-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Distinct Functions for the piRNA Pathway in Genome Maintenance and Telomere Protection
Transposons and other selfish DNA elements can be found in all phyla, and mobilization of these elements can compromise genome integrity. The piRNA (PIWI-interacting RNA) pathway silences transposons in the germline, but it is unclear if this pathway has additional functions during development. Here we show that mutations in the Drosophila piRNA pathway genes, armi, aub, ago3, and rhi, lead to extensive fragmentation of the zygotic genome during the cleavage stage of embryonic divisions. Additionally, aub and armi show defects in telomere resolution during meiosis and the cleavage divisions; and mutations in lig-IV, which disrupt non-homologous end joining, suppress these fusions. By contrast, lig-IV mutations enhance chromosome fragmentation. Chromatin immunoprecipitation studies show that aub and armi mutations disrupt telomere binding of HOAP, which is a component of the telomere protection complex, and reduce expression of a subpopulation of 19 - to 22-nt telomere-specific piRNAs. Mutations in rhi and ago3, by contrast, do not block HOAP binding or production of these piRNAs. These findings uncover genetically separable functions for the Drosophila piRNA pathway. The aub, armi, rhi, and ago3 genes silence transposons and maintain chromosome integrity during cleavage-stage embryonic divisions. However, the aub and armi genes have an additional function in assembly of the telomere protection complex.
Published in the journal: . PLoS Genet 6(12): e32767. doi:10.1371/journal.pgen.1001246
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001246Summary
Transposons and other selfish DNA elements can be found in all phyla, and mobilization of these elements can compromise genome integrity. The piRNA (PIWI-interacting RNA) pathway silences transposons in the germline, but it is unclear if this pathway has additional functions during development. Here we show that mutations in the Drosophila piRNA pathway genes, armi, aub, ago3, and rhi, lead to extensive fragmentation of the zygotic genome during the cleavage stage of embryonic divisions. Additionally, aub and armi show defects in telomere resolution during meiosis and the cleavage divisions; and mutations in lig-IV, which disrupt non-homologous end joining, suppress these fusions. By contrast, lig-IV mutations enhance chromosome fragmentation. Chromatin immunoprecipitation studies show that aub and armi mutations disrupt telomere binding of HOAP, which is a component of the telomere protection complex, and reduce expression of a subpopulation of 19 - to 22-nt telomere-specific piRNAs. Mutations in rhi and ago3, by contrast, do not block HOAP binding or production of these piRNAs. These findings uncover genetically separable functions for the Drosophila piRNA pathway. The aub, armi, rhi, and ago3 genes silence transposons and maintain chromosome integrity during cleavage-stage embryonic divisions. However, the aub and armi genes have an additional function in assembly of the telomere protection complex.
Introduction
Drosophila piRNAs have been implicated in transposon silencing and maintenance of genome integrity during female germline development. However, piRNA pathway mutations lead to complex developmental phenotypes [1], [2], [3], [4], and piRNAs have been implicated in control of gene expression [5], [6], [7], [8]. Furthermore, the majority of piRNAs in other systems, including mouse testes, are not derived from repeated elements [9], [10], [11], [12], [13]. The full extent of piRNA functions thus remains to be explored.
Mutations in the majority of Drosophila piRNA pathway genes disrupt asymmetric localization of RNAs along the axes of the oocyte, and lead to maternal effect embryonic lethality [1], [2], [3], [4]. The axis specification defects linked to several of piRNA pathway mutations are dramatically suppressed by a null mutation in mnk, which encodes a Checkpoint kinase 2 (Chk2) homolog required for DNA damage signaling, indicating that the loss of asymmetric RNA localization is downstream of DNA damage [1], [2]. Oocyte patterning defects generally lead to embryonic lethality, but the mnk allele that suppresses the axis specification defects associated with piRNA mutations does not suppress embryonic lethality [1], [2], [3]. piRNAs thus have an essential function during embryogenesis that is independent of Chk2 activation and DNA damage signaling. To gain insight into potential new functions for the piRNA pathway, we have characterized the embryonic lethality associated with four piRNA pathway mutations. These studies reveal a novel function for a subset of piRNA genes in assembly of the telomere protection complex, and suggest that this process is directed by a subpopulation of 19–22 nt piRNAs.
Results/Discussion
The armi and aub genes encode a putative RNA helicase and a piRNA binding PIWI Argonaute protein, and recent studies suggest that they have distinct functions in piRNA biogenesis [2], [8], [14], [15] Mutations in aub dramatically reduce piRNA species that overlap by 10 nt, which is characteristic of ping-pong amplification, while armi mutations reduce total piRNA production but enhance the ping-pong signature [15]. Mutations in aub and armi lead to maternal-effect embryonic lethality, however, suggesting that these genes share an essential function. To gain insight into the lethality associated with these mutations, we first analyzed DNA break accumulation during oogenesis. Germline-specific DNA breaks normally form during early oogenesis, as meiosis is initiated [16]. In several piRNA mutants, however, DNA breaks persist, which could compromise the female pronucleus and thus lead to genetic instability in the early zygote [2], [14]. DNA breaks trigger phosphorylation of histone H2Av, producing γ-H2Av foci near the break sites [17]. In wild-type ovaries, γ-H2Av foci begin to accumulate in region 2 of the germarium, as meiotic breaks are formed [16]. These foci are significantly reduced in stage 2 egg chambers, which have completed meiotic repair and budded from the germarium. Later in oogenesis, γ-H2Av foci accumulate in the nurse cell nuclei, which undergo endoreduplication. However, these foci remain undetectable in the oocyte [16]. In ovaries mutant for aub or armi, γ-H2Av foci appear in germarium region 2, but persist in nurse cells and the oocyte through stage 4. By stage 5, however, γ-H2Av foci are undetectable in 50% of armi and aub mutant oocytes, and are significantly reduced in the remaining oocytes (Figure S1 and data not shown). Both armi and aub mutations thus increase DNA damage during early oogenesis, but most of the damage in the oocyte appears to be repaired as oogenesis proceeds.
As wild type oocytes mature and initiate meiotic spindle assembly, the major chromosomes form a single mass at the spindle equator and the non-exchange 4th chromosomes move toward the poles [18], [19]. In OregonR, we observed distinct 4th chromosomes in 79% of stage 13 oocytes. In stage 13 aub and armi mutants, by contrast, distinct 4th chromosomes were observed in only 11% and 18% of stage 13 oocytes, respectively (Figure S2, Table S1). However, a single primary mass of chromatin was always observed. These observations are consistent with γ-H2Av data suggesting that DNA breaks formed during early oogenesis are often repaired as the oocyte matures. In addition, both aub and armi mutations appear to inhibit separation of the small 4th chromosomes, although it is also possible that this small chromosome is fragmented and thus difficult to detect cytologically.
Drosophila oocytes are activated as they pass through the oviduct, which triggers completion of the meiotic divisions. The first meiotic division is completed in the oviduct, but meiosis II can be observed in freshly laid eggs and is characterized by four well-separated meiotic products on tandem spindles (Figure 1A). In aub and armi mutant embryos, the meiotic chromatin was either stretched across the paired meiotic spindles, or fragmented and spread over both spindles (Figure 1A). No wild type meiotic figures were observed. Breaks thus appear to persist in some stage 14 oocytes, although this does not disrupt the karyosome organization during earlier stages. However, other oocytes appear to have intact chromosomes that fail to resolve during the meiotic divisions.
Fig. 1. Chromatin organization in piRNA mutant embryos. 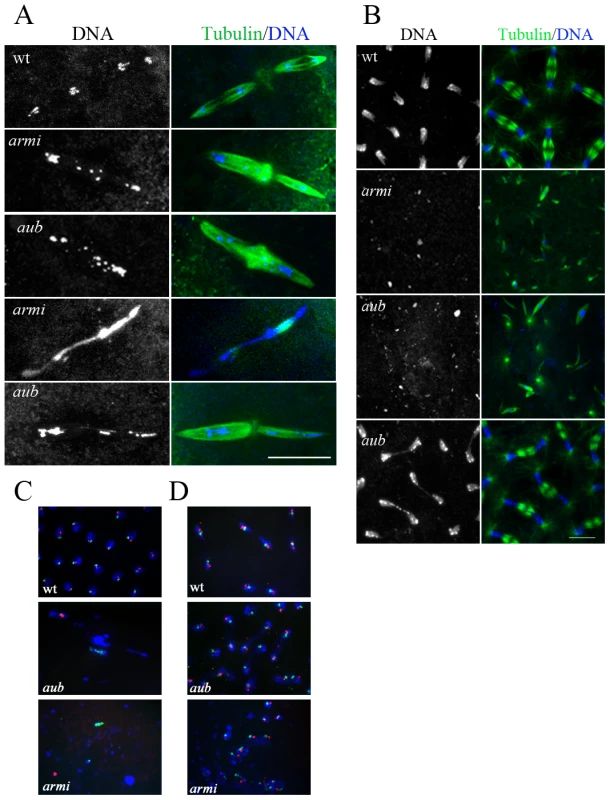
A. Immunostaining for α-tubulin (green) and DNA (blue) in 0–30-min-old embryos showing chromatin fragmentation and chromatin fusions in aub and armi mutant embryos during meiosis II. Scale bar is 15 µM. B. Cross-section of 0–3-hr-old embryos during syncytial mitotic divisions showing DNA fragmentation and chromatin bridges during segregation in aub and armi mutants. Scale bar is 10 µM. C, D. Dual-label FISH for two Y-chromosome-specific satellites, (AATAC)n in green and (AATAAAC)n in red, with DNA in blue showing mis-segregation of these repeats in aub and armi embryos (C). In contrast, embryos undergoing cleavage mitotic divisions show both the labels in most of the segregating chromatids in aub (D). Compromised zygotic genomic integrity in piRNA mutants
Fertilization and pronuclear fusion initiate 13 rapid cleavage stage mitotic divisions [16]. These divisions are syncytial, but membranes surround the cortical nuclei to form cells following mitosis 13 [20]. 0 to 3-hr old cleavage stage aub and armi mutant embryos showed two distinct phenotypes. 60% of aub mutant embryos and 90% of armi mutant embryos contained dispersed chromatin fragments that were often associated with small spindle-like microtubule bundles (Figure 1B, Table S2). The remaining embryos appeared to be progressing through cleavage divisions, and some cellularization and gastrulation stage embryos were observed. However, chromosome bridges/lagging chromosomes were present in 50% to 70% of the cleavage stage anaphase and early telophase figures (Figure 1B and Figure 2C).
Fig. 2. ligIV–dependent telomere fusions in piRNA mutants. 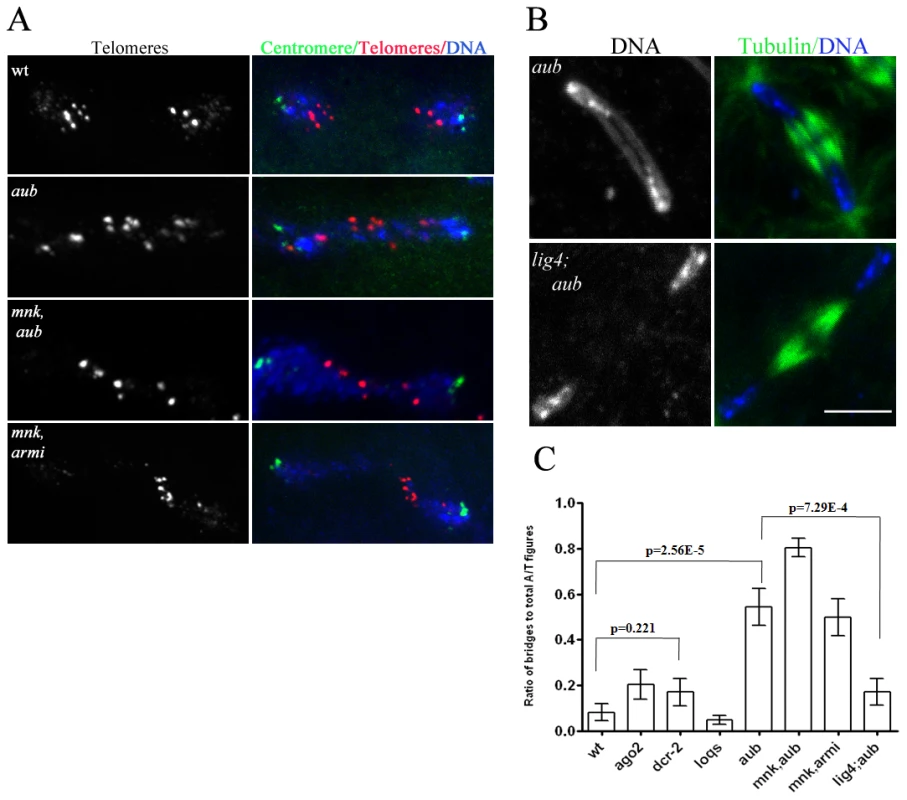
A. Two-color FISH for a pair of daughter nuclei in anaphase, labeled for centromeric dodeca satellite (green) and telomeric transposon, HeT-A (red) with DNA (blue) showing telomeres are fused in piRNA mutants. B. Immunostaining for microtubules (green) and DNA (blue) in 0–3 hr-old embryos showing suppression of chromatin bridge formation in ligIV;aub embryos. Scale bar is 10 µM. C. Ratio of anaphase/telophase bridges to total anaphase/telophase figures in different genotypes. The data for multiple samples were compared using Anova test, and sample mean was plotted with standard error of mean (SEM) as error bars. A two-tailed t-test was performed for certain pairs and p-values are noted on the graph. Chromatin fragmentation could result from replication of broken chromosomes inherited from the female, or from post-fertilization fragmentation of the zygotic genome. To directly assay zygotic genome integrity, mutant females were mated to wild type males and dual-label FISH was used to monitor physically separate regions of the Y chromosome. In male embryos derived from wild type females, the two Y chromosome probes always co-segregated through anaphase and telophase (Figure 1C, 1D). Mutant embryos showing chromatin fragmentation, by contrast, contained chromatin clusters that did not label for either Y chromosome probe, or that labeled for only one of the two probes (Figure 1C). In mutant embryos that proceeded through cleavage stage mitotic cycles, the majority of segregating chromatids retained both Y chromosome markers, indicating that chromosome continuity had been maintained. Chromatids with only one of two markers were observed, however, indicating that breaks had separated regions on a Y chromosome arm from the centromere (Figure 1D). The axial patterning defects associated with piRNA mutations are suppressed by mutations in mnk [1], [2], but mnk did not suppress either the chromatin fragmentation or segregation defects linked to aub and armi (Table S2, Figure S3). Mutations in aub and armi thus destabilize the genome of the zygote and disrupt chromosome resolution during the cleavage divisions through processes that are independent of DNA damage signaling.
Mutations in the armi and aub genes disrupt piRNA production and transposon silencing, but have also been reported to inhibit homology dependent target cleavage by siRNAs [21], [22]. In addition, null mutations in argonaute2 (ago2), which block siRNA based silencing, have been reported to disrupt mitosis during the syncytial blastoderm stage [23]. These observations raise the possibility that chromatin fragmentation and fusion in aub and armi mutants result from defects in the siRNA pathway. We therefore analyzed cleavage in embryos from females homozygous for null mutations in ago2 and dcr2, which block siRNA production and silencing [24]. Consistent with previous studies, we find that embryos from ago2 and dcr2 mutant females are viable [23], [24]. However, we did not observe chromosome fragmentation or a statistically significant increase in anaphase bridge formation relative to wild type controls (Figure S4, Figure 2C). The loquacious (loqs) gene encodes a Dicer-1 binding protein required for miRNA production [25], and we find that embryos from loqs mutant females also proceed through normal cleavage stage divisions (Figure S4, Figure 2C). Chromosome segregation and maintenance of zygotic genome integrity during early embryogenesis thus appear to be independent of the siRNA and miRNA pathways, but require at least two components of the piRNA pathway.
Telomere fusions in aub and armi embryos
In S. pombe, mutations in ago1, dcr1 and rdp1 disrupt kinetochore assembly and thus lead to lagging mitotic chromosomes due to defects in centromere movement to the spindle poles [26]. To determine if Drosophila piRNA mutations disrupt kinetochore assembly, we performed dual label FISH for centromeric dodeca-satellite sequences [27] and the telomere-specific transposon HeT-A. In aub and armi mutants, centromeric sequences segregated to the spindle poles in essentially every anaphase figure, but telomere specific sequences were consistently present at the chromatin bridges (Figure 2A). These observations indicate that armi and aub are not required for kinetochore assembly, but are needed for telomere resolution.
Telomeres are protected from recognition as DNA double strand breaks by the telomere-protection complex (TPC), and defects in telomere protection thus lead to covalent ligation of chromosome ends by the non-homologous end-joining (NHEJ) pathway [28], [29]. DNA Ligase IV is required for NHEJ, and ligase IV mutations suppress fusions that result from covalent joining of unprotected chromosome ends [28], [29]. To determine if chromosome fusions in aub and armi are due to NHEJ, we generated ligIV;aub and ligIV;armi double mutant females and analyzed chromosome segregation in the resulting embryos. In aub single mutant embryos, 50% of anaphase figures show bridges, but anaphase bridges are present in only 15% of ligIV;aub double mutants (Figure 2B, 2C). By contrast, the fraction of embryos showing chromosome fragmentation increases in ligIV;aub double mutants (Table S2). Chromosome fragmentation also increased in ligIV;armi mutant embryos, and as a result morphologically normal anaphase figures could not be observed (Table S2). These findings strongly suggest that lagging chromosomes result from covalent ligation of chromosome ends by the NHEJ pathway, while chromatin fragmentation results from DNA breaks that are repaired by NHEJ. Mutations in armi and aub lead to significant over-expression of transposable elements [8], [14], [30], including DNA elements that are mobilized by a “cut and paste” mechanism that directly produces double strand breaks [31]. In addition, NHEJ pathway has been implicated in repair of gapped retroviral integration intermediates [32]. Chromosome fragmentation may therefore result from transposon over-expression and mobilization, which induces breaks that overwhelm the NHEJ pathway. Telomere fusions, by contrast, appear to result from defects in telomere protection, which lead to chromosome end recognition by the NHEJ pathway.
Assembly of the telomere protection complex
The Drosophila TPC includes HOAP and Modigliani (Moi), which may function only at chromosome ends, and HP1a and the MRN complex, which have additional roles in heterochromatic silencing and DNA repair [33], [34], [35], [36]. To directly assay for TPC recruitment, we used chromatin immunoprecipitation (ChIP) to measure HP1a and HOAP binding to the telomere specific transposon HeT-A (Figure 3B, 3C). In wild type ovaries, HOAP and HP1a bind to multiple regions of HeT-A (Figure 3B, 3C). In armi and aub mutants, by contrast, HOAP and HP1a binding to the Het-A 5′-UTR and ORF are significantly reduced (Figure 3B, 3C). The 5′end of Het-A is oriented toward the chromosome end, and is therefore likely to lie at the telomere. Ovarian tissue consists of germ cells with a surrounding layer of somatic cells, which complicates interpretation of these biochemical studies. However, ChIP on 0–3 hour old embryos from aub and mnk,aub mutant females revealed significant reduction in HOAP binding at the HeT-A 5′-UTR (Figure S5). The aub and armi genes thus appear to be required for TPC recruitment, consistent with ligation of chromosome ends in mutant embryos.
Fig. 3. Mutations in aub and armi disrupt assembly of the telomere protection complex. 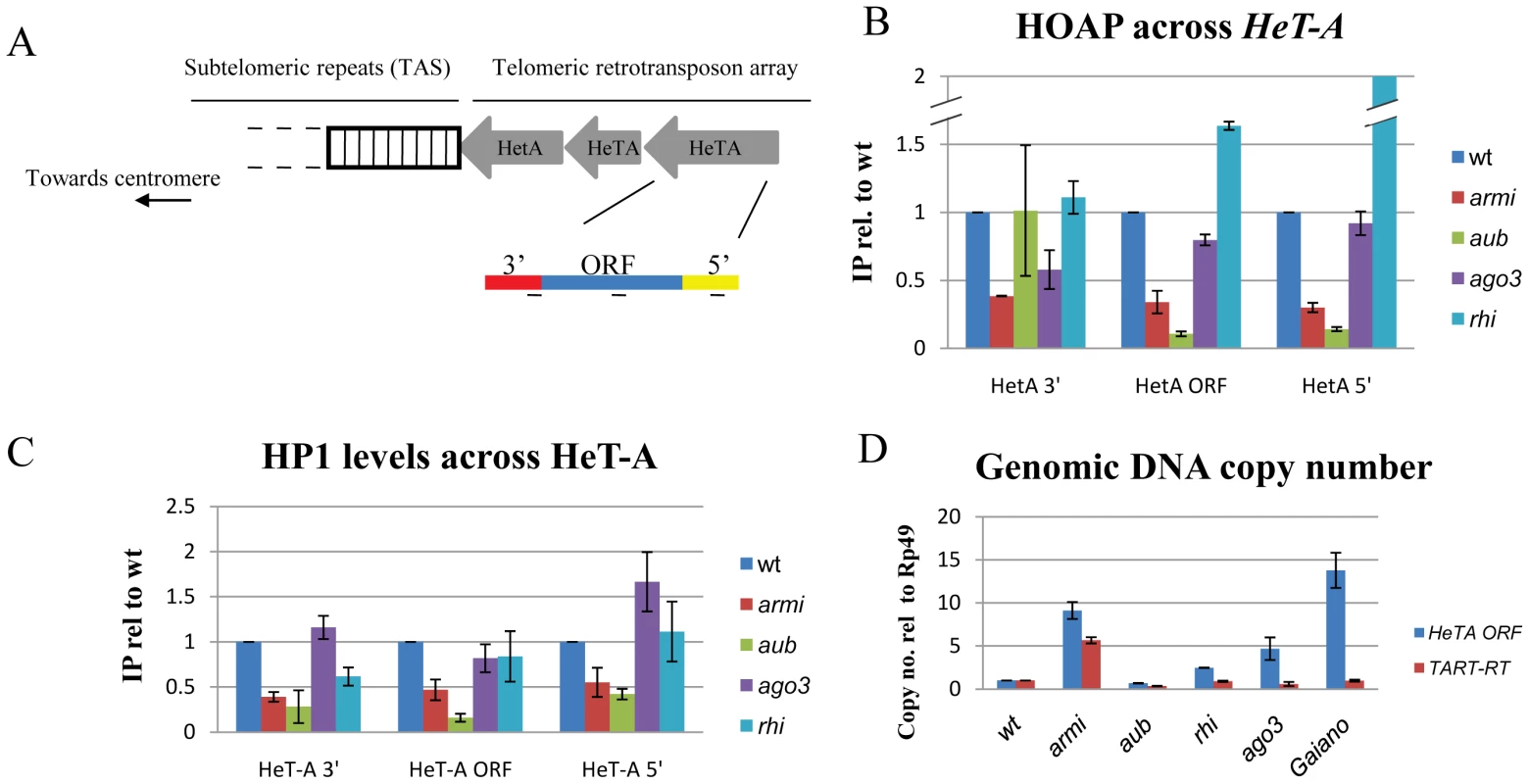
A. Schematic showing transposon arrays at Drosophila telomeres. The HeT-A transposon 3′ and 5′-UTRs are in red and yellow respectively, and the ORF is in blue. B, C. Binding of the telomere protection complex proteins HOAP and HP1 to HeT-A. Chromatin Immunoprecipitation (ChIP) was used to recover bound DNA, and the percent of input chromatin precipitated was determined by qPCR. Fold change in binding relative to wild type is shown, and was calculated by dividing mutant by wild type (wt) values. D. Genomic copy number for HeT-A and TART. Copy number was determined by qPCR, using the single copy Rp49 gene as an internal standard. Gaiano is a wild-type stock previously shown to carry additional telomeric transposon repeats. To determine if other piRNA pathway mutations disrupt telomere protection, we analyzed the cleavage stage embryonic divisions in ago3 and rhi mutants. The ago3 locus encodes a PIWI clade protein that primarily binds sense strand piRNAs, and rhi encodes a rapidly evolving HP1 homologue required for production of precursor RNAs from a subset of piRNA clusters [14], [30]. Essentially all of the rhi and ago3 mutant embryos showed chromatin fragmentation, as observed in the majority of aub and armi mutants (Figure S6). We therefore biochemically assayed for TPC assembly in ovarian chromatin using ChIP for HOAP and HP1a. Surprisingly, neither ago3 nor rhi mutations disrupt HOAP or HP1a binding to Het-A, and rhi mutants show greater than wild type levels of HOAP binding to Het-A (Figure 3B, 3C). By contrast, these rhi alleles reduce total piRNA production by 10 fold [14]. The ago3 mutations appear to be null, and the rhi mutations are strong hypomorphc alleles. Assembly of the TPC in the ago3 and rhi mutants is therefore unlikely to be mediated by residual protein. Instead, these findings strongly suggest that aub and armi have a function in telomere protection that is not shared by ago3 or rhi.
In Drosophila, chromosome breaks can be converted to stable telomeres [37], called terminal deletions, which accumulate additional copies of the telomeric elements HeT-A and TART. When terminal deletions are passaged in animals heterozygous for aub or the piRNA pathway gene spnE, the number of terminal TART repeats increase[38]. The defects in TPC assembly in aub and armi could therefore be triggered by increased HeT-A and TART copy number, which could titrate TPC components. We therefore assayed telomeric transposon copy number in aub and armi mutants, which show defects in TPC assembly, and in rhi and ago3 mutants, which do not. We also assayed telomeric transposon copy number and mitotic chromosome segregation in a wild-type variant, Gaiano, that has been reported to carry additional HeT-A repeats [39]. Consistent with previous reports, we find that Gaiano has 10 to 15 fold more HeT-A copies than OregonR controls (Figure 3D). Despite the increase in telomere length, this stock is viable and fertile, and we did not observe telomere fusions or lagging chromosomes during the cleavage stage embryonic divisions (Figure S6). In addition, we found that aub mutants that show defects in TPC assembly do not accumulate additional copies of HeT-A or TART, while rhi and ago3 mutants that are wild type for TPC binding show an increase in telomere-specific transposon copy number (Figure 3D). Assembly of the TPC is therefore independent of telomere specific transposon copy number (Figure S6).
Aub and Armi are required for production of a subpopulation of 19–22 nt piRNAs
piRNAs are proposed to direct PIWI clade proteins to targets through sequence specific interactions. Our observations raised the possibility that armi and aub promote production of piRNAs that direct the telomere protection complex to transposons that make up chromsome ends. We therefore analyzed published small RNA deep sequencing data[14], [15], [30] for species derived from a fourth chromosome cluster, defined by a high density of uniquely mapping piRNAs, containing multiple repeats of the telomeric transposons [40]. Our bioinformatic analysis showed that 70–80% of telomere specific piRNAs match this cluster (Figure 4, Table S3). Figure 4 shows length histograms for small RNAs from wt, rhi, ago3, aub and armi mutant ovaries that map to this cluster. Data are normalized to sequencing depth, and small RNAs mapping to the plus genomic strand are represented in blue and RNAs mapping to the minus strand are in red. Significantly, aub and armi mutations lead to a preferential loss of shorter piRNAs mapping to the minus genomic strand (Figure 4B, 4C). Loss of these shorter RNAs highlights the peak at 21 nt, which is retained in all of the mutants and likely represent endogenous siRNAs (Figure 4A, black arrow). The telomeric elements (HeT-A and TART) are almost exclusively on the minus genomic strand in this cluster, and the RNAs that are lost in aub and armi thus correspond to the sense strand of the target elements. Ovaries mutant for ago3 and rhi, by contrast, retain these shorter sense strand RNAs.
Fig. 4. piRNAs linked to a 4th chromosome cluster containing telomeric transposon fragments. 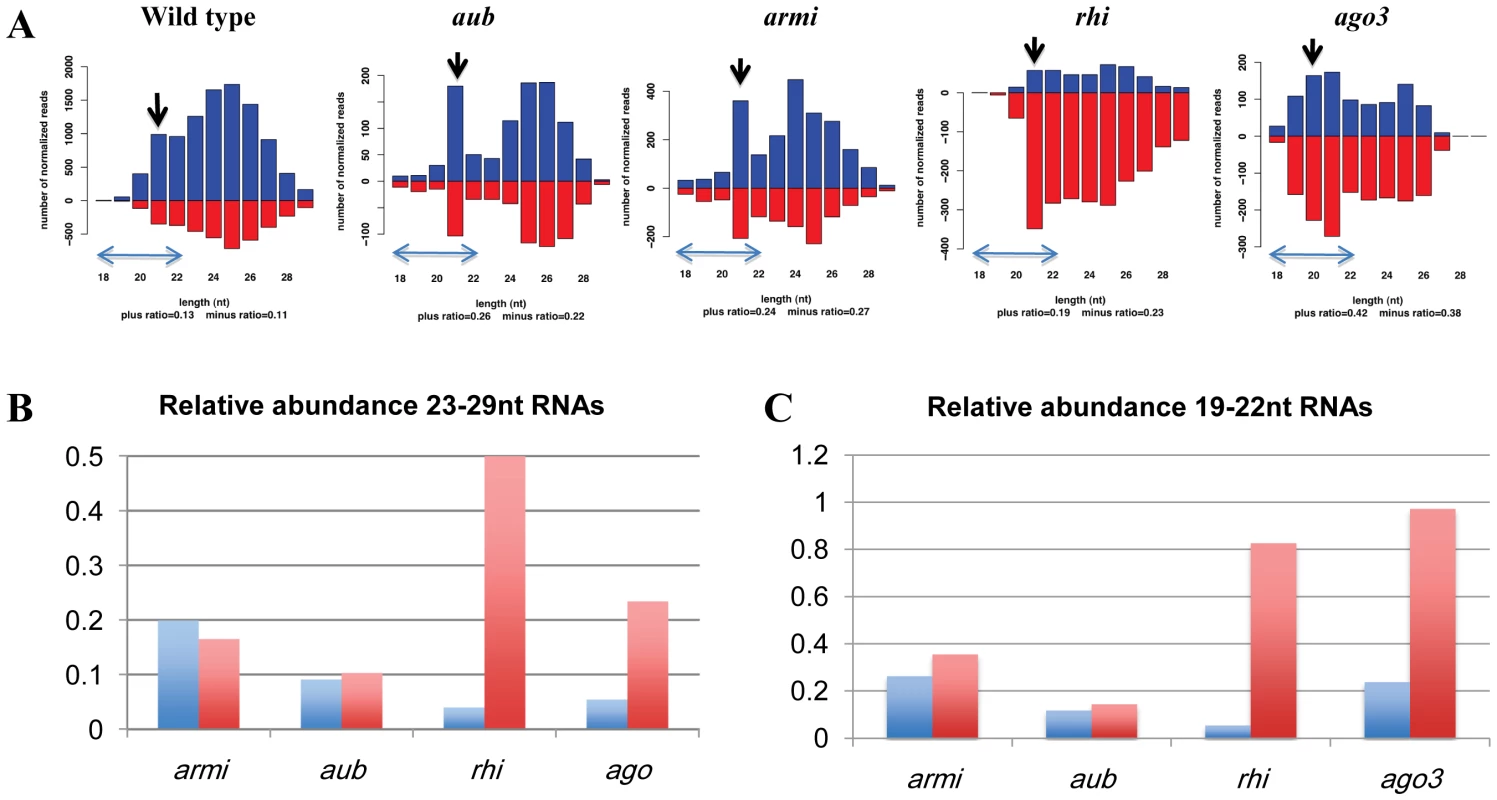
A. Length histograms showing plus genomic strand (blue) and minus genomic strand (red) mapping piRNAs in wt, armi, aub, rhi and ago3 mutants. The relative abundance is normalized to sequencing depth and is plotted on the y-axis. Note that sense strand of the transposon fragments in this cluster are on the minus genomic strand, and that the scales differ. Preferential loss of shorter piRNAs from aub and armi leads to a prominent endo-siRNA peak at 21 nt (marked by a black arrow). B. Abundance of longer (23–29 nt) plus strand (blue) and minus strand (red) piRNAs in the indicated mutants relative to their respective wild-type controls. All four mutations reduce plus strand piRNAs, which are anti-sense to the telomeric transposons. C. 19–22 nt genomic plus and minus strand piRNAs in the indicated mutants. All four mutations reduce plus strand RNAs. However, minus strand species are retained at near wild type levels in both rhi and ago3 mutants. For panels B and C, bars show normalized reads in mutants divided by normalized reads in wild-type controls. We quantified the relative abundance of typical 23–29nt long piRNAs and the shorter 19–22nt species, excluding the 21nt endo-siRNA peak. All four mutations significantly reduce 23 to 29 nt piRNAs, although rhi mutants retain approximately 50% of wild type minus strand species. Loss of these piRNAs is consistent with over-expression of transposons matching this cluster in all four mutants (Figure S8). By contrast, the shorter minus strand RNAs are reduced by 3 to 10 fold in armi and aub, but are expressed at 80% to 95% of wild type levels in ago3 and rhi (Figure 4B, 4C). In addition, short piRNA species from the telomeric cluster co-immunoprecipitate with Piwi protein [15], [30], which localizes to the nucleus and is a likely effector of chromatin functions for the piRNA pathway (Figure S7). Binding of this subpopulation of piRNAs by Piwi is retained in ago3 mutants, which assemble the TPC, but significantly reduced in armi mutants, which block assembly of the TPC (Figure S7).
Taken together, these observations suggest that the piRNA pathway has two genetically distinct functions during oogenesis and early embryogenesis. The pathway prevents DNA damage during oogenesis and maintains the integrity of the zygotic genome during the embryonic cleavage divisions, which likely reflects the established role for piRNAs in transposon silencing [2], [8], [14], [30]. This function requires aub, armi, rhi and ago3, which are also required for wild type piRNA production. In addition, our studies reveal a novel function for the piRNA genes aub and armi in telomere protection, whch may be mediated by a novel class of short RNAs that bind to Piwi. Consistent with this hypothesis, it has been reported that germline clones of piwi null alleles do not significantly disrupt oogenesis, but lead to maternal effect embryonic lethality and severe chromosome segregation defects during the cleavage divisions [41]. A subpopulation of Piwi-bound piRNAs may therefore direct assembly of the TPC.
Materials and Methods
Fly stocks
Flies were reared at 25°C on standard corn meal medium. OregonR and w1118 were used as controls. Stocks carrying the following alleles were obtained from the Bloomington Stock Center: ago251B, ago2Df, aubHN2, aubQC42, dcr2L811fsX, mnkP6, ligIV5, rhi02086 and rhiKG00910 . ago251B is an imprecise P-element induced deletion of the first two exons of ago2 locus. aubHN2 and aubQC42are both EMS-induced point mutations [42], [43]. dcr2L811fsX is an EMS-induced loss-of-function allele described in [24]. rhi02086 and rhiKG00910 are both P-element insertion alleles, which act as strong hypomorphs [44]. Both armi1and armi72.1alleles are strong hypomorphic alleles which produce armi transcript at low levels [4]. mnkP6,aubHN2 and mnkP6,aubQC42 [2] recombinants were generated using standard genetic procedures. The loqsf00791 and loqsKO alleles were from Bloomington and Dennis McKearin [25], respectively. Stocks carrying ago34931and ago33658, which are loss-of-function alleles with premature stop codons [30], were obtained from the Zamore lab (University of Massachusetts Medical School).
Immunostaining and fluorescence in situ hybridization
0–30-min-old or 0–3-hr-old embryos were fixed in methanol and immunostained for α-tubulin (Dm1α, Sigma Chemical Co., 1∶300) and 0.2 µM TOTO-3 (Molecular Probes) using standard procedures [45]. For staining of egg chambers, the ovaries were dissected in Robb's medium and fixed in 4% formaldehyde as described [2]. γ-H2Av antibody was generously provided by Kim McKim (Rutgers) and was used at 1∶500 dilution. The dodeca-satellite probe for the fluorescent in situ hybridization was made by 3′ end labeling using terminal deoxynucleotidyl transferase (Roche), followed by direct fluorophore conjugation using ARES DNA labeling kit as described by the manufacturer (Molecular Probes). The dodeca satellite sequence from the pBK6E218 plasmid was amplified using T3 and T7 primers [27]. The telomeric probe was made by indirect substitution of DIG-dUTP using the PCR DIG probe synthesis kit (Roche). The sequence was amplified from genomic DNA using the following primers - telF - 5′-GACAATGCACGACAGAGGAA-3′ and telR - 5′-GTCTTTTTGGGTTTGCGGTA-3′. The Y-chromosome satellites (AATAC)n and (AATAAAC)n were purchased as oligos with direct conjugation of FAM and Cy-3 fluorophores at the 3′end (IDT). Hybridization was performed as described previously [46]. Fluorescently labeled samples were imaged using a Leica TCS-SP inverted scanning confocal microscope or a Nikon TE-2000E2 inverted microscope and captured using Metamorph software (Universal Imaging). All images were processed using Image J (Rasband, W.S., ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/, 1997–2006) and Adobe Photoshop.
Chromatin bridges quantification
To quantify chromatin bridges, the ratio of anaphase/telophase (A/T) bridges to total A/T figures was calculated for 10 to 30 embryos. The mean bridge frequency was determined by designating each embryo as an independent experiment, and the standard error was determined using an Anova test. Two-tailed t-tests were also used to compare specific data sets, using α = 0.05. P-values are noted on the graphs.
Chromatin Immunoprecipitation and quantitative PCR (qPCR)
Whole ovaries were dissected from 2–5-day old flies and fixed using 1.8% formaldehyde for 10 minutes at room temperature. For ChIP using embryos, 0–3 hr old embryos were collected and fixed using 1.8% formaldehyde for 20 minutes at room temperature. The ChIP assay was performed as per manufacturer's instructions (Invitrogen) and as previously described with some modifications [14]. Immunoprecipitation was done using HOAP polyclonal serum previously described [14] or the monoclonal HP1 antibody (Developmental Studies Hybridoma Bank, IA). The purified DNA was subjected to qPCR using Applied Biosystems 7500 system, and data was analyzed by calculating the % of immunoprecipitated DNA compared to the input DNA sample. All ChIPs were performed at least twice and the data presented is an average of two different biological replicates with technical triplicates for each of them. The data was plotted with error bars representing standard deviations for individual samples. The difference between primer efficiencies was calculated by preparing standard curves and was taken into consideration while calculating % IP values. The primer sequences are available upon request.
Sequence extraction and annotation
For each sequence read, the first occurrence of the 6-mer perfectly matching the 5′-end of the 3′-linker was identified. Sequences without a match were discarded. The extracted inserts for sequences that contained the 3′-linker were then mapped to the female Drosophila melanogaster genome (Release R5.5, excluding chromosome YHet). Inserts that matched fully to a genomic sequence were collected using Bowtie (Langmead et al., 2009) and the corresponding genomic coordinates were determined for downstream functional analysis. Sequences corresponding to pre-miRNAs or non-coding RNAs (ncRNAs) were identified and removed. For analyis of the telomeric cluster, small RNA length distributions were determined for reads that mapping to chr4 : 1280000–1350999, normalizing for sequencing depth (genome mapping reads excluding ncRNAs).
Supporting Information
Zdroje
1. ChenY
PaneA
SchüpbachT
2007
Cutoff and aubergine mutations result in retrotransposon upregulation and checkpoint activation in Drosophila.
Current biology: CB
17
637
642
2. KlattenhoffC
BratuDP
McGinnis-SchultzN
KoppetschBS
CookHA
2007
Drosophila rasiRNA pathway mutations disrupt embryonic axis specification through activation of an ATR/Chk2 DNA damage response.
Developmental cell
12
45
55
3. PaneA
WehrK
SchüpbachT
2007
zucchini and squash encode two putative nucleases required for rasiRNA production in the Drosophila germline.
Developmental cell
12
851
862
4. CookH
KoppetschB
WuJ
TheurkaufW
2004
The Drosophila SDE3 Homolog armitage Is Required for oskar mRNA Silencing and Embryonic Axis Specification.
Cell
116
817
829
5. AravinAA
NaumovaNM
TulinAV
VaginVV
RozovskyYM
2001
Double-stranded RNA-mediated silencing of genomic tandem repeats and transposable elements in the D. melanogaster germline.
Curr Biol
11
1017
1027
6. NishidaKM
SaitoK
MoriT
KawamuraY
Nagami-OkadaT
2007
Gene silencing mechanisms mediated by Aubergine piRNA complexes in Drosophila male gonad.
RNA (New York, NY)
13
1911
1922
7. SaitoK
InagakiS
MituyamaT
KawamuraY
OnoY
2009
A regulatory circuit for piwi by the large Maf gene traffic jam in Drosophila.
Nature
461
1296
1299
8. VaginVV
SigovaA
LiC
SeitzH
GvozdevV
2006
A distinct small RNA pathway silences selfish genetic elements in the germline.
Science
313
320
324
9. GrimsonA
SrivastavaM
FaheyB
WoodcroftB
ChiangHR
2008
Early origins and evolution of microRNAs and Piwi-interacting RNAs in animals.
Nature
455
1193
1197
10. GrivnaST
BeyretE
WangZ
LinH
2006
A novel class of small RNAs in mouse spermatogenic cells.
Genes and Development
20
1709
1714
11. BatistaPJ
RubyJG
ClaycombJM
ChiangR
FahlgrenN
2008
PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans.
Molecular cell
31
67
78
12. Aravin
GaidatzisD
PfefferS
Lagos-QuintanaM
LandgrafP
2006
A novel class of small RNAs bind to MILI protein in mouse testes.
Nature
442
203
207
13. GirardAl
SachidanandamR
HannonG
CarmellM
2006
A germline-specific class of small RNAs binds mammalian Piwi proteins.
Nature
442
199
202
14. KlattenhoffC
XiH
LiC
LeeS
XuJ
2009
The Drosophila HP1 Homolog Rhino Is Required for Transposon Silencing and piRNA Production by Dual-Strand Clusters.
Cell
138
1137
1149
15. MaloneCD
BrenneckeJ
DusM
StarkA
McCombieWR
2009
Specialized piRNA pathways act in germline and somatic tissues of the Drosophila ovary.
Cell
137
522
535
16. McKimKS
JangJK
ManheimEA
2002
Meiotic recombination and chromosome segregation in Drosophila females.
Annu Rev Genet
36
205
232
17. MadiganJP
ChotkowskiHL
GlaserRL
2002
DNA double-strand break-induced phosphorylation of Drosophila histone variant H2Av helps prevent radiation-induced apoptosis.
Nucleic acids research
30
3698
3705
18. TheurkaufWE
HawleyRS
1992
Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein.
J Cell Biol
116
1167
1180
19. GillilandWD
HughesSF
ViettiDR
HawleyRS
2009
Congression of achiasmate chromosomes to the metaphase plate in Drosophila melanogaster oocytes.
Developmental biology
325
122
128
20. FoeVE
OdellGM
EdgarBA
1993
Mitosis and morphogenesis in the Drosophila embryo: point and counterpoint.
Michael BateAMA
: CSHL Press
The Development of Drosophila melanogaster
149
300
21. TomariY
DuT
HaleyB
SchwarzD
BennettR
2004
RISC Assembly Defects in the Drosophila RNAi Mutant armitage.
Cell
116
831
841
22. KennerdellJR
YamaguchiS
CarthewRW
2002
RNAi is activated during Drosophila oocyte maturation in a manner dependent on aubergine and spindle-E.
Genes Dev
16
1884
1889
23. DeshpandeG
CalhounG
SchedlP
2005
Drosophila argonaute-2 is required early in embryogenesis for the assembly of centric/centromeric heterochromatin, nuclear division, nuclear migration, and germ-cell formation.
Genes & development
19
1680
1685
24. LeeYS
NakaharaK
PhamJW
KimK
HeZ
2004
Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways.
Cell
117
69
81
25. ParkJK
LiuX
StraussTJ
McKearinDM
LiuQ
2007
The miRNA pathway intrinsically controls self-renewal of Drosophila germline stem cells.
Current biology: CB
17
533
538
26. HallIM
NomaK
GrewalSI
2003
RNA interference machinery regulates chromosome dynamics during mitosis and meiosis in fission yeast.
Proc Natl Acad Sci U S A
100
193
198
27. AbadJP
CarmenaM
BaarsS
SaundersRD
GloverDM
1992
Dodeca satellite: a conserved G+C-rich satellite from the centromeric heterochromatin of Drosophila melanogaster.
Proceedings of the National Academy of Sciences of the United States of America
89
4663
4667
28. BiX
SrikantaD
FantiL
PimpinelliS
BaduguR
2005
Drosophila ATM and ATR checkpoint kinases control partially redundant pathways for telomere maintenance.
Proceedings of the National Academy of Sciences of the United States of America
102
15167
15172
29. SmogorzewskaA
KarlsederJ
Holtgreve-GrezH
JauchA
de LangeT
2002
DNA ligase IV-dependent NHEJ of deprotected mammalian telomeres in G1 and G2.
Current biology: CB
12
1635
1644
30. LiC
VaginVV
LeeS
XuJ
MaS
2009
Collapse of germline piRNAs in the absence of Argonaute3 reveals somatic piRNAs in flies.
Cell
137
509
521
31. WickerT
SabotF
Hua-VanA
BennetzenJ
CapyP
2007
A unified classification system for eukaryotic transposable elements.
Nature reviews Genetics
8
973
982
32. LiL
OlveraJM
YoderKE
MitchellRS
ButlerSL
2001
Role of the non-homologous DNA end joining pathway in the early steps of retroviral infection.
The EMBO journal
20
3272
3281
33. CenciG
SiriacoG
RaffaG
KellumR
GattiM
2003
The Drosophila HOAP protein is required for telomere capping.
Nature cell biology
5
82
84
34. BiX
WeiS-CD
RongYS
2004
Telomere protection without a telomerase; the role of ATM and Mre11 in Drosophila telomere maintenance.
Current biology: CB
14
1348
1353
35. PerriniB
PiacentiniL
FantiL
AltieriF
ChichiarelliS
2004
HP1 controls telomere capping, telomere elongation, and telomere silencing by two different mechanisms in Drosophila.
Molecular cell
15
467
476
36. RaffaGD
SiriacoG
CugusiS
CiapponiL
CenciG
2009
The Drosophila modigliani (moi) gene encodes a HOAP-interacting protein required for telomere protection.
Proceedings of the National Academy of Sciences of the United States of America
106
2271
2276
37. BiessmannH
MasonJM
FerryK
d'HulstM
ValgeirsdottirK
1990
Addition of telomere-associated HeT DNA sequences “heals” broken chromosome ends in Drosophila.
Cell
61
663
673
38. SavitskyM
KwonD
GeorgievP
KalmykovaA
GvozdevV
2006
Telomere elongation is under the control of the RNAi-based mechanism in the Drosophila germline.
Genes & development
20
345
354
39. SiriacoGM
CenciG
HaoudiA
ChampionLE
ZhouC
2002
Telomere elongation (Tel), a new mutation in Drosophila melanogaster that produces long telomeres.
Genetics
160
235
245
40. BrenneckeJ
AravinAA
StarkA
DusM
KellisM
2007
Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila.
Cell
128
1089
1103
41. CoxDN
ChaoA
LinH
2000
piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells.
Development
127
503
514
42. SchupbachT
WieschausE
1991
Female sterile mutations on the second chromosome of Drosophila melanogaster. II. Mutations blocking oogenesis or altering egg morphology.
Genetics
129
1119
1136
43. HarrisAN
MacdonaldPM
2001
Aubergine encodes a Drosophila polar granule component required for pole cell formation and related to eIF2C.
Development
128
2823
2832
44. VolpeAM
HorowitzH
GraferCM
JacksonSM
BergCA
2001
Drosophila rhino encodes a female-specific chromo-domain protein that affects chromosome structure and egg polarity.
Genetics
159
1117
1134
45. TheurkaufWE
1994
Immunofluorescence analysis of the cytoskeleton during oogenesis and early embryogenesis.
Methods Cell Biol
44
489
505
46. BlumenstielJP
FuR
TheurkaufWE
HawleyRS
2008
Components of the RNAi machinery that mediate long-distance chromosomal associations are dispensable for meiotic and early somatic homolog pairing in Drosophila melanogaster.
Genetics
180
1355
1365
Štítky
Genetika Reprodukční medicína
Článek Genome-Wide Interrogation of Mammalian Stem Cell Fate Determinants by Nested Chromosome DeletionsČlánek Season of Conception in Rural Gambia Affects DNA Methylation at Putative Human Metastable EpiallelesČlánek A Quantitative Systems Approach Reveals Dynamic Control of tRNA Modifications during Cellular StressČlánek Reduction of Protein Translation and Activation of Autophagy Protect against PINK1 Pathogenesis inČlánek The Loss of PGAM5 Suppresses the Mitochondrial Degeneration Caused by Inactivation of PINK1 inČlánek Cleavage of Phosphorothioated DNA and Methylated DNA by the Type IV Restriction Endonuclease ScoMcrAČlánek Competitive Repair by Naturally Dispersed Repetitive DNA during Non-Allelic Homologous Recombination
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 12- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Genome-Wide Interrogation of Mammalian Stem Cell Fate Determinants by Nested Chromosome Deletions
- Whole-Genome and Chromosome Evolution Associated with Host Adaptation and Speciation of the Wheat Pathogen
- Association of Variants at 1q32 and with Ankylosing Spondylitis Suggests Genetic Overlap with Crohn's Disease
- Initiator Elements Function to Determine the Activity State of BX-C Enhancers
- Identification of Genes Required for Neural-Specific Glycosylation Using Functional Genomics
- A Young Duplicate Gene Plays Essential Roles in Spermatogenesis by Regulating Several Y-Linked Male Fertility Genes
- The EpsE Flagellar Clutch Is Bifunctional and Synergizes with EPS Biosynthesis to Promote Biofilm Formation
- Histone H2A C-Terminus Regulates Chromatin Dynamics, Remodeling, and Histone H1 Binding
- Season of Conception in Rural Gambia Affects DNA Methylation at Putative Human Metastable Epialleles
- A Quantitative Systems Approach Reveals Dynamic Control of tRNA Modifications during Cellular Stress
- GC-Rich Sequence Elements Recruit PRC2 in Mammalian ES Cells
- A Single Enhancer Regulating the Differential Expression of Duplicated Red-Sensitive Opsin Genes in Zebrafish
- Investigation and Functional Characterization of Rare Genetic Variants in the Adipose Triglyceride Lipase in a Large Healthy Working Population
- Reduction of Protein Translation and Activation of Autophagy Protect against PINK1 Pathogenesis in
- Noisy Splicing Drives mRNA Isoform Diversity in Human Cells
- The Loss of PGAM5 Suppresses the Mitochondrial Degeneration Caused by Inactivation of PINK1 in
- Thymus-Associated Parathyroid Hormone Has Two Cellular Origins with Distinct Endocrine and Immunological Functions
- An ABC Transporter Mutation Is Correlated with Insect Resistance to Cry1Ac Toxin
- Role of Individual Subunits of the CSN Complex in Regulation of Deneddylation and Stability of Cullin Proteins
- The C-Terminal Domain of the Bacterial SSB Protein Acts as a DNA Maintenance Hub at Active Chromosome Replication Forks
- The DNA Damage Response Pathway Contributes to the Stability of Chromosome III Derivatives Lacking Efficient Replicators
- Cleavage of Phosphorothioated DNA and Methylated DNA by the Type IV Restriction Endonuclease ScoMcrA
- LaeA Control of Velvet Family Regulatory Proteins for Light-Dependent Development and Fungal Cell-Type Specificity
- Competitive Repair by Naturally Dispersed Repetitive DNA during Non-Allelic Homologous Recombination
- Distinct Functions for the piRNA Pathway in Genome Maintenance and Telomere Protection
- MOS11: A New Component in the mRNA Export Pathway
- Self-Mating in the Definitive Host Potentiates Clonal Outbreaks of the Apicomplexan Parasites and
- A Role for ATF2 in Regulating MITF and Melanoma Development
- Ancestral Regulatory Circuits Governing Ectoderm Patterning Downstream of Nodal and BMP2/4 Revealed by Gene Regulatory Network Analysis in an Echinoderm
- Cancer and Neurodegeneration: Between the Devil and the Deep Blue Sea
- Functional Comparison of Innate Immune Signaling Pathways in Primates
- Linking Crohn's Disease and Ankylosing Spondylitis: It's All about Genes!
- Genomics Meets Glycomics—The First GWAS Study of Human N-Glycome Identifies HNF1α as a Master Regulator of Plasma Protein Fucosylation
- Continuous and Periodic Expansion of CAG Repeats in Huntington's Disease R6/1 Mice
- Expression of Linear and Novel Circular Forms of an -Associated Non-Coding RNA Correlates with Atherosclerosis Risk
- Endocytic Sorting and Recycling Require Membrane Phosphatidylserine Asymmetry Maintained by TAT-1/CHAT-1
- Histone Deacetylases Suppress CGG Repeat–Induced Neurodegeneration Via Transcriptional Silencing in Models of Fragile X Tremor Ataxia Syndrome
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Functional Comparison of Innate Immune Signaling Pathways in Primates
- Expression of Linear and Novel Circular Forms of an -Associated Non-Coding RNA Correlates with Atherosclerosis Risk
- Genome-Wide Interrogation of Mammalian Stem Cell Fate Determinants by Nested Chromosome Deletions
- Histone H2A C-Terminus Regulates Chromatin Dynamics, Remodeling, and Histone H1 Binding
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



