-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Response Regulator VxrB Controls Colonization and Regulates the Type VI Secretion System
Pathogenic bacteria experience varying conditions during infection of human hosts and often use two-component signal transduction systems (TCSs) to monitor their environment. TCS consists of a histidine kinase (HK), which senses environmental signals, and a corresponding response regulator (RR), which mediates a cellular response. The genome of the human pathogen V. cholerae contains a multitude of genes encoding HKs and RRs proteins. In the present study, we systematically analyzed the role of each V. cholerae RR for its role in pathogenesis. We identified a previously uncharacterized RR, VxrB, as a new virulence factor. We demonstrated that VxrB controls expression of the type VI secretion system (T6SS), a virulence nanomachine that directly translocates effectors into bacterial or host cells, thereby facilitating colonization by competing with sister cells and intestinal microbiota. This study represents the first systematic analysis of the role of all RRs in V. cholerae pathogenesis and provides a foundation for understanding the signal transduction pathways controlling V. cholerae pathogenesis.
Published in the journal: . PLoS Pathog 11(5): e32767. doi:10.1371/journal.ppat.1004933
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004933Summary
Pathogenic bacteria experience varying conditions during infection of human hosts and often use two-component signal transduction systems (TCSs) to monitor their environment. TCS consists of a histidine kinase (HK), which senses environmental signals, and a corresponding response regulator (RR), which mediates a cellular response. The genome of the human pathogen V. cholerae contains a multitude of genes encoding HKs and RRs proteins. In the present study, we systematically analyzed the role of each V. cholerae RR for its role in pathogenesis. We identified a previously uncharacterized RR, VxrB, as a new virulence factor. We demonstrated that VxrB controls expression of the type VI secretion system (T6SS), a virulence nanomachine that directly translocates effectors into bacterial or host cells, thereby facilitating colonization by competing with sister cells and intestinal microbiota. This study represents the first systematic analysis of the role of all RRs in V. cholerae pathogenesis and provides a foundation for understanding the signal transduction pathways controlling V. cholerae pathogenesis.
Introduction
Vibrio cholerae causes the diarrheal disease cholera that affects 3 to 5 million people worldwide every year, resulting in 100,000–120,000 deaths annually [1]. V. cholerae produces a number of virulence factors which facilitate colonization of the intestine and subsequent disease. Major virulence factors are cholera toxin (CT), which is responsible for production of profuse watery diarrhea, and a type IV pilus called the toxin-coregulated pilus (TCP), which is required for intestinal colonization [2]. V. cholerae virulence factors are well known to be under extensive transcriptional control. CT and TCP production are controlled by the transcriptional activator ToxT [3, 4]. Expression of toxT, in turn, is controlled by a virulence regulatory cascade involving the membrane-bound transcriptional activators ToxRS and TcpPH. These two regulators activate toxT transcription directly [5–7]. TcpPH expression is activated by the transcriptional activators AphA and AphB [8, 9]. The quorum sensing (QS) regulatory system is also linked to the virulence gene regulatory cascade through HapR, the master QS regulator, which represses aphA expression [10].
Recently, the type VI secretion system (T6SS) has been identified as a new virulence factor in V. cholerae [11, 12]. T6SSs deliver effector proteins into both eukaryotic and bacterial cells in a contact-dependent manner [12, 13]. V. cholerae has one T6SS system with multiple T6SS effectors: VrgG1 and VrgG3 (valine-glycine repeat protein G), which have actin cross-linking activity and peptidoglycan-degrading activity, respectively [14–17]; TseL, which has lipase activity [15, 18]; and VasX, which perturbs the cytoplasmic membrane of target cells [15, 19]. Activity of these effectors is antagonized by corresponding immunity proteins: TsiV3, TsiV1, and TsiV2, respectively, to prevent killing by strains bearing these proteins [15, 16, 20, 21].
The T6SS can be divided into functional sections consisting of the core structural components, the T6SS effector and immunity proteins, and transcriptional regulators. The base of the T6SS apparatus spans the cell envelope, and is a tube within a tube. The inner tube is composed of polymers of the hemolysin coregulated protein (Hcp). The outer tube, also called the contractile sheath, is formed by polymers of VipA and VipB [14, 22]. The Hcp inner tube is capped with a spike complex of trimeric VgrG proteins. The effectors are delivered by contraction of the VipA/VipB sheath, which in turn results in ejection of the inner tube along with VgrG and the effectors towards the target cell [12].
The genes encoding the T6SS components are organized into one large cluster (VCA0105-VCA0124) and two auxiliary clusters (VCA0017-VCA0022 and VC1415-VC1421) [11, 23]. A key positive transcriptional regulator of the V. cholerae T6SS is VasH (VCA0117), which is related to enhancer binding proteins that activate transcription in a σ54 (RpoN) dependent manner [24, 25]. VasH acts on the T6SS auxiliary clusters and vgrG3 of the large cluster, but does not affect expression of the structural genes encoded in the large T6SS gene cluster [24, 26]. Additionally, Hcp production is positively regulated by the master quorum sensing regulator HapR and the global regulator cyclic AMP (cAMP) receptor protein CRP, and negatively regulated by QS regulator LuxO and by global regulator TsrA, a protein homologous to heat-stable nucleoid-structuring (H-NS) [27, 28]. These studies have thus shown that numerous global regulators control T6SS expression, as well as one specific regulator (VasH).
V. cholerae T6SS studies have mainly focused on the V. cholerae O37 serogroup V52 strain because it assembles a T6SS apparatus constitutively [11]. In this strain, the T6SS is required for cytotoxicity towards Dictyostelium discoideum and J774 macrophages, and induces inflammatory diarrhea in the mouse model [29]. In V. cholerae O1 strain C6706, the T6SS is not constitutively produced and conditions that promote T6SS production are unknown. However, production of T6SS can be achieved in other O1 strains by inactivating mutations in genes encoding the LuxO and TsrA negative regulators. In O1 strains, the T6SS translocates T6SS effectors into macrophages, and increases fecal diarrhea and intestinal inflammation in infant rabbits [27]. It was also shown that the V. cholerae O1 C6706 strain T6SS mediates antagonistic interbacterial interactions during intestinal colonization. A strain unable to produce the TsiV3 immunity protein, which provides immunity against the effector VgrG3, exhibited an intestinal colonization defect only when co-infected with strains harboring an intact T6SS locus and VrgG3 [30]. Although T6SS is regulated and expressed differently between V. cholerae strains, production of this system in multiple strains promotes virulence against both eukaryotic and bacterial cells, suggesting the function is largely conserved but the regulation varies.
Pathogenic bacteria experience varying conditions during infection of human hosts and often use two-component signal transduction systems (TCSs) to monitor their environments [31]. TCSs play important roles in the regulation of virulence factors, metabolic adaptation to host environments, and response to numerous environmental stresses including pH, osmolarity, oxygen availability, bile salts, and antimicrobial peptides [32]. TCS rely on a phosphorelay-based signal transduction system. The prototypical TCS consists of a membrane-bound histidine kinase (HK), which senses environmental signals, and a corresponding response regulator (RR), which mediates a cellular response. Response regulators are typically multi-domain proteins harboring a conserved receiver domain (REC) and C-terminal output domain such as DNA-binding, diguanylate cyclase, or methyltransferase [33–35]. Upon environmental stimulation, the HK catalyzes an ATP-dependent autophosphorylation reaction on a conserved histidine residue. The phosphoryl group is transferred from the HK to a conserved aspartate residue on the RR, eliciting a conformation change and subsequent cellular response [32, 34, 35].
The V. cholerae genome reference genome of O1 EL Tor N16961 strain is predicted to encode 43 HK and 49 RR (http://www.ncbi.nlm.nih.gov/Complete_Genomes/RRcensus.html and http://www.p2cs.org). We also included 3 additional RRs (VpsT, VpsR, QstR) which were not annotated in these databases. Thirteen of these 52 putative RRs have been previously characterized and eight have a role in virulence factor production and host colonization: VarA, LuxO, VieA, PhoB, ArcA, FlrC, CarR, and CheY-3 [36–44]. VarA and LuxO repress production of quorum sensing regulator HapR, which represses expression of aphA and, in turn, TCP and CT production [36, 37], VieA regulates ctxAB expression indirectly by affecting production of ToxT through cyclic diguanylate (c-di-GMP) signaling [38, 39]. The RR for phosphate limitation, PhoB, directly controls expression of a key transcriptional regulator, TcpPH, which activates toxT transcription [40]. The RR ArcA controls adaptation to low oxygen environment of the intestine and positively controls the expression of toxT [41]. CarR regulates glycine and diglycine modification of lipid A, confers polymyxin B resistance, and is required for intestinal colonization, although this phenotype is strain dependent [42]. FlrC controls flagellar biosynthesis and CheY-3 is needed for control of chemotactic motility [43, 44]. Both motility and chemotaxis are known colonization factors for V. cholerae [43]. Together, these results show that RRs shown play a role in intestinal colonization have three basic targets: known virulence regulators and concomitant CT and TCP production; lipid A modification enzymes; or motility and chemotaxis. 39/52, however, were not yet analyzed at the time of this study.
To systematically evaluate the role of V. cholerae TCSs in intestinal colonization, we generated in-frame deletion mutants of each RR gene and analyzed the in vivo colonization phenotypes of the resulting mutants. We found 12 RR were required for wild-type intestinal colonization. One RR in particular had a very strong defect, encoded by genomic locus VCA0566. We determined that VCA0566 (now termed Vibrio type six secretion regulator, vxrB) controls expression of several genes including the T6SS genes. We used multiple methods to substantiate that VxrB is required for expression of the T6SS in vitro and in vivo. Lastly, we report that the T6SS contributes to colonization of the V. cholerae O1 strain used in this study.
Results
Multiple RRs impact intestinal colonization
We have a limited understanding of the V. cholerae TCSs and their role in colonization and adaptation to host environments. To evaluate the importance of the 52 TCS RRs in colonization, we generated in-frame deletion mutants of the 40 RRs. For this analysis, we excluded 12 RR that were either predicted to be involved in chemotaxis (11 CheY, CheV, and CheB proteins) or that we were unable to mutate (VC2368, ArcA) [43, 45]. We then analyzed the ability of 40 RR deletion mutants to colonize the small intestine in an in vivo competition assay where in vivo fitness of a mutant strain is compared to that of wild type strain using the infant mouse infection model (Fig 1A) [46]. While the vast majority of mutants—28—were not different from wild type, we identified 12 RR mutants that had a statistically significant colonization difference as compared to wild type (Fig 1A). We focused on 8 mutants with a statistically significant colonization difference and exhibited at least 1.2-fold difference in CI (Fig 1B). Consistent with previous studies, we identified that ΔVC0719 (phoB), ΔVC1021 (luxO), ΔVC1213 (varA), and ΔVC2135 (flrC) were defective in colonization [36, 37, 40, 44]. The competitive indices (CI) for ΔphoB, ΔluxO, ΔvarA, and ΔflrC were 0.01, 0.02, 0.16, and 0.43, respectively (Fig 1B).
Fig. 1. Identification of RRs impacting colonization in the infant mouse infection model. 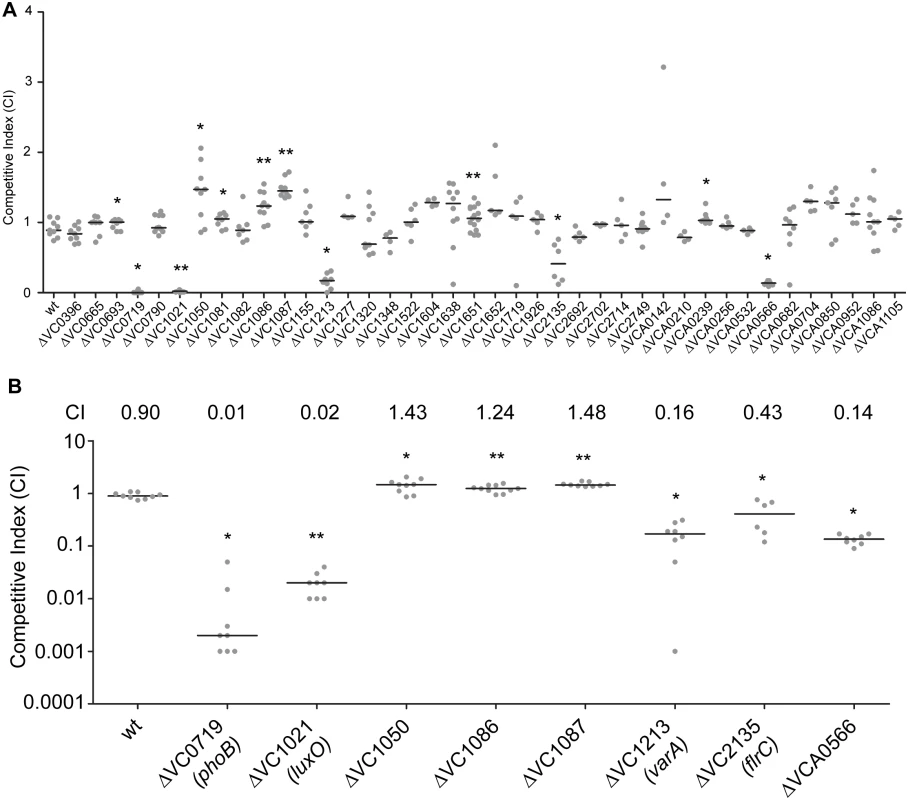
(A) Ability of 40 ΔRR mutants in V. cholerae strain A1552 to colonize the infant mouse intestine was analyzed using a competition assay with the isogenic wild-type strain. (B) Same data presented in Fig 1A, expanded to highlight the mutants showing a statistically significant difference in colonization p<0.05 and a minimum 1.2-fold change in colonization ability. Competitive index (CI) is defined as the output ratio of mutant to wild-type bacteria divided by the input ratio of mutant to wild-type bacteria. Each symbol represents the CI in an individual mouse; horizontal bars indicate the median. Statistical analysis was carried out using Wilcoxon Signed Rank Test, comparing the CI of each strain to the CI of wt lacZ+ / wt lacZ- (shown as wt) (*, p<0.05; **, p<0.01). Additionally, we identified a set of genes whose absence slightly but statistically significantly enhanced colonization (at least 1.2 fold higher CI), suggesting that inhibition of their expression and activity may be needed for wild-type colonization. These mutants were ΔVC1050, ΔVC1086, and ΔVC1087, which exhibited subtle and enhanced colonization phenotypes with CIs of 1.43, 1.24, and 1.48, respectively (Fig 1B). VC1050 is classified as an Hnr-type RR, [47] but its function is yet to be determined. VC1086 and VC1087 are part of a predicted eight gene operon encompassing VC1080-VC1087. Both VC1086 and VC1087 have domains that suggest they function in cyclic guanylate (c-di-GMP) regulation. Specifically, VC1086 contains an EAL domain with conserved residues required for enzymatic function, while VC1087 harbors an HD-GYP domain, but this domain lacks the conserved residues required for enzymatic activity.
We also identified one RR that was defective for colonization that had not been previously characterized. This mutant, ΔVCA0566, had a colonization defect with a CI of 0.14 (Fig 1A and 1B). Because this uncharacterized RR was important for colonization, we focused the rest of our studies on this protein.
ΔVCA0566/VxrB impacts colonization
VCA0566 is the second gene of a predicted five gene operon and had been previously annotated as a RR of the OmpR family. The encoded protein, which we named VxrB for reasons described below, is 245 amino acids in length with an N-terminal REC domain and a C-terminal winged helix-turn-helix DNA-binding domain (Fig 2B). Previously characterized members of the OmpR family in V. cholerae include PhoB, CarR, and ArcA [40–42]. Amino acid sequence alignment of the V. cholerae RRs in the OmpR family and the previously characterized E. coli OmpR [48] was used to identify the aspartate residue that is predicted to be phosphorylated in the REC domain (Fig 2A). Since the phosphorylation state of a RR is likely to determine its activity, we mutated the aspartate residue in the REC domain of VxrB to mimic constitutively active (D78E) and inactive (D78A) versions, as used in other work [48], and replaced the wild-type gene in the chromosome with these altered genes. These mutants were competed against wild type in the infant mouse colonization assay to determine if the phosphorylation state of VxrB is important for colonization. In accordance with our initial colonization screen, ΔvxrB displayed a CI of 0.15 (Fig 2B). Somewhat surprisingly, the CI for vxrB::D78A (inactive form) was 0.53, indicating a modest defect in colonization. This result indicates that the “inactive” form of VxrB does not phenocopy the ΔvxrB mutant, suggesting that VxrB harboring D78A substitution is not fully inactive. The CI for vxrB::D78E (active form) is 1.07, suggesting that constitutive activation of VxrB does not significantly impair V. cholerae (Fig 2B). Collectively, these findings suggest that in vivo phosphorylation of VxrB at D78 is likely to be important for its colonization function, but apparently not absolutely required. It is also likely that VxrB may not function by conventional phosphorylation-dependent signal transduction [49].
Fig. 2. The role of the phosphorylation state of VxrB in colonization. 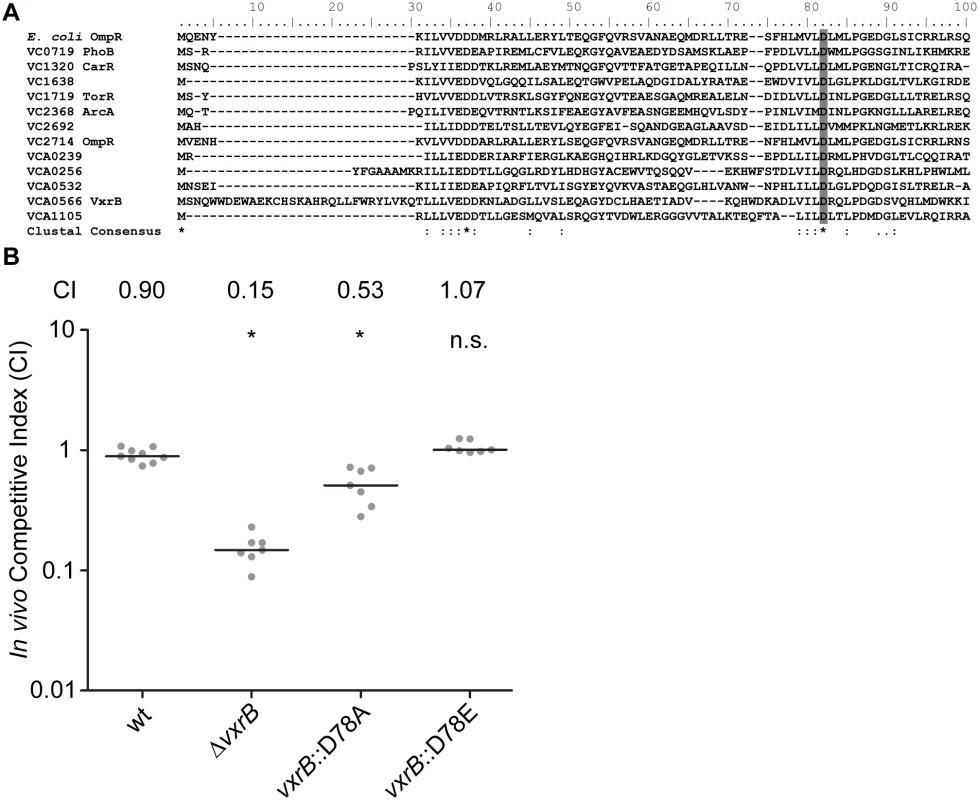
(A) Amino acid sequence alignment of the REC domains of the RR proteins belonging to the OmpR family using ClustalW. Numbers above the sequence correspond to the amino acid number of each protein. VC numbers indicate the RR encoded in V. cholerae genome. OmpR from E. coli was used as a reference to align the aspartate residue that has been shown to be important for phosphorylation (D55A) [48]. Highlighted region in gray indicate the aspartate residue that is predicted to be the site of phosphorylation. B) In vivo competition assay of a strain harboring the mutated version of vxrB on the chromosome. The vxrB gene was mutated to convert the aspartate residue predicted to be important for phosphorylation to emulate the inactive (D78A) or active (D78E) state of VxrB. *, p<0.05 by the Wilcoxon Signed Rank Test as compared to the CI of wt. All members of the vxr operon contribute to in vivo colonization
The first gene of the vxr loci, VCA0565, is annotated as an HK. The other three genes (VCA0567-69) are predicted to encode proteins of unknown function (Fig 3A). We now termed these genes Vibrio type six secretion regulator (vxr) ABCDE and determined that these genes are co-transcribed using RT-PCR and RNAseq analysis (Fig 3A and S1 Fig). Both the genomic context and organization is conserved in the Vibrio species (S2–S4 Figs) and vxr gene products do not share significant sequence similarity with previously characterized proteins.
Fig. 3. Role of the vxrABCDE operon in colonization. 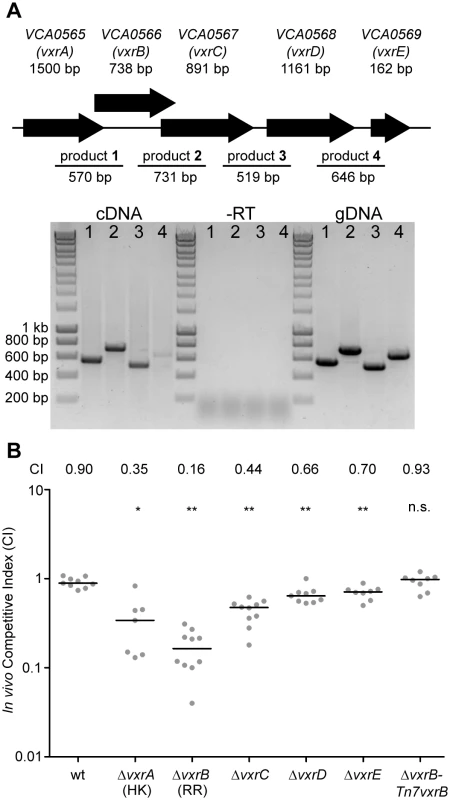
(A) The top panels shows a schematic representation of the genomic organization of the vxrABCDE locus The bottom panel shows the results of RT-PCR analysis designed to determine if vxrABCDE genes are cotranscribed. RT-PCR primers designed to amplify intergenic regions are indicated. Products were detected for VCA0565-66 (product 1), VCA0566-67 (product 2), VCA0667-68 (product 3), and VCA0568-69 (product 4) V. cholerae genomic DNA template was used for PCR to evaluate primers and amplified product sizes. RT-PCR reaction without reverse transcriptase (-RT) was used as a negative control. (B) Ability of A1552 ΔvxrA, ΔvxrB, ΔvxrC, ΔvxrD, ΔvxrE, and ΔvxrB-Tn7vxrB complementation strains to colonize the infant mouse intestine was analyzed using a competition assay with wild-type. Each symbol represents the CI in an individual mouse; horizontal bars indicate the median. *, p<0.05; **, p<0.01 by the Wilcoxon Signed Rank Test as compared to the CI of wt. To gain a better understanding of the role of the vxr operon in colonization, we investigated whether the cognate HK and other genes in the vxr operon also contributed to mouse colonization. In-frame unmarked deletion mutants of vxrA, vxrB, vxrC, vxrD, and vxrE (Fig 3B) were generated and analyzed in an in vivo competition assay. Each mutant was outcompeted, with CIs of 0.35, 0.16, 0.44, 0.66, and 0.70, respectively (Fig 3B). These findings suggest that while vxrA and vxrB genes are critical for colonization in the infant mouse model, contribution of vxrCDE genes appears to be minor.
To further confirm the phenotype of ΔvxrB colonization defect, a wild type copy of vxrB whose expression was driven from its native promoter was inserted into the Tn7 site (located between VC0487 and VC0488) on the chromosome of ΔvxrB. In vivo competition assay of ΔvxrB-Tn7vxrB had a CI of 0.93, similar to wild type levels, where ΔvxrB had a CI of 0.16 (Fig 3B). Thus, the ΔvxrB colonization defect is restored to wild-type levels by introduction of the wild-type copy of vxrB.
VxrB regulates T6SS gene expression
To gain a better understanding of the contribution of VxrB to V. cholerae pathogenesis, we performed high throughput transcriptome sequencing (RNA-seq) analysis to identify the V. cholerae genes controlled by VxrB. We used cells grown under virulence inducing AKI conditions, to mimic the intestinal conditions encountered when we know VxrB is important. 149 genes showed statistically significant differences in gene expression between the wild type and mutant (S2 and S3 Tables). Of these, 80 genes were expressed to greater levels in the ΔvxrB mutant relative to the wild type (S2 Table), while 69 were expressed to lower levels in the ΔvxrB mutant relative to wild type (S3 Table). Of particular interest was the observation that message abundance of most of the T6SS genes in both the large cluster (VCA0105-VCA0124) and the two auxiliary clusters (VCA0017-VCA0022 and VC1415-VC1421) were less in the VxrB mutant relative to wild type (Table 1) (S5 Fig). This finding suggests that VxrB activates expression of the T6SS genes.
Tab. 1. Expression of Type VI secretion genes in the Δ<i>vxrB</i> mutant relative to wild type. 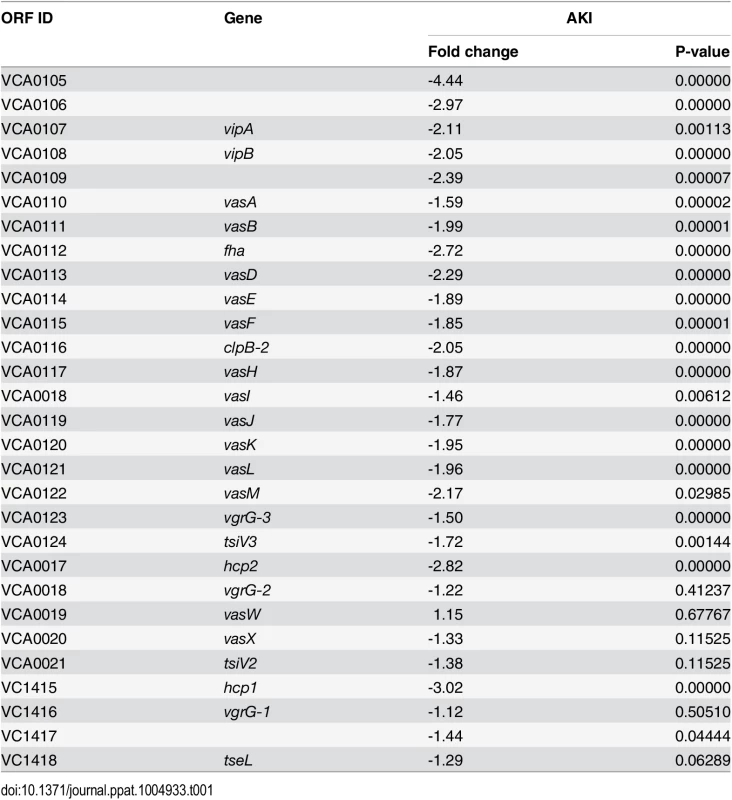
VxrB regulates production of T6SS
To further analyze the role of vxrB in T6SS expression and function, we compared the levels of the major T6SS structural component, Hcp, between wild type and ΔvxrB mutant V. cholerae. Quantitative real-time PCR analysis of hcp revealed that the transcript abundance of hcp was decreased by 3.7-fold under AKI conditions and 4.1 - fold under LB conditions in the ΔvxrB mutant relative to wild type (Fig 4A). This finding supports that VxrB regulates expression of hcp and is consistent with the RNA-seq analysis. Additionally the levels of the Hcp protein in ΔvxrB were lower than wild type, in both whole cell samples and culture supernatants (Fig 4B). We also determined that complementation of the vxrB mutation (ΔvxrB-Tn7vxrB) restored Hcp to wild-type levels. Because we found lower amounts of Hcp in the supernatant as well as in whole cells, this finding suggests that VxrB is needed to express and secrete Hcp. As negative controls, we included a Δhcp1Δhcp2 mutant because it is unable to produce the Hcp proteins [11, 50]. As expected, no Hcp production was observed in this mutant. Furthermore, complementation of hcp1 in the Δhcp1Δhcp2 mutant partially restored Hcp levels (Fig 4B). Overall these findings suggest that Hcp production is decreased in ΔvxrB mutant.
Fig. 4. Analysis of Hcp production and secretion in the vxrB mutant. 
(A) Quantitative real-time PCR analysis of hcp using total RNA isolated from wild-type and ΔvxrB grown in AKI and LB. Experiments were performed using two independent biological replicates, each performed in quadruplicate. The Pfaffl method was used to compare expression levels of hcp to 16s rRNA and relative expression was calculated by comparing expression in ΔvxrB with that of wt. *, p<0.05 by Student’s t-test. (B) Hcp production and secretion was analyzed in whole cells and culture supernatants in wild type, ΔvxrB, ΔvxrB::Tn7vxrB, Δhcp1Δhcp2, and Δhcp1Δhcp2::Tn7hcp1 strains by immunoblot analysis. Equal amounts of total protein (determined by BCA assay) were loaded onto a SDS 13% polyacrylamide gel. Prior to TCA precipitation and total protein quantification, 100μg/ml of BSA was added to the supernatant. After the blot was probed for Hcp, the blot was stripped of all antibodies using Western Blot Stripping Buffer (GM Biosciences, MD) and the same blot was used to probe for CRP and BSA in the whole cell and supernatant, respectively. The data shown is representative of the results of three independent experiments. (C) Interbacterial killing was analyzed by mixing V. cholerae strains and prey E. coli strain MC4100 in a 10:1 ratio, followed by incubation on LB agar plates for 4 hours at 30°C and determination of surviving E. coli MC4100. The data represent averages and standard deviations of three independent experiments. *, p<0.05 by the Student’s t-test as compared to the values for interbacterial killing of the wild-type strain. Next we analyzed whether VxrB was needed for T6SS function, by examining T6SS-mediated interbacterial killing. Killing assays between the V. cholerae and the target E. coli K-12 strain MC4100 showed that wild-type V. cholerae decreased the numbers of E. coli compared to control experiments. This killing was dependent on the T6SS, as shown by greater numbers of E. coli obtained when incubated with V. cholerae Δhcp1Δhcp2 mutant and ΔvasH mutants, consistent with the findings reported by Ishikawa et al. (Fig 4C) [50]. This phenotype was complemented by introduction of either hcp1 or hcp2 into the Tn7 site on the chromosome. Consistent with our transcriptional and protein analysis presented above, we found that ΔvxrB mutants mediated less E. coli killing. These findings suggest that T6SS regulation by VxrB contributes to interbacterial killing.
Since VxrB regulates T6SS expression and is required to for intestinal colonization, we next asked whether the T6SS itself is required for intestinal colonization. We performed in vivo competition assays of a T6SS null mutant (Δhcp1Δhcp2) against wild type in the infant mouse model. We found that the in-vivo CI for Δhcp1Δhcp2 was 0.17 (Fig 5A). In addition, ΔvgrG3 also had an in-vivo CI of 0.26 suggesting that T6SS components are important for intestinal colonization (Fig 5A). This suggests that structural components of the T6SS are needed to colonize the intestine. Furthermore, this finding also suggests that the colonization defect associated with the ΔvxrB mutant could be caused by diminished T6SS production. To evaluate this possibility, we tested the in vivo competition of Δhcp1Δhcp2 against ΔvxrB and found that these strains competed nearly equally with each other (Fig 5C). Furthermore, in-vivo CI of ΔvxrBΔhcp1Δhcp2 triple mutant against ΔvxrB was 0.07 and ΔvxrBΔhcp1Δhcp2 against wt was 0.10. This finding suggests that the colonization defect by ΔvxrB was not solely due to altered expression of T6SS genes and other factors regulated by VxrB also contribute to colonization. It is also likely that T6SS expression is not completely abolished by the vxrB mutation. Indeed, western analysis (Fig 4B) shows that in vxrB mutant Hcp production is reduced but not completely eliminated. Similarly in vitro killing assay shows that vxrB mutant’s interbacterial killing ability is not identical to that of the strain lacking T6SS.
Fig. 5. Analysis of the role of T6SS in colonization. 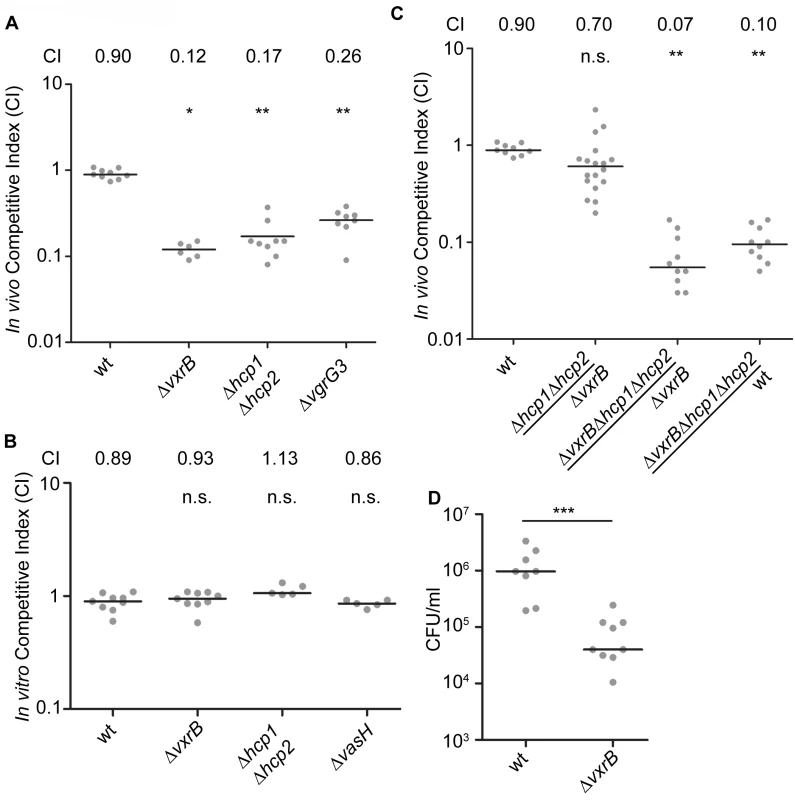
(A) Ability of ΔvxrB, ΔvxrB-Tn7vxrB, Δhcp1Δhcp2, ΔvgrG3, to colonize the infant mouse intestine was analyzed using a competition assay with isogenic wild-type strain. *, p<0.05; **, p<0.01 by the Wilcoxon Signed Rank Test as compared to wt (CI of 0.90). (B) In vitro competition assay of ΔvxrB, Δhcp1Δhcp2, and ΔvasH. Strains were grown in LB at 30°C for 20 hours. Statistical analysis was carried out using Wilcoxon Signed Rank Test, comparing the CI of each strain to the CI of wt lacZ+ / wt lacZ- (shown as wt) (CI of 0.98). (C) Ability of Δhcp1Δhcp2 to colonize the infant mouse intestine was analyzed using a competition assay with ΔvxrB. (D) Ability of wild type and ΔvxrB to colonize the infant mouse intestine in single-strain infections. Each symbol represents the CI in an individual mouse; horizontal bars indicate the median. ***, p<0.001 by the Student’s t-test as compared to the values for colonization of the wild-type strain. n.s. indicates mutants were not significantly different from the wt. We next asked whether VxrB plays a role in growth in vitro, by performing an in vitro competition assay. ΔvxrB mutants grew equally well as wild type, suggesting that neither had a competitive advantage over the other in vitro (Fig 5B). This outcome suggests that there may be an in vivo signal produced in the infant mouse that triggers T6SS activity and colonization. We also performed single-strain colonization assays in the infant mouse model with ΔvxrB. There was a 12.7-fold decrease in colonization for ΔvxrB compared to wild type (Fig 5D). This finding suggests that the colonization defect by ΔvxrB was not solely dependent on wild type, and possibly could be caused by competition with the normal flora or ability of the mutant to adapt to the infection microenvironment.
Discussion
Systematic mutational phenotypic characterization of TCSs has been performed in only a few bacteria, including Vibrio fischeri, E. coli, Bacillus subtilis, Streptococcus pneumoniae, and Enterococcus faecalis [51–55]. In this study, we systematically analyzed the role of all V. cholerae TCS in colonization of the infant mouse small intestine and identified the RRs that play roles in mouse intestinal colonization. Specifically, ΔVC0719 (phoB), ΔVC1021 (luxO), ΔVC1213 (varA), and ΔVC2135 (flrC), and ΔVCA0566 (vxrB) exhibited intestinal colonization defects while ΔVC1050, ΔVC1086, and ΔVC1087 showed enhanced colonization. Many of the RRs had either no statistically significant defect or minor defects in the infant mouse colonization assay. It remains possible, however, that these RRs have a role in colonization in other infection models.
In vivo transcriptome analysis has been performed on different strains of V. cholerae in the infant mouse and rabbit ileal loop infection models. The analysis of the whole genome expression of V. cholerae O1 El Tor C6706 cells accumulating in the ceca of orally infected infant rabbits and the intestines of orally infected infant mice revealed that expression of the genes encoding RRs is altered during in vivo growth conditions as compared to in vitro growth in nutrient broth and that in vivo expression of TCS also differed between the model systems [56]. In the infant rabbit infection model, expression of seven RR (VC1081, VC1082, VC1155, vieA, VC2702 (cbrR), VCA0210, and VCA1105) was increased and 1 RR (carR) was decreased by more than 2-fold significantly in comparison to V. cholerae cells grown in vitro in nutrient broth. In the infant mouse infection model, expression of 17 RR (vpsR, VC1050, VC1081, VC1082, VC1086, VC1087, VC1155, VC1522, flrC, cbrR, ompR, dct-D2, vxrB, uhpA, vpsT, VCA1086, and VCA1105) and 9 RR (qstR, phoB, VC1348, VC1638, vieB, cpxR, ntrC, VCA0532, pgtA) were either decreased and increased significantly by more than 2-fold, respectively, in comparison to V. cholerae cells grown in vitro in nutrient broth [56]. vxrA and vxrB transcript levels were decreased 2 and 3-fold, respectively, in the experiments reported by Mandlik and colleagues, but did not reach statistical significance [56]. This work all used the V. cholerae O1 El Tor strain C6706, and so it is yet unknown whether vxrB expression is similarly regulated in the O1 El Tor A1552 strain used here.
There have been two other studies that analyzed V. cholerae infection phenotypes on a global scale, although they did not specifically target RR. Together, these studies and ours suggests there is a set of genes required for intestinal colonization across multiple models. Fu et al. used random transposon mutants coupled with insertion site sequencing (Tn-seq) in a rabbit model [30]. They identified insertions in two genes—VC1021 (luxO) and VC1155—that showed 8-15-fold reduction in colonization, while strains harboring insertions into RRs VC1348, vieA, vieB, arcA, VCA0256, uhpA, and pgtA had less than a 5-fold reduction in colonization (p<0.001). Another Tn-seq study using the infant rabbit model identified defects associated with luxO and arcA as above, and additionally phoB and varA [57]. Combining the results of these studies with ours identifies luxO, phoB, and varA, as required for in vivo fitness, and others that are variably identified. Because the Tn-seq work used transposon libraries, it is not known whether all RR were eliminated, so it is possible that their studies missed some critical RR. There are hints in their data, however, that the vxr locus is necessary in these other models as well. While Fu et al. did not identify vxrA or vxrB mutants, they did determine that a strain with an insertion into VCA0567 (vxrC) exhibited a 9-fold reduction in colonization (p<0.0001) [30]. Additionally, Kamp et al. found that a strain with a transposon insertion in VCA0565 (vxrA) had a disadvantage in fitness (mean fitness value of 0.6) when the bacteria from rabbit cecum fluid was placed into pond water for 48 hours at 30°C [57]. Collectively, these studies suggest that the Vxr genes play important roles in V. cholerae colonization and environmental dissemination.
Our study revealed that the RR VxrB plays a significant role in colonization and in vitro inter-bacterial competition through its ability to regulate expression of T6SS genes. Neither vxrB nor any of the vxrABCDE operon members show similarity to previously characterized proteins. The vxr loci are conserved among the Vibrio species Vibrio parahaemolyticus, Vibrio vulnificus, Vibrio harveyi, and V. fischeri. BLAST analysis revealed that VxrA protein exhibits 67–80%, VxrB 79–84%, VxrC 56–68%, VxrD 58–74%, and VxrE 68–81% sequence similarity to the same proteins in other Vibrio species (S2–S4 Figs). We also analyzed the predicted structure and function of the VxrCDE proteins using the protein homology/analogy recognition engine (Phyre) [58]. While VxrC and VxrE could not be modeled with high confidence and sufficient coverage, VxrD exhibited structural similarity to outer membrane protein transport proteins (100% confidence, 90% coverage). These analyses suggest that vxr genomic loci are a part of the ancestral Vibrio genome, and therefore likely have an evolutionarily conserved role in Vibrio biology.
Expression and production of T6SS are tightly regulated at the transcriptional and posttranscriptional levels in a variety of bacterial systems [12, 13, 59]. Environmental signals such as iron limitation, thermoregulation, salinity, envelope stress, indole, and growth on surfaces regulate T6SS expression [59]. In V. cholerae A1552, the strain used here, T6SS genes are expressed when cell are grown in high-osmolarity and low temperature conditions [50]. A recent study revealed that the V. cholerae A1552 T6SS genes are part of the competence regulon and their expression is induced when the bacterium grows on chitinous surfaces in a TfoX-, HapR-, and QstR-dependent manner [60]. Our work presented here identified VxrB as a regulator of the T6SS large gene cluster and the two auxiliary clusters. The predicted cognate HK of VxrB, VxrA, does not exhibit similarity to previously characterized sensory domains. The signals that govern expression and activity of VxrAB and how the VxrAB TCS is integrated into the T6SS regulatory network of V. cholerae are yet to be determined. We determined that while the wild-type strain has a competitive advantage in vivo over ΔvxrB, neither strain had a competitive advantage over the other in vitro. Furthermore, single infection studies showed that ΔvxrB had a significant colonization defect compared to wild type, suggesting that VxrB could be involved in competition with normal flora and that ΔvxrB could have a reduced fitness in infection environment. These observations also suggest that there may be an in vivo signal produced in the infant mouse that triggers T6SS activity and colonization. Our studies thus provided significant new insights into the regulation of T6SS in V. cholerae and provided further support that the T6SS is critical for V. cholerae virulence.
Materials and Methods
Ethics statement
All animal procedures used were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals [61] and were approved by the UC Santa Cruz Institutional Animal Care and Use Committee (Yildf1206).
Bacterial strains, plasmids, and culture conditions
The bacterial strains and plasmids used in this study are listed in S1 Table. Escherichia coli CC118λpir strains were used for DNA manipulation, and E. coli S17-1λpir strains were used for conjugation with V. cholerae. In-frame deletion mutants of V. cholerae were generated as described earlier [62]. All V. cholerae and E. coli strains were grown aerobically, at 30°C and 37°C, respectively, unless otherwise noted. All cultures were grown in Luria-Bertani (LB) broth (1% Tryptone, 0.5% Yeast Extract, 1% NaCl), pH 7.5, unless otherwise stated. LB agar medium contains 1.5% (wt/vol) granulated agar (BD Difco, Franklin Lakes, NJ). AKI medium contains 0.5% NaCl, 0.3% NaHCO3, 0.4% Yeast Extract, and 1.5% Peptone, as previously described [63]. Antibiotics were used at the following concentrations: ampicillin 100 μg/ml; rifampicin 100 μg/ml; gentamicin 50 μg/ml; streptomycin 50 μg/ml.
DNA manipulations
An overlapping PCR method was used to generate in-frame deletion constructs of each RR genes using previously published methods [62]. Briefly, a 500–600 bp 5’ flanking sequence of the gene, including several nucleotides of the coding region, was PCR amplified using del-A and del-B primers. del-C and del-D primers were used to amplify the 3’ region of the gene including 500–600 bp of the downstream flanking sequence. The two PCR products were joined using the splicing overlap extension technique [64, 65] and the resulting PCR product, which lacks 80% of amino acids, was digested with two restriction enzymes and ligated to similarly-digested pGP704sacB28 suicide plasmid. Construction of vxrB plasmid harboring point mutations were performed using a similar technique [66] with the following alterations: primers containing the new sequence harboring the point mutations were used in place of the del-B and del-C primers. The deletion constructs were sequenced (UC Berkeley DNA Sequencing Facility, Berkeley, CA) and the clones without any undesired mutations were used. The deletion constructs are listed in S1 Table.
Generation of in-frame deletion mutants and Tn7 complementation strains
The deletion plasmids were maintained in E. coli CC118λpir. Biparental matings were carried out with the wild type V. cholerae and an E. coli S17λpir strain harboring the deletion plasmid. Selection of deletion mutants were done as described [64] and were verified by PCR. The Tn7 complementation V. cholerae strains were generated by triparental matings with donor E. coli S17λpir carrying pGP704-Tn7 with gene of interest, helper E. coli S17λpir harboring pUX-BF13, and V. cholerae strains. Transconjugants were selected on thiosulfate-citrate-bile salts-sucrose (TCBS) (BD Difco, Franklin Lakes, NJ) agar medium containing gentamicin at 30°C. The Tn7 complementation V. cholerae strains were verified by PCR.
Intestinal colonization assay
An in vivo competition assay for intestinal colonization was performed as described previously [46]. Briefly, each of the V. cholerae mutant strains (lacZ+) and the fully virulent reference strain (lacZ-otherwise wild-type)) were grown to stationary phase at 30°C with aeration in LB broth. Mutant strains and wild-type were mixed at 1 : 1 ratios in 1x Phosphate Buffered Saline (PBS). The inoculum was plated on LB agar plates containing 5-bromo-4-chloro-3-indoyl-β-D-galactopyranoside (X-gal) to differentiate wild-type and mutant colonies and to determine the input ratios. Approximately, 106–107 cfu were intragastrically administered to groups of 5–7 anesthetized 5-day old CD-1 mice (Charles River Laboratories, Hollister, CA). After 20 hours of incubation, the small intestine was removed, weighed, homogenized, and plated on appropriate selective and differential media to enumerate mutant and wild-type cells recovered and to obtain the output ratios. In vivo competitive indices were calculated by dividing the small intestine output ratio by the inoculum input ratio of mutant to wild-type strains. For single strain infections, 107 cfu of each strain, including otherwise wild type (lacZ-) strain, were intragastrically administrated to 5-day old CD-1 mice. After 20 hours of incubation, the small intestine was harvested and plated on selective media as previously described above. Statistical analyses for competition infections were performed using Wilcoxon Signed Rank Test. Statistical analyses were performed using Prism 5 software (GraphPad Software, Inc., San Diego, CA) using Wilcoxon Signed Rank Test. P values of <0.05 were determined to be statistically significant.
Reverse transcription-PCR
RNA was isolated as described below. The reverse transcription reaction to generate cDNA was carried out using the SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions at 25°C for 5 min, 50°C for 1 h, and 70°C for 15 min using 1 μg of RNA in a 20 μl final volume. The product was used in a PCR using suitable primers, and RNA without RT treatment was used as a negative control.
Quantitative real time (qRT) PCR
For qRT-PCR expression analysis, RNA was isolated as described below. cDNA was synthesized using iScript cDNG Synthesis Kit (Bio-Rad, Hercules, CA) from 1 μg of total RNA. Real-time PCR was performed using a Bio-Rad CFX1000 thermal cycler and Bio-RAD CFX96 real-time imager with specific primer pairs (designed within the coding region of the target gene) and SsoAdvanced SYBR green supermix (Bio-Rad, Hercules, CA). Results are from two independent experiments performed in quadruplicate. All samples were normalized to the expression of the housekeeping gene 16S using the Pfaffl method [67]. Relative expression was calculated by normalizing expression at ΔvxrB by that of wt. Statistical analysis was performed using two-tailed student’s t test.
RNA isolation
V. cholerae cells were grown aerobically overnight in LB at 37°C, then diluted 1 : 100 in fresh 10 ml AKI media in borosilicate glass test tubes (diameter, 15mm; height, 150 mm) and incubated at 37°C without shaking for 4 hours. After 4 hours, 10 ml cultures were transferred to 125 ml flasks (for maximal aerated growth on an orbital shaker (250 rpm) for 2 hours. Aliquots (2 ml) of the cultures were collected and centrifuged for 2 min at room temperature. The cell pellets were immediately resuspended in 1 ml of TRIzol (Invitrogen, Carlsbad, CA) and stored at -80°C. Total RNA was isolated according to the manufacturer’s instructions. To remove contaminating DNA, total RNA was incubated with RNase-free DNase I (Ambion, Grand Island, NY), and an RNeasy mini kit (Qiagen, Valencia, CA) was used to clean up RNA after DNase digestion. Five micrograms of total RNA was treated with a MICROBExpress Kit (Ambion, Grand Island, NY) to remove ribosomal RNA, and the efficiency was confirmed by Bioanalyzer analysis (Agilent Technologies, Santa Clara, CA). Three biological replicates were generated for each condition.
cDNA library construction and Illumina HiSeq sequencing
Libraries for RNA-seq were prepared using NEBNext Ultra Directional RNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA). Twelve indexed samples were sequenced per single lane using the HiSeq2500 Illumina sequencing platform for 50 bp single reads (UC Davis Genome Center, UC Davis, CA) and subsequently analyzed and visualized via the CLC Genomics Workbench version 7.5 (Qiagen, Valencia, CA). Samples were mapped to the V. cholerae genome N16961. Differentially regulated genes were identified as those displaying a fold change with an absolute value of 1.5-fold or greater. Statistical significance was determined by Empirical analysis of Digital Gene Expression (edgeR) test where p<0.05 was deemed significant [68].
Analysis of Hcp production and secretion
V. cholerae strains were grown to an OD600 of 2.0, and the culture (25 ml) was centrifuged at 20,000 g for 10 min to obtain whole cell pellets. The culture supernatant containing secreted proteins were filtered through 0.22 μ membranes (Millipore, Billerica, Massachusetts) and secreted proteins in the culture supernatant were precipitated with 13% trichloroacetic acid (TCA) overnight at 4°C, pelleted by centrifugation at 47,000 g for 30 min at 4°C, wash with ice cold acetone and resuspended in 1x PBS containing Complete protease inhibitor (Roche, Basel, Switzerland). Bovine serum albumin (BSA, 100 μg/ml) was added to the culture supernatant prior to TCA precipitation as a control. Protein pellets from whole cell were suspended in 2% sodium dodecyl sulfate (SDS) and protein concentrations were estimated using a Pierce BCA protein assay kit (Thermo Scientific, Rockford, IL). Equal amounts of total protein (20 μg) were loaded onto a SDS 13% polyacrylamide gel electrophoresis (SDS-PAGE). Western blot analyses were performed as described [69] using anti-Hcp polyclonal antiserum provided by the Sun Wai [28], anti-CRP (Neoclone Inc., Madison, WI), and anti-BSA (Santa Cruz Biotech, Santa Cruz, CA). OneMinute Western Blot Stripping Buffer (GM Biosciences, Frederick, MD) was used to remove the Hcp antibodies and the same blot was used again to probe for CRP or BSA. These experiments were conducted with at least three biological replicates.
Bacterial killing assay
Killing assays were performed as described previously [20]. Briefly, bacterial strains were grown overnight on LB plates and resuspended in LB broth containing 340 mM NaCl, as V. cholerae strain A1552 displayed enhanced interbacterial virulence towards E. coli under high osmolarity [50]. V. cholerae and E. coli MC4100 were mixed at a 10 : 1 ratio and 25 μl was spotted onto LB agar plates containing 340 mM NaCl and incubated at 37°C for 4 hours. Spots were harvested, serially diluted, and plated onto LB plates containing 50 μg/ml of streptomycin to enumerate surviving E. coli prey cells.
In vitro competition assay
The following assay was performed similarly as the intestinal colonization assay except no animal models were used. The V. cholerae mutant strains with wild-type lacZ allele (lacZ+) and reference strain (lacZ-) were grown to stationary phase at 30°C with aeration in LB broth. Mutant strains and wild-type were mixed at 1 : 1 ratios in 1x PBS. The inoculum was plated on LB agar plates containing X-gal to differentiate colonies formed by the wild-type and mutant strains and to determine the input ratios. The inoculum (50 μl) was spotted on to a LB agar plate and incubated at 37°C. After 20–24 hours of incubation, the 50 μl spots were scraped off the agar plate and resuspended in 1x PBS. The resuspension was serially diluted and plated on appropriate selective and differential media to enumerate mutant and wild type cells recovered and to obtain the output ratios. In vitro competitive indices were calculated by dividing the output ratio by the inoculum input ratio of mutant to wild type strains. Statistical analyses were performed using Wilcoxon Signed Rank Test.
Supporting Information
Zdroje
1. Ali M, Lopez AL, You YA, Kim YE, Sah B, Maskery B, et al. The global burden of cholera. Bulletin of the World Health Organization. 2012 Mar 1;90(3):209–18A. doi: 10.2471/BLT.11.093427 22461716
2. Kaper JB, Morris JG Jr., Levine MM. Cholera. Clin Microbiol Rev. 1995 Jan;8(1):48–86. 7704895
3. DiRita VJ, Parsot C, Jander G, Mekalanos JJ. Regulatory cascade controls virulence in Vibrio cholerae. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5403–7. 2052618
4. Higgins DE, Nazareno E, DiRita VJ. The virulence gene activator ToxT from Vibrio cholerae is a member of the AraC family of transcriptional activators. J Bacteriol. 1992 Nov;174(21):6974–80. 1400247
5. Higgins DE, DiRita VJ. Transcriptional control of toxT, a regulatory gene in the ToxR regulon of Vibrio cholerae. Mol Microbiol. 1994 Oct;14(1):17–29. 7830555
6. Hase CC, Mekalanos JJ. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci U S A. 1998 Jan 20;95(2):730–4. 9435261
7. Krukonis ES, Yu RR, Dirita VJ. The Vibrio cholerae ToxR/TcpP/ToxT virulence cascade: distinct roles for two membrane-localized transcriptional activators on a single promoter. Mol Microbiol. 2000 Oct;38(1):67–84. 11029691
8. Kovacikova G, Lin W, Skorupski K. Vibrio cholerae AphA uses a novel mechanism for virulence gene activation that involves interaction with the LysR-type regulator AphB at the tcpPH promoter. Mol Microbiol. 2004 Jul;53(1):129–42. 15225309
9. Skorupski K, Taylor RK. A new level in the Vibrio cholerae ToxR virulence cascade: AphA is required for transcriptional activation of the tcpPH operon. Mol Microbiol. 1999 Feb;31(3):763–71. 10048021
10. Kovacikova G, Skorupski K. Regulation of virulence gene expression in Vibrio cholerae by quorum sensing: HapR functions at the aphA promoter. Mol Microbiol. 2002 Nov;46(4):1135–47. 12421317
11. Pukatzki S, Ma AT, Sturtevant D, Krastins B, Sarracino D, Nelson WC, et al. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci U S A. 2006 Jan 31;103(5):1528–33. 16432199
12. Ho BT, Dong TG, Mekalanos JJ. A view to a kill: the bacterial type VI secretion system. Cell host & microbe. 2014 Jan 15;15(1):9–21.
13. Silverman JM, Brunet YR, Cascales E, Mougous JD. Structure and regulation of the type VI secretion system. Annu Rev Microbiol. 2012;66 : 453–72. doi: 10.1146/annurev-micro-121809-151619 22746332
14. Pukatzki S, Ma AT, Revel AT, Sturtevant D, Mekalanos JJ. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc Natl Acad Sci U S A. 2007 Sep 25;104(39):15508–13. 17873062
15. Dong TG, Ho BT, Yoder-Himes DR, Mekalanos JJ. Identification of T6SS-dependent effector and immunity proteins by Tn-seq in Vibrio cholerae. Proc Natl Acad Sci U S A. 2013 Feb 12;110(7):2623–8. doi: 10.1073/pnas.1222783110 23362380
16. Brooks TM, Unterweger D, Bachmann V, Kostiuk B, Pukatzki S. Lytic activity of the Vibrio cholerae type VI secretion toxin VgrG-3 is inhibited by the antitoxin TsaB. J Biol Chem. 2013 Mar 15;288(11):7618–25. doi: 10.1074/jbc.M112.436725 23341465
17. Ma AT, McAuley S, Pukatzki S, Mekalanos JJ. Translocation of a Vibrio cholerae type VI secretion effector requires bacterial endocytosis by host cells. Cell host & microbe. 2009 Mar 19;5(3):234–43.
18. Russell AB, LeRoux M, Hathazi K, Agnello DM, Ishikawa T, Wiggins PA, et al. Diverse type VI secretion phospholipases are functionally plastic antibacterial effectors. Nature. 2013 Apr 25;496(7446):508–12. doi: 10.1038/nature12074 23552891
19. Miyata ST, Kitaoka M, Brooks TM, McAuley SB, Pukatzki S. Vibrio cholerae requires the type VI secretion system virulence factor VasX to kill Dictyostelium discoideum. Infect Immun. 2011 Jul;79(7):2941–9. doi: 10.1128/IAI.01266-10 21555399
20. Miyata ST, Unterweger D, Rudko SP, Pukatzki S. Dual expression profile of type VI secretion system immunity genes protects pandemic Vibrio cholerae. PLoS Pathog. 2013;9(12):e1003752. doi: 10.1371/journal.ppat.1003752 24348240
21. Unterweger D, Miyata ST, Bachmann V, Brooks TM, Mullins T, Kostiuk B, et al. The Vibrio cholerae type VI secretion system employs diverse effector modules for intraspecific competition. Nature communications. 2014;5 : 3549. doi: 10.1038/ncomms4549 24686479
22. Basler M, Pilhofer M, Henderson GP, Jensen GJ, Mekalanos JJ. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature. 2012 Mar 8;483(7388):182–6. doi: 10.1038/nature10846 22367545
23. Zheng J, Ho B, Mekalanos JJ. Genetic analysis of anti-amoebae and anti-bacterial activities of the type VI secretion system in Vibrio cholerae. PloS one. 2011;6(8):e23876. doi: 10.1371/journal.pone.0023876 21909372
24. Kitaoka M, Miyata ST, Brooks TM, Unterweger D, Pukatzki S. VasH is a transcriptional regulator of the type VI secretion system functional in endemic and pandemic Vibrio cholerae. J Bacteriol. 2011 Dec;193(23):6471–82. doi: 10.1128/JB.05414-11 21949076
25. Bernard CS, Brunet YR, Gavioli M, Lloubes R, Cascales E. Regulation of type VI secretion gene clusters by sigma54 and cognate enhancer binding proteins. J Bacteriol. 2011 May;193(9):2158–67. doi: 10.1128/JB.00029-11 21378190
26. Dong TG, Mekalanos JJ. Characterization of the RpoN regulon reveals differential regulation of T6SS and new flagellar operons in Vibrio cholerae O37 strain V52. Nucleic Acids Res. 2012 Sep;40(16):7766–75. doi: 10.1093/nar/gks567 22723378
27. Zheng J, Shin OS, Cameron DE, Mekalanos JJ. Quorum sensing and a global regulator TsrA control expression of type VI secretion and virulence in Vibrio cholerae. Proc Natl Acad Sci U S A. 2010 Dec 7;107(49):21128–33. doi: 10.1073/pnas.1014998107 21084635
28. Ishikawa T, Rompikuntal PK, Lindmark B, Milton DL, Wai SN. Quorum sensing regulation of the two hcp alleles in Vibrio cholerae O1 strains. PloS one. 2009;4(8):e6734. doi: 10.1371/journal.pone.0006734 19701456
29. Ma AT, Mekalanos JJ. In vivo actin cross-linking induced by Vibrio cholerae type VI secretion system is associated with intestinal inflammation. Proc Natl Acad Sci U S A. 2010 Mar 2;107(9):4365–70. doi: 10.1073/pnas.0915156107 20150509
30. Fu Y, Waldor MK, Mekalanos JJ. Tn-Seq analysis of Vibrio cholerae intestinal colonization reveals a role for T6SS-mediated antibacterial activity in the host. Cell host & microbe. 2013 Dec 11;14(6):652–63.
31. Beier D, Gross R. Regulation of bacterial virulence by two-component systems. Curr Opin Microbiol. 2006 Apr;9(2):143–52. 16481212
32. Krell T, Lacal J, Busch A, Silva-Jimenez H, Guazzaroni ME, Ramos JL. Bacterial sensor kinases: diversity in the recognition of environmental signals. Annu Rev Microbiol. 2010;64 : 539–59. doi: 10.1146/annurev.micro.112408.134054 20825354
33. Galperin MY. Diversity of structure and function of response regulator output domains. Curr Opin Microbiol. 2010 Apr;13(2):150–9. doi: 10.1016/j.mib.2010.01.005 20226724
34. Gao R, Stock AM. Biological insights from structures of two-component proteins. Annu Rev Microbiol. 2009;63 : 133–54. doi: 10.1146/annurev.micro.091208.073214 19575571
35. Laub MT, Goulian M. Specificity in two-component signal transduction pathways. Annual review of genetics. 2007;41 : 121–45. 18076326
36. Jang J, Jung KT, Park J, Yoo CK, Rhie GE. The Vibrio cholerae VarS/VarA two-component system controls the expression of virulence proteins through ToxT regulation. Microbiology. 2011 May;157(Pt 5):1466–73. doi: 10.1099/mic.0.043737-0 21330435
37. Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci U S A. 2002 Mar 5;99(5):3129–34. 11854465
38. Tischler AD, Camilli A. Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect Immun. 2005 Sep;73(9):5873–82. 16113306
39. Tischler AD, Lee SH, Camilli A. The Vibrio cholerae vieSAB locus encodes a pathway contributing to cholera toxin production. J Bacteriol. 2002 Aug;184(15):4104–13. 12107127
40. Pratt JT, Ismail AM, Camilli A. PhoB regulates both environmental and virulence gene expression in Vibrio cholerae. Mol Microbiol. 2010 Sep;77(6):1595–605. doi: 10.1111/j.1365-2958.2010.07310.x 20659293
41. Sengupta N, Paul K, Chowdhury R. The global regulator ArcA modulates expression of virulence factors in Vibrio cholerae. Infect Immun. 2003 Oct;71(10):5583–9. 14500477
42. Bilecen K, Fong JC, Cheng A, Jones CJ, Zamorano-Sanchez D, Yildiz FH. Polymyxin B Resistance and biofilm formation in Vibrio cholerae is controlled by the response regulator CarR. Infect Immun. 2015 Jan 12.
43. Butler SM, Camilli A. Both chemotaxis and net motility greatly influence the infectivity of Vibrio cholerae. Proc Natl Acad Sci U S A. 2004 Apr 6;101(14):5018–23. 15037750
44. Correa NE, Lauriano CM, McGee R, Klose KE. Phosphorylation of the flagellar regulatory protein FlrC is necessary for Vibrio cholerae motility and enhanced colonization. Mol Microbiol. 2000 Feb;35(4):743–55. 10692152
45. Butler SM, Camilli A. Going against the grain: chemotaxis and infection in Vibrio cholerae. Nature reviews Microbiology. 2005 Aug;3(8):611–20. 16012515
46. Taylor RK, Miller VL, Furlong DB, Mekalanos JJ. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–7. 2883655
47. Galperin MY. Structural classification of bacterial response regulators: diversity of output domains and domain combinations. J Bacteriol. 2006 Jun;188(12):4169–82. 16740923
48. Delgado J, Forst S, Harlocker S, Inouye M. Identification of a phosphorylation site and functional analysis of conserved aspartic acid residues of OmpR, a transcriptional activator for ompF and ompC in Escherichia coli. Mol Microbiol. 1993 Dec;10(5):1037–47. 7934854
49. Ma S, Selvaraj U, Ohman DE, Quarless R, Hassett DJ, Wozniak DJ. Phosphorylation-independent activity of the response regulators AlgB and AlgR in promoting alginate biosynthesis in mucoid Pseudomonas aeruginosa. J Bacteriol. 1998 Feb;180(4):956–68. 9473053
50. Ishikawa T, Sabharwal D, Broms J, Milton DL, Sjostedt A, Uhlin BE, et al. Pathoadaptive conditional regulation of the type VI secretion system in Vibrio cholerae O1 strains. Infect Immun. 2012 Feb;80(2):575–84. doi: 10.1128/IAI.05510-11 22083711
51. Hussa EA, O'Shea TM, Darnell CL, Ruby EG, Visick KL. Two-component response regulators of Vibrio fischeri: identification, mutagenesis, and characterization. J Bacteriol. 2007 Aug;189(16):5825–38. 17586650
52. Kobayashi K, Ogura M, Yamaguchi H, Yoshida K, Ogasawara N, Tanaka T, et al. Comprehensive DNA microarray analysis of Bacillus subtilis two-component regulatory systems. J Bacteriol. 2001 Dec;183(24):7365–70. 11717295
53. Lange R, Wagner C, de Saizieu A, Flint N, Molnos J, Stieger M, et al. Domain organization and molecular characterization of 13 two-component systems identified by genome sequencing of Streptococcus pneumoniae. Gene. 1999 Sep 3;237(1):223–34. 10524254
54. Throup JP, Koretke KK, Bryant AP, Ingraham KA, Chalker AF, Ge Y, et al. A genomic analysis of two-component signal transduction in Streptococcus pneumoniae. Mol Microbiol. 2000 Feb;35(3):566–76. 10672179
55. Yamamoto K, Hirao K, Oshima T, Aiba H, Utsumi R, Ishihama A. Functional characterization in vitro of all two-component signal transduction systems from Escherichia coli. J Biol Chem. 2005 Jan 14;280(2):1448–56. 15522865
56. Mandlik A, Livny J, Robins WP, Ritchie JM, Mekalanos JJ, Waldor MK. RNA-Seq-based monitoring of infection-linked changes in Vibrio cholerae gene expression. Cell host & microbe. 2011 Aug 18;10(2):165–74.
57. Kamp HD, Patimalla-Dipali B, Lazinski DW, Wallace-Gadsden F, Camilli A. Gene fitness landscapes of Vibrio cholerae at important stages of its life cycle. PLoS Pathog. 2013;9(12):e1003800. doi: 10.1371/journal.ppat.1003800 24385900
58. Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nature protocols. 2009;4(3):363–71. doi: 10.1038/nprot.2009.2 19247286
59. Bernard CS, Brunet YR, Gueguen E, Cascales E. Nooks and crannies in type VI secretion regulation. J Bacteriol. 2010 Aug;192(15):3850–60. doi: 10.1128/JB.00370-10 20511495
60. Borgeaud S, Metzger LC, Scrignari T, Blokesch M. Bacterial evolution. The type VI secretion system of Vibrio cholerae fosters horizontal gene transfer. Science. 2015 Jan 2;347(6217):63–7. doi: 10.1126/science.1260064 25554784
61. National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.). Guide for the care and use of laboratory animals. 8th ed. Washington, D.C.: National Academies Press; 2011. xxv, 220 p. p.
62. Lim B, Beyhan S, Meir J, Yildiz FH. Cyclic-diGMP signal transduction systems in Vibrio cholerae: modulation of rugosity and biofilm formation. Mol Microbiol. 2006 Apr;60(2):331–48. 16573684
63. Iwanaga M, Yamamoto K, Higa N, Ichinose Y, Nakasone N, Tanabe M. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiology and immunology. 1986;30(11):1075–83. 3543624
64. Fong JC, Yildiz FH. The rbmBCDEF gene cluster modulates development of rugose colony morphology and biofilm formation in Vibrio cholerae. J Bacteriol. 2007 Mar;189(6):2319–30. 17220218
65. Lefebvre B, Formstecher P, Lefebvre P. Improvement of the gene splicing overlap (SOE) method. BioTechniques. 1995 Aug;19(2):186–8. 8527132
66. Beyhan S, Bilecen K, Salama SR, Casper-Lindley C, Yildiz FH. Regulation of rugosity and biofilm formation in Vibrio cholerae: comparison of VpsT and VpsR regulons and epistasis analysis of vpsT, vpsR, and hapR. J Bacteriol. 2007 Jan;189(2):388–402. 17071756
67. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001 May 1;29(9):e45. 11328886
68. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010 Jan 1;26(1):139–40. doi: 10.1093/bioinformatics/btp616 19910308
69. Berk V, Fong JC, Dempsey GT, Develioglu ON, Zhuang X, Liphardt J, et al. Molecular architecture and assembly principles of Vibrio cholerae biofilms. Science. 2012 Jul 13;337(6091):236–9. doi: 10.1126/science.1222981 22798614
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary TuberculosisČlánek Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic PlagueČlánek Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host SpeciesČlánek Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR SelectionČlánek Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus GenomesČlánek Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from FerrioxamineČlánek Remembering MumpsČlánek Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling PathwayČlánek Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 5- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Parasites and Their Heterophagic Appetite for Disease
- The Elusive Role of the Prion Protein and the Mechanism of Toxicity in Prion Disease
- Intestinal Colonization Dynamics of
- Activation of Typhi-Specific Regulatory T Cells in Typhoid Disease in a Wild-Type . Typhi Challenge Model
- The Engineering of a Novel Ligand in gH Confers to HSV an Expanded Tropism Independent of gD Activation by Its Receptors
- Neutrophil-Derived MMP-8 Drives AMPK-Dependent Matrix Destruction in Human Pulmonary Tuberculosis
- Group Selection and Contribution of Minority Variants during Virus Adaptation Determines Virus Fitness and Phenotype
- Phosphatidic Acid Produced by Phospholipase D Promotes RNA Replication of a Plant RNA Virus
- A Ribonucleoprotein Complex Protects the Interleukin-6 mRNA from Degradation by Distinct Herpesviral Endonucleases
- Characterization of Transcriptional Responses to Different Aphid Species Reveals Genes that Contribute to Host Susceptibility and Non-host Resistance
- Circumventing . Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic Plague
- Natural Killer Cell Sensing of Infected Cells Compensates for MyD88 Deficiency but Not IFN-I Activity in Resistance to Mouse Cytomegalovirus
- Manipulation of the Xanthophyll Cycle Increases Plant Susceptibility to
- Ly6C Monocytes Regulate Parasite-Induced Liver Inflammation by Inducing the Differentiation of Pathogenic Ly6C Monocytes into Macrophages
- Admixture in Humans of Two Divergent Populations Associated with Different Macaque Host Species
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Experimental Evolution of an RNA Virus in Wild Birds: Evidence for Host-Dependent Impacts on Population Structure and Competitive Fitness
- Inhibition and Reversal of Microbial Attachment by an Antibody with Parasteric Activity against the FimH Adhesin of Uropathogenic .
- The EBNA-2 N-Terminal Transactivation Domain Folds into a Dimeric Structure Required for Target Gene Activation
- Human and Murine Clonal CD8+ T Cell Expansions Arise during Tuberculosis Because of TCR Selection
- The NLRP3 Inflammasome Is a Pathogen Sensor for Invasive via Activation of α5β1 Integrin at the Macrophage-Amebae Intercellular Junction
- Sequential Conformational Changes in the Morbillivirus Attachment Protein Initiate the Membrane Fusion Process
- A Two-Component DNA-Prime/Protein-Boost Vaccination Strategy for Eliciting Long-Term, Protective T Cell Immunity against
- cAMP-Signalling Regulates Gametocyte-Infected Erythrocyte Deformability Required for Malaria Parasite Transmission
- Response Regulator VxrB Controls Colonization and Regulates the Type VI Secretion System
- Evidence for a Novel Mechanism of Influenza Virus-Induced Type I Interferon Expression by a Defective RNA-Encoded Protein
- Dust Devil: The Life and Times of the Fungus That Causes Valley Fever
- TNF-α Induced by Hepatitis C Virus via TLR7 and TLR8 in Hepatocytes Supports Interferon Signaling via an Autocrine Mechanism
- The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen,
- L-Rhamnosylation of Wall Teichoic Acids Promotes Resistance to Antimicrobial Peptides by Delaying Interaction with the Membrane
- Rapid Sequestration of by Neutrophils Contributes to the Development of Chronic Lesion
- Selective Recruitment of Nuclear Factors to Productively Replicating Herpes Simplex Virus Genomes
- The Expression of Functional Vpx during Pathogenic SIVmac Infections of Rhesus Macaques Suppresses SAMHD1 in CD4 Memory T Cells
- Fob1 and Fob2 Proteins Are Virulence Determinants of via Facilitating Iron Uptake from Ferrioxamine
- TRAF1 Coordinates Polyubiquitin Signaling to Enhance Epstein-Barr Virus LMP1-Mediated Growth and Survival Pathway Activation
- Vaccine-Elicited Tier 2 HIV-1 Neutralizing Antibodies Bind to Quaternary Epitopes Involving Glycan-Deficient Patches Proximal to the CD4 Binding Site
- Remembering Mumps
- The Role of Horizontal Gene Transfer in the Evolution of the Oomycetes
- Advances and Challenges in Computational Prediction of Effectors from Plant Pathogenic Fungi
- Investigating Fungal Outbreaks in the 21st Century
- Systems Biology for Biologists
- How Does the Dinoflagellate Parasite Outsmart the Immune System of Its Crustacean Hosts?
- FCRL5 Delineates Functionally Impaired Memory B Cells Associated with Exposure
- Phospholipase D1 Couples CD4 T Cell Activation to c-Myc-Dependent Deoxyribonucleotide Pool Expansion and HIV-1 Replication
- Influenza A Virus on Oceanic Islands: Host and Viral Diversity in Seabirds in the Western Indian Ocean
- Geometric Constraints Dominate the Antigenic Evolution of Influenza H3N2 Hemagglutinin
- Widespread Recombination, Reassortment, and Transmission of Unbalanced Compound Viral Genotypes in Natural Arenavirus Infections
- Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity
- A Single Protein S-acyl Transferase Acts through Diverse Substrates to Determine Cryptococcal Morphology, Stress Tolerance, and Pathogenic Outcome
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
- Mechanisms of Stage-Transcending Protection Following Immunization of Mice with Late Liver Stage-Arresting Genetically Attenuated Malaria Parasites
- The Myelin and Lymphocyte Protein MAL Is Required for Binding and Activity of ε-Toxin
- Genome-Wide Identification of the Target Genes of AP2-O, a AP2-Family Transcription Factor
- An Atypical Mitochondrial Carrier That Mediates Drug Action in
- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Helminth Infection and Commensal Microbiota Drive Early IL-10 Production in the Skin by CD4 T Cells That Are Functionally Suppressive
- Circulating Pneumolysin Is a Potent Inducer of Cardiac Injury during Pneumococcal Infection
- ExoT Induces Atypical Anoikis Apoptosis in Target Host Cells by Transforming Crk Adaptor Protein into a Cytotoxin
- Discovery of a Small Non-AUG-Initiated ORF in Poleroviruses and Luteoviruses That Is Required for Long-Distance Movement
- Induces the Premature Death of Human Neutrophils through the Action of Its Lipopolysaccharide
- Varicella Viruses Inhibit Interferon-Stimulated JAK-STAT Signaling through Multiple Mechanisms
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Recovery of Recombinant Crimean Congo Hemorrhagic Fever Virus Reveals a Function for Non-structural Glycoproteins Cleavage by Furin
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway
- Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis
- Expression in the Fat Body Is Required in the Defense Against Parasitic Wasps in
- Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



