-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Conserved Spirosomes Suggest a Single Type of Transformation Pilus in Competence
Streptococcus pneumoniae often escapes prevention and treatment through rapid horizontal gene transfer via natural transformation. Uptake of exogenous DNA requires expression of a transformation pilus but two markedly different models for pilus assembly and function have been proposed. We previously reported a long, Type 4 pilus-like appendage on the surface of competent pneumococci that binds extracellular DNA as initial receptor, while a separate study proposed that secreted short, ‘plaited’ transformation pili act simply as peptidoglycan drills to open DNA gateways. Here we show that the ‘plaited’ structures are not competence-specific or related to transformation. We further demonstrate that these are macromolecular assemblies of the metabolic enzyme acetaldehyde-alcohol dehydrogenase—or spirosomes—broadly conserved across the bacterial kingdom.
Published in the journal: . PLoS Pathog 11(4): e32767. doi:10.1371/journal.ppat.1004835
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004835Summary
Streptococcus pneumoniae often escapes prevention and treatment through rapid horizontal gene transfer via natural transformation. Uptake of exogenous DNA requires expression of a transformation pilus but two markedly different models for pilus assembly and function have been proposed. We previously reported a long, Type 4 pilus-like appendage on the surface of competent pneumococci that binds extracellular DNA as initial receptor, while a separate study proposed that secreted short, ‘plaited’ transformation pili act simply as peptidoglycan drills to open DNA gateways. Here we show that the ‘plaited’ structures are not competence-specific or related to transformation. We further demonstrate that these are macromolecular assemblies of the metabolic enzyme acetaldehyde-alcohol dehydrogenase—or spirosomes—broadly conserved across the bacterial kingdom.
Introduction
Despite medical advances and vaccination campaigns, respiratory tract invasion by Streptococcus pneumoniae remains a leading mortality cause worldwide [1–3]. A particular challenge in the prevention and treatment of pneumococcal infections lies in the bacterium’s striking genomic plasticity, as it allows for efficient antibiotic resistance development, capsular serotype switching and vaccine escape [4]. Horizontal gene transfer and chromosomal rearrangements typically result from the avid uptake and recombination of exogenous DNA known as natural transformation. A strictly regulated event, it occurs during a transitory state of the bacterium’s life cycle—competence—and requires the timed expression of a dedicated set of genes [5]. Among these are the genes of the comG operon, which are conserved among naturally competent Gram-positive bacteria and are homologous to the ones encoding Type 4 pili (T4P) and Type 2 secretion system (T2SS) pseudo-pili components in Gram-negative bacteria [6,7]. Although mechanistic studies of structural determinants for DNA uptake—such as putative transformation-specific cellular appendages—hold promise for the development of novel antiinfectives and helper compounds, there have been only limited and contradictory reports on the initial steps of this important biological process [8–10].
As until recently no pilus-like structure had been observed in any transformable Gram-positive bacterium, it had been postulated that the pneumococcal comG operon encodes a short T2SS-like pseudo-pilus that serves to destabilize the cell wall peptidoglycan for DNA entry [6,9,11]. The main experimental evidence for this model comes from a different transformable organism, Bacillus subtilis, where pilus length was indirectly deduced from biochemical data [9].
Our team identified a long, micrometer-sized, T4P-like pilus protruding on the surface of competent cells from different pneumococcal strains with wild-type genotype (Fig 1A) [10]. Among these are two highly transformable laboratory strains of different genetic background—R6 and TCP1251—as well as a capsulated clinical isolate—the G54 strain [10]. We showed that major constituent of the transformation pilus is the ComGC pilin and that the pilus is sensitive to mechanical stress, which can lead to its detachment from the cell (shearing) [10,12]. Finally, we showed that this transformation pilus binds extracellular DNA and proposed that it acts as the initial DNA receptor on the surface of competent pneumococci [10].
Fig. 1. Pneumococcal transformation pilus controversy: Long, T4P-like transformation pilus versus short, ‘plaited’ polymers. 
(A) Long, T4P-like transformation pilus protruding on the surface of competence-induced S. pneumoniae strain R1501 [10]. (B) High-resolution structural model of a T2SS-peudopilus in side (left) and top (right) views [15], visualized in PyMOL (Schrödinger). (C) Scaled electron density reprojections of the T2SS-pseudopilus model (left), compared to class averages of the long T4P-like transformation pilus reported in [10] (center) and the short, ‘plaited’ filaments reported in [8] (right). Scale bars 5 nm. (D) Coexistence of the T4P-like pilus (black arrowhead) and ‘plaited’ filaments (white arrowhead) in competence-induced S. pneumoniae culture. (E) Immunogold labeling of major pilin ComGC in a strain carrying an additional FLAG-tagged ectopic copy of the comGC gene. A T4P-like pilus and a ‘plaited’ filament are indicated by black and white arrowheads, respectively. (F) and (G) Unabolished release of ‘plaited’ filaments in non-competent, pilus-deficient ΔcomGB [17] and ΔcomGA S. pneumoniae, respectively. A subsequent study visualized completely different structures—short, ‘plaited’ polymers—in the medium of competence-induced S. pneumoniae [8]. Biochemical observation of significant ComGC release in the medium during competence convinced the authors that the ‘plaited’ structures corresponded to secreted transformation pili. After failing to immunolabel these structures, they expressed heterologously the whole comG operon in Escherichia coli and visualized the release of similar polymers [8]. Finally, they proposed a model, which is consistent with the classical but speculative model of transformation pseudo-pili: rather than acting as a DNA receptor, the pneumococcal transformation pilus acts as a peptidoglycan-drilling device whose release leaves a gateway for transforming DNA to find the uptake machinery [8,10].
Here we show definitive experimental evidence that the short ‘plaited’ filaments are not transformation pili or other structural determinants of natural transformation. We further identify the structures as fermentative spirosomes, or macromolecular complexes of the acetaldehyde-alcohol dehydrogenase enzyme AdhE, which is widely conserved across the bacterial kingdom. Being aware of the limited view and resolution that observation by electron microscopy provides, we underscore the need for thorough validation by orthogonal approaches. Finally, we briefly synthesize the present-day published collective knowledge by proposing an updated model of pneumococcal transformation.
Results
T4P-like pilus vs ‘plaited’ filaments: Morphological differences
Perhaps the most intriguing aspect of the Balaban et al. study is the distinctive morphology of the reported ‘plaited’ filaments themselves [8]. As the authors point out, the genetic makeup of the comG operon resembles significantly that of operons encoding T4P or T2SS components in Gram-negative bacteria [5,7,8,13]. This includes from sequence homology of the individual genes through their intraoperon organization to the putative bioassembly platform and post-translational modifications of the encoded components. The structure of both T4 pili and T2SS pseudo-pili has been extensively studied [14,15]. Generally T4 pilins pack tightly into thin but extremely strong and several micrometers long surface-attached helical filaments [14]. Typical dimensions vary from 5–6 nm width for the T4aP of many bacteria (Pseudomonas aeurginosa, Neisseria gonorrhoeae and others) to the thicker, about 8 nm wide T4bP of enteropathogens such as Vibrio cholerae and Salmonella enterica serovar Typhi [14]. Conversely, structural models of T2SS pseudopili, which normally act as short, protein ejecting pistons in the periplasm, present an architecture that is very similar to that of gonococcal T4aP (Fig 1B) [15]. Both T4P and T2SS pseudo-pili feature a grooved surface with relatively small protuberations characteristic of the pilin helical packing [14,15].
The characteristic structural features of T4P were conserved in the transformation pili that we visualized previously on the surface of competence-induced pneumococci from several different wild-type strains [10]. In contrast, the ‘plaited’ structures visualized subsequently represent short, 40–200 nm long structures that are significantly thicker (~ 10 nm) and present large protuberant domains on their helical surface (Fig 1C) [8]. The authors proposed that the filaments are ‘plaited’, i.e. composed of two interlaced transformation pili [8]. Given the tight pilin packing in known T4P structures, however, we found it quite striking that homologous pili could sustain such a significant deformation to form an interlaced dimer. Although molecular dynamics simulations revealed that T2SS pseudo-pili can adopt a wide range of helical twist angles, they were not shown to have a propensity for short-range bending [16]. Moreover, while many T4P can form bundles, those can be hundreds of nanometers thick, contain a large number of pili and present much more limited distortion at the level of individual filaments [14].
T4P-like and plaited filaments co-exist in competent S. pneumoniae cultures
Intrigued by the striking new features of the ‘plaited’ filaments we wanted to see whether the transformation pili we previously reported could form similar structures. We were able to visualize the ‘plaited’ polymers along with the T4P-like long transformation pili in wild-type pneumococci (Fig 1D). However, in a strain with an additional FLAG-tagged ectopic copy of comGC gene for the major pilin [10] (S1 Table), we were able to immunolabel only the long, surface-attached pili using an anti-FLAG antibody (Fig 1E). Similar results were reported by Balaban and colleagues who failed to immunolabel the ‘plaited’ structures with a different antibody raised against ComGC [8].
The ‘plaited’ filaments observed in S. pneumoniae cultures are not related to pilus biogenesis or competence
As negative immunolabeling results are difficult to interpret and can be due to a variety of technical and structural factors, we proceeded to investigate the role of the plaited filaments in competence. We tested three negative control strains carrying either single-gene deletions for essential pilus biogenesis components (S1 Table)—the assembly platform protein ComGB [17] or the associated powering ATP-ase ComGA—or expressing a preprocessing incompetent ComGC variant (ComGCE20V, or ComGCE5V in mature pilin residue numbering). As shown previously, although these mutants can express monomeric pilins, they cannot assemble surface exposed pili and are transformation-incompetent [8,10,17]. In all three ΔcomGA, ΔcomGB and comGCE20V strains we still detected release of ‘plaited’ filaments while expression of the long, T4P-like transformation pilus was abolished (Fig 1F and Fig 1G and S1). Finally, we observed significant release of these structures even in the absence of competence induction (Fig 2A), further confirming that they are not related to pilus biogenesis during natural transformation.
Fig. 2. Identification of the ‘plaited’ filaments as AdhE spirosomes. 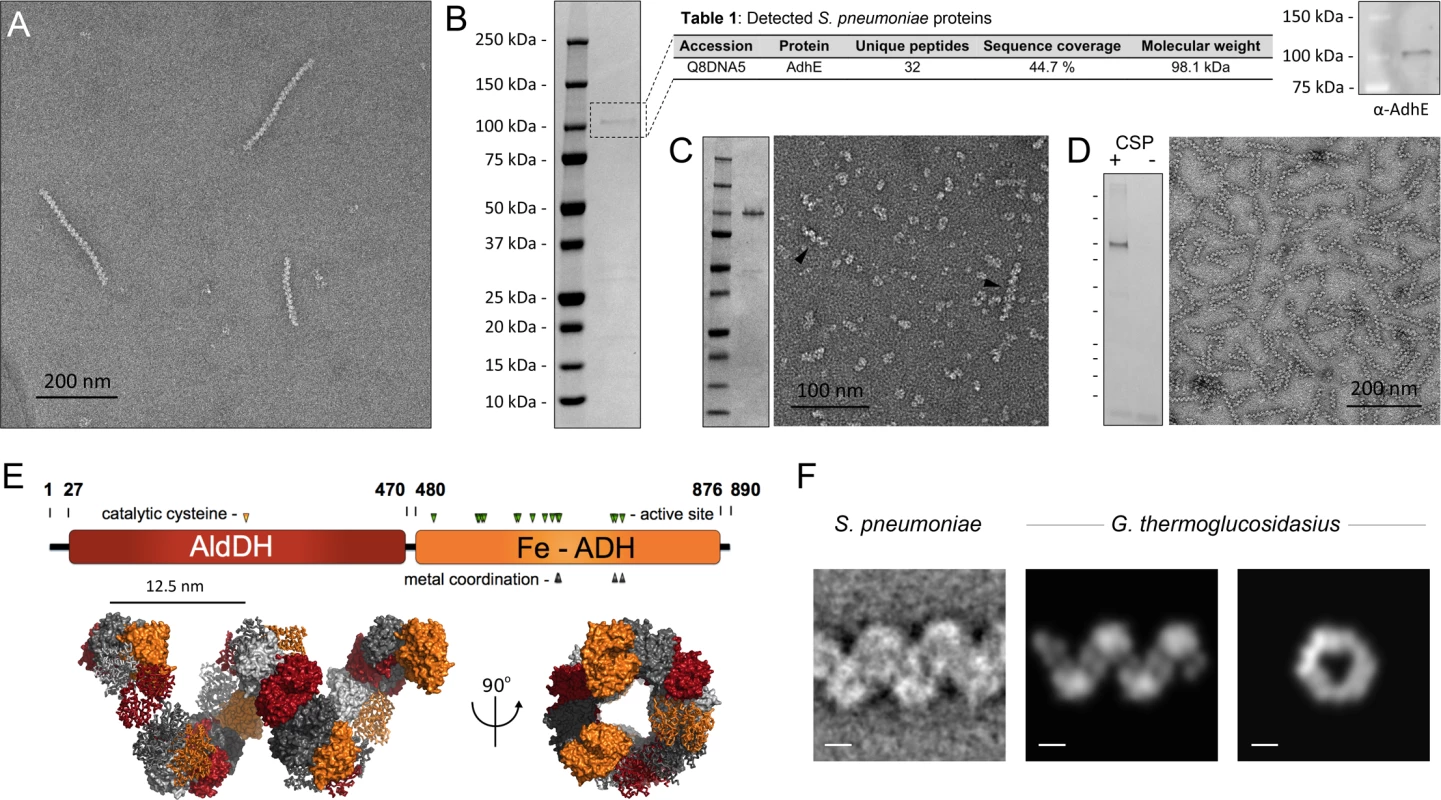
(A) Negatively stained purified ‘plaited’ filaments. (B) Left, coomassie stained SDS-PAGE on the purified filament fraction. Center, proteomic analysis of the predominant protein band; Posterior Error Probability (PEP) value for AdhE is 0. Right, Western blot validation using an anti-AdhE antibody (Agrisera). (C) Heterologous expression in E. coli and purification of AdhES.pneumoniae. Left, coomassie stained SDS-PAGE of the affinity purified protein (S2 Table). Right, negatively stained electron microscopy of the purified protein with black arrowheads indicating spontaneously formed spirosomes. (D) Immunopurification of S. pneumoniae spirosomes from a strain carrying an additional competence-inducible FLAG-tagged ectopic copy of the adhE gene (SO007): left, coomassie-stained SDS-PAGE of the eluted fraction in CSP-induced and-uninduced cells (CSP, competence stimulating peptide); right, negative-stain EM on the eluted fraction (E) Top, AdhE domain organization; bottom, high-resolution structural model of a G. thermoglucosidasius spirosome [19], visualized in PyMOL (Schrödinger). The color of the individual protomers alternate between grayscale and domain-coded color representation; successive AdhE dimers alternate between ribbon and surface representation (F) A representative class average of the ‘plaited’ filaments compared to electron density reprojections of the G. thermoglucosidasius spirosome model [19]. Scale bars 5nm. The ‘plaited’ filaments observed in S. pneumoniae cultures are spirosomes—Macromolecular complexes of the fermentative enzyme AdhE
To identify the building subunits of the ‘plaited’ filaments, we developed an enrichment and purification protocol based on differential ultracentrifugation, microfiltration and size exclusion chromatography. Electron microscopy imaging of the purified polymers showed a homogeneous sample composed primarily of the characteristic coiled polymers with an average length of 100–300 nm (Fig 2A). SDS-PAGE analysis of the corresponding fraction showed the presence of a predominant protein species with a molecular weight of ~100 kDa. LC-MS/MS proteomic analyses on the excised, trypsin-digested gel band, as well as on the total purified filaments fraction (S2 Table), unambiguously identified the predominant protein as bifunctional acetaldehyde-alcohol dehydrogenase AdhE, and the result was validated biochemically by Western blot detection using an anti-AdhE antibody (Fig 2B). Heterologous expression of the S. pneumoniae adhE gene in Escherichia coli and affinity purification of the expressed protein showed spontaneous coiled filament formation in the eluted fraction (Fig 2C). Finally, the protein composition of the ‘plaited’ polymers was validated by affinity pull-down with anti-FLAG antibody-conjugated resin on samples from a S. pneumoniae strain carrying an additional ectopic adhE gene copy for competence-inducible expression of a C-terminally FLAG-tagged protein (Fig 2D).
AdhE is a 98 kDa protein with an N-terminal acetylating aldehyde dehydrogenase domain (AldDH) and a C-terminal Fe-dependent alcohol dehydrogenase domain (Fe-ADH) (Fig 2E) [18]. Homologous dual domain proteins are common among fermentative bacteria and are reported to catalyze the NADH-dependent conversion of acetyl-CoA to ethanol via an aldehyde intermediate. Most importantly, in many species AdhE has been shown to polymerize into fine helical filaments called spirosomes [19–26] that are morphologically consistent with the ‘plaited’ filaments discussed here and reported as self-secreting, ‘plaited’ transformation pili by Balaban and colleagues [8]. A high resolution structural model of spirosome assembly by the closely homologous AdhE of Geobacillus thermoglucosidasius shows multimeric arrangement of the individual subunits into a right-handed spiral filament with six protomers per helical turn and overall pitch and width parameters consistent with negatively stained class averages of its pneumococcal counterpart (Fig 2E and 2F) [19]. It is also important to note that the proposed spirosome structure—which is based on crystallographic and in-solution biophysical data, homology modeling and in silico macromolecular docking—corresponds to a single-start helix rather than a ‘plaited’ polymer of two or more interlaced filaments [19].
AdhE is not required for natural transformation
To examine a putative role of AdhE in natural transformation, we first followed the protein’s expression over the course of competence induction that was verified by the detection of a competence-inducible FLAG-tagged ectopic copy of ComGC. While we have shown competence-specific ComGC expression in wild-type genetic background previously [10], AdhE protein levels remained stable over the course of the experiment (Fig 3A). We next constructed an adhE-null Streptococcus pneumoniae R6 mutant (ΔadhE) and examined its transformation efficiency for uptake of resistance-encoding DNA cassette under challenge with the corresponding antibiotic. While the ΔadhE mutant shows slightly decreased transformation efficiency (~ 2-fold), this change is negligible compared to typical results under comGC disruption (~ 10 000-fold) and can be due to reduced metabolic fitness under the microaerobic conditions of the experiment (Fig 3B). Our data are consistent with a previous genome-wide study aiming to identify genes essential for natural transformation in Streptococcus pneumoniae, which have failed to identify AdhE as a requirement for DNA uptake [27]. Finally, while no spirosome release was detected for the competence-induced ΔadhE pneumococci, typical T4P-like transformation pili were observed (Fig 3C and 3D).
Fig. 3. AdhE during competence induction. 
(A) Expression levels of AdhE and ComGC-FLAG in the RL001 strain following competence induction using an anti-AdhE and anti-FLAG antibodies, respectively. Samples were normalized for total protein content prior to loading on the gel. (B) Transformation efficiency of an adhE-null (strain AD001) mutant relative to the reference strain (strain R1501). The data are representative of two biological replicates, with two repetitions each. An asterisk indicates typical transformation efficiency upon ComGC disruption [10]. (C-D) T4P-like transformation pili assembled by the ΔadhE strain. Spirosome formation is a conserved metabolic phenomenon across the bacterial kingdom
Formation of spirosomes has been reported in a variety of Gram-positive and Gram-negative bacteria, with the first studies dating back several decades and refering to the building protein, AdhE, as spirosin [19–26]. AdhE conservation across representative species with confirmed spirosome formation shows significant sequence homology even among relatively distant taxa (Table 1). Nevertheless, sequence similarity mapping along the AdhEG.thermoglucosidasius structural model reveals that highly conserved residues cluster in only few surface-exposed patches [19,28]. These correspond to the deep active site clefts of the two dehydrogenase domains, as well as sites at or near the interdomain linker. The latter would likely remain buried in the context of mature spirosomes, as they stabilize embrace-like interactions between AdhE monomers in the high-resolution structural model of G. thermoglucosidasius spirosomes [19] (S2 Fig). Thus the exposed spirosome surface would retain significant variability, which in turn could translate into differences in spirosome morphology and stability across species. Moreover, earlier reports have demonstrated that spirosome helix parameters can vary significantly depending on the presence and type of small molecule and metal ion cofactors [20,21].
Tab. 1. AdhE sequence conservation in representative Gram-positive (+) and Gram-negative (-) species with confirmed spirosome formation [<em class="ref">19</em>–<em class="ref">26</em>]. ![AdhE sequence conservation in representative Gram-positive (+) and Gram-negative (-) species with confirmed spirosome formation [<em class="ref">19</em>–<em class="ref">26</em>].](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image/bd9e41d1ab3ad77cb838d91c1e512fee.png)
In addition to S. pneumoniae, we observed spirosome release in cultures of Clostridium difficile, Streptococcus sanguinis, and E. coli (Fig 4A–4C and Table 1) [29,30]. An adhE null strain of S. sanguinis [30] showed no release of morphologically consistent filaments, serving as an additional control for correct target identification. For the two Gram-positive species, C. difficile and S. sanguinis, spirosome morphology was practically indistinguishable from that of S. pneumoniae. The helical filaments we observed in E. coli cultures, on the other hand, were visibly more tightly coiled (Fig 4D) To verify that those correspond to AdhE macromolecular complexes we purified an enriched spirosome fraction and validated its major component as the bifunctional dehydrogenase using proteomic and biochemical methods (Fig 4D and S3 Table). Thus, although we expect morphological variations to be commonplace across species and even sample handling protocols, we are confident that spirosome assembly and extracellular release can be detected in many more environmental, clinically isolated, or genetically engineered bacterial strains.
Fig. 4. Cross-species conservation of AdhE spirosomes. 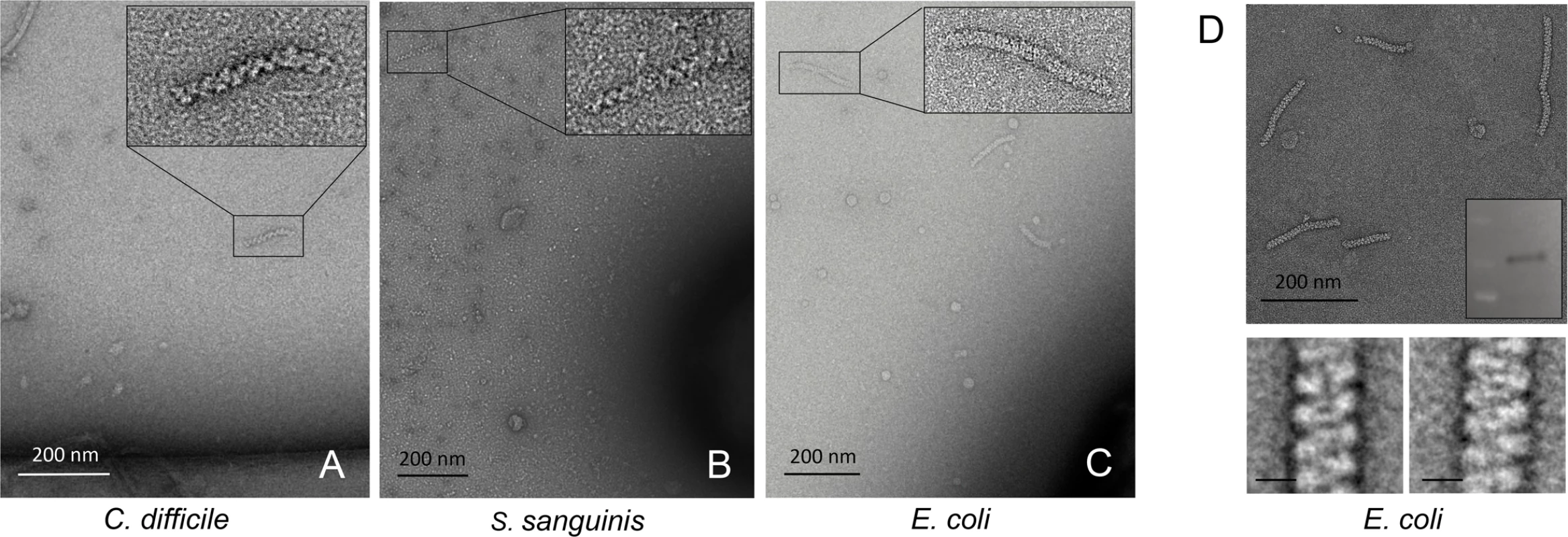
(A-C) Negative-stain electron microscopy on spirosomes released in cultures of C. difficile, S. sanguinis and E. coli strains [29,30]. (D) Purification (top), AdhE immunodetection (inset) and representative class averages of E. coli spirosomes; scale bars 10 nm (bottom). Discussion
S. pneumoniae and E. coli release helical AdhE spirosomes and not plaited transformation pili
The major horizontal gene transfer mechanism in S. pneumoniae—natural transformation—requires regulated expression of the comG pilus biogenesis operon, homologous to operons encoding T4P and T2SS pseudopili in Gram-negative bacteria [5,7,8,13]. Recent studies have reported conflicting results regarding the morphology and function of pneumococcal transformation pili. One proposed mechanism is that S. pneumoniae expresses a long, DNA-binding, T4P-like cell surface appendage to ‘fish’ extracellular transforming DNA [10], while an alternative hypothesis argues that competent pneumococci express short, self-secreting T2SS plaited pili that perforate the cell wall peptidoglycan to allow for DNA entry [8].
Here we show that the ‘plaited’ filamentous polymers are not related to natural transformation or pilus biogenesis but are instead widely conserved and well documented macromolecular complexes of the fermentative enzyme bifunctional acetaldehyde-alcohol dehydrogenase AdhE. Its tandem domain architecture secures the two-step NADH-dependent reduction of acetyl-CoA to ethanol via an aldehyde intermediate [19–25,31]. Although the biological significance of AdhE polymerization in such massive structures remains enigmatic, it is plausible that spirosome assembly delivers spatially localized metabolic flux to limit diffusion of the highly reactive aldehyde species and secure optimized conversion kinetics [32]. In addition, the high resolution structural model of G. thermoglucosidasius spirosomes shows that polymer assembly buries ~ 6 500 Å2 of surface area per monomer, which could have dramatic effects on protein stability and function [19,31].
Extracellular spirosome release by cultured bacteria appears to be the result of random cell lysis as no biological function or secretion mechanism can be assigned to the phenomenon. As expected for a fermentative enzyme and consistent with reports in the literature, AdhE expression and spirosome assembly is expected to increase under microaerobic and anaerobic conditions as opposed to aerobic cultures [23,33]. Anaerobic growth and increased cell lysis are both common in cultures of competence-induced pneumococci, where the signaling process of fratricide kills non-competent cells to release extracellular DNA available for uptake [17,34]. This can explain why the spirosomes were initially associated to natural transformation in S. pneumoniae and reported to represent ‘plaited’ transformation pili [8]. Conversely, in their study Balaban and colleagues attempted to reconstitute expression of pneumococcal transformation pili in E. coli by heterologous expression of the entire comG operon [8]. As a result, extracellular release of spirosomes was readily detected and the AdhE polymers were again labeled erroneously. It remains unclear why the authors failed to detect spirosomes in their negative control culture. One possibility is that the structures were omitted in the limited observation field that high-magnification electron microscopy experiments provide. Conversely, it is possible that overexpression of several non-native proteins—among which a macromolecular complex targeted to the inner membrane—could have had destabilizing effects on the expression strain, leading to increased cell lysis and spirosome release [35,36].
S. pneumoniae spirosomes share significant structural similarity with other bacterial proteinaceous filaments
In our quest to identify the structures observed by Balaban and colleagues [8], we initially hypothesized that they were randomly released RecA nucleofilaments due to their striking similarity to polymerized RecA homologs from other bacterial and eukaryotic species. Such structures have been extensively documented in the literature: forming characteristic helical coils, RecA filaments can be more or less extended depending on the presence and type of DNA and small-molecule ligands [37–39]. Essential but not exclusive to natural transformation, cytosolic RecA is massively expressed during competence and its polymerization on the incoming single-stranded DNA is key to DNA protection and subsequent integration in the genome (Fig 5) [40]. Nevertheless, while ΔrecA cells display normal DNA uptake during competence, RecA-based recombination plays pivotal role in DNA repair throughout the bacterial life cycle [40,41]. This indicates that pilus-dependent DNA uptake and RecA polymerization are intrinsically uncoupled and could have explained the continuous release of ‘plaited’ filaments in the pilus-defficient strains. We were indeed able to detect RecA release in the medium of competence-induced cultures of both wild-type and ΔcomGB cells (S3 Fig panel A). Also, although with slightly different parameters in terms of helical width and pitch, the ‘plaited’ filaments were structurally similar to both the coiled structure of a eukaryotic RecA homolog (S3 Fig panel B) [37], as well as to in vitro reconstituted RecAS.pneumoniae nucleofilaments (S3 Fig panel C). Nevertheless, release of the characteristic polymers persisted in a ΔrecA strain (S3 Fig panel D) and we were unable to detect the protein in the filament-enriched fractions following purification (S1 Table).
Fig. 5. Comprehensive model of pneumococcal natural transformation [5–7,10,61]: upon competence induction a transformation pilus is expressed on the cell surface. ![Comprehensive model of pneumococcal natural transformation [<em class="ref">5</em>–<em class="ref">7</em>,<em class="ref">10</em>,<em class="ref">61</em>]: upon competence induction a transformation pilus is expressed on the cell surface.](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image/e225aecc292087b9a6ba262eaa185620.png)
It is a ~ 5–6 nanometers wide, several micrometers long helical filament, morphologically and compositionally similar to T4P in Gram-negative bacteria. While its major component is the ComGC pilin, minor pilins ComGD-G as well as the biosynthetic ComGA-ComGB platform are required for pilus biogenesis. Upon expression, pilins are processed by the prepilin peptidase PilD. The mature pilus would serve as an initial ‘trap’ for exogenous DNA or, alternatively, self-secrete. In the former case, transforming DNA would then be guided to the uptake machinery either through pilus retraction, charge-based gliding on the pilus helical lattice (one-dimensional diffusion), or another as yet uncharacterized mechanism. In a hypothetical self-secretion scenario, transforming DNA would have to find the uptake machinery through a three-dimensional diffusion process, while avoiding inhibitory interactions with the secreted pili. Cell-surface binding by the DNA receptor ComEA and single-strand conversion by the nuclease EndA would then guide transforming DNA to the ComEC transporter. The translocase ComFA in partnership with cofactors would power transmembrane transport through ATP hydrolysis. Upon entry in the cytosol the single-stranded DNA would be immediately protected and prepared for recombination. It is therefore important to note that macromolecular organization in helical filaments is not uncommon among proteins from both the bacterial and other taxa. These include but are not limited to nucleic acid-binding proteins, cytoskeletal elements, building blocks of cell surface appendages and phage capsid subunits [39,42–44]. This, together with the markedly different helical parameters of the highly conserved E. coli spirosomes shown here underscores the fact that limited-view, low-resolution morphology imaging and bulk biochemical experiments alone are often insufficient to deduce the nature of macromolecular assemblies. Rather, a combination of orthogonal approaches that spans the different resolution levels and integrates genetic, biochemical and structural data in a meaningful way is generally warranted to avoid false-positive or otherwise erroneous results.
S. pneumoniae requires a long, T4P-like transformation pilus for DNA uptake
Taken together, our data rule out the existence of short ‘plaited’ transformation pili in competent pneumococci and reassert the expression of a long, 5–6 nanometer wide appendage, structurally and compositionally similar to T4P in Gram-negative bacteria [10]. This finding bridges Gram-negative and Gram-positives DNA uptake systems and provides a comprehensive picture of this major lateral gene transfer event. Indeed, a recent study showed the existence of a T4-pilus on competent V. cholerae, which shares many features with the pneumococcal transformation pilus: competence-induced expression, prerequisite for DNA uptake, and roughly a single copy per cell [45].
Apart from morphology alone, however, it is interesting to discuss the probable mechanism through which the transformation pili secure DNA entry into competent pneumococci. Although expression of any type of pilus would require overcoming the physical barriers of cell-wall peptidoglycan and overlaying capsule—and thus possibly facilitate DNA entry—the similarities among transformation pili of Gram-positive and Gram-negative bacteria suggest that naturally transformable species might have evolved a conserved and more sophisticated mechanism of pilus function than simple cell-wall destabilization.
In agreement with a long-standing ‘pseudo-pilus’ hypothesis, Balaban and colleagues proposed a model in which the transformation pili self-secrete in the medium of competent S. pneumoniae, thus opening gateways in the cell wall peptidoglycan for passive exogenous DNA entry [8,9]. Their hypothesis was supported by the observation that ComGC found in the supernatant of different S. pneumoniae strains after centrifugation correlates with the peak of transformation efficiency [8]. Since we previously showed that transformation pilus expression is absolutely required for DNA uptake, it is not surprising to observe correlation between extracellular ComGC and transformation efficiency [10]. However, ComGC release in culture supernatants can be a result from both cell lysis and/or compromised pilus integrity. As we showed previously, pneumococcal transformation pili are fairly sensitive to mechanical stress and short vortexing and centrifugation are routinely used for their shearing and isolation [10,12]. Such mechanical forces, however, are unlikely to be exerted in nature, where competent pneumococci are typically cushioned in protective biofilm matrix [46].
As we have conducted only single time point visualization experiments, it is theoretically possible that the expresses transformation pili eventually detach from the cell to open entry pores for transforming DNA (Fig 5). However, the sheer size and ATP-dependent assembly of the transformation pilus makes such self-secretion hypothesis unlikely: the observed long native pili would be energetically taxing on the cells if their sole function were to be ejected prior to DNA uptake. Finally, it has been previously reported that native transformation pili bind and co-purify with DNA already present in the cell culture and that DNA binding at the surface of competent pneumococci is abolished in a pilus-deficient strain [10,47]. DNA-binding is also conserved in the homologous T4P of Gram-negative bacteria [14,48,49]. In such a DNA-binding context, pilus release would actually inhibit transformation by titrating out DNA available for uptake (Fig 5). This once again argues against a self-secreting mechanism of function and reinforces a cell-surface attached role for the pilus in transformation.
While no mechanistic or quantitative data on DNA binding by the pilus are available, electron microscopy showed extensive contact interfaces between the long transformation pili and DNA chains [10]. It is therefore plausible that multiple weak interactions along the helical pilus lattice stabilize this interaction and allow its reversal upon DNA uptake. Such a scenario would also explain why no DNA binding to a non-polymerizing ComGC truncation has ever been detected [8,50]. Even more interesting, however, is the question of how pilus-bound DNA gets brought to the DNA uptake machinery in the cell membrane. In Gram-negative bacteria, T4P-bound DNA is proposed to be actively hauled to the cell by rapid bottom-up pilus depolymerization powered by a dedicated retraction ATPase [14]. Although a similar mechanism has been proposed for S. pneumoniae and other transformable Gram-positive bacteria, pneumococci lack homologous retraction ATPase and are likely to utilize a distinct mechanism for DNA entry. In addition, transforming DNA uptake occurs at much lower speeds in Gram-positive bacteria than Gram-negative T4P retraction [51,52].
Many sequence-specific DNA binding proteins can scan DNA for their target sites at speeds several orders of magnitude higher than the upper limit for a three-dimensional diffusion-controlled process [53]. This can generally be achieved by at least two passive mechanisms, which involve sequence non-specific DNA binding and subsequent translocation of the protein along the DNA: 1) charge-based protein sliding, where the protein engages in a one-dimensional random walk along the DNA in search of its target, and 2) direct intersegment transfer, where the protein can bind and hop between two remote regions on the DNA without losing the non-specifically bound state [53]. Although we can not exclude the involvement of an unidentified retraction ATPase or additional receptor proteins in exogenous DNA uptake, we favor a model where the pneumococcal transformation pilus provides a similar facilitated diffusion framework (Fig 5). By preserving multiple dynamic non-specific interactions with the pilus, transforming DNA would overcome the thermodynamic limitations of a three-dimensional diffusion process until it passively finds the membrane associated uptake machinery and becomes actively pumped in the cell (Fig 5).
Materials and Methods
Spirosome release visualization by electron microscopy (EM)
Streptococcus pneumoniae spirosomes were observed in both competent and non-competent cells. For competence induction cells were grown in microaerobic conditions, without agitation, at 37°C in Casamino Acid-Tryptone (CAT) medium supplemented with 0.2% glucose, 15mM dipotassium phosphate, 3mM sodium hydroxide and 1mM calcium chloride and adjusted to pH 7.8. Competence was triggered by the addition of Competence Stimulating Peptide (CSP) at OD600 = 0.15 for 10–30 min. Non-competent pneumococci and Escherichia coli cells were grown similarly in LB to OD600 = 0.3 and OD600 = 0.6, respectively. Clostridium difficile cells were grown at 37°C under strict anaerobic conditions on Tryptone-Yeast extract-Glucose (TYG) plates supplemented with 0.1% thioglycolate. Streptococcus sanguinis cells were grown anaerobically, without agitation, at 37°C in CAT medium to OD600 = 0.3. For spirosome visualization cells were scraped off the plates or pelleted by centrifugation and resuspended in TBS (50 mM Tris-HCl pH 7.6, 150 mM NaCl) at ~ 5 μl TBS per milliliter of culture at OD600 = 0.3. 5 μl drops of each suspension were then placed directly on glow discharged carbon coated grids (EMS, USA) for 1 minute. The grids were then blot-dried on filter paper, washed on a drop of ultrapure water, and negatively stained with 2% uranyl acetate in water. Specimens were examined on an FEI Tecnai T12 BioTWIN LaB6 electron microscope operating at 120 kV at nominal magnifications of 18500–68000 and 1–3 μm defocus. Images were recorded on a Gatan Ultrascan 4000 CCD camera.
Transformation efficiency assay
An adhE deletion (strain AD001) was introduced in the R1501 genetic background by transformation with a DNA cassette carrying a kanamycin resistance gene inserted between two ~1000 base pair fragments corresponding to the S. pneumoniae genomic regions flanking adhE. Briefly, the genomic region upstream from the AdhE open reading frame was amplified using forward and reverse primers 5’-ACA TGG CAA TCC GAT TCA TAA GGG G-3’ and 5’-GCC ATC TAT GTG TCG GAA CGA TAT CCT TTG TTA ATT TTT TCA CAA GTT TAT TAT AAC G-3’, respectively, while the genomic region downstream of the adhE gene was amplified the following primer pair 5’-AAA ATG TGT TTT TCT TTG TTT TGT TTA TCA GTC TAG AAG CAA GAC AAA AAC TCA A-3’ and 5’-TTG CTA TTT ATG CAT GCA GAA GAC CAA ATG-3’. A third pcr reaction was used to amplify a kanamycin-resistance gene using the pR411 plasmid as template DNA [54] and forward and reverse primers 5’-AGG ATA TCG TTC CGA CAC ATA GAT GGC GTC GCT AGT-3’ and 5’-GCT TCT AGA CTG ATA AAC AAA ACA AAG AAA AAC ACA TTT TTT TGT CAA AAT TCG TTT-3’, carrying complementarity to the 3’-end of the adhE-upstream and 5’-end of the adhE-downstream fragments, respectively. The three pcr products were then assembled using overlap extension PCR and the purified DNA cassette was used for transformation of competence-induced S. pneumoniae R1501 cells. adhE-null mutants (strain AD001) were positively selected by growth in the presence of kanamycin (60 μg/ml) and adhE deletion was confirmed independently by DNA sequencing and western blot detection using an anti-AdhE antibody. For all transformation experiments, competence was triggered as above at OD600 = 0.15 for 10 minutes, followed by DNA addition and 20 minute incubation at 30°C. Transformants were selected on Columbia Agar supplemented with 5% horse blood and appropriate antibiotics. For the transformation efficiency assays, cells were transformed with 100 ng of a DNA cassette, amplified from S. pneumoniae R304 genomic DNA and containing the streptomycin resistance gene str41. Bacteria were plated in the presence and absence of streptomycin (100 μg/ml) and incubated at 37°C overnight before colony counting.
Spirosome enrichment and purification
All steps of the purification protocol were performed at 4°C. 8L of S. pneumoniae culture grown in LB to OD600 = 0.3 or 4L of E. coli culture grown to OD600 = 0.6 were pelleted by centrifugation (20 min at 5000 g) and resuspended in 6 ml of cold TBS, vortexed briefly and centrifuged to remove the bulk of intact cells and debris (10 min at 12000 g followed by 15 min at 50000 g). Triton x-100 was added to the supernatant at final concentration of 0.25%. Following 30 minute agitation for solubilization of remaining membrane fragments, the samples were filtered through a 0.45 μm cellulose acetate filter (Corning) and centrifuged for 1h at 125000 g for spirosome pelleting. After careful removal of the supernatant, the pellet was resuspended in 50 μl TBS, re-filtered and loaded on a Superose 6 3.2/300 size exclusion column (GE Healthcare). Spirosome enriched fractions were found to elute with the void volume. Sample preparation for electron microscopy was performed as above.
Proteomics analysis
Trypsin digestion was performed as described previously [55] and the digests were analyzed under standard conditions on an LTQ-Orbitrap Velos (Thermo Fisher, Bremen) equipped with Ultimate 3000 nano-HPLC (Dionex). Briefly, tryptic peptides were desalted and separated on a C-18 nano-HPLC column under a gradient of acetonitrile. Minimum signal threshold for triggering an MS/MS event was set to 5000 counts. After a survey scan, the 10 most intense precursor ions were selected for CID fragmentation (top10). Raw files were processed with MaxQuant 1.4.1.2 [56]. Protein identification was done using Andromeda against a Streptococcus pneumoniae (strain ATCC BAA-255 / R6) (Taxonomy 171101–1947 proteins) or Escherichia coli (strain K12 / MC4100 / BW2952) (Taxonomy 595496–4043 proteins) database. A false-discovery rate of 1% was used for both peptide and protein identification. Reverse and contaminant proteins were excluded and only proteins identified with a minimum of 2 peptides were considered.
Single-particle EM data processing
Spirosomes and transformation pili were visualized by EM as above at nominal magnifications of 49000 and 68000, respectively. The contrast transfer function parameters were assessed using CTFFIND3 [57], and the phase flipping was done in SPIDER [58]. Linear filament segments were boxed with e2helixboxer and single particle stacks were generated for each dataset (EMAN2 [59]): 4309 particles for the transformation pilus (134x134 pixel box, 1.5 Å/pixel), 728 particles for the S. pneumoniae spirosomes (180x180 pixel box, 2.2 Å/pixel) and 555 particles for the E. coli spirosomes (180x180 pixel box, 2.2 Å/pixel). Normalization, centering, multi-reference alignment, multi-statistical analysis, and classification (15–40 particles per class) were done in IMAGIC-4D (Image Science Software, GmbH). IMAGIC-4D was also used for generation of two-dimensional reprojections of the T2SS pilus and the G. thermoglucosidasius spirosome.
Heterologous expression and purification of AdhES.pneumoniae
The coding sequence for AdhES.pneumoniae was cloned in-frame in a pET21a vector for heterologous expression of C-terminally hexahistidine-tagged protein in E. coli. Briefly, the AdhES.pneumoniae open reading frame was PCR-amplified from S. pneumoniae R1501 genomic DNA using forward and reverse primers 5’-CAT ATG AAA GCT ATG GAG GAA AAT ATG GCT G-3’ and 5’-GCG GCC GCT TTA CGG CGT CCT GGT CTT TCT TTG-3’, respectively. The pET21a vector was pcr-amplified using forward and reverse primers 5’-GGA CGC CGT AAA GCG GCC GCA CTC GAG CAC CAC CAC-3’ and 5’-CAT ATT TTC CTC CAT AGC TTT CAT ATG TAT ATC TCC TTC TTA-3’, respectively. 90 ng of linearized vector DNA were incubated in 1 : 1 molar ratio with the AdhE pcr product in an In-Fusion cloning reaction (Clontech) and transformed into Top10 cells (Invitrogen). Plasmid DNA was purified from individual clones, verified for AdhE coding region insertion and used for transformation in BL21 (DE3) cells (Invitrogen). For expression, transformed BL21 (DE3) cells were grown under agitation at 37°C in LB to OD600 = 0.6 and expression was induced with 0.7 mM IPTG for 2h. Cells were pelleted by centrifugation (4500 g for 20 min), resuspended in TBS supplemented with cOmplete Protease Inhibitor Cocktail (Roche) and disrupted by sonication. Cellular debris were removed by centrifugation (20 000 g for 30 min) and the lysates were incubated with batch Talon metal affinity resin (Clontech) for 30 min at room temperature. The resin was extensively washed (TBS, 15 mM imidazole) and bound protein was eluted with TBS supplemented with 140 mM Imidazole. For EM observation eluted protein was immediately applied on glow-discharged continuous carbon grids, stained, and imaged as above.
Immunopurification of spirosomes
For AdhE spirosome pull-down, a strain carrying an additional competence-inducible FLAG-tagged ectopic copy of the adhE gene was constructed. Briefly, the adhE gene was PCR-amplified using S. pneumoniae R1501 genomic DNA as template and primer pair 5’-GAG GAA GAA ACC ATG TTG AAA GCT ATG GAG GAA AAT ATG GCT GAT AAA AAA AC-3’ and 5’-AAA ATC AAA CGG ATC TTA CTT GTC ATC GTC ATC CTT GTA ATC TTT ACG GCG TCC TGG TCT TTC TTT G-3’, the latter designed to add a C-terminal FLAG tag to the encoded protein. In parallel, pCEPx vector DNA [60] was digested with NcoI and BamHI restriction enzymes. 90ng of the linearized vector were incubated in 1 : 1 molar ratio with the adhE-FLAG pcr product in an In-Fusion cloning reaction (Clontech) and transformed into Top10 E. coli cells (Invitrogen). Plasmid DNA was purified from individual clones, verified for AdhE-FLAG coding region insertion, and transformed into competence-induced R1501 cells, followed by selection with kanamycin (Kan). The resulting SO007 strain was sequence-verified for the pCEPx-derived adhE-FLAG–KanR cassette recombination [60] and cultured in CAT medium to OD600 = 0.15. CSP-induced and non-induced cells were pelleted by centrifugation and resuspended in TBS by brief vortexing in the presence of millimeter-sized glass beads for increased cell lysis. Cells were pelleted again, and the supernatants were incubated with anti-FLAG M2 affinity resin (Sigma-Aldrich A2220) for 1h at RT and under agitation. After washing with TBS, the resin-bound fraction was eluted by the addition of 100 μg/mL 3X FLAG peptide (Sigma Aldrich F4799) and mixing for additional 15 min at 4°C. The samples were then subjected to SDS-PAGE and EM analyses.
In vitro reconstitution of RecA filaments
2.5 μM purified RecAS. pneumoniae was incubated with 50 mM KCl, 0.5 mM DTT, 10 mM Tris-HCl pH 7.5, 2 mM magnesium acetate, and 2 mM ATP-γ-S for 15 minutes at 37°C. RecA nucleofilament formation was induced by the addition of a 54-nucleotide-long single-stranded DNA fragment or single-stranded M13mp18 DNA (New England Biolabs) at nucleotide:RecA monomer ratio of 3 : 1. After additional 15 minutes at 37°C, the samples were prepared for EM observation as above.
Supporting Information
Zdroje
1. Bogaert D, De Groot R, Hermans PW (2004) Streptococcus pneumoniae colonisation: the key to pneumococcal disease. The Lancet Infectious diseases 4 : 144–154. 14998500
2. Campbell GD Jr., Silberman R (1998) Drug-resistant Streptococcus pneumoniae. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 26 : 1188–1195.
3. Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, et al. (2013) Global burden of childhood pneumonia and diarrhoea. Lancet 381 : 1405–1416. doi: 10.1016/S0140-6736(13)60222-6 23582727
4. Hiller NL, Ahmed A, Powell E, Martin DP, Eutsey R, et al. (2010) Generation of genic diversity among Streptococcus pneumoniae strains via horizontal gene transfer during a chronic polyclonal pediatric infection. PLoS pathogens 6: e1001108. doi: 10.1371/journal.ppat.1001108 20862314
5. Johnston C, Martin B, Fichant G, Polard P, Claverys JP (2014) Bacterial transformation: distribution, shared mechanisms and divergent control. Nature reviews Microbiology 12 : 181–196. doi: 10.1038/nrmicro3199 24509783
6. Chen I, Dubnau D (2004) DNA uptake during bacterial transformation. Nature reviews Microbiology 2 : 241–249. 15083159
7. Johnston C, Campo N, Berge MJ, Polard P, Claverys JP (2014) Streptococcus pneumoniae, le transformiste. Trends in microbiology 22 : 113–119. doi: 10.1016/j.tim.2014.01.002 24508048
8. Balaban M, Battig P, Muschiol S, Tirier SM, Wartha F, et al. (2014) Secretion of a pneumococcal type II secretion system pilus correlates with DNA uptake during transformation. Proceedings of the National Academy of Sciences of the United States of America 111: E758–765. doi: 10.1073/pnas.1313860111 24550320
9. Chen I, Provvedi R, Dubnau D (2006) A macromolecular complex formed by a pilin-like protein in competent Bacillus subtilis. The Journal of biological chemistry 281 : 21720–21727. 16751195
10. Laurenceau R, Pehau-Arnaudet G, Baconnais S, Gault J, Malosse C, et al. (2013) A type IV pilus mediates DNA binding during natural transformation in Streptococcus pneumoniae. PLoS pathogens 9: e1003473. doi: 10.1371/journal.ppat.1003473 23825953
11. Dubnau D (1999) DNA uptake in bacteria. Annual review of microbiology 53 : 217–244. 10547691
12. Nunn D, Bergman S, Lory S (1990) Products of three accessory genes, pilB, pilC, and pilD, are required for biogenesis of Pseudomonas aeruginosa pili. Journal of bacteriology 172 : 2911–2919. 1971619
13. Mann JM, Carabetta VJ, Cristea IM, Dubnau D (2013) Complex formation and processing of the minor transformation pilins of Bacillus subtilis. Molecular microbiology 90 : 1201–1215. doi: 10.1111/mmi.12425 24164455
14. Craig L, Pique ME, Tainer JA (2004) Type IV pilus structure and bacterial pathogenicity. Nature reviews Microbiology 2 : 363–378. 15100690
15. Campos M, Nilges M, Cisneros DA, Francetic O (2010) Detailed structural and assembly model of the type II secretion pilus from sparse data. Proceedings of the National Academy of Sciences of the United States of America 107 : 13081–13086. doi: 10.1073/pnas.1001703107 20616068
16. Nivaskumar M, Bouvier G, Campos M, Nadeau N, Yu X, et al. (2014) Distinct docking and stabilization steps of the Pseudopilus conformational transition path suggest rotational assembly of type IV pilus-like fibers. Structure 22 : 685–696. doi: 10.1016/j.str.2014.03.001 24685147
17. Havarstein LS, Martin B, Johnsborg O, Granadel C, Claverys JP (2006) New insights into the pneumococcal fratricide: relationship to clumping and identification of a novel immunity factor. Molecular microbiology 59 : 1297–1307. 16430701
18. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. Journal of molecular biology 215 : 403–410. 2231712
19. Extance J, Crennell SJ, Eley K, Cripps R, Hough DW, et al. (2013) Structure of a bifunctional alcohol dehydrogenase involved in bioethanol generation in Geobacillus thermoglucosidasius. Acta crystallographica Section D, Biological crystallography 69 : 2104–2115. doi: 10.1107/S0907444913020349 24100328
20. Kawata T, Masuda K, Nomura S (1982) Superprecipitation-like phenomenon and destruction induced by adenosine 5'-triphosphate in spirosomes isolated from Lactobacillus brevis. Microbiology and immunology 26 : 979–983. 6298583
21. Kessler D, Herth W, Knappe J (1992) Ultrastructure and pyruvate formate-lyase radical quenching property of the multienzymic AdhE protein of Escherichia coli. The Journal of biological chemistry 267 : 18073–18079. 1325457
22. Matayoshi S, Oda H (1985) Detection of fine spiral structures (spirosomes) by weak sonication in some bacterial strains. Microbiology and immunology 29 : 13–20. 3990585
23. Matayoshi S, Oda H, Sarwar G (1989) Relationship between the production of spirosomes and anaerobic glycolysis activity in Escherichia coli B. Journal of general microbiology 135 : 525–529. 2695595
24. Nomura S, Masuda K, Kawata T (1989) Comparative characterization of spirosomes isolated from Lactobacillus brevis, Lactobacillus fermentum, and Lactobacillus buchneri. Microbiology and immunology 33 : 23–34. 2733612
25. Ueki Y, Masuda K, Kawata T (1982) Purification and characterization of spirosomes in Lactobacillus brevis. Microbiology and immunology 26 : 199–211. 7109979
26. Yamato M, Takahashi Y, Tomotake H, Ota F, Hirota K, et al. (1994) Monoclonal antibodies to spirosin of Yersinia enterocolitica and analysis of the localization of spirosome by use of them. Microbiology and immunology 38 : 177–182. 7521508
27. Burghout P, Bootsma HJ, Kloosterman TG, Bijlsma JJ, de Jongh CE, et al. (2007) Search for genes essential for pneumococcal transformation: the RADA DNA repair protein plays a role in genomic recombination of donor DNA. Journal of bacteriology 189 : 6540–6550. 17631629
28. Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, et al. (2004) UCSF Chimera—a visualization system for exploratory research and analysis. Journal of computational chemistry 25 : 1605–1612. 15264254
29. Sebaihia M, Wren BW, Mullany P, Fairweather NF, Minton N, et al. (2006) The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nature genetics 38 : 779–786. 16804543
30. Xu P, Ge X, Chen L, Wang X, Dou Y, et al. (2011) Genome-wide essential gene identification in Streptococcus sanguinis. Scientific reports 1 : 125. doi: 10.1038/srep00125 22355642
31. Krissinel E, Henrick K (2007) Inference of macromolecular assemblies from crystalline state. Journal of molecular biology 372 : 774–797. 17681537
32. Zhang YH (2011) Substrate channeling and enzyme complexes for biotechnological applications. Biotechnology advances 29 : 715–725. doi: 10.1016/j.biotechadv.2011.05.020 21672618
33. Encheva V, Shah HN, Gharbia SE (2009) Proteomic analysis of the adaptive response of Salmonella enterica serovar Typhimurium to growth under anaerobic conditions. Microbiology 155 : 2429–2441. doi: 10.1099/mic.0.026138-0 19389776
34. Eldholm V, Johnsborg O, Haugen K, Ohnstad HS, Havarstein LS (2009) Fratricide in Streptococcus pneumoniae: contributions and role of the cell wall hydrolases CbpD, LytA and LytC. Microbiology 155 : 2223–2234. doi: 10.1099/mic.0.026328-0 19389766
35. Gubellini F, Verdon G, Karpowich NK, Luff JD, Boel G, et al. (2011) Physiological response to membrane protein overexpression in E. coli. Molecular & cellular proteomics: MCP 10: M111 007930.
36. Wagner S, Baars L, Ytterberg AJ, Klussmeier A, Wagner CS, et al. (2007) Consequences of membrane protein overexpression in Escherichia coli. Molecular & cellular proteomics: MCP 6 : 1527–1550.
37. Okorokov AL, Chaban YL, Bugreev DV, Hodgkinson J, Mazin AV, et al. (2010) Structure of the hDmc1-ssDNA filament reveals the principles of its architecture. PloS one 5: e8586. doi: 10.1371/journal.pone.0008586 20062530
38. Williams RC, Spengler SJ (1986) Fibers of RecA protein and complexes of RecA protein and single-stranded phi X174 DNA as visualized by negative-stain electron microscopy. Journal of molecular biology 187 : 109–118. 2937923
39. Yu X, VanLoock MS, Yang S, Reese JT, Egelman EH (2004) What is the structure of the RecA-DNA filament? Current protein & peptide science 5 : 73–79.
40. Berge M, Mortier-Barriere I, Martin B, Claverys JP (2003) Transformation of Streptococcus pneumoniae relies on DprA - and RecA-dependent protection of incoming DNA single strands. Molecular microbiology 50 : 527–536. 14617176
41. Cox MM (1999) Recombinational DNA repair in bacteria and the RecA protein. Progress in nucleic acid research and molecular biology 63 : 311–366. 10506835
42. Mizuno N, Dramicanin M, Mizuuchi M, Adam J, Wang Y, et al. (2013) MuB is an AAA+ ATPase that forms helical filaments to control target selection for DNA transposition. Proceedings of the National Academy of Sciences of the United States of America 110: E2441–2450. doi: 10.1073/pnas.1309499110 23776210
43. Shih YL, Rothfield L (2006) The bacterial cytoskeleton. Microbiology and molecular biology reviews: MMBR 70 : 729–754. 16959967
44. Stubbs G, Kendall A (2012) Helical viruses. Advances in experimental medicine and biology 726 : 631–658. doi: 10.1007/978-1-4614-0980-9_28 22297534
45. Seitz P, Blokesch M (2013) DNA-uptake machinery of naturally competent Vibrio cholerae. Proceedings of the National Academy of Sciences of the United States of America 110 : 17987–17992. doi: 10.1073/pnas.1315647110 24127573
46. Vidal JE, Howery KE, Ludewick HP, Nava P, Klugman KP (2013) Quorum-sensing systems LuxS/autoinducer 2 and Com regulate Streptococcus pneumoniae biofilms in a bioreactor with living cultures of human respiratory cells. Infection and immunity 81 : 1341–1353. doi: 10.1128/IAI.01096-12 23403556
47. Berge M, Moscoso M, Prudhomme M, Martin B, Claverys JP (2002) Uptake of transforming DNA in Gram-positive bacteria: a view from Streptococcus pneumoniae. Molecular microbiology 45 : 411–421. 12123453
48. Cehovin A, Simpson PJ, McDowell MA, Brown DR, Noschese R, et al. (2013) Specific DNA recognition mediated by a type IV pilin. Proceedings of the National Academy of Sciences of the United States of America 110 : 3065–3070. doi: 10.1073/pnas.1218832110 23386723
49. van Schaik EJ, Giltner CL, Audette GF, Keizer DW, Bautista DL, et al. (2005) DNA binding: a novel function of Pseudomonas aeruginosa type IV pili. Journal of bacteriology 187 : 1455–1464. 15687210
50. Provvedi R, Dubnau D (1999) ComEA is a DNA receptor for transformation of competent Bacillus subtilis. Molecular microbiology 31 : 271–280. 9987128
51. Kurre R, Maier B (2012) Oxygen depletion triggers switching between discrete speed modes of gonococcal type IV pili. Biophysical journal 102 : 2556–2563. doi: 10.1016/j.bpj.2012.04.020 22713571
52. Maier B, Chen I, Dubnau D, Sheetz MP (2004) DNA transport into Bacillus subtilis requires proton motive force to generate large molecular forces. Nature structural & molecular biology 11 : 643–649.
53. von Hippel PH, Berg OG (1989) Facilitated target location in biological systems. The Journal of biological chemistry 264 : 675–678. 2642903
54. Prudhomme M, Camilli A, Claverys J-P (2007) In vitro mariner mutagenesis of Streptococcus pneumoniae: tools and traps. In: Hakenbeck R, Chhatwal GS, editors. The Molecular Biology of Streptococci Norwich, UK: Horizon Scientific Press. pp. 511–517.
55. Wilm M, Shevchenko A, Houthaeve T, Breit S, Schweigerer L, et al. (1996) Femtomole sequencing of proteins from polyacrylamide gels by nano-electrospray mass spectrometry. Nature 379 : 466–469. 8559255
56. Cox J, Matic I, Hilger M, Nagaraj N, Selbach M, et al. (2009) A practical guide to the MaxQuant computational platform for SILAC-based quantitative proteomics. Nature protocols 4 : 698–705. doi: 10.1038/nprot.2009.36 19373234
57. Mindell JA, Grigorieff N (2003) Accurate determination of local defocus and specimen tilt in electron microscopy. Journal of structural biology 142 : 334–347. 12781660
58. Shaikh TR, Gao H, Baxter WT, Asturias FJ, Boisset N, et al. (2008) SPIDER image processing for single-particle reconstruction of biological macromolecules from electron micrographs. Nature protocols 3 : 1941–1974. doi: 10.1038/nprot.2008.156 19180078
59. Tang G, Peng L, Baldwin PR, Mann DS, Jiang W, et al. (2007) EMAN2: an extensible image processing suite for electron microscopy. Journal of structural biology 157 : 38–46. 16859925
60. Martin B, Granadel C, Campo N, Henard V, Prudhomme M, et al. (2010) Expression and maintenance of ComD-ComE, the two-component signal-transduction system that controls competence of Streptococcus pneumoniae. Molecular microbiology 75 : 1513–1528. doi: 10.1111/j.1365-2958.2010.07071.x 20180906
61. Claverys JP, Martin B, Polard P (2009) The genetic transformation machinery: composition, localization, and mechanism. FEMS microbiology reviews 33 : 643–656. doi: 10.1111/j.1574-6976.2009.00164.x 19228200
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Relay of Herpes Simplex Virus between Langerhans Cells and Dermal Dendritic Cells in Human SkinČlánek Does the Arthropod Microbiota Impact the Establishment of Vector-Borne Diseases in Mammalian Hosts?Článek The Ebola Epidemic Crystallizes the Potential of Passive Antibody Therapy for Infectious DiseasesČlánek Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting PolypeptideČlánek A Redox Regulatory System Critical for Mycobacterial Survival in Macrophages and Biofilm Development
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Pathogens as Biological Weapons of Invasive Species
- Selection and Spread of Artemisinin-Resistant Alleles in Thailand Prior to the Global Artemisinin Resistance Containment Campaign
- Endopeptidase-Mediated Beta Lactam Tolerance
- Prospective Large-Scale Field Study Generates Predictive Model Identifying Major Contributors to Colony Losses
- Relay of Herpes Simplex Virus between Langerhans Cells and Dermal Dendritic Cells in Human Skin
- Structural Determinants of Phenotypic Diversity and Replication Rate of Human Prions
- Sigma Factor SigB Is Crucial to Mediate Adaptation during Chronic Infections
- EphrinA2 Receptor (EphA2) Is an Invasion and Intracellular Signaling Receptor for
- Toxin-Induced Necroptosis Is a Major Mechanism of Lung Damage
- Heterologous Expression in Remodeled . : A Platform for Monoaminergic Agonist Identification and Anthelmintic Screening
- Novel Disease Susceptibility Factors for Fungal Necrotrophic Pathogens in Arabidopsis
- Interleukin 21 Signaling in B Cells Is Required for Efficient Establishment of Murine Gammaherpesvirus Latency
- Phosphorylation at the Homotypic Interface Regulates Nucleoprotein Oligomerization and Assembly of the Influenza Virus Replication Machinery
- Human Papillomaviruses Activate and Recruit SMC1 Cohesin Proteins for the Differentiation-Dependent Life Cycle through Association with CTCF Insulators
- Ubiquitous Promoter-Localization of Essential Virulence Regulators in
- TGF-β Suppression of HBV RNA through AID-Dependent Recruitment of an RNA Exosome Complex
- The Immune Adaptor ADAP Regulates Reciprocal TGF-β1-Integrin Crosstalk to Protect from Influenza Virus Infection
- Antagonism of miR-328 Increases the Antimicrobial Function of Macrophages and Neutrophils and Rapid Clearance of Non-typeable (NTHi) from Infected Lung
- The Epigenetic Regulator G9a Mediates Tolerance to RNA Virus Infection in
- Does the Arthropod Microbiota Impact the Establishment of Vector-Borne Diseases in Mammalian Hosts?
- Hantaan Virus Infection Induces Both Th1 and ThGranzyme B+ Cell Immune Responses That Associated with Viral Control and Clinical Outcome in Humans
- Viral Inhibition of the Transporter Associated with Antigen Processing (TAP): A Striking Example of Functional Convergent Evolution
- Plasma Membrane Profiling Defines an Expanded Class of Cell Surface Proteins Selectively Targeted for Degradation by HCMV US2 in Cooperation with UL141
- Optineurin Regulates the Interferon Response in a Cell Cycle-Dependent Manner
- IFIT1 Differentially Interferes with Translation and Replication of Alphavirus Genomes and Promotes Induction of Type I Interferon
- The EBNA3 Family of Epstein-Barr Virus Nuclear Proteins Associates with the USP46/USP12 Deubiquitination Complexes to Regulate Lymphoblastoid Cell Line Growth
- Hepatitis C Virus RNA Replication Depends on Specific and -Acting Activities of Viral Nonstructural Proteins
- A Neuron-Specific Antiviral Mechanism Prevents Lethal Flaviviral Infection of Mosquitoes
- The Aspartate-Less Receiver (ALR) Domains: Distribution, Structure and Function
- Global Genome and Transcriptome Analyses of Epidemic Isolate 98-06 Uncover Novel Effectors and Pathogenicity-Related Genes, Revealing Gene Gain and Lose Dynamics in Genome Evolution
- The Ebola Epidemic Crystallizes the Potential of Passive Antibody Therapy for Infectious Diseases
- Ebola Virus Entry: A Curious and Complex Series of Events
- Conserved Spirosomes Suggest a Single Type of Transformation Pilus in Competence
- Spatial Structure, Transmission Modes and the Evolution of Viral Exploitation Strategies
- Bacterial Cooperation Causes Systematic Errors in Pathogen Risk Assessment due to the Failure of the Independent Action Hypothesis
- Transgenic Fatal Familial Insomnia Mice Indicate Prion Infectivity-Independent Mechanisms of Pathogenesis and Phenotypic Expression of Disease
- Cerebrospinal Fluid Cytokine Profiles Predict Risk of Early Mortality and Immune Reconstitution Inflammatory Syndrome in HIV-Associated Cryptococcal Meningitis
- Utilize Host Actin for Efficient Maternal Transmission in
- Borna Disease Virus Phosphoprotein Impairs the Developmental Program Controlling Neurogenesis and Reduces Human GABAergic Neurogenesis
- An Effector Peptide Family Required for Toll-Mediated Immunity
- Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting Polypeptide
- A Redox Regulatory System Critical for Mycobacterial Survival in Macrophages and Biofilm Development
- Quadruple Quorum-Sensing Inputs Control Virulence and Maintain System Robustness
- Leukocyte-Derived IFN-α/β and Epithelial IFN-λ Constitute a Compartmentalized Mucosal Defense System that Restricts Enteric Virus Infections
- A Strategy for O-Glycoproteomics of Enveloped Viruses—the O-Glycoproteome of Herpes Simplex Virus Type 1
- Macrocyclic Lactones Differ in Interaction with Recombinant P-Glycoprotein 9 of the Parasitic Nematode and Ketoconazole in a Yeast Growth Assay
- Neofunctionalization of the α1,2fucosyltransferase Paralogue in Leporids Contributes to Glycan Polymorphism and Resistance to Rabbit Hemorrhagic Disease Virus
- The Extracytoplasmic Linker Peptide of the Sensor Protein SaeS Tunes the Kinase Activity Required for Staphylococcal Virulence in Response to Host Signals
- Murine CMV-Induced Hearing Loss Is Associated with Inner Ear Inflammation and Loss of Spiral Ganglia Neurons
- Dual miRNA Targeting Restricts Host Range and Attenuates Neurovirulence of Flaviviruses
- GATA-Dependent Glutaminolysis Drives Appressorium Formation in by Suppressing TOR Inhibition of cAMP/PKA Signaling
- Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Infection
- Genetic Analysis Using an Isogenic Mating Pair of Identifies Azole Resistance Genes and Lack of Locus’s Role in Virulence
- A Temporal Gate for Viral Enhancers to Co-opt Toll-Like-Receptor Transcriptional Activation Pathways upon Acute Infection
- Neutrophil Recruitment to Lymph Nodes Limits Local Humoral Response to
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Toxin-Induced Necroptosis Is a Major Mechanism of Lung Damage
- Transgenic Fatal Familial Insomnia Mice Indicate Prion Infectivity-Independent Mechanisms of Pathogenesis and Phenotypic Expression of Disease
- Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Infection
- EphrinA2 Receptor (EphA2) Is an Invasion and Intracellular Signaling Receptor for
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



