-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Loss of PGAM5 Suppresses the Mitochondrial Degeneration Caused by Inactivation of PINK1 in
PTEN-induced kinase 1 (PINK1), which is required for mitochondrial homeostasis, is a gene product responsible for early-onset Parkinson's disease (PD). Another early onset PD gene product, Parkin, has been suggested to function downstream of the PINK1 signalling pathway based on genetic studies in Drosophila. PINK1 is a serine/threonine kinase with a predicted mitochondrial target sequence and a probable transmembrane domain at the N-terminus, while Parkin is a RING-finger protein with ubiquitin-ligase (E3) activity. However, how PINK1 and Parkin regulate mitochondrial activity is largely unknown. To explore the molecular mechanism underlying the interaction between PINK1 and Parkin, we biochemically purified PINK1-binding proteins from human cultured cells and screened the genes encoding these binding proteins using Drosophila PINK1 (dPINK1) models to isolate a molecule(s) involved in the PINK1 pathology. Here we report that a PINK1-binding mitochondrial protein, PGAM5, modulates the PINK1 pathway. Loss of Drosophila PGAM5 (dPGAM5) can suppress the muscle degeneration, motor defects, and shorter lifespan that result from dPINK1 inactivation and that can be attributed to mitochondrial degeneration. However, dPGAM5 inactivation fails to modulate the phenotypes of parkin mutant flies. Conversely, ectopic expression of dPGAM5 exacerbated the dPINK1 and Drosophila parkin (dParkin) phenotypes. These results suggest that PGAM5 negatively regulates the PINK1 pathway related to maintenance of the mitochondria and, furthermore, that PGAM5 acts between PINK1 and Parkin, or functions independently of Parkin downstream of PINK1.
Published in the journal: . PLoS Genet 6(12): e32767. doi:10.1371/journal.pgen.1001229
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001229Summary
PTEN-induced kinase 1 (PINK1), which is required for mitochondrial homeostasis, is a gene product responsible for early-onset Parkinson's disease (PD). Another early onset PD gene product, Parkin, has been suggested to function downstream of the PINK1 signalling pathway based on genetic studies in Drosophila. PINK1 is a serine/threonine kinase with a predicted mitochondrial target sequence and a probable transmembrane domain at the N-terminus, while Parkin is a RING-finger protein with ubiquitin-ligase (E3) activity. However, how PINK1 and Parkin regulate mitochondrial activity is largely unknown. To explore the molecular mechanism underlying the interaction between PINK1 and Parkin, we biochemically purified PINK1-binding proteins from human cultured cells and screened the genes encoding these binding proteins using Drosophila PINK1 (dPINK1) models to isolate a molecule(s) involved in the PINK1 pathology. Here we report that a PINK1-binding mitochondrial protein, PGAM5, modulates the PINK1 pathway. Loss of Drosophila PGAM5 (dPGAM5) can suppress the muscle degeneration, motor defects, and shorter lifespan that result from dPINK1 inactivation and that can be attributed to mitochondrial degeneration. However, dPGAM5 inactivation fails to modulate the phenotypes of parkin mutant flies. Conversely, ectopic expression of dPGAM5 exacerbated the dPINK1 and Drosophila parkin (dParkin) phenotypes. These results suggest that PGAM5 negatively regulates the PINK1 pathway related to maintenance of the mitochondria and, furthermore, that PGAM5 acts between PINK1 and Parkin, or functions independently of Parkin downstream of PINK1.
Introduction
Parkinson's disease (PD (OMIM #168600)) is a neurodegenerative disease that affects the maintenance of dopaminergic (DA) neurons. PD prevalence is estimated at ∼1% among people over the age of 65 and increases with age. Clinical features of PD include motor abnormalities (tremor, rigidity, akinesia), autonomic disturbances, psychiatric disability and cognitive impairment. The recent identification of PD-associated genes has advanced our understanding the molecular mechanisms underlying PD. Two of these genes, PINK1 (PARK6, OMIM #605909, Gene ID: 65018) and parkin (PARK2, OMIM #600116, Gene ID: 5071), are associated with early-onset autosomal recessive PD, in which loss-of-function (LOF) of a single gene product results in the clinical manifestation of Parkinsonism [1], [2]. The PINK1 gene encodes a serine/threonine kinase with a predicted mitochondrial target sequence and a probable transmembrane domain at the N-terminus [3]. The gene product of the parkin gene encodes a protein with an E3 activity [4]–[6]. Recent genetic studies in Drosophila have reported that dPINK1 (Gene ID: 31607) acts as an upstream regulator of dParkin (Gene ID: 40336) in a common pathway that influences mitochondrial maintenance in a subset of tissues, including the flight muscle and DA neurons [7]–[9]. LOF of the dPINK1 or the dparkin genes results in enlarged or swollen mitochondria, a phenotype that can be partially rescued by heterozygosity for LOF mutations of the mitochondrial fusion-promoting components Optic atrophy 1 (OPA1) and Mitofusin (Mfn), or by increased mitochondrial fission activity via increased dosage of the dynamin-related protein 1 (drp1) gene [10]–[12]. Studies in mammalian or Drosophila cultured cells report that PINK1 is required to recruit Parkin to damaged depolarized mitochondria, and promotes their degradation through an autophagic event called mitophagy [13]–[16]. Thus, there is strong evidence to support an important role for PINK1 and Parkin in regulating mitochondrial homeostasis. However, little is known about how PINK1 regulates mitochondrial integrity and turnover through Parkin. Indeed, the precise means by which PINK1 exerts an effect on Parkin is not clear.
Here we show that a mitochondrial protein, phosphoglycerate mutase 5 (PGAM5, Gene ID: 192111), which was previously reported to be localized at the outer mitochondrial membrane and to lack a phosphoglycerate mutase activity [17], [18], is involved in the PINK1 pathway, and that loss of PGAM5 activity improves mitochondrial defects caused by PINK1 inactivation in Drosophila.
Results
Isolation of PGAM5 as a PINK1-Binding Protein
We and others have previously demonstrated that PINK1 is genetically upstream of parkin [7]–[9]. To further investigate the relationship between PINK1 and Parkin, we searched for PINK1-binding proteins using a combination of biochemical purification and mass spectrometric analysis. We affinity-purified human PINK1 with a FLAG tag at its C-terminus (hPINK1-FLAG) from lysate of human embryonic kidney (HEK) 293 cells stably expressing hPINK1-FLAG using an anti-FLAG column, and determined proteins specifically presented in the hPINK1-FLAG elution fractions, which include cytoskeleton-related proteins (MAP1B (GeneID: 4131), KIF11 (GeneID: 3832), Tubulin GeneID: 602530, 191130)), proteasome subunits (PSMD1 (GeneID: 5707), PSMD2 (GeneID: 5708), PSMC6 (GeneID: 5706)), PRKDC (GeneID: 5591), Hsp70 (1A, GeneID: 3303; 1B, GeneID: 3304), Hsp90 (GeneID: 3320), Cdc37 (GeneID: 11140), Insulin substrate-4 (IRS-4, GeneID: 8471) and PGAM5 (Figure 1A). PRKDC is one of proteins non-specifically associated with FLAG-tagged proteins in our proteomic analyses (data not shown). The roles of Hsp90, Cdc37 and the proteasome for PINK1 have been characterized previously [15], [19]–[22]. We therefore chose IRS-4 and PGAM5 and tested whether these proteins modulate the dPINK1 LOF phenotypes by Drosophila genetics. Our initial in vivo tests revealed that a mutant allele for dPGAM5 (CG14816, GeneID: 31143), PGAM5NP0568 significantly suppressed the abnormal wing postures observed in dPINK1 knockdown flies [9] (Figure 1B), while it failed to improve the viability (Figure 1C). Reducing the dose of chico (GeneID: 64880), which encodes a Drosophila orthologue of IRS-4, significantly suppresses the short lifespan phenotype caused by dPINK1 knockdown, without affecting wing posture (Figure 1D and 1E). Inhibition of chico activity has previously been reported to extend the lifespan of Drosophila, such that we reasoned that the effect on lifespan we observed might reflect a general phenomenon rather than reflecting a specific interaction with dPINK1 [23]. Thus for subsequent studies, we chose to focus on PGAM5.
Fig. 1. Identification of PINK1-binding proteins that modulate the phenotypes of dPINK1 knockdown fly. 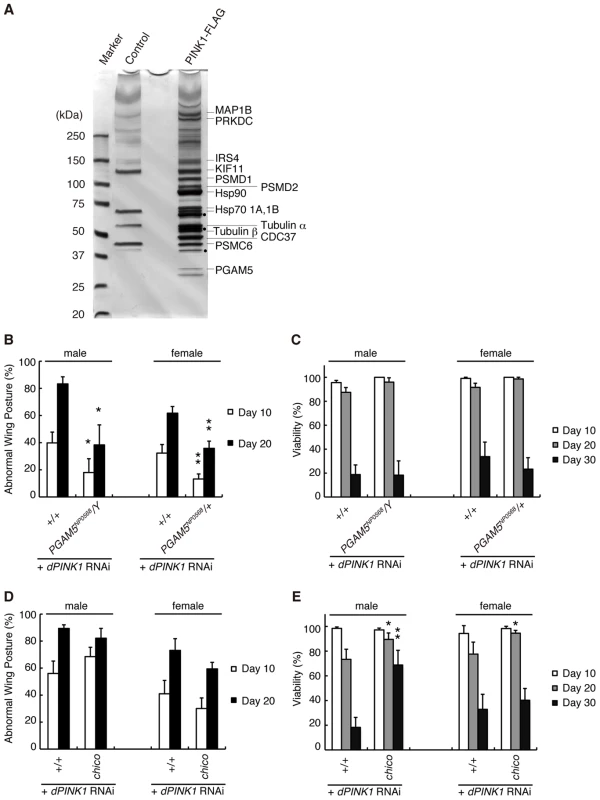
(A) Silver-stained polyacrylamide gel to visualize hPINK1-binding proteins. FLAG elution fractions purified from cells stably expressing hPINK1-FLAG (PINK1-FLAG lane) and parental cells (Control lane) are separated on a gel (For details of the procedure, see Materials and Methods). Bands corresponding to hPINK1 (dots) and representative co-purified proteins are indicated. (B, C) The wing phenotype typical of 10- and 20-day-old dPINK1 RNAi flies [9] (B) was suppressed by the PGAM5NP0568 mutant allele, whereas viability of 10-, 20- and 30-day-old adult flies was not improved (C). *, p<0.05; **, p<0.01 vs. age-matched dPINK1 RNAi group in Student's t-test. The genotypes are as follows: MHC-GAL4> dPINK1RNAi (+/+), PGAM5NP0568/Y; MHC-GAL4> dPINK1RNAi (PGAM5NP0568/Y), PGAM5NP0568/+; MHC-GAL4> dPINK1RNAi (PGAM5NP0568/+). MHC-GAL4, a muscle-specific driver. Flies were raised at 29°C as the RNAi-induced dPINK1 defects are more pronounced when flies are raised at that temperature. (D, E) Removal of one copy of the IRS4 ortholog chico had no effect on the wing phenotype of dPINK1 RNAi flies (D) but improved viability (E). *, p<0.05; **, p<0.01 vs. age-matched dPINK1 RNAi group. The genotypes are: MHC-GAL4> dPINK1RNAi (+/+), chico/+; MHC-GAL4> dPINK1RNAi (chico). Flies were raised at 29°C. The results of co-immunoprecipitation confirmed that C-terminally Myc-tagged human PGAM5 (hPGAM5-Myc) specifically binds to hPINK1-FLAG in transfected HEK293 cells (Figure 2A). Moreover, we found that hPGAM5 and hPINK1 immunoreactivity co-localizes with mitochondria in transfected HeLa cells, consistent with the previous finding that PGAM5 is localized to the mitochondria (Figure 2B and 2C) [24]. To test if endogenous hPGAM5 interacts with hPINK1, we first generated an anti-hPGAM5 antibody (Figure 2D). Next, we used a previously established anti-PINK1 antibody to immunoprecipitate PINK1 from HEK293 cell lysate, then probed with anti-PGAM5 to detect endogenous hPGAM5. As shown in Figure 2E, endogenous hPGAM5 was detectable in the fraction immunoprecipitated using anti-hPINK1 antibody but not a control antibody, confirming the results of mass spectrometric analysis. Physical association of dPINK1 with dPGAM5 was also observed in Drosophila S2 cells (Figure 2F), suggesting that their functional interaction is conserved between human and Drosophila.
Fig. 2. PGAM5 associates with PINK1 at mitochondria. 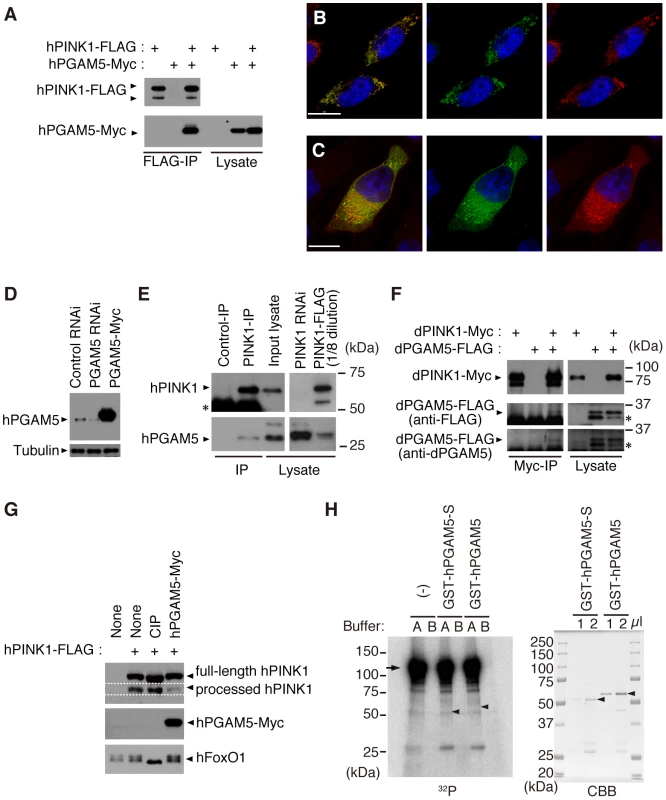
(A) hPGAM5 binds to hPINK1 in HEK293 cells. Lysate expressing C-terminally Myc-tagged hPGAM5 (hPGAM5-Myc) and FLAG-tagged hPINK1 (hPINK1-FLAG) was subjected to immunoprecipitation with anti-FLAG antibody (FLAG-IP), and analyzed by immunoblotting with anti-tag antibodies. (B) hPGAM5 is localized to the mitochondria. HeLa cells transfected with hPGAM5-Myc were visualized with anti-Myc (green). Mitochondria were visualized with MitoTracker (red) and nuclei with DAPI (blue). Regions of co-localization of hPGAM5 with mitochondria appear in yellow in the merged image. (C) hPGAM5 and hPINK1 co-localize at mitochondria. HeLa cells co-transfected with hPINK1 and hPGAM5-Myc were stained with anti-PINK1 (green) and anti-Myc (red). (D) Anti-hPGAM5 antibody specifically recognizes ∼30 kDa bands in extract from HEK293 cells, which were reduced in lysates from cells treated with siRNAs directed against hPGAM5. Lysate expressing hPGAM5-Myc and anti-tubulin signals served as a positive control and a loading control, respectively. (E) Endogenous hPGAM5 is associated with hPINK1. An anti-PINK1 (PINK1-IP) or an antibody against the unrelated protein Delta (Control-IP) was used for immunoprecipitation of proteins in HEK293 cells. Cell lysate in which hPINK1 was knocked down by RNAi (PINK1 RNAi) and lysate from cells that overexpressed hPINK1-FLAG (PINK1-FLAG) served as additional controls. The PINK1-FLAG lysate was diluted eight-fold with loading buffer to reduce the strong signal present in that sample. Asterisk, bands attributable to detection of the antibodies themselves, which may mask lower molecular weight hPINK1 bands (∼52 kDa). (F) dPGAM5 is associated with dPINK1 in Drosophila S2 cells. S2 cell lysate expressing dPINK1-Myc and dPGAM5-FLAG was subjected to immunoprecipitation with anti-Myc antibody (Myc-IP), and analyzed by immunoblotting with anti-tag or anti-dPGAM5 antibodies. Asterisks, a putative processed form of dPGAM5. (G) HEK 293 cell lysate expressing hPINK1-FLAG together with hPGAM5-Myc was subjected to Phos-tag immunoblotting [43]. hPINK1-FLAG lysate treated with alkaline phosphatase (CIP) was used as a positive control. A phospho-protein FoxO1 was efficiently dephosphorylated by the CIP treatment. (H) An in vitro kinase assay was performed using 2x GST-dPINK1 and GST-hPGAM5. Recombinant 2x GST-dPINK1 purified from bacteria was used as a kinase source. Recombinant GST-hPGAM5 short form (GST-hPGAM5-S) or GST-hPGAM5 was purified from bacteria and 1 and 2 µl of the purified fractions were separated by SDS-PAGE and stained with Coomassie Brilliant Blue (CBB, right-hand panel; arrowheads, GST-hPGAM5-S or GST-hPGAM5). A total of 100 or 400 ng of GST-hPGAM5-S or GST-hPGAM5, respectively, were incubated with 100 ng of 2x GST-dPINK1 in kinase reaction buffer A (100 mM Tris-HCl [pH 7.5], 240 mM NaCl, 30 µM ATP, 10 mM MgCl2, 2 mM CaCl2, 5 µCi γ-32P ATP) or buffer B (100 mM Tris-HCl [pH7.5], 240 mM NaCl, 30 µM ATP, 10 mM EDTA, 5 µCi γ-32P ATP) for 30 min at 30°C. The reaction mixture was suspended in SDS sample buffer and then subjected to SDS-PAGE and autoradiography (Left, 32P; the arrow and arrowheads represent expected migration positions of 2x GST-dPINK1 and GST-hPGAM5/GST-hPGAM5-S, respectively). No specific signals corresponding hPGAM5 or hPGAM5-S were observed. Note that 2x GST-dPINK1 lacks kinase activity in the buffer B, suggesting that activation of PINK1 requires divalent cations such as Mg2+ and Ca2+. Scale bars = 15 µm in (B and C). Previous findings that PINK1 and PGAM5 possess kinase and phosphatase activities, respectively [18], [25], [26], prompted us to test the possibility of their enzyme-substrate relationships. A mobility shift assay to monitor the status of PINK1 phosphorylation suggested that overexpression of hPGMA5 has little effect on hPINK1 phosphorylation (Figure 2G). On the other hand, an in vitro kinase assay using recombinant dPINK1 failed to show a possibility that PGAM5 is a substrate for PINK1, or that PGAM5 modifies hPINK1 kinase activities (Figure 2H).
dPGAM5 Alters Mitochondrial Morphology in Drosophila
The Drosophila genome appears to have two orthologs of mammalian PGAM5, one on the X (CG14816) and the other on the second chromosome (CG15874, GeneID: 37899). We have renamed CG14816 and CG15874 as dPGAM5 and dPGAM5-2, respectively. Our initial in vivo genetic study and most subsequent analyses were performed using dPGAM5 mutant and transgenic animals because dPGAM5 is more similar to hPGAM5 than is dPGAM5-2 (dPGAM5 vs. hPGAM5, 44% amino acid identity, and dPGAM5-2 vs. hPGAM5, 38% identity, as determined using ClustalW v1.4 to align the sequences), and because the results of high-throughput analysis of transcript abundance suggest that the dPGAM5-2 transcript is expressed at very low levels at the adult stage, if at all (see http://flybase.org/reports/FBgn0035004.html).
We determined the P-element insertion allele PGAM5NP0568 as a hypomorph allele, which showed a reduction of dPGAM5 transcript levels to about 25% of normal levels (Figure S1). We then generated a dPGAM5 null allele PGAM51, in which the expression of dPGAM5 completely disappeared at both the transcript and protein levels (Figure S1B and S1C). The PGAM51 homozygous animal is viable, fertile and grossly normal. However, it displayed longer lifespan (Figure 3A). By contrast, overexpression of dPGAM5 or dPGAM5-2 resulted in shorter longevity (Figure 3B). Since a previous report described that overexpression of human PGAM5 affects the mitochondrial morphology or mobility in the cultured cells [24], we observed the mitochondria in the dPGAM5 null and transgenic flies. Although inactivation of dPGAM5 gene function did not cause mitochondrial degeneration, the morphology of the mitochondria appears to be moderately altered (Figure 3D and 3H compared to Figure 3C and 3G). The mitochondria in the indirect flight muscles of the PGAM5 mutant flies were longer in the long-axis direction compared to control animals (Figure 3K). A similar tendency was seen in DA neurons of the adult brain although the difference did not reach statistical significance (Figure 3L and 3M). In addition, we frequently observed constrictions in the mitochondria (see broken lines in Figure 3D). In contrast, transgenic expression of dPGAM5 or dPGAM5-2 in Drosophila leads to fragmentation of mitochondria, with cristae well-preserved in the indirect flight muscles (Figure 3E, 3F, 3I, and 3J) and in the tyrosine-hydroxylase (TH)-positive neurons of the adult fly brain (Figure 3N and 3O). These results suggested that dPGAM5 is likely to promote the mitochondrial fission process in Drosophila.
Fig. 3. dPGAM5 is dispensable for normal development, but affects lifespan in Drosophila. 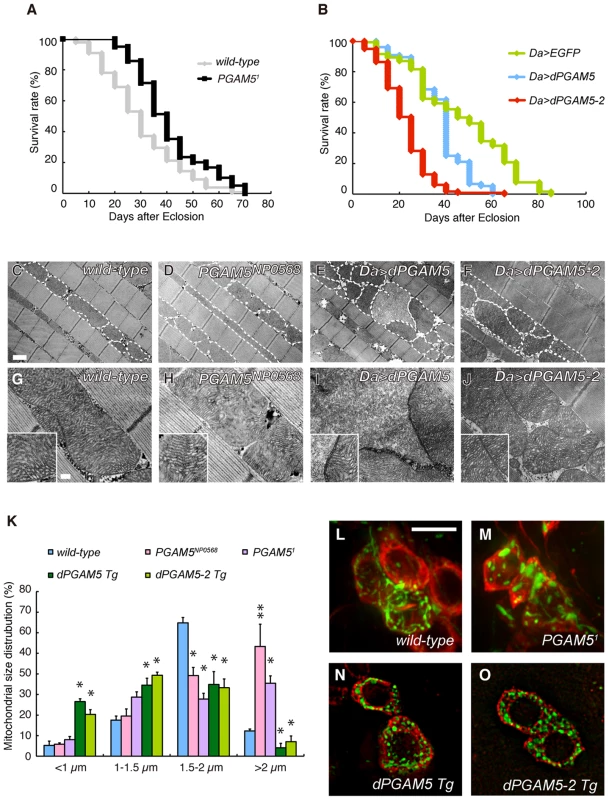
(A) Loss of dPGAM5 genes extends the lifespan. Adult male wild-type (yw/Y; n = 125) vs. dPGAM5 null (y, PGAM51/Y; n = 125) flies, p<0.001 by log rank test. (B) Overexpression of dPGAM5 or dPGAM5-2 in Drosophila causes shorter lifespan. Overexpression of the transgenes was induced using the ubiquitous daughterless (Da)-GAL4 driver. Lifespan of adult male EGFP (n = 130), dPGAM5 (n = 76) and dPGAM5-2 (n = 117) flies. EGFP vs. dPGAM5, p<0.001; EGFP vs. dPGAM5-2, p<0.001; by log rank test. (C–J) Transmission electron microscopy (TEM) analysis of the indirect flight muscle and morphology of mitochondria in 2-day-old adult flies with the indicated genotypes. In C–F, we outlined some mitochondria with broken lines to highlight morphology. The insets in G–J show representative mitochondria matrixes. A revertant, PINK1RV, was used as a wild-type comparison [8]. The genotypes are: PINK1RV/Y (C, G), PGAM5NP0568/Y (D, H), Da-GAL4> UAS-dPGAM5 (E, I), Da-GAL4> UAS-dPGAM5-2 (F, J). Scale bars = 1 µm in C–F and 200 nm in G–J. (K) Quantification of the percentage of mitochondrial size distribution in the indirect muscle tissue from wild-type (n = 136 from 5 adult flies), PGAM5NP0568 (n = 155 from 5), PGAM51 (n = 87 from 5), dPGAM5 Tg (n = 143 from 5) and dPGAM5-2 Tg flies (n = 147 from 5) as shown in (C–J). The length of the mitochondria in the direction of the myofibrils was measured. Data are shown as means ± SE (* p<0.05, **p<0.01 vs. wild-type). (L–O) Brain tissues of 5-day-old adult flies were stained with anti-TH antibody (red). Mitochondria labeled with mitoGFP (green) were observed in the PPL1 TH-positive neurons of the indicated genotypes. The genotypes are as follows: TH-GAL4> mitoGFP (wild-type), PGAM51/Y; TH-GAL4>UAS-mitoGFP (PGAM51), UAS-dPGAM5/TH-GAL4> UAS-mitoGFP (dPGAM5 Tg), UAS-dPGAM5-2/TH-GAL4> UAS-mitoGFP (dPGAM5-2 Tg). TH-GAL4, a DA neuron-specific driver. Scale bar = 5 µm. The Relationship between PGAM5 and the Mitochondrial Fission/Fusion Machinery
Evolutionarily-conserved GTPases Mfn and OPA1 promote the mitochondrial fusion event while another GTPase Drp1 regulates the mitochondrial fission [27], [28]. To determine the role of PGAM5 in the mitochondrial fission pathway, we manipulated the activities of the genes that are involved in mitochondrial fission/fusion in dPGAM5 null flies. Decreased Mfn activity resulted in fragmented mitochondria in the indirect muscle tissues, which was not affected by removal of the dPGAM5 gene (Figure 4A–4D and 4G, Figure S2). Conversely, an increased mitochondrial fission activity by introducing an extra copy of the drp1 gene was not suppressed in the dPGAM5 null genetic background (Figure 4E–4G). These results suggested that dPGAM5 may function upstream of Mfn or Drp1, or that the mechanism of the mitochondrial morphological changes by dPGAM5 is independent of that of the known fusion/fission components.
Fig. 4. Relationship between dPGAM5 and the mitochondrial fusion/fission genes. 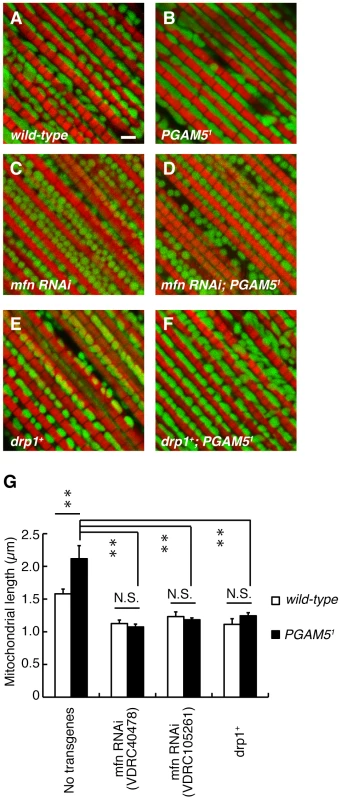
(A–F) dPGAM5 inactivation failed to rescue the mitochondrial fragmentation caused by mfn knockdown (mfn RNAi) or introduction of an extra copy of the drp1 gene (drp1+). To visualize the mitochondria under a fluorescence microscopy, we used the muscle-specific MHC-GAL4 driver to induce expression of a mitoGFP (green) transgene in 5-day-old adult flies with the indicated genotypes. Muscle tissue was counterstained with phalloidin (red). Scale bar = 2 µm. (G) The average length of the mitochondria in the direction of the myofibrils was measured from wild-type (n = 343 from 7 adult flies), PGAM51 (n = 390 from 8), mfn RNAi (VDRC40478, n = 305 from 6; VDRC105261, n = 372 from 8), mfn RNAi (VDRC40478); PGAM51 (n = 355 from 7), mfn RNAi (VDRC105261); PGAM51 (n = 237 from 5), drp1+ (n = 245 from 5) and drp1+; PGAM51 (n = 247 from 5) as shown in (A–F). Data are shown as means ± SE (**p<0.01; N.S., not significant). The genotypes are as follows: +/Y; MHC-GAL4>mitoGFP (A, wild-type), PGAM51/Y; MHC-GAL4>UAS-mitoGFP (B, PGAM51), +/Y; MHC-GAL4>UAS-mitoGFP; UAS-mfn RNAi (VDRC40478) (C, mfn RNAi), PGAM51/Y; MHC-GAL4>UAS-mitoGFP; UAS-mfn RNAi (VDRC40478) (D, mfn RNAi; PGAM51), +/Y; MHC-GAL4>mitoGFP; drp1+ (E, drp1+), PGAM51/Y; MHC-GAL4>mitoGFP; drp1+ (F, drp1+; PGAM51). dPGAM5 Modulates Phenotypes Caused by dPINK1 Inactivation in Drosophila
We next confirmed that the results of the genetic tests in Figure 1B and 1C using a LOF allele for dPINK1, PINK1B9 to exclude off-target effects due to RNAi (Figure 5). Adult PINK1B9 flies often have abnormal thoraces with dents in the mid-anterior region, which is likely due to degeneration of the muscle tissues lining the inside of the thorax (Figure 5B) [8]. This thorax phenotype seen in PINK1B9 flies can be suppressed by introduction of the PGAM5NP0568 or the PGAM51 allele (Figure 5A and 5D). We then examined the effects of dPGAM5 inactivation on dPINK1 mutant phenotypes that progressively increase over time. As described above, loss of dPINK1 activity leads to the appearance of abnormal wing postures, which is indicative of flight muscle degeneration, and the percent of affected flies increases with advancing age (Figure 5C and 5F) [8]. Introduction of the dPGAM5 mutant alleles dramatically suppresses this phenotype (Figure 5E and 5F), whereas ectopic expression of dPGAM5 enhances the phenotype (Figure 5G). Progressive loss of climbing ability and the shorter lifespan of PINK1B9 flies are additional prominent phenotypes that may represent dysfunction of DA neurons of the central nervous system and muscle degeneration. The dPGAM5 mutant alleles also significantly improved these phenotypes (Figure 5H and 5J). Conversely, overexpression of dPGAM5 worsened the phenotypes (Figure 5I and 5K). Transmission electron microscopy (TEM) sections from one day-old adult PINK1B9 mutant flies reveal that mitochondria in the indirect flight muscles are abnormally fused with one another and that the structures of the mitochondrial cristae are unclear (i.e. the cristae have lost the normal electron density seen by TEM) as compared to those of a dPINK1 revertant line (Figure 6A and 6D compared to Figure 3C and 3G). Importantly, the mitochondrial hyperfusion and loss of cristae usually observed in dPINK1 mutant animals can be partly suppressed by introduction of the PGAM5NP0568 or the PGAM51 allele (Figure 6B, 6E, and 6G). In sharp contrast, transgenic expression of dPGAM5 further promoted mitochondrial degeneration (Figure 6C and 6F). Similar results were obtained when mitochondria in DA neurons of the adult brain (Figure 6H, 6I, and 6J) and in the indirect muscle tissues (Figure S3) were visualized using a version of GFP with a mitochondrial targeting signal (mitoGFP). Mitochondrial morphology in DA neurons in wild-type flies showed a long tubular network in the cytoplasm (Figure 3L). As previously reported, DA neurons in PINK1B9 flies form spherical aggregates of mitochondria (Figure 6I). Removal of dPGAM5 from PINK1B9 flies led to an increase in the number of small fragmented or tubular mitochondria (Figure 6J). These results suggest that excessive mitochondrial aggregation, which is modulated by dPGAM5 inactivation, is indicative of a functional failure of mitochondria in DA neurons.
Fig. 5. Loss of dPGAM5 suppresses dPINK1 mutant phenotypes in Drosophila. 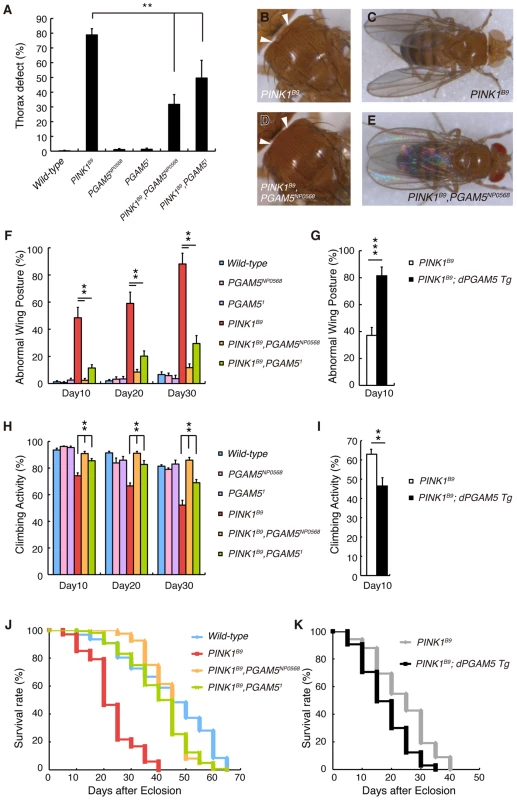
A thorax defect (B, arrowheads) and abnormal wing posture (C) caused by loss of dPINK1 activity are suppressed in dPGAM5 mutant genetic backgrounds (A, D and E). (F) Percentage of 10-, 20- and 30-day-old male flies showing abnormal wing postures. Error bars show S.E. from three experiments. (G) Percentage of 10-day-old male PINK1B9 and PINK1B9 ubiquitously overexpressing dPGAM5 flies showing abnormal wing postures. Error bars show S.E. from three experiments. (H, I) Percentage of locomotor activity. Error bars show S.E. from three repeated experiments. (J) Lifespan of adult male flies. Loss of dPGAM5 partially improved the reduced lifespan seen in PINK1B9 fly (PINK1B9 vs. PINK1B9, PGAM5NP0568 or PINK1B9, PGAM51, p<0.001; wild-type vs. PINK1B9, PGAM5NP0568 or PINK1B9, PGAM51, p<0.01 by the log rank test). (K) Lifespan of adult male PINK1B9 and PINK1B9 ubiquitously overexpressing dPGAM5 flies. Overexpression of dPGAM5 further reduced the lifespan (PINK1B9 vs. PINK1B9; dPGAM5 Tg, p<0.001). The same files were used in (A–F, H and J) and in (G, I and K). The genotypes and the number used in the assays are; wild-type (PINK1RV/Y, n = 161), PGAM5NP0568 (PGAM5NP0568/Y, n = 161), PGAM51 (PGAM51/Y, n = 161), PINK1B9 (PINK1B9/Y, n = 101), PINK1B9, PGAM5NP0568 (PINK1B9, PGAM5NP0568/Y, n = 162) and PINK1B9, PGAM51 (PINK1B9, PGAM51/Y, n = 160) in (A–F, H and J), PINK1B9 (PINK1B9/Y; Da-GAL4/+, n = 162) and PINK1B9, dPGAM5 Tg (PINK1B9/Y; Da-GAL4> UAS-dPGAM5, n = 161) in (G, I and K). Fig. 6. Loss of dPGAM5 improves degeneration of the mitochondria and DA neurons caused by dPINK1 inactivation in Drosophila. 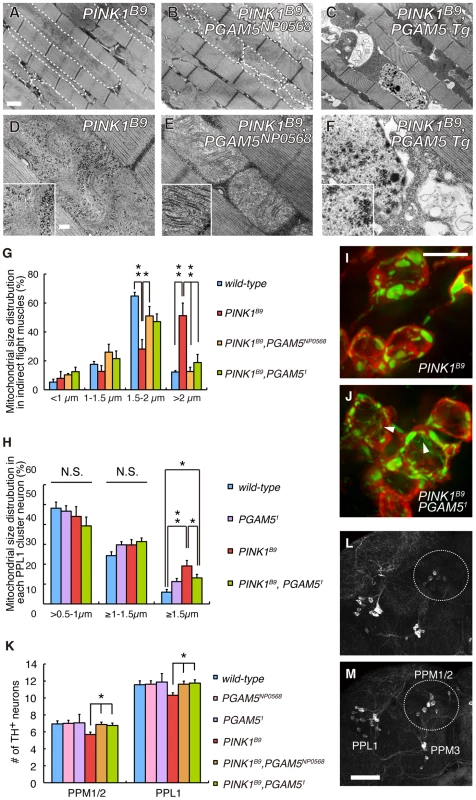
(A–F) TEM analysis of the indirect flight muscle and morphology of mitochondria in 2-day-old adult flies with the indicated genotypes. In A and B, some mitochondria are outlined with broken lines. The insets in D–F show representative mitochondria matrixes. Scale bars = 1 µm in A–C and 200 nm in D–F. (G) Quantification of the percentage of mitochondrial size distribution in the indirect muscle tissue from wild-type (n = 136 from 5 adult flies), PINK1B9 (n = 96 from 5), PINK1B9, PGAM5NP0568 (n = 116 from 5), PINK1B9, PGAM51 (n = 111 from 5) as shown in Figure 3K. Data are shown as means ± SE (* p<0.05, **p<0.01). (H–J) Quantification of the percentage of cytoplasmic mitochondrial aggregates with diameter of 0.5–1.0, 1.0–1.5 or ≥1.5 µm in each PPL1 TH+ neuron from wild-type (n = 373 from 18 adult flies), PGAM51 (n = 356 from 18), PINK1B9 (n = 231 from 11), PINK1B9PGAM51 flies (n = 235 from 13). Mitochondrial morphology was revealed by mitoGFP as shown in Figure 3L–3O. Data are shown as means ± SE (*, p<0.05;**, p<0.01; N.S., not significant). Tubular or reticular mitochondria were excluded from the estimation due to difficulty in the counting. However, the ratio of mitochondria with that morphology was also increased in PINK1B9PGAM51 flies (J) compared with that in PINK1B9 flies (I). Arrowheads in (J) indicate representative tubular or reticular mitochondria. Scale bar in (I) = 5 µm. (K) Quantification of TH+ DA neuron number in the PPM1, PPM2 and PPL1 clusters in 25-day-old males. PPM1 and PPM2 cluster neurons were counted together. Data are shown as means ± SE (*, p<0.05; n = 16). (L, M) Representative images of PPM1/2, PPM3 and PPL1 clusters of PINK1B9 (L) and PINK1B9, PGAM5NP0568 flies (M) visualized with anti-TH antibody. Scale bar in (M) = 50 µm. The genotypes are: PINK1RV/Y (wild-type), PINK1B9/Y (PINK1B9), PGAM5NP0568/Y (PGAM5NP0568), PGAM51/Y (PGAM51), PINK1B9, PGAM5NP0568/Y (PINK1B9, PGAM5NP0568), PINK1B9, PGAM51/Y (PINK1B9, PGAM51), PINK1B9/Y; Da-GAL4> dPGAM5 (PINK1B9, PGAM5 Tg), in (A–G, K–M), PINK1RV/Y; TH-GAL4> mitoGFP (wild-type), PINK1B9/Y; TH-GAL4> mitoGFP (PINK1B9), PGAM51/Y; TH-GAL4> mitoGFP (PGAM51), PINK1B9, PGAM51/Y; TH-GAL4> mitoGFP (PINK1B9, PGAM51) in (H–J). Consistent with the beneficial effects of dPGAM5 inactivation on the mitochondrial degeneration seen in PINK1B9 flies, we observed that dPGAM5 inactivation suppresses the loss of DA neurons in the protocerebral posterior lateral 1 (PPL1) and protocerebral posterior medial 1 and 2 (PPM1/2) clusters of aged flies (Figure 6K–6M).
Removal of dPGAM5 Fails to Suppress Phenotypes Resulting from dparkin Inactivation
Previous studies in Drosophila suggested that dPINK1 is genetically associated with dparkin and furthermore, that dPINK1 functions upstream of dparkin [7]–[9]. In addition, dparkin null mutations cause mitochondrial degeneration of a subset of tissues in Drosophila, which phenocopies dPINK1 inactivation [29], [30]. Given the evidence that PGAM5 is involved in the PINK1 pathway, we next asked if dPGAM5 also affects the in vivo mitochondrial phenotypes associated with mutations in dParkin. Introduction of PGAM5NP0568 in the parkin hypomorphic genetic background (parkinP21) had little effect on abnormal wing postures (Figure 7A–7C) [29], [30]. Consistent with the result in the wing phenotype, loss of dPGAM5 activity failed to rescue the age-dependent motor defects and shorter lifespan observed in parkinP21flies (Figure 7D and 7F). In the same settings, overexpression of dPGAM5 further enhanced both motor defect and reduced lifespan phenotype (Figure 7E and 7G). Loss of dParkin activity results in an elongated morphology in mitochondria of the adult indirect flight muscle tissues, a phenotype that was suppressed by loss of the dPGAM5 gene (Figure 7H, 7I, and 7L). However, the crista structures of the mitochondria were not restored by inactivation of the dPGAM5 gene (Figure 7J and 7K). Taken together, these data suggest that dPGAM5 lies genetically upstream of dparkin, or functions independently of dparkin downstream of dPINK1 in Drosophila.
Fig. 7. Disruption of dPGAM5 fails to suppress the mitochondrial phenotype caused by dParkin inactivation in Drosophila. 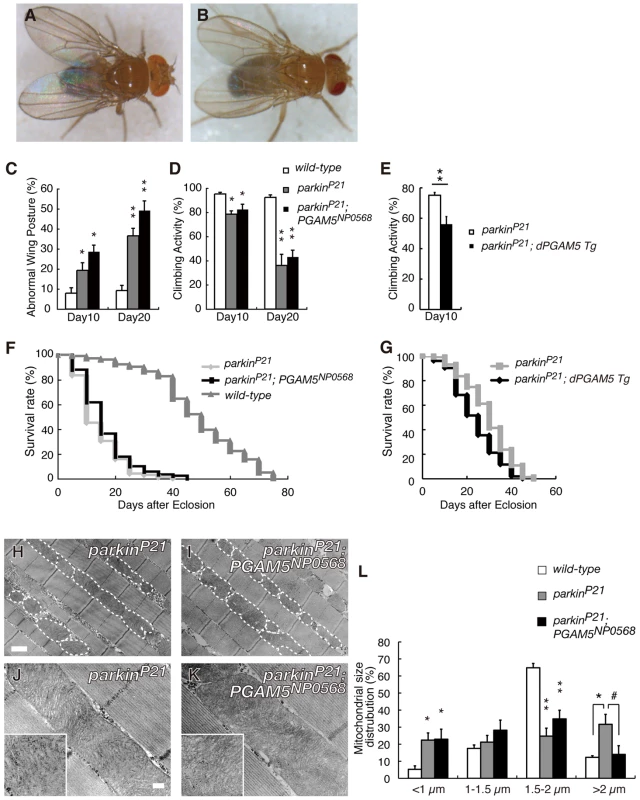
The abnormal wing posture caused by a homozygous dParkin mutation (A) was not suppressed by removal of the dPGAM5 gene (B). (C) Percentage of flies with abnormal wing posture among 10- and 20-day-old male wild-type (n = 105), parkinP21 (n = 102) and PGAM5NP0568; parkinP21 (n = 109) flies. Error bars show S.E. from three repeated experiments. *, p<0.05; **, p<0.01 vs. dParkin(+/+). (D) Percentage of flies showing locomotor activity among 10- and 20-day-old male parkinP21 (n = 86), parkinP21 (n = 73) and PGAM5NP0568; parkinP21 (n = 78) flies. Error bars show S.E. from twenty repeated experiments. *, p<0.05; **, p<0.01 vs. dParkin(+/+). (E) Locomotor activity of 10-day-old male parkinP21 (n = 153) and parkinP21 ubiquitously overexpressing dPGAM5 flies (parkinP21; dPGAM5 Tg, n = 155) flies. Error bars show S.E. from twenty repeated experiments. **, p<0.01. (F) Lifespan of adult male wild-type (n = 104), parkinP21 (n = 102) and PGAM5NP0568; parkinP21 (n = 91) flies. PGAM5NP0568; parkinP21 vs. parkinP21, p = 0.191; wild-type vs. parkinP21, p<0.001 by log rank test. (G) Lifespan of adult male parkinP21 (n = 153) and parkinP21; dPGAM5 Tg flies (n = 155) flies. parkinP21 vs. parkinP21; dPGAM5 Tg, p<0.001 by log rank test. (H–K) TEM analysis of the indirect flight muscle and mitochondrial morphology in tissue from flies of the indicated genotypes. The long tubular mitochondrial phenotype seen in parkinP21 flies can be rescued by dPGAM5 inactivation (H and I). However, the mitochondrial matrix still appears degenerated (insets in J and K). Scale bars = 1 µm in H and I and 200 nm in J and K. (L) Quantification of the percentage of mitochondrial size distribution in the indirect muscle tissue from wild-type (n = 136 from 5 adult flies), parkinP21 (n = 89 from 5) and parkinP21; PGAM5NP0568 flies (n = 84 from 5) as shown in (H–K). The length of the mitochondria in the direction of the myofibrils was measured. Data are shown as means ± SE (* p<0.05, **p<0.01 vs. wild-type; # p<0.05 vs. parkinP21; PGAM5NP0568). The genotypes are: +/Y (wild-type), +/Y; parkinP21/parkinP21 (parkinP21), PGAM5NP0568/Y; parkinP21/parkinP21 (parkinP21; PGAM5NP0568). Activation of a Redox Control Pathway Improves Viability of dPINK1 Mutant Flies
PGAM5 was previously reported to interact with Keap1 (Gene ID: 9817), a substrate adaptor protein for a Cullin-3-dependent E3 complex [17]. In a normal redox state, the Keap1 complex suppresses activity of a bZIP transcription factor, Nrf2, through ubiquitin/proteasome-dependent protein degradation [31]. Oxidative stress impairs inhibition of Nrf2 by Keap1 [31]. Nrf2 thus becomes stabilized and activates oxidative stress protective genes, restoring cellular redox homeostasis. Although we confirmed the association of PGAM5 with Keap1 in human cultured cells, the proposed Keap1-binding motif in PGAM5, NXESGE, was not conserved in dPGAM5 (Figure S1). On the other hand, Keap1/Nrf2 signaling does appear to be conserved in Drosophila [32]. We tested if Keap1/Nrf2 signaling modulates PINK1 phenotypes. Removal of a copy of the keap1 gene (Gene ID: 42062) in dPINK1 knockdown flies, wherein the dPINK1 RNAi was expressed in the muscle tissues, failed to rescue the abnormal wing posture (Figure 8A). However, Keap1 heterozygosity is beneficial to survival of aging dPINK1 knockdown files, supporting a previous report suggesting that oxidative stress is partly involved in the PINK1 pathology (Figure 8B) [33], [34].
Fig. 8. Reduction of Keap1 activity improves the lifespan of dPINK1 RNAi flies. 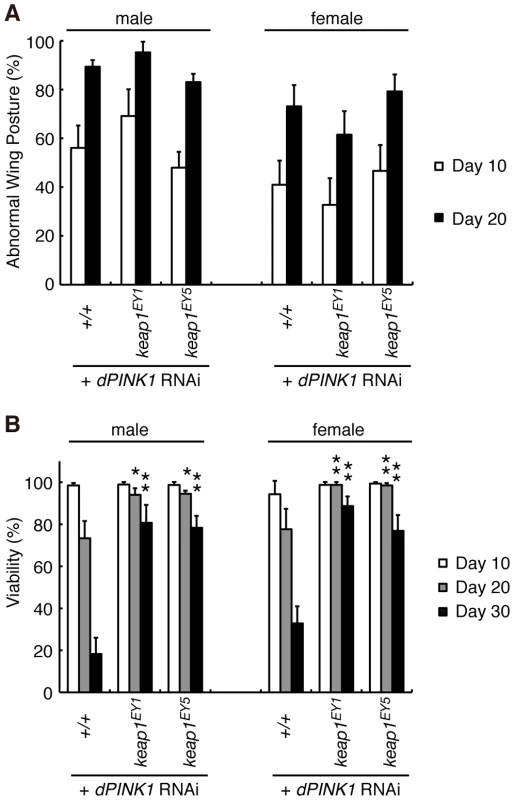
Removal of one copy of Drosophila keap1 had no effects on the wing phenotype of dPINK1 RNAi flies (A) but improved viability (B). *, p<0.05; **, p<0.01 vs. age-matched dPINK1 RNAi group. The genotypes are as follows: MHC-GAL4> dPINK1RNAi (+/+), MHC-GAL4> dPINK1RNAi/KeapEY1 (KeapEY1), MHC-GAL4> dPINK1RNAi/KeapEY5 (KeapEY5). Flies were raised at 29°C. Discussion
The event of fusion/fission is required for maintenance of a healthy mitochondrial population. Mitochondrial fusion is believed to require the interchange of a set of internal components, including copies of the mitochondrial genome, respiratory proteins and metabolic products. Mitochondrial fission has been proposed to play a role in disposal of damaged mitochondria, such as those with a reduced mitochondrial membrane potential, via mitophagy [35]. A role for PINK1 in the regulation of mitochondrial fission/fusion dynamics has recently been demonstrated in Drosophila [10]–[12]. The PINK1/Parkin pathway appears to promote fission and/or inhibits fusion, likely through an indirect mechanism. Indeed, loss of dPINK1 or dParkin produces swollen or enlarged mitochondria in tissues with high-energy demands, such as the muscles, which is suppressed by reduced fusion activity or increased fission activity after genetic manipulation of the mitochondrial fission/fusion machinery. Namely, either reducing the activity of the mitochondrial fusion proteins OPA1 and Mfn, or increasing the activity of a mitochondrial fission protein, Drp1, can partially rescue PINK1 and parkin mutant phenotypes.
We identified PGAM5 as a PINK1-binding protein and went on to show that dPGAM5 can modulate dPINK1 mutant phenotypes. Loss of dPGAM5 activity had little effect on the lifespan of a dPINK1 RNAi fly strain in our initial in vivo test (Figure 1C). However, we found that loss of dPGAM5 does significantly extend lifespan of PINK1B9 mutant flies (Figure 5J). We speculate that continuous expression of the short hairpin RNA in the RNAi-based test confers additional toxicity, leading to a shorter lifespan in a sequence-independent manner, such that the suppressive effect of dPGAM5 mutations cannot be detected in the PINK1 RNAi flies.
dPGAM5 appears to be dispensable for mitochondrial homeostasis in Drosophila, as overall, flies homozygous for a null allele of dPGAM5, PGAM51, appear to be normal. It has previously been reported that ectopic expression of PGAM5 leads to perinuclear aggregation or small fragmentation of mitochondria in mammalian cultured cells, which suggested that PGAM5 has a role in regulation of mitochondrial fission/fusion process or mobility [24]. Our study also observed alteration of mitochondrial morphology in Drosophila with different dPGAM5 activities. Transgenic expression of dPGAM5 or dPGAM5-2 leads to fragmentation of mitochondria both in the TH+ neurons and indirect flight muscles (Figure 3E, 3F, 3I and 3J). By contrast, dPGAM5 LOF moderately increases mitochondria with a longer tubular or a swollen morphology (Figure 3D and 3K). Our genetic tests with the known mitochondrial fusion/fission machinery suggested that PGAM5 acts upstream of them or in an independent pathway (Figure 4). Given that PGAM5 is involved in mitochondrial fission, loss of PGAM5 would be expected to enhance the PINK1 mutant phenotype in Drosophila, similar to the interaction between PINK1 and the mitochondrial fusion/fission machinery [10]–[12]. Interestingly, the number of large aggregated mitochondria, which are frequently seen in PINK1B9 flies, was mildly decreased in TH+ neurons of PINK1B9PGAM51 flies (Figure 6H–6J). Moreover, loss of dPGAM5 also modulated the mitochondrial morphology of dParkin mutant fly without suppressing the mitochondrial degeneration (Figure 7H–7L). Based on these observations, it could be speculated that PGAM5 does not directly regulate mitochondrial fission but instead, modulates the PINK1 pathway in a different way. Recent studies have proposed two different models for the mechanism of mitochondrial morphological changes through the PINK1/Parkin pathway in Drosophila and mammals. Ziviani et al. and Poole et al. have demonstrated that dParkin promotes degradation of Mfn in a dPINK1-dependent manner, which leads to mitochondrial fragmentation in Drosophila [16], [36]. Our current results demonstrated that the loss of dPGAM5 activity does not affect mitochondrial fragmentation caused by reduction of Mfn activity, suggesting that dPGAM5 might not contribute to the proposed PINK1/Parkin pathway (Figure 4). Sandebring et al. have proposed that accumulation of damaged mitochondria by PINK1 inactivation results in mitochondrial calcium efflux, which activates Drp1 through Calcineurin-mediated dephosphorylation of Drp1 in human cells [37]. This model well explains the observation that loss of PINK1 indirectly promotes mitochondrial fragmentation in mammalian cells and the indication that PINK1 is not a core component of the fusion/fission machinery in a Drosophila study [11]. However, most of Drosophila studies do not support a result that loss of PINK1 leads to mitochondrial fragmentation in mammals. Thus, it still remains a question for further investigation how PGAM5 modulates the mitochondrial dynamics.
Although the property of PGAM5 to physically interact with PINK1 appears to be conserved between human and Drosophila, the functional significance of this binding remains to be established. PINK1 and PGAM5 have kinase and phosphatase activities, respectively. However, there is no evidence to suggest that PINK1 directly phosphorylates PGAM5, or that PGAM5 dephosphorylates PINK1 (Figure 2G and 2H) [18], [25], [26]. The PINK1 protein levels are maintained at very low level under steady-state conditions by constitutive processing and subsequent degradation through the ubiquitin-proteasome pathway [15]. Recent studies suggested that PINK1 selectively translocates from cytosol to mitochondria with low membrane potential, at which PINK1 is stabilized [14], [15], [38]. The accumulated PINK1 on the depolarized mitochondria further recruits Parkin to induce mitophagy [13]–[15], [38]. However, Parkin does not seem to be the target of PINK1 kinase activity [15], and PINK1 does not seem to activate Parkin E3 activity directly (data not shown). Based on these findings, it is possible that PGAM5 may promote a selective recruitment of PINK1 to the outer membrane of the damaged mitochondria, or that PGAM5 may regulate PINK1 stabilization. Our molecular analysis, however, did not support the idea that PGAM5 stabilizes PINK1 (data not shown). In addition, because loss of dPGAM5 partially suppresses dPINK1 null phenotypes, it seems likely that PINK1 negatively regulates PGAM5 function (Figure 9). PGAM5 was originally identified as a Bcl-xL-binding protein, and in itself can be toxic to cells, promoting mitochondrial fragmentation, when expressed at high levels (Figure 3B). Therefore, it seems possible that PGAM5 modulates a cell protective or a mitochondrial morphogenetic activity of the Bcl-2 family member Bcl-xL downstream of PINK1 but in a pathway that is independent from Parkin (Figure 9A) [39]. In this context, PINK1 may suppress the cell toxic action of PGAM5 through an indirect mechanism where an unidentified substrate of PINK1 inactivates PGAM5. Interestingly, a recent report suggests that the dPINK1 phenotype can be partially suppressed by transgenic expression of Drosophila Bcl-2 protein Buffy [8]. Alternatively, PGAM5 may be one of components of a negative regulator complex against Parkin E3, downstream of PINK1 (Figure 9B). Matsuda et al. have reported that Parkin E3 activity is activated only at the depolarized mitochondria, suggesting the existence of its negative regulator(s) [38]. E3 activity of Parkin may be released when PINK1 associates with the negative regulator complex via PGAM5 and suppresses its function by phosphorylation of another complex component(s). This idea might be partly supported by our observation that loss of dPGAM5 had little effect on dparkin mutant flies.
Fig. 9. Schematic of the proposed PINK1/PGAM5 pathways in Drosophila. 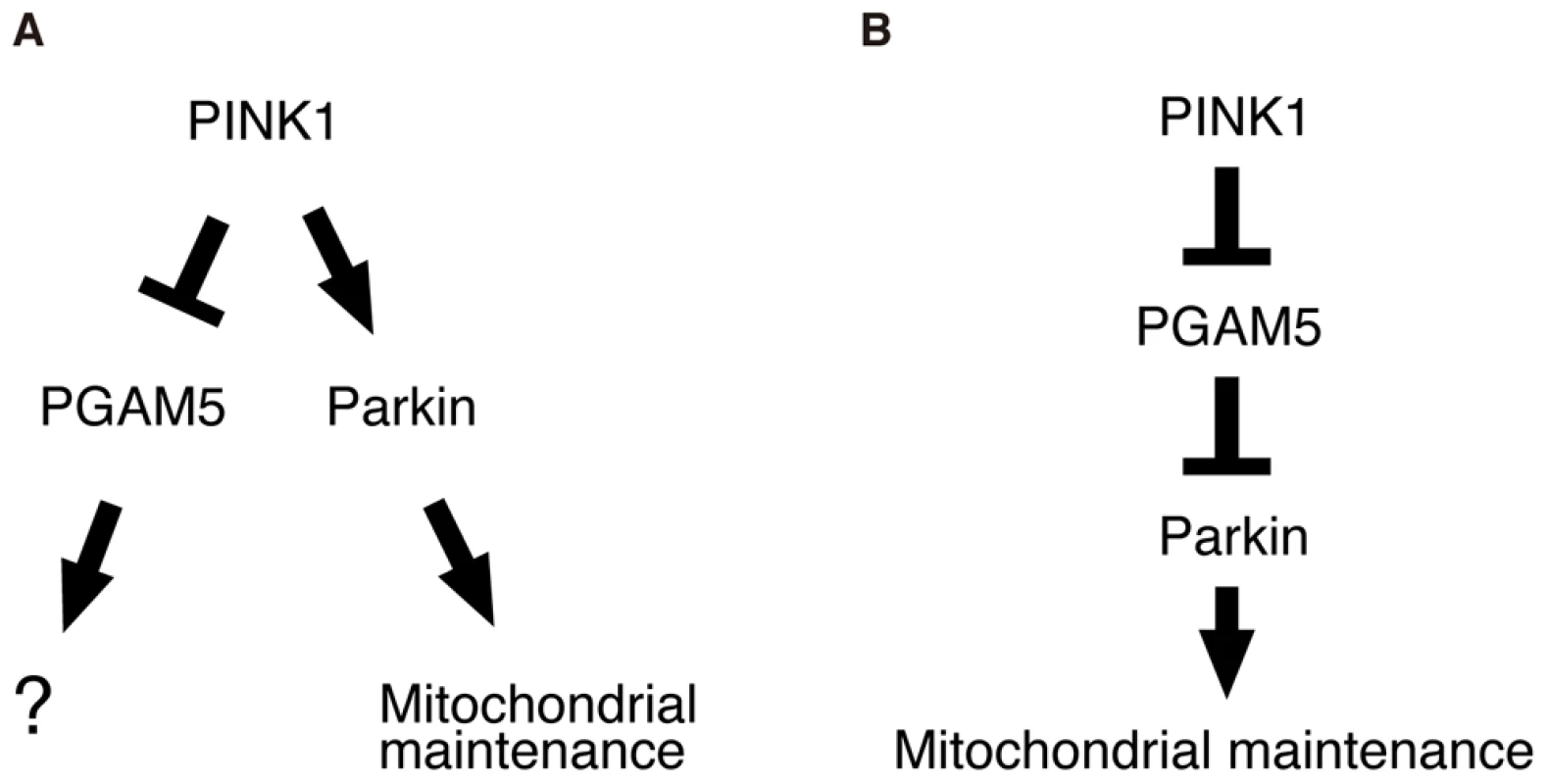
(A) PGAM5 has a role in mitochondrial activities independently of Parkin downstream of PINK1. (B) PGAM5 negatively regulates Parkin downstream of PINK1. Although the primary cause of the mitochondrial degeneration by the loss of PINK1 remains less obvious, the growing evidence suggests that PINK1 eliminates oxidatively damaged mitochondria in cooperation with Parkin, the failure of which leads to tissue degeneration. Supporting for this hypothesis, the mitochondrial phenotypes of dPINK1 and dParkin mutant flies are primarily exhibited in similar tissues that require higher energy demands [7]–[9]. Although dPAGM5 might not regulate Keap1 function in Drosophila, the Keap1/Nrf2 pathway appears to be conserved in Drosophila [32], and activation of the Keap1/Nrf2 pathway by genetic manipulation effectively suppressed the short-lifespan phenotype by dPINK1 inactivation. This finding may also support the above idea that the accumulation of oxidatively damaged mitochondria leads to degeneration of specific tissues, providing a hint of therapeutic strategies for PINK1-associated PD.
In conclusion, the results of our genetic study demonstrate that the mitochondrial-localized protein PGAM5 modulates the PINK1 pathway in Drosophila. However, further work will be required to determine how PGAM5 regulates the PINK1 pathway at the molecular level, as well as to determine if manipulation of PGAM5 activity might provide a therapeutic advantage in treatment of PINK1-associated PD.
Materials and Methods
Purification of PINK1-Binding Proteins
HEK293 cells stably expressing hPINK1-FLAG or parent cells were grown in suspension culture (Joklik-modified Eagle's minimum essential medium with 5% fetal bovine serum). The cell pellet (2.4×108 cells) was homogenized in lysis buffer (50 mM Tris pH 7.4, 120 mM NaCl, 5 mM EDTA, 10% glycerol, 1% Trion-X100) supplemented with Complete Protease Inhibitors (Roche Diagnostics). The soluble fraction of the suspension was immunoprecipitated with anti-FLAG M2 agarose (Sigma-Aldrich) and washed five times in lysis buffer. The fractions eluted with 200 µg/ml 3x FLAG peptide were resolved by SDS-PAGE. Specific bands detected by silver staining were excised for in-gel digestion. The digest extracted from the gel was subjected to online HPLC-MS/MS, followed by informatics-based identification of the proteins (Nippon Proteomics).
Drosophila Genetics
Fly culture and crosses were performed on standard fly food containing yeast, cornmeal and molasses, and flies were raised at 25°C unless otherwise indicated. To generate UAS-dPGAM5 transgenic lines, cDNA for dPGAM5 and dPGAM5-2 obtained by RT-PCR from adult Drosophila total RNA was subcloned into the pUAST vector. Introduction of transgenes into Drosophila germ line and establishment of transgenic lines into a w– background were performed by BestGene Inc. (Chino Hills, CA). A P-element insertion line for dPGAM5 mutant, PGAM5NP0568 obtained from Kyoto Drosophila Genetic Resource Center, expresses a reduced level (∼25%) of mRNA, in which NP0568 element is integrated 50 bp downstream of the dPGAM5 translational start site (Figure S1B). We could not detect any dPGAM5 protein signal in PGAM5NP0568 homozygous flies (Figure S1C). The PGAM5NP0568 line was backcrossed to w– for six generations to remove background mutations, and used for most experiments as a dPGAM5 mutant line. UAS-dPGAM5 RNAi (VDRC 51655) and UAS-mfn RNAi (VDRC105261 and VDRC40478) strains were obtained from the Vienna Drosophila RNAi Center. To generate PGAM51, the KG09727 P-element insertion (obtained from the Bloomington Drosophila Stock Center) was mobilized using Δ2–3 transposase, and the entire dPGAM5 coding region was deleted by imprecise excision, as shown in Figure S1A. All other fly stocks and GAL4 lines used in this study were obtained from the Bloomington Drosophila Stock Center and have been previously described: UAS-dPINK1 RNAi [9]; PINK1B9 and revertant PINK1RV [8]; parkinP21 [30]; keap1EY1and keap1EY5 [32]; Drp12 and Drp1+[40].
RT-PCR and Plasmids
For quantitative RT-PCR analysis, reverse transcription and PCR reactions with total RNA extracted from fly heads were performed using a Superscript VILO cDNA synthesis kit (Invitrogen) and SYBR GreenER qPCR SuperMix (Invitrogen), respectively. Full-length cDNAs corresponding to hPGAM5 (GenBank NP_001170543) and a short isoform of hPGAM5 (hPGAM5-S, GenBank NM_138575) were amplified by RT-PCR from total RNA purified from HEK293 cells or a cDNA clone (RIKEN clone ID: IRAK003D15), and was cloned in pcDNA3-Myc, pGEX6P-1 and pGEX4T-1 vectors. Expression plasmids for hPINK1-FLAG and pUAST-dPINK1-Myc have been reported elsewhere [9], [20].
Antibodies
Rabbit anti-human PGAM5 polyclonal antibody was raised against recombinant GST-tagged PGAM5 domain (89–289 aa) produced in the E. coli strain BL21(DE3)pLysS (Novagen), and was affinity-purified against the antigen. Rabbit anti-dPGAM5 polyclonal antibody was raised against the peptide ELLTNRIPRDVKNVV. Anti-hPINK1 antibody (BC100-494), anti-Myc (4A6) and anti-FLAG (M2) antibodies were purchased from Novus, Millipore and Sigma-Aldrich, respectively. Mouse anti-TH monoclonal antibody was purchased from ImmunoStar, and rabbit anti-Drosophila TH polyclonal antibody was described previously [9].
Cell Culture, Immunoprecipitation, and Immunoblot Analysis
Transfection of mammalian cultured cell, immunopurification of FLAG-protein from transfected cell lysate and immunoblot analysis was performed as described previously [4], [41]. For the hPGAM5 RNAi experiment, HEK293 cell lysate transfected with 20 µM stealth RNAi reagent against hPGAM5 or a control RNAi reagent (Invitrogen), was analyzed 72 hrs after transfection. To detect an endogenous interaction between PINK1 and PGAM5, we treated HEK293 cells (2×107 cells) with 20 µM carbonyl cyanide 3-chlorophenylhydrazone for 24 hrs to induce a sufficient level of human PINK1 protein for the study. The treated cells were subjected to immunoprecipitation using a Rabbit TrueBlot kit combined with rabbit anti-human PINK1 or rabbit anti-Delta (Santa Cruz) as a species-matched control. For the preparation of fly samples for immunoblot analysis, fly heads were directly homogenized in 20 µl/head of SDS sample buffer using a motor-driven pestle. After centrifugation at 16,000 g for 10 min, the supernatant was used in SDS-PAGE.
In Vitro Phosphorylation Assay
Recombinant 2x GST-dPINK1 (153–709 aa), which has an N-terminal GST-tag and a C-terminal GST/6x His tag, was produced in the E. coli strain pG-KJE8/BL21 (TAKARA) and purified by a sequential purification with Ni-NTA agarose and glutathione sepharose. GST-hPGAM5-S (1–255 aa) and GST-hPGAM5 (1–289 aa) were incubated with 2x GST-dPINK1 as described in Figure 2.
Whole-Mount Immunostaining and Transmission Electron Microscopic (TEM) Analysis
Counting of TH-positive neurons was performed by whole-mount immunostaining of brain samples as described previously [9]. TEM images were obtained at the Biomedical Research Core of Tohoku University Graduate School of Medicine. All histochemical analyses were performed using DeltaVision microscope system (Applied Precision) or LSM5 PASCAL laser scanning microscope system (Carl Zeiss). The images obtained by DeltaVision system were deconvolved through 10 iterations using the DeltaVision deconvolution software (Applied Precision). Area calculation of the mitochondria was performed following established criteria for classification [12] using softWoRx (Applied Precision) or Image J software from the US National Institute of Health (http://rsb.info.nih.gov/ij/).
Lifespan Assay and Quantification of Wing Phenotypes and Climbing Ability
For lifespan studies, twenty female adult flies per vial were maintained at 25°C, transferred to fresh fly food, and scored for survival every 4 or 5 days. To control for isogeny, the PGAM5NP0568, PINK1B9 and PGAM51, PINK1B9 alleles were backcrossed to PINK1B9 for six generations, and parkinP21 and PGAM5NP0568; parkinP21 were backcrossed to w− wild-type background for six generations, UAS-dPGAM5 transgenic flies were generated in the w− genetic background and thus have matched genetic backgrounds. The lifespan of PGAM51 was compared in the y− genetic background. The number of flies exhibiting defective abnormal wing posture (held-up or drooped) was determined for each genotype [9]. A climbing assay was performed as described previously [42].
Statistical Analysis
One-way repeated measures ANOVA was performed to determine significant differences among multiple groups unless otherwise indicated. If a significant result was achieved (p<0.05), the mean of the control and the specific test groups was analyzed using the Tukey-Kramer test. For lifespan assays, the Kaplan-Meier analysis with log-rank test was performed.
Supporting Information
Zdroje
1. KitadaT
AsakawaS
HattoriN
MatsumineH
YamamuraY
1998 Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392 605 608
2. ValenteEM
Abou-SleimanPM
CaputoV
MuqitMM
HarveyK
2004 Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 304 1158 1160
3. UnokiM
NakamuraY
2001 Growth-suppressive effects of BPOZ and EGR2, two genes involved in the PTEN signaling pathway. Oncogene 20 4457 4465
4. ImaiY
SodaM
TakahashiR
2000 Parkin suppresses unfolded protein stress-induced cell death through its E3 ubiquitin-protein ligase activity. J Biol Chem 275 35661 35664
5. ShimuraH
HattoriN
KuboS
MizunoY
AsakawaS
2000 Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet 25 302 305
6. ZhangY
GaoJ
ChungKK
HuangH
DawsonVL
2000 Parkin functions as an E2-dependent ubiquitin - protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc Natl Acad Sci U S A 97 13354 13359
7. ClarkIE
DodsonMW
JiangC
CaoJH
HuhJR
2006 Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441 1162 1166
8. ParkJ
LeeSB
LeeS
KimY
SongS
2006 Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441 1157 1161
9. YangY
GehrkeS
ImaiY
HuangZ
OuyangY
2006 Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci U S A 103 10793 10798
10. PooleAC
ThomasRE
AndrewsLA
McBrideHM
WhitworthAJ
2008 The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A 105 1638 1643
11. DengH
DodsonMW
HuangH
GuoM
2008 The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A 105 14503 14508
12. YangY
OuyangY
YangL
BealMF
McQuibbanA
2008 Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci U S A 105 7070 7075
13. Vives-BauzaC
ZhouC
HuangY
CuiM
de VriesRL
2010 PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A 107 378 383
14. GeislerS
HolmstromKM
SkujatD
FieselFC
RothfussOC
2010 PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 12 119 131
15. NarendraDP
JinSM
TanakaA
SuenDF
GautierCA
2010 PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 8 e1000298 doi:10.1371/journal.pbio.1000298
16. ZivianiE
TaoRN
WhitworthAJ
2010 Drosophila Parkin requires PINK1 for mitochondrial translocation and ubiquitinates Mitofusin. Proc Natl Acad Sci U S A 107 5018 5023
17. LoSC
HanninkM
2006 PGAM5, a Bcl-XL-interacting protein, is a novel substrate for the redox-regulated Keap1-dependent ubiquitin ligase complex. J Biol Chem 281 37893 37903
18. TakedaK
KomuroY
HayakawaT
OguchiH
IshidaY
2009 Mitochondrial phosphoglycerate mutase 5 uses alternate catalytic activity as a protein serine/threonine phosphatase to activate ASK1. Proc Natl Acad Sci U S A 106 12301 12305
19. WeihofenA
OstaszewskiB
MinamiY
SelkoeDJ
2008 Pink1 Parkinson mutations, the Cdc37/Hsp90 chaperones and Parkin all influence the maturation or subcellular distribution of Pink1. Hum Mol Genet 17 602 616
20. MoriwakiY
KimYJ
IdoY
MisawaH
KawashimaK
2008 L347P PINK1 mutant that fails to bind to Hsp90/Cdc37 chaperones is rapidly degraded in a proteasome-dependent manner. Neurosci Res 61 43 48
21. LinW
KangUJ
2008 Characterization of PINK1 processing, stability, and subcellular localization. J Neurochem 106 464 474
22. TakatoriS
ItoG
IwatsuboT
2008 Cytoplasmic localization and proteasomal degradation of N-terminally cleaved form of PINK1. Neurosci Lett 430 13 17
23. ClancyDJ
GemsD
HarshmanLG
OldhamS
StockerH
2001 Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 292 104 106
24. LoSC
HanninkM
2008 PGAM5 tethers a ternary complex containing Keap1 and Nrf2 to mitochondria. Exp Cell Res 314 1789 1803
25. BeilinaA
Van Der BrugM
AhmadR
KesavapanyS
MillerDW
2005 Mutations in PTEN-induced putative kinase 1 associated with recessive parkinsonism have differential effects on protein stability. Proc Natl Acad Sci U S A 102 5703 5708
26. SilvestriL
CaputoV
BellacchioE
AtorinoL
DallapiccolaB
2005 Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum Mol Genet 14 3477 3492
27. OkamotoK
ShawJM
2005 Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet 39 503 536
28. ChanDC
2006 Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 22 79 99
29. GreeneJC
WhitworthAJ
KuoI
AndrewsLA
FeanyMB
2003 Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci U S A 100 4078 4083
30. PesahY
PhamT
BurgessH
MiddlebrooksB
VerstrekenP
2004 Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development 131 2183 2194
31. NguyenT
NioiP
PickettCB
2009 The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284 13291 13295
32. SykiotisGP
BohmannD
2008 Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell 14 76 85
33. MaiS
KlinkenbergM
AuburgerG
Bereiter-HahnJ
JendrachM
2010 Decreased expression of Drp1 and Fis1 mediates mitochondrial elongation in senescent cells and enhances resistance to oxidative stress through PINK1. J Cell Sci 123 917 926
34. GandhiS
Wood-KaczmarA
YaoZ
Plun-FavreauH
DeasE
2009 PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell 33 627 638
35. TwigG
ElorzaA
MolinaAJ
MohamedH
WikstromJD
2008 Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27 433 446
36. PooleAC
ThomasRE
YuS
VincowES
PallanckL
2010 The mitochondrial fusion-promoting factor mitofusin is a substrate of the PINK1/parkin pathway. PLoS ONE 5 e10054 doi:10.1371/journal.pone.0010054
37. SandebringA
ThomasKJ
BeilinaA
van der BrugM
ClelandMM
2009 Mitochondrial alterations in PINK1 deficient cells are influenced by calcineurin-dependent dephosphorylation of dynamin-related protein 1. PLoS ONE 4 e5701 doi:10.1371/journal.pone.0005701
38. MatsudaN
SatoS
ShibaK
OkatsuK
SaishoK
2010 PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 189 211 221
39. BermanSB
ChenYB
QiB
McCafferyJM
RuckerEB3rd
2009 Bcl-x L increases mitochondrial fission, fusion, and biomass in neurons. J Cell Biol 184 707 719
40. VerstrekenP
LyCV
VenkenKJ
KohTW
ZhouY
2005 Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 47 365 378
41. ImaiY
SodaM
InoueH
HattoriN
MizunoY
2001 An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 105 891 902
42. ImaiY
GehrkeS
WangHQ
TakahashiR
HasegawaK
2008 Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J 27 2432 2443
43. KinoshitaE
Kinoshita-KikutaE
TakiyamaK
KoikeT
2006 Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol Cell Proteomics 5 749 757
Štítky
Genetika Reprodukční medicína
Článek Genome-Wide Interrogation of Mammalian Stem Cell Fate Determinants by Nested Chromosome DeletionsČlánek Season of Conception in Rural Gambia Affects DNA Methylation at Putative Human Metastable EpiallelesČlánek A Quantitative Systems Approach Reveals Dynamic Control of tRNA Modifications during Cellular StressČlánek Reduction of Protein Translation and Activation of Autophagy Protect against PINK1 Pathogenesis inČlánek Cleavage of Phosphorothioated DNA and Methylated DNA by the Type IV Restriction Endonuclease ScoMcrAČlánek Competitive Repair by Naturally Dispersed Repetitive DNA during Non-Allelic Homologous Recombination
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 12- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Akutní intermitentní porfyrie
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Genome-Wide Interrogation of Mammalian Stem Cell Fate Determinants by Nested Chromosome Deletions
- Whole-Genome and Chromosome Evolution Associated with Host Adaptation and Speciation of the Wheat Pathogen
- Association of Variants at 1q32 and with Ankylosing Spondylitis Suggests Genetic Overlap with Crohn's Disease
- Initiator Elements Function to Determine the Activity State of BX-C Enhancers
- Identification of Genes Required for Neural-Specific Glycosylation Using Functional Genomics
- A Young Duplicate Gene Plays Essential Roles in Spermatogenesis by Regulating Several Y-Linked Male Fertility Genes
- The EpsE Flagellar Clutch Is Bifunctional and Synergizes with EPS Biosynthesis to Promote Biofilm Formation
- Histone H2A C-Terminus Regulates Chromatin Dynamics, Remodeling, and Histone H1 Binding
- Season of Conception in Rural Gambia Affects DNA Methylation at Putative Human Metastable Epialleles
- A Quantitative Systems Approach Reveals Dynamic Control of tRNA Modifications during Cellular Stress
- GC-Rich Sequence Elements Recruit PRC2 in Mammalian ES Cells
- A Single Enhancer Regulating the Differential Expression of Duplicated Red-Sensitive Opsin Genes in Zebrafish
- Investigation and Functional Characterization of Rare Genetic Variants in the Adipose Triglyceride Lipase in a Large Healthy Working Population
- Reduction of Protein Translation and Activation of Autophagy Protect against PINK1 Pathogenesis in
- Noisy Splicing Drives mRNA Isoform Diversity in Human Cells
- The Loss of PGAM5 Suppresses the Mitochondrial Degeneration Caused by Inactivation of PINK1 in
- Thymus-Associated Parathyroid Hormone Has Two Cellular Origins with Distinct Endocrine and Immunological Functions
- An ABC Transporter Mutation Is Correlated with Insect Resistance to Cry1Ac Toxin
- Role of Individual Subunits of the CSN Complex in Regulation of Deneddylation and Stability of Cullin Proteins
- The C-Terminal Domain of the Bacterial SSB Protein Acts as a DNA Maintenance Hub at Active Chromosome Replication Forks
- The DNA Damage Response Pathway Contributes to the Stability of Chromosome III Derivatives Lacking Efficient Replicators
- Cleavage of Phosphorothioated DNA and Methylated DNA by the Type IV Restriction Endonuclease ScoMcrA
- LaeA Control of Velvet Family Regulatory Proteins for Light-Dependent Development and Fungal Cell-Type Specificity
- Competitive Repair by Naturally Dispersed Repetitive DNA during Non-Allelic Homologous Recombination
- Distinct Functions for the piRNA Pathway in Genome Maintenance and Telomere Protection
- MOS11: A New Component in the mRNA Export Pathway
- Self-Mating in the Definitive Host Potentiates Clonal Outbreaks of the Apicomplexan Parasites and
- A Role for ATF2 in Regulating MITF and Melanoma Development
- Ancestral Regulatory Circuits Governing Ectoderm Patterning Downstream of Nodal and BMP2/4 Revealed by Gene Regulatory Network Analysis in an Echinoderm
- Cancer and Neurodegeneration: Between the Devil and the Deep Blue Sea
- Functional Comparison of Innate Immune Signaling Pathways in Primates
- Linking Crohn's Disease and Ankylosing Spondylitis: It's All about Genes!
- Genomics Meets Glycomics—The First GWAS Study of Human N-Glycome Identifies HNF1α as a Master Regulator of Plasma Protein Fucosylation
- Continuous and Periodic Expansion of CAG Repeats in Huntington's Disease R6/1 Mice
- Expression of Linear and Novel Circular Forms of an -Associated Non-Coding RNA Correlates with Atherosclerosis Risk
- Endocytic Sorting and Recycling Require Membrane Phosphatidylserine Asymmetry Maintained by TAT-1/CHAT-1
- Histone Deacetylases Suppress CGG Repeat–Induced Neurodegeneration Via Transcriptional Silencing in Models of Fragile X Tremor Ataxia Syndrome
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Functional Comparison of Innate Immune Signaling Pathways in Primates
- Expression of Linear and Novel Circular Forms of an -Associated Non-Coding RNA Correlates with Atherosclerosis Risk
- Genome-Wide Interrogation of Mammalian Stem Cell Fate Determinants by Nested Chromosome Deletions
- Histone H2A C-Terminus Regulates Chromatin Dynamics, Remodeling, and Histone H1 Binding
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



