-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
The considerable uncertainty regarding cancer risks associated with inherited mutations of BRCA2 is due to unknown factors. To investigate whether common genetic variants modify penetrance for BRCA2 mutation carriers, we undertook a two-staged genome-wide association study in BRCA2 mutation carriers. In stage 1 using the Affymetrix 6.0 platform, 592,163 filtered SNPs genotyped were available on 899 young (<40 years) affected and 804 unaffected carriers of European ancestry. Associations were evaluated using a survival-based score test adjusted for familial correlations and stratified by country of the study and BRCA2*6174delT mutation status. The genomic inflation factor (λ) was 1.011. The stage 1 association analysis revealed multiple variants associated with breast cancer risk: 3 SNPs had p-values<10−5 and 39 SNPs had p-values<10−4. These variants included several previously associated with sporadic breast cancer risk and two novel loci on chromosome 20 (rs311499) and chromosome 10 (rs16917302). The chromosome 10 locus was in ZNF365, which contains another variant that has recently been associated with breast cancer in an independent study of unselected cases. In stage 2, the top 85 loci from stage 1 were genotyped in 1,264 cases and 1,222 controls. Hazard ratios (HR) and 95% confidence intervals (CI) for stage 1 and 2 were combined and estimated using a retrospective likelihood approach, stratified by country of residence and the most common mutation, BRCA2*6174delT. The combined per allele HR of the minor allele for the novel loci rs16917302 was 0.75 (95% CI 0.66–0.86, ) and for rs311499 was 0.72 (95% CI 0.61–0.85, ). FGFR2 rs2981575 had the strongest association with breast cancer risk (per allele HR = 1.28, 95% CI 1.18–1.39, ). These results indicate that SNPs that modify BRCA2 penetrance identified by an agnostic approach thus far are limited to variants that also modify risk of sporadic BRCA2 wild-type breast cancer.
Published in the journal: . PLoS Genet 6(10): e32767. doi:10.1371/journal.pgen.1001183
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001183Summary
The considerable uncertainty regarding cancer risks associated with inherited mutations of BRCA2 is due to unknown factors. To investigate whether common genetic variants modify penetrance for BRCA2 mutation carriers, we undertook a two-staged genome-wide association study in BRCA2 mutation carriers. In stage 1 using the Affymetrix 6.0 platform, 592,163 filtered SNPs genotyped were available on 899 young (<40 years) affected and 804 unaffected carriers of European ancestry. Associations were evaluated using a survival-based score test adjusted for familial correlations and stratified by country of the study and BRCA2*6174delT mutation status. The genomic inflation factor (λ) was 1.011. The stage 1 association analysis revealed multiple variants associated with breast cancer risk: 3 SNPs had p-values<10−5 and 39 SNPs had p-values<10−4. These variants included several previously associated with sporadic breast cancer risk and two novel loci on chromosome 20 (rs311499) and chromosome 10 (rs16917302). The chromosome 10 locus was in ZNF365, which contains another variant that has recently been associated with breast cancer in an independent study of unselected cases. In stage 2, the top 85 loci from stage 1 were genotyped in 1,264 cases and 1,222 controls. Hazard ratios (HR) and 95% confidence intervals (CI) for stage 1 and 2 were combined and estimated using a retrospective likelihood approach, stratified by country of residence and the most common mutation, BRCA2*6174delT. The combined per allele HR of the minor allele for the novel loci rs16917302 was 0.75 (95% CI 0.66–0.86, ) and for rs311499 was 0.72 (95% CI 0.61–0.85, ). FGFR2 rs2981575 had the strongest association with breast cancer risk (per allele HR = 1.28, 95% CI 1.18–1.39, ). These results indicate that SNPs that modify BRCA2 penetrance identified by an agnostic approach thus far are limited to variants that also modify risk of sporadic BRCA2 wild-type breast cancer.
Introduction
After more than a decade of clinical testing for mutations of BRCA1 and BRCA2, there remains considerable uncertainty regarding cancer risks associated with inherited mutations of these genes. This variable penetrance is most striking for BRCA2 [1]–[4], and it affects medical management [5]. Women with the same BRCA2 mutation may develop breast, ovarian or other cancers at different ages or not at all [6]. In a segregation analysis of families identified through breast cancer cases diagnosed before age 55, the residual familial clustering after accounting for BRCA1 and BRCA2 mutations could be explained by a large number of low penetrance genes with multiplicative effects on breast cancer risk [7], [8]. A candidate gene approach in BRCA2 mutation carriers led to the discovery of loci that modify the penetrance of BRCA2 mutations, such as RAD51 135 G>C [9] and perhaps CASP8 [10], [11] and IGFBP2 [12], if replicated. To investigate whether other common single nucleotide polymorphisms (SNP), copy number variants (CNV), or copy number polymorphisms (CNP) modify penetrance for BRCA2 mutation carriers, we undertook a two-staged genome-wide association study (GWAS) in BRCA2 mutation carriers from the international Consortium for Investigators of Modifiers of BRCA1/2 (CIMBA) and other international studies. We hypothesized that an agnostic search for breast cancer loci in an enriched population of BRCA2 mutation carriers, the first among this high risk population, would provide greater power than a sporadic population of equal number, and would yield associations specific to BRCA2 carriers and/or the general population.
Results
Stage 1 and Stage 2 Genotyping
In stage 1, genotype data were available for 899 young (<40 years) affected and 804 older (>40 years) unaffected carriers of European ancestry after quality control filtering and removal of ethnic outliers (Figure S1). A total of 592,163 filtered SNPs genotyped using the Affymetrix Genome-Wide Human SNP Array 6.0 platform passed quality control assessment. In stage 1, comparison of the observed and expected distributions (quantile-quantile plot: Figure S2) showed little evidence for an inflation of the test statistics (genomic inflation factor λ = 1.01), thereby excluding the possibility of significant hidden population substructure, cryptic relatedness among subjects or differential genotype calling between BRCA2 affected and BRCA2 unaffected carriers. Multiple variants were found to be associated with breast cancer risk (Figure S3): 3 SNPs had p<10−5 and 39 SNPs had p<10−4. The most significant association () was observed for FGFR2 rs2981582 (Table 1), a variant previously shown to be associated with increased risk of BRCA2-related breast cancer [13]. A positive association was also observed with rs3803662 (Table 1), near TOX3, which has also been associated with sporadic breast cancer risk [13].
Tab. 1. Estimates of breast cancer association for loci (two confirmatory loci at FGFR2 and TOX3, and two novel loci with stage 1 and 2 combined of p<10−4) among BRCA2 mutation carriers in a two-staged genome-wide association study. 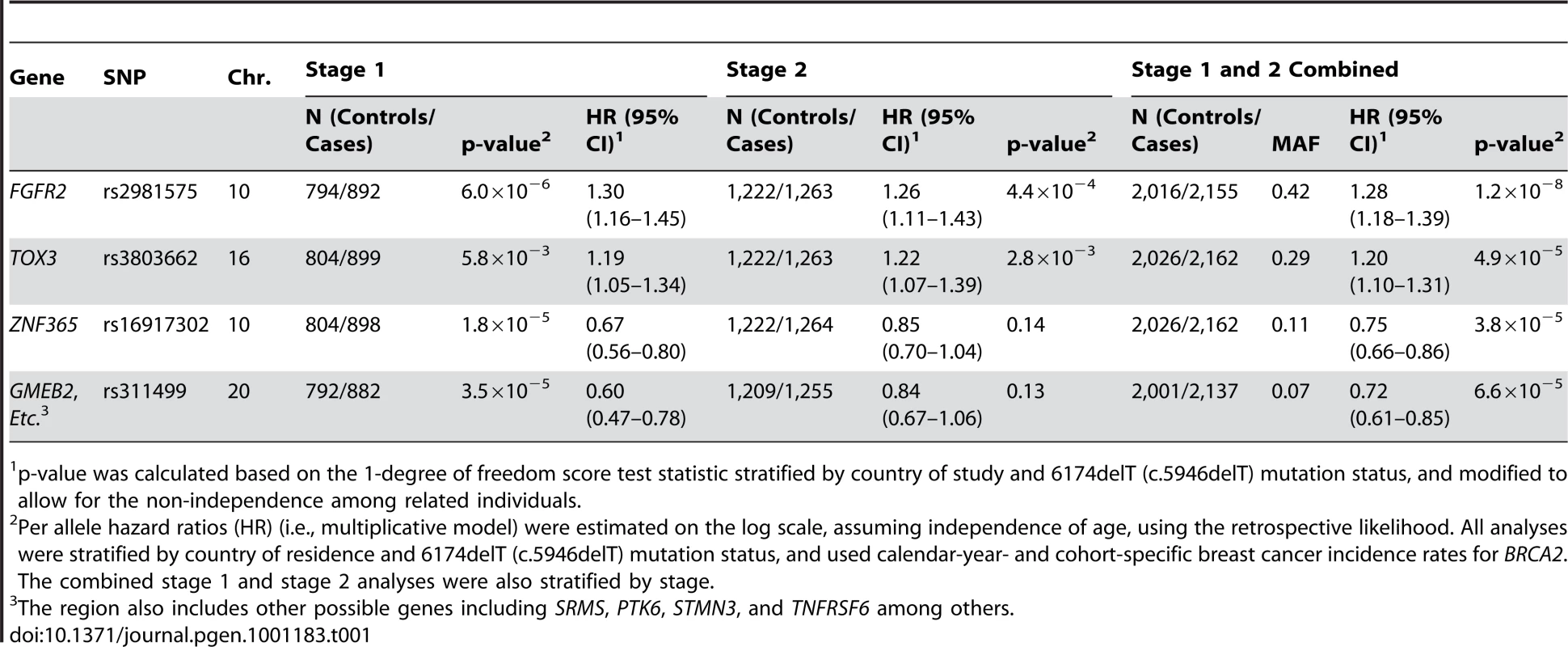
p-value was calculated based on the 1-degree of freedom score test statistic stratified by country of study and 6174delT (c.5946delT) mutation status, and modified to allow for the non-independence among related individuals. Using the stage 1 data, we also performed a GSEA as implemented in MAGENTA [14] to evaluate whether a functionally-related set of genes relevant to BRCA2 function (Table S1) was enriched for relative risk associations (see Statistical Methods). The 59 genes selected are related to the Fanconi anemia pathway [15] as well as other pathways reported in the literature to regulate or interact with BRCA1/2 [16]. These showed no enrichment of associations with the breast cancer risk (p = 0.56). In addition, eight of 125 known cancer susceptibility alleles identified by previous GWAS of other cancers [17] were associated with BRCA2 modification in the current study, a number not greater than expected (Kolmogorv-Smirnov p = 0.60) by chance alone. Of the 113 most significantly associated SNPs (p<10−3) in our study, three showed significant association (p<0.05) with BRCA1-associated breast cancer risk in a complimentary GWAS [18].
In the combined stage 1 and stage 2 results, four independent SNPs (pairwise ) were associated with increased risk of breast cancer risk with p-values<10−4 (Table 1). Previously identified breast cancer susceptibility loci [13], [19], [20] had the most significant associations among BRCA2 mutation carriers (FGFR2: per allele and TOX3: per allele ). Novel loci, rs16917302 on chromosome 10 and rs311499 on chromosome 20, had HRs in stage 2 that were in the same direction as those observed for stage 1 (Figure 1, Table 1), but were smaller in magnitude (HR = 0.67 (95% CI:0.56–0.80) vs. 0.85 (95% CI: 0.70–1.04) for rs16917302; HR = 0.60 (95%CI:0.50–0.78) vs. 0.84 (95%CI: 0.67–1.06) for rs311499) perhaps reflecting a “winner's curse” effect” [21]. The associations for these SNPs were not statistically significant in stage 2 (Table 1). In the combined stage 1 and stage 2 dataset, the C allele of rs16917302 was associated with lower risk of breast cancer (per allele HR = 0.75, 95% CI 0.66–0.86; ; Table 1), and the C allele of rs311499 was associated with a reduced risk (per allele HR = 0.72, 95% CI 0.61–0.85; ; Table 1). A full list of stage 2 results can be found in Table S2. Using the combined stage 1 and stage 2 data, there was no evidence that the HR for SNP rs16917302 changes with age (p = 0.63), but there was some evidence that the per-allele HR for rs311499 may increase with age (p = 0.034).
Fig. 1. Association signals, genetic structure, and linkage disequilibrium of the novel modifier loci of BRCA2 penetrance in the regions surrounding rs1691730 on chromosome 10 and rs311499 on chromosome 20. 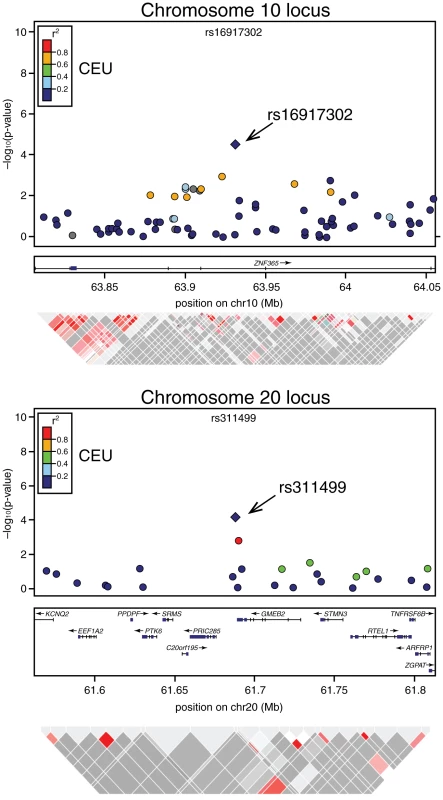
The color of the dots indicates linkage disequilibrium (LD; based on r2 values) in the CEU population (as per scale). Triangle plots below represent LD from actual data of BRCA2 carries in the study. Copy Number Variant Analysis
We also examined the association of both high-frequency CNPs and low-frequency CNVs to case-control status using the stage 1 data. After performing standard quality control measures including a minor allele frequency (MAF) threshold of 5%, we identified 191 polymorphisms with reliable genotypes. No associations were found between CNVs and the phenotype; there was no inflation or deflation of the test statistic, and the best p-value was . We similarly assessed less common CNPs, and found neither the overall burden of events (or any subclass thereof, such as large deletions overlapping genes) nor any specific locus associated with breast cancer risk (Figure S4).
Excess Sharing in Genetic Isolates and Outbred Populations Analyzed
Because of the prior evidence of significant LD extent around the 6174delT (c.5946delT) founder mutation in the Ashkenazi Jewish population [22], we explored the potential excess sharing of the genome compared to the BRCA2 region in both Ashkenazi Jewish and non-Jewish European ancestries. Using GERMLINE [23], shared segments of greater than 5 cM were computed based on the imputed genotype dataset. In the BRCA2 region, we observed a significant excess of sharing amongst both Ashkenazi (n = 304) and non-Jewish (n = 1331) individuals compared to samples from an autism study (n = 808) suggesting common founders for BRCA2 mutations. Examining sites across the genome every 2.5 cM (excluding telomere and centromere regions), we observed possible pairs share segments greater than 5 cM that on average 0.005% (u = 50.17, s.d = 55.5, max = 491) for non-Jewish individuals and 0.12% (u = 141.11, s.d = 57.32, max = 525) for Ashkenazi Jewish individuals. Comparing cases and controls, we did not observe a significant difference in number of pairs of samples sharing segments greater than 5cM across the genome excluding chromosome 13. That is, there was no evidence of overall excess sharing across the genome other than for the BRCA2 locus within the Ashkenazi Jewish and non-Ashkenazi Jewish populations in the study.
Discussion
In this GWAS of BRCA2 mutation carriers, the first in this high risk population, we found previously identified breast cancer susceptibility loci modified risk of BRCA2-associated breast cancer with similar magnitude of association. Although FGFR2 (rs2981575) was the only locus to reach genome-wide statistical significance, novel loci, rs16917302 and rs10509168 were each associated with breast cancer risk.
rs16917302 is located on chromosome 10, in the zinc finger protein 365 gene (ZNF365). A recent multistage GWAS of 15,992 sporadic breast cancer cases and 16,891 controls also observed an inverse association (per allele OR = 0.82, 95% CI 0.82–0.91, ) between breast cancer risk and rs10509168, a SNP 18kb from rs16917302 (pairwise ) and located in intron 4 of ZNF365 [24]. Of the 3,659 cases and 4,897 controls in phase 1 of that study, imputation revealed that the locus identified in our BRCA2 study, rs16917302, was significantly associated with risk for breast cancer (p = 0.02) (Easton DF, personal communication). The second novel SNP in the current study, rs311499, is located on chromosome 20, within a region containing several possible candidate genes including GMEB2, SRMS, PTK6, STMN3, and TNFRSF6. The functional significance of both of these regions with breast carcinogenesis is unknown; further research is warranted.
There was some evidence that the HR associated with rs311499 may change with age. We also observed that the stage 1 HR for this SNPs was larger in magnitude compared to the stage 2 HR, consistent with a winner's curse effect [21]. Since stage 1 of our experiment included mostly BRCA2 mutation carriers diagnosed at a young age, and stage 2 mutation carriers diagnosed an older age, the “winner's curse” and age-specific effects are confounded and may be difficult to distinguish. Fitting the age-dependent HR model for SNP rs311499 using the stage 2 data yielded no significant variation in the HR by age (p = 0.47), but the sample size for this analysis was relatively small. Future larger studies should aim to clarify this.
Mutations in known genes (BRCA1, BRCA2, TP53, CHEK2, PTEN, and ATM) explain only 20–25% of the familial clustering of breast cancer; the residual familial clustering may be explained by the existence of multiple common, low-penetrance alleles (‘polygenes’) [25]. Perhaps because the majority of BRCA2-associated breast tumors are estrogen receptor (ER)-positive, as are the majority of non-hereditary breast cancers [26], risk alleles for sporadic breast cancer are more likely to be modifiers of risk of BRCA2-associated hereditary breast cancer. Of the seven GWAS-identified breast cancer-associated SNPs examined in a BRCA2 background [13], [19], [20], SNPS in FGFR2 (rs2981575), TOX3 (rs3803662), MAP3K1 (rs889312), and LSP1 (rs3817198) have been shown to modify BRCA2 penetrance, in contrast with BRCA1 tumors, in which only two of these same SNPs (based on a 2 degrees of freedom model) modified risk of these largely ER-negative tumors [26]. As previously noted [13], [20], the stage 1 HRs among BRCA2 mutation carriers, reported here, were nearly identical to odds ratio estimates observed in sporadic breast cancer studies, consistent with a simple multiplicative interaction between the BRCA2 mutant alleles and the common susceptibility SNPs. If replicated, the two additional SNPs identified here would only explain about 1.7% of the variance in breast cancer risk among BRCA2 mutation carriers. Taken together, the combined effects of all the common and putative risk modifiers in this study only account for ∼4% of the variance of BRCA2 mutations, compared with 1.1% for the single RAD51 135 G>C variant, which is rare and biologically-linked to BRCA2 function, as shown by candidate gene studies [9]. Thus, the common alleles that modify risk in BRCA1 and BRCA2 backgrounds appear to have comparable associated risks in sporadic ER-positive and ER-negative tumors, respectively [18]. While individual SNPs are unlikely to be used to guide radiographic screening and risk-reducing surgical strategies, the combined effect of these SNPs may ultimately be used for the tailor management of subsets of BRCA mutation carriers [5].
While we took great efforts to collect all of the possible known BRCA2 mutation carriers, there were insufficient numbers to stratify by race and BRCA2 mutations with the exception of BRCA2*6174delT mutations. Due to the small numbers of women of non-European ancestry who have participated in the individual studies represented here, the current analysis was based only on women who had genetic backgrounds consistent with HapMap CEU samples. While we expect that SNPs identified among women of European ancestry might also be applicable to women of other genetic backgrounds, additional research in these populations will be needed. Similarly, the observed associations represented across all types of mutations, and specifically a weighted average of BRCA2*6174delT and non-delT mutations. It is possible that the observed associations may only modify the penetrance of specific BRCA2 mutations due to differential effects on function or differences in genetic background. Our analysis was stratified on the basis of the most common BRCA2 mutation, BRCA2*6174delT, which is prevalent in individuals with an Ashkenazi Jewish ancestry. Large numbers of mutation carriers will be necessary to calculate mutation-specific estimates. In addition, there was a drop-out of SNPs in the two phases of this study. While we were able to achieve a representative coverage of the genome, it is also possible that additional studies using denser arrays may provide further information.
As expected, we observed associations with some of the major common genetic variants seen in genome-wide scans of breast cancer in a non-BRCA1/2 mutation background. However, we found no evidence for loci with stronger effects than FGFR2. Although we observed an association with a novel locus at ZNF365 that appears also to be a risk factor for sporadic breast cancer, overall, our results suggest that there are no common variants with major effects (i.e., OR>2.0) that are specific in BRCA2 carriers. Similarly, in a recent report of SNPs from sporadic breast cancer GWAS genotyped in a restricted set of BRCA1/2 carriers [27], loci in LOC134997 (rs9393597: per allele HR = 1.55, 95% CI 1.25–1.92, ) and FBXL7 (rs12652447: HR = 1.37, 95% CI 1.16–1.62, ) were associated with BRCA2 breast cancer risk with p-values weaker than FGFR2 reported here (per allele ), although the magnitudes of the associations were slightly stronger than FGFR2 (HR = 1.28). Although these SNPs were not in our genotyped panel of SNPs at stage 1, imputation results indicate that SNP rs9393597 has a p-value of 0.008 and SNP rs12652447 a p-value of 0.04 for association with breast cancer risk for the BRCA2 mutation carriers in our stage1. However, there is substantial overlap between our study and the study of Wang et al. [27].
Replication in larger datasets will be necessary to precisely estimate the magnitude of the associations of suspected loci identified from our study, candidate gene analysis [10]–[12], and other selection approaches [27]. It is of interest, however, that when utilizing an agnostic approach in BRCA2 mutation carriers in this study, the major determinants of risk variation in mutation carriers are those that also modify risk in subsets of sporadic, BRCA1/2 wild type, breast cancer. However, it remains possible that unique variants with smaller effects, or rarer variants (not evaluated in this experiment), may be specific modifiers of breast cancer risk in BRCA2 carriers. Their detection would require study populations much larger than the current analysis, which is presently the largest such cohort assembled.
Materials and Methods
Study Subjects
Ethics statement
All carriers were recruited to studies (Table 2) at the host institutions under IRB-approved protocols.
Tab. 2. Description of affected and unaffected carriers selected for <i>BRCA2</i> GWAS Stage 1 and 2. 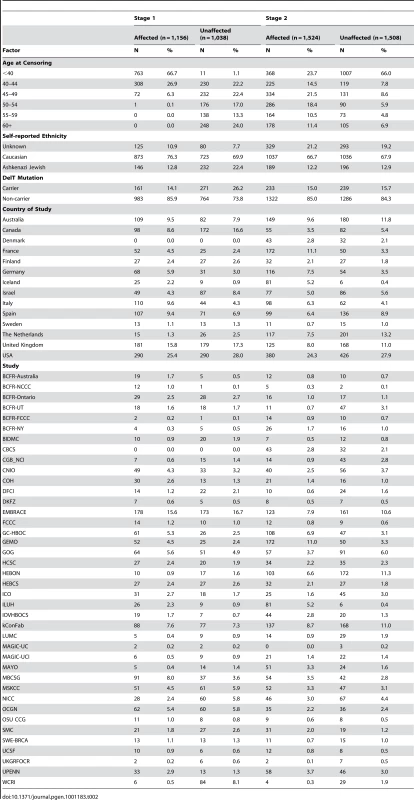
Selection of affected individuals and controls
A total of 6,272 BRCA2 carriers from 39 studies (Table 2) and 14 countries contributed DNA samples for this project. With the exception of NICC, all studies are members of the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA) [28]. Recruitment of carriers were conducted predominantly through cancer genetics clinics, and enrolled through national or regional efforts. Other studies were recruited through population-based or community-based ascertainments. All subjects provided written informed consent. Eligible female carriers were aged 18 years or older, were self-reported ‘white’, and had mutations in BRCA2. Data were available on age at study recruitment, age at cancer diagnosis, age of bilateral prophylactic mastectomy, BRCA1/2 mutation description, and self-reported ethnicity. Only a limited number of cases had detailed information on tumor characteristics (e.g., estrogen and progesterone receptor status); therefore, subtype analyses were not performed at this stage.
Genotyping and Quality Control
Stage 1 Affymetrix genotyping
All eligible DNA samples provided by participating centers were subjected to a rigorous quality control assessment, including measures of overall DNA quality and quantity. A total of 1,156 young (≤50 years) affected women and 1,038 unaffected women with high quality DNA samples were selected (Table 2). For time efficiency, stage 1 genotyping occurred in two phases: phase 1 included 421 cases and 404 controls and phase 2 included 735 cases and 634 controls.
Prior to the genome-wide scan, we genotyped five SNPs previously genotyped by the CIMBA study centers as a pre-filter for sample identification. Thirty-one samples (Figure S1) were discordant in the two genotyping rounds and were excluded from further analysis.
The genotyping for the stage 1 GWAS was performed on 2,163 eligible carriers using the Affymetrix 6.0 GeneChip array that included 906,622 SNPs (Figure S1). To further monitor the identity of the DNA samples, a fingerprinting panel of 14 SNPs with a minor allele frequency >10% in HapMap European individuals were genotyped on all samples, using Sequenom iPLEX, before and after Affymetrix genotyping. The AMG gender assay was used for gender assessment. As an additional quality control measure, cases and controls were interleafed on each plate to eliminate technical bias. Each plate also included one HapMap CEU DNA sample.
The DNA samples and genotyping calls for both phases of stage 1 were filtered through a series of data quality control parameters using the Birdseed module of the Birdsuite software developed at Broad Institute [29]. Among the 2,163 samples genotyped in the stage 1 GWAS, 253 failed to hybridize to the chip due to poor DNA quality and were excluded (Figure S1). Fifty-five samples were dropped with call rates <95%. Three samples were contaminated, 43 were identified by genotyping to be duplicates, and 4 were male; all were dropped from analyses.
SNPs were also filtered using Birdseed and were removed if monomorphic or >10% missing (n = 38,962), genotype call rates <95% (n = 50,810), minor allele frequencies <1% (n = 104,792), departures from Hardy-Weinberg Equilibrium (p<10−6; n = 1,090), differential missingness with respect to phenotype (p<10−3; n = 275), and differential missingness with respect to nearby SNPs (p<10−10; n = 22,065). A total of 6,212 SNPs had different missingness patterns in phase 1 compared to phase 2, and were excluded. Since we found that significant missingness correlated to SNPs mapping to longer fragments of Affymetrix 6.0 digestion products, we also removed the SNPs on fragments longer than 1000bp (n = 85,990).
With the remaining 1,805 carriers and 596,426 SNPs, an iterative process proceeded to drop all individuals with low call rates (<95%), high autosomal heterozygosity rates (false discovery rate <0.1%), and high identity by descent scores (≥0.95) and to drop all SNPs with minor allele frequencies <1% and SNP call rates <95% until the final run contained individuals above the individual and SNP filter thresholds (n = 1,747 samples and 592,566 SNPs). A more stringent HWE filter (p<10−7) was then applied and 403 additional SNPs were dropped. Nine individuals with missing mutation descriptions were removed.
Finally, principal components analysis was used to identify the ethnic outliers (Figure S5). A total of 1,743 BRCA2 mutation carriers and the HapMap3 data for 210 individuals of European (CEU), Han Chinese (CHB), and Yoruba (YRI) African descent were available for multidimensional scaling using the genomic kinship matrix estimated using a set of 53,641 autosomal and uncorrelated SNPs. A cut-off of >11% was used to exclude samples with non-CEU ancestry (n = 35). Genotype-phenotype association analyses were based on 1,703 (899 young affected and 804 unaffected) BRCA2 mutation carriers and 592,163 SNPs, covering 85% of the common HapMap 3 SNPs (imputed with (see below), including 64% of the markers that were removed in the QC process).
Where directly genotyped data were not available, probabilities were imputed with Beagle.3.0.2 (using the default parameters) using CEU+TSI samples on HapMap3 release2 B36 as the reference panel (410 chromosomes, 1.4 M SNPs).
Stage 2 Sequenom iPLEX genotyping
The primary SNP selection strategy was based on the results of the kinship-adjusted score test of 592,163 GWAS genotyped SNPs. From stage 1, a total of 79 top independent regions () with pairwise r2 values<0.80 were selected for genotyping in stage 2 (Figure S6). For the top 10 SNPs if available, an additional correlated SNP (pairwise ; n = 5) was selected to serve as genotyping backup. The remaining SNPs for stage 2 were selected based on two alternate strategies. First, we added the 14 (as well as FGFR2 counted in the top 10 SNPs above) confirmed breast cancer SNPs from prior independent GWAS of sporadic breast cancer. Second, we also selected the 15 top independent regions (pairwise ) based on the ranking of the p-values from a logistic regression analysis of 1.5 million imputed SNPs. In total for stage 2 replication phase, we selected 113 SNPs and 1,524 breast cancer carriers and 1,508 control carriers (Table 2) for genotyping using the Sequenom iPLEX platform.
Samples were excluded for call rates ≤95% (n = 476), duplication in stage 2 (identity by state (IBS)∼1.0; n = 43), duplication in stage 1 and 2 (IBS; n = 25), lack of complete phenotype data (n = 1), and insufficient country-specific numbers (n = 1; Figure S6). A total of 100 SNPs were successfully multiplexed into three pools; the remaining 13 SNPs were not genotyped. Genotyping QC filters excluded 15 SNPs due to call rates ≤90% (n = 14) and MAF<1% (n = 1). In summary, the final association analyses in stage 2 were based on 2,486 carriers (1,264 affected and 1,222 unaffected carriers) and 85 SNPs.
Statistical Methods
Defining time at risk
Carriers were censored at the first breast or ovarian cancer or bilateral prophylactic mastectomy, whichever occurred first. Carriers who developed any cancer were censored at time of bilateral prophylactic mastectomy if it occurred more than a year prior to the cancer diagnosis (to avoid censoring at bilateral mastectomies related to diagnosis in which rounded ages were used). The remaining carriers were censored at the age of last observation. This was defined either by the age/date at interview or age at follow-up depending on the information provided by the participating center. Carriers censored at diagnosis of breast cancer were considered cases in the analysis. Mutation carriers censored at ovarian cancer diagnosis were considered unaffected. Carriers with a censoring/last follow-up age older than age 80 were censored at age 80 because there are no reliable cancer incidence rates for BRCA1/2 carriers beyond age 80.
Genotype–phenotype associations
Analyses, based on 1,703 BRCA2 mutation carriers and 592,163 SNPs, were performed within a survival analysis framework. Since the mutation carriers were not selected at random with respect to their disease status, standard methods of survival (e.g., Cox regression) may lead to biased estimates of relative risk [30]. Therefore, analyses were conducted by modeling the retrospective likelihood of the observed genotypes conditional on the disease phenotypes. The associations between genotype and breast cancer risk at both stages were assessed using the 1-degree of freedom score test statistic based on this retrospective likelihood, as previously described [9], [18]. All models were stratified by country of study and 6174delT (c.5946delT) mutation status, the most common BRCA2 mutation in this study and a marker of the Ashkenazi Jewish population among Ashkenazi Jewish women [31]–[33]. Since the linkage disequilibrium structure among Ashkenazi Jewish people may differ from other mutation carriers [34], stratifying by the *6174delT provides additional control for population stratification. To allow for the non-independence among related individuals, an adjusted version of the score test was used in which the variance of the score was derived by taking into account the correlation between the genotypes [35], [36]. Analyses were performed in R using the GenABEL libraries [37] and custom written software.
To estimate the magnitude of the associations, the effect of each SNP was modelled either as a per allele hazard ratio (HR) (i.e., multiplicative model) or as separate HRs for heterozygotes and homozygotes, and these were estimated on the log scale. The HRs were assumed to be independent of age (i.e. we used a Cox proportional-hazards model). For the most significant novel associations this assumption was verified by adding a genotype-by-age interaction term to the model to fit models in which the HR changed with age. The retrospective likelihood was implemented in the pedigree-analysis software MENDEL [38] as previously described [9]. All analyses were stratified by country of residence and 6174delT (c.5946delT) mutation status, and used calendar-year - and cohort-specific breast cancer incidence rates for BRCA2 [25]. The combined stage 1 and stage 2 analyses were also stratified by stage. Parameter estimates were obtained by maximising the retrospective likelihood. To allow for the non-independence among related mutation carriers, we used a robust variance estimation approach in order to obtain standard errors for the parameters [39], [40]. Related individuals were identified through a unique family identifier.
Copy number variant analysis
We also examined the association of both high-frequency and low-frequency copy number variants (CNV) to the age of diagnosis of breast cancer as a dichotomous trait using the stage 1 data [29]. We called known, common variants (copy number polymorphisms, CNPs) with Canary [29]. CNP alleles lower than 1% in frequency were removed, to maximize the number of the CNPs that were bi-allelic instead of multi-alleleic. CNPs were removed that had for call rate <95%, differential missingness by genotype (p<10−3), or departure from Hardy-Weinberg proportions (p<10−3). Post-QC, we had 191 high-quality genotyped polymorphisms. We used PLINK to assess association using logistic regression and the same ancestry covariates of no interest as with SNPs. We similarly assessed less common CNVs discovered by Birdseye [29] for association with age at diagnosis using PLINK [41]. Finally, we also looked specifically at CNVs overlapping the BRCA2 gene itself using LOD scores and Birdseye.
Haplotype sharing analysis
We looked for evidence of excess sharing across the genome and the BRCA2 region. Using GERMLINE [23], shared segments of greater than 5 cM were computed based on the imputed genotype dataset among both Ashkenazi (n = 304) and non-Jewish (n = 1,331) samples compared to samples from an autism study (n = 808) (Figure S3). Examining sites across the genome every 2.5 cM (excluding telomere and centromere regions), we computed the mean of the proportion, standard deviation, and the maximum values for non-Jewish and Ashkenazi women, respectively.
Gene Set Enrichment Analysis
We tested whether 59 genes known to regulate or interact with BRCA2 [16] (Table S1) were enriched for associations with age of onset of breast cancer in BRCA2 mutation carriers, using a new implementation of Gene Set Enrichment Analysis (GSEA) called Meta-Analysis Gene-Set Enrichment of variaNT Associations (MAGENTA) [14]. The 59 genes were compiled using a Pubmed abstract mining software, Chilibot [42], and were selected if they were related to the Fanconi anemia pathway [15] as well as others reported from literature to regulate or interact with BRCA1/2 [43]. An association p-value was calculated for each gene in the genome, defined as the most-significant association p-value of all genotyped SNPs that lie within 110 kb upstream and 40 kb downstream to the gene's most extreme transcript boundaries, followed by correction for gene score confounders (gene size, number of SNPs per gene and linkage disequilibrium related properties). SNP association p-values were taken from the stage 1 GWAS. To compute a GSEA p-value for the BRCA gene set, the fraction of genes with an association p-value more significant than the 95 percentile of all gene p-values in the genome was compared to a null distribution, generated by randomly sampling gene-sets of identical size from the genome 10,000 times. Of the 59 BRCA interactors, two genes were assigned the same most significant SNP due to physical proximity in the genome. To prevent potential over-estimation of gene set enrichment due to physical clustering of genes in a gene set, we retained only one gene of each subset of genes assigned the same best SNP (the gene with the most significant gene p-value) for the analysis of both the real and permuted gene sets.
Supporting Information
Zdroje
1. TryggvadottirL
SigvaldasonH
OlafsdottirGH
JonassonJG
JonssonT
2006 Population-based study of changing breast cancer risk in Icelandic BRCA2 mutation carriers, 1920–2000. J Natl Cancer Inst 98 116 122
2. SimchoniS
FriedmanE
KaufmanB
Gershoni-BaruchR
Orr-UrtregerA
2006 Familial clustering of site-specific cancer risks associated with BRCA1 and BRCA2 mutations in the Ashkenazi Jewish population. Proc Natl Acad Sci U S A 103 3770 3774
3. BeggCB
HaileRW
BorgA
MaloneKE
ConcannonP
2008 Variation of breast cancer risk among BRCA1/2 carriers. JAMA 299 194 201
4. AntoniouA
PharoahPD
NarodS
RischHA
EyfjordJE
2003 Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 72 1117 1130
5. RobsonM
OffitK
2007 Clinical practice. Management of an inherited predisposition to breast cancer. N Engl J Med 357 154 162
6. OffitK
2006 BRCA mutation frequency and penetrance: new data, old debate. J Natl Cancer Inst 98 1675 1677
7. AntoniouAC
PharoahPD
McMullanG
DayNE
StrattonMR
2002 A comprehensive model for familial breast cancer incorporating BRCA1, BRCA2 and other genes. Br J Cancer 86 76 83
8. AntoniouAC
PharoahPD
McMullanG
DayNE
PonderBA
2001 Evidence for further breast cancer susceptibility genes in addition to BRCA1 and BRCA2 in a population-based study. Genet Epidemiol 21 1 18
9. AntoniouAC
SinilnikovaOM
SimardJ
LeoneM
DumontM
2007 RAD51 135G→C modifies breast cancer risk among BRCA2 mutation carriers: results from a combined analysis of 19 studies. Am J Hum Genet 81 1186 1200
10. CatucciI
VerderioP
PizzamiglioS
ManoukianS
PeisselB
The CASP8 rs3834129 polymorphism and breast cancer risk in BRCA1 mutation carriers. Breast Cancer Res Treat
11. Palanca SuelaS
Esteban CardenosaE
Barragan GonzalezE
de Juan JimenezI
Chirivella GonzalezI
CASP8 D302H polymorphism delays the age of onset of breast cancer in BRCA1 and BRCA2 carriers. Breast Cancer Res Treat 119 87 93
12. NeuhausenSL
BrummelS
DingYC
SingerCF
PfeilerG
2009 Genetic variation in insulin-like growth factor signaling genes and breast cancer risk among BRCA1 and BRCA2 carriers. Breast Cancer Res 11 R76
13. AntoniouAC
SpurdleAB
SinilnikovaOM
HealeyS
PooleyKA
2008 Common breast cancer-predisposition alleles are associated with breast cancer risk in BRCA1 and BRCA2 mutation carriers. Am J Hum Genet 82 937 948
14. SegreAV
GroopL
MoothaVK
DalyMJ
AltshulerD
2010 Common inherited variation in mitochondrial genes is not enriched for associations with type 2 diabetes or related glycemic traits. PLoS Genet 6 e1001058 doi:10.1371/journal.pgen.1001058
15. D'AndreaAD
Susceptibility pathways in Fanconi's anemia and breast cancer. N Engl J Med 362 1909 1919
16. O'DonovanPJ
LivingstonDM
2010 BRCA1 and BRCA2: breast/ovarian cancer susceptibility gene products and participants in DNA double-strand break repair. Carcinogenesis 31 961 967
17. StadlerZ
ThomP
RobsonME
WeitzelJN
KauffND
2010 Genome-wide Association Studies of Cancer. J Clin Oncol in press
18. AntoniouAC
2010 A genome-wide association study identified a 19p13 locus that modifies the risk of breast cancer in BRCA1 mutation carriers and is associated with estrogen receptor negative and triple negative breast cancer in the general population. Nat Genet under review
19. KirchhoffT
ChenZQ
GoldB
PalP
GaudetMM
2009 The 6q22.33 Locus and Breast Cancer Susceptibility. Cancer Epidemiol Biomarkers Prev
20. AntoniouAC
SinilnikovaOM
McGuffogL
HealeyS
NevanlinnaH
2009 Common variants in LSP1, 2q35 and 8q24 and breast cancer risk for BRCA1 and BRCA2 mutation carriers. Hum Mol Genet
21. ZollnerS
PritchardJK
2007 Overcoming the winner's curse: estimating penetrance parameters from case-control data. Am J Hum Genet 80 605 615
22. EllisNA
KirchhoffT
MitraN
YeTZ
ChuaiS
2006 Localization of breast cancer susceptibility loci by genome-wide SNP linkage disequilibrium mapping. Genet Epidemiol 30 48 61
23. GusevA
LoweJK
StoffelM
DalyMJ
AltshulerD
2009 Whole population, genome-wide mapping of hidden relatedness. Genome Res 19 318 326
24. TurnbullC
AhmedS
MorrisonJ
PernetD
RenwickA
Genome-wide association study identifies five new breast cancer susceptibility loci. Nat Genet
25. AntoniouAC
CunninghamAP
PetoJ
EvansDG
LallooF
2008 The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer 98 1457 1466
26. FoulkesWD
StefanssonIM
ChappuisPO
BeginLR
GoffinJR
2003 Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst 95 1482 1485
27. WangX
PankratzVS
FredericksenZ
TarrellR
KarausM
Common variants associated with breast cancer in genome-wide association studies are modifiers of breast cancer risk in BRCA1 and BRCA2 mutation carriers. Hum Mol Genet 19 2886 2897
28. Chenevix-TrenchG
MilneRL
AntoniouAC
CouchFJ
EastonDF
2007 An international initiative to identify genetic modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: the Consortium of Investigators of Modifiers of BRCA1 and BRCA2 (CIMBA). Breast Cancer Res 9 104
29. KornJM
KuruvillaFG
McCarrollSA
WysokerA
NemeshJ
2008 Integrated genotype calling and association analysis of SNPs, common copy number polymorphisms and rare CNVs. Nat Genet 40 1253 1260
30. AntoniouAC
GoldgarDE
AndrieuN
Chang-ClaudeJ
BrohetR
2005 A weighted cohort approach for analysing factors modifying disease risks in carriers of high-risk susceptibility genes. Genet Epidemiol 29 1 11
31. ToninP
WeberB
OffitK
CouchF
RebbeckTR
1996 Frequency of recurrent BRCA1 and BRCA2 mutations in Ashkenazi Jewish breast cancer families. Nat Med 2 1179 1183
32. NeuhausenS
GilewskiT
NortonL
TranT
McGuireP
1996 Recurrent BRCA2 6174delT mutations in Ashkenazi Jewish women affected by breast cancer. Nat Genet 13 126 128
33. NeuhausenSL
MazoyerS
FriedmanL
StrattonM
OffitK
1996 Haplotype and phenotype analysis of six recurrent BRCA1 mutations in 61 families: results of an international study. Am J Hum Genet 58 271 280
34. OlshenAB
GoldB
LohmuellerKE
StruewingJP
SatagopanJ
2008 Analysis of genetic variation in Ashkenazi Jews by high density SNP genotyping. BMC Genet 9 14
35. AminN
van DuijnCM
AulchenkoYS
2007 A genomic background based method for association analysis in related individuals. PLoS ONE 2 e1274 doi:10.1371/journal.pone.0001274
36. LeuteneggerAL
PrumB
GeninE
VernyC
LemainqueA
2003 Estimation of the inbreeding coefficient through use of genomic data. Am J Hum Genet 73 516 523
37. AulchenkoYS
RipkeS
IsaacsA
van DuijnCM
2007 GenABEL: an R library for genome-wide association analysis. Bioinformatics 23 1294 1296
38. LangeK
WeeksD
BoehnkeM
1988 Programs for Pedigree Analysis: MENDEL, FISHER, and dGENE. Genet Epidemiol 5 471 472
39. HuberPJ
1967 The behaviour of maximum maximum likelihood estimates under non-standard conditions. Proceedings of the Fifth Berkeley Symposium in Mathematical Statistics and Probability 1 221 233
40. LinDY
WeiLJ
1989 The robust inference for the cox-proportional hazards model. J Am Stat Assoc 84 1074 1078
41. PurcellS
NealeB
Todd-BrownK
ThomasL
FerreiraMA
2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 559 575
42. ChenH
SharpBM
2004 Content-rich biological network constructed by mining PubMed abstracts. BMC Bioinformatics 5 147
43. O'DonovanP
LivingstonDM
2010 BRCA1 and BRCA2: breast/ovarian cancer susceptibility gene products and participants in DNA double strand break repair. Carcinogenesis 6 961 7
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 10- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- FSHD: A Repeat Contraction Disease Finally Ready to Expand (Our Understanding of Its Pathogenesis)
- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- The Meiotic Recombination Checkpoint Suppresses NHK-1 Kinase to Prevent Reorganisation of the Oocyte Nucleus in
- Actin Depolymerizing Factors Cofilin1 and Destrin Are Required for Ureteric Bud Branching Morphogenesis
- DSIF and RNA Polymerase II CTD Phosphorylation Coordinate the Recruitment of Rpd3S to Actively Transcribed Genes
- Continuous Requirement for the Clr4 Complex But Not RNAi for Centromeric Heterochromatin Assembly in Fission Yeast Harboring a Disrupted RITS Complex
- Genome-Wide Association Study of Blood Pressure Extremes Identifies Variant near Associated with Hypertension
- The Cytosine Methyltransferase DRM2 Requires Intact UBA Domains and a Catalytically Mutated Paralog DRM3 during RNA–Directed DNA Methylation in
- β-Actin and γ-Actin Are Each Dispensable for Auditory Hair Cell Development But Required for Stereocilia Maintenance
- Genetic Association Study Identifies as a Risk Gene for Idiopathic Dilated Cardiomyopathy
- Evidence for a Xer/ System for Chromosome Resolution in Archaea
- Four Novel Loci (19q13, 6q24, 12q24, and 5q14) Influence the Microcirculation
- Lifespan Extension by Preserving Proliferative Homeostasis in
- Ancient and Recent Adaptive Evolution of Primate Non-Homologous End Joining Genes
- Loss of the p53/p63 Regulated Desmosomal Protein Perp Promotes Tumorigenesis
- Altering a Histone H3K4 Methylation Pathway in Glomerular Podocytes Promotes a Chronic Disease Phenotype
- Characterization of LINE-1 Ribonucleoprotein Particles
- Conserved Genes Act as Modifiers of Invertebrate SMN Loss of Function Defects
- Alternative Splicing at a NAGNAG Acceptor Site as a Novel Phenotype Modifier
- Tight Regulation of the Gene of the KplE1 Prophage: A New Paradigm for Integrase Gene Regulation
- Conjugative DNA Transfer Induces the Bacterial SOS Response and Promotes Antibiotic Resistance Development through Integron Activation
- Nasty Viruses, Costly Plasmids, Population Dynamics, and the Conditions for Establishing and Maintaining CRISPR-Mediated Adaptive Immunity in Bacteria
- Stress-Induced Activation of Heterochromatic Transcription
- H3K27me3 Profiling of the Endosperm Implies Exclusion of Polycomb Group Protein Targeting by DNA Methylation
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
- Characterising and Predicting Haploinsufficiency in the Human Genome
- Dual Functions of ASCIZ in the DNA Base Damage Response and Pulmonary Organogenesis
- Pervasive Cryptic Epistasis in Molecular Evolution
- Transition from Positive to Neutral in Mutation Fixation along with Continuing Rising Fitness in Thermal Adaptive Evolution
- Comprehensive Analysis Reveals Dynamic and Evolutionary Plasticity of Rab GTPases and Membrane Traffic in
- Regulates Tissue-Specific Mitochondrial DNA Segregation
- Role for the Mammalian Swi5-Sfr1 Complex in DNA Strand Break Repair through Homologous Recombination
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



