-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Regulates Tissue-Specific Mitochondrial DNA Segregation
Mitochondrial DNA (mtDNA) sequence variants segregate in mutation and tissue-specific manners, but the mechanisms remain unknown. The segregation pattern of pathogenic mtDNA mutations is a major determinant of the onset and severity of disease. Using a heteroplasmic mouse model, we demonstrate that Gimap3, an outer mitochondrial membrane GTPase, is a critical regulator of this process in leukocytes. Gimap3 is important for T cell development and survival, suggesting that leukocyte survival may be a key factor in the genetic regulation of mtDNA sequence variants and in modulating human mitochondrial diseases.
Published in the journal: . PLoS Genet 6(10): e32767. doi:10.1371/journal.pgen.1001161
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001161Summary
Mitochondrial DNA (mtDNA) sequence variants segregate in mutation and tissue-specific manners, but the mechanisms remain unknown. The segregation pattern of pathogenic mtDNA mutations is a major determinant of the onset and severity of disease. Using a heteroplasmic mouse model, we demonstrate that Gimap3, an outer mitochondrial membrane GTPase, is a critical regulator of this process in leukocytes. Gimap3 is important for T cell development and survival, suggesting that leukocyte survival may be a key factor in the genetic regulation of mtDNA sequence variants and in modulating human mitochondrial diseases.
Introduction
Mammalian mitochondrial DNA (mtDNA) is a maternally inherited high copy genome. Copy number ranges from 102 to 105 depending upon the cell type, and typically, there is a single haplotype or sequence variant in a cell (homoplasmy) [1]–[3]. Germline or somatic cell mutations in mtDNA lead to the co-occurrence of two or more sequence variants in a cell, a state known as heteroplasmy. In the absence of selection, the segregation of mtDNA sequence variants can be modeled as a random walk using two parameters: copy number and rate of turnover [4]. However, in some cases there is preferential selection for one mtDNA sequence variant over another, which depends upon the variant, tissue, and nuclear background.
Most human pathogenic mtDNA mutations are heteroplasmic, and typically oxidative phosphorylation function is impaired when the proportion of mutant mtDNA exceeds a critical threshold in the cell [5], [6], leading to a wide spectrum of clinical disorders, generally affecting tissues with a high aerobic demand [1]. Transmission of most mutations through the female germline is stochastic [7]; however, in somatic tissues, mtDNA mutations can have skewed segregation patterns depending upon the mutation, tissue, and pedigree [6]–[14]. For instance, there is negative selection for the A3243G mutation in tRNAleu usually associated with MELAS (Mitochondrial Encephalomyopathy, Lactic Acidosis, Stroke-like episodes) in peripheral blood, but not in other tissues [15], [16]. However, this segregation pattern is not observed for other mitochondrial tRNA mutations, such as A8344G associated with MERRF (Myoclonic Epilepsy with Ragged-red fibers) [13], [17]. Thus, while both tRNA mutations impair mitochondrial translation, genetically these mutations are treated differently in the same cell types. To investigate the molecular basis for tissue-specific mtDNA segregation, we have used a heteroplasmic mouse model segregating two neutral mtDNA haplotypes derived from two old inbred mouse strains, BALB and NZB [18]. Transmission of these haplotypes through the female germline is neutral [18]; however, in post-natal life, the BALB mtDNA haplotype accumulates in hematopoietic tissues, while in the kidney and liver there is selection for the NZB haplotype [19]. In every other tissue investigated there is no preference for either mtDNA haplotype. The mechanisms for this mtDNA selection between tissues are apparently completely different [20], [21]. Previously, we demonstrated that nuclear-encoded genes regulate this selection process and mapped the quantitative trait loci (QTL) involved [22]. Further, we showed that selection for the BALB mtDNA haplotype in hematopoietic tissues can be completely eliminated in certain nuclear backgrounds [21]. In this study, we show that Gimap3 is a critical gene for regulating mtDNA segregation hematopoietic tissues in this model.
Results
Selection for the BALB mtDNA haplotype in hematopoietic tissues with age is rapid, proportional to the starting heteroplasmy level, and can be modeled as an exponential function [21]. The phenotype is robust, being found in a number of Mus musculus domesticus strains (DBA, 129Sv, NZB, C3H, C57BL/6J). In contrast, on the CAST/Ei mouse nuclear background, selection for the BALB mtDNA haplotype in hematopoietic tissues is completely abolished [21], suggesting that a combination of nuclear genes can completely regulate this process. To identify the genetic basis underlying this binary mtDNA segregation phenotype in hematopoietic tissues, we outcrossed heteroplasmic BALB/c females with CAST/Ei males to generate an F2 intercross (BALB/c X CAST/Ei). Mice were grouped into two phenotypes, based on either the absence or presence of mtDNA selection in the spleen (Figure 1). Mice were classified as having no mtDNA selection, if the % NZB mtDNA in hematopoietic tissues was similar to that of neutral tissues ie. those in which only random segregation is observed. All other mice were classified as positive for mtDNA selection, regardless of the rate of selection. In F2 mice, we found age-dependent regulation of this mtDNA segregation phenotype. At three months of age, approximately 40% of the F2 mice showed no mtDNA selection in the spleen, while at 12 months of age only 6% of F2 mice maintained the same phenotype. There was no difference between males and females.
Fig. 1. MtDNA segregation in hematopoietic tissues of 12-month-old heteroplasmic F2 (BALB/c X CAST/Ei) mice. 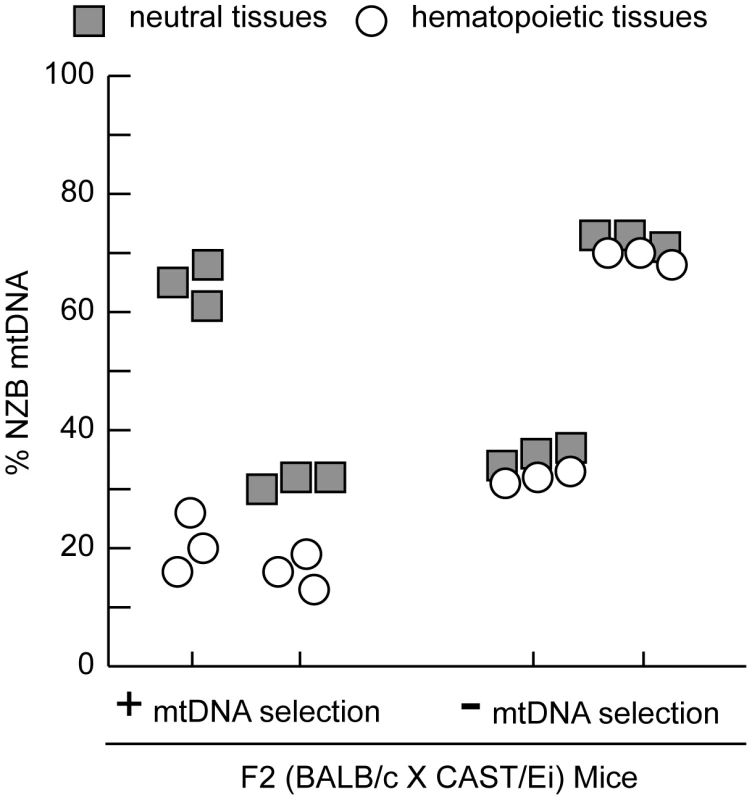
A representative profile of mtDNA heteroplasmy levels in hematopoietic (spleen, peripheral blood, and bone marrow) and neutral tissues (heart, brain and skeletal muscle) from four 12-month-old F2 (BALB/c X CAST/Ei) mice illustrates the mtDNA segregation phenotypes. Mice were classified as having no (-) mtDNA selection if the % NZB mtDNA in hematopoietic tissues was similar to neutral tissues or having (+) mtDNA selection. Data is presented from mice with high (>60%) or moderate (35%) levels of NZB heteroplasmy in their neutral tissues. Clearly the genetic regulation of this binary mtDNA segregation phenotype is complex, yet we reasoned that at 12 months of age, two fully penetrant recessive loci could account for the frequency of such a phenotype. We performed a genome-wide linkage scan on 12 month old F2 mice (n = 168) using 680 SNPs to map loci regulating the absence of mtDNA segregation. We identified an 11 Mb interval on chromosome 6 (37.4–48.99 Mb) significantly linked to the loss of mtDNA selection (LOD 4.6, genome-wide p = 0.007 with 10, 000 permutations, Figure 2). No other loci across the genome reached statistically significant levels after the permutation analysis. However, we did detect two suggestive loci (p<0.63) [23], one on chromosome 11 (p = 0.310) and another on chromosome 13 (p = 0.557). We had previously mapped this same chromosome 6 locus as Smdq - 3, a QTL controlling the rate of mtDNA selection in the spleen at 12 months of age [22]. Together, these results confirms that chromosome 6 contains a gene(s) critical for the regulation of mtDNA segregation in hematopoietic tissues.
Fig. 2. A chromosome 6 locus significantly affects mtDNA segregation in hematopoietic tissues. 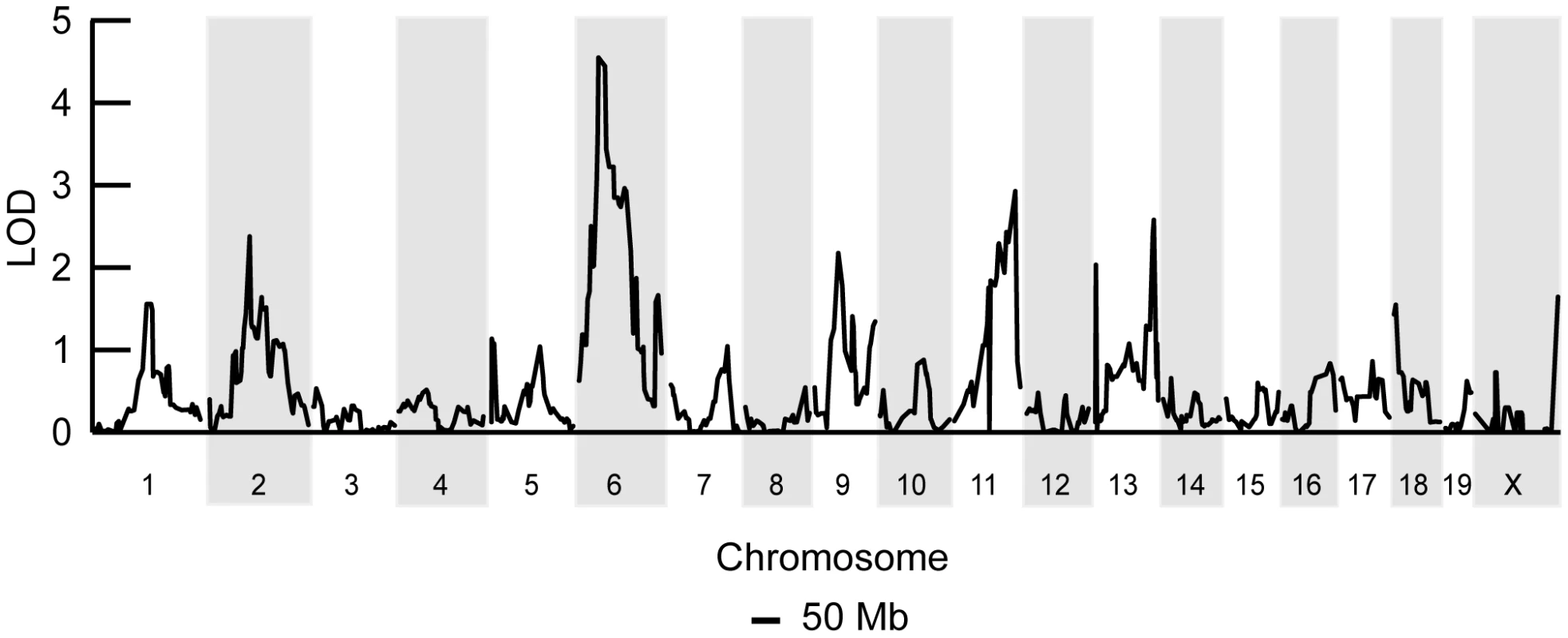
Genome-wide linkage analysis from 168 F2 (BALB/c X CAST/Ei) mice searching for loci regulating the loss of mtDNA selection. Only the chromosome 6 locus was significantly linked to this phenotype (LOD 4.6, genome-wide p = 0.007 with 10,000 permutations). To identify candidate genes within this 11 Mb interval, we searched for those annotated with a putative role in mitochondrial biology (GO:0005739 - mitochondrion) and found six matching this criterion (Table 1). Evaluating candidate genes for a tissue-specific role in mitochondrial biology is a difficult process, because most mitochondrial genes tend to be ubiquitously expressed. Gstk1, Ndufb2 and Mrps33 are ubiquitously expressed, and the latter two would presumably affect oxidative phosphorylation function. However, we and others have shown that there is no difference in respiratory chain function between NZB and BALB mtDNA haplotypes [20], [24]. Little is known of the putative kinase Adck2. In contrast, Gimap3 and Gimap5, paralogues with 84% identity at the amino acid level (Figure 3A), have immune-related functions and make particularly attractive candidate genes because the mtDNA segregation phenotype occurs only in hematopoietic tissues.
Fig. 3. Gimap3 and Gimap5 protein sequences. 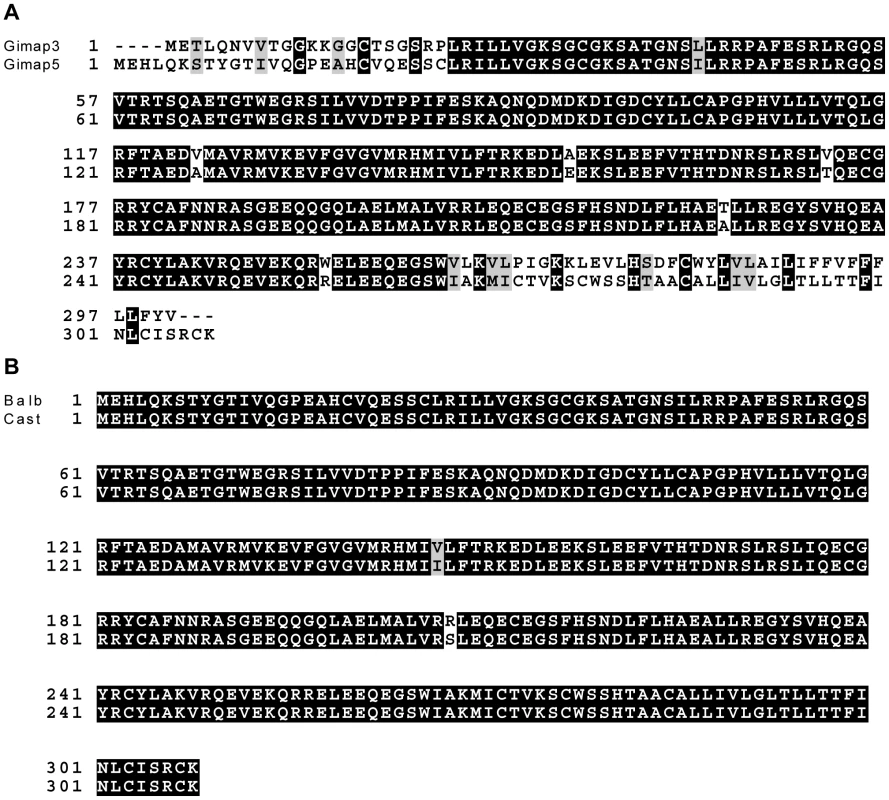
A. ClustalW alignment of BALB Gimap3 and Gimap5 protein sequences. B. ClustalW alignment of BALB and CAST Gimap5. Tab. 1. Chromosome 6 genes located between 37–49 Mb with a role in mitochondrial biology (GO:0005739). 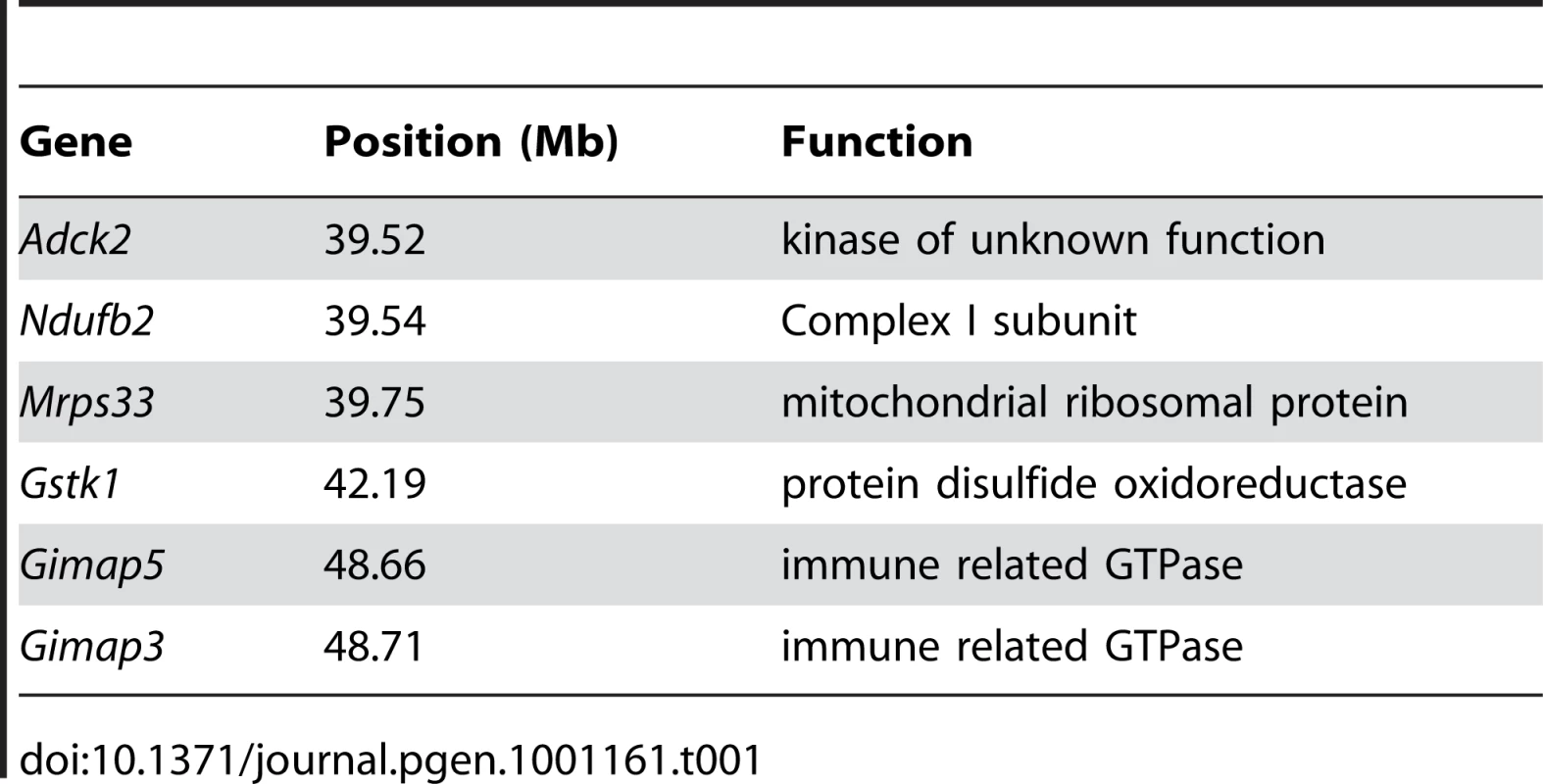
We sequenced the full-length cDNA of Gimap3 and Gimap5 from total RNA extracted from BALB/c and CAST/Ei spleens. Gimap5 contained two missense changes (Val to Ile and Arg to Ser) between BALB and CAST variants (Figure 3B). Neither of these amino acid variants are evolutionarily conserved in other Gimap family members [25]. However, for Gimap3 we found differential exon splicing with the CAST/Ei variant missing one of five exons. Gimap3 consists of five exons with two in frame AUG start sites in exons 3 and 4. Exon 4 also contains a stop codon upstream of the second AUG start site, so when all five exons are spliced together, the second start is used for translation of the mature protein (Figure 4). In the CAST/Ei Gimap3 mRNA, exon 4 is missing so translation starts from the first AUG (Figure 4), thereby altering the reading frame to produce a mature protein with an extra 58 amino acids at the N-terminus (Figure 4). We sequenced across exon 4 in genomic DNA from BALB/c and CAST/Ei and discovered a G to A transition in the splice acceptor site of exon 4 in CAST/Ei that prevents splicing of this exon into the mRNA (Figure 4). Since this mtDNA segregation phenotype is conserved among a variety of Mus musculus domesticus strains [22], we sequenced across exon 4 in four of these strains and found that the genomic sequence was identical to that of BALB/c (Figure 4). This altered mRNA splicing for the CAST/Ei allele changes considerably the Gimap3 protein sequence in the soluble domain of the protein, but does not affect the C-terminal transmembrane domain (Figure 4), which anchors and localizes it to the outer mitochondrial membrane [26].
Fig. 4. Gimap3 gene structure and protein sequence in BALB/c and CAST/Ei mouse strains. 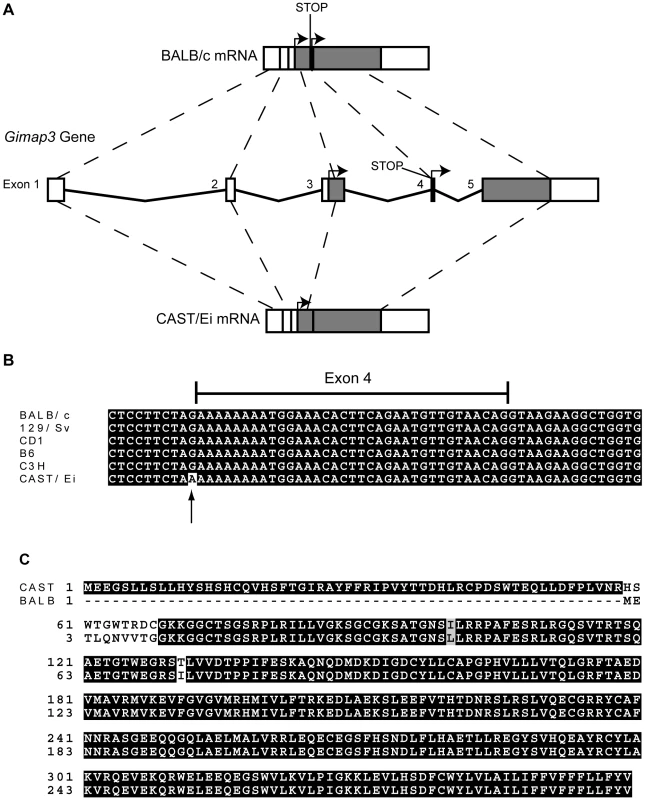
A. Exon structure and splicing of Gimap3. An AUG start codon is present in both exon 3 and exon 4. In exon 4, upstream of AUG start codon is a stop codon. In the BALB/c allele all 5 exons are spliced together, so translation of the mature protein initiates at the second AUG start codon, with a predicted size of 34 kDa. In the CAST/Ei allele, a G-A transition in the splice acceptor site of exon 4 prevents its splicing into the mature mRNA, so exon 4 is missing and translation initiates from the first AUG start codon, predicting a protein of 41 kDa. B. Alignment of the Gimap3 exon 4 and flanking intronic sequence from 5 Mus musculus domesticus strains, all of which are indistinguishable in the phenotype for mtDNA segregation compared to the Mus musculus castaneus CAST/Ei strain. C. ClustalW alignment of CAST and BALB Gimap3 protein sequences. MtDNA segregation in hematopoietic tissues is age-dependent, but it is unclear what role Gimap3 has in younger mice. To test whether there was an association of the CAST/Ei allele with the loss of mtDNA selection in the spleen, we genotyped the Gimap3 locus in three month old F2 mice (n = 145). Indeed, we observed a significant enrichment for the CAST/Ei allele in mice with no mtDNA selection and loss of the CAST/Ei allele in mice with mtDNA selection (Figure 5). This data suggests Gimap3 plays an important role in mtDNA segregation in hematopoietic tissues independent of age and made Gimap3 an attractive candidate gene.
Fig. 5. Enrichment of the CAST/Ei Gimap3 allele in three-month-old F2 mice with no mtDNA selection. 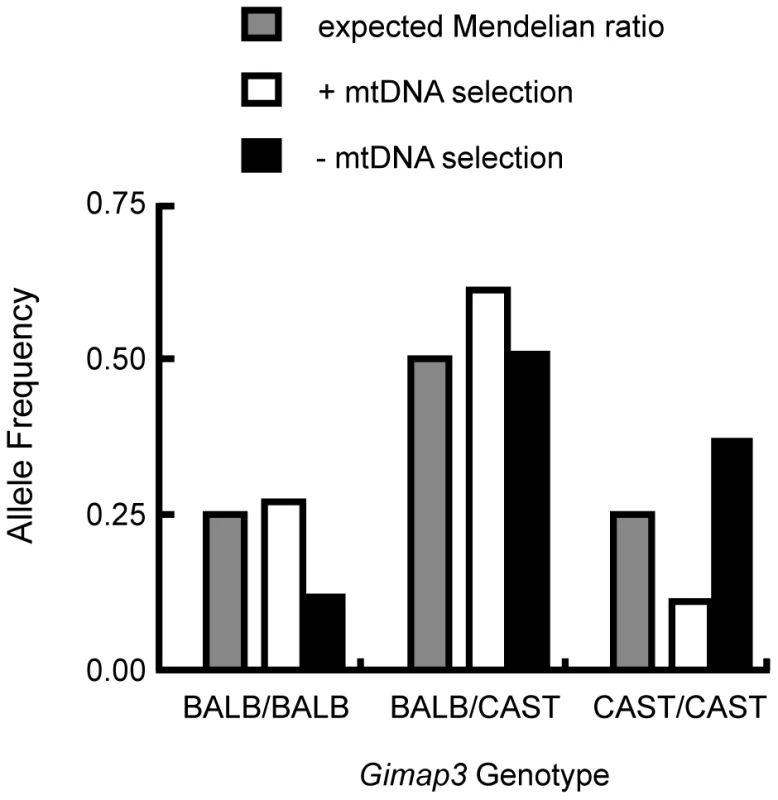
Three-month-old F2 (BALB/c X CAST/Ei) mice (n = 145) were classified into two groups, presence (+) (n = 88, 0.61) or absence (-) (n = 57, 0.39) of mtDNA selection, and then genotyped for their Gimap3 alleles. Distributions were compared to the expected Mendelian ratios by Chi-square analysis (p = 0.00033). To definitively test for a role of Gimap3 in regulating mtDNA segregation, we generated transgenic mice overexpressing the Cast/Ei Gimap3 cDNA driven off the ubiquitous ROSA26 promoter (Figure 6). The transgene was expressed ubiquitously, as expected from this promoter, and at a higher level than the endogenous Gimap3 in the spleen (Figure 6). Transgenic males were crossed to heteroplasmic females and progeny sampled at three months of age. Our previous QTL mapping results demonstrated that the Smdq-3 locus on chromosome 6 has an additive genetic effect, so our expectation was the CAST/Ei Gimap 3 allele would slow the rate of mtDNA segregation in the spleen. Consistent with our expectation, overexpression of the CAST/Ei Gimap3 in the spleen significantly slowed the rate of mtDNA segregation compared to littermate controls and our heteroplasmic mouse model on the BALB/c nuclear background (Figure 6). These results confirm Gimap3 is an important regulator of hematopoietic mtDNA segregation.
Fig. 6. Transgenic expression of CAST/Ei Gimap3 cDNA in heteroplasmic mice slows the rate of splenic mtDNA segregation. 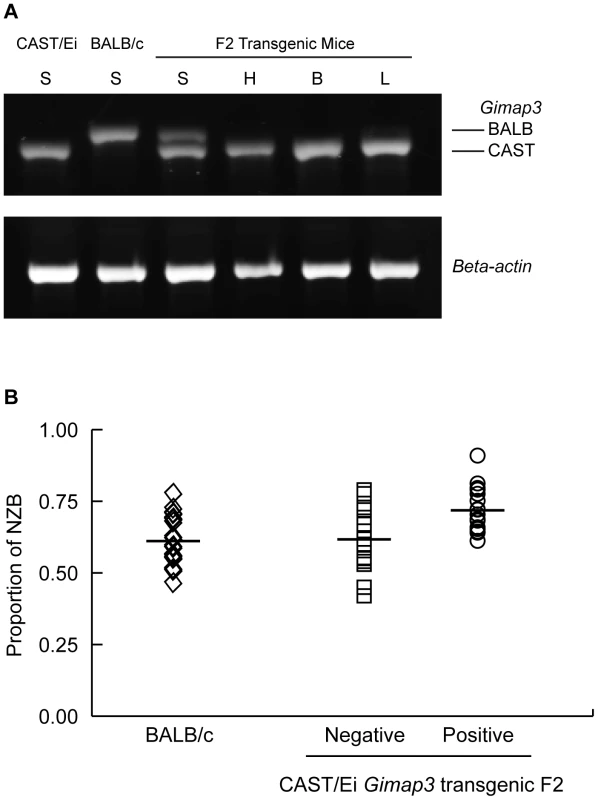
A. CAST/Ei Gimap3 transgene expression across a number of mouse tissues (B-brain; L-lung; H-heart; S-spleen) driven off the ROSA26 promoter. Endogenous Gimap3 expression from BALB/c or CAST/Ei spleen was loaded as a control. Equal amounts of total RNA were amplified by RT-PCR under the same conditions in each tissue. Beta-actin was used as a control. B. CAST/Ei Gimap3 transgene expression in three-month-old mice significantly slows down the mean rate of mtDNA segregation in the spleen compared to littermate controls and the BALB/c heteroplasmic mouse model (ANOVA, p = 0.0011). Data are presented as a scatter plot with means indicated (bar). Transgene negative (n = 16) and positive (n = 17); BALB/c (n = 23). Our results further support the hypothesis that the pathways regulating mtDNA segregation are indeed tissue or cell-specific. In our heteroplasmic mouse model, mtDNA selection for the NZB haplotype in the liver and kidney is regulated by different genes and with different kinetics [20], [22]. Ectopic expression of the CAST Gimap3 transgene had no effect on NZB mtDNA selection in the liver or kidney (Figure 7), nor had any effect on mtDNA segregation in tissues which are neutral for selection in our mouse model, such as the brain, heart, lung, and skeletal muscle. Consistent with this finding, retroviral overexpression of the CAST Gimap3 in heteroplasmic murine embryonic fibroblasts had no effect on heteroplasmy levels (Figure 7). These results imply that a cell-specific context or pathway is also required to alter mtDNA segregation.
Fig. 7. Ectopic expression of Gimap3 has no effect on mtDNA segregation. 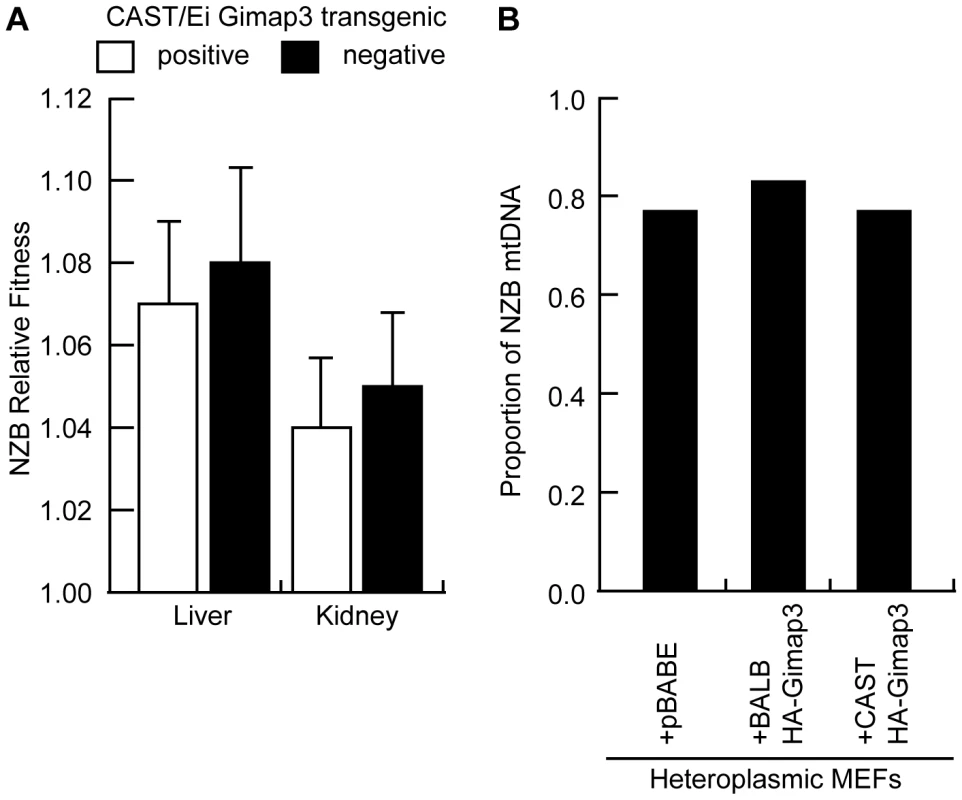
A. Relative fitness of NZB mtDNA in the liver and kidney of F2 transgenic littermates positive or negative for the CAST/Ei Gimap3 cDNA. Data are presented as means ± SD (transgene negative, n = 16; and positive, n = 17). B. Cultured heteroplasmic murine embryonic fibroblasts were transduced with BALB Gimap3 or CAST Gimap3 containing an N-terminal HA tag in pBABE, or with empty vector (pBABE). Cells were grown continuously in culture for 1 month. The change in NZB heteroplasmy in the bulk culture was determined comparing the level after 1 month of culture to the initial level before retroviral transduction. Mitochondrial genome copy number regulation has been proposed to influence the segregation of mtDNA haplotypes and human mtDNA mutations. To test whether changes in mtDNA copy number regulate mtDNA segregation in hematopoietic tissues, we measured the copy number in the spleen of F2 mice and found no difference between mice with either absence or presence of mtDNA selection (Figure 8). These data demonstrate that copy number regulation per se is not a major determinant for this particular mtDNA segregation phenotype, and that Gimap3 expression has no role in regulating mtDNA copy number.
Fig. 8. MtDNA copy number regulation in the spleen has no effect on mtDNA segregation. 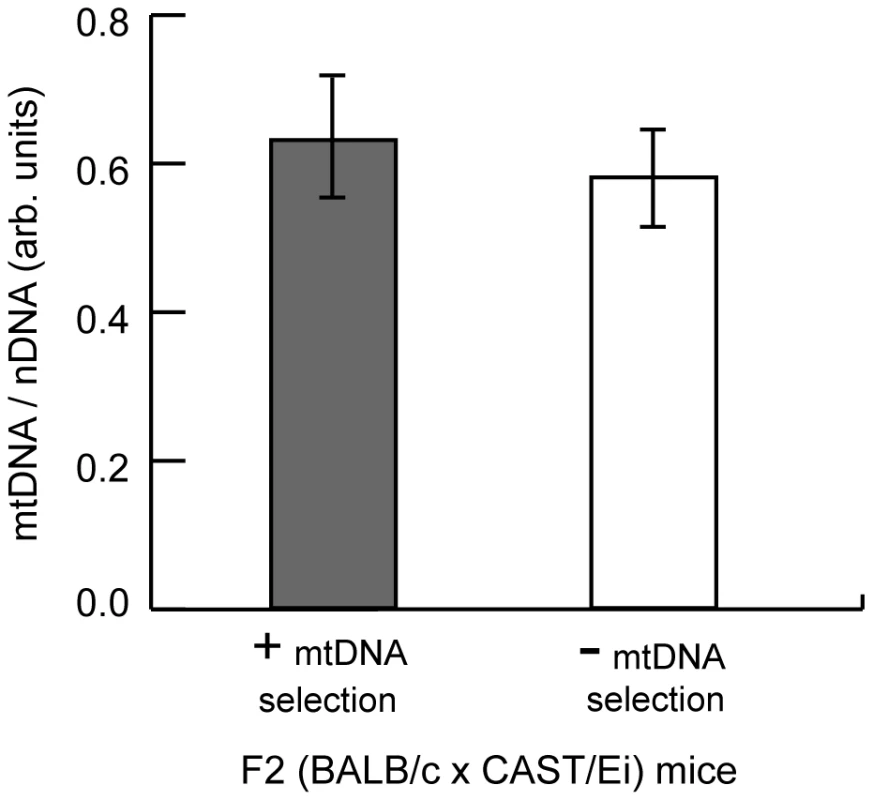
MtDNA copy number relative to nuclear DNA was measured in the spleen of three month F2 (BALB/c X CAST/Ei) mice with or without mtDNA selection. Data are presented as means ± SD (+ mtDNA selection, n = 32; - mtDNA selection, n = 13). Discussion
In this study, we identify the first nuclear-encoded gene that influences mtDNA segregation in mammals. Gimap3 is an outer mitochondrial membrane GTPase, which we show genetically can regulate the rate of mtDNA segregation in hematopoietic tissues. We also demonstrate that segregation of mtDNA haplotypes in mouse hematopoietic tissues is a complex genetic trait regulated with age but independent of mtDNA copy number. Variation in Gimap3 alone does not account for the entire segregation phenotype for the following reasons. At three months of age, some mice homozygous for the CAST allele still exhibit mtDNA selection, the phenotype is age-dependent, and in our transgenic mice, overexpression of the CAST Gimap3 had a quantitative effect on the rate of mtDNA segregation. These observations suggest other genes, such as the two suggestive loci detected on chromosome 11 and 13 in the linkage analysis are involved in the regulation of mtDNA segregation.
MtDNA selection in hematopoietic tissues of both humans and mice can be modeled as an exponential function, however, the rates are significantly different, up to 70 times faster in our mouse model than in humans [17]. In humans carrying the A3243G MELAS mutation, there is depletion of mtDNA independent of heteroplasmy level and age, which might be a secondary effect of the mutation and a driver for selection of wild type mtDNA [16]. However, the mechanism that leads to a decreased copy number and drives selection for the wild type mtDNA remains unknown. Rajasimha et al. [17] have postulated that selection against the A3243G MELAS mutation likely occurs in the stem cell population of rapidly dividing cells. Data from our mouse model do not support this mechanism of segregation, even though in our BALB/c heteroplasmic mouse model, selection for mtDNA occurs in leukocytes from both lymphoid and myeloid lineages [21]. In rapidly dividing colonic crypts there is no selection for mtDNA haplotypes [18], and, in our F2 (BALB/c X CAST/Ei) cross the frequency of mice that have lost mtDNA selection changes with age.
Gimap3 is a member of the conserved Gimap (GTPase of immunity-associated protein) gene cluster found only in vertebrates, with an orthologue in angiosperm plants [25]–[28]. Both Gimap3 and Gimap5 (a paralogue of Gimap3) contain a G1 to G5 switch GTPase, two coiled-coil motifs, and a hydrophobic conserved box [25]. In humans, only GIMAP5 is a functional gene producing two splice variants with predicted molecular masses of 34.8 and 39.5 kDa, while GIMAP3 appears to be a pseudogene [25]. Very little is known about protein function, and in particular the role of the GTPase domain and the conserved box remain an enigma. These two proteins are critical for T cell development and cell survival, and shown to interact with anti-apoptotic Bcl-2 family members, but the mechanisms are not understood [27]. Gimap5 was originally identified as the factor responsible for the severe T cell lymphopenia in the diabetes prone BioBreeding rat [29], although in mice loss of Gimap5 function produces a broader and more severe phenotype, which includes a leukocyte developmental defect, liver dysfunction, and lethality (median age of death around 14–15 weeks) [30]. The CAST/Ei variant of Gimap3 only differs at the N-terminus, leaving intact all of the known functional domains of the protein, including the C-terminus required for membrane insertion and localization. How these extra 58 amino acids in the CAST/Ei Gimap3 variant affect protein function requires further characterization of Gimap3 in leukocytes.
How can an outer mitochondrial membrane protein regulate mtDNA segregation in hematopoietic tissues? Selection for mtDNA haplotypes can only be directed at two levels, either at the DNA sequence itself or at the proteins encoded within it. Analysis of Gimap3 protein sequence does not support a direct physical interaction with mtDNA, because the protein does not appear to span both mitochondrial membranes into the matrix space in order to facilitate such an interaction. One possibility is that Gimap3 acts as a node or switch on the outer membrane for a retrograde signaling cascade involving mitochondrial peptide export, a process that occurs across eukaryotes [31]–[34]. Bacteria use peptide export-import as a control circuit to regulate processes, such as nutrient uptake and sporulation [35]. Further work on Gimap3 will establish its function within mtDNA segregation and whether peptide export or cell survival are involved.
Methods
Ethics Statement
These studies were approved by the McGill University Animal Care Committee and The Regional State Administrative Agency of Southern Finland (ESAVI).
Mice and Breeding
To produce mice for the genome scan, female BALB/c mice heteroplasmic for the BALB and NZB mtDNA haplotypes were outcrossed to male CAST/Ei mice to generate an F1, which were then intercrossed to obtain F2 progeny. Transgenic mice were made by cloning the CAST/Ei cDNA of Gimap3 into the EcoRI site of pBroad3 (Invivogen), which was then microinjected into fertilized FVB embryos. Founders were screened for the transgene, germline transmission, and autosomal inheritance. Transgenic mice were crossed to BALB/c, and the resulting F1 males crossed to heteroplasmic BALB/c females to generate heteroplasmic littermates.
Phenotyping
Tissues were collected from mice at 3 and 12 months of age and DNA extracted by conventional methods. Heteroplasmy levels were determined across tissues and the mtDNA segregation phenotype in hematopoietic tissues done according to Battersby et al. [21]. Only animals with an initial level of NZB heteroplasmy above 20% were included in the analysis. Relative fitness values for NZB mtDNA in the kidney and liver were calculated as previously described [22].
MtDNA Quantitation
Relative levels of mtDNA to nuclear DNA were determined using SYBR Green (Kapa Biosystems) on a Bio-Rad CFX96 thermal cycler with primers for mtDNA (forward 5′ - GAGCATCTTATCCACGCTTCC, reverse 5′-GGTGGTACTCCCGCTGTAAA) and the single copy nuclear-encoded gene beta-2 microglobulin (forward 5-TGTCAGATATGTCCTTCAGCAAGG, reverse 5-TGCTTAACTCTGCAGGCGTATG). Samples and standards were run in triplicate and used only after comparing the post-run amplification efficiencies.
Genotyping
The Illumina Medium Density Linkage Panel was used for SNP genotyping of mouse heart genomic DNA. From a total of 1449 markers on the panel, 680 SNPs were informative between BALB/c and CAST/Ei. The Gimap3 allele was genotyped in genomic DNA by PCR using primers (forward 5′ - ACGTGCACAGACCCATTTCT, reverse 5′ - GTGCTGGAGGGAAGTTTGTC) and then digested Hpy188III and separated on agarose gels. Mice were screened for the presence of the transgene using a PCR assay that amplified the Gimap3 CAST/Ei cDNA and the BALB/c gene (forward 5′-CATACCGTCACACCATCTGC, reverse 5′-CTTTTACCGCAGCCAGATTT), which amplifies a 320 bp fragment from the cDNA and a 1700 bp fragment from the gene.
RNA Analysis
All tissues sampled were frozen in liquid nitrogen and stored at −80°C. Total RNA was extracted with Trizol (Invitrogen) then treated with DNaseI to eliminate potential DNA contamination. Gimap3 cDNAs were amplified from BALB/c and CAST/Ei spleens by RT-PCR (Qiagen) with primers (forward 5′ - TCCTGCCTGAGAGACTGTTG, reverse 5′ - TGTGAGTGATCCCAATCCAC). Transgene and endogenous Gimap3 expression was measured by RT-PCR using equal amounts of total RNA with primers for Gimap3 (forward 5′ - TGGACTTCCCATTGGTAAACA, reverse 5′-ACCCCAAAGACCTCCTTCAC) and beta-actin (forward 5′ -TCACCCACACTGTGCCCATCTAC, reverse 5′ -GAGTACTTGCGCTCAGGAGGAGC).
Retroviral Constructs
Full-length cDNAs were cloned into a Gateway (Invitrogen) converted pBABE-puro retroviral expression vector and transfected into the Phoenix amphotropic packaging line to transiently produce virus, which was then used to infect NIH3T3 or heteroplasmic murine embryonic fibroblasts.
Statistical Analysis
Linkage analysis was carried out by regression at the markers under a logistic regression model and an allele dosage mode of inheritance. The genome wide corrected p-values were based on a 10,000 permutation sample. Allele distributions of Gimap3 in three month old F2 mice were analyzed by Chi-Square analysis comparing to an expected Mendelian distribution. The effect of the CAST/Ei transgene on mtDNA segregation was analyzed in datasets first for normality, followed by ANOVA and posthoc testing.
Zdroje
1. TaylorRW
TurnbullDM
2005 Mitochondrial DNA mutations in human disease. Nat Rev Genet 6 389 402
2. WaiT
TeoliD
ShoubridgeEA
2008 The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat Genet 40 1484 1488
3. CreeLM
SamuelsDC
de Sousa LopesSC
RajasimhaHK
WonnapinijP
2008 A reduction of mitochondrial DNA molecules during embryogenesis explains the rapid segregation of genotypes. Nat Genet 40 249 254
4. ChinneryPF
SamuelsDC
1999 Relaxed replication of mtDNA: A model with implications for the expression of disease. Am J Hum Genet 64 1158 1165
5. HayashiJ
OhtaS
KikuchiA
TakemitsuM
GotoY
1991 Introduction of disease-related mitochondrial DNA deletions into HeLa cells lacking mitochondrial DNA results in mitochondrial dysfunction. Proc Natl Acad Sci U S A 88 10614 10618
6. BouletL
KarpatiG
ShoubridgeEA
1992 Distribution and threshold expression of the tRNA(Lys) mutation in skeletal muscle of patients with myoclonic epilepsy and ragged-red fibers (MERRF). Am J Hum Genet 51 1187 1200
7. ChinneryPF
ThorburnDR
SamuelsDC
WhiteSL
DahlHM
2000 The inheritance of mitochondrial DNA heteroplasmy: random drift, selection or both? Trends Genet 16 500 505
8. LarssonNG
HolmeE
KristianssonB
OldforsA
TuliniusM
1990 Progressive increase of the mutated mitochondrial DNA fraction in Kearns-Sayre syndrome. Pediatr Res 28 131 136
9. KawakamiY
SakutaR
HashimotoK
FujinoO
FujitaT
1994 Mitochondrial myopathy with progressive decrease in mitochondrial tRNA(Leu)(UUR) mutant genomes. Ann Neurol 35 370 373
10. DunbarDR
MooniePA
JacobsHT
HoltIJ
1995 Different cellular backgrounds confer a marked advantage to either mutant or wild-type mitochondrial genomes. Proc Natl Acad Sci U S A 92 6562 6566
11. FuK
HartlenR
JohnsT
GengeA
KarpatiG
1996 A novel heteroplasmic tRNAleu(CUN) mtDNA point mutation in a sporadic patient with mitochondrial encephalomyopathy segregates rapidly in skeletal muscle and suggests an approach to therapy. Hum Mol Genet 5 1835 1840
12. WeberK
WilsonJN
TaylorL
BrierleyE
JohnsonMA
1997 A new mtDNA mutation showing accumulation with time and restriction to skeletal muscle. Am J Hum Genet 60 373 380
13. ChinneryPF
HowellN
LightowlersRN
TurnbullDM
1997 Molecular pathology of MELAS and MERRF. The relationship between mutation load and clinical phenotypes. Brain 120 Pt 10 1713 1721
14. ChinneryPF
ZwijnenburgPJ
WalkerM
HowellN
TaylorRW
1999 Nonrandom tissue distribution of mutant mtDNA. Am J Med Genet 85 498 501
15. RahmanS
PoultonJ
MarchingtonD
SuomalainenA
2001 Decrease of 3243 A–G mtDNA mutation from blood in MELAS syndrome: a longitudinal study. Am J Hum Genet 68 238 240
16. PyleA
TaylorRW
DurhamSE
DeschauerM
SchaeferAM
2007 Depletion of mitochondrial DNA in leucocytes harbouring the 3243A->G mtDNA mutation. J Med Genet 44 69 74
17. RajasimhaHK
ChinneryPF
SamuelsDC
2008 Selection against pathogenic mtDNA mutations in a stem cell population leads to the loss of the 3243A–>G mutation in blood. Am J Hum Genet 82 333 343
18. JenuthJP
PetersonAC
FuK
ShoubridgeEA
1996 Random genetic drift in the female germline explains the rapid segregation of mammalian mitochondrial DNA. Nat Genet 14 146 151
19. JenuthJP
PetersonAC
ShoubridgeEA
1997 Tissue-specific selection for different mtDNA genotypes in heteroplasmic mice. Nat Genet 16 93 95
20. BattersbyBJ
ShoubridgeEA
2001 Selection of a mtDNA sequence variant in hepatocytes of heteroplasmic mice is not due to differences in respiratory chain function or efficiency of replication. Hum Mol Genet 10 2469 2479
21. BattersbyBJ
RedpathME
ShoubridgeEA
2005 Mitochondrial DNA segregation in hematopoietic lineages does not depend on MHC presentation of mitochondrially encoded peptides. Hum Mol Genet 14 2587 2594
22. BattersbyBJ
Loredo-OstiJC
ShoubridgeEA
2003 Nuclear genetic control of mitochondrial DNA segregation. Nat Genet 33 183 186
23. AbiolaO
AngelJM
AvnerP
BachmanovAA
BelknapJK
2003 The nature and identification of quantitative trait loci: a community's view. Nat Rev Genet 4 911 916
24. Moreno-LoshuertosR
Acin-PerezR
Fernandez-SilvaP
MovillaN
Perez-MartosA
2006 Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nat Genet 38 1261 1268
25. KruckenJ
SchroetelRM
MullerIU
SaidaniN
MarinovskiP
2004 Comparative analysis of the human gimap gene cluster encoding a novel GTPase family. Gene 341 291 304
26. DaheronL
ZenzT
SiracusaLD
BrennerC
CalabrettaB
2001 Molecular cloning of Ian4: a BCR/ABL-induced gene that encodes an outer membrane mitochondrial protein with GTP-binding activity. Nucleic Acids Res 29 1308 1316
27. NittaT
NasreenM
SeikeT
GojiA
OhigashiI
2006 IAN family critically regulates survival and development of T lymphocytes. PLoS Biol 4 e103 doi:10.1371/journal.pbio.0040103
28. NittaT
TakahamaY
2007 The lymphocyte guard-IANs: regulation of lymphocyte survival by IAN/GIMAP family proteins. Trends Immunol 28 58 65
29. MacMurrayAJ
MoralejoDH
KwitekAE
RutledgeEA
Van YserlooB
2002 Lymphopenia in the BB rat model of type 1 diabetes is due to a mutation in a novel immune-associated nucleotide (Ian)-related gene. Genome Res 12 1029 1039
30. SchulteisRD
ChuH
DaiX
ChenY
EdwardsB
2008 Impaired survival of peripheral T cells, disrupted NK/NKT cell development, and liver failure in mice lacking Gimap5. Blood 112 4905 4914
31. LovelandB
WangCR
YonekawaH
HermelE
LindahlKF
1990 Maternally transmitted histocompatibility antigen of mice: a hydrophobic peptide of a mitochondrially encoded protein. Cell 60 971 980
32. ShawarSM
VyasJM
RodgersJR
CookRG
RichRR
1991 Specialized functions of major histocompatibility complex class I molecules. II. Hmt binds N-formylated peptides of mitochondrial and prokaryotic origin. J Exp Med 174 941 944
33. HaynesCM
YangY
BlaisSP
NeubertTA
RonD
2010 The matrix peptide exporter HAF-1 signals a mitochondrial UPR by activating the transcription factor ZC376.7 in C. elegans. Mol Cell 37 529 540
34. YoungL
LeonhardK
TatsutaT
TrowsdaleJ
LangerT
2001 Role of the ABC transporter Mdl1 in peptide export from mitochondria. Science 291 2135 2138
35. PeregoM
1997 A peptide export-import control circuit modulating bacterial development regulates protein phosphatases of the phosphorelay. Proc Natl Acad Sci U S A 94 8612 8617
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 10- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Hypogonadotropní hypogonadismus u žen a vliv na výsledky reprodukce po IVF
- Molekulární vyšetření pro stanovení prognózy pacientů s chronickou lymfocytární leukémií
- Prof. Petr Urbánek: Potřebujeme najít pacienty s nediagnostikovanou akutní intermitentní porfyrií
-
Všechny články tohoto čísla
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- FSHD: A Repeat Contraction Disease Finally Ready to Expand (Our Understanding of Its Pathogenesis)
- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- The Meiotic Recombination Checkpoint Suppresses NHK-1 Kinase to Prevent Reorganisation of the Oocyte Nucleus in
- Actin Depolymerizing Factors Cofilin1 and Destrin Are Required for Ureteric Bud Branching Morphogenesis
- DSIF and RNA Polymerase II CTD Phosphorylation Coordinate the Recruitment of Rpd3S to Actively Transcribed Genes
- Continuous Requirement for the Clr4 Complex But Not RNAi for Centromeric Heterochromatin Assembly in Fission Yeast Harboring a Disrupted RITS Complex
- Genome-Wide Association Study of Blood Pressure Extremes Identifies Variant near Associated with Hypertension
- The Cytosine Methyltransferase DRM2 Requires Intact UBA Domains and a Catalytically Mutated Paralog DRM3 during RNA–Directed DNA Methylation in
- β-Actin and γ-Actin Are Each Dispensable for Auditory Hair Cell Development But Required for Stereocilia Maintenance
- Genetic Association Study Identifies as a Risk Gene for Idiopathic Dilated Cardiomyopathy
- Evidence for a Xer/ System for Chromosome Resolution in Archaea
- Four Novel Loci (19q13, 6q24, 12q24, and 5q14) Influence the Microcirculation
- Lifespan Extension by Preserving Proliferative Homeostasis in
- Ancient and Recent Adaptive Evolution of Primate Non-Homologous End Joining Genes
- Loss of the p53/p63 Regulated Desmosomal Protein Perp Promotes Tumorigenesis
- Altering a Histone H3K4 Methylation Pathway in Glomerular Podocytes Promotes a Chronic Disease Phenotype
- Characterization of LINE-1 Ribonucleoprotein Particles
- Conserved Genes Act as Modifiers of Invertebrate SMN Loss of Function Defects
- Alternative Splicing at a NAGNAG Acceptor Site as a Novel Phenotype Modifier
- Tight Regulation of the Gene of the KplE1 Prophage: A New Paradigm for Integrase Gene Regulation
- Conjugative DNA Transfer Induces the Bacterial SOS Response and Promotes Antibiotic Resistance Development through Integron Activation
- Nasty Viruses, Costly Plasmids, Population Dynamics, and the Conditions for Establishing and Maintaining CRISPR-Mediated Adaptive Immunity in Bacteria
- Stress-Induced Activation of Heterochromatic Transcription
- H3K27me3 Profiling of the Endosperm Implies Exclusion of Polycomb Group Protein Targeting by DNA Methylation
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
- Characterising and Predicting Haploinsufficiency in the Human Genome
- Dual Functions of ASCIZ in the DNA Base Damage Response and Pulmonary Organogenesis
- Pervasive Cryptic Epistasis in Molecular Evolution
- Transition from Positive to Neutral in Mutation Fixation along with Continuing Rising Fitness in Thermal Adaptive Evolution
- Comprehensive Analysis Reveals Dynamic and Evolutionary Plasticity of Rab GTPases and Membrane Traffic in
- Regulates Tissue-Specific Mitochondrial DNA Segregation
- Role for the Mammalian Swi5-Sfr1 Complex in DNA Strand Break Repair through Homologous Recombination
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



