-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Selection and Spread of Artemisinin-Resistant Alleles in Thailand Prior to the Global Artemisinin Resistance Containment Campaign
The Plasmodium falciparum parasites that cause malaria are evolving resistance to our most effective and potent anti-malarial drugs available, called artemisinins. Currently, artemisinin resistance is emerging in a number of countries in the Greater Mekong Subregion, including Cambodia, Thailand, Myanmar, and Vietnam. Historically, the Thai-Cambodia border region has been an epicenter of resistance to several anti-malarial drugs. To prevent the spread of artemisinin resistant parasites from the Greater Mekong Subregion, a global artemisinin resistance project was initiated in 2009. Here, we show that artemisinin resistance associated mutation in the K13 gene were widely present throughout Thailand, as early as 2007, primarily along the Thai-Cambodia and Thai-Myanmar border regions. Additional data based on microsatellite markers suggests that the most commonly found K13 C580Y allele may have two recent independent origins in Thailand, on the borders of Cambodia and Myanmar.
Published in the journal: . PLoS Pathog 11(4): e32767. doi:10.1371/journal.ppat.1004789
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004789Summary
The Plasmodium falciparum parasites that cause malaria are evolving resistance to our most effective and potent anti-malarial drugs available, called artemisinins. Currently, artemisinin resistance is emerging in a number of countries in the Greater Mekong Subregion, including Cambodia, Thailand, Myanmar, and Vietnam. Historically, the Thai-Cambodia border region has been an epicenter of resistance to several anti-malarial drugs. To prevent the spread of artemisinin resistant parasites from the Greater Mekong Subregion, a global artemisinin resistance project was initiated in 2009. Here, we show that artemisinin resistance associated mutation in the K13 gene were widely present throughout Thailand, as early as 2007, primarily along the Thai-Cambodia and Thai-Myanmar border regions. Additional data based on microsatellite markers suggests that the most commonly found K13 C580Y allele may have two recent independent origins in Thailand, on the borders of Cambodia and Myanmar.
Introduction
Artemisinin combination therapy (ACT) has been adopted globally as the first-line treatment for uncomplicated Plasmodium falciparum malaria and has contributed to the reduction in malaria related mortality and morbidity. However, resistance to artemisinin poses a threat to the global effort to control malaria. In 2008–2009 the first verified cases of artemisinin resistance, characterized by delayed parasite clearance, were observed in western Cambodia [1]. Prior to those cases, instances of reduced parasite susceptibility to artemisinin were reported in parts of Thailand bordering Cambodia (Chantaburi, Trat, Sakaew, Sisaket, Burirum, and Surin provinces) and Myanmar (Tak province) as early as 2003 [2]. The Thai-Cambodian border region has historically been the epicenter of multi-drug resistant (MDR) malaria [3]. As resistance to earlier anti-malarial drugs spread from this region to Africa and other parts of Asia through parasite migration, there is a serious concern that a similar scenario may occur with artemisinin resistance [4]. The ACT artesunate-mefloquine (ASMQ) has been used as first-line therapy in Thailand since 1995, beginning in areas where multi-drug resistance had evolved. Use of ASMQ was extended to the rest of the country after the World Health Organization (WHO) recommended ACT for global use in the early 2000s [5]. In Thailand, ASMQ was initially introduced as a two-day regimen and in 2008 was extended to three days (three days of artesunate and two days of mefloquine, but with the same total dose as the two-day regimen) [6].
The ASMQ regimen has remained generally effective in Thailand despite high levels of mefloquine resistance with a cure rate of greater than 90% [5]. However, in several locations in Cambodia and Thailand, treatment failure rates over 10% have also been observed [7]. To date, the strongest evidence of artemisinin resistance was initially reported in western Cambodia, and subsequently other parts of Southeast Asia [1, 7–13].
To prevent the spread of artemisinin-resistant P. falciparum, the WHO and other partners initiated an artemisinin resistance containment project for the Greater Mekong Subregion in 2009 [14]. The goal was to identify and prevent artemisinin-resistant parasites from spreading outside of documented hotspot regions along the Thai-Cambodian border by ensuring proper diagnosis and full treatment of reported malaria cases [15]. Subsequently, the WHO, along with other partners, initiated the Global Plan for Artemisinin Resistance Containment and Emergency Response to artemisinin resistance in the Greater Mekong Subregion [5].
Currently, therapeutic efficacy studies (TES) are considered the gold standard for determining antimalarial drug efficacy [16]. However, the WHO recommends that TES results be complimented using molecular marker studies [17]. Therefore, it was desirable to identify a molecular marker for artemisinin resistance. Initial studies using a genome-wide association approach found two loci on P. falciparum chromosomes 10 and 13 to be associated with artemisinin resistance [12, 18]. After a long search to pinpoint a specific gene associated with artemisinin resistance, the K13 gene (PF3D7_1343700) on chromosome 13 was identified as a potential molecular marker [19]. The study identified mutations in the propeller domain of the K13 gene that were associated with artemisinin resistance as measured by ex vivo ring stage survival assays and delayed parasite clearance times [19]. Specifically, the study identified 18 non-synonymous single nucleotide polymorphisms (SNPs) in the K13 propeller domain, of which three mutations (C580Y, R539T and Y493H) were strongly associated with increased ring stage survival and delayed parasite clearance rates. The C580Y allele accounted for about 85% of all mutant K13 alleles observed in 2011–2012 in western Cambodia [19]. Most recently, a two year multi-site project by Ashley et al. further confirmed that multiple SNPs in the propeller domain of K13 were predictive of slow parasite clearance and that these mutations were found in multiple countries in the Greater Mekong Subregion [20]. That study, along with two other recent studies by Takala-Harrison et al. and Miotto et al. [13, 21], showed that the C580Y allele was the predominant allele in Cambodia, Myanmar, and Vietnam. The authors further demonstrated that the C580Y allele may have emerged independently in Cambodia and Myanmar.
Although Thailand has historically been an epicenter of resistance to several antimalarial drugs, currently there are limited data on the artemisinin resistance-associated K13 propeller mutations in this region. Here, using historical samples collected during 2007 from ten different sites in Thailand, we set forth to answer the following questions: (1) Were artemisinin resistance-associated K13 mutant alleles present in Thailand prior to the implementation of the artemisinin resistance containment projects? (2) If so, what were the prevalence and distribution of the K13 propeller mutations in Thailand? (3) What are the evolutionary histories of the different K13 mutant and wild type alleles? (4) Are the K13 mutant alleles evolving locally or are particular mutants spreading across Thailand? and (5) Is there evidence for selection of resistant K13 alleles?
Results
Distribution and frequency of K13 propeller mutations in Thailand during 2007
All 417 patient samples were either wild type or had a single mutation in the K13 propeller domain. Twelve percent (50/417) carried one of seven mutant alleles (N458Y, R539T, E556D, P574L, R575K, C580Y, S621F) in the K13 propeller domain, including two mutations (R575K and S621F) that have not been reported previously in Thailand. The C580Y mutant allele, which is a predominant allele in Cambodia, accounted for 52% (26/50) of all mutant alleles identified in our study population. The C580Y allele frequencies were higher along the Thai-Cambodian border, in Chanthaburi (N = 5/10, 50% C580Y), Trat (N = 5/12, 42% C580Y), and Sisaket (N = 8/13, 62% C580Y) provinces compared to the provinces along the Thai-Myanmar border, Chumporn (N = 2/12, 17% C580Y), Ranong (N = 3/40, 8% C580Y), Kanchanaburi (N = 6/40, 15% C580Y), and Tak (N = 1/171, 1% C580Y). Interestingly, the R539T alleles were only found in eastern Thailand near the Cambodian border in Trat (N = 1/12, 8% R539T) and Sisaket (N = 2/13, 16% R539T) provinces. Besides the C580Y mutation, four previously identified mutations (R575K, P574L, E556D, and N458Y) as well as one novel K13 propeller allele not reported yet (S621F), were found in western parts of Thailand. The R575K and S621F alleles were only present along the Thai-Myanmar border in Prachuap (N = 6/33, 18% R575K), Kanchanaburi (N = 4/40, 10% R575K), and Tak (N = 1/132, 1% S621F), provinces. The P574L allele was present in Ranong (N = 4/40, 10%), followed by 8% (N = 1/12) prevalence in Chumporn and 3% (N = 1/33) in Prachuap. All parasite isolates from the northwestern province of Mae Hong Son (N = 42/42) and southeastern province of Yala (N = 40/40) carried the wild type K13 propeller allele. Overall, these results show the presence of parasites harboring single non-synonymous mutations in the K13 propeller domain as early as 2007 in eight Thai provinces (Fig 1).
Fig. 1. Geographic distribution of the K13 propeller alleles in Thailand in 2007. 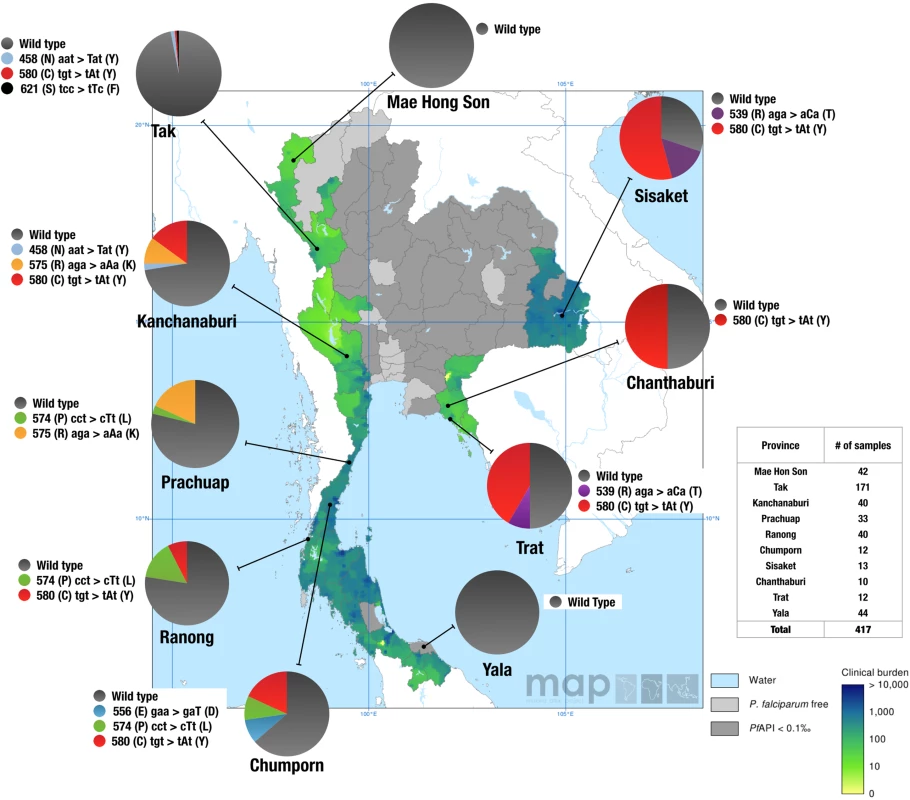
Pie charts show K13 propeller allele frequencies among 417 parasite isolates in 10 Thailand provinces. The different alleles are color coded. The results are shown on top of the clinical burden map of P. falciparum in Thailand in 2007 (Malaria Atlas Project) [34]. Light grey areas are P. falciparum malaria free and dark grey areas have an unstable risk of malaria transmission (i.e. annual case incidence, or API, is reported at less than 1 per 10,000). Map shows mean estimate for the clinical burden in the range from 0 (light green) to 10,000 (dark green/blue) clinical cases per year. The clinical burden predictions are based on a Bayesian geostatistical model. Emergence and spread of the C580Y allele in Thailand
Microsatellites flanking K13 were used to infer the evolutionary histories of the C580Y alleles. Parasites with the C580Y alleles from eastern and western Thailand shared a similar genetic profile in most loci, with the exception of the 8.6kb locus downstream of the gene (Fig 2). This microsatellite locus clearly separates the C580Y alleles based on geography (east versus west). Moreover, the C580Y alleles in the eastern region had a 194 bp allele size at locus 31.5kb, whereas in the western Thailand most of the C580Y alleles had a 198 bp allele size with the exception of three isolates (Fig 2). In addition, there is a high degree of microsatellite identity among infections with the C580Y allele as compared to the wild type K13 haplotypes both upstream and downstream from K13 (S1 Dataset).
Fig. 2. K13 flanking microsatellites of parasites from Thailand. 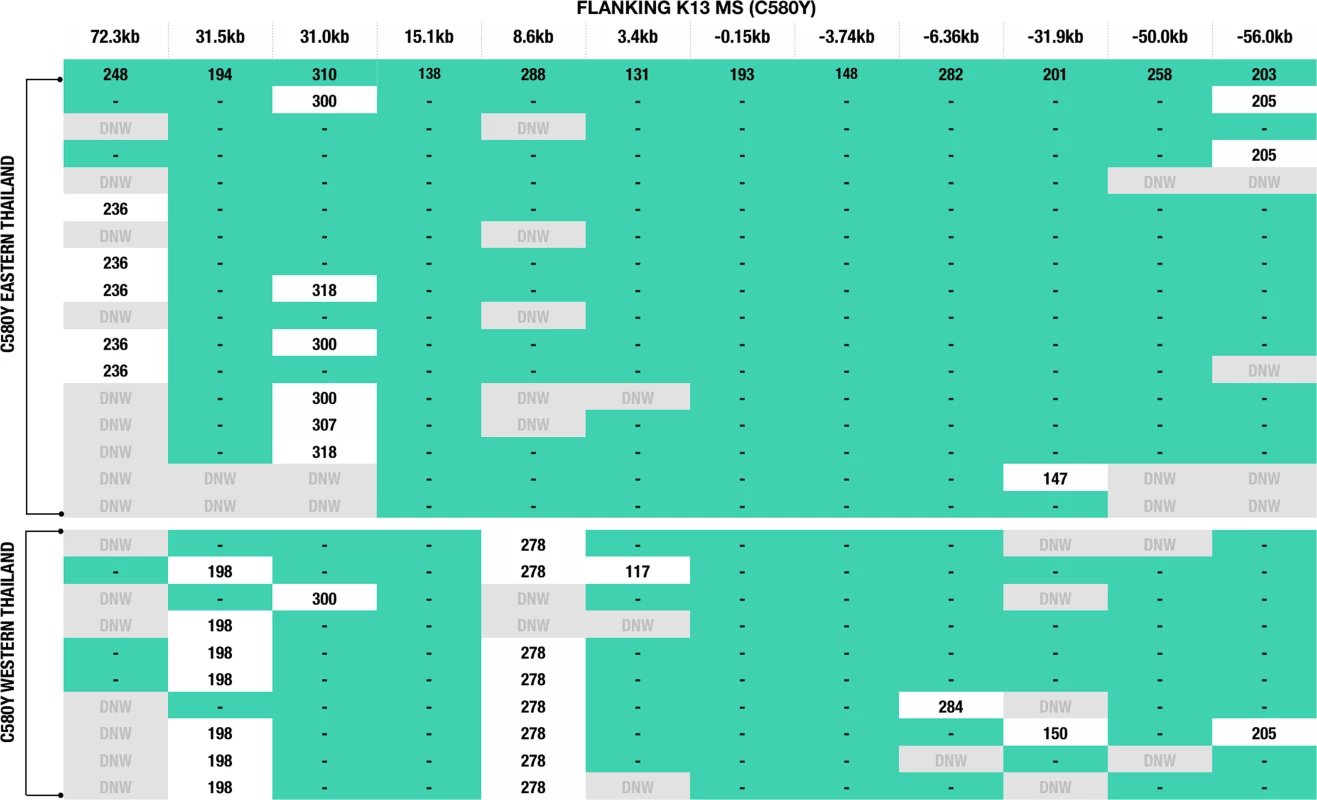
The figure comprises data from 16 western and 10 eastern Thai parasites with the C580Y mutation. Allele lengths are shown for 12 microsatellites positioned at: 72.3kb, 31.5kb, 31.0kb, 15.1kb, 8.6kb, 3.4kb, -0.15kb, -3.74kb, -6.36kb, -31.9kb, -50.0kb, and -56.0kb. Teal green shading and lines indicate identical allele sizes. DNW (in grey) = indicates no successful amplification after a third attempt or not enough DNA was available to repeat the analysis. Reduced genetic heterozygosity of the C580Y K13 propeller allele
Using the nine microsatellite loci flanking the K13 propeller gene, expected heterozygosity (He) was calculated for the C580Y and wild type alleles (Fig 3). The N458Y, R539T, P574L, R575K and S621F alleles were excluded as there were limited samples to carry out the analysis. The C580Y allele (N = 26, mean He = 0.3526 ± 0.08) showed a 56% reduction (p = 0.0046) in heterozygosity as compared to wild type alleles (N = 22, mean He = 0.6246 ± 0.06) (Fig 3A). No significant difference (p = 0.2240) in heterozygosity was found when comparing western C580Y alleles (N = 10, mean He = 0.4360 ± 0.03) to eastern C580Y alleles (N = 15, mean He = 0.2755 ± 0.05) (Fig 3B). Mean He between the wild type and different mutant alleles were compared using the Mann-Whitney U test.
Fig. 3. Heterozygosity valley around K13 propeller alleles in Thailand. 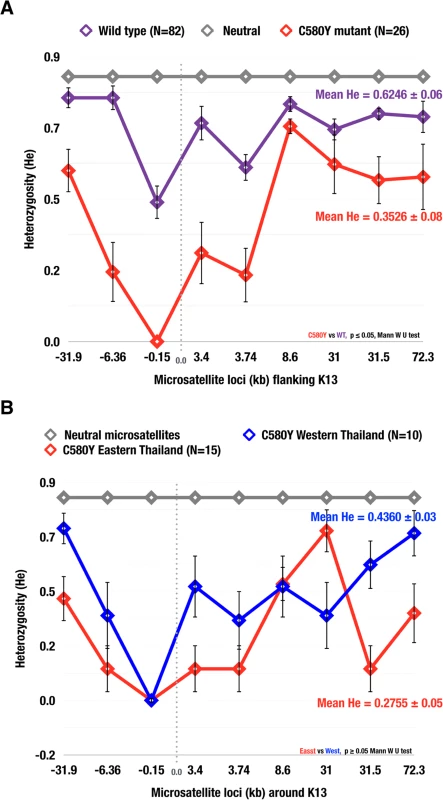
The expected heterozygosity of parasite isolates with: (A) the C580Y mutation (N = 26) and wild type alleles (N = 82); (B) the C580Y from the east (N = 10) and west (N = 16). Diversity was reduced at all 9 K13 propeller microsatellite loci for C580Y compared to wild type alleles. The mean He in (A) for C580Y (0.3526 ± 0.08), wild type (0.6246 ± 0.06), neutral (0.7650 ± 0.05); (B) C580Y east (0.2755 ± 0.05) and C580Y west (0.4360 ± 0.03 ± 0.03). The error bars indicate ± standard deviation (SD). Principal component analysis and regional separation of C580Y alleleic haplotypes
The results of the principal component analysis of the neutral and flanking microsatellite data for the C580Y mutants and wild type isolates are plotted in Fig 4. When considering the flanking microsatellite data for all C580Y mutants, the first principal component separated the isolates into those from the western (Kanchanaburi, Ranong, and Chumporn) and eastern (Chanthanburi, Trat, and Sisaket) provinces (Fig 4A), with the exception of a single isolate. No clear geographical separation is observed when looking at the flanking K13 microsatellites in the wild type population (Fig 4B). Interestingly, some of the parasites from Sisaket Province clustered together suggesting a recent clonal expansion of these parasites (Fig 4C). Similar results were obtained by a neighbor joining tree analysis (S1 Fig).
Fig. 4. Population structure by geography. 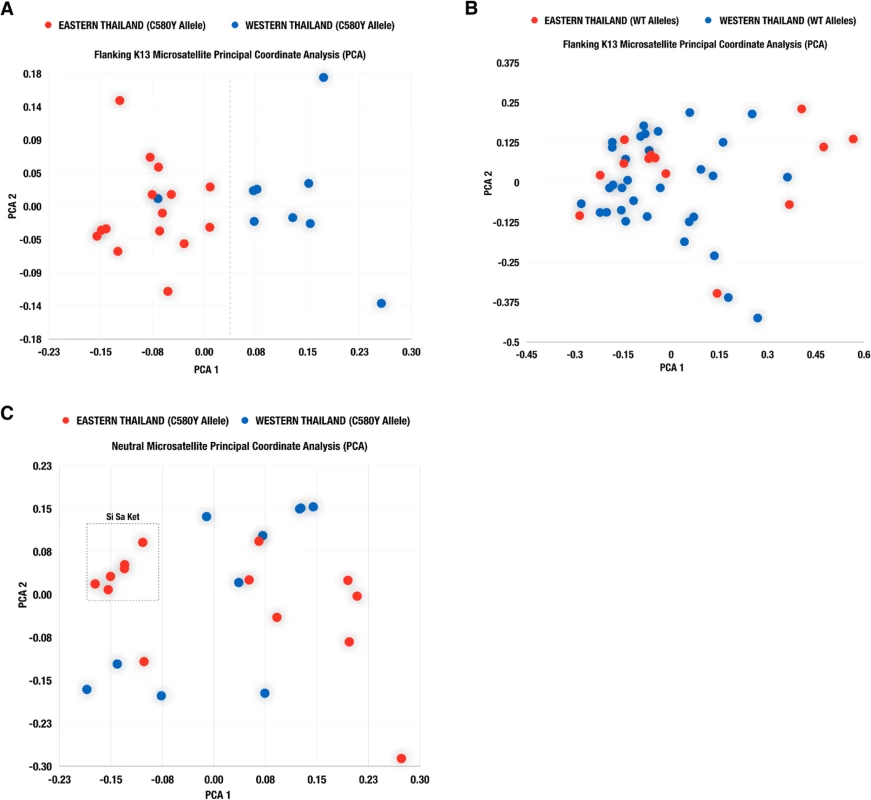
Scatter plots from principal component analysis (PCA) based on flanking K13 microsatellite analysis of parasite isolates with the C580Y allele (A) and wild type allele (B). Results of PCA based on neutral microsatellites for parasite isolates carrying the C580Y allele, are shown in (C). Association of genetic dissimilarity with genetic distance
The genetic dissimilarity between the K13 flanking microsatellites of C580Y mutants was highly associated (Pearson's correlation coefficient: 0.44, 95% CI: 0.34–0.52) with the geographic distance between the sites where the isolates were collected. Average genetic dissimilarity was lower between pairs of C580Y mutants than between pairs of wild type parasites (t-test p-value < 0.001), and the association between genetic dissimilarity and geographic distance was stronger for C580Y mutants than for wild type parasites (Fig 5).
Fig. 5. Association between genetic dissimilarity and geographic distance. 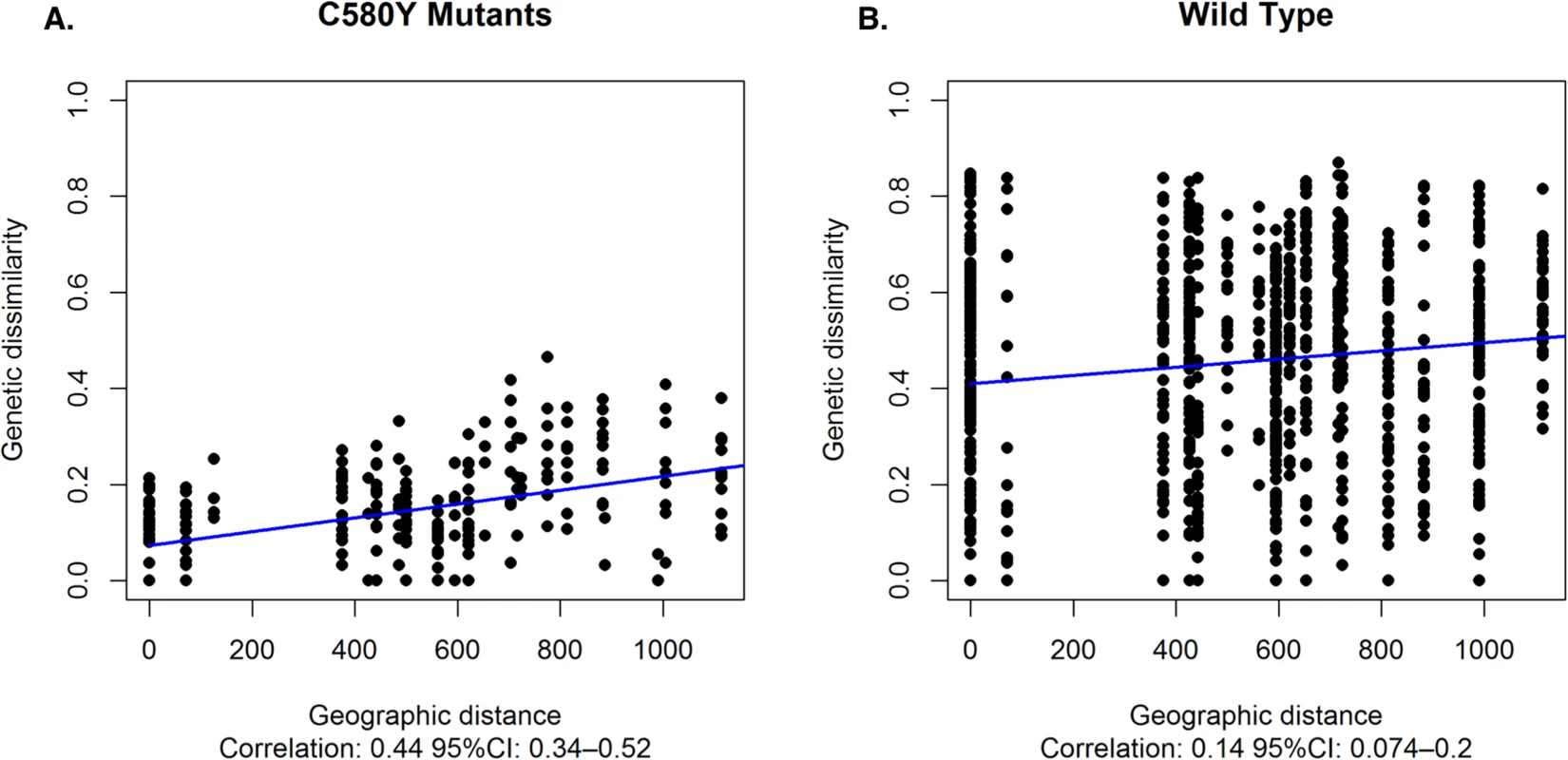
(A) Genetic dissimilarity and geographic distance between pairs of C580Y mutants (A) and wild type parasites (B). Genetic dissimilarity of flanking microsatellites for C580Y is highly associated with geographic distance (Pearson's correlation coefficient: 0.44, 95% CI:0.34–0.52). Average genetic dissimilarity is lower between C580Y and wild type parasites (t-test p-value < 0.001). Discussion
To our knowledge, this study is one of the first reports to systematically analyze the artemisinin resistance K13 propeller mutations and flanking microsatellite loci in parasites collected in numerous sites in Thailand shortly before the implementation of the artemisinin resistance containment project in 2009. The samples were collected from across ten provinces, including the containment zones and areas at highest risk for malaria. This study provides evidence that (1) artemisinin resistance alleles were present in 8 out of 10 Thai provinces sampled, including two mutant alleles (R575K and S621F) not previously reported in Thailand, (2) artemisinin resistance-associated K13 alleles had evolved along the Thai-Cambodia and Thai-Myanmar border regions at least two years prior to the implementation of the artemisinin resistance containment project, (3) the artemisinin resistance-associated C580Y mutant alleles were the most common and widespread in Thailand, (4) there are clear differences in microsatellites that differentiate the C580Y mutant alleles from eastern and western parts of Thailand, and (5) the C580Y alleles appear to have had two recent, independent origins.
Our study provides insight into the prevalence and distribution of K13 mutations in Thailand as early as 2007. Interestingly, the prevalence of mutant K13 alleles reported in Pailin, Cambodia during 2007 for the C580Y (45%) and R539T (5%) alleles [19] is consistent with our findings. Across the border from Pailin, in the provinces of Chanthaburi, Sisaket and Trat, we observed that more than 42% of the parasites screened carried the C580Y mutant allele (Fig 1). The C580Y allele has since been associated with delayed parasite clearance in this region [19, 20]. The highest prevalence of the C580Y (62%) and R539T (16%) mutations is seen in the province of Sisaket, just north of Cambodia. These results are in agreement with the recent study by Ashley et al [20], which provided compelling evidence that the C580Y and R539T mutations are associated with delayed parasite clearance in both Sisaket and Ranong. Moreover, 78% (7/9) of the parasite isolates from Sisaket showed a similar clonal genetic profile, suggesting that this may have been a recent clonal expansion event (Figs 3C and S1). Similar findings were reported by Miotto et al [22], who demonstrated that three subpopulations associated with clinical resistance to artemisinin may have recently expanded in Cambodia and elsewhere in the region.
In eastern Thailand, C580Y and R539T were the only mutations observed; however, this may be due to the limited number of samples analyzed. In contrast, the provinces bordering Myanmar had the following mutations: S621F, C580Y, R575K, P574L, E556D, and N458Y. C580Y, P574L and R575K were the most commonly found alleles along the Thai-Myanmar border region (i.e. from Kanchanaburi to Chumporn). Interestingly, the C580Y and R575K mutations have recently been reported near the Thai-Myanmar border as well [23, 24]. Other mutant alleles (N458Y, S621F, and E556D) were rare and restricted to one or two sites (Fig 1), suggesting that these K13 mutations may have arisen independently. Although previous studies have confirmed a strong association between select K13 propeller domain mutations and delayed parasite clearance [19, 20, 25], it remains to be determined whether the remaining mutant alleles will have a similar association. Furthermore, it remains to be seen whether the C580Y mutation will trend towards fixation in Thailand, as was seen between 2001 and 2012 in Pailin, Cambodia [19]. Our data show that artemisinin-resistant K13 alleles did not spread to or evolve in the southernmost Yala province or the northern Mae Hong Son province during 2007. In Yala, the parasites had identical flanking and neutral microsatellite haplotypes, which is consistent with our previously published results [26], indicating a closely related clonal population.
Population differentiation analysis further reveals that parasites with the C580Y allele group together by geography (Fig 3A). Analysis of raw microsatellite haplotype data for these parasites revealed that alleles circulating in the east and west comprise two distinct lineages marked by differences in the 8.6kb locus downstream of the K13 gene (Fig 2). These data suggest the C580Y mutations may have arisen independently along the Thai-Cambodia and Thai-Myanmar borders. The reduced pattern of heterozygosity of C580Y alleles compared to wild type alleles (Fig 3) further suggests recent independent origins along the Thai-Cambodia and Thai-Myanmar borders. This interpretation of our data is consistent with the recently published work by Miotto et al. [21]. The study provided compelling evidence for the selection of the C580Y allele in the Greater Mekong Subregion [21], which is consistent with our data, and suggested that the selection process may have been under way on both sides of Thailand at the time of this study. One possible interpretation of our findings would be that the parasites migrated across Thailand prior to the independent C580Y emergence events. Recent findings by Takala-Harrison et al. and Miotto et al. are consistent with the independent emergence of the C580Y allele, which was also observed along the Myanmar-Thai border and the lower Mekong region [13, 21]. The strong association between the genetic dissimilarity and geographic distance of the C580Y mutants further supports the hypothesis that this mutation may have emerged independently in eastern and western Thailand (Fig 5).
Given the history of population movements within this region, some of the mutant alleles in the Thai-Myanmar region (C580Y and P574L) and Thai-Cambodia region (C580Y and R539T) may share common ancestry. Interestingly, in the work by Miotto et al. [21], parasites with the most common K13 mutant alleles (C580Y, I543T, R539T, and Y493H) were found in multiple countries in the region, indicating that parasite cross-border movement may have already occurred. It remains to be determined if the P574L and R575K alleles, which have been found in Myanmar [13, 23], originated in either Thailand or Myanmar.
It has been suggested that in the absence of drug pressure, parasites with some resistant mutations are less fit than their ancestral wild type counterparts [27]. However, with continued drug pressure one might expect resistant alleles, such as the C580Y mutation, to eventually become fixed in the population as has occurred with other resistance mutations in the past. The work by Ariey et al., which identified the K13 propeller as a molecular marker of artemisinin resistance, demonstrated that over the course of 11 years (2001–2012), the C580Y allele prevalence increased from 40% to 90% in Pailin, Cambodia [19]. This would suggest that the parasites carrying the C580Y mutation may be nearing fixation in the population, and therefore, no sensitive parasites will remain to outcompete them in the absence of ACT. This is very worrisome, as ACT is one of our last working treatment options for drug resistant P. falciparum.
In summary, it is evident from our study that artemisinin-resistant K13 alleles have been evolving along both the Thai-Cambodian border and Thai-Myanmar border long before the artemisinin containment project was implemented. It is further evident that the most commonly found C580Y allele had two distinct haplotypes, suggesting different patterns of origin and migration along the Thai-Cambodia and Thai-Myanmar regions.
Materials and Methods
Ethics statement
This protocol was approved by the Thailand Ministry of Public Health. CDC Human Research Protection Office provided approval for retrospective testing using anonymized samples. Study participants and/or their guardians provided written informed consent.
Study sites and samples
A total of 417 Plasmodium falciparum infected blood samples were used in this study. The samples were collected in 2007 as part of a malaria surveillance study conducted by the Thailand Ministry of Public Health [28]. Finger prick blood samples were collected from ten malaria-endemic provinces of Thailand. Six of these provinces (Mae Hong Son, Tak, Kanchanaburi, Prachuap, Chumporn, and Ranong) are on the Myanmar border, and three (Sisaket, Chanthaburi, and Trat) are on the Cambodian border, while one (Yala) is in southern Thailand bordering Malaysia. In 2007, the reported malaria incidence rates were 17.2, 9.2, 8.7, and 8.5 cases per 1,000 residents in Yala, Mae Hong Son, Tak, and Ranong, respectively, and 3.9, 2.9, 1.7 and 1.2 per 1,000 population in Chumporn, Prachuap, Chanthaburi and Kanchanaburi, respectively [29].
PCR amplification and sequencing of Plasmodium falciparum K13 gene
The K13 gene was amplified using a nested PCR method that was modified from a previous study [19]. New secondary primers that are species specific for P. falciparum were developed and used. For the primary PCR the same primers as in [19] were used (K13P1 5’-GGGAATCTGGTGGTAACAGC-3’ and K13R1 5’-CGGAGTGACCAAATCTGGGA-3’). For the secondary PCR, new primers were designed (K13S1, 5’ GTAAAGTGAAGCCTTGTTG-3’ and K13S2 5'-TTCATTTGTATCTGGTGAAAAG -3’). Two μl of genomic DNA was amplified using 0.5 μM of each primer, 0.2 mM dNTP, 3 and 2 mM MgCl2 for the primary and secondary reactions, respectively, and 1 U Expand High Fidelity Taq (Roche). For the primary reaction, the following cycling parameters were used: 5 min at 94°C, 40 cycles of 94°C for 30 s, 60°C for 90s, 72°C for 90s, and final extension for 10 min at 72°C. For the nested PCR, 1 μl of the primary PCR product was used as a template. For the nested PCR reaction, the following cycling parameters were used: 2 min at 94°C, 40 cycles of 94°C for 30 s, 55°C for 30s, 72°C for 90s, and final extension for 10 min at 72°C. PCR products were separated and visualized using 2% agarose gel electrophoresis and Gel red (Biotium, Hayward CA). Sanger sequencing of PCR products was performed using ABI 3730 (Applied Biosystems, Foster City, CA). Sequences were deposited to Genbank (Accession Numbers:KP334284—KP334700).
Microsatellite loci genotyping
Twenty-five microsatellite loci flanking the K13 gene on chromosome 13 (PF3D7_1343700) were tested for evidence of selection as indicated by a reduction in heterozygosity around the K13 gene. Three of the loci, L4_165 (72.3 kb), LM_173 (-3.74 kb) and B1_P1 (-31.9 kb) were previously described [18], and the remaining 22 were newly designed for this study. Only 12 out of 25 loci were informative and further analyzed to study the selective sweeps and genetic lineages of resistance K13 alleles (downstream: 3.4kb, 8.6kb, 15.1kb, 31.0kb, 31.5kb, 72.3kb; upstream: -0.15kb, -3.7kb, -6.36kb, -31.9kb, -50.0kb, -56.0kb). The primers used are shown in S1 Table. In addition, eight putatively neutral microsatellite loci located on chromosome 2 (GenBank UniSTS C2M27, C2M29, C2M34, and C2M33) and chromosome 3 (GenBank UniSTS C3M40, C3M88, C3M69, and C3M39) were used as previously described [28]. A previously described protocol [30] for cycling was modified for this study. Briefly, primer pairs with annealing temperature in the 50–60°C range were designed and cycling conditions were adjusted according to each primer pairs melting temperatures (TMs). The sizes of the amplification products were assayed by capillary electrophoresis on an Applied Biosystems 3130 xl sequencer (Applied Biosystems, Foster City CA).
Estimating genetic diversity
To determine genetic diversity, the expected heterozygosity (He) was estimated using all K13 flanking or neutral microsatellite loci using the Excel Microsatellite Toolkit, version 3.1.1 [31]. He was calculated using the formula [n/ (n-1)][1-Ʃpi2] for He; and 2(n-1)/n3 {2(n-2) [Ʃ(pi3-(Ʃpi2)2]}for variance, where n is the number of samples genotyped for any locus and pi is the frequency of the ith allele. Any locus for which an allele could not be amplified after two attempts was assigned as DNW, indicating no amplification. Mean He between the wild type and C580Y mutant alleles were compared using the Mann-Whitney U test. Statistical significance was defined as p ≤ 0.05.
Microsatellite and SNP analysis
Sanger sequences were analyzed using Geneious Pro R8 (www.geneious.com) to identify specific SNP combinations. A custom pipeline was created using the Geneious workflow feature to automate the SNP analysis (shared @GitHub). Briefly, by selecting a user defined sequence list (select all raw sequences > create list) and reference sequence as an input, the workflow will automatically map the input sequences to the reference sequence, identify all SNPs, and export the final SNP calls. Each step creates a sub-folder allowing the user to check the results. SNPs were only called if both the forward and reverse strands had the mutation. Microsatellite fragment analysis was performed using the Geneious Pro R8 microsatellite plugin. For determining genetic lineages of the K13 alleles, the POLYSAT R package was used [32]. Using the built-in functions in POLYSAT R, a pairwise distance matrix and principal component analysis (PCA) matrix was calculated using a stepwise mutation model for the flanking and neutral microsatellite markers. The pairwise distance matrix was used to construct a neighbor joining tree via the T-REX web server [33]. The resulting neighbor joining tree was imported into Geneious Pro R8 and colored according to geography. The geographic distance between the ten sampling sites was calculated, and genetic dissimilarity between each pair of isolates using the flanking microsatellites was plotted against the geographic distance between the sites where they were collected. Pearson’s correlation coefficient was calculated to assess the association between genetic dissimilarity and geographic distance. The genetic dissimilarity scatter plots were created and visualized using R 3.1.1. All R code used to run the analysis has been uploaded to: GitHub
Accession numbers
Sequences were deposited to Genbank (Accession Numbers:KP334284—KP334700).
Supporting Information
Zdroje
1. Noedl H., et al., Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med, 2008. 359(24): p. 2619–20. doi: 10.1056/NEJMc0805011 19064625
2. Vijaykadga S., et al., In vivo sensitivity monitoring of mefloquine monotherapy and artesunate-mefloquine combinations for the treatment of uncomplicated falciparum malaria in Thailand in 2003. Trop Med Int Health, 2006. 11(2): p. 211–9. 16451346
3. Wongsrichanalai C., et al., Epidemiology of drug-resistant malaria. Lancet Infect Dis, 2002. 2(4): p. 209–18. 11937421
4. Wootton J.C., et al., Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature, 2002. 418(6895): p. 320–3. 12124623
5. Satimai W., et al., Artemisinin resistance containment project in Thailand. II: Responses to mefloquine-artesunate combination therapy among falciparum malaria patients in provinces bordering Cambodia. Malar J, 2012. 11: p. 300. 22929621
6. Alker A.P., et al., Pfmdr1 and in vivo resistance to artesunate-mefloquine in falciparum malaria on the Cambodian-Thai border. Am J Trop Med Hyg, 2007. 76(4): p. 641–7. 17426163
7. Phyo A.P., et al., Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet, 2012. 379(9830): p. 1960–6. doi: 10.1016/S0140-6736(12)60484-X 22484134
8. Dondorp A.M., et al., Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med, 2009. 361(5): p. 455–67. doi: 10.1056/NEJMoa0808859 19641202
9. Amaratunga C., et al., Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: a parasite clearance rate study. Lancet Infect Dis, 2012. 12(11): p. 851–8. doi: 10.1016/S1473-3099(12)70181-0 22940027
10. Carrara V.I., et al., Malaria burden and artemisinin resistance in the mobile and migrant population on the Thai-Myanmar border, 1999–2011: an observational study. PLoS Med, 2013. 10(3): p. e1001398. doi: 10.1371/journal.pmed.1001398 23472056
11. Kyaw M.P., et al., Reduced susceptibility of Plasmodium falciparum to artesunate in southern Myanmar. PLoS One, 2013. 8(3): p. e57689. doi: 10.1371/journal.pone.0057689 23520478
12. Takala-Harrison S., et al., Genetic loci associated with delayed clearance of Plasmodium falciparum following artemisinin treatment in Southeast Asia. Proc Natl Acad Sci U S A, 2013. 110(1): p. 240–5. doi: 10.1073/pnas.1211205110 23248304
13. Takala-Harrison S., et al., Independent Emergence of Artemisinin Resistance Mutations Among Plasmodium falciparum in Southeast Asia. J Infect Dis, 2014. 211(5):670–9. doi: 10.1093/infdis/jiu491 25180241
14. WHO, Strategic Plan to Strengthen Malaria Control and Elimination in the Greater Mekong Sub region: 2010–2014. 2010: The World Health Organizaiton. Geneva.
15. Khamsiriwatchara A., et al., Artemisinin resistance containment project in Thailand. (I): Implementation of electronic-based malaria information system for early case detection and individual case management in provinces along the Thai-Cambodian border. Malar J, 2012. 11: p. 247. doi: 10.1186/1475-2875-11-247 22839508
16. WHO, Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations. 2008: World Health Organization. Geneva.
17. WHO, Global report on antimalarial drug efficacy and drug resistance: 2000–2010. 2010: World Health Organization. Geneva.
18. Cheeseman I.H., et al., A major genome region underlying artemisinin resistance in malaria. Science, 2012. 336(6077): p. 79–82. doi: 10.1126/science.1215966 22491853
19. Ariey F., et al., A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature, 2014. 505(7481): p. 50–5. doi: 10.1038/nature12876 24352242
20. Ashley E.A., et al., Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med, 2014. 371(5): p. 411–23. doi: 10.1056/NEJMoa1314981 25075834
21. Miotto O., et al., Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat Genet, 2015. 47(3):226–34 doi: 10.1038/ng.3189 25599401
22. Miotto O., et al., Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat Genet, 2013. 45(6): p. 648–55. doi: 10.1038/ng.2624 23624527
23. Nyunt, M.H., et al., Molecular Assessment of Artemisinin Resistance Markers, Polymorphisms in the K13 Propeller, and a Multidrug-Resistance Gene in the Eastern and Western Border Areas of Myanmar. Clin Infect Dis, 2014. pii: ciu1160. [Epub ahead of print]
24. Tun, K.M., et al., Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: a cross-sectional survey of the K13 molecular marker. Lancet Infect Dis, 2015. pii: S1473-3099(15)70032-0.
25. Straimer J., et al., Drug resistance. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science, 2015. 347(6220): p. 428–31. doi: 10.1126/science.1260867 25502314
26. Pumpaibool T., et al., Genetic diversity and population structure of Plasmodium falciparum in Thailand, a low transmission country. Malar J, 2009. 8: p. 155. doi: 10.1186/1475-2875-8-155 19602241
27. Anderson T.J. and Roper C., The origins and spread of antimalarial drug resistance: lessons for policy makers. Acta Trop, 2005. 94(3): p. 269–80. 15878153
28. Alam M.T., et al., Tracking origins and spread of sulfadoxine-resistant Plasmodium falciparum dhps alleles in Thailand. Antimicrob Agents Chemother, 2011. 55(1): p. 155–64. doi: 10.1128/AAC.00691-10 20956597
29. WHO, Malaria in the greater Mekong subregion: regional and country profiles. 2008: The World Health Organization. Geneva.
30. Alam M.T., et al., Selective sweeps and genetic lineages of Plasmodium falciparum drug-resistant alleles in Ghana. J Infect Dis, 2011. 203(2): p. 220–7. doi: 10.1093/infdis/jiq038 21288822
31. Su X., et al., A genetic map and recombination parameters of the human malaria parasite Plasmodium falciparum. Science, 1999. 286(5443): p. 1351–3. 10558988
32. Clark L.V. and Jasieniuk M., POLYSAT: an R package for polyploid microsatellite analysis. Mol Ecol Resour, 2011. 11(3): p. 562–6. doi: 10.1111/j.1755-0998.2011.02985.x 21481215
33. Boc A., Diallo A.B., and Makarenkov V., T-REX: a web server for inferring, validating and visualizing phylogenetic trees and networks. Nucleic Acids Res, 2012. 40(Web Server issue): p. W573–9. doi: 10.1093/nar/gks485 22675075
34. Hay S.I., et al., Developing global maps of the dominant anopheles vectors of human malaria. PLoS Med, 2010. 7(2): p. e1000209. doi: 10.1371/journal.pmed.1000209 20161718
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Relay of Herpes Simplex Virus between Langerhans Cells and Dermal Dendritic Cells in Human SkinČlánek Does the Arthropod Microbiota Impact the Establishment of Vector-Borne Diseases in Mammalian Hosts?Článek The Ebola Epidemic Crystallizes the Potential of Passive Antibody Therapy for Infectious DiseasesČlánek Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting PolypeptideČlánek A Redox Regulatory System Critical for Mycobacterial Survival in Macrophages and Biofilm Development
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Pathogens as Biological Weapons of Invasive Species
- Selection and Spread of Artemisinin-Resistant Alleles in Thailand Prior to the Global Artemisinin Resistance Containment Campaign
- Endopeptidase-Mediated Beta Lactam Tolerance
- Prospective Large-Scale Field Study Generates Predictive Model Identifying Major Contributors to Colony Losses
- Relay of Herpes Simplex Virus between Langerhans Cells and Dermal Dendritic Cells in Human Skin
- Structural Determinants of Phenotypic Diversity and Replication Rate of Human Prions
- Sigma Factor SigB Is Crucial to Mediate Adaptation during Chronic Infections
- EphrinA2 Receptor (EphA2) Is an Invasion and Intracellular Signaling Receptor for
- Toxin-Induced Necroptosis Is a Major Mechanism of Lung Damage
- Heterologous Expression in Remodeled . : A Platform for Monoaminergic Agonist Identification and Anthelmintic Screening
- Novel Disease Susceptibility Factors for Fungal Necrotrophic Pathogens in Arabidopsis
- Interleukin 21 Signaling in B Cells Is Required for Efficient Establishment of Murine Gammaherpesvirus Latency
- Phosphorylation at the Homotypic Interface Regulates Nucleoprotein Oligomerization and Assembly of the Influenza Virus Replication Machinery
- Human Papillomaviruses Activate and Recruit SMC1 Cohesin Proteins for the Differentiation-Dependent Life Cycle through Association with CTCF Insulators
- Ubiquitous Promoter-Localization of Essential Virulence Regulators in
- TGF-β Suppression of HBV RNA through AID-Dependent Recruitment of an RNA Exosome Complex
- The Immune Adaptor ADAP Regulates Reciprocal TGF-β1-Integrin Crosstalk to Protect from Influenza Virus Infection
- Antagonism of miR-328 Increases the Antimicrobial Function of Macrophages and Neutrophils and Rapid Clearance of Non-typeable (NTHi) from Infected Lung
- The Epigenetic Regulator G9a Mediates Tolerance to RNA Virus Infection in
- Does the Arthropod Microbiota Impact the Establishment of Vector-Borne Diseases in Mammalian Hosts?
- Hantaan Virus Infection Induces Both Th1 and ThGranzyme B+ Cell Immune Responses That Associated with Viral Control and Clinical Outcome in Humans
- Viral Inhibition of the Transporter Associated with Antigen Processing (TAP): A Striking Example of Functional Convergent Evolution
- Plasma Membrane Profiling Defines an Expanded Class of Cell Surface Proteins Selectively Targeted for Degradation by HCMV US2 in Cooperation with UL141
- Optineurin Regulates the Interferon Response in a Cell Cycle-Dependent Manner
- IFIT1 Differentially Interferes with Translation and Replication of Alphavirus Genomes and Promotes Induction of Type I Interferon
- The EBNA3 Family of Epstein-Barr Virus Nuclear Proteins Associates with the USP46/USP12 Deubiquitination Complexes to Regulate Lymphoblastoid Cell Line Growth
- Hepatitis C Virus RNA Replication Depends on Specific and -Acting Activities of Viral Nonstructural Proteins
- A Neuron-Specific Antiviral Mechanism Prevents Lethal Flaviviral Infection of Mosquitoes
- The Aspartate-Less Receiver (ALR) Domains: Distribution, Structure and Function
- Global Genome and Transcriptome Analyses of Epidemic Isolate 98-06 Uncover Novel Effectors and Pathogenicity-Related Genes, Revealing Gene Gain and Lose Dynamics in Genome Evolution
- The Ebola Epidemic Crystallizes the Potential of Passive Antibody Therapy for Infectious Diseases
- Ebola Virus Entry: A Curious and Complex Series of Events
- Conserved Spirosomes Suggest a Single Type of Transformation Pilus in Competence
- Spatial Structure, Transmission Modes and the Evolution of Viral Exploitation Strategies
- Bacterial Cooperation Causes Systematic Errors in Pathogen Risk Assessment due to the Failure of the Independent Action Hypothesis
- Transgenic Fatal Familial Insomnia Mice Indicate Prion Infectivity-Independent Mechanisms of Pathogenesis and Phenotypic Expression of Disease
- Cerebrospinal Fluid Cytokine Profiles Predict Risk of Early Mortality and Immune Reconstitution Inflammatory Syndrome in HIV-Associated Cryptococcal Meningitis
- Utilize Host Actin for Efficient Maternal Transmission in
- Borna Disease Virus Phosphoprotein Impairs the Developmental Program Controlling Neurogenesis and Reduces Human GABAergic Neurogenesis
- An Effector Peptide Family Required for Toll-Mediated Immunity
- Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting Polypeptide
- A Redox Regulatory System Critical for Mycobacterial Survival in Macrophages and Biofilm Development
- Quadruple Quorum-Sensing Inputs Control Virulence and Maintain System Robustness
- Leukocyte-Derived IFN-α/β and Epithelial IFN-λ Constitute a Compartmentalized Mucosal Defense System that Restricts Enteric Virus Infections
- A Strategy for O-Glycoproteomics of Enveloped Viruses—the O-Glycoproteome of Herpes Simplex Virus Type 1
- Macrocyclic Lactones Differ in Interaction with Recombinant P-Glycoprotein 9 of the Parasitic Nematode and Ketoconazole in a Yeast Growth Assay
- Neofunctionalization of the α1,2fucosyltransferase Paralogue in Leporids Contributes to Glycan Polymorphism and Resistance to Rabbit Hemorrhagic Disease Virus
- The Extracytoplasmic Linker Peptide of the Sensor Protein SaeS Tunes the Kinase Activity Required for Staphylococcal Virulence in Response to Host Signals
- Murine CMV-Induced Hearing Loss Is Associated with Inner Ear Inflammation and Loss of Spiral Ganglia Neurons
- Dual miRNA Targeting Restricts Host Range and Attenuates Neurovirulence of Flaviviruses
- GATA-Dependent Glutaminolysis Drives Appressorium Formation in by Suppressing TOR Inhibition of cAMP/PKA Signaling
- Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Infection
- Genetic Analysis Using an Isogenic Mating Pair of Identifies Azole Resistance Genes and Lack of Locus’s Role in Virulence
- A Temporal Gate for Viral Enhancers to Co-opt Toll-Like-Receptor Transcriptional Activation Pathways upon Acute Infection
- Neutrophil Recruitment to Lymph Nodes Limits Local Humoral Response to
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Toxin-Induced Necroptosis Is a Major Mechanism of Lung Damage
- Transgenic Fatal Familial Insomnia Mice Indicate Prion Infectivity-Independent Mechanisms of Pathogenesis and Phenotypic Expression of Disease
- Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Infection
- EphrinA2 Receptor (EphA2) Is an Invasion and Intracellular Signaling Receptor for
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



