and the Recombination Landscape
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
- Polyadenylation-Dependent Control of Long Noncoding RNA Expression by the Poly(A)-Binding Protein Nuclear 1
- A Unified Method for Detecting Secondary Trait Associations with Rare Variants: Application to Sequence Data
- Genetic and Biochemical Dissection of a HisKA Domain Identifies Residues Required Exclusively for Kinase and Phosphatase Activities
- Informed Conditioning on Clinical Covariates Increases Power in Case-Control Association Studies
- Biochemical Diversification through Foreign Gene Expression in Bdelloid Rotifers
- Genomic Variation and Its Impact on Gene Expression in
- Spastic Paraplegia Mutation N256S in the Neuronal Microtubule Motor KIF5A Disrupts Axonal Transport in a HSP Model
- Lamin B1 Polymorphism Influences Morphology of the Nuclear Envelope, Cell Cycle Progression, and Risk of Neural Tube Defects in Mice
- A Targeted Glycan-Related Gene Screen Reveals Heparan Sulfate Proteoglycan Sulfation Regulates WNT and BMP Trans-Synaptic Signaling
- Dopaminergic D2-Like Receptors Delimit Recurrent Cholinergic-Mediated Motor Programs during a Goal-Oriented Behavior
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



