-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaStructural Determinants of Phenotypic Diversity and Replication Rate of Human Prions
Sporadic Creutzfeldt-Jakob disease (sCJD) represents ~90% of all human prion diseases worldwide. This neurodegenerative disease, which is transmissible and invariably fatal, is characterized by variable progression rates and remarkable diversity of clinical and pathological traits. The infectious sCJD prions propagating the pathology mainly in the brain are assemblies of abnormally folded isoform (PrPSc) of a host-encoded prion protein (PrPC). The structure and replication mechanisms of human prions are unknown, and whether the PrPSc subtypes identified by proteolytic fragmentation represent distinct strains of sCJD prions has been debated. Here, we isolated sCJD prions from patients with two very distant phenotypes of the disease, compared their structural organization using recently developed biophysical techniques, and investigated their replication in vitro. Our data indicate that these sCJD prions are characterized by different secondary structure organization and quaternary packing arrangements, and that these structural differences are responsible for distinct prion replication rates and unique phenotypic characteristics of the disease. Furthermore, our analysis reveals that, contrary to previous observations for yeast prions, the replication tempo of sCJD prions is determined not so much by their conformational stability but by specific structural features that control the growth speed of prion particles.
Published in the journal: . PLoS Pathog 11(4): e32767. doi:10.1371/journal.ppat.1004832
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004832Summary
Sporadic Creutzfeldt-Jakob disease (sCJD) represents ~90% of all human prion diseases worldwide. This neurodegenerative disease, which is transmissible and invariably fatal, is characterized by variable progression rates and remarkable diversity of clinical and pathological traits. The infectious sCJD prions propagating the pathology mainly in the brain are assemblies of abnormally folded isoform (PrPSc) of a host-encoded prion protein (PrPC). The structure and replication mechanisms of human prions are unknown, and whether the PrPSc subtypes identified by proteolytic fragmentation represent distinct strains of sCJD prions has been debated. Here, we isolated sCJD prions from patients with two very distant phenotypes of the disease, compared their structural organization using recently developed biophysical techniques, and investigated their replication in vitro. Our data indicate that these sCJD prions are characterized by different secondary structure organization and quaternary packing arrangements, and that these structural differences are responsible for distinct prion replication rates and unique phenotypic characteristics of the disease. Furthermore, our analysis reveals that, contrary to previous observations for yeast prions, the replication tempo of sCJD prions is determined not so much by their conformational stability but by specific structural features that control the growth speed of prion particles.
Introduction
Prions are a novel class of infectious agents that are composed solely of self-replicating misfolded protein aggregates [1]. In mammals, prions cause a group of invariably fatal and rapidly progressive neurodegenerative diseases, originally described as transmissible spongiform encephalopathies (TSEs) [1,2]. The most common of the human prion diseases is sporadic Creutzfeldt-Jakob disease (sCJD) [3], accounting for ~90% of all CJD cases worldwide [4]. One of the most intriguing features of these diseases is their vast phenotypic heterogeneity [1,4]. In patients homozygous for methionine in the PRNP gene, there are two major subtypes of sCJD: MM1 and MM2. These types differ with regard to the progression rate of the disease, pattern of proteinase K (PK)-resistant fragments of infectious prion protein aggregates PrPSc, (Fig 1a), neuropathological characteristics of brain lesions, and transmissibility properties in transgenic mice [4–10].
Fig. 1. Schematic representation of PK-resistant fragments in rPrPSc corresponding to Type 1 (MM1) and Type 2 (MM2) sCJD prions and molecular characteristics of purified human rPrPSc used in structural studies. 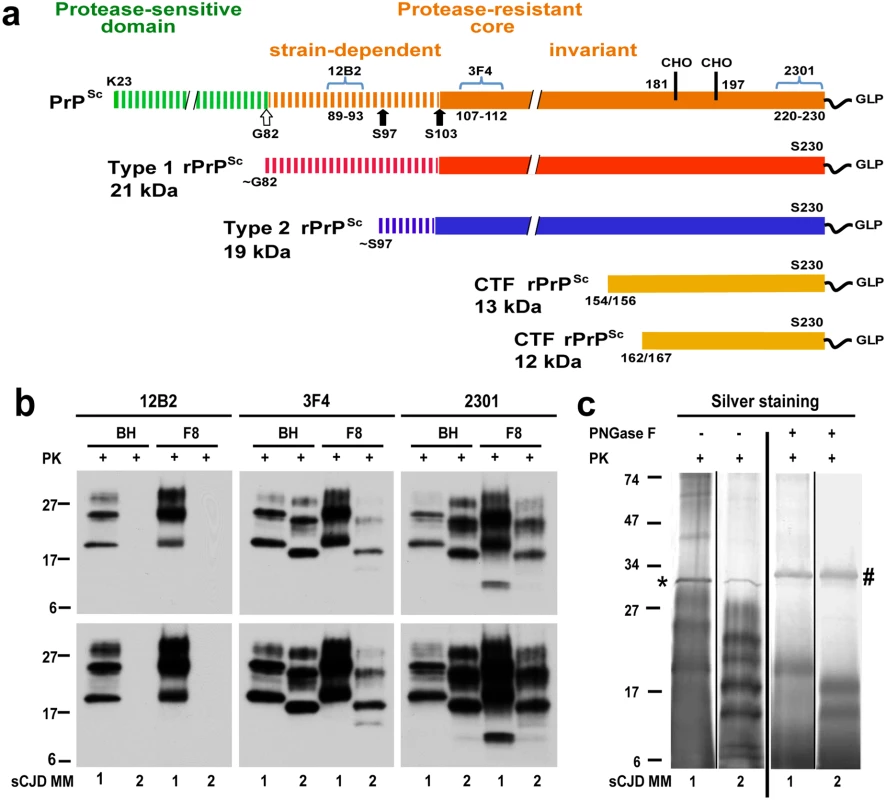
(a) Outline of classification of Type 1 and Type 2 human prions based on proteolytic fragmentation of PrPSc [5,52]. Major cleavage sites by PK are indicated by arrows; GLP—glycolipid; CHO- complex N-glycosylation chains. The codes above light blue brackets represent monoclonal antibodies used in differentiation of various domains of human prions, and the numbers below these brackets indicate linear epitopes recognized by these antibodies. (b) Distinct glycosylation patterns and electrophoretic mobilities of purified human Type 1 (MM1) and Type 2 (MM2) sCJD rPrPSc (homozygous for methionine (M) in codon 129) used in structural studies. To differentiate Type 1, Type 2 prions, and their C-terminal fragments, Western blots of purified rPrPSc (fraction 8; F8) from the brain homogenate (BH) of type MM1 and MM2 sCJD were developed with mAb 12B2 (epitope residues 89–93) [53], mAb 3F4 (epitope residues 107–112) [54], and rabbit polyclonal antibody 2301 (epitope residues 220–231) [55]. The lower panels correspond to prolonged exposure of the same WB to detect less abundant low molecular weight fragments of rPrPSc. (c) Distinct fragmentation patterns of purified MM1 and MM2 sCJD prions in silver stained SDS-PAGE before and after deglycosylation with PNGase F. The symbols (*) and (#) indicate bands corresponding to PK and PNGase F, respectively. The molecular weights of marker proteins are in kDa. A substantial progress has been made in recent years in prion research using laboratory rodent-adapted, cloned prion strains. These studies revealed, among others, that phenotypic variability of these model prions is directly linked to (and likely encoded in) structural differences of PrPSc, and suggested that prion replication rates are inversely proportional to conformational stability of rodent PrPSc (as defined by the concentration of denaturant needed to dissociate/unfold PrPSc aggregates) [11,12]. By contrast, our understanding of the molecular basis of human prions such as those causing sCJD is far less advanced. These prions are present in human brain at a very low concentration (approximately 100-fold lower compared to that in a prion-infected rodent brain) and, thus, are much more difficult to purify and characterize. In fact, no direct structural data are available for PrPSc present in sCJD brains beyond the evidence that the N-terminus is variably resistant to denaturation and proteolytic digestion (Fig 1a) [5,7,13–17]. Even though earlier studies suggest that phenotypic diversity in human prion disease is somehow related to distinct PrPSc isoforms, conformational spectrum of these isoforms and the issue of strains of human prions are poorly understood, hindering efforts to develop generally accepted international classification of human prion disease. Moreover, the classical approach—isolation and definition of a full repertoire of sCJD prion strains in transgenic mice models with uniform genetic background—had not been successful due to the constrains imposed by the extensive phenotypic and genetic diversity of sCJD [4] and very long incubation time and/or limited transmissibility to transgenic mice [6,8–10]. The characterization of human prions is further complicated by the frequent co-existence of diverse prion particles [18,19] and prion adaptation and evolution in a new host [9,19].
To bridge some of these gaps, here we purified sCJD prions from two cases of phenotypically very distant sCJD types, determined their replication tempo as well as characterized structural organization using recently emerged approaches based on mass spectrometry-detected hydrogen/deuterium exchange. Our data provide direct experimental evidence that different phenotypes of sCJD are associated with structurally distinct PrPSc aggregates. Furthermore, these data suggest that, in contrast to the observations for murine prion strains [11,12], the replication rate of sCJD prions is not a pure function of conformational stability but is rather dictated by specific structural features of PrPSc.
Results and Discussion
From the collection of samples obtained from 340 patients with an unequivocal diagnosis of Type 1 (MM1) and Type 2 (MM2) sCJD, we selected one case that is representative of each neuropathology group (S1 Fig) and displayed ≥99% pure Type 1 or Type 2 proteinase K-resistant PrPSc (rPrPSc), as detected by both conformation dependent immunoassay (CDI) and Western blots [13,14,20]. The disease duration in these representative cases, as well as biochemical characteristics of brain PrPSc associated with them (levels of total PrPSc and rPrPSc, size of PrPSc particles, conformational stability of PrPSc) correspond to the respective median values reported previously for each group [13,14] (Table 1).
Tab. 1. Source, biophysical characteristics, and replication rate of Type 1 and Type 2 sCJD prions. 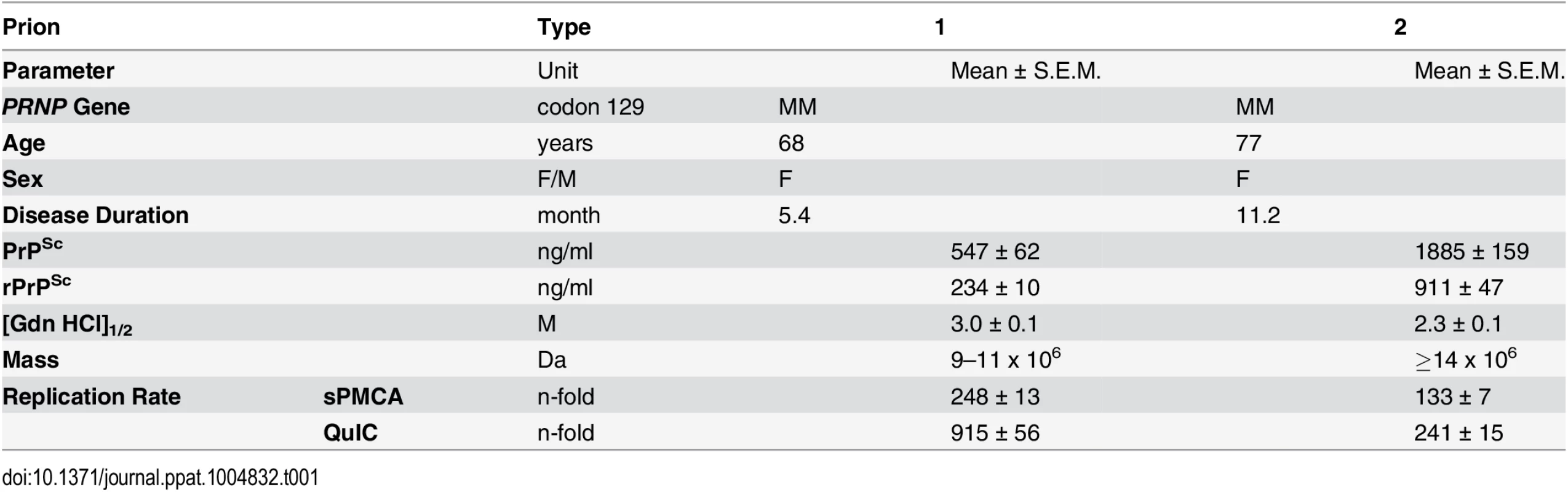
The native prion particles containing rPrPSc from these two cases were purified for structural studies with a scaled up protocol we developed previously for purification of infectious and structurally intact Sc237 prions from Syrian hamster brains [21]. The Western blot patterns of purified MM1 and MM2 rPrPSc in the final fraction 8 (F8) and in the original brain homogenates (BH) were superimposable, documenting complete qualitative recovery of rPrPSc from brain homogenates (Fig 1b). As expected [22], the mass of unglycosylated fragments was ~21 kDa in Type 1 and ~19 kDa in Type 2 rPrPSc, and Type 2 rPrPSc was not detectable with mAb 12B2 due to the missing N-terminal epitope (Fig 1b and S2b Fig). The 12–13 kDa C-terminal fragments were more abundant in Type 1 rPrPSc and detectable in Type 2 after longer exposure (Fig 1b). The silver-stained gels demonstrated the pattern of rPrPSc corresponding to the major bands on Western blots, and the isolated rPrPSc was ~90% pure (Fig 1c). These patterns were highly reproducible upon purification of rPrPSc from different cortical areas of the same brain (S2 Fig).
To investigate the prion size, we separated sCJD prion particles according to sedimentation velocity in sucrose gradient [13]. Consistent with previous data, the peak sedimentation velocity of MM1 rPrPSc was found to be substantially slower than that of MM2 rPrPSc [13] (Fig 2a). Based on calibration with standard proteins [13], we estimate that the majority of MM1 rPrPSc particles have a molecular mass of 9-11x106 Da (~380–460 monomers), whereas the respective value for MM2 rPrPSc particles is ≥14x106 Da (≥600 monomers) (Fig 2a and Table 1).
Fig. 2. Sedimentation velocity, conformational stability, and seeding potency of isolated sCJD prions. 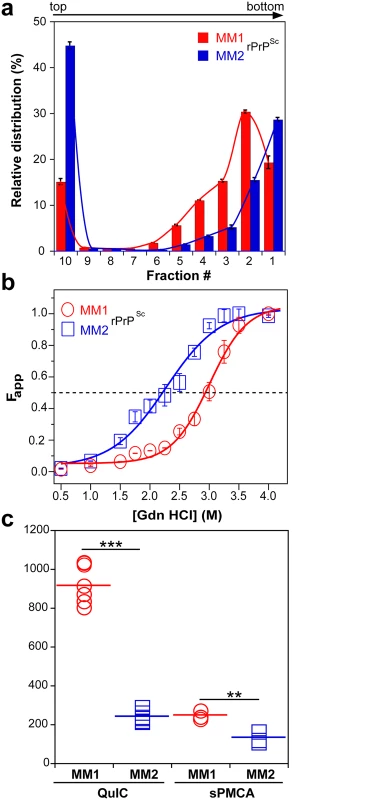
(a) Distinct sedimentation velocity profiles of MM1 and MM2 prions. The samples were fractionated by ultracentrifugation in sucrose gradient and fractions were collected from the bottom of the tubes and analyzed for rPrPSc by CDI. The bars represent average ± SEM; CDI was performed on each sCJD sample in triplicate. (b) The conformational stability of MM1 and MM2 rPrPSc. The curves represent best fit to a sigmoidal function. The values of apparent fractional change (Fapp) are mean ± SEM obtained from four batches of purified MM1 and MM2 prions, each determined in triplicate measurements. (c) Amplification of MM1 and MM2 sCJD prions by QuIC using recombinant human PrP(23–231, 129M) substrate and by sPMCA using brain homogenate of Tg mice expressing human PrPC (129M). The amplification index is the ratio between the concentration of PrPSc before and after PMCA measured with CDI. The data points represent results of six QuIC and three sPMCA experiments, each measured in triplicate with CDI. The mean values are indicated by horizontal lines. *** P<0.001, ** P<0.005 determined by ANOVA. Using CDI, we compared the conformational stability of rPrPSc obtained from different brain cortex areas in four independent purification rounds from each sCJD case (Fig 2b). The average stability of MM1 rPrPSc against denaturation by GdnHCl was significantly higher than that of MM2 rPrPSc, with GdnHCl concentration corresponding to midpoint denaturation of 3.0 and 2.3 M, respectively (Fig 2b and Table 1).
Next, we assessed the seeding efficacy (amplification index) of MM1 rPrPSc and MM2 rPrPSc in vitro using two different methods, QuIC and sPMCA. The amplification index (potency) of different seeds is expressed as a ratio between the concentration of the PrPSc conformers produced with PMCA or QuIC divided by the concentration of PrPSc in the seed after subtracting the background obtained in control unseeded samples. Detailed protocols of these methods and control experiments showing lack of spontaneous prion protein conversion in the unseeded reactions have been described previously [13]. In both assays, the seeding efficacy of MM1 rPrPSc was markedly higher compared to that of MM2 rPrPSc (Fig 2c and Table 1). This higher replication efficacy of MM1 prions from the case selected for the present structural studies is consistent with ~4-fold higher median replication potency of MM1 prions compared to MM2 prions we previously observed (using both QuIC and sPMCA techniques) for prions from ten MM1 and ten MM2 sCJD cases (Supplemental S2 Fig in [13]). These data in vitro are also in accord with available bioassay data that demonstrate higher transmission rates and significantly shorter incubation times of MM1 sCJD prions in transgenic mice expressing human PrPC (129M) or human/mouse PrPC chimeras [8,9]. The higher replication efficiency of the conformationally more stable MM1 rPrPSc is both intriguing and unexpected, as some previous experiments with mouse prion strains suggest that there is an inverse correlation between prion replication rate and conformational stability of total PrPSc (i.e., the less stable conformers should replicate faster) [11,12]. Our present data indicate that this previously suggested relationship does not apply to sCJD rPrPSc.
Clearly, understanding the molecular basis of phenotypic variability in sCJD requires structural characterization of PrPSc that goes beyond relatively crude assays such as proteolytic fragmentation followed by Western blotting or conformational stability measurements. This is a challenging task because most of the methods developed for structural studies of protein aggregates are not applicable to brain-derived PrPSc, as they require isotopic labeling or introduction of other spectroscopic probes. However, new opportunities in this regard are offered by two mass spectrometry based methods: backbone amide hydrogen/deuterium exchange coupled with mass spectrometry (HXMS) [23] and histidine hydrogen/deuterium exchange mass spectrometry (His-HXMS) [24]. Here, we used these two methods for structural comparison of MM1 rPrPSc and MM2 rPrPSc.
The HXMS method measures the rate of H/D exchange of protein backbone amide hydrogen atoms. Since the exchange rates are much faster for protein segments that are unstructured as compared to those that are involved in H-bonded structures such as α-helices or β-sheets, these measurements provide a sensitive tool for conformational analysis. This approach, which we recently successfully used for structural analysis of strain-specific differences in murine prions [23], is especially useful for studying amyloids and related protein aggregates, as the exchange rates within the β-sheet cores of these aggregates are exceptionally slow [25–30].
The first step in HXMS analysis is the generation of peptic fragments that can be separated by ultrahigh performance liquid chromatography (UHPLC) and identified by MS. Both for MM1 and MM2 rPrPSc, we were able to identify 27 peptic fragments that give rise to MS spectra with a signal-to-noise ratio sufficient for reliable calculation of deuterium incorporation. These fragments (some of them partially overlapping) cover ~85% of the C-terminal region 117–224, with the only significant gap for the segment 169–181 that contains one of the glycosylation sites (likely due to a very low concentration of peptic fragment(s) derived from the nonglycosylated component of rPrPSc). No peptic fragments could be analyzed from the N-terminal region up to residue 116, presumably due to the ragged N-terminus of human rPrPSc. The extent of deuterium incorporation for MM1 rPrPSc and MM2 rPrPSc after 5 min and 240 h incubation in D2O is shown in Fig 3. For both rPrPSc types, the region of relatively little protection against deuterium incorporation maps to residues ~145–160. This is in striking contrast to murine prion strains studied to date, in which case this central region is characterized by high degree of protection against H/D exchange [23]. Thus, it appears that the ~145–160 region of sCJD prions is structurally less ordered than the same region in cloned murine prion strains.
Fig. 3. Deuterium incorporation for peptic fragments derived from MM1 rPrPSc (red) and MM2 rPrPSc(blue). 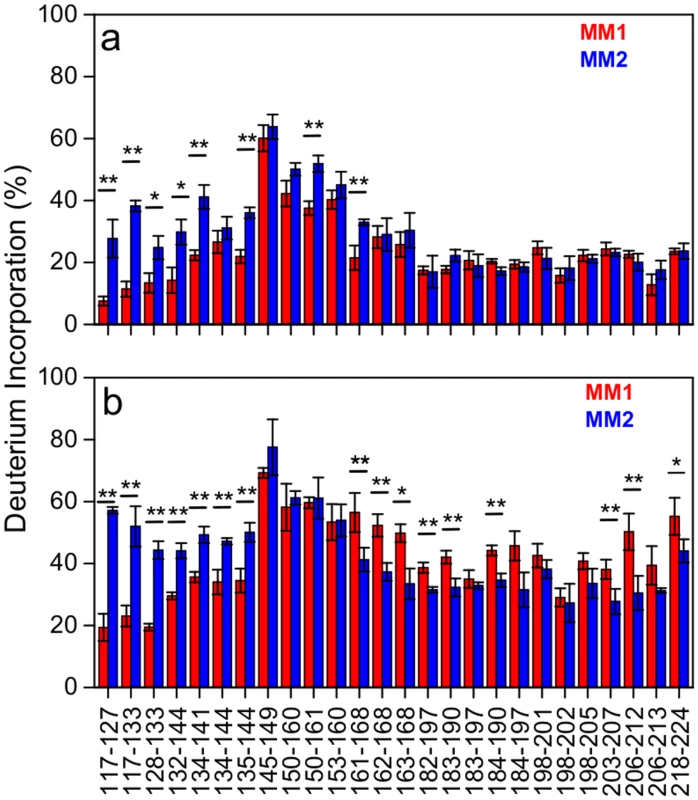
(a) 5 min incubation in D2O. (b) 240 h incubation in D2O. Error bars indicate standard deviation (3 independent experiments). *, P<0.05; **, P<0.02. In contrast to similar protection against deuterium incorporation in the ~145–160 regions of MM1 and MM2 rPrPSc, there are substantial differences in other parts of rPrPSc corresponding to distinct sCJD phenotypes. This is especially evident for the 117–144 region, as peptic fragments derived from this part of MM1 rPrPSc consistently show higher degree of H/D exchange as compared to those corresponding to the same region in MM2 rPrPSc. The difference is particularly striking for the 117–133 region, in which case the degree of deuterium incorporation after 240 h exchange is 2.5–3 fold lower for MM1 rPrPSc, indicating markedly higher level of structural order in this part of MM1 rPrPSc as compared to MM2 rPrPSc. An opposite trend is observed for the C-terminal region ~161–224, where higher protection against H/D exchange is observed for MM2 rPrPSc (Fig 3). Altogether, these data clearly demonstrate substantial structural differences between rPrPSc corresponding to two different phenotypes of sCJD. The resolution of HXMS alone is not sufficient to propose any specific structural model that could account for these differences. However, within the context of the frequently considered model based on the parallel in-register β-structure motif [31,32], region-specific differences in resistance to H/D exchange observed between MM1 PrPSc and MM2 PrPSc could likely reflect factors such as different proportions in these regions of residues involved in β-strands and loops between them and/or packing differences between individual β-strands. As in the case of murine prions [23], high level of protection against H/D exchange in the C-terminal region of sCJD PrPSc is not compatible with the structural model proposing that residues ~89–175 form left-handed β-helices, with the C-terminal region retaining the native-like α-helical conformation of PrPC [33]. However, the present data alone do not exclude the possibility that the entire PK-resistant region of PrPSc could form a β-helix-like structure.
Structural properties of sCJD prions were further probed using the recently developed approach of His-HXMS which measures the rate of H/D exchange of C2 protons in histidine side chains [34–36]. Information provided by this method is complementary to that obtained from amide HXMS measurements: while amide HXMS probes protein structural organization and dynamics at the level of the polypeptide backbone, His-HXMS probes the microenvironment (water accessibility) of specific His side chains [34–36]. As shown in a recent study with recombinant prion protein amyloid fibrils, the latter approach can be particularly useful in probing quaternary structure of ordered protein aggregates, providing information about the packing arrangement and interfaces between β-sheets [31].
There are six His residues in the PK-resistant region of human PrPSc (His99, His111, His140, His150, His177 and His187). MS signal for the peptide fragment containing His99 was too weak to allow reliable measurements. However, high quality H/D exchange data could be obtained for five other His residues. In the native structure of the PrPC monomer, all these His side chains are fully exposed to water. Thus, as expected for unprotected histidines [34,35], the half-times of exchange are about 2–3 days. In the rPrPSc structures, these half-times are substantially longer, indicating that all His side chains are located in at least partially water-protected environment (Fig 4). However, the degree of this protection for individual His side chains varies greatly between MM1 rPrPSc and MM2 rPrPSc. For example, in MM1 rPrPSc, His177 is still in a relatively water accessible environment (exchange half-time of 9 days), whereas in MM2 PrPSc, this side chain is much more protected from water (exchange half-time of 56 days). An opposite situation is observed for His111, in which the environment of the side chain is much more water-protected in the structure of MM1 rPrPSc than that of the MM2 counterpart (exchange half-times of 67 and 16 days, respectively).
Fig. 4. Histidine H/D exchange for monomeric PrPC (black), MM1 rPrPSc (red) and MM2 rPrPSc (blue). 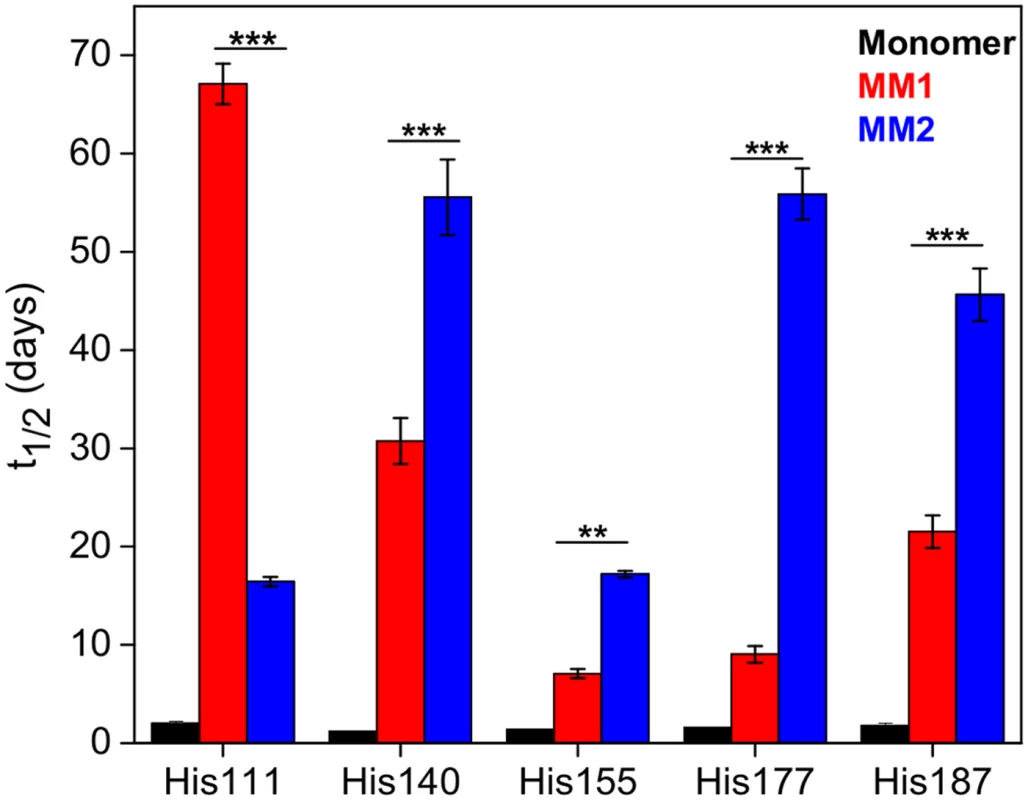
The parameter t1/2 represents the half-time of exchange reaction for individual His residues. Error bars indicate standard deviation (3 independent experiments). **, P<0.01; ***, P<0.001. Recent crystallographic studies with amyloidogenic peptides identified two types of interfaces between β-sheets in amyloid structures: one that is highly water protected with strong interdigitation of side chains (“dry” steric zipper) and one that is more accessible to water [37,38]. Within this context, the differences in water exposure of individual His side chains observed between MM1 rPrPSc and MM2 rPrPSc could be explained by distinct packing arrangements of β-sheets in these two structures (i.e., the same His side chain being in dry or wet interface depending on the rPrPSc type). It should be noted, however, that even for the most protected His side chains in rPrPSc, the exchange half-times are substantially shorter than those recently observed in synthetic amyloid fibrils prepared from the recombinant PrP (hundreds of hours), suggesting that steric zippers in brain-derived rPrPSc might be less perfect than those in synthetic amyloid fibrils. This is not entirely surprising given that rPrPSc particles contain glycosylated isoforms, and glycans may interfere with packing between β-sheets.
Experiments with two strains of yeast prion [PSI+] demonstrated that, in this case, the critical determinant of the strength of prion phenotype is the susceptibility of prion aggregates to fragmentation (that creates new ends for monomer recruitment), with the less stable structure corresponding to the stronger phenotype [39]. This fragmentation and maintenance of the yeast prion state in vivo is believed to be mediated by the molecular chaperone Hsp104 [39,40]. Even though there are no known mammalian homologs of the disaggregating chaperone HsP104, the general hypothesis that less stable prions are more virulent has been adopted in the field of mammalian prions, and this model appeared to be supported by studies in vivo with some rodent prion strains [11,12,41], even though the results of these studies could also be explained by strain-dependent differences in prion clearance rates. Furthermore, an inverse correlation was found between the replication tempo in vitro and the conformational stability of the protease-sensitive sCJD PrPSc (but not the protease-resistant component, rPrPSc) [13]. However, data for some other rodent prion strains appear to be inconsistent with this model [42,43]. The picture is further complicated by the fact that there are no reliable direct assays to probe fragmentation susceptibility of PrPSc aggregates, and their stability is typically assessed by measuring resistance to denaturation with SDS or chaotropes; the relationship between the latter property and fragility is not necessarily straightforward.
Our present data clearly demonstrate that, in contrast to the observations for some murine prions, lower conformational stability of sCJD rPrPSc does not result in higher replication rate of these prions. Thus, at least in the case of human prions, conformational stability of rPrPSc (as defined by resistance to denaturation with SDS or chaotropes) is definitely not a reliable predictor of the incubation period of the disease. Importantly, our structural studies allowed us to identify substantial differences between the molecular organization of MM1 and MM2 rPrPSc, both at the level of the polypeptide backbone as well as the quaternary packing arrangements. As shown in our recent study with recombinant PrP amyloid fibrils[24], the differences in packing between β-sheets may result in distinct conformational stabilities. However, it appears that it is not the conformational stability per se that controls the replication rate of rPrPSc, as the observed faster replication of MM1 sCJD prions when compared to MM2 counterparts would imply a paradoxical scenario in which higher stability of rPrPSc results in a faster replication tempo. Instead, our data strongly suggest that distinct replication rates of MM1 and MM2 sCJD prions are dictated by specific structural features of corresponding rPrPSc aggregates, features that control the intrinsic growth rate of these aggregates (i.e., the rate of templated conformational conversion of the PrPC substrate). Thus, the balance of factors controlling strain-specific replication tempo of sCJD prions appears to be diametrically different from that described for yeast prions [PSI+] that are associated with aggregation of Sup35 protein. In the latter case, the intrinsic elongation rate of Sup35 amyloid fibrils Sc4 corresponding to the stronger (faster replicating) prion phenotype is slower than that of fibrils Sc37 corresponding to the weaker phenotype, but this is more than compensated by lower stability (and thus higher effective concentration of ends) for Sc4 fibrils [39]. By contrast, the faster replicating strain of sCJD prion is characterized by higher conformational stability, implying that, in this case, the dominant factor in controlling the replication tempo is not prion stability but the intrinsic growth rate (i.e., the rate of the conversion of PrPC monomers).
Considerable structural differences between type 1 and type 2 PrPSc in sCJD are especially intriguing given frequent coexistence of these two prion strains in affected individuals [20]. Whether this strain coexistence is the result of a primordial spontaneous misfolding or conformational evolution due to the template flipping during passage through cells expressing different post-translationally modified PrPC remains to be determined [19]. It should also be noted that type 1 and type 2 sCJD prions represent only a small fraction of the spectrum of human prions. It is likely that the structural variability among PrPSc corresponding to different familial forms of human prion diseases might be even larger than the extent of structural differences described herein for type 1 and type 2 sCJD prions.
Materials and Methods
Ethics statement and clinicopathologic characteristics of sCJD cases
All procedures were performed under protocols approved by the Institutional Review Board at Case Western Reserve University. In all cases, written informed consent for research was obtained from patients or legal guardians and the material used had appropriate ethical approval for use in this project. All patients’ data and samples were coded and handled according to NIH guidelines to protect patients’ identities. We selected two representative subjects from a group of 340 patients with definitive diagnosis of sCJD. The criteria for inclusion were: (1) availability of clinical diagnosis of CJD according to WHO criteria [44,45] and clearly determined and dated initial symptoms upon neurologic examination to ascertain the disease duration; (2) methionine homozygous at codon 129 of the human prion protein (PrP) gene (PRNP); (3) unequivocal classification as pure Type 1 or Type 2 sCJD according to WB pattern; (4) unequivocal classification of pathology as definite Type 1 or 2 at the National Prion Disease Pathology Surveillance Center (NPDPSC) in Cleveland, Ohio; (5) demographic data distribution within 95% confidence interval of the whole group, resulting in no difference between selected cases and the whole group in any of the statistically followed parameters.
Purification of prions from sCJD human brains
The purification of rPrPSc from human brains was performed as described previously for 263K prions from Syrian hamster brains [21] with the following additional steps. The partially purified samples containing ~10 μg of human PrPSc were resuspended in PBS, pH7.4 containing 2 mM CaCl2 and 2% Sarkosyl, sonicated in a sonication bath (3 x 5 s), and incubated with 70 μg/ml of Collagenase (Worthington Biochemical Corporation) with shaking at 600 rpm in Eppendorf Thermomixer for 4 h at 37°C. After adding MgCl2 to a final concentration of 5 mM, the samples were incubated with 50 IU/ml of Benzonase (Novagen/EMP) for additional 1 h at 37°C, followed by 1 h incubation with 100 μg/ml of proteinase K (Amresco, Solon, OH/ Invitrogen) at 37°C. The PK was blocked with protease inhibitor (PI) cocktail containing 0.5mM PMSF, and 5 μg/ml of aprotinin and leupeptin, respectively. The pellet obtained after centrifugation (30 min, 18,000 x g, 4°C) in Allegra centrifuge equipped with F2402H rotor was resuspended in 400 μl of 10% NaCl containing 1% Sarkosyl and PI cocktail, and spun again. The final pellet was resuspended in PBS containing 2% Sarkosyl and PI cocktail (1 : 1000, v/v), and delipidated overnight with four volumes of Methanol/Chloroform (2 : 1, v/v) at -20°C. Finally, the sample was collected by centrifugation, resuspended in water containing 0.1% Sarkosyl and stored at -80°C.
Physicochemical properties and molecular characteristics of purified sCJD prions
The purified rPrPSc was analyzed by SDS PAGE followed by silver staining and/or western blots, and by conformation-dependent immunoassay (CDI). The latter assay was performed as described previously [6,14] with the following minor modifications. First, we used white Lumitrac 600 High Binding Plates (E&K Scientific, Santa Clara, California) coated with mAb 8H4 (epitope 175–185)[46] in 200 mM NaH2PO4 containing 0.03% (w/v) NaN3, pH 7.5. Second, aliquots of 20 μl from each fraction containing 0.007% (v/v) of Patent Blue V (Sigma) were directly loaded into wells of white strip plates prefilled with 200μl of Assay Buffer (Perkin Elmer, Waltham, Massachusetts). Finally, the captured PrP was detected by a europium-conjugated [47] anti-PrP mAb 3F4 (epitope 107–112) and the time-resolved fluorescence (TRF) signals were measured by the multi-mode microplate reader PHERAstar Plus (BMG LabTech, Durham, North Carolina). The recHuPrP(90–231,129M) and PrP(23–231,129M) used as a calibrant in the CDI was prepared and purified as described previously [48]. The conformational stability of rPrPSc was determined with CDI as described previously [14,47] and the raw CDI signal was converted into the apparent fractional change and fitted by least square method with a sigmoidal transition model to determine GdnHCl concentration where 50% of PrPSc is unfolded ([Gdn HCl]1/2) [14]. The sedimentation velocity and mass of sCJD prions was determined with calibrated sucrose gradient ultracentrifugation as described [13].
Replication rate of sCJD prions measured in vitro
The Quaking-induced Conversion (QuIC) [49] and sonication-driven serial Protein Misfolding Cyclic Amplification (sPMCA) [50] procedures were performed essentially as described previously [13,14]. Briefly, rhuPrP(23–231,129M) used as a substrate in QuIC was expressed, purified, and refolded to α-helical conformation as described previously [48], and its initial concentration was calculated from absorbance at 280 nm using the molar extinction coefficient 56650 M-1cm-1. The stock of rhuPrP(23–231) in 10 mM sodium acetate buffer, pH 4.0, was pretreated with 12 mM HCl [rhuPrP:HCl ratio (v/v) of 1 : 3.9] for 7.5 min and immediately diluted to a final concentration of 0.1 mg/ml into the reaction buffer composed of 20 mM NaH2PO4, 130 mM NaCl, pH 6.9, and containing 0.1% SDS, 0.1% Triton X-100, and 1 : 5000 (v/v) N2 (Invitrogen, Carlsbad, California). The QuIC was performed with final volume of 100 μl per well in a sterile V-bottom, low-binding polypropylene 96-well plate (VWR, Arlington Heights, Illinois) equipped with a 3 mm diameter PTFE bead (Fisher Scientific, Pittsburgh, Pennsylvania) in each well. The aliquots of sCJD brain homogenates were diluted into the complete QuIC reaction buffer to obtain final 10-4 dilution of sCJD prions, and the plates were sealed with sterile AxyMat Silicone Sealing Mat (VWR, Arlington Heights, Illinois). The QuIC was performed in samples seeded with sCJD PrPSc at 55°C for 20 hrs in an Eppendorf Thermomixer (Eppendorf, Hauppauge, New York) set for 1 min shaking at 1400 rpm, followed by 1 min incubation. The reaction was stopped by adding to each well 50 μl of PBS (pH 6.9) containing 3% (w/v) Sarkosyl and Proteinase K (PK; Amresco, Solon, Ohio) to obtain the final Sarkosyl concentration of 1% (w/v) and PrP/PK ratio of 10 : 1 (w/w). The plates were incubated for 1 h at 37°C with shaking at 1200 rpm on the Eppendorf Thermomixer with 1 min intervals. The PK was blocked in each well with protease inhibitors (0.5 mM PMSF, 5 ug/ml of aprotinin and leupeptin) and the PK-resistant PrP was measured with CDI [13,14].
Sonication-driven serial Protein Misfolding Cyclic Amplification (sPMCA) of sCJD samples was performed as described [50] with the following modifications. Human PrPSc was replicated using brains of transgenic mice overexpressing human PrP with methionine at position 129 [51]. The 10% brain homogenates from sCJD patients were diluted 1000-fold into 10% normal brain homogenate and 100 μl was transferred into 0.2 ml PCR tubes equipped with 2.38 mm diameter PTFE ball (K-mac Plastics, Wyoming, Michigan). Tubes were positioned on an adaptor placed on the plate holder of a microsonicator (Misonix Model 3000, Farmingdale, New York) programmed to perform cycles of 60 min incubation at 32°C followed by a 30 s pulse of sonication set at 80% power. Samples were incubated, without shaking, and immersed in the water of the sonicator bath. After a round of 24 cycles, a 10 μl aliquot of the amplified material was diluted into 90 μl of normal transgenic mouse brain homogenate and a new round of 24 PMCA cycles was performed. This procedure was repeated four times to reach a final 106-fold dilution of the initial sCJD brain homogenate, and the replication rate was calculated from PrPSc content measured before and after sPMCA with CDI [13,14,19].
Backbone amide hydrogen/deuterium exchange mass spectrometry experiments (HXMS)
To initiate deuterium labeling, 10 μl aliquots of purified sCJD rPrPSc (~1.8 μg) were collected by centrifugation (21000×g, 30 min, 4°C) and added to 100 μl of 10 mM phosphate buffer (pH 7.3) in D2O. After incubation at room temperature for different time periods, samples were collected by centrifugation and dissociated into monomers by adding 20 μl of ice cold 100 mM phosphate buffer (pH 2.5) containing 7 M GdnHCl and 0.1 M Tris (2-carboxyethyl) phosphine hydrochloride. After 5 minutes incubation (~0°C), the solution was diluted 10 times with ice cold 0.05% trifluoracetic acid and digested for 5 min with pepsin as described previously [23]. The peptic fragments were collected in a C18 trap column (Symmetry C18 NanoEase, Waters, USA), washed to remove salts, and eluted on a UPLC BEH-C18 HPLC column (Waters, USA) using a gradient of 2–45% acetonitrile at a flow rate of 23 μl/min. Peptides separated on the column were analyzed by an LTQ Orbitrap XL mass spectrometer (ThermoElectron, San Jose, CA). To minimize back-exchange, both the trap and the analytical column were placed in a cooled chamber (~2°C) integrated with a LEAP TriValve system (LEAP Technologies, USA). The extent of deuterium incorporation in each peptic fragment was determined from mass spectra (with a correction for back-exchange) as described previously [23].
Histidine hydrogen/deuterium exchange (His-HXMS) experiments
For these measurements, samples of purified sCJD rPrPSc from human brain (~3 μg) were suspended in D2O buffer (10 mM sodium phosphate, 10 μM EDTA, 50 μM Pefabloc, 1 ug/ml Aprotinin, pH 9.0). After incubation for 5 days at 37°C, samples were collected by centrifugation and deglycosylated with PNGase F. To obtain fragments containing single His residues, samples were then digested with immobilized pepsin, followed by digestion with immobilized trypsin. Finally, the peptic fragments were separated on an UPLC column and analyzed by mass spectrometry as described above for HXMS experiments. The pseudo-first-order rate constant (k) of His hydrogen exchange reaction was determined by the equation: k = -ln{1-[((R(t)—R(0))/((1 + R(t)—R(0))] x 1/P}/t, where P is the fractional D2O content in the solvent, R is the ratio of M+1/M isotopic peak of a given peptide before (time = 0) and after the H/X reaction (time = t). The half-life (t1/2, days) of His exchange reaction was calculated using the equation: t1/2 (day) = ln2/k/24, where k (hour-1) is the rate constant at the alkaline conditions (pH = 9) [34,35].
Supporting Information
Zdroje
1. Prusiner SB, Scott MR, DeArmond SJ, Carlson G (2004) Transmission and replication of prions. In: Prusiner SB, editor. Prion Biology and Diseases. 2nd ed. Cold Spring Harbor: Cold Spring Harbor Laboratory Press. pp. 187–242.
2. Gajdusek DC, Gibbs CJ Jr., Alpers M (1966) Experimental transmission of a kuru-like syndrome to chimpanzees. Nature 209 : 794–796. 5922150
3. Gibbs CJ Jr., Gajdusek DC, Asher DM, Alpers MP, Beck E, et al. (1968) Creutzfeldt-Jakob disease (spongiform encephalopathy): transmission to the chimpanzee. Science 161 : 388–389. 5661299
4. Puoti G, Bizzi A, Forloni G, Safar JG, Tagliavini F, et al. (2012) Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol 11 : 618–628. doi: 10.1016/S1474-4422(12)70063-7 22710755
5. Gambetti P, Kong Q, Zou W, Parchi P, Chen SG (2003) Sporadic and familial CJD: classification and characterisation. Br Med Bull 66 : 213–239. 14522861
6. Safar JG, Geschwind MD, Deering C, Didorenko S, Sattavat M, et al. (2005) Diagnosis of human prion disease. Proc Natl Acad Sci USA 102 : 3501–3506. 15741275
7. Safar JG (2012) Molecular Mechanisms Encoding Quantitative and Qualitative Traits of Prion Strains. In: Gambetti P, editor. Prions and Diseases. New York: Springer Verlag.
8. Bishop MT, Will RG, Manson JC (2010) Defining sporadic Creutzfeldt-Jakob disease strains and their transmission properties. Proc Natl Acad Sci U S A 107 : 12005–12010. doi: 10.1073/pnas.1004688107 20547859
9. Giles K, Glidden DV, Patel S, Korth C, Groth D, et al. (2010) Human prion strain selection in transgenic mice. Ann Neurol 68 : 151–161. doi: 10.1002/ana.22104 20695008
10. Wadsworth JD, Joiner S, Linehan JM, Desbruslais M, Fox K, et al. (2008) Kuru prions and sporadic Creutzfeldt-Jakob disease prions have equivalent transmission properties in transgenic and wild-type mice. Proc Natl Acad Sci U S A 105 : 3885–3890. doi: 10.1073/pnas.0800190105 18316717
11. Legname G, Nguyen H-OB, Peretz D, Cohen FE, DeArmond SJ, et al. (2006) Continuum of prion protein structures enciphers a multitude of prion isolate-specified phenotypes. Proc Natl Acad Sci USA 103 : 19105–19110. 17142317
12. Colby DW, Giles K, Legname G, Wille H, Baskakov IV, et al. (2009) Design and construction of diverse mammalian prion strains. Proc Natl Acad Sci U S A 106 : 20417–20422. doi: 10.1073/pnas.0910350106 19915150
13. Kim C, Haldiman T, Surewicz K, Cohen Y, Chen W, et al. (2012) Small Protease Sensitive Oligomers of PrP(Sc) in Distinct Human Prions Determine Conversion Rate of PrP(C). PLoS Pathog 8: e1002835. doi: 10.1371/journal.ppat.1002835 22876179
14. Kim C, Haldiman T, Cohen Y, Chen W, Blevins J, et al. (2011) Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate. PLoS Pathog 7: e1002242. doi: 10.1371/journal.ppat.1002242 21931554
15. Uro-Coste E, Cassard H, Simon S, Lugan S, Bilheude JM, et al. (2008) Beyond PrP9res) type 1/type 2 dichotomy in Creutzfeldt-Jakob disease. PLoS Pathog 4: e1000029. doi: 10.1371/journal.ppat.1000029 18389084
16. Wadsworth JD, Collinge J (2011) Molecular pathology of human prion disease. Acta Neuropathol 121 : 69–77. doi: 10.1007/s00401-010-0735-5 20694796
17. Gambetti P, Cali I, Notari S, Kong Q, Zou WQ, et al. (2011) Molecular biology and pathology of prion strains in sporadic human prion diseases. Acta Neuropathol 121 : 79–90. doi: 10.1007/s00401-010-0761-3 21058033
18. Polymenidou M, Stoeck K, Glatzel M, Vey M, Bellon A, et al. (2005) Coexistence of multiple PrPSc types in individuals with Creutzfeldt-Jakob disease. Lancet Neurol 4 : 805–814. 16297838
19. Haldiman T, Kim C, Cohen Y, Chen W, Blevins J, et al. (2013) Coexistence of Distinct Prion Types Enables Conformational Evolution of Human PrPSc by Competitive Selection. J Biol Chem 288 : 29846–29861. doi: 10.1074/jbc.M113.500108 23974118
20. Cali I, Castellani R, Alshekhlee A, Cohen Y, Blevins J, et al. (2009) Co-existence of scrapie prion protein types 1 and 2 in sporadic Creutzfeldt-Jakob disease: its effect on the phenotype and prion-type characteristics. Brain 132 : 2643–2658. doi: 10.1093/brain/awp196 19734292
21. Safar J, Roller PP, Gajdusek DC, Gibbs CJ Jr. (1993) Conformational transitions, dissociation, and unfolding of scrapie amyloid (prion) protein. J Biol Chem 268 : 20276–20284. 8104185
22. Parchi P, de Boni L, Saverioni D, Cohen ML, Ferrer I, et al. (2012) Consensus classification of human prion disease histotypes allows reliable identification of molecular subtypes: an inter-rater study among surveillance centres in Europe and USA. Acta Neuropathol 124 : 517–529. doi: 10.1007/s00401-012-1002-8 22744790
23. Smirnovas V, Baron GS, Offerdahl DK, Raymond GJ, Caughey B, et al. (2011) Structural organization of brain-derived mammalian prions examined by hydrogen-deuterium exchange. Nat Struct Mol Biol 18 : 504–506. doi: 10.1038/nsmb.2035 21441913
24. Cobb NJ, Apostol MI, Chen S, Smirnovas V, Surewicz WK (2014) Conformational stability of mammalian prion protein amyloid fibrils is dictated by a packing polymorphism within the core region. J Biol Chem 289 : 2643–2650. doi: 10.1074/jbc.M113.520718 24338015
25. Del Mar C, Greenbaum EA, Mayne L, Englander SW, Woods VL Jr. (2005) Structure and properties of alpha-synuclein and other amyloids determined at the amino acid level. Proc Natl Acad Sci U S A 102 : 15477–15482. 16223878
26. Lu X, Wintrode PL, Surewicz WK (2007) Beta-sheet core of human prion protein amyloid fibrils as determined by hydrogen/deuterium exchange. Proc Natl Acad Sci U S A 104 : 1510–1515. 17242357
27. Smirnovas V, Kim JI, Lu X, Atarashi R, Caughey B, et al. (2009) Distinct Structures of Scrapie Prion Protein (PrPSc)-seeded Versus Spontaneous Recombinant Prion Protein Fibrils Revealed by Hydrogen/Deuterium Exchange. J Biol Chem 284 : 24233–24241. doi: 10.1074/jbc.M109.036558 19596861
28. Toyama BH, Kelly MJ, Gross JD, Weissman JS (2007) The structural basis of yeast prion strain variants. Nature 449 : 233–237. 17767153
29. Toyama BH, Weissman JS (2011) Amyloid structure: conformational diversity and consequences. Annu Rev Biochem 80 : 557–585. doi: 10.1146/annurev-biochem-090908-120656 21456964
30. Miller MB, Wang DW, Wang F, Noble GP, Ma J, et al. (2013) Cofactor molecules induce structural transformation during infectious prion formation. Structure 21 : 2061–2068. doi: 10.1016/j.str.2013.08.025 24120764
31. Cobb NJ, Sonnichsen FD, McHaourab H, Surewicz WK (2007) Molecular architecture of human prion protein amyloid: a parallel, in-register beta-structure. Proc Natl Acad Sci U S A 104 : 18946–18951. 18025469
32. Groveman BR, Dolan MA, Taubner LM, Kraus A, Wickner RB, et al. (2014) Parallel in-register intermolecular beta-sheet architectures for prion-seeded prion protein (PrP) amyloids. J Biol Chem 289 : 24129–24142. doi: 10.1074/jbc.M114.578344 25028516
33. Govaerts C, Wille H, Prusiner SB, Cohen FE (2004) Evidence for assembly of prions with left-handed beta-helices into trimers. Proc Natl Acad Sci U S A 101 : 8342–8347. 15155909
34. Miyagi M, Nakazawa T (2008) Determination of pK(a) values of individual histidine residues in proteins using mass spectrometry. Anal Chem 80 : 6481–6487. doi: 10.1021/ac8009643 18665614
35. Miyagi M, Wan Q, Ahmad MF, Gokulrangan G, Tomechko SE, et al. (2011) Histidine Hydrogen-Deuterium Exchange Mass Spectrometry for Probing the Microenvironment of Histidine Residues in Dihydrofolate Reductase. PLoS One 6.
36. Tran DT, Banerjee S, Alayash AI, Crumbliss AL, Fitzgerald MC (2012) Slow histidine H/D exchange protocol for thermodynamic analysis of protein folding and stability using mass spectrometry. Anal Chem 84 : 1653–1660. doi: 10.1021/ac202927p 22185579
37. Nelson R, Sawaya MR, Balbirnie M, Madsen AO, Riekel C, et al. (2005) Structure of the cross-beta spine of amyloid-like fibrils. Nature 435 : 773–778. 15944695
38. Sawaya MR, Sambashivan S, Nelson R, Ivanova MI, Sievers SA, et al. (2007) Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature 447 : 453–457. 17468747
39. Tanaka M, Collins SR, Toyama BH, Weissman JS (2006) The physical basis of how prion conformations determine strain phenotypes. Nature 442 : 585–589. 16810177
40. Uptain SM, Lindquist S (2002) Prions as protein-based genetic elements. Annu Rev Microbiol 56 : 703–741. 12142498
41. Bett C, Joshi-Barr S, Lucero M, Trejo M, Liberski P, et al. (2012) Biochemical properties of highly neuroinvasive prion strains. PLoS Pathog 8: e1002522. doi: 10.1371/journal.ppat.1002522 22319450
42. Peretz D, Scott M, Groth D, Williamson A, Burton D, et al. (2001) Strain-specified relative conformational stability of the scrapie prion protein. Protein Sci 10 : 854–863. 11274476
43. Ayers JI, Schutt CR, Shikiya RA, Aguzzi A, Kincaid AE, et al. (2011) The strain-encoded relationship between PrP replication, stability and processing in neurons is predictive of the incubation period of disease. PLoS Pathog 7: e1001317. doi: 10.1371/journal.ppat.1001317 21437239
44. World Health Organization (1999) WHO infection control guidelines for transmissible spongiform encephalopathies. Geneva. 38 p.
45. Geschwind MD, Shu H, Haman A, Sejvar JJ, Miller BL (2008) Rapidly progressive dementia. Ann Neurol 64 : 97–108. doi: 10.1002/ana.21430 18668637
46. Zanusso G, Liu D, Ferrari S, Hegyi I, Yin X, et al. (1998) Prion protein expression in different species: Analysis with a panel of new mAbs. Proc Natl Acad Sci USA 95 : 8812–8816. 9671761
47. Safar J, Wille H, Itri V, Groth D, Serban H, et al. (1998) Eight prion strains have PrPSc molecules with different conformations. Nat Med 4 : 1157–1165. 9771749
48. Morillas M, Swietnicki W, Gambetti P, Surewicz WK (1999) Membrane environment alters the conformational structure of the recombinant human prion protein. J Biol Chem 274 : 36859–36865. 10601237
49. Atarashi R, Wilham JM, Christensen L, Hughson AG, Moore RA, et al. (2008) Simplified ultrasensitive prion detection by recombinant PrP conversion with shaking. Nat Methods 5 : 211–212. doi: 10.1038/nmeth0308-211 18309304
50. Castilla J, Morales R, Saa P, Barria M, Gambetti P, et al. (2008) Cell-free propagation of prion strains. EMBO J 27 : 2557–2566. doi: 10.1038/emboj.2008.181 18800058
51. Telling GC, Scott M, Mastrianni J, Gabizon R, Torchia M, et al. (1995) Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell 83 : 79–90. 7553876
52. Parchi P, Capellari S, Chen SG, Petersen RB, Gambetti P, et al. (1997) Typing prion isoforms. Nature 386 : 232–233. 9069279
53. Langeveld JP, Jacobs JG, Erkens JH, Bossers A, van Zijderveld FG, et al. (2006) Rapid and discriminatory diagnosis of scrapie and BSE in retro-pharyngeal lymph nodes of sheep. BMC Vet Res 2 : 19. 16764717
54. Kascsak RJ, Rubenstein R, Merz PA, Tonna-DeMasi M, Fersko R, et al. (1987) Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J Virol 61 : 3688–3693. 2446004
55. Chen SG, Teplow DB, Parchi P, Teller JK, Gambetti P, et al. (1995) Truncated forms of the human prion protein in normal brain and in prion diseases. J Biol Chem 270 : 19173–19180. 7642585
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Relay of Herpes Simplex Virus between Langerhans Cells and Dermal Dendritic Cells in Human SkinČlánek Does the Arthropod Microbiota Impact the Establishment of Vector-Borne Diseases in Mammalian Hosts?Článek The Ebola Epidemic Crystallizes the Potential of Passive Antibody Therapy for Infectious DiseasesČlánek Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting PolypeptideČlánek A Redox Regulatory System Critical for Mycobacterial Survival in Macrophages and Biofilm Development
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Pathogens as Biological Weapons of Invasive Species
- Selection and Spread of Artemisinin-Resistant Alleles in Thailand Prior to the Global Artemisinin Resistance Containment Campaign
- Endopeptidase-Mediated Beta Lactam Tolerance
- Prospective Large-Scale Field Study Generates Predictive Model Identifying Major Contributors to Colony Losses
- Relay of Herpes Simplex Virus between Langerhans Cells and Dermal Dendritic Cells in Human Skin
- Structural Determinants of Phenotypic Diversity and Replication Rate of Human Prions
- Sigma Factor SigB Is Crucial to Mediate Adaptation during Chronic Infections
- EphrinA2 Receptor (EphA2) Is an Invasion and Intracellular Signaling Receptor for
- Toxin-Induced Necroptosis Is a Major Mechanism of Lung Damage
- Heterologous Expression in Remodeled . : A Platform for Monoaminergic Agonist Identification and Anthelmintic Screening
- Novel Disease Susceptibility Factors for Fungal Necrotrophic Pathogens in Arabidopsis
- Interleukin 21 Signaling in B Cells Is Required for Efficient Establishment of Murine Gammaherpesvirus Latency
- Phosphorylation at the Homotypic Interface Regulates Nucleoprotein Oligomerization and Assembly of the Influenza Virus Replication Machinery
- Human Papillomaviruses Activate and Recruit SMC1 Cohesin Proteins for the Differentiation-Dependent Life Cycle through Association with CTCF Insulators
- Ubiquitous Promoter-Localization of Essential Virulence Regulators in
- TGF-β Suppression of HBV RNA through AID-Dependent Recruitment of an RNA Exosome Complex
- The Immune Adaptor ADAP Regulates Reciprocal TGF-β1-Integrin Crosstalk to Protect from Influenza Virus Infection
- Antagonism of miR-328 Increases the Antimicrobial Function of Macrophages and Neutrophils and Rapid Clearance of Non-typeable (NTHi) from Infected Lung
- The Epigenetic Regulator G9a Mediates Tolerance to RNA Virus Infection in
- Does the Arthropod Microbiota Impact the Establishment of Vector-Borne Diseases in Mammalian Hosts?
- Hantaan Virus Infection Induces Both Th1 and ThGranzyme B+ Cell Immune Responses That Associated with Viral Control and Clinical Outcome in Humans
- Viral Inhibition of the Transporter Associated with Antigen Processing (TAP): A Striking Example of Functional Convergent Evolution
- Plasma Membrane Profiling Defines an Expanded Class of Cell Surface Proteins Selectively Targeted for Degradation by HCMV US2 in Cooperation with UL141
- Optineurin Regulates the Interferon Response in a Cell Cycle-Dependent Manner
- IFIT1 Differentially Interferes with Translation and Replication of Alphavirus Genomes and Promotes Induction of Type I Interferon
- The EBNA3 Family of Epstein-Barr Virus Nuclear Proteins Associates with the USP46/USP12 Deubiquitination Complexes to Regulate Lymphoblastoid Cell Line Growth
- Hepatitis C Virus RNA Replication Depends on Specific and -Acting Activities of Viral Nonstructural Proteins
- A Neuron-Specific Antiviral Mechanism Prevents Lethal Flaviviral Infection of Mosquitoes
- The Aspartate-Less Receiver (ALR) Domains: Distribution, Structure and Function
- Global Genome and Transcriptome Analyses of Epidemic Isolate 98-06 Uncover Novel Effectors and Pathogenicity-Related Genes, Revealing Gene Gain and Lose Dynamics in Genome Evolution
- The Ebola Epidemic Crystallizes the Potential of Passive Antibody Therapy for Infectious Diseases
- Ebola Virus Entry: A Curious and Complex Series of Events
- Conserved Spirosomes Suggest a Single Type of Transformation Pilus in Competence
- Spatial Structure, Transmission Modes and the Evolution of Viral Exploitation Strategies
- Bacterial Cooperation Causes Systematic Errors in Pathogen Risk Assessment due to the Failure of the Independent Action Hypothesis
- Transgenic Fatal Familial Insomnia Mice Indicate Prion Infectivity-Independent Mechanisms of Pathogenesis and Phenotypic Expression of Disease
- Cerebrospinal Fluid Cytokine Profiles Predict Risk of Early Mortality and Immune Reconstitution Inflammatory Syndrome in HIV-Associated Cryptococcal Meningitis
- Utilize Host Actin for Efficient Maternal Transmission in
- Borna Disease Virus Phosphoprotein Impairs the Developmental Program Controlling Neurogenesis and Reduces Human GABAergic Neurogenesis
- An Effector Peptide Family Required for Toll-Mediated Immunity
- Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting Polypeptide
- A Redox Regulatory System Critical for Mycobacterial Survival in Macrophages and Biofilm Development
- Quadruple Quorum-Sensing Inputs Control Virulence and Maintain System Robustness
- Leukocyte-Derived IFN-α/β and Epithelial IFN-λ Constitute a Compartmentalized Mucosal Defense System that Restricts Enteric Virus Infections
- A Strategy for O-Glycoproteomics of Enveloped Viruses—the O-Glycoproteome of Herpes Simplex Virus Type 1
- Macrocyclic Lactones Differ in Interaction with Recombinant P-Glycoprotein 9 of the Parasitic Nematode and Ketoconazole in a Yeast Growth Assay
- Neofunctionalization of the α1,2fucosyltransferase Paralogue in Leporids Contributes to Glycan Polymorphism and Resistance to Rabbit Hemorrhagic Disease Virus
- The Extracytoplasmic Linker Peptide of the Sensor Protein SaeS Tunes the Kinase Activity Required for Staphylococcal Virulence in Response to Host Signals
- Murine CMV-Induced Hearing Loss Is Associated with Inner Ear Inflammation and Loss of Spiral Ganglia Neurons
- Dual miRNA Targeting Restricts Host Range and Attenuates Neurovirulence of Flaviviruses
- GATA-Dependent Glutaminolysis Drives Appressorium Formation in by Suppressing TOR Inhibition of cAMP/PKA Signaling
- Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Infection
- Genetic Analysis Using an Isogenic Mating Pair of Identifies Azole Resistance Genes and Lack of Locus’s Role in Virulence
- A Temporal Gate for Viral Enhancers to Co-opt Toll-Like-Receptor Transcriptional Activation Pathways upon Acute Infection
- Neutrophil Recruitment to Lymph Nodes Limits Local Humoral Response to
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Toxin-Induced Necroptosis Is a Major Mechanism of Lung Damage
- Transgenic Fatal Familial Insomnia Mice Indicate Prion Infectivity-Independent Mechanisms of Pathogenesis and Phenotypic Expression of Disease
- Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Infection
- EphrinA2 Receptor (EphA2) Is an Invasion and Intracellular Signaling Receptor for
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



