-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Aspiration pneumonia after cerebrovascular stroke: a comparison between patients with and without dysphagia
Aspirační pneumonie po cévní mozkové příhodě: srovnání pacientů s dysfagií a bez ní
Úvod: Pneumonie se objevuje u 11 až 21 procent pacientů s anamnézou akutní cévní mozkové příhody (CMP) a zhoršují jejich celkový stav. Hlavní příčinou pneumonie je aspirace a existuje souvislost mezi dysfagií, která se vyskytuje u patologických stavů, a poruchami vědomí. Cílem této práce bylo zhodnotit a porovnat míru prevalence aspirační pneumonie u pacientů s mrtvicí, kteří trpěli dysfagií, s těmi, kteří dysfagii neměli.
Metoda: Retrospektivně jsme určili pacienty, kteří prodělali CMP od 1. března 2018 do 31. prosince 2021 pomocí dat z jejich dokumentace. Následně jsme srovnali skupinu s dysfagií (pacienti, u nichž se po CMP objevila dysfagie) se skupinou bez dysfagie (pacienti, kteří dysfagií nikdy netrpěli). Pacienti byli zhodnoceni s ohledem na charakteristiky studie a statistická analýza byla provedena za použití softwaru SPSS.
Výsledky: Do studie bylo zařazeno celkem 380 pacientů s CMP, včetně 150 s dysfagií a 230 bez dysfagie. Prevalence dysfagie byla signifikantně vyšší u pacientů s krvácením do mozku v porovnání se skupinou s ischemickou cévní mozkovou příhodou. Z 29 pacientů s hemoragickou cévní mozkovou příhodou se dysfagie neobjevila pouze u 5, což se signifikantně lišilo od odpovídajícího počtu ve skupině s ischemickou cévní mozkovou příhodou (225 z 351 pacientů). Prevalence pneumonie byla signifikantně vyšší mezi pacienty, u nichž se objevila dysfagie (50,6 % oproti 6,5 %).
Závěr: Naše studie svědčí o tom, že dysfagie má při vzniku aspirační pneumonie významnou roli. Zároveň jsme pozorovali signifikantně vyšší výskyt dysfagie u pacientů s krvácením do mozku.
Klíčová slova:
cévní mozková příhoda – dysfagie – aspirační pneumonie
Authors: N. A. Moulaei 1,2,3; N. Ramroodi 1,2; A. S. M. N. Tabatabaie 1,2; H. A. Danesh 1,2; H. A. Khazaie 1,2
Authors place of work: Zahedan University of Medical Sciences, Zahedan, Iran 1; Clinical Immunology Research Center, Zahedan University of Medical Sciences, Zahedan, Iran 2; Infectious Diseases and Tropical Medicine Research Center, Zahedan University of Medical Sciences, Zahedan, Iran 3
Published in the journal: Anest. intenziv. Med., 33, 2022, č. 3-4, s. 148-152
Category: Původní práce
doi: https://doi.org/10.36290/aim.2022.023Summary
Introduction: Pneumonia develops in 11%-21% of patients with a history of acute stroke and weakens their system conditions. The main cause of pneumonia is aspiration, and there is a relationship between dysphagia, which occurs in pathological conditions, and disorders of consciousness. This research aims to evaluate and compare the prevalence rate of aspiration pneumonia in stroke patients with and without dysphagia.
Methods: We retrospectively identified patients who had experienced a stroke from March 1, 2018, to December 31, 2021 through their documented data. Then, we compared the dysphagia group (patients who developed dysphagia after stroke) with the non‑dysphagia group (patients who had never experienced dysphagia). The patients were evaluated regarding the study characteristics and the statistical analysis was done using the SPSS software.
Results: A total number of 380 stroke patients including 150 dysphagia and 230 non‑dysphagia patients were included in the present study. The prevalence of dysphagia was significantly higher among cerebral hemorrhage patients compared to the ischemic stroke group. Out of 29 patients with hemorrhagic stroke, only 5 did not develop dysphagia, which was significantly different from the corresponding numbers among the ischemic stroke group (225 out of 351 patients). The prevalence of pneumonia was significantly higher among the patients who developed dysphagia (50.6% versus 6.5%).
Conclusion: Our study suggests the substantial role of dysphagia in developing aspiration pneumonia. On the other hand, we observed a significantly higher rate of dysphagia among patients with cerebral hemorrhage.
Keywords:
dysphagia – aspiration pneumonia – cerebrovascular stroke
Introduction
Pneumonia develops in 11%–21% of patients with a history of acute stroke and weakens their general conditions [1, 2]. The main cause of pneumonia is aspiration [3], and there is a relationship between dysphagia, which occurs in pathological conditions, and disorders of consciousness. Dysphagia is observed during the critical period after stroke in 45% to 65% of the patients, and it can lead to an abnormality in absorption of food nutrients, malnutrition, and aspiration pneumonia after the stroke [4,5]. Dysphagia is considered the major risk factor for the development of pneumonia. Patients with dysphagia are more than three times at risk of developing pneumonia (RR: 3.17), and aspiration adds to this risk (RR: 11.56) [6]. Moreover, sensory nerve damage to the throat, which is common after stroke, is associated with an increased risk of aspiration pneumonia [7]. Pneumonia is the third leading cause of death in the first month after a stroke [8]. Aspiration pneumonia can happen 1 to 2 weeks after the stroke attack [9]. In 70% of all cases of stroke‑related pneumonia (SAP) [10], early‑onset pneumonia (EOP) occurs within 72 hours after admission. It has been reported that in 18.7% of such cases, the probability of dysphagia during stroke is 30–70% [11–13]. A GCS of less than 9 was reported. At this point, since dysphagia may occur, aspiration pneumonia should be prevented. Although measures such as oral care [15] and patient positioning [16] are taken, they are not completely effective to prevent the condition. Patients with disorders of consciousness suffer from voluntary swallowing. Even in a unilateral supranuclear lesion, the returning of food due to dysphagia may temporarily decrease. This causes permanent discharges in the (pear‑shaped) pyriform sinus around the laryngeal opening. As for the relationship between pharyngeal secretions and aspiration in patients with dysphagia, it has been reported that silent aspiration can occur when secretions in the pyriform sinuses (pear‑shaped) increase and enter the trachea chronically [17]. An increase in secretions, which is associated with silent aspiration, causes aspiration pneumonia. Passive suctioning is necessary in order to remove these secretions to reduce the risk of this pneumonia. Some reports on the development of low‑pressure sinus suction devices for the pyriform sinuses have been published [18, 19]. This research aims to evaluate and compare the prevalence rate of aspiration pneumonia in stroke patients with and without dysphagia.
Methods
Participants (patients in research)
We retrospectively identified primary patients who had experienced a stroke from March 1, 2018, to December 31, 2021 through their documented data. Then, we compared the dysphagia group (patients who developed dysphagia after stroke) with the non‑dysphagia group (patients who had never experienced dysphagia). Dysphagia was defined as difficulty swallowing occurring in the mouth or throat which is referred to as oropharyngeal dysphagia or upper dysphagia, as well. The diagnostic criteria were considered according to Martino et al’s study, consisting of lung inflammation in addition to apparent aspiration, high level of suspicion by the physician for the presence of aspiration, and dysphagia. They also argued that, due to the fact that the absolute diagnosis of apparent aspiration is difficult, parameters such as elevated peripheral white blood cell count (> 10,000/μL) and infiltrative shadows on chest radiography should be considered as the basis for identifying aspiration pneumonia [37]. Selection of the treatment method was based on the patient’s preference and the physician’s experience. Our treatment options included head flexion, head rotation, head tilt, bolus viscosity, texture, and volume modifications, supraglottic swallow, super ‑ supraglottic swallow, effortful swallow, Mendelsohn maneuver, and some specific exercises. Regarding the nutrition type, mild dysphagia patients received a dysphagia diet and those with severe dysphagia received nutrients by tube. Out of all patients, 194 used mechanical ventilation and the rest did not require it.
The groups (1 : 1) were matched based on age, gender, other auxiliary variables, inclination score, and index year. The date of the stroke diagnosis for each patient was defined as an index. Individuals under 20 and over 99 years old or the cases whose follow‑up date was incomplete were excluded.
Diagnosis of aspiration pneumonia
The main contribution of this research was the diagnosis of aspiration pneumonia (inflammation of lung tissue induced by oropharyngeal contents). Patients were followed up from the index date to the onset of pneumonia aspiration, death, cancellation of National Health Insurance registration, or the end of the research date (December 31, 2021) or whichever was earlier.
Residues in the depressions and pyriform sinuses were defined as a clear barium envelope in the throat after deglutition and were classified into 4 levels: level 1 (never); level 2 (lowest) < 10% of the mass; level 3 (average) from 10% to 50% of the mass; and level 4 (highest) 50% of the mass [20].
The longest time for time parameters was selected of three concentrated liquid deglutition. The depression and pyriform sinus were measured based on the maximum amount of food remaining.
Statistical analysis
Tests such as Pearson’s chi‑squared or Fisher’s exact test were performed to assess the differences in contract data between patients in the dysphagia and non‑dysphagia groups. In addition, an independent samples t‑test was performed in the pneumonia and non‑pneumonia groups to assess differences between the two groups in terms of continuous data and Cox’s relative risk regression analysis to assess mortality. We conducted a comparison in terms of aspiration pneumonia risk over a 2-year period from the index date in the dysphagia and non‑dysphagia groups. In the subgroup analysis, we divided subtypes of stroke into cerebral hemorrhage (ICH) and other types to compare the relationship between the subtype and dysphagia during the follow‑up period.
Demographic information, comorbidity, risk factors, and residues were obtained. We used the Kaplan-Meier estimator and the log-rank test, respectively, to estimate the probability of aspiration pneumonia and mortality and to evaluate the differences between the dysphagia and non-dysphagia groups. All statistical operations were done using the SAS 9.3 statistical package: all P values were two-sided, and P values less than < 0.001 were considered statistically very significant.
Results
A total number of 380 stroke patients including 150 dysphagia and 230 non‑dysphagia patients were included in the present study. The mean age of patients who developed dysphagia (56.3 ± 8.3) was higher than that in the non‑dysphagia group (55.4 ± 4.7). However, this difference was not significant. Most of our patients, specifically those with dysphagia, were hospitalized in the ICU, while the remaining patients received treatment in the neurology ward. The frequency distribution of genders was not significantly different between the two groups, as well. The prevalence of dysphagia was significantly higher among the cerebral hemorrhage patients than in the ischemic stroke group. Out of 29 patients with hemorrhagic stroke, only 5 did not develop dysphagia, which was significantly different from the corresponding numbers in the ischemic stroke group (225 out of 351 patients). The prevalence of pneumonia was significantly higher among the patients who developed dysphagia (50.6% versus 6.5%) (Table 1).
Tab. 1. General characteristics of patients with and without dysphagia 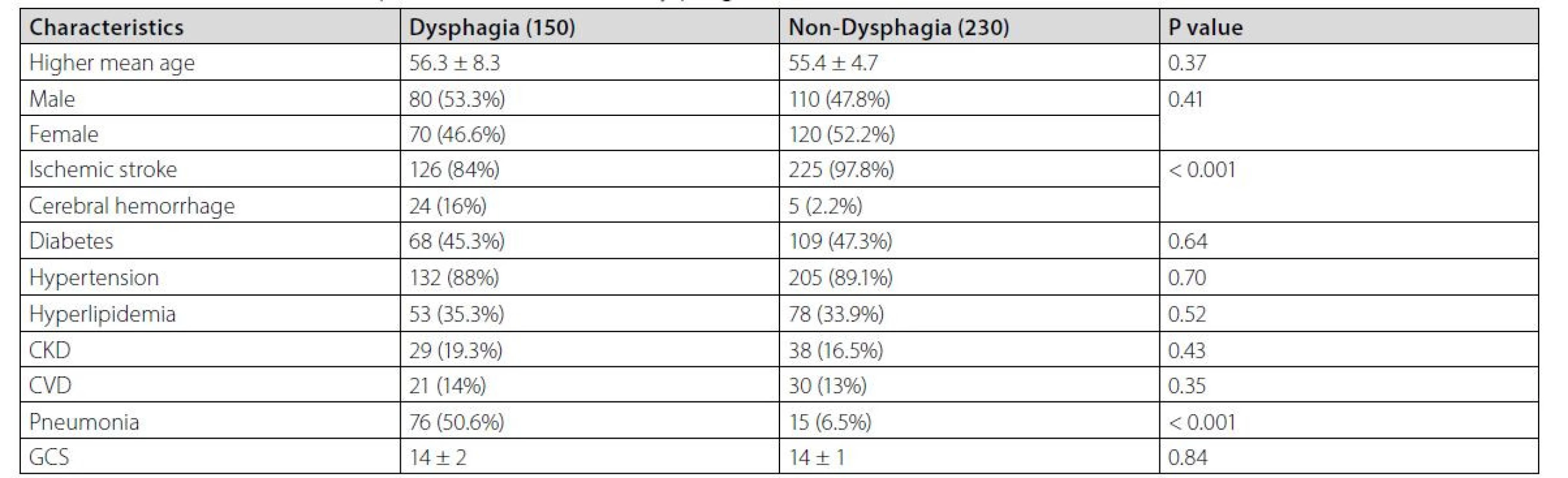
When comparing the patients who developed pneumonia with those who did not, we found a significantly higher mean age among those in the pneumonia group. The vallecular and pyriform sinus residues were also significantly higher in the pneumonia group. However, smoking and positive medical history of chronic diseases such as diabetes and hypertension did not make a significant difference in the prevalence of pneumonia (Table 2).
Tab. 2. General characteristics of patients with and without pneumonia 
CPMD = cricopharyngeal muscle dysfunction; CVA = cerebrovascular accident; FOIS = functional oral intake scale; GERD = gastroesophageal
reflux disease; HL_comp = hyolaryngeal complex movement; HNC = head and neck cancer; HPtt = hypopharyngeal transit time (sec); IQR = interquartile range; PAS = Penetration Aspiration Scale; PCR = pharyngeal constriction ratio; PES-AP = pharyngoesophageal segment in the anterior–posterior view (cm); PES-L = Pharyngoesophageal segment in the lateral view (cm); SD, standard deviationOur results showed that most cases of aspiration pneumonia occurred on days 8, 7, and 6 after hospitalization, and we observed no cases of aspiration pneumonia on the first day (Table 3).
Tab. 3. The prevalence of aspiration pneumonia based on hospitalization 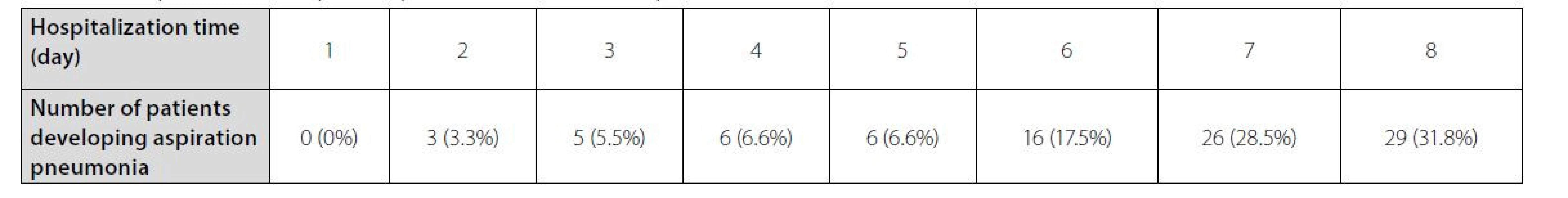
Discussion
Although many patients with dysphagia recover within a week after a stroke, approximately 50% of patients with dysphagia have difficulty swallowing. Dysphagia has a high prevalence after stroke, ranging from approximately 30% to 65% [21, 22]. The risk of developing aspiration pneumonia, disability, malnutrition, and death increases in patients with dysphagia. Post‑stroke pneumonia is a common malignant infection that affects one‑third of stroke patients, especially when it co‑occurs with dysphagia [23, 24]. The risk of pneumonia is higher in patients with dysphagia. In addition, patients with dysphagia are more than three times at risk of developing pneumonia after stroke, and the risk increases 11-fold in those with confirmed aspiration [25]. Our findings are consistent with the previous research concerning the very high prevalence of aspiration pneumonia in patients with dysphagia after stroke (50.6% to 6.5%). According to a report, the incidence of dysphagia ‑ related aspiration pneumonia was significantly higher in the first year in patients who experienced a hemorrhagic attack compared to those with an ischemic attack in the general population. Thus, in this group, there is a small amount of dysphagia with its side effects, that is, aspiration pneumonia [26]. On the other hand, our study showed that out of 29 cerebral hemorrhage cases only 5 did not develop dysphagia, while the corresponding rate for ischemic stroke patients was 225 out of 351. We also examined some of the influencing factors for pneumonia development in patients with stroke ‑ induced dysphagia. Patients who developed pneumonia had a significantly higher mean age, which was in line with the study conducted by Feng et al. who reported a significant association between age and the risk of pneumonia development [27]. In addition, Perry et al. in their study indicated that advanced age could significantly predict mortality [28]. Although the older age in our research was associated with an increased risk for pneumonia, previous research showed conflicting results [29–31]. One possibility is that the elderly are more sensitive to the spread of pneumonia due to a lower functional status, age ‑ related changes in respiratory function, age ‑ related swallowing changes, and decreased respiratory clearance [32, 33]. In our research, there was no significant relationship between the presence of tracheostomy and the spread of pneumonia. However, Feng and colleagues reported a significant relationship between these two factors [27]. The amounts of vallecular residue and pyriform sinus residue were significantly higher among patients who developed pneumonia, which was in line with the results of Ko et al. [34]. Larger amounts of food left in the throat for a long time may increase the likelihood of lung aspiration [35]. On the other hand, our study was in line with that by Xu et al. who indicated a significant relationship between developing pneumonia and the length of hospital stay [36]. It appears that in people with stroke and, as a result, dysphagia experience, there is a strong positive relationship between malnutrition and aspiration pneumonia in prognosis and mortality. Recent evidence suggests that rehabilitating dysphagia in a successful way and early prevention may reduce both malnutrition and pneumonia in stroke patients with dysphagia. As an example, patients who receive a dysphagia rehabilitation program based on strenuous exercise are less likely to develop malnutrition and aspiration pneumonia than those who receive dietary and compensatory changes or those who are untested [19]. This research has some limitations. This is a review study based on data from medical records and patient reports (in which recall bias may occur). Moreover, we recognize that the definitions related to pneumonia may vary in clinical reports and may be integrated with other respiratory infections. It is also possible that a follow‑up period of more than 2 years will lead to disagreements in results, especially in patients with a medical history of degenerative conditions.
Conclusion
Our study suggests the substantial role of dysphagia in developing aspiration pneumonia. On the other hand, we observed a significantly higher rate of dysphagia among patients with cerebral hemorrhage.
Zdroje
1. Hilker R, Poetter C, Findeisen N, Sobesky J, Jacobs A, Neveling M, Heiss WD. Nosocomial pneumonia after acute stroke: implications for neurosurgical intensive care medicine. Stroke. 2003;34(4):975-81.
2. Brogan E, Langdon C, Brookes K, Budgeon C, Blacker D. Dysphagia and factors associated with respiratory infections in the first week post stroke. Neuroepidemiology. 2014;43(2):140-4. 3.
3. Teramoto S. Novel preventive and therapeutic strategy for poststroke pneumonia. Expert Rev Neurother. 2009;9(8):1187-200.
4. Khazaei HA, Khazaei B, Dashtizadeh GA, Mohammadi M. Cigarette smoking and skin prick test in patients with allergic rhinitis. International Journal of High Risk Behaviors & Addiction. 2015 Sep;4(3).
5. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 2005; 36(12): 2756-63.
6. Kidd D, Lawson J, Nesbitt R, MacMahon J. Aspiration in acute stroke: a clinical study with videofluoroscopy. Q J Med 3371993;86(12):825-9.
7. Bokaeian M, Khazaei HA, Javadimehr M. Nasopharyngeal carriage, antibiotic resistance and serotype distribution of Streptococcus pneumoniae among healthy adolescents in Zahedan. Iranian Red Crescent Medical Journal. 2011 May;13(5):328.
8. Altman KW, Yu GP, Schaefer SD. Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg 2010; 136(8):784-9.
9. Aslanyan S, Weir CJ, Diener HC, Kaste M, Lees KR. Pneumonia and urinary tract infection after acute ischaemic stroke: a tertiary analysis of the GAIN International trial. Eur J Neurol. 2004;11(1):49-53.
10. Dziewas R, Ritter M, Schilling M, Konrad C, Oelenberg S, Nabavi DG, Stögbauer F, Ringelstein EB, Lu¨demann P. Pneumonia in acute stroke patients fed by nasogastric tube. J Neurol Neurosurg Psychiatry. 2004;75(6):852-6.
11. Smithard DG, O’Neill PA, England RE, Park CL, Wyatt R, Martin DF, Morris J. The natural history of dysphagia following a stroke. Dysphagia. 1997;12(4):188–93.
12. Mann G, Hankey GJ, Cameron D. Swallowing function after stroke: prognosis and prognostic factor at 6 months. Stroke. 1999;30(4):744-8.
13. Gordon C, Hewer RL, Wade TD. Dysphagia in acute stroke. Br Med J. 1987;295(6595):411–4.
14. Paciaroni M, Mazzotta G, Corea F, Caso V, Venti M, Milia P, Silvestrelli G, Palmerini F, Parnetti L, Gallai V. Dysphagia following stroke. Eur Neurol. 2004;51(3):162-7.
15. Lawrence ES, Coshall C, Dundas R, Stewart J, Rudd AG, Howard R, Wolfe CD. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke. 2001;32(6):1279-84.
16. Sørensen RT, Rasmussen RS, Overgaard K, Lerche A, Johansen AM. Lindhardt. Dysphagia screening and intensified oral hygiene reduce pneumonia after stroke. J Neurosci Nurs. 2013;45(3):139-46.
17. Palazzo P, Brooks A, James D, Moore R, Alexandrov AV, Alexandrov AW. Risk of pneumonia associated with zero‑degree head positioning in acute ischemic stroke patients treated with intravenous tissue plasminogen activator. Brain Behav. 2016;6(2):e00425.
18. Eisenhuber E, Schima W, Schober E, Pokieser P, Stadler A, Scharitzer M, Oschatz E. Videofluoroscopic assessment of patients with dysphagia: pharyngeal retention is a predictive factor for aspiration. AJR Am J Roentgenol. 2002;178(2):393-8.
19. Belafsky PC, Mehdizadeh OB, Ledgerwood L, Kuhn M. Evaluation of hypopharyngeal suction to eliminate aspiration: the Retro‑Esophageal Suction (REScue) catheter. Dysphagia. 2015;30(1):74-9.
20. Han TR, Paik NJ, Park JW. Quantifying swallowing function after stroke: a functional dysphagia scale based on videofluoroscopic studies. Arch Phys Med Rehabil. 2001;82 : 677-82.
21. Paciaroni M, Mazzotta G, Corea F, et al. Dysphagia following stroke. Eur Neurol 2004;51 : 162-167.
22. Mann G, Hankey GJ, Cameron D. Swallowing disorders following acute stroke: prevalence and diagnostic accuracy. Cerebrovasc Dis 2000;10 : 380-386.
23. Masiero S, Pierobon R, Previato C, et al. Pneumonia in stroke patients with oropharyngeal dysphagia: a six‑month follow‑up study. Neurol Sci 2008;29 : 139-145.
24. Sellars C, Bowie L, Bagg J, et al. Risk factors for chest infection in acute stroke: a prospective cohort study. Stroke 2007;38 : 2284-2291.
25. Martino R, Foley N, Bhogal S, et al. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 2005;36 : 2756-2763.
26. Koton S, Schneider L, Rosamond WD, et al. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA 2014;312 : 259-268.
27. Feng MC, Lin YC, Chang YH, Chen CH, Chiang HC, Huang LC, Yang YH, Hung CH. The mortality and the risk of aspiration pneumonia related with dysphagia in stroke patients. Journal of Stroke and Cerebrovascular Diseases. 2019 May 1;28(5):1381-7.
28. Perry SE, Miles A, Fink JN, Huckabee ML. The dysphagia in stroke protocol reduces aspiration pneumonia in patients with dysphagia following acute stroke: a clinical audit. Translational stroke research. 2019 Feb;10(1):36-43.
29. Bock JM, Varadarajan V, Brawley MC, Blumin JH. Evaluation of the natural history of patients who aspirate. Laryngoscope 2017;127:S1-s10.
30. Langmore SE, Terpenning MS, Schork A, et al. Predictors of aspiration pneumonia: how important is dysphagia? Dysphagia 1998;13 : 69-81.
31. Manabe T, Teramoto S, Tamiya N, Okochi J, Hizawa N. Risk factors for aspiration pneumonia in older adults. PLoS One 2015;10:e0140060.
32. Lowery EM, Brubaker AL, Kuhlmann E, Kovacs EJ. The aging lung. Clin Interv Aging 2013;8 : 1489-1496.
33. Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging 2006;1 : 253-260.
34. Ko JY, Shin DY, Kim TU, Kim SY, Hyun JK, Lee SJ. Predictors of Aspiration Pneumonia in the Elderly With Swallowing Dysfunction: Videofluoroscopic Swallowing Study. Annals of Rehabilitation Medicine. 2021 Apr;45(2):99.
35. Logemann JA. Evaluation and treatment of swallowing disorders. 2nd ed. Austin, TX: Pro‑ED; 1998
36. Xu Z, Gu Y, Li J, Wang C, Wang R, Huang Y, Zhang J. Dysphagia and aspiration pneumonia in elderly hospitalization stroke patients: risk factors, cerebral infarction area comparison. Journal of Back and Musculoskeletal Rehabilitation. 2019 Jan 1;32(1):85-91.
37. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, an
Štítky
Anesteziologie a resuscitace Intenzivní medicína
Článek vyšel v časopiseAnesteziologie a intenzivní medicína
Nejčtenější tento týden
2022 Číslo 3-4- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Neodolpasse je bezpečný přípravek v krátkodobé léčbě bolesti
- Metamizol v léčbě různých bolestivých stavů – kazuistiky
- Léčba akutní pooperační bolesti z pohledu ortopeda
-
Všechny články tohoto čísla
- Sdělení redakční rady
- Covid (nejen) z pohledu intenzivní medicíny
- Je etické použití ECMO za účelem „udržení“ dárce orgánů?
- The effect of two different anesthesia regimens on annexin V levels and early postoperative complications in CABG
- Evaluation of the Utility of the Advance Care Planning and Decision‑Making Supportive Tool
- Infekční komplikace po etomidátu versus propofolu v souvislosti s indukcí do celkové anestezie v kardiochirurgii
- Aspiration pneumonia after cerebrovascular stroke: a comparison between patients with and without dysphagia
- Monitoring of processed EEG under anesthesia II
- Specificity léčby bolesti u kriticky nemocných dospělých pacientů s BMI ≥ 40
- Rocuronium‑induced anaphylactic shock in pregnancy successfully treated with sugammadex
- Dlouhodobý covid-19 syndrom a perspektivy
- Septický šok a vitamin C?
- VV‑ECMO in organ donor after brain death – case report and review of the issue
- Eighty years since the clinical use of d‑tubocurarine and 70 years since the clinical use of succinylcholine. History of muscle relaxants
- Barva moči z pohledu klinické fyziologie
- Zpráva z kongresu SSAIM
- Informace o vzniku výzkumné iniciativy pro paliativní medicínu a etiku v intenzivní péči : Research Initiative in Palliative Care and Ethics in the Intensive Care Unit (RIPE‑ ICU)
- Mechanická síla – jak vlastně souvisí s traumatizací plic při umělé ventilaci?
- Abstrakty z XXVIII. kongresu ČSARIM
- Anesteziologie a intenzivní medicína
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Barva moči z pohledu klinické fyziologie
- Monitoring of processed EEG under anesthesia II
- Rocuronium‑induced anaphylactic shock in pregnancy successfully treated with sugammadex
- Aspiration pneumonia after cerebrovascular stroke: a comparison between patients with and without dysphagia
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



