-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
Mouse Embryonic Stem (ES) cells express a unique set of microRNAs (miRNAs), the miR-290-295 cluster. To elucidate the role of these miRNAs and how they integrate into the ES cell regulatory network requires identification of their direct regulatory targets. The difficulty, however, arises from the limited complementarity of metazoan miRNAs to their targets, with the interaction requiring as few as six nucleotides of the miRNA seed sequence. To identify miR-294 targets, we used Dicer1-null ES cells, which lack all endogenous mature miRNAs, and introduced just miR-294 into these ES cells. We then employed two approaches to discover miR-294 targets in mouse ES cells: transcriptome profiling using microarrays and a biochemical approach to isolate mRNA targets associated with the Argonaute2 (Ago2) protein of the RISC (RNA Induced Silencing Complex) effector, followed by RNA–sequencing. In the absence of Dicer1, the RISC complexes are largely devoid of mature miRNAs and should therefore contain only transfected miR-294 and its base-paired targets. Our data suggest that miR-294 may promote pluripotency by regulating a subset of c-Myc target genes and upregulating pluripotency-associated genes such as Lin28.
Published in the journal: . PLoS Genet 6(10): e32767. doi:10.1371/journal.pgen.1001163
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001163Summary
Mouse Embryonic Stem (ES) cells express a unique set of microRNAs (miRNAs), the miR-290-295 cluster. To elucidate the role of these miRNAs and how they integrate into the ES cell regulatory network requires identification of their direct regulatory targets. The difficulty, however, arises from the limited complementarity of metazoan miRNAs to their targets, with the interaction requiring as few as six nucleotides of the miRNA seed sequence. To identify miR-294 targets, we used Dicer1-null ES cells, which lack all endogenous mature miRNAs, and introduced just miR-294 into these ES cells. We then employed two approaches to discover miR-294 targets in mouse ES cells: transcriptome profiling using microarrays and a biochemical approach to isolate mRNA targets associated with the Argonaute2 (Ago2) protein of the RISC (RNA Induced Silencing Complex) effector, followed by RNA–sequencing. In the absence of Dicer1, the RISC complexes are largely devoid of mature miRNAs and should therefore contain only transfected miR-294 and its base-paired targets. Our data suggest that miR-294 may promote pluripotency by regulating a subset of c-Myc target genes and upregulating pluripotency-associated genes such as Lin28.
Introduction
Embryonic stem cells, which are derived from the inner cell mass of the blastocyst, hold great clinical promise because of their unique capacity to both self-renew and differentiate into potentially any cell type. Understanding the molecular controls of pluripotency is key to realising their therapeutic potential. While the general importance of small RNAs in gene regulation has been recognised in plants and animals, much remains to be understood about the specific role of small RNAs in ES cells.
miRNAs and small interfering RNAs (siRNAs) are a class of small (≈20–25 nucleotide) non-coding RNAs that direct sequence-specific post-transcriptional repression of target mRNAs. Mature miRNAs and siRNAs are generated from double stranded RNA (dsRNA) precursors by the RNase III enzyme Dicer [1], [2]. The mature small RNA is then incorporated into a protein of the Argonaute family [3], [4]. This RNA-protein complex forms the core of the effector complex referred to as the RNA-induced silencing complex (RISC). Within the RISC, the small RNA acts a guide to direct Argonaute proteins to complementary target transcripts to elicit the cleavage, degradation or translational repression of their targets depending on their degree of complementarity [5].
Several studies implicate miRNAs in the control of early embryonic development and maintenance of the pluripotent stem cell state. Disruption of the single Dicer1 gene in mice leads to early embryonic lethality around E7.5 [6]. Dicer1 mutant embryos have greatly reduced expression of Oct-4 in the epiblast, implying a lack of pluripotent cells, and it is not possible to derive ES cells from Dicer1-null blastocysts. However, conditional deletion of Dicer1 from established ES cells results in an impaired capacity to differentiate, as well as a profound initial proliferation defect that is overcome with time [7], [8]. Moreover, large scale cloning and sequencing efforts have revealed a subset of miRNAs that are unique to ES cells [9]–[11]. The miR-290-295 cluster (Figure 1A) consists of 6 miRNAs that share a similar 5′ region from nucleotides 2–8, known as the ‘seed’ sequence, which is thought to be the primary specificity determinant for target recognition in most miRNAs [12]. The miR-290-295 cluster accounts for the majority of all miRNAs expressed in undifferentiated ES cells but decreases after ES cells differentiate [9]. Recent evidence suggests that there are functional differences between miRNAs from the miR-290-295 cluster. Only miR-291-3p, miR-294 and miR-295 can promote the G1-S transition of the cell cycle and the induction of pluripotency [13], [14]. Furthermore, miR-293 expression and seed sequence differs markedly from the other members of this family, indicating the need to re-examine previous inferences based on whole miR-290-295 overexpression studies [15]–[17].
Fig. 1. Microarray analysis of Dicer1-null ES cells transfected with miR-294. 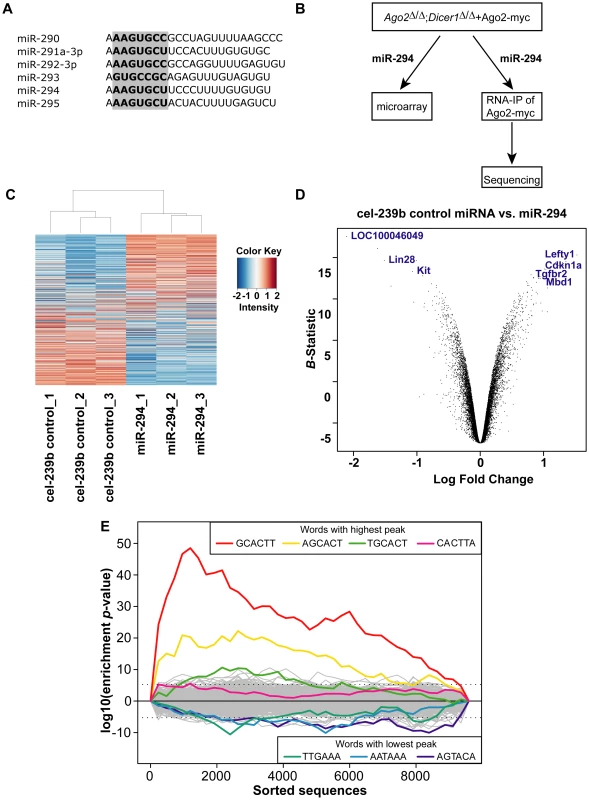
(A) Sequences of predominantly expressed mature miRNAs from the miR-290-295 cluster with their seed sequence highlighted in bold. (B) Experimental scheme to identify miR-294 targets: miR-294 was transfected into Ago2Δ/Δ;Dicer1Δ/Δ ES cells transgenic for Ago2-myc; the transcriptome profile was analysed by microarray; and miR-294-programmed Ago2-myc was subjected to RNA-immunoprecipitation and deep sequencing of the bound mRNA. (C) Heatmap showing differentially expressed genes between miR-294-transfected and cel-239b-transfected ES cells. The heat map was colour coded using red for downregulation and blue for upregulation (with respect to the mean signal in all the six samples). (D) Volcano plot to set threshold for differentially expressed genes. A negative log Fold Change on the x-axis corresponds to upregulated genes in miR-294 transfected cells whereas, a positive log Fold Change corresponds to downregulated genes in miR-294 transfected cells. (E) Overrepresentation of miR-294 seed matches in the most downregulated genes of the microarray. The motif-discovery tool Sylamer was used. The x-axis represents the sorted gene list from most downregulated (left) to most upregulated (right). The y-axis shows the significance for each word. To identify targets and study the function of individual miRNAs in ES cells, we used Dicer1-null ES cells in which we replaced endogenous Ago2 with a transgenic Ago2-myc (Dicer1Δ/Δ+Ago2-myc) (Figure 1B). In this Dicer1-null background, there are no mature miRNAs [11], which enables the reintroduction of any single miRNA at a time. To investigate the role of miR-294 in ES cells, we profiled the transcriptome of Dicer1-null ES cells transfected with miR-294. In addition, we used a biochemical approach that exploits the direct interaction of the mature miRNA to its mRNA target within the Ago protein of the RISC complex [18]. Immunoprecipitation of Ago2-myc from Dicer1-null ES transfected with miR-294, followed by RNA-sequencing of associated RNAs should lead to identification of direct miR-294 targets. Our strategy overcomes the problem of immunoprecipitating RISC complexes that contain many different miRNAs with their corresponding targets [19]–[21]. This approach is applicable to the study of any single miRNA or combination of miRNAs, potentially in any cell type. We describe here the results of the microarray and RNA-Sequencing analyses, and how miR-294 integrates into the ES cell regulatory network.
Results
Transcriptome analysis of miR-294–transfected Dicer1-null ES cells
First, we established Dicer1-null ES cells that lack all mature miRNAs (Figure S1B, S1C), into which we can reintroduce one specific miRNA of interest at a time. Previous studies have shown that the miR-290-295 cluster might have an important role in the pluripotency of ES cells [14], [16], [17] amongst which miR-294 is one of the most abundantly expressed miRNAs (Figure S1A) [11]. We decided to investigate the potential role of miR-294 in pluripotency.
Metazoan miRNAs typically bind to partially complementary sites in the 3′ untranslated region (3′ UTRs) of their target mRNAs to direct translational repression or mRNA destabilisation [22]–[24]. Overexpression of a miRNA can affect hundreds of mRNAs and messages that are downregulated tend to have significant enrichment of sequences complementary to the corresponding seed of the miRNA [16], [17], [24]. To identify the global effects of miR-294 in ES cells, we analysed the transcriptomes of Dicer1Δ/Δ+Ago2-myc ES cells transfected with miR-294 compared to Dicer1Δ/Δ+Ago2-myc ES cells transfected with a cel-239b control miRNA, which has minimal sequence identity to mouse miRNAs. The transcriptomes were profiled using Illumina microarrays (GEO accession code: GSE20048). The relative signal intensities of each of the 15735 probes across the six samples were plotted as a heat map (Figure 1C), which revealed global downregulation and upregulation of probes in each sample transfected with miR-294. To select differentially expressed genes, probes were ranked by the log odds ratio (B-statistic) of each probe showing differential expression, and plotted against the log fold change in a ‘volcano’ plot (Figure 1D). A B-statistic >5 (and corresponding to an adjusted p-value <0.0001) was selected as a cut-off for differential expression (Figure 1C). Using this conservative cut-off gives, with very high confidence, 162 upregulated (Table S1) and 248 downregulated differentially expressed genes upon miR-294 transfection.
To determine whether the downregulated transcripts contain miR-294 seed matches, we used the k-mer composition analysis tool Sylamer [25] to search for overrepresentation of sequence motifs in the 3′ UTRs. Each gene on the array (for which a 3′ UTR sequence was annotated) was ranked from most downregulated to most upregulated according to log fold change. If enrichment of the seed sequence in the 3′ UTRs correlates with the ranking of genes according to their fold change, then one would expect to observe a sharp peak in overrepresentation of the miRNA seed for the top-ranked genes. The resulting enrichment analysis plot shows that a strong signal is evident for 6-mer words corresponding to the seed region of miR-294 (Figure 1E), peaking approximately at gene 1000 in the ranked list. Conversely, this sequence is depleted in upregulated genes. The maximum of the first enrichment peak can be chosen as a threshold, above which, genes can be considered candidate targets if they contain the appropriate seed sequences matches. This yielded a more relaxed list of 487 predicted targets, which have at least one 6-mer present in their 3′ UTR (Table S2). This Sylamer list contains all of the conservative list of predicted targets (127 out of 248 have at least one 6-mer in their 3′ UTR) selected using a B-statistic >5 (Table S2). In summary, there was overrepresentation of the miR-294 seed sequence in the most downregulated genes, indicating that many of the observed gene expression changes are likely to be consequences of miR-294 expression.
RNA–IP and RNA–sequencing of Ago2-myc–bound mRNAs
To discover direct targets of miR-294, we used a biochemical approach to isolate mRNAs targeted by miRNAs within the Ago2 protein of the RISC. To facilitate the immunoprecipitation of Ago2, we replaced endogenous Ago2 with a myc-epitope tagged Ago2. We also constructed a catalytically-inactive mutant Ago2-myc (Ago2-myc-MUT) by point-mutating residues Q633R and H634A in the PIWI domain to capture the subset of target mRNAs that would otherwise be sliced by Ago2 [26], [27]. Point mutations Q633R and H634A were previously shown to abolish mouse Ago2 cleavage-activity without affecting siRNA binding [27]. First, we confirmed the specificity of Ago2-myc immunoprecipitation (IP) (Figure S2A), and that immunoprecipitation of Ago2-myc from Ago2flox/flox;Dicer1flox/flox ES cells does retain miRNAs (Figure S2B). We then assessed the enrichment of known targets using Dicer1-null ES cells transfected with miR-294. We found enrichment of Cdkn1a, a known miR-294 target [13], in immunoprecipitated RNA from Dicer1Δ/Δ+Ago2-myc ES cells transfected with miR-294, compared to cel-239b (Figure S2C). Following RNA-immunoprecipitation of miR-294-programmed Ago2-myc, RNA from the INPUT (total RNA) and IP (Figure S3A) were subjected to library preparation and sequenced by SOLiD (GEO accession code: GSE20199).
A comparison of the global gene expression changes detected by microarray and by RNA-Sequencing of the INPUT revealed a similar trend (Figure 2A). Furthermore, there was overrepresentation of the miR-294 seed sequence in the most downregulated genes of the sequenced INPUT (Figure 2B), indicating that miR-294 was functional and that the transfection efficiency was sufficiently high (Figure S3B) to bring about gene expression changes. An examination of the IP vs. INPUT ratios for each sample revealed an overall greater dynamic range of enrichment for samples transfected with miR-294 compared to cel-239b (Figure S4). The pattern was similar for the catalytically-inactive Ago2-myc. This result was expected since miRNA-directed slicing of the target mRNA is thought to occur rarely in metazoans [12]. However, the overall extra enrichment could not be accounted for by the overrepresentation of miR-294 seed sequence matches in the 3′ UTRs of enriched genes (Figure 3A). There is a surprising general tendency for hexamers with a higher GC content to be overrepresented in the 3′ UTRs of enriched transcripts (Figure 3A and 3B). This effect is also observed in samples transfected with the control cel-239b (Figure 3C). However, the GC effect is markedly stronger in the samples transfected with miR-294.
Fig. 2. Global gene expression changes in the INPUT. 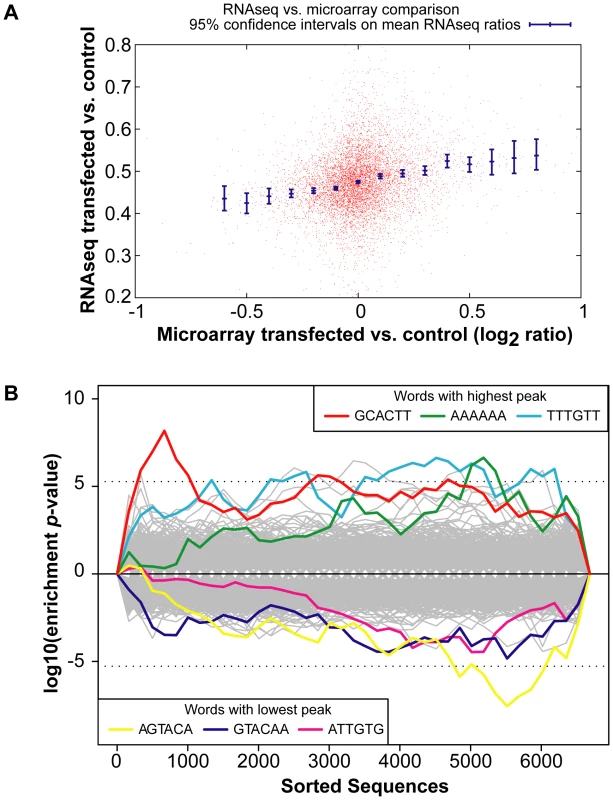
(A) RNA-Sequencing (RNA-Seq) of the INPUT (Total RNA) follows the microarray trend in gene expression. The y-axis represents the RNA-Seq ratio of the INPUT from Dicer1Δ/Δ+Ago2-myc ES cells transfected with miR-294 vs. cel-239b. Higher values mean enriched in cel-239b vs. miR-294. The x-axis represents the fold change in gene expression as measured by microarray. A positive value indicates upregulation of genes in miR-294-transfected cells and a negative value indicates downregulation of genes in miR-294-transfected cells. (B) Overrepresentation of 6-mer (position 2-7) seed matches to miR-294 in the INPUT of Dicer1Δ/Δ+Ago2-myc ES cells transfected with miR-294. The x-axis represents the sorted gene list from most downregulated (left) to most upregulated (right). The y-axes show the significance for each word. Fig. 3. Hexamer composition of enriched 3′ UTRs. 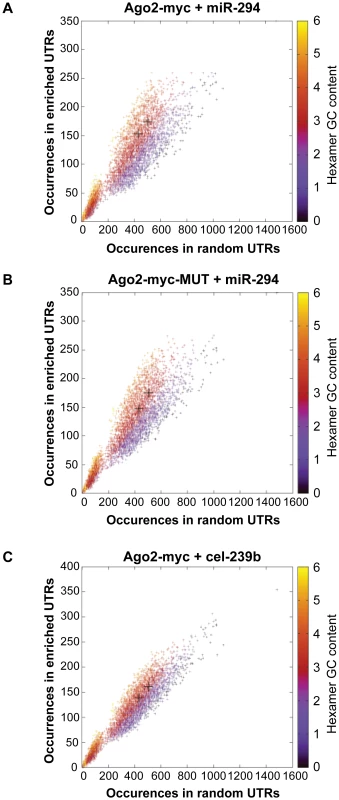
Each point represents a hexamer and the color corresponds to its GC content. This plot compares the frequency of hexamers present in ≈500 top-enriched 3′ UTRs vs. ≈2000 non-enriched 3′ UTRs for (A) Ago2-myc transfected with miR-294 (B) Ago2-myc-MUT transfected with miR-294 (C) Ago2-myc transfected with cel-239b. The black crosses indicate the three hexamers contained within the miR-294 seed 8-mer. To ascertain whether this extra IP enrichment contains miR-294 targets, the correlation between our data and computational miR-294 target predictions was tested. The fraction of genes ranked by p-value (based on the likelihood of enrichment) that have a TargetScan prediction was calculated. For samples transfected with miR-294, approximately one-tenth (0.1) are predicted targets by TargetScan for the top 1000-most enriched genes (Figure 4A). For samples transfected with cel-239b, the cumulative fraction is considerably lower (0.04–0.06) (Figure 4A). Thus, there is a correspondence between the degree of observed enrichment and the TargetScan computational target predictions. In contrast, there was no correlation with miRanda [28] target predictions (Figure 4B).
Fig. 4. Comparison of genes enriched in the IP with computational and microarray predicted targets. 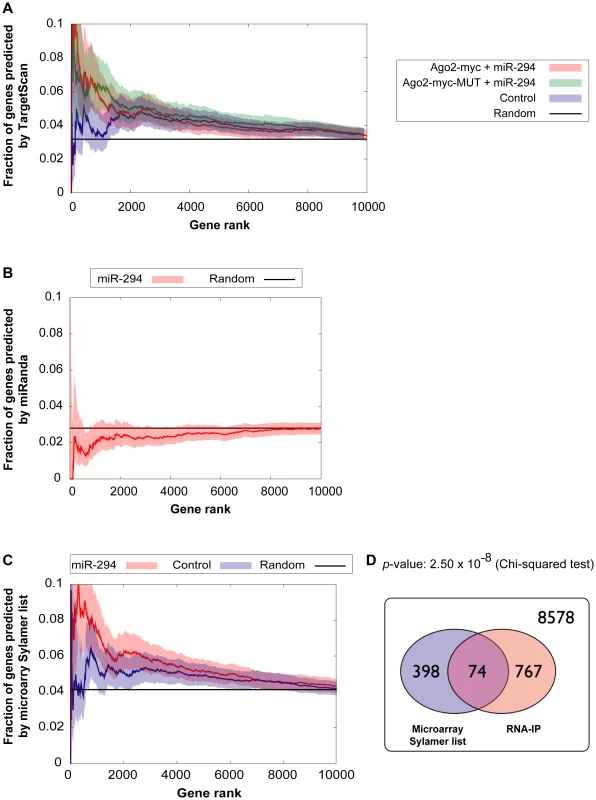
(A,B) Correlation between enriched genes in the IP and computational target predictions. The x-axes represent fraction of genes ranked by increasing p-value for the likelihood of enrichment in the IP. The y-axes represent the fraction of those genes, which have a (A) TargetScan prediction (B) miRanda prediction. Red indicates Ago2-myc samples transfected with miR-294; green indicates Ago2-myc-MUT samples transfected with miR-294; blue indicates samples transfected with cel-239b control miRNA. The black line shows the expected outcome if enrichment and target predictions are independent from one another. (C) Correlation between enriched genes in the IP and microarray target predictions (selected from the Sylamer-based cut-off). The x-axis represents the fraction of genes ranked by p-value for the likelihood of enrichment in the IP. The threshold for selecting enrichment was a p-value <0.003 from the binomial model. (D) Venn diagram overlap between Sylamer microarray target predictions and enriched genes in the IP. 8578 represents the number of genes which were considered (both microarray and RNA-IP data was available) but which were neither depleted on the array nor enriched in the IP. To have a genome-wide benchmark for identifying miR-294 targets, we compared enriched genes in the IP (selected using a p-value <0.003 cut-off) with our microarray-predicted targets. There is a statistically significant correlation between IP enrichment and the microarray-predicted targets (Figure 4C and 4D). The trend is similar to the correlation between IP enrichment and TargetScan predictions (Figure 4A). Thus, miR-294 targets are enriched in the IP but there is also a non-seed-match-specific binding effect that correlates with hexamer GC content.
In summary, a substantial proportion of enriched genes with miR-294 seed matches are not, however, detected in the microarray target predictions. These genes could be direct targets whose transcript levels remain unchanged by miR-294 overexpression and are therefore, not detected by microarray analysis. Alternatively, the basis of this extra enrichment could represent novel targets with non-canonical seed matches, non-specific associations, or technical limitations.
miR-294 may promote pluripotency through regulation of shared c-Myc targets and upregulation of Lin28
To gain an insight into the biological role of miR-294, we performed a Gene Ontology (GO) analysis on the upregulated and downregulated genes (with at least one 6-mer in their 3′ UTR) selected from the microarray (B-statistic >5). The top ten terms included enrichment for genes involved in cell cycle (regulation of the G1-S transition), and development and transcription (Figure 5A). The miR-290-295 cluster has been described as a ‘Trojan horse’ inside ES cells to bring about differentiation [29]. If this were indeed the case, then one would expect to find an enrichment of differentiation-associated terms in the upregulated genes. This is not the case. Instead, the majority of terms associated with differentiation were more enriched in the downregulated genes. In addition, Lin28 was upregulated by miR-294 transfection into Dicer1Δ/Δ+Ago2-myc ES cells (Figure 5C). Lin28 is considered to be important for stem cell maintenance by blocking the processing of let-7 [30], [31], a critical miRNA involved in differentiation [32]. Furthermore, Lin28, in conjunction with Nanog, Oct-4 and Sox2 can reprogram human fibroblasts into pluripotent cells [33]. No pluripotency genes were detected amongst the downregulated genes. This is consistent with a potential role of miR-294 in the maintenance of the pluripotent state because co-expression of miRNAs that directly target the pluripotency factors would be detrimental to ES cells.
Fig. 5. Functional enrichment of genes differentially expressed upon miR-294 transfection. 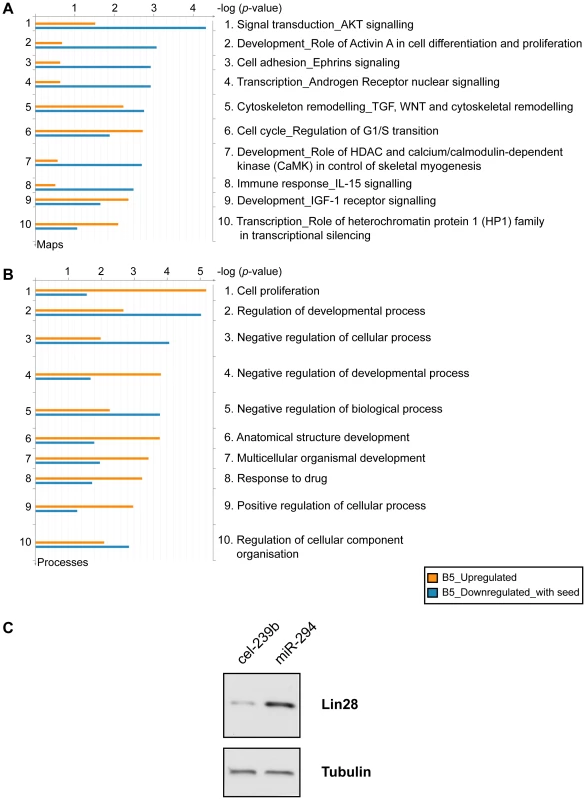
Genes identified with differential expression from the microarray analysis were assigned functions based on Gene Ontology (GO) annotations using the GeneGo tool (GeneGo Inc). Functional ontologies are represented by (A) GeneGo canonical pathway maps (B) GO processes. The top ten terms are shown for downregulated genes (B-statistic >5) with at least one 6-mer site in its 3′ UTR (orange) and upregulated genes (B-statistic >5) (blue). (C) Western blot of Lin28 expression in Dicer1Δ/Δ+Ago2-myc ES cells transfected with cel-239b control miRNA or miR-294. In keeping with cell proliferation as the top-ranking functional GO category (Figure 5B), miR-294 has previously been reported to be able to substitute for c-Myc, but not Oct-4, Sox2 or Klf4, in the reprogramming of mouse embryonic fibroblasts (MEFs) into induced Pluripotent Stem (iPS) cells [14]. The authors posited that miR-294 acts as a downstream target of c-Myc, which binds to the promoter region of mir-290-295. To test the alternative (and not exclusive) possibility that miR-294 may also substitute for c-Myc by regulating shared targets, a GeneGo (GeneGo Inc) network was generated using the downregulated and upregulated genes (with a B-statistic >5) from the microarray as an input list. The resulting networks revealed enrichment for a subset of targets of the c-Myc network (Figure 6). c-Myc was a central node with direct regulatory connections to target genes, but it was not itself a target of miR-294. However, many c-Myc target genes were downregulated directly or upregulated indirectly by miR-294. This suggests that miR-294 and c-Myc regulate an overlapping set of target genes, which is consistent with their comparable roles during iPS induction [14]. Thus, from the GeneGo network, miR-294 appears to act synergistically with c-Myc on a subset of targets. However, miR-294 may also repress a subset of targets that are induced by c-Myc. Since the reprogramming process is not identical when miR-294 is substituted for c-Myc [14], this might perhaps explain some of the observed differences between them during the derivation of iPS cells. This network motif, in which c-Myc both activates a target, and inhibits it via miR-294, is described as “incoherent feedforward” regulation [34]. Our network also includes a previously hypothesised incoherent feedforward loop involving Oct-4, miR-294 and Lefty1/Lefty2 [35]. The functional significance of the incoherent feedforward loop has not been fully delineated but it can accelerate the response following a stimulus by decreasing the target concentration, fine tune steady state levels and buffer against perturbation [34]. In conclusion, miR-294 may promote pluripotency through regulating a shared subset of c-Myc target genes rather than simply being a downstream effector of c-Myc, and through the upregulation of pluripotency-associated genes, such as Lin28.
Fig. 6. c-Myc and Oct-4 networks. 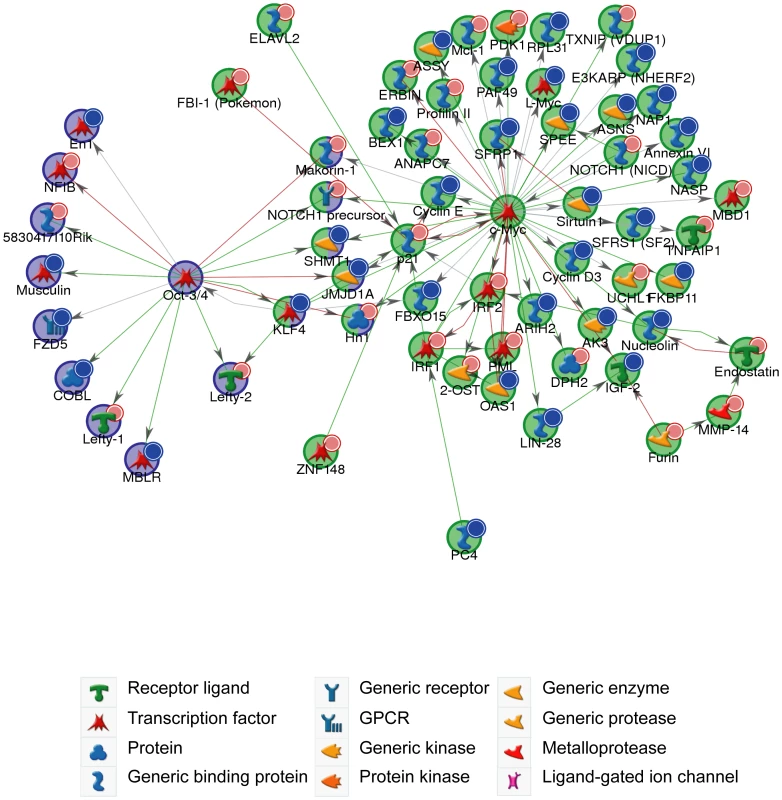
GeneGo network generated from microarray gene expression data. Downregulated genes (B-statistic >5) with at least one 6-mer site in its 3′ UTR are marked with red circles; upregulated (B-statistic >5) with blue circles. c-Myc network (p-value <1.70×10−130); Oct-4 network (p-value <8.20×10−35). Green arrows indicate an activating effect; red arrows an inhibitory effect and grey is an unspecified effect. Discussion
Our findings show that miR-294 regulates a subset of c-Myc target genes, and upregulates Lin28. We did not observe substantial overlap between Oct-4, Nanog or Sox2 regulated genes and miR-294-regulated transcripts. Thus, the effects on c-Myc and Lin28 are distinct from the other core pluripotency factors, consistent with previous data that proposed functional differences between targets of Myc and that of the other core pluripotency factors [36]–[38]. Furthermore, our conclusions are supported by a recent study [39]. However, we did not detect upregulation of c-Myc mRNA by miR-294 in our microarray results, but rather Mycl1, which belongs to the Myc family of transcription factors. This difference could be a result of the different microarray platforms used for the transcriptome analyses.
Indeed, we identified other networks that were enriched for miR-294-regulated transcripts, which included Sp1, Esr1, Hnf4-alpha, p53 and Stat3. The integration of miRNAs into transcriptional networks offers versatility to the regulatory outputs: it enables miRNAs to affect the dynamical properties of transcription factor targets, modulate the strength of transcription factors, and increase the robustness of transcription factor networks. With respect to c-Myc, this model may provide insights on how the initial proliferation defects of Dicer1-null ES cells can be compensated with time [7], [8], and indicates a resetting of the transcriptional network in the absence of miRNAs.
This is the first study to use Dicer1-null ES cells combined with a biochemical approach to identify targets of a single miRNA at a time. A surprising finding was that there was a GC bias in hexamer composition in the 3′ UTRs of enriched transcripts. This effect was much greater for samples transfected with miR-294 than with cel-239b, implying that there is a biological basis for this association. Such transcripts with no predicted canonical seed matches may reflect binding to miRNAs that don't follow the rules of seed-driven target recognition or non-specific associations. Recently, a role for seed sequence-independent regulation has emerged for miR-328, which acts through its C-rich region to titrate a translational inhibitor, hnRNP E2 protein, from its target mRNA [40]. However, the possibility of non-canonical targets would require careful verification.
A pertinent issue for the biochemical approach is the ability of miRNAs to induce mRNA destabilisation independently of Ago2-catalysed slicing, as this could hamper the isolation of mRNAs that are being degraded. One possible way to address this in future work is to explore different Ago2 mutants that cannot interact with factors that bring about mRNA destabilisation, such as GW182 [41]. The use of such a mutant might improve the likelihood of retaining intact mRNAs and increase the efficiency of the pull down.
Despite a large discrepancy between miRNAs found in human and mouse ES cells, there are homologs of the miR-290-295 cluster in human ES cells (miR-371, miR-372 and miR-373) [10], alluding to an important role of these miRNAs in the pluripotency of mammalian ES cells. Given the expression of miR-294 in primordial germ cells [42] and its function in pluripotency, it will be interesting to determine the effects of miR-290-295 conditional deletion upon the germline.
Materials and Methods
Cell culture
Mice carrying floxed alleles of Ago2 or Dicer1 were described previously [43], [44]. Ago2flox/flox;Dicer1flox/flox ES cells were derived from Ago2flox/flox;Dicer1flox/flox blastocysts and cultured on feeders in media supplemented with leukaemia inhibitory factor. Ago2flox/flox;Dicer1flox/flox ES cells were transfected with linearised plasmids of pCAG-Ago2-myc, pCAG-Ago2-myc-MUT or pCAG-myc, and selected with G418. To excise the floxed alleles of Ago2 and Dicer1 in Ago2flox/flox;Dicer1flox/flox stable clones, ES cells were transfected with Cre-GFP plasmid (Addgene). After 24 hr, GFP-positive cells were flow-sorted (FACSAria, BD Biosciences). 2000 or 4000 cells/10 cm dish were plated to enable picking of single colonies. The genotype of individual clones was determined by PCR using primers: Dicer-FN 5′-GGT TAC ATG GCT AGA CTC AAA GC-3′; Dicer-RN 5′-AGG TGC CTT TCG TTT AGG AAC-3′; Dicer-FWF 5′-AAA GCA GAA CTC TAA TGC CCC-3′. It was further confirmed by profiling the expression of mature miRNAs from the miR-290-295 cluster (Figure S1C). ES cells were maintained in ES cell medium, in the absence of feeders on gelatinised tissue culture plates. ES medium consisted of:: DMEM/F12 (Invitrogen), 15% FCS (Gibco), 2 mM L-glutamine (Gibco), 0.1 mM non-essential amino acids (Gibco), 1 mM sodium pyruvate (Sigma), 0.12% sodium bicarbonate (Sigma), 10 µM β-mercaptoethanol (Gibco), 50 µg/ml penicillin/streptomycin (Gibco), and 2×103 U/ml LIF (Chemicon).
Plasmids
The Ago2 coding sequence was amplified from 129Sv/Ev mouse ES cell cDNA by PCR using primers: Ago2-5′ 5′ - AGA ATT CAT GTA CTC GGG AGC CGG CCC CGT TCT T-3′; Ago2-3′ 5′-ATG CGG CCG CTC ACA GAT CCT CTT CTG AGA TGA GTT TTT GTT CAG CAA AGT ACA TGG TGC GCA G-3′ to contain a carboxy-terminal myc-epitope tag. Q633R and H634A point-mutations were introduced by site-directed mutagenesis using the QuiKChange kit (Stratagene). For the mammalian expression constructs, wild-type and point-mutated Ago2-myc in pCAGIG were digested with Sal1-Not1 to include the CAG promoter upstream of the Ago2-myc coding sequence and ligated into pEGFP-1 (Clontech), which was pre-digested with Sal1-Not1 to remove the EGFP coding sequence.
Transfection
Transfections were performed using the Mouse ES Cell Nucleofection Kit (Lonza), and program A23 of Nucleofactor I apparatus (Lonza), as specified by the manufacturer's instructions. Transfections of miRNA mimics mmu-miR-294 and cel-239b (Dharmacon) was performed as described in [17]. Briefly, for microarray analysis, approximately 4×106 Dicer1-null ES cells were resuspended in 90 µl mouse ES cell Nucleofection Solution. 300 pmol of miR-294 and 1 µg pEGFP-1 (Clontech) plasmid (which served as a control for transfection efficiency) were diluted in 10 µl Nucleofection Solution, mixed with the cells and transferred to a Lonza cuvette. For RNA-IP experiments, cells were transfected with 300 pmol of miR-294 or cel-239b control miRNA (Dharmacon) and harvested 12–16 hr after transfection for immunoprecipitation.
Western blotting
Cells were lysed in lysis buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, and protease inhibitors). Equal concentration of the lysed proteins were separated on polyacrylamide-SDS gels, transferred onto PVDF Hybond membrane and probed with the following primary antibodies: anti-Ago2 (Abnova, 1∶1000), Lin28 (Cell Signaling Technology, 1∶1000), Tubulin (Sigma, 1∶5000). This was followed by incubation with horseradish peroxidase-conjugated secondary antibodies. ECL kits (Amersham) were used for detection.
Immunofluorescence
Cells were grown on Lab-Tek chamber slides and fixed with 4% paraformaldehyde in PBS for 15 minutes at room temperature. Cells were blocked in 1% BSA/0.1% Triton X-100 in PBS and incubated O/N at 4°C with the following primary antibodies: anti-Eomes (eBioscience, 1∶200), anti-Oct3/4 (BDBiosciences, 1∶200). This was followed by incubation with AlexaFluor secondary antibodies (1∶500; Molecular Probes) and DAPI (1∶1000; Sigma), for 1 hr at room temperature.
miRNA – and RNA–immunoprecipitation
miRNA - and RNA-immunoprecipitation was performed in native conditions as described [45]–[47]. 2.5×107 cells were pelleted and resuspended in 220 µl ice-cold Polysomal Lysis Buffer [100 mM KCl, 5 mM MgCl2, 10 mM HEPES (pH 7.0; Gibco), 0.5% NP40, 1 mM DTT, 200 U Recombinant RNasin Ribonuclease Inhibitor (Promega), 200 U SUPERase•In RNase inhibitor, and Protease Inhibitor Cocktail]. The lysate was passed through a 27G needle five times, incubated on ice for 30 min at 4°C and then transferred to –80°C to promote lysis. The lysate was then thawed on ice, centrifuged (15 min, maximum speed, 4°C), and the supernatant was transferred to a new tube. 400 U DNase (Roche) was added and incubated on 30 min at 4°C. 20 µl of the lysate was saved for the INPUT at –80°C. The remaining lysate was diluted in 800 µl NT2 buffer [50 mM Tris-Hcl (pH 7.4), 150 mM NaCl, 1 mM MgCl2, 0.05% NP40, Protease Inhibitor Cocktail, 200 U Recombinant RNasin Ribonuclease Inhibitor] and pre-cleared for 2 hr at 4°C with 20 µl Dynabeads-Protein A (Invitrogen), which had been pre-blocked in 0.5% BSA +1 mg/ml yeast tRNA (Ambion). The supernatant was incubated with 20 µl anti-myc (Cell Signaling Technology) antibody O/N at 4°C and 200 U Recombinant RNasin Ribonuclease Inhibitor. The next day, the RNA/antibody complex was precipitated by addition of 50 µl Dynabeads-Protein A for 2 hr at 4°C. The beads were washed with 1 ml NT2 buffer four times and then resuspended in 100 µl NT2 buffer and transferred to a fresh tube. 80 µl of NT2 buffer was added to 20 µl of the stored INPUT. RNA was extracted with 1 ml Trizol according to the manufacturer's protocol and RNA was resuspended in 20 µl H2O. To amplify mRNA 20 cycles for sequencing, 1 µl of IP (10–40 ng/µl) and 1 µl of INPUT (diluted to 0.5–2.5 ng/µl) were used. For sequencing of mRNAs, the single cell method was modified according to [48]. For 220-plex microRNA expression profiling, 220 miRNAs were reverse transcribed, amplified and then analysed by Q-PCR, as described [49], [50].
Real-time quantitative PCR
Quantitative PCR was performed on an ABI PRISM 7000 sequence detection system (Applied Biosystems). For SYBR Green fluorescent nucleic acid dye, primers were designed in order to achieve product lengths between 50 and 100 bp. TaqMan probes were used for detection of mature miRNAs by Q-PCR. The reaction conditions were: 95°C 10 min, 40× (95°C 15 s, 60°C 1 min). Data was normalised to HPRT, or INPUT RNA amount in the case of RNA - and miRNA-immunoprecipitation experiments, using the ΔΔCT method [51].
Microarray
Feeder-free Ago2Δ/Δ;Dicer1Δ/Δ+Ago2-myc ES cells were transfected in triplicate with 1 µg of pEGFP-1 and 300 pmol of miR-294 mimic or C. elegans cel-239b control miRNA and harvested 24 hr later. Cells were sorted for GFP expression and total RNA was isolated using the RNeasy Mini kit (Qiagen). 1 µg of total RNA from each triplicate (six samples in total) was sent to Cambridge Genomic Services, Cambridge University for sample processing and hybridisation to MouseWG-6 v2.0 Illumina microarrays. Cambridge Genomic Services performed the quality control analysis, gene selection, and data normalisation. Microarray data analysis was carried out using the R language with Bioconductor packages [52] and custom-written code. GO and network analysis were performed using the online software MetaCore from GeneGo Inc. Microarray data were deposited in the Gene Expression Omnibus (GEO) database (Accession Code: GSE20048).
RNA–Sequencing
The amplified cDNAs from the IP and INPUT were subjected to SOLiD library preparation by ABI. The cDNAs were then sequenced by ABI's next-generation sequencing SOLiD system. RNA-Sequencing reads were mapped to RefSeq transcripts using ABI's pipeline, as described [48]. To visualise the data, we typically plotted the fraction of reads for a given gene which came from the IP library:
Differential representation was measured as the probability that was drawn from a binomial distribution withand
Sequencing data were deposited in the GEO database (Accession Code: GSE20199).
Supporting Information
Zdroje
1. HutvágnerG
McLachlanJ
PasquinelliAE
BálintE
TuschlT
2001 A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 293 834 838
2. KettingRF
FischerSE
BernsteinE
SijenT
HannonGJ
2001 Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes & Development 15 2654 2659
3. HammondSM
BernsteinE
BeachD
HannonGJ
2000 An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404 293 296
4. HammondSM
BoettcherS
CaudyAA
KobayashiR
HannonGJ
2001 Argonaute2, a link between genetic and biochemical analyses of RNAi. Science 293 1146 1150
5. HutvágnerG
ZamorePD
2002 A microRNA in a multiple-turnover RNAi enzyme complex. Science 297 2056 2060
6. BernsteinE
KimSY
CarmellMA
MurchisonEP
AlcornH
2003 Dicer is essential for mouse development. Nat Genet 35 215 217
7. KanellopoulouC
MuljoSA
KungAL
GanesanS
DrapkinR
2005 Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes & Development 19 489 501
8. MurchisonEP
PartridgeJF
TamOH
CheloufiS
HannonGJ
2005 Characterization of Dicer-deficient murine embryonic stem cells. Proc Natl Acad Sci USA 102 12135 12140
9. HoubaviyHB
MurrayMF
SharpPA
2003 Embryonic stem cell-specific MicroRNAs. Developmental Cell 5 351 358
10. SuhM-R
LeeY
KimJY
KimS-K
MoonS-H
2004 Human embryonic stem cells express a unique set of microRNAs. Developmental Biology 270 488 498
11. CalabreseJM
SeilaAC
YeoGW
SharpPA
2007 RNA sequence analysis defines Dicer's role in mouse embryonic stem cells. Proc Natl Acad Sci USA 104 18097 18102
12. BartelDP
2009 MicroRNAs: target recognition and regulatory functions. Cell 136 215 233
13. WangY
BaskervilleS
ShenoyA
BabiarzJE
BaehnerL
2008 Embryonic stem cell-specific microRNAs regulate the G1-S transition and promote rapid proliferation. Nat Genet 40 1478 1483
14. JudsonRL
BabiarzJE
VenereM
BlellochR
2009 Embryonic stem cell-specific microRNAs promote induced pluripotency. Nature Biotechnology 27 459 461
15. CiaudoC
ServantN
CognatV
SarazinA
KiefferE
2009 Highly dynamic and sex-specific expression of microRNAs during early ES cell differentiation. PLoS Genet 5 e1000620 doi:10.1371/journal.pgen.1000620
16. BenettiR
GonzaloS
JacoI
MuñozP
GonzalezS
2008 A mammalian microRNA cluster controls DNA methylation and telomere recombination via Rbl2-dependent regulation of DNA methyltransferases. Nature Structural & Molecular Biology 15 268 279
17. SinkkonenL
HugenschmidtT
BerningerP
GaidatzisD
MohnF
2008 MicroRNAs control de novo DNA methylation through regulation of transcriptional repressors in mouse embryonic stem cells. Nature Structural & Molecular Biology 15 259 267
18. WangY
ShengG
JuranekS
TuschlT
PatelDJ
2008 Structure of the guide-strand-containing argonaute silencing complex. Nature 456 209 213
19. BeitzingerM
PetersL
ZhuJY
KremmerE
MeisterG
2007 Identification of human microRNA targets from isolated argonaute protein complexes. RNA Biol 4 76 84
20. HendricksonDG
HoganDJ
HerschlagD
FerrellJE
BrownPO
2008 Systematic identification of mRNAs recruited to argonaute 2 by specific microRNAs and corresponding changes in transcript abundance. PLoS ONE 3 e2126 doi:10.1371/journal.pone.0002126
21. ChiSW
ZangJB
MeleA
DarnellRB
2009 Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature 460 479 486
22. LeeRC
FeinbaumRL
AmbrosV
1993 The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75 843 854
23. WightmanB
HaI
RuvkunG
1993 Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75 855 862
24. LimLP
LauNC
Garrett-EngeleP
GrimsonA
SchelterJM
2005 Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433 769 773
25. Van DongenS
Abreu-GoodgerC
EnrightAJ
2008 Detecting microRNA binding and siRNA off-target effects from expression data. Nat Meth 5 1023 1025
26. JinekM
DoudnaJA
2009 A three-dimensional view of the molecular machinery of RNA interference. Nature 457 405 412
27. LiuJ
CarmellMA
RivasFV
MarsdenCG
ThomsonJM
2004 Argonaute2 is the catalytic engine of mammalian RNAi. Science 305 1437 1441
28. BetelD
WilsonM
GabowA
MarksDS
SanderC
2008 The microRNA.org resource: targets and expression. Nucleic Acids Research 36 D149053
29. GangarajuVK
LinH
2009 MicroRNAs: key regulators of stem cells. Nature Reviews Molecular Cell Biology 10 116 125
30. ViswanathanSR
DaleyGQ
GregoryRI
2008 Selective blockade of microRNA processing by Lin28. Science 320 97 100
31. HeoI
JooC
KimY-K
HaM
YoonM-J
2009 TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 138 696 708
32. BüssingI
SlackFJ
GrosshansH
2008 let-7 microRNAs in development, stem cells and cancer. Trends in molecular medicine 14 400 409
33. YuJ
VodyanikMA
Smuga-OttoK
Antosiewicz-BourgetJ
FraneJL
2007 Induced pluripotent stem cell lines derived from human somatic cells. Science 318 1917 1920
34. AlonU
2007 Network motifs: theory and experimental approaches. Nat Rev Genet 8 450 461
35. MarsonA
LevineSS
ColeMF
FramptonGM
BrambrinkT
2008 Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell 134 521 533
36. ChenX
XuH
YuanP
FangF
HussM
2008 Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133 1106 1117
37. KimJ
ChuJ
ShenX
WangJ
OrkinSH
2008 An extended transcriptional network for pluripotency of embryonic stem cells. Cell 132 1049 1061
38. SridharanR
TchieuJ
MasonMJ
YachechkoR
KuoyE
2009 Role of the murine reprogramming factors in the induction of pluripotency. Cell 136 364 377
39. MeltonC
JudsonRL
BlellochR
2010 Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature 463 621 626
40. EiringAM
HarbJG
NevianiP
GartonC
OaksJJ
2010 miR-328 Functions as an RNA Decoy to Modulate hnRNP E2 Regulation of mRNA Translation in Leukemic Blasts. Cell 140 652 665
41. TillS
LejeuneE
ThermannR
BortfeldM
HothornM
2007 A conserved motif in Argonaute-interacting proteins mediates functional interactions through the Argonaute PIWI domain. Nature Structural & Molecular Biology 14 897 903
42. HayashiK
Chuva De Sousa LopesSM
KanedaM
TangF
HajkovaP
2008 MicroRNA biogenesis is required for mouse primordial germ cell development and spermatogenesis. PLoS ONE 3 e1738 doi:10.1371/journal.pone.0001738
43. O'CarrollD
MecklenbraukerI
DasPP
SantanaA
KoenigU
2007 A Slicer-independent role for Argonaute 2 in hematopoiesis and the microRNA pathway. Genes & Development 21 1999 2004
44. YiR
O'carrollD
PasolliHA
ZhangZ
DietrichFS
2006 Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat Genet 38 356 362
45. PeritzT
ZengF
KannanayakalTJ
KilkK
EiríksdóttirE
2006 Immunoprecipitation of mRNA-protein complexes. Nature Protocols 1 577 580
46. KeeneJD
KomisarowJM
FriedersdorfMB
2006 RIP-Chip: the isolation and identification of mRNAs, microRNAs and protein components of ribonucleoprotein complexes from cell extracts. Nature Protocols 1 302 307
47. ZhaoJ
SunBK
ErwinJA
SongJ-J
LeeJT
2008 Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 322 750 756
48. TangF
BarbacioruC
WangY
NordmanE
LeeC
2009 mRNA-Seq whole-transcriptome analysis of a single cell. Nat Meth 6 377 382
49. TangF
HajkovaP
BartonSC
LaoK
SuraniMA
2006 MicroRNA expression profiling of single whole embryonic stem cells. Nucleic Acids Research 34 e9
50. TangF
HajkovaP
BartonSC
O'carrollD
LeeC
2006 220-plex microRNA expression profile of a single cell. Nature Protocols 1 1154 1159
51. NolanT
HandsRE
BustinSA
2006 Quantification of mRNA using real-time RT-PCR. Nature Protocols 1 1559 1582
52. GentlemanRC
CareyVJ
BatesDM
BolstadB
DettlingM
2004 Bioconductor: open software development for computational biology and bioinformatics. Genome Biology 2008 9 : 210 5 R80
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 10- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Intrauterinní inseminace a její úspěšnost
- Délka menstruačního cyklu jako marker ženské plodnosti
-
Všechny články tohoto čísla
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- FSHD: A Repeat Contraction Disease Finally Ready to Expand (Our Understanding of Its Pathogenesis)
- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- The Meiotic Recombination Checkpoint Suppresses NHK-1 Kinase to Prevent Reorganisation of the Oocyte Nucleus in
- Actin Depolymerizing Factors Cofilin1 and Destrin Are Required for Ureteric Bud Branching Morphogenesis
- DSIF and RNA Polymerase II CTD Phosphorylation Coordinate the Recruitment of Rpd3S to Actively Transcribed Genes
- Continuous Requirement for the Clr4 Complex But Not RNAi for Centromeric Heterochromatin Assembly in Fission Yeast Harboring a Disrupted RITS Complex
- Genome-Wide Association Study of Blood Pressure Extremes Identifies Variant near Associated with Hypertension
- The Cytosine Methyltransferase DRM2 Requires Intact UBA Domains and a Catalytically Mutated Paralog DRM3 during RNA–Directed DNA Methylation in
- β-Actin and γ-Actin Are Each Dispensable for Auditory Hair Cell Development But Required for Stereocilia Maintenance
- Genetic Association Study Identifies as a Risk Gene for Idiopathic Dilated Cardiomyopathy
- Evidence for a Xer/ System for Chromosome Resolution in Archaea
- Four Novel Loci (19q13, 6q24, 12q24, and 5q14) Influence the Microcirculation
- Lifespan Extension by Preserving Proliferative Homeostasis in
- Ancient and Recent Adaptive Evolution of Primate Non-Homologous End Joining Genes
- Loss of the p53/p63 Regulated Desmosomal Protein Perp Promotes Tumorigenesis
- Altering a Histone H3K4 Methylation Pathway in Glomerular Podocytes Promotes a Chronic Disease Phenotype
- Characterization of LINE-1 Ribonucleoprotein Particles
- Conserved Genes Act as Modifiers of Invertebrate SMN Loss of Function Defects
- Alternative Splicing at a NAGNAG Acceptor Site as a Novel Phenotype Modifier
- Tight Regulation of the Gene of the KplE1 Prophage: A New Paradigm for Integrase Gene Regulation
- Conjugative DNA Transfer Induces the Bacterial SOS Response and Promotes Antibiotic Resistance Development through Integron Activation
- Nasty Viruses, Costly Plasmids, Population Dynamics, and the Conditions for Establishing and Maintaining CRISPR-Mediated Adaptive Immunity in Bacteria
- Stress-Induced Activation of Heterochromatic Transcription
- H3K27me3 Profiling of the Endosperm Implies Exclusion of Polycomb Group Protein Targeting by DNA Methylation
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
- Characterising and Predicting Haploinsufficiency in the Human Genome
- Dual Functions of ASCIZ in the DNA Base Damage Response and Pulmonary Organogenesis
- Pervasive Cryptic Epistasis in Molecular Evolution
- Transition from Positive to Neutral in Mutation Fixation along with Continuing Rising Fitness in Thermal Adaptive Evolution
- Comprehensive Analysis Reveals Dynamic and Evolutionary Plasticity of Rab GTPases and Membrane Traffic in
- Regulates Tissue-Specific Mitochondrial DNA Segregation
- Role for the Mammalian Swi5-Sfr1 Complex in DNA Strand Break Repair through Homologous Recombination
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



