-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Lifespan Extension by Preserving Proliferative Homeostasis in
Regenerative processes are critical to maintain tissue homeostasis in high-turnover tissues. At the same time, proliferation of stem and progenitor cells has to be carefully controlled to prevent hyper-proliferative diseases. Mechanisms that ensure this balance, thus promoting proliferative homeostasis, are expected to be critical for longevity in metazoans. The intestinal epithelium of Drosophila provides an accessible model in which to test this prediction. In aging flies, the intestinal epithelium degenerates due to over-proliferation of intestinal stem cells (ISCs) and mis-differentiation of ISC daughter cells, resulting in intestinal dysplasia. Here we show that conditions that impair tissue renewal lead to lifespan shortening, whereas genetic manipulations that improve proliferative homeostasis extend lifespan. These include reduced Insulin/IGF or Jun-N-terminal Kinase (JNK) signaling activities, as well as over-expression of stress-protective genes in somatic stem cell lineages. Interestingly, proliferative activity in aging intestinal epithelia correlates with longevity over a range of genotypes, with maximal lifespan when intestinal proliferation is reduced but not completely inhibited. Our results highlight the importance of the balance between regenerative processes and strategies to prevent hyperproliferative disorders and demonstrate that promoting proliferative homeostasis in aging metazoans is a viable strategy to extend lifespan.
Published in the journal: . PLoS Genet 6(10): e32767. doi:10.1371/journal.pgen.1001159
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001159Summary
Regenerative processes are critical to maintain tissue homeostasis in high-turnover tissues. At the same time, proliferation of stem and progenitor cells has to be carefully controlled to prevent hyper-proliferative diseases. Mechanisms that ensure this balance, thus promoting proliferative homeostasis, are expected to be critical for longevity in metazoans. The intestinal epithelium of Drosophila provides an accessible model in which to test this prediction. In aging flies, the intestinal epithelium degenerates due to over-proliferation of intestinal stem cells (ISCs) and mis-differentiation of ISC daughter cells, resulting in intestinal dysplasia. Here we show that conditions that impair tissue renewal lead to lifespan shortening, whereas genetic manipulations that improve proliferative homeostasis extend lifespan. These include reduced Insulin/IGF or Jun-N-terminal Kinase (JNK) signaling activities, as well as over-expression of stress-protective genes in somatic stem cell lineages. Interestingly, proliferative activity in aging intestinal epithelia correlates with longevity over a range of genotypes, with maximal lifespan when intestinal proliferation is reduced but not completely inhibited. Our results highlight the importance of the balance between regenerative processes and strategies to prevent hyperproliferative disorders and demonstrate that promoting proliferative homeostasis in aging metazoans is a viable strategy to extend lifespan.
Introduction
Lifespan of many organisms can be increased by optimizing both genetic and environmental conditions, including reducing calorie intake [1]–[3], increasing oxidative stress protection [4], [5] and reducing Insulin/IGF1 signaling (IIS) [6]–[8]. These different interventions are likely to be acting through related mechanisms, notably by increasing stress-protective gene expression in differentiated somatic cells, prolonging their functional lifespan and delaying tissue degeneration [7], [9]–[12]. In addition to such stress-protective mechanisms, metazoans also maintain tissue homeostasis through regenerative processes that rely on the long-term maintenance of a functional population of somatic stem and progenitor cells. For these cells, a similar, and perhaps more significant, relationship between stress protection and lifespan is expected, as their long-term maintenance is critical to conserve regenerative capacity. This relationship is complicated, however, by the fact that such cells are mitotically active, and their deregulation thus has the potential to promote dysplasia and increase the incidence of cancer [13], [14]. Accordingly, mammalian stem cells generally exhibit a robust intrinsic ability to limit and repair intracellular damage [15]–[19], yet also employ strong anti-proliferative mechanisms that prevent cancer, but limit the regenerative capacity of stem cells in old age [18], [20]–[22]. The regenerative decline of many tissues is thus caused by oxidative stress and DNA damage in stem and progenitor cells, as well as by cell-autonomous up-regulation of cell cycle inhibitors like p16, and by changes in the systemic environment [13], [20], [23]–[27]. Accordingly, processes that maintain the regenerative capacity of stem and progenitor cell populations, but prevent hyper-proliferation and cancer (i.e. processes that promote proliferative homeostasis), are expected to significantly influence longevity of the organism [28].
Recent studies in mouse hematopoietic stem cells (HSCs) indicate that the IIS pathway and its downstream transcription factor Foxo constitute an important regulatory system that controls stem cell stress protection while also influencing proliferation [18], [29]–[32]. Foxo (Daf-16 in C.elegans) is repressed by IIS and is required for the lifespan extension observed when IIS activity is reduced either systemically, or specifically in adipose tissue [6]–[8]. Foxo induces the expression of genes involved in scavenging reactive oxygen species (ROS) and repairing damage to DNA and proteins, while also inducing cell cycle inhibitors [33]–[37]. Loss of Foxo in HSCs therefore results in increased proliferation of the HSC population, while boosting ROS levels and increasing apoptosis. As a consequence, the long term repopulating ability of HSCs is reduced [18], [29], [30], [32].
Drosophila is emerging as a genetically tractable model to assess the importance of regeneration in lifespan and aging [38]–[43]. Recently identified somatic stem cells in Drosophila include intestinal stem cells (ISCs) in the posterior midgut epithelium, as well as stem cells in malpighian tubules [44] and the hindgut [45], [46]. ISCs are critical for regeneration and maintenance of the midgut epithelium [47]–[50]. These cells are characterized by the expression of the marker genes escargot and Delta, and divide asymmetrically to give rise to a new ISC and an Enteroblast (EB) that differentiates into one of two cell types: Enterocytes (ECs) and Enteroendocrine cells (EEs). In contrast to the mammalian lineage, no transit amplifying cell population exists in Drosophila; ISC being the only dividing cell type in the midgut epithelium [47]–[49]. In young animals, ISCs divide rarely, as less than 5 mitoses can be observed at any given timepoint in the intestine [42], [47]–[49]. In response to stressful challenges, however, ISC proliferation is strongly increased, a regenerative response that allows restoring large parts of the intestinal epithelium in response to damaging agents, such as pathogens, genotoxins, or ROS inducing compounds [42], [43], [50]–[53]. Interestingly, this regenerative function of ISCs can have deleterious consequences for the organism, as excessive proliferation of ISCs in response to stress is accompanied by the accumulation of mis-differentiated cells in the intestine, which ultimately disrupts epithelial integrity with a dysplastic phenotype [43]. In the aging gut, such dysplasia is widely observed under normal culture conditions, suggesting that an age-related over-proliferation of ISCs (due to either elevated or chronic oxidative stress or to pervasive inflammation) contributes to the loss of intestinal function and to the increased mortality of aging flies [42], [43], [54]. This phenotype is caused by an age-related increase in the activity of the stress-responsive Jun-N-terminal Kinase (JNK) signaling pathway [43], [54].
The Drosophila intestine thus constitutes an accessible model system to study whether preserving proliferative homeostasis of aging tissues can influence overall lifespan of metazoans. We initiate such studies here by assessing the effects of intestinal dysplasia on lifespan. Our results reveal a significant correlation between the loss of proliferative homeostasis in the intestinal epithelium and fly lifespan. Importantly, we show that limiting proliferation rates by moderately reducing IIS or JNK activities in the somatic stem cells lineages is sufficient to extend lifespan. We further find that the beneficial effects of reducing IIS activity in this lineage can be recapitulated by selectively over-expressing stress-protective Foxo target genes. In such flies, intestinal dysplasia is delayed, accompanied by improved maintenance of metabolic health, and by increased lifespan. Our results demonstrate that promoting proliferative homeostasis in somatic tissues is sufficient to extend lifespan in metazoans.
Results
Intestinal regeneration influences lifespan
Recent studies suggest a significant influence of intestinal regeneration on fly viability. Flies in which intestinal dysplasia is accelerated are short lived [43], while animals with impaired ISC proliferation or daughter cell differentiation die faster when infected by enteropathogenic bacteria than wild-type flies [50], . These observations indicated that genetic conditions in which intestinal homeostasis is preserved might result in increased lifespan.
To start testing this hypothesis, we first tested the requirement of ISC-mediated tissue renewal for optimal lifespan. Ectopic activation of Notch signaling in ISCs was previously shown to irreversibly impair their function by promoting differentiation [43], [49]. In order to abolish ISC function, we thus transiently expressed an activated form of Notch (IntraCellular Domain; NICD) in ISCs and EBs using the esgGal4 driver. In young adult esgGal4 heterozygous flies, Gal4 activity is restricted to ISCs and EBs in the intestine, to malpighian tubule stem cells, as well as to the testis and salivary glands, and is not detected in other tissues (Figure S1). To prevent developmental effects of the expression of UAS-driven transgenes, we used a heat-inducible system in which esgGal4 is combined with a temperature-sensitive Gal80 (TARGET system, [55]; Figure S2D). Transient expression of NICD for 7 days in young flies, significantly shortens lifespan (Figure S2A), supporting the notion that maintaining somatic stem cell function is critical for optimal lifespan. Importantly, longevity is not significantly affected when these flies are kept at a permissive temperature throughout life, confirming that lifespan shortening is caused by transient adult expression of NICD, and not by ectopic expression of the protein during development (Figure S2B; further confirming the selective inducibility of the employed TARGET system, UAS-linked transgene expression is detectable in esgG4, tubGal80ts flies only at the restrictive temperature, 29°C, Figure S2D, S2E).
We next assessed the relationship between ISC proliferation rates, intestinal dysplasia and lifespan (Figure 1). In wild-type flies, the number of dividing ISCs detectable at a given timepoint (as measured by the number of pH3+, phosphorylated Histone H3 positive, cells in the gut) increases 10-fold between 3 days and 30 days of age when reared at 25°C or between 3 days and 18 days of age when reared at 29°C (Figure 1A, [42]). This increase is accompanied by a progressive accumulation of polyploid, mis-differentiated cells that accumulate at the basal membrane of the epithelium and can be visualized by their continuous expression of the ISC/EB marker escargot (esg; [42], [43], [54]). This dysplastic phenotype is readily observed in old flies expressing GFP under the control of the esgGal4 driver, and can be classified into four distinct categories that correlate with the frequency of pH3+ cells per gut and thus serve as an accessible quantitative criterion for intestinal dysplasia within a fly population (Figure 1B and Text S1). Importantly, age-related dysplasia is not accompanied by aberrant proliferation of ISC daughter cells, as confirmed by analysis of pH3+ cell frequencies and clonal growth rates in individual marked ISC lineages in old flies or in stress conditions (Figure S3). This is consistent with previous ISC lineage analysis demonstrating that no transit amplifying population of cells exist in the Drosophila midgut [48]. The frequency of pH3+ cell numbers in the gut is thus a direct measure of ISC proliferation rates.
Fig. 1. Intestinal homeostasis and tissue regeneration is critical for normal lifespan. 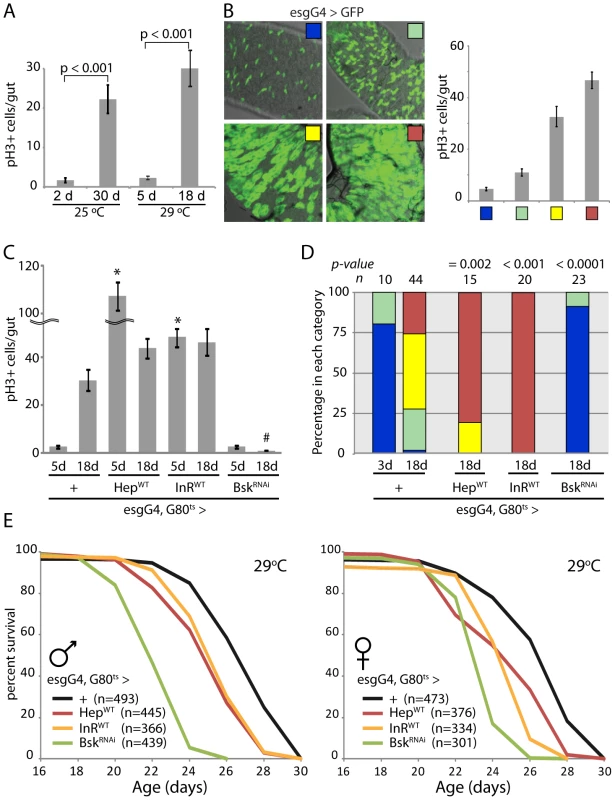
A. Age-related increase in the frequency of pH3+ cells in the aging intestine of wild-type flies (Average and SEM is shown). ISC over-proliferation is accelerated at higher temperature (29°C). Intestines were dissected at the indicated age and phosphorylated Histone H3 was detected by immunohistochemistry. B. The size of GFP+ cell clusters can be used to evaluate dysplasia in esgGal4, UAS-GFP flies (see also Text S1). The 4 categories defined visually in the panels on the left correlate with the frequency of pH3+ cells in the gut (right). C. Activation of JNK and IIS pathways in ISC (esg>Hep and esg>inR respectively) induces over-proliferation as early as 5 days, while inhibition of JNK (esg>BskRNAi) prevents tissue regeneration as shown by much reduced frequency of pH3+ cells. The TARGET system was used to prevent developmental effects of esg-driven transgenes expression (Genotypes: w1118;esgGal4,UAS-GFP/+;tubGal80ts, w1118;esgGal4,UAS-GFP/UAS-HepWT;tG80ts, w1118;esgGal4,UAS-GFP/UAS-InRWT;tG80ts, and w1118;esgGal4,UAS-GFP/UAS-BskRNAi;tG80ts). Flies were reared at 18°C and then aged at 29°C to restrict expression of transgenes to adulthood. Averages and SEM are shown. * p<0.001 compared to Control at 5 days; # p<0.001 compared to Control at 18 days using Student's t-test. D. Intestinal dysplasia in the flies described above was monitored using the method described in Figure 1B (see also Text S1) after 18 days. Activation of JNK and IIS pathways causes accelerated dysplasia, reduction of JNK signaling leads to a complete prevention of tissue regeneration. p-value from Pearson XiSquare test. E. Flies with impaired intestinal homeostasis and tissue regeneration are short-lived. The mortality of the flies described above was recorded at 29°C. Detailed lifespan analysis is shown in Table S1. To influence intestinal proliferation rates in aging flies, we modulated the activities of the JNK or IIS pathways in ISCs and EBs using the esgGal4 driver. Activation of both pathways with this driver increases ISC proliferation [43], [53], [54]. We activated or inhibited JNK by expressing the JNK Kinase Hemipterous (Hep) or dsRNA against the JNK Bsk (BskRNAi), respectively, or activated IIS by expressing the Insulin receptor (InR). We used the heat-inducible esgGal4, tubGal80ts system to prevent expression of transgenes before adulthood, and therefore assessed age-related changes in ISC proliferation and intestinal homeostasis at 18 days of age at 29°C (Figure 1C and 1D). Consistent with previous findings, activating JNK or insulin signaling activity resulted in dramatically increased dysplasia at 18 days and elevated proliferation rates even in young flies (5 days of induction), while when JNK signaling was impaired in the ISC lineage, dysplasia was almost entirely prevented and ISC proliferation was strongly impaired.
Interestingly, both of these extreme conditions, accelerated dysplasia and strongly inhibited ISC proliferation, resulted in significant lifespan shortening, supporting our hypothesis that intestinal homeostasis and the regenerative capacity of the intestinal epithelium are critical for fly lifespan (Figure 1E, Table S1). Maintaining intestinal proliferation rates at levels that preserve regenerative capacity while limiting dysplasia might thus influence lifespan positively.
Reduced age-associated dysplasia in long-lived IIS loss-of-function conditions
To test this idea, we first evaluated proliferative homeostasis in long-lived fly populations (Figure 2). Intestinal dysplasia in non-labeled intestines can be quantified by defining categories based on the extent of BrdU incorporation in the intestinal epithelium (reflecting both ISC divisions and endoreplication of daughter cells), and the loss of tissue architecture observed when staining with the membrane marker armadillo (arm, Figure 2A, see Text S1). Since reduced IIS activity extends lifespan in flies, we assessed whether long-lived fly lines with reduced IIS activity would exhibit delayed dysplasia. Indeed, limiting IIS activity systemically reduces the age-associated increase in the frequency of pH3+ cells, as well as the increase in intestinal BrdU incorporation and the loss of epithelial architecture in the gut (Figure 2B and 2C). IIS activity was reduced by ablating insulin-producing cells (IPCs) through expression of the pro-apoptotic gene reaper (rpr) under the control of dilp2Gal4, by reducing the genedose of the insulin receptor substrate-homologue Chico, or in trans-heterozygotes for the insulin receptor loss-of-function alleles InRE19 and InR05545. Flies with all three genetic conditions are robustly long-lived [56]–[59], suggesting that the reduction in intestinal dysplasia observed here is associated with longevity.
Fig. 2. Reduced IIS activity delays tissue degeneration in the intestine. 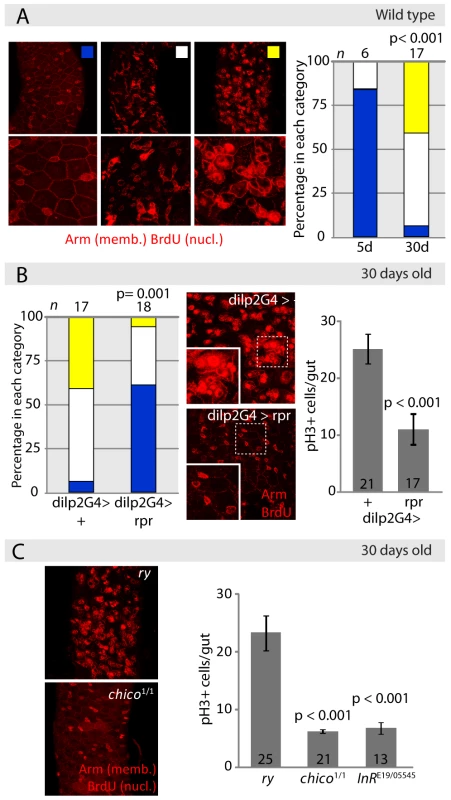
A. Evaluation of intestinal dysplasia in aging wild-type flies. BrdU incorporation identifies proliferating cells (nuclear, red), while immunohistochemistry with anti-Armadillo antibodies detects changes in epithelial structure (membrane, red). Flies were aged at 25°C, and fed BrdU for 48 hrs. B. Reducing systemic insulin signaling by ablation of Insulin Producing Cells (IPCs) delays aging-associated dysplasia. Representative pictures of the midgut from aging (30 days old at 25°C) control flies (w1118;dilp2Gal4>+) and flies with ablated IPC's (w1118;dilp2Gal4>rpr) are shown in the center. Scoring was performed based on the classification shown in B. Significant delay of dysplasia in dilp2Gal4>rpr can be observed compared to dilp2G>+ controls (left panel, Pearson Xi Square test). This correlates with reduced numbers of pH3+ cells in dilp2Gal4>rpr (Average and SEM; Student's t-test). C. Frequency of pH3+ cells in aging chico1/1 homozygotes and InrE19/05545 compared to isogenic wild-type controls (ry506) (Average and SEM, Student's t- test). Representative BrdU/Armadillo-stained midguts from aging chico1 mutant flies and sibling controls (ry) are shown on the left. Interestingly, in these long-lived lines, IIS activity is reduced, but not absent, since the insulin receptor can signal directly to PI3K, bypassing the requirement for Chico [60], and ablation of IPCs results in loss of selected insulin-like peptides, whereas insulin-like peptides expressed in other tissues, such as the fatbody and germline, are retained [61]–[64]. Accordingly, the average number of pH3+ cells decreased significantly, but moderately, indicating that in these long-lived animals, proliferative homeostasis is preserved without negatively impacting regenerative capacity (Figure 2C and 2D).
Repression of IIS in the ISC lineage inhibits ISC proliferation and shortens lifespan
The Insulin signaling pathway has wide-ranging functions in growth, metabolism, and reproduction [6]–[8], [65]–[68]. The observed correlation between the extent of age-related dysplasia and lifespan in the genetic conditions tested above could thus be a secondary consequence of other physiological changes. To test more directly whether impairing IIS activity in the ISC lineage would influence age-related dysplasia and affect lifespan, we over-expressed a dominant-negative Insulin receptor (InRDN; [69], dominant-negative PI3Kinase (DP110DN [70]), a dsRNA targeting the IIS downstream kinase Akt (AktRNAi, Figure S4), as well as wild-type Foxo under the control of heat-inducible esgGal4 (esgGal4, tubGal80ts). In all four cases, we observed strongly reduced age-related dysplasia of the intestinal epithelium, confirming that IIS activity in ISCs is required for the age-related over-proliferation of these cells (Figure 3A). However, this reduction was as strong as when JNK was repressed by expression of BskRNAi, suggesting that impaired regeneration in these guts might also limit viability and reduce lifespan. We tested this prediction and found indeed that expression of Foxo, AktRNAi, or DP110DN under the control of esgGal4, Gal80ts (at 29°C) or of Foxo under the control of esgGal4 (25°C) caused significant lifespan shortening (Figure 3B–3D, Figure S5, Table S2, Table S3).
Fig. 3. Strong reduction of insulin signaling in the somatic stem cell lineages delays age-related dysplasia and shortens lifespan. 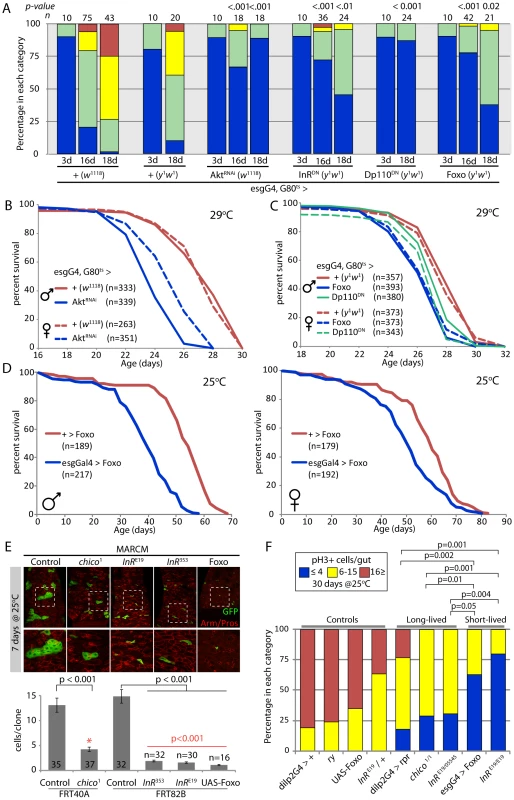
A. Intestinal degeneration was monitored in aging control flies (w1118;esgGal4,GFP;Gal80ts and y1w1;esgGal4,GFP;Gal80ts) and flies with impaired insulin signaling activity in ISCs (w1118;esgGal4,GFP;Gal80ts/UAS-AktRNAi, y1w1;esgGal4,GFP;Gal80ts/UAS-Dp110DN, y1w1;esgGal4,GFP;Gal80ts/UAS-InRDN, y1w1;esgGal4,GFP;Gal80ts/UAS-Dp110DN, y1w1;esgGal4,GFP/UAS-Foxo;Gal80ts). Strong inhibition of IIS in ISC prevents age-related intestinal dysplasia. p-value from Pearson XiSquare test. B,C. Flies with impaired intestinal homeostasis and tissue regeneration are short-lived. The mortality of the flies described above was recorded at 29°C. Detailed lifespan analysis is shown in Table S2. D. EsgGal4 was used to express Foxo in the ISC lineage. Fly lines were backcrossed into the w1118 background (10 generations) and sibling populations derived from crosses of w1118;esgGal4/+ females with y1w1;UAS-Foxo/UAS-Foxo males were compared. Flies were reared at 18°C to minimize driver activity during development, and adults were maintained at 25°C. Lifespan is significantly shortened in flies expressing Foxo under the control of esgGal4. A detailed analysis of the mortality, as well as the mortality of isogenic controls (w1118/y1w1), flies is shown in Table S3. E. Growth of InR and chico1 homozygous mutant ISC clones and clones over-expressing Foxo in the intestinal epithelium. Clones were induced using the MARCM system by heat-shock at three days of age and clone size was evaluated at 7 days after heat shock. chico1 clones exhibit reduced, but not absent growth, while InR mutant clones and Foxo over-expressing clones remain mostly single cells. Representative images are shown in top panels (Green: GFP; Red: Armadillo/Prospero). Clone size quantification is shown in lower graphic (Averages and SEM). p-values from Student's t-test. InR homozygous mutant clones and clones over-expressing Foxo grow significantly less than chico1 mutant clones (p-values in red). F. Comparison of the proliferation rate in the intestine of controls, long-lived and short-lived flies, after 30 days at 25°C, suggesting that long-lived mutants achieve the proper balance between tissue dysplasia and absence of regeneration. p-value from Pearson XiSquare test. Reduced IIS activity in the ISC lineage thus shortens lifespan most likely by preventing regeneration. Supporting this interpretation, we found that ISC clones (induced by somatic recombination using the MARCM system; [71], [72] homozygous for InRE19 or InR353, or over-expressing Foxo, have a strongly reduced ability to grow (and thus to generate newly differentiated ECs and EEs, Figure 3E). The extent of this growth repression was significantly more severe than in chico1 homozygous mutant clones (Figure 3E). Interestingly, InRE19 homozygous mutant flies are short-lived (as opposed to InRE19/InR05545 transheterozygotes; [56]), further strengthening the notion that impaired regeneration of the intestinal epithelium of these flies is associated with shorter lifespan.
Lifespan extension by limiting IIS and JNK signaling in somatic stem cells
Taken together, the results described above support the notion of a critical relationship between proliferative homeostasis, regeneration and lifespan: reduced ISC proliferation, and thus limited age-related dysplasia (as in dilp>rpr, chico1 homozygotes and InRE19/InR5545 transheterozygotes), is beneficial, while impaired ISC proliferation, and thus reduced regenerative capacity (as in InRE19 homozygotes or in flies over-expressing Foxo in the ISC lineage), shortens lifespan. This relationship can be illustrated by comparing relative lifespan with the fraction of flies with low, intermediate or high frequencies of ISC proliferation at 30 days (reared at 25°C) for the genotypes discussed above (Figure 3F).
To test this model more directly, and to confirm that improved proliferative homeostasis is sufficient to extend lifespan, we repressed IIS and JNK activities in ISCs and EBs using 5961 Geneswitch-Gal4 (5961GS; Figure 4, [73]). This RU486-inducible driver recapitulates the esgGal4 expression pattern in the intestine, albeit at much lower levels, allowing moderate repression of IIS and JNK activities in an RU486-dependent manner ([73], Figure 4A–4C; we assessed GFP expression under the control of 5961GS in various tissues, and observed weak RU486-dependent induction only in the intestine, Figure S6). Female flies expressing InRDN, DP110DN, AktRNAi, BskDN, or BskRNAi under the control of this driver show moderately, but significantly, reduced intestinal proliferation at old age (Figure 4D–4J). Importantly, these flies are significantly longer lived when exposed to RU486 than isogenic siblings exposed to mock treatment (median lifespan extended at least 10% for all conditions); whereas control flies show almost no RU486-dependent change in longevity (1% change in median lifespan; Figure 4D–4K and Table S4; expression of the same transgenes in males resulted in no significant lifespan effect, not shown). All together, these results strongly support the model outlined above.
Fig. 4. Moderate inhibition of IIS and JNK pathways in somatic stem cell lineages extends lifespan. 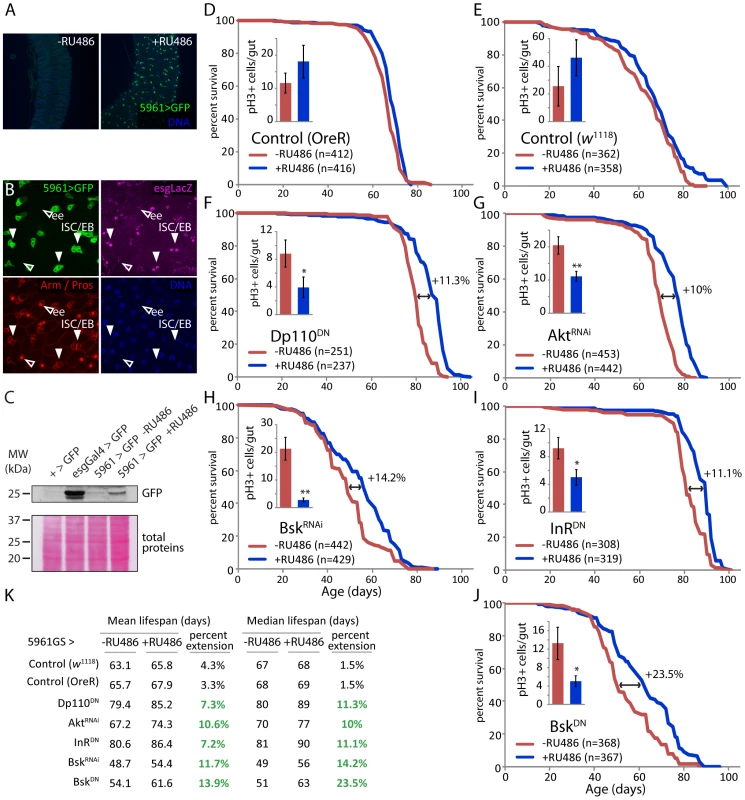
A. The 5961GS driver is expressed in the intestine and responsive to RU486. GFP can be detected in the posterior midgut of 5961GS>GFP flies after RU486 exposure, no GFP is detected when flies are kept on control food. B. In the intestine, the activity of the 5961GS driver is restricted to ISC and EB. Only LacZ-positive cells express GFP in 5961GS>GFP/esg-LacZ flies. The expression of the reporter esg-LacZ identifies ISC and EB, immunostaining against prospero identifies EE. C. Western-blot analysis of total extract from dissected guts shows that GFP can be detected in the intestine of 5961GS>GFP flies after RU486 exposure. However, the expression level remains much lower than in the intestine of esgGal4>GFP flies. D–J. Moderate reduction of the IIS (F, G, I) and JNK (H, J) pathways using 5961GS extends lifespan. The mortality of sibling flies of the indicated genotypes placed on control food (-RU486) or food supplemented with RU486 (+RU486) was compared at 25°C. The treatment has minimal effect on the longevity of control flies (5961GS,UAS-GFP>+ in w1118 and OreR background), but causes significant increase in longevity of flies with reduced IIS and JNK pathways (5961GS,UAS-GFP>UAS-InRDN, 5961GS,UAS-GFP>UAS-Dp110DN, 5961GS,UAS-GFP>UAS-AktRNAi, 5961GS,UAS-GFP>UAS-BskDN, 5961GS,UAS-GFP>UAS-BskRNAi). The relative extension of the median lifespan is shown for each genetic condition. For each condition, the reduction of ISC proliferation by the treatment was confirmed, as measured by the number of pH3+ cells in the intestinal epithelium, in 50 to 70 days old females (n>12 guts; Averages and SEM; p-values from Student's t-test * p<0.05, ** p<0.01). K. Summary of lifespan statistics including mean and median lifespan (days) for all conditions. Detailed lifespan analysis is shown in Table S4. Expression of stress-protective genes in the ISC lineage limits age-associated dysplasia
IIS and JNK signaling activities thus have to be carefully balanced to maintain intestinal homeostasis and regenerative capacity. This balance will ultimately influence the expression of Foxo target genes, which encode stress-protective proteins as well as cell cycle inhibitors and pro-apoptotic factors that are expected to have antagonistic consequences for stem and progenitor cell maintenance and proliferation. Accordingly, a critical and pleiotropic function of Foxo proteins in stress-protection, proliferation and apoptotic control of stem cells has been described for the hematopoietic system in mice [18], [29]–[32]. Selectively increasing the expression of stress-protective Foxo target genes in the ISC lineage might thus be sufficient to limit age-related dysplasia without impairing regeneration, thus recapitulating the consequences of organism-wide moderate reduction of IIS activity, and potentially extending lifespan.
To test this hypothesis, we used esgGal4 to express Hsp68, a heatshock protein that extends lifespan when expressed in the whole fly [74], and Jafrac1, a peroxiredoxin that detoxifies ROS and can increase lifespan when expressed in the brain [75], [76]. Both genes are Foxo targets (Figure S7; [75]) and strikingly, we found that both caused a significant delay in dysplasia (both in the posterior midgut, as well as when assessing invasion of the proventriculus by GFP-positive cells; Figure 5A and Figure S8), accompanied by moderate reduction in the frequency of pH3+ cells in the gut (Figure 5B). Since dysplasia in aging intestinal epithelia is accompanied by increased expression of Dl [43], we further tested the expression of Dl in these intestines and found a significant decrease in Dl accumulation compared to wild-type animals, confirming that the accumulation of mis-differentiated ISC progeny in these flies is reduced (Figure 5C).
Fig. 5. Overexpression of stress-protective genes in the somatic stem cell lineages delays intestinal degeneration and limits metabolic decay. 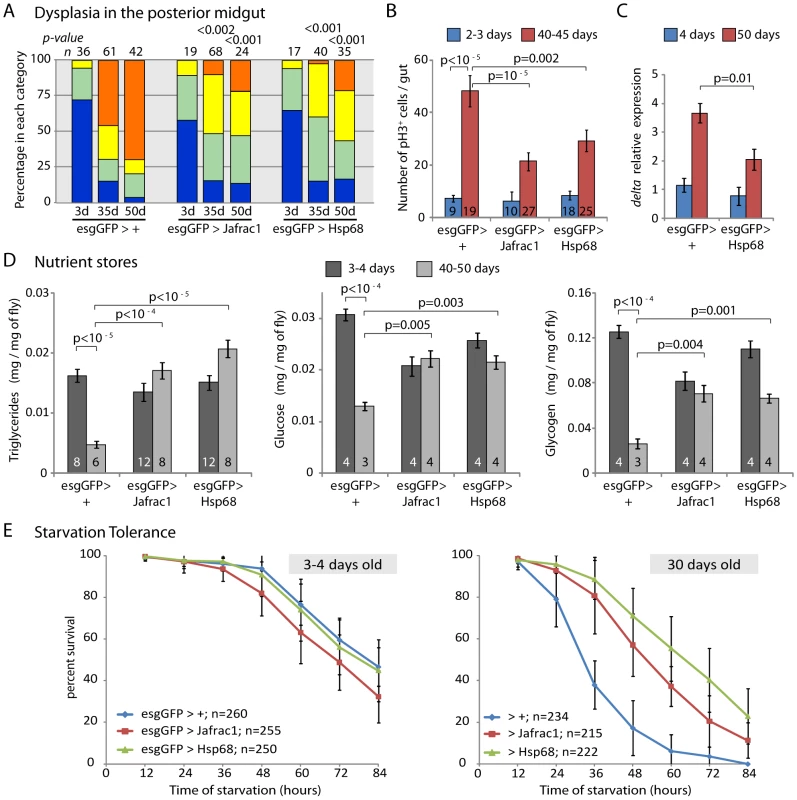
A. Overexpression of Jafrac1 or Hsp68 delays age-related loss of intestinal architecture. Intestinal degeneration in aging (3, 35, and 50 days) control flies (esgGFP>+) and flies overexpressing cytoprotective genes in the ISCs (esgGFP>Jafrac1 and esgGFP>Hsp68) was scored in the posterior midgut. p- values from Pearson Xi Square test. B. Overexpression of Jafrac1 or Hsp68 under the control of esgGal4 also limits the increase in the frequency of pH3+ cells in aging intestines (Averages and SEM; Student's t-test). C. Dl expression relative to rp49 in the aging intestine measured by real-time RT-PCR (Averages and SEM; Student's t-test). D. Over-expression of Jafrac1 or Hsp68 delays age-related changes in nutrient levels. Triglycerides, free glucose and glycogens were measured in young (3–4 days old) or old (40–50 days old) flies. Concentration is shown as mg nutrient per mg fresh fly. The number at the bottom of each bar represents the number of samples. All error bars represent standard deviation, p-value from Student's t-test. E. Jafrac1 and Hsp68 expression in ISCs increases starvation tolerance in old flies. Wet starvation resistance was determined in the indicated populations of flies aged for three days (left) or for 30 days (right). Improved metabolic homeostasis in flies expressing stress-protective genes in somatic stem cell lineages
Dysplasia in the aging intestinal epithelium is expected to cause defects in nutrient absorption, resulting in deficient nutrient stores in the organism and disrupting metabolic homeostasis. Since hsp68 and jafrac1 expression in ISCs and their daughter cells significantly delays intestinal dysplasia, we tested whether the maintenance of metabolic homeostasis was improved in these flies. The amount of free glucose, triglycerides and glycogen stored by old wild-type flies is significantly reduced compared to young animals (Figure 5D). When jafrac1 or hsp68 were expressed under the control of esgGal4, however, high levels of these nutrient stores were maintained in aging flies. This rescue of metabolic homeostasis correlates with increased starvation tolerance (Figure 5E), further supporting the idea that maintenance of intestinal homeostasis by protecting somatic stem cells is critical for metabolic health of aging flies.
Lifespan extension by stress-protective gene expression in stem cell lineages
To assess whether over-expressing stress-protective genes using the esg-Gal4 driver would be sufficient to extend lifespan, we compared demographies of multiple independent populations of flies expressing jafrac1 and hsp68 under the control of esgGal4 to isogenic wild-type controls. Strikingly, we observed consistent and significant lifespan extension in both males and females when jafrac1 and hsp68 were expressed (Figure 6A and 6B; Table S5). To exclude that the esgGal4-driven expression of stress-protective genes in salivary glands is causing the observed lifespan extension, we also tested the lifespan of flies in which hsp68 was expressed using GMR-Gal4, an eye-specific driver that also expresses Gal4 in salivary glands, and found no effect (Figures S1B, S1C, S9). We further confirmed the beneficial consequences of Jafrac1 and Hsp68 expression on lifespan using the weaker 5961GS driver, and found moderate but significant extension of lifespan in flies expressing both transgenes (Figure 6C, Table S6). Evidently, expressing selected stress-protective Foxo target genes in the ISC lineage is sufficient to recapitulate the effects of reducing IIS or JNK activity in these cells.
Fig. 6. Over-expression of stress-protective genes in the somatic stem cell lineages extends lifespan. 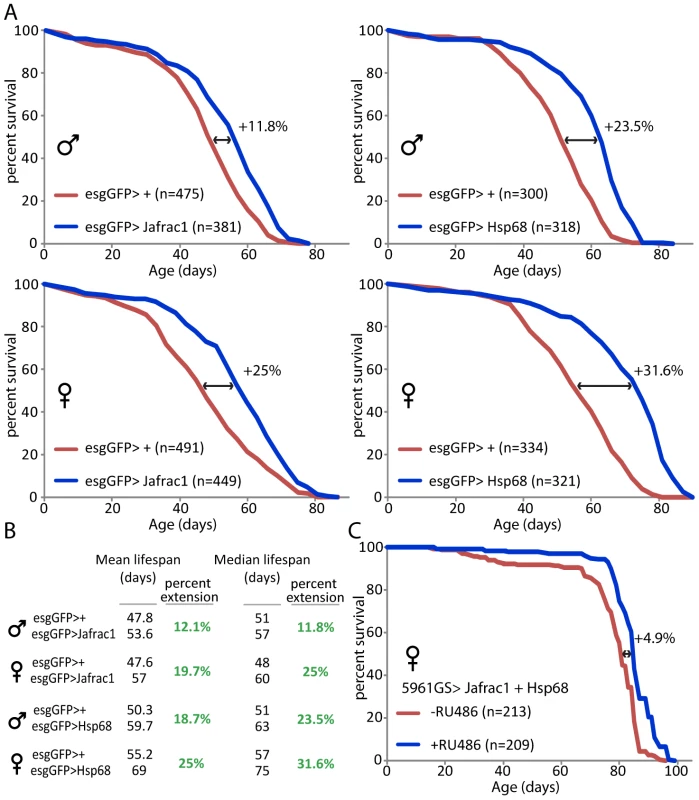
A. Survival curves of esgGFP>Jafrac1 and esgGFP>Hsp68 flies compared to their respective wild type isogenic controls. UAS lines were backcrossed 10 generation into w1118 background. Wild type and UAS siblings were crossed to esgGFP and mortality of the progeny was recorded at 25°C. B. Summary of lifespan statistics including mean and median lifespan (days). C. Over-expression of Jafrac1 and Hsp68 using the 5961GS driver moderately extends lifespan. The mortality of sibling flies (5961GS,GFP>UAS-Jafrac1,UAS-Hsp68) placed on control food or food supplemented with RU486 was compared at 25°C. Due to the weak activity of the 5961GS driver, both UAS-Jafrac1 and UAS-Hsp68 transgenes were combined to observe a significant effect. The relative extension of the median lifespan is shown for all curves. Detailed lifespan analysis is shown in Table S5 and S6. Discussion
Our results indicate that proliferative homeostasis in high turnover tissues is limiting for Drosophila lifespan and highlight the importance of mechanisms that balance pro - and anti-mitotic activities. In the ISC lineage, this balance involves fine-tuning the activities of the pro-mitotic IIS and JNK signaling pathways to ensure appropriate supply of newly formed ISC daughter cells while limiting dysplasia. Accordingly, we observe moderately reduced intestinal proliferation rates in long-lived IIS mutants, as well as lifespan extension when IIS or JNK signaling are moderately reduced in the ISC lineage. This association between proliferative activity in the intestinal epithelium and lifespan is illustrated in Figure 7. Strikingly, intestinal proliferation rates correlate with relative lifespan over a wide range of genotypes.
Fig. 7. A model for the impact of regenerative capacity on lifespan. 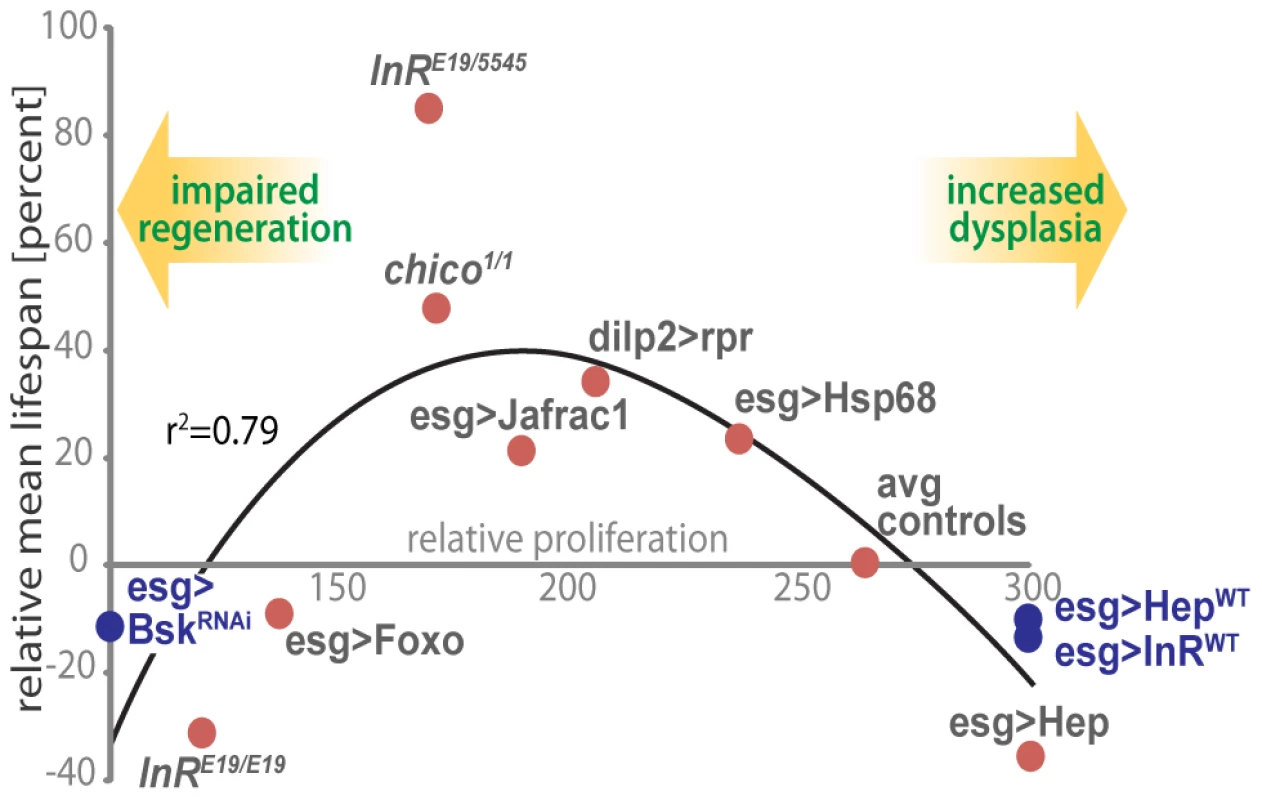
Genetic conditions that moderately decrease ISC proliferation (thus limiting dysplasia) are associated with increased lifespan, while strong repression of ISC proliferation is deleterious for regeneration and shortens lifespan. The association of lifespan and regenerative capacity of the intestine in aging flies is illustrated by comparing ISC proliferation rates and lifespan. Relative ISC proliferation rates were calculated for each genotype using the three categories defined in Figure 3F (low, intermediate and high frequencies of pH3+ cells) and the following formula: × = (proportion in cat.1) + 2*(proportion in cat.2) + 3*(proportion in cat.3). This model includes lifespan and intestinal proliferation analysis from experiments conducted at 25°C (red dots) or 29°C (blue dots). The relative proliferation data is from this study. These data are plotted against lifespan changes in the respective genotypes relative to corresponding isogenic controls, from this work and from published studies [56], [57], [59]. Polynomial regression curve (3rd degree) was fitted using Excel. Our results further show that the stress-protective components of the Foxo-regulated gene expression program are sufficient to maintain proliferative homeostasis, extending lifespan of the organism. Reduction of IIS activity, which extends lifespan in many organisms, is thus accompanied by the preservation of regenerative processes. While reducing IIS activity or activating Foxo in adipose tissue is sufficient to extend lifespan of flies, mice and worms [65]–[68], our results suggest that the anti-proliferative and stress-protective consequences of Foxo activation in high-turnover tissues also contribute to lifespan extension in IIS loss-of-function conditions. Interestingly, a tumor-suppressing role for Foxo in mice and C.elegans has been reported [77]–[79], while Foxo regulates redox homeostasis in mouse HSCs [18], [29], [30], [32], [77]. Reduced IIS activity thus optimizes somatic maintenance, metabolism and regenerative processes in complex metazoans, and all three physiologic consequences of IIS repression seem to contribute to achieve maximum lifespan.
It remains to be tested whether the lifespan extension commonly observed in flies exposed to dietary restriction (DR) is also associated with delayed intestinal dysplasia. Reduced IIS activity contributes to lifespan extension in DR conditions [80]–[82] suggesting that reduced intestinal dysplasia might contribute to DR-induced lifespan extension.
Interestingly, the effects of JNK signaling on lifespan are more complex. JNK can extend lifespan when activated in the brain by repressing the expression of insulin-like peptides [37], [74], [83], [84], thus systemically repressing IIS activity [37], [74]. Our findings reported here, however, show that JNK activation in the ISC lineage can have deleterious effects and needs to be limited to ensure longevity. Such pleiotropic consequences of JNK have also been reported in other contexts and have significant implications for the development of therapies targeting this pathway [85]–[87].
The importance of anti-oxidant Foxo target genes in regulating proliferative homeostasis highlights the challenging environment to which the intestinal epithelium is exposed. Apart from extraneous toxins and oxidants, the intestinal epithelium also mounts strong oxidative responses to inflammation, potentially exposing ISCs and daughter cells to high levels of oxidative stress [50], [52], [88]–[91]. Our results show that over-expressing stress-protective proteins in the ISC lineage is sufficient to limit and optimize cellular responses to these challenges, thus preserving intestinal homeostasis longer (but not indefinitely, as a significant fraction of these animals do develop dysplasia at older ages).
Intestinal dysplasia is caused by over-proliferation of ISCs in concert with mis-differentiation of ISC progeny, and in long-lived animals both processes are prevented. Due to technical limitations of the Gal4 drivers used, however, we cannot exclude that the expression of stress-protective genes, or of IIS or JNK repressors with esgGal4 or 5961GS affects primarily the differentiation process of EBs rather than the ISC itself. While the inability of IIS mutant clones to grow, and the significant reduction in the number of pH3+ cells in IIS and JNK loss-of-function conditions and in Hsp68 and Jafrac1 over-expressing flies, demonstrates that ISC proliferation is indeed influenced by these manipulations, it is conceivable that this effect might be mediated by indirect, non-cell-autonomous limitation of ISC proliferation by EBs in these conditions. Such a feedback control of ISC division would be interesting, and further studies are needed to test this possibility.
The pattern of esgGal4 and of 5961GS expression further requires considering effects of IIS and JNK activities in other tissues on lifespan: While we can exclude the testes and salivary glands as sources of the observed effects (lifespan effects are observed in both males and females using esgGal4, expression of Jafrac1 and Hsp68 in salivary glands has no effect on lifespan, and 5961GS is not expressed in salivary glands), we cannot currently exclude a contribution of malpighian tubule stem cells. These cells also appear to respond to proliferative signals such as Hep or InR over-expression, and over-proliferate in stressed flies (GFP-labeled cells accumulate in malpighian tubules in these flies; Biteau, unpublished), but the exact mechanism of regeneration and a potential age-related dysplastic phenotype in this tissue remain unexplored. Importantly, a contribution of this somatic stem cell population to the lifespan effects reported here would further support our model of the importance of proliferative homeostasis in high-turnover tissues for Drosophila lifespan.
It is interesting that using the weaker 5961GS driver, lifespan extension in IIS and JNK loss-of-function conditions is only observed in females. This sexual dimorphism might be a consequence of a slight difference in driver activity between the sexes (no significant difference in driver activity can be observed, however), or might indicate selective sensitivity of females to intestinal dysplasia. Interestingly, intestinal turnover rates in females are higher than in males [50], indicating a potential reason for such a selective sensitivity. Accordingly, lifespan extension by esg-mediated expression of Hsp68 and Jafrac1 is also stronger in females than in males.
Based on the highly conserved regulation of regenerative processes in flies and vertebrates [13], [14], [25], [47]–[49], our findings suggest that interventions that focus on maintaining regenerative capacity by improving stem and progenitor cell stress-protection hold significant promise for slowing aging in higher organisms, including humans. Interestingly, vertebrates seem to have evolved more efficient and extensive cell autonomous anti-proliferative mechanisms in stem cells than flies [20]–[22], resulting in longer-lasting maintenance of homeostasis in high-turnover tissues. The rapid decay of intestinal homeostasis in flies indicates that such control mechanisms have not been acquired in these short-lived animals, yet our data also suggest the potential for active control of proliferation rates in the intestinal epithelium by systemic insulin-like peptide levels. Interestingly, the regulation of stem cell proliferation by IIS and Foxo is conserved in mammalian systems, suggesting that similar systemic control of stem cell proliferation could be harnessed to regulate regenerative capacity and lifespan in vertebrates [18], [31]. How the maintenance of intestinal homeostasis is influenced by environmental parameters that affect systemic IIS activity is an interesting subject of further studies.
Materials and Methods
Drosophila stocks and culture
The following strains were obtained from the Bloomington Drosophila Stock Center: w1118, ry506, y1w1, UAS-InRDN, UAS-Dp110DN, UAS-rpr, and tub-Gal80ts. UAS-AktRNAi and UAS-BskRNAi were obtained from the Vienna Drosophila RNAi Center (transformant ID 2902 and 34138). esg-Gal4 was kindly provided by S. Hayashi; chico1 and UAS-FoxoTM by M. Tatar; dilp2-Gal4 by E.Rulifson; UAS-Hep and sep-Gal4 by M. Mlodzik; UAS-NICD by N.Perrimon. MARCM stocks were gifts from N. Perrimon (hsFlp; tub-Gal4,UAS-GFP;FRT82B tubGal80) and B.Ohlstein (hsFlp; FRT40A tub-Gal80; tub-Gal4,UAS-GFP). 5961GS was a gift from B.Ohlstein. FRT chromosomes were kindly provided by D. Drummond-Barbosa (FRT40A chico1, FRT82B InRE19 and FRT82B InR353). The UAS-Foxo and UAS-Hsp68 were described previously [37], [74]. The UAS-Jafrac1 transgene was constructed by cloning the coding sequence of the jafrac1 gene, amplified from cDNA by using the following primers: 5′-ATGCCCCAGCTACAGAAGC-3′ and 5′-TTAGGAGGTGGTCTCGAAG-3′, into a pUAST vector. Transgenic flies were generated using standard procedures.
All flies were raised on the following food: 1 liter distilled water, 13.8 g agar, 22 g molasses, 80 g malt extract, 18 g Brewer's yeast, 80 g corn flour, 10 g soy flour, 6.25 mL propionic acid, 2 g methyl-p-benzoate, 7.2 mL of Nipagin (20% in EtOH). Flies were kept at 25°C and 65% humidity, on a 12 h light/dark cycle, unless otherwise indicated.
Conditional expression of UAS-linked transgenes
The TARGET system was used to conditionally express UAS-linked transgenes in ISCs [55]; the esg-Gal4 driver was combined with a ubiquitously expressed temperature-sensitive Gal80 inhibitor (esg-Gal4;tub-Gal80ts). Crosses and flies were kept at room temperature (permissive temperature), then shifted to 29°C to allow expression of the transgenes.
Generation of marked homozygous mutant clones
chico and InR mutant clones were generated by somatic recombination using the MARCM stocks described above and FRT40A and FRT82B chromosomes carrying chico1 and InR mutations, respectively. Using Flp/FRT-mediated somatic recombination with a repressible cell marker, MARCM allows generating homozygous mutant clones of cells that are positively marked (by GFP in this case). 2–4 days old flies were heat-shocked for 45 minutes at 37°C to induce somatic recombination. Clones resulting from mitotic recombination were observed 7 days after induction.
Immunostaining and microscopy
Intact guts were fixed at room temperature for 45 minutes in 100 mM glutamic acid, 25 mM KCl, 20 mM MgSO4, 4 mM Sodium Phosphate, 1 mM MgCl2, 4% formaldehyde. All subsequent incubations were done in PBS, 0.5% BSA, 0.1% TritonX-100 at 4°C.
The following primary antibodies were used: mouse anti-BrdU (Becton Dickson) 1∶200; mouse anti-Prospero and anti-Armadillo (Developmental Studies Hybridoma Bank) 1∶250 and 1∶100; rabbit anti-pH3 (Upstate) 1∶1000. Fluorescent secondary antibodies were obtained from Jackson Immunoresearch. Hoechst was used to stain DNA.
Confocal images were collected using a Leica SP5 confocal system and processed using the Leica software and Adobe Photoshop.
BrdU incorporation
Flies were cultured on standard food supplemented with BrdU (final concentration 0.2 mg/ml) for 2 days. Intact guts were fixed as previously described and DNA was denatured by incubating tissue in 3M HCl for 30 minutes. Samples were then processed for immunostaining as described above.
Metabolite measurements
4 to 5 females (without the head) were homogenized in 150 µl of buffer (10 mM KH2PO4, 1 mM EDTA, pH 7.4). 10 µl of cleared extract was used to measure triglycerides, glucose and glycogen concentrations according to the manufacturer instructions (Triglyceride Liquicolor, Stanbio; Glucose and Starch Assay Kits, Sigma).
Lifespan analysis
For lifespan experiments at 29°C using the TARGET system, virgin females (esgGal4, UAS-GFP; tubGal80ts) were crossed to the following UAS transgenes or the respective wild-type controls: UAS-Hepwt, UAS-BskRNAi, and UAS-AktRNAi (back-crossed at least 10 generations into w1118); UAS-Foxowt and UAS-Dp110DN (in y1,w1 background); and UAS-InR (in w1118 background). Crosses were kept at room temperature. After collection and sorting (60–100 flies/cage), flies were placed at 29°C to age.
The UAS-Jafrac1 and UAS-Hsp68 transgenes were backcrossed 10 times into the w1118 background and kept as an unbalanced stock. Of this stock, 10 to 15 homozygous males (+/+ or UAS/UAS) were independently crossed to 40 yw; esgGal,UASGFP/CyO virgins. To control for the effect of over-expressing Hsp68 in salivary glands, males (+/+ or UAS/UAS) from the same backcrossed UAS-Hsp68 stock were independently crossed to w; GMR-Gal4,UAS-GFP homozygous virgins. Crosses and progeny were kept at all times at 25°C.The progeny of these crosses was collected 2 days after hatching and allowed to mate in bottles for 3 days. Flies were finally separated according to their sex and genotype into cages (50–100 flies/cage).
To test the effect of Foxo over-expression using the esgGal4 driver on lifespan, the driver was backcrossed 10 times into the w1118 background and kept as an unbalanced stock. 40 w1118; esgGal4/+ virgins were crossed to 10–15 yw; UAS-Foxo homozygous males. Crosses were kept at 18°C to minimize driver expression and potential developmental defects associated with Foxo expression. The progeny of these crosses was collected 4 to 5 days after the first fly hatched. Flies were allowed to mate in bottles for 2 days at room temperature. Siblings were finally separated according to their sex and genotype into cages (20–50 flies/cage) and transferred at 25°C.
For RU486 food supplementation, 100 µl of a 5 mg/ml solution of RU486 or vehicle (ethanol 80%) were deposited on top of a food vial and dried for at least 16 hours to ensure complete evaporation, resulting in a 0.2 mg/ml concentration of RU486 in the food accessible to flies (determined using a dye control as previously described for drug treatments [92].
For all populations, plastic cages (175 ml volume, 5 cm diameter from Greiner bio-one) were used for lifespan experiments. Food, changed every 2 days, was provided in vials inserted into a foam plug (4.9 cm in diameter, 3 cm thick from Greiner bio-one), dead flies were visually identified (flies not moving, not responding to mechanical stimulation and laying on their side or back were deemed dead), and the number of dead flies was recorded. Cages were replaced after 20 days (flies were transferred into new cages without anesthesia). Survival of the different populations was analyzed using the SAS JMP7 statistical software.
The driver lines (esgGal4 and 5961) used to collect females for all lifespan studies are Wolbachia negative, the lifespan effects observed in flies with reduced JNK or IIS activity in somatic stem cells are thus Wolbachia independent.
Analysis of GFP expression by western blot
Analysis of esgGal4, 5961GS and GMR-Gal4 expression pattern by western blot 5 females (esgGal4>UAS-GFP, 5961GS>UAS-GFP, GMRGal4>UAS-GFP or OregonR) were dissected into heads, guts, salivary glands, ovaries and the carcasses of the thorax and abdomen. Tissues were homogenized in protein sample buffer; proteins were separated by SDS-PAGE and transferred to nitrocellulose membrane using standard procedures. GFP was detected using rabbit anti-GFP antibody (Invitrogen; 1∶5000), HRP-conjugated anti-rabbit and chemi-luminescence, according to manufacturer instructions. Total proteins, detected using Ponceau staining, or Heterochromatin Protein 1 (detected by immune-staining, anti-HP1, DSHB; 1∶5000) are used as loading controls.
Analysis of gene expression
Total RNA from 5 guts, from embryos, or from dissected 3rd instar larval eye imaginal discs, was extracted using Trizol and cDNA synthesized using Superscript II (Invitrogen). Real time PCR was performed using SYBR Green, a Biorad IQ5 apparatus and the following primers pairs (Delta: 5′-TGA GCA CTT TCT CCT CGC ACA TCT-3′and 5′-AGG CTT GTA CTG CAA CCA GGA TCT-3′; Rp49 : 5′-TCC TAC CAG CTT CAA GAT GAC-3′ and 5′-CAC GTT GTG CAC CAG GAA CT-3′; Akt: 5′-AAG CGT TTG GGA GGT GGA AAG GAT-3′ and 5′ - TCA ACT CCA CAC TCT CTC CCG TAA-3′; Actin: 5′-CTC GCC ACT TGC GTT TAC AGT-3′ and 5′ - TCC ATA TCG TCC CAG TTG GTC-3′; Jafrac1 : 5′-CAA GTT GAG CGA CTA CAA GG-3′ and 5′-TCA TCG AGC ACT CCA TAG TC-3′). Data was calculated using the ΔCt method and normalized to actin levels. Results are average +/ − standard deviation of at least 3 independent biological samples run in triplicate.
Supporting Information
Zdroje
1. SohalRS
WeindruchR
1996 Oxidative stress, caloric restriction, and aging. Science 273 59 63
2. GuarenteL
PicardF
2005 Calorie restriction—the SIR2 connection. Cell 120 473 482
3. KoubovaJ
GuarenteL
2003 How does calorie restriction work? Genes Dev 17 313 321
4. StadtmanER
2001 Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci 928 22 38
5. FinkelT
HolbrookNJ
2000 Oxidants, oxidative stress and the biology of ageing. Nature 408 239 247
6. TatarM
BartkeA
AntebiA
2003 The endocrine regulation of aging by insulin-like signals. Science 299 1346 1351
7. KenyonC
2005 The plasticity of aging: insights from long-lived mutants. Cell 120 449 460
8. RussellSJ
KahnCR
2007 Endocrine regulation of ageing. Nat Rev Mol Cell Biol 8 681 691
9. VijgJ
CampisiJ
2008 Puzzles, promises and a cure for ageing. Nature 454 1065 1071
10. PartridgeL
GemsD
2002 Mechanisms of ageing: public or private? Nat Rev Genet 3 165 175
11. GuarenteL
KenyonC
2000 Genetic pathways that regulate ageing in model organisms. Nature 408 255 262
12. KenyonC
2001 A conserved regulatory system for aging. Cell 105 165 168
13. RadtkeF
CleversH
2005 Self-renewal and cancer of the gut: two sides of a coin. Science 307 1904 1909
14. RadtkeF
CleversH
RiccioO
2006 From gut homeostasis to cancer. Curr Mol Med 6 275 289
15. Van ZantG
LiangY
2003 The role of stem cells in aging. Exp Hematol 31 659 672
16. RossiDJ
BryderD
SeitaJ
NussenzweigA
HoeijmakersJ
2007 Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447 725 729
17. NijnikA
WoodbineL
MarchettiC
DawsonS
LambeT
2007 DNA repair is limiting for haematopoietic stem cells during ageing. Nature 447 686 690
18. TothovaZ
KolliparaR
HuntlyBJ
LeeBH
CastrillonDH
2007 FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128 325 339
19. RossiDJ
BryderD
ZahnJM
AhleniusH
SonuR
2005 Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A 102 9194 9199
20. SharplessNE
DePinhoRA
2007 How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol 8 703 713
21. WeissmanIL
2000 Stem cells: units of development, units of regeneration, and units in evolution. Cell 100 157 168
22. ReyaT
MorrisonSJ
ClarkeMF
WeissmanIL
2001 Stem cells, cancer, and cancer stem cells. Nature 414 105 111
23. RandoTA
2006 Stem cells, ageing and the quest for immortality. Nature 441 1080 1086
24. RossiDJ
JamiesonCH
WeissmanIL
2008 Stems cells and the pathways to aging and cancer. Cell 132 681 696
25. CasaliA
BatlleE
2009 Intestinal stem cells in mammals and Drosophila. Cell Stem Cell 4 124 127
26. WagersAJ
ConboyIM
2005 Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell 122 659 667
27. RossiDJ
BryderD
WeissmanIL
2007 Hematopoietic stem cell aging: mechanism and consequence. Exp Gerontol 42 385 390
28. CampisiJ
SedivyJ
2009 How does proliferative homeostasis change with age? What causes it and how does it contribute to aging? J Gerontol A Biol Sci Med Sci 64 164 166
29. MiyamotoK
MiyamotoT
KatoR
YoshimuraA
MotoyamaN
2008 FoxO3a regulates hematopoietic homeostasis through a negative feedback pathway in conditions of stress or aging. Blood 112 4485 4493
30. TothovaZ
GillilandDG
2007 FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell 1 140 152
31. MayackSR
ShadrachJL
KimFS
WagersAJ
2010 Systemic signals regulate ageing and rejuvenation of blood stem cell niches. Nature 463 495 500
32. MiyamotoK
ArakiKY
NakaK
AraiF
TakuboK
2007 Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell 1 101 112
33. MurphyCT
McCarrollSA
BargmannCI
FraserA
KamathRS
2003 Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424 277 283
34. GreerEL
BrunetA
2005 FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 24 7410 7425
35. GershmanB
PuigO
HangL
PeitzschRM
TatarM
2007 High-resolution dynamics of the transcriptional response to nutrition in Drosophila: a key role for dFOXO. Physiol Genomics 29 24 34
36. JungerMA
RintelenF
StockerH
WassermanJD
VeghM
2003 The Drosophila Forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J Biol 2 20
37. WangMC
BohmannD
JasperH
2005 JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 121 115 125
38. BoyleM
WongC
RochaM
JonesDL
2007 Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell 1 470 478
39. PanL
ChenS
WengC
CallG
ZhuD
2007 Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell 1 458 469
40. WallenfangMR
NayakR
DiNardoS
2006 Dynamics of the male germline stem cell population during aging of Drosophila melanogaster. Aging Cell 5 297 304
41. ChengJ
TurkelN
HematiN
FullerMT
HuntAJ
2008 Centrosome misorientation reduces stem cell division during ageing. Nature 456 599 604
42. ChoiNH
KimJG
YangDJ
KimYS
YooMA
2008 Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell 7 318 334
43. BiteauB
HochmuthCE
JasperH
2008 JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell 3 442 455
44. SinghSR
LiuW
HouSX
2007 The adult Drosophila Malpighian Tubules are maintained by multipotent stem cells. Cell Stem Cell 1 191 203
45. TakashimaS
MkrtchyanM
Younossi-HartensteinA
MerriamJR
HartensteinV
2008 The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature 454 651 655
46. FoxDT
SpradlingAC
2009 The Drosophila hindgut lacks constitutively active adult stem cells but proliferates in response to tissue damage. Cell Stem Cell 5 290 297
47. MicchelliCA
PerrimonN
2006 Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439 475 479
48. OhlsteinB
SpradlingA
2006 The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439 470 474
49. OhlsteinB
SpradlingA
2007 Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science 315 988 992
50. JiangH
PatelPH
KohlmaierA
GrenleyMO
McEwenDG
2009 Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137 1343 1355
51. CroninSJ
NehmeNT
LimmerS
LiegeoisS
PospisilikJA
2009 Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science 325 340 343
52. BuchonN
BroderickNA
PoidevinM
PradervandS
LemaitreB
2009 Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe 5 200 211
53. AmcheslavskyA
JiangJ
IpYT
2009 Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell 4 49 61
54. BuchonN
BroderickNA
ChakrabartiS
LemaitreB
2009 Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev 23 2333 2344
55. McGuireSE
LePT
OsbornAJ
MatsumotoK
DavisRL
2003 Spatiotemporal rescue of memory dysfunction in Drosophila. Science 302 1765 1768
56. TatarM
KopelmanA
EpsteinD
TuMP
YinCM
2001 A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292 107 110
57. ClancyDJ
GemsD
HarshmanLG
OldhamS
StockerH
2001 Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 292 104 106
58. WessellsRJ
FitzgeraldE
CypserJR
TatarM
BodmerR
2004 Insulin regulation of heart function in aging fruit flies. Nat Genet 36 1275 1281
59. BroughtonSJ
PiperMD
IkeyaT
BassTM
JacobsonJ
2005 Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci U S A 102 3105 3110
60. BohniR
Riesgo-EscovarJ
OldhamS
BrogioloW
StockerH
1999 Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell 97 865 875
61. BrogioloW
StockerH
IkeyaT
RintelenF
FernandezR
2001 An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol 11 213 221
62. IkeyaT
GalicM
BelawatP
NairzK
HafenE
2002 Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol 12 1293 1300
63. OkamotoN
YamanakaN
YagiY
NishidaY
KataokaH
2009 A fat body-derived IGF-like peptide regulates postfeeding growth in Drosophila. Dev Cell 17 885 891
64. SlaidinaM
DelanoueR
GronkeS
PartridgeL
LeopoldP
2009 A Drosophila insulin-like peptide promotes growth during nonfeeding states. Dev Cell 17 874 884
65. GiannakouME
GossM
JungerMA
HafenE
LeeversSJ
2004 Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science 305 361
66. LibinaN
BermanJR
KenyonC
2003 Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 115 489 502
67. HwangboDS
GershamB
TuMP
PalmerM
TatarM
2004 Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 429 562 566
68. BluherM
KahnBB
KahnCR
2003 Extended longevity in mice lacking the insulin receptor in adipose tissue. Science 299 572 574
69. WuQ
ZhangY
XuJ
ShenP
2005 Regulation of hunger-driven behaviors by neural ribosomal S6 kinase in Drosophila. Proc Natl Acad Sci U S A 102 13289 13294
70. LeeversSJ
WeinkoveD
MacDougallLK
HafenE
WaterfieldMD
1996 The Drosophila phosphoinositide 3-kinase Dp110 promotes cell growth. Embo J 15 6584 6594
71. LeeT
LuoL
2001 Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci 24 251 254
72. WuJS
LuoL
2006 A protocol for mosaic analysis with a repressible cell marker (MARCM) in Drosophila. Nat Protoc 1 2583 2589
73. MathurD
BostA
DriverI
OhlsteinB
2010 A transient niche regulates the specification of Drosophila intestinal stem cells. Science 327 210 213
74. WangMC
BohmannD
JasperH
2003 JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev Cell 5 811 816
75. LeeKS
Iijima-AndoK
IijimaK
LeeWJ
LeeJH
2009 JNK/FOXO-mediated neuronal expression of fly homologue of peroxiredoxin II reduces oxidative stress and extends lifespan. J Biol Chem 284 29454 29461
76. RadyukSN
KlichkoVI
SpinolaB
SohalRS
OrrWC
2001 The peroxiredoxin gene family in Drosophila melanogaster. Free Radic Biol Med 31 1090 1100
77. PaikJH
KolliparaR
ChuG
JiH
XiaoY
2007 FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128 309 323
78. Pinkston-GosseJ
KenyonC
2007 DAF-16/FOXO targets genes that regulate tumor growth in Caenorhabditis elegans. Nat Genet 39 1403 1409
79. PinkstonJM
GariganD
HansenM
KenyonC
2006 Mutations that increase the life span of C. elegans inhibit tumor growth. Science 313 971 975
80. ClancyDJ
GemsD
HafenE
LeeversSJ
PartridgeL
2002 Dietary restriction in long-lived dwarf flies. Science 296 319
81. KatewaSD
KapahiP
Dietary restriction and aging, 2009. Aging Cell 9 105 112
82. ZidBM
RogersAN
KatewaSD
VargasMA
KolipinskiMC
2009 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell 139 149 160
83. KarpacJ
Hull-ThompsonJ
FalleurM
JasperH
2009 JNK signaling in insulin-producing cells is required for adaptive responses to stress in Drosophila. Aging Cell 8 288 295
84. KarpacJ
JasperH
2009 Insulin and JNK: optimizing metabolic homeostasis and lifespan. Trends Endocrinol Metab 20 100 106
85. ManningAM
DavisRJ
2003 Targeting JNK for therapeutic benefit: from junk to gold? Nat Rev Drug Discov 2 554 565
86. KarinM
GallagherE
2005 From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life 57 283 295
87. LiuJ
LinA
2005 Role of JNK activation in apoptosis: a double-edged sword. Cell Res 15 36 42
88. HaEM
LeeKA
SeoYY
KimSH
LimJH
2009 Coordination of multiple dual oxidase-regulatory pathways in responses to commensal and infectious microbes in drosophila gut. Nat Immunol 10 949 957
89. HaEM
LeeKA
ParkSH
KimSH
NamHJ
2009 Regulation of DUOX by the Galphaq-phospholipase Cbeta-Ca2+ pathway in Drosophila gut immunity. Dev Cell 16 386 397
90. RyuJH
HaEM
OhCT
SeolJH
BreyPT
2006 An essential complementary role of NF-kappaB pathway to microbicidal oxidants in Drosophila gut immunity. EMBO J 25 3693 3701
91. HaEM
OhCT
BaeYS
LeeWJ
2005 A direct role for dual oxidase in Drosophila gut immunity. Science 310 847 850
92. GroverD
FordD
BrownC
HoeN
ErdemA
2009 Hydrogen peroxide stimulates activity and alters behavior in Drosophila melanogaster. PLoS One 4 e7580 doi:10.1371/journal.pone.0007580
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 10- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- FSHD: A Repeat Contraction Disease Finally Ready to Expand (Our Understanding of Its Pathogenesis)
- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- The Meiotic Recombination Checkpoint Suppresses NHK-1 Kinase to Prevent Reorganisation of the Oocyte Nucleus in
- Actin Depolymerizing Factors Cofilin1 and Destrin Are Required for Ureteric Bud Branching Morphogenesis
- DSIF and RNA Polymerase II CTD Phosphorylation Coordinate the Recruitment of Rpd3S to Actively Transcribed Genes
- Continuous Requirement for the Clr4 Complex But Not RNAi for Centromeric Heterochromatin Assembly in Fission Yeast Harboring a Disrupted RITS Complex
- Genome-Wide Association Study of Blood Pressure Extremes Identifies Variant near Associated with Hypertension
- The Cytosine Methyltransferase DRM2 Requires Intact UBA Domains and a Catalytically Mutated Paralog DRM3 during RNA–Directed DNA Methylation in
- β-Actin and γ-Actin Are Each Dispensable for Auditory Hair Cell Development But Required for Stereocilia Maintenance
- Genetic Association Study Identifies as a Risk Gene for Idiopathic Dilated Cardiomyopathy
- Evidence for a Xer/ System for Chromosome Resolution in Archaea
- Four Novel Loci (19q13, 6q24, 12q24, and 5q14) Influence the Microcirculation
- Lifespan Extension by Preserving Proliferative Homeostasis in
- Ancient and Recent Adaptive Evolution of Primate Non-Homologous End Joining Genes
- Loss of the p53/p63 Regulated Desmosomal Protein Perp Promotes Tumorigenesis
- Altering a Histone H3K4 Methylation Pathway in Glomerular Podocytes Promotes a Chronic Disease Phenotype
- Characterization of LINE-1 Ribonucleoprotein Particles
- Conserved Genes Act as Modifiers of Invertebrate SMN Loss of Function Defects
- Alternative Splicing at a NAGNAG Acceptor Site as a Novel Phenotype Modifier
- Tight Regulation of the Gene of the KplE1 Prophage: A New Paradigm for Integrase Gene Regulation
- Conjugative DNA Transfer Induces the Bacterial SOS Response and Promotes Antibiotic Resistance Development through Integron Activation
- Nasty Viruses, Costly Plasmids, Population Dynamics, and the Conditions for Establishing and Maintaining CRISPR-Mediated Adaptive Immunity in Bacteria
- Stress-Induced Activation of Heterochromatic Transcription
- H3K27me3 Profiling of the Endosperm Implies Exclusion of Polycomb Group Protein Targeting by DNA Methylation
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
- Characterising and Predicting Haploinsufficiency in the Human Genome
- Dual Functions of ASCIZ in the DNA Base Damage Response and Pulmonary Organogenesis
- Pervasive Cryptic Epistasis in Molecular Evolution
- Transition from Positive to Neutral in Mutation Fixation along with Continuing Rising Fitness in Thermal Adaptive Evolution
- Comprehensive Analysis Reveals Dynamic and Evolutionary Plasticity of Rab GTPases and Membrane Traffic in
- Regulates Tissue-Specific Mitochondrial DNA Segregation
- Role for the Mammalian Swi5-Sfr1 Complex in DNA Strand Break Repair through Homologous Recombination
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



