-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity
Nearly 1 million deaths occur annually as a result of complications associated with P. falciparum infection, with children younger than 5 being the most susceptible age group. Earlier studies have demonstrated that children co-infected with P. falciparum and Epstein-Barr virus (EBV) have impaired immune responses to control EBV, and this can result in the development of a jaw tumor called endemic Burkitt’s lymphoma (eBL). It is not known if there is any impact of acute EBV infection on the generation of anti-malarial immunity. We have used mouse models of EBV [murine gammaherpesvirus 68 (MHV68)] and malaria (P. yoelii XNL) to demonstrate that acute gammaherpesvirus infection can impair the generation of antibodies that control Plasmodium parasitemia, in turn causing a non-lethal P. yoelii XNL infection to become lethal. We identify a critical role for the MHV68 M2 protein in mediating the suppressive effect of acute MHV68 infection on the generation of humoral immunity to a secondary malaria infection. This work demonstrates that gammaherpesvirus infections can suppress the generation of an effective anti-malaria immune response and suggests that acute EBV infection should be investigated as a risk factor for the development of severe malaria in young children.
Published in the journal: . PLoS Pathog 11(5): e32767. doi:10.1371/journal.ppat.1004858
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004858Summary
Nearly 1 million deaths occur annually as a result of complications associated with P. falciparum infection, with children younger than 5 being the most susceptible age group. Earlier studies have demonstrated that children co-infected with P. falciparum and Epstein-Barr virus (EBV) have impaired immune responses to control EBV, and this can result in the development of a jaw tumor called endemic Burkitt’s lymphoma (eBL). It is not known if there is any impact of acute EBV infection on the generation of anti-malarial immunity. We have used mouse models of EBV [murine gammaherpesvirus 68 (MHV68)] and malaria (P. yoelii XNL) to demonstrate that acute gammaherpesvirus infection can impair the generation of antibodies that control Plasmodium parasitemia, in turn causing a non-lethal P. yoelii XNL infection to become lethal. We identify a critical role for the MHV68 M2 protein in mediating the suppressive effect of acute MHV68 infection on the generation of humoral immunity to a secondary malaria infection. This work demonstrates that gammaherpesvirus infections can suppress the generation of an effective anti-malaria immune response and suggests that acute EBV infection should be investigated as a risk factor for the development of severe malaria in young children.
Introduction
Nearly 1 million individuals die annually as a result of severe malaria, largely children under the age of 5 [1]. In regions that are endemic for Plasmodium falciparum transmission, mathematical modeling data suggests that immunity to severe non-cerebral malaria requiring hospitalization in children may be attained after 1–2 infections [2]. However, it is not fully understood why some children are unable to acquire immunity to severe lethal disease. Multiple factors may account for this (reviewed in [3,4]) and the presence of co-infecting pathogens in the host could be one such factor. In Sub-Saharan Africa, infants are often co-infected with Epstein-Barr virus (EBV), a gammaherpesvirus that infects B cells and maintains latency throughout the lifetime of the host [5]. Children are often seropositive to EBV by the age of 6 months in this region of the world [6] and it is well established that children infected with EBV living in areas endemic for transmission of P. falciparum have increased chances of developing endemic Burkitt’s Lymphoma (eBL). eBL is the most lethal of childhood cancers in equatorial Africa, with the highest prevalence in children aged 5–9 years old. eBL is characterized by a c-myc translocation that results in over-expression of the oncogene (reviewed in [7]). It is postulated that repeated infections with P. falciparum results in a weakened anti-viral CD8 T-cell response that allows for the outgrowth of transformed B cells [8–11].
Despite the compelling evidence indicating a role for P. falciparum in modulating the immune responses that control EBV infection, little is known regarding the impact of acute EBV infection on the development and functionality of the immune responses that control P. falciparum infection. It is well appreciated that the humoral response is protective during Plasmodium infection. Passive immunization of children in The Gambia [12] and adults in Thailand [13] with P. falciparum hyper-immune serum from adult donors living in Sub-Saharan Africa allowed for control of peripheral parasitemia. Additionally, numerous studies in humans have identified a role for increased breadth and diversity in the anti-Plasmodium humoral response that provides a protective advantage during clinical malaria [14–17]. Although acute EBV infection is generally asymptomatic in young children [18], virus-induced humoral immune deficiencies have been observed in one case of co-infection with a secondary pathogen [19] and in young adults experiencing a primary EBV infection and manifesting symptoms of Infectious Mononucleosis (IM) [20,21]. Although there are few reports of this phenomenon, these documented cases provide key evidence of the ability of EBV to suppress humoral responses during the acute phase of infection. This data, combined with the known role of antibody in resolution of P. falciparum parasitemia (refs), suggests that overlapping acute EBV infection could suppress anti-malarial humoral responses in some children and thus be a contributing factor in the development of severe malarial disease.
Acute murine gammaherpesvirus 68 (MHV68) infections of mice, like acute EBV infections in humans, can induce a transient immune suppression of the humoral response during secondary antigenic challenge [22]. Using MHV68 as a model for acute EBV infection, we have investigated whether gammaherpesvirus infection can suppress the humoral immune response to a secondary malarial infection. We have used the well-established non-lethal murine models of malaria infection P. yoelii XNL and P. chabaudi AS, and determined that acute gammaherpesvirus infection can suppress the anti-malarial humoral response during co-infection with either of these Plasmodium infections. This suppression results in loss of control of peripheral parasitemia in P. yoelii XNL, but not P. chabaudi AS, infection and transforms the non-lethal infection into a lethal one. This is in agreement with the course of infection in B cell deficient μMT mice where P. chabaudi, but not P. yoelii, parasitemia is controlled [23]. The reduced anti-malarial antibody response during co-infection was accompanied with a virus induced failure to maintain the T follicular helper cells subset in the spleen. As such, loss of this critical T helper cell in the germinal center follicle resulted in loss of germinal center B cells and a failure to develop sufficient plasma cells to produce anti-malarial antibody.
We have identified that the MHV68-derived latency associated protein M2 is essential for the failure of co-infected animals to mount anti-malarial humoral responses and that this effect in the mouse model lasted up to 30 days. This identifies the acute phase of infection as necessary for virus-mediated immune suppression of the humoral response. In terms of EBV infection in humans, one case study identified the asymptomatic acute phase of EBV infection to induce immune suppression that can last up to 4 weeks [19]. This potentially gives a 4 week time window of humoral suppression that could substantially influence the outcome of a P. falciparum infection. As such, our data provides novel and compelling evidence for a need to evaluate primary acute EBV infections as a potential risk factor in the development of non-cerebral severe malaria.
Results
Acute MHV68 infection impairs the development of malaria specific antibody responses
The humoral response is generally considered to be a critical effector mechanism for controlling peripheral parasitemia in both human and mouse malaria infection [23]. To understand the impact of acute MHV68 infection on the humoral response to a Plasmodium infection, we infected C57BL/6 mice with 1000 PFU of MHV68 intra-nasally (IN) on day -7 and 105 parasitized red blood cells (pRBCs) of P. yoelii XNL or P. chabaudi AS intra-peritoneally (IP) on day 0 (Fig 1A). Single infection with either of the Plasmodium species was non-lethal but, in the context of an MHV68 infected mouse, P. yoelii XNL, but not P. chabaudi AS, caused 100% lethality (Fig 1B). This corroborates a previous observation by Haque et al. who also observed lethality during MHV68 and P. yoelii XNL co-infection [24]. Knowing the importance of a robust humoral response in protection during Plasmodium infection [12,13], we hypothesized that MHV68 may impair the generation of an effective antibody response to control P. yoelii XNL parasitemia. Total IgM levels were reduced in co-infected animals relative to singly infected animals in P. yoelii XNL co-infection at day 23 post malaria infection (Mann Whitney-U test p<0.05) and at days 11 and 15 post malaria infection in P. chabaudi AS co-infection (both Mann Whitney-U test p<0.05) (Fig 1B and 1C). Total IgG levels were similarly affected and reduced at day 23 post malaria infection in P. yoelii XNL co-infected animals and at day 11 post malaria infection in P. chabaudi AS co-infected animals (both Mann Whitney U-test p<0.05; Fig 1B and 1D). This reduction in total IgG was mirrored in parasite-reactive IgG in both co-infection models compared with the relevant singly-infected animals (both Mann Whitney U-test p<0.05; Fig 1E and 1F). This observation shows that MHV68 acute infection can suppress the humoral response to malaria infection in mice. In one of the mouse malaria models tested (P. yoelii XNL), this suppression is correlated with the transformation of a non-lethal malaria infection into a lethal one (Fig 1B). This observation prompted us to evaluate how suppression of the anti-malaria humoral response impacts the control of peripheral parasitemia and to investigate why the acute phase of MHV68 co-infection impacted the virulence of P. yoelii XNL, but not P. chabaudi AS infection.
Fig. 1. MHV68 co-infection with the non-lethal P. yoelii XNL in C57BL/6 results in lethal malarial disease and suppressed Plasmodium specific IgG response. 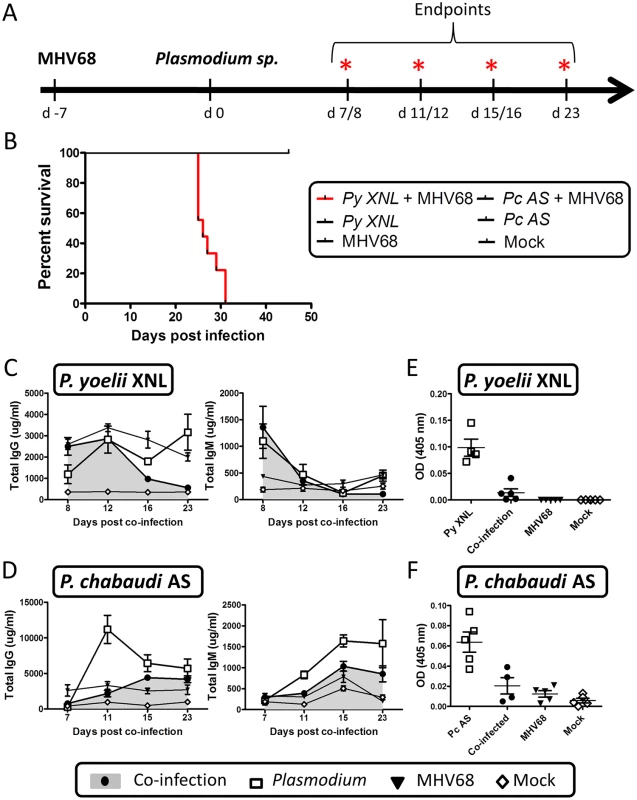
(A) Timeline of infection. 6–8 week old C57BL/6 mice were infected with 1000 PFU of MHV68 on day -7 followed by infection with 105pRBCs of non-lethal P. yoelii XNL or P. chabaudi AS. Infections consisted of 5 experimental groups: MHV68 + Plasmodium, Plasmodium, MHV68 or mock infected. Each experimental group consisted of n = 5 and was repeated twice. Animals were sacrificed at days 8, 12, 16 and 23 post P. yoelii XNL infection or day 7, 11, 15 and 23 post P. chabaudi AS infection for collection of spleen, lung and blood. (B) Survival analysis of animals co-infected with MHV68 and P. yoelii XNL or P. chabaudi AS. Total IgG and IgM levels in serum in (C) P. yoelii XNL (Day 23 IgG—P. yoelii vs co-infected: p<0.05 Mann Whitney U-test) or (D) P. chabaudi AS co-infection model (Day 11 IgG—P. chabaudi vs co-infected: p<0.05 Mann Whitney U-test). Parasite specific IgG levels in serum during (E) P. yoelii XNL (day 23 post infection, P. yoelii vs co-infected: p<0.05 Mann Whitney U-test) or (F) P. chabaudi AS (day 11 post infection, P. chabaudi vs co-infected: p<0.05 Mann Whitney U-test) co-infection. Acute MHV68 co-infection leads to loss of control of P. yoelii XNL, but not P. chabaudi AS parasitemia
To extend the above observations, we evaluated the impact of an acute MHV68 infection on clearance of the primary peak of parasitemia during secondary challenge with Plasmodium. During the initial stages of malaria infection, MHV68 and P. yoelii XNL co-infected animals had comparable peripheral parasitemia when compared with P. yoelii XNL singly infected animals (Fig 2A). However, by day 17 post-infection, singly infected animals began to control peripheral parasitemia while co-infected animals were unable to do so (Fig 2A; Mann Whitney-U test p<0.05). There was a trend for co-infected animals to have more severe malarial anemia during P. yoelii XNL and MHV68 co-infection compared to P. yoelii XNL singly infected animals, but this did not reach statistical significance (Fig 2B; Mann Whitney-U test on the area above the curve p = 0.056). There was no difference in P. chabaudi AS parasitemia or anemia in singly infected or MHV68 co-infected groups (Mann Whitney-U test on the area under or above the curve respectively p>0.05) (Fig 2A and 2B).
Fig. 2. P. yoelii XNL requires Plasmodium specific IgG response to clear primary peak of parasitemia. 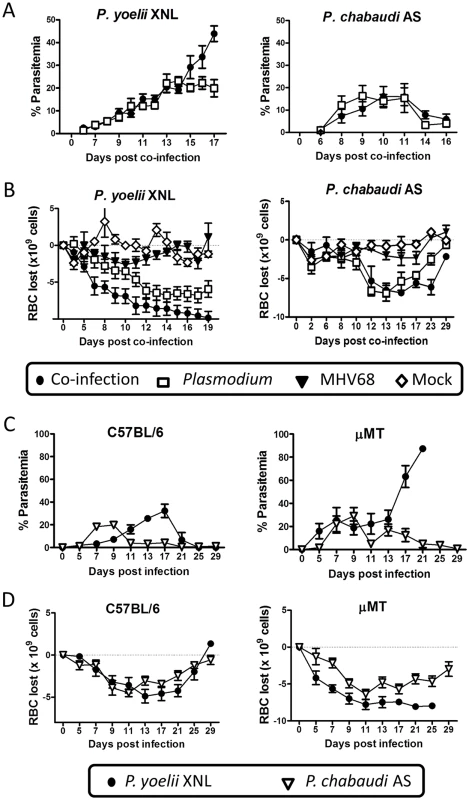
(A) Percent parasitemia in the periphery during P. yoelii XNL (p<0.05; area under curve, Mann Whitney U-test) or P. chabaudi AS co-infection models (p>0.05; area under the curve, Mann Whitney U-test). (B) Anemia during P. yoelii XNL (p>0.05; area over curve, Mann Whitney U-test, P. yoelii vs. co-infected) or P. chabaudi AS co-infection (p>0.05; area over curve, Mann Whitney U-test, P. chabaudi vs. co-infected). (C) Percent parasitemia in periphery during infection of single P. yoelii XNL or P. chabaudi AS in C57BL/6 or μMT (B cell-deficient) mice. (D) Anemia during infection of single P. yoelii XNL or P. chabaudi AS in C57BL/6 or μMT mice. It is possible that elevated persistent replication of MHV68 in the lungs of co-infected mice could contribe to the lethality of P. yoelii XNL infection. However, although we observed that levels of preformed virus were significantly higher in the co-infected animals compared to singly infected animals (Mann Whitney-U test on area under the curve p <0.05 at day 23 post P. yoelii XNL co-infection and day 15 post P. chabaudi AS co-infection MHV68 infection; S1 Fig, panel A), we also observed a similar increase in viral titer in the lungs of P. chabaudi AS and MHV68 co-infected animals which was not lethal (S1 Fig, panel B). The elevated persistent viral replication in the lungs of co-infected animals correlated to a decrease in virus-specific IgG in co-infected mice compared to mice infected with MHV68 alone (S1 Fig, panels C & D). Notably, the virus specific antibody response is critical in long term control of viral replication [25]. Assessment of lung tissue at day 23 post co-infection with P. yoelii XNL and MHV68 indicated increased Type II hyperplasia, which is indicative of interstitial pneumonia (S1 Fig, panel E) and hemosiderin deposition, as compared to animals singly infected with MHV68. In contrast, single MHV68 infection caused greater levels of inflammation in the lung as defined by larger numbers of histiocytes (macrophages and dendritic cells) in the lung tissue (S1 Fig, panel E). Animals co-infected with P. chabaudi AS and MHV68 showed little to no obvious lung tissue damage as compared to single MHV68 infection (S1 Fig, panel E). Thus, at this point we cannot rule out the possibility that increased persistent replication of MHV68 contributes to the lethality of P. yoelii in co-infected mice.
Based on previously published work [23,26], we hypothesized that the reason why the abolishment of the anti-malarial humoral immunity is lethal for P. yoelii XNL, but not P. chabaudi AS infected mice, is because each infection has a differential requirement for a parasite specific antibody response to control the primary peak of parasitemia. To test this hypothesis, we compared the course of infection for both species of rodent malaria in μMT (B cell-deficient) and C57BL/6 mice. We observed that, in the absence of B cells, P. chabaudi AS infected animals could control the primary peak of parasitemia, whereas, P. yoelii XNL infected animals developed fulminant parasitemia (Fig 2C). This was also mirrored in the development of a more severe SMA in P. yoelii XNL co-infected animals (Fig 2D). This data supports the hypothesis that suppression of the anti-Plasmodium humoral response in MHV68 co-infected animals is a key factor in why MHV68 co-infection alters the lethality of P. yoelii XNL, but not P. chabaudi AS, malaria infection.
MHV68 impairs the formation of plasma cells in response to secondary malaria infection
We hypothesized that the impairment of the anti-malarial humoral response in MHV68 infected animals was due to a defect in the generation or function of plasma cells upon infection with malaria. We assessed the populations of plasma cells and germinal center (GC) B cells (a precursor of memory and plasma cells) in the spleen at different times post-infection with malaria. Mice that were co-infected with MHV68 and malaria had a comparable number of GC B cells compared to singly infected animals at day 7–8 post infection with malaria (S2 and S3 Figs; co-infected compared with singly infected animals Mann Whitney-U test p>0.05 in both models). However, by day 12 post-infection with P. yoelii XNL or day 15 post-infection with P. chabaudi AS, GC B cell numbers were significantly reduced as compared to a single Plasmodium infection (both Mann Whitney-U test p<0.05). At day 8 post co-infection, the GC B cells present were located in T cell-containing germinal centers in representative P. yoelii XNL singly infected and MHV68 co-infected animals (Fig 3C), suggesting that the defect was in the maintenance of the germinal center rather than a follicular structural defect. The impaired GC response correlated with greatly reduced numbers of plasma cells by day 11/12 post-infection with malaria in MHV68 co-infected animals compared with malaria singly infected animals (Mann Whitney-U test p<0.05 in both cases). This observation suggests that the defect in anti-malarial antibody responses to the Plasmodium infection in MHV68 co-infected animals is likely due to a defect in the generation and/or maintenance of GC B cells.
Fig. 3. MHV68 suppresses splenic B cell responses during co-infection with Plasmodium. 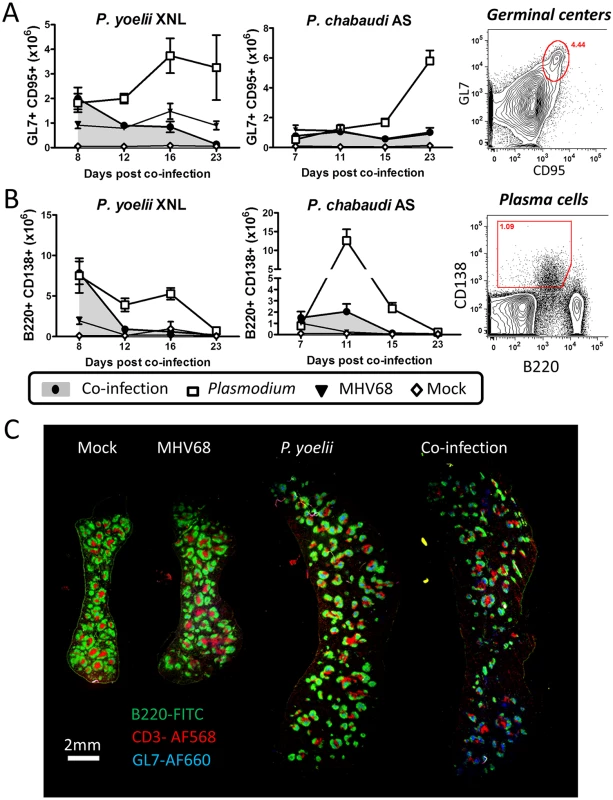
The timeline and experimental set up was identical to that shown in Fig 1A. (A) Absolute numbers of splenic GC B cell populations (B220+ GL7+ CD95+) during P. yoelii XNL and P. chabaudi AS co-infection models with representative gating strategy (Day 12 post P. yoelii or Day 15 post P. chabaudi; Plasmodium vs. co-infected, p<0.05, Mann Whitney U-test). (B) Absolute numbers of splenic plasma cell populations (CD3- B220int CD138+) during P. yoelii XNL AND P. chabaudi AS co-infection models with representative gating strategy (Day 12 post P. yoelii or Day 11 post P. chabaudi; Plasmodium vs. co-infected, p<0.05, Mann Whitney U-test). (C) Spleen section for mock infected, MHV68 infected, P. yoelii XNL infected and MHV68 and P. yoelii XNL co-infected animals at day 8 post infection with P. yoelii XNL (or day 15 post-infection with MHV68). Green: B220-FITC (B cells), Blue: GL7-AF660 (Germinal center B cells) and Red: CD3-AF568 (T cells). Impaired germinal center maintenance is correlated with reduced Tfh survival
Since germinal center formation and maintenance is dependent on CD4+ T follicular helper cells (Tfh) (reviewed in [27]), we hypothesized that GC B cell numbers may not be sustained if there are detrimental changes in the splenic Tfh population. One notable observation from the representative spleen sections shown in Fig 3C is that the day 8 co-infected mouse appears to have a reduced number of CD3+ T cells within the germinal center follicles compared to the day 8 P. yoelii XNL singly infected mouse (Fig 3C). Thus, although levels of GC B cells are comparable to a single P. yoelii XNL infection at this early time point (Fig 3A), a reduction in CD3+ T cells, which would include the Tfh subset, may explain the subsequent decay of the splenic GC population. As such, we next evaluated the T cell repertoire in the spleen that is required for germinal center formation and survival.
We analyzed how total Tfh cells (CD4+ CXCR5+ PD-1+), activated/antigen specific Tfh (CD4+ PD-1+ CD44hi CXCR5+) and germinal center Tfh (GL7+ CXCR5+) cells (Fig 4A) changed over time in the spleen. It was evident that by 23 days post-malaria infection there were defects in the maintenance of all three Tfh subsets in co-infected animals when compared to P. yoelii XNL singly infected animals (Fig 4B; Mann Whitney-U test p<0.05 in all cases). This was mirrored in the MHV68 and P. chabaudi co-infected animals (Fig 4C). The MHV68 and P. yoelii XNL co-infected animals displayed defects in the total and activated Tfh subsets as early as 12 days post co-infection (Fig 4B; Mann Whitney-U test p<0.05 in all cases) indicating that MHV68 co-infected animals are capable of generating Tfh responses within the first week after co-infection, but they failed to maintain this cellular subset. The MHV68 induced defect in the Tfh population by day 12 post P. yoelii XNL co-infection (Fig 4B) also corresponds to the time point at which the GC B cell population begins to decline (Fig 3A). This also applies to the decrease in the Tfh population by day 15 post P. chabaudi AS challenge (Fig 4C) in MHV68 co-infected animals compared with P. chabaudi AS singly-infected animals and the corresponding reduction in GC B cell numbers (Fig 3A). This correlation supports the hypothesis that the failure to maintain the population of GC B cells in MHV68 co-infected mice is correlated with a failure to maintain a Tfh cell population.
Fig. 4. MHV68 and Plasmodium co-infection results in defective splenic T follicular helper (Tfh) response. 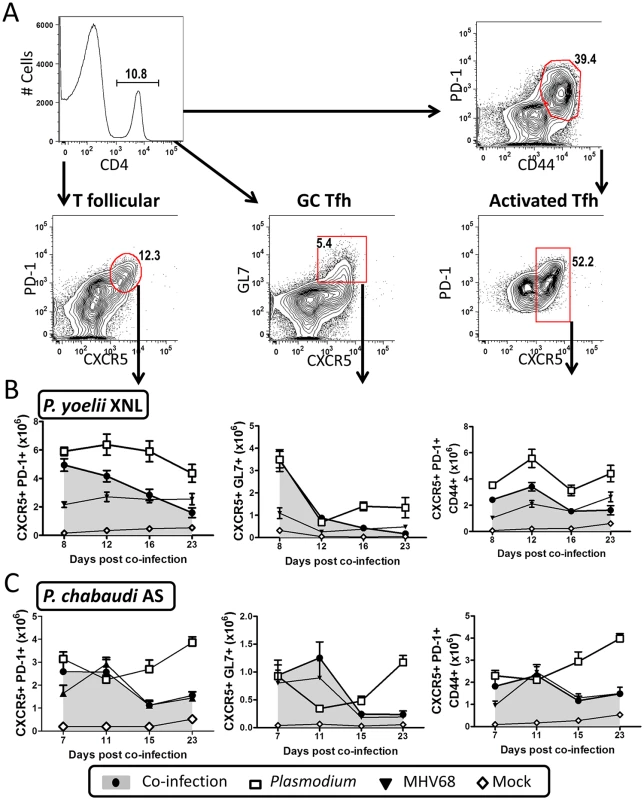
The timeline and experimental set up was identical to that shown in Fig 1A. (A) Representative flow plots for gating strategies used to define the global Tfh population (CD4+ PD-1+ CXCR5+), germinal center Tfh (CD4+ GL7+ CXCR5+) and activated/antigen specific Tfh (CD4+ CD44+ PD-1+ CXCR5+). (B) Absolute values for all three Tfh subsets are plotted for the P. yoelii XNL (Day 23, all Tfh subsets, P. yoelii vs. co-infected, p<0.05 Mann Whitney U-test) or (C) P. chabaudi co-infection models at multiple time points (Day 23, all Tfh subsets, P. chabaudi vs. co-infected, p<0.05 Mann Whitney U-test). The defective anti-malarial humoral response induced by an acute pre-existing MHV68 infection may have been a result of alteration of other T cell subsets known to be involved in generating an antibody response, or in the control of parasitemia during malaria infection. To address this, we enumerated numbers of regulatory T cells (Tregs) that can negatively regulate the Tfh response [28] and CD4+T cells that co-express IFN-γ and IL-10 which play an important role in the control of P. yoelii parasitemia [29]. Acute MHV68 co-infection did not lead to an increase in these subsets in response to malaria infection (S3 and S4 Figs). In fact, Treg numbers were significantly decreased by MHV68 co-infection at day 15/16 post-infection with malaria in both models (S3 Fig, panel B; Mann Whitney-U test p<0.05 in both cases), which we reasoned should enhance, rather than suppress the Tfh response [28], making it an unlikely explanation for suppression of anti-malarial humoral immunity in MHV68 co-infected mice. To further assess the consequences of Tfh deficiency on P. yoelii XNL compared with P. chabaudi AS malaria infection, we infected IL-21R-/ - mice which can generate comparable levels of Tfh cells and germinal center responses during the early stages of an LCMV infection (around 15 days), but fail to maintain both Tfh and germinal center responses after 2 weeks of infection [30], recapitulating the immunological phenotype of MHV68 and malaria co-infected animals. Similar to μMT mice, P. yoelii XNL infection of IL21R-/ - mice was lethal and this was associated with impaired control of parasitemia and a concomitant increase in the severity of SMA (S5 Fig, panels A and B). In contrast, IL-21R deficiency did not affect the kinetics of a P. chabaudi AS single infection (S5 Fig). Collectively, this data supports the hypothesis that the failure of MHV68 co-infected animals to maintain the Tfh cellular subset in the spleen is associated with a defective humoral response against a secondary malaria infection, which in the case of a P. yoelii XNL infection, results in lethality.
Acute, but not latent MHV68 infection, is required for exacerbated malarial disease during co-infection
Given that all children in an endemic area would likely be co-infected with EBV and malaria by the time they are 2 years of age, we hypothesized that gammaherpesvirus induced suppression of the establishment of an anti-malarial humoral responses would depend on the timing of co-infection. Gammaherpesvirus infections, such as EBV and MHV68, can be divided into 2 distinct phases: a lytic phase in which there is acute virus replication and dissemination, followed by the establishment of viral latency in B cells and some other cell types. Latent infection consists of a quiescent phase of viral gene expression, but still results in an underlying inflammatory response [5]. Therefore we investigated whether latent infection as well as acute infection resulted in a defect in the development of the anti-malarial humoral response during co-infection. C57BL/6 mice were infected with MHV68 at 60 (latency), 30 (latency), 15 (late lytic - acute) or 7 (early lytic - acute) days prior to being co-infected with P. yoelii XNL (Fig 5A). We measured the number of GC B cells and plasma cells (Fig 5B), the levels of circulating P. yoelii XNL specific IgG responses (Fig 5C) and the numbers of Tfh cellular subsets in the spleen (Fig 5D).
Fig. 5. Acute, but not latent, MHV68 infection results in suppressed humoral response. 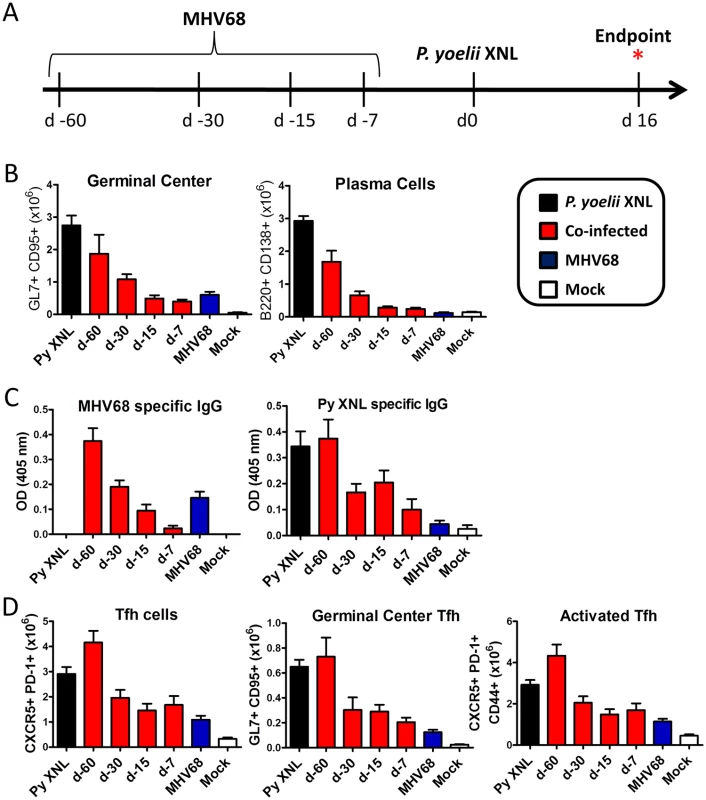
(A) Timeline of infection. C57BL/6 mice were infected with 1000 PFU of MHV68 IN at day -60, -30, -15 or -7 and challenged with 105 pRBCs on day 0. Absolute number of (B) splenic GC B cell (B220+ GL7+ CD95+) and plasma cell (CD3- B220int CD138+) populations at day 16 post P. yoelii XNL infection (For GC and PC: Day -7 and Day -15 co-infected vs. P. yoelii, Kruskal Wallis p<0.05; Dunn’s pairwise comparison test p<0.05/ Day -30 co-infected vs. P. yoelii, Kruskal Wallis p<0.05; Dunn’s pairwise comparison test p>0.05). (C) MHV68 and P. yoelii XNL specific IgG responses at day 16 post P. yoelii XNL infection (Day -7 and Day -15 co-infected vs. P. yoelii, Kruskal Wallis p<0.05; Dunn’s pairwise comparison test p<0.05/ Day -30 co-infected vs. P. yoelii, Kruskal Wallis p<0.05; Dunn’s pairwise comparison test p>0.05). (D) Global Tfh population (CD4+ PD-1+ CXCR5+), germinal center Tfh (CD4+ GL7+ CXCR5+) and activated/antigen specific Tfh (CD4+ CD44+ PD-1+ CXCR5+) in the spleen at day 16 post P. yoelii XNL infection. As expected, animals infected at day -7 prior to P. yoelii XNL infection showed a marked reduction in all of these parameters compared to mice singly infected with P. yoelii XNL (Kruskal-Wallis p<0.05; Dunn’s pairwise comparison p<0.05 in all cases). This pattern was repeated in animals infected with MHV68 at 15 days prior to P. yoelii XNL infection (Kruskal-Wallis p<0.05; Dunn’s pairwise comparison p<0.05 in all cases) and there was a trend towards this pattern in mice infected with MHV68 for 30 days prior to P. yoelii XNL infection that did not reach significance (Kruskal-Wallis p<0.05; Dunn’s pairwise comparison p>0.05 in all cases). However, it is clear that the suppressive effects of an acute MHV68 infection were not present in mice that were latently infected with MHV68 60 days prior to infection with P. yoelii XNL. This data suggests that an established latent MHV68 infection does not suppress the generation of the humoral immune response to an incoming malaria infection in mice.
The mechanism by which acute MHV68 infection can suppress the generation of a humoral response to malaria during co-infection is unclear, but GC B cells from animals with MHV68 co-infection 15 or 7 days prior to P. yoelii XNL infection had increased expression of PD-L1 (S6 Fig), a ligand for PD-1 that negatively regulates Tfh expansion [31], relative to P. yoelii XNL singly-infected animals (Kruskal-Wallis p<0.05; Dunn’s pairwise comparison p>0.05 in both cases). Thus, one possibility is that virus induced PD-L1 expression on GC B cells may contribute to the loss of Tfh functionality during co-infection.
The MHV68 M2 gene product plays a role in suppression of anti-parasitic humoral responses
The data presented above clearly points to a suppressed humoral response as being a critical mediator of lethality during P. yoelii XNL co-infection with MHV68. As such, we hypothesized that if we could restore the parasite specific humoral response, we could rescue mice from lethality caused by an MHV68 and P. yoelii XNL co-infection. It has previously been shown that the M2 gene product of MHV68 can induce significant levels of IL-10 production from B cells and modulate the surface phenotype of infected B cells [32,33]. IL-10 is known to have multiple immunomodulatory roles, one of which is to negatively regulate T cell responses [34,35]. We hypothesized that one reason Tfh cells did not function correctly could be due to M2-induced suppression, a hypothesis supported by published work showing that in the absence of M2, mice are able to mount enhanced virus specific CD8+ T cell responses [33]. Given that the downstream effect of MHV68 induced immunosuppression was a result of impaired anti-malarial antibody responses, we initially asked whether levels of virus specific IgG responses were enhanced in the absence of M2 expression. To avoid a known defect in the establishment of splenic infection following intranasal inoculation of M2-deficient MHV68 mutants [36]we opted to infect mice with the same dose of virus (1000 PFU) as used in the previous experiments, but via the intraperitoneal route—a route and dose of virus which allow the M2-deficient mutant to efficiently infect the spleen [36]. As a proper control, the marker rescue virus (i.e, a recombinant MHV68 in which the genetic mutation introduced into the M2 null mutant was restored to the wild type virus sequence) [37], was also administered via the IP route. Notably, we have extensively compared IN versus IP MHV68 co-infection with P. yoelii and found there to be no difference in outcome. M2.Stop (M2 null virus; M2.St) infection in a C57BL/6 mouse induced a nearly 2-fold higher MHV68 specific IgG response as compared to infection with the marker rescue control virus (M2.MR; MR) (Fig 6A; day 21 post-infection Mann Whitney-U test p<0.05). It is important to note that day 21 post-MHV68 infection in this experiment corresponds to day 14 post-co-infection with P. yoelii XNL. The time point at which the virus specific humoral response is suppressed overlaps with the timing at which parasite specific IgG responses become severely compromised during co-infection (Fig 1C and 1E). This observation suggests that M2 may be mediating the virus-induced suppression of anti-malarial humoral immune responses.
Fig. 6. The MHV68 M2 gene product is necessary for virus mediated humoral suppression and lethality during Plasmodium co-infection. 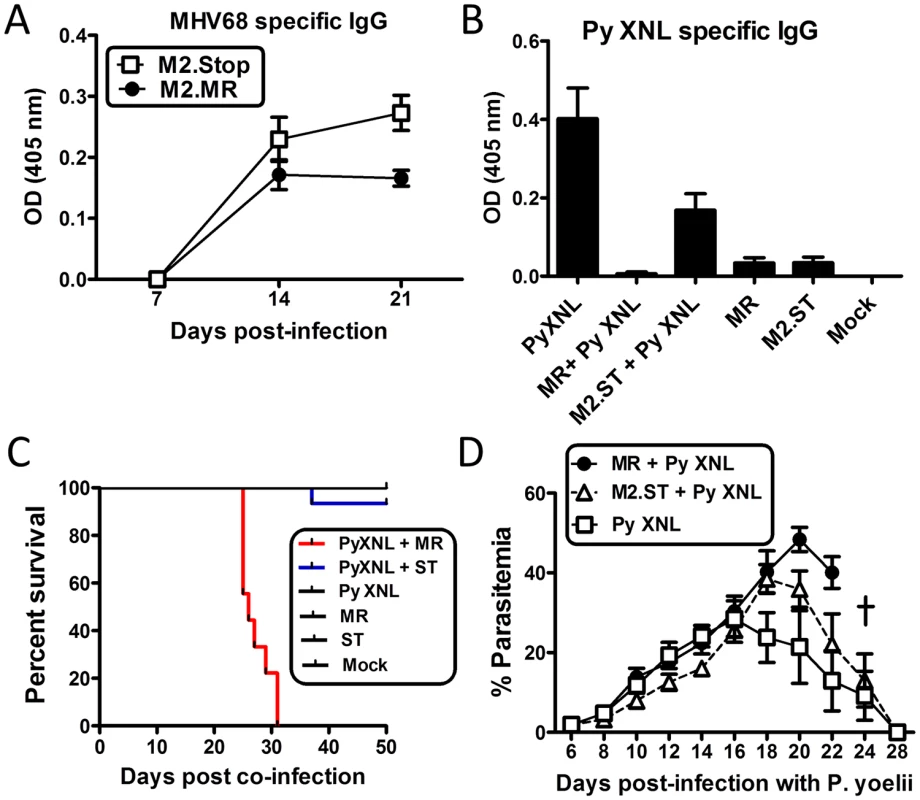
(A) MHV68 specific IgG titers from serum of animals infected with the MR (M2.Marker Rescue) or M2.Stop (ST, M2-null) viruses. Serum was collected and analyzed on days 7, 14 and 21 post infection with either virus (n = 10/ virus) (Day 21, MR vs. M2.Stop, Kruskal Wallis p<0.05; Dunn’s pairwise comparison test p<0.05). (B) P. yoelii XNL specific IgG response during P. yoelii XNL co-infection with either the M2.MR or M2.Stop virus. Serum was collected at day 20 post infection with P. yoelii XNL (WT + P. yoelii co-infected vs. P. yoelii, Kruskal Wallis p<0.05; Dunn’s pairwise comparison test p<0.05/ WT + P. yoelii co-infected vs. M2.Stop + P. yoelii, Kruskal Wallis p<0.05; Dunn’s pairwise comparison test p>0.05). (C) Survival curve during P. yoelii XNL co-infection with either the M2.MR or M2.Stop virus. Note: data representing P. yoelii XNL + MHV68 co-infection is the identical data set to that in Fig 1B. It was added in panel C for comparative purposes. (D) % parasitemia in the periphery during P. yoelii XNL, P. yoelii XNL +MR and P. yoelii XNL + M2.Stop infection. To evaluate whether loss of M2 expression could alleviate the MHV68 induced suppression of the anti-malarial humoral response, we infected mice with either the M2 null mutant (M2.Stop) or the marker rescue virus control (MR) 7 days prior to challenge with P. yoelii XNL. While co-infection with the MR virus suppressed the generation of anti-P. yoelii XNL specific IgG (Kruskal Wallis p<0.05; Dunn’s pairwise comparison test p<0.05), mice that were co-infected with M2.Stop showed a 28-fold increase in P. yoelii XNL specific IgG at day 23 post co-infection relative to the lethal MHV68 and P. yoelii XNL co-infection model (Kruskal Wallis p<0.05; Dunn’s pairwise comparison test p>0.05) (Fig 6B). Importantly, 94% of mice co-infected with M2.Stop and P. yoelii XNL survived (1 out of 15 mice died) (Fig 6C) compared to 100% lethality in mice co-infected with MR and P. yoelii XNL. Consistent with this observation, circulating parasitemia was not detectable by microscopy at day 28 post co-infection in the M2.Stop and P. yoelii XNL co-infected mice (Fig 6D). This data clearly establishes a strong link between MHV68 mediated suppression of the humoral response to P. yoelii XNL and survival. In addition, it argues for a role of the MHV68 M2 protein in mediating the suppression of the anti-malarial humoral response in MHV68 co-infected mice.
Discussion
The risk factors postulated to contribute to disease severity in young children infected with malaria are numerous and include co-infection with other pathogens [4]. Many children in Equatorial African countries are seropositive for EBV by the age of 6 months as protective maternal antibodies wane [6,38], indicating that primary infection with EBV coincides with the time at which the risk of developing severe malaria is greatest [6,18]. Acute infection with EBV is asymptomatic in young children [18] and results in a latent infection that persists for the life time of the host. Children who experience recurrent infection with P. falciparum while latent for EBV show an impairment in virus specific CD8 T cell responses [8,39–42] which contributes to an increased risk of developing eBL [reviewed in [7]]. While the impact of repeated P. falciparum infections can abate an EBV-specific adaptive immune response, little has been known regarding the impact of acute EBV infection on severe malarial disease during childhood.
The risk of co-infection with P. falciparum before the age of 1 is extremely high for children living in Sub-Saharan Africa [6,18] and for some children it is likely that their primary infections of EBV and P. falciparum will overlap. There are several reports that asymptomatic EBV infection can have suppressive effects on the host’s humoral adaptive response. One prominent example involves a case study on a child aged 2 ½ years presenting with a recurrent case of otitis media and pneumonia. It was established that this child exhibited suppressed humoral responses when immunized with bacteriophage ɸX174 or Keyhole limpet hemocyanin (KLH) during an asymptomatic infection with EBV. Increased EBV specific antibody titers correlated with a suppression in secondary humoral responses to unrelated antigens [19]. The same observation has been documented in young adults experiencing a primary EBV infection and manifesting symptoms of Infectious Mononucleosis (IM) [20,21]. Similarly, Holder et al. recently described a role for acute EBV infection in attenuating vaccine specific antibody responses in Gambian children as compared to children who had concurrent CMV infection [43]. These observations were also extended to the marmoset model where Wedderburn et al. demonstrate that acute co-infection of EBV and P. brasilianum resulted in severe morbidity and death [44]. Collectively, these documented cases provide key evidence of the immune suppressive nature of an acute EBV infection on the development of humoral immunity. More importantly, several studies have shown a correlation between non-cerebral severe disease (particularly severe malarial anemia [SMA]) and attenuated parasite specific antibody responses [14,45].
Here, using well characterized mouse models, we provide evidence that acute gammaherpesvirus infection can suppress the development of humoral immunity to a secondary Plasmodium infection in two different non-lethal models of rodent malaria (Fig 1). Interestingly, this defect correlated with a transformation of a non-lethal malaria infection into a lethal one in the case of P. yoelii XNL co-infection, but had no obvious impact on the pathogenesis of P. chabaudi AS infection (Fig 2). This result is likely to be due to the differential role of antibody mediated parasite clearance mechanisms in controlling the primary peak of parasitemia in each of these models (Figs 2 and S5) [23,26], although the effects of a primary acute MHV68 infection on other cells types such as macrophages cannot be ruled out. The reason why P. yoelii XNL infection is more dependent on antibodies for the control of parasitemia than P. chabaudi AS is unknown, but could be related to the kinetics of infection. In our hands, P. yoelii XNL parasitemia in intact C57BL/6 mice peaked significantly later than P. chabaudi AS infection (15.5 ± 1.5 days compared with 8.2 ±0.5 days; Mann Whitney-U test p<0.05). T cells, in particular IFN-γ-producing T cells [46–49], have been implicated in orchestrating the control of peripheral parasitemia in both models, whereas IL-10 producing T cells have been shown to exacerbate P. yoelii XNL parasitemia [29]. Therefore, it is possible that MHV68 infection resulted in an alteration of T cell phenotypes generated against a secondary malaria infection that impacted the pathogenesis of the infection. However, these splenic CD4+ T cell populations measured at different time points post-infection were not significantly altered (S4 Fig), and IL-10-producing CD4+T cells were less in number during MHV68 and P. yoelii XNL co-infected animals as compared to P. yoelii XNL singly infected animals (S4 Fig, panel B), which theoretically should lead to better control of peripheral parasitemia [29]. Furthermore, T cells have been shown to play a critical role in the control of the primary peak of P. chabaudi AS infection [48,49], yet MHV68 co-infection did not alter the peripheral parasitemia in this model suggesting that the relevant defect lies within the failure to mount an appropriate humoral response.
Despite the differential outcome of the two co-infection models, the impact of acute viral infection on the suppression of the humoral response is a common feature of MHV68 and malaria co-infection in C57BL/6 mice. Our data suggests that co-infected mice have a profound defect in the ability to form antibody producing plasma cells (Fig 3). GC B cells are precursors of plasma cells and are dependent on the T follicular helper subset for development and maintenance (reviewed in [50,51]). Analysis of germinal center formation at day 8 post-infection with P. yoelii XNL demonstrates that germinal centers can form in the spleens of co-infected mice, but are not maintained (Fig 3). This may be due to a defective ability of GC B cells to communicate with Tfh cells due to elevated expression of the suppressive ligand PD-L1 (S6 Fig). PD-L1 mediates its inhibitory role by ligating the PD-1 (Programmed Death-1) receptor on T cells. Recent studies suggest that the reduction in HIV specific antibody responses during chronic infection is correlated with an up-regulation of PD-L1 expression on germinal center B cells [52]. It is interesting that the possible cause of the Tfh impairment may be associated with a change in the surface phenotype of the GC B cell. This is particularly significant in the case of MHV68 infection, since B cells are the primary cell infected by this virus. However, it is unclear whether MHV68 directly affects the GC B cell, or whether soluble mediators of infection suppress the function of Tfh cells in their ability to support the transformation of GC B cells to plasma cells. Our data does not support a role for suppressive effects of FoxP3+ Tregs (S3 Fig) in mediating this effect since the expansion of Tregs is comparable during co-infection compared to a single Plasmodium infection. (S3 Fig, Mann Whitney-U test p<0.05 in both cases).
The suppression of the anti-malarial humoral response is evident during acute, but not latent, MHV68 infection (Fig 5). MHV68 has evolved elaborate immune evasion strategies to survive the potent innate inflammatory responses that it induces during acute infection [53]. One interesting observation previously made in the Speck laboratory noted that loss of M2 expression in vivo resulted in a more robust anti-viral CD8 T cell response [33]. M2 is a unique viral protein expressed by MHV68 which shares some functional homology with the LMP1 and LMP2a EBV gene products—which mimic CD40 and BCR signaling, respectively [54,55]. M2 is able to promote signals downstream of the BCR receptor [56,57], induces IL-10 production from B cells [33,37] and promotes differentiation of infected B cells into plasma cells (note that ≤ 1% of B cells are MHV68 infected at the peak of latency) [32]. One notable effect of M2 expression in vivo is the dramatic increase of IL-10 levels in the serum of infected mice [33]. IL-10 is an immune-modulatory cytokine which is capable of suppressing T cell activation [35]. M2 mediated reduction of the anti-viral CD8 T cell response likely reflects an evolutionary viral adaptation that allows for the evasion of the immune response, and more importantly, allows for establishment of viral latency. Since the humoral response is dependent on a robust T cell response, we predicted that M2 may also influence the generation of a virus specific IgG response, a critical branch of the adaptive response involved in long term clearance of the virus [25]. Our data shows that in the absence of M2 expression, the development of an anti-viral humoral response was enhanced 2-fold (Fig 6). Additionally, co-infection with M2.Stop mutant virus and P. yoelii XNL showed a 28-fold increase in the anti-malarial IgG response, which also correlated with survival during co-infection. We compared virus specific IgG levels in mice infected with another unrelated viral mutant that is null for M1 protein expression. Both the M1.Stop and M1.MR viruses showed equivalent levels of virus specific IgG responses over 2 months of infection (S7 Fig), suggesting an M2-specific role in suppressing the virus specific humoral response. This further corroborates data shown by Getahun et al. [22], which demonstrated that infection with M1.Stop or M3.Stop (a viral chemokine) could not alleviate the virus-induced immune suppression. We currently do not understand the mechanism by which M2 is mediating this effect, although we hypothesize that increased IL-10 production from B cells in the splenic follicle may negatively regulate Tfh survival and consequently affect germinal center maintenance and development. Although B cell expansion is beneficial for seeding viral latency and persistence, it is evident that the virus also negatively regulates the production of virus specific IgG responses, a key immune evasion mechanism which would support viral persistence. Although M2 expression is associated with IL-10 production, we cannot rule out the possibility that M2 is mediating its effect in a non-IL-10 dependent manner or that M2-driven IL-10 expression may impact other aspects of the host response that may contribute to the observed lethality of MHV68 and P. yoelii co-infection. However, our novel discovery implicating M2 in mediating the virus induced humoral suppression against secondary parasitic infection is a key observation for future efforts in dissecting the mechanism behind this observed phenotype.
As with every model system, certain limitations exist. Our studies rely on infection of 6–8 week old mice since this age group has been extensively studied. The limited studies in neonatal mice suggest that BALB/c mice, and not C57BL/6 mice, are more susceptible to infection and may develop myocarditis and neurologic disorders [58,59]. It appears that neonate C57BL/6 mice, which is the background used in our studies, do not experience altered immune responses compared to adults. As such, there is little premise to suggest that younger mice would react differently to acute MHV68 infection. However, the impact of co-infection on malarial disease severity in neonate mice has not been explored and is worth pursuing in other studies. Another important aspect worth noting is that the isolated system used to model human co-infection cannot encompass the myriad of factors influencing malarial disease severity in humans. It has been extensively demonstrated that various other viral, bacterial, and helminth co-infections impact malarial disease [60–65]. Additionally, factors such as parasite virulence, nutrition, host health and genetics can also contribute to the variation in malarial disease severity [3,4]. As such, acute EBV infection is unlikely to be the sole contributor in modulating malarial disease. However, the results reported in this manuscript aim to elucidate previously neglected co-infections, such as ubiquitous asymptomatic EBV infection, in altering non-cerebral malarial disease severity. More importantly, multiple human reports indicate that asymptomatic acute EBV infection has the ability to alter the generation of a humoral response during secondary pathogen challenge [19–21,43,44] and demonstrated by us and others [22] during MHV68 acute infection. Collectively, our observations in the mouse model and supporting literature of human studies provide a strong premise for investigating the role of acute EBV in malarial disease. Undoubtedly, detailed longitudinal studies are required in humans to conclusively establish this correlation.
In conclusion, our work provides compelling evidence that acute gammaherpesvirus infection can negatively modulate the humoral immune response to malaria infection. This data provides justification to investigate how EBV infection might impact the development of P. falciparum humoral immunity in young children living in malaria endemic areas. If found to be a risk factor for developing severe malaria, tackling EBV infection via the development and use of an EBV vaccine or anti-viral therapies in malaria endemic areas, may provide some relief in the development of non-cerebral severe disease during childhood malaria infection.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Emory University Institutional Animal Care and Use Committee, and in accordance with established guidelines and policies at Emory University School of Medicine (Protocol Number: YER-2002245-031416GN).
Mice and infections
Female C57BL/6 mice (6–8 weeks) were purchased from Jackson Laboratories. μMT and RAG2-/ - mice were purchased from Jackson laboratories and bred in house. IL21R-/ - mice were a gift of Dr. Warren J. Leonard and were bred in house. Mice were infected with 1000 Plaque Forming Units (PFU) of MHV68 in DMEM without fetal bovine serum (FBS) either Intra-Nasally (IN) or Intra-Peritoneally (IP). Animals are infected IN with MHV68 throughout the manuscript, unless where otherwise noted. Frozen stabilates of P. yoelii XNL or P. chabaudi AS were administered IP in Kreb’s saline with glucose (KSG) into donor mice. After day 7–9 after infection of the donor mouse, experimental mice were infected at a dose of 1 x 105 parasitized Red Blood Cells (pRBCs) via the IP route in KSG. Anemia was measured by counting RBCs from tail blood diluted in KSG using a haemocytometer [66]. Parasitemia was enumerated from Giemsa stained thin blood smears.
Limiting dilution analysis of viral lung titer and lung tissue histology
The left lung was collected for analysis of viral replication using a limiting dilution analysis as previously described [67]. Briefly, lungs were homogenized in 1ml of complete DMEM media and 1.0 mm Zirconia/Silicon beads (BioSpec products) using a BioSpec mini bead-beater 16. Samples were homogenized 4 times (1 minute homogenization followed by 1 minute rest on ice). Samples were then transferred to a new tube with 0.5 mm Zirconia/Silicon beads and homogenization was repeated as above. Homogenate was then plated on Mouse Embryonic Fibroblasts (MEFs) in 96 well plates in serial 2 fold dilutions, up to 12 dilutions. Plates were incubated for 2 weeks in a low evaporation incubator (5% CO2, 37°C) and analyzed for Cytopathic effect (CPE). Results are plotted as percent of wells displaying CPE at each plated dilution. For lung tissue histology, the left lung tissue was collected for lung histology analysis. Whole tissue was fixed in 10% (v/v) Normal Buffered formaldehyde for 24 hours at room temperature. Tissue was then put into 70% ethanol solution until samples were sectioned. Samples were paraffin embedded and prepared for Hematoxylin and Eosin Staining as previously described [68].
Flow cytometry
Splenocytes were blocked with anti-CD16/32 (BD bioscience). Surface stains were performed in PBS-2% FBS - 1mM EDTA for 20 minutes on ice. Markers used: CD138-PE (BD bioscience), B220-Pacific Blue (Biolegend) or Pacific Orange (Invtirogen), CD95-PE-Cy7 (BD biosciences), GL7-Alexa Fluor 660 or FITC (eBioscience), CD3-PerCP/ Pacific Blue (BD bioscience), CD4-PerCP (BD Bioscience), CD8-Pacific Orange (Invitrogen), IL-10-PE (Biolegend), IFN-γ-APC (ebioscience), IL-2 PE-Cy7 (ebioscience) or FITC (Biolegend), purified anti-CXCR5 (BD biosciences), Biotin-SP-AffiniPure F(ab')2 Fragment Goat Anti-Rat IgG (H+L) (Jackson immunoresearch), Streptavidin-APC (Molecular probe), CD279-PE (Biolegend), CD44-Pacific Blue (Biolegend), CD25-Pacific Blue (Biolegend), FoxP3-APC (ebioscience) and CD19-FITC/PE/PE-Cy7/PerCP/ APC/Pacific Blue (BD bioscience). Intracellular cytokine stains for IL-2, IL-10 and IFN-γ were performed using the BD bioscience Cytofix/Cytoperm staining kit. Cells were stimulated (5 hours, 5% CO2, 37°C) in anti-CD3 coated 96 wells tissue culture plates and supplemented with soluble anti-CD28 and Brefeldin A. Intracellular staining for FoxP3 was performed using the eBioscience FoxP3 staining kit. Staining for Tfh cells was performed as previously described [69]. Fixable live dead stains were purchased in FITC from Life Technologies and Zombie Yellow (Pacific Orange) from Biolegend and used according to manufacturer’s guidelines. Stained splenocytes were fixed in 2% formaldehyde prior to analysis. Samples were read on a BD LSRII. Data was analyzed using FACS Diva and FloJo software.
Blood collection and ELISA
For serum samples, blood was collected by cardiac puncture during terminal bleeds. Blood was allowed to clot at 4°C for 1 hour. Tubes were spun at 4°C at 14,000 rpm for 2 minutes. Serum was transferred to a fresh tube and stored at -80°C. For plasma collection, 100μl of blood was collected from the tail vein into Lithium-heparin coated tubes (BD microtainer). Tubes were spun at 4°C at 10,000 rpm for 10 minutes. Plasma was transferred to a fresh tube and stored at -80°C. ELISA assays were performed as previously described [70]. Briefly, 96 well Nunc ImmunoMaxisorp ELISA plates were coated with 0.5 ug/well of goat anti-mouse IgG or IgM antibody (Southern Biotech) or sucrose gradient purified MHV68 in PBS. Serum was serially diluted (3 fold, beginning at 1 : 100) and 6 dilutions were plated for each sample. Alkaline Phosphatase conjugated goat anti-mouse IgG or IgM (Southern Biotech) was used as a secondary antibody. For parasite specific ELISAs, mice were infected with 105 pRBC of P. yoelii XNL or P. chabaudi AS. Blood was harvested and pooled from infected mice. pRBCs were purified using a Percoll gradient. Purified pRBCs were then cultured for schizont maturation for 4 hours in a shaking 37°C water bath in RPMI media supplemented with 10% FBS, 100ug/ml Streptomycin, 100U/ml Penicillin, L-Glutamine (2 mM), HEPES (6 mM), β-mercaptoethanol (50 μM) and sodium pyruvate (0.5 mM). pRBC schizonts were then spun out of culture and lysed with lysis buffer (50 mM Tris/HCl + 1 mM EDTA + 0.5% SDS). Optical Density (OD) of homogenate was read at 206 nm. Plates were coated with homogenate at an OD of 0.1–0.2. ELISA protocol for the parasite specific response was performed as previously reported [71] Color was developed using p-nitrophenyl phosphate (Sigma) in a diethanolamine substrate buffer. Absorbance at 405 nm was read on a Biotek Synergy HT reader. Data is represented as absorbance at 405 nm.
Immunofluorescence staining and microscopy
Whole spleen was collected at day 8 (post parasite infection) from animals that were MHV68, P. yoelii XNL, MHV68 + P. yoelii XNL or mock infected. The whole spleen was embedded in Tissue-Tek OCT media (Sakura-Finetek) and frozen in chilled Isopentane. 7 μm tissue sections were mounted on slides and allowed to dry at room temperature for 12 hours after which they were frozen at -80°C for long term storage. For staining, slides were allowed to equilibrate to room temperature then rehydrated in PBS for 10 minutes. Sections were stained using B220-FITC (BD bioscience), GL7-AF660 (eBioscience) and a primary purified biotinylated CD3 (eBioscience) with secondary anti-Streptavidin in AF-568 (Life Technologies). Sections were blocked in 5% normal mouse serum in PBS for 20 minutes at room temperature. Primary stains were incubated in block solution for 1 hour and secondary stains for 30 minutes. Sections were washed 3 times with PBS and then mounted with Prolong anti-fade without DAPI (Cell signaling) and #1.5 Fisherbrand microscope slides. Mounted sections were allowed to cure in the dark at room temperature for 24 hours. Fluorescence images were taken on an Olympus Fluoview FV1000 with a 10X 0.3 NA objective and utilizing the multi-area time lapse (MATL) xy-stitching functions. The confocal pinhole was opened to 300 μm to increase the thickness of the optical section facilitating the single plane image. Entire spleen sections required ~100 to 200 images, 1600 x 1600 pixels (~850 x 850 μm) at a zoom of 1.5 and zero overlap. This exceeds the 15000 x 15000 stitching pixel limit of the Fluoview software, and as such a Fiji plugin was written to convert MATL log files for use with the stitching plugin within Fiji [link to http://ici.emory.edu/Resources/plugins.html].
Supporting Information
Zdroje
1. World Health Organization. Global Malaria Programme. (2012) World malaria report 2012. Geneva: World Health Organization. xxxiv, 249 p. p.
2. Gupta S, Snow RW, Donnelly CA, Marsh K, Newbold C (1999) Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med 5 : 340–343. 10086393
3. Miller LH, Baruch DI, Marsh K, Doumbo OK (2002) The pathogenic basis of malaria. Nature 415 : 673–679. 11832955
4. Greenwood B, Marsh K, Snow R (1991) Why do some African children develop severe malaria? Parasitol Today 7 : 277–281. 15463389
5. Speck SH, Ganem D (2010) Viral latency and its regulation: lessons from the gamma-herpesviruses. Cell Host Microbe 8 : 100–115. doi: 10.1016/j.chom.2010.06.014 20638646
6. Piriou E, Asito AS, Sumba PO, Fiore N, Middeldorp JM, et al. (2012) Early age at time of primary Epstein-Barr virus infection results in poorly controlled viral infection in infants from Western Kenya: clues to the etiology of endemic Burkitt lymphoma. J Infect Dis 205 : 906–913. doi: 10.1093/infdis/jir872 22301635
7. Rochford R, Cannon MJ, Moormann AM (2005) Endemic Burkitt's lymphoma: a polymicrobial disease? Nat Rev Microbiol 3 : 182–187. 15685227
8. Whittle HC, Brown J, Marsh K, Greenwood BM, Seidelin P, et al. (1984) T-cell control of Epstein-Barr virus-infected B cells is lost during P. falciparum malaria. Nature 312 : 449–450. 6095104
9. Njie R, Bell AI, Jia H, Croom-Carter D, Chaganti S, et al. (2009) The effects of acute malaria on Epstein-Barr virus (EBV) load and EBV-specific T cell immunity in Gambian children. J Infect Dis 199 : 31–38. doi: 10.1086/594373 19032105
10. Moormann AM, Chelimo K, Sumba PO, Tisch DJ, Rochford R, et al. (2007) Exposure to holoendemic malaria results in suppression of Epstein-Barr virus-specific T cell immunosurveillance in Kenyan children. J Infect Dis 195 : 799–808. 17299709
11. Moormann AM, Heller KN, Chelimo K, Embury P, Ploutz-Snyder R, et al. (2009) Children with endemic Burkitt lymphoma are deficient in EBNA1-specific IFN-gamma T cell responses. Int J Cancer 124 : 1721–1726. doi: 10.1002/ijc.24014 19089927
12. Cohen S, Mc GI, Carrington S (1961) Gamma-globulin and acquired immunity to human malaria. Nature 192 : 733–737. 13880318
13. Sabchareon A, Burnouf T, Ouattara D, Attanath P, Bouharoun-Tayoun H, et al. (1991) Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am J Trop Med Hyg 45 : 297–308. 1928564
14. Osier FH, Fegan G, Polley SD, Murungi L, Verra F, et al. (2008) Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun 76 : 2240–2248. doi: 10.1128/IAI.01585-07 18316390
15. Rono J, Osier FH, Olsson D, Montgomery S, Mhoja L, et al. (2013) Breadth of anti-merozoite antibody responses is associated with the genetic diversity of asymptomatic Plasmodium falciparum infections and protection against clinical malaria. Clin Infect Dis 57 : 1409–1416. doi: 10.1093/cid/cit556 23983244
16. Rovira-Vallbona E, Moncunill G, Bassat Q, Aguilar R, Machevo S, et al. (2012) Low antibodies against Plasmodium falciparum and imbalanced pro-inflammatory cytokines are associated with severe malaria in Mozambican children: a case-control study. Malar J 11 : 181. doi: 10.1186/1475-2875-11-181 22646809
17. Dobano C, Rogerson SJ, Mackinnon MJ, Cavanagh DR, Taylor TE, et al. (2008) Differential antibody responses to Plasmodium falciparum merozoite proteins in Malawian children with severe malaria. J Infect Dis 197 : 766–774. doi: 10.1086/527490 18260767
18. Henle G, Henle W (1970) Observations on childhood infections with the Epstein-Barr virus. J Infect Dis 121 : 303–310. 4313278
19. Bowen TJ, Wedgwood RJ, Ochs HD, Henle W (1983) Transient immunodeficiency during asymptomatic Epstein-Barr virus infection. Pediatrics 71 : 964–967. 6304613
20. Junker AK, Ochs HD, Clark EA, Puterman ML, Wedgwood RJ (1986) Transient immune deficiency in patients with acute Epstein-Barr virus infection. Clin Immunol Immunopathol 40 : 436–446. 3015461
21. Provisor AJ, Iacuone JJ, Chilcote RR, Neiburger RG, Crussi FG, et al. (1975) Acquired agammaglobulinemia after a life threatening illness with clinical and laboratory features of Infectious Mononucleosis in three related male children. The New England Journal of Medicine 293 : 62–65. 165416
22. Getahun A, Smith MJ, Kogut I, van Dyk LF, Cambier JC (2012) Retention of anergy and inhibition of antibody responses during acute gamma herpesvirus 68 infection. J Immunol 189 : 2965–2974. doi: 10.4049/jimmunol.1201407 22904300
23. van der Heyde HC, Huszar D, Woodhouse C, Manning DD, Weidanz WP (1994) The resolution of acute malaria in a definitive model of B cell deficiency, the JHD mouse. J Immunol 152 : 4557–4562. 8157969
24. Haque A, Rachinel N, Quddus MR, Haque S, Kasper LH, et al. (2004) Co-infection of malaria and gamma-herpesvirus: exacerbated lung inflammation or cross-protection depends on the stage of viral infection. Clin Exp Immunol 138 : 396–404. 15544614
25. Weck KE, Kim SS, Virgin HI, Speck SH (1999) B cells regulate murine gammaherpesvirus 68 latency. J Virol 73 : 4651–4661. 10233924
26. Grun JL, Weidanz WP (1983) Antibody-independent immunity to reinfection malaria in B-cell-deficient mice. Infect Immun 41 : 1197–1204. 6350181
27. Crotty S (2011) Follicular helper CD4 T cells (TFH). Annu Rev Immunol 29 : 621–663. doi: 10.1146/annurev-immunol-031210-101400 21314428
28. Belkaid Y, Rouse BT (2005) Natural regulatory T cells in infectious disease. Nat Immunol 6 : 353–360. 15785761
29. Couper KN, Blount DG, Wilson MS, Hafalla JC, Belkaid Y, et al. (2008) IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog 4: e1000004. doi: 10.1371/journal.ppat.1000004 18401464
30. Rasheed MA, Latner DR, Aubert RD, Gourley T, Spolski R, et al. (2013) Interleukin-21 is a critical cytokine for the generation of virus-specific long-lived plasma cells. J Virol 87 : 7737–7746. doi: 10.1128/JVI.00063-13 23637417
31. Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26 : 677–704. doi: 10.1146/annurev.immunol.26.021607.090331 18173375
32. Liang X, Collins CM, Mendel JB, Iwakoshi NN, Speck SH (2009) Gammaherpesvirus-driven plasma cell differentiation regulates virus reactivation from latently infected B lymphocytes. PLoS Pathog 5: e1000677. doi: 10.1371/journal.ppat.1000677 19956661
33. Siegel AM, Herskowitz JH, Speck SH (2008) The MHV68 M2 protein drives IL-10 dependent B cell proliferation and differentiation. PLoS Pathog 4: e1000039. doi: 10.1371/journal.ppat.1000039 18389062
34. Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A (2001) Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19 : 683–765. 11244051
35. de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, te Velde A, et al. (1991) Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med 174 : 915–924. 1655948
36. Jacoby MA, Virgin HWt, Speck SH (2002) Disruption of the M2 gene of murine gammaherpesvirus 68 alters splenic latency following intranasal, but not intraperitoneal, inoculation. J Virol 76 : 1790–1801. 11799175
37. Rangaswamy US, Speck SH (2014) Murine Gammaherpesvirus M2 Protein Induction of IRF4 via the NFAT Pathway Leads to IL-10 Expression in B Cells. PLoS Pathog 10: e1003858. doi: 10.1371/journal.ppat.1003858 24391506
38. Biggar RJ, Henle W, Fleisher G, Bocker J, Lennette ET, et al. (1978) Primary Epstein-Barr virus infections in African infants. I. Decline of maternal antibodies and time of infection. Int J Cancer 22 : 239–243. 212369
39. Moss DJ, Burrows SR, Castelino DJ, Kane RG, Pope JH, et al. (1983) A comparison of Epstein-Barr virus-specific T-cell immunity in malaria-endemic and-nonendemic regions of Papua New Guinea. Int J Cancer 31 : 727–732. 6305850
40. Whittle HC, Brown J, Marsh K, Blackman M, Jobe O, et al. (1990) The effects of Plasmodium falciparum malaria on immune control of B lymphocytes in Gambian children. Clin Exp Immunol 80 : 213–218. 1972671
41. Lam KM, Syed N, Whittle H, Crawford DH (1991) Circulating Epstein-Barr virus-carrying B cells in acute malaria. Lancet 337 : 876–878. 1672968
42. Chene A, Nylen S, Donati D, Bejarano MT, Kironde F, et al. (2011) Effect of acute Plasmodium falciparum malaria on reactivation and shedding of the eight human herpes viruses. PLoS One 6: e26266. doi: 10.1371/journal.pone.0026266 22039454
43. Holder B, Miles DJ, Kaye S, Crozier S, Mohammed NI, et al. (2010) Epstein-Barr virus but not cytomegalovirus is associated with reduced vaccine antibody responses in Gambian infants. PLoS One 5: e14013. doi: 10.1371/journal.pone.0014013 21103338
44. Wedderburn N, Davies DR, Mitchell GH, Desgranges C, de The G (1988) Glomerulonephritis in common marmosets infected with Plasmodium brasilianum and Epstein-Barr virus. J Infect Dis 158 : 789–794. 2844917
45. Leoratti FM, Durlacher RR, Lacerda MV, Alecrim MG, Ferreira AW, et al. (2008) Pattern of humoral immune response to Plasmodium falciparum blood stages in individuals presenting different clinical expressions of malaria. Malar J 7 : 186. doi: 10.1186/1475-2875-7-186 18816374
46. Su Z, Stevenson MM (2000) Central role of endogenous gamma interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infect Immun 68 : 4399–4406. 10899836
47. De Souza JB, Williamson KH, Otani T, Playfair JH (1997) Early gamma interferon responses in lethal and nonlethal murine blood-stage malaria. Infect Immun 65 : 1593–1598. 9125535
48. Langhorne J, Mombaerts P, Tonegawa S (1995) alpha beta and gamma delta T cells in the immune response to the erythrocytic stages of malaria in mice. Int Immunol 7 : 1005–1011. 7577794
49. Sayles PC, Rakhmilevich L (1996) Exacerbation of Plasmodium chabaudi malaria in mice by depletion of TCR alpha beta+ T cells, but not TCR gamma delta+ T cells. Immunology 87 : 29–33. 8666432
50. Nutt SL, Tarlinton DM (2011) Germinal center B and follicular helper T cells: siblings, cousins or just good friends? Nat Immunol 12 : 472–477. 21739669
51. Vinuesa CG, Linterman MA, Goodnow CC, Randall KL (2010) T cells and follicular dendritic cells in germinal center B-cell formation and selection. Immunol Rev 237 : 72–89. doi: 10.1111/j.1600-065X.2010.00937.x 20727030
52. Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, et al. (2013) Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med 19 : 494–499. doi: 10.1038/nm.3109 23475201
53. Paludan SR, Bowie AG, Horan KA, Fitzgerald KA (2011) Recognition of herpesviruses by the innate immune system. Nat Rev Immunol 11 : 143–154. doi: 10.1038/nri2937 21267015
54. Uchida J, Yasui T, Takaoka-Shichijo Y, Muraoka M, Kulwichit W, et al. (1999) Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science 286 : 300–303. 10514374
55. Caldwell RG, Wilson JB, Anderson SJ, Longnecker R (1998) Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity 9 : 405–411. 9768760
56. Pires de Miranda M, Alenquer M, Marques S, Rodrigues L, Lopes F, et al. (2008) The Gammaherpesvirus m2 protein manipulates the Fyn/Vav pathway through a multidocking mechanism of assembly. PLoS One 3: e1654. doi: 10.1371/journal.pone.0001654 18301737
57. Pires de Miranda M, Lopes FB, McVey CE, Bustelo XR, Simas JP (2013) Role of Src homology domain binding in signaling complexes assembled by the murid gamma-herpesvirus M2 protein. J Biol Chem 288 : 3858–3870. doi: 10.1074/jbc.M112.439810 23258536
58. Hausler M, Sellhaus B, Scheithauer S, Engler M, Alberg E, et al. (2005) Murine gammaherpesvirus-68 infection of mice: A new model for human cerebral Epstein-Barr virus infection. Ann Neurol 57 : 600–603. 15786475
59. Hausler M, Sellhaus B, Scheithauer S, Gaida B, Kuropka S, et al. (2007) Myocarditis in newborn wild-type BALB/c mice infected with the murine gamma herpesvirus MHV-68. Cardiovasc Res 76 : 323–330. 17658501
60. Graham AL, Lamb TJ, Read AF, Allen JE (2005) Malaria-filaria coinfection in mice makes malarial disease more severe unless filarial infection achieves patency. J Infect Dis 191 : 410–421. 15633101
61. Brooker S, Akhwale W, Pullan R, Estambale B, Clarke SE, et al. (2007) Epidemiology of plasmodium-helminth co-infection in Africa: populations at risk, potential impact on anemia, and prospects for combining control. Am J Trop Med Hyg 77 : 88–98. 18165479
62. Hasang W, Dembo EG, Wijesinghe R, Molyneux ME, Kublin JG, et al. (2014) HIV-1 infection and antibodies to Plasmodium falciparum in adults. J Infect Dis 210 : 1407–1414. doi: 10.1093/infdis/jiu262 24795481
63. Thursz MR, Kwiatkowski D, Torok ME, Allsopp CE, Greenwood BM, et al. (1995) Association of hepatitis B surface antigen carriage with severe malaria in Gambian children. Nat Med 1 : 374–375. 7585070
64. Berkley JA, Bejon P, Mwangi T, Gwer S, Maitland K, et al. (2009) HIV infection, malnutrition, and invasive bacterial infection among children with severe malaria. Clin Infect Dis 49 : 336–343. doi: 10.1086/600299 19548833
65. Otieno RO, Ouma C, Ong'echa JM, Keller CC, Were T, et al. (2006) Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS 20 : 275–280. 16511422
66. Spence PJ, Jarra W, Levy P, Reid AJ, Chappell L, et al. (2013) Vector transmission regulates immune control of Plasmodium virulence. Nature 498 : 228–231. doi: 10.1038/nature12231 23719378
67. Krug LT, Evans AG, Gargano LM, Paden CR, Speck SH (2013) The absence of M1 leads to increased establishment of murine gammaherpesvirus 68 latency in IgD-negative B cells. J Virol 87 : 3597–3604. doi: 10.1128/JVI.01953-12 23302876
68. Cardiff RD, Miller CH, Munn RJ (2014) Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc 2014 : 655–658. doi: 10.1101/pdb.prot073411 24890205
69. Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, et al. (2009) Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325 : 1006–1010. doi: 10.1126/science.1175870 19608860
70. Sangster MY, Topham DJ, D'Costa S, Cardin RD, Marion TN, et al. (2000) Analysis of the virus-specific and nonspecific B cell response to a persistent B-lymphotropic gammaherpesvirus. J Immunol 164 : 1820–1828. 10657630
71. Harris JV, Bohr TM, Stracener C, Landmesser ME, Torres V, et al. (2012) Sequential Plasmodium chabaudi and Plasmodium berghei infections provide a novel model of severe malarial anemia. Infect Immun 80 : 2997–3007. doi: 10.1128/IAI.06185-11 22689817
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary TuberculosisČlánek Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic PlagueČlánek Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host SpeciesČlánek Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR SelectionČlánek Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus GenomesČlánek Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from FerrioxamineČlánek Remembering MumpsČlánek Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling PathwayČlánek Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 5- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Parasites and Their Heterophagic Appetite for Disease
- The Elusive Role of the Prion Protein and the Mechanism of Toxicity in Prion Disease
- Intestinal Colonization Dynamics of
- Activation of Typhi-Specific Regulatory T Cells in Typhoid Disease in a Wild-Type . Typhi Challenge Model
- The Engineering of a Novel Ligand in gH Confers to HSV an Expanded Tropism Independent of gD Activation by Its Receptors
- Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis
- Group Selection and Contribution of Minority Variants during Virus Adaptation Determines Virus Fitness and Phenotype
- Phosphatidic Acid Produced by Phospholipase D Promotes RNA Replication of a Plant RNA Virus
- A Ribonucleoprotein Complex Protects the Interleukin-6 mRNA from Degradation by Distinct Herpesviral Endonucleases
- Characterization of Transcriptional Responses to Different Aphid Species Reveals Genes that Contribute to Host Susceptibility and Non-host Resistance
- Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic Plague
- Natural Killer Cell Sensing of Infected Cells Compensates for MyD88 Deficiency but Not IFN-I Activity in Resistance to Mouse Cytomegalovirus
- Manipulation of the Xanthophyll Cycle Increases Plant Susceptibility to
- Ly6C Monocytes Regulate Parasite-Induced Liver Inflammation by Inducing the Differentiation of Pathogenic Ly6C Monocytes into Macrophages
- Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host Species
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Experimental Evolution of an RNA Virus in Wild Birds: Evidence for Host-Dependent Impacts on Population Structure and Competitive Fitness
- Inhibition and Reversal of Microbial Attachment by an Antibody with Parasteric Activity against the FimH Adhesin of Uropathogenic .
- The EBNA-2 N-Terminal Transactivation Domain Folds into a Dimeric Structure Required for Target Gene Activation
- Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR Selection
- The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive via Activation of α5β1 Integrin at the Macrophage-Amebae Intercellular Junction
- Sequential Conformational Changes in the Morbillivirus Attachment Protein Initiate the Membrane Fusion Process
- A Two-Component DNA-Prime/Protein-Boost Vaccination Strategy for Eliciting Long-Term, Protective T Cell Immunity against
- cAMP-Signalling Regulates Gametocyte-Infected Erythrocyte Deformability Required for Malaria Parasite Transmission
- Response Regulator VxrB Controls Colonization and Regulates the Type VI Secretion System
- Evidence for a Novel Mechanism of Influenza Virus-Induced Type I Interferon Expression by a Defective RNA-Encoded Protein
- Dust Devil: The Life and Times of the Fungus That Causes Valley Fever
- TNF-α Induced by Hepatitis C Virus via TLR7 and TLR8 in Hepatocytes Supports Interferon Signaling via an Autocrine Mechanism
- The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen,
- L-Rhamnosylation of Wall Teichoic Acids Promotes Resistance to Antimicrobial Peptides by Delaying Interaction with the Membrane
- Rapid Sequestration of by Neutrophils Contributes to the Development of Chronic Lesion
- Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus Genomes
- The Expression of Functional Vpx during Pathogenic SIVmac Infections of Rhesus Macaques Suppresses SAMHD1 in CD4 Memory T Cells
- Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from Ferrioxamine
- TRAF1 Coordinates Polyubiquitin Signaling to Enhance Epstein-Barr Virus LMP1-Mediated Growth and Survival Pathway Activation
- Vaccine-Elicited Tier 2 HIV-1 Neutralizing Antibodies Bind to Quaternary Epitopes Involving Glycan-Deficient Patches Proximal to the CD4 Binding Site
- Remembering Mumps
- The Role of Horizontal Gene Transfer in the Evolution of the Oomycetes
- Advances and Challenges in Computational Prediction of Effectors from Plant Pathogenic Fungi
- Investigating Fungal Outbreaks in the 21st Century
- Systems Biology for Biologists
- How Does the Dinoflagellate Parasite Outsmart the Immune System of Its Crustacean Hosts?
- FCRL5 Delineates Functionally Impaired Memory B Cells Associated with Exposure
- Phospholipase D1 Couples CD4 T Cell Activation to c-Myc-Dependent Deoxyribonucleotide Pool Expansion and HIV-1 Replication
- Influenza A Virus on Oceanic Islands: Host and Viral Diversity in Seabirds in the Western Indian Ocean
- Geometric Constraints Dominate the Antigenic Evolution of Influenza H3N2 Hemagglutinin
- Widespread Recombination, Reassortment, and Transmission of Unbalanced Compound Viral Genotypes in Natural Arenavirus Infections
- Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity
- A Single Protein S-acyl Transferase Acts through Diverse Substrates to Determine Cryptococcal Morphology, Stress Tolerance, and Pathogenic Outcome
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
- Mechanisms of Stage-Transcending Protection Following Immunization of Mice with Late Liver Stage-Arresting Genetically Attenuated Malaria Parasites
- The Myelin and Lymphocyte Protein MAL Is Required for Binding and Activity of ε-Toxin
- Genome-Wide Identification of the Target Genes of AP2-O, a AP2-Family Transcription Factor
- An Atypical Mitochondrial Carrier That Mediates Drug Action in
- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Helminth Infection and Commensal Microbiota Drive Early IL-10 Production in the Skin by CD4 T Cells That Are Functionally Suppressive
- Circulating Pneumolysin Is a Potent Inducer of Cardiac Injury during Pneumococcal Infection
- ExoT Induces Atypical Anoikis Apoptosis in Target Host Cells by Transforming Crk Adaptor Protein into a Cytotoxin
- Discovery of a Small Non-AUG-Initiated ORF in Poleroviruses and Luteoviruses That Is Required for Long-Distance Movement
- Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
- Varicella Viruses Inhibit Interferon-Stimulated JAK-STAT Signaling through Multiple Mechanisms
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Recovery of Recombinant Crimean Congo Hemorrhagic Fever Virus Reveals a Function for Non-structural Glycoproteins Cleavage by Furin
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



