-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Ly6C Monocytes Regulate Parasite-Induced Liver Inflammation by Inducing the Differentiation of Pathogenic Ly6C Monocytes into Macrophages
The liver is not only a central organ for efficient metabolism of nutrients and for toxin clearance, but also for immune surveillance, including elimination of intravascular infections. However, excess of nutrients like fat or of toxins like alcohol and certain medications, as well as infections can trigger overactive immune responses which destroy the liver. Such chronic inflammations are major worldwide human health problem with often lethal consequences. Thus, understanding the particular function of various liver immune cells could provide original concepts to alleviate damages in this vital organ. Here, we dissected the heterogeneity, dynamics and function of the myeloid/monocytic cell compartment in the liver of mice infected with Trypanosoma congolense parasite. We established that infiltration of Ly6C+ monocyte subset initiated liver injury in infected mice. More importantly, we revealed that another myeloid cell subset for which the role in liver injury remained elusive, the Ly6C - monocyte subset, exerted hepatoprotective function in infected mice by secreting the anti-inflammatory cytokine IL-10 and by inducing, through cell-contact, the differentiation of pathogenic Ly6C+ monocytes into macrophages expressing genes coding for anti-inflammatory molecules. Thus, augmenting Ly6C - monocyte accumulation or functionality may represent a useful intervention strategy complementing anti-infective medication in conditions of liver injury due to chronic infections.
Published in the journal: . PLoS Pathog 11(5): e32767. doi:10.1371/journal.ppat.1004873
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004873Summary
The liver is not only a central organ for efficient metabolism of nutrients and for toxin clearance, but also for immune surveillance, including elimination of intravascular infections. However, excess of nutrients like fat or of toxins like alcohol and certain medications, as well as infections can trigger overactive immune responses which destroy the liver. Such chronic inflammations are major worldwide human health problem with often lethal consequences. Thus, understanding the particular function of various liver immune cells could provide original concepts to alleviate damages in this vital organ. Here, we dissected the heterogeneity, dynamics and function of the myeloid/monocytic cell compartment in the liver of mice infected with Trypanosoma congolense parasite. We established that infiltration of Ly6C+ monocyte subset initiated liver injury in infected mice. More importantly, we revealed that another myeloid cell subset for which the role in liver injury remained elusive, the Ly6C - monocyte subset, exerted hepatoprotective function in infected mice by secreting the anti-inflammatory cytokine IL-10 and by inducing, through cell-contact, the differentiation of pathogenic Ly6C+ monocytes into macrophages expressing genes coding for anti-inflammatory molecules. Thus, augmenting Ly6C - monocyte accumulation or functionality may represent a useful intervention strategy complementing anti-infective medication in conditions of liver injury due to chronic infections.
Introduction
Hosts can develop two different strategies to control pathogen infections, resistance and tolerance. During resistance, the host reduces the pathogen burden by activating and recruiting immune cells to the site of infection that mount a pro-inflammatory immune response. Tolerance refers to the action whereby the host repairs the tissue damage, i.e the pathogenicity, caused by the inflammatory immune cells that mediate the resistance [1, 2]. African trypanosomes are extracellular protozoan parasites causing sleeping sickness in humans and Nagana disease in cattle in sub-Saharan Africa. In experimental Trypanosoma congolense infection, C57BL/6 mice are considered as "trypanotolerant", being resistant and tolerant to the disease. The resistance of these animals results from their capacity to develop IFN-γ and MyD88-dependent CD11b+ myeloid cells, i.e. M1-type myeloid cells, including CCR2-dependent Ly6C+ monocytes and macrophages that secrete trypanotoxic molecules like TNF and NO and exert phagocytic activity to control the parasitemia [3–9]. This control of parasite growth occurs mainly in the liver [4, 10]. However, the M1-activated Ly6C+ monocyte subpopulation negatively affects the tolerance to T. congolense infection. Indeed, T. congolense-infected mice die from a systemic inflammatory response syndrome that is initiated by the engulfment of parasite components by liver myeloid cells, and culminates in apoptosis of liver myeloid cells and necrosis of liver parenchymal cells that reduce the host survival [3, 4]. In this respect, Ly6C+ monocytes have been shown to trigger liver cell apoptosis/necrosis, resulting in organ failure and early death by perpetuating a TNF-mediated pro-inflammatory immune response [7, 11]. On the other hand, IL-10 does not influence the resistance but is an essential contributor to the tolerance to T. congolense infection. This cytokine has been shown to down-regulate the Ly6C+ monocyte-induced pathogenicity and to induce regulatory, M2-type myeloid cells expressing a number of genes that could contribute to tissue healing, including maintenance of liver homeostasis. Both regulatory T cells and CD11b+ myeloid cells have been identified as sources of IL-10 during T. congolense infection in trypanotolerant animals [7, 10, 11]. Yet, within the heterogeneous CD11b+ myeloid cell population, the subset responsible for the IL-10 mediated anti-inflammatory immune response, thus for trypanotolerance, remained to be identified. In this study, we reveal the mobilization of IL-10-expressing Ly6C- monocytes and macrophages after the control of the first peak of parasitemia when a M2-type regulatory immune response arises in the liver of T. congolense—infected mice. In particular, Ly6C- monocytes exerted regulatory functions through the suppression of TNF production by Ly6C+ monocytes and by promoting the differentiation of the latter monocytes into macrophages. Hereby, Ly6C- monocytes contributed to the trypanotolerance by dampening liver damage caused by T. congolense—induced pro-inflammatory immune response. Thus, while the liver protective function of IL-10-producing regulatory T cells is amply documented [12–14], we have now established a similar function for Ly6C- monocytes.
Results
Trypanosome infection induces the sequential mobilization of distinct myeloid cell subsets in the liver
The phenotype of intrahepatic CD11b+ myeloid cells during parasite-induced liver inflammation was investigated in CX3CR1-GFP+/- C57BL/6 mice infected with T. congolense at day 7, 14 and 21 post infection (pi). Based on FACS analysis (Fig 1A, S1 Fig in S1 Text), three main cell subsets were identified in the liver of infected mice: Ly6C+ 'inflammatory' monocytes (CX3CR1int CD11bhi CD115hi MHC-II - to int CD62Lhi F4/80int Mertk- CD64lo CD11c- Mar-1-), Ly6C- 'patrolling' monocytes (CX3CR1hi CD11bhi CD115hi MHC-II - to lo CD11ahi F4/80lo Mertk- CD64- CD11cint Mar-1-) and macrophages (Ly6C- CD11bint CX3CR1int F4/80hi Mertk+ MHC-IIhi CD115lo CD64hi CD11c- Mar-1-) [15–19]. When addressing the dynamics of these three distinct liver myeloid cell subsets, Ly6C+ monocytes were found to be recruited predominantly at day 7 pi (Fig 1A) when a M1-type inflammatory immune response is mounted to control the first peak of parasitemia [4, 6], while the Ly6C- monocytes and the macrophages accumulated in the late stage of infection at day 21 pi (Fig 1A), when a M2-type/regulatory immune response develops and controls the liver damage caused by the M1-type response [7, 11]. Moreover, the Ly6C+ monocytes and the macrophages mobilized in the liver of infected mice were prominently MHC-II - to int and MHC-IIint to hi, respectively, while the MHC-II - to lo fraction of the Ly6C- monocytes accumulated (Fig 1B, S2 Fig in S1 Text). As compared to blood monocytes, liver Ly6C+ monocytes showed an increased F4/80 and MHC-II expression and a decreased CD115 expression (Fig 1C, S2 Fig in S1 Text), suggesting their maturation upon entering the liver of T. congolense-infected mice. Blood Ly6C- monocytes only exhibited decreased CD115 expression upon entry in the liver (Fig 1C, S2 Fig in S1 Text).
Fig. 1. Distinct liver myeloid cell subsets are sequentially mobilized in T. congolense-infected mice. 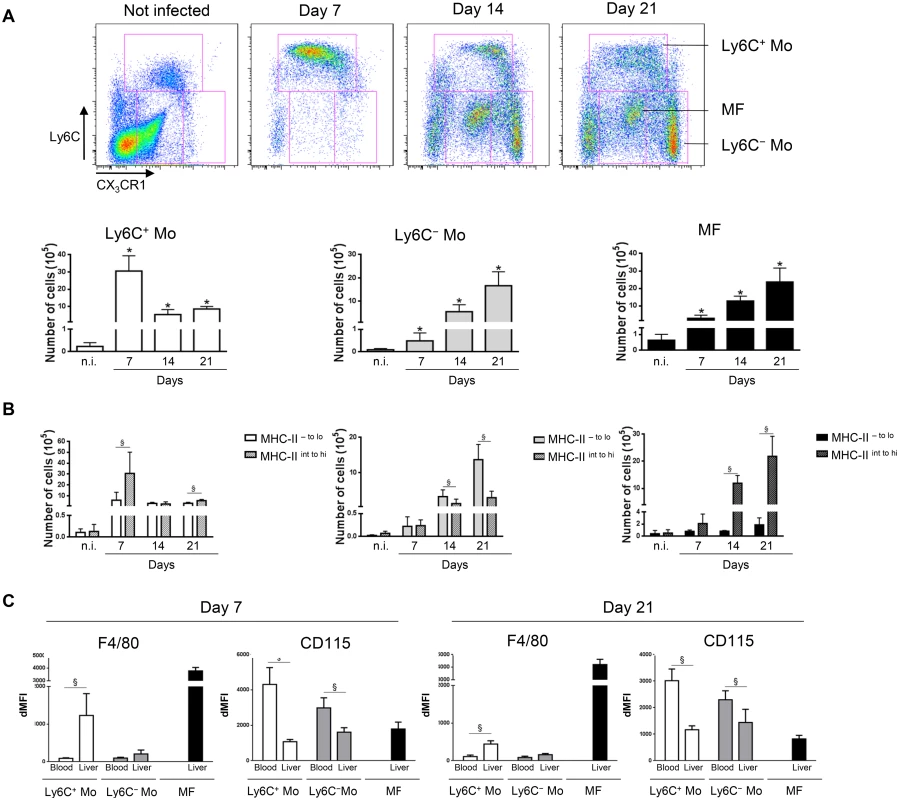
Ly6C+ monocytes (Ly6C+ Mo), Ly6C- monocytes (Ly6C- Mo) and macrophages (MF) were gated based on Ly6C and CX3CR1/GFP expression as illustrated in S1A Fig in S1 Text in non-infected (n.i) and infected CX3CR1-GFP+/- mice at day 7, 14 and 21 pi. Numbers of (A) the 3 myeloid cell subsets and (B) of the MHC-II- to lo and MHC-IIint to hi fraction of Ly6C+ monocytes, Ly6C- monocytes and macrophages within liver non-parenchymal cell population were determined. Data are shown as mean + SD of 3 individual mice from one representative out of four independent experiments. * p< 0.05 compared to non-infected mice; § p<0.05 comparing populations linked by horizontal bar. (C) F4/80 or CD115 expression on Ly6C+ monocytes, Ly6C- monocytes and macrophages from the blood or the liver at day 7 and 14 pi determined as mean fluorescence intensity difference (dMFI) between anti-F/80 or anti-CD115 and isotype control antibodies. Ly6C+ monocytes differentiate into macrophages in the liver of T. congolense-infected mice
In view of the sequential accumulation of myeloid cell subsets in the liver of infected mice (Fig 1), the turnover of Ly6C+ and Ly6C- monocytes and their relationship with the macrophage pool was investigated. When BrdU was continuously administered in infected mice, blood Ly6C+ monocytes became more rapidly BrdU+ than liver Ly6C+ monocytes concomitant with the acquisition of Ki67 expression (S3 Fig in S1 Text), indicating a rapid turnover and/or proliferation rate of the circulating Ly6C+ monocytes. In comparison, liver macrophages and even more blood and liver Ly6C- monocytes showed a longer lag-phase in BrdU incorporation and a KI67lo and Ki67- profile, respectively, pointing to a lower turnover and/or proliferation rate of the 2 latter cell populations (S3 Fig in S1 Text). These data suggest that BrdU+ Ly6C+ monocytes can differentiate into a macrophage and/or a Ly6C- monocyte population in the liver of infected mice. Accordingly, when Ly6C+ monocytes isolated from the liver of T. congolense—infected CD45.2 LysM-GFP mice (in which myeloid cells that express LysM are labeled with GFP; [20]) were transferred in infected CD45.1 wild-type (WT) mice, they mainly acquired within 72 h, a CD11bint Ly6C- MHC-II+ F4/80hi CD64lo Mertkhi but CD11c- and Mar-1- profile (Fig 2A) representing macrophages (S1 Fig in S1 Text; [16, 18, 19]). In contrast, Ly6C- monocytes from T. congolense—infected CX3CR1-GFP+/- CD45.2 mice transferred in CD45.1 mice maintained within 96 h post-transfer a CX3CR1hi Ly6C- profile and did not acquire F4/80 and MHC-II expression (S4 Fig in S1 Text). A minor fraction of the transferred GFP+ Ly6C+ monocytes acquired a Ly6C- F4/80lo MHC-II- phenotype (Fig 2A), similar to the Ly6C- monocyte phenotype (S1 Fig in S1 Text). These data and the longest lag-phase in BrDU incorporation for Ly6C- monocytes observed in S3 Fig in S1 Text, suggested that Ly6C+ monocytes could differentiate into Ly6C- monocytes during T. congolense-induced inflammation. Thus, we investigated whether the reduction of circulating and liver Ly6C+ monocytes occurring in CCR2-/- mice infected with African trypanosome [21] affected the presence of the other liver myeloid cell subsets. The absence of GFP reporter gene in the CCR2-/- mice at our disposition required the setting of an alternative gating strategy to distinguish the Ly6C+ monocyte, the Ly6C- monocyte and the macrophage cell subsets (S5A Fig in S1 Text). This strategy was (i) based on the differential expression of CD115, Ly6C, MHC-II and F4/80 molecules by these three cell populations reported in S1 Fig in S1 Text, and (ii) validated using CX3CR1-GFP+/- mice (S5B Fig in S1 Text). At day 7 and 21 pi, T. congolense-infected CCR2-/- mice exhibited not only a lower amount of Ly6C+ monocytes, but also of Ly6C- monocytes in the blood and the liver (S5C Fig in S1 Text), confirming that some Ly6C+ monocytes can differentiate into Ly6C- monocytes in infected mice. The reduced amount of monocytes in infected CCR2-/- mice coincided with a trend towards lower amount of liver macrophages (S5CFig in S1 Text). Together, these data indicate that Ly6C+ monocytes differentiate mainly into macrophages but also into Ly6C- monocytes in the liver of T. congolense-infected mice. In contrast, Ly6C- monocytes did not acquire macrophage differentiation markers.
Fig. 2. Ly6C+ monocytes differentiate into macrophages in T. congolense-infected mice. 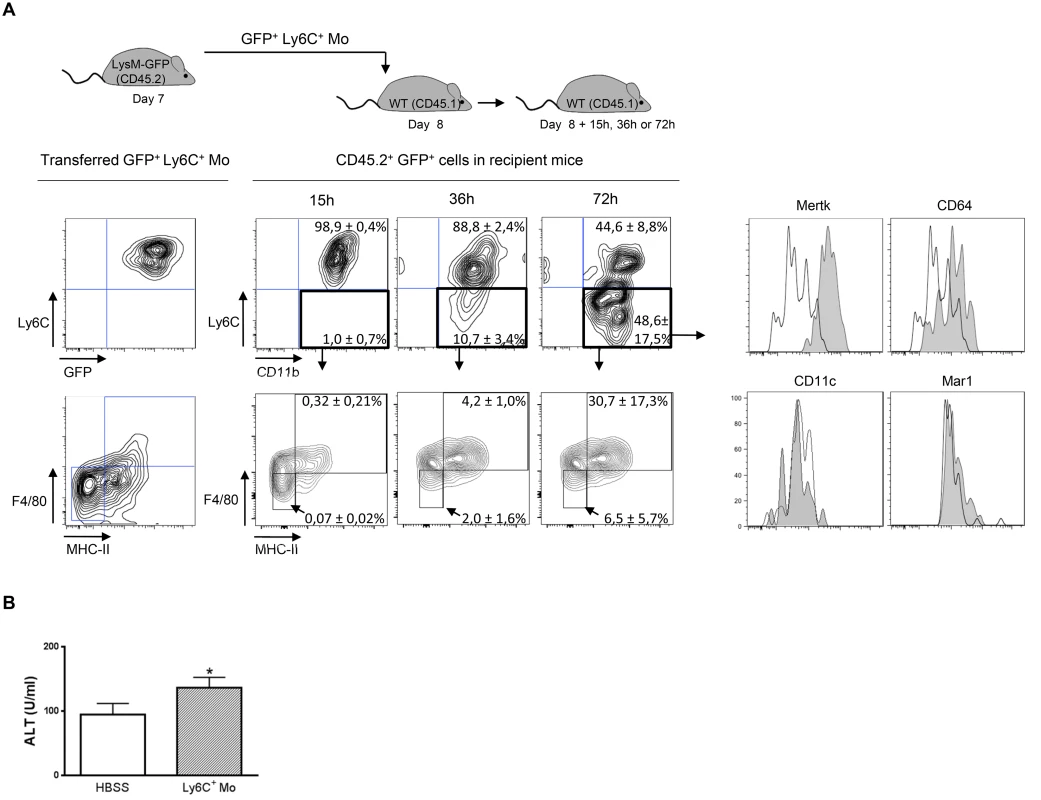
(A) Liver GFP+ Ly6C+ monocytes (Ly6C+ Mo) purified from CD45.2 LysM-GFP mice at day 7 pi were transferred in CD45.1 WT mice at d8 pi. After 15, 36 and 72 h, liver CD45.2+ GFP+ cells from recipient mice were analysed for CD11b and Ly6C expression. MHC-II, F4/80, Mertk, CD64, CD11c and Mar1 expression was then investigated in Ly6C- CD11b+ cells. FACS profiles are representative of 1 out of 9 mice tested in three independent experiments. Percentages of cells in indicated gates are as shown as mean ± SD of 3 individual mice of one representative out of three independent experiments. (B) ALT levels in mice treated with Ly6C+ monocytes or with HBSS as control. Data are shown as mean + SD of 3 individual mice of one representative out of three independent experiments. * p<0.05 compared to control mice. Liver myeloid cell subsets from trypanosome-infected mice exhibit different activation status
The transfer of Ly6C+ monocytes at day 6 pi increased liver injury reflected by increased alanine aminotransferase (ALT) serum levels in T. congolense-infected mice (Fig 2B), confirming the pathogenic nature of these cells [21]. These cells were the major source of TNF in the liver of infected mice (Fig 3A). Our previous research established that liver CD11b+ Ly6G- myeloid cells contributed to IL-10 production and expressed genes associated with a M2-type activation, hereby limiting the production of hepatotoxic TNF by Ly6C+ monocytes [7, 10, 21]. In view of the expansion of both the Ly6C- monocyte and the macrophage populations within liver CD11b+ myeloid cells at this stage of T. congolense infection (Fig 1A), their relative contribution to IL-10 production and M2-type activation was investigated at day 21 pi. As compared to non-fractionated CD11b+ myeloid cells, the Il10 gene expression was similarly increased in Ly6C- monocytes and macrophages, while down-regulated in Ly6C+ monocytes (Fig 3B). Moreover, investigating the expression of genes that are induced by IL-10 in M2-type non-fractionated CD11b+ cells during African trypanosome infection [7, 10, 22], showed that Folr2, Ctss, Mgl2 and Sepp1 expression was upregulated in macrophages but not in the other myeloid cell subsets from T. congolense—infected mice; Ngfb and Arg1 expression was induced to similar levels in the three myeloid cell subpopulations; and F13a1 expression was induced at higher level in Ly6C+ monocytes than in the other myeloid cell subpopulations (S6A Fig in S1 Text). Concerning M1-type genes triggered in non-fractionated CD11b+ myeloid from T. congolense—infected mice [7, 10], Tnf and to lower extent Nos2 expression was upregulated mainly in Ly6C+ monocytes, and Ccl2, Cxcl9 and Cxcl10 expression was induced in macrophages. With the exception of Ccl2, none of the tested genes showed induced expression in Ly6C- monocytes compared to the non-fractionated CD11b+ myeloid cell population (S6B Fig in S1 Text). These data suggest that Ly6C- monocytes and macrophages were the main IL-10 producers within liver CD11b+ myeloid cell subsets in the late stage of T. congolense infection. Moreover, the three myeloid cell subsets showed differential activation status, Ly6C+ monocytes expressing mainly M1-type genes, Ly6C- monocytes expressing mainly IL-10 and macrophages expressing a mix of M1/M2-type genes.
Fig. 3. Ly6C+ monocytes are the main producers of TNF while Ly6C- monocytes and macrophages are the major source of IL-10 in T. congolense-infected mice. 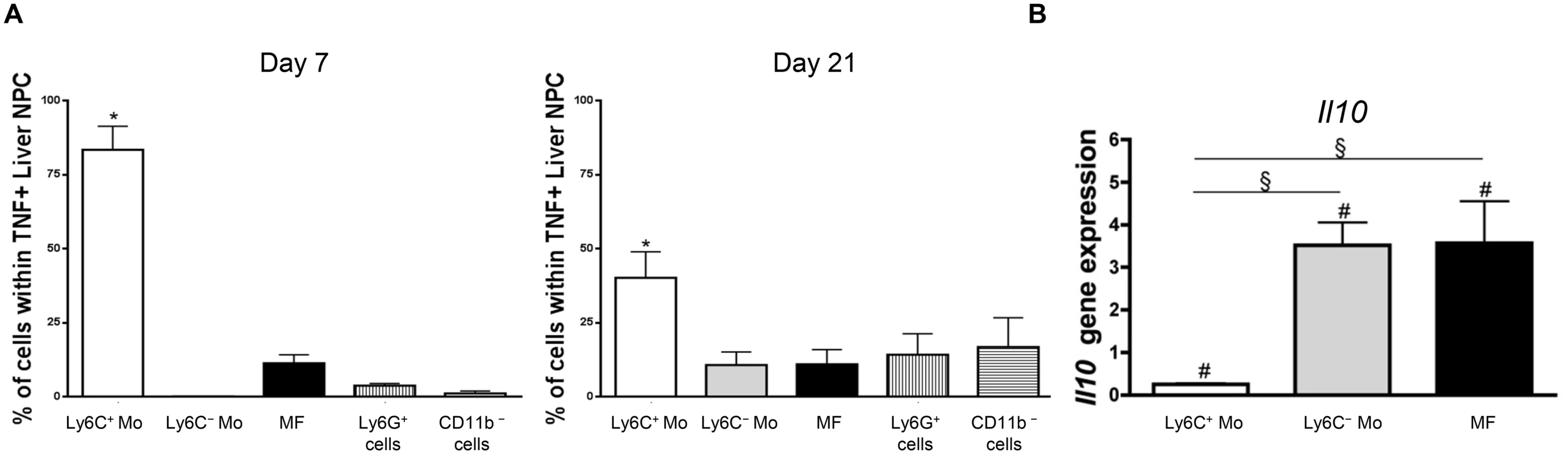
(A) Intracellular TNF+ cells were gated in liver non-parenchymal cells from CX3CR1-GFP+/- mice at day 7 and 21 pi. The percentage of Ly6C+ monocytes (Ly6C+ Mo), Ly6C- monocytes (Ly6C- Mo), macrophages (MF) gated as in Fig 1A, and of CD11b+ Ly6G+ neutrophils and CD11b- cell was determined within TNF+ cells. Data are shown as mean + SD of 3 individual mice from one representative out of three independent experiments. * p<0.05 compared to other populations (B) Il10 gene expression in liver Ly6C+ monocytes, Ly6C- monocytes and macrophages purified from CX3CR1-GFP+/- mice at day 21 pi was normalized against S12 gene expression and expressed relatively to gene expression in non-fractionated CD11b+ liver cells. Data are shown as mean + SD of 3 individual mice from one representative out of three independent experiments. # p<0.05 compared to non-fractionated CD11b+ myeloid cells; § p<0.05 comparing populations linked by horizontal bar. Ly6C- monocytes limit the production of TNF by Ly6C+ monocytes during trypanosome-induced liver injury
The expansion of IL-10-producing Ly6C- monocytes and the decline of pathogenic TNF-producing Ly6C+ monocytes in late stage T. congolense—infected mice prompted us to investigate whether the former monocytes exerted anti-inflammatory/hepatoprotective activity. We focused on the role of the MHC-II - to lo Ly6C- monocyte fraction since this population was found to accumulate in the course of infection (Fig 1B; S2 Fig in S1 Text).
In in vitro co-cultures, Ly6C- monocytes purified from the liver at day 21 pi were found to reduce the production of TNF by liver Ly6C+ monocytes isolated at day 7 pi (Fig 4A), when their production of TNF was maximal [7, 10, 11]. To validate these data in vivo, Ly6C- monocytes were sorted from T. congolense-infected CD45.2 CX3CR1-GFP+/- mice at day 21 pi and transferred in CD45.1 WT mice at day 4 and 6 pi. At day 7 pi, Ly6C+ monocytes from recipient mice expressed a higher intracellular level of TNF than the CD11b+ Ly6C- cell fraction (Fig 4B) that includes macrophages and Ly6C- monocytes (S1 Fig in S1 Text). The two latter cell populations are thus marginal contributors to TNF production as compared to Ly6C+ monocytes in infected mice (in agreement with Fig 3A). More importantly, the intracellular production of TNF decreased in the Ly6C+ monocyte but not in the CD11b+ Ly6C- cell fraction in recipient mice treated with Ly6C- monocytes as compared to control-treated mice (Fig 4B). The decrease in TNF production in Ly6C+ monocytes from recipient mice treated with Ly6C- monocytes coincided with a reduced production of TNF in non-parenchymal cell culture supernatants and reduced liver damage (Fig 4C and 4D). These data suggest that Ly6C- monocytes restrained TNF production by Ly6C+ monocytes and hereby exerted hepatoprotective function in T. congolense—infected mice. To support these data obtained by augmenting the Ly6C- population in infected animals through adoptive transfer experiments, we adopted a reverse strategy, using Nr4a1-/- mice in which Ly6C- monocytes are almost absent from the circulation due to apoptosis in the bone marrow [23]. Non-parenchymal liver cells from Nr4a1-/- and WT mice cultured ex-vivo secreted similar amount of TNF. Moreover, in both mouse strains, the percentage of TNF producing Ly6C+ monocytes and macrophages as well as their respective TNF production were comparable (S7 Fig in S1 Text), confirming that the Nr4a1 transcription factor did not affect the capacity of Ly6C+ monocytes and macrophages to secrete TNF [24]. In T. congolense—infected Nr4a1-/- mice, the reduction of the liver Ly6C- monocyte population coincided with the increased accumulation of Ly6C+ monocytes (Fig 5A). These cells produced more TNF (Fig 5B), resulting in increased production of TNF in non-parenchymal cell culture supernatants as well as in the blood (Fig 5C and 5D), coupled with increased ALT serum levels (Fig 5E). Moreover, although the percentage of liver macrophages decreased in Nr4a1-/- mice (S8 Fig in S1 Text), no difference in their absolute number was observed (Fig 5A) due to the higher amount of liver non-parenchymal isolated from infected Nr4a1-/- mice. The TNF production by CD11b+ Ly6C- cells that include mainly macrophages marginally increased (Fig 5B). Furthermore, the lack of Ly6C- monocytes and the decrease in percentage of the macrophage population in the liver of infected Nr4a1-/- mice associated with reduced IL-10 levels in cell culture supernatants and in blood (Fig 5F and 5G), confirming these two cell types as potential sources of IL-10 in T. congolense—infected mice (Fig 3B).
Fig. 4. Ly6C- monocytes limit TNF production by Ly6C+ monocytes in T. congolense-infected mice. 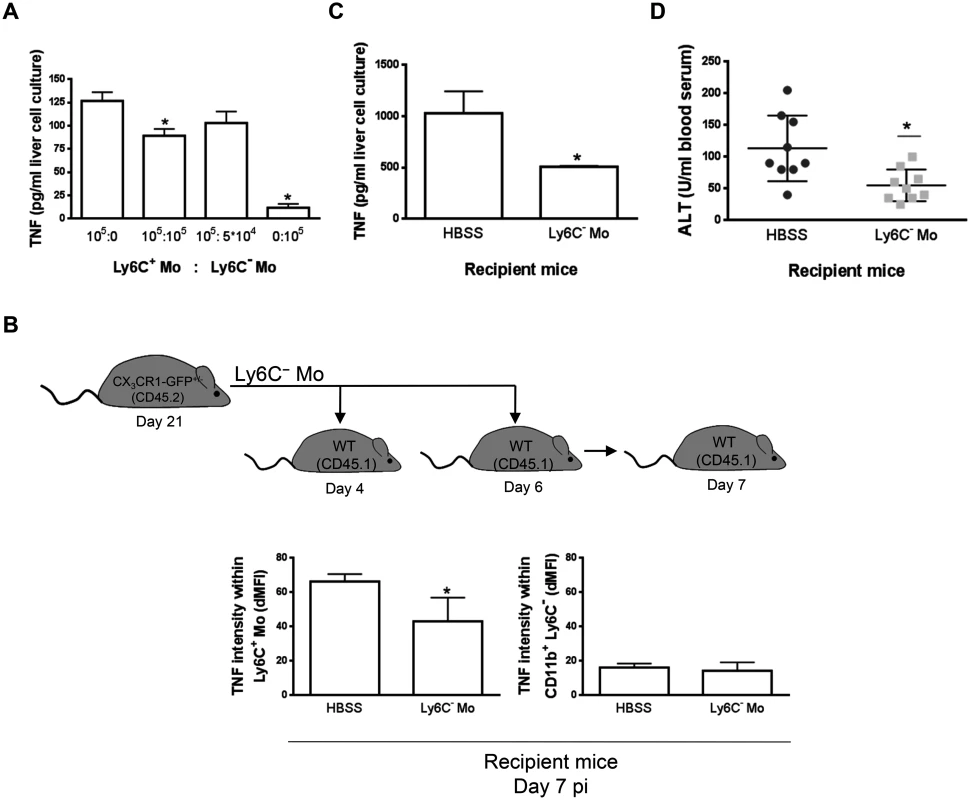
(A) Liver Ly6C+ monocytes (Ly6C+ Mo) and MHC-II- to lo Ly6C- monocytes (Ly6C- Mo) purified from CX3CR1-GFP+/- mice at day 7 pi and 21 pi, respectively, were cultured at indicated ratio. TNF concentration in cell supernatants was measured. Data are shown as mean + SD of 1 representative out of five independent experiments. * p<0.05 compared to Ly6C+ monocytes cultured alone. (B-D) Liver MHC-II- to lo Ly6C- monocytes purified from CD45.2 CX3CR1-GFP+/- mice at day 21 pi were transferred in CD45.1 WT mice at day 4 and day 6 pi. Control mice received HBSS. Different parameters were evaluated in recipient mice at d7 pi: (B) Spontaneous TNF levels in liver Ly6C+ monocytes (left panel) and CD11b+ Ly6C- cells (right panel) from control or Ly6C- monocyte-treated mice determined as mean fluorescence intensity difference (dMFI) between anti-TNF and isotype control antibodies. (C) Spontaneous TNF concentration in supernatants of liver non-parenchymal cell cultures and (D) serum alanine aminotransferase (ALT) levels. Data are shown as mean + SD of 3 individual mice of one representative out of three independent experiments. * p<0.05 compared to control mice. Fig. 5. Depletion of Ly6C- monocytes in Nr4a1-/- mice increases TNF production and liver damage in T. congolense—infected mice. 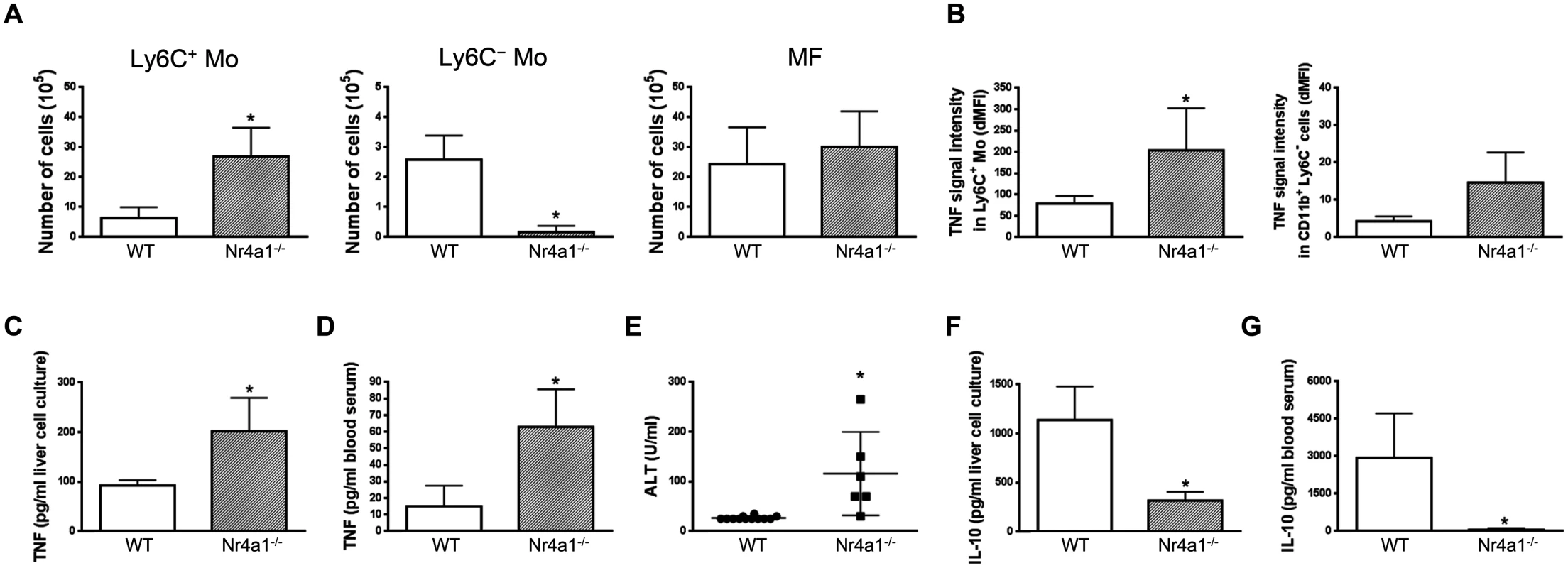
Different parameters were evaluated in WT and Nr4a1-/- mice at day 21 pi. Liver Ly6C+ monocytes (Ly6C+ Mo), Ly6C- monocytes (Ly6C- Mo) and macrophages (MF) were gated as in S5A Fig in S1 Text. (A) Numbers of Ly6C+ monocytes, Ly6C- monocytes and macrophages within liver non-parenchymal cell population. (B) Spontaneous TNF levels in Ly6C+ monocytes and CD11b+ Ly6C- cells determined as mean fluorescence intensity difference (dMFI) between anti-TNF and isotype control antibodies. (C,D) TNF and (F,G) IL-10 concentration in (C,F) supernatants of liver non-parenchymal cell cultures or (D,G) in blood serum. (E) ALT levels in blood serum. Data are shown as mean + SD of 3 individual mice of one representative out of five independent experiments. *p<0.05 compared to WT mice. IL-10 contributes to the Ly6C- monocyte-regulatory activity during trypanosome infection
Ly6C- monocytes could contribute to the production of IL-10 in T. congolense—infected mice (Fig 3; Fig 5F and 5G) and limit the production of TNF by Ly6C+ monocytes (Fig 4A–4C; Fig 5B–5D). Therefore, we investigated whether the limitation of TNF production by Ly6C- monocytes was mediated by IL-10. Two types of experiments were performed.
First, TNF production was monitored in co-cultures containing liver Ly6C- and Ly6C+ monocytes sorted from mice at day 21 and day 7 pi, respectively, in the presence of neutralizing anti-IL-10R antibody (Fig 6A). Blocking the IL-10R signaling ablated the limitation of TNF production observed when Ly6C+ monocytes were cultured with Ly6C- monocytes. In contrast, IL-10R signaling neutralization had no effect on the production of TNF when Ly6C+ monocytes were cultured alone.
Fig. 6. Ly6C- monocytes through IL-10 production limit TNF production by Ly6C+ monocytes in T. congolense-infected mice. 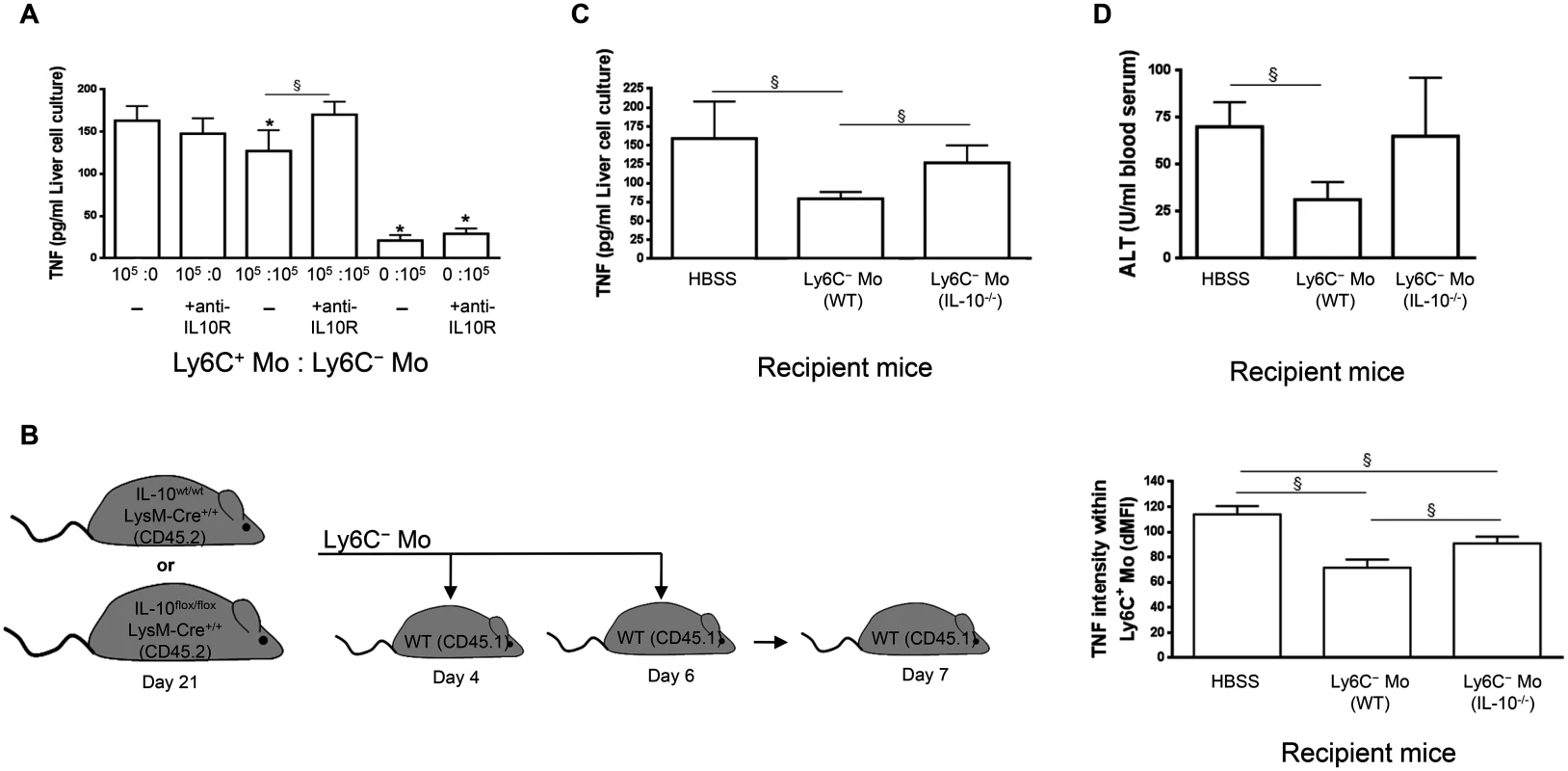
(A) Liver Ly6C+ monocytes (Ly6C+ Mo) and MHC-II- to lo Ly6C- monocytes (Ly6C- Mo) purified from CX3CR1-GFP+/- mice at day 7 pi and 21 pi, respectively, were cultured alone or together at indicated ratio in presence of control (-) or neutralising anti-IL-10R antibody. TNF concentration in cell supernatants was measured. Data are shown as mean + SD of 1 out of five independent experiments. * p<0.05 compared to Ly6C+ monocytes cultured alone, § p<0.05 comparing populations linked by horizontal bar. (B-D) Liver MHC-II- to lo Ly6C- monocytes gated as described in Fig 5A in S1 Text purified from IL-10wt/wt LysM-cre+/+ or IL-10flox/flox LysM-cre+/+ mice at day 21 pi were transferred in CD45.1 WT mice at day 4 and day 6 pi. Control mice received HBSS. Different parameters were evaluated in recipient mice at day 7 pi: (B) TNF levels in liver Ly6C+ monocytes determined as mean fluorescence intensity difference (dMFI) between anti-TNF and isotype control antibodies, (C) Spontaneous TNF concentration in liver non-parenchymal cell culture supernatants, and (D) ALT levels in blood serum. Data are shown as mean + SD of 3 individual mice of one representative out of three independent experiments. § p<0.05 comparing populations linked by horizontal bar. Second, liver Ly6C- monocytes were isolated at day 21 pi from CD45.2 T. congolense—infected IL-10flox/flox LysM-cre+/+ mice, which lack IL-10 production specifically in LysM-expressing myeloid cells and hereby exhibit increased TNF levels and increased liver damage [7, 25]. These cells were adoptively transferred in CD45.1 WT mice at day 4 and 6 pi and the intracellular production of TNF by liver Ly6C+ monocytes was investigated at day 7 pi. As shown in Fig 6B, TNF production was impaired to a greater extent in recipient mice treated with Ly6C- monocytes from control IL-10wt/wt LysM-cre+/+ mice than in recipient mice treated with Ly6C- monocytes from IL-10flox/flox LysM-cre+/+ mice. Moreover, the secretion of TNF was reduced in cell culture supernatants of recipient mice treated with Ly6C- monocytes from control IL-10wt/wt LysM-cre+/+ mice but not from IL-10flox/flox LysM-cre+/+ mice (Fig 6C). Finally, ALT levels were reduced in recipient mice treated with Ly6C- monocytes from control IL-10wt/wt LysM-cre+/+ mice but not from IL-10flox/flox LysM-cre+/+ mice (Fig 6D). Together these data suggest that Ly6C- monocytes could hamper TNF production by Ly6C+ monocytes partly via their IL-10 production and hereby reduce liver injury in T. congolense—infected mice.
Ly6C- monocytes promote the differentiation of Ly6C+ monocytes into macrophages during trypanosome-induced liver inflammation
Transfer experiment of Ly6C+ monocytes in recipient mice suggested that a fraction of the liver macrophages in T. congolense—infected mice originate from Ly6C+ monocytes (Fig 2A). Moreover, the liver macrophage population reduced while the Ly6C+ cell population increased in infected Nr4a1-/- mice lacking Ly6C- monocytes (S8 Fig in S1 Text). In contrast, in infected mice treated with Ly6C- monocytes, the Ly6C- CX3CR1int macrophage population increased (Fig 7A). Therefore, we investigated whether Ly6C- monocytes shaped by T. congolense infection could favor the differentiation of Ly6C+ monocytes into macrophages. We used Ly6C- monocytes from day 21 pi because their clear accumulation at that stage of infection coincided with increased macrophage accumulation (Fig 1A) and with a shift from a pathogenic to anti-pathogenic immune response required for trypanotolerance [7, 10, 11]. GFP+ Ly6C+ monocytes isolated from LysM-GFP mice at day 7 pi were adoptively transferred, with or without Ly6C- monocytes isolated from CD45.2 mice at day 21 pi, in recipient CD45.1 WT mice at day 12 pi. Liver CD45.2+ GFP+ cells were characterized 48 h later (Fig 7B). In mice that were not treated with Ly6C- monocytes, 45 ± 4.6% of the GFP+ monocytes differentiated into Ly6C- CD11bint MHC-II+ cells (Fig 7B) that represented macrophages (S1 Fig in S1 Text). These percentages increased to 73 ± 8.7% in mice treated with Ly6C- monocytes (Fig 7B). TNF production decreased and IL-10 production increased in non-parenchymal cell culture supernatants from mice treated with Ly6C- monocytes, and serum ALT level decreased (Fig 7C–7E). These data suggest that in the liver of T. congolense—infected mice, Ly6C- monocytes promote the differentiation of Ly6C+ monocytes into macrophages and switch the cytokine milieu from hepatodestructive to hepatoprotective. This finding was confirmed by transferring GFP+ Ly6C+ monocytes or GFP+ Ly6C+ monocytes plus Ly6C- monocytes isolated from infected WT mice in Nr4a1-/- recipients that do not produce Ly6C- monocytes (Fig 8A). In mice co-treated with Ly6C- monocytes, the percentage of liver GFP+ cells that gained F4/80 and MHC-II expression and lowered their expression of CD11b, reflecting a differentiation towards macrophages, increased and coincided with reduced TNF and ALT level as well as with increased IL-10 level (Fig 8A–8D).
Fig. 7. Ly6C- monocytes induce differentiation of Ly6C+ monocytes into macrophages in T. congolense-infected WT mice. 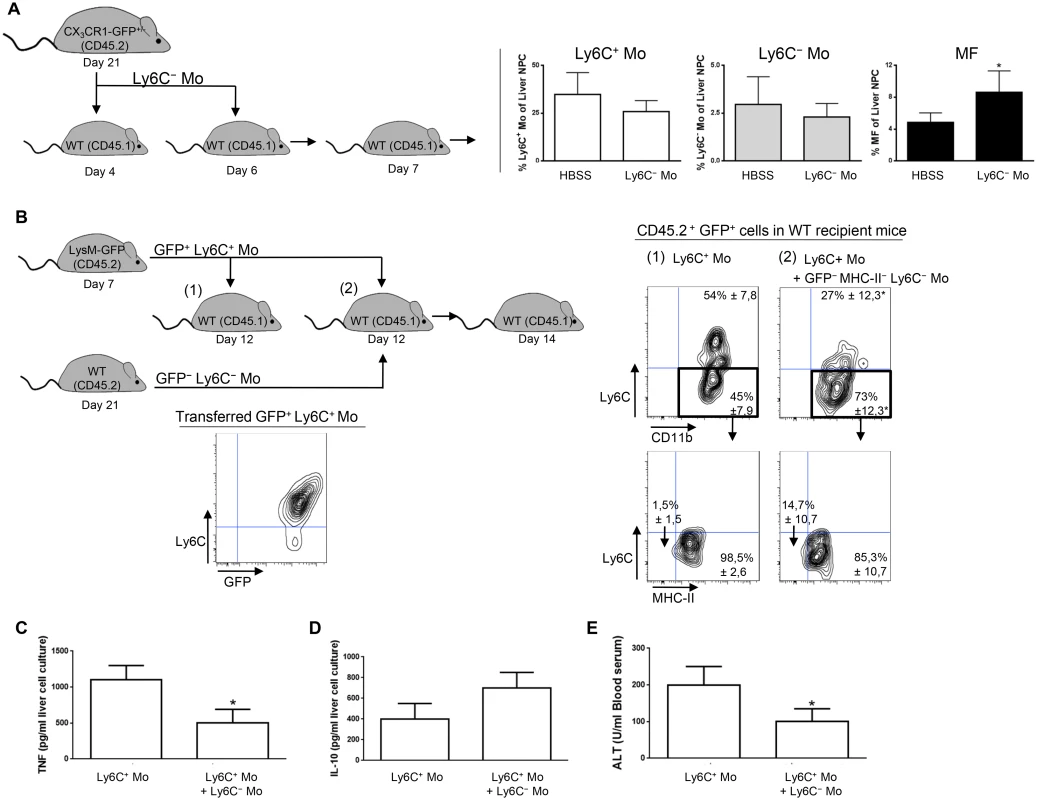
(A) Liver MHC-II- to lo Ly6C- monocytes (Ly6C- Mo) purified from CX3CR1-GFP+/- mice at day 21 pi were transferred in CD45.1 WT mice at day 4 and day 6 pi. Control mice received HBSS. At day 7 pi, percentages of Ly6C+ monocytes (Ly6C+ Mo), Ly6C- monocytes (Ly6C- Mo) and macrophages (MF) within liver non-parenchymal cells were determined in recipient mice. Data are shown as mean + SD of 3 individual mice of one representative out of three independent experiments. *p<0.05 compared to control mice. (B) GFP+ Ly6C+ monocytes purified from CD45.2 LysM-GFP mice at day 7 pi were transferred in CD45.1 WT mice at day 12 pi with or without MHC-II-to lo Ly6C- monocytes gated as described in S5A Fig in S1 Text and purified from CD45.2 WT mice at day 21 pi. After 48 h, liver CD45.2+ GFP+ cells in recipient mice were analyzed for CD11b and Ly6C expression. MHC-II expression was then investigated in Ly6C- CD11b+ cells. FACS profiles are representative of 1 out of 9 mice tested in three independent experiments. Percentages of cells in indicated gates are shown as mean ± SD of 3 individual mice of one representative out of three independent experiments. (C) TNF and (D) IL-10 concentration in supernatants of liver non-parenchymal cell cultures from recipient mice. (E) ALT levels in blood serum from recipient mice. Data are shown as mean + SD of 3 individual mice of one representative out of three independent experiments. *p<0.05 compared to recipient mice receiving only Ly6C+ monocytes. Fig. 8. Ly6C- monocytes induce differentiation of Ly6C+ monocytes into macrophages in T. congolense-infected Nr4a1-/- mice that lack endogenous Ly6C- monocytes. 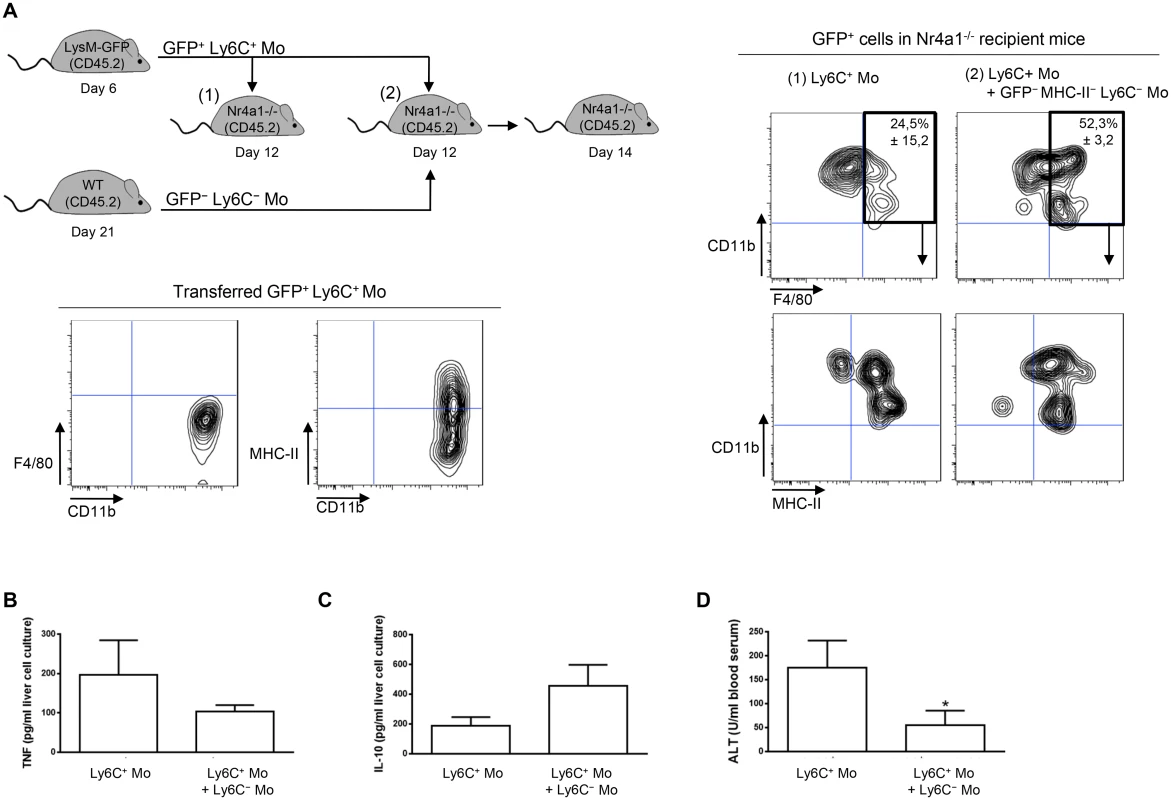
(A) GFP+ Ly6C+ monocytes (Ly6C+ Mo) purified from CD45.2 LysM-GFP mice at day 7 pi were transferred in CD45.2 Nr4a1-/- mice at day 12 pi with or without MHC-II-to lo Ly6C- monocytes (Ly6C- Mo) gated as described in Fig 5A in S1 Text and purified from CD45.2 WT mice at day 21 pi. After 48 h, liver GFP+ cells in recipient mice were analyzed for CD11b and F4/80 expression. MHC-II expression was then investigated in CD11b+ F4/80+ cells. FACS profiles are representative of 1 out of 9 mice tested in three independent experiments. Percentages of cells in indicated gates are shown as mean ± SD of 3 individual mice of one representative out of three independent experiments. (B) TNF and (C) IL-10 concentration in supernatants of liver non-parenchymal cell cultures from recipient mice. (D) ALT levels in blood serum from recipient mice. Data are shown as mean + SD of 3 individual mice of one representative out of three independent experiments. *p<0.05 compared to recipient mice receiving only Ly6C+ monocytes. To address a mechanistic basis for how Ly6C- monocytes could promote the differentiation of Ly6C+ monocytes into macrophages, Ly6C+ monocytes and GFP+ Ly6C- monocytes purified from T. congolense-infected mice at day 6 and 21 pi, respectively, were co-cultured with and without a transwell plate. The Ly6C+ cell differentiation to macrophages was investigated after 7 days, gating out the GFP+ cells (Fig 9A). The GFP- cell population exhibited reduced expression of Ly6C and increased expression of F4/80, MerTK, CD64 and MHC-II, reflecting macrophage differentiation of the Ly6C+ monocytes, yet only when the two monocyte populations were in contact. The capacity of Ly6C- monocytes to inhibit the production of TNF by Ly6C+ monocytes was also partially ablated when the two cell populations were separated from each other (Fig 9B). Thus, a cell contact between the Ly6C+ and the Ly6C- monocytes contributed to the differentiation of Ly6C+ cells into macrophages and to the inhibition of TNF production.
Fig. 9. Differentiation of Ly6C+ monocytes into macrophages requires a physical contact with Ly6C- monocytes. 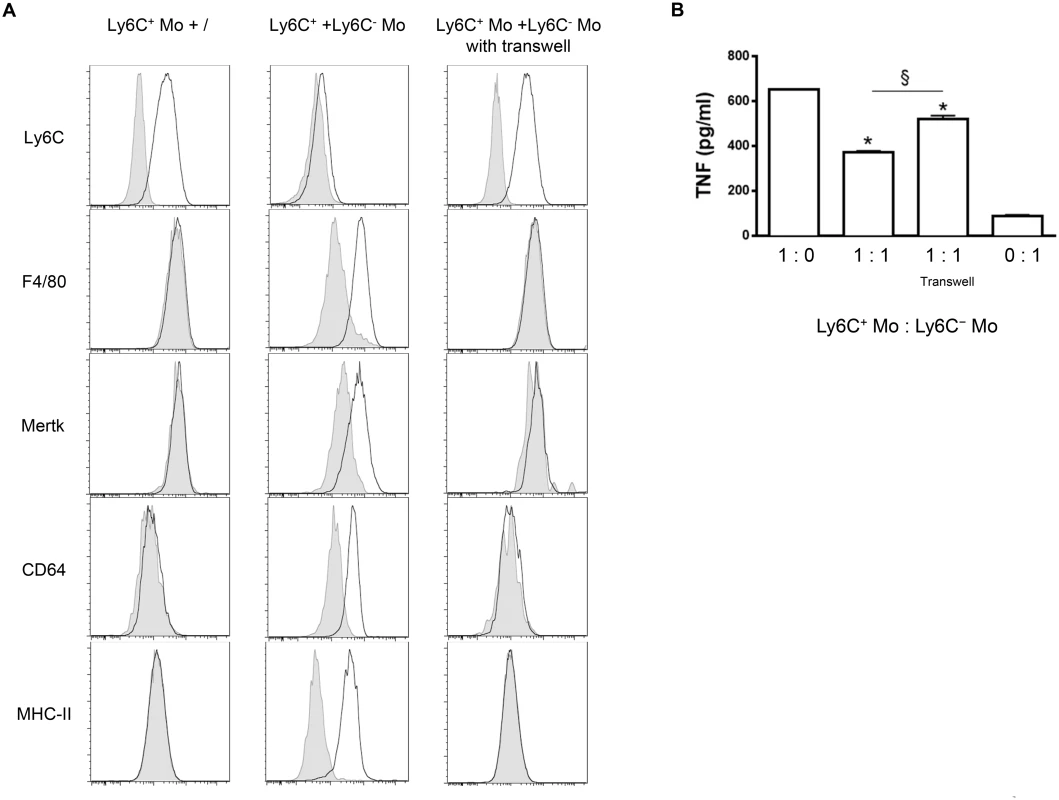
(A) Ly6C+ monocytes (Ly6C+ Mo) purified from T. congolense-infected CD45.2 WT mice at day 6 pi were cultured alone or with MHC-II- to lo Ly6C- monocytes (Ly6C- Mo) purified from infected CD45.2 LysM-GFP mice at day 21 pi (1: 1 ratio). Similar cultures were performed in transwell plates. Expression of Ly6C, F4/80, Mertk, CD64 or MHC-II (white line) was investigated 7 days later on GFP- Ly6C+ monocyte-derived cells gated as in S5A Fig in S1 Text (grey line: isotype control). Results are representative of 1 out of 9 mice tested in 3 independent experiments. (B) TNF concentration in cell culture supernatants was measured. Data are shown as mean + SD of 1 representative out of three independent experiments. * p<0.05 compared to Ly6C+ monocytes cultured alone; § p<0.05 comparing populations linked by horizontal bar. Discussion
Dynamics of liver myeloid cells in infected mice
As in other life-threatening liver inflammation models [26–28], liver cell necrosis is thought to be mainly mediated by TNF-producing Ly6C+ monocytes during experimental T. congolense infection in trypanotolerant C57BL/6 mice [7]. However, in trypanotolerant mice infected with this intravascular parasite, an IL-10-dependent, Foxp3+ Treg and CD11b+ myeloid cell-mediated immune response develops after the control of the first peak of parasitemia to regulate the inflammatory condition, hereby preserving liver integrity and preventing early death of the host. In this regard, our reported data suggest that myeloid cell-derived IL-10 only deactivates the pathogenic activity of M1-type Ly6C+ monocytes while Tregs affected both pathogenic T cells and myeloid cells [7, 11, 29]. Here, the heterogeneity and dynamic of liver myeloid cells in T. congolense-infected mice was further detailed. We now report that the mounted anti-inflammatory immune response was concomitant with the mobilization/accumulation in the liver of Ly6C- monocytes (CX3CR1hi CD11bhi CD115hi CD11ahi F4/80lo CD64- CD11cint) and macrophages (CX3CR1int CD11bint F4/80hi CD115lo CD64hi CD11c-) coupled with a decreased presence of Ly6C+ monocytes (CX3CR1int CD11bhi CD115hi CD62Lhi F4/80int CD64lo CD11c-). The Ly6C+ monocytes and the macrophages mobilized in the liver of infected mice were prominently MHC-II - to int and MHC-IIint to hi, while the MHC-II - to lo fraction of Ly6C- monocytes mounted up. The switch from Ly6C+ monocytes to Ly6C- monocytes and macrophages in the course of T. congolense infection reflected the change from a TNF, hepatodestructive to an IL-10, hepatoprotective milieu [11]. Accordingly, we show that Ly6C+ monocytes were the main producers of TNF in infected mice while Ly6C- monocytes and macrophages contribute to IL-10 gene and protein expression. A similar sequential mobilization of TNF+ Ly6C+ and IL-10+ Ly6C- myeloid cell subsets in the acute and chronic phase of various inflammation models has been highlighted [30–33].
Fate and function of Ly6C- monocytes in infected mice
It is commonly agreed that circulating Ly6C+ and Ly6C- monocytes—depending on the pathogenic insult, their fate, timing of recruitment and location—exert tissue beneficial or detrimental functions. Hereby, Ly6C+ monocytes can infiltrate a variety of inflamed tissues, where they down-regulate their Ly6C expression and differentiate into M1 - or M2-type macrophages that exert destructive or protective function in the initiation or resolving phase of inflammation, respectively [31–37]. Similarly, tissue-accumulating Ly6C- monocytes can exert healing or damaging activity in different models of sterile and non-sterile inflammation [38–41]. While it was proposed that Ly6C- monocytes can differentiate into macrophages or DCs in inflamed tissues [32, 38–43], it is now commonly agreed that even in steady state condition Ly6C- monocytes are not bona fide monocytes but represent terminally differentiated "housekeeper" blood-resident macrophages crawling along the endothelium of the vessels [19, 38, 42, 44–47].
Using adoptive transfer experiments, we evidenced the differentiation of Ly6C+ monocytes mainly into Ly6C- F4/80hi Mertkhi MHC-II+ CD11c- Mar-1- macrophages in the liver of T. congolense trypanotolerant mice (Fig 10). This differentiation pathway thus differed from the more commonly acknowledged differentiation of Ly6C+ monocytes into TIP DCs evidenced in trypanosusceptible mice or in bacterial infection [48, 49]. Similarly, in fibrotic liver, fibrogenic Ly6C+ monocytes rapidly differentiate into Ly6C- "restorative" macrophages [33, 50]. Moreover, using BudU labelling, adoptive transfer experiments and CCR2-/- mice, we confirmed that circulating CCR2-dependent Ly6C+ monocytes could also partially give rise to Ly6C- monocytes in this inflammatory setting [19, 42, 44, 45]. The Ly6C- monocytes that expanded during T. congolense infection did not seem to differentiate into macrophages or DCs as suggested by their lower ex situ expression of Pu1, Cmaf and Mafb genes and their low level of MHC-II molecule expression, as well as by maintenance of their F4/80- MHC-II - to lo phenotype after transfer in infected animals. However, we did not exclude that these cells were terminally differentiated macrophages [18, 19, 42, 44].
Fig. 10. Overview of the interactions between Ly6C+ monocytes, Ly6C- monocytes and macrophages contributing to trypanotolerance. 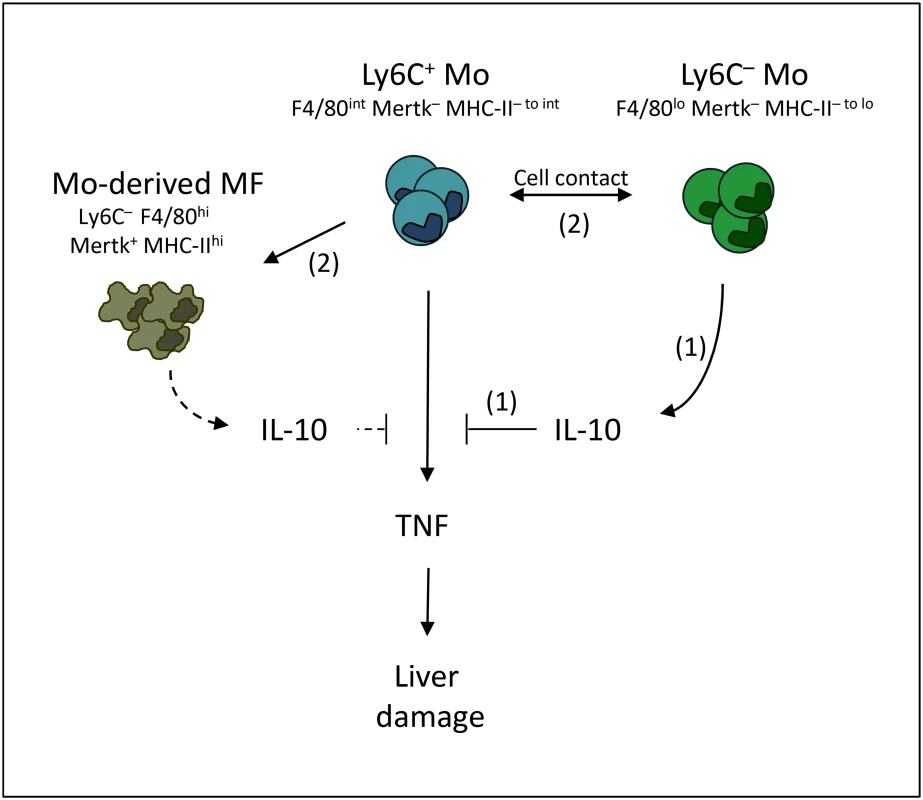
TNF-producing Ly6C+ monocytes contribute to liver injury in T. congolense-infected mice. Ly6C- monocytes hamper the pathogenic activity of Ly6C+ monocytes through (1) IL-10 production and (2) cell contact that trigger the differentiation of Ly6C+ monocytes into restorative macrophages. Whether IL-10 possibly produced by monocyte-derived macrophages (dashed lines) contribute to hepatoprotective activity is not elucidated. Very limited data exist about the functional role of Ly6C- monocytes in the outcome of liver inflammation [51]. These cells do not seem to be mobilized in the liver during fibrosis progression [50], but a recent report suggests that the intrahepatic Ly6C- monocyte population increases in a model of non-alcoholic fatty liver disease (NAFLD) provided mice receive a drug treatment that ameliorates the disease by decreasing inflammation [52]. We report here that that the liver Ly6C- monocyte population expanded during T. congolense infection and exerted a hepatoprotective/restorative function. Indeed, the absence of Ly6C- monocytes in infected Nr4a1-/- mice resulted in higher TNF production and increased liver damage. In contrast, treatment of infected WT or Nr4a1-/- mice with Ly6C- monocytes triggered the differentiation of Ly6C+ monocytes into macrophages, limited TNF production, resulting in hepatoprotection. The limiting effect of Ly6C- monocytes on TNF production by Ly6C+ monocytes was partially mediated by cell contact and by IL-10 (Fig 10). Indeed, the inhibition of TNF production was partially restored when the Ly6C+ monocytes and Ly6C- monocytes were physically separated. Moreover, the treatment of infected mice with Ly6C- monocytes from control IL-10wt/wt LysM-cre+/+ mice reduced the production of TNF by Ly6C+ monocytes and the liver damage more efficiently than the treatment with Ly6C- monocytes from IL-10flox/flox LysM-cre+/+ mice lacking IL-10 gene expression in myeloid cells. Furthermore, the capacity of Ly6C- monocytes to differentiate Ly6C+ monocytes into macrophages also required a cell contact. In this regard, PD-L1 or PD-1 surface expression by Ly6C- monocytes was reported to trigger tolerogenic immune response [53, 54]. Moreover, soluble mediators like M-CSF, GM-CSF, IL-6, TGF-β, PGE2 and retinoic acid could contribute to the differentiation of Ly6C+ monocytes into macrophages and/or to inhibition of TNF production [32, 55–60].
The Ly6C+ monocytes, Ly6C- monocytes and macrophages from T. congolense-infected mice exhibited a differential activation status. Ly6C+ monocytes expressed mainly M1-type genes, Ly6C- monocytes expressed mainly IL-10 and macrophages expressed a mix of M1 - and M2-type genes. Such a mixed expression of M1 - and M2-type genes was previously associated with the restorative phenotype of anti-fibrotic liver macrophages [33]. The distinct gene profile of Ly6C- monocytes and macrophages suggested that these cells exerted different functions in protecting the liver against T. congolense-induced injury. In this regard, macrophages expanding in the liver of T. congolense—infected mice expressed higher level than Ly6C- monocytes of the M2-type gene selenoprotein P (Sepp1), that we documented to exert anti-oxidant hepatoprotective function during infection [10]. We could not ascertain directly that Ly6C+ monocyte-derived macrophages developed a restorative activation program in vivo because the low amount of adoptively transferred cells homing in the liver of infected mice did not consent their purification. Of note, it has become evident in the recent years that upon sterile and non-sterile inflammation, monocyte subsets originating from bone marrow and their derived macrophages complement the long-lived tissue-resident macrophage pool that originate from embryonic precursors [44, 61–64]. The current view is that in inflamed tissue, the resident macrophage pool restores homeostasis by local proliferation, by adopting a M2-type activation status and by secreting IL-10 [65–67]. Thus, besides monocyte-derived macrophages, tissue-resident macrophages are candidates to develop a restorative activation program during the late stage of T. congolense infection. However, no definitive reporter mice currently allow to discriminate the liver resident macrophages from the recruited monocyte-derived macrophages, hampering investigating their particular role, including M1/M2-type activation and proliferative status, in tissue pathogenicity.
In conclusion, we reveal that in the liver of T. congolense-infected mice Ly6C- monocytes and macrophages were major sources of the anti-pathogenic cytokine IL-10. Ly6C- monocytes exerted a hepatoprotective function through their IL-10 production and through cell-contact by limiting the TNF production by pathogenic Ly6C+ monocytes and by inducing the differentiation of the latter cells into restorative macrophages.
Materials and Methods
Ethic statement
Experiments, maintenance and care of mice complied with guidelines of the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (CETS n°123) and were approved by the Ethical Committee for Animal Experiments of the Vrije Universiteit Brussel, Brussels, Belgium (laboratory accreditation number 121 0220, project number 11-220-12).
Mice, parasites, infections
CD45.2 C57BL/6 mice: CX3CR1-GFP+/+ mice were bred in house with CD45.2 WT mice purchased from Janvier, France to obtain CX3CR1-GFP+/- mice. IL-10flox/flox mice kindly donated by Dr. A. Roers (Department of Immunology, Technical University of Dresden, Germany) were crossed with IL-10wt/wt LysM-cre+/+ mice (Jackson Laboratory, USA) to generate IL-10flox/flox LysM - cre+/+ mice. LysM-GFP mice, kindly provided by Dr. T. Graf (Center for Genomic Regulation, Barcelona, Spain) and Nr4a1 (Nur77)-/- mice were bred in house. CD45.1 WT C57BL/6 mice were bred in-house.
T. congolense variant antigen type 13 (Tc13, kindly provided by Dr. H. Tabel, University of Saskatchewan, Canada) stored at -80°C were injected in cyclophosphamide-treated WT C57BL/6 mice. At day 4 post infection, mice were bled, parasites were purified by diethyl-aminoethyl-cellulose chromatography and used to infect experimental female, age-matched (8–14 weeks) mice (2000 parasites/mouse, ip).
Isolation of liver non-parenchymal immune cells and adoptive transfer of monocytes
Animals were euthanized (CO2) and livers were perfused through the portal vein with 10 ml of 120 U/ml collagenase type III (Worthington Biochemical Corporation) in HBSS (Gibco). Then, liver was disrupted mechanically and incubated in 10 ml of a 100 U/ml collagenase III solution in HBSS (25 min, 37°C). The resulting cell suspension was passed through a 70-μm nylon mesh filter and washed by adding 40 ml of HBSS supplemented with 10% FCS and 2 mM EDTA (HBSS/FCS/EDTA) followed by centrifugation (300 g, 8 min, 4°C). After erythrocyte lysis (0.83% NH4Cl in 0.01M Tris-HCl, pH 7.2), the pellet was resuspended in 10 ml of Lymphoprep (Lucron Bioproducts) and overlaid with 10 ml HBSS/FCS/EDTA. After centrifugation (430 g, 30 min, 20°C), the layer of low-density cells at the interface containing liver non-parenchymal cells was harvested, washed with HBSS containing 10% FCS (HBSS/FCS) and centrifuged (300 g, 10 min, 4°C). Cells were resuspended in HBSS/FCS, counted while excluding dead cells using trypan blue and diluted to working concentrations (5 x 106/ml). Liver immune cells and buffers were kept on ice during isolation protocols and subsequent analysis. CD11b+ Ly6C+ Ly6G+ granulocytes were not retained within the non-parenchymal fractions due to their high-density characteristic.
At indicated time-points post infection, unfractionated CD11b+ liver immune cells were isolated using magnetic separation columns according to the manufacturer’s protocol (Miltenyi Biotec). BD FACSAria II (BD Biosciences) was then used to sort from CD11b+ cells, Ly6C- monocytes from CX3CR1-GFP+/- (CD11bhi MHC-II- CX3CR1hi), Ly6C- monocytes from WT, IL-10wt/wt LysM-cre+/+ or IL-10flox/flox LysM-Cre+/+ mice (CD11bhi CD115+ MHC-II-) or Ly6C+ monocytes from CX3CR1-GFP+/-, WT or LysM-GFP mice (CD11bhi CD115+). Macrophages were sorted from CD11b+ cells of CX3CR1-GFP+/- mice as CD115+ F4/80hi Ly6C- CX3CR1int cells.
For adoptive transfer experiments, recipient mice were treated iv with Ly6C- monocytes or Ly6C+ monocytes (8 x 105 cells/mouse). Control mice were treated with HBSS. One day after the last transfer, liver immune cells were isolated and intracellular spontaneous production of TNF in Ly6C+ CD11b+ monocytes and Ly6C- CD11b+ cells was analyzed by FACS. In addition, liver immune cells were cultured for 24 h and TNF concentration in supernatants was measured.
Cell cultures and supernatants
At indicated time-points post infection, liver immune cells (2 x 106/ml, 24-well plates) were cultured in RPMI 1640 (Gibco) supplemented with 10% FCS, 100 U/ml penicillin, 100 mg/ml streptomycin, 0.1 mM non-essential amino acids, 2 mM L-glutamine (all from Invitrogen Life Technologies). Alternatively, Ly6C+ and Ly6C- monocytes were cultured at indicated ratio in 200 μl (96-well plates). Cell supernatants were collected 1 day later. When required, cultures were treated with 3 μg/ml of anti-IL10R antibody (clone 1B1.3a, kindly provided by Dr. O. Leo, ULB, Gosselies, Belgium) or control antibody (rat IgG1, BD Biosciences). For in vitro macrophage differentiation test, liver sorted Ly6C+ monocytes (2 x 105) and GFP+ Ly6C- monocytes (2 x 105) were cultured without adding M-CSF in 200 μl medium. Cells and supernatants were harvested 7 days later. When required, Ly6C+ monocytes and GFP+ Ly6C- monocytes were plated in the bottom and upper compartments of transwell plates (Costar, 0.4 μm), respectively.
Quantification of cytokines
TNF and IL-10 was quantified in cell supernatants or in blood serum using specific sandwich ELISAs (R&D Systems), in accordance to the manufacturers’ protocols.
Extracellular and intracellular FACS analysis
For extracellular stainings, liver immune cells (106) were transferred in FACS tubes and stained for 20 min in ice-cold HBSS supplemented with 0.5% FCS using conventional protocols. Cells were pre-incubated with anti-FcγR antibody before adding commercial labeled surface marker antibodies (S1 Table in S1 Text). For intracellular stainings, cells (Ly6C+ CD11b+ monocytes, Ly6C- CD11b+ cells) were cultured 4 h with brefeldin A (BD Biosciences), subsequently fixed and permeabilized following the manufacturer's instructions (BD Biosciences). Cells were then stained with PE-conjugated anti-TNF (clone MP6-XT22) or IgG1κ isotype control (R3-34) antibody and washed with ice-cold PBS. Cells were analyzed on a FACSCanto II (BD Biosciences). Delta-Median Fluorescence Intensity (dMFI) was calculated as difference between MFI of anti-TNF and isotype control antibody staining. Analyses were performed using FlowJo software.
Quantitative real-time PCR
RNA extracted using the RNeasy plus mini kit (Qiagen) was reverse-transcribed with oligo(dT) and SuperScript II RT (Invitrogen), following the manufacturer's instructions. Quantitative real-time PCR was performed in an iCycle, with iQ SYBR Green Supermix (Bio-Rad). Primer sequences are listed in S2 Table in S1 Text. PCR cycles consisted of 1-minute denaturation at 94°C, 45-second annealing at 55°C, and 1-minute extension at 72°C. Gene expression was normalized using ribosomal S12 (Mrps12) protein as a housekeeping gene.
Alanine aminotransferase (ALT) levels
ALT levels were measured in serum samples using a commercial kit (Boehringer).
Statistical analysis
All comparisons were tested for statistical significance using the unpaired t test from GraphPad Prism 4.0 software.
Supporting Information
Zdroje
1. Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335 : 936–941. doi: 10.1126/science.1214935 22363001
2. Sears BF, Rohr JR, Allen JE, Martin LB. The economy of inflammation: when is less more? Trends Parasitol. 2011;27 : 382–387. doi: 10.1016/j.pt.2011.05.004 21680246
3. Shi M, Pan W, Tabel H. Experimental African trypanosomiasis: IFN-gamma mediates early mortality. Eur J Immunol. 2003;33 : 108–118. 12594839
4. Shi M, Wei G, Pan W, Tabel H. Trypanosoma congolense infections: antibody-mediated phagocytosis by Kupffer cells. J Leukoc Biol. 2004;76 : 399–405. 15136584
5. Magez S, Radwanska M, Drennan M, Fick L, Baral TN, Brombacher F, et al. Interferon-gamma and nitric oxide in combination with antibodies are key protective host immune factors during trypanosoma congolense Tc13 Infections. J Infect Dis. 2006;193 : 1575–1583. 16652287
6. Magez S, Radwanska M, Drennan M, Fick L, Baral TN, Allie N, et al. Tumor necrosis factor (TNF) receptor-1 (TNFp55) signal transduction and macrophage-derived soluble TNF are crucial for nitric oxide-mediated Trypanosoma congolense parasite killing. J Infect Dis. 2007;196 : 954–962. 17703428
7. Bosschaerts T, Morias Y, Stijlemans B, Hérin M, Porta C, Sica A, et al. IL-10 limits production of pathogenic TNF by M1 myeloid cells through induction of nuclear NF-κB p50 member in Trypanosoma congolense infection-resistant C57BL/6 mice. Eur J Immunol. 2011;41 : 3270–3280. doi: 10.1002/eji.201041307 21805465
8. Lu W, Wei G, Pan W, Tabel H. Trypanosoma congolense Infections: Induced Nitric Oxide Inhibits Parasite Growth In Vivo. J Parasitol Res. 2011;2011 : 316067. doi: 10.1155/2011/316067 21584233
9. Salmon D, Vanwalleghem G, Morias Y, Denoeud J, Krumbholz C, Lhommé F, et al. Adenylate cyclases of Trypanosoma brucei inhibit the innate immune response of the host. Science. 2012;337 : 463–466. doi: 10.1126/science.1222753 22700656
10. Bosschaerts T, Guilliams M, Noel W, Hérin M, Burk RF, Hill KE, et al. Alternatively activated myeloid cells limit pathogenicity associated with African trypanosomiasis through the IL-10 inducible gene selenoprotein P. J Immunol. 2008;180 : 6168–6175. 18424738
11. Guilliams M, Oldenhove G, Noel W, Hérin M, Brys L, Loi P, et al. African trypanosomiasis: naturally occurring regulatory T cells favor trypanotolerance by limiting pathology associated with sustained type 1 inflammation. J Immunol. 2007;179 : 2748–2757. 17709488
12. Bourdi M, Masubuchi Y, Reilly TP, Amouzadeh HR, Martin JL, George JW, et al. Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology. 2002;35 : 289–298. 11826401
13. Erhardt A, Biburger M, Papadopoulos T, Tiegs G. IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. Hepatology. 2007;45 : 475–485. 17256743
14. Carambia A, Herkel J. CD4 T cells in hepatic immune tolerance. J Autoimmun. 2010;34 : 23–28. doi: 10.1016/j.jaut.2009.08.006 19720498
15. Langlet C, Tamoutounour S, Henri S, Luche H, Ardouin L, Grégoire C, et al. CD64 expression distinguishes monocyte-derived and conventional dendritic cells and reveals their distinct role during intramuscular immunization. J Immunol. 2012;188 : 1751–1760. doi: 10.4049/jimmunol.1102744 22262658
16. Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity. 2013;38 : 322–335. doi: 10.1016/j.immuni.2012.10.016 23352232
17. Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19 : 71–82. 12871640
18. Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13 : 1118–1128. doi: 10.1038/ni.2419 23023392
19. Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE, et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39 : 599–610. doi: 10.1016/j.immuni.2013.08.007 24012416
20. Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood. 2000;96 : 719–726. 10887140
21. Bosschaerts T, Guilliams M, Stijlemans B, Morias Y, Engel D, Tacke F, et al. Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-gamma and MyD88 signaling. PLoS Pathog. 2010;6(8): e1001045. doi: 10.1371/journal.ppat.1001045 20714353
22. Ghassabeh GH, De Baetselier P, Brys L, Noël W, Van Ginderachter JA, Meerschaut S, et al. Identification of a common gene signature for type II cytokine-associated myeloid cells elicited in vivo in different pathologic conditions. Blood. 2006;108 : 575–583. 16556895
23. Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, et al. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C - monocytes. Nat Immunol. 2011;12 : 778–785. doi: 10.1038/ni.2063 21725321
24. Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, et al. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110 : 416–427. doi: 10.1161/CIRCRESAHA.111.253377 22194622
25. Siewe L, Bollati-Fogolin M, Wickenhauser C, Krieg T, Müller W, Roers A. Interleukin-10 derived from macrophages and/or neutrophils regulates the inflammatory response to LPS but not the response to CpG DNA. Eur J Immunol. 2006;36 : 3248–3255. 17111348
26. Tacke F, Luedde T, Trautwein C. Inflammatory pathways in liver homeostasis and liver injury. Clin Rev Allergy Immunol. 2009;36 : 4–12. doi: 10.1007/s12016-008-8091-0 18600481
27. Helk E, Bernin H, Ernst T, Ittrich H, Jacobs T, Heeren J, et al. TNFα-mediated liver destruction by Kupffer cells and Ly6Chi monocytes during Entamoeba histolytica infection. PLoS Pathog. 2013;9(1):e1003096. doi: 10.1371/journal.ppat.1003096 23300453
28. Perry BC, Soltys D, Toledo AH, Toledo-Pereyra LH. Tumor necrosis factor-α in liver ischemia/reperfusion injury. J Invest Surg. 2011;24 : 178–188. doi: 10.3109/08941939.2011.568594 21675854
29. Shi MQ, Wei GJ, Tabel H. Trypanosoma congolense infections: MHC class II-restricted immune responses mediate either protection or disease, depending on IL-10 function. Parasite Immunol. 2007;29 : 107–111. 17241399
30. Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res. 2013;112 : 1624–1633. doi: 10.1161/CIRCRESAHA.113.300890 23743228
31. Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204 : 1057–1069. 17485518
32. Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013;38 : 555–569. doi: 10.1016/j.immuni.2013.02.012 23477737
33. Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109: E3186–195. doi: 10.1073/pnas.1119964109 23100531
34. Hamers AA, Vos M, Rassam F, Marinković G, Marincovic G, Kurakula K, et al. Bone marrow-specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circ Res. 2012;110 : 428–438. doi: 10.1161/CIRCRESAHA.111.260760 22194623
35. Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117 : 185–194. 17200718
36. Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327 : 656–661. doi: 10.1126/science.1178331 20133564
37. Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325 : 612–616. doi: 10.1126/science.1175202 19644120
38. Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, et al. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153 : 362–375. doi: 10.1016/j.cell.2013.03.010 23582326
39. Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317 : 666–670. 17673663
40. Amano H, Amano E, Santiago-Raber ML, Moll T, Martinez-Soria E, Fossati-Jimack L, et al. Selective expansion of a monocyte subset expressing the CD11c dendritic cell marker in the Yaa model of systemic lupus erythematosus. Arthritis Rheum. 2005;52 : 2790–2798. 16142734
41. Misharin AV, Cuda CM, Saber R, Turner JD, Gierut AK, Haines GK, et al. Nonclassical Ly6C(-) Monocytes Drive the Development of Inflammatory Arthritis in Mice. Cell Rep. 2014;9 : 591–604. doi: 10.1016/j.celrep.2014.09.032 25373902
42. Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38 : 79–91. doi: 10.1016/j.immuni.2012.12.001 23273845
43. Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37 : 1076–1090. doi: 10.1016/j.immuni.2012.08.026 23219392
44. Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14 : 392–404. doi: 10.1038/nri3671 24854589
45. Sunderkötter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172 : 4410–4417. 15034056
46. Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204 : 171–180. 17190836
47. Michaud JP, Bellavance MA, Préfontaine P, Rivest S. Real-time in vivo imaging reveals the ability of monocytes to clear vascular amyloid beta. Cell Rep. 2013;5 : 646–653. doi: 10.1016/j.celrep.2013.10.010 24210819
48. Guilliams M, Movahedi K, Bosschaerts T, VandenDriessche T, Chuah MK, Hérin M, et al. IL-10 dampens TNF/inducible nitric oxide synthase-producing dendritic cell-mediated pathogenicity during parasitic infection. J Immunol. 2009;182 : 1107–1118. 19124754
49. Serbina NV, Shi C, Pamer EG. Monocyte-mediated immune defense against murine Listeria monocytogenes infection. Adv Immunol. 2012;113 : 119–134. doi: 10.1016/B978-0-12-394590-7.00003-8 22244581
50. Karlmark KR, Weiskirchen R, Zimmermann HW, Gassler N, Ginhoux F, Weber C, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50 : 261–274. doi: 10.1002/hep.22950 19554540
51. Tacke F. Functional role of intrahepatic monocyte subsets for the progression of liver inflammation and liver fibrosis in vivo. Fibrogenesis Tissue Repair. 2012;5(Suppl 1):S27. 23259611
52. McMahan RH, Wang XX, Cheng LL, Krisko T, Smith M, El Kasmi K, et al. Bile acid receptor activation modulates hepatic monocyte activity and improves nonalcoholic fatty liver disease. J Biol Chem. 2013;288 : 11761–1170. doi: 10.1074/jbc.M112.446575 23460643
53. Peng Y, Latchman Y, Elkon KB. Ly6C(low) monocytes differentiate into dendritic cells and cross-tolerize T cells through PDL-1. J Immunol. 2009;182 : 2777–2785. doi: 10.4049/jimmunol.0803172 19234172
54. Ka MB, Gondois-Rey F, Capo C, Textoris J, Million M, Raoult D, et al. Imbalance of circulating monocyte subsets and PD-1 dysregulation in Q fever endocarditis: the role of IL-10 in PD-1 modulation. PLoS One. 2014;9(9):e107533. doi: 10.1371/journal.pone.0107533 25211350
55. Chomarat P, Banchereau J, Davoust J, Karolina Palucka A. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1 : 510–514. 11101873
56. Heusinkveld M, de Vos van Steenwijk PJ, Goedemans R, Ramwadhdoebe TH, Gorter A, Welters MJ, et al. M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J Immunol. 2011;187 : 1157–1165. doi: 10.4049/jimmunol.1100889 21709158
57. den Hartog G, van Altena C, Savelkoul HF, van Neerven RJ. The mucosal factors retinoic acid and TGF-β1 induce phenotypically and functionally distinct dendritic cell types. Int Arch Allergy Immunol. 2013;162 : 225–236. doi: 10.1159/000353243 24022014
58. Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33 : 119–126. doi: 10.1016/j.it.2011.12.001 22277903
59. Nascimento M, Huang SC, Smith A, Everts B, Lam W, Bassity E, et al. Ly6Chi monocyte recruitment is responsible for Th2 associated host-protective macrophage accumulation in liver inflammation due to schistosomiasis. PLoS Pathog. 2014;10(8):e1004282. doi: 10.1371/journal.ppat.1004282 25144366
60. Gasson JC. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood. 1991;77 : 1131–1145. 2001448
61. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496 : 445–455. doi: 10.1038/nature12034 23619691
62. Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38 : 792–804. doi: 10.1016/j.immuni.2013.04.004 23601688
63. Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209 : 1167–1181. doi: 10.1084/jem.20120340 22565823
64. Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336 : 86–90. doi: 10.1126/science.1219179 22442384
65. Davies LC, Rosas M, Jenkins SJ, Liao CT, Scurr MJ, Brombacher F, et al. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat Commun. 2013;4 : 1886. doi: 10.1038/ncomms2877 23695680
66. Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332 : 1284–1288. doi: 10.1126/science.1204351 21566158
67. Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19 : 1166–1172. doi: 10.1038/nm.3258 23933982
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary TuberculosisČlánek Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic PlagueČlánek Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host SpeciesČlánek Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR SelectionČlánek Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus GenomesČlánek Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from FerrioxamineČlánek Remembering MumpsČlánek Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling PathwayČlánek Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 5- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Parasites and Their Heterophagic Appetite for Disease
- The Elusive Role of the Prion Protein and the Mechanism of Toxicity in Prion Disease
- Intestinal Colonization Dynamics of
- Activation of Typhi-Specific Regulatory T Cells in Typhoid Disease in a Wild-Type . Typhi Challenge Model
- The Engineering of a Novel Ligand in gH Confers to HSV an Expanded Tropism Independent of gD Activation by Its Receptors
- Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis
- Group Selection and Contribution of Minority Variants during Virus Adaptation Determines Virus Fitness and Phenotype
- Phosphatidic Acid Produced by Phospholipase D Promotes RNA Replication of a Plant RNA Virus
- A Ribonucleoprotein Complex Protects the Interleukin-6 mRNA from Degradation by Distinct Herpesviral Endonucleases
- Characterization of Transcriptional Responses to Different Aphid Species Reveals Genes that Contribute to Host Susceptibility and Non-host Resistance
- Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic Plague
- Natural Killer Cell Sensing of Infected Cells Compensates for MyD88 Deficiency but Not IFN-I Activity in Resistance to Mouse Cytomegalovirus
- Manipulation of the Xanthophyll Cycle Increases Plant Susceptibility to
- Ly6C Monocytes Regulate Parasite-Induced Liver Inflammation by Inducing the Differentiation of Pathogenic Ly6C Monocytes into Macrophages
- Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host Species
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Experimental Evolution of an RNA Virus in Wild Birds: Evidence for Host-Dependent Impacts on Population Structure and Competitive Fitness
- Inhibition and Reversal of Microbial Attachment by an Antibody with Parasteric Activity against the FimH Adhesin of Uropathogenic .
- The EBNA-2 N-Terminal Transactivation Domain Folds into a Dimeric Structure Required for Target Gene Activation
- Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR Selection
- The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive via Activation of α5β1 Integrin at the Macrophage-Amebae Intercellular Junction
- Sequential Conformational Changes in the Morbillivirus Attachment Protein Initiate the Membrane Fusion Process
- A Two-Component DNA-Prime/Protein-Boost Vaccination Strategy for Eliciting Long-Term, Protective T Cell Immunity against
- cAMP-Signalling Regulates Gametocyte-Infected Erythrocyte Deformability Required for Malaria Parasite Transmission
- Response Regulator VxrB Controls Colonization and Regulates the Type VI Secretion System
- Evidence for a Novel Mechanism of Influenza Virus-Induced Type I Interferon Expression by a Defective RNA-Encoded Protein
- Dust Devil: The Life and Times of the Fungus That Causes Valley Fever
- TNF-α Induced by Hepatitis C Virus via TLR7 and TLR8 in Hepatocytes Supports Interferon Signaling via an Autocrine Mechanism
- The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen,
- L-Rhamnosylation of Wall Teichoic Acids Promotes Resistance to Antimicrobial Peptides by Delaying Interaction with the Membrane
- Rapid Sequestration of by Neutrophils Contributes to the Development of Chronic Lesion
- Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus Genomes
- The Expression of Functional Vpx during Pathogenic SIVmac Infections of Rhesus Macaques Suppresses SAMHD1 in CD4 Memory T Cells
- Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from Ferrioxamine
- TRAF1 Coordinates Polyubiquitin Signaling to Enhance Epstein-Barr Virus LMP1-Mediated Growth and Survival Pathway Activation
- Vaccine-Elicited Tier 2 HIV-1 Neutralizing Antibodies Bind to Quaternary Epitopes Involving Glycan-Deficient Patches Proximal to the CD4 Binding Site
- Remembering Mumps
- The Role of Horizontal Gene Transfer in the Evolution of the Oomycetes
- Advances and Challenges in Computational Prediction of Effectors from Plant Pathogenic Fungi
- Investigating Fungal Outbreaks in the 21st Century
- Systems Biology for Biologists
- How Does the Dinoflagellate Parasite Outsmart the Immune System of Its Crustacean Hosts?
- FCRL5 Delineates Functionally Impaired Memory B Cells Associated with Exposure
- Phospholipase D1 Couples CD4 T Cell Activation to c-Myc-Dependent Deoxyribonucleotide Pool Expansion and HIV-1 Replication
- Influenza A Virus on Oceanic Islands: Host and Viral Diversity in Seabirds in the Western Indian Ocean
- Geometric Constraints Dominate the Antigenic Evolution of Influenza H3N2 Hemagglutinin
- Widespread Recombination, Reassortment, and Transmission of Unbalanced Compound Viral Genotypes in Natural Arenavirus Infections
- Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity
- A Single Protein S-acyl Transferase Acts through Diverse Substrates to Determine Cryptococcal Morphology, Stress Tolerance, and Pathogenic Outcome
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
- Mechanisms of Stage-Transcending Protection Following Immunization of Mice with Late Liver Stage-Arresting Genetically Attenuated Malaria Parasites
- The Myelin and Lymphocyte Protein MAL Is Required for Binding and Activity of ε-Toxin
- Genome-Wide Identification of the Target Genes of AP2-O, a AP2-Family Transcription Factor
- An Atypical Mitochondrial Carrier That Mediates Drug Action in
- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Helminth Infection and Commensal Microbiota Drive Early IL-10 Production in the Skin by CD4 T Cells That Are Functionally Suppressive
- Circulating Pneumolysin Is a Potent Inducer of Cardiac Injury during Pneumococcal Infection
- ExoT Induces Atypical Anoikis Apoptosis in Target Host Cells by Transforming Crk Adaptor Protein into a Cytotoxin
- Discovery of a Small Non-AUG-Initiated ORF in Poleroviruses and Luteoviruses That Is Required for Long-Distance Movement
- Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
- Varicella Viruses Inhibit Interferon-Stimulated JAK-STAT Signaling through Multiple Mechanisms
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Recovery of Recombinant Crimean Congo Hemorrhagic Fever Virus Reveals a Function for Non-structural Glycoproteins Cleavage by Furin
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



