-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Parallel Evolution of a Type IV Secretion System in Radiating Lineages of the Host-Restricted Bacterial Pathogen
Adaptive radiation is the rapid origination of multiple species from a single ancestor as the result of concurrent adaptation to disparate environments. This fundamental evolutionary process is considered to be responsible for the genesis of a great portion of the diversity of life. Bacteria have evolved enormous biological diversity by exploiting an exceptional range of environments, yet diversification of bacteria via adaptive radiation has been documented in a few cases only and the underlying molecular mechanisms are largely unknown. Here we show a compelling example of adaptive radiation in pathogenic bacteria and reveal their genetic basis. Our evolutionary genomic analyses of the α-proteobacterial genus Bartonella uncover two parallel adaptive radiations within these host-restricted mammalian pathogens. We identify a horizontally-acquired protein secretion system, which has evolved to target specific bacterial effector proteins into host cells as the evolutionary key innovation triggering these parallel adaptive radiations. We show that the functional versatility and adaptive potential of the VirB type IV secretion system (T4SS), and thereby translocated Bartonella effector proteins (Beps), evolved in parallel in the two lineages prior to their radiations. Independent chromosomal fixation of the virB operon and consecutive rounds of lineage-specific bep gene duplications followed by their functional diversification characterize these parallel evolutionary trajectories. Whereas most Beps maintained their ancestral domain constitution, strikingly, a novel type of effector protein emerged convergently in both lineages. This resulted in similar arrays of host cell-targeted effector proteins in the two lineages of Bartonella as the basis of their independent radiation. The parallel molecular evolution of the VirB/Bep system displays a striking example of a key innovation involved in independent adaptive processes and the emergence of bacterial pathogens. Furthermore, our study highlights the remarkable evolvability of T4SSs and their effector proteins, explaining their broad application in bacterial interactions with the environment.
Published in the journal: . PLoS Genet 7(2): e32767. doi:10.1371/journal.pgen.1001296
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001296Summary
Adaptive radiation is the rapid origination of multiple species from a single ancestor as the result of concurrent adaptation to disparate environments. This fundamental evolutionary process is considered to be responsible for the genesis of a great portion of the diversity of life. Bacteria have evolved enormous biological diversity by exploiting an exceptional range of environments, yet diversification of bacteria via adaptive radiation has been documented in a few cases only and the underlying molecular mechanisms are largely unknown. Here we show a compelling example of adaptive radiation in pathogenic bacteria and reveal their genetic basis. Our evolutionary genomic analyses of the α-proteobacterial genus Bartonella uncover two parallel adaptive radiations within these host-restricted mammalian pathogens. We identify a horizontally-acquired protein secretion system, which has evolved to target specific bacterial effector proteins into host cells as the evolutionary key innovation triggering these parallel adaptive radiations. We show that the functional versatility and adaptive potential of the VirB type IV secretion system (T4SS), and thereby translocated Bartonella effector proteins (Beps), evolved in parallel in the two lineages prior to their radiations. Independent chromosomal fixation of the virB operon and consecutive rounds of lineage-specific bep gene duplications followed by their functional diversification characterize these parallel evolutionary trajectories. Whereas most Beps maintained their ancestral domain constitution, strikingly, a novel type of effector protein emerged convergently in both lineages. This resulted in similar arrays of host cell-targeted effector proteins in the two lineages of Bartonella as the basis of their independent radiation. The parallel molecular evolution of the VirB/Bep system displays a striking example of a key innovation involved in independent adaptive processes and the emergence of bacterial pathogens. Furthermore, our study highlights the remarkable evolvability of T4SSs and their effector proteins, explaining their broad application in bacterial interactions with the environment.
Introduction
Adaptation to different ecological niches can lead to rapid diversification of a single ancestor into an array of distinct species or ecotypes. This process, called adaptive radiation, typically occurs after the arrival of a founding population in a novel environment with unoccupied ecological niches (‘ecological opportunity’) and/or by the acquisition of a novel trait (‘evolutionary key innovation’) allowing the exploitation of so far unapproachable niches [1]. Spectacular examples of adaptive radiation come from different metazoan lineages with the cichlid fishes of the East African Great Lakes and the Darwin finches on Galapagos Islands representing the most prominent examples [2], [3]. Although known from a few cases only, bacterial lineages also underwent adaptive radiation - as documented in natural settings as well as in evolution experiments [4]–[6]. It remains a fundamental problem to biology to understand why and how certain lineages diversified; adaptive radiations, and in particular the genetic and genomic basis thereof, provide an ideal set-up to address this question [3], [7].
One of the most fascinating aspects of adaptive radiation is the frequent occurrence of evolutionary parallelism resulting in independent adaptation to same ecological niches [8]–[10]. Such evolutionary parallelisms are excellent examples for the action of similar, yet independent, selective forces and, hence, for the key role of natural selection in evolution [11]. Furthermore, parallel adaptive radiations in a single group indicate the existence of traits conferring a high degree of adaptability allowing the group members to efficiently occupy distinct environments. Therefore, lineages that radiate in parallel are of great value to study the molecular basis of adaptation and their independent evolutionary trajectories [1], [9]. This is of particular interest in case of host-adapted bacteria differentiating into divergent ecological niches and potentially resulting in the emergence of new pathogens. Species of the α-proteobacterial genus Bartonella are specifically adapted to distinct mammalian reservoir hosts where they cause intra-erythrocytic infections [12]. Different animal models revealed that Bartonella upon reservoir host infection colonizes a primary cellular niche from where the bacteria get seeded into the bloodstream adhering to and invading erythrocytes [13]–[15]. In most cases, infections of the reservoir host do not lead to disease symptoms suggesting a highly specific adaptation to the corresponding host niche. The transmission between host individuals is mediated by blood sucking arthropods.
An integrative genome-wide analysis showed that most factors essential for Bartonella to colonize their mammalian reservoir hosts are found within the core genome of this genus [16]. This is not surprising as it reflects the common strategy used by divergently adapted species to colonize their hosts. However, this study also revealed that two type IV secretion systems (T4SS), Trw and VirB, which are essential for host interaction at different stages of the infection cycle represent the few colonization factors exclusively found in the most species rich sub-lineages of bartonellae. It was assumed that the horizontal acquisition of these T4SS substantially refined the infection strategy of Bartonella facilitating concurrent adaptation to a wide range of different hosts [16]. The VirB T4SS translocates a cocktail of evolutionarily related effector proteins into host cells of the primary infection niche where they modulate various cellular processes [17]–[21]. The Trw T4SS is involved in the erythrocyte invasion by binding to the erythrocytic surface with its manifold variants of pilus subunits [22]–[24].
Here, we study the evolutionary relationship of Bartonella species adapted to distinct reservoir hosts and investigate the genetic mechanisms underlying adaptive radiation in different lineages. We uncover two parallel adaptive radiations in the genus Bartonella. Our genome-wide analysis revealed a remarkable evolutionary parallelism in the horizontally acquired VirB T4SS in the two radiating lineages. This parallelism is characterized at the molecular level by the lineage-specific chromosomal integration of the virB loci and the independent origination of versatile sets of effector proteins for the interaction with host cells. Providing an arsenal of host-subverting functions that can be efficiently modulated, the VirB T4SS thus seems to represent an evolutionary key innovation triggering the independent radiations of the two lineages. Our study provides detailed insights into the molecular mechanisms underlying parallel adaptive radiations in a bacterial pathogen. Furthermore, many of the diversified T4SS effector proteins carry a FIC domain recently shown to mediate ‘AMPylation’, a lately recognized post-translational modification [25], [26]. FIC domains are highly conserved in evolution and the diversified variants of the Bartonella effector proteins may display a suitable model to study their activity spectrum in the future.
Results/Discussion
Genome sequencing of ecologically distinct Bartonella strains
To study the adaptive evolution of Bartonella on a genomic level, we aimed for a set of genome sequences from species adapted to distinct mammalian reservoir hosts. To this end, we included in our analysis the published genome sequences of Bartonella bacilliformis (Bb), Bartonella grahamii (Bg), Bartonella henselae (Bh), Bartonella quintana (Bq), and Bartonella tribocorum (Bt) [16], [27], [28]. These five species are adapted to human (Bb and Bq), cat (Bh), mouse (Bg), and rat (Bt). Further, we sequenced the complete genome of Bartonella clarridgeiae (Bc) and generated draft sequences of Bartonella schoenbuchensis (Bs), Bartonella rochalimae (Br), Bartonella sp. AR 15-3 (BAR15), and Bartonella sp. 1-1C (B1-1C). Bs was selected as representative of a solely ruminant-infecting clade [16]. Bc, Br, BAR15, and B1-1C were previously shown to be closely related [29]–[31]. However, they were isolated from different mammalian reservoir hosts and therefore display a suitable set of species to study adaptive processes on the genomic level. BAR15 and B1-1C were recently isolated from American red squirrel and rat, respectively [30], [31], whereas Br was predominantly recovered from canidae like dogs or foxes, and Bc from cats [32]–[34].
Genome sequencing by 454-pyrosequencing resulted in an average sequence coverage of >35x. The single chromosome of the completely assembled genome of Bc was found to be 1,522,743 bp in size and thus belongs to the smaller genomes of Bartonella (Table 1). The draft genomes of Br, BAR15, B1-1C, and Bs consist of 13 to 19 contigs with total genome sizes similar to the one of Bc. On average, 99% of all 454-sequencing reads were assembled into the analyzed 13 to 19 contigs indicating that our draft genomes did not miss essential sequence data for subsequent analysis. Genomic features of the strains used in this study are summarized in Table 1.
Tab. 1. Genomic features of analyzed Bartonella species and information about their reservoir host and phylogenetic lineage (see also Figure 1). 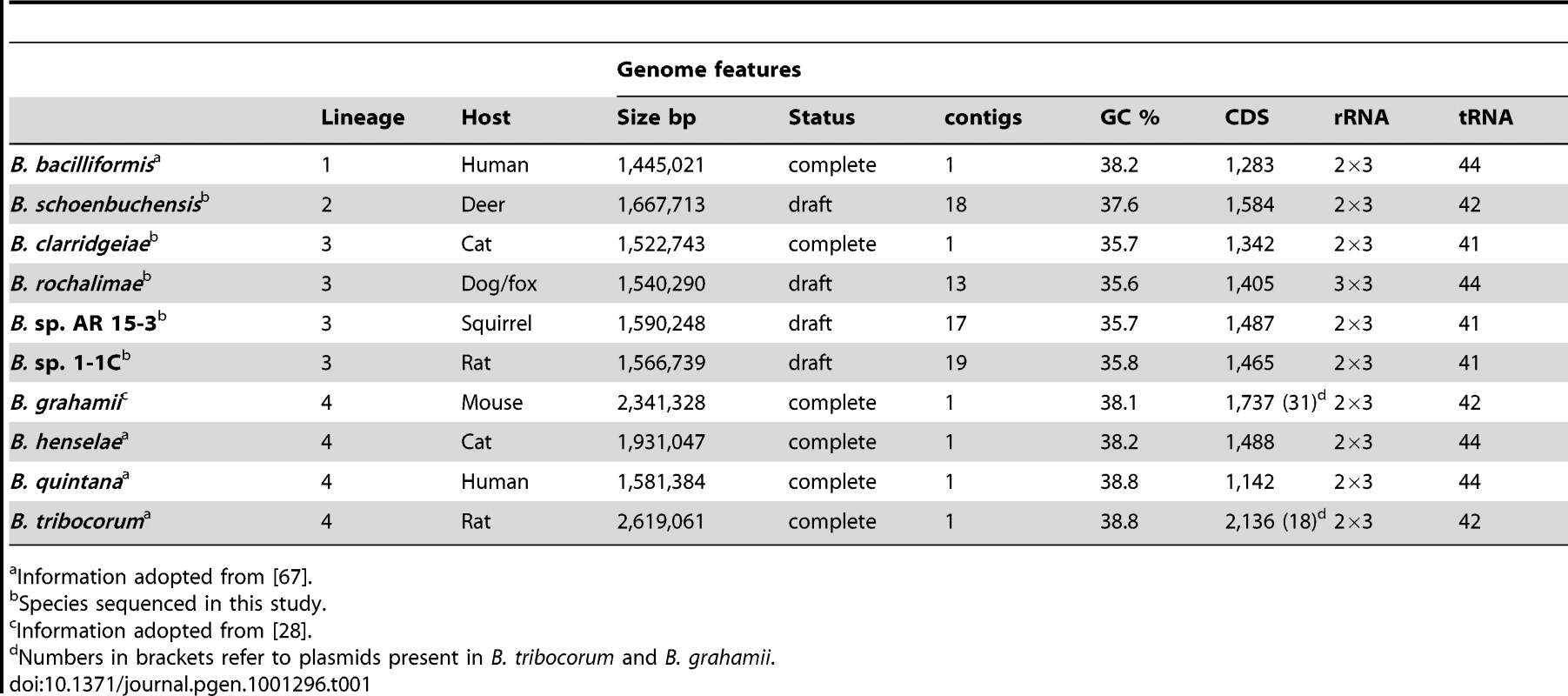
Information adopted from [67]. A genome-wide phylogeny reveals parallel adaptive radiations in two sister clades of Bartonella
We inferred a robust species tree of the genus Bartonella based on 478 core genome genes of the ten available Bartonella genomes sequenced (Figure S1). To exclude that recombination or horizontal gene transfer within this set of core genome genes was affecting our phylogenetic analysis, we reconstructed single gene trees of the entire data set and performed a recombination analysis using the GARD algorithm [35]. 471 of the 478 genes revealed the same overall topology as our genome-wide phylogeny with the two monophyletic clades of lineage 3 and lineage 4 (see Figure 1). Further, the GARD analysis detected significant recombination breakpoints with a p-value<0.01 in only two out of the 478 core genome genes. Together these analyses show that our genomic data set is suitable for inferring a consistent species tree.
Fig. 1. Phylogeny of Bartonella based on a genome-wide dataset. 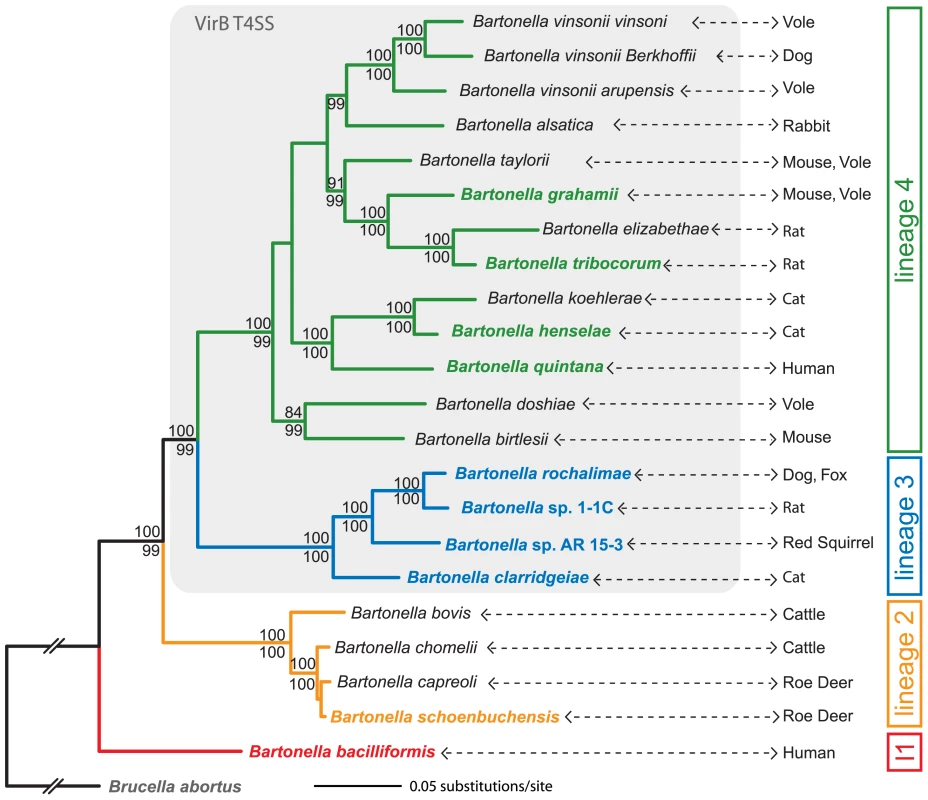
Maximum likelihood analysis using an alignment of 478 genes (515,751 nt) of ten sequenced Bartonella species (indicated by bold and color type) and Brucella abortus. Based on sequence data from rpoB, gltA, ribC, and groEL genes, additional Bartonella species were included in the analysis. Numbers above the branches represent maximum likelihood bootstraps (100 replicates); numbers below represent Bayesian posterior probabilities. Values ≥80% are shown. Lineages harboring the VirB T4SS [16] are shaded in gray; the primary mammalian hosts are indicated for each species. Phylogenetic trees inferred from either the genomic data set excluding non-sequenced bartonellae or the sequences from only rpoB, gltA, ribC, and groEL genes revealed the same four lineages for the Bartonella ingroup (Figure S1). Estimates of average evolutionary divergence over sequence pairs within lineages and between lineages are presented in Table S1. l1, lineage 1. Based on available sequence information for the housekeeping genes rpoB, gltA, ribC, and groEL, we included most other Bartonella species in the analysis resulting in a so-called supertree phylogeny (Figure 1) [36]. Just as the analysis based on the 478 core genome genes of the ten sequences species alone, this supertree revealed four major clades in the monophyletic bartonellae: ancestral lineage 1 represented by the highly virulent human pathogen Bb [37]; lineage 2 comprising of Bs and three other ruminant-infecting species; lineage 3 consisting of the closely related Bc, Br, BAR15, and B1-1C; and the most species-rich lineage 4 with 13 species including Bg, Bh, Bq, and Bt (Figure 1). A phylogeny based on only the four housekeeping genes resulted in the same clustering of these taxa into the four different Bartonella lineages (Figure S1).
In contrast to the ancestral lineage 1, lineages 2, 3, and 4 are ramifying to different degrees comprising species isolated from various hosts. While the species of lineage 2 are limited to infect ruminants and have overlapping host range [38], the diversification of lineage 3 and 4 seems to result from the specific adaptation to distinct mammalian hosts [12]. To substantiate the ecological divergence within these two lineages, we analyzed the genotype-host correlation of Bartonella isolates sampled from diverse mammals. Based on gltA sequences, this analysis revealed clustering of strains isolated from same or similar hosts in lineage 3 and 4 (Figure S2). Further support for the host specific adaptation of different Bartonella species comes from recently published laboratory infections [39], [40] and from our own rat infection experiments with the strains of lineage 3 (Figure S3). It is to mention that some Bartonella species can incidentally be transmitted to other hosts like humans [12]. These so-called zoonotic Bartonella species do not cause intraerythrocytic bacteremia in the accidental human host reflecting the lack of specific adaptation. However, such accidental transmissions might facilitate the emergence of new specificity resulting in host switches and the origination of new species. In particular, several species of lineage 3 and 4 are known to display such zoonotic pathogens, whereas for lineage 1 or 2 to our knowledge no such case has been reported so far [12], [29].
In summary, our genome-wide phylogenetic analysis shows that the sister lineages 3 and 4 have evolved by adaptive radiations into same or similar ecological niches (i.e. hosts). Long internal branches separating the two lineages from each other and preceding the radiations are evidence for their independent occurrence (Figure 1). Due to the lack of calibration time points, the exact timing of these independent radiations cannot be deduced. However, the phylogenetic tree in Figure 1 might suggest that lineage 3 diversified more recently compared to lineage 4. This is supported by the mean p-distances inferred for the sequenced taxa of these two lineages: lineage 3 = 0.07±0.0002, lineage 4 = 0.12±0.0003 (see also Table S1). Two alternative explanations for the observed differences in lineage diversification could be (i) a sampling bias, i.e. the full diversity of lineage 3 was not captured or (ii) smaller population sizes for species of lineage 4 over lineage 3 leading to faster evolution at purifying sites. Significant differences in population size might be rather unlikely as Bartonella species are thought to share a common life style in their respective reservoir host. Whether sampling of Bartonella species in animal populations was exhaustive enough is difficult to assess. However, a newly discovered species would only change the coalescent point of a lineage if it would hold a more ancestral position than the already known species of this lineage. In contrast to these alternative hypothesis, epidemiological studies rather seem to support the scenario of a more ancient onset of the radiation in lineage 4: (i) lineage 4 comprises a much wider range of divergently adapted species and (ii) they represent the most frequently found Bartonella species in natural host populations [30], [41]. In contrast, except for Bc, taxa of lineage 3 were only recently detected and so far only sampled at low prevalence [29]–[32].
The VirB T4SS displays an adaptive trait specific to the radiating lineages of Bartonella
The evolutionary parallelism of the radiating Bartonella lineages provides an ideal setting to study independent evolutionary processes linked to the adaptation to divergent niches. An important driving force for these adaptive radiations might be the presence of ecological opportunities. The niche of Bartonella in the mammalian reservoir host, the bloodstream, displays a privileged environment in which other resource-competing microbes are typically absent. Thus, the adoption of the characteristic intra-erythrocytic infection strategy together with the vector-borne transmission route might have enabled adaptive radiations of Bartonella by the specialization to different hosts. However, not all Bartonella lineages appear to have diversified to the same extent (see Figure 1) suggesting that the availability of such an ecological opportunity alone is not sufficient to explain the pronounced radiation of lineages 3 and 4. Supposedly, key innovations, i.e. lineage-specific traits underlying the adaptation to the mammalian host niches are responsible for the adaptive radiations. Potential adaptive traits would have to be involved in species-environment interactions, such as molecular factors responsible for causing bacteremia. Further, in analogy to the modulation of the adaptive traits in metazoan radiations [2], [3], any molecular factor used to exploit distinct environments in a specific manner should be divergent among niche-specialized species [1]. Molecular evolutionary analyses provide the means to identify divergent adaptive traits as the genes encoding them are expected to show signs of adaptive evolution, i.e. an excess of non-synonymous (dn) over synonymous (ds) substitutions as the result of positive selection. We performed a genome-wide natural selection analysis in the two radiating lineages to detect genes (and therefore traits) with divergent evolution. To this end, we analyzed all orthologous genes from the available genomes of the radiating lineage 3 (Bc, Br, BAR15, and B1-1C) and lineage 4 (Bh, Bg, Bq, and Bt) for signs of adaptive sequence evolution by inferring the natural selection of orthologs by estimation of ω, the ratio of non-synonymous (dn, amino acid change) to synonymous (ds, amino acid conservation) substitution rates (ω = dn/ds). Generally, ω<1, ω = 1, ω>1 represent purifying, neutral, and positive selection (adaptive evolution), respectively [42].
We first calculated “gene-wide” dn/ds for all orthologous genes of the two lineages (lineage 3 : 1,097 genes, lineage 4 : 1,091 genes). We excluded one gene from this analysis for each of the two lineages, because the GARD analysis detected statistically significant recombination breakpoints. As adaptive evolution is typically affecting only a few sites of a gene rather than the entire gene sequence [42], we first looked for genes exhibiting an elevated value of ω≥0.25 over the entire gene length. This analysis revealed 133 (12%) and 86 (8%) genes in lineage 3 and lineage 4, respectively, under relaxed purifying selection indicating signs of adaptive evolution (Figure 2). To have an additional measurement for adaptive evolution, we subjected our genomic data sets to a maximum likelihood analysis for the detection of site-specific positive selection. To this end, we used the CodeML module implemented in the PAML package. CodeML compares the likelihoods of different evolutionary models for each analyzed gene alignment. When comparing model M2a (PositiveSelection) vs. model M1a (NearlyNeutral), we detected 62 or 34 genes for lineage 3 and 26 or 14 genes for lineage 4 harboring sites under positive selection with a p-value of <0.05 or <0.01, respectively. A large fraction of these genes (29 for lineage 3 and 12 for lineage 4) exhibited also gene-wide dn/ds values ≥0.25 indicating them as good candidates for encoding adaptive traits (Table 2). Comprehensive lists of the genes identified to have dn/ds values ≥0.25 and/or exhibiting site-specific positive selection in the CodeML analysis are provided in Table S2 and Table S3 for lineage 3 and lineage 4, respectively. As non-synonymous mutations accumulate over time, the higher number of genes identified for lineage 3 could be a further indication for its more recent radiation, or alternatively, the effect of larger population sizes compared to lineage 4. Irrespectively, these findings render the dataset derived from lineage 3 to be more sensitive to the detection of adaptive sequence evolution (Figure 2, Table 2).
Fig. 2. Gene-wide dn/ds analysis of the core genomes of the two radiating lineages. 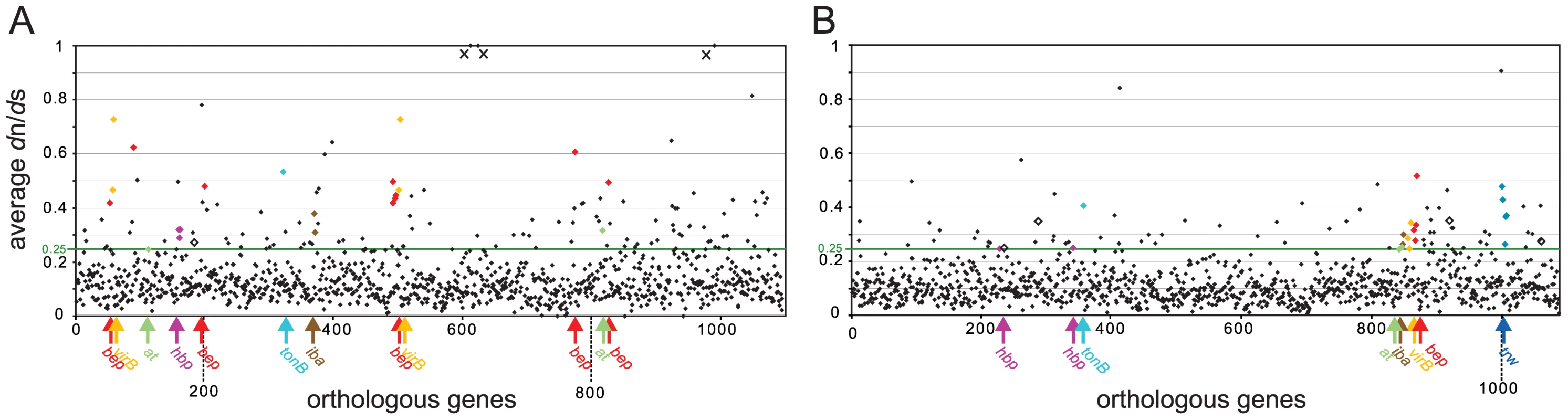
dn/ds analysis for lineage 3 (A) and for lineage 4 (B). The mean values plotted on the y-axis were obtained from the dn/ds values of pairs of orthologs. Genes are ordered according to their position in the genome of Bc (A) and Bq (B). Genes with elevated dn/ds values homologous to known colonization factors of Bartonella (based on previously published results [16], [43]–[45]), are depicted by colors. at, putative autotransporter, bep, Bartonella effector protein, hbp, hemin-binding protein, iba, inducible Bartonella autotransporter, virB, VirB T4SS protein, trw, Trw T4SS protein. Genes encoding hypothetical proteins known to be important for colonization are depicted by open black squares (one in lineage 3, four in lineage 4). In lineage 3, three genes exhibit average dn/ds values ≫1 (marked by crosses). This is explained by the fact that between Br and B1-1C, only non-synonymous mutations but no synonymous mutations have been detected in these three genes. As all three genes revealed ω<1 in the other pair-wise comparisons, they were treated as outliers and excluded from further analysis. Tab. 2. Number of genes revealing significant positive selection in the CodeML analysis for lineage 3 and lineage 4. 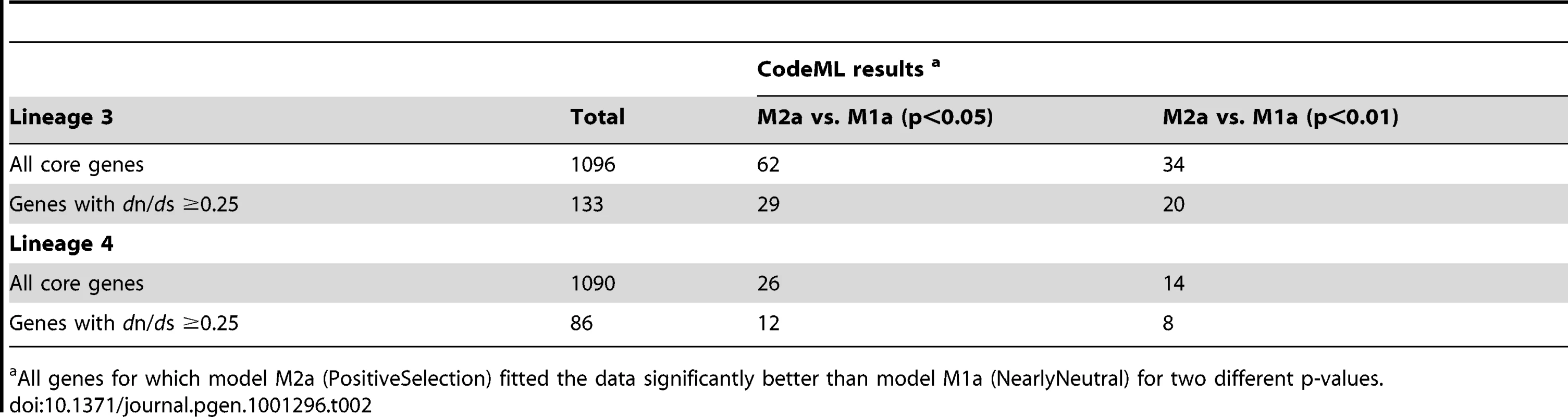
All genes for which model M2a (PositiveSelection) fitted the data significantly better than model M1a (NearlyNeutral) for two different p-values. Interestingly, there was a marked number of genes with ω≥0.25 over the entire gene length that overlapped in both lineages (Table 3). Among those were genes encoding autotransporters, hemin-binding proteins, and different components of the VirB T4SS. They all constitute important host colonization factors [43]–[45] and thus are likely to display adaptive traits of Bartonella. For many of these genes, also our analysis of site-specific natural selection detected positive selection (Table 3). Most remarkably, all analyzed Bartonella effector protein (bep) genes of the VirB T4SS were among the genes with ω≥0.25. Particularly in lineage 3, they showed strong signs of adaptive evolution by exhibiting ω>0.4 over the entire gene length. Further, in eight out of nine analyzed bep genes of lineage 3, we detected site-specific positive selection (Table 3). Being exclusively found in the radiating lineages and showing strong signs of adaptive evolution, the VirB system and its effector proteins, thus, fulfill the criteria of an evolutionary key innovation likely contributing to the parallel adaptive radiations of Bartonella. Autotransporters and hemin-binding proteins could represent further adaptive traits important for radiations in Bartonella. They exhibited strong positive selection in our analysis and are known to be important factors for host colonization. Further, their conservation throughout the genus Bartonella indicates an important role for the life style and infection strategy of this pathogen (Table S2, Table S3). However, factors conserved in radiating and non-radiating lineages appear unlikely to represent specific key innovations, unless other factors, such as the absence of ecological opportunities or ecological separation, prevented certain lineages to radiate and to colonize more divergent niches.
Tab. 3. Common genes of lineage 3 and lineage 4 with average dn/ds values (ω) ≥0.25 and results of the CodeML analysis. 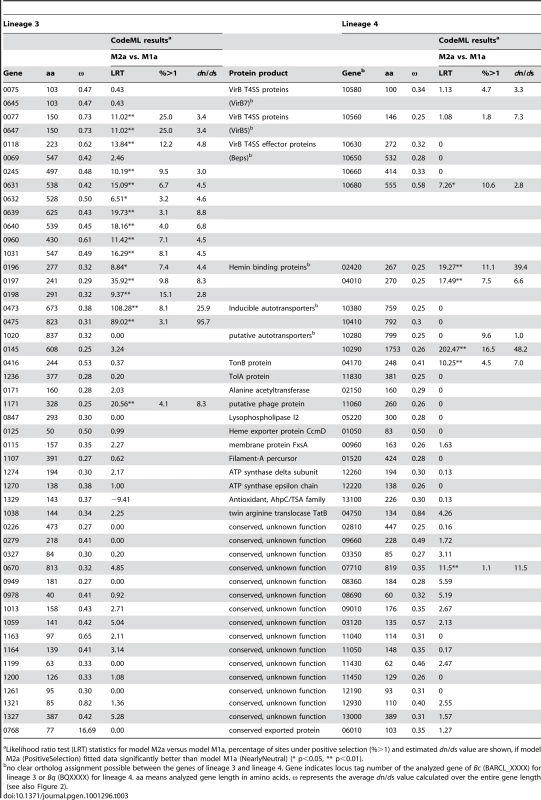
Likelihood ratio test (LRT) statistics for model M2a versus model M1a, percentage of sites under positive selection (%>1) and estimated dn/ds value are shown, if model M2a (PositiveSelection) fitted data significantly better than model M1a (NearlyNeutral) (* p<0.05, ** p<0.01). Importantly, our analysis revealed some lineage-specific colonization factors to carry signs of adaptive evolution. Among others, surface-exposed pilus-components of the Trw T4SS exclusively present in lineage 4 were found to exhibit elevated dn/ds values and sites under positive selection (Table S3). This is in agreement with previous studies and appears to reflect the adaptation of this putative adhesion factor to the erythrocytic surface of different host species [22], [24]. As the Trw T4SS is only present in lineage 4, it might have specifically contributed to the radiation of this most species-rich clade of Bartonella. Factors known to be important for colonization and exclusively present in lineage 3 were not identified by our analysis.
We cannot exclude that the selective pressure imposed by the immune-system might have contributed to the adaptive evolution detected in our genome-wide analysis. It was previously reported that the arms race between host and pathogen can drive the diversification of secretion system - and effector protein-encoding genes [46], [47]. However, in case of the Trw T4SS, recently published in vitro infections with erythrocytes isolated from different mammals demonstrated that the Trw-dependent binding and invasion of Bartonella is host-specific [22]. Although experimental data is not yet available, our data suggest that the VirB T4SS and its effector proteins evolved by similar mechanisms. Together with the previous finding that the VirB T4SSs belong to the few colonization factors specific to the radiating lineages [16], this analysis reveals these horizontally acquired host interacting systems as potential key innovations facilitating adaptation to new hosts and therefore driving the radiations of Bartonella.
Independent evolutionary history of the VirB T4SS in the two parallel adaptive radiations of Bartonella
To further assess the role of the VirB T4SS for the independent adaptive radiations of Bartonella, we compared the chromosomal organization of the VirB and effector protein-encoding genes of the two lineages. Remarkably, our analysis uncovered independent evolutionary scenarios for the chromosomal incorporation of this horizontally acquired trait. In the genomes of lineage 4 (Bg, Bh, Bq, and Bt), the virB T4SS genes, virB2-virB11 and the coupling protein gene virD4, are encoded at the same chromosomal location (Figure 3, Figure S4). Also, the bep genes are encoded in this region. In contrast, the genome sequences of lineage 3 (Bc, Br, BAR15, and B1-1C) revealed marked differences in organization, copy number, and chromosomal localization of the genes encoding the VirB T4SS. In the completely assembled genome of Bc, we found three copies of the virB2-virB10 genes encoded at two different chromosomal locations (Figure 3, Figure S4). Two copies are encoded at the same locus and belong to inverted repeats of ∼10kb. They are separated by several bep genes and the gene virD4. A third copy of the virB2-virB10 cluster including an additional bep gene is encoded in another genomic region highly conserved across different Bartonella lineages (Figure 3).
Fig. 3. Genomic organization of the virB T4SS and bep gene loci in the two radiating lineages. 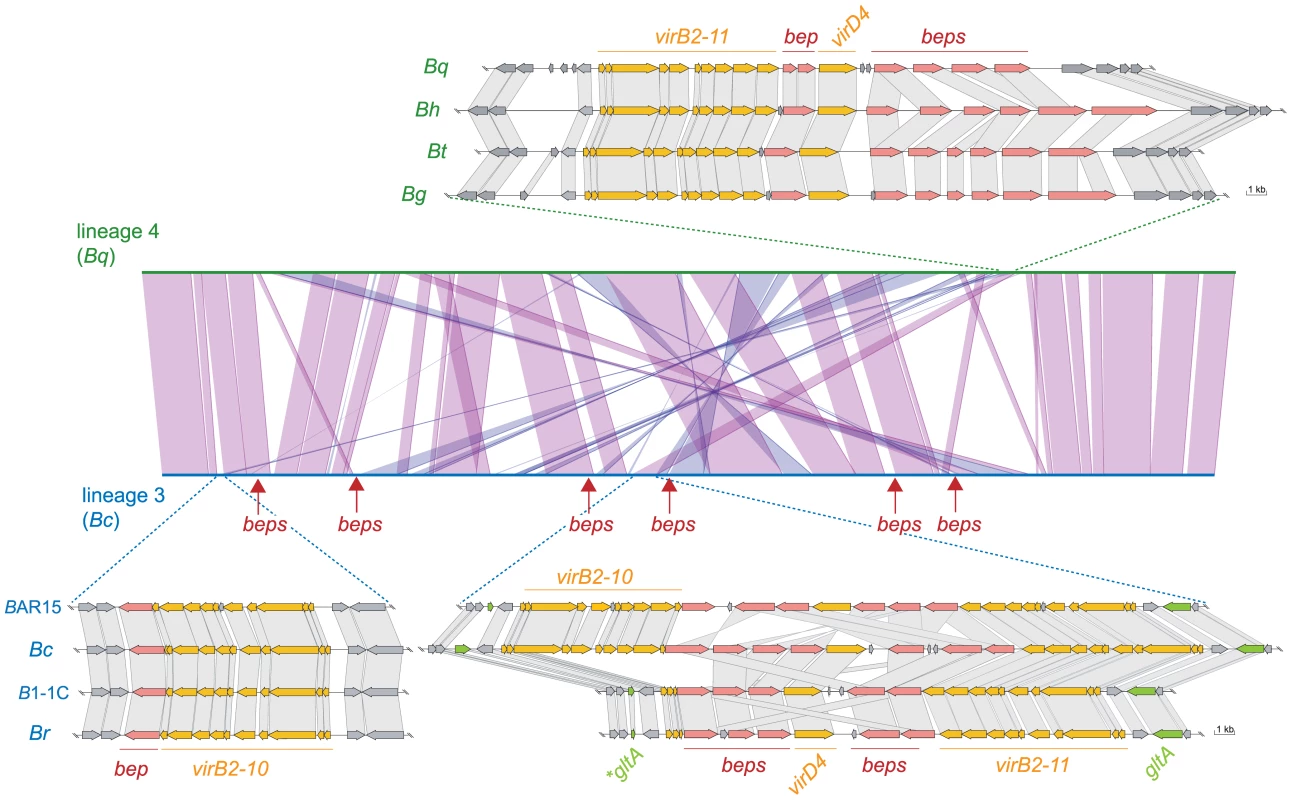
Synteny plot of Bc (lineage 3) and Bq (lineage 4) generated in MaGe [58] and genetic organization of the virB T4SS loci. Syntenic relationships comprising at least five genes are indicated by violet and blue lines for genes found on the same or the opposite strand, respectively. The genomic integration sites of virB loci are indicated. Additional bep loci in lineage 3 are marked by arrows. For the depicted genomic loci, orthologous genes are connected via gray boxes. For bep genes, connections are drawn if they belong to the same Bep clade (Figure 4) or if they are top blast hits of one another. The glutamine syntethase I gene (glnA) and its fragments which are flanking the two inverted virB T4SS copies are colored in green. The fragments are indicated by an asterisk. The same chromosomal integration and amplification of the virB T4SS genes was found in the other three genomes of lineage 3. However, in a common ancestor of Br and B1-1C, one of the three copies must have been partially deleted, as only virB2, virB3, and a remnant of the virB4 gene were found in the corresponding region of these two genomes (Figure 3, Figure S4). Interestingly, the different copies of virB2-virB10 are identical to each other within one species, but divergent across different species indicating the presence of an intra-chromosomal homogenization process. The fact that duplicated components of another T4SS, Trw, also evolved in concert, and the finding of several other identical genes or gene clusters in different Bartonella genomes [24] suggests that sequence homogenization is a common mechanism in Bartonella to conserve paralogous gene copies. The inverted organization of the two virB T4SS gene clusters seems to result from a duplication event subsequent to the integration of a first copy. Evidence comes from a remnant of the glutamine synthetase I gene (glnA) flanking the entire locus at its upstream end. The full-length copy of this vertically inherited housekeeping gene is located directly downstream of the integration site (Figure 3, Figure S4).
In addition to the effector genes adjacently located to the virB genes (as in lineage 4), we found six additional loci encoding bep genes in lineage 3 (Figure S4). These effector genes are not entirely conserved throughout lineage 3, and the existence of gene remnants provides evidence of their deterioration in certain species. Altogether, we identified 12 to 16 bep genes in lineage 3, whereas only five to seven bep genes are present in lineage 4.
Incomplete synteny in the corresponding regions may hinder comparison between the two different lineages, however, no gene remnants could be found at the different integration sites across the two lineages. We cannot fully exclude that massive genomic recombination events resulted in the different chromosomal locations and the lineage-specific dissemination of the virB and bep genes. Yet, such a scenario appears unlikely, as the overall genomic backbone is largely conserved (Figure 3) and the flanking regions of the virB T4SS integration sites do not encode vertically-inherited orthologs across the two lineages. Furthermore, the absence of mobile elements adjacent to the virB T4SS genes such as recombinases, transposases, or integrases is not supportive of an intra-chromosomal mobilization of this genomic locus. T4SS are ancestrally related to conjugation machineries [48]. Thus, the virB genes might have been transferred from a conjugative plasmid into the chromosome by independent events after the divergence of lineage 3 and lineage 4. In Bg, a closely related T4SS, the Vbh, is encoded on a plasmid in addition to a chromosomally integrated copy [28]. This indicates that these horizontally acquired elements can be maintained on extra-chromosomal replicons within Bartonella from where they are integrated into the chromosome. Similarly, pathogenic Escherichia coli strains from different phylogenetic clades were shown to have evolved in parallel by the independent incorporation of virulence traits from mobile genetic elements [49].
Bartonella effector proteins of the two radiating lineages evolved by independent gene amplification and diversification processes
As the chromosomal organization implicates different evolutionary histories of the VirB T4SS in the two radiating lineages, we investigated the relation among the effector proteins translocated by this secretion system. It was previously shown that the bep genes have evolved from a single ancestor by duplication, diversification, and reshuffling of domains resulting in modular gene architectures [17]. The C-terminal BID (Bartonella intracellular delivery) domain is shared by all Beps as it constitutes the secretion signal for the transport via the VirB T4SS. In their N-terminal part, Beps either harbor a FIC (filamentation-induced by cAMP) domain or repeats of additional BID domains (Figure S5). We assessed the evolutionary relationship among the bep genes by inferring phylogenetic trees on the basis of either the BID or the FIC domain, or the entire gene sequence. This revealed that the bep genes of lineage 3 and lineage 4 form two separate clades (Figure 4, Figure S6). Apparently, consecutive rounds of lineage-specific duplications of an ancestral effector gene resulted in the parallel emergence of two distinct arsenals of bep genes. These duplication events preceded the adaptive radiation in both lineages as phylogenetic clusters of effector genes (Bep clades in Figure 4) comprise positional orthologs present in all or a subset of the analyzed genomes of the corresponding lineage (Figure S4). Gene duplications frequently display the primary adaptive response after the acquisition of beneficial factors, because they occur at much higher frequency than other adaptive mutations [50]. This might have been the initial selective pressure for the independent amplification processes. However, in both lineages, the duplicated bep genes subsequently diversified by accumulating mutations as indicated by different branch lengths separating bep genes in Figure 4.
Fig. 4. Gene tree of Bartonella effector proteins. 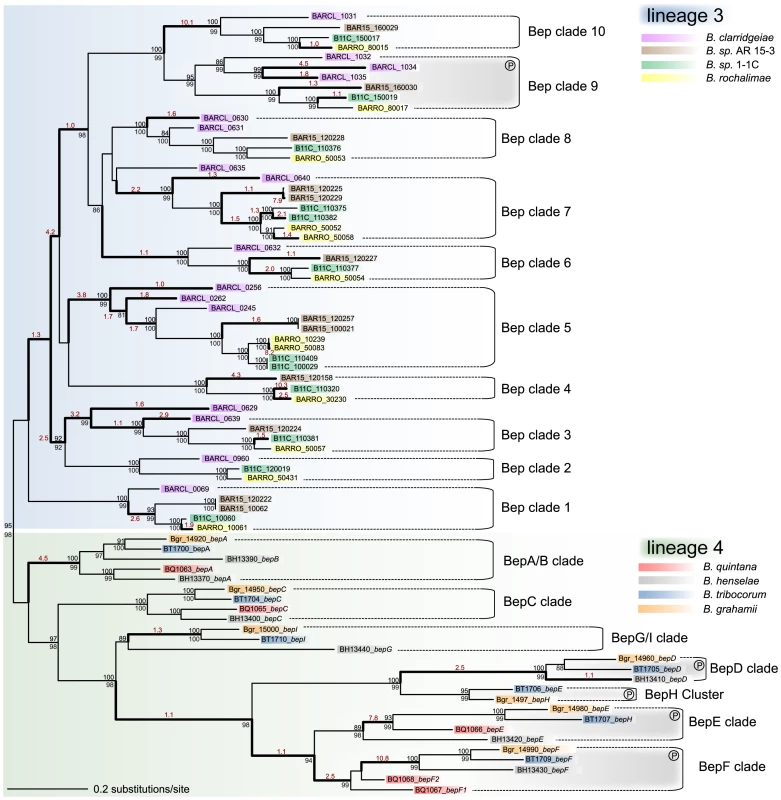
The maximum likelihood tree is based on the BID domain and the C-terminal extension of all bep genes. Numbers above the branches represent maximum likelihood bootstraps (100 replicates); numbers below Bayesian posterior probabilities. Values ≥80% are shown. The independent clustering of the bep genes from lineage 3 and lineage 4 is indicated by blue and green shading, respectively. Different bep genes are named with locus_tag and gene name (if existing) and they are color-coded species-specifically according to the legend. Bold type highlights phylogenetic branches with dn/ds values >1 (the estimated dn/ds value is indicated in red color type). Sub-clades comprising orthologous bep genes are indicated (Bep clades). In lineage 3, BARCL_0629 and BARCL_0635 are not included in any clade since their phylogenetic position is neither supported by bootstraps nor posterior probabilities. In both lineages, clades of bep genes encoding tandem-repeated tyrosine-phosphorylation motifs are indicated by gray shading and an encircled ‘P’ symbol. To analyze the sequence evolution during the parallel amplification and diversification processes, we used a branch test for positive selection. Since positive selection is not continuously acting during evolution, this analysis allows the detection of episodic adaptive evolution on single phylogenetic branches. We detected positive selection on many of the internal branches suggesting that subsequent to their duplication different Bep clades have undergone adaptive sequence evolution in both lineages (Figure 4, Figure S6). For Bartonella, experimental studies showed that effector proteins exhibit distinct phenotypic properties on host cells indicating that the evolutionary diversification of the duplicated effectors was substantially driven by the acquisition of novel functions [18]–[21]. Not all branches exhibit dn/ds values >1, though, suggesting episodic changes in the selection pressure acting on different effector gene copies. For example, functional redundancy of paralogous effector copies could have resulted in neutral drift, whereas conservation of an advantageous function might have led to purifying selection on certain branches.
The basis for the functional versatility seems to lie in the adaptability of the domains encoded by bep genes. In case of the FIC domain, recently published work showed that this domain mediates a new post-translational modification by transferring an AMP moiety onto a target protein [25]. Proteins ‘AMPylated’ by FIC domains belong to the family of GTPases. The diversity of these targets and their numerous functions in cellular processes might allow the diversified Beps to target and subvert a variety of host cell functions by target-specific ‘AMPylation’. The high degree of conservation of the FIC domain in different kingdoms of life provides further evidence for the remarkable versatility of this domain [51]. Interestingly, also the BID domain, constituting part of the translocation signal of the effector proteins, seems to be capable of adopting various functions in the host cell [17]. In case of BepA, it was shown that the BID domain is sufficient to mediate the anti-apoptotic property of this effector protein [18]. BID domains of other Beps with the same domain constitution as BepA do not exhibit this phenotype indicating specific adaptive modulation of this domain for BepA. This functional adaptability might also explain why certain effector genes carry more than one BID domain.
At last, tandem-repeated tyrosine-phosphorylation motifs found in a subset of effector proteins confer another multifaceted molecular mechanism to modulate cellular processes. Phosphorylated effector proteins are thought to recruit cellular binding partners resulting in the formation of signaling scaffolds that interfere with specific host cell signaling pathways [21]. For several effector proteins of Bh (lineage 4), tyrosine phosphorylation by host cells has been reported and the targeted host interaction partners studied [17], [21]. Beside Bartonella, a number of other pathogens, as E. coli (EPEC), Helicobacter pylori, or Chlamydia trachomatis are using tyrosine-phosphorylation of effector proteins to modulate their hosts in very distinct ways demonstrating the versatility of this type of host subversion [21]. In Bartonella, the tyrosine-phosphorylated effector proteins seem to display an important functionality of the VirB-mediated host modulation as we found effector proteins of this type in both radiating lineages (see below).
Parallel evolution of Bartonella effector proteins harboring tandem-repeated tyrosine-phosphorylation motifs
Our analyses suggest that the domain structure of the ancestral effector gene consisted of an N-terminal FIC and a C-terminal BID domain (FIC-BID). In both lineages, the FIC-BID domain structure displays the most abundant effector protein type. In lineage 3, only effector genes of Bep clade 9 consist of domain architectures different than FIC-BID (Figure S5). The gene tree in Figure 4 shows that bep genes with the shortest evolutionary distance across the two lineages are the ones harboring the FIC-BID structure (BepA clade and Bep clade 1). bep genes with different domain architecture constitute more distantly related clades across the two lineages indicating that they derived by independent recombination from the ancestral domain structure. Furthermore, the distantly related Vbh T4SS of Bg and Bs encodes an effector protein consisting of the FIC-BID domain structure [28].
As mentioned above, it was shown for lineage 4 that some of the derived bep genes become phosphorylated by host cell kinases at conserved tandem-repeated tyrosine-phosphorylation motifs leading to the interference with specific host cell pathways [21]. Strikingly, we found that bep genes with derived domain architecture in lineage 3 also harbour regions with tandem-repeated tyrosine motifs (Figure 5, Figure S5). In silico predictions of tyrosine-phosphorylation sites with three different programs [52]–[54] consistently revealed a high number of potentially phosphorylated motifs within these repetitive regions (Figure 5, Table S4). We ectopically expressed these effector proteins in HEK293T cells and showed that they are indeed phosphorylated within eukaryotic cells by tyrosine kinases implicating their functional importance for host interaction (Figure 5). Interestingly, the motifs found in lineage 3 are clearly different from the ones present in lineage 4 and are also less conserved as depicted by their consensus sequences in Figure 5. This suggests that the motifs in lineage 3 may generally be under weaker purifying selection than in lineage 4, because they target either less conserved or even different pathways in their hosts. Further, the lower degree of conservation and the higher number of motifs per effector found in lineage 3 could also indicate that these proteins and particularly their motifs are under positive selection and evolved more recently than their equivalents in lineage 4.
Fig. 5. Parallel evolution of Bartonella effector proteins harboring tandem-repeated tyrosine motifs phosphorylated in host cells. 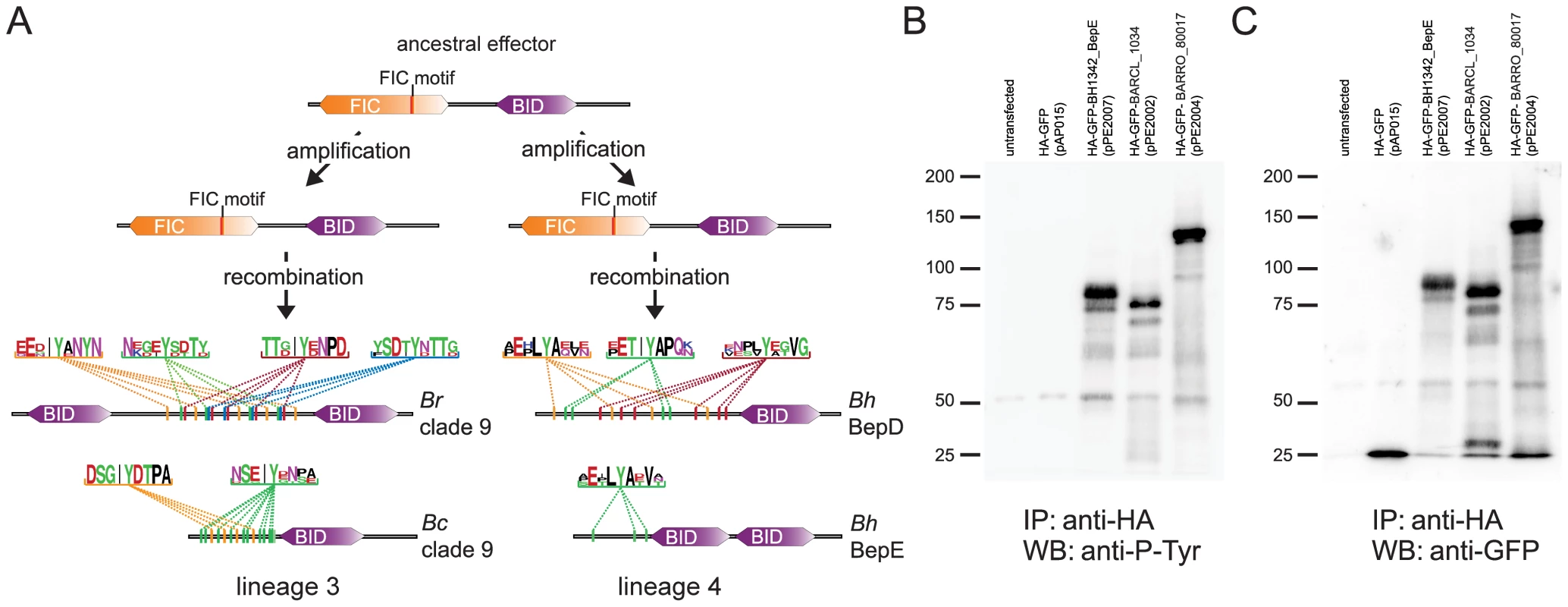
(A) The lineage-specific amplification of effector proteins harboring the ancestral domain structure was followed by secondary recombination events in both lineages. This resulted in the independent emergence of Beps with a derived domain structure (see also Figure S5). In silico predictions for tyrosine-phosphorylation motifs consistently detected high numbers of tandem-repeated phosphorylation sites in independently derived Beps of both lineages (here shown for two Beps of Bep clade 9 [BARRO_80017 of Br and BARCL_1034 of Bc] from lineage 3 and BepD and BepE of Bh from lineage 4). The depicted motifs were identified with NetPhos2.0 with a score ≥0.8 (for predictions of all motifs in lineage 3 see Table S4). The consensus sequence of tandem-repeated motifs (color-coded) is shown for each of the four Beps by WebLogos (http://weblogo.berkeley.edu/logo.cgi). (B) and (C), Immunoprecipitation/Western blot analysis of Beps with predicted tyrosine-phosphorylation motifs ectopically expressed in HEK293T cells. Immunoprecipitations were performed with HA antibody-coated agarose beads. (B) Western blot analysis was performed with anti-phosphotyrosine antibody. (C) Western blot analysis was performed with anti-GFP antibody. The tested constructs correspond to the Beps of Bep clade 9 (BARCL_1034, 70.8 kDa, and BARRO_80017, 113.1 kDa) and BepE of B. henselae (BH13420, 81.4 kDa) shown in (A). HA-GFP was used as a control (28.7 kDa). Together with the fact that tandem-repeated phosphorylation motifs are only found in bep genes with derived domain architecture, our findings, thus, suggest parallel evolution of this class of effector proteins within the two radiating lineages. Whether similar pathways are targeted by these effectors in the two lineages remains unknown. Yet, the striking parallelism in the molecular evolution of this class of effector proteins indicates their central role in the VirB T4SS mediated host modulation by Bartonella.
Conclusions
Emerging infectious diseases are frequently caused by zoonotic pathogens which are incidentally transmitted to humans from their reservoir niche (e.g. other animal hosts). Therefore, the understanding of the mechanisms driving diversification of host-adapted bacteria in nature is of relevance for human health. In the present study, we explored the adaptive diversification of host-restricted bartonellae. Our genome-wide phylogeny revealed that two sister clades of this α-proteobacterial pathogen have evolved by parallel adaptive radiations (lineage 3 and lineage 4 in Figure 1). Both lineages comprise species adapted to same or similar reservoir hosts including zoonotic (e.g. Bh and Br) or human specific (Bq) pathogens. The more recent diversification of lineage 3 including the recently recognized incidental human pathogen Br [29] underlines the importance to study the molecular basis of such lineage diversifications.
In line with the ‘ecological’ parallelism of their radiations, our comparative genomic analyses between lineage 3 and lineage 4 uncover striking evolutionary parallelisms at the molecular level of a likely key innovation - the VirB T4SS – essentially involved in the infection of the mammalian hosts. Chromosomal fixation of this horizontally transferred trait occurred by independent evolutionary events. In both lineages, the arsenal of effector proteins translocated via the VirB T4SS was shaped independently by gene duplications and positive selection of diversified gene copies. This amplification process mostly occurred before the onset of the radiations. Strikingly, beside the diversification of effector proteins encoding the evolutionary conserved ‘AMPylase’ domain (FIC), both lineages have convergently evolved a novel effector class with derived domain structure and tandem-repeated tyrosine-phosphorylation motifs. By these evolutionary processes, large reservoirs of distinct biological functions were invented from a single ancestral effector gene. This functional versatility provides the framework for the adaptive potential of the VirB T4SS. Apparently, the plasticity of the underlying genomic loci seems to have favored the parallel occurrence of these adaptive processes in two distinct lineages, thereby essentially contributing to the parallel radiations of Bartonella.
Materials and Methods
Ethics statement
Animals were handled in strict accordance with good animal practice as defined by the relevant European (European standards of welfare for animals in research), national (Information and guidelines for animal experiments and alternative methods, Federal Veterinary Office of Switzerland) and/or local animal welfare bodies. Animal work was approved by the Veterinary Office of the Canton Basel City on June 2003 (licence no. 1741).
Bacterial strains and growth conditions
Bc strain 73 [34], B1-1C [31], and Br strain ATCC BAA-1498 [29] were grown routinely for 3–5 days on tryptic soy agar containing 5% defibrinated sheep-blood in a water-saturated atmosphere with 5% CO2 at 35°C. BAR15 [30] and Bs strain R1 [55] were grown under the same conditions on heart infusion agar and Colombia base agar, respectively.
Genome sequencing, assembly, and annotation
Using the QIAGEN Genomic DNA Isolation kit (Qiagen), DNA was isolated from bacteria grown from single colonies. For 454-sequencing, the DNA was prepared with an appropriate kit supplied by Roche Applied Science and sequenced on a Roche GS-FLX [56]. To assemble the reads, Newbler standard running parameters with ace file output were used. Newbler assemblies were considerably improved by linking overlapping contigs on the basis of the “_to” and “_from” information appended to the read name in the ace files. For the assemblies of Bc, BAR15, B1-1C, Br, and Bs, we obtained a 454-sequence coverage of 35x, 37x, 39x, 39x, and 29x, respectively (for details on 454-sequencing see Table S5). Repeats were identified by analyzing the coverage of each Newbler contig. If the link between two contigs was ambiguous, PCR and long-range PCR were used to confirm contig joins. For the complete assembly of the Bc genome, a library of 35 kb inserts was generated using the CopyControl Fosmid Kit (Epicentre). By end-sequencing of library clones with Sanger technology, 983 high-quality reads were obtained and mapped onto the 454-sequencing-based assembly. Remaining sequence gaps were closed by PCR. The final singular contig was fully covered by staggered fosmid clones indicating a correct assembly of the circular chromosome of Bc. Gene predictions of the genome of Bc and the draft genomes of Bs, Br, BAR15, and B1-1 were performed using AMIGene software [57]. Automated functional gene annotation was conducted with the genome annotation system MaGe [58]. For orthologous genes, the annotation was adopted from the manually annotated genome of Bt [16]. Manual validation of the annotation was performed for the virB and bep genes. By using the “FusionFission” tool of MaGe [58] fragmented genes were identified and the corresponding sequences subsequently examined for 454-sequencing errors. After correcting these errors, the updated sequences were re-annotated as described above. The sequence data of the genome of Bc and the contigs of the draft genomes of Br, BAR15, B1-1C, and Bs is stored on the web-based interface MaGe (Bartonella2Scope, https://www.genoscope.cns.fr/agc/mage/bartonella2Scope) and has been deposited in the EMBL Nucleotide Sequence Database under accession numbers FN645454–FN645524.
Phylogenetic analyses
Phylogenetic trees were based on nucleotide sequence data. Alignments were generated on protein sequences with ClustalW [59] and back-translated into aligned DNA sequences using MEGA4 [60]. Tree topologies were calculated with maximum likelihood and Bayesian inference methods as implemented in the programs PAUP* [61] and MrBayes [62], respectively. The genome-wide phylogeny of Bartonella was calculated on the basis of 478 orthologous genes of the ten sequenced Bartonella genomes and the genome of Brucella abortus (bv. 1 str. 9-941). Orthologs were determined by using the “PhyloProfile Synteny” tool of MaGe [58] with a threshold of 60% protein identity over at least 80% of the length of proteins being directional best hits of each other. The alignments of the 478 identified genes were concatenated resulting in a total of 515,751 aligned nucleotide sites. Tree topology and branch lengths were obtained by maximum likelihood analysis using the HKY85 model. Bootstrap support values were calculated for 100 replicates. For Bayesian inference, the program MrBayes [62] was run for one million iterations with standard parameters (two runs with four heated Monte-Carlo Markov chains in parallel; number of substitutions = 6; burnin = 25%). For the Bartonella ingroup, single gene trees were calculated with maximum likelihood and tree topology congruency assessed with PAUP*. 471 of the 478 single gene trees revealed the same monophyletic clustering of the eight taxa into lineage 3 and lineage 4 as the genome-wide phylogeny. Further, we performed a recombination analysis for each of the 478 single gene alignments using the GARD algorithm as implemented in the HYPHY package [35]. The GARD analysis was run with the GTR model using a general discrete distribution with three rate classes. To identify statistical significant recombination breakpoints in our alignments, we used the Kishino-Hasegawa test as implemented in the GARDProcess.bf algorithm of the HYPHY package. To include non-sequenced Bartonella species in the genome-wide phylogeny, we used available sequence data from the gltA, groEL, ribC, and rpoB genes (7731 aligned sites). Trees were obtained as described above. MrBayes [62] was run for five million iterations. Branch lengths for tip branches of non-sequenced taxa are calculated on the basis of the four housekeeping genes. Branch lengths for tip branches of sequenced taxa and internal branches separating sequenced and non-sequenced taxa are based on the genomic data set. The maximum likelihood tree only based on the gltA, groEL, ribC, and rpoB genes was inferred as described for the genome-wide phylogeny. Bep gene trees were inferred from nucleotide alignments of either the most C-terminal BID domain including the C-terminus (948 sites), the FIC domain including the N-terminal extension (1,305 sites), or the entire bep sequence of genes harboring FIC domains (3,972 sites). To select an appropriate substitution model, the Akaike information criterion of Modeltest 3.7 [63] and MrModeltest 2.0 [64] was used for the maximum likelihood and Bayesian inference analysis, respectively. For the alignments based on the BID domain or the entire bep gene sequence, we obtained the GTR+G+I model with both programs. For the alignments based on the FIC domain, the TVM+I+G model (Modeltest 3.7) and GTR+G+I model (MrModeltest 2.0) were selected. Trees were inferred with the parameters provided by these models as described above. MrBayes [62] was run for one million iterations. The Neighbor-joining phylogeny of different Bartonella isolates in Figure S2 was inferred from a 242 nt segment of the gltA gene with the program MEGA4 [60]. Bootstrap values were calculated for 1,000 replicates.
Natural selection analyses
Based on the four available genomes, orthologous genes for each of the two lineages 3 and 4 were determined by using the “PhyloProfile Synteny” tool of MaGe [58]. The threshold was set to 30% protein identity over at least 60% of the length of proteins being directional best hits of each other. The same tool was used to detect genes without orthologs. By comparing these automatically identified orthologs and non-orthologs, genes present in neither of the two lists were detected and manually assigned to one of the two lists. Alignments were generated and a GARD recombination analysis conducted as described above. To obtain the average dn/ds value (ω) of each ortholog, the arithmetic mean of pair-wise dn/ds values (calculated by the method of Yang and Nielsen implemented in PAML 4.1 [65]) was used. Site tests of positive selection were performed with PAML 4.1 using the CodeML module [65]. To detect positive selection model M1a (NearlyNeutral) vs. model M2a (PositiveSelection) and model M7 (beta) vs. model M8 (beta+ω) were analyzed. PAUP* [61] was used to infer maximum likelihood trees for each set of orthologs. For the CodeML control file, standard parameters were used. The relative significance of model M2a (PositiveSelection) vs. model M1a (NearlyNeutral) and model M8 (beta+ω) vs. model M7 (beta) was assessed using likelihood-ratio-tests (two degrees of freedom). Genes for which significant positive selection was detected were inspected for alignment errors potentially affecting the results of this analysis. If necessary, the alignments were manually modified and the CodeML analysis repeated. Phylogenetic branches were tested for positive selection by using the TestBranchDNDS.bf module implemented as standard analysis tool in HyPhy [66].
Rat infections
Ten weeks old female WISTAR rats obtained from RCC-Füllinsdorf were housed in an BSL2-animal facility for two weeks prior to infection allowing acclimatization. For inoculation, bacterial strains were grown as described above, harvested in phosphate-buffered saline (PBS), and diluted to OD595 = 1. Rats were anesthetized with a 2–3% Isuflurane/O2 mixture and infected with 10 µl of the bacterial suspension in the dermis of the right ear. Blood samples were taken at the tail vein and immediately mixed with PBS containing 3.8% sodium-citrate to avoid coagulation. After freezing to −70°C and subsequent thawing, undiluted and diluted blood samples were plated on tryptic soy agar and heart infusion agar containing 5% defibrinated sheep-blood. CFUs were counted after 8–12 days of growth.
Nucleotide distances
Nucleotide distances were calculated with the program MEGA4 [60] for the alignments based on the genome-wide dataset and the four housekeeping genes. The numbers of base substitutions per site from averaging over all sequence pairs within and between groups were calculated. Codon positions included were 1st, 2nd, and 3rd. All positions containing gaps and missing data were eliminated from the dataset (Complete deletion option).
Cloning of plasmids for expression of HA-GFP-Bep fusion proteins
To construct the plasmids pPE2002 and pPE2004, bep genes BARCL_1034 (Bc) and BARRO_80017 (Br) were amplified from genomic DNA with primer pairs containing flanking BamHI/NotI sites: prPE453 (ATAAGAATGCGGCCGCGATGAAAAC-CCATAACACTCCTG)/prPE454 (CGGG-ATCCTTAATGTGTTATAACCATCGTTC) and prPE455 (ATAAGAATGCGGCCGCG-ATGAATTTTGGAGAAAAGAAAAAAATG)/prPE456 (CGGGATCCTTAAATAGC-TACAGCTAACGATTTTTTC), respectively. PCR products were digested with the enzymes BamHI and NotI and ligated into the BamHI/NotI sites of the backbone of plasmid pAP013 (kindly provided by Arto Pulliainen). The resulting constructs pPE2001 (BARCL_1034) and pPE2003 (BARRO_80017) were cut with NotI and ligated with a GFP fragment obtained from NotI digested pAP013. The plasmid pPE2007 was constructed by cutting bepE of B. henselae from plasmid pRO1100 (kindly provided by Rusudan Okujava) with NotI and BamHI and ligating it into pAP013. All plasmid DNA isolations and PCR purifications were performed with Macherey-Nagel and Promega columns according to manufacturer's instructions.
Immunoprecipitation and Western blot analysis of HA-GFP-Bep fusion proteins
The protocol for growth and transfection of HEK293T was performed as described previously [18]. 36 h after transfection, cells were incubated for 10 minutes with 10 ml Pervanadate medium (5 ml PBS containing 100 mM orthovanadate and 200 mM H2O2, incubated for 10 min with 500 µl Catalase [2 mg/ml in PBS] before 45 ml M199 medium were added). After washing three times with 7 ml of PBS at room temperature, cells were scraped off and resuspended in 1 ml of ice-cold PBS containing 1 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM orthovanadate, 1 mM leupeptin, and 1 mM pepstatin and collected by centrifugation (3,000g at 4°C for 60 sec). The resulting pellet was lysed in 300 µl of ice cold modified RIPA buffer (50 mM Tris-HCl [pH 7.4], 75 mM NaCl, 1 mM EDTA, 1 mM orthovanadate, 1 mM leupeptin, 1 mM pepstatin) for 1 hour at 4°C. The lysate was centrifuged (16,000g at 4°C for 15 min) and 12 µl of anti-HA-agarose (Sigma) added to the supernatant. After 150 min of incubation at 4°C on a slowly turning rotation shaker, the agarose was washed three times with 300 µl of modified RIPA buffer (3,000g for 10 sec). The affinity-gel pellet was then resuspended in 20 µl of modified RIPA buffer, 20 µl of SDS-sample buffer (2×) were added, and the sample was heated for 5 min at 95°C. Proteins were separated on a 10% SDS-polyacrylamide gel, blotted on a nitrocellulose membrane (Hybond-C Extra, Amersham Pharmacia), and examined for tyrosine phosphorylation by using monoclonal antibody 4G10 (Millipore) and anti-mouse IgG-horseradish peroxidase (HRP) afterwards. The HRP-conjugated antibody was visualized by enhanced chemiluminescence (PerkinElmer). For visualization of the signal from GFP-fusion proteins, the membrane was subsequently incubated in 4% PBS-Tween containing 0.02% NaN3 and anti-GFP antibody (Invitrogen), followed by incubation with anti-mouse IgG-HRP and visualization by enhanced chemiluminescence.
Supporting Information
Zdroje
1. SchluterD
2000 The ecology of adaptive radiation Oxford Oxford University Press viii, 288
2. GrantPR
GrantBR
2008 How and why species multiply : the radiation of Darwin's finches Princeton Princeton University Press xix, 218
3. SalzburgerW
2009 The interaction of sexually and naturally selected traits in the adaptive radiations of cichlid fishes. Mol Ecol 18 169 185
4. JohnsonZI
ZinserER
CoeA
McNultyNP
WoodwardEM
2006 Niche partitioning among Prochlorococcus ecotypes along ocean-scale environmental gradients. Science 311 1737 1740
5. RaineyPB
TravisanoM
1998 Adaptive radiation in a heterogeneous environment. Nature 394 69 72
6. SikorskiJ
NevoE
2005 Adaptation and incipient sympatric speciation of Bacillus simplex under microclimatic contrast at “Evolution Canyons” I and II, Israel. Proc Natl Acad Sci U S A 102 15924 15929
7. BrakefieldPM
RoskamJC
2006 Exploring evolutionary constraints is a task for an integrative evolutionary biology. Am Nat 168 Suppl 6 S4 13
8. DuponchelleF
ParadisE
RibbinkAJ
TurnerGF
2008 Parallel life history evolution in mouthbrooding cichlids from the African Great Lakes. Proc Natl Acad Sci U S A 105 15475 15480
9. KronforstMR
KapanDD
GilbertLE
2006 Parallel genetic architecture of parallel adaptive radiations in mimetic Heliconius butterflies. Genetics 174 535 539
10. LososJB
RicklefsRE
2009 Adaptation and diversification on islands. Nature 457 830 836
11. Harvey P.H.PMD
1991 The comparative method in evolutionary biology London/New York/Oxford Oxford University Press
12. DehioC
2005 Bartonella-host-cell interactions and vascular tumour formation. Nat Rev Microbiol 3 621 631
13. MarignacG
BarratF
ChomelB
Vayssier-TaussatM
GandoinC
2010 Murine model for Bartonella birtlesii infection: New aspects. Comp Immunol Microbiol Infect Dis 33 95 107
14. SchuleinR
SeubertA
GilleC
LanzC
HansmannY
2001 Invasion and persistent intracellular colonization of erythrocytes. A unique parasitic strategy of the emerging pathogen Bartonella. J Exp Med 193 1077 1086
15. ZhangP
ChomelBB
SchauMK
GooJS
DrozS
2004 A family of variably expressed outer-membrane proteins (Vomp) mediates adhesion and autoaggregation in Bartonella quintana. Proc Natl Acad Sci U S A 101 13630 13635
16. SaenzHL
EngelP
StoeckliMC
LanzC
RaddatzG
2007 Genomic analysis of Bartonella identifies type IV secretion systems as host adaptability factors. Nat Genet 39 1469 1476
17. SchuleinR
GuyeP
RhombergTA
SchmidMC
SchroderG
2005 A bipartite signal mediates the transfer of type IV secretion substrates of Bartonella henselae into human cells. Proc Natl Acad Sci U S A 102 856 861
18. SchmidMC
ScheideggerF
DehioM
Balmelle-DevauxN
SchuleinR
2006 A translocated bacterial protein protects vascular endothelial cells from apoptosis. PLoS Pathog 2 e115 doi:10.1371/journal.ppat.0020115
19. RhombergTA
TruttmannMC
GuyeP
EllnerY
DehioC
2009 A translocated protein of Bartonella henselae interferes with endocytic uptake of individual bacteria and triggers uptake of large bacterial aggregates via the invasome. Cell Microbiol 11 927 945
20. ScheideggerF
EllnerY
GuyeP
RhombergTA
WeberH
2009 Distinct activities of Bartonella henselae type IV secretion effector proteins modulate capillary-like sprout formation. Cell Microbiol 11 1088 1101
21. SelbachM
PaulFE
BrandtS
GuyeP
DaumkeO
2009 Host cell interactome of tyrosine-phosphorylated bacterial proteins. Cell Host Microbe 5 397 403
22. Vayssier-TaussatM
Le RhunD
DengHK
BivilleF
CescauS
2010 The Trw type IV secretion system of Bartonella mediates host-specific adhesion to erythrocytes. PLoS Pathog 6 e1000946 doi:10.1371/journal.ppat.1000946
23. SeubertA
HiestandR
de la CruzF
DehioC
2003 A bacterial conjugation machinery recruited for pathogenesis. Mol Microbiol 49 1253 1266
24. NystedtB
FrankAC
ThollessonM
AnderssonSG
2008 Diversifying selection and concerted evolution of a type IV secretion system in Bartonella. Mol Biol Evol 25 287 300
25. YarbroughML
LiY
KinchLN
GrishinNV
BallHL
2009 AMPylation of Rho GTPases by Vibrio VopS disrupts effector binding and downstream signaling. Science 323 269 272
26. YarbroughML
OrthK
2009 AMPylation is a new post-translational modiFICation. Nat Chem Biol 5 378 379
27. AlsmarkCM
FrankAC
KarlbergEO
LegaultBA
ArdellDH
2004 The louse-borne human pathogen Bartonella quintana is a genomic derivative of the zoonotic agent Bartonella henselae. Proc Natl Acad Sci U S A 101 9716 9721
28. BerglundEC
FrankAC
CalteauA
Vinnere PetterssonO
GranbergF
2009 Run-off replication of host-adaptability genes is associated with gene transfer agents in the genome of mouse-infecting Bartonella grahamii. PLoS Genet 5 e1000546 doi:10.1371/journal.pgen.1000546
29. EremeevaME
GernsHL
LydySL
GooJS
RyanET
2007 Bacteremia, fever, and splenomegaly caused by a newly recognized Bartonella species. N Engl J Med 356 2381 2387
30. InoueK
MaruyamaS
KabeyaH
HagiyaK
IzumiY
2009 Exotic small mammals as potential reservoirs of zoonotic Bartonella spp. Emerg Infect Dis 15 526 532
31. LinJW
ChenCY
ChenWC
ChomelBB
ChangCC
2008 Isolation of Bartonella species from rodents in Taiwan including a strain closely related to ‘Bartonella rochalimae’ from Rattus norvegicus. J Med Microbiol 57 1496 1501
32. HennJB
GabrielMW
KastenRW
BrownRN
KoehlerJE
2009 Infective endocarditis in a dog and the phylogenetic relationship of the associated “Bartonella rochalimae” strain with isolates from dogs, gray foxes, and a human. J Clin Microbiol 47 787 790
33. KordickDL
HilyardEJ
HadfieldTL
WilsonKH
SteigerwaltAG
1997 Bartonella clarridgeiae, a newly recognized zoonotic pathogen causing inoculation papules, fever, and lymphadenopathy (cat scratch disease). J Clin Microbiol 35 1813 1818
34. HellerR
ArtoisM
XemarV
De BrielD
GehinH
1997 Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J Clin Microbiol 35 1327 1331
35. Kosakovsky PondSL
PosadaD
GravenorMB
WoelkCH
FrostSD
2006 GARD: a genetic algorithm for recombination detection. Bioinformatics 22 3096 3098
36. Bininda-EmondsOR
2005 Supertree construction in the genomic age. Methods Enzymol 395 745 757
37. IhlerGM
1996 Bartonella bacilliformis: dangerous pathogen slowly emerging from deep background. FEMS Microbiol Lett 144 1 11
38. ChomelBB
BoulouisHJ
BreitschwerdtEB
KastenRW
Vayssier-TaussatM
2009 Ecological fitness and strategies of adaptation of Bartonella species to their hosts and vectors. Vet Res 40 29
39. ChomelBB
HennJB
KastenRW
NietoNC
FoleyJ
2009 Dogs are more permissive than cats or guinea pigs to experimental infection with a human isolate of Bartonella rochalimae. Vet Res 40 27
40. KosoyMY
SaitoEK
GreenD
MarstonEL
JonesDC
2000 Experimental evidence of host specificity of Bartonella infection in rodents. Comp Immunol Microbiol Infect Dis 23 221 238
41. BerglundEC
EllegaardK
GranbergF
XieZ
MaruyamaS
2010 Rapid diversification by recombination in Bartonella grahamii from wild rodents in Asia contrasts with low levels of genomic divergence in Northern Europe and America. Mol Ecol 19 2241 2255
42. YangZ
NielsenR
2002 Codon-substitution models for detecting molecular adaptation at individual sites along specific lineages. Mol Biol Evol 19 908 917
43. CarrollJA
ColemanSA
SmithermanLS
MinnickMF
2000 Hemin-binding surface protein from Bartonella quintana. Infect Immun 68 6750 6757
44. DehioC
2004 Molecular and cellular basis of Bartonella pathogenesis. Annu Rev Microbiol 58 365 390
45. MinnickMF
SappingtonKN
SmithermanLS
AnderssonSG
KarlbergO
2003 Five-member gene family of Bartonella quintana. Infect Immun 71 814 821
46. McCannHC
GuttmanDS
2008 Evolution of the type III secretion system and its effectors in plant-microbe interactions. New Phytol 177 33 47
47. MaW
DongFF
StavrinidesJ
GuttmanDS
2006 Type III effector diversification via both pathoadaptation and horizontal transfer in response to a coevolutionary arms race. PLoS Genet 2 e209 doi:10.1371/journal.pgen.0020209
48. FrankAC
AlsmarkCM
ThollessonM
AnderssonSG
2005 Functional divergence and horizontal transfer of type IV secretion systems. Mol Biol Evol 22 1325 1336
49. OguraY
OokaT
IguchiA
TohH
AsadulghaniM
2009 Comparative genomics reveal the mechanism of the parallel evolution of O157 and non-O157 enterohemorrhagic Escherichia coli. Proc Natl Acad Sci U S A 106 17939 17944
50. SandegrenL
AnderssonDI
2009 Bacterial gene amplification: implications for the evolution of antibiotic resistance. Nat Rev Microbiol 7 578 588
51. KinchLN
YarbroughML
OrthK
GrishinNV
2009 Fido, a novel AMPylation domain common to fic, doc, and AvrB. PLoS ONE 4 e5818 doi:10.1371/journal.pone.0005818
52. BlomN
GammeltoftS
BrunakS
1999 Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol 294 1351 1362
53. HuangHD
LeeTY
TzengSW
HorngJT
2005 KinasePhos: a web tool for identifying protein kinase-specific phosphorylation sites. Nucleic Acids Res 33 W226 229
54. ObenauerJC
CantleyLC
YaffeMB
2003 Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res 31 3635 3641
55. DehioC
LanzC
PohlR
BehrensP
BermondD
2001 Bartonella schoenbuchii sp. nov., isolated from the blood of wild roe deer. Int J Syst Evol Microbiol 51 1557 1565
56. MarguliesM
EgholmM
AltmanWE
AttiyaS
BaderJS
2005 Genome sequencing in microfabricated high-density picolitre reactors. Nature 437 376 380
57. BocsS
CruveillerS
VallenetD
NuelG
MedigueC
2003 AMIGene: Annotation of MIcrobial Genes. Nucleic Acids Res 31 3723 3726
58. VallenetD
LabarreL
RouyZ
BarbeV
BocsS
2006 MaGe: a microbial genome annotation system supported by synteny results. Nucleic Acids Res 34 53 65
59. ThompsonJD
HigginsDG
GibsonTJ
1994 CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22 4673 4680
60. TamuraK
DudleyJ
NeiM
KumarS
2007 MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24 1596 1599
61. WilgenbuschJC
SwoffordD
2003 Inferring evolutionary trees with PAUP*. Curr Protoc Bioinformatics Chapter 6 Unit 6.4.1 6.4.28
62. HuelsenbeckJP
RonquistF
2001 MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17 754 755
63. PosadaD
2003 Using MODELTEST and PAUP* to select a model of nucleotide substitution. Curr Protoc Bioinformatics Chapter 6 Unit 6.5.1 6.5.14
64. NylanderJAA
2004 MrModeltest v2 Program distributed by the author
65. YangZ
2007 PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24 1586 1591
66. PondSL
FrostSD
MuseSV
2005 HyPhy: hypothesis testing using phylogenies. Bioinformatics 21 676 679
67. EngelP
DehioC
2009 Genomics of Host-Restricted Pathogens of the Genus Bartonella. Genome Dyn 6 158 169
68. ArvandM
RaoultD
FeilEJ
2010 Multi-locus sequence typing of a geographically and temporally diverse sample of the highly clonal human pathogen Bartonella quintana. PLoS ONE 5 e9765 doi:10.1371/journal.pone.0009765
69. BaiY
KosoyMY
CullyJF
BalaT
RayC
2007 Acquisition of nonspecific Bartonella strains by the northern grasshopper mouse (Onychomys leucogaster). FEMS Microbiol Ecol 61 438 448
70. BaiY
KosoyMY
LerdthusneeK
PeruskiLF
RichardsonJH
2009 Prevalence and genetic heterogeneity of Bartonella strains cultured from rodents from 17 provinces in Thailand. Am J Trop Med Hyg 81 811 816
71. BemisDA
KaniaSA
2007 Isolation of Bartonella sp. from sheep blood. Emerg Infect Dis 13 1565 1567
72. BerglundEC
EhrenborgC
Vinnere PetterssonO
GranbergF
NaslundK
2010 Genome dynamics of Bartonella grahamii in micro-populations of woodland rodents. BMC Genomics 11 152
73. BermondD
BoulouisHJ
HellerR
Van LaereG
MonteilH
2002 Bartonella bovis Bermond et al. sp. nov. and Bartonella capreoli sp. nov., isolated from European ruminants. Int J Syst Evol Microbiol 52 383 390
74. BermondD
HellerR
BarratF
DelacourG
DehioC
2000 Bartonella birtlesii sp. nov., isolated from small mammals (Apodemus spp.). Int J Syst Evol Microbiol 50 1973 1979
75. BirtlesRJ
HarrisonTG
SaundersNA
MolyneuxDH
1995 Proposals to unify the genera Grahamella and Bartonella, with descriptions of Bartonella talpae comb. nov., Bartonella peromysci comb. nov., and three new species, Bartonella grahamii sp. nov., Bartonella taylorii sp. nov., and Bartonella doshiae sp. nov. Int J Syst Bacteriol 45 1 8
76. BirtlesRJ
HazelSM
BennettM
BownK
RaoultD
2001 Longitudinal monitoring of the dynamics of infections due to Bartonella species in UK woodland rodents. Epidemiol Infect 126 323 329
77. BirtlesRJ
RaoultD
1996 Comparison of partial citrate synthase gene (gltA) sequences for phylogenetic analysis of Bartonella species. Int J Syst Bacteriol 46 891 897
78. CelebiB
CarhanA
KilicS
BaburC
2010 Detection and Genetic Diversity of Bartonella vinsonii Subsp. berkhoffii Strains Isolated from Dogs in Ankara, Turkey. J Vet Med Sci 72 969 73
79. ChamberlinJ
LaughlinLW
RomeroS
SolorzanoN
GordonS
2002 Epidemiology of endemic Bartonella bacilliformis: a prospective cohort study in a Peruvian mountain valley community. J Infect Dis 186 983 990
80. ChangCC
ChomelBB
KastenRW
HellerRM
UenoH
2000 Bartonella spp. isolated from wild and domestic ruminants in North America. Emerg Infect Dis 6 306 311
81. ClarridgeJE3rd
RaichTJ
PirwaniD
SimonB
TsaiL
1995 Strategy to detect and identify Bartonella species in routine clinical laboratory yields Bartonella henselae from human immunodeficiency virus-positive patient and unique Bartonella strain from his cat. J Clin Microbiol 33 2107 2113
82. DillonB
ValenzuelaJ
DonR
BlanckenbergD
WigneyDI
2002 Limited diversity among human isolates of Bartonella henselae. J Clin Microbiol 40 4691 4699
83. EllisBA
RegneryRL
BeatiL
BacellarF
RoodM
1999 Rats of the genus Rattus are reservoir hosts for pathogenic Bartonella species: an Old World origin for a New World disease? J Infect Dis 180 220 224
84. GundiVA
DavoustB
KhamisA
BoniM
RaoultD
2004 Isolation of Bartonella rattimassiliensis sp. nov. and Bartonella phoceensis sp. nov. from European Rattus norvegicus. J Clin Microbiol 42 3816 3818
85. GurfieldAN
BoulouisHJ
ChomelBB
KastenRW
HellerR
2001 Epidemiology of Bartonella infection in domestic cats in France. Vet Microbiol 80 185 198
86. HarrusS
Bar-GalGK
GolanA
Elazari-VolcaniR
KosoyMY
2009 Isolation and genetic characterization of a Bartonella strain closely related to Bartonella tribocorum and Bartonella elizabethae in Israeli commensal rats. Am J Trop Med Hyg 81 55 58
87. HellerR
RiegelP
HansmannY
DelacourG
BermondD
1998 Bartonella tribocorum sp. nov., a new Bartonella species isolated from the blood of wild rats. Int J Syst Bacteriol 48 Pt 4 1333 1339
88. HennJB
ChomelBB
BoulouisHJ
KastenRW
MurrayWJ
2009 Bartonella rochalimae in raccoons, coyotes, and red foxes. Emerg Infect Dis 15 1984 1987
89. HofmeisterEK
KolbertCP
AbdulkarimAS
MageraJM
HopkinsMK
1998 Cosegregation of a novel Bartonella species with Borrelia burgdorferi and Babesia microti in Peromyscus leucopus. J Infect Dis 177 409 416
90. HolmbergM
MillsJN
McGillS
BenjaminG
EllisBA
2003 Bartonella infection in sylvatic small mammals of central Sweden. Epidemiol Infect 130 149 157
91. InoueK
KabeyaH
KosoyMY
BaiY
SmirnovG
2009 Evolutional and geographical relationships of Bartonella grahamii isolates from wild rodents by multi-locus sequencing analysis. Microb Ecol 57 534 541
92. InoueK
MaruyamaS
KabeyaH
YamadaN
OhashiN
2008 Prevalence and genetic diversity of Bartonella species isolated from wild rodents in Japan. Appl Environ Microbiol 74 5086 5092
93. JardineC
AppleyardG
KosoyMY
McCollD
Chirino-TrejoM
2005 Rodent-associated Bartonella in Saskatchewan, Canada. Vector Borne Zoonotic Dis 5 402 409
94. KosoyM
MurrayM
GilmoreRDJr
BaiY
GageKL
2003 Bartonella strains from ground squirrels are identical to Bartonella washoensis isolated from a human patient. J Clin Microbiol 41 645 650
95. KosoyMY
RegneryRL
TzianabosT
MarstonEL
JonesDC
1997 Distribution, diversity, and host specificity of Bartonella in rodents from the Southeastern United States. Am J Trop Med Hyg 57 578 588
96. LiDM
MengFX
SongXP
QinZJ
YangXR
2006 [Study on Bartonella vinsonii berkhoffii isolated from blood of native dogs in China]. Zhonghua Liu Xing Bing Xue Za Zhi 27 333 338
97. LydySL
EremeevaME
AsnisD
PaddockCD
NicholsonWL
2008 Isolation and characterization of Bartonella bacilliformis from an expatriate Ecuadorian. J Clin Microbiol 46 627 637
98. MaillardR
RiegelP
BarratF
BouillinC
ThibaultD
2004 Bartonella chomelii sp. nov., isolated from French domestic cattle (Bos taurus). Int J Syst Evol Microbiol 54 215 220
99. MarquezFJ
2010 Molecular Detection of Bartonella alsatica in European Wild Rabbits (Oryctolagus cuniculus) in Andalusia (Spain). Vector Borne Zoonotic Dis 10 731 4
100. MarstonEL
FinkelB
RegneryRL
WinotoIL
GrahamRR
1999 Prevalence of Bartonella henselae and Bartonella clarridgeiae in an urban Indonesian cat population. Clin Diagn Lab Immunol 6 41 44
101. PretoriusAM
BeatiL
BirtlesRJ
2004 Diversity of bartonellae associated with small mammals inhabiting Free State province, South Africa. Int J Syst Evol Microbiol 54 1959 1967
102. RolainJM
FournierPE
RaoultD
BonerandiJJ
2003 First isolation and detection by immunofluorescence assay of Bartonella koehlerae in erythrocytes from a French cat. J Clin Microbiol 41 4001 4002
103. TeaA
Alexiou-DanielS
PapoutsiA
PapaA
AntoniadisA
2004 Bartonella species isolated from rodents, Greece. Emerg Infect Dis 10 963 964
104. YingB
KosoyMY
MaupinGO
TsuchiyaKR
GageKL
2002 Genetic and ecologic characteristics of Bartonella communities in rodents in southern China. Am J Trop Med Hyg 66 622 627
105. ZeaiterZ
FournierPE
OgataH
RaoultD
2002 Phylogenetic classification of Bartonella species by comparing groEL sequences. Int J Syst Evol Microbiol 52 165 171
Štítky
Genetika Reprodukční medicína
Článek Break to Make a ConnectionČlánek A New Testing Strategy to Identify Rare Variants with Either Risk or Protective Effect on DiseaseČlánek The Architecture of Gene Regulatory Variation across Multiple Human Tissues: The MuTHER Study
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 2- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Intrauterinní inseminace a její úspěšnost
- Růst a vývoj dětí narozených pomocí IVF
- Hypogonadotropní hypogonadismus u žen a vliv na výsledky reprodukce po IVF
-
Všechny články tohoto čísla
- Break to Make a Connection
- A New Testing Strategy to Identify Rare Variants with Either Risk or Protective Effect on Disease
- Single-Tissue and Cross-Tissue Heritability of Gene Expression Via Identity-by-Descent in Related or Unrelated Individuals
- Pervasive Adaptive Protein Evolution Apparent in Diversity Patterns around Amino Acid Substitutions in
- The Architecture of Gene Regulatory Variation across Multiple Human Tissues: The MuTHER Study
- MiRNA Control of Vegetative Phase Change in Trees
- New Functions of Ctf18-RFC in Preserving Genome Stability outside Its Role in Sister Chromatid Cohesion
- Genome-Wide Association Studies of the PR Interval in African Americans
- Mapping of the Disease Locus and Identification of As a Candidate Gene in a Canine Model of Primary Open Angle Glaucoma
- Mapping a New Spontaneous Preterm Birth Susceptibility Gene, , Using Linkage, Haplotype Sharing, and Association Analysis
- A Population Genetic Approach to Mapping Neurological Disorder Genes Using Deep Resequencing
- and Genes Modulate the Switch between Attraction and Repulsion during Behavioral Phase Change in the Migratory Locust
- Targeted Sister Chromatid Cohesion by Sir2
- Correlated Evolution of Nearby Residues in Drosophilid Proteins
- Parallel Evolution of a Type IV Secretion System in Radiating Lineages of the Host-Restricted Bacterial Pathogen
- Lipophorin Receptors Mediate the Uptake of Neutral Lipids in Oocytes and Imaginal Disc Cells by an Endocytosis-Independent Mechanism
- Genome-Wide Association Study of Coronary Heart Disease and Its Risk Factors in 8,090 African Americans: The NHLBI CARe Project
- The Evolution of Host Specialization in the Vertebrate Gut Symbiont
- Genome-Wide Association of Familial Late-Onset Alzheimer's Disease Replicates and and Nominates in Interaction with
- Risk Alleles for Systemic Lupus Erythematosus in a Large Case-Control Collection and Associations with Clinical Subphenotypes
- Association between Common Variation at the Locus and Changes in Body Mass Index from Infancy to Late Childhood: The Complex Nature of Genetic Association through Growth and Development
- AID Induces Double-Strand Breaks at Immunoglobulin Switch Regions and Causing Chromosomal Translocations in Yeast THO Mutants
- A Study of CNVs As Trait-Associated Polymorphisms and As Expression Quantitative Trait Loci
- Whole-Genome Comparison Reveals Novel Genetic Elements That Characterize the Genome of Industrial Strains of
- Prevalence of Epistasis in the Evolution of Influenza A Surface Proteins
- Srf1 Is a Novel Regulator of Phospholipase D Activity and Is Essential to Buffer the Toxic Effects of C16:0 Platelet Activating Factor
- Two Frizzled Planar Cell Polarity Signals in the Wing Are Differentially Organized by the Fat/Dachsous Pathway
- Phosphoinositide Regulation of Integrin Trafficking Required for Muscle Attachment and Maintenance
- Pathogenic VCP/TER94 Alleles Are Dominant Actives and Contribute to Neurodegeneration by Altering Cellular ATP Level in a IBMPFD Model
- Meta-Analysis of Genome-Wide Association Studies in Celiac Disease and Rheumatoid Arthritis Identifies Fourteen Non-HLA Shared Loci
- A Genome-Wide Study of DNA Methylation Patterns and Gene Expression Levels in Multiple Human and Chimpanzee Tissues
- Nucleosomes Containing Methylated DNA Stabilize DNA Methyltransferases 3A/3B and Ensure Faithful Epigenetic Inheritance
- Mutations in Zebrafish Result in Adult-Onset Ocular Pathogenesis That Models Myopia and Other Risk Factors for Glaucoma
- [], the Prion Formed by the Chromatin Remodeling Factor Swi1, Is Highly Sensitive to Alterations in Hsp70 Chaperone System Activity
- Characterization of Transcriptome Remodeling during Cambium Formation Identifies and As Opposing Regulators of Secondary Growth
- The Cardiac Transcription Network Modulated by Gata4, Mef2a, Nkx2.5, Srf, Histone Modifications, and MicroRNAs
- Epistatic Interaction Maps Relative to Multiple Metabolic Phenotypes
- Quantitative Models of the Mechanisms That Control Genome-Wide Patterns of Transcription Factor Binding during Early Development
- Genome-Wide Transcript Profiling of Endosperm without Paternal Contribution Identifies Parent-of-Origin–Dependent Regulation of
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Meta-Analysis of Genome-Wide Association Studies in Celiac Disease and Rheumatoid Arthritis Identifies Fourteen Non-HLA Shared Loci
- MiRNA Control of Vegetative Phase Change in Trees
- Risk Alleles for Systemic Lupus Erythematosus in a Large Case-Control Collection and Associations with Clinical Subphenotypes
- The Cardiac Transcription Network Modulated by Gata4, Mef2a, Nkx2.5, Srf, Histone Modifications, and MicroRNAs
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



