-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Association of Variants at 1q32 and with Ankylosing Spondylitis Suggests Genetic Overlap with Crohn's Disease
Ankylosing spondylitis (AS) is a common inflammatory arthritic condition. Overt inflammatory bowel disease (IBD) occurs in about 10% of AS patients, and in addition 70% of AS cases may have subclinical terminal ileitis. Spondyloarthritis is also common in IBD patients. We therefore tested Crohn's disease susceptibility genes for association with AS, aiming to identify pleiotropic genetic associations with both diseases. Genotyping was carried out using Sequenom and Applied Biosystems TaqMan and OpenArray technologies on 53 markers selected from 30 Crohn's disease associated genomic regions. We tested genotypes in a population of unrelated individual cases (n = 2,773) and controls (n = 2,215) of white European ancestry for association with AS. Statistical analysis was carried out using a Cochran-Armitage test for trend in PLINK. Strong association was detected at chr1q32 near KIF21B (rs11584383, P = 1.6×10−10, odds ratio (OR) = 0.74, 95% CI:0.68–0.82). Association with disease was also detected for 2 variants within STAT3 (rs6503695, P = 4.6×10−4. OR = 0.86 (95% CI:0.79–0.93); rs744166, P = 2.6×10−5, OR = 0.84 (95% CI:0.77–0.91)). Association was confirmed for IL23R (rs11465804, P = 1.2×10−5, OR = 0.65 (95% CI:0.54–0.79)), and further associations were detected for IL12B (rs10045431, P = 5.2×10−5, OR = 0.83 (95% CI:0.76–0.91)), CDKAL1 (rs6908425, P = 1.1×10−4, OR = 0.82 (95% CI:0.74–0.91)), LRRK2/MUC19 (rs11175593, P = 9.9×10−5, OR = 1.92 (95% CI: 1.38–2.67)), and chr13q14 (rs3764147, P = 5.9×10−4, OR = 1.19 (95% CI: 1.08–1.31)). Excluding cases with clinical IBD did not significantly affect these findings. This study identifies chr1q32 and STAT3 as ankylosing spondylitis susceptibility loci. It also further confirms association for IL23R and detects suggestive association with another 4 loci. STAT3 is a key signaling molecule within the Th17 lymphocyte differentiation pathway and further enhances the case for a major role of this T-lymphocyte subset in ankylosing spondylitis. Finally these findings suggest common aetiopathogenic pathways for AS and Crohn's disease and further highlight the involvement of common risk variants across multiple diseases.
Published in the journal: . PLoS Genet 6(12): e32767. doi:10.1371/journal.pgen.1001195
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001195Summary
Ankylosing spondylitis (AS) is a common inflammatory arthritic condition. Overt inflammatory bowel disease (IBD) occurs in about 10% of AS patients, and in addition 70% of AS cases may have subclinical terminal ileitis. Spondyloarthritis is also common in IBD patients. We therefore tested Crohn's disease susceptibility genes for association with AS, aiming to identify pleiotropic genetic associations with both diseases. Genotyping was carried out using Sequenom and Applied Biosystems TaqMan and OpenArray technologies on 53 markers selected from 30 Crohn's disease associated genomic regions. We tested genotypes in a population of unrelated individual cases (n = 2,773) and controls (n = 2,215) of white European ancestry for association with AS. Statistical analysis was carried out using a Cochran-Armitage test for trend in PLINK. Strong association was detected at chr1q32 near KIF21B (rs11584383, P = 1.6×10−10, odds ratio (OR) = 0.74, 95% CI:0.68–0.82). Association with disease was also detected for 2 variants within STAT3 (rs6503695, P = 4.6×10−4. OR = 0.86 (95% CI:0.79–0.93); rs744166, P = 2.6×10−5, OR = 0.84 (95% CI:0.77–0.91)). Association was confirmed for IL23R (rs11465804, P = 1.2×10−5, OR = 0.65 (95% CI:0.54–0.79)), and further associations were detected for IL12B (rs10045431, P = 5.2×10−5, OR = 0.83 (95% CI:0.76–0.91)), CDKAL1 (rs6908425, P = 1.1×10−4, OR = 0.82 (95% CI:0.74–0.91)), LRRK2/MUC19 (rs11175593, P = 9.9×10−5, OR = 1.92 (95% CI: 1.38–2.67)), and chr13q14 (rs3764147, P = 5.9×10−4, OR = 1.19 (95% CI: 1.08–1.31)). Excluding cases with clinical IBD did not significantly affect these findings. This study identifies chr1q32 and STAT3 as ankylosing spondylitis susceptibility loci. It also further confirms association for IL23R and detects suggestive association with another 4 loci. STAT3 is a key signaling molecule within the Th17 lymphocyte differentiation pathway and further enhances the case for a major role of this T-lymphocyte subset in ankylosing spondylitis. Finally these findings suggest common aetiopathogenic pathways for AS and Crohn's disease and further highlight the involvement of common risk variants across multiple diseases.
Introduction
Ankylosing spondylitis (AS) is one of a group of common inflammatory rheumatic diseases known as spondyloarthritidies in which involvement of the spine and sacroiliac joints is prominent [1]. Heritability of the disease assessed by twin studies has been determined to be >90% [2]. Apart from the well known HLA-B27 association, recent genetic studies have identified ERAP1, IL23R and 2 intergenic regions at chr2p15 and chr21q22 as genes/loci associated with AS [3], [4]. However, these alleles only explain a fraction of the overall genetic risk for AS, and other loci are also expected to contribute to susceptibility.
There is increasing interest in the genetics community in the study of genetic findings from related diseases to identify pleiotropic genes, as an efficient method to identify further disease-associated variants. Findings from genome-wide association (GWA) studies have identified susceptibility genes common to different diseases, particularly in autoimmune conditions [5]. For example, variants in PTPN22 are associated with rheumatoid arthritis (RA), type-1-diabetes (T1D) and Crohn's disease (CD). Thus far, only the gene IL23R (associated with AS) has also been found to be associated with inflammatory bowel disease (IBD) and psoriasis, although the three conditions commonly occur in the same patients, and are co-familial. About 10% of AS patients have overt IBD, and in addition about 70% of AS cases have subclinical terminal ileitis [6]. Gut inflammation is frequent in patients with spondylarthritis, and one-quarter of patients who have chronic spondyloarthritis have some features of CD [7]. Spondyloarthritis is also common in IBD patients. Axial and peripheral arthritis can occur in up to 30% of patients with IBD [8]. The prevalence of axial involvement in IBD is 10–20% for sacroiliitis and 3–12% for spondylitis [9], while radiographic evidence of sacroiliitis is reported in 10–51% of patients with IBD [10]. A study of families of AS probands (n = 205) and of healthy controls (n = 1,352) in the Icelandic population demonstrated evidence to support a common genetic component for AS and IBD [11]. In addition to confirming the known familiality of both conditions, the study demonstrated a risk ratio of 3.0 and 2.1 in 1st and 2nd-degree relatives respectively, for the occurrence of AS in families of probands with IBD, and with the occurrence of IBD in families of patients with AS. It therefore seems likely that common pathogenic pathways may act in the development of both diseases and may be major players in chronic inflammatory disorders. We therefore sought to investigate CD risk variants for association with AS in order to explain the co-occurrence of both conditions.
Results
This study has 80% power to detect an additive association (P = 0.05) with odds ratios of 1.15–1.23 for markers with minor allele frequencies of 0.5–0.1 respectively. These values are based on a disease prevalence of 0.4% in the general population and linkage disequilibrium between markers of r2 = 0.8.
In phase 1 of the genotyping experiment MAFs for 53 markers genotyped on cases were compared to MAF calculated from historical controls from the 1958 BBC (genotyped and imputed SNPs). Eight SNPs were excluded from further analysis; two variants failed to meet imputation QC thresholds, four were excluded based on their MAF and two failed the call rate criterion. Of the remaining markers, 23 SNPs achieved a nominal P value of 0.1 (Table S1). This is significantly more markers than would be expected by chance (P = 4.8×10−10). Of these, one failed assay design and 22 were taken forward for further genotyping in phase 2 of the experiment. A further marker failed QC thresholds in phase 2 and was excluded from further analysis.
Considering the combined phase 1 and 2 data, experiment-wise association (P<9.4×10−4) was observed with seven genomic regions (eight variants) and nominal association (P<0.05) was detected for 18 markers (Table 1). Associations were tested in both the overall cohort, and comparing cases with no clinical IBD (n = 2613) with healthy controls. The frequency of AS cases with coexistent IBD in our dataset is 5.8%. No significant difference was noted in any of the findings having excluded cases with clinical IBD, supporting these associations being relevant to the pathogenesis of AS, rather than just coexistent IBD.
Tab. 1. Association analysis findings of SNPs achieving P<0.1 in discovery cohort and successfully genotyped in the replication cohort. 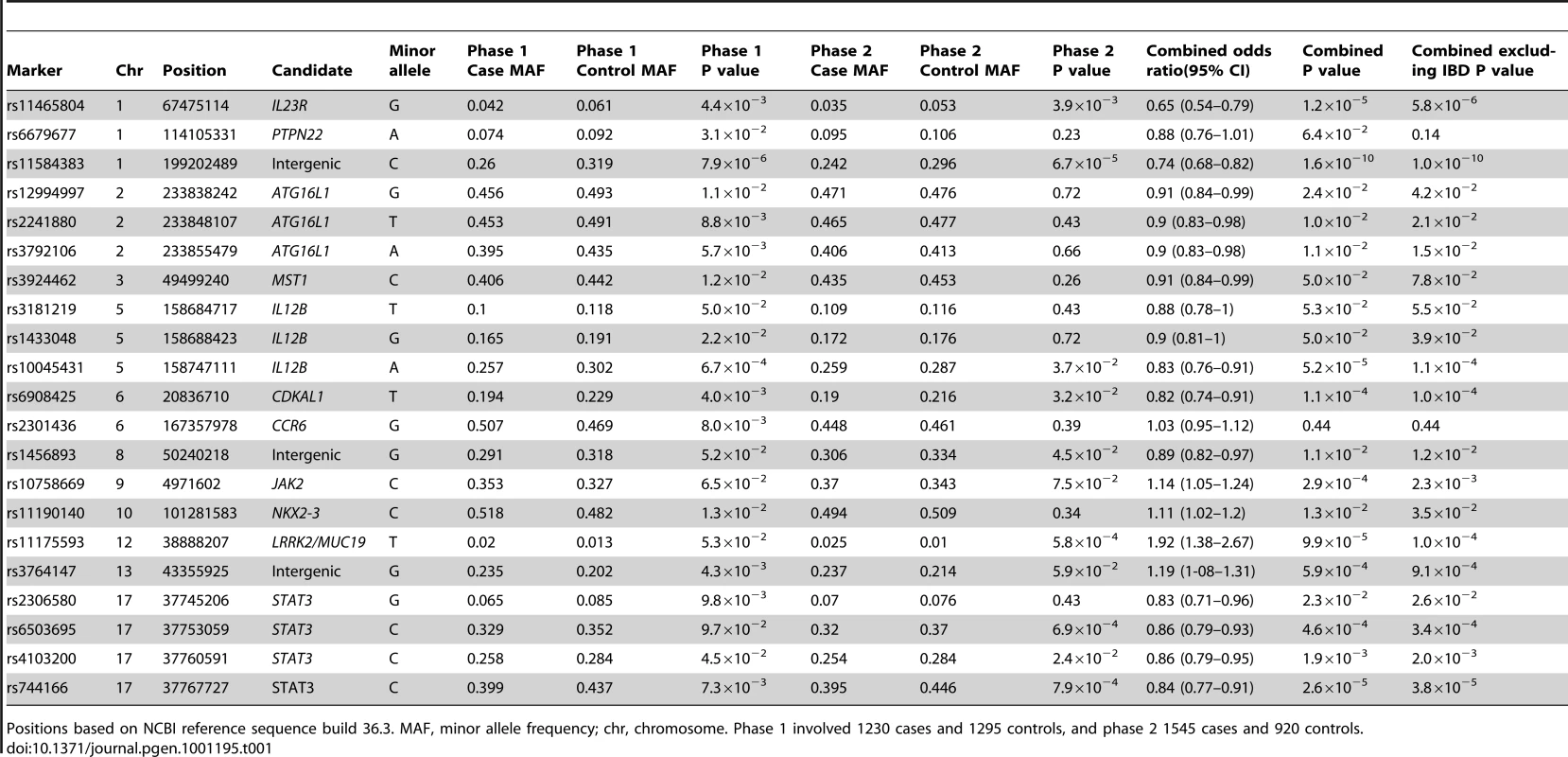
Positions based on NCBI reference sequence build 36.3. MAF, minor allele frequency; chr, chromosome. Phase 1 involved 1230 cases and 1295 controls, and phase 2 1545 cases and 920 controls. The strongest association was identified at genome-wide significance for rs11584383 at chr1q32 (P = 1.6×10−10) with OR = 0.74 (95% CI:0.68–0.82). This marker is downstream of and flanked by KIF21B and the putative open reading frame C1orf106. Convincing evidence of association was also identified for STAT3. Association in STAT3 was detected at experiment-wise significance for 2 markers (rs744166, P = 2.6×10−5, OR = 0.84 (95% CI:0.77–0.91); rs6503695, P = 4.6×10−4, OR = 0.86 (95% CI:0.79–0.93)). Another 2 variants within the gene showed more modest association (rs2306580, P = 0.023; rs4103200, P = 1.9×10−3) but rs4103200 demonstrated nominal association in both phases of the genotyping experiment.
Variants within or near CDKAL1 (rs6908425, P = 1.1×10−4, OR = 0.82 (95% CI:0.74–0.91)), IL12B (rs10045431, P = 5.2×10−5, OR = 0.83 (95% CI:0.76–0.91)), LRRK2/MUC19 (rs11175593, P = 9.9×10−5, OR = 1.92 (95% CI:1.38–2.67)) and at chr13q14 within C13orf31 (rs3764147, P = 5.9×10−4, OR = 1.19 (95% CI:1-08–1.31)) also demonstrated experiment-wise significant association in this study. The function of CDKAL1 is poorly understood but the gene has been associated with type-2-diabetes [12], [13], and could also possibly be a psoriasis risk locus [14].
Other nominal associations detected in this study include 3 variants within ATG16L1 and single SNPs at MST1, JAK2, NKX2-3 and chr8q24. Further studies will be required to robustly establish the significance of these findings. No association was observed at TNFSF15 (rs4263839, P = 0.3), consistent with previous findings in AS [3], [4], contrasting with findings in undifferentiated spondyloarthritis [15], in which suggestive but not genome-wide significant association has been reported (rs4979459, P = 4.9×10−5). The peak IBD and spondyloarthritis associations are with different SNPs that lie 85kb apart, and thus if this gene is truly spondyloarthritis-associated, it is likely that different genetic variants are associated with IBD and spondyloarthritis.
This study also confirms the IL23R association with AS at rs11465804 (P = 1.2×10−5), OR = 0.65 (95% CI:0.54–0.79). In a recent study, IL23R association with AS was confirmed with a peak signal within the gene at rs11209026 (P = 9.1×10−14) [4]. However, evidence of association at rs11465804 (3,432 bp away from rs11209026) is much weaker, and was previously reported at P = 2×10−4 in a study involving 1471 AS cases and 2125 matched control individuals [3].
Discussion
The study presented here has identified new loci associated with AS. The strongest of these associations was within an intergenic region at chr1q32, near the gene KIF21B. The protein encoded by this gene belongs to a family of kinesin motor proteins. Kinesins are used for the transport of essential components along axonal and dendritic microtubules by neurons. KIF5A has been associated with rheumatoid arthritis, type-1-diabetes, and is close to a locus recently reported to be associated with multiple sclerosis [16]. It is possible that KIF5A is not the key associated gene at this chromosome 12q13-14 locus. However, if confirmed as the true disease-susceptibility gene for these autoimmune diseases, this would strongly suggest alternate functions for the kinesin protein family.
The STAT3 association is particularly significant because of its role, along with IL23R, in the Th17 pathway. In response to cytokine signaling through the IL-23R, STAT3 is activated by phosphorylation and is translocated to the nucleus where it acts as a transcriptional activator. Loss of function mutations of STAT3 result in Job's syndrome, in which an absence of Th17 lymphocytes leads to recurrent severe infections, particularly with extracellular bacteria [17].
The association with IL12B is of particular interest given the associations of IL23R and STAT3 with AS. This gene has also been shown to be associated with psoriasis in Caucasian and Chinese populations [18], [19]. IL12B encodes the p40 subunit common to both IL-12 and IL-23 and again highlights the involvement of Th17 cells in disease development. Another marker (rs1433048) within the gene was also nominally associated (P<0.05) in the combined analysis of the study. It is not clear at the LRRK2/MUC19 locus which is the key associated gene with Crohn's disease, although a recent study suggests that LRRK2 is the more likely to be truly disease-associated [20]. LRRK2, a member of the leucine-rich repeat kinase family, is thought to be involved in the process of autophagy. MUC19 encodes a mucin involved in epithelial lining protection; altered intestinal permeability has long been thought to be important in the pathogenesis of AS.
This study also provides further evidence of pleiotropic effects in human disease pathology. A notable example of this is the association of PTPN22 with several autoimmune conditions including RA, T1D, CD and SLE. One of the 1st AS risk loci identified, IL23R, is also associated with both forms of IBD (ulcerative colitis and Crohn's disease) as well as psoriasis. In this study we provide further evidence for previously and newly identified pleiotropic genes in autoimmune diseases. Given the delicate nature of the immune system and the tight control of the different cell populations it is not surprising that risk alleles of important immune response genes may be associated across a number of different conditions. These findings support the use of study designs focusing on genes previously identified as being associated with related conditions as being an efficient method for identifying further genetic disease-associations.
This study of genes associated with Crohn's disease has identified definite genome-wide significant association with AS of SNPs at chromosome 1q32 near KIF21B, and experiment-wise association at five other novel-AS loci including STAT3, IL12B, CDKAL1, LRRK2/MUC19, and at chr13q14. This confirms that genes play an important part in the co-familiality of Crohn's disease and AS, and highlights the value of studies of potentially pleiotropic genes in related diseases.
Materials and Methods
Cases included in the study were unrelated individuals with AS of white European ancestry from the UK, USA, Canada and Australia. The diagnosis of AS met the modified New York definition criteria [21]. Ethnically matched unrelated control individuals were selected from the 1958 British Birth Cohort (BBC) and an Oxfordshire (UK) healthy blood donor cohort. All patients gave informed, written consent, and the study was approved by the relevant ethics review committees.
SNPs chosen for the study were selected from previously reported risk loci identified in a CD GWA study [22]. Three candidate loci of high interest were studied in greater depth (STAT3, IL12B and ATG16L1). For these genes, tagging SNPs (r2≥0.8) were selected using Tagger in Haploview (http://www.broadinstitute.org/haploview/haploview) and using the CEU population as a reference panel from the International HapMap Project database (http://www.hapmap.org/). Whenever assay design for selected SNPs failed, perfect proxy SNPs (r2 = 1) were selected.
Genotyping was carried out in two phases. In phase 1, 53 SNPs from 30 distinct genomic regions were selected for genotyping in 1,230 cases. Case genotype results were compared with historical genotypes from 1,295 unrelated individuals from the 1958 British Birth Cohort, which had been typed with both the Illumina HumanHap 550 and Affymetrix SNP Array 5.0 microarrays. Whenever selected markers were missing from the control genotypes, imputation was carried out using MACH (http://www.sph.umich.edu/csg/abecasis/MACH/index.html) against the HapMap CEU dataset (reference panel). Imputed markers with low overall quality scores (Q<0.95) and/or in low LD with typed markers (r2<0.3) were excluded from further analysis. Only genotypes with quality scores ≥0.95 were included in the study. All nominally associated markers (P<0.1) were taken forward for genotyping in phase 2 on 1,543 cases and 920 controls.
In phase 2, genotyping was performed using Sequenom (Sequenom Inc., San Diego, USA), and Applied Biosystems TaqMan and OpenArray technologies (Life Technologies, Carlsbad, USA). For Sequenom, SNPs were assayed and typed using iPLEX chemistry on a matrix assisted laser desorption/ionization time-of-flight (MALDI_TOF) mass spectrometer. PCR reactions, cycling conditions and post-PCR extension reactions were all performed as recommended by the manufacturer. The iPLEX reaction products were desalted and spotted on SpectroChip and were processed and analysed in a compact mass spectrometer (MassARRAY Workstation). MassARRAY Typer 4.0 software was used for automated and manual genotype calling. OpenArray is a new genotyping platform technology from Applied Biosystems for medium-throughput experiment. SNPs were typed using TaqMan genotyping chemistry supported on a metal-based array. DNA samples were loaded and amplified on arrays as recommended by the manufacturer. Arrays were scanned on the OpenArray NT imager and genotypes were called using the OpenArray SNP Genotyping analysis software. Whenever assay design or the genotyping assay failed, markers were then genotyped using single TaqMan probe technology as recommended by the manufacturer.
Association statistics were calculated using PLINK software [23]. Analysis was carried out using the Cochran-Armitage test for trend, excluding markers failing the following criteria; missingness rate >0.1, minor allele frequency (MAF)<0.01 and exact Hardy-Weinberg equilibrium P<5×10−5. We also excluded individuals with a missing genotype rate >0.1 from the analysis. Experiment-wise significance (P = 9.4×10−4) was determined by Bonferroni correction based on the total number of markers genotyped in phase1 of the study.
Supporting Information
Zdroje
1. BraunJ
SieperJ
2007 Ankylosing spondylitis. Lancet 369 1379 1390
2. BrownMA
KennedyLG
MacGregorAJ
DarkeC
DuncanE
1997 Susceptibility to ankylosing spondylitis in twins: the role of genes, HLA, and the environment. Arthritis Rheum 40 1823 1828
3. BurtonPR
ClaytonDG
CardonLR
CraddockN
DeloukasP
2007 Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet 39 1329 1337
4. ReveilleJD
SimsAM
DanoyP
EvansDM
LeoP
2010 Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat Genet 42 123 127
5. HindorffLA
SethupathyP
JunkinsHA
RamosEM
MehtaJP
2009 Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A 106 9362 9367
6. MielantsH
VeysEM
CuvelierC
De VosM
GoemaereS
1995 The evolution of spondyloarthropathies in relation to gut histology. II. Histological aspects. J Rheumatol 22 2273 2278
7. Leirisalo-RepoM
TurunenU
StenmanS
HeleniusP
SeppalaK
1994 High frequency of silent inflammatory bowel disease in spondylarthropathy. Arthritis Rheum 37 23 31
8. OrchardTR
HoltH
BradburyL
HammersmaJ
McNallyE
2009 The prevalence, clinical features and association of HLA-B27 in sacroiliitis associated with established Crohn's disease. Aliment Pharmacol Ther 29 193 197
9. Schorr-LesnickB
BrandtLJ
1988 Selected rheumatologic and dermatologic manifestations of inflammatory bowel disease. Am J Gastroenterol 83 216 223
10. Dekker-SaeysBJ
MeuwissenSG
Van Den Berg-LoonenEM
De HaasWH
AgenantD
1978 Ankylosing spondylitis and inflammatory bowel disease. II. Prevalence of peripheral arthritis, sacroiliitis, and ankylosing spondylitis in patients suffering from inflammatory bowel disease. Ann Rheum Dis 37 33 35
11. ThjodleifssonB
GeirssonAJ
BjornssonS
BjarnasonI
2007 A common genetic background for inflammatory bowel disease and ankylosing spondylitis: a genealogic study in Iceland. Arthritis Rheum 56 2633 2639
12. SaxenaR
VoightBF
LyssenkoV
BurttNP
de BakkerPI
2007 Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316 1331 1336
13. ZegginiE
WeedonMN
LindgrenCM
FraylingTM
ElliottKS
2007 Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316 1336 1341
14. LiY
LiaoW
ChangM
SchrodiSJ
BuiN
2009 Further genetic evidence for three psoriasis-risk genes: ADAM33, CDKAL1, and PTPN22. J Invest Dermatol 129 629 634
15. ZinovievaE
BourgainC
KadiA
LetourneurF
IzacB
2009 Comprehensive linkage and association analyses identify haplotype, near to the TNFSF15 gene, significantly associated with spondyloarthritis. PLoS Genet 5 e1000528 doi:10.1371/journal.pgen.1000528
16. Australia and New Zealand Multiple Sclerosis Genetics Consortium (ANZgene) 2009 Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet 41 824 828
17. HollandSM
DeLeoFR
ElloumiHZ
HsuAP
UzelG
2007 STAT3 mutations in the hyper-IgE syndrome. N Engl J Med 357 1608 1619
18. NairRP
DuffinKC
HelmsC
DingJ
StuartPE
2009 Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet 41 199 204
19. ZhangXJ
HuangW
YangS
SunLD
ZhangFY
2009 Psoriasis genome-wide association study identifies susceptibility variants within LCE gene cluster at 1q21. Nat Genet 41 205 210
20. PhillipsAM
NimmoER
LimbergenJV
DrummondHE
SmithL
2009 Detailed haplotype-tagging study of germline variation of MUC19 in inflammatory bowel disease. Inflamm Bowel Dis
21. van der LindenS
ValkenburgHA
CatsA
1984 Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27 361 368
22. BarrettJC
HansoulS
NicolaeDL
ChoJH
DuerrRH
2008 Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet 40 955 962
23. PurcellS
NealeB
Todd-BrownK
ThomasL
FerreiraMA
2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 559 575
Štítky
Genetika Reprodukční medicína
Článek Genome-Wide Interrogation of Mammalian Stem Cell Fate Determinants by Nested Chromosome DeletionsČlánek Season of Conception in Rural Gambia Affects DNA Methylation at Putative Human Metastable EpiallelesČlánek A Quantitative Systems Approach Reveals Dynamic Control of tRNA Modifications during Cellular StressČlánek Reduction of Protein Translation and Activation of Autophagy Protect against PINK1 Pathogenesis inČlánek The Loss of PGAM5 Suppresses the Mitochondrial Degeneration Caused by Inactivation of PINK1 inČlánek Cleavage of Phosphorothioated DNA and Methylated DNA by the Type IV Restriction Endonuclease ScoMcrAČlánek Competitive Repair by Naturally Dispersed Repetitive DNA during Non-Allelic Homologous Recombination
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 12- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Genome-Wide Interrogation of Mammalian Stem Cell Fate Determinants by Nested Chromosome Deletions
- Whole-Genome and Chromosome Evolution Associated with Host Adaptation and Speciation of the Wheat Pathogen
- Association of Variants at 1q32 and with Ankylosing Spondylitis Suggests Genetic Overlap with Crohn's Disease
- Initiator Elements Function to Determine the Activity State of BX-C Enhancers
- Identification of Genes Required for Neural-Specific Glycosylation Using Functional Genomics
- A Young Duplicate Gene Plays Essential Roles in Spermatogenesis by Regulating Several Y-Linked Male Fertility Genes
- The EpsE Flagellar Clutch Is Bifunctional and Synergizes with EPS Biosynthesis to Promote Biofilm Formation
- Histone H2A C-Terminus Regulates Chromatin Dynamics, Remodeling, and Histone H1 Binding
- Season of Conception in Rural Gambia Affects DNA Methylation at Putative Human Metastable Epialleles
- A Quantitative Systems Approach Reveals Dynamic Control of tRNA Modifications during Cellular Stress
- GC-Rich Sequence Elements Recruit PRC2 in Mammalian ES Cells
- A Single Enhancer Regulating the Differential Expression of Duplicated Red-Sensitive Opsin Genes in Zebrafish
- Investigation and Functional Characterization of Rare Genetic Variants in the Adipose Triglyceride Lipase in a Large Healthy Working Population
- Reduction of Protein Translation and Activation of Autophagy Protect against PINK1 Pathogenesis in
- Noisy Splicing Drives mRNA Isoform Diversity in Human Cells
- The Loss of PGAM5 Suppresses the Mitochondrial Degeneration Caused by Inactivation of PINK1 in
- Thymus-Associated Parathyroid Hormone Has Two Cellular Origins with Distinct Endocrine and Immunological Functions
- An ABC Transporter Mutation Is Correlated with Insect Resistance to Cry1Ac Toxin
- Role of Individual Subunits of the CSN Complex in Regulation of Deneddylation and Stability of Cullin Proteins
- The C-Terminal Domain of the Bacterial SSB Protein Acts as a DNA Maintenance Hub at Active Chromosome Replication Forks
- The DNA Damage Response Pathway Contributes to the Stability of Chromosome III Derivatives Lacking Efficient Replicators
- Cleavage of Phosphorothioated DNA and Methylated DNA by the Type IV Restriction Endonuclease ScoMcrA
- LaeA Control of Velvet Family Regulatory Proteins for Light-Dependent Development and Fungal Cell-Type Specificity
- Competitive Repair by Naturally Dispersed Repetitive DNA during Non-Allelic Homologous Recombination
- Distinct Functions for the piRNA Pathway in Genome Maintenance and Telomere Protection
- MOS11: A New Component in the mRNA Export Pathway
- Self-Mating in the Definitive Host Potentiates Clonal Outbreaks of the Apicomplexan Parasites and
- A Role for ATF2 in Regulating MITF and Melanoma Development
- Ancestral Regulatory Circuits Governing Ectoderm Patterning Downstream of Nodal and BMP2/4 Revealed by Gene Regulatory Network Analysis in an Echinoderm
- Cancer and Neurodegeneration: Between the Devil and the Deep Blue Sea
- Functional Comparison of Innate Immune Signaling Pathways in Primates
- Linking Crohn's Disease and Ankylosing Spondylitis: It's All about Genes!
- Genomics Meets Glycomics—The First GWAS Study of Human N-Glycome Identifies HNF1α as a Master Regulator of Plasma Protein Fucosylation
- Continuous and Periodic Expansion of CAG Repeats in Huntington's Disease R6/1 Mice
- Expression of Linear and Novel Circular Forms of an -Associated Non-Coding RNA Correlates with Atherosclerosis Risk
- Endocytic Sorting and Recycling Require Membrane Phosphatidylserine Asymmetry Maintained by TAT-1/CHAT-1
- Histone Deacetylases Suppress CGG Repeat–Induced Neurodegeneration Via Transcriptional Silencing in Models of Fragile X Tremor Ataxia Syndrome
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Functional Comparison of Innate Immune Signaling Pathways in Primates
- Expression of Linear and Novel Circular Forms of an -Associated Non-Coding RNA Correlates with Atherosclerosis Risk
- Genome-Wide Interrogation of Mammalian Stem Cell Fate Determinants by Nested Chromosome Deletions
- Histone H2A C-Terminus Regulates Chromatin Dynamics, Remodeling, and Histone H1 Binding
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



