-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Pervasive Cryptic Epistasis in Molecular Evolution
The functional effects of most amino acid replacements accumulated during molecular evolution are unknown, because most are not observed naturally and the possible combinations are too numerous. We created 168 single mutations in wild-type Escherichia coli isopropymalate dehydrogenase (IMDH) that match the differences found in wild-type Pseudomonas aeruginosa IMDH. 104 mutant enzymes performed similarly to E. coli wild-type IMDH, one was functionally enhanced, and 63 were functionally compromised. The transition from E. coli IMDH, or an ancestral form, to the functional wild-type P. aeruginosa IMDH requires extensive epistasis to ameliorate the combined effects of the deleterious mutations. This result stands in marked contrast with a basic assumption of molecular phylogenetics, that sites in sequences evolve independently of each other. Residues that affect function are scattered haphazardly throughout the IMDH structure. We screened for compensatory mutations at three sites, all of which lie near the active site and all of which are among the least active mutants. No compensatory mutations were found at two sites indicating that a single site may engage in compound epistatic interactions. One complete and three partial compensatory mutations of the third site are remote and lie in a different domain. This demonstrates that epistatic interactions can occur between distant (>20Å) sites. Phylogenetic analysis shows that incompatible mutations were fixed in different lineages.
Published in the journal: . PLoS Genet 6(10): e32767. doi:10.1371/journal.pgen.1001162
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001162Summary
The functional effects of most amino acid replacements accumulated during molecular evolution are unknown, because most are not observed naturally and the possible combinations are too numerous. We created 168 single mutations in wild-type Escherichia coli isopropymalate dehydrogenase (IMDH) that match the differences found in wild-type Pseudomonas aeruginosa IMDH. 104 mutant enzymes performed similarly to E. coli wild-type IMDH, one was functionally enhanced, and 63 were functionally compromised. The transition from E. coli IMDH, or an ancestral form, to the functional wild-type P. aeruginosa IMDH requires extensive epistasis to ameliorate the combined effects of the deleterious mutations. This result stands in marked contrast with a basic assumption of molecular phylogenetics, that sites in sequences evolve independently of each other. Residues that affect function are scattered haphazardly throughout the IMDH structure. We screened for compensatory mutations at three sites, all of which lie near the active site and all of which are among the least active mutants. No compensatory mutations were found at two sites indicating that a single site may engage in compound epistatic interactions. One complete and three partial compensatory mutations of the third site are remote and lie in a different domain. This demonstrates that epistatic interactions can occur between distant (>20Å) sites. Phylogenetic analysis shows that incompatible mutations were fixed in different lineages.
Introduction
In a half century of molecular phylogenetics there never has been a systematic investigation of the functional and fitness effects of amino acid replacements in evolution. Experimental studies focus on those few mutations that change protein function [1]. Of the remaining thousands of replacements nothing is said – they may or may not be of functional consequence. Sequence analyses use statistical approaches to explore modes of evolution [2], [3]. Rarely does fitting alternative evolutionary models to observed data disallow alternative explanations. For example, to some [4]–[6] an elevated ratio of amino acid replacements to silent substitutions between species (dn/ds) suggests evidence for the action of positive selection. To others [7] it suggests relaxed selection against slightly deleterious amino acid replacements during population bottlenecks. Both interpretations are viable.
Non-additive interactions among mutations (epistasis) are critical to protein structure and function [1], [8] and consequently to speciation [9], the evolution of sex [10], recombination [11], dominance [12], robustness [13] and human disease [14]. Non-additive interactions force sites to functionally co-vary during evolution [15], [16]. Computational methods that ignore phylogenetic structure [17]–[29] fail to distinguish between co-variation arising from functional causes and co-variation arising though shared common ancestry. The latter, an ineluctable product of shared history, is reflected in the bifurcating hierarchy of a phylogenetic tree (a tree must collapse to a star burst if no sites co-vary). Computational methods that account for phylogenetic structure [30]–[38] have identified sites likely to functionally co-evolve [32], [36]. The relative scarcity of such sites accords with the observation that most amino acid replacements occur at the surfaces of proteins where solvent exposed side chains are less likely to interact [39], [40]. On the other hand it may simply reflect a lack of statistical power in many, though not all [32], of computational methods used. An alternative approach identifies pathogenic missense mutations in one species that have no obvious detrimental effect in a related species [41], [42]. This approach does not detect deleterious mutations of minor phenotypic effect.
Computationally derived predictions need empirical verification [1]. Gloor et al. [43] used site directed mutagenesis to confirm the epistasis predicted between co-evolving residues in yeast phosphoglycerate kinase. Experiments with yeast iso-2-cytochrome c [44] also identified epistatic interactions between sites. However, two other studies, one with game bird lysozymes [45], [46] and one with vertebrate p53 domains [47], failed to find any evidence of epistasis. Several other site directed mutagenesis studies identified epistatic interactions among positively selected replacements in TEM-1 β-lacatamase [48], vertebrate steroid receptors [49] and visual pigments [50] and in coral red fluorescent proteins [51]. In no case, however, have experiments been designed to explore the prevalence of epistasis in molecular evolution in general.
Here, we explore the prevalence of epistasis in molecular evolution from the distribution of functional effects caused by individual mutations introduced to one sequence from a homologue in another species. We studied the leuB encoded β-isopropylmalate dehydrogenase (IMDH) because: 1) the enzyme has a conserved well defined role in leucine biosynthesis [52], [53]; 2) high resolution x-ray crystallography of divergent IMDHs (<35% identical) reveals a conserved protein fold [54]–[57]; sequence alignments show that divergent IMDHs rarely differ by more than a few insertions and deletions; and 4) the relationship between enzyme performance (kcat/Km.NAD) and fitness has been determined using Escherichia coli as a model system [58]. The IMDHs from two mesophyles, E. coli and P. aeruginosa, differ at 168 of 365 sites including six small indels (Figure S1) located in flexible loops external to the core structure. Conserved in fold and function, E. coli and P. aeruginosa IMDHs provide excellent material with which to investigate protein evolution arising through sequence divergence in the absence of major changes in structure and function.
Results/Discussion
Functional Effects of Single Amino Acid Replacements
We constructed 168 site directed mutants of E. coli leuB (each with a single mutation from P. aeruginosa leuB) and then expressed and purified each enzyme and determined its kinetic parameters. The resulting distribution of enzyme performances (kcat/Km.NAD) is strongly skewed to the left and only a single outlier with increased performance lies on the right (Figure 1A). The error distribution, obtained by repeatedly assaying wild-type E. coli IMDH, is Gaussian, , (Figure 1B). This same distribution is expected of amino acid replacements that do not affect function. The 52 mutants with relative performances above E. coli wild-type form a half-Gaussian distribution, , , similar to the error distribution. This suggests mutations have no detectable effect on enzyme performance, 63 reduce it, and one increases it. Pair-wise t-tests (for unequal replication and unequal variances [59]) combine with a 5% false discovery rate [60] to identify 61 mutants of changed performance: 56 have decreased performance and 5 have increased performance, with only the single outlier having performance increased by more than 15%.
Fig. 1. Cryptic epistasis in IMDH. 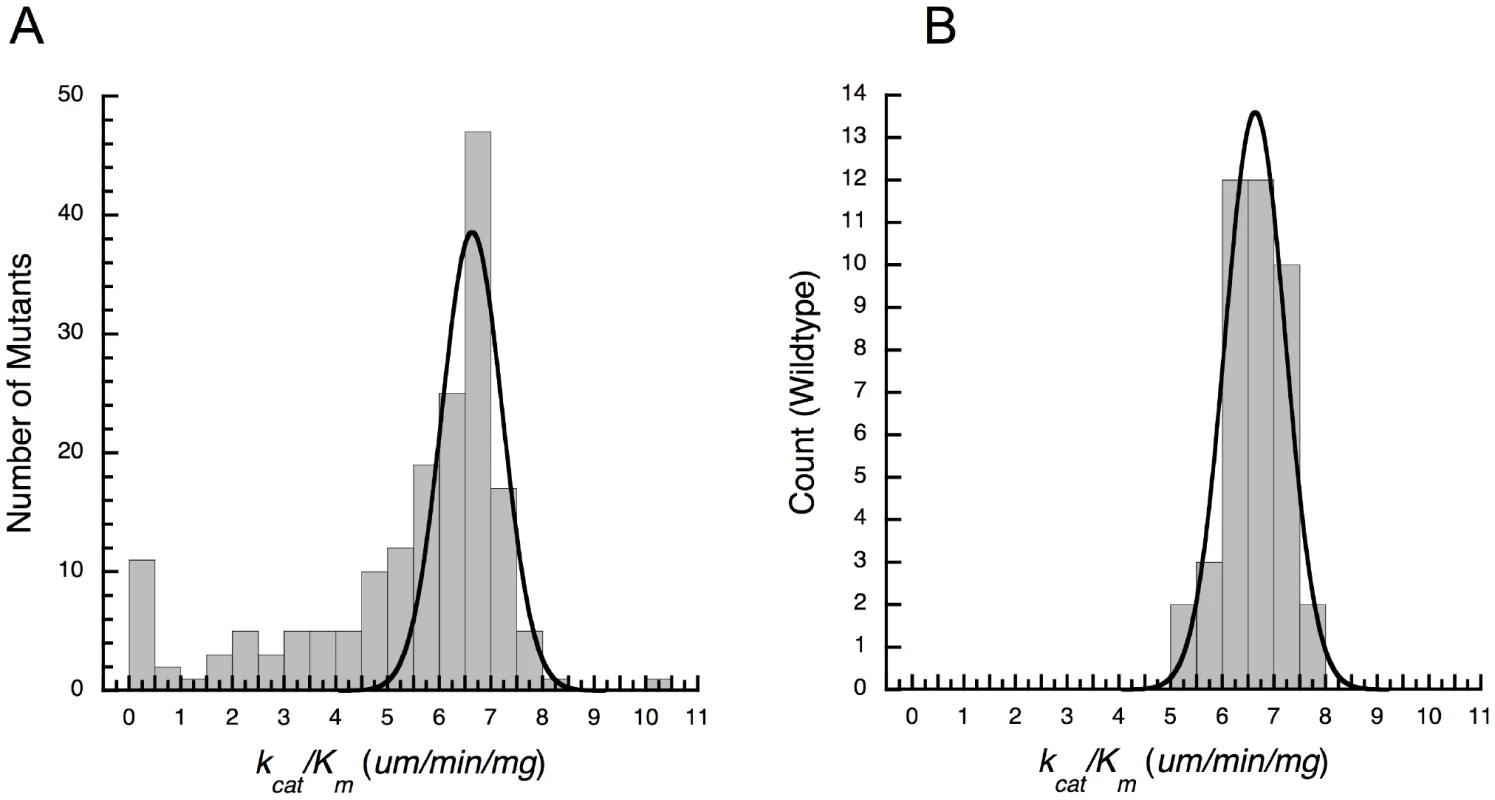
(A) Distribution of functional effects produced upon moving single amino acids and indels from P. aeruginosa IMDH into E. coli IMDH. The shoulder on the left indicates the presence of extensive epistasis. (B) Error distribution obtained by repeatedly assaying wild-type E. coli IMDH. Gaussian distributions fitted as described in the text. The assumption that mutations act independently, and hence additively, leads to a predicted performance for P. aeruginosa IMDH that is clearly wrong. The sum of the individual mutational effects and the E. coli kcat/Km.NAD is negative: . This is a physical impossibility. The assumption that mutations act multiplicatively is also wrong. In simple transition state theory , where ΔG′ is the difference in free energy between the ground state and the transition state, R is the gas constant and T is °Kelvin [61]. The difference in free energy between the transition states of the mutant and wild-type enzymes is . With five mutants completely inactive the sum of the ΔΔG′ and is minus infinity and the predicted performance is . In fact P. aeruginosa IMDH has a , , slightly lower than that of E. coli IMDH which has a , . The inescapable conclusion is that amino acid replacements at many sites interact. IMDH evolution is characterized by rampant epistasis that remains cryptic until revealed by experiment.
That only 64 of the 168 sites affect function certainly underestimates the number that interact epistatically. To understand this, consider two cases in which two sites are involved in a simple pair-wise interaction (Figure 2). In each case, only one of the two sites reduces function when an amino acid in one species is mutated to that found in another. Mutating the second site restores the ancestral functional state. Mutations at both sites may reduce function if three or more amino acid replacements arose during the course of evolution (Figure S2A) – although this is not guaranteed. In a simple network of pair-wise interactions only two of three sites might be identified (Figure S2B). We expect some of the remaining 104 sites to engage in epistatic interactions.
Fig. 2. The evolution of cryptic epistasis. 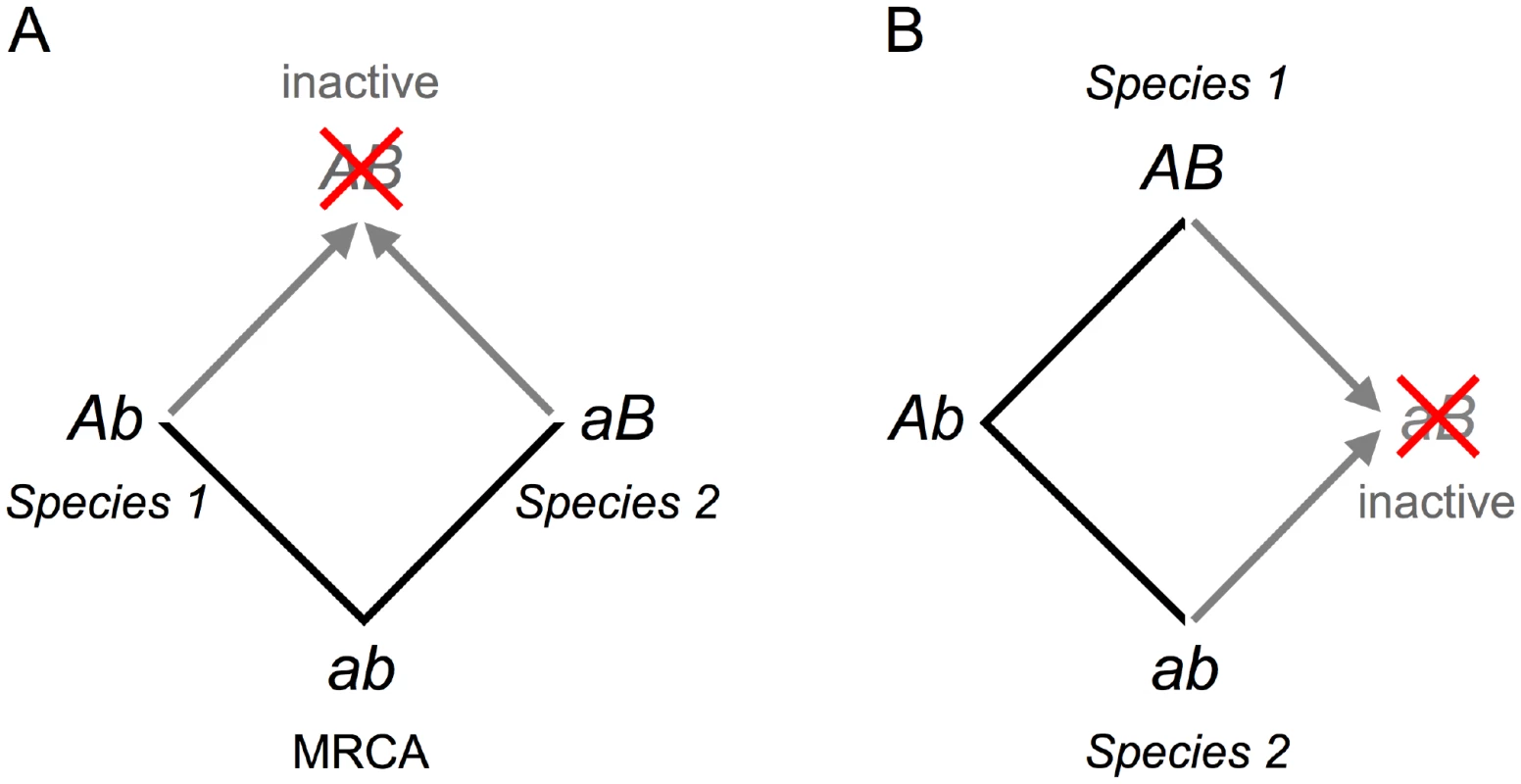
(A) Two lineages diverge from the most recent common ancestor (MRCA), genotype ab. Each fixes a mutation to produce genotypes Ab and aB. The presence of either mutation prevents the second becoming fixed because the double mutant, genotype AB, is deleterious. Cryptic epistasis is revealed upon moving mutation A from species 1 into species 2 or moving mutation B from species 2 into species 1 (gray arrows). Moving mutations a or b to form genotype ab restores the ancestral functional state. (B) Cryptic epistasis can also occur when both mutations arise in the same lineage leading to species 1. In this case cryptic epistasis is revealed upon moving mutation a into species 1 or mutation B into species 2 (gray arrows). Moving mutations A or b to form genotype Ab regenerates a functional evolutionary intermediate. Zuckerkandl [15] first proposed that amino acid replacements at one site in a protein might influence the acceptability of amino acid replacements at other sites. Fitch and Markowitz [16] suggested that as species diverge from a common ancestor their sets of variable sites also diverge to explore different regions of sequence space. Mutating a currently invariant site in one species by introducing an amino acid from a homologous protein in another species risks producing a loss-of-function mutant. The many functionally compromised mutants in this study amply confirm the insight of these early pioneers.
Location of Deleterious Amino Acid Replacements
Mutations affecting function (<80% wild-type performance) are scattered throughout the IMDH structure (Figure S3). Solvent accessibility, distance to the catalytic center (Asp 251), secondary structure and rate of amino acid replacement per site do not correlate significantly with performance for the mutants analyzed here. Only one mutation, F73L, likely affects catalysis by contacting a substrate directly (Figure S4).
Identifying Compensatory Mutations
We screened for compensatory mutations of F73L, A94D and A284C, all of which lie near the active site and all of which are among the least active mutants. We combined each deleterious mutation with each of the 167 remaining mutations, expressed and purified each double mutant, and assayed their activities. Four mutations compensate the F73L mutation (Table 1). F120A, identified in the original screen because it produces a 50% increase in wild-type performance, now produces a 6-fold increase in performance so that the F73L,F120A double mutant, while not as active as the E. coli wild-type enzyme, is as active as the P. aeruginosa enzyme. Three other compensatory mutants, F132L C136I and I179V, do not individually affect wild-type performance. Performance is completely restored to E. coli wild-type levels in the F73L,I179V double mutant. Performance is partly restored in the F73L,F132L and F73L,C136I double mutants. No mutations were found to restore function to A94D and A284C.
Tab. 1. Performance of mutants towards NAD. 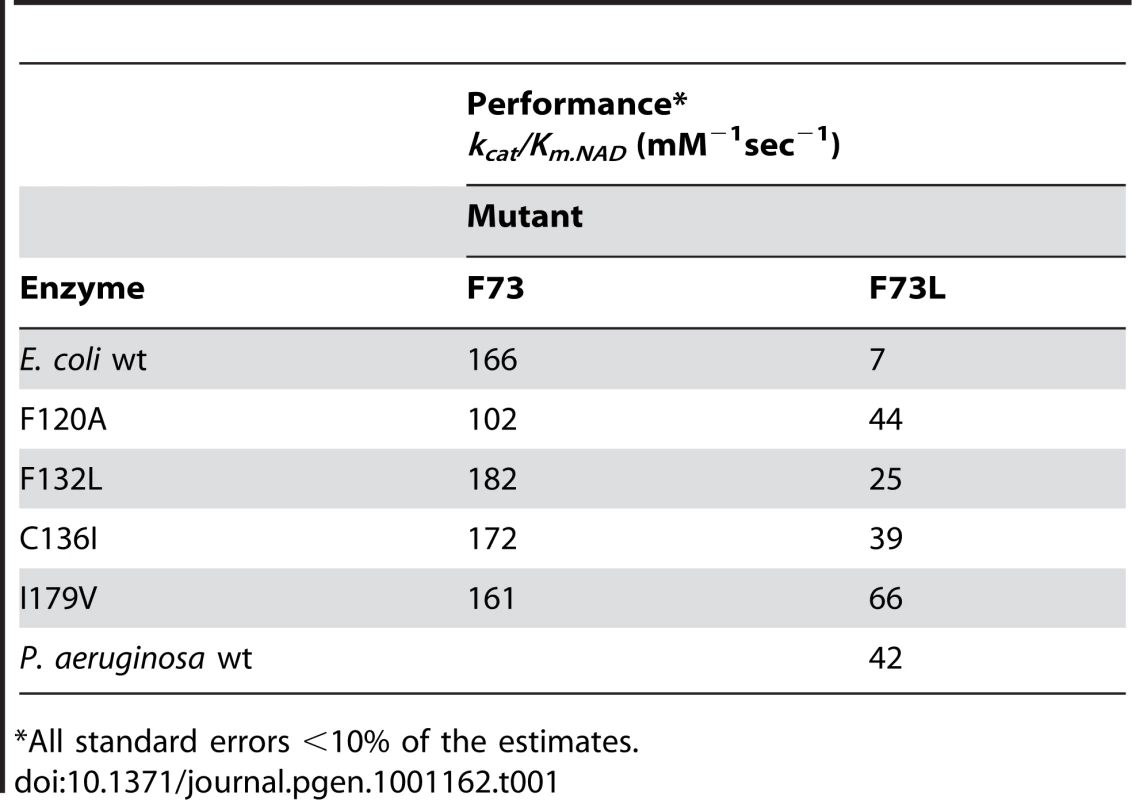
*All standard errors <10% of the estimates. Our results are compatible with several types of interactions. Using E. coli IMDH performance as a standard suggests a simple pair-wise interaction between L73 and I179 because only L73F and I179V are fully compensatory. Using the lower P. aeruginosa IMDH performance as a standard suggests a high-order interaction with function compromised only when residues L73, F120, C136, I179 are combined. Replacing any one amino acid, L73F, F120A, C136I, or I179V destroys the 4-way interaction to restore full performance. That no mutation restores function to A94D and A284C demonstrates several compensatory mutations are essential; at least two sites (A and B) must each interact with each original mutation (X) to form a simple chain (A-X-B).
Whereas previous experimental studies [41]–[45] assumed interacting residues would be in close physical contact, all compensatory mutations for F73L in the large domain lie more than 20 Å distant in the small domain, close to a hinge in the b-sheet on which the two domains swivel (Figure 3). This suggests a common mode of action, possibly related to repositioning the F73L-shifted nicotinamide ring for catalysis. Our experimental strategy, of moving replacements from one homologue into another and screening for compensatory mutations, is useful in that it provides a general means to identify interacting sites regardless of the mechanisms involved.
Fig. 3. Locations of compensatory mutations for F73L. 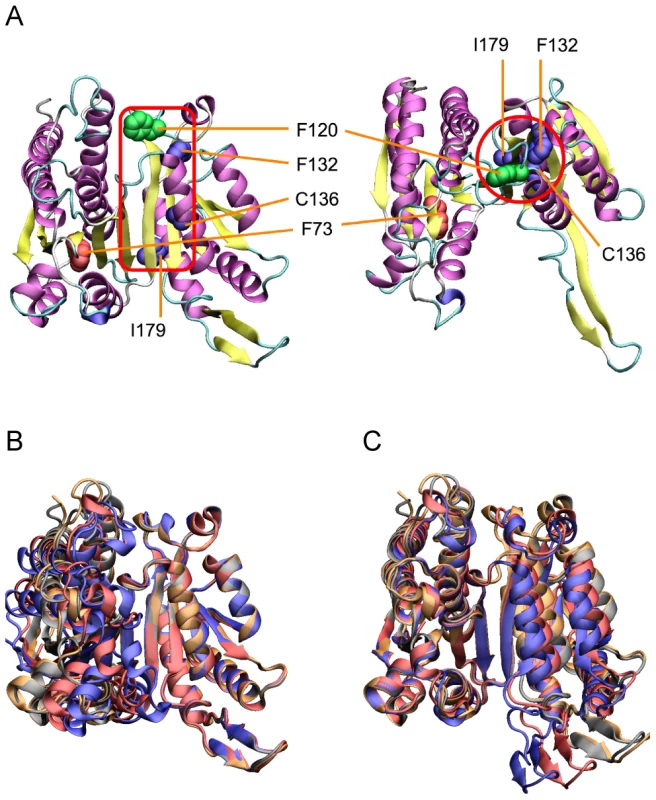
Compensatory mutations for the deleterious effects of F73L in the large domain are located in the small domain close to the hinge in the b-sheet. (A) Two views of the locations of the compensatory mutations for F73L in E. coli IMDH (space filling: brown for F73L, green for F120A and blue for F132L, C136I and I179V). (B) The two domains of IMDH are built on the β-sheet and move as rigid bodies. Superimposing the small right-hand domains of E. coli and S. typhimurium IMDHs reveals rigid body movements of the large domains on the left. (C) Superimposing the large left-hand domains reveals rigid body movements of the small domains on the right. The hinge region lies in the β-sheet between the two domains. Structures from E. coli (pdb 1CM7) and S. typhimurium IMDHs (pdb 1CNZ). Predicted Fitness Effects
The predicted fitness effects of most mutations are tiny. Previous work [56] established that wild-type E. coli IMDH lies on a fitness plateau (Figure 4A). In this limit of adaptation [62], increases in performance do not improve fitness and even large reductions in IMDH performance produce small fitness effects (Figure 4B). Indeed, 65% of the selection coefficients are predicted to be less than 10−5/generation. Selection during starvation growth with glucose as the sole limiting resource is far greater than in nature where leucine is both widely available and abundant [63]. Many mutations, including the one with increased performance, are likely selectively neutral, or very nearly so.
Fig. 4. Large changes in IMDH performance cause small changes in fitness. 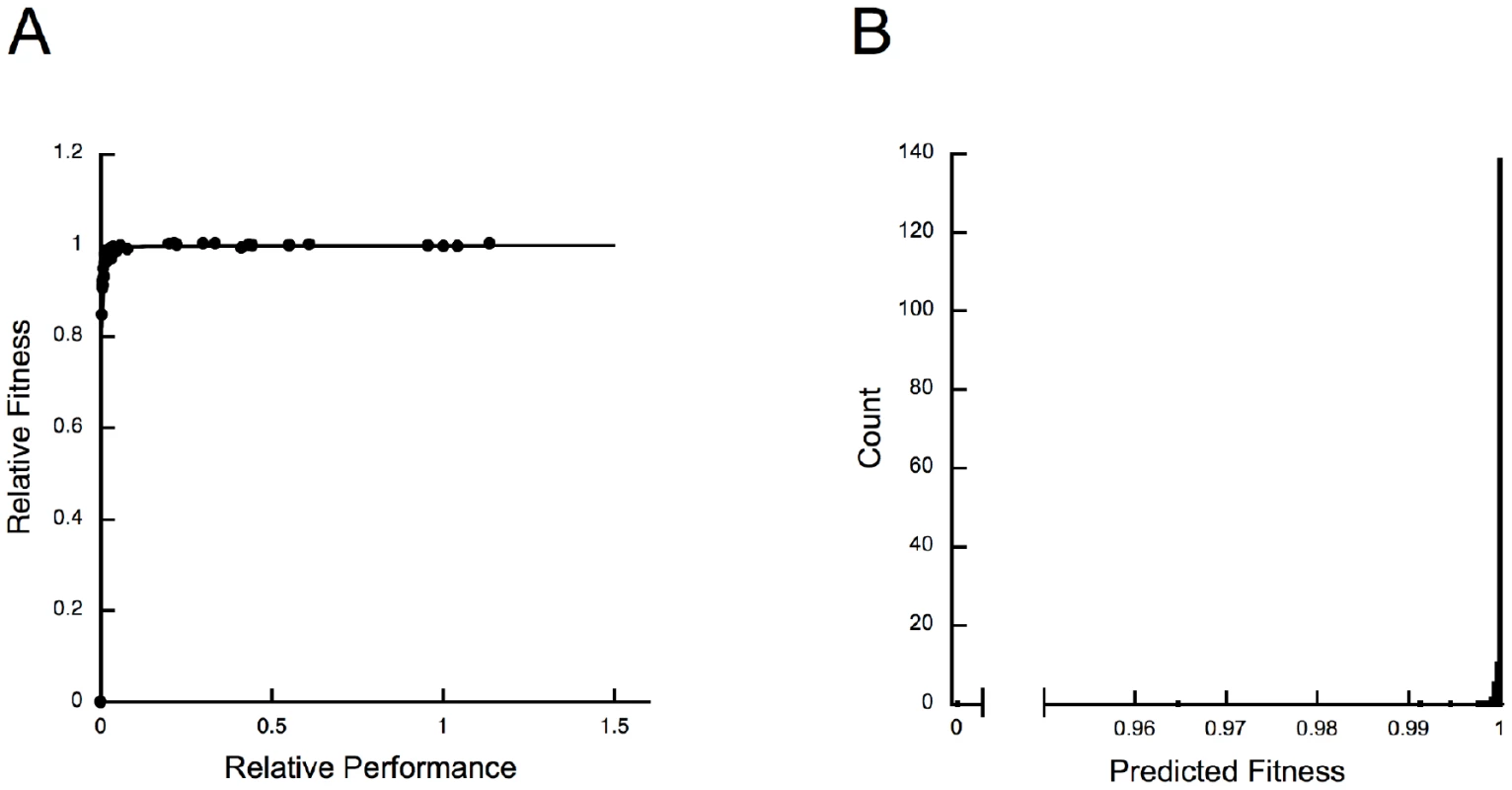
(A) Relationship between fitness and performance, which conforms to a dominance curve [12], shows that E. coli wild-type IMDH lies far into a fitness plateau [58], [62]. (B) As a consequence, even large reductions in enzyme activity are predicted to have very small fitness effects. The predicted fitnesses were calculated using a hyperbola, y = 1.0005x/(0.00032+x), fitted to the data in (A). Two Models of Neutral Evolution
The cryptic epistasis we revealed is consistent with two modes of neutral evolution: the covarion process [64] and the nearly neutral process [65]. In the covarion process, neutral and/or beneficial mutations are fixed in different lineages that, when brought together in the same protein, are deleterious (Figures 2, Figure S2). In the nearly neutral process successive slightly deleterious alleles are fixed by random genetic drift (particularly during population bottlenecks) until a compensatory mutation arises that, on restoring full activity, is fixed by positive selection (particularly after a population expands). The concave fitness function for E. coli IMDH (Figure S4), typical of dominance curves [12], provides the fitness plateau on which fitness could gradually drift downwards as slightly deleterious mutations sequentially fix before a beneficial compensatory mutation restores full activity.
The two processes can be distinguished by determining the order in which mutations arise during the course of evolution. Phylogenetic analysis suggests that the most recent common ancestor (MRCA) of E. coli and P. aeruginosa had amino acids FAFVV at sites 73, 120, 132, 136 and 179 (Figure 5). On the lineage leading to E. coli mutations V136C and V179I arose first (the order is indeterminate) before mutation A120F. Each mutation is compatible with F73. On the lineage leading to P. aeruginosa mutations F73L and F132V arose (the order is indeterminate) before mutation V132L and finally mutation V136I. The presence of V179 is expected to compensate the potentially deleterious interaction between L73 and F132 in the event that the F73L mutation arose first. Hence, the pattern of replacements supports the nearly neutral process because a potentially deleterious mutation never arose before a compensatory mutation in the same lineage.
Fig. 5. Incompatible mutations arise in different lineages. 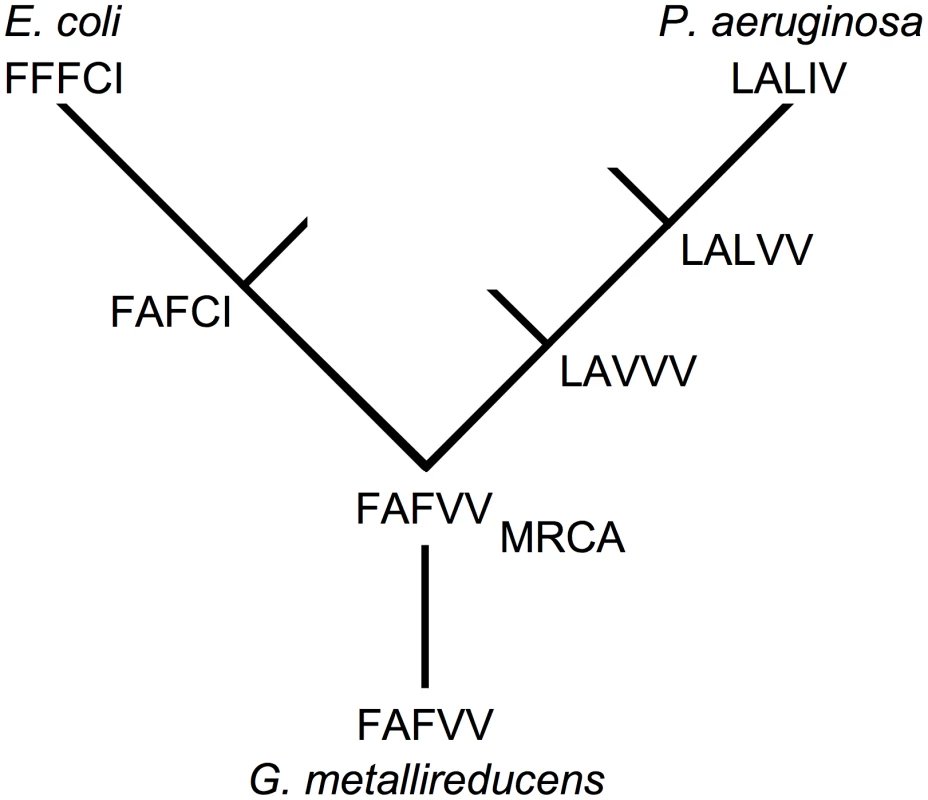
Each genotype is designated by five amino acids (single amino acid code) arranged in order of sites 73, 120, 132, 136 and 179. Three mutations on the lineage leading from the MCRA (defined using Geobacter metallireducens as the outgroup) to E. coli are needed to complete the interaction: A120F, V136C and V179I. Genotype FAFCI on the E. coli lineage and engineered into E. coli IMDH is as active as wild-type. Neither F132V nor C136V affect the performance of E. coli IMDH. Simplified from a full tree of 537 taxa. Implications of Cryptic Epistasis for Molecular Evolution
Our demonstration of rampant cryptic epistasis in IMDH is entirely in accord with a recent insightful analysis of protein evolution that invoked extensive epistasis to account for the retarded divergence seen in ancient proteins [66]. There the case was made for a rugged fitness landscape characterized by multidimensional sign epistasis that forces sites to be conserved for billions of years until the right combination of amino acids at other sites to allows them to evolve. Our failure to identify compensatory mutations for A94D and A284C is indicative of multidimensional sign epistasis. That a single replacement is sufficient to compensate the F73L mutation demonstrates that epistasis need not always be multidimensional, however.
In an earlier study [67], a mutant library in which 52 natural amino acid replacements from 15 subtilisin orthologues had been recombined was screened for function. Sequence comparisons of the unscreened and the screened libraries suggested that almost all possible pair-wise combinations of amino acids can coexist and that functional co-dependencies are rare. These conclusions seemingly stand in contradiction to ours.
The subtilisin experiment suggests that 7 of pairs compromise function for . In other words about a half percent of pair-wise interactions are deleterious. For E. coli IMDH, the probability, f, that introducing an amino acid from an orthologue has no effect on function is , where D is the number of residues that differ between the two sequences. For E. coli IMDH we have , and hence . The two-fold difference between the two estimates of p is small considering the differences between the enzymes and the experimental methods employed. The take-home lesson is that epistatic interactions may be rare individually, but their cumulative impact on evolution rapidly increases with divergence, D. The phenomenon is akin to the snowball effect describing the accumulation of Dobzhanski-Muller incompatibilities during speciation [68].
The simplest model of sequence evolution is a Poisson process in which each site accumulates mutations at a constant rate λ. The expected number of mutations accumulated at time t is simply λt and the variance in the number of substitutions at time t is also λt. This gives the Poisson molecular clock a characteristic variance to mean ratio of . However, sequence analyses show that the molecular clock is the over-dispersed with [69]–[75]. Various hypotheses have been proposed to explain this over-dispersion including episodic bursts of selection, increased rates of fixation of deleterious alleles during population bottlenecks, fluctuating neutral spaces and variable mutation rates [73], [76]–[84]. Cryptic epistasis, in causing constraints at sites to vary and hence substitution rates at sites to vary, undoubtedly contributes to over-dispersion in the molecular clock.
Simulations show that ignoring changes in substitution rates (heterotachy) can induce systematic errors in phylogenetic reconstruction, including topological inaccuracies, long-branch biases and other effects [85]–[91]. Simulations also show that ignoring co-dependencies among sites causes the amount of evolution to be underestimated, particularly on branches deep in a tree [92]. The resulting impression of rapid ancient radiations with an indeterminate branching order makes identifying the origins of some taxonomic groups difficult [93], [94]. Taking explicit account of co-dependencies within data has been shown to aid phylogenetic inference [92], [95]. While recent advances accommodate temporal variability in substitution rates within sites [87], even going so far as to model pair-wise interactions between sites in close proximity using predefined statistical potentials calculated from structural data [88], general phylogenetic practice does not [89]. The extensive cryptic epistasis we have revealed suggests that the usual practice of ignoring co-dependencies among sites needs reconsidering.
Ancestral sequence resurrection is a popular experimental approach to explore ancient phenotypes and adaptations [1]. Accurately inferred ancestral sequences are essential, otherwise there can be little confidence in the experimental results. Caution is warranted when interpreting functional patterns that mimic in silico reconstruction biases [96]. Current methods ignore functional co-dependencies among sites; the consequences for the accuracy of inferred ancestral sequences is largely unexplored. On the one hand, coupling between sites represents a loss of degrees of freedom (knowing the residue at one site allows inferences to be made about the residues at coupled sites) that leads to overconfidence in reconstructed trees [97]. This is particularly problematic if attempting to reconstruct ancestral sequences during a supposed rapid ancient radiation. On the other hand, the same loss of degrees of freedom means that fewer inferences are made, which should improve accuracy. Simulations suggest that the conditions producing phylogenetic uncertainty also make the ancestral state identical across plausible trees [98]. This helps make ancestral sequence reconstructions robust to phylogenetic uncertainty.
Our dissection of epistatic interactions with site 73 shows that amino acid replacements accumulated during evolution can interact without affecting protein function. Nevertheless, cryptic epistasis may impact functional evolution. Reconstructing ancestral proteins on either side of an ancient functional change neglects epistatic interactions that earlier prevented the change and that later prevented the new function reverting [99] or changing in response to a new selective pressure. Such canalizing epistasis both retards functional evolution and thwarts attempts to engineer enzymes rationally [100]. Protein breeding experiments commonly use mutant libraries, generated either by recombining related sequences [101] or by allowing sequences to accumulate ‘neutral drift’ mutations [102], to circumvent the canalizing effects of cryptic epistasis. We speculate that the rampant cryptic epistasis, inferred by computational methods [66] and detected in experiments on IMDH, might be sufficiently extensive to resist functional changes on evolutionary time scales. Only when rare neutral mutations relieve its canalizing effects can new functions evolve. This model potentially explains why protein evolution is characterized by long periods of functional stasis punctuated by rapid functional shifts.
Materials and Methods
Strains, Media, and Chemicals
E. coli K12 strains MG1655, JW5807 (Keio Collection) [103], MM294D and BL21-gold-DleuB::kanr have been previously described [53], [58]. A derivative of E. coli strain BL21-gold (Stratagene) was constructed by P1 transduction [104] of the ΔleuB-leuC::kanr construct from strain JW5807. LB medium was supplemented with 15 g/l Bacto agar for plates [104]. TALON Superflow metal affinity resin and TALON xTractor Buffer were purchased from Takara Bio USA (Madison, WI). Unless specified otherwise, chemicals were purchased from Sigma-Aldrich (St. Louis) and restriction enzymes were purchased from New England Biolabs (Ipswich, MA) and Fermentas (Canada). dl-threo-3-isopropylmalic acid was purchased from Wako Pure Chemical Industries (Japan).
Sequencing
All mutants sequenced at the BioMedical Genomics Center, University of Minnesota.
Constructing Plasmid pLeuB
The leu operon, from mid leuA through leuC, was acquired by genomic PCR from MG1655. The genomic PCR product and pMML22KBA-KYVY [58] were digested with restriction enzymes RsrII and SphI. The vector and insert were ligated by quick ligation (Fermentas), to create pLeuB7. The construct was transformed by RbCl transformation [105] into MM294D and selected on LB/Amp(100 µg/ml), overnight at 37°C.
Primer Design
The 5′-primer is designed with 15–20 bases, then the bases to be mutated, followed by a minimum of 12 bases at the 3′ end. The 3′-primer is complementary to the first 15–20 bases 5′-primer. Thus, the primers are staggered and only the 5′-primer encodes the mutations to be introduced.
Plasmid Methylation
Cytosine residues in plasmid pLeuB7 were methylated by the CpG Methyltransferase M.SssI (New England Biolabs) according to the manufacture's instructions [106]. Methylated DNA and a nonmethylated control were diluted 1∶25, and 2 µl transformed into E. coli strain MM294D by using the RbCl/CaCl2 method [105]. After transformation cells were plated on LB/ampicillin (100 µg/ml) and incubated overnight at 37°C.
Mutagenesis
Restriction sites (Figure S5) and the 168 single mutants were introduced into wild-type E. coli leuB using the protocol in Table 2 [107]. Five microliters of each finished reaction was run on a 1% agarose gel to verify the PCR worked, and 2 µl was transformed [105] into MM294D, plated on LB/ampicillin (100 µg/ml) and incubated overnight at 37°C. The presence of mutations was confirmed by sequencing. Mutant enzymes with kinetic characteristics different from wild-type had their entire leuB gene re-sequenced to confirm that no other mutations had inadvertently been introduced.
Tab. 2. PCR reaction mixture and cycling protocol. 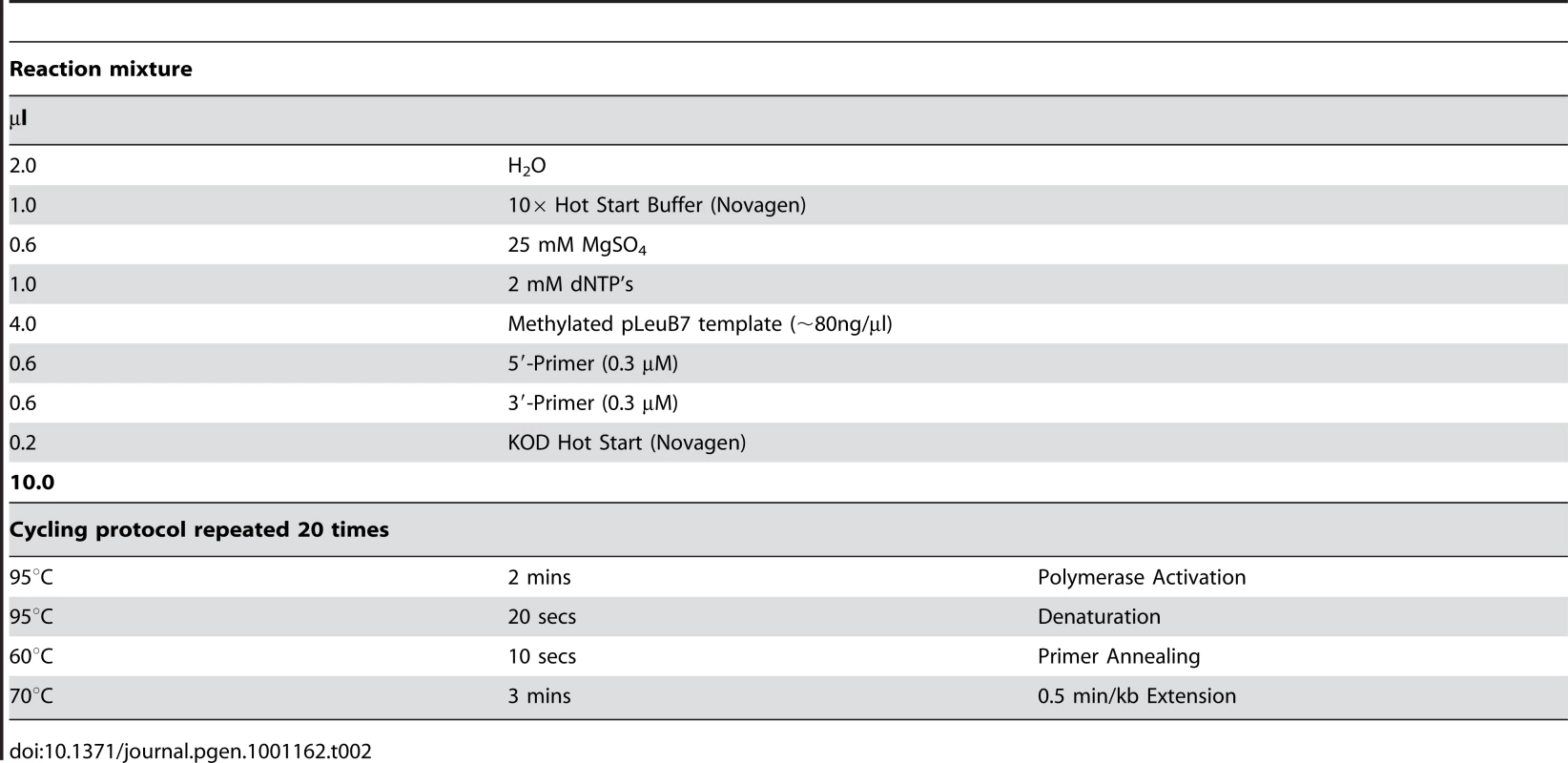
Double mutants incorporating F73L, A94D and A284C with other mutations were constructed by restriction digestion and ligation using strain MM294D as a host. F73L, A94D, A284C were restriction digested and inserts with L24V, S156E, and Y360A ligated to form parent vectors F73L,L24V, F73L,S156E, A94D,L24V, A94D,S156E, A284C,S156E, and A284C,Y360A. L24V removes the AflII site, S156E removes the BamHI site, and Y360A removes the SnaBI site. Parent vectors were then restriction digested and inserts, obtained from restriction digests of other single mutants, were ligated in. After transformation [105] cells were plated on LB/ampicillin (100 µg/ml) and incubated overnight at 37°C. Colonies were grown in LB/ampicillin (100 µg/ml) and the plasmids purified. Double mutants were identified by the presence of a restored AflII, BamHI or SnaBI restriction site. Those remaining mutations close to F73L, A94D and A284C that could not be introduced by restriction digestion and ligation were introduced by PCR mutagenesis and the entire gene sequenced.
In all 694 mutants were constructed: 17 restriction sites were introduced into pLeuB7, 170 single mutants were made (the exact position of one single amino acid deletion could not be reliably identified and so three mutates deleting residues 150, 151 and 152, were constructed), and 3×169 double mutants were made.
Protein Expression
Mutant IMDHs were over-expressed from plasmids in a derivative of E. coli strain BL21-gold (Stratagene) formed by P1 transduction [104] of the ΔleuB-leuC::kanr construct from strain JW5807. Transformed cells were grown overnight at 37°C in 5 mL of LB containing ampicillin (100 µg/ml) and 0.2 mM IPTG. Following centrifugation, cells were resuspended in 1 mL of BD TALON xTractor Buffer (Becton-Dickenson). After 10 min rocking at room temperature, the sample was then centrifuged for 20 min at 11,200×g and the supernatant transferred to a TALON 2 mL disposable gravity column containing 2 mL of equilibrated BD TALON Superflow metal affinity resin. The protein was then eluted following the manufacture's protocol with the exception that potassium salts were substituted for sodium salts. All enzymes were purified to homogeneity as judged using Coomassie stained SDS-PAGE gels.
Screening Double Mutants
Double mutants were screened for compensatory mutations at 37°C in 25 mM MOPS, 100 mM KCl, 1 mM DTT, pH 7.3 in the presence of fixed concentrations of 0.2 mM dl-threo-3-isopropylmalic acid and 5 mM MgCl2 and 0.1 mM NAD. The concentration of NAD lies far below the Kms of the single mutants (459±51 mM for F73L, 687±35 mM for A94D, 777±34 mM for A284C). With each mutant unsaturated the rate of the reaction is proportional to kcat/Km making improvements in performance readily detectable.
Enzyme Kinetics
Kinetics were performed at 37°C in 25 mM MOPS, 100 mM KCl, 1 mM DTT, pH 7.3 in the presence of fixed concentrations of 0.2 mM dl-threo-3-isopropylmalic acid and 5 mM MgCl2, and with concentrations of NAD varied from 1/4 to 10× the apparent Km. Reactions were initiated by adding 10 µL of mutant IMDH (diluted in 50 mM potassium phosphate, 300 mM KCl, 150 mM imidazole, 10 mM β-mercaptoethanol, pH 7.0) to 1 ml of the reaction mix in a 1 cm semi-UV (methylacrylate) cuvette (Fisher Scientific). Reaction rates were determined spectrophotometrically by measuring the production of NADH at 340 nm using a molar extinction coefficient of 6220 M−1 cm−1, in a thermostated Cary 300 Bio with a 6×6 Peltier block (Varian). Inhibition constants were determined in the presence of varying fixed concentrations of reduced coenzyme. Kinetic parameters Vmax, Km and Vmax/Km were determined using nonlinear regression as implemented in JMP (SAS Institute). Maximum turnover rates, , were calculated with enzyme concentrations, [E], determined spectrophotometrically by Bradford assay [108] (Bio-Rad) using bovine IgG as the standard.
Each single mutant was independently expressed, purified and kinetically characterized twice.
Phylogenetics
A total of 537 amino acid sequences (downloaded from GenBank via the NCBI web site http://www.ncbi.nlm.nih.gov/) were aligned using ClustalW software [109]. X-ray structures (IMDHs 1HEX, 1CNZ, 1CM7, IV53, 1W0D, 1WPW, 1VLC, and 1A05) were downloaded from the PDB web site (http://www.pdb.org/pdb/home/home.do) and superimposed using Swiss-Pdb Viewer software [110]. Superpositioned structures were used as a guide to adjust the alignments of highly divergent sequences. A bootstrapped neighbor joining tree was constructed with PHYLIP [111] using a JTT [112] substitution matrix with deep branches swapped and assessed by maximum likelihood. A consensus tree was generated with Mr.Bayes [113] based on a gamma distributed, mixed model of amino acid evolution. The MCMC was run for 75000 generations sampling every 50 generations with a burn-in of 500. Both trees produced similar results when ancestral sites were reconstructed by fastml [114]. With Bayesian posterior probabilities <0.9 accounting for <15% of sites (mostly in flexible loops), the amino acid identities at most sites in the deduced sequence of the most recent common ancestor are reliably inferred.
Supporting Information
Zdroje
1. DeanAM
ThorntonJW
2007 Mechanistic approaches to the study of evolution: the functional synthesis. Nat Rev Genet 8 675 688
2. FelsensteinJ
2004 Inferring phylogenies Sunderland Sinaur Assoc Inc
3. NeiM
KumarS
2000 Molecular evolution and phylogenetics Oxford Oxford University Press
4. SmithNG
Eyre-WalkerA
2002 Adaptive protein evolution in Drosophila. Nature 415 1022 1024
5. CharlesworthJ
Eyre-WalkerA
2006 The rate of adaptive evolution in enteric bacteria. Mol Biol Evol 23 1348 1356
6. SawyerSA
KulathinalRJ
BustamanteCD
HartlDL
2003 Bayesian analysis suggests that most amino acid replacements in Drosophila are driven by positive selection. J Mol Evol 57 154 164
7. HughesAL
2008 Near neutrality: leading edge of the neutral theory of molecular evolution. Ann NY Acad Sci 1133 162 179
8. DePristoMA
WeinreichDM
HartlDL
2007 Missense meanderings in sequences space: a biophysical view of protein evolution. Nat Rev Genet 6 678 687
9. PresgravesDC
2010 The molecular evolutionary basis of species formation. Nat Rev Genet 11 175 180
10. BartonNH
CharlesworthB
1998 Why sex and recombination? Science 281 1986 1990
11. KondrashovAS
1988 Deleterious mutations and the evolution of sexual reproduction. Nature 336 435 440
12. KacserH
BurnsJA
1981 The molecular basis of dominance. Genetics 97 639 666
13. JasnosL
KoronaM
2007 Epistatic buffering of fitness loss in yeast double deletion strains. Nat Genet 39 550 554
14. CordellHJ
2009 Detecting gene-gene interactions that underlie human diseases. Nat Rev Genet 10 392 404
15. ZuckerkandlE
1963 Perspectives in molecular anthropology.
WashburnSL
Classification and human evolution Chicago Aldine 243 272
16. FitchWM
MarkowitzE
1970 An improved method for determining codon variability in a gene and its application to the rate of fixation of mutations in evolution. Biochem Genet 4 579 593
17. KorberBT
FarberRM
WolpertDH
LapedesAS
1993 Covariation of mutations in the V3 loop of human immunodeficiency virus type 1 envelope protein: an information theoretic analysis. Proc Natl Acad Sci USA 90 7176 7180
18. GöbelU
SanderC
SchneiderR
Valencia
1994 A Correlated mutations and residue contacts in proteins. Proteins 18 309 317
19. ShindyalovIN
KolchanovNA
SanderC
1994 Can three-dimensional contacts in protein structures be predicted by analysis of correlated mutations? Protein Eng 7 349 358
20. TaylorWR
FloresTP
OrengoCA
1994 Multiple protein structure alignment. Protein Sci 3 1858 1870
21. LocklessSW
RanganathanR
1999 Evolutionarily conserved pathways of energetic connectivity in protein families. Science 286 295 299
22. PritchardL
BladonPMO
MitchellJJ
DuftonM
2001 Evaluation of a novel method for the identification of coevolving protein residues. Protein Eng 14 549 555
23. ValdarWS
2002 Scoring residue conservation. Proteins 48 227 241
24. SüelGM
LocklessSW
WallMA
RanganathanR
2003 Evolutionarily conserved networks of residues mediate allosteric communication in proteins. Nat Struct Biol 10 59 69
25. SocolichM
LocklessSW
RussWP
LeeH
GardnerKH
2005 Evolutionary information for specifying a protein fold. Nature 437 512 518
26. RussWP
LoweryDM
MishraP
YaffeMB
RanganathanR
2005 Natural-like function in artificial WW domains. Nature 437 579 583
27. GloorGB
MartinLC
WahlLM
DunnSD
2005 Mutual information in protein multiple sequence alignments reveals two classes of coevolving positions. Biochemistry 44 7156 7165
28. AnéC
BurleighJG
McMahonMM
SandersonMJ
2005 Covarion structure in plastid genome evolution: a new statistical test. Mol Biol Evol 22 914 924
29. WangK
SamudralaR
2006 Incorporating background frequency improves entropy - based residue conservation measures. BMC Bioinformatics 17 385
30. TuffleyC
SteelMA
1997 Modelling the covarion hypothesis of nucleotide substitution. Math Biosci 147 63 91
31. LockhartPJ
SteelMA
BarbrookAC
HusonDH
CharlestonMA
1998 A covariotide model explains apparent phylogenetic structure of oxygenic photosynthetic lineages. Mol Biol Evol 15 1183 1188
32. PollockDD
TaylorWR
GoldmanN
1999 Coevolving protein residues: Maximum likelihood identification and relationship to structure. J Mol Biol 287 187 198 (1999)
33. GaltierN
2001 Maximum-likelihood phylogenetic analysis under a covarion-like model. Mol Biol Evol 18 866 873
34. HuelsenbeckJP
2002 Testing a covariotide model of DNA substitution. Mol Biol Evol 19 698 707
35. DutheilJ
PupkoT
Jean-MarieA
GaltierN
2005 A model-based approach for detecting coevolving positions in a molecule. Mol Biol Evol 22 1919 1928
36. FaresMA
TraversSAA
2006 A Novel Method for detecting intramolecular coevolution: Adding a further dimension to selective constraints analyses. Genetics 173 9 23
37. WangHC
SpencerM
SuskoE
RogerAJ
2007 Testing for covarion-like evolution in protein sequences. Mol Biol Evol 24 294 305
38. RodrigueN
KleinmanCL
PhilippeH
LartillotN
2009 Computational methods for evaluating phylogenetic models of coding sequence evolution with dependence between codons. Mol Biol Evol 26 1663 1676
39. DeanAM
GoldingGB
2000 Enzyme evolution explained (sort of).
AltmanRB
DunkerAK
HunterL
LauderdaleK
KleinTE
The Pacific symposium on bioinformatics 2000: Singapore World Scientific
40. DeanAM
NeuhauserC
GrenierC
GoldingGB
2002 The pattern of amino acid replacements in α/β-barrels. Mol Biol Evol 19 1846 1864
41. KondrashovAS
SunyaevS
KondrashovFA
2002 Dobzhansky-Muller incompatibilities in protein evolution. Proc Natl Acad Sci USA 99 14878 14883
42. KulathinalRJ
BettencourtBR
HartlDL
2004 Compensated deleterious mutations in insect genomes. Science 306 1553 1554
43. GloorGB
TyagiG
AbrassartDM
KingstonAJ
FernandesAD
2010 Functionally compensating, coevolving positions are neither homoplasic nor conserved in clades. Mol Biol Evol 27 1181 1191
44. FisherA
ShiY
RitterA
FerrettiJA
Perez-LamboyG
2000 Functional correlation in amino acid residue mutations of yeast iso-2-cytochrome c that is consistent with the prediction of the concomitantly variable codon theory in cytochrome c evolution. Biochem Genet 38 181 200
45. MalcolmBA
WilsonKP
MatthewsBW
KirschJF
WilsonAC
1990 Ancestral lysozymes reconstructed, neutrality tested, and thermostability linked to hydrocarbon packing. Nature 345 86 89
46. WilsonKP
MalcomBA
MatthewsBW
1992 Structural and thermodynamic analysis of compensating mutations within the core of chicken egg white lysozyme. J Biol Chem 267 10842 10849
47. MateuMG
FershtA
1999 Mutually compensatory mutations during evolution of the tetramerization domain of tumor suppressor p53 lead to impaired hetero-oligomerization. Proc Natl Acad Sci USA 96 3595 3599
48. WeinreichDM
DelaneyNF
DePristoMA
HartlDL
2006 Darwinian evolution can follow only very mutational paths to fitter proteins. Science 312 111 114
49. BridghamJT
CarrollSM
ThorntonJW
2006 Evolution of hormone-receptor complexity by molecular exploitation. Science 312 97 101
50. YokoyamaS
TadaT
ZhangH
BrittL
2008 Elucidation of phenotypic adaptations: molecular analyses of dim-light vision proteins in vertebrates. Proc Natl Acad Sci USA 105 13480 13485
51. FieldSF
MatzMV
2010 Retracing evolution of red fluorescence in GFP-like proteins from Faviina corals. Mol Biol Evol 27 225 233
52. StryerL
1995 Biochemistry New York W. H. Freeman & Co
53. MillerSP
LunzerM
DeanAM
2006 Direct demonstration of an adaptive constraint. Science 314 458 461
54. ImadaK
SatoM
TanakaN
KatsubeY
MatsuuraY
1991 Three-dimensional structure of a highly thermostable enzyme, 3-isopropylmalate dehydrogenase of Thermus thermophilus at 2.2Å resolution. J Mol Biol 222 725 738
55. WallonG
KrygerG
LovettST
OshimaT
RingeD
1997 Crystal structures of Escherichia coli and Salmonella typhimurium 3-isopropylmalate dehydrogenase and comparison with their thermophilic counterpart from Thermus thermophilus. J Mol Biol 266 1016 1031
56. ImadaK
InagakiK
MatsunamiH
KawaguchiH
TanakaH
1998 Structure of 3 - isopropylmalate dehydrogenase in complex with 3-isopropylmalate at 2.0 Å resolution: the role of Glu88 in the unique substrate-recognition mechanism. Structure 6 971 982
57. TsuchiyaD
SekiguchiT
TakenakaA
1997 Crystal structure of 3-isopropylmalate dehydrogenase from the moderate facultative thermophile, Bacillus coagulans: two strategies for thermostabilization of protein structures. J Biochem (Tokyo) 122 1092 104
58. LunzerM
MillerSP
FelsheimR
DeanAM
2005 The biochemical architecture of an ancient adaptive landscape. Science 310 499 501
59. SokalRR
RohlfFJ
1995 Biometry: The Principles and Practices of Statistics in Biological Research, 3rd ed NY WH Freeman
60. BenjaminiY
HochbergY
1995 Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc B 57 289 300
61. FershtA
1998 Structure and Mechanism in Protein Science: A Guide to Enzyme Catalysis and Protein Folding NY WH Freeman
62. HartlDL
DykhuizenDE
DeanAM
1985 Limits of adaptation: the evolution of selective neutrality. Genetics 111 655 674
63. KuikenKA
LymanCM
1948 The availability of amino acids in some foods. J Nutr 36 359 368
64. FitchWM
MarkowitzE
1970 An improved method for determining codon variability in a gene and its application to the rate of fixation of mutations in evolution. Biochem Genet 4 579 593
65. OhtaT
1992 The nearly neutral theory of molecular evolution. Annu Rev Ecol Syst 23 263 286
66. PovolotskayaIS
KondrashovFA
2010 Sequence space and the ongoing expansion of the protein universe. Nature 465 922 927
67. GovindarajanS
NessJE
KimS
MundorffEC
MinshullJ
2003 Systematic variation of amino acid substitutions for stringent assessment of pairwise covariation. J Mol Biol 328 1061 1069
68. OrrHA
1995 The population genetics of speciation: the evolution of hybrid incompatibilities. Genetics 139 1805 1813
69. OhtaT
KimuraM
1971 On the constancy of the evolutionary rate of cistrons. J Mol Evol 1 18 25
70. LangleyCH
FitchWM
1973 The constancy of evolution: a statistical analysis of α and β haemoglobins, cytochrome c, and point fibrinopeptide A.
MortonNE
Genetic Structure of Populations Honolulu University of Hawaii Press 246 262
71. LangleyCH
FitchWM
1974 An examination of the constancy of the rate of molecular evolution. J Mol Evol 3 161 177
72. GillespieJH
LangleyCH
1979 Are evolutionary rates really variable? J Mol Evol 13 27 34
73. GillespieJH
1991 The causes of molecular evolution Oxford, UK Oxford University Press
74. OhtaT
1995 Synonymous and nonsynonymous substitutions in mammalian genes and the nearly neutral theory. J Mol Evol 40 56 63
75. BedfordT
WapinskiI
HartlDL
2008 Overdispersion of the molecular clock varies between yeast, Drosophila and mammals. Genetics 179 977 984
76. GillespieJH
1984 The molecular clock may be an episodic clock. Proc Natl Acad Sci USA 81 8009 8013
77. GillespieJH
1984 Molecular evolution over the mutational landscape. Evolution 38 1116 1129
78. TakahataN
1987 On the overdispersed molecular clock. Genetics 116 169 179
79. OhtaT
TachidaT
1990 Theoretical study of near neutrality. I. Heterozygosity and rate of mutant substitution. Genetics 126 219 229
80. TachidaH
1991 A study on a nearly neutral mutation model in finite populations. Genetics 128 183 192
81. IwasaY
1993 Overdispersed molecular evolution in constant environments. J Theor Biol 164 373 393
82. TakahataN
1991 Statistical models of the over-dispersed molecular clock. Theoret Popul Biol 39 329 344
83. ArakiH
TachidaH
1997 Bottleneck effect on evolutionary rate in the nearly neutral mutation model. Genetics 147 907 914
84. CutlerDJ
2000 Understanding the over-dispersed molecular clock. Genetics 154 1403 1417
85. KolaczkowskiB
ThorntonJW
2004 Performance of maximum parsimony and likelihood phylogenetics when evolution is heterogeneous. Nature 431 980 984
86. GruenheitN
LockhartPJ
SteelM
MartinW
2008 Difficulties in testing for covarion - like properties of sequences under the confounding influence of changing proportions of variable sites. Mol Biol Evol 25 1512 1520
87. WangHC
SuskoE
RogerAJ
2009 PROCOV: maximum likelihood estimation of protein phylogeny under covarion models and site-specific covarion patter analysis. BMC Evol Biol 9 225
88. RodrigueN
KleinmanCL
PhilippeH
LartillotN
2009 Computational methods for evaluating phylogenetic models of coding sequence evolution with dependence between codons. Mol Biol Evol 26 1663 1676
89. FelsensteinJ
2004 Inferring phylogenies Sunderland Sinaur Assoc Inc
90. KolaczkowskiB
ThorntonJW
2008 A mixed branch length model of heterotachy improves phylogenetic accuracy. Mol Biol Evol 25 1054 1066
91. KolaczkowskiB
ThorntonJW
2009 Long-branch attraction bias and inconsistency in Bayesian phylogenetics. PLoS ONE 4 e7891 doi:10.1371/journal.pone.0007891
92. WhelanS
2008 The genetic code can cause systematic bias in simple phylogenetic models. Phil Trans Roy Soc B 363 4003 4011
93. RokasA
KrügerD
CarrollSB
2005 Animal evolution and the molecular signature of radiations compressed in time. Science 310 1933 1938
94. RokasA
CarrollSB
2006 Bushes in the tree of life. PLoS Biol 4 e352 doi:10.1371/journal.pbio.0040352
95. SchönigerM
von HaeselerA
1994 A stochastic model for the evolution of autocorrelated DNA sequences. Mol Phylogenet Evol 3 240 247
96. WilliamsPD
PollockDD
BlackburneBP
GoldsteinRA
2006 Assessing the accuracy of ancestral protein reconstruction methods. PLoS Comput Biol 2 e69 doi:10.1371/journal.pcbi.0020069
97. TillierERM
CollinsRA
1995 Neighbor Joining and Maximum Likelihood with RNA sequences: addressing the interdependence of sites. Mol Biol Evol 12 7 15
98. Hanson-SmithV
KolaczkowskiB
ThorntonJW
2010 Robustness of ancestral sequence reconstruction to phylogenetic uncertainty. Mol Biol Evol 2010 Apr 5. [Epub ahead of print]
99. BridghamJT
OrtlundEA
ThorntonJW
2009 An epistatic ratchet constrains the direction of glucocorticoid receptor evolution. Nature 461 515 519
100. TokurikiN
StricherF
SerranoL
TawfikDS
2008 How protein stability and new functions trade off. PLoS Comput Biol 4 e1000002 doi:10.1371/journal.pcbi.1000002
101. StemmerWP
1994 Rapid evolution of a protein in vitro by DNA shuffling. Nature 370 389 391
102. BershteinS
GoldinK
TawfikDS
2008 Intense neutral drifts yield robust and evolvable consensus proteins. J Mol Biol 379 1029 1044
103. BabaT
AraT
HasegawaM
TakaiY
OkumuraY
2006 Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2 2006.0008
104. MillerJH
1992 A short course in bacterial genetics Cold Spring Harbor Cold Spring Harbor Laboratory Press
105. HanahanD
JesseeJ
BloomFR
1991 Plasmid transformation of E. coli and other bacteria. Methods Enzymol 204 63 113
106. New England Biolabs 1994 The NEB Transcript 6 7
107. Novagen 2009 User protocol TB506 Rev. A 0408
108. BradfordMM
1976 A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248 254
109. ThompsonJD
HigginsDG
GibsonTJ
1994 CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position - specific gap penalties and weight matrix choice. Nucleic Acids Res 22 4673 4680
110. GuexN
PeitschMC
1997 SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18 2714 2723
111. FelsensteinJ
1994 PHYLIP Version 3.5 Seattle University of Washington, WA
112. JonesDT
TaylorWR
ThorntonJM
1992 The rapid generation of mutation data matrices from protein sequences. Comp Appl Biosci 8 275 282
113. RonquistF
HuelsenbeckJP
2003 MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19 1572 1574
114. PupkoT
Pe'erI
GraurD
HasegawaM
FriedmanN
2002 A branch-and-bound algorithm for the inference of ancestral amino-acid sequences when the replacement rate varies among sites: application to the evolution of five gene families. Bioinformatics 18 1116 1123
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 10- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Hypogonadotropní hypogonadismus u žen a vliv na výsledky reprodukce po IVF
- Molekulární vyšetření pro stanovení prognózy pacientů s chronickou lymfocytární leukémií
- Prof. Petr Urbánek: Potřebujeme najít pacienty s nediagnostikovanou akutní intermitentní porfyrií
-
Všechny články tohoto čísla
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- FSHD: A Repeat Contraction Disease Finally Ready to Expand (Our Understanding of Its Pathogenesis)
- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- The Meiotic Recombination Checkpoint Suppresses NHK-1 Kinase to Prevent Reorganisation of the Oocyte Nucleus in
- Actin Depolymerizing Factors Cofilin1 and Destrin Are Required for Ureteric Bud Branching Morphogenesis
- DSIF and RNA Polymerase II CTD Phosphorylation Coordinate the Recruitment of Rpd3S to Actively Transcribed Genes
- Continuous Requirement for the Clr4 Complex But Not RNAi for Centromeric Heterochromatin Assembly in Fission Yeast Harboring a Disrupted RITS Complex
- Genome-Wide Association Study of Blood Pressure Extremes Identifies Variant near Associated with Hypertension
- The Cytosine Methyltransferase DRM2 Requires Intact UBA Domains and a Catalytically Mutated Paralog DRM3 during RNA–Directed DNA Methylation in
- β-Actin and γ-Actin Are Each Dispensable for Auditory Hair Cell Development But Required for Stereocilia Maintenance
- Genetic Association Study Identifies as a Risk Gene for Idiopathic Dilated Cardiomyopathy
- Evidence for a Xer/ System for Chromosome Resolution in Archaea
- Four Novel Loci (19q13, 6q24, 12q24, and 5q14) Influence the Microcirculation
- Lifespan Extension by Preserving Proliferative Homeostasis in
- Ancient and Recent Adaptive Evolution of Primate Non-Homologous End Joining Genes
- Loss of the p53/p63 Regulated Desmosomal Protein Perp Promotes Tumorigenesis
- Altering a Histone H3K4 Methylation Pathway in Glomerular Podocytes Promotes a Chronic Disease Phenotype
- Characterization of LINE-1 Ribonucleoprotein Particles
- Conserved Genes Act as Modifiers of Invertebrate SMN Loss of Function Defects
- Alternative Splicing at a NAGNAG Acceptor Site as a Novel Phenotype Modifier
- Tight Regulation of the Gene of the KplE1 Prophage: A New Paradigm for Integrase Gene Regulation
- Conjugative DNA Transfer Induces the Bacterial SOS Response and Promotes Antibiotic Resistance Development through Integron Activation
- Nasty Viruses, Costly Plasmids, Population Dynamics, and the Conditions for Establishing and Maintaining CRISPR-Mediated Adaptive Immunity in Bacteria
- Stress-Induced Activation of Heterochromatic Transcription
- H3K27me3 Profiling of the Endosperm Implies Exclusion of Polycomb Group Protein Targeting by DNA Methylation
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
- Characterising and Predicting Haploinsufficiency in the Human Genome
- Dual Functions of ASCIZ in the DNA Base Damage Response and Pulmonary Organogenesis
- Pervasive Cryptic Epistasis in Molecular Evolution
- Transition from Positive to Neutral in Mutation Fixation along with Continuing Rising Fitness in Thermal Adaptive Evolution
- Comprehensive Analysis Reveals Dynamic and Evolutionary Plasticity of Rab GTPases and Membrane Traffic in
- Regulates Tissue-Specific Mitochondrial DNA Segregation
- Role for the Mammalian Swi5-Sfr1 Complex in DNA Strand Break Repair through Homologous Recombination
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



