-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Helminth Infection and Commensal Microbiota Drive Early IL-10 Production in the Skin by CD4 T Cells That Are Functionally Suppressive
The skin is a major barrier protecting the host from pathogen infection, but is also a site for immune regulation. Using a murine model of repeated percutaneous exposure to infectious Schistosoma mansoni cercariae, we show that, in the skin, CD4+ T cells that do not express markers of conventional regulatory T cells are the main early source of immunoregulatory IL-10 and are functionally suppressive of adaptive immune responses. We demonstrate that the production of regulatory IL-10 in the skin is greatly enhanced after repeated schistosome infection compared to levels present after a single infection and that it limits both neutrophil recruitment and local CD4+ T cell proliferation, thereby preventing excessive inflammation and tissue damage. Initially (day 1), IL-10 producing CD4+ T cells are reactive towards skin commensal bacteria, although over succeeding days they progressively become specific for schistosome antigens. Consequently, our findings highlight a role for early IL-10 produced by dermal CD4+ T cells to mediate immune regulation in advance of later stage chronic infection conventionally associated with the presence of IL-10. Our work provides a mechanistic insight into the triggers of early IL-10 production at barrier sites like the skin, and suggests how tolerance and pathogen clearance might be co-regulated early after exposure to infectious agents.
Published in the journal: . PLoS Pathog 11(5): e32767. doi:10.1371/journal.ppat.1004841
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004841Summary
The skin is a major barrier protecting the host from pathogen infection, but is also a site for immune regulation. Using a murine model of repeated percutaneous exposure to infectious Schistosoma mansoni cercariae, we show that, in the skin, CD4+ T cells that do not express markers of conventional regulatory T cells are the main early source of immunoregulatory IL-10 and are functionally suppressive of adaptive immune responses. We demonstrate that the production of regulatory IL-10 in the skin is greatly enhanced after repeated schistosome infection compared to levels present after a single infection and that it limits both neutrophil recruitment and local CD4+ T cell proliferation, thereby preventing excessive inflammation and tissue damage. Initially (day 1), IL-10 producing CD4+ T cells are reactive towards skin commensal bacteria, although over succeeding days they progressively become specific for schistosome antigens. Consequently, our findings highlight a role for early IL-10 produced by dermal CD4+ T cells to mediate immune regulation in advance of later stage chronic infection conventionally associated with the presence of IL-10. Our work provides a mechanistic insight into the triggers of early IL-10 production at barrier sites like the skin, and suggests how tolerance and pathogen clearance might be co-regulated early after exposure to infectious agents.
Introduction
The skin provides an important first line of defence against infectious pathogens which can gain entry via open wounds/abrasions (e.g. Staphylococcus aureus [1]), following injection via insect bites (e.g. Leishmania sp. protozoa [2] or filarial nematodes [3]), or via direct percutaneous penetration (e.g. soil transmitted hookworms [4], or the helminth Schistosoma sp [5]). As one of the body’s largest tissues, the skin is equipped with a several types of cells with immune function, including myeloid cells [6], epidermal γδ T cells [7] and innate lymphoid cells [8], which alongside other types of cell operate in concert against pathogens, but also provide a mechanism of immune regulation to prevent excessive inflammation and ensure tolerance of commensal microorganisms [9–11]. Regulation of the immune response in the skin is particularly important as this organ is host to at least one billion commensal bacteria per square inch [12,13]. One type of cell in the skin that has been most often associated with immunomodulation are conventional αβ regulatory CD4+ T cells, such as Tr1 [14] and FoxP3+ cells [15], which are thought to be important following skin exposure, for example, to Leishmania [16] and Plasmodium [17].
Following percutaneous infection of the skin by schistosome parasites, a balance must be established between providing immune protection for the host whilst preventing excessive tissue damage and promoting wound healing [18–20]. The larval parasite (cercaria) gains entry into the skin aided by the release of excretory/secretory (E/S) products from the pre-and post-acetabular glands [21], which have stimulatory, as well as regulatory effects on cells of the host’s innate immune system [22–24]. Indeed, cercarial E/S antigens specifically promote production of regulatory IL-10 by antigen presenting cells [23,25] (Sanin & Mountford, manuscript in preparation), and by cultures of whole blood cells obtained from infected individuals from endemic areas for schistosomiasis [26].
IL-10 is often linked to the development of immune regulation following chronic infection with both protozoan, as well as helminth parasites [27]. It has a well-characterized role in limiting liver pathology and mediating resistance to the chronic stage of schistosome disease where eggs released by adult worms act as a major stimulus [28–31]. However, the vast majority of experimental studies of schistosome infection focus upon immune events after a single infection despite the knowledge that for many human residents of schistosome-endemic regions, repeated exposure to cercariae is likely to occur during daily domestic contact with contaminated water sources [32]. A recent study using a murine model of repeated exposures to schistosome cercariae prior to the onset of chronic egg deposition, revealed that the skin infection site becomes rich in Th2-associated IL-4, but also immune regulatory IL-10 [18]. Moreover, CD4+ T cells in the skin-draining lymph nodes of these repeatedly infected mice became hypo-responsive to schistosome antigens [18], which was alleviated in the absence of IL-10 [33]. These findings suggest that the suppression of early immune responses to cutaneous infection can be elicited by repeated pathogen exposure and could be mediated by IL-10 at the skin site of infection.
In the current study, we show that skin inflammation following repeated schistosome infection is increased in the absence of IL-10, and that the primary cellular source of IL-10 in the skin was from CD3+ CD4+ T cells. Moreover, IL-10+ dermal CD4+ T cells from repeatedly exposed mice were functionally suppressive as they were able to reduce the proliferation of responsive skin-draining lymph node (sdLN) CD4+ T cells from mice exposed to a single dose of cercariae. Finally, the production of IL-10 in the skin, which derived from a combination of schistosome-specific and commensal microbiota-specific CD4+ T cell responses, contributed towards limiting inflammation and tissue damage following infection. Thus, we propose that in the skin, IL-10+ CD4+ T cells that do not express markers of conventional
T regulatory cells have an important role in immune regulation after repeated percutaneous exposure to schistosome cercariae. This has relevance for a range of other pathogens which infect their hosts through the skin.
Results
Absence of IL-10 promotes proliferation of dermal CD4+ T cells
Repeated percutaneous exposure to 4 doses (4x) of infective S. mansoni cercariae causes enhanced production of IL-10 by pinnae skin biopsies compared to those recovered from singly (1x) exposed pinnae (Fig 1A, p<0.001) and was accompanied by increased thickening of the skin at the site of infection when compared to skin exposed to a single dose of cercariae (Fig 1B, p<0.001). However, 4x mice that were deficient for IL-10 (i.e. IL-10-/-), had an even greater increase in pinnae thickness compared to 4x wild type (WT) animals (Fig 1B, p<0.0001). This was reflected by the recovery of greater numbers of dermal exudate cells (DEC) from the biopsies of 4x compared to 1x WT skin, which were even more numerous from 4x IL-10-/- compared to 4x WT pinnae (Fig 1C, p<0.05). Skin biopsies from 4x IL-10-/- mice also released significantly more of the pro-inflammatory cytokine IL-12p40 than 1x IL-10-/- and 4x WT skin (Fig 1D, both p<0.0001).
Fig. 1. IL-10 produced in the skin following repeated exposures to S. mansoni cercariae limits CD4+ T cell proliferation and mediates recruitment of granulocytes. 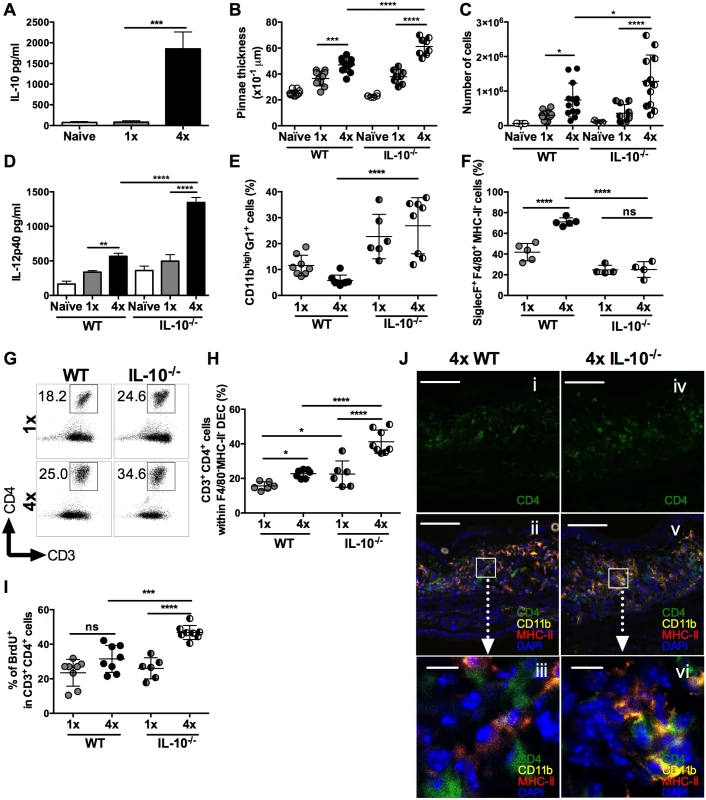
(A) IL-10 released from overnight in vitro cultures of skin (pinnae) biopsies from naïve, 1x or 4x infected mice measured by ELISA. Data are means +SEM (6–8 pinnae per group). (B) Pinnae thickness (x10-1 μm) (n = 8 pinnae) and (C) DEC numbers (n = 6–13 pinnae) for naïve, 1x and 4x infected WT, or IL-10-/- mice, on day 4 post-final exposure. Symbols are values for individual tissue samples; horizontal bars are the means ±SEM. (D) IL-12p40 detected in overnight cultures of skin biopsies from naive, infected 1x, or 4x WT and IL-10-/- mice. Values are means +SEM (n = 8 pinnae). (E) CD11bhighGr1+ neutrophils as a proportion of DEC recovered from the skin infection site of 1x, or 4x infected WT and IL-10-/- mice. Symbols are values for independent tissue samples; horizontal bars are the means ±SEM; n = 6–8 pinnae. (F) Mean percentage ±SEM of SiglecF+F4/80+MHC-II- eosinophils in DEC recovered from infected 1x, or 4x WT and IL-10-/- mice. Symbols are values for cells obtained from independent tissue samples; horizontal bars are the means ±SEM; n = 4–5 pinnae. (G) Representative flow cytometry dot plots and (H) mean percentages ± SEM of dermal CD3+CD4+ T cells (gated on CD11b-F4/80-MHC-II- DEC) from 1x, or 4x infected WT and IL-10-/- mice on day 4 post-final exposure. (I) Percentage of proliferating BrdU+ dermal CD3+CD4+ T cells obtained from 1x, or 4x infected WT and IL-10-/- mice. Symbols are values for cells obtained from independent tissue samples; horizontal bars are the means ± SEM; n = 6–8 pinnae. ANOVA and multiple comparisons tests (Bonferroni’s, Sidak’s and Tukey’s) were performed to compare the means of selected groups (* = p<0.05; ** = p<0.01; *** = p<0.001; **** = p<0.0001; ns = p>0.05). (J) Merged confocal Z-stack images (9) from cryosections of pinnae incubated with mAbs specific for CD4 (green), MHC-II (red) and CD11b (yellow), and counterstained with DAPI nuclear stain (blue), from (i, ii & iii) 4x WT or (iv, v & vi) 4x IL-10-/- skin. Scale bar = 100μm (i, ii, iv & v); = 10μm (iii & vi). Flow cytometric analysis of myeloid DEC based on F4/80 and MHC-II expression (defined in S1A Fig), combined with mAbs against CD11b and Gr1, or CD4 and CD3 (S1B Fig), revealed marked differences in the leukocyte populations recovered from the skin of WT and IL-10-/- mice (Fig 1E and 1F). The proportion of DEC that were CD11bhighGr1+ neutrophils increased significantly in 4x IL-10-/- compared to 4x WT skin (Fig 1E, p<0.0001), and when combined with the total numbers of DEC (Fig 1C), the number of neutrophils was 4.74-fold greater in 4x compared to 1x IL-10-/- mice. Conversely, fewer SiglecF+F4/80+MHC-II- eosinophils were present in DEC from 4x IL-10-/- compared to 4x WT skin samples (Fig 1F and S2A Fig, p<0.0001). There were no differences between the proportions of MHC-IImid, or MHC-IIhigh cells with putative antigen presenting function between 4x WT and 4x IL-10-/- skin samples (S2B, S2C and S2D Fig, p>0.05), although there were decreased proportions in 4x compared to 1x skin. Thus, the absence of IL-10 in 4x infected mice resulted in increased inflammation evident as exacerbated thickening of the skin, increased recruitment of neutrophils, and greater levels of IL-12p40.
In addition to changes in innate immune cell populations, the proportion of CD3+CD4+ (F4/80-MHC-II-) T lymphocytes in the DEC population was increased in 4x WT compared to 1x WT skin samples (Fig 1G and 1H), and they were much more abundant as a proportion of total DEC recovered from 4x IL-10-/- compared to 4x WT skin (Fig 1H). Furthermore, the proliferation of CD3+CD4+ DEC, as measured by the in vivo incorporation of BrdU, was markedly higher in 4x IL-10-/- pinnae compared to all other groups of infected mice (Fig 1I, p<0.001–0.0001), illustrating that there is greater proliferation of dermal CD4+ T cells in IL-10 deficient skin.
The increase in CD4+ T cell numbers in 4x infected skin compared to a single exposure quantified by flow cytometry was confirmed qualitatively using immunohistochemistry and confocal microscopy. Pinnae from 4x WT mice had increased numbers of CD4+ T cells and CD11b+MHC-II+ cells compared to 1x WT mice (S3A and S3B Fig). Furthermore, increased numbers of CD4+ T cells were apparent in the site of infection of 4x IL-10-/- compared to 4x WT mice (Fig 1Ji versus 1Jiv, and S3C Fig). In addition, whilst CD4+ T cells in 4x WT skin, were distributed fairly evenly throughout the tissue section (Fig 1Jii and 1Jiii), in 4x IL-10-/- pinnae CD4+ T cells were localized in close proximity to CD11b+MHC-II+ cells (Fig 1Jv and 1Jvi).
CD4+ T cells in the skin are the main source of IL-10
Using IL-10 reporter mice [34] the proportion of IL-10GFP+ DEC recovered from skin exposed to S. mansoni cercariae was found to be significantly increased in 4x compared to 1x mice (Fig 2A and 2B, p<0.0001). Further characterization of IL-10GFP+ DEC (gating strategy in S1 Fig), revealed two IL-10GFP+ populations identified as F4/80+MHC-IIhigh tissue resident macrophages (R4A gate), and CD3+CD4+ T cells (from F4/80-MHC-II- gate (R1)) in both 1x and 4x pinnae (Fig 2C and 2D). In naïve skin, IL-10GFP+ DEC were almost exclusively within the F4/80+MHC-IIhigh R4A cell population (S4A Fig). However, one day after infection, the proportion of IL-10 producing cells was equally split between CD4+ T cells and F4/80+MHC-IIhigh macrophages (S4B Fig). By day 4 after infection, CD4+ T cells became the predominant source of IL-10 in both 1x (Fig 2E) and 4x (Fig 2F) infected pinnae. Importantly, the proportion of IL-10GFP+ cells that were CD4+ T cells was greater in 4x compared to 1x DEC (Fig 2G, p<0.01).
Fig. 2. CD4+ T cells are the predominant source of IL-10 in DEC. 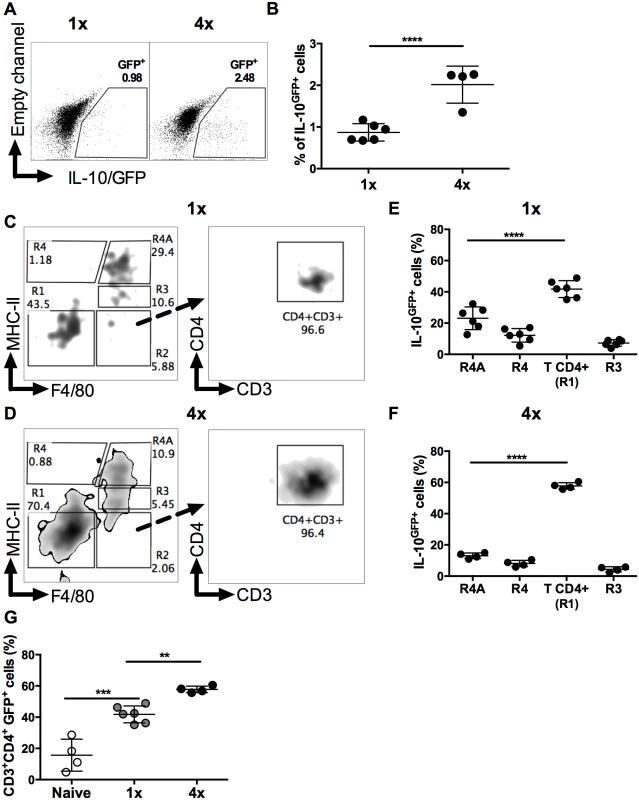
(A) Representative flow cytometry dot plots, and (B) percentages ± SEM (n = 4–6 pinnae), of IL-10GFP+ DEC from 1x and 4x infected mice; gate shows proportions IL-10GFP+ cells. Representative flow cytometry dot plots of IL-10GFP+ DEC from infected (C) 1x and (D) 4x IL-10+/GFP mice on day 4 after final exposure to infectious cercariae. Density plots show initial F4/80 vs. MHC-II gating strategy (left-hand plots) to yield R1 (double negative F4/80-MHC-II-), R2 (F4/80+MHC-II-), R3 (F4/80+MHC-IImid), R4 (F4/80-MHC-IIhigh), and R4A (F4/80+MHC-IIhigh) cell populations. R1 was then gated according to CD4 vs. CD3 expression (right-hand plot). Proportions of IL-10GFP+ DEC from infected (E) 1x and (F) 4x mice. (G) Proportion of IL-10GFP+ CD3+CD4+ T cells of total IL-10GFP+ cells in DEC from naïve, 1x or 4x infected mice on day 4 post-final infection. Symbols are values for cells obtained from independent tissue samples; horizontal bars are the means ± SEM; n = 4–6 pinnae. ANOVA and multiple comparisons tests (Sidak’s and Tukey’s) were performed to compare the means of selected groups (** = p<0.01; *** = p<0.001; **** = p<0.0001; ns = p>0.05). Conventional CD4+ T regulatory cells in the skin are not a major source of IL-10 in 4x infected mice
Several types of CD4+ T cells can produce IL-10, including regulatory T cells [35], but the proportion of FoxP3+ thymic T regulatory cells (tTreg), identified by their co-expression of Helios and Nrp1 in DEC (Fig 3A left panel), was significantly lower in CD3+CD4+ DEC recovered from 4x compared to 1x mice (Fig 3B, p<0.0001), although the absolute number of tTreg remained unchanged between these two groups of mice (Fig 3C). The proportions and numbers of peripheral Treg (pTreg), expressing FoxP3 but not Helios (Fig 3A middle panel), were not significantly different between 1x and 4x DEC (Fig 3B and 3C, p>0.05). Conversely, Type 1 regulatory T (Tr1) cells, which co-express CD223 and CD49b (Fig 3A, right panel), were slightly increased in 4x compared to 1x DEC as a proportion (Fig 3B, p<0.05), but the difference in the total number of Tr1 cells between the infection groups did not reach statistical significance (Fig 3C, p>0.05). Nevertheless, CD4+ T cells that did not fall into any of these three categories of conventional regulatory T cells were the most abundant type of CD4+ cells in both groups of infected skin (~70% of all CD4+ DEC, Fig 3B and 3C), whilst their proportion and number in 4x pinnae was significantly greater than in 1x skin (Fig 3B and 3C, p<0.0001).
Fig. 3. IL-10 producing dermal CD4+ cells do not express markers of conventional regulatory T cells. 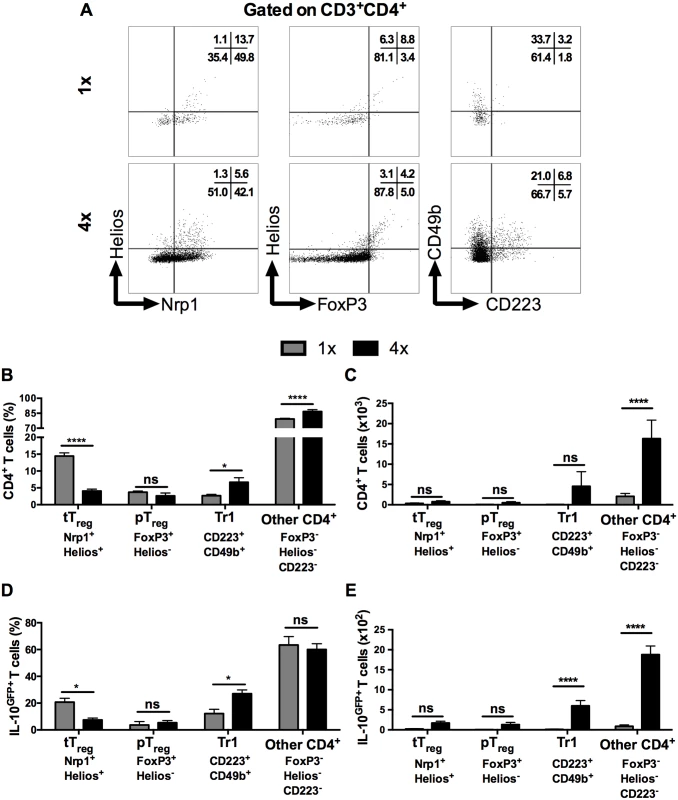
(A) Representative flow cytometry dot plots for thymic (Nrp1+Helios+) Treg cells, peripheral (FoxP3+Helios-) Treg cells, and Tr1 (CD223+CD49b+) cells. (B) Mean proportion +SEM and (C) absolute numbers of dermal CD4+ thymic and peripheral Treg cells, as well as Tr1, and other CD3+CD4+ (Helios-FoxP3-, not co-expressing CD223 plus CD49b) dermal cell populations recovered from 1x (grey), or 4x (black) infected mice on day 4 post-final exposure. (D) Mean proportions +SEM and (E) absolute numbers of dermal T cells defined in A gated on IL-10GFP+ CD4+ T cells from 1x or 4x infected mice. ANOVA and Sidak’s multiple comparisons test were performed to compare the means of selected groups (* = p<0.05; **** = p<0.0001; ns = p>0.05). Analysis of IL-10GFP+ CD4+ DEC showed that IL-10 producing pTreg were not a prominent source of IL-10 (<5% of all IL-10GFP+ cells) in either 1x or 4x infected skin (Fig 3D). Their number also did not differ significantly between the two groups of mice (Fig 3E). Furthermore, tTreg contributed proportionally less IL-10 relative to other CD4+ cell types in 4x skin (Fig 3D), and their number was not significantly different between the two infection groups (Fig 3E). Conversely, the proportion of Tr1 cells in 4x pinnae that were IL-10GFP+ increased (Fig 3D, p<0.05), and the difference in their number between 1x and 4x mice was significantly greater (Fig 3E). However, CD4+ T cells that fell in none of these categories were by far the predominant CD4+ cell population that were IL-10GFP+ in the skin after infection, representing more than 60% of all IL-10GFP+ CD4+ T cells in both 1x and 4x DEC (Fig 3D), as well as increasing in number significantly in 4x DEC (Fig 3E, p<0.0001).
Dermal CD4+ T cells are specific for S. mansoni antigen and skin commensal microbiota
To verify the antigen specificity of dermal CD4+ T cells, carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) labelled DEC cultures were stimulated with soluble antigen derived from parasite larvae (i.e. SSAP). CD4+ DEC obtained from 1x mice on day 1 after infection exhibited very low levels of proliferation to parasite antigen, which was similar to those in DEC cultured without antigen (no-antigen controls; Fig 4A left and Fig 4B). In contrast, CD4+ DEC from 1x mice recovered on day 4, as well as those from 4x mice recovered on day 1 and day 4, all exhibited enhanced proliferation in response to SSAP (Fig 4A and 4B, p<0.0001). The proliferative responses to SSAP of dermal CD4+ T cells recovered on day 4 after infection, from both 1x and 4x mice, were significantly greater than for cells recovered on day 1 (Fig 4B, p<0.0001).
Fig. 4. Dermal CD4+ T cells from skin exposed to S. mansoni cercariae respond in vitro to parasite and skin commensal antigens. 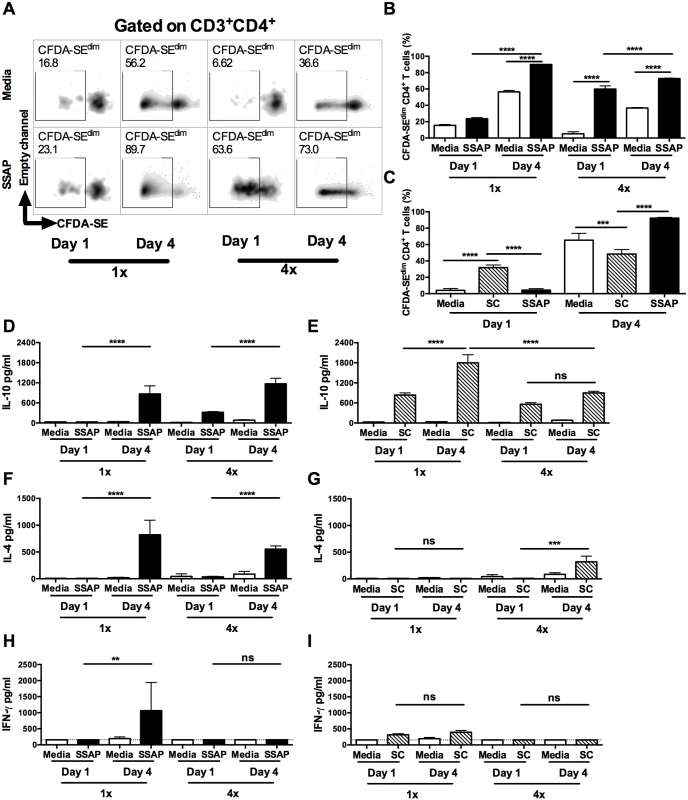
(A) Representative flow cytometry density plots of CFDA-SE labelled CD3+CD4+ dermal T cells from 1x or 4x infected WT mice recovered on day 1 or 4 after infection; DEC were obtained from skin biopsies and stimulated in vitro for 96h in the presence, or absence of parasite antigen (SSAP). Gate is set for CFDA-SEdim cells previously gated on CD3+CD4+cells. (B) Percent CFDA-SEdim CD3+CD4+ cells (mean +SEM; n = 4–5). (C) Mean percentage +SEM of CFDA-SEdim CD3+CD4+ T cells from DEC cultures in the presence or absence of SSAP, or antigen from skin commensals (SC), from 1x infected mice on day 1 or 4 after infection. Production of (D & E) IL-10, (F & G) IL-4 and (H & I) IFN-γ in culture supernatants of skin biopsies from 1x or 4x infected mice obtained on day 1 and 4 post-final infection, cultured in the presence, or absence of SSAP or SC antigen; bars are means + SEM, n = 4–5. ANOVA and Sidak’s multiple comparisons test analysis show statistically significant differences between selected groups (* = p<0.05; ** = p<0.01; *** = p<0.001; **** = p<0.0001; ns = p>0.05). Although dermal CD4+ T cells from 1x pinnae recovered on day 1 after infection did not proliferate in response to SSAP, a proportion of them were positive for IL-10 compared to naive mice (S4B and S5A Figs). As it is unlikely that sufficient time will have elapsed for parasite antigen-specific CD4+ T cells to have been primed in the lymph node and recruited to the skin site of infection, it was thought possible that these CD4+ T cells were responding to other foreign antigens. Commensal microbiota resident on the skin surface might gain access to the dermis as the parasite embarks on percutaneous penetration, thus commensals are a possible source of antigen. Indeed, dermal CD4+ T cells from 1x mice recovered on day 1 proliferated in vitro to antigen derived from skin commensal microbes (SC), and the level of proliferation was significantly greater compared to that in response to parasite SSAP antigen (Fig 4C, p<0.0001). In contrast, dermal CD4+ T cells from 1x mice recovered on day 4 after infection proliferated vigorously to parasite SSAP antigen, to a much greater level than in response to SC (Fig 4C, p<0.0001). CD4+ T cell proliferation where no antigen was added is likely to be a result of in situ priming of DEC antigen presenting cells with schistosome antigen already present in the skin of repeatedly exposed mice, as larvae can remain in this tissue for up to 4 days post-exposure.
Stimulation of DEC from 1x and 4x infected mice with parasite SSAP antigen resulted in the production of significantly greater quantities of IL-10 compared to unstimulated 1x and 4x DEC, whilst the levels of IL-10 were significantly greater for both groups of mice on day 4 than day 1 (Fig 4D, p<0.0001). High levels of IL-10 were also detected in the supernatants of DEC stimulated with SC antigen, and were at their greatest on day 4 in 1x mice (Fig 4E, p<0.0001). On the other hand, whilst IL-4 was produced by DEC from both 1x and 4x mice in response to SSAP (Fig 4F), detectable levels of IL-4 were only produced by 4x DEC recovered on day 4 in response to SC antigen (Fig 4G, p<0.001). Furthermore, whilst 1x DEC on day 4 produced significant quantities of IFN-γ to SSAP antigen (Fig 4H), only very low levels of this cytokine were detected in DEC supernatants from 1x and 4x mice at both time points in response to SC antigen (Fig 4I).
There were significantly more IL-10GFP+ CD4+ T cells in DEC recovered on day 1 after S. mansoni infection than in naïve skin (S5A Fig). However, dermal CD4+ T cells in naïve skin might be exposed to commensal microorganisms during the course of the host’s development [36]. Consequently, although very few CD4+ T cells were recoverable from naïve skin, we show that proliferation of these cells in vitro to SC antigen was comparable to that of dermal CD4+ T cells recovered on day 1 after exposure to schistosome cercariae (S5B Fig). Furthermore, cultures of DEC from naive mice stimulated with SC antigen also produced significantly elevated levels of IL-10 (S5C Fig), but did not produce IL-4 (S5D Fig), and only non-significant quantities of IFN-γ (S5E Fig). DEC from naïve mice did not respond to SSAP. Thus, we demonstrate that a number of CD4+ T cells with specificity to commensal antigens are present in naïve skin and we conclude that infection with schistosome cercariae enhances the exposure of CD4+ T cells to SC antigen resulting in the observed IL-10 production.
IL-10 production from CD4+ T cells is required for regulation of the dermal immune response
As the production of IL-10 in our model derived predominantly from dermal CD4+ T cells, the effect of their absence was examined in repeatedly infected Rag2-/- mice. CD3-B220+ B cells and CD3+CD8+ lymphocytes, which are also absent in Rag2-/- mice, were not abundant in S. mansoni infected skin (S6 Fig). As CD3+CD8+ lymphocytes were significantly reduced in number in 4x DEC (p<0.001), and CD3-B220+ B cell numbers remained unchanged, we conclude that differences observed in Rag2-/- mice would be predominantly due to the absence of CD4+ T cells. Indeed, the absence of CD4+ T cells in 4x Rag2-/- mice resulted in an increase in the proportion of CD11b+Gr1+ neutrophils compared to 4x WT controls (Fig 5A, p<0.05), whereas there was a significant reduction in the proportion of SiglecF+F4/80+MHC-II- eosinophils (Fig 5B, p<0.001), reminiscent of the findings for infected IL-10-/- mice (Fig 1E and 1F). The production of IL-10 (Fig 5C, p<0.05) and IL-4 (Fig 5D, p<0.05) by cultured skin biopsies was also impaired in 4x Rag2-/- mice. Collectively, our data are compatible with the notion that the dermal CD4+ T cell population is the main source of IL-10 in S. mansoni infected skin and that other IL-10+ myeloid cells (such as those defined in Fig 2) are not sufficient to recapitulate the immune response observed in 4x WT mice.
Fig. 5. Lack of CD4+ T cells ablates IL-10 production induced in skin exposed to S. mansoni cercariae. 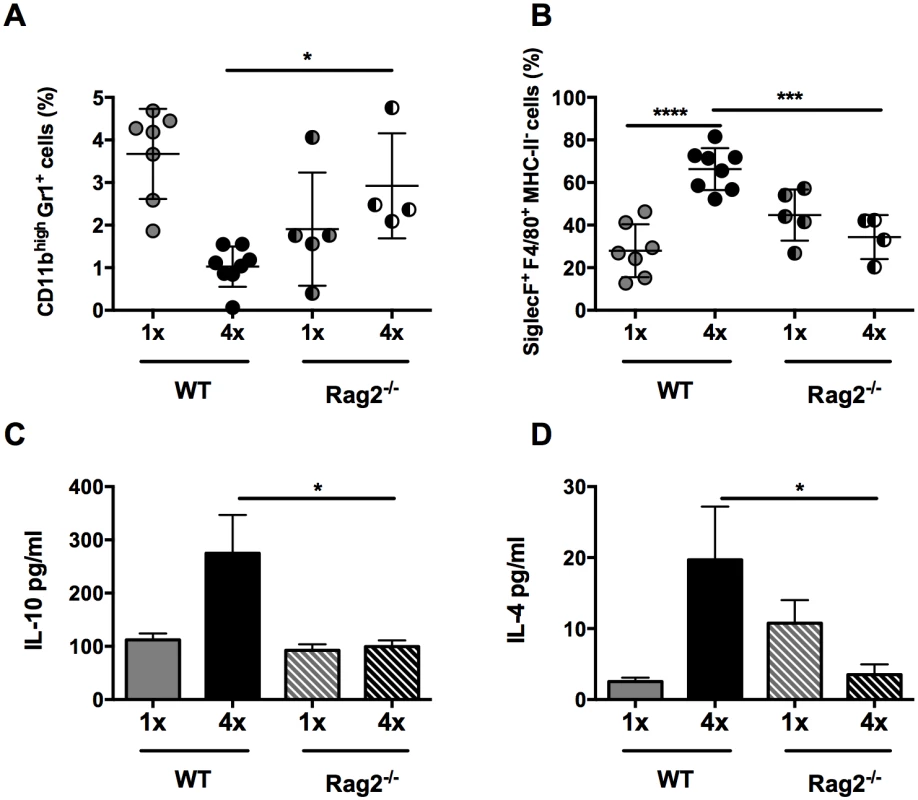
(A) Proportions of CD11bhighGr1+ neutrophils and (B) SiglecF+F4/80+MHC-II- eosinophils in total DEC recovered from 1x, or 4x infected WT and Rag2-/- mice on day 4 post infection. Symbols are values for cells obtained from independent tissue samples; horizontal bars are the means ± SEM; n = 4–6 pinnae per group. Production of (C) IL-10 and (D) IL-4 in overnight culture supernatants of skin biopsies from 1x or 4x infected WT and Rag2-/- mice obtained on day 4 post-final infection; bars are means + SEM, n = 4–6 pinnae per group, as measured by ELISA. Significant differences means were compared between of selected groups by ANOVA and post-hoc tests (Sidak’s and Tukey’s) (* = p<0.05; ** = p<0.01; *** = p<0.001; **** = p<0.0001; ns = p>0.05). IL-10+ dermal CD4+ T cells suppress proliferation of CD4+ T cells in the skin draining lymph node
Since the presence of IL-10 in the skin is able to regulate the proliferation of CD4+ T cells in situ in the skin site of infection (Fig 1I), we sought to determine whether dermal IL-10 producing cells from 4x mice were able to inhibit in vitro proliferation of CD4+ T cells recovered from sdLN of mice exposed to 1x infection.
As expected, sdLN CD4+ cells from 1x mice were responsive and proliferated in vitro to parasite SSAP antigen (Fig 6A, left). The addition of dermal CD4+ T cells from 4x infected IL-10-/- mice did not significantly affect the ability of 1x sdLN CD4+ cells to proliferate (Fig 6B, p>0.05). However, addition of IL-10 competent dermal CD4+ T cells from 4x WT mice significantly inhibited proliferation of 1x sdLN CD4+ cells (Fig 6B, p<0.05). Moreover, the difference between adding dermal CD4+ T cells from 4x WT and 4x IL-10-/- mice was statistically significant (Fig 6B, p<0.001).
Fig. 6. IL-10GFP+CD4+ T cells from the skin of mice infected with S. mansoni suppress proliferation of CD4+ T cells in the skin draining lymph nodes. 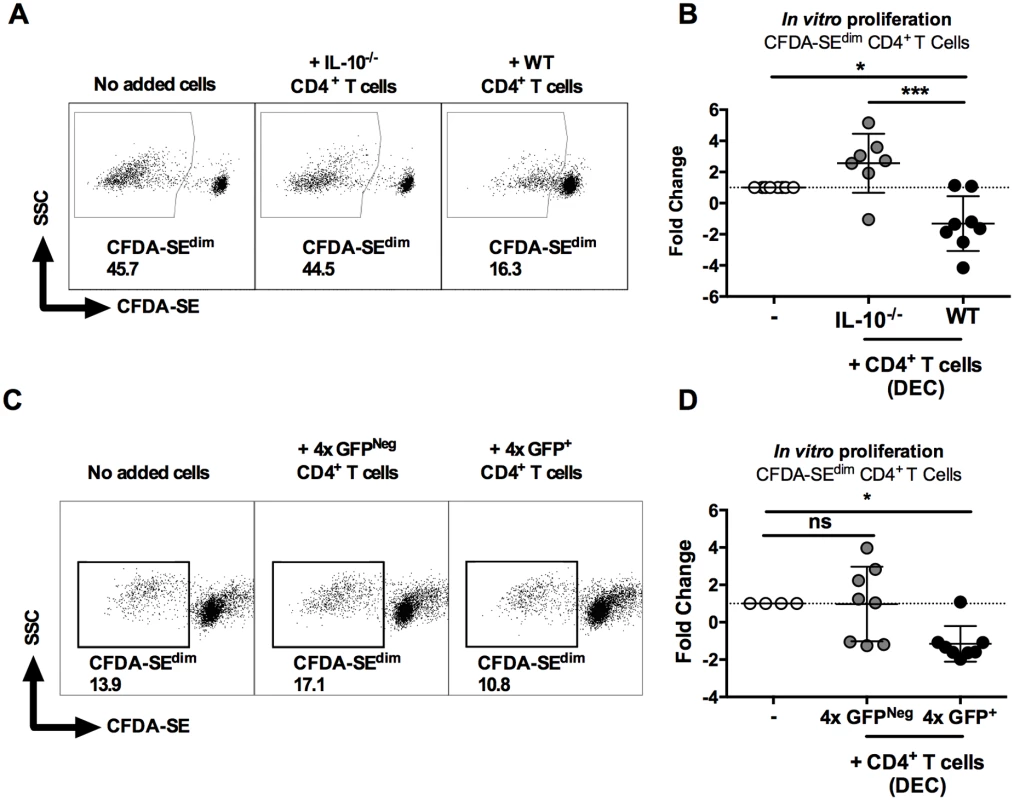
(A) Representative flow cytometry dot plots of CFDA-SEdim CD3+CD4+ cells from the sdLN of 1x infected mice after co-culture for 96h with dermal CD4+ T cells from 4x infected WT or IL-10-/- mice in the presence of parasite antigen SSAP. (B) Data is given as sdLN CD3+CD4+ T cell proliferation expressed as the fold-change observed in each of seven corresponding mice co-cultured in the presence of dermal CD4+ T cells from either WT or IL-10-/- mice relative to sdLN CD3+CD4+ T cells cultured without added dermal CD4+ T cells. Symbols are individual mice; horizontal bars are means ± SEM; n = 7. (C) Representative flow plots and (D) fold change ± SEM of CFDA-SEdim sdLN CD3+CD4+ T cells from 1x infected mice (n = 8) co-cultured with GFP+, or GFPNeg dermal CD4+ T cells from 4x infected mice in the presence of SSAP. Differences between the means of selected groups were compared by ANOVA and Tukey’s multiple comparisons test analysis (* = p<0.05; ** = p<0.01; *** = p<0.001; **** = p<0.0001; ns = p>0.05). Furthermore, IL-10GFP+ dermal CD4+ T cells sorted from the DEC of 4x infected mice significantly inhibited proliferation of 1x sdLN CD4+ cells in response to parasite SSAP antigen (Fig 6C and 6D, p<0.05), whereas GFPNeg dermal CD4+ T cells from the same 4x mice did not significantly alter the proliferation of 1x sdLN CD4+ cells (Fig 6C and 6D, p>0.05). Combined, these data demonstrate that IL-10 production from dermal CD4+ T cells is responsible for regulating the responsiveness of sdLN CD4+ T cells.
Discussion
The role of IL-10 in maintaining immune homeostasis and resolving inflammation is critical for host survival and function [16,37–39], a phenomenon that has been well studied in the pathogenesis of chronic schistosomiasis [28–31] but is not understood, or documented, at the early stage of percutaneous schistosome infection. Here, we show that repeated exposures of the skin to infective S. mansoni cercariae results in an early increase in IL-10 production by CD4+ T cells at the skin site of infection. The production of IL-10 by these cells occurs prior to the late stage production of IL-10 conventionally associated with chronic schistosome infection after the onset of egg deposition [30,40]. Single infection with S. mansoni cercariae leads to transient IL-10 production in the skin, which returns to naïve levels after 14 days [41], whilst repeated infection leads to sustained production of IL-10 between days 1–8 after exposure [18]. In our model, early IL-10 production by dermal CD4+ T cells was important in limiting the extent of inflammation at the site of infection by reducing skin thickening and neutrophil recruitment. Moreover, dermal CD4+ T cells were able to limit local proliferation of CD4+ T cells in the dermis and suppress the proliferation of normally responsive CD4+ T cells from proximal sdLN of singly infected animals.
In the current study, CD4+ T cells were the main producers of IL-10 after repeated exposure to S. mansoni cercariae supporting the findings of others, who report that CD4+ T cells are important contributors to the production of IL-10 in a range of disease settings [16,42–46], as well as being an important target of IL-10 [47]. In the mesenteric lymph nodes and the spleen during chronic S. mansoni infection, CD25+CD4+ and CD25-CD4+ T cells were the main producers of IL-10 [48], and it is well known that IL-10 is important in limiting increased granuloma formation around eggs deposited in the liver, leading to decreased host survival [31,49]. However, here we demonstrate that production of IL-10 by dermal CD4+ T cells occurs very early during the initial stages of S. mansoni infection, well in advance of egg deposition, which is a hallmark of chronic/long term infection. We show that early IL-10 was instrumental in limiting inflammation after primary but particularly following repeated exposures to infectious cercariae. Local proliferation of CD4+ T cells, plus the recruitment of neutrophils were regulated by this early production of IL-10 in the skin. This suggests that during natural infection, where repeated exposures to infective cercariae are likely to be common for residents of schistosome-endemic areas [32], IL-10 could play an important role in limiting leukocyte mediated tissue damage associated with migration of larvae through the skin. Indeed, whole blood cultures of infected individuals in endemic regions produced enhanced levels of IL-10 in response to cercarial E/S antigens [26], which supports the hypothesis that this cytokine is triggered by the early stage of the parasite.
Multiple subsets of T helper cells can make IL-10 [16,42–46], including T helper (Th) 2, thymic Nrp1+ Helios+ Treg (tTreg), peripheral FoxP3+ Helios- Treg (pTreg), and Tr1 cells [27,50,51], and previous studies in the skin have highlighted a role of regulatory T cells in the prevention of excessive inflammation during exposure to protozoan parasites [15–17,52]. However in our studies, the proportions of both tTreg and pTreg decreased in 4x infected mice, and as they were a scarce source of IL-10, there was limited evidence for these regulatory CD4+ T cell subtypes being responsible for early IL-10 in the skin of mice repeatedly exposed to larvae of the Schistosoma helminth. Although CD223+ CD49b+ Tr1 cells, which are thought to exert their function through the production of IL-10 [14,50], were slightly increased as a proportion of all CD4+ T cells in 4x mice, and as a proportion and number of IL-10+ CD4+ T cells in the skin, most of the IL-10+ CD4+ T cells in the skin expressed none of the markers associated with conventional ‘regulatory’ phenotypes despite being functionally suppressive. Nevertheless, as several of these functionally suppressive IL-10+ CD4+ T cells co-expressed Nrp1 and CD49b, both of which are up-regulated in activated T cells [53,54], our findings do not rule out the possibility that they arise from conventional regulatory T cells which have lost expression of FoxP3, Helios, or CD223 (LAG3), as reported by others [55,56].
A major difference that distinguishes our work from those cited above [15–17,52] is that they were based upon the study of immune responses to a single pathogen exposure leading to persistent/chronic infection. In contrast, in our study, repeated exposures to cercariae led to regulation being evident early after infection, thus emphasizing the early role of antigen-specific CD4+ T cells in mediating immune regulation in sites of initial infection, such as the skin. The functionally suppressive CD4+ T cells had a putative Th2 bias, as they secreted IL-4, but not IFN-γ, which is in line with our previous studies showing the production of elevated levels of IL-4, but not Th1 associated cytokines in skin exposed to multiple doses rather than a single dose of cercariae [18]. CD4+ cells were confirmed as the source of IL-4 since this cytokine was not detected in T cell deficient skin (i.e. Rag2-/-). The role of Th2 cells in limiting skin inflammation has been suggested during disorders such as atopic dermatitis, where innate immune cells are regulated by cytokines from Th2 cells [13,57]. However, recent literature highlights the possibility that CD4+ T cells could be tissue resident memory cells (TRM) as identified primarily in pulmonary and gastrointestinal mucosal tissue sites [58,59]. Whilst CD69 and CD103 expressing CD4+ cells have also been reported in skin [9,60–62], it is not clear whether they are circulating, or are tissue resident, and we did not observe dermal CD69+ CD4+ T, or CD103+ CD4+ T cells in our model. Finally, although CD4+ TRM cells can produce IFNγ/IL-17 [58], and IL-4/IL-13 following helminth infection [63], their production of IL-10 has not previously been reported. Consequently, further investigation into the possible definition of IL-10+ CD4+ population in the dermis is warranted.
Myeloid cells such as F4/80+MHC-IIhigh tissue resident macrophages were also a source of IL-10 in the skin after S. mansoni infection, most likely in response to cercarial E/S products [25] (Sanin & Mountford, manuscript in preparation). We showed previously that after repeated infection, M2-like dermal APCs conditioned within the skin infection site, which is rich in IL-4 and IL-10, were associated with decreased CD4+ T cell responsiveness in the skin draining lymph node [18]. However, our current findings show that proliferation of dermal CD4+ T lymphocytes is not impaired in repeatedly infected skin and that IL-10 production is predominantly from CD4+ T cells in the skin rather than myeloid cells. This accords with recent findings that IL-10 is the main driver of CD4+ T cell hypo-responsiveness in the lymph node [33]. In the present study, the role of F4/80+MHC-IIhigh macrophage derived IL-10 might be important for the initial polarization of dermal CD4+ T cells towards their subsequent production of IL-10, as shown in another recent study of helminth infection [64]. Indeed, IL-10 is able to enhance its own expression through the activation of STAT3 [65]. The clusters of MHC-II+ cells and CD4+ T cells observed in schistosome-infected skin may represent how myeloid cells activate CD4+ T cells after S. mansoni infection, as suggested in other infection models [66,67]. IL-10 production in T cells can be regulated by several cytokines (e.g. IL-4, IL-6, IL-12, and IL-27), as well as multiple transcription factors (i.e. STAT1, STAT3, STAT4, STAT6, GATA3, and c-MAF) [68]. In particular, IL-27 has been reported to drive IL-10 production by CD4+ T cells in dermal lesions caused by infection with Leishmania parasites [69], although IL-27 was not detected in the culture supernatants of skin biopsies in our schistosome infection model. However, full exploration of these cytokines and transcription factors was beyond the scope of the current study.
In the absence of T lymphocytes (i.e. Rag2-/-), very low levels of IL-10 were noted and the dermal immune response was equivalent to that in IL-10-/- mice, supporting our conclusion that myeloid and/or CD4negative lymphoid cells are not a major source of IL-10. Other lymphocytes, which are absent in Rag2-/- skin, such as B cells and CD8+ T cells were rare in infected skin (<1000 cells) and did not expand in number, or proportion after repeated infection, nor did they produce IL-10. Therefore, IL-10 derived from dermal CD4+ T cells, rather than myeloid cells [18], appears to be responsible for inducing CD4+ lymphocyte hypo-responsiveness in the sdLN, as well as conditioning the dermal immune environment.
Although functionally suppressive IL-10+ CD4+ T cells in the dermis had specificity for schistosome antigen, a population of dermal CD4+ T cells produced IL-10 in response to antigens from commensal microbiota. Indeed, bacterial commensals in barrier tissues such as the skin can influence the immune response [12,13,70], and can exacerbate immune pathology by altering the balance between regulatory and effector T cells by triggering IL-17 and IFN-γ, although a role for IL-10 has not been established [36,71,72]. Here we show that CD4+ T cells in the skin produce IL-10 in a response that was initially directed against commensal microbiota. We speculate that skin commensals gain access to the dermis as the parasites invade the skin and therefore stimulate resident T cells with specificity for antigens from commensal microbiota as early as day 1 after schistosome infection. These early responding T cells could include Tr1 cells, which are a known source of IL-10 [14,50], especially as their antigen specificity can be to commensal microbiota [52]. The early production of IL-10 triggered by commensals as a result of penetrating S. mansoni cercariae, therefore could be a strategy adopted by the parasite to limit anti-parasite immune responses from the host. This might be particularly relevant in the context of the enhanced neutrophilia observed here in the absence of IL-10. The use of germ-free animals could provide direct evidence of the role of skin commensals in S. mansoni infection, as topical antibiotic treatment of skin to eliminate skin microbiota is likely to have an adverse effect on cercarial penetration, as seen with the use of soap [73]. However, the impact of commensal microorganisms on the dermal immune response to Schistosoma infection in humans in the face of concurrent antibiotic treatment requires further investigation.
In summary, we demonstrate that repeated exposures of the skin to infective S. mansoni cercariae leads to an early increase in IL-10 production by S. mansoni specific CD4+ T cells in the dermis, with a putative Th2 bias, at the site of infection. This is accompanied by a previously unreported bystander response to commensal microbiota that gain access to the dermis as cercariae penetrate the skin. IL-10 production by these dermal CD4+ T cells is important in limiting the extent of skin inflammation, leukocyte proliferation and recruitment. Critically, these dermal CD4+ T cells are able to suppress the proliferation of CD4+ T cells from the draining lymph nodes which could explain the development of hypo-responsiveness should they migrate in vivo to proximal lymph nodes. Collectively our findings demonstrate the importance of early IL-10 production by functionally suppressive CD4+ T cells in the skin in response to S. mansoni cercariae and highlights a possible role of these cells in maintaining host fitness in populations that inhabit areas endemic for schistosomiasis, and other helminth larvae that penetrate via the skin [74,75]. Finally, our data show that functionally suppressive CD4+ T helper cells, that are not conventional regulatory CD4+ T cells, are important as modulatory cells in the skin after repeated exposure to pathogens. We suggest that the role of IL-10 in controlling early immune responses to schistosomes, may act as a prelude to the subsequent development of IL-10 mediated immune regulation conventionally associated with chronic helminth infections.
Materials and Methods
Ethics statement
Female mice aged between 6–10 weeks were used for all experiments carried out in accordance with the UK Animal’s Scientific Procedures Act 1986 and with approval of the University of York Ethics Committee (PPL #60/4340).
Animals
Wild type C57BL/6 (WT) and IL-10 deficient (IL-10-/-) mice [76], as well as transgenic mice lacking RAG2 (Rag2-/-), or IL-10 reporter knockin (tiger) animals (IL-10+/GFP) [34] were bred and housed at the University of York.
Infection protocol and parasites
Mice were percutaneously exposed via each pinna to four doses (4x) of 150 S. mansoni cercariae at weekly intervals from day 0 to day 21 as previously described (repeated infection) [18,77]. Age and sex-matched cohorts were exposed in parallel to a single dose (1x) of 150 cercariae on day 21. Using this infection protocol via the pinna, a 50% penetration rate is observed [77] amounting to ~75 cercariae per pinna at each time-point. Inflammation of pinnae was measured using a dial gauge micrometer (Mitutoyo, Japan). In most experiments, pinnae were harvested 4 days after the last infection, or in selected experiments obtained on day one. Auricular lymph nodes draining the skin site of infection (sdLN) were also harvested in specific experiments on day 4 post-final infection.
Dermal exudate cells
Pinnae from naïve, and infected mice (on day 1 or 4 post-final exposure to cercariae) were removed, briefly sterilized with ethanol, air-dried and split along the central cartilage into two halves to obtain the population of dermal exudate cells (DEC) as described previously [18,78]. Split pinnae were floated overnight at 37°C 5% CO2 in the absence of added antigen on RPMI-1640 media (Gibco, Paisley, UK) containing 10% heat inactivated FCS (Biosera, Uckfield, UK), 2mM L-Glutamine solution, 1% Pen/Strep (both Gibco), and 50μM 2-mercaptoethanol (Sigma-Aldrich, Gillingham, UK) (complete RPMI) in non-adherent 24 well tissue culture plates (Greiner Labortechnik, Frickenhausen, Germany). Floating pinnae tissues were removed and the remaining culture supernatants containing DEC spun at 1000g for 7min at 4°C before being re-suspended in complete RPMI and live cells enumerated using a hemocytometer. Cell-free culture supernatants were recovered and stored at -20°C before being analyzed by cytokine-specific ELISA.
In vitro antigen stimulation of DEC
In order to stimulate DEC for an antigen-specific recall response, soluble schistosomula antigen preparation (SSAP) was prepared from in vitro cultured mechanically-transformed larvae as described previously [79]. Skin commensal antigen (SC) was prepared by culturing skin swabs taken from female WT C57BL/6 mice overnight in liquid broth medium at 37°C. Recovered microbes were washed in sterile PBS, sonicated at full power in PBS for 5 min (30s on / 30s off), and inactivated for 0.5h under UV light. DEC were then cultured at 5x105 cells/ml in complete RPMI in the presence, or absence, of 50 μg/ml SSAP, or 5 μg/ml SC, for 96 hours at 37°C. Proliferation was measured after labelling DEC with 3 μM CFDA-SE (Invitrogen, Paisley, UK) [18]. DEC were subsequently labelled with specific monoclonal antibodies (mAb) detailed below and proliferation determined by flow cytometry according to the decrease in the fluorescence of CFDA-SE.
Flow cytometry
DEC were incubated with 1 μg anti-CD16/32 mAb (eBioscience, Hatfield, UK) in goat-serum (Sigma-Aldrich) to block non-specific uptake of antibodies and then subsequently labelled with LIVE/DEAD Fixable Aqua Dead Cell Stain (Life Technologies, Paisley, UK) according to the manufacturer’s instructions, plus the following mAbs conjugated to various fluorescent labels: anti-CD4 (clone RM4-5), anti-CD3 (clone 17A2), anti-MHC-II (IA-IE) (clone M5/114), anti-Nrp1 (clone 3DS304M), anti-CD11b (clone M1/70), anti-F4/80 (clone BM8), anti-Gr1 (clone RB6-8C5), anti-SiglecF (clone eBio440c), anti-CD45 (clone 2D1) (all eBioscience), anti-CD49b (clone R1-2) (BioLegend, London, UK) and anti-CD223 (LAG3) (clone C9B7W) (BD Bioscience, Oxford, UK). For intracellular staining, cells were washed with and fixed with 2% paraformaldehyde for 1 hour at 4°C before being washed and incubated for 1 hour in 1x permeabilization buffer (eBioscience) for anti-FoxP3 (clone NRRF-30) and anti-Helios (clone 22F6) (eBioscience). All flow cytometry was acquired using the Cyan ADP analyser (DakoCytomation, Stockport, UK), or BD LSR Fortessa analyser (BD Biosciences, Oxford, UK). Data was analysed using FlowJo software v7.6.5 (Tree Star, Inc, Ashland, Oregon, USA).
Detection of intracellular IL-10 in DEC
Detection of IL-10 production by different cell types in DEC was achieved using IL-10+/GFP mice. WT and IL-10+/GFP mice were infected and pinnae harvested as described above. Split pinnae were incubated with complete RPMI for 12 hours prior to the addition of 1x Brefeldin A (eBioscience) following the manufacturer’s instructions. After a further 8 hours, DEC were prepared for flow cytometric analysis, by washing once with PBS.
Sorting of dermal CD4+ T cells and in vitro co-culture with sdLN cells
DEC obtained from 4x infected WT, IL-10+/GFP and IL-10-/- mice were labelled with mAb against CD4, CD3, CD45 and MHC-II as described above. Dermal CD45+CD3+CD4+ T cells were recovered following FACS (MoFlo Astrios, Beckman Coulter, London, UK) from WT and IL-10-/- mice. For IL-10+/GFP mice, dermal CD45+CD3+CD4+ IL-10GFP+ T cells and CD45+CD3+CD4+ IL-10GFP- T cells were obtained. CFDA-SE labelled single cell suspensions from the sdLN were co-cultured at 2.5x105 cell/ml in complete RPMI with, or without, 2x103 sorted dermal T cells in the presence, or absence, of 50 μg/ml SSAP for 96 hours at 37°C. Antigen-specific proliferation of sdLN CD4+ cells was measured by a decrease in levels of CFDA-SE after 72 hours as described above compared to cells stimulated the absence of antigen.
Cytokine analysis by ELISA
Culture supernatants were collected from overnight skin biopsies, or from sdLN cell cultures after 96 hours, for cytokine analysis as previously described [80]. IL-4, IL-10 and IL-12p40 were quantified by DuoSet ELISA (R&D Systems, Abingdon, UK), whilst IFN-γ was measured using specific capture and detection antibodies (BD Pharmingen, Oxford, UK) [77].
In vivo cell proliferation
Mice received 1 mg BrdU (Sigma-Aldrich) i.p. daily for the final 4 days before harvest of pinnae in order to determine in vivo cell proliferation. DEC were then recovered, and blocked with 1 μg anti-CD16/32 mAb in goat-serum. Subsequently, DEC were labelled for surface expression of CD3, CD4, MHC-II, F4/80 and CD11b (all eBioscience) in PBS supplemented with 1% FCS. Cells were then washed and incubated in 1x Fixation/Permeabilisation buffer (eBioscience) for 1 hour at 4°C before being washed and incubated for 1 hour at 37°C in 100 μg DNase (Sigma-Aldrich). Finally, cells were labelled for 45 minutes at room temperature with anti-BrdU APC mAb, or rat IgG1 APC (eBioscience), in 1x Permeabilisation buffer as per the manufacturer’s protocol.
Confocal microscopy and immunofluorescence
Freshly recovered pinnae were fixed in PBS/4% paraformaldehyde on ice for 30 min then transferred to PBS/15% sucrose for a further 1h on ice. Fixed pinnae were then embedded in OCT medium (Sakura Finetek, Netherlands), and frozen at -80°C. Cryosections (6μm) obtained from frozen pinnae were simultaneously blocked and permeabilised in PBS supplemented with 5% goat serum (Sigma-Aldrich) and 0.05% saponin (Sigma-Aldrich) for 30min at room temperature. Sections were incubated in PBS/5% goat serum/0.05% saponin for 1h at room temperature with mAbs directly conjugated to various fluorescent labels: anti-CD4 (clone RM4-5), anti-MHC-II (IA-IE) (clone M5/114) and anti-CD11b (clone M1/70), or suitable isotype controls (all eBioscience) and then washed in PBS/0.05% saponin (3x, 5min each). Finally, sections were counter-stained with 2μg/ml DAPI (Life Technologies) for 5min and rinsed with distilled water. Slides were mounted in Prolong Gold AntiFade reagent (Life Technologies) prior to analysis using a Zeiss 710 inverse confocal microscope (Carl Zeiss, Cambridge, UK). All images were analysed using identical acquisition settings in Zeiss ZEN software. Image handling (including contrast adjustment) was performed on ImageJ (National Institute of Health).
Statistics
Analysis of variance (ANOVA) and then multiple comparisons tests (Bonferroni’s, Tukey’s, Sidak’s and Dunnett’s) were performed to establish significant differences between the groups (* = p<0.05, ** = p<0.01; *** = p<0.001, **** = p<0.0001) using the software package GraphPad Prism (GraphPad Software, Inc., La Jolla, California, USA). Similarly, unpaired two-tailed T tests were performed in selected experiments to compare experimental groups. Error bars represent the standard error of the mean (SEM), based on technical replicates for in vitro experiments, or biological replicates for in vivo experiments.
Accession numbers
Supporting Information
Zdroje
1. Rudikoff D, Lebwohl M (1998) Atopic dermatitis. Lancet 351 : 1715–1721. 9734903
2. Kedzierski L, Evans KJ (2014) Immune responses during cutaneous and visceral leishmaniasis. Parasitology: 1–19.
3. Hoerauf A, Pfarr K, Mand S, Debrah AY, Specht S (2011) Filariasis in Africa—treatment challenges and prospects. Clin Microbiol Infect 17 : 977–985. doi: 10.1111/j.1469-0691.2011.03586.x 21722251
4. Loukas A, Prociv P (2001) Immune responses in hookworm infections. Clin Microbiol Rev 14 : 689–703, table of contents. 11585781
5. Paveley RA, Aynsley SA, Cook PC, Turner JD, Mountford AP (2009) Fluorescent imaging of antigen released by a skin-invading helminth reveals differential uptake and activation profiles by antigen presenting cells. PLoS Negl Trop Dis 3: e528. doi: 10.1371/journal.pntd.0000528 19829705
6. Loser K, Beissert S (2007) Dendritic cells and T cells in the regulation of cutaneous immunity. Adv Dermatol 23 : 307–333. 18159907
7. Fahl SP, Coffey F, Wiest DL (2014) Origins of gammadelta T Cell Effector Subsets: A Riddle Wrapped in an Enigma. J Immunol 193 : 4289–4294. doi: 10.4049/jimmunol.1401813 25326547
8. Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, et al. (2013) Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol 14 : 564–573. doi: 10.1038/ni.2584 23603794
9. Heath WR, Carbone FR (2013) The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat Immunol 14 : 978–985. doi: 10.1038/ni.2680 24048119
10. Pasparakis M, Haase I, Nestle FO (2014) Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol 14 : 289–301. doi: 10.1038/nri3646 24722477
11. Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ (2009) Skin immune sentinels in health and disease. Nat Rev Immunol 9 : 679–691. doi: 10.1038/nri2622 19763149
12. Belkaid Y, Naik S (2013) Compartmentalized and systemic control of tissue immunity by commensals. Nat Immunol 14 : 646–653. doi: 10.1038/ni.2604 23778791
13. Grice EA, Segre JA (2011) The skin microbiome. Nat Rev Microbiol 9 : 244–253. doi: 10.1038/nrmicro2537 21407241
14. Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, et al. (2013) Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med 19 : 739–746. doi: 10.1038/nm.3179 23624599
15. Campanelli AP, Roselino AM, Cavassani KA, Pereira MS, Mortara RA, et al. (2006) CD4+CD25+ T cells in skin lesions of patients with cutaneous leishmaniasis exhibit phenotypic and functional characteristics of natural regulatory T cells. J Infect Dis 193 : 1313–1322. 16586370
16. Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL (2002) CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420 : 502–507. 12466842
17. Couper KN, Blount DG, Wilson MS, Hafalla JC, Belkaid Y, et al. (2008) IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog 4: e1000004. doi: 10.1371/journal.ppat.1000004 18401464
18. Cook PC, Aynsley SA, Turner JD, Jenkins GR, Van Rooijen N, et al. (2011) Multiple helminth infection of the skin causes lymphocyte hypo-responsiveness mediated by Th2 conditioning of dermal myeloid cells. PLoS Pathog 7: e1001323. doi: 10.1371/journal.ppat.1001323 21445234
19. Jenkins SJ, Hewitson JP, Jenkins GR, Mountford AP (2005) Modulation of the host's immune response by schistosome larvae. Parasite Immunol 27 : 385–393. 16179032
20. Mountford AP, Trottein F (2004) Schistosomes in the skin: a balance between immune priming and regulation. Trends Parasitol 20 : 221–226. 15105022
21. Dorsey CH, Cousin CE, Lewis FA, Stirewalt MA (2002) Ultrastructure of the Schistosoma mansoni cercaria. Micron 33 : 279–323. 11742750
22. Jenkins SJ, Hewitson JP, Ferret-Bernard S, Mountford AP (2005) Schistosome larvae stimulate macrophage cytokine production through TLR4-dependent and-independent pathways. Int Immunol 17 : 1409–1418. 16186163
23. Jenkins SJ, Mountford AP (2005) Dendritic cells activated with products released by schistosome larvae drive Th2-type immune responses, which can be inhibited by manipulation of CD40 costimulation. Infect Immun 73 : 395–402. 15618177
24. Bourke CD, Prendergast CT, Sanin DE, Oulton TE, Hall RJ, et al. (2015) Epidermal keratinocytes initiate wound healing and pro-inflammatory immune responses following percutaneous schistosome infection. Int J Parasitol.
25. Sanin DE, Mountford AP (2015) Sm16, a major component of Schistosoma mansoni cercarial excretory/secretory products, prevents macrophage classical activation and delays antigen processing. Parasit Vectors 8 : 1. 25561160
26. Turner JD, Meurs L, Dool P, Bourke CD, Mbow M, et al. (2013) Schistosome infection is associated with enhanced whole-blood IL-10 secretion in response to cercarial excretory/secretory products. Parasite Immunol 35 : 147–156. doi: 10.1111/pim.12028 23398537
27. Redpath SA, Fonseca NM, Perona-Wright G (2014) Protection and pathology during parasite infection: IL-10 strikes the balance. Parasite Immunol 36 : 233–252. doi: 10.1111/pim.12113 24666543
28. Wilson MS, Cheever AW, White SD, Thompson RW, Wynn TA (2011) IL-10 Blocks the Development of Resistance to Re-Infection with Schistosoma mansoni. PLoS Pathog 7: e1002171. doi: 10.1371/journal.ppat.1002171 21829367
29. Mentink-Kane MM, Cheever AW, Wilson MS, Madala SK, Beers LM, et al. (2011) Accelerated and progressive and lethal liver fibrosis in mice that lack interleukin (IL)-10, IL-12p40, and IL-13Ralpha2. Gastroenterology 141 : 2200–2209. doi: 10.1053/j.gastro.2011.08.008 21864478
30. Hesse M, Piccirillo CA, Belkaid Y, Prufer J, Mentink-Kane M, et al. (2004) The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol 172 : 3157–3166. 14978122
31. Wynn TA, Cheever AW, Williams ME, Hieny S, Caspar P, et al. (1998) IL-10 regulates liver pathology in acute murine Schistosomiasis mansoni but is not required for immune down-modulation of chronic disease. J Immunol 160 : 4473–4480. 9574553
32. Scott JT, Diakhate M, Vereecken K, Fall A, Diop M, et al. (2003) Human water contacts patterns in Schistosoma mansoni epidemic foci in northern Senegal change according to age, sex and place of residence, but are not related to intensity of infection. Trop Med Int Health 8 : 100–108. 12581433
33. Prendergast CT, Sanin DE, Cook PC, Mountford AP (2015) CD4+ T cell hypo-responsiveness after repeated exposure to Schistosoma mansoni larvae is dependent upon interleukin-10. Infect Immun.
34. Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara-Tejero M, et al. (2006) Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity 25 : 941–952. 17137799
35. Saraiva M, O'Garra A (2010) The regulation of IL-10 production by immune cells. Nat Rev Immunol 10 : 170–181. doi: 10.1038/nri2711 20154735
36. Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, et al. (2012) Compartmentalized control of skin immunity by resident commensals. Science 337 : 1115–1119. doi: 10.1126/science.1225152 22837383
37. Couper KN, Blount DG, Riley EM (2008) IL-10: the master regulator of immunity to infection. J Immunol 180 : 5771–5777. 18424693
38. Kullberg MC, Rothfuchs AG, Jankovic D, Caspar P, Wynn TA, et al. (2001) Helicobacter hepaticus-induced colitis in interleukin-10-deficient mice: cytokine requirements for the induction and maintenance of intestinal inflammation. Infect Immun 69 : 4232–4241. 11401959
39. Kane MM, Mosser DM (2001) The role of IL-10 in promoting disease progression in leishmaniasis. J Immunol 166 : 1141–1147. 11145695
40. Sadler CH, Rutitzky LI, Stadecker MJ, Wilson RA (2003) IL-10 is crucial for the transition from acute to chronic disease state during infection of mice with Schistosoma mansoni. Eur J Immunol 33 : 880–888. 12672053
41. Hogg KG, Kumkate S, Mountford AP (2003) IL-10 regulates early IL-12-mediated immune responses induced by the radiation-attenuated schistosome vaccine. Int Immunol 15 : 1451–1459. 14645154
42. Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, et al. (2009) Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity 31 : 209–219. doi: 10.1016/j.immuni.2009.05.012 19646904
43. Shoemaker J, Saraiva M, O'Garra A (2006) GATA-3 directly remodels the IL-10 locus independently of IL-4 in CD4+ T cells. J Immunol 176 : 3470–3479. 16517715
44. Chang HD, Helbig C, Tykocinski L, Kreher S, Koeck J, et al. (2007) Expression of IL-10 in Th memory lymphocytes is conditional on IL-12 or IL-4, unless the IL-10 gene is imprinted by GATA-3. Eur J Immunol 37 : 807–817. 17304625
45. Xu J, Yang Y, Qiu G, Lal G, Wu Z, et al. (2009) c-Maf regulates IL-10 expression during Th17 polarization. J Immunol 182 : 6226–6236. doi: 10.4049/jimmunol.0900123 19414776
46. Anderson CF, Oukka M, Kuchroo VJ, Sacks D (2007) CD4(+)CD25(-)Foxp3(-) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med 204 : 285–297. 17283207
47. Joss A, Akdis M, Faith A, Blaser K, Akdis CA (2000) IL-10 directly acts on T cells by specifically altering the CD28 co-stimulation pathway. Eur J Immunol 30 : 1683–1690. 10898505
48. Scheer S, Gross S, Mouahid G, Mone H, Lamers MC (2014) A novel tool to identify the relative contribution of lymphoid cell types that contribute to IL-10 production during the infection with Schistosoma mansoni: The TIGER index. J Immunol Methods.
49. Hoffmann KF, Cheever AW, Wynn TA (2000) IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol 164 : 6406–6416. 10843696
50. Satoguina J, Mempel M, Larbi J, Badusche M, Loliger C, et al. (2002) Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis). Microbes Infect 4 : 1291–1300. 12443893
51. McKee AS, Pearce EJ (2004) CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J Immunol 173 : 1224–1231. 15240714
52. Belkaid Y (2008) Role of Foxp3-positive regulatory T cells during infection. Eur J Immunol 38 : 918–921. doi: 10.1002/eji.200738120 18395860
53. Kassiotis G, Gray D, Kiafard Z, Zwirner J, Stockinger B (2006) Functional specialization of memory Th cells revealed by expression of integrin CD49b. J Immunol 177 : 968–975. 16818752
54. Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, et al. (2012) Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med 209 : 1723–1742, S1721. 22966001
55. Coomes SM, Pelly VS, Wilson MS (2013) Plasticity within the alphabeta(+)CD4(+) T-cell lineage: when, how and what for? Open Biol 3 : 120157. doi: 10.1098/rsob.120157 23345540
56. Kim BS, Kim IK, Park YJ, Kim YS, Kim YJ, et al. (2010) Conversion of Th2 memory cells into Foxp3+ regulatory T cells suppressing Th2-mediated allergic asthma. Proc Natl Acad Sci U S A 107 : 8742–8747. doi: 10.1073/pnas.0911756107 20421479
57. Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, et al. (2003) Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol 171 : 3262–3269. 12960356
58. Turner DL, Farber DL (2014) Mucosal resident memory CD4 T cells in protection and immunopathology. Front Immunol 5 : 331. doi: 10.3389/fimmu.2014.00331 25071787
59. Turner DL, Bickham KL, Thome JJ, Kim CY, D'Ovidio F, et al. (2014) Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol 7 : 501–510. doi: 10.1038/mi.2013.67 24064670
60. Bos JD, Hagenaars C, Das PK, Krieg SR, Voorn WJ, et al. (1989) Predominance of "memory" T cells (CD4+, CDw29+) over "naive" T cells (CD4+, CD45R+) in both normal and diseased human skin. Arch Dermatol Res 281 : 24–30. 2525009
61. Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, et al. (2006) The vast majority of CLA+ T cells are resident in normal skin. J Immunol 176 : 4431–4439. 16547281
62. Carbone FR, Mackay LK, Heath WR, Gebhardt T (2013) Distinct resident and recirculating memory T cell subsets in non-lymphoid tissues. Curr Opin Immunol 25 : 329–333. doi: 10.1016/j.coi.2013.05.007 23746791
63. Thawer SG, Horsnell WG, Darby M, Hoving JC, Dewals B, et al. (2014) Lung-resident CD4(+) T cells are sufficient for IL-4Ralpha-dependent recall immunity to Nippostrongylus brasiliensis infection. Mucosal Immunol 7 : 239–248. doi: 10.1038/mi.2013.40 23778354
64. Ziegler T, Rausch S, Steinfelder S, Klotz C, Hepworth MR, et al. (2015) A Novel Regulatory Macrophage Induced by a Helminth Molecule Instructs IL-10 in CD4+ T Cells and Protects against Mucosal Inflammation. J Immunol.
65. Staples KJ, Smallie T, Williams LM, Foey A, Burke B, et al. (2007) IL-10 induces IL-10 in primary human monocyte-derived macrophages via the transcription factor Stat3. J Immunol 178 : 4779–4785. 17404258
66. Mueller SN (2014) Skin DCs cluster for efficient T cell activation. Nat Immunol 15 : 1004–1005. doi: 10.1038/ni.3012 25329181
67. Macleod BL, Bedoui S, Hor JL, Mueller SN, Russell TA, et al. (2014) Distinct APC subtypes drive spatially segregated CD4+ and CD8+ T-cell effector activity during skin infection with HSV-1. PLoS Pathog 10: e1004303. doi: 10.1371/journal.ppat.1004303 25121482
68. Gabrysova L, Howes A, Saraiva M, O'Garra A (2014) The regulation of IL-10 expression. Curr Top Microbiol Immunol 380 : 157–190. doi: 10.1007/978-3-662-43492-5_8 25004818
69. Anderson CF, Stumhofer JS, Hunter CA, Sacks D (2009) IL-27 regulates IL-10 and IL-17 from CD4+ cells in nonhealing Leishmania major infection. J Immunol 183 : 4619–4627. doi: 10.4049/jimmunol.0804024 19748991
70. Bollrath J, Powrie FM (2013) Controlling the frontier: regulatory T-cells and intestinal homeostasis. Semin Immunol 25 : 352–357. doi: 10.1016/j.smim.2013.09.002 24184013
71. Nakamizo S, Egawa G, Honda T, Nakajima S, Belkaid Y, et al. (2014) Commensal bacteria and cutaneous immunity. Semin Immunopathol.
72. Shen W, Li W, Hixon JA, Bouladoux N, Belkaid Y, et al. (2014) Adaptive immunity to murine skin commensals. Proc Natl Acad Sci U S A 111: E2977–2986. doi: 10.1073/pnas.1401820111 25002505
73. Okwuosa VN, Osuala FO (1993) Toxicity of washing soaps to Schistosoma mansoni cercariae and effects of sublethal concentrations on infectivity in mice. Appl Parasitol 34 : 69–75. 8508221
74. de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, et al. (2003) Soil-transmitted helminth infections: updating the global picture. Trends Parasitol 19 : 547–551. 14642761
75. Kolarova L, Horak P, Skirnisson K, Mareckova H, Doenhoff M (2013) Cercarial dermatitis, a neglected allergic disease. Clin Rev Allergy Immunol 45 : 63–74. doi: 10.1007/s12016-012-8334-y 22915284
76. Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75 : 263–274. 8402911
77. Mountford AP, Hogg KG, Coulson PS, Brombacher F (2001) Signaling via interleukin-4 receptor alpha chain is required for successful vaccination against schistosomiasis in BALB/c mice. Infect Immun 69 : 228–236. 11119510
78. Mendez S, Reckling SK, Piccirillo CA, Sacks D, Belkaid Y (2004) Role for CD4(+) CD25(+) regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity. J Exp Med 200 : 201–210. 15263027
79. Mountford AP, Harrop R, Wilson RA (1995) Antigens derived from lung-stage larvae of Schistosoma mansoni are efficient stimulators of proliferation and gamma interferon secretion by lymphocytes from mice vaccinated with attenuated larvae. Infect Immun 63 : 1980–1986. 7729911
80. Hogg KG, Kumkate S, Anderson S, Mountford AP (2003) Interleukin-12 p40 secretion by cutaneous CD11c+ and F4/80+ cells is a major feature of the innate immune response in mice that develop Th1-mediated protective immunity to Schistosoma mansoni. Infect Immun 71 : 3563–3571. 12761141
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary TuberculosisČlánek Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic PlagueČlánek Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host SpeciesČlánek Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR SelectionČlánek Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus GenomesČlánek Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from FerrioxamineČlánek Remembering MumpsČlánek Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling PathwayČlánek Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 5- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Parasites and Their Heterophagic Appetite for Disease
- The Elusive Role of the Prion Protein and the Mechanism of Toxicity in Prion Disease
- Intestinal Colonization Dynamics of
- Activation of Typhi-Specific Regulatory T Cells in Typhoid Disease in a Wild-Type . Typhi Challenge Model
- The Engineering of a Novel Ligand in gH Confers to HSV an Expanded Tropism Independent of gD Activation by Its Receptors
- Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis
- Group Selection and Contribution of Minority Variants during Virus Adaptation Determines Virus Fitness and Phenotype
- Phosphatidic Acid Produced by Phospholipase D Promotes RNA Replication of a Plant RNA Virus
- A Ribonucleoprotein Complex Protects the Interleukin-6 mRNA from Degradation by Distinct Herpesviral Endonucleases
- Characterization of Transcriptional Responses to Different Aphid Species Reveals Genes that Contribute to Host Susceptibility and Non-host Resistance
- Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic Plague
- Natural Killer Cell Sensing of Infected Cells Compensates for MyD88 Deficiency but Not IFN-I Activity in Resistance to Mouse Cytomegalovirus
- Manipulation of the Xanthophyll Cycle Increases Plant Susceptibility to
- Ly6C Monocytes Regulate Parasite-Induced Liver Inflammation by Inducing the Differentiation of Pathogenic Ly6C Monocytes into Macrophages
- Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host Species
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Experimental Evolution of an RNA Virus in Wild Birds: Evidence for Host-Dependent Impacts on Population Structure and Competitive Fitness
- Inhibition and Reversal of Microbial Attachment by an Antibody with Parasteric Activity against the FimH Adhesin of Uropathogenic .
- The EBNA-2 N-Terminal Transactivation Domain Folds into a Dimeric Structure Required for Target Gene Activation
- Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR Selection
- The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive via Activation of α5β1 Integrin at the Macrophage-Amebae Intercellular Junction
- Sequential Conformational Changes in the Morbillivirus Attachment Protein Initiate the Membrane Fusion Process
- A Two-Component DNA-Prime/Protein-Boost Vaccination Strategy for Eliciting Long-Term, Protective T Cell Immunity against
- cAMP-Signalling Regulates Gametocyte-Infected Erythrocyte Deformability Required for Malaria Parasite Transmission
- Response Regulator VxrB Controls Colonization and Regulates the Type VI Secretion System
- Evidence for a Novel Mechanism of Influenza Virus-Induced Type I Interferon Expression by a Defective RNA-Encoded Protein
- Dust Devil: The Life and Times of the Fungus That Causes Valley Fever
- TNF-α Induced by Hepatitis C Virus via TLR7 and TLR8 in Hepatocytes Supports Interferon Signaling via an Autocrine Mechanism
- The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen,
- L-Rhamnosylation of Wall Teichoic Acids Promotes Resistance to Antimicrobial Peptides by Delaying Interaction with the Membrane
- Rapid Sequestration of by Neutrophils Contributes to the Development of Chronic Lesion
- Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus Genomes
- The Expression of Functional Vpx during Pathogenic SIVmac Infections of Rhesus Macaques Suppresses SAMHD1 in CD4 Memory T Cells
- Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from Ferrioxamine
- TRAF1 Coordinates Polyubiquitin Signaling to Enhance Epstein-Barr Virus LMP1-Mediated Growth and Survival Pathway Activation
- Vaccine-Elicited Tier 2 HIV-1 Neutralizing Antibodies Bind to Quaternary Epitopes Involving Glycan-Deficient Patches Proximal to the CD4 Binding Site
- Remembering Mumps
- The Role of Horizontal Gene Transfer in the Evolution of the Oomycetes
- Advances and Challenges in Computational Prediction of Effectors from Plant Pathogenic Fungi
- Investigating Fungal Outbreaks in the 21st Century
- Systems Biology for Biologists
- How Does the Dinoflagellate Parasite Outsmart the Immune System of Its Crustacean Hosts?
- FCRL5 Delineates Functionally Impaired Memory B Cells Associated with Exposure
- Phospholipase D1 Couples CD4 T Cell Activation to c-Myc-Dependent Deoxyribonucleotide Pool Expansion and HIV-1 Replication
- Influenza A Virus on Oceanic Islands: Host and Viral Diversity in Seabirds in the Western Indian Ocean
- Geometric Constraints Dominate the Antigenic Evolution of Influenza H3N2 Hemagglutinin
- Widespread Recombination, Reassortment, and Transmission of Unbalanced Compound Viral Genotypes in Natural Arenavirus Infections
- Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity
- A Single Protein S-acyl Transferase Acts through Diverse Substrates to Determine Cryptococcal Morphology, Stress Tolerance, and Pathogenic Outcome
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
- Mechanisms of Stage-Transcending Protection Following Immunization of Mice with Late Liver Stage-Arresting Genetically Attenuated Malaria Parasites
- The Myelin and Lymphocyte Protein MAL Is Required for Binding and Activity of ε-Toxin
- Genome-Wide Identification of the Target Genes of AP2-O, a AP2-Family Transcription Factor
- An Atypical Mitochondrial Carrier That Mediates Drug Action in
- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Helminth Infection and Commensal Microbiota Drive Early IL-10 Production in the Skin by CD4 T Cells That Are Functionally Suppressive
- Circulating Pneumolysin Is a Potent Inducer of Cardiac Injury during Pneumococcal Infection
- ExoT Induces Atypical Anoikis Apoptosis in Target Host Cells by Transforming Crk Adaptor Protein into a Cytotoxin
- Discovery of a Small Non-AUG-Initiated ORF in Poleroviruses and Luteoviruses That Is Required for Long-Distance Movement
- Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
- Varicella Viruses Inhibit Interferon-Stimulated JAK-STAT Signaling through Multiple Mechanisms
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Recovery of Recombinant Crimean Congo Hemorrhagic Fever Virus Reveals a Function for Non-structural Glycoproteins Cleavage by Furin
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




