-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genome-Wide Association Studies of the PR Interval in African Americans
The PR interval on the electrocardiogram reflects atrial and atrioventricular nodal conduction time. The PR interval is heritable, provides important information about arrhythmia risk, and has been suggested to differ among human races. Genome-wide association (GWA) studies have identified common genetic determinants of the PR interval in individuals of European and Asian ancestry, but there is a general paucity of GWA studies in individuals of African ancestry. We performed GWA studies in African American individuals from four cohorts (n = 6,247) to identify genetic variants associated with PR interval duration. Genotyping was performed using the Affymetrix 6.0 microarray. Imputation was performed for 2.8 million single nucleotide polymorphisms (SNPs) using combined YRI and CEU HapMap phase II panels. We observed a strong signal (rs3922844) within the gene encoding the cardiac sodium channel (SCN5A) with genome-wide significant association (p<2.5×10−8) in two of the four cohorts and in the meta-analysis. The signal explained 2% of PR interval variability in African Americans (beta = 5.1 msec per minor allele, 95% CI = 4.1–6.1, p = 3×10−23). This SNP was also associated with PR interval (beta = 2.4 msec per minor allele, 95% CI = 1.8–3.0, p = 3×10−16) in individuals of European ancestry (n = 14,042), but with a smaller effect size (p for heterogeneity <0.001) and variability explained (0.5%). Further meta-analysis of the four cohorts identified genome-wide significant associations with SNPs in SCN10A (rs6798015), MEIS1 (rs10865355), and TBX5 (rs7312625) that were highly correlated with SNPs identified in European and Asian GWA studies. African ancestry was associated with increased PR duration (13.3 msec, p = 0.009) in one but not the other three cohorts. Our findings demonstrate the relevance of common variants to African Americans at four loci previously associated with PR interval in European and Asian samples and identify an association signal at one of these loci that is more strongly associated with PR interval in African Americans than in Europeans.
Published in the journal: . PLoS Genet 7(2): e32767. doi:10.1371/journal.pgen.1001304
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001304Summary
The PR interval on the electrocardiogram reflects atrial and atrioventricular nodal conduction time. The PR interval is heritable, provides important information about arrhythmia risk, and has been suggested to differ among human races. Genome-wide association (GWA) studies have identified common genetic determinants of the PR interval in individuals of European and Asian ancestry, but there is a general paucity of GWA studies in individuals of African ancestry. We performed GWA studies in African American individuals from four cohorts (n = 6,247) to identify genetic variants associated with PR interval duration. Genotyping was performed using the Affymetrix 6.0 microarray. Imputation was performed for 2.8 million single nucleotide polymorphisms (SNPs) using combined YRI and CEU HapMap phase II panels. We observed a strong signal (rs3922844) within the gene encoding the cardiac sodium channel (SCN5A) with genome-wide significant association (p<2.5×10−8) in two of the four cohorts and in the meta-analysis. The signal explained 2% of PR interval variability in African Americans (beta = 5.1 msec per minor allele, 95% CI = 4.1–6.1, p = 3×10−23). This SNP was also associated with PR interval (beta = 2.4 msec per minor allele, 95% CI = 1.8–3.0, p = 3×10−16) in individuals of European ancestry (n = 14,042), but with a smaller effect size (p for heterogeneity <0.001) and variability explained (0.5%). Further meta-analysis of the four cohorts identified genome-wide significant associations with SNPs in SCN10A (rs6798015), MEIS1 (rs10865355), and TBX5 (rs7312625) that were highly correlated with SNPs identified in European and Asian GWA studies. African ancestry was associated with increased PR duration (13.3 msec, p = 0.009) in one but not the other three cohorts. Our findings demonstrate the relevance of common variants to African Americans at four loci previously associated with PR interval in European and Asian samples and identify an association signal at one of these loci that is more strongly associated with PR interval in African Americans than in Europeans.
Introduction
The electrocardiogram is an important clinical tool that provides a graphical representation of the electrical activity of the heart as captured by skin surface electrodes. The electrocardiographic PR interval represents conduction through the atria and AV node, and impaired conduction through these tissues is a central pathogenic mechanism in many cardiac arrhythmias. Prolongation of the PR interval is a risk factor for long-term atrial fibrillation, heart block, and all-cause mortality [1]. A substantial proportion of the variability of the PR interval is explained by genetic factors with heritability estimates ranging between 30 and 50% in populations of European and Asian ancestry [2]–[7]. Identification of the specific alleles underlying the heritability of the PR interval might provide novel insights into molecular electrophysiology, lead to novel drug targets for arrhythmia treatment, and facilitate genetic prediction of arrhythmia risk and response to antiarrhythmics. Genome-wide association (GWA) studies have identified nine loci associated with PR interval duration in populations of European [6], [8] or South Asian ancestry [9]. Four of these associations have been replicated [6], [9]. However, there is a general paucity of GWA studies performed in populations of African ancestry [10] and none have been performed for PR interval duration which has been suggested to be longer in individuals of African than European ancestry [11]. Samples of African ancestry typically have shorter-range linkage disequilibrium (LD) than samples of European or Asian ancestry, potentially reducing genomic coverage in GWA studies using fixed arrays. However, samples of African ancestry offer greater genetic diversity; European and Asian populations have experienced a loss of allelic diversity since the out-of-Africa founding events [12], [13]. Thus, unique alleles influencing complex traits may be present in samples of African ancestry and shorter-range LD can facilitate narrowing of association signals. We studied the association of genetic ancestry with PR interval and performed a GWA study in 6,247 African Americans from four cohorts as part of the Candidate-gene Association Resource (CARe) consortium including the Atherosclerosis Risk in Communities (ARIC) study, the Cleveland Family Study (CFS), the Jackson Heart Study (JHS) and the Multi-Ethnic Study of Atherosclerosis (MESA). Replication of genome-wide significant associations was attempted by direct genotyping in 2,022 African Americans from the Health, Aging, and Body Composition Study (Health ABC) and the Healthy Aging in Neighborhoods of Diversity across the Life Span Study (HANDLS).
Results
Phenotype modeling
Baseline characteristics of the study populations are shown in Table 1. After adjustment for covariates, residual standard deviations for PR interval ranged from 22.1 to 26.9 msec in the four cohorts. The proportion of variation in phenotype explained by covariates (r2) was modest, ranging from 0.04 to 0.15, as shown in Table 1.
Tab. 1. Description of African American study samples. 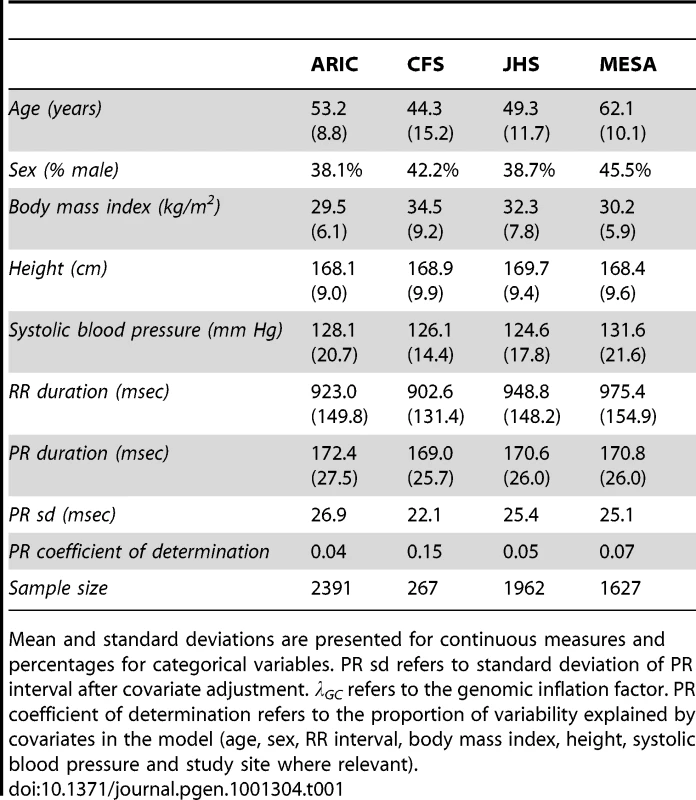
Mean and standard deviations are presented for continuous measures and percentages for categorical variables. PR sd refers to standard deviation of PR interval after covariate adjustment. λGC refers to the genomic inflation factor. PR coefficient of determination refers to the proportion of variability explained by covariates in the model (age, sex, RR interval, body mass index, height, systolic blood pressure and study site where relevant). Genetic ancestry and PR interval
The distribution of European ancestry relative to African ancestry was similar across cohorts; ARIC (median 0.15, IQR 0.11–0.22), CFS (median 0.18, IQR 0.13–0.26), JHS (median 0.16, IQR 0.12–0.21) and MESA (median 0.19, IQR 0.12–0.30). African ancestry was associated with longer PR interval than European ancestry in ARIC (13.3 msec longer for complete African compared to complete European ancestry, p = 0.009) but not in CFS (−13.2 msec, p = 0.44), JHS (1.0 msec, p = 0.89) or MESA (7.2 msec, p = 0.11).
Meta-analysis of genome-wide association studies
Genomic inflation was minimal in ARIC (λGC = 1.02) and MESA (λGC = 1.01) and modest in the family-based CFS (λGC = 1.10) and JHS (λGC = 1.08) studies, likely due to relatedness of some participants. Quantile-quantile plots of p-values were consistent with null distributions at all but the lowest p-values (Figure S1). Genomic control was applied to test statistics in all cohorts. Genomic inflation in the meta-analysis was modest (λGC = 1.04); genomic control was applied again (Figure 1). The distribution of association results across the genome is shown in Figure 2. Twenty-four single nucleotide polymorphisms (SNPs) at four genomic loci reached genome-wide significance as shown in Table S1. We clustered these SNPs by pairwise correlation in the HapMap phase II YRI panel and observed five weakly correlated linkage disequilibrium bins (all pairwise r2 were <0.3). Results for the SNP with the lowest p-value in each cluster are shown in Table 2 and the correlation of these SNPs to SNPs reported in previous GWA studies of European and Asian ancestry are shown in Table 3.
Fig. 1. Quantile-quantile plot for meta-analysis of genome-wide association studies of PR interval duration in African Americans. 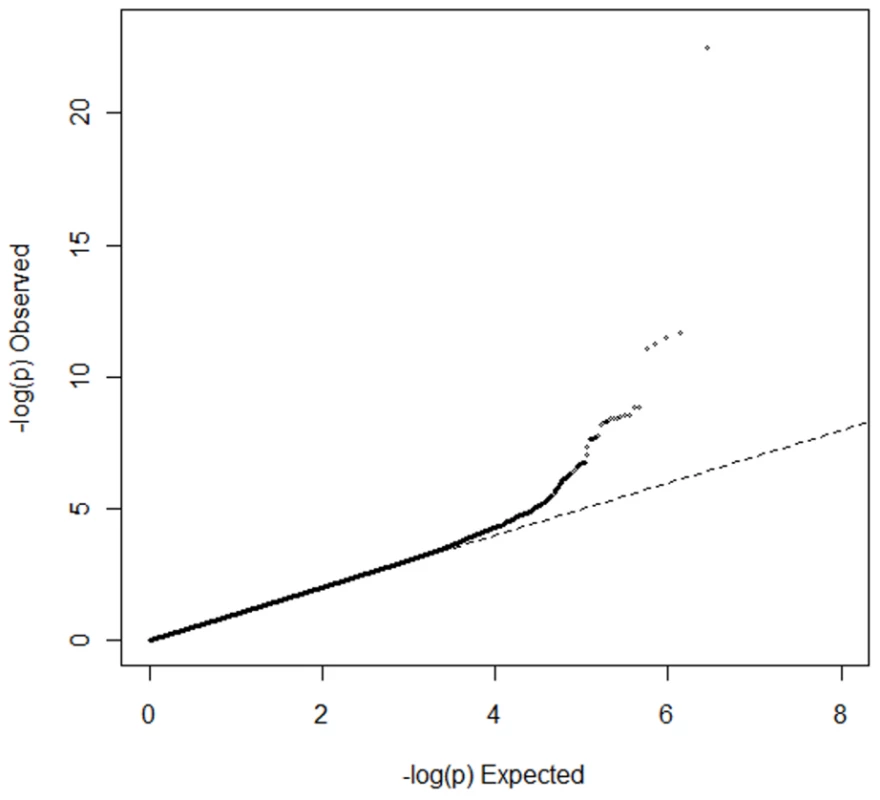
Plotted on the x-axis are expected p-values under the null hypothesis and on the y-axis the observed p-values after genomic control has been applied. Fig. 2. PR interval association results for 2.8 million SNPs across 22 autosomes. 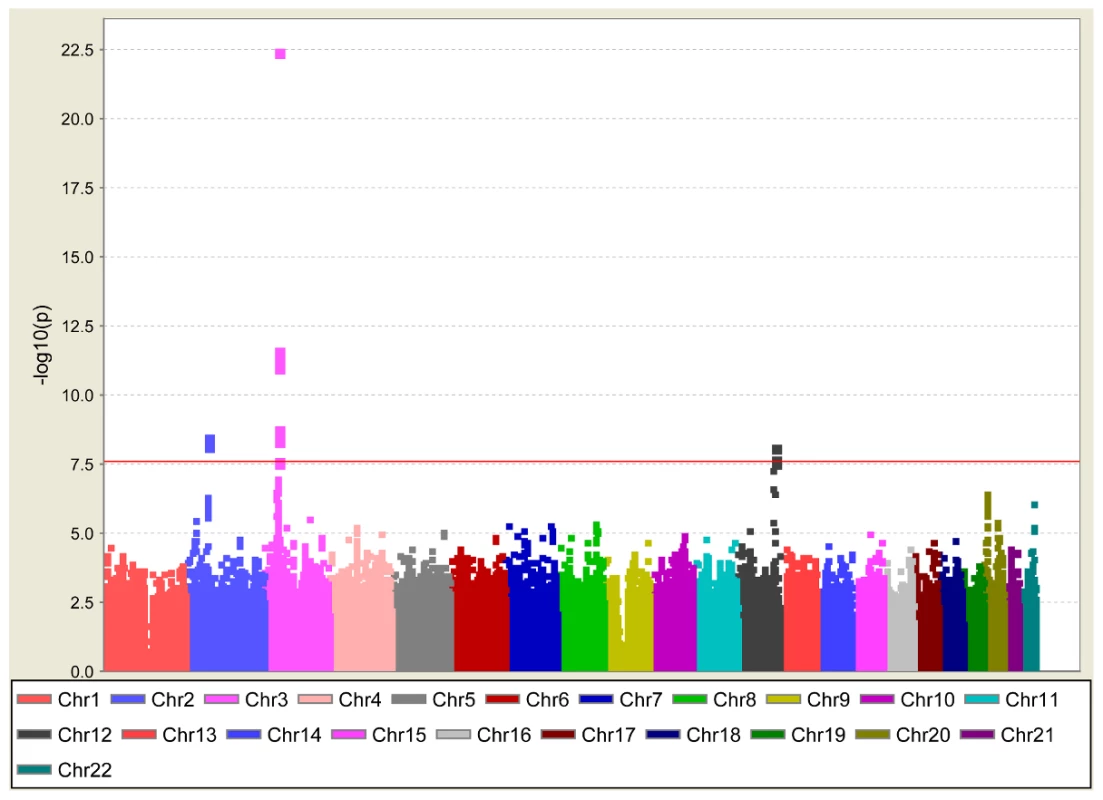
Each dot represents one SNP. Plotted on the x-axis is physical position by chromosome and on the y-axis –log10(p-value). Tab. 2. SNPs with the lowest p-values in each cluster of SNPs reaching genome-wide significance (p<2.5×10−8). 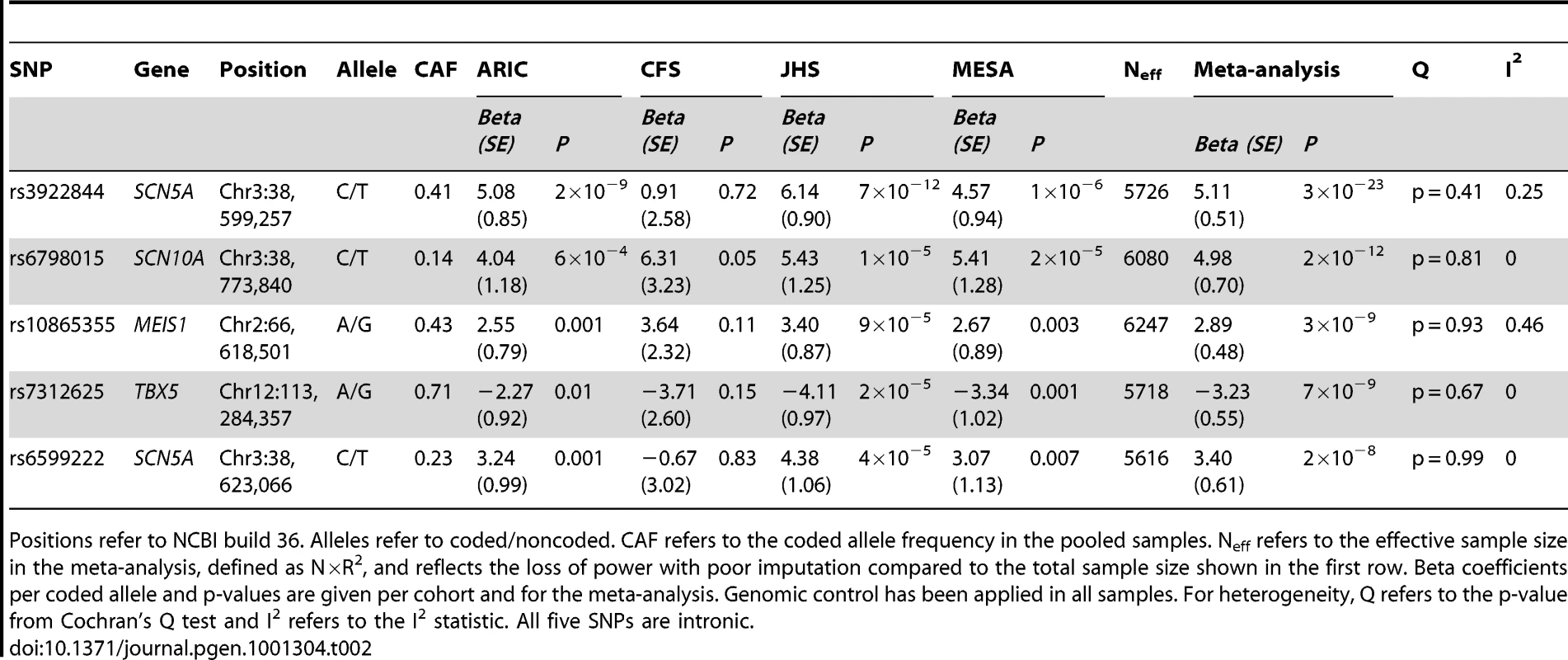
Positions refer to NCBI build 36. Alleles refer to coded/noncoded. CAF refers to the coded allele frequency in the pooled samples. Neff refers to the effective sample size in the meta-analysis, defined as N×R2, and reflects the loss of power with poor imputation compared to the total sample size shown in the first row. Beta coefficients per coded allele and p-values are given per cohort and for the meta-analysis. Genomic control has been applied in all samples. For heterogeneity, Q refers to the p-value from Cochran's Q test and I2 refers to the I2 statistic. All five SNPs are intronic. Tab. 3. Correlation of the SNPs with the lowest p-value in each cluster of SNPs reaching genome-wide significance in CARe to SNPs reaching genome-wide significance in previous European and Asian studies. 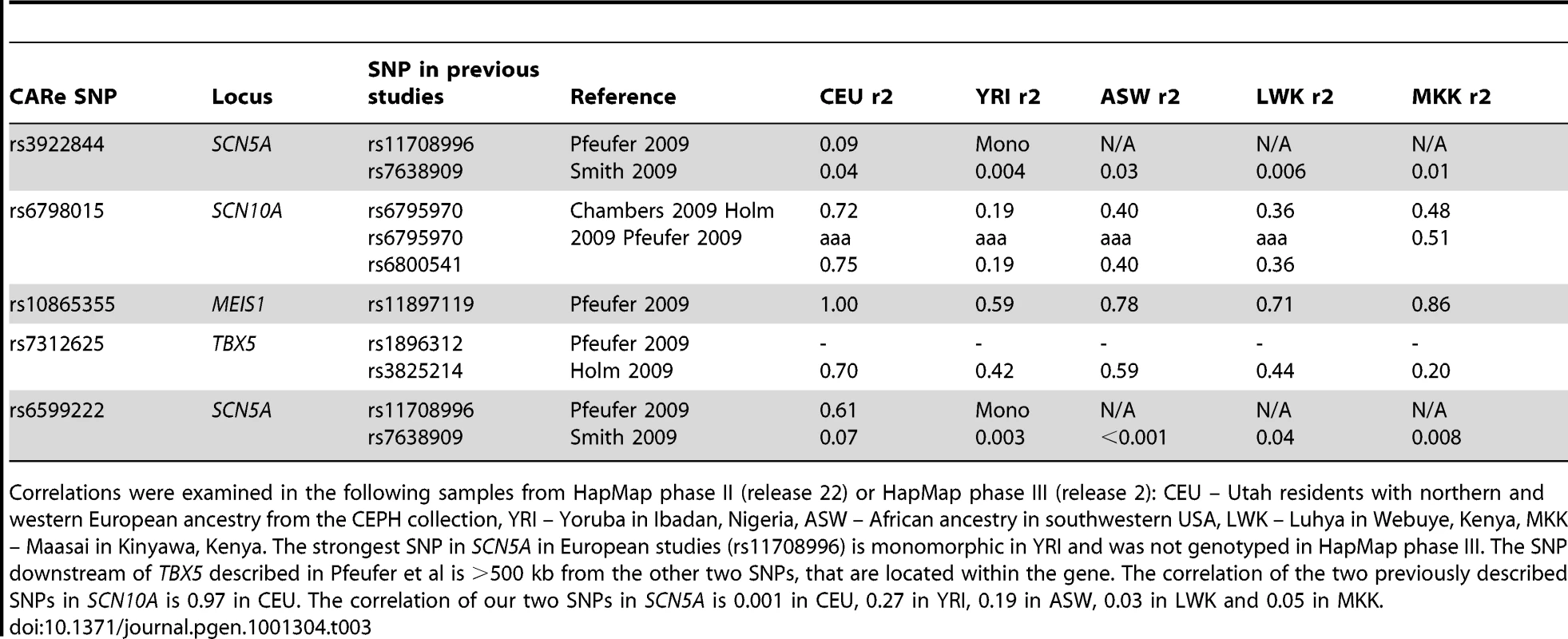
Correlations were examined in the following samples from HapMap phase II (release 22) or HapMap phase III (release 2): CEU – Utah residents with northern and western European ancestry from the CEPH collection, YRI – Yoruba in Ibadan, Nigeria, ASW – African ancestry in southwestern USA, LWK – Luhya in Webuye, Kenya, MKK – Maasai in Kinyawa, Kenya. The strongest SNP in SCN5A in European studies (rs11708996) is monomorphic in YRI and was not genotyped in HapMap phase III. The SNP downstream of TBX5 described in Pfeufer et al is >500 kb from the other two SNPs, that are located within the gene. The correlation of the two previously described SNPs in SCN10A is 0.97 in CEU. The correlation of our two SNPs in SCN5A is 0.001 in CEU, 0.27 in YRI, 0.19 in ASW, 0.03 in LWK and 0.05 in MKK. The top association result was for rs3922844 at SCN5A (5.1 msec per minor allele, 95% CI = 4.1–6.1, p = 3×10−23), explaining 2% of variation in PR interval. The SNP (rs3922844) reached genome-wide significance (p<2.5×10−8) in the larger cohorts, ARIC and JHS, considered individually. The SNP had a large effect estimate (5.1–6.1 msec or 0.19–0.24 standard deviations per C-allele) consistently across ARIC, JHS and MESA as shown in Table 2. The effect estimate was smaller in CFS, but CFS had a smaller sample of African Americans and hence wider confidence intervals than was observed in other larger samples. We observed no evidence of heterogeneity across the four African American cohorts (p for heterogeneity = 0.41). The SNP was well imputed in all cohorts as shown in Table S2. It was not correlated in HapMap CEU or YRI panels with the SNPs at this locus that have been identified in previous GWA studies of European or Asian ancestry (r2<0.10, Table 3).
We observed an additional association signal in SCN5A (rs6599222, p = 2×10−8), for which the strongest SNP was in LD with the SNP identified in a previous European GWA study (rs11708996, r2 = 0.61) and with an effect size (+3.4 msec per minor allele, 95%CI = 2.2–4.6) that was similar to the European study [8]. However, when analyzed conditional on rs3922844, the SNP rs6599222 was only borderline significantly associated with PR interval as shown in Table S3. The SNPs in SCN5A previously reported to be associated with PR interval in a cohort of Micronesians [5] were not associated with PR interval in African Americans (p = 0.57). The association results, correlations and locations of all studied SNPs in SCN5A are plotted against recombination rates in Figure 3.
Fig. 3. Regional association plots for PR interval duration at the SCN5A locus. 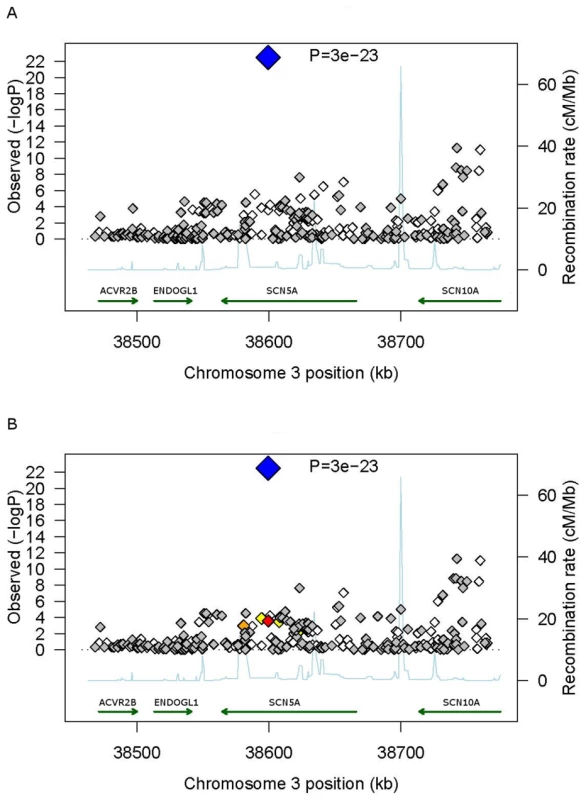
The plot covers the genomic region from 100 kb upstream of SCN5A to 100 kb downstream of SCN5A. White diamonds represent genotyped SNPs and grey diamonds represent imputed SNPs. The large blue diamond represents the SNP with the lowest p-value (rs3922844). Diamond color represents pairwise correlation between directly genotyped SNPs with the strongest SNP; red indicates strong correlation (r2>0.8), orange indicates moderate correlation (0.8>r2>0.5) and yellow indicates weak correlation (0.5>r2>0.2). Recombination rate is plotted in the background and known genes are shown in the bottom of the plot. Positions refer to NCBI build 36. SNP correlations and estimates of recombination rates were obtained from HapMap phase II. Panel A uses LD patterns from the YRI sample and Panel B from the CEU sample for results in individuals of African American ancestry. We further observed significant associations for a cluster of ten highly correlated SNPs in SCN10A that included the missense SNP (rs6795970) reported previously in GWA studies of individuals of European and Asian ancestry [6], [8], [9]. The missense SNP did not have the lowest p-value in the cluster but had the highest effect estimate, the lowest minor allele frequency (Table 2) and a somewhat lower imputation quality than other SNPs. Significant associations also were seen with SNPs inTBX5 and MEIS1. These correlated with the strongest reported SNPs from the GWA study performed in individuals of European ancestry at the loci shown in Table 3. The SNPs with the lowest p-values in each cluster were all well imputed as shown in Table S2. We observed no evidence for heterogeneity of effect across cohorts for any SNP reported in Table 2 (p>0.05).
As shown in Table 4, additional adjustment for local ancestry estimates did not impact any of the genome-wide significant associations.
Tab. 4. Replication and local ancestry analyses of SNPs with the lowest p-value in each cluster of SNPs reaching genome-wide significance. 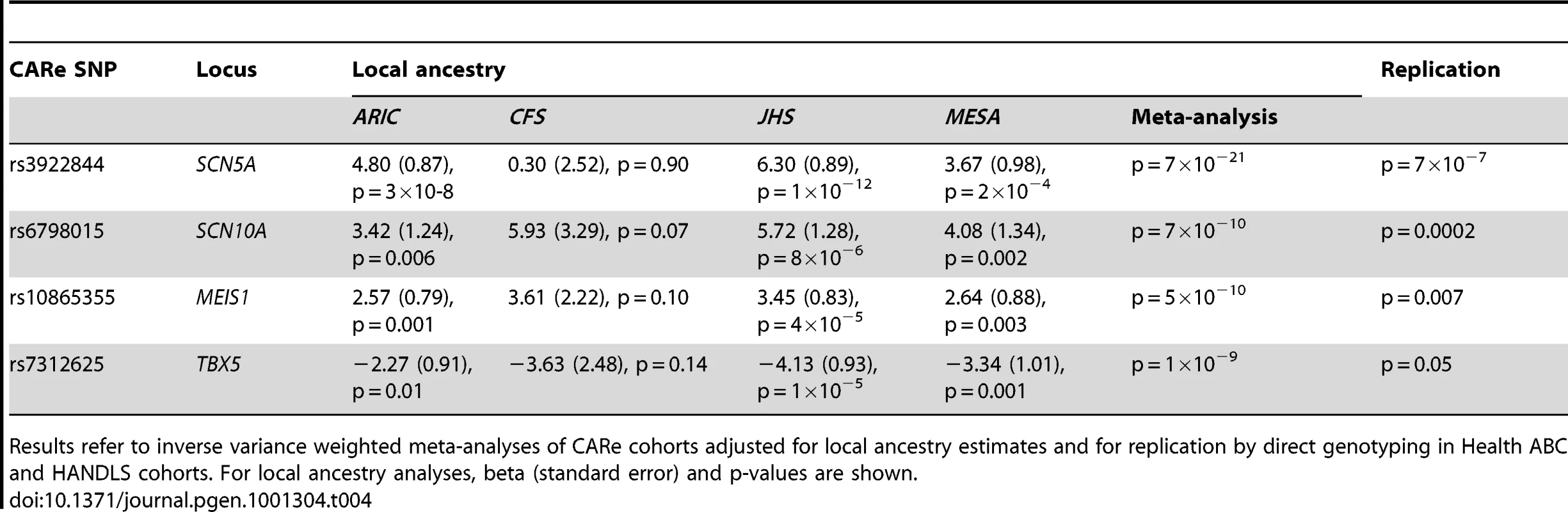
Results refer to inverse variance weighted meta-analyses of CARe cohorts adjusted for local ancestry estimates and for replication by direct genotyping in Health ABC and HANDLS cohorts. For local ancestry analyses, beta (standard error) and p-values are shown. Of the other SNPs reaching genome-wide significance in previous studies [6], [8], rs3807989 in CAV1 showed some evidence of association in the same direction (2.1 msec per A allele, p = 6×10−5) as did rs7692808 (−1.7 msec per A allele, p = 0.004) and rs7660702 (−1.2 msec per C allele, p = 0.02) in ARHGAP24 but not rs251253 near NKX2-5 (p = 0.98), rs4944092 in WNT11 (p = 0.93) or rs11047543 in SOX5 (p = 0.20).
Replication in African Americans
The strongest SNPs at loci reaching genome-wide significance were genotyped in Health ABC and HANDLS. As shown in Table 4, we observed evidence of replication for the top SNPs in SCN5A, SCN10A and MEIS1 and borderline significant replication for TBX5.
Association study in individuals of European ancestry
We examined the result for rs3922844 in a large, European GWA study (CHARGE-PR) [8], where it was imputed in four cohorts (n = 14,042). We observed a significant association with the PR interval (beta 2.40 msec per major allele, 95% CI = 1.83–2.97, p = 3×10−16). As in HapMap reference panels, allele frequencies were reversed in Europeans compared to African Americans, with a minor allele frequency of 0.30 for the T-allele. The effect size was significantly different in CHARGE-PR as compared to CARe (p for heterogeneity <0.001, I2 = 0.99).
Discussion
We performed a meta-analysis of GWA studies of the PR interval duration in individuals of African ancestry from four cohorts. We used a stringent significance threshold of 2.5×10−8, as previous genetic analyses have suggested twice as many independent SNPs in samples of African ancestry compared to European ancestry [14].
In two cohorts and in the meta-analysis we observed a genome-wide significant association with the SNP rs3922844 in SCN5A that explains approximately 2% of variability in PR interval duration. The SNP was not correlated with rs11708996, another SNP in SCN5A associated with PR interval duration in prior GWA studies of subjects of European ancestry [8], and had a larger effect estimate than rs11708996. We, therefore, examined the result for rs3922844 in a previously reported GWA study of PR interval in subjects of European ancestry [8] and observed genome-wide significant association but with a smaller effect estimate as compared to our study (5.1 msec, 95% CI = 4.1–6.1 versus 2.4 msec, 95% CI = 1.8–3.0, p for heterogeneity <0.001).Thus, we have evaluated genetic variants associated with PR interval duration in subjects of African American ancestry and identified an association signal from SCN5A with a larger effect size in individuals of African American compared to European ancestry.
We consider direct prolongation of the PR interval by rs3922844 unlikely given the differing effect sizes in individuals of African American and European ancestry. No SNP catalogue, including HapMap, used as reference panel for imputation in our study, includes all common sequence variants. We consider it more likely that rs3922844 is correlated with one or several causal variants and that the varying effect sizes result from differences in LD patterns across populations. For example, two variants with opposing effects could exist at the locus, with overlapping signals in Europeans but with isolation of individual signals in African Americans due to breakdown of linkage disequilibrium. The SNP rs3922844 was not directly genotyped in our samples but was imputed with high imputation quality as measured by the average ratio of observed variance to that expected by the Hardy-Weinberg equilibrium. We note that no SNPs included on our genotyping platform had an r2>0.2 with rs3922844 in the African (YRI) HapMap panel. A small cluster of genotyped SNPs were well correlated with rs3922844 in the European (CEU) HapMap panel (r2 = 0.6–0.8) as shown in Figure 3 but none of these reached a p-value less than 10−4. These observations suggest that the association was detected due to correlation of rs3922844 with multiple, weakly correlated, genotyped SNPs. Alternatively, a strong gene-environment interaction for rs3922844 could result in large differences in effect estimates across populations subject to differential environmental exposures or other genetic effects. Large-scale resequencing or imputation from large-scale resequencing efforts such as the 1000 Genomes Project, and functional experiments will ultimately be necessary to identify the causal variants.
Although allelic heterogeneity between families is well established for monogenic diseases, little is known about common variants across different populations. Recently identified findings include different association signals at the locus containing KCNQ1 with type 2 diabetes in European and Asian cohorts [10], at MYH9 with focal segmental glomerulosclerosis [15] and a MYBPC3 variant that causes cardiomyopathy and is only polymorphic in Asian populations [16]. Allelic heterogeneity is conceptually important and highlights the value of genetic association studies spanning diverse racial and ethnic populations. Studies across multiple populations may identify novel disease loci and genetic factors underlying differences in risk by race. Our results do not provide support for allelic heterogeneity across populations for PR interval, although limited power may have failed to detect such effects.
Genetic admixture studies have estimated that, on average, modern African American genomes have approximately 80–90% African and 10–20% European ancestry [17], which is well in line with our global ancestry analyses. It is important to note that there is great genetic diversity across the African continent. Our study included African Americans, who predominantly have an ancestral origin in Central and West Africa. It is possible that GWA studies in other samples of African ancestry might identify additional association signals.
The clinical impact of our findings merit further exploration. For example, a missense variant in SCN5A, S1103Y (rs7626962), which is polymorphic in African Americans but very rare in Europeans, has been associated with a markedly increased risk of ventricular arrhythmias [18]. This variant was not correlated with rs3922844 in the YRI panel of HapMap phase III (r2 = 0.04). Furthermore, African Americans have been shown to have a lower incidence of atrial fibrillation than Europeans. Longer PR interval duration (difference in means 11.8 msec) has also been reported in African Americans compared to Europeans in the ARIC study [11]. We were able to confirm this observation in ARIC using genetic ancestry, with a similar magnitude (13.3 msec longer with complete African compared to complete European ancestry, p = 0.009). However, we did not observe differences by genetic ancestry in CFS, JHS or MESA. This discrepancy could be due to differences in trans-African ancestry or other factors. Our results further indicate that common variants are unlikely to explain a large part of any ancestry-related variation in atrial and atrioventricular nodal conduction. The strongest variant observed here explained 2% of PR interval variability. It seems more likely that any ancestry-related differences in atrial conduction are due to rare variants, but this awaits further testing. Additional studies in populations with atrial fibrillation or sudden cardiac death will be necessary to determine the impact of the SNPs identified in our study on clinical outcomes.
In our meta-analysis, we also observed genome-wide significant association signals of SNPs in SCN10A, TBX5 and MEIS1, which are correlated with SNPs reported in previous GWA studies in individuals of European ancestry. The lowest p-value in the SCN10A cluster of SNPs was not observed in the previously described missense variant (rs6795970) [6], [9] but in an intronic SNP (rs6798015). The effect estimate was highest for the missense SNP but it also had the lowest minor allele frequency in the cluster, a lower allele frequency than in European (CEU) and Asian (JPT and CHB) HapMap panels. No evidence that the missense variant explains the association with PR interval has been published yet. Although it is possible that another SNP mediates the association, we find it more likely that the weaker p-value for the intronic SNP in our meta-analysis reflects differences in imputation quality or chance difference in the strength of the observed association. To ultimately identify the causal variants in each population, large-scale resequencing and functional experiments will be necessary.
Four genome-wide association studies for PR interval duration have been published previously. The first study identified an association with SNPs in SCN5A in a Micronesian founder population, but no SNP reached genome-wide significance [5]. A large study in individuals of European ancestry identified nine loci of genome-wide significance [8], including ARHGAP24, CAV1/CAV2, MEIS1, NKX2-5, SCN5A, SCN10A, SOX5, TBX5/TBX3 and WNT11. A third study in Icelanders identified and replicated significant associations with four of these loci [6]; ARHGAP24, CAV1, SCN10A and TBX5/TBX3. Finally, a study in individuals of Asian ancestry identified and replicated association with SNPs in SCN10A [9]. Here, we have reported genome-wide significant associations in African Americans with SNPs in strong LD with the previously reported SNPs at four of these loci; MEIS1, SCN5A, SCN10A and TBX5/TBX3. These findings demonstrate the relevance of these common alleles to individuals of African ancestry.
Our study has a number of limitations. All GWA studies suffer risk of false positive associations due to multiple testing, which is higher in samples of African ancestry where two million independent SNPs have been described [14]. We therefore applied a stringent significance threshold (2.5×10−8) to account for the approximately two million common variant tests estimated to exist across the genome. Large sample sizes are required to achieve such stringent significance levels. Our significant findings are thus unlikely to be false positives, especially as association signals at the same loci have been observed in European studies. However, even in the meta-analysis our study was underpowered to detect both common variants of small effect and rare variants. Measurement error in PR duration and heterogeneity between cohorts may further contribute to loss of power. Another potential problem encountered in genetic association studies is population stratification or confounding by ancestry. We sought to address this issue by filtering out individuals with cryptic relatedness or unlikely to be of African ancestry, adjusting for principal components of genome-wide data that describe within-population genetic variation, using family-based methods where appropriate and applying genomic control.
In conclusion, our results suggest that genetic loci underlying complex traits may be in large part the same across ancestral populations. However, association signals may show different effects across ethnic and racial populations and multiple association signals may exist at these loci. We anticipate that large-scale resequencing and functional experiments will ultimately identify the causal variants specific to each population.
Materials and Methods
Ethics statement
The study was approved by the Institutional Review Board at all participating institutions. Only individuals who provided informed consent to genetic testing were included.
Study samples
Individuals of self-reported African American ancestry from four cohort studies were genotyped as part of the Candidate-gene Association Resource (CARe) [19] (Lettre G et al, submitted). Detailed descriptions of these cohorts have been published previously [20]–[23]. Additional details are shown in Table S4. Details on replication samples (Health ABC, HANDLS) are shown in Table S5.
ARIC
The Atherosclerosis Risk in Communities (ARIC) study is a prospective population-based study of atherosclerosis and cardiovascular disease that included 15,792 men and women between 45 and 64 years from four US communities, 4,314 of whom were self-reported black Americans from two of the four communities (Jackson, MS and Forsyth County, NC) [20]. Electrocardiographic recordings used in the present study were performed at baseline examinations between 1987–1989. After exclusions, data on 2,391 black individuals with genotypes, information on all covariates and informed consent remained for analyses.
CFS
The Cleveland Family Study (CFS) is a family-based, longitudinal study designed to study the risk factors for sleep apnea [21]. The 632 African Americans with available DNA were genotyped as part of CARe. Electrocardiographic recordings used for the present study were performed at the final exam cycle conducted in a Clinical Research Unit between 2001–2006. After exclusions, 267 individuals remained for analyses.
JHS
The Jackson Heart Study (JHS) is a prospective, community-based study of the causes of the high prevalence of cardiovascular disease in African Americans that includes 5,301 self-reported African Americans recruited between 2000–2004 [22]. The cohort comprises residents of a tri-county area near Jackson, MS (Hinds County, Rankin County and Madison County). The study includes a subsample of unrelated participants (35–84 years) and a nested family-based subcohort (≥21 years). Electrocardiographic recordings used in the present study and blood collection for DNA extraction were performed at baseline examinations between 2000–2004. Genotype data were available in 3,030 individuals, including 885 who were also included in the ARIC study. After exclusions, 1962 individuals with ECG data and not included in ARIC were included in the present analysis.
MESA
The Multi-Ethnic Study of Atherosclerosis (MESA) is a population-based study of the characteristics of subclinical cardiovascular disease that included 6,814 individuals (28% African Americans) free from known cardiovascular disease between 45–84 years recruited from 6 field centers in the US [23]. Electrocardiographic recordings used in the present study and blood sampling for DNA extraction were performed at baseline visits between 2000–2002. African Americans constituted 28% of the total sample, of whom 1627 with genotype and phenotype data remained after exclusions.
ECG recordings
12-lead electrocardiograms with standard lead placements were recorded during ten seconds in all cohorts using Marquette MAC PC, MAC6 or MAC1200 machines (GE Healthcare, Milwaukee, WI, USA). PR interval duration was measured electronically using either the Marquette 12SL algorithm or the MC MEANS algorithm (Table S4).
Genotyping and quality control
All genotyping was performed at the Broad Institute of Harvard and MIT using the Affymetrix Genome-Wide Human SNP Array 6.0, which interrogates 906,600 SNPs, according to the manufacturers recommendations. Sample processing and quality control has been described previously [19] (Lettre G et al, submitted). Briefly, 1 ug of genomic DNA was equally interleaved on 96-well master plates. Quantity of double stranded DNA was ascertained using PicoGreen (Molecular Probes, Oregon, USA). Restriction enzyme digestion and PCR amplification using universal primers was performed to generate fragments of between 200–1,100 base pairs in length which were further fragmented to 25–50 bp and labeled with biotinylated nucleotides. Labeled fragments were hybridized to the microarray, washed and detected. Genotypes were called using Birdseed v1.33 and quality control steps were performed in PLINK, EIGENSTRAT and PREST-Plus [24]–[28]. To confirm sample identity, genotype concordance between 24 SNPs genotyped in all samples both on the microarrays and using Sequenom iPlex were monitored. DNA samples with genotyping success rate <95% were excluded. Autosomal heterozygosity rates were estimated to identify and exclude samples with poor DNA quality or contamination. Duplicated or contaminated samples were identified from identity by descent (IBD) estimates and excluded. Samples that were present in both JHS and ARIC were excluded based on IBD concordance. We also excluded samples unlikely to be African American (ARIC = 8), based on principal component analyses incorporating carefully curated reference populations of 1,178 European Americans and 756 Nigerians in EIGENSTRAT as previously described (Lettre G et al, submitted).
We excluded SNPs with genotyping success rate <90%, minor allele frequency <1%, SNPs that map to multiple locations, SNPs where missingness could be predicted from surrounding haplotypes and SNPs associated with chemistry plates (i. e. differential genotyping by plate). SNPs with unusually high numbers of Mendelian errors were excluded from family-based samples. No exclusions were performed based on Hardy-Weinberg equilibrium given admixture of the sample. In total, about 800,000 SNPs passed quality control in all cohorts.
Imputation and quality control
Imputation of additional ungenotyped SNPs was performed using hidden Markov models as implemented in MACH v1.16 [29] for a total of 2,801,419 million SNPs. Phased reference haplotypes from combined HapMap phase II CEU and YRI panels were used with equal proportions. Imputation was performed in two steps: in the first step, related individuals (as identified by proportion of genomic variation shared IBS/IBD (pi hat)>0.05) were filtered and error and recombination rates were estimated per sample in MACH using 30 iterations. In the second step, estimated rates were used to estimate allele dosage for all samples in one iteration. The average ratio of observed variance over that expected under Hardy-Weinberg equilibrium per SNP was also estimated as quality metric (R2). All samples were genotyped for 50,000 SNPs using the ITMAT-Broad-CARe (IBC) array [30] and the concordance rate (1–1/2× [imputed dosage – chip dosage]) of imputed genotypes with IBC genotypes was high (∼95%) in all samples. We excluded poorly imputed SNPs (ratio of observed over expected variance <0.3), SNPs with minor allele frequency<1% and non-autosomal SNPs. Sample outliers from missingness clustering analyses in PLINK were also removed.
Phenotype modeling
We excluded from all analyses individuals with missing covariates, <18 years, pacemaker implant, Wolf-Parkinson-White pattern, extreme PR values (<80 ms and >320 ms), prevalent atrial fibrillation, heart failure, myocardial infarction, or 2nd or 3rd degree AV block. PR interval duration was regressed linearly on age, sex, RR interval, BMI, height, systolic blood pressure and study site where relevant using SAS 9.2 (SAS Institute, Cary, North Carolina). Residuals were used as phenotype for association analyses.
Genome-wide association analyses and meta-analysis
For all cohorts except CFS, GWA analysis was performed in PLINK, using a linear regression model with an additive genetic model. The family-based CFS study was analyzed using linear mixed-effects models as implemented in the GWAF package for R [31]. Although JHS contains a family-based subcohort, little effect on inflation of using family-based methods were observed in preliminary analyses for a set of traits. Pedigrees for CFS were confirmed using identity by state (IBS) or IBD estimates from PREST-Plus. In all association analyses, we adjusted for population stratification by including the first ten principal components in addition to genome-wide SNP data. Cohort-specific GWA results were combined using fixed effects meta-analysis with inverse variance weights. Genomic inflation factors were evaluated in each cohort prior to meta-analysis and in the combined results [32]. We prespecified a genome-wide significance threshold of 2.5×10−8 as suggested for populations of African ancestry by Pe'er et al [14], assuming 2 million independent common variant tests in genomes of individuals of African American ancestry with p<0.05 genome-wide. Heterogeneity across samples was assessed by Cochran's χ2 test of heterogeneity with 4 degrees of freedom. To identify independent signals at loci we clustered all genome-wide significant hits by pairwise correlations ≥0.5 in the YRI HapMap panel. All analyses were performed using PLINK or R version 2.10.1 (R Foundation for Statistical Computing, Vienna, Austria). We examined the results for any SNPs of genome-wide significance that were uncorrelated with signals previously reported in other ancestral populations in a large published GWA study of PR interval in Europeans ancestry [8].
Genetic ancestry analyses
Individual estimates of global ancestry (proportion of European ancestors relative to African ancestors) and local ancestry were calculated using ANCESTRYMAP [33] and HAPMIX [34], as previously described (Lettre G et al, submitted). To study the association of genetic ancestry and PR interval, global ancestry estimates were regressed linearly on PR residuals. To assess the impact of local ancestry on SNP associations, all associations with genome-wide significance were adjusted for local ancestry.
Supporting Information
Zdroje
1. ChengS
KeyesMJ
LarsonMG
McCabeEL
Newton-ChehC
2009 Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. JAMA 301 2571 2577
2. HavlikRJ
GarrisonRJ
FabsitzR
FeinleibM
1980 Variability of heart rate, P-R, QRS and Q-T durations in twins. J Electrocardiol 13 45 48
3. Newton-ChehC
GuoCY
WangTJ
O'Donnell CJ
LevyD
2007 Genome-wide association study of electrocardiographic and heart rate variability traits: the Framingham Heart Study. BMC Med Genet 8 Suppl 1 S7
4. PiliaG
ChenWM
ScuteriA
OrruM
AlbaiG
2006 Heritability of cardiovascular and personality traits in 6,148 Sardinians. PLoS Genet 2 e132 doi:10.1371/journal.pgen.0020132
5. SmithJG
LoweJK
KovvaliS
MallerJB
SalitJ
2009 Genome-wide association study of electrocardiographic conduction measures in an isolated founder population: Kosrae. Heart Rhythm 6 634 641
6. HolmH
GudbjartssonDF
ArnarDO
ThorleifssonG
ThorgeirssonG
2010 Several common variants modulate heart rate, PR interval and QRS duration. Nat Genet 42 117 122
7. HansonB
TunaN
BouchardT
HestonL
EckertE
1989 Genetic factors in the electrocardiogram and heart rate of twins reared apart and together. Am J Cardiol 63 606 9
8. PfeuferA
van NoordC
MarcianteKD
ArkingDE
LarsonMG
2010 Genome-wide association study of PR interval. Nat Genet 42 153 159
9. ChambersJC
ZhaoJ
TerraccianoCM
BezzinaCR
ZhangW
2010 Genetic variation in SCN10A influences cardiac conduction. Nat Genet 42 149 152
10. RosenbergNA
HuangL
JewettEM
SzpiechZA
JankovicI
2010 Genome-wide association studies in diverse populations. Nat Rev Genet 11 356 366
11. SolimanEZ
PrineasRJ
CaseLD
ZhangZM
GoffDCJr
2009 Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke 40 1204 11
12. The International HapMap Investigators 2005 A haplotype map of the human genome. Nature 437 1299 1320
13. TishkoffSA
WilliamsSM
2002 Genetic analysis of African populations: human evolution and complex disease. Nat Rev Genet 3 611 621
14. Pe'erI
YelenskyR
AltshulerD
DalyMJ
2008 Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet Epidemiol 32 381 385
15. KoppJB
SmithMW
NelsonGW
JohnsonRC
FreedmanBI
2008 MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet 40 1175 1184
16. DhandapanyPS
SadayappanS
XueY
PowellGT
RaniDS
2009 A common MYBPC3 (cardiac myosin binding protein C) variant associated with cardiomyopathies in South Asia. Nat Genet 41 187 191
17. TishkoffSA
Reed FloydA
FriedlaenderFR
EhretC
RanciaroA
2009 The genetic structure of Africans and African Americans. Science 324 1035 44
18. SplawskiI
TimothyKW
TateyamaM
ClancyCE
MalhotraA
2002 Variant of SCN5A sodium channel implicated in risk of cardiac arrhythmia. Science 297 1333 1336
19. MusunuruK
LettreG
YoungT
FarlowDN
PirruccelloJP
2010 Candidate Gene Association Resource (CARe): Design, Methods, and Proof of Concept. Circ Cardiovasc Genet 3 267 75
20. The ARIC Investigators 1989 The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 129 687 702
21. RedlineS
TishlerPV
TostesonTD
WilliamsonJ
KumpK
1995 The familial aggregation of obstructive sleep apnea. Am J Respir Crit Care Med 151 682 7
22. TaylorHAJr
WilsonJG
JonesDW
SarpongDF
SrinivasanA
2005 Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis 15 S6 4-17
23. BildDE
BluemkeDA
BurkeGL
DetranoR
Diez RouxAV
2002 Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 156 871 881
24. KornJM
KuruvillaFG
McCarrollSA
WysokerA
NemeshJ
2008 Integrated genotype calling and association analysis of SNPs, common copy number polymorphisms and rare CNVs. Nat Genet 40 1253 1260
25. McCarrollSA
KuruvillaFG
KornJM
CawleyS
NemeshJ
2008 Integrated detection and population-genetic analysis of SNPs and copy number variation. Nat Genet 40 1166 1174
26. PurcellS
NealeB
Todd-BrownK
ThomasL
FerreiraMA
2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 559 575
27. PriceAL
PattersonNJ
PlengeRM
WeinblattME
ShadickNA
2006 Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38 904 909
28. McPeekMS
SunL
2000 Statistical tests for detection of misspecified relationships by use of genome-screen data. Am J Hum Genet 66 1076 1094
29. LiY
WillerC
SannaS
AbecasisG
2009 Genotype imputation. Annu Rev Genomics Hum Genet 10 387 406
30. KeatingBJ
TischfieldS
MurraySS
BhangaleT
PriceTS
2008 Concept, design and implementation of a cardiovascular gene-centric 50 k SNP array for large-scale genomic association studies. PLoS ONE 3 e3583 doi:10.1371/journal.pone.0003583
31. ChenMH
YangQ
2010 GWAF: an R package for genome-wide association analyses with family data. Bioinformatics 26 580 581
32. DevlinB
RoederK
1999 Genomic control for association studies. Biometrics 55 997 1004
33. PattersonN
HattangadiN
LaneB
LohmuellerKE
HaflerDA
2004 Methods for high-density admixture mapping of disease genes. Am J Hum Genet 74 979 1000
34. PriceAL
TandonA
PattersonN
BarnesKC
RafaelsN
2009 Sensitive detection of chromosomal segments of distinct ancestry in admixed populations. PLoS Genet 5 e1000519 doi:10.1371/journal.pgen.1000519
Štítky
Genetika Reprodukční medicína
Článek Break to Make a ConnectionČlánek A New Testing Strategy to Identify Rare Variants with Either Risk or Protective Effect on DiseaseČlánek The Architecture of Gene Regulatory Variation across Multiple Human Tissues: The MuTHER Study
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 2- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Break to Make a Connection
- A New Testing Strategy to Identify Rare Variants with Either Risk or Protective Effect on Disease
- Single-Tissue and Cross-Tissue Heritability of Gene Expression Via Identity-by-Descent in Related or Unrelated Individuals
- Pervasive Adaptive Protein Evolution Apparent in Diversity Patterns around Amino Acid Substitutions in
- The Architecture of Gene Regulatory Variation across Multiple Human Tissues: The MuTHER Study
- MiRNA Control of Vegetative Phase Change in Trees
- New Functions of Ctf18-RFC in Preserving Genome Stability outside Its Role in Sister Chromatid Cohesion
- Genome-Wide Association Studies of the PR Interval in African Americans
- Mapping of the Disease Locus and Identification of As a Candidate Gene in a Canine Model of Primary Open Angle Glaucoma
- Mapping a New Spontaneous Preterm Birth Susceptibility Gene, , Using Linkage, Haplotype Sharing, and Association Analysis
- A Population Genetic Approach to Mapping Neurological Disorder Genes Using Deep Resequencing
- and Genes Modulate the Switch between Attraction and Repulsion during Behavioral Phase Change in the Migratory Locust
- Targeted Sister Chromatid Cohesion by Sir2
- Correlated Evolution of Nearby Residues in Drosophilid Proteins
- Parallel Evolution of a Type IV Secretion System in Radiating Lineages of the Host-Restricted Bacterial Pathogen
- Lipophorin Receptors Mediate the Uptake of Neutral Lipids in Oocytes and Imaginal Disc Cells by an Endocytosis-Independent Mechanism
- Genome-Wide Association Study of Coronary Heart Disease and Its Risk Factors in 8,090 African Americans: The NHLBI CARe Project
- The Evolution of Host Specialization in the Vertebrate Gut Symbiont
- Genome-Wide Association of Familial Late-Onset Alzheimer's Disease Replicates and and Nominates in Interaction with
- Risk Alleles for Systemic Lupus Erythematosus in a Large Case-Control Collection and Associations with Clinical Subphenotypes
- Association between Common Variation at the Locus and Changes in Body Mass Index from Infancy to Late Childhood: The Complex Nature of Genetic Association through Growth and Development
- AID Induces Double-Strand Breaks at Immunoglobulin Switch Regions and Causing Chromosomal Translocations in Yeast THO Mutants
- A Study of CNVs As Trait-Associated Polymorphisms and As Expression Quantitative Trait Loci
- Whole-Genome Comparison Reveals Novel Genetic Elements That Characterize the Genome of Industrial Strains of
- Prevalence of Epistasis in the Evolution of Influenza A Surface Proteins
- Srf1 Is a Novel Regulator of Phospholipase D Activity and Is Essential to Buffer the Toxic Effects of C16:0 Platelet Activating Factor
- Two Frizzled Planar Cell Polarity Signals in the Wing Are Differentially Organized by the Fat/Dachsous Pathway
- Phosphoinositide Regulation of Integrin Trafficking Required for Muscle Attachment and Maintenance
- Pathogenic VCP/TER94 Alleles Are Dominant Actives and Contribute to Neurodegeneration by Altering Cellular ATP Level in a IBMPFD Model
- Meta-Analysis of Genome-Wide Association Studies in Celiac Disease and Rheumatoid Arthritis Identifies Fourteen Non-HLA Shared Loci
- A Genome-Wide Study of DNA Methylation Patterns and Gene Expression Levels in Multiple Human and Chimpanzee Tissues
- Nucleosomes Containing Methylated DNA Stabilize DNA Methyltransferases 3A/3B and Ensure Faithful Epigenetic Inheritance
- Mutations in Zebrafish Result in Adult-Onset Ocular Pathogenesis That Models Myopia and Other Risk Factors for Glaucoma
- [], the Prion Formed by the Chromatin Remodeling Factor Swi1, Is Highly Sensitive to Alterations in Hsp70 Chaperone System Activity
- Characterization of Transcriptome Remodeling during Cambium Formation Identifies and As Opposing Regulators of Secondary Growth
- The Cardiac Transcription Network Modulated by Gata4, Mef2a, Nkx2.5, Srf, Histone Modifications, and MicroRNAs
- Epistatic Interaction Maps Relative to Multiple Metabolic Phenotypes
- Quantitative Models of the Mechanisms That Control Genome-Wide Patterns of Transcription Factor Binding during Early Development
- Genome-Wide Transcript Profiling of Endosperm without Paternal Contribution Identifies Parent-of-Origin–Dependent Regulation of
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Meta-Analysis of Genome-Wide Association Studies in Celiac Disease and Rheumatoid Arthritis Identifies Fourteen Non-HLA Shared Loci
- MiRNA Control of Vegetative Phase Change in Trees
- Risk Alleles for Systemic Lupus Erythematosus in a Large Case-Control Collection and Associations with Clinical Subphenotypes
- The Cardiac Transcription Network Modulated by Gata4, Mef2a, Nkx2.5, Srf, Histone Modifications, and MicroRNAs
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



