-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Single-Tissue and Cross-Tissue Heritability of Gene Expression Via Identity-by-Descent in Related or Unrelated Individuals
Family studies of individual tissues have shown that gene expression traits are genetically heritable. Here, we investigate cis and trans components of heritability both within and across tissues by applying variance-components methods to 722 Icelanders from family cohorts, using identity-by-descent (IBD) estimates from long-range phased genome-wide SNP data and gene expression measurements for ∼19,000 genes in blood and adipose tissue. We estimate the proportion of gene expression heritability attributable to cis regulation as 37% in blood and 24% in adipose tissue. Our results indicate that the correlation in gene expression measurements across these tissues is primarily due to heritability at cis loci, whereas there is little sharing of trans regulation across tissues. One implication of this finding is that heritability in tissues composed of heterogeneous cell types is expected to be more dominated by cis regulation than in tissues composed of more homogeneous cell types, consistent with our blood versus adipose results as well as results of previous studies in lymphoblastoid cell lines. Finally, we obtained similar estimates of the cis components of heritability using IBD between unrelated individuals, indicating that transgenerational epigenetic inheritance does not contribute substantially to the “missing heritability” of gene expression in these tissue types.
Published in the journal: . PLoS Genet 7(2): e32767. doi:10.1371/journal.pgen.1001317
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001317Summary
Family studies of individual tissues have shown that gene expression traits are genetically heritable. Here, we investigate cis and trans components of heritability both within and across tissues by applying variance-components methods to 722 Icelanders from family cohorts, using identity-by-descent (IBD) estimates from long-range phased genome-wide SNP data and gene expression measurements for ∼19,000 genes in blood and adipose tissue. We estimate the proportion of gene expression heritability attributable to cis regulation as 37% in blood and 24% in adipose tissue. Our results indicate that the correlation in gene expression measurements across these tissues is primarily due to heritability at cis loci, whereas there is little sharing of trans regulation across tissues. One implication of this finding is that heritability in tissues composed of heterogeneous cell types is expected to be more dominated by cis regulation than in tissues composed of more homogeneous cell types, consistent with our blood versus adipose results as well as results of previous studies in lymphoblastoid cell lines. Finally, we obtained similar estimates of the cis components of heritability using IBD between unrelated individuals, indicating that transgenerational epigenetic inheritance does not contribute substantially to the “missing heritability” of gene expression in these tissue types.
Introduction
The genome contains a complex set of instructions for the assembly and maintenance of an organism. A fundamental goal in biology is to understand the relationship between genotype and phenotype. This goal can be achieved in part by studying the genetic basis of gene expression, as many genotype-phenotype correlations are a consequence of genetically driven variation in gene expression [1]. A number of studies have mapped individual cis and trans regulatory variants in humans, and recent work has suggested that the majority of regulators act in trans [2]-[5]; regulation of gene expression has also been widely studied in animal models [6]-[9]. However, the bulk of variability in gene expression remains unexplained. Heritability analyses can shed light on the genetic basis of gene expression. Several previous studies have demonstrated substantial overall heritability of gene expression in family data sets, and heritability approaches have also been broadly applied to other phenotypes [10]-[14].
In this study, we used gene expression measurements [11] and genome-wide single nucleotide polymorphism (SNP) data [15] from 722 Icelanders from family cohorts to examine the heritability of gene expression in blood and adipose tissue. By studying more than one tissue type, we were able to analyze the regulation of gene expression both within and across tissues. Our goal was to answer three key questions about gene expression heritability. First, can heritability be partitioned into cis and trans components using local and genome-wide IBD between pairs of individuals? Second, to what extent are heritable components of variance shared across tissues? Third, to what extent does heritability extend to distantly related individuals inheriting IBD segments from distant ancestors?
We sought to partition the heritability of gene expression into cis versus trans components by comparing the effects of IBD at the genome-wide level (trans) to those of IBD at the local level (cis), defined as the number of chromosomes (0, 1 or 2) shared IBD at the genomic location containing the expressed gene. Our results show a substantially higher proportion of heritability due to cis regulation, 37% in blood and 24% in adipose tissue, than the 12% reported in a previous ancestry-based study of lymphoblastoid cell lines (LCL) in African Americans [16]. One possible explanation for this discrepancy is transgenerational epigenetic inheritance, which is one of the explanations proposed to account for the “missing heritability” in genetic studies of human traits [17]-[23]. Epigenetic inheritance would regulate gene expression at the cis locus, and would be expected to contribute to cis heritability in family-based analyses but not in ancestry-based analyses, given that this mode of inheritance persists over a relatively short time scale. However, by using IBD in distantly related individuals to produce similar estimates of cis heritability, we were able to rule out this hypothesis. Instead, our analyses indicate that the proportion of heritability attributable to cis regulation is tissue-specific, and that similarities in gene expression across tissues are primarily due to heritable cis effects. Thus, the proportion of gene expression heritability attributable to cis regulation is expected to increase as a function of the number of different cell types present in the tissue being assayed, consistent with results obtained from blood, adipose tissue and LCL.
Methods
Ethics statement
This research was approved by the Data Protection Commission of Iceland and the National Bioethics Committee of Iceland. The appropriate informed consent was obtained for all sample donors.
Icelandic Family Blood cohort
Relative abundances of 23,720 transcripts were obtained for blood samples from each of 1,001 individuals from the IFB cohort, as described previously [11] (see Web Resources). Values were adjusted for sex and age. We removed 4,985 transcripts that either had >5% missing data, did not map to an autosomal chromosome, or mapped to more than one genomic location. We removed 16 individuals with >5% missing data and 269 individuals for which long-range phased SNP data were not available. This left 18,735 transcripts and 716 individuals. Most of out analyses focused on 2,233 related pairs (individuals from the same family pedigree with genome-wide IBD >0.05) spanning a subset of 687 individuals.
Icelandic Family Adipose cohort
Relative abundances of 23,720 transcripts were obtained for adipose tissue samples from each of 673 individuals from the IFA cohort, as described previously [11] (see Web Resources). Values were adjusted for sex, age and body mass index (BMI), restricting to 638 individuals with BMI data. We removed 4,621 transcripts that either had >5% missing data, did not map to an autosomal chromosome, or mapped to more than one genomic location. We removed 2 individuals with >5% missing data and 67 individuals for which long-range phased SNP data were not available. This left 19,099 transcripts and 569 individuals. Most of our analyses focused on 1,700 related pairs (individuals from the same family pedigree with genome-wide IBD >0.05) spanning a subset of 531 individuals.
Local IBD estimates
Individuals were genotyped using the Illumina 300K chip. Owing to the sensitive nature of genotype data, access to these data can only be granted at the headquarters of deCODE Genetics in Iceland. Given long-range phased Illumina 300K data [15] for a pair of individuals, we partitioned the genome into 2cM blocks and for each block performed 2×2 = 4 comparisons between haplotypes from the two individuals. We declared two haplotypes to be IBD if they matched at >95% of alleles in the block, non-IBD if they matched at <85% of alleles, and unknown-IBD otherwise. We excluded SNPs with missing data in one or both individuals, so that lack of a match implies a mismatch, and set IBD status to unknown for pairs of haplotypes with >5% of SNPs excluded. We defined local IBD as the total number of comparisons producing a match. We verified that this approach infers 0∶1∶2 copies IBD between parent-child pairs with probabilities 0.2%∶99.3%∶0.4% and 0∶1∶2: copies IBD between sibling pairs with probabilities 24.9%∶50.1%∶24.9%, excluding from this computation the 7% of pairs and blocks for which inferred IBD was unknown. These numbers are a function of the thresholds we used to define IBD and non-IBD; the thresholds were largely chosen for specificity rather than sensitivity since for our application it does not matter that inferred IBD is sometimes unknown. The numbers are very close to the expected theoretical probabilities (for parent-child pairs, 2 copies IBD is expected to occasionally occur due to IBD in “unrelated” parents). This validates our use of long-range phased SNP genotypes to compute local IBD estimates. We computed genome-wide IBD estimates as the average of local IBD estimates across all 2cM blocks.
Heritability estimates using genome-wide IBD only
We applied variance-components methods to estimate narrow-sense heritability [14], [24]. The source code used in our heritability analyses is available for download (see Web Resources). Let egs denote the gene expression for gene g and individual s, normalized to have mean 0 and variance 1 across individuals. Let θst denote the genome-wide IBD between individuals s and t (0≤θst≤1) and be the N×N matrix of genome-wide IBD, where N is the number of individuals. Let Vg denote the covariance matrix of normalized gene expression for gene g. We consider the model and fit hg2, the heritability of gene g, to the observed normalized gene expression values egs by maximizing the likelihood , where . Values of egs, hg and Vg vary with tissue type, but we view tissue type as an implicit index rather than an explicit index for simplicity of notation. For both blood and adipose tissue, the estimated values of hg2 were ∼80% correlated to values that were computed previously using similar methods [11], despite the fact that the current analysis was restricted to a subset of individuals for which long-range phased SNP data was available for local IBD inference. We declare hg2>0 to be nominally significant (P<0.05) if hg2 is larger than each value analogously estimated from 19 data sets with sample labels randomly permuted (thus, 5% of genes will be nominally significant even in the absence of a true effect). We estimated average h2 as the average of hg2 across genes g. We computed standard errors on h2 by performing independent runs with sample labels randomly permuted; we obtained identical standard errors using either five permutations or 20 permutations. Similar procedures were used in the cis vs. trans and cross-tissue analyses described below.
Heritability estimates using both cis and trans IBD
We extended the variance-components approach to cis and trans heritability via the model , where is the N×N matrix of local (cis) IBD between individuals s and t at the genomic location proximal to gene g. We used the midpoint of the gene expression probe to define genomic location, but the value of γgst is not sensitive to this choice as local IBD segments between related individuals span many megabases. We scale γgst to have value 0.0, 0.5, or 1.0 (for 0, 1, or 2 copies shared). We fit the cis heritability hg,cis2 and trans heritability hg,trans2 by maximizing the usual likelihood, fitting hg2 = hg,cis2+hg,trans2 and hg,cis2 in turn. We average across genes g to estimate hcis2 and htrans2. We define the proportion of heritable gene expression variation that is due to cis regulation as πcis = hcis2/(hcis2+htrans2). As above, all values vary with tissue type, which we view as an implicit index.
Cross-tissue analysis
The cross-tissue correlation ρ was computed as the correlation between normalized expression levels in blood and adipose tissue across genes and individuals. Due to the normalization, this is equal to the average of gene-specific correlations ρg. We computed standard errors of both gene-specific and average cross-tissue correlations via jackknife, repeating the computation with each individual removed in turn and estimating the standard error as times the standard deviation of the N estimates. We now describe our estimation of cross-tissue heritability. Let and denote normalized expression levels for gene g and individual s in blood and adipose tissue, respectively. Let Wg denote the covariance matrix of the vector (ebg,eag) of length 2N. Here the relevant equations are where ξ2 denotes cross-tissue heritability, ρ denotes cross-tissue correlation, and denotes the tensor product of a 2×2 matrix with an N×N matrix to form a 2N×2N matrix. For example, the first term of Wg has entries hbg,cis2γgst in the upper left N×N block, ξg,cis2γgst in the upper right N×N block, and so on. This generalization of the variance-components approach to cross-phenotype analyses has been previously described (for the case of genome-wide IBD) in an analysis of two height phenotypes, self-reported height and clinically measured height [25]. The likelihood is defined in the usual way, replacing Vg with Wg and eg with (ebg,eag). We fit hbg2 = hbg,cis2+hbg,trans2, hbg,cis2, hag2 = hag,cis2+hag,trans2, hag,cis2, ρg, ξg2 = ξg,cis2+ξg,trans2 and ξg,cis2 in turn. For each of the parameters estimated, we compute average values by averaging across genes g.
Web Resources
-
http://www.ncbi.nlm.nih.gov/geo/ (Gene Expression Omnibus). Gene expression data sets have been deposited into the GEO database under accession numbers GSE7965 and GPL3991, as described previously [11].
-
http://www.hsph.harvard.edu/faculty/alkes-price/software/. The source code used in our heritability analyses is available for download, along with the results presented in Table S1.
Results
Overall heritability of gene expression
For the analysis of gene expression in blood, we analyzed normalized intensity values for 18,735 mRNA transcripts. Analysis was restricted to 687 individuals from the IFB cohort for whom long-range phased SNP data were available (see Methods). For each pair of individuals, we used the long-range phased SNP data to compute the number of chromosomes shared IBD at each location in the genome, and computed the genome-wide IBD as an average of these values (Figure 1; see Methods). Our initial analyses focused on 2,233 related pairs with genome-wide IBD >0.05. For the analysis of gene expression in adipose tissue, we similarly analyzed 19,099 mRNA transcripts of 531 individuals from the IFA cohort, focusing on 1,700 related pairs with genome-wide IBD >0.05 (see Methods). The IFA cohort largely overlaps the IFB cohort, with 496 of the 722 individuals analyzed appearing in both cohorts.
Fig. 1. Local and genome-wide IBD. 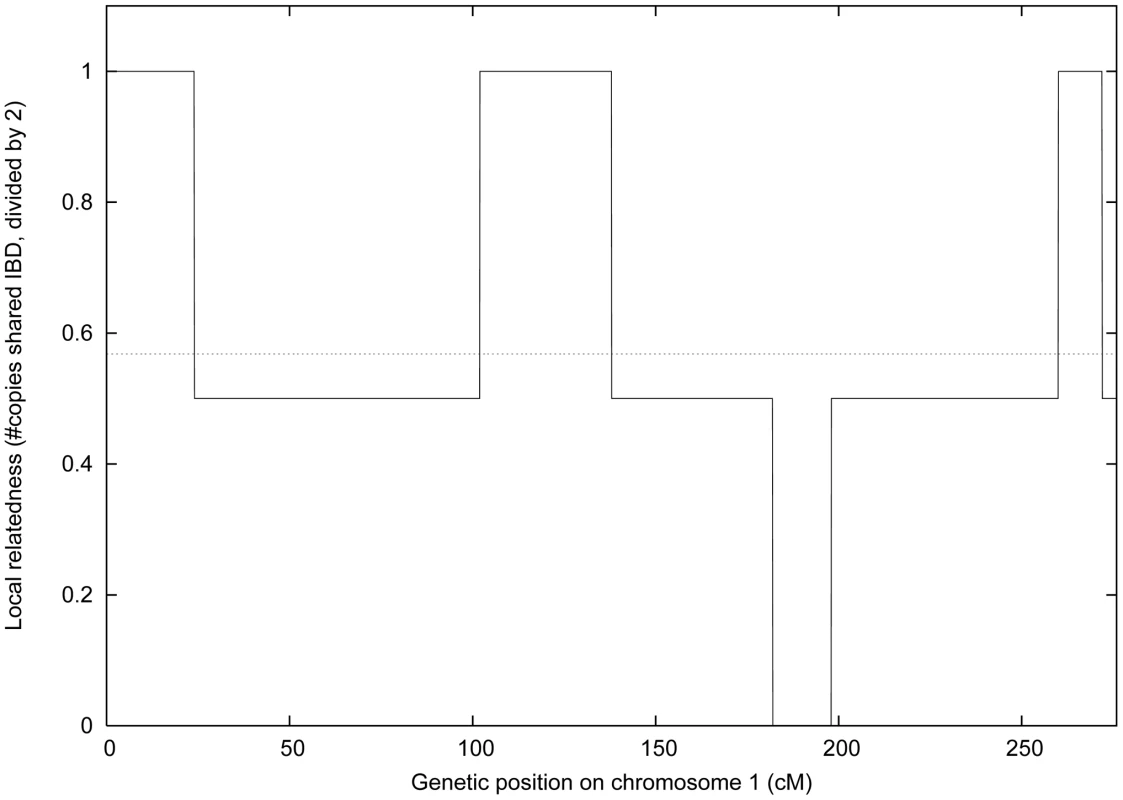
We plot the local relatedness (0, 1 or 2 copies IBD) between two siblings from the IFB cohort at each 2cM block on chromosome 1. The dotted line represents their genome-wide relatedness of 0.568, which is within the expected range for siblings [46]. We estimated the overall heritability hg2 for each gene g using variance-component methods [14] (see Methods). Although estimates for each gene g are statistically noisy at these sample sizes, histograms show a clear positive bias for both IFB and IFA cohorts (Figure S1 and Table S1), and hg2>0 was nominally significant (P = 0.05; see Methods) for an excess of genes: 42% for IFB and 63% for IFA. We computed the average h2 as the average of hg2 across genes g. A relevant question is whether or not to allow negative values of hg2 when computing this average [26]. Such values have no biological interpretation (except in the case of negative correlation among siblings in traits that depend on birth order). However, because values close to zero may be either increased or decreased by statistical noise—leading to negative estimates of hg2 for 3,031 of 18,735 genes for IFB and 1,038 of 19,099 genes for IFA—we elected to allow negative values in our main computations so as to produce an unbiased estimate of average h2. We obtained estimates of h2 = 0.150 for blood and h2 = 0.234 for adipose tissue. We obtained similar results when using a regression-based approach to estimate average h2 (Text S1), which more readily lends itself to visualization (Figure 2A and 2B). (When clipping negative hg2 values to zero, we obtained h2 = 0.159 for blood and h2 = 0.237 for adipose tissue.) Our results are consistent with previous analyses reporting that expression levels of a substantial fraction of genes are significantly heritable at the level of h2 = 0.3 or higher [10]-[13], [26].
Fig. 2. Family heritability in the IFB and IFA cohorts. 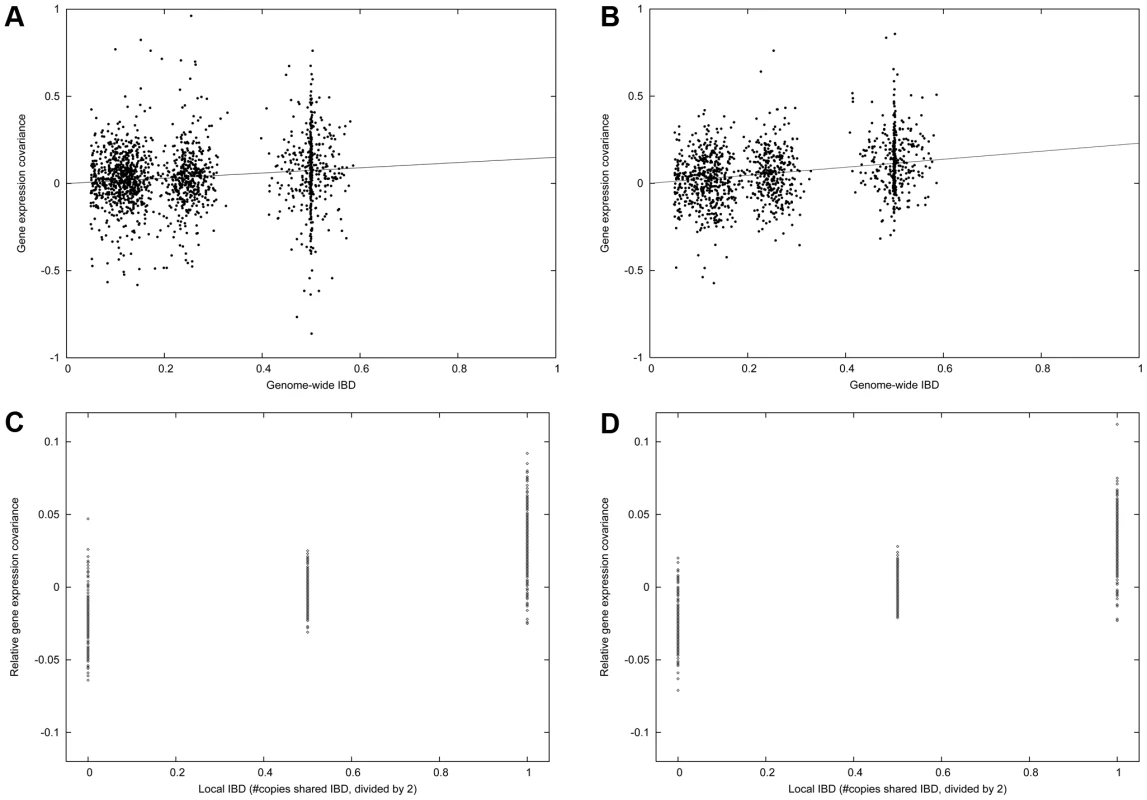
(A) Gene expression covariance (average value of product of normalized gene expression measurements) between related individuals in the IFB cohort varies with genome-wide IBD. Each point represents one pair of related individuals. The slope of this plot corresponds to the regression-based estimate of h2. (B) Same as (A), for IFA cohort. (C) Gene expression covariance between siblings for genes with 0, 1 or 2 copies IBD at the cis locus, minus total covariance as displayed above. The slope of this plot corresponds to the regression-based estimate of hcis2. The signal to noise ratio is higher in this plot due to reduced effects of systematic noise covariance. (D) Same as (C), for IFA cohort. Cis versus trans heritability of gene expression
While estimates of overall heritability are based on genome-wide IBD, it is possible to estimate cis versus trans heritability by extending variance components to consider both local (cis) IBD at the genomic location close to the expressed gene, and genome-wide (trans) IBD (see Methods). As before, analyses were restricted to 2,233 and 1,700 related pairs from the IFB and IFA cohorts, respectively. Histograms of hg,cis2 and hg,trans2 estimates for each gene g show a clear positive bias for both IFB and IFA cohorts (Figure S2 and Table S1), with an excess of nominally significant (P<0.05) genes for IFB (hg,cis2>0 : 16%; hg,trans2>0 : 19%) and IFA (hg,cis2>0 : 16%; hg,trans2>0 : 30%). For IFB, we obtained average cis and trans heritability estimates of hcis2 = 0.055 and htrans2 = 0.095, respectively, which sum to h2 = 0.150. This leads to the conclusion that the proportion of heritability of expression due to cis variants in blood is πcis = 37%. For IFA, we obtained estimates of hcis2 = 0.057 and htrans2 = 0.177, which sum to h2 = 0.234. This yields an estimate of πcis = 24% in adipose tissue. The values of h2 and htrans2 in adipose tissue are significantly higher than for blood, but hcis2 is similar, leading to a lower value of πcis. We obtained similar results when using a regression-based approach to estimate average hcis2 and htrans2 (Text S1; Figure 2C and 2D). We note that there is considerably less statistical uncertainty in estimates of hcis2 (Figure 2C and 2D) than in estimates of h2 (Figure 2A and 2B). Indeed, we obtained standard errors of h2 = 0.150±0.011, hcis2 = 0.055±0.001 and htrans2 = 0.095±0.010 for blood and h2 = 0.234±0.011, hcis2 = 0.057±0.002 and htrans2 = 0.177±0.010 for adipose tissue (see Methods). These standard errors are 7-100 times lower than standard errors for single-gene heritability estimates, which are inadequate for estimating πcis (see Text S1). The much lower standard errors for hcis2 are a consequence of variation in cis IBD across the genome that decouples the estimation of this parameter from the systematic noise covariance structure across all pairs of individuals (see Text S1). Based on these standard errors for hcis2 and htrans2, πcis has little statistical uncertainty, although results may be affected by modeling uncertainty.
Our heritability model does not account for the possibility of phenotypic similarity in related individuals due to shared environment, which can confound estimates of heritability [14]. We note that such effects would inflate estimates of h2 and htrans2, but have a negligible impact on hcis2, since the extent of shared environment would be related to genome-wide (trans) rather than local (cis) IBD. To investigate the possibility of confounding due to shared environment, we computed the average correlation in gene expression between spouses, who are genetically unrelated but have a shared environment. We observed average correlations of 0.074±0.042 in 33 IFB spouse pairs and 0.076±0.035 in 28 IFA spouse pairs, which are similar in magnitude to correlations between sib-sib or parent-child pairs that correspond to the average heritabilities reported above (see Text S1 and Table S2). Thus, there is strong evidence that shared environment can lead to similarity in gene expression phenotypes. We further investigated whether the gene by gene signature of correlations in spouse pairs matches the signature of correlations in sib-sib or parent-child pairs or estimates of hg2, but found that it does not (see Text S1 and Table S3). Thus, we hypothesize that the correlations in spouse pairs are due to very recent shared environment (e.g. diet) arising from sharing the same household, whereas the correlations in sib-sib and parent-child pairs in this study (who are unlikely to share the same household, since only adult individuals were sampled) are due to genetic heritability. However, we cannot rule out a small amount of inflation in h2 and htrans2 estimates due to shared environment in related individuals.
Assessing the impact of epigenetic inheritance on cis heritability
Our family-based estimates of πcis in blood and adipose tissue are considerably greater than a previous estimate of 12±3% obtained using lymphoblastoid cell lines (LCL) from African-Americans, in which local versus genome-wide European ancestry was used to infer the relative contribution of cis versus trans heritability [16]. An analogous ancestry-based analysis of LCL gene expression data [27] from admixed HapMap 3 Mexican-Americans [28] has produced a similarly low value of πcis = 13±9%. One possible explanation for the lower values as compared to family-based estimates could be the epigenetic inheritance of cis-acting factors other than DNA sequence that are transmitted from parent to offspring. Given the relatively short time scale of epigenetic inheritance, this would be expected to have a much greater impact on family-based estimates of πcis than those based on ancestry [22]-[23].
To further explore the epigenetic hypothesis, we repeated the cis versus trans analysis using subsets of unrelated or distantly related individuals (genome-wide IBD <0.01) from the IFB and IFA cohorts. The mean genome-wide IBD for all such pairs of individuals was 0.0044, with a standard deviation of 0.0018, consistent with the known properties of distant relatedness between “unrelated” individuals from Iceland as well as other world populations [29]-[31]. We independently generated five random subsets of IFB individuals (85, 87, 92, 93, 91 individuals) and five random subsets of IFA individuals (127, 85, 92, 95, 89 individuals) with genome-wide IBD <0.01 between each pair of individuals in each subset, such that each subset was maximal subject to this constraint. The resulting estimates of hcis2 were 0.057±0.008 for blood and 0.067±0.005 for adipose tissue (mean ± standard deviation across five subsets). These estimates of hcis2 were close to our previous estimates based on closely related pairs, thereby ruling out a substantial contribution of epigenetic inheritance to cis heritability (see Discussion). However, we did not obtain meaningful estimates of htrans2 using distantly related individuals, due to the systematic noise covariance structure (see Text S1), and therefore πcis could not be estimated. We note that similar results for distantly related pairs were obtained using different IBD estimation algorithms (see Text S1).
Cross-tissue analysis
We conducted a cross-tissue analysis of expression heritability in blood and adipose tissue in 496 individuals who overlapped between the IFB and IFA cohorts. We determined that an individual’s blood expression for a particular gene is slightly but significantly correlated to the same individual’s adipose expression for the same gene, with an average correlation of ρ = 0.041±0.005 (mean ± standard error) (see Methods). Although estimates for each gene g are statistically noisy at these sample sizes, histograms show a clear positive bias in ρg (Figure S3), and ρg>0 was nominally significant (P = 0.05) for 20% of genes, a significant excess.
We next investigated the relationship between an individual’s blood expression and a related individual’s adipose expression, using variance-components methods (see Methods). This revealed that cross-tissue similarity varies with the level of family relatedness, with an average cross-tissue heritability estimate of ξ2 = 0.030±0.006. Analogous to the analyses for single tissues, we partitioned the cross-tissue heritability into cis and trans components, yielding values of ξcis2 = 0.031±0.001 and ξtrans2 = -0.001±0.006. We obtained similar results using regression-based approaches (Text S1; Figure 3A and 3B). Histograms of cross-heritability estimates for each gene g show a positive bias for ξg2 and ξg,cis2, but not ξg,trans2, for which the histogram is symmetric about zero (Figure S4). While our estimate of ξtrans2 is not significantly different from zero, ξcis2 is highly significant and explains the bulk of our estimate of ρ. This implies that the extent to which gene expression in blood and adipose tissue is similar across genes and individuals is dominated by heritable effects at the cis locus.
Fig. 3. Cross-tissue heritability in the IFB and IFA cohorts. 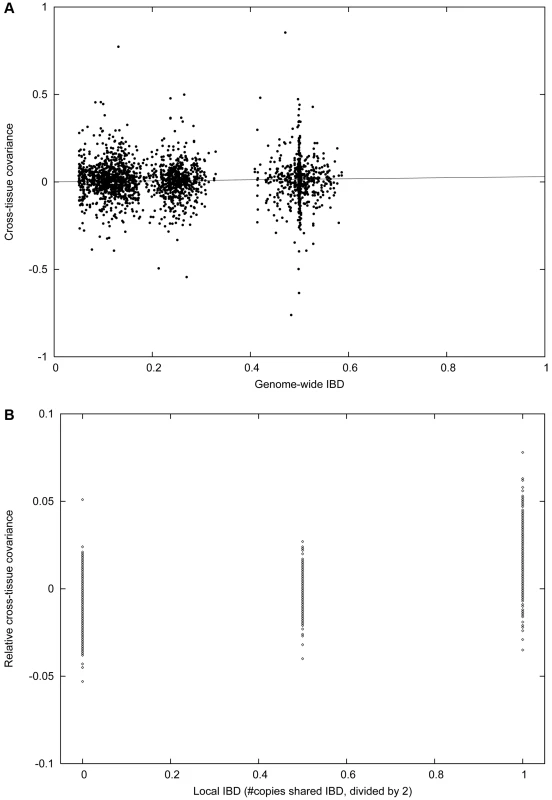
Plots are analogous to those in Figure 2, except that we analyze the covariance between related individuals in different tissues, instead of between related individuals in the same tissue. (A) Cross-tissue covariance between related individuals in the intersection of IFB and IFA varies with genome-wide IBD. The slope of this plot corresponds to the regression-based estimate of ξ2. (B) Cross-tissue covariance between siblings in the intersection of IFB and IFA with 0, 1 or 2 copies IBD at the cis locus, minus total covariance as displayed in (A). The slope of this plot corresponds to ξcis2. Averaging across cell types with shared cis effects increases the value of πcis
Our finding that cross-tissue similarities are dominated by heritable cis effects leads to the mathematical result that πcis is expected to increase with tissue heterogeneity: as the number of cell types represented in a tissue increases, the strongly correlated cis effects will add linearly but the uncorrelated trans effects will be diluted. In detail, let x and y denote cells types and suppose that Cov(exgs,exgt) = Cov(eygs,eygt) = hcis2γgst+htrans2θst for all genes g and individuals s≠t, and that all cis effects (but no trans or non-genetic effects) are shared across cell types. Thus, Cov(exgs,eygt) = hcis2γgst. Now consider a tissue z containing cell types x and y. Up to a normalization constant, Cov(ezgs,ezgt) = Cov(0.5(exgs+eygs),0.5(exgt+eygt)) = hcis2γgst+0.5htrans2θst, so that πcis,z = hcis2/(hcis2+0.5htrans2) is larger than πcis,x = πcis,y = hcis2/(hcis2+htrans2).
We verified this theoretical result empirically by defining ezgs = ebgs + eags as the average of normalized gene expression in blood and adipose tissue, normalized to mean 0 and variance 1. For synthetic tissue z, we obtained the value πcis = 0.41, which is larger than the value of πcis for either blood or adipose tissue, and similar to the predicted value of 0.055/(0.055+0.25(0.095+0.177)) = 0.45 based on hcis2 and htrans2 (πcis < 0.45 is actually expected since not all cis effects are shared). Thus, the variability in πcis across tissue types (0.12 for LCL, 0.24 for adipose, 0.37 for blood) is consistent with the fact that LCL represent a single cell type, whereas adipose tissue and blood contain many cell types: adipose tissue contains smooth muscle cells, fibroblasts, adipocytes, mast-cells and endothelial cells, while blood contains erythrocytes, thrombocytes, neutrophils, lymphocytes, monocytes, eosinophils and basophils in proportions that vary across individuals [32]-[34]. This also explains why studies of individual cell types have been more successful in identifying trans eQTLs than studies of whole tissues, and why most replications across tissue types occur at cis eQTLs [11], [34]-[37].
Discussion
In this study, we observed a greater contribution of cis regulation in blood and adipose tissue than in a previous ancestry-based analysis of LCL in African-Americans [16]. This result is not sensitive to sample size, because although estimates for individual genes are statistically noisy, we considered averages across genes. We also observed that cross-tissue similarity between blood and adipose expression is genetically heritable and dominated by cis effects. These two results are highly concordant. Due to the dilution of trans effects that are not shared across cell types, cis regulation is expected to explain a greater proportion of heritability in tissue types that are heterogeneous in their cell composition, such as blood and adipose tissue—particularly blood, in which cell type proportions may vary among individuals. This highlights the importance of considering different tissue types [16]. However, other explanations for the higher contribution of cis regulation in this study than in the ancestry-based analysis are also possible. For example, epistasis between two neighboring cis variants would be included in cis heritabilities estimated via IBD, but not in the ancestry-based analysis in which ancestry is a partial proxy for SNP genotype but a very poor proxy for both genotypes of two interacting SNPs. In addition, epistatic interactions involving multiple loci may potentially be important, and may confound estimates of narrow-sense heritability, but are outside the scope of this study. A further possibility is that trans effects in LCL could be overstated due to genetically heritable variation of in vitro factors such as the response to EBV virus, which would mimic trans regulation in heritability analyses but does not reflect true biological trans regulation [38]. Distinguishing between these possibilities is an important direction of future work.
Efforts to understand cis regulation are likely to benefit from combining information from many cell or tissue types, since underlying mechanisms can be either shared or cell-type specific. Indeed, our finding that on average roughly half single-tissue cis heritability (hcis2) is shared across tissues (ξcis2) is consistent with a recent study focusing on cis eQTLs, which reported that 54%, 50% and 54% of cis eQTLs in fibroblasts, LCLs and T cells, respectively, are cell-type specific [36]. Those percentages would be expected to be higher when considering only two cell types, but lower at larger sample sizes. On the other hand, studies of trans regulation should focus on a single cell type to avoid diluting trans effects that are not shared across cell types. New technologies to assay cell type-specific gene expression in complex tissues may also prove valuable [39]. Future experiments will shed light on whether similarity between tissues other than blood and adipose is also predominantly explained by heritable cis effects. Results may vary by organism as well as tissue type. Recent studies of fat, kidney, adrenal and heart tissues in rat recombinant inbred strains also observed reduced trans effects in more heterogeneous tissues, but reported some evidence of cross-tissue regulation in trans as well as in cis [8]-[9].
The similarity of cis heritability results using IBD in closely related versus distantly related individuals has significant implications. It has been suggested that epigenetic inheritance, defined as the transmission across generations of epigenetic changes not due to variation in DNA sequence, is a potential source of the “missing heritability” in genetic association studies [17]-[21]. Epigenetic inheritance would be expected to influence expression at the cis locus, and would be expected to contribute to cis heritability between closely related individuals but not between distantly related individuals, given that this mode of inheritance persists over a relatively short time scale [22]-[23]. Our failure to observe any such discordance suggests that transgenerational epigenetic inheritance is unlikely to play a major role in the missing heritability of gene expression and other traits, although it does not rule out a very small aggregate effect across all genes or large effects at certain metastable epialleles [40]-[41], nor does it shed light on the importance of mitotically conserved epigenetic effects that are not transmitted from parent to offspring.
Our results highlight the utility of using IBD in distantly related individuals to make inferences about heritability. This approach will be particularly valuable as sample sizes increase, since the number of pairs of individuals increases quadratically with sample size. Indeed, IBD in distantly related individuals has already proven useful for mapping specific loci [42], and heritability-related analyses using identity-by-state (IBS) instead of IBD have also yielded important insights [43]-[45]. By using IBD segments shorter than those analyzed here to consider IBD sharing at different distances from genes, it may even be possible draw conclusions about the distribution of genomic distances at which cis regulation contributes to heritability.
Supporting Information
Zdroje
1. CooksonW
LiangL
AbecasisG
MoffattM
LathropM
2009 Mapping complex disease traits with global gene expression. Nat Rev Genet 10 184 194
2. SchadtEE
MonksSA
DrakeTA
LusisAJ
CheN
2003 Genetics of gene expression surveyed in maize, mouse and man. Nature 422 297 302
3. MorleyM
MolonyCM
WeberTM
DevlinJL
EwensKG
2004 Genetic analysis of genome-wide variation in human gene expression. Nature 430 743 747
4. GiladY
RifkinSA
PritchardJK
2008 Revealing the architecture of gene regulation: the promise of eQTL studies. Trends Genet 24 408 415
5. CheungVG
NayakRR
WangIX
ElwynS
CousinsSM
MorleyM
SpielmanRS
Polymorphic cis - and trans-regulation of human gene expression. PLoS Biol 8 e1000480 doi:10.1371/journal.pbio.1000480
6. CheslerEJ
LuL
ShouS
QuY
GuJ
2005 Complex trait analysis of gene expression uncovers polygenic and pleiotropic networks that modulate nervous system function. Nat Genet 37 233 242
7. WilliamsRB
CotsapasCJ
CowleyMJ
ChanE
NottDJ
2006 Normalization procedures and detection of linkage signal in genetical-genomics experiments. Nat Genet 38 855 856; author reply 856-859
8. PetrettoE
MangionJ
DickensNJ
CookSA
KumaranMK
2006 Heritability and tissue specificity of expression quantitative trait loci. PLoS Genet 2 e172 doi:10.1371/journal.pgen.0020172
9. PetrettoE
BottoloL
LangleySR
HeinigM
McDermott-RoeC
2010 New insights into the genetic control of gene expression using a Bayesian multi-tissue approach. PLoS Comput Biol 6 e1000737 doi:10.1371/journal.pcbi.1000737
10. MonksSA
LeonardsonA
ZhuH
CundiffP
PietrusiakP
2004 Genetic inheritance of gene expression in human cell lines. Am J Hum Genet 75 1094 1105
11. EmilssonV
ThorleifssonG
ZhangB
LeonardsonAS
ZinkF
2008 Genetics of gene expression and its effect on disease. Nature 452 423 428
12. StrangerBE
NicaAC
ForrestMS
DimasA
BirdCP
2007 Population genomics of human gene expression. Nat Genet 39 1217 1224
13. DixonAL
LiangL
MoffattMF
ChenW
HeathS
2007 A genome-wide association study of global gene expression. Nat Genet 39 1202 1207
14. VisscherPM
HillWG
WrayNR
2008 Heritability in the genomics era--concepts and misconceptions. Nat Rev Genet 9 255 266
15. KongA
MassonG
FriggeML
GylfasonA
ZusmanovichP
2008 Detection of sharing by descent, long-range phasing and haplotype imputation. Nat Genet 40 1068 1075
16. PriceAL
PattersonN
HancksDC
MyersS
ReichD
2008 Effects of cis and trans genetic ancestry on gene expression in African Americans. PLoS Genet 4 e1000294 doi:10.1371/journal.pgen.1000294
17. McCarthyMI
HirschhornJN
2008 Genome-wide association studies: potential next steps on a genetic journey. Hum Mol Genet 17 R156 165
18. MaherB
2008 Personal genomes: The case of the missing heritability. Nature 456 18 21
19. ManolioTA
CollinsFS
CoxNJ
GoldsteinDB
HindorffLA
2009 Finding the missing heritability of complex diseases. Nature 461 747 753
20. EichlerEE
FlintJ
GibsonG
KongA
LealSM
2010 Missing heritability and strategies for finding the underlying causes of complex disease. Nat Rev Genet 11 446 450
21. KaminskyZA
TangT
WangSC
PtakC
OhGH
2009 DNA methylation profiles in monozygotic and dizygotic twins. Nat Genet 41 240 245
22. YoungsonNA
WhitelawE
2008 Transgenerational epigenetic effects. Annu Rev Genomics Hum Genet 9 233 257
23. JablonkaE
RazG
2009 Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. Q Rev Biol 84 131 176
24. AmosCI
1994 Robust variance-components approach for assessing genetic linkage in pedigrees. Am J Hum Genet 54 535 543
25. MacgregorS
CornesBK
MartinNG
VisscherPM
2006 Bias, precision and heritability of self-reported and clinically measured height in Australian twins. Hum Genet 120 571 580
26. McRaeAF
MatigianNA
VadlamudiL
MulleyJC
MowryB
2007 Replicated effects of sex and genotype on gene expression in human lymphoblastoid cell lines. Hum Mol Genet 16 364 373
27. StrangerBE
MontgomerySB
DimasA
PartsL
IngleCE
Patterns of cis regulatory variation in diverse human populations. Submitted
28. The International HapMap 3 Consortium 2010 An integrated haplotype map of rare and common genetic variation in diverse human populations. Nature 467 52 8
29. HelgasonA
PalssonS
GudbjartssonDF
KristjanssonT
StefanssonK
2008 An association between the kinship and fertility of human couples. Science 319 813 816
30. The International HapMap Consortium 2007 A second generation human haplotype map of over 3.1 million SNPs. Nature 449 851 61
31. GusevA
LoweJK
StoffelM
DalyMJ
AltshulerD
2009 Whole population, genome-wide mapping of hidden relatedness. Genome Res 19 318 326
32. AstoriG
VignatiF
BardelliS
TubioM
GolaM
2007 "In vitro" and multicolor phenotypic characterization of cell subpopulations identified in fresh human adipose tissue stromal vascular fraction and in the derived mesenchymal stem cells. J Transl Med 5 55
33. JosephsonB
DahlbergG
1952 Variations in the cell-content and chemical composition of the human blood due to age, sex and season. Scand J Clin Lab Invest 4 216 236
34. CheungVG
SpielmanRS
2009 Genetics of human gene expression: mapping DNA variants that influence gene expression. Nat Rev Genet 10 595 604
35. SchadtEE
MolonyC
ChudinE
HaoK
YangX
2008 Mapping the genetic architecture of gene expression in human liver. PLoS Biol 6 e107 doi:10.1371/journal.pbio.0060107
36. DimasAS
DeutschS
StrangerBE
MontgomerySB
BorelC
2009 Common regulatory variation impacts gene expression in a cell type-dependent manner. Science 325 1246 1250
37. IdaghdourY
CzikaW
ShiannaKV
LeeSH
VisscherPM
2010 Geographical genomics of human leukocyte gene expression variation in southern Morocco. Nat Genet 42 62 67
38. ChoyE
YelenskyR
BonakdarS
PlengeRM
SaxenaR
2008 Genetic analysis of human traits in vitro: drug response and gene expression in lymphoblastoid cell lines. PLoS Genet 4 e1000287 doi:10.1371/journal.pgen.1000287
39. Shen-OrrSS
TibshiraniR
KhatriP
BodianDL
StaedtlerF
2010 Cell type-specific gene expression differences in complex tissues. Nat Methods 7 287 289
40. MorganHD
SutherlandHG
MartinDI
WhitelawE
1999 Epigenetic inheritance at the agouti locus in the mouse. Nat Genet 23 314 318
41. RakyanVK
ChongS
ChampME
CuthbertPC
MorganHD
2003 Transgenerational inheritance of epigenetic states at the murine Axin(Fu) allele occurs after maternal and paternal transmission. Proc Natl Acad Sci U S A 100 2538 2543
42. KennyEE
GusevA
RiegelK
LutjohannD
LoweJK
2009 Systematic haplotype analysis resolves a complex plasma plant sterol locus on the Micronesian Island of Kosrae. Proc Natl Acad Sci U S A 106 13886 13891
43. KangHM
SulJH
ServiceSK
ZaitlenNA
KongSY
2010 Variance component model to account for sample structure in genome-wide association studies. Nat Genet 42 348 354
44. YangJ
BenyaminB
McEvoyBP
GordonS
HendersAK
2010 Common SNPs explain a large proportion of the heritability for human height. Nat Genet 42 565 569
45. PowellJE
VisscherPM
GoddardME
2010 Reconciling the analysis of IBD and IBS in complex trait studies. Nat Rev Genet
46. VisscherPM
MedlandSE
FerreiraMA
MorleyKI
ZhuG
2006 Assumption-free estimation of heritability from genome-wide identity-by-descent sharing between full siblings. PLoS Genet 2 e41 doi:10.1371/journal.pgen.0020041
Štítky
Genetika Reprodukční medicína
Článek Break to Make a ConnectionČlánek A New Testing Strategy to Identify Rare Variants with Either Risk or Protective Effect on DiseaseČlánek The Architecture of Gene Regulatory Variation across Multiple Human Tissues: The MuTHER Study
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 2- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Break to Make a Connection
- A New Testing Strategy to Identify Rare Variants with Either Risk or Protective Effect on Disease
- Single-Tissue and Cross-Tissue Heritability of Gene Expression Via Identity-by-Descent in Related or Unrelated Individuals
- Pervasive Adaptive Protein Evolution Apparent in Diversity Patterns around Amino Acid Substitutions in
- The Architecture of Gene Regulatory Variation across Multiple Human Tissues: The MuTHER Study
- MiRNA Control of Vegetative Phase Change in Trees
- New Functions of Ctf18-RFC in Preserving Genome Stability outside Its Role in Sister Chromatid Cohesion
- Genome-Wide Association Studies of the PR Interval in African Americans
- Mapping of the Disease Locus and Identification of As a Candidate Gene in a Canine Model of Primary Open Angle Glaucoma
- Mapping a New Spontaneous Preterm Birth Susceptibility Gene, , Using Linkage, Haplotype Sharing, and Association Analysis
- A Population Genetic Approach to Mapping Neurological Disorder Genes Using Deep Resequencing
- and Genes Modulate the Switch between Attraction and Repulsion during Behavioral Phase Change in the Migratory Locust
- Targeted Sister Chromatid Cohesion by Sir2
- Correlated Evolution of Nearby Residues in Drosophilid Proteins
- Parallel Evolution of a Type IV Secretion System in Radiating Lineages of the Host-Restricted Bacterial Pathogen
- Lipophorin Receptors Mediate the Uptake of Neutral Lipids in Oocytes and Imaginal Disc Cells by an Endocytosis-Independent Mechanism
- Genome-Wide Association Study of Coronary Heart Disease and Its Risk Factors in 8,090 African Americans: The NHLBI CARe Project
- The Evolution of Host Specialization in the Vertebrate Gut Symbiont
- Genome-Wide Association of Familial Late-Onset Alzheimer's Disease Replicates and and Nominates in Interaction with
- Risk Alleles for Systemic Lupus Erythematosus in a Large Case-Control Collection and Associations with Clinical Subphenotypes
- Association between Common Variation at the Locus and Changes in Body Mass Index from Infancy to Late Childhood: The Complex Nature of Genetic Association through Growth and Development
- AID Induces Double-Strand Breaks at Immunoglobulin Switch Regions and Causing Chromosomal Translocations in Yeast THO Mutants
- A Study of CNVs As Trait-Associated Polymorphisms and As Expression Quantitative Trait Loci
- Whole-Genome Comparison Reveals Novel Genetic Elements That Characterize the Genome of Industrial Strains of
- Prevalence of Epistasis in the Evolution of Influenza A Surface Proteins
- Srf1 Is a Novel Regulator of Phospholipase D Activity and Is Essential to Buffer the Toxic Effects of C16:0 Platelet Activating Factor
- Two Frizzled Planar Cell Polarity Signals in the Wing Are Differentially Organized by the Fat/Dachsous Pathway
- Phosphoinositide Regulation of Integrin Trafficking Required for Muscle Attachment and Maintenance
- Pathogenic VCP/TER94 Alleles Are Dominant Actives and Contribute to Neurodegeneration by Altering Cellular ATP Level in a IBMPFD Model
- Meta-Analysis of Genome-Wide Association Studies in Celiac Disease and Rheumatoid Arthritis Identifies Fourteen Non-HLA Shared Loci
- A Genome-Wide Study of DNA Methylation Patterns and Gene Expression Levels in Multiple Human and Chimpanzee Tissues
- Nucleosomes Containing Methylated DNA Stabilize DNA Methyltransferases 3A/3B and Ensure Faithful Epigenetic Inheritance
- Mutations in Zebrafish Result in Adult-Onset Ocular Pathogenesis That Models Myopia and Other Risk Factors for Glaucoma
- [], the Prion Formed by the Chromatin Remodeling Factor Swi1, Is Highly Sensitive to Alterations in Hsp70 Chaperone System Activity
- Characterization of Transcriptome Remodeling during Cambium Formation Identifies and As Opposing Regulators of Secondary Growth
- The Cardiac Transcription Network Modulated by Gata4, Mef2a, Nkx2.5, Srf, Histone Modifications, and MicroRNAs
- Epistatic Interaction Maps Relative to Multiple Metabolic Phenotypes
- Quantitative Models of the Mechanisms That Control Genome-Wide Patterns of Transcription Factor Binding during Early Development
- Genome-Wide Transcript Profiling of Endosperm without Paternal Contribution Identifies Parent-of-Origin–Dependent Regulation of
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Meta-Analysis of Genome-Wide Association Studies in Celiac Disease and Rheumatoid Arthritis Identifies Fourteen Non-HLA Shared Loci
- MiRNA Control of Vegetative Phase Change in Trees
- Risk Alleles for Systemic Lupus Erythematosus in a Large Case-Control Collection and Associations with Clinical Subphenotypes
- The Cardiac Transcription Network Modulated by Gata4, Mef2a, Nkx2.5, Srf, Histone Modifications, and MicroRNAs
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



